
Exhibit 99.1 PRINT® Drug Delivery Technology: Bringing Small Molecule Chemistry to Retinal Disease Casey Kopczynski, PhD Chief Scientific Officer @ OIS ASRS 2021Exhibit 99.1 PRINT® Drug Delivery Technology: Bringing Small Molecule Chemistry to Retinal Disease Casey Kopczynski, PhD Chief Scientific Officer @ OIS ASRS 2021

Important Information The information in this presentation does not contain all of the information that a potential investor should review before investing in Aerie shares. The descriptions of Aerie Pharmaceuticals, Inc. (the “Company” or “Aerie”) in this presentation are qualified in their entirety by reference to reports filed with the SEC. Certain information in this presentation has been obtained from outside sources or is anecdotal in nature. While such information is believed to be reliable for the purposes used herein, no representations are made as to the accuracy or completeness thereof and we take no responsibility for such information. ® ® Any discussion of the potential use or expected success of Rhopressa (netarsudil ophthalmic solution) 0.02% or Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%, with respect to foreign approval or additional indications, and our current or any future product candidates, including AR-1105, AR-13503, AR-14034, AR-6121 and ® ® AR-15512, is subject to regulatory approval. In addition, any discussion of U.S. Food and Drug Administration (“FDA”) approval of Rhopressa or Rocklatan does not guarantee ® ® ® ® successful commercialization of Rhopressa or Rocklatan . For more information on Rhopressa , including prescribing information, refer to the full Rhopressa product label at ® ® www.rhopressa.com. For more information on Rocklatan , including prescribing information, refer to the full Rocklatan product label at www.rocklatan.com. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. This presentation shall not constitute an offer to sell, nor a solicitation of an offer to buy, any of Aerie’s securities. Certain statements in this presentation, including any guidance or timelines presented herein, are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. For example, uncertainties around the duration and severity of the current global COVID-19 pandemic including its possible impact on our clinical and commercial operations and our global supply chain could cause our actual results to be materially different ® than those expressed in our forward-looking statements. In particular, these statements include any discussion of potential commercial sales, placement or utilization of Rocklatan ® ® ® or Rhopressa in the United States or any other market. Likewise, FDA approval of Rhopressa and Rocklatan does not constitute approval of any future product candidates. Any top line data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may ® ® ® ® be disclosed at any time. FDA approval of Rhopressa and Rocklatan also does not constitute regulatory approval of Rhopressa or Rocklatan in jurisdictions outside the United ® ® States and there can be no assurance that we will receive regulatory approval for Rhopressa or Rocklatan in jurisdictions outside the United States. In addition, any discussion in this presentation about preclinical activities or opportunities associated with our products or discussions involving the potential for our dry eye or retinal product candidates are preliminary and the outcome of any studies may not be predictive of the outcome of later trials and ultimate regulatory approval. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. Any statements regarding Aerie’s future liquidity, cash balances or financing transactions also constitute forward-looking statements as are discussions of the possibility of, or possible results of, any commercial transactions or collaborations. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law. 2 For Investor UseImportant Information The information in this presentation does not contain all of the information that a potential investor should review before investing in Aerie shares. The descriptions of Aerie Pharmaceuticals, Inc. (the “Company” or “Aerie”) in this presentation are qualified in their entirety by reference to reports filed with the SEC. Certain information in this presentation has been obtained from outside sources or is anecdotal in nature. While such information is believed to be reliable for the purposes used herein, no representations are made as to the accuracy or completeness thereof and we take no responsibility for such information. ® ® Any discussion of the potential use or expected success of Rhopressa (netarsudil ophthalmic solution) 0.02% or Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%, with respect to foreign approval or additional indications, and our current or any future product candidates, including AR-1105, AR-13503, AR-14034, AR-6121 and ® ® AR-15512, is subject to regulatory approval. In addition, any discussion of U.S. Food and Drug Administration (“FDA”) approval of Rhopressa or Rocklatan does not guarantee ® ® ® ® successful commercialization of Rhopressa or Rocklatan . For more information on Rhopressa , including prescribing information, refer to the full Rhopressa product label at ® ® www.rhopressa.com. For more information on Rocklatan , including prescribing information, refer to the full Rocklatan product label at www.rocklatan.com. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. This presentation shall not constitute an offer to sell, nor a solicitation of an offer to buy, any of Aerie’s securities. Certain statements in this presentation, including any guidance or timelines presented herein, are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. For example, uncertainties around the duration and severity of the current global COVID-19 pandemic including its possible impact on our clinical and commercial operations and our global supply chain could cause our actual results to be materially different ® than those expressed in our forward-looking statements. In particular, these statements include any discussion of potential commercial sales, placement or utilization of Rocklatan ® ® ® or Rhopressa in the United States or any other market. Likewise, FDA approval of Rhopressa and Rocklatan does not constitute approval of any future product candidates. Any top line data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may ® ® ® ® be disclosed at any time. FDA approval of Rhopressa and Rocklatan also does not constitute regulatory approval of Rhopressa or Rocklatan in jurisdictions outside the United ® ® States and there can be no assurance that we will receive regulatory approval for Rhopressa or Rocklatan in jurisdictions outside the United States. In addition, any discussion in this presentation about preclinical activities or opportunities associated with our products or discussions involving the potential for our dry eye or retinal product candidates are preliminary and the outcome of any studies may not be predictive of the outcome of later trials and ultimate regulatory approval. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. Any statements regarding Aerie’s future liquidity, cash balances or financing transactions also constitute forward-looking statements as are discussions of the possibility of, or possible results of, any commercial transactions or collaborations. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law. 2 For Investor Use
Aerie Overview Aerie IOP–Reducing Products (IP 2030+) ® ® • Rhopressa and Rocklatan commercialized in the United States • Glaucoma Franchise Approved in Europe • Globalization Plan Under Way Key Pipeline Activities • AR-15512 TRPM8 agonist for Dry Eye (advancing to Phase 3) • Retina Sustained-Release Implant Platform: • AR-1105 (Dexamethasone; DME Phase 3 preparations underway) • AR-13503 (ROCKi; First-in-human wAMD/DME study in progress) • AR-14034 (Pan-VEGF inhibitor; Preclinical) • AR-6121 ROCKi-linked steroid anti-inflammatory (preclinical) ® ® Rhopressa and Rocklatan have not been approved by any regulatory authority other than the FDA and EMA. AR-15512, AR-1105, AR-13503, AR-14034 and AR-6121 are development stage product candidates and are not approved by any regulatory agency. Dex: Dexamethasone 3 For Investor UseAerie Overview Aerie IOP–Reducing Products (IP 2030+) ® ® • Rhopressa and Rocklatan commercialized in the United States • Glaucoma Franchise Approved in Europe • Globalization Plan Under Way Key Pipeline Activities • AR-15512 TRPM8 agonist for Dry Eye (advancing to Phase 3) • Retina Sustained-Release Implant Platform: • AR-1105 (Dexamethasone; DME Phase 3 preparations underway) • AR-13503 (ROCKi; First-in-human wAMD/DME study in progress) • AR-14034 (Pan-VEGF inhibitor; Preclinical) • AR-6121 ROCKi-linked steroid anti-inflammatory (preclinical) ® ® Rhopressa and Rocklatan have not been approved by any regulatory authority other than the FDA and EMA. AR-15512, AR-1105, AR-13503, AR-14034 and AR-6121 are development stage product candidates and are not approved by any regulatory agency. Dex: Dexamethasone 3 For Investor Use
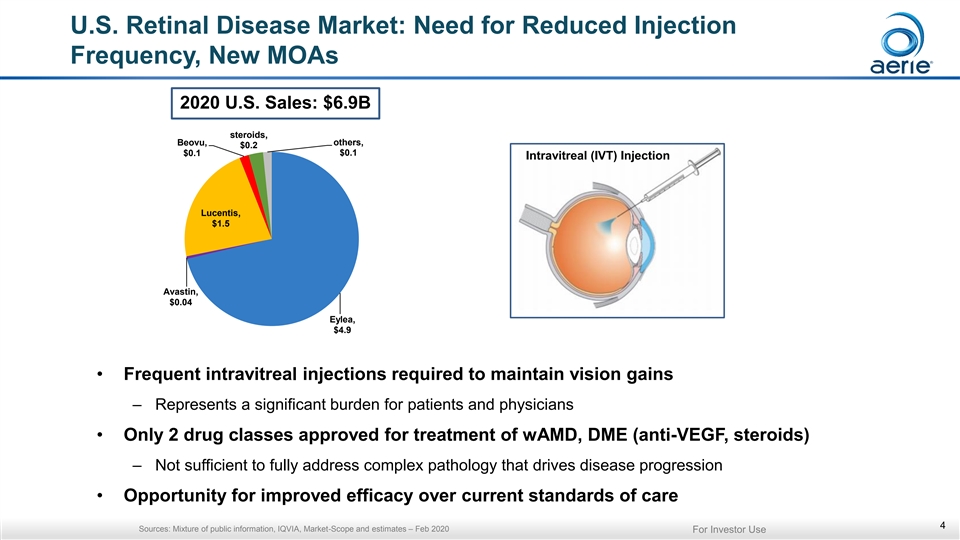
U.S. Retinal Disease Market: Need for Reduced Injection Frequency, New MOAs 2020 U.S. Sales: $6.9B steroids, others, Beovu, $0.2 $0.1 $0.1 Intravitreal (IVT) Injection Lucentis, $1.5 Avastin, $0.04 Eylea, $4.9 • Frequent intravitreal injections required to maintain vision gains – Represents a significant burden for patients and physicians • Only 2 drug classes approved for treatment of wAMD, DME (anti-VEGF, steroids) – Not sufficient to fully address complex pathology that drives disease progression • Opportunity for improved efficacy over current standards of care 4 Sources: Mixture of public information, IQVIA, Market-Scope and estimates – Feb 2020 For Investor UseU.S. Retinal Disease Market: Need for Reduced Injection Frequency, New MOAs 2020 U.S. Sales: $6.9B steroids, others, Beovu, $0.2 $0.1 $0.1 Intravitreal (IVT) Injection Lucentis, $1.5 Avastin, $0.04 Eylea, $4.9 • Frequent intravitreal injections required to maintain vision gains – Represents a significant burden for patients and physicians • Only 2 drug classes approved for treatment of wAMD, DME (anti-VEGF, steroids) – Not sufficient to fully address complex pathology that drives disease progression • Opportunity for improved efficacy over current standards of care 4 Sources: Mixture of public information, IQVIA, Market-Scope and estimates – Feb 2020 For Investor Use
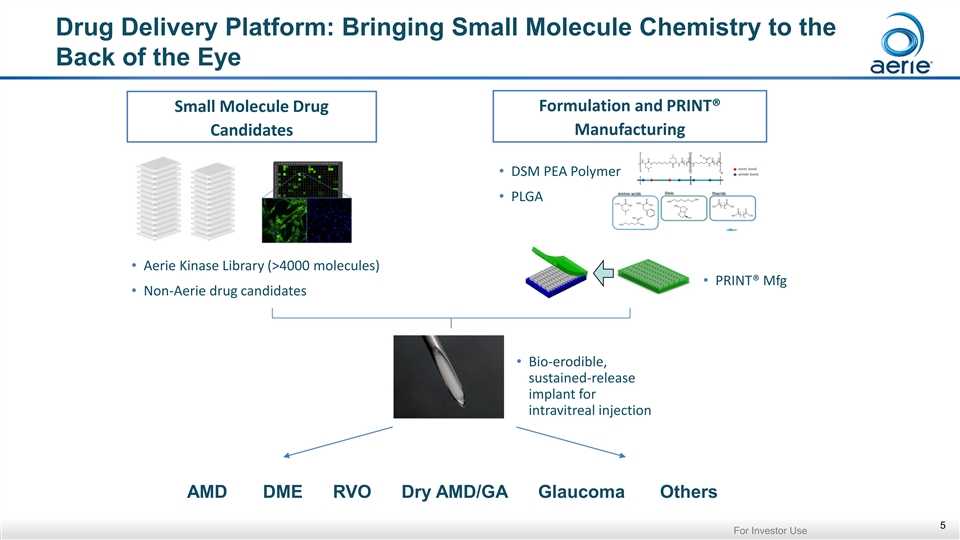
Drug Delivery Platform: Bringing Small Molecule Chemistry to the Back of the Eye Formulation and PRINT® Small Molecule Drug Manufacturing Candidates • DSM PEA Polymer • PLGA • Aerie Kinase Library (>4000 molecules) • PRINT® Mfg • Non-Aerie drug candidates • Bio-erodible, sustained-release implant for intravitreal injection AMD DME RVO Dry AMD/GA Glaucoma Others 5 For Investor UseDrug Delivery Platform: Bringing Small Molecule Chemistry to the Back of the Eye Formulation and PRINT® Small Molecule Drug Manufacturing Candidates • DSM PEA Polymer • PLGA • Aerie Kinase Library (>4000 molecules) • PRINT® Mfg • Non-Aerie drug candidates • Bio-erodible, sustained-release implant for intravitreal injection AMD DME RVO Dry AMD/GA Glaucoma Others 5 For Investor Use
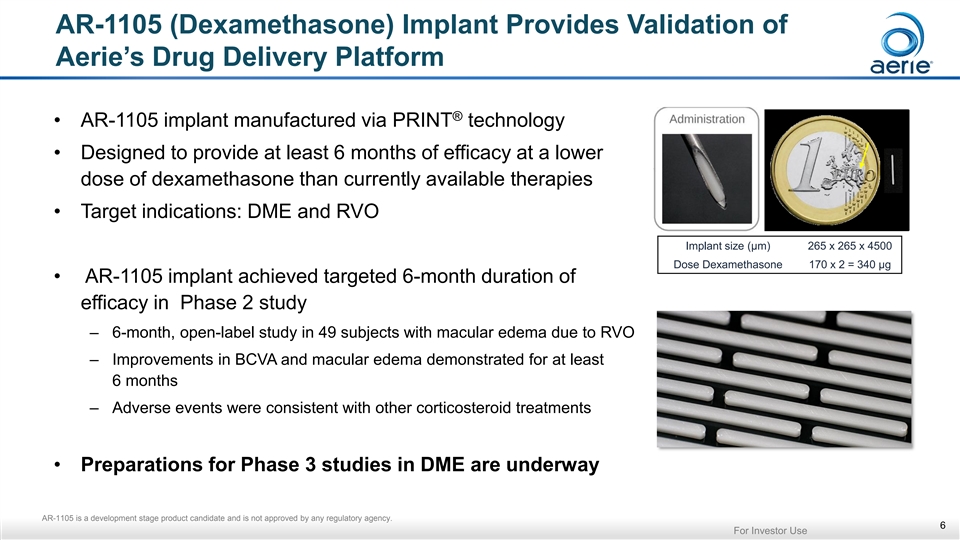
AR-1105 (Dexamethasone) Implant Provides Validation of Aerie’s Drug Delivery Platform ® • AR-1105 implant manufactured via PRINT technology • Designed to provide at least 6 months of efficacy at a lower dose of dexamethasone than currently available therapies • Target indications: DME and RVO Implant size (µm) 265 x 265 x 4500 Dose Dexamethasone 170 x 2 = 340 µg • AR-1105 implant achieved targeted 6-month duration of efficacy in Phase 2 study – 6-month, open-label study in 49 subjects with macular edema due to RVO – Improvements in BCVA and macular edema demonstrated for at least 6 months – Adverse events were consistent with other corticosteroid treatments • Preparations for Phase 3 studies in DME are underway AR-1105 is a development stage product candidate and is not approved by any regulatory agency. 6 For Investor UseAR-1105 (Dexamethasone) Implant Provides Validation of Aerie’s Drug Delivery Platform ® • AR-1105 implant manufactured via PRINT technology • Designed to provide at least 6 months of efficacy at a lower dose of dexamethasone than currently available therapies • Target indications: DME and RVO Implant size (µm) 265 x 265 x 4500 Dose Dexamethasone 170 x 2 = 340 µg • AR-1105 implant achieved targeted 6-month duration of efficacy in Phase 2 study – 6-month, open-label study in 49 subjects with macular edema due to RVO – Improvements in BCVA and macular edema demonstrated for at least 6 months – Adverse events were consistent with other corticosteroid treatments • Preparations for Phase 3 studies in DME are underway AR-1105 is a development stage product candidate and is not approved by any regulatory agency. 6 For Investor Use
AR-1105 Opportunity • The DME market is growing in the United States and abroad – currently over $100M in the U.S. and nearly $300M in Europe • Potential benefits of the 6-month sustained efficacy of AR-1105, if approved: – Highly competitive dosing frequency for both the U.S. and European steroid markets – Opportunity for market expansion as a more favorable treatment alternative for anti-VEGF non-responders – May also benefit physician productivity and overall health economics ® • Aerie’s exclusive PRINT platform may allow for low-cost production and significant pricing flexibility Positive AR-1105 Ph2 topline sustained efficacy data supports advancement as a potentially significant pipeline asset for Aerie, of particular value in Europe 7 AR-1105 is a development stage product candidate and is not approved by any regulatory agency For Investor UseAR-1105 Opportunity • The DME market is growing in the United States and abroad – currently over $100M in the U.S. and nearly $300M in Europe • Potential benefits of the 6-month sustained efficacy of AR-1105, if approved: – Highly competitive dosing frequency for both the U.S. and European steroid markets – Opportunity for market expansion as a more favorable treatment alternative for anti-VEGF non-responders – May also benefit physician productivity and overall health economics ® • Aerie’s exclusive PRINT platform may allow for low-cost production and significant pricing flexibility Positive AR-1105 Ph2 topline sustained efficacy data supports advancement as a potentially significant pipeline asset for Aerie, of particular value in Europe 7 AR-1105 is a development stage product candidate and is not approved by any regulatory agency For Investor Use

AR-13503 Implant: A First-in-Class ROCK/PKC Inhibitor For Retinal Disease AR-13503 Implants Mouse OIR Model in Rabbit Eye Esterases Netarsudil AR-13503 ROCK2 Ki = 1 nM ROCK2 Ki = 0.2 nM • AR-13503 is active metabolite of netarsudil • New MOA has potential to improve outcomes by targeting vascular defects, inflammation, fibrosis and neurodegeneration • Effective as monotherapy and as adjunct to anti-VEGF therapy in preclinical models • Expect implant to provide durable treatment effect with injection frequency of once every 4 – 6 months Ding, J. et al. Invest. Ophthalmol. Vis. Sci. 2019;60(9):5387. Data on File. AR-13503 is an investigational drug not yet approved by any regulatory authority. 8 For Investor UseAR-13503 Implant: A First-in-Class ROCK/PKC Inhibitor For Retinal Disease AR-13503 Implants Mouse OIR Model in Rabbit Eye Esterases Netarsudil AR-13503 ROCK2 Ki = 1 nM ROCK2 Ki = 0.2 nM • AR-13503 is active metabolite of netarsudil • New MOA has potential to improve outcomes by targeting vascular defects, inflammation, fibrosis and neurodegeneration • Effective as monotherapy and as adjunct to anti-VEGF therapy in preclinical models • Expect implant to provide durable treatment effect with injection frequency of once every 4 – 6 months Ding, J. et al. Invest. Ophthalmol. Vis. Sci. 2019;60(9):5387. Data on File. AR-13503 is an investigational drug not yet approved by any regulatory authority. 8 For Investor Use

AR-13503 Implant: 6-month Phase 1 Clinical Study Initiated, Successfully Advanced to Stage 2 Stage 1: Safety in wAMD and DME Stage 2: Safety in DME https://clinicaltrials.gov/ct2/show/NCT03835884?term=AR-13503&draw=2&rank=1. AR-13503 is an investigational drug not yet approved by any regulatory authority. 9 For Investor UseAR-13503 Implant: 6-month Phase 1 Clinical Study Initiated, Successfully Advanced to Stage 2 Stage 1: Safety in wAMD and DME Stage 2: Safety in DME https://clinicaltrials.gov/ct2/show/NCT03835884?term=AR-13503&draw=2&rank=1. AR-13503 is an investigational drug not yet approved by any regulatory authority. 9 For Investor Use
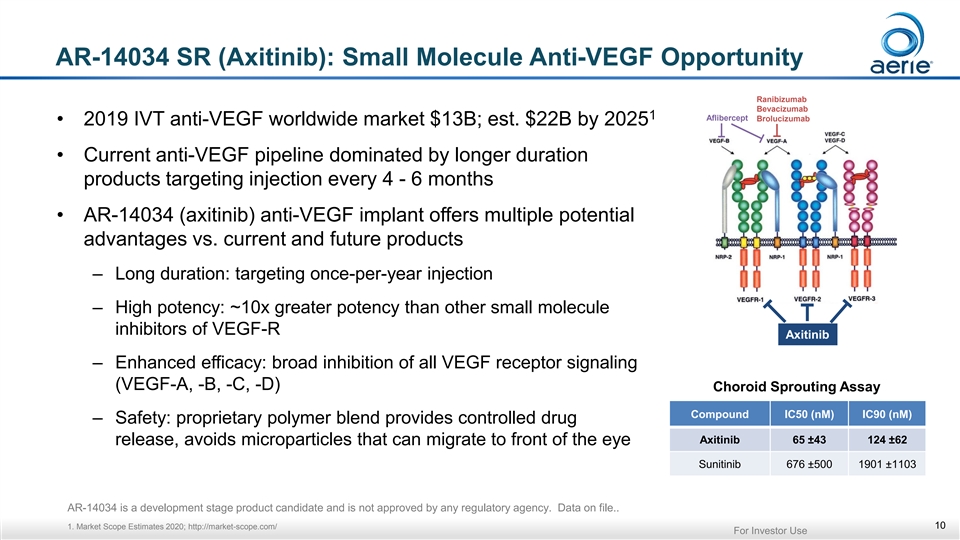
AR-14034 SR (Axitinib): Small Molecule Anti-VEGF Opportunity Ranibizumab Bevacizumab 1 Aflibercept Brolucizumab • 2019 IVT anti-VEGF worldwide market $13B; est. $22B by 2025 • Current anti-VEGF pipeline dominated by longer duration products targeting injection every 4 - 6 months • AR-14034 (axitinib) anti-VEGF implant offers multiple potential advantages vs. current and future products – Long duration: targeting once-per-year injection – High potency: ~10x greater potency than other small molecule inhibitors of VEGF-R Axitinib – Enhanced efficacy: broad inhibition of all VEGF receptor signaling (VEGF-A, -B, -C, -D) Choroid Sprouting Assay Compound IC50 (nM) IC90 (nM) – Safety: proprietary polymer blend provides controlled drug Axitinib 65 ±43 124 ±62 release, avoids microparticles that can migrate to front of the eye Sunitinib 676 ±500 1901 ±1103 AR-14034 is a development stage product candidate and is not approved by any regulatory agency. Data on file.. 1. Market Scope Estimates 2020; http://market-scope.com/ 10 For Investor UseAR-14034 SR (Axitinib): Small Molecule Anti-VEGF Opportunity Ranibizumab Bevacizumab 1 Aflibercept Brolucizumab • 2019 IVT anti-VEGF worldwide market $13B; est. $22B by 2025 • Current anti-VEGF pipeline dominated by longer duration products targeting injection every 4 - 6 months • AR-14034 (axitinib) anti-VEGF implant offers multiple potential advantages vs. current and future products – Long duration: targeting once-per-year injection – High potency: ~10x greater potency than other small molecule inhibitors of VEGF-R Axitinib – Enhanced efficacy: broad inhibition of all VEGF receptor signaling (VEGF-A, -B, -C, -D) Choroid Sprouting Assay Compound IC50 (nM) IC90 (nM) – Safety: proprietary polymer blend provides controlled drug Axitinib 65 ±43 124 ±62 release, avoids microparticles that can migrate to front of the eye Sunitinib 676 ±500 1901 ±1103 AR-14034 is a development stage product candidate and is not approved by any regulatory agency. Data on file.. 1. Market Scope Estimates 2020; http://market-scope.com/ 10 For Investor Use

AR-14034 SR (Axitinib): Preclinical Results Support Up to 12 Months Duration in Clinic Cumulative Cumulative Drug Release In Vitro Release Rate in 1X PBS w/0.5% TWEEN 20 100 In vitro: In vivo Comparison 80 Percent drug released Time 60 In vitro In rabbits Day 14/16 10% 6-9% 40 Day 29/31 17% 14% 20 Month 5 60% 50-60% 0 Days • Proprietary polymer blend produces optimal elution rate over time • Drug release rate in rabbits predicts up to 12-months duration (once a year injection) in clinic – Aerie rabbit data accurately predicted AR-1105 6-month duration in clinic IND-enabling preclinical studies underway; IND filing planned for 2H 2022 AR-14034 is a development stage product candidate and is not approved by any regulatory agency. 11 Data on File For Investor Use 0 28 56 84 112 140 168 196 224 252 280 % ReleasedAR-14034 SR (Axitinib): Preclinical Results Support Up to 12 Months Duration in Clinic Cumulative Cumulative Drug Release In Vitro Release Rate in 1X PBS w/0.5% TWEEN 20 100 In vitro: In vivo Comparison 80 Percent drug released Time 60 In vitro In rabbits Day 14/16 10% 6-9% 40 Day 29/31 17% 14% 20 Month 5 60% 50-60% 0 Days • Proprietary polymer blend produces optimal elution rate over time • Drug release rate in rabbits predicts up to 12-months duration (once a year injection) in clinic – Aerie rabbit data accurately predicted AR-1105 6-month duration in clinic IND-enabling preclinical studies underway; IND filing planned for 2H 2022 AR-14034 is a development stage product candidate and is not approved by any regulatory agency. 11 Data on File For Investor Use 0 28 56 84 112 140 168 196 224 252 280 % Released
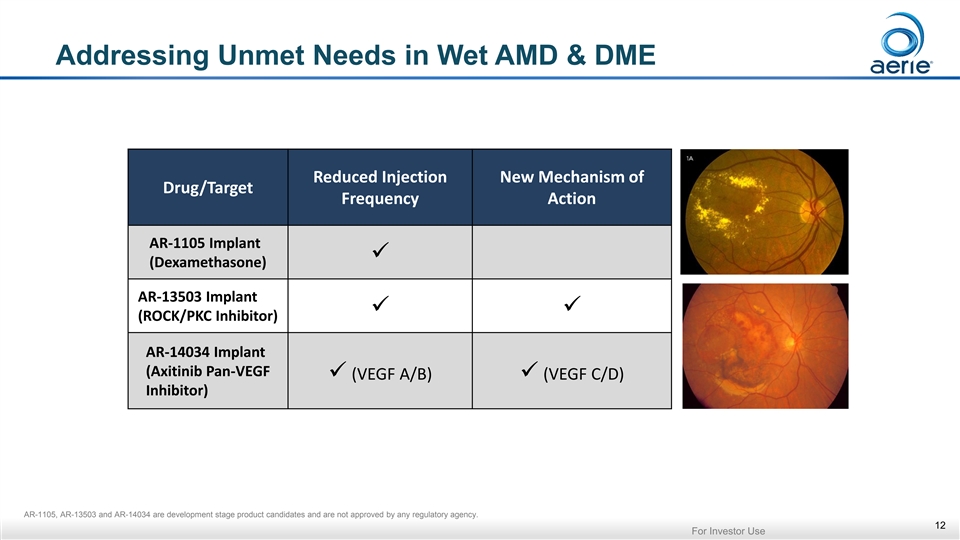
Addressing Unmet Needs in Wet AMD & DME Reduced Injection New Mechanism of Drug/Target Frequency Action AR-1105 Implant ✓ (Dexamethasone) AR-13503 Implant ✓✓ (ROCK/PKC Inhibitor) AR-14034 Implant (Axitinib Pan-VEGF ✓ (VEGF A/B)✓ (VEGF C/D) Inhibitor) AR-1105, AR-13503 and AR-14034 are development stage product candidates and are not approved by any regulatory agency. 12 For Investor UseAddressing Unmet Needs in Wet AMD & DME Reduced Injection New Mechanism of Drug/Target Frequency Action AR-1105 Implant ✓ (Dexamethasone) AR-13503 Implant ✓✓ (ROCK/PKC Inhibitor) AR-14034 Implant (Axitinib Pan-VEGF ✓ (VEGF A/B)✓ (VEGF C/D) Inhibitor) AR-1105, AR-13503 and AR-14034 are development stage product candidates and are not approved by any regulatory agency. 12 For Investor Use

THANK YOUTHANK YOU












