UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE TO
TENDER OFFER STATEMENT
under Section 14(d)(1) or Section 13(e)(1)
of the Securities Exchange Act of 1934
AMBIT BIOSCIENCES CORPORATION
(Name of Subject Company)
CHARGE ACQUISITION CORP.
a wholly-owned subsidiary of
DAIICHI SANKYO COMPANY, LIMITED
(Names of Filing Persons — Offeror)
Common Stock, Par Value $0.001 per share
(Title of Class of Securities)
02318X100
(CUSIP Number of Class of Securities)
Seth Flaum
Daiichi Sankyo, Inc.
2 Hilton Ct.
Parsippany, NJ 07054-4410
Telephone: (973) 944-2600
(Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications on Behalf of Filing Persons)
with copy to:
Patrick Naughton
Simpson Thacher & Bartlett LLP
425 Lexington Avenue
New York, New York 10017-3954
Telephone: (212) 455-2000
CALCULATION OF FILING FEE
| | |
| Transaction Valuation* | | Amount of Filing Fee* |
| Not Applicable | | Not Applicable |
|
| * | A filing fee is not required in connection with this filing as it relates solely to preliminary communications made before the commencement of a tender offer. |
| ¨ | Check box if any part of the fee is offset as provided by Rule 0-11(a)(2) and identify the filing with which the offsetting fee was previously paid. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | |
| Amount Previously Paid: Not applicable. | | Filing Party: Not applicable. |
| Form or Registration No.: Not applicable. | | Date Filed: Not applicable. |
| x | Check the box if the filing relates solely to preliminary communications made before the commencement of a tender offer. |
Check the appropriate boxes below to designate any transactions to which the statement relates:
| | x | third-party tender offer subject to Rule 14d-1. |
| | ¨ | issuer tender offer subject to Rule 13e-4. |
| | ¨ | going-private transaction subject to Rule 13e-3. |
| | ¨ | amendment to Schedule 13D under Rule 13d-2. |
Check the following box if the filing is a final amendment reporting the results of the tender offer. ¨
On September 28, 2014, Ambit Biosciences Corporation (“Ambit”) and Daiichi Sankyo Company, Limited (“Daiichi Sankyo”) issued a joint press release announcing the execution of an Agreement and Plan of Merger (the “Merger Agreement”). Pursuant to the Merger Agreement, Charge Acquisition Corp., a wholly-owned subsidiary of Daiichi Sankyo (“Purchaser”), will commence a tender offer (the “Offer”) to purchase all of the issued and outstanding shares of Ambit common stock for (a) $15.00 per share in cash, plus (b) one non-transferrable contingent value right for each share of Ambit common stock, which represents the contractual right to receive up to $4.50 per share upon the achievement of certain commercialization-related milestones. If successful, the Offer will be followed by a merger of Purchaser with and into Ambit (the “Merger”).
This Schedule TO filing consists of the following documents relating to the proposed Offer and Merger:
(i) Presentation by employees of Daiichi Sankyo at a meeting of Ambit Biosciences employees on September 30, 2014.
|
Jeff Warmke, PhD Sr. Vice President External Scientific Affairs Town Hall Meeting September 30, 2014 |
|
2 Today is the start of a new future for us… …because Ambit Biosciences and Daiichi Sankyo have entered into an agreement for Daiichi Sankyo to acquire Ambit Biosciences The agreement establishes a new opportunity for us to meet our mutual goal of delivering new medicines to patients with unmet needs Why is Daiichi Sankyo (DS) interested in Ambit Biosciences? • Our missions are similar • Ambit’s strategy to seek partnerships to accelerate development of compounds aligns with DS’s strategy to explore mutually beneficial partnerships and executing strategic acquisitions • • DS wants to be a leader in oncology • DS has great respect for the people and the accomplishments of Ambit The quizartinib Ph2 data are impressive, and quizartinib will fit seamlessly into our oncology pipeline |
|
About Daiichi Sankyo One of the Top pharmaceutical companies in Japan Ranked among the top 20 global pharmaceutical companies Worldwide Presence: Ground presence in more than 50 countries Manufacturing locations in 13 countries R&D locations in Japan, US, Germany, UK, India, and etc. Consolidated net sales – JPY 997.9 Bn (FY2012) = 9.9 Bn US$ * >32,000 employees globally represented by 50 nationalities Business model encompassing; Innovative and Established pharmaceuticals, OTC, and Vaccines *Currency rate : JPY/USD=100.0 2014 September 30 Ambit Town hall 3 |
|
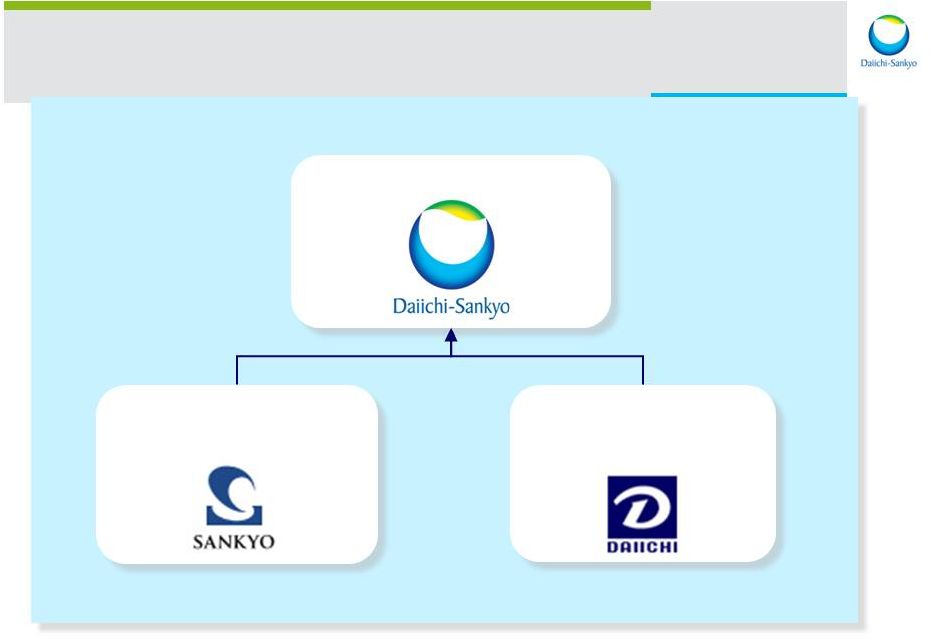
Daiichi Sankyo Co., Ltd. SANKYO CO., LTD. DAIICHI SANKYO CO., LTD. DAIICHI PHARMACEUTICAL CO., LTD. Merger 2007 Founded 1899 Founded 1918 4 |
|
Our Values and Commitments 1. To create first-in-class and best-in-class drugs 2. To take a global perspective, and respect local values 3. To foster intellectual curiosity and strategic insight Innovation Innovation Our Imperative 4. To provide the highest quality medical information 5. To provide a stable supply of top-quality pharmaceutical products 6. To be an ethical, trusted, and respectful partner Integrity Integrity Our Strength 7. To be accountable for achieving our goals 8. To demonstrate professionalism, respect for others and teamwork Accountability Accountability Our Culture To Contribute to the Enrichment of Quality of Life Around the World Through the Creation of Innovative Pharmaceuticals, and Through the Provision of Pharmaceuticals Addressing Diverse Medical Needs Mission 5 |
|
Tradition of innovation and giving Dr. Takamine isolated adrenaline, and developed Taka- Diatase for medicinal use Dr. Jokichi Takamine, Sankyo’s first president Dr. Takamine funded the gift of cherry trees from Tokyo to Washington, DC in 1912. 7 |
|
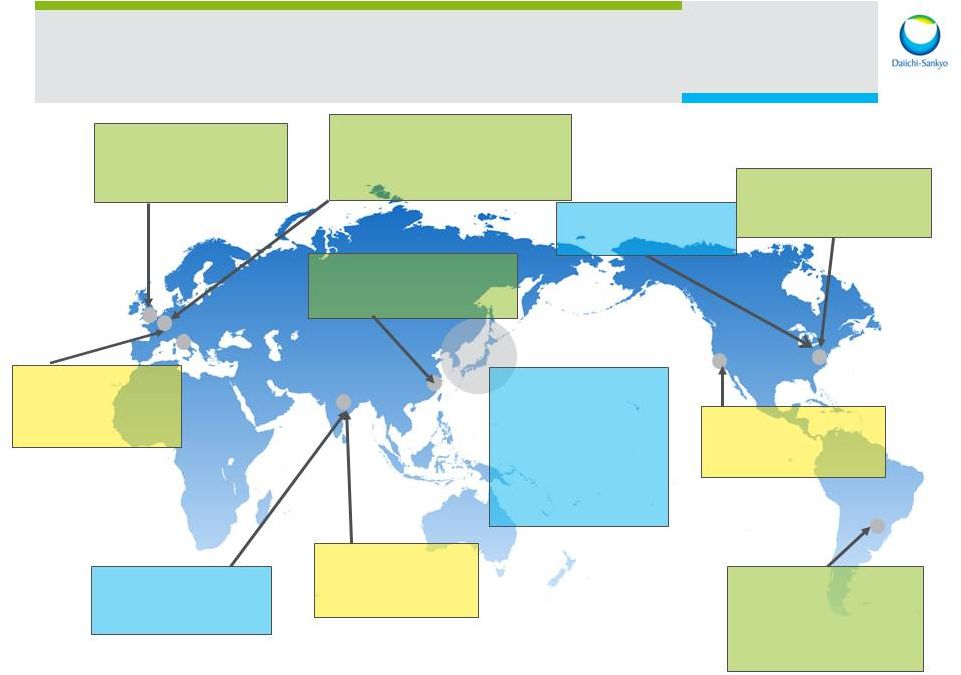
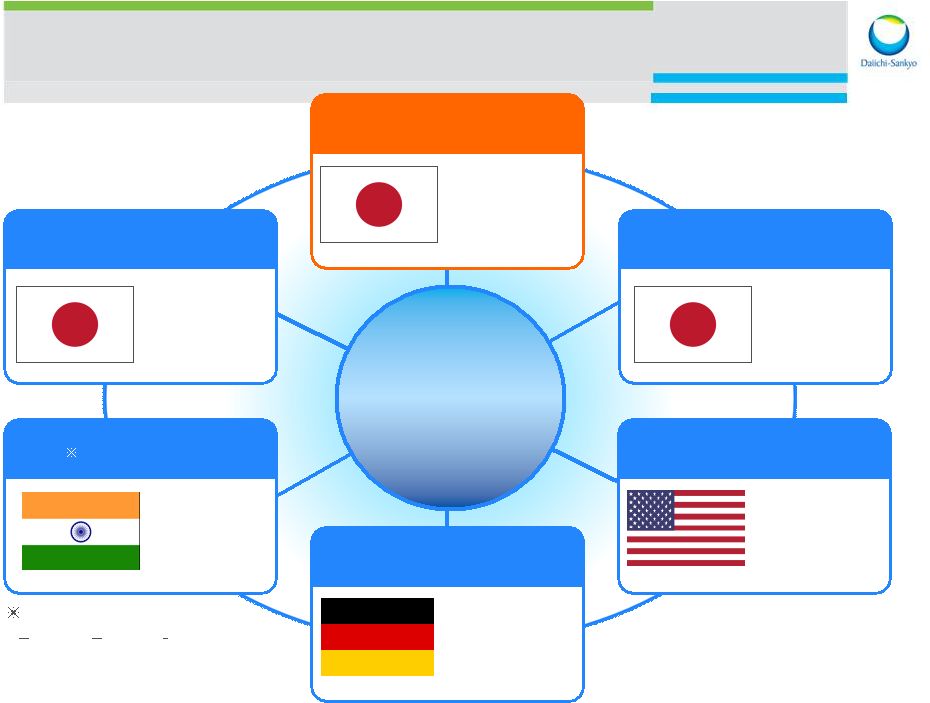
Daiichi Sankyo is a global company 30,000 employees worldwide 3,000 employees in the U.S. Daiichi Sankyo US Daiichi Sankyo Europe Daiichi Sankyo Japan Latin American Affiliates Venezuela, Brazil Asia Affiliates China, Taiwan, Korea, Thailand India 8 |
|
3 Daiichi Sankyo Co., Ltd R&D Division Daiichi Sankyo RD Novare Asubio Pharma (R&D) Daiichi Sankyo Inc. Edison, NJ (Development) Daiichi Sankyo Development London (Development) Daiichi Sankyo Europe GmbH Munich (Development, Pharmaceutical technology) Daiichi Sankyo (China) (Development) U3 Pharma Munich (oncology, mAB) Daiichi Sankyo Life Science Research Center India Ranbaxy Laboratories (R&D) Daiichi Sankyo Brazil (Development) Daiichi Sankyo Venezuela (Development) Plexxikon Berkeley, CA (oncology) Global R&D Locations Asubio (Development) Edison, NJ 9 |
|
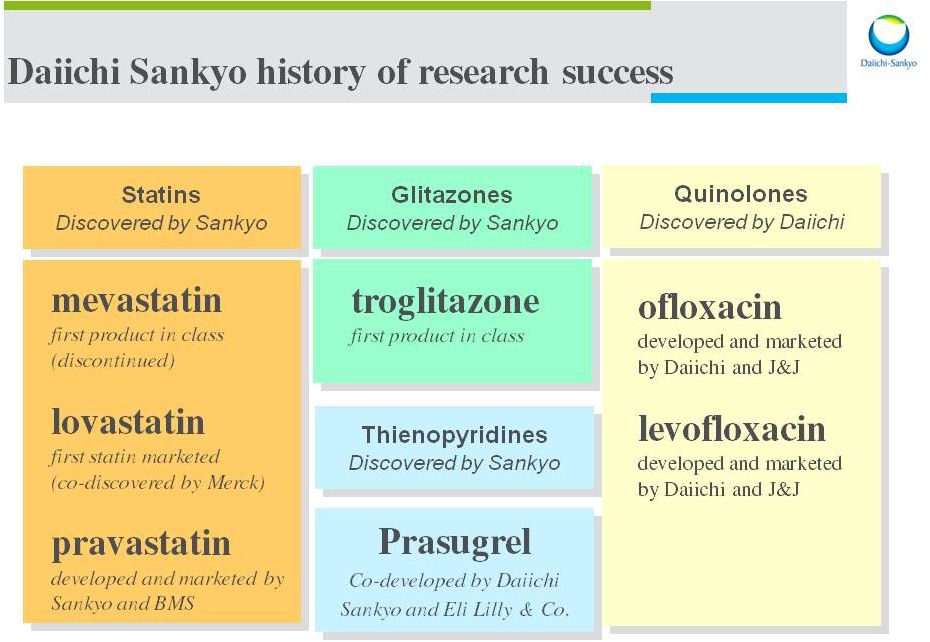
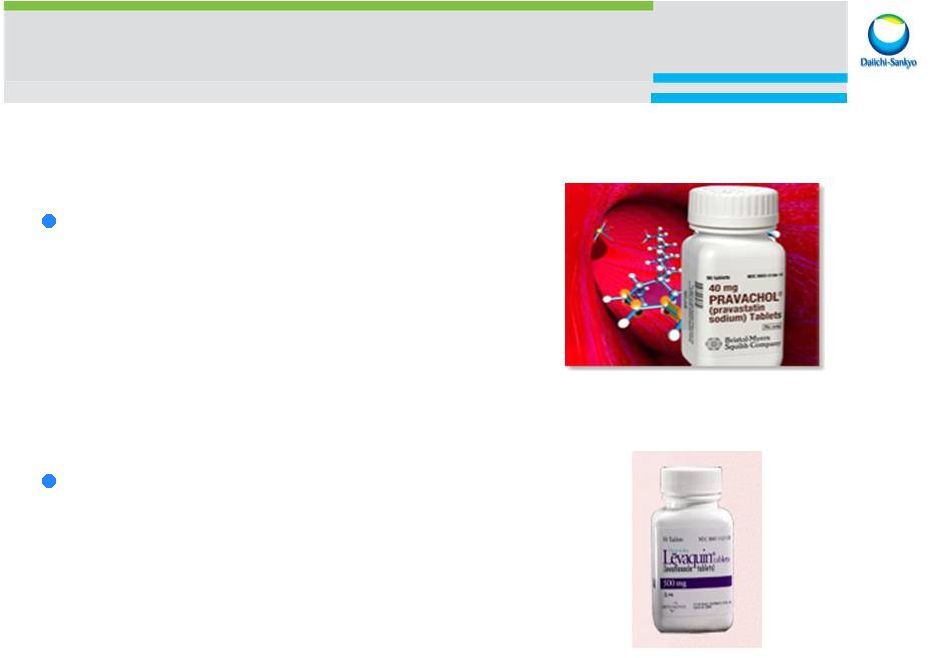
Our innovation history Pravastatin : HMG-CoA inhibitor Anti-cholesterol launched in 1989 licensed to BMS Pravachol Levofloxacin : Broad spectrum anti-biotic quinolone launched in 1993 licensed to J&J Levaquin The innovation of Taka-diastase by Dr. Jokichi Takamine, 1 st president of ex-Sankyo, continues today 2014 September 30 Ambit Town hall 10 |
|
11 Our innovation history Olmesartan : Blocker (ARB) Anti-hypertensive launched in 2002 Benicar ® , Benicar HCT ® Azor ® , Tribenzor ® Prasugrel : ADP receptor inhibitor Anti-platelet launched in 2009 co-promotes with Eli Lilly & Co. launch in Japan this year Effient ® Edoxaban : FXa inhibitor Anti-coagulant Approved in Japan (VTE, AF) as Lixiana NDA globally 2014 September 30 Ambit Town hall Angiotensin Receptor ® |
|
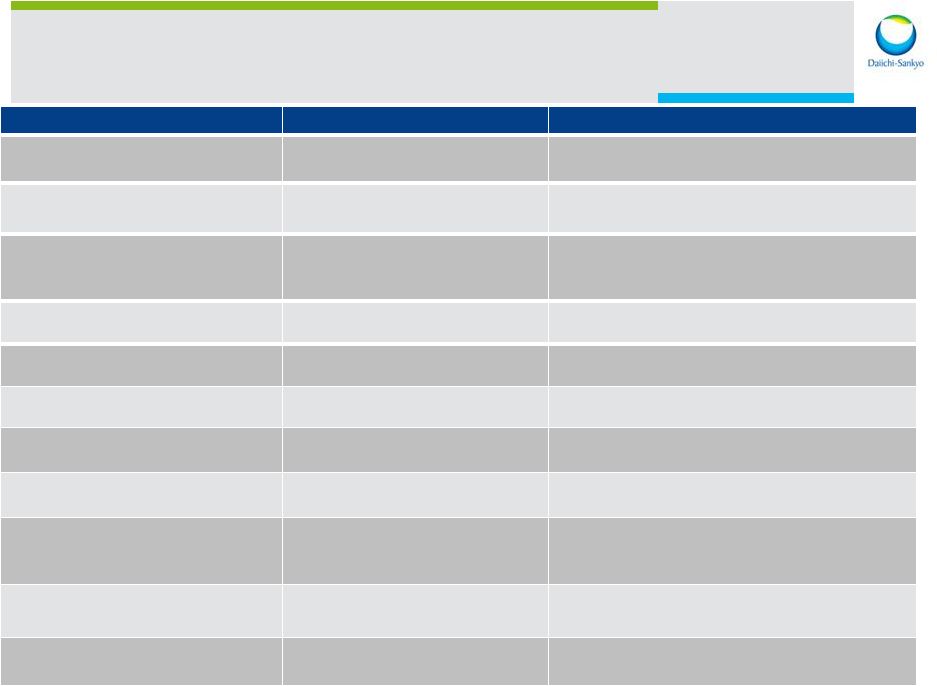
12 Representative Business Development Activity Partner Description Strategic Rationale Charleston Labs (2014) Development and Commercialization Collaboration Collaboration to develop and commercialize novel hydrocodone combination products for acute pain UCSF – Institute for Neurodegenerative Diseases(2014) Drug Discovery Collaboration Discovery of novel therapeutics and diagnostics for multiple neurodegenerative diseases Sanford Burnham Medical Research Institute (2014) Research and Development Collaboration Enhance internal drug discovery in CV & Metabolic diseases; leverage their Translational Medicine expertise Virtici and Celdara Medical (2013) Research and Development Collaboration Enhance internal drug discovery in oncology and other therapeutic areas Amplimmune (2012) Research and Development Collaboration Enhance internal drug discovery in autoimmune disease NGM Biopharmaceuticals (2012) Research Collaboration Enhance internal drug discovery in diabetes Mitsubishi Tanabe (2012) Strategic Sales Alliance in Japan Leverage sales/marketing infrastructure, improve near term pipeline Coherus (2012) Research and Development Collaboration; Equity Investment Collaboration to develop and commercialize bio-similars Plexxikon (2011) Acquisition Broaden pipeline and expand small molecule research programs (oncology, immunology, CVM); add personalized medicine approach U3 Pharma (2008) (2008) Acquisition Broaden oncology pipeline and expand antibody research programs ArQule (2008) Research, Development and Commercial Alliance Broaden oncology pipeline and support drug discovery efforts |
|
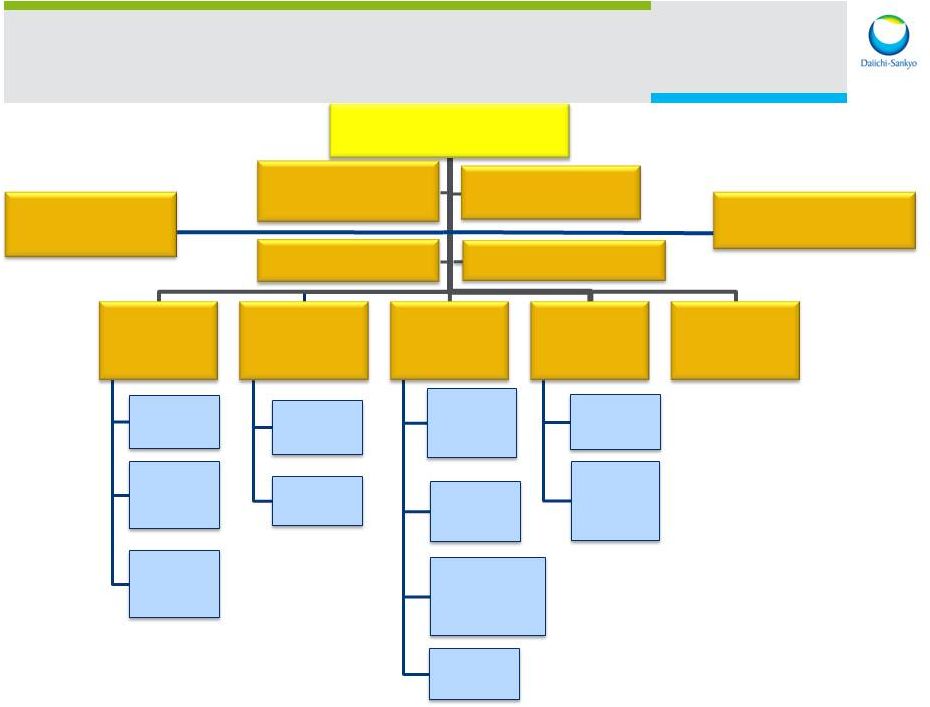
R&D Unit as of April 2014 GRDHRC Goddu Global Head of R&D Glenn Gormley Asubio Pharma Global Head Research RCI TCRM ROF RD Novare Global Head Biologics U3 Pharma BOF Global Head Development Ghazzi Japan DSPD (US) Ghazzi DSD (EU) Asia Plexxikon Venture Science Lab. Global Head R&D Planning GRDFC GRaDiC External Scientific Affairs Warmke Global Head Project Management 13 |
|
R&D Focus Therapeutic Areas Discovery Priority Areas - Oncology - Cardiovascular-Metabolics New Areas - Drug creation based on disease mechanisms, developing products with new mechanisms of action that address heavily unmet needs Thrombotic disorders Hypertension Bacterial infections Hyperlipidemia 14 2014 September 30 Ambit Town hall |
|
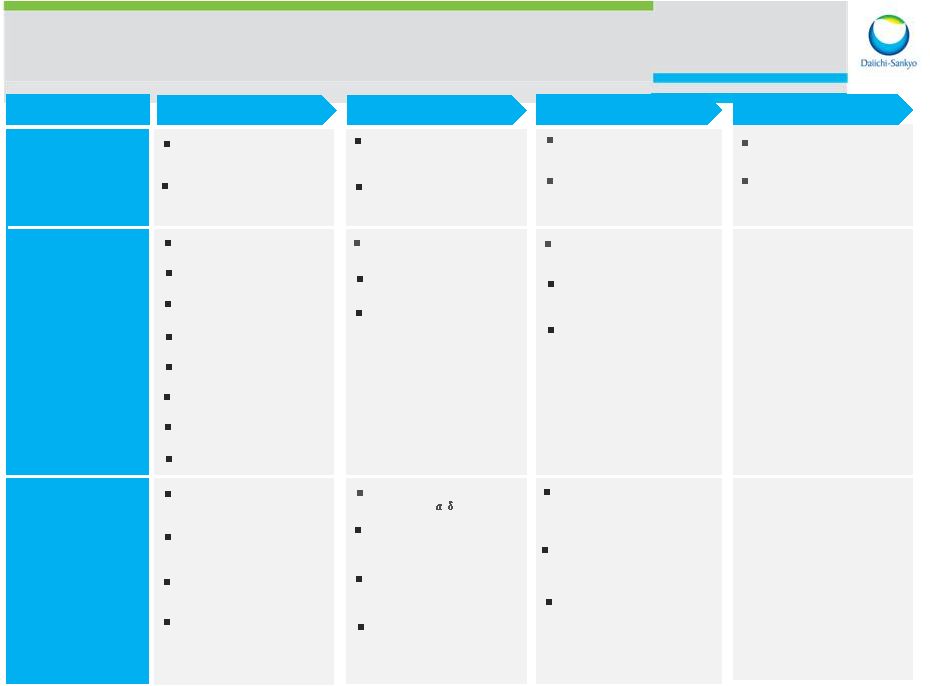
15 Phase 1 Phase 2 Phase 3 Application Therapeutic area Cardiovascular- Metabolics Oncology Others Prasugrel(JP) (CS-747 prasugrel / ischemic stroke / anti-platelet agent) DU-176b (US/EU) (edoxaban / AF / oral factor Xa inhibitor) DU-176b (US/EU) (edoxaban / VTE / oral factor Xa inhibitor) Tivantinib (US/EU) (ARQ 197/ HCC / Met inhibitor) Denosumab (JP) (AMG 162 / breast cancer adjuvant / anti-RANKL antibody) Denosumab (JP) (AMG 162 / rheumatoid arthritis / anti-RANKL anti-body) DS-5565 (Global) (Chronic pain / 2 ligand) Daiichi Sankyo R&D Pipeline Levofloxacin (JP) (DR-3355 / anti-infection / New quinolone) Nimotuzamab (JP) DE-766/ Gastric cancer / anti-EGFR antibody) Prasugrel (US) (CS-747 / Sickle Cell Disease / anti-platelet agent) CS-3150 (JP) (Anti-hypertensive/DM nephropathy / MR antagonist) U3-1287 (US/EU) (patritumab / anti-HER3 antibody) PLX4032 (US/EU) (vemurafenib / BRAF inhibitor) PLX3397 (US) (Fms/Kit/Flt3-ITD inhibitor) DS-7309 (Anti-diabetes / Glucokinase activator) DS-8500 (Anti-diabetes / GPR119 agonist) DS-1040 (Acute ischemic stroke / TAFIa inhibitor) U3-1565 (US/JP) (Anti-HB-EGF antibody) DS-7423 (US/JP) (PI3K/mTOR inhibitor) DS-3078 (US/EU) (mTOR inhibitor) CS-8958 (US/EU) (laninamivir / anti-influenza / Outlicensing with Biota) PLX5622 (Rheumatoid arthritis / FMS kinase inhibitor) Hydromorphone (DS 7113 / Narcotic analgesic / opioid mu-receptor regulator) SUN13837 (US/EU) (Spinal cord injury / Modulator of bFGF signaling system) DS-3032 (US) (MDM2 inhibitor) PLX7486(US) (Fms/Trk inhibitor) DS-1093 (Anemia of chronic kidney disease/HIF-PH inhibitor) As of July 2014 DS-8895 (JP) (Anti-EPHA2 antibody)) DS-8273 (US) (Anti DR5 antibody) PLX8384 US) (BRAF inhibitor) DS- 3801 (Chronic obstipation/GPR 38 agonist) DS-1971 (Chronic pain) Ioforminol (JP) (GE 1451 x-ray contrast media/ Angiography) 2014 September 30 Ambit Town hall |
|
16 Global Clinical Committee Global Clin Operations Committee Global Regulatory Committee Biostat & Data Mgmt Committee Global TMCP Committee DS Japan DS Americas Ghazzi DS Europe DS Asia Global Head of Development Ghazzi Regions Regions Functions Functions Chief Medical Advisor Global Development Leadership Team (GDLT) Global Development Leadership Team (GDLT) |
|
Global Development Organization Europe DSD: UK DSID, India Japan DS, Tokyo DS RD Novare Asubio, Kobie Americas DSPD, Edison, NJ Plexxikon, Berkeley, CA Ambit Biosciences, La Jolla, CA Asia Shanghai, China Beijing, China Taipei, Taiwan Seoul, Korea 17 |
|
18 Global R&D Network Daiichi Sankyo Life Science Research Centre in India Asubio Pharma Plexxikon RCI U3 Pharma Daiichi Daiichi Sankyo Sankyo Global Global Research Research Functions Functions PLX4032 PLX3397 PLX8394 PLX7486 PLX5622 U3-1287 U3-1565 SUN13837 Research Center In India DS RD Novare Co., Ltd R&D Partnership with DS Venture Science Laboratory New Organization Effective April 2013 2014 September 30 Ambit Town hall |
|
19 HER3 EGFR DR5 c-Met HB-EGF U3-1565 Nimotuzumab DE-766 ARQ-197 Tigatuzamab CS-1008 Daiichi Sankyo Oncology Research Targets BRAF Zelboraf® FLT3-ITD Japanese Journal of Clinical Oncology. Jan. 2014. 00(0) 1-7 Denosumab U3-1287 Quizartinib RANKL RANK Osteoclast Bone Metastasis 19 |
|
Making a difference in patients’ lives… Our mutual goals to find medicines to improve patient’s live is a common objective of both Ambit Biosciences and Daiichi Sankyo. So our first priority will be to ensure that there are no delays in the development activities for quizartinib. And for that, we need your help. We respect that you may have many questions about your role moving forward. Daiichi Sankyo commits to addressing your questions and needs – and as soon as our HR organization have had time to think through the integrations and future steps, we will share that with you. 20 |
|
21 What we can share with you today • Until closing, each company will continue as a separate, independent company • Integration planning will begin immediately • Anticipate impact to employees pre deal-close will be minimal • Post deal-close anticipate little change to current HR plans and policies (See your HR representative for details) • As integration planning progresses, a clearer understanding of individual employment impact will become clearer • Each employee will be treated as an individual |
|
22 Research team members Communication within and between Discovery and Development globally Development team members Global R&D culture supports: • Importance of “open discussion” and “debate” • Respectful “challenge” for new ideas and innovation • Cross-functional, cross-cultural, and diverse ideas |
|
23 An Integration Planning Steering Committee (IPSC) will be formed DS co-leaders will be Jeff Warmke and Kym Goddu DS and Ambit members of the IPSC and sub-teams will be appointed and confirmed Communications regarding post-close integration plans will be shared as soon as we can for implementation post-close Integration Planning Next Steps DS and Ambit HR teams will be meeting to address questions from both organizations and understand our cultures Integrations take time, they can be frustrating and not so simple Patience and mutual respect are key |
|
24 Important Information This presentation is for informational purposes only and is not an offer to buy or the solicitation of an offer to sell any shares of Ambit Biosciences common stock. The tender offer described herein has not yet been commenced. On the commencement date of the tender offer, an offer to purchase, a letter of transmittal and related documents will be filed with the Securities and Exchange Commission (SEC). The solicitation of offers to buy shares of Ambit Biosciences common stock will only be made pursuant to the offer to purchase, the letter of transmittal and related documents. Investors and Ambit Biosciences securityholders are strongly advised to read both the tender offer statement and the solicitation/recommendation statement that will be filed by Ambit Biosciences regarding the tender offer when they become available as they will contain important information. Investors and securityholders may obtain free copies of these statements (when available) and other documents filed with respect to the tender offer at the SEC's website at www.sec.gov. In addition, copies of the tender offer statement and related materials (when available) may be obtained for free by directing such requests to the information agent for the tender offer or by directing such requests to the Daiichi Sankyo Group investor relations at the e-mail address below. The solicitation/recommendation statement and related documents (when available) may be obtained by directing such requests to Ambit Biosciences investor relations at the phone number or e-mail address below. Contacts: Daiichi Sankyo Tokyo Media and Investor Relations: Yasuki Minobe Daiichi Sankyo Co., Ltd. minobe.yasuki.eg@daiichisankyo.co.jp +81-3-6225-1126 US Media Relations: Kimberly Wix (US) Daiichi Sankyo, Inc. kwix@dsi.com +973 944 2338 |