
FY 2023 Results & Business Update March 14, 2024 Exhibit 99.2

The takeover offer described in this communication (the “Takeover Offer”) has not yet commenced. This communication is neither an offer to purchase nor a solicitation of an offer to sell shares of MorphoSys AG (the “Company”). The final terms and further provisions regarding the Takeover Offer will be in the offer document once the publication of the offer document by Novartis BidCo AG (formerly known as Novartis data42 AG) (the “Bidder”) has been approved by the German Federal Financial Supervisory Authority (the “BaFin”), after which the offer document will be filed with the U.S. Securities and Exchange Commission (the “SEC”). A solicitation and an offer to buy shares of the Company will be made only pursuant the offer document. In connection with the Takeover Offer, the Bidder and Novartis AG will file a Tender Offer Statement on Schedule TO with the SEC (together with the offer document, an Offer to Purchase including the means to tender and other related documents, the “Takeover Offer Documents”), the Company’s management board and supervisory board will issue a joint reasoned statement in accordance with sec. 27 of the German Securities Acquisition and Takeover Act and the Company will file a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC (together with the joint reasoned statement, the “Recommendation Statements”). THE COMPANY’S STOCKHOLDERS AND OTHER INVESTORS ARE URGED TO READ THE TAKEOVER OFFER DOCUMENTS AND THE RECOMMENDATION STATEMENTS BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION WHICH SHOULD BE READ CAREFULLY BEFORE ANY DECISION IS MADE WITH RESPECT TO THE TAKEOVER OFFER. The Takeover Offer Documents and the Recommendation Statements will be distributed to all stockholders of the Company in accordance with German and U.S. securities laws. The Tender Offer Statement on Schedule TO and the Solicitation/Recommendation Statement on Schedule 14D-9 will be made available for free at the SEC’s website at www.sec.gov. Additional copies may be obtained for free by contacting the Bidder or the Company. Free copies of these materials and certain other offering documents will be made available on the Company’s website in English at morphosys.com/en/investors/Novartis-TakeoverOffer and in German at morphosys.com/de/investoren/Novartis-TakeoverOffer, by mail to MorphoSys AG, Semmelweisstrasse 7, 82152 Planegg, Germany or by phone at +49 89 8992 7179. In addition to the Offer to Purchase, including the means to tender and certain other Takeover Offer Documents, as well as the Solicitation/Recommendation Statement, the Company files other information with the SEC. The Company’s filings with the SEC are also available for free to the public from commercial document-retrieval services and at the website maintained by the SEC at www.sec.gov and are also available free of charge under the “SEC Filings” section of the Company’s website at www.morphosys.com/en/investors. In order to reconcile certain areas where German law and U.S. law conflict, Novartis AG and the Bidder expect to request no-action and exemptive relief from the SEC to conduct the Takeover Offer in the manner described in the offer document. Acceptance of the Takeover Offer by stockholders residing outside Germany and the United States of America may be subject to further legal requirements. With respect to the acceptance of the Takeover Offer outside Germany and the United States, no responsibility is assumed for the compliance with such legal requirements applicable in the respective jurisdiction. Additional Information and Where to Find It © MorphoSys – FY 2023 Results

This communication contains certain forward-looking statements concerning the Company, the Bidder and the Takeover Offer that involve substantial risks and uncertainties. Forward-looking statements include any statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “goal,” “may,” “might,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” and similar expressions. In this communication, the Company’s forward-looking statements include statements about the parties’ ability to satisfy the conditions to the consummation of the Takeover Offer; statements about the expected timetable for the consummation of the Takeover Offer; the Company’s plans, objectives, expectations and intentions; and the financial condition, results of operations and business of the Company and Novartis AG. The forward-looking statements contained in this communication represent the judgment of the Company as of the date of this communication and involve known and unknown risks and uncertainties, which might cause the actual results, financial condition and liquidity, performance or achievements of the Company, or industry results, to be materially different from any historic or future results, financial conditions and liquidity, performance or achievements expressed or implied by such forward-looking statements. In addition, even if the Company's results, performance, financial condition and liquidity, and the development of the industry in which it operates are consistent with such forward-looking statements, they may not be predictive of results or developments in future periods. Those risks and uncertainties that could cause the actual results to differ from expectations contemplated by forward-looking statements include, among other things: uncertainties as to the timing of the Takeover Offer; uncertainties as to how many of the Company’s stockholders will tender their stock in the Takeover Offer; the possibility that competing offers will be made; the possibility that various conditions for the Takeover Offer may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the Takeover Offer; the effects of the Takeover Offer on relationships with employees, other business partners or governmental entities; that the Bidder and Novartis AG may not realize the potential benefits of the Takeover Offer; transaction costs associated with the Takeover Offer; that the Company’s expectations may be incorrect; the inherent uncertainties associated with competitive developments, clinical trial and product development activities and regulatory approval requirements; the Company's reliance on collaborations with third parties; estimating the commercial potential of the Company’s development programs; and other risks indicated in the risk factors included in the Company’s filings with the SEC, including the Company’s Annual Report on Form 20-F, as well as the Solicitation/Recommendation Statement on Schedule 14D-9 to be filed by the Company and the Tender Offer Statement on Schedule TO and related Takeover Offer Documents to be filed by the Bidder and Novartis AG. Given these uncertainties, the reader is advised not to place any undue reliance on such forward-looking statements. These forward-looking statements speak only as of the date of publication of this communication. The Company and the Bidder expressly disclaim any obligation to update any such forward-looking statements in this communication to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements, unless specifically required by law or regulation. Forward-Looking Statements © MorphoSys – FY 2023 Results

Agenda 01 FY 2023 Highlights & 2024 Outlook Jean-Paul Kress, M.D., Chief Executive Officer (CEO) 02 Development Update Tim Demuth, M.D., Ph.D., Chief Research & Development Officer (CR&DO) 03 Financial Results & Update Lucinda Crabtree, Ph.D., Chief Financial Officer (CFO) 04 Q&A Jean-Paul Kress, Lucinda Crabtree, Tim Demuth © MorphoSys – FY 2023 Results

FY 2023 Highlights & 2024 Outlook 01 © MorphoSys – FY 2023 Results Jean-Paul Kress, M.D. CEO

Exceptional Progress in 2023, Resulting in Proposed Acquisition by Novartis Pelabresib and tulmimetostat are investigational medicines and have not yet been evaluated or approved by regulatory authorities. The development of pelabresib was funded in part by The Leukemia and Lymphoma Society®. © MorphoSys – FY 2023 Results Advanced Potential Best and First-in- Class Pipeline Entered into Agreement to Be Acquired by Novartis Pelabresib combination represents potential paradigm shift in myelofibrosis treatment, with opportunities to expand into new indications Tulmimetostat has best- and first-in-class potential in array of advanced cancers Provides attractive, immediate and certain cash value to shareholders Accelerates potential of pelabresib on global scale

3 4 MorphoSys’ Key First-Half 2024 Priorities MorphoSys and Novartis will continue to act as two separate companies through expected close in first half of 2024 FDA, Food and Drug Administration; EMA, European Medicines Agency Close proposed Novartis acquisition 1 2 Prepare and submit filing for the approval of pelabresib combination in first-line myelofibrosis to the FDA and EMA Complete tafasitamab transition to Incyte Diligently manage cash runway and maintain business continuity © MorphoSys – FY 2023 Results

Development Update 02 © MorphoSys – FY 2023 Results Tim Demuth, M.D., Ph.D. CR&DO
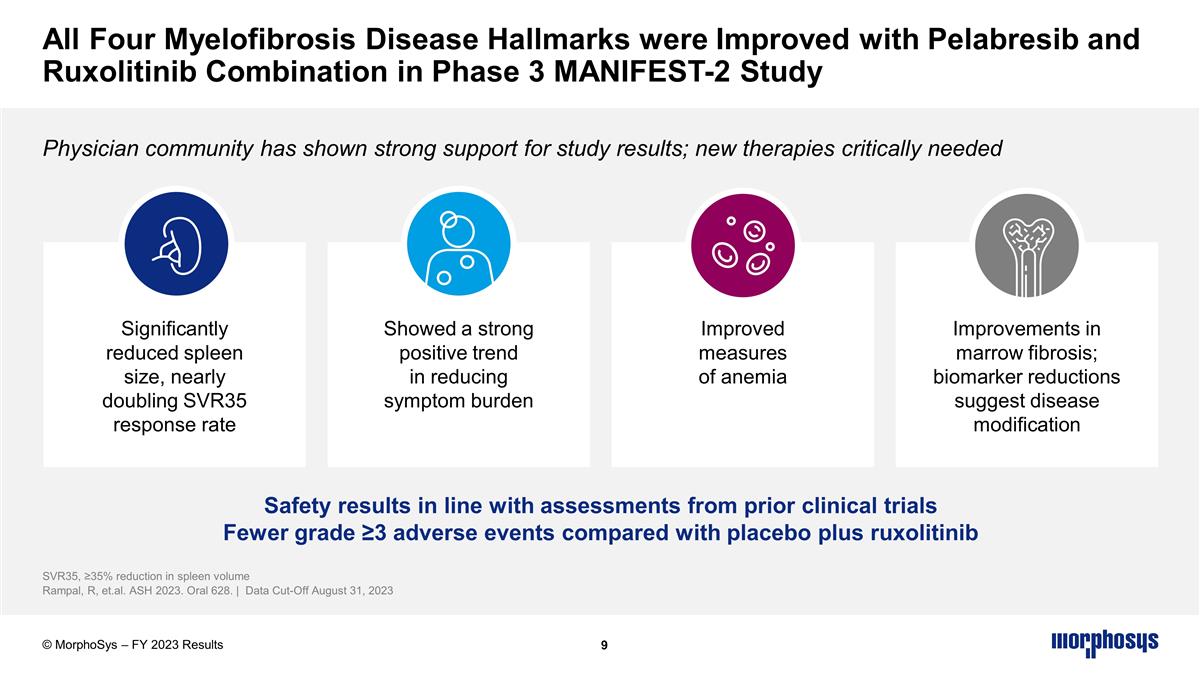
Significantly reduced spleen size, nearly doubling SVR35 response rate Improved measures of anemia Improvements in marrow fibrosis; biomarker reductions suggest disease modification Showed a strong positive trend in reducing symptom burden Safety results in line with assessments from prior clinical trials Fewer grade ≥3 adverse events compared with placebo plus ruxolitinib All Four Myelofibrosis Disease Hallmarks were Improved with Pelabresib and Ruxolitinib Combination in Phase 3 MANIFEST-2 Study Physician community has shown strong support for study results; new therapies critically needed SVR35, ≥35% reduction in spleen volume Rampal, R, et.al. ASH 2023. Oral 628. | Data Cut-Off August 31, 2023 © MorphoSys – FY 2023 Results
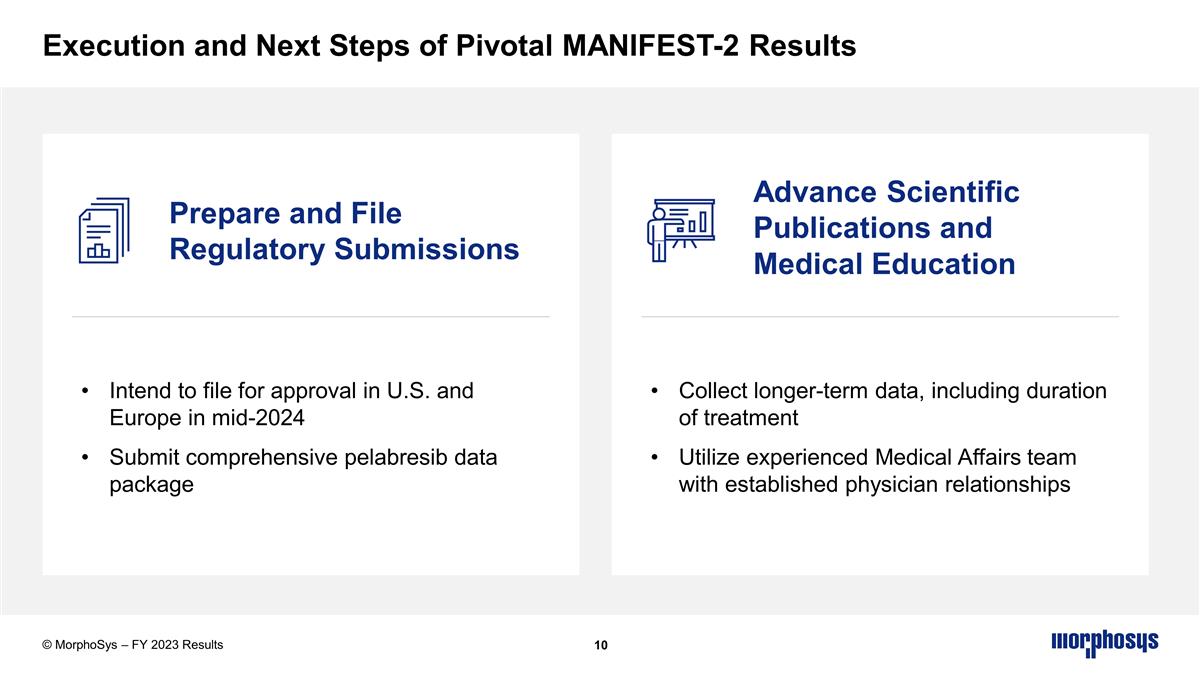
Execution and Next Steps of Pivotal MANIFEST-2 Results © MorphoSys – FY 2023 Results Collect longer-term data, including duration of treatment Utilize experienced Medical Affairs team with established physician relationships Prepare and File Regulatory Submissions Advance Scientific Publications and Medical Education Intend to file for approval in U.S. and Europe in mid-2024 Submit comprehensive pelabresib data package

03 Financial Results & Update © MorphoSys – FY 2023 Results Lucinda Crabtree, Ph.D. CFO
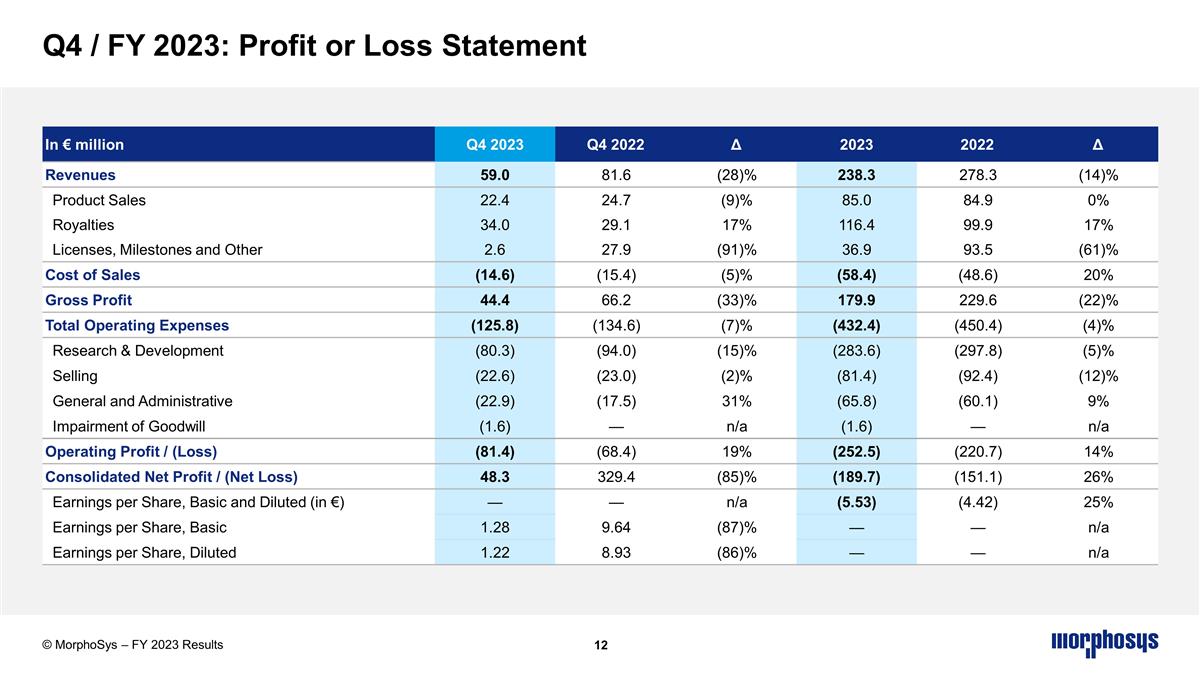
In € million Q4 2023 Q4 2022 Δ 2023 2022 Δ Revenues 59.0 81.6 (28)% 238.3 278.3 (14)% Product Sales 22.4 24.7 (9)% 85.0 84.9 0% Royalties 34.0 29.1 17% 116.4 99.9 17% Licenses, Milestones and Other 2.6 27.9 (91)% 36.9 93.5 (61)% Cost of Sales (14.6) (15.4) (5)% (58.4) (48.6) 20% Gross Profit 44.4 66.2 (33)% 179.9 229.6 (22)% Total Operating Expenses (125.8) (134.6) (7)% (432.4) (450.4) (4)% Research & Development (80.3) (94.0) (15)% (283.6) (297.8) (5)% Selling (22.6) (23.0) (2)% (81.4) (92.4) (12)% General and Administrative (22.9) (17.5) 31% (65.8) (60.1) 9% Impairment of Goodwill (1.6) — n/a (1.6) — n/a Operating Profit / (Loss) (81.4) (68.4) 19% (252.5) (220.7) 14% Consolidated Net Profit / (Net Loss) 48.3 329.4 (85)% (189.7) (151.1) 26% Earnings per Share, Basic and Diluted (in €) — — n/a (5.53) (4.42) 25% Earnings per Share, Basic 1.28 9.64 (87)% — — n/a Earnings per Share, Diluted 1.22 8.93 (86)% — — n/a Q4 / FY 2023: Profit or Loss Statement © MorphoSys – FY 2023 Results
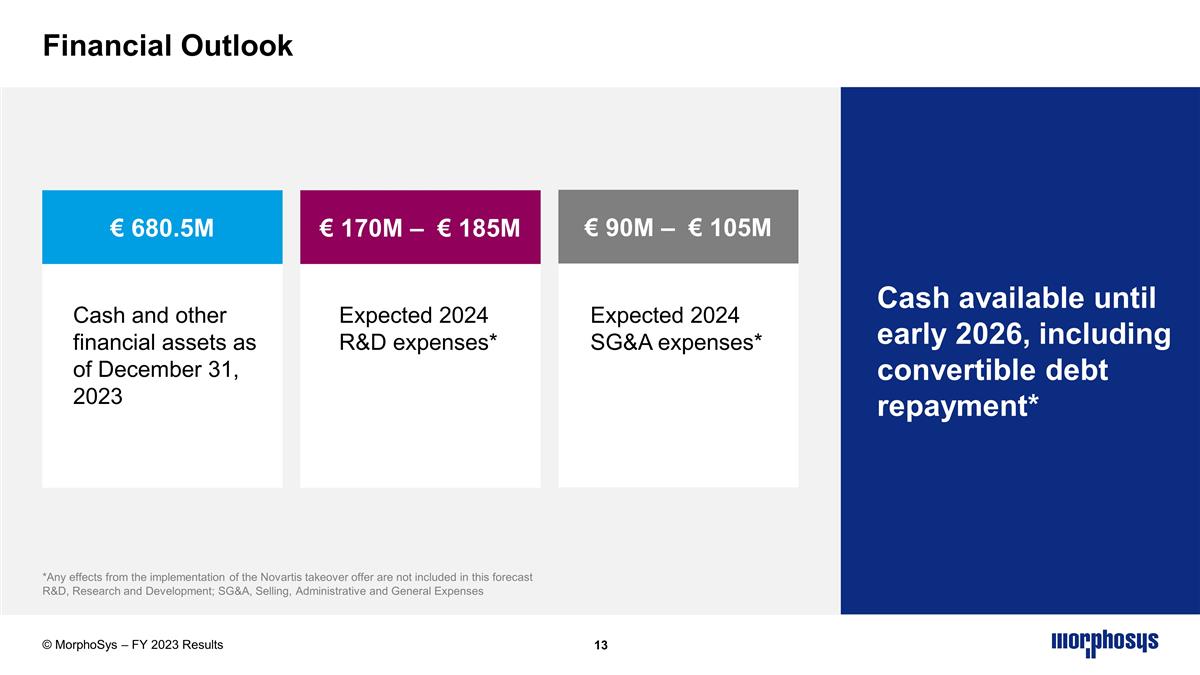
Financial Outlook *Any effects from the implementation of the Novartis takeover offer are not included in this forecast R&D, Research and Development; SG&A, Selling, Administrative and General Expenses © MorphoSys – FY 2023 Results Cash and other financial assets as of December 31, 2023 Expected 2024 R&D expenses* Cash available until early 2026, including convertible debt repayment* € 680.5M € 170M – € 185M € 90M – € 105M Expected 2024 SG&A expenses*

Q&A Tim Demuth, M.D., Ph.D. CR&DO 04 Jean-Paul Kress, M.D. CEO © MorphoSys – FY 2023 Results Lucinda Crabtree, Ph.D. CFO

Thank you! www.MorphoSys.com © MorphoSys – FY 2023 Results
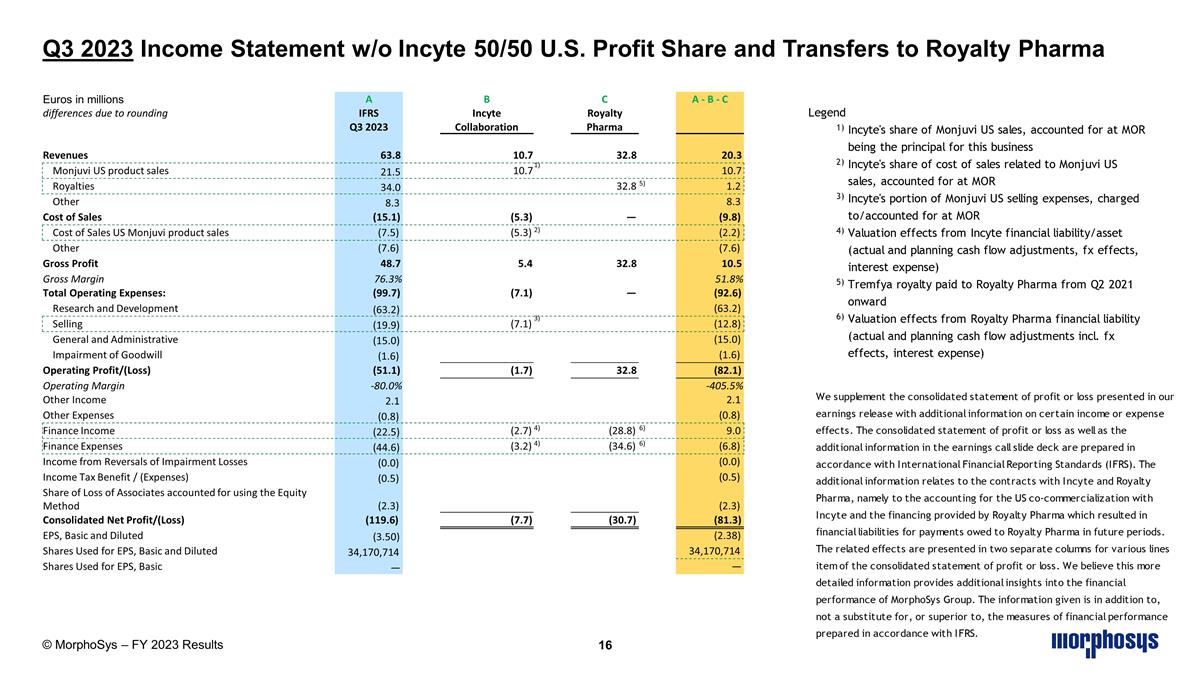
Q3 2023 Income Statement w/o Incyte 50/50 U.S. Profit Share and Transfers to Royalty Pharma Euros in millions A B C A - B - C differences due to rounding IFRS Incyte Royalty Q3 2023 Collaboration Pharma Revenues 63.8 10.7 32.8 20.3 Monjuvi US product sales 21.5 10.7 1) 10.7 Royalties 34.0 32.8 5) 1.2 Other 8.3 8.3 Cost of Sales (15.1) (5.3) — (9.8) Cost of Sales US Monjuvi product sales (7.5) (5.3) 2) (2.2) Other (7.6) (7.6) Gross Profit 48.7 5.4 32.8 10.5 Gross Margin 76.3% 51.8% Total Operating Expenses: (99.7) (7.1) — (92.6) Research and Development (63.2) (63.2) Selling (19.9) (7.1) 3) (12.8) General and Administrative (15.0) (15.0) Impairment of Goodwill (1.6) (1.6) Operating Profit/(Loss) (51.1) (1.7) 32.8 (82.1) Operating Margin -80.0% -405.5% Other Income 2.1 2.1 Other Expenses (0.8) (0.8) Finance Income (22.5) (2.7) 4) (28.8) 6) 9.0 Finance Expenses (44.6) (3.2) 4) (34.6) 6) (6.8) Income from Reversals of Impairment Losses (0.0) (0.0) Income Tax Benefit / (Expenses) (0.5) (0.5) Share of Loss of Associates accounted for using the Equity Method (2.3) (2.3) Consolidated Net Profit/(Loss) (119.6) (7.7) (30.7) (81.3) EPS, Basic and Diluted (3.50) (2.38) Shares Used for EPS, Basic and Diluted 34,170,714 34,170,714 Shares Used for EPS, Basic — — © MorphoSys – FY 2023 Results
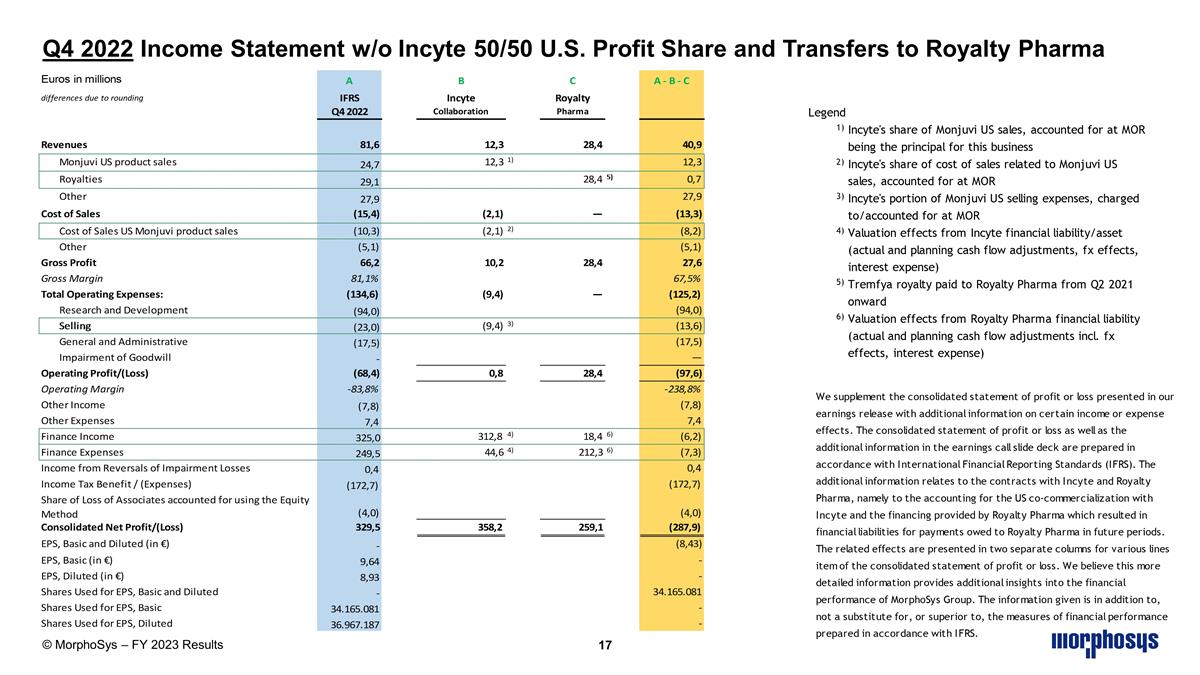
Q4 2022 Income Statement w/o Incyte 50/50 U.S. Profit Share and Transfers to Royalty Pharma © MorphoSys – FY 2023 Results

Q4 2023 Income Statement w/o Incyte 50/50 U.S. Profit Share and Transfers to Royalty Pharma © MorphoSys – FY 2023 Results
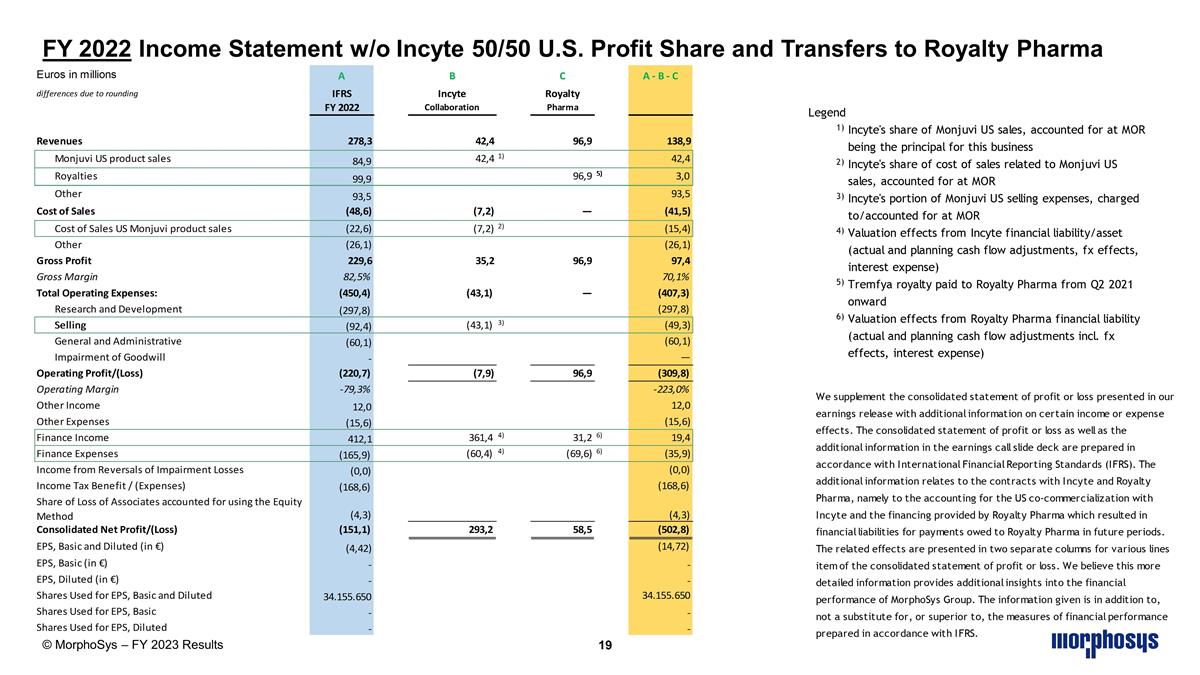
FY 2022 Income Statement w/o Incyte 50/50 U.S. Profit Share and Transfers to Royalty Pharma © MorphoSys – FY 2023 Results
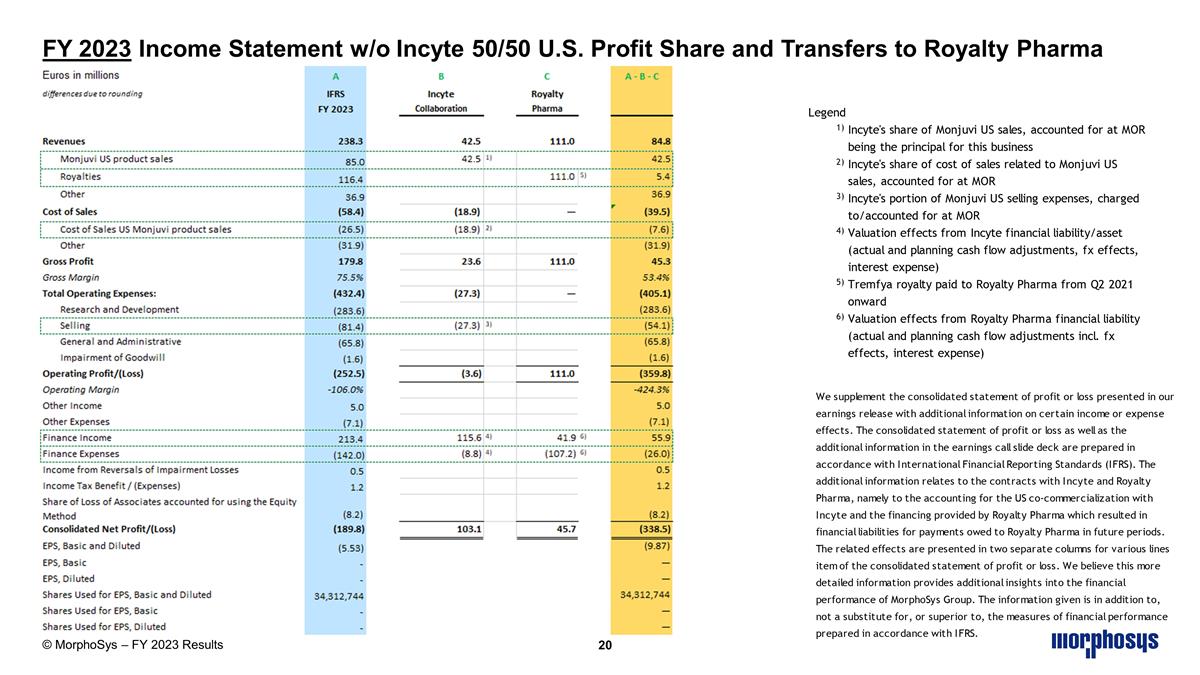
FY 2023 Income Statement w/o Incyte 50/50 U.S. Profit Share and Transfers to Royalty Pharma © MorphoSys – FY 2023 Results



















