
Exhibit 99.1 AERIE An Emerging Leader in Ophthalmology Ap r i l 2 0 2 2 For Investor Use Only 1

Disclaimer The information in this presentation regarding Aerie Pharmaceuticals, Inc. (the “Company” or “Aerie”) does not contain all of the information that a potential investor should review before investing in Aerie shares. The descriptions of Aerie in this presentation are qualified in their entirety by reference to the Company’s reports filed with the Securities and Exchange Commission (SEC). Certain information in this presentation has been obtained from outside sources or is anecdotal in nature. While such information is believed to be reliable for the purposes used herein, no representations are made as to the accuracy or completeness thereof and we take no responsibility for such information. ® ® Any discussion of the potential use or expected success of Rhopressa (netarsudil ophthalmic solution) 0.02% or Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%, with respect to foreign approval or additional indications, and our current or any future product candidates, including AR-1105, AR-13503, AR-14034, AR-6121 and AR-15512, is subject to regulatory approval. In addition, any ® ® ® ® discussion of U.S. Food and Drug Administration (“FDA”) approval of Rhopressa or Rocklatan does not guarantee successful commercialization of Rhopressa or Rocklatan . For more information on ® ® ® Rhopressa , including prescribing information, refer to the full Rhopressa product label at www.rhopressa.com. For more information on Rocklatan , including prescribing information, refer to the full ® Rocklatan product label at www.rocklatan.com. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise, except as otherwise required by law. We are not making any representation or warranty that the information in this presentation is accurate or complete. This presentation shall not constitute an offer to sell, nor a solicitation of an offer to buy, any of Aerie’s securities. This presentation contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “exploring,” “pursuing” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Forward-looking statements in this presentation include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our commercial franchise and our pipeline, any guidance or timelines, cash burn rate, future liquidity, cash balances or financing transactions, our ongoing and anticipated preclinical studies and clinical trials, FDA or other regulatory approvals and effectiveness of any product, product candidates or future product candidates. In addition, any top line data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may be disclosed at any time. Furthermore, any discussion in this presentation about preclinical activities or opportunities associated with our products or discussions involving the potential for our product candidates are preliminary and the outcome of any studies may not be predictive of the outcome of later trials and ultimate regulatory approval. Any future clinical trial results may not demonstrate safety and efficacy sufficient to obtain regulatory approval related to the preclinical research findings discussed in this presentation. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, industry change and other factors beyond our control, and depend on regulatory approvals and economic and other environmental circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. We discuss many of these risks in greater detail under the heading “Risk Factors” in the quarterly and annual reports that we file with the Securities and Exchange Commission (SEC). Forward-looking statements are not guarantees of future performance and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements ® ® contained in this presentation. In particular, FDA and European Medicines Agency (EMA) approval of Rhopressa and Rocklatan , and Medicines and Healthcare products Regulatory Agency (MHRA) ® ® ® ® authorization of Roclanda does not guarantee regulatory approval of Rhopressa , Rocklatan or Roclanda in other jurisdictions, and there can be no assurance that we will receive regulatory approval for ® ® ® ® ® Rhopressa , Rocklatan or Roclanda in such other jurisdictions. In addition, FDA approval of Rhopressa and Rocklatan does not guarantee FDA approval of our product candidates or any future product candidates and there can be no assurance that we will receive FDA approval for our product candidates or any future product candidates. Furthermore, the acceptance of the Investigational New Drug Applications by the FDA for our product candidates does not guarantee FDA approval of such product candidates and the outcomes of later clinical trials for our product candidates may not be sufficient to submit a New Drug Application (NDA) with the FDA or to receive FDA approval. Any forward-looking statements that we make in this presentation speak only as of the date of this presentation. We assume no obligation to update our forward-looking statements whether as a result of new information, future events or otherwise, after the date of this presentation. 2 For Investor Use Only

An Emerging Leader in Ophthalmology ® ® Rhopressa /Rocklatan Growing Glaucoma franchise: Commercial-Stage • U.S.: 2021 Net Product Revenue of $112MM; 2022 guidance: $130-140MM Ophthalmology Company • ROW: Partnership with Santen: EU: Approved, Japan: Phase 3 Two Phase 3-ready programs (in Dry Eye and in DME) Differentiated Pipeline ® Developing an early-stage pipeline including leveraging PRINT implant technology Ex-U.S. partnering deals strengthen balance sheet Partnering & Manufacturing State-of-the-art manufacturing, with potential for improving efficiencies $140MM in cash* as of 12/31/2021 Funded to Execute on Plan $90MM received from Santen in January 2022 *Cash is comprised of cash, cash equivalents and short-term investments, per the Q4: 2021 10K filing. 3 For Investor Use Only
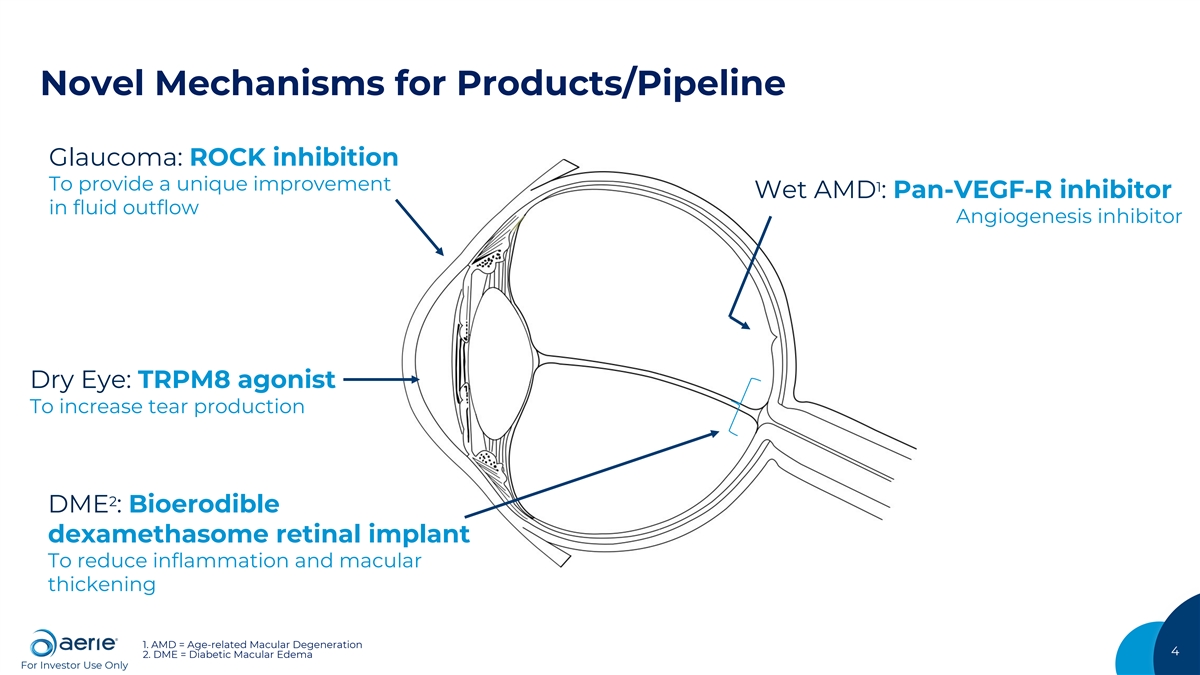
Novel Mechanisms for Products/Pipeline Glaucoma: ROCK inhibition To provide a unique improvement 1 Wet AMD : Pan-VEGF-R inhibitor in fluid outflow Angiogenesis inhibitor Dry Eye: TRPM8 agonist To increase tear production 2 DME : Bioerodible dexamethasome retinal implant To reduce inflammation and macular thickening 1. AMD = Age-related Macular Degeneration 4 2. DME = Diabetic Macular Edema For Investor Use Only

Aerie Product Pipeline Targets Attractive Commercial Markets ® Two Phase 3 Ready Programs plus “PRINT ” Technology Platform Product/Indication R&D Phase 1 Phase 2 Phase 3 Marketed Status/Next Steps ® Rhopressa / On the Market in U.S. ® Rocklatan : Approved in EU, Phase 3 in Japan Glaucoma AR-15512 Phase 3 program to begin in Q2:22 Dry Eye AR-1105 Evaluate options for Phase 3 DME development AR-14034 IND Filing planned in H2:22 Wet AMD 5 For Investor Use Only

Aerie 2.0: Strategic pillars for long-term success Growing Strong Financial Robust Pipeline Commercial Position Business Reducing our annual cash burn Driving sustainable growth of Making smart choices with rate in maintaining a solid the commercial business capital in advancing our pipeline financial position and focus on ROIC Attracting the right talent and leadership to support sustainable growth 6 For Investor Use Only
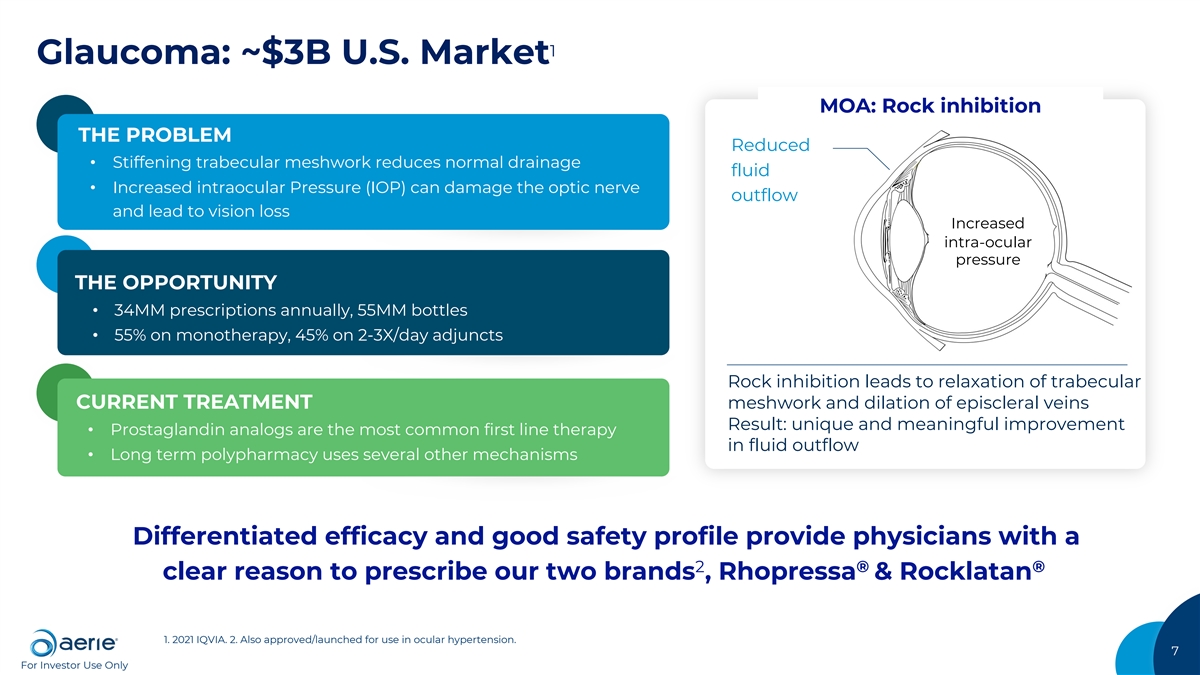
1 Glaucoma: ~$3B U.S. Market MOA: Rock inhibition THE PROBLEM Reduced • Stiffening trabecular meshwork reduces normal drainage fluid • Increased intraocular Pressure (IOP) can damage the optic nerve outflow and lead to vision loss Increased intra-ocular pressure THE OPPORTUNITY • 34MM prescriptions annually, 55MM bottles • 55% on monotherapy, 45% on 2-3X/day adjuncts Rock inhibition leads to relaxation of trabecular CURRENT TREATMENT meshwork and dilation of episcleral veins Result: unique and meaningful improvement • Prostaglandin analogs are the most common first line therapy in fluid outflow • Long term polypharmacy uses several other mechanisms Differentiated efficacy and good safety profile provide physicians with a 2 ® ® clear reason to prescribe our two brands , Rhopressa & Rocklatan 1. 2021 IQVIA. 2. Also approved/launched for use in ocular hypertension. 7 For Investor Use Only
Glaucoma Franchise • Consistently lower IOP by 20% • Regardless of baseline pressure Refreshed Branding Strategy: First Line or First Switch 8 For Investor Use Only
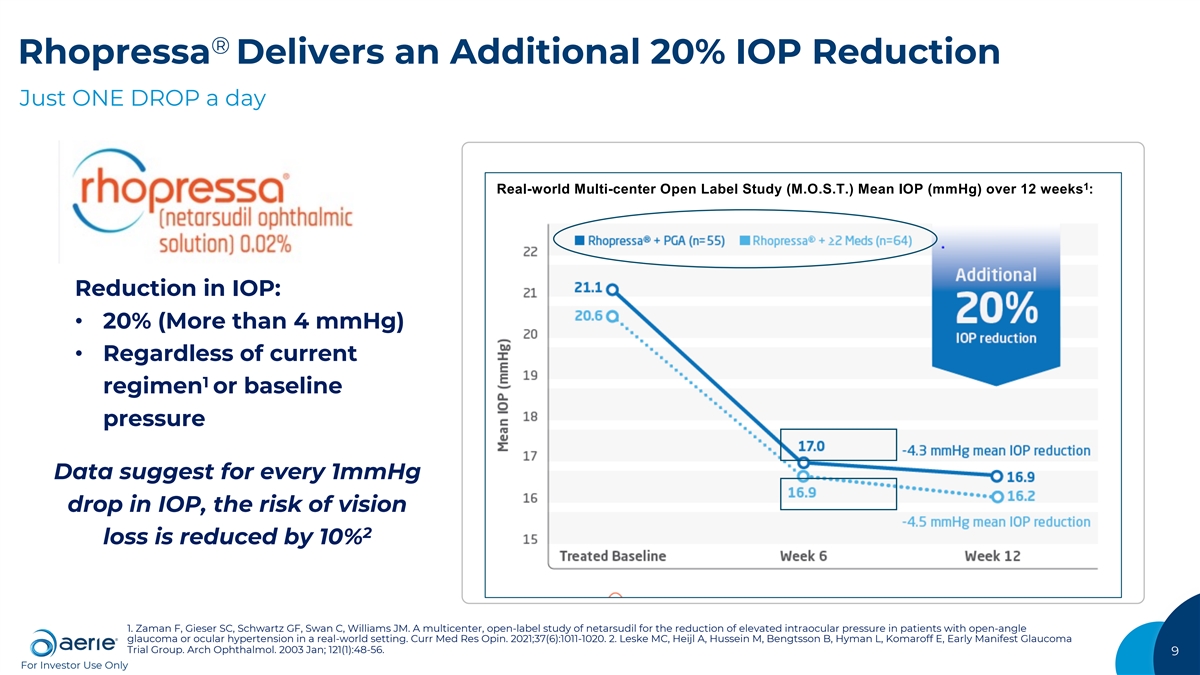
® Rhopressa Delivers an Additional 20% IOP Reduction Just ONE DROP a day 1 Real-world Multi-center Open Label Study (M.O.S.T.) Mean IOP (mmHg) over 12 weeks : Reduction in IOP: • 20% (More than 4 mmHg) • Regardless of current 1 regimen or baseline pressure Data suggest for every 1mmHg drop in IOP, the risk of vision 2 loss is reduced by 10% 1. Zaman F, Gieser SC, Schwartz GF, Swan C, Williams JM. A multicenter, open-label study of netarsudil for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension in a real-world setting. Curr Med Res Opin. 2021;37(6):1011-1020. 2. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, Early Manifest Glaucoma Trial Group. Arch Ophthalmol. 2003 Jan; 121(1):48-56. 9 For Investor Use Only

® Rocklatan Refreshed branding as first-line/first switch ® Rocklatan (netarsudil/latanoprost combo) is the only 1 once-daily, fixed-dose combination treatment in the U.S. 1,2 Achieves superior IOP reduction vs. latanoprost-- the 2 world’s most prescribed glaucoma treatment Effective, regardless of current regimen. No reported 3 systemic effects ® ® 4 Patent coverage (Rocklatan & Rhopressa ) through 2034 ® 1. Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% Prescribing Information, Aerie Pharmaceuticals, Inc., Irvine, Calif. 2020. 2. Asrani, S., Bacharach, J., Holland, E. et al. Fixed-Dose Combination of Netarsudil and Latanoprost in Ocular Hypertension and Open-Angle Glaucoma: Pooled Efficacy/Safety Analysis of Phase 3 MERCURY-1 and -2. Adv Ther 37, 10 1620–1631 (2020). For Investor Use Only
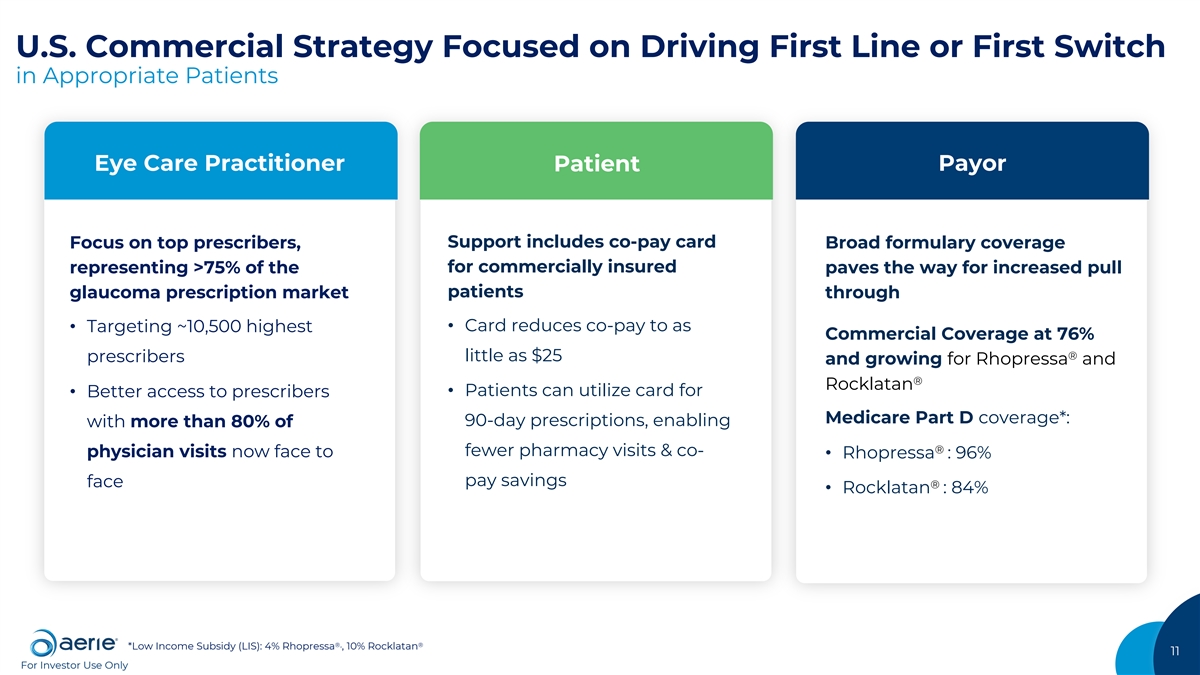
U.S. Commercial Strategy Focused on Driving First Line or First Switch in Appropriate Patients Eye Care Practitioner Patient Payor Support includes co-pay card Focus on top prescribers, Broad formulary coverage for commercially insured representing >75% of the paves the way for increased pull patients glaucoma prescription market through • Card reduces co-pay to as • Targeting ~10,500 highest Commercial Coverage at 76% ® little as $25 prescribers and growing for Rhopressa and ® Rocklatan • Patients can utilize card for • Better access to prescribers Medicare Part D coverage*: 90-day prescriptions, enabling with more than 80% of ® fewer pharmacy visits & co- physician visits now face to • Rhopressa : 96% pay savings face ® • Rocklatan : 84% ®, ® *Low Income Subsidy (LIS): 4% Rhopressa , 10% Rocklatan 11 For Investor Use Only

Global Studies and Medical Affairs Program Intended to support generation of meaningful clinical data and improved patient outcomes nd 2 Phase 3 Registration Trial Underway in Japan by Santen: st • Positive results from 1 Phase 3 trial reported by Santen in October 2021 nd • A 2 confirmatory Phase 3 trial is underway Upcoming Medical Meetings Presentations: • 3 abstracts to be presented by Aerie at ARVO and ASCRS • Plus 10 abstracts related to Investigator Initiated Trials and Independent Trials ® Rocklatan Phase 4 Program: ® • Designed to demonstrate Rocklatan as a highly effective single bottle 1X/day therapy • Initiated in the U.S. in March 2022 12 For Investor Use Only

1 Dry Eye: ~$1.6B U.S. Market MOA: TRPM8 agonism Inadequate tear THE PROBLEM production • The need for a chronic treatment that can provide rapid and more & quality effective relief of Dry Eye symptoms TRPM8 receptor THE OPPORTUNITY • 30MM patients (18MM diagnosed) • <10% treated by Rx TRPM8 agonism leads to stimulation of cold sensing receptors on the cornea and eyelid CURRENT TREATMENT Result: increased natural tear production; • OTC artificial tears, Rx anti-inflammatories and nasal tear cooling sensation on the ocular surface stimulants AR-15512 is a differentiated, novel, first-in-class product candidate for the treatment of the signs and symptoms of Dry Eye. Phase 3 Program to begin in Q2:2022 1. Market Scope 2021 Dry Eye Product Market Review 13 AR-15512 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only
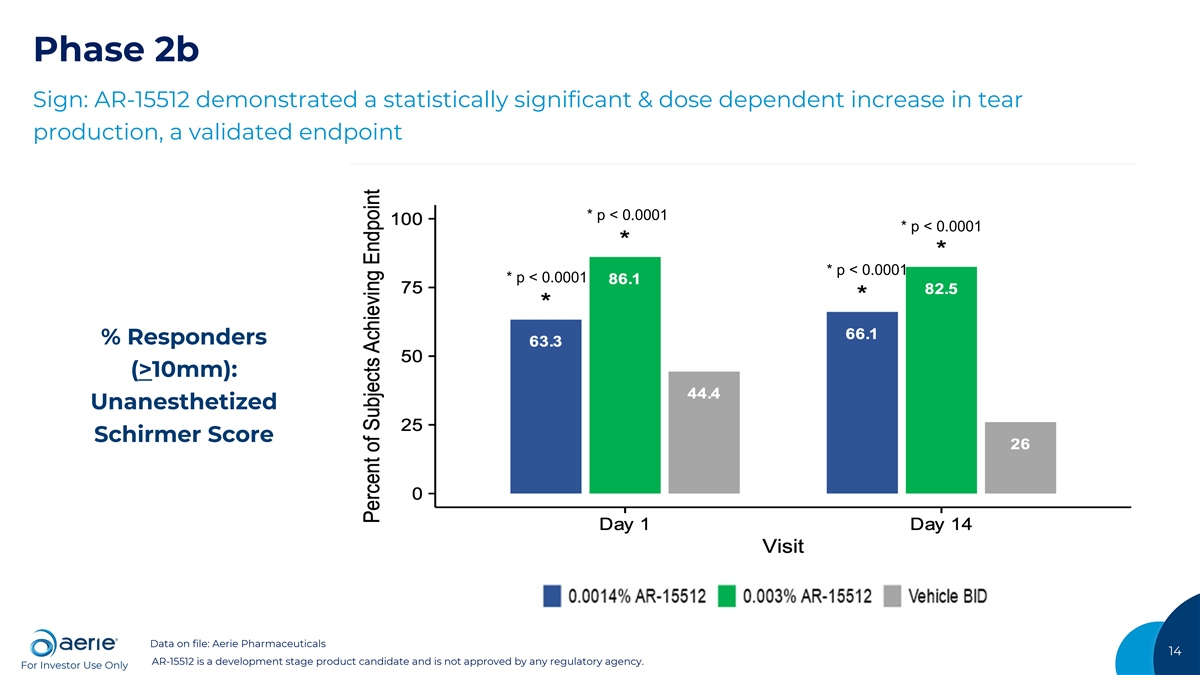
Phase 2b Sign: AR-15512 demonstrated a statistically significant & dose dependent increase in tear production, a validated endpoint * p < 0.0001 * p < 0.0001 * p < 0.0001 * p < 0.0001 % Responders (>10mm): Unanesthetized Schirmer Score Data on file: Aerie Pharmaceuticals 14 AR-15512 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only
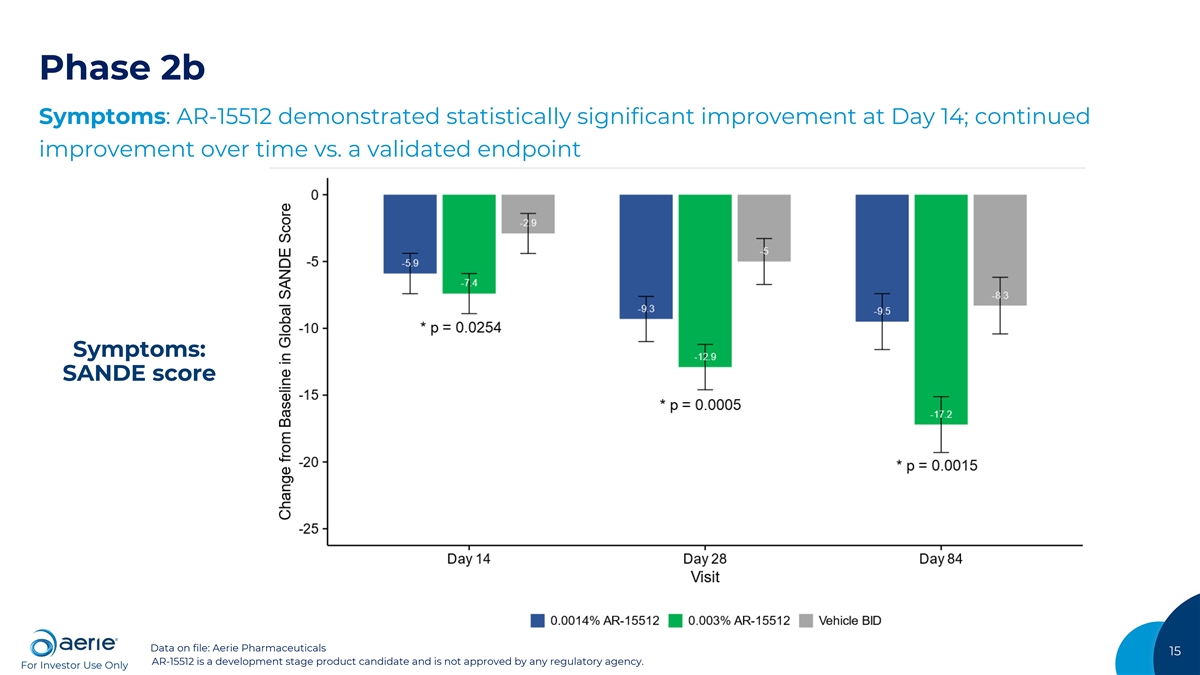
Phase 2b Symptoms: AR-15512 demonstrated statistically significant improvement at Day 14; continued improvement over time vs. a validated endpoint Symptoms: SANDE score Data on file: Aerie Pharmaceuticals 15 AR-15512 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only

Totality of AR-15512 Phase 2b Data Support Next Steps Statistically significant, dose-dependent improvements seen across multiple validated sign, symptom and Quality of Life endpoints: o Sign: a statistically significant and very robust increase in tear production as early as Day 1. The magnitude stayed nearly identical at Day 14 Phase 2b Results* o Symptoms: a statistically significant improvement, in favor of AR-15512, found as early as Day 14, based on SANDE score o Statistically significant improvements in ocular discomfort, eye dryness and numerous measure of daily living activities Good tolerability profile, low discontinuations (no difference across groups) Brief, mild sensation immediately after dosing in ~40% of treated subjects Phase 2b Safety Data No systemic or serious adverse events attributed to study medication Successful alignment meeting with the FDA. Phase 3 program to include: 2 Next Steps efficacy studies and a 12-month long term safety study. The first study is expected to begin in Q2:22 16 *Primary endpoints not achieved. AR-15512 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only

1 MOA: bioerodible DME: ~$2B U.S. Market dexamethasone retinal impact Macular THE PROBLEM edema • Inflammation and thickening of the macula (macular edema) • Can lead to loss of vision CURRENT TREATMENT • Angiogenesis inhibitors & steroids. Patients & providers are dissatisfied with frequent high-cost intravitreal injections required to maintain vision gains The first product to use Aerie’s ® exclusive PRINT delivery platform AR-1105 is the only bioerodible dexamethasone retinal implant to demonstrate 6 months of efficacy in treating macular edema which provides reduced injection frequency 1. 2021 Market Scope Retina Report. 17 AR-1105 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only
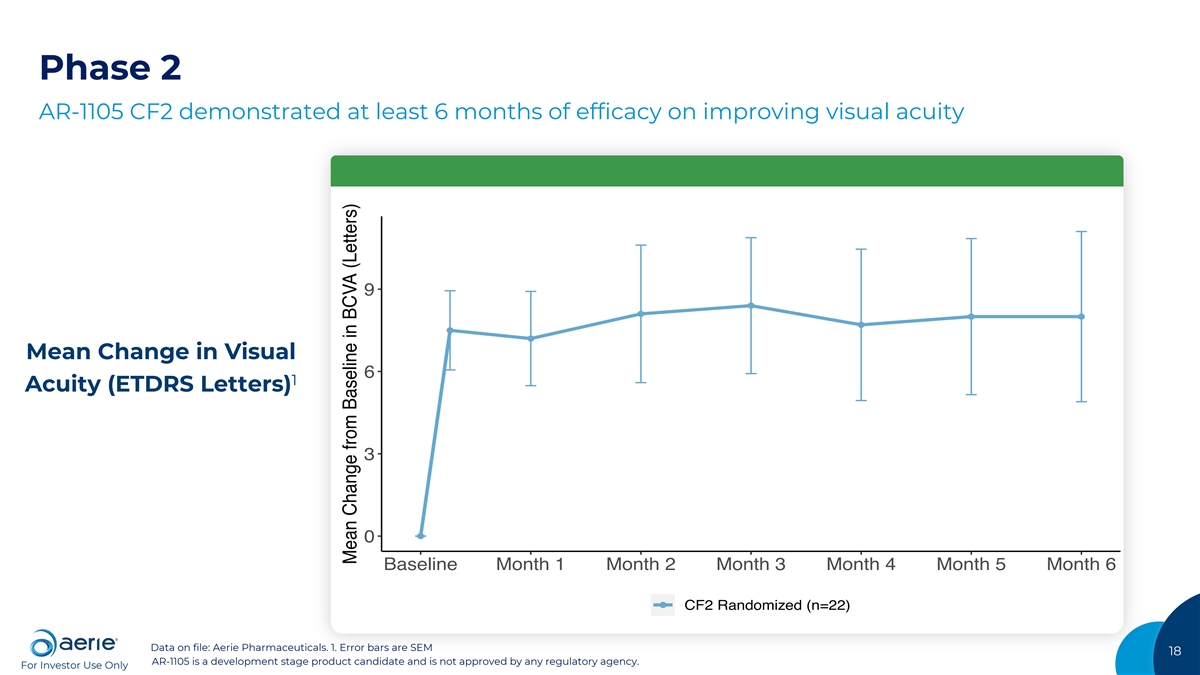
Phase 2 AR-1105 CF2 demonstrated at least 6 months of efficacy on improving visual acuity 9 Mean Change in Visual 6 1 Acuity (ETDRS Letters) 3 0 Baseline Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 CF2 Randomized (n=22) Data on file: Aerie Pharmaceuticals. 1. Error bars are SEM 18 AR-1105 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only Source: Table 14.2.1.2.1; error bars are SEM Mean Change from Baseline in BCVA (Letters)
AR-1105 Phase 2 Proof-of-Concept Data Support Next Steps At least 6 months efficacy: improved visual acuity and reducing macular edema Phase 2 Efficacy Data Both formulations well tolerated; no unexpected findings Phase 2 Safety Data Adverse events consistent with other corticosteroids & intravitreal injections Next Step Evaluate options for Phase 3 development AR-1105 is a development stage product candidate and is not approved by any regulatory agency. 19 For Investor Use Only
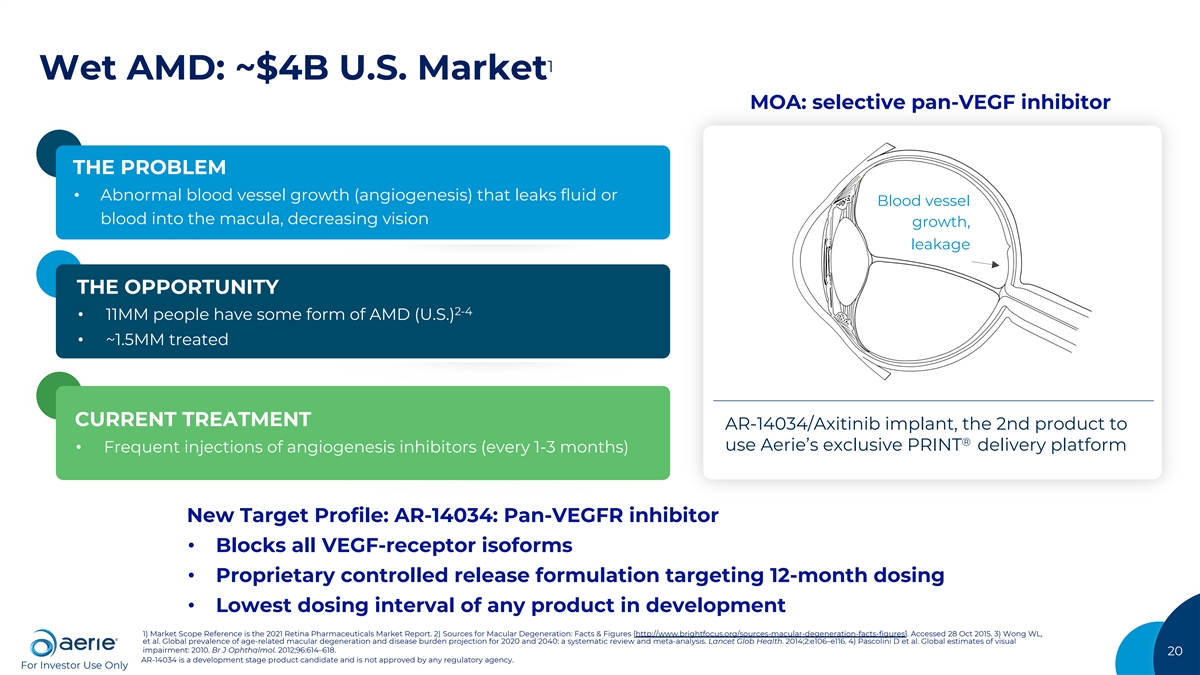
1 Wet AMD: ~$4B U.S. Market MOA: selective pan-VEGF inhibitor THE PROBLEM • Abnormal blood vessel growth (angiogenesis) that leaks fluid or Blood vessel blood into the macula, decreasing vision growth, leakage THE OPPORTUNITY 2-4 • 11MM people have some form of AMD (U.S.) • ~1.5MM treated CURRENT TREATMENT AR-14034/Axitinib implant, the 2nd product to ® use Aerie’s exclusive PRINT delivery platform • Frequent injections of angiogenesis inhibitors (every 1-3 months) New Target Profile: AR-14034: Pan-VEGFR inhibitor • Blocks all VEGF-receptor isoforms • Proprietary controlled release formulation targeting 12-month dosing • Lowest dosing interval of any product in development 1) Market Scope Reference is the 2021 Retina Pharmaceuticals Market Report. 2) Sources for Macular Degeneration: Facts & Figures [http://www.brightfocus.org/sources-macular-degeneration-facts-figures]. Accessed 28 Oct 2015. 3) Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. 4) Pascolini D et al. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. 20 AR-14034 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only
Positive AR-14034 Preclinical Data Support Planned IND Filing H2:22 ® Axitinib is a highly-selective Pan-VEGF blocker (Pfizer’s INLYTA ) Preclinical POC Data Preclinical data supports target 12-month release profile Pre-clinical Safety Data No ocular safety concerns Status IND-enabling studies underway Regulatory Targeting an H2:2022 IND filing 21 AR-14034 is a development stage product candidate and is not approved by any regulatory agency. For Investor Use Only
Partnership Opportunities Santen Glaucoma Agreement ® ® • Rhopressa /Rocklatan • Japan, East Asia, Europe, China, India, CIS* countries, parts of Latin America and Oceania Other Ex-U.S. Out-Licensing Opportunities • AR-15512, AR-1105, AR-14034 Leverage Manufacturing • Improve Athlone, Ireland capacity utilization Strengthening Balance Sheet • Upfront/milestone payments *CIS: Commonwealth of Independent States 22 For Investor Use Only
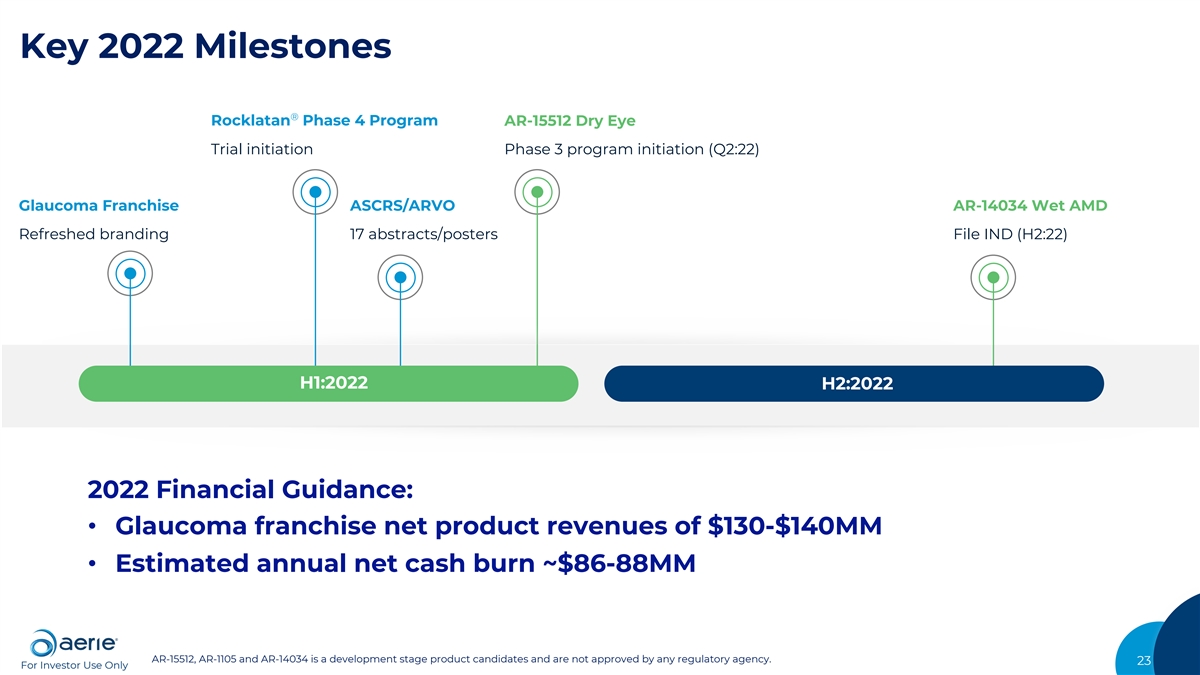
Key 2022 Milestones ® Rocklatan Phase 4 Program AR-15512 Dry Eye Trial initiation Phase 3 program initiation (Q2:22) Glaucoma Franchise ASCRS/ARVO AR-14034 Wet AMD Refreshed branding 17 abstracts/posters File IND (H2:22) H1:2022 H2:2022 2022 Financial Guidance: • Glaucoma franchise net product revenues of $130-$140MM • Estimated annual net cash burn ~$86-88MM AR-15512, AR-1105 and AR-14034 is a development stage product candidates and are not approved by any regulatory agency. 23 For Investor Use Only

Aerie 2.0: Strategic pillars for long-term success Growing Strong Financial Robust Pipeline Commercial Position Business Reducing our annual cash burn Driving sustainable growth of Making smart choices with rate in maintaining a solid the commercial business capital in advancing our pipeline financial position and focus on ROIC Attracting the right talent and leadership to support sustainable growth 24 For Investor Use Only
An Emerging Leader in Ophthalmology Carolyn McAuliffe Hans Vitzthum ir@aeriepharma.com www.aeriepharma.com For Investor Use Only
























