Exhibit 99.1
Can an Insomnia Therapy Treat Sleep Maintenance without Suppressing Arousability?
Effects of Doxepin 1 mg, 3 mg, and 6 mg across Phase 3 Trials
H. Heith Durrence, PhD, Philip Jochelson, MD, Roberta Rogowski, BSN
Somaxon Pharmaceuticals, Inc.
ABSTRACT
Introduction:This report reviews the relationship between sleep maintenance efficacy and arousability from three Phase 3 trials evaluating doxepin (DXP 1 mg, 3 mg, 6 mg), a selective H1 antagonist at the doses studied, in adult and elderly populations with either primary or transient insomnia.
Methods:Sleep maintenance endpoints from three double-blind placebo-controlled trials are reported. In two trials, subjects meeting DSM-IV-TR criteria for primary insomnia were randomized for up to 12 weeks of treatment. Study A was a 12-week trial of elderly subjects (N=240; DXP 1 mg and 3 mg versus placebo (PBO)); Study B was a 5-week trial of adult subjects (N=221; DXP 3 mg and 6 mg versus PBO). Study C was a single-night trial that used a model of transient insomnia to simulate sleep disturbance in healthy adults (N=565; DXP 6 mg versus PBO). Efficacy was evaluated with polysomnography. Sleep maintenance endpoints included wake after sleep onset (WASO) and number of awakenings (NAW). Data from the first and final night (N; Study A=N85; Study B=N29) of the study are reported.
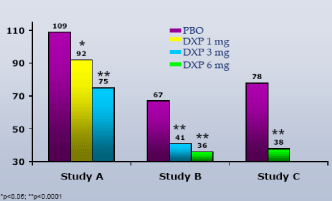
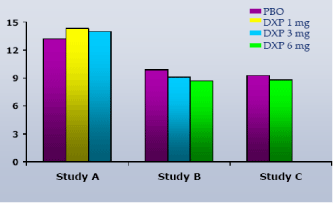
Results:DXP 1 mg (Study A; p<0.01), 3 mg (Study A and B; p<0.0001) and 6 mg (Study B and C; p<0.0001) significantly improved WASO on N1 of all three trials, with improvements vs. PBO ranging from 18 (Study A, 1 mg) to 39 minutes (Study C, 6 mg). These significant improvements were maintained at the final time point. There was no change in the NAW versus PBO at any dose or time point in any trial.
Conclusions:DXP 1 mg, 3 mg and 6 mg demonstrated significant improvement in WASO across three Phase 3 trials that was maintained at the final time point. Interestingly, sleep maintenance efficacy was not accompanied by reductions in NAW, a finding inconsistent with the existing literature involving GABA-mediated hypnotic medication. These pharmacodynamic differences may be attributed to histamine antagonism versus GABA mediation. These data suggest DXP is effective at treating sleep maintenance insomnia in both transient and chronic insomnia populations, and in adult and elderly populations. Additionally, these data suggest that DXP 1 mg, 3 mg and 6 mg may reduce time spent awake after nighttime arousals without suppressing arousability, though further evaluation is necessary.
INTRODUCTION AND METHODS
The most frequent complaint of patients with chronic insomnia is difficulty staying asleep, otherwise known as sleep maintenance. Data on the efficacy of sleep maintenance treatments are, however, relatively limited compared with sleep onset. The available data suggest that medications currently approved to treat insomnia do not appear to consistently maintain sleep. Additionally, in virtually all trials there was a strong positive relationship between the two primary sleep maintenance variables, WASO and NAW, with either both being significant or both not being significant.
DXP, a potent and selective H1 antagonist at doses of 1 mg, 3 mg, and 6 mg,1,2 has demonstrated improvement in sleep maintenance that persists throughout the night.3 This report reviews the sleep maintenance efficacy from three Phase 3 trials evaluating DXP (1 mg, 3 mg, 6 mg) in adult and elderly populations with either primary or transient insomnia.
Objective and Study Design
Study A was a randomized, double-blind, placebo-controlled, parallel-group, long-term (12 weeks) trial designed to assess the efficacy and safety of DXP 1 mg and 3 mg in an elderly population. Study B was a randomized, double-blind, placebo-controlled, parallel-group trial designed to assess the efficacy and safety of DXP 3 mg and 6 mg in adults across a 5-week treatment period. Study C was a single-night trial that used a model of transient insomnia to simulate sleep disturbance in healthy adults.
Subjects Subjects in Study A and B were required to have at least a 3-month history of primary insomnia according to DSM-IV-TR, and to self-report a certain level of sleep impairment; subjects meeting these requirements were additionally required to meet PSG sleep impairment criteria. Study C recruited healthy adults 25 to 55 years of age (inclusive), with no current or previous sleep problems.
Assessment of Efficacy and Safety In Study A, subjects underwent one night of 8-hour PSG recording at each study visit (N1, N15, N29, N57 and N85) during the double-blind (DB) period. In Study B, subjects had 2 nights of 8-hour PSG recording at each study visit (N1 and N2; N15 and N16; and N29 and N30) during the DB treatment period. In Study C, subjects underwent a single night of PSG assessment. Sleep maintenance endpoints included WASO and NAW. WASO and NAW were analyzed globally and in each of the 8 hours of PSG assessment. Data from N1 are reported for all trials, with N85 reported for Study A and N29 for Study B.
STATISTICS
Efficacy analyses were conducted using the Intent-To-Treat (ITT) analysis set, which in all trials included all randomized subjects who received at least one dose of double-blind study drug. Analysis of covariance (ANCOVA) methods were used to compare mean values for each PSG variable between PBO and the DXP group(s) in all studies, using a model that included main effects for treatment and study center with the baseline value as the covariate.
DEMOGRAPHICS AND DISPOSITION
In Study A, a total of 240 subjects met eligibility criteria and were randomized into the study (81 PBO, 77 DXP 1 mg, and 82 DXP 3 mg), with 214 subjects (89%) completing the study. In Study B, a total of 229 subjects met eligibility criteria and were randomized into the study, 221 of which were included in the ITT population (73 PBO, 75 DXP 3 mg, 73 DXP 6 mg), with 203 subjects (92%) completing the study. In Study C, a total of 565 randomized subjects (282 PBO, 283 DXP 6 mg) completed the study.
The mean age in Study A was 71.4 years and the study included more women (65%) than men (35%). Subjects were Caucasian (80%), African American (9%), Hispanic (9%), and Other (2%). In Study B, the mean age was 44.5 years and the study included more women (73%) than men (27%). Most patients were Caucasian (48%), followed by African American (33%), Hispanic (16%), Asian (1%), and Other (2%). In Study C, the mean age was 35.5 years and the study included more women (55%) than men (45%). Most of the patients were Caucasian (50%), followed by Hispanic (32%), African American (15%), Asian (1%), Native Hawaiian or other Pacific Islander (<1%), and Other (1%).
EFFICACY RESULTS
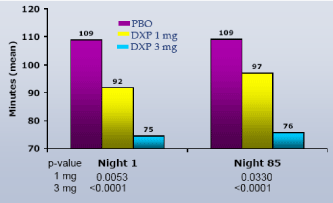
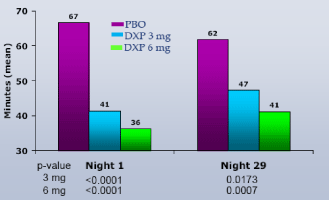
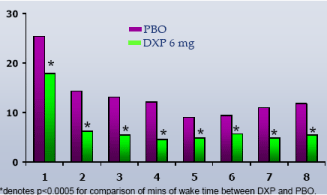
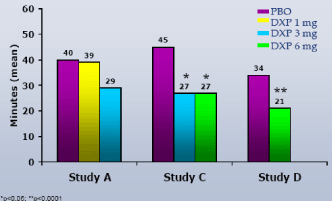
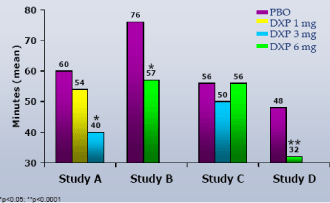
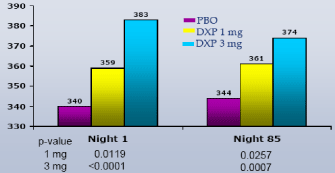
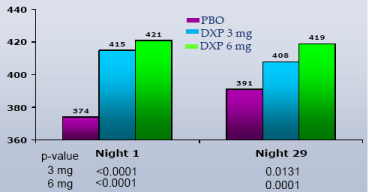
WASO.On N1, DXP 1 mg (Study A;Figure 1), 3 mg (Study A and B;Figures 1 and 2), and 6 mg (Study B and C;Figures 2 and 3) significantly improved 8-hour WASO in all three trials (Figure 3), with improvements versus PBO ranging from 18 (Study A, 1 mg) to 40 minutes (Study C, 6 mg). These significant improvements were all maintained at the final time point in both Study A and Study B (Figures 1 and 2).
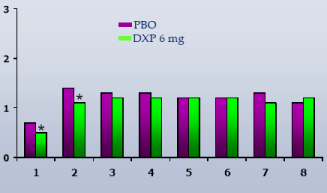
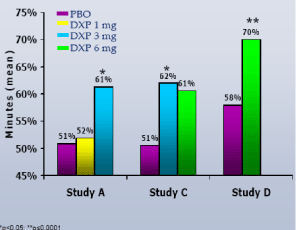
NAW.In contrast to WASO, overall NAW were no different versus PBO at any dose or time point in any trial (Figure 4).
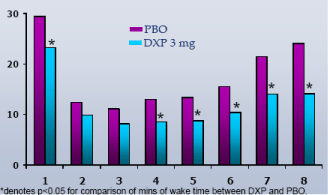
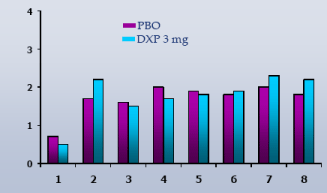
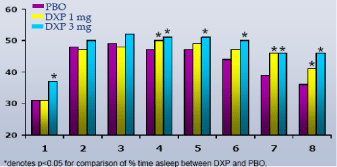
Wake Time and NAW by Hour.In Study A, DXP 3 mg significantly improved wake time by hour in the majority of hours on N1 (Figure 5(3 mg)); NAW were not significantly decreased at any hour of the night (Figure 6(3 mg)). In Study B, DXP 3 mg and 6 mg significantly improved wake time by hour in the majority of hours on N1; NAW were significantly less at only one time point for each dose (Hour 3 for DXP 3 mg; Hour 2 for DXP 6 mg) versus PBO. In Study C, DXP 6 mg significantly improved wake time in all 8 hours (p<0.0005) on N1 (Figure 7); NAW were significantly less at only two time points (Hours 1 and 2) versus PBO (Figure 8). A similar pattern of improvement in wake time with virtually no change in NAW was seen at the final night in both Study A and Study B.
Figure 1. WASO on N1 and N85 from Long-term Elderly Insomnia Trial (Study A)
Figure 2. WASO on N1 and N29 from Adult Insomnia Trial (Study B)
Figure 3. WASO on N1 across Phase 3 Trials
Figure 4. Number of Awakenings (NAW) on N1 across Phase 3 Trials
Figure 5. Wake Time by Hour on N1 from Long-term Elderly Insomnia Trial (Study A); DXP 3 mg vs PBO
Figure 6. NAW by Hour on N1 from Long-term Elderly Insomnia Trial (Study A); DXP 3 mg vs PBO
Figure 7. Wake Time by Hr on N1 from Transient Insomnia Trial (Study C)
Figure 8. NAW by Hour on N1 from Transient Insomnia Trial (Study C)
RESULTS SUMMARY
In these three Phase 3 trials:
| • | | DXP 1 mg, 3 mg, and 6 mg significantly improved sleep maintenance, as evidence by significantly reduced WASO and wake time by hour |
| | – | | WASO improvements occurred globally, and wake time improvements occurred in most hours of the night for DXP 3 mg and 6 mg |
| |
| | – | | The consistent and robust improvements in WASO were maintained at final time point |
| |
| | – | | Improvements began in the 1st hour of the night for highest doses, with strongest effect generally in the last half of the night |
| • | | DXP 1 mg, 3 mg, and 6 mg did not significantly reduce NAW, either globally or by hour |
| | – | | Improvement in WASO not due to reducing the frequency of awakenings |
| • | | Pattern of WASO improvement without an accompanying decrease in awakenings was consistent both globally and by hour |
CONCLUSIONS
In all of the Phase 3 trials, DXP 1 mg, 3 mg and 6 mg demonstrated significant improvement in WASO globally, and DXP 3 mg and 6 mg demonstrated improvement in wake time by hour at most hours of the night; these improvements were maintained at the final time point in the 5- and 12-week trials. Interestingly, sleep maintenance efficacy was not accompanied by reductions in number of awakenings (NAW), a finding inconsistent with the existing literature involving GABA-mediated hypnotic medication. These data suggest DXP is effective at treating sleep maintenance insomnia in both transient and chronic insomnia populations, and in adult and elderly populations, with the strongest effect in the last half of the night. Additionally, these data suggest that DXP 1 mg, 3 mg and 6 mg may reduce time spent awake after nighttime arousals without suppressing arousability, though further evaluation is necessary.
The effect on sleep maintenance without a change in arousability suggests that at the doses of DXP studied, patients may be able to wake up naturally during the night if necessary, while simultaneously preventing any such arousal from lasting an extended period of time. The apparent lack of effect on arousability in the presence of sleep maintenance efficacy is consistent with the hypothesis of histamine selectivity for these doses of DXP, the likely mechanism of action for the sleep effects. In contrast to the majority of agents used in practice that act via the GABA receptor complex, low-dose DXP does not globally suppress wake-promoting systems, but appears to selectively block one of a number of parallel wake promoting systems, thus avoiding the potential negative effects associated with non-arousability.
REFERENCES
| 1. | | Richelson E. Pharmacology of Antidepressants. Mayo Clin Proc 2001;76:511-527 |
| |
| 2. | | Somaxon Pharmaceuticals Inc. In Vitro Pharmacological Profile of Doxepin... Data on file |
| |
| 3. | | Roth T, Rogowski R, Hull S, et al. Efficacy and Safety of Doxepin 1, 3 and 6 mg...Sleep 2007;30: 1555-1561 |
Supported by funding from Somaxon Pharmaceuticals, Inc.
Night 1/Week 1 Effects of Doxepin 1 mg, 3 mg, and 6 mg on Sleep Onset Across Phase 3 Trials of Transient and Chronic Insomnia
Roberta Rogowski, BSN, Philip Jochelson, MD, H. Heith Durrence, PhD
Somaxon Pharmaceuticals, Inc.
ABSTRACT
Introduction:This report reviews sleep onset efficacy from four Phase 3 trials evaluating doxepin (DXP 1 mg, 3 mg, 6 mg), a selective H1 antagonist at the doses studied, in adult and elderly patient populations with either primary or transient insomnia.
Methods:Sleep onset endpoints from four randomized, double-blind, placebo-controlled trials are reported. In three trials, patients meeting DSM-IV-TR criteria for primary insomnia were randomized for up to 12 weeks of treatment. Study A was a 12-week polysomnography (PSG) trial of elderly patients (N=240; DXP 1 mg and 3 mg versus placebo (PBO)); Study B was a 4-week outpatient trial also with elderly patients (N=254; DXP 6 mg versus PBO); Study C was a 5-week PSG trial of adult patients (N=221; DXP 3 mg and 6 mg versus PBO). The fourth trial (Study D) used a model of transient insomnia to simulate sleep onset disturbance in healthy adults (N=565; DXP 6 mg versus PBO). Efficacy was evaluated with PSG and patient reports. Endpoints of sleep onset included sleep efficiency (SE) in Hour 1 (Studies A, C and D), latency to persistent sleep (LPS; Studies A, C and D), and patient-reported latency to sleep onset (LSO; Studies A, B, C and D). Data from the first assessment point are reported; this corresponds to Night (N) 1 for all LPS measurements; Week 1 for LSO in Study A; N1 for LSO in Study B.
Results:In Study A, DXP 3 mg significantly improved SE in Hour 1 and LSO. In Study B, DXP 6 mg significantly improved LSO. In Study C, DXP 3 mg significantly improved SE in Hour 1 and LPS; DXP 6 mg significantly improved LPS. In Study D, DXP 6 mg significantly improved all three onset variables.
Conclusion:DXP 3 mg and 6 mg significantly improved the majority of objective and subjective sleep onset parameters across four Phase 3 trials on Night One/Week 1. These data suggest that DXP 3 and 6 mg can be effective at treating insomnia characterized by sleep onset difficulty in both transient and chronic insomnia populations, in both adult and elderly patient populations on the first assessment.
INTRODUCTION AND METHODS
DXP, a potent and selective H1 antagonist at doses of 1 mg, 3 mg, and 6 mg,1,2 has demonstrated improvement in sleep maintenance that persists throughout the night.3 Though neither of those Phase 2 trials were powered or enriched for sleep onset difficulties, there was a trend towards efficacy in these variables. This report reviews the Night 1/Week 1 sleep onset efficacy from four Phase 3 trials evaluating DXP (1 mg, 3 mg, 6 mg) in adult and elderly patient populations with either primary or transient insomnia.
Objective and Study Design
Study A was a randomized, double-blind, placebo-controlled, parallel-group, long-term (12 weeks) trial designed to assess the efficacy and safety of DXP 1 mg and 3 mg in an elderly population. Study B was a randomized, double-blind, placebo-controlled, parallel-group, outpatient trial designed to assess the efficacy and safety of DXP 6 mg across a 4-week treatment period in an elderly population. Study C was a randomized, double-blind, placebo-controlled, parallel-group trial designed to assess the efficacy and safety of DXP 3 mg and 6 mg in adults across a 5-week treatment period. Study D was a single-night trial that used a model of transient insomnia to simulate sleep disturbance in healthy adults.
Subjects Subjects in Study A , B and C were required to have at least a 3-month history of primary sleep maintenance insomnia according to DSM-IV-TR, and to self-report a certain level of sleep impairment; subjects meeting these requirements were additionally required to meet PSG sleep impairment criteria in Study A and C. Study D recruited healthy adults 25 to 55 years of age (inclusive), with no current or previous sleep problems.
Assessment of Efficacy and Safety In Study A, subjects underwent one night of 8-hour PSG recording at each study visit during the double-blind (DB) period. In Study B, subjects made weekly visits to the study center during the DB treatment period. In Study C, subjects had 2 nights of 8-hour PSG recording at each study visit during the DB treatment period. In Study D, subjects underwent a single night of PSG assessment. Sleep onset endpoints included LPS, LSO, and SE in Hour 1. Data from the first assessment point are reported; this corresponds to night 1 for all LPS measurements and SE in Hour 1; week 1 for LSO in Study A; and night 1 for LSO in Study B. Data from N1 for LPS and LSO are additionally integrated across all chronic insomnia Phase 3 trials for which such endpoints were measured.
STATISTICS
Efficacy analyses were conducted using the Intent-To-Treat (ITT) analysis set, which in all trials included all randomized subjects who received at least one dose of double-blind study drug. Analysis of covariance (ANCOVA) methods were used to compare mean values for each variable between PBO and the relevant DXP group(s) in all studies, using a model that included main effects for treatment and study center with the baseline value as the covariate. LSO data were log-transformed for all studies, while LPS data were log-transformed for all except Study D.
DEMOGRAPHICS AND DISPOSITION
In Study A, a total of 240 subjects met eligibility criteria and were randomized into the study (81 PBO, 77 DXP 1 mg, and 82 DXP 3 mg), with 214 subjects (89%) completing the study. In Study B, a total of 254 subjects met eligibility criteria and were randomized into the study (124 PBO, 130 DXP 6 mg), with 237 subjects (93%) completing the study. In Study C, a total of 229 subjects met eligibility criteria and were randomized into the study, 221 of which were included in the ITT population (73 PBO, 75 DXP 3 mg, 73 DXP 6 mg), with 203 subjects (92%) completing the study. In Study D, a total of 565 randomized subjects (282 PBO, 283 DXP 6 mg) completed the study.
The mean age in Study A was 71.4 years and the study included more women (65%) than men (35%). Subjects were Caucasian (80%), African American (9%), Hispanic (9%), and Other (2%). In Study B, the mean age was 72.5 years and the study included more women (65%) than men (35%). Subjects were Caucasian (87%), African American (7%), Hispanic (3%), and Other (3%). In Study C, the mean age was 44.5 years and the study included more women (73%) than men (27%). Most patients were Caucasian (48%), followed by African American (33%), Hispanic (16%), Asian (1%), and Other (2%). In Study D, the mean age was 35.5 years and the study included more women (55%) than men (45%). Most of the patients were Caucasian (50%), followed by Hispanic (32%), African American (15%), Asian (1%), Native Hawaiian or other Pacific Islander (<1%), and Other (1%).
EFFICACY RESULTS
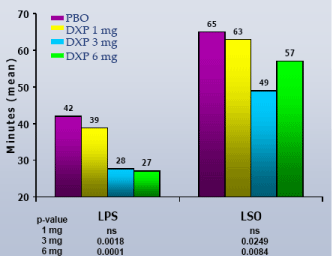
LPS on N1.LPS was significantly improved in Study C (DXP 3 mg and 6 mg;Figure 1) and Study D (6 mg;Figure 1). LPS was not assessed in Study B, and was not significantly improved for either dose in Study A.
LSO on N1/ Week 1.LSO was significantly improved in Study A (DXP 3 mg only;Figure 2), Study B (6 mg;Figure 2), and Study D (6 mg;Figure 2). LSO was not significantly improved for either dose in Study C (Figure 2).
SE % in Hour 1 on N1.SE % in Hour 1 was significantly improved in Study A (DXP 3 mg only;Figure 3), Study C (DXP 3 mg only;Figure 3) and Study D (6 mg;Figure 3). SE % in Hour 1 was not assessed in Study B.
Integrated LPS and LSO.DXP 3 mg and 6 mg significantly improved LPS (Figure 4) and LSO (Figure 4) in the integrated Phase 3 dataset. DXP 1 mg was not significantly improved for either variable.
Figure 1. LPS on N1 from all Phase 3 PSG Trials
Figure 2. LSO on N1/Week 1 from all Phase 3 Trials
Figure 3. SE % in Hour 1 on N1 from all Phase 3 PSG Trials
Figure 4. LPS and LSO from Phase 3 Integrated Dataset
RESULTS SUMMARY
In these four Phase 3 trials:
| • | | DXP 3 mg and 6 mg significantly improved sleep onset compared with PBO in the majority of parameters at the first assessment |
| |
| • | | Strongest improvements appeared to be to SE % in Hour 1 |
CONCLUSIONS
DXP 3 mg and 6 mg significantly improved the majority of objective and subjective sleep onset parameters as assessed on Night 1/Week 1 across four Phase 3 trials. Statistical significance was generally not observed at later time points in these clinical trials. These results are noteworthy considering that the chronic insomnia trials (Studies A, B, and C) were neither powered nor enriched for sleep onset difficulties. These data suggest that DXP 3 and 6 mg can be effective at treating insomnia characterized by sleep onset difficulty in both transient and chronic insomnia populations, in both adult and elderly patient populations.
Historically in the field of sleep medicine, the objective variable typically examined to determine sleep onset efficacy was LPS. This approach makes intuitive sense when examining agents acting via the GABA receptor complex. These drugs work via global suppression of wake promoting systems, and thus a discrete cutoff for determining the first occurrence of PSG sleep seems warranted given the rapid transition from wake to sleep. With agents like low-dose DXP, however, this approach does not seem as appropriate. DXP, a potent and selective H1 antagonist, appears to work at these doses by selectively blocking one of a number of parallel wake promoting systems as opposed to global suppression. With this type of agent it may be equally important to view the transition from wake to sleep as more of a dynamic process, suggesting that variables like SE % in Hour 1 may also be useful in examining sleep onset efficacy.
REFERENCES
| 1. | | Richelson E. Pharmacology of Antidepressants. Mayo Clin Proc 2001;76:511-527 |
| |
| 2. | | Somaxon Pharmaceuticals Inc. In Vitro Pharmacological Profile of Doxepin... Data on file |
| |
| 3. | | Roth T, Rogowski R, Hull S, et al. Efficacy and Safety of Doxepin 1, 3 and 6 mg...Sleep 2007;30: 1555-1561 |
Supported by funding from Somaxon Pharmaceuticals, Inc.
Efficacy of Doxepin 1 mg and 3 mg on Early Morning Awakenings in Elderly Adults with Primary Insomnia
Philip Jochelson, MD,1 Elizabeth Ludington, PhD,1 H. Heith Durrence, PhD,1 Roberta Rogowski, BSN,1 Thomas Roth, PhD2
1Somaxon Pharmaceuticals, Inc.,2Henry Ford Sleep Disorders Center
ABSTRACT
Introduction:Early morning awakenings, waking too early and being unable to fall back to sleep, is often a key symptom of many with chronic insomnia, with a disproportionate number of elderly adults suffering from it. Though it is a core symptom of DSM-IV diagnosed insomnia, it is seldom addressed in clinical trials examining medication effects on sleep parameters. The present analysis examined the impact of doxepin (DXP 1 mg, 3 mg), a selective H1 antagonist at the doses studied, on EMA in an elderly population with primary insomnia.
Methods:Selected endpoints from a randomized, double-blind, placebo-controlled study of elderly adults with DSM-IV-TR defined primary insomnia are reported. Subjects were randomized to 12 weeks of DXP 1 mg (N=77), 3 mg (N=82), or placebo (PBO; N=81). Efficacy was evaluated with polysomnography (PSG) data from the first and last time points of the study, Nights 1 (N1) and 85 (N85). PSG endpoints of early morning awakenings included sleep efficiency (SE) in the last third-of-the-night (SE-LTN), SE last quarter-of-the-night (SE-LQN), and SE in Hours 7 and 8. Next-day residual effects were assessed using the Digit Symbol Substitution Test (DSST), Symbol Copying Test (SCT), and a Visual Analog Scale (VAS) for sleepiness.
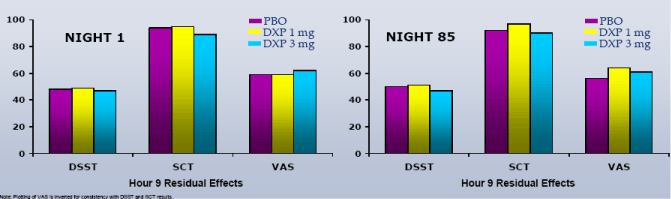
Results:On N1, DXP 1 mg and 3 mg significantly improved SE-LTN (p≤0.0007), SE-LQN (p≤0.0011) and SE in hours 7 (p≤0.0028) and 8 (p≤0.0211), when respectively compared with PBO. These improvements were generally sustained at N85, with significance versus PBO maintained for 3 mg on all parameters except SE in Hour 8 (p=0.06). There were no significant group differences in the DSST, SCT, or VAS at any time point during the trial.
Conclusions:In elderly adults with chronic insomnia, DXP 1 mg and 3 mg significantly improved PSG parameters associated with early morning awakenings, a prevalent, bothersome, but neglected symptom. These improvements were sustained through the final hour of the night with no observed next-day residual effects. These data suggest that DXP 1 mg and 3 mg are effective at treating early morning awakenings without causing next-day residual effects.
INTRODUCTION AND METHODS
Chronic insomnia often is accompanied by waking too early and being unable to return to sleep. Though early morning awakenings are a core symptom of DSM-IV insomnia and occur frequently in the elderly (~25% prevalence in elderly adults),1 it is seldom addressed in sleep trials. This dearth of research on a frequent complaint is problematic, particularly given that difficulty with early morning awakenings is a pervasive problem across all age groups, and not only the elderly.1
DXP, a potent and selective H1 antagonist at doses of 1 mg, 3 mg, and 6 mg,2,3 has demonstrated significant efficacy in preventing early morning awakenings at these low doses.4 The present analyses examined the efficacy of DXP 1 mg and 3 mg on parameters associated with early morning awakenings in elderly adults with chronic insomnia.
Objective and Study Design
This was a randomized, double-blind, placebo-controlled, parallel-group study designed to assess the efficacy and safety of two dose levels of DXP, 1 mg and 3 mg, in elderly adults with chronic primary insomnia.
Subjects Subjects were ≥65 years of age with at least a 3-month history of primary insomnia, who self-reported experiencing ≥60 minutes of wake time after sleep onset, ≥30 minutes of latency to sleep onset, and ≤6.5 hours of total sleep time (TST) on at least 4 of 7 consecutive nights prior to PSG screening. PSG screening criteria included a mean wake-time-during-sleep of ≥60 minutes on both PSG screening nights, with neither night <45 minutes, a TST >240 minutes and ≤390 minutes on both nights, and an LPS >10 minutes on both nights.
Assessment of Efficacy and Safety At each study visit during the DB period, subjects underwent one night of 8-hour PSG recording (N1, N15, N29, N57, and N85). Selected efficacy and safety parameters from the first and last time points in this study that may be associated with early morning awakenings are reported. PSG variables associated with early morning awakenings included SE-LTN, SE-LQN, and SE in Hours 7 and 8. Safety assessments included vital signs, physical examinations, neurological assessment, 12-lead electrocardiograms, clinical laboratory assessments, and assessments of adverse events (AEs). Because efficacy in preventing early morning awakenings by definition occurs predominantly at the end of the night, next-day residual effects are presented. These assessments included the DSST, SCT, and a VAS scale assessing sleepiness. These residual effects were assessed the following morning, approximately 1 hour after the end of PSG recording (Hour 9).
STATISTICS
Efficacy analyses were conducted using the Intent-To-Treat analysis set; this set included all randomized subjects who received at least one dose of DB study drug. Analysis of covariance (ANCOVA) methods were used to compare mean values for each PSG variable between PBO, DXP 1 mg, or DXP 3 mg, using a model that included main effects for treatment and study center with the baseline value as the covariate. For the DSST, SCT, and VAS measures, the mean changes from pre-dose in the sleep laboratory (N1 and N85) to the following morning (Day 2 and Day 86) were compared among treatments using an ANCOVA model that included main effects for treatment and study center with the pre-dose value as a covariate.
DEMOGRAPHICS AND DISPOSITION
Of subjects screened for participation, 240 met all entry criteria and were randomized to DXP 1 mg (n=77), DXP 3 mg (n=82), or PBO (n=81), with 214 (89%) completing the study. Early discontinuation rates (Table 1) and baseline characteristics were comparable across treatment groups. The mean age of subjects was 71.4 years and the study included more women (65%) than men (35%). Subjects were Caucasian (80%), African American (9%), Hispanic (9%), and Other (2%).
Table 1. Summary of Subject Disposition with Key Reasons for Withdrawal
| | | | | | | | | | | | | |
| Disposition | | Placebo | | DXP 1 mg | | DXP 3 mg |
| (Overall N=240) | | (N=81) | | (N=77) | | (N=82) |
| | | | | | | | | | | | | |
Completed study | | | 86 | % | | | 91 | % | | | 90 | % |
| |
| | | | | | | | | | | | | |
Discontinued from study | | | 14 | % | | | 9 | % | | | 10 | % |
| |
| | | | | | | | | | | | | |
Adverse Event | | | 4 | % | | | 1 | % | | | 4 | % |
| |
| | | | | | | | | | | | | |
Consent Withdrawn | | | 7 | % | | | 0 | % | | | 2 | % |
| |
| | | | | | | | | | | | | |
Other | | | 2 | % | | | 8 | % | | | 4 | % |
| |
EFFICACY RESULTS
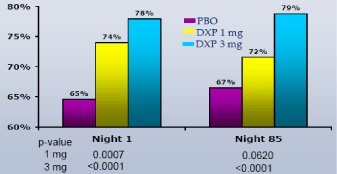
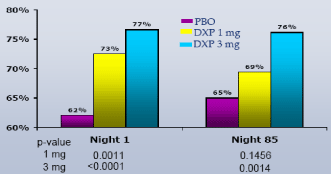
On N1, DXP 1 mg and 3 mg significantly improved SE-LTN (p≤0.0007; Figure 1) and SE-LQN (p≤0.0011; Figure 2), and DXP 3 mg significantly improved SE in hours 7 (p≤0.0028; Figure 3 (3 mg)) and 8 (p≤0.0211; Figure 3 (3 mg)), in each case compared with PBO. These improvements were generally sustained at N85 (Figures 1, 2 and 4), with significance versus PBO maintained for 3 mg on all parameters except SE in hour 8 (p=0.06).
Figure 1. SE Last Third-of-the-night on N1 and N85
Figure 2. SE Last Quarter-of-the-night on N1 and N85
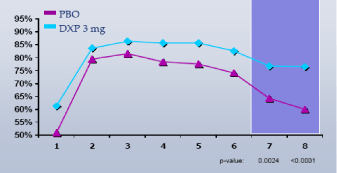
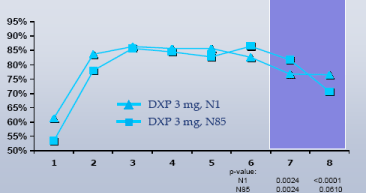
Figure 3. Sleep Efficiency % by Hour on Night 1
Figure 4. Sleep Efficiency % by Hour on Night 1 and Night 85; DXP 3 mg only
SAFETY
Rates of treatment-emergent AEs were lower in subjects treated with DXP compared with placebo, with 52% of subjects in the placebo group, 40% of subjects in the DXP 1 mg group, and 38% of subjects in the DXP 3 mg group experiencing an AE. The most common AEs were headache (PBO 14%; DXP 1 mg 3%; DXP 3 mg 6%) and somnolence (PBO 5%; DXP 1 mg 5%; DXP 3 mg 2%). There were no reports of complex sleep behaviors, memory impairment or cognitive disorders in any doxepin-treated subject. There were no clinically relevant changes in laboratory parameters, vital signs, physical examinations, or ECGs.
In terms of next-day residual effects, there were no significant group differences in the DSST, SCT, or VAS at any time point during the trial, including N1 and N85 (Figure 5).
Figure 5. Summary of Next-day Residual Effects on N1 and N85
RESULTS SUMMARY
In this study:
| • | | DXP 1 mg and 3 mg demonstrated efficacy in preventing early morning awakenings as measured by SE in the last third- and last quarter-of-the-night, and SE in Hours 7 and 8 |
| | – | | Improvements were generally sustained at N85, with significance maintained for 3 mg on all except SE in Hour 8 (p=0.06) |
| • | | The efficacy in preventing early morning awakenings was not accompanied by residual effects the following morning at Hour 9 |
| |
| • | | DXP 1 mg and 3 mg were well-tolerated, with an overall incidence of AEs lower than PBO in this long-term trial |
| | – | | Discontinuation rates were low considering the duration of the trial, with lower rates overall in the two DXP groups vs PBO |
| • | | No reports of complex sleep behaviors, memory impairment, or anticholinergic effects, and no evidence of weight gain |
CONCLUSIONS
In this study of elderly adults with chronic primary insomnia, administration of DXP 1 mg and 3 mg resulted in significant improvements on PSG parameters associated with early morning awakenings. The efficacy in preventing EMA in this study is notable, given that this component of chronic primary insomnia is prevalent but seldom addressed. These sleep improvements were sustained through the final hour with no evidence of next-day residual effects.
The absence of residual effects at Hour 9 combined with efficacy through Hour 8 is an intriguing finding. In light of the evidence of the endogenous opponent processes that are theorized to influence the sleep-wake cycle,5 the results of this study suggest that the efficacy of DXP at these doses may be due to the effects that the selectivity and potency of DXP for H1 have on such processes. Both doses were well-tolerated, with no reports of complex sleep behaviors, amnesia or anticholinergic effects. These data suggest that DXP 1 mg and 3 mg reduce the duration of and may prevent early morning awakenings, a novel finding in the insomnia treatment literature for a drug with no observed next-day residual effects.
REFERENCES
| 1. | | National Sleep Foundation. 2005 Sleep in America Poll |
| |
| 2. | | Richelson E. Pharmacology of Antidepressants. Mayo Clin Proc 2001;76:511-527 |
| |
| 3. | | Somaxon Pharmaceuticals Inc. In Vitro Pharmacological Profile of Doxepin... Data on file |
| |
| 4. | | Roth T, Rogowski R, Hull S, et al. Efficacy and Safety of Doxepin 1, 3 and 6 mg...Sleep 2007;30: 1555-1561 |
| |
| 5. | | Edgar D, Dement W, Fuller C. Effect of SCN lesions...Evidence for opponent processes in sleep-wake regulation. J Neurosci 1993;13:1065-79 |
Supported by funding from Somaxon Pharmaceuticals, Inc.
Effects of Doxepin 1 mg, 3 mg, and 6 mg on Sleep Efficiency by Hour
from Two Long-term Trials of Chronic Insomnia
Alan Lankford, PhD,1 H. Heith Durrence, PhD,2 Philip Jochelson, MD,2 Roberta Rogowski, BSN2
1Sleep Disorders Center of Georgia,2Somaxon Pharmaceuticals, Inc.
ABSTRACT
Introduction:Currently approved insomnia medications that act as GABA modulators have not generally demonstrated efficacy lasting into the final hours of the night without significant next-day residual effects. This report reviews time spent asleep by hour and residual effects data from two long-term polysomnography (PSG) trials evaluating doxepin (DXP), a selective H1 antagonist at the doses studied (1 mg, 3 mg, 6 mg), for the treatment of insomnia.
Methods:Time asleep was evaluated in two double-blind, placebo-controlled trials; a 12-week trial of elderly patients (Study A; N=240; DXP 1 and 3 mg vs. placebo (PBO)) and a 5-week trial of adult patients (Study B; N=221; DXP 3 mg and 6 mg vs PBO). Total sleep time (TST) was analyzed globally, in each of the 8 hours of PSG assessment, and in the final third- and quarter-of-the-night. Next-day residual effects were assessed at Hour 9 using the Digit Symbol Substitution Test (DSST) and the Symbol Copying Test (SCT). Data from the first (Night 1) and the final assessment time point are reported.
Results:DXP 1 mg (Study A), 3 mg (Study A and B) and 6 mg (Study B) significantly improved overall TST on Night 1 (N1) in both trials compared with PBO. Overall TST was also significantly improved on the final assessment night (Study A, N85; Study B, N29) in both trials. Significant improvements in the % of time asleep in the final third- and quarter-of-the-night, in the final hour, and in the majority of other hours across the night were also observed. In terms of next-day residual effects, there were no significant differences in the DSST or SCT at any dose in either trial.
Conclusions:In adult and elderly patients with chronic insomnia, DXP 1 mg, 3 mg and 6 mg significantly improved the % of time asleep both globally and at most hours throughout the night, with the strongest effect in the last part of the night. Importantly, though low-dose DXP increased the amount of time asleep through the final hour of assessment (Hour 8), efficacy was not accompanied by evidence of next-day residual sedation at Hour 9. These data suggest histamine may be an integral part of a gating mechanism in the arousal system that allows transition from sleep to wake without residual sedation.
INTRODUCTION AND METHODS
Chronic insomnia is characterized by difficulty getting to sleep, staying asleep, and waking too early and being unable to return to sleep. Though insomnia is clearly a disorder that persists across the entire night for many people, none of the currently available insomnia treatments have demonstrated the ability to work throughout the night without significant next-day residual effects.
DXP, a potent and selective H1 antagonist at doses of 1 mg, 3 mg, and 6 mg,1,2 has demonstrated improvement in sleep that persists throughout the night.3 This report reviews time spent asleep by hour and residual effects data from two long-term polysomnography (PSG) trials evaluating DXP 1 mg, 3 mg, and 6 mg for the treatment of insomnia.
Objective and Study DesignStudy A was a randomized, double-blind, placebo-controlled, parallel-group, long-term (12 weeks) trial designed to assess the efficacy and safety of DXP 1 mg and 3 mg using polysomnography. Study B was a randomized, double-blind, placebo-controlled, parallel-group, trial designed to assess the efficacy and safety of DXP 3 mg and 6 mg across a 5-week treatment period. Both trials recruited subjects with chronic primary insomnia, with Study A including elderly adults ≥65 years old and Study B including adults 18-64 years old.
Subjects:Subjects were required to have at least a 3-month history of primary insomnia according to DSM-IV-TR. Both trials required subjects to self-report a certain degree of sleep impairment; subjects meeting these requirements were additionally required to meet PSG sleep impairment criteria.
Assessment of Efficacy and Safety:In Study A, subjects underwent one night of 8-hour PSG recording at each study visit (N1, N15, N29, N57 and N85) during the double-blind (DB) period. In Study B, subjects had 2 nights of 8-hour PSG recording at each study visit (N1 and N2; N15 and N16; and N29 and N30) during the DB treatment period. Data from N1 are reported for both trials, with N85 reported for Study A and N29 for Study B. PSG-determined TST was analyzed globally, in the final third- and quarter-of-the-night, and in each of the 8 hours of PSG assessment. Data averaged across the DB period (N1, N15, N29, N57 and N85) are reported for some of the variables from the long-term trial (Study A). Safety parameters included vital signs, physical examinations, neurological assessments, 12-lead electrocardiograms (ECGs), clinical laboratory data, and evaluation of adverse events (AEs). Next-day residual effects were assessed in both studies using the Digit Symbol Substitution Test (DSST), the Symbol Copying Test (SCT), and a Visual Analog Scale (VAS) assessing sleepiness.
STATISTICS
Efficacy analyses were conducted using the Intent-To-Treat (ITT) analysis set, which in both trials included all randomized subjects who received at least one dose of double-blind (DB) study drug. Analysis of covariance (ANCOVA) methods were used to compare mean values for each PSG variable between PBO and the two DXP groups in both studies, using a model that included main effects for treatment and study center with the baseline value as the covariate. For the DSST, SCT, and VAS measures, the mean changes from pre-dose in the sleep laboratory to the following morning were compared among treatments using an ANCOVA model that included main effects for treatment and study center with the pre-dose value as a covariate.
DEMOGRAPHICS AND DISPOSITION
In Study A, a total of 240 subjects met eligibility criteria and were randomized into the study (81 PBO, 77 DXP 1 mg, and 82 DXP 3 mg), with 214 subjects (89%) completing the study. In Study B, a total of 229 subjects met eligibility criteria and were randomized into the study, 221 of which were included in the ITT population (73 PBO, 75 DXP 3 mg, 73 DXP 6 mg), with 203 subjects (92%) completing the study. Baseline characteristics and early termination rates were comparable in both studies, with lower overall discontinuation rates for the DXP groups versus PBO. The mean age in Study A was 71.4 years and the study included more women (65%) than men (35%). Subjects were Caucasian (80%), African American (9%), Hispanic (9%), and Other (2%). In Study B, the mean age was 44.5 years and the study included more women (73%) than men (27%). Most patients were Caucasian (48%), followed by African American (33%), Hispanic (16%), Asian (1%), and Other (2%).
EFFICACY RESULTS
Overall TST.On N1, DXP 1 mg (Study A;Figure 1), 3 mg (Study A and B;Figures 1 and 2), and 6 mg (Study B;Figure 2) significantly improved TST in both trials compared with PBO. TST was also significantly improved on the final assessment night (Study A, N85; Study B, N29) in both trials.
Sleep Time Final Third and Quarter.There were significant improvements in the % of time asleep in the final third- and quarter-of-the-night on N1 of both studies and at all doses (p<0.05).These improvements were sustained at N85 (Study A) and N29 (Study B), with significance versus PBO maintained for the highest dose in both trials.
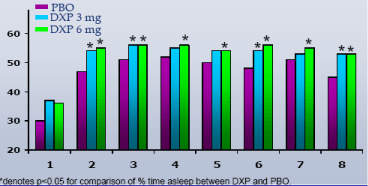
Sleep Time by Hour.DXP 1 mg (Study A;Figure 3), 3 mg (Study A and B;Figures 3 and 4), and 6 mg (Study B;Figure 4) significantly improved % of time asleep in the final hour, and in the majority of other hours across the night on N1. These improvements were sustained for most hours across the night at N85 (Study A) and N29 (Study B).
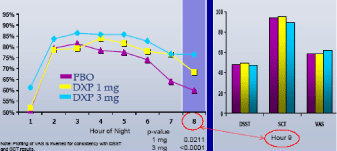
Next-day Residual Effects.The significant efficacy in the final hour (Hour 8) of both trials (Figures 5 and 6) was not accompanied by next-day residual sedation at Hour 9 in either Study A (Figure 5) or Study B (Figure 6). There were no significant group differences in the DSST, SCT, or VAS at any time point during either trial.
Figure 1. TST on N1 and N85 from Long-term Trial (Study A)
Figure 2. TST on N1 and N29 (Study B)
Figure 3. Sleep Time by Hour on Night 1 from Long-term Trial (Study A)
Figure 4. Sleep Time by Hour on Night 1 from Adult Insomnia Trial (Study B)
Figure 5. Sleep Efficiency % by Hour on Night 1 from Long-term Trial with Next-day Residual Effects at Hour 9 (Study A)
Figure 6. Sleep Efficiency % by Hour on Night 1 with Next-day Residual Effects at Hour 9 (Study B)
SAFETY
Rates of treatment-emergent AEs were comparable across groups in both Study A and Study B (Table 1). There were no clinically relevant changes in laboratory parameters, vital signs, physical examinations, or ECGs in either trial. There were no reports of complex sleep behaviors, memory impairment or cognitive disorders in either trial. There was also no evidence of weight gain in either trial.
Table 3. Summary of Adverse Events in Study A and Study B
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 12-WEEK ELDERLY TRIAL (STUDY A) | | 5-WEEK ADULT TRIAL (STUDY B) |
| | | PBO | | DXP 1 mg | | DXP 3 mg | | PBO | | DXP 3 mg | | DXP 6 mg |
| |
OVERALL | | | 52 | % | | | 40 | % | | | 38 | % | | | 27 | % | | | 35 | % | | | 32 | % |
Central Nervous System | | | 20 | % | | | 8 | % | | | 11 | % | | | 14 | % | | | 12 | % | | | 12 | % |
Headache | | | 14 | % | | | 3 | % | | | 6 | % | | | 10 | % | | | 5 | % | | | 0 | % |
Somnolence/Sedation | | | 5 | % | | | 5 | % | | | 3 | % | | | 5 | % | | | 9 | % | | | 8 | % |
Dizziness | | | 2 | % | | | 0 | % | | | 2 | % | | | 1 | % | | | 0 | % | | | 0 | % |
Memory Impairment | | | 1 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
Gastrointestinal | | | 12 | % | | | 5 | % | | | 6 | % | | | 4 | % | | | 4 | % | | | 8 | % |
Respiratory | | | 6 | % | | | 3 | % | | | 5 | % | | | 3 | % | | | 1 | % | | | 1 | % |
Psychiatric | | | 1 | % | | | 1 | % | | | 2 | % | | | 1 | % | | | 1 | % | | | 3 | % |
Other Events of Interest | | | | | | | | | | | | | | | | | | | | | | | | |
Dry Mouth | | | 2 | % | | | 1 | % | | | 2 | % | | | 0 | % | | | 0 | % | | | 0 | % |
| |
Note: These data are derived from the safety analysis set, which is defined as all randomized subjects who received study drug.
RESULTS SUMMARY
In these two trials:
| • | | DXP 1 mg, 3 mg, and 6 mg demonstrated efficacy in increasing time asleep both globally and at most hours throughout the night |
| | – | | Strongest effect was in the last half of the night, including the final third-, quarter-, and hour-of-the-night |
| |
| | – | | Improvements began on Night 1 and were generally sustained at final time point, with significance maintained for most parameters |
| • | | Increased sleep time through the final hour (Hour 8) was not accompanied by residual effects the following morning (Hour 9) |
| |
| • | | DXP 1 mg, 3 mg and 6 mg were well-tolerated, with an overall incidence of AEs lower than PBO in the long-term elderly trial (Study A) |
| | – | | No evidence of complex sleep behaviors, memory impairment, or anticholinergic effects, and no evidence of weight gain |
CONCLUSIONS
In these studies of adult and elderly patients with chronic insomnia, administration of DXP 1 mg, 3 mg, and 6 mg significantly improved the % of time asleep both globally and at most hours throughout the night, with the strongest effect in the last part of the night. Importantly, though low-dose DXP increased the amount of time asleep through the final hour of assessment (Hour 8), efficacy was not accompanied by evidence of next-day residual sedation at Hour 9. All doses were well-tolerated, with no reports of complex sleep behaviors, amnesia or anticholinergic effects.
These results suggest that the efficacy of DXP at these doses may be due in part to a combination of the potency and selectivity of DXP for H1, and further suggest that histamine may be an integral part of a gating mechanism in the arousal system that allows transition from sleep to wake without residual sedation. In summary, these data indicate that adult and elderly patients taking DXP at doses of 1 mg, 3 mg, and 6 mg may experience an increase in time spent asleep that lasts throughout the night without evidence of next-day residual sedation.
REFERENCES
| 1. | | Richelson E. Pharmacology of Antidepressants. Mayo Clin Proc 2001;76:511-527 |
| |
| 2. | | Somaxon Pharmaceuticals Inc. In Vitro Pharmacological Profile of Doxepin... Data on file |
| |
| 3. | | Roth T, Rogowski R, Hull S, et al. Efficacy and Safety of Doxepin 1, 3 and 6 mg...Sleep 2007;30: 1555-1561 |
Supported by funding from Somaxon Pharmaceuticals, Inc.