QuickLinks -- Click here to rapidly navigate through this documentAs filed with the Securities and Exchange Commission on February 13, 2006
Registration No. 333-130470
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2
to
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ACCELLENT INC.
(Exact name of Registrant as specified in its charter)
Maryland
(State or other jurisdiction of
incorporation or organization) | | 3841
(Primary Standard Industrial
Classification Code Number) | | 84-1507827
(I.R.S. Employer
Identification Number) |
(see following pages for additional registrants)
100 Fordham Road
Wilmington, Massachusetts 01887
(978) 570-6900
(Address, including zip code, and telephone number, including
area code, of Registrant's principal executive offices)
Stewart A. Fisher
Chief Financial Officer, Executive Vice President, Treasurer and Secretary
Accellent Inc.
100 Fordham Road
Wilmington, Massachusetts 01887
(978) 570-6900
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies of all communications, including communications sent to agent for service, should be sent to:
Joseph H. Kaufman, Esq.
Simpson Thacher & Bartlett LLP
425 Lexington Avenue
New York, NY 10017
(212) 455-2000
Approximate date of commencement of proposed sale to public:
As soon as practicable after this Registration Statement becomes effective.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of
Securities to be Registered
| | Amount to
be Registered
| | Proposed Maximum
Offering Price
Per Unit(1)
| | Proposed Maximum
Aggregate
Offering Price(1)
| | Amount of
Registration Fee
|
|---|
|
| 101/2% Senior Subordinated Exchange Notes due 2013.. | | $305,000,000 | | 100% | | $305,000,000 | | $32,635.00(2) |
|
| Guarantees of 101/2% Senior Subordinated Exchange Notes due 2013(3) | | N/A(4) | | (4) | | (4) | | (4) |
|
- (1)
- Estimated solely for purposes of computing the registration fee pursuant to Rule 457(f) under the Securities Act of 1933, as amended (the "Securities Act").
- (2)
- Previously paid.
- (3)
- See inside facing page for additional registrant guarantors.
- (4)
- Pursuant to Rule 457(n) under the Securities Act, no separate filing fee is required for the guarantees.
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
TABLE OF ADDITIONAL REGISTRANT GUARANTORS
Exact Name of Registrant as Specified in its Charter(1)
| | State or Other
Jurisdiction of
Incorporation or
Organization
| | I.R.S. Employer
Identification
Number
| | Industrial
Classification Code
Number
|
|---|
| Accellent Corp. | | Colorado | | 91-2054669 | | 3841 |
| American Technical Molding, Inc. | | California | | 99-0266738 | | 3841 |
| Brimfield Acquisition Corp. | | Delaware | | 51-0386457 | | 3841 |
| Brimfield Precision, LLC | | Delaware | | 04-3457459 | | 3841 |
| CE Huntsville Holdings Corp. | | Delaware | | 54-2181917 | | 3841 |
| Cycam, Inc. | | Pennsylvania | | 25-1567669 | | 3841 |
| ELX, Inc. | | Pennsylvania | | 25-1711485 | | 3841 |
| G&D, Inc. d/b/a Star Guide Corporation | | Colorado | | 84-0718817 | | 3841 |
| Hayden Precision Industries, LLC | | Delaware | | 16-1564447 | | 3841 |
| Kelco Acquisition, LLC | | Delaware | | 52-2139676 | | 3841 |
| Machining Technology Group, LLC | | Tennessee | | 62-1755768 | | 3841 |
| MedSource Technologies, Inc. | | Delaware | | 52-2094496 | | 3841 |
| MedSource Technologies, LLC | | Delaware | | 41-1934170 | | 3841 |
| MedSource Technologies, Newton Inc. | | Delaware | | 41-1990432 | | 3841 |
| MedSource Technologies Pittsburgh, Inc. | | Delaware | | 04-3710128 | | 3841 |
| MedSource Trenton, Inc. | | Delaware | | 32-0000036 | | 3841 |
| Micro-Guide, Inc. | | California | | 95-1866997 | | 3841 |
| National Wire & Stamping, Inc. | | Colorado | | 84-0485552 | | 3841 |
| Noble-Met, Ltd. | | Virginia | | 54-1480585 | | 3841 |
| Portlyn, LLC | | Delaware | | 02-0506852 | | 3841 |
| Spectrum Manufacturing, Inc. | | Nevada | | 36-2997517 | | 3841 |
| Tenax, LLC | | Delaware | | 06-1567572 | | 3841 |
| Texcel, Inc. | | Massachusetts | | 04-2973748 | | 3841 |
| Thermat Acquisition Corp. | | Delaware | | 52-2235950 | | 3841 |
| UTI Corporation | | Pennsylvania | | 23-1721795 | | 3841 |
| UTI Holding Company | | Delaware | | 51-0407158 | | 3841 |
| Venusa, Ltd. | | New York | | 13-3029017 | | 3841 |
- (1)
- The address and telephone number of each co-registrant's principal executive offices is 100 Fordham Road, Wilmington, Massachusetts 01887, (978) 570-6900.
The information in this prospectus is not complete and may be changed. We may not sell the securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED FEBRUARY 13, 2006
$305,000,000

Accellent Inc.
Offer to Exchange all outstanding $305,000,000 101/2% Senior Subordinated Notes due 2013 for an equal amount of 101/2% Senior Subordinated Exchange Notes due 2013, which have been registered under the Securities Act.
The Exchange Offer
- •
- We will exchange all outstanding notes that are validly tendered and not validly withdrawn for an equal principal amount of exchange notes that are freely tradeable.
- •
- You may withdraw tenders of outstanding notes at any time prior to the expiration of the exchange offer.
- •
- The exchange offer expires at 5:00 p.m., New York City time, on , 2006, unless extended. We do not currently intend to extend the expiration date.
- •
- The exchange of outstanding notes for exchange in the exchange offer will not be a taxable event for U.S. federal income tax purposes.
- •
- We will not receive any proceeds from the exchange offer.
The Exchange Notes
- •
- The exchange notes are being offered in order to satisfy certain of our obligations under the registration rights agreement entered into in connection with the placement of the outstanding notes.
- •
- The terms of the exchange notes to be issued in the exchange offer are substantially identical to the outstanding notes, except that the exchange notes will be freely tradeable.
- •
- Each of Accellent Inc.'s domestic subsidiaries initially jointly and severally, irrevocably and unconditionally guarantee, on an unsecured senior subordinated basis, the performance and full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of Accellent Inc. under the outstanding notes, exchange notes and the indenture governing the notes.
Results of Exchange Notes
- •
- The exchange notes may be sold in the over-the-counter market, in negotiated transactions or through a combination of such methods. We do not plan to list the exchange notes on a national market.
Any broker-dealer who holds notes acquired for its own account as a result of market-making activities or other trading activities, and who receives exchange notes pursuant to the exchange offer, may be an "underwriter" within the meaning of the Securities Act. If you are a broker-dealer and you receive exchange notes for your own account, you must acknowledge that you will deliver a prospectus in connection with any resale of such exchange notes. By making such acknowledgment, you will not be deemed to admit that you are an "underwriter" under the Securities Act. Broker-dealers may use this prospectus in connection with any resale of exchange notes received in exchange for outstanding notes where such outstanding notes were acquired by the broker-dealer as a result of market-making activities or trading activities. We have agreed that, for a period of 180 days after the expiration of the exchange offer or until any broker-dealer has sold all registered notes held by it, we will make this prospectus available to such broker-dealer for use in connection with any such resale. A broker-dealer may not participate in the exchange offer with respect to outstanding notes acquired other than as a result of market-making activities or trading activities. See "Plan of Distribution."
If you are an affiliate of Accellent Inc. or are engaged in, or intend to engage in, or have an agreement or understanding to participate in, a distribution of the exchange notes, you cannot rely on the applicable interpretations of the Securities and Exchange Commission and you must comply with the registration requirements of the Securities Act in connection with any resale transaction.
You should consider carefully the risk factors beginning on page 19 of this prospectus before participating in the exchange offer.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the exchange notes to be distributed in the exchange offer or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2006.
TABLE OF CONTENTS
| | Page
|
|---|
| Summary | | 1 |
| Risk Factors | | 19 |
| Disclosure Regarding Forward-Looking Statements | | 34 |
| Use of Proceeds | | 36 |
| Capitalization | | 37 |
| Selected Historical Consolidated Financial Data | | 38 |
| Management's Discussion and Analysis of Financial Condition and Results of Operations | | 44 |
| Business | | 69 |
| Management | | 85 |
| Principal Stockholders | | 96 |
| The Transactions | | 98 |
| Certain Relationships and Related Party Transactions | | 99 |
| Description of Other Indebtedness | | 101 |
| The Exchange Offer | | 104 |
| Description of the Exchange Notes | | 114 |
| Book-Entry; Delivery and Form | | 173 |
| United States Federal Income Tax Consequences of the Exchange Offer | | 176 |
| Certain ERISA Considerations | | 177 |
| Plan of Distribtuion | | 179 |
| Legal Matters | | 180 |
| Experts | | 180 |
| Available Information | | 180 |
| Index to Financial Statements | | F-1 |
| Unaudited Pro Forma Condensed Combined Financial Statements | | P-1 |
| Financial Statement Schedules | | S-1 |
This prospectus does not constitute an offer to sell, or a solicitation of an offer to buy, any exchange notes offered hereby in any jurisdiction where, or to any person to whom, it is unlawful to make such offer or solicitation. The information contained in this prospectus speaks only as of the date of this prospectus unless the information specifically indicates that another date applies. No dealer, salesperson or other person has been authorized to give any information or to make any representations other than those contained in this prospectus in connection with the offer contained herein and, if given or made, such information or representations must not be relied upon as having been authorized by Accellent Inc. Neither the delivery of this prospectus nor any sales made hereunder shall under any circumstances create an implication that there has been no change in our affairs or that of our subsidiaries since the date hereof.
i
SUMMARY
This summary highlights information appearing elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you should consider before participating in the exchange offer. You should carefully read the entire prospectus, including the financial data and related notes and section entitled "Risk Factors." Unless the context otherwise requires, references in this prospectus to "Accellent," "we," "our," "us" and "the company" refer to Accellent Inc. and its consolidated subsidiaries, which were acquired pursuant to the Transactions (as described below), and references to the "Issuer" refer to Accellent Inc., the issuer of the notes. Financial information identified in this prospectus as "pro forma" gives effect to the acquisitions of MedSource Technologies, Inc., Campbell Engineering, Inc. and Machining Technology Group, LLC (referred to collectively as the "Acquisitions") and financial information identified in this prospectus as "pro forma as adjusted" gives effect to the Acquisitions and the closing of the Transactions.
Industry and market data used throughout this prospectus is based on independent industry publications, government publications, reports by market research firms, including the research report that was commissioned by us and prepared by the Millennium Research Group entitled "Global Markets for Medical Device Outsourcing 2005, Millennium Research Group", and other published independent sources. Some data is also based on our good faith estimates, which are derived from our review of internal surveys and independent sources.
Our Company
Overview
We are the largest provider of outsourced precision manufacturing and engineering services to the medical device industry according to market share comparisons by the Millennium Research Group. We focus on what we believe are three of the largest and fastest growing segments of the medical device market: cardiology, endoscopy and orthopaedics. Our customers are the leading medical device companies in the world, including Abbott Laboratories, Boston Scientific, Guidant, Johnson & Johnson, Medtronic, Smith & Nephew, St. Jude, Stryker, Tyco and Zimmer. We provide our customers with reliable, high-quality, cost-efficient, integrated outsourcing solutions that span the complete supply chain spectrum.
Our design and engineering, precision component manufacturing, device assembly and supply chain management services provide multiple strategic benefits to our customers. We help speed our customers' products to market, lower their manufacturing costs, provide capabilities that they do not possess internally, and enable our customers to concentrate resources on clinical education, research, sales and marketing.
We have developed long-term relationships with our largest customers and work closely with them in the designing, testing, prototyping and manufacturing of their products. Based on discussions with our customers, we believe we are considered a preferred strategic supplier by a majority of our top ten customers, and often become the sole supplier of the manufacturing and engineering services that we provide to our customers. Many of the end products we produce for our customers are regulated by the U.S. Food & Drug Administration, or the FDA, which has stringent quality standards for manufacturers of medical devices. Complying with these requirements involves significant investments of money and time, which results in stronger relationships with our customers.
We generate significant recurring revenues from a diverse range of products that generally have long product life cycles. Moreover, the majority of our revenues comes from high value, single use products that are either regulated for one-time use, implanted into the body or are considered too critical to be re-used. We currently work with our customers on over 10,000 stock keeping units, providing us with tremendous product diversity across our customer base.
1
We expect our future growth to come from a combination of factors, including market growth for cardiology, endoscopy and orthopaedic devices, increased outsourcing of existing and new products by our customers to us, and increasing our market share of the overall outsourcing market. Our ability to grow our business however, is subject to certain risks, including those specific to the medical device industry.
On November 22, 2005, we completed a series of transactions resulting in the acquisition of approximately 71% of our capital stock by affiliates of Kohlberg Kravis Roberts & Co. L.P., or KKR, approximately 24% of our capital stock by entities affiliated with Bain Capital, or Bain, and the remainder held by certain members of management. We refer to KKR and Bain as the "Sponsors" in this prospectus. See "—The Transactions."
Industry Background
The medical device industry enjoys favorable industry dynamics, with projected revenue growth in our key market segments of approximately 11% annually from 2004 through 2009, according to the Millennium Research Group.
We focus on what we believe are three of the largest and fastest growing segments of the medical device industry: cardiology, endoscopy and orthopaedics, which together account for a $58.5 billion market according to the Millennium Research Group. The outsourcing opportunities for these three targeted end markets are expected to grow at 14.4% from $4.0 billion in 2004 to $7.9 billion in 2009, according to the Millennium Research Group. We believe that these end markets are attractive based on their large size, significant volume growth, relatively high customer profit margins, strong product pipelines and a demonstrable need for our high-quality manufacturing and engineering services.
We target these three end markets by focusing on the 15 leading medical device companies that operate in one or more of these markets. We believe these leading medical device companies will generate outsourcing opportunities similar to the end markets in which they operate.
Our target market is expected to grow through a combination of growth in our customers' end markets and an increase in the amount of manufacturing and engineering services outsourced to third party providers. As a result, the anticipated growth of the outsourced market will outpace growth in the overall end markets as customers continue to shift resources towards clinical education, research, sales and marketing.
Many of the medical device companies in our end markets are increasingly utilizing third party manufacturing and engineering providers as part of their business and manufacturing strategies. Medical device companies are choosing their strategic outsourcing partners based on the partner's ability to provide comprehensive precision manufacturing and engineering capabilities. Based on industry experience and customer feedback, management believes that the key decision making criteria for customers are product quality and reliability of delivery. We believe medical device companies will continue to outsource manufacturing to third party providers based on:
- •
- the desire of medical device companies to accelerate their time to market;
- •
- the increasing complexity of manufacturing medical device products;
- •
- the rationalization of medical device companies' existing manufacturing facilities;
- •
- the increasing focus by medical device companies on clinical education, research, sales and marketing; and
- •
- the desire of medical device companies to reduce product development and manufacturing costs.
2
Competitive Strengths
Our competitive strengths make us a preferred strategic partner for many of the leading medical device companies and position us for profitable growth. Our preferred provider status is evident through our long-term customer relationships, sole source agreements and/or by official designations.
- •
- Market Leader. We are the largest provider of outsourced precision manufacturing and engineering services in our target markets according to market share comparisons by the Millennium Research Group. We also believe we are approximately two to three times as large as our nearest direct competitors. Our size enables us to invest significant resources across a broad set of capabilities to build our infrastructure, including manufacturing facilities, engineering expertise, company-wide quality systems and sales capabilities.
- •
- Strong Long-Term Strategic Partnerships With Targeted Customers. Based on discussions with our customers, we believe we are considered a preferred strategic supplier to a majority of our top ten customers and often become the sole supplier of manufacturing and engineering services for a significant portion of the products we provide to our customers. We have a highly focused sales force dedicated to serving the leading medical device manufacturers, many of which we have had relationships with for at least ten years. Within these large customers, we generate diversified revenue streams across separate divisions and multiple products. As a result of our strong relationships, we are well-positioned to compete for a majority of our customers' outsourcing needs and benefit as our customers seek to reduce their supplier base.
- •
- Breadth of Manufacturing and Engineering Capabilities. We provide a comprehensive range of manufacturing and engineering services, including design, testing, prototyping, production and device assembly, as well as global supply chain management services. Our facilities have areas of expertise and capabilities which allow us to provide proprietary manufacturing services. Our breadth of capabilities is becoming increasingly important as customers continue to seek integrated supply chain solutions.
- •
- Reputation for Quality. We believe our reputation and experience as a high quality manufacturer provide us with an advantage in winning new business as large medical device companies want to partner with successful, proven manufacturers who have the systems and capabilities necessary to deliver a high level of quality that is comparable to their own.
- •
- Strategic Locations. We believe that the location of our design, prototyping and engineering centers near our major customers and the location of certain of our facilities in advantageous manufacturing centers provide us with a competitive advantage. Our strategic locations allow us to facilitate speed to market, rapid prototyping, low cost assembly and overall customer familiarity.
- •
- Strong Financial Profile. We believe that as large medical device companies look to partner with suppliers of significant scale and stability, we are favorably positioned by having consistently demonstrated solid historical revenue growth and steadily improving margins. It is our belief that our strong operating earnings combined with modest capital expenditures and working capital requirements will continue to generate significant free cash flow.
- •
- Experienced and Committed Management Team. We have a highly experienced management team at both the corporate and operational levels. Our senior management team, led by President and Chief Executive Officer Ron Sparks, has an average of over 20 years of industry experience. Our management team invested approximately $30 million of equity in connection with the Transactions.
Despite these competitive strengths, we face operational risks and challenges. For example, our manufacturing, design and engineering processes may become obsolete if we do not respond to changes
3
in technology and our reputation for quality may be harmed if our suppliers and subcontractors are unable to meet our volume and quality requirements. You should carefully consider these risks and challenges, all the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth under "Risk Factors" in deciding whether to invest in the notes.
Business Strategy
Our objective is to grow profitably and strengthen our position as the leading provider of outsourced precision manufacturing and engineering services to the medical device industry through the following:
- •
- Increase Share Within Target Market Leaders. We are focused on increasing our share of revenues from the leading companies within our target markets. We believe the strength of our customer relationships and our customer-focused sales teams, in combination with the breadth of our capabilities and manufacturing expertise, put us in a preferred position to capture an increasing percentage of new business.
- •
- Increase Manufacturing Efficiencies. We will continue to implement quality and manufacturing programs across all of our facilities to improve the cost structure of our manufacturing through the reduction of labor and overhead costs, tighter inventory controls and process improvement. In that regard, we have introduced our "Lean Manufacturing" program which is designed to improve manufacturing cycle times and reduce costs. In addition, our internal research and development team continues to develop proprietary techniques that often enable us to manufacture products at lower costs than our customers and competitors.
- •
- Expand Design and Prototyping Capabilities and Presence. We intend to grow revenues from design and prototyping services by continuing to invest in selected strategic locations and equipment. We believe being involved in the initial design and prototyping of medical devices positions us favorably to capture the ongoing manufacturing business of these devices as they move to full production.
- •
- Provide an Integrated Supply Chain Solution. We are constantly adding strategic capabilities in order to provide a continuum of service for our customers throughout their product life cycles, thereby allowing them to reduce the number of vendors they deal with and focus their resources on speed to market. These capabilities range from concept validation and design and development, through manufacturing, warehousing and distribution.
- •
- Selectively Pursue Complementary Acquisitions. The fragmented nature of the medical device outsourcing industry presents opportunities for us to selectively pursue complementary acquisitions, which would allow us to expand our scope and scale to further enhance our offering to our customers.
Recent Acquisitions
On September 12, 2005, we acquired substantially all of the assets of Campbell Engineering, Inc., or Campbell, a Huntsville, Alabama based manufacturing and engineering firm. Campbell had revenue of $10.9 million for the year ended December 31, 2004, and is engaged in the business of design, analysis, precision fabrication, assembly and testing of primarily orthopaedic implants and instruments.
On October 6, 2005, we acquired 100% of the outstanding membership interests in Machining Technology Group, LLC, or MTG, an Arlington, Tennessee based privately held manufacturing and engineering company. MTG had revenue of $11.3 million for the year ended December 31, 2004 and specializes in rapid prototyping and manufacturing of specialized orthopaedic implants and instruments.
In this prospectus, we refer to these acquisitions as the "2005 Acquisitions."
4
Risk Related to Our Business
Our ability to successfully operate our business is subject to certain risks, including those that are generally associated with the medical devices industry. For example:
- •
- Because a significant portion of our net sales comes from a few large customers, any decrease in sales to these large customers could harm our operating results;
- •
- We may not be able to continue to grow our business if the trend by medical device companies to outsource their manufacturing activities does not continue or if our customers decide to manufacture internally products that we currently provide;
- •
- Our industry is very competitive; we may face competition from, and we may be unable to compete successfully against, new entrants and established companies with greater resources;
- •
- If we do not respond to changes in technology, our manufacturing, design and engineering processes may become obsolete and we may experience reduced sales and lose customers; and
- •
- We depend on outside suppliers and subcontractors, and our production and reputation could be harmed if they are unable to meet our volume and quality requirements and alternative sources are not available.
Any of these factors and other factors described under "Risk Factors" may restrict our future growth.
The Transactions
On October 7, 2005, we entered into an agreement and plan of merger with Accellent Acquisition Corp., or AAC, an entity controlled by affiliates of KKR, pursuant to which Accellent Merger Sub Inc., a wholly-owned subsidiary of AAC, has merged with and into Accellent Inc., with Accellent Inc. being the surviving entity (the "Merger"). As a result of the Merger:
- •
- our capital stock and other equity interests outstanding immediately prior to the Merger (other than a portion of the shares of A-9 Preferred Stock held by certain existing stockholders which has been converted into equity of Accellent Holdings Corp.) and the options to receive our common stock outstanding immediately prior to the Merger (other than certain options held by members of management which have been rolled over into options to purchase shares of Accellent Holdings Corp. ("Rollover Options")) have been cancelled and converted into the right to receive aggregate cash consideration of approximately $830 million less accrued interest on our existing indebtedness plus cash on hand at the time of closing and less approximately $17 million of cash bonuses to certain employees in connection with the change of control that occurred upon consummation of the Merger;
- •
- entities affiliated with KKR own approximately 71% of our outstanding common stock immediately following the Merger, entities affiliated with Bain own approximately 24% of our common stock; and
- •
- certain members of management will own approximately 5% of our outstanding common stock, of which approximately 3% includes the value of Accellent Inc. stock options exchanged for Accellent Holdings Corp. stock options, and approximately 2% which represents shares of common stock owned by management.
In connection with the Merger, entities affiliated with KKR and entities affiliated with Bain made an equity investment in Accellent Holdings Corp. of approximately $611 million, with approximately $30 million of additional equity rolled over by 58 members of management. Equity rolled over by management includes approximately $19 million of equity in stock options of Accellent Inc. that was
5
exchanged for stock options in Accellent Holdings Corp., approximately $1 million of after-tax stock option proceeds used by management to acquire common stock of Accellent Holdings Corp, and $10 million of preferred stock of Accellent Inc. exchanged for $10 million of common stock of Accellent Holdings Corp. The equity rolled over by management in the form of stock options included approximately $14 million of equity rolled over by our executive officers, which is comprised of 8 individuals. In addition, in connection with the Merger, we:
- •
- entered into a senior secured credit facility, consisting of a $400 million senior secured term loan facility and a $75 million senior secured revolving credit facility;
- •
- issued $305 million aggregate principal amount of senior subordinated notes, resulting in net proceeds of approximately $301 million after approximately $4 million original issue discount;
- •
- repaid approximately $409 million of our indebtedness, including pursuant to a tender offer for Accellent Corp.'s $175 million 10% senior subordinated notes due 2012; and
- •
- paid approximately $73 million of transaction fees and expenses, including tender premiums.
In connection with the Merger, Accellent Holdings Corp. granted new options to purchase shares of Accellent Holdings Corp. to certain members of management. All equity positions currently held by our named executive officers are in the form of stock options.
Our tender offer to purchase all of the outstanding 10% senior subordinated notes due 2012 of Accellent Corp. expired November 21, 2005 and our consent solicitation to amend the indenture governing the notes to eliminate substantially all of the restrictive covenants and effect certain other amendments to the indenture expired on November 3, 2005. We received tenders and the requisite consents for 100% of the 10% senior subordinated notes and, as a result, we accepted for payment and paid for all notes validly tendered and executed a supplemental indenture effecting the proposed amendments, which supplemental indenture has become operative.
The Merger and related financing transactions are referred to collectively in this prospectus as the "Transactions."
About Kohlberg Kravis Roberts & Co. L.P.
KKR is one of the world's oldest and most experienced private equity firms specializing in management buyouts. KKR's investment approach is focused on acquiring attractive business franchises and working closely with management over the long term to design and implement value-creating strategies. Over the past 29 years, KKR has invested in 140 transactions with a total value of over $185 billion.
6
Ownership and Corporate Structure
The chart below illustrates our ownership and corporate structure upon completion of the Transactions.
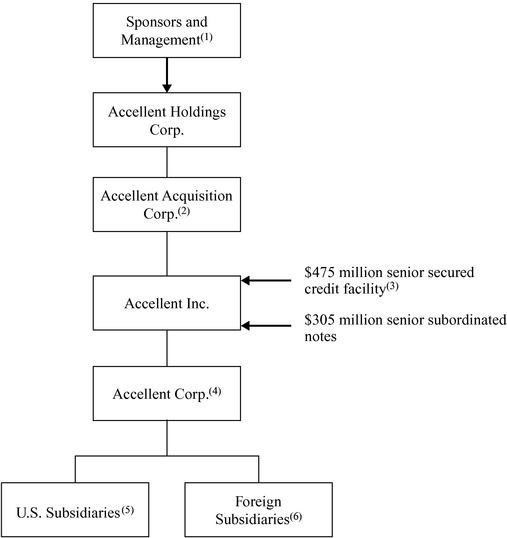
- (1)
- Entities affiliated with KKR own approximately 71% of the common stock of Accellent Holdings Corp., entities affiliated with Bain own approximately 24% of the common stock of Accellent Holdings Corp., with the remaining 5% held by certain members of management.
- (2)
- Accellent Acquisition Corp. guarantees all amounts outstanding under our senior secured credit facility on a senior secured basis.
- (3)
- Consists of a $400 million seven-year term loan B facility and a $75 million six-year revolving credit facility. Our senior secured credit facility is secured by substantially all of our assets and substantially all assets of Accellent Acquisition Corp. and of each of our current and future domestic subsidiaries, including all of our capital stock and the capital stock of each of our existing and future direct and indirect subsidiaries, except that with respect to foreign subsidiaries such lien and pledge is limited to 65% of the voting stock of first-tier foreign subsidiaries. The notes are subordinated to our senior secured credit facility and are unsecured.
- (4)
- We received tenders for 100% of the 10% senior subordinated notes due 2012 in connection with the tender offer we commenced for Accellent Corp.'s existing notes. See "The Transactions." Accellent Corp. guarantees all amounts outstanding under our senior secured credit facility on a senior secured basis and the notes on a senior subordinated basis.
- (5)
- All of these entities guarantee all amounts outstanding under our senior secured credit facility on a senior secured basis and the notes on a senior subordinated basis.
- (6)
- As noted in footnote (3) above, we have granted a security interest to the lenders under our senior secured credit facility in 65% of the voting stock of first-tier foreign subsidiaries.
7
Summary of Terms of the Exchange Offer
On November 22, 2005, Accellent Inc. completed the private offering of the outstanding notes. References to the "notes" in this prospectus are references to both the outstanding notes and the exchange notes. This prospectus is part of a registration statement covering the exchange of the outstanding notes for the exchange notes.
Accellent Inc. and the guarantors entered into a registration rights agreement with the initial purchasers in the private offering in which Accellent Inc. and the guarantors agreed to deliver to you this prospectus as part of the exchange offer and agreed to use all commercially reasonable efforts to have the registration statement covering the exchange to be declared effective on or prior to the date 210 days after the closing of the private offering. You are entitled to exchange in the exchange offer your outstanding notes for exchange notes which are identical in all material respects to the outstanding notes except:
- •
- the exchange notes have been registered under the Securities Act;
- •
- the exchange notes are not entitled to certain registration rights which are applicable to the outstanding notes under the registration rights agreement; and
- •
- certain special interest rate provisions are no longer applicable.
The Exchange Offer |
|
We are offering to exchange up to $305,000,000 aggregate principal amount of our 101/2% Senior Subordinated Exchange Notes due 2013, which we refer to in this prospectus as the exchange notes, for up to $305,000,000 aggregate principal amount of our 101/2% Senior Subordinated Notes due 2013, which we refer to in this prospectus as the outstanding notes. Outstanding notes may be exchanged only in denominations of $2,000 and integral multiples of $1,000 in excess of $2,000. |
Resale |
|
Based on an interpretation by the staff of the SEC set forth in no-action letters issued to third parties, we believe that the exchange notes issued pursuant to the exchange offer in exchange for outstanding notes may be offered for resale, resold and otherwise transferred by you (unless you are an "affiliate" of Accellent Inc., within the meaning of Rule 405 under the Securities Act) without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that you are acquiring the exchange notes in the ordinary course of your business and that you are not engaged in, do not intend to engage in, and have no arrangement or understanding with any person to participate in, a distribution of the exchange notes. |
|
|
Each participating broker-dealer that receives exchange notes for its own account pursuant to the exchange offer in exchange for outstanding notes that were acquired as a result of market-making or other trading activity must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. See "Plan of Distribution." |
|
|
Any holder of outstanding notes who: |
| | | | | |
8
|
|
• |
|
is an affiliate of the Issuer; |
|
|
• |
|
does not acquire exchange notes in the ordinary course of business; or |
|
|
• |
|
tenders in the exchange offer with the intention to participate, or for the purpose of participating, in a distribution of exchange notes; |
|
|
cannot rely on the position of the staff of the SEC enunciated in Exxon Capital Holdings Corporation, Morgan Stanley & Co. Incorporated or similar no-action letters and, in the absence of an exemption therefrom, must comply with the registration and prospectus delivery requirement of the Securities Act in connection with the resale of the exchange notes. |
Expiration Date; Withdrawal of Tender |
|
The exchange offer will expire at 5:00 p.m., New York City time, on , 2006, unless extended by us. We do not currently intend to extend the expiration date. A tender of outstanding notes pursuant to the exchange offer may be withdrawn at any time prior to the expiration date. Any outstanding notes not accepted for exchange for any reason will be returned without expense to the tendering holder promptly after the expiration or termination of the exchange offer. |
Certain Conditions to the Exchange Offer |
|
The exchange offer is subject to customary conditions, which we may waive. Please read the section captioned "The Exchange Offer—Certain Conditions to the Exchange Offer" of this prospectus for more information regarding the conditions to the exchange offer. |
Procedures for Tendering Outstanding Notes |
|
If you wish to accept the exchange offer, you must complete, sign and date the accompanying letter of transmittal, or a facsimile of the letter of transmittal, according to the instructions contained in this prospectus and the letter of transmittal. You must also mail or otherwise deliver the letter of transmittal, or a facsimile of the letter of transmittal, together with the outstanding notes and any other required documents, to the exchange agent at the address set forth on the cover page of the letter of transmittal. If you hold outstanding notes through The Depository Trust Company, or DTC, and wish to participate in the exchange offer, you must comply with the Automated Tender Offer Program procedures by DTC, by which you will agree to be bound by the letter of transmittal. By signing, or agreeing to be bound by the letter of transmittal, you will represent to us that, among other things: |
| | | | | |
9
|
|
• |
|
any exchange notes that you receive will be acquired in the ordinary course of business; |
|
|
• |
|
you have no arrangement or understanding with any person or entity to participate in a distribution of the exchange notes; |
|
|
• |
|
if you are a broker-dealer that will receive exchange notes for your own account in exchange for outstanding notes that were acquired as a result of market-making activities or other trading activity, that you will deliver a prospectus, as required by law, in connection with any resale of such exchange notes; and |
|
|
• |
|
you are not an "affiliate," as defined in Rule 405 of the Securities Act, of Accellent Inc. or, if you are an affiliate, you will comply with any applicable registration and prospectus delivery requirements of the Securities Act. |
Special Procedures for Beneficial Owners |
|
If you are a beneficial owner of outstanding notes which are registered in the name of a broker, dealer, commercial bank, trust company or other nominee, and you wish to tender such outstanding notes in the exchange offer, you should contact such registered holder promptly and instruct such registered holder to tender on your behalf. If you wish to tender on your own behalf, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either make appropriate arrangements to register ownership of the outstanding notes in your name or obtain a properly completed bond power from the registered holder. The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date. |
Guaranteed Delivery Procedures |
|
If you wish to tender your outstanding notes and your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other documents required by the letter of transmittal or comply with the applicable procedures under DTC's Automated Tender Offer Program prior to the expiration date, you must tender your outstanding notes according to the guaranteed delivery procedures set forth in this prospectus under "The Exchange Offer—Guaranteed Delivery Procedures." |
| | | | | |
10
Effect on Holders of Outstanding Notes |
|
As a result of the making of, and upon acceptance for exchange of all validly tendered outstanding notes pursuant to the terms of the exchange offer, we will have fulfilled a covenant contained in the registration rights agreement and, accordingly, there will be no increase in the interest rate on the outstanding notes under the circumstances described in the registration rights agreement. If you are a holder of outstanding notes and you do not tender your outstanding notes in the exchange offer, you will continue to hold such outstanding notes and you will be entitled to all the rights and limitations applicable to the outstanding notes in the indenture, except for any rights under the registration rights agreement that by their terms terminate upon the consummation of the exchange offer. To the extent that outstanding notes are tendered and accepted in the exchange offer, the trading market for outstanding notes could be adversely affected. |
Consequences of Failure
to Exchange |
|
All untendered outstanding notes will continue to be subject to the restrictions on transfer provided for in the outstanding notes and in the indenture. In general, the outstanding notes may not be offered or sold, unless registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offer, we do not currently anticipate that we will register the outstanding notes under the Securities Act. |
Certain United States Federal Income Tax Consequences |
|
The exchange of outstanding notes for exchange notes in the exchange offer will not be a taxable event for the United States federal income tax purposes. See "United States Federal Income Tax Consequences of the Exchange Offer." |
Use of Proceeds |
|
We will not receive any cash proceeds from the issuance of exchange notes pursuant to the exchange offer. |
Exchange Agent |
|
The Bank of New York is the exchange agent for the exchange offer. The address and telephone number of the exchange agent are set forth in the section captioned "The Exchange Offer—Exchange Agent" of this prospectus. |
11
Summary of Terms of the Exchange Notes
| Issuer | | Accellent Inc. |
| Notes Offered | | $305,000,000 aggregate principal amount of 101/2% Senior Subordinated Exchange Notes due 2013 |
| Maturity Date | | December 1, 2013 |
| Interest Payment Dates | | June 1 and December 1, beginning on June 1, 2006. |
| Ranking | | The notes will be our unsecured senior subordinated obligations and will: |
| | | • | | be subordinated in right of payment to our existing and future senior debt, including our senior secured credit facility; |
| | | • | | rank equally in right of payment to all of our future senior subordinated debt; |
| | | • | | be effectively subordinated in right of payment to all of our existing and future secured debt (including our senior secured credit facility) to the extent of the value of the assets securing such debt, and be structurally subordinated to all obligations of each of our subsidiaries that is not a guarantor of the notes; and |
| | | • | | rank senior in right of payment to all of our future debt and other obligations that are, by their terms, expressly subordinated in right of payment to the senior subordinated notes. |
| | | Similarly, the senior subordinated note guarantees will be unsecured senior subordinated obligations of the guarantors and will: |
| | | • | | be subordinated in right of payment to all of the applicable guarantor's existing and future senior debt, including such guarantor's guarantee under our senior secured credit facility; |
| | | • | | rank equally in right of payment to all of the applicable guarantor's future senior subordinated debt; |
| | | • | | be effectively subordinated in right of payment to all of the applicable guarantor's existing and future secured debt (including such guarantor's guarantee under our senior secured credit facility) to the extent of the value of the assets securing such debt, and be structurally subordinated to all obligations of any subsidiary of a guarantor if that subsidiary is not also a guarantor of the notes; and |
| | | • | | rank senior in right of payment to all of the applicable guarantor's future subordinated debt and other obligations that are, by their terms, expressly subordinated in right of payment to the notes. |
| | | | | |
12
| | | As of September 30, 2005, on a pro forma as adjusted basis, the notes and related guarantees (1) would have ranked effectively junior to approximately $400 million of senior indebtedness and (2) would have been structurally subordinated to $5.4 million of total liabilities, including trade payables but excluding intercompany obligations, of our non-guarantor subsidiaries. |
| Guarantees | | All payments on the notes are initially jointly and severally and unconditionally guaranteed on an unsecured senior subordinated basis by all of Accellent Inc.'s domestic subsidiaries. On a pro forma as adjusted basis, our non-guarantor subsidiaries would have accounted for approximately 1.0% of our assets as of September 30, 2005 and generated approximately 3.6% of our net sales for the year ended December 31, 2004. |
| Optional Redemption | | Prior to December 1, 2009, we will have the option to redeem some or all of the notes for cash at a redemption price equal to 100% of their principal amount plus an applicable make-whole premium (as described in "Description of the Exchange Notes—Optional Redemption") plus accrued and unpaid interest to the redemption date. Beginning on December 1, 2009, we may redeem some or all of the notes at the redemption prices listed under "Description of the Exchange Notes—Optional Redemption" plus accrued interest on the notes to the date of redemption. |
| Optional Redemption After Certain Equity Offerings | | Until December 1, 2008, we may redeem up to 35% of the aggregate principal amount of the notes with the proceeds of certain public equity offerings. |
| Change of Control Offer | | Upon the occurrence of a change of control, you will have the right, as holders of the notes, to require us to repurchase some or all of your notes at 101% of their face amount, plus accrued and unpaid interest to the repurchase date. See "Description of the Exchange Notes—Repurchase at the Option of Holders—Change of Control." |
| | | We may not be able to pay you the required price for notes you present to us at the time of a change of control because: |
| | | • | | we may not have enough funds at that time; or |
| | | • | | terms of our senior debt may prevent us from making such payment. |
| Certain Indenture Provisions | | The indenture governing the notes contains covenants limiting our ability and the ability of our restricted subsidiaries to: |
| | | • | | incur additional debt or issue certain preferred shares; |
| | | • | | pay dividends on or make distributions in respect of our capital stock or make other restricted payments; |
| | | • | | make certain investments; |
| | | • | | sell certain assets; |
| | | • | | create liens on certain assets to secure debt; |
| | | | | |
13
| | | • | | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; |
| | | • | | enter into certain transactions with our affiliates; and |
| | | • | | designate our subsidiaries as unrestricted subsidiaries. |
| | | These covenants are subject to a number of important limitations and exceptions. See "Description of the Exchange Notes." |
| ERISA Considerations | | The notes may, subject to certain restrictions described in "Certain ERISA Considerations" herein, be sold and transferred to ERISA Plans and Plans. |
| No Prior Market | | The exchange notes will be new securities for which there is currently no market. Although the initial purchasers in the private offering of the outstanding notes have informed us that they intend to make a market in the outstanding notes and, if issued, in the exchange notes, they are not obligated to do so and they may discontinue market making activities at any time without notice. Accordingly, we cannot assure you that a liquid market for the outstanding notes or exchange notes will develop or be maintained. |
| Listing | | The notes have been made eligible for trading in The PORTALsm Market. |
| Use of Proceeds | | There will be no cash proceeds to us from the exchange offer. |
| Risk Factors | | Investing in the notes involves substantial risks. See "Risk Factors" for a description of some of the risks relating to the exchange offer. |
Our principal executive offices are located at 100 Fordham Road, Wilmington, Massachusetts 01887 and our telephone number there is (978) 570-6900. We are incorporated in the State of Maryland. Our website is located at www.accellent.com.The information on our website is not part of this prospectus and is not being incorporated by reference herein.
14
SUMMARY UNAUDITED PRO FORMA COMBINED FINANCIAL DATA
The following table contains summary unaudited pro forma combined financial data for the nine months ended September 30, 2004 and 2005 and for the twelve months ended December 31, 2004 derived from the financial information set forth in our "Unaudited Pro Forma Condensed Combined Financial Statements" included elsewhere in this prospectus. The summary unaudited pro forma condensed combined statements of operations data for the nine months ended September 30, 2004 and 2005 and for the twelve months ended December 31, 2004 give effect to the MedSource Transactions (as defined below) and the 2005 Acquisitions as if they had occurred on January 1, 2004. The summary unaudited pro forma condensed combined balance sheet data gives effect to the MTG acquisition as if it had occurred on September 30, 2005. The summary unaudited pro forma combined financial data are intended for informational purposes only and do not purport to present our actual financial position or the results of operations that actually would have occurred or that may be obtained in the future if the transactions described had occurred as presented. In addition, future results may vary significantly from the results reflected in such statements due to certain factors beyond our control.
The MedSource Transactions, which occurred on June 30, 2004, include:
- •
- the acquisition of MedSource for $205.4 million in cash (net of $14.3 million of cash acquired);
- •
- the payment in cash of MedSource's indebtedness and accrued interest of $37.0 million;
- •
- the payment in cash of Accellent Corp.'s old senior secured credit facility, Accellent Corp.'s old senior subordinated indebtedness, our senior indebtedness, and accrued interest of $154.1 million;
- •
- the borrowings under Accellent Corp.'s old senior secured credit facility of $194.0 million;
- •
- the offering of the 10% senior subordinated notes due 2012, which generated $175.0 million in cash; and
- •
- the payment of fees in connection with Accellent Corp.'s old secured credit facility and senior subordinated notes which will be amortized to interest expense over the life of each respective instrument.
The MedSource acquisition and these other related transactions are referred to collectively in this prospectus as the "MedSource Transactions."
The 2005 Acquisitions include:
- •
- the acquisition of Campbell for approximately $18.2 million in cash;
- •
- the acquisition of MTG for approximately $50.2 million, paid in cash of $34.0 million and shares of our Class A-9 Convertible Preferred Stock of $16.2 million;
- •
- additional borrowings of $42.0 million under the old credit facility, which include additional terms loans of $12.5 million and revolving credit facility borrowings of $29.5 million; and
- •
- the repayment of all existing indebtedness of MTG concurrent with the closing of our acquisition of MTG.
The summary unaudited pro forma as adjusted combined balance sheet data has been adjusted to give effect to the Transactions as if these events occurred as of September 30, 2005. The summary unaudited pro forma as adjusted combined financial data are for informational purposes only and do not purport to present what our results of operations and financial condition would have been had the Transactions actually occurred on these earlier dates, nor do they project our results of operations for any future period or our financial condition at any future date.
15
The unaudited pro forma combined data account for the MedSource, Campbell and MTG acquisitions using the purchase method of accounting, which requires that we adjust assets and liabilities to their fair values. The valuation of Campbell and MTG is based upon available information and certain assumptions that we believe are reasonable. The total purchase price for Campbell and MTG was allocated to our net assets based on preliminary estimates of fair value. The final purchase price allocation will be based on a formal valuation analysis and may include adjustments to the amounts shown here. The summary unaudited pro forma as adjusted combined financial data account for the Transactions using the purchase method of accounting which requires that we adjust all of our assets and liabilities to their fair values as of the date of the Merger. The valuation of our assets and liabilities is based upon available information and certain assumptions that we believe are reasonable. A final valuation is in process. The result of the final allocation could be materially different from the preliminary allocation set forth in the unaudited pro forma condensed combined financial statements. In particular, we have initiated an appraisal of all property, plant and equipment acquired from MTG, and to be acquired from us in the Merger. The results of the final appraisal and the final allocation could be materially different from the preliminary allocation set forth in the unaudited pro forma condensed combined financial statements.
You should read the summary pro forma data set forth in the following table in conjunction with "Use of Proceeds," "Capitalization," "Selected Historical Consolidated Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "The Transactions," the consolidated financial statements of Accellent, MedSource, Campbell and MTG and the related notes thereto and the Unaudited Pro Forma Condensed Combined Financial Statements and the related notes thereto included elsewhere in this prospectus. In addition, future results may vary significantly from the results reflected in such statements due to certain factors beyond our control. See "Risk Factors."
16
| | Pro Forma for twelve months ended
| | Pro Forma for nine
months ended
| |
|---|
| | December 31, 2004
| | September 30,
2004
| | September 30,
2005
| |
|---|
| | (In thousands)
| |
|---|
| STATEMENT OF OPERATIONS DATA: | | | | | | | | | | |
| Net sales | | $ | 436,164 | | $ | 323,023 | | $ | 359,835 | |
| Cost of Sales | | | 318,444 | | | 235,611 | | | 246,576 | |
| | |
| |
| |
| |
| Gross profit | | | 117,720 | | | 87,412 | | | 113,259 | |
| Selling General and Administrative Expense | | | 62,602 | | | 46,986 | | | 50,618 | |
| Research and Development Expenses | | | 2,849 | | | 2,204 | | | 2,294 | |
| Restructuring and Other Charges | | | 5,934 | | | 4,441 | | | 3,824 | |
| Amortization of Intangibles | | | 7,634 | | | 5,672 | | | 5,737 | |
| | |
| |
| |
| |
| Income from Operations | | | 38,701 | | | 28,109 | | | 50,786 | |
| Other Income (Expense) | | | | | | | | | | |
| | Interest Expense, Net | | | (32,101 | ) | | (23,910 | ) | | (25,857 | ) |
| | Other Income (Expense) | | | 56 | | | 70 | | | (182 | ) |
| | |
| |
| |
| |
| Total Other Expense | | | (32,045 | ) | | (23,840 | ) | | (26,039 | ) |
| | |
| |
| |
| |
| Income (Loss) Before Income Taxes | | | 6,656 | | | 4,269 | | | 24,747 | |
| Income Tax Expense | | | 3,919 | | | 2,573 | | | 10,714 | |
| | |
| |
| |
| |
| Net Income (Loss) | | $ | 2,737 | | $ | 1,696 | | $ | 14,033 | |
| | |
| |
| |
| |
OTHER FINANCIAL DATA: |
|
|
|
|
|
|
|
|
|
|
| Capital Expenditures | | $ | 22,019 | | $ | 15,504 | | $ | 19,499 | |
| EBITDA(1) | | | 62,286 | | | 46,198 | | | 68,762 | |
| | Twelve months ended
December 31, 2004
| | At and for the
nine months ended
September 30, 2005
|
|---|
| | Pro forma
as adjusted
| | Pro forma
| | Pro forma
as adjusted
|
|---|
| BALANCE SHEET DATA: | | | | | | | | | |
| Cash and Cash Equivalents | | | | | $ | 6,038 | | $ | — |
| Total Assets | | | | | | 661,071 | | | 1,427,850 |
| Total Debt | | | | | | 408,624 | | | 700,948 |
| Redeemable and Convertible Preferred Stock | | | | | | — | | | — |
| Stockholders' Equity | | | | | | 169,210 | | | 638,263 |
PRO FORMA CREDIT STATISTICS: |
|
|
|
|
|
|
|
|
|
| Cash interest expense(2) | | $ | 57,800 | | $ | 23,855 | | $ | 43,350 |
| Ratio of earnings to fixed charges | | | — | | | 1.9 | x | | — |
| Deficiency of earnings to fixed charges | | | 32,231 | | | — | | | 2,596 |
- (1)
- We define EBITDA as net income (loss) before net interest expense, income tax expense, depreciation and amortization. Since EBITDA may not be calculated the same by all companies, this measure may not be comparable to similarly titled measures by other companies. We use EBITDA as a supplemental measure of our performance and to provide additional information to investors about the calculation of certain financial covenants in the indenture governing the notes and under our senior secured credit facility. EBITDA has limitations as an analytical tool, and you should not consider them in isolation, or as a substitute for analysis of our results as reported under GAAP. See "Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Other Key Indicators of Financial Condition and
17
Operating Performance" for a discussion of our use of EBITDA and certain limitations of EBITDA as a financial measure. EBITDA is calculated as follows for the periods presented:
| | Pro Forma for twelve months ended
| | Pro Forma for nine
months ended
|
|---|
| | December 31, 2004
| | September 30,
2004
| | September 30,
2005
|
|---|
| | (In thousands)
|
|---|
| Consolidated Pro Forma EBITDA: | | | | | | | | | |
| Net income (loss) | | $ | 2,737 | | $ | 1,696 | | $ | 14,033 |
| Interest expense, net | | | 32,101 | | | 23,910 | | | 25,857 |
| Income tax expense | | | 3,919 | | | 2,573 | | | 10,714 |
| Depreciation and amortization | | | 23,529 | | | 18,019 | | | 18,158 |
| | |
| |
| |
|
| EBITDA | | $ | 62,286 | | $ | 46,198 | | $ | 68,762 |
| | |
| |
| |
|
- (2)
- Cash interest expense does not include any amortization of capitalized debt issuance cost.
18
RISK FACTORS
You should carefully consider the risk factors set forth below as well as the other information contained in this prospectus before you decide to tender outstanding notes in the exchange offer. Any of the following risks could materially and adversely affect our business, financial condition or results of operations. In such a case, you may lose all or part of your original investment.
Risks Related to the Exchange Offer
If you choose not to exchange your outstanding notes, the present transfer restrictions will remain in force and the market price of your outstanding notes could decline.
If you do not exchange your outstanding notes for exchange notes in the exchange offer, then you will continue to be subject to the transfer restrictions on the outstanding notes as set forth in the offering circular distributed in connection with the private offering of the outstanding notes. In general, the outstanding notes may not be offered or sold unless they are registered or exempt from registration under the Securities Act and applicable state securities laws. Except as required by the registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act. You should refer to "Summary—Summary of Terms of the Exchange Offer" and "The Exchange Offer" for information about how to tender your outstanding notes.
The tender of outstanding notes under the exchange offer will reduce the principal amount of the outstanding notes outstanding, which may have an adverse effect upon, and increase the volatility of, the market price of the outstanding notes due to reduction in liquidity.
Risks Related to Our Indebtedness and the Exchange Notes
We have a substantial amount of indebtedness which may adversely affect our cash flow and our ability to operate our business, remain in compliance with debt covenants and make payments on our indebtedness, including the notes.
On a pro forma as adjusted basis as of September 30, 2005, our total indebtedness would have been $701 million, including the notes. We also would have had an additional $75 million available for borrowing under the revolving portion of our senior secured credit facility at that date. We also are permitted to incur up to an additional $100 million of senior secured debt under our senior secured term loan facility at the option of participating lenders subject to certain conditions. The following chart shows our level of indebtedness and certain other information on a pro forma as adjusted basis as of September 30, 2005.
| | Pro Forma As Adjusted as of
September 30, 2005
|
|---|
| | (Dollars in millions)
|
|---|
| Revolving credit facility(1) | | $ | — |
| Term loan facilities(2) | | | 400.0 |
| Senior subordinated notes, net of discount | | | 300.9 |
| | |
|
| | Total indebtedness | | $ | 700.9 |
| | |
|
- (1)
- Upon the closing of the Transactions, we entered into a $75 million senior secured revolving credit facility with a six-year maturity.
- (2)
- Upon the closing of the Transactions, we entered into a $400 million senior secured term loan facility with a seven-year maturity.
Our substantial indebtedness could have important consequences for you, including:
- •
- making it more difficult for us to make payments on the notes;
- •
- increasing our vulnerability to general economic and industry conditions;
19
- •
- requiring a substantial portion of cash flow from operations to be dedicated to the payment of principal and interest on our indebtedness, therefore reducing our ability to use our cash flow to fund our operations, capital expenditures and future business opportunities;
- •
- exposing us to the risk of increased interest rates as certain of our borrowings, including borrowings under our senior secured credit facility, will be at variable rates of interest;
- •
- restricting us from making strategic acquisitions or causing us to make non-strategic divestitures;
- •
- limiting our ability to obtain additional financing for working capital, capital expenditures, debt service requirements, acquisitions and general corporate or other purposes; and
- •
- placing us at a disadvantage compared to our competitors who have less debt.
We and our subsidiaries may be able to incur substantial additional indebtedness in the future, subject to the restrictions contained in our senior secured credit facility and the indenture governing the notes. If new indebtedness is added to our current debt levels, the related risks that we now face could intensify.
Our debt agreements contain restrictions that limit our flexibility in operating our business.
Our senior secured credit facility and the indenture governing the notes contain various covenants that limit our ability to engage in specified types of transactions. These covenants limit our and our restricted subsidiaries' ability to, among other things:
- •
- incur additional indebtedness;
- •
- pay dividends on, repurchase or make distributions in respect of our capital stock or make other restricted payments;
- •
- make certain investments;
- •
- sell certain assets;
- •
- create liens;
- •
- consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; and
- •
- enter into certain transactions with our affiliates.
In addition, under the senior secured credit facility, we will be required to satisfy and maintain specified financial ratios and other financial condition tests. Our ability to meet those financial ratios and tests can be affected by events beyond our control, and we cannot assure you that we will meet those ratios and tests. A breach of any of these covenants could result in a default under the senior secured credit facility. Upon the occurrence of an event of default under the senior secured credit facility, the lenders could elect to declare all amounts outstanding under the senior secured credit facility to be immediately due and payable and terminate all commitments to extend further credit. If we were unable to repay those amounts, the lenders under the senior secured credit facility could proceed against the collateral granted to them to secure that indebtedness. We have pledged substantially all of our assets as collateral under the senior secured credit facility. If the lenders under the senior secured credit facility accelerate the repayment of borrowings, we cannot assure you that we will have sufficient assets to repay the senior secured credit facility and the notes.
These covenants also may restrict our ability to pursue complementary acquisitions in the future, which has been a significant portion of our growth strategy. As a result, our business, operating results, financial condition or growth prospects could be adversely affected, particularly if other medical device companies consolidate to create new companies with greater market power.
We may not be able to generate sufficient cash to service all of our indebtedness, including the notes, and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We cannot assure
20
you that we will maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness, including the notes.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, or to sell assets, seek additional capital or restructure or refinance our indebtedness, including the notes. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. Our senior secured credit facility and the indenture governing the notes restrict our ability to dispose of assets and use the proceeds from the disposition. We may not be able to consummate those dispositions or to obtain the proceeds that we could realize from them and these proceeds may not be adequate to meet any debt service obligations then due.
Your right to receive payments on the notes and the guarantees is effectively junior to those lenders who have a security interest in our assets.
Our obligations under the notes and our guarantors' obligations under their guarantees of the notes are contractually subordinated and unsecured, but our obligations under our senior secured credit facility are secured by substantially all of our assets and substantially all of our assets of our current and future domestic subsidiaries, including all of our capital stock and the capital stock of each of our existing and future direct and indirect subsidiaries, except that with respect to foreign subsidiaries such lien and pledge is limited to 65% of the voting stock of such foreign subsidiary.
As of September 30, 2005, on a pro forma as adjusted basis, we would have had $400 million of senior secured indebtedness, all of which would have been indebtedness under our senior secured credit facility and which would not have included availability under our revolving credit facility. We also are permitted to incur up to an additional $100 million of senior secured debt under our senior secured term loan facility at the option of participating lenders subject to certain conditions, which additional term loans will have the same security and guarantees as the $400 million term loan B facility under our senior secured credit facility. The indenture governing the notes permits us and our restricted subsidiaries to incur substantial additional indebtedness in the future, including senior secured indebtedness. See "Description of Other Indebtedness."
Because the notes and the guarantees are unsecured obligations, your right of repayment may be compromised if any of the following situations occur:
- •
- we enter into bankruptcy, liquidation, reorganization, or other winding-up proceedings;
- •
- there is a default in the payment under the senior secured credit facility or other secured indebtedness; or
- •
- there is an acceleration of any indebtedness under the senior secured credit facility or other secured indebtedness.
If any of these events occurs, the secured lenders could sell those of our assets in which they have been granted a security interest, to your exclusion, even if an event of default exists under the indenture at such time. As a result, upon the occurrence of any of these events, there may not be sufficient funds to pay amounts due on the notes and the guarantees.
Only certain of our subsidiaries guarantee the notes, and the assets of our non-guarantor subsidiaries may not be available to make payments on the notes.
The notes are not guaranteed by any of our non-U.S. subsidiaries. Accordingly, claims of holders of the notes will be subordinate to the claims of creditors of these non-guarantor subsidiaries, including trade creditors. All obligations of our non-guarantor subsidiaries will have to be satisfied before any of the assets of such subsidiaries would be available for distribution, upon a liquidation or otherwise, to us or a guarantor of the notes.
21
On a pro forma basis, our non-guarantor subsidiaries would have accounted for approximately $15.8 million, or 3.6%, of our net sales for the twelve months ended December 31, 2004, and approximately $13.9 million, or 1.0%, of our total assets, and approximately $5.4 million, or 0.7%, of our total liabilities, in each case as of September 30, 2005.
Your right to receive payments on the notes and the guarantees will be junior to the rights of the lenders under our senior secured credit facility and any of our and the guarantors' future senior indebtedness.
The notes and the guarantees rank in right of payment behind all of our and the guarantors' existing and future senior indebtedness. On a pro forma basis, as of September 30, 2005, we would have had approximately $400 million of senior indebtedness. We are permitted to incur up to an additional $100 million of senior secured debt under our senior secured term loan facility at the option of participating lenders subject to certain conditions, which additional term loans will have the same security and guarantees as the $400 million term loan B facility under our senior secured credit facility. We are also permitted to incur substantial additional indebtedness, including senior indebtedness, in the future.
We and the guarantors may not pay principal, premium, if any, interest or other amounts on account of the notes or the guarantees in the event of a payment default or certain other defaults in respect of certain of our senior indebtedness, including debt under the senior secured credit facility, unless the senior indebtedness has been paid in full or the default has been cured or waived. In addition, in the event of certain other defaults with respect to the senior indebtedness, we or the guarantors may not be permitted to pay any amount on account of the notes or the guarantees for a designated period of time. See "Description of the Exchange Notes—Subordination of the Senior Subordinated Notes."
Because of the subordination provisions in the notes and the guarantees, in the event of our bankruptcy, liquidation or dissolution, our or the guarantors' assets will not be available to pay obligations under the notes or the applicable guarantee until we or the guarantors have made all payments in cash on its senior indebtedness. We cannot assure you that sufficient assets will remain after all these payments have been made to make any payments on the notes, including payments of principal or interest when due.
Repayment of our debt, including the notes, is dependent on cash flow generated by our subsidiaries.
We are a holding company, and all of our assets will be owned by our subsidiaries. Repayment of our indebtedness, including the notes, is dependent on the generation of cash flow by our subsidiaries and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Unless they are guarantors of the notes, our subsidiaries do not have any obligation to pay amounts due on the notes or to make funds available for that purpose. Our subsidiaries may not be able to, or be permitted to, make distributions to enable us to make payments in respect of our indebtedness, including the notes. Each of our subsidiaries is a distinct legal entity and, under certain circumstances, legal and contractual restrictions may limit our ability to obtain cash from our subsidiaries. While the indenture governing the notes limits the ability of our subsidiaries to incur consensual restrictions on their ability to pay dividends or make other intercompany payments to us, these limitations are subject to important qualifications and exceptions. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness, including the notes.
We may not be able to repurchase the notes upon a change of control.
Upon the occurrence of specific kinds of change of control events, we will be required to offer to repurchase all outstanding notes at 101% of their principal amount plus accrued and unpaid interest. The senior secured credit facility provides that certain change of control events (including a change of control as defined in the indenture governing the notes) constitute a default. Any future credit agreement or other agreements relating to senior indebtedness to which we become a party may
22
contain similar provisions. If we experience a change of control that triggers a default under our senior secured credit facility, we could seek a waiver of such default or seek to refinance our senior secured credit facility. In the event we do not obtain such a waiver or refinance the senior secured credit facility, such default could result in amounts outstanding under our senior secured credit facility being declared due and payable. The source of funds for any such purchase of the notes will be our available cash or cash generated from our subsidiaries' operations or other sources, including borrowings, sales of assets or sales of equity. We may not be able to repurchase the notes upon a change of control because we may not have sufficient financial resources to purchase all of the notes that are tendered upon a change of control. In addition, the change of control covenant in the indenture does not cover all corporate reorganizations, mergers or similar transactions and may not provide you with protection in a highly leveraged transaction. See "Description of the Exchange Notes—Certain Covenants."
The optional redemption feature of the notes is likely to affect the market value of the notes and any optional redemption may affect the actual yield of your investment.
During any period in which such notes are subject to redemption at our option, their market value generally will not rise substantially above the redemption price because of the increased likelihood of redemption by us, and this also may be true prior to any such period. We may redeem all or part of the notes in circumstances where our cost of borrowing is lower than the interest rate on the notes. At such times, you generally would not be able to reinvest redemption proceeds at an effective interest rate which is as high as the interest rate on the notes, and such reinvestment might only be at a significantly lower rate. A partial redemption of the notes also may adversely affect liquidity for the remaining outstanding notes.
Because any redemption would occur prior to maturity, the repayment of principal would occur at times other than those you may have expected. In addition, if we choose to redeem all or part of the notes prior to December 1, 2009, the redemption price may be tied to United States Treasury securities. As a result, changes in the price of these securities may affect the actual yield of your investment.
Federal and state fraudulent transfer laws may permit a court to void the guarantees, and, if that occurs, you may not receive any payments on the notes.
Federal and state fraudulent transfer and conveyance statutes may apply to the issuance of the notes and the incurrence of the guarantees. Under federal bankruptcy law and comparable provisions of state fraudulent transfer or conveyance laws, which may vary from state to state, the notes or guarantees could be voided as a fraudulent transfer or conveyance if (1) we or any of the guarantors, as applicable, issued the notes or incurred the guarantees with the intent of hindering, delaying or defrauding creditors or (2) we or any of the guarantors, as applicable, received less than reasonably equivalent value or fair consideration in return for either issuing the notes or incurring the guarantees and, in the case of (2) only, one of the following is also true at the time thereof:
- •
- we or any of the guarantors, as applicable, were insolvent or rendered insolvent by reason of the issuance of the notes or the incurrence of the guarantees;
- •
- the issuance of the notes or the incurrence of the guarantees left us or any of the guarantors, as applicable, with an unreasonably small amount of capital to carry on the business;
- •
- we or any of the guarantors intended to, or believed that we or such guarantor would, incur debts beyond our or such guarantor's ability to pay as they mature; or
- •
- we or any of the guarantors was a defendant in an action for money damages, or had a judgment for money damages docketed against us or such guarantor if, in either case, after final judgment, the judgment is unsatisfied.
As a general matter, value is given for a transfer or an obligation if, in exchange for the transfer or obligation, property is transferred or an antecedent debt is secured or satisfied. A debtor will generally not be considered to have received value in connection with a debt offering if the debtor uses the
23
proceeds of that offering to make a dividend payment or otherwise retire or redeem equity securities issued by the debtor. In other instances, courts have found that a debtor did not receive reasonably equivalent value or fair consideration if, in a leveraged transaction, the proceeds of the issuance were paid to the debtor's stockholders, although we cannot predict how a court would rule in this case.
We cannot be certain as to the standards a court would use to determine whether or not we or the guarantors were solvent at the relevant time or, regardless of the standard that a court uses, that the issuance of the guarantees would not be further subordinated to our or any of our guarantors' other debt. Generally, however, an entity would be considered insolvent if, at the time it incurred indebtedness:
- •
- the sum of its debts, including contingent liabilities, was greater than the fair saleable value of all its assets; or
- •
- the present fair saleable value of its assets was less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or
- •
- it could not pay its debts as they become due.
If a court were to find that the issuance of the notes or the incurrence of the guarantee was a fraudulent transfer or conveyance, the court could void the payment obligations under the notes or such guarantee or further subordinate the notes or such guarantee to presently existing and future indebtedness of ours or of the applicable guarantor, or require the holders of the notes to repay any amounts received with respect to such guarantee. In the event of a finding that a fraudulent transfer or conveyance occurred, you may not receive any repayment on the notes. Further, the voidance of the notes could result in an event of default with respect to our and our subsidiaries' other debt that could result in acceleration of such debt.
Your ability to transfer the notes may be limited by the absence of an active trading market, and there is no assurance that any active trading market will develop for the notes.
We are offering the exchange notes to the holders of the outstanding notes. The outstanding notes were offered and sold in November 2005 to a small number of institutional investors and are eligible for trading in the PORTAL Market.
The initial purchasers have advised us that they intend to make a market in the outstanding notes, and the exchange notes, if issued, as permitted by applicable laws and regulations; however, the initial purchasers are not obligated to make a market in any of the outstanding notes or the exchanges notes and they may discontinue their market-making activities at any time without notice. Therefore, we cannot assure you that an active market for any of the outstanding notes or exchange notes will develop or, if developed, that it will continue. Historically, the market for non investment-grade debt has been subject to disruptions that have caused substantial volatility in the prices of securities similar to the notes. We cannot assure you that the market, if any, for any of the outstanding notes or exchange notes will be free from similar disruptions or that any such disruptions may not adversely affect the prices at which you may sell your notes. In addition, subsequent to their initial issuance, the outstanding notes or exchange notes may trade at a discount from their initial offering price, depending upon prevailing interest rates, the market for similar notes, our performance and other factors.
Risks Related to Our Business
Quality problems with our processes, products and services could harm our reputation for producing high quality products and erode our competitive advantage.
Quality is extremely important to us and our customers due to the serious and costly consequences of product failure. Many of our customers require us to adopt and comply with specific quality standards, and they periodically audit our performance. Our quality certifications are critical to the marketing success of our products and services. If we fail to meet these standards, our reputation could be damaged, we could lose customers and our revenue could decline. Aside from specific customer
24
standards, our success depends generally on our ability to manufacture to exact tolerances precision engineered components, subassemblies and finished devices from multiple materials. If our components fail to meet these standards or fail to adapt to evolving standards, our reputation as a manufacturer of high quality components could be harmed, our competitive advantage could be damaged, and we could lose customers and market share.
If we experience decreasing prices for our products and services and we are unable to reduce our expenses, our results of operations will suffer.
We may experience decreasing prices for the products and services we offer due to:
- •
- pricing pressure experienced by our customers from managed care organizations and other third party payors;
- •
- increased market power of our customers as the medical device industry consolidates; and
- •
- increased competition among medical engineering and manufacturing services providers.
If the prices for our products and services decrease and we are unable to reduce our expenses, our results of operations will be adversely affected.
Because a significant portion of our net sales comes from a few large customers, any decrease in sales to these customers could harm our operating results.
The medical device industry is concentrated, with relatively few companies accounting for a large percentage of sales in the cardiology, endoscopy and orthopaedic markets that we target. Accordingly, our net sales and profitability are highly dependent on our relationships with a limited number of large medical device companies. Pro forma for the nine months ended September 30, 2005, our top 15 customers accounted for approximately 70% of our net sales. In particular, Johnson & Johnson, Boston Scientific and Medtronic each accounted for more than 10% of our net sales for this period on a pro forma basis. We are likely to continue to experience a high degree of customer concentration, particularly if there is further consolidation within the medical device industry. We cannot assure you that net sales from customers that have accounted for significant net sales in the past, either individually or as a group, will reach or exceed historical levels in any future period. For example, Boston Scientific is currently transferring a number of products currently assembled by us to its own assembly operation. Based on preliminary estimates and our experience to date with this customer, we expect net sales from Boston Scientific to decrease annually by approximately $40 million, with the substantial majority of this net sales decrease commencing in 2006. We can provide no assurance that we will replace such business and that the loss will not adversely affect our operating results in 2006 and thereafter. For a detailed discussion of the Boston Scientific relationship, see "Management's Discussion and Analysis of Financial Condition and Results of Operations—Overview." The loss or a significant reduction of business from any of our major customers would adversely affect our results of operations.
We may not be able to continue to grow our business if the trend by medical device companies to outsource their manufacturing activities does not continue or if our customers decide to manufacture internally products that we currently provide.
Our design, manufacturing and assembly business has grown partly as a result of the increase over the past several years in medical device companies outsourcing these activities. We view the increasing use of outsourcing by medical device companies as an important component of our future growth strategy. While industry analysts expect the outsourcing trend to increase, our current and prospective customers continue to evaluate our capabilities against the merits of internal production. As previously discussed, Boston Scientific is currently transferring a number of products currently assembled by us to its own assembly operation. Protecting intellectual property rights and maximizing control over regulatory compliance are among factors that may influence medical device companies to keep production in-house. Any substantial slowing of growth rates or decreases in outsourcing by medical
25
device companies could cause our revenue to decline, and we may be limited in our ability or unable to continue to grow our business.
Our operating results may fluctuate, which may make it difficult to forecast our future performance.
Fluctuations in our operating results may cause uncertainty concerning our performance and prospects or may result in our failure to meet expectations. Our operating results have fluctuated in the past and are likely to fluctuate significantly in the future due to a variety of factors, which include, but are not limited to:
- •
- the fixed nature of a substantial percentage of our costs, which results in our operations being particularly sensitive to fluctuations in revenue;
- •
- changes in the relative portion of our revenue represented by our various products, which could result in reductions in our profits if the relative portion of our revenue represented by lower margin products increases;
- •
- introduction and market acceptance of our customers' new products and changes in demand for our customers' existing products;
- •
- the accuracy of our customers' forecasts of future production requirements;
- •
- timing of orders placed by our principal customers that account for a significant portion of our revenues;
- •
- timing of payments by customers;
- •
- price concessions as a result of pressure to compete;
- •
- cancellations by customers as a result of which we may recover only our costs plus our target markup;
- •
- availability of raw materials, including nitinol, elgiloy, tantalum, stainless steel, columbium, zirconium, titanium, gold, silver and platinum;
- •
- increased costs of raw materials, supplies or skilled labor;
- •
- effectiveness in managing our manufacturing processes; and
- •
- changes in competitive and economic conditions generally or in our customers' markets.
Investors should not rely on results of operations in any past period as an indication of what our results will be for any future period.
Our industry is very competitive; we may face competition from, and we may be unable to compete successfully against, new entrants and established companies with greater resources.
The market for outsourced manufacturing and engineering services to the medical device industry is very competitive and includes thousands of companies. As more medical device companies seek to outsource more of the design, prototyping and manufacturing of their products, we will face increasing competitive pressures to grow our business in order to maintain our competitive position, and we may encounter competition from and lose customers to other companies with design, technological and manufacturing capabilities similar to ours. Some of our potential competitors may have greater name recognition, greater operating revenues, larger customer bases, longer customer relationships and greater financial, technical, personnel and marketing resources than we have. If we are unsuccessful competing with our competitors for our existing and prospective customers' business, we could lose business and our financial results could suffer.
As we rationalize manufacturing capacity and shift production to more economical facilities, our customers may choose to reallocate their outsource requirements among our competitors or perform such functions internally.
As we integrate acquired operations and rationalize manufacturing capability and shift production to more economical facilities, our customers may evaluate their outsourcing requirements and decide to use the services of our competitors or move design and production work back to their own internal
26
facilities. For some customers, geographic proximity to the outsourced design or manufacturing facility may be an important consideration and our reallocation may cause them to no longer use our services for future work. If our customers reallocate work among outsourcing vendors or complete design or production in their own facilities, we would lose business, which could impair our growth and operating results. Further, unanticipated delays or difficulties in facility consolidation and rationalization of our current and future facilities could cause interruptions in our services which could damage our reputation and relationships with our customers and could result in a loss of customers and market share.
If we do not respond to changes in technology, our manufacturing, design and engineering processes may become obsolete and we may experience reduced sales and lose customers.
We use highly engineered, proprietary processes and highly sophisticated machining equipment to meet the critical specifications of our customers. Without the timely incorporation of new processes and enhancements, particularly relating to quality standards and cost-effective production, our manufacturing, design and engineering capabilities will likely become outdated, which could cause us to lose customers and result in reduced revenues or profit margins. In addition, new or revised technologies could render our existing technology less competitive or obsolete or could reduce demand for our products and services. It is also possible that finished medical device products introduced by our customers may require fewer of our components or may require components that we lack the capabilities to manufacture or assemble. In addition, we may expend resources on developing new technologies that do not result in commercially viable processes for our business, which could adversely impact our margins and operating results.
Inability to obtain sufficient quantities of raw materials and production feedstock could cause delays in our production.
Our business depends on a continuous supply of raw materials and production feedstock. Raw materials and production feedstock needed for our business are susceptible to fluctuations in price and availability due to transportation costs, government regulations, price controls, change in economic climate or other unforeseen circumstances. Failure to maintain our supply of raw materials and production feedstock could cause production delays resulting in a loss of customers and a decline in revenue. Due to the supply and demand fundamentals of raw material and production feedstock used by us, we have occasionally experienced extended lead times on purchases and deliveries from our suppliers. Consequently, we have had to adjust our delivery schedule to customers. In addition, fluctuations in the cost of raw materials and production feedstock may increase our expenses and affect our operating results. The principal raw materials and production feedstock used in our business include stainless steel, tantalum, columbium, zirconium, titanium, nitinol, elgiloy, gold, silver, platinum, hydrogen, natural gas and electricity. In particular, tantalum and nitinol are in limited supply. For wire fabrication, we purchase most of our stainless steel wire from an independent, third party supplier. The loss of this supplier could interrupt production and harm our business.
Our international operations are subject to a variety of risks that could adversely affect those operations and thus our profitability and operating results.
We have international manufacturing operations in Europe and Mexico. We also receive a portion of our net sales from international sales, approximately two-thirds of which is generated by exports from our facilities in the United States and the remaining of which is generated by sales from our international facilities. Although we take measures to minimize risks inherent to our international operations, the following risks may have a negative effect on our profitability and operating results, impair the performance of our foreign operations or otherwise disrupt our business:
- •
- fluctuations in the value of currencies could cause exchange rates to change and impact our profitability;
27
- •
- changes in labor conditions and difficulties in staffing and managing foreign operations, including labor unions, could lead to delays or disruptions in production or transportation of materials or our finished products;
- •
- greater difficulty in collecting accounts receivable and longer payment cycles, which can be more common in our international operations, could adversely impact our operating results over a particular fiscal period; and
- •
- changes in foreign regulations, export duties, taxation and limitations on imports or exports could increase our operational costs, impose fines or restrictions on our ability to carry on our business or expand our international operations.
We may expand into new markets and products and our expansion may not be successful.
We may expand into new markets through the development of new product applications based on our existing specialized manufacturing, design and engineering capabilities and services. These efforts could require us to make substantial investments, including significant research, development, engineering and capital expenditures for new, expanded or improved manufacturing facilities which would divert resources from other aspects of our business. Expansion into new markets and products may be costly without resulting in any benefit to us. Specific risks in connection with expanding into new markets include the inability to transfer our quality standards into new products, the failure of customers in new markets to accept our products and price competition in new markets. If we choose to expand into new markets and are unsuccessful, our financial condition could be adversely affected and our business harmed.
We are subject to a variety of environmental laws that could be costly for us to comply with, and we could incur liability if we fail to comply with such laws or if we are responsible for releases of contaminants to the environment.
Federal, state and local laws impose various environmental controls on the management, handling, generation, manufacturing, transportation, storage, use and disposal of hazardous chemicals and other materials used or generated in the manufacturing of our products. If we fail to comply with any present or future environmental laws, we could be subject to fines, corrective action, other liabilities or the suspension of production. We have in the past paid civil penalties for violations of environmental laws. To date, such matters have not had a material adverse impact on our business or financial condition. We cannot assure you, however, that such matters will not have a material impact on us in the future.
In addition, conditions relating to our operations may require expenditures for clean-up of releases of hazardous chemicals into the environment. For example, we were required and continue to perform remediation as a result of leaks from underground storage tanks at our Collegeville, Pennsylvania facility. In addition, we may have future liability with respect to contamination at our current or former properties or with respect to third party disposal sites. Although we do not anticipate that currently pending matters will have a material adverse effect on our results of operations and financial condition, we cannot assure you that these matters or others that arise in the future will not have such an effect.
Changes in environmental laws may result in costly compliance requirements or otherwise subject us to future liabilities. In addition, to the extent these changes affect our customers and require changes to their devices, our customers could have a reduced need for our products and services, and, as a result, our revenue could suffer.
28
Our inability to protect our intellectual property could result in a loss of our competitive advantage, and infringement claims by third parties could be costly and distracting to management.
We rely on a combination of patent, copyright, trade secret and trademark laws, confidentiality procedures and contractual provisions to protect our intellectual property. The steps we have taken or will take to protect our proprietary rights may not adequately deter unauthorized disclosure or misappropriation of our intellectual property, technical knowledge, practice or procedures.
We may be required to spend significant resources to monitor our intellectual property rights, we may be unable to detect infringement of these rights and we may lose our competitive advantage associated with our intellectual property rights before we do so. If it became necessary for us to resort to litigation to protect our intellectual property rights, any proceedings could be burdensome and costly and we may not prevail. Although we do not believe that any of our products, services or processes infringe the intellectual property rights of third parties, historically, patent applications in the United States and some foreign countries have not been publicly disclosed until the patent is issued (or as of recently, until publication, which occurs eighteen months after filing), and we may not be aware of currently filed patent applications that relate to our products or processes. If patents later issue on these applications, we may in the future be notified that we are infringing patent or other intellectual property rights of third parties and we may be liable for infringement at that time. In the event of infringement of patent or other intellectual property rights, we may not be able to obtain licenses on commercially reasonable terms, if at all, and we may end up in litigation. The failure to obtain necessary licenses or other rights or the occurrence of litigation arising out of infringement claims could disrupt our business and impair our ability to meet our customers' needs which, in turn, could have a negative effect on our financial condition and results of operations. Infringement claims, even if not substantiated, could result in significant legal and other costs and may be a distraction to management. We also may be subject to significant damages or injunctions against development and sale of our products.
In addition, any infringement claims, significant charges or injunctions against our customers' products that incorporate our components may result in our customers not needing or having a reduced need for our capabilities and services.
Our earnings and financial condition could suffer if we or our customers become subject to product liability claims or recalls. We may also be required to spend significant time and money responding to investigations or requests for information related to end-products of our customers, including for example, responding to the subpoena we received in the investigation of Guidant Corporation described below in which we have been informed we are a witness.
The manufacture and sale of products that incorporate components manufactured or assembled by us exposes us to potential product liability claims and product recalls, including those that may arise from misuse or malfunction of, or design flaws in, our components or use of our components with components or systems not manufactured or sold by us. Product liability claims or product recalls with respect to our components or the end-products of our customers into which our components are incorporated, whether or not such problems relate to the products and services we have provided and regardless of their ultimate outcome, could require us to pay significant damages or to spend significant time and money in litigation or responding to investigations or requests for information. We manufacture polyimide products for Guidant in accordance with Guidant's design specifications. On October 25, 2005, Guidant publicly announced that the U.S. Attorney's Office in Minneapolis, Minnesota had issued a subpoena to Guidant requesting documents relating to its Ventak Prizm 2 and Contak Renewal 1 and 2 defibrillator devices. We received a subpoena dated October 28, 2005 from the Minneapolis office of the U.S. Attorney in connection with this investigation. The subpoena requests documents relating to polyimide products which we manufacture for use in implanted medical devices and also documents regarding the risks related to the use of polyimide in any medical device implanted in the human body. We have been orally advised by the office of the U.S. Attorney in
29
Minneapolis that we are providing this information as a witness to this investigation. We intend to cooperate fully in responding to the subpoena.
We may also lose revenue from the sale of components if the commercialization of a product that incorporates our components or subassemblies is limited or ceases as a result of such claims or recalls. For example, two of MedSource's products were subject to recalls in 2001 and 2002. As a result of such product recalls, our customer redesigned the manufacturing process and decided to manufacture the device internally, resulting in lost annual revenues of approximately $5.0 million and $2.0 million for 2001 and 2002, respectively. In addition, certain finished medical devices into which our components were incorporated have been subject to product recalls. Expenditures on litigation or damages, to the extent not covered by insurance, and declines in revenue could impair our earnings and our financial condition. Also, if, as a result of claims or recalls our reputation is harmed, we could lose customers, which would also negatively affect our business.
We cannot assure you that we will be able to maintain our existing insurance, which is currently insured at an aggregate level of $25 million per year, or to do so at reasonable cost and on reasonable terms. In addition, if our insurance coverage is not sufficient to cover any costs we may incur or damages we may be required to pay if we are subject to product liability claims or product recalls, we will have to use other resources to satisfy our obligations.
We and our customers are subject to various political, economic and regulatory changes in the healthcare industry that could force us to modify how we develop and price our components, manufacturing capabilities and services and could harm our business.
The healthcare industry is highly regulated and is influenced by changing political, economic and regulatory factors. Federal and state legislatures have periodically considered programs to reform or amend the United States healthcare system at both the federal and state levels. Regulations affecting the healthcare industry in general, and the medical device industry in particular, are complex, change frequently and have tended to become more stringent over time. In addition, these regulations may contain proposals to increase governmental involvement in healthcare, lower reimbursement rates or otherwise change the environment in which healthcare industry participants, including medical device companies, operate. While we are not aware of any legislation or regulations specifically targeting the medical device industry that are currently pending, any such regulations could impair our ability to operate profitably. In addition, any failure by us to comply with applicable government regulations could also result in the cessation of portions or all of our operations, impositions of fines and restrictions on our ability to carry on or expand our operations.
Consolidation in the healthcare industry could have an adverse effect on our revenues and results of operations.
Many healthcare industry companies, including medical device companies, are consolidating to create new companies with greater market power. As the healthcare industry consolidates, competition to provide products and services to industry participants will become more intense. These industry participants may try to use their market power to negotiate price concessions or reductions for medical devices that incorporate components produced by us. If we are forced to reduce our prices because of consolidation in the healthcare industry, our revenues would decrease and our business, financial condition and results of operations would suffer.
Our business is indirectly subject to healthcare industry cost containment measures that could result in reduced sales of medical devices containing our components.
Our customers and the healthcare providers to whom our customers supply medical devices rely on third party payors, including government programs and private health insurance plans, to reimburse some or all of the cost of the procedures in which medical devices that incorporate components manufactured or assembled by us are used. The continuing efforts of government, insurance companies and other payors of healthcare costs to contain or reduce those costs could lead to patients being
30
unable to obtain approval for payment from these third party payors. If that were to occur, sales of finished medical devices that include our components may decline significantly, and our customers may reduce or eliminate purchases of our components. The cost containment measures that healthcare providers are instituting, both in the United States and internationally, could harm our ability to operate profitably. For example, managed care organizations have successfully negotiated volume discounts for pharmaceuticals. While this type of discount pricing does not currently exist for medical devices, if managed care or other organizations were able to affect discount pricing for devices, it may result in lower prices to our customers from their customers and, in turn, reduce the amounts we can charge our customers for our design and manufacturing services.
Accidents at one of our facilities could delay production and could subject us to claims for damages.
Our business involves complex manufacturing processes and hazardous materials that can be dangerous to our employees. We employ safety procedures in the design and operation of our facilities; however, there is a risk that an accident or death could occur in one of our facilities. Any accident could result in significant manufacturing delays, disruption of operations or claims for damages resulting from injuries, which could result in decreased sales and increased expenses. To date, we have not incurred any such significant delays, disruptions or claims. The potential liability resulting from any accident or death, to the extent not covered by insurance, would require us to use other resources to satisfy our obligations and could cause our business to suffer.
A substantial amount of our assets represents goodwill, and our net income will be reduced if our goodwill becomes impaired.
As of September 30, 2005, on a pro forma as adjusted basis, our estimated goodwill, net represented approximately $883.5 million, or 61.9%, of our total assets. Goodwill is generated in our acquisitions when the cost of an acquisition exceeds the fair value of the net tangible and identifiable intangible assets we acquire. Goodwill is subject to an impairment analysis at least annually based on the fair value of the reporting unit. We could be required to recognize reductions in our net income caused by the write-down of goodwill, which if significantly impaired, could materially and adversely affect our results of operations.
Our inability to access additional capital could have a negative impact on our growth strategy.
Our growth strategy will require additional capital for, among other purposes, completing acquisitions, managing acquired companies, acquiring new equipment and maintaining the condition of existing equipment. If cash generated internally is insufficient to fund capital requirements, or if funds are not available under our senior secured credit facility, we will require additional debt or equity financing. Adequate financing may not be available or, if available, may not be available on terms satisfactory to us. If we fail to obtain sufficient additional capital in the future, we could be forced to curtail our growth strategy by reducing or delaying capital expenditures and acquisitions, selling assets or restructuring or refinancing our indebtedness. See "Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources."
Our industrial operations are highly cyclical.
We have established customer relationships with companies outside of the medical device market, pursuant to which these customers incorporate our products and services into their products such as high density discharge lamps, fiber optics, motion sensors and power generators. For the nine months ended September 30, 2005, on a pro forma basis, our industrial operations accounted for approximately 8.2% of our net sales. Historically, net sales from these operations have been highly cyclical. We believe volatility in this area of our operations is due in part to lower sales to customers servicing the electronics, power generation, telecommunication, aerospace and industrial markets due to the economic downturn. Accordingly, we cannot predict when volatility will occur and how severely it will impact our results of operations.
31
We face risks associated with the implementation of our new Enterprise Resource Planning System.
We are in the process of installing a third party enterprise resource planning system, or ERP System, across our facilities, which will enable the sharing of customer, supplier and engineering data across our company. The installation and integration of the ERP System may divert the attention of our information technology professionals and certain members of management from the management of daily operations to the integration of the ERP System. Further, we may experience unanticipated delays in the implementation of the ERP System, difficulties in the integration of the ERP System across our facilities or interruptions in service due to failures of the ERP System. Continuing and uninterrupted performance of our ERP System is critical to the success of our business strategy. Any damage or failure that interrupts or delays operations may dissatisfy customers and could have a material adverse effect on our business, financial condition, results of operations and cash flow.
We license the ERP software from a third party. If these licenses are discontinued, or become invalid or unenforceable, there can be no assurance that we will be able to develop substitutes for this software independently or to obtain alternative sources at acceptable prices or in a timely manner. Any delays in obtaining or developing substitutes for licensed software could have a material adverse effect on our operations.
The loss of the services of any members of our senior management could adversely affect our business.
Our success depends upon the retention of our senior management, including Ron Sparks, our President and Chief Executive Officer. We cannot assure you that we would be able to find qualified replacements for the individuals who make up our senior management if their services were no longer available. The loss of services of one or more members of our senior management team could have a material adverse effect on our business, financial condition and results of operations. We have entered into employment agreements with Ron Sparks, Stewart A. Fisher, Gary D. Curtis, Daniel C. Croteau and John Konsin. We do not currently maintain key-man life insurance for any of our employees.
Our business may suffer if we are unable to recruit and retain the experienced engineers and management personnel that we need to compete in the medical device industry.
Our future success depends upon our ability to attract, retain and motivate highly skilled engineers and management personnel. We may not be successful in attracting new engineers or management personnel or in retaining or motivating our existing personnel, which may lead to increased recruiting, relocation and compensation costs for such personnel. These increased costs may reduce our profit margins or make hiring new engineers impracticable. Some of our manufacturing processes are highly technical in nature. Our ability to maintain, expand or renew existing engagements with our customers, enter into new engagements and provide additional services to our existing customers depends on our ability to hire and retain engineers with the skills necessary to keep pace with continuing changes in the medical device industry. We compete with other companies in the medical device industry to recruit engineers. Our inability to hire additional qualified personnel may also require an increase in the workload for both existing and new personnel.
We depend on outside suppliers and subcontractors, and our production and reputation could be harmed if they are unable to meet our volume and quality requirements and alternative sources are not available.
Our current capabilities do not include all elements that are required to satisfy all of our customers' requirements. As we position ourselves to provide our customers with a single source solution, we may rely increasingly on third party suppliers, subcontractors and other outside sources for components or services. Manufacturing problems may occur with these third parties. A supplier may fail to develop and supply products and components to us on a timely basis, or may supply us with products and components that do not meet our quality, quantity or cost requirements. If any of these problems occur, we may be unable to obtain substitute sources of these products and components on a timely basis or on terms acceptable to us, which could harm our ability to manufacture our own products and components profitably or on time. In addition, if the processes that our suppliers use to
32
manufacture products and components are proprietary, we may be unable to obtain comparable components from alternative suppliers.
We have engaged in several acquisitions during the last several years as part of our growth strategy and may selectively pursue complementary acquisitions in the future, but, because of the uncertainty involved, we may not be able to locate suitable acquisition candidates and may not successfully integrate acquired businesses into our business and operations.
We may selectively pursue complementary acquisitions. However, we may not be able to identify potential acquisition candidates that we think could complement our business or may not be able to negotiate acceptable terms for any acquisition candidates we are able to identify. As a result, we may not be able to realize this element of our growth strategy. In addition, even if we are successful in acquiring any businesses, we may experience material negative consequences to our business, financial condition or results of operations if we cannot successfully integrate the operations of any acquired businesses with ours. The integration of companies that have previously been operated separately involves a number of risks, including, but not limited to:
- •
- demands on management related to the significant increase in the size of the business for which they are responsible;
- •
- diversion of management's attention from the management of daily operations to the integration of operations;
- •
- management of employee relations across facilities;
- •
- difficulties in the assimilation of different corporate cultures and practices, as well as in the assimilation and retention of broad and geographically dispersed personnel and operations;
- •
- difficulties and unanticipated expenses related to the integration of departments, systems (including accounting systems), technologies, books and records, procedures and controls (including internal accounting controls, procedures and policies), as well as in maintaining uniform standards, including environmental management systems;
- •
- expenses of any undisclosed or potential liabilities; and
- •
- ability to maintain our and our acquired companies' customers after the acquisitions.
Successful integration of acquired operations with ours depends on our ability to manage the combined operations, to realize opportunities for revenue growth presented by broader product offerings and expanded geographic coverage and to eliminate redundant and excess costs. If our integration efforts are not successful, we may not be able to maintain the levels of revenues, earnings or operating efficiency that we and the acquired companies achieved or might achieve separately.
Our Sponsors may have interests that conflict with yours.
The Sponsors control our affairs and policies. Circumstances may occur in which the interests of the Sponsors could be in conflict with the interests of the holders of the notes. In addition, the Sponsors may have an interest in pursuing acquisitions, divestitures or other transactions that, in their judgment, could enhance their equity investment, even though such transactions might involve risks to the holders of the notes if the transactions resulted in our being more leveraged or significantly changed the nature of our business operations or strategy. In addition, if we encounter financial difficulties, or we are unable to pay our debts as they mature, the interests of the Sponsors might conflict with those of the holders of the notes. In that situation, for example, the holders of the notes might want us to raise additional equity from the Sponsors or other investors to reduce our leverage and pay our debts, while the Sponsors might not want to increase their investment in us or have their ownership diluted and instead choose to take other actions, such as selling our assets. Additionally, the Sponsors and certain of their affiliates are in the business of making investments in companies and may from time to time in the future acquire interests in businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours. Further, if the Sponsors pursue such acquisitions or make further investments in our industry, those acquisition and investment opportunities may not be available to us. So long as the Sponsors continue to indirectly own a significant amount of our equity, even if such amount is less than 50%, they will continue to be able to influence or effectively control our decisions.
33
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains "forward-looking statements" within the meaning of the federal securities laws, which involve risks and uncertainties. You can identify forward-looking statements because they contain words such as "believes," "expects," "may," "will," "should," "seeks," "approximately," "intends," "plans," "estimates," or "anticipates" or similar expressions that concern, among other things, our results of operations, financial condition, liquidity, prospects, growth, strategies and the industry in which we operate. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of their dates. These forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain and subject to a number of risks and uncertainties. These risks and uncertainties include, without limitation, those identified under "Risk Factors" and elsewhere in this prospectus. All subsequent written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by the cautionary statements.
The following list represents some, but not necessarily all, of the factors that could cause actual results to differ from historical results or those anticipated or predicted by these forward-looking statements:
- •
- our substantial indebtedness following the consummation of the Transactions, as described herein;
- •
- quality problems with our processes, products and services;
- •
- decreases in prices for our products and services;
- •
- reliance on a few large customers for a significant portion of our net sales;
- •
- unpredictable product cycles of the medical device manufacturing industry and uncertain demand for our manufacturing, design and engineering capabilities and related services;
- •
- competition from other companies;
- •
- change in industry demand for outsourced medical device manufacturing;
- •
- loss of significant customers or customer relationships due to the customers' shift to internal production or the relocation of our production facilities;
- •
- failure to respond to changes in technology which may cause our manufacturing, design and engineering process to become obsolete;
- •
- inability to obtain sufficient quantities of raw materials and production feedstock that could cause delays in production;
- •
- our international operations' exposure to foreign currency fluctuations, exchange rates, laws and regulations, longer payment cycles and greater difficulty in collecting accounts receivables;
- •
- environmental regulations;
- •
- loss of intellectual properties;
- •
- possibility of product liability claims;
- •
- various political, economic and regulatory changes in the healthcare industry;
- •
- consolidation in the healthcare industry;
- •
- accidents at our facilities that results in production delay or claims for damages;
- •
- risk of implementing our new Enterprise Resource Planning System;
34
- •
- inability to locate suitable acquisition candidates and to integrate acquisitions into our business and operations;
- •
- control by our controlling shareholder; and
- •
- the other factors set forth under "Risk Factors."
We caution you that the foregoing list of important factors may not contain all of the material factors that are important to you. In addition, in light of these risks and uncertainties, the matters referred to in the forward-looking statements contained in this prospectus may not in fact occur. We undertake no obligation to publicly update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
35
USE OF PROCEEDS
We will not receive any cash proceeds from the issuance of the exchange notes. In consideration for issuing the exchange notes as contemplated in this prospectus, we will receive in exchange a like principal amount of outstanding notes, the terms of which are identical in all material respects to the exchange notes, except that the exchange notes are registered under the Securities Act, are not entitled to the registration rights which are applicable to the outstanding notes, and are not subject to certain special interest rate provisions applicable to the outstanding notes. The outstanding notes surrendered in exchange for the exchange notes will be retired and canceled and cannot be reissued. Accordingly, issuance of the exchange notes will not result in any change in our capitalization.
36
CAPITALIZATION
The following table sets forth our capitalization as of September 30, 2005:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to the MTG acquisition; and
- •
- on a pro forma as adjusted basis to give further effect to the Transactions.
The information in this table should be read in conjunction with "Selected Historical Consolidated Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "The Transactions" and the consolidated financial statements and related notes and the Unaudited Pro Forma Condensed Combined Financial Statements and the related notes included elsewhere in this prospectus.
| | As of September 30, 2005
|
|---|
| | Historical
| | Pro Forma
| | Pro Forma
As Adjusted
|
|---|
| | (In millions)
|
|---|
| Debt: | | | | | | | | | |
| Existing senior secured credit facility(1): | | | | | | | | | |
| | Revolving credit facility | | $ | 8.0 | | $ | 29.5 | | $ | — |
| | Term loan facility | | | 191.6 | | | 204.1 | | | — |
| Senior secured credit facility(2): | | | | | | | | | |
| | Revolving credit facility | | | — | | | — | | | — |
| | Term loan B facility | | | — | | | — | | | 400.0 |
| 10% Senior Subordinated Notes of Accellent Corp. | | | 175.0 | | | 175.0 | | | — |
| 101/2% Senior Subordinated Notes of Accellent Inc., net of discount | | | — | | | — | | | 300.9 |
| Equipment loans and capital leases | | | — | | | — | | | — |
| | |
| |
| |
|
| | Total debt | | $ | 374.6 | | $ | 408.6 | | $ | 700.9 |
| Total stockholder's equity | | | 153.0 | | | 169.2 | | | 638.3 |
| | |
| |
| |
|
| | Total capitalization | | $ | 527.6 | | $ | 577.8 | | $ | 1,339.2 |
| | |
| |
| |
|
- (1)
- Consists of a six-year $194.0 million term facility and a five-year $40.0 million revolving credit facility. In addition, Accellent Corp. may borrow up to $50.0 million in additional term loans under the facility, with approval of participating lenders. As of September 30, 2005, Accellent Corp. had approximately $5.8 million of letters of credit outstanding.
- (2)
- In connection with the Transactions, we entered into a senior secured credit facility, which consists of a $400.0 million seven-year term loan B facility and a $75.0 million six-year revolving credit facility. Our outstanding letters of credit remain approximately $5.8 million subsequent to the Transactions, which reduce the amounts available under the revolving credit portion of our senior secured credit facility.
37
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
The following presents our selected historical consolidated financial data for each of the five full fiscal years in the period ended December 31, 2004 and for each of the nine month periods ended September 30, 2004 and 2005. The operating data for each of the three years in the period ended December 31, 2004 and the balance sheet data as of December 31, 2003 and 2004 were derived from our audited consolidated financial statements included elsewhere in this prospectus. The balance sheet data as of December 31, 2002 were derived from our audited financial statements that are not included in this prospectus. The balance sheet data as of December 31, 2001 and 2000, and the operating data for the years ended December 31, 2001 and 2000 were derived from our unaudited financial statements that are not included in this prospectus. Our operating data for the nine month periods ended September 30, 2004 and 2005 and our balance sheet data as of September 30, 2004 and 2005 were derived from our unaudited consolidated financial statements that are included elsewhere in this prospectus. Our unaudited consolidated financial statements have been prepared on the same basis as our audited consolidated financial statements. In our opinion, the unaudited consolidated financial statements from which the data below is derived contain all adjustments, which consist only of normal and recurring adjustments, necessary for a fair presentation of our results for those periods. The results of operations for any period are not necessarily indicative of the results to be expected for any future period.
We acquired UTI Corporation, a Pennsylvania corporation and our wholly owned subsidiary that we refer to as UTI, in June 2000. Although we were the acquiring corporation, UTI was substantially larger than us at the time of the acquisition and is viewed as our corporate predecessor for financial accounting purposes. In accordance with applicable SEC rules, we have presented separately the selected historical consolidated financial data of UTI for the five month period ended May 31, 2000 (the date immediately preceding our acquisition of UTI), in order to show a full five years of selected historical financial data. The operating data for UTI for the five months ended May 31, 2000 and the balance sheet data for UTI as of May 31, 2000 are derived from the audited consolidated financial statements of UTI that are not included in this prospectus.
We consummated the MedSource acquisition on June 30, 2004 and, as a result, the assets and liabilities of MedSource are recorded on our balance sheet as of the date of the MedSource acquisition and the results of operations of MedSource for June 30, 2004 are included in our results for that day. We consummated the Campbell acquisition on September 12, 2005, and the MTG acquisition on October 6, 2005. As a result, the assets and liabilities of MTG are not recorded on our balance sheet at September 30, 2005. The results of operations of Campbell and MTG will be reflected in our results for the day of the acquisition and each day thereafter.
The information presented below should be read in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes thereto included elsewhere in this prospectus.
38
Our Company
| | Twelve Months Ended December 31,
| | Nine Months Ended
September 30,
| |
|---|
| | 2000(1)
| | 2001(1)
| | 2002(1)
| | 2003(1)
| | 2004(1)
| | 2004(1)
| | 2005
| |
|---|
| | (In thousands)
| |
|---|
| STATEMENT OF OPERATIONS DATA: | | | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 77,965 | | $ | 137,488 | | $ | 135,841 | | $ | 174,223 | | $ | 320,169 | | $ | 212,485 | | $ | 340,253 | |
| Cost of sales | | | 54,403 | | | 88,974 | | | 96,740 | | | 121,029 | | | 234,396 | | | 155,155 | | | 234,122 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Gross profit | | | 23,562 | | | 48,514 | | | 39,101 | | | 53,194 | | | 85,773 | | | 57,330 | | | 106,131 | |
| Selling, general and administrative expenses | | | 19,055 | | | 27,040 | | | 23,548 | | | 28,612 | | | 45,912 | | | 30,681 | | | 49,539 | |
| Research and development expenses | | | 1,321 | | | 2,106 | | | 2,380 | | | 2,603 | | | 2,668 | | | 2,023 | | | 2,294 | |
| Restructuring and other charges(2) | | | — | | | — | | | 2,440 | | | 1,487 | | | 3,600 | | | 2,107 | | | 3,824 | |
| Impairment of goodwill and intangibles(3) | | | — | | | — | | | 21,725 | | | — | | | — | | | — | | | — | |
| Amortization of intangibles(3) | | | 8,140 | | | 10,067 | | | 4,703 | | | 4,828 | | | 5,539 | | | 3,937 | | | 4,660 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | (4,954 | ) | | 9,301 | | | (15,695 | ) | | 15,664 | | | 28,054 | | | 18,582 | | | 45,814 | |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Interest expense, net | | | (11,363 | ) | | (17,802 | ) | | (16,923 | ) | | (16,587 | ) | | (26,879 | ) | | (19,397 | ) | | (23,731 | ) |
| | Other(4) | | | 35 | | | (1 | ) | | 61 | | | (9 | ) | | (3,312 | ) | | (3,284 | ) | | (105 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Total other expense | | | (11,328 | ) | | (17,803 | ) | | (16,862 | ) | | (16,596 | ) | | (30,191 | ) | | (22,681 | ) | | (23,836 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) before income taxes | | | (16,282 | ) | | (8,502 | ) | | (32,557 | ) | | (932 | ) | | (2,137 | ) | | (4,099 | ) | | 21,978 | |
| Income tax expense (benefit) | | | (5,404 | ) | | (1,504 | ) | | (5,145 | ) | | 13,872 | | | 3,483 | | | 2,341 | | | 10,045 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net income (loss) | | $ | (10,878 | ) | $ | (6,998 | ) | $ | (27,412 | ) | $ | (14,804 | ) | $ | (5,620 | ) | $ | (6,440 | ) | $ | 11,933 | |
OTHER FINANCIAL DATA: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flows provided by (used in): | | | | | | | | | | | | | | | | | | | | | | |
| | Operating activities | | $ | 6,779 | | $ | 9,362 | | $ | 14,022 | | $ | 14,392 | | $ | 22,231 | | $ | 6,179 | | $ | 21,470 | |
| | Investing activities | | | (204,916 | ) | | (14,163 | ) | | (9,446 | ) | | (20,370 | ) | | (227,376 | ) | | (221,317 | ) | | (35,623 | ) |
| | Financing activities | | | 205,349 | | | (439 | ) | | (1,517 | ) | | 3,977 | | | 217,071 | | | 218,583 | | | 5,701 | |
| Capital expenditures | | | 3,145 | | | 6,497 | | | 6,218 | | | 6,371 | | | 13,900 | | | 8,718 | | | 15,586 | |
| Depreciation and amortization | | | 11,902 | | | 15,455 | | | 10,858 | | | 11,591 | | | 16,152 | | | 11,289 | | | 16,062 | |
| EBITDA(5) | | | 6,983 | | | 24,755 | | | (4,776 | ) | | 27,246 | | | 40,894 | | | 26,587 | | | 61,771 | |
| Ratio of earnings to fixed charges(6) | | | — | | | — | | | — | | | — | | | — | | | — | | | 1.9 | x |
| Deficiency of earnings to fixed charges | | | 16,282 | | | 8,502 | | | 32,557 | | | 932 | | | 2,137 | | | 4,099 | | | — | |
BALANCE SHEET DATA (at period end): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents | | $ | 8,058 | | $ | 2,818 | | $ | 5,877 | | $ | 3,974 | | $ | 16,004 | | $ | 7,403 | | $ | 7,388 | |
| Total assets | | | 266,350 | | | 262,081 | | | 235,775 | | | 279,135 | | | 600,229 | | | 601,690 | | | 608,668 | |
| Total debt | | | 140,020 | | | 140,189 | | | 144,411 | | | 136,246 | | | 368,052 | | | 368,543 | | | 374,624 | |
| Redeemable and convertible preferred stock | | | 540 | | | 540 | | | 540 | | | 12,593 | | | 30 | | | 30 | | | — | |
| Total stockholder's equity | | | 91,861 | | | 82,072 | | | 64,219 | | | 56,813 | | | 137,461 | | | 136,146 | | | 153,010 | |
39
UTI
| | Five Months Ended
May 31, 2000
| |
|---|
| | (In thousands)
| |
|---|
| STATEMENT OF OPERATIONS DATA: | | | | |
| Net sales | | $ | 35,661 | |
| Cost of sales | | | 23,567 | |
| | |
| |
| Gross profit | | | 12,094 | |
| Selling, general and administrative expenses | | | 27,732 | |
| Research and development expenses | | | 702 | |
| Amortization of intangibles | | | 188 | |
| | |
| |
| Loss from operations | | | (16,528 | ) |
| Interest expense, net | | | 257 | |
| Other | | | 55 | |
| | |
| |
| Loss before income taxes | | | (16,840 | ) |
| Income tax expense | | | 234 | |
| | |
| |
| Net loss | | $ | (17,074 | ) |
| | |
| |
| OTHER FINANCIAL DATA: | | | | |
| Cash flows provided by (used in): | | | | |
| | Operating activities | | $ | 5,497 | |
| | Investing activities | | | 1,333 | |
| | Financing activities | | | (6,580 | ) |
| Deficiency of earnings to fixed charges | | $ | 16,840 | |
BALANCE SHEET DATA (at period end): |
|
|
|
|
| Cash and cash equivalents | | | 2,366 | |
| Total assets | | | 54,992 | |
| Total debt | | | 5,483 | |
| Stockholder's equity | | | 29,492 | |
- (1)
- We acquired G&D, Inc. d/b/a/ Star Guide, Inc. on July 6, 1999 and Noble-Met, Ltd. on January 11, 2000, and we acquired UTI on June 1, 2000, American Technical Molding, Inc. on December 22, 2000, Micro-Guide, Inc. on October 31, 2001, Venusa, Ltd and Venusa de Mexico, S.A. de C.V. (together, "Venusa") on February 28, 2003, and MedSource on June 30, 2004. All acquisitions were accounted for using the purchase method of accounting. Accordingly the assets acquired and liabilities assumed were recorded in our financial statements at their fair market value and the operating results of the acquired companies are reflected since the date of acquisition.
- (2)
- During 2002, we implemented two restructuring plans focused on consolidating our U.S. operations. During the second quarter of 2002, we announced the relocation of the majority of operations in our South Plainfield, New Jersey facility to the Collegeville, Pennsylvania facility. A restructuring charge of $0.5 million was recognized that consisted of $0.1 million related to severance and $0.4 million associated with the write-down of assets and other closure costs at the South Plainfield, New Jersey facility.
- During the fourth quarter of 2002, we announced the consolidation of our machining capabilities into our Wheeling, Illinois facility and the closing of our Miramar, Florida plant. As a result, we recognized a restructuring charge of $1.4 million consisting of: $0.1 million related to stay-on bonuses earned through December 31, 2002; $0.5 million related to the write-down of assets; and $0.8 million related to lease obligations. In 2003, the relocation was completed and we recognized
40
a restructuring charge of $1.8 million consisting of: $0.5 million related to stay-on and relocation bonuses earned through the relocation date; $0.3 million related to the relocation of equipment and plant clean-up; $0.7 million of other exit costs; and $0.3 million related to excess inventory discarded (included in cost of sales in the consolidated statements of operations).
- During the third quarter of 2002, we decided not to proceed with the construction of a new technology center and recognized a loss of $0.5 million related to the write-down of previously capitalized costs.
- In connection with the MedSource acquisition, we identified $17.2 million of costs associated with eliminating duplicate positions and plant consolidations, which is comprised of $9.7 million in severance payments, and $7.5 million in lease and other contract termination costs. Severance payments relate to approximately 520 employees in manufacturing, selling and administration which are expected to be paid by the end of fiscal year 2007. All other costs are expected to be paid by 2018. The costs of these plant consolidations was reflected in the purchase price of MedSource in accordance with the FASB Emerging Issues Task Force ("EITF") No. 95-3,Recognition of Liabilities in Connection with a Purchase Business Combination. These costs include estimates to close facilities and consolidate manufacturing capacity, and are subject to change based on the actual costs incurred to close these facilities.
- We recognized $3.6 million of restructuring charges and acquisition integration costs during fiscal year 2004, including $1.7 million of severance, facility closure and relocation costs incurred as part of a MedSource manufacturing facility closure plan which existed at the time of the acquisition, and $1.0 million of salary related costs due to the elimination of positions we deemed to be redundant as a result of the MedSource acquisition. In addition to the $2.7 million in restructuring charges incurred during fiscal year 2004, we incurred $0.9 million of costs for the integration of MedSource comprised of outside professional services and salary related cost and incentive compensation earned by members of an integration team.
- We recognized $3.8 million of restructuring charges and acquisition integration costs during the first nine months of 2005, including $1.2 million of severance costs and $2.1 million of other exit costs including costs to move production processes from five facilities that are in the process of being closed to our other production facilities. In addition, we incurred $0.5 million of costs for the integration of MedSource.
41
- The following table summarizes the recorded accruals and activity related to the restructuring and other charges (in thousands):
| | Employee Costs
| | Other Exit
Costs
| | Total
| |
|---|
| Restructuring and other charges | | $ | 230 | | $ | 2,210 | | $ | 2,440 | |
| | Less: cash payments | | | (80 | ) | | (143 | ) | | (223 | ) |
| | Less: non-cash Items | | | — | | | (1,262 | ) | | (1,262 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2002 | | | 150 | | | 805 | | | 955 | |
| Restructuring charge | | | 471 | | | 1,016 | | | 1,487 | |
| Inventory discarded | | | — | | | 322 | | | 322 | |
| | Less: cash payments | | | (613 | ) | | (1,559 | ) | | (2,172 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2003 | | | 8 | | | 584 | | | 592 | |
| Restructuring charge | | | 1,307 | | | 2,293 | | | 3,600 | |
| Plant closure and severance costs for MedSource integration | | | 11,559 | | | 9,927 | | | 21,486 | |
| | Less: cash payments | | | (5,110 | ) | | (2,891 | ) | | (8,001 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2004 | | | 7,764 | | | 9,913 | | | 17,677 | |
| Adjustment to planned plant closure and severance costs for the MedSource integration | | | (1,932 | ) | | (2,387 | ) | | (4,319 | ) |
| Restructuring and integration charges incurred | | | 1,191 | | | 2,633 | | | 3,824 | |
| | Less: cash payments | | | (3,886 | ) | | (3,080 | ) | | (6,966 | ) |
| | |
| |
| |
| |
| Balance September 30, 2005 | | $ | 3,137 | | $ | 7,079 | | $ | 10,216 | |
| | |
| |
| |
| |
- (3)
- Effective January 1, 2002, we adopted SFAS No, 142, "Goodwill and Other Intangible Assets." Accordingly, we no longer amortize goodwill. For the year ended 2000 and 2001, we amortized $3.2 million and $5.4 million, respectively, of goodwill. As a result of a loss of significant customers during 2002, goodwill impairment was determined to exist in one of our three reporting units. Accordingly, an impairment of goodwill charge of $17.5 million was recognized. In addition, related intangible assets of developed technology and know how and customer base were reduced to their estimated fair value based on projected cash flow by $2.2 million and $2.0 million, respectively.
- (4)
- For the nine months ended September 30, 2004 other income (expense) includes $3.3 million of pre-payment fees associated with the retirement of Accellent Corp.'s old senior subordinated indebtedness and our senior indebtedness.
- (5)
- We define EBITDA as net income (loss) before net interest expense, income tax expense, depreciation and amortization. Since EBITDA may not be calculated the same by all companies, this measure may not be comparable to similarly titled measures by other companies. We use EBITDA as a supplemental measure of our performance and to provide additional information to investors about the calculation of certain financial covenants in the indenture governing the notes and under our senior secured credit facility. EBITDA has limitations as an analytical tool, and you should not consider EBITDA in isolation, or as a substitute for analysis of our results as reported under GAAP. See "Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Other Key Indicators of Financial Condition and Operating Performance" for a discussion of our use of EBITDA and certain limitations of
42
EBITDA as a financial measure. The following table sets forth a reconciliation of net income to EBITDA for the periods indicated:
| | Twelve Months Ended December 31,
| | Nine Months Ended
September 30,
|
|---|
| | 2000
| | 2001
| | 2002
| | 2003
| | 2004
| | 2004
| | 2005
|
|---|
| | (In thousands)
| |
| |
|
|---|
| RECONCILIATION OF NET INCOME TO EBITDA: | | | | | | | | | | | | | | | | | | | | | |
| | Net income (loss) | | $ | (10,878 | ) | $ | (6,998 | ) | $ | (27,412 | ) | $ | (14,804 | ) | $ | (5,620 | ) | $ | (6,440 | ) | $ | 11,933 |
| | Interest expense | | | 11,363 | | | 17,802 | | | 16,923 | | | 16,587 | | | 26,879 | | | 19,397 | | | 23,731 |
| | (Benefit) Provision for income taxes | | | (5,404 | ) | | (1,504 | ) | | (5,145 | ) | | 13,872 | | | 3,483 | | | 2,341 | | | 10,045 |
| | Depreciation and amortization | | | 11,902 | | | 15,455 | | | 10,858 | | | 11,591 | | | 16,152 | | | 11,289 | | | 16,062 |
| | |
| |
| |
| |
| |
| |
| |
|
| EBITDA | | $ | 6,983 | | $ | 24,755 | | $ | (4,776 | ) | $ | 27,246 | | $ | 40,894 | | $ | 26,587 | | $ | 61,771 |
| | |
| |
| |
| |
| |
| |
| |
|
- (6)
- For purposes of calculating the ratio of earnings to fixed charges, earnings consist of income before income taxes plus fixed charges. Fixed charges include: interest expense, whether expensed or capitalized; amortization of debt issuance cost; and the portion of rental expense representative of the interest factor.
43
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with the "Selected Historical Consolidated Financial Data," the consolidated financial statements and related notes thereto and the Unaudited Pro Forma Condensed Combined Financial Statements and related notes thereto included elsewhere in this prospectus. This discussion contains forward-looking statements and involves numerous risks and uncertainties, including those described in the "Risk Factors" section of this prospectus. Actual results may differ materially from those contained in any forward-looking statements.
Overview
We are the largest provider of outsourced precision manufacturing and engineering services in our target markets of the medical device industry according to market share comparisons by the Millennium Research Group. We offer our customers design and engineering, precision component manufacturing, device assembly and supply chain management services. We have extensive resources focused on providing our customers reliable, high quality, cost-efficient, integrated outsourced solutions. Based on discussions with our customers, we believe we often become the sole supplier of manufacturing and engineering services for the products we provide to our customers.
We primarily focus on the leading companies in three large and growing markets within the medical device industry: cardiology, endoscopy, and orthopaedics. Our customers include many of the leading medical device companies, including Abbott Laboratories, Boston Scientific, Guidant, Johnson & Johnson, Medtronic, Smith & Nephew, St. Jude Medical, Stryker, Tyco International and Zimmer. During 2004, our top 10 customers accounted for approximately 55% of net sales with two customers each accounting for greater than 10% of net sales. During the first nine months of 2005, our top 10 customers accounted for approximately 61% of net sales with three customers each accounting for greater than 10% of net sales. Although we expect net sales from our largest customers to continue to constitute a significant portion of our net sales in the future, Boston Scientific is currently transferring a number of products currently assembled by us to its own assembly operation. As of September 30, 2005, net sales have not been adversely impacted by the pending Boston Scientific production transfer. Based on preliminary estimates and our experience to date with this customer, we expect net sales from Boston Scientific to decrease annually by approximately $40 million, with the substantial majority of the net sales decrease taking place in 2006. While we believe that the transferred business can be replaced with new business from existing and potential new customers to offset the loss, there is no assurance that we will replace such business and the loss will not adversely affect our operating results in 2006 and thereafter. While net sales are aggregated by us to the ultimate parent of a customer, we typically generate diversified revenue streams within these large customers across separate divisions and multiple products.
Recent Acquisitions
On September 12, 2005, we, through our wholly-owned subsidiary, CE Huntsville Holdings Corp., acquired substantially all of the assets of Campbell Engineering, Inc., or Campbell. The Campbell acquisition was accounted for as a purchase and accordingly our results of operations include Campbell's results beginning September 12, 2005. Campbell was a privately held engineering and manufacturing firm providing design, analysis, precision fabrication, assembly and testing of primarily orthopaedic implants and instruments. Subject to the terms of the asset purchase agreement, we and our wholly-owned subsidiary CE Huntsville Holdings Corp. agreed to pay, in the aggregate, a cash purchase price for the assets (including the shareholder real property) of up to approximately $30.1 million, with approximately $18.1 million of which (before certain closing adjustments) was payable at the closing. The actual amount paid at closing after closing adjustments was $17.7 million,
44
plus estimated closing costs of $0.5 million, for a total initial purchase price of $18.2 million. The remaining portion of the purchase price will be paid, if at all, pursuant to an earnout arrangement payable contingent upon the 2005 and 2006 full year financial performance of the acquired business. If earned, the 2005 and 2006 earnout payments would be expected to be made in the second quarters of 2006 and 2007, respectively. The closing cash payment was funded with a combination of cash on hand and approximately $8.0 million of proceeds received from a draw on our existing revolving credit facility, or the Revolving Loan. The Revolving Loan is a euro dollar rate loan accruing interest at an annual rate equal to LIBOR plus 3.00%. The Revolving Loan matures on June 30, 2010. In connection with the transaction, CE Huntsville Holdings Corp. became a subsidiary guarantor of Accellent Corp.'s 10% senior subordinated notes due 2012 and of Accellent Corp.'s obligations under its existing senior secured credit facility.
Subsequent to our third quarter of 2005, on October 6, 2005, we purchased 100% of the outstanding membership interests in Machining Technology Group, LLC, or MTG, an Arlington, Tennessee based privately held manufacturing and engineering company specializing in rapid prototyping and manufacturing of specialized orthopaedic implants and instruments for the orthopaedic industry. The acquisition was consummated pursuant to an Interest Purchase Agreement, dated as of October 6, 2005, by and among Accellent Corp., Gary Stavrum and Timothy Hanson, the members of MTG (the "Interest Purchase Agreement"). Subject to the terms of the Interest Purchase Agreement, we agreed to pay, in the aggregate, approximately $50.2 million consisting of (i) approximately $33.0 million in cash (before certain closing adjustments) which was paid at the closing, (ii) $16.2 million which was paid at the closing by us by the issuance of 407,407 shares of our Class A-9 5% Convertible Preferred Stock, and (iii) estimated closing costs of $1.0 million. An additional $6.0 million will be paid, if at all, pursuant to an earnout arrangement payable contingent upon the 2006 full year financial performance of MTG. If the earnout arrangement is earned, the earnout payment would be expected to be made in the second quarter of 2007. The closing cash payment was funded with a combination of approximately $21.5 million of proceeds received from a draw on Accellent Corp.'s existing revolving credit facility, and approximately $12.5 million of proceeds received from the utilization of the term loan facility provided for under Accellent Corp.'s existing senior secured credit facility.
The Transactions
On October 7, 2005, we entered into an agreement and plan of merger (the "Merger Agreement") with Accellent Acquisition Corp., or AAC, an entity controlled by affiliates of Kohlberg Kravis Roberts & Co. L.P. pursuant to which Accellent Merger Sub Inc., a wholly-owned subsidiary of AAC, has merged with and into us, with the company being the surviving entity (the "Merger"). In addition, we and certain of our stockholders executed a voting agreement in which the stockholders agreed to vote their shares in favor of the Merger.
On October 21, 2005, we commenced a cash tender offer (the "Offer") and consent solicitation (the "Solicitation") for any and all of Accellent Corp.'s outstanding 10% senior subordinated notes due 2012. The consents of the holders of the outstanding senior subordinated notes were solicited in the Solicitation to eliminate substantially all of the restrictive and reporting covenants, certain events of default and certain other provisions in the Indenture. The Offer expired at 5:00 p.m., New York City time, on November 21, 2005. The Solicitation expired at 5:00 p.m., New York City time, on November 3, 2005. We have received tenders and the requisite consents for 100% of the 10% senior subordinated notes. As a result, we have accepted for payment and paid for all notes validly tendered and have executed a supplemental indenture effecting the proposed amendments, which supplemental indenture has become operative.
45
Accounting Policy Overview
We primarily recognize product net sales upon shipment, when title passes to the customer or, if products are shipped on consignment to a particular customer, when the customer uses the product. For services, we recognize net sales during the period in which our services are rendered. We primarily generate our net sales domestically. In 2004, approximately 85% of our net sales were sold to customers located in the United States. Since a substantial majority of the leading medical device companies are located in the United States, we expect our net sales to U.S.-based companies to remain a high percentage of our net sales in the future.
Our operations are based on purchase orders that typically provide for 30 to 90 days delivery from the time the purchase order is received, but which can provide for delivery within 30 days or up to 180 days, depending on the product and the customer's ability to forecast requirements.
Cost of goods sold includes raw materials, labor and other manufacturing costs associated with the products we sell. Some products incorporate precious metals, such as gold, silver and platinum. Changes in prices for those commodities are generally passed through to our customers.
Selling, general and administrative expenses include salaries, sales commissions, and other selling and administrative costs.
Amortization of intangible assets is primarily related to our acquisitions of G&D, Inc. d/b/a Star Guide, Noble-Met, Ltd., UTI, American Technical Molding, Inc., Venusa, Ltd. and Venusa de Mexico, S.A. de C.V. (together with Venusa, Ltd., "Venusa"), MedSource and Campbell Engineering, Inc. Interest expense is primarily related to indebtedness incurred to finance our acquisitions.
Concurrent with our acquisition of MedSource on June 30, 2004, we aligned our management by the three medical device market segments which we serve. As a result of this alignment, we have three operating segments: cardiology, endoscopy, and orthopaedics. We have determined that all of our operating segments meet the aggregation criteria of paragraph 17 of SFAS No. 131, and are treated as one reportable segment.
In connection with the MedSource acquisition, we identified $17.2 million of costs associated with eliminating duplicate positions and plant consolidations, which is comprised of $9.7 million in severance payments and $7.5 million in lease termination and other contract termination costs. Severance payments relate to approximately 520 employees in manufacturing, selling and administration and are expected to be paid by the end of fiscal year 2007. All other costs are expected to be paid by 2018. The costs of these plant consolidations were reflected in the purchase price of MedSource in accordance with the FASB Emerging Issues Task Force ("EITF") No. 95-3,Recognition of Liabilities in Connection with a Purchase Business Combination. These costs include estimates to close facilities and consolidate manufacturing capacity, and are subject to change based on the actual costs incurred to close these facilities. Changes to these estimates could increase or decrease the amount of the purchase price allocated to goodwill in the near term. During the first nine months of fiscal year 2005, we decreased the estimated liability for the MedSource facilities consolidation by $4.3 million, resulting in a decrease to the purchase price allocation to goodwill in the same amount.
46
The following table summarizes the recorded accruals and activity related to the restructuring activities (in thousands):
| | Employee costs
| | Other costs
| | Total
| |
|---|
| Balance as of December 31, 2004 | | $ | 7,764 | | $ | 9,913 | | $ | 17,677 | |
| Adjustment to planned plant closure and severance costs for the MedSource integration | | | (1,932 | ) | | (2,387 | ) | | (4,319 | ) |
| Restructuring and integration charges incurred | | | 1,191 | | | 2,633 | | | 3,824 | |
| Paid year-to-date | | | (3,886 | ) | | (3,080 | ) | | (6,966 | ) |
| | |
| |
| |
| |
| Balance September 30, 2005 | | $ | 3,137 | | $ | 7,079 | | $ | 10,216 | |
| | |
| |
| |
| |
Results of Operations
The following table sets forth percentages derived from the consolidated statements of operations for the nine months ended September 30, 2005 and 2004, presented as a percentage of net sales.
| | Nine months ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| STATEMENT OF OPERATIONS DATA: | | | | | |
| Net Sales | | 100.0 | % | 100.0 | % |
| Cost of Sales | | 68.8 | | 73.0 | |
| Gross Profit | | 31.2 | | 27.0 | |
| Selling, General and Administrative Expenses | | 14.5 | | 14.4 | |
| Research and Development Expenses | | 0.7 | | 1.0 | |
| Restructuring and Other Charges | | 1.1 | | 1.0 | |
| Amortization of Intangibles | | 1.4 | | 1.9 | |
| | |
| |
| |
| Income from Operations | | 13.5 | % | 8.7 | % |
| | |
| |
| |
Nine Months Ended September 30, 2005 Compared to Nine Months Ended September 30, 2004
Net Sales
Net sales for the first nine months of 2005 were $340.3 million, an increase of $127.8 million or 60% compared to net sales of $212.5 million for the first nine months of 2004. Higher net sales was due to the acquisition of MedSource, which increased net sales by $89.8 million, a $49.3 million increase due to the higher unit volume of shipments and $0.7 million from the acquisition of Campbell. These increases were partially offset by our facility rationalization program, which included the closing or sale of select facilities, resulting in a reduction in net sales attributable to former MedSource facilities of $12.0 million for the first nine months of 2005. Three customers, Boston Scientific, Johnson & Johnson and Medtronic each accounted for greater than 10% of net sales for the first nine months of 2005. Two customers, Boston Scientific and Johnson & Johnson each accounted for greater than 10% of net sales for the first nine months of 2004.
Gross Profit
Gross profit for the first nine months of 2005 was $106.1 million compared to $57.3 million for the first nine months of 2004. The $48.8 million increase in gross profit was caused by the MedSource acquisition which increased gross margin by $26.3 million, and unit volume increases which increased gross margin by $19.2 million. Additionally, gross profit for the first nine months of 2004 included a
47
$3.4 million write-off of the step-up of inventory related to the acquisition of MedSource, and gross profit for the first nine months of 2005 included a $0.1 million write-off of the step-up of inventory related to the acquisition of Campbell.
Gross margin was 31.2% of net sales for the first nine months of 2005 compared to 27.0% of net sales for the first nine months of 2004. The increase in gross margins is due to increased sales, which lead to improved leverage of our fixed cost of sales. Also, gross margin for the first nine months of 2004 included the write-off of the step-up of inventory related to the acquisition of MedSource which reduced gross margin by 1.6% in that period.
Selling, General and Administration Expenses
SG&A expenses, were $49.5 million for the first nine months of 2005 compared to $30.7 million for the first nine months of 2004. The increase in SG&A costs were caused by the MedSource acquisition which increased SG&A cost by $15.0 million in the 2005 period as compared to the 2004 period, and $3.5 million in increased charges for stock-based compensation. The increase in stock-based compensation is due to our increased liability for phantom stock plans due to the increase in value of our common stock, and to amortize compensation expense associated with restricted stock and stock options granted during the third quarter of 2005. In addition, our SG&A expenses for the first nine months of 2005 include $0.6 million of expenses related to the Merger
In July 2005, our Board of Directors approved the grant of 609,237 shares of our restricted stock to certain members of our and Accellent Corp.'s management. Each share vests 100% on the four year anniversary from the date of grant. We are recording compensation expense of $10.0 million over the vesting period of the restricted stock, or 48 months, resulting in a quarterly compensation charge to SG&A expense of $624,000 starting in July 2005. Also in July 2005, our Board of Directors approved the grant of 281,152 stock options to certain members of our and Accellent Corp.'s management at option prices that are below the fair market value of the underlying common stock. We are recording compensation expense of $2.3 million over the vesting period of the stock options, or 60 months, resulting in a quarterly compensation charge to SG&A of $115,000 starting in July 2005. As a result of the completion of the Merger, the restricted stock and stock options granted in July 2005 fully vested, resulting in an acceleration of all remaining unamortized non-cash stock-based compensation.
SG&A expenses were 14.5% of net sales for the first nine months of 2005 versus 14.4% of net sales for the first nine months of 2004. The higher 2005 percentage was impacted by increased charges for stock-based compensation in the 2005 period, which amounted to 1.1% of net sales for the 2005 period as compared to 0.1% in the 2004 period.
Research and Development Expenses
R&D expenses for the first nine months of 2005 were $2.3 million or 0.7% of net sales, compared to $2.0 million or 1.0% of net sales for the first nine months of 2004. The lower 2005 percentage was driven by sales growth, which lead to improved leverage of our fixed R&D costs.
Restructuring and Other Charges
We recognized $3.8 million of restructuring charges and acquisition integration costs during the first nine months of fiscal year 2005, including $1.2 million of severance costs and $2.1 million of other exit costs including costs to move production processes from five facilities that are closing to our other production facilities. In addition to the $3.3 million in restructuring charges incurred during first nine months of fiscal year 2005, we incurred $0.5 million of costs for the integration of MedSource.
48
Amortization
Amortization was $4.7 million for the first nine months of 2005 compared to $3.9 million for the first nine months of 2004. The higher amortization was due to the acquisition of MedSource.
Interest Expense, net
Interest expense, net was $23.7 million for the first nine months of 2005 compared to $19.4 million for the first nine months of 2004. The increase was due to increased debt incurred to acquire MedSource. This increase was partially offset by $4.5 million of accelerated amortization of debt discounts and deferred financing costs incurred during the first nine months of 2004 due to the refinancing of our subsidiary's senior secured credit facility and various senior subordinated indebtedness.
Other expense
Other expense was $0.1 million for the first nine months of 2005 compared to $3.3 million for the first nine months of 2004. The decrease is due to debt prepayment penalties of $3.3 million incurred during the first nine months of 2004.
Income Tax Expense
Income tax expense for the nine months ended September 30, 2005 was $10.0 million on pre-tax income of $22.0 million, or 45.7% of pre-tax income. The effective rate is higher than the statutory rate due to $6.5 million in charges for non-cash deferred income taxes, including $5.2 million for tax benefits acquired from MedSource that have been credited to goodwill and not benefited in the statement of operations and $1.3 million of charges for the different book and tax treatment for goodwill. The remaining $3.5 million of income tax expense for the nine months ended September 30, 2005 includes $3.3 million for certain state and foreign income taxes and $0.2 million for domestic federal income taxes. For the nine months ended September 30, 2004, we recorded a tax provision of $2.3 million on a pre-tax net loss of $4.1 million, due to provisions for certain state and foreign income taxes.
2004 Compared to 2003
Net Sales
Net sales for 2004 were $320.2 million, an increase of $146.0 million or 84% compared to net sales of $174.2 million for 2003. The higher net sales were caused by the June 30, 2004 acquisition of MedSource, which increased net sales by $94.9 million, and the inclusion of Venusa for the full year in 2004, which increased net sales by $3.9 million, as well as a $47.2 million increase related to higher unit volume of shipments. Two customers, Boston Scientific and Johnson & Johnson, accounted for greater than 10% of net sales for 2004. One customer, Boston Scientific, accounted for greater than 10% of net sales for 2003.
Gross Profit
Gross profit for 2004 was $85.8 million as compared to $53.2 million for 2003. The $32.6 million increase in gross profit was caused by the acquisition of MedSource, which after excluding the expense for inventory step-up and inventory obsolescence, increased gross margin by $21.2 million, and higher unit shipments which increased gross margin by $16.7 million, partially offset by a $5.3 million charge for inventory acquired in the MedSource acquisition, which is including $3.4 million relating to the step up in value of the acquired inventory and $1.9 million for inventory obsolescence.
49
Gross margin was 26.8% of net sales for 2004 as compared to 30.5% of net sales for 2003. As a result of the acquisition of MedSource, acquired inventories were stepped up in value by $3.4 million. This step up in inventory reduced gross margin by $3.4 million, or 1.1%, for 2004. Additionally, we incurred a charge for inventory valuation related to certain MedSource facilities of $1.9 million, or 0.6% during 2004. Further, the MedSource gross margins have historically been lower than gross margins attained by us before the acquisition. The lower MedSource gross margins impacted our gross margins during 2004 by $6.3 million, or 2.0%.
Selling, General and Administration Expenses
SG&A expenses were $46.0 million for 2004 compared to $28.6 million for 2003. The increase in SG&A costs was due to the acquisition of MedSource, which added $11.5 million of SG&A costs during 2004, including $0.6 million increase to bad debt expense for certain acquired MedSource related accounts receivable.
SG&A expenses were 14.4% of net sales for 2004 versus 16.4% of net sales for 2003. The lower 2004 percentage was driven by sales growth resulting in improved absorption of our SG&A cost structure. Additionally, in 2003 we incurred $1.6 million of costs associated with executive officer transition.
Research and Development Expenses
R&D expenses for 2004 were $2.7 million or 0.8% of net sales, compared to $2.6 million or 1.5% of net sales for 2003. The lower 2004 percentage was driven by sales growth in combination with leveraging the R&D cost structure.
Restructuring and Other Charges
We recognized $3.6 million of restructuring charges and acquisition integration costs during fiscal year 2004, including $1.7 million of severance, facility closure and relocation costs incurred as part of a MedSource manufacturing facility closure plan which existed at the time of the acquisition, and $1.0 million of salary related costs due to the elimination of positions we deemed to be redundant as a result of the MedSource acquisition. In addition to the $2.7 million in restructuring charges incurred during fiscal year 2004, we incurred $0.9 million of costs for the integration of MedSource comprised of outside professional services and salary related cost and incentive compensation earned by members of an integration team.
The following table summarizes the recorded accruals and activity related to the restructuring (in thousands):
| | Employee costs
| | Other costs
| | Total
| |
|---|
| Balance as of December 31, 2003 | | $ | 8 | | $ | 584 | | $ | 592 | |
| Restructuring charge | | | 1,307 | | | 2,293 | | | 3,600 | |
| Plant closure and severance costs for MedSource integration | | | 11,559 | | | 9,927 | | | 21,486 | |
| Less: cash payments | | | (5,110 | ) | | (2,891 | ) | | (8,001 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2004 | | $ | 7,764 | | $ | 9,913 | | $ | 17,677 | |
| | |
| |
| |
| |
Interest Expense, net
Interest expense, net increased $10.3 million to $26.9 million for fiscal year 2004 versus $16.6 million for fiscal year 2003 due to the refinancing of Accellent Corp.'s senior secured credit
50
facility and various senior subordinated indebtedness, which resulted in $4.5 million of accelerated amortization of debt discounts and deferred financing costs during the second quarter of fiscal year 2004, and interest expense on the increased debt incurred to acquire MedSource. Interest expense, net includes interest income of approximately $94,000 and $19,000 for fiscal years 2004 and 2003, respectively.
Other income (expense)
For the fiscal year 2004, other income (expense) includes $3.3 million of pre-payment fees associated with the retirement of Accellent Corp.'s old senior subordinated indebtedness and our senior indebtedness.
Income Tax Expense (Benefit)
Income tax expense was $3.5 million for fiscal year 2004 as compared to $13.9 million for fiscal year 2003. The expense incurred for fiscal year 2004 is as a result of certain state and foreign taxes which cannot be offset by losses in other jurisdictions, and a provision for deferred taxes related to the different book and tax treatment for goodwill. During the fourth quarter of fiscal year 2003, we determined that it is more likely than not that our deferred tax asset will not be realized, and we provided a valuation allowance equal to the full amount of the deferred tax asset.
Amortization
Amortization was $5.5 million for fiscal year 2004 compared to $4.8 million for fiscal year 2003. The higher amortization was due to the acquisition of MedSource, which added $0.7 million in fiscal year 2004.
Dividends on Redeemable and Convertible Preferred Stock
In connection with the MedSource acquisition, we repurchased $18.8 million of redeemable preferred stock with a carrying value on our balance sheet of $12.6 million. We recorded the difference between the repurchase price and the carrying value of $6.2 million as an accretive dividend. In addition, we declared and paid a dividend of $2.0 million on these redeemable shares.
2003 Compared to 2002
Net Sales
Net sales for 2003 were $174.2 million, an increase of $38.4 million or 28% compared to net sales of $135.8 million for 2002. The higher net sales were the result of the February 28, 2003 acquisition of Venusa which increased 2003 net sales by $36.5 million. Approximately 75% of the net sales of Venusa are from a single customer, which combined with existing sales from this customer, made this our largest customer generating approximately 25% of our total net sales for 2003.
Assuming the Venusa acquisition occurred on January 1, 2002, net sales from products used in medical markets for 2003 were $148.3 million, an increase of $22.3 million or 17.7% compared to net sales from products used in medical markets of $126.0 million for 2002. The increase in net sales from medical products was due to the manufacturing ramp-up of several product lines for endoscopy customers. Net sales from products sold into the industrial markets for 2003 were $29.8 million, a decrease of $2.0 million or 6.3% compared to net sales from products sold into the industrial markets of $31.8 million for 2002.
51
Gross Profit
Gross profit for 2003 was $53.2 million as compared to $39.1 million for 2002. The increase in gross profit was the result of the acquisition of Venusa in 2003 which increased gross profit by $10.0 million, and a $5.0 million charge related to obsolete inventory in 2002.
Gross margin for 2003 increased to 30.5% from 28.8% in the prior year. The increase was due to the $5.0 million charge in 2002, partially offset by the acquisition of Venusa. The $5.0 million charge was to reduce inventory to its expected net realizable value. Throughout 2002 and 2003 approximately $3.3 million of the written down inventory was discarded. The inventory charge negatively impacted gross margin for 2002 by 3.7%. The acquisition of Venusa negatively impacted gross margin for 2003 by 0.9%.
Selling, General and Administrative Expenses
SG&A expenses for 2003 were $28.6 million compared to $23.5 million in 2002. The increase was due to the acquisition of Venusa which increased SG&A expenses by $3.9 million, and $1.9 million of severance and relocation charges incurred in connection with executive officer transitions, which took place in the third and fourth quarter of 2003.
Selling, general and administrative expenses were 16.4% of net sales in 2003 versus 17.3% of net sales in 2002. The lower percentage in 2003 was driven by strong net sales growth in combination with leveraging the selling, general and administrative cost structure, partially offset by the executive officer transition charges.
Research and Development Expenses
Research and development expenses for 2003 were $2.6 million or 1.5% of net sales, compared to $2.4 million or 1.8% of net sales in 2002.
Restructuring and Other Charges
In 2003 we completed the consolidation of machining capabilities into the Wheeling, Illinois facility and the closing of our Miramar, Florida plant and recognized a restructuring charge of $1.8 million, including $0.3 million related to excess inventory included in cost of sales, $0.5 million of employee-related costs and $1.0 million of other exit costs.
During 2002, we implemented two restructuring plans focused on consolidating our U.S. operations. We relocated the majority of operations in the South Plainfield, New Jersey facility to the Collegeville, Pennsylvania facility. A restructuring charge of $0.5 million was recognized. We announced the consolidation of machining capabilities into the Wheeling, Illinois facility and the closing of our Miramar, Florida plant. A restructuring charge of $1.4 million was recognized. Also in 2002, we decided not to proceed with construction of a new technology center and recognized a loss of $0.5 million related to the write-down of previously capitalized costs. Employee-related costs included in the restructuring charge were $0.2 million and the remaining $2.2 million consisted of other exit costs.
52
The following table summarizes the recorded accruals and activity related to the restructuring and other charges (in thousands):
| | Employee Costs
| | Other Exit Costs
| | Total
| |
|---|
| Restructuring and other charges | | $ | 230 | | $ | 2,210 | | $ | 2,440 | |
| Less cash payments | | | (80 | ) | | (143 | ) | | (223 | ) |
| Less non cash items | | | — | | | (1,262 | ) | | (1,262 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2002 | | | 150 | | | 805 | | | 955 | |
| Restructure charge | | | 471 | | | 1,016 | | | 1,487 | |
| Inventory discarded | | | — | | | 322 | | | 322 | |
| Less cash payments | | | (613 | ) | | (1,559 | ) | | (2,172 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2003 | | $ | 8 | | $ | 584 | | $ | 592 | |
| | |
| |
| |
| |
Impairment of Goodwill and Intangibles
During the third quarter of fiscal year 2002, one of our reporting units experienced a decline in sales due to the loss of two key customers. Net sales for this reporting unit declined $3.8 million for fiscal year 2002 as compared to fiscal year 2001, with most of this decline occurring during the second half of fiscal year 2002. The annualized amount of sales lost from these two customers was approximately $6.0 million. We had earned high profit margins on the business that was lost, resulting in the elimination of all positive cash flow from this reporting unit. We immediately implemented cost reduction programs to mitigate the impact on cash flow, however, annual cash flow was still expected to drop in excess of $3.0 million per year after the impact of all cost reduction initiatives. The valuation of this reporting unit was based on discounted cash flows, including a five year cash flow projection plus a residual value calculation to value the cash flows beyond the years that are discretely forecast. The reduction in cash flows significantly reduced the value of this reporting unit, and lead to a goodwill impairment charge of $17.5 million and an intangible asset impairment charge of $4.2 million recorded in the fourth quarter of fiscal year 2002.
Amortization
Amortization in 2003 was $4.8 million compared to $4.7 million in 2002.
Interest Expense, net
Interest expense, net decreased $0.3 million to $16.6 million for 2003 from $16.9 million for 2002, mainly due to a decrease in outstanding debt. Interest expense, net includes $19,000 and $78,000 of interest income for years 2003 and 2002, respectively.
Income Tax Expense (Benefit)
Income tax expense was $13.9 million for 2003 as compared to an income tax benefit of $5.1 million for 2002. The expense incurred for 2003 was mainly due to the provision of an allowance against our deferred tax asset. In accordance with SFAS 109, Accounting for Income Taxes, projected future taxable income generally cannot be used as a basis for recovering deferred tax assets if there are cumulative losses during recent years. During the fourth quarter of 2003 we determined that income from our operations would not be sufficient to cover our interest and financing costs. Accordingly, we provided a valuation allowance equal to the full amount of our net deferred tax asset.
Liquidity and Capital Resources
Our principal sources of liquidity have been cash flows from operations and borrowings under Accellent Corp.'s senior secured credit facility, entered into in conjunction with our June 30, 2004
53
acquisition of MedSource, which included a five-year $40.0 million revolving credit facility and a six-year $194.0 million term facility. Additionally, we were able to borrow up to $50.0 million in additional term loans, with the approval of participating lenders.
At September 30, 2005, our subsidiary had $5.8 million of letters of credit outstanding and $8.0 million of revolving loans that reduced the amounts available under the revolving credit portion of Accellent Corp.'s senior secured credit facility to $26.2 million. Our subsidiary received $12.5 million from additional term loans in October 2005 in connection with its acquisition of MTG. In addition, our subsidiary drew an additional $21.5 million in revolving loans on its senior secured credit facility in October 2005 in connection with its acquisition of MTG, leaving $4.7 million available under the revolving portion of its senior secured credit facility.
During the first nine months of 2005, cash provided by operating activities was $21.5 million compared to $6.2 million for the first nine months of 2004. The increase in cash provided by operations is due to increased profitability which generated an increase of $27.6 million in cash flow from operations. The increase in profitability has been offset by a $12.3 million increase in working capital. The decreased cash flow due to working capital was caused in part by the timing of interest payments which negatively impacted cash flow by $6.8 million, and increased payments related to our restructuring programs of $3.5 million.
During the first nine months of 2005, cash used in investing activities totaled $35.6 million compared to $221.3 million for the first nine months of 2004. The decrease in cash used in investing activities is attributable to the acquisition of MedSource, which used $204.4 million of cash during the first nine months of 2004, and an earn-out payment relating to our acquisition of Venusa, which used $9.6 million of cash during the first nine months of 2004. These costs were partially offset by the acquisition of Campbell for $17.9 million in cash during the first nine months of 2005, the final Venusa earn-out payment of $2.2 million during the first nine months of 2005, and increased capital expenditures of $6.9 million during the first nine months of 2005 due to the acquisition of MedSource and increased demand for our products.
During the first nine months of 2005, cash provided by financing activities was $5.7 million and consisted of $8.0 million in proceeds from long-term debt to partially fund our Campbell acquisition, $1.5 million of scheduled debt payments pursuant to Accellent Corp.'s senior secured credit facility, and $0.8 million of deferred financing fees incurred in connection with the amendment of Accellent Corp.'s senior secured credit facility in March 2005. Cash provided by financing activities was $218.6 million for the first nine months of 2004 and relate to the following financing transactions, which took place in conjunction with our June 30, 2004 acquisition of MedSource:
- •
- The issuance of $369.0 million of indebtedness consisting of Accellent Corp.'s senior secured credit facility, which is a $194.0 million six-year term facility, and $175.0 million of 10% senior subordinated notes due July 15, 2012. Our subsidiary incurred $17.1 million of fees related to the new debt.
- •
- The repayment of all previously outstanding debt, which included our subsidiary's credit facility of $83.5 million, our subsidiary's senior subordinated notes of $21.5 million and our senior notes of $38.3 million.
- •
- The repayment of all MedSource debt and capital leases totaling $36.1 million.
- •
- The payment of $22.2 million of dividends.
- •
- The repurchase of $18.8 million of our Class C Redeemable Preferred Stock.
- •
- The issuance of 7,568,980 shares of our Class A-8 5% Convertible Preferred stock for approximately $88.0 million, net of $1.8 million of fees.
54
For 2004, cash provided by operating activities was $22.2 million compared to $14.4 million for 2003. The increase in cash generated from operations is due to improved profitability which increased cash from operations by $6.2 million, and the increase in accrued bonuses and profit sharing of $3.4 million as a result of the increase in operating performance. The bonus and profit sharing accruals were paid during the first quarter of 2005.
During 2004, cash used in investing activities totaled $227.4 million compared to $20.4 million for fiscal year 2003. The increase in cash used in investing activities was attributable to the acquisition of MedSource, for which we paid $205.4 million during fiscal year 2004, and increased capital spending due to the MedSource acquisition.
During fiscal year 2004, financing activities generated $217.1 million of cash compared to $4.0 million of cash for fiscal year 2003. Cash provided by financing activities in 2004 was related to the financing transactions described above, which took place in conjunction with our June 30, 2004 acquisition of MedSource.
Cash provided by operating activities was approximately $14.4 million for 2003 compared to approximately $14.0 million for 2002. Cash provided by operating activities in 2003 was related to a net loss of approximately $14.8 million, plus depreciation and amortization of approximately $11.6 million, non-cash interest expense of approximately $7.1 million and deferred income taxes of approximately $12.3 million. Cash provided by operating activities in 2002 was related to a net loss of approximately $27.4 million, plus depreciation and amortization of approximately $10.9 million, non-cash interest expense of approximately $6.2 million, an impairment charge of approximately $21.7 million, partially offset by a change in deferred income taxes of approximately $5.2 million. In addition, cash was provided by inventory reduction of approximately $3.5 million.
Cash used in investing activities was approximately $20.4 million for 2003 compared to approximately $9.4 million for 2002. Cash used in investing activities in 2003 included approximately $6.4 million associated with capital spending and approximately $14.4 million (net of acquired cash) associated with the acquisition of Venusa. Cash used in investing activities in 2002 included approximately $6.2 million associated with capital spending and approximately $3.3 million (net of acquired cash) primarily associated with the earn-out related to an acquisition.
Cash provided by financing activities was approximately $4.0 million for 2003 compared to cash used in financing activities of approximately $1.5 million for the year 2002. Cash provided by financing activities was comprised of approximately $18.7 million of proceeds from the sale of our Class C Redeemable Preferred Stock, partially offset by approximately $14.1 million of net debt reduction and approximately $0.7 million of deferred financing fees. Cash used in financing activities in 2002 was comprised of approximately $1.0 million of net debt reduction and approximately $0.5 million of deferred financing fees.
Capital Expenditures. We anticipate that we will spend approximately $30 million to $35 million on capital expenditures for 2006. Our senior secured credit facility contains restrictions on our ability to make capital expenditures. We may make capital expenditures in each fiscal year in an amount not exceeding the greater of (i) 6% of consolidated net sales for such fiscal year and (ii) for the 2006 fiscal year, $39,000,000; for the 2007 fiscal year, $40,000,000; for the 2008 fiscal year, $42,500,000; for the 2009 fiscal year, $45,000,000; for the 2010 fiscal year, $47,500,000; for the 2011 fiscal year, $52,500,000; and for the 2012 fiscal year, $55,000,000. The senior secured credit facility also allows us to carry forward unused amounts and to carry back future permitted amounts, in each case on a limited basis. Based on current estimates, our management believes that the amount of capital expenditures permitted to be made under our senior secured credit facility will be adequate to grow our business according to our business strategy and to maintain our continuing operations.
Other Expenditures. In connection with our acquisition of Venusa in February of 2003, we are obligated to pay contingent consideration based on agreed upon earnings targets for fiscal years 2002,
55
2003 and 2004. During the second quarter of 2005, we paid $2.2 million in cash, $3.6 million in our Class A-7 5% Convertible Preferred Stock and $0.2 million in our phantom stock in connection with Venusa achieving fiscal year 2004 earn out targets. During the second quarter of 2004, we paid $9.6 million in cash, $26.0 million in our Class A-7 5% Convertible Preferred Stock, and $1.3 million in our phantom stock in connection with Venusa achieving fiscal year 2002 and 2003 earnout targets.
Other Long-Term Liabilities. Other long-term liabilities increased $1.6 million from $23.7 million at December 31, 2004 to $25.3 million at September 30, 2005. The increase was due to a $2.8 million increase in our phantom stock liability due to the increased value of our stock, and a $1.3 million increase in our deferred tax liability due to the different book and tax treatment for goodwill. These increases were partially offset by a $2.5 million decrease in the long-term portion of our estimated costs to close certain MedSource facilities. Other long-term liabilities increased from $13.3 million as of December 31, 2003 to $23.7 million as of December 31, 2004. This increase is due to a $10.9 million increase in long-term restructuring accruals for lease and severance costs related to the planned closure of certain MedSource facilities. Also in connection with the acquisition of MedSource, we increased our long-term environmental accrual by $0.9 million for identified potential clean up obligations of MedSource as described in greater detail in the following paragraph. These increases on other long-term liabilities were partially offset by the payment of long-term accrued interest expense of $4.4 million during fiscal year 2004.
As of September 30, 2005, we have provided a liability of $4.7 million for environmental clean up matters. The United States Environmental Protection Agency, or EPA, issued an Administrative Consent Order in July 1988 requiring UTI, our subsidiary, to study and, if necessary, remediate the groundwater and soil beneath and around its plant in Collegeville, Pennsylvania. Since that time, UTI has implemented and is operating successfully a contamination treatment system approved by the EPA. We expect to incur approximately $0.2 million of ongoing operating costs during fiscal year 2005 relating to the Collegeville remediation effort. Our environmental accrual at December 31, 2004 includes $3.8 million related to Collegeville. We have identified potential additional clean up obligations in connection with our acquisition of MedSource, which we estimate will result in remediation costs up to between $0.8 to $1.1 million, for which we have accrued $0.9 million based on our best estimate of the total remediation costs. Due to the early stage of the MedSource investigation and remediation process, we cannot estimate the timing of the costs to be incurred. We believe that the clean up of these identified environmental matters will not have a material adverse effect upon our liquidity, capital resources, business or consolidated financial position. However, one or more of such environmental matters could have a significant negative impact on our consolidated financial results for a particular reporting period.
Our ability to make payments on our indebtedness and to fund planned capital expenditures, other expenditures and long-term liabilities, and necessary working capital will depend on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. For example, Boston Scientific has informed us that it intends to transfer a number of products currently assembled by us to its own assembly operation. Based on our current level of operations, we believe our cash flow from operations and available borrowings under our senior secured credit facility will be adequate to meet our liquidity requirements for the next 12 months and the foreseeable future. No assurance can be given, however, that this will be the case.
Indebtedness. The following is a description of our material indebtedness as of September 30, 2005:
On June 30, 2004, Accellent Corp. issued $175.0 million in aggregate principal amount of 10% senior subordinated notes due 2012. The notes were initially purchased by Credit Suisse First Boston LLC and Wachovia Capital Markets, LLC and were resold to various qualified institutional buyers and
56
non-U.S. persons pursuant to Rule 144A and Regulation S, respectively, under the Securities Act. Interest on the notes is payable semi-annually on January 15 and July 15 of each year, and the notes mature on July 15, 2012. The notes are guaranteed by all of Accellent Corp.'s existing domestic subsidiaries and by all of its future domestic subsidiaries that are not designated by it as unrestricted subsidiaries. The notes are Accellent Corp.'s and the applicable guarantor's senior subordinated unsecured obligations and rank behind its and the applicable guarantors' obligations under Accellent Corp.'s senior secured credit facility and any future senior indebtedness.
Accellent Corp. has the option to redeem the notes, in whole or in part, at any time on or after July 15, 2008, at redemption prices declining from 105% of their principal amount on July 15, 2008 to 100% of their principal amount on or after July 15, 2010, plus accrued and unpaid interest. At any time on or prior to July 15, 2007, Accellent Corp. may also redeem up to 35% of the aggregate principal amount of the notes at a redemption price of 110% of their principal amount, plus accrued and unpaid interest, within 90 days of the closing of (a) an underwritten public offering or (b) a private placement (other than to an affiliate) resulting in net cash proceeds of $50.0 million or more, in each case, of our stock or its stock to the extent that the cash proceeds of the sale of our stock are used as a capital contribution to Accellent Corp. Upon a change of control, as defined in the indenture pursuant to which the notes were issued, Accellent Corp. is required to offer to repurchase the notes at a purchase price equal to 101% of their principal amount, plus accrued and unpaid interest.
The indenture limits its and its subsidiaries' ability to, among other things:
- •
- pay dividends;
- •
- redeem capital stock and make other restricted payments and investments;
- •
- incur additional debt or issue preferred stock;
- •
- enter into agreements that restrict our subsidiaries from paying dividends or other distributions;
- •
- make loans or otherwise transfer assets to us or to any other subsidiaries;
- •
- create liens on assets;
- •
- engage in transactions with affiliates;
- •
- sell assets, including capital stock of subsidiaries; and
- •
- merge, consolidate or sell all or substantially all of our assets and the assets of our subsidiaries.
The indenture contains customary events of default including, but not limited to:
- •
- failure to pay any installment of interest on the notes as and when the same becomes due and payable;
- •
- failure to pay all or any part of the principal, or premium, if any, on the notes when and as the same becomes due and payable at maturity, redemption, by acceleration or otherwise;
- •
- its failure or the failure by any of its subsidiaries to observe or perform any other covenant or agreement contained in the notes or the indenture;
- •
- the occurrence of certain events of bankruptcy, insolvency or reorganization in respect of its or any of its significant subsidiaries as defined in Regulation S-X of the Securities Act;
- •
- the occurrence of a default in its indebtedness or the indebtedness of any of its subsidiaries with an aggregate amount outstanding in excess of $15.0 million (a) resulting from the failure to pay principal at maturity or (b) as a result of which the maturity of such Indebtedness has been accelerated prior to its stated maturity;
- •
- the occurrence of any final unsatisfied judgments not covered by insurance aggregating in excess of $15.0 million, at any one time rendered against it or any of its subsidiaries; and
57
- •
- any guarantee of a guarantor that is a significant subsidiary ceases to be in full force and effect or becomes unenforceable or invalid or is declared null and void (other than in accordance with the terms of the guarantee and the indenture) or any guarantor denies or disaffirms its obligations under its guarantee.
In August 2004, Accellent Corp. filed a registration statement (Registration No. 333-118675) under the Securities Act pursuant to a registration rights agreement entered into in connection with the notes offering. The registration statement was declared effective by the SEC on February 11, 2005, enabling the eligible holders of the notes to exchange their notes for notes registered under the Securities Act. The transaction closed on March 15, 2005 and 100% of the notes were exchanged for registered notes. Accellent Corp. did not receive any proceeds from the transaction.
As of the expiration date of the tender offer described elsewhere in this prospectus, 100% of the outstanding principal amount of the notes were tendered. Accellent Corp. has accepted for payment and paid for all notes validly tendered on or prior to the expiration date of the tender offer.
On June 30, 2004, Accellent Corp. entered into a senior secured credit facility with a syndicate of financial institutions, including Credit Suisse, Cayman Islands Branch, as sole lead arranger, sole book runner, collateral agent and administrative agent, and Wachovia Bank, National Association, as syndication agent. The senior secured credit facility provides for aggregate borrowings by Accellent Corp. of up to $234.0 million and consists of a six-year $194.0 million term facility and a five-year $40.0 million revolving credit facility. Up to $15.0 million of the revolving credit facility is available as a letter of credit sub-facility and up to $5.0 million as a swingline sub-facility. Accellent Corp. amended the senior secured credit facility on March 25, 2005 to lower the interest rate applicable to term loans, to increase the amount of potential additional term loans from $40.0 million to $50.0 million and to allow increased flexibility in, and funds for, acquisitions. Additional term loans require the approval of participating lenders. Principal payments will continue to be due in the amounts of $1.9 million per year plus, beginning in 2006, 75% of Excess Cash Flow, as defined by the senior secured credit facility. The balance is due June 30, 2010.
On June 30, 2004, Accellent Corp. borrowed $194.0 million under the term facility under the senior secured credit facility. The borrowings under the senior secured credit facility were used to provide a portion of the proceeds required to consummate the MedSource acquisition. The revolving credit facility is used for our working capital and general corporate requirements. At September 30, 2005, we had $5.8 million of letters of credit outstanding and $8.0 million of revolving loans that reduced the amounts available under our revolving credit facility to $26.2 million. We received $12.5 million from additional term loans in October 2005 in connection with our acquisition of MTG. In addition, we drew an additional $21.5 million in revolving loans on our revolving credit facility in October 2005 in connection with our acquisition of MTG, leaving $4.7 million available under the revolving portion of the senior secured credit facility.
The senior secured credit facility is fully and unconditionally guaranteed on a joint and several basis by us and by Accellent Corp.'s existing and future, direct and indirect domestic subsidiaries. The senior secured credit facility and guarantees are secured by first priority security interests in, and mortgages on, substantially all of Accellent Corp.'s and its direct and indirect domestic subsidiaries' and our tangible and intangible assets, including first priority pledges of all the equity interests owned by us in Accellent Corp. and owned by Accellent Corp. and the guarantors in its existing and future direct and indirect domestic subsidiaries and up to 65% of the equity interests owned by it in its and the guarantors' existing and future first tier foreign subsidiaries.
Term loan borrowings under the senior secured credit facility, as amended, generally bear interest, at Accellent Corp.'s option, at either the base rate (generally the applicable prime lending rate of Credit Suisse First Boston, as announced from time to time) plus 1.25% or LIBOR plus 2.25%. At
58
September 30, 2005, the interest rate on the term loan borrowings was 6.09%. Revolving loan borrowings under the senior secured credit facility bear interest, at our option, at either the base rate plus a margin or at LIBOR plus a margin. In either case, the margin will vary depending on our leverage ratio, which is the amount of our total consolidated debt (calculated in accordance with the senior secured credit facility) as of the end of each fiscal quarter divided by our consolidated adjusted EBITDA (as calculated in accordance with the senior secured credit facility) for the four fiscal quarters then ended. The margins vary from 3.50% per annum for LIBOR revolving loans and 2.50% per annum for base rate revolving loans if our leverage ratio is greater than 6.00-to-1, down to 2.50% per annum for LIBOR revolving loans and 1.50% per annum for base rate revolving loans if our leverage ratio is less than 3.50-to-1. Accellent Corp. may have several revolving loans outstanding at any time bearing interest at a combination of the base rate and LIBOR rates having interest rate periods from one to 12 months.
Accellent Corp. is permitted to voluntarily prepay principal amounts outstanding or reduce commitments under the senior secured credit facility at any time, in whole or in part, without premium or penalty, upon providing proper notice and subject to minimum amount requirements. In addition, subject to certain exceptions, Accellent Corp. is required to prepay outstanding amounts under the senior secured credit facility with a portion of its excess cash flow, the net proceeds of certain asset dispositions, casualty insurance and condemnation recovery events and upon the issuance of certain equity securities or debt.
The senior secured credit facility contains customary and appropriate affirmative and negative covenants for financings of its type (and subject to negotiated exceptions). The financial covenants include:
- •
- a limitation on capital expenditures;
- •
- a maximum leverage ratio test; and
- •
- a minimum interest coverage and fixed charge coverage ratio test.
Other covenants, among other things, limit its ability to:
- •
- incur liens or other encumbrances;
- •
- make investments;
- •
- make acquisitions;
- •
- incur additional debt;
- •
- enter into sale leaseback transactions;
- •
- incur certain contingent liabilities;
- •
- make certain restricted junior payments and other similar distributions;
- •
- enter into mergers, consolidations and similar combinations;
- •
- sell assets or engage in similar transfers;
- •
- amend certain material agreements, including the indenture governing the 10% senior subordinated notes due 2012; and
- •
- engage in transactions with affiliates.
The senior secured credit facility contains customary events of default including, but not limited to:
- •
- failure to make payments when due;
- •
- defaults under other material agreements or instruments of indebtedness;
- •
- noncompliance with covenants;
59
- •
- breaches of representations and warranties;
- •
- bankruptcy events;
- •
- judgments in excess of specified amounts;
- •
- failure of any guaranty or security agreement supporting the senior secured credit facility to be in full force and effect; and
- •
- a change of control (as such term is defined in the senior secured credit facility).
As of September 30, 2005, we and Accellent Corp. were in compliance with their respective covenants under the senior secured credit facility.
In connection with the Transactions, on November 22, 2005, Accellent Corp. repaid all outstanding obligations under its senior secured credit facility.
Other Key Indicators of Financial Condition and Operating Performance
EBITDA, Adjusted EBITDA (including pro forma presentations thereof) and the related ratios presented in this prospectus are supplemental measures of our performance that are not required by, or presented in accordance with GAAP. EBITDA and Adjusted EBITDA are not measurements of our financial performance under GAAP and should not be considered as alternatives to net income or any other performance measures derived in accordance with GAAP, or as an alternative to cash flow from operating activities as a measure of our liquidity.
EBITDA represents net income (loss) before net interest expense, income tax expense, depreciation and amortization. Adjusted EBITDA is defined as EBITDA further adjusted to give effect to unusual items, non-cash items and other adjustments, all of which are required in calculating covenant ratios and compliance under the indenture governing the exchange notes offered hereby and under our senior secured credit facility.
We believe that the inclusion of EBITDA and Adjusted EBITDA in this prospectus is appropriate to provide additional information to investors about the calculation of certain financial covenants in the indenture governing the exchange notes offered hereby and under our senior secured credit facility. Adjusted EBITDA is a material component of these covenants. For instance, the indenture governing the exchange notes offered hereby and our senior secured credit facility contain financial covenant ratios, specifically total leverage and interest coverage ratios, that are calculated by reference to Adjusted EBITDA. Non-compliance with the financial ratio maintenance covenants contained in our senior secured credit facility could result in the requirement to immediately repay all amounts outstanding under such facility, while non-compliance with the debt incurrence ratios contained in the indenture governing the exchange notes offered hereby would prohibit us from being able to incur additional indebtedness other than pursuant to specified exceptions.
We also present EBITDA because we consider it an important supplemental measure of our performance and believe it is frequently used by securities analysts, investors and other interested parties in the evaluation of high yield issuers, many of which present EBITDA when reporting their results. Measures similar to EBITDA are also widely used by us and others in our industry to evaluate and price potential acquisition candidates. We believe EBITDA facilitates operating performance comparison from period to period and company to company by backing out potential differences caused by variations in capital structures (affecting relative interest expense), tax positions (such as the impact on periods or companies of changes ineffective tax rates or net operating losses) and the age and book depreciation of facilities and equipment (affecting relative depreciation expense).
In calculating Adjusted EBITDA, as permitted by the terms of our indebtedness, we eliminate the impact of a number of items we do not consider indicative of our ongoing operations and for the other reasons noted above. For the reasons indicated herein, you are encouraged to evaluate each adjustment
60
and whether you consider it appropriate. In addition, in evaluating Adjusted EBITDA, you should be aware that in the future we may incur expenses similar to the adjustments in the presentation of Adjusted EBITDA. Our presentation of Adjusted EBITDA should not be construed as an inference that our future results will be unaffected by unusual or non-recurring items.
EBITDA and Adjusted EBITDA have limitations as analytical tools, and you should not consider them in isolation, or as a substitute for analysis of our results as reported under GAAP. Some of these limitations are:
- •
- they do not reflect our cash expenditures for capital expenditure or contractual commitments;
- •
- they do not reflect changes in, or cash requirements for, our working capital requirements;
- •
- they do not reflect the significant interest expense, or the cash requirements necessary to service interest or principal payments on our indebtedness;
- •
- although depreciation and amortization are non-cash charges, the assets being depreciated and amortized will often have to be replaced in the future, and EBITDA and Adjusted EBITDA do not reflect cash requirements for such replacements;
- •
- Adjusted EBITDA does not reflect the impact of earnings or charges resulting from matters we consider not to be indicative of our ongoing operations, as discussed in our presentation of "Adjusted EBITDA" in this prospectus; and
- •
- other companies, including other companies in our industry, may calculate these measures differently than we do, limiting their usefulness as a comparative measure.
Because of these limitations, EBITDA and Adjusted EBITDA should not be considered as measures of discretionary cash available to us to invest in the growth of our business or reduce our indebtedness. We compensate for these limitations by relying primarily on our GAAP results and using EBITDA and Adjusted EBITDA only supplementally. For more information, see our consolidated financial statements and the notes to those statements included elsewhere in this prospectus.
The following table sets forth a reconciliation of net income to EBIDTA for the periods indicated:
| | Twelve Months Ended December 31,
| | Nine Months Ended
September 30,
|
|---|
| | 2000
| | 2001
| | 2002
| | 2003
| | 2004
| | 2004
| | 2005
|
|---|
| | (In thousands)
| |
| |
|
|---|
| RECONCILIATION OF NET INCOME TO EBITDA: | | | | | | | | | | | | | | | | | | | | | |
| | Net income (loss) | | $ | (10,878 | ) | $ | (6,998 | ) | $ | (27,412 | ) | $ | (14,804 | ) | $ | (5,620 | ) | $ | (6,440 | ) | $ | 11,933 |
| | Interest expense | | | 11,363 | | | 17,802 | | | 16,923 | | | 16,587 | | | 26,879 | | | 19,397 | | | 23,731 |
| | (Benefit) Provision for income taxes | | | (5,404 | ) | | (1,504 | ) | | (5,145 | ) | | 13,872 | | | 3,483 | | | 2,341 | | | 10,045 |
| | Depreciation and amortization | | | 11,902 | | | 15,455 | | | 10,858 | | | 11,591 | | | 16,152 | | | 11,289 | | | 16,062 |
| | |
| |
| |
| |
| |
| |
| |
|
| EBITDA | | $ | 6,983 | | $ | 24,755 | | $ | (4,776 | ) | $ | 27,246 | | $ | 40,894 | | $ | 26,587 | | $ | 61,771 |
| | |
| |
| |
| |
| |
| |
| |
|
61
The following table sets forth a reconciliation of EBITDA to Adjusted EBITDA for the periods indicated:
| | Twelve Months Ended
December 31,
| | Nine Months Ended
September 30,
| |
|---|
| | 2003
| | 2004
| | 2004
| | 2005
| |
|---|
| EBITDA | | $ | 27,246 | | $ | 40,894 | | $ | 26,587 | | $ | 61,771 | |
| | Adjustments: | | | | | | | | | | | | | |
| | | Restructuring and other charges(a) | | | 1,653 | | | 3,600 | | | 2,107 | | | 3,824 | |
| | | Stock-based compensation(b) | | | (791 | ) | | 266 | | | 161 | | | 3,556 | |
| | | Executive severance(c) | | | — | | | 232 | | | — | | | 384 | |
| | | Executive relocation(d) | | | — | | | 67 | | | — | | | 507 | |
| | | Executive officer transition(e) | | | 1,572 | | | — | | | — | | | — | |
| | | Write-off of step-up of acquired inventory at date of acquisitions(f) | | | — | | | 3,397 | | | 3,397 | | | 120 | |
| | | Write off acquired A/R and inventory(g) | | | — | | | 2,479 | | | — | | | (336 | ) |
| | | Losses incurred by closed facilities(h) | | | — | | | 2,046 | | | 844 | | | 829 | |
| | | Gain on sale of building(i) | | | — | | | (118 | ) | | (118 | ) | | — | |
| | | Management fees to existing stockholders(j) | | | 500 | | | 700 | | | 475 | | | 675 | |
| | | Debt prepayment penalty(k) | | | — | | | 3,295 | | | 3,295 | | | — | |
| | | Costs related to the Transactions(l) | | | — | | | — | | | — | | | 558 | |
| | |
| |
| |
| |
| |
| Adjusted EBITDA | | $ | 30,180 | | $ | 56,858 | | $ | 36,748 | | $ | 71,888 | |
| | |
| |
| |
| |
| |
- (a)
- Set forth below is a reconciliation of restructuring and other charges as shown on the selected historical consolidated financial data to the amount shown above as an adjustment to EBITDA:
| | Twelve months Ended
December 31,
| | Nine months ended
September 30,
|
|---|
| | 2003
| | 2004
| | 2004
| | 2005
|
|---|
| Restructuring and other | | $ | 1,487 | | $ | 3,600 | | $ | 2,107 | | $ | 3,824 |
| Inventory charges in cost of sales | | | 322 | | | — | | | — | | | — |
| Less: depreciation in restructuring and other | | | (156 | ) | | — | | | — | | | — |
| | |
| |
| |
| |
|
| | | $ | 1,653 | | $ | 3,600 | | $ | 2,107 | | $ | 3,824 |
| | |
| |
| |
| |
|
- (b)
- We have incurred non-cash charges for stock-based compensation relating to stock options granted during the twelve months ended December 31, 2000 and 2001, non-cash dividends on phantom stock issued to employees in connection with certain acquisitions, an increase in the value of phantom stock, and charges for restricted stock and stock options granted to
62
| | Twelve months Ended
December 31,
| | Nine months ended
September 30,
|
|---|
| | 2003
| | 2004
| | 2004
| | 2005
|
|---|
| 2000 & 2001 stock options | | $ | 191 | | $ | 170 | | $ | 114 | | $ | 115 |
| Phantom stock market value adjustment | | | (617 | ) | | — | | | — | | | 2,747 |
| Award of redeemable preferred stock | | | — | | | 20 | | | — | | | — |
| July 2005 stock options and restricted stock | �� | | — | | | — | | | — | | | 616 |
| Adjustment of redeemable preferred to stock to market value | | | (500 | ) | | — | | | — | | | |
| Phantom stock non-cash dividends | | | 135 | | | 76 | | | 47 | | | 78 |
| | |
| |
| |
| |
|
| | | $ | (791 | ) | $ | 266 | | $ | 161 | | $ | 3,556 |
| | |
| |
| |
| |
|
- (c)
- We have incurred executive severance costs due to contractual severance obligations for certain executives of $0.2 million during the twelve months ended December 31, 2004, and $0.4 million during the nine months ended September 30, 2005.
- (d)
- We have aligned our management structure along the three target markets that we serve: cardiology, endoscopy, and orthopaedics. In connection with this alignment, we have created new divisional management offices and relocated employees to these offices. We incurred executive relocation costs of $0.1 million during the twelve months ended December 31, 2004 and $0.5 million during the nine months ended September 30, 2005, including $0.1 million which we incurred during our third quarter of 2005.
- (e)
- During the twelve months ended December 31, 2003, we incurred $1.6 million of costs due to a change in our chief executive officer, consisting primarily of severance.
- (f)
- We record the assets and liabilities of acquired companies at their respective fair values upon the date of acquisition. Inventories are recorded at fair value at the acquisition date, with the difference between the cost of the inventory and fair value charged to cost of sales as the inventory is sold. During the twelve months ended December 31, 2004, we incurred $3.4 million of costs to write off the step-up of inventory acquired from MedSource. During the nine months ended September 30, 2005, we incurred $0.1 million of costs to write off a portion of the step-up of inventory acquired from Campbell, all of which was incurred during our third quarter of 2005.
- (g)
- We incurred charges of $2.5 million in the fourth quarter of 2004 to write down $1.9 million of inventory and $0.6 million of receivables acquired from MedSource. During our first quarter of 2005, we settled a $0.3 million MedSource receivable that had previously been written off, and, as a result, recorded a reduction of the original EBITDA adjustment.
- (h)
- In connection with our acquisition of MedSource, we implemented a plan to close certain MedSource facilities. We closed the Newton, Massachusetts facility in January 2005, sold the Norwell, Massachusetts facility in February 2005, and closed the Navojoa, Mexico facility in April 2005. We have added back losses incurred by these facilities during the twelve months ended December 31, 2004 and the nine months ended September 30, 2004 and 2005 of $2.0 million, $0.8 million and $0.8 million, respectively.
- (i)
- We recorded a gain of $0.1 million on the sale of a manufacturing facility located in South Plainfield, New Jersey during the nine months ended September 30, 2004 and the twelve months ended December 31, 2004.
- (j)
- We incurred management fees to one of our equity sponsors, KRG Capital Partners, LLC of $0.5 million per year during the twelve months ended December 31, 2003. Effective July 1, 2004, we began to incur an additional management fee to another equity sponsor, DLJ Merchant Banking of $0.4 million annually. In connection with the Transactions, such arrangements were terminated and we entered into a management services agreement with KKR that provides for a $1.0 million annual payment, such amount to increase by 5% per year. See "Certain Relationships and Related Party Transactions."
63
- (k)
- In connection with the MedSource acquisition, we repaid existing indebtedness and as a result incurred $3.3 million of prepayment penalties.
- (l)
- In connection with the Transactions, we have incurred expenses, primarily professional fees, totaling $0.6 million. All of the fees incurred were recorded during the nine months ended September 30, 2005, specifically during our third quarter of 2005.
Off-Balance Sheet Arrangements
We do not have any "off-balance sheet arrangements" (as such term is defined in Item 303 of Regulation S-K) that are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Contractual Obligations and Commitments
The following table sets forth our long-term contractual obligations as of December 31, 2004 (in thousands):
| | Payment due by Period
|
|---|
Contractual Obligations
| | Total
| | Less than 1 year
| | 1–3 years
| | 3–5 years
| | More than 5 years
|
|---|
| Senior secured credit facility | | $ | 193,030 | | $ | 1,940 | | $ | 3,880 | | $ | 3,880 | | $ | 183,330 |
| Senior subordinated notes | | | 175,000 | | | — | | | — | | | — | | | 175,000 |
| Capital leases | | | 22 | | | 21 | | | 1 | | | — | | | — |
| Operating leases(1) | | | 42,515 | | | 6,165 | | | 10,282 | | | 7,580 | | | 18,488 |
| Purchase obligations | | | 31,967 | | | 31,967 | | | — | | | — | | | — |
| Other long-term obligations(2) | | | 14,386 | | | 296 | | | 2,068 | | | 213 | | | 11,809 |
| | |
| |
| |
| |
| |
|
| | Total | | $ | 456,920 | | $ | 40,389 | | $ | 16,231 | | $ | 11,673 | | $ | 388,627 |
| | |
| |
| |
| |
| |
|
- (1)
- Includes lease extension options which we expect will be exercised.
- (2)
- Other long-term obligations include environmental remediation obligations of $4.8 million, accrued severance benefits of $1.8 million, accrued compensation and pension benefits of $4.4 million and deferred income taxes of $3.4 million. Accrued future rental obligations of $9.3 million included in other long-term liabilities on our consolidated balance sheet as of December 31, 2004 are included in our operating leases in the table of contractual obligations.
In connection with the 2005 Acquisitions, an additional $12.0 million of consideration will be paid, if at all, pursuant to an earnout arrangement contingent upon the 2005 and 2006 full year financial performance of Campbell. If the Campbell earnout arrangement is earned, the payment would be made in the second quarters of 2006 and 2007. Also, an additional $6.0 million of consideration will be paid, if at all, pursuant to an earnout arrangement payable contingent upon 2006 full year financial performance of MTG. If the MTG earnout arrangement is earned, the payment would be made in the second quarter of 2007.
Effect of the Transactions
We intend to fund ongoing operations through cash generated by operations and availability under the revolving portion of our senior secured credit facility. As part of the Transactions, we have incurred substantial debt, including under our senior secured credit facility and the notes, with interest payments on this indebtedness increasing our liquidity requirements. See "Risk Factors—Risks Related to our Indebtedness and the Exchange Notes."
64
Our senior secured credit facility consists of a $400 million term loan B facility due in 2012 and a $75 million revolving credit facility due in 2011. Our outstanding letters of credit remain approximately $5.8 million subsequent to the Transactions, which reduce the amounts available under the revolving credit portion of our senior secured credit facility. We drew down on our revolving credit facility to fund working capital requirements soon after the closing of the Transactions.
Borrowings under our term loan B facility bear interest at our option at either LIBOR plus an applicable margin or the alternate base rate plus an applicable margin.
Our senior secured credit facility requires us to meet a maximum total leverage ratio, a minimum interest coverage ratio and a maximum capital expenditures limitation. In addition, the senior secured credit facility contains certain restrictive covenants which, among other things, limit the incurrence of additional indebtedness, dividends, prepayments of other indebtedness, investments, mergers and consolidations, changes in business, liens and other matters customarily restricted in such agreements. It also contains certain customary events of default, subject to grace periods, as appropriate. See "Description of Other Indebtedness."
Future principal debt payments are expected to be paid out of cash flows from operations, borrowings under the revolving portion of our senior secured credit facility, and future refinancing of our debt.
Our ability to make scheduled payments of principal, or to pay the interest or special interest, if any, on, or to refinance our indebtedness, to fund planned capital expenditures and acquisitions will depend on our future performance, which, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. Based upon the current level of operations, we believe that cash flow from operations and available cash, together with borrowings under our senior secured credit facility, will be adequate to meet our future liquidity needs throughout the remainder of 2005 and 2006. There can be no assurance that we will generate sufficient cash flow from operations, that anticipated revenue growth and operating improvements will be realized or that future borrowings will be available under the senior secured credit facility in an amount sufficient to enable us to service our indebtedness, including the notes, or to fund other liquidity needs. In addition, there can be no assurance that we will be able to effect any future refinancing of our debt on commercially reasonable terms or at all.
The following table sets forth our long-term contractual obligations as of December 31, 2004 (in thousands) on an as adjusted pro forma basis:
| | Payment due by Period
|
|---|
Contractual Obligations
| | Total
| | Less than
1 year
| | 1–3 years
| | 3–5 years
| | More than
|
|---|
| Senior secured credit facility | | $ | 400,000 | | $ | 3,000 | | $ | 8,000 | | $ | 8,000 | | $ | 381,000 |
| 101/2% senior subordinated notes | | | 305,000 | | | — | | | — | | | — | | | 305,000 |
| Capital leases | | | 22 | | | 21 | | | 1 | | | — | | | — |
| Operating leases(1) | | | 42,515 | | | 6,165 | | | 10,282 | | | 7,580 | | | 18,488 |
| Purchase obligations | | | 31,967 | | | 31,967 | | | — | | | — | | | — |
| Other long-term obligations(2) | | | 14,386 | | | 296 | | | 2,068 | | | 213 | | | 11,809 |
| | |
| |
| |
| |
| |
|
| | Total | | $ | 793,890 | | $ | 41,449 | | $ | 20,351 | | $ | 15,793 | | $ | 716,297 |
| | |
| |
| |
| |
| |
|
- (1)
- Includes lease extension options which we expect will be exercised.
- (2)
- Other long-term obligations include environmental remediation obligations of $4.8 million, accrued severance benefits of $1.8 million, accrued compensation and pension benefits of $4.4 million and deferred income taxes of $3.4 million. Accrued future rental obligations of $9.3 million included in other long-term liabilities on our consolidated balance sheet as of December 31, 2004 are included in our operating leases in the table of contractual obligations.
65
Critical Accounting Policies
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We base our estimates on historical experience, current conditions and various other assumptions that are believed to be reasonable under the circumstances. Estimates and assumptions are reviewed on an ongoing basis and the effects of revisions are reflected in the consolidated financial statements in the period they are determined to be necessary. Actual results could differ materially from those estimates under different assumptions or conditions. We believe the following critical accounting policies impact our judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition. The amount of product revenue recognized in a given period is impacted by our judgments made in establishing our reserve for potential future product returns. We provide a reserve for our estimate of future returns against revenue in the period the revenue is recorded. Our estimate of future returns is based on such factors as historical return data and the timing of historical returns as compared to the sale date. The amount of revenue we recognize will be directly impacted by our estimates made to establish the reserve for potential future product returns. Our provision for sales returns was $1.1 million, $0.8 million and $0.3 million at September 30, 2005, December 31, 2004 and December 31, 2003, respectively.
Allowance for Doubtful Accounts. We estimate the collectibility of our accounts receivable and the related amount of bad debts that may be incurred in the future. The allowance for doubtful accounts results from an analysis of specific customer accounts, historical experience, credit ratings and current economic trends. Based on this analysis, we provide allowances for specific accounts where collectibility is not reasonably assured.
Provision for Inventory Valuation. Inventory purchases and commitments are based upon future demand forecasts. Excess and obsolete inventory are valued at their net realizable value, which may be zero. We periodically experience variances between the amount of inventory purchased and contractually committed to and our demand forecasts, resulting in excess and obsolete inventory valuation charges.
Valuation of Goodwill. Goodwill is the excess of the purchase price over the fair value of identifiable net assets acquired in business combinations. In accordance with SFAS No. 142, goodwill is assigned to the reporting unit expected to benefit from the synergies of the combination. We have assigned our goodwill to three reporting units. Goodwill for each reporting unit is subject to an annual impairment test, or more often if impairment indicators arise, using a fair-value-based approach. In assessing the fair value of goodwill, we make projections regarding future cash flow and other estimates, and may utilize third party appraisal services. If these projections or other estimates for one or all of these reporting units change, we may be required to record an impairment charge.
Valuation of Long-lived Assets. Long-lived assets are comprised of property, plant and equipment and intangible assets with finite lives. We assess the impairment of long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable through projected undiscounted cash flows expected to be generated by the asset. When we determine that the carrying value of intangible assets and fixed assets may not be recoverable, we measure impairment by the amount by which the carrying value of the asset exceeds the related fair value. Estimated fair value is generally based on projections of future cash flows and other estimates, and guidance from third party appraisal services.
66
Self Insurance Reserves. We accrue for costs to provide self insured benefits under our workers' compensation and employee health benefits programs. With the assistance of third party workers' compensation experts, we determine the accrual for workers' compensation losses based on estimated costs to resolve each claim. We accrue for self insured health benefits based on historical claims experience. We maintain insurance coverage to prevent financial losses from catastrophic workers' compensation or employee health benefit claims. Our financial position or results of operations could be impacted in a fiscal quarter due to a material increase in claims. Our accruals for self insured workers compensation and employee health benefits at September 30, 2005 and December 31, 2004 were $3.9 million and $3.3 million, respectively.
Environmental Reserves. We accrue for environmental remediation costs when it is probable that a liability has been incurred and a reasonable estimate of the liability can be made. Our remediation cost estimates are based on the facts known at the current time including consultation with a third party environmental specialist and external legal counsel. Changes in environmental laws, improvements in remediation technology and discovery of additional information concerning known or new environmental matters could affect our operating results.
Pension and Other Employee Benefits. Certain assumptions are used in the calculation of the actuarial valuation of our defined benefit pension plans. These assumptions include the weighted average discount rate, rates of increase in compensation levels and expected long-term rates of return on assets. If actual results are less favorable than those projected by management, additional expense may be required.
Income Taxes. We estimate our income taxes in each of the jurisdictions in which we operate. This process involves estimating our actual current tax exposure together with assessing temporary differences resulting from differing treatment of items, such as goodwill amortization, for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. We then assess the likelihood that our deferred tax assets will be recovered from future taxable income and to the extent we believe that recovery is not likely, we establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we increase or decrease our income tax provision in our consolidated statement of operations. If any of our estimates of our prior period taxable income or loss prove to be incorrect, material differences could impact the amount and timing of income tax benefits or payments for any period.
New Accounting Pronouncements
In November 2004, the FASB issued SFAS No. 151, "Inventory Costs, an amendment of ARB No. 43, Chapter 4." SFAS No. 151 amends Accounting Research Bulletin ("ARB") No. 43, Chapter 4, to clarify that abnormal amounts of idle facility expense, freight, handling costs and wasted materials (spoilage) should be recognized as current-period charges. In addition, SFAS No. 151 requires that allocation of fixed production overhead to inventory be based on the normal capacity of the production facilities. SFAS No. 151 is effective for inventory costs incurred during fiscal years beginning after June 15, 2005. We are currently assessing the impact that SFAS No. 151 will have on our results of operations, financial position or cash flows.
On December 16, 2004, the FASB issued SFAS No. 123 (Revised 2004), "Share-Based Payment." SFAS 123R requires that compensation cost related to share-based payment transactions be recognized in the financial statements. Share-based payment transactions within the scope of SFAS 123R include stock options, restricted stock plans, performance-based awards, stock appreciation rights, and employee share purchase plans. The provisions of SFAS 123R are effective for our first interim period that begins after December 31, 2005. Accordingly, we will implement the revised standard in the first quarter of fiscal year 2006. Currently, we account for share-based payment transactions under the
67
provisions of APB 25, which does not necessarily require the recognition of compensation cost in the financial statements. We are assessing the implications of this revised standard, which may materially impact our results of operations in the first quarter of fiscal year 2006 and thereafter.
Quantitative and Qualitative Disclosures About Market Risk
Market Risk
We are subject to market risk associated with change in interest rates and foreign currency exchange rates.
Interest Rate Risk
We are subject to market risk associated with change in the London Interbank Offered Rate (LIBOR) and the Federal Funds Rate published by the Federal Reserve Bank of New York in connection with our senior secured credit facility. Based on the outstanding balance at September 30, 2005, a hypothetical 10% change in rates under the senior secured credit facility would result in a change to our annual interest expense of approximately $1.2 million.
Foreign Currency Risk
We operate some facilities in foreign countries. At September 30, 2005, approximately $7.4 million of long-lived assets were located in foreign countries. Our principal currency exposures relate to the Euro, British pound and Mexican pesos. We consider the market risk to be low, as the majority of the transactions at our European locations are denominated in the Euro or British Pound, and our exposure to date in the Mexican peso has not been significant.
68
BUSINESS
Overview
We are the largest provider of outsourced precision manufacturing and engineering services to the medical device industry according to market share comparisons by the Millennium Research Group. We focus on what we believe are three of the largest and fastest growing segments of the medical device market: cardiology, endoscopy and orthopaedics. Our customers are the leading medical device companies in the world, including Abbott Laboratories, Boston Scientific, Guidant, Johnson & Johnson, Medtronic, Smith & Nephew, St. Jude, Stryker, Tyco and Zimmer. We provide our customers with reliable, high-quality, cost-efficient, integrated outsourcing solutions that span the complete supply chain spectrum.
Our design and engineering, precision component manufacturing, device assembly and supply chain management services provide multiple strategic benefits to our customers. We help speed our customers' products to market, lower their manufacturing costs, provide capabilities that they do not possess internally, and enable our customers to concentrate resources on clinical education, research, sales and marketing.
We have developed long-term relationships with our largest customers and work closely with them in the designing, testing, prototyping, validation and production of their products. In many cases, we have been partnering with our key customers for over ten years. Based on discussions with our customers, we believe we are considered a preferred strategic supplier by a majority of our top ten customers, and often become the sole supplier of the manufacturing and engineering services that we provide to our customers. Many of the end products we produce for our customers are regulated by the FDA, which has stringent quality standards for manufacturers of medical devices. Complying with these requirements involves significant investments of money and time, which results in stronger relationships with our customers. Because of these stringent standards, multiple validations of our manufacturing process are required by the FDA to ensure high quality, reliable production. The joint investment of time and process validation by us and our customers, along with the possibility of supply disruptions and quality fluctuations associated with moving a product line, often create high switching costs for transferring product lines once a product begins production. Typically, once our customers have begun production of a certain product with us, they do not move their products to another supplier. Further, validation requirements encourage customers to consolidate business with preferred suppliers such as us, whose processes have been validated in the past.
We generate significant recurring revenues from a diverse range of products that generally have long product life cycles. Moreover, the majority of our revenues comes from high value, single use products that are either regulated for one-time use, implanted into the body or are considered too critical to be re-used. We currently work with our customers on over 10,000 stock keeping units, providing us with tremendous product diversity across our customer base.
We expect our future growth to come from a combination of factors, including market growth for cardiology, endoscopy and orthopaedic devices, increased outsourcing of existing and new products by our customers to us, and increasing our market share of the overall outsourcing market. This growing revenue base is made up of a diversified product mix with limited technology or product obsolescence risk. We manufacture many products that have been used in medical devices for over ten years, such as biopsy instruments, joint implants, pacemakers and surgical instruments. Even as our customers' end market products experience new product innovation, we continue to supply the base products and
69
services across end market product cycles. For the nine months ended September 30, 2005, on a pro forma basis, our net sales by end market were:
| | Pro
Forma
| |
|---|
| Endoscopy | | 43 | % |
| Cardiology | | 33 | % |
| Orthopaedic | | 16 | % |
| Industrial | | 8 | % |
| | |
| |
| | | 100 | % |
| | |
| |
On April 27, 2004, we entered into an Agreement and Plan of Merger pursuant to which, on June 30, 2004, MedSource and its subsidiaries became our wholly owned subsidiaries. As a result of the merger, we became the largest provider of manufacturing and engineering services to the medical device industry. We have successfully integrated the former UTI and MedSource into a single customer-focused organization. The size and scope of our business following the merger has enabled us to invest in differentiated design, prototyping and manufacturing capabilities and quality and information systems as well as a focused sales force. We have essentially completed the operational integration and have also substantially completed our planned facility rationalizations.
We have subsequently made two small acquisitions to complement our capabilities in the orthopaedics market, Campbell of Huntsville, Alabama and MTG of Arlington, Tennessee.
On October 7, 2005, we entered into a merger agreement that resulted in approximately 71% of our capital stock owned by affiliates of KKR, approximately 24% of our capital stock owned by affiliates of Bain and the remainder held by certain members of management. See "The Transactions."
Industry Background
Medical Device End Markets. The medical device industry enjoys favorable industry dynamics, with projected revenue growth in our key market segments of approximately 11% annually from 2004 through 2009, according to the Millennium Research Group.
We focus on what we believe are three of the largest and fastest growing segments of the medical device industry: cardiology, endoscopy and orthopaedics, which together account for a $58.5 billion market according to the Millennium Research Group. The outsourcing opportunities for these three targeted end markets are expected to grow at 14.4% from $4.0 billion in 2004 to $7.9 billion in 2009, according to the Millennium Research Group. We believe that these end markets are attractive based on their large size, significant volume growth, relatively high customer profit margins, strong product pipelines, competitive environment and a demonstrable need for our high-quality manufacturing and engineering services.
We target these three end markets by focusing on the 15 leading medical device companies that operate in one or more of these markets. We believe these leading medical device companies will generate outsourcing opportunities similar to the end markets in which they operate.
We believe this demand is being driven primarily as a result of:
- •
- Aging Population. The average age of the U.S. population is expected to increase significantly over the next decade. According to U.S. Census data, the total U.S. population is projected to grow approximately 5% from 2005 to 2010, while the number of individuals in the United States over the age of 55 is projected to grow approximately 14% during the same period. As the average age of the population increases, the demand for medical products and services, including medical devices, is expected to increase as well.
70
- •
- Active Lifestyles. As people are living longer, more active lives, the adoption of medical devices such as orthopaedic implants and arthroscopy devices has grown. In addition, in order to maintain this active lifestyle, patients demand more functional, higher technology devices.
- •
- Advances in Medical Device Technology. The development of new medical device technology is driving growth in the medical device market. Examples include neurostimulation, drug-eluting stents and innovative pacemakers, which are experiencing rapid adoption in the medical community because of the significant demonstrated patient benefits.
- •
- Increased Global Utilization. The global medical device market is largely concentrated in North America, Western Europe and Japan. As the United States is the major global supplier of medical devices, the aging of the European and Japanese populations and the increased global utilization of medical devices further add to medical device volume growth. Emerging countries in Asia, South America and Eastern Europe are also increasing their consumption of medical devices due to enhanced awareness and increasing financial flexibility.
- •
- Increase in Minimally Invasive Technologies. The medical device market is witnessing a major shift away from invasive or open surgical procedures to minimally invasive procedures and technologies. Minimally invasive procedures have been developed to reduce the pain, trauma, recovery time and overall costs resulting from open and more invasive procedures. The continued adoption of such minimally invasive technologies is expected to continue driving growth in the overall medical device market.
The chart below provides examples of customer products in our targeted markets and the products and services that we provide for each of the customer end products.
Market
| |
| | Customer Devices/Products
| |
| | Our Products and Services
|
|---|
| Cardiology | | | | | | | | |
| | Interventional Cardiology | | • | | Stents | | • | | Stent tubing, stents, mandrels |
| | | • | | Rotational artery clearing device | | • | | Ground guidewires, tubular drive components |
| | | • | | Guidewires, delivery systems | | • | | Marker bands, catheter shafts, tooling mandrels, corewires, guidewire assemblies |
| |
Cardiac Rhythm Management |
|
• |
|
Pacemakers |
|
• |
|
Implantable electrodes, |
| | | | | | | | | connector blocks, lugs |
| | | • | | Implantable defibrillators | | • | | Electrodes & leads |
| |
Cardiac Surgery |
|
• |
|
Heart immobilization devices |
|
• |
|
Machined tubing |
| | | • | | Heart valves | | • | | Machined valve bodies and leaflets |
| |
Interventional Neurology |
|
• |
|
Implantable coils |
|
• |
|
Wire coiling |
| | | • | | Catheters/delivery systems | | • | | Guidewires |
| |
Peripheral Vascular |
|
• |
|
Stents/Delivery systems |
|
• |
|
Stents, stent tubing, guidewires, |
| | | | | | | | | hypotubing |
| Endoscopy | | | | | | | | |
| | Laparoscopy/Gynecology | | • | | Harmonic scalpel blades | | • | | Blade assemblies |
| | | • | | Breast biopsy devices | | • | | Tubular components, MIM jaws |
| | | | | | | | | and anvils, stamped components |
| | | • | | Trocars | | • | | Tubular components and complete assemblies |
| | | • | | Birth control devices | | • | | Tubular machined components |
| | | | | | | • | | Complete finished devices |
| |
Arthroscopy |
|
• |
|
Shaver blades |
|
• |
|
Blade assemblies |
| | | • | | Arthroscopes | | • | | Arthroscope tubing |
| | | • | | Suture anchors | | • | | Machined anchors, drivers |
| | | | | | | | | |
71
| |
Urology |
|
• |
|
Stone retrieval baskets |
|
• |
|
Wire grinding |
| | | • | | Thermal tumor shrinkage | | • | | Catheter design and fabrication |
| | | • | | Bladder stapling devices | | • | | Finished goods |
| |
Gastrointestinal |
|
• |
|
Biopsy forceps |
|
• |
|
Complete assembly, supply chain management |
| | | | | | | • | | MIM jaws |
| | | | | | | • | | Plastic catheters |
| | | | | | | • | | Plastic injection molded assemblies |
| |
Ophthalmology |
|
• |
|
Ultrasonic tips |
|
• |
|
Micro tube drawing and machining |
| |
Drug Delivery |
|
• |
|
Drug pumps |
|
• |
|
Case stamping |
| | | | | | | • | | Hypotube needle fabrication |
| |
Wound Closure |
|
• |
|
Stapling devices |
|
• |
|
Stamped components |
| | | | | | | • | | Anvils |
| | | | | | | • | | Tubular components |
| Orthopaedics | | | | | | | | |
| |
Joint Replacement |
|
• |
|
Artificial hip, knee & extremity implants |
|
• |
|
Hip stems & sleeves, acetabular shells & liners, tibial trays, posts, inserts & wedges, femoral components, glenoids, shoulder stems, ankle, finger & toe components. Forgings, surface treatment & engineering services to support customer implant programs |
| | | • | | Hip and knee trials | | • | | Same as implants above, but machined from plastic, aluminum and stainless steel. Used for sizing implants |
| | | • | | Procedure specific instruments | | • | | Impactors, implant holders, reamers, drills, taps, tamps, alignment guides, cutting blocks, screw drivers, torque wrenches |
| |
Spinal |
|
• |
|
Fusion components, artificial discs, and dynamic stabilization system implants |
|
• |
|
Plates, screws, rods, cross-connectors, metal discs, polyurethane inserts, surface treatments |
|
|
• |
|
Procedure specific instruments |
|
• |
|
Screwdrivers, torque wrenches, gages, implant holders, reamers, cutting guides |
| | | • | | Orthobiologics | | • | | Single use sterile delivery system design, manufacture & assembly & packaging, Machined synthetic bone substitutes and packaging |
| |
Trauma |
|
• |
|
Implants |
|
• |
|
Maxillofacial plates & screws, compression plates & screws, IM nail, fixation screws |
| | | • | | External Fixation | | • | | Clamps, hooks, screws |
|
|
• |
|
Procedure specific instruments |
|
• |
|
Screwdrivers, drills, taps, reamers, alignment guides |
72
Medical Device Companies in Our Key Target End Markets Are Outsourcing Manufacturing, Design and Engineering. As medical devices have become more technically complex, the demand for precision manufacturing capabilities and related engineering services has increased significantly. Many of the leading medical device companies in our end markets are increasingly utilizing third-party manufacturing and engineering providers as part of their business and manufacturing strategies. Outsourcing allows medical device companies to take advantage of the manufacturing technologies, economies of scale and supply chain management expertise of third-party manufacturers. Outsourcing also enables medical device companies to concentrate resources on clinical education, research, sales and marketing.
Medical device companies carefully select their manufacturing and engineering outsourcing partners primarily based on quality and reliability. Medical devices companies require stringent validation processes and manufacturing standards to ensure high quality production and reliable delivery. The validation and approval process for third-party manufacturing requires a significant amount of time and engineering resources that often result in long-term relationships. These processes may also include inspection by the FDA of the manufacturing facilities in connection with products undergoing premarket regulatory review. As a result, we believe that medical device companies increasingly seek to reduce the number of suppliers they use by consolidating with a limited number of strategic partners with demonstrated track records. We believe medical device companies are choosing their strategic outsourcing partners based on the partner's ability to:
- •
- Provide comprehensive precision manufacturing and engineering capabilities
- •
- Deliver consistently high quality and highly reliable products at competitive prices
- •
- Assist in rapid time-to-market and time-to-volume manufacturing requirements
- •
- Manage a global supply chain
Growth in Outsourced Manufacturing Services. We believe our target market is represented by the amount of manufacturing and engineering services outsourced by the leading medical device companies to third-party manufacturers. Our target market is growing through a combination of growth in our customers' end markets and an increase in the amount of manufacturing and engineering services outsourced to third-party providers. Within the medical device outsourcing market, our specific target markets—cardiology, endoscopy and orthopaedics—are projected to grow annually at a rate of 15.3%, 14.3% and 13.7%, respectively, through 2009 according to the Millennium Research Group.
We believe our current target market will continue to increase due to both the growth in medical device end markets and an increase in outsourcing by medical device companies. Key factors driving increased penetration in outsourcing include:
- •
- Desire to Accelerate Time-to-Market. The leading medical device companies are focused on clinical education, research, sales and marketing in order to maximize the commercial potential from new products. For these new products, the medical device companies are attempting to reduce development time in order to bring products to market faster and compete more effectively. Outsourcing enables medical device companies to accelerate time-to-market and clinical adoption.
- •
- Increasing Complexity of Manufacturing Medical Device Products. As medical device companies seek to provide additional functionality in their products, the complexity of the technologies and processes involved in producing medical devices has increased. Medical device outsourcing companies have invested in facilities with comprehensive services and experienced personnel to deliver precision manufacturing services for these increasingly complex products.
73
Competitive Strengths
Our competitive strengths make us a preferred strategic partner for many of the leading medical device companies and position us for profitable growth. Our preferred provider status is evidenced through our long-term customer relationships, sole source agreements and/or by official designations.
- •
- Market Leader. We are the largest provider of outsourced precision manufacturing and engineering services in our target markets according to market share comparisons by the Millennium Research Group. We also believe we are approximately two to three times as large as our nearest direct competitors. We continue to invest in information technology and quality systems that enable us to meet or exceed the increasingly rigorous standards of our customers and differentiate us from our competitors.
- •
- Strong Long-Term Strategic Partnerships With Targeted Customers. Based on discussions with our customers, we believe we are considered a preferred strategic supplier to a majority of our top ten customers and often become the sole supplier of manufacturing and engineering services for a significant portion of the products we provide to our customers. We have a highly focused sales force dedicated to serving the leading medical device manufacturers, many of which we have had relationships with for at least ten years. Within these large customers, we generate diversified revenue streams across separate divisions and multiple products. As a result of our strong relationships, we are well-positioned to compete for a majority of our customers' outsourcing needs and benefit as our customers seek to reduce their supplier base.
- •
- Breadth of Manufacturing and Engineering Capabilities. We provide a comprehensive range of manufacturing and engineering services, including design, testing, prototyping, production and device assembly, as well as global supply chain management services. We have over 200 engineers available to help design, prototype and test feasibility and manufacturability. We have made significant investments in precision manufacturing equipment, information technology and quality systems. Our facilities have areas of expertise and capabilities which allow us to provide proprietary manufacturing services. In addition, our internal R&D team has developed innovative automation techniques that create economies of scale that can reduce production costs and often enable us to manufacture products at lower costs than our customers and competitors.
74
- •
- Reputation for Quality. We believe we have a reputation as a high quality manufacturer. Most of our strategic facilities follow a single uniform quality system and are ISO 13485 certified, a quality standard that is specific to medical devices and the most advanced level attainable. Due to the patient-critical and highly regulated nature of the products our customers provide, strong quality systems are an important factor in our customers' selection of a strategic manufacturing partner. As a result, our reputation and experience provide us with an advantage in winning new business as large medical device companies want to partner with successful, proven manufacturers who have the systems and capabilities necessary to deliver a high level of quality that is comparable to their own.
- •
- Strategic Locations. We believe that the location of our design, prototyping and engineering centers near our major customers and the location of certain of our facilities in advantageous manufacturing centers provide us with a competitive advantage. Our strategic locations allow us to facilitate speed to market, rapid prototyping, low cost assembly and overall customer familiarity. For example, our design, prototyping and engineering centers in Boston, Massachusetts; Minneapolis, Minnesota; and near Memphis, Tennessee; and our manufacturing center in Galway, Ireland, are strategically located near our major customers. In addition, our Juarez, Mexico facility provides our customers with a low-cost manufacturing and assembly solution.
- •
- Strong Financial Profile. We believe that as large medical device companies look to partner with suppliers of significant scale and stability, we are favorably positioned by having consistently demonstrated solid historical revenue growth and steadily improving margins. It is our belief that our strong operating earnings combined with modest capital expenditures and working capital requirements will continue to generate significant free cash flow. Furthermore, we have accumulated a significant net operating loss carryforward which will meaningfully reduce our cash tax payments in the next few years, thereby increasing free cash flow available to reduce indebtedness.
- •
- Experienced and Committed Management Team. We have a highly experienced management team at both the corporate and operational levels. Our senior management team, led by our President and Chief Executive Officer Ron Sparks, has an average of over 20 years of industry experience. Members of our management team also have extensive experience in mergers, acquisitions and integrations. Our management team is highly motivated and committed to the success of our company and invested approximately $30 million of equity in connection with the Transactions.
Business Strategy
Our objective is to follow a focused and profitable growth strategy, and to strengthen our position as the leading provider of outsourced precision manufacturing and engineering services to our target markets through the following:
- •
- Increase Share Within Target Market Leaders. We are focused on increasing our share of revenues from the leading companies within our target markets. We intend to strengthen our close relationships with the top companies in our target markets by continuing to deliver high quality products and services. We believe the strength of our customer relationships and our customer-focused sales teams, in combination with the breadth of our capabilities and manufacturing expertise, put us in a preferred position to capture an increasing percentage of new business.
- •
- Increase Manufacturing Efficiencies. We recently completed the registration of the majority of our strategic facilities under a single quality management system. We have implemented a new quality policy and have established new company wide policies and procedures and a corporate
75
management review process. Additionally, we have introduced our "Lean Manufacturing" program designed to improve manufacturing cycle times and reduce costs. The program consists of customized training, process mapping, and the implementation of process improvements. Several of our facilities completed the Lean Process Excellence assessment in the third quarter of 2005. Since initiating the implementation of process improvements, certain facilities have experienced improved response and delivery times with customers. These benefits are expected to increase as we further implement the continuous improvement initiative. Due to the early stage of the program, we are currently unable to quantify expected future cost savings.
- •
- Expand Design and Prototyping Capabilities and Presence. We intend to grow revenues from design and prototyping services by continuing to invest in selected strategic locations and equipment. We currently have design facilities for cardiology in Minneapolis, Minnesota, endoscopy in Boston, Massachusetts, and orthopaedics near Memphis, Tennessee. We believe being involved in the initial design and prototyping of medical devices positions us favorably to capture the ongoing manufacturing business of these devices as they move to full production.
- •
- Provide an Integrated Supply Chain Solution. We are constantly adding strategic capabilities in order to provide a continuum of service for our customers throughout their product life cycles, thereby allowing them to reduce the number of vendors they deal with and focus their resources on speed to market. These capabilities range from concept validation and design and development, through manufacturing, warehousing and distribution.
- •
- Selectively Pursue Complementary Acquisitions. The fragmented nature of the medical device outsourcing industry presents opportunities for us to selectively pursue complementary acquisitions, which would allow us to expand our scope and scale to further enhance our offering to our customers.
Capabilities
As medical device companies' outsourcing continues to grow, we believe that our customers' reliance upon the breadth of our capabilities increases. Our capabilities include Design and Engineering, Precision Component Manufacturing, Device Assembly and Supply Chain Management.
Design and Engineering. We offer design and engineering services that include product design engineering, design for manufacturability, analytical engineering, rapid prototyping and pilot production. We focus on providing design solutions to meet our customers' functional and cost needs by incorporating reliable manufacturing and assembly methods. Through our engineering design services, we engage our customers early in the product development to reduce their manufacturing costs and accelerate the development cycle.
Capability
| | Description & Customer Application
|
|---|
| Product Design Engineering | | Computer Aided Design (CAD) tool used to model design concepts which supports the design portion of the project, freeing the customer's staff for additional research |
| Design for Manufacturability | | Experience in manufacturing and process variation analysis ensures reliability and ongoing quality are designed in from the onset which eliminates customers' need for duplicate quality assurance measures, provides for continuous improvement and assures long-term cost control objectives are met |
| | | |
76
| Analytical Engineering | | Finite Element Analysis (FEA) and Failure Mode and Effect Analysis (FMEA) tools verify function and reliability of a device prior to producing clinical builds which shortens the design cycle allowing products reach the market faster and more cost effectively |
| Physical Models | | Computer Aided Manufacturing (CAM), Stereolithography and "Soft Tooling" concepts which permit rapid prototyping to provide customers with assurance that they have fulfilled the needs of their clinical customers and confirms a transition from design to production |
| Pilot Production | | Short run manufacturing in a controlled environment utilizing significant engineering support to optimize process prior to production transfer, which provides opportunity to validate manufacturing process before placement in a full scale manufacturing environment |
Precision Component Manufacturing. We utilize a broad array of manufacturing processes to produce metal and plastic based medical device components. These include metal forming, machining and molding and polymer molding, machining and extrusion processes.
Capability
| | Description & Customer Application
|
|---|
| Tube Drawing | | Process to manufacture miniature finished tubes or tubular parts used in stents, cardio catheters, endoscopy instruments & orthopaedic implants |
| Wire Drawing | | Specialized clad wires utilized in a variety of cardiology and neurological applications |
| Wire Grinding & Coiling | | Secondary processing of custom wires to create varying thicknesses, or shapes (springs) used in "guide-wires" and catheters for angioplasty and as components in neurological applications |
| Micro-Laser Cutting | | Process involves using a laser to remove material in tubular components resulting in tight tolerances and the ability to create the "net like" shapes used in both cardiology and peripheral stents |
| CNC Swiss Machining | | Machining process using a predetermined computer controlled path to remove metal or plastic material thereby producing a three dimensional shape. Used in orthopaedic implants such as highly specialized bone screws and miniature components used in cardiac rhythm management |
| High Speed CNC multi-axis Profile Machining | | Machining process using a predetermined computer controlled path to remove metal or plastic material thereby producing a three dimensional shape. Used to produce orthopaedic implants where precise mating surfaces are required |
| Electrical Discharge Machining (EDM) | | Machining process using thermal energy from an electrical discharge to create very accurate, thin delicate shapes and to manufacture complete components used in arthroscopy, laparoscopy and other surgical procedures |
| | | |
77
| Plastic Injection Molding | | Melted plastic flows into a mold which has been machined in the mirror image of the desired shape; process is used throughout the medical industry to create components of assemblies and commonly combined with metal components |
| Metal Injection Molding | | Metal powders bound by a polymer are injected into a mold to produce a metal part of the desired shape; used in higher volume metal applications to reduce manufacturing costs in orthopaedics, endoscopy, arthroscopy and other procedures |
| Plastic Extrusion | | Process that forces liquid polymer material between a shaped die and mandrel to produce a continuous length of plastic tubing; used in cardiology catheter applications |
| Alloy Development | | Product differentiation in the medical device industry is commonly driven by the use of alternative materials; we work with our customers to develop application-specific materials that offer marketable features and demonstrable benefits |
| Forging | | Process using heat and impact to "hammer" metal shapes and forms. Secondary processing needed to bring to finished form. Most often to fabricate surgical instruments within the medical industry |
Device Assembly. Device assembly is being driven by the medical device companies' focus on more products being released in shorter timeframes. To fulfill this growing need, we provide contract manufacturing services for complete/finished medical devices at our U.S., Mexico and Ireland facilities. We provide the full range of assembly capabilities defined by our customers' needs, including packaging, labeling, kitting and sterilization.
Capability
| | Description & Customer Application
|
|---|
| Mechanical Assembly | | Uses a variety of sophisticated attachment methods such as laser, plasma, ultrasonic welding, or adhesives to join components into complete medical device assemblies |
| Electro-Mechanical Assembly | | Uses a combination of electrical devices such as printed circuit boards, motors and graphical displays with mechanical sub-assemblies to produce a finished medical device |
| Marking/Labeling & Sterile Packaging | | Use of laser or ink jet marking, or pad printing methods for product identification, branding, and regulatory compliance; applying packaging methods such as form-fill-seal or pouch-fill-seal to package individual medical products for sterilization and distribution |
Supply Chain Management. Our supply chain management services encompass the complete order fulfillment process from raw material to finished devices for entire product lines. This category of capabilities is an umbrella for the capabilities listed above, not only including design and engineering, component manufacturing and device assembly but also raw materials sourcing, quality control/sterilization and warehousing and delivery, which are described below.
Capability
| | Description & Customer Application
|
|---|
| Raw Materials Sourcing | | Procurement and consulting on the choice of raw materials, allow design and materials suitability |
| | | |
78
| Quality Control/Sterilization | | The ability to design and validate quality control systems that meet or exceeds customer requirements. In addition, we provide validated sterilization services |
| Warehousing and Delivery | | The ability to provide customer storage and distribution services, including end user distribution |
Business Segments
Cardiology. Diseases involving disorders of the heart and blood vessels are one of the leading causes of death in developed countries. Interventional Cardiology, Electrophysiology and the Cardiac Rhythm Management (CRM) markets are the largest revenue generators in the cardiology segment. In response to these growing markets, medical device companies are developing innovative and often less invasive products such as cardiac resynchronization and drug eluting stents.
We have a long history of service to the cardiology market. We design, develop, manufacture and assemble implants and instruments for CRM, interventional cardiology, cardiac surgery and peripheral vascular markets. These precision products are typically produced from plastic materials, electronic components, specialized metals and precious metals. We provide a diverse range of products to this market, including stent tubing, guidewires & delivery systems, implantable pacemaker electrodes, marker bands, header assemblies for pacing and defibrillator units and machined heart valve assemblies.
Endoscopy. The medical device market is shifting away from open surgical procedures to minimally invasive procedures and technologies to reduce pain, trauma and recovery time. The continued adoption of such minimally invasive technologies is expected to continue driving growth in the overall medical device market.
In order to take advantage of these market trends, we design, develop, manufacture and assemble implants, instruments and equipment for use in minimally invasive procedures. Our experience encompasses the electrosurgical, laparoscopic, wound closure, opthalmic surgery, gynecology and arthroscopic markets. Endoscopy devices are produced from high quality materials suited for each individual device performance requirements and application, including precision metals and plastics processing technology. Our extensive design and development expertise, precision component manufacturing and advanced assembly competencies offer our customers comprehensive endoscopy device manufacturing services.
Future growth in the endoscopy segment is expected to be driven by high growth specialty markets within the greater endoscopy market. For instance, the gynecology market, which includes permanent female birth control devices, assisted reproductive implants, endometrial resections, and incontinence, is projected to grow at an annual rate of 14.8% from 2004 through 2009 according to the Millennium Research Group.
Orthopaedics. Growth in the orthopaedic market is being driven by demographics and technological advances. The aging population and an accelerating interest in an active lifestyle are leading to an increased incidence of orthopaedic injuries. Additionally, innovation, especially in less invasive surgical techniques, has reduced the recovery time and minimized patient discomfort improving post-operative outcomes.
Orthopaedic companies are demanding more from their supply chain and we meet those challenges with a comprehensive portfolio of services aimed at addressing the critical needs of the orthopaedic industry. We develop, manufacture and assemble implants and instruments for the reconstructive, spinal, trauma, and sports medicine segments. Our capabilities are enhanced by a new robotic forging facility as well as our proprietary CHEMTEX® surface treatment to facilitate bone in-growth. With multiple manufacturing sites and hundreds of CNC machines, we have the scale and resources to simplify our customers' supply chain.
79
The spine and knee market, as well as orthobiologics which includes bioabsorbables and biomaterials, are expected to offer very strong growth opportunities over the next several years. The 2005 Acquisitions focus primarily on these high-growth markets. The acquisitions provide (i) complementary product offerings that can be easily integrated into our existing product portfolio and (ii) new line extensions in product areas where we currently do not compete.
Sales and Marketing
We market and sell our products directly to our customers through our sales team of approximately 41 individuals. With respect to our sales force, 36 individuals are based in the United States, while five individuals are based in Europe.
Our sales force targets the top medical device customers in each of the target markets that we serve. Each of these top accounts is assigned dedicated Corporate Account Teams based upon the target markets in which they participate. The primary mission of our sales force is to increase our market share with these top customers by expanding our relationships and securing new business. Our end market focus allows us to better understand our customers' specific needs.
The engineering expertise of our sales force allows us to provide technical assistance and advice to our customers in the field. This assistance and advice strengthens our close working relationships between our sales personnel and our customers.
Customers
Our customers include the top worldwide medical device manufacturers that concentrate primarily in the cardiology, endoscopy and orthopaedics markets. We have built strong relationships with our customers by delivering highly customized and engineered components, assemblies and finished goods for their markets. Pro forma for the nine months ended September 30, 2005, approximately 92% of our net sales was derived from our medical device customers.
Our strategy is to focus on the top 15 medical device companies, which we believe represent a substantial portion of the overall market opportunity. For the nine months ended September 30, 2005, on a pro forma basis, these top 15 medical device companies accounted for approximately 70% of our net sales. In particular, Johnson & Johnson, Boston Scientific and Medtronic each accounted for more than 10% of our net sales for the nine months ended September 30, 2005 on a pro forma basis. We provide a multitude of products and services to our customers across their various divisions.
We also have established customer relationships with companies outside of the medical device market. Our industrial customers service the electronic, computer, industrial equipment and consumer markets. We provide them with high quality, complex components for use in high density discharge lamps, fiber optics, motion sensors and power generation.
International Operations
On a pro forma basis for the nine months ended September 30, 2005, approximately 15% of our sales were international sales. There are additional risks associated with our international sales than with domestic sales, including those resulting from currency fluctuations, duties and taxation, foreign legal and regulatory requirements, changing labor conditions and longer payment cycles.
Information Technology
We are in the process of installing the Oracle 11i enterprise resource planning, or ERP, system across our facilities. Building upon a core existing deployment of Oracle ERP at several of our sites, this project will upgrade and deploy a full implementation of ERP functionality at all of our facilities. We believe our ERP platform and related information technology systems will enable us to better serve our customers by aiding us in predicting customer demand, utilizing latest production planning methodologies, taking advantage of economies of scale in purchasing, providing greater flexibility to
80
move product from design to manufacturing at various sites and improving the accuracy of capturing and estimating our manufacturing and engineering costs. In addition, we utilize computer aided design, CAD, and computer aided manufacturing, or CAM, software at our facilities which allows us to improve our product quality and enhance the interactions between our engineers and our customers. We focus our systems to provide direct business benefits to us, our customers and our suppliers.
Quality
Due to the patient-critical and highly regulated nature of the products our customers provide, strong quality systems are an important factor in our customers' selection of a strategic manufacturing partner. In order for our customers to outsource manufacturing to us, our quality program must meet or exceed customer requirements.
Our Quality Management System is based on the standards developed by the International Organization for Standardization (ISO) and the FDA's Quality System Regulation. These standards specify the requirements necessary for a quality management system to consistently provide product that meets or exceeds customer requirements. Also included are requirements for processes to ensure continual improvement and continued effectiveness of the system. Compliance to ISO Standards is assessed by independent audits from an accredited third party (a Registrar) and through internal and customer audits of the quality system at each facility.
We have registered the majority of our facilities under a single Quality Management System which conforms to ISO 13485:2003, "Medical Devices—Quality management systems—Requirements for regulatory purposes", and there are plans in place to add facilities to this list.
Additionally we are deploying our "lean manufacturing" program throughout the company. The lean manufacturing program supports the quality system and drives continuous improvement by utilizing six sigma principles. The principles of the six sigma methodology (Define, Measure, Analyze, Improve and Control) allow the sources of variation in a process to be identified, systematically reduced and controlled to maintain the improvements.
Supply Arrangements
We have established relationships with many of our materials providers. However, most of the raw materials that are used in our products are subject to fluctuations in market price. In particular, the prices of stainless steel, titanium and platinum have historically fluctuated, and the prices that we pay for these materials, and, in some cases, their availability, are dependent upon general market conditions. In most cases we have pass-through pricing arrangements with our customers that purchase precious metal components.
When manufacturing and assembling medical devices, we may subcontract manufacturing services that we cannot perform in-house. As we provide our customers with a fully integrated supply chain solution, we will continue to rely on third-party suppliers, subcontractors and outside sources for components or services that we cannot provide through our internal resources.
To date, we have not experienced any material difficulty obtaining necessary raw materials or subcontractor services.
Intellectual Property
The products that we manufacture are made to order based on the customers' specifications and may be designed using our design and engineering services. Generally, our customers retain ownership of and the rights to their products' design while we retain the rights to any of our proprietary manufacturing processes.
We continue to develop intellectual property primarily in the areas of process engineering and materials development for the purpose of internal proprietary utilization. The intellectual property
81
developed helps enhance our production capabilities and improve margins in our manufacturing processes while providing a competitive differentiator. Examples of technologies developed include improvements in micro profile grinding, polymer micro tube manufacturing, metal injection molding, and surface enhancement methods for surgical implants.
We also continue to develop intellectual property for the purpose of licensing certain technologies to our medical device customers. Use of these technologies by our medical device customers in their finished design, component or material solution results in additional royalty revenues. Examples of licensed technologies include improvements to catheter based applications, gastrointestinal surgical devices, and vascular stents.
In addition, we are a party to several license agreements with third parties pursuant to which we have obtained, on varying terms, non-exclusive rights to practice inventions in patents held by third parties in connection with precision metal injection manufacturing technology.
Competition
The medical device manufacturing and engineering services industry has traditionally been highly fragmented with several thousand companies that have limited manufacturing capabilities and limited sales and marketing expertise. We believe that very few companies offer the scope of manufacturing capabilities and services that we provide to medical device companies, however, we may compete in the future against companies that assemble broad manufacturing capabilities and related services. We compete with different companies depending on the type of product or service offered or the geographic area served. We are not aware of a single competitor that operates in all of our target markets or offers the same range of products and services that we offer.
Our existing or potential competitors include suppliers with different subsets of our manufacturing capabilities, suppliers that concentrate on niche markets, and suppliers that have, are developing, or may in the future develop, broad manufacturing capabilities and related services. We compete for new business at all phases of the product lifecycle, which includes development of new products, the redesign of existing products and transfer of mature product lines to outsourced manufacturers. Competition is generally based on reputation, quality, delivery, responsiveness, breadth of capabilities, including design and engineering support, price, customer relationships, and increasingly the ability to provide complete supply chain management rather than individual components.
Lastly, many of our customers also have the capability to manufacture similar products in house, if they so choose.
Government Regulation
Our business is subject to governmental requirements, including those federal, state and local environmental laws and regulations governing the emission, discharge, use, storage and disposal of hazardous materials and the remediation of contamination associated with the release of these materials at or from our facilities or off-site disposal locations. Many of our manufacturing processes involve the use and subsequent regulated disposal of hazardous materials. We monitor our compliance with all federal and state environmental regulations, and have in the past paid civil penalties and taken corrective measures for violations of environmental laws. To date, such matters have not had a material adverse impact on our business or financial condition. We cannot assure you, however, that such matters will not have a material impact on us in the future.
In several instances, we and certain MedSource subsidiaries have entered into settlements arising from alleged liability as potentially responsible parties for the off-site disposal or treatment of hazardous substances. None of those settlements have had a material adverse impact on our business or financial condition.
82
Environmental laws have been interpreted to impose strict, joint and several liability on owners and operators of contaminated facilities and parties that arrange for the off-site disposal or treatment of hazardous materials. Pursuant to such laws in 2001, the United States Environmental Protection Agency, or EPA, approved a Final Design Submission submitted by UTI, our wholly owned subsidiary, to the EPA in respect of a July 1988 Administrative Consent Order issued by the EPA. The Administrative Consent Order alleged that hazardous substances had been released into the environment from UTI's Collegeville, Pennsylvania plant and required UTI to study and, if necessary, remediate the groundwater and soil beneath and around the plant. Since that time, UTI has implemented and is operating successfully a contamination treatment system approved by the EPA. MedSource's subsidiaries also operate or formerly operated facilities located on properties where environmental contamination may have occurred or be present.
At September 30, 2005, we recorded a long-term liability of $4.7 million related to the potential MedSource remediation and the Collegeville remediation. We have prepared estimates of our potential liability for these properties, if any, based on available information. Changes in EPA standards, improvement in cleanup technology and discovery of additional information, however, could affect the estimated costs associated with these matters in the future.
We are a medical device and component manufacturing and engineering services provider. Some of the products that we manufacture may be considered by the FDA to be finished medical devices. The manufacturing processes used in the production of these finished medical devices are subject to FDA regulatory-inspection, and must comply with FDA regulations, including its Quality System Regulation, or QSR. The QSR requires manufacturers of finished medical devices to follow elaborate design, testing, control, documentation and other quality assurance procedures during the finished device manufacturing process. The QSR governs manufacturing activities broadly defined to include activities such as product design, manufacture, testing, packaging, labeling, distribution and installation. Some of our customers may also require by contractual agreement that we comply with the QSR when manufacturing their device components. Our FDA registered facilities are subject to FDA inspection at any time for compliance with the QSR and other FDA regulatory requirements. Failure to comply with these regulatory requirements may result in civil and criminal enforcement actions, including financial penalties, seizures, injunctions and other measures. In some cases, failure to comply with the QSR could prevent or delay our customers from gaining approval to market their products. Our products must also comply with state and foreign regulatory requirements.
In addition, the FDA and state and foreign governmental agencies regulate many of our customers' products as medical devices. FDA approval/clearance is required for those products prior to commercialization in the U.S., and approval of regulatory authorities in other countries may also be required prior to commercialization in those jurisdictions. Moreover, in the event that we build or acquire additional facilities outside the U.S., we will be subject to the medical device manufacturing regulations of those countries. Our Mexico facility must comply with U.S. FDA regulations, which we believe are more stringent than the local regulatory requirements our facility must also comply with. Some other countries may rely upon compliance with U.S. regulations or upon ISO certification as sufficient to satisfy certain of their own regulatory requirements for a product or the manufacturing process for a product.
In order to comply with regulatory requirements, our customers may wish to audit our operations to evaluate our quality systems. Accordingly, we routinely permit audits by our customers.
83
Facilities
We have 21 leased facilities and 7 owned facilities. Our principal executive office is located at 100 Fordham Road, Wilmington, Massachusetts 01887. We believe that our current facilities are adequate for our operations. Certain information about our facilities is set forth below:
Location
| | Approximate Square
Footage
| | Own/Lease
|
|---|
| Arlington, Tennessee | | 29,000 | | Own |
| Arvada, Colorado | | 45,000 | | Lease |
| Brimfield, Massachusetts | | 30,000 | | Own |
| Brooklyn Park, Minnesota | | 95,000 | | Lease |
| Collegeville, Pennsylvania | | 179,000 | | Own |
| Corry, Pennsylvania | | 67,000 | | Lease |
| El Paso, Texas | | 20,000 | | Lease |
| Englewood, Colorado | | 27,000 | | Lease |
| Hamburg, New York | | 18,000 | | Lease |
| Huntsville, Alabama | | 44,000 | | Own |
| Laconia, New Hampshire | | 41,000 | | Lease |
| Minneapolis, Minnesota(1) | | 7,000 | | Lease |
| Orchard Park, New York | | 41,000 | | Lease |
| Pittsburgh, Pennsylvania | | 68,000 | | Own |
| Salem, Virginia | | 64,000 | | Lease |
| South Plainfield, New Jersey | | 6,000 | | Lease |
| Sturbridge, Massachusetts | | 18,000 | | Lease |
| Trenton, Georgia | | 13,000 | | Lease |
| Trenton, Georgia | | 31,000 | | Own |
| Upland, California | | 50,000 | | Lease |
| Watertown, Connecticut | | 44,000 | | Lease |
| Wheeling, Illinois | | 48,000 | | Own |
| Wheeling, Illinois | | 51,000 | | Lease |
| Wilmington, Massachusetts | | 22,000 | | Lease |
| Aura, Germany | | 63,000 | | Lease |
| Galway, Ireland | | 17,000 | | Lease |
| Juarez, Mexico | | 101,000 | | Lease |
| Manchester, England | | 10,000 | | Lease |
| | |
| | |
| Total | | 1,249,000 | | |
| | |
| | |
- (1)
- We have subleased this facility for the remainder of its lease term.
Employees
As of September 30, 2005, we had 3,777 employees. We also employ a number of temporary employees to assist with various projects. Other than some employees at our facility in Aura, Germany, our employees are not represented by any union. We have never experienced a work stoppage or strike and believe that we have good relationships with our employees.
Legal Proceedings
From time to time, we are involved in legal proceedings in the ordinary course of our business. We are not currently involved in any pending legal proceedings that we believe could have a material adverse effect on our financial position or results of operations. Please see "Government Regulation" above for a description of certain environmental remediation matters which are incorporated by reference herein.
84
MANAGEMENT
Our executive officers and the directors, and their respective ages as of September 30, 2005, are as follows:
Name(1)
| | Age
| | Position
|
|---|
| Ron Sparks | | 50 | | President, Chief Executive Officer and Director |
| Stewart A. Fisher | | 44 | | Chief Financial Officer, Executive Vice President, Treasurer, Secretary and Director |
| Daniel C. Croteau | | 40 | | Executive Vice President, General Manager, Orthopaedic Division |
| Gary D. Curtis | | 49 | | Executive Vice President, Sales and Marketing |
| Daniel B. DeSantis | | 49 | | Executive Vice President of Human Resources |
| Jeffrey M. Farina | | 48 | | Executive Vice President of Technology and Chief Technology Officer |
| John Konsin | | 47 | | Executive Vice President, General Manager, Endoscopy Division |
| Michael W. Michelson | | 54 | | Director |
| Kenneth W. Freeman | | 55 | | Director |
| James C. Momtazee | | 33 | | Director |
| Steven Barnes | | 45 | | Director |
- (1)
- KKR has the right to designate an additional member to our Board in connection with the Transactions.
Ron Sparks has served as our President, Chief Executive Officer and Director since September, 2003. Prior to joining us, Mr. Sparks most recently served from 1998 to 2003 as President of Smith & Nephew, Inc., Endoscopy Division, a subsidiary of Smith & Nephew plc, which is in the principal business of design, manufacture and sales of endoscopy medical devices to healthcare professionals. Prior to that appointment, he served from 1995 to 1998 as President of the Wound Management Division, which is in the principal business of design, manufacture and sales of advanced wound care products to healthcare professionals. In addition, he was a member of the Group Executive Committee of Smith & Nephew plc, having been appointed in 1999. Mr. Sparks is a graduate of the University of Massachusetts and INSEAD in Fountainebleau, France.
Stewart A. Fisher has served as our Chief Financial Officer, Executive Vice President, Treasurer and Secretary since October, 2001. Prior to joining us, Mr. Fisher was Chief Financial Officer and Vice President of GenTek, Inc., a global manufacturer of telecommunications equipment and other industrial products from April 2000 to September 2001. Mr. Fisher was Chief Financial Officer and Vice President of The General Chemical Group, a predecessor company to GenTek, Inc., from April 1999 to March 2000. GenTek, Inc. filed for bankruptcy under Chapter 11 of the United States Bankruptcy Code on October 11, 2002, more than one year after Mr. Fisher resigned from GenTek, Inc. to join us. Earlier in his career, Mr. Fisher was a Manager of Corporate Finance and Capital Markets in the Treasurer's Office of General Motors Corporation. Mr. Fisher holds a B.S. degree in accounting, summa cum laude, from Lehigh University and an M.B.A. with distinction from the Wharton School of the University of Pennsylvania.
Daniel C. Croteau has served as our Executive Vice President, General Manager, Orthopaedic Division since July 2004. Prior to the MedSource acquisition in June 2004, Mr. Croteau served in
85
various executive positions with MedSource including the Senior Vice President of Corporate Development from July 2002 to June 2004, as the Vice President of Corporate Development from December 2001 to July 2002, as the Vice President of Business Development from January 2000 to December 2001 and as the Director of Business Development from August 1999 to January 2000. Prior to joining MedSource, Mr. Croteau served at Booz Allen & Hamilton, a strategy consulting firm in Sydney, Australia from 1997 to 1999. Previously, Mr. Croteau worked with General Electric Corporation and two start-up companies in various technical marketing, sales, business development and general management roles. Mr. Croteau has a B.S. in Mechanical Engineering from the University of Vermont and an M.B.A. from Harvard Business School.
Gary D. Curtis has served as our Executive Vice President, Sales and Marketing since April 2003. Prior to joining us, Mr. Curtis was unaffiliated with any business entity from November 2002 until April 2003 and was employed by US Surgical/Tyco Healthcare, a medical device manufacturer, from January 1999 to November 2002. While at US Surgical/Tyco Healthcare, Mr. Curtis served as the Vice President—Distribution Sales from December 2000 to November 2002, and as the Vice President—Sales Operations from January 1999 to December 2000. Mr. Curtis has a B.S. in Marketing from Millikin University.
Daniel B. DeSantis has served as our Executive Vice President of Human Resources since May 2005. Prior to joining us, Mr. DeSantis served as the Global Director of Human Resources for the Business Services group at American Standard Companies, Inc. from January 2003 to May 2005. Mr. DeSantis was unaffiliated with any business entity from May 2002 until January 2003. Mr. DeSantis served as the Vice President of Human Resource Operations of IMS Health from February 1998 to May 2002. Mr. DeSantis holds an M.S. in human resources from Rutgers University, an M.B.A. from Fairleigh Dickinson University, as well as a B.S. in chemical engineering from Rensselaer Polytechnic Institute.
Jeffrey M. Farina has served as our Executive Vice President of Technology and Chief Technology Officer since June 2004, and from June 2000 to June 2004 our Vice President of Engineering. Mr. Farina joined UTI in 1989, serving as a Project Manager and Engineering Manager in its Uniform Tubes division and then as its Vice President of Engineering. Mr. Farina has B.S. and M.S. degrees in mechanical engineering from Drexel University.
John Konsin joined us as Executive Vice President, General Manager, Endoscopy Division in October 2005. Prior to joining us, Mr. Konsin served as the Vice President & General Manager—Gynecology & Vascular for the Endoscopy Division of Smith & Nephew, Inc. from November 2004 to October 2005. Mr. Konsin served as the Vice President—Marketing for the Endoscopy Division of Smith & Nephew, Inc. from April 1999 to November 2004. Prior to joining Smith & Nephew, Inc. Mr. Konsin worked in senior management positions with Chiron Corporation and Advanced Technology Laboratories. Mr. Konsin has a B.S. in Business Administration from Duquesne University and an M.B.A. from the University of Colorado.
Michael W. Michelson has been a member of the limited liability company which serves as the general partner of KKR since 1996. Prior thereto, he was a general partner of KKR. Mr. Michelson is also a director of Alliance Imaging and Jazz Pharmaceuticals, Inc.
Kenneth W. Freeman has been a managing director of KKR since May 2005. Prior to joining KKR, Mr. Freeman served as Chairman and CEO of Quest Diagnostics Incorporated and its predecessor company from 1995 through 2004. Mr. Freeman retired from Quest Diagnostics in December 2004. Previously he served in financial and general management roles at Corning Incorporated for more than twenty years. Mr. Freeman is also a director of Alliance Imaging and President and CEO of Masonite International Corporation.
86
James C. Momtazee has been an executive of KKR beginning in 1996. From 1994 to 1996, Mr. Momtazee was with Donaldson, Lufkin & Jenrette in its investment banking department. Mr. Momtazee is also a director of Accuride Corporation, Alliance Imaging and Jazz Pharmaceuticals, Inc.
Steven Barnes has been associated with Bain Capital since 1988 and has been a Managing Director since 2000. In addition to working for Bain Capital, he also held senior operating roles of several Bain Capital portfolio companies including Chief Executive Officer of Dade Behring, Inc., President of Executone Business Systems, Inc., and President of Holson Burnes Group, Inc. Mr. Barnes presently serves on several boards including Sigma Kalon, Sealy and Unisource.
Section 16(a) Beneficial Ownership Reporting Compliance
None of our directors, executive officers or any beneficial owner of more than 10% of our equity securities is required to file reports pursuant to Section 16(a) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), with respect to their relationship with us because we do not have any equity securities registered pursuant to Section 12 of the Exchange Act.
Code of Business Conduct and Ethics
Our board adopted a code of business conduct and ethics applicable to directors, officers and employees to establish standards and procedures related to the compliance with laws, rules and regulations, treatment of confidential information, conflicts of interest, competition and fair dealing and reporting of violations of the code; and includes a requirement that we make prompt disclosure of any waiver of the code for executive officers or directors made by our board. A copy of the code of business conduct and ethics is available in print without charge to any person who sends a request to the office of the Secretary of the Company at 100 Fordham Road, Wilmington, Massachusetts 01887.
Executive Compensation
The following table sets forth information concerning all compensation awarded to, earned by or paid to the individuals who served as our chief executive officer during the fiscal year ended December 31, 2005 and our other four most highly compensated executive officers who were serving as such at the end of our most recently completed fiscal year. We refer to these individuals as our named executive officers. All compensation reported for our named executive officers, other than options to purchase securities of us, is the compensation they received in their capacities as executive officers of Accellent Corp. and Accellent. Our named executive officers did not receive any additional compensation from us or our subsidiaries during the years shown.
87
Summary Compensation Table
| |
| |
| |
| |
| | Long Term Compensation Awards
| |
|
|---|
| |
| | Annual Compensation
| |
|
|---|
| |
| |
| | Securities
Underlying
Options
(#)
| |
|
|---|
Name and Principal Position
| | Year
| | Salary
($)
| | Bonus
($)(6)
| | Other Annual
Compensation
($)
| | Restricted
Stock Awards
($)(7)
| | All Other
Compensation
($)
|
|---|
| Ron Sparks(1) | | 2005 | | 408,875 | | — | | — | | 2,589,620 | | 3,093,333 | | 6,680,000 |
| | President and Chief Executive | | 2004 | | 344,773 | | 405,093 | | — | | — | | — | | 1,773 |
| | Officer | | 2003 | | 96,023 | | 85,333 | | — | | — | | 540,000 | | — |
Stewart A. Fisher(2) |
|
2005 |
|
333,333 |
|
— |
|
— |
|
2,491,280 |
|
1,950,000 |
|
5,024,000 |
| | Chief Financial Officer, | | 2004 | | 314,090 | | 298,018 | | — | | — | | — | | 6,150 |
| | Executive Vice President, | | 2003 | | 301,500 | | 284,150 | | — | | — | | 337,500 | | 6,000 |
| | Treasurer and Secretary | | | | | | | | | | | | | | |
Daniel C. Croteau(3) |
|
2005 |
|
263,333 |
|
— |
|
— |
|
360,170 |
|
165,000 |
|
849,000 |
| | Executive Vice President, | | 2004 | | 126,760 | | 224,500 | | — | | — | | 80,000 | | 5,998 |
| | General Manager, Orthopaedic Division | | 2003 | | — | | — | | — | | — | | — | | — |
Gary D. Curtis(4) |
|
2005 |
|
222,500 |
|
— |
|
— |
|
360,170 |
|
201,046 |
|
848,350 |
| | Executive Vice President, | | 2004 | | 204,697 | | 167,574 | | 130,310 | | — | | 55,000 | | 5,858 |
| | Sales & Marketing | | 2003 | | 139,280 | | 71,270 | | 127,973 | | — | | 25,000 | | — |
Jeffrey M. Farina(5) |
|
2005 |
|
193,333 |
|
— |
|
— |
|
395,819 |
|
197,502 |
|
849,000 |
| | Executive Vice President | | 2004 | | 185,000 | | 148,092 | | — | | — | | 5,000 | | 9,172 |
| | of Technology and Chief Technology Officer | | 2003 | | 157,841 | | 79,221 | | — | | — | | — | | 4,135 |
- (1)
- Mr. Sparks became our President and Chief Executive Officer in September 2003. The amount reported under the bonus column for 2004 includes $20,000 for 200,000 fully vested shares of our Class B-2 Convertible Preferred Stock awarded to Mr. Sparks in May of 2004. The amount reported as all other compensation for 2005 represents a management bonus earned in connection with the change of control that occurred upon the consummation of the Merger. The amount reported as all other compensation for 2004 reflect employer contributions to a 401(k) plan.
- (2)
- Mr. Fisher serves as our Chief Financial Officer, Executive Vice President, Treasurer and Secretary. The amount reported as all other compensation for 2005 represents a management bonus of $5,010,000 earned in connection with the change of control that occurred upon the consummation of the Merger, and $14,000 for employer contributions to a 401(k) plan. The amounts reported as all other compensation in 2004 and 2003 reflect employer contributions to a 401(k) plan.
- (3)
- Mr. Croteau became our Executive Vice President & General Manager of our Orthopaedic Division in September 2004. The amount reported under the bonus column for 2004 includes a $75,000 signing bonus relating to Mr. Croteau's entering into an employment agreement with us. The amount reported as all other compensation for 2005 represents a management bonus of $835,000 earned in connection with the change of control that occurred upon the consummation of the Merger, and $14,000 for employer contributions to a 401(k) plan. The amounts reported in 2004 as all other compensation reflect employer contributions to a 401(k) plan.
- (4)
- Mr. Curtis became our Executive Vice President of Sales & Marketing in April 2003. The amount reported as other annual compensation for 2004 includes $69,386 for relocation reimbursements, $45,091 of reimbursements for taxes incurred as a result of the relocation and a $15,833 relocation bonus. The amount reported as other annual compensation for 2003 includes $92,888 of relocation reimbursements and $35,085 of reimbursements for taxes incurred as a result of the relocation reimbursements. The amount reported as all other compensation for 2005 represents a management bonus of $835,000 earned in connection with the change of control that occurred upon the consummation of the Merger, and $13,350 for employer contributions to a 401(k) plan. The amounts reported as all other compensation in 2004 reflect employer contributions to a 401(k) plan.
- (5)
- Mr. Farina serves as our Executive Vice President of Technology and Chief Technology Officer. The amounts reported as all other compensation reflect a management bonus of $835,000 in 2005 earned in connection with the change of control that occurred upon the consummation of the Merger, employer contributions to a
88
401(k) plan of $14,000, $5,550 and $4,735 in 2005, 2004 and 2003, respectively, and employer contributions to a deferred profit sharing plan of $9,172 and $4,135 in 2004 and 2003, respectively.
- (6)
- The amount of bonuses earned by each named executive officer for 2005 does not include annual bonuses which will be determined and paid during March 2006.
- (7)
- All shares of restricted stock granted during 2005 were valued at $16.39 per share as of the grant date, and were sold at a price of $23.32 in connection with the change of control that occurred upon consummation of the Merger.
89
Option Grants in Last Fiscal Year
The following table sets forth information related to each grant of stock options under our and Accellent Holdings Corp. option plans to our named executive officers for the fiscal year ended December 31, 2005:
| |
| | Individual Grants
| |
| |
| |
|
|---|
| |
| |
| | Potential Realizable Value At Assumed Annual Rates of Stock Price Appreciation for Option Term
|
|---|
| | Number of
Securities
Underlying
Options
Granted
| | Percent of
Total Options
Granted to
Employees In
Fiscal Year
| |
| |
| |
|
|---|
Name
| | Exercise
Price per
Share
| | Market
Price on
Grant Date
| | Expiration
Date
|
|---|
| | 5%
| | 10%
|
|---|
| Ron Sparks | | 3,093,333 | (1) | 38 | % | $ | 5.00 | | $ | 5.00 | | 11/22/15 | | $ | 9,726,903 | | $ | 24,649,881 |
| Stewart A. Fisher | | 1,950,000 | (1) | 24 | % | $ | 5.00 | | $ | 5.00 | | 11/22/15 | | $ | 6,131,723 | | $ | 15,538,989 |
| Daniel C. Croteau | | 25,000 | (2) | 1 | % | $ | 12.29 | | $ | 23.32 | | 9/30/15 | | $ | 642,396 | | $ | 1,204,902 |
| | | 140,000 | (1) | 2 | % | $ | 5.00 | | $ | 5.00 | | 11/22/15 | | $ | 440,226 | | $ | 1,115,620 |
| Gary D. Curtis | | 201,046 | (1) | 3 | % | $ | 5.00 | | $ | 5.00 | | 11/22/15 | | $ | 632,184 | | $ | 1,602,078 |
| Jeffrey M. Farina | | 32,700 | (2) | 1 | % | $ | 8.18 | | $ | 16.39 | | 7/15/15 | | $ | 605,525 | | $ | 1,122,638 |
| | | 164,802 | (1) | 2 | % | $ | 5.00 | | $ | 5.00 | | 11/22/15 | | $ | 518,215 | | $ | 1,313,260 |
- (1)
- Options granted under the Accellent Holdings Corp. option plan.
- (2)
- Options granted under our stock option plan. Upon the consummation of the Merger, all options under our plans were either purchased or exchanged for options in Accellent Holdings Corp.
Aggregate Option Exercises in Last Fiscal Year and Fiscal Year End Option Values
The following table provides summary information for each of our named executive officers with respect to Accellent Holdings Corp. stock options held as of December 31, 2005. The value of unexercised in-the-money options shown below have been calculated on the basis of $5.00 per share, less the applicable exercise price per share, multiplied by the number of shares underlying these options.
| |
| |
| | Number of Securities Underlying Unexercised Options as of December 31, 2005
| | Value of Unexercised In-The-Money Options as of December 31, 2005
|
|---|
Name
| | Shares Acquired on Exercise(1)
| | Value
Realized(1)
|
|---|
| | Unexercisable
| | Exercisable(2)
| | Unexercisable
| | Exercisable(2)
|
|---|
| Ron Sparks | | 156,811 | | $ | 2,373,509 | | 3,093,333 | | 1,546,667 | | $ | — | | $ | 5,800,000 |
| Stewart A. Fisher | | 175,198 | | $ | 2,435,820 | | 1,950,000 | | 1,200,000 | | $ | — | | $ | 4,500,000 |
| Daniel C. Croteau | | 65,360 | | $ | 886,543 | | 140,000 | | 160,000 | | $ | — | | $ | 600,000 |
| Gary D. Curtis | | 23,075 | | $ | 349,266 | | 201,046 | | 229,767 | | $ | — | | $ | 861,626 |
| Jeffrey M. Farina | | 107,546 | | $ | 1,791,771 | | 164,802 | | 188,346 | | $ | — | | $ | 706,298 |
- (1)
- The shares acquired on exercise and value realized represents amounts earned by our named executive officers from the sale of our stock options in connection with the change of control upon consummation of the Merger.
- (2)
- Exercisable shares represent options received by our named executive officers as a result of their exchange of fully vested Accellent Inc. options into fully vested options of Accellent Holdings Corp. in connection with the change of control upon consummation of the Merger.
90
Pension Plan Table
We maintain a Supplemental Executive Retirement Pension Program (SERP) for certain of our senior executives, including some of our named executive officers. Benefits under the SERP are shown in the tables below. All participants fall into the 30-year accrual category, which provides them with the following benefits based on years of service:
PENSION PLAN TABLE—30-YEAR ACCRUAL
| | Years of Service
|
|---|
Remuneration
|
|---|
| | 15
| | 20
| | 25
| | 30
| | 35
|
|---|
| $ | 150,000 | | $ | 37,500 | | $ | 50,000 | | $ | 62,500 | | $ | 75,000 | | $ | 75,000 |
| | 175,000 | | | 43,750 | | | 58,333 | | | 72,917 | | | 87,500 | | | 87,500 |
| | 200,000 | | | 50,000 | | | 66,667 | | | 83,333 | | | 100,000 | | | 100,000 |
| | 225,000 | | | 56,250 | | | 75,000 | | | 93,750 | | | 112,500 | | | 112,500 |
| | 250,000 | | | 62,500 | | | 83,333 | | | 104,167 | | | 125,000 | | | 125,000 |
| | 300,000 | | | 75,000 | | | 100,000 | | | 125,000 | | | 150,000 | | | 150,000 |
| | 400,000 | | | 100,000 | | | 133,333 | | | 166,667 | | | 200,000 | | | 200,000 |
| | 450,000 | | | 112,500 | | | 150,000 | | | 187,500 | | | 225,000 | | | 225,000 |
| | 500,000 | | | 125,000 | | | 166,667 | | | 208,333 | | | 250,000 | | | 250,000 |
Compensation covered by the SERP is calculated by determining the average of a participant's highest five consecutive years of compensation. Generally, compensation is determined on the basis of the total taxable compensation of a participant. The table below identifies the five year average annual compensation covered by the SERP as of the year ended December 31, 2005 for each of our named executive officers covered by the SERP, and the respective years of service for benefit accrual purposes.
Executive
| | Average
Covered
Compensation
| | Years of
Service
|
|---|
| Ron Sparks | | $ | 586,879 | | 2 |
| Stewart A. Fisher | | | 522,549 | | 4 |
| Daniel C. Croteau | | | — | | — |
| Gary D. Curtis | | | 384,550 | | 3 |
| Jeffrey M. Farina | | | 254,757 | | 17 |
Director Compensation
Prior to the Transactions, we reimbursed our directors for reasonable out-of-pocket expenses related to attending board of directors meetings. Following the Transactions, we intend to pay all of our non-management directors an annual retainer of $30,000 in cash for their service as members of the board of directors and to reimburse all directors for any out-of-pocket expenses incurred by them in connection with services provided in such capacity.
Accellent Holdings Corp. has established a Directors' Deferred Compensation Plan (the "Plan") for all non-employee directors. The Plan allows each non-employee director to elect to defer receipt of all or a portion of his or her annual directors' fees to a future date or dates. Any amounts deferred under the Plan are credited to a phantom stock account. The number of phantom shares of common stock of Accellent Holdings Corp. credited to the director's phantom stock account will be determined based on the amount of the compensation deferred during any given year, divided by the then "fair market value" (as such term if defined in the Plan) per share of Accellent Holdings Corp.'s common stock. If there has been no public offering of Accellent Holdings Corp.'s common stock, the "fair market value" of the common stock will be determined in the good faith discretion of the Accellent Holdings Corp. Board of Directors. Upon a separation from service on the Accellent Holdings Corp. Board of Directors or the occurrence of a "change of control" of Accellent Holdings Corp. (as such
91
term is defined in the Plan), each director will receive (or commence receiving, depending upon whether the director has elected to receive distributions from his or her phantom stock account in a lump sum or in installments over time) a distribution of his or her phantom stock account, in either cash or stock of Accellent Holdings Corp. (subject to the prior election of each such director). The Plan may be amended or terminated at any time by the Board of Directors, and in form and operation is intended to be compliant with Section 409A of the Internal Revenue Code.
Employment Contracts and Termination of Employment and Change-In-Control Arrangements
We have entered into employment agreements with certain named executive officers, which provide for their employment as executive officers of us and our subsidiaries. We have also entered into separation agreements with former executive officers. The terms of these employment agreements and separation agreements are set forth below. Accellent Corp. has an employment agreement with Jeffrey M. Farina.
On September 15, 2003, we entered into an employment agreement with Ron Sparks to serve as our President and Chief Executive Officer. In connection with the Transactions, the agreement was assigned to Accellent Holdings Corp. The term of the agreement is three years. Under the agreement, Mr. Sparks is entitled to an annual salary of $325,000, subject to subsequent annual adjustment. In addition, we granted Mr. Sparks an option to purchase 540,000 shares of our common stock, which option vests over a five-year term and has an exercise price of $8.18 per share. In the event of a change of control, or upon the closing of an initial public offering of our securities, all of such options shall become immediately exercisable. Upon the closing of an initial public offering of our securities, we shall grant Mr. Sparks an additional option to purchase an amount of our common stock equal to one percent of the number of shares of our common stock outstanding as of the closing of such initial public offering at an exercise price equal to the initial public offering price. Mr. Sparks is also eligible to receive a cash bonus each year based on the achievement of certain performance objectives. Mr. Sparks also has the right to participate in our car plan, pursuant to the terms of such plan. If Mr. Sparks is terminated without cause or decides to leave his employment for good reason, then, in consideration for the execution by Mr. Sparks of a release, we shall pay to Mr. Sparks a severance payment equal to eighteen times his monthly base salary then in effect and we shall continue to provide health insurance benefits and life insurance for Mr. Sparks for eighteen months from the date of termination of employment. Under those circumstances, he is also entitled to receive, pro-rated to the date of termination, any bonus he would have received for that year. If Mr. Sparks is terminated for cause or decides to leave his employment without good reason, his rights to base salary, benefits and bonuses immediately terminate. In May, 2004, we issued Mr. Sparks, in a one-time grant, 200,000 shares of Class B-2 Convertible Preferred Stock in consideration of Mr. Sparks' performance since his appointment as our President and Chief Executive Officer.
On April 1, 2004, we entered into an employment agreement with Gary Curtis. Under the agreement, Mr. Curtis was offered an annual salary of $210,000 and is eligible to receive a bonus each year based primarily on our financial performance. In addition, we granted Mr. Curtis an option to purchase 50,000 shares of our common stock, which option vests over a five-year term and has an exercise price of $8.18 per share. Mr. Curtis also has the right to participate in our car plan, pursuant to the terms of such plan. In the event Mr. Curtis is terminated without cause, then, in consideration for the execution by Mr. Curtis of a release, we shall pay to Mr. Curtis a severance payment equal to twelve times his monthly base salary then in effect and we shall continue to provide health insurance benefits for twelve months following his termination. On July 19, 2004, we entered into an employment letter with Mr. Curtis to confirm his integration team role. Under the employment letter, Mr. Curtis is eligible to receive an integration team bonus with an award potential of $25,000 for each six-month performance period. In addition, we granted Mr. Curtis an option to purchase 5,000 shares of our common stock, which option vests over a five-year term and has an exercise price of $8.18 per share.
92
Mr. Curtis is also subject to our trade secrets and non-disclosure, non-solicitation, non-competition and invention assignment agreement under which he agrees not to compete with us for a period of one-year after termination of his employment with us.
In September, 2001, we entered into an employment agreement with Stewart Fisher to serve as our Chief Financial Officer. In connection with the Transactions, the agreement was assigned to Accellent Holdings Corp. The term of the agreement is five years. Under the agreement, Mr. Fisher is entitled to an annual salary of $300,000, subject to subsequent annual adjustment. In addition, we granted Mr. Fisher an option to purchase 135,000 shares of our common stock, which option vests over a five-year term and has an exercise price of $9.78 per share. Mr. Fisher is also eligible to receive a cash bonus each year based on the achievement of certain performance objectives. The employment agreement provides Mr. Fisher with reimbursement of reasonable and necessary relocation expenses and a bonus of $150,000 to reimburse him for benefits forfeited from his previous employer. If Mr. Fisher is terminated without cause or decides to leave his employment for good reason, then, in consideration for the execution by Mr. Fisher of a release, we shall continue to pay Mr. Fisher his base salary then in effect and we shall continue to provide health, dental and vision insurance through the earlier of the date Mr. Fisher obtains other full-time employment or eighteen months from the date of termination of employment. Under those circumstances, he is also entitled to receive, pro-rated to the date of termination, any bonus he would have received for that year. If Mr. Fisher is terminated for cause or decides to leave his employment without good reason, his rights to base salary, benefits and bonuses shall immediately terminate. In the event of a change of control, all options granted to Mr. Fisher shall become immediately exercisable. Mr. Fisher is also subject to a noncompete agreement under which he agrees not to compete with us for a period ending on the later of October 1, 2006 or one-year after the termination of Mr. Fisher's employment with us.
On July 1, 2004, we entered into an employment agreement with Daniel C. Croteau to serve as its Executive Vice President, General Manager, Orthopaedic Division. The term of the agreement is three years. Under the agreement, Mr. Croteau is entitled to an annual salary of $240,000, subject to subsequent annual adjustment. In addition, we granted Mr. Croteau an option to purchase 75,000 shares of our common stock, at an exercise price of $8.18 per share. Mr. Croteau shall also receive an option to purchase an additional 25,000 shares of our common stock, effective as of July 1, 2005, at an exercise price equal to the then current fair market value. The options shall vest over a five-year term. Mr. Croteau is also eligible to receive a cash bonus each year based on the achievement of certain individual and company performance objectives. For the 2004 performance year, Mr. Croteau was entitled to a minimum bonus of $125,000. For the 2005 performance year, Mr. Croteau is entitled to a minimum bonus of $75,000. The employment agreement provides Mr. Croteau with reimbursement of reasonable and necessary relocation expenses and a payment of $75,000 to reimburse him for benefits forfeited from his previous employment contract with MedSource prior to our acquisition of MedSource. Mr. Croteau has the right to participate in our car plan, pursuant to the terms of such plan. If Mr. Croteau is terminated without cause, then, in consideration for the execution by Mr. Croteau of a release, we shall continue to pay Mr. Croteau his base salary then in effect and we shall continue to provide medical benefits for a period of twelve months from the date of termination of employment. If Mr. Croteau is terminated for cause or decides to leave his employment without good reason, his rights to base salary, benefits and bonuses shall immediately terminate. In the event of a change of control, all options granted to Mr. Croteau shall become immediately exercisable. Mr. Croteau is also subject to our non-disclosure, non-solicitation, non-competition and invention assignment agreement.
Employee Benefit Plans
Generally, our employees, including certain of our directors and named executive officers, participate in our various employee benefit plans, including a stock option and incentive plan which
93
provides for grants of incentive stock options, nonqualified stock options, restricted stock and restricted stock units. We maintain pension plans which provide benefits at a fixed rate for each month of service, 401(k) plans, and profit sharing plans which are available to employees at several of our locations. As described above under "Executive Compensation." We have a Supplemental Executive Retirement Pension Program (SERP), a non-qualified, unfunded deferred compensation plan that covers certain executives. In addition, we and certain of our subsidiaries maintain phantom stock plans, which provide grants of phantom stock to our eligible employees as part of retention plans. Holders of phantom stock under these plans have no voting rights, no stockholder rights and no employment rights. They are, however, entitled to receive dividends on phantom stock and redemption of the phantom shares within ten years of issuance or upon the death of the holder. Six of our subsidiaries maintain defined contribution plans for the benefit of their eligible employees pursuant to which employees who participate in the plans may make elective deferrals of a portion of their salary and we may make discretionary profit sharing or employer matching contributions to the plans. Two of our subsidiaries maintain profit sharing plans for the benefit of their eligible employees pursuant to which we may make discretionary profit sharing contributions to the plans.
Management Bonus Plan
On October 7, 2005, our board of directors adopted, and our shareholders approved the Accellent Inc. Management Bonus Plan (the "Bonus Plan"). The Bonus Plan provided that certain of our employees receive cash bonuses in connection with the change in control that occurred upon the consummation of the Merger. The aggregate bonus payments under the Bonus Plan totaled $16,700,000 and were paid upon the consummation of the Merger.
Equity Plan
We have adopted the 2005 Equity Plan for Key Employees of Accellent Holdings Corp. and Its Subsidiaries and Affiliates, which provides for the grant of incentive stock options (within the meaning of Section 422 of the Internal Revenue Code), options that are not incentive stock options, and various other stock-based grants, including the shares of common stock of Accellent Holdings Corp. sold to, and options granted to the executive officers and other key employees. As of the date of this prospectus, we have granted under the Equity Plan certain options as non-incentive stock options. The options are generally granted as follows: 50% vest and become exercisable over the passage of time, which we refer to as "time options," assuming the optionee continues to be employed by us, and 50% vest and become exercisable over time based upon the achievement of certain performance targets, which we refer to as "performance options."
Exercise Price. The exercise price of the options is the fair market value of the shares underlying the options on the date of the grant of the option.
Vesting of Time Options. Time options generally become exercisable by the holder of the option in installments of 20% on each of the first five anniversaries of the grant date.
Vesting of Performance Options. Performance options generally become exercisable over the first five fiscal years occurring after the grant date upon the achievement of certain performance targets. In the event that performance targets are not achieved in any given fiscal year but are achieved in a subsequent year, the performance option will become exercisable as to the previously unexercisable percentage of the performance options from the missed years, as well as with respect to the percentage of the performance options in respect of the fiscal year in which the performance targets are achieved.
Effect of Change in Control of Accellent Holdings Corp. In addition, immediately prior to a change in control of Accellent Holdings Corp., as defined in the Equity Plan, (i) the exercisability of the time options will automatically accelerate with respect to 100% of the shares of common stock of Accellent
94
Holdings Corp. subject to the time options and (ii) a percentage of the unvested performance options will automatically vest if certain internal rate of return targets have been achieved.
Effect of Disposition of Shares of Common Stock of Accellent Holdings Corp. In addition, based upon Accellent Holdings LLC's disposition of its shares of common stock of Accellent Holdings Corp., the exercisability of the time and performance options will automatically accelerate with respect to a percentage of the shares of common stock of Accellent Holdings Corp. subject to the time and performance options.
Miscellaneous. The options will only be transferable by will or pursuant to applicable laws of descent and distribution upon the death of the optionee. The Equity Plan may be amended or terminated by Accellent Holdings Corp.'s board of directors at any time.
Excise Tax Protection Agreement
Following the completion of the Transactions, Accellent Holdings Corp. entered into agreements with members of management providing such individuals with excise tax protection from excise taxes imposed on such member of management under Section 4999 of the Internal Revenue Code of 1986, as amended, in the event of a change in control (as defined in the agreement) following a public offering (as defined in the agreement) and if certain conditions are met, prior to a public offering.
95
PRINCIPAL STOCKHOLDERS
Accellent Acquisition Corp. owns 100% of the capital stock of Accellent Inc., and Accellent Holdings Corp. owns 100% of the capital stock of Accellent Acquisition Corp.
The following table and accompanying footnotes show information regarding the beneficial ownership of Accellent Holdings Corp. common stock as of November 22, 2005 by (i) each person known by us to beneficially own more than 5% of the outstanding shares of Accellent Holdings Corp. common stock, (ii) each of our directors, (iii) each named executive officer and (iv) all directors and executive officers as a group. Unless otherwise indicated, the address of each person named in the table below is c/o Accellent Inc., 100 Fordham Road, Wilmington, Massachusetts 01887.
Name and Address of Beneficial Owner
| | Beneficial Ownership of
Accellent Holdings Corp.
Common Stock(1)
| | Percentage of
Accellent Holdings Corp.
Common Stock
| |
|---|
| KKR Millennium GP LLC(2) | | 91,650,000 | | 71.5 | % |
| Bain Capital(3) | | 30,550,000 | | 23.8 | % |
| Ron Sparks(4) | | 1,546,667 | | 1.2 | % |
| Stewart A. Fisher(5) | | 1,200,000 | | | * |
| Daniel C. Croteau(6) | | 160,000 | | | * |
| Gary D. Curtis(7) | | 229,767 | | | * |
| Jeffrey M. Farina(8) | | 188,346 | | | * |
| Michael W. Michelson(2) | | 91,650,000 | | 71.5 | % |
| Kenneth W. Freeman(2) | | 91,650,000 | | 71.5 | % |
| James C. Momtazee(2) | | 91,650,000 | | 71.5 | % |
| Steven Barnes(3) | | 30,550,000 | | 23.8 | % |
| Directors and executive officers as a group (9 persons)(9) | | 125,920,439 | | 98.2 | % |
- *
- Less than one percent
- (1)
- The amounts and percentages of Accellent Holdings Corp. common stock beneficially owned are reported on the basis of regulations of the SEC governing the determination of beneficial ownership of securities. Under the rules of the SEC, a person is deemed to be a "beneficial owner" of a security if that person has or shares "voting power," which includes the power to vote or to direct the voting of such security, or "investment power," which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Under these rules, more than one person may be deemed to be a beneficial owner of such securities as to which such person has an economic interest.
- (2)
- Shares shown as beneficially owned by KKR Millennium GP LLC reflect shares of common stock owned of record by KKR Millennium Fund L.P., KKR Partners III, L.P. and KKR Financial Corp. through their investment vehicle, Accellent Holdings LLC. KKR Millennium GP LLC is the general partner of KKR Associates Millennium L.P., which is the general partner of the KKR Millennium Fund L.P. Messrs. Henry R. Kravis, George R. Roberts, James H. Greene, Jr., Paul E. Raether, Michael W. Michelson, Perry Golkin, Johannes P. Huth, Todd A. Fisher, Alexander Navab, Marc Lipschultz, Jacques Garaialde, Michael Calbert and Scott Nuttall, as members of KKR Millennium GP LLC, may be deemed to share beneficial ownership of any shares beneficially owned by KKR Millennium GP LLC, but disclaim such beneficial ownership. Mr. Michelson is a member of, and Messrs. Freeman and Momtazee are executives of, KKR and each is a director of Accellent Holdings Corp. and Accellent Inc. They disclaim beneficial ownership of any Accellent Holdings Corp. shares beneficially owned by KKR. The address of KKR Millennium GP LLC and each individual listed above is c/o Kohlberg Kravis Roberts & Co. L.P., 9 West 57th Street, New York, New York 10019.
- (3)
- Shares shown as beneficially owned by Bain Capital and Mr. Steven Barnes reflect the aggregate number of shares of common stock held, or beneficially held, by Bain Capital Integral Investors, LLC ("Bain") and BCIP TCV, LLC ("BCIP"). Mr. Barnes is a Managing Director of Bain Capital Investors, LLC ("BCI"), which is the administrative member of each of Bain and BCIP. Accordingly, Mr. Barnes and BCI may each be deemed to beneficially own shares owned by Bain and BCIP. Mr Barnes is a director of Accellent Holdings Corp. and Accellent Inc. Mr. Barnes and BCI disclaim beneficial ownership of any such shares in which they do not have a pecuniary interest. The address of Bain, BCIP, BCI and Mr. Barnes is c/o Bain Capital, LLC, 111 Huntington Avenue, Boston, Massachusetts 02199.
- (4)
- Consists of 1,546,667 shares of common stock underlying outstanding stock options that are exercisable within 60 days.
96
- (5)
- Consists of 1,200,000 shares of common stock underlying outstanding stock options that are exercisable within 60 days.
- (6)
- Consists of 160,000 shares of common stock underlying outstanding stock options that are exercisable within 60 days.
- (7)
- Consists of 229,767 shares of common stock underlying outstanding stock options that are exercisable within 60 days.
- (8)
- Consists of 188,346 shares of common stock underlying outstanding stock options that are exercisable within 60 days.
- (9)
- Includes 3,720,439 shares of common stock underlying outstanding stock options that are exercisable within 60 days.
97
THE TRANSACTIONS
On October 7, 2005, we entered into an agreement and plan of merger with Accellent Acquisition Corp., or AAC, an entity controlled by affiliates of Kohlberg Kravis Roberts & Co. L.P., which we refer to collectively as KKR, pursuant to which Accellent Merger Sub Inc., a wholly-owned subsidiary of AAC, merged with and into Accellent Inc., with Accellent Inc. being the surviving entity (the "Merger"). As a result of the Merger:
- •
- our capital stock and other equity interests outstanding immediately prior to the Merger (other than a portion of the shares of A-9 Preferred Stock held by certain existing stockholders which have been converted into equity of Accellent Holdings Corp.) and the options to receive our common stock outstanding immediately prior to the Merger (other than Rollover Options) have been cancelled and converted into the right to receive aggregate cash consideration of approximately $830 million less accrued interest on our existing indebtedness plus cash on hand at the time of closing and less approximately $17 million of cash bonuses to certain employees in connection with the change of control that occurred upon consummation of the merger;
- •
- entities affiliated with KKR own approximately 71% of our outstanding common stock immediately following the Merger, entities affiliated with Bain own approximately 24% of our common stock; and
- •
- certain members of management will own approximately 5% of our outstanding common stock, of which approximately 3% includes the value of Accellent Inc. stock options exchanged for Accellent Holdings Corp. stock options, and approximately 2% which represents shares of common stock owned by management.
In connection with the Merger, entities affiliated with KKR and entities affiliated with Bain made an equity investment in Accellent Holdings Corp. of approximately $611 million, with approximately $30 million of additional equity rolled over by 58 members of management. Equity rolled over by management includes approximately $19 million of equity in stock options of Accellent Inc. that was exchanged for stock options in Accellent Holdings Corp., $1 million of after-tax stock option proceeds used by management to acquire common stock of Accellent Holdings Corp, and $10 million of preferred stock of Accellent Inc. exchanged for $10 million of common stock of Accellent Holdings Corp. The equity rolled over by management in the form of stock options included approximately $14 million of equity rolled over by our executive officers, which is comprised of 8 individuals. In addition, in connection with the Merger, we
- •
- entered into a senior secured credit facility, consisting of a $400 million senior secured term loan facility and a $75 million senior secured revolving credit facility;
- •
- issued $305 million aggregate principal amount of senior subordinated notes, resulting in net proceeds of approximately $301 million after approximately $4 million original issue discount;
- •
- repaid approximately $409 million of our indebtedness, including pursuant to a tender offer for Accellent Corp.'s $175 million 10% senior subordinated notes due 2012;
- •
- paid approximately $73 million of transaction fees and expenses, including tender premiums.
In connection with the Merger, Accellent Holdings Corp. granted new options to purchase shares of Accellent Holdings Corp. to certain members of management. All equity positions currently held by our named executive officers are in the form of stock options.
Our tender offer to purchase all of the outstanding 10% senior subordinated notes due 2012 of Accellent Corp. expired on November 21, 2005 and our consent solicitation to amend the indenture governing the notes to eliminate substantially all of the restrictive covenants and effect certain other amendments to the indenture expired on November 3, 2005. We received tenders and the requisite consents for 100% of the 10% senior subordinated notes. As a result, we have accepted for payment and paid for all notes validly tendered and executed a supplemental indenture effecting the proposed amendments, which supplemental indenture has become operative.
98
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Management Services Agreement with KKR
In connection with the Transactions, we entered into a management services agreement with KKR pursuant to which KKR will provide certain structuring, consulting and management advisory services to us. Pursuant to this agreement, KKR received an aggregate transaction fee of $13.0 million paid upon the closing of the Transactions and will receive an advisory fee of $1.0 million payable annually, such amount to increase by 5% per year. We indemnify KKR and its affiliates, directors, officers and representatives for losses relating to the services contemplated by the management services agreement and the engagement of KKR pursuant to, and the performance by KKR of the services contemplated by, the management services agreement.
Registration Rights Agreement
In connection with the Transactions, we entered into a registration rights agreement with entities affiliated with KKR and entities affiliated with Bain Capital (each a "Sponsor Entity" and together the "Sponsor Entities") pursuant to which the Sponsor Entities are entitled to certain demand rights with respect to the registration and sale of their shares of common stock.
Management Bonus Plan
On October 7, 2005, our board of directors adopted, and our shareholders approved the Accellent Inc. Management Bonus Plan (the "Bonus Plan"). The Bonus Plan provided that certain of our employees receive cash bonuses in connection with the change in control that occurred upon the consummation of the Merger. The aggregate bonus payments under the Bonus Plan totaled $16,700,000 and were paid upon the consummation of the Merger. Please see "Management—Executive Compensation" for a description of cash bonuses received by our named executive officers.
Management Stockholder's Agreement
In connection with retaining the Rollover Options, the grant of options under the new option plan and, in certain cases, the purchase of shares of common stock of Accellent Holdings Corp., certain of our members of management entered into a management stockholder's agreement with us. The management stockholder's agreement generally restricts the ability of the management stockholders to transfer shares held by them for five years after the closing of the Transactions.
If a management stockholder's employment is terminated prior to the fifth anniversary of the closing of the Transactions, we have the right to purchase the shares and options held by such person on terms specified in the management stockholder's agreement. If, prior to a public offering of Accellent Holdings Corp's common stock, a management stockholder's employment is terminated as a result of death or disability, such stockholder or, in the event of such stockholder's death, the estate of such stockholder has the right to force us to purchase his shares and options, on terms specified in the management stockholder's agreement. In addition, if, prior to a public offering of Accellent Holdings Corp's common stock, a management stockholder's employment is terminated by us without cause (as defined in the management stockholder's agreement) or by the management stockholder for good reason (as defined in the management stockholder's agreement), such stockholder has the right to force us to exercise his or her Rollover Options and then purchase all or a portion of the shares underlying such Rollover Options, but only the number of shares equal to the remaining tax liability (above the minimum required withholding tax liability) incurred upon exercise of such options. If, prior to a public offering of Accellent Holdings Corp's common stock, a management stockholder's employment is terminated by the management stockholder without good reason, such stockholder has the right to force us to exercise his or her Rollover Options and then purchase all or a portion of the shares underlying such Rollover Options, but only if the amount of applicable withholding taxes which we are
99
required to withhold in respect of income recognized as a consequence of the exercise of such options (the "Statutory Withholding") is less than the actual tax liability that would have been incurred on the original value of the Rollover Options (the "Original Liability Amount") and then we are only required to purchase that number of shares equal to the difference between the Original Liability Amount and the Statutory Withholding. If, prior to a public offering of Accellent Holdings Corp.'s common stock, a management stockholder receives a notice from the Internal Revenue Service that taxes are due and payable in connection with his or her Rollover Options (other than in connection with the exercise or lapse of restrictions thereof) (the "Rollover Tax Liability"), such stockholder has the right to force us to exercise his or her Rollover Options and then purchase all or a portion of the shares underlying such Rollover Options, but only the number of shares equal to the Rollover Tax Liability.
The management stockholder's agreement also permits these members of management under certain circumstances to participate in registrations by us of our equity securities. Such registration rights would be subject to customary limitations.
Sale Participation Agreement
Each management stockholder entered into a sale participation agreement, which grants to the management stockholder the right to participate in any sale of shares of common stock by the Sponsor Entities occurring prior to the fifth anniversary of our initial public offering on the same terms as the Sponsor Entities. In order to participate in any such sale, the management stockholder may be required, among other things, to become a party to any agreement under which the common stock is to be sold, and to grant certain powers with respect to the proposed sale of common stock to custodians and attorneys-in-fact.
Existing Agreements
All of our existing agreements, which include a securities purchase agreement, registration rights agreement, shareholders' agreement, anti-dilution agreement, management agreement and subscription agreement, with certain entities affiliated with KRG/CMS L.P. and DLJ Merchant Banking Partners III, L.P., have been terminated in connection with the Transactions.
100
DESCRIPTION OF OTHER INDEBTEDNESS
Senior Secured Credit Facility
In connection with the Transactions, we entered into a senior secured credit facility with JPMorgan Chase Bank, N.A., as administrative agent, consisting of a term loan facility and a revolving credit facility, with a syndicate of lenders. The terms of the senior facility are set forth below. This description of the senior facility does not purport to be complete.
Borrowings
The senior facility provides for a $400 million term loan facility and a $75 million revolving credit facility, which includes a letter of credit subfacility. The proceeds of the term loan were used to fund a portion of the Merger, to repay all outstanding indebtedness under Accellent Corp.'s old senior secured credit facility and to pay certain fees, expenses and other costs associated with the Merger. The proceeds of the revolving credit facility and the letters of credit will be used for general corporate purposes.
The full amount of the term loans was borrowed on the closing date. The term loans will amortize in 27 quarterly installments of 0.25% of the original principal amount of the term loans, with the balance payable on the seventh anniversary of the closing date. Amounts prepaid or repaid with respect to the term loans may not be reborrowed. The senior facility provides that up to $100 million of additional term loans may be incurred under the term facility, with the average life to maturity and final maturity date of such additional term loans to be no earlier than the average life to maturity and final maturity date, respectively, of the initial term loans and with pricing to be agreed.
Revolving loans may be borrowed, repaid and re-borrowed after the closing date until the sixth anniversary of the closing date, and any revolving loans outstanding on the sixth anniversary of the closing date shall be repaid on such date. Approximately $69.2 million of the revolving facility was available on the closing date.
Voluntary prepayments of the term loans and revolving commitment reductions are permitted in whole or in part, subject to minimum prepayment requirements. Voluntary prepayments of LIBOR loans on a date other than the last day of the relevant interest period are also subject to payment of customary breakage costs, if any. We are required to prepay the loans with the net proceeds of certain incurrences of indebtedness, a certain percentage of excess cash flow and, subject to certain reinvestment rights, certain asset sales.
Interest
The interest rates under the senior facility are based in the case of the term loans, at our option, on either LIBOR plus 2.00% or the alternative base rate plus 1.00%, and, in the case of the revolving loans, at our option, on either LIBOR plus 2.25% or the alternate base rate plus 1.25%, which applicable margins are in each case subject to reduction based upon the attainment of certain leverage ratios. Overdue principal bears interest at a rate per annum equal to 2.0% above the rate then applicable to such principal amount. Overdue interest and other amounts bear interest at a rate per annum equal to 2.0% above the rate then applicable to alternate base rate loans under the term facility. With respect to LIBOR loans, each interest period will have a duration of, at our option, either one, two, three or six months, or, if available to all relevant lenders, nine or twelve months, and interest is payable in arrears at the end of each such interest period and, in any event, at least every three months. With respect to alternate base rate loans, interest is payable quarterly in arrears on the last day of each calendar quarter. Calculations of interest are based on a 360-day year (or 365/366 days, in the case of certain base rate loans) for actual days elapsed.
101
Fees
The senior facility provides for the payment to the lenders of a commitment fee equal to 0.50% per annum on the average daily unused portion of the available commitments under the revolving credit facility, payable quarterly in arrears and upon termination of the commitments, which commitment fee is subject to reduction based upon the attainment of a certain leverage ratio. The senior facility also provides for the payment to the lenders of a letter of credit fee on the average daily stated amount of all outstanding letters of credit equal to the then-applicable spread for LIBOR loans under the revolving credit facility, and a payment to JPMorgan Chase Bank, N.A., as letter of credit issuer, of a letter of credit fronting fee on the average daily stated amount of all outstanding letters of credit at 0.125% per annum, in each case payable quarterly in arrears and upon termination of the commitments under the revolving facility.
Collateral and guarantees
The loans under the senior facility and certain hedging obligations owing to senior facility lenders or their affiliates are guaranteed by Accellent Acquisition Corp. and by all of our existing and future direct and indirect wholly-owned domestic subsidiaries. The loans, the guarantees and such hedging arrangements are secured by a first priority perfected lien, subject to certain exceptions, on substantially all of our and the guarantors' existing and future properties and tangible and intangible assets, including a pledge of all of the capital stock held by such persons (other than certain capital stock of foreign subsidiaries).
Representations, warranties and covenants
The senior facility contains certain customary representations and warranties. In addition, we are required to maintain certain ratings in effect with respect to the senior facility, and the senior facility contains customary covenants restricting the ability our and certain of our subsidiaries' ability to, among other things:
- •
- declare dividends and redeem capital stock;
- •
- incur additional indebtedness (including guarantees of indebtedness);
- •
- create liens;
- •
- engage in mergers, consolidations, acquisitions and asset sales;
- •
- change the nature of its business;
- •
- make investments, loans and advances;
- •
- enter into sale-leaseback transactions;
- •
- engage in certain transactions with affiliates;
- •
- make prepayments of subordinated debt and amend subordinated debt documents;
- •
- change its fiscal year; and
- •
- make capital expenditures.
In addition, the senior facility requires us to maintain a maximum ratio of consolidated net debt to consolidated EBITDA and a minimum ratio of consolidated EBITDA to consolidated interest expense.
Events of Default
Events of default under the senior facility include but not be limited to:
- •
- failure to pay principal, interest, fees or other amounts when due;
102
- •
- material breach of any representation or warranty;
- •
- covenant defaults;
- •
- cross defaults to other material indebtedness;
- •
- events of bankruptcy;
- •
- invalidity of any guarantee or security interest;
- •
- a change of control; and
- •
- other customary events of default.
Other Long-Term Debt
As of September 30, 2005, our obligations under capital leases totaled $49,000.
103
THE EXCHANGE OFFER
General
We hereby offer, upon the terms and subject to the conditions set forth in this prospectus and in the accompanying letter of transmittal (which together constitute the exchange offer), to exchange up to $305 million aggregate principal amount of our 101/2% Senior Subordinated Notes due 2013, which we refer to in this prospectus as the outstanding notes, for a like aggregate principal amount of our 101/2% Senior Subordinated Exchange Notes due 2013, which we refer to in this prospectus as the exchange notes, properly tendered on or prior to the expiration date and not withdrawn as permitted pursuant to the procedures described below. The exchange offer is being made with respect to all of the outstanding notes.
As of the date of this prospectus $305 million aggregate principal amount of the outstanding notes is outstanding. This prospectus, together with the letter of transmittal, is first being sent on or about , to all holders of outstanding notes known to us. Our obligation to accept outstanding notes for exchange pursuant to the exchange offer is subject to certain conditions set forth under "—Certain Conditions to the Exchange Offer" below. We currently expect that each of the conditions will be satisfied and that no waivers will be necessary.
Purpose and Effect of the Exchange Offer
We and the guarantors have entered into a registration rights agreement with the initial purchasers of the outstanding notes in which we and the guarantors agreed, under some circumstances, to file a registration statement relating to an offer to exchange the outstanding notes for exchange notes. We also agreed to use all commercially reasonable efforts to cause the exchange offer registration statement to become effective under the Securities Act no later than 210 days after the closing date and to keep the exchange offer open for a period of 30 days. The exchange notes will have terms substantially identical to the outstanding notes, except that the exchange notes will not contain terms with respect to transfer restrictions, registration rights and special interest for failure to observe certain obligations in the registration rights agreement. The outstanding notes were issued on November 22, 2005.
Under certain circumstances set forth in the registration rights agreement, we will use all commercially reasonable efforts to cause the SEC to declare effective a shelf registration statement with respect to the resale of the outstanding notes and keep the registration statement effective for up to two years after the date on which such shelf registration statement becomes effective.
If we fail to comply with certain obligations under the registration rights agreement, we will be required to pay special interest to holders of the outstanding notes.
Each holder of outstanding notes that wishes to exchange outstanding notes for transferable exchange notes in the exchange offer will be required to make the following representations:
- •
- any exchange notes will be acquired in the ordinary course of its business;
- •
- the holder will have no arrangements or understanding with any person to participate in the distribution of the exchange notes within the meaning of the Securities Act;
- •
- the holder is not an "affiliate," as defined in Rule 405 of the Securities Act, of the Issuer or if it is an affiliate, that it will comply with applicable registration and prospectus delivery requirements of the Securities Act to the extent applicable;
- •
- if the holder is not a broker-dealer, that it is not engaged in, and does not intend to engage in, the distribution of the exchange notes; and
- •
- if the holder is a broker-dealer, that it will receive exchange notes for its own account in exchange for outstanding notes that were acquired as a result of market-making activities or
104
Resale of Exchange Notes
Based on interpretations of the SEC staff set forth in no action letters issued to unrelated third parties, we believe that exchange notes issued under the exchange offer in exchange for outstanding notes may be offered for resale, resold and otherwise transferred by any exchange note holder without compliance with the registration and prospectus delivery provisions of the Securities Act, if:
- •
- the holder is not an "affiliate" of the Issuer within the meaning of Rule 405 under the Securities Act;
- •
- the exchange notes are acquired in the ordinary course of the holder's business; and
- •
- the holder does not intend to participate in the distribution of the exchange notes.
Any holder who tenders in the exchange offer with the intention of participating in any manner in a distribution of the exchange notes:
- •
- cannot rely on the position of the staff of the SEC set forth inMorgan Stanley & Co. Incorporated (available June 5, 1991) andExxon Capital Holdings Corporation (available May 13, 1988), as interpreted in the SEC's letter toShearman & Sterling, dated July 2, 1993, or similar no-action letters; and
- •
- must comply with the registration and prospectus delivery requirements of the Securities Act in connection with a secondary resale transaction.
This prospectus may be used for an offer to resell, for the resale or for other retransfer of exchange notes only as specifically set forth in this prospectus. With regard to broker-dealers, only broker-dealers that acquired the outstanding notes as a result of market-making activities or other trading activities may participate in the exchange offer. Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where the outstanding notes were acquired by the broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. Please read the section captioned "Plan of Distribution" for more details regarding the transfer of exchange notes.
Terms of the Exchange Offer
Upon the terms and subject to the conditions set forth in this prospectus and in the accompanying letter of transmittal, we will accept for exchange any outstanding notes properly tendered and not withdrawn prior to the expiration date. We will issue $2,000 principal amount of exchange notes in exchange for each $2,000 principal amount of outstanding notes surrendered under the exchange offer, and we will issue $1,000 principal amount of exchange notes in exchange for each $1,000 principal amount of outstanding notes in excess of $2,000 surrendered under the exchange offer. Outstanding notes may be tendered only in denominations of $2,000 and integral multiples of $1,000 in excess of $2,000.
The form and terms of the exchange notes will be substantially identical to the form and terms of the outstanding notes except the exchange notes will be registered under the Securities Act, will not bear legends restricting their transfer and will not provide for any additional amounts upon our failure to fulfill our obligations under the registration rights agreement to file, and cause to be effective, a registration statement. The exchange notes will evidence the same debt as the outstanding notes. The exchange notes will be issued under and entitled to the benefits of the same indenture that authorized the issuance of the outstanding notes. Consequently, the outstanding notes and the exchange notes will be treated as a single class of debt securities under the indenture.
105
The exchange offer is not conditioned upon any minimum aggregate principal amount of outstanding notes being tendered for exchange.
As of the date of this prospectus, $305 million aggregate principal amount of the outstanding notes are outstanding. This prospectus and a letter of transmittal are being sent to all registered holders of outstanding notes. There will be no fixed record date for determining registered holders of outstanding notes entitled to participate in the exchange offer.
We intend to conduct the exchange offer in accordance with the provisions of the registration rights agreement, the applicable requirements of the Securities Act and the Securities Exchange Act of 1934, or Securities Exchange Act, and the rules and regulations of the SEC. Outstanding notes that are not tendered for exchange in the exchange offer will remain outstanding and continue to accrue interest and will be entitled to the rights and benefits the holders have under the indenture relating to the outstanding notes and the registration rights agreement, except for any rights under the registration rights agreement that by their terms terminate upon the consummation of the exchange offer.
We will be deemed to have accepted for exchange properly tendered outstanding notes when we have given oral or written notice of the acceptance to the exchange agent. The exchange agent will act as agent for the tendering holders for the purposes of receiving the exchange notes from us and delivering exchange notes to the holders. Under the terms of the registration rights agreement, we reserve the right to amend or terminate the exchange offer, and not to accept for exchange any outstanding notes not previously accepted for exchange, upon the occurrence of any of the conditions specified below under the caption "—Certain Conditions to the Exchange Offer."
Holders who tender outstanding notes in the exchange offer will not be required to pay brokerage commissions or fees or, subject to the instructions in the letter of transmittal, transfer taxes with respect to the exchange of outstanding notes. We will pay all charges and expenses, other than certain applicable taxes described below, in connection with the exchange offer. It is important that you read the section labeled "—Fees and Expenses" below for more details regarding fees and expenses incurred in the exchange offer.
Expiration Date; Extensions; Amendments
The exchange offer will expire at 5:00 p.m., New York City time on , 2006, unless in our sole discretion we extend it. In order to extend the exchange offer, we will notify the exchange agent orally or in writing of any extension. We will notify the registered holders of outstanding notes of the extension by press release or other public announcement no later than 9:00 a.m., New York City time, on the business day after the previously scheduled expiration date.
We reserve the right, in our sole discretion:
- •
- to delay accepting for exchange any outstanding notes due to an extension of the exchange offer;
- •
- to extend the exchange offer or to terminate the exchange offer and to refuse to accept outstanding notes not previously accepted if any of the conditions set forth below under "—Certain Conditions to the Exchange Offer" have not been satisfied, by giving oral or written notice of the delay, extension or termination to the exchange agent; or
- •
- under the terms of the registration rights agreement, to amend the terms of the exchange offer in any manner provided, that in the event of a material change in the exchange offer, including the waiver of a material condition, we will extend the offer period so that at least five business days remain in the exchange offer following notice of the material change.
Any delay in acceptance, extension, termination, or amendment will be followed as promptly as practicable by oral or written notice to the registered holders of outstanding notes. If we amend the exchange offer in a manner that we determine constitutes a material change, we will promptly disclose
106
the amendment in a manner reasonably calculated to inform the holder of outstanding notes of the amendment.
Without limiting the manner in which we may choose to make public announcements of any delay in acceptance, extension, termination or amendment of the exchange offer, we will have no obligation to publish, advertise, or otherwise communicate any public announcement, other than by making a timely release to a financial news service.
Certain Conditions to the Exchange Offer
Despite any other term of the exchange offer, we will not be required to accept for exchange, or issue any exchange notes for, any outstanding notes, and we may terminate the exchange offer as provided in this prospectus if prior to expiration of the exchange offer:
- •
- the exchange notes to be received will not be tradable by the holder without restriction under the Securities Act, the Securities Exchange Act, and without material restrictions under the blue sky or securities laws of substantially all of the states of the United States;
- •
- the exchange offer, or the making of any exchange by a holder of outstanding notes, violates applicable law or any applicable interpretation of the staff of the SEC; or
- •
- any action or proceeding has been instituted or threatened in any court or by or before any governmental agency with respect to the exchange offer that, in our judgment, would reasonably be expected to impair our ability to proceed with the exchange offer.
In addition, we will not be obligated to accept for exchange the outstanding notes of any holder that has not made to us:
- •
- the representations described under "—Purpose and Effect of the Exchange Offer," "—Procedures for Tendering" and "Plan of Distribution"; and
- •
- such other representations as may be reasonably necessary under applicable SEC rules, regulations or interpretations to make available to it an appropriate form for registration of the exchange notes under the Securities Act.
We expressly reserve the right, at any time or at various times, to extend the period of time during which the exchange offer is open. Consequently, we may delay acceptance of any outstanding notes by giving oral or written notice of the extension to their holders. During any such extensions, all notes previously tendered will remain subject to the exchange offer, and we may accept them for exchange. We will return any outstanding notes that we do not accept for exchange for any reason without expense to their tendering holder promptly after the expiration or termination of the exchange offer.
We expressly reserve the right to amend or terminate the exchange offer, and to reject for exchange any outstanding notes not previously accepted for exchange, upon the occurrence of any of the conditions of the exchange offer specified above. We will give oral or written notice of any extension, amendment, nonacceptance, or termination to the holders of the outstanding notes as promptly as practicable. In the case of any extension, such notice will be issued no later than 9:00 a.m., New York City time, on the business day after the previously scheduled expiration date.
These conditions are for our sole benefit and we may assert them regardless of the circumstances that may give rise to them or waive them in whole or in part at any or at various times in our sole discretion. If we fail at any time to exercise any of the foregoing rights, this failure will not constitute a waiver of this right. Each right will be deemed an ongoing right that we may assert at any time or at various times.
In addition, we will not accept for exchange any outstanding notes tendered, and will not issue exchange notes in exchange for any outstanding notes, if at the time any stop order will be threatened or in effect with respect to the registration statement of which this prospectus constitutes a part or the qualification of the indenture under the Trust Indenture Act of 1939.
107
Procedures for Tendering
Only a holder of outstanding notes may tender the outstanding notes in the exchange offer. To tender in the exchange offer, a holder must:
- •
- complete, sign and date the accompanying letter of transmittal, or a facsimile of the letter of transmittal; have the signature on the letter of transmittal guaranteed if the letter of transmittal so requires; and mail or deliver the letter of transmittal or facsimile to the exchange agent prior to the expiration date; or
- •
- comply with DTC's Automated Tender Offer Program procedures described below.
In addition, either:
- •
- the exchange agent must receive certificates for the outstanding notes along with the accompanying letter of transmittal prior to the expiration date; or
- •
- the exchange agent must receive, prior to the expiration date, a timely confirmation of book-entry transfer of the outstanding notes into the exchange agent's account at DTC according to the procedures for book-entry transfer described below or a properly transmitted agent's message; or
- •
- the holder must comply with the guaranteed delivery procedures described below.
To be tendered effectively, the exchange agent must receive any physical delivery of a letter of transmittal and other required documents at the address set forth below under "—Exchange Agent" prior to the expiration date.
The tender by a holder that is not withdrawn prior to the expiration date will constitute an agreement between the holder and us in accordance with the terms and subject to the conditions set forth in this prospectus and in the accompanying letter of transmittal.
The method of delivery of outstanding notes, the letter of transmittal and all other required documents to the exchange agent is at the holder's election and risk. Rather than mail these items, we recommend that holders use an overnight or hand delivery service. In all cases, holders should allow sufficient time to assure delivery to the exchange agent before the expiration date. Holders should not send the letter of transmittal or outstanding notes to us. Holders may request their respective brokers, dealers, commercial banks, trust companies or other nominees to effect the above transactions for them.
Any beneficial owner whose outstanding notes are registered in the name of a broker, dealer, commercial bank, trust company or other nominee and who wishes to tender should contact the registered holder promptly and instruct it to tender on the owner's behalf. If the beneficial owner wishes to tender on its own behalf, it must, prior to completing and executing the accompanying letter of transmittal and delivering its outstanding notes either:
- •
- make appropriate arrangements to register ownership of the outstanding notes in such owner's name; or
- •
- obtain a properly completed bond power from the registered holder of outstanding notes.
The transfer of registered ownership may take considerable time and may not be completed prior to the expiration date.
Signatures on a letter of transmittal or a notice of withdrawal described below must be guaranteed by a member firm of a registered national securities exchange or of the National Association of Securities Dealers, Inc., a commercial bank or trust company having an office or correspondent in the
108
United States or another "eligible institution" within the meaning of Rule 17Ad-15 under the Exchange Act, unless the outstanding notes are tendered:
- •
- by a registered holder who has not completed the box entitled "Special Issuance Instructions" or "Special Delivery Instructions" on the accompanying letter of transmittal; or
- •
- for the account of an eligible institution.
If the accompanying letter of transmittal is signed by a person other than the registered holder of any outstanding notes listed on the outstanding notes, the outstanding notes must be endorsed or accompanied by a properly completed bond power.
The bond power must be signed by the registered holder as the registered holder's name appears on the outstanding notes and an eligible institution must guarantee the signature on the bond power.
If the accompanying letter of transmittal or any outstanding notes or bond powers are signed by trustees, executors, administrators, guardians, attorneys-in-fact, officers of corporations or others acting in a fiduciary or representative capacity, these persons should so indicate when signing. Unless waived by us, they should also submit evidence satisfactory to us of their authority to deliver the accompanying letter of transmittal.
The exchange agent and DTC have confirmed that any financial institution that is a participant in DTC's system may use DTC's Automated Tender Offer Program to tender. Participants in the program may, instead of physically completing and signing the accompanying letter of transmittal and delivering it to the exchange agent, transmit their acceptance of the exchange offer electronically. They may do so by causing DTC to transfer the outstanding notes to the exchange agent in accordance with its procedures for transfer. DTC will then send an agent's message to the exchange agent. The term "agent's message" means a message transmitted by DTC, received by the exchange agent and forming part of the book-entry confirmation, to the effect that:
- •
- DTC has received an express acknowledgment from a participant in its Automated Tender Offer Program that is tendering outstanding notes that are the subject of the book-entry confirmation;
- •
- the participant has received and agrees to be bound by the terms of the accompanying letter of transmittal, or, in the case of an agent's message relating to guaranteed delivery, that the participant has received and agrees to be bound by the applicable notice of guaranteed delivery; and
- •
- the agreement may be enforced against that participant.
We will determine in our sole discretion all outstanding questions as to the validity, form, eligibility, including time or receipt, acceptance of tendered outstanding notes and withdrawal of tendered outstanding notes. Our determination will be final and binding. We reserve the absolute right to reject any outstanding notes not properly tendered or any outstanding notes the acceptance of which would, in the opinion of our counsel, be unlawful. We also reserve the right to waive any defects, irregularities or conditions of tender as to particular outstanding notes. Our interpretation of the terms and conditions of the exchange offer, including the instructions in the accompanying letter of transmittal, will be final and binding on all parties. Unless waived, any defects or irregularities in connection with tenders of outstanding notes must be cured within such time as we will determine. Although we intend to notify holders of defects or irregularities with respect to tenders of outstanding notes, neither we, the exchange agent, nor any other person will incur any liability for failure to give the notification. Tenders of outstanding notes will not be deemed made until any defects or irregularities have been cured or waived. Any outstanding notes received by the exchange agent that are not properly tendered and as to which the defects or irregularities have not been cured or waived will be returned to the exchange agent without cost to the tendering holder, unless otherwise provided in the letter of transmittal, as soon as practicable following the expiration date.
109
In all cases, we will issue exchange notes for outstanding notes that we have accepted for exchange under the exchange offer only after the exchange agent timely receives:
- •
- outstanding notes or a timely book-entry confirmation of the outstanding notes into the exchange agent's account at DTC; and
- •
- a properly completed and duly executed letter of transmittal and all other required documents or a properly transmitted agent's message.
By signing the accompanying letter of transmittal or authorizing the transmission of the agent's message, each tendering holder of outstanding notes will represent or be deemed to have represented to us that, among other things:
- •
- any exchange notes that the holder receives will be acquired in the ordinary course of its business;
- •
- the holder has no arrangement or understanding with any person or entity to participate in the distribution of the exchange notes;
- •
- if the holder is not a broker-dealer, that it is not engaged in and does not intend to engage in the distribution of the exchange notes;
- •
- if the holder is a broker-dealer that will receive exchange notes for its own account in exchange for outstanding notes that were acquired as a result of market-making activities or other trading activities, that it will deliver a prospectus, as required by law, in connection with any resale of any exchange notes. See "Plan of Distribution"; and
- •
- the holder is not an "affiliate," as defined in Rule 405 of the Securities Act, of ours or, if the holder is an affiliate, it will comply with any applicable registration and prospectus delivery requirements of the Securities Act.
Book-Entry Transfer
The exchange agent will make a request to establish an account with respect to the outstanding notes at DTC for purposes of the exchange offer promptly after the date of this prospectus. Any financial institution participating in DTC's system may make book-entry delivery of outstanding notes by causing DTC to transfer the outstanding notes into the exchange agent's account at DTC in accordance with DTC's procedures for transfer. Holders of outstanding notes who are unable to deliver confirmation of the book-entry tender of their outstanding notes into the exchange agent's account at DTC or all other documents required by the letter of transmittal to the exchange agent on or prior to the expiration date must tender their outstanding notes according to the guaranteed delivery procedures described below.
Guaranteed Delivery Procedures
Holders wishing to tender their outstanding notes but whose outstanding notes are not immediately available or who cannot deliver their outstanding notes, the accompanying letter of transmittal or any other available required documents to the exchange agent or comply with the applicable procedures under DTC's Automated Tender Offer Program prior to the expiration date may tender if:
- •
- the tender is made through an eligible institution;
- •
- prior to the expiration date, the exchange agent receives from the eligible institution either a properly completed and duly executed notice of guaranteed delivery, by facsimile transmission,
110
Upon request to the exchange agent, a notice of guaranteed delivery will be sent to holders who wish to tender their outstanding notes according to the guaranteed delivery procedures set forth above.
Withdrawal of Tenders
Except as otherwise provided in this prospectus, holders of outstanding notes may withdraw their tenders at any time prior to the expiration date.
For a withdrawal to be effective:
- •
- the exchange agent must receive a written notice of withdrawal, which notice may be by telegram, telex, facsimile transmission or letter of withdrawal at one of the addresses set forth below under "—Exchange Agent", or
- •
- holders must comply with the appropriate procedures of DTC's Automated Tender Offer Program system.
Any notice of withdrawal must:
- •
- specify the name of the person who tendered the outstanding notes to be withdrawn;
- •
- identify the outstanding notes to be withdrawn, including the principal amount of the outstanding notes; and
- •
- where certificates for outstanding notes have been transmitted, specify the name in which the outstanding notes were registered, if different from that of the withdrawing holder.
If certificates for outstanding notes have been delivered or otherwise identified to the exchange agent, then, prior to the release of the certificates, the withdrawing holder must also submit:
- •
- the serial numbers of the particular certificates to be withdrawn; and
- •
- a signed notice of withdrawal with signatures guaranteed by an eligible institution unless the holder is an eligible institution.
If outstanding notes have been tendered pursuant to the procedure for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at DTC to be credited with the withdrawn outstanding notes and otherwise comply with the procedures of that facility. We will determine all questions as to the validity, form and eligibility, including time of receipt, of the notices, and our determination will be final and binding on all parties. We will deem any outstanding notes so withdrawn not to have been validly tendered for exchange for purposes of the exchange offer. Any outstanding notes that have been tendered for exchange but that are not exchanged for any reason will be returned to their holder without cost to the holder, or, in the case of
111
outstanding notes tendered by book-entry transfer into the exchange agent's account at DTC according to the procedures described above, the outstanding notes will be credited to an account maintained with DTC for outstanding notes, as soon as practicable after withdrawal, rejection of tender or termination of the exchange offer. Properly withdrawn, outstanding notes may be retendered by following one of the procedures described under "—Procedures for Tendering" above at any time on or prior to the expiration date.
Exchange Agent
The Bank of New York has been appointed as exchange agent for the exchange offer. You should direct questions and requests for assistance, requests for additional copies of this prospectus or for the letter of transmittal and requests for the notice of guaranteed delivery to the exchange agent as follows:
| By Mail or Overnight Delivery: | | By Facsimile: | | By Hand Delivery: |
| | | | | |
The Bank of New York
Reorganization Unit
101 Barclay Street—7 East
New York, NY 10286
Attention: Randolph Holder | | The Bank of New York
Reorganization Unit
101 Barclay Street—7 East
New York, NY 10286
Attention: Randolph Holder
(212) 298-1915
Confirm Receipt of
Facsimile by telephone
(212) 815-5098 | | The Bank of New York
Reorganization Unit
101 Barclay Street
Lobby Level—Corp. Trust Window
New York, NY 10286
Attention: Randolph Holder |
Delivery of the letter of transmittal to an address other than as set forth above or transmission via facsimile other than as set forth above does not constitute a valid delivery of the letter of transmittal.
Fees and Expenses
We will bear the expenses of soliciting tenders. The principal solicitation is being made by mail; however, we may make additional solicitations by telephone or in person by our officers and regular employees and those of our affiliates.
We have not retained any dealer-manager in connection with the exchange offer and will not make any payments to broker-dealers or others soliciting acceptance of the exchange offer. We will, however, pay the exchange agent reasonable and customary fees for its services and reimburse it for its related reasonable out-of-pocket expenses.
We will pay the cash expenses to be incurred in connection with the exchange offer. The expenses are estimated in the aggregate to be approximately $425,000. They include:
- •
- SEC registration fees;
- •
- fees and expenses of the exchange agent and Trustee;
- •
- accounting and legal fees and printing costs; and
- •
- related fees and expenses.
112
Transfer Taxes
We will pay all transfer taxes, if any, applicable to the exchange of outstanding notes under the exchange offer. The tendering holder, however, will be required to pay any transfer taxes, whether imposed on the registered holder or any other person, if:
- •
- certificates representing outstanding notes for principal amounts not tendered or accepted for exchange are to be delivered to, or are to be issued in the name of, any person other than the registered holder of outstanding notes tendered;
- •
- tendered outstanding notes are registered in the name of any person other than the person signing the letter of transmittal; or
- •
- a transfer tax is imposed for any reason other than the exchange of outstanding notes under the exchange offer. If satisfactory evidence of payment of the taxes is not submitted with the letter of transmittal, the amount of the transfer taxes will be billed to that tendering holder.
Consequences of Failure to Exchange
Holders of outstanding notes who do not exchange their outstanding notes for exchange notes under the exchange offer will remain subject to the restrictions on transfer of the outstanding notes:
- •
- as set forth in the legend printed on the notes as a consequence of the issuance of the outstanding notes under the exemption from, or in transactions not subject to, the registration requirements of the Securities Act and applicable state securities laws; and
- •
- otherwise as set forth in the offering memorandum distributed in connection with the private offering of the outstanding notes.
In general, you may not offer or sell the outstanding notes unless they are registered under the Securities Act, or if the offer or sale is exempt from registration under the Securities Act and applicable state securities laws. Except as required by the registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act. Based on interpretations of the SEC staff, exchange notes issued in the exchange offer may be offered for resale, resold or otherwise transferred by their holders (other than any holder that is our "affiliate" within the meaning of Rule 405 under the Securities Act) without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that the holders acquired the exchange notes in the ordinary course of the holders' business and the holders have no arrangement or understanding with respect to the distribution of the exchange notes to be acquired in the exchange offer. Any holder who tenders in the exchange offer for the purpose of participating in a distribution of the exchange notes:
- •
- cannot rely on the applicable interpretations of the SEC; and
- •
- must comply with the registration and prospectus delivery requirements of the Securities Act in connection with a secondary resale transaction.
Accounting Treatment
We will record the exchange notes in our accounting records at the same carrying value as the outstanding notes, which is the aggregate principal amount, as reflected in our accounting records on the date of exchange. Accordingly, we will not recognize any gain or loss for accounting purposes in connection with the exchange offer. We will record the expenses of the exchange offer as incurred.
Other
Participation in the exchange offer is voluntary, and you should carefully consider whether to accept. You are urged to consult your financial and tax advisors in making your own decision on what action to take.
We may in the future seek to acquire untendered outstanding notes in open market or privately negotiated transactions, through subsequent exchange offers or otherwise. We have no present plans to acquire any outstanding notes that are not tendered in the exchange offer or to file a registration statement to permit resales of any untendered outstanding notes.
113
DESCRIPTION OF THE EXCHANGE NOTES
General
Certain terms used in this description are defined under the subheading "Certain Definitions." In this description, (i) the terms "we," "our" and "us" each refer to Accellent Inc. ("Accellent") and its consolidated Subsidiaries and (ii) the term "Issuer" refers only to Accellent Inc. and not any of its Subsidiaries. The term "Senior Subordinated Notes" refers to the outstanding notes and the exchange notes.
The outstanding notes were, and the exchange notes will be, issued under an indenture dated November 22, 2005 (the "Indenture") among the Issuer, the Guarantors and The Bank of New York, as trustee (the "Trustee"). Except as set forth herein, the terms of the Senior Subordinated Notes are substantially identical and include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act.
The following description is only a summary of the material provisions of the Indenture, does not purport to be complete and is qualified in its entirety by reference to the provisions of those agreements, including the definitions therein of certain terms used below. We urge you to read the Indenture because it, not this description, defines your rights as Holders of the Senior Subordinated Notes. You may request copies of the Indenture at our address set forth under the heading "Summary."
Brief Description of Senior Subordinated Notes
The Senior Subordinated Notes:
- •
- are unsecured senior subordinated obligations of the Issuer;
- •
- are subordinated in right of payment to all existing and future Senior Indebtedness (including the Senior Credit Facilities) of the Issuer;
- •
- are effectively subordinated to all secured Indebtedness of the Issuer (including the Senior Credit Facilities);
- •
- are senior in right of payment to any future Subordinated Indebtedness (as defined with respect to the Senior Subordinated Notes) of the Issuer;
- •
- are guaranteed on an unsecured senior subordinated basis by each Restricted Subsidiary that is a Domestic Subsidiary (other than special purpose Restricted Subsidiaries formed in connection with Receivables Facilities) and that guarantees other Indebtedness of the Issuer and its Restricted Subsidiaries, including the Senior Credit Facilities; and
- •
- are subject to registration with the SEC pursuant to a Registration Rights Agreement.
Guarantees
The Guarantors, as primary obligors and not merely as sureties, initially jointly and severally irrevocably and unconditionally guarantee, on an unsecured senior subordinated basis, the performance and full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Issuer under the Indenture and the Senior Subordinated Notes, whether for payment of principal of or interest on or Additional Interest in respect of the Senior Subordinated Notes, expenses, indemnification or otherwise, on the terms set forth in the Indenture by executing the Indenture.
As of the date of the Indenture, all of the Restricted Subsidiaries that are Domestic Subsidiaries guarantee the Senior Subordinated Notes. Each of the Guarantees of the Senior Subordinated is a
114
general unsecured obligation of each Guarantor, is subordinated in right of payment to all existing and future Senior Indebtedness of each such entity and is effectively subordinated to all secured Indebtedness of each such entity. The Senior Subordinated Notes are structurally subordinated to Indebtedness of Subsidiaries of the Issuer that do not Guarantee the Senior Subordinated Notes.
Not all of the Issuer's Subsidiaries Guarantee the Senior Subordinated Notes. In the event of a bankruptcy, liquidation or reorganization of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Issuer. Only our Restricted Subsidiaries that are Domestic Subsidiaries and that guarantee other Indebtedness of the Issuer and its Restricted Subsidiaries are required to guarantee the Notes. For the twelve months ended December 31, 2004, on a pro forma as adjusted basis, the non-guarantor Subsidiaries generated 3.6% and 4.8% of our net sales and Adjusted EBITDA, respectively. In addition, as of September 30, 2005, on a pro forma as adjusted basis, the non-guarantor Subsidiaries held 1.0% of our assets.
The obligations of each Guarantor under its Guarantees are limited as necessary to prevent the Guarantees from constituting a fraudulent conveyance under applicable law.
Any entity that makes a payment under its Guarantee is entitled upon payment in full of all guaranteed obligations under the Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor's pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor's liability on its Guarantee could be reduced to zero. See "Risk Factors—Risks Related to Our Indebtedness and the Notes—Federal and state fraudulent transfer laws may permit a court to void the guarantees, and, if that occurs, you may not receive any payment on the notes."
A Guarantee by a Guarantor shall provide by its terms that it shall be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of the Capital Stock of such Guarantor (including any sale, exchange or transfer), after which the applicable Guarantor is no longer a Restricted Subsidiary or all or substantially all the assets of such Guarantor, which sale, exchange or transfer is made in compliance with the applicable provisions of the Indenture;
(b) the release or discharge of such Guarantor from its guarantee of Indebtedness under the Senior Credit Facilities or the guarantee that resulted in the obligation of such Guarantor to guarantee the Notes, if such Guarantor would not then otherwise be required to guarantee the Notes pursuant to the covenant described under "Certain Covenants—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries" (treating any guarantees of such Guarantor that remain outstanding as incurred at least 30 days prior to such release or discharge);
(c) the proper designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary; or
(d) the Issuer exercising its legal defeasance option or covenant defeasance option as described under "Legal Defeasance and Covenant Defeasance" or the Issuer's obligations under the Indenture being discharged in accordance with the terms of the Indenture; and
(2) such Guarantor delivering to the Trustee an Officer's Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Indenture relating to such transaction have been complied with.
115
Ranking
Senior Indebtedness Versus the Senior Subordinated Notes
The payment of the principal of, premium, if any, and interest on the Senior Subordinated Notes and the payment of any Guarantee is subordinate in right of payment to the prior payment in cash in full of all Senior Indebtedness of the Issuer or the relevant Guarantor, as the case may be, including the obligations of the Issuer and such Guarantor under the Senior Credit Facilities.
The Senior Subordinated Notes are subordinated in right of payment to all of the Issuer's and the Guarantor's existing and future Senior Indebtedness and effectively subordinated to all of the Issuer's and the Guarantor's existing and future Secured Indebtedness to the extent of the value of the assets securing such Indebtedness. As of September 30, 2005, on apro forma as adjusted basis, we would have had $400 million of Senior Indebtedness (all of which would have been secured Indebtedness, consisting entirely of secured Indebtedness under the Senior Credit Facilities).
Although the Indenture contains limitations on the amount of additional Indebtedness that the Issuer and the Guarantors may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Indebtedness. See "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock."
Liabilities of Subsidiaries versus Notes
All of our operations are conducted through our subsidiaries. Our existing foreign subsidiaries are not Guaranteeing the notes, and, as described above under "Guarantees", Guarantees may be released under certain circumstances. In addition, our future subsidiaries may not be required to Guarantee the notes. Claims of creditors of any non-guarantor Subsidiaries, including trade creditors and creditors holding indebtedness or Guarantees issued by such non-guarantor Subsidiaries, and claims of preferred stockholders of such non-guarantor Subsidiaries generally will have priority with respect to the assets and earnings of such non-guarantor Subsidiaries over the claims of our creditors, including holders of the notes, even if such claims do not constitute Senior Indebtedness. Accordingly, the notes will be effectively subordinated to creditors (including trade creditors) and preferred stockholders, if any, of such non-guarantor Subsidiaries.
As of September 30, 2005, on a pro forma as adjusted basis, the total liabilities of our foreign subsidiaries were approximately $5.4 million, including trade payables. Although the Indenture limits the incurrence of Indebtedness and preferred stock by certain of our subsidiaries, such limitation is subject to a number of significant exceptions and qualifications and the Indebtedness incurred in compliance with the covenants could be substantial. Moreover, the Indenture does not impose any limitation on the incurrence by such subsidiaries of liabilities that are not considered Indebtedness under the Indenture. See "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock."
Other Senior Subordinated Notes versus Notes
Only Indebtedness of the Issuer or a Guarantor that is Senior Indebtedness rank senior to the notes and the relevant Guarantee in accordance with the provisions of the Indenture. The notes and each Guarantee in all respects rank pari passu with all other Senior Subordinated Indebtedness of the Issuer and the relevant Guarantor, respectively.
We and the Guarantors have agreed in the Indenture that we and they will not incur any Indebtedness that is subordinate or junior in right of payment to our Senior Indebtedness or the Senior Indebtedness of such Guarantors, unless such Indebtedness is Senior Subordinated Indebtedness of the applicable Person or is expressly subordinated in right of payment to Senior Subordinated Indebtedness
116
of such Person. The Indenture does not treat (i) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (ii) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Paying Agent and Registrar for the Senior Subordinated Notes
The Issuer maintains one or more paying agents for the Senior Subordinated Notes in the Borough of Manhattan, City of New York. The initial paying agent for the Senior Subordinated Notes is the Trustee.
The Issuer maintains a registrar with offices in the Borough of Manhattan, City of New York. The initial registrar is the Trustee. The registrar maintains a register reflecting ownership of the Senior Subordinated Notes outstanding from time to time and will make payments on and facilitate transfer of Senior Subordinated Notes on behalf of the Issuer.
The Issuer may change the paying agents or the registrars without prior notice to the Holders. The Issuer or any of its Subsidiaries may act as a paying agent or registrar.
Subordination of the Senior Subordinated Notes
Only Indebtedness of the Issuer or a Guarantor that is Senior Indebtedness rank senior to the Senior Subordinated Notes and the Guarantees in accordance with the provisions of the Indenture. The Senior Subordinated Notes and Guarantees in all respects rankpari passu with all other Senior Subordinated Indebtedness of the Issuer and the relevant Guarantor, respectively.
The Issuer and the Guarantors have agreed in the Indenture that the Issuer and the Guarantors will not incur any Indebtedness that is subordinate or junior in right of payment to the Senior Indebtedness of such Person, unless such Indebtedness is Senior Subordinated Indebtedness of the applicable Person or is expressly subordinated in right of payment to Senior Subordinated Indebtedness of such Person. The Indenture does not treat (i) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (ii) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Neither the Issuer nor any Guarantor is permitted to pay principal of, premium, if any, or interest on the Senior Subordinated Notes (or pay any other obligations relating to the Senior Subordinated Notes, including Additional Interest, fees, costs, expenses, indemnities and rescission or damage claims) or make any deposit pursuant to the provisions described under "Legal Defeasance and Covenant Defeasance" or "Satisfaction and Discharge" below and may not purchase, redeem or otherwise retire any Senior Subordinated Notes (collectively, "pay the notes") (except in the form of Permitted Junior Securities) if either of the following occurs (a "Payment Default"):
(1) any Obligation on any Designated Senior Indebtedness of the Issuer is not paid in full in cash when due (after giving effect to any applicable grace period); or
(2) any other default on Designated Senior Indebtedness of the Issuer occurs and the maturity of such Designated Senior Indebtedness is accelerated in accordance with its terms;
unless, in either case, the Payment Default has been cured or waived and any such acceleration has been rescinded or such Designated Senior Indebtedness has been paid in full in cash. Regardless of the foregoing, the Issuer is permitted to pay the Senior Subordinated Notes if the Issuer and the Trustee receive written notice approving such payment from the Representatives of all Designated Senior Indebtedness with respect to which the Payment Default has occurred and is continuing.
117
During the continuance of any default (other than a Payment Default) (a "Non-Payment Default") with respect to any Designated Senior Indebtedness pursuant to which the maturity thereof may be accelerated without further notice (except such notice as may be required to effect such acceleration) or the expiration of any applicable grace periods, the Issuer is not permitted to pay the Senior Subordinated Notes (except in the form of Permitted Junior Securities) for a period (a "Payment Blockage Period") commencing upon the receipt by the Trustee (with a copy to the Issuer) of written notice (a "Blockage Notice") of such Non-Payment Default from the Representative of such Designated Senior Indebtedness specifying an election to effect a Payment Blockage Period and ending 179 days thereafter. The Payment Blockage Period will end earlier if such Payment Blockage Period is terminated:
(1) by written notice to the Trustee and the Issuer from the Person or Persons who gave such Blockage Notice;
(2) because the default giving rise to such Blockage Notice is cured, waived or otherwise no longer continuing; or
(3) because such Designated Senior Indebtedness has been discharged or repaid in full in cash.
Notwithstanding the provisions described above, unless the holders of such Designated Senior Indebtedness or the Representative of such Designated Senior Indebtedness have accelerated the maturity of such Designated Senior Indebtedness, the Issuer and related Guarantors are permitted to resume paying the Senior Subordinated Notes after the end of such Payment Blockage Period. The Senior Subordinated Notes shall not be subject to more than one Payment Blockage Period in any consecutive 360-day period irrespective of the number of defaults with respect to Designated Senior Indebtedness during such period;provided that if any Blockage Notice is delivered to the Trustee by or on behalf of the holders of Designated Senior Indebtedness of the Issuer (other than the holders of Indebtedness under the Senior Credit Facilities), a Representative of holders of Indebtedness under the Senior Credit Facilities may give another Blockage Notice within such period. However, in no event may the total number of days during which any Payment Blockage Period or Periods on the Senior Subordinated Notes is in effect exceed 179 days in the aggregate during any consecutive 360-day period, and there must be at least 181 days during any consecutive 360-day period during which no Payment Blockage Period is in effect. Notwithstanding the foregoing, however, no default that existed or was continuing on the date of delivery of any Blockage Notice to the Trustee will be, or be made, the basis for a subsequent Blockage Notice unless such default has been waived for a period of not less than 90 days (it being acknowledged that any subsequent action, or any breach of any financial covenants during the period after the date of delivery of a Blockage Notice, that, in either case, would give rise to a Non-Payment Default pursuant to any provisions under which a Non-Payment Default previously existed or was continuing shall constitute a new Non-Payment Default for this purpose).
In connection with the Senior Subordinated Notes, in the event of any payment or distribution of the assets of the Issuer upon a total or partial liquidation or dissolution or reorganization of or similar proceeding relating to the Issuer or its property:
(1) the holders of Senior Indebtedness of the Issuer will be entitled to receive payment in full in cash of such Senior Indebtedness before the Holders of the Senior Subordinated Notes are entitled to receive any payment;
(2) until the Senior Indebtedness of the Issuer is paid in full in cash, any payment or distribution to which Holders of the Senior Subordinated Notes would be entitled but for the subordination provisions of the Indenture will be made to holders of such Senior Indebtedness as their interests may appear, except that Holders of Senior Subordinated Notes may receive Permitted Junior Securities; and
(3) if a distribution is made to Holders of the Senior Subordinated Notes that, due to the subordination provisions, should not have been made to them, such Holders of the Senior Subordinated
118
Notes are required to hold it in trust for the holders of Senior Indebtedness of the Issuer and pay it over to them as their interests may appear.
The subordination and payment blockage provisions described above will not prevent a Default from occurring under the Indenture upon the failure of the Issuer to pay interest or principal with respect to the Senior Subordinated Notes when due by their terms. If payment of the Senior Subordinated Notes is accelerated because of an Event of Default, the Issuer must promptly notify the holders of Designated Senior Indebtedness or the Representative of such Designated Senior Indebtedness of the acceleration. So long as there shall remain outstanding any Senior Indebtedness under the Senior Credit Facilities, a Blockage Notice may be given only by the administrative agent thereunder unless otherwise agreed to in writing by the requisite lenders named therein. If any Designated Senior Indebtedness of the Issuer is outstanding, neither the Issuer nor any Guarantor may pay the Senior Subordinated Notes until five Business Days after the Representatives of all the issuers of such Designated Senior Indebtedness receive notice of such acceleration and, thereafter, may pay the Senior Subordinated Notes only if the Indenture otherwise permits payment at that time.
Each Guarantor's obligations under its Guarantee are senior subordinated obligations of that Guarantor. As such, the rights of Holders to receive payment pursuant to such Guarantee are subordinated in right of payment to the rights of holders of Senior Indebtedness of such Guarantor. The terms of the subordination and payment blockage provisions described above with respect to the Issuer's obligations under the Senior Subordinated Notes apply equally to the obligations of such Guarantor under its Guarantee.
A Holder by its acceptance of Senior Subordinated Notes agrees to be bound by such provisions and authorizes and expressly directs the Trustee, on its behalf, to take such action as may be necessary or appropriate to effectuate the subordination provided for in the Indenture and appoints the Trustee its attorney-in-fact for such purpose.
By reason of the subordination provisions contained in the Indenture, in the event of a liquidation or insolvency proceeding, creditors of the Issuer or a Guarantor who are holders of Senior Indebtedness of the Issuer or such Guarantor, as the case may be, may recover more, ratably, than the Holders of the Senior Subordinated Notes, and creditors who are not holders of Senior Indebtedness may recover less, ratably, than holders of Senior Indebtedness and may recover more, ratably, than the Holders of the Senior Subordinated Notes.
The terms of the subordination provisions described above do not apply to payments from money or the proceeds of Government Securities held in trust by the Trustee for the payment of principal of and interest on the Senior Subordinated Notes pursuant to the provisions described under "Legal Defeasance and Covenant Defeasance" or "Satisfaction and Discharge," if the foregoing subordination provisions were not violated at the time the applicable amounts were deposited in trust pursuant to such provisions.
Transfer and Exchange
A Holder may transfer or exchange Senior Subordinated Notes in accordance with the Indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of Senior Subordinated Notes. Holders will be required to pay all taxes due on transfer. The Issuer is not required to transfer or exchange any Senior Subordinated Note selected for redemption. Also, the Issuer is not required to transfer or exchange any Senior Subordinated Note for a period of 15 days before a selection of Senior Subordinated Notes to be redeemed.
119
Principal, Maturity and Interest
The Issuer will issue $305,000,000 of Senior Subordinated Notes in this offering. The Senior Subordinated Notes will mature on December 1, 2013. Subject to compliance with the covenant described below under the caption "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock," the Issuer may issue additional Senior Subordinated Notes from time to time after this offering under the Indenture ("Additional Senior Subordinated Notes"). The Senior Subordinated Notes offered by the Issuer and any Additional Senior Subordinated Notes subsequently issued under the Indenture will be treated as a single class for all purposes under the Indenture, including waivers, amendments, redemptions and offers to purchase. Unless the context requires otherwise, references to "Senior Subordinated Notes" for all purposes of the Indenture and this "Description of the Notes" include any Additional Senior Subordinated Notes that are actually issued.
Interest on the Senior Subordinated Notes accrues at the rate of 101/2% per annum and is payable semi-annually in arrears on June 1 and December 1, commencing on June 1, 2006 to the Holders of Senior Subordinated Notes of record on the immediately preceding May 15 and November 15. Interest on the Senior Subordinated Notes accrues from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest on the Senior Subordinated Notes is computed on the basis of a 360-day year comprised of twelve 30-day months.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuer is not required to make any mandatory redemption or sinking fund payments with respect to the Senior Subordinated Notes. However, under certain circumstances, the Issuer may be required to offer to purchase Senior Subordinated Notes as described under the caption "Repurchase at the Option of Holders." We may at any time and from time to time purchase Senior Subordinated Notes in the open market or otherwise.
Optional Redemption
Except as set forth below, the Issuer is not be entitled to redeem the Senior Subordinated Notes at its option prior to December 1, 2009.
At any time prior to December 1, 2009 the Issuer may redeem all or a part of the Senior Subordinated Notes, upon not less than 30 nor more than 60 days' prior notice mailed by first class mail to the registered address of each Holder, at a redemption price equal to 100% of the principal amount of Senior Subordinated Notes redeemed plus the Applicable Premium as of, and accrued and unpaid interest and Additional Interest, if any, to the date of redemption (the "Redemption Date"), subject to the rights of Holders on the relevant record date to receive interest due on the relevant interest payment date.
On and after December 1, 2009 the Issuer may redeem the Senior Subordinated Notes, in whole or in part, upon notice as described under the heading "Repurchase at the Option of Holders—Selection and Notice" at the redemption prices (expressed as percentages of principal amount of the Senior Subordinated Notes to be redeemed) set forth below, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on December 1 of each of the years indicated below:
Year
| | Percentage
| |
|---|
| 2009 | | 105.250 | % |
| 2010 | | 102.625 | % |
| 2011 and thereafter | | 100.000 | % |
120
In addition, until December 1, 2008, the Issuer may, at its option, redeem up to 35% of the aggregate principal amount of Senior Subordinated Notes issued by it at a redemption price equal to 110.50% of the aggregate principal amount thereof, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of Senior Subordinated Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds of one or more Public Equity Offerings;provided that at least 65% of the sum of the aggregate principal amount of Senior Subordinated Notes originally issued under the Indenture and any Additional Senior Subordinated Notes that are Senior Subordinated Notes issued under the Indenture after the Issue Date remains outstanding immediately after the occurrence of each such redemption;provided further that each such redemption occurs within 90 days of the date of closing of each such Public Equity Offering.
Notice of any redemption upon any Public Equity Offering may be given prior to the redemption thereof, and any such redemption or notice may, at the Issuer's discretion, be subject to one or more conditions precedent, including, but not limited to, completion of the related Public Equity Offering.
The Trustee shall select the Senior Subordinated Notes to be purchased in the manner described under "Repurchase at the Option of Holders—Selection and Notice."
Repurchase at the Option of Holders
Change of Control
The Senior Subordinated Notes will provide that if a Change of Control occurs, unless the Issuer has previously or concurrently mailed a redemption notice with respect to all the outstanding Senior Subordinated Notes as described under "Optional Redemption," the Issuer will make an offer to purchase all of the Senior Subordinated Notes pursuant to the offer described below (the "Change of Control Offer") at a price in cash (the "Change of Control Payment") equal to 101% of the aggregate principal amount thereof plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase, subject to the right of Holders of the Senior Subordinated Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will send notice of such Change of Control Offer by first-class mail, with a copy to the Trustee, to each Holder of Senior Subordinated Notes to the address of such Holder appearing in the security register with a copy to the Trustee, with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled "Change of Control," and that all Senior Subordinated Notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Issuer;
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is mailed (the "Change of Control Payment Date");
(3) that any Senior Subordinated Note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuer defaults in the payment of the Change of Control Payment, all Senior Subordinated Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Senior Subordinated Notes purchased pursuant to a Change of Control Offer will be required to surrender such Senior Subordinated Notes, with the form entitled "Option of Holder to Elect Purchase" on the reverse of such Senior Subordinated Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
121
(6) that Holders will be entitled to withdraw their tendered Senior Subordinated Notes and their election to require the Issuer to purchase such Senior Subordinated Notes,provided that the paying agent receives, not later than the close of business on the 30th day following the date of the Change of Control notice, a telegram, telex, facsimile transmission or letter setting forth the name of the Holder of the Senior Subordinated Notes, the principal amount of Senior Subordinated Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Senior Subordinated Notes and its election to have such Senior Subordinated Notes purchased;
(7) that if the Issuer is redeeming less than all of the Senior Subordinated Notes, the Holders of the remaining Senior Subordinated Notes will be issued new Senior Subordinated Notes and such new Senior Subordinated Notes will be equal in principal amount to the unpurchased portion of the Senior Subordinated Notes surrendered. The unpurchased portion of the Senior Subordinated Notes must be equal to $2,000 or an integral multiple of $1,000 in excess of $2,000; and
(8) the other instructions, as determined by us, consistent with the covenant described hereunder, that a Holder must follow.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Senior Subordinated Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
On the Change of Control Payment Date, the Issuer will, to the extent permitted by law,
(1) accept for payment all Senior Subordinated Notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer,
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Senior Subordinated Notes or portions thereof so tendered, and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the Senior Subordinated Notes so accepted together with an Officer's Certificate to the Trustee stating that such Senior Subordinated Notes or portions thereof have been tendered to and purchased by the Issuer.
The Senior Credit Facilities limit, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may prohibit or limit, the Issuer from purchasing any Senior Subordinated Notes as a result of a Change of Control. In the event a Change of Control occurs at a time when the Issuer is prohibited from purchasing the Senior Subordinated Notes, the Issuer could seek the consent of its lenders to permit the purchase of the Senior Subordinated Notes or could attempt to refinance the borrowings that contain such prohibition. If the Issuer does not obtain such consent or repay such borrowings, the Issuer will remain prohibited from purchasing the Senior Subordinated Notes. In such case, the Issuer's failure to purchase tendered Senior Subordinated Notes would constitute an Event of Default under the Indenture. If, as a result thereof, a default occurs with respect to any Senior Indebtedness, the subordination provisions in the Indenture would restrict payments to the Holders of Senior Subordinated Notes under certain circumstances. The Senior Credit Facilities provide that certain change of control events with respect to the Issuer would constitute a default thereunder (including a Change of Control under the Indenture). If we experience a change of control that triggers a default under our Senior Credit Facilities, we could seek a waiver of such default or seek to refinance our Senior Credit Facilities. In the event we do not obtain such a waiver or refinance the Senior Credit Facilities, such default could result in amounts outstanding under our Senior Credit Facilities being declared due and payable.
122
Our ability to pay cash to the Holders of Senior Subordinated Notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the Senior Subordinated Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. After the Issue Date, we have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Indenture, but that could increase the amount of indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock" and "Certain Covenants—Liens." Such restrictions in the Indenture can be waived only with the consent of the Holders of a majority in principal amount of the Senior Subordinated Notes then outstanding. Except for the limitations contained in such covenants, however, the Indenture does not contain any covenants or provisions that may afford Holders of the Senior Subordinated Notes protection in the event of a highly leveraged transaction.
We are not be required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by us and purchases all Senior Subordinated Notes validly tendered and not withdrawn under such Change of Control Offer. Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of "Change of Control" includes a disposition of all or substantially all of the assets of the Issuer to any Person. Although there is a limited body of case law interpreting the phrase "substantially all," there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of "all or substantially all" of the assets of the Issuer. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Senior Subordinated Notes may require the Issuer to make an offer to repurchase the Senior Subordinated Notes as described above.
The provisions under the Indenture relative to the Issuer's obligation to make an offer to repurchase the Senior Subordinated Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Subordinated Notes.
Asset Sales
The Indenture provides that the Issuer will not, and will not permit any of its Restricted Subsidiaries to, cause, make or suffer to exist an Asset Sale, unless:
(1) the Issuer or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value (as determined in good faith by the Issuer) of the assets sold or otherwise disposed of; and
123
(2) except in the case of a Permitted Asset Swap, at least 75% of the consideration therefor received by the Issuer or such Restricted Subsidiary, as the case may be, is in the form of cash or Cash Equivalents;provided that the amount of:
(a) any liabilities (as shown on the Issuer's or such Restricted Subsidiary's most recent balance sheet or in the footnotes thereto) of the Issuer or such Restricted Subsidiary, other than liabilities that are by their terms subordinated to the Senior Subordinated Notes, that are assumed by the transferee of any such assets and for which the Issuer and all of its Restricted Subsidiaries have been validly released by all creditors in writing,
(b) any securities received by the Issuer or such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale, and
(c) any Designated Non-cash Consideration received by the Issuer or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed 2.5% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value,
shall be deemed to be cash for purposes of this provision and for no other purpose.
Within 365 days after the receipt of any Net Proceeds of any Asset Sale, the Issuer or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce:
(a) Obligations under Senior Indebtedness, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Senior Subordinated Indebtedness (and to correspondingly reduce commitments with respect thereto);provided that the Issuer shall equally and ratably reduce Obligations under the Senior Subordinated Notes as provided under "Optional Redemption," through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of Senior Subordinated Notes to purchase their Senior Subordinated Notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Senior Subordinated Notes that would otherwise be prepaid, or
(c) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Issuer or another Restricted Subsidiary,
(2) to make (a) an Investment in any one or more businesses,provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business, or
(3) to make an investment in (a) any one or more businesses,provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in
124
each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Issuer, or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment within 180 days of such commitment (an "Acceptable Commitment") and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Issuer or such Restricted Subsidiary enters into another Acceptable Commitment (a "Second Commitment") within 180 days of entering into the first such Acceptable Commitment;provided further that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the first sentence of the preceding paragraph will be deemed to constitute "Excess Proceeds." When the aggregate amount of Excess Proceeds exceeds $20.0 million, the Issuer shall make an offer to all Holders of the Senior Subordinated Notes and, if required by the terms of any Indebtedness that ispari passu with the Senior Subordinated Notes ("Pari Passu Indebtedness"), to the holders of such Pari Passu Indebtedness (an "Asset Sale Offer"), to purchase the maximum aggregate principal amount of the Senior Subordinated Notes and such Pari Passu Indebtedness that is $2,000 or an integral multiple of $1,000 in excess of $2,000 that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100% of the principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Indenture. The Issuer will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $20.0 million by mailing the notice required pursuant to the terms of the Indenture, with a copy to the Trustee.
To the extent that the aggregate amount of Senior Subordinated Notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Issuer may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Indenture. If the aggregate principal amount of Senior Subordinated Notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the Senior Subordinated Notes and such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the Senior Subordinated Notes or such Pari Passu Indebtedness tendered. Upon completion of any such Asset Sale Offer, the amount of Excess Proceeds shall be reset at zero.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the Indenture.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Senior Subordinated Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
The Senior Credit Facilities limit, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may prohibit or limit, the Issuer from purchasing any Senior Subordinated Notes pursuant to this Asset Sales covenant. In the event the
125
Issuer is prohibited from purchasing the Senior Subordinated Notes, the Issuer could seek the consent of its lenders to the purchase of the Senior Subordinated Notes or could attempt to refinance the borrowings that contain such prohibition. If the Issuer does not obtain such consent or repay such borrowings, it will remain prohibited from purchasing the Senior Subordinated Notes. In such case, the Issuer's failure to purchase tendered Senior Subordinated Notes would constitute an Event of Default under the Indenture. If, as a result thereof, a default occurs with respect to any Senior Indebtedness, the subordination provisions in the Indenture would restrict payments to the Holders of the Senior Subordinated Notes under certain circumstances.
Selection and Notice
If the Issuer is redeeming less than all of the Senior Subordinated Notes issued by it at any time, the Trustee will select the Senior Subordinated Notes to be redeemed (a) if the Senior Subordinated Notes are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange on which the Senior Subordinated Notes are listed or (b) on a pro rata basis, by lot or by such other method as the Trustee shall deem fair and appropriate.
Notices of purchase or redemption shall be mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the purchase or redemption date to each Holder of Senior Subordinated Notes at such Holder's registered address, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Senior Subordinated Notes or a satisfaction and discharge of the Indenture. If any Senior Subordinated Note is to be purchased or redeemed in part only, any notice of purchase or redemption that relates to such Senior Subordinated Note shall state the portion of the principal amount thereof that has been or is to be purchased or redeemed.
The Issuer will issue a new Senior Subordinated Note in a principal amount equal to the unredeemed portion of the original Senior Subordinated Note in the name of the Holder upon cancellation of the original Senior Subordinated Note. Senior Subordinated Notes called for redemption become due on the date fixed for redemption. On and after the redemption date, interest ceases to accrue on Senior Subordinated Notes or portions of them called for redemption.
Certain Covenants
Set forth below are summaries of certain covenants contained in the Indenture.
Limitation on Restricted Payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Issuer's, or any of its Restricted Subsidiaries' Equity Interests, including any dividend or distribution payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Issuer payable solely in Equity Interests (other than Disqualified Stock) of the Issuer; or
(b) dividends or distributions by a Restricted Subsidiary so long as, in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Issuer or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
126
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Issuer or any direct or indirect parent of the Issuer, including in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than:
(a) Indebtedness permitted under clauses (7) and (8) of the covenant described under "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock"; or
(b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment
(all such payments and other actions set forth in clauses (I) through (IV) above being collectively referred to as "Restricted Payments"), unless, at the time of such Restricted Payment:
(1) no Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on apro forma basis, the Issuer could incur $1.00 of additional Indebtedness under the provisions of the first paragraph of the covenant described under "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock"; and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries after the Issue Date (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (4), (6)(c), (9) and (14) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period) beginning October 1, 2005, to the end of the Issuer's most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100% of such deficit;plus
(b) 100% of the aggregate net cash proceeds and the fair market value, as determined in good faith by the Issuer, of marketable securities or other property received by the Issuer since immediately after the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock") from the issue or sale of:
(i) (A) Equity Interests of the Issuer, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value, as determined in good faith by the Issuer, of marketable securities or other property received from the sale of:
(x) Equity Interests to members of management, directors or consultants of the Issuer, any direct or indirect parent company of the Issuer and the Issuer's Subsidiaries after the Issue Date to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
127
and (B) to the extent such net cash proceeds are actually contributed to the Issuer, Equity Interests of the Issuer's direct or indirect parent companies (excluding contributions of the proceeds from the sale of Designated Preferred Stock of such companies or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Issuer that have been converted into or exchanged for such Equity Interests of the Issuer;
provided,however, that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock (as defined below), (X) Equity Interests or convertible debt securities of the Issuer sold to a Restricted Subsidiary, as the case may be, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions;plus
(c) 100% of the aggregate amount of cash and the fair market value, as determined in good faith by the Issuer, of marketable securities or other property contributed to the capital of the Issuer following the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock") (other than by a Restricted Subsidiary and other than by any Excluded Contributions);plus
(d) 100% of the aggregate amount received in cash and the fair market value, as determined in good faith by the Issuer, of marketable securities or other property received by means of:
(i) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of Restricted Investments made by the Issuer or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Issuer or its Restricted Subsidiaries and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments by the Issuer or its Restricted Subsidiaries, in each case after the Issue Date; or
(ii) the sale (other than to the Issuer or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary after the Issue Date;plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after the Issue Date, the fair market value of the Investment in such Unrestricted Subsidiary, as determined by the Issuer in good faith or if, in the case of an Unrestricted Subsidiary, such fair market value may exceed $15.0 million, in writing by an Independent Financial Advisor, at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary other than an Unrestricted Subsidiary to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
The foregoing provisions will not prohibit:
(1) the payment of any dividend within 60 days after the date of declaration thereof, if at the date of declaration such payment would have complied with the provisions of the Indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests ("Treasury Capital Stock") or Subordinated Indebtedness of the Issuer or any Equity Interests of any
128
direct or indirect parent company of the Issuer, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent contributed to the Issuer (in each case, other than any Disqualified Stock) ("Refunding Capital Stock") and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividends thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividends on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Issuer) in an aggregate amount per year no greater than the aggregate amount of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the redemption, repurchase or other acquisition or retirement of Subordinated Indebtedness of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuer or a Guarantor, as the case may be, which is incurred in compliance with "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock" so long as:
(a) the principal amount of such new Indebtedness does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium required to be paid under the terms of the instrument governing the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness;
(b) such new Indebtedness is subordinated to the Senior Subordinated Notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so purchased, exchanged, redeemed, repurchased, acquired or retired for value;
(c) such new Indebtedness has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired; and
(d) such new Indebtedness has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Issuer or any of its direct or indirect parent companies held by any future, present or former employee, director or consultant of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement;provided,however, that the aggregate Restricted Payments made under this clause (4) do not exceed in any calendar year $10.0 million (with unused amounts in any calendar year being carried over to succeeding calendar years subject to a maximum (without giving effect to the following proviso) of $20.0 million in any calendar year);provided further that such amount in any calendar year may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Issuer and, to the extent contributed to the Issuer, Equity Interests of any of the Issuer's direct or indirect parent companies, in each case to members of management, directors or consultants of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph;plus
129
(b) the cash proceeds of key man life insurance policies received by the Issuer or its Restricted Subsidiaries after the Issue Date;less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
andprovided further that cancellation of Indebtedness owing to the Issuer from members of management of the Issuer, any of the Issuer's direct or indirect parent companies or any of the Issuer's Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Indenture; provided, however, in the case of this clause (4), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of such Restricted Payment, the Issuer and its Restricted Subsidiaries on a consolidated basis would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00; and provided further that the aggregate amount of Restricted Payments made pursuant to this clause (4) shall not exceed $50.0 million;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Issuer or any of its Restricted Subsidiaries issued in accordance with the covenant described under "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock" to the extent such dividends are included in the definition of "Fixed Charges";
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Issuer after the Issue Date;
(b) the declaration and payment of dividends to a direct or indirect parent company of the Issuer, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) of such parent corporation issued after the Issue Date,provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Issuer from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided,however, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on apro forma basis, the Issuer and its Restricted Subsidiaries on a consolidated basis would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed the greater of (x) $20.0 million and (y) 1.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(8) repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the declaration and payment of dividends on the Issuer's common stock (or the payment of dividends to any direct or indirect parent entity to fund a payment of dividends on such entity's common stock), following the first public offering of the Issuer's common stock or the common stock
130
of any of its direct or indirect parent companies after the Issue Date, of up to 6% per annum of the net cash proceeds received by or contributed to the Issuer in or from any such public offering, other than public offerings with respect to the Issuer's common stock registered on Form S-8 and other than any public sale constituting an Excluded Contribution; provided, however, in the case of this clause (9), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of the declaration of such dividend, after giving effect to such dividend on a pro forma basis, the Issuer and its Restricted Subsidiaries on a consolidated basis would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(10) Restricted Payments that are made with Excluded Contributions;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed $25.0 million;
(12) distributions or payments of Receivables Fees;
(13) any Restricted Payment used to fund the Transaction and the fees and expenses related thereto or owed to Affiliates, in each case to the extent permitted by the covenant described under "—Transactions with Affiliates";
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to the provisions similar to those described under the captions "Repurchase at the Option of Holders—Change of Control" and "Repurchase at the Option of Holders—Asset Sales";provided that all Senior Subordinated Notes tendered by Holders in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed or acquired for value;
(15) the declaration and payment of dividends by the Issuer to, or the making of loans to, any direct or indirect parent in amounts required for any direct or indirect parent companies to pay, in each case without duplication,
(a) franchise taxes and other fees, taxes and expenses required to maintain their corporate existence;
(b) federal, state and local income taxes, to the extent such income taxes are attributable to the income of the Issuer and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries;provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer and its Restricted Subsidiaries would be required to pay in respect of federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(c) customary salary, bonus and other benefits payable to officers and employees of any direct or indirect parent company of the Issuer to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Issuer to the extent such costs and expenses are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries; and
(e) fees and expenses other than to Affiliates of the Issuer related to any unsuccessful equity or debt offering of such parent entity; and
(16) the distribution, dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Issuer or a Restricted Subsidiary by Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are cash and/or Cash Equivalents);
131
provided,however, that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the Issue Date, all of the Issuer's Subsidiaries are Restricted Subsidiaries. The Issuer will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the last sentence of the definition of "Unrestricted Subsidiary." For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Issuer and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the last sentence of the definition of "Investment." Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10), (11) or (16) of the second paragraph of this covenant, or pursuant to the definition of "Permitted Investments," and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries are not subject to any of the restrictive covenants set forth in the Indenture.
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, "incur" and collectively, an "incurrence") with respect to any Indebtedness (including Acquired Indebtedness) and the Issuer will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock;provided,however, that the Issuer may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and any of its Restricted Subsidiaries may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio on a consolidated basis for the Issuer and its Restricted Subsidiaries' most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on apro forma basis (including apro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period,provided,however, that Restricted Subsidiaries that are not Guarantors may not incur Indebtedness or issue Disqualified Stock or Preferred Stock if, after givingpro forma effect to such incurrence or issuance (including apro forma application of the net proceeds therefrom), more than an aggregate of $50.0 million of Indebtedness or Disqualified Stock or Preferred Stock of Restricted Subsidiaries that are not Guarantors is outstanding pursuant to this paragraph at such time.
The foregoing limitations will not apply to:
(1) the incurrence of Indebtedness under Credit Facilities by the Issuer or any of its Restricted Subsidiaries and the issuance and creation of letters of credit and bankers' acceptances thereunder (with letters of credit and bankers' acceptances being deemed to have a principal amount equal to the face amount thereof), up to the following aggregate principal amounts: (a) up to $75.0 million of Indebtedness under any revolving credit facility outstanding at any time pursuant to this clause (1)(a), (b) up to $400.0 million of Indebtedness in the form of term loans outstanding at any time pursuant to this clause (1)(b), provided that if, at the time of incurrence under this clause (1)(b), the Issuer's Senior Leverage Ratio, determined on apro forma basis (including apro forma application of the net proceeds therefrom) is greater than the Applicable Senior Leverage Ratio, such amount shall be reduced by the amount of any mandatory principal payments actually made by the borrower thereunder in respect of Indebtedness thereunder with Net Proceeds from an Asset Sale or series of related Asset Sales that constitutes the sale, transfer, conveyance or other disposition of all or substantially all of a
132
segment (as defined under GAAP) of the Issuer (other than any segment predominantly composed of assets acquired by the Issuer or its Restricted Subsidiaries subsequent to the Issue Date), and (c) up to $100.0 million of Indebtedness in the form of term loans outstanding at any time pursuant to this clause (1)(c), provided that at the time of incurrence under this clause (1)(c), the Issuer's Senior Leverage Ratio, determined on apro forma basis (including apro forma application of the net proceeds therefrom) is less than or equal to the Applicable Senior Leverage Ratio;
(2) the incurrence by the Issuer and any Guarantor of Indebtedness represented by the Senior Subordinated Notes (including any Guarantee) (other than any Additional Senior Subordinated Notes);
(3) Indebtedness of the Issuer and its Restricted Subsidiaries in existence on the Issue Date, including the 10% Senior Subordinated Notes due 2015 of Accellent Corp. (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations), Disqualified Stock and Preferred Stock incurred by the Issuer or any of its Restricted Subsidiaries, to finance the purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets up to an aggregate amount which, when aggregated with the principal amount of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (4) and including all Refinancing Indebtedness incurred to refund, refinance or replace any other Indebtedness, Disqualified Stock and Preferred Stock incurred pursuant to this clause (4), does not exceed the greater of (x) $25.0 million and (y) 1.5% of Total Assets;
(5) Indebtedness incurred by the Issuer or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers' compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers' compensation claims;provided,however, that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Issuer or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition;provided,however, that
(a) such Indebtedness is not reflected on the balance sheet of the Issuer, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (6)(a)); and
(b) the maximum assumable liability in respect of all such Indebtedness shall at no time exceed the gross proceeds including non-cash proceeds (the fair market value of such non-cash proceeds being measured at the time received and without giving effect to any subsequent changes in value) actually received by the Issuer and its Restricted Subsidiaries in connection with such disposition;
(7) Indebtedness of the Issuer to a Restricted Subsidiary;provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the Senior Subordinated Notes;provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in any Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness;
133
(8) Indebtedness of a Restricted Subsidiary to the Issuer or another Restricted Subsidiary;provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Senior Subordinated Notes of such Guarantor;provided further that any subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Issuer or another Restricted Subsidiary,provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Issuer or another of its Restricted Subsidiaries) shall be deemed in each case to be an issuance of such shares of Preferred Stock;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness permitted to be incurred pursuant to "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock," exchange rate risk or commodity pricing risk;
(11) obligations in respect of performance, bid, appeal and surety bonds and completion guarantees provided by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary equal to 100.0% of the net cash proceeds received by the Issuer since immediately after the Issue Date from the issue or sale of Equity Interests of the Issuer or cash contributed to the capital of the Issuer (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Issuer or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of "—Limitation on Restricted Payments" to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of "—Limitation on Restricted Payments" or to make Permitted Investments (other than Permitted Investments specified in clauses (1) and (3) of the definition thereof) and (b) Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference, which when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed $60.0 million (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence by the Issuer or any Restricted Subsidiary of Indebtedness, Disqualified Stock or Preferred Stock which serves to refund or refinance any Indebtedness, Disqualified Stock or Preferred Stock incurred as permitted under the first paragraph of this covenant and clauses (2), (3) and (12)(a) above, this clause (13) and clause (14) below or any Indebtedness, Disqualified Stock or Preferred Stock issued to so refund or refinance such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith
134
(the "Refinancing Indebtedness") prior to its respective maturity;provided,however, that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of the Indebtedness, Disqualified Stock or Preferred Stock being refunded or refinanced,
(b) to the extent such Refinancing Indebtedness refinances (i) Indebtedness subordinated orpari passu to the Senior Subordinated Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated orpari passu to the Senior Subordinated Notes or the Guarantee at least to the same extent as the Indebtedness being refinanced or refunded or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively, and
(c) shall not include:
- (i)
- Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of the Issuer;
- (ii)
- Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor; or
- (iii)
- Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary;
(14) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Issuer or any Restricted Subsidiary or merged into the Issuer or a Restricted Subsidiary in accordance with the terms of the Indenture;provided that after giving effect to such acquisition or merger, either:
(a) the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of this covenant, or
(b) the Fixed Charge Coverage Ratio of the Issuer and the Restricted Subsidiaries is greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business,provided that such Indebtedness is extinguished within two Business Days of its incurrence;
(16) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Issuer or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Indenture, or
(b) any guarantee by a Restricted Subsidiary of Indebtedness of the Issuerprovided that such guarantee is incurred in accordance with the covenant described below under "—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries";
135
(18) Indebtedness of Foreign Subsidiaries of the Issuer incurred not to exceed at any one time outstanding and together with any other Indebtedness incurred under this clause (18) 5.0% of the Total Assets of the Foreign Subsidiaries (it being understood that any Indebtedness incurred pursuant to this clause (18) shall cease to be deemed incurred or outstanding for purposes of this clause (18) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (18));
(19) Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred to finance or assumed in connection with an acquisition in a principal amount not to exceed $20.0 million in the aggregate at any one time outstanding together with all other Indebtedness, Disqualified Stock and/or Preferred Stock issued under this clause (19) (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (19) shall cease to be deemed incurred or outstanding for purposes of this clause (19) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (19)); and
(20) Indebtedness of the Issuer or any of its Restricted Subsidiaries consisting of (i) the financing of insurance premiums or (ii) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business.
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (21) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses;provided that all Indebtedness outstanding under the Credit Facilities on the Issue Date will be treated as incurred on the Issue Date under clause (1) of the preceding paragraph; and
(2) at the time of incurrence, the Issuer will be entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above.
Accrual of interest, the accretion of accreted value and the payment of interest in the form of additional Indebtedness, Disqualified Stock or Preferred Stock will not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt;provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency
136
exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
Liens
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, create, incur, assume or suffer to exist any Lien (except Permitted Liens) that secures obligations under any Indebtedness rankingpari passu with or subordinated to the Senior Subordinated Notes or any related Guarantee, on any asset or property of the Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the Senior Subordinated Notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; or
(2) in all other cases, the Senior Subordinated Notes or the Guarantees are equally and ratably secured, except that the foregoing shall not apply to (a) Liens securing the Senior Subordinated Notes and the related Guarantees and (b) Liens securing Senior Indebtedness of the Issuer or any Guarantor.
Merger, Consolidation or Sale of All or Substantially All Assets
The Issuer may not consolidate or merge with or into or wind up into (whether or not the Issuer is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) the Issuer is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation organized or existing under the laws of the jurisdiction of organization of the Issuer or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person, as the case may be, being herein called the "Successor Company");
(2) the Successor Company, if other than the Issuer, expressly assumes all the obligations of the Issuer under the Senior Subordinated Notes pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Trustee;
(3) immediately after such transaction, no Default exists;
(4) immediately after givingpro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Successor Company would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of the covenant described under "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock," or
(b) the Fixed Charge Coverage Ratio for the Successor Company, the Issuer and its Restricted Subsidiaries would be greater than such Ratio for the Issuer and its Restricted Subsidiaries immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case clause (b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person's obligations under the Indenture, the Senior Subordinated Notes and the Registration Rights Agreement; and
137
(6) the Issuer shall have delivered to the Trustee an Officer's Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture.
The Successor Company will succeed to, and be substituted for the Issuer, as the case may be, under the Indenture, the Guarantees and the Senior Subordinated Notes, as applicable. Notwithstanding the foregoing clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Issuer, and
(2) the Issuer may merge with an Affiliate of the Issuer, as the case may be, solely for the purpose of reincorporating the Issuer in a State of the United States so long as the amount of Indebtedness of the Issuer and its Restricted Subsidiaries is not increased thereby.
Subject to certain limitations described in the Indenture governing release of a Guarantee upon the sale, disposition or transfer of a guarantor, no Guarantor will, and the Issuer will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not the Issuer or Guarantor is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation organized or existing under the laws of the jurisdiction of organization of such Guarantor, as the case may be, or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Guarantor or such Person, as the case may be, being herein called the "Successor Person");
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Indenture and such Guarantor's related Guarantee pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Trustee;
(c) immediately after such transaction, no Default exists; and
(d) the Issuer shall have delivered to the Trustee an Officer's Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture; or
(2) the transaction is made in compliance with the covenant described under "Repurchase at the Option of Holders—Asset Sales."
Subject to certain limitations described in the Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Indenture and such Guarantor's Guarantee. Notwithstanding the foregoing, any Guarantor may merge into or transfer all or part of its properties and assets to another Guarantor or the Issuer.
Transactions with Affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer (each
138
of the foregoing, an "Affiliate Transaction") involving aggregate payments or consideration in excess of $5.0 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm's-length basis; and
(2) the Issuer delivers to the Trustee with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $10.0 million, a resolution adopted by the majority of the board of directors of the Issuer approving such Affiliate Transaction and set forth in an Officer's Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Issuer or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Indenture described above under the covenant "—Limitation on Restricted Payments" and the definition of "Permitted Investments";
(3) the payment of management, consulting, monitoring and advisory fees and related expenses to the Investors;
(4) the payment of reasonable and customary fees paid to, and indemnities provided on behalf of, officers, directors, employees or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Issuer or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Issuer or such Restricted Subsidiary from a financial point of view or stating that the terms are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm's-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter;provided,however, that the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous to the Holders when taken as a whole;
(8) the Transaction and the payment of all fees and expenses related to the Transaction, in each case as disclosed in this prospectus;
(9) transactions with customers, clients, suppliers, or purchasers or sellers of goods or services, in each case in the ordinary course of business and otherwise in compliance with the terms of the Indenture which are fair to the Issuer and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Issuer or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Issuer to any Permitted Holder or to any director, officer, employee or consultant;
139
(11) sales of accounts receivable, or participations therein, in connection with any Receivables Facility;
(12) payments by the Issuer or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Issuer in good faith;
(13) payments or loans (or cancellation of loans) to employees or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries and employment agreements, stock option plans and other similar arrangements with such employees or consultants which, in each case, are approved by the Issuer in good faith; and
(14) investments by the Investors in securities of the Issuer or any of its Restricted Subsidiaries so long as (i) the investment is being offered generally to other investors on the same or more favorable terms and (ii) the investment constitutes less than 5% of the proposed or outstanding issue amount of such class of securities.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries that are not Guarantors to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any such Restricted Subsidiary to:
(1) (a) pay dividends or make any other distributions to the Issuer or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(2) make loans or advances to the Issuer or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Issuer or any of its Restricted Subsidiaries,
except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation;
(b) the Indenture and the Senior Subordinated Notes;
(c) purchase money obligations for property acquired in the ordinary course of business that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Issuer or any of its Restricted Subsidiaries in existence at the time of such acquisition (but not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person and its Subsidiaries, or the property or assets of the Person and its Subsidiaries, so acquired;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Issuer pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock
140
and Preferred Stock" and "—Liens" that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under "—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock";
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases or licenses of intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (k) above;provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Issuer, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing; and
(m) restrictions created in connection with any Receivables Facility that, in the good faith determination of the Issuer are necessary or advisable to effect such Receivables Facility.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Issuer will not permit any of its Restricted Subsidiaries that is a Domestic Subsidiary, other than a Guarantor or a special-purpose Restricted Subsidiary formed in connection with Receivables Subsidiaries, to guarantee the payment of any Indebtedness of the Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Issuer or any Guarantor:
(a) if the Senior Subordinated Notes or such Guarantor's Guarantee are subordinated in right of payment to such Indebtedness, the Guarantee under the supplemental indenture shall be subordinated to such Restricted Subsidiary's guarantee with respect to such Indebtedness substantially to the same extent as the Senior Subordinated Notes are subordinated to such Indebtedness; and
(b) if such Indebtedness is by its express terms subordinated in right of payment to the Senior Subordinated Notes or such Guarantor's Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Senior Subordinated Notes;
(2) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Issuer or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee; and
141
(3) such Restricted Subsidiary shall deliver to the Trustee an Opinion of Counsel to the effect that:
(a) such Guarantee has been duly executed and authorized; and
(b) such Guarantee constitutes a valid, binding and enforceable obligation of such Restricted Subsidiary, except insofar as enforcement thereof may be limited by bankruptcy, insolvency or similar laws (including, without limitation, all laws relating to fraudulent transfers) and except insofar as enforcement thereof is subject to general principles of equity;
provided that this covenant shall not be applicable to any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary.
Limitation on Layering
The Indenture provides that the Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is subordinate in right of payment to any Senior Indebtedness of the Issuer or such Guarantor, as the case may be, unless such Indebtedness is either:
(1) equal in right of payment with the Senior Subordinated Notes or such Guarantor's Guarantee of the Senior Subordinated Notes, as the case may be; or
(2) expressly subordinated in right of payment to the Senior Subordinated Notes or such Guarantor's Guarantee of the Senior Subordinated Notes, as the case may be.
The Indenture will not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Reports and Other Information
Notwithstanding that the Issuer may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Indenture requires the Issuer to file with the SEC (and make available to the Trustee and Holders of the Senior Subordinated Notes (without exhibits), without cost to any Holder, within 15 days after it files them with the SEC) from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days after the end of each of the first three fiscal quarters of each fiscal year, reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Issuer would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
142
in each case, in a manner that complies in all material respects with the requirements specified in such form;provided that the Issuer shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Issuer will make available such information to prospective purchasers of Senior Subordinated Notes, in addition to providing such information to the Trustee and the Holders of the Senior Subordinated Notes, in each case within 15 days after the time the Issuer would be required to file such information with the SEC, if it were subject to Sections 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Issuer agrees that, for so long as any Senior Subordinated Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
In the event that any direct or indirect parent company of the Issuer becomes a guarantor of the Senior Subordinated Notes, the Indenture permits the Issuer to satisfy its obligations in this covenant with respect to financial information relating to the Issuer by furnishing financial information relating to such parent;provided that the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Issuer and its Restricted Subsidiaries on a standalone basis, on the other hand.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offer or the effectiveness of the shelf registration statement by the filing with the SEC of the exchange offer registration statement or shelf registration statement, and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act.
Events of Default and Remedies
The Indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the Senior Subordinated Notes (whether or not prohibited by the subordination provisions of the Indenture);
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the Senior Subordinated Notes (whether or not prohibited by the subordination provisions of the Indenture);
(3) failure by the Issuer or any Guarantor for 60 days after receipt of written notice given by the Trustee or the Holders of not less 30% in principal amount of the Senior Subordinated Notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clauses (1) and (2) above) contained in the Indenture or the Senior Subordinated Notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Issuer or any of its Restricted Subsidiaries, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Senior Subordinated Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
143
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $20.0 million or more at any one time outstanding;
(5) failure by the Issuer or any Significant Subsidiary to pay final judgments aggregating in excess of $20.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Issuer or any Significant Subsidiary; or
(7) the Guarantee of any Significant Subsidiary shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary, as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Indenture or the release of any such Guarantee in accordance with the Indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Indenture, the Trustee or the Holders of at least 30% in principal amount of the then total outstanding Senior Subordinated Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Senior Subordinated Notes to be due and payable immediately;provided,however, that so long as any Indebtedness permitted to be incurred under the Indenture as part of the Senior Credit Facilities shall be outstanding, no such acceleration shall be effective until the earlier of:
(1) acceleration of any such Indebtedness under the Senior Credit Facilities; or
(2) five Business Days after the giving of written notice of such acceleration to the Issuer and the administrative agent under the Senior Credit Facilities.
Upon the effectiveness of such declaration, such principal and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Senior Subordinated Notes will become due and payable without further action or notice. The Indenture provides that the Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee shall have no obligation to accelerate the Senior Subordinated Notes if in the best judgment of the Trustee acceleration is not in the best interest of the Holders of the Senior Subordinated Notes.
The Indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding Senior Subordinated Notes by notice to the Trustee may on behalf of the Holders of all of the Senior Subordinated Notes waive any existing Default and its consequences under the Indenture except a continuing Default in the payment of interest on, premium, if any, or the principal of any Senior Subordinated Note held by a non-consenting Holder. In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Senior Subordinated Notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged; or
144
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
Subject to the provisions of the Indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request or direction of any of the Holders of the Senior Subordinated Notes unless the Holders have offered to the Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Senior Subordinated Note may pursue any remedy with respect to the Indenture or the Senior Subordinated Notes unless:
(1) such Holder has previously given the Trustee notice that an Event of Default is continuing;
(2) Holders of at least 30% in principal amount of the total outstanding Senior Subordinated Notes have requested the Trustee to pursue the remedy;
(3) Holders of the Senior Subordinated Notes have offered the Trustee reasonable security or indemnity against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding Senior Subordinated Notes have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the Indenture the Holders of a majority in principal amount of the total outstanding Senior Subordinated Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the Indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of a Senior Subordinated Note or that would involve the Trustee in personal liability.
The Indenture provides that the Issuer is required to deliver to the Trustee annually a statement regarding compliance with the Indenture, and the Issuer is required, within five Business Days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of the Issuer or any Guarantor or any of their parent companies shall have any liability for any obligations of the Issuer or the Guarantors under the Senior Subordinated Notes, the Guarantees or the Indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting Senior Subordinated Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Senior Subordinated Notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such a waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuer and the Guarantors under the Indenture will terminate (other than certain obligations) and will be released upon payment in full of all of the Senior Subordinated Notes. The Issuer may, at its option and at any time, elect to have all of its obligations discharged with respect
145
to the Senior Subordinated Notes and have the Issuer and each Guarantor's obligation discharged with respect to its Guarantee ("Legal Defeasance") and cure all then existing Events of Default except for:
(1) the rights of Holders of Senior Subordinated Notes to receive payments in respect of the principal of, premium, if any, and interest on the Senior Subordinated Notes when such payments are due solely out of the trust created pursuant to the Indenture;
(2) the Issuer's obligations with respect to Senior Subordinated Notes concerning issuing temporary Senior Subordinated Notes, registration of such Senior Subordinated Notes, mutilated, destroyed, lost or stolen Senior Subordinated Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer's obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Indenture.
In addition, the Issuer may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to certain covenants that are described in the Indenture ("Covenant Defeasance") and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Senior Subordinated Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuer) described under "Events of Default and Remedies" will no longer constitute an Event of Default with respect to the Senior Subordinated Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Senior Subordinated Notes:
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the Senior Subordinated Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Senior Subordinated Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Senior Subordinated Notes and the Issuer must specify whether such Senior Subordinated Notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuer has received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the Senior Subordinated Notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Senior Subordinated Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the Senior Subordinated Notes will not recognize income, gain or loss for
146
U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make such deposit and the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the Indenture) to which, the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound;
(6) the Issuer shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuer shall have delivered to the Trustee an Officer's Certificate stating that the deposit was not made by the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or any Guarantor or others; and
(8) the Issuer shall have delivered to the Trustee an Officer's Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Satisfaction and Discharge
The Indenture will be discharged and will cease to be of further effect as to all Senior Subordinated Notes, when either:
(1) all Senior Subordinated Notes theretofore authenticated and delivered, except lost, stolen or destroyed Senior Subordinated Notes which have been replaced or paid and Senior Subordinated Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
(2) (a) all Senior Subordinated Notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Issuer and the Issuer or any Guarantor have irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the Senior Subordinated Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the Senior Subordinated Notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption;
(b) no Default (other than that resulting from borrowing funds to be applied to make such deposit) with respect to the Indenture or the Senior Subordinated Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the Indenture) to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound;
(c) the Issuer has paid or caused to be paid all sums payable by it under the Indenture; and
147
(d) the Issuer has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the Senior Subordinated Notes at maturity or the redemption date, as the case may be.
In addition, the Issuer must deliver an Officer's Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
Except as provided in the next two succeeding paragraphs, the Indenture, any Guarantee and the Senior Subordinated Notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the Senior Subordinated Notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Senior Subordinated Notes, and any existing Default or compliance with any provision of the Indenture or the Senior Subordinated Notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding Senior Subordinated Notes, other than Senior Subordinated Notes beneficially owned by the Issuer or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Senior Subordinated Notes).
The Indenture provides that, without the consent of each affected Holder of Senior Subordinated Notes, an amendment or waiver may not, with respect to any Senior Subordinated Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Senior Subordinated Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Senior Subordinated Note or alter or waive the provisions with respect to the redemption of such Senior Subordinated Notes (other than provisions relating to the covenants described above under the caption "Repurchase at the Option of Holders");
(3) reduce the rate of or change the time for payment of interest on any Senior Subordinated Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the Senior Subordinated Notes, except a rescission of acceleration of the Senior Subordinated Notes by the Holders of at least a majority in aggregate principal amount of the Senior Subordinated Notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the Indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any Senior Subordinated Note payable in money other than that stated therein;
(6) make any change in the provisions of the Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest on the Senior Subordinated Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or interest on such Holder's Senior Subordinated Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder's Senior Subordinated Notes;
(9) make any change in the subordination provisions thereof that would adversely affect the Holders; or
(10) except as expressly permitted by the Indenture, modify the Guarantees of any Significant Subsidiary in any manner adverse to the Holders of the Senior Subordinated Notes.
148
Notwithstanding the foregoing, the Issuer, any Guarantor (with respect to a Guarantee or the Indenture to which it is a party) and the Trustee may amend or supplement the Indenture and any Guarantee or Senior Subordinated Notes without the consent of any Holder;
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Senior Subordinated Notes in addition to or in place of certificated Senior Subordinated Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide the assumption of the Issuer's or any Guarantor's obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the Indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuer or any Guarantor;
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the Indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to add a Guarantor under the Indenture;
(11) to conform the text of the Indenture, Guarantees or the Senior Subordinated Notes to any provision of this "Description of the Exchange Notes" to the extent that such provision in this "Description of the Exchange Notes" was intended to be a verbatim recitation of a provision of the Indenture, Guarantee or Senior Subordinated Notes; or
(12) making any amendment to the provisions of the Indenture relating to the transfer and legending of Senior Subordinated Notes as permitted by the Indenture, including, without limitation to facilitate the issuance and administration of the Senior Subordinated Notes;provided,however, that (i) compliance with the Indenture as so amended would not result in Senior Subordinated Notes being transferred in violation of the Securities Act or any applicable securities law and (ii) such amendment does not materially and adversely affect the rights of Holders to transfer Senior Subordinated Notes.
The consent of the Holders is not necessary under the Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication will be deemed given on the first date on which publication is made and notices given by first- class mail, postage prepaid, will be deemed given five calendar days after mailing.
Concerning the Trustee
The Indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Issuer, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee will be permitted to
149
engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The Indenture provides that the Holders of a majority in principal amount of the outstanding Senior Subordinated Notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee will be required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee will be under no obligation to exercise any of its rights or powers under the Indenture at the request of any Holder of the Senior Subordinated Notes, unless such Holder shall have offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Governing Law
The Indenture, the Senior Subordinated Notes and any Guarantee is governed by and construed in accordance with the laws of the State of New York.
Certain Definitions
Set forth below are certain defined terms used in the Indenture. For purposes of the Indenture, unless otherwise specifically indicated, the term "consolidated" with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
"Acquired Indebtedness" means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
"Acquisition" means the transactions contemplated by the Transaction Agreement.
"Additional Interest" means all additional interest then owing pursuant to the Registration Rights Agreement.
"Affiliate" of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, "control" (including, with correlative meanings, the terms "controlling," "controlled by" and "under common control with"), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
"Applicable Premium" means, with respect to any Senior Subordinated Note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Senior Subordinated Note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Senior Subordinated Note at December 1, 2009 (each such redemption price being set forth in the table appearing above under the caption "Optional Redemption"), plus (ii) all required interest payments due on such Senior Subordinated Note through December 1, 2009 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury
150
Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such Senior Subordinated Note.
"Applicable Senior Leverage Ratio" means (1) for any date prior to December 1, 2007, 4.0 to 1.0, and (2) for any date on or after December 1, 2007, 3.75 to 1.0.
"Asset Sale" means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions, of property or assets (including by way of a Sale and Lease-Back Transaction) of the Issuer or any of its Restricted Subsidiaries (each referred to in this definition as a "disposition"); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary, whether in a single transaction or a series of related transactions;
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Issuer in a manner permitted pursuant to the provisions described above under "Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets" or any disposition that constitutes a Change of Control pursuant to the Indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under "Certain Covenants—Limitation on Restricted Payments";
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of transactions with an aggregate fair market value of less than $10.0 million;
(e) any disposition of property or assets or issuance of securities by a Restricted Subsidiary of the Issuer to the Issuer or by the Issuer or a Restricted Subsidiary of the Issuer to another Restricted Subsidiary of the Issuer;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures on assets;
(j) sales of accounts receivable, or participations therein, in connection with any Receivables Facility; and
(k) any financing transaction with respect to property built or acquired by the Issuer or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Indenture.
"beneficial ownership" has the meaning assigned to such term in Rule 13d-3 and Rule 13d-5 under the Exchange Act, except that in calculating the beneficial ownership of any particular "person" (as
151
such term is used in Section 13(d)(3) of the Exchange Act), such "person" shall be deemed to have beneficial ownership of all securities that such "person" has the right to acquire, whether such right is currently exercisable or is exercisable only upon the occurrence of a subsequent condition.
"Business Day" means each day which is not a Legal Holiday.
"Campbell Acquisition" means the acquisition of Campbell Engineering, Inc. on September 12, 2005.
"Capital Stock" means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person.
"Capitalized Lease Obligation" means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) in accordance with GAAP.
"Cash Equivalents" means:
(1) United States dollars;
(2) (a) euro, or any national currency of any participating member state of the EMU; or
(b) in the case of any Foreign Subsidiary that is a Restricted Subsidiary, such local currencies held by them from time to time in the ordinary course of business;
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of such government with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of one year or less from the date of acquisition, bankers' acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3) and (4) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-1 by Moody's or at least A-1 by S&P and in each case maturing within 24 months after the date of creation thereof;
(7) marketable short-term money market and similar securities having a rating of at least P-2 or A-2 from either Moody's or S&P, respectively (or, if at any time neither Moody's nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof;
(8) investment funds investing 95% of their assets in securities of the types described in clauses (1) through (7) above;
152
(9) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody's or S&P with maturities of 24 months or less from the date of acquisition;
(10) Indebtedness or Preferred Stock issued by Persons with a rating of "A" or higher from S&P or "A2" or higher from Moody's with maturities of 24 months or less from the date of acquisition and;
(11) Investments with average maturities of 12 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody's.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above,provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
"Change of Control" means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Issuer becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than the Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership of 50% or more of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies holding directly or indirectly 100% of the total voting power of the Voting Stock of the Issuer.
"Consolidated Depreciation and Amortization Expense" means with respect to any Person for any period, the total amount of depreciation and amortization expense, including the amortization of deferred financing fees of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
"Consolidated Interest Expense" means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations, and (e) net payments, if any, pursuant to interest rate Hedging Obligations with respect to Indebtedness, and excluding (w) any Additional Interest, (x) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (y) any expensing of bridge, commitment and other financing fees and (z) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Receivables Facility); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
153
(3) interest income for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
"Consolidated Net Income" means, with respect to any Person for any period, the aggregate of the Net Income, of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP;provided,however, that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the Transaction), severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans shall be excluded,
(2) the Net Income for such period shall not include the cumulative effect of a change in accounting principles during such period,
(3) any after-tax effect of income (loss) from disposed or discontinued operations and any net after-tax gains or losses on disposal of disposed, abandoned or discontinued operations shall be excluded,
(4) any after-tax effect of gains or losses (less all fees and expenses relating thereto) attributable to asset dispositions other than in the ordinary course of business, as determined in good faith by the Issuer, shall be excluded,
(5) the Net Income for such period of any Person that is not a Subsidiary, or is an Unrestricted Subsidiary, or that is accounted for by the equity method of accounting, shall be excluded;provided that Consolidated Net Income of the Issuer shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the referent Person or a Restricted Subsidiary thereof in respect of such period,
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of "Certain Covenants—Limitation on Restricted Payments," the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded if the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination wholly permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived,provided that Consolidated Net Income of the Issuer will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein,
(7) any impairment charge or asset write-off pursuant to Financial Accounting Standards Board Statement No. 142 and No. 144 and the amortization of intangibles arising pursuant to No. 141 shall be excluded,
(8) effects of adjustments in any line item in such Person's consolidated financial statements required or permitted by the Financial Accounting Standards Board Statement Nos. 141 and 142 resulting from the application of purchase accounting in relation to the Transaction, the Campbell Acquisition and the MTG Acquisition or any acquisition that is consummated after the Issue Date, net of taxes, shall be excluded,
154
(9) any after-tax effect of income (loss) from the early extinguishment of Indebtedness or Hedging Obligations or other derivative instruments shall be excluded, and
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights shall be excluded.
Notwithstanding the foregoing, for the purpose of the covenant described under "Certain Covenants—Limitation on Restricted Payments" only (other than clause (3)(d) thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Issuer and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Issuer and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Issuer or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
"Contingent Obligations" means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness ("primary obligations") of any other Person (the "primary obligor") in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor,
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor, or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
"Credit Facilities" means, with respect to the Issuer or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities, or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock") or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
"Default" means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
"Designated Non-cash Consideration" means the fair market value of non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer's Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Issuer, less the amount of cash or Cash
155
Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
"Designated Preferred Stock" means Preferred Stock of the Issuer or any parent corporation thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Issuer or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer's Certificate executed by the principal financial officer of the Issuer or the applicable parent corporation thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of the "Certain Covenants—Limitation on Restricted Payments" covenant.
"Designated Senior Indebtedness" means:
(1) any Indebtedness outstanding under the Senior Credit Facilities; and
(2) any other Senior Indebtedness permitted under the Indenture, the principal amount of which is $50.0 million or more and that has been designated by the Issuer as "Designated Senior Indebtedness."
"Disqualified Stock" means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the maturity date of the Senior Subordinated Notes or the date the Senior Subordinated Notes are no longer outstanding;provided,however, that if such Capital Stock is issued to any plan for the benefit of employees of the Issuer or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations.
"Domestic Subsidiary" means, with respect to any Person, any Restricted Subsidiary of such Person other than a Foreign Subsidiary.
"EBITDA" means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by:
(a) provision for taxes based on income or profits or capital, including, without limitation, state, franchise and similar taxes (such as the Pennsylvania capital tax) and foreign withholding taxes of such Person paid or accrued during such period deducted (and not added back) in computing Consolidated Net Income;plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk and (y) costs of surety bonds in connection with financing activities, in each case, to the extent included in Fixed Charges) to the extent the same was deducted (and not added back) in calculating such Consolidated Net Income;plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period to the extent the same were deducted (and not added back) in computing Consolidated Net Income;plus
156
(d) any expenses or charges (other than depreciation or amortization expense) related to any Equity Offering, Permitted Investment, acquisition, disposition, recapitalization or the incurrence of Indebtedness permitted to be incurred by the Indenture (including a refinancing thereof) (whether or not successful), including (i) such fees, expenses or charges related to the offering of the Senior Subordinated Notes and the Credit Facilities and (ii) any amendment or other modification of the Senior Subordinated Notes, and, in each case, deducted (and not added back) in computing Consolidated Net Income;plus
(e) the amount of any restructuring charge or reserve deducted (and not added back) in such period in computing Consolidated Net Income, including any one-time costs incurred in connection with acquisitions after the Issue Date and costs related to the closure and/or consolidation of facilities;plus
(f) any other non-cash charges, including any write offs or write downs, reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period);plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly Owned Subsidiary deducted (and not added back) in such period in calculating Consolidated Net Income;plus
(h) the amount of management, monitoring, consulting and advisory fees and related expenses paid in such period to the Investors to the extent otherwise permitted under "Certain Covenants—Transactions with Affiliates";plus
(i) the amount of loss on sale of receivables and related assets to the Receivables Subsidiary in connection with a Receivables Facility;plus
(j) any costs or expense incurred by the Issuer or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Issuer or net cash proceeds of an issuance of Equity Interest of the Issuer (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under "Certain Covenants—Limitation on Restricted Payments";
(2) decreased by (without duplication) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period, and
(3) increased or decreased by (without duplication):
(a) any net gain or loss resulting in such period from Hedging Obligations and the application of Statement of Financial Accounting Standards No. 133;plus orminus, as applicable,
(b) any net gain or loss resulting in such period from currency translation gains or losses related to currency remeasurements of Indebtedness (including any net loss or gain resulting from hedge agreements for currency exchange risk), plus or minus, as applicable
(c) without duplication, the Historical Adjustments incurred in such period.
"EMU" means economic and monetary union as contemplated in the Treaty on European Union.
"Equity Interests" means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
157
"Equity Offering" means any public or private sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer's or any direct or indirect parent company's common stock registered on Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private sale that constitutes an Excluded Contribution.
"euro" means the single currency of participating member states of the EMU.
"Exchange Act" means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
"Excluded Contribution" means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from
(1) contributions to its common equity capital, and
(2) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of the Issuer) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Issuer,
in each case designated as Excluded Contributions pursuant to an officer's certificate executed by the principal financial officer of the Issuer on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under "Certain Covenants—Limitation on Restricted Payments."
"Fixed Charge Coverage Ratio" means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Issuer or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the "Fixed Charge Coverage Ratio Calculation Date"), then the Fixed Charge Coverage Ratio shall be calculated givingpro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (as determined in accordance with GAAP) that have been made by the Issuer or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on apro forma basis assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or disposed operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated givingpro forma effect thereto for such period as if such Investment, acquisition, disposition, merger,
158
consolidation or disposed operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, wheneverpro forma effect is to be given to a transaction, thepro formacalculations shall be made in good faith by the chief financial officer of the Issuer. If any Indebtedness bears a floating rate of interest and is being givenpro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Issuer to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on apro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
"Fixed Charges" means, with respect to any Person for any period, the sum of:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock during such period; and
(3) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
"Foreign Subsidiary" means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof and any Restricted Subsidiary of such Foreign Subsidiary.
"GAAP" means generally accepted accounting principles in the United States which are in effect on the Issue Date.
"Government Securities" means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt;provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
"guarantee" means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of
159
credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
"Guarantee" means the guarantee by any Guarantor of the Issuer's Obligations under the Indenture.
"Guarantor" means, each Restricted Subsidiary that Guarantees the Senior Subordinated Notes in accordance with the terms of the Indenture.
"Hedging Obligations" means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
"Historical Adjustments" means, with respect to the Issuer and its Restricted Subsidiaries, without duplication, the following items to the extent incurred prior to the Issue Date and disclosed in the adjustments to "Adjusted EBITDA" in the Offering Circular:
(1) restructuring costs associated with the acquisition of MedSource;
(2) non-cash charges for stock-based compensation;
(3) executive severance costs due to contractual severance obligations;
(4) executive relocation costs associated with the creation of new divisional management offices;
(5) write-off of the step-up of inventory acquired from MedSource;
(6) write-downs of receivables and inventory acquired from MedSource and Campbell;
(7) losses associated with closed facilities in connection with the acquisition of MedSource;
(8) gain on the sale of a manufacturing facility;
(9) management fees paid to KRG Capital Partners, LLC and DLJ Merchant Banking;
(10) prepayment penalty relating to Accellent Corp.'s 2004 debt refinancing; and
(11) costs related to the Transaction.
"Holder" means the Person in whose name a Senior Subordinated Note is registered on the registrar's books.
"Indebtedness" means, with respect to any Person, without duplication:
(1) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers' acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations), except (i) any such balance that constitutes a trade payable or similar obligation to a trade or similar creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP; or
(d) representing any Hedging Obligations;
160
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided,however, that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Receivables Facilities.
"Independent Financial Advisor" means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Issuer, qualified to perform the task for which it has been engaged.
"Initial Purchasers" means Credit Suisse First Boston LLC, J.P. Morgan Securities Inc., and
Bear, Stearns & Co. Inc.
"Investment Grade Rating" means a rating equal to or higher than Baa3 (or the equivalent) by Moody's and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
"Investment Grade Securities" means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Issuer and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
"Investments" means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers, commission, travel and similar advances to officers and employees, in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Issuer in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of "Unrestricted Subsidiary" and the covenant described under "Certain Covenants—Limitation on Restricted Payments":
(1) "Investments" shall include the portion (proportionate to the Issuer's equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Issuer at the time that such Subsidiary is designated an Unrestricted Subsidiary;provided,however, that upon a redesignation of
161
such Subsidiary as a Restricted Subsidiary, the Issuer shall be deemed to continue to have a permanent "Investment" in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Issuer "Investment" in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Issuer equity interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer, in each case as determined in good faith by the Issuer.
"Investors" means Kohlberg Kravis Roberts & Co. L.P. and Bain Capital and their respective Affiliates but not including, however, any portfolio companies of any of the foregoing.
"Issue Date" means November 22, 2005.
"Issuer" has the meaning set forth in the first paragraph under "General".
"Legal Holiday" means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York.
"Lien" means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction;provided that in no event shall an operating lease be deemed to constitute a Lien.
"Moody's" means Moody's Investors Service, Inc. and any successor to its rating agency business.
"MTG Acquisition" means the acquisition of Machining Technology Group, LLC on October 6, 2005.
"Net Income" means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
"Net Proceeds" means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of "Repurchase at the Option of Holders—Asset Sales") to be paid as a result of such transaction and any deduction of appropriate amounts to be provided by the Issuer or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
"Obligations" means any principal, interest (including any interest accruing subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), penalties, fees, indemnifications, reimbursements (including
162
reimbursement obligations with respect to letters of credit and banker's acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
"Officer" means the Chairman of the Board, the Chief Executive Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Issuer.
"Officer's Certificate" means a certificate signed on behalf of the Issuer by an Officer of the Issuer, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of the Issuer, that meets the requirements set forth in the Indenture.
"Opinion of Counsel" means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer or the Trustee.
"Permitted Asset Swap" means the concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and cash or Cash Equivalents between the Issuer or any of its Restricted Subsidiaries and another Person;provided, that any cash or Cash Equivalents received must be applied in accordance with the "Repurchase at the Option of Holders—Asset Sales" covenant.
"Permitted Holders" means each of the Investors and members of management of the Issuer (or its direct parent) who are holders of Equity Interests of the Issuer (or any of its direct or indirect parent companies) on the Issue Date and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members;provided, that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors and members of management, collectively, have beneficial ownership of more than 50% of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies.
"Permitted Investments" means:
(1) any Investment in the Issuer or any of its Restricted Subsidiaries;
(2) any Investment in cash and Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Issuer or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Issuer or a Restricted Subsidiary,
and, in each case, any Investment held by such Person;provided, that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets not constituting cash, Cash Equivalents or Investment Grade Securities and received in connection with an Asset Sale made pursuant to the provisions of "Repurchase at the Option of Holders—Asset Sales" or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date, including any earn-out obligation in respect of the Campbell Acquisition and the MTG Acquisition (as in effect on the Issue Date);
163
(6) any Investment acquired by the Issuer or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Issuer or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable; or
(b) as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock"
(8) any Investment in a Similar Business having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed the greater of (x) $20.0 million and (y) 1.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(9) Investments the payment for which consists of Equity Interests (exclusive of Disqualified Stock) of the Issuer, or any of its direct or indirect parent companies;provided,however, that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in "Certain Covenants—Limitations on Restricted Payments";
(10) guarantees of Indebtedness permitted under the covenant described in "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock";
(11) any transaction to the extent it constitutes an Investment that is permitted and made in accordance with the provisions of the second paragraph of the covenant described under "Certain Covenants—Transactions with Affiliates" (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment;
(13) additional Investments having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (x) $20.0 million and (y) 1.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(14) Investments relating to a Receivables Subsidiary that, in the good faith determination of the Issuer are necessary or advisable to effect any Receivables Facility;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $10.0 million outstanding at any one time, in the aggregate; and
(16) loans and advances to officers, directors and employees for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person's purchase of Equity Interests of the Issuer or any direct or indirect parent company thereof.
"Permitted Junior Securities" means:
(1) Equity Interests in the Issuer, any Guarantor or any direct or indirect parent of the Issuer; or
164
(2) unsecured debt securities that are subordinated to all Senior Indebtedness (and any debt securities issued in exchange for Senior Indebtedness) to substantially the same extent as, or to a greater extent than, the Senior Subordinated Notes and the related Guarantees are subordinated to Senior Indebtedness under the Indenture;
provided that the term "Permitted Junior Securities" shall not include any securities distributed pursuant to a plan of reorganization if the Indebtedness under the Senior Credit Facilities is treated as part of the same class as the Senior Subordinated Notes for purposes of such plan of reorganization;provided further that to the extent that any Senior Indebtedness of the Issuer or the Guarantors outstanding on the date of consummation of any such plan of reorganization is not paid in full in cash on such date, the holders of any such Senior Indebtedness not so paid in full in cash have consented to the terms of such plan of reorganization.
"Permitted Liens" means, with respect to any Person:
(1) pledges or deposits by such Person under workmen's compensation laws, unemployment insurance laws or similar legislation, or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers', warehousemen's and mechanics' Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4), (12)(b), (18) or (19) of the second paragraph under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock";provided that Liens securing Indebtedness permitted to be incurred pursuant to clause (18) extend only to the assets of Foreign Subsidiaries and Liens securing Indebtedness permitted to be incurred pursuant to clause (19) are solely on acquired property or the assets of the acquired entity, as the case may be;
(7) Liens existing on the Issue Date;
165
(8) Liens on property or shares of stock of a Person at the time such Person becomes a Subsidiary;provided,however, such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary;provided,further,however, that such Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(9) Liens on property at the time the Issuer or a Restricted Subsidiary acquired the property, including any acquisition by means of a merger or consolidation with or into the Issuer or any of its Restricted Subsidiaries;provided,however, that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition;provided,further,however, that the Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Issuer or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock";
(11) Liens securing Hedging Obligations so long as related Indebtedness is, and is permitted to be under the Indenture, secured by a Lien on the same property securing such Hedging Obligations;
(12) Liens on specific items of inventory of other goods and proceeds of any Person securing such Person's obligations in respect of bankers' acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Issuer or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Issuer and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Issuer or any Guarantor;
(16) Liens on equipment of the Issuer or any of its Restricted Subsidiaries granted in the ordinary course of business to the Issuer's clients;
(17) Liens on accounts receivable and related assets incurred in connection with a Receivables Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (6), (7), (8) and (9);provided,however, that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (6), (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations incurred in the ordinary course of business which obligations do not exceed $15.0 million at any one time outstanding;
(21) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under the caption "Events of Default and Remedies" so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the
166
review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(22) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(23) Liens (i) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection, (ii) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (iii) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens deemed to exist in connection with Investments in repurchase agreements permitted under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock";provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(25) Liens encumbering reasonable customary initial deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes; and
(26) Liens that are contractual rights of set-off (i) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (ii) relating to pooled deposit or sweep accounts of the Issuer or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries or (iii) relating to purchase orders and other agreements entered into with customers of the Issuer or any of its Restricted Subsidiaries in the ordinary course of business.
For purposes of this definition, the term "Indebtedness" shall be deemed to include interest on such Indebtedness.
"Person" means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
"Preferred Stock" means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
"Public Equity Offering" means any public sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer's or any direct or indirect parent company's common stock registered on Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public sale that constitutes an Excluded Contribution.
"Qualified Proceeds" means assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business;provided that the fair market value of any such assets or Capital Stock shall be determined by the Issuer in good faith.
"Rating Agencies" means Moody's and S&P or if Moody's or S&P or both shall not make a rating on the Senior Subordinated Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuer which shall be substituted for Moody's or S&P or both, as the case may be.
167
"Receivables Facility" means any of one or more receivables financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Issuer or any of its Restricted Subsidiaries (other than a Receivables Subsidiary) pursuant to which the Issuer or any of its Restricted Subsidiaries sells its accounts receivable to either (a) a Person that is not a Restricted Subsidiary or (b) a Receivables Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
"Receivables Fees" means distributions or payments made directly or by means of discounts with respect to any accounts receivable or participation interest therein issued or sold in connection with, and other fees paid to a Person that is not a Restricted Subsidiary in connection with, any Receivables Facility.
"Receivables Subsidiary" means any Subsidiary formed for the purpose of, and that solely engages only in one or more Receivables Facilities and other activities reasonably related thereto.
"Registration Rights Agreement" means the Registration Rights Agreement with respect to the Senior Subordinated Notes dated as of the Issue Date, among the Company, the Guarantors and the Initial Purchasers.
"Related Business Assets" means assets (other than cash or Cash Equivalents) used or useful in a Similar Business,provided that any assets received by the Issuer or a Restricted Subsidiary in exchange for assets transferred by the Issuer or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
"Representative" means any trustee, agent or representative (if any) for an issue of Senior Indebtedness of the Issuer.
"Restricted Investment" means an Investment other than a Permitted Investment.
"Restricted Subsidiary" means, at any time, any direct or indirect Subsidiary of the Issuer (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary;provided,however, that upon the occurrence of an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of "Restricted Subsidiary."
"S&P" means Standard & Poor's, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
"Sale and Lease-Back Transaction" means any arrangement providing for the leasing by the Issuer or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Issuer or such Restricted Subsidiary to a third Person in contemplation of such leasing.
"SEC" means the U.S. Securities and Exchange Commission.
"Secured Indebtedness" means any Indebtedness of the Issuer or any of its Restricted Subsidiaries secured by a Lien.
"Securities Act" means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
"Senior Credit Facilities" means the Credit Facility under the Credit Agreement entered into as of the Issue Date by and among the Issuer, the lenders party thereto in their capacities as lenders thereunder, JPMorgan Chase Bank, N.A., as Administrative Agent, and Credit Suisse First Boston, as Syndication Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions,
168
renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock" above).
"Senior Indebtedness" means:
(1) all Indebtedness of the Issuer or any Guarantor outstanding under the Senior Credit Facilities and related Guarantees (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuer or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuer or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into),provided that such Hedging Obligations are permitted to be incurred under the terms of the Indenture;
(3) any other Indebtedness of the Issuer or any Guarantor permitted to be incurred under the terms of the Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is on a parity with or subordinated in right of payment to the Senior Subordinated Notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3);
provided,however, that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuer or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Indenture;provided,however that such Indebtedness shall be deemed not to have been incurred in violation of the Indenture for purposes of this clause if such Indebtedness consists of Designated Senior Indebtedness, and the holder(s) of such Indebtedness of their agent or representative (a) had no actual knowledge at the time of incurrence that the incurrence of such Indebtedness violated the Indenture and (b) shall have receive a certificate from an officer of the Issuer to the effect that the incurrence of such Indebtedness does not violate the provisions of the Indenture.
"Senior Leverage Ratio" means, with respect to the Issuer as of any date of determination (the "Determination Date"), the ratio of (a) the sum of (1) the aggregate amount of Senior Indebtedness of the Issuer and the Guarantors on the Determination Date, plus (2) the aggregate amount of
169
Indebtedness, Disqualified Stock and Preferred Stock of all Restricted Subsidiaries of the Issuer that are not Guarantors on the Determination Date, to (b) EBITDA of the Issuer and its Restricted Subsidiaries on a consolidated basis for the most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the Determination Date.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (as determined in accordance with GAAP) that have been made by the Issuer or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to the Determination Date shall be calculated on a pro forma basis assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or disposed operation that would have required adjustment pursuant to this definition, then the Senior Leverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or disposed operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to a transaction, the pro forma calculations shall be made in good faith by the chief financial officer of the Issuer.
"Senior Subordinated Indebtedness" means:
(1) with respect to the Issuer, Indebtedness which ranks equal in right of payment to the Senior Subordinated Notes issued by the Issuer; and
(2) with respect to any Guarantor, Indebtedness which ranks equal in right of payment to the Guarantee of such entity of Senior Subordinated Notes.
"Significant Subsidiary" means any Restricted Subsidiary that would be a "significant subsidiary" as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
"Similar Business" means any business conducted or proposed to be conducted by the Issuer and its Restricted Subsidiaries on the Issue Date or any business that is similar, reasonably related, incidental or ancillary thereto.
"Subordinated Indebtedness" means, with respect to the Senior Subordinated Notes,
(1) any Indebtedness of the Issuer which is by its terms subordinated in right of payment to the Senior Subordinated Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Senior Subordinated Notes.
"Subsidiary" means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
170
- (x)
- more than 50% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
- (y)
- such Person or any Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
"Total Assets" means the total assets of the Issuer and its Restricted Subsidiaries on a consolidated basis, as shown on the most recent balance sheet of the Issuer or such other Person as may be expressly stated.
"Transaction" means the transactions contemplated by the Transaction Agreement, the issuance of the Senior Subordinated Notes and borrowings under the Senior Credit Facilities as in effect on the Issue Date.
"Transaction Agreement" means the Agreement and Plan of Merger, dated as of October 7, 2005 between Accellent Inc. and Accellent Acquisition Corp. as the same may be amended prior to the Issue Date.
"Treasury Rate" means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to December 1, 2009;provided,however, that if the period from the Redemption Date to December 1, 2009 is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
"Trust Indenture Act" means the Trust Indenture Act of 1939, as amended (15 U.S.C §§ 77aaa-777bbbb).
"Unrestricted Subsidiary" means:
(1) any Subsidiary of the Issuer which at the time of determination is an Unrestricted Subsidiary (as designated by the Issuer, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated);provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Issuer;
(2) such designation complies with the covenants described under "Certain Covenants—Limitation on Restricted Payments"; and
(3) each of:
171
(b) its Subsidiaries
has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Issuer or any Restricted Subsidiary.
The Issuer may designate any Unrestricted Subsidiary to be a Restricted Subsidiary;provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Issuer could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test described in the first paragraph under "Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock"; or
(2) the Fixed Charge Coverage Ratio for the Issuer its Restricted Subsidiaries would be greater than such ratio for the Issuer and its Restricted Subsidiaries immediately prior to such designation,
in each case on apro forma basis taking into account such designation.
Any such designation by the Issuer shall be notified by the Issuer to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Issuer or any committee thereof giving effect to such designation and an Officer's Certificate certifying that such designation complied with the foregoing provisions.
"Voting Stock" of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
"Weighted Average Life to Maturity" means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
"Wholly-Owned Subsidiary" of any Person means a Subsidiary of such Person, 100% of the outstanding Equity Interests of which (other than directors' qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
172
BOOK-ENTRY; DELIVERY AND FORM
Except as set forth below, the exchange notes will be issued in registered, global form in minimum denominations of $2,000 and integral multiples of $1,000 in excess of $2,000.
The exchange notes will be represented by one or more notes in registered, global form without interest coupons (collectively, the "Global Exchange Notes"). The Global Exchange Notes will be deposited upon issuance with the trustee as custodian for The Depository Trust Company ("DTC"), in New York, New York, and registered in the name of DTC or its nominee, in each case for credit to an account of a direct or indirect participant in DTC as described below.
Except as set forth below, the Global Exchange Notes may be transferred, in whole and not in part, only to another nominee of DTC or to a successor of DTC or its nominee. Beneficial interests in the Global Exchange Notes may not be exchanged for notes in certificated form except in the limited circumstances described below. See "—Exchange of Global Exchange Notes for Certificated Notes." Except in the limited circumstances described below, owners of beneficial interests in the Global Exchange Notes will not be entitled to receive physical delivery of notes in certificated form.
Depository Procedures
The following description of the operations and procedures of DTC, Euroclear and Clearstream are provided solely as a matter of convenience. These operations and procedures are solely within the control of the respective settlement systems and are subject to changes by them. We take no responsibility for these operations and procedures and urge investors to contact the system or their participants directly to discuss these matters.
DTC has advised us that DTC is a limited-purpose trust company created to hold securities for its participating organizations (collectively, the "Participants") and to facilitate the clearance and settlement of transactions in those securities between the Participants through electronic book-entry changes in accounts of its Participants. The Participants include securities brokers and dealers (including the Initial Purchasers), banks, trust companies, clearing corporations and certain other organizations. Access to DTC's system is also available to other entities such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a Participant, either directly or indirectly (collectively, the "Indirect Participants"). Persons who are not Participants may beneficially own securities held by or on behalf of DTC only through the Participants or the Indirect Participants. The ownership interests in, and transfers of ownership interests in, each security held by or on behalf of DTC are recorded on the records of the Participants and Indirect Participants.
DTC has also advised us that, pursuant to procedures established by it:
(1) upon deposit of the Global Exchange Notes, DTC will credit the accounts of the Participants designated by the Initial Purchasers with portions of the principal amount of the Global Exchange Notes; and
(2) ownership of these interests in the Global Exchange Notes will be shown on, and the transfer of ownership of these interests will be effected only through, records maintained by DTC (with respect to the Participants) or by the Participants and the Indirect Participants (with respect to other owners of beneficial interest in the Global Exchange Notes).
Investors in the Global Exchange Notes who are Participants may hold their interests therein directly through DTC. All interests in a Global Exchange Note, including those held through Euroclear or Clearstream, may be subject to the procedures and requirements of DTC. Those interests held through Euroclear or Clearstream may also be subject to the procedures and requirements of such systems. The laws of some states require that certain Persons take physical delivery in definitive form of securities that they own. Consequently, the ability to transfer beneficial interests in a Global Exchange
173
Note to such Persons will be limited to that extent. Because DTC can act only on behalf of the Participants, which in turn act on behalf of the Indirect Participants, the ability of a Person having beneficial interests in a Global Exchange Note to pledge such interests to Persons that do not participate in the DTC system, or otherwise take actions in respect of such interests, may be affected by the lack of a physical certificate evidencing such interests.
Except as described below, owners of interest in the Global Exchange Notes will not have notes registered in their names, will not receive physical delivery of notes in certificated form and will not be considered the registered owners or "Holders" thereof under the indenture for any purpose.
Payments in respect of the principal of, and interest and premium, if any, and additional interest, if any, on a Global Exchange Note registered in the name of DTC or its nominee will be payable to DTC in its capacity as the registered holder under the indenture. Under the terms of the indenture, we and the trustee will treat the Persons in whose names the notes, including the Global Exchange Notes, are registered as the owners of the notes for the purpose of receiving payments and for all other purposes. Consequently, neither we, the trustee nor any of our agents or any agent of the trustee has or will have any responsibility or liability for:
(1) any aspect of DTC's records or any Participant's or Indirect Participant's records relating to or payments made on account of beneficial ownership interest in the Global Exchange Notes or for maintaining, supervising or reviewing any of DTC's records or any Participant's or Indirect Participant's records relating to the beneficial ownership interests in the Global Exchange Notes; or
(2) any other matter relating to the actions and practices of DTC or any of its Participants or Indirect Participants.
DTC has advised us that our current practice, upon receipt of any payment in respect of securities such as the notes (including principal and interest), is to credit the accounts of the relevant Participants with the payment on the payment date unless DTC has reason to believe it will not receive payment on such payment date. Each relevant Participant is credited with an amount proportionate to its beneficial ownership of an interest in the principal amount of the relevant security as shown on the records of DTC. Payments by the Participants and the Indirect Participants to the beneficial owners of notes will be governed by standing instructions and customary practices and will be the responsibility of the Participants or the Indirect Participants and will not be the responsibility of DTC, the trustee or us. Neither we nor the trustee will be liable for any delay by DTC or any of the Participants or the Indirect Participants in identifying the beneficial owners of the notes, and the Company and the trustee may conclusively rely on and will be protected in relying on instructions from DTC or its nominee for all purposes.
Transfers between the Participants will be effected in accordance with DTC's procedures, and will be settled in same-day funds, and transfers between participants in Euroclear and Clearstream will be effected in accordance with their respective rules and operating procedures.
DTC has advised us that it will take any action permitted to be taken by a holder of notes only at the direction of one or more Participants to whose account DTC has credited the interests in the Global Notes and only in respect of such portion of the aggregate principal amount of the notes as to which such Participant or Participants has or have given such direction. However, if there is an Event of Default under the notes, DTC reserves the right to exchange the Global Exchange Notes for legended notes in certificated form, and to distribute such notes to its Participants.
Although DTC has agreed to the foregoing procedures to facilitate transfers of interests in the Global Exchange Notes among participants in DTC it is under no obligation to perform or to continue to perform such procedures, and may discontinue such procedures at any time. Neither we nor the trustee nor any of their respective agents will have any responsibility for the performance by DTC or its
174
respective participants or indirect participants of their respective obligations under the rules and procedures governing their operations.
Exchange of Global Exchange Notes for Certificated Notes
A Global Exchange Note is exchangeable for definitive notes in registered certificated form ("Certificated Notes") if:
(1) DTC (a) notifies us that it is unwilling or unable to continue as depositary for the Global Exchange Notes or (b) has ceased to be a clearing agency registered under the Exchange Act and, in either case, we fail to appoint a successor depositary;
(2) Subject to the procedures of DTC, we, at our option, notify the trustee in writing that we elect to cause the issuance of the Certificated Notes; or
(3) there has occurred and is continuing an Event of Default with respect to the notes.
In addition, beneficial interests in a Global Exchange Note may be exchanged for Certificated Notes upon prior written notice given to the trustee by or on behalf of DTC in accordance with the indenture. In all cases, Certificated Notes delivered in exchange for any Global Exchange Note or beneficial interests in Global Exchange Notes will be registered in the names, and issued in any approved denominations, requested by or on behalf of the depositary (in accordance with its customary procedures).
Exchange of Certificated Notes for Global Notes
Certificated Notes may not be exchanged for beneficial interests in any Global Exchange Note unless the transferor first delivers to the trustee a written certificate (in the form provided in the indenture) to the effect that such transfer will comply with the appropriate transfer restrictions applicable to such notes.
Same Day Settlement and Payment
We will make payments in respect of the notes represented by the Global Exchange Notes (including principal, premium, if any, interest and additional interest, if any) by wire transfer of immediately available funds to the accounts specified by the Global Exchange Note holder. We will make all payments of principal, interest and premium, if any, and additional interest, if any, with respect to Certificated Notes by wire transfer of immediately available funds to the accounts specified by the holders of the Certificated Notes or, if no such account is specified, by mailing a check to each such holder's registered address. The notes represented by the Global Exchange Notes are expected to be eligible to trade in The PORTAL Market and to trade in DTC's Same-Day Funds Settlement System, and any permitted secondary market trading activity in such notes will, therefore, be required by DTC to be settled in immediately available funds. We expect that secondary trading in any Certificated Notes will also be settled in immediately available funds.
175
UNITED STATES FEDERAL INCOME
TAX CONSEQUENCES OF THE EXCHANGE OFFER
The exchange of outstanding notes for exchange notes in the exchange offer will not constitute a taxable event to holders for United States federal income tax purposes. Consequently, no gain or loss will be recognized by a holder upon receipt of an exchange note, the holding period of the exchange note will include the holding period of the outstanding note exchanged therefor and the basis of the exchange note will be the same as the basis of the outstanding note immediately before the exchange.
In any event, we encourage persons considering the exchange of outstanding notes for exchange notes to consult their own tax advisors concerning the United States federal income tax consequences in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction.
176
CERTAIN ERISA CONSIDERATIONS
The following is a summary of certain considerations associated with the purchase and holding of the exchange notes by employee benefit plans that are subject to Title I of the U.S. Employee Retirement Income Security Act of 1974, as amended ("ERISA"), plans, individual retirement accounts and other arrangements that are subject to Section 4975 of the Code or provisions under any federal, state, local, non-U.S. or other laws, rules or regulations that are similar to such provisions of ERISA or the Code (collectively, "Similar Laws"), and entities whose underlying assets are considered to include "plan assets" of such plans, accounts and arrangements (each, a "Plan").
General Fiduciary Matters
ERISA and the Code impose certain duties on persons who are fiduciaries of a Plan subject to Title I of ERISA or Section 4975 of the Code (an "ERISA Plan") and prohibit certain transactions involving the assets of an ERISA Plan and its fiduciaries or other interested parties. Under ERISA and the Code, any person who exercises any discretionary authority or control over the administration of such an ERISA Plan or the management or disposition of the assets of such an ERISA Plan, or who renders investment advice for a fee or other compensation to such an ERISA Plan, is generally considered to be a fiduciary of the ERISA Plan.
In considering an investment in the exchange notes of a portion of the assets of any Plan, a fiduciary should determine whether the investment is in accordance with the documents and instruments governing the Plan and the applicable provisions of ERISA, the Code or any Similar Law relating to a fiduciary's duties to the Plan including, without limitation, the prudence, diversification, delegation of control and prohibited transaction provisions of ERISA, the Code and any other applicable Similar Laws.
Prohibited Transaction Issues
Section 406 of ERISA and Section 4975 of the Code prohibit ERISA Plans from engaging in specified transactions involving plan assets with persons or entities who are "parties in interest," within the meaning of ERISA, or "disqualified persons," within the meaning of Section 4975 of the Code, unless an exemption is available. A party in interest or disqualified person who engages in a nonexempt prohibited transaction may be subject to excise taxes and other penalties and liabilities under ERISA and the Code. In addition, the fiduciary of the ERISA Plan that engages in such a nonexempt prohibited transaction may be subject to penalties and liabilities under ERISA and the Code. The acquisition and/or holding of the exchange notes by an ERISA Plan with respect to which we or the initial purchasers are considered a party in interest or disqualified person may constitute or result in a direct or indirect prohibited transaction under Section 406 of ERISA and/or Section 4975 of the Code, unless the investment is acquired and is held in accordance with an applicable statutory, class or individual prohibited transaction exemption. In this regard, the United States Department of Labor has issued prohibited transaction class exemptions ("PTCEs") that may apply to the acquisition and holding of the exchange notes. These class exemptions include, without limitation, PTCE 84-14 respecting transactions determined by independent qualified professional asset managers, PTCE 90-1 respecting insurance company pooled separate accounts, PTCE 91-38 respecting bank collective investment funds, PTCE 95-60 respecting life insurance company general accounts and PTCE 96-23 respecting transactions determined by in-house asset managers, although there can be no assurance that all of the conditions of any such exemptions will be satisfied. Because of the foregoing, the exchange notes should not be purchased or held by any person investing "plan assets" of any Plan, unless such purchase and holding will not constitute a non-exempt prohibited transaction under ERISA and the Code or similar violation of any applicable Similar Laws.
177
Representation
Accordingly, by acceptance of an exchange note, each purchaser and subsequent transferee will be deemed to have represented and warranted that either (i) no portion of the assets used by such purchaser or transferee to acquire and hold the exchange notes constitutes assets of any Plan or (ii) the purchase and holding of the exchange notes by such purchaser or transferee will not constitute a non-exempt prohibited transaction under Section 406 of ERISA or Section 4975 of the Code or similar violation under any applicable Similar Laws.
The foregoing discussion is general in nature and is not intended to be all-inclusive. Due to the complexity of these rules and the penalties that may be imposed upon persons involved in non-exempt prohibited transactions, it is particularly important that fiduciaries or other persons considering purchasing and holding the exchange notes on behalf of, or with the assets of, any Plan, consult with their counsel regarding the potential applicability of ERISA, Section 4975 of the Code and any Similar Laws to such transactions and whether an exemption would be applicable to the purchase and holding of the exchange notes.
178
PLAN OF DISTRIBUTION
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for outstanding notes where the outstanding notes were acquired as a result of market-making activities or other trading activities. To the extent any such broker-dealer participates in the exchange offer and so notifies the Issuer, or causes the Issuer to be so notified in writing, the Issuer has agreed that for a period of 180 days after the date of this prospectus, it will make this prospectus, as amended or supplemented, available to such broker-dealer for use in connection with any such resale, and will promptly send additional copies of this prospectus and any amendment or supplement to this prospectus to any broker-dealer that requests such documents in the letter of transmittal.
We will not receive any proceeds from any exchange of outstanding notes for exchange notes or from any sale of exchange notes by broker-dealers. Exchange notes received by broker-dealers for their own accounts pursuant to the exchange offer may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the exchange notes or a combination of these methods of resale, at market prices prevailing at the time of resale, at prices related to the prevailing market prices or negotiated prices. Any resale may be made directly to purchasers or to or through brokers or dealers who may receive compensation in the form of commissions or concessions from any broker-dealer or the purchasers of any exchange notes. Any broker-dealer that resells exchange notes that were received by it for its own account pursuant to the exchange offer and any broker or dealer that participates in a distribution of the exchange notes may be deemed to be an "underwriter" within the meaning of the Securities Act and any profit on any resale of exchange notes and any commissions or concessions received by these persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an "underwriter" within the meaning of the Securities Act.
We have agreed to pay all expenses incident to the exchange offer other than commissions or concessions of any brokers or dealers and, except in certain circumstances, the expenses of counsel and other advisors of the holders and will indemnify the holders of outstanding notes, including any broker-dealers, against certain liabilities, including liabilities under the Securities Act.
179
LEGAL MATTERS
The validity of the exchange notes offered hereby will be passed upon for us by Simpson Thacher & Bartlett LLP, New York, New York. Certain partners of Simpson Thacher & Bartlett LLP, members of their families, related persons and others have an indirect interest, through limited partnerships that are investors in KKR Millennium Fund L.P., in less than 1% of our common stock.
EXPERTS
The consolidated financial statements of Accellent Inc. and its subsidiaries as of December 31, 2004 and 2003 and for each of the three years in the period ended December 31, 2004 included in this prospectus have been so incorporated in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The consolidated financial statements of MedSource Technologies, Inc. at June 30, 2003 and 2002 and for each of the three years in the period ended June 30, 2003, appearing in this prospectus and registration statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of Campbell at December 31, 2003 and 2004 and for each of the two years in the period ended December 31, 2004, appearing in this prospectus and the registration statement with respect to which this prospectus forms a part have been audited by Beason & Nalley, Inc., an independent public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of MTG at December 31, 2003 and 2004 and for each of the two years in the period ended December 31, 2004, appearing in this prospectus and registration statement with respect to which this prospectus forms a part have been audited by Lenahan, Smith & Bargiachi, PC, an independent public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
AVAILABLE INFORMATION
Accellent Inc. and its guarantors have filed with the SEC a registration statement on Form S-4 under the Securities Act with respect to the exchange notes being offered hereby. This prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement. For further information with respect to us and the exchange notes, reference is made to the registration statement. Statements contained in this prospectus as to the contents of any contract or other document are not necessarily complete, and, where such contract or other document is an exhibit to the registration statement, each such statement is qualified by the provisions in such exhibit, to which reference is hereby made. Following the offering of the exchange notes, Accellent Inc. and its guarantors will be subject to the informational requirements of the Exchange Act, and, in accordance therewith, will file reports and other information with the SEC. The registration statement, such reports and other information can be inspected and copied at the Public Reference Room of the SEC located at 100 F Street, N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any portion of the registration statement, can be obtained from the Public Reference Room of the SEC at prescribed rates. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room. Such materials may also be accessed electronically by means of the SEC's home page on the Internet (http://www.sec.gov).
180
So long as Accellent Inc. and its guarantors are subject to the periodic reporting requirements of the Exchange Act, Accellent Inc. and its guarantors are required to furnish the information required to be filed with the SEC to the trustee and the holders of the outstanding notes and the exchange notes. Accellent Inc. and its guarantors have agreed that, even if Accellent Inc. and its guarantors are not required under the Exchange Act to furnish such information to the SEC, we will nonetheless continue to furnish information that would be required to be furnished by Accellent Inc. and its guarantors by Section 13 of the Exchange Act.
181
INDEX TO FINANCIAL STATEMENTS
| Accellent Inc. Consolidated Financial Statements | | |
Report of Independent Registered Public Accounting Firm |
|
F-3 |
| Consolidated Balance Sheets As of December 31, 2004 and 2003 | | F-4 |
Consolidated Statements of Operations for the years ended December 31, 2004, 2003
and 2002 | | F-5 |
| Consolidated Statements of Stockholders' Equity for the years ended December 31, 2004, 2003 and 2002 | | F-6 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2004, 2003 and 2002 | | F-7 |
| Notes to Consolidated Financial Statements | | F-8 |
| Unaudited Condensed Consolidated Balance Sheets As of September 30, 2005 and December 31, 2004 | | F-46 |
| Unaudited Condensed Consolidated Statements of Operations for the nine months ended September 30, 2005 and 2004 | | F-47 |
| Unaudited Condensed Consolidated Statements of Cash Flows for the nine months ended September 30, 2005 and 2004 | | F-48 |
| Notes to Unaudited Condensed Consolidated Financial Statements | | F-49 |
MedSource Technologies, Inc. Consolidated Financial Statements |
|
|
Report of Independent Registered Public Accounting Firm |
|
F-64 |
| Consolidated Balance Sheets As of June 30, 2003 and 2002 | | F-65 |
| Consolidated Statements of Operations for the years ended June 30, 2003, 2002 and 2001 | | F-66 |
| Consolidated Statements of Changes in Mandatory Redeemable Convertible Stock and Stockholders' Equity (Deficit) for the years ended June 30, 2003, 2002 and 2001 | | F-67 |
| Consolidated Statements of Cash Flows for the years ended June 30, 2003, 2002 and 2001 | | F-69 |
| Notes to Consolidated Financial Statements | | F-70 |
| Unaudited Consolidated Balance Sheets As of March 28, 2004 and June 30, 2003 | | F-93 |
| Unaudited Consolidated Statements of Operations for the three and nine months ended March 28, 2004 and March 30, 2003 | | F-94 |
| Unaudited Consolidated Statements of Cash Flows for the nine months ended March 28, 2004 and March 30, 2003 | | F-95 |
| Notes to Unaudited Consolidated Financial Statements | | F-96 |
Campbell Engineering, Inc. Financial Statements |
|
|
Independent Auditors' Report |
|
F-101 |
| Balance Sheets As of December 31, 2004 and 2003 | | F-102 |
| Statements of Income for the years ended December 31, 2004 and 2003 | | F-103 |
| Statements of Changes in Stockholders' Equity for the years ended December 31, 2004 and 2003 | | F-104 |
| Statements of Cash Flows for the years ended December 31, 2004 and 2003 | | F-105 |
| Notes to Financial Statements | | F-106 |
| Unaudited Balance Sheets As of June 30, 2005 and December 31, 2004 | | F-112 |
| Unaudited Statements of Income for the six months ended June 30, 2005 and 2004 | | F-113 |
| Unaudited Statements of Changes in Stockholders' Equity for the six months ended June 30, 2005 | | F-114 |
| Unaudited Statements of Cash Flows for the six months ended June 30, 2005 and 2004 | | F-115 |
| Notes to Unaudited Financial Statements | | F-116 |
| | | |
F-1
Machining Technology Group, LLC Financial Statements |
|
|
Independent Auditors' Report |
|
F-122 |
| Balance Sheets As of December 31, 2004 and 2003 | | F-123 |
| Statements of Income for the years ended December 31, 2004 and 2003 | | F-124 |
| Statements of Changes in Members' Equity for the years ended December 31, 2004 and 2003 | | F-125 |
| Statements of Cash Flows for the years ended December 31, 2004 and 2003 | | F-126 |
| Notes to Financial Statements | | F-127 |
| Unaudited Balance Sheets As of September 30, 2005 and December 31, 2004 | | F-132 |
| Unaudited Statements of Income for the nine months ended September 30, 2005 and 2004 | | F-133 |
| Unaudited Statements of Changes in Members' Equity | | F-134 |
| Unaudited Statements of Cash Flows for the nine months ended September 30, 2005 and 2004 | | F-135 |
| Notes to Unaudited Financial Statements | | F-136 |
Unaudited Pro Forma Condensed Combined Financial Statements |
|
|
Unaudited Pro Forma Condensed Combined Financial Statements |
|
P-1 |
| Unaudited Pro Forma Condensed Combined Balance Sheet as of September 30, 2005 | | P-4 |
| Notes to Unaudited Pro Forma Condensed Combined Balance Sheet | | P-5 |
| Unaudited Pro Forma Condensed Combined Statement of Operations for the nine months ended September 30, 2004 | | P-9 |
| Unaudited Pro Forma Condensed Combined Statement of Operations for the twelve months ended December 31, 2004 | | P-10 |
| Unaudited Pro Forma Condensed Combined Statement of Operations for the nine months ended September 30, 2005 | | P-11 |
| Notes to Unaudited Pro Forma Condensed Combined Statements of Operations | | P-12 |
Financial Statement Schedules |
|
|
Schedule II—Valuation and Qualifying Accounts for the three years ended December 31, 2004 |
|
S-1 |
F-2
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Accellent Inc.:
In our opinion, the accompanying consolidated balance sheets and related consolidated statements of operations, stockholders' equity and cash flows present fairly, in all material respects, the financial position of Accellent Inc. and its subsidiaries ("the Company") at December 31, 2004 and December 31, 2003, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2004 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
PricewaterhouseCoopers LLP
Philadelphia, PA
May 31, 2005, except for Note 17,
as to which the date
is December 16, 2005
F-3
ACCELLENT INC.
Consolidated Balance Sheets
As of December 31, 2004 and 2003
(In thousands, except share data)
| | 2004
| | 2003
| |
|---|
| Assets | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 16,004 | | $ | 3,974 | |
| | Receivables, net of allowance for doubtful accounts and return reserves of $2,909 and $974, respectively | | | 48,354 | | | 20,661 | |
| | Inventories | | | 58,014 | | | 28,776 | |
| | Prepaid expenses and other | | | 3,471 | | | 1,764 | |
| | |
| |
| |
| Total current assets | | | 125,843 | | | 55,175 | |
| Property, plant and equipment, net | | | 85,945 | | | 39,258 | |
| Goodwill | | | 289,461 | | | 113,855 | |
| Intangible assets, net | | | 81,874 | | | 68,813 | |
| Deferred financing costs and other assets, net | | | 17,106 | | | 2,034 | |
| | |
| |
| |
| Total assets | | $ | 600,229 | | $ | 279,135 | |
| | |
| |
| |
Liabilities and Stockholders' equity |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
| | Current portion of long term debt | | $ | 1,961 | | $ | 12,370 | |
| | Accounts payable | | | 20,447 | | | 7,574 | |
| | Accrued payroll and benefits | | | 13,011 | | | 5,784 | |
| | Accrued interest | | | 10,575 | | | 692 | |
| | Accrued expenses, other | | | 26,986 | | | 46,119 | |
| | |
| |
| |
| Total current liabilities | | | 72,980 | | | 72,539 | |
| Note payable and long term debt | | | 366,091 | | | 123,876 | |
| Other long term liabilities | | | 23,667 | | | 13,314 | |
| | |
| |
| |
| Total liabilities | | | 462,738 | | | 209,729 | |
| Commitments and contingencies | | | | | | | |
| Redeemable and convertible preferred stock | | | 30 | | | 12,593 | |
| Stockholders' equity: | | | | | | | |
| | Capital stock: | | | | | | | |
| | | Convertible preferred stock, aggregate liquidation preference of $240,752 | | | 166 | | | 72 | |
| | | Common stock, par value $0.01 per share, 50,000,000 shares authorized and 429,578 shares issued and outstanding | | | 4 | | | 4 | |
| | Accumulated other comprehensive income | | | 1,716 | | | 1,324 | |
| | Additional paid in capital | | | 211,249 | | | 125,467 | |
| | Retained earnings (deficit) | | | (75,674 | ) | | (70,054 | ) |
| | |
| |
| |
| Total stockholders' equity | | | 137,461 | | | 56,813 | |
| | |
| |
| |
| Total liabilities and stockholders' equity | | $ | 600,229 | | $ | 279,135 | |
| | |
| |
| |
The accompanying notes are an integral part of these financial statements.
F-4
ACCELLENT INC.
Consolidated Statements of Operations
For the years ended December 31, 2004, 2003 and 2002
(In thousands)
| | 2004
| | 2003
| | 2002
| |
|---|
| Net sales | | $ | 320,169 | | $ | 174,223 | | $ | 135,841 | |
| Cost of sales | | | 234,396 | | | 121,029 | | | 96,740 | |
| | |
| |
| |
| |
| Gross profit | | | 85,773 | | | 53,194 | | | 39,101 | |
| Selling, general and administrative expenses | | | 45,912 | | | 28,612 | | | 23,548 | |
| Research and development expenses | | | 2,668 | | | 2,603 | | | 2,380 | |
| Restructuring and other charges | | | 3,600 | | | 1,487 | | | 2,440 | |
| Impairment of goodwill | | | — | | | — | | | 17,523 | |
| Impairment of intangible assets | | | — | | | — | | | 4,202 | |
| Amortization of intangible assets | | | 5,539 | | | 4,828 | | | 4,703 | |
| | |
| |
| |
| |
| Income (loss) from operations | | | 28,054 | | | 15,664 | | | (15,695 | ) |
| Other income (expense): | | | | | | | | | | |
| | Interest expense, net | | | (26,879 | ) | | (16,587 | ) | | (16,923 | ) |
| | Other (expense) income, including debt prepayment penalty of $3,295 in 2004 | | | (3,312 | ) | | (9 | ) | | 61 | |
| | |
| |
| |
| |
| Total other expense | | | (30,191 | ) | | (16,596 | ) | | (16,862 | ) |
| | |
| |
| |
| |
| Loss before income taxes | | | (2,137 | ) | | (932 | ) | | (32,557 | ) |
| Income tax expense (benefit) | | | 3,483 | | | 13,872 | | | (5,145 | ) |
| | |
| |
| |
| |
| Net loss | | | (5,620 | ) | | (14,804 | ) | | (27,412 | ) |
| Dividends on redeemable and convertible preferred stock | | | (8,201 | ) | | — | | | — | |
| | |
| |
| |
| |
| Net loss available to common stockholders | | $ | (13,821 | ) | $ | (14,804 | ) | $ | (27,412 | ) |
| | |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
F-5
ACCELLENT INC.
Consolidated Statements of Stockholders' Equity
For the years ended December 31, 2004, 2003 and 2002
(In thousands, except share data)
| | Convertible Preferred Stock
| | Common Stock
| | Accumulated other
comprehensive income (loss)
| |
| |
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Additional
paid in
capital
| | Cumulative
translation
adjustment
| | Minimum
pension
liability
| | Gain (loss) on
derivative
instruments
| | Retained
earnings
(deficit)
| | Total
Stockholders'
equity
| |
|---|
| Balance, January 1, 2002 | | 7,186,962 | | $ | 72 | | 429,578 | | $ | 4 | | $ | 119,302 | | $ | (212 | ) | $ | (121 | ) | $ | (1,369 | ) | $ | (27,838 | ) | $ | 89,838 | |
| | Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | — | | | — | | | (27,412 | ) | | (27,412 | ) |
| | | Cumulative translation adjustment | | — | | | — | | — | | | — | | | — | | | 959 | | | — | | | — | | | — | | | 959 | |
| | | Net gain on derivative instruments (net of tax expense of $475) | | — | | | — | | — | | | — | | | — | | | — | | | — | | | 712 | | | — | | | 712 | |
| | | Minimum pension liability | | — | | | — | | — | | | — | | | — | | | — | | | (100 | ) | | — | | | — | | | (100 | ) |
| | Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | (25,841 | ) |
| | Amortization of deferred stock based compensation | | — | | | — | | — | | | — | | | 222 | | | — | | | — | | | — | | | — | | | 222 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
Balance, December 31, 2002 |
|
7,186,962 |
|
|
72 |
|
429,578 |
|
|
4 |
|
|
119,524 |
|
|
747 |
|
|
(221 |
) |
|
(657 |
) |
|
(55,250 |
) |
|
64,219 |
|
| | Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| �� | | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | — | | | — | | | (14,804 | ) | | (14,804 | ) |
| | | Cumulative translation adjustment | | — | | | — | | — | | | — | | | — | | | 836 | | | — | | | — | | | — | | | 836 | |
| | | Net gain on derivative instruments (net of tax expense of $438) | | — | | | — | | — | | | — | | | — | | | — | | | — | | | 657 | | | — | | | 657 | |
| | | Minimum pension liability | | — | | | — | | — | | | — | | | — | | | — | | | (38 | ) | | — | | | — | | | (38 | ) |
| | Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | (13,349 | ) |
| | Value of warrants issued in connection with Series C Redeemable Preferred Stock | | — | | | — | | — | | | — | | | 6,164 | | | — | | | — | | | — | | | — | | | 6,164 | |
| | Exercise of anti-dilution agreement | | 31,620 | | | — | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| | Compensation credit associated with phantom stock plans | | — | | | — | | — | | | — | | | (412 | ) | | — | | | — | | | — | | | — | | | (412 | ) |
| | Amortization of deferred stock based compensation | | — | | | — | | — | | | — | | | 191 | | | — | | | — | | | — | | | — | | | 191 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
Balance, December 31, 2003 |
|
7,218,582 |
|
|
72 |
|
429,578 |
|
|
4 |
|
|
125,467 |
|
|
1,583 |
|
|
(259 |
) |
|
— |
|
|
(70,054 |
) |
|
56,813 |
|
| | Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Net loss | | — | | | — | | — | | | — | | | — | | | — | | | — | | | — | | | (5,620 | ) | | (5,620 | ) |
| | | Cumulative translation adjustment | | — | | | — | | — | | | — | | | — | | | 571 | | | — | | | — | | | — | | | 571 | |
| | | Minimum pension liability | | — | | | — | | — | | | — | | | — | | | — | | | (179 | ) | | — | | | — | | | (179 | ) |
| | | Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | (5,228 | ) |
| | | Stock issued in connection with acquisitions | | 1,767,548 | | | 18 | | — | | | — | | | 25,793 | | | — | | | — | | | — | | | — | | | 25,811 | |
| | | Proceeds from sale of Class A-8 5% Convertible Preferrred Stock | | 7,568,980 | | | 76 | | — | | | — | | | 87,972 | | | — | | | — | | | — | | | — | | | 88,048 | |
| | | Compensation expense associated with phantom stock plans | | — | | | — | | — | | | — | | | 141 | | | — | | | — | | | — | | | — | | | 141 | |
| | | Amortization of deferred stock based compensation | | — | | | — | | — | | | — | | | 170 | | | — | | | — | | | — | | | — | | | 170 | |
| | Dividends on preferred stock | | — | | | — | | — | | | — | | | (28,294 | ) | | — | | | — | | | — | | | — | | | (28,294 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, December 31, 2004 | | 16,555,110 | | $ | 166 | | 429,578 | | $ | 4 | | $ | 211,249 | | $ | 2,154 | | $ | (438 | ) | $ | — | | $ | (75,674 | ) | $ | 137,461 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
F-6
ACCELLENT INC.
Consolidated Statements of Cash Flows
For the years ended December 31, 2004, 2003 and 2002
(In thousands)
| | 2004
| | 2003
| | 2002
| |
|---|
| Cash flows from operating activities: | | | | | | | | | | |
| | Net loss | | $ | (5,620 | ) | $ | (14,804 | ) | $ | (27,412 | ) |
| | | Adjustments to reconcile net loss to net cash flows from operating activities—Depreciation and amortization of intangibles | | | 16,152 | | | 11,591 | | | 10,858 | |
| | | | Amortization of debt discounts and non cash interest accrued | | | 7,557 | | | 7,095 | | | 6,166 | |
| | | | Restructuring and other charges, net of cash expended | | | 148 | | | (363 | ) | | 1,152 | |
| | | | Impairment charge | | | — | | | — | | | 21,725 | |
| | | | Loss (gain) on disposal of property and equipment | | | (74 | ) | | 10 | | | 719 | |
| | | | Deferred income taxes | | | 1,514 | | | 12,324 | | | (5,239 | ) |
| | | | Non cash compensation charge | | | 266 | | | (309 | ) | | 222 | |
| | | | Increase in inventory reserves | | | 2,434 | | | 623 | | | 3,629 | |
| | | Changes in operating assets and liabilities excluding effects of acquisitions—Receivables | | | (2,911 | ) | | (565 | ) | | 12 | |
| | | | Inventories | | | (3,835 | ) | | (5,718 | ) | | 3,465 | |
| | | | Prepaid expenses and other | | | (1,005 | ) | | (695 | ) | | (228 | ) |
| | | | Accounts payable and accrued expenses | | | 7,022 | | | 5,203 | | | (381 | ) |
| Other, net | | | 583 | | | — | | | (666 | ) |
| | |
| |
| |
| |
| Net cash provided by operating activities | | | 22,231 | | | 14,392 | | | 14,022 | |
| | |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | |
| | Capital expenditures | | | (13,900 | ) | | (6,371 | ) | | (6,218 | ) |
| | Proceeds from sale of equipment | | | 1,413 | | | 93 | | | 398 | |
| | Acquisitions, net of cash acquired | | | (214,982 | ) | | (14,390 | ) | | (3,316 | ) |
| | Other noncurrent assets | | | 93 | | | 298 | | | (310 | ) |
| | |
| |
| |
| |
| Net cash used in investing activities | | | (227,376 | ) | | (20,370 | ) | | (9,446 | ) |
| | |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | |
| | Indebtedness— | | | | | | | | | | |
| | | Proceeds from long-term debt | | | 372,000 | | | 8,000 | | | 11,500 | |
| | | Principal payments on long-term debt | | | (185,039 | ) | | (22,067 | ) | | (12,470 | ) |
| | Repurchase of redeemable and convertible preferred stock | | | (12,583 | ) | | — | | | — | |
| | Dividends paid on redeemable and convertible preferred stock | | | (28,294 | ) | | — | | | — | |
| | Deferred financing fees | | | (17,061 | ) | | (673 | ) | | (547 | ) |
| | Proceeds from sale of redeemable and convertible preferred stock | | | 88,048 | | | 18,717 | | | — | |
| | |
| |
| |
| |
| Net cash provided by (used in) financing activities | | | 217,071 | | | 3,977 | | | (1,517 | ) |
| | |
| |
| |
| |
| Effect of exchange rate changes in cash | | | 104 | | | 98 | | | — | |
| | |
| |
| |
| |
| Net increase (decrease) in cash and cash equivalents | | | 12,030 | | | (1,903 | ) | | 3,059 | |
| Cash and cash equivalents, beginning of year | | | 3,974 | | | 5,877 | | | 2,818 | |
| | |
| |
| |
| |
| Cash and cash equivalents, end of year | | $ | 16,004 | | $ | 3,974 | | $ | 5,877 | |
| | |
| |
| |
| |
| Supplemental disclosure: | | | | | | | | | | |
| | Cash paid for interest | | $ | 9,546 | | $ | 9,985 | | $ | 10,178 | |
| | Cash paid for income taxes | | | 1,136 | | | 275 | | | 283 | |
| Supplemental disclosure of non cash investing activities: | | | | | | | | | | |
| | Cash paid for businesses acquired: | | | | | | | | | | |
| | | Working capital net of cash acquired of $14,304, $1,166 and $0, respectively | | $ | 7,482 | | $ | 1,892 | | $ | — | |
| | | Property, plant and equipment | | | 44,392 | | | 1,272 | | | — | |
| | | Goodwill, intangible and other assets | | | 188,142 | | | 49,231 | | | — | |
| | | Long term liabilities | | | (34,717 | ) | | (969 | ) | | — | |
| | | Change in accrued expenses for acquisitions related to earn out and expense payments | | | 9,683 | | | (37,036 | ) | | 3,316 | |
| | |
| |
| |
| |
| | | $ | 214,982 | | $ | 14,390 | | $ | 3,316 | |
| | |
| |
| |
| |
| Supplemental disclosure of non cash financing activities: | | | | | | | | | | |
| | Stock issued in connection with acquisitions | | $ | 25,811 | | $ | — | | $ | — | |
The accompanying notes are an integral part of these financial statements.
F-7
ACCELLENT INC.
Notes to Consolidated Financial Statements
1. Summary of Significant Accounting Policies
The consolidated financial statements include the accounts of Accellent Inc. and its wholly owned subsidiaries (collectively, the "Company"). All significant intercompany transactions have been eliminated.
The Company is engaged in providing product development and design, custom manufacturing of components, assembly of finished devices and supply chain manufacturing services primarily for the medical device industry. Sales are focused in both domestic and European markets.
Concurrent with the acquisition of MedSource Technologies, Inc. ("MedSource") on June 30, 2004, the Company realigned its management to focus on the three main medical device markets which it serves. As a result of this realignment, the Company has three operating segments: endoscopy, cardiology and orthopaedic. The Company has determined that all of its operating segments meet the aggregation criteria of paragraph 17 of SFAS No. 131, and are treated as one reportable segment.
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of accounts receivable. A significant portion of the Company's customer base is comprised of companies within the medical industry. The Company does not require collateral from its customers. The Company's two largest customers represented approximately 20% and 14% of consolidated net sales for fiscal year 2004. Sales to these two customers are comprised of different products, shipping to several locations, which thus reduce the Company's exposure to the loss of the entire business with this customer. One customer represented approximately 25% of consolidated net sales for fiscal year 2003, and one customer represented approximately 10% of consolidated net sales for fiscal year 2002. The loss of one or more of the Company's largest customers would most likely have a negative short-term impact on the Company's results of operations.
Boston Scientific has informed the Company that it intends to transfer a number of products currently assembled by the Company to its own assembly operation. Based on preliminary estimates and our experience to date with this customer, we expect net sales from Boston Scientific to decrease by approximately $35 million, with the substantial majority of the decrease taking place in 2006. While the Company believes that the transferred business can be replaced with new business from existing and potential new customers to offset the loss, there is no assurance that the Company will replace such business and the loss will not adversely affect operating results in 2005 and thereafter.
The Company has established manufacturing subsidiaries in Europe and Mexico. The functional currency of each of these subsidiaries is the respective local currency. Assets and liabilities of the Company's foreign subsidiaries are translated into U.S. dollars using the current rate of exchange existing at period-end, while revenues and expenses are translated at average monthly exchange rates. Translation gains and losses are recorded as a component of accumulated other comprehensive income (loss) within stockholder's equity. Transaction gains and losses are included in other income (expense),
F-8
net. Currency transaction gains and losses included in operating results for the years ended December 31, 2004, 2003 and 2002 were not significant.
Cash and cash equivalents consist of all highly liquid investments with an original or remaining maturity of 90 days or less. Cash and cash equivalents are carried at cost, which approximates fair value.
Inventories are stated at the lower of cost (on first-in, first-out basis) or market and include the cost of materials, labor and manufacturing overhead. Scrap resulting from the manufacturing process is valued in inventory at the estimated price which will be received from the refinery.
Property, Plant and Equipment
Property, plant and equipment consist of (in thousands):
| | December 31
| |
|---|
| | 2004
| | 2003
| |
|---|
| Land | | $ | 2,045 | | $ | 1,775 | |
| Buildings and improvements | | | 19,388 | | | 9,617 | |
| Machinery and equipment | | | 91,126 | | | 47,979 | |
| Construction in progress | | | 4,923 | | | 1,138 | |
| | |
| |
| |
| | | | 117,482 | | | 60,509 | |
| Less—Accumulated depreciation | | | (31,537 | ) | | (21,251 | ) |
| | |
| |
| |
| Property, plant and equipment, net | | $ | 85,945 | | $ | 39,258 | |
| | |
| |
| |
Property, plant and equipment are stated at cost. Expenditures for maintenance and repairs are charged to expense as incurred. Expenditures which significantly increase value or extend useful lives are capitalized and replaced properties are retired. Depreciation is calculated principally by the use of straight-line method over the estimated useful lives of depreciable assets. Accelerated methods are used for tax purposes. Amortization of leasehold improvements is calculated by use of the straight-line method over the shorter of the lease terms, including renewal options expected to be exercised, or estimated useful lives of the equipment. Useful lives of depreciable assets, by class, are as follows:
| Buildings and improvements | | 20 years |
| Machinery and equipment | | 3 to 12 years |
| Leasehold improvements | | 3 to 17 years |
| Computer equipment and software | | 2 to 5 years |
The Company evaluates the useful lives and potential impairment of property, plant and equipment whenever events or changes in circumstances indicate that either the useful life or carrying value may be impaired. Events and circumstances which may indicate impairment include a change in the use or condition of the asset, regulatory changes impacting the future use of the asset, historical or
F-9
projected operating or cash flow losses for the operating segment utilizing the asset, or an expectation that an asset could be disposed of prior to the end of its useful life. If the carrying value of the asset is not recoverable based on an analysis of cash flow, a charge for impairment is recorded equal to the amount by which the carrying value of the asset exceeds the related fair value. Estimated fair value is generally based on projections of future cash flows and discount rates that reflect risks associated with achieving future cash flows. Additionally, we analyze the remaining useful life of potential impaired assets and adjust these lives when appropriate.
Cost and accumulated depreciation for property retired or disposed of are removed from the accounts, and any gain or loss on disposal is credited or charged to earnings. Capitalized interest in connection with constructing property and equipment was not significant. Depreciation expense was $10.6 million, $6.8 million and $6.2 million for the years ended December 31, 2004, 2003 and 2002, respectively.
Goodwill represents the excess of cost over fair value of the net assets of acquired businesses. On January 1, 2002, the Company adopted Statement of Financial Accounting Standards (SFAS) No. 142, "Goodwill and Other Intangible Assets". As required by SFAS No. 142, the Company discontinued amortizing the remaining balance of goodwill. Prior to January 1, 2002, goodwill had been amortized over 20 years on a straight-line basis.
Upon the adoption of SFAS 142, the Company assigned acquired goodwill to three reporting units based on the expected benefit from the synergies of the combination. In connection with the acquisition of MedSource in June of 2004, the Company realigned its management to focus on the three main medical device markets which it serves. As a result of this realignment, the Company reallocated its goodwill to the three reporting units created as a result of this realignment. Goodwill for each of the reporting units is subject to an annual impairment test (or more often if impairment indicators arise), using a fair value-based approach. Fair value of each reporting unit is determined based on the discounted projected cash flows of the reporting unit. If the carrying amount of the reporting unit, including goodwill, exceeds its fair value, additional impairment tests are performed to quantify the impairment, if any. The amount of the impairment is based on the implied fair value of goodwill. To determine the implied fair value of goodwill, the Company allocates the fair value of the reporting unit to all of its assets and liabilities. This allocation utilizes cash flow estimates and discount rates that reflect the risks associated with achieving future cash flows in order to determine the fair values of each asset and liability. The excess of the fair value of the reporting unit over the amounts assigned to the assets and liabilities equals the implied fair value of goodwill. If the implied fair value of goodwill is less than the carrying amount, an impairment loss is recorded. The Company has elected October 31st as the annual impairment assessment date for all reporting units, and will perform additional impairment tests when triggering events occur.
Other intangible assets primarily include developed technology and know how, customer contracts and customer base obtained in connection with the acquisitions. The valuations were based on appraisals based on assumptions made by management using estimated future operating results and cash flows of the underlying business operations.
F-10
Amortization periods are as follows:
| | Amortization Period
|
|---|
| Developed technology and know how | | 10 to 20 years |
| Customer contracts and relationships | | 6 to 20 years |
| Non compete agreements and other | | 3–5 years |
The Company evaluates the potential impairment of intangible assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable through projected undiscounted cash flows expected to be generated by the asset. If the carrying value of intangible assets is not recoverable, a charge for impairment is recorded equal to the amount by which the carrying value of the asset exceeds the related fair value. Estimated fair value is generally based on projections of future cash flows and discount rates that reflect the risks associated with achieving future cash flows.
Research and development costs are expensed as incurred.
The Company recognizes all derivatives on the balance sheet at fair value. On the date the derivative instrument is entered into, the Company determines the hedge designation. Cash flow hedge designation is given to derivatives that hedge a forecasted transaction or the variability of cash flows to be received or paid related to a recognized asset or liability. Changes in the fair value of a derivative that is designated as, and meets all the required criteria for, a cash flow hedge are recorded in accumulated other comprehensive income and reclassified into earnings as the underlying hedged item affects earnings. Also, changes in the entire fair value of a derivative that is not designated as a hedge are recorded immediately in earnings. The Company formally documents all relationships between hedging instruments and hedged items, as well as its risk-management objective and strategy for undertaking various hedge transactions. This process includes relating all derivatives that are designated as cash flow hedges to specific assets and liabilities on the balance sheet or to specific firm commitments or forecasted transactions.
The Company currently has no outstanding interest rate swap agreements. At December 31, 2002, the Company had three outstanding interest rate swap agreements to effectively convert LIBOR-based variable rate debt to fixed rate debt. At December 31, 2002, the notional amount of the contracts in place was $36.0 million. The contracts matured on July 10, 2003. The Company received variable rate payments (equal to the three month LIBOR rate) from third parties during the term of the contracts and was obligated to pay fixed interest rate payments (7.13%) to the third parties during the term of the contracts.
In 2004, 2003 and 2002, the net loss resulting from cash flow hedge ineffectiveness was not significant. There are no transactions or other events that will result in the reclassification into earnings of gains or losses that are reported in accumulated other comprehensive income (loss) within the next twelve months.
F-11
The Company accounts for income taxes under the provisions of SFAS No. 109 "Accounting for Income Taxes," which requires the use of the liability method in accounting for deferred taxes. If it is more likely than not that some portion, or all, of a deferred tax asset will not be realized, a valuation allowance is recognized.
The cost of obtaining financing has been deferred and is being amortized on a straight-line basis over the life of the associated obligations. Additionally, the Company capitalizes and defers direct and incremental costs associated with proposed business combinations, primarily consisting of fees paid to outside legal counsel and accounting advisors and other third parties, related to due diligence performed on the target companies. Upon the successful closure of an acquisition, the Company includes capitalized costs as part of the overall purchase price. Deferred acquisition costs where the Company has determined that it is unlikely that the business combination will be completed are written off when such determination is made.
The Company accounts for stock options issued to employees of the Company using the intrinsic value method of Accounting Principles Board (APB) Opinion No. 25, "Accounting for Stock Issued to Employees." The Company's accounting method for recording stock-based compensation expense does not require any initial accounting entries for the deferred compensation determined based upon the initial measurement of compensation. Rather, when stock options are granted at an exercise price less than the current fair market value of the underlying stock, the Company records compensation expense to the future periods expected to benefit with a corresponding credit to additional paid-in capital.
For options granted at the end of 2001 and 2002, the grant date market value was greater than the exercise price. The difference between the grant date market value and the exercise price is recorded as compensation expense over the vesting period of the options, or 3 to 5 years. Expenses of $169,505, $191,361 and $222,809 were recorded in 2004, 2003 and 2002, respectively. Total unearned stock-based compensation as of December 31, 2004 was $251,691, and will be amortized to compensation expense in the amounts of $156,750 and $94,941 for the fiscal years ending December 31, 2005 and 2006, respectively. A corresponding increase to additional paid-in capital is recorded for the amounts charged to compensation expense. Had compensation expense for the stock option plans been determined
F-12
consistent with the provisions of SFAS No. 123, the Company's net loss would have been the pro forma amounts indicated below (in thousands):
| | Year ended December 31
| |
|---|
| | 2004
| | 2003
| | 2002
| |
|---|
| Net loss as reported | | $ | (5,620 | ) | $ | (14,804 | ) | $ | (27,412 | ) |
| Add total stock compensation expense, net of tax, included in net loss as reported | | | 110 | | | 117 | | | 136 | |
| Less total stock compensation expense—fair value method net of tax | | | (1,711 | ) | | (835 | ) | | (516 | ) |
| | |
| |
| |
| |
| Pro forma net loss | | $ | (7,221 | ) | $ | (15,522 | ) | $ | (27,792 | ) |
| | |
| |
| |
| |
The weighted average fair value of each option grant in 2004, 2003 and 2002 was estimated to be $3.91, $4.20 and $9.61, respectively. The fair value was determined on the date of grant using the Black-Scholes option pricing model with the following range of assumptions used for the option grants which occurred during the years ended December 31, 2004, 2003 and 2002:
| | Year ended December 31
| |
|---|
| | 2004
| | 2003
| | 2002
| |
|---|
| Volatility | | 33.74 | % | 38.75 | % | 45.76 | % |
| Risk free interest rate | | 4.38 | % | 4.15 | % | 4.95 | % |
| Expected life in years | | 8 | | 8 | | 8 | |
| Dividend yield | | 0 | | 0 | | 0 | |
The Company records revenue in compliance with SAB 104, which requires that the following criteria are met: (a) persuasive evidence of an arrangement exists, (b) delivery has occurred or services have been rendered, (c) the price from the buyer is fixed or determinable, and (d) collectibility is reasonably assured. The Company records revenue based on written arrangements or purchase orders with the customer, and upon transfer of title of the product or rendering of the service. Revenue for product sold on consignment is recognized when the customer uses the product.
Amounts billed for shipping and handling fees are classified as sales in the consolidated income statement. Costs incurred for shipping and handling are classified as cost of sales.
The Company provides a reserve for estimated future returns against revenue in the period revenue is recorded. The estimate of future returns is based on such factors as known pending returns and historical return data.
The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the
F-13
date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
In December 2003, the FASB issued SFAS No. 132 (revised 2003) "Employers' Disclosures about Pensions and Other Post-retirement Benefits." This standard increases the existing disclosure requirements by requiring more details about pension plan assets, benefit obligations, cash flows, benefit costs and related information. The expanded disclosures require that plan assets be segregated by category, such as debt, equity and real estate, and that disclosures on certain expected rates of return be incorporated. SFAS No. 132 (R) also will require the Company to disclose various elements of pension and post-retirement benefit costs in interim-period financial statements. The Company adopted SFAS No. 132 (R) on January 1, 2004. The adoption did not have an impact on the Company's consolidated statements of operations, financial position or its cash flows.
In November 2004, the FASB issued SFAS No. 151, "Inventory Costs, an amendment of ARB No. 43, Chapter 4." SFAS No. 151 amends Accounting Research Bulletin ("ARB") No. 43, Chapter 4, to clarify that abnormal amounts of idle facility expense, freight, handling costs and wasted materials (spoilage) should be recognized as current-period charges. In addition, SFAS No. 151 requires that allocation of fixed production overhead to inventory be based on the normal capacity of the production facilities. SFAS No. 151 is effective for inventory costs incurred during fiscal years beginning after June 15, 2005. The Company is currently assessing the impact that SFAS No. 151 will have on the results of operations, financial position or cash flows.
On December 16, 2004, the FASB issued SFAS No. 123 (Revised 2004), "Share-Based Payment." SFAS 123R requires that compensation cost related to share-based payment transactions be recognized in the financial statements. Share-based payment transactions within the scope of SFAS 123R include stock options, restricted stock plans, performance-based awards, stock appreciation rights, and employee share purchase plans. The provisions of SFAS 123R are effective for the Company's first interim period that begins after December 31, 2005. Accordingly, the Company will implement the revised standard in the first quarter of fiscal year 2006. Currently, the Company accounts for share-based payment transactions under the provisions of APB 25, which does not necessarily require the recognition of compensation cost in the financial statements. The Company is assessing the implications of this revised standard, which may materially impact the Company's results of operations in the first quarter of fiscal year 2006 and thereafter.
Certain prior years' amounts have been reclassified to conform to the current year's presentation.
2. Acquisitions
On June 30, 2004, the Company acquired MedSource Technologies, Inc. The acquisition was accounted for as a purchase and accordingly the results of operations include MedSource's results beginning June 30, 2004. MedSource is an engineering and manufacturing services provider to the medical device industry. The Company acquired MedSource in order to create one of the largest providers of outsourced precision manufacturing and engineering services in the Company's target markets of the medical device industry. The purchase price was $219.2 million, consisting of
F-14
$208.8 million in cash for the purchase of common stock and the cash out of options and warrants, and $10.4 million of transaction fees. The purchase was financed by a combination of new debt and equity as discussed in notes 6 and 10. In addition, the then existing indebtedness of MedSource equal to $36.1 million plus related accrued interest was repaid in conjunction with the acquisition. The Company estimates that it will incur $21.7 million for integration and other liabilities.
The purchase price for the MedSource acquisition was allocated as follows (in thousands):
| Inventories | | $ | 27,838 | |
| Accounts receivable | | | 24,782 | |
| Prepaid expenses and other current assets | | | 702 | |
| Property and equipment | | | 44,392 | |
| Goodwill | | | 169,542 | |
| Intangible and other assets | | | 19,938 | |
| Current liabilities | | | (34,688 | ) |
| Debt assumed | | | (36,131 | ) |
| Other long term liabilities | | | (10,976 | ) |
| | |
| |
| Cash paid, net of cash acquired of $14,304 | | $ | 205,399 | |
| | |
| |
The amounts allocated to intangible assets for the MedSource acquisition by intangible asset class, and the respective weighted average amortization period is as follows:
| | Amount
| | Weighted average
useful life
|
|---|
| | (in thousands)
| | (in years)
|
|---|
| Customer base | | $ | 16,300 | | 15.0 |
| Developed technology | | | 2,300 | | 10.0 |
| | |
| |
|
| | | $ | 18,600 | | 14.1 |
| | |
| |
|
Approximately $45.7 million of the total $188.1 million allocated to goodwill and other intangible assets for MedSource will be amortizable and deductible for tax purposes.
The Company determines the value and potential purchase price of target acquisition companies based on multiples of future cash flow. These cash flow projections may include an estimate of improved cash flow performance as compared to historical performance of the target acquisition company based on expected future growth and/or cost reduction opportunities. The value of the acquired company based on our cash flow analysis may differ significantly from the carrying value of the acquired net assets, resulting in an allocation of a significant portion of the purchase price to goodwill and other intangible assets.
On February 28, 2003, the Company acquired all of the shares of capital stock of Venusa, Ltd. and Venusa de Mexico, S.A. de C.V. ("Venusa") with facilities located in El Paso, Texas and Juarez, Mexico for approximately $18.5 million, including transaction costs of approximately $1.0 million. Venusa is a contract manufacturer of proprietary medical devices. The acquisition added assembly capabilities to the Company. Venusa has several contracts with renewable extensions. The purchase price was negotiated on the basis of current and expected future cash flows from Venusa. The purchase price was
F-15
paid in cash of $15.7 million and $2.8 million to be paid in 2004 in a combination of cash, the Class A-7 5% Convertible Preferred Stock and phantom stock. The payment was required to have been made in at least 25% cash and 25% stock, with the balance paid in any combination of cash or stock, at the Company's determination. The Stock Purchase Agreement also provides for certain earn-out provisions which resulted in additional consideration of $34.1 million as a result of Venusa's 2003 earnings, as defined, which was paid in 2004 in a combination of approximately 25% in cash and 75% in Class A-7 5% Convertible Preferred Stock, as described previously. In addition, as a result of Venusa's 2004 earnings, as defined, additional consideration of $6.0 million will be paid during fiscal year 2005 in a combination of approximately 25% in cash and 75% in Class A-7 5% Convertible Preferred Stock. The Company records contingent consideration based on the value of that consideration as of the date the consideration is deemed earned. The Company determined the value of the equity portion of the contingent consideration earned as of December 31, 2003 and 2004 based on arms length negotiations between the Company and Venusa, and arms length negotiations between the Company and potential investors in the Company's equity securities. The total fair value of the stock issued, phantom stock issued and cash paid for additional consideration is recorded as additional goodwill.
The purchase was funded with proceeds from the issuance of 1,136,364 shares of the Company's Class C Redeemable Preferred Stock at $16.50 per share. Each holder of Class C Redeemable Preferred Stock also received a warrant to purchase one share of the Company's Class AB Convertible Preferred Stock at an exercise price of $0.01 per share for each share of Class C Redeemable Preferred Stock they received. The Class C Redeemable Preferred Stock earns cumulative dividends, when and if declared, at an annual rate of 8% on the liquidation value of $16.50 per share. The Company repurchased its Class C Redeemable Preferred Stock in June of 2004.
The purchase price for the Venusa acquisition was allocated as follows (in thousands):
| Inventories | | $ | 1,299 | |
| Other current assets | | | 4,704 | |
| Property and equipment | | | 1,272 | |
| Customer contracts and relationships | | | 3,000 | |
| Other intangibles | | | 100 | |
| Goodwill | | | 12,052 | |
| Current liabilities | | | (2,945 | ) |
| Long term liabilities | | | (969 | ) |
| | |
| |
| | | $ | 18,513 | |
| | |
| |
The following unaudited pro forma consolidated financial information reflects the purchase of Venusa assuming the acquisition had occurred as of January 1, 2002, and the purchase of MedSource assuming the acquisition had occurred as of January 1, 2003. This unaudited pro forma information has been provided for information purposes only and is not necessarily indicative of the results of the
F-16
operations or financial condition that actually would have been achieved if the acquisition had been on the date indicated, or that may be reported in the future (in thousands):
| | 2004
| | 2003
| | 2002
| |
|---|
| | (unaudited)
| |
|---|
| Revenues | | $ | 413,943 | | $ | 357,547 | | $ | 157,823 | |
| Net loss | | | (614 | ) | | (63,382 | ) | | (26,116 | ) |
The pro forma net loss for fiscal year 2002 includes goodwill and intangible asset impairment charges of $21.7 million. The pro forma net loss for fiscal year 2003 includes an intangible impairment charge of $40.0 million recorded by MedSource.
In 2002, the Company made previously accrued payments for prior acquisitions of $3.3 million.
All of the Company's acquisitions were accounted for using the purchase method of accounting. Accordingly the assets acquired and liabilities assumed were recorded in the financial statements at their fair market values and the operating results of the acquired companies are reflected in the accompanying consolidated financial statements since the date of acquisition.
3. Restructuring and Other Charges
During 2002, the Company implemented two restructuring plans focused on consolidating the Company's U.S. operations. During the second quarter of 2002, the Company announced the relocation of the majority of operations in its South Plainfield, New Jersey facility to the Collegeville, Pennsylvania facility. A restructuring charge of $0.5 million was recognized that consisted of $0.1 million related to severance and $0.4 million associated with the write-down of assets and other closure costs at the South Plainfield, New Jersey facility.
During the fourth quarter of 2002, the Company announced the consolidation of its machining capabilities into its Wheeling, Illinois facility and the closing of its Miramar, Florida plant. As a result, the Company recognized a restructuring charge of $1.4 million consisting of: $0.1 million related to stay-on bonuses earned through December 31, 2002; $0.5 million related to the write-down of assets; and $0.8 million related to lease obligations. In 2003, the relocation was completed and the Company recognized a restructuring charge of $1.8 million consisting of: $0.5 million related to stay-on and relocation bonuses earned through the relocation date; $0.3 million related to the relocation of equipment and plant clean-up; $0.7 million of other exit costs; and $0.3 million related to excess inventory discarded (included in cost of sales in the consolidated statements of operations).
During the third quarter of 2002, the Company decided not to proceed with the construction of a new technology center and recognized a loss of $0.5 million related to the write-down of previously capitalized costs.
In connection with the MedSource acquisition, the Company identified $21.5 million of costs associated with eliminating duplicate positions and plant consolidations, which is comprised of $11.6 million in severance payments, and $9.9 million in lease and other contract termination costs. Severance payments relate to approximately 590 employees in manufacturing, selling and administration which are expected to be paid by the end of fiscal year 2006. All other costs are expected to be paid by 2018. The costs of these plant consolidations was reflected in the purchase price of MedSource in accordance with the FASB Emerging Issues Task Force ("EITF") No. 95-3,Recognition of Liabilities in
F-17
Connection with a Purchase Business Combination. These costs include estimates to close facilities and consolidate manufacturing capacity, and are subject to change based on the actual costs incurred to close these facilities.
The Company recognized $3.6 million of restructuring charges and acquisition integration costs during fiscal year 2004, including $1.7 million of severance, facility closure and relocation costs incurred as part of a MedSource manufacturing facility closure plan which existed at the time of the acquisition, and $1.0 million of salary related costs due to the elimination of positions deemed to be redundant as a result of the MedSource acquisition. In addition to the $2.7 million in restructuring charges incurred during fiscal year 2004, the Company incurred $0.9 million of costs for the integration of MedSource comprised of outside professional services and salary related cost and incentive compensation earned by members of an integration team.
The following table summarizes the recorded accruals and activity related to the restructuring and other charges (in thousands):
| | Employee Costs
| | Exit Costs and
Other
| | Total
| |
|---|
| Restructuring and other charges | | $ | 230 | | $ | 2,210 | | $ | 2,440 | |
| | Less: cash payments | | | (80 | ) | | (143 | ) | | (223 | ) |
| | Less: non cash items | | | — | | | (1,262 | ) | | (1,262 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2002 | | | 150 | | | 805 | | | 955 | |
| Restructuring charge | | | 471 | | | 1,016 | | | 1,487 | |
| Inventory discarded | | | — | | | 322 | | | 322 | |
| | Less: cash payments | | | (613 | ) | | (1,559 | ) | | (2,172 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2003 | | | 8 | | | 584 | | | 592 | |
| Restructuring charge | | | 1,307 | | | 2,293 | | | 3,600 | |
| Plant closure and severance costs for MedSource integration | | | 11,559 | | | 9,927 | | | 21,486 | |
| | Less: cash payments | | | (5,110 | ) | | (2,891 | ) | | (8,001 | ) |
| | |
| |
| |
| |
| Balance as of December 31, 2004 | | $ | 7,764 | | $ | 9,913 | | $ | 17,677 | |
| | |
| |
| |
| |
4. Inventories
Inventories consisted of the following at December 31, 2004 and 2003 (in thousands):
| | December 31
|
|---|
| | 2004
| | 2003
|
|---|
| Raw materials | | $ | 20,939 | | $ | 8,184 |
| Work in process | | | 24,068 | | | 11,865 |
| Finished goods | | | 13,007 | | | 8,727 |
| | |
| |
|
| | | $ | 58,014 | | $ | 28,776 |
| | |
| |
|
F-18
In connection with the purchase of certain precious metals for anticipated manufacturing requirements, the Company enters into consignment agreements with a third party, whereby the Company purchases the precious metal from the consignor at the time when an external sale is made at the prevailing market price. The prevailing price at the time of sale is passed through to the customer. These contracts are used to help protect against volatility in certain precious metals prices.
5. Goodwill and Other Intangible Assets
Upon the adoption of SFAS 142, the Company assigned acquired goodwill to three reporting units based on the expected benefit from the synergies of the combination. In connection with the acquisition of MedSource in June of 2004, the Company realigned its management to focus on the three main medical device markets which it serves. As a result of this realignment, the Company reallocated its goodwill to the three reporting units created as a result of this realignment. The Company has elected October 31 as the annual impairment assessment date for all reporting units.
The Company acquired American Technical Molding, Inc. ("ATM") in December 2000 for $27.6 million, plus additional contingent consideration of $3.0 million paid in fiscal year 2002 based on earnings targets achieved by ATM for fiscal year 2001. The total purchase price of $30.6 million was allocated in accordance with the provisions of APB 16. Amounts allocated to intangible assets were as follows: developed technology and know-how, $7.6 million, customer base, $3.3 million and assembled workforce, $0.2 million. The valuation of the intangibles was based upon a discounted cash flow analysis which utilized annual sales growth of 10% to 15% and a discount rate of 15%. The excess of the purchase price over the fair value of assets acquired and liabilities assumed was $20.4 million, and was recorded as goodwill.
During the third quarter of fiscal year 2002, ATM experienced a reduction in net revenues and cash flow due to the loss of two customers which were significant to ATM. When assessing the fair value of the ATM reporting unit, the Company took into consideration the impact of reduced net revenues on future cash flow projections. The ATM reporting unit was valued at $8.9 million based on a valuation utilizing both a market approach and an income approach. The market approach assumptions included an EBITDA multiple of 6.2x and a sales multiple of 0.89x based on a peer group. The income approach utilized a discount rate of 14.5%, and a significant reduction in annual cash flows due to the loss of two customers. The carrying value of the ATM reporting unit was $30.6 million on October 31, 2002. Since the carrying value of the ATM reporting unit exceeded its fair value, an additional test was performed to quantify the amount of the impairment. As part of the allocation process, the Company determined that the intangible assets of the ATM reporting unit were impaired. Based on a revised cash flow analysis which took into consideration the reduction in future cash flow due to lower revenues, the Company determined that the intangible assets were impaired by $4.2 million.
The Company allocated the fair value of the ATM reporting unit as of October 31, 2002 to all of its assets and liabilities. The excess of the fair value of the reporting unit over the amounts assigned to the assets and liabilities equals the implied value of goodwill. As a result of this allocation, goodwill of the ATM reporting unit was determined to be impaired as of October 31, 2002. The total impairment
F-19
of goodwill and intangible assets for the ATM reporting unit in fiscal 2002 was as follows (in thousands):
| | Carrying Value
| | Fair Value
| | Impairment
|
|---|
| Goodwill | | $ | 19,496 | | $ | 1,973 | | $ | 17,523 |
| Developed technology and know how | | | 6,577 | | | 4,359 | | | 2,218 |
| Customer base | | | 2,967 | | | 983 | | | 1,984 |
| | |
| |
| |
|
| | | $ | 29,040 | | $ | 7,315 | | $ | 21,725 |
| | |
| |
| |
|
The consolidated statement of operations of the Company for fiscal 2002 includes a charge for the impairment of goodwill of $17,523,000 and a charge for the impairment of intangible assets of $4,202,000 due to the expected reduction in cash flows of the ATM reporting unit.
In fiscal 2002, with the adoption of SFAS No. 142, the Company reclassified $5,995,946, net of $1,904,054 of amortization, of the value of assembled workforce to goodwill. Previously assembled workforce had been amortized over 5-7 years on a straight line basis.
The following table summarizes the changes in goodwill (in thousands):
| Balance December 31, 2001 | | $ | 79,049 | |
| Transfer of assembled workforce with adoption of SFAS No. 142 | | | 5,996 | |
| Acquisitions | | | 158 | |
| Impairment charge | | | (17,523 | ) |
| | |
| |
| Balance December 31, 2002 | | | 67,680 | |
| Acquisitions | | | 46,175 | |
| | |
| |
| Balance December 31, 2003 | | | 113,855 | |
| Acquisitions | | | 175,606 | |
| | |
| |
| Balance December 31, 2004 | | $ | 289,461 | |
| | |
| |
Intangible assets as of December 31, 2004 are comprised of (in thousands):
| | Gross Carrying
Amount
| | Accumulated
Amortization
| | Net Carrying
Amount
|
|---|
| Developed technology and know how | | $ | 64,039 | | $ | 15,346 | | $ | 48,693 |
| Customer contracts and relationships | | | 39,193 | | | 6,075 | | | 33,118 |
| Non compete agreements and other | | | 425 | | | 362 | | | 63 |
| Customer backlog | | | 60 | | | 60 | | | — |
| | |
| |
| |
|
| | | $ | 103,717 | | $ | 21,843 | | $ | 81,874 |
| | |
| |
| |
|
F-20
Intangible assets as of December 31, 2003 are comprised of (in thousands):
| | Gross Carrying
Amount
| | Accumulated
Amortization
| | Net Carrying
Amount
|
|---|
| Developed technology and know how | | $ | 61,739 | | $ | 11,885 | | $ | 49,854 |
| Customer contracts and relationships | | | 22,893 | | | 4,037 | | | 18,856 |
| Non compete agreements and other | | | 425 | | | 322 | | | 103 |
| Customer backlog | | | 60 | | | 60 | | | — |
| | |
| |
| |
|
| | | $ | 85,117 | | $ | 16,304 | | $ | 68,813 |
| | |
| |
| |
|
Intangible asset amortization expense was $5,538,754, $4,827,605 and $4,703,330 in 2004, 2003 and 2002, respectively. Estimated intangible asset amortization expense for each of the five succeeding years approximates $6.1 million.
Other long-term assets include $15,860,893 and $2,033,833 in 2004 and 2003, respectively, for deferred financing and transaction costs, net of accumulated amortization of $1,193,010 and $2,332,813 in 2004 and 2003, respectively.
6. Short-Term and Long-Term Borrowings
Long-term debt at December 31, 2004 and 2003 consisted of the following (in thousands):
| | December 31
| |
|---|
| | 2004
| | 2003
| |
|---|
Credit and guaranty agreement, interest at 5.28% at
December 31, 2004 | | $ | 193,030 | | $ | — | |
| Senior subordinated notes maturing July 15, 2012, interest at 10% | | | 175,000 | | | — | |
| Revolving credit facility | | | — | | | 11,500 | |
| Term loan A with a bank, 4.67% at December 31, 2003 | | | — | | | 22,219 | |
| Term loan B with a bank, 5.00% at December 31, 2003 | | | — | | | 43,425 | |
| Term loan C with a bank, 5.21% at December 31, 2003 | | | — | | | 7,920 | |
| Capital lease obligations | | | 22 | | | 49 | |
| Senior subordinated notes maturing June 1, 2007, interest at 13.5% less unamortized discount of $1,260 at December 31, 2003 | | | — | | | 20,239 | |
| Senior notes maturing June 1, 2008, subject to partial mandatory redemption on June 1, 2006, interest at 15.563% through June 1, 2005, 16.101% thereafter less unamortized discount and prepayment premium of $2,288 at December 31, 2003. | | | — | | | 30,894 | |
| | |
| |
| |
| Total debt | | | 368,052 | | | 136,246 | |
| Less—current portion | | | (1,961 | ) | | (12,370 | ) |
| | |
| |
| |
| Long term debt, excluding current portion | | $ | 366,091 | | $ | 123,876 | |
| | |
| |
| |
F-21
Annual principal repayments are as follows (in thousands):
Fiscal year
| | Amount
|
|---|
| 2005 | | $ | 1,961 |
| 2006 | | | 1,941 |
| 2007 | | | 1,940 |
| 2008 | | | 1,940 |
| 2009 | | | 1,940 |
| 2010 and thereafter | | | 358,330 |
| | |
|
| Total debt | | $ | 368,052 |
| | |
|
On June 30, 2004, the Company's subsidiary, Accellent Corp., formerly known as Medical Device Manufacturing, Inc. entered into a new Credit and Guaranty Agreement (the "Credit Agreement") which included $194.0 million of term loans and up to $40.0 million available under the revolving credit facility. The term loans bear interest at variable rates. At December 31, 2004, the interest rate was 5.28%. Over the next five years the principal payments will be $1.9 million per year plus, beginning in 2006, 75% of excess cash flow, as defined by the Credit Agreement. The balance is due June 30, 2010. As of December 31, 2004, $7.3 million of the revolving credit facility was supporting the Company's letters of credit, leaving $32.7 million available.
On June 30, 2004, Accellent Corp. issued $175.0 million of 10% Senior Subordinated Notes (the "Senior Subordinated Notes—2012") due July 15, 2012. Interest is payable on January 15 and July 15 each year beginning January 15, 2005.
On June 30, 2004, the Company repaid Accellent Corp.'s debt under the its previously outstanding credit agreement of $83.5 million, MDMI's 13.5% senior subordinated notes due June 1, 2007 (the "Senior Subordinated Notes—2007") of $21.5 million and its senior notes due June 1, 2008 (the "Senior Notes") of $38.3 million. The Company also paid prepayment penalties in the aggregate of $4.7 million on the Senior Subordinated Notes—2007 and the Senior Notes. As a result of these transactions the Company also recognized interest expense of $2.9 million for the write-off of unamortized debt discounts and $1.6 million for the write-off of unamortized deferred financing fees related to the retired debt. The Company incurred $17.1 million of expenses for the new debt including $11.5 million in investment banking and bank arrangement fees, and $5.6 million in legal, accounting and other professional fees. These costs will be amortized over the lives of the new debt.
Accellent Corp.'s debt agreements contain various covenants, including minimum cash flow (as defined), debt service coverage ratios and maximum capital spending limits. In addition, the debt agreements restrict Accellent Corp. from paying dividends and making certain investments. The convenants and restrictions of the Indenture governing Accellent Corp.'s Senior Subordinated Notes—2012 apply only to Accellent Corp. and not to the Company. All covenants and restrictions under the Credit Agreement apply to Accellent Corp., and the covenants and restrictions other than the financial covenants apply to the Company.
As of December 31, 2004, the Company and Accellent Corp. were in compliance with their respective covenants under the Credit Agreement and the Senior Subordinated Notes—2012.
At December 31, 2003 accrued interest related to the Senior Notes of $4.4 million was included in other long-term liabilities on the Consolidated Balance Sheet.
F-22
7. Employee Benefit Plans
The Company has pension plans covering employees at two facilities. Benefits at one facility are provided at a fixed rate for each month of service. The Company's funding policy is consistent with the funding requirements of federal law and regulations. Plan assets consist of cash equivalents, bonds and certain equity securities. The Company's German facility has an unfunded frozen pension plan covering employees hired before 1993.
The change in projected benefit obligation (in thousands):
| | 2004
| | 2003
| |
|---|
| Benefit obligation at beginning of year | | $ | 2,333 | | $ | 1,708 | |
| | Service cost | | | 77 | | | 58 | |
| | Interest cost | | | 130 | | | 115 | |
| | Actuarial loss | | | 349 | | | 291 | |
| | Currency translation | | | 137 | | | 220 | |
| | Benefits paid | | | (70 | ) | | (59 | ) |
| | |
| |
| |
| Benefit obligation at end of year | | $ | 2,956 | | $ | 2,333 | |
| | |
| |
| |
The change in plan assets (in thousands):
| | 2004
| | 2003
| |
|---|
| Fair value of plan assets at beginning of year | | $ | 804 | | $ | 561 | |
| | Actual return on plan assets | | | 57 | | | 98 | |
| | Employer contribution | | | 36 | | | 183 | |
| | Benefits paid | | | (41 | ) | | (38 | ) |
| | |
| |
| |
| Fair value of plan assets at end of year | | $ | 856 | | $ | 804 | |
| | |
| |
| |
Reconciliation of the accrued benefit cost recognized in the financial statements (in thousands):
| | 2004
| | 2003
| |
|---|
| Funded status | | $ | (2,100 | ) | $ | (1,528 | ) |
| Unrecognized net actuarial loss | | | 680 | | | 479 | |
| Currency translation | | | 24 | | | 16 | |
| | |
| |
| |
| Accrued benefit obligation | | | (1,396 | ) | | (1,033 | ) |
| | |
| |
| |
| | Presented as Prepaid expenses and other | | | 204 | | | 224 | |
| | Presented as Other long term liabilities | | | (1,600 | ) | | (1,257 | ) |
| | |
| |
| |
| | | Total | | $ | (1,396 | ) | $ | (1,033 | ) |
| | |
| |
| |
F-23
Components of net periodic benefit cost for years ended December 31 (in thousands):
| | 2004
| | 2003
| | 2002
| |
|---|
| Service Cost | | $ | 77 | | $ | 58 | | $ | 56 | |
| Interest Cost | | | 130 | | | 115 | | | 100 | |
| Expected return of plan assets | | | (56 | ) | | (43 | ) | | (44 | ) |
| Amortization of transaction obligation | | | — | | | 25 | | | 22 | |
| Recognized net actuarial loss | | | 23 | | | 23 | | | 8 | |
| | |
| |
| |
| |
| | | $ | 174 | | $ | 178 | | $ | 142 | |
| | |
| |
| |
| |
Assumptions for benefit obligations at December 31:
| | 2004
| | 2003
|
|---|
| Discount rate | | 5.66%–4.50% | | 6.02%–5.50% |
| Rate of compensation increase | | 3% | | 5% |
Assumptions for net periodic benefit costs for years ended December 31:
| | 2004
| | 2003
| | 2002
|
|---|
| Discount rate | | 6.02%–4.50% | | 6.75%–6.50% | | 7.25%–6.50% |
| Expected long term return on plan assets | | 7.00% | | 7.00% | | 7.00% |
| Rate of compensation increase | | 3.0% | | 5.0% | | 5.0% |
To develop the expected long-term rate of return on plan assets assumption, the Company considered the historical returns and the future expectations for returns for each asset class, as well as the target asset allocation of the pension portfolio and the payment of plan expenses from the pension trust. This resulted in the selection of the 7.0% expected long-term rate of return on plan assets assumption.
The pension plans have the following asset allocations, as of their measurement dates:
| | Actual Percentage of
Plan Assets at
December 31,
| |
|---|
| | 2004
| | 2003
| |
|---|
| Asset Category | | | | | |
| Equity Securities—Domestic | | 59.6 | % | 61.3 | % |
| Debt Securities | | 39.5 | % | 38.2 | % |
| Cash and Other | | 0.9 | % | 0.5 | % |
| | |
| |
| |
| | Total | | 100.0 | % | 100.0 | % |
| | |
| |
| |
F-24
As of December 31, 2004, the Pension Plans' target asset allocation was as follows:
Asset Class
| | Target
Allocation %
| |
|---|
| Fixed Income | | 40.0 | % |
| Domestic Equity | | 60.0 | % |
The asset allocation policy was developed in consideration of the long-term investment objective of ensuring that there is an adequate level of assets to support benefit obligations to plan participants. A secondary objective is minimizing the impact of market fluctuations on the value of the plans' assets.
In addition to the broad asset allocation described above, the following policies apply to individual asset classes:
Fixed income investments are oriented toward investment grade securities rated "BBB" or higher. They are diversified among individual securities and sectors. The average maturity was 6.6 years as of December 31, 2004.
Equity investments are diversified among individual securities, industries and economic sectors. Most securities held are issued by companies with medium to large market capitalizations.
The Company has 401(k) plans available for most employees. An employee may contribute up to 10–15% of gross salary to the 401(k) plan, depending upon the specific plan. The Company's Board of Directors determines annually what contribution, if any, the Company shall make to the 401(k) plan.
The employees' contributions vest immediately, while the Company's contributions vest over an immediate to six-year period. The Company matches 25 - 50% of the employee's contributions to this plan up to certain maximums, depending upon the specific plan. The Company's contributions for matching totaled approximately $1.1 million, $0.7 million and $0.6 million for the years ended December 31, 2004, 2003 and 2002, respectively.
The Company has profit sharing plans available to employees at several of its locations. The Company's Board of Directors determines annually what contribution, if any, the Company shall make to the profit sharing plan. The Company's contributions vest over an immediate to six-year period. The Company expensed $3.4 million, $2.9 million and $1.7 million for these plans for the years ended December 31, 2004, 2003 and 2002, respectively.
The Company has a Supplemental Executive Retirement Pension Program (SERP) that covers certain of its executives. The SERP is a non-qualified, unfunded deferred compensation plan. Expenses incurred by the Company related to the SERP, which are actuarially determined, were $33,282, $139,186 and $117,700 for the years ended December 31, 2004, 2003 and 2002, respectively. The liability for the plan was $0.3 million and $1.1 million as of December 31, 2004 and 2003, respectively, and was included in other long-term liabilities of the Company.
F-25
8. Stock Grants and Options and Stock Based Plans
The Company has a stock option and incentive plan which provides for grants of incentive stock options, nonqualified stock options, restricted stock and restricted stock units. The total number of shares authorized under the plan is 3,600,000 at December 31, 2004. The plan generally requires exercise of options within ten years of grant. Vesting is determined in the applicable stock option agreement and generally occurs in equal annual installments over five years. All stock options granted by the Company have been granted to directors and employees of the Company.
Stock grant and option transactions are summarized as follows:
Shares under option
| | Number of shares
| | Weighted average
exercise price
|
|---|
| Outstanding at December 31, 2001 | | 1,298,117 | | $ | 7.09 |
| | Granted | | 9,000 | | | 10.08 |
| | Exercised | | — | | | — |
| | Forfeited | | (50,076 | ) | | 9.78 |
| | |
| | | |
| Outstanding at December 31, 2002 | | 1,257,041 | | | 7.00 |
| | |
| | | |
| | Granted | | 1,008,050 | | | 8.18 |
| | Exercised | | — | | | — |
| | Forfeited | | (128,050 | ) | | 9.50 |
| | |
| | | |
| Outstanding at December 31, 2003 | | 2,137,041 | | | 7.41 |
| | Granted | | 645,700 | | | 8.18 |
| | Exercised | | — | | | — |
| | Forfeited | | (192,131 | ) | | 8.78 |
| | |
| | | |
| Outstanding at December 31, 2004 | | 2,590,610 | | | 7.50 |
| | |
| | | |
Options exercisable at: |
|
|
|
|
|
| | December 31, 2002 | | 726,250 | | $ | 5.16 |
| | December 31, 2003 | | 811,024 | | $ | 5.62 |
| | December 31, 2004 | | 1,097,985 | | $ | 6.41 |
Below is additional information related to the Company's stock option grants to its employees which are outstanding and exercisable at December 31, 2004:
| | Options outstanding
| |
| |
|
|---|
| | Options exercisable
|
|---|
| | Number of
options
outstanding at
December 31,
2004
| | Weighted
average
remaining
contractual
life in years
| |
|
|---|
Range of exercise prices
| | Weighted
average
exercise price
(per share)
| | Number
exercisable at
December 31,
2004
| | Weighted
average
exercise price
(per share)
|
|---|
| $2.22 | | 425,977 | | 5.4 | | $ | 2.22 | | 425,977 | | $ | 2.22 |
| $6.08–6.67 | | 18,641 | | 5.1 | | | 6.10 | | 18,513 | | | 6.10 |
| $8.18 | | 1,563,750 | | 8.9 | | | 8.18 | | 207,929 | | | 8.18 |
| $8.89–9.78 | | 582,242 | | 5.9 | | | 9.58 | | 445,566 | | | 9.61 |
| | |
| | | |
| |
| |
|
| | | 2,590,610 | | 7.6 | | | 7.50 | | 1,097,985 | | | 6.41 |
| | |
| | | |
| |
| |
|
At December 31, 2004, 1,009,390 shares are available to grant under the Company's stock option plan.
F-26
The Star Guide Phantom Stock Plan provides for grants of phantom stock to eligible employees of Star Guide, a subsidiary of the Company. The Company granted 29,708 phantom shares on July 6, 1999 under this plan. All shares of phantom stock granted under this plan are immediately and fully vested. Holders of phantom stock under this plan have no voting rights, no stockholder rights and no employment rights. They are, however, entitled to receive the greater of the value of the Company's Class A-1 5% Convertible Preferred Stock or $10.94 per share (pre-conversion) upon redemption. Redemption of phantom shares will occur upon the earlier of certain changes in the Company's ownership, certain qualified public offerings, ten years after issuance or death of the holder. In addition, dividends are cumulative at the rate of 5% per share per annum, do not require declaration by the Board of Directors and are paid no later than upon redemption of the phantom shares. The redemption payment can be paid, as determined by the Company, in any combination of cash or discounted stock options. A holder of phantom stock may not transfer or assign phantom stock, other than by will or the laws of descent and distribution. The Company records compensation expense (credit) to adjust for the redemption value and accumulated dividends upon redemption since the redemption is certain to occur.
The 2000 Employee Phantom Stock Plan provides grants to eligible employees of the Company as determined by the Board of Directors. Up to a total of 229,167 phantom shares may be granted. The Company granted 38,268 phantom shares on March 15, 2001 under this plan in connection with the payment of contingent consideration owed in respect of the Noble-Met acquisition. All shares of phantom stock granted under the plan were immediately and fully vested. Holders of phantom stock under this plan have no voting rights, no stockholder rights and no employment rights. They are, however, entitled to receive the greater of the value of the Company's Class A-2 5% Convertible Preferred Stock or $12.00 per share (pre-conversion) upon redemption. Redemption of phantom shares will occur upon the earlier of certain changes in the Company's ownership, certain qualified public offerings, ten years after issuance or death of the holder. In addition, dividends are cumulative at the rate of 5% per share per annum, do not require declaration by the Board of Directors and are paid no later than upon redemption of the phantom shares. The redemption payment can be paid, as determined by the Company, in any combination of cash or discounted stock options. A holder of phantom stock may not transfer or assign phantom stock, other than by will or the laws of descent and distribution. The Company records compensation expense (credit) at the end of each accounting period to adjust for the redemption value and accumulated dividends upon redemption since the redemption is certain to occur.
The Venusa earn-out plan provides grants of phantom stock to certain employees of Venusa who were employed at Venusa at the time of the acquisition. The Company granted 86,523 phantom shares on May 31, 2004 based on the achievement of certain earnings targets for fiscal years 2002 and 2003. The Company will grant an additional 14,266 phantom shares during fiscal year 2005 based on the achievement of certain earnings targets for fiscal year 2004. All shares of phantom stock granted under this plan are immediately and fully vested. Holders of phantom stock under this plan have no voting rights, no stockholder rights and no employment rights. They are, however, entitled to receive the value of 1.8 shares of the Company's common stock for each share of phantom stock upon redemption. Redemption of the phantom shares will occur upon the earlier of certain changes in the Company's ownership, certain qualified public offerings, ten years after issuance or death of the holder. In addition, dividends are cumulative at the rate of 5% per share per annum, do not require declaration
F-27
by the Board of Directors and are paid no later than upon redemption of the phantom shares. The redemption will be paid in cash. A holder of phantom stock may not transfer or assign phantom stock, other than by will or the laws of descent and distribution. The Company records the issuance of phantom stock under this plan as an increase to the cost to acquire Venusa. The Company records compensation expense (credit) at the end of each accounting period to adjust for the redemption value and accumulated dividends upon redemption since the redemption is certain to occur.
9. Income Taxes
The provision for income taxes includes federal, state and foreign taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. The components of the provision for income taxes for the years ended December 31, 2004, 2003 and 2002 are as follows (in thousands):
| | 2004
| | 2003
| | 2002
| |
|---|
| Current | | | | | | | | | | |
| | Federal | | $ | — | | $ | — | | $ | — | |
| | State | | | 1,228 | | | 997 | | | — | |
| | Foreign | | | 741 | | | 383 | | | 160 | |
| Deferred | | | | | | | | | | |
| | Federal | | | 1,386 | | | 11,020 | | | (5,214 | ) |
| | State | | | 128 | | | 1,472 | | | (91 | ) |
| | |
| |
| |
| |
| Total provision | | $ | 3,483 | | $ | 13,872 | | $ | (5,145 | ) |
| | |
| |
| |
| |
Loss before income taxes included income (loss) from foreign operations of $3,362,000, $1,607,000 and $(333,000) in 2004, 2003 and 2002, respectively.
Major differences between income taxes at the federal statutory rate and the amount recorded on the accompanying consolidated statements of operations for the years ended December 31, 2004, 2003 and 2002 are as follows (in thousands):
| | 2004
| | 2003
| | 2002
| |
|---|
| Tax at statutory rate | | $ | (748 | ) | $ | (326 | ) | $ | (11,395 | ) |
| Valuation allowance on deferred tax assets | | | 3,931 | | | 13,554 | | | 1,058 | |
| State taxes, net of federal benefit | | | 787 | | | 1,212 | | | (106 | ) |
| Nondeductible goodwill | | | — | | | 5 | | | 6,133 | |
| Other, net | | | (487 | ) | | (573 | ) | | (835 | ) |
| | |
| |
| |
| |
| Tax provision | | $ | 3,483 | | $ | 13,872 | | $ | (5,145 | ) |
| | |
| |
| |
| |
F-28
The following is a summary of the significant components of the Company's deferred tax assets and liabilities as of December 31, 2004 and 2003 (in thousands):
| | 2004
| | 2003
| |
|---|
| Deferred tax assets (liabilities): | | | | | | | |
| | Operating loss and tax credit carryforwards | | $ | 36,746 | | $ | 12,493 | |
| | Compensation | | | 2,702 | | | 2,212 | |
| | Environmental | | | 3,251 | | | 2,031 | |
| | Inventory and accounts receivable reserves | | | 4,281 | | | 4,016 | |
| | Restructuring costs | | | 7,210 | | | 240 | |
| | Other | | | 2,635 | | | 2,625 | |
| | Valuation allowances | | | (43,072 | ) | | (15,413 | ) |
| Deferred tax liabilities: | | | | | | | |
| | Depreciation | | | (3,924 | ) | | (3,493 | ) |
| | Intangibles | | | (13,260 | ) | | (6,628 | ) |
| | |
| |
| |
| Net deferred tax assets (liabilities) | | $ | (3,431 | ) | $ | (1,917 | ) |
| | |
| |
| |
During the fourth quarter of 2003, the Company determined that income from its operations would not be sufficient to cover its interest and financing costs. Accordingly, the Company provided a valuation allowance of $13.6 million equal to the full amount of the Company's net deferred tax asset. Approximately $93.2 million of taxable income is needed to fully realize deferred tax assets. The loss carryforwards expire beginning in the year 2020 and ending in the year 2024. The future utilization of the loss carryforwards may be limited in future years by the provisions of internal revenue code section 382 due to change in control occurrences experienced by both the Company and MedSource.
As of December 31, 2004, the Company has not provided for withholding or U.S. federal income taxes on undistributed earnings of foreign subsidiaries since such earnings are expected to be reinvested indefinitely.
10. Capital Stock
The Company's Board of Directors has authorized an aggregate number of common shares for issuance equal to 50,000,000, $0.01 par value per share and 50,000,000 shares of preferred stock, $0.01 par value per share.
At December 31, 2004 and 2003, the Company had 429,578 shares of common stock outstanding. Holders of the Company's common stock are entitled to vote on all matters submitted to the Company's stockholders for a vote together with the holders of the Company's preferred stock, all voting together as a single class.
F-29
The Company has ten classes of convertible preferred stock as summarized below:
| |
| | Balance December 31,
|
|---|
| |
| | 2004
| | 2003
| | 2002
|
|---|
| | | Shares | | | 868,372 | | | 868,372 | | | 868,372 |
| Class A-1 | | Amount | | $ | 8,684 | | $ | 8,684 | | $ | 8,684 |
| | | Shares | | | 1,156,250 | | | 1,156,250 | | | 1,156,250 |
| Class A-2 | | Amount | | $ | 11,563 | | $ | 11,563 | | $ | 11,563 |
| | | Shares | | | 26,456 | | | 26,456 | | | 26,456 |
| Class A-3 | | Amount | | $ | 264 | | $ | 264 | | $ | 264 |
| | | Shares | | | 3,437,500 | | | 3,437,500 | | | 3,437,500 |
| Class A-4 | | Amount | | $ | 34,375 | | $ | 34,375 | | $ | 34,375 |
| | | Shares | | | 995,469 | | | 995,469 | | | 995,469 |
| Class A-5 | | Amount | | $ | 9,955 | | $ | 9,955 | | $ | 9,955 |
| | | Shares | | | 187,033 | | | 187,033 | | | 187,033 |
| Class A-6 | | Amount | | $ | 1,870 | | $ | 1,870 | | $ | 1,870 |
| | | Shares | | | 1,767,548 | | | — | | | — |
| Class A-7 | | Amount | | $ | 17,675 | | | — | | | — |
| | | Shares | | | 7,568,980 | | | — | | | — |
| Class A-8 | | Amount | | $ | 75,690 | | | — | | | — |
| | | Shares | | | 547,502 | | | 547,502 | | | 515,882 |
| Class AA | | Amount | | $ | 5,475 | | $ | 5,475 | | $ | 5,159 |
| | | Shares | | | — | | | — | | | — |
| Class AB | | Amount | | | — | | | — | | | — |
| | | | |
| |
| |
|
| Total | | Shares | | | 16,555,110 | | | 7,218,582 | | | 7,186,962 |
| | | | |
| |
| |
|
| | | Amount | | $ | 165,551 | | $ | 72,186 | | $ | 71,870 |
| | | | |
| |
| |
|
Each series of Class A Convertible Preferred Stock, which includes the Class AA and Class AB Convertible Preferred Stock unless otherwise noted, rank senior to the common stock and the Class B Convertible Preferred Stock described in Note 11, junior to the Class C Redeemable Preferred Stock described in Note 11, andpari passu with each other series of Class A Convertible Preferred Stock in priority with respect to the payment of dividends and the right to receive payment in connection with any liquidation, dissolution or winding up of the Company. Each series of Class A Convertible Preferred Stock is subject to, and the holders of shares of each such series are entitled to, the following rights, privileges, preferences and priorities:
Dividends. The holders of shares of Class A Convertible Preferred Stock, other than holders of Class AA and Class AB Convertible Preferred Stock, are entitled to be paid cumulative dividends at the rate of 5% of the liquidation value of such shares in preference to payment of dividends on common stock or other classes of junior capital stock of the Company when, as, and if declared by the Company's Board of Directors. After payment of all dividends on the eligible series of Class A Convertible Preferred Stock, the holders of all series of Class A Convertible Preferred Stock are entitled to participate, on an as converted basis, with the outstanding shares of the Company's common
F-30
stock as to any dividends payable on such common stock when, as, and if declared by the Company's Board of Directors.
Voting Rights. The holders of all series of Class A Convertible Preferred Stock are entitled to vote on all matters submitted to the Company's stockholders for a vote together with the holders of the Company's common stock, all voting together as a single class. Each holder of Class A Convertible Preferred Stock is entitled to a number of votes (rounded to the nearest whole number) equal to the number of shares of the Company's common stock into which such shares are convertible immediately after the close of business on the record date fixed for taking such vote.
Special Voting Rights. The consent of the holders of at least two-thirds of each affected series of Class A Convertible Preferred Stock, each such series voting as a separate class, is required for any action that (a) authorizes or issues shares of any class of capital stock having any preference or priority superior to any preference or priority of a series of Class A Convertible Preferred Stock other than Class AA or Class AB Convertible Preferred Stock or (b) changes the number of shares or class of capital stock into which shares of such affected series are convertible. With respect to the Class AA and Class AB Convertible Preferred Stock, the consent of the holders of at least two-thirds of the Class A Convertible Preferred Stock, voting together as a single class, is required for any action that authorizes or issues shares of any class of capital stock having any preference or priority superior to any preference or priority of the Class A Convertible Preferred Stock.
Conversion. Shares of Class A Convertible Preferred Stock are convertible at the holder's option at a rate of 1.8 shares of the Company's common stock per share of Class A Convertible Preferred Stock. The Company may at any time require the conversion of all of the outstanding Class A Convertible Preferred Stock upon the closing of a firmly underwritten public offering of shares of the Company's common stock. Any declared dividends are payable upon conversion.
Class A-1 5% Convertible Preferred Stock
Shares of Class A-1 5% Convertible Preferred Stock were issued in connection with a 1999 acquisition. The carrying value of Class A-1 5% Convertible Preferred Stock differs from its original issuance value by $2.6 million due to valuing certain shares issued to the selling shareholders in the 1999 acquisition at their carryover basis. At December 31, 2004 and 2003, there were 2,500,000 shares authorized and 868,372 shares issued and outstanding with a liquidation preference of $9.5 million.
Class A-2 5% Convertible Preferred Stock
At December 31, 2004 and 2003, there were 1,400,000 shares authorized and 1,156,250 issued and outstanding with a liquidation preference of $13.9 million.
Class A-3 5% Convertible Preferred Stock
At December 31, 2004 and 2003, there were 26,456 shares authorized, issued and outstanding with a liquidation preference of $0.5 million.
F-31
Class A-4 5% Convertible Preferred Stock
At December 31, 2004 and 2003, there were 6,250,000 shares authorized and 3,437,500 issued and outstanding with a liquidation preference of $55.0 million.
Class A-5 5% Convertible Preferred Stock
At December 31, 2004 and 2003, there were 1,500,000 shares authorized and 995,469 shares issued and outstanding with a liquidation preference of $15.9 million.
Class A-6 5% Convertible Preferred Stock
On December 20, 2001, the Company sold 187,033 shares of its Class A-6 5% Convertible Preferred Stock for $3.1 million. The proceeds were used to fund the $3.0 million earn-out for a 2000 acquisition. The shares are convertible into common stock valued at $4.8 million. The value of the common stock was based on the expected offering price of $14.40 for the initial public offering of the Company's common stock during 2001. The excess of the fair value of the common stock conversion rights over the price paid for the Class A-6 5% Convertible Preferred Stock was recorded as a beneficial conversion feature which was reflected on the Company's statement of operations as a reduction of net income available to common stockholders. The initial public offering of the Company's common stock was never consummated. At December 31, 2004 and 2003, there were 300,000 shares of Class A-6 5% Convertible Preferred Stock authorized and 187,033 shares issued and outstanding with a liquidation preference of $3.1 million.
Class A-7 5% Convertible Preferred Stock
On May 31, 2004, the Company issued 1,767,548 shares of its Class A-7 5% Convertible Preferred Stock valued at $26.0 million as partial payment of its obligation under the Venusa acquisition's earn-out provision. The liquidation value of the Class A-7 5% Convertible Preferred Stock is $14.72. The Company determined the value of the Class A-7 5% Convertible Preferred Stock and phantom stock based on arms length negotiations between the Company and Venusa, and arms length negotiations between the Company and potential investors in the Company's equity securities. The Company will issue approximately 260,000 additional Class A-7 shares during fiscal year 2005 in connection with Venusa achieving certain earn-out targets for fiscal year 2004. There are no additional earn-out provisions requiring the Company to issue additional Class A-7 shares. At December 31, 2003 and 2004 there were 2,000,000 shares authorized. At December 31, 2004 there were 1,767,548 shares issued and outstanding with a liquidation preference of $26.0 million. In May 2005, the Company amended its Articles of Incorporation to increase the number of authorized shares of its Class A-7 5% Convertible Preferred Stock to issue the additional Class A-7 shares in connection with the Venusa earn-out. There were no shares issued and outstanding at December 31, 2003.
Class A-8 5% Convertible Preferred Stock
On June 30, 2004, the Company sold 7,568,980 shares of its Class A-8 5% Convertible Preferred Stock for $88.0 million, net of approximately $1.8 million in fees. The liquidation value of the Class A-8 5% Convertible Preferred Stock is $14.28. In connection with the sale of these shares, the Company issued warrants to the Class A-8 shareholders which provided for the issuance of additional shares in the event Venusa achieved earn-out targets which entitle Venusa shareholders to additional shares of
F-32
Class A-7 5% Convertible Preferred Stock. Venusa achieved the earn-out targets for fiscal year 2004 which will require the Company to issue approximately 172,000 additional Class A-8 shares during fiscal year 2005. There are no additional earn-out provisions requiring the Company to issue additional Class A-8 shares. At December 31, 2004, there were 9,000,000 shares authorized and 7,568,980 shares issued and outstanding with a liquidation preference of $108.1 million. There were no shares authorized, issued or outstanding at December 31, 2003.
Class AA Convertible Preferred Stock
On May 31, 2000, the Company entered into a Securities Purchase Agreement with certain investors for the issuance of an aggregate of 515,882 shares of Class AA Convertible Preferred Stock in connection with the issuance of the Senior Notes and Senior Subordinated Notes—2007. The Class AA Convertible Preferred Stock was valued at $6.9 million and the Senior Notes and the Senior Subordinated Notes—2007 have been discounted for the amount attributed to the stock. On May 28, 2003 pursuant to an anti-dilution agreement, certain investors exercised their rights and acquired 31,620 shares of Class AA Convertible Preferred Stock at $0.01 per share. At each of December 31, 2004 and 2003, there were 1,000,000 shares authorized and 547,502 shares issued and outstanding, with a liquidation preference of $8.8 million. Other investors have the right to acquire 2,380 shares of Class AA Convertible Preferred Stock at $0.01 per share.
Class AB Convertible Preferred Stock
On February 24, 2003, the Company increased the number of authorized Class AB Convertible Preferred shares from 750,000 to 1,200,000 and decreased their liquidation value from $17.75 per share to $16.50 per share. As of December 31, 2004 and 2003, none were issued and outstanding.
On February 28, 2003, the Company sold 1,136,364 shares of Class C Redeemable Preferred Stock for $16.50 per share. Each share of Class C Redeemable Preferred Stock was issued a warrant to purchase one share of the Company's Class AB Convertible Preferred Stock at the exercise price of $0.01 per share. The warrants have been valued at $6.2 million based on the present value of the expected return on investment assuming a three year return on investment of a discount rate of 30%. At December 31, 2004, warrants to purchase 1,136,364 shares of Class AB Convertible Preferred Stock were outstanding and unexercised.
11. Redeemable and Convertible Preferred Stock
At December 31, 2003, there were 300,000 shares authorized, issued and outstanding. These shares were redeemed during fiscal year 2004 at liquidation value, or $30,000.
At December 31, 2004 and 2003, there were 300,000 and 100,000 shares authorized, issued and outstanding, respectively. In May 2004, the Company issued 200,000 shares of its Class B-2 Convertible Preferred Stock at a value equal to the liquidation value of $0.10 per share to the Chief Executive
F-33
Officer of the Company in respect of services performed. Liquidation value was determined to be the most appropriate method to record the Class B-2 Convertible Preferred Stock based on the likelihood that redemption will occur.
The Class B Convertible Preferred Stock has a liquidation value equal to $.10 per share, is subordinate to all classes of Class A Convertible Preferred Stock, and is not entitled to receive cumulative dividends. The holders of shares of Class B Convertible Preferred Stock are entitled to participate, on an as converted basis, with the holders of the Company's common stock as to any dividends declared and paid on common stock. Shares of Class B Convertible Preferred Stock are convertible into the Company's voting common stock based on a conversion formula under which portions of the Class B Convertible Preferred Stock are convertible when the Company realizes certain internal rates of return, as calculated in accordance with the Articles of Incorporation. All Class B Convertible Preferred Stock will be redeemed at liquidation value if not previously converted into common stock, on July 1, 2004 with respect to Class B-1 Convertible Preferred shares and May 31, 2005 with respect to the Class B-2 Convertible Preferred shares. The Class B-1 Convertible Preferred shares were redeemed during fiscal year 2004. The Company may at any time require the conversion of all of the outstanding Class B Convertible Preferred shares upon the closing of a firmly underwritten public offering of its common stock. The holders of Class B Convertible Preferred shares are entitled to notice of all shareholder meetings but do not have voting rights. However, the consent of the holders of at least two-thirds of each class of Class B Convertible Preferred Stock, voting together as a single class, shall be required for any action that (a) authorizes or issues shares of any class of stock having any preference or priority superior to any such preference or priority of such class of Class B Convertible Preferred Stock; or (b) changes the number of shares or class of stock into which such class of Class B Convertible Preferred Stock is convertible.
On February 28, 2003, the Company sold 1,136,364 shares of Class C Redeemable Preferred Stock for $18.8 million. The proceeds were used to fund the acquisition of Venusa. The shares are valued at $12.6 million. The difference of $6.2 million represents the portion of the proceeds attributable to the value of the warrants issued for each share of Class C Redeemable Preferred Stock to purchase one share of Class AB Convertible Preferred Stock at the exercise price of $0.01 per share. The Company repurchased the Class C Redeemable Preferred Stock in June of 2004 at liquidation value, or $18.8 million.
The Company's Consolidated Statement of Operations includes $28.3 million of dividends on redeemable and convertible preferred stock which includes the $6.2 million difference between the carrying value of the Class C Redeemable Preferred Stock repurchased by the Company, and $22.1 million of dividends declared and paid by the Company.
As of December 31, 2004 and 2003, there were 63,636 shares and 1,200,000 shares, respectively, of Class C Redeemable Preferred Stock authorized. As of December 31, 2003, there were 1,136,364 shares issued and outstanding with a liquidation preference of $18.8 million. As of December 31, 2004, there were no shares issued and outstanding.
F-34
12. Related-Party Transactions
The Company pays fees to KRG Capital Partners, LLC, which, as the general partner of the general partner of KRG/CMS L.P. beneficially owns a significant portion of the Company's stock. During the years ended December 31, 2004, 2003 and 2002, the Company expensed KRG management fees, plus expenses, of $0.5 million, $0.6 million and $0.5 million, respectively. Additionally, the Company incurred KRG transaction related fees, plus expenses, of $2.3 million for fiscal year 2004 and $0.2 million for fiscal year 2003 which were included as a part of the cost of the related acquisitions. During fiscal year 2004, the Company paid preferred stock dividends to KRG/CMS L.P. of $10.2 million, and repurchased $8.3 million of Class C Redeemable Preferred Stock from KRG/CMS L.P.
DLJ Merchant Banking Partners III, L.P., and certain related funds including DLJ Merchant Banking III, Inc., own a significant portion of the Company's stock. During fiscal year 2004, the Company expensed management fees, plus expenses of $0.3 million to DLJ Merchant Banking III, Inc. Additionally, the Company paid DLJ Merchant Banking III, Inc. $1.8 million in connection with the sale of Class A-8 5% Convertible Preferred Stock. During fiscal year 2004, the Company paid $0.5 million of preferred stock dividends and repurchased $1.3 million of Class C Redeemable Preferred Stock from DLJ Investment Partners II, L.P., DLJ Investment Funding II, Inc., DLJ ESC II L.P., DLJ Investment Partners, L.P., and their permitted successors and transferees, which are affiliates of DLJ Merchant Banking Partners III, L.P.
An affiliate of DLJ Merchant Banking Partners III, Inc. acted as the sole lead arranger, sole book runner, collateral agent and administrative agent under the Credit Agreement, and as an initial purchaser of the Senior Subordinated Notes—2012, for which it received customary fees and expenses during the fiscal year ended December 31, 2004 of $8.8 million.
The Company leases a facility from a stockholder and director as further explained in Note 16.
13. Environmental Matters
At December 31, 2004, the Company had a long-term liability of $4.8 million related to the potential MedSource remediation and the Collegeville remediation. At December 31, 2003, the Company had a long-term liability of $4.0 million related to the Collegeville remediation. The increase in the liability from December 31, 2003 relates primarily to the MedSource acquisition. The Company has prepared estimates of its potential liability for these properties, if any, based on available information. Changes in EPA standards, improvement in cleanup technology and discovery of additional information, however, could affect the estimated costs associated with these matters in the future. The clean up of these identified environmental matters is not expected to have a material adverse effect upon the liquidity, capital resources, business or consolidated financial position of the Company. However, one or more of such environmental matters could have a significant negative impact on consolidated financial results for a particular reporting period.
In 2001, the United States Environmental Protection Agency, or EPA, approved a Final Design Submission submitted by UTI Corporation, a Pennsylvania corporation and a wholly owned subsidiary of the Company ("UTI Pennsylvania"), to the EPA in respect of a July 1988 Administrative Consent Order issued by the EPA requiring UTI Pennsylvania to study and, if necessary, remediate the groundwater and soil beneath and around its plant in Collegeville, Pennsylvania. Since that time, UTI Pennsylvania has implemented and is operating successfully a contamination treatment system approved by the EPA. MedSource's subsidiaries also operate or formerly operated facilities located on properties where environmental contamination may have occurred or be present.
F-35
14. Fair value of Financial Instruments
The estimated fair value of financial instruments has been determined by the Company using available market information and appropriate methodologies; however, considerable judgment is required in interpreting market data to develop these estimates. Accordingly, the estimates presented herein are not necessarily indicative of the amounts that the Company could realize in a current market exchange. Certain of these financial instruments are with major financial institutions and expose the Company to market and credit risks and may at times be concentrated with certain counterparties or groups of counterparties. The creditworthiness of counterparties is continually reviewed, and full performance is anticipated.
The methods and assumptions used to estimate the fair value of each class of financial instruments are set forth below:
- •
- Cash and cash equivalents, accounts receivable and accounts payable—The carrying amounts of these items are a reasonable estimate of their fair values.
- •
- Borrowings under the Credit Agreement—Borrowings under the credit arrangements have variable rates that reflect currently available terms and conditions for similar debt. The carrying amount of this debt is a reasonable estimate of its fair value.
- •
- Borrowings under the Senior Subordinated Notes—2012—Borrowings under the Senior Subordinated Notes—2012 have a fixed rate that reflect currently available terms and conditions for similar debt. The carrying amount of this debt is a reasonable estimate of is fair value.
15. Business Segments
Concurrent with the acquisition of MedSource on June 30, 2004, the Company realigned its management to focus on the three main medical device markets which it serves. As a result of this realignment, the Company has three operating segments: endoscopy, cardiology and orthopaedic. The Company has determined that all of its operating segments meet the aggregation criteria of paragraph 17 of SFAS No. 131, and are treated as one reportable segment.
The following table presents net sales by country or geographic region based on the location of the customer for the years ended December 31, 2004, 2003 and 2002 (in thousands):
| | 2004
| | 2003
| | 2002
|
|---|
| Net sales: | | | | | | | | | |
| | United States | | $ | 270,513 | | $ | 143,634 | | $ | 107,782 |
| | Ireland | | | 16,620 | | | 7,270 | | | 5,734 |
| | Germany | | | 11,160 | | | 8,271 | | | 7,607 |
| | Netherlands | | | 5,218 | | | 3,969 | | | 3,564 |
| | United Kingdom | | | 3,442 | | | 2,312 | | | 2,806 |
| | Other Western Europe | | | 7,799 | | | 4,023 | | | 3,746 |
| | Asia Pacific | | | 1,793 | | | 1,799 | | | 1,859 |
| | Eastern Europe | | | 1,641 | | | 1,346 | | | 1,091 |
| | Central and South America | | | 1,173 | | | 1,022 | | | 724 |
| | Other | | | 810 | | | 577 | | | 928 |
| | |
| |
| |
|
| Total | | $ | 320,169 | | $ | 174,223 | | $ | 135,841 |
| | |
| |
| |
|
F-36
The following table presents long-lived assets based on the location of the asset (in thousands):
| | December 31
|
|---|
| | 2004
| | 2003
|
|---|
| Long lived assets: | | | | | | |
| | United States | | $ | 467,157 | | $ | 184,898 |
| | England | | | 510 | | | 592 |
| | Germany | | | 2,229 | | | 2,169 |
| | Ireland | | | 2,102 | | | 1,480 |
| | Mexico | | | 2,388 | | | 715 |
| | |
| |
|
| Total | | $ | 474,386 | | $ | 189,854 |
| | |
| |
|
The table above includes goodwill and intangibles and other assets of $371,334,590 and $150,595,603 in 2004 and 2003, respectively which are included in U.S. long-lived assets.
16. Commitments and Contingencies
The Company is obligated on various lease agreements for office space, automobiles and equipment, expiring through 2021, which are accounted for as operating leases.
The Company leases an office and manufacturing facility from a stockholder and director under a 4-year operating lease through July 2007. The lease requires payments of approximately $35,000 per month, which represents prevailing market rates.
Aggregate rental expense for the years ended December 31, 2004, 2003 and 2002 was $5,679,107, $3,131,878 and $2,298,689, respectively. The future minimum rental commitments under all operating leases are as follows (in thousands):
Year
| | Amount
|
|---|
| 2005 | | $ | 6,701 |
| 2006 | | | 4,458 |
| 2007 | | | 3,433 |
| 2008 | | | 2,601 |
| 2009 | | | 1,419 |
| Thereafter | | | 6,447 |
| | |
|
| Total | | $ | 25,059 |
| | |
|
The Company is involved in various legal proceedings in the ordinary course of business. In the opinion of management, after consultation with legal counsel, the outcome of such proceedings will not have a materially adverse effect on the Company's financial position or results of operations or cash flows.
The Company has various purchase commitments for materials, supplies, machinery and equipment incident to the ordinary conduct of business. Such purchase commitments are generally for a period of less than one year, often cancelable and able to be rescheduled and not at prices in excess of current market prices.
F-37
The Company declared and paid $22.1 million of accrued dividends on its Class A 5% Convertible Preferred Stock and its Class C Redeemable Preferred Stock on June 30, 2004. Concurrently, the Company repurchased all of its outstanding Class C Redeemable Preferred Stock at liquidation value, or $18.8 million. Since June 30, 2004, dividends have not been declared or accrued on Class A 5% Convertible Preferred Stock. To the extent all cumulative dividends would have been declared, a liability of $5.8 million and $18.8 million would have been recorded as of December 31, 2004 and 2003, respectively.
17. Supplemental Guarantor Condensed Consolidating Financial Statements
On November 22, 2005, the Company issued $305,000,000 in principal amount of 101/2% Senior Subordinated Notes due 2013. In connection with the issuance, all of its domestic subsidiaries have guaranteed (the "Subsidiary Guarantors") on a joint and several, full and unconditional basis. Certain foreign subsidiaries (the "Non Guarantor Subsidiaries") have not guaranteed such debt.
The following tables present the condensed consolidating balance sheets of the Company ("Parent"), the Subsidiary Guarantors and the Non Guarantor Subsidiaries as of December 31, 2004 and December 31, 2003 and the related condensed consolidating statements of operations and cash flows for each year in the three-year period ended December 31, 2004.
F-38
Condensed Consolidating Balance Sheets
December 31, 2004 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
|
|---|
| Cash and cash equivalents | | $ | — | | $ | 14,705 | | $ | 1,299 | | $ | — | | $ | 16,004 |
| Receivables, net | | | — | | | 46,805 | | | 1,549 | | | — | | | 48,354 |
| Inventories | | | — | | | 55,686 | | | 2,328 | | | — | | | 58,014 |
| Prepaid expenses and other | | | — | | | 3,299 | | | 172 | | | — | | | 3,471 |
| | |
| |
| |
| |
| |
|
| | Total current assets | | | — | | | 120,495 | | | 5,348 | | | — | | | 125,843 |
| Property, plant and equipment, net | | | — | | | 80,907 | | | 5,038 | | | — | | | 85,945 |
| Intercompany receivable (payable) | | | 49,072 | | | — | | | 524 | | | (49,596 | ) | | — |
| Investment in subsidiaries | | | 77,966 | | | 6,571 | | | — | | | (84,537 | ) | | — |
| Goodwill | | | — | | | 289,461 | | | — | | | — | | | 289,461 |
| Intangibles, net | | | — | | | 81,874 | | | — | | | — | | | 81,874 |
| Other assets, net | | | — | | | 17,135 | | | (29 | ) | | — | | | 17,106 |
| | |
| |
| |
| |
| |
|
| | Total assets | | $ | 127,038 | | $ | 596,443 | | $ | 10,881 | | $ | (134,133 | ) | $ | 600,229 |
| | |
| |
| |
| |
| |
|
| Current portion of long-term debt | | $ | — | | $ | 1,961 | | $ | — | | $ | — | | $ | 1,961 |
| Accounts payable | | | — | | | 19,360 | | | 1,087 | | | — | | | 20,447 |
| Accrued liabilities | | | (10,453 | ) | | 59,433 | | | 1,592 | | | — | | | 50,572 |
| | |
| |
| |
| |
| |
|
| | Total current liabilities | | | (10,453 | ) | | 80,754 | | | 2,679 | | | — | | | 72,980 |
| Note payable and long-term debt | | | — | | | 366,091 | | | — | | | — | | | 366,091 |
| Other long-term liabilities | | | — | | | 71,632 | | | 1,631 | | | (49,596 | ) | | 23,667 |
| | |
| |
| |
| |
| |
|
| | Total liabilities | | | (10,453 | ) | | 518,477 | | | 4,310 | | | (49,596 | ) | | 462,738 |
| Redeemable and convertible preferred stock | | | 30 | | | — | | | — | | | — | | | 30 |
| Equity | | | 137,461 | | | 77,966 | | | 6,571 | | | (84,537 | ) | | 137,461 |
| | |
| |
| |
| |
| |
|
| | Total liabilities and equity | | $ | 127,038 | | $ | 596,443 | | $ | 10,881 | | $ | (134,133 | ) | $ | 600,229 |
| | |
| |
| |
| |
| |
|
F-39
Condensed Consolidating Balance Sheets
December 31, 2003 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
|
|---|
| Cash and cash equivalents | | $ | — | | $ | 3,408 | | $ | 566 | | $ | — | | $ | 3,974 |
| Receivables, net | | | — | | | 19,076 | | | 1,664 | | | (79 | ) | | 20,661 |
| Inventories | | | — | | | 26,811 | | | 1,965 | | | — | | | 28,776 |
| Prepaid expenses and other | | | — | | | 1,686 | | | 78 | | | — | | | 1,764 |
| | |
| |
| |
| |
| |
|
| | Total current assets | | | — | | | 50,981 | | | 4,273 | | | (79 | ) | | 55,175 |
| Property, plant and equipment, net | | | — | | | 34,894 | | | 4,364 | | | — | | | 39,258 |
| Deferred income taxes | | | — | | | 29 | | | (29 | ) | | — | | | — |
| Intercompany receivable (payable) | | | — | | | 1,520 | | | — | | | (1,520 | ) | | — |
| Investment in subsidiaries | | | 79,008 | | | 3,822 | | | — | | | (82,830 | ) | | — |
| Goodwill | | | — | | | 113,855 | | | — | | | — | | | 113,855 |
| Intangibles, net | | | — | | | 68,813 | | | — | | | — | | | 68,813 |
| Other assets, net | | | — | | | 2,034 | | | — | | | — | | | 2,034 |
| | |
| |
| |
| |
| |
|
| | Total assets | | $ | 79,008 | | $ | 275,948 | | $ | 8,608 | | $ | (84,429 | ) | $ | 279,135 |
| | |
| |
| |
| |
| |
|
| Current portion of long-term debt | | $ | — | | $ | 12,370 | | $ | — | | $ | — | | $ | 12,370 |
| Accounts payable | | | — | | | 7,037 | | | 612 | | | (75 | ) | | 7,574 |
| Accrued liabilities | | | (7,873 | ) | | 59,075 | | | 1,392 | | | 1 | | | 52,595 |
| | |
| |
| |
| |
| |
|
| | Total current liabilities | | | (7,873 | ) | | 78,482 | | | 2,004 | | | (74 | ) | | 72,539 |
| Note payable and long-term debt | | | 13,085 | | | 110,791 | | | — | | | — | | | 123,876 |
| Other long-term liabilities | | | 4,390 | | | 7,667 | | | 2,782 | | | (1,525 | ) | | 13,314 |
| | |
| |
| |
| |
| |
|
| | Total liabilities | | | 9,602 | | | 196,940 | | | 4,786 | | | (1,599 | ) | | 209,729 |
| Redeemable and convertible preferred stock | | | 12,593 | | | — | | | — | | | — | | | 12,593 |
| Equity | | | 56,813 | | | 79,008 | | | 3,822 | | | (82,830 | ) | | 56,813 |
| | |
| |
| |
| |
| |
|
| | Total liabilities and equity | | $ | 79,008 | | $ | 275,948 | | $ | 8,608 | | $ | (84,429 | ) | $ | 279,135 |
| | |
| |
| |
| |
| |
|
F-40
Condensed Consolidating Statements of Operations
Year ended December 31, 2004 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net sales | | $ | — | | $ | 305,275 | | $ | 15,793 | | $ | (899 | ) | $ | 320,169 | |
| Cost of sales | | | — | | | 224,689 | | | 10,606 | | | (899 | ) | | 234,396 | |
| Selling, general and administrative expenses | | | 189 | | | 43,043 | | | 2,680 | | | — | | | 45,912 | |
| Research and development expenses | | | — | | | 2,426 | | | 242 | | | — | | | 2,668 | |
| Restructuring and other charges | | | — | | | 3,600 | | | — | | | — | | | 3,600 | |
| Amortization of intangibles | | | — | | | 5,539 | | | — | | | — | | | 5,539 | |
| | |
| |
| |
| |
| |
| |
| | Income (loss) from operations | | | (189 | ) | | 25,978 | | | 2,265 | | | — | | | 28,054 | |
| Interest expense (income) | | | 4,940 | | | 21,680 | | | 259 | | | — | | | 26,879 | |
| Other expense (income) | | | 1,731 | | | 2,388 | | | (807 | ) | | — | | | 3,312 | |
| Equity in earnings (losses) of affiliates | | | (1,435 | ) | | 2,485 | | | — | | | (1,050 | ) | | — | |
| Income tax expense (benefit) | | | (2,675 | ) | | 5,830 | | | 328 | | | — | | | 3,483 | |
| | |
| |
| |
| |
| |
| |
| | Net income (loss) | | $ | (5,620 | ) | $ | (1,435 | ) | $ | 2,485 | | $ | (1,050 | ) | $ | (5,620 | ) |
| | |
| |
| |
| |
| |
| |
Condensed Consolidating Statements of Operations
Year ended December 31, 2003 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net sales | | $ | — | | $ | 163,231 | | $ | 11,190 | | $ | (198 | ) | $ | 174,223 | |
| Cost of sales | | | — | | | 113,291 | | | 7,936 | | | (198 | ) | | 121,029 | |
| Selling, general and administrative expenses | | | 191 | | | 26,408 | | | 2,013 | | | — | | | 28,612 | |
| Research and development expenses | | | — | | | 2,405 | | | 198 | | | — | | | 2,603 | |
| Restructuring and other charges | | | — | | | 1,487 | | | — | | | — | | | 1,487 | |
| Amortization of intangibles | | | — | | | 4,828 | | | — | | | — | | | 4,828 | |
| | |
| |
| |
| |
| |
| |
| | Income from operations | | | (191 | ) | | 14,812 | | | 1,043 | | | — | | | 15,664 | |
| Interest expense (income) | | | 5,823 | | | 10,510 | | | 254 | | | — | | | 16,587 | |
| Other expense (income) | | | — | | | 522 | | | (513 | ) | | — | | | 9 | |
| Equity in earnings (losses) of affiliates | | | (11,148 | ) | | 1,009 | | | — | | | 10,139 | | | — | |
| Income tax expense | | | (2,358 | ) | | 15,937 | | | 293 | | | — | | | 13,872 | |
| | |
| |
| |
| |
| |
| |
| | Net income (loss) | | $ | (14,804 | ) | $ | (11,148 | ) | $ | 1,009 | | $ | 10,139 | | $ | (14,804 | ) |
| | |
| |
| |
| |
| |
| |
F-41
Condensed Consolidating Statements of Operations
Year ended December 31, 2002 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net sales | | $ | — | | $ | 127,566 | | $ | 8,354 | | $ | (79 | ) | $ | 135,841 | |
| Cost of sales | | | — | | | 89,990 | | | 6,829 | | | (79 | ) | | 96,740 | |
| Selling, general and administrative expenses | | | 223 | | | 21,681 | | | 1,644 | | | — | | | 23,548 | |
| Research and development expenses | | | — | | | 2,220 | | | 160 | | | — | | | 2,380 | |
| Restructuring and other charges | | | — | | | 2,440 | | | — | | | — | | | 2,440 | |
| Impairment of goodwill | | | — | | | 17,523 | | | — | | | — | | | 17,523 | |
| Impairment of intangible assets | | | — | | | 4,202 | | | — | | | — | | | 4,202 | |
| Amortization of intangibles | | | — | | | 4,703 | | | — | | | — | | | 4,703 | |
| | |
| |
| |
| |
| |
| |
| | Loss from operations | | | (223 | ) | | (15,193 | ) | | (279 | ) | | — | | | (15,695 | ) |
| Interest expense (income) | | | 5,166 | | | 11,423 | | | 334 | | | — | | | 16,923 | |
| Other income | | | — | | | (61 | ) | | — | | | — | | | (61 | ) |
| Equity in earnings (losses) of affiliates | | | (24,122 | ) | | (657 | ) | | — | | | 24,779 | | | — | |
| Income tax expense (benefit) | | | (2,099 | ) | | (3,090 | ) | | 44 | | | — | | | (5,145 | ) |
| | |
| |
| |
| |
| |
| |
| | Net income (loss) | | $ | (27,412 | ) | $ | (24,122 | ) | $ | (657 | ) | $ | 24,779 | | $ | (27,412 | ) |
| | |
| |
| |
| |
| |
| |
F-42
Condensed Consolidating Statements of Cash Flows
Year ended December 31, 2004 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net cash provided by (used for) operating activities | | $ | (4,797 | ) | $ | 23,196 | | $ | 3,832 | | $ | — | | $ | 22,231 | |
| | |
| |
| |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| | Capital expenditures | | | — | | | (12,774 | ) | | (1,126 | ) | | — | | | (13,900 | ) |
| | Proceeds form sale of equipment | | | — | | | 1,413 | | | — | | | — | | | 1,413 | |
| | Acquisitions, net of cash acquired | | | — | | | (214,982 | ) | | — | | | — | | | (214,982 | ) |
| | Other noncurrent assets | | | — | | | 93 | | | — | | | — | | | 93 | |
| | |
| |
| |
| |
| |
| |
| Net cash used in investing activities | | | — | | | (226,250 | ) | | (1,126 | ) | | — | | | (227,376 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Borrowings | | | — | | | 372,000 | | | — | | | — | | | 372,000 | |
| | Repayments | | | (74,478 | ) | | (110,561 | ) | | — | | | — | | | (185,039 | ) |
| | Repurchase of redeemable and convertible preferred stock | | | (12,583 | ) | | — | | | — | | | — | | | (12,583 | ) |
| | Dividends paid on redeemable and convertible preferred stock | | | (28,294 | ) | | — | | | — | | | — | | | (28,294 | ) |
| | Deferred financing fees | | | — | | | (17,061 | ) | | — | | | — | | | (17,061 | ) |
| | Proceeds from sale of stock | | | 88,048 | | | — | | | — | | | — | | | 88,048 | |
| | Intercompany advances | | | 32,104 | | | (30,063 | ) | | (2,041 | ) | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| Cash flows provided by (used for) financing activities | | | 4,797 | | | 214,315 | | | (2,041 | ) | | — | | | 217,071 | |
| | |
| |
| |
| |
| |
| |
| Effect of exchange rate changes in cash | | | — | | | 36 | | | 68 | | | — | | | 104 | |
| Net increase (decrease) in cash and cash equivalents | | | — | | | 11,297 | | | 733 | | | — | | | 12,030 | |
| Cash and cash equivalents, beginning of year | | | — | | | 3,408 | | | 566 | | | — | | | 3,974 | |
| | |
| |
| |
| |
| |
| |
| Cash and cash equivalents, end of year | | $ | — | | $ | 14,705 | | $ | 1,299 | | $ | — | | $ | 16,004 | |
| | |
| |
| |
| |
| |
| |
F-43
Condensed Consolidating Statements of Cash Flows
Year ended December 31, 2003 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net cash provided by (used for) operating activities | | $ | (2,262 | ) | $ | 14,911 | | $ | 1,766 | | $ | (23 | ) | $ | 14,392 | |
| | |
| |
| |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| | Capital expenditures | | | — | | | (5,193 | ) | | (1,178 | ) | | — | | | (6,371 | ) |
| | Transferred assets | | | — | | | 140 | | | (140 | ) | | — | | | — | |
| | Proceeds form sale of equipment | | | — | | | 79 | | | 14 | | | — | | | 93 | |
| | Acquisitions, net of cash acquired | | | — | | | (14,442 | ) | | 52 | | | — | | | (14,390 | ) |
| | Other noncurrent assets | | | — | | | 298 | | | — | | | — | | | 298 | |
| | |
| |
| |
| |
| |
| |
| Net cash used in investing activities | | | — | | | (19,118 | ) | | (1,252 | ) | | — | | | (20,370 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Borrowings | | | — | | | 8,000 | | | — | | | — | | | 8,000 | |
| | Repayments | | | — | | | (22,067 | ) | | — | | | — | | | (22,067 | ) |
| | Deferred financing fees | | | — | | | (673 | ) | | — | | | — | | | (673 | ) |
| | Intercompany advances | | | (16,455 | ) | | 16,899 | | | (455 | ) | | 11 | | | — | |
| | Proceeds from redeemable and convertible preferred stock | | | 18,717 | | | — | | | — | | | — | | | 18,717 | |
| | |
| |
| |
| |
| |
| |
| Cash flows provided by (used for) financing activities | | | 2,262 | | | 2,159 | | | (455 | ) | | 11 | | | 3,977 | |
| | |
| |
| |
| |
| |
| |
| Effect of exchange rate changes in cash | | | — | | | 46 | | | 52 | | | — | | | 98 | |
| Net increase (decrease) in cash and cash equivalents | | | — | | | (2,002 | ) | | 111 | | | (12 | ) | | (1,903 | ) |
| Cash and cash equivalents, beginning of year | | | — | | | 5,410 | | | 455 | | | 12 | | | 5,877 | |
| | |
| |
| |
| |
| |
| |
| Cash and cash equivalents, end of year | | $ | — | | $ | 3,408 | | $ | 566 | | $ | — | | $ | 3,974 | |
| | |
| |
| |
| |
| |
| |
F-44
Condensed Consolidating Statements of Cash Flows
Year ended December 31, 2002 (in 000s)
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net cash provided by operating activities | | $ | — | | $ | 12,681 | | $ | 1,329 | | $ | 12 | | $ | 14,022 | |
| | |
| |
| |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| | Capital expenditures | | | — | | | (5,533 | ) | | (685 | ) | | — | | | (6,218 | ) |
| | Transferred assets | | | — | | | 22 | | | (22 | ) | | — | | | — | |
| | Proceeds form sale of equipment | | | — | | | 398 | | | — | | | — | | | 398 | |
| | Acquisitions, net of cash acquired | | | — | | | (3,316 | ) | | — | | | — | | | (3,316 | ) |
| | Other noncurrent assets | | | — | | | (310 | ) | | — | | | — | | | (310 | ) |
| | |
| |
| |
| |
| |
| |
| Net cash used in investing activities | | | — | | | (8,739 | ) | | (707 | ) | | — | | | (9,446 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Borrowings | | | — | | | 11,500 | | | — | | | — | | | 11,500 | |
| | Repayments | | | — | | | (12,470 | ) | | — | | | — | | | (12,470 | ) |
| | Deferred financing fees | | | — | | | (547 | ) | | — | | | — | | | (547 | ) |
| | Intercompany advances | | | — | | | 457 | | | (457 | ) | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| Cash flows used in financing activities | | | — | | | (1,060 | ) | | (457 | ) | | — | | | (1,517 | ) |
| | |
| |
| |
| |
| |
| |
| Net increase in cash and cash equivalents | | | — | | | 2,882 | | | 165 | | | 12 | | | 3,059 | |
| Cash and cash equivalents, beginning of year | | | — | | | 2,528 | | | 290 | | | — | | | 2,818 | |
| | |
| |
| |
| |
| |
| |
| Cash and cash equivalents, end of year | | $ | — | | $ | 5,410 | | $ | 455 | | $ | 12 | | $ | 5,877 | |
| | |
| |
| |
| |
| |
| |
F-45
ACCELLENT INC.
Unaudited Condensed Consolidated Balance Sheets
As of September 30, 2005 and December 31, 2004
(In thousands)
| | September 30,
2005
| | December 31,
2004
| |
|---|
| Assets | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 7,388 | | $ | 16,004 | |
| | Accounts receivable, net of allowances of $2,067 and $2,909, respectively | | | 56,561 | | | 48,354 | |
| | Inventories | | | 61,393 | | | 58,014 | |
| | Prepaid expenses and other | | | 2,706 | | | 3,471 | |
| | |
| |
| |
| | | Total current assets | | | 128,048 | | | 125,843 | |
| Property and equipment, net | | | 96,102 | | | 85,945 | |
| Goodwill | | | 284,879 | | | 289,461 | |
| Intangibles, net | | | 83,654 | | | 81,874 | |
| Deferred financing costs and other assets | | | 15,985 | | | 17,106 | |
| | |
| |
| |
| | | Total assets | | $ | 608,668 | | $ | 600,229 | |
| | |
| |
| |
Liabilities and Stockholders' equity |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
| | Current portion of long term debt | | $ | 1,961 | | $ | 1,961 | |
| | Accounts payable | | | 20,218 | | | 20,447 | |
| | Accrued payroll and benefits | | | 12,143 | | | 13,011 | |
| | Accrued interest | | | 3,741 | | | 10,575 | |
| | Accrued expenses, other | | | 19,677 | | | 26,986 | |
| | |
| |
| |
| | | Total current liabilities | | | 57,740 | | | 72,980 | |
| Notes payable and long term debt | | | 372,663 | | | 366,091 | |
| Other long term liabilities | | | 25,255 | | | 23,667 | |
| | |
| |
| |
| | | Total liabilities | | | 455,658 | | | 462,738 | |
| Redeemable and convertible preferred stock | | | — | | | 30 | |
| Stockholders' equity: | | | | | | | |
| | Convertible preferred stock, aggregate liquidation preference of $265,739 | | | 171 | | | 166 | |
| | Common stock, par value $.01 per share, 50,000,000 shares authorized and 432,626 shares issued and outstanding | | | 4 | | | 4 | |
| | Additional paid in capital | | | 215,646 | | | 211,249 | |
| | Accumulated other comprehensive income | | | 928 | | | 1,716 | |
| | Accumulated deficit | | | (63,739 | ) | | (75,674 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 153,010 | | | 137,461 | |
| | |
| |
| |
| | | Total liabilities and stockholders' equity | | $ | 608,668 | | $ | 600,229 | |
| | |
| |
| |
The accompanying notes are an integral part of these financial statements.
F-46
ACCELLENT INC.
Unaudited Condensed Consolidated Statements of Operations
For the nine months ended September 30, 2005 and 2004
(In thousands)
| | Nine Months Ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| Net sales | | $ | 340,253 | | $ | 212,485 | |
| Cost of sales | | | 234,122 | | | 155,155 | |
| | |
| |
| |
| Gross profit | | | 106,131 | | | 57,330 | |
| Selling, general and administrative expenses | | | 49,539 | | | 30,681 | |
| Research and development expenses | | | 2,294 | | | 2,023 | |
| Restructuring and other charges | | | 3,824 | | | 2,107 | |
| Amortization of intangibles | | | 4,660 | | | 3,937 | |
| | |
| |
| |
| Income from operations | | | 45,814 | | | 18,582 | |
| Interest expense, net | | | 23,731 | | | 19,397 | |
| Other (income) expense, including debt prepayment penalties of $3,295 for the nine months ended September 30, 2004 | | | 105 | | | 3,284 | |
| | |
| |
| |
| Income (loss) before income taxes | | | 21,978 | | | (4,099 | ) |
| Income tax expense | | | 10,045 | | | 2,341 | |
| | |
| |
| |
| Net income (loss) | | | 11,933 | | | (6,440 | ) |
| Dividends on redeemable and convertible preferred stock | | | — | | | (8,201 | ) |
| | |
| |
| |
| Net income (loss) available to common stockholders | | $ | 11,933 | | $ | (14,641 | ) |
| | |
| |
| |
The accompanying notes are an integral part of these financial statements.
F-47
ACCELLENT INC.
Unaudited Condensed Consolidated Statements of Cash Flows
For the nine months ended September 30, 2005 and 2004
(In thousands)
| | Nine Months Ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| OPERATING ACTIVITIES: | | | | | | | |
| Net income (loss) | | $ | 11,933 | | $ | (6,440 | ) |
| Cash provided by operating activities: | | | | | | | |
| | Depreciation and amortization | | | 16,062 | | | 11,289 | |
| | Amortization of debt discounts and non cash interest accrued | | | 2,002 | | | 6,942 | |
| | Deferred income taxes | | | 6,532 | | | 993 | |
| | Non cash compensation charge | | | 3,556 | | | 161 | |
| | Loss (gain) on disposal of assets | | | 310 | | | (114 | ) |
| | Changes in operating assets and liabilities, net of business acquired: | | | | | | | |
| | | Increase in accounts receivable | | | (7,379 | ) | | (5,816 | ) |
| | | Increase in inventories | | | (2,462 | ) | | (6,435 | ) |
| | | Decrease in prepaid expenses and other | | | 790 | | | 898 | |
| | | (Decrease) increase in accounts payable and accrued expenses | | | (9,874 | ) | | 4,701 | |
| | |
| |
| |
| Net cash provided by operating activities | | | 21,470 | | | 6,179 | |
| | |
| |
| |
INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
| Purchase of property, plant & equipment | | | (15,586 | ) | | (8,718 | ) |
| Proceeds from sale of assets | | | 61 | | | 1,402 | |
| Acquisition of business | | | (20,098 | ) | | (214,001 | ) |
| | |
| |
| |
| Net cash used in investing activities | | | (35,623 | ) | | (221,317 | ) |
| | |
| |
| |
FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
| Proceeds from long term debt | | | 8,000 | | | 372,000 | |
| Principal payments on long term debt | | | (1,473 | ) | | (184,547 | ) |
| Proceeds from sale of convertible preferred stock | | | — | | | 88,048 | |
| Proceeds from exercise of stock options | | | 30 | | | — | |
| Redemption and repurchase of redeemable and convertible preferred stock | | | (30 | ) | | (12,583 | ) |
| Dividends paid on redeemable and convertible preferred stock | | | — | | | (28,367 | ) |
| Deferred financing fees | | | (826 | ) | | (15,968 | ) |
| | |
| |
| |
| Net cash provided by financing activities | | | 5,701 | | | 218,583 | |
| | |
| |
| |
| EFFECT OF EXCHANGE RATE CHANGES IN CASH: | | | (164 | ) | | (16 | ) |
| | |
| |
| |
| | | (Decrease) increase in cash and cash equivalents | | | (8,616 | ) | | 3,429 | |
| Cash and cash equivalents at beginning of period | | | 16,004 | | | 3,974 | |
| | |
| |
| |
| | | Cash and cash equivalents at end of period | | $ | 7,388 | | $ | 7,403 | |
| | |
| |
| |
Supplemental disclosure of non-cash investing activities: |
|
|
|
|
|
|
|
| | Cash paid for businesses acquired | | | | | | | |
| | | Working capital net of cash acquired of $14,304 in 2004 | | $ | 1,888 | | $ | 3,489 | |
| | | Property, plant and equipment | | | 5,241 | | | 44,822 | |
| | | Goodwill and intangible assets | | | 11,102 | | | 198,047 | |
| | | Long-term liabilities | | | (43 | ) | | (41,506 | ) |
| | | Change in accrued expenses for acquisitions related to earn-out and expense payments | | | 1,910 | | | 9,149 | |
| | |
| |
| |
| | | $ | 20,098 | | $ | 214,001 | |
| | |
| |
| |
| Property, plant and equipment acquired through long-term lease | | $ | 392 | | $ | — | |
| | |
| |
| |
The accompanying notes are an integral part of these financial statements.
F-48
ACCELLENT INC.
Notes to Unaudited Condensed Consolidated Financial Statements
1. Summary of Significant Accounting Policies
The accompanying unaudited condensed consolidated financial statements of Accellent Inc. ("the Company") have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial statements. Accordingly, they do not include all information and footnotes required by accounting principles generally accepted in the United States for complete financial statements. In the opinion of management, all adjustments (consisting only of normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for interim periods are not necessarily indicative of the results that may be expected for the fiscal year as a whole. For further information, refer to the Company's consolidated financial statements and footnotes thereto for the year ended December 31, 2004 included elsewhere herein.
The Company accounts for stock options issued to employees using the intrinsic value method of Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees", and related interpretations. Had the Company elected to recognize compensation expense for the granting of stock options based on the fair values at the grant dates consistent with the methodology prescribed by Statement of Financial Accounting Standards No. 148, "Accounting for Stock-Based Compensation," pro forma net income (loss) would have been reported as follows (in thousands):
| | Nine months ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| Net income (loss): | | | | | | | |
| | As reported | | $ | 11,933 | | $ | (6,440 | ) |
| | Add total stock compensation expense, net of tax, included in income (loss) as reported | | | 3,477 | | | 127 | |
| | Less total stock compensation expense—fair value method net of tax | | | (4,698 | ) | | (1,127 | ) |
| | |
| |
| |
| | Pro forma net income (loss) | | $ | 10,712 | | $ | (7,440 | ) |
| | |
| |
| |
Stock compensation expense, net of tax, included in net income as reported for the three and nine month periods ended September 30, 2005 includes a charge of $2,001,000 and $2,747,000, respectively, to increase the Company's liability for phantom stock plans due to the increase in value of the Company's common stock.
2. Acquisitions
On June 30, 2004, the Company acquired MedSource Technologies, Inc. ("MedSource"). The MedSource acquisition was accounted for as a purchase and accordingly the results of operations include MedSource's results beginning June 30, 2004. MedSource is an engineering and manufacturing services provider to the medical device industry. The Company acquired MedSource in order to create one of the largest providers of outsourced precision manufacturing and engineering services in the Company's target markets of the medical device industry. The purchase price was $219.7 million, consisting of $208.8 million in cash for the purchase of common stock and the cash out of options and
F-49
warrants, and $10.9 million of transaction fees. The purchase was financed by a combination of new debt and equity as discussed in notes 6 and 8. In addition, the then existing indebtedness of MedSource equal to $36.1 million plus related accrued interest was repaid in conjunction with the acquisition. The Company estimates that it will incur $17.4 million for integration and other liabilities.
The purchase price for the MedSource acquisition was allocated as follows (in thousands):
| Inventories | | $ | 27,707 | |
| Accounts receivable | | | 24,782 | |
| Prepaid expenses and other current assets | | | 702 | |
| Property and equipment | | | 44,144 | |
| Goodwill | | | 160,297 | |
| Intangible and other assets | | | 19,938 | |
| Current liabilities | | | (30,304 | ) |
| Debt assumed | | | (36,131 | ) |
| Other long term liabilities | | | (5,736 | ) |
| | |
| |
| Cash paid, net of cash acquired of $14,304 | | $ | 205,399 | |
| | |
| |
The decrease in goodwill for the nine months ended September 30, 2005 is primarily related to the reduction in accrued integration and restructuring costs for MedSource as discussed in Note 3, and the expected utilization of tax benefits acquired from MedSource as discussed in Note 7.
On September 12, 2005, the Company acquired substantially all of the assets of Campbell Engineering, Inc. ("Campbell") and certain real property owned by the shareholders of Campbell and used by Campbell in the conduct of its business. The Campbell acquisition was accounted for as a purchase and accordingly the results of operations include Campbell's results beginning September 12, 2005. Campbell is a privately held engineering and manufacturing firm providing design, analysis, precision fabrication, assembly and testing of primarily orthopaedic implants and instruments. The Company acquired Campbell to expand product offerings and manufacturing capabilities in the orthopaedic medical device market. The purchase price was $18.2 million, all of which was paid in cash. An additional $12.0 million of consideration will be paid, if at all, pursuant to an earnout arrangement contingent upon the 2005 and 2006 full year financial performance of the acquired business.
The Company has preliminarily identified the tangible and intangible assets as well as the liabilities assumed in the Campbell acquisition. The final purchase price allocation could vary from the
F-50
preliminary allocation. The preliminary purchase price allocation for the Campbell acquisition was as follows (in thousands):
| Inventories | | $ | 1,401 | |
| Accounts receivable | | | 1,111 | |
| Prepaid expenses and other current assets | | | 21 | |
| Property and equipment | | | 5,241 | |
| Goodwill | | | 4,662 | |
| Intangible and other assets | | | 6,440 | |
| Current liabilities | | | (645 | ) |
| Debt assumed | | | (43 | ) |
| | |
| |
| Total purchase price | | $ | 18,188 | |
| | |
| |
The amounts allocated to intangible assets for the Campbell acquisition by intangible asset class, and the respective weighted average amortization period is as follows:
| | Amount
| | Weighted
average useful life
|
|---|
| | (in thousands)
| | (in years)
|
|---|
| Customer relationships | | $ | 6,410 | | 13.5 |
| Non-compete | | | 30 | | 5.0 |
| | |
| |
|
| | | $ | 6,440 | | 13.4 |
| | |
| |
|
The entire amount allocated to goodwill and other intangible assets for the Campbell acquisition will be amortizable and deductible for tax purposes.
The Company determines the value and potential purchase price of target acquisition companies based on multiples of future cash flow. These cash flow projections may include an estimate of improved cash flow performance as compared to historical performance of the target acquisition company based on expected future growth and/or cost reduction opportunities. The value of the acquired company based on our cash flow analysis may differ significantly from the carrying value of the acquired net assets, resulting in an allocation of a significant portion of the purchase price to goodwill and intangible assets.
The following unaudited pro forma consolidated financial information reflects the purchase of MedSource and Campbell assuming the acquisitions had occurred as of the beginning of each period. This unaudited pro forma information has been provided for informational purposes only and is not necessarily indicative of the results of operations or financial condition that actually would have been achieved if the acquisitions had been on the date indicated, or that may be reported in the future (in thousands):
| | Nine months ended
September 30, 2005
| | Nine months ended
September 30, 2004
|
|---|
| Net sales | | $ | 348,542 | | $ | 314,080 |
| Net income | | | 12,530 | | | 115 |
F-51
The pro forma net income for the nine months ended September 30, 2004 includes $2.3 million of restructuring charges recognized by MedSource.
3. Restructuring and Other Charges
In connection with the MedSource acquisition, the Company identified $17.2 million of costs associated with eliminating duplicate positions and plant consolidations, which is comprised of $9.7 million in severance payments and $7.5 million in lease termination and other contract termination costs. Severance payments relate to approximately 520 employees in manufacturing, selling and administration and are expected to be paid by the end of fiscal year 2007. All other costs are expected to be paid by 2018. The costs of these plant consolidations were reflected in the purchase price of MedSource in accordance with the FASB Emerging Issues Task Force ("EITF") No. 95-3,Recognition of Liabilities in Connection with a Purchase Business Combination. These costs include estimates to close facilities and consolidate manufacturing capacity, and are subject to change based on the actual costs incurred to close these facilities. Changes to these estimates could increase or decrease the amount of the purchase price allocated to goodwill in the near term.
The Company determined that one facility identified as a closure site would likely not be closed due to the nature of product manufactured at that site and the expected costs and benefits to transfer production to another manufacturing location. The Company reduced the MedSource integration accrual by approximately $3.0 million to eliminate the cost to close this facility. Additionally, the completion of detailed closure plans resulted in a change in projected closure dates to later dates for certain facilities. The Company reduced the MedSource integration accrual by approximately $0.7 million to lower the projected costs to exit leased facilities due to the revised closure dates. Other reductions to the MedSource integration accrual during the first nine months of fiscal year 2005 included the settlement of a contract termination in connection with a closed facility for approximately $0.3 million less than originally projected. During the first nine months of fiscal year 2005, the total reductions to the MedSource integration accrual were $4.3 million, resulting in a decrease to the purchase price allocation to goodwill in the same amount.
The Company recognized $3.8 million of restructuring charges and acquisition integration costs during the first nine months of fiscal year 2005, including $1.2 million of severance costs and $2.1 million of other exit costs including costs to move production processes from five facilities that are closing to other production facilities of the Company. In addition to the $3.3 million in restructuring charges incurred during first nine months of fiscal year 2005, the Company incurred $0.5 million of costs for the integration of MedSource.
F-52
The following table summarizes the recorded accruals and activity related to the restructuring activities (in thousands):
| | Employee costs
| | Other costs
| | Total
| |
|---|
| Balance as of December 31, 2004 | | $ | 7,764 | | $ | 9,913 | | $ | 17,677 | |
| Adjustment to planned plant closure and severance costs for the MedSource integration | | | (1,932 | ) | | (2,387 | ) | | (4,319 | ) |
| Restructuring and integration charges incurred | | | 1,191 | | | 2,633 | | | 3,824 | |
| Paid year to date | | | (3,886 | ) | | (3,080 | ) | | (6,966 | ) |
| | |
| |
| |
| |
| Balance September 30, 2005 | | $ | 3,137 | | $ | 7,079 | | $ | 10,216 | |
| | |
| |
| |
| |
4. Comprehensive Income
Comprehensive income (loss) represents net income plus the results of any stockholders' equity changes related to currency translation. For the three and nine months ended September 30, 2005 and 2004, the Company reported comprehensive income of (in thousands):
| | Nine months ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| Net income (loss) | | $ | 11,933 | | $ | (6,440 | ) |
| Cumulative translation adjustments | | | (788 | ) | | (91 | ) |
| | |
| |
| |
| Comprehensive income (loss) | | $ | 11,145 | | $ | (6,531 | ) |
| | |
| |
| |
5. Inventories
Inventories at September 30, 2005 and December 31, 2004 consisted of the following (in thousands):
| | September 30, 2005
| | December 31, 2004
|
|---|
| Raw materials | | $ | 22,174 | | $ | 20,939 |
| Work in process | | | 23,979 | | | 24,068 |
| Finished goods | | | 15,240 | | | 13,007 |
| | |
| |
|
| Total | | $ | 61,393 | | $ | 58,014 |
| | |
| |
|
F-53
6. Short-Term and Long-Term Debt:
Long-term debt at September 30, 2005 and December 31, 2004 consisted of the following (in thousands):
| | September 30, 2005
| | December 31, 2004
| |
|---|
| Senior secured credit facility term loans, interest at 6.09% at September 30, 2005 and 5.28% at December 31, 2004 | | $ | 191,575 | | $ | 193,030 | |
| Senior secured credit facility revolving credit facility loans, interest at 6.84% at September 30, 2005 | | | 8,000 | | | — | |
| Senior subordinated notes maturing July 15, 2012, interest at 10% | | | 175,000 | | | 175,000 | |
| Capital lease obligations. | | | 49 | | | 22 | |
| | |
| |
| |
| Total debt | | | 374,624 | | | 368,052 | |
| Less current portion | | | (1,961 | ) | | (1,961 | ) |
| | |
| |
| |
| Long term debt, excluding current portion | | $ | 372,663 | | $ | 366,091 | |
| | |
| |
| |
The Company's subsidiary, Accellent Corp., entered into a Senior Secured Credit Facility dated June 30, 2004 (the "Credit Agreement") which includes $194.0 million of term loans and up to $40.0 million available under the revolving credit facility. Additionally, the Company may borrow up to $40.0 million in additional term loans, with the approval of participating lenders. On March 25, 2005, Accellent Corp. amended its Credit Agreement to lower the interest rate applicable to the term loans as follows: on base rate loans from base rate (generally the applicable prime lending rate of Credit Suisse First Boston, as announced from time to time) plus 2.00% to base rate plus 1.25%, on euro dollar rate loans from LIBOR plus 3.00% to LIBOR plus 2.25%. In addition, the amendment increased the amount of potential additional term loans from $40.0 million to $50.0 million and allowed increased flexibility in, and funds for, acquisitions. Additional term loans require the approval of participating lenders. Principal payments will continue to be due in the amounts of $1.9 million per year plus, beginning in 2006, 75% of Excess Cash Flow, as defined by the Credit Agreement. The balance is due June 30, 2010. As of September 30, 2005, $5.8 million of the revolving credit facility was supporting the Accellent Corp.'s letters of credit, and $8.0 million was borrowed leaving $26.2 million available.
In connection with the acquisition of Campbell on September 12, 2005, the Accellent Corp. used $8.0 million of its revolving credit facility (the "Revolving Loan") under the Credit Agreement to fund part of the acquisition. The Revolving Loan is a euro dollar rate loan accruing interest at an annual rate equal to LIBOR plus 3.00%. The Revolving Loan matures on June 30, 2010.
Accellent Corp. incurred $0.8 million in fees in connection with the amendment of the Credit Agreement during the first nine months of fiscal year 2005, which will be amortized to interest expense over the remaining term of the Credit Agreement.
Also in connection with the amendment, the Company wrote off deferred financing costs resulting in a charge to interest expense of $0.2 million for the first six months of fiscal year 2005.
On June 30, 2004, Accellent Corp. issued $175.0 million in aggregate principal amount of 10% senior subordinated notes due 2012 (the "Senior Subordinated Notes"). Interest on the Senior Subordinated Notes is payable on January 15th and July 15th of each year.
F-54
Accellent Corp.'s debt agreements contain various covenants, including minimum cash flow (as defined therein), debt service coverage ratios and maximum capital spending limits. In addition, the debt agreements restrict Accellent Corp. from paying dividends and making certain investments. The covenants and restrictions of the indenture governing the Senior Subordinated Notes apply only to Accellent Corp. and not the Company. All covenants and restrictions under the Credit Agreement apply to Accellent Corp., and the covenants and restrictions other than financial covenants apply to the Company.
As of September 30, 2005, the Company and Accellent Corp. were in compliance with their respective covenants under the Credit Agreement and the Senior Subordinated Notes.
7. Income Taxes
Income tax expense for the nine months ended September 30, 2005 was $10.0 million on pre-tax income of $22.0 million, or 45.7% of pre-tax income. The effective rate is higher than the statutory rate primarily due to $6.5 million in charges for non-cash deferred income taxes, including $5.2 million for tax benefits acquired from MedSource which have been credited to goodwill and not benefited in the statement of operations and $1.3 million of charges for the different book and tax treatment for goodwill. The remaining $3.5 million of income tax expense for the nine months ended September 30, 2005 includes $3.3 million for certain state and foreign income taxes and $0.2 million for domestic federal income taxes. The Company expects to offset most of its 2005 domestic federal income taxes with net operating loss carryforwards. For the nine months ended September 30, 2004, the Company recorded a tax provision of $2.3 million on a pre-tax net loss of $4.1 million, primarily due to provisions for certain state and foreign income taxes.
8. Capital Stock and Redeemable Preferred Stock
The Company has 50,000,000 shares of common stock authorized and 432,626 shares issued and outstanding, $.01 per value per share.
For a more detailed discussion of the capital stock and redeemable stock of the Company, refer to footnotes 10 and 11 of the Company's consolidated financial statements and footnotes included elsewhere herein.
F-55
The following table summarizes the amounts recorded as redeemable and convertible preferred stock, convertible preferred stock and additional paid-in capital for the nine months ended September 30, 2005 (in thousands):
| | Redeemable
and convertible
preferred stock
| | Convertible
preferred stock
| | Additional
paid-in capital
|
|---|
| Beginning balance, January 1, 2005 | | $ | 30 | | $ | 166 | | $ | 211,249 |
| Amortization of stock-based compensation | | | — | | | — | | | 729 |
| Compensation charge associated with phantom stock plans | | | — | | | — | | | 29 |
| Issuance of convertible preferred stock in connection with acquisition | | | | | | 5 | | | 3,599 |
| Exercise of employee stock options | | | — | | | | | | 30 |
| Adjustment to reduce accrued dividend | | | | | | | | | 10 |
| Redemption of redeemable and convertible preferred stock | | | (30 | ) | | — | | | — |
| | |
| |
| |
|
| Ending balance, September 30, 2005 | | $ | — | | $ | 171 | | $ | 215,646 |
| | |
| |
| |
|
During the nine months ended September 30, 2005, the outstanding shares of Class B-2 Redeemable and Convertible Preferred Stock were redeemed for the carrying value of $30,000. In addition, the Company issued 244,832 shares of its Class A-7 5% Convertible Preferred Stock to the former owners of Venusa in connection with Venusa achieving certain earn-out targets for fiscal year 2004. The Company recorded a contribution to capital of $3.6 million to reflect the issuance of these shares to the former owners of Venusa. There are no additional earn-out provisions relating to the Venusa acquisition.
In July 2005, the Board of Directors approved the grant of 609,237 shares of its restricted stock to certain members of the Company's management. The shares vest 100% on the four year anniversary from the date of grant. The Company is recording compensation expense of $10.0 million over the vesting period of the restricted shares, or 48 months, resulting in a quarterly compensation charge to Selling, General and Administrative expense, or SG&A, of $624,000 starting in July 2005. Also in July 2005, the Board of Directors approved the grant of 281,152 stock options to certain members of the Company's management at option prices that are below the fair market value of the underlying common stock. The Company is recording compensation expense of $2.3 million over the vesting period of the stock options, or 60 months, resulting in a quarterly compensation charge to SG&A of $115,000 starting in July 2005.
9. Pension Plans
Effective January 1, 2004, the Company adopted SFAS No. 132 (revised 2003),Employers' Disclosures about Pensions and Other Postretirement Benefits. This standard requires the disclosure of the
F-56
components of net periodic benefit cost recognized during interim periods. Components on net periodic pension cost for the nine months ended September 30, 2005 and 2004 were as follows (in thousands):
| | Nine months ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| Service cost | | $ | 70 | | $ | 57 | |
| Interest cost | | | 104 | | | 97 | |
| Expected return of plan assets | | | (44 | ) | | (42 | ) |
| Recognized net actuarial loss | | | 35 | | | 17 | |
| | |
| |
| |
| | | $ | 165 | | $ | 129 | |
| | |
| |
| |
Assuming that the actual return on plan assets is consistent with the expected annualized rate of 7.0% for the remainder of fiscal year 2005, and that interest rates remain constant, the Company would be required to make total contributions to its pension plans of $74,000 for fiscal year 2005.
10. Subsequent Events
On October 6, 2005, the Company purchased 100% of the outstanding membership interests in Machining Technology Group, LLC ("MTG"), an Arlington, Tennessee based privately held manufacturing and engineering company specializing in rapid prototyping and manufacturing of specialized orthopaedic implants and instruments for the orthopaedic industry. The purchase price was $50.2 million which was paid in cash of $34.0 million and Class A-9 5% Convertible Preferred stock of the Company valued at $16.2 million. An additional $6.0 million of consideration will be paid, if at all, pursuant to an earn out arrangement payable contingent upon the 2006 full year financial performance of MTG. If the earn out arrangement is earned, the payment would be expected to be made in the second quarter of 2007. The closing cash payment was funded with a combination of approximately $21.5 million of proceeds received from a draw on Accellent Corp.'s existing revolving credit facility pursuant to its Credit Agreement, and approximately $12.5 million of proceeds received from the utilization of the incremental term loan facility provided for under the Credit Agreement. Following the acquisition, the total amount available to be drawn on the revolving credit facility was approximately $4.7 million, net of approximately $5.8 million of letters of credit outstanding that reduced the amounts available under the revolving credit facility.
On October 7, 2005, the Company's signed a definitive agreement and plan of merger (the "Merger Agreement") with an affiliate of Kohlberg Kravis Roberts & Co. L.P. (the "KKR Affiliate"). In addition, the KKR Affiliate, the Company and certain of its stockholders executed a voting agreement in which the stockholders agreed to vote their shares in favor of the merger. The Company anticipates that it will refinance existing debt, including Accellent Corp.'s Credit Facility and existing Senior Subordinated Notes, in connection with the closing of the merger.
On October 21, 2005, Accellent Corp. commenced a cash tender offer and consent solicitation for any and all of Accellent Corp.'s outstanding Senior Subordinated Notes (the "Offer"). The consents of the holders of the outstanding Senior Subordinated Notes are being solicited to eliminate substantially all of the restrictive and reporting covenants, certain events of default and certain other provisions in
F-57
the indenture governing the Senior Subordinated Notes. The Company has received tenders and the requisite consents for 100% of the Senior Subordinated Notes.
The pending merger with the KKR Affiliate, or the "Merger", is subject to various customary conditions, including adoption of the merger agreement by the Company's stockholders, the expiration or early termination of any applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, the absence of certain legal impediments, the receipt of certain regulatory approvals and the receipt of debt and equity financings. If all of the other conditions to the Merger are satisfied, under the terms of the existing financing commitment letters, the Company can consummate the Merger even if the Offer is not consummated and the requisite consents, as defined in the Offer, are not obtained through a covenant defeasance of the Senior Subordinated Notes in accordance with the terms of the Senior Subordinated Notes. If the Merger is not consummated, Accellent Corp. will not purchase any of the Senior Subordinated Notes tendered pursuant to the Offer or cause the proposed amendments in the Offer to become operative.
11. Supplemental Guarantor Condensed Consolidating Financial Statements:
On November 22, 2005, the Company issued $305,000,000 in principal amount of 101/2% Senior Subordinated Notes due 2013. In connection with the issuance, all of its domestic subsidiaries have guaranteed (the "Subsidiary Guarantors") on a joint and several, full and unconditional basis. Certain foreign subsidiaries (the "Non Guarantor Subsidiaries") have not guaranteed such debt.
The following tables present the unaudited condensed consolidating statements of operations for the nine months ended September 30, 2005 and September 30, 2004 of the Company ("Parent"), the Subsidiary Guarantors and the Non-Guarantor Subsidiaries, the unaudited condensed consolidating balance sheets as of September 30, 2005 and December 31, 2004, and cash flows for the nine months ended September 30, 2005 and September 30, 2004.
Condensed Consolidating Statements of Operations
Nine months ended September 30, 2005 (in thousands):
| | Parent
| | Subsidiary
Guarantors
| | Non-Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
|
|---|
| Net sales | | $ | — | | $ | 326,707 | | $ | 14,006 | | $ | (460 | ) | $ | 340,253 |
| Cost of sales | | | — | | | 226,176 | | | 8,406 | | | (460 | ) | | 234,122 |
| Selling, general and administrative expenses | | | 729 | | | 47,067 | | | 1,743 | | | — | | | 49,539 |
| Research and development expenses | | | — | | | 2,023 | | | 271 | | | — | | | 2,294 |
| Restructuring and other charges | | | — | | | 3,697 | | | 127 | | | — | | | 3,824 |
| Amortization of intangibles | | | — | | | 4,660 | | | — | | | — | | | 4,660 |
| | |
| |
| |
| |
| |
|
| | Income (loss) from operations | | | (729 | ) | | 43,084 | | | 3,459 | | | — | | | 45,814 |
| Interest expense | | | — | | | 23,724 | | | 7 | | | — | | | 23,731 |
| Other expense (income) | | | — | | | 315 | | | (210 | ) | | — | | | 105 |
| Equity in earnings of affiliates | | | 12,662 | | | 2,457 | | | — | | | (15,119 | ) | | — |
| Income tax expense | | | — | | | 8,840 | | | 1,205 | | | — | | | 10,045 |
| | |
| |
| |
| |
| |
|
| | Net income (loss) | | $ | 11,933 | | $ | 12,662 | | $ | 2,457 | | $ | (15,119 | ) | $ | 11,933 |
| | |
| |
| |
| |
| |
|
F-58
Consolidating Statements of Operations
Nine months ended September 30, 2004 (in thousands):
| | Parent
| | Subsidiary
Guarantors
| | Non-Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net sales | | $ | — | | $ | 201,637 | | $ | 11,577 | | $ | (729 | ) | $ | 212,485 | |
| Cost of sales | | | — | | | 148,134 | | | 7,750 | | | (729 | ) | | 155,155 | |
| Selling, general and administrative expenses | | | 127 | | | 28,612 | | | 1,942 | | | — | | | 30,681 | |
| Research and development expenses | | | — | | | 1,824 | | | 199 | | | — | | | 2,023 | |
| Restructuring and other charges | | | — | | | 2,107 | | | — | | | — | | | 2,107 | |
| Amortization of intangibles | | | — | | | 3,937 | | | — | | | — | | | 3,937 | |
| | |
| |
| |
| |
| |
| |
| | Income (loss) from operations | | | (127 | ) | | 17,023 | | | 1,686 | | | — | | | 18,582 | |
| Interest expense | | | 4,940 | | | 14,250 | | | 207 | | | — | | | 19,397 | |
| Other expense (income) | | | 1,731 | | | 1,631 | | | (78 | ) | | — | | | 3,284 | |
| Equity in earnings of affiliates | | | (2,293 | ) | | 1,420 | | | — | | | 873 | | | — | |
| Income tax expense (benefit) | | | (2,651 | ) | | 4,855 | | | 137 | | | — | | | 2,341 | |
| | |
| |
| |
| |
| |
| |
| | Net income (loss) | | $ | (6,440 | ) | $ | (2,293 | ) | $ | 1,420 | | $ | 873 | | $ | (6,440 | ) |
| | |
| |
| |
| |
| |
| |
F-59
Condensed Consolidating Balance Sheets
September 30, 2005 (in thousands):
| | Parent
| | Subsidiary
Guarantors
| | Non-Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
|
|---|
| Cash and cash equivalents | | $ | — | | $ | 6,074 | | $ | 1,314 | | $ | — | | $ | 7,388 |
| Receivables, net | | | — | | | 54,195 | | | 2,425 | | | (59 | ) | | 56,561 |
| Inventories | | | — | | | 58,685 | | | 2,708 | | | — | | | 61,393 |
| Prepaid expenses and other | | | — | | | 2,554 | | | 152 | | | — | | | 2,706 |
| | |
| |
| |
| |
| |
|
| | Total current assets | | | — | | | 121,508 | | | 6,599 | | | (59 | ) | | 128,048 |
| Property, plant and equipment, net | | | — | | | 90,995 | | | 5,107 | | | — | | | 96,102 |
| Intercompany receivable (payable) | | | 52,620 | | | 80 | | | 2,143 | | | (54,843 | ) | | — |
| Investment in subsidiaries | | | 89,841 | | | 8,430 | | | — | | | (98,271 | ) | | — |
| Goodwill | | | — | | | 284,879 | | | — | | | — | | | 284,879 |
| Intangibles, net | | | — | | | 83,654 | | | — | | | — | | | 83,654 |
| Deferred financing costs and other assets | | | — | | | 15,960 | | | 25 | | | — | | | 15,985 |
| | |
| |
| |
| |
| |
|
| | Total assets | | $ | 142,461 | | $ | 605,506 | | $ | 13,874 | | $ | (153,173 | ) | $ | 608,668 |
| | |
| |
| |
| |
| |
|
| Current portion of long-term debt | | $ | — | | $ | 1,961 | | $ | — | | $ | — | | $ | 1,961 |
| Accounts payable | | | — | | | 19,159 | | | 1,118 | | | (59 | ) | | 20,218 |
| Accrued liabilities | | | (10,549 | ) | | 43,351 | | | 2,809 | | | (50 | ) | | 35,561 |
| | |
| |
| |
| |
| |
|
| | Total current liabilities | | | (10,549 | ) | | 64,471 | | | 3,927 | | | (109 | ) | | 57,740 |
| Note payable and long-term debt | | | — | | | 372,663 | | | — | | | — | | | 372,663 |
| Other long-term liabilities | | | — | | | 78,531 | | | 1,517 | | | (54,793 | ) | | 25,255 |
| | |
| |
| |
| |
| |
|
| | Total liabilities | | | (10,549 | ) | | 515,665 | | | 5,444 | | | (54,902 | ) | | 455,658 |
| Equity | | | 153,010 | | | 89,841 | | | 8,430 | | | (98,271 | ) | | 153,010 |
| | |
| |
| |
| |
| |
|
| | Total liabilities and equity | | $ | 142,461 | | $ | 605,506 | | $ | 13,874 | | $ | (153,173 | ) | $ | 608,668 |
| | |
| |
| |
| |
| |
|
F-60
11. Supplemental Guarantor Condensed Consolidating Financial Statements (Continued):
Condensed Consolidating Balance Sheets
December 31, 2004 (in thousands):
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
|
|---|
| Cash and cash equivalents | | $ | — | | $ | 14,705 | | $ | 1,299 | | $ | — | | $ | 16,004 |
| Receivables, net | | | — | | | 46,805 | | | 1,549 | | | — | | | 48,354 |
| Inventories | | | — | | | 55,686 | | | 2,328 | | | — | | | 58,014 |
| Prepaid expenses and other | | | — | | | 3,299 | | | 172 | | | — | | | 3,471 |
| | |
| |
| |
| |
| |
|
| | Total current assets | | | — | | | 120,495 | | | 5,348 | | | — | | | 125,843 |
| Property, plant and equipment, net | | | — | | | 80,907 | | | 5,038 | | | — | | | 85,945 |
| Intercompany receivable (payable) | | | 49,072 | | | — | | | 524 | | | (49,596 | ) | | — |
| Investment in subsidiaries | | | 77,966 | | | 6,571 | | | — | | | (84,537 | ) | | — |
| Goodwill | | | — | | | 289,461 | | | — | | | — | | | 289,461 |
| Intangibles, net | | | — | | | 81,874 | | | — | | | — | | | 81,874 |
| Other assets, net | | | — | | | 17,135 | | | (29 | ) | | — | | | 17,106 |
| | |
| |
| |
| |
| |
|
| | Total assets | | $ | 127,038 | | $ | 596,443 | | $ | 10,881 | | $ | (134,133 | ) | $ | 600,229 |
| | |
| |
| |
| |
| |
|
| Current portion of long-term debt | | $ | — | | $ | 1,961 | | $ | — | | $ | — | | $ | 1,961 |
| Accounts payable | | | — | | | 19,360 | | | 1,087 | | | — | | | 20,447 |
| Accrued liabilities | | | (10,453 | ) | | 59,433 | | | 1,592 | | | — | | | 50,572 |
| | |
| |
| |
| |
| |
|
| | Total current liabilities | | | (10,453 | ) | | 80,754 | | | 2,679 | | | — | | | 72,980 |
| Note payable and long-term debt | | | — | | | 366,091 | | | — | | | — | | | 366,091 |
| Other long-term liabilities | | | — | | | 71,632 | | | 1,631 | | | (49,596 | ) | | 23,667 |
| | |
| |
| |
| |
| |
|
| | Total liabilities | | | (10,453 | ) | | 518,477 | | | 4,310 | | | (49,596 | ) | | 462,738 |
| Redeemable and convertible preferred stock | | | 30 | | | — | | | — | | | — | | | 30 |
| Equity | | | 137,461 | | | 77,966 | | | 6,571 | | | (84,537 | ) | | 137,461 |
| | |
| |
| |
| |
| |
|
| | Total liabilities and equity | | $ | 127,038 | | $ | 596,443 | | $ | 10,881 | | $ | (134,133 | ) | $ | 600,229 |
| | |
| |
| |
| |
| |
|
F-61
11. Supplemental Guarantor Condensed Consolidating Financial Statements (Continued):
Consolidating Statements of Cash Flows
Nine months ended September 30, 2005 (in thousands):
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net cash provided by operating activities | | $ | — | | $ | 18,635 | | $ | 2,835 | | $ | — | | $ | 21,470 | |
| | |
| |
| |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | | | — | | | | |
| | Capital expenditures | | | — | | | (14,692 | ) | | (894 | ) | | — | | | (15,586 | ) |
| | Transferred assets | | | — | | | 201 | | | (201 | ) | | — | | | — | |
| | Proceeds from sale of equipment | | | — | | | 61 | | | — | | | — | | | 61 | |
| | Acquisitions, net of cash acquired | | | — | | | (20,098 | ) | | — | | | — | | | (20,098 | ) |
| | |
| |
| |
| |
| |
| |
| Net cash used in investing activities | | | — | | | (34,528 | ) | | (1,095 | ) | | — | | | (35,623 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Proceeds from debt | | | — | | | 8,000 | | | — | | | — | | | 8,000 | |
| | Repayments | | | — | | | (1,473 | ) | | — | | | — | | | (1,473 | ) |
| | Intercompany advances | | | — | | | 1,619 | | | (1,619 | ) | | — | | | — | |
| | Proceeds from exercise of stock options | | | 30 | | | — | | | — | | | — | | | 30 | |
| | Redemption of redeemable and convertible preferred stock | | | (30 | ) | | — | | | — | | | — | | | (30 | ) |
| | Deferred financing fees | | | — | | | (826 | ) | | — | | | — | | | (826 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows (used in) provided by financing activities | | | — | | | 7,320 | | | (1,619 | ) | | — | | | 5,701 | |
| | |
| |
| |
| |
| |
| |
| Effect of exchange rate changes in cash | | | — | | | (58 | ) | | (106 | ) | | — | | | (164 | ) |
| | |
| |
| |
| |
| |
| |
| Net (decrease) increase in cash and cash equivalents | | | — | | | (8,631 | ) | | 15 | | | — | | | (8,616 | ) |
| Cash and cash equivalents, beginning of period | | | — | | | 14,705 | | | 1,299 | | | — | | | 16,004 | |
| | |
| |
| |
| |
| |
| |
| Cash and cash equivalents, end of period | | $ | — | | $ | 6,074 | | $ | 1,314 | | $ | — | | $ | 7,388 | |
| | |
| |
| |
| |
| |
| |
F-62
11. Supplemental Guarantor Condensed Consolidating Financial Statements (Continued):
Consolidating Statements of Cash Flows
Nine months ended September 30, 2004 (in thousands):
| | Parent
| | Subsidiary
Guarantors
| | Non-
Guarantor
Subsidiaries
| | Eliminations
| | Consolidated
| |
|---|
| Net cash provided by (used in) operating activities | | $ | (4,793 | ) | $ | 9,294 | | $ | 1,678 | | $ | — | | $ | 6,179 | |
| | |
| |
| |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | | | — | | | | |
| | Capital expenditures | | | — | | | (7,778 | ) | | (940 | ) | | — | | | (8,718 | ) |
| | Transferred assets | | | — | | | (8 | ) | | 8 | | | — | | | — | |
| | Proceeds from sale of equipment | | | — | | | 1,402 | | | — | | | — | | | 1,402 | |
| | Acquisitions, net of cash acquired | | | — | | | (214,001 | ) | | — | | | — | | | (214,001 | ) |
| | |
| |
| |
| |
| |
| |
| Net cash used in investing activities | | | — | | | (220,385 | ) | | (932 | ) | | — | | | (221,317 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| | Borrowing | | | — | | | 372,000 | | | — | | | — | | | 372,000 | |
| | Repayments | | | (74,477 | ) | | (110,070 | ) | | — | | | — | | | (184,547 | ) |
| | Intercompany advances | | | 32,172 | | | (32,024 | ) | | (148 | ) | | — | | | — | |
| | Proceeds from sale of stock | | | 88,048 | | | — | | | — | | | — | | | 88,048 | |
| | Repurchase of redeemable and convertible preferred stock | | | (12,583 | ) | | — | | | — | | | — | | | (12,583 | ) |
| | Dividends paid on redeemable and convertible preferred stock | | | (28,367 | ) | | — | | | — | | | — | | | (28,367 | ) |
| | Deferred financing fees | | | — | | | (15,968 | ) | | — | | | — | | | (15,968 | ) |
| | |
| |
| |
| |
| |
| |
| Cash flows provided by (used in) financing activities | | | 4,793 | | | 213,938 | | | (148 | ) | | — | | | 218,583 | |
| | |
| |
| |
| |
| |
| |
| Effect of exchange rate changes in cash | | | — | | | 4 | | | (20 | ) | | — | | | (16 | ) |
| | |
| |
| |
| |
| |
| |
| Net increase in cash and cash equivalents | | | — | | | 2,851 | | | 578 | | | — | | | 3,429 | |
| Cash and cash equivalents, beginning of year | | | — | | | 3,408 | | | 566 | | | — | | | 3,974 | |
| | |
| |
| |
| |
| |
| |
| Cash and cash equivalents, end of year | | $ | — | | $ | 6,259 | | $ | 1,144 | | $ | — | | $ | 7,403 | |
| | |
| |
| |
| |
| |
| |
F-63
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors
MedSource Technologies, Inc.
We have audited the accompanying consolidated balance sheets of MedSource Technologies, Inc. as of June 30, 2003 and 2002, and the related consolidated statements of operations, changes in mandatory redeemable convertible stock and stockholders' equity (deficit), and cash flows for each of the three years in the period ended June 30, 2003. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of MedSource Technologies, Inc. at June 30, 2003 and the consolidated results of their operations and their cash flows for each of the three years in the period ended June 30, 2003, in conformity with U.S. generally accepted accounting principles.
Minneapolis, Minnesota
July 28, 2003
F-64
MEDSOURCE TECHNOLOGIES, INC.
Consolidated Balance Sheets
As of June 30, 2003 and 2002
(In thousands, except share data)
| | June 30, 2003
| | June 30, 2002
| |
|---|
| Assets | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 10,781 | | $ | 38,268 | |
| | Accounts receivable, net of allowance of $818 in 2003 and $646 in 2002 | | | 23,710 | | | 24,031 | |
| | Inventories | | | 25,617 | | | 20,503 | |
| | Prepaid expenses and other current assets | | | 4,318 | | | 2,402 | |
| | |
| |
| |
| | | Total current assets | | | 64,426 | | | 85,204 | |
| Property, plant, and equipment, net | | | 52,752 | | | 42,045 | |
| Goodwill | | | 96,582 | | | 113,113 | |
| Other identifiable intangible assets, net | | | 1,432 | | | 4,092 | |
| Deferred financing costs | | | 1,682 | | | 1,971 | |
| Other assets | | | 1,343 | | | 1,404 | |
| | |
| |
| |
| | | Total assets | | $ | 218,217 | | $ | 247,829 | |
| | |
| |
| |
Liabilities and stockholders' equity |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
| | Accounts payable | | $ | 10,868 | | $ | 7,924 | |
| | Accrued compensation and benefits | | | 5,498 | | | 5,352 | |
| | Other accrued expenses | | | 2,293 | | | 3,491 | |
| | Reserve for restructuring | | | 958 | | | 2,381 | |
| | Current portion of obligations under capital leases | | | 1,326 | | | 439 | |
| | Current portion of long term debt | | | 6,427 | | | 5,500 | |
| | |
| |
| |
| | | Total current liabilities | | | 27,370 | | | 25,087 | |
| Obligations under capital leases | | | 3,962 | | | 1,467 | |
| Long term debt, less current portion | | | 30,073 | | | 34,500 | |
| Other long term liabilities | | | 731 | | | 455 | |
| Stockholders' equity: | | | | | | | |
| | 6% Series E preferred stock, par value $0.01 per share: | | | | | | | |
| | | Authorized shares | | | — | | | 6,000 | |
| | | Issued and outstanding shares—none at 2003 and 1,935 at 2002 | | | — | | | 1,974 | |
| | Common stock, par value $0.01 per share: | | | | | | | |
| | | Authorized shares—70,000,000 at 2003 and 2002 | | | | | | | |
| | | Issued shares—28,905,719 at 2003 and 26,918,533 at 2002 | | | 289 | | | 269 | |
| | Additional paid in capital | | | 277,791 | | | 268,455 | |
| | Treasury stock, 113,696 at 2003 and 97,576 at 2002 | | | (1,463 | ) | | (1,282 | ) |
| | Accumulated other comprehensive loss | | | (288 | ) | | — | |
| | Accumulated deficit | | | (118,326 | ) | | (83,025 | ) |
| | Unearned compensation | | | (1,922 | ) | | (71 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 156,081 | | | 186,320 | |
| | |
| |
| |
| | | Total liabilities and stockholders' equity | | $ | 218,217 | | $ | 247,829 | |
| | |
| |
| |
See accompanying notes.
F-65
MEDSOURCE TECHNOLOGIES, INC.
Consolidated Statements of Operations
For the years ended June 30, 2003, 2002, 2001
(In thousands, except share and per share amounts)
| | Fiscal Year Ended June 30,
| |
|---|
| | 2003
| | 2002
| | 2001
| |
|---|
| Revenues | | $ | 177,298 | | $ | 158,899 | | $ | 128,462 | |
| Cost and expenses: | | | | | | | | | | |
| | Cost of products sold | | | 131,970 | | | 117,089 | | | 94,386 | |
| | Selling, general, and administrative expense | | | 33,495 | | | 29,876 | | | 26,199 | |
| | Amortization of goodwill and other intangibles | | | 338 | | | 340 | | | 5,640 | |
| | Impairment of intangible assets | | | 40,000 | | | — | | | — | |
| | Restructuring charges | | | 3,724 | | | — | | | 11,464 | |
| | |
| |
| |
| |
| | Total cost and expenses | | | 209,527 | | | 147,305 | | | 137,689 | |
| | |
| |
| |
| |
| Operating (loss) income | | | (32,229 | ) | | 11,594 | | | (9,227 | ) |
| Interest expense, net | | | (2,669 | ) | | (7,671 | ) | | (10,213 | ) |
| Loss on debt extinguishment | | | — | | | (6,857 | ) | | — | |
| Other (expense) income | | | (100 | ) | | (4,782 | ) | | 53 | |
| | |
| |
| |
| |
| Loss before income taxes | | | (34,998 | ) | | (7,716 | ) | | (19,387 | ) |
| Income tax (expense) benefit | | | (267 | ) | | 118 | | | (70 | ) |
| | |
| |
| |
| |
| Net loss | | | (35,265 | ) | | (7,598 | ) | | (19,457 | ) |
| Preferred stock dividends and accretion of discount on preferred stock | | | — | | | (31,168 | ) | | (9,688 | ) |
| | |
| |
| |
| |
| Net loss attributed to common stockholders | | $ | (35,265 | ) | $ | (38,766 | ) | $ | (29,145 | ) |
| | |
| |
| |
| |
| Net loss per share attributed to common stockholders — basic and diluted | | $ | (1.28 | ) | $ | (3.50 | ) | $ | (5.55 | ) |
| | |
| |
| |
| |
| Weighted average common shares outstanding — basic and diluted | | | 27,602,806 | | | 11,086,103 | | | 5,252,749 | |
| | |
| |
| |
| |
See accompanying notes.
F-66
MEDSOURCE TECHNOLOGIES, INC.
Consolidated Statements of Changes in Mandatory Redeemable
Convertible Stock and Stockholders' Equity (Deficit)
For the years ended June 30, 2003, 2002 and 2001
(In thousands)
| | Mandatory Redeemable Convertible Stock
| | Stockholders' Equity (Deficit)
| |
|---|
| | Series B
Preferred
Stock
| | Series C
Preferred
Stock
| | Series D
Preferred
Stock
| | Series F
Preferred
Stock
| | Series A
Convertibl
Preferred
Stock
| | Series E
Convertibl
Preferred
Stock
| | Series Z
Preferred
Stock
| |
|---|
| Balance at July 1, 2000 | | | 22,293 | | | — | | | — | | | — | | | — | | | — | | | 1 | |
| Cumulative effect change due to implementation of SFAS No. 133 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Change in fair value of interest rate swaps | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net loss for the year | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Comprehensive loss for the period | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Sale and issuance of Series C preferred stock, net of costs of $3,061 | | | — | | | 37,239 | | | — | | | — | | | — | | | — | | | — | |
| Issuance of Series D preferred stock and options for acquired business | | | — | | | — | | | 31,575 | | | — | | | — | | | — | | | — | |
| Issuance of stock pursuant to option exercises | | | — | | | — | | | 374 | | | — | | | — | | | — | | | — | |
| Accretion of discounts on mandatory redeemable convertible preferred stock | | | 2,379 | | | 275 | | | 391 | | | — | | | — | | | — | | | — | |
| Accrued dividends on mandatory redeemable convertible preferred stock | | | 1,617 | | | 1,676 | | | 1,048 | | | — | | | — | | | — | | | — | |
| Amortization of unearned compensation | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2001 | | | 26,289 | | | 39,190 | | | 33,388 | | | — | | | — | | | — | | | 1 | |
| Change in fair value of interest rate swaps | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net loss for the period | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Comprehensive loss for the period | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Issuance of stock pursuant to option exercises | | | — | | | — | | | 48 | | | — | | | — | | | — | | | — | |
| Sale of Series E preferred stock and common stock purchase warrants | | | — | | | — | | | — | | | — | | | — | | | 4,168 | | | — | |
| Accretion of discounts on mandatory redeemable convertible preferred stock | | | 177 | | | 310 | | | 598 | | | — | | | — | | | — | | | — | |
| Accrued dividends on mandatory redeemable convertible preferred stock | | | 1,276 | | | 2,415 | | | 1,571 | | | — | | | — | | | — | | | — | |
| Amortization of unearned compensation | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Issuance of preferred and common stock for acquired business | | | — | | | — | | | — | | | 3,636 | | | — | | | — | | | — | |
| Conversion of Series Z to common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | (1 | ) |
| Conversion of Series A to common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Conversion of Series B to common stock | | | (22,980 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
| Conversion of Series D to common stock | | | — | | | — | | | (35,605 | ) | | — | | | — | | | — | | | — | |
| Conversion of Series C to common stock | | | — | | | (41,915 | ) | | — | | | — | | | — | | | — | | | — | |
| Payment of Series B dividend | | | (4,762 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
| Sale of common stock, net of issuance costs | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Amortization of discount on redeemable preferred stock | | | — | | | — | | | — | | | 364 | | | — | | | 1,832 | | | — | |
| Amortization of dividend on redeemable preferred stock | | | — | | | — | | | — | | | 63 | | | — | | | 119 | | | — | |
| Return on Series C preferred stock | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Termination of interest rate swap | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Redemption of Series F preferred stock | | | — | | | — | | | — | | | (4,063 | ) | | — | | | — | | | — | |
| Redemption of Series E preferred stock | | | — | | | — | | | — | | | — | | | — | | | (4,145 | ) | | — | |
| Shares received from sale of business | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2002 | | | — | | | — | | | — | | | — | | | — | | | 1,974 | | | — | |
| Change in fair value of interest rate cap | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Net loss for the period | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Comprehensive loss for the period | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Common stock issued for acquired business | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Redemption of Series E preferred stock | | | — | | | — | | | — | | | — | | | — | | | (1,974 | ) | | — | |
| Sale of common stock, net of issuance costs | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Issuance of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Forfeiture of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Amortization of unearned compensation | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2003 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes.
F-67
MEDSOURCE TECHNOLOGIES, INC.
Consolidated Statements of Changes in Mandatory Redeemable
Convertible Stock and Stockholders' Equity (Deficit)
For the years ended June 30, 2003, 2002 and 2001
(In thousands)
| | Stockholders' Equity (Deficit)
| |
|---|
| | Number of
Common
Shares
| | Common
Stock
| | Additional
Paid In
Capital
| | Treasury
Stock
| | Accumulated
Other
Comprehensive
Loss
| | Accumulated
Deficit
| | Unearned
Compensation
| | Total
Stockholders'
Equity
(Deficit)
| |
|---|
| Balance at July 1, 2000 | | 5,235 | | | 52 | | | 33,591 | | | — | | | — | | | (18,572 | ) | | — | | | 15,072 | |
| Cumulative effect change due to implementation of SFAS No. 133 | | — | | | — | | | — | | | — | | | 1,097 | | | — | | | — | | | 1,097 | |
| Change in fair value of interest rate swaps | | — | | | — | | | — | | | — | | | (2,657 | ) | | — | | | — | | | (2,657 | ) |
| Net loss for the year | | — | | | — | | | — | | | — | | | — | | | (19,457 | ) | | — | | | (19,457 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (22,114 | ) |
| Sale and issuance of Series C preferred stock, net of costs of $3,061 | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Issuance of Series D preferred stock and options for acquired business | | — | | | — | | | — | | | — | | | — | | | — | | | (286 | ) | | (286 | ) |
| Issuance of stock pursuant to option exercises | | 21 | | | — | | | 284 | | | — | | | — | | | — | | | — | | | 284 | |
| Accretion of discounts on mandatory redeemable convertible preferred stock | | — | | | — | | | — | | | — | | | — | | | (3,045 | ) | | — | | | (3,045 | ) |
| Accrued dividends on mandatory redeemable convertible preferred stock | | — | | | — | | | — | | | — | | | — | | | (4,341 | ) | | — | | | (4,341 | ) |
| Amortization of unearned compensation | | — | | | — | | | — | | | — | | | — | | | — | | | 72 | | | 72 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2001 | | 5,256 | | | 52 | | | 33,875 | | | — | | | (1,560 | ) | | (45,415 | ) | | (214 | ) | | (13,261 | ) |
| Change in fair value of interest rate swaps | | — | | | — | | | — | | | — | | | (374 | ) | | — | | | — | | | (374 | ) |
| Net loss for the period | | — | | | — | | | — | | | — | | | — | | | (7,598 | ) | | — | | | (7,598 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | | ��� | | | — | | | — | | | — | | | — | | | — | | | (7,972 | ) |
| Issuance of stock pursuant to option exercises | | — | | | — | | | 19 | | | — | | | — | | | — | | | — | | | 19 | |
| Sale of Series E preferred stock and common stock purchase warrants | | — | | | — | | | 1,832 | | | — | | | — | | | — | | | — | | | 6,000 | |
| Accretion of discounts on mandatory redeemable convertible preferred stock | | — | | | — | | | — | | | — | | | — | | | (1,085 | ) | | — | | | (1,085 | ) |
| Accrued dividends on mandatory redeemable convertible preferred stock | | — | | | — | | | — | | | — | | | — | | | (5,262 | ) | | — | | | (5,262 | ) |
| Amortization of unearned compensation | | — | | | — | | | — | | | — | | | — | | | — | | | 143 | | | 143 | |
| Issuance of preferred and common stock for acquired business | | 824 | | | 8 | | | 9,883 | | | — | | | — | | | — | | | — | | | 9,891 | |
| Conversion of Series Z to common stock | | 650 | | | 7 | | | (6 | ) | | — | | | — | | | — | | | — | | | — | |
| Conversion of Series A to common stock | | 1,919 | | | 19 | | | (19 | ) | | — | | | — | | | — | | | — | | | — | |
| Conversion of Series B to common stock | | 3,327 | | | 33 | | | 22,947 | | | — | | | — | | | — | | | — | | | 22,980 | |
| Conversion of Series D to common stock | | 1,770 | | | 18 | | | 35,587 | | | — | | | — | | | — | | | — | | | 35,605 | |
| Conversion of Series C to common stock | | 3,906 | | | 39 | | | 41,876 | | | — | | | — | | | — | | | — | | | 41,915 | |
| Payment of Series B dividend | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Sale of common stock, net of issuance costs | | 9,266 | | | 93 | | | 101,174 | | | — | | | — | | | — | | | — | | | 101,267 | |
| Amortization of discount on redeemable preferred stock | | — | | | — | | | — | | | — | | | — | | | (2,196 | ) | | — | | | (364 | ) |
| Amortization of dividend on redeemable preferred stock | | — | | | — | | | — | | | — | | | — | | | (182 | ) | | — | | | (63 | ) |
| Return on Series C preferred stock | | — | | | — | | | 21,287 | | | — | | | — | | | (21,287 | ) | | — | | | — | |
| Termination of interest rate swap | | — | | | — | | | — | | | — | | | 1,934 | | | — | | | — | | | 1,934 | |
| Redemption of Series F preferred stock | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Redemption of Series E preferred stock | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (4,145 | ) |
| Shares received from sale of business | | — | | | — | | | — | | | (1,282 | ) | | — | | | — | | | — | | | (1,282 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2002 | | 26,918 | | | 269 | | | 268,455 | | | (1,282 | ) | | — | | | (83,025 | ) | | (71 | ) | | 186,320 | |
| Change in fair value of interest rate cap | | — | | | — | | | — | | | — | | | (288 | ) | | — | | | — | | | (288 | ) |
| Net loss for the period | | — | | | — | | | — | | | — | | | — | | | (35,265 | ) | | — | | | (35,265 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (35,553 | ) |
| Common stock issued for acquired business | | 667 | | | 7 | | | 5,997 | | | — | | | — | | | — | | | — | | | 6,004 | |
| Redemption of Series E preferred stock | | — | | | — | | | — | | | — | | | — | | | (36 | ) | | — | | | (2,010 | ) |
| Sale of common stock, net of issuance costs | | 430 | | | 4 | | | 926 | | | — | | | — | | | — | | | — | | | 930 | |
| Issuance of restricted common stock | | 875 | | | 9 | | | 2,413 | | | — | | | — | | | — | | | (2,422 | ) | | — | |
| Forfeiture of restricted common stock | | 16 | | | — | | | — | | | (181 | ) | | — | | | — | | | 159 | | | (22 | ) |
| Amortization of unearned compensation | | — | | | — | | | — | | | — | | | — | | | — | | | 412 | | | 412 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2003 | | 28,906 | | $ | 289 | | $ | 277,791 | | $ | (1,463 | ) | $ | (288 | ) | $ | (118,326 | ) | $ | (1,922 | ) | $ | 156,081 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes.
F-68
MEDSOURCE TECHNOLOGIES, INC.
Consolidated Statements of Cash Flows
For the years ended June 30, 2003, 2002 and 2001
(In thousands)
| | Fiscal Year Ended June 30,
| |
|---|
| | 2003
| | 2002
| | 2001
| |
|---|
| Operating activities | | | | | | | | | | |
| Net loss | | $ | (35,265 | ) | $ | (7,598 | ) | $ | (19,457 | ) |
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | | | | |
| | Depreciation | | | 8,648 | | | 7,791 | | | 6,555 | |
| | Amortization of goodwill and other intangibles | | | 338 | | | 340 | | | 5,640 | |
| | Amortization of deferred financing costs and discount on long term debt | | | 658 | | | 6,157 | | | 1,122 | |
| | Amortization of unearned compensation | | | 412 | | | 143 | | | 72 | |
| | Impairment of intangible assets | | | 40,000 | | | — | | | — | |
| | Restructuring charges | | | — | | | — | | | 11,464 | |
| | Loss (gain) on sale of equipment | | | 926 | | | — | | | (29 | ) |
| | Changes in operating assets and liabilities, net of effect of businesses acquired: | | | | | | | | | | |
| | | Accounts and notes receivable | | | 1,221 | | | (1,682 | ) | | (4,296 | ) |
| | | Inventories | | | (4,676 | ) | | (6,889 | ) | | (1,775 | ) |
| | | Prepaid expenses and other current assets | | | (1,494 | ) | | 768 | | | (836 | ) |
| | | Interest escrow fund | | | — | | | 1,849 | | | 2,500 | |
| | | Accounts payable, accrued compensation and benefits, accrued expenses, and other | | | (926 | ) | | (7,514 | ) | | 476 | |
| | | Other | | | (484 | ) | | (1,120 | ) | | (183 | ) |
| | |
| |
| |
| |
| Net cash provided by (used in) operating activities | | | 9,358 | | | (7,755 | ) | | 1,253 | |
| Investing activities | | | | | | | | | | |
| Acquisition of businesses, net of cash acquired | | | (22,617 | ) | | (6,312 | ) | | (378 | ) |
| Other additions to plant and equipment, net | | | (12,783 | ) | | (8,598 | ) | | (11,491 | ) |
| Proceeds from sale of equipment | | | 80 | | | 245 | | | 242 | |
| | |
| |
| |
| |
| Net cash used in investing activities | | | (35,320 | ) | | (14,665 | ) | | (11,627 | ) |
| Financing activities | | | | | | | | | | |
| Proceeds from sale and leaseback of equipment | | | 3,818 | | | — | | | — | |
| Proceeds from issuance of long term debt, net of financing costs, and interest escrow fund | | | 8,000 | | | 37,939 | | | 105 | |
| Payments of long term debt | | | (12,307 | ) | | (91,890 | ) | | (5,549 | ) |
| Proceeds from sale of Series C and D preferred stock, net of costs | | | — | | | — | | | 37,897 | |
| Proceeds from sale of Series E preferred stock and common stock, net of costs | | | 974 | | | 107,286 | | | — | |
| Payments of Series B dividends | | | — | | | (4,762 | ) | | — | |
| Redemption of Series E and F preferred stock | | | (2,010 | ) | | (8,208 | ) | | — | |
| Net payments on lines of credit | | | — | | | — | | | (4,000 | ) |
| Other | | | — | | | 34 | | | — | |
| | |
| |
| |
| |
| Net cash (used in) provided by financing activities | | | (1,525 | ) | | 40,399 | | | 28,453 | |
| | |
| |
| |
| |
| (Decrease) increase in cash and cash equivalents | | | (27,487 | ) | | 17,979 | | | 18,079 | |
| Cash and cash equivalents at beginning of period | | | 38,268 | | | 20,289 | | | 2,210 | |
| | |
| |
| |
| |
| Cash and cash equivalents at end of period | | $ | 10,781 | | $ | 38,268 | | $ | 20,289 | |
| | |
| |
| |
| |
| Supplemental disclosure of cash flow information | | | | | | | | | | |
| Cash paid for interest | | $ | 2,672 | | $ | 7,018 | | $ | 9,319 | |
| | |
| |
| |
| |
| Cash paid for income taxes | | $ | — | | $ | 108 | | $ | 150 | |
| | |
| |
| |
| |
| Preferred and common stock issued for acquisitions | | $ | 6,004 | | $ | 13,527 | | $ | 31,289 | |
| | |
| |
| |
| |
See accompanying notes.
F-69
MEDSOURCE TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
1. Organization and Description of Business
MedSource Technologies, Inc. (the "Company") was formed as a Delaware corporation on April 14, 1998. For the period from April 14, 1998 through March 30, 1999 (inception of operations), the Company had no employees or other operations.
The Company and its subsidiaries operate in one business segment and provide product development and design services, precision metal and plastic part manufacturing, product assembly services and supply chain management primarily for the medical device industry. The Company's operations and customer base are located primarily in North America.
2. Significant Accounting Policies
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated.
Cash equivalents include money market mutual funds and other highly liquid investments purchased with maturities of three months or less. The cash equivalents are carried at cost, which approximates market.
We specifically analyze our accounts receivable, historical bad debts, customer concentrations, customer credit-worthiness, and current economic trends, when evaluating the adequacy of our allowance for doubtful accounts. The reserve requirements are based on the best facts available to us and are reevaluated and adjusted as additional information is received. We are not able to predict changes in our customers' financial condition. If the condition of our customers deteriorates we may have to update our estimates, which could have a material adverse effect on our financial condition. As of June 30, 2003, we had $0.8 million reserved against our accounts receivable.
Inventories include material, labor and overhead and are stated at the lower of cost, using the FIFO (first-in, first-out) method, or market. In assessing the ultimate realization of inventories, we are required to make judgments as to future demand requirements compared to current or committed inventory levels. Our reserve requirements generally increase as demand decreases due to changes in among other things, market conditions, product life cycles, and technological obsolescence.
Property, Plant, and Equipment
Property, plant, and equipment are recorded at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Amortization of capital leases and leasehold improvements is provided on a straight-line basis over the lives of the related assets or the life of the lease, whichever is shorter, and is included with depreciation expense.
F-70
Goodwill represents the cost in excess of the fair value of the tangible and identifiable intangible assets of the businesses acquired and, prior to July 1, 2001, was being amortized on a straight-line basis over 20 years based on the operating histories and market niches of these businesses. Effective July 1, 2001, the Company adopted Statement of Financial Accounting Standard (SFAS) No. 142, Goodwill and Other Intangible Assets. Under SFAS No. 142, goodwill must be tested for impairment annually, or more often if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount, using a two-step approach. Step one is to compare the fair value of a reporting unit with its carrying amount, including goodwill. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test will be performed to measure the amount of impairment loss, if any. Step two compares the implied fair value of the reporting unit's goodwill with the carrying amount of that goodwill. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination is determined. If the carrying amount of a reporting unit's goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The Company has one operating segment consisting of multiple manufacturing facilities with similar economic characteristics producing goods for a similar set of customers (i.e., the medical device industry). Thus the Company has concluded that it currently has one reporting unit for purposes of the goodwill impairment test. The Company estimates the present value of its estimated future cash flows or other market valuation techniques to measure the fair value of the reporting unit.
The identifiable intangible assets consist mainly of patents and are amortized over the life of the patents.
See Note 6—Goodwill and Other Intangible Assets for a more detailed discussion of the fiscal 2003 impairment test and $40.0 million impairment charge.
The Company evaluates long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the Company plans to continue to use the assets in ongoing operations, the estimated future cash flows (undiscounted and without interest charges) from the use of the assets are compared to the current carrying amount. If the current carrying value of the assets is greater than the assets' fair market value, an impairment charge is recognized equal to the difference. If the Company plans to dispose of the assets via sale, estimates of fair market value are taken from appropriate external sources. If the asset's carrying value exceeds the asset's fair market value an impairment charge is recognized equal to the difference, and the asset is reclassified on the consolidated balance sheet to the assets held for sale category.
Costs incurred in connection with arranging the Company's long-term debt agreements are capitalized and amortized over the life of the related debt issuance using the effective interest method. Accumulated amortization was $0.7 million at June 30, 2003, $0.1 million at June 30, 2002 and $1.9 million at June 30, 2001. See Note 7—Long-Term Debt for a detailed description of our long-term debt transactions.
F-71
Deferred income taxes are determined using the liability method, which gives consideration to the future tax consequences associated with differences between the financial accounting and tax basis of assets and liabilities. This method also gives immediate effect to changes in income tax laws.
The Company recognizes revenue when persuasive evidence of a final agreement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured. Revenue from product sales is primarily recognized at the time of shipment. Product shipments are supported by purchase orders from customers that indicate the price for each product. For services, we recognize revenue primarily on a time and materials basis. Service revenues are supported by customer orders or contracts that indicate the price for the services being rendered. For fiscal 2003, 2002, and 2001 service revenues were less than 10% of total revenues.
The Company includes shipping and handling costs in the cost of products sold.
The Company has elected to follow Accounting Principles Board Opinion No. 25, Accounting for Stock Issued to Employees, in the primary financial statements and to provide the supplemental disclosures required by SFAS No. 148, Accounting for Stock-Based Compensation—Transition and Disclosure. Stock compensation is awarded to key employees in the form of stock options and restricted stock. All stock options are issued at fair market value on the date of grant. Accordingly, we did not recognize stock compensation expenses for stock options granted during the periods presented. In determining the fair value of options granted during fiscal 2003, the Company used the Black-Scholes option-pricing model with the following weighted average assumptions: expected volatility factor of 94.5%; risk-free interest rate of 1.975%; dividend yield of zero; and an expected option life of four years. For options granted prior to fiscal 2003, we determined the fair value of options granted using the minimum value option pricing model with the following weighted average assumptions: risk-free interest rate of 5.5%; dividend yield of zero; and an expected option life of four years. The change in option pricing methodologies in fiscal 2003 is the result of being a public traded company during the entire fiscal year. The minimum value-option pricing model is not permitted for publicly traded companies under SFAS No. 123. The following table summarizes what our operating results would have been if we had utilized the fair value method of accounting for stock options (in thousands):
| | FY2003
| | FY2002
| | FY2001
| |
|---|
| Net loss as reported | | $ | (35,265 | ) | $ | (38,766 | ) | $ | (29,145 | ) |
| Stock compensation expense—fair value based method | | | (1,721 | ) | | (1,022 | ) | | (482 | ) |
| | |
| |
| |
| |
| Pro forma net loss | | $ | (36,986 | ) | $ | (39,788 | ) | $ | (29,627 | ) |
| | |
| |
| |
| |
| Loss per share as reported (basic and diluted) | | $ | (1.28 | ) | $ | (3.50 | ) | $ | (5.55 | ) |
| | |
| |
| |
| |
| Pro forma loss per share (basic and diluted) | | $ | (1.34 | ) | $ | (3.59 | ) | $ | (5.64 | ) |
| | |
| |
| |
| |
F-72
We have issued restricted stock as part of employee incentive plans. The fair market value of the restricted stock is amortized over the projected remaining vesting period. During the fiscal year ended June 30, 2003, we incurred $0.3 million of non-cash stock compensation expenses related to restricted stock issuances. No such charges were incurred in fiscal 2002 and fiscal 2001. (see Note 10—Mandatory Redeemable Convertible Stock and Stockholders' Equity).
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash equivalents and accounts receivable. The Company performs ongoing credit evaluations of its customers, does not generally require collateral or other security, and maintains an allowance for potential credit losses.
Customers that accounted for more than 10% of consolidated revenues are as follows:
| | Year Ended June 30,
| |
|---|
| | 2003
| | 2002
| | 2001
| |
|---|
| Customer A | | 27 | % | 25 | % | 18 | % |
| Customer B | | 12 | | 12 | | 12 | |
| Customer C | | 11 | | — | | — | |
At June 30, 2003 and 2002 receivables from these customers represented 37% and 17% respectively, of total accounts receivable.
The Company's financial instruments consist primarily of cash equivalents, accounts receivable, accounts payable, and debt instruments. The carrying amounts of financial instruments other than the debt instruments are representative of their fair values due to their short maturities. The Company's principal long-term debt agreements bear interest at market rates; thus, management believes their carrying amounts approximate fair value. Management believes the carrying amount of the remaining loans is not materially different from estimated fair value.
Net loss per common share attributed to common stockholders is based on the net loss for the period adjusted for dividend requirements on all preferred stock and accretion of discounts on mandatory redeemable preferred stock. The resulting net loss attributed to common stockholders is divided by the weighted average number of shares of common stock outstanding during the period to arrive at the basic net loss per share attributed to common stockholders. For all periods presented, the impact of the assumed exercise of certain options, warrants, and unvested restricted stock was anti-dilutive, and those securities were therefore excluded from the computation.
F-73
The Company accounts for its derivative financial instruments in accordance with SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities. The statement requires the Company to recognize all derivatives on the balance sheet at fair value. Derivatives that are not hedges must be adjusted to fair value through earnings. If the derivative is a hedge, depending on the nature of the hedge, changes in the fair value of derivatives will either be offset against the change in fair value of the hedged assets, liabilities, or firm commitments through earnings or recognized in other comprehensive income until the hedged item is recognized in earnings. The ineffective portion of a derivative's change in fair value will be immediately recognized in earnings.
We have a derivative financial instrument to hedge our exposure to changes in interest rates. The financial instrument is marked-to-market each financial statement date. The change in the hedge's fair market value was recognized in other comprehensive income. Upon payment of the underlying note payable the unrealized gain or loss recognized in accumulated comprehensive income will be reclassified as a realized gain or loss in our operating results. (See Note 7—Long-Term Debt.)
Certain prior year amounts have been reclassified to conform with the current year presentation.
The preparation of financial statements in conformity with U.S. generally accepted accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
In October 2001, the FASB issued SFAS No. 144, "Accounting for the Impairment of Disposal of Long-Lived Assets," which replaced SFAS No. 121, "Accounting for the Impairment of Long-Lived Assets and Long-Lived Assets to Be Disposed Of." Though SFAS No. 144 retained the basic guidance of SFAS No. 121, regarding when and how to measure an impairment loss, it provides additional implementation guidelines. The Company adopted this statement in the first quarter of fiscal 2003 and its adoption did not have a material impact on the Company's financial statements.
In April 2002, the FASB issued SFAS No. 145 "Rescission of FASB Statement No. 4, 44, and 62, Amendment of FASB Statement No. 13, and Technical corrections. SFAS No. 145 required us to reclassify the fiscal 2002 $6.9 million loss on debt extinguishment as a loss from continuing operations rather than an extraordinary item. The provisions of SFAS No. 145 are effective for fiscal years beginning after May 15, 2002. Accordingly, we adopted this standard beginning in the first quarter of fiscal 2003. The reclassification had no impact on our net loss, cash flows or financial position.
In June 2002, the FASB issued SFAS No. 146 "Accounting for Costs Associated With Exit or Disposal Activities." SFAS No. 146 superseded Emerging Issues Task Force (EITF) Issue No. 94-3, "Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring)." The principal difference between SFAS No. 146
F-74
and EITF 94-3 relates to when an entity can recognize a liability related to exit or disposal activities. SFAS No. 146 requires that a liability be recognized for cost associated with an exit or disposal activity when the liability is incurred. EITF 94-3 allowed a liability related to an exit or disposal activity to be recognized on the date the entity commits to an exit plan. We adopted this standard on January 1, 2003, which was the standard's effective date. The standard did not materially impact our consolidated financial results or financial position upon adoption, but will affect the timing of when we recognize expected restructuring charges in future periods.
In November of 2003, the FASB issued Interpretation No. 45, "Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of the Indebtedness of Others," which requires a guarantor to recognize and measure certain types of guarantees at fair value. In addition, Interpretation No. 45 requires the guarantor to make new disclosures for these guarantees and other types of guarantees that are not subject to the initial recognition and initial measurement provisions. The disclosure requirements are effective for interim or annual periods ended after December 31, 2002, while the recognition and measurement provisions are applicable on a prospective basis to guarantees issued or modified after December 31, 2002. We adopted the initial recognition and measurement provisions as well as the disclosure provisions of Interpretation No. 45 during the third quarter of fiscal 2003. The initial recognition and measurement provisions did not have a material impact on our consolidated financial results or financial condition.
In December of 2002, the FASB issued SFAS No. 148, "Accounting for Stock-Based Compensation—Transition and Disclosure." The provisions of SFAS No. 148 amend SFAS No. 123, "Accounting for Stock Based Compensation," to provide alternative methods of transitioning to a fair value-based method of accounting for stock-based compensation. In addition, SFAS No. 148 also expands the disclosure requirements of SFAS No. 123 by requiring more detailed disclosure in both annual and interim financial statements. The transition provisions of SFAS No. 148 did not have a material impact on our financial results, as we did not adopt the fair value-based accounting provisions of SFAS No. 123, which is commonly referred to as expensing of stock options. The disclosure provisions of SFAS No. 148 are effective for interim periods beginning after December 15, 2002. Accordingly, we adopted the disclosure provisions during the third quarter if fiscal 2003.
3. Acquisitions
On September 4, 2002, the Company acquired Cycam, Inc. ("Cycam"), a company located in Houston, Pennsylvania that manufactures reconstructive implants and instruments. The total purchase price was approximately $24.4 million, which included $18.4 million in cash and 667,175 shares of common stock valued at $6.0 million. The fair market value of the shares issued in connection with the Cycam acquisition was based on the market price of our common stock on the date of issuance. The acquisition was recorded using the purchase method of accounting. The purchase price allocation was $5.9 million to net tangible assets and $18.5 million to goodwill. In conjunction with the acquisition, the Company drew $8.0 million from the acquisition line under the Company's old credit facility. The effect of the acquisition on our historical financial position and results of operations is not material, and therefore no pro forma data of this acquisition is presented. Cycam's operating results have been included in our consolidated operating results since the date of acquisition.
F-75
The acquisition of Cycam expanded our capacity and capabilities in the metal machining of orthopaedic reconstructive implants. In addition, Cycam provided us with the complimentary capabilities of plastic machining, surface coatings, near net shape forging, and sterilized packaging and kitting of orthopaedic implants. Cycam also had strong relationships with several leading orthopaedic companies where we had only a minor presence.
During our fiscal year ended June 30, 2002, the Company completed the acquisition of HV Technologies, Inc. ("HV Technologies"), a company located in Trenton, Georgia that manufactures polyimide and composite micro-tubing used in interventional and minimally invasive catheters, delivery systems and instruments. The total purchase price was approximately $19.1 million, including cash of $5.6 million, 4,000 shares of Series F preferred stock valued at $3.6 million, and 824,255 shares of common stock valued at $9.9 million. The results of HVT are included in the Company's consolidated Statement of Operations from the date of acquisition and were not material.
To help provide financing for the acquisition, the Company issued 6,000 shares of its 6% Series E preferred stock (the "Series E preferred stock") and warrants to purchase an aggregate of 200,000 shares of its common stock. The Company had agreed that the total number of shares issuable upon exercise of the warrants would increase on each of the first five anniversaries of the date of issuance of the Series E Preferred Stock by an aggregate of 45,000 shares per year for each year that the Series E preferred stock remained outstanding. However, as discussed below, the Company redeemed all of the Series E preferred stock before the first anniversary of its issuance. The Company recorded a discount of $1.8 million to the carrying value of the Series E preferred stock equal to the consideration allocated to the warrants.
The Series E preferred stock and the Series F preferred stock accrued dividends at $60 per year during the first year from issuance, payable at the discretion of the Board of Directors, and at the rate of $160 per year on a retroactive basis after the first anniversary of issuance. The Company redeemed the Series F preferred stock and 4,065 shares of the Series E preferred stock in April 2002, at a price equal to $1,000 per share plus accrued and unpaid dividends. During fiscal 2003, the Company redeemed the remaining 1,935 shares of Series E preferred stock at a price equal to $1,000 per share plus accrued and unpaid dividends.
In connection with the Company's issuance of Series E preferred stock, the Company obtained the consent of the holders of its $20.0 million of Senior Subordinated Promissory Notes (the "Notes") to complete the acquisition of HV Technologies and changed some of the covenants to which the Company was subject under an agreement between the Company and those holders. At the same time, the Company agreed to increase by $1.0 million the amount payable by the Company upon redemption of the Notes. As of April 2, 2002 the Company repaid the Notes, including the prepayment fees.
The Company allocated the purchase price as follows: $2.7 million for fair value of tangible assets acquired, net of liabilities assumed, and deferred taxes and $16.4 million for goodwill.
During fiscal 2001, the Company completed the acquisition of ACT Medical, Inc., a Massachusetts company with additional facilities in Santa Clara, California and a contract for production and assembly
F-76
services in Navojoa, Mexico. The acquisition was completed by the issuance of 33,423 shares of 6% Series D cumulative convertible redeemable preferred stock, rollover of options for an additional 6,920 shares of Series D preferred stock, and cash payments of $1.0 million to stockholders electing to receive cash instead of stock. The rollover of options represented replacement of outstanding options to purchase the common stock of ACT Medical that had been issued under a plan sponsored by ACT Medical with options to purchase the Series D preferred stock of the Company. The fair value of the options to purchase the Series D preferred stock of the Company of $3.4 million was included in the purchase price, and the intrinsic value related to the unvested options was recorded as unearned compensation. The acquisition was recorded using the purchase method of accounting, and the operating results are included in the Company's consolidated statements of operations since the date of acquisition (December 30, 2000). The total purchase price was allocated as follows (in thousands):
| Fair value of tangible assets acquired, net of liabilities assumed, and deferred taxes | | $ | 1,649 |
| Identifiable intangible assets, net of deferred taxes | | | 3,648 |
| Goodwill | | | 28,440 |
| | |
|
| | | $ | 33,737 |
| | |
|
The following unaudited pro forma summary presents the consolidated results of operations as if the acquisition had occurred at the beginning of the fiscal 2001 (in thousands, except per share):
| Net revenues | | $ | 141,248 | |
| Loss before taxes | | | (20,750 | ) |
| Net loss | | | (20,820 | ) |
| Net loss attributed to common stockholders | | | (31,825 | ) |
| | |
| |
| Net loss per share attributed to common stockholders | | $ | (6.06 | ) |
| | |
| |
4. Inventories
Inventories consist of the following (in thousands):
| | June 30,
2003
| | June 30,
2002
|
|---|
| Raw materials | | $ | 13,806 | | $ | 10,638 |
| Work in progress | | | 8,389 | | | 6,529 |
| Finished goods | | | 3,422 | | | 3,336 |
| | |
| |
|
| Total | | $ | 25,617 | | $ | 20,503 |
| | |
| |
|
F-77
5. Property, Plant, and Equipment
Property, plant, and equipment consists of the following (in thousands):
| | Estimated
Useful Lives
(Years)
| | June 30, 2003
| | June 30, 2002
| |
|---|
| Land | | | | $ | 569 | | $ | 169 | |
| Buildings and improvements | | 1 to 20 | | | 2,106 | | | 46 | |
| Leasehold improvements | | 2 to 20 | | | 7,461 | | | 6,320 | |
| Machinery and equipment | | 3 to 15 | | | 52,880 | | | 39,796 | |
| Furniture and fixtures | | 1 to 7 | | | 10,259 | | | 6,136 | |
| Automobiles | | 2 to 3 | | | 34 | | | 82 | |
| Software | | 3 to 8 | | | 1,016 | | | 626 | |
| Construction in progress | | | | | 5,986 | | | 6,410 | |
| | | | |
| |
| |
| Total | | | | | 80,311 | | | 59,585 | |
| Less accumulated depreciation and amortization | | | | | (27,559 | ) | | (17,540 | ) |
| | | | |
| |
| |
| Net property, plant, and equipment | | | | $ | 52,752 | | $ | 42,045 | |
| | | | |
| |
| |
As of June 30, 2003 capital leases with a gross amount of $6.3 million were included in machinery and equipment. The assets have $0.7 million of accumulated depreciation and are reported at a net book value of $5.6 million.
6. Goodwill and Other Identifiable Intangible Assets
In June 2001, the FASB issued SFAS No. 141, Business Combinations, and No. 142, Goodwill and Other Intangible Assets, effective for fiscal years beginning after December 15, 2001 with early adoption permitted for companies with fiscal years beginning after March 15, 2001. Under the new rules, goodwill and intangible assets deemed to have indefinite lives are not amortized but instead are subject to an impairment test annually or at other times if indicators of impairment exist. Other intangible assets will continue to be amortized over their useful lives.
The Company adopted these standards beginning in the first quarter of fiscal 2002. Amounts previously recorded as separately identifiable intangibles for acquired work force and customer base have been subsumed to goodwill in accordance with SFAS No. 141, increasing goodwill by $34.5 million as of the date of adoption. Effective with the July 1, 2002 adoption of SFAS No. 142, goodwill is no longer amortized but is instead subject to an impairment test annually or at other times if indicators of impairment exist.
During fiscal 2003, we reduced our revenue forecast. Since the reduction in forecasted revenue indicated that our goodwill and intangible assets might be impaired we performed an impairment test using the income approach. We compared the present value of the estimated future cash flows of our business to the carrying value of net assets. This analysis indicated that our goodwill and intangible assets were impaired. Therefore, with the help of external sources, we evaluated the fair value of our net assets as if we were being purchased in a business combination. We recognized a $37.7 million goodwill impairment charge and a $2.3 million impairment charge related to our intangible assets. Goodwill represents a substantial portion of our assets. Therefore, future adverse changes in business or market conditions may result in additional goodwill impairment charges, which could have a material adverse effect on our financial results and financial position.
F-78
Goodwill and other identifiable intangible assets resulting from acquisitions of businesses and the formation of the Company consist of the following (in thousands):
| | June 30, 2003
| | June 30, 2002
| |
|---|
| Goodwill at beginning of period | | $ | 113,113 | | $ | 62,210 | |
| | SFAS No. 142 reclassification | | | — | | | 34,530 | |
| | Acquisitions | | | 21,130 | | | 16,373 | |
| | Impairment charges | | | (37,661 | ) | | — | |
| | |
| |
| |
| Goodwill at end of period | | $ | 96,582 | | $ | 113,113 | |
| | |
| |
| |
| Other identifiable intangibles: | | | | | | | |
| | Patents and intellectual properties | | | 1,441 | | | 4,383 | |
| | Covenants not to compete | | | — | | | 476 | |
| | |
| |
| |
| | | | 1,441 | | | 4,859 | |
| Less accumulated amortization | | | (9 | ) | | (767 | ) |
| | |
| |
| |
| | | $ | 1,432 | | $ | 4,092 | |
| | |
| |
| |
The $2.7 million net decrease in other identifiable intangibles was primarily related to the impairment charges mentioned above. Of the $37.7 million goodwill impairment charge, $30.0 million was recognized during the third quarter of fiscal 2003 and the remainder was recognized during the fourth quarter of fiscal 2003. The entire $2.7 million patent impairment was recognized during the fourth quarter of fiscal 2003. The patents and intellectual properties have a remaining estimated useful life of 12 years. We expect to incur approximately $0.1 million of amortization expense per year over the remaining life of the intangible assets.
With the adoption of SFAS No. 142, the Company ceased amortization of goodwill as of July 1, 2001. The following table presents the results of the Company for all periods presented on a comparable basis (in thousands, except per share data):
| | Fiscal Year Ended
| |
|---|
| | June 30,
2003
| | June 30,
2002
| | June 30,
2001
| |
|---|
| Net loss attributed to common stockholders, as reported | | $ | (35,265 | ) | $ | (38,766 | ) | $ | (29,145 | ) |
| Add back goodwill, workforce, and customer base amortization (net of tax) | | | — | | | — | | | 5,268 | |
| | |
| |
| |
| |
| Adjusted net loss attributed to common stockholders | | $ | (35,265 | ) | $ | (38,766 | ) | $ | (23,877 | ) |
| | |
| |
| |
| |
| Basic and diluted net loss per share: | | | | | | | | | | |
| Net loss attributed to common stockholders, as reported | | $ | (1.28 | ) | $ | (3.50 | ) | $ | (5.55 | ) |
| Goodwill, workforce, and customer base amortization (net of tax) | | | — | | | — | | | 1.00 | |
| | |
| |
| |
| |
| Adjusted net loss attributed to common stockholders | | $ | (1.28 | ) | $ | (3.50 | ) | $ | (4.55 | ) |
| | |
| |
| |
| |
F-79
7. Long-Term Debt
Long-term debt consists of the following (in thousands):
| | June 30,
2003
| | June 30,
2002
| |
|---|
| Notes payable | | $ | 29,455 | | $ | 40,000 | |
| Acquisition loans | | | 7,045 | | | — | |
| Obligations under capital leases | | | 5,288 | | | 1,906 | |
| | |
| |
| |
| | | | 41,788 | | | 41,906 | |
| Less: | | | | | | | |
| | Current portion | | | (7,753 | ) | | (5,939 | ) |
| | |
| |
| |
| | | $ | 34,035 | | $ | 35,967 | |
| | |
| |
| |
In fiscal 2003, we amended our old senior credit facility. We originally entered into the old senior credit facility in fiscal 2002 after paying off the entire outstanding balance of our former credit facility with proceeds received from our initial public offering ("IPO"). The amendment reduced our revolving credit facility to $15.0 million from $25.0 million. Prior to the amendment, we also had a $40.0 million term loan and an $8.0 million acquisition loan under the old senior credit facility (earlier in fiscal 2003, we had borrowed $8.0 million under the acquisition line (which is no longer available) to finance our acquisition of Cycam). In conjunction with the amendment of the credit facility, we made a payment of $7.5 million. Of the $7.5 million payment, $6.5 million represented a payment on the $40.0 million term loan, and the remainder was applied toward the acquisition line term loan. All loans under our amended old senior credit facility will mature in fiscal 2007.
During fiscal 2004, we will be required to make payments under the term loan of $5.0 million, payable in quarterly installments. During fiscal 2005, fiscal 2006, and fiscal 2007, we will be required to make payments of $7.4 million, $9.2 million, and $7.9 million, respectively, each payable in quarterly installments. During fiscal 2004, we will also be required to make payments under the acquisition loan of $1.4 million, also payable in quarterly installments. During fiscal 2005, fiscal 2006, and fiscal 2007, we will be required to make payments of $1.6 million, $2.2 million, and $1.8 million on the acquisition line term loan, each also payable in quarterly installments.
Concurrent with receipt of funds from the Company's IPO on April 2, 2002, the Company repaid the entire $66.3 million outstanding under its former senior credit facility and the entire $21.4 million outstanding (including a $1.4 million pre-payment fee) under its former Senior Subordinated Notes. As a result of repaying our former senior subordinated debt the Company recognized a loss of $6.9 million, inclusive of a $5.5 million write-off of unamortized financing costs and discount and a $1.4 million prepayment fee. The Company also recognized a charge of $1.9 million for terminating the interest rate swap agreements that was recognized as interest expense.
At the Company's option, interest rates applicable to loans under its new senior credit facility will be either:
- •
- The greater of the bank's prime rate plus a margin, which depends upon the Company's leverage ratio, ranging from 50 to 175 basis points, or
- •
- LIBOR plus a margin, which depends upon our leverage ratio, ranging from 225 to 350 basis points.
F-80
The Company has entered into an interest rate cap agreement to protect against interest rate fluctuations with respect to the term loans outstanding under its new senior credit facility. This agreement, executed with a highly-rated financial counter-party, requires the counter-party to make payments to the Company in the event that a reference floating rate index exceeds an agreed upon fixed rate. Changes in the fair value of the cap agreement are accounted for as a cash flow hedge.
The amended senior credit facility contains affirmative and negative covenants and limitations, including, but not limited to, required minimum coverage of the Company's obligations to pay interest and incur fixed charges, restrictions on its ability to pay dividends, make other payments and enter into sale transactions, limitations on liens, limitations on its ability to incur additional indebtedness and agreements that the Company use excess cash on hand and proceeds from future equity issuances to pay down its senior credit facility. In addition, the Company also needs the lender's consent to make any acquisition in which it pays more than $10.0 million (including more than $5.0 million in cash, deferred payments and the assumption of debt) or to pay more than $20.0 million (including more than $10.0 million in cash, deferred payments and the assumption of debt) for all of the acquisitions that the Company completes during any fiscal year.
The senior credit facility is secured by all of the Company's assets and contains various events of default, including, but not limited to, defaults upon the occurrence of a change of control of the Company and defaults for non-payment of principal interest or fees, breaches of warranties or covenants, bankruptcy or insolvency, ERISA violations and cross-defaults to other indebtedness.
We are involved in several capital leases related to our machinery and equipment. As of June 30, 2003, we have obligations totaling $5.3 million under capital leases, of which $1.3 million will be paid before the end of fiscal 2004. These leases will expire in fiscal 2007. The interest rate incurred on these capital leases ranges from 7.1%-9.2%. The leases expire beginning in fiscal 2005 through fiscal 2007.
Maturities of long-term debt outstanding and obligations under capital leases at June 30, 2003, are summarized by fiscal year as follows (in thousands):
| 2004 | | $ | 7,753 |
| 2005 | | | 10,292 |
| 2006 | | | 12,724 |
| 2007 | | | 11,019 |
| | |
|
| | | $ | 41,788 |
| | |
|
8. Related-Party Transactions
The Company had entered into management services agreements ("MSAs") with entities associated with certain of the Company's directors and stockholders whereby the Company paid fees plus reimbursement of out-of-pocket expenses for management services rendered. As of April 2002, all MSAs were terminated. Fees incurred for the years ended June 30, 2002, and 2001 totaled $1.5 million, and $1.7 million, respectively. In addition, pursuant to the MSAs, the Company paid fees based on a percentage of the aggregate consideration of each future business acquisition, plus reimbursement of out-of-pocket expenses. Such fees and expenses totaled $0.3 million and $0.6 million, relating to the acquisitions made in the years ended June 30, 2002 and 2001, respectively.
F-81
Certain of the Company's subsidiaries lease their facilities from related parties as a result of acquisitions. Total rent expense under these leases for the years ended June 30, 2003, June 30, 2002 and July 1, 2001 was approximately $0.2 million, $0.6 million, and $0.7 million, respectively. Future minimum lease commitments at June 30, 2003 in connection with these related-party leases are approximately $0.2 million per year with a total future commitment of $1.8 million.
9. Income Taxes
Income tax benefit (expense) consists of the following (in thousands):
| | Year Ended June 30,
| |
|---|
| | 2003
| | 2002
| | 2001
| |
|---|
| Current: | | | | | | | | | | |
| | Federal | | $ | — | | $ | 208 | | $ | — | |
| | State | | | (267 | ) | | (90 | ) | | (70 | ) |
| | |
| �� |
| |
| |
| | | | (267 | ) | | 118 | | | (70 | ) |
| Deferred: | | | | | | | | | | |
| | Federal | | | — | | | — | | | — | |
| | State | | | — | | | — | | | — | |
| | |
| |
| |
| |
| | | | — | | | — | | | — | |
| | |
| |
| |
| |
| | | $ | (267 | ) | $ | 118 | | $ | (70 | ) |
| | |
| |
| |
| |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of deferred tax assets and liabilities are as follows (in thousands):
| | June 30, 2003
| | June 30, 2002
| | June 30, 2001
| |
|---|
| Deferred tax assets: | | | | | | | | | | |
| | Organization costs | | $ | 516 | | $ | 889 | | $ | 1,798 | |
| | Nondeductible reserves and current liabilities | | | 1,189 | | | 900 | | | 1,335 | |
| | Restructuring reserve | | | 373 | | | 1,330 | | | 3,704 | |
| | Net operating loss carryforwards | | | 18,005 | | | 16,838 | | | 8,708 | |
| | Valuation reserve | | | (11,143 | ) | | (12,805 | ) | | (6,147 | ) |
| | |
| |
| |
| |
| | | Total deferred tax assets | | | 8,940 | | | 7,132 | | | 9,398 | |
| Deferred tax liabilities: | | | | | | | | | | |
| | Identified intangible assets | | | (485 | ) | | (1,450 | ) | | (6,187 | ) |
| | Property, plant, and equipment | | | (2,860 | ) | | (1,666 | ) | | (1,530 | ) |
| | Goodwill | | | (5,394 | ) | | (4,013 | ) | | (1,662 | ) |
| | Other | | | (201 | ) | | (3 | ) | | (19 | ) |
| | |
| |
| |
| |
| | | Total deferred tax liabilities | | | (8,940 | ) | | (7,132 | ) | | (9,398 | ) |
| | |
| |
| |
| |
| Net deferred tax liabilities | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
F-82
The Company has U.S. net operating loss carryforwards of approximately $45.0 million, subject to certain limitations, which expire at different times beginning in 2019 and extending through 2023.
A reconciliation between the income tax benefit computed at the federal statutory rate and the recorded income tax benefit (expense) is as follows (in thousands):
| | June 30,
2003
| | June 30,
2002
| | June 30,
2001
| |
|---|
| Income tax benefit computed at the federal statutory rate | | $ | 12,250 | | $ | 2,698 | | $ | 6,785 | |
| State income taxes, net of federal benefit | | | 1,482 | | | 315 | | | 899 | |
| Goodwill impairment, not deductible for tax purposes | | | (15,058 | ) | | — | | | — | |
| Restructuring reserve, portion not deductible for tax purposes. | | | — | | | — | | | (882 | ) |
| Amortization of goodwill, not deductible for tax purposes | | | — | | | — | | | (401 | ) |
| Valuation reserve | | | 1,069 | | | (3,108 | ) | | (6,462 | ) |
| Other | | | (10 | ) | | 213 | | | (9 | ) |
| | |
| |
| |
| |
| Income tax benefit (expense) | | $ | (267 | ) | $ | 118 | | $ | (70 | ) |
| | |
| |
| |
| |
10. Mandatory Redeemable Convertible Stock and Stockholders' Equity Mandatory Redeemable Convertible Stock
On March 30, 1999, the Company sold 300,000 shares of 6% Series B Cumulative Convertible Redeemable Preferred Stock (the "Series B preferred stock"), $0.01 par value per share, for cash in a private placement. On May 14, 1999, the Company sold an additional 32,728 shares for cash in a private placement. All Series B preferred stock was converted in connection with the Company's IPO on April 2, 2002. As a result of the conversion, the Company paid $4.8 million in accrued dividends.
On October 24, 2000, the Company sold 40,000 shares of 6% Series C Cumulative Convertible Redeemable Preferred Stock (the "Series C preferred stock"), $0.01 par value per share, for cash in a private placement. On April 18, 2001, the Company sold an additional 300 shares for cash in a private placement. In addition to the shares purchased at a price of $1,000 per share, each purchaser also received an option to purchase an additional .2857 shares at a price of $1,000 per share for each share acquired. The option was exercisable prior to or coincident with the earlier to occur of (i) a qualified public offering (as defined) and (ii) October 24, 2001. The options were not exercised and expired on October 24, 2001. All Series C preferred stock was converted in connection with the Company's IPO on April 2, 2002. Additionally, on February 27, 2001, the Company issued a warrant to purchase 525 shares of Series C preferred stock for $1,000 per share to the placement agent it had used in connection with the issuance of the Series C preferred stock on October 24, 2000. The warrant was exercised upon the completion of the IPO.
In connection with the Company's initial public offering, our Series C preferred stock converted into a number of shares of our common stock based upon the initial public offering price of our common stock. The Company's net loss for the fiscal year ending June 30, 2002 includes a deemed preferred stock dividend of approximately $21.3 million for the value of the additional shares of our common stock issued to the holders of our Series C preferred stock upon conversion and to reflect the beneficial conversion feature.
F-83
In conjunction with the acquisition of ACT Medical, Inc. (see Note 3), the Company issued 33,423 shares of 6% Series D Cumulative Convertible Redeemable Preferred Stock (the "Series D preferred stock"), $0.01 par value per share, and rolled over options for an additional 6,920 shares of Series D preferred stock. All Series D preferred stock was converted in connection with the Company's IPO on April 2, 2002.
On March 30, 1999, the Company issued 37,440 shares of Series A Preferred Stock (the "Series A preferred stock"), $.01 par value per share, in connection with the acquisition of businesses. In addition, the Company also issued 600 shares of Series A preferred stock to key employees of an acquired company in conjunction with the acquisition. The fair value of these shares totaled approximately $201,000 and was included in the Company's organization and start-up costs in the period ended July 3, 1999. Subsequent to March 30, 1999, the Company sold an additional 330 shares of Series A preferred stock to key employees for cash at fair value as determined at March 30, 1999. All Series A preferred stock was converted in connection with the Company's IPO on April 2, 2002.
On March 30, 1999, the Company sold 65,000 shares of Series Z Convertible Nominal Value Redeemable Preferred Stock (the "Series Z preferred stock"), $0.01 par value per share, for cash in a private placement. The Series Z preferred stock had no dividend rights and was senior only to the common stock with respect to rights on liquidation. The Series Z preferred stock was convertible at the option of the holder into common stock at any time. The initial conversion rate was one share of Series Z preferred stock for 10 shares of common stock, subject to anti-dilution provisions. All Series Z preferred stock were converted in connection with the Company's IPO on March 27, 2002.
On fiscal 2002, the Company issued 6,000 shares of its 6% Series E Preferred Stock (the "Series E preferred stock"), $.01 par value per share, for cash in a private placement (see Note 3—Acquisitions). The Series E preferred stock accrues dividends at $60 per year during the first year from issuance, payable at the discretion of the Board of Directors, and at the rate of $160 per year on a retroactive basis after the first anniversary of issuance. During April 2002, 4,065 shares of the Series E preferred stock were redeemed for $1,000 per share plus accumulated dividends. During fiscal 2003, the remaining 1,935 shares of Series E preferred stock were redeemed.
On April 14, 1998, the Company was incorporated with the sale of 100 shares of common stock at $1 per share. In February 1999, an additional 65 shares were sold to individuals at $1,000 per share. On March 30, 1999, in conjunction with the acquisitions and the commencement of business operations, the stock was split 2,209-for-1 (adjusting the pre-split shares to 363,594 shares) and an additional 38,706 shares were sold for cash to existing stockholders. The amount paid for the common stock was based on fair value. Also on March 30, 1999, 42,500 shares of common stock were issued in connection with
F-84
the acquisition of a business. In January 2000, the Company's common stock was split 10-for-1 and all share references to common stock have been adjusted to give effect to the split. The adjusted post-split outstanding common shares was 4,448,000.
On March 27, 2002, the Company commenced its IPO in which it initially sold 8,340,000 shares of common stock at a price of $12.00 per share. The net proceeds of the IPO, which the Company received on April 2, 2002, after deducting underwriting discounts, were approximately $93.1 million. The Company used these proceeds to pay down senior debt in the amount of $66.3 million, extinguish senior subordinated debt in the amount of $21.4 million (including a $1.4 million pre-payment fee), redeem Series E preferred stock in the amount of $4.1 million (including dividends), redeem Series F preferred stock in the amount of $4.1 million (including dividends), pay accrued and unpaid dividends on Series B preferred stock in the amount of $4.8 million and to pay fees under service agreements with Kidd & Co. and Whitney Mezzanine Management Company. The Company also incurred approximately $2.0 million in other expenses related to the IPO. Additionally, in April 2002, the Company's underwriters exercised their option to purchase an additional 1,251,000 shares of common stock at $12.00 per share, with 926,000 shares sold by the Company and 325,000 shares sold by two stockholders. This resulted in additional net proceeds, which the Company received on April 17, 2002, of $10.3 million after deducting underwriting discounts.
Upon consummation of the IPO all shares of the Company's Series A, Series B, Series C, Series D and Series Z preferred stock converted to common stock. The conversion amounts of common shares were as follows:
| Series A | | 1,918,500 |
| Series B | | 3,327,279 |
| Series C | | 3,934,870 |
| Series D | | 1,769,549 |
| Series Z | | 650,000 |
| Series C Warrant | | 2,916 |
During fiscal 2003, the remaining 1,935 shares of Series E Preferred Stock were redeemed for $1,000 per share plus accumulated unpaid dividends. Therefore, as of June 30, 2003 there were no accumulated unpaid dividends.
F-85
The Company originally had reserved 4,430,000 shares of its common stock for issuance to directors, officers, employees, and consultants under the 1999 Stock Plan (the Plan). The table below shows the activity in the Plan:
| | Options
Outstanding
| | Shares Reserved
| | Weighted Average
Initial Exercise
Price
|
|---|
| Balance at July 1, 2000 | | 1,441,070 | | 308,930 | | | |
| | Reserved | | — | | 1,680,000 | | | |
| | Granted | | 1,555,660 | | (1,555,660 | ) | $ | 17.13 |
| | Exercised | | (20,308 | ) | — | | | 13.99 |
| | Canceled | | (401,038 | ) | 401,038 | | | 14.40 |
| | |
| |
| | | |
| Balance at June 30, 2001 | | 2,575,384 | | 834,308 | | | 15.54 |
| | Reserved | | — | | 1,000,000 | | | |
| | Granted | | 739,624 | | (739,624 | ) | | 16.96 |
| | Exercised | | (400 | ) | — | | | 12.00 |
| | Canceled | | (461,996 | ) | 461,996 | | | 17.58 |
| | |
| |
| | | |
| Balance at June 30, 2002 | | 2,852,612 | | 1,556,680 | | | 15.58 |
| | Reserved | | — | | — | | | — |
| | Granted | | 671,750 | | (671,750 | ) | | 10.33 |
| | Exercised | | — | | — | | | — |
| | Canceled | | (3,082,149 | ) | 3,082,149 | | | 15.00 |
| | |
| |
| | | |
| Balance at June 30, 2003 | | 442,213 | | 3,967,079 | | $ | 13.00 |
| | |
| |
| | | |
The options outstanding at June 30, 2003 include 34,000 options with an initial exercise price of $2.25 per share, 222,018 options with an initial exercise price of $8.25-$12.00 per share, 100,305 options with an exercise price of $13.19-$16.24 per share, and 85,890 options with an exercise price of $17.00-$20.00. Options granted through June 30, 2003 are exercisable for 10 years from date of grant and vest 25% each year. The initial exercise price of the options applies to the options vesting at the first anniversary date. The initial exercise price remains fixed for all options granted after June 30, 2001. Prior to June 30, 2001, all options had variable exercise prices equal to 110%, 121% and 131.1% of the initial exercise price on the second, third and fourth grant date anniversary, respectively, except options granted at $16.24 in fiscal 2001, which had a fixed exercise price. There were 201,337 options outstanding with an initial exercise price between $12.00-$14.00 per share and 75,560 options outstanding with an initial exercise price between $16.24-$20.00 per share that were fully vested and exercisable at June 30, 2003. The weighted average grant date fair values of options to purchase common stock granted in fiscal years 2003, 2002 and 2001 were $6.91, $3.35 and $3.38, respectively.
During fiscal 2003, we implemented a stock option exchange program. The exchange program was offered to all employees and non-employee directors. Under the stock option exchange program, employees were eligible to cancel outstanding stock options under the 1999 stock plan in exchange for new options that will be awarded six months and a day after the cancellation date, which was June 6,
F-86
2003. The number of shares subject to the new option will be equal to one half the number of shares subjected to cancelled options. The new options will vest in four annual installments, with respect to employees, or three annual installments, with respect to non-employee directors. A total of 2,243,786 options were cancelled on June 6, 2003 in conjunction with the stock option exchange program.
The Company also has 106,978 options outstanding that were assumed in connection with the ACT Medical acquisition (see Note 3—Acquisitions).
All stock options and unvested restricted stock were antidilutive due to the net loss incurred in fiscal 2003. In addition, if we had net income certain shares of common stock and unvested restricted stock would have been antidilutive because they had an exercise price greater than the average market price during the year. If we had net income for fiscal 2003 diluted weighted average commons shares outstanding would have increased by 647,414 shares.
In addition to stock options, we have issued restricted stock as part of employee incentive plans. The fair market value of the restricted stock is amortized over the vesting period. During the fiscal year ended June 30, 2003 we incurred $0.3 million of non-cash stock compensation expenses related to restricted stock issuances. No such charges were incurred during fiscal 2002 and 2001. During fiscal 2003 we issued 824,442 and 50,407 shares of restricted stock at a grant date market value of $2.25 and $11.25 per share, respectively. As of June 30, 2003, we had 854,952 shares of restricted stock outstanding, all of which were granted under the 1999 Stock Plan.
The Company has reserved the following shares of common stock as of June 30, 2003:
| 1999 Stock Plan | | 3,548,550 |
| Series D Options | | 106,978 |
| Series E Warrants | | 66,329 |
| Employee Stock Purchase Plan | | 273,773 |
| | |
|
| Total | | 3,995,630 |
| | |
|
The number of shares reserved for issuance under the Employee Stock Purchase Plan is subject to an annual increase on the first day of each fiscal year equal to the lower of 750,000 shares of common stock, 2.5% of the number of shares of common stock outstanding on that date or such lesser amount that may be determined by the Company's board of directors.
The 3,548,550 shares of common stock reserved for issuance under the 1999 Stock Plan as of June 30, 2003, reflects the 860,742 shares of restricted stock that were issued under the plan during fiscal 2003, net of forfeited shares due to employee terminations.
We have an employee stock purchase plan that is available to substantially all employees. Eligible employees may purchase our common stock through payroll deductions. Employees can purchase our common stock at a price equal to the lower of 85% of the closing market price of our common stock at the beginning or end of each stock purchase period. We issued 0.2 million shares of common stock
F-87
during fiscal 2003 in connection with our employee stock purchase plan. Prior to fiscal 2003, no common stock was issued in connection with the employee stock purchase plan.
11. Employee Benefits
The Company offers their qualified employees the opportunity to participate in a defined contribution retirement plan qualifying under the provisions of Section 401(k) of the Internal Revenue Code. Expenses recorded by the Company with respect to 401(k) plan for the years ended June 30, 2003, June 30, 2002 and June 30, 2001 were $1.2 million, $1.6 million, and $0.6 million, respectively.
12. Leases
The Company has operating leases relating principally to its buildings. Total rent expense the for the years ended June 30, 2003, June 30, 2002, and June 30, 2001 (including amounts to related parties—see Note 8—Related-Party Transactions) was approximately $3.9 million, $2.5 million and $3.5 million, respectively. Future minimum lease commitments at June 30, 2003, for leases with initial or remaining terms of more than one year, including amounts due to related parties, are summarized by fiscal year as follows (in thousands):
| 2004 | | $ | 3,329 |
| 2005 | | | 2,923 |
| 2006 | | | 2,384 |
| 2007 | | | 2,253 |
| 2008 | | | 2,144 |
| Thereafter | | | 12,657 |
| | |
|
| | | $ | 25,690 |
| | |
|
13. Restructuring Charge
In June 2002, the FASB issued SFAS No. 146 "Accounting for Costs Associated With Exit or Disposal Activities." SFAS No. 146 superseded Emerging Issues Task Force ("EITF") Issue No. 94-3, "Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring)." The principle difference between SFAS No. 146 and EITF 94-3 relates to when an entity can recognize a liability related to exit or disposal activities. SFAS No. 146 requires that a liability be recognized for costs associated with an exit or disposal activity when the liability is incurred. EITF 94-3 allowed a liability related to an exit or disposal activity to be recognized on the date the entity commits to an exit plan. We adopted this standard on January 1, 2003, which was the standard's effective date. The standard did not materially impact our consolidated financial results or financial position upon adoption, but did affect the timing of when we recognized restructuring charges related to the fiscal 2003 restructuring plan.
F-88
In fiscal 2003, we implemented our second restructuring plan to reconfigure our resources in an effort to meet our customer's needs and lower our cost of operations. The restructuring plan includes facility consolidations, employee terminations, and other activities. Based on an evaluation of the unique and common characteristics of the various facilities, management determined that it could achieve overall cost savings by closing several facilities, thus improving capacity utilization and efficiency at the remaining facilities. Criteria in this evaluation included: current capacity utilization; uniqueness of manufacturing capabilities; current operating costs; difficulty and cost associated with relocation and revalidation of key processes and equipment; and customer supply requirements. We estimate the total cost of this restructuring plan will be $15.0-$20.0 million and to complete the plan by the end of fiscal 2005. In addition to these charges, we will invest $1.7 million in plant and equipment to ensure the facility consolidation does not disrupt operations. The estimate is based on the best available facts at this time. These estimates may change as new information becomes available. The following table contains detailed information about the charges incurred to date, recorded in accordance with SFAS No. 146, related to the fiscal 2003 restructuring plan (in millions):
Description
| | Estimated
Charges
| | Fiscal
2003
Charges
| | Estimate
Remaining
Charges
|
|---|
| Employee termination | | $ | 4.8 | | $ | 0.7 | | $ | 4.1 |
| Facility consolidation | | | 4.6 | | | — | | | 4.6 |
| Property, plant and equipment disposals | | | 2.2 | | | — | | | 2.2 |
| Other direct costs | | | 6.7 | | | 0.6 | | | 6.1 |
| | |
| |
| |
|
| Total | | $ | 18.3 | | $ | 1.3 | | $ | 17.0 |
| | |
| |
| |
|
Employee termination charges represent the cost of reducing our workforce in conjunction with facility consolidations. During fiscal 2003 we terminated 43 people in as a result of the fiscal 2003 restructuring plan. We estimate that a total of approximately 270 people will be terminated as a result of this restructuring plan. Facility consolidation charges represent the direct costs of moving property, plant and equipment to new facilities. Property, plant and equipment disposals represents the write-off of redundant assets that will no longer be used in ongoing operations as a result of our facility consolidation initiative.
The following table contains information regarding our restructuring liability as of June 30, 2003 (in millions):
Description
| | Beginning
Balance
| | Additions
| | Payments
| | Ending
Balance
|
|---|
| Employee termination | | $ | — | | $ | 0.7 | | $ | 0.2 | | $ | 0.5 |
| Facility consolidation | | | — | | | — | | | — | | | — |
| Property, plant and equipment disposals | | | — | | | — | | | — | | | — |
| Other direct costs | | | — | | | 0.6 | | | 0.6 | | | — |
| | |
| |
| |
| |
|
| Total | | $ | — | | $ | 1.3 | | $ | 0.8 | | $ | 0.5 |
| | |
| |
| |
| |
|
We estimate that we will incur $5.8-$8.3 million and $7.9-$10.4 million of charges in fiscal 2004 and fiscal 2005, respectively, related to the fiscal 2003 restructuring plan.
F-89
In June 2001, the Company completed a strategic review of its manufacturing operations and support functions. Based on this review and with approval of the Board of Directors, management implemented its first restructuring plan and began actions to eliminate redundant facilities. We recognized an $11.5 million restructuring charge in accordance with EITF 94-3 "Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring)."
Information relating to the fiscal 2001 restructuring charges is as follows (in millions):
| | Initial
Accrual
| | Reclassification
| | Additional
Accrual
| | Payments
through
June 30,
2003
| | Balance at
June 30, 2003
|
|---|
| Impairment of goodwill and other intangibles | | $ | 3.6 | | $ | — | | $ | — | | $ | 3.6 | | $ | — |
| Impairment of property, plant and equipment | | | 1.9 | | | 2.3 | | | — | | | 4.2 | | | — |
| Employee termination benefits | | | 3.8 | | | (1.2 | ) | | 1.9 | | | 4.0 | | | 0.5 |
| Other direct costs | | | 2.2 | | | (1.1 | ) | | 0.5 | | | 1.6 | | | — |
| | |
| |
| |
| |
| |
|
| | | $ | 11.5 | | $ | — | | $ | 2.4 | | $ | 13.4 | | $ | 0.5 |
| | |
| |
| |
| |
| |
|
Facilities at Danbury, Connecticut, Pittsfield, Massachusetts, and East Longmeadow, Massachusetts were identified to be closed or sold with production absorbed into existing facilities in Pennsylvania, Minnesota, New Hampshire, and Mexico. During fiscal 2002 the Company sold the Pittsfield and East Longmeadow facilities. The Danbury facility was closed during fiscal 2003. In addition, management decided to close its Redwood City, California facility as part of this restructuring plan. This decision led to additional restructuring charges, which are reflected in the "additions" column in the table above.
Because management expected that it would not retain all of the customers served by these four facilities, a portion of the customer base intangible asset ($0.5 million) was written off as well as the entire remaining acquired workforce intangible for each facility ($0.5 million). In addition, because management believed the residual goodwill recorded at each acquisition was significantly related to the local operations, it concluded that goodwill was impaired by the closure of the facilities and wrote off the related goodwill ($2.6 million). Other recorded charges related to the restructuring include employee termination benefits expected to be paid based on the Company's announced termination benefits policy ($3.3 million), costs of plant and equipment not expected to be recovered ($4.2 million), and other exit costs ($1.6 million), including costs related to lease terminations, facilities restoration, equipment dismantlement and disposal, legal costs, and other costs. Costs related to realignment of leadership positions in the corporate support organization also were accrued at June 30, 2002 ($1.2 million). A reclassification in the allocation of the reserve as shown in the table above was a result of selling the Pittsfield and East Longmeadow facilities as opposed to closing them.
Employee termination benefits consist of payments to employees based on the Company's severance policy of two weeks pay for each year of credited service with a minimum of six weeks payment and outplacement consultation services. The $3.3 million accrual for employee termination benefits was based on approximately 225 individuals estimated to be affected, actual credited service,
F-90
and actual compensation. The $1.2 million accrual for corporate management severance benefits included salary continuation, outplacement consultation services and legal cost for seven individuals employed by the Company's corporate headquarters operations whose positions were eliminated as a result of the Company-wide restructuring. The charge for other direct costs which aggregated $1.6 million was comprised of estimated costs for (1) lease terminations, real estate taxes and property insurance of $0.5 million, (2) plant shut down costs and restoration of facilities to pre-lease conditions of $0.5 million, (3) dismantlement and disposal of obsolete equipment of $0.3 million, (4) legal costs of $0.2 million and (5) other related shut down costs of $0.1 million.
14. Comprehensive Income (Loss)
Comprehensive income (loss) represents net loss attributed to common stockholders plus the results of any stockholders' equity changes related to the Company's previous interest rate swaps and current interest rate cap agreements. For fiscal 2003, 2002 and 2001 comprehensive loss, net-of-tax, was $35.6 million, $8.0 million, and $22.1 million, respectively.
F-91
15. Quarterly Financial Data (Unaudited) (In thousands, except share and per share amounts)
| | First
Quarter
| | Second
Quarter
| | Third
Quarter
| | Fourth
Quarter
| | Total
| |
|---|
| 2003 | | | | | | | | | | | | | | | | |
| Net sales | | $ | 41,003 | | $ | 44,621 | | $ | 44,511 | | $ | 47,163 | | $ | 177,298 | |
| Cost of sales | | | 30,812 | | | 33,170 | | | 34,007 | | | 33,981 | | | 131,970 | |
| Income (loss) before income taxes | | | 1,681 | | | 2,707 | | | (30,413 | ) | | (8,973 | ) | | (34,998 | ) |
| Income tax (expense) benefit | | | (2 | ) | | (13 | ) | | (12 | ) | | (240 | ) | | (267 | ) |
| | |
| |
| |
| |
| |
| |
| Net loss | | $ | 1,679 | | $ | 2,694 | | $ | (30,425 | ) | $ | (9,213 | ) | $ | (35,265 | ) |
| | |
| |
| |
| |
| |
| |
| Average common shares outstanding basic | | | 27,135,481 | | | 27,652,413 | | | 27,639,127 | | | 27,856,085 | | | 27,602,806 | |
| | |
| |
| |
| |
| |
| |
| Average common shares outstanding diluted | | | 27,453,441 | | | 27,862,127 | | | 27,639,127 | | | 27,856,085 | | | 27,602,806 | |
| | |
| |
| |
| |
| |
| |
| Income (loss) per share basic and diluted | | $ | 0.06 | | $ | 0.10 | | $ | (1.10) | (1) | $ | (0.33) | (2) | $ | (1.28 | ) |
| | |
| |
| |
| |
| |
| |
| 2002 | | | | | | | | | | | | | | | | |
| Net sales | | $ | 33,865 | | $ | 38,290 | | $ | 42,150 | | $ | 44,594 | | $ | 158,899 | |
| Cost of sales | | | 26,107 | | | 28,509 | | | 31,483 | | | 30,990 | | | 117,089 | |
| Loss before income taxes | | | (1,223 | ) | | (400 | ) | | (2,836 | ) | | (3,257 | ) | | (7,716 | ) |
| Income tax benefit | | | — | | | — | | | 3 | | | 115 | | | 118 | |
| | |
| |
| |
| |
| |
| |
| Net loss | | $ | (1,223 | ) | $ | (400 | ) | $ | (2,833 | ) | $ | (3,142 | ) | $ | (7,598 | ) |
| Net loss attributed to common stockholders | | | (3,884 | ) | | (3,061 | ) | | (28,650 | ) | | (3,171 | ) | | (38,766 | ) |
| | |
| |
| |
| |
| |
| |
| Average common shares outstanding—basic and diluted | | | 5,255,755 | | | 5,256,155 | | | 7,146,444 | | | 26,779,727 | | | 11,086,103 | |
| | |
| |
| |
| |
| |
| |
| Loss per share basic and diluted | | $ | (0.74 | ) | $ | (0.58 | ) | $ | (4.01 | ) | $ | (0.12 | ) | $ | (3.50 | ) |
| | |
| |
| |
| |
| |
| |
- (1)
- Includes $30.0 million goodwill impairment charge, and $1.9 million restructuring charges.
- (2)
- Includes $10.0 million goodwill and other intangible asset impairment charges, and $1.8 million restructuring charges.
F-92
MEDSOURCE TECHNOLOGIES, INC.
Unaudited Consolidated Balance Sheets
As of March 28, 2004 and June 30, 2003
(In thousands)
| | March 28,
2004
| | June 30,
2003
| |
|---|
| ASSETS | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 13,978 | | $ | 10,781 | |
| | Accounts receivable, net | | | 23,075 | | | 23,710 | |
| | Inventories | | | 24,292 | | | 25,617 | |
| | Prepaid expenses and other current assets | | | 4,002 | | | 4,318 | |
| | |
| |
| |
| | | Total current assets | | | 65,347 | | | 64,426 | |
| Property, plant, and equipment, net | | | 50,551 | | | 52,752 | |
| Goodwill | | | 96,637 | | | 96,582 | |
| Other identifiable intangible assets, net | | | 1,327 | | | 1,432 | |
| Deferred financing costs | | | 1,364 | | | 1,682 | |
| Other assets | | | 1,338 | | | 1,343 | |
| | |
| |
| |
| | | Total assets | | $ | 216,564 | | $ | 218,217 | |
| | |
| |
| |
LIABILITIES & STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
| | Accounts payable | | $ | 10,579 | | $ | 10,868 | |
| | Accrued compensation and benefits | | | 4,279 | | | 5,498 | |
| | Other accrued expenses | | | 2,916 | | | 2,293 | |
| | Reserve for restructuring | | | 489 | | | 958 | |
| | Current portion of obligations under capital lease | | | 1,290 | | | 1,326 | |
| | Current portion of long term debt | | | 7,955 | | | 6,427 | |
| | |
| |
| |
| | | Total current liabilities | | | 27,508 | | | 27,370 | |
| Obligations under capital leases, less current portion | | | 2,999 | | | 3,962 | |
| Long term debt, less current portion | | | 25,877 | | | 30,073 | |
| Other long term liabilities | | | 602 | | | 731 | |
| Stockholders' equity: | | | | | | | |
| | Common stock | | | 292 | | | 289 | |
| | Additional paid in capital | | | 278,192 | | | 277,791 | |
| | Treasury stock | | | (1,500 | ) | | (1,463 | ) |
| | Accumulated other comprehensive loss | | | (217 | ) | | (288 | ) |
| | Accumulated deficit | | | (115,676 | ) | | (118,326 | ) |
| | Unearned compensation | | | (1,513 | ) | | (1,922 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 159,578 | | | 156,081 | |
| | |
| |
| |
| | | | Liabilities & stockholders' equity | | $ | 216,564 | | $ | 218,217 | |
| | |
| |
| |
See Notes to Consolidated Financial Statements
F-93
MEDSOURCE TECHNOLOGIES, INC.
Unaudited Consolidated Statements of Operations
For the three and nine months ended March 28, 2004 and March 30, 2003
(In thousands except share and per share amounts)
| | Three months ended
| | Nine months ended
| |
|---|
| | March 28,
2004
| | March 30,
2003
| | March 28,
2004
| | March 30,
2003
| |
|---|
| Revenues | | $ | 46,027 | | $ | 44,511 | | $ | 136,258 | | $ | 130,135 | |
| Costs and expenses: | | | | | | | | | | | | | |
| | Cost of product sold | | | 35,037 | | | 34,007 | | | 104,107 | | | 97,989 | |
| | Selling, general and administrative expense | | | 7,692 | | | 8,379 | | | 23,224 | | | 24,475 | |
| | Restructuring charges | | | 1,100 | | | 1,948 | | | 3,989 | | | 1,948 | |
| | Goodwill impairment | | | — | | | 30,000 | | | — | | | 30,000 | |
| | |
| |
| |
| |
| |
| Operating income (loss) | | | 2,198 | | | (29,823 | ) | | 4,938 | | | (24,277 | ) |
| Interest expense, net | | | (668 | ) | | (590 | ) | | (2,027 | ) | | (1,748 | ) |
| | |
| |
| |
| |
| |
| Income (loss) before income taxes | | | 1,530 | | | (30,413 | ) | | 2,911 | | | (26,025 | ) |
| Income tax expense | | | 56 | | | 12 | | | 261 | | | 27 | |
| | |
| |
| |
| |
| |
| Net income (loss) | | $ | 1,474 | | $ | (30,425 | ) | $ | 2,650 | | $ | (26,052 | ) |
| | |
| |
| |
| |
| |
| Net income (loss) per (basic and diluted) | | $ | 0.05 | | $ | (1.10 | ) | $ | 0.09 | | $ | (0.95 | ) |
| | |
| |
| |
| |
| |
| Weighted average common shares outstanding | | | | | | | | | | | | | |
| | Basic | | | 28,125,901 | | | 27,639,127 | | | 28,044,846 | | | 27,413,489 | |
| | Diluted | | | 29,046,182 | | | 27,639,127 | | | 28,753,689 | | | 27,413,489 | |
See Notes to Consolidated Financial Statements
F-94
MEDSOURCE TECHNOLOGIES, INC.
Unaudited Consolidated Statements of Cash Flows
For the nine months ended March 28, 2004 and March 30, 2003
(In thousands)
| | For the Nine Months Ended
| |
|---|
| | March 28, 2004
| | March 30, 2003
| |
|---|
| Cash flows from operating activities: | | | | | | | |
| | Net income | | $ | 2,650 | | $ | (26,052 | ) |
| | Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | |
| | | Depreciation | | | 7,045 | | | 6,324 | |
| | | Non cash stock compensation | | | 461 | | | 71 | |
| | | Goodwill impairment | | | — | | | 30,000 | |
| | | Amortization of other intangibles | | | 105 | | | 253 | |
| | | Amortization of deferred financing costs and discount on long term debt | | | 342 | | | 305 | |
| | | Loss on retirement of equipment | | | 491 | | | 1,122 | |
| | Changes in operating assets and liabilities, net of effect of business acquired: | | | | | | | |
| | | Accounts receivable | | | 635 | | | 145 | |
| | | Inventories | | | 1,325 | | | (3,306 | ) |
| | | Prepaid expenses and other current assets | | | 316 | | | (590 | ) |
| | Accounts payable, accrued compensation and benefits, accrued expenses and other | | | (1,353 | ) | | (2,806 | ) |
| | | Other | | | (113 | ) | | (280 | ) |
| | |
| |
| |
| | | | Net cash provided by operating activities | | | 11,904 | | | 5,186 | |
| Cash flows from investing activities: | | | | | | | |
| | Acquisition of businesses, net of cash acquired | | | — | | | (22,591 | ) |
| | Proceeds from sale of equipment | | | 348 | | | 80 | |
| | Additions to plant and equipment, net | | | (5,685 | ) | | (10,623 | ) |
| | |
| |
| |
| | | | Net cash used in investing activities | | | (5,418 | ) | | (33,134 | ) |
| Cash flows from financing activities: | | | | | | | |
| | Payments of long term debt | | | (3,715 | ) | | (2,997 | ) |
| | Proceeds of long term debt | | | — | | | 8,000 | |
| | Redemption of Series E preferred stock | | | — | | | (2,010 | ) |
| | Proceeds from sale of common stock, net of costs | | | 345 | | | 813 | |
| | |
| |
| |
| | | | Net cash (used in) provided by financing activities | | | (3,370 | ) | | 3,806 | |
| | |
| |
| |
| Increase (decrease) in cash and cash equivalents | | | 3,197 | | | (24,142 | ) |
| Cash and cash equivalents at beginning of period | | | 10,781 | | | 38,268 | |
| | |
| |
| |
| Cash and cash equivalents at end of period | | $ | 13,978 | | $ | 14,126 | |
| | |
| |
| |
See Notes To Consolidated Financial Statements
F-95
MEDSOURCE TECHNOLOGIES, INC.
Notes to Unaudited Consolidated Financial Statements
1. Interim Financial Statements
MedSource Technologies, Inc. ("we" or the "Company") has prepared the unaudited interim consolidated financial statements presented herein in accordance with accounting principles generally accepted in the United States for interim financial statements and in accordance with the instructions to Form 10-Q and Regulation S-X. Accordingly, they do not include all information and footnotes required by accounting principles generally accepted in the United States for complete financial statements. The consolidated financial statements are unaudited but reflect all adjustments, consisting of normal recurring adjustments and accruals, which, in the opinion of management, are considered necessary for a fair presentation of our financial position and results of operations and cash flows for the interim periods presented. Results of operations for interim periods are not necessarily indicative of the results that may be expected for the full fiscal year.
The consolidated financial statements should be read in conjunction with the summary of significant accounting policies and notes to consolidated financial statements included in the Company's annual report for its fiscal year ended June 30, 2003.
Preparation of the Company's financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
The Company accounts for its stock-based employee compensation plans using the intrinsic value method in accordance with Accounting Principles Board Opinion (APB) No. 25, "Accounting for Stock Issued to Employees," and related interpretations. Stock compensation is awarded to key employees in the form of stock options and restricted stock. All stock options are issued with exercise prices equal to the fair market value of the related shares on the date of issuance. Accordingly, as provided by APB No. 25, we did not recognize any stock compensation expense for stock options granted during the periods presented. The following table summarizes what our operating results would have been if the Company had applied the fair value recognition provisions of Statement of Financial Accounting
F-96
Standards (SFAS) No.148, "Accounting for Stock-Based Compensation," to its stock based employee compensation (in thousands except share and per share amounts):
| | For The Three Months Ended
| | For the Nine Months Ended
| |
|---|
| | March 28, 2004
| | March 30, 2003
| | March 28, 2004
| | March 30, 2003
| |
|---|
| Net income (loss) as reported | | $ | 1,474 | | $ | (30,425 | ) | $ | 2,650 | | $ | (26,052 | ) |
| Stock compensation expense—fair value based method | | | (287 | ) | | (272 | ) | | (956 | ) | | (325 | ) |
| | |
| |
| |
| |
| |
| Pro forma net income | | $ | 1,187 | | $ | (30,697 | ) | $ | 1,694 | | $ | (26,377 | ) |
| | |
| |
| |
| |
| |
| Net income (loss) per share as reported (basic and diluted) | | $ | 0.05 | | $ | (1.10 | ) | $ | 0.09 | | $ | (0.95 | ) |
| | |
| |
| |
| |
| |
| Pro forma net income (loss) per share (basic and diluted) | | $ | 0.04 | | $ | (1.11 | ) | $ | 0.06 | | $ | (0.96 | ) |
| | |
| |
| |
| |
| |
| Weighted average shares outstanding—basic | | | 28,125,901 | | | 27,639,127 | | | 28,044,846 | | | 27,413,489 | |
| Effect of dilutive securities: | | | | | | | | | | | | | |
| | Stock option plans | | | 327,543 | | | — | | | 153,292 | | | — | |
| | Restricted stock | | | 569,781 | | | — | | | 532,600 | | | — | |
| | Stock warrants | | | 22,957 | | | — | | | 22,951 | | | — | |
| | Dilutive potential common shares | | | 920,281 | | | — | | | 708,843 | | | — | |
| | |
| |
| |
| |
| |
| | Weighted average shares outstanding—diluted | | | 29,046,182 | | | 27,639,127 | | | 28,753,689 | | | 27,413,489 | |
| | |
| |
| |
| |
| |
We have issued restricted stock as part of employee incentive plans. The fair market value of the restricted stock is amortized over the projected remaining vesting period. During the three months and nine months ended March 28, 2004, we incurred $0.2 million and $0.5 million of non-cash stock compensation expenses related to restricted stock issuances. During the three and nine months ended March 30, 2003, we incurred $0.0 million and $0.1 million of non-cash stock compensation expenses related to restricted stock issuances.
2. Acquisition
On September 4, 2002, the Company acquired Cycam, Inc. ("Cycam"), a company located in Houston, Pennsylvania that manufactures reconstructive implants and instruments. The total purchase price was approximately $24.4 million, which included $18.4 million in cash and 667,175 shares of common stock valued at $6.0 million. The fair market value of the shares issued in connection with the Cycam acquisition was based on the market price of our common stock on the date of issuance. The acquisition was recorded using the purchase method of accounting. The purchase price allocation was $6.0 million to net tangible assets and $18.4 million to goodwill. During the nine months ended March 28, 2003, the purchase price allocation was finalized and resulted in an increase of $0.1 million to the allocation to goodwill. In conjunction with the acquisition, the Company drew $8.0 million from the acquisition line under the Company's old credit facility. The effect of the acquisition on our historical financial position and results of operations is not material, and therefore no pro forma data
F-97
of this acquisition is presented. Cycam's operating results have been included in our consolidated operating results since the date of acquisition.
The acquisition of Cycam expanded our capacity and capabilities in the metal machining of orthopaedic reconstructive implants. In addition, Cycam provided us with the complimentary capabilities of plastic machining, surface coatings, near net shape forging, and sterilized packaging and kitting of orthopaedic implants. Cycam also had strong relationships with several leading orthopaedic companies where we had only a minor presence prior to the acquisition.
3. Inventories
Inventories consisted of the following (in thousands):
| | March 28, 2004
| | June 30, 2003
|
|---|
| | (Unaudited)
| |
|
|---|
| Raw material | | $ | 11,345 | | $ | 13,806 |
| Work in progress | | | 8,590 | | | 8,389 |
| Finished goods | | | 4,357 | | | 3,422 |
| | |
| |
|
| | Total | | $ | 24,292 | | $ | 25,617 |
| | |
| |
|
4. Goodwill
During the nine months ended March 28, 2004, goodwill increased by $0.1 million resulting from purchase accounting adjustments related to the Cycam and Midwest Plastics acquisitions that occurred in the year ended June 30, 2003.
5. Other Identifiable Intangible Assets, net
During the nine months ended March 28, 2004, other identifiable intangible assets, net, decreased by $0.1 million resulting from amortization.
6. Comprehensive Income (Loss)
Comprehensive income (loss) represents net income (loss) attributed to common stockholders plus the results of any stockholders' equity changes relating to the Company's previous interest rate swaps and current interest rate cap agreements. For the three and nine months ended March 28, 2004 comprehensive income was $1.5 million and $2.7 million, respectively. For the three and nine months ended March 30, 2003, comprehensive loss was $30.5 million and $26.3 million, respectively.
7. Restructuring Charges
During the three and nine months ended March 28, 2004, we continued our fiscal 2003 restructuring plan to reconfigure our resources in an effort to meet our customer's needs and lower our cost of operations. The restructuring plan includes facility consolidations, employee terminations, and other activities. We estimate the total cost of this restructuring plan will be $15.0-$20.0 million and that the plan will be completed by the end of fiscal 2005. The total cost estimate includes an investment of $1.7 million in plant and equipment to ensure the facility consolidation does not disrupt operations.
F-98
The estimate is based on the best available information at this time. These estimates may change as new information becomes available. The following table contains detailed information about the charges incurred to date related to the fiscal 2003 restructuring plan (in millions), recorded in accordance with SFAS No. 146, "Accounting for Costs Associated With Exit or Disposal Activities":
Description
| | Estimated
Charges
| | Incurred
through
March 28,
2004
| | Estimated
Remaining
Charges
|
|---|
| Employee termination | | $ | 4.8 | | $ | 2.1 | | $ | 2.7 |
| Facility consolidation | | | 4.6 | | | 1.6 | | | 3.0 |
| Property, plant and equipment disposals | | | 2.2 | | | 0.4 | | | 1.8 |
| Other direct costs | | | 6.7 | | | 0.9 | | | 5.8 |
| | |
| |
| |
|
| Total | | $ | 18.3 | | $ | 5.0 | | $ | 13.3 |
| | |
| |
| |
|
We estimate that we will incur $4.9-$7.4 million and $7.1-$9.6 million of charges in fiscal 2004 and fiscal 2005, respectively, related to the fiscal 2003 restructuring plan.
Employee termination charges represent the cost of reducing our workforce in conjunction with facility consolidations and other downsizing activities. Facility consolidation charges represent the direct costs of moving property, plant and equipment to different facilities. Property plant and equipment disposals represents the write-off of redundant assets that will no longer be used in ongoing operations as a result of our facility consolidation initiative, net of disposal proceeds.
The following table contains information regarding our fiscal 2003 restructuring plan liability as of March 28, 2004 (in millions):
Description
| | Balance at
June 30, 2003
| | Additions
| | Payments
| | Balance at
March 28, 2004
|
|---|
| Employee termination | | $ | 0.5 | | $ | 1.2 | | $ | 1.2 | | $ | 0.5 |
| Facility consolidation | | | — | | | 1.1 | | | 1.1 | | | — |
| Property, plant and equipment disposals | | | — | | | 0.5 | | | 0.5 | | | — |
| Other direct costs | | | — | | | 0.3 | | | 0.3 | | | — |
| | |
| |
| |
| |
|
| Total | | $ | 0.5 | | $ | 3.1 | | $ | 3.1 | | $ | 0.5 |
| | |
| |
| |
| |
|
During the three and nine months ended March 28, 2004, the Company continued to finalize the fiscal 2001 restructuring plan. All activities associated with the fiscal 2001 restructuring plan have been
F-99
finalized. The following table contains information regarding our fiscal 2001 restructuring plan liability as of March 28, 2004 (in millions):
Description
| | Initial
Accrual
| | Reclassification
| | Additional
Accrual
| | Payments through
March 28, 2004
| | Balance at
March 28, 2004
|
|---|
| Impairment of goodwill and other intangibles | | $ | 3.6 | | $ | — | | $ | — | | $ | 3.6 | | $ | — |
| Impairment of property, plant and equipment | | | 1.9 | | | 2.3 | | | 0.1 | | | 4.3 | | | — |
| Employee termination benefits | | | 3.8 | | | (1.2 | ) | | 1.9 | | | 4.5 | | | — |
| Other direct costs | | | 2.2 | | | (1.1 | ) | | 0.7 | | | 1.8 | | | — |
| | |
| |
| |
| |
| |
|
| | | $ | 11.5 | | $ | — | | $ | 2.7 | | $ | 14.2 | | $ | — |
| | |
| |
| |
| |
| |
|
8. Income Taxes
The effective income tax rate for the three months and nine months ended March 28, 2004, differs from the statutory rate due to the utilization of net operating loss carryovers.
9. Subsequent Event
On April 27, 2004, the Company entered into an Agreement and Plan of Merger with Medical Device Manufacturing, Inc., a wholly owned subsidiary of UTI Corporation, ("Purchaser") and Pine Merger Corporation ("Merger Sub"), pursuant to which Merger Sub will merge with and into the Company with the Company being the surviving corporation and becoming a wholly owned subsidiary of Purchaser. The merger is conditioned upon, among other things, the approval of the merger by the Company's stockholders, any required antitrust clearance and the receipt by Purchaser of the proceeds contemplated by financing commitments.
F-100

Independent Auditors' Report
To the Board of Directors
Campbell Engineering, Inc.
Huntsville, Alabama
We have audited the accompanying balance sheets of Campbell Engineering, Inc. as of December 31, 2004 and 2003, and the related statements of income, changes in stockholders' equity and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Campbell Engineering, Inc. as of December 31, 2004 and 2003, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
CERTIFIED PUBLIC ACCOUNTANTS
August 31, 2005
(October 3, 2005 as to Note 12)
| |
|
|---|
| | | Beason & Nalley, Inc.
101 Monroe Street
Huntsville, Alabama
35801-4829 |
| | | (256) 533-1720
(800) 416-1946
(256) 534-8558 Fax |
| | | www.beasonnalley.com |
F-101
CAMPBELL ENGINEERING, INC.
Balance Sheets
As of December 31, 2004 and 2003
| | 2004
| | 2003
|
|---|
| Current Assets: | | | | | | |
| Cash | | $ | 395,022 | | $ | 90,556 |
| Marketable securities | | | 104,785 | | | — |
Accounts receivable, net of allowance for doubtful accounts of $2,846 and $0 at December 31, 2004 and 2003, respectively |
|
|
1,840,032 |
|
|
659,159 |
| Other receivables | | | 10,993 | | | 28,684 |
| Inventory | | | 1,051,477 | | | 635,166 |
| Prepaid expenses | | | 20,961 | | | 13,053 |
| | |
| |
|
| Total Current Assets | | | 3,423,270 | | | 1,426,618 |
| Note receivable related party | | | — | | | 211,447 |
| Property and equipment, net | | | 2,759,719 | | | 1,624,550 |
| | |
| |
|
| Total Assets | | $ | 6,182,989 | | $ | 3,262,615 |
| | |
| |
|
| Current Liabilities: | | | | | | |
| Accounts payable | | $ | 371,068 | | $ | 254,938 |
| Accrued expenses | | | 286,987 | | | 178,773 |
| Other current liabilities | | | 13,092 | | | 9,101 |
| Current portion of notes payable | | | 416,277 | | | 167,656 |
| Current portion of capital lease obligations | | | 44,783 | | | 33,364 |
| | |
| |
|
| Total Current Liabilities | | | 1,132,207 | | | 643,832 |
| Long-Term Liabilities: | | | | | | |
| Notes payable, net of current portion | | | 981,017 | | | 346,258 |
| Capital lease obligations, net of current portion | | | 83,086 | | | 102,384 |
| | |
| |
|
| Total Long-Term Liabilities | | | 1,064,103 | | | 448,642 |
| | |
| |
|
| Total Liabilities | | | 2,196,310 | | | 1,092,474 |
| | |
| |
|
| Stockholders' Equity | | | | | | |
| Common stock, $1 par value; 1,000 shares authorized, issued and outstanding | | | 1,000 | | | 1,000 |
| Paid-in capital | | | 10,000 | | | 10,000 |
| Retained earnings | | | 3,969,900 | | | 2,159,141 |
| Accumulated other comprehensive income | | | 5,779 | | | — |
| | |
| |
|
| Total Stockholders' Equity | | | 3,986,679 | | | 2,170,141 |
| | |
| |
|
Total Liabilities and Stockholders' Equity |
|
$ |
6,182,989 |
|
$ |
3,262,615 |
| | |
| |
|
The accompanying notes are an integral part of the financial statements.
F-102
CAMPBELL ENGINEERING, INC.
Statements of Income
For the years ended December 31, 2004 and 2003
| | 2004
| | 2003
|
|---|
| Sales | | $ | 10,899,271 | | $ | 5,403,960 |
| Cost of goods sold | | | 7,037,197 | | | 4,199,910 |
| | |
| |
|
| Gross Profit | | | 3,862,074 | | | 1,204,050 |
| General and administrative expenses | | | 481,719 | | | 410,348 |
| | |
| |
|
| Income From Operations | | | 3,380,355 | | | 793,702 |
| Other Expenses: | | | | | | |
| Interest expense, net | | | 40,795 | | | 35,104 |
| Other expense | | | 25,801 | | | 7,047 |
| | |
| |
|
| Total Other Expense | | | 66,596 | | | 42,151 |
| | |
| |
|
Net Income |
|
$ |
3,313,759 |
|
$ |
751,551 |
| | |
| |
|
The accompanying notes are an integral part of the financial statements.
F-103
CAMPBELL ENGINEERING, INC.
Statements of Changes in Stockholders' Equity
For the years ended December 31, 2004 and 2003
| | Common
Stock
Shares
| | Common
Stock
Amount
| | Paid-in
Capital
| | Retained
Earnings
| | Accumulated
Other
Comprehensive
Income
| | Total
| |
|---|
| Balance, January 1, 2003 | | 1,000 | | $ | 1,000 | | $ | 10,000 | | $ | 1,622,587 | | $ | — | | $ | 1,633,587 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | |
| Net income | | — | | | — | | | — | | | 751,551 | | | — | | | 751,551 | |
| Total comprehensive income | | — | | | — | | | — | | | — | | | — | | | 751,551 | |
| Distributions | | — | | | — | | | — | | | (214,997 | ) | | — | | | (214,997 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance, December 31, 2003 | | 1,000 | | | 1,000 | | | 10,000 | | | 2,159,141 | | | — | | | 2,170,141 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | |
| Net income | | — | | | — | | | — | | | 3,313,759 | | | — | | | 3,313,759 | |
| Unrealized gain on marketable securities | | — | | | — | | | — | | | — | | | 5,779 | | | 5,779 | |
| Total comprehensive income | | — | | | — | | | — | | | — | | | — | | | 3,319,538 | |
| Distributions | | — | | | — | | | — | | | (1,503,000 | ) | | — | | | (1,503,000 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance, December 31, 2004 | | 1,000 | | $ | 1,000 | | $ | 10,000 | | $ | 3,969,900 | | $ | 5,779 | | $ | 3,986,679 | |
| | |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of the financial statements.
F-104
CAMPBELL ENGINEERING, INC.
Statements of Cash Flows
For the years ended December 31, 2004 and 2003
| | 2004
| | 2003
| |
|---|
| Cash Flows From Operating Activities: | | | | | | | |
| Net Income | | $ | 3,313,759 | | $ | 751,551 | |
| Adjustments to reconcile net income to net cash provided (used) by operating activities: | | | | | | | |
| Depreciation expense | | | 383,589 | | | 251,005 | |
| (Gain) loss on disposal of property and equipment | | | (3,187 | ) | | 4,974 | |
| Provision for doubtful accounts | | | 2,846 | | | — | |
| Change in: | | | | | | | |
| Accounts receivable | | | (1,183,719 | ) | | 1,157,327 | |
| Other receivables | | | 17,691 | | | (15,637 | ) |
| Inventory | | | (416,311 | ) | | (635,166 | ) |
| Prepaid expenses | | | (7,908 | ) | | 4,887 | |
| Accounts payable | | | 116,130 | | | 90,168 | |
| Accrued expenses | | | 108,214 | | | 47,761 | |
| Other current liabilities | | | 3,991 | | | (7,371 | ) |
| | |
| |
| |
| Total adjustments | | | (978,664 | ) | | 897,948 | |
| | |
| |
| |
| Net Cash Provided by Operating Activities | | | 2,335,095 | | | 1,649,499 | |
| | |
| |
| |
| Cash Flows From Investing Activities: | | | | | | | |
| Purchase of property and equipment | | | (1,490,975 | ) | | (421,375 | ) |
| Proceeds from sale of property and equipment | | | 3,187 | | | — | |
| Purchase of marketable securities | | | (99,006 | ) | | — | |
| | |
| |
| |
| Net Cash Used by Investing Activities | | | (1,586,794 | ) | | (421,375 | ) |
| | |
| |
| |
| Cash Flows From Financing Activities: | | | | | | | |
| Net payments on line of credit | | | — | | | (877,121 | ) |
| Principal payments on notes payable | | | (595,200 | ) | | (340,350 | ) |
| Proceeds from issuance of notes payable | | | 1,478,580 | | | 303,388 | |
| Principal payments on capital lease obligations | | | (35,662 | ) | | (27,853 | ) |
| Payments received on notes receivable — related party | | | 211,447 | | | 2,672 | |
| Distributions to stockholders | | | (1,503,000 | ) | | (214,997 | ) |
| | |
| |
| |
| Net Cash Used by Financing Activities | | | (443,835 | ) | | (1,154,261 | ) |
| | |
| |
| |
| Net Increase in Cash | | | 304,466 | | | 73,863 | |
| Cash, beginning of year | | | 90,556 | | | 16,693 | |
| | |
| |
| |
| Cash, end of year | | $ | 395,022 | | $ | 90,556 | |
| | |
| |
| |
| Supplemental Information: | | | | | | | |
| Cash paid for: | | | | | | | |
| Interest | | $ | 50,404 | | $ | 43,561 | |
| Supplemental Schedule of Noncash Investing and Financing Activities: | | | | | | | |
| Property and equipment purchased under capital lease | | $ | 27,783 | | $ | 34,071 | |
Unrealized gain on marketable securities |
|
$ |
5,779 |
|
$ |
— |
|
The accompanying notes are an integral part of the financial statements.
F-105
CAMPBELL ENGINEERING, INC.
Notes to Financial Statements
As of December 31, 2004 and 2003
1. Summary of Significant Accounting Policies
Nature of Business—Campbell Engineering, Inc. (the Company) was incorporated in Alabama in 1979. The Company provides engineering, design, precision machining and assembly of aerospace hardware and commercial products. The Company maintains its offices in Huntsville, Alabama. The Company's customers are located throughout the continental United States.
Marketable Securities—Available-for-sale securities are stated at fair value, and unrealized holding gains and losses are reported as a separate component of stockholders' equity.
Accounts Receivable—Customers are typically commercial entities, the US. Government, various government agencies and subcontractors. Bad debts arising from contracts with commercial customers are estimated using the allowance method.
Inventory—Inventoried costs are stated at the lower of average cost or estimated net realizable value. Costs include actual production cost, allocable overhead, factory administrative costs, initial tooling and other related non-recurring costs incurred to-date with revenue recognized on units delivered. All inventory is classified as work in process.
Property and equipment—Property and equipment are carried at cost less accumulated depreciation. Depreciation is provided on a straight-line basis over the estimated useful life.
Expenditures for maintenance and repairs are charged to operations as incurred. Expenditures for renewals and betterments are capitalized and written off through depreciation and amortization charges. Property retired or sold is removed from the asset and related accumulated depreciation accounts, and any profit or loss resulting is reflected in the statement of income.
Leased equipment meeting certain criteria is capitalized and the present value of the related lease payments is recorded as a liability. Amortization of leased equipment is included in depreciation.
Revenue Recognition—Revenue in which the Company produces units of a basic product in a continuous or sequential production process to the buyers' specifications under valid purchase orders is recognized upon transfer of title of the product to the customer.
Amounts billed to customers for shipping and handling costs are included in sales in the statement of income with the associated costs included as a component of costs of goods sold.
Income Taxes—The Company has elected to be taxed under the provisions of Subchapter S of the Internal Revenue Code. Under those provisions, the Company does not pay corporate income taxes on its taxable income. Instead, the stockholders are liable for individual income taxes on their respective share of the Company's taxable income.
Concentration of Credit Risk—Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and accounts receivable. The Company maintains cash in accounts with high quality, federally insured financial institutions. At times, the balances in these accounts may be in excess of federally insured limits. The Company also extends credit to its customers, including commercial entities and governmental agencies.
Use of Estimates—Management uses estimates and assumptions in preparing financial statements in accordance with accounting principles generally accepted in the United States of America. Those estimates and assumptions affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities, and the reported revenues and expenses. Significant estimates used in preparing these financial statements include those assumed in computing the allowance for doubtful
F-106
accounts. It is at least reasonably possible that the significant estimates used will change within the next year.
Comprehensive Income—Statement of Financial Accounting Standards No. 130,Reporting Comprehensive Income, governs the financial statement presentation of changes in stockholders' equity resulting from non-owner sources. Accumulated other comprehensive income as reported in the accompanying balance sheet represents unrealized gains and losses on available for sale securities.
2. Marketable Securities
The Company held the following marketable securities as of December 31, 2004:
| | 2004
|
|---|
| | Amortized Cost
| | Gross Unrealized Gains
| | Fair Value
|
|---|
| Corporate equity securities | | $ | 99,006 | | $ | 5,779 | | $ | 104,785 |
| | |
| |
| |
|
| Total equity securities | | $ | 99,006 | | $ | 5,779 | | $ | 104,785 |
| | |
| |
| |
|
3. Accounts Receivable
Accounts receivable, net as of December 31, 2004 and 2003, are comprised of the following:
| | 2004
| | 2003
|
|---|
| Commercial Customers | | | | | | |
| Amounts billed | | $ | 1,745,918 | | $ | 588,666 |
| U.S. Government: | | | | | | |
| Amounts billed | | | 94,114 | | | 70,493 |
| | |
| |
|
| Total accounts receivable, net | | $ | 1,840,032 | | $ | 659,159 |
| | |
| |
|
4. Property and Equipment
A summary of property and equipment as of December 31, 2004 and 2003, is as follows:
| | 2004
| | 2003
|
|---|
| Land | | $ | 70,250 | | $ | 70,250 |
| Building and improvements | | | 634,491 | | | 634,491 |
| Machinery and equipment | | | 5,035,808 | | | 3,562,267 |
| Furniture and fixtures | | | 31,253 | | | 31,253 |
| Other capital assets | | | 68,277 | | | 48,277 |
| | |
| |
|
| | | | 5,840,079 | | | 4,346,538 |
| Less accumulated depreciation | | | 3,080,360 | | | 2,721,988 |
| | |
| |
|
Property and equipment, net |
|
$ |
2,759,719 |
|
$ |
1,624,550 |
| | |
| |
|
F-107
The capitalized cost of leased equipment as of December 31, 2004 and 2003 is $224,140 and $196,814, respectively. Amortization of capitalized lease equipment is computed on the straight-line basis over the shorter of the term of the lease or the estimated life of the equipment. Total accumulated amortization expense of capitalized lease equipment as of December 31, 2004 and 2003 is $79,382 and $49,336, respectively. Amortization expense of capital leases totaled $30,046 and $22,472 for the years ended December 31, 2004 and 2003, respectively.
5. Line of Credit
The Company has an open revolving line-of-credit at a local financial institution that permits the Company to borrow up to $1,500,000 and $1,000,000 at December 31, 2004 and 2003, respectively. Interest is payable monthly at 5.25% and 4.00% at December 31, 2004 and 2003, respectively. The variable interest rate is based on LIBOR. The line is scheduled to expire on May 15, 2006. The outstanding balance was zero as of December 31, 2004 and 2003. The financial institution has a security interest in all of the Company's business assets and a second lien on the Company's main facilities. Up to $1,000,000 of the line is personally guaranteed by the Company's president and secretary/treasurer. In 2003 the financial institution required that the Company comply with various financial and reporting covenants.
6. Notes Payable
Notes payable as of December 31, 2004 and 2003, consists of the following:
| | 2004
| | 2003
|
|---|
| Note payable to a local financial institution, requiring monthly payments of $5,339 including interest at a variable rate, which is based on the prime rate, of 4.00% at December 31, 2003. The note, maturing on November 18, 2007, has a security interest in a building and was paid in full during 2004. The note was guaranteed by the president and secretary/treasurer of the Company. | | $ | — | | $ | 115,836 |
| Note payable to a local financial institution, requiring monthly payments of $3,429 including interest at a variable rate, which is based on the prime rate, of 5.25% at December 31, 2004. The note, maturing on June 30, 2007, has a security interest in a piece of equipment. | | | 103,388 | | | — |
| Note payable to a local financial institution, requiring monthly payments of $3,803 including interest at a variable rate, which is based on the bank's base rate, of 4.00% at December 31, 2003, respectively. The note, maturing on May 25, 2006, has a security interest in accounts and contracts receivable and property and equipment and was paid in full during 2004. The note was guaranteed by the president and secretary/treasurer of the Company. | | | — | | | 92,988 |
| Note payable to a local financial institution, requiring monthly payments of $11,116 including interest at a variable rate, which is based on the prime rate, of 5.25% at December 31, 2004. The note, maturing on October 5, 2007, has a security interest in equipment. | | | 353,366 | | | — |
| | | | | | | |
F-108
| Note payable to a finance company, requiring monthly payments of $722 including interest at a fixed rate of 3.9% at December 31, 2003. The note, maturing on October 18, 2005, has a security interest in a vehicle and was paid in full during 2004. | | | — | | | 14,638 |
| Note payable to a local financial institution, requiring monthly payments of $14,115 including interest at a fixed rate of 5.98% at December 31, 2004. The note, maturing on December 20, 2008, has a security interest in a building and property and equipment. The note was guaranteed by the president and secretary/treasurer of the Company. | | | 600,000 | | | — |
| Note payable to a finance company, requiring monthly payments of $517 including interest at a fixed rate of 4.95% at December 31, 2003. The note, maturing on April 29, 2008, has a security interest in a vehicle and was paid in full during 2004. | | | — | | | 24,536 |
| Note payable to a local financial institution, requiring monthly payments of $3,005 including interest at a variable rate of 4.00% at December 31, 2003. The note, maturing on October 14, 2008, has a security interest in accounts and contracts receivable and property and equipment and was paid in full during 2004. The note was guaranteed by the president and secretary/treasurer of the Company. | | | — | | | 158,031 |
| Note payable to a local financial institution, requiring monthly payments of $2,084 including interest at a variable rate, which is based on the bank's base rate, of 4.00% at December 31, 2003. The note, maturing on September 5, 2008, has a security interest in accounts and contracts receivable and property and equipment and was paid in full during 2004. The note was guaranteed by the president and secretary/treasurer of the Company. | | | — | | | 107,885 |
| Note payable to a local financial institution, requiring monthly payments of $11,603 including interest at a variable rate, which is based on the prime rate, of 5.25% at December 31, 2004. The note, maturing on July 15, 2007, has a security interest in equipment. The note was guaranteed by the president and secretary/treasurer of the Company. | | | 340,540 | | | — |
| | |
| |
|
| Total notes payable | | | 1,397,294 | | | 513,914 |
| Less: current portion of notes payable | | | 416,277 | | | 167,656 |
| | |
| |
|
| Notes payable, net of current portion | | $ | 981,017 | | $ | 346,258 |
| | |
| |
|
F-109
Annual maturities of notes payable at December 31, 2004 are as follows:
| 2005 | | $ | 416,277 |
| 2006 | | | 439,177 |
| 2007 | | | 380,043 |
| 2008 | | | 161,797 |
| | |
|
|
|
$ |
1,397,294 |
| | |
|
At December 31, 2004 and 2003, the fair values of these borrowings approximated their carrying values based on the variable nature of the interest rates.
7. Capital Lease Obligations
The Company leases certain office equipment and manufacturing machinery under long-term lease agreements. Capital lease obligations as of December 31, 2004 and 2003, consists of the following
| | 2004
| | 2003
|
|---|
| Capital lease obligation, requiring monthly payments of $2,553, including interest at a rate of 6.9% per annum. | | $ | 76,603 | | $ | 107,801 |
| Capital lease obligation, requiring monthly payments of $627, including interest at a rate of 4.0% per annum. | | | 30,119 | | | 37,648 |
| Capital lease obligation, requiring monthly payments of $778, including interest at a rate of 0.1% per annum. | | | 25,672 | | | — |
| Capital lease obligation, requiring monthly payments of $61, including interest at a rate of 14.5% per annum. | | | — | | | 143 |
| Capital lease obligation, requiring monthly payments of $235, including interest at a rate of 13.0% per annum. | | | 4,944 | | | 7,792 |
| | |
| |
|
| Total capital lease obligations, including interest | | | 137,338 | | | 153,384 |
| Less: amount representing interest | | | 9,469 | | | 17,636 |
| | |
| |
|
| Total capital lease obligations | | | 127,869 | | | 135,748 |
| Less: current portion of capital lease obligations | | | 44,783 | | | 33,364 |
| | |
| |
|
| Capital lease obligations, net of current portion | | $ | 83,086 | | $ | 102,384 |
| | |
| |
|
F-110
Future minimum lease payments under the capital lease obligations at December 31, 2004 are as follows:
| 2005 | | $ | 50,331 |
| 2006 | | | 49,625 |
| 2007 | | | 29,852 |
| 2008 | | | 7,530 |
| | |
|
| Total minimum lease payments | | | 137,338 |
| Less: amount representing interest | | | 9,469 |
| | |
|
| Capital lease obligations | | $ | 127,869 |
| | |
|
8. Operating Leases
Total rent expense under operating lease agreements, including lease agreements with expiration dates of less than one year, for the year ended December 31, 2004 and 2003 was $109,584 and $121,110, respectively.
9. Profit Sharing Plan
The Company has established a 401(k) profit sharing plan, for all eligible employees. The plan provides for employee contributions ranging from 0% to 10% of their earnings. Employer contributions to the plan are made on a discretionary basis and employees become wholly vested after 5 years of service. The Company had a total of $25,806 and $2,155 related to contribution and plan expenses for the years ended December 31, 2004 and 2003, respectively.
10. Related Party Transactions
The note receivable account represents unsecured advances to Company stockholders and are payable on demand. Interest is calculated on these loans at the base rate charged by the Company's local financial institution. The base rate was 4.00% at December 31, 2003.
The Company leases an office building from a stockholder of the Company. Lease expense for the building was $96,000 and $84,000 for 2004 and 2003, respectively.
11. Concentration of Credit Risk and Major Customer
One customer accounted for 79% and 64% of total revenues in 2004 and 2003, respectively, and 89% and 89% of Accounts Receivable at December 31, 2004 and 2003, respectively. A second customer accounted for 19% and 33% of total revenues in 2004 and 2003, respectively, and 5% and 10% of Accounts Receivable at December 31, 2004 and 2003, respectively. No other customers accounted for greater than 10% of total revenues in either 2004 or 2003.
12. Subsequent Event
Effective September 12, 2005, the Company was sold to Accellent Corp.
F-111
Campbell Engineering, Inc.
Unaudited Balance Sheets
As of June 30, 2005 and December 31, 2004
| | June 30, 2005
| | December 31, 2004
|
|---|
| Current Assets: | | | | | | |
| Cash | | $ | 443,096 | | $ | 395,022 |
| Marketable securities | | | 106,343 | | | 104,785 |
Accounts receivable, net of allowance for doubtful accounts of $55,176 and $2,846 at June 30, 2005 and December 31, 2004, respectively |
|
|
1,916,011 |
|
|
1,840,032 |
| Other receivables | | | 12,441 | | | 10,993 |
| Inventory | | | 1,341,027 | | | 1,051,477 |
| Prepaid expenses | | | 8,000 | | | 20,961 |
| | |
| |
|
| Total Current Assets | | | 3,826,918 | | | 3,423,270 |
| Property and equipment, net | | | 3,868,332 | | | 2,759,719 |
| | |
| |
|
| Total Assets | | $ | 7,695,250 | | $ | 6,182,989 |
| | |
| |
|
| Current Liabilities: | | | | | | |
| Accounts payable | | $ | 270,797 | | $ | 371,068 |
| Accrued expenses | | | 299,352 | | | 286,987 |
| Other current liabilities | | | 11,047 | | | 13,092 |
| Current portion of notes payable | | | 673,074 | | | 416,277 |
| Current portion of capital lease obligations | | | 46,028 | | | 44,783 |
| | |
| |
|
| Total Current Liabilities | | | 1,300,298 | | | 1,132,207 |
| Long-Term Liabilities: | | | | | | |
| Notes payable, net of current portion | | | 1,442,588 | | | 981,017 |
| Capital lease obligations, net of current portion | | | 59,756 | | | 83,086 |
| | |
| |
|
| Total Long-Term Liabilities | | | 1,502,344 | | | 1,064,103 |
| | |
| |
|
| Total Liabilities | | | 2,802,642 | | | 2,196,310 |
| | |
| |
|
| Stockholders' Equity: | | | | | | |
| Common stock, $1 par value; 1,000 shares authorized, issued and outstanding | | | 1,000 | | | 1,000 |
| Paid-in capital | | | 10,000 | | | 10,000 |
| Retained earnings | | | 4,879,129 | | | 3,969,900 |
| Accumulated other comprehensive income | | | 2,479 | | | 5,779 |
| | |
| |
|
| Total Stockholders' Equity | | | 4,892,608 | | | 3,986,679 |
| | |
| |
|
Total Liabilities and Stockholders' Equity |
|
$ |
7,695,250 |
|
$ |
6,182,989 |
| | |
| |
|
The accompanying notes are an integral part of the financial statements.
F-112
Campbell Engineering, Inc.
Unaudited Statements of Income
For the six months ended June 30, 2005 and 2004
| | 2005
| | 2004
|
|---|
| Sales | | $ | 6,643,241 | | $ | 5,014,398 |
| Cost of goods sold | | | 4,713,493 | | | 3,054,030 |
| | |
| |
|
| Gross Profit | | | 1,929,748 | | | 1,960,368 |
| General and administrative expenses | | | 304,155 | | | 216,880 |
| | |
| |
|
| Income From Operations | | | 1,625,593 | | | 1,743,488 |
| Other Expenses: | | | | | | |
| Interest expense, net | | | 57,466 | | | 17,854 |
| Other expense | | | 12,798 | | | 6,314 |
| | |
| |
|
| Total Other Expense | | | 70,264 | | | 24,168 |
| | |
| |
|
| Net Income | | $ | 1,555,329 | | $ | 1,719,320 |
| | |
| |
|
The accompanying notes are an integral part of the financial statements.
F-113
Campbell Engineering, Inc.
Unaudited Statements of Changes in Stockholders' Equity
For the six months ended June 30, 2005
| | Common
Stock
Shares
| | Common
Stock
Amount
| | Paid-in
Capital
| | Retained
Earnings
| | Accumulated
Other
Comprehensive
Income
| | Total
| |
|---|
| Balance, January 1, 2005 | | 1,000 | | $ | 1,000 | | $ | 10,000 | | $ | 3,969,900 | | $ | 5,779 | | $ | 3,986,679 | |
Comprehensive income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income | | — | | | — | | | — | | | 1,555,329 | | | — | | | 1,555,329 | |
| Unrealized gain on marketable securities | | — | | | — | | | — | | | — | | | (3,300 | ) | | (3,300 | ) |
| | | | | | | | | | | | | | | | |
| |
| Total comprehensive income | | — | | | — | | | — | | | — | | | — | | | 1,552,029 | |
| Distributions | | — | | | — | | | — | | | (646,100 | ) | | — | | | (646,100 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance, June 30, 2005 | | 1,000 | | $ | 1,000 | | $ | 10,000 | | $ | 4,879,129 | | $ | 2,479 | | $ | 4,892,608 | |
| | |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of the financial statements.
F-114
Campbell Engineering, Inc.
Unaudited Statements of Cash Flows
For the six months ended June 30, 2005 and 2004
| | 2005
| | 2004
| |
|---|
| Cash Flows From Operating Activities: | | | | | | | |
| Net Income | | $ | 1,555,329 | | $ | 1,719,320 | |
Adjustments to reconcile net income to net cash provided (used) by operating activities: |
|
|
|
|
|
|
|
| Depreciation expense | | | 279,676 | | | 160,274 | |
| Realized gain on sale of investments | | | (4,209 | ) | | — | |
| Loss on disposal of property and equipment | | | 4,880 | | | — | |
| Provision for doubtful accounts | | | 52,330 | | | — | |
Change in: |
|
|
|
|
|
|
|
| Accounts receivable | | | (128,309 | ) | | (1,074,659 | ) |
| Other receivables | | | (1,448 | ) | | (400 | ) |
| Inventory | | | (289,550 | ) | | (29,684 | ) |
| Prepaid expenses | | | 12,961 | | | 792 | |
| Accounts payable | | | (100,271 | ) | | 418,796 | |
| Accrued expenses | | | 12,365 | �� | | 44,803 | |
| Other | | | (2,045 | ) | | 61,355 | |
| | |
| |
| |
| Total adjustments | | | (163,620 | ) | | (418,723 | ) |
| | |
| |
| |
| Net Cash Provided by Operating Activities | | | 1,391,709 | | | 1,300,597 | |
| | |
| |
| |
Cash Flows From Investing Activities: |
|
|
|
|
|
|
|
| Purchase of property and equipment | | | (1,405,169 | ) | | (909,854 | ) |
| Proceeds from sale of property and equipment | | | 12,000 | | | 2,187 | |
| Purchase of marketable securities | | | (56,208 | ) | | — | |
| Proceeds from sale of marketable securities | | | 55,559 | | | — | |
| | |
| |
| |
| Net Cash Used by Investing Activities | | | (1,393,818 | ) | | (907,667 | ) |
| | |
| |
| |
Cash Flows From Financing Activities: |
|
|
|
|
|
|
|
| Principal payments on notes payable | | | (492,712 | ) | | (189,931 | ) |
| Proceeds from issuance of notes payable | | | 1,211,080 | | | 432,960 | |
| Principal payments on capital lease obligations | | | (22,085 | ) | | (13,355 | ) |
| Distributions | | | (646,100 | ) | | (443,000 | ) |
| | |
| |
| |
| Net Cash Provided (Used) by Financing Activities | | | 50,183 | | | (213,326 | ) |
| | |
| |
| |
| Net Increase in Cash | | | 48,074 | | | 179,604 | |
| Cash, beginning of year | | | 395,022 | | | 90,556 | |
| | |
| |
| |
| Cash, at end of period | | $ | 443,096 | | $ | 270,160 | |
| | |
| |
| |
Supplemental Information: |
|
|
|
|
|
|
|
Cash paid for: |
|
|
|
|
|
|
|
| Interest | | $ | 61,005 | | $ | 17,855 | |
Supplemental Schedule of Noncash Investing and Financing Activities: |
|
|
|
|
|
|
|
| Unrealized Gain on marketable securities | | $ | 909 | | $ | — | |
The accompanying notes are an integral part of the financial statements.
F-115
Campbell Engineering, Inc.
Notes to Unaudited Financial Statements
As of June 30, 2005
1. Summary of Significant Accounting Policies
Nature of Business—Campbell Engineering, Inc. (the Company) was incorporated in Alabama in 1979. The Company provides engineering, design, precision machining and assembly of aerospace hardware and commercial products. The Company maintains its offices in Huntsville, Alabama. The Company's customers are located throughout the continental United States.
Marketable Securities—Available-for-sale securities are stated at fair value, and unrealized holding gains and losses are reported as a separate component of stockholders' equity.
Accounts Receivable—Customers are typically commercial entities, the U.S. Government, various government agencies and subcontractors. Bad debts arising from contracts with commercial customers are estimated using the allowance method.
Inventory—Inventoried costs are stated at the lower of average cost or estimated net realizable value. Costs include actual production cost, allocable overhead factory administrative costs, initial tooling and other related non-recurring costs incurred to-date with revenue recognized on units delivered. All inventory is classified as work in process.
Property and equipment—Property and equipment are carried at cost less accumulated depreciation. Depreciation is provided on a straight-line basis over the estimated useful life.
Expenditures for maintenance and repairs are charged to operations as incurred. Expenditures for renewals and betterments are capitalized and written off through depreciation and amortization charges. Property retired or sold is removed from the asset and related accumulated depreciation accounts, and any profit or loss resulting is reflected in the statement of income.
Leased equipment meeting certain criteria is capitalized and the present value of the related lease payments is recorded as a liability. Amortization of leased equipment is included in depreciation.
Revenue Recognition—Revenue in which the Company produces units of a basic product in a continuous or sequential production process to the buyers' specifications under valid purchase orders is recognized upon transfer of title of the product to the customer.
Amounts billed to customers for shipping and handling costs are included in sales in the statement of income with the associated costs included as a component of costs of goods sold.
Income Taxes—The Company has elected to be taxed under the provisions of Subchapter S of the Internal Revenue Code. Under those provisions, the Company does not pay corporate income taxes on its taxable income. Instead, the stockholders are liable for individual income taxes on their respective share of the Company's taxable income.
Concentration of Credit Risk—Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and accounts receivable. The Company maintains cash in accounts with high quality, federally insured financial institutions. At times, the balances in these accounts may be in excess of federally insured limits. The Company also extends credit to its customers, including commercial entities and governmental agencies.
Use of Estimates—Management uses estimates and assumptions in preparing financial statements in accordance with accounting principles generally accepted in the United States of America. Those estimates and assumptions affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities, and the reported revenues and expenses. Significant estimates used in preparing these financial statements include those assumed in computing the allowance for doubtful
F-116
accounts. It is at least reasonably possible that the significant estimates used will change within the next year.
Comprehensive Income—Statement of Financial Accounting Standards No. 130,Reporting Comprehensive Income, governs the financial statement presentation of changes in stockholders' equity resulting from non-owner sources. Accumulated other comprehensive income as reported in the accompanying balance sheet represents unrealized gains and losses on available for sale securities.
2. Marketable Securities
The Company held the following marketable securities as of June 30, 2005 and December 31, 2004:
| | June 30, 2005
|
|---|
| | Amortized Cost
| | Gross
Unrealized Gains
| | Fair Value
|
|---|
| Corporate equity securities | | $ | 103,864 | | $ | 2,479 | | $ | 106,343 |
| | |
| |
| |
|
| Total equity securities | | $ | 103,864 | | $ | 2,479 | | $ | 106,343 |
| | |
| |
| |
|
| | December 31, 2004
|
|---|
| | Amortized Cost
| | Gross
Unrealized Gains
| | Fair Value
|
|---|
| Corporate equity securities | | $ | 99,006 | | $ | 5,779 | | $ | 104,785 |
| | |
| |
| |
|
| Total equity securities | | $ | 99,006 | | $ | 5,779 | | $ | 104,785 |
| | |
| |
| |
|
3. Accounts Receivable
Accounts receivable as of June 30, 2005 and December 31, 2004, are comprised of the following:
| | June 30,
2005
| | December 31,
2004
|
|---|
| Commercial Customers: | | | | | | |
| Amounts billed | | $ | 1,833,917 | | $ | 1,745,918 |
| U.S. Government: | | | | | | |
| Amounts billed | | | 82,094 | | | 94,114 |
| | |
| |
|
| Total accounts receivable, net | | $ | 1,916,011 | | $ | 1,840,032 |
| | |
| |
|
F-117
4. Property and Equipment
A summary of property and equipment as of June 30, 2005 and December 31, 2004, is as follows:
| | June 30,
2005
| | December 31,
2004
|
|---|
| Land | | $ | 70,250 | | $ | 70,250 |
| Building and improvements | | | 634,491 | | | 634,491 |
| Machinery and equipment | | | 6,410,331 | | | 5,035,808 |
| Furniture and fixtures | | | 31,253 | | | 31,253 |
| Other capital assets | | | 68,277 | | | 68,277 |
| | |
| |
|
| | | | 7,214,602 | | | 5,840,079 |
| Less accumulated depreciation | | | 3,346,270 | | | 3,080,360 |
| | |
| |
|
| Property and equipment, net | | $ | 3,868,332 | | $ | 2,759,719 |
| | |
| |
|
The capitalized cost of leased equipment as of June 30, 2005 and December 31, 2004 is $224,140. Amortization of capitalized lease equipment is computed on the straight-line basis over the shorter of the term of the lease or the estimated life of the equipment. Total accumulated amortization expense of capitalized lease equipment as of June 30, 2005 and December 31, 2004 is $98,457 and $79,382, respectively. Amortization expense of capital leases totaled $19,075 and $22,472 for the six months ended June 30, 2005 and year ended December 31, 2004, respectively.
5. Line of Credit
The Company has an open revolving line-of-credit at a local financial institution that permits the Company to borrow up to $1,500,000 at June 30, 2005 and December 31, 2004. Interest is payable monthly at 6.25% and 5.25% at June 30, 2005 and December 31, 2004, respectively. The variable interest rate is based on LIBOR. The line is scheduled to expire on May 15, 2006. The outstanding balance was zero as of June 30, 2005 and December 31, 2004. The financial institution has a security interest in all of the Company's business assets and a second lien on the Company's main facilities. Up to $1,000,000 of the line is personally guaranteed by the Company's president and secretary/treasurer.
6. Notes Payable
Notes payable as of June 30, 2005 and December 31, 2004, consists of the following:
| | June 30,
2005
| | December 31,
2004
|
|---|
| Note payable to a local financial institution, requiring monthly payments of $9,430 including interest at a variable rate, which is based on the prime rate, of 6.46% at June 30, 2005. The note, maturing on April 15, 2008, has a security interest in equipment. The note was guaranteed by the president and secretary/treasurer of the Company. | | $ | 285,847 | | $ | — |
| | | | | | | |
F-118
| Note payable to a local financial institution, requiring monthly payments of $3,429 including interest at a variable rate, which is based on the prime rate, of 5.25% at June 30, 2005. The note, maturing on June 30, 2007, has a security interest in a piece of equipment and was paid in full during 2005. | | | — | | | 103,388 |
| Note payable to a local financial institution, requiring monthly payments of $5,940 including interest at a fixed rate of 6.46% at June 30, 2005. The note, maturing on February 22, 2009, has a security interest in equipment. The note was guaranteed by the president and secretary/treasurer of the Company. | | | 231,559 | | | — |
| Note payable to a local financial institution, requiring monthly payments of $11,116 including interest at a variable rate, which is based on the prime rate, of 6.25% and 5.25% at June 30, 2005 and December 31, 2004. The note, maturing on October 5, 2007, has a security interest in equipment. | | | 285,097 | | | 353,366 |
| Note payable to a local financial institution, requiring monthly payments of $6,155 including interest at a fixed rate of 6.8% at June 30, 2005. The note, maturing on April 18, 2009, has a security interest in equipment. | | | 248,015 | | | — |
| Note payable to a local financial institution, requiring monthly payments of $14,115 including interest at a fixed rate of 5.98% at June 30, 2005 and December 31, 2004. The note, maturing on December 20, 2008, has a security interest in a building and property and equipment. The note was guaranteed by the president and secretary/treasurer of the Company. | | | 520,886 | | | 600,000 |
| Note payable to a local financial institution, requiring monthly payments of $9,335 including interest at a fixed rate of 6.24% at June 30, 2005. The note, maturing on January 20, 2009, has a security interest in equipment. | | | 366,006 | | | — |
| Note payable to a local financial institution, requiring monthly payments of $11,603 including interest at a variable rate, which is based on the prime rate, of 5.25% at June 30, 2005 and December 31, 2004. The note, maturing on July 15, 2007, has a security interest in equipment. The note was guaranteed by the president and secretary/treasurer of the Company. | | | 178,252 | | | 340,540 |
| | |
| |
|
| Total notes payable | | | 2,115,662 | | | 1,397,294 |
| Less: current portion of notes payable | | | 673,074 | | | 416,277 |
| | |
| |
|
| Notes payable, net of current portion | | $ | 1,442,588 | | $ | 981,017 |
| | |
| |
|
F-119
Annual maturities of notes payable at June 30, 2005 are as follows:
| 2006 | | $ | 673,074 |
| 2007 | | | 698,488 |
| 2008 | | | 507,845 |
| 2009 | | | 236,255 |
| | |
|
| | | $ | 2,115,662 |
| | |
|
At June 30, 2005 and December 31, 2004, the fair values of these borrowings approximated their carrying values based on the variable nature of the interest rates.
7. Capital Lease Obligations
The Company leases certain office equipment and manufacturing machinery under long-term lease agreements. Capital lease obligations as of June 30, 2005 and December 31, 2004, consists of the following:
| | June 30,
2005
| | December 31,
2004
|
|---|
| Capital lease obligation, requiring monthly payments of $2,553, including interest at a rate of 6.9% per annum. | | $ | 61,282 | | $ | 76,603 |
| Capital lease obligation, requiring monthly payments of $627, including interest at a rate of 4.0% per annum. | | | 26,354 | | | 30,119 |
| Capital lease obligation, requiring monthly payments of $778, including interest at a rate of 0.1% per annum. | | | 21,004 | | | 25,672 |
| Capital lease obligation, requiring monthly payments of $235, including interest at a rate of 13.0% per annum. | | | 3,532 | | | 4,944 |
| | |
| |
|
| Total capital lease obligations, including interest | | | 112,172 | | | 137,338 |
| Less: amount representing interest | | | 6,388 | | | 9,469 |
| | |
| |
|
| Total capital lease obligations | | | 105,784 | | | 127,869 |
| Less: current portion of capital lease obligations | | | 46,028 | | | 44,783 |
| | |
| |
|
Capital lease obligations, net of current portion |
|
$ |
59,756 |
|
$ |
83,086 |
| | |
| |
|
F-120
Future minimum lease payments under the capital lease obligations at June 30, 2005 are as follows:
| 2006 | | $ | 50,331 |
| 2007 | | | 48,212 |
| 2008 | | | 9,863 |
| 2009 | | | 3,766 |
| | |
|
| Total minimum lease payments | | | 112,172 |
| Less: amount representing interest | | | 6,388 |
| | |
|
| Capital lease obligations | | $ | 105,784 |
| | |
|
8. Operating Leases
Total rent expense under operating lease agreements, including lease agreements with expiration dates of less than one year, for the six months ended June 30, 2005 and 2004 was $48,717 and $62,877, respectively.
9. Profit Sharing Plan
The Company has established a 401(k) profit sharing plan, for all eligible employees. The plan provides for employee contributions ranging from 0% to 10% of their earnings. Employer contributions to the plan are made on a discretionary basis and employees become fully vested after 5 years of service. The Company had a total of $19,584 and $5,155 related to contribution and plan expenses for the six months ended June 30, 2005 and 2004, respectively.
10. Related Party Transactions
The Company leases an office building from a stockholder of the Company. Lease expense for the building was $48,000 for the six months ended June 30, 2005 and 2004.
11. Concentration of Credit Risk and Major Customer
One customer accounted for 78% and 75% of total revenues for the six months ended June 30, 2005 and 2004, respectively, and 80% and 89% of Accounts Receivable at June 30, 2005 and December 31, 2004, respectively. A second customer accounted for 12% and 20% of total revenues for the six months ended June 30, 2005 and 2004, respectively, and 4% and 5% of Accounts Receivable at June 30, 2005 and December 31, 2004, respectively. No other customers accounted for greater than 10% of total revenues in either the six month period ended June 30, 2005 and 2004.
12. Subsequent Event
Effective September 12, 2005, the Company was sold to Accellent Corp.
F-121

Independent Auditors' Report
To the Members
Machining Technology Group, LLC
Arlington, Tennessee
We have audited the accompanying balance sheets of Machining Technology Group, LLC as of December 31, 2004 and 2003, and the related statements of operations, members' capital, and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to in the first paragraph present fairly, in all material respects, the financial position of Machining Technology Group, LLC as of December 31, 2004 and 2003, and the results of its operations and its cash flows for the years then ended in conformity with generally accepted accounting principles in the United States of America.
Lenahan, Smith & Bargiachi, P.C.
Memphis, Tennessee
September 22, 2005
F-122
MACHINING TECHNOLOGY GROUP, LLC
Balance Sheets
As of December 31, 2004 and 2003
| | December 31,
|
|---|
| | 2004
| | 2003
|
|---|
| Assets | | | | | | |
Current Assets |
|
|
|
|
|
|
| Cash and cash equivalents | | $ | 819,013 | | $ | 2,212 |
| Accounts receivable—trade | | | 529,945 | | | 709,274 |
| Accounts receivable—fixed asset sale | | | 30,780 | | | — |
| Inventories | | | 475,133 | | | 516,190 |
| Prepaid expenses | | | 140,869 | | | 73,673 |
| Prepaid state taxes | | | 983 | | | — |
| | |
| |
|
| | | | 1,996,723 | | | 1,301,349 |
| Property and Equipment, net | | | 4,393,964 | | | 3,044,108 |
| Other Assets | | | | | | |
| Deferred tax asset | | | 73,271 | | | — |
| | |
| |
|
| Total Assets | | $ | 6,463,958 | | $ | 4,345,457 |
| | |
| |
|
Liabilities and Members' Capital |
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
| Current portion of long-term debt | | $ | 329,789 | | $ | 175,969 |
| Customer deposits | | | 102,843 | | | 45,949 |
| Accounts payable | | | 264,405 | | | 377,876 |
| Accrued vacation & payroll | | | 90,912 | | | 17,741 |
| Accrued profit sharing expense | | | 84,794 | | | 120,000 |
| Accrued state taxes | | | — | | | 6,275 |
| | |
| |
|
| | | | 872,743 | | | 743,810 |
| Long-Term Debt, less current maturities | | | 1,241,491 | | | 627,857 |
| Deferred Income Taxes | | | 80,213 | | | 15,996 |
| Members' Capital | | | 4,269,511 | | | 2,957,794 |
| | |
| |
|
| Total Liabilities and Members' Capital | | $ | 6,463,958 | | $ | 4,345,457 |
| | |
| |
|
See notes to financial statements and accountants' report.
F-123
MACHINING TECHNOLOGY GROUP, LLC
Statements of Income
For the years ended December 31, 2004 and 2003
| | For Years Ended
December 31,
| |
|---|
| | 2004
| | 2003
| |
|---|
| Product Sales, net | | $ | 11,321,689 | | $ | 8,444,178 | |
| Cost of Sales | | | 5,923,825 | | | 4,053,733 | |
| | |
| |
| |
| Gross Profit | | | 5,397,864 | | | 4,390,445 | |
| General and Administrative Expenses | | | 850,729 | | | 636,741 | |
| | |
| |
| |
| Income from operations | | | 4,547,135 | | | 3,753,704 | |
| Other Income (Expense) | | | | | | | |
| | Interest expense | | | (41,060 | ) | | (29,357 | ) |
| | Interest income | | | 2,022 | | | — | |
| | Gain on sale of equipment | | | 26,742 | | | — | |
| | |
| |
| |
| Total other expense | | | (12,296 | ) | | (29,357 | ) |
| | |
| |
| |
| Income before income taxes | | | 4,534,839 | | | 3,724,347 | |
| Income tax benefit (expense) | | | | | | | |
| | State tax benefit (expense) | | | 12,556 | | | (5,321 | ) |
| | Deferred state tax benefit (expense) | | | 9,054 | | | (15,996 | ) |
| | |
| |
| |
| Total income tax benefit (expense) | | | 21,610 | | | (21,317 | ) |
| | |
| |
| |
| Net Income | | $ | 4,556,449 | | $ | 3,703,030 | |
| | |
| |
| |
See notes to financial statements and accountants' report.
F-124
MACHINING TECHNOLOGY GROUP, LLC
Statements of Changes in Members' Equity
For the years ended December 31, 2004 and 2003
| | For Years Ended
December 31,
| |
|---|
| | 2004
| | 2003
| |
|---|
| Members' Capital—Beginning of Year | | $ | 2,957,794 | | $ | 1,679,622 | |
| Net Income for the Year | | | 4,556,449 | | | 3,703,030 | |
| Capital Account Distributions | | | (3,244,732 | ) | | (2,424,858 | ) |
| | |
| |
| |
| Members' Capital—End of Year | | $ | 4,269,511 | | $ | 2,957,794 | |
| | |
| |
| |
See notes to financial statements and accountants' report.
F-125
MACHINING TECHNOLOGY GROUP, LLC
Statements of Cash Flows
For the years ended December 31, 2004 and 2003
| | For Years Ended
December 31,
| |
|---|
| | 2004
| | 2003
| |
|---|
| Cash flows from operating activities: | | | | | | | |
| Net Income | | $ | 4,556,449 | | $ | 3,703,030 | |
| Adjustments to reconcile net income to net cash flows from operating activities: | | | | | | | |
| Depreciation and amortization of equipment | | | 441,518 | | | 297,570 | |
| (Increase) decrease in accounts receivable | | | 179,331 | | | (436,710 | ) |
| (Increase) decrease in inventory | | | 41,057 | | | (337,928 | ) |
| (Increase) decrease in prepaid expenses | | | (67,196 | ) | | (73,673 | ) |
| Increase (decrease) in accounts payable | | | (113,471 | ) | | 265,856 | |
| Increase (decrease) in other payables | | | 56,894 | | | (142,963 | ) |
| Increase (decrease) in accrued vacation | | | 73,171 | | | 17,741 | |
| Increase (decrease) in accrued profit sharing contribution | | | (35,206 | ) | | 60,000 | |
| Increase (decrease) in accrued taxes | | | (7,258 | ) | | 1,396 | |
| Increase (decrease) in deferred tax liability | | | 64,217 | | | 15,996 | |
| Decrease in deferred tax asset | | | (73,271 | ) | | — | |
| Increase in gain on sale of equipment | | | (26,742 | ) | | — | |
| | |
| |
| |
Net cash provided by operating activities |
|
|
5,089,493 |
|
|
3,370,315 |
|
| | |
| |
| |
Cash flows from investing activities: |
|
|
|
|
|
|
|
Capital expenditures |
|
|
(1,870,832 |
) |
|
(1,470,798 |
) |
| Sale of equipment | | | 75,420 | | | — | |
| | |
| |
| |
Net cash used in investing activities |
|
|
(1,795,412 |
) |
|
(1,470,798 |
) |
| | |
| |
| |
Cash flows from financing activities: |
|
|
|
|
|
|
|
| Proceeds from long term-debt | | | 1,010,228 | | | 683,717 | |
| Principal payments on long-term debt | | | (242,776 | ) | | (147,937 | ) |
| Distributions of member capital | | | (3,244,732 | ) | | (2,424,858 | ) |
| | |
| |
| |
Net cash used in financing activities |
|
|
(2,477,280 |
) |
|
(1,889,078 |
) |
| | |
| |
| |
Net Increase in Cash |
|
|
816,801 |
|
|
10,439 |
|
Cash at beginning of year |
|
|
2,212 |
|
|
(8,227 |
) |
| | |
| |
| |
| Cash at end of year | | $ | 819,013 | | $ | 2,212 | |
| | |
| |
| |
See notes to financial statements and accountants' report.
F-126
MACHINING TECHNOLOGY GROUP, LLC
Notes to Financial Statements
For the years ended December 31, 2004 and 2003
1. Summary of significant accounting policies
The Company, located in Arlington, Tennessee, was established in 1998. The Company manufactures surgical tools and implants used in the medical industry which are sold to customers located in the United States.
This limited liability company shall dissolve and its affairs wound up in accordance with the Act and the Company Agreement on December 31, 2025, unless the term shall be extended by Amendment to the Company Agreement and the Articles of Organization.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Actual results could differ from those estimates.
The Company records revenue in compliance with SAB 104, which requires that the following criteria are met: (a) persuasive evidence of an arrangement exists, (b) delivery has occurred or services have been rendered, (c) the price from the buyer is fixed or determinable, and (d) collectibility is probable. The Company records revenue based on written arrangements or purchase orders with customers, and upon transfer of title of the product or rendering of the service.
Amounts billed for shipping and handling fees are classified as sales in the income statement. Costs incurred for shipping and handling are classified as cost of sales.
Cash and cash equivalents consist primarily of cash in bank.
Inventories are stated at lower of cost or market (on first in, first out basis) and include the cost of materials, labor, and manufacturing overhead.
Property, Plant, and Equipment
Property, plant, and equipment are carried at cost. Expenditures for maintenance and repairs are charged to expense as incurred. Expenditures which significantly increase value or extend useful lives are capitalized and replaced properties are retired. Depreciation is calculated principally by the use of straight-line method over the estimated useful lives of depreciable assets. Accelerated methods are used for tax purposes. Amortization of leasehold improvements is calculated by use of the straight-line method over the estimated useful life. Plant equipment and machinery are depreciated over an estimated useful life of 5 to 10 years. Leasehold improvements are depreciated over an estimated useful life of 5 to 40 years, computers and software over an estimated useful life of 3 to 5 years,
F-127
vehicles over an estimated useful life of 3 years, and office furniture and fixtures over an estimated useful life of 7 years. Depreciation expense for the years ended December 31, 2004 and 2003 was $441,518 and $297,570, respectively. Federal tax depreciation for the years ended December 31, 2004 and 2003 was $1,527,101 and $970,853.
The accompanying financial statements do not include a provision or liability for federal income taxes because the Company is taxed as a partnership and the members are taxed individually on their share of company earnings. Net state income tax benefit for 2004 was $12,556, and net state tax expense for 2003 was $5,321. Net deferred state tax benefit was $9,054 for 2004, and net deferred state tax expense for 2003 was $15,996.
Deferred tax liabilities were the result of a book and state tax depreciation difference and a change in certain accrued expenses. Deferred tax assets were the result of state tax credits. The availability of these credits upon transfer of company ownership is not known.
Tennessee Industrial Machinery Tax Credits and Jobs Tax Credits resulted in a reduction of state taxes in the amount of $29,934 for 2004 and $27,000 for 2003. Amounts reported on the financial statements are net of these credits. Below is a schedule of tax credits available for carryover:
| | Jobs Tax Credit
| | Industrial
Machinery Credit
| |
|---|
| Beginning balance | | $ | 60,017 | | $ | — | |
| 2004 credit increases | | | 26,000 | | | 17,188 | |
| 2004 benefit | | | (15,284 | ) | | (14,650 | ) |
| | |
| |
| |
| Carryover to 2005 | | $ | 70,733 | | $ | 2,538 | |
| | |
| |
| |
Of the $70,733 of Jobs Tax Credits, $44,733 will expire in December 2017 and $26,000 will expire in December 2019. The Industrial Machinery Tax Credit will expire in December 2019.
The Company has accrued a liability for compensated absences. Employees of the Company are entitled to paid vacation and paid sick days depending on length of service. The Company's policy is to recognize the cost of compensated absences at the end of each period.
Based on historical collections, the Company does not believe an allowance for doubtful accounts is necessary.
The fair value of the Company's financial instruments, comprised of cash, accounts receivable, accounts and notes payable, approximates recorded value.
F-128
2. Related party transactions
The Company leases its facilities from a related company, GT Management, LLC. There is no lease agreement but rents are paid monthly. Rent expense paid to GT Management, LLC for the years ended December 31, 2004 and 2003 is $170,000 and $165,483, respectively.
3. Profit sharing plan
The Company has an established salary deferral plan (401k) and profit sharing plan. For 2004, the company matched employee 401(k) withholding in the amount of $35,206. The Company also accrued a profit sharing expense of $84,794. Total expenses for the 401(k) match and profit sharing expense were $120,000 for 2004. The profit sharing plan contribution is made at the discretion of management. For 2003 there was no employer match available under the salary deferral plan but the Company did contribute to the profit sharing plan. Profit sharing plan expense for 2003 was $120,000.
4. Operating Leases
The Company leases some of its machinery and equipment for various terms under long-term, non-cancelable operating lease agreements. The leases expire at various dates through 2006 and provide for renewal and purchase options at the fair market renewal value on a month to month basis.
Lease expense totaled $123,066 for the year ended December 31, 2004 and $86,244 for the year ended December 31, 2003. The following is a schedule by year of future minimum rental payments required under the operating lease agreements:
Year Ending December 31,
| | Amount
|
|---|
| 2005 | | $ | 103,829 |
| 2006 | | | 59,945 |
| 2007 | | | 16,351 |
| Thereafter | | | 18,262 |
| | |
|
| | | $ | 198,387 |
| | |
|
5. Capital Leases
The Company leases certain machinery and equipment under agreements that are classified as capital leases. The cost of equipment under capital leases is included in the Balance Sheet as property, plant, and equipment and was $178,500 at December 31, 2004. Accumulated amortization of the leased equipment at December 31, 2004 was $98,787 and $85,116 at December 31, 2003. Amortization of assets under capital leases is included in depreciation expense.
The final capital lease was paid in full in April 2004, therefore there are no minimum lease payment obligations.
6. Major customers and concentrations of credit risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of accounts receivable. A significant portion of the Company's customer base is comprised of companies within the medical industry. The Company does not require collateral from its customers. For the year ended December 31, 2004, sales to one major customer amounted to approximately 82% of net sales. For the year ended December 31, 2003, sales to one major customer amounted to
F-129
approximately 94% of net sales. Sales to this customer were comprised of different products shipped to different locations. The loss of this customer would most likely have a negative impact on the Company's results of operations.
Five major customers accounted for 36%, 22%, 15%, 11% and 10% of accounts receivable net of customer deposits for the year ended December 31, 2004. For the year ended December 31, 2003, one major customer accounted for 98% of accounts receivable net of customer deposits.
The Company maintains its cash in bank in excess of the federally insured limits at times throughout the year.
7. Inventories
Inventories consisted of the following:
| | December 31
|
|---|
| | 2004
| | 2003
|
|---|
| Raw materials | | $ | 67,615 | | $ | 103,196 |
| Work in process | | | 350,466 | | | 399,016 |
| Finished goods | | | 57,052 | | | 13,978 |
| | |
| |
|
| Total inventory | | $ | 475,133 | | $ | 516,190 |
| | |
| |
|
8. Short-term and long-term debt obligations
Long-term debt at December 31, 2004 and 2003 consisted of the following:
| | 2004
| | 2003
| |
|---|
| Note payable to vendor in monthly installments of $1,104, non-interest bearing, due in November 2004, secured by vehicle | | $ | — | | $ | 11,041 | |
| Note payable to bank in monthly installments of $916, including interest at 6.5%, due April 2004, secured by machinery | | | — | | | 4,505 | |
| Note payable to bank in monthly installments of $4,704, including interest at 6%, due in December 2006, secured by machinery | | | 106,044 | | | 154,430 | |
| Note payable to bank in monthly installments of $11,120, including interest at 5.95%, due in January 2010, secured by machinery | | | 573,228 | | | — | |
| Note payable to bank in monthly installments of $7,421, including interest at 5.6%, due in October 2009, secured by machinery | | | 370,195 | | | — | |
| Note payable to bank in monthly installments of $774, non-interest bearing, due in May 2008, secured by vehicle | | | 31,718 | | | 41,002 | |
| Note payable to bank in monthly installments of $3,815, including interest at 4.5%, due in August 2007, secured by machinery | | | 298,197 | | | 329,782 | |
| Note payable to bank in monthly installments of $6,786, including interest at 4.45%, due in June 2007, secured by machinery | | | 191,898 | | | 263,066 | |
| | |
| |
| |
| Total debt | | | 1,571,280 | | | 803,826 | |
| Less: current portion | | | (329,789 | ) | | (175,969 | ) |
| | |
| |
| |
| Long-term debt, less current portion | | $ | 1,241,491 | | $ | 627,857 | |
| | |
| |
| |
F-130
Future maturities of long term debt are as follows as of December 31, 2004:
| 2005 | | $ | 329,789 |
| 2006 | | | 352,316 |
| 2007 | | | 471,939 |
| 2008 | | | 207,672 |
| 2009 | | | 200,174 |
| Thereafter | | | 9,390 |
| | |
|
| | | $ | 1,571,280 |
| | |
|
9. Line of credit
The Company has a demand bank line of credit totaling $400,000, including letters of credit, under which the Company may borrow on an unsecured basis at the bank's prime rate. There were no amounts outstanding under this line of credit at December 31, 2004 and 2003.
10. Supplementary Disclosures of Statement of Cash Flows
Cash paid during the year for:
| | December 31,
|
|---|
| | 2004
| | 2003
|
|---|
| Income taxes—net of refunds | | $ | 18,916 | | $ | 12,879 |
| Interest | | $ | 41,060 | | $ | 29,357 |
F-131
MACHINING TECHNOLOGY GROUP, LLC
Unaudited Balance Sheets
As of September 30, 2005 and December 31, 2004
| | September 30, 2005
| | December 31, 2004
|
|---|
| | (as restated)
| |
|
|---|
| Assets | | | | | | |
| Current Assets | | | | | | |
| Cash and cash equivalents | | $ | 427,079 | | $ | 819,013 |
| Accounts receivable—trade | | | 1,967,956 | | | 529,945 |
| Accounts receivable—other | | | 2,260 | | | 30,780 |
| Inventories | | | 752,402 | | | 475,133 |
| Prepaid expenses | | | 21,622 | | | 140,869 |
| Prepaid state taxes | | | 1,369 | | | 983 |
| | |
| |
|
| | | | 3,172,688 | | | 1,996,723 |
| Property and Equipment, net | | | 6,922,049 | | | 4,393,964 |
| Other Assets | | | | | | |
| Deferred tax asset | | | 85,464 | | | 73,271 |
| | |
| |
|
| Total Assets | | $ | 10,180,201 | | $ | 6,463,958 |
| | |
| |
|
Liabilities and Members' Capital |
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
| Current portion of long-term debt | | $ | 517,304 | | $ | 329,789 |
| Customer deposits | | | 399,999 | | | 102,843 |
| Accounts payable | | | 429,739 | | | 264,405 |
| Accrued vacation & payroll | | | 218,085 | | | 90,912 |
| Accrued profit sharing expense | | | — | | | 84,794 |
| Accrued expenses | | | 47,490 | | | — |
| Accrued state taxes | | | 4,773 | | | — |
| | |
| |
|
| | | | 1,617,390 | | | 872,743 |
| Long-Term Debt, less current maturities | | | 1,260,246 | | | 1,241,491 |
| Deferred Income Taxes | | | 103,196 | | | 80,213 |
| Minority Interest | | | 1,481,938 | | | — |
| Members' Capital | | | 5,717,431 | | | 4,269,511 |
| | |
| |
|
| Total Liabilities and Members' Capital | | $ | 10,180,201 | | $ | 6,463,958 |
| | |
| |
|
See notes to financial statements.
F-132
MACHINING TECHNOLOGY GROUP, LLC
Unaudited Statements of Income
For the nine months ended September 30, 2005 and 2004
| | For the nine months ended September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| | (as restated)
| |
| |
|---|
| Product Sales net, | | $ | 11,362,439 | | $ | 8,942,526 | |
| Cost of Sales | | | 5,668,430 | | | 4,311,551 | |
| | |
| |
| |
| Gross Profit | | | 5,694,009 | | | 4,630,975 | |
| General and Administrative Expenses | | | 857,325 | | | 585,999 | |
| | |
| |
| |
| Income from operations | | | 4,836,684 | | | 4,044,976 | |
| Other Income (expense) | | | | | | | |
| | Minority interest | | | (58,982 | ) | | — | |
| | Interest expense | | | (79,165 | ) | | (26,467 | ) |
| | Interest income | | | 750 | | | 676 | |
| | Miscellaneous income | | | 2,152 | | | — | |
| | |
| |
| |
| Total other income/(expense) | | | (135,245 | ) | | (25,791 | ) |
| | |
| |
| |
| Income before income taxes | | | 4,701,439 | | | 4,019,185 | |
| Income tax benefit (expense) | | | | | | | |
| | State tax benefit (expense) | | | — | | | 6,098 | |
| | Deferred state tax benefit (expense) | | | (10,790 | ) | | 5,008 | |
| | |
| |
| |
| Total income tax benefit (expense) | | | (10,790 | ) | | 11,106 | |
| | |
| |
| |
| Net Income | | $ | 4,690,649 | | $ | 4,030,291 | |
| | |
| |
| |
See notes to financial statements.
F-133
MACHINING TECHNOLOGY GROUP, LLC
Unaudited Statements of Changes in Members' Equity
For the nine months ended September 30, 2005 and 2004
| | For nine months ended September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| | (as restated)
| |
| |
|---|
| Members' Capital—Beginning of Year | | $ | 4,269,511 | | $ | 2,957,794 | |
| Capital Account Adjustment | | | (15,667 | ) | | — | |
| Net Income for the Year | | | 4,690,649 | | | 4,030,291 | |
| Capital Account Distributions | | | (3,227,062 | ) | | (2,449,779 | ) |
| | |
| |
| |
| Members' Capital—End of Year | | $ | 5,717,431 | | $ | 4,538,306 | |
| | |
| |
| |
See notes to financial statements.
F-134
MACHINING TECHNOLOGY GROUP, LLC
Unaudited Statement of Cash Flows
| | For Nine Months Ended
September 30,
| |
|---|
| | 2005
| | 2004
| |
|---|
| | (as restated)
| |
| |
|---|
| Cash flows from operating activities: | | | | | | | |
| | Net Income | | $ | 4,690,649 | | $ | 4,030,291 | |
| | | Adjustments to reconcile net income to net cash flows from operating activities: | | | | | | | |
| | | | Depreciation and amortization of equipment | | | 573,019 | | | 315,357 | |
| | | | (Increase) in accounts receivable | | | (1,440,271 | ) | | (99,672 | ) |
| | | | (Increase) in inventory | | | (277,269 | ) | | (58,796 | ) |
| | | | Decrease in prepaid expenses | | | 118,861 | | | 52,842 | |
| | | | Increase (decrease) in accounts payable | | | 165,444 | | | (170,588 | ) |
| | | | Increase in other payables | | | 344,037 | | | 59,298 | |
| | | | Increase in accrued vacation & payroll | | | 127,173 | | | 108,086 | |
| | | | (Decrease) in accrued profit sharing contribution | | | (84,794 | ) | | (120,000 | ) |
| | | | Increase in accrued taxes | | | 4,773 | | | (3,033 | ) |
| | | | Increase (decrease) in deferred tax liability | | | 22,983 | | | 50,911 | |
| | | | (Increase) decrease in deferred tax asset | | | (12,193 | ) | | (55,919 | ) |
| | | | Minority interest | | | 58,982 | | | — | |
| | |
| |
| |
| Net cash provided by operating activities | | | 4,291,394 | | | 4,108,777 | |
| | |
| |
| |
| Cash flows from investing activities: | | | | | | | |
| | Capital expenditures | | | (2,359,720 | ) | | (523,181 | ) |
| | Sale of equipment | | | 30,780 | | | — | |
| | |
| |
| |
| Net cash used in investing activities | | | (2,328,940 | ) | | (523,181 | ) |
| | |
| |
| |
| Cash flows from financing activities: | | | | | | | |
| | Advances from line of credit | | | 700,000 | | | 50,000 | |
| | Proceeds from long-term debt | | | 297,860 | | | — | |
| | Principal payments on long-term debt | | | (1,064,304 | ) | | (185,026 | ) |
| | Distributions of member capital | | | (3,227,063 | ) | | (2,449,780 | ) |
| | Contributions of member capital | | | 927,063 | | | — | |
| | |
| |
| |
| Net cash used in financing activities | | | (2,366,444 | ) | | (2,584,806 | ) |
| | |
| |
| |
| Net Increase (Decrease) in Cash | | | (403,990 | ) | | 1,000,790 | |
| Cash at beginning of period | | | 819,013 | | | 2,212 | |
| Cash at beginning of period—Minority Interest | | | 12,056 | | | — | |
| | |
| |
| |
| Cash at end of period | | $ | 427,079 | | $ | 1,003,002 | |
| | |
| |
| |
See notes to financial statements.
F-135
MACHINING TECHNOLOGY GROUP, LLC
Notes to Unaudited Financial Statements
As of September 30, 2005
1. Summary of significant accounting policies
The financial statement of Machining Technology Group, LLC and GT Management, LLC are combined for September 30, 2005. All significant intercompany accounts and transactions have been eliminated in the combination.
Financial Accounting Standards Board Interpretation No 46 (FIN 46) (Revised December 2003), Consolidation of Variable Interest Entities, requires that if an enterprise is the primary beneficiary of a variable interest entity, the assets, liabilities, and results of operations of the variable interest entity should be included in the consolidated financial statements of the enterprise. The Company leases its building and land from GT Management, LLC. The Company believes it is the primary beneficiary and that the lessor is a variable interest entity. Under FIN 46, the lessor is required to be consolidated in the Company's balance sheet as of January 1, 2005. The building and land will be recorded as an asset and the related debt will be recorded as a liability in the Company's balance sheet. The impact of the Company's future statement of operations will be increased depreciation and interest expense, which will be partially offset by decreased rent expense. The members' capital of GT Management, LLC is reflected on the Company's balance sheet as a minority interest. The cost of the buildings and associated land at January 1, 2005 was $678,556 and the related debt was $160,755. As of the balance sheet date of September 30, 2005, the cost of the buildings, land, and expansions was $1,652,226 and related debt was $124,682. Accumulated depreciation was $57,037 and $33,965 at September 30, 2005 and December 31, 2004 respectively.
The Company, located in Arlington, Tennessee, was established in 1998. The Company manufactures surgical tools and implants used in the medical industry which are sold to customers located in the United States.
This limited liability company shall dissolve and its affairs wound up in accordance with the Act and the Company Agreement on December 31, 2025, unless the term shall be extended by Amendment to the Company Agreement and the Articles of Organization.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Actual results could differ from those estimates.
The Company records revenue in compliance with SAB 104, which requires that the following criteria are met: (a) persuasive evidence of an arrangement exists, (b) delivery has occurred or services have been rendered, (c) the price from the buyer is fixed or determinable, and (d) collectibility is probable. The Company records revenue based on written arrangements or purchase orders with customers, and upon transfer of title of the product or rendering of the service.
F-136
Amounts billed for shipping and handling fees are classified as sales in the income statement. Costs incurred for shipping and handling are classified as cost of sales.
Cash and cash equivalents consist primarily of cash in bank.
Inventories are stated at lower of cost or market (on first in, first out basis) and include the cost of materials, finished goods, and work in progress . Interim statements are valued at an average hourly rate of work-in-process hours based on historical rates for September 30, 2005.
Property, Plant, and Equipment
Property, plant, and equipment are carried at cost. Expenditures for maintenance and repairs are charged to expense as incurred. Expenditures which significantly increase value or extend useful lives are capitalized and replaced properties are retired. Depreciation is calculated principally by the use of the straight-line method over the estimated useful lives of depreciable assets. Accelerated methods are used for tax purposes. Amortization of leasehold improvements is calculated by use of the straight-line method over the estimated useful life. Plant equipment and machinery are depreciated over an estimated useful life of 5 to 10 years. Leasehold improvements are depreciated over an estimated useful life of 5 to 40 years, computers and software over an estimated useful life of 3 to 5 years, vehicles over an estimated useful life of 3 years, and office furniture and fixtures over an estimated useful life of 7 years.
The accompanying financial statements do not include a provision or liability for federal income taxes because the Company is taxed as a partnership and the members are taxed individually on their share of company earnings.
Deferred tax liabilities were the result of a book and state tax depreciation difference and a change in certain accrued expenses. Deferred tax assets were the result of state tax credits. The availability of these credits upon transfer of company ownership is not known.
Tennessee Industrial Machinery Tax Credits and Jobs Tax Credits resulted in a reduction of state taxes in the amount of $29,934 for 2004 and $5,431 for the nine months ended September 30, 2005. Amounts reported on the financial statements are net of these credits. Below is a schedule of tax credits available for carryover.
F-137
Due to the current tax laws for limited liability companies in Tennessee there is some doubt as to whether the Company will incur the deferred excise tax liability of $103,196 recorded on the balance sheet. If not for the pending sale of the company, this liability would not be recorded.
| | Jobs Tax Credit
| | Industrial Machinery Credit
| |
|---|
| Beginning balance | | $ | 60,017 | | $ | — | |
| 2004 credit increases | | | 26,000 | | | 17,188 | |
| 2004 benefit | | | (15,284 | ) | | (14,650 | ) |
| | |
| |
| |
| Carryover to 2005 | | $ | 70,733 | | $ | 2,538 | |
| 1/1/05 thru 9/30/05 credit increases | | | — | | | 8,860 | |
| Current period benefit | | | (5,431 | ) | | — | |
| | |
| |
| |
| | | $ | 65,302 | | $ | 11,398 | |
| | |
| |
| |
Of the $65,302 of Jobs Tax Credits, $39,302 will expire in December 2017 and $26,000 will expire in December 2019. Regarding the Industrial Machinery Tax Credit, $2,538 will expire in December 2019 and the balance of $8,860 will expire in December 2020.
The Company has accrued a liability for compensated absences. Employees of the Company are entitled to paid vacation and paid sick days depending on length of service. The Company's policy is to recognize the cost of compensated absences at the end of each period.
Based on historical collections, the Company does not believe an allowance for doubtful accounts is necessary.
The fair value of the Company's financial instruments, comprised of cash, accounts receivable, accounts and notes payable, approximates recorded value.
The Company has restated the unaudited financial statements for the nine months ended September 30, 2005 to correct an error in the method used to value the September 30, 2005 inventories. The effect of this change is a $349,934 increase in inventory, net income, and members'
F-138
capital and a $349,934 decrease in cost of goods sold. The impact on each respective line of the unaudited financial statements for the nine months ended September 30, 2005 is noted below:
| | As Reported
| | Adjustment
| | As Restated
| |
|---|
| Balance Sheet: | | | | | | | | | | |
| Inventory | | $ | 402,468 | | $ | 349,934 | | $ | 752,402 | |
| Total Current Assets | | | 2,822,754 | | | 349,934 | | | 3,172,688 | |
| Total Assets | | | 9,830,267 | | | 349,934 | | | 10,180,201 | |
| Members' Capital | | | 5,367,497 | | | 349,934 | | | 5,717,431 | |
| Total Liabilities and Members' Capital | | | 9,830,267 | | | 349,934 | | | 10,180,201 | |
Statement of Income: |
|
|
|
|
|
|
|
|
|
|
| Cost of Sales | | | 6,018,364 | | | (349,934 | ) | | 5,668,430 | |
| Gross Profit | | | 5,344,075 | | | 349,934 | | | 5,694,009 | |
| Income from operations | | | 4,486,750 | | | 349,934 | | | 4,836,684 | |
| Income before income taxes | | | 4,351,505 | | | 349,934 | | | 4,701,439 | |
| Net Income | | | 4,340,715 | | | 349,934 | | | 4,690,649 | |
Statement of Changes in Members' Capital: |
|
|
|
|
|
|
|
|
|
|
| Net Income for the Year | | | 4,340,715 | | | 349,934 | | | 4,690,649 | |
| Members' Capital—End of Year | | | 5,367,497 | | | 349,934 | | | 5,717,431 | |
Statement of Cash Flow: |
|
|
|
|
|
|
|
|
|
|
| Net Income | | | 4,340,715 | | | 349,934 | | | 4,690,649 | |
| (Increase) in inventory | | | 72,665 | | | (349,934 | ) | | (277,269 | ) |
2. Related party transactions
The Company leases its facilities from a related company, GT Management, LLC. There is no lease agreement but rents are paid monthly.
3. Profit sharing plan
The Company has an established salary deferral plan (401k) and profit sharing plan. The profit sharing plan contribution is made at the discretion of management.
4. Capital Leases
The Company leases certain machinery and equipment under agreements that are classified as capital leases. The cost of equipment under capital leases is included in the Balance Sheet as property, plant, and equipment and was $178,500 at December 31, 2004 and $501,000 at September 30, 2005. Accumulated amortization of the leased equipment at December 31, 2004 was $98,787 and $386,758 at September 30, 2005.
F-139
The future minimum lease payments required under the capital leases as of September 30, 2005, are as follows:
For the twelve months ending September 30,
| | Amount
|
|---|
| 2006 | | $ | 65,265 |
| 2007 | | | — |
| Thereafter | | | — |
| | |
|
| | | $ | 65,265 |
| | |
|
The two remaining capital leases will be paid in full in August 2006, therefore the total minimum lease payments are all current obligations and any reduction to reduce net minimum lease payments to present value would not be material.
5. Major customers and concentrations of credit risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of accounts receivable. A significant portion of the Company's customer base is comprised of companies within the medical industry. The Company does not require collateral from its customers. Five major customers accounted for 36%, 22%, 15%, 11% and 10% of accounts receivable net of customer deposits for the year ended December 31, 2004. Two major customers accounted for 76% and 11% of accounts receivable, net of customer deposits, for the period ended September 30, 2005. The loss of any one of these customers would most likely have a negative short-term impact on the Company's results of operations.
The Company maintains its cash in bank in excess of the federally insured limits at times throughout the year.
6. Inventories
Inventories of the Company consisted of the following at:
| | December 30, 2004
| | September 30, 2005
|
|---|
| Raw materials | | $ | 67,615 | | $ | 103,593 |
| Work in process | | | 350,466 | | | 167,873 |
| Finished goods | | | 57,052 | | | 131,002 |
| | |
| |
|
| Total inventory | | $ | 475,133 | | $ | 402,468 |
| | |
| |
|
F-140
7. Short-term and long-term debt obligations
Long-term debt at December 31, 2004 and September 30, 2005 consisted of the following:
| | December 31, 2004
| | September 30, 2005
| |
|---|
| Note payable to bank in monthly installments of $4,704, including interest at 6%, due in December 2006, secured by machinery | | $ | 106,044 | | $ | 67,829 | |
| Note payable to bank in monthly installments of $11,120, including interest at 5.95%, due in January 2010, secured by machinery | | | 573,228 | | | 507,577 | |
| Note payable to bank in monthly installments of $7,421, including interest at 5.6%, due in October 2009, secured by machinery | | | 370,195 | | | 323,869 | |
| Note payable to bank in monthly installments of $774, non-interest bearing, due in May 2008, secured by vehicle | | | 31,718 | | | — | |
| Note payable to bank in monthly installments of $3,815, including interest at 4.5%, due in August 2007, secured by machinery | | | 298,197 | | | 273,916 | |
| Note payable to bank in monthly installments of $6,786, including interest at 4.45%, due in June 2007, secured by machinery | | | 191,898 | | | 136,409 | |
| Note payable to bank in monthly installments of $4,692, including interest at 7.4%, due in March 2008, secured by real estate | | | — | | | 124,682 | |
| Note payable to bank in monthly installments of$4,964, non interest bearing, due in May 2010, secured by machinery | | | — | | | 278,003 | |
| Note payable to bank in monthly installments of$3,340, including interest at 12.78%, due in August 2006, secured by machinery | | | — | | | 37,433 | |
| Note payable to bank in monthly installments of$3,128, including interest at 2.79%, due in May 2006, secured by machinery | | | — | | | 27,832 | |
| | |
| |
| |
| Total debt | | | 1,571,280 | | | 1,777,550 | |
| Less: current portion | | | (329,789 | ) | | (517,304 | ) |
| | |
| |
| |
| Long-term debt, less current portion | | $ | 1,241,491 | | $ | 1,260,246 | |
| | |
| |
| |
Future maturities of long term debt are as follows as of September 30, 2005:
| 2006 | | $ | 517,304 |
| 2007 | | | 610,281 |
| 2008 | | | 288,564 |
| 2009 | | | 272,428 |
| 2010 | | | 88,973 |
| Thereafter | | | — |
| | |
|
| | | $ | 1,777,550 |
| | |
|
8. Line of credit
The Company has a demand bank line of credit totaling $400,000 at December 31, 2004 and $750,000 at September 30, 2005, including letters of credit, under which the Company may borrow on an unsecured basis at the bank's prime rate. There were no amounts outstanding under this line of credit at December 31, 2004 and September 30, 2005.
9. Subsequent Events
Effective October 6, 2005 the Company and the variable interest entity were sold to Accellent Corp.
F-141
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
The following unaudited pro forma condensed combined financial statements contain unaudited historical financial data for the twelve month period ended December 31, 2004 and the nine month periods ended September 30, 2004 and 2005 that are derived from our, MedSource Technologies, Inc.'s ("MedSource"), Campbell Engineering, Inc.'s ("Campbell") and Machining Technology Group, LLC's ("MTG") audited and unaudited consolidated financial statements. We consummated the MedSource acquisition on June 30, 2004 and, as a result, the assets and liabilities of MedSource are recorded on our balance sheet as of the date of the MedSource acquisition and the results of operations of MedSource for June 30, 2004 are included in our results for that day and each day thereafter. We consummated the acquisition of Campbell's assets on September 12, 2005, and the MTG acquisition on October 6, 2005. As a result, the assets and liabilities of MTG are not recorded on our balance sheet at September 30, 2005. The results of operations of Campbell are and MTG will be reflected in our results for the day of the acquisition and each day thereafter. The unaudited pro forma condensed combined statements of operations for the twelve month period ended December 31, 2004 and the nine month periods ended September 30, 2004 and 2005 give effect to the MedSource, Campbell asset and MTG acquisitions as if they had occurred on January 1, 2004. The unaudited pro forma condensed consolidated balance sheet at September 30, 2005 gives effect to the MTG acquisition as if it had occurred on September 30, 2005. The unaudited pro forma condensed combined financial statements are intended for informational purposes only and do not purport to present our actual financial position or the results of operations that actually would have occurred or that may be obtained in the future if the transactions described had occurred as presented. In addition, future results may vary significantly from the results reflected in such statements due to certain factors beyond our control.
The MedSource Transactions, which occurred on June 30, 2004, include:
- •
- the acquisition of MedSource for $205.4 million in cash (net of $14.3 million of cash acquired);
- •
- the payment in cash of MedSource's indebtedness and accrued interest of $37.0 million;
- •
- the payment in cash of Accellent Corp.'s old senior secured credit facility, Accellent Corp.'s old senior subordinated indebtedness, our senior indebtedness, and accrued interest of $154.1 million;
- •
- the borrowings under Accellent Corp.'s old senior secured credit facility of $194.0 million;
- •
- the offering of the 10% senior subordinated notes due 2012, which generated $175.0 million in cash; and
- •
- the payment of fees in connection with Accellent Corp.'s old senior secured credit facility and senior subordinated notes which will be amortized to interest expense over the life of each respective instrument.
The 2005 Acquisitions include:
- •
- the acquisition of Campbell for approximately $18.2 million in cash;
- •
- the acquisition of MTG for approximately $50.2 million, paid in cash of $34.0 million and shares of our Class A Convertible Preferred Stock of $16.2 million;
- •
- additional borrowings of $42.0 million under the old credit facility, which include additional terms loans of $12.5 million and revolving credit facility borrowings of $29.5 million; and
- •
- the repayment of all existing indebtedness of MTG concurrent with the closing of our acquisition of MTG.
P-1
The Transactions, which occurred on November 22, 2005, include the following:
- •
- the acquisition of a majority of our capital stock by affiliates of KKR and entities affiliated with Bain for approximately $611 million;
- •
- the roll over of stock options and stock owned by existing management valued at approximately $30 million;
- •
- entering into a senior secured credit facility, consisting of a $400 million senior secured term loan facility and a $75 million senior secured revolving credit facility;
- •
- issuance of $305 million aggregate principal amount of senior subordinated notes, resulting in net proceeds of approximately $301 million after approximately $4 million of original issue discount;
- •
- repayment of approximately $409 million of our indebtedness, including pursuant to a tender offer for Accellent Corp.'s $175 million 10% senior subordinated notes due 2012; and
- •
- payment of approximately $73 million of transaction fees and expenses, including tender premiums.
The unaudited pro forma as adjusted combined statements of operations have been adjusted to give effect to the Transactions as if these events occurred as of January 1, 2004. The unaudited pro forma as adjusted combined balance sheet has been adjusted to give effect to the Transactions as if these events occurred as of September 30, 2005. The unaudited pro forma as adjusted combined financial data are for informational purposes only and do not purport to present what our results of operations and financial condition would have been had the Transactions actually occurred on these earlier dates, nor do they project our results of operations for any future period or our financial condition at any future date.
Our acquisitions of Campbell and MTG are considered business combinations in accordance with the guidance of SFAS 141. As part of the Transactions, all of our capital stock was acquired by affiliates of KKR, entities affiliated with Bain and existing management. The acquisition of our capital stock was structured as a reverse acquisition whereby a newly formed entity, Accellent Merger Sub Inc., was merged into us as part of the Transactions. Reverse acquisitions are outside the scope of EITF 88-16 as the legal structure does not result in a surviving new entity. Therefore, SFAS 141 will apply to the acquisition of us as part of the Transactions resulting in a 100% step up of our assets to fair value. In accordance with SFAS 141, we allocate the cost of each acquisition to the fair value of the assets and liabilities acquired. Excess of the purchase price over the identifiable assets and liabilities was recorded as goodwill. Costs incurred to issue debt in connection with the Transactions have been recorded as deferred financing costs and will be expensed to interest expense over the term of the debt. Stock options rolled over by management will be valued at fair value in accordance with the guidance of paragraph 17 of FASB Interpretation No. 44.
The valuation of Campbell and MTG is based upon available information and certain assumptions that we believe are reasonable. The total purchase price for Campbell and MTG was allocated to our net assets based on preliminary estimates of fair value. We used different methods to value the different categories of intangible assets. Customer base intangible assets were valued based on an excess earnings method which isolates the excess return attributable to this category of intangible asset. Proprietary or developed technology intangible assets were valued based on a royalty savings approach to estimate what an unrelated party would pay as a percentage of revenue for the use of the technology. Trade name and trademark intangible assets also used a royalty savings approach to estimate what an unrelated party would pay as a percentage of revenue for use of the trade name or trademark. Preliminary appraisals have been performed to assist management in the valuation of all identifiable intangible assets of Campbell and MTG as well as the valuation of Campbell's fixed assets.
P-2
In addition, a preliminary appraisal of MTG's fixed assets is in process. Although we are not aware of any material adjustments to the preliminary allocation, the final purchase price allocation of Campbell and MTG may include adjustments to the amounts shown here.
The unaudited pro forma financial data account for the acquisition of us as part of the Transactions using the purchase method of accounting which requires that we adjust all of our assets and liabilities to their fair values as of the date of the Merger. Our preliminary valuation included in the unaudited pro forma financial data is based on our experience with prior acquisitions and the guidance provided by SFAS 141, paragraph A14. Although we are not aware of any material adjustments to the preliminary allocation, the final allocation relating to the Transactions may include adjustments to the amounts shown here.
We expect to finalize the valuations of Campbell and MTG, as well as the valuation of us in connection with the sale of a majority of our capital stock as part of the Transactions when we issue our audited financial statements for the year ended December 31, 2005.
You should read the following in conjunction with the consolidated financial statements of Accellent, MedSource, Campbell and MTG and the related notes thereto included elsewhere in this prospectus. In addition, future results may vary significantly from the results reflected in such statements due to certain factors beyond our control. See "Risk Factors."
P-3
ACCELLENT INC.
Unaudited Pro Forma Condensed Combined Balance Sheet
As of September 30, 2005
(In thousands)
| | Accellent
| | MTG
| | Pro Forma
Acquisition
Adjustments
| | Pro Forma
Accellent
Including
Acquisitions
| | Pro Forma
Transactions
Adjustments
| | Pro Forma
As Adjusted
Accellent
|
|---|
| Assets: | | | | | | | | | | | | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents | | $ | 7,388 | | $ | 427 | | $ | (1,777 | )(a) | $ | 6,038 | | $ | (6,038 | )(b) | $ | — |
| Receivables, net | | | 56,561 | | | 1,968 | | | — | | | 58,529 | | | — | | | 58,529 |
| Inventories | | | 61,393 | | | 753 | | | 329 | (a) | | 62,475 | | | 16,691 | (c) | | 79,166 |
| Prepaid expenses and other | | | 2,706 | | | 25 | | | — | | | 2,731 | | | — | | | 2,731 |
| | |
| |
| |
| |
| |
| |
|
| Total current assets | | | 128,048 | | | 3,173 | | | (1,448 | ) | | 129,773 | | | 10,653 | | | 140,426 |
| Property and equipment, net | | | 96,102 | | | 6,922 | | | — | | | 103,024 | | | — | | | 103,024 |
| Goodwill | | | 284,879 | | | — | | | 30,082 | (a) | | 314,961 | | | 568,532 | (c) | | 883,493 |
| Intangibles, net | | | 83,654 | | | — | | | 13,940 | (a) | | 97,594 | | | 181,906 | (c) | | 279,500 |
| Deferred financing costs and other assets | | | 15,985 | | | 85 | | | — | | | 16,070 | | | 5,337 | (d) | | 21,407 |
| | |
| |
| |
| |
| |
| |
|
| Total assets | | $ | 608,668 | | $ | 10,180 | | $ | 42,574 | | $ | 661,422 | | $ | 766,428 | | $ | 1,427,850 |
| | |
| |
| |
| |
| |
| |
|
Liabilities and Stockholders' Equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current portion of long term debt | | $ | 1,961 | | $ | 517 | | $ | (517 | )(a) | $ | 1,961 | | $ | 1,039 | (b) | $ | 3,000 |
| Accounts payable | | | 20,218 | | | 430 | | | — | | | 20,648 | | | — | | | 20,648 |
| Accrued payroll and benefits | | | 12,143 | | | 218 | | | — | | | 12,361 | | | — | | | 12,361 |
| Accrued interest | | | 3,741 | | | — | | | — | | | 3,741 | | | — | | | 3,741 |
| Accrued expenses, other | | | 19,677 | | | 452 | | | 1,000 | (a) | | 21,129 | | | — | | | 21,129 |
| | |
| |
| |
| |
| |
| |
|
| Total current liabilities | | | 57,740 | | | 1,617 | | | 483 | | | 59,840 | | | 1,039 | | | 60,879 |
| Revolving credit facility | | | — | | | — | | | — | | | — | | | — | (b) | | — |
| Term Loan B | | | — | | | — | | | — | | | — | | | 397,000 | (b) | | 397,000 |
| Senior subordinated notes | | | — | | | — | | | — | | | — | | | 300,948 | (b) | | 300,948 |
| Senior Subordinated Bridge Facility | | | — | | | — | | | — | | | — | | | — | (b) | | — |
| Long term debt—existing | | | 372,663 | | | 1,260 | | | 32,740 | (a) | | 406,663 | | | (406,663 | )(b) | | — |
| Other long term liabilities | | | 25,255 | | | 103 | | | — | | | 25,358 | | | 5,402 | (c) | | 30,760 |
| | |
| |
| |
| |
| |
| |
|
| Total liabilities | | | 455,658 | | | 2,980 | | | 33,223 | | | 491,861 | | | 297,726 | | | 789,587 |
| | |
| |
| |
| |
| |
| |
|
| Minority interest | | | — | | | 1,482 | | | (1,482 | )(a) | | — | | | — | | | — |
| | |
| |
| |
| |
| |
| |
|
| Total Stockholders' Equity | | | 153,010 | | | 5,718 | | | 10,833 | (a) | | 169,561 | | | 468,702 | (e) | | 638,263 |
| | |
| |
| |
| |
| |
| |
|
| Total liabilities and Stockholders' Equity | | $ | 608,668 | | $ | 10,180 | | $ | 42,574 | | $ | 661,422 | | $ | 766,428 | | $ | 1,427,850 |
| | |
| |
| |
| |
| |
| |
|
See accompanying Notes to Unaudited Pro Forma Balance Sheet
P-4
Accellent Inc.
Notes to the Unaudited Pro Forma Condensed Combined Balance Sheet
- (a)
- Reflects the following based on the fair value of the assets acquired and liabilities assumed for the MTG acquisition. The total purchase price for the MTG acquisition was allocated to our net assets based on preliminary estimates of fair value. The preliminary valuation of the customer relationship intangibles was based upon information gathered by us during the due diligence process, and discounted future cash flows from the customer relationships utilizing an excess earnings method. A preliminary appraisal was utilized to validate cash flow assumptions. Although we are not aware of any material adjustments to the preliminary allocation, the final purchase price allocation could differ from these preliminary amounts.
| Purchase price: | | | | |
| Borrowed under Senior Credit Facility | | $ | 34,000 | |
| Class A-9 5% Convertible Preferred Stock | | | 16,200 | |
| | |
| |
| Total Purchase price | | $ | 50,200 | |
| | |
| |
| Inventory adjustment to fair value less cost to sell | | $ | 329 | |
| Customer relationship intangibles | | | 13,940 | |
| Goodwill | | | 30,082 | |
| Accrued transaction costs | | | (1,000 | ) |
| Minority interest | | | 1,482 | |
| Historical MTG equity | | | 5,367 | |
| | |
| |
| | | $ | 50,200 | |
| | |
| |
Concurrent with the acquisition of MTG, the Company repaid all outstanding indebtedness of MTG of $1,831, resulting in a net adjustment to debt totaling $32,169, of which $517 is included as a reduction to the current portion of long-term and a pro-forma reduction to cash of $1,777.
- (b)
- Reflects the estimated sources and uses of cash for the Transactions as follows (dollars in thousands):
| Sources | | | | |
| Revolving credit facility(1) | | $ | — | |
| Term Loan B(2) | | | 400,000 | |
| Senior subordinated notes, net of discount | | | 300,948 | |
| Senior Subordinated Bridge Facility | | | — | |
| Equity contribution(3) | | | 611,000 | |
| | |
| |
| Total sources | | $ | 1,311,948 | |
| | |
| |
| Uses | | | | |
| Purchase price to existing stockholders(4) | | $ | 836,563 | |
| Repayment of existing debt | | | 408,624 | |
| Estimated transaction costs(5) | | | 42,838 | |
| Estimated tender fees(5) | | | 29,961 | |
| | |
| |
| Total uses | | | 1,317,986 | |
| | |
| |
| Pro forma net adjustment to cash(4) | | $ | (6,038 | ) |
| | |
| |
- (1)
- The revolving credit facility provides for borrowing up to $75 million.
- (2)
- Upon the closing of the Transactions, we entered into a $400 million senior secured term loan facility.
P-5
- (3)
- Represents the amount invested in equity securities of Accellent Inc. by investments funds associated with or designated by the Sponsors. Excludes approximately $29.7 million of equity rolled over by certain members of management. See "Summary—Ownership and Corporate Structure" and "The Transactions".
- (4)
- The amount paid to purchase outstanding equity was reduced by accrued interest on our existing indebtedness at the time of closing.
- (5)
- Consists of $20.0 million of estimated financing fees, that will be capitalized and amortized over the related terms of the financings; $2.4 million of bridge loan fees that was expensed prior to or upon consummation of the Merger and reflected as an adjustment to historical equity, $20.4 million of direct acquisition costs, and $30.0 million in estimated fees in connection with the tender of the 10% senior subordinated notes due 2012 to be recognized as a loss upon the extinguishment of the notes.
- (c)
- The following table sets forth the calculation and adjustments made related to the preliminary allocation of purchase price with respect to the Transactions (dollars in thousands):
| Purchase price(1) | | | | $ | 866,263 | |
| Transaction fess and expenses directly related to the Transactions(2) | | | | | 20,425 | |
| | | | |
| |
| Total | | | | | 886,688 | |
| Net assets acquired before adjustments | | 169,561 | | | | |
| Existing goodwill | | (314,961 | ) | | | |
| Existing deferred financing costs | | (14,638 | ) | | | |
| Existing customer relationship and proprietary technology intangible assets | | (97,594 | ) | | | |
| Estimated tender premium | | (29,962 | ) | | | |
| | |
| | | | |
| Net assets acquired before adjustments | | | | | (287,594 | ) |
| | | | |
| |
| Estimated purchase price in excess of net assets acquired | | | | | 1,174,282 | |
| Adjustments to net assets acquired: | | | | | | |
| Inventory(3) | | 16,691 | | | | |
| Identifiable intangible assets(3) | | 279,500 | | | | |
| Fixed assets(3) | | — | | | | |
| | |
| | | | |
| Subtotal | | 296,191 | | | | |
| Deferred income taxes(4) | | (5,402 | ) | | | |
| | |
| | | | |
| Preliminary adjustments to net assets acquired | | | | | 290,789 | |
| | | | |
| |
| Pro-forma goodwill | | | | | 883,493 | |
| Existing goodwill | | | | | (314,961 | ) |
| | | | |
| |
| Pro-forma adjustment to goodwill | | | | $ | 568,532 | |
| | | | |
| |
P-6
The pro forma adjustment to intangible assets was as follows(3):
| Fair value of identifiable intangible assets: | | | | |
| | Customer base and relationships | | $ | 229,500 | |
| | Proprietary technology | | | 17,000 | |
| | Trademarks and tradenames | | | 33,000 | |
| | |
| |
| | | | 279,500 | |
| | Less: existing carrying of intangible assets | | | (97,594 | ) |
| | |
| |
| Pro forma adjustment to intangible assets | | $ | 181,906 | |
| | |
| |
We were acquired as part of a competitive bidding process whereby multiple potential acquirers submitted bids to buy us. Potential acquirers made bids to acquire us based on their assessment of our future cash flows. Since our 2003 fiscal year, we have experienced significant growth in our income from operations due to the successful integration of acquisitions and growth in the target markets of the medical device markets that we serve. The growth in our target markets is expected to continue. Our recent improved financial performance and favorable industry conditions resulted in an expectation by potential acquirers that our current level of cash flow would continue, and potentially improve. As a result, the price to acquire us was significantly higher than the net book value of our assets. We expect a significant portion of the purchase price to be allocated to goodwill and other intangible assets because:
- •
- We will incur $30 million in costs to retire existing indebtedness, write off approximately $14 million in deferred financing fees related to existing indebtedness; incur approximately $36 million of transaction costs consisting primarily of investment banking and equity sponsor fees and pay $17 million of management bonuses upon the consummation of the Merger, all of which will further reduce the carrying value of our net assets;
- •
- Prior to the acquisition of us, our goodwill and other intangible assets were already a significant portion of our total assets; and
- •
- The Company has acquired many businesses since its inception, and based on our acquisition experience, the fair value of our property, plant and equipment is likely to be close to the carrying value of those assets.
Appendix A of SFAS 141, paragraph A14 provides an illustrative listing of potential intangible assets that meet the criteria to be recognized apart from goodwill. Based on the examples provided by this guidance, and our experience with prior acquisitions, the intangible assets to be valued by us are expected to include our customer base and relationships, developed technology, in-process research and development as well as trademarks and trade names. Our most significant intangible asset is our customer base and relationships due to the significant amount of cash flow associated with this asset over a 15 year estimated life. We expect to allocate a portion of the purchase price to proprietary technology and in-process research and development. However, our customers own a significant portion of the intellectual property associated with the products that we manufacture. Approximately 10% of our net sales are generated by our proprietary technology. As a result, the portion of the purchase price allocated to our technology based intangible assets and in-process research and development will likely not be significant.
Although we are not aware of any material revisions to our estimates, our final valuation could result in an allocation that differs materially than that presented in these unaudited pro forma condensed combined financial statements. We expect the final valuation of our net assets to be complete when we issue our audited financial statements for the year ended December 31, 2005.
P-7
- (1)
- Represents both the cash investment of $836.6 million and the management participation and rollover equity of $29.7 million.
- (2)
- Represents estimated expenses related primarily to legal, accounting, investment banking and Sponsors fees.
- (3)
- Reflects the estimated impact on deferred income taxes related purchase accounting adjustments totaling $198.9 million calculated at a 38.25% statutory tax rate, for a total gross deferred tax liability of $76.1 million. The gross deferred tax liability has been reduced by $70.7 million of deferred tax assets, relating to net operating loss carryforwards available to the company.
- (d)
- Reflects the capitalization of $20.0 million of estimated financing costs that we incurred with the senior credit facilities and the outstanding notes, net of $14.6 million of unamortized deferred financing costs eliminated in purchase accounting.
- (e)
- Adjustments to stockholders' equity consists of the following (dollars in thousands):
| Sponsors cash equity contribution | | $ | 611,000 | |
| Management roll over equity and cash contribution | | | 29,700 | |
| | |
| |
| Total equity contribution | | | 640,700 | |
| Less transaction costs charged to expense | | | (2,437 | ) |
| | |
| |
| Pro forma equity balance | | | 638,263 | |
| | |
| |
| Less historic equity | | | (169,561 | ) |
| | |
| |
| Net adjustment to equity | | $ | 468,702 | |
| | |
| |
P-8
ACCELLENT INC.
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Nine Months Ended September 30, 2004
Amounts in thousands
| | Accellent
nine months
ended
September 30,
2004
| | Medsource
interim
period
ended
June 29,
2004
| | Campbell
nine months
ended
September 30,
2004
| | MTG nine
months
ended
September 30,
2004
| | Pro Forma
Acquisition
Adjustments
| | Pro Forma
Accellent
Including
Acquisitions
| | Pro Forma
Transaction
Adjustments
| | Pro Forma
as Adjusted
Accellent
| |
|---|
| Net sales | | $ | 212,485 | | $ | 94,301 | | $ | 7,821 | | $ | 8,943 | | $ | (527 | )(a) | $ | 323,023 | | $ | — | | $ | 323,023 | |
| Cost of sales | | | 155,155 | | | 71,612 | | | 5,027 | | | 4,312 | | | (495 | )(b) | | 235,611 | | | — | | | 235,611 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| | Gross profit | | | 57,330 | | | 22,689 | | | 2,794 | | | 4,631 | | | (32 | ) | | 87,412 | | | — | | | 87,412 | |
| Selling, general and administrative expenses | | | 30,681 | | | 15,024 | | | 365 | | | 586 | | | 330 | (b) | | 46,986 | | | — | | | 46,986 | |
| Research and development expenses | | | 2,023 | | | 181 | | | — | | | — | | | — | | | 2,204 | | | — | | | 2,204 | |
| Restructuring and other charges | | | 2,107 | | | 2,334 | | | — | | | — | | | — | | | 4,441 | | | — | | | 4,441 | |
| Amortization of intangibles | | | 3,937 | | | 60 | | | — | | | — | | | 1,675 | (c) | | 5,672 | | | 7,238 | (g) | | 12,910 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Income from operations | | | 18,582 | | | 5,090 | | | 2,429 | | | 4,045 | | | (2,037 | ) | | 28,109 | | | (7,238 | ) | | 20,871 | |
| Other income (expense) | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Interest (expense) income, net | | | (19,397 | ) | | (1,278 | ) | | (25 | ) | | (26 | ) | | (3,184 | )(d) | | (23,910 | ) | | (22,052 | )(h) | | (45,962 | ) |
| | Other (expense) income, including debt prepayment penalties of $3,295 in 2004 | | | (3,284 | ) | | 71 | | | (12 | ) | | — | | | 3,295 | (e) | | 70 | | | — | | | 70 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Total Other income (expense) | | | (22,681 | ) | | (1,207 | ) | | (37 | ) | | (26 | ) | | 111 | | | (23,840 | ) | | (22,052 | ) | | (45,892 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) before income taxes | | | (4,099 | ) | | 3,883 | | | 2,392 | | | 4,019 | | | (1,926 | ) | | 4,269 | | | (29,290 | ) | | (25,021 | ) |
| Income tax expense (benefit) | | | 2,341 | | | 243 | | | — | | | (11 | ) | | — | (f) | | 2,573 | | | — | (i) | | 2,573 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Net income (loss)(j) | | $ | (6,440 | ) | $ | 3,640 | | $ | 2,392 | | $ | 4,030 | | $ | (1,926 | ) | $ | 1,696 | | $ | (29,290 | ) | $ | (27,594 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes to unaudited pro forma condensed combined statements of operations.
P-9
ACCELLENT INC.
Unaudited Pro Forma Condensed Combined Statement of Operations
For the twelve months ended December 31, 2004
(In thousands)
| | Accellent twelve months ended
December 31, 2004
| | MedSource interim period ended
June 29, 2004
| | Campbell twelve months ended
December 31, 2004
| | MTG twelve months ended
December 31, 2004
| | Pro Forma
Acquisition
Adjustments
| | Pro Forma
Accellent
Including
Acquisitions
| | Pro Forma
Transactions
Adjustments
| | Pro Forma
as Adjusted
Accellent
| |
|---|
| Net Sales | | $ | 320,169 | | $ | 94,301 | | $ | 10,899 | | $ | 11,322 | | $ | (527 | )(a) | $ | 436,164 | | $ | — | | $ | 436,164 | |
| Cost of sales | | | 234,396 | | | 71,612 | | | 7,037 | | | 5,924 | | | (525 | )(b) | | 318,444 | | | — | | | 318,444 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Gross profit | | | 85,773 | | | 22,689 | | | 3,862 | | | 5,398 | | | (2 | ) | | 117,720 | | | — | | | 117,720 | |
| Selling, general and administrative expense | | | 45,912 | | | 15,024 | | | 482 | | | 851 | | | 333 | (b) | | 62,602 | | | — | | | 62,602 | |
| Research and development expense | | | 2,668 | | | 181 | | | — | | | — | | | — | | | 2,849 | | | — | | | 2,849 | |
| Restructuring and other charges | | | 3,600 | | | 2,334 | | | — | | | — | | | — | | | 5,934 | | | — | | | 5,934 | |
| Amortization of intangibles | | | 5,539 | | | 60 | | | — | | | — | | | 2,035 | (c) | | 7,634 | | | 9,666 | (g) | | 17,300 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | 28,054 | | | 5,090 | | | 3,380 | | | 4,547 | | | (2,370 | ) | | 38,701 | | | (9,666 | ) | | 29,035 | |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (26,879 | ) | | (1,278 | ) | | (41 | ) | | (39 | ) | | (3,864 | )(d) | | (32,101 | ) | | (29,221 | )(h) | | (61,322 | ) |
| Other | | | (3,312 | ) | | 71 | | | (25 | ) | | 27 | | | 3,295 | (e) | | 56 | | | — | | | 56 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Total other income (expense) | | | (30,191 | ) | | (1,207 | ) | | (66 | ) | | (12 | ) | | (569 | ) | | (32,045 | ) | | (29,221 | ) | | (61,266 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) before income taxes | | | (2,137 | ) | | 3,883 | | | 3,314 | | | 4,535 | | | (2,939 | ) | | 6,656 | | | (38,887 | ) | | (32,231 | ) |
| Income tax expense (benefit) | | | 3,483 | | | 243 | | | — | | | (22 | ) | | 215 | (f) | | 3,919 | | | — | (i) | | 3,919 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Net income (loss) | | $ | (5,620 | ) | $ | 3,640 | | $ | 3,314 | | $ | 4,557 | | $ | (3,154 | ) | $ | 2,737 | | $ | (38,887 | ) | $ | (36,150 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes to unaudited pro forma condensed combined statements of operations.
P-10
ACCELLENT INC.
Unaudited Pro Forma Condensed Combined Statement of Operations
For the nine months ended September 30, 2005
(In thousands)
| | Accellent nine months ended
September 30,
2005
| | Campbell interim period ended September 11, 2005
| | MTG nine months ended September 30, 2005
| | Pro Forma
Acquisition
Adjustments
| | Pro Forma
Accellent
Including
Acquisitions
| | Pro Forma
Transaction
Adjustments
| | Pro Forma
as Adjusted
Accellent
| |
|---|
| Net Sales | | $ | 340,253 | | $ | 8,220 | | $ | 11,362 | | $ | — | | $ | 359,835 | | | — | | $ | 359,835 | |
| Cost of sales | | | 234,122 | | | 6,710 | | | 5,668 | | | 76 | (b) | | 246,576 | | | — | | | 246,576 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Gross profit | | | 106,131 | | | 1,510 | | | 5,694 | | | (76 | ) | | 113,259 | | | — | | | 113,259 | |
| Selling, general and administrative expense | | | 49,539 | | | 452 | | | 857 | | | (230 | )(b) | | 50,618 | | | — | | | 50,618 | |
| Research and development expense | | | 2,294 | | | — | | | — | | | — | | | 2,294 | | | — | | | 2,294 | |
| Restructuring and other charges | | | 3,824 | | | — | | | — | | | — | | | 3,824 | | | — | | | 3,824 | |
| Amortization of intangibles | | | 4,660 | | | — | | | — | | | 1,077 | (c) | | 5,737 | | | 7,238 | (g) | | 12,975 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | 45,814 | | | 1,058 | | | 4,837 | | | (923 | ) | | 50,786 | | | (7,238 | ) | | 43,548 | |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (23,731 | ) | | (88 | ) | | (78 | ) | | (1,960 | )(d) | | (25,857 | ) | | (20,105 | )(h) | | (45,962 | ) |
| Other | | | (105 | ) | | (20 | ) | | (57 | ) | | — | | | (182 | ) | | — | | | (182 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Total other income (expense) | | | (23,836 | ) | | (108 | ) | | (135 | ) | | (1,960 | ) | | (26,039 | ) | | (20,105 | ) | | (46,144 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) before income taxes | | | 21,978 | | | 950 | | | 4,702 | | | (2,883 | ) | | 24,747 | | | (27,343 | ) | | (2,596 | ) |
| Income tax expense (benefit) | | | 10,045 | | | — | | | 11 | | | 658 | | | 10,714 | | | (5,813 | )(i) | | 4,901 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net income (loss) | | $ | 11,933 | | $ | 950 | | $ | 4,691 | | $ | (3,541 | ) | $ | 14,033 | | $ | (21,530 | ) | $ | (7,497 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes to unaudited pro forma condensed combined statements of operations.
P-11
ACCELLENT INC.
Notes to Unaudited Pro Forma Condensed Combined Statements of Operations
- (a)
- Represents the elimination of sales by the Company to MedSource and sales by MedSource to the Company totaling $527,000 during the twelve months ended December 31, 2004.
- (b)
- The Company had incurred an annual monitoring fee to DLJ Merchant Banking III, Inc., in connection with the MedSource acquisition and increased salaries for certain employees in connection with the MTG acquisition. In connection with the Campbell and MTG acquisitions, the Company acquired real estate which was previously leased to the acquired companies by related parties. As a result, the Company will incur increased depreciation and less rent expense. The Company will not incur transaction costs previously incurred by Campbell and MTG in connection with the sale of those businesses to the Company. These adjustments are as follows (in thousands):
| | Nine months
ended
September 30,
2004
| | Twelve months
ended
December 31,
2004
| | Nine months
ended
September 30,
2005
| |
|---|
| Elimination of intercompany sales | | $ | (527 | ) | $ | (527 | ) | $ | — | |
| Salaries adjustment | | | 130 | | | 133 | | | 106 | |
| Depreciation expense increase | | | 102 | | | 135 | | | 42 | |
| Rent expense reduction | | | (200 | ) | | (266 | ) | | (72 | ) |
| | |
| |
| |
| |
| Total adjustments to cost of sales | | $ | (495 | ) | $ | (525 | ) | $ | 76 | |
| | |
| |
| |
| |
Salaries adjustment |
|
|
130 |
|
|
133 |
|
|
106 |
|
| Transaction expenses | | | — | | | — | | | (336 | ) |
| Additional monitoring fee to DLJ | | | 200 | | | 200 | | | — | |
| | |
| |
| |
| |
| Total adjustments to selling, general and administrative expenses | | $ | 330 | | $ | 333 | | $ | (230 | ) |
| | |
| |
| |
| |
- (c)
- The pro forma adjustment for amortization expense was based on the identifiable intangible assets and useful lives assigned to each. The actual amount of identifiable intangible assets of MedSource and the estimated identifiable intangible assets of Campbell and MTG, and the respective lives of each areas follows:
| | Amount
| | Useful life
|
|---|
| | (in thousands)
| | (in years)
|
|---|
| Customer base—MedSource | | $ | 16,300 | | 15 |
| Developed technology—MedSource | | | 2,300 | | 10 |
| Customer relationship—Campbell | | | 6,050 | | 15 |
| Customer relationship—Campbell | | | 360 | | 5 |
| Non-compete—Campbell | | | 30 | | 5 |
| Customer relationship—MTG | | | 13,600 | | 15 |
| Customer relationship—MTG | | | 340 | | 7 |
| | |
| |
|
| | | $ | 38,980 | | 14 |
| | |
| |
|
- We assigned a 15 year life to a significant portion of the customer base intangible assets acquired from MedSource, Campbell and MTG due to a history of limited customer attrition for the underlying customers. For many of our subsidiaries, our top customers have changed minimally over the past 10-20 years. In addition, medical device customers can incur significant costs to move production due to the costs to re-develop manufacturing processes with a new vendor. Certain customer relationship intangibles of MTG and Campbell were assigned shorter lives due to
P-12
potentially lower probabilities of renewal. The useful life of the MedSource developed technology intangible asset was determined based on an analysis of market competitive trends and the rate of technological change in the industry for the technologies associated with this intangible, resulting in an estimated life of 10 years. Non-compete intangibles were assigned a life based on the terms of the non-compete arrangement and the time required to develop a competing product offering, resulting in an estimated useful life of 5 years.
The valuations of the identifiable intangible assets of Campbell and MTG are expected to be completed when we issue our audited financial statements for the year ended December 31, 2005. An increase or decrease in the amount of purchase price allocated to amortizable assets would impact the amount of amortization expense. Identifiable intangible assets have been amortized on a straight-line basis in the unaudited pro forma condensed combined statements of operations.
Based on the 14 year estimated weighted average useable life of the intangible assets described above, each increase of $1.0 million of purchase price allocated to amortizable intangible assets would result in an annual decrease to pro forma income from operations of approximately $0.1 million. Based on the $39.0 million of intangible assets described above, a decrease of 1, 2 and 3 yeas in the weighted average useful life would result in annual decreases to pro forma income from operations of approximately $0.2 million, $0.5 million and $0.7 million, respectively.
- (d)
- Consists of (in thousands):
| | Nine months
ended
September 30,
2004
| | Twelve months
ended
December 31,
2004
| | Nine months
ended
September 30,
2005
| |
|---|
| Elimination of interest on retired indebtedness at the Company | | $ | 11,970 | | $ | 11,970 | | $ | — | |
| Elimination of interest on retired indebtedness at MedSource, Campbell and MTG | | | 1,329 | | | 1,358 | | | 166 | |
| Interest on borrowings under the senior credit facility and the notes | | | (13,164 | ) | | (13,164 | ) | | — | |
| Amortization of deferred financing fees of the Company | | | (1,193 | ) | | (1,193 | ) | | — | |
| Interest on debt to fund Campbell and MTG acquisitions | | | (2,126 | ) | | (2,835 | ) | | (2,126 | ) |
| | |
| |
| |
| |
| | | $ | (3,184 | ) | $ | (3,864 | ) | $ | (1,960 | ) |
| | |
| |
| |
| |
- (e)
- Represents the elimination of the one-time expenses for prepayment fees associated with the retired indebtedness of the Company.
- (f)
- Campbell was a S Corporation prior to the acquisition, and therefore not subject to corporate income taxes. MTG was a limited liability company subject only to state corporate income taxes. Adjustments represent additional corporate level income taxes since both entities will be subject to federal and state income tax after the acquisition. Only incremental state taxes were provided for the year ended December 31, 2004 due to the federal taxable net operating loss incurred. Only incremental state taxes and certain non-cash federal taxes were provided for the nine month period ending September 30, 2005 due to the net operating loss carryforward available for federal tax purposes.
P-13
- (g)
- Represents change in amortization based upon preliminary estimates of the fair values and useful lives of identifiable intangible assets and the respective lives of each category of identifiable intangible asset is as follows (in thousands):
| | Amount
| | Life (years)
|
|---|
| Customer Base & Relationships | | $ | 229,500 | | 15.00 |
| Proprietary Technology | | | 17,000 | | 8.50 |
| | |
| |
|
| | | $ | 246,500 | | 14.25 |
| | |
| |
|
| Indefinite life intangibles—Trademarks & Tradenames | | $ | 33,000 | | |
| | |
| | |
- We assigned a 15 year life to our customer base and relationship intangible asset due to our history of limited customer attrition. For many of our subsidiaries, our top customers have changed minimally over the past 10-20 years. In addition, medical device customers can incur significant costs to move production due to the costs to re-develop manufacturing processes with a new vendor. The useful life of the proprietary technology intangible was based on an analysis of market competitive trends and the rate of technological change in the industry for the technologies associated with this intangible asset, resulting in a range of preliminary useful lives of 7 to 10 years.
- We invested significant resources to develop the Accellent trademark and trade name during 2004. All of our divisions extensively use our trademark and trade name. We have initiated external programs to build our brand name through our Accellent Advantage program which builds brand awareness of our capabilities with senior management of our target customer base. We also have launched advertising programs in trade publications under our new brand name, and opened our initial Accellent Idea Center, which will provide design and engineering services to our target customer base. The legal registration of our trademark is near completion in the United States, and underway in Canada, China, Mexico and the European Union. We intend to maintain the legal rights to our trademark indefinitely. We have applied an indefinite life to the Accellent trademark and trade name based on our intent to use this intangible asset indefinitely. Therefore, in accordance with SFAS 142, the Accellent trademark and trade name intangible asset will not be amortized until its useful life is no longer indefinite. Our trademark and trade name intangible will be tested for impairment annually, along with goodwill, or more frequently, if events or changes in circumstances suggest that the asset might be impaired.
- Although we are not aware of any material adjustments to the preliminary allocation, the final purchase price allocation may result in a materially different allocation for tangible and intangible assets than that presented in these unaudited pro forma condensed combined financial statements. An increase or decrease in the amount of purchase price allocated to amortizable assets would impact the amount of annual amortization expense. Identifiable intangible assets have been amortized on a straight-line basis in the unaudited pro forma condensed combined statements of income. The following table shows the decrease to pro forma income from operations for every $10.0 million of purchase price allocated to amortizable intangible assets at an estimated 14.2 year life:
| | Twelve months
| | Nine months
|
|---|
| Decrease in operating income | | $ | 702 | | $ | 526 |
P-14
- The following table shows the decrease in pro forma income from operations for every 1 year decrease in the weighted average useful life of the estimated identifiable intangible assets described above:
| | Twelve months
| | Nine months
|
|---|
| Decrease by 1 year | | 1,306 | | 980 |
| Decrease by 2 years | | 2,824 | | 2,118 |
| Decrease by 3 years | | 4,613 | | 3,460 |
- (h)
- Reflects pro forma interest expense resulting from our new capital structure, using 3-month LIBOR rates at November 17, 2005, as follows (in thousands):
| | Nine months
ended
September 30,
2004
| | Twelve months
ended
December 31,
2004
| | Nine months
ended
September 30,
2005
| |
|---|
| Revolving credit facility | | $ | — | | $ | — | | $ | — | |
| Term loan credit facility | | | 19,050 | | | 25,400 | | | 19,050 | |
| 101/2% senior subordinated notes | | | 24,019 | | | 32,025 | | | 24,019 | |
| Bank commitment fees | | | 281 | | | 375 | | | 281 | |
| | |
| |
| |
| |
| Total cash interest expense | | | 43,350 | | | 57,800 | | | 43,350 | |
| Amortization of capitalized debt issuance costs and note discount | | | 2,612 | | | 3,522 | | | 2,612 | |
| | |
| |
| |
| |
| Total pro forma interest expense | | | 45,962 | | | 61,322 | | | 45,962 | |
| Less historical interest expense | | | (23,910 | ) | | (32,101 | ) | | (25,857 | ) |
| | |
| |
| |
| |
| Net adjustment to interest expense | | $ | 22,052 | | $ | 29,221 | | $ | 20,105 | |
| | |
| |
| |
| |
- A 0.125% change in interest rates on the new debt issuance would change cash interest expense for the twelve-month and nine-month periods for each new debt security as follows (in thousands):
| | Twelve month period
| | Nine month period
|
|---|
| Term loan credit facilities | | $ | 500 | | $ | 375 |
| 101/2% senior subordinated notes | | | 381 | | | 286 |
| | |
| |
|
| Total | | $ | 881 | | $ | 661 |
| | |
| |
|
- (i)
- Represents the elimination of non-cash taxes recorded in connection with the usage of net operating losses acquired from MedSource. Based on the pro forma pre-tax net income, these non-cash taxes would not be recorded. No additional benefit is recorded due to net operating loss carryforwards available for both the year ended December 31, 2004 and the nine month period ending September 30, 2005.
- (j)
- Net income does not include the following non-recurring charges which will be recorded during our fourth quarter of 2005: $14.6 million write-off of existing deferred financing fees, $30.0 million of estimated tender premiums related to the redemption of the 10% senior subordinated notes due 2012, $2.4 million of bridge loan costs to be expensed prior to or upon the closing, $36.4 million of transaction costs consisting primarily of investment banking and equity sponsor fees incurred by
P-15
the Company to be expensed prior to or upon the Closing, $16.7 million of bonuses payable to certain employees in connection with the Merger to be expensed prior to or upon the Closing, and a preliminary estimated charge for in-process research and development costs of $8.2 million. We also expect to record a $16.7 million increase in our inventory to record inventories at fair value. We will amortize this increased value of our inventory over our inventory turns resulting in charges to cost of sales of approximately $10.0 million during the fourth quarter of 2005 and $6.7 million during the first quarter of 2006. All of the expenses incurred in connection with the Transactions are non-recurring, and have been excluded from the pro forma statements of operations.
The preliminary charge for in-process research and development includes numerous projects. Our projects focus on the use of new production materials, including biomaterials, as well as new methods for fabricating metals, polyimide and other materials. The first category of projects, which we refer to as our Incremental IPR&D, is comprised of 14 individual projects which are in various stages of completion. Projects that build on our current core capabilities are included in this category. All projects in this category are estimated to be complete by 2009. Costs to complete the Incremental IPR&D are estimated to be $2.8 million. Net cash flows from Incremental IPR&D are estimated to be positive by 2008. We refer to a second category of projects as our Platform IPR&D, which is comprised of 13 individual projects which are in various stages of completion. Projects that offer new and unique production capabilities to our current product offerings are included in this category. All projects in this category are estimated to be complete by 2009. Costs to complete the Platform IPR&D are estimated to be $6.4 million. Net cash flows from Platform IPR&D are estimated to be positive by 2009. We refer to our last category of projects as our Breakthrough IPR&D, which is comprised of 4 individual projects which are in the early stages of completion. Projects that involve entirely new manufacturing processes and are likely to entirely replace existing manufacturing methods are included in this category. All projects in this category are estimated to be complete by 2010. Costs to complete the Breakthrough IPR&D are estimated to be $4.0 million. Net cash flows from Breakthrough IPR&D are estimated to be positive by 2010. The fair value of each project category was determined based on an excess earnings method which isolates the excess return attributable to each project category. Cash flows were discounted using a rate of 13% over a period of 15 years. Probability factors were applied to revenue streams based on the associated risk for each category. Breakthrough IPR&D was assigned a probability of 60%, Platform IPR&D was assigned a probability of 70% and Incremental IPR&D was assigned a probability of 80%.
P-16
ACCELLENT INC.
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
For the Three Years Ended December 31, 2004 (In thousands)
Amounts in Thousands
| | Balance at
Beginning of
Period
| | Additions
Charged to
Expense
| | Other(a)
| | Amounts
Written Off
| | Balance at
End of
Period
|
|---|
| Allowance for doubtful accounts | | | | | | | | | | | | | | | |
| December 31, 2004 | | $ | 670 | | $ | 850 | | $ | 851 | | $ | (305 | ) | $ | 2,066 |
| December 31, 2003 | | | 830 | | | 172 | | | — | | | (332 | ) | | 670 |
| December 31, 2002 | | | 581 | | | 392 | | | — | | | (143 | ) | | 830 |
Amounts in Thousands
| | Balance at
Beginning of
Period
| | Additions
Charged to
Expense
| | Other
| | Returns
Processed
| | Balance at
End of
Period
|
|---|
| Reserve for returns: | | | | | | | | | | | | | | | |
| December 31, 2004 | | $ | 304 | | $ | 7,124 | | $ | — | | $ | (6,585 | ) | $ | 843 |
| December 31, 2003 | | | — | | | 3,847 | | | — | | | (3,543 | ) | | 304 |
| December 31, 2002 | | | — | | | 2,860 | | | — | | | (2,860 | ) | | — |
- (a)
- Reserve balance for MedSource as of the acquisition date of June 30, 2004.
S-1

PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 20. Indemnification of Directors and Officers.
The following is a summary of the statutes, charter and bylaw provisions or other arrangements under which the registrant's directors and officers are insured or indemnified against liability in their capacities as such.
The registrant is organized under the laws of the State of Maryland. Maryland law permits a Maryland corporation to include in its charter a provision limiting the liability of its directors and officers to the corporation and its stockholders for money damages, except for liability resulting from:
- •
- actual receipt of an improper benefit or profit in money, property or services; or
- •
- active and deliberate dishonesty established by a final judgment as being material to the cause of action.
Article Tenth of the registrant's Third Articles of Amendment and Restatement, as amended, provides that the liability of any director or officer of the registrant to the registrant or its stockholders for money damages shall be limited to the sum of ten dollars, provided nothing in Article Tenth shall limit the liability of a director or officer (i) to the extent that it is proved that such person actually received an improper benefit or profit in money, property or services, for the amount of the benefit or profit in money property or services actually received, or (ii) to the extent that a judgment or other final adjudication, adverse to such person is entered in a proceeding based on a finding in the proceeding that such person's action, or failure to act, was the result of active and deliberate dishonesty and was material to the cause of action adjudicated in the proceeding.
Article VI of the registrant's Bylaws provides that the registrant shall indemnify, to the fullest extent permitted by the laws of the State of Maryland, any present or former director or officer of the registrant, or any person who serves or served another corporation, partnership, joint venture, trust or other enterprise in one of such capacities at the request of the registrant, who, by reason of such position, was, is, or is threatened to be made a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative.
Maryland law requires a corporation (unless its charter provides otherwise, which our charter does not) to indemnify a director or officer who has been successful, on the merits or otherwise, in the defense of any proceeding to which he or she is made, or threatened to be made, a party by reason of his or her service in such capacity. Maryland law permits a corporation to indemnify its present and former directors and officers, among others, against judgments, penalties, fines, settlements and reasonable expenses actually incurred by them in connection with any proceeding to which they may be made, or are threatened to be made, a party by reason of their service in those or other capacities unless it is established that:
- •
- the act or omission of the director or officer was material to the matter giving rise to the proceeding and (i) was committed in bad faith or (ii) was the result of active and deliberate dishonesty; or
- •
- the director or officer actually received an improper personal benefit in money, property or services; or
- •
- in the case of any criminal proceeding, the director or officer had reasonable cause to believe that the act or omission was unlawful.
However, under Maryland law, a Maryland corporation may not indemnify for an adverse judgment in a suit by or in the right of the corporation or for a judgment of liability on the basis that
II-1
personal benefit was improperly received, unless in either case a court orders indemnification and then only for expenses. In addition, Maryland law permits a corporation to advance reasonable expenses to a director or officer upon the corporation's receipt of:
- •
- a written affirmation by the director or officer of his or her good faith belief that he or she has met the standard of conduct necessary for indemnification by the corporation; and
- •
- a written undertaking by him or her or on his or her behalf to repay the amount paid or reimbursed by the corporation if it is ultimately determined that the standard of conduct was not met.
The registrant maintains directors' and officers' liability insurance which insures against liabilities that directors or officers of the registrant may incur in such capacities.
Item 21. Exhibits and Financial Statement Schedules.
See Exhibit Index.
Item 22. Undertakings.
(a) The undersigned registrants hereby undertake:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the Registration Statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the Registration Statement. Notwithstanding the foregoing, any increase or decrease in the volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective Registration Statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the Registration Statement or any material change to such information in the Registration Statement;
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fide offering thereof; and
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser, if the registrants are subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of
II-2
the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use; and
(5) That, for the purpose of determining liability of the registrants under the Securities Act to any purchaser in the initial distribution of the securities: The undersigned registrants undertake that in a primary offering of securities of the undersigned registrants pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrants will be sellers to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrants relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrants or used or referred to by the undersigned registrants;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrants or their securities provided by or on behalf of the undersigned registrants; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrants to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrants pursuant to the foregoing provisions, or otherwise, the registrants have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrants of expenses incurred or paid by a director, officer or controlling person of the registrants in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrants will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of that issue.
(b) The undersigned registrants hereby undertake to respond to requests for information that is incorporated by reference into the prospectus pursuant to Items 4, 10(b), 11 or 13 of this form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the Registration Statement through the date of responding to the request.
(c) The undersigned registrants hereby undertake to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the Registration Statement when it became effective.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, the registrant has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | ACCELLENT INC. |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Executive Vice President, Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | President, Chief Executive Officer and Director (Principal Executive Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Executive Vice President, Treasurer, Secretary and Director (Principal Financial Officer and Principal Accounting Officer) |
*
(Michael W. Michelson) |
|
Director |
*
(Kenneth W. Freeman) |
|
Director |
*
(James C. Momtazee) |
|
Director |
*
(Steven Barnes) |
|
Director |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, each registrant listed below has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | ACCELLENT CORP.
AMERICAN TECHNICAL MOLDING, INC.
BRIMFIELD ACQUISITION CORP.
CE HUNTSVILLE HOLDINGS CORP.
G&D, INC. D/B/A STAR GUIDE CORPORATION
MEDSOURCE TECHNOLOGIES, INC.
MEDSOURCE TECHNOLOGIES, NEWTON INC.
MEDSOURCE TECHNOLOGIES PITTSBURGH, INC.
MEDSOURCE TRENTON, INC.
MICRO-GUIDE, INC.
NATIONAL WIRE & STAMPING, INC.
NOBLE-MET, LTD.
SPECTRUM MANUFACTURING, INC.
TEXCEL, INC.
THERMAT ACQUISITION CORP.
UTI HOLDING COMPANY
VENUSA, LTD. |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Vice President,
Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | President, Chief Executive Officer and Director (Principal Executive Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Vice President, Treasurer, Secretary and Director (Principal Financial Officer and Principal Accounting Officer) |
*
(Timothy Mathews) |
|
Director |
*
(Thomas F. Lemker) |
|
Director of National Wire & Stamping, Inc. and Texcel, Inc. |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, UTI Corporation has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | UTI CORPORATION |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Vice President, Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | President and Director (Principal Executive Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Vice President, Treasurer, Secretary and Director (Principal Financial Officer and Principal Accounting Officer) |
*
(Timothy Mathews) |
|
Director |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-6
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, each of Cycam, Inc. and ELX, Inc. has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | CYCAM, INC.
ELX, INC. |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Vice President, Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | President, Chief Executive Officer and Director (Principal Chief Financial Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Vice President, Treasurer, Secretary and Director (Principal Financial Officer and Principal Accounting Officer) |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-7
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, Machining Technology Group, LLC has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | MACHINING TECHNOLOGY GROUP, LLC |
|
By: |
|
Accellent Corp. and UTI Holding Company |
|
Its: |
|
Members |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Vice President, Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | Chief Manager and President (Principal Executive Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Vice President, Treasurer and Secretary (Principal Financial Officer and Principal Accounting Officer) |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, each registrant listed below has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | BRIMFIELD PRECISION, LLC
HAYDEN PRECISION INDUSTRIES, LLC
KELCO ACQUISITION, LLC
MACHINING TECHNOLOGY GROUP, LLC
PORTLYN, LLC
TENAX, LLC |
|
By: |
|
MedSource Technologies, LLC |
|
Its: |
|
Sole Member |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Vice President, Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | President and Chief Executive Officer (Principal Executive Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Vice President, Treasurer and Secretary (Principal Financial Officer and Principal Accounting Officer) |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-9
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, MedSource Technologies, LLC has duly caused this Amendment No. 2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, County of Cambridge and State of Massachusetts, on this 13th day of February, 2006.
| | MEDSOURCE TECHNOLOGIES, LLC |
|
By: |
|
MedSource Technologies, Inc. |
|
Its: |
|
Sole Member |
|
By: |
|
/s/ STEWART A. FISHER
|
| | | | Name: | | Stewart A. Fisher |
| | | | Title: | | Chief Financial Officer, Vice President, Treasurer and Secretary |
Pursuant to the requirements of the Securities Act, this Amendment No. 2 has been signed below by the following persons in the capacities indicated on February 13, 2006.
Signature
| | Capacity
|
|---|
|
|
|
*
(Ron Sparks) | | President and Chief Executive Officer (Principal Executive Officer) |
/s/ STEWART A. FISHER
(Stewart A. Fisher) |
|
Chief Financial Officer, Vice President, Treasurer and Secretary (Principal Financial Officer and Principal Accounting Officer) |
*By: |
|
/s/ STEWART A. FISHER
Attorney-in-fact |
|
|
|
|
II-10
EXHIBIT INDEX
EXHIBIT
NUMBER
| | EXHIBIT DESCRIPTION
|
|---|
| 2.1 | | Agreement and Plan of Merger, dated as of October 7, 2005, by and between Accellent Inc. and Accellent Acquisition Corp. (incorporated by reference to Exhibit 99.2 to Accellent Corp.'s Current Report on Form 8-K, filed on October 11, 2005). |
| 2.2 | | Voting Agreement, dated as of October 7, 2005, by and among Accellent Inc., Accellent Acquisition Corp. and certain stockholders of Accellent Inc. (incorporated by reference to Exhibit 99.2 to Accellent Corp.'s Current Report on Form 8-K, filed on October 11, 2005). |
| 3.1** | | Third Articles of Amendment and Restatement, as amended, of Accellent Inc. |
| 3.2** | | Amended and Restated Bylaws of Accellent Inc. |
| 3.3* | | Articles of Incorporation, as amended, of Accellent Corp. |
| 3.4* | | Bylaws of Accellent Corp. |
| 3.5* | | Restated Articles of Incorporation of American Technical Molding, Inc. |
| 3.6* | | Restated Bylaws of American Technical Molding, Inc. |
| 3.7* | | Certificate of Incorporation of Brimfield Acquisition Corp. |
| 3.8* | | Bylaws of Brimfield Acquisition Corp. |
| 3.9* | | Certificate of Formation, as amended, of Brimfield Precision, LLC |
| 3.10* | | Limited Liability Company Agreement of Brimfield Precision, LLC |
| 3.11** | | Certificate of Incorporation of CE Huntsville Holdings Corp. |
| 3.12** | | Bylaws of CE Huntsville Holdings Corp. |
| 3.13* | | Articles of Incorporation, as amended, of Cycam, Inc. |
| 3.14* | | Bylaws of Cycam, Inc. |
| 3.15* | | Articles of Incorporation of ELX, Inc. |
| 3.16* | | Amended and Restated Bylaws of ELX, Inc. |
| 3.17* | | Articles of Incorporation, as amended, of G&D, Inc. d/b/a Star Guide Corporation |
| 3.18* | | Bylaws, as amended, of G&D, Inc. d/b/a Star Guide Corporation |
| 3.19* | | Certificate of Formation, as amended, of Hayden Precision Industries, LLC |
| 3.20* | | Limited Liability Company Agreement of Hayden Precision Industries, LLC |
| 3.21* | | Certificate of Formation of Kelco Acquisition, LLC |
| 3.22* | | Amended and Restated Limited Liability Company Agreement of Kelco Acquisition, LLC |
| 3.23** | | Articles of Organization of Machining Technology Group, LLC |
| 3.24** | | Second Amended and Restated Operating Agreement of Machining Technology Group, LLC |
| 3.25* | | Certificate of Incorporation of MedSource Technologies, Inc. |
| 3.26* | | Bylaws of MedSource Technologies, Inc. |
| 3.27* | | Certificate of Formation of MedSource Technologies, LLC |
| 3.28* | | Limited Liability Company Agreement of MedSource Technologies, LLC |
| 3.29* | | Certificate of Incorporation, as amended, of MedSource Technologies, Newton Inc. |
| 3.30* | | Bylaws of MedSource Technologies, Newton Inc. |
| | | |
II-11
| 3.31* | | Certificate of Incorporation, as amended, of MedSource Technologies Pittsburgh, Inc. |
| 3.32* | | Bylaws of MedSource Technologies Pittsburgh, Inc. |
| 3.33* | | Certificate of Incorporation of MedSource Trenton, Inc. |
| 3.34* | | Bylaws of MedSource Trenton, Inc. |
| 3.35* | | Articles of Incorporation, as amended, of Micro-Guide, Inc. |
| 3.36* | | Amended and Restated Bylaws of Micro-Guide, Inc. |
| 3.37* | | Articles of Incorporation, as amended, of National Wire & Stamping, Inc. |
| 3.38* | | Bylaws of National Wire & Stamping, Inc. |
| 3.39* | | Articles of Incorporation, as amended, of Noble-Met, Ltd. |
| 3.40* | | Bylaws, as amended, of Noble-Met, Ltd. |
| 3.41* | | Certificate of Formation, as amended, of Portlyn, LLC |
| 3.42* | | Limited Liability Company Agreement of Portlyn, LLC |
| 3.43* | | Articles of Incorporation of Spectrum Manufacturing, Inc. |
| 3.44* | | Bylaws, as amended, of Spectrum Manufacturing, Inc. |
| 3.45* | | Certificate of Formation of Tenax, LLC |
| 3.46* | | Limited Liability Company Agreement of Tenax, LLC |
| 3.47* | | Restated Articles of Organization of Texcel, Inc. |
| 3.48* | | Amended and Restated Bylaws of Texcel, Inc. |
| 3.49* | | Certificate of Incorporation of Thermat Acquisition Corp. |
| 3.50* | | Bylaws of Thermat Acquisition Corp. |
| 3.51* | | Amended and Restated Articles of Incorporation, as amended, of UTI Corporation |
| 3.52* | | Bylaws of UTI Corporation |
| 3.53* | | Certificate of Incorporation of UTI Holding Company |
| 3.54* | | Bylaws of UTI Holding Company |
| 3.55* | | Certificate of Incorporation of Venusa, Ltd. |
| 3.56* | | Bylaws of Venusa, Ltd. |
| 4.1 | | Indenture, dated as of November 22, 2005, among Accellent Inc., the subsidiary guarantors named therein and The Bank of New York, as trustee (incorporated by reference to Exhibit 4.1 to Accellent Corp.'s Current Report on Form 8-K, filed on November 29, 2005). |
| 4.2 | | Exchange and Registration Rights Agreement, dated November 22, 2005, among Accellent Inc., the subsidiary guarantors named therein and Credit Suisse First Boston LLC, J.P. Morgan Securities Inc. and Bear, Stearns & Co. Inc. (incorporated by reference to Exhibit 4.2 to Accellent Corp.'s Current Report on Form 8-K, filed on November 29, 2005). |
| 4.3 | | Form of Exchange Note (included in Exhibit 4.1) |
| 5.1** | | Opinion of Simpson Thacher & Bartlett LLP |
| 5.2** | | Opinion of Bass, Berry & Sims PLC. |
| 5.3** | | Opinion of Hogan & Hartson L.L.P. |
| 5.4† | | Opinion of Mintz Levin Cohn Ferris Glovsky and Popeo, P.C. |
| 5.5** | | Opinion of Saul Ewing LLP. |
| | | |
II-12
| 5.6** | | Opinion of Snell & Wilmer L.L.P. |
| 5.7** | | Opinion of Venable LLP. |
| 10.1 | | Credit Agreement, dated November 22, 2005, among Accellent Acquisition Corp., Accellent Merger Sub Inc., Accellent Inc., the lenders party thereto, JPMorgan Chase Bank, N.A., as administrative agent, Credit Suisse First Boston, as syndication agent and Lehman Commercial Paper Inc., as documentation agent (incorporated by reference to Exhibit 10.1 to Accellent Corp.'s Current Report on Form 8-K, filed on November 29, 2005). |
| 10.2 | | Guarantee, dated as of November 22, 2005, among Accellent Acquisition Corp., the subsidiaries named therein and JPMorgan Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.2 to Accellent Corp.'s Current Report on Form 8-K, filed on November 29, 2005). |
| 10.3 | | Pledge Agreement, dated as of November 22, 2005, among Accellent Acquisition Corp., Accellent Merger Sub Inc., Accellent Inc., the subsidiaries named therein and JPMorgan Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.3 to Accellent Corp.'s Current Report on Form 8-K, filed on November 29, 2005). |
| 10.4 | | Security Agreement, dated as of November 22, 2005, among Accellent Holdings Corp., Accellent Merger Sub Inc., Accellent Inc., the subsidiaries named therein and JPMorgan Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.4 to Accellent Corp.'s Current Report on Form 8-K, filed on November 29, 2005). |
| 10.5** | | 2005 Equity Plan for Key Employees of Accellent Holdings Corp. and Its Subsidiaries and Affiliates. |
| 10.6** | | Management Services Agreement, dated November 22, 2005, between Accellent Inc. and Kohlberg Kravis Roberts & Co. L.P. |
| 10.7** | | Form of Rollover Agreement, dated November 22, 2005, between Accellent Holdings Corp. and certain members of management. |
| 10.8** | | Form of Management Stockholder's Agreement, dated November 22, 2005, between Accellent Holdings Corp. and certain members of management. |
| 10.9** | | Form of Sale Participation Agreement, dated November 22, 2005, between Accellent Holdings LLC and certain members of management. |
| 10.10** | | Registration Rights Agreement, dated November 22, 2005, between Accellent Holdings Corp. and Accellent Holdings LLC. |
| 10.11** | | Stock Subscription Agreement, dated November 16, 2005, between Bain Capital Integral Investors LLC and Accellent Holdings Corp. |
| 10.12** | | Stockholders' Agreement, dated as of November 16, 2005 by and among Accellent Holdings Corp., Bain Capital Integral Investors, LLC, BCIP TCV, LLC and Accellent Holdings LLC. |
| 10.13 | | Asset Purchase Agreement, dated as of September 12, 2005 by and among Accellent Corp., CE Huntsville Holdings Corp., Campbell Engineering, Inc. and the shareholders of Campbell Engineering, Inc. (incorporated by reference to Exhibit 10.2 to Accellent Corp.'s Quarterly Report on Form 10-Q, filed on November 1, 2005). |
| 10.14 | | Agreement and Plan of Merger, dated as of April 27, 2004, among MedSource Technologies, Inc., Medical Device Manufacturing, Inc. and Pine Merger Corporation (incorporated by reference to Exhibit 2.1 to MedSource Technologies, Inc.'s Current Report on Form 8-K, filed on April 28, 2004). |
| 10.15 | | Employment Agreement, dated as of September 15, 2003, between Accellent Inc. and Ron Sparks (incorporated by reference to Exhibit 10.1 of Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2004). |
| | | |
II-13
| 10.16 | | Employment letter dated July 19, 2004 between Accellent Inc. and Gary Curtis (incorporated by reference to Exhibit 10.2 of Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2004). |
| 10.17** | | Interest Purchase Agreement, dated as of October 6, 2005, by and among Accellent Corp., Gary Stavrum and Timothy Hanson, the members of Machining Technology Group, LLC. |
| 10.18 | | Employment Agreement, dated as of September 2001, between Accellent Inc. and Stewart Fisher (incorporated by reference to Exhibit 10.3 of Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2004). |
| 10.19 | | Employment Agreement, dated July 1, 2004, between Accellent Inc. and Daniel C. Croteau (incorporated by reference to Exhibit 10.5 of Accellent Corp.'s Annual Report on Form 10-K, filed on March 15, 2005). |
| 10.20 | | Trade Secrets Agreement and Employment Contract, dated April 7, 2003, between Accellent Inc. and Gary D. Curtis (incorporated by reference to Exhibit 10.7 of Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2004). |
| 10.21 | | Non-Disclosure, Non-Solicitation, Non-Competition and Invention Assignment Agreement, dated July 22, 2003, between Accellent Inc. and Gary D. Curtis (incorporated by reference to Exhibit 10.8.1 of Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2004). |
| 10.22 | | Non-Competition Agreement, dated September, 2001, between Accellent Inc. and Stewart Fisher (incorporated by reference to Exhibit 10.8.2 of Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2004). |
| 10.23 | | Non-Disclosure, Non-Solicitation, Non-Competition and Invention Assignment Agreement, dated September 15, 2004, between Accellent Inc. and Daniel C. Croteau (incorporated by reference to Exhibit 10.8.4 of Accellent Corp.'s Annual Report on Form 10-K, filed on March 15, 2005). |
| 10.24 | | Accellent Inc. Supplemental Executive Retirement Pension Program (incorporated by reference to Exhibit 10.11 to Accellent Inc.'s Registration Statement on Form S-1, filed on February 14, 2001) |
| 10.25** | | Form of Stock Option Agreement, dated November 22, 2005, between Accellent Holdings Corp. and certain members of management. |
| 10.26** | | Accellent Holdings Corp. Directors' Deferred Compensation Plan. |
| 12.1** | | Statement of Computation of Ratio of Earnings to Fixed Charges. |
| 21.1** | | Subsidiaries of Accellent Inc. |
| 23.1† | | Consent of PricewaterhouseCoopers LLP. |
| 23.2† | | Consent of Ernst & Young LLP. |
| 23.3† | | Consent of Beason & Nalley, Inc. |
| 23.4† | | Consent of Lenahan, Smith & Bargiachi, PC. |
| 23.5 | | Consent of Simpson Thacher & Bartlett LLP (included in Exhibit 5.1). |
| 23.6 | | Consent of Bass, Berry & Sims PLC (included in Exhibit 5.2). |
| 23.7 | | Consent of Hogan & Hartson L.L.P. (included in Exhibit 5.3). |
| 23.8 | | Consent of Mintz Levin Cohn Ferris Glovsky and Popeo, P.C. (included in Exhibit 5.4). |
| 23.9 | | Consent of Saul Ewing LLP (included in Exhibit 5.5). |
| 23.10 | | Consent of Snell & Wilmer L.L.P. (included in Exhibit 5.6). |
| | | |
II-14
| 23.11 | | Consent of Venable LLP (included in Exhibit 5.7). |
| 23.12† | | Consent of Millennium Research Group. |
| 24.1** | | Power of Attorney of Accellent Inc. |
| 25.1** | | Form T-1 Statement of Eligibility and Qualification under the Trust Indenture Act of 1939, as amended, of The Bank of New York, as trustee. |
| 99.1** | | Form of Letter of Transmittal. |
| 99.2** | | Form of Notice of Guaranteed Delivery. |
| 99.3** | | Form of Letter to Broker-Dealers |
| 99.4** | | Form of Letter to Clients |
- †
- Filed herewith.
- *
- Incorporated by reference to Accellent Corp.'s Registration Statement on Form S-4, filed on August 30, 2005.
- **
- Previously filed.
II-15
QuickLinks
TABLE OF ADDITIONAL REGISTRANT GUARANTORSTABLE OF CONTENTSSUMMARYOur CompanyRisk Related to Our BusinessThe TransactionsAbout Kohlberg Kravis Roberts & Co. L.P.Ownership and Corporate StructureSummary of Terms of the Exchange OfferSummary of Terms of the Exchange NotesSUMMARY UNAUDITED PRO FORMA COMBINED FINANCIAL DATARISK FACTORSRisks Related to the Exchange OfferRisks Related to Our Indebtedness and the Exchange NotesRisks Related to Our BusinessDISCLOSURE REGARDING FORWARD-LOOKING STATEMENTSUSE OF PROCEEDSCAPITALIZATIONSELECTED HISTORICAL CONSOLIDATED FINANCIAL DATAMANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSBUSINESSMANAGEMENTSummary Compensation TablePENSION PLAN TABLE—30-YEAR ACCRUALPRINCIPAL STOCKHOLDERSTHE TRANSACTIONSCERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONSDESCRIPTION OF OTHER INDEBTEDNESSTHE EXCHANGE OFFERDESCRIPTION OF THE EXCHANGE NOTESBOOK-ENTRY; DELIVERY AND FORMUNITED STATES FEDERAL INCOME TAX CONSEQUENCES OF THE EXCHANGE OFFERCERTAIN ERISA CONSIDERATIONSPLAN OF DISTRIBUTIONLEGAL MATTERSEXPERTSAVAILABLE INFORMATIONINDEX TO FINANCIAL STATEMENTSReport of Independent Registered Public Accounting FirmACCELLENT INC. Consolidated Balance Sheets As of December 31, 2004 and 2003 (In thousands, except share data)ACCELLENT INC. Consolidated Statements of Operations For the years ended December 31, 2004, 2003 and 2002 (In thousands)ACCELLENT INC. Consolidated Statements of Cash Flows For the years ended December 31, 2004, 2003 and 2002 (In thousands)ACCELLENT INC. Notes to Consolidated Financial StatementsACCELLENT INC. Unaudited Condensed Consolidated Balance Sheets As of September 30, 2005 and December 31, 2004 (In thousands)ACCELLENT INC. Unaudited Condensed Consolidated Statements of Operations For the nine months ended September 30, 2005 and 2004 (In thousands)ACCELLENT INC. Unaudited Condensed Consolidated Statements of Cash Flows For the nine months ended September 30, 2005 and 2004 (In thousands)ACCELLENT INC. Notes to Unaudited Condensed Consolidated Financial StatementsREPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMMEDSOURCE TECHNOLOGIES, INC. Consolidated Balance Sheets As of June 30, 2003 and 2002 (In thousands, except share data)MEDSOURCE TECHNOLOGIES, INC. Consolidated Statements of Operations For the years ended June 30, 2003, 2002, 2001 (In thousands, except share and per share amounts)MEDSOURCE TECHNOLOGIES, INC. Consolidated Statements of Changes in Mandatory Redeemable Convertible Stock and Stockholders' Equity (Deficit) For the years ended June 30, 2003, 2002 and 2001 (In thousands)MEDSOURCE TECHNOLOGIES, INC. Consolidated Statements of Changes in Mandatory Redeemable Convertible Stock and Stockholders' Equity (Deficit) For the years ended June 30, 2003, 2002 and 2001 (In thousands)MEDSOURCE TECHNOLOGIES, INC. Consolidated Statements of Cash Flows For the years ended June 30, 2003, 2002 and 2001 (In thousands)MEDSOURCE TECHNOLOGIES, INC. Notes to Consolidated Financial StatementsMEDSOURCE TECHNOLOGIES, INC. Unaudited Consolidated Balance Sheets As of March 28, 2004 and June 30, 2003 (In thousands)MEDSOURCE TECHNOLOGIES, INC. Unaudited Consolidated Statements of Operations For the three and nine months ended March 28, 2004 and March 30, 2003 (In thousands except share and per share amounts)MEDSOURCE TECHNOLOGIES, INC. Unaudited Consolidated Statements of Cash Flows For the nine months ended March 28, 2004 and March 30, 2003 (In thousands)MEDSOURCE TECHNOLOGIES, INC. Notes to Unaudited Consolidated Financial StatementsCAMPBELL ENGINEERING, INC. Balance Sheets As of December 31, 2004 and 2003CAMPBELL ENGINEERING, INC. Statements of Income For the years ended December 31, 2004 and 2003CAMPBELL ENGINEERING, INC. Statements of Changes in Stockholders' Equity For the years ended December 31, 2004 and 2003CAMPBELL ENGINEERING, INC. Statements of Cash Flows For the years ended December 31, 2004 and 2003CAMPBELL ENGINEERING, INC. Notes to Financial Statements As of December 31, 2004 and 2003Campbell Engineering, Inc. Unaudited Balance Sheets As of June 30, 2005 and December 31, 2004Campbell Engineering, Inc. Unaudited Statements of Income For the six months ended June 30, 2005 and 2004Campbell Engineering, Inc. Unaudited Statements of Changes in Stockholders' Equity For the six months ended June 30, 2005Campbell Engineering, Inc. Unaudited Statements of Cash Flows For the six months ended June 30, 2005 and 2004Campbell Engineering, Inc. Notes to Unaudited Financial Statements As of June 30, 2005Independent Auditors' ReportMACHINING TECHNOLOGY GROUP, LLC Balance Sheets As of December 31, 2004 and 2003MACHINING TECHNOLOGY GROUP, LLC Statements of Income For the years ended December 31, 2004 and 2003MACHINING TECHNOLOGY GROUP, LLC Statements of Changes in Members' Equity For the years ended December 31, 2004 and 2003MACHINING TECHNOLOGY GROUP, LLC Statements of Cash Flows For the years ended December 31, 2004 and 2003MACHINING TECHNOLOGY GROUP, LLC Notes to Financial Statements For the years ended December 31, 2004 and 2003MACHINING TECHNOLOGY GROUP, LLC Unaudited Balance Sheets As of September 30, 2005 and December 31, 2004MACHINING TECHNOLOGY GROUP, LLC Unaudited Statements of Income For the nine months ended September 30, 2005 and 2004MACHINING TECHNOLOGY GROUP, LLC Unaudited Statements of Changes in Members' Equity For the nine months ended September 30, 2005 and 2004MACHINING TECHNOLOGY GROUP, LLC Unaudited Statement of Cash FlowsMACHINING TECHNOLOGY GROUP, LLC Notes to Unaudited Financial Statements As of September 30, 2005UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTSACCELLENT INC. Unaudited Pro Forma Condensed Combined Balance Sheet As of September 30, 2005 (In thousands)Accellent Inc. Notes to the Unaudited Pro Forma Condensed Combined Balance SheetACCELLENT INC. Unaudited Pro Forma Condensed Combined Statement of Operations For the Nine Months Ended September 30, 2004 Amounts in thousandsACCELLENT INC. Unaudited Pro Forma Condensed Combined Statement of Operations For the twelve months ended December 31, 2004 (In thousands)ACCELLENT INC. Unaudited Pro Forma Condensed Combined Statement of Operations For the nine months ended September 30, 2005 (In thousands)ACCELLENT INC. Notes to Unaudited Pro Forma Condensed Combined Statements of OperationsACCELLENT INC. SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS For the Three Years Ended December 31, 2004 (In thousands)PART II INFORMATION NOT REQUIRED IN THE PROSPECTUSSIGNATURESSIGNATURESSIGNATURESSIGNATURESSIGNATURESSIGNATURESSIGNATURESEXHIBIT INDEX