Exhibit 99.1
For Immediate Release
Company name: DAIICHI SANKYO COMPANY, LIMITED
Representative: Takashi Shoda, President and Representative Director
(Code no.: 4568, First Section, Tokyo, Osaka and Nagoya Stock Exchanges)
Please address inquiries to Toshio Takahashi, Corporate Officer in Charge,
Corporate Communications Department
Telephone: +81-3-6225-1126
http://www.daiichisankyo.co.jp/
Sales of Intrathecal Baclofen Therapy Products Begin
Tokyo, April 4, 2006 –DAIICHI SANKYO COMPANY, LIMITED has announced that its subsidiary Daiichi Pharmaceutical Co., Ltd. (President: Kiyoshi Morita) launches Intrathecal Baclofen therapy products in Japan with the listing of the SynchroMed® EL pump and the InDura® catheter for national health insurance (NHI) reimbursement on April 1, 2006. Daiichi Pharmaceutical starts to market the SynchroMed infusion system, consisting of an implantable pump and catheter, in combination with its anti-spasticity agent Intrathecal Gabalon® (generic name: baclofen), which has already been approved for manufacture and sales, along with associated accessories. Medtronic Japan Co., Ltd. (President: Stephen R. LA NEVE) obtained approval for the SynchroMed infusion system.
Intrathecal Gabalon, along with the SynchroMed infusion system, is indicated for patients with severe spasticity unresponsive to conventional treatments. The SynchroMed programmer and catheter passer are also used with ITB therapy. The pump is implanted in subcutaneous tissue of the lower abdominal region. The catheter delivers Intrathecal Gabalon directly from the pump to the spinal cord, allowing long-term drug delivery to suppress strong spasticity experienced by patients. The reservoir of Intrathecal Gabalon in the pump can be replenished every 2–3 months.
Clinical trials conducted in Japan have confirmed that Intrathecal Gabalon, directly administered to the intrathecal space of the spinal cord, markedly reduces severe spasticity with much smaller doses than oral medicines. The Intrathecal Gabalon dose can be adjusted according to the patient’s condition and duration of treatment, in order to reduce side effects and increase treatment efficacy. This allows treatment to be optimized to suit each individual patient.
The SynchroMed infusion system and Intrathecal Gabalon have been used to treat some 50,000 patients in 22 countries around the world. In Japan, Intrathecal Gabalon and the SynchroMed infusion system were granted orphan drug and device status. Daiichi Pharmaceutical and Medtronic Japan began joint clinical trials in February 2002, and approval for manufacturing and sales was obtained for the SynchroMed infusion system in March 2005 and for Intrathecal Gabalon in April 2005. Intrathecal Gabalon was added to the NHI drug price list on September 16, 2005, and launched on December 12, 2005, but marketing activities were put on hold until the pump and catheter gained NHI coverage on April 1, 2006.
Daiichi Pharmaceutical’s goal is to contribute to the quality of life of the many patients suffering from severe spasticity who are unresponsive to conventional treatments by providing a new treatment option.
Reference materials
Product summaries
Intrathecal Gabalon
| | | | |
| Therapeutic category | | Anti-spastic (Japan standard product code 871249) |
| Brand name | | Intrathecal Gabalon® (generic name: baclofen) |
| Indication | | Severe spasticity resulting from cerebrospinal diseases (only where existing treatments are inadequate) |
| Storage and shelf life | | Store at room temperature; shelf life of 3 years |
| Regulatory classification | | Powerful drug, designated drug, prescription drug |
| Date of approval | | April 11, 2005 |
| Re-evaluation period | | 10 years after approval |
| NHI drug price | | Intrathecal infusion 0.005% 1 mL: |
| | | 1 ampoule of 0.05 mg/1 mL | | 1,107 yen |
| | | Intrathecal infusion 0.05% 20 mL: | | |
| | | 1 ampoule of 10 mg/20 mL | | 22,119 yen |
| | | Intrathecal infusion 0.2% 5 mL: | | |
| | | 1 ampoule of 10 mg/5 mL | | 22,119 yen |
| | |
SynchroMed EL pump | | | | |
| |
| Brand name | | SynchroMed® EL pump |
| |
| Purpose of use | | Drug infusion pump used for the intrathecal administration of Intrathecal Baclofen for patients with severe spasticity resulting from cerebrospinal diseases (only where existing treatments are inadequate) |
| |
| Dimensions (diameter x thickness); weight | | 70.4 mm x 27.5 mm; 205 g |
| |
| Reservoir capacity | | 18 mL |
| |
Flow: Infusion rate Infusion accuracy | | Minimum: 0.048 mL/day; Maximum: 0.9 mL/ hour ±15% |
| Re-evaluation period | | 7 years after approval |
| |
InDura catheter | | |
| |
Brand name | | InDura® catheter |
Purpose of use | | Intrathecal drug delivery catheter for connection to an implantable drug infusion pump |
Catheter length | | Distal segment: 381 mm; Proximal segment: 660 mm |
Materials | | X-ray opaque silicon rubber |
| | |
| Catheter passer | | | | |
| | |
| Model number | | 8591-38 | | 8591-60 |
| Purpose of use | | Tunneling rod to pass the InDura catheter through subcutaneous tissue |
| Length | | 38 cm | | 60 cm |
| Materials | | Stainless steel/polypropylene |
|
| SynchroMed programmer |
| |
Brand name (model number) | | SynchroMed® programmer (8821) |
| Purpose of use | | Programmer for non-invasive programming of an implantable drug infusion pump |
| Dimensions (h x w x d); weight | | 14.1 cm x 38.5 cm x 32.1 cm; 6.6 kg |
| * | All the above medical devices are manufactured and sold by Medtronic Japan and represented by its designated sales agent Daiichi Pharmaceutical. |
ITB therapy products and diagram showing implantation site in the body
Intrathecal Gabalon (0.005%, 0.05%, and 0.2%)
| | |
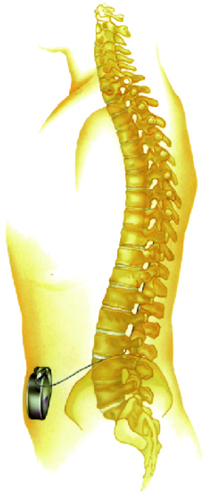
In vivo implantation of the pump (schematic diagram) | | 
Pump and catheter |
| | 
Programmer |
Glossary
Severe spasticity
Refers to the condition where injury to the nerves in the brain or spinal cord leads to enhancement of nerve transmission in the spinal cord, resulting in strong muscle spasticity (muscles become tight and stiff). In most cases, the condition is accompanied by spasms, clonus, pain, and a tight sensation in the thoracic and abdominal regions. Oral anti-spastic agents are used to treat mild to moderate spasticity, but these drugs produce little improvement with severe spasticity and there have been almost no effective treatments for this condition. Spasticity is associated with such primary conditions as spinal cord injury, brain injury, cerebral palsy, stroke, and degenerative diseases of the spinal cord, including multiple sclerosis and spinocerebellar degeneration.
Terms of approval
Intrathecal Baclofen, along with the SynchroMed infusion system, was approved under the following conditions. 1) Over a certain period, post-marketing studies of all patients in which the drug is used should be carried out to assess treatment outcomes, and appropriate steps are taken to understand all serious adverse events. 2) Before this product is delivered to the hospital, appropriate measures are taken, such as product training to ensure that physicians have a sufficient understanding of the product’s safety and efficacy profiles including those for the SynchroMed infusion system and only physicians with sufficient knowledge and experience in the procedure use the product.
Medtronic Japan
Medtronic Inc. is a medical device manufacturer headquartered in Minneapolis, Minnesota, USA that is a global leader in cardiac pacemakers, defibrillators, and other implantable devices. Medtronic Inc. established Medtronic Japan as its Japanese subsidiary in 1975. Medtronic Inc. achieved sales of US$10bn (approx. 1.15trn yen) in the fiscal year ending April 2005 and employs around 32,000 staff worldwide.



