
2021 Annual Report A nn ua l R ep or t 20 21

Our Clinical Pipeline Clinical Programs Developed by Partners (Selection) Most advanced development stage Program / Partner Indication Gantenerumab / Roche Alzheimer’s disease Otilimab (MOR103/GSK3196165) / GlaxoSmithKline Rheumatoid arthritis Severe pulmonary COVID-19 related disease Felzartamab1 / I-Mab Multiple myeloma Abelacimab (MAA868) / Anthos Therapeutics Atrial fibrillation Setrusumab (BPS804) / Mereo/Novartis/Ultragenyx Brittle bone syndrome NOV-8 (CMK389) / Novartis Pulmonary sarcoidosis NOV-9 (LKA651) / Novartis Diabetic eye diseases Most advanced development stage Program Indication Tafasitamab1 L-MIND / Relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) B-MIND / r/r DLBCL firstMIND / First-line DLBCL frontMIND / First-line DLBCL inMIND / r/r follicular lymphoma / marginal zone lymphoma Pelabresib MANIFEST-2 / Myelofibrosis MANIFEST / Myelofibrosis Most advanced development stage Program Indication Felzartamab IGNAZ / Immunoglobulin A nephropathy M-PLACE / Anti-PLA2R-positive membranous nephropathy New-PLACE / Anti-PLA2R-positive membranous nephropathy CPI-0209 Advanced solid tumors / Hematologic malignancies Most advanced development stage Program / Partner Indication Ianalumab (VAY736) / Novartis Inflammation Bimagrumab / Novartis / Versanis Bio Type 2 diabetes Utomilumab (PF-05082566) / Pfizer Cancer (multiple indications) NOV-14 (CSJ117) / Novartis Asthma MOR2102 / I-Mab r/r advanced solid tumors © MorphoSys, March 2022 P H A S E 1 P H A S E 2 P H A S E 3 L A U N C H E D P H A S E 1 P H A S E 2 P H A S E 3 L A U N C H E D P H A S E 1 P H A S E 2 P H A S E 3 L A U N C H E D P H A S E 1 P H A S E 2 P H A S E 3 L A U N C H E D 1 Global Collaboration and License Agreement with Incyte Corporation; co-commer- cialization in the U.S.; Incyte has exclusive commercialization rights outside the U.S. 2 Not conducted, as not necessary. Pipeline products are under clinical investigation and there is no guarantee any investigational product will be approved by regulatory authorities. 1 Sublicensed to I-Mab for development in China, Hong Kong, Macao and Taiwan. 2 Sublicensed to I-Mab for development in China, Hong Kong, Macao, Taiwan and South Korea. 2
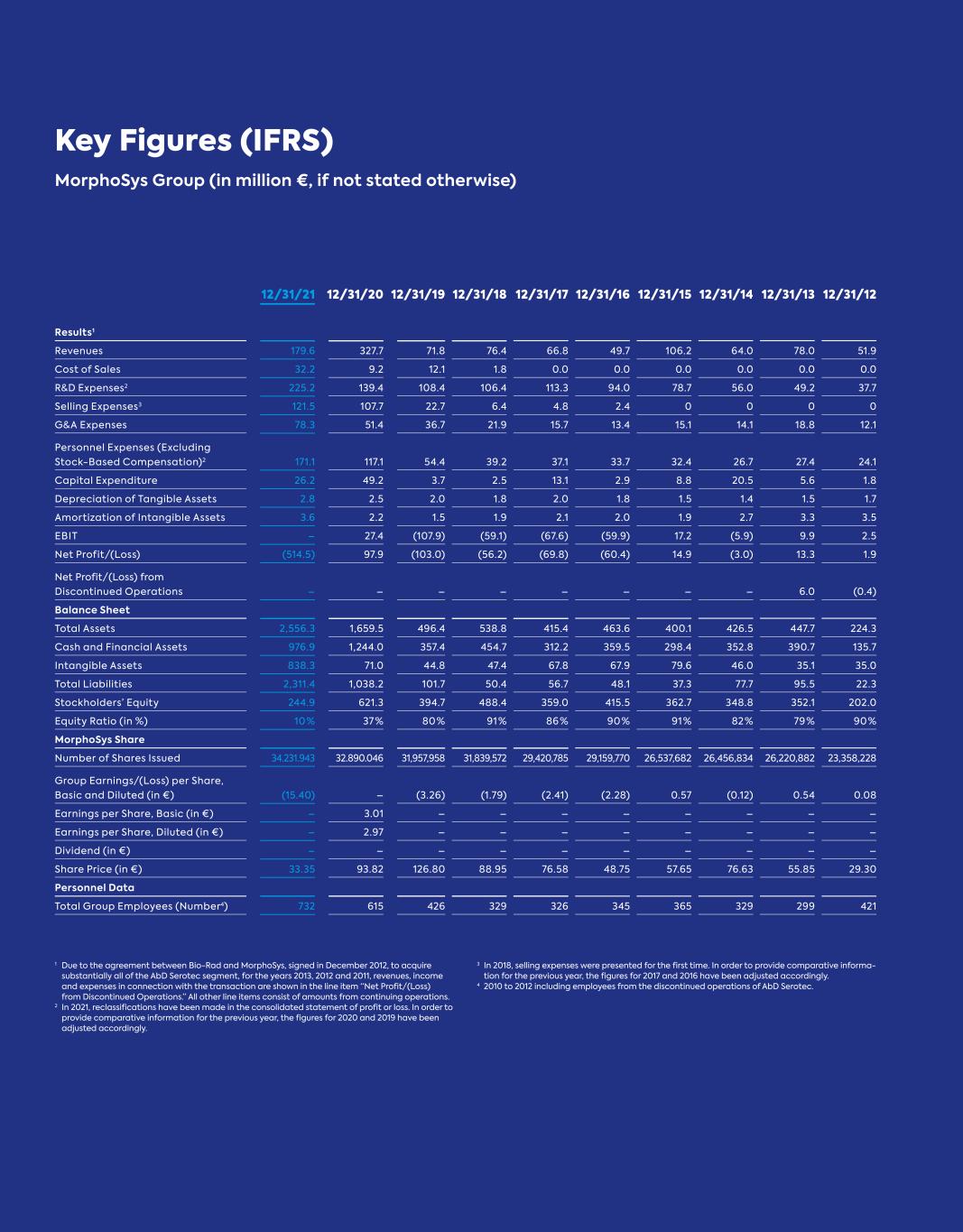
Key Figures (IFRS) MorphoSys Group (in million €, if not stated otherwise) 1 Due to the agreement between Bio-Rad and MorphoSys, signed in December 2012, to acquire substantially all of the AbD Serotec segment, for the years 2013, 2012 and 2011, revenues, income and expenses in connection with the transaction are shown in the line item “Net Profit/(Loss) from Discontinued Operations.” All other line items consist of amounts from continuing operations. 2 In 2021, reclassifications have been made in the consolidated statement of profit or loss. In order to provide comparative information for the previous year, the figures for 2020 and 2019 have been adjusted accordingly. 3 In 2018, selling expenses were presented for the first time. In order to provide comparative informa- tion for the previous year, the figures for 2017 and 2016 have been adjusted accordingly. 4 2010 to 2012 including employees from the discontinued operations of AbD Serotec. 12/31/21 12/31/20 12/31/19 12/31/18 12/31/17 12/31/16 12/31/15 12/31/14 12/31/13 12/31/12 Results1 Revenues 179.6 327.7 71.8 76.4 66.8 49.7 106.2 64.0 78.0 51.9 Cost of Sales 32.2 9.2 12.1 1.8 0.0 0.0 0.0 0.0 0.0 0.0 R&D Expenses2 225.2 139.4 108.4 106.4 113.3 94.0 78.7 56.0 49.2 37.7 Selling Expenses3 121.5 107.7 22.7 6.4 4.8 2.4 0 0 0 0 G&A Expenses 78.3 51.4 36.7 21.9 15.7 13.4 15.1 14.1 18.8 12.1 Personnel Expenses (Excluding Stock-Based Compensation)2 171.1 117.1 54.4 39.2 37.1 33.7 32.4 26.7 27.4 24.1 Capital Expenditure 26.2 49.2 3.7 2.5 13.1 2.9 8.8 20.5 5.6 1.8 Depreciation of Tangible Assets 2.8 2.5 2.0 1.8 2.0 1.8 1.5 1.4 1.5 1.7 Amortization of Intangible Assets 3.6 2.2 1.5 1.9 2.1 2.0 1.9 2.7 3.3 3.5 EBIT – 27.4 (107.9) (59.1) (67.6) (59.9) 17.2 (5.9) 9.9 2.5 Net Profit/(Loss) (514.5) 97.9 (103.0) (56.2) (69.8) (60.4) 14.9 (3.0) 13.3 1.9 Net Profit/(Loss) from Discontinued Operations – – – – – – – – 6.0 (0.4) Balance Sheet Total Assets 2,556.3 1,659.5 496.4 538.8 415.4 463.6 400.1 426.5 447.7 224.3 Cash and Financial Assets 976.9 1,244.0 357.4 454.7 312.2 359.5 298.4 352.8 390.7 135.7 Intangible Assets 838.3 71.0 44.8 47.4 67.8 67.9 79.6 46.0 35.1 35.0 Total Liabilities 2,311.4 1,038.2 101.7 50.4 56.7 48.1 37.3 77.7 95.5 22.3 Stockholders’ Equity 244.9 621.3 394.7 488.4 359.0 415.5 362.7 348.8 352.1 202.0 Equity Ratio (in %) 10 % 37 % 80 % 91 % 86 % 90 % 91 % 82 % 79 % 90 % MorphoSys Share Number of Shares Issued 34.231.943 32.890.046 31,957,958 31,839,572 29,420,785 29,159,770 26,537,682 26,456,834 26,220,882 23,358,228 Group Earnings/(Loss) per Share, Basic and Diluted (in €) (15.40) – (3.26) (1.79) (2.41) (2.28) 0.57 (0.12) 0.54 0.08 Earnings per Share, Basic (in €) – 3.01 – – – – – – – – Earnings per Share, Diluted (in €) – 2.97 – – – – – – – – Dividend (in €) – – – – – – – – – – Share Price (in €) 33.35 93.82 126.80 88.95 76.58 48.75 57.65 76.63 55.85 29.30 Personnel Data Total Group Employees (Number4) 732 615 426 329 326 345 365 329 299 421
Annual Report https://reports.morphosys.com/2021 Non-Financial Report https://csr.morphosys.com/2021 2021 2021 See our latest reports online You can also find our Group’s current annual and non-financial reports in both English and German online. Simply go to our website. We look forward to your visit. Annual Report Non-Financial Report

Th e C o m p a ny C o nt en ts At MorphoSys, our ambition is to redefine how cancer is treated. As a commercial-stage biopharmaceutical company, we are driven by the urgency to deliver groundbreaking medicines. Guided by our mission: more life for people with cancer.

We are an emerging leader in hematology/oncology and are committed to making a profound im- pact in the lives of patients - with the ongoing commercialization of Monjuvi® and the clinical development of pelabresib in myelofibrosis.

Th e C o m p a ny C o nt en ts

Th e C o m p a ny C o nt en ts Monjuvi Since launch, approximately 2,000 patients have been treated with our cancer immuno- therapy in the U.S. Monjuvi, in combination with lenalidomide, provides a paradigm shift in treating adult patients with relapsed or refrac- tory diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplant. Beyond the current indication, approved under accelerated approval, we are developing Monjuvi in additional hemato- logical indications.

Pelabresib Our aspiration for our most exciting new pipe- line asset is to potentially change the standard of care in myelofibrosis, a bone marrow cancer for which only limited treatment options are available. Pelabresib is currently being explored in a phase 3 trial, and we expect the data in the first half of 2024.

Th e C o m p a ny C o nt en ts

Contents 01 02 The Company Letter to the Shareholders 14 Report of the Supervisory Board 18 Supervisory Board of MorphoSys AG 22 MorphoSys on the Capital Market 25 Non-Financial Group Report 28 Fundamentals of the MorphoSys Group 34 Macroeconomic and Sector-Specific Conditions 51 Analysis of Net Assets, Financial Position and Results of Operations 52 Outlook and Forecast 69 Risk and Opportunity Report 73 Subsequent Events 84 Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance 85 Group Management Report 8 Contents

Th e C o m p a ny03 Consolidated Statement of Profit or Loss (IFRS) 106 Consolidated Statement of Comprehensive Income (IFRS) 107 Consolidated Balance Sheet (IFRS) 108 Consolidated Statement of Changes in Stockholders’ Equity (IFRS) 110 Consolidated Statement of Cash Flows (IFRS) 112 Notes 114 Responsibility Statement 172 Independent Auditor’s Report 173 Executive Committee of MorphoSys AG 180 Glossary 182 List of Figures and Tables 184 Financial Statements Additional Information C o nt en ts 9Contents

See our latest reports online You can also find our Group’s current annual and non-financial reports in both English and German online. Simply go to our website. We look forward to your visit. Annual Report https://reports.morphosys.com/2021 Non-Financial Report https://csr.morphosys.com/2021 10 The Company Contents

01Contents Th e C o m p a nyThe Company Letter to the Shareholders 14 Report of the Supervisory Board 18 Supervisory Board of MorphoSys AG 22 MorphoSys on the Capital Market 25 Non-Financial Group Report 28 Contents The Company 11

With the acquisition of Constellation Pharmaceuticals, we accelerated our transformation into a global biopharmaceutical company and strengthened our late-stage pipeline. 12 The Company

G ro up M a na g em en t R ep o rt The Management Board Malte Peters, M.D. Chief Research and Development Officer Jean-Paul Kress, M.D. Chief Executive Officer Sung Lee Chief Financial Officer from left to right: The Management Board The Company 13

Letter to the Shareholders Dear Ladies and Gentlemen, Dear Shareholders, In 2020, with the accelerated U.S. approval and launch of Monjuvi® (tafasitamab-cxix), we became an integrated commercial biopharma- ceutical company. Building on this achievement, in 2021 we made significant steps towards our ambition of becoming a leader in the hematology/oncology space. We continue to make progress with the Monjuvi launch, and 2021 has been the first full year as a commer- cial enterprise. We have seen encouraging sales growth over the past quarters and approximately 2,000 patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) have been treated with our immunotherapy in the U.S. since the approval in 2020. In 2021 we made an important strategic move with the acquisition of Constellation Pharmaceuticals which added pelabresib to our late stage pipeline. Pelabresib, like tafasitamab, has the potential to change how cancer patients are treated. 2021 also reflected the ongoing challenges we faced with the COVID-19 pandemic. Despite the incredible achievements in medicine, the dis- ease continues to affect us all on both a personal and business level. We are just at the beginning of the opportunity with Monjuvi® Monjuvi received accelerated approval in July 2020 in the U.S. for adult patients with r/r DLBCL and who are not eligible for autologous stem cell transplant. DLBCL is an aggressive disease and the most common type of non-Hodgkin’s lymphoma (NHL) worldwide. Monjuvi is the first and only FDA-approved second-line treatment for non-transplant eligible patients, and we believe this cancer immunotherapy has the potential to transform the standard of care in DLBCL. The adoption of new treatment regimens, especially in DLBCL, can take time. While the pandemic restricted our ability to meet with doctors in Letter to the Shareholders 14 The Company Letter to the Shareholders

G ro up M a na g em en t R ep o rt person for the majority of the first half of 2021, the gradual opening up of facilities and continued education about the benefits of Monjuvi in the second half led to a broadening of the prescriber base and in- creased treatments of second-line patients. During the year, we presented compelling new data for Monjuvi: The three-year long-term data from the L-MIND study not only showed a durable response and consistent safety profile, but also suggested that the combination with lenalidomide could potentially lead to durable remission. We also presented real-world evidence at a medi- cal conference comparing the tafasitamab-lenalidomide combi- nation with other treatments currently used for relapsed or refrac- tory DLBCL. The tafasitamab opportunity has broad potential applicability in NHL. Looking beyond the U.S. market, we were delighted to announce with our partner, Incyte, conditional approvals in 2021 in the European Union and Canada. Incyte has exclusive commercialization rights to tafasitamab outside the United States. We also made important progress in potentially broadening the indi- cations for tafasitamab. In the spring, we initiated a pivotal phase 3 trial, called frontMIND, in first-line DLBCL. We started this trial based on encouraging early-stage data in DLBCL patients previously un- treated for their disease, and the trial is already enrolling with signifi- cant interest from the medical community. With our partner Incyte, we initiated a phase 3 trial in other types of NHL: In April, we dosed the first patient in the inMIND study, evaluating tafasitamab in combina- tion with lenalidomide and rituximab in patients with r/r follicular lym- phoma (FL) or marginal zone lymphoma (MZL). Acquisition of Constellation Pharmaceuticals represents transforma- tional growth opportunity An important part of our growth strategy has been to expand our late-stage clinical pipeline, especially in our focus area of hematology/ oncology. The Constellation acquisition bolstered our position in he- matology/oncology, supporting our aspiration to become a leader in this space. It added another phase 3 product candidate, pelabresib, to our pipeline that is currently being evaluated in combination with ruxolitinib, the current standard of care, in patients with myelofibrosis. Our vision here is to change the standard of care. With the acquisition also came a promising mid-stage oncology clinical program, CPI-0209, an EZH2 inhibitor. Letter to the Shareholders The Company 15

To fund this acquisition, we entered into a funding agreement with Royalty Pharma that could potentially provide more than US$ 2 billion of capital. We understand there are questions about the rationale of monetizing royalty streams in order to finance the development of clinical programs. We did this because of the high conviction we have in pelabresib, the lead product candidate of Constellation. Moreover, we fundamentally believe we can create greater long-term share- holder value by focusing on our proprietary drug development and commercialization. Our vision for pelabresib is to change the treatment paradigm for myelofibrosis A major growth driver over the intermediate and long-term is pelabresib which we believe could have the potential to change the standard of care in myelofibrosis. It is currently being evaluated in a pivotal phase 3 clinical trial (MANIFEST-2) to treat myelofibrosis – a type of bone marrow cancer for which there is no cure and treatment options are limited. The latest interim data from the MANIFEST phase 2 trial were presented in late 2021 at the American Society of Hematology Annual Meeting. These data underpin our strong conviction in pelabresib and in the ongoing phase 3 trial where we have applied our clinical development expertise to optimize the trial design. We expect the data to readout in the first half of 2024 and we are continuing our efforts to ensure operational excellence. “In 2021 we took bold moves towards realizing our long- term goal of becoming not just a player but a leader in the hema tology/oncology space.” Jean-Paul Kress, M.D., Chief Executive Officer 16 The Company Letter to the Shareholders

G ro up M a na g em en t R ep o rt Growing the clinical pipeline behind our lead programs Beyond tafasitamab and pelabresib, we are building an attractive and robust pipeline. We acquired CPI-0209 through the acquisition of Constellation. CPI-0209 is a small molecule designed to promote anti- tumor activity by specifically inhibiting EZH2. We believe that targeting EZH2 has the potential for a broad therapeutic application in a variety of tumor types. CPI-0209 is currently being assessed in a phase 2 study, and we expect to report proof-of-concept data in 2022. Felzartamab is a CD38 targeting antibody that has the potential to deplete CD38 positive plasma cells. The clinical development of felz- artamab in two autoimmune diseases affecting the kidneys – membra- nous nephropathy and immunoglobulin A nephropathy is progressing. The enrollment for the studies in membranous nephropathy has been completed and topline data are expected in the second half of 2022. We thank you for helping MorphoSys to achieve our ambitious goals and build a bright future MorphoSys is entering an exciting, new era. We took bold steps this year to strengthen our position for future success. We are very well positioned to develop and deliver innovative cancer medicines that could have a profound impact on the lives of cancer patients and drive growth in the years ahead. We are proud of our accomplishments this year and remain highly focused on successfully delivering and executing our strategy. I am truly excited about what lies ahead for our Company. I would like to thank everyone who has played an important role in help- ing us bring life-saving therapies to patients. Thank you to our employees for their hard work, dedication and flexibility during a time of uncer- tainty and change. Thank you to the clinicians, patients and their loved ones who participate in our clinical trials. And thank you to our share- holders who believe in our Company and our ability to achieve our goals. We look forward to continuing to make progress in the year ahead and making a difference for people with cancer. Sincerely, Jean-Paul Kress, M.D. Chief Executive Officer Letter to the Shareholders The Company 17

Report of the Supervisory Board Cooperation of the Management Board and Supervisory Board During the 2021 financial year, the Supervisory Board compre- hensively performed the duties assigned to it by law, the Articles of Association, Rules of Procedure and the recommendations of the German Corporate Governance Code in its version dated De- cember 16, 2019 (hereinafter referred to as the “Code”) with two justified exceptions. We regularly advised and continually over- saw the Management Board in its management of the Company and dealt extensively with the operational and strategic develop- ment of the Group. The Management Board fulfilled its duty to inform and furnish us with periodic written and verbal reports containing timely and detailed information on all business trans- actions and events of significant relevance to the Company. The Management Board prepared these reports in collaboration with the respective departments. In our Committee meetings and ple- nary sessions, we had the opportunity to discuss the Manage- ment Board’s reports and the proposed resolutions in full. The Management Board answered our questions on strategic topics affecting the Company with a great level of detail and submitted the relevant documents in a timely manner. Any deviations from the business plan were thoroughly explained to us and we were directly involved at an early stage in all decisions relevant to the Company. An appropriate resolution was passed when the Supervisory Board’s approval for individual actions was required by law, the Articles of Association or the Rules of Procedure. The Supervisory Board members approved all actions by the Management Board requiring Supervisory Board approval based on the documenta- tion provided in advance by the Management Board. When neces- sary, the Supervisory Board received the support of the relevant Committees and, together with the Management Board, discussed any projects requiring decision. All matters requiring approval were submitted for review by the Management Board to the Super- visory Board on a timely basis. Outside of the meetings of the Supervisory Board plenum and the Committees, the chairman of the Supervisory Board regularly exchanged information and ideas with the Management Board and especially the Chief Executive Officer, Dr. Jean-Paul Kress. The Supervisory Board chairman was always kept promptly in- formed of the current business situation and any significant busi- ness transactions. The Chairs of the Committees have also had regular contact with the Management Board members in their respective areas of responsibility and individual Management Board members on demand. Supervisory Board Meetings in the 2021 Financial Year and Key Items of Discussion A total of 11 Supervisory Board meetings were held in the 2021 financial year. The Supervisory Board regularly held closed ses- sions without participation of the Management Board as part of their Supervisory Board meetings. All Supervisory Board mem- bers were present at all Supervisory Board meetings. A detailed overview of the participation of all Supervisory Board members in the respective Supervisory Board and Committee meetings can be found in the “Statement on Corporate Governance,” which is available on the Company’s website under the heading “Inves- tors > Corporate Governance > Statement on Corporate Gover- nance,” and in the Annual Report on pages 90 to 91. In urgent cases occurring outside of meetings, the Supervisory Board passed resolutions by written procedure. In addition to the above, a one-day strategy meeting took place in November 2021 that primarily addressed: • the Company’s corporate strategy & financial outlook; • the development strategy for the clinical and pre-clinical assets of the MorphoSys Group; • review of the research portfolio and strategy for the research assets of the MorphoSys Group; and • organizational effectiveness. During the 2021 financial year, the Supervisory Board paid par- ticular attention to the following topics and passed resolutions on these topics after a thorough review and discussion: • acquisition of Constellation Pharmaceuticals Inc.; • entering into the strategic funding partnership with Royalty Pharma Inc. including capital increase to allow for agreed upon equity investment by Royalty Pharma; • approval of various study and supply-related contracts exceed- ing EUR 10 Mio.; • evaluation of the achievement of the Company goals 2020 and setting the Company goals for 2021 and 2022; • approval of the terms and conditions of the Performance Share Unit Program 2021 as well as an amendment to the terms and conditions of the Performance Share Unit Program 2020 and defi- nition of the number of performance share units to be granted to the members of the Management Board under these plans; • approval of the terms and conditions of the Restricted Stock Units Program 2021 and the Stock Option Program 2021 for US beneficiaries; • agenda and proposed resolutions for the 2021 Annual General Meeting; Report of the Supervisory Board 18 The Company Report of the Supervisory Board

G ro up M a na g em en t R ep o rt • confirmation of Dr. Marc Cluzel as chair of the Supervisory Board and re-establishment and re-staffing of the Committees in the Board’s constituent meeting following the 2021 Annual General Meeting; • appointment of the new Chief Financial Officer, Sung Lee, and conclusion of a corresponding service agreement; • re-appointment of Dr. Jean-Paul Kress as Chief Executive Officer and Dr. Malte Peters as Chief Research & Development Officer and conclusion of corresponding service agreements; • revision of the rules of procedure of the Management Board in- cluding schedules of responsibilities; • resolution upon a remuneration system for the Management Board and for the Supervisory Board as well as development of a new remuneration system 2022 and the remuneration report 2021; • conclusion of a release agreement with the former Chief Operat- ing Officer, Dr. Roland Wandeler, in the course of his stepping down as of December 31, 2021; • update of the declaration of conformity 2020 and new declara- tion of conformity 2021; and • budget for the 2022 financial year. We commissioned an independent remuneration consultant to confirm the appropriateness of the Management Board’s compen- sation and its comparison to the remuneration of various levels of employees. We discussed and agreed on the key performance indicators for the long-term incentive plans for the Management Board and other employees in key positions. Further, we approved the new remuneration system for the members of the Manage- ment Board, which is in line with the new provisions of the Ger- man Stock Corporation Act (AktG) and the Code, and which was submitted for approval to the 2021 Annual General Meeting. In addition, we developed a new remuneration system which shall be submitted for approval to the 2022 Annual General Meeting. Furthermore, we approved the financial statements for the finan- cial year 2020, acknowledged the half-year results for 2021 and the first and third quarter reports as well as dealt with the State- ment on Corporate Governance and the Corporate Governance Report. Our regular discussions in the Supervisory Board’s plenary meet- ings were focused on MorphoSys’ long-term strategy, Monjuvi® sales performance, revenue and cash development as well as the regular financial reports including the communication to the in- vestor community and share price development. Further focal points of discussion were results and progress of the Company’s clinical programs for the development of proprietary drugs and research activities as well as the acquisition and subsequent inte- gration of Constellation Pharmaceuticals, which included a review of the combined research and development portfolio and future organizational R&D set-up. Furthermore, we reviewed the finan- cial outlook for the 2023/2024 financial years and deliberated on MorphoSys’ associated future potential financing needs. In addition, we carried out an evaluation on how effective the Super- visory Board and its committees fulfill their tasks, which was performed via a questionnaire that included a joint self-evaluation of the Supervisory Board, its Committees and also the Manage- ment Board. Furthermore, we kept ourselves regularly informed with respect to the Company’s risk management system, internal audit results as well as the internal control and compliance man- agement system. Conflicts of Interest within the Supervisory Board No conflicts of interest arose within the Supervisory Board in the 2021 financial year. Activities and Meetings of Supervisory Board Committees To ensure that its duties are performed efficiently, the Supervisory Board has established three permanent committees – the Audit Committee, the Remuneration and Nomination Committee and the Science and Technology Committee – to prepare the issues that fall within the Supervisory Board’s respective areas of responsi- bility for the Supervisory Board plenum. In each Supervisory Board meeting, the chairs of the Committees report to the Super- visory Board on the Committees’ work. The minutes of the Com- mittee meetings are made available to all Supervisory Board members. The composition of these committees can be found in the “Statement on Corporate Governance,” which is available on the Company’s website under the heading “Investors > Corporate Governance > Statement on Corporate Governance,” and in the Annual Report on pages 87 to 92. The Audit Committee met on 5 occasions in the 2021 financial year. All Committee members were present at all Audit Committee meetings. The Committee dealt mainly with accounting issues, quarterly reports, annual financial statements and consolidated financial statements. The Committee discussed these topics with the Management Board and recommended the approval of the financial statements to the Supervisory Board. The auditor took part in four Audit Committee meetings and informed its members of the audit results. Based on the Auditors Reform Act and the requirements for the external and internal rotation of the auditor, the Audit Committee had carried out in 2020 a public tender for the 2021 annual audit and half-year review. As a result, the Audit Committee made a recommendation to the Supervisory Board with respect to the Supervisory Board’s proposal at the Annual Gen- eral Meeting for the election of the independent auditor for the 2021 financial year. In addition, the Audit Committee dealt with the annual update of a list of permitted and pre-approved non-audit services of the auditor. The Committee also discussed the risk management system, the compliance management system and the results of the internal audit conducted in the 2021 financial year, as well as specific accounting issues under International Report of the Supervisory Board The Company 19

Financial Reporting Standards (IFRS) relevant to the Company. Furthermore, the Committee regularly discussed the Company’s asset management policy and the investment recommendations made by the Management Board. The Committee also discussed in depth the 2022 budget and the financial outlook for the 2023/2024 financial years. Furthermore, the Committee moni- tored the further development and adaptation to new processes and transactions of the system of Internal Control over Financial Reporting (ICoFR) to ensure continuous SOX compliance by end of 2021. To increase efficiency, there is a joint Remuneration and Nomina- tion Committee, which deliberates on matters relating to remu- neration and nomination. The Committee met on 8 occasions in the 2021 financial year. All Committee members participated at all Committee meetings. In its function as a remuneration com- mittee, the Committee mainly dealt with the Management Board’s remuneration system and level of compensation. In particular, the Committee dealt with the implementation of a new remuneration system 2021 for the members of the Management Board, which was submitted to the 2021 Annual General Meeting for approval. In addition, the Committee developed a new remuneration system 2022 for the members of the Management Board, which shall be submitted to the 2022 Annual General Meeting for approval, and dealt with the preparation of the 2021 remuneration report. Fur- ther, the Committee also commissioned an independent remuner- ation expert to verify the (horizontal and vertical) appropriateness of the Management Board’s remuneration. Based on this report, the Committee prepared a recommendation on the Management Board’s compensation and submitted this to the Supervisory Board for approval. In addition, the Committee gave careful con- sideration to the Company goals as a basis for the Management Board’s short-term variable remuneration and offered appropriate recommendations to the Supervisory Board for resolution. The Committee discussed the key performance indicators of the long- term incentive plans for the Management Board and other em- ployees in key positions. Further, this Committee prepared the release agreement with the Chief Operating Officer, Dr. Roland Wandeler. In addition, this Committee dealt with succession plan- ning within the Company, in particular as regards the succession of the departed Management Board member Jens Holstein. In this context, the Committee recommended the appointment and pre- pared the respective Management Board contract of Sung Lee as the new Chief Financial Officer, who has been appointed as mem- ber of the Management Board by the Supervisory Board. The Science and Technology Committee was held on 6 occasions during the 2021 financial year. All Committee members partici- pated in all Committee meetings. The Committee dealt mainly with the Company’s research activities as well as overall strategy to expand the proprietary drug pipeline, the development of novel technologies, the Company’s drug development plans and future development strategy, progress in the clinical trials as well as required budget resources. One major focus was the acquisi- tion and integration of Constellation Pharmaceuticals, including the overall strategy and opportunity related to the expansion of the research pipeline and the development in myelofibrosis, the development of the BET Inhibitor pelabresib, as well as the second generation EZH2 Inhibitor (CPI-0209). The Committee further deliberated about the future research organizational set-up. Moreover, the development of tafasitamab with its expansion into other indications and lines of therapy, in combination with estab- lished or novel anti-cancer agents, was examined. The Committee also evaluated the execution of the frontMIND and firstMIND studies to complement the forementioned development. Addition- ally, the Committee also addressed and reviewed the further de- velopment of felzartamab in autoimmune diseases. The members of the Science and Technology Committee also serve as members of the ad-hoc deal committee, which meets in this function when necessary and which dealt with the acquisi- tion of Constellation Pharmaceuticals Inc. during the financial year 2021. Corporate Governance The Supervisory Board devoted its attention to the further devel- opment of MorphoSys’ corporate governance, taking into consid- eration the Code. The Corporate Governance Statement according to Section 289f HGB (German Commercial Code), including the detailed Corporate Governance Report, and the Group Statement on Corporate Governance according to Section 315d HGB, can be found on the Company’s website under the heading “Investors > Corporate Governance > Corporate Governance Report” and in the Annual Report on pages 85 to 104. We also discussed with the Management Board the Company’s compliance with the Code’s recommendations and in two justified cases approved an exception to the recommendations of the Code. Based on this consultation, the Management Board and the Super- visory Board submitted the annual Declaration of Conformity on November 29, 2021. The current version of the Declaration of Conformity can be found in this Annual Report and is perma- nently available on the Company’s website under the heading “Investors > Corporate Governance > Declaration of Conformity.” Changes in the Composition of the Management Board and Supervisory Board By decision of the Supervisory Board on January 18, 2021, Sung Lee was appointed as Chief Financial Officer for a term of three years from February 2, 2021, until January 31, 2024. Further, the Chief Operating Officer, Dr. Roland Wandeler, resigned as mem- ber of the Management Board and COO in November 2021 with effect as of December 31, 2021. No further changes in the compo- sition of the Management Board took place during the 2021 finan- cial year. No changes in the composition of the Supervisory Board took place during the 2021 financial year. 20 The Company Report of the Supervisory Board

G ro up M a na g em en t R ep o rt Audit of the Annual Financial Statements and Consolidated Financial Statements For the 2021 financial year, the Company commissioned Pricewater- houseCoopers GmbH Wirtschaftsprüfungsgesellschaft, Munich (“PwC”) as its auditor. The audit contract was awarded by the Supervisory Board in accordance with the resolution of the Annual General Meeting on May 19, 2021. The Supervisory Board ob- tained a declaration of independence from the auditor in advance. The consolidated financial statements and the annual financial statements of MorphoSys AG, as well as the Group Management Report and the Management Report for the 2021 financial year, were properly audited by PwC and issued with an unqualified audit opinion. The key topics of the audit for the consolidated and annual financial statements for the 2021 financial year were man- agement override of controls and fraud in revenue recognition, the risk of fraud in revenue recognition due to potential fictitious manual adjustments to revenue, the gross-to net accounting re- lated to Monjuvi®, sales, the subsequent valuation of the financial liability from collaborations, the valuation of the financial liabili- ties arising from the agreements with Royalty Pharma, the tax treatment of the Royalty Pharma agreements, the purchase price allocation for the business combination with Constellation Pharma- ceuticals, the goodwill impairment test related to Constellation Pharmaceuticals and for statutory purposes the valuation of the investment in MorphoSys US Inc., as well as the assessment of the design and effectiveness of internal controls in accordance with SOX404. In addition, the auditor confirmed that the Manage- ment Board had established an appropriate early risk detection system. The audit reports and documents relating to the consolidated financial statements and the annual financial statements were provided on a timely basis to all Supervisory Board members for review. The audit report, the consolidated financial statements, the Group Management Report of the MorphoSys Group and the audit report, the annual financial statements and the Manage- ment Report of MorphoSys AG were discussed in detail at the Audit Committee meeting on March 14, 2022, and the meeting of the Supervisory Board on March 15, 2022. The auditor attended all meetings concerning the consolidated and annual financial state- ments, the half-year report and quarterly interim statements and reported on the key results of his audit and review, respectively. The auditor also explained the scope and focus of the audit and review and was available to the Audit Committee and the Super- visory Board to answer questions and provide further information. The Audit Committee discussed the audit results in detail and recommended to the Supervisory Board that it approves the consolidated and annual financial statements prepared by the Management Board. The Supervisory Board also took note of the audit results and, in turn, reviewed the consolidated and annual financial statements and Management Reports in accordance with the statutory provisions. Following its own examination, the Supervisory Board also determined that it sees no cause for objec- tion. The consolidated and annual financial statements as well as the Group Management Report and the Management Report as prepared by the Management Board and audited by the auditor, were subsequently approved by the Supervisory Board. Thus, the annual financial statements were adopted. The Company has to prepare a remuneration report in accordance with Sec. 162 of the German Stock Corporation Act (AktG) and a separate non-financial report for the fiscal year 2021. The Super- visory Board has commissioned PwC with a voluntary material review of the remuneration report and to review the separate non-financial report by way of a review with limited assurance. All members of the Supervisory Board received the remuneration report and the separate non-financial report and the independent auditor’s report on the audit in a timely manner. PwC’s report and the audit opinion were discussed at the Supervisory Board’s ple- nary meeting on March 15, 2022. PwC’s auditor participated in this discussion and presented the audit results. The Supervisory Board took note of the results of the audit with approval. Recognition for Dedicated Service On behalf of the entire Supervisory Board, I would like to thank the members of the Management Board and the employees of MorphoSys for their achievements, their dedicated service and the inspirational work environment witnessed during this past financial year. Through their efforts, MorphoSys’ portfolio has continued to mature and expand, and important milestones have been achieved. The Supervisory Board would also like to thank the departed Management Board member Dr. Roland Wandeler for his contri- butions to the Executive Committee, the launch of Monjuvi® and the establishment of our commercial operations. Planegg, March 15, 2022 Dr. Marc Cluzel Chairman of the Supervisory Board Report of the Supervisory Board The Company 21

Supervisory Board of MorphoSys AG Supervisory Board of MorphoSys AG Marc Cluzel, M.D., Ph.D. Chairman, Montpellier, France Member of the Supervisory Board of: Griffon Pharmaceuticals Inc., Canada (Member of the Board of Directors) Moleac Pte. Ltd., Singapore (Member of the Board of Directors) George Golumbeski, Ph.D. Deputy Chairman, Far Hills, NJ, USA Member of the Supervisory Board of: Ananke Therapeutics, Inc., Boston, MA, USA (Chairman of the Board of Directors) Carrick Therapeutics Ltd., Dublin, Ireland (Chairman of the Board of Directors) Sage Therapeutics, Inc., Cambridge, MA, USA (Member of the Board of Directors) Shattuck Labs, Inc., Austin, TX, USA (Chairman of the Board of Directors) Krisja Vermeylen Board Member, Herentals, Belgium Member of the Supervisory Board of: Diaverum AB, Lund, Sweden (Member of the Board of Directors) 22 The Company Supervisory Board of MorphoSys AG

G ro up M a na g em en t R ep o rt The CVs of our Supervisory Board Members can be found on the Company’s website under the heading “About us > Leadership.” Sharon Curran Board Member, Dublin, Ireland Member of the Supervisory Board of: CAT Capital Topco Ltd., Saint Peter Port, Guernsey (Member of the Board of Directors) CAT Capital Bidco Ltd., Dublin, Ireland (Member of the Board of Directors) Clinigen Group plc., Burton upon Trent, United Kingdom (Member of the Board of Directors) Circassia Pharmaceuticals plc., Oxford, United Kingdom (Member of the Board of Directors) Wendy Johnson Board Member, San Diego, CA, USA Member of the Supervisory Board of: Exagen, Inc., Vista, CA, USA (Member of the Board of Directors) Michael Brosnan Board Member, Osterville, MA, USA Member of the Supervisory Board of: Daimler Truck AG, Stuttgart, Germany (Member of the Board of Directors) Daimler Truck Holding AG, Stuttgart, Germany (Member of the Board of Directors) Supervisory Board of MorphoSys AG The Company 23

2021 Non-Financial Report Sustainability at MorphoSys We are aware of our responsibility to current and future generations and believe that sustainable action is a prerequisite for long-term business success. Read more on this topic in our Group’s 2021 Non-Financial Report. You can find our 2021 Non-Financial Report online at: https://csr.morphosys.com/2021 24 The Company Sustainability at MorphoSys

G ro up M a na g em en t R ep o rt MorphoSys on the Capital Market Index Memberships, Stock Market Environment and MorphoSys Share Performance MorphoSys AG shares have been trading on the Frankfurt Stock Exchange since 1999. In 2018, MorphoSys issued American De- positary Shares (ADSs*) on the U.S. NASDAQ exchange based on MorphoSys’ common stock. The Company’s ticker symbol is “MOR” on both exchanges. MorphoSys AG is part of the SDAX index (since September 2021, before MDAX) and of the TecDAX index. MorphoSys is also a com- ponent of the NASDAQ Composite Index through its ADS program and is included in various other indices, such as the NASDAQ Health Care Index, the Loncar Cancer Immunotherapy Index and the S-Network Medical Breakthrough Index. In 2021 the biotech sector underperformed compared to the overall markets. The overall negative sentiment, according to BioCentury, was driven by several factors. Generalist investors fled life sciences for sectors that would benefit from an economic recovery, such as consumer-heavy cyclicals. Also, while in 2020, COVID-19 vaccines lifted the sector as a whole, only a few selected companies benefited in 2021. Indeed, BioCentury’s analysis of NASDAQ-listed biotech found that at year-end 2021, nearly one- third of the 816 companies with a market cap above US$ 25 mil- lion were within 10% of their 52-week low, while almost two- thirds were within 25%. In contrast, only 7% of companies were within 10% of their 52-week high. MorphoSys’ shares opened the 2021 trading year on Xetra at € 93.82 and closed the year at € 33.35. While the NASDAQ Bio- technology Index had a negative return in 2021 and closed the year about 1% lower than at the beginning of the year, the decline of the MorphoSys’ share price in 2021 was much steeper. During the year, MorphoSys made a bold strategic move to set the Com- pany up for long-term value creation, the results of which we expect to play out in the next few years. With the acquisition of Constellation Pharmaceuticals, Inc. (“Con- stellation”), we expanded the pipeline with two clinical-stage can- cer drug candidates. This acquisition was financed through an agreement with Royalty Pharma. We are now well positioned to deliver on our ambition to become a leader in hematology/oncol- ogy with multiple commercial products by 2025 and to create long-term shareholder value. We have all the pieces including a compelling pipeline, development and commercial expertise and a strong balance sheet to execute on our strategic priorities. ›› see figure 01 – Performance of the MorphoSys Share in 2021 (page 26) ›› see figure 02 – Performance of the MorphoSys Share 2017–2021 (page 26) Liquidity The average daily trading volume of the MorphoSys share across all regulated trading platforms decreased to € 27.5 million in 2021 (previous year: € 33.5 million), corresponding to a year-on- year decrease of 18%. For the TecDAX and SDAX selection indices, trading volumes were down year-on-year by 11% and 14%, respec- tively. In the TecDAX, MorphoSys ranked 29th in terms of market capitalization* at year-end 2021 (previous year: 13th). In the SDAX Index, MorphoSys ranked 48th in terms of market capitalization at year-end. * see glossary – page 182 In addition to the trading on the regulated platforms, an average of approximately 258,000 of MorphoSys’ shares with a value of approximately € 15.0 million were traded daily on alternative trading venues (“dark pools”) in 2021 (2020: 217,000 shares; € 22.4 million). This figure corresponds to a year-on-year de- crease in trading outside of the regulated markets of approximately 33%. The MorphoSys ADSs reached a volume of US$ 1.5 million per trading day in the reporting year (previous year: US$ 3 mil- lion), corresponding to a decrease of approximately 50%. Capital Structure The Company’s common stock increased to 34,231,943 shares or € 34,231,943 in the reporting year due to the purchase of shares by Royalty Pharma, created from a capital increase, as well as the exercise of stock options granted to the Management Board and certain Company employees in 2017. A detailed description of the capital increase and convertible bond program can be found in Notes 5.20.1* and 6.1.1*. * cross-reference to page 145 and page 147 MorphoSys on the Capital Market MorphoSys on the Capital Market MorphoSys on the Capital Market The Company 25
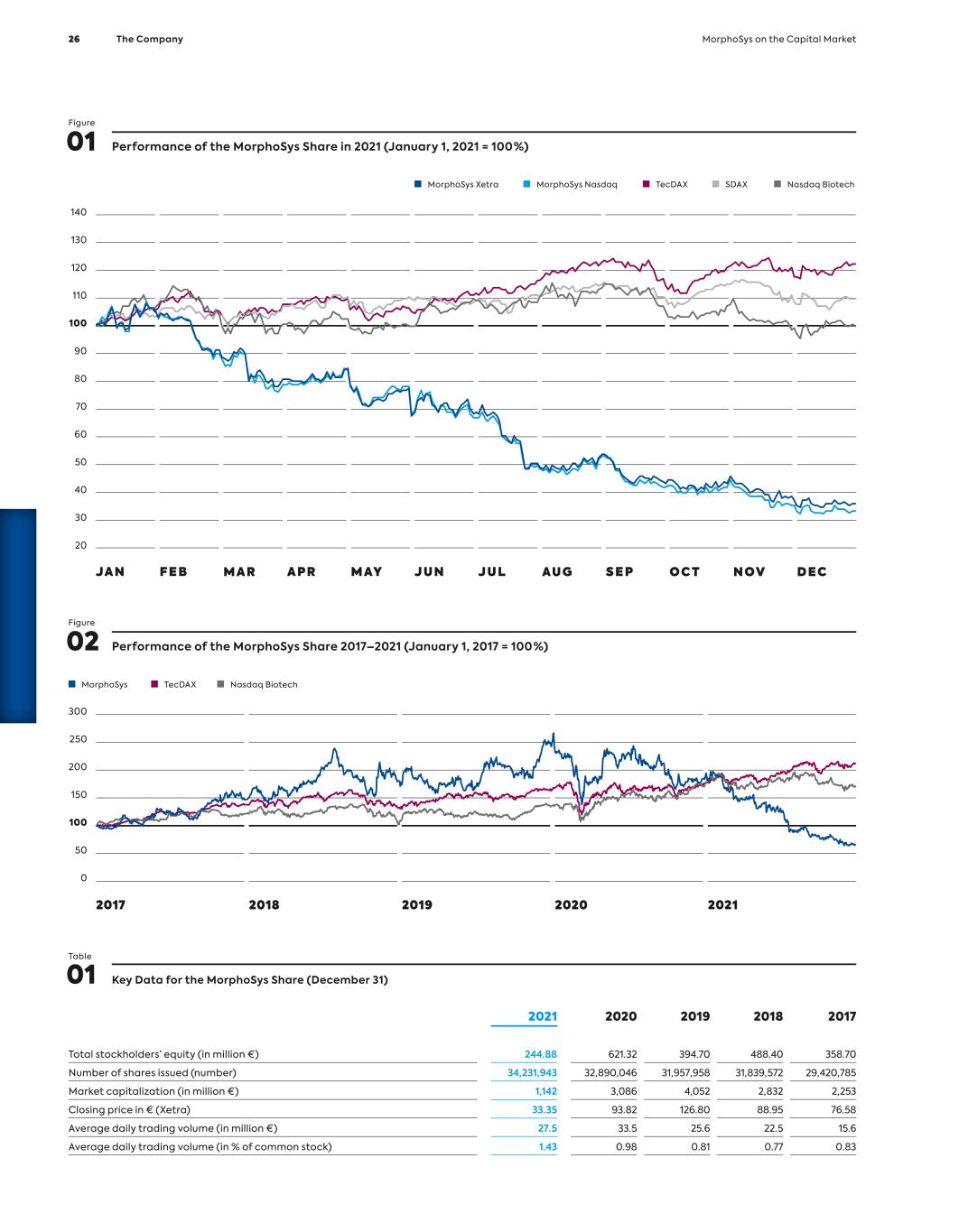
JAN FE B MAR APR MAY JUN JUL AUG SE P OC T NOV DEC TecDAX MorphoSys Nasdaq MorphoSys Xetra SDAX Nasdaq Biotech TecDAX MorphoSys Nasdaq Biotech 140 130 120 110 100 90 80 70 60 50 40 30 20 300 250 200 150 100 50 0 2017 2018 2019 2020 2021 Figure 01 Performance of the MorphoSys Share in 2021 (January 1, 2021 = 100 %) Figure 02 Performance of the MorphoSys Share 2017–2021 (January 1, 2017 = 100 %) Table 01 Key Data for the MorphoSys Share (December 31) 2021 2020 2019 2018 2017 Total stockholders’ equity (in million €) 244.88 621.32 394.70 488.40 358.70 Number of shares issued (number) 34,231,943 32,890,046 31,957,958 31,839,572 29,420,785 Market capitalization (in million €) 1,142 3,086 4,052 2,832 2,253 Closing price in € (Xetra) 33.35 93.82 126.80 88.95 76.58 Average daily trading volume (in million €) 27.5 33.5 25.6 22.5 15.6 Average daily trading volume (in % of common stock) 1.43 0.98 0.81 0.77 0.83 26 The Company MorphoSys on the Capital Market

G ro up M a na g em en t R ep o rt Various voting rights notifications were made pursuant to Section 33 (1) of the German Securities Trading Act (WpHG) during the reporting year. The notifications were published on the MorphoSys website under Investors – Stock Information – Voting Rights. At the end of the reporting year, the free float in MorphoSys AG shares, as per the definition of Deutsche Börse, was 99.76%. Dividend Policy We have not distributed dividends since our inception, and we do not expect to set or distribute any cash dividends in the foresee- able future. It is our intention to invest any future profits in the growth and development of our business. Unless otherwise re- quired by law, the future determination of any cash dividends will be at the sole discretion of the Management Board and Super- visory Board and will depend on our net assets, financial position, results of operations, capital requirements and other factors that the Management Board and Supervisory Board deem relevant. Investor Relations Activities Since March 2020, with the onset of the COVID-19 pandemic, interactions with shareholders, investors and analysts have been taking place digitally to a much greater extent than before. The impact of this has been particularly evident on investor confer- ences, whose added value has always been the direct personal exchange with a broad spectrum of market participants and resulting networking. While the pandemic revealed that increas- ing digitalization saves travel time and costs, it also showed that established processes and contacts needed to adapt even better to the changed digital environment. During the reporting year, MorphoSys participated in 20 international investor conferences and investment banking events. The year 2021 began with the J.P. Morgan Healthcare Conference, where the medical and com- mercial potential of tafasitamab were presented together with an outlook for the rest of the pipeline. The vast majority of investor conferences in 2021 were held using various virtual formats. MorphoSys held conference calls in the reporting year with the publication of its annual, half-year and quarterly reports. These calls could be followed over the Internet. During these calls, the Management Board reported on business developments and an- swered questions from participants. At the analyst and investor meetings, the main topics addressed were the advances made in Monjuvi®’s commercialization, the further progress of tafasitamab’s clinical development, the acqui- sition of Constellation and, its lead product pelabresib, and cash runway. On June 2, 2021, MorphoSys hosted a conference call and webcast on the acquisition of Constellation. During the call, management presented the clinical development candidates pelabresib and CPI-0209 and explained their medical and commercial potential in a number of oncology indications. At the end of 2021, 18 analysts were monitoring and evaluating the performance of MorphoSys shares (previous year: 20). These analysts had the following recommendations at the end of 2021: Table 02 Analyst Recommendations (December 31, 2021) Buy/Overweight/Market Outperform Hold/Neutral Reduce/Underperform 9 9 0 Buy/Overweight/Market Outperform = buy/positive; Hold/Neutral = neutral; Reduce/Underperform = sell/negative. Further detailed information on MorphoSys’ shares, key financial figures, events and conferences, can be found on the Company’s website under Investors. MorphoSys on the Capital Market The Company 27

Non-Financial Group Report https://csr.morphosys.com/2021 Non-Financial Group Report We are conscious of the responsibility we share for present and future generations and see sustainable action as a prerequisite for long-term business success. MorphoSys is dedicated to the discovery, development and commercialization of outstanding, innovative therapies for patients, with a focus on cancer and auto- immune diseases. To ensure sustainable business success, we incorporate Environmental, Social and Governance (ESG) into our daily business and base our business model on sustainable growth that is aligned with the interests of stakeholders. We are focused on creating long-term value and weigh our actions in terms of their impact on the environment, society, patients and employees. A detailed explanation of our view of sustainable corporate gover- nance and the specific measures we have taken during the re- porting year can be found in the separate “Non-Financial Group Report,” available on our website under https://csr.morphosys. com/2021. Non-Financial Group Report 28 The Company Non-Financial Group Report

02Contents Group Management Report Fundamentals of the MorphoSys Group 34 Macroeconomic and Sector-Specific Conditions 51 Analysis of Net Assets, Financial Position and Results of Operations 52 Outlook and Forecast 69 Risk and Opportunity Report 73 Subsequent Events 84 Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance 85 G ro up M a na g em en t R ep o rt Group Management Report 29

Summary In 2021, MorphoSys continued its work to discover, develop and commercialize, innovative therapies for patients, with a focus on cancer and autoimmune diseases. In July 2021, we completed the acquisition of Constellation Pharmaceuticals Inc. (“Constel- lation”). The addition of Constellation offers a transformational growth opportunity for MorphoSys, expanding our pipeline in a meaningful way with two clinical-stage cancer drug candidates. In addition to this major corporate event, we continued to make progress with our existing programs. At the time of the Constella- tion acquisition, we also entered into a funding agreement with Royalty Pharma plc. Our lead program, tafasitamab, is already on the market in the U.S. under the brand name Monjuvi (tafasitamab-cxix). Monjuvi (tafasitamab-cxix) has been approved under accelerated approval by the U.S. FDA in July 2020. Together with Incyte, we are co-promoting Monjuvi in the U.S. Incyte holds exclusive rights for development and commercialization outside the U.S. In August 2021, the European Commission (EC) granted condi- tional marketing authorization for Minjuvi® (tafasitamab) in Europe in combination with lenalidomide, followed by Minjuvi monotherapy, for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplant (ASCT). Also in August 2021, Health Canada granted Incyte a Notice of Com- pliance with conditions for Minjuvi (tafasitamab) in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low- grade lymphoma, who are not eligible for ASCT. In January 2021, a marketing authorization application for tafasitamab was accepted for review by regulatory authorities in Switzerland. 30 Group Management Report

Fi na nc ia l S ta te m en ts To further broaden the tafasitamab opportunity, during 2021, MorphoSys initiated the frontMIND study, a pivotal phase 3 trial in patients with first-line DLBCL. Also, MorphoSys and Incyte announced the first patient dosed in inMIND, a phase 3 study in patients with r/r follicular lymphoma or r/r marginal zone lym- phoma (MZL). In June 2021, MorphoSys and Incyte announced new three-year follow-up data from the phase 2 L-MIND study of tafasitamab in combination with lenalidomide in adult pa- tients with r/r DLBCL. The new results built on previous findings showing durable responses and a consistent safety profile of ta- fasitamab in combination with lenalidomide followed by tafasi- tamab monotherapy. In December 2021, additional results from the RE-MIND2 dataset comparing tafasitamab and lenalido- mide outcomes to those observed in matched cohorts of 1) po- latuzumab vedotin plus bendamustine and rituximab (pola-BR), 2) rituximab plus lenalidomide (R2); and 3) CAR-T therapies were presented. The findings suggested that tafasitamab plus lena- lidomide may improve health outcomes compared to pola-BR and R2, with a prolonged survival benefit for r/r DLBCL patients. Comparable overall survival (OS) between tafasitamab and lenalidomide and CAR-T therapies was observed. Pelabresib is a late-stage proprietary program that MorphoSys acquired through the Constellation acquisition. We are currently conducting two clinical trials of pelabresib for the treatment of myelofibrosis (MF): MANIFEST, our ongoing, open-label phase 2 clinical trial evaluating pelabresib both as a monotherapy and in combination with ruxolitinib, and MANIFEST-2, our global, dou- ble-blinded, randomized pivotal phase 3 study evaluating pela- bresib in combination with ruxolitinib versus placebo in JAK-in- hibitor-naïve MF patients. Exploratory data from the MANIFEST trial were presented at the European Hematology Association (EHA) Annual Meeting in June 2021. Translational data across all Summary Group Management Report 31

three arms of the study illustrated the effect of pelabresib on key cytokines associated with MF and its impact on bone mar- row fibrosis. Taken together, these data support our hypothesis that pelabresib may have a potential disease-modifying activ- ity in MF. At the American Society of Hematology (ASH) Annual Meeting in December 2021, we presented updated interim clini- cal and translational data from the ongoing MANIFEST trial, which included 54 more patients and longer-term follow-up than previously reported data. We believe that the latest interim data from MANIFEST underscore the potential of pelabresib in the treatment of MF. With respect to MANIFEST-2, MorphoSys has optimized the study design and implemented measures to accelerate patient recruitment since the acquisition of Constel- lation. Progress was also made with programs in earlier stages of clinical development. Interim data from the phase 1/2 proof- of-concept M-PLACE trial that evaluates felzartamab in an- ti-PLA2R antibody positive membranous nephropathy (MN) were presented in November 2021. Early efficacy data were pre- sented: of the 27 treated patients with evaluable results, 24 showed an initial rapid reduction of anti-PLA2R antibody levels one week after the first treatment. The safety profile was shown to be consistent with the proposed mechanism of action of felz- artamab. The trial was fully enrolled in November 2021. Two ad- ditional trials with felzartamab were initiated during 2021 – New-PLACE, a phase 2 study evaluating different treatment schedules to identify the regimen for a pivotal study in patients with anit-PLA2R-antibody positive MN, and the phase 2 IGNAZ trial evaluating felzartamab in patients with immunoglobulin A nephropathy (IgAN). 32 Group Management Report Summary

Fi na nc ia l S ta te m en ts CPI-0209 is another product candidate acquired through Con- stellation. CPI-0209 is a small molecule designed to promote anti-tumor activity by specifically inhibiting the enzymatic function of enhancer of zeste homolog 2 (EZH2) protein. We are currently conducting a phase 1/2 clinical trial of CPI-0209 in pa- tients with solid tumors and hematological malignancies. Our partners responsible for clinical development of licensed programs also continued their activities during 2021. For exam- ple, in June I-Mab announced that the Center for Drug Evalua- tion (CDE) of the China National Medical Products Administra- tion (NMPA) had approved the Investigational New Drug (IND) application to initiate a phase 1b study with felzartamab in pa- tients with systemic lupus erythematosus (SLE), the most com- mon form of lupus. In October, licensing partner Roche received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) for gantenerumab for the treatment of people living with Alzheimer’s disease. Also in October 2021, li- censing partner GSK announced that it had made the decision not to further explore otilimab as a potential treatment for se- vere pulmonary COVID-19- related disease in patients aged of 70 years and older. Clinical development of otilimab in rheuma- toid arthritis remains ongoing. In 2021, MorphoSys continued to transform the Company with activities focused on advancing and positioning the Company for long-term success. We grew sales of Monjuvi, while also broadening our pipeline through a transformative acquisition. Summary Group Management Report 33

Fundamentals of the MorphoSys Group Organizational Structure and Business Model MorphoSys AG, as the ultimate parent company, is located in Planegg, near Munich. MorphoSys AG has one wholly owned sub- sidiary, MorphoSys US Inc. (Boston, Massachusetts, USA). MorphoSys US Inc. in turn has a wholly owned subsidiary - Con- stellation Pharmaceuticals, Inc. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. also has a wholly owned subsidiary, Constellation Securities Corp. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. and Constellation Securities Corp. are collectively referred to as “Con- stellation”, and all entities constitute the “MorphoSys Group” or “Group”. Following the acquisition on July 15, 2021, Constellation Pharma- ceuticals Inc. was merged into MorphoSys Development Inc., which was incorporated as a wholly owned subsidiary of MorphoSys US Inc. on May 28, 2021, in accordance with the merger agreement. From this upward merger, Constellation Phar- maceuticals Inc. remained as a wholly owned subsidiary of MorphoSys US Inc. The Planegg site MorphoSys AG houses the central corporate functions such as accounting, controlling, human resources, le- gal, patents, purchasing, corporate communications and investor relations, as well as the scientific research departments and labo- ratories. MorphoSys US Inc. is responsible for advancing tafasi- tamab’s* commercialization. Constellation focuses its activities on the clinical development of drug candidates and the related administrative departments. Further information on the Group’s overall structure can be found in Note 2.2.1*. * cross-reference to page 117 Legal Structure of the Morphosys Group: Group Management and Supervision The parent company of the MorphoSys Group is MorphoSys AG, a German stock corporation listed in the Prime Standard segment of the Frankfurt Stock Exchange and on the NASDAQ Global Mar- ket. In accordance with the German Stock Corporation Act, the Company has a dual management structure with the Manage- ment Board as the governing body. The four members of the Man- agement Board are appointed and supervised by the Supervisory Board. Following the departure of Roland Wandeler, Ph.D., Chief Operating Officer, effective December 31, 2021, the Management Board consists of only three members. The Supervisory Board of MorphoSys AG is elected by the Annual General Meeting and cur- rently consists of six members. Detailed information on the Group’s management and supervision and its corporate gover- nance principles can be found in the Corporate Governance Re- port. Targets and Strategy MorphoSys mission is to discover, develop and commercialize in- novative therapies for patients. MorphoSys is a fully integrated commercial biopharmaceutical company. Its activities in 2021 focused on hematology/oncology and autoimmune diseases. The Company aims to realize intermediate- and long-term growth through its focus on proprietary drug development and commer- cialization. Through the acquisition of Constellation, the Com- pany has rapidly expanded its pipeline in the hematology/oncol- ogy area. Our priority is on the Company’s lead development candidates pelabresib and tafasitamab; continuing to make progress with the commercialization of Monjuvi and obtaining approvals in addi- tional indications; bringing pelabresib to the market as well as continuing to develop other clinical candidates. Fundamentals of the MorphoSys Group 34 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts MorphoSys is now primarily advancing the clinical development of its own compounds, with further antibody candidates being clinically developed by partners. During the clinical phases, deci- sions are made on a case-by-case basis as to whether and at what point a partnership for further development and commercializa- tion should be pursued. Drug candidates can be either fully out-li- censed, developed on a proprietary basis or with a partner (co-de- velopment). The development of drug candidates on behalf of other companies is no longer a focus of MorphoSys’ business activities. As such, the previous segment reporting for the Proprietary Development and Partnered Discovery segments was discontinued as of the first quarter of 2021. The development of drug candidates is broadly based on MorphoSys’ innovative technologies. These include our estab- lished antibody and technology platforms HuCAL®*, Ylanthia®* and Slonomics®*, as well as the bispecific technology CyCAT®. Under the agreement signed with Cherry Biolabs in November 2020, MorphoSys was granted exclusive access to the Hemibody technology* for several targets*, which will be used to develop a novel multispecific antibody technology for effector cell recruit- ment (T-cell* engager). We continue to leverage our resources and know-how to expand and develop these technologies. This may include the expansions of our portfolio not only through our own proprietary research and development activities but also through in-licensing and acquisitions. * see glossary – page 182 Group Management and Performance Indicators MorphoSys uses financial indicators to steer the Group. These in- dicators help to monitor the success of strategic decisions and give the Group the opportunity to take quick corrective action when necessary. The Company’s management also monitors and evaluates selected early indicators so that it can thoroughly as- sess a project’s progress and promptly take the appropriate ac- tions should a problem occur. No most important non-financial performance indicators are used for steering the Company. Mate- rial non-financial aspects are taken into account in a separate “Non-Financial Group Report”, which is available on our website. Financial Performance Indicators The development of the significant financial performance indica- tors in the reporting year is described in detail in the chapter “Analysis of Net Assets, Financial Position and Results of Opera- tions”. The key financial indicators used to measure the Compa- ny’s operating performance are revenues, operating expenses and percentage of research and development expenses included therein. Starting 2022, Monjuvi U.S. net product sales, the gross margin of Monjuvi U.S. net product sales, Research and Development ex- penses, as well as total combined expenses for Selling and Gen- eral & Administrative will be used as key financial performance indicators, since these indicators are the most significant for steering MorphoSys Group. These indicators are routinely ana- lyzed and evaluated. As an additional factor, the liquidity position (presented in the following balance sheet items: “Cash and cash equivalents” as well as “Other financial assets (current and non-current)” is also regularly analyzed and evaluated. Liquidity position is not con- sidered to be part of the key financial performance indicators. The budget for the respective financial year is approved by the Management Board and Supervisory Board. Subsequent to the approval of the budget, a forecast is made two times within the year to assess if the Company is on track to achieve its financial goals and progress towards financial guidance. The forecast in- forms decision-making and enables management to take actions to achieve its goals. Fundamentals of the MorphoSys Group Group Management Report 35
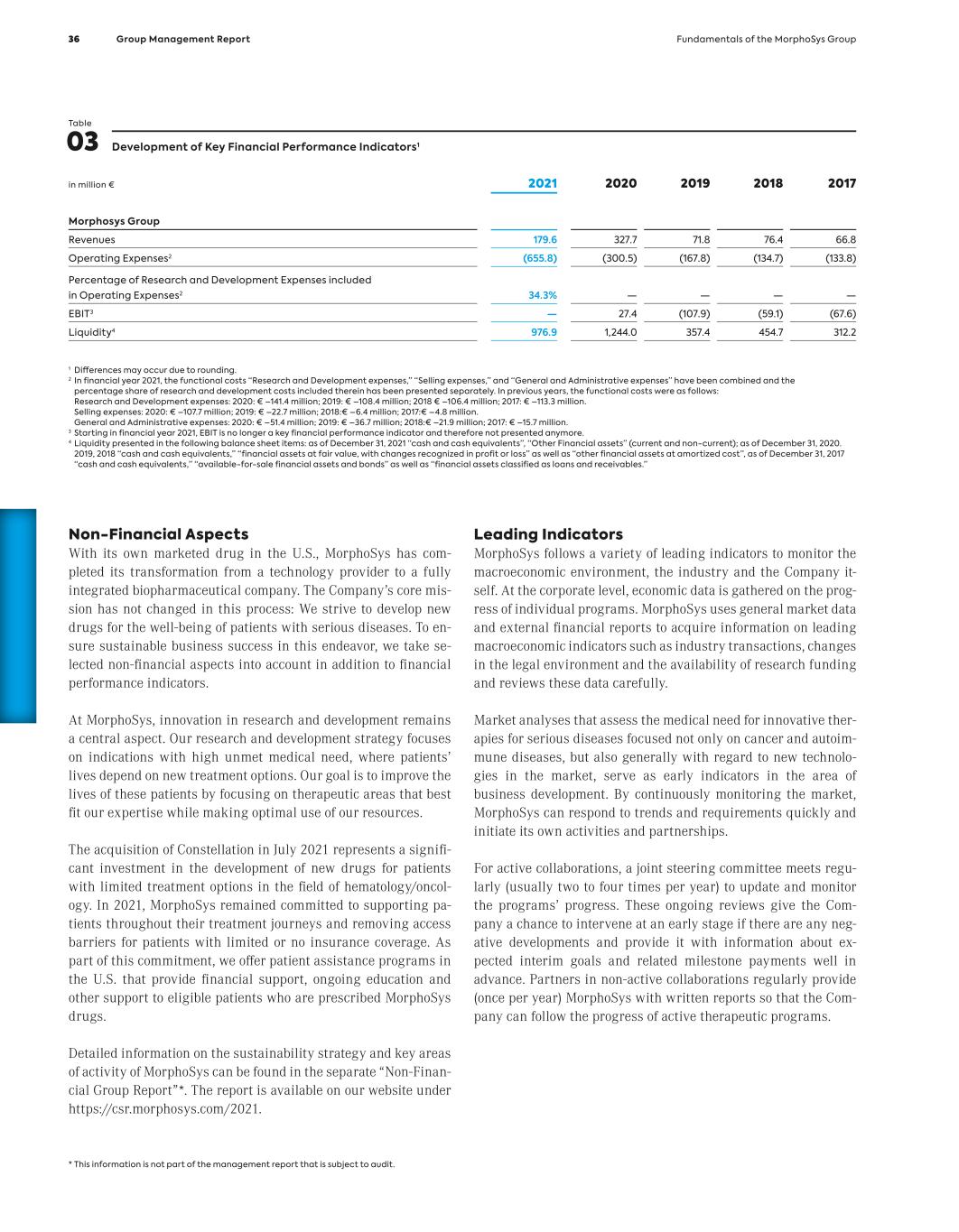
Table 03 Development of Key Financial Performance Indicators1 in million € 2021 2020 2019 2018 2017 Morphosys Group Revenues 179.6 327.7 71.8 76.4 66.8 Operating Expenses2 (655.8) (300.5) (167.8) (134.7) (133.8) Percentage of Research and Development Expenses included in Operating Expenses2 34.3% — — — — EBIT3 — 27.4 (107.9) (59.1) (67.6) Liquidity4 976.9 1,244.0 357.4 454.7 312.2 1 Differences may occur due to rounding. 2 In financial year 2021, the functional costs “Research and Development expenses,” “Selling expenses,” and “General and Administrative expenses” have been combined and the percentage share of research and development costs included therein has been presented separately. In previous years, the functional costs were as follows: Research and Development expenses: 2020: € –141.4 million; 2019: € –108.4 million; 2018 € –106.4 million; 2017: € –113.3 million. Selling expenses: 2020: € –107.7 million; 2019: € –22.7 million; 2018:€ –6.4 million; 2017:€ –4.8 million. General and Administrative expenses: 2020: € –51.4 million; 2019: € –36.7 million; 2018:€ –21.9 million; 2017: € –15.7 million. 3 Starting in financial year 2021, EBIT is no longer a key financial performance indicator and therefore not presented anymore. 4 Liquidity presented in the following balance sheet items: as of December 31, 2021 “cash and cash equivalents”, “Other Financial assets” (current and non-current); as of December 31, 2020. 2019, 2018 “cash and cash equivalents,” “financial assets at fair value, with changes recognized in profit or loss” as well as “other financial assets at amortized cost”, as of December 31, 2017 “cash and cash equivalents,” “available-for-sale financial assets and bonds” as well as “financial assets classified as loans and receivables.” Non-Financial Aspects With its own marketed drug in the U.S., MorphoSys has com- pleted its transformation from a technology provider to a fully integrated biopharmaceutical company. The Company’s core mis- sion has not changed in this process: We strive to develop new drugs for the well-being of patients with serious diseases. To en- sure sustainable business success in this endeavor, we take se- lected non-financial aspects into account in addition to financial performance indicators. At MorphoSys, innovation in research and development remains a central aspect. Our research and development strategy focuses on indications with high unmet medical need, where patients’ lives depend on new treatment options. Our goal is to improve the lives of these patients by focusing on therapeutic areas that best fit our expertise while making optimal use of our resources. The acquisition of Constellation in July 2021 represents a signifi- cant investment in the development of new drugs for patients with limited treatment options in the field of hematology/oncol- ogy. In 2021, MorphoSys remained committed to supporting pa- tients throughout their treatment journeys and removing access barriers for patients with limited or no insurance coverage. As part of this commitment, we offer patient assistance programs in the U.S. that provide financial support, ongoing education and other support to eligible patients who are prescribed MorphoSys drugs. Detailed information on the sustainability strategy and key areas of activity of MorphoSys can be found in the separate “Non-Finan- cial Group Report”*. The report is available on our website under https://csr.morphosys.com/2021. Leading Indicators MorphoSys follows a variety of leading indicators to monitor the macroeconomic environment, the industry and the Company it- self. At the corporate level, economic data is gathered on the prog- ress of individual programs. MorphoSys uses general market data and external financial reports to acquire information on leading macroeconomic indicators such as industry transactions, changes in the legal environment and the availability of research funding and reviews these data carefully. Market analyses that assess the medical need for innovative ther- apies for serious diseases focused not only on cancer and autoim- mune diseases, but also generally with regard to new technolo- gies in the market, serve as early indicators in the area of business development. By continuously monitoring the market, MorphoSys can respond to trends and requirements quickly and initiate its own activities and partnerships. For active collaborations, a joint steering committee meets regu- larly (usually two to four times per year) to update and monitor the programs’ progress. These ongoing reviews give the Com- pany a chance to intervene at an early stage if there are any neg- ative developments and provide it with information about ex- pected interim goals and related milestone payments well in advance. Partners in non-active collaborations regularly provide (once per year) MorphoSys with written reports so that the Com- pany can follow the progress of active therapeutic programs. * This information is not part of the management report that is subject to audit. 36 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts Commercialization In July 2018, MorphoSys established a subsidiary in the United States – MorphoSys US Inc. – in preparation for the potential mar- keting approval of tafasitamab. The subsidiary’s registered office is located in Boston, Massachusetts, USA. At the end of 2021, MorphoSys US Inc. had 93 people employed as part of, or to sup- port, its commercial structure. MorphoSys’ commercial activities are currently focused on Monjuvi in the United States; the Com- pany is co-commercializing this product with Incyte. On July 31, 2020, Monjuvi (tafasitamab-cxix)* in combination with lenalidomide was approved under acceleratd approval by the U.S. FDA* for the treatment of adult patients with relapsed or re- fractory (r/r*) diffuse large B-cell lymphoma (DLBCL*) not other- wise specified, including DLBCL arising from low-grade lym- phoma, and who are not eligible for autologous stem cell transplant (ASCT*). This was the first U.S. FDA approval of a second-line treatment for adult patients with r/r DLBCL in the U.S. The safety and tolerability profile supports a paradigm shift towards treat- ing patients to progression, which could enable long-term disease control. Monjuvi is accessible to patients in both community care and academic settings as an off-the-shelf intravenous infusion that does not require hospitalization or heavy monitoring. Upon approval, MorphoSys and Incyte launched ‘My Mission Support’, a robust patient support program offering financial assistance, ongoing education and other resources to eligible patients who are prescribed Monjuvi in the U.S. The program was launched to support patients throughout their treatment journeys and to help lower patient access barriers. Monjuvi has been included in the National Comprehensive Can- cer Network® Clinical Practice Guidelines (NCCN Guidelines®) in Oncology for B-cell Lymphomas since August 2020. The NCCN Guidelines in the United States were updated to include Monjuvi in combination with lenalidomide with a Category 2A designation as an option for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) not other- wise specified, including DLBCL arising from low-grade lym- phoma, and who are not eligible for ASCT. Inclusion in these guidelines increases awareness of a product within the oncology community and also drives certain formulary decisions. As of April 1, 2021, Monjuvi was granted a J-code, further simplifying reimbursement for some treatment centers. In the first half of 2021, commercial performance was adversely impacted by the COVID-19 pandemic. As vaccination rates in- creased there was a gradual easing of restrictions at sites of care, which allowed sales teams to engage in more face-to-face meet- ings with physicians and contributed to the positive momentum we observed in the second half of 2021. Many larger facilities, however, opened at a slower pace than community health centers. In 2021, MorphoSys and Incyte continued to see high penetration in the community setting which drove approximately 70% of Mon- juvi prescriptions. Since launch, the Company, along with partner Incyte, has in aggregate received orders from more than 1,000 treatment sites. During the fourth quarter, more than 570 ac- counts placed orders, with over 70% of those accounts representing repeat accounts. The proportion of accounts that reordered has been consistent from the third quarter through the fourth quarter. The partnership has also made tremendous strides in educating our customers of the value of combination treatment of Monjuvi and lenalidomide for people with r/r DLBCL not otherwise speci- fied, and who are ineligible for ASCT. Approximately 2,000 pa- tients have been treated with Monjuvi in the U.S. since its launch. Operating Business Performance During 2021, MorphoSys focused on commercializing its marketed product and in advancing product candidates at various stages of development. The acquisition of Constellation represents a trans- formation for MorphoSys, expanding its clinical development pipe- line and positioning the Company for long-term sustainable growth. The key measures of value for MorphoSys’ research and develop- ment activities include: • Project launches and the advancement of individual develop- ment programs • Clinical and preclinical research results • Regulatory guidance of healthcare authorities for the approval of individual therapeutic programs • Collaborations, partnerships and M&A activities with other companies to expand the technology base and expand the drug pipeline, as well as to commercialize the therapeutic programs • Strong patent protection to secure MorphoSys’ market position MorphoSys announced on June 2, 2021, its plans to acquire Con- stellation Pharmaceuticals Inc. for US$ 34.00 (equivalent to € 28.79) per share in cash, representing a total equity value of US$1,635.2 million (equivalent to € 1,384.7 million). The transac- tion was unanimously approved by the Management Board and Supervisory Board of MorphoSys as well as by the Board of Direc- tors of Constellation. The acquisition was completed on July 15, 2021. Constellation is a clinical-stage biopharmaceutical com- pany that discovers and develops novel product candidates to ad- dress serious unmet medical needs in patients with cancers asso- ciated with abnormal gene expression or drug resistance. Constellation’s two lead product candidates, pelabresib (CPI- 0610*), a BET inhibitor, and CPI-0209, a second-generation EZH2* inhibitor, are in mid- to late-stage clinical development. MorphoSys expects the following benefits from the acquisition: • Accelerates growth strategy with promising mid- to late-stage product candidates: The transaction accelerates MorphoSys’ strategy to grow through proprietary drug development and commercialization. Constellation’s lead product candidates, pelabresib and CPI-0209, are in phase 3 and phase 2, respec- tively, and may offer broad potential for a range of oncology in- dications. They fit well with MorphoSys’ proven clinical devel- opment, regulatory and commercial capabilities, and MorphoSys is well positioned to rapidly advance and unlock the potential of the Constellation portfolio. * see glossary – page 182 Fundamentals of the MorphoSys Group Group Management Report 37

• Strengthens position in hematology/oncology and expands into solid tumors: Constellation adds an attractive, complementary pipeline of highly innovative mid- to late-stage cancer therapy candidates, augmenting MorphoSys’ existing pipeline in hema- tologic malignancies and expanding into potential therapies for solid tumors. At the time of the Constellation acquisition, MorphoSys also en- tered into a funding agreement with Royalty Pharma plc (“Roy- alty Pharma”). Under the terms of this agreement, Royalty Pharma made a US$ 1,425.0 million (€ 1,206.7 million) upfront payment to MorphoSys and also provided MorphoSys with access to up to US$ 350.0 million (€ 296.4 million) in development fund- ing bonds with the flexibility to draw over a one-year period. MorphoSys agreed to place a minimum of US$ 150.0 million (€ 127.0 million) of development funding bonds. Royalty Pharma also invested US$ 100.0 million (€ 84.7 million) in a cash capital increase of MorphoSys under an authorization to exclude sub- scription rights of existing shareholders and will make additional payments of up to US$ 100.0 million (€ 84.7 million) upon reach- ing clinical, regulatory and commercial milestones for otilimab*, gantenerumab and pelabresib. Royalty Pharma gained rights to receive 100% of MorphoSys’ royalties* on net sales of Tremfya®, 80% of future royalties and 100% of future milestone payments on otilimab, 60% of future royalties on gantenerumab, and 3% on fu- ture net sales of Constellation’s clinical-stage assets, pelabresib and CPI-0209. Research and Development As of December 31, 2021, MorphoSys’ research and development activities are currently focused on the following clinical candi- dates: • Tafasitamab (MOR208, formerly XmAb5574) is a humanized Fc-modified monoclonal antibody directed against CD19*. CD19 is selectively expressed on the surface of B-cells*, which belong to a group of white blood cells. CD19 enhances B-cell receptor signaling, which is an important factor in B-cell survival and growth. CD19 is a potential target structure for the treatment of B-cell malignancies. • Pelabresib* (CPI-0610) is an investigational selective small mol- ecule BET inhibitor with an epigenetic mechanism of action that has been designed to promote anti-tumor activity by specif- ically inhibiting the function of BET proteins, which normally enhance target gene expression. The FDA and EMA granted orphan drug designation to pelabresib for the treatment of my- elofibrosis in November 2019 and February 2020 respectively. We believe there is an opportunity to address serious unmet medical needs in patients with myelofibrosis. As part of MorphoSys’ agreement with Royalty Pharma, Royalty Pharma is entitled to receive 3% of future net sales of pelabresib. • Felzartamab* (MOR202/TJ202) is an investigational human monoclonal HuCAL-IgG1-antibody directed against a unique epitope of the target molecule CD38*. CD38 is a surface anti- gen* broadly expressed on malignant myeloma cells as well as on antibody-producing plasmablasts and plasma cells, the latter playing an important role in the pathogenesis of antibody-medi- ated autoimmune diseases. • CPI-0209 is an investigational small molecule, second-genera- tion EZH2 inhibitor with an epigenetic mechanism of action that has been designed to achieve comprehensive target cover- age through increased on-target residence time. Data from in vitro preclinical models of multiple cancer types suggested that CPI-0209 may bind to EZH2 more durably and with higher af- finity than first-generation EZH2 inhibitors. CPI-0209 was de- signed to eliminate auto-induction of metabolism, which has been an issue with other EZH2 inhibitors. Royalty Pharma is entitled to receive 3% of future net sales of CPI-0209. In addition to MorphoSys’ own pipeline, the following programs, among others, are being further developed by MorphoSys’ part- ners: • Felzartamab (see above) is also being further developed by I-Mab for mainland China, Taiwan, Hong Kong and Macao, where, if approved, it may also be commercialized. I-Mab is cur- rently pursuing development in multiple myeloma (MM*) and systemic lupus erythematosus (SLE). • Gantenerumab, a HuCAL antibody targeting amyloid beta*, is being developed by Roche as a potential treatment for Alzhei- mer’s disease. As part of the agreement with Royalty Pharma, MorphoSys will retain 40% of future royalties on gantenerumab and will provide Royalty Pharma with 60% of future royalties. • Otilimab* (formerly MOR103/GSK3196165) is a HuCAL anti- body directed against granulocyte-monocyte colony-stimulat- ing factor (GM-CSF*). Due to its diverse functions in the im- mune system, GM-CSF can be considered a target for a broad range of anti-inflammatory therapies such as rheumatoid ar- thritis* (RA). Otilimab was fully out-licensed to GlaxoSmith- Kline (GSK) in 2013. MorphoSys will retain 20% of future royal- ties on otilimab and, as part of the agreement with Royalty Pharma, will provide Royalty Pharma with 80% of future royal- ties and 100% of future milestone payments. • Tremfya is a HuCAL antibody targeting the p19 subunit of IL-23 that is being developed and commercialized by Janssen. It is the first commercial product based on MorphoSys’ proprietary technology. Royalty Pharma is entitled to receive 100% of MorphoSys’ royalties on net sales of Tremfya, commencing with the second quarter of 2021. • MOR210/TJ210 is an antibody directed against C5aR*, derived from MorphoSys’ HuCAL library. C5aR, the receptor of comple- ment factor C5a*, is being investigated as a potential new drug target in the fields of immuno-oncology, immune and chronic inflammatory diseases. In November 2018, MOR210/TJ210 was out-licensed to I-Mab for Greater China and South Korea. • In addition to the programs listed above, MorphoSys and its partners are pursuing several programs in various stages of research and clinical development. 38 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts Proprietary Clinical Development Tafasitamab Overview Tafasitamab (MOR208, formerly XmAb5574) is a humanized Fc-modified monoclonal antibody directed against CD19. CD19 is selectively expressed on the surface of B-cells, which belong to a group of white blood cells. CD19 enhances B-cell receptor signal- ing, which is an important factor in B-cell survival and growth, making CD19 a potential target structure for the treatment of B-cell malignancies. The clinical development of tafasitamab is currently focused on B-cell non-Hodgkin’s lymphoma (NHL*), par- ticularly diffuse large B-cell lymphoma (DLBCL), follicular lym- phoma (FL*) and marginal zone lymphoma (MZL*). In addition, we will initiate the MINDway study, in which we are investigating an optimized treatment regimen to reduce the frequency of drug ad- ministration and thereby reduce the burden on the patient. Lymphomas collectively represent approximately 5% of all can- cers diagnosed in the United States. The group of NHL diseases is the most prevalent of all lymphoproliferative diseases. According to the National Cancer Institute, there were an estimated 81,560 new cases in the United States in 2021 and an estimated 20,720 deaths due to this disease (“Cancer Stat Facts 2021: Non-Hod- gkin’s Lymphoma”). DLBCL is the most common type of NHL in adults and accounts for approximately one-third of all NHL cases globally. The current first-line treatment of B-cell lymphomas, in- cluding DLBCL, most commonly consists of a combination chemo- therapy regimen plus the antibody rituximab, also referred to commonly as R-CHOP* (R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone). Yet, despite the thera- peutic success of frontline R-CHOP in DLBCL, up to 40% of pa- tients either do not respond to the treatment (are refractory) or relapse after initial treatment with fast disease progression. The market research and consulting firm GlobalData expects the therapeutic market for non-Hodgkin’s lymphoma (NHL) to reach approximately € 8 billion (approximately US$ 9 billion) in 2024 (report “B-cell NHL: Opportunity Analysis 2017–2027”). We currently forecast an opportunity as a second- and later-line treatment in r/r DLBCL of approximately 10,000 eligible patients per year in the U.S. and approximately 14,000 eligible patients per year in Europe who are not eligible for high-dose chemotherapy (HDC*) and ASCT. As a potential first-line treatment in DLBCL, we believe there is currently a market opportunity of 30,000 pa- tients in the U.S. and 40,000 patients in Europe. Operational Development Tafasitamab is being developed pursuant to a collaboration and license agreement entered into with Xencor, Inc. (Xencor) in June 2010. Under this agreement, Xencor granted MorphoSys an exclu- sive worldwide license to tafasitamab for all indications. MorphoSys also has a collaboration and license agreement for the global further development and commercialization of tafasitamab with Incyte, signed in January 2020. Under the terms of the agreement, MorphoSys and Incyte will develop tafasitamab broadly in relapsed or refractory (r/r) DLBCL and first-line DLBCL, as well as in additional indications beyond DLBCL, such as follicular lym- phoma (FL), and marginal zone lymphoma (MZL). MorphoSys is responsible for conducting frontMIND*, a pivotal phase 3 study in first-line DLBCL. Incyte is responsible for conducting inMIND*, a pivotal phase 3 study in r/r FL/MZL. Incyte is also responsible for conducting a phase 1b combination study of its PI3K delta inhibi- tor parsaclisib with tafasitamab in various r/r B-cell malignan- cies. MorphoSys and Incyte share responsibility for initiating additional global clinical trials*. * see glossary – page 182 MorphoSys and Incyte are co-commercializing Monjuvi in the United States. Monjuvi in combination with lenalidomide was ap- proved in the U.S. in July 2020 for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DL- BCL) not otherwise specified, including DLBCL arising from low- grade lymphoma, and who are not eligible for autologous stem cell transplantation (ASCT). This was the first FDA approval of a sec- ond-line therapy for adult patients with r/r DLBCL in the United States. Monjuvi was approved by the FDA under an accelerated approval process based on overall response rate. Continued ap- proval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). On August 26, 2021, MorphoSys and Incyte announced that the European Commission (EC) had granted conditional marketing authorization for tafasitamab (brand name Minjuvi) in combina- tion with lenalidomide, followed by tafasitamab monotherapy, for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) who are not eligible for ASCT. In July 2021, the Committee for Orphan Medicinal Prod- ucts (COMP) confirmed the orphan drug designation status of Minjuvi, agreeing that sufficient justification had been provided that Minjuvi may be of significant benefit to patients with this disease. On August 24, 2021, Health Canada granted conditional market- ing authorization to Incyte for Minjuvi in combination with lena- lidomide for the treatment of adults with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) not otherwise speci- fied, including DLBCL arising from low-grade lymphoma, who are not eligible for ASCT. Related to these ex-U.S. regulatory approvals, in the third quarter 2021, MorphoSys received, for the first time, royalty revenue for Minjuvi sales outside of the U.S. pursuant to the agreement with Incyte. On January 5, 2021, MorphoSys and Incyte announced that the Swiss Agency for Therapeutic Products (Swissmedic) had ac- cepted the marketing authorization application (MAA*) for tafasi- tamab. The MAA seeks approval for tafasitamab, in combination with lenalidomide, followed by tafasitamab monotherapy, for the treatment of adult patients with r/r DLBCL, including DLBCL aris- ing from low-grade lymphoma, who are not candidates for ASCT. The MAA is being reviewed as part of the U.S. Food and Drug Fundamentals of the MorphoSys Group Group Management Report 39

Administration’s (FDA) modified Project Orbis, which provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators. Collaboration among international regulators may allow patients with cancer to receive earlier access to products in other countries. On August 17, 2021, Incyte announced that it had entered into a collaboration and license agreement with a subsidiary of Inno- Care for tafasitamab in Greater China. Under the terms of the agreement, InnoCare received the rights to develop and exclu- sively commercialize tafasitamab in hematology/oncology in mainland China, Hong Kong, Macao and Taiwan. Incyte holds the development and commercialization rights for tafasitamab out- side the U.S., and MorphoSys receives tiered royalties on ex-U.S. net sales. Studies of Tafasitamab The clinical development of tafasitamab is focused on non-Hod- gkin’s lymphoma (NHL). In DLBCL, MorphoSys aims to position tafasitamab as a backbone therapy for patients suffering from this disease, regardless of treatment line or potential combination therapy. Treatment options for patients with r/r DLBCL who are not candidates for high-dose chemotherapy (HDC) and ASCT were limited prior to the U.S. approval of tafasitamab. In June 2021, MorphoSys and Incyte announced new three-year follow-up data from the ongoing phase 2 L-MIND* study of tafasi- tamab (Monjuvi) in combination with lenalidomide in adult pa- tients with r/r DLBCL. The new results, based on an October 30, 2020 data cut-off, built on previous findings showing durable re- sponses and a consistent safety profile of tafasitamab in combina- tion with lenalidomide followed by tafasitamab monotherapy. A total of 80 out of 81 enrolled study patients receiving tafasitamab plus lenalidomide were included in the efficacy analysis at ap- proximately three years follow-up (≥35 months). The long-term analysis, as assessed by an independent review committee (IRC), showed that patients treated with tafasitamab plus lenalidomide had an overall response rate (ORR*) of 57.5%, including a com- plete response (CR*) rate of 40%. Additionally, the median dura- tion of response (DoR*) was 43.9 months, with a median overall survival (OS*) of 33.5 months and median progression-free sur- vival (PFS*) of 11.6 months. In December 2021, additional results from the RE-MIND2* study were presented at the 2021 American Society of Hematology (ASH) Annual Meeting. The study matched L-MIND trial patients receiv- ing tafasitamab in combination with lenalidomide followed by ta- fasitamab monotherapy with real-world adult patients who re- ceived the most frequently used treatments for r/r DLBCL. These treatments included 1) polatuzumab vedotin plus bendamustine and rituximab (pola-BR*); 2) rituximab plus lenalidomide (R2); and 3) CD19 chimeric antigen receptor T-cell (CAR-T*) therapies. Specifically, the study showed the following results: • A significant improvement in median overall survival (OS) was observed for tafasitamab plus lenalidomide with 20.1 months compared to pola-BR with 7.2 months (p = 0.038), and 24.6 months for tafasitamab plus lenalidomide compared to 7.4 months for R2* (p = 0.014). • A comparable median OS benefit was observed with tafasi- tamab plus lenalidomide with 22.5 months compared to CAR-T with 15 months however, these results were not statistically significant. • ORR, a key secondary endpoint, was statistically significantly higher for tafasitamab plus lenalidomide at 63.6% versus R2 at 30.3% (p = 0.013). • Tafasitamab plus lenalidomide also achieved a significantly higher CR rate, a key secondary endpoint, with 39.4% versus 15.2% for R2 (p = 0.0514). • While safety endpoints were not included in this study, the most common adverse events (AEs) associated with tafasitamab plus lenalidomide were feeling tired or weak, diarrhea, cough, fever, swelling of lower legs or hands, respiratory tract infection and decreased appetite. Warnings and Precautions for Monjuvi included infusion-related reactions (6%), serious or severe mye- losuppression (including neutropenia (50%), thrombocytopenia (18%), and anemia (7%)), infections (73%) and embryo-fetal tox- icity. Neutropenia led to treatment discontinuation in 3.7% of patients. The most common adverse reactions (≥ 20%) were neu- tropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, pyrexia, peripheral edema, respiratory tract infection, and de- creased appetite. The phase 2/3 study, B-MIND* is evaluating the safety and effi- cacy of tafasitamab in combination with the chemotherapeutic agent bendamustine in comparison to rituximab plus bendamus- tine in patients with r/r DLBCL who are not candidates for HDC and ASCT. The study has been fully recruited as of June 2021. The regulatory significance of the B-MIND study has decreased as both FDA and EMA* have approved Monjuvi and Minjuvi, respec- tively, based on L-MIND data. Long-term safety data of B-MIND are required by the EMA as an obligation for the conditional marketing authorization. As such, the event-driven primary anal- ysis has been removed from the planned analyses; all final analy- ses of primary and secondary endpoints will be performed in mid-2024. In addition to clinical development in r/r DLBCL, on May 11, 2021 MorphoSys announced that the first patient had been dosed in frontMIND, a pivotal phase 3 trial of tafasitamab in first-line DL- BCL: frontMIND is evaluating tafasitamab and lenalidomide in combination with R-CHOP compared to R-CHOP alone as first-line treatment for high-intermediate and high-risk patients with un- treated DLBCL. The study is planned to enroll up to 880 patients. Updated preliminary data presented at ASH, 2021 from first- MIND*, a phase 1b, open-label, randomized study on the safety and efficacy of R-CHOP plus either tafasitamab or tafasitamab plus lenalidomide for patients with newly diagnosed DLBCL, showed a preliminary overall response rate of 90.9% versus 93.9%, respectively in a patient population that had an overall 40 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts poor prognosis. The combination of tafasitamab, lenalidomide and R-CHOP had an acceptable and manageable safety profile. These results supported further investigation of the tafasitamab plus lenalidomide combination in the frontMIND study. On November 11, 2021, MorphoSys provided an update on the frontMIND study, indicating that enrollment was going well and that additional sites were being added in the United States to satisfy investigator and patient interests. Topline data from the trial are expected in the second half of 2025. On April 19, 2021, MorphoSys and Incyte announced that the first patient had been dosed in the phase 3 inMIND study. InMIND is a global, double-blind, placebo-controlled, randomized phase 3 study evaluating whether tafasitamab and lenalidomide as an add-on to rituximab provides improved clinical benefit compared with lenalidomide alone as an add-on to rituximab in patients with r/r FL Grade 1 to 3a or r/r nodal, splenic or extranodal MZL. The study is expected to enroll over 600 adult patients with r/r FL or r/r MZL. The primary endpoint of the study is PFS in the FL population, and the key secondary endpoints are PFS and OS in the overall population as well as positron emission tomography complete response (PET-CR) at the end of treatment (EOT) in the FL population. Topline data from the inMIND trial are expected in the second half of 2023. Initiated in late 2021 and sponsored by Incyte, the topMIND* trial is a single-arm, open-label, phase 1b/2a, multicenter basket study to evaluate whether tafasitamab and parsaclisib can be safely combined at the recommended phase 2 dose and dosing regimen that was established for each of the two compounds as a treatment option for adult participants with r/r B-cell malignan- cies. Participants will be assigned to disease-specific cohorts based on the histology of their underlying disease: Cohort 1: r/r DLBCL, Cohort 2: r/r MCL, Cohort 3: r/r FL, Cohort 4: r/r MZL, and Cohort 5: r/r CLL*/SLL*. The primary outcomes of the phase 1b part of the trial will be the number of TEAEs* and incidence of dose-limiting toxicities. Key secondary objectives include ORR for the phase 2a part and various PK measures. * see glossary – page 182 Pelabresib Overview Pelabresib, also known as CPI-0610, is a small molecule designed to promote anti-tumor activity by selectively inhibiting the func- tion of BET proteins to decrease the expression of abnormally ex- pressed genes in cancer. The clinical development of pelabresib is currently focused on myelofibrosis (MF). MF is a form of bone marrow cancer that disrupts the body’s normal production of blood cells. It causes fibrosis (scarring) of the bone marrow, lead- ing to severe anemia as well as thrombocytopenia. MF have en- larged spleens as well as many other physical symptoms, includ- ing abdominal discomfort, bone pain and extreme fatigue. Approximately 4–6 per 100,000 people in the U.S. are diagnosed with MF, most of whom are intermediate- or high-risk patients. There are limited treatment options for patients with MF. We be- lieve there are approximately 30,000 to 35,000 intermediate- or high-risk MF patients in the United States and Europe that are eligible for systemic treatment, including ruxolitinib. Incyte, which markets ruxolitinib (Jakafi®), has estimated that about half of these eligible patients in the United States receive treatment with ruxolitinib. Ruxolitinib, a JAK1/2 inhibitor, is the current stan- dard of care for intermediate- and high-risk MF patients. Many of these eligible patients do not initially receive treatment with rux- olitinib. For example, patients with low red blood cell or platelet counts are ineligible to receive ruxolitinib. Fedratinib is a second JAK1/2 inhibitor approved for use in treating MF. Patients who be- come refractory to, or discontinue therapy with, ruxolitinib and fedratinib generally have a poor survival prognosis. Currently ap- proved drugs for the treatment of patients suffering from MF offer symptomatic improvement and are generally not considered to be disease-modifying. As part of MorphoSys’ agreement with Royalty Pharma, Royalty Pharma is entitled to receive 3% of future net sales of pelabresib. Studies of Pelabresib Pelabresib is currently in two clinical trials for the treatment of MF, the phase 2 MANIFEST trial and the phase 3 MANIFEST-2 trial. MANIFEST is a global, multicenter, open-label phase 2 study that evaluates pelabresib as monotherapy or in combination with ruxolitinib, the current standard of care. In Arm 3 of this study, pelabresib is being evaluated in combination with ruxoli- tinib, in JAK-inhibitor-naïve MF patients, with a primary end- point of the proportion of patients with a ≥35% spleen volume re- duction from baseline (SVR35) after 24 weeks of treatment. Pelabresib is also being evaluated in a second-line setting (2L) either as a monotherapy in patients who are resistant to, intoler- ant of, or ineligible for ruxolitinib and no longer on the drug (Arm 1), or as add-on therapy to ruxolitinib in patients with a sub-opti- mal response to ruxolitinib or MF progression (Arm 2). Patients in Arms 1 and 2 are being stratified based on transfusion-depen- dent (TD) status. The primary endpoint for the patients in cohorts 1A and 2A, who were TD at baseline, is conversion to transfusion independence for 12 consecutive weeks. The primary endpoint for patients in cohorts 1B and 2B, who were not TD at baseline, is the proportion of patients with a SVR35 after 24 weeks of treatment. On June 11, 2021, Constellation announced that interim data from the MANIFEST trial were presented at the European Hematology Association (EHA) annual meeting. The data were based on a data cut-off of September 29, 2020. In Arm 3 of the study, an interim efficacy subgroup analysis in JAK-inhibitor-naïve patients was presented. Forty-two of 63 evaluable patients (67%) achieved a SVR35 at 24 weeks, achieving the primary endpoint for Arm 3. Thirty-four of 60 evaluable patients (57%) achieved a ≥50% reduc- tion in Total Symptom Scores (TSS50) at 24 weeks. Strong re- sponse was observed with pelabresib, irrespective of baseline risk status or demographic and disease characteristics. Central pathology review of 27 1L patient bone marrow samples showed at least a one-grade improvement in bone marrow fibrosis in 9 out of 27 patients (33%); in all of these patients, improvement was observed within six months of starting treatment. Sixteen out of 27 patients (59%) showed stabilization of bone marrow fibrosis, Fundamentals of the MorphoSys Group Group Management Report 41

while only one out of 27 patients (4%) showed worsening. An in- terim analysis of Arms 1 and 2 suggested that pelabresib mono- therapy in JAK-inhibitor-experienced or -ineligible patients, and with pelabresib in combination with ruxolitinib in ruxolitinib-ex- perienced patients, may result in improvements in anemia. In December 2021, updated data from MANIFEST were presented at the 2021 ASH Annual Meeting. At this meeting, the latest in- terim data from Arm 3 of MANIFEST evaluating pelabresib as a first-line combination with ruxolitinib for patients with MF who had not previously been treated with a JAK inhibitor (JAK inhibi- tor-naïve) were presented. As of September 10, 2021, the data cut- off, a total of 84 JAK inhibitor-naïve patients had been enrolled in Arm 3 and received the combination. Based on the interim data, 68% (n = 57) of patients treated with the combination achieved an SVR35 response at week 24 and 60% (n = 47) had SVR35 response at week 48. Most patients also saw their symptoms reduced, with 56% (n = 46) achieving TSS50 from baseline at week 24. At the time of the data cut-off, 53 patients (63% of the 84 patients) were still on treatment. No new safety signals were identified in the study. The most common hematologic adverse events were throm- bocytopenia (12%, grade 3/4) and anemia (34%, grade 3/4). Non-hematological events included dyspnea (5%, grade 3) and re- spiratory tract infections (8%, grade 3/4). Additional data from Arm 1 of the ongoing MANIFEST trial were also presented in an oral presentation at the 2021 ASH Annual Meeting: pelabresib is being evaluated as a monotherapy in pa- tients with advanced MF who are ineligible to receive, intolerant of, or refractory to JAK inhibitors, a population with very limited therapeutic options. Patients were divided into two cohorts, TD and non-TD. For the TD cohort, the primary endpoint was conver- sion to transfusion independence for 12 consecutive weeks. In the non-TD cohort, the primary endpoint was SVR35 at week 24. At week 24, 11% (n = 7) of patients reached SVR35. In addition, 31% of patients had a spleen volume reduction of 25% or more (n = 20) at week 24. Across all cohorts, 28% (n = 18) of patients achieved TSS50. No new safety signals were identified in the study. The most common hematologic adverse events were thrombocytope- nia (23%, grade 3/4) and anemia (15%, grade 3). Non-hematologi- cal events included diarrhea (6%, grade 3) and respiratory tract infections (5%, grade 3). MANIFEST-2, a global, double-blinded, randomized phase 3 clin- ical study, is evaluating pelabresib plus ruxolitinib versus pla- cebo plus ruxolitinib in JAK-inhibitor-naïve patients with pri- mary MF or post-essential thrombocythemia (post-ET) or post-polycythemia (post-PV) MF who have splenomegaly* and symptoms requiring therapy. Since the acquisition of Constella- tion, MorphoSys has optimized the study’s design by increasing the number of trial participants to 400 patients. Measures have also been taken to improve the speed of enrollment, including adding new contract research organizations (CROs), improving the interaction with investigators, and expanding the number of countries and sites, as well as other measures. With these activi- ties in place, MorphoSys expects to report primary analysis data from this study in the first half of 2024. Felzartamab Overview Felzartamab is an investigational human monoclonal Hu- CAL-IgG1-antibody directed against a unique epitope of the tar- get molecule CD38. CD38 is a surface antigen broadly expressed on malignant myeloma cells as well as on antibody-producing plasmablasts and plasma cells, the latter playing an important role in the pathogenesis of antibody-mediated autoimmune dis- eases. Preclinical and clinical results suggest that felzartamab may have therapeutic activity in autoantibody-mediated autoim- mune diseases, and clinical trials are ongoing in two such dis- eases – membranous nephropathy (MN*) and immunoglobulin A nephropathy (IgAN*). MN occurs when the small blood vessels in a part of the kidney called glomeruli, which filter wastes from the blood, become in- flamed and thickened. Around 80% of MN cases are primary and mediated by autoantibodies, with phospholipase A2 receptor (PLA2R) antibody positive MN accounting for up to 85% of all primary MN (Trujillo, 2019; Pozdizk, 2018; Couser 2017). MN is a leading cause of nephrotic syndrome in adults worldwide (Couser, 2017). Nephrotic syndrome results from excreting too much pro- tein in urine due to a kidney disorder. Although 30–40% of MN patients may experience spontaneous remission, 30% of patients experience persistent proteinuria with long-term preservation of renal function, and another 30–50% progress to renal failure within 10–15 years (Trujillo, 2019; Heaf, 1999; Troyanov, 2004). Even if patients do not progress to renal failure, they have an in- creased risk of life-threatening thromboembolic and cardiovas- cular events, and are subject to infections (Wagner, 1983; Heaf, 1999; Lee, 2016). In the U.S., the incidence of MN is estimated at 1.2 per 100,000; about 3,000 adults are newly diagnosed every year (McGrogan, 2011). There currently is no approved standard treatment for MN. IgAN is the most common form of glomerulonephritis, a group of renal disorders that causes damage to the glomeruli, hindering their ability to carry out their essential functions. In IgAN, a com- bination of genetic and environmental factors causes patients to produce galactose-deficient IgA (Gd-IgA), whereupon the pa- tients’ immune system reacts by producing specific autoantibod- ies. The binding of these IgG autoantibodies to Gd-IgA leads to the formation of immune complexes in the circulation. The im- mune complexes then accumulate in the glomerular mesangium where they induce local inflammation, mesangial proliferation, glomerulosclerosis and loss of renal function. Patients with IgAN may experience different symptoms including blood and/or pro- tein leaking into the urine, high blood pressure, interstitial lung disease, glomerulosclerosis (scarring of the kidneys’ blood ves- sels) and a slow progression to chronic kidney disease. About 40% of patients with IgAN progress to end-stage renal disease within 20 years of diagnosis. Worldwide IgAN incidence is estimated at 2.5 per 100,000. Currently there are no approved treatments that can specifically prevent the production of Gd IgA nor its corre- sponding autoantibody. 42 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts According to Data Bridge Market Research, the U.S. membranous nephropathy market is projected to grow at a CAGR of 5.0% be- tween 2021 and 2028 and is expected to reach US$ 153.1 million (€ 135.2 million) by 2028. According to Research and Markets, the IgAN market in the seven major markets (United States, Ger- many, Spain, Italy, France, United Kingdom and Japan) was US$ 109.3 million (€ 96.5 million) in 2020 and the prevalence has been shown to increase over time. Studies of Felzartamab In October 2019, MorphoSys initiated a phase 1/2 trial in an- ti-PLA2R antibody positive MN*. The proof-of-concept trial called M-PLACE* is an open-label, multicenter trial primarily assessing the safety and tolerability of felzartamab. On November 4, 2021, MorphoSys presented interim results from M-PLACE at the 2021 Annual Meeting of the American Society of Nephrology (ASN). The study included 31 patients with primarily medium or high levels of anti-PLA2R antibody titers at baseline and/or patients who were refractory to previous treatments. Of the 27 treated pa- tients with evaluable results, 24 showed an initial rapid reduction of anti-PLA2R antibody levels one week after the first treatment. After 12 weeks of treatment, most patients showed a substantial reduction in autoantibody titer. The observed titer reduction was independent of cohort and suggests successful depletion of CD38-positive plasma cells. The safety profile was consistent with the proposed mechanism of action of felzartamab. An early assess- ment of urine protein: creatinine ratio (UPCR) results at six months of treatment showed a decrease in six of ten patients, with four patients having a decrease of >=50% from baseline. The first pa- tient who had already reached the 12-month time point showed a complete immunologic response and a partial clinical response. Also in November 2021, MorphoSys reported that the M-PLACE trial was fully enrolled. Additional data from the study are ex- pected to be available in the second half of 2022. In February 2021, the first patient was dosed in the New-PLACE* study, a phase 2 study evaluating different treatment schedules to identify the regimen for a pivotal study in patients with an- ti-PLA2R-antibody positive MN. Enrollment in this study was completed at the end of 2021, and topline data are expected in the second half of 2022. In October 2021, the first patient was dosed in the phase 2 IG- NAZ* trial evaluating felzartamab in patients with IgAN. This multicenter, randomized, double-blind, parallel-group, place- bo-controlled trial is planned to enroll approximately 48 patients and is designed to assess the efficacy, safety and pharmacokinet- ics (PK)/pharmacodynamics (PD) of felzartamab in patients with IgAN. The primary objective of this study is to evaluate the effi- cacy of felzartamab compared to placebo. The primary endpoint is the relative change in UPCR and will be assessed for each patient nine months after treatment initiation. Study sites are located in Europe, North America and Asia-Pacific, excluding Greater China. Proof-of-concept data from the IGNAZ trial are expected in the fourth quarter of 2022. CPI-0209 Overview CPI-0209 is a small molecule designed to promote anti-tumor ac- tivity by specifically inhibiting EZH2, an enzyme that suppresses target gene expression. We believe that targeting EZH2 may have the potential for therapeutic application in various tumor types. Royalty Pharma is entitled to receive 3% of future net sales of CPI-0209. Studies of CPI-0209 Patient enrollment in a phase 1/2 clinical trial of CPI-0209 is on- going. The phase 1 portion of the trial evaluated CPI-0209 as a monotherapy in patients with advanced solid tumors. After deter- mining the recommended phase 2 dose of 350 mg (oral, once- daily), patients are currently being dosed in the phase 2 expan- sion cohorts in select tumor indications, (urothelial carcinoma (ARID1A mutant), ovarian clear cell carcinoma (ARID1A mutant), endometrial carcinoma (ARID1A mutant), lymphoma, mesotheli- oma, metastatic castration resistant prostate cancer), and data from this part of the trial are expected in 2022. As of the data cut-off of March 9, 2021, of the 4 BAP1 loss meso- thelioma patients, one patient had a PR* after four cycles of treat- ment and two had SD*. The high levels of target engagement ob- served preclinically were corroborated clinically. All 40 patients were evaluated for safety. Across all dose cohorts, 43% of patients had at least one Grade 3 or greater treatment emergent adverse event (TEAE), 28% of patients had at least one serious adverse event (SAE*). The most common TEAEs (≥15%) included thrombo- cytopenia (reversible and dose dependent), diarrhea, asthenic conditions, nausea, anemia, dysgeusia, abdominal pain and alo- pecia. 23% of patients reported a TEAE that led to dose reduction or interruption. Four patients discontinued treatment because of TEAEs. One patient in the highest dose cohort (375mg) experi- enced Grade 4 thrombocytopenia, and one patient experienced a Grade 5 adverse event due to progressive disease. Based on this preliminary data, CPI-0209 appeared to be generally well toler- ated. We expect to report additional results from the trial in 2022. * see glossary – page 182 Clinical Development through Partners The most advanced programs being developed by partners are outlined below. Felzartamab Overview MorphoSys has an exclusive regional licensing agreement for fel- zartamab with I-Mab for Greater China, where development is currently focused on multiple myeloma (MM), a blood cancer that develops in mature plasma cells in the bone marrow. MM is the second most common form of blood cancer worldwide. According to GLOBOCAN 2020 statistics, there were an estimated 4.6 million cancer cases, more than 21,000 MM cases and more than 16,000 deaths in China in 2020. In China, the incidence of MM is pro- jected to continue to increase at least through 2040. Current ther- apies are associated with serious side effects and limited efficacy. Fundamentals of the MorphoSys Group Group Management Report 43

Regional Agreement with I-Mab MorphoSys has an exclusive regional licensing agreement for fel- zartamab with I-Mab. Under the terms of the agreement, signed in November 2017, I-Mab has the exclusive rights to develop and commercialize felzartamab in mainland China, Taiwan, Hong Kong and Macao. Upon signing the agreement, MorphoSys re- ceived an immediate upfront payment of US$ 20 million (€ 18 mil- lion). MorphoSys is also entitled to receive additional suc- cess-based clinical and commercial milestone payments from I-Mab of up to US$ 100 million (€ 88 million), as well as tiered double-digit royalties on net sales of felzartamab in the agreed regions. Studies of Felzartamab I-Mab is conducting a phase 3 clinical trial in Greater China to evaluate felzartamab in combination with lenalidomide plus dexamethasone in patients with r/r MM. This study is a random- ized, open-label, parallel-controlled, multi-center study to evalu- ate the efficacy and safety of the combination of felzartamab, lenalidomide and dexamethasone versus the combination of lena- lidomide and dexamethasone in patients with r/r MM who have received at least one prior line of treatment. The study was initi- ated in April 2019 at sites in Taiwan and started in mainland China in April 2020 as part of a coordinated effort to accelerate the study. In October 2021, I-Mab announced that patient enroll- ment in this pivotal phase 3 trial has been completed. I-Mab is also evaluating felzartamab as a third-line therapy in patients with r/r MM in a pivotal phase 2 trial that started in March 2019. At the end of August 2021, I-Mab announced that topline data met primary and secondary endpoints. On June 25, 2021, I-Mab announced that the Center for Drug Eval- uation (CDE) of the China National Medical Products Administra- tion (NMPA) had approved the Investigational New Drug (IND*) application to initiate a phase 1b study with felzartamab in pa- tients with systemic lupus erythematosus (SLE). SLE, the most common type of lupus, is an autoimmune disease in which the immune system attacks its own tissues, causing widespread in- flammation and tissue damage in the affected organs. It can affect the joints, skin, brain, lungs, kidneys and blood vessels. There is no cure for SLE. The phase 1b multi-center trial is evaluating the safety, tolerability, pharmacokinetics (PK) and pharmacodynam- ics (PD) of felzartamab in patients with SLE in China. The SLE study start date is scheduled for Q1 2022. Gantenerumab Overview Gantenerumab is a HuCAL antibody targeting amyloid beta, and is being developed by licensing partner Roche as a potential treatment for Alzheimer’s disease (AD). Amyloid beta refers to a group of peptides that play an important role in Alzheimer’s dis- ease as they are the main component of the amyloid plaques found in the brain of Alzheimer’s patients. Gantenerumab binds to the N-terminus and a section in the middle of the amyloid beta peptide. The antibody removes amyloid beta via microglia-medi- ated phagocytosis. It has been designed to promote clearance of amyloid plaques in the brain, a pathological hallmark of AD, and has shown downstream effects on multiple biomarkers of AD pa- thology and neurodegeneration in clinical trials. According to the market research and consulting company Decision Resources, the value of the global market for the treatment of Alzheimer’s disease is expected to reach approximately € 35 billion ( approxi- mately US$ 40 billion) in 2030 (report titled “Market Forecast Assumption Alzheimer’s Disease 2020–2030”). According to figures from the Alzheimer’s Association, more than six million people in the United States live with Alzheimer’s disease. Deaths from Alzheimer’s disease increased 16% during the COVID-19 pandemic (https://www.alz.org/alzheimers-de- mentia/facts-figures). In 2019, Alzheimer’s disease was the sixth-leading cause of death in the United States (https://www. cdc.gov/nchs/fastats/leading-causes-of-death.htm). Operational Development Under the agreement announced in June 2021 between MorphoSys and Royalty Pharma, Royalty Pharma has the right to receive 60% of future royalties on gantenerumab. On October 11, 2021, MorphoSys announced that Roche had re- ceived Breakthrough Therapy Designation by the U.S. FDA for gantenerumab for the treatment of Alzheimer’s disease. This des- ignation was based on data showing that gantenerumab signifi- cantly reduced brain amyloid plaque, a pathological hallmark of this disease, in the SCarlet RoAD and Marguerite RoAD open-la- bel extension trials, as well as other studies. Studies of Gantenerumab In June 2018, Roche initiated a new phase 3 development program for patients with Alzheimer’s disease. The program consists of the GRADUATE 1 and GRADUATE 2 phase 3 trials. The two mul- ticenter, randomized, double-blinded, placebo-controlled studies are investigating the efficacy and safety of gantenerumab in more than 2,000 patients with early (prodromal to mild) Alzheimer’s disease and follow them for over two years. The primary endpoint for both studies is the assessment of the signs and symptoms of dementia, measured as the clinical dementia rating sum of boxes (CDR-SOB) score. Learnings from the SCarlet RoAD and Margue- rite RoAD studies were incorporated into the optimized design of the phase 3 GRADUATE trials, with patients receiving a signifi- cantly higher dose of gantenerumab as a subcutaneous injection than in Roche’s previous trials. The GRADUATE 1 and 2 trials are expected to be completed in Q4 2022. Otilimab Overview Otilimab (formerly MOR103/GSK3196165) is a HuCAL-IgG1-anti- body directed against granulocyte-macrophage colony-stimulat- ing factor (GM-CSF). Due to its diverse functions in the immune 44 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts system, GM-CSF can be considered a target for a broad spectrum of anti-inflammatory therapies, such as those in rheumatoid ar- thritis (RA). RA is a chronic inflammatory disease that affects the synovial membrane of the joints and is accompanied by painful swelling that can lead to bone destruction and joint deformity. MorphoSys discovered and advanced otilimab to clinical develop- ment. In June 2013, MorphoSys announced that it had entered into a worldwide agreement with GSK for the development and commercialization of otilimab. Under the terms of the agreement, GSK assumes responsibility for all further development and com- mercialization of the compound. Under the terms of the agree- ment, MorphoSys received an upfront payment of € 22.5 million. Depending on the achievement of certain development, regula- tory, commercial and revenue milestones, MorphoSys is eligible to receive further payments from GSK of up to € 423 million, as well as tiered double-digit royalties on net sales. Under the agree- ment between MorphoSys and Royalty Pharma, Royalty Pharma is entitled to 80% of future royalties and 100% of future milestone payments for otilimab. The total market for RA drugs is growing steadily. According to the market research and consulting firm Decision Resources, the market for RA drugs was projected to reach € 28 billion (US$ 32 billion) in 2022 in G7 countries (report titled “Market Forecast Assumptions Rheumatoid Arthritis 2020–2030”). Studies of Otilimab In July 2019, GSK launched a phase 3 program for RA called Con- tRAst. The treatment of the first patient in this program triggered a milestone payment of € 22 million to MorphoSys. Data from the ContRAst program studies are expected by the end of 2022. GSK also initiated a clinical trial (OSCAR) in 2020 to evaluate the efficacy and safety of otilimab in patients with severe pulmonary COVID-19-associated disease. The event of the first patient dosed in the expanded OSCAR study triggered milestone payments totaling € 16 million to MorphoSys in financial year 2021. In October 2021, GSK provided an update that it had made the decision not to further explore otilimab as a potential treatment for severe pulmonary COVID-19 related disease in patients aged of 70 years and older. Tremfya® (Guselkumab) Overview Tremfya is a human HuCAL antibody targeting the p19 subunit of IL-23 that is being developed and commercialized by Janssen. It is the first commercial product based on MorphoSys’ proprietary technology. It is approved for the treatment of patients with mod- erate to severe psoriasis* (plaque psoriasis) in the United States, Canada, the European Union (EU), Japan, China and a number of other countries. In the U.S. and elsewhere, it is also approved for the treatment of adults with active psoriatic arthritis* and in the EU for the treatment of adult patients with active psoriatic arthri- tis who have had an inadequate response or have not tolerated prior disease-modifying antirheumatic drug (DMARD) therapy. In Japan, Tremfya is approved for the treatment of patients with various forms of psoriasis, psoriatic arthritis and palmoplantar pustulosis. * see glossary – page 182 Under an agreement with Janssen, MorphoSys receives royalties on net sales of Tremfya and is also entitled to milestone payments on selected future development activities. Under the agreement between MorphoSys and Royalty Pharma, Royalty Pharma is en- titled to 100% of future Tremfya royalties starting with royalties for the second quarter of 2021. MOR210/TJ210 Overview MOR210/TJ210 is a human antibody directed against C5aR, de- rived from MorphoSys’ HuCAL technology. C5aR, the receptor of complement factor C5a, is being investigated as a potential new drug target in the fields of immuno-oncology and autoimmune diseases. Tumor cells generate high levels of C5a, which is be- lieved to contribute to an immuno-suppressive and, consequently, tumor growth-promoting microenvironment by recruiting and activating myeloid suppressor cells (MDSCs). MOR210/TJ210 is engineered to neutralize the immuno-suppressive function of MDSCs by blocking the interaction between C5a and its receptor and enabling the immune system to fight the tumor. Regional Agreement with I-Mab In November 2018, MorphoSys announced that the Company had entered into an exclusive strategic collaboration and regional li- censing agreement with I-Mab. Under the agreement, I-Mab has exclusive rights to develop and commercialize MOR210/TJ210 in mainland China, Hong Kong, Macao, Taiwan and South Korea, while MorphoSys retains rights in the rest of the world. The agreement deepened the existing partnership with I-Mab and built on the existing collaboration to develop MOR210/TJ210. Un- der the agreement, I-Mab has exclusive rights to develop and commercialize MOR210/TJ210 in the territories covered by the agreement. With MorphoSys’ support, I-Mab is to conduct and fund all worldwide development activities for MOR210/TJ210, in- cluding clinical trials in China and the U.S., up to proof-of-con- cept in oncology. Study of MOR210/TJ210 On January 25, 2021, MorphoSys and I-Mab announced the dos- ing of the first patient in the U.S. in a phase 1 dose-finding study evaluating the safety, tolerability, PK and PD of MOR210/TJ210 as monotherapy in patients with r/r advanced solid tumors. The phase 1 clinical trial is an open-label, multiple-dose group, dose-finding study in various centers across the U.S. I-Mab has announced another phase 1 clinical trial to evaluate the dose-finding and safety for the treatment of patients with ad- vanced solid tumors in 2022 in China. Fundamentals of the MorphoSys Group Group Management Report 45

Other Business Activities Technologies MorphoSys has developed a number of technologies that provide direct access to human antibodies for the treatment of diseases. MorphoSys has historically used these technologies for proprietary and partnered programs but is now primarily focused on expand- ing its own pipeline with these and other technologies. MorphoSys’ most important technologies include HuCAL, a collection of sev- eral billion fully human antibodies, and a system for their optimi- zation. Another important and, compared to HuCAL, further opti- mized platform is Ylanthia: a large antibody library* representing the next generation of antibody technologies. Ylanthia is based on an innovative concept for generating highly specific and fully hu- man antibodies. With Ylanthia, MorphoSys has set a new stan- dard in therapeutic antibody development and will continue to preferentially use this technology to identify antibody candidates for its proprietary pipeline. With Slonomics, MorphoSys has a pat- ent-protected, fully automated gene synthesis and modification technology to generate highly diverse gene libraries in a con- trolled process, for example to improve antibody properties. * see glossary – page 182 MorphoSys also has a licensing agreement with Cherry Biolabs, a spin-off of the University Hospital of Würzburg, Germany, grant- ing MorphoSys the rights to apply Cherry Biolabs’ innovative, multispecific Hemibody technology to six exclusive targets. Com- bined with MorphoSys’ expertise in antibody technologies, the Hemibody technology offers the potential to generate novel T-cell-engaging medicines with higher precision and better safety profiles for the treatment of cancer patients. MorphoSys intends to further develop the Hemibody technology in the con- text of MorphoSys’ CyCAT dual-targeting platform to advance novel Hemibody-based treatment options for patients with hema- tological and solid cancers. Drug Development MorphoSys has a broad development pipeline and develops drugs using its own research and development and in collaboration with pharmaceutical and biotechnology partners as well as academic institutions. Our core business is the development of new therapies for pa- tients suffering from serious diseases. Our first proprietary pro- gram to receive marketing approval is tafasitamab – brand name Monjuvi, which was first approved in the U.S. in July 2020 in com- bination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DL- BCL) not otherwise specified, including DLBCL arising from low- grade lymphoma, and who are not eligible for ASCT. Tafasitamab under the brand name Minjuvi has also been approved for mar- keting in the EU and Canada. We have become a fully integrated biopharmaceutical company developing and commercializing proprietary medicines. Our ac- tivities focus on cancer treatments, but we also conduct select programs in inflammatory diseases. The ability of monoclonal antibodies to bind to specific antigens on tumors or activate the immune system against cancer to un- leash a therapeutic effect in patients has led to their dominant role in targeted cancer therapies. According to the report “2021 Global Oncology Trends” published by the IQVIA Institute, the surge in innovation treatments in recent years, accompanied by a strong focus across health systems to increase early diagnosis and expanded patient access to treatments, has resulted in global spending on oncology drugs reaching US$ 164 billion in 2020 and an estimated US$ 269 billion by 2025 even as annual growth rates ease to about 10%. Chronic inflammatory and autoimmune diseases affect millions of patients worldwide and impose an enormous social and economic burden. MorphoSys’ most advanced proprietary clinical programs are de- scribed in the Research and Development section. Clinical-stage programs developed through partners are entirely under the control of our partners. These programs include not only those in our core area of oncology but also in indications where we have not established proprietary expertise. Programs, which are the most advanced, are outlined in the Research and Development section. Influential Factors Good public medical care is a political goal in many countries. The need for new forms of therapy is growing as a result of demo- graphic change. Certain cost containment measures in Europe and the U.S. risk limiting access to innovation for patients and could slow the industry’s investment in the development of new therapies. Regulatory approval processes in the U.S., Europe and elsewhere are lengthy, time-consuming and largely unpredictable. Approv- al-related laws, regulations and policies and the type and amount of information necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. MorphoSys recognized the impact of the global COVID-19 pan- demic on healthcare systems and society worldwide, as well as the resulting potential impact on preclinical and clinical pro- grams, specifically clinical trials. In spring 2020, MorphoSys ac- tivated its existing business continuity plans to minimize any disruptions to ongoing operations caused by the COVID-19 pan- demic and to take the necessary actions to protect its employees. In addition, MorphoSys continuously monitors the situation as a whole as well as each clinical program individually and decides on the necessary course of action to ensure the safety of patients, personnel and other stakeholders, as well as on the collection of data. The Company makes adjustments where necessary to com- ply with regulatory, institutional and governmental require- ments and guidelines related to COVID-19. The top priority is the safety of all clinical program participants and ensuring that the studies in which they participate are conducted in accordance with the study protocol. Despite the rapid changes in conditions worldwide and the potential impact they may have on clinical 46 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts trials, MorphoSys continues to work diligently to maintain its drug development plans. The continuation of the commercializa- tion of Monjuvi had incorporated the use of digital channels. In addition, the sales and medical teams are using a combination of virtual and face-to-face communication to market Monjuvi, which enables them to take the right response to the uncertainty caused by the COVID-19 pandemic in the U.S. MorphoSys continuously monitors the development of the global COVID-19 pandemic and the emergence of any new virus vari- ants and decides on a case-by-case basis on the necessary course of action and measures to ensure the safety of employees and patients. Patents Our proprietary technologies and resulting drug candidates are our most valuable assets. It is therefore crucial to our success that we protect these assets with appropriate measures such as pat- ents and patent applications. This is the only way we can exclu- sively utilize these assets and the reason our Intellectual Prop- erty (IP) department seeks out the most optimal strategy to protect our products and technologies. The rights of third parties are also actively monitored and respected. Our core technologies form the basis of the Company’s success. All our technologies are protected by numerous patent families. For our Ylanthia antibody library, patents have been granted in all major territories, including Europe, the U.S. and Asian mar- kets. For other technologies, such as the dual targeting-based Cy- CAT concept, patents have been in-licensed to ensure we have freedom to act. Our development programs are also protected by numerous pat- ent families. In addition to patents protecting the drug candidates themselves, we have filed further patent applications that cover other aspects of the programs. The relevant patents for our devel- opment candidates otilimab (out-licensed to GSK) and felzartamab (out-licensed to I-Mab for China, Hong Kong, Macao and Taiwan) will not expire before 2026. This date does not take into account potential added protection of up to five years that could be gained through supplementary protection certificates or patent term ex- tensions. The main patents for pelabresib run until 2032 (U.S.) and 2031 (Europe), not including possible extension through supplemental protection certificates or term extensions. In addition, the use of pelabresib for the treatment of myelofibrosis is patent-protected in the U.S. until 2039. The main patents for CPI-0209 have a term until 2039. Here, too, a possible extension through supplementary protection certifi- cates or term extensions is not included. The tafasitamab program is also protected by diverse patents. The core patents are scheduled to expire in 2029 (U.S.) and 2027 (Eu- rope), without taking into account the additional protection of up to five years available through supplementary protection certifi- cates or patent term extensions. A corresponding application for patent term extension (PTE) has been filed to extend the patent term in the U.S., while an application for a supplementary protec- tion certificate (SPC) in Europe is being prepared based on the European approval and will be filed in early 2022. The patents for the tafasitamab program are being advanced in close coordina- tion with our partner Incyte. Regulatory exclusivities are also in place for all development programs. The programs that are co-developed with or for partner compa- nies are also extensively patent-protected. Our patent department works closely with the relevant partners. The patents for these drug development programs have terms that far exceed the terms of the underlying technology patents. We also monitor our com- petitors’ activities so we can take action when necessary. In the 2021 financial year, we continued to reinforce the patent protection of our development programs and growing technology portfolio, which represent the core value drivers of our Company. We have more than 110 different proprietary patent families worldwide, in addition to the numerous patent families we are pursuing in collaboration with our partners. Corporate Developments In 2021, MorphoSys continuously monitored the development of the global COVID-19 pandemic and the emergence of new viral variants, and decided on a case-by-case basis the measures nec- essary to ensure the safety of employees and patients. This al- lowed MorphoSys to continue to maintain its drug development plans despite the rapidly changing conditions worldwide and their potential impact on clinical trials. On January 6, 2021, MorphoSys announced the appointment of Sung Lee as the Company’s Chief Financial Officer (CFO), effec- tive February 2, 2021. Mr. Lee has more than 20 years of financial leadership experience in the biopharmaceutical and technology businesses. As a member of the Management Board of MorphoSys AG, he is responsible for all financial areas of the Company. He is based in Planegg, Germany. On May 19, 2021, MorphoSys AG’s Annual General Meeting re- elected Marc Cluzel, M.D., Ph.D., Sharon Curran and Krisja Ver- meylen to the Company’s Supervisory Board. Due to the ongoing COVID-19 restrictions, the 2021 Annual General Meeting was also held as a virtual meeting without the physical attendance of shareholders or their proxies and made available as an audio/ video broadcast on the Internet to registered shareholders. Fundamentals of the MorphoSys Group Group Management Report 47

615 326 329 426 Total Employees 2017 2018 2019 2020 2021 732 Figure 03 Total Headcount of the MorphoSys Group (December 31) (Number) 122 351 142 127 504 94 7 Administration R & D Sales Production Employees by Function 2020 2021 48 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts On June 2, 2021, MorphoSys entered into an agreement with Con- stellation under which MorphoSys would acquire Constellation for US$34.00 (€ 28.79) per share in cash, representing a total eq- uity value of US$ 1,635.2 million (€ 1,384.7 million). The transac- tion was unanimously approved by the Management and Supervi- sory Boards of MorphoSys and Constellation’s Board of Directors. MorphoSys also announced it had entered into a financing agree- ment with Royalty Pharma in conjunction with the Constellation transaction. On July 15, 2021, MorphoSys announced the successful comple- tion of its all-cash tender offer, announced on June 2, 2021, to ac- quire all outstanding shares of Constellation for US$ 34.00 per share, net in cash. 42,811,957 shares of Constellation were validly tendered and not validly withdrawn in the tender offer, represent- ing approximately 89% of Constellation’s outstanding shares on the date of the tender offer’s expiration. Under the terms of the merger agreement between Constellation, MorphoSys and MorphoSys Development Inc., all shares validly tendered and not validly withdrawn were accepted for payment. MorphoSys imme- diately completed the acquisition of Constellation in a second step merger of MorphoSys Development Inc. with Constellation, result- ing in Constellation surviving as an indirect wholly owned sub- sidiary of MorphoSys. On July 16, 2021, MorphoSys announced that the Management Board, with the consent of the Supervisory Board, had resolved an increase in the share capital of MorphoSys AG by issuing 1,337,552 new ordinary shares from Authorized Capital 2021-II. The capital increase excluded the preemptive rights of existing shareholders to enable the purchase of 1,337,552 new ordinary shares by Royalty Pharma. Following the capital increase, the new ordinary shares represented 3.9% of MorphoSys’ registered share capital. The share purchase from Royalty Pharma totaling US$ 100 million is part of the financing agreement with MorphoSys for the completed acquisition of Constellation. The agreement be- came effective upon the merger’s closing on July 15, 2021. On July 26, 2021, MorphoSys announced an update of its 2021 fi- nancial guidance as well as a reduction in financial liabilities. Based on its preliminary unaudited consolidated results for the first six months of 2021, MorphoSys updated the guidance for Group revenues to € 155 million to € 180 million (previously: € 150 million to € 200 million, published on March 15, 2021 and reiterated on May 5, 2021). The updated revenue guidance pri- marily reflected revised product sales expectations for Monjuvi. MorphoSys also updated its expected operating expenses, con- sisting of R&D, selling, general and administrative expenses, to a range of € 435 million to € 465 million (previously: € 355 million to € 385 million). R&D expenses were expected to account for 52–57% of the Group’s operating expenses (previously: 45–50%), excluding one-time transaction-related costs. The updated guid- ance for Group operating expenses mainly reflected the acquisi- tion of Constellation, which had been completed on July 15, 2021. On November 9, 2021, MorphoSys announced the decision of Ro- land Wandeler, Ph.D., to step down as Chief Operating Officer (COO) and Management Board member of MorphoSys, effective December 31, 2021, in order to pursue new opportunities. Follow- ing his departure, the ongoing management of the marketing and sales organization remain with General Manager Joe Horvat in the U.S., who reports directly to the Chief Executive Officer, Jean- Paul Kress, M.D. Group Headcount Development On December 31, 2021, the MorphoSys Group had 732 employees (December 31, 2020: 615). The MorphoSys Group employed an av- erage of 678 employees in 2021 (2020: 564). Of the current 732 employees, 7 worked in production, 504 in re- search and development, 127 in general and administrative posi- tions and 94 in sales and marketing. All of these employees are based at our locations in Germany and the United States. We do not have collective wage agreements with our employees, and there were no employee strikes during the reporting year. At the end of the reporting year 2021, our workforce comprised employees representing 43 different nationalities (2020: 39). ›› see figure 03 – Total Headcount of the MorphoSys Group (page 48) ›› see figure 04 – Employees By Gender (page 50) To compete successfully for the top talent, MorphoSys conducts an annual comparison of the Company’s compensation with that paid by other companies in the biotech industry and similar sec- tors and adjusts the salary structure when necessary. The remu- neration system consists of fixed compensation and a variable annual bonus linked to the achievement of corporate targets. In- dividual targets promote the employees’ personal development and the achievement of overriding corporate goals. A “spot bo- nus” is also awarded on the spot to employees for exceptional per- formance. This instrument was used frequently again to reward employees during the reporting year. Fundamentals of the MorphoSys Group Group Management Report 49

2020 48 2021 2020 58 59 2021 4142 Total Employees (in %) Executives (Number) Trainees (Number) 56 Figure 04 Employees by Gender (December 31) 33 46 2020 44 32 2021 50 Group Management Report Fundamentals of the MorphoSys Group

Fi na nc ia l S ta te m en ts Macroeconomic and Sector-Specific Conditions Changes in the Business Environment In January 2022, the International Monetary Fund (IMF) forecast that the global economy would grow by 4.4% for 2021 (report “World Economic Outlook Update January 2022”). Following from the IMF’s previous report in October 2021, supply disruptions continued into the fourth quarter, hindering global manufactur- ing, especially in Europe and the United States. A resurgence in COVID-19 cases (particularly in Europe) also held back a broader recovery. Although there were signs of a global turnaround in November with a pickup in international trade and upside sur- prises for services activity and industrial production data, this only partially offset earlier declines. The IMF’s growth forecast for the advanced economies in 2021 was +5.0%, compared to a decline of 4.5% in 2020, and the forecast for the emerging and developing economies was +6.5% (2020: –2.0%). The IMF’s forecast for growth in the euro area in 2021 was +5.2% (2020: –6.4%), compared to +2.7% for Germany (2020: –4.6%); +5.6% for the U.S. (2020: –3.4%); +8.1% for China (2020: +2.3%); +4.5% for Russia (2020: –2.7%) and +4.7% for Brazil (2020: –3.9%). When managing its business activities, MorphoSys takes a num- ber of potential macroeconomic risks and opportunities into con- sideration. Currency Development The EUR/USD exchange rate has fluctuated between 1.22 and 1.12 over the last year, currently around 1.12, with inflation ex- pectations and interest rate differences being the main drivers, in addition to trade conflicts and ongoing geopolitical tensions. The majority of our business transactions are conducted in euros and U.S. dollars. With the acquisition of Constellation we have significantly expanded our footprint in the US. Primarily driven by the additional ongoing clinical studies, U.S. dollar expenses are expected to exceed the U.S. dollar revenues for the next finan- cial year. Therefore, strengthening of the U.S. dollar against the euro, all other things remaining equal, would have a negative impact on our operating result. We manage this risk through var- ious mechanisms, such as optimizing our U.S. dollar assets against our U.S. dollar liabilities and maintaining an adequate (currently around 20%) amount of U.S. dollars in our bank ac- counts. Macroeconomic and Sector-Specific Conditions Macroeconomic and Sector-Specific Conditions Group Management Report 51

Analysis of Net Assets, Financial Position and Results of Operations This report on the net assets, financial position and results of op- erations should be read in conjunction with the annual consoli- dated financial statements and the notes thereto, which also form part of this annual report. In addition to historical financial infor- mation, the following report contains forward-looking statements that reflect our plans, estimates and opinions. Our actual results may differ materially from these forward-looking statements. Fac- tors that could cause or contribute to these differences or cause our actual results or the timing of selected events to differ mate- rially from those anticipated in these forward-looking statements include those set forth under “Risk Factors,” “Special Note Regard- ing Forward-Looking Statements” and elsewhere in this report. Our consolidated financial statements comply with both the IFRSs* published by the International Accounting Standards Board (IASB) and those adopted by the EU. The consolidated financial state- ments also take into account the supplementary provisions under commercial law, which must be applied in accordance with Sec- tion 315e (1) of the German Commercial Code (Handelsgesetz- buch – HGB). * see glossary – page 182 Results of Operations Revenues Revenues in the reporting year decreased by 45% or € 148.1 mil- lion to € 179.6 million (2020: € 327.7 million). This decrease re- sulted first and foremost from revenues of € 236.1 million in 2020 stemming from the execution of the collaboration and license agreement with Incyte. Revenues from royalties on net sales amounted to € 65.6 million (2020: € 42.5 million). Revenues from Monjuvi product sales totaled € 66.9 million (2020: € 18.5 mil- lion) in its first full year after receiving marketing authorization in August 2020. In 2021, milestone payments of € 20.0 million (2020: € 4.8 million) were recognized, which were mainly com- prised of a single milestone payment from GSK. On a regional basis, revenues from biotechnology and pharmaceu- tical companies in the U.S. and Canada decreased by 51%, or € 162.8 million, from € 319.1 million in 2020 to € 156.3 million in the reporting year. This development was driven primarily by revenue from the collaboration and license agreement with In- cyte. Revenues with customers in Europe and Asia increased by more than 100%, or € 14.7 million, to € 23.3 million in 2021 (2020: € 8.6 million). This increase resulted mainly from the recognition of a milestone payment from GSK of € 16.0 million in 2021. In 2021, a total of 59% of the revenues generated were attributable to activities with partners Janssen, Incyte and GSK. In 2020, 93% of the revenues generated were attributable to activities with partners Incyte, Janssen and I-Mab Biopharma. In 2019, 89% of the revenues generated were attributable to activities with part- ners Janssen, GSK and I-Mab Biopharma. Revenues in 2020 increased by more than 100%, or € 255.9 mil- lion, to € 327.7 million (2019: € 71.8 million). This increase re- sulted first and foremost from revenues of € 255.8 million stem- ming from the collaboration and license agreement with Incyte. Revenues from royalties on net sales of Tremfya amounted to € 42.5 million (2019: € 31.8 million). Revenues from Monjuvi product sales totaled € 18.5 million, which were recognized for the first time after receiving marketing authorization in August 2020. Revenues in the 2019 financial year were primarily attrib- utable to royalties of € 31.8 million from Janssen on the net sales of Tremfya and a milestone payment of € 22.0 million from GSK triggered by the dosing of the first patient upon the initiation of a phase 3 clinical development program. On a regional basis, revenues from biotechnology and pharmaceu- tical companies in the U.S. and Canada increased by more than 100%, or € 286.8 million, from € 32.3 million in 2019 to € 319.1 mil- lion in 2020. This development was driven primarily by revenue from the collaboration and license agreement with Incyte in fi- nancial year 2020. Revenues with customers in Europe and Asia decreased by 78%, or € 30.9 million, to € 8.6 million in 2020 (2019: € 39.5 million). This decrease resulted from the recognition of a milestone payment from GSK of € 22.0 million in 2019. ›› see figure 05 – Revenues by Region (page 53) ›› see figure 06 – Sales by Categories (page 54) ›› see figure 07 – Monjuvi Product Sales (page 54) Analysis of Net Assets, Financial Position and Results of Operations 52 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations
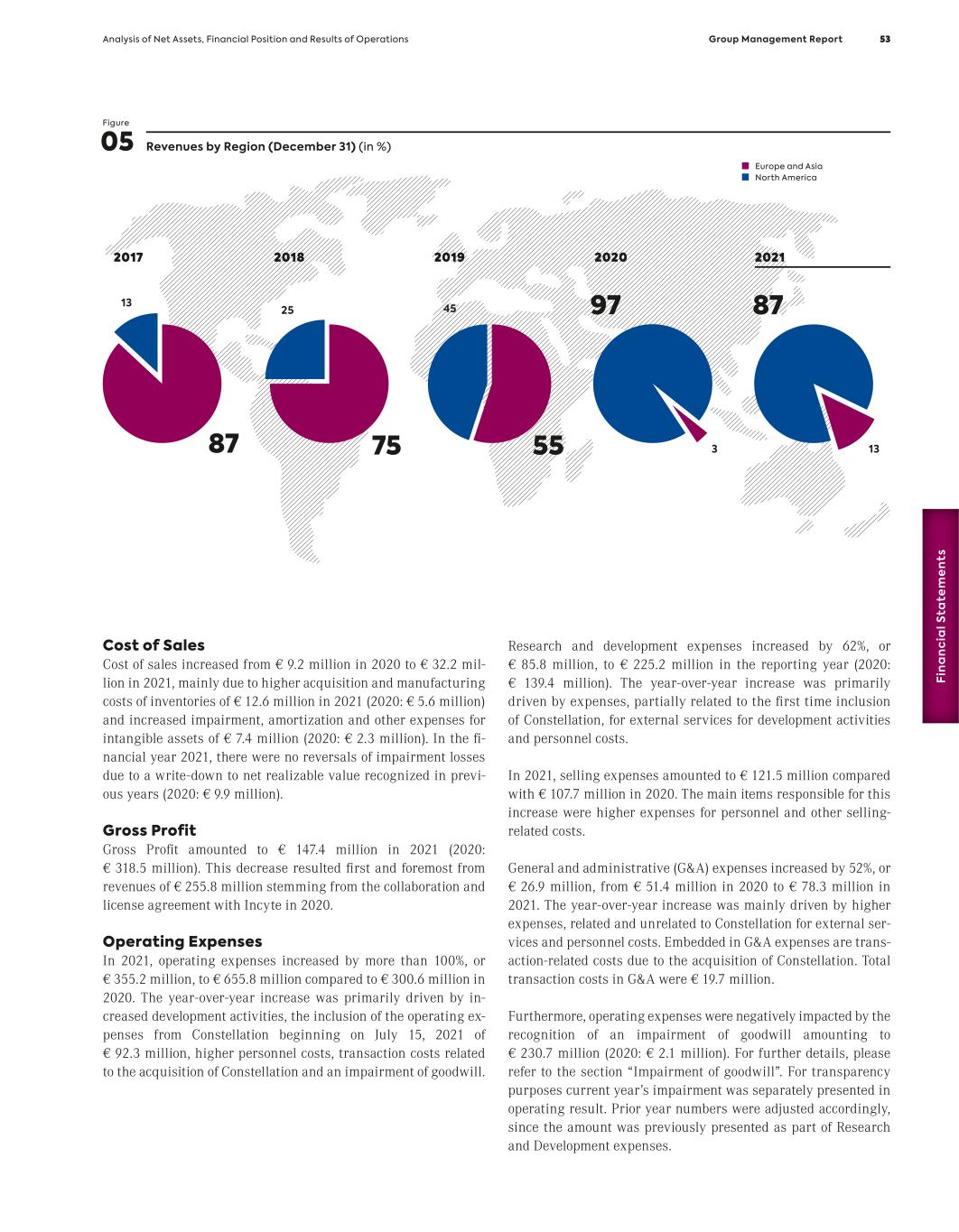
Europe and Asia North America Figure 05 Revenues by Region (December 31) (in %) 13 25 87 75 55 3 20212020201920182017 45 97 13 87 Fi na nc ia l S ta te m en ts Cost of Sales Cost of sales increased from € 9.2 million in 2020 to € 32.2 mil- lion in 2021, mainly due to higher acquisition and manufacturing costs of inventories of € 12.6 million in 2021 (2020: € 5.6 million) and increased impairment, amortization and other expenses for intangible assets of € 7.4 million (2020: € 2.3 million). In the fi- nancial year 2021, there were no reversals of impairment losses due to a write-down to net realizable value recognized in previ- ous years (2020: € 9.9 million). Gross Profit Gross Profit amounted to € 147.4 million in 2021 (2020: € 318.5 million). This decrease resulted first and foremost from revenues of € 255.8 million stemming from the collaboration and license agreement with Incyte in 2020. Operating Expenses In 2021, operating expenses increased by more than 100%, or € 355.2 million, to € 655.8 million compared to € 300.6 million in 2020. The year-over-year increase was primarily driven by in- creased development activities, the inclusion of the operating ex- penses from Constellation beginning on July 15, 2021 of € 92.3 million, higher personnel costs, transaction costs related to the acquisition of Constellation and an impairment of goodwill. Research and development expenses increased by 62%, or € 85.8 million, to € 225.2 million in the reporting year (2020: € 139.4 million). The year-over-year increase was primarily driven by expenses, partially related to the first time inclusion of Constellation, for external services for development activities and personnel costs. In 2021, selling expenses amounted to € 121.5 million compared with € 107.7 million in 2020. The main items responsible for this increase were higher expenses for personnel and other selling- related costs. General and administrative (G&A) expenses increased by 52%, or € 26.9 million, from € 51.4 million in 2020 to € 78.3 million in 2021. The year-over-year increase was mainly driven by higher expenses, related and unrelated to Constellation for external ser- vices and personnel costs. Embedded in G&A expenses are trans- action-related costs due to the acquisition of Constellation. Total transaction costs in G&A were € 19.7 million. Furthermore, operating expenses were negatively impacted by the recognition of an impairment of goodwill amounting to € 230.7 million (2020: € 2.1 million). For further details, please refer to the section “Impairment of goodwill”. For transparency purposes current year’s impairment was separately presented in operating result. Prior year numbers were adjusted accordingly, since the amount was previously presented as part of Research and Development expenses. Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 53
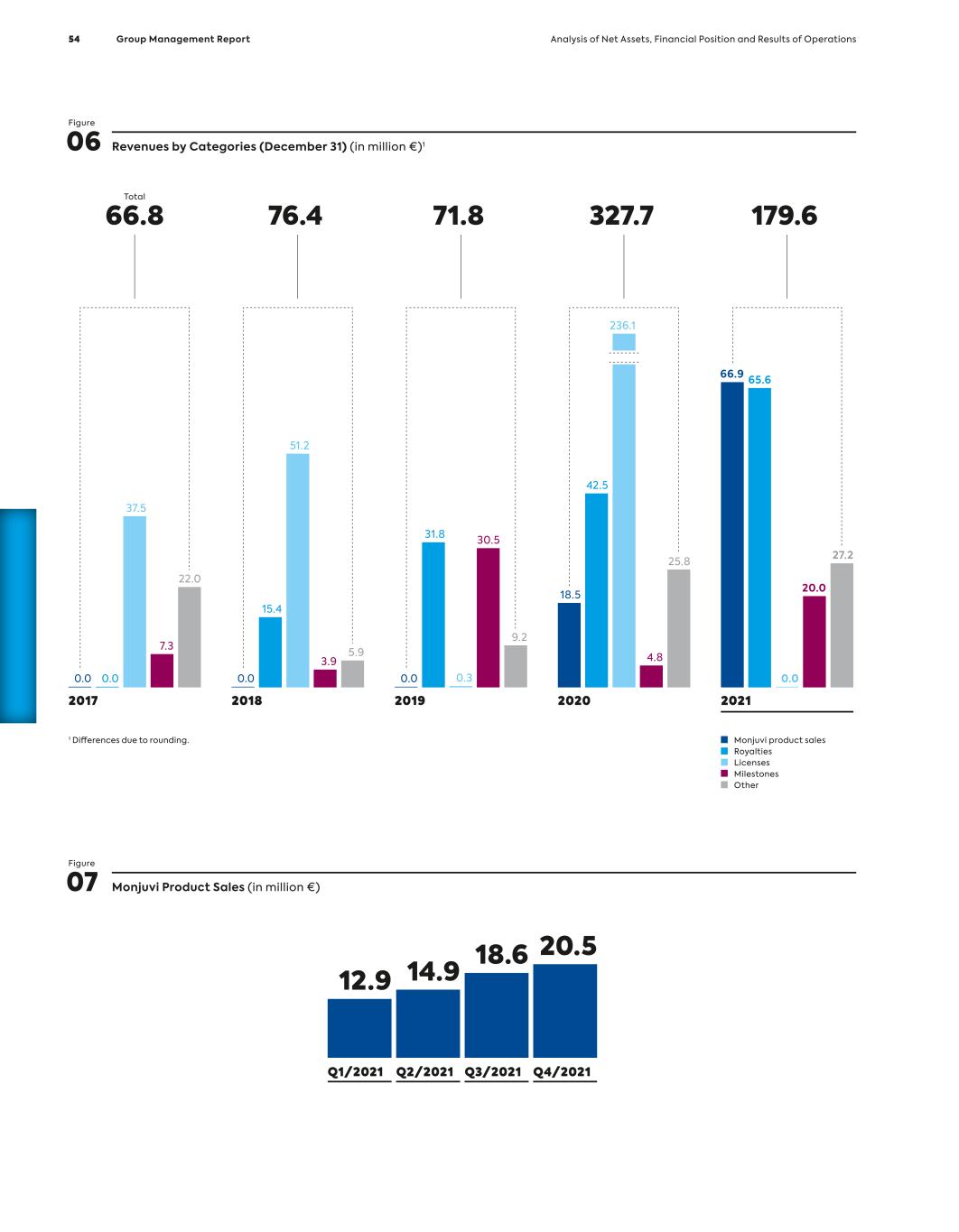
1 Diff erences due to rounding. 0.0 0.0 0.0 18.5 66.9 0.0 15.4 31.8 42.5 65.6 37.5 51.2 0.3 236.1 0.0 7.3 3.9 30.5 4.8 20.0 22.0 5.9 9.2 25.8 27.2 Figure 06 Revenues by Categories (December 31) (in million €)1 2017 2018 2019 2020 66.8 76.4 71.8 327.7 Total 2021 179.6 12.9 14.9 18.6 20.5 Monjuvi product sales Royalties Licenses Milestones Other Figure 07 Monjuvi Product Sales (in million €) Q1/2021 Q2/2021 Q3/2021 Q4/2021 54 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations

Fi na nc ia l S ta te m en ts In 2020, operating expenses increased by 79%, or € 132.8 million, from € 167.8 million in 2019 to € 300.6 million in 2020. An in- crease in research and development expenses, selling expenses and general and administrative expenses contributed to this development. Research and development expenses increased by 29%, or € 30.9 million, to € 139.4 million in 2020 (2019: € 108.4 mil- lion). In 2020, selling expenses amounted to € 107.7 million com- pared with € 22.7 million in 2019. The main items responsible for this increase were higher expenses for personnel and external services as the company prepared for the launch of Monjuvi in 2020. G&A expenses increased by 40%, or € 14.7 million, from € 36.7 million in 2019 to € 51.4 million in 2020, which was also largely due to increased personnel expenses and expenses for ex- ternal services. ›› see figure 08 – Selected R&D Expenses (page 56) Research and Development Research and development expenses increased by 62%, or € 85.8 million, to € 225.2 million in 2021 (2020: € 139.4 million) mainly due to higher expenses for external laboratory services. Expenses for external laboratory services and legal and scientific consulting services increased from € 77.8 million in the previous year to € 131.5 million in the reporting year, mainly due to higher expenses for external laboratory services in connection with the development of tafasitamab and felzartamab. Research and Devel- opment expenses related to Constellation’s lead compounds pela- bresib, CPI-0209 and personnel were recorded for the first time starting from July 15, 2021 and onwards. Overall, personnel ex- penses were higher, rising from € 32.3 million in the previous year to € 65.9 million in the reporting year, partially driven by the addition of Constellation. Expenses for intangible assets amounted to € 7.9 million in 2021 (2020: € 18.1 million). In 2020, these were influenced by impair- ment losses of € 11.7 million in connection with an impairment of the MOR107 in-process research and development program. De- preciation, amortization and other expenses for infrastructure increased from € 8.7 million in 2020 to 11.8 million in 2021, mainly due to higher lease expenses and utilities. Other expenses increased from € 2.5 million in 2020 to € 4.1 million in 2021. Ex- penses for consumables increased from € 3.2 million in the previ- ous year to € 4.1 million in 2021. In 2020, research and development expenses increased by 29%, or € 30.9 million, to € 139.4 million (2019: € 108.4 million). This increase was mainly the result of higher expenses for external laboratory services. Expenses for external laboratory services and legal and scientific consulting services increased from € 62.4 million in 2019 to € 77.8 million in 2020, mainly due to higher expenses for external laboratory services in connection with the development of tafasitamab. Personnel expenses were also higher, rising from € 28.5 million in 2019 to € 32.3 million in 2020. Expenses for intangible assets amounted to € 18.1 million in 2020 (2019: € 5.6 million). In 2020, these were influenced by impair- ment losses of € 11.7 million in connection with an impairment of the MOR107 in-process research and development program. De- preciation, amortization and other expenses for infrastructure increased from € 5.9 million in 2019 to € 8.7 million in 2020, mainly due to higher expenses for insurance. Other expenses de- creased from € 3.1 million in 2019 to € 2.5 million in 2020. Ex- penses for consumables increased from € 2.9 million in 2019 to € 3.2 million in 2020. Selling Selling expenses increased by 13%, or € 13.8 million, to € 121.5 million in 2021 (2020: € 107.7 million). The increase re- sults mainly from higher personnel expenses (2021: € 63.5 mil- lion; 2020: € 52.8 million) and other operating costs (2021: € 5.7 million; 2020; € 3.4 million). The personnel expenses in- creased by € 10.7 million to € 63.5 million in 2021 due to the full year impact of marketing activities for Monjuvi. Other operating costs primarily increased due to database subscriptions. Addi- tional selling expenses of € 1.3 million related to Constellation were recorded since the acquisition date. In 2020, selling expenses increased by more than 100% or € 85.1 million to € 107.7 million (2019: € 22.7 million). This was mainly due to higher expenses for external services and person- nel expenses. The expenses for external services increased by € 36.4 million to € 50.7 million in 2020 due to the commercializa- tion of Monjuvi (2019: € 14.3 million). Personnel expenses in- creased to € 52.8 million (2019: € 6.8 million) due to the market- ing activities for Monjuvi. General and Administrative (G&A) G&A expenses increased by 52%, or € 26.9 million, in 2021 and amounted to € 78.3 million (2020: € 51.4 million). The year-over- year increase was mainly driven by higher expenses, partially related Constellation inclusion, for external services and person- nel costs. Embedded in G&A expenses were transaction related costs due to the acquisition of Constellation. Total transaction costs in G&A were € 19.7 million. Costs relating to the acquisition of Constellation were the main driver for the increase from € 15.6 million in the previous year to € 35.9 million of external services in the reporting year. Personnel expenses increased from € 29.9 million in the previous year to € 32.6 million in the reporting year. Higher expenses for salaries, retention and sever- ance payments were primarily responsible for this increase, which was partially offset by lower deferred compensation ex- penses. Depreciation, amortization and other expenses for infra- structure increased from € 4.1 million in the previous year to € 6.9 million in 2021, mainly resulting from increased insurance costs. Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 55

External Laboratory Funding Personnel Consumables Figure 08 Selected R&D Expenses (December 31) (in million €) Total Other (includes expenses for intangible assets, technical infrastructure and external services) 2017 2018 2019 2020 2021 61.1 47.9 62.4 77.8 131.5 28.5 25.3 28.5 32.3 2.6 2.3 2.9 3.2 4.1 21.1 30.9 14.6 26.1 23.7 65.9 113.3 106.4 108.4 139.4 225.2 G&A expenses increased by 40%, or € 14.7 million, in 2020 and amounted to € 51.4 million (2019: € 36.7 million). The main rea- sons for this increase were higher personnel expenses and ex- penses for external services. Personnel expenses increased from € 22.6 million in 2019 to € 29.9 million in 2020. Higher expenses for salaries were primarily responsible for this increase. Ex- penses for external services increased from € 10.0 million in 2019 to € 15.6 million in 2020, which was particularly related to the commercialization of Monjuvi. Other expenses decreased from € 1.9 million in 2019 to € 1.3 million in 2020, mainly due to lower travel expenses. Impairment of Goodwill Goodwill resulting from the Constellation acquisition are com- prised of assets which are not separately recognizable, such as workforce, preclinical studies, increasing probabilities of success rates for clinical stage assets, market share, access to new indica- tions as well as the Constellation epigenetics platform (early pipe- line). MorphoSys decided to focus its research efforts on the most ad- vanced discovery and technology programs and also to centralize all laboratory activities at its German research hub in Planegg, Germany. 56 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations

Fi na nc ia l S ta te m en ts Consequently, all US-based activities relating to discovery biol- ogy and drug discovery departments were abandoned. Therefore, any early pipeline projects cannot be realized anymore and the expected cash flows from these projects will not materialize ac- cordingly. Since the early pipeline was part of the goodwill ac- quired as of July 15, 2021, an impairment test was performed as of December 31, 2021, based on the latest cashflow projections, which resulted in a need for impairment on the goodwill in the amount of € 230.7 million. In 2020, an impairment of goodwill in the amount of € 2.1 million was recorded on the goodwill associated with the acquisition of Sloning BioTechnology GmbH in 2010. For transparency purposes current year’s impairment was sepa- rately presented in the operating result. Prior year numbers were adjusted accordingly since the amount was previously presented as part of Research and Development expenses. Other Income Other income decreased by 44%, or € 6.4 million, to € 8.2 million in the reporting year (2020: € 14.6 million) and mainly resulted from exchange rate gains of € 7.6 million (2020: € 13.7 million). Other income increased by more than 100%, or € 13.8 million, to € 14.6 million in 2020 (2019: € 0.8 million) and mainly resulted from exchange rate gains from operating activities of € 13.7 mil- lion (2019: € 0.2 million). In 2020, one-off gains from the disposal of the Lanthio companies amounted to € 0.4 million Other Expenses In the 2021 reporting year, other expenses increased by 23%, or € 1.2 million, from € 5.2 million in 2020 to € 6.4 million in 2021. This increase was mainly the result of currency losses of € 5.9 million (2020: € 4.6 million) and other expenses of € 0.4 mil- lion (2020: € 0.6 million). Other expenses increased by more than 100%, or € 4.5 million, from € 0.6 million in 2019 to € 5.2 million in 2020. This increase was mainly the result of currency losses of € 4.6 million (2019: € 0.4 mil- lion) and other expenses of € 0.6 million (2019: € 0.2 million). Finance Income Finance income increased by 5%, or € 4.6 million, to € 96.6 mil- lion in the reporting year (2020: € 92.0 million) and resulted from items amounting to € 75.7 million (2020: € 82.0 million) in con- nection with the changes in plan assumptions of financial assets and financial liabilities from collaborations. These items included effects from differences between planning assumptions and ac- tual figures and the fair value measurement (refer to Note 5.18* titled “Financial Assets and Liabilities from Collaborations” con- tained in the Notes to the Consolidated Financial Statements). Also included is finance income from the investment of cash and cash equivalents and foreign currency translation gains from in- vesting of funds amounting to € 20.9 million (2020: € 9.4 million). No income from financial derivatives was recognized in 2021 (2020: € 0.7 million). * cross-reference to page 143 Finance income increased by more than 100%, or € 89.2 million, to € 92.0 million in 2020 (2019: € 2.8 million) and resulted from items amounting to € 82.0 million (2019: € 0 million) in connec- tion with the measurement of financial assets and financial liabil- ities from collaborations. These items included effects from cur- rency translation and fair value measurement (refer to Note 5.18* titled “Financial Assets and Liabilities from Collaborations” con- tained in the Notes to the Consolidated Financial Statements). Also included is finance income from the investment of cash and cash equivalents and foreign currency translation gains from in- vesting of funds amounting to € 9.4 million (2019: € 1.3 million). Income of € 0.7 million (2019: € 1.5 million) from financial deriv- atives was also recognized. * cross-reference to page 143 Finance Expenses Finance expenses increased by 89%, or € 85.2 million, to € 181.5 million in the reporting year (2020: € 96.2 million). This increase was mainly due to the effects from Financial Liabilities from future payments to Royalty Pharma of € 94.7 million (2020: € 0) resulting from differences between planning assumptions and actual figures, foreign currency effects and the application of the effective interest method (also refer to note 5.19* “Financial Liabilities from Future Payments to Royalty Pharma” contained in the Notes to the Consolidated Financial Statements). Further- more, the finance expense effects from Financial Liabilities from Collaborations of € 59.7 million (2020: € 45.4 million), specifically from the fx revaluation effects as well as the application of the effective interest method, contributed to the increase (refer to Note 5.18* titled “Financial Assets and Liabilities from Collabora- tions” contained in the Notes to the Consolidated Financial State- ments). Furthermore, this line item included finance expenses from the investment of cash and cash equivalents and foreign currency translation losses from financing activities of € 11.4 mil- lion (2020: € 46.1 million). This included losses of € 3.5 million (2020: € 5.0 million) from financial derivatives. Other finance ex- penses amounted to € 15.6 million (2020: € 4.6 million) in 2021, mainly relating to interest on the convertible bond issued in Octo- ber 2020 in the amount of € 12.1 million (2020: € 2.5 million) as well as € 1.2 million (2020: € 1.2 million) in interest expenses from the compounding of non-current lease liabilities were also recognized in the reporting year. * cross-reference to page 144 and page 143 Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 57

Finance expenses increased by more than 100%, or € 93.9 mil- lion, to € 96.2 million in 2020 (2019: € 2.3 million). This increase was mainly due to the effects of financial assets and financial lia- bilities from collaborations of € 45.4 million (2019: € 0 million) and specifically from the difference in the planning assumptions versus the actual results. The application of the effective interest method and foreign currency valuation (refer to Note 5.18* “Fi- nancial Assets and Liabilities from Collaborations” contained in the Notes to the Consolidated Financial Statements) also contrib- uted to the increase. Furthermore, this line item included finance expenses from the investment of cash and cash equivalents and foreign currency translation losses from financing activities of € 46.1 million (2019: € 1.0 million). This included losses of € 5.0 million (2019: € 0.2 million) from financial derivatives. € 1.2 million (2019: € 0.9 million) in interest expenses from the compounding of non-current lease liabilities were also recog- nized in 2020. * cross-reference to page 143 Income Tax Expenses The Group recorded total income tax benefits of € 76.6 million in 2021 (2020: income tax benefits of € 75.4 million), which con- sisted of current tax income of € 1.2 million, mainly due to a loss carry back, (2020: expense € 67.1 million) and deferred tax in- come of € 75.4 million (2020: € 142.5 million ). The effective in- come tax rate equaled 13% in the reporting year (2020: (335.2)%). The difference compared to the expected tax rate of 26.7% is pri- marily due to the non-recognition of deferred tax assets on tempo- rary differences and current year tax losses for the US tax group, whereas in 2020 the variance between effective and expected tax rate was mainly due to the recognition of deferred tax assets on prior year losses and temporary differences. Consolidated Net Profit/Loss For The Period In 2021, the consolidated net loss amounted to € 514.5 million (2020: consolidated net profit of € 97.9 million; 2019: consolidated net loss of € 103.0 million). 58 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations
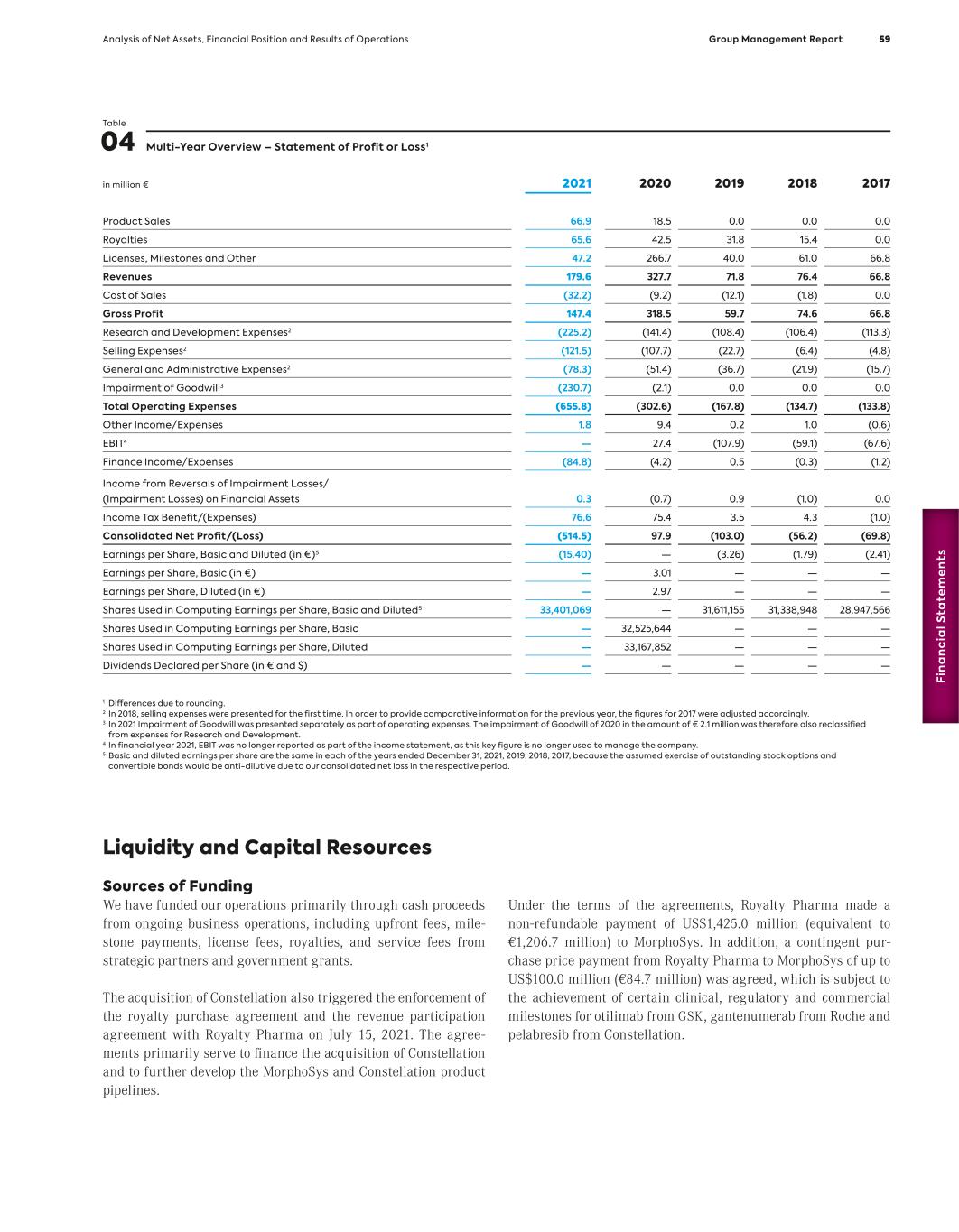
Fi na nc ia l S ta te m en ts Table 04 Multi-Year Overview – Statement of Profit or Loss1 in million € 2021 2020 2019 2018 2017 Product Sales 66.9 18.5 0.0 0.0 0.0 Royalties 65.6 42.5 31.8 15.4 0.0 Licenses, Milestones and Other 47.2 266.7 40.0 61.0 66.8 Revenues 179.6 327.7 71.8 76.4 66.8 Cost of Sales (32.2) (9.2) (12.1) (1.8) 0.0 Gross Profit 147.4 318.5 59.7 74.6 66.8 Research and Development Expenses2 (225.2) (141.4) (108.4) (106.4) (113.3) Selling Expenses2 (121.5) (107.7) (22.7) (6.4) (4.8) General and Administrative Expenses2 (78.3) (51.4) (36.7) (21.9) (15.7) Impairment of Goodwill3 (230.7) (2.1) 0.0 0.0 0.0 Total Operating Expenses (655.8) (302.6) (167.8) (134.7) (133.8) Other Income/Expenses 1.8 9.4 0.2 1.0 (0.6) EBIT4 — 27.4 (107.9) (59.1) (67.6) Finance Income/Expenses (84.8) (4.2) 0.5 (0.3) (1.2) Income from Reversals of Impairment Losses/ (Impairment Losses) on Financial Assets 0.3 (0.7) 0.9 (1.0) 0.0 Income Tax Benefit/(Expenses) 76.6 75.4 3.5 4.3 (1.0) Consolidated Net Profit/(Loss) (514.5) 97.9 (103.0) (56.2) (69.8) Earnings per Share, Basic and Diluted (in €)5 (15.40) — (3.26) (1.79) (2.41) Earnings per Share, Basic (in €) — 3.01 — — — Earnings per Share, Diluted (in €) — 2.97 — — — Shares Used in Computing Earnings per Share, Basic and Diluted5 33,401,069 — 31,611,155 31,338,948 28,947,566 Shares Used in Computing Earnings per Share, Basic — 32,525,644 — — — Shares Used in Computing Earnings per Share, Diluted — 33,167,852 — — — Dividends Declared per Share (in € and $) — — — — — 1 Differences due to rounding. 2 In 2018, selling expenses were presented for the first time. In order to provide comparative information for the previous year, the figures for 2017 were adjusted accordingly. 3 In 2021 Impairment of Goodwill was presented separately as part of operating expenses. The impairment of Goodwill of 2020 in the amount of € 2.1 million was therefore also reclassified from expenses for Research and Development. 4 In financial year 2021, EBIT was no longer reported as part of the income statement, as this key figure is no longer used to manage the company. 5 Basic and diluted earnings per share are the same in each of the years ended December 31, 2021, 2019, 2018, 2017, because the assumed exercise of outstanding stock options and convertible bonds would be anti-dilutive due to our consolidated net loss in the respective period. Liquidity and Capital Resources Sources of Funding We have funded our operations primarily through cash proceeds from ongoing business operations, including upfront fees, mile- stone payments, license fees, royalties, and service fees from strategic partners and government grants. The acquisition of Constellation also triggered the enforcement of the royalty purchase agreement and the revenue participation agreement with Royalty Pharma on July 15, 2021. The agree- ments primarily serve to finance the acquisition of Constellation and to further develop the MorphoSys and Constellation product pipelines. Under the terms of the agreements, Royalty Pharma made a non-refundable payment of US$1,425.0 million (equivalent to €1,206.7 million) to MorphoSys. In addition, a contingent pur- chase price payment from Royalty Pharma to MorphoSys of up to US$100.0 million (€84.7 million) was agreed, which is subject to the achievement of certain clinical, regulatory and commercial milestones for otilimab from GSK, gantenumerab from Roche and pelabresib from Constellation. Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 59

In return, MorphoSys has agreed in the royalty purchase agree- ment to pass on the following to Royalty Pharma: 100% of MorphoSys’ entitlement since April 1, 2021, for royalties from net sales of Tremfya from Janssen, 80% of future royalties as well as 100% of the future milestone payments for otilimab from GSK and 60% of future royalties for gantenerumab from Roche. Constella- tion will pass on 3% of future net sales of clinical-stage com- pounds (pelabresib and CPI-0209) to Royalty Pharma based on the revenue participation agreement. If revenues based on net sales of pelabresib exceed US$ 30.0 million (€ 25.4 million) in any fiscal year, an additional purchase price of US$50.0 million (€42.3 million) will be due. However, the rights to the underlying intel- lectual property of pelabresib and CPI-0209 will remain with MorphoSys. The future license income in the form of royalties and milestones of Tremfya, otilimab, gantenerumab and of shares of future net sales of the product candidates pelabresib and CPI-0209 will not result in a cash inflow and outflow at MorphoSys, as the agreed royalty percentages and milestones are paid directly by Janssen, GSK and Roche to Royalty Pharma. The associated royalties, mile- stones and net sales will still be presented as revenues in MorphoSys profit or loss statement. On July 15, 2021, the development funding bond agreement with Royalty Pharma became effective. Under the terms of this agree- ment, MorphoSys must draw at least US$150.0 million (equiva- lent to €127.0 million) and can draw down a maximum of US$350.0 million (equivalent to €296.4 million) within one year. Repayment will be made at 2.2 times the amount drawn accord- ing to a fixed payment schedule within ten years and nine months after the first drawdown without any repayment in the first two years after a drawdown. To date, no partial amount of the bond has been called. Cash and Other Financial Assets (previously referred to as “Li- quidity”) is defined as the sum of the balance sheet items “cash and cash equivalents” and “other financial assets”. On December 31, 2021, cash and cash equivalents amounted to € 123.2 million and other financial assets amounted to € 853.7 mil- lion. On December 31, 2020, cash and cash equivalents amounted to € 109.8 million and other financial assets amounted to € 937.7 million. Cash in excess of immediate working capital requirements is in- vested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Investments are pri- marily made in money market funds, corporate bonds and term deposits with fixed or variable interest. Our functional currency is the euro. Nevertheless, we have li- quidity in U.S. dollars, which could lead to exchange rate gains or losses in our financial results depending on the fluctuation of the euro/U.S. dollar exchange rate. We are not subject to any operating covenants or capital require- ments. Uses of Funding We primarily use cash and other financial assets to fund the re- search and development costs related to the development of our product candidates and to commercialize Monjuvi. Our primary future funding requirements include the development and com- mercialization of our proprietary clinical pipeline, particularly in relation to tafasitamab and pelabresib and, to a lesser extent, fel- zartamab and CPI-0209. We believe that we have sufficient existing cash and other finan- cial assets (including cash invested in various financial assets as described above) to cover our expected operating expenses for at least the next twelve months. We have based this estimate on assumptions that may prove to be incorrect, and it is possible that we may utilize our capital re- sources more quickly than anticipated. The process of investigat- ing product candidates in clinical trials and their commercializa- tion is fundamentally an expensive process. Both the timing and progress of development trials as well as the success of commer- cialization cannot be predicted with certainty. As our product candidates are in various stages of development and the outcome of our activities is uncertain, we cannot estimate the amounts required in their entirety to successfully complete the development and commercialization of our product candidates. Additional capital may be required in the short term to implement our various projects, particularly proprietary development pro- grams, as well as in-licensing and potential M&A transactions. If we cannot generate revenue quickly enough to cover pipeline de- velopments, we may rely in the short to medium term on non-di- lutive capital measures such as out-licensing for financing. Gen- erally, we take public and private equity and bond issues, including convertible bonds, into consideration when funding our future financing needs. Additional capital may not be available at reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the develop- ment or commercialization of one or more of our product candi- dates. If we issue debt or equity instruments to raise additional capital, it may result in the dilution of our existing shareholders, increase our fixed payment obligations, or result in securities that have rights senior to those of our ordinary shares or ADSs. If we incur debt, we could become subject to covenants restricting our operations and potentially impairing our competitiveness, such as limitations on our ability to incur additional debt, acquire, 60 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations

Fi na nc ia l S ta te m en ts sell or license intellectual property rights or other operational restrictions that could adversely impact our ability to conduct business. Cash Flows Net Cash Provided by/(Used in) Operating Activities In 2021, net cash used by operating activities amounted to € 481.4 million and was mainly attributable to the consolidated net loss of € 514.5 million and changes in operating assets and liabilities, including income taxes paid, totaling € 177.6 million. This was offset by non-cash items totaling € 210.6 million. The consoli- dated net loss of € 514.5 million resulted mainly from expenses incurred to finance MorphoSys’ ongoing operations, specifically cost of sales, research and development expenses, selling ex- penses, and general and administrative expenses. Prior year’s net profit resulted mainly from revenues from the collaboration and license agreement with Incyte, which was not recurring in 2021. Non-cash items included mainly income tax benefits in the amount of € 76.6 million (2020: € 75.4 million) and the net change in financial assets / liabilities from collaborations in the amount of € 16.0 million (2020: € 36.6 million). These were offset by the net change in financial liabilities from future payments to Roy- alty Pharma in the amount of € 42.8 million (2020: € 0), scheduled depreciation and amortization as well as impairments of tangible and intangible assets and right-of-use assets amounting to € 246.0 million (2020: € 24.8 million) and the full year non cash effective change of bonds amounting to € 12.1 million (2020: € 2.5 million). Changes in operating assets and liabilities in 2021 mainly included an increase in inventories, prepaid expenses and other assets of € 30.3 million (2020: increase of € 8.5 million), partially offset by a decrease in accounts receivable of € 10.5 mil- lion (2020: decrease of € 69.6 million). Accounts payable and ac- crued liabilities decreased by € 90.8 million (2020: increase of € 77.5 million). The main reason for this decline relates to ac- counts payable and accrued expenses of Constellation, which were included for the first time due to the acquisition on July 15, 2021. The accrued expenses and accounts payable of Constella- tion mainly comprised share-based payment obligations to Con- stellation’s employees that became due on the date of the acquisi- tion by MorphoSys as well as accrued transaction costs. Their subsequent payment in 2021 led to the decrease presented in this cash flow item. The year-on-year decrease in accounts receivable was mainly due to lower outstanding receivables at the end of the year 2021. The increase in inventories, prepaid expenses and other assets was due in particular to the higher inventories for the commercialization of Monjuvi in the U.S. Furthermore, MorphoSys paid € 64.6 million of income taxes in financial year 2021 due to net profit in 2020 (2020: € 0.3 million). In the previous year, net cash provided by operating activities amounted to € 35.3 million and was mainly attributable to the consolidated net profit of € 97.9 million. This was offset by non- cash income totaling € 62.6 million. The consolidated net profit of € 97.9 million resulted mainly from revenues from the collabora- tion and license agreement with Incyte, which was largely offset by expenses incurred to finance MorphoSys’ ongoing operations, specifically cost of sales, research and development expenses, selling expenses, and general and administrative expenses. Non- cash income included income tax benefits in the amount of € 75.4 million, income from the reversal of impairment of inventory in the amount of € 13.3 million related to the receipt of regulatory approval for Monjuvi and the net change in financial assets / lia- bilities from collaborations in the amount of € 36.6 million. These were offset by scheduled and unscheduled depreciation and amor- tization of tangible and intangible assets and rights of use amounting to € 24.8 million, net losses from other financial assets amounting to € 21.8 million, net losses from derivative financial instruments amounting to € 4.3 million and expenses for share- based incentive programs amounting to € 9.0 million. Changes in operating assets and liabilities in 2020 mainly included an in- crease in accounts receivable of € 69.6 million and in inventories, prepaid expenses and other assets of € 8.5 million. Accounts pay- able and accrued liabilities increased by € 77.5 million. The year- over-year increase in accounts receivable was mainly due to lower outstanding receivables at the end of the year. The increase in inventories, prepaid expenses and other assets was due in par- ticular to the recognition of inventories as a result of the market- ing authorization for Monjuvi in the U.S. The increase in external laboratory services outstanding at yearend, in particular related to tafasitamab, was the main reason for the higher trade payables and accrued liabilities. In 2019, the net cash used in operating activities amounted to € 81.1 million, primarily driven by the consolidated net loss of € 103.0 million, which was partially offset by non-cash expenses of € 9.5 million, and changes in operating assets and liabilities and taxes paid of € 12.4 million. The consolidated net loss of € 103.0 million was largely due to expenses we incurred to fund our ongoing operations, particularly the cost of sales, research and development expenses, selling expenses, and general and administrative expenses. The main contributors to non-cash charges were expenses for share-based payment of € 6.7 million and depreciation and amortization of tangible and intangible as- sets and of right-of-use assets of € 6.2 million, offset by income tax benefits of € 3.5 million. Changes in operating assets and lia- bilities for 2019 consisted primarily of an increase in accounts payable and accruals by € 13.2 million as well as a decrease in accounts receivable by € 2.7 million. This was offset by an in- crease in prepaid expenses and other assets by € 4.4 million. The increase in external laboratory services outstanding at the end of 2019, primarily related to tafasitamab, was the primary driver of the higher trade payables and accrued liabilities. The contract li- ability incurred during the year was largely related to prepay- ments received from contract partners. The decrease in accounts Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 61

receivable was due to a comparatively lower level of receivables outstanding at year-end 2019. The increase in prepaid expenses and other assets stemmed mainly from higher prepayments and higher receivables due from tax authorities from input tax surplus. Net Cash Provided by/(Used in) Investing Activities In 2021, net cash used in investing activities amounted to € 831.0 million, primarily driven by payments to acquire other financial assets amounting to € 2,188.3 million. These were offset by pro- ceeds from the sale of other financial assets amounting to € 2,592.0 million. This net cash outflow from investing activities was mainly due to a shift in the composition of our investment portfolio, as securities matured and were sold and new, compara- ble securities were acquired. The cash outflow relating to the ac- quisition of 100% shares in Constellation, net of acquired cash, in 2021 amounted to € 1,206.6 million. In addition, € 22.3 million was used for the acquisition of intangible assets in 2021. In 2020, net cash used in investing activities amounted to € 879.6 million, primarily driven by payments to acquire other financial assets amounting to € 1,745.7 million. These were offset by pro- ceeds from the sale of other financial assets amounting to € 900.8 million. The cash outflow from investing activities was mainly due to a shift in the composition of our investment portfolio, as securities matured and were sold and new, comparable securities were acquired. In addition, € 44.9 million was used for the acqui- sition of intangible assets in 2020. In 2019, net cash provided by investing activities was € 79.5 mil- lion, primarily driven by proceeds from the sale of other financial assets in the amount of € 371.9 million, partially offset by the purchase of other financial assets in the amount of € 274.8 mil- lion. Cash provided by investing activities primarily related to shifts in the composition in our investment portfolio as financial assets matured and were sold and new, similar financial assets were purchased. Additionally, in 2019, € 15.0 million were used to purchase a minority interest of 13.4% in Vivoryon Therapeutics AG. Net Cash Provided by/(Used in) Financing Activities Net cash provided by financing activities amounted to € 1,322.9 million in 2021 and consisted primarily of the cash receipts from the contracts with Royalty Pharma in the amount of € 1,206.7 million and the proceeds from the issuance of shares of € 84.7 million to Royalty Pharma as well as proceeds of € 40.0 million from financing collaborations from Incyte. Net cash provided by financing activities amounted to € 907.2 million in 2020 and consisted primarily of proceeds in the amount of € 80.6 million from the issuance of shares, as well as proceeds of € 510.2 million from financing collaborations, both in connec- tion with the collaboration and license agreement with Incyte. Further proceeds came from the issuance of convertible bonds in the amount of € 319.9 million, which were partially offset by lease payments of € 2.8 million and interest payments of € 1.4 million. In 2019, net cash provided by financing activities was € 0.4 mil- lion and mainly related to proceeds from the exercise of convert- ible bonds by related parties in the amount of € 3.7 million offset by lease and interest payments in the amount of € 3.4 million. Investments In 2021, MorphoSys invested € 3.7 million in property, plant and equipment (2020: € 4.3 million), mainly laboratory equipment (i.e., machinery) and tenant fixtures. Depreciation of property, plant and equipment in 2021 increased to € 2.8 million (2020: € 2.5 million). MorphoSys invested € 22.5 million in intangible assets in the re- porting year (2020: € 44.9 million). Of this amount, € 11.5 million was spent on internally generated intangible assets and € 10.4 million on in-process R&D programs. Amortization of in- tangible assets amounted to € 3.6 million in 2021 (2020: € 2.2 mil- lion). In 2020, impairment losses of € 14.0 million were recog- nized on intangible assets, thereof € 11.7 million on in-process R&D programs. In the course of the acquisition of Constellation in 2021, MorphoSys acquired € 719.4 million in intangible assets and € 1.6 million in property, plant and equipment. 62 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations
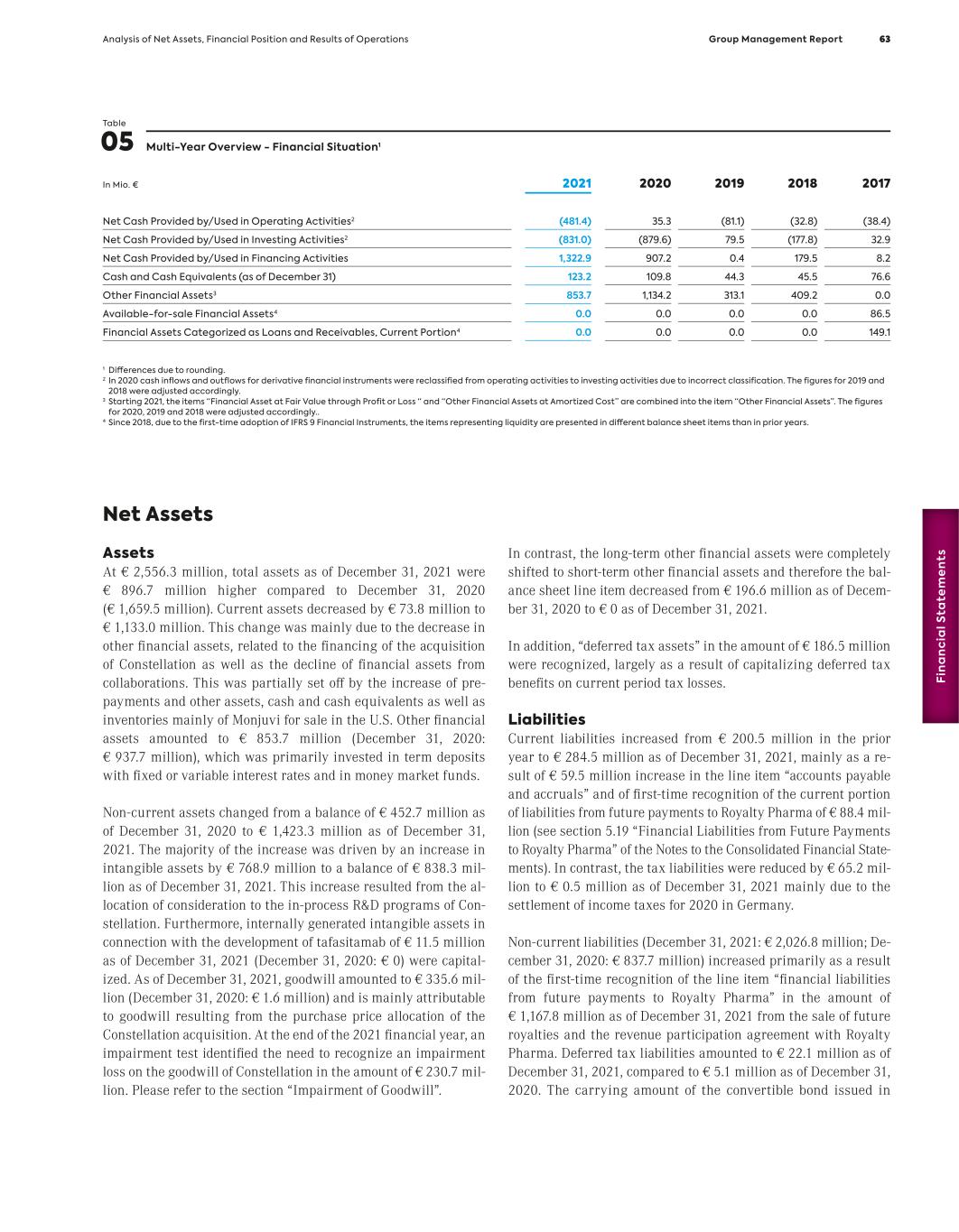
Fi na nc ia l S ta te m en ts Table 05 Multi-Year Overview - Financial Situation1 In Mio. € 2021 2020 2019 2018 2017 Net Cash Provided by/Used in Operating Activities2 (481.4) 35.3 (81.1) (32.8) (38.4) Net Cash Provided by/Used in Investing Activities2 (831.0) (879.6) 79.5 (177.8) 32.9 Net Cash Provided by/Used in Financing Activities 1,322.9 907.2 0.4 179.5 8.2 Cash and Cash Equivalents (as of December 31) 123.2 109.8 44.3 45.5 76.6 Other Financial Assets3 853.7 1,134.2 313.1 409.2 0.0 Available-for-sale Financial Assets4 0.0 0.0 0.0 0.0 86.5 Financial Assets Categorized as Loans and Receivables, Current Portion4 0.0 0.0 0.0 0.0 149.1 1 Differences due to rounding. 2 In 2020 cash inflows and outflows for derivative financial instruments were reclassified from operating activities to investing activities due to incorrect classification. The figures for 2019 and 2018 were adjusted accordingly. 3 Starting 2021, the items “Financial Asset at Fair Value through Profit or Loss “ and “Other Financial Assets at Amortized Cost” are combined into the item “Other Financial Assets”. The figures for 2020, 2019 and 2018 were adjusted accordingly.. 4 Since 2018, due to the first-time adoption of IFRS 9 Financial Instruments, the items representing liquidity are presented in different balance sheet items than in prior years. Net Assets Assets At € 2,556.3 million, total assets as of December 31, 2021 were € 896.7 million higher compared to December 31, 2020 (€ 1,659.5 million). Current assets decreased by € 73.8 million to € 1,133.0 million. This change was mainly due to the decrease in other financial assets, related to the financing of the acquisition of Constellation as well as the decline of financial assets from collaborations. This was partially set off by the increase of pre- payments and other assets, cash and cash equivalents as well as inventories mainly of Monjuvi for sale in the U.S. Other financial assets amounted to € 853.7 million (December 31, 2020: € 937.7 million), which was primarily invested in term deposits with fixed or variable interest rates and in money market funds. Non-current assets changed from a balance of € 452.7 million as of December 31, 2020 to € 1,423.3 million as of December 31, 2021. The majority of the increase was driven by an increase in intangible assets by € 768.9 million to a balance of € 838.3 mil- lion as of December 31, 2021. This increase resulted from the al- location of consideration to the in-process R&D programs of Con- stellation. Furthermore, internally generated intangible assets in connection with the development of tafasitamab of € 11.5 million as of December 31, 2021 (December 31, 2020: € 0) were capital- ized. As of December 31, 2021, goodwill amounted to € 335.6 mil- lion (December 31, 2020: € 1.6 million) and is mainly attributable to goodwill resulting from the purchase price allocation of the Constellation acquisition. At the end of the 2021 financial year, an impairment test identified the need to recognize an impairment loss on the goodwill of Constellation in the amount of € 230.7 mil- lion. Please refer to the section “Impairment of Goodwill”. In contrast, the long-term other financial assets were completely shifted to short-term other financial assets and therefore the bal- ance sheet line item decreased from € 196.6 million as of Decem- ber 31, 2020 to € 0 as of December 31, 2021. In addition, “deferred tax assets” in the amount of € 186.5 million were recognized, largely as a result of capitalizing deferred tax benefits on current period tax losses. Liabilities Current liabilities increased from € 200.5 million in the prior year to € 284.5 million as of December 31, 2021, mainly as a re- sult of € 59.5 million increase in the line item “accounts payable and accruals” and of first-time recognition of the current portion of liabilities from future payments to Royalty Pharma of € 88.4 mil- lion (see section 5.19 “Financial Liabilities from Future Payments to Royalty Pharma” of the Notes to the Consolidated Financial State- ments). In contrast, the tax liabilities were reduced by € 65.2 mil- lion to € 0.5 million as of December 31, 2021 mainly due to the settlement of income taxes for 2020 in Germany. Non-current liabilities (December 31, 2021: € 2,026.8 million; De- cember 31, 2020: € 837.7 million) increased primarily as a result of the first-time recognition of the line item “financial liabilities from future payments to Royalty Pharma” in the amount of € 1,167.8 million as of December 31, 2021 from the sale of future royalties and the revenue participation agreement with Royalty Pharma. Deferred tax liabilities amounted to € 22.1 million as of December 31, 2021, compared to € 5.1 million as of December 31, 2020. The carrying amount of the convertible bond issued in Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 63
October 2020 was € 282.8 million as of December 31, 2021. The long-term portion of the financial liabilities from collaborations declined from € 516.4 million ending 2020 to € 513.3 million as of December 31, 2021. Stockholders’ Equity As of December 31, 2021, Group equity totaled € 244.9 million compared to € 621.3 million on December 31, 2020. The Compa- ny’s equity ratio as of December 31, 2021 amounted to 10% com- pared to 37% on December 31, 2020. This decrease in the equity ratio resulted mainly from the first-time recognition of a financial liability from future payments to Royalty Pharma in 2021 under the royalty purchase agreement and the revenue participation agreement with Royalty Pharma as well as the consolidated net loss of the financial year 2021, which was mainly influenced by the impairment of goodwill. The number of shares issued totaled 34,231,943 as of Decem- ber 31, 2021, of which 34,148,789 shares were outstanding (De- cember 31, 2020: 32,890,046 shares issued and 32,758,632 shares outstanding). Common stock was higher as a result of the pur- chase of 1,337,552 shares by Royalty Pharma, as well as the exer- cise of 4,345 stock options from employees. On December 31, 2021, the Company held 83,154 treasury shares with a value of € 3,085,054 – a decrease of € 1,783,690 compared to December 31, 2020 (131,414 shares, € 4,868,744). The reason for this decrease was the transfer of 45,891 treasury shares amounting to € 1,696,131 to the Management Board and selected employees of the Company (beneficiaries) from the 2017 Long- Term Incentive Plan (LTI Plan). The vesting period for this LTI Plan expired on April 1, 2021 and offered beneficiaries a six- month period until October 13, 2021 to receive a total of 45,891 shares. In addition, 2,369 treasury shares for an amount of € 87,558 from the 2019 Long-Term Incentive Plan were trans- ferred to certain employees of MorphoSys US Inc. Table 06 Multi-Year Overview – Balance Sheet Structure1 in million € 12/31/2021 12/31/2020 12/31/2019 12/31/2018 12/31/2017 Assets Current Assets 1,133.0 1,206.8 303.7 388.9 340.7 Non-Current Assets 1,423.3 452.7 192.7 149.9 74.7 Total 2,566.6 1,659.5 496.4 538.8 415.4 Liabilities and Stockholders’ Equity Current Liabilities 284.5 200.5 61.6 45.9 47.7 Non-Current Liabilities 2,026.8 837.7 40.2 4.5 9.0 Stockholders’ Equity2 244.9 621.3 394.7 488.4 358.7 Total 2,556.3 1,659.5 496.5 538.8 415.4 1 Differences due to rounding. 2 Includes common stock as of December 31, 2021: € 34,231,943; December 31, 2020: € 32,890,046; December 31, 2019: € 31,957,958; December 31, 2018: € 31,839,572; December 31, 2017: € 29,420,785 64 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations
Fi na nc ia l S ta te m en ts Contractual Obligations The following table summarizes our contractual obligations as of December 31, 2021: Table 07 Contractual Obligations (December 31, 2021) Payments due by period (in € thousands) Total Less than 1 Year 1 to 3 years 3 to 5 years More than 5 Years Leases 49,565 4,256 8,375 8,375 28,559 Other 16,602 578 16,024 0 0 The item “Other” consists of future minimum payments under performance share unit programs and contracts for insurance and other services. Lease Obligations We enter into long-term leases for facilities, company cars and equipment. The majority of these leasing contracts can be re- newed on a yearly or quarterly basis, and some agreements may be terminated prematurely. Other Commitments Other commitments may become due for future payments for out- sourced studies. As of December 31, 2021, we expected to incur approximately € 236.5 million of expenses for outsourced stud- ies, of which approximately € 138.9 million will be paid in the next 12 months. If certain milestones are achieved by MorphoSys (for example, submitting an investigational new drug (IND) application for spe- cific target molecules), this may trigger milestone payments to licensors of up to an aggregate of US$ 236.5 million (approxi- mately € 208.8 million) related to regulatory events or the achievement of sales targets. Off-Balance-Sheet Arrangements We do not currently have any off-balance-sheet arrangements and did not have such arrangements in the years 2021 or 2020 that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial con- dition, revenues or expenses, results of operations, liquidity, cash requirements or capital resources. Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 65

Comparison of Actual Business Results versus Forecasts A detailed comparison of the Company’s forecasts versus the ac- tual results can be found in Table 08. Table 08 Comparison of Actual Business Results Versus Forecasts 2021 Targets 2021 Results Financial targets Group revenues of € 155-180 million (initial forecast of € 150-200 mil- lion; adjustment on July 26, 2021, driven by Monjuvi US sales of the first six months and the expectations for the remaining months of 2021) Group revenues of € 179.6 million Operating expenses of € 435-465 million (initial forecast of € 355-385 million; adjustment on July 26, 2021, driven by operating expenses of Constellation and one-time transaction-related costs of € 36 mil- lion) Operating expenses of € – 655.8 million; one-time transaction-re- lated costs of € 37.3 million Impairment of Constellation goodwill of EUR 230.7 million Research and development expenses of 52-57% of total operating expenses, excluding one-time transaction-related costs (initial fore- cast of 45–50%; adjustment on July 26, 2021) Research and development expenses of 34% of total operating ex- penses Proprietary Clini- cal Development Tafasitamab • Continue phase 1b study with tafasitamab in previously untreated DLBCL (firstMIND) • Initiate pivotal phase 3 study of tafasitamab in previously un- treated DLBCL (frontMIND) • Initiate pivotal phase 3 study (inMIND) of tafasitamab in patients with indolent lymphoma (r/r FL/MZL) • Evaluate (in collaboration with Incyte and Xencor) tafasitamab, plamotamab and lenalidomide in patients with relapsed or refrac- tory diffuse large B-cell lymphoma (DLBCL), first-line DLBCL and relapsed or refractory follicular lymphoma (FL) • Continue L-MIND study of tafasitamab and evaluate long-term ef- ficacy and safety data • Continue pivotal phase 3 trial (B-MIND) with tafasitamab in combi- nation with bendamustine for r/r DLBCL • Continue phase 2 COSMOS* study with tafasitamab in CLL/SLL in combination with idelalisib and venetoclax • Support Incyte in its initiated regulatory submissions to the EMA, Swissmedic and Health Canada for tafasitamab in combination with lenalidomide for r/r DLBCL • Support Incyte in submitting marketing authorization applications in additional markets Tafasitamab • Phase 1b study firstMIND continued as planned • The first patient in the pivotal phase 3 frontMIND study was dosed in May • The first patient was dosed in the pivotal phase 3 inMIND study in April • Preparations continued for a possible study (in collaboration with Incyte and Xencor) of tafasitamab, plamotamab and lena- lidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), first-line DLBCL and relapsed or re- fractory follicular lymphoma (FL) • Three-year results from the phase 2 L-MIND study of tafasi- tamab in combination with lenalidomide for the treatment of re- lapsed or refractory DLBCL were presented in June at the Ameri- can Society of Clinical Oncology (ASCO) Annual Meeting 2021 • The phase 3 B-MIND trial continued and has been fully recruited as of June; the significance of the B-MIND study has decreased as both FDA and EMA have approved Monjuvi based on L-MIND data. Consequently, the event-driven primary analysis has been removed from the planned analyses • The COSMOS study was completed • Tafasitamab in combination with lenalidomide was conditionally approved for r/r DLBCL by the EMA and Health Canada in August • Support provided to Incyte for its initiated regulatory submission to Swissmedic for tafasitamab in combination with lenalidomide for r/r DLBCL 66 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations

Fi na nc ia l S ta te m en ts 2021 Targets 2021 Results Felzartamab • Generate data from the phase 1/2 M-PLACE (proof-of-concept) study of felzartamab for the treatment of anti-PLA2R-antibody- positive membranous nephropathy • Continue dose schedule finding study (New-PLACE) in membra- nous nephropathy Felzartamab • Interim results from the M-PLACE study of felzartamab were pre- sented at the Annual Meeting of the American Society of Nephrol- ogy in November • The New-PLACE study was continued in 2021 Continue and/or initiate development programs for antibody identi- fication and preclinical development Early-stage drug discovery programs continued Clinical Develop- ment Through Partners Felzartamab/I-Mab • Support partner I-Mab in the regulatory filing (BLA*) for felz- artamab for multiple myeloma in China Felzartamab/I-Mab • Continue to support partner I-Mab in the regulatory filing (BLA) for felzartamab for multiple myeloma in China Otilimab/GSK • Publish preliminary results of OSCAR study of otilimab for the treat- ment of severe pulmonary COVID-19-associated disease by part- ner GSK in February 2021 Otilimab/GSK • GSK announced preliminary results from the OSCAR trial of otilimab for the treatment of severe pulmonary COVID-19-re- lated disease in March • In October 2021, GSK provided an update that it had made the decision not to further explore otilimab as a potential treatment for severe pulmonary COVID-19 related disease in patients aged of 70 years and older The Management Board’s General Assessment of Business Performance The 2021 financial year was a transformative year for MorphoSys and its employees. We worked together to transform MorphoSys from a research partner to the pharmaceutical industry into a fully integrated biopharmaceutical company. As part of this pro- cess, the corporate activities in 2021 focused on three important areas: • Executing on the commercialization of Monjuvi in the U.S. • Expanding the development pipeline by acquiring Constellation • Advancing the clinical development of our proprietary product candidates The co-commercialization of tafasitamab with Incyte in the U.S. gained momentum during the year after headwinds from the COVID-19 pandemic in the first quarter. The number of prescribing health centers grew steadily through the year, treating approxi- mately 2,000 patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) since launch in August 2020. Monjuvi’s net product sales for full-year 2021 equaled US$ 79.1 million. Further milestones were also achieved with tafasitamab. In Au- gust, Health Canada granted conditional marketing approval for Minjuvi (tafasitamab) in combination with lenalidomide for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma. The European Commission also granted conditional marketing approval for Minjuvi (tafasitamab) in combination with lenalidomide, followed by monotherapy with tafasitamab, for the treatment of adult DLBCL patients who are ineligible for autol- ogous stem cell transplantation (ASCT). With the completion of the Constellation acquisition in mid-July 2021, MorphoSys strengthened its position in hematology/oncol- ogy. Constellation’s lead product candidate, pelabresib (CPI-0610), a BET inhibitor, is currently in a phase 3 clinical trial for the treat- ment of myelofibrosis (MF). Following the acquisition by MorphoSys, improvements were made to the study design of the phase 3 trial, and measures were taken to accelerate recruitment. * see glossary – page 182 Analysis of Net Assets, Financial Position and Results of Operations Group Management Report 67

In December 2021, MorphoSys presented the latest data from the MANIFEST phase 2 study evaluating the potential of pelabresib in the treatment of myelofibrosis at the 63rd Annual Meeting of the American Society of Hematology. These data supported the further development of pelabresib in combination with ruxoli- tinib in MANIFEST-2, a global, phase 3, randomized, double-blind study in JAK-inhibitor-naïve MF patients. The following advances were made in our proprietary clinical de- velopment programs during the financial year: In January 2021, the U.S. Food and Drug Administration (FDA) granted orphan drug designation to tafasitamab for the treatment of follicular lymphoma (FL), with the first patient dosed in the phase 3 inMIND study in April 2021. The study is evaluating the safety and efficacy of tafasitamab compared to placebo in combi- nation with lenalidomide and rituximab in patients with relapsed or refractory follicular lymphoma (FL) or marginal zone lym- phoma (MZL). In 2021, the phase 1b firstMIND study of tafasitamab, initiated in December 2019, continued as planned. The study is evaluating the safety and preliminary efficacy of tafasitamab in combination with R-CHOP compared to tafasitamab and lenalidomide in addi- tion to R-CHOP in adult patients with newly diagnosed, previ- ously untreated DLBCL. In May 2021, the first patient was dosed in the pivotal phase 3 frontMIND study. The study is evaluating tafasitamab plus lena- lidomide in combination with R-CHOP compared to R-CHOP as first-line treatment for patients in moderate to high-risk groups with untreated diffuse large B-cell lymphoma (DLBCL). In June 2021, three-year results from the phase 2 L-MIND trial of tafasitamab in combination with lenalidomide for the treatment of relapsed or refractory DLBCL were presented at the 2021 Annual Meeting of the American Society of Clinical Oncology (ASCO). MorphoSys and Incyte presented real-world evidence results in December from the RE-MIND2 study of tafasitamab (Monjuvi) in combination with lenalidomide for the treatment of relapsed or refractory diffuse large B-cell lymphoma at the American Society of Hematology Annual Meeting (ASH 2021). RE-MIND2 compares outcomes of patients from the pivotal L-MIND trial to comparable patient populations treated with NCCN/ESMO recommended therapies. The results of the retrospective cohort analysis showed a significant improvement in overall survival compared to po- la-BR and R2, two other treatments for DLBCL, and should further increase Monjuvi’s acceptance among treating doctors and pa- tients. The CD38 antibody felzartamab, a proprietary development based on our HuCAL antibody technology, could be used against autoim- mune diseases, among others. In the first half of 2021, first data from the phase 1/2 M-PLACE (proof-of-concept) study in membra- nous nephropathy (MN) were presented at the Annual Meeting of the American Society of Nephrology. In this study, felzartamab demonstrated the potential to rapidly and significantly reduce an- ti-PLA2R antibody levels in patients with anti-PLA2R-antibody positive membranous nephropathy. In October 2021, the first patient was treated in a new phase 2 trial (IGNAZ) evaluating felzartamab in patients with immuno- globulin A nephropathy (IgAN). IgAN is a chronic and debilitat- ing autoimmune disease that affects the kidneys and is the most common glomerular disease worldwide. In conclusion, MorphoSys made major strides forward in advanc- ing its business during 2021. This included the first full year of commercializing Monjuvi in the U.S. with our partner Incyte, while working together to further broaden the opportunity for this important product via new market approvals and clinical tri- als in areas beyond the lead indication. We completed a transfor- mative M&A deal, acquiring Constellation to grow our late-stage oncology pipeline with compelling product candidates that fit well with our existing pipeline, which we also moved forward during the year. We are well positioned to continue to advance and grow our business in the months and years ahead. In the 2021 financial year, Group revenues amounted to € 179.6 million. Revenues consisted primarily of € 66.9 million in revenues from Monjuvi U.S. sales and royalty revenues from Tremfya in the amount of € 65.6 million. In the 2021 financial year, operating expenses were recognized in the amount of € 655.8 million, of which 34% accounted for research and develop- ment. Cash used in operating activities amounted to € 481.4 mil- lion, mainly as a result of the consolidated net loss. Our cash and investments of € 976.9 million are a confirmation of the strength of the Company’s financial resources. MorphoSys recognized the impact of the global COVID-19 pan- demic on healthcare systems and society worldwide, as well as the resulting potential impact on preclinical and clinical pro- grams, specifically clinical trials. In spring 2020, MorphoSys ac- tivated its existing business continuity plans to minimize any disruptions to ongoing operations caused by the COVID-19 pan- demic and to take the necessary actions to protect its employees. In addition, MorphoSys continuously monitors the situation as a whole as well as each clinical program individually and decides on the necessary course of action to ensure the safety of patients, personnel and other stakeholders, as well as on the collection of data. 68 Group Management Report Analysis of Net Assets, Financial Position and Results of Operations

Fi na nc ia l S ta te m en ts Outlook and Forecast The acquisition of Constellation was an important step on MorphoSys’ path to becoming a leading biopharmaceutical com- pany in hematology/oncology. To be successful, MorphoSys must rapidly develop new therapies with first-in-class and/or best-in- class potential and make them available to patients. In order to accomplish this, MorphoSys plans to prioritize its capital alloca- tion to late stage clinical studies. MorphoSys’ own development activities focus mainly on thera- pies for the treatment of blood cancers, which the Company in- tends to bring to market maturity and commercialize. Other drug candidates, such as felzartamab, which MorphoSys is currently testing in clinical trials for autoimmune diseases, could be fur- ther developed with partners or fully out-licensed due to its focus on hematology/oncology. General Statement on Expected Development MorphoSys has defined three strategic value drivers: • Revenues from the commercialization of Monjuvi • New marketing approvals for advanced drug candidates • Further development of mid-stage drug candidates with the op- tion to partner or out-license them The Management Board expects the following to be among the developments taking place in 2022: • Higher sales of Monjuvi in the U.S., with commercialization driven by the Company’s own capabilities and its partner In- cyte • Expand Monjuvi to additional disease indications and advance proprietary clinical development of product candidates: pela- bresib, felzartamab and CPI-0209 The expected developments and progress of the pipeline are pre- sented in detail below in the section “Future Research and Devel- opment and Expected Business Performance.” Strategic Outlook MorphoSys invests a significant portion of its financial resources in the clinical development of its own drug candidates. The Com- pany is focused on diseases in the hematology/oncology area. The Management Board believes a focus on proprietary drug develop- ment and commercialization offers the best path to achieving a sustainable increase in the Company’s value. The Management Board has prioritized the further clinical devel- opment of tafasitamab and pelabresib as well as the financing of pivotal clinical trials. To this end, revenues from the commercial- ization of Monjuvi are expected to contribute as well as partner- ships to leverage the full potential of the Company’s own develop- ment candidates. Increasing direct revenues from the commercialization of Mon- juvi is a core component of MorphoSys’ value creation strategy. Following the 2020 approval and launch of Monjuvi in the U.S., the processes are also underway for launches in Europe and Can- ada through MorphoSys’ partner Incyte. Additional regions are expected to follow, such as Switzerland, where Incyte could also commercialize tafasitamab, with MorphoSys entitled to royalties on sales. MorphoSys and Incyte have also identified significant unmet medical need and commercial opportunities for tafasitamab out- side of DLBCL in non-Hodgkin’s lymphoma. The Management Board believes tafasitamab could offer considerable future poten- tial, not only as a first-line therapy in DLBCL, but also in other indications such as r/r follicular lymphoma (FL) and r/r marginal zone lymphoma (MZL). Tafasitamab could become the future backbone in DLBCL therapy and other therapies. Pelabresib is viewed by the Management Board, as well as by leading medical experts, as a promising drug that may have the potential to fundamentally improve the treatment of myelofibro- sis. In ongoing clinical trials, pelabresib is demonstrating that the mechanism of action of the BET inhibitor has significant ame- liorative effects on all four major disease characteristics in myelo- fibrosis, such as spleen size, anemia, fibrosis of bone marrow and the patient’s overall physical condition. Outlook and Forecast Outlook and Forecast Group Management Report 69

With felzartamab, MorphoSys has another proprietary develop- ment candidate in advanced clinical development studies in the field of autoimmune diseases. MorphoSys’ focus on hematology/ oncology agents may lead to the Company choosing not to con- tinue developing and commercializing felzartamab on its own but instead develop it further within the framework of a partnership or by out-licensing it to another company with expertise in the autoimmune field. Partnerships can also help generate value through milestone pay- ments and royalties in the event of market approval (revenue sharing). Partnered programs such as gantenerumab with Roche, felzartamab in multiple myeloma with I-Mab or otilimab with GSK are the next candidates that could reach the market. In order to accomplish the overriding aim of being a leader in hematology/oncology, continually investing in the Company’s further development is not only sensible, but also essential. Expected Economic Development In its January 2022 report, the International Monetary Fund (IMF) projected global economic growth of 4.4% in 2022, com- pared to 5.9% for the year 2021. According to the IMF, the global economy entered 2022 in a weaker position than previously ex- pected. As the new COVID-19 variant Omicron has spread, coun- tries have reimposed mobility restrictions. Additionally, rising energy prices and supply disruptions have resulted in higher and more broad-based inflation than anticipated, notably in the United States and many emerging markets and developing economies. Uncertainty remains for the year ahead. The emergence of new COVID-19 variants could prolong the pandemic and induce re- newed economic disruptions. Moreover, supply chain disruptions, energy price volatility, and localized wage pressures translate into a high level of uncertainty around inflation and policy paths. Adding to the unknown, geopolitical tensions remain high, par- ticularly with the recent invasion of Ukraine by Russia. This con- flict is expected to take a strong human and economic toll far be- yond the Ukrainian border, particularly in Europe. Given these many challenges, international cooperation is critical, including developing an effective global health strategy to address the now two-year-old pandemic. Growth in advanced economies is antici- pated to reach 3.9% in 2022, compared to 5.0% for 2021. The IMF expects growth in the euro area to be 3.9% in 2022 compared to 5.2% for 2021. Growth in Germany is anticipated to rise to 3.8% in 2022 (2021: 2.7%), and the IMF projection for U.S. economic growth in 2022 is 4.0% (2021: 5.6%). The IMF’s 2022 growth fore- cast for emerging and developing countries is 4.8% (2021: 6.5%), and growth in China in the coming year is projected at 4.8% (2021: 8.1%). Russia’s economy is anticipated to grow by 2.8% in 2022 (2021: 4.5%), and Brazil is expected to barely grow at 0.3% for 2022 (2021: 4.7%). MorphoSys AG has implemented a business continuity plan to largely prevent the collapse of critical business processes and en- sure their resumption in the case of a natural disaster, public health emergency such as the novel coronavirus, or other serious events. However, depending on the severity of the situation, it may be difficult or, in some cases, impossible to avoid an interrup- tion in our business for a significant period of time. Our contin- gency plans for disaster recovery and business continuity may prove inadequate in the event of a serious disaster or similar event, and we may incur substantial costs that could have a mate- rial adverse effect on our business. Expected Development of the Life Sciences Sector In mid-January 2022, BioCentury published an article “Valua- tions could get investors, acquirers hunting for opportunities,” interviewing 15 investors and bankers to get their views on the year ahead. With biotech valuations beaten down in 2021 and a number of new companies debuting, investors believe there are plenty of buying opportunities heading into 2022. However, the IPO flow is predicted to slow as poor IPO performance translates to less crossover activity. The article reports that sentiment largely remains negative for the sector, but the low valuations could help change this. The conditions for M&A remain strong given the lower valuations and the large amounts of cash that large biopharma companies have on hand. In a related article, “Buysiders eye 2022 as the year of immu- no-oncology’s next big act,” investors indicated that cancer im- munotherapy is poised for long-awaited breakthroughs in 2022, which promises a full slate of regulatory and clinical milestones that could reignite buysider excitement while drawing in general- ists. Both checkpoint inhibitors as well as bispecific antibodies could emerge from a seven-year regulatory lull in 2022. Investors also expect interest in neurology — ignited by the Aduhelm (adu- canumab) approval last year — to carry over into 2022, with mul- tiple catalysts anticipated in this area. The high level of innovation in the biotechnology sector is re- flected in the number of new product approvals in 2021. Despite the ongoing challenges posed by the COVID-19 crisis, 50 new compounds were approved by the U.S. FDA in 2021, down slightly from the 53 approved in 2020. In addition, there were ten Biolog- ics License Application (BLA) approvals in 2021. In the EU, a re- cord 52 new drugs and vaccines were authorized for marketing in 2021, compared to 42 in 2020. The record in 2021 was in part due to the approval of four new vaccines and three new therapeutics for COVID-19. 70 Group Management Report Outlook and Forecast

Fi na nc ia l S ta te m en ts According to the report by PricewaterhouseCoopers (PwC), enti- tled: “Pharmaceutical & Life Sciences: Deals 2022 Outlook,” M&A activity in 2022 is projected to be between US$ 350–400 billion for the year, driven by all subsectors. This would be an increase compared to 2021, when the total value of deals was US$ 269.4 bil- lion, a 46% increase compared to 2020. Biotech deals in the range of US$ 5 billion to US$ 15 billion, combined with mid-sized pharma and medical device deals, are expected to drive signifi- cant investment money into M&A activity. PwC also predicts that the sector could see a large (US$ 100 billion or higher) deal that is part of a “transact-to-transform” strategy. Tailwinds around the need to invest in growth as well as access to capital are ex- pected to more than offset potential negative factors such as drug pricing, a recently active U.S. Federal Trade Commission agenda and tax rate increases. Future Research and Development and Expected Business Performance MorphoSys will continue to invest in research and development, with the majority of funds directed towards developing the Com- pany’s proprietary drug candidates tafasitamab, pelabresib, felz- artamab and CPI-0209. Most of these funds will be used in the short to medium term for advancing the broad clinical develop- ment of tafasitamab and pelabresib. Investments aimed at identi- fying target molecules, developing the corresponding antibodies, and in technology are also possible. The investments planned in proprietary drug candidates and technologies are expected to continue to lead to the progressive maturity of the pipeline’s product candidates. The following events and development activities, among others, are planned in 2022: • First proof-of-concept data from the ongoing clinical phase 2 study of CPI-0209 in solid tumors and blood cancer; • Additional data from the phase 1/2 M-PLACE (proof-of-concept) study of felzartamab for the treatment of anti-PLA2R-antibody positive membranous nephropathy (MN); • First data from the phase 2 study (IGNAZ) to evaluate felz- artamab in patients with immunoglobulin A nephropathy (IgAN); • MorphoSys’ partner Roche expects a pivotal data readout of the GRADUATE 1 and GRADUATE 2 trials with gantenerumab in the second half of 2022. Roche initiated these phase 3 develop- ment program for patients with Alzheimer’s disease in 2018; • Initiation of a combination study (in collaboration with Incyte and Xencor) of tafasitamab, plamotamab and lenalidomide in patients with relapsed or refractory diffuse large B-cell lym- phoma (DLBCL), first-line DLBCL and relapsed or refractory fol- licular lymphoma (FL). The following events and development activities, among others, are planned beyond 2022: • The primary analysis data from the pivotal phase 3 study (in- MIND) of tafasitamab in patients with indolent lymphoma (r/r FL/MZL) in the second half of 2023; • The primary analysis data from the pivotal phase 3 study (MANIFEST-2) of pelabresib in myelofibrosis (MF) in the first half of 2024; • The primary analysis data from the pivotal phase 3 study (front- MIND) of tafasitamab in previously untreated DLBCL in the second half of 2025. We also expect individual product candidates developed by part- ners to continue to mature in programs where MorphoSys bene- fits from royalties and milestone payments if successful. Whether, when and to what extent any news is published after the studies’ primary completion is solely at the discretion of our partners. Expected Development of the Financial Position and Liquidity MorphoSys transformed from a research- and technology-driven company to a commercial biopharmaceutical company with the launch of its first product in 2020. With the change in our busi- ness model and a certain degree of added complexity to our in- come statement from our partnership with Incyte and the agree- ment with Royalty Pharma, our focus is on the forecast parameters that are the most important to our external stakeholders. These parameters highlight our key business drivers: revenue growth from product sales, ongoing investment in expanding our pipe- line, and supporting the launch of Monjuvi. In concrete terms, these parameters focus on Monjuvi’s net sales in the U.S., the as- sociated gross margin, and the R&D and SG&A expenses. In the 2022 financial year, the Management Board expects Mon- juvi’s U.S. net product sales to range from US$ 110 million to US$ 135 million1, accompanied by a gross margin of 75% to 80%. This revenue guidance does not include royalty income, mile- stone payments or other revenues from partners as these revenue sources are not under our direct control. Royalty revenues for the sales of Tremfya will be transferred to Royalty Pharma and will therefore not result in any cash inflow for MorphoSys. MorphoSys expects to receive royalties for Minjuvi sales outside the U.S., but does not provide a prognosis for this royalty stream as MorphoSys does not receive a sales forecast from its partner Incyte. This guidance is subject to a number of uncertainties, including the potential for variability from Monjuvi, the ongoing COVID-19 pandemic and its impact on our business and that of our partners. 1 This would translate into Monjuvi sales of € 95.7 million to € 117.4 million at a EUR/USD exchange rate of 1.15. Outlook and Forecast Group Management Report 71

In 2022, the Group expects R&D expenses to range from €��300 million to € 325 million. R&D expenses primarily represent our investments in the development of tafasitamab, pelabresib, felzartamab and CPI-0209. R&D expenses are expected to in- crease compared to the prior year predominantly due to invest- ment in three late-stage studies. This increase is partly offset by the consolidation of research activities across the company. SG&A, including Incyte’s share of Monjuvi’s selling costs, are ex- pected to range from € 155 million to € 170 million. This reflects savings from synergies after the acquisition from Constellation as well as a redefined commercial structure. The overall guidance is subject to a number of uncertainties, in- cluding but not limited to the ongoing COVID-19 pandemic and its impact on MorphoSys’ business. Milestone payments and royalties related to the achievement of market maturity of HuCAL and Ylanthia antibodies could have an impact on the Company’s net assets and financial position in the years ahead. Such events could lead to exceeding our financial targets. Failures in drug development could also have an adverse effect on the MorphoSys Group. Negative effects from another COVID-19-like pandemic or COVID-19 variants are also possible, and cannot be excluded. Revenue growth in the short to medium term will depend on the Company’s ability to successfully con- tinue to commercialize Monjuvi. At the end of the 2021 financial year, MorphoSys had cash and investments of € 976.9 million (December 31, 2020: € 1,244.0 mil- lion). The liquid funds are predominantly required to finance and advance the development of the proprietary portfolio to key clini- cal milestones, including pivotal data readouts for tafasitamab and pelabresib. Dividend The separate financial statements of MorphoSys AG, prepared in accordance with German Generally Accepted Accounting Princi- ples (German Commercial Code), show an accumulated deficit, which prevents the Company from distributing a dividend for the 2021 financial year. In view of the anticipated losses in 2022, the Company expects to continue to report an accumulated loss for the 2022 financial year. MorphoSys plans to invest further in the development of proprietary drugs and commercialization of Mon- juvi. Based on these plans, MorphoSys does not expect to pay a dividend in the foreseeable future. This outlook takes into account all known factors at the time of preparing this report and is based on the Management Board’s assumptions about events that could affect the Company’s busi- ness in 2022 and beyond. Future results may differ from the ex- pectations described in the section “Outlook and Forecast.” The most significant risks are described in the risk report. 72 Group Management Report Outlook and Forecast

Fi na nc ia l S ta te m en ts Risk and Opportunity Report We operate in an industry characterized by constant change and innovation. The challenges and opportunities in the pharmaceuti- cal and biotechnology industry are influenced by a variety of fac- tors. Global demographic changes, medical advances and the de- sire to improve the quality of life offer excellent growth opportunities. Companies must also, however, grapple with the growing regulatory requirements in the areas of drug develop- ment and commercialization, as well as the cost pressures weigh- ing on healthcare systems. We make every effort to systematically identify new opportuni- ties and leverage our business success to generate a sustainable increase in the Company’s enterprise value. In our industry, en- trepreneurial success is not achievable without conscious risk-taking. Our integrated risk and opportunity management system identifies the relevant issues, assesses them and takes suitable action to avert threats so we can achieve our corporate objectives. A periodic strategy review ensures there is a balance between risks and opportunities. We assume a risk only when it involves an opportunity to increase the Company’s value. Principles of Integrated Risk and Opportunity Management We continually encounter both risks and opportunities that could have a potential material impact on our net assets and financial position, as well as a direct effect on intangible assets, such as our image in the sector or our brand name. We define risk as internal or external events that could have a direct adverse impact on the achievement of our corporate objec- tives. Opportunities represent positive deviations from our corpo- rate planning and are in direct relation to risks. Our integrated risk and opportunity management system is therefore an integral part of our corporate governance practices and ensures adher- ence to the principles of good corporate governance and compli- ance with the regulatory requirements. We have a comprehensive system in place to recognize, assess, communicate and manage our risks, and to identify our opportu- nities at an early stage. The Group-wide integrated risk and op- portunity management system focuses on major risks which alone or in combination with other risks could potentially jeopar- dize the Company. Risks and opportunities that do not meet this criterion are deliberately excluded by the system and managed and monitored on a decentralized basis at the level of the respec- tive organizational unit. The integrated risk and opportunity management system is described in a risk manual containing all the key elements of the process. During the 2021 financial year, we continued to develop our risk and opportunity management system to better reflect the busi- ness model of an integrated biopharmaceutical company and our associated internationalization. Table 09* provides an overview of significant changes in comparison to prior years. * cross-reference to page 74 Organization of Integrated Risk and Opportunity Management Our Management Board is responsible for the integrated risk and opportunity management system and ensures that all risks and opportunities are evaluated, monitored and presented in their en- tirety. The system’s Group-wide coordination, implementation and further development are the responsibility of the central Global Risk Management function, which is part of the Group Fi- nancial Planning & Analysis (FP&A) Department and reports di- rectly to the Chief Financial Officer. The Supervisory Board has tasked the Audit Committee with monitoring the effectiveness of our risk management system. The Audit Committee reports its findings to the entire Supervisory Board and the Management Board twice a year. Risk ownership is generally assigned at the level of the respective Executive Committee member as well as to selected executives with overriding responsibility. This group is defined as “risk own- ers.” As part of the integrated risk and opportunity management process, risk owners receive support from “risk agents.” Risk agents are experienced employees and generally members of the Global Leadership Group. They identify the risks in their respec- tive areas in close coordination with the central Global Risk Man- agement function. The distinction between the responsibilities of risk owners and risk agents is based on MorphoSys’ global man- agement and operating model. Risk and Opportunity Report Risk and Opportunity Report Group Management Report 73

Process Element Old Process New Process Risk responsibility Cost center managers are assigned risk responsibility and report risks with the support of a risk manager. Risk responsibility is generally assigned at the level of the respective Executive Committee member (as well as to selected executives). Selected „risk agents“ are responsible for identifying risks in combined areas (e.g., for clinical development risks at the program level) in coordination with central risk management. Opportunity and risk identifi cation Threshold values are not used in risk identifi cation by risk owners. Risk offi cers and risk managers identify risks largely independently without involving the central risk management department in their content. Risks, including strategic risks, are identifi ed and assessed for a period of one year and three years, respectively. A minimum threshold for reporting risks is put in place as part of the Group-wide integrated opportunity & risk management system. Signifi cant risks are already updated in advance of the semi-annual identifi cation process of the central Global Risk Management function based on the insights gathered from discussions with the Executive Committee and other executives. To ensure completeness, a structured query of all functional areas still continues to be carried out via the risk agents. In order to take the special features of multi-year clinical development cycles into consideration, a distinction is made between short-term risks (observation period of up to 12 months) and long-term/strategic risks. The latter include all risks that extend beyond a 12-month period. Opportunity and risk assessment Risk offi cers and risk managers assess risks largely independently without involving the central risk management department in terms of content. The central function of Global Risk Management reviews all identifi ed risks and subjects them to an initial quality assurance review in relation to the initial risk assessment and possible interdependencies with other risks. The main strategic risks identifi ed in this process are discussed in a workshop with selected risk agents and other executives to ensure an objective assessment. 09 09 Comparison of Old and New Integrated Opportunity & Risk Management System Risk and Opportunity Management System at MorphoSys Table Figure Supervision Reporting Monitoring Global Risk Management Global Internal Controls Global Compliance Departmental and divisional management Corporate Internal Audit Reporting Supervisory Board / Audit Committee Monitoring the eff ectiveness of the integrated CRM Executive Committee Overall responsibility for the set-up and execution of the integrated CRM Submission of results Performance of audit in line with audit plan Risk message Specifi cation of guide- lines and monitoring 1st line 2nd line 3rd line The central Global Risk Management function initiates and di- rects the ystematic risk identification process. Its organizational integration into the Group’s FP&A unit ensures that there is a tight link between risk and opportunity management and corpo- rate planning. Global Risk Management plays an important role in analyzing the interdependencies of risks and objectifying risk assessment. The Corporate Internal Audit Department is closely involved in the risk and opportunity management process. In addition to an ongoing exchange with the Global Risk Management function, the Internal Audit Department receives the audit reports in order to incorporate the findings into its risk-based audit plan. In accor- dance with this plan, the Internal Audit Department also con- ducts audits relating to integrated risk and opportunity manage- ment at irregular intervals. Figure 09 provides an overview of the organization and responsi- bilities of our integrated risk and opportunity management sys- tem, which is based on the globally recognized “Three Lines of Defense” model and meets the statutory requireme ts for the re- sponsibilities of the Management Board and supervisory bodies. ›› see figure 09 – Risk and Opportunity Management System at MorphoSys (page 75) Process and Reporting of Integrated Risk and Opportunity Management As part of our integrated risk and opportunity management pro- cess, all our major risks are identified and assessed by the rele- vant departments and reported in a structured form to Global Risk Management. This routine process takes place twice a year as what is called a “risk run”. For significant changes in material risks between the risk runs, the risk owners and risk agents are required to submit their respective reports to Global Risk Man- agement via an ad hoc process. Various quality assurance mea- sures have been implemented to ensure that the departments in- volved initially assess and record the risks as objectively as possible. These measures include a kick-off meeting to present the key aspects of the integrated risk and opportunity manual, as well as the close monitoring of the reporting process by Global Risk Management. After receiving the feedback from the risk agents, Global Risk Management carries out an initial review to identify the principal risks and highlight the interdependencies between identified risks. A workshop is held with selected risk agents in which the key risks and opportunities are calibrated based on the initial feedback and the key statements, and detailed in the report to the Management Board and supervisory bodies. 74 Group Management Report Risk and Opportunity Report

Process Element Old Process New Process Risk responsibility Cost center managers are assigned risk responsibility and report risks with the support of a risk manager. Risk responsibility is generally assigned at the level of the respective Executive Committee member (as well as to selected executives). Selected „risk agents“ are responsible for identifying risks in combined areas (e.g., for clinical development risks at the program level) in coordination with central risk management. Opportunity and risk identifi cation Threshold values are not used in risk identifi cation by risk owners. Risk offi cers and risk managers identify risks largely independently without involving the central risk management department in their content. Risks, including strategic risks, are identifi ed and assessed for a period of one year and three years, respectively. A minimum threshold for reporting risks is put in place as part of the Group-wide integrated opportunity & risk management system. Signifi cant risks are already updated in advance of the semi-annual identifi cation process of the central Global Risk Management function based on the insights gathered from discussions with the Executive Committee and other executives. To ensure completeness, a structured query of all functional areas still continues to be carried out via the risk agents. In order to take the special features of multi-year clinical development cycles into consideration, a distinction is made between short-term risks (observation period of up to 12 months) and long-term/strategic risks. The latter include all risks that extend beyond a 12-month period. Opportunity and risk assessment Risk offi cers and risk managers assess risks largely independently without involving the central risk management department in terms of content. The central function of Global Risk Management reviews all identifi ed risks and subjects them to an initial quality assurance review in relation to the initial risk assessment and possible interdependencies with other risks. The main strategic risks identifi ed in this process are discussed in a workshop with selected risk agents and other executives to ensure an objective assessment. 09 09 Comparison of Old and New Integrated Opportunity & Risk Management System Risk and Opportunity Management System at MorphoSys Table Figure Supervision Reporting Monitoring Global Risk Management Global Internal Controls Global Compliance Departmental and divisional management Corporate Internal Audit Reporting Supervisory Board / Audit Committee Monitoring the eff ectiveness of the integrated CRM Executive Committee Overall responsibility for the set-up and execution of the integrated CRM Submission of results Performance of audit in line with audit plan Risk message Specifi cation of guide- lines and monitoring 1st line 2nd line 3rd line Fi na nc ia l S ta te m en ts The risk assessment is derived from an evaluation of the risk’s probability of occurrence and impact using a five-point scale, as shown in table 10*. In terms of impact, MorphoSys distinguishes between financial and non-financial impact. Financial impact is defined as a negative deviation from the budgeted operating re- sult, whereby the impact on liquidity is equally considered for non-profit items. Both the short-term (12 months) and medi- um-term (three years) financial impact is taken into consider- ation. Non-financial risks in our integrated opportunity and risk management system are circumstances and defined as those hav- ing no initial direct impact on the operating result or liquidity during the planning period, but still have a negative impact on the achievement of the Company’s targets. Examples include the loss of reputation or key employees, both of which can have a sustained impact on the Company’s potential for success. An- other example specific to our industry is the impact of delays in patient recruitment for clinical trials. Such delays initially lead to lower costs, which from a purely mechanical standpoint represent an opportunity when compared to initial planning, but in the long term have a negative effect causing a delay in the development plan, which outweigh the short-term benefit of lower costs. In view of the increasing importance clinical programs have on the Company’s value, we continued to evolve our integrated opportu- nity and risk management system in the reporting year to opti- mally reflect these Company- and industry-specific characteris- tics. The integrated opportunity and risk management system addresses both opportunities and risks of the MorphoSys Group, with systematic quantification and aggregation only for risks. * cross-reference to page 76 Accounting-Related Internal Control System To ensure accurate bookkeeping and accounting and maintain reliable financial reporting in the consolidated financial state- ments and group management report, we use internal controls through our financial reporting, which we have expanded pursu- ant to the provisions of Section 404 of the Sarbanes-Oxley Act of 2002 (SOX* 404), in addition to Group-wide reporting guidelines and other measures, such as employee training and ongoing pro- fessional education. This essential component of Group account- ing consists of preventative, monitoring and detection measures intended to ensure adequate security and control in accounting and operating functions. * see glossary – page 182 Further detailed information about the internal control system for financial reporting can be found in the Corporate Governance Re- port. Risk and Opportunity Report Group Management Report 75

10 Risk Assessment Categories Table Probability of occurrence > 50% Low Moderate Medium High High 30% – < 50% Low Moderate Moderate Medium High 20% – < 30% Low Low Moderate Moderate Medium 10% – < 20% Low Low Low Moderate Moderate < 10% Low Low Low Low Low Financial impact* 1-year view < € 2 million € 2 – 5 million € 5 – 10 million € 10 – 25 million > € 25 million 3-year view < € 6 million € 6 – 15 million € 15 – 30 million € 30 – 75 million > € 75 million Qualitative equivalents Marginal impact on value creation potential, e.g., delays in projects in the research area Low impact on value creation potential, e.g., failure of projects in the research area Medium impact on value creation potential, e.g., delays or failures of stud- ies in early or mid-stages of clinical development Strong impact on value creation potential, e.g., delays in clinical trials for major programs Signifi cant impact on value creation potential, e.g., failure of clinical trials in major programs Marginal impact on reputation and ability to continue operations, e.g., general critical reporting or internal processes that need optimization Low impact on reputa- tion and ability to con- tinue operations, e.g., unexpected departure of key employees Medium impact on repu- tation and ability to continue operations, e.g., potential diffi culty in communicating with academia and institutions Severe impact on reputa- tion and ability to con- tinue operations, e.g., re- ports of compromised patient safety or a signif- icant cybersecurity at- tack Signifi cant impact on reputation and ability to continue operations, e.g., loss of approvals due to security threats or opera- tional catastrophic events Signifi cant risks * based on operating income and liquidity Description of Key Opportunities Increasing life expectancy in industrialized countries and the changes in income and lifestyle in the emerging markets are ex- pected to drive the demand for new and innovative treatments and advanced technologies. Progress in science and medicine has led to a better understanding of the biological processes of dis- ease. This, in turn, paves the way for new therapeutic approaches in bi- and multispecific antibody development and other technol- ogy platforms. Advanced antibody discovery and protein engineering technolo- gies and a broad portfolio of validated clinical programs have made us one of the globally recognized biotechnology companies in the therapeutic antibody field. The class of monoclonal antibod- ies is one of today’s most successful, top-selling therapies in can- cers and immune diseases. Our key opportunities are described in table 11 and ranked ac- cording to their expected potential value contribution and strate- gic relevance. Table 11 Summary of MorphoSys’ Key Opportunities Opportunities Full realization of pelabresib’s potential in product development Full realization of tafasitamab’s potential in product development and commercialization Further advancement of current phase 2 studies of felzartamab and CPI-0209 Additional income from milestones and royalties from partnered programs Maximization of value proposition from promising investigational compounds 76 Group Management Report Risk and Opportunity Report

Fi na nc ia l S ta te m en ts Full Realization of Pelabresib’s Potential in Product Development We believe pelabresib has the potential to become the standard of care in myelofibrosis. This assessment was underlined by the pre- sentation of confirmatory phase 2 data (MANIFEST) at the Amer- ican Society of Hematology conference at the end of the last finan- cial year. The approval of pelabresib could unlock significant positive and transformative potential for MorphoSys in an indica- tion where there is a high need for improved treatment options for approximately 30,000 to 35,000 patients in the U.S. and Europe. To intensify further product development, MorphoSys has already adapted the study’s design and plans to enroll more patients in the active phase 3 study. One of the Company-wide strategic pri- orities, in addition to the activities already completed, is to ensure the active study’s smooth and prompt completion. In addition, the Company is contemplating expanding the geographic availabil- ity, although the clear focus is on the U.S. and European markets. Full Realization of Potential of Monjuvi (Tafasitamab) in Product Development and Commercialization Monjuvi (tafasitamab-cxix) is our first commercial product and represents a significant opportunity as it is currently the only FDA-approved drug in the second line setting for patients with r/r DLBCL in combination with lenalidomide. MorphoSys is focusing on commercializing Monjuvi in the U.S. market with its partner Incyte. MorphoSys will receive royalties for the commercializa- tion outside the U.S., which will be handled by Incyte. Data from the L-MIND study published in 2021 have underpinned the exist- ing long-term treatment outcomes, and approximately 2,000 pa- tients have been treated with Monjuvi in the U.S. since its launch. We are therefore concentrating our efforts on Monjuvi’s further commercialization where we believe many more patients could benefit from treatment, which would directly lead to higher sales revenues. In addition to the focus on Monjuvi’s commercialization, we are also prioritizing further development in DLBCL and beyond, par- ticularly within the scope of our active phase 3 trial in first-line DLBCL, tafasitamab’s development in FL, and through combina- tion studies with other promising drugs. If approval is granted in important markets after completion of the clinical phases, there is a chance of a significant increase in medium- and long-term sales potential. Further Advancement of Current Phase 2 Studies of Felzartamab and CPI-0209 The two phase 2 compounds, felzartamab and CPI-0209, comple- ment MorphoSys’ proprietary clinical pipeline. CPI-0209 is a potentially best-in-class EZH2 inhibitor currently in phase 2 development for advanced solid tumors and blood can- cer. Results from the ongoing feasibility study are expected in 2022. Felzartamab is a CD38 antibody that, based on data from two active phase 2 studies, may have strong therapeutic potential for autoimmune diseases. The focus for both compounds is to continue their development and gain further insight from the data generated. Further in- house development, co-development with a partner and out-li- censing are all conceivable options to accomplish this. Additional Income from Milestones and Royalties from Partnered Programs As previously described, our business focus during the past few years has significantly shifted away from traditional contract re- search towards proprietary product development and commer- cialization, especially since our acquisition of Constellation and the agreement with Royalty Pharma. Due to programs partnered in the past, however, MorphoSys may still be entitled to substan- tial cash inflows from milestones and/or licensing income in the future. This is the case, for example, for otilimab and gan- tenerumab that are covered under the agreement with Royalty Pharma, despite the fact that a portion of the royalties for these product candidates has been assigned. MorphoSys’ partners, such as Novartis, with whom the Company has had a long-stand- ing research collaboration, also have other drugs in development. Maximization of Value of Promising Investiga- tional Compounds in the Discovery Phase We have already demonstrated our ability to develop promising drugs and technologies. Our in-house research department could help us in the future to increase the pace and success rate of our proprietary drug development programs, and new technologies could open up new disease areas with completely novel modes of action. The Company’s technology development is driven by a team of scientists focused on advancing our technologies. In addi- tion to our in-house technology development, we also rely on ex- ternal sources to bolster our efforts in this area. One example is our licensing agreement with Cherry Biolabs, which grants us the rights to apply their innovative, multispecific Hemibody technol- ogy in the context of our CyCAT dual-targeting platform. Interest- ing development candidates are discussed regularly by portfolio management committees and the Executive Committee. Description of Key Risks In this report describing the key risks, we explain the financial and non-financial risks that we consider to be most relevant for the achievement of the Company’s targets in 2022 and beyond. The risk and opportunity profile of the MorphoSys Group has changed significantly in the past year as a result of the acquisi- tion of Constellation on July 15, 2021, and the associated financ- ing agreement with Royalty Pharma. We have therefore revised our allocation of risks to risk categories compared to the previous year’s presentation to better reflect the evolution of our business model. The following overview provides an explanation and summary of the different risk categories and a description of the items gener- ally included in these categories. Risk and Opportunity Report Group Management Report 77

Category Explanation Change in Presentation vs. the Previous Year Strategic risks This category encompasses mainly those risks resulting from a deviation in the progress of our proprietary clinical develop- ment programs from the clinical development plan, as well as the potential impact of the COVID-19 pandemic situation on the recruitment of study participants. Also included in this category are risks arising from the general business strategy, such as the risks associated with current or potential collaborations. Focus is on risks related to key value drivers. Operational risks Risks in this category consist of those material risks that are attributable to the Company‘s operations, particularly those related to the execution of processes, which also includes ensuring business operations in the event of disruptions such as catastrophe situations or cybersecurity incidents. Risks in connection with the integration of Constellation Pharmaceuticals are also part of this category. This category now includes risks related to securing operations that were reported under “organizational risks” in the prior year. Risks related to the provision of active ingredients for clinical trials as well as related to commercial marketing were reported last year under “strategic risks” but are now sub- sumed in this category. Commercial risks Commercial risks are those related to the marketing of ap- proved products. In the forecast period, this comprises mainly the sales performance of Monjuvi/Minjuvi®, as well as the po- tential eff ects of the COVID-19 pandemic situation on the sales potential generated from marketing. Risks from commercialization were reported under the cate- gory “strategic risks” in the previous year. Financial risks This category groups together risks that are directly related to the organization‘s fi nances. Examples include exchange rate risks, the access to and securing of adequate fi nancing, as well as tax-related risks. This category includes corporate fi nance risk, which was previ- ously reported under “strategic risks”. Legal & compliance risks Legal & compliance-related risks include risks arising from compliance with laws and equivalent regulations. Particularly relevant are industry-specifi c regulations in the area of health- care compliance and GxP-relevant issues and risks relating to safeguarding intellectual property (IP). Risks that were assigned to the category “GxP-relevant risks” in the previous year have now been assigned to this category as they also represent compliance risks. In addition, risks relating to the securing of intellectual property rights that were previ- ously assigned to the “external risks” and other categories are now reported in their entirety under this category. 12 Overview of Risk Categories Table The assessment of risk relevance is not distinguished according to category, but instead by impact and probability of occurrence. For this reason, the major risks listed in table 13* do not always include risks from all five categories. * cross-reference to page 79 MorphoSys, like most companies, is fundamentally affected by developments related to the COVID-19 pandemic. However, in contrast to the broad impact felt in many industries, the direct impact on MorphoSys is largely limited to its access to treatment facilities and patients, which affects not only the commercializa- tion of approved products, but also clinical study recruitment and operation. In the detailed presentation of material risks, the risks arising from developments associated with the COVID-19 pan- demic are therefore assigned to the corresponding general cate- gories. Strategic Risk Strategic risks are those risks that affect the long-term viability of our current and future business success. In line with our busi- ness model, these risks are primarily those that arise when the progress of our own major development programs deviates from the clinical development plan. This includes the potential impact of the COVID-19 pandemic on patient recruitment for clinical studies. Generally speaking, interim results from clinical trials may result in a study’s discontinuation or a modification in its design. There is also a possibility that regulatory authorities may not accept our proposed clinical development strategy or our ap- plication based on the data and/or may not grant approval or with- draw the granted approval under specific circumstances. Risks could also arise from current or future collaborations or other business development activities, which can negatively af- fect our potential to create strategic added value. 78 Group Management Report Risk and Opportunity Report

Risk Category Change vs. the Previous Year Assessment Risks in the clinical development of pelabresib Strategic Medium Risks in the clinical development of tafasitamab Strategic Medium Restriction in access to patients Commercial Moderate Competitive and market risks Commercial Moderate Supply chain-related risks Operational Moderate Personnel risks Operational Moderate Risks in connection with the integra- tion of Constellation Operational Moderate Currency risks Financial Moderate Tax risks Financial Medium 13 Overview of MorphoSys’ Most Signifi cant Risks Table Fi na nc ia l S ta te m en ts Pelabresib Development Risk As outlined in the description of opportunities, we believe that pelabresib has the potential to become the standard of care in myelofibrosis. Our view, however, is based on the assumption that the clinical endpoints of the MANIFEST-2 pivotal study are met, which is an inherent risk of clinical development and only par- tially under MorphoSys’ control. One of the necessary prerequi- sites for successful development is our ability to recruit a suffi- cient number of patients to generate meaningful data. Immediately following our acquisition of Constellation, we established a task force reporting directly to the Chief Research and Development Officer to ensure we achieve this. We also set up additional loca- tions for our clinical studies. Nevertheless, despite these mea- sures, there is still a risk that the clinical endpoints are not met, or met only to a limited extent, or that there is a delay in compar- ison to the original development plan, any of which could have a significant impact on the Company’s potential for future value creation. Tafasitamab Development Risk Similar risks exist for clinical trials in other indications as well as for approvals for tafasitamab, which we are working on together with our collaboration partner Incyte. We initiated new studies in the reporting year and have implemented measures to ensure that we can promptly enroll patients. The achievement of the clin- ical endpoint is again beyond MorphoSys’ control and is an inher- ent risk of clinical development. Development Risks Associated with Other Clinical Programs In addition to our two main clinical programs, we have two other programs, CPI-0209 and felzartamab, in phase 2 clinical develop- ment. These active studies are considered proof-of-concept stud- ies, which means there are further opportunities for clinical de- velopment if the outcome is successful. These studies also carry the risk that the clinical endpoints are not achieved to a satisfac- tory extent. Business Development Risk Due to the high cost of clinical trials, we are not able to conduct all scientifically feasible development projects independently and need to prioritize our investments based on business decision models despite our strong liquidity. Collaborations with other partners may be an alternative for development projects investi- gating our product candidates in new indications. Should such collaborations fail to materialize, there is a risk that we will not be able to realize the Company’s potential to create value. How- ever, this does not represent a risk compared to our forecast, as the latter does not include such an assumption due to the uncer- tainty of the conclusion or the conditions of possible collabora- tions. Risk and Opportunity Report Group Management Report 79

Commercial Risk In July 2020, MorphoSys received accelerated FDA approval for the commercialization of Monjuvi in the U.S. Since that time, the relative importance of revenues generated from our own commer- cialization of the product with our partner Incyte has been steadily increasing. From a risk standpoint, we distinguish the short-term risk arising from potential COVID-19 restrictions from the more mid- to long-term risk of the market and competitive environment. Pandemic-Related Limitations in Access to Patients and Facilities The ebb and flow in COVID-19 infections since the start of the pandemic have impacted patient access to treatment facilities in the U.S. Therapies planned in “hot spot” regions, for example, may be postponed due to a lack of capacity. Furthermore, safety protocols implemented at various sites of care may restrict the ability of our sales force to engage in-person with medical person- nel. As a result, there is a risk that we will not achieve the reve- nue planned from the sale of Monjuvi in the U.S. We assess any potential impact of this risk, however, as moderate. Competitive and Market Risk Despite our innovative products, we operate in a competitive en- vironment not only for existing therapies but also unapproved therapeutic alternatives still in clinical research. We meet these challenges through a combination of education about our product and additional data from ongoing clinical studies. Nevertheless, there is a risk that the therapies preferred change over time, com- petitive products are approved, or existing therapies gain market share at our expense. There is also significant pressure to contain healthcare costs in the European and North American markets, and payers have taken actions that may result in access restrictions or lead di- rectly and indirectly to price reductions for our products. We ex- pect these efforts to increase and expand over time and continu- ously monitor the related discussions. However, due to the political situation in the U.S., our core sales market, we do not expect any significant impact from such regulatory measures during the forecast period. Operational Risk Operational risk includes material risks that are attributable to the Company’s operations, specifically those related to the execu- tion of processes such as maintaining business operations in the event of catastrophic events or cybersecurity incidents. Risks in connection with the integration of Constellation are also included in this category. Supply Chain Risk MorphoSys does not produce its own active pharmaceutical in- gredients but outsources this manufacturing to contract manu- facturing organizations (“CMOs”), which is typical for a number of comparable companies in our industry. MorphoSys addresses the risk of material procurement through contractual agreements and continual monitoring. The impact of the COVID-19 pandemic on global supply chains has resulted in an increased risk of bot- tlenecks in the availability of consumables and raw materials compared to prior years. MorphoSys is addressing this risk by securing a safety backlog, resulting in the risk from delays in the supply of products for active clinical trials and commercial use during the forecast period being assessed as low risk. Personnel Risk MorphoSys’ key asset is its employees. The Company’s future success largely depends on its ability to attract, develop and re- tain key employees over time. This risk has increased due to the outbreak of the COVID-19 pandemic and the higher demand for personnel in pharmaceutical companies that has resulted. MorphoSys has offices in the U.S. and in Germany, two countries with a high demand for personnel and a correspondingly large number of competing biotechnology companies. To maintain its image as an attractive employer for skilled personnel, MorphoSys offers competitive compensation and a range of options for per- sonnel development. Succession planning for key positions en- sures that there is no significant risk arising from the level of employee turnover that is typical for the industry and the Compa- ny’s location. Integration Risk A “risk run” in the fall of 2021 identified a short-term, moderate organizational risk related to the operational integration of Con- stellation into the MorphoSys Group. Should MorphoSys be un- able to integrate the acquired company into the Group’s struc- tures and processes within a reasonable period of time, there is a risk that potential synergies may fail to be realized as planned. This risk also includes the potential departure of employees in key positions with specific background knowledge. To mitigate this risk, a project team has been formed consisting of experi- enced Constellation and MorphoSys employees from various de- partments that is focused on key aspects of the integration. By the end of the 2021 financial year, significant progress had al- ready been made in integrating the companies’ operations. A global operating model was rolled out to manage major functions across locations and facilitate the business and decision-making processes. While the measures taken have greatly reduced inte- gration risk, a financial risk exists that potential synergies will not be leveraged as planned. IT and Cybersecurity Risk Cyber risks encompass all risks to computer and information net- works, IT infrastructure and IT-based business and production processes from exposure to sabotage, espionage or other criminal acts. Should the established security measures fail, MorphoSys could suffer reputational damage as well as payment obligations arising from contractual and legal claims from customers, con- tractual partners and authorities. An increase in the professional- ization of cyberattacks has become evident in the past several years, with social engineering techniques increasingly being used in addition to purely technological attacks. MorphoSys has implemented extensive safeguards in information technology 80 Group Management Report Risk and Opportunity Report

Fi na nc ia l S ta te m en ts and cybersecurity. Internal controls and quality assurance proce- dures have been rolled out across all major applications and un- derlying networks and infrastructure. We have advanced sys- tems to prevent unauthorized intrusions and support the timely monitoring of attacks on our IT systems. A qualified Computer Emergency Response Team (CERT) has also been established in addition to extensive preventive training and awareness-raising measures for employees. Further details on our IT and cybersecurity measures can also be found in the Information Technology section in the Corporate Governance Report. Business Continuity Risk MorphoSys has implemented a business continuity plan to pre- vent the widespread collapse of critical business processes and ensure their resumption should a natural disaster, health-related crisis (such as the coronavirus), or other serious event occur. However, depending on the severity, it may be difficult or impos- sible for us to continue our business for a significant length of time. Our disaster recovery and business continuity plans may prove inadequate should a severe disaster or similar event occur. We also may incur significant costs that could have a material adverse effect on our business. In almost two years since the on- set of the coronavirus pandemic, mobile working has allowed op- erations to continue at MorphoSys. Except for a few tasks that re- quire an on-site presence, business can continue off-site without significant restrictions. As a result, business continuity risk is classified as low. Financial Risk Our financial risk management aims to mitigate financial risks and balance these risks with the needs arising from our business activities. As part of our financial risk management, we continu- ously monitor current developments in the tax legislation of our sales markets and operating sites so that we can identify and ad- dress tax risks at an early stage. Liquidity Risk Unexpected fluctuations in revenues, unplanned adverse develop- ments in expenses and external events and changes in the busi- ness environment can all have a negative impact on our short- to medium-term liquidity and profitability. To ensure our short-term liquidity, we invest a sufficient share of our financial assets in short-term financial instruments. We also have a comprehensive liquidity plan based on our corporate planning that includes the simulated effects of various scenarios so we can determine our medium- and long-term liquidity requirements. To further reduce our financial risk, we take the outcome of the liquidity plan into account when prioritizing research and development projects and determining the financing requirements and the resulting con- crete financing activities of the Management Board. MorphoSys also has access to other non-dilutive financing options, such as utilizing the development funding bonds provided by Royalty Pharma. Currency Risk MorphoSys generates a large percentage of its revenues in U.S. dollars, and this percentage is expected to increase with the ex- pected increase in revenues from Monjuvi. U.S. commercializa- tion costs and research and development costs are also incurred in U.S. dollars, and the proportion of these costs have increased following the acquisition of Constellation. As long as the costs in U.S. dollars exceed U.S. dollar revenues, a further depreciation in the EUR/USD exchange rate represents a short- and medium-term risk for MorphoSys. The fluctuation in macroeconomic develop- ment in recent months has caused this risk to increase compared to the previous year. The Financial Planning & Analysis and Cor- porate Treasury departments continuously monitor changes in the EUR/USD exchange rate. A strategy for investing in U.S. dol- lar financial products has been developed in consultation with the Chief Financial Officer and in line with the internal guidelines for investing in financial products. Interest Rate and Default Risk As a result of the ongoing, tense economic situation in Europe, the potential insolvency of banking institutions continues to rep- resent a financial risk. We are therefore continuing to invest, when possible, only in funds and products of banks that are con- sidered safe and have a high rating or are backed by a strong partner. We diversify and invest in lower-risk money market funds in order to limit our exposure to individual financial insti- tutions. A strategy that excludes all risks of potential bank insol- vencies would be too expensive and impractical. German govern- ment bonds, for example, are a very safe investment, but currently trade at a negative interest rate. On the other hand, earning ade- quate interest on financial investments poses a risk, particularly as the key interest rate is at a negative level. We have limited in- vestment options if we want to avoid negative interest rates while staying within the Company’s investment guidelines. We invest, when possible, in instruments that yield positive interest, but there is no guarantee that such investments will remain avail- able. Tax Risks The accounting treatment of the payment that MorphoSys AG re- ceived from Royalty Pharma in the third quarter of 2021 could be examined by the tax authorities under German tax law in the context of a future tax audit. This examination is considered stan- dard given the amount of the payment. Based on the Company’s knowledge of German tax law and supported by tax experts, the Company has concluded that the tax risk assessment is medium in accordance with the Company ś internal risk valuation system. Consequently, due to the remaining uncertainty and the signifi- cance, a contingent income tax liability in the amount of € 223.1 million is reported (refer to Note 7.2* in the notes). * cross-reference to page 161 Risk and Opportunity Report Group Management Report 81

Regulatory and Compliance Risk Regulatory and compliance-related risks include risks arising from failing to comply with laws and equivalent regulations. Of particular relevance are risks related to industry-specific regula- tions in the area of healthcare compliance, GxP*-relevant issues, and risks concerning the protection of intellectual property (IP). MorphoSys has implemented extensive systems and processes to minimize these risks. Due to the implemented countermeasures, these risks in the financial year were classified as low overall. Compliance Risk In the area of healthcare compliance, the focus is on combating bribery and corruption and the key regulations governing com- mercialization activities in the U.S., such as the Anti-Kickback Statute, False Claims Act, Open Payments Act, and the Food, Drug, and Cosmetic Act. A relevant compliance risk is that the Company fails to fully grasp operational challenges and, as a re- sult, the compliance management program (CMP) is not estab- lished in accordance with regulatory requirements and industry standards. To address this risk, we have implemented a risk- based compliance management program that takes into account all of the current trends and applicable requirements, including the Code of Conduct; the Global Anti-Bribery Policy; the Global Policy on Interactions with Healthcare Professionals, Healthcare Organizations, Patients and Patient Organizations; the Global Fair Market Value Policy; the Global Policy on Transparency and Disclosure of Transfers of Value to Healthcare Professionals, Healthcare Organizations, Patients and Patient Organizations; and the relevant U.S. and German guidelines. We also have a global Compliance Committee that meets quar- terly and makes informed decisions on the further development of the CMP. Regular training sessions are held, which are aimed at all employees as well as specific employee groups. A guide for the sales force has also been developed to help the sales team imple- ment the guidelines in their daily work. An extensive onboarding program is offered to new employees in both Germany and the U.S. A compliance risk assessment is conducted annually, in which feedback is gathered from more than 60 of the Company’s executives to evaluate and minimize risks. Our control activities feed into our training and communication priorities. All these measures would not be possible without a clear message from the management: Our Management Board members empha- size the importance of compliance regularly, including at events during the annual Compliance Week, which took place again in the reporting year. Further details on our CMP can be found in the Corporate Gover- nance Report in the section “Compliance management program.” GxP-Related Risk Companies that research, develop and produce drugs and active ingredients for commercial use are subject to comprehensive reg- ulations known as GxP regulations. Compliance with these regu- lations is essential to receive approval from regulatory authori- ties. GxP-relevant risks can arise from a number of business areas if quality standards are not met. To counter these risks, we are committed to meeting the highest quality standards in our business operations, as outlined in our separate “Non-Financial Group Report.”* Certain risks may arise when the internal qual- ity management system fails to meet legal requirements or fails to implement internal systems to detect quality issues. If internal controls are unable to detect guideline violations of Good Manu- facturing Practice (GMP*), Good Clinical Practice (GCP*), Good Laboratory Practice (GLP*), Good Distribution Practice (GDP*), or Good Pharmacovigilance Practice (GVP*), this would also repre- sent a compliance risk. To minimize risk, the internal quality management system is also regularly reviewed by external ex- perts and subjected to recurring audits by an internal, indepen- dent quality assurance department. * see glossary – page 182 Intellectual Property Risk The patent protection of our proprietary technologies and active ingredients is vitally important to realizing the expected bene- fits. To mitigate risks in this area, we monitor new patents as well as patent applications and analyze the corresponding results. We also develop strategies to ensure that third-party patents and pat- ent applications do not restrict our own activities. In doing so, we try to safeguard our freedom of action with regard to our propri- etary technology platforms and products as much as possible. Risks in this area can arise from the potential for third-party patents or patent applications to fail to be recognized or incor- rectly assessed. Risks may also arise from enforcing our property rights against third parties. The respective processes may in- volve high costs and require considerable resources. There is also a risk that a third party may file a counterclaim. A further risk may also arise from a changing regulatory environment. We min- imize this risk through the ongoing training of the relevant groups and discussions with external experts. It is also conceiv- able that competitors may attack our patents, or that our patents or patent families are infringed upon, which in turn could lead us to take legal action against competitors. Such proceedings are associated with high costs and represent a significant financial risk, particularly in the U.S. By letter dated June 10, 2021, MorphoSys was notified by a licen- sor of the initiation of arbitration proceedings in the United States. The licensor alleges breach of contract and claims dam- ages for the licensor’s argued loss of revenues. Despite the patent expiry in 2018 confirmed by the licensor at the time, this is now disputed and a significantly longer patent term is assumed. Tak- ing into account the associated legal and consulting costs, the * This information is not part of the management report that is subject to audit. 82 Group Management Report Risk and Opportunity Report

Fi na nc ia l S ta te m en ts potential amount in dispute in the proceedings is in the low dou- ble-digit Euro million range and also includes a currently unspec- ified share of royalty income. A decision by the arbitration court is expected in the fourth quarter 2022. Based on the current as- sessment of the facts, MorphoSys believes that the arguments presented are unfounded and that the arbitration will likely be decided in MorphoSys’ favor. There was no arbitration decision and no other new developments in the third and fourth quarter of 2021. The Management Board’s Evaluation of the Group’s Overall Risk Situation Our Management Board considers our overall risk as manageable and trusts in the effectiveness of the integrated risk and opportu- nity management system to keep up with changes in the environ- ment and the needs of the ongoing business. It is the Manage- ment Board’s view that the Group’s continued existence is not jeopardized. This assessment applies to the Group as a whole, as well as to each Group company. This statement also applies in the unlikely event that several of the material risks occur cumula- tively, as even in such a scenario the risk-bearing capacity de- fined by the Board of Management is not undercut. The Management Board’s conclusion is based on the following considerations: • The Group’s high liquidity base • The Management Board’s conviction that the Group is well-po- sitioned to cope with any adverse events that may occur • The Group’s comprehensive portfolio of proprietary preclinical and clinical programs • The Group’s extensive portfolio of partnerships with a number of large pharmaceutical companies and a base of technologies to expand its proprietary portfolio Despite these factors, it is impossible to influence, control or rule out risk in its entirety. Risk and Opportunity Report Group Management Report 83

Subsequent Events A detailed description of the subsequent events can be found in the Notes to the Consolidated Financial Statements (Note 7.9*). * cross-reference to page 171 Subsequent Events 84 Group Management Report Subsequent Events

Fi na nc ia l S ta te m en ts Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance The Statement on Corporate Governance and the Group Statement on Corporate Governance, as well as the Report on Corporate Gov- ernance, are available on our website under Investors – Corporate Governance. Statement on Corporate Gover- nance pursuant to Section 289f HGB and Group Statement on Corporate Governance pursuant To Section 315d HGB for the 2021 Financial Year In the Statement on Corporate Governance pursuant to Section 289f of the German Commercial Code (HGB) and the Group State- ment on Corporate Governance pursuant to Section 315d HGB, the Management Board and the Supervisory Board present informa- tion on the most essential components of our corporate gover- nance. The components include the annual Declaration of Confor- mity pursuant to Section 161 of the German Stock Corporation Act (AktG), the relevant information on corporate governance practices and other aspects of corporate governance that include, above all, a description of the working practices of the Manage- ment Board and Supervisory Board. Declaration of Conformity of the Management Board and Supervisory Board of MorphoSys AG with regard to the German Corporate Gover- nance Code (“Code”) The Management Board and the Supervisory Board of MorphoSys AG declare pursuant to Section 161 of the German Stock Corporation Act: 1. From November 29, 2020, the date of its most recent Declara- tion of Conformity in the version as amended and updated on March 11, 2021, MorphoSys AG has complied – with the excep- tions described below – with the recommendations of the “Gov- ernment Commission on the German Corporate Governance Code” in the Code version dated December 16, 2019 (“GCGC 2020”): • MorphoSys AG does not comply with the recommendation C.4 of the GCGC 2020, according to which a Supervisory Board member, who is not a member of any Management Board of a listed company, shall not accept more than five Supervisory Board mandates at non-group listed companies or comparable functions (in a listed or non-listed company), with an appointment as chair of the Supervisory Board be- ing counted twice. The member of the Supervisory Board Dr. George Golumbeski currently holds in aggregate four com- parable functions in pharmaceutical and biotechnological companies in Ireland and the United States of America, thereof two functions as chairman of the board of directors. Dr. Golumbeski’s positions have at no time in the past af- fected the fulfillment of his duties as a member of the Super- visory Board of MorphoSys AG. MorphoSys AG continuously ensures that Dr. Golumbeski’s positions will not distract his focus on MorphoSys AG’s business and that Mr. Golumbeski has sufficient time to perform his duties as a member of the Supervisory Board of MorphoSys AG with due regularity and care. • MorphoSys AG does not comply with the recommendation C.5 of the GCGC 2020, according to which members of the Management Board of a listed company shall not accept the chairmanship of a Supervisory Board in a non-group listed company. The Chief Executive Officer (CEO) of MorphoSys AG, Dr. Jean-Paul Kress, holds a position as chairman of the Board of Directors of a French biopharmaceutical company, which he had already accepted prior to his appointment as a member of the Management Board of MorphoSys AG and which has at no time in the past affected the fulfillment of his duties as CEO of MorphoSys AG. MorphoSys AG continu- ously ensures that Dr. Kress’ position as chairman of the Board of Directors of such company will not distract his fo- cus on MorphoSys AG’s business and that Dr. Kress has suf- ficient time to perform his duties as CEO of MorphoSys AG with due regularity and care. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corpo- rate Governance Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 85

2. MorphoSys AG will continue to comply – with the exceptions described above under item 1 – with the recommendations of the GCGC 2020. Planegg, this November 29, 2021 MorphoSys AG For the For the Management Board: Supervisory Board: Dr. Jean-Paul Kress Dr. Marc Cluzel Chief Executive Officer Chairman of the Supervisory Board Relevant Information on Corporate Governance Practices We ensure compliance with the law and the highest ethical stan- dards in particular through the Group-wide enforcement of the Code of Conduct, the Compliance Management Handbook, and other internal policies and guidelines. Our Code of Conduct sets out the fundamental principles and key policies and practices for business behavior. The Code is a valu- able tool for our employees and executives, particularly in busi- ness, legal and ethical dilemmas. The Code of Conduct reinforces our transparent and sound management principles and fosters the trust placed in us by the public, business partners, employees and financial markets. Compliance with the Code of Conduct is carefully monitored. The Group-wide implementation of the Code is overseen by the Global Compliance Committee. The Code of Conduct itself is routinely reviewed and updated, provided to all new employees, and can be downloaded in German or English from our website under the section Investors – Corporate Gover- nance. The Compliance Management Handbook describes our compli- ance management program (CMP) and is intended to ensure com- pliance with all regulations and prescribe high ethical standards that apply to both the management and all employees. The Man- agement Board has overall responsibility for the CMP and is re- quired to report regularly to the Audit Committee of the Supervi- sory Board. In carrying out its compliance responsibility, the Management Board has assigned the relevant tasks to various functions at MorphoSys. The Global Compliance Committee consists of our three members of the Management Board (Chief Executive Officer, Chief Research and Development Officer, and Chief Financial Officer) and senior representatives from various departments. In 2021 the Chief Inte- gration Officer and Site Head of Constellation has also been in- cluded as a member of the Global Compliance Committee to en- sure gradient integration of Constellation into MorphoSys’ Compliance Management Program. It meets quarterly and sup- ports the Head of Global Compliance in implementing and moni- toring the CMP. The Global Compliance Committee is specifically responsible for the identification and discussion of all compli- ance-relevant issues, and thus makes it possible for the Head of Global Compliance and the other members of the Global Compli- ance Committee to periodically verify our compliance status and, if necessary, update the CMP. 86 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts The Head of Global Compliance monitors our existing CMP and updates it in accordance with the decisions of the Management Board and Global Compliance Committee. Compliance colleagues are the first point of contact for all employees regarding all com- pliance matters. For more information on our compliance management program, please see the Report on Corporate Governance. Composition of the Management Board and Supervisory Board Management Board In the 2021 financial year, the Management Board of MorphoSys AG consisted of a Chief Executive Officer and three other members. By resolution of the Supervisory Board on Janu- ary 18, 2021, Sung Lee was appointed as a member of the Man- agement Board and Chief Financial Officer effective February 2, 2021. Roland Wandeler, Ph.D., resigned from the MorphoSys AG Management Board effective December 31, 2021. Therefore, as of January 1, 2022, the Management Board consists of a Chief Exec- utive Officer and two additional members. The various areas of responsibility are currently defined in the business allocation plan as follows: • Jean-Paul Kress, M.D., Chief Executive Officer, responsible for the areas of Strategy & Planning, Business Development & Alli- ance Management, Human Resources, Legal, Compliance & In- tellectual Property, Corporate Communications, Technical Op- erations, Information Technology & Facilities, Quality Assurance & Internal Audit, global responsibility for U.S. oper- ations, Strategic Marketing & Market Access; Forecasting & In- sights, coordination of responsibilities of Management Board members; representative of Management Board to the Supervi- sory Board and the public • Sung Lee, Chief Financial Officer (as of February 2, 2021), re- sponsible for Accounting & Taxes, Global Controlling & Internal Controls, Corporate Development & M&A, Central Purchasing & Logistics, Investor Relations, and Environmental Social Gover- nance (ESG) • Malte Peters, M.D., Chief Research and Development Officer, re- sponsible for Research, Preclinical Development, Clinical De- velopment, Clinical Operations, Biostatistics & Data Manage- ment, Drug Safety & Pharmacovigilance, Regulatory Affairs, Medical Affairs, and Global Program Teams • Roland Wandeler, Ph.D., Chief Operating Officer (until Decem- ber 31, 2021), responsible globally for U.S. operations, Strategic Marketing & Market Access, and Forecasts & Insights Supervisory Board Our Supervisory Board consists of six members who oversee and advise the Management Board. Sharon Curran, Krisja Vermeylen and Marc Cluzel were reelected as members of the Supervisory Board at the 2021 Annual General Meeting. The current Supervisory Board consists of professionally quali- fied members who represent our shareholders. The Chair of the Supervisory Board, Marc Cluzel, M.D., Ph.D., coordinates the Board’s activities, chairs the Supervisory Board meetings and represents the interests of the Supervisory Board externally. All Supervisory Board members are independent as per the defini- tion in the German Corporate Governance Code (“Code”) and the NASDAQ Listing Rules and have many years of experience in the biotechnology and pharmaceutical industries. The Chair of the Supervisory Board is not a former member of our Management Board. The detailed composition of the Supervisory Board, includ- ing its members and committees, is listed in the tables below. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 87
Table 14 Composition of the Supervisory Board until Termination of the 2021 Annual General Meeting Name Position Initial Appoint- ment End of Term Audit Committee Remuneration and Nomination Committee Science and Technology Committee Marc Cluzel, M.D., Ph.D. Chairman 2012 2021 George Golumbeski, Ph.D. Deputy Chairman 2018 2023 Krisja Vermeylen Member 2017 2021 Michael Brosnan Member 2018 2023 Wendy Johnson Member 2015 2022 Sharon Curran Member 2019 2021 Independent financial expert Chairperson Member Table 15 Composition of the Supervisory Board since Termination of the 2021 Annual General Meeting Name Position Initial Appoint- ment End of Term Audit Committee Remuneration and Nomination Committee Science and Technology Committee Marc Cluzel, M.D., Ph.D. Chairman 2012 2024 George Golumbeski, Ph.D. Deputy Chairman 2018 2023 Krisja Vermeylen Member 2017 2024 Michael Brosnan Member 2018 2023 Wendy Johnson Member 2015 2022 Sharon Curran Member 2019 2024 Independent financial expert Chairperson Member 88 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts Working Practices of the Management Board, Supervisory Board and Executive Committee To ensure good corporate governance, a guiding principle of the cooperation between our Management Board and our Supervi- sory Board is the open, comprehensive and regular communica- tion of information. The dual board system prescribed by the Ger- man Stock Corporation Act clearly differentiates between the Company’s management and its supervision. The responsibility of both Boards is clearly stipulated by the legislator and the Arti- cles of Association as well as the Boards’ rules of procedure. The boards work closely together to make decisions and take actions for the Company’s benefit. Their stated objective is to sustainably increase the Company’s value. Management Board members have their own separate areas of responsibility, as defined in the schedule of responsibilities, and regularly report to the other Management Board members. Coop- eration among Management Board members is governed by the rules of procedure. The Supervisory Board approves both the schedule of responsibilities and the rules of procedure. The Company has also established the so-called “Executive Com- mittee.” Under the leadership of the Chief Executive Officer, the Executive Committee is responsible for the development of the strategy, for the commercialization, the operational management of the Company and the achievement of its targets and results. The Executive Committee prepares the decisions for the Manage- ment Board’s resolutions and adopts resolutions jointly with the Management Board, provided this is not the sole responsibility of the Management Board by law or by resolution of the Supervisory Board. The Executive Committee consists of the members of the Management Board and senior executives from the Company’s core areas such as Business Development & Licensing and Alli- ance Management, Technical Operations, Human Resources, Le- gal, and Compliance & Intellectual Property. In addition to the members of the Management Board, the current members of the Executive Committee are Barbara Krebs-Pohl, Ph.D. (Senior VP, Head of Global BD&L and Alliance Management), Daniel Palmacci (Senior VP, Global Head of Technical Operations), Maria Cas- tresana (Senior VP, Global Head of Human Resources), Charlotte Lohmann (Senior VP, General Counsel, Legal, Compliance & IP) and Joe Horvat (US General Manager) . Executive Committee meetings are generally held weekly and at least once every two weeks and when necessary in the interest of the Company. Separate Management Board meetings are gener- ally held when this is in the interest of the Company or legally required. During these meetings, resolutions are passed con- cerning measures and transactions that, under the rules of proce- dure of the Management Board, require the approval of the entire Management Board. At least half of the Management Board’s members must be present to pass a resolution. Management Board resolutions are passed by a simple majority and, in the event of a tied vote, the Chief Executive Officer’s vote decides. In case of material events, each Management Board or Supervisory Board member can call an extraordinary meeting of the entire Management Board. Management Board resolutions can also be adopted outside of meetings orally, by telephone or in writing (also by e-mail). Written minutes are taken for each meeting of the full Management Board and Executive Committee and are submitted for approval to the full Management Board and Execu- tive Committee, as well as for the signature of the Chief Executive Officer, at the following meeting. The Management Board promptly and comprehensively informs the Supervisory Board in writing and at Supervisory Board meet- ings about planning, business development, the Group’s position, risk management and other compliance issues. Extraordinary meetings of the Supervisory Board are also convened in case of material events. The Management Board involves the Supervi- sory Board in the strategy, planning and all fundamental Com- pany issues. The Management Board’s rules of procedure specify that material business transactions require the approval of the Supervisory Board. Detailed information on the cooperation of the Management Board and Supervisory Board and important items of discussion during the 2021 financial year can be found in the Report of the Supervisory Board. The Supervisory Board holds a minimum of two meetings during each calendar half-year. In addition to the Articles of Association, the Supervisory Board has adopted rules of procedure for the Su- pervisory Board. In accordance with these rules of procedure, the Chairperson of the Supervisory Board coordinates the activities of the Supervisory Board, chairs the Supervisory Board meetings and represents the interests of the Supervisory Board externally. The Supervisory Board generally adopts its resolutions in meet- ings, but resolutions may also be passed outside of meetings in writing (also by e-mail), by telephone or video conference. The Supervisory Board has a quorum when at least two-thirds of its members participate in the vote. Resolutions of the Supervi- sory Board are generally passed with a simple majority. In the event of a tied vote, the Chairperson’s vote decides. The Supervisory Board meetings are recorded in minutes. Reso- lutions passed outside of meetings are also documented in writ- ing. A copy of the Supervisory Board’s minutes is made available to all Supervisory Board members. In accordance with recom- mendation D.13 of the Code, the Supervisory Board assesses at regular intervals how effective the Supervisory Board in its en- tirety and its committees perform their tasks. The members of the Management Board also participate in this review. The last review was carried out by the Supervisory Board in December 2021 and was based on a questionnaire completed by the mem- bers of both the Supervisory Board and the Management Board. The results were then discussed and evaluated in a subsequent Supervisory Board meeting. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 89

Composition and Working Practices of the Management Board and Supervisory Board Committees The Management Board has not formed any committees. The Supervisory Board has three permanent committees: the Au- dit Committee, the Remuneration and Nomination Committee, and the Science and Technology Committee. The members of the three committees formed by the Supervisory Board are profes- sionally qualified. Table 16 Participation of Supervisory Board Members Supervisory Board Meetings Video confer- ence Video confer- ence On-site On-site Video confer- ence Video confer- ence Video confer- ence Video confer- ence On-site On-site On-site1 Video confer- ence Name 01/18/ 2021 03/11/ 2021 05/18/ 2021 05/19/ 2021 05/26/ 2021 06/01/ 2021 06/02/ 2021 07/16/ 2021 07/27/ 2021 11/08/ 2021 11/09/ 2021 12/14/ 2021 Marc Cluzel, M.D., Ph.D. Wendy Johnson Krisja Vermeylen George Golumbeski, Ph.D. Michael Brosnan Sharon Curran 1 strategy meeting Meetings of the Audit Committee Video conference Video conference On-site Video conference On-site Name 03/10/2021 05/04/2021 07/27/2021 09/30/2021 11/08/2021 Krisja Vermeylen Michael Brosnan Sharon Curran 90 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance
Fi na nc ia l S ta te m en ts Meetings of the Remuneration and Nomination Committee Video conference Video conference Video conference On-site Video conference Video conference Video conference Video conference Name 01/15/2021 03/01/2021 05/05/2021 05/18/2021 07/22/2021 09/22/2021 10/29/2021 12/13/2021 Marc Cluzel, M.D., Ph.D. Krisja Vermeylen Wendy Johnson George Golumbeski, Ph.D.* – – – – Meetings of the Science and Technology Committee Video conference Video conference Video conference On-site On-site On-site Name 03/09/2021 03/11/2021 04/30/2021 05/17/2021 07/26/2021 11/08/2021 Wendy Johnson George Golumbeski, Ph.D. Michael Brosnan* – – – – Marc Cluzel, M.D., Ph.D.* – – – – – * Guest participation. attended in person participation via video Audit Committee The main task of the Audit Committee is to support the Supervi- sory Board in fulfilling its supervisory duties with respect to the accuracy of the annual and consolidated financial statements, the activities of the auditor and internal control functions, such as risk management, compliance and internal auditing. The Audit Committee submits a recommendation to the Supervisory Board for the election at the Annual General Meeting of an independent auditor. The members of the Audit Committee are Michael Bros- nan (Chair), Sharon Curran and Krisja Vermeylen. Currently, Mi- chael Brosnan meets the prerequisite of an independent financial expert. Remuneration and Nomination Committee The Remuneration and Nomination Committee is responsible for the preparation and the annual review of the Management Board’s remuneration system prior to its final approval. When necessary, the Committee searches for suitable candidates to appoint to the Management Board and Supervisory Board and submits appoint- ment proposals to the Supervisory Board. The Committee also prepares the service agreements with Management Board mem- bers. The members of the Remuneration and Nomination Commit- tee are Krisja Vermeylen (Chair), Marc Cluzel, M.D., Ph.D., and Wendy Johnson. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 91

Science and Technology Committee The Science and Technology Committee advises the Supervisory Board on matters concerning proprietary drug and technology development and prepares the relevant Supervisory Board resolu- tions. The members of the Science and Technology Committee are George Golumbeski, Ph.D. (Chair) and Wendy Johnson. Ad Hoc Deal Committee The members of the Science and Technology Committee also serve as members of the Ad Hoc Deal Committee, which meets in this capacity as required. In 2021, the Ad Hoc Deal Committee dealt with the acquisition of Constellation. Pursuant to recommendation C.14 of the Code, the curriculum vitae of the members of the Supervisory Board are published on our website under About us – Leadership – Supervisory Board. Remuneration System and Compensation of the Members of the Management Board and Super- visory Board On March 11, 2021, the Supervisory Board resolved a remunera- tion system for the members of the Management Board which is in line with the requirements for management board remunera- tion as amended by the Act Implementing the Second Sharehold- ers’ Rights Directive (ARUG II) and the Code in its version as amended on December 16, 2019. The remuneration system does not yet apply to any member of the Management Board. The remuneration report pursuant to Section 162 AktG, including the auditor’s report, can be found on our website under Investors – Corporate Governance. The applicable remuneration system for the members of the Management Board and the most recent reso- lution of the Annual General Meeting 2021 on the remuneration of Supervisory Board members can be found on our website under Investors – Annual General Meeting 2021. Report on Corporate Governance At MorphoSys, responsible, sustainable and value-oriented corpo- rate governance is a high priority. Good corporate governance is an essential aspect of our corporate management and forms the framework for the Group’s management and supervision, includ- ing the Group’s organization, commercial principles and tools for its guidance and control. The Code provides a standard for the transparent monitoring and management of companies that strongly emphasizes shareholder interests. The German Federal Ministry of Justice originally pub- lished the Code in 2002. On December 16, 2019, the Government Commission on the German Corporate Governance Code adopted a new version of the Code, which entered into force upon its pub- lication in the German Federal Gazette on March 20, 2020. The Code contains recommendations and suggestions with regard to the management and supervision of German companies listed on a stock exchange. It is based on domestic and internationally rec- ognized standards for good and responsible corporate gover- nance. The Code aims to make the German system of corporate governance transparent for investors. It contains recommenda- tions and suggestions on corporate governance with regard to shareholders and the Annual General Meeting, the Management Board and Supervisory Board, transparency, accounting and val- uation principles, and auditing. There is no obligation to comply with the recommendations and suggestions of the Code. The German Stock Corporation Act only requires the management boards and supervisory boards of listed German companies to publish a declaration each year, (i) either confirming that the company has complied with the recom- mendations of the Code or (ii) listing the recommendations the company has not complied with and the reasons for the deviation from the recommendations of the Code. In addition, a listed com- pany must also state in its annual declaration whether it intends to comply with the recommendations or must list the recommen- dations it does not intend to comply with in the future. These declarations must be published permanently on the company’s website. If the company changes its position on certain recom- mendations between two annual declarations, it must disclose this fact and state the reasons for the deviation from the recom- mendations. If suggestions from the Code are not complied with, this does not have to be disclosed. Many of the corporate governance principles contained in the Code have been practiced at MorphoSys for many years. Our cor- porate governance principles are outlined in the Statement on Corporate Governance pursuant to Sections 289f and 315d HGB. The statement also contains the annual Declaration of Confor- mity, relevant information on corporate governance practices and a description of the Management Board and Supervisory Board’s working practices. Additional information can be found in this Report on Corporate Governance. Communication with the Capital Market A key principle of corporate communication at MorphoSys is to simultaneously and fully inform institutional investors, private shareholders, financial analysts, employees and all other stake- holders of the Company’s situation through regular, transparent and timely communication. The Company is firmly committed to following a fair information policy. 92 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts Regular meetings with analysts and investors in the context of roadshows and individual meetings play a central role in investor relations at MorphoSys. Conference calls are publicly webcast and follow the publications of quarterly and annual results and give analysts an immediate opportunity to ask questions about the Company’s development. Presentations from conferences and similar events are made available to those interested on the MorphoSys website, as are visual and audio recordings of other important events. The Company’s website www.morphosys.com/en serves as a cen- tral platform for current information on the Company and its de- velopment. Financial reports, analyst meetings and conference presentations, as well as press releases and ad hoc statements, are also available. The important regularly scheduled publica- tions and events (annual reports, interim reports, annual general meetings and press and analyst conferences) are published in the Company’s financial calendar well in advance. With the commercialization of Monjuvi in the U.S., the website www.morphosys.com/en accommodates the specific information needs of U.S. users and positions MorphoSys as an emerging leader in the hematology/oncology market. Competence Profile, Diversity Concept and Objectives for the Composition The Company’s Supervisory Board has updated its competence profile and objectives for its composition based on the new Code recommendations and has prepared a diversity concept in accor- dance with Section 289f (2) no. 6 of the German Commercial Code. According to this concept, the Supervisory Board of MorphoSys AG shall be composed in such a way that the Supervi- sory Board in its entirety possesses the knowledge, skills and professional experience necessary to perform its duties properly and ensure that it appropriately supervises and advises the Man- agement Board of MorphoSys AG while taking diversity into ac- count. When electing Supervisory Board members, the candi- dates who are proposed to the Annual General Meeting fulfill the overall competence profile based on their professional compe- tence, experience, integrity, commitment, independence and character. Proposals to the Annual General Meeting also take the objectives for the composition of the Supervisory Board into con- sideration. Competence Profile The members of the Supervisory Board shall in its entirety pos- sess the professional competence and experience to fulfill the tasks of the Supervisory Board of MorphoSys AG as an interna- tionally operating biopharmaceutical company. The Supervisory Board in particular considers the following skills and expertise to be essential for the composition of the Su- pervisory Board of MorphoSys AG: • Members should have a general knowledge of the industry in which the Company operates in order to make sufficient and substantive contributions at Supervisory Board meetings • At least one member must have experience in drug develop- ment • At least one member must have experience in commercializa- tion • At least one member must have expertise in the fields of ac- counting or auditing (Section 100 (5) AktG) • At least one member must have experience with personnel is- sues concerning Management Board matters Diversity Concept for the Supervisory Board of MorphoSys AG The Supervisory Board strives to ensure an appropriate level of diversity with respect to age, gender, internationality and profes- sional background, as well as regarding professional expertise, experience and personality, in order to achieve a diverse compo- sition of the Supervisory Board and enable it, in its entirety, to base its decisions on different cultural and professional perspec- tives and wide experiences. The Supervisory Board gives particular consideration to the fol- lowing criteria: • At least two members of the Supervisory Board shall have ex- tensive international experience or an international back- ground • At least one member of the Supervisory Board shall be under the age of 60 at the time of the member’s appointment • At least two members of the Supervisory Board shall have dif- ferent professional backgrounds and experience With respect to the proportion of women on the Supervisory Board, the Supervisory Board has set target figures as well as deadlines for their achievement in accordance with Section 111 (5) AktG, to which reference is made. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 93

Other Targets in the Composition of the Supervisory Board Age Limit At the time of their appointment by the Annual General Meeting, Supervisory Board members should not be more than 70 years of age. The Supervisory Board may, however, decide to make an ex- ception in specific cases. Duration of Appointment The uninterrupted length of the term of office of a Supervisory Board member shall generally not exceed 12 years. However, the Supervisory Board may resolve an exception to this rule in cer- tain cases. Independence The Supervisory Board of MorphoSys AG considers a number of at least four independent members to be an appropriate number of independent members, taking into account the shareholder struc- ture. According to the Code, a Supervisory Board member is con- sidered to be independent of MorphoSys AG, its Management Board and any controlling shareholders when he or she has no personal or business relationship with the Company, the Manage- ment Board or a controlling shareholder. The Supervisory Board’s assessment of the independence of Supervisory Board members is, amongst others, based on the recommendations of the Code. Consequently, a Supervisory Board member is generally not con- sidered independent if such member, or a close member of his or her family • was a member of the Management Board of MorphoSys AG in the two years preceding his or her appointment to the Supervi- sory Board of MorphoSys AG; • maintains or has maintained a material business relationship (directly or indirectly) with MorphoSys AG or a Group company of MorphoSys AG in the year preceding his or her appointment; • is a close family member of a Management Board member; or • has been a member of the Supervisory Board for more than 12 years. Significant and lasting conflicts of interest should be avoided, particularly those resulting from functions carried out for major competitors. It must be taken into account, however, that certain conflicts of interest cannot generally be excluded. Possible con- flicts of interest must be disclosed to the Chairperson of the Su- pervisory Board and will be resolved by appropriate measures. This could lead to the termination of the Supervisory Board man- date of the member concerned if the conflict of interest is not merely temporary. Availability All members of the Supervisory Board must ensure that they have sufficient time available to properly perform their Supervi- sory Board duties at MorphoSys AG. Therefore, as a rule, it is re- quired that: • the Supervisory Board member is able to personally attend at least four ordinary Supervisory Board meetings per year, for which a reasonable amount of preparation time is required in each case; in the event of exceptional circumstances to be deter- mined by the Supervisory Board’s Chairperson, the participa- tion of one or more Supervisory Board members in ordinary Supervisory Board meetings by other means (such as video conference) shall also be sufficient; • the Supervisory Board member is able to attend extraordinary meetings of the Supervisory Board, if necessary, to deal with specific topics; • the Supervisory Board member is able to attend the Annual General Meeting; • the Supervisory Board member has sufficient time to review the annual and consolidated financial statements; and • the Supervisory Board member allocates additional time to pre- pare for and attend committee meetings, in accordance with his or her membership in one or more of the Supervisory Board’s current three permanent committees. Current Composition of the Supervisory Board The Supervisory Board of MorphoSys AG is composed in accor- dance with the above objectives. It is composed of an appropriate number of independent members with an international back- ground. As the Supervisory Board as a whole currently has six members, of which three are women, an appropriate proportion of women has been achieved. Target Values for the Proportion of Women In the Supervisory Board The Supervisory Board of MorphoSys AG consists of six members, three of whom are women, representing a proportion of 50%. The Supervisory Board of MorphoSys AG has set the target value for the proportion of women on the Supervisory Board at 33.33%, meaning at least two out of six members shall be women. This target figure shall apply until June 30, 2025. In the Management Board The Management Board of MorphoSys AG consisted of four male members until December 31, 2021, and has consisted of three male members since January 1, 2022. As a result, the current pro- portion of women on the Company’s Management Board is 0%. Since the Supervisory Board of MorphoSys AG is of the opinion that, despite the continuing efforts to increase the proportion of women within the Management Board, the best possible qualifi- cation of a candidate for the Management Board must be assessed according to a variety of applied diversity criteria, in July 2020, the Supervisory Board has set the target value for the proportion 94 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts of women on the Company’s Management Board at 0%. This target value shall apply until June 30, 2023. The Supervisory Board nev- ertheless aims to increase the proportion of women within the Management Board. In the course of the next resolution upon a target value for the proportion of women within the Management Board, the Supervisory Board will take this objective into ac- count. In the First and Second Management Level below the Management Board 1. Target value for the first management level below the Manage- ment Board In 2020, the Management Board confirmed its resolution for a target value of 30% of women in the first management level below the Management Board as of July 2017 and intends to maintain a minimum proportion of women of 30% in the first management level below the Management Board until June 30, 2025. As of the date of the resolution on the target value, the first management level below the Management Board of MorphoSys AG (line managers reporting directly to the Man- agement Board) consisted of 21 members, of which nine are women, corresponding to a proportion of women of 42.86%. MorphoSys AG continuously complied with this requirement in the reporting year. 2. Target value for the second management level below the Man- agement Board In 2020, the Management Board confirmed its resolution for a target value of 30% women in the second management level below the Management Board as of July 2017 and intends to maintain a minimum proportion of women of 30% in the second management level below the Management Board until June 30, 2025. As of the date of the resolution on the target, the second management level below the Management Board of MorphoSys AG (line managers reporting directly to the first management level below the Management Board) consisted of 53 members, 22 of whom are women, corresponding to a pro- portion of women of 41.51%. MorphoSys AG continuously com- plied with this requirement in the reporting year. Diversity Concept for the Management Board of MorphoSys AG Pursuant to Section 289f (2) No. 6 of the German Commercial Code, the Supervisory Board has determined the following diver- sity concept for the composition of the Management Board of MorphoSys AG. The aim of the diversity concept for the Management Board is to consciously use diversity for the further success of the Company. The Supervisory Board believes that diversity in terms of differ- ent perspectives, competencies and backgrounds of experience is an important prerequisite for competitiveness and sustainable corporate success. Together with the Management Board, the Supervisory Board en- sures long-term succession planning for the Management Board. In the search for candidates for the position of a member of the Management Board of MorphoSys AG, the decisive selection crite- ria include, amongst others, professional qualifications for the position to be taken over, leadership qualities, previous perfor- mance, and acquired skills and knowledge of the business of MorphoSys AG. In the composition of the Management Board, the Supervisory Board also particularly takes the following aspects into account: • The members of the Management Board shall, in their entirety, have the necessary knowledge, skills and professional experi- ence required for their tasks. • Where possible, the members of the Management Board should have different levels of educational and professional experience. • The members of the Management Board shall, in their entirety, be familiar with the market environment, the individual busi- ness fields and the market segment in which MorphoSys AG operates. • The members of the Management Board shall, in their entirety, have relevant experience in leading a publicly listed company. • There should be a sufficient age mix among the members of the Management Board. • With regard to the proportion of women on the Management Board, the Supervisory Board has set target values, as well as deadlines for their achievement, in accordance with Section 111 (5) AktG, to which reference is made. The above criteria were taken into account in the appointment of the Management Board members. Other Targets in the Composition of the Management Board Age Limit At the time of their appointment, Management Board members should not be more than 67 years of age. The Supervisory Board may, however, decide to make an exception in specific cases. The age limit of 67 is currently complied with. Managers’ Transactions The members of the Management Board and the Supervisory Board of MorphoSys AG, as well as persons closely associated with them, are required to disclose trading in MorphoSys shares in accordance with the requirements set forth in the relevant le- gal provisions (Article 19 (1a) of the Market Abuse Regulation (MAR)). During the reporting year, MorphoSys received notifications pur- suant to Article 19 (1a) MAR, which are shown in the table below. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 95

Table 17 Managers’ Transactions 2021 Party Subject to the Notification Requirement Function Date of Transaction Type of Transaction Aggregated Share Price Aggregated Volume Place of Transaction Sung Lee Chief Financial Officer 12/03/2021 Acquisition of shares US$ 9.36 US$ 14,040.00 Nasdaq Sung Lee Chief Financial Officer 11/23/2021 Acquisition of shares US$ 9.55 US$ 4,775.00 Nasdaq Sung Lee Chief Financial Officer 11/22/2021 Acquisition of shares US$ 9.80 US$ 19,600.00 Nasdaq Sung Lee Chief Financial Officer 10/01/2021 Acquisition of shares US$ 11.67 US$ 23,340.00 Nasdaq Sung Lee Chief Financial Officer 08/02/2021 Acquisition of shares US$ 13.70 US$ 27,400.00 Nasdaq Michael Brosnan Member of the Supervisory Board 07/30/2021 Acquisition of shares US$ 13.96 US$ 69.788,58 Nasdaq Krisja Vermeylen Member of the Supervisory Board 07/29/2021 Acquisition of shares € 47.25 € 16,538.66 Xetra Michael Brosnan Member of the Supervisory Board 06/03/2021 Acquisition of shares US$ 19.58 US$ 97,906.28 Nasdaq Malte Peters, M.D. Chief Research and Development Officer 04/14/2021 Allocation of 4,143 shares as part of his remuneration as member of the Management Board (Performance Share Plan 2017) (issuer’s own shares) not numerable not numerable Outside a trading venue Sung Lee Chief Financial Officer 03/23/2021 Acquisition of shares US$ 22.75 US$ 22,750.00 Nasdaq Wendy Johnson Member of the Supervisory Board 03/17/2021 Acquisition of shares US$ 23.35 US$ 5,837.50 Nasdaq Krisja Vermeylen Member of the Supervisory Board 03/17/2021 Acquisition of shares € 75.90 € 22,770.00 Xetra C&F Consulting EURL Person closely associated 03/16/2021 Acquisition of shares € 76.00 € 19,000.00 Xetra Michael Brosnan Member of the Supervisory Board 03/16/2021 Acquisition of shares US$ 22.73 US$ 227,300.00 Nasdaq Avoiding Conflicts of Interest The members of the Management Board and the Supervisory Board are obligated to refrain from actions that could lead to con- flicts of interest with their responsibilities at MorphoSys AG. Such transactions or sideline activities of the Management Board must be disclosed to the Supervisory Board without undue delay and require the Supervisory Board’s approval. The Supervisory Board, in turn, must inform the Annual General Meeting of any conflicts of interest that arise and disclose how they were dealt with. No conflict of interest arose in the Supervisory Board in the 2021 financial year. Share Repurchases By resolution of the Annual General Meeting on May 23, 2014, MorphoSys was authorized, in accordance with Section 71 (1) no. 8 of the German Stock Corporation Act (AktG), to repurchase trea- sury shares in an amount of up to 10% of the existing share capi- tal up to and including April 30, 2019. Following the authoriza- tion’s expiry, no new authorization was proposed to the Annual General Meeting; therefore, no such authorization currently exists. Information Technology The transition from working remotely due to COVID-19 to a hy- brid and highly flexible work model was accompanied by an inte- grative technology update of our physical and virtual meeting rooms and a new collaboration and booking platform. We began with the technical integration and consolidation of IT systems following the acquisition of Constellation and will com- plete this process in 2022. In 2021, the focus was on the interop- erability of all systems to ensure the fastest possible collabora- tion. This year, we will exploit the full potential of all synergies and establish a global, harmonized IT landscape. The specialist IT teams have already been combined to form a joint IT organiza- tion. A special focus was placed on the further digitalization and auto- mation of business processes. With the introduction of electronic signatures using DocuSign™, we were able to significantly accel- erate signature circulation and automate processes. A new, global learning management system forms the basis for the digital edu- cation strategy, which relies on e-learning and remote training. 96 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts We are advancing our innovation using artificial intelligence through tools such as Aily™, which will make it possible to fore- see ways to optimize recruitment for clinical trials. We are also investing in the expansion of our Veeva™ system landscape for unified management of quality and regulatory information, which is crucial for rapidly launching products (e.g., pelabresib) and maintaining their marketing approval. In the area of IT security, we continued to optimize our cyber de- fense measures and progressed through the integration phase with Constellation with increased awareness. An automated pen- etration testing and validation platform was deployed to review our technical security controls and identify potential vulnerabili- ties. We continued to train and raise our employees’ awareness of their own contributions to the Company’s IT security. Our Computer Emergency Response Team (CERT) has not de- tected any serious security incidents during the reporting year. Information on the Internal Control and Risk Management System with regard to the Accounting Process under Section 289 (4) and Section 315 (4) HGB In the 2021 reporting year, we completed a routine update of the documentation for our existing internal control and risk manage- ment system for maintaining adequate internal control over fi- nancial reporting, which we have expanded based on the provi- sions of Section 404 of the Sarbanes-Oxley Act of 2002 (SOX 404). This ensures the existence of essential controls designed to re- port financial figures as precisely and accurately as possible. Our internal controls over financial reporting are based on the glob- ally recognized COSO 2013 Internal Control – Integrated Frame- work, defined by the COSO organization (Committee of Sponsor- ing Organizations of the Treadway Commission). We use this framework, which is the most commonly used framework for the internal control over financial reporting. System constraints make it impossible to give absolute assurance that internal controls will always prevent or completely detect all misrepresentations made in the context of financial reporting. Internal controls can only provide sufficient assurance that finan- cial reporting is reliable and verify that the financial statements were prepared in accordance with the applicable IFRS standards endorsed by the European Union (EU) for external purposes. The consolidated financial statements and the interim financial statements are subject to a number of preparation, auditing and control processes to ensure that they are submitted to the market and the shareholders in a timely, complete and quality manner. All internal controls over financial reporting are defined and rolled out for all companies by the central Global Internal Con- trols function in close coordination with the departments in- volved. These process-integrated measures include the separa- tion of planning, posting and execution of financial transactions within the framework of a strict four eyes principle. The separa- tion of functions is significantly enhanced by the appropriate al- location rights for the IT systems. Internal guidelines and proce- dures also exist to regulate the implementation of process activities and controls and must be complied with at all times by the employees involved. The transactional controls are flanked by target/actual comparisons and further downstream plausibility checks. The control mechanisms described apply both to the ac- counting processes of the consolidated companies and the pro- cess of closing the consolidated financial statements, which in- cludes consolidation. In addition to internal controls integrated into the processes, a separate independent monitoring process is also carried out by the Internal Audit Department, which is bundled in the Corporate Internal Audit central function. Due to the obligations of SOX 404 and in order to comply with the requirements of Section107 (3) of the German Stock Corporation Act, Internal Audit performs an annual independent audit of all significant internal controls for financial reporting, supported by a qualified and independent ex- ternal service provider. As part of its regular communication with the supervisory bodies, the Internal Audit Department reports every six months to the Chief Financial Officer and the Audit Committee on the results of the structural and functional audits of the accounting-related internal control system. Predictions of future events in the narrower sense are not part of our internal control and risk management system. Nevertheless, we have implemented a risk management system that ensures early identification and assessment of business-specific risks. Ap- propriate countermeasures are taken to eliminate identified risks or at least reduce them to an acceptable level. Particular attention is paid to those risks which could endanger the existence of the company. The Management Board ensures that risks are dealt with responsibly on an ongoing basis and keeps the Supervisory Board informed of existing risks and their development. Detailed information on our opportunities and risks can be found in the “Risk and Opportunity Report.” Accounting and External Audit We prepare our annual financial statements in accordance with the provisions of the German Commercial Code (HGB) and the German Stock Corporation Act (AktG). The consolidated financial statements are prepared in accordance with International Financial Reporting Standards (IFRS) and in compliance with the recommendations of the International Finan- cial Reporting Standards Interpretations Committee (IFRS IC). We have applied all standards and interpretations that were in force on December 31, 2021, and adopted by the EU into European law. As of December 31, 2021, there were no standards or inter- pretations with an impact on our consolidated financial state- ments as of December 31, 2021 and 2020 that had entered into force but had not yet been adopted into European law. Therefore, Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 97

Head of Global Compliance reports to reports to Chief Executive Offi cer General Counsel, Member of the Executive Committee Credo Code of Conduct Anti-Bribery, Due Diligence of Third Parties Trainings & Awareness Integrity Line Compliance Documents Compliance Committee Compliance Management Program CMP reports, if required, to Chairperson of the Audit Committee leading the global CMP and managing the interfaces between diff erent compliance streams Compliance Risk Management Transparency & Disclosure Review and Approval of Key Initiatives Monitoring & Continuous Improvement 10 Compliance Management Program (CMP) Figureour consolidated financial statements comply with both the IFRS published by the International Accounting Standards Board (IASB) and the IFRS adopted by the EU. In addition, our consoli- dated financial statements take into account the supplementary provisions of German commercial law that are to be applied in accordance with Section 315e (1) HGB). For the election of our auditor, the Audit Committee of the Super- visory Board submits a nomination proposal to the Supervisory Board. At the 2021 Annual General Meeting, PricewaterhouseC- oopers GmbH Wirtschaftsprüfungsgesellschaft was appointed as auditor for the 2021 financial year. As proof of its independence, the auditor submitted an Independence Declaration to the Super- visory Board. The lead auditor of these consolidated financial statements was Holger Lutz, who has audited the consolidated fi- nancial statements since 2019. PricewaterhouseCoopers GmbH has been our auditor since the 2011 financial year. Information on audit-related fees and all other fees provided by PricewaterhouseCoopers GmbH to us during the 2021 financial year can be found in Note 5.13*. * cross-reference to page 141 Compliance Management Program The separate “Non-Financial Group Report”* sets out the basic mechanisms of our compliance management program (CMP). The report is available on our website under https://csr.morphosys. com/2021. The identification and assessment of compliance risks are an im- portant part of the CMP and are incorporated into the program’s overall strategic development. Our main compliance-relevant risk areas are evaluated using a systematic approach and taking into account our current business strategy and priorities. During the reporting year, we carried out an annual compliance risk assess- ment that included anti-bribery and other relevant risk areas. Risk mitigation measures were initiated for the areas of action identified. Within the scope of the CMP, employees are given the opportunity to report potential compliance issues within the MorphoSys Group in a protected and, if desired, anonymous man- ner through the MorphoSys Integrity Line reporting system. In addition to an annual compliance risk analysis, we have devel- oped other appropriate guidelines and have monitored compli- ance. In order to prevent compliance breaches, employees were routinely trained in topics relevant for compliance. Traditional compliance refresher trainings have been provided to the employ- ees, as well as newly developed trainings on thoughtful commu- nications and investigator-initiated trials. In November 2021, MorphoSys held a Compliance Week that in- cluded a number of engaging activities for employees of MorphoSys AG, MorphoSys US Inc. and, for the first time, Constel- lation under the motto “Integrity in All We Do.” Compliance-related discussions and analyses at all levels of the Company lead to a continuous improvement in managing and mitigating risk at MorphoSys. In conjunction with the EU General Data Protection Regulation (Regulation [EU] 2016/679 – “GDPR”), which entered into force on May 25, 2018, we have implemented various procedures since 2018 to ensure compliance with the GDPR. More details can be found in the separate “Non-Financial Group Report.”* ›› see figure 10 – Compliance Management Program (CMP) (page 99) Internal Audit Department Our Internal Audit department is an essential element of the Cor- porate Governance structure. The department assists us in ac- complishing our objectives by prescribing a systematic approach to evaluating and improving the effectiveness of our risk manage- ment, internal control and other corporate governance processes. The activities of the Internal Audit function are supported by co-sourcing partner Protiviti, an independent consulting firm with expertise in internal audit, risk and compliance. The Internal Audit department executes a risk-based audit plan that includes the requirements and recommendations of the Man- agement Board, as well as those of the Supervisory Board’s Audit Committee. The Internal Audit department is also responsible for performing management testing in accordance with the require- ments of the U.S. Sarbanes-Oxley Act, Section 404 (SOX). This procedure involves independently testing the appropriateness and effectiveness of internal controls in the business processes relevant to financial reporting. The outcome of each internal audit is communicated to the CEO and the relevant members of the Executive Committee. In addi- tion, the Head of Internal Audit reports to the Audit Committee of the Supervisory Board on the results of the internal audits and SOX management testing twice a year or immediately if neces- sary. Three audits were carried out in the year 2021. Some areas for action were identified, resulting in the adoption of corresponding corrective plans of action. The internal audit plan for 2022 envis- ages four audits, which will cover the activities of all entities of the MorphoSys Group. * This information is not part of the management report that is subject to audit. 98 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Head of Global Compliance reports to reports to Chief Executive Offi cer General Counsel, Member of the Executive Committee Credo Code of Conduct Anti-Bribery, Due Diligence of Third Parties Trainings & Awareness Integrity Line Compliance Documents Compliance Committee Compliance Management Program CMP reports, if required, to Chairperson of the Audit Committee leading the global CMP and managing the interfaces between diff erent compliance streams Compliance Risk Management Transparency & Disclosure Review and Approval of Key Initiatives Monitoring & Continuous Improvement 10 Compliance Management Program (CMP) Figure Fi na nc ia l S ta te m en ts Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 99

Disclosures pursuant to Section 289a (1), Section 315a (1) HGB and Explan- atory Report of the Management Board pursuant to Section 176 (1) Sentence 1 AktG Composition of Share Capital On December 31, 2021, the Company’s share capital amounted to € 34,231,943, divided into 34,231,943 no-par-value bearer shares. With the exception of the 83,154 treasury shares held by the Com- pany, these bearer shares possess voting rights, with each share granting one vote at the Annual General Meeting. As of Decem- ber 31, 2021, the Company’s registered share capital amounted to € 34,227,598, divided into 34,227,598 no-par-value bearer shares. This amount of registered share capital does not yet reflect the increase in share capital and the number of shares resulting from the exercise of 4,345 subscription rights to shares in the Com- pany (stock options) in 2021. On January 17, 2022, the Supervi- sory Board of the Company resolved to amend the wording of the Articles of Association to reflect the higher share capital of € 34,231,943 and filed this amendment for entry in the Commer- cial Register. The entry in the Commercial Register was made on January 29, 2022. Restrictions Affecting Voting Rights and the Transfer of Shares Our Management Board is not aware of any restrictions that may affect voting rights or the transfer of shares, or any restrictions that may emerge from agreements between shareholders. Voting rights restrictions may also arise from the provisions of the German Stock Corporation Act (AktG), such as those pursuant to Section 136 AktG, or the provisions for treasury shares pursu- ant to Section 71b AktG. Interests in Share Capital Exceeding 10% of Voting Rights We have not been made aware or notified of any direct or indirect interests in the Company’s share capital that exceed 10% of the voting rights. Shares with Special Rights Conferring Powers of Control Shares with special rights conferring powers of control do not exist. Control over Voting Rights with regard to Employee Ownership of Capital Employees who hold shares in the Company exercise their voting rights directly in accordance with the statutory provisions and the Articles of Association, as do other shareholders. Appointment and Dismissal of Management Board Members and Amendments to the Articles of Association In accordance with Section 6 of the Articles of Association and Section 84 of the German Stock Corporation Act (AktG), the Su- pervisory Board determines the number of members on the Man- agement Board, appoints and revokes members and nominates the Chairman. Until December 31, 2021, the Management Board consisted of the Chairman and three further members. Since the departure of Roland Wandeler, Ph.D., the Management Board con- sists only of the Chairman of the Management Board and two further members. Members of the Management Board can be ap- pointed for a maximum term of five years. Reappointments and extensions in the term of office are allowed for a maximum term of five years in each case. The Supervisory Board may revoke the appointment of a Management Board member or Chairman of the Management Board for good cause as defined by Section 84 (3) AktG. Where the Management Board lacks a required member, a Management Board member will be appointed by the court in ur- gent cases pursuant to Section 85 AktG. As a rule, the Articles of Association can only be amended by a resolution of the General Meeting in accordance with Section 179 (1) sentence 1 AktG. Pursuant to Section 179 (2) sentence 2 AktG in conjunction with Section 20 of the Articles of Association, our Annual General Meeting resolves on amendments to the Articles of Association generally with a simple majority of the votes cast and a simple majority of the share capital represented. If the law stipulates a higher mandatory majority of votes or capital, this shall apply. Amendments to the Articles of Association that only affect their wording can be resolved by the Supervisory Board in accordance with Section 179 (1) sentence 2 AktG in conjunction with Section 12 (3) of the Articles of Association. Power of the Management Board to Issue Shares The Management Board’s power to issue shares is granted under Section 5 (5) through (6i) of the Company’s Articles of Association and the statutory provisions. The Supervisory Board is autho- rized to amend the wording of the Articles of Association in accor- dance with the scope of the capital increase from conditional or authorized capital. 100 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts 1. Authorized Capital In the case of an authorized capital increase, the Management Board is authorized with the consent of the Supervisory Board to determine the further details of the capital increase and its implementation. a. Pursuant to Article 5 (5) of the Articles of Association, the Management Board is authorized with the consent of the Su- pervisory Board to increase the Company’s share capital against contribution in cash and/or contribution in kind on one or several occasions by a total of up to € 4,861,376 by issuing up to 4,861,376 new, no-par-value bearer shares un- til and including May 18, 2026 (Authorized Capital 2021-I). b. In case of capital increases, shareholders are principally en- titled to subscription rights. The shares may also be sub- scribed to by one or several credit institutions with the obli- gation to offer the shares to shareholders for subscription. The Management Board with the Supervisory Board’s con- sent is, however, authorized to exclude shareholders’ sub- scription rights in the following cases: aa) in the case of a capital increase against contribution in cash, to the extent necessary to avoid fractional amounts; or bb) in the case of a capital increase against contribution in kind; or cc) in the case of a capital increase against contribution in cash to the extent the new shares shall be placed on a foreign stock exchange in the context of an IPO. The total number of shares to be issued by way of a capital increase against contribution in cash and/or in kind, exclud- ing subscription rights and based on the above authoriza- tions, shall not exceed 10% of the share capital, calculated either based on the authorizations’ effective date or the time they are exercised, whichever amount is lower. The 10% limit mentioned above shall take into account (i) treasury shares sold with the exclusion of subscription rights after the effective date of these authorizations, (ii) shares issued on the basis of other authorized capital with the exclusion of subscription rights during the period in which these autho- rizations are in effect, and (iii) shares to be issued to service convertible bonds and/or bonds with warrants, insofar as the convertible bonds and/or bonds with warrants have been issued with the exclusion of shareholders’ subscription rights while these authorizations are in effect but in respect of items (i), (ii) and/or (iii) in each case only insofar as the shares are not used to service claims by members of govern- ing bodies and/or employees of the Company and/or its affil- iated companies under employee participation programs. b) Pursuant to Section 5 (6) of the Articles of Association, the Management Board is authorized with the consent of the Su- pervisory Board to increase the Company’s share capital against contribution in cash on one or several occasions by a total of up to € 1,951,452 by issuing up to 1,951,452 new no- par-value bearer shares until and including May 18, 2026 (Authorized Capital 2021-II). In case of capital increases, shareholders are principally en- titled to subscription rights. The shares may also be sub- scribed to by one or several credit institutions with the obli- gation to offer the shares to shareholders for subscription. The Management Board is, however, authorized to exclude shareholder subscription rights, with the Supervisory Board’s consent, in the following cases: aa) to the extent such exclusion is necessary to avoid frac- tional amounts; or bb) if the issue price of the new shares is not significantly below the market price of shares of the same class al- ready listed and the total number of shares issued against contribution in cash, excluding subscription rights, during the term of this authorization does not ex- ceed 10% of the share capital on the date this authoriza- tion takes effect or at the time it is exercised, in accor- dance with or in the respective application of Section 186 (3) sentence 4 AktG. This 10% limit shall take into account treasury shares of the Company, which are sold during the term of this authorization with the exclusion of shareholders’ subscription rights in accordance with section 71 para. 1 no. 8 sentence 5 clause 2 AktG in con- junction with section 186 para. 3 sentence 4 AktG. Fur- thermore, shares issued or to be issued to service con- vertible bonds and/or bonds with warrants shall be included in this 10% limit of the share capital, provided that these convertible bonds and/or bonds with war- rants were issued during the term of this authorization with the exclusion of subscription rights in the respec- tive application of section 186 para. 3 sentence 4 AktG. In addition, shares issued excluding shareholders’ sub- scription rights during the term of this authorization on the basis of other capital measures in direct or mutatis mutandis application of section 186 para. 3 sentence 4 AktG shall be included in this 10% limit of the share cap- ital. The maximum limit reduced in accordance with the above sentences of this paragraph shall be increased again when a new authorization to exclude shareholders’ subscription rights resolved by the Annual General Meeting takes effect in accordance with section 186 para. 3 sentence 4 AktG after the reduction, in the amount of the new authorization, up to a maximum of 10% of the share capital in accordance with the require- ments of sentence 1 of this paragraph bb). Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 101

The total number of shares to be issued by way of a capital increase against contribution in cash, excluding subscrip- tion rights and based on the authorizations mentioned above shall not exceed 10% of the share capital when calculated based on the authorizations’ effective date or exercise, whichever amount is lower. The aforementioned 10% limit shall include (i) treasury shares sold with exclusion of sub- scription rights after the effective date of these authoriza- tions, (ii) shares issued on the basis of other authorized cap- ital with the exclusion of subscription rights during the period in which these authorizations are in effect and (iii) shares to be issued to service convertible bonds and/or bonds with warrants, insofar as the convertible bonds and/ or bonds with warrants have been issued with the exclusion of shareholders’ subscription rights while these authoriza- tions are in effect but in respect of items (i), (ii) and/or (iii) in each case only insofar as the shares are not used to service claims of members of the Management Board and/or employ- ees of the Company and/or its affiliated companies under employee participation programs. The maximum limit re- duced in accordance with the above sentences of this para- graph shall be increased again when a new authorization to exclude shareholders’ subscription rights resolved by the Annual General Meeting takes effect after the reduction, in the amount of the new authorization, up to a maximum of 10% of the share capital in accordance with the require- ments of sentence 1 of this paragraph. c. Pursuant to Article 5 (6a) of the Articles of Association, the Management Board is authorized with the consent of the Su- pervisory Board to increase the Company’s share capital against contribution in cash and/or contribution in kind on one or several occasions up to and including May 18, 2026 by up to a total of € 315,000 by issuing up to 315,000 new no- par-value bearer shares (Authorized Capital 2021-III). The subscription rights of shareholders are excluded. The Autho- rized Capital 2021-III serves the purpose of delivering shares of the Company against the contribution of payment claims resulting from Restricted Stock Units (RSUs) in order to fulfill RSUs that were granted in accordance with the terms and conditions of the Restricted Stock Unit Program 2021 of the Company (RSUP 2021) exclusively to senior man- agers and employees (including directors and officers) of MorphoSys US Inc. The issue price of the new shares must amount to at least € 1.00 and can be paid either by way of a cash contribution and/or contribution in kind, including in particular the contribution of claims against the Company under the RSUP 2021. The Management Board is authorized to determine the further details of the capital increase and its implementation with the consent of the Supervisory Board; this also includes the determination of the profit par- ticipation of the new shares, which may, in deviation from Section 60 (2) AktG, also participate in the profit of an al- ready completed financial year, provided that no resolution on the appropriation of profits has yet been adopted for the fiscal year in question. d. Pursuant to Article 5 (6h) of the Articles of Association, the Management Board is authorized with the consent of the Su- pervisory Board to increase the Company’s share capital on one or several occasions by a total of up to € 159,197 by issu- ing up to 159,197 new no-par-value bearer shares against cash contribution and/or contribution in kind until and in- cluding April 30, 2024 (Authorized Capital 2019-I). The subscription rights of shareholders are excluded. The Authorized Capital 2019-I serves the purpose of delivering shares of the Company against the contribution of payment claims resulting from Restricted Stock Units (RSUs) in order to fulfill RSUs that were granted in accordance with the terms and conditions of the Company’s Restricted Stock Unit Program (RSUP) exclusively to senior managers and employ- ees (including directors and officers) of MorphoSys US Inc. The issue price of the new shares must amount to at least € 1.00 and may be paid either by way of a cash contribution and/or contribution in kind, including in particular the con- tribution of claims against the Company under the RSUP. The Management Board is authorized with the consent of the Supervisory Board to determine the further details of the capital increase and its implementation; this also includes the determination of the profit participation of the new shares, which may, in deviation from Section 60 para. 2 AktG, also participate in the profit of an already completed financial year, provided that no resolution on the appropria- tion of profits has yet been adopted for the fiscal year in question. 102 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

Fi na nc ia l S ta te m en ts 2. Conditional Capital a. Pursuant to Article 5 (6b) of the Articles of Association, the Company’s share capital is conditionally increased by up to € 2,475,437 through the issue of up to 2,475,437 no-par- value bearer shares (Conditional Capital 2016-I). The condi- tional capital increase serves solely as a means to grant new shares to the holders of conversion or warrant rights, which will be issued by the company or companies in which the Company has a direct or indirect majority interest according to the authorizing resolution of the Annual General Meeting on June 2, 2016, under Agenda Item 7 letter a). The shares will be issued at the respective conversion or exercise price to be determined in accordance with the resolution above. The conditional capital increase will only be carried out to the extent that the holders of conversion or warrant rights exercise these rights or fulfill conversion obligations under such bonds. The shares will be entitled to dividends as of the beginning of the previous financial year, provided they were issued before the start of the Company’s Annual General Meeting, or as of the beginning of the financial year in which they were issued. b. Pursuant to Article 5 (6c) of the Articles of Association, the Company’s share capital is conditionally increased by up to € 3,289,004 by issuing up to 3,289,004 new no-par-value bearer shares (Conditional Capital 2021-I). The conditional capital increase serves exclusively to grant new shares to the holders of conversion or warrant rights issued by the Company or by companies in which the Company directly or indirectly holds a majority interest in accordance with the authorization resolution of the Annual General Meeting of May 19, 2021 under Agenda Item 10 a). The shares shall be issued at the conversion or warrant price to be determined in each case in accordance with the aforementioned resolution. The conditional capital increase shall only be carried out to the extent that the holders of conversion or warrant rights exercise their conversion or warrant rights or fulfill conver- sion obligations under such bonds. The shares shall partici- pate in profits – to the extent they come into existence by the beginning of the Annual General Meeting of the Company – from the beginning of the preceding financial year, other- wise from the beginning of the financial year in which they come into existence. c. Pursuant to Article 5 (6g) of the Articles of Association, the share capital is increased conditionally by up to € 741,390 through the issue of up to 741,390 new no-par-value bearer shares of the Company (Conditional Capital 2016-III). The conditional capital serves to meet the obligations of sub- scription rights that have been issued and exercised based on the authorization resolved by the Annual General Meet- ing of June 2, 2016 under Agenda Item 9 letter a). The condi- tional capital increase will only be executed to the extent that holders of subscription rights exercise their right to subscribe to shares of the Company. The shares will be is- sued at the exercise price set in each case as the issue price in accordance with Agenda Item 9 letter a) subparagraph (8) of the Annual General Meeting’s resolution dated June 2, 2016; Section 9 (1) AktG remains unaffected. The new shares are entitled to dividends for the first time for the financial year for which there has been no resolution by the Annual General Meeting on the appropriation of profits at the time of the shares’ issue. The Management Board, and the Supervi- sory Board where members of the Management Board are concerned, is authorized to determine the additional detail of the conditional capital increase and its execution. d. Pursuant to Article 5 (6i) of the Articles of Association, the Company’s share capital is increased conditionally by up to € 1,314,615 by issuing up to 1,314,615 new no-par-value bearer shares (Conditional Capital 2020-I). The conditional capital serves to fulfill subscription rights that were issued and exercised on the basis of the authorization resolved by the Annual General Meeting on May 27, 2020, under Agenda Item 11, letter a). The conditional capital increase will only be implemented to the extent that holders of subscription rights exercise their subscription rights to subscribe to shares of the Company. The shares will be issued at the ex- ercise price determined in accordance with the resolution of the Annual General Meeting of May 27, 2020, under Agenda Item 11, letter a) subparagraph (8) as the issue price; Sec- tion9 (1) AktG remains unaffected. The new shares are enti- tled to dividends for the first time for the financial year for which, at the time of their issue, no resolution by the Annual General Meeting on the appropriation of profits has yet been passed. The Management Board, or, insofar as members of the Management Board are affected, the Supervisory Board are authorized to determine the further details of the condi- tional capital increase and its implementation. Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance Group Management Report 103

Power of Management Board to Repurchase Shares The Management Board is currently not authorized to repurchase the Company’s shares. Material Agreements Made by the Company that fall under the Condition of a Change of Control after a Takeover Bid A change of control as a result of a takeover bid could have an impact on our convertible bond issued in October 2020, the under- lying contract of which contains customary change-of-control clauses. According to these clauses, bondholders can demand early repayment of the outstanding amounts in the event of a change of control. The Company has not entered into any further material agree- ments that are subject to a change of control following a takeover bid. Compensation Agreements Concluded by the Company with Management Board Members and Employees in the Event of a Takeover Bid In accordance with the service agreements in force in the report- ing period, the Management Board members may terminate their contract following a change of control and claim the compensa- tion still outstanding up to the regular end of the service contract, but at least the compensation for two years, as a severance pay- ment. In the case of Sung Lee, severance payments in the event of premature termination of the service contract due to a change of control are capped at 200% of the annual compensation in line with the new requirements of the Code. In addition, the plan terms of the long-term variable compensation programs provide that, in the event of a change of control, all granted stock options, performance shares and other comparable direct or indirect inter- ests in MorphoSys with compensation character vest with imme- diate effect and can be exercised after the expiry of the statutory waiting periods. Following a change of control, some executives may terminate their service contracts and demand a severance payment in the amount of one annual gross fixed salary and the full contractual bonus for the calendar year in which the termination is effected. A target achievement rate of 100% is applied. In such a case, all stock options and performance shares granted will vest immedi- ately and may be exercised after the statutory vesting periods and blackout periods have expired. The following cases are con- sidered to be a change of control: (i) MorphoSys transfers all or substantially all of its corporate assets to a non-affiliated com- pany, (ii) MorphoSys merges with a non-affiliated company, (iii) MorphoSys AG, as a controlled company, becomes a party to an agreement pursuant to Section 291 of the German Stock Corpora- tion Act (AktG), or MorphoSys is integrated in accordance with Section 319 of the German Stock Corporation Act (AktG), or (iv) a shareholder or third party directly or indirectly holds 30% or more of the voting rights of MorphoSys, or at least 30% of the voting rights are attributed to the shareholder or third party. 104 Group Management Report Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance

03Contents Financial Statements Fi na nc ia l S ta te m en ts Consolidated Statement of Profit or Loss (IFRS) 106 Consolidated Statement of Comprehensive Income (IFRS) 107 Consolidated Balance Sheet (IFRS) 108 Consolidated Statement of Changes in Stockholders’ Equity (IFRS) 110 Consolidated Statement of Cash Flows (IFRS) 112 Notes General Information 114 Summary of Significant Accounting Policies 114 Business Combination 126 Notes to the Profit or Loss Statement 128 Notes to the Balance Sheet 135 Remuneration System for the Management Board and Employees of the Group 147 Additional Notes 161 Financial Statements 105
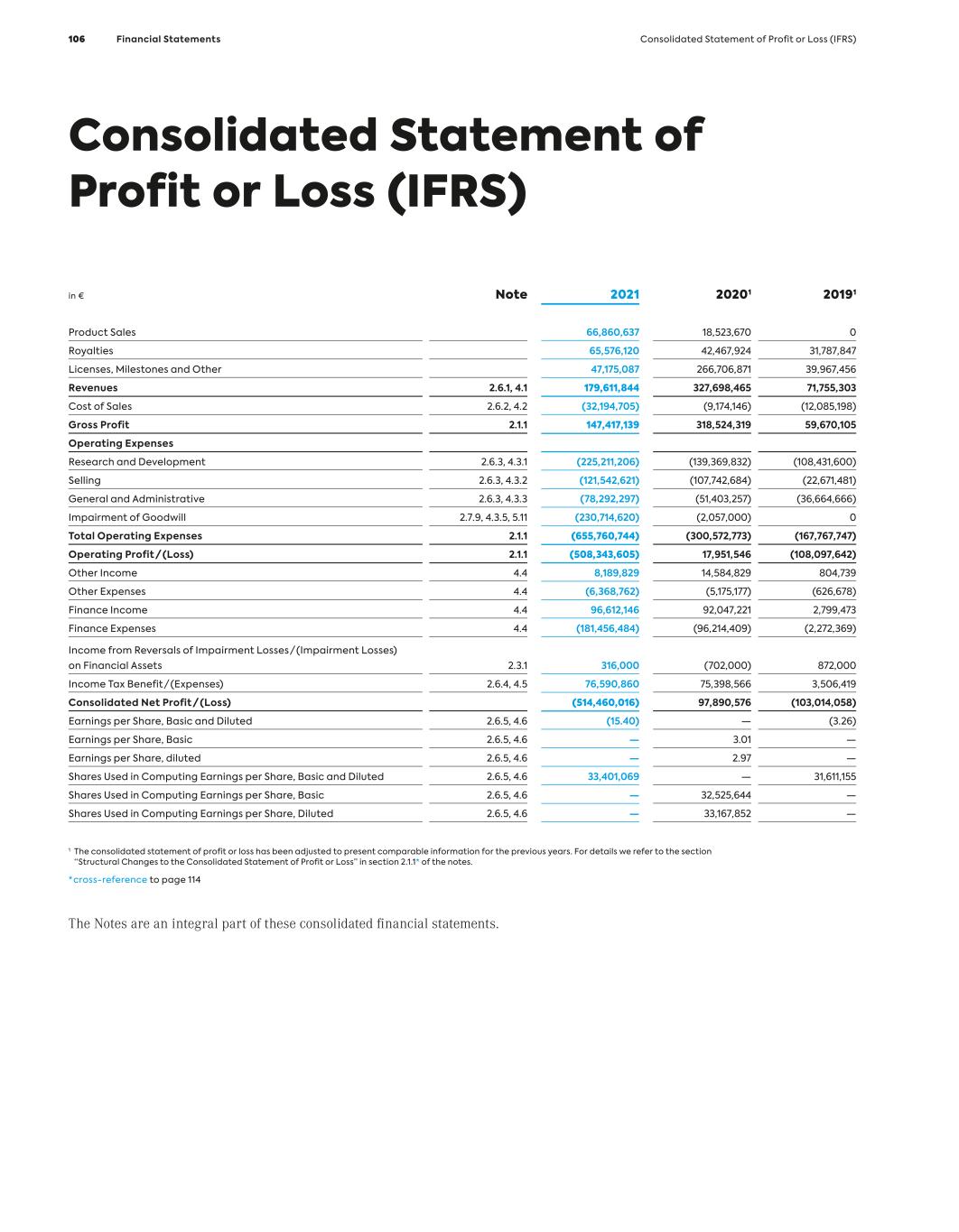
Consolidated Statement of Profit or Loss (IFRS) in € Note 2021 20201 20191 Product Sales 66,860,637 18,523,670 0 Royalties 65,576,120 42,467,924 31,787,847 Licenses, Milestones and Other 47,175,087 266,706,871 39,967,456 Revenues 2.6.1, 4.1 179,611,844 327,698,465 71,755,303 Cost of Sales 2.6.2, 4.2 (32,194,705) (9,174,146) (12,085,198) Gross Profit 2.1.1 147,417,139 318,524,319 59,670,105 Operating Expenses Research and Development 2.6.3, 4.3.1 (225,211,206) (139,369,832) (108,431,600) Selling 2.6.3, 4.3.2 (121,542,621) (107,742,684) (22,671,481) General and Administrative 2.6.3, 4.3.3 (78,292,297) (51,403,257) (36,664,666) Impairment of Goodwill 2.7.9, 4.3.5, 5.11 (230,714,620) (2,057,000) 0 Total Operating Expenses 2.1.1 (655,760,744) (300,572,773) (167,767,747) Operating Profit / (Loss) 2.1.1 (508,343,605) 17,951,546 (108,097,642) Other Income 4.4 8,189,829 14,584,829 804,739 Other Expenses 4.4 (6,368,762) (5,175,177) (626,678) Finance Income 4.4 96,612,146 92,047,221 2,799,473 Finance Expenses 4.4 (181,456,484) (96,214,409) (2,272,369) Income from Reversals of Impairment Losses / (Impairment Losses) on Financial Assets 2.3.1 316,000 (702,000) 872,000 Income Tax Benefit / (Expenses) 2.6.4, 4.5 76,590,860 75,398,566 3,506,419 Consolidated Net Profit / (Loss) (514,460,016) 97,890,576 (103,014,058) Earnings per Share, Basic and Diluted 2.6.5, 4.6 (15.40) — (3.26) Earnings per Share, Basic 2.6.5, 4.6 — 3.01 — Earnings per Share, diluted 2.6.5, 4.6 — 2.97 — Shares Used in Computing Earnings per Share, Basic and Diluted 2.6.5, 4.6 33,401,069 — 31,611,155 Shares Used in Computing Earnings per Share, Basic 2.6.5, 4.6 — 32,525,644 — Shares Used in Computing Earnings per Share, Diluted 2.6.5, 4.6 — 33,167,852 — 1 The consolidated statement of profit or loss has been adjusted to present comparable information for the previous years. For details we refer to the section “Structural Changes to the Consolidated Statement of Profit or Loss” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. Consolidated Statement of Profit or Loss (IFRS) * cross-reference to page 114 106 Financial Statements Consolidated Statement of Profit or Loss (IFRS)
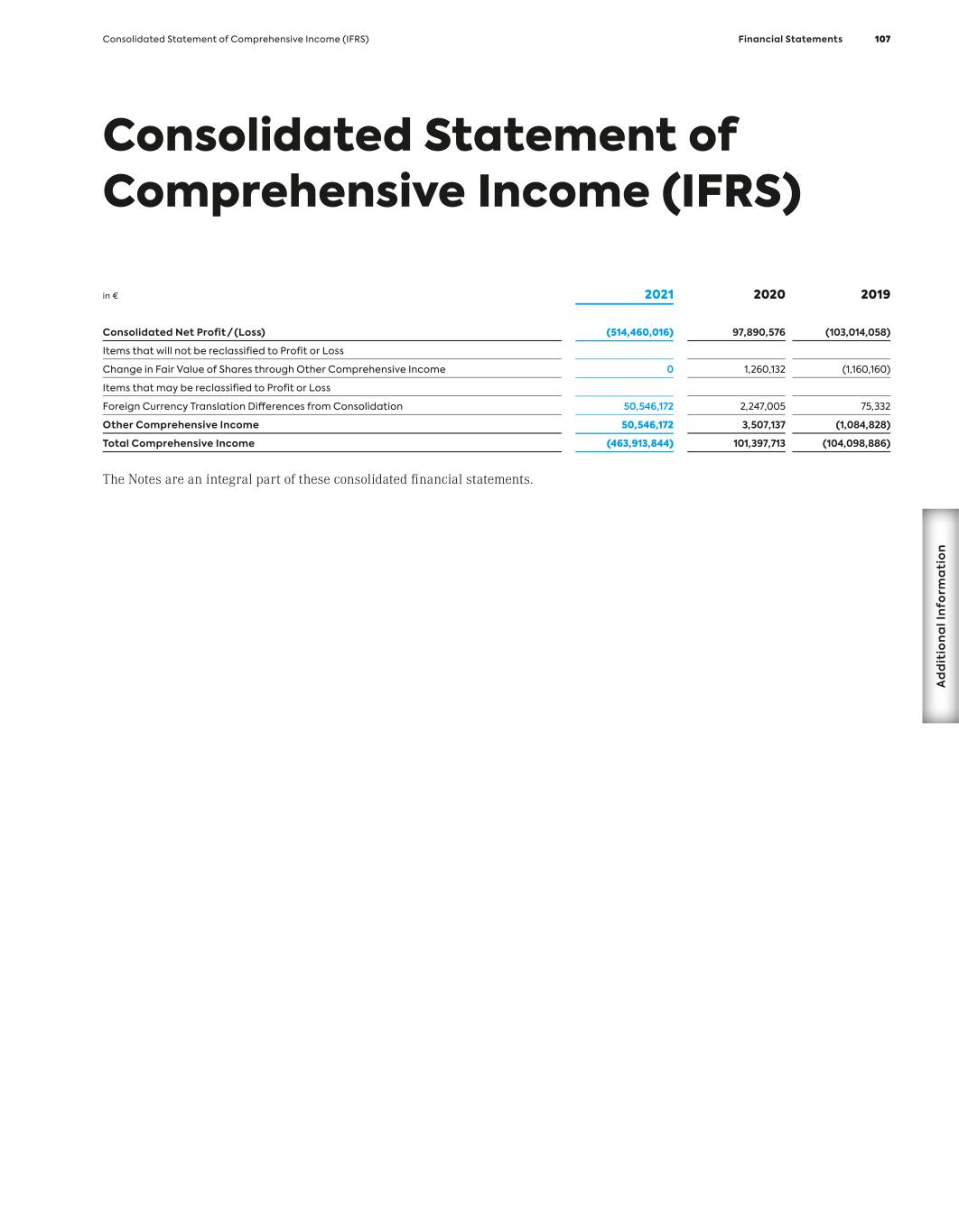
Consolidated Statement of Comprehensive Income (IFRS) in € 2021 2020 2019 Consolidated Net Profit / (Loss) (514,460,016) 97,890,576 (103,014,058) Items that will not be reclassified to Profit or Loss Change in Fair Value of Shares through Other Comprehensive Income 0 1,260,132 (1,160,160) Items that may be reclassified to Profit or Loss Foreign Currency Translation Differences from Consolidation 50,546,172 2,247,005 75,332 Other Comprehensive Income 50,546,172 3,507,137 (1,084,828) Total Comprehensive Income (463,913,844) 101,397,713 (104,098,886) The Notes are an integral part of these consolidated financial statements. Consolidated Statement of Comprehensive Income (IFRS) Consolidated Statement of Comprehensive Income (IFRS) Financial Statements 107

Consolidated Balance Sheet (IFRS) in € Note 12/31/2021 12/31/20201 Assets Current Assets Cash and Cash Equivalents 2.7.1, 5.1 123,248,256 109,794,680 Other Financial Assets 2.7.1, 5.2 853,686,102 937,651,314 Accounts Receivable 2.7.1, 5.3 75,911,054 83,354,276 Financial Assets from Collaborations 2.7.1, 5.18 16,729,924 42,870,499 Income Tax Receivables 2.7.2, 5.6 1,089,078 401,826 Other Receivables 2.7.2, 5.4 2,226,912 2,159,475 Inventories 2.7.3, 5.5 20,755,187 9,962,657 Prepaid Expenses and Other Assets 2.7.4, 5.7 39,323,437 20,621,493 Total Current Assets 1,132,969,950 1,206,816,220 Non-Current Assets Property, Plant and Equipment 2.7.5, 5.8 7,106,783 6,323,753 Right-of-Use Assets 2.7.6, 5.9 42,485,275 44,417,767 Intangible Assets 2.7.7, 5.10 838,322,389 69,375,149 Goodwill 2.7.8, 5.11 335,574,009 1,619,233 Other Financial Assets 2.7.1, 5.2 0 196,587,542 Deferred Tax Asset 2.7.13, 4.5, 5.12 186,545,176 132,806,097 Prepaid Expenses and Other Assets 2.7.4, 5.7 13,250,634 1,567,259 Total Non-Current Assets 1,423,284,266 452,696,800 Total Assets 2,556,254,216 1,659,513,020 1 The consolidated balance sheet has been adjusted to present comparable information for the previous year. For details we refer to the section “Structural Changes to the Consolidated Balance Sheet” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. Consolidated Balance Sheet (IFRS) * cross-reference to page 114 108 Financial Statements Consolidated Balance Sheet (IFRS)
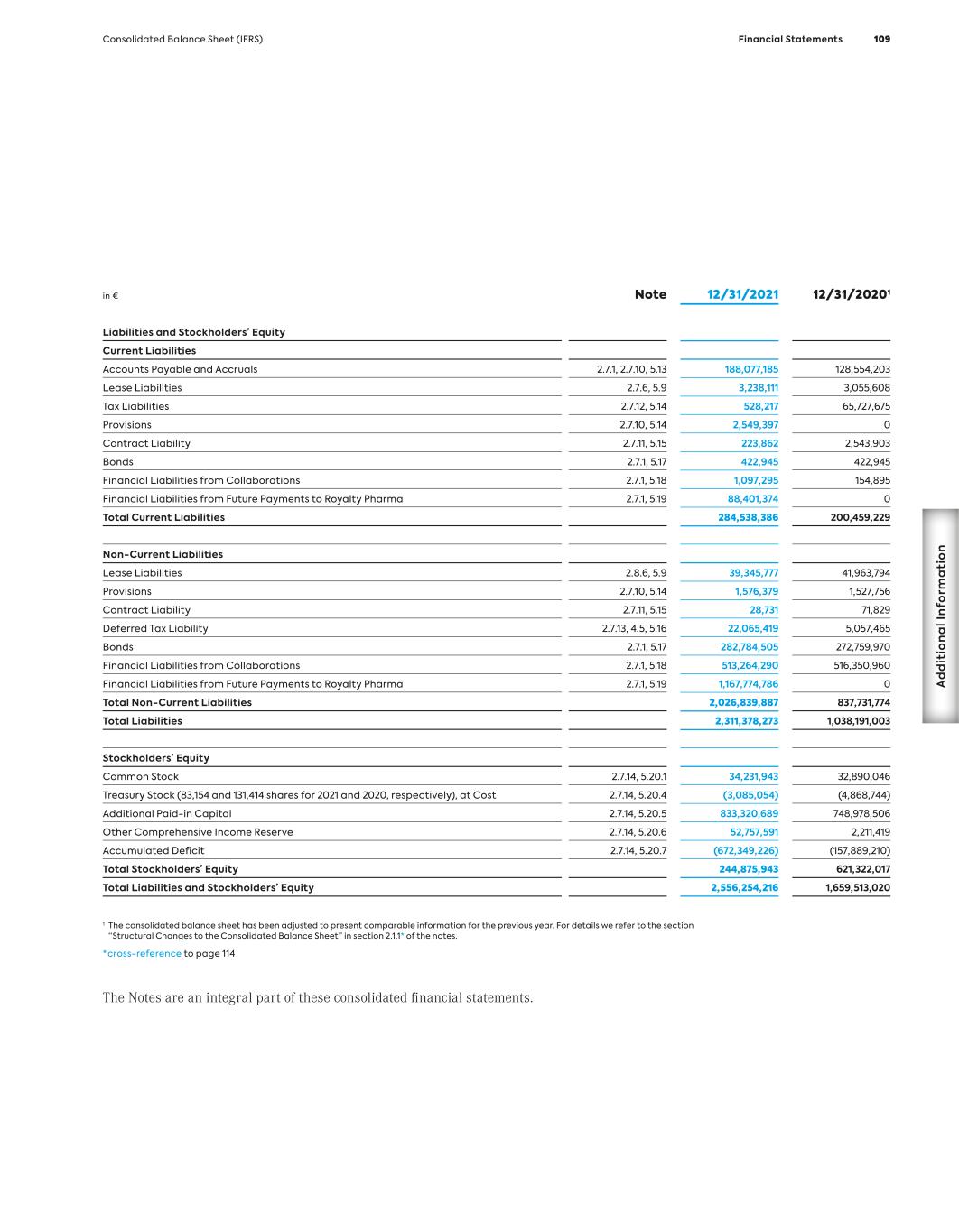
in € Note 12/31/2021 12/31/20201 Liabilities and Stockholders’ Equity Current Liabilities Accounts Payable and Accruals 2.7.1, 2.7.10, 5.13 188,077,185 128,554,203 Lease Liabilities 2.7.6, 5.9 3,238,111 3,055,608 Tax Liabilities 2.7.12, 5.14 528,217 65,727,675 Provisions 2.7.10, 5.14 2,549,397 0 Contract Liability 2.7.11, 5.15 223,862 2,543,903 Bonds 2.7.1, 5.17 422,945 422,945 Financial Liabilities from Collaborations 2.7.1, 5.18 1,097,295 154,895 Financial Liabilities from Future Payments to Royalty Pharma 2.7.1, 5.19 88,401,374 0 Total Current Liabilities 284,538,386 200,459,229 Non-Current Liabilities Lease Liabilities 2.8.6, 5.9 39,345,777 41,963,794 Provisions 2.7.10, 5.14 1,576,379 1,527,756 Contract Liability 2.7.11, 5.15 28,731 71,829 Deferred Tax Liability 2.7.13, 4.5, 5.16 22,065,419 5,057,465 Bonds 2.7.1, 5.17 282,784,505 272,759,970 Financial Liabilities from Collaborations 2.7.1, 5.18 513,264,290 516,350,960 Financial Liabilities from Future Payments to Royalty Pharma 2.7.1, 5.19 1,167,774,786 0 Total Non-Current Liabilities 2,026,839,887 837,731,774 Total Liabilities 2,311,378,273 1,038,191,003 Stockholders’ Equity Common Stock 2.7.14, 5.20.1 34,231,943 32,890,046 Treasury Stock (83,154 and 131,414 shares for 2021 and 2020, respectively), at Cost 2.7.14, 5.20.4 (3,085,054) (4,868,744) Additional Paid-in Capital 2.7.14, 5.20.5 833,320,689 748,978,506 Other Comprehensive Income Reserve 2.7.14, 5.20.6 52,757,591 2,211,419 Accumulated Deficit 2.7.14, 5.20.7 (672,349,226) (157,889,210) Total Stockholders’ Equity 244,875,943 621,322,017 Total Liabilities and Stockholders’ Equity 2,556,254,216 1,659,513,020 1 The consolidated balance sheet has been adjusted to present comparable information for the previous year. For details we refer to the section “Structural Changes to the Consolidated Balance Sheet” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. * cross-reference to page 114 Consolidated Balance Sheet (IFRS) Financial Statements 109
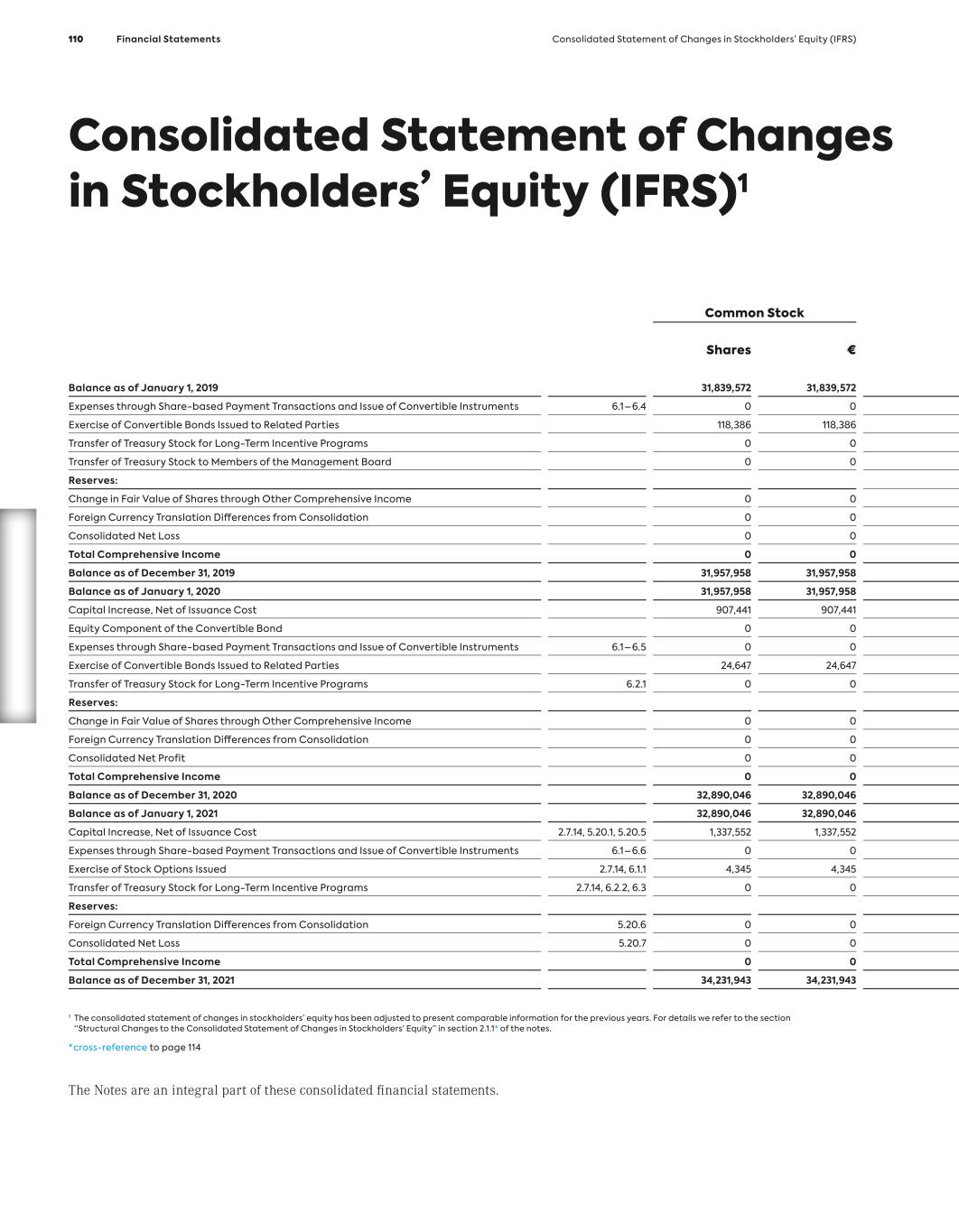
Consolidated Statement of Changes in Stockholders’ Equity (IFRS)1 Common Stock Treasury Stock Additional Paid-in Capital € Other Com- prehensive In- come Reserve € Accumulated Deficit € Total Stockholders’ Equity €Shares € Shares € Balance as of January 1, 2019 31,839,572 31,839,572 281,036 (10,398,773) 619,908,453 (210,890) (152,765,728) 488,372,634 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6.1 – 6.4 0 0 0 0 6,654,470 0 0 6,654,470 Exercise of Convertible Bonds Issued to Related Parties 118,386 118,386 0 0 3,655,168 0 0 3,773,554 Transfer of Treasury Stock for Long-Term Incentive Programs 0 0 (52,328) 1,934,043 (1,934,043) 0 0 0 Transfer of Treasury Stock to Members of the Management Board 0 0 (2,908) 107,480 (107,480) 0 0 0 Reserves: Change in Fair Value of Shares through Other Comprehensive Income 0 0 0 0 0 (1,160,160) 0 (1,160,160) Foreign Currency Translation Differences from Consolidation 0 0 0 0 0 75,332 0 75,332 Consolidated Net Loss 0 0 0 0 0 0 (103,014,058) (103,014,058) Total Comprehensive Income 0 0 0 0 0 (1,084,828) (103,014,058) (104,098,886) Balance as of December 31, 2019 31,957,958 31,957,958 225,800 (8,357,250) 628,176,568 (1,295,718) (255,779,786) 394,701,772 Balance as of January 1, 2020 31,957,958 31,957,958 225,800 (8,357,250) 628,176,568 (1,295,718) (255,779,786) 394,701,772 Capital Increase, Net of Issuance Cost 907,441 907,441 0 0 79,590,657 0 0 80,498,098 Equity Component of the Convertible Bond 0 0 0 0 36,483,050 0 0 36,483,050 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6.1 – 6.5 0 0 0 0 7,455,761 0 0 7,455,761 Exercise of Convertible Bonds Issued to Related Parties 24,647 24,647 0 0 760,976 0 0 785,623 Transfer of Treasury Stock for Long-Term Incentive Programs 6.2.1 0 0 (94,386) 3,488,506 (3,488,506) 0 0 0 Reserves: Change in Fair Value of Shares through Other Comprehensive Income 0 0 0 0 0 1,260,132 0 1,260,132 Foreign Currency Translation Differences from Consolidation 0 0 0 0 0 2,247,005 0 2,247,005 Consolidated Net Profit 0 0 0 0 0 0 97,890,576 97,890,576 Total Comprehensive Income 0 0 0 0 0 3,507,137 97,890,576 101,397,713 Balance as of December 31, 2020 32,890,046 32,890,046 131,414 (4,868,744) 748,978,506 2,211,419 (157,889,210) 621,322,017 Balance as of January 1, 2021 32,890,046 32,890,046 131,414 (4,868,744) 748,978,506 2,211,419 (157,889,210) 621,322,017 Capital Increase, Net of Issuance Cost 2.7.14, 5.20.1, 5.20.5 1,337,552 1,337,552 0 0 83,301,053 0 0 84,638,605 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6.1 – 6.6 0 0 0 0 2,587,931 0 0 2,587,931 Exercise of Stock Options Issued 2.7.14, 6.1.1 4,345 4,345 0 0 236,889 0 0 241,234 Transfer of Treasury Stock for Long-Term Incentive Programs 2.7.14, 6.2.2, 6.3 0 0 (48,260) 1,783,690 (1,783,690) 0 0 0 Reserves: Foreign Currency Translation Differences from Consolidation 5.20.6 0 0 0 0 0 50,546,172 0 50,546,172 Consolidated Net Loss 5.20.7 0 0 0 0 0 0 (514,460,016) (514,460,016) Total Comprehensive Income 0 0 0 0 0 50,546,172 (514,460,016) (463,913,844) Balance as of December 31, 2021 34,231,943 34,231,943 83,154 (3,085,054) 833,320,689 52,757,591 (672,349,226) 244,875,943 1 The consolidated statement of changes in stockholders’ equity has been adjusted to present comparable information for the previous years. For details we refer to the section “Structural Changes to the Consolidated Statement of Changes in Stockholders’ Equity” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. Consolidated Statement of Changes in Stockholders’ Eq- uity (IFRS) * cross-reference to page 114 110 Financial Statements Consolidated Statement of Changes in Stockholders’ Equity (IFRS)

Consolidated Statement of Changes in Stockholders’ Equity (IFRS)1 Common Stock Treasury Stock Additional Paid-in Capital € Other Com- prehensive In- come Reserve € Accumulated Deficit € Total Stockholders’ Equity €Shares € Shares € Balance as of January 1, 2019 31,839,572 31,839,572 281,036 (10,398,773) 619,908,453 (210,890) (152,765,728) 488,372,634 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6.1 – 6.4 0 0 0 0 6,654,470 0 0 6,654,470 Exercise of Convertible Bonds Issued to Related Parties 118,386 118,386 0 0 3,655,168 0 0 3,773,554 Transfer of Treasury Stock for Long-Term Incentive Programs 0 0 (52,328) 1,934,043 (1,934,043) 0 0 0 Transfer of Treasury Stock to Members of the Management Board 0 0 (2,908) 107,480 (107,480) 0 0 0 Reserves: Change in Fair Value of Shares through Other Comprehensive Income 0 0 0 0 0 (1,160,160) 0 (1,160,160) Foreign Currency Translation Differences from Consolidation 0 0 0 0 0 75,332 0 75,332 Consolidated Net Loss 0 0 0 0 0 0 (103,014,058) (103,014,058) Total Comprehensive Income 0 0 0 0 0 (1,084,828) (103,014,058) (104,098,886) Balance as of December 31, 2019 31,957,958 31,957,958 225,800 (8,357,250) 628,176,568 (1,295,718) (255,779,786) 394,701,772 Balance as of January 1, 2020 31,957,958 31,957,958 225,800 (8,357,250) 628,176,568 (1,295,718) (255,779,786) 394,701,772 Capital Increase, Net of Issuance Cost 907,441 907,441 0 0 79,590,657 0 0 80,498,098 Equity Component of the Convertible Bond 0 0 0 0 36,483,050 0 0 36,483,050 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6.1 – 6.5 0 0 0 0 7,455,761 0 0 7,455,761 Exercise of Convertible Bonds Issued to Related Parties 24,647 24,647 0 0 760,976 0 0 785,623 Transfer of Treasury Stock for Long-Term Incentive Programs 6.2.1 0 0 (94,386) 3,488,506 (3,488,506) 0 0 0 Reserves: Change in Fair Value of Shares through Other Comprehensive Income 0 0 0 0 0 1,260,132 0 1,260,132 Foreign Currency Translation Differences from Consolidation 0 0 0 0 0 2,247,005 0 2,247,005 Consolidated Net Profit 0 0 0 0 0 0 97,890,576 97,890,576 Total Comprehensive Income 0 0 0 0 0 3,507,137 97,890,576 101,397,713 Balance as of December 31, 2020 32,890,046 32,890,046 131,414 (4,868,744) 748,978,506 2,211,419 (157,889,210) 621,322,017 Balance as of January 1, 2021 32,890,046 32,890,046 131,414 (4,868,744) 748,978,506 2,211,419 (157,889,210) 621,322,017 Capital Increase, Net of Issuance Cost 2.7.14, 5.20.1, 5.20.5 1,337,552 1,337,552 0 0 83,301,053 0 0 84,638,605 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6.1 – 6.6 0 0 0 0 2,587,931 0 0 2,587,931 Exercise of Stock Options Issued 2.7.14, 6.1.1 4,345 4,345 0 0 236,889 0 0 241,234 Transfer of Treasury Stock for Long-Term Incentive Programs 2.7.14, 6.2.2, 6.3 0 0 (48,260) 1,783,690 (1,783,690) 0 0 0 Reserves: Foreign Currency Translation Differences from Consolidation 5.20.6 0 0 0 0 0 50,546,172 0 50,546,172 Consolidated Net Loss 5.20.7 0 0 0 0 0 0 (514,460,016) (514,460,016) Total Comprehensive Income 0 0 0 0 0 50,546,172 (514,460,016) (463,913,844) Balance as of December 31, 2021 34,231,943 34,231,943 83,154 (3,085,054) 833,320,689 52,757,591 (672,349,226) 244,875,943 1 The consolidated statement of changes in stockholders’ equity has been adjusted to present comparable information for the previous years. For details we refer to the section “Structural Changes to the Consolidated Statement of Changes in Stockholders’ Equity” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. Consolidated Statement of Changes in Stockholders’ Equity (IFRS) Financial Statements 111
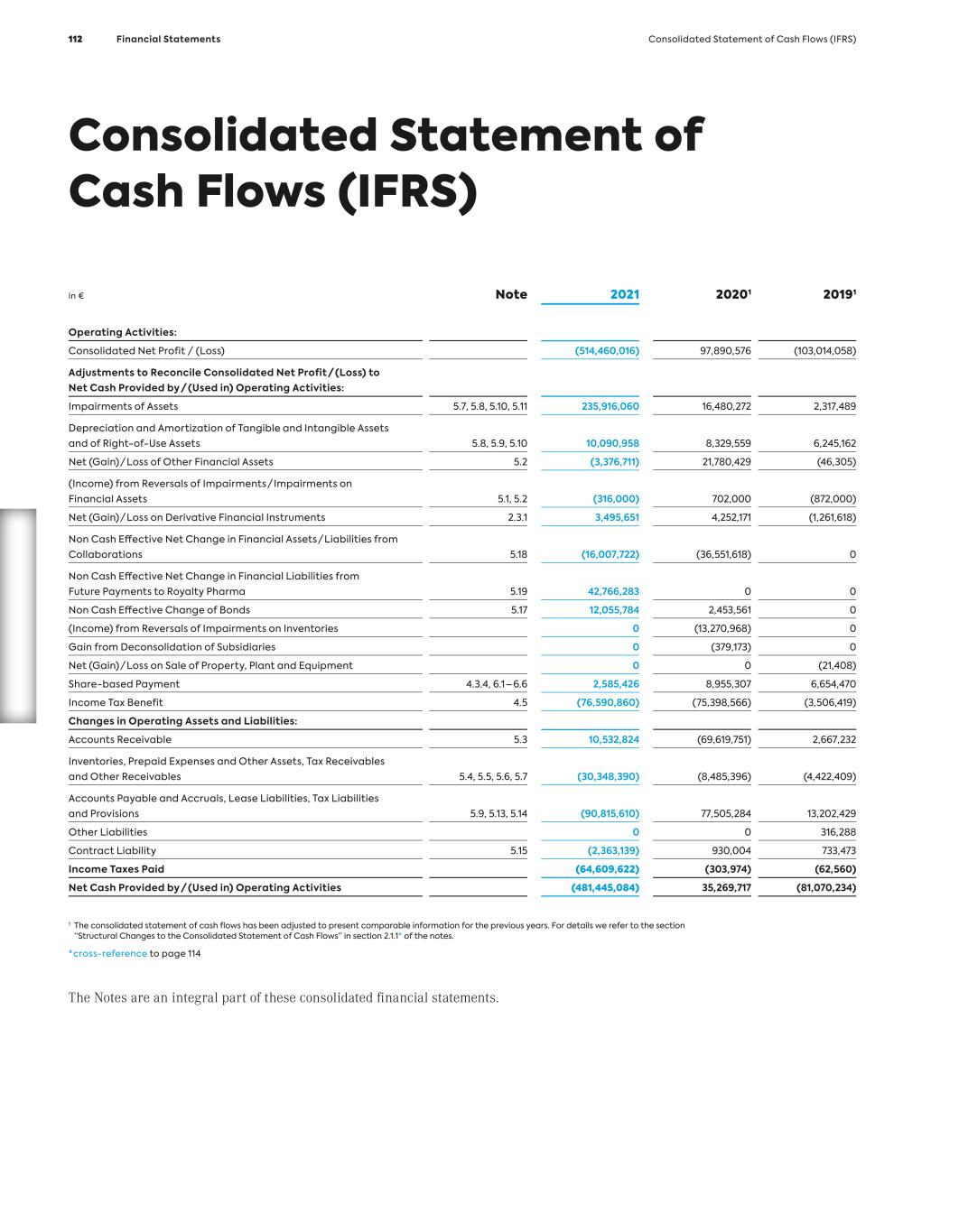
Consolidated Statement of Cash Flows (IFRS) in € Note 2021 20201 20191 Operating Activities: Consolidated Net Profit / (Loss) (514,460,016) 97,890,576 (103,014,058) Adjustments to Reconcile Consolidated Net Profit / (Loss) to Net Cash Provided by / (Used in) Operating Activities: Impairments of Assets 5.7, 5.8, 5.10, 5.11 235,916,060 16,480,272 2,317,489 Depreciation and Amortization of Tangible and Intangible Assets and of Right-of-Use Assets 5.8, 5.9, 5.10 10,090,958 8,329,559 6,245,162 Net (Gain) / Loss of Other Financial Assets 5.2 (3,376,711) 21,780,429 (46,305) (Income) from Reversals of Impairments / Impairments on Financial Assets 5.1, 5.2 (316,000) 702,000 (872,000) Net (Gain) / Loss on Derivative Financial Instruments 2.3.1 3,495,651 4,252,171 (1,261,618) Non Cash Effective Net Change in Financial Assets / Liabilities from Collaborations 5.18 (16,007,722) (36,551,618) 0 Non Cash Effective Net Change in Financial Liabilities from Future Payments to Royalty Pharma 5.19 42,766,283 0 0 Non Cash Effective Change of Bonds 5.17 12,055,784 2,453,561 0 (Income) from Reversals of Impairments on Inventories 0 (13,270,968) 0 Gain from Deconsolidation of Subsidiaries 0 (379,173) 0 Net (Gain) / Loss on Sale of Property, Plant and Equipment 0 0 (21,408) Share-based Payment 4.3.4, 6.1 – 6.6 2,585,426 8,955,307 6,654,470 Income Tax Benefit 4.5 (76,590,860) (75,398,566) (3,506,419) Changes in Operating Assets and Liabilities: Accounts Receivable 5.3 10,532,824 (69,619,751) 2,667,232 Inventories, Prepaid Expenses and Other Assets, Tax Receivables and Other Receivables 5.4, 5.5, 5.6, 5.7 (30,348,390) (8,485,396) (4,422,409) Accounts Payable and Accruals, Lease Liabilities, Tax Liabilities and Provisions 5.9, 5.13, 5.14 (90,815,610) 77,505,284 13,202,429 Other Liabilities 0 0 316,288 Contract Liability 5.15 (2,363,139) 930,004 733,473 Income Taxes Paid (64,609,622) (303,974) (62,560) Net Cash Provided by / (Used in) Operating Activities (481,445,084) 35,269,717 (81,070,234) 1 The consolidated statement of cash flows has been adjusted to present comparable information for the previous years. For details we refer to the section “Structural Changes to the Consolidated Statement of Cash Flows” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. Consolidated Statement of Cash Flows (IFRS) * cross-reference to page 114 112 Financial Statements Consolidated Statement of Cash Flows (IFRS)
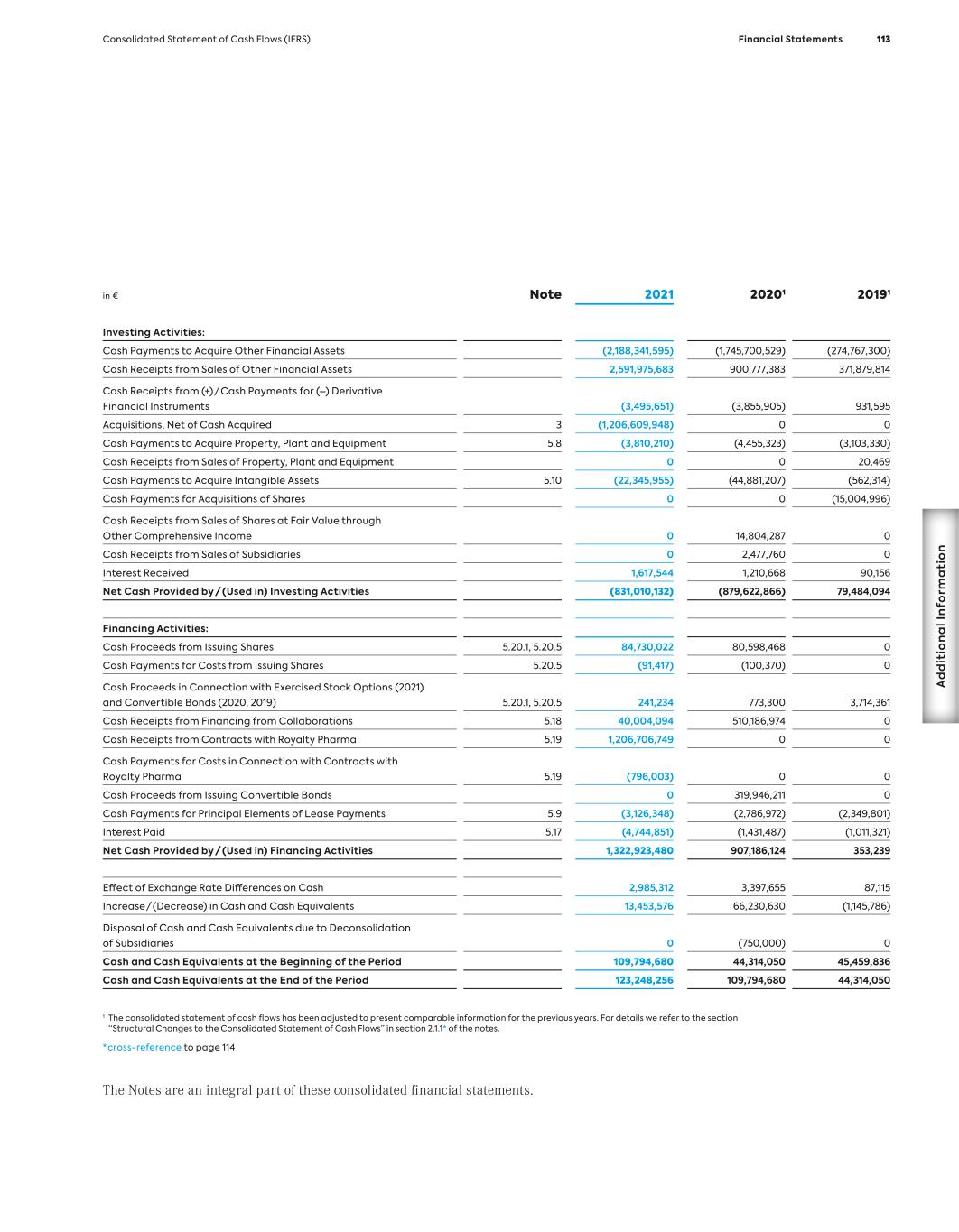
in € Note 2021 20201 20191 Investing Activities: Cash Payments to Acquire Other Financial Assets (2,188,341,595) (1,745,700,529) (274,767,300) Cash Receipts from Sales of Other Financial Assets 2,591,975,683 900,777,383 371,879,814 Cash Receipts from (+) / Cash Payments for (–) Derivative Financial Instruments (3,495,651) (3,855,905) 931,595 Acquisitions, Net of Cash Acquired 3 (1,206,609,948) 0 0 Cash Payments to Acquire Property, Plant and Equipment 5.8 (3,810,210) (4,455,323) (3,103,330) Cash Receipts from Sales of Property, Plant and Equipment 0 0 20,469 Cash Payments to Acquire Intangible Assets 5.10 (22,345,955) (44,881,207) (562,314) Cash Payments for Acquisitions of Shares 0 0 (15,004,996) Cash Receipts from Sales of Shares at Fair Value through Other Comprehensive Income 0 14,804,287 0 Cash Receipts from Sales of Subsidiaries 0 2,477,760 0 Interest Received 1,617,544 1,210,668 90,156 Net Cash Provided by / (Used in) Investing Activities (831,010,132) (879,622,866) 79,484,094 Financing Activities: Cash Proceeds from Issuing Shares 5.20.1, 5.20.5 84,730,022 80,598,468 0 Cash Payments for Costs from Issuing Shares 5.20.5 (91,417) (100,370) 0 Cash Proceeds in Connection with Exercised Stock Options (2021) and Convertible Bonds (2020, 2019) 5.20.1, 5.20.5 241,234 773,300 3,714,361 Cash Receipts from Financing from Collaborations 5.18 40,004,094 510,186,974 0 Cash Receipts from Contracts with Royalty Pharma 5.19 1,206,706,749 0 0 Cash Payments for Costs in Connection with Contracts with Royalty Pharma 5.19 (796,003) 0 0 Cash Proceeds from Issuing Convertible Bonds 0 319,946,211 0 Cash Payments for Principal Elements of Lease Payments 5.9 (3,126,348) (2,786,972) (2,349,801) Interest Paid 5.17 (4,744,851) (1,431,487) (1,011,321) Net Cash Provided by / (Used in) Financing Activities 1,322,923,480 907,186,124 353,239 Effect of Exchange Rate Differences on Cash 2,985,312 3,397,655 87,115 Increase / (Decrease) in Cash and Cash Equivalents 13,453,576 66,230,630 (1,145,786) Disposal of Cash and Cash Equivalents due to Deconsolidation of Subsidiaries 0 (750,000) 0 Cash and Cash Equivalents at the Beginning of the Period 109,794,680 44,314,050 45,459,836 Cash and Cash Equivalents at the End of the Period 123,248,256 109,794,680 44,314,050 1 The consolidated statement of cash flows has been adjusted to present comparable information for the previous years. For details we refer to the section “Structural Changes to the Consolidated Statement of Cash Flows” in section 2.1.1* of the notes. The Notes are an integral part of these consolidated financial statements. * cross-reference to page 114 Consolidated Statement of Cash Flows (IFRS) Financial Statements 113

1 General Information Business Activities and the Company MorphoSys AG (“the Company” or “MorphoSys”) is a commercial-stage biopharmaceutical company dedicated to the discovery, development and commercialization of therapeutic antibodies for patients suffering from cancer and autoimmune diseases. The Company has a proprietary portfo- lio of compounds and a pipeline of compounds developed with partners from the pharmaceutical and biotechnology industry. MorphoSys was founded as a German limited liability company in July 1992. In June 1998, MorphoSys became a German stock corporation. In March 1999, the Com- pany completed its initial public offering on Germany’s “Neuer Markt”: the segment of the Deutsche Börse designated, at that time, for high- growth companies. On January 15, 2003, MorphoSys AG was admitted to the Prime Standard segment of the Frankfurt Stock Exchange. On April 18, 2018, MorphoSys completed an IPO on the Nasdaq Global Mar- ket through the issue of American Depositary Shares (ADS). Each ADS represented 1/4 of a MorphoSys ordinary share. MorphoSys AG’s regis- tered office is located in Planegg (district of Munich), and the registered business address is Semmelweisstrasse 7, 82152 Planegg, Germany. The MorphoSys AG consolidated and separate financial statements can be viewed at this address. The Company is registered in the Commercial Register B of the District Court of Munich under the number HRB 121023. 2 Summary of Significant Accounting Policies 2.1 Basis of and Changes in Accounting Standards 2.1.1 Basis of Application These consolidated financial statements were prepared in accordance with the International Financial Reporting Standards (“IFRS”), taking into account the recommendations of the International Financial Report- ing Standards Interpretations Committee (IFRS IC). We have applied all standards and interpretations that were in force as of December 31, 2021 and adopted by the European Union (EU). As of December 31, 2021, there were no standards or interpretations that affected our consolidated finan- cial statements for the years ended December 31, 2021, 2020 and 2019 that were in effect, but not yet endorsed into European law. As a result, our consolidated financial statements comply with both the IFRSs pub- lished by the International Accounting Standards Board (IASB) and those adopted by the EU. These consolidated financial statements also take into account the supplementary provisions under commercial law, which must be applied in accordance with Section 315e (1) of the German Com- mercial Code (Handelsgesetzbuch – HGB). In accordance with the regula- tions of the United States Securities and Exchange Commission, the statement of profit or loss is presented for a comparative period of three years. This extends beyond the comparative period of two years in accor- dance with the requirements of IFRS as adopted by the EU. The consolidated financial statements as of the reporting dates of Decem- ber 31, 2021 and 2020, as well as the periods from January 1 through December 31 for the years 2021, 2020 and 2019, comprise MorphoSys AG and its subsidiaries (collectively, the “MorphoSys Group” or the “Group”). MorphoSys AG prepares the consolidated financial statements for the largest and the smallest consolidated group. All figures in this report were rounded to the nearest euro, thousand eu- ros or million euros. By virtue of MorphoSys’ business model, the COVID-19 pandemic has had a limited impact on MorphoSys’ net assets and financial position in 2021. The ongoing COVID-19 pandemic, however, has had a negative im- pact on the results of operations especially in 2021, specifically on lower than expected sales of Monjuvi. Furthermore, this also extends the un- certainties of planning assumptions relating to future Monjuvi-sales, which have significant effects on the financial assets and liabilities from collaborations. In addition, the adherence to the time schedule of the clin- ical studies was associated with higher expenses. There have been no material asset impairments that have been recognized in connection with COVID-19. Structural Changes to the Segment Reporting As of the first quarter of 2021, MorphoSys no longer presents the previ- ous segment information for the Proprietary Development and Partnered Discovery segments as part of the regular internal reporting to the Man- agement Board as the Company’s chief operating decision-maker. Inter- nal reporting focuses exclusively on the key value drivers of future reve- nues from product sales, further market approvals for tafasitamab, and Group royalties. The previous segment reporting was published for the last time for external purposes as of December 31, 2020. Reporting now comprises only the consolidated statement of profit or loss and no longer includes separate segment reporting. With the acquisition of Constella- tion, efforts related to the marketing approvals of pelabresib and CPI- 0209 will expand, but this does not result in any changes in the assess- ment of the segment reporting. Notes General Information Summary of Significant Accounting Policies 114 Financial Statements Notes

Structural Changes to the Consolidated Statement of Profit or Loss The change in the Company’s internal management and corresponding financial guidance for the 2021 financial year also prompted changes in the presentation of the consolidated statement of profit or loss. The follow- ing changes were implemented for the first time with the reporting of 2021: • Presentation of the components of revenue “Product Sales”, “Royalties” and “Licenses, Milestones and Other” • Introduction of the item “Gross Profit” on the statement of profit or loss as the difference between revenues and cost of sales • “Operating Expenses” include research and development, as well as selling, general and administrative expenses. In this context, total op- erating expenses for 2020 were adjusted by € 9.2 million (2019: € 12.1 million) as the cost of sales are no longer included in this sum line item in order to provide comparable prior year information. • Introduction of the item “Impairment of goodwill” on the statement of profit or loss as a component of “Operating expenses”. In this context, research and development expenses for 2020 have been adjusted by € 2.1 million to provide comparable information for the comparative period. • The item “Earnings before Interest and Taxes” (EBIT) on the statement of profit or loss has been discontinued • Introduction of the item “Operating Profit (+) / Loss (–)” on the state- ment of profit or loss as the difference between the statement’s items “Gross Profit” and “Operating Expenses” The prior year’s presentation of the figures has been adjusted accord- ingly in order to provide comparable information for the previous years. Structural Changes to the Consolidated Balance Sheet To improve readability, adjustments were made to the presentation in the consolidated balance sheet. The following changes were implemented for the first time for the reporting in 2021: • Consolidation of the asset items “Financial Asset at Fair Value through Profit or Loss” and “Other Financial Assets at Amortized Cost” into the item “Other Financial Assets” • Asset items “Inventories, Net”, “Property, Plant and Equipment, Net” and “Right-of-Use Assets, Net” have the term “Net”, “Prepaid Expenses and Other Current Assets” has the term “Current” and “Prepaid Ex- penses and Other Assets, Net of Current Portion” has the term “Net of Current Portion” deleted • Consolidation of the asset items “Patents, net”, “Licenses, net”, “Li- censes for Marketed Products”, “In-process R&D Programs” and “Soft- ware, net” into the item “Intangible Assets” • Change in the name of current and non-current liability line items “Other Provisions” to “Provisions” and “Convertible Bond” to “Bonds” • Deletion of the term “current portion” for all current liability items and “excluding current portion” for all non-current liability items The prior year’s presentation of the figures has been adjusted accord- ingly in order to provide comparable information for the previous years. Structural Changes to the Consolidated Statement of Changes in Stock- holders’ Equity To improve readability, adjustments were made to the consolidated state- ment of changes in stockholders’ equity. The following changes were im- plemented for the first time for the reporting in 2021: • Change in the name of “Compensation Related to the Grant of Stock Options and Performance Shares” to “Expenses through Share-based Payment Transactions and Issue of Convertible Instruments” • Change in the name of “Transfer of Treasury Stock to Related Parties” to “Transfer of Treasury Stock to Members of the Management Board” and of “Exercise of Convertible Bonds Issued” to “Exercise of Convert- ible Bonds Issued to Related Parties” The prior year’s presentation of the figures has been adjusted accord- ingly in order to provide comparable information for the previous years. Structural Changes to the Consolidated Statement of Cash Flows The aggregation of items and changes in the titles of balance sheet items resulted in corresponding changes in the cash flow statement: • Consolidation of the items “Net (Gain) / Loss of Financial Assets at Fair Value through Profit or Loss” and “Net (Gain) / Loss of Financial Assets at Amortized Cost” into the item “Net (Gain) / Loss of Other Financial Assets” • Consolidation of the items “Recognition of Contract Liability” and “Con- tract Liability” into the item “Contract Liability” • Consolidation of the items “Cash Payments to Acquire Financial Assets at Fair Value through Profit or Loss” and “Cash Payments to Acquire Other Financial Assets at Amortized Cost” into the item “Cash Pay- ments to Acquire Other Financial Assets” • Consolidation of the items “Cash Receipts from Sales of Financial As- sets at Fair Value through Profit or Loss” and “Cash Receipts from Sales of Other Financial Assets at Amortized Cost” into the item “Cash Re- ceipts from Sales of Other Financial Assets” • Split of the item “Cash Receipts from (+) / Cash Payments for (–) Deriv- ative Financial Instruments” into “Cash Receipts from Derivative Fi- nancial Instruments” and “Cash Payments for Derivative Financial In- struments” • Change in the name of “Non Cash Effective Change of Financial Liabil- ities at Amortized Cost” to “Non Cash Effective Change of Bonds”, “Ac- counts Payable and Accruals, Lease Liabilities, Tax Liabilities and Other Provisions” to “Accounts Payable and Accruals, Lease Liabilities, Tax Liabilities and Provisions”, of “Cash Payments for Acquisitions of Shares at Fair Value through Other Comprehensive Income” to Cash Payments for Acquisitions” and of “Cash Proceeds in Connection with Convertible Bonds Granted to Related Parties” to “Cash Proceeds in Connection with Exercised Stock Options and Convertible Bonds” The prior year’s presentation of the figures has been adjusted accord- ingly in order to provide comparable information for the previous years. Unless stated otherwise, the accounting policies set out below were ap- plied consistently to all periods presented in these consolidated financial statements. Notes Financial Statements 115
2.1.2 Changes in Accounting Standards and Disclosures The accounting standards applied generally correspond to the policies used in the prior year. New or Revised Standards and Interpretations Adopted for the first Time in the Financial Year Standard / Interpretation Mandatory Application for financial years starting on Adopted by the European Union Possible Impact on MorphoSys IFRS 16 (A) Covid-19-Related Rent Concessions beyond 30 June 2021 1/1/2021 yes none IFRS 9, IAS 39, IFRS 7, IFRS 4 and IFRS 16 (A) Interest Rate Benchmark Reform – Phase 2 1/1/2021 yes yes IFRS 4 (A) Deferral of IFRS 9 1/1/2021 yes none (A) Amendments The impact on the consolidated financial statements from the amendment to IFRS 9, IAS 39, IFRS 7, IFRS 4 and IFRS 16 is not considered to be ma- terial and are therefore not explained separately. Standards with the re- mark “none” do not have an impact on the consolidated financial state- ments. New or Revised Standards and Interpretations not yet Mandatorily Appli- cable The following new or revised standards that were not yet mandatory in the reporting period or have not yet been adopted by the European Union, have not been applied prematurely. The effects on the consolidated finan- cial statements of standards marked with “yes” are considered probable and are currently being examined by the Group. Only significant effects are described in more detail. The impact on the consolidated financial statements of the amendments to IAS 1, IAS 8 and IAS 12 are not consid- ered material and, therefore, not explained separately. Standards with the comment “none” are not expected to have a material impact on the consolidated financial statements. Standard / Interpretation Mandatory Application for financial years starting on Adopted by the European Union Possible Impact on MorphoSys IFRS 3 (A) Reference to the Conceptual Framework 1/1/2022 yes none IFRS 17 and IFRS 17 (A) Insurance Contracts and Amendments to IFRS 17 1/1/2023 yes none IFRS 17 (A) Initial Application of IFRS 17 and IFRS 9 — Comparative Information 1/1/2023 no none IAS 1 (A) Classification of Liabilities as Current or Non-current 1/1/2023 no yes IAS 1 (A) Disclosure of Accounting Policies 1/1/2023 no yes IAS 8 (A) Definition of Accounting Estimates 1/1/2023 no yes IAS 12 (A) Deferred Tax related to Assets and Liabilities arising from a Single Transaction 1/1/2023 no yes IAS 16 (A) Property, Plant and Equipment — Proceeds before Intended Use 1/1/2022 yes none IAS 37 (A) Amended by Onerous Contracts — Cost of Fulfilling a Contract 1/1/2022 yes none Annual Improvements to International Financial Reporting Stan- dards, 2018 – 2020 1/1/2022 yes none (A) Amendments 116 Financial Statements Notes
2.2 Consolidation Principles 2.2.1 Consolidated Companies and Scope of Consolidation MorphoSys AG, as the ultimate parent company, is located in Planegg, near Munich. MorphoSys AG has one wholly owned subsidiary, MorphoSys US Inc. (Boston, Massachusetts, USA). MorphoSys US Inc. in turn has a wholly owned subsidiary – Constellation Pharmaceuticals Inc. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. also has a wholly owned subsidiary, Constellation Securities Corp. (Cam- bridge, Massachusetts, USA). Constellation Pharmaceuticals Inc. and Constellation Securities Corp. are collectively referred to as “Constella- tion”, and all entities constitute the “MorphoSys Group” or “Group”. Following the acquisition on July 15, 2021, Constellation Pharmaceuticals Inc. was merged into MorphoSys Development Inc., which was incorpo- rated as a wholly owned subsidiary of MorphoSys US Inc. on May 28, 2021, in accordance with the merger agreement. From this upward merger, Constellation Pharmaceuticals Inc. remained as a wholly owned subsidiary of MorphoSys US Inc. The consolidated financial statements as of December 31, 2021, were pre- pared by the Management Board on March 15, 2022, by resolution of the Management Board, authorized for issue, and forwarded to the Supervi- sory Board for review and approval. The members of the Group’s Manage- ment Board are Jean-Paul Kress, M.D., as Chief Executive Officer (Chair of the Management Board), Sung Lee as Chief Financial Officer and Malte Peters, M.D., as Chief Research and Development Officer. Sung Lee assumed the position as Chief Financial Officer on February 2, 2021. Roland Wandeler, Ph.D., stepped down as a member of the Management Board with effect from the end of December 31, 2021. 2.2.2 Consolidation Methods The following Group subsidiaries are included in the scope of consolida- tion, as shown in the table below. Company Purchase of Shares / Establishment Included in Basis of Consolidation since Constellation Pharmaceuticals Inc., Cambridge, Massachusetts, USA July 2021 15/7/2021 Constellation Securities Corp., Cambridge, Massachusetts, USA July 2021 15/7/2021 MorphoSys US Inc., Boston, Massachusetts, USA July 2018 2/7/2018 These subsidiaries are fully consolidated as they are direct or indirect wholly owned subsidiaries. MorphoSys controls the subsidiaries due to its full power over the investees. Additionally, MorphoSys is subject to risk exposure and has rights to variable returns from its involvement with the investees. MorphoSys also has unlimited capacity to exert power over the investees to influence its returns. The Group does not have any entities consolidated as joint ventures using the equity method, nor does it exercise a significant influence. The assets and liabilities of the fully consolidated international entities are recognized using Group-wide uniform accounting and valuation methods. The consolidation methods applied have not changed from the previous year. Upon consolidation, the carrying amounts of the parent company’s in- vestments in each subsidiary are offset against the parent’s share in the equity of each subsidiary. Inter-company assets and liabilities, income and expenses, and profits or losses arising from transactions between Group companies are eliminated in full. 2.3 Principles of Foreign Currency Translation The Group’s consolidated financial statements are presented in euros, which is also the parent company’s functional currency. For each entity, the Group determines the functional currency. The items included in the financial statements of each entity are measured using that functional currency. Transactions and Balances Transactions in foreign currencies are initially recorded by the Group’s entities at their respective functional currency spot rates at the date the transaction first qualifies for recognition. Monetary assets and liabilities denominated in foreign currencies are translated at the functional cur- rency spot rates of exchange at the reporting date. Differences arising on settlement or translation of monetary items are recognized in other in- come or expenses. For monetary items relating to investing and financ- ing activities, differences are recognized in finance income or finance expenses. Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using the exchange rates at the dates of the initial transactions. Group Companies On consolidation, the assets and liabilities of foreign operations are translated into euros at the rate of exchange prevailing at the reporting date and their statements of profit or loss are translated at average ex- change rates. The exchange differences arising on translation for consol- idation are recognized in “Other Comprehensive Income Reserve” (eq- uity). 2.4 Key Estimates and Assumptions In preparing the consolidated financial statements, it is necessary to make estimates and assumptions that affect the carrying amounts of as- sets, liabilities and contingent liabilities at the balance sheet date and the amounts of income and expense recognized in the period under report. The actual results may differ from these estimates. The estimates and underlying assumptions are subject to continuous review and are based on historical experience and other factors, including the expectation of future events that are believed to be realistic under the prevailing cir- cumstances. Any changes in estimates are recognized in the period in which the changes are made and in all relevant future periods. The re- sulting accounting-related estimates will, by definition, seldom corre- spond to the actual results. The estimates and assumptions that carry a significant risk of causing material adjustments to the carrying amounts of assets and liabilities in the next financial year are addressed below. Notes Financial Statements 117

Revenues Revenues from product sales, royalties, license fees, milestones are sub- ject to assumptions regarding variable consideration components, proba- bilities of occurrence and individual selling prices within the scope of the accounting and measurement principles explained in Note 2.6.1.* Accru- als in connection with revenues from product sales are also affected by estimates and assumptions. * cross-reference to page 119 Impairment of Financial Assets Impairment losses on financial assets in the form of debt instruments and accounts receivable are based on assumptions about credit risk. The Group exercises discretion in making these assumptions and in selecting the inputs to calculate the impairment based on past experience, current market conditions and forward-looking estimates at the end of each re- porting period. Financial Assets and Liabilities from Collaborations For details on estimates and assumptions in connection with financial assets and liabilities from collaborations refer to Note 5.18*. * cross-reference to page 143 Leases In determining the lease term, all facts and circumstances are consid- ered that create an economic incentive to exercise an extension option. Extension options are only included in the lease term if the lease is rea- sonably certain to be extended. Licenses for Marketed Products The acquired licenses are amortized over their estimated useful life. An impairment loss is recognized when events or changes in circumstances indicate that the licenses are impaired. Intangible assets not yet available for use and Goodwill The Group performs an annual review to determine whether in-process R&D programs (intangible assets not yet available for use) or goodwill is subject to impairment in accordance with the accounting policies dis- cussed in Note 2.7.9*. The recoverable amounts from in-process R&D pro- grams and cash-generating units have been determined using value-in- use calculations and are subjected to a sensitivity analysis. These calculations require the use of estimates (see Notes 5.10* and 5.11*). * cross-reference to page 125, page 139 and page 140 Accruals The Group has entered into various research and development contracts with research institutions and other companies. These agreements are generally cancellable, and related costs are recorded as research and de- velopment expenses as incurred. The Group recognizes accruals for esti- mated ongoing research costs that have been incurred. When evaluating the appropriateness of the deferred expenses, the Group analyzes the progress of the studies, including the phase and completion of events, invoices received and contractually agreed costs. Significant judgments and estimates are made in determining the deferred balances at the end of any reporting period. Actual results may differ from the Group’s esti- mates. The Group’s historical accrual estimates have not been materially different from the actual costs. Financial Liabilities from Future Payments to Royalty Pharma For details on estimates and assumptions in connection with the finan- cial liabilities from future payment to Royalty Pharma refer to Note 5.19*. * cross-reference to page 144 Income Taxes Income taxes comprise taxes levied in the individual countries on tax- able profit and changes in deferred taxes. The income taxes reported are recognized on the basis of the statutory regulations in force or enacted as of the reporting date in the amount in which they are expected to be paid or refunded. Deferred taxes are recognized for tax-deductible or tempo- rary taxable differences between the carrying amounts of assets and lia- bilities in the IFRS balance sheet and the tax base, as well as for tax ef- fects arising from consolidation measures and tax reduction claims arising from loss carryforwards that are likely to be realized in subse- quent years. Goodwill is excluded. The assessment of the recoverability of deferred tax assets considers the currently achieved total results of a legal entity as well as the expected future taxable results, derived from the corporate planning. The recogni- tion of deferred tax assets on tax loss carryforwards requires manage- ment to make estimates and judgments about the amount of future tax- able profit available against which the tax loss carryforwards can be utilized. Deferred tax assets on loss carryforwards are only recognized to the extent that sufficient taxable income is expected in the future. Uncertain tax positions are analyzed on an ongoing basis and, if taxes are sufficiently probable, risk provisions are recognized in an appropri- ate amount in each case. Uncertainties arise, among other things, from matters that are being discussed in ongoing tax audits but have not yet resulted in final findings or are under discussion due to disputed legal situations or new case law. As the estimates can change over time, for example, as a result of find- ings in the course of the tax audit or current case law, there will also be a corresponding effect on the amount of the required assessment of the risk provision. The amount of the expected tax liability or tax receivable reflects the amount representing the best estimate or the expected value, taking into account any existing tax uncertainties. For the assessment of the impairment of deferred tax assets, the plan- ning assumptions are influenced by key estimates and mainly include the profit forecasts of the respective legal entities. Effects of Climate Change on Financial Reporting In fiscal year 2021, the company analyzed potential sustainability risks in the areas of climate change and water scarcity. In both areas, the Com- pany has not identified any material risks to its business model. There- fore, the Company does not currently expect any material impact of sus- tainability risks on its financial reporting. 2.5 Business Combinations The acquisition method of accounting is used to account for all business combinations, regardless of whether equity instruments or other assets are acquired. The consideration transferred for the acquisition of a sub- sidiary comprises the fair values of the assets transferred. Identifiable assets acquired and liabilities assumed in a business combi- nation are measured initially at their fair values at the acquisition date. Acquisition related costs are expensed in the general and administrative expenses as incurred. The excess of the consideration transferred over the fair value of the net identifiable assets acquired is recorded as goodwill and allocated to a cash-generating unit. If those amounts are less than the fair value of the net identifiable assets of the business acquired, the difference is recog- nized directly in profit or loss as a bargain purchase. 118 Financial Statements Notes

2.6 Accounting Policies applied to Line Items of the Statement of Profit or Loss 2.6.1 Revenues Recognizing revenue from contracts with customers requires the follow- ing five-stage approach: • Identification of the contract • Identification of performance obligations • Determination of the transaction price • Allocation of the transaction price • Revenue recognition The Group’s revenues typically include revenue from product sales, roy- alties, license fees, milestone payments and service fees. Revenues from Product Sales Revenues from the sale of MorphoSys products are recognized at the transaction price at the time the customer obtains control of the product. This is defined as the point at which the customer receives the product. As a result, revenues are recognized based on a specific point in time. The transaction price represents the consideration expected by MorphoSys in exchange for the product and takes into account variable components. The variable consideration is only included in the transac- tion price if it is highly probable that there will not be a subsequent ma- terial adjustment to the transaction price. The most common elements of variable consideration related to product sales at MorphoSys are listed below and are determined according to the expected value approach. • Rebates and discounts agreed with government agencies, buying groups, specialty distributors and specialty pharmacies are accrued and deducted from revenues at the time the related revenues are recog- nized. They are calculated based on actual discounts and rebates granted, specific regulatory requirements, specific terms in individual agreements, product pricing and/or the anticipated sales channel mix. Because the Company recognizes revenue upon transfer of control of the product to specialty distributors and specialty pharmacies, and not upon transfer to the end-user (patient), for certain rebates the Com- pany is required to estimate the mix of product sales between its sales channels in determining the amount of rebate that will ultimately be paid. • Discounts offered to customers are intended to encourage prompt pay- ment and are deferred and recognized as revenue deductions at the time the related revenues are recognized. • Accruals for product returns are recognized as revenue deductions at the time the corresponding revenues are recognized. Variable consideration is deducted from trade receivables, in case these are directly paid to the direct customer. In case payments are to be made to another party, these are presented as accruals. Accruals for revenue deductions are adjusted to the actual amounts when rebates and dis- counts and cash discounts are realized. The accruals represent estimates of the related obligations, meaning that management’s judgment is re- quired in estimating the impact of these revenue deductions. Royalties Revenue recognition for royalties (income based on a percentage of sales of a marketed product) is based on the same revenue recognition princi- ples that apply to sales-based milestones, as described below. License Fees and Milestone Payments The Group recognizes revenues from license fees for intellectual prop- erty (IP) both at a point in time and over a period of time. The Group must make an assessment as to whether such a license represents a right-to- use the IP (at a point in time) or a right to access the IP (over time). Reve- nue for a right-to-use license is recognized by the Group when the licensee can use and benefit from the IP after the license term begins, e.g., the Group has no further obligations in the context of the out-licens- ing of a drug candidate or technology. A license is considered a right to access the intellectual property when the Group undertakes activities during the license term that significantly affect the IP, the customer is directly exposed to any positive or negative effects of these activities, and these activities do not result in the transfer of a good or service to the customer. Revenues from the right to access the IP are recognized on a straight-line basis over the license term. Milestone payments for research and development are contingent upon the occurrence of a future event and represent variable consideration. The Group’s management estimates at the contract’s inception that the most likely amount for milestone payments is zero. The most likely amount method of estimation is considered the most predictive for the outcome since the outcome is binary; e.g. achieving a specific success in clinical development (or not). The Group includes milestone payments in the total transaction price only to the extent that it is highly probable that a significant reversal of accumulated revenue will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Sales-based milestone payments included in contracts for IP licenses are considered by the Group to be sales-based license fees because they are solely determined by the sales of an approved drug. Accordingly, such milestones are recognized as revenue once the sales of such drugs occur or at a later point if the performance obligation has not been fulfilled. Service Fees Service fees for the assignment of personnel to research and development collaborations are recognized as revenues in the period the services were provided. If a Group company acts as an agent, revenues are recognized on a net basis. Agreements with multiple Performance Obligations A Group company may enter into agreements with multiple performance obligations that include both licenses and services. In such cases, an as- sessment must be made as to whether the license is distinct from the services (or other performance obligations) provided under the same agreement. The transaction price is allocated to separate performance obligations based on the relative stand-alone selling price of the perfor- mance obligations in the agreement. The Group company estimates stand-alone selling prices for goods and services not sold separately on the basis of comparable transactions with other customers. The residual approach is the method used to estimate a stand-alone selling price when the selling price for a good or service is highly variable or uncertain. Principle-Agent Relationships In agreements involving two or more independent parties who contribute to the provision of a specific good or service to a customer, the Group company assesses whether it has promised to provide the specific good or service itself (the company acting as a principal) or to arrange for this specific good or service to be provided by another party (the company acting as an agent). Depending on the result of this assessment, the Group company recognizes revenues on a gross (principal) or net (agent) basis. A Group company is an agent and recognizes revenue on a net ba- sis if its obligation is to arrange for another party to provide goods or Notes Financial Statements 119

services, i.e., the Group company does not control the specified good or service before it is transferred to the customer. Indicators to assist a com- pany in determining whether it does not control the good or service be- fore it is provided to a customer and is, therefore, an agent, include, but are not limited to, the following criteria: • Another party is primarily responsible for fulfilling the contract. • The company does not have inventory risk. • The company does not have discretion in establishing the price. No single indicator is determinative or weighted more heavily than other indicators. However, some indicators may provide stronger evidence than others, depending on the individual facts and circumstances. A Group company’s control needs to be substantive; obtaining the legal title to a good or service only momentarily before it is transferred to the customer does not necessarily indicate that a Group company is a principal. Gener- ally, an assessment as to whether a Group company is acting as a principal or an agent in a transaction requires a considerable degree of judgment. Based on the relevant facts and circumstances, the assessment of an agreement may lead to the conclusion that the counterparty is a coopera- tion partner or partner rather than a customer because the contract par- ties share equally in the risk of co-developing a drug and in the future profits from the marketing of the approved drug. 2.6.2 Cost of Sales The cost of sales includes the acquisition and production cost of invento- ries recognized as an expense, personnel expenses, inventory write- downs, reversals of inventory write-downs, impairments and scheduled depreciation and other expenses for intangible assets, costs for external services as well as other costs. Cost of sales are recognized as an expense as incurred. 2.6.3 Operating Expenses Operating expenses are allocated to the functional costs on the basis of cost centers or percentage allocation keys. Research and Development Expenses Research costs are expensed in the period in which they occur. Develop- ment costs are generally expensed as incurred. Development costs are recognized as an intangible asset when the criteria such as the probabil- ity of expected future economic benefits, as well as the reliability of cost measurement, are met. This line item contains personnel expenses, consumable supplies, im- pairment charges, impairment reversals, amortization and other costs related to intangible assets (additional information can be found in Note 5.10*), costs for external services, infrastructure costs and depreci- ation as well as other costs. * cross-reference to page 139 Selling Expenses The line item includes personnel costs, consumable supplies, amortiza- tion of intangible assets (software; additional information can be found in Note 5.10*), costs for external services, infrastructure costs and depreci- ation as well as other costs. This item also includes all expenses for ser- vices provided by Incyte in connection with the joint US sales activities. * cross-reference to page 139 General and Administrative Expenses The line item includes personnel costs, consumable supplies, amortiza- tion of intangible assets (software; additional information can be found in Note 5.10*), costs for external services, infrastructure costs and depreci- ation as well as other costs. * cross-reference to page 139 Expenses through Share-based Payment Transactions and Issue of Con- vertible Instruments The Group spreads the compensation expenses from the estimated fair values of share-based payments on the reporting date over the period in which the beneficiaries provide the services that triggered the granting of the share-based payments. Personnel expense is recognized in the re- spective functional area to which the beneficiary is allocated. Share-based compensation is considered when the Group acquires goods or services in exchange for shares or stock options (“settlement in equity instruments”) or other assets that represent the value of a specific num- ber of shares or stock options (“cash settlement”). Additional information can be found in Note 6*. * cross-reference to page 147 2.6.4 Income Tax Expenses / Benefits Current income taxes are calculated based on the respective local taxable income and local tax rules for the period. In addition, current income taxes presented for the period include adjustments for uncertain tax pay- ments or tax refunds for periods not yet finally assessed, excluding inter- est expenses and penalties on the underpayment of taxes. In the event that amounts included in the tax returns are considered unlikely to be accepted by the tax authorities (uncertain tax positions), a provision for income taxes is recognized. Tax refund claims from uncertain tax posi- tions are recognized when it is probable that they can be realized. Cur- rent taxes reflect the expected tax liability on the taxable income for the year, based on the enacted or substantially enacted tax rates, as well as adjustments to the tax liability for previous years. Deferred tax assets or liabilities are calculated for temporary differences between the tax bases and the financial statement carrying amounts, including differences from consolidation, unused tax loss carryforwards, and unused tax credits. Measurement is based on enacted or substan- tively enacted tax rates and tax rules. Deferred tax assets are offset against deferred tax liabilities when the taxes are levied by the same taxation authority, and the entity has a le- gally enforceable right to offset current tax assets against current tax li- abilities according to their maturity. Assessments as to the recoverability of deferred tax assets require the use of judgment regarding assumptions related to estimated future tax- able profits. This includes the amounts of taxable future profits, the peri- ods in which those profits are expected to occur, and the availability of tax planning opportunities. The Group records a deferred tax asset only when it is probable that a corresponding amount of taxable profit will be available against which the deductible temporary differences relating to the same taxation authority and the same taxable entity can be utilized. The analysis and forecasting required in this process are performed for individual jurisdictions by qualified local tax and financial professionals. Given the potential significance surrounding the underlying estimates and assumptions, group-wide policies and procedures have been de- signed to ensure consistency and reliability around the recoverability assessment process. Forecast operating results are based upon approved business plans, which are themselves subject to a well-defined process of control. As a matter of policy, especially strong evidence supporting the recognition of deferred tax assets is required if an entity has suffered a loss in either the current or the preceding period. Changes in deferred tax assets and liabilities are generally recognized through profit and loss in the consolidated statement of profit or loss, ex- cept for changes recognized directly in equity, and changes recognized in connection with a business combination, where the purchase price al- 120 Financial Statements Notes

location results in deferred tax assets and liabilities being recognized as an offset against goodwill. Deferred tax assets are recognized only to the extent that it is likely that there will be future taxable income to offset. Deferred tax assets are reduced by the amount that the related tax benefit is no longer expected to be realized. 2.6.5 Earnings per Share The Group reports basic and diluted earnings per share. Basic earnings per share are computed by dividing the net profit or loss attributable to parent company shareholders by the weighted-average number of ordi- nary shares outstanding for the reporting period. Diluted earnings per share are calculated in the same manner with the exception that the net profit or loss attributable to parent company shareholders and the weight- ed-average number of ordinary shares outstanding are adjusted for any dilutive effects resulting from stock options and restricted stock units granted to the Management Board and employees and convertible bonds. The potentially dilutive shares are excluded from the calculation of the dilutive earnings per share, if the dilutive effect would result in a decline in the loss per share for the respective year. 2.7 Accounting Policies applied to Line Items of the Balance Sheet The balance sheet is presented on the basis of the current/non-current distinction. Current assets and liabilities are those that are due within a period of one year. Regardless of their maturity, accounts receivable, ac- counts payable and inventories are also deemed to be current if they are due or sold within the normal course of a business cycle, which can be longer than one year. Deferred taxes are presented as non-current assets and liabilities. 2.7.1 Financial Instruments A financial instrument is a contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity. Financial assets and liabilities comprise non-derivative and derivative receivables and payables. The Group recognizes financial instruments at the point in time when it becomes the contractual party of the instrument. A normal market pur- chase or sale of financial assets is recognized on the trade date, i.e. the date on which the obligation to buy or sell the asset was entered into. On initial recognition, the Group measures financial assets and financial liabilities at fair value, with the exception of trade receivables without a significant financing component, which are measured at the transaction price specified in section 2.6.1*. * cross-reference to page 119 When the financial asset is not subsequently measured at fair value in profit or loss, transaction costs directly attributable to the acquisition of that asset will be added to the fair value. Transaction costs of financial assets measured at fair value through profit or loss are recognized as expenses in profit or loss. Direct attributable transaction costs are deducted from the fair value if they are attributable to financial liabilities measured at amortized cost. Transaction costs are recognized directly in profit or loss if they are re- lated to the issue of financial liabilities measured at fair value. Financial assets and liabilities are offset only when it is currently legally enforceable to offset the amounts and there is an intention to do so. The Group does not perform offsetting. Financial Assets Classification, Measurement and Disclosure The Group’s financial assets include both debt instruments and equity instruments. A debt instrument is a contractual right to receive cash or another financial asset from another entity or to exchange financial as- sets or financial liabilities with another entity under conditions that are potentially favorable to the entity. An equity instrument is any contract that evidences a residual interest in the assets of an entity after deduct- ing all of its liabilities. The classification of financial assets (debt instruments) for subsequent measurement depends on the Group’s business model for managing the financial assets and the asset’s cash flow characteristics. The business model reflects how the Group manages its financial assets to generate cash flows. The business model determines whether cash flows will result from collecting contractual cash flows, selling the financial assets, or both. A financial asset can give rise to cash flows that are ‘solely pay- ments of principal and interest (SPPI)’ on the principal amount outstand- ing. This SPPI test involves an assessment of whether the cash flows of the instrument consist solely of payments of interest and principal. Inter- est is typically consideration for the time value of money and credit risk. Payments of principal are payments on the principal amount outstand- ing. Assets that are held in order to collect the contractual cash flows and for which these cash flows represent interest and principal payments only are measured at amortized cost (AC). Interest income from these finan- cial assets is recognized in finance income using the effective interest method. Negative interests are recognized in Finance Expense. Gains and losses upon derecognition are recognized directly in profit or loss and recorded in the finance result. Impairment losses are recognized as a separate line item in profit or loss. The Group’s financial assets at amor- tized cost comprise the balance sheet item “Cash and Cash Equivalents”, part of the balance sheet item “Other Financial Assets” (term deposits), the balance sheet item “Accounts Receivable” and part of the balance sheet item “Prepaid Expenses and Other Assets” (restricted cash for e.g. rental deposits). The Group considers all bank balances, cash in hand and short-term de- posits with a maturity of three months or less from the date of acquisition to be cash and cash equivalents. Assets that are held to collect the contractual cash flows and to sell the financial assets and where the cash flows represent principal and interest payments only are measured at fair value through other comprehensive income (FVOCI). The Group does not hold any financial assets that are measured at fair value through other comprehensive income. Assets that do not meet the criteria of the categories “at amortized cost” or “at fair value through other comprehensive income” are allocated to the category “at fair value through profit or loss” (FVTPL) Gains and losses on debt instruments that are subsequently measured at fair value through profit or loss are recognized in other income/expenses or the fi- nance result in the period in which they occur. The Group’s financial as- sets measured at fair value through profit or loss include part of the bal- ance sheet item “Other Financial Assets” (money market funds) and the balance sheet item “Financial Assets from Collaborations”. Derivatives with a positive fair value are recorded in the balance sheet item “Other Receivables” and derivatives with a negative fair value are recorded in the balance sheet item “Other Liabilities”. MorphoSys does not apply hedge accounting. Notes Financial Statements 121

The Group reclassifies debt instruments only in case when there is a change in the business model for managing such assets. For investments in equity instruments that are not held for trading, clas- sification depends on whether the Group has irrevocably elected, at the time of initial recognition when the instrument is acquired, to measure the equity instruments at fair value through other comprehensive in- come. If this option is not exercised, equity instruments are measured at fair value through profit or loss. The Group has exercised the option to measure all equity instruments held at fair value through other compre- hensive income. As a result, after derecognition of such an instrument, no subsequent reclassification of these gains and losses to the consoli- dated income statement takes place. Dividends from such instruments continue to be recognized in profit or loss under other income when the Group’s right to receive payment is established. Equity instruments in- clude the equity investments made by the Group. Impairment and Reversal of Impairment Financial assets in the categories measured at amortised cost (AC) and at fair value through other comprehensive income (FVOCI) require the cal- culation of an impairment loss, which is recognized on the basis of ex- pected credit losses. A distinction is made between a general and a sim- plified impairment model. Impairment losses on financial instruments are reported under impair- ment losses on financial assets. Reversals of impairment are recognized in income from reversals of impairment losses. Impairment losses on trade receivables are reported in other expenses. Amounts, which were written off previously, but are received in subse- quent periods, are recognized in other income. Financial Instruments according to General Expected Credit Loss Model The Group assesses on a forward-looking basis the expected credit losses associated with its debt instruments carried at amortized cost. When a debt instrument is recognized for the first time, an impairment loss is recognized in the amount of the expected loss for twelve months. The impairment method applied depends on whether there has been a signif- icant increase in credit risk. If at the reporting date, the credit risk of a financial instrument has not increased significantly since initial recogni- tion, the Group measures the loss allowance for that financial instrument at an amount equal to twelve-month expected credit losses (Level 1). Where the expected lifetime of an asset is less than twelve months, ex- pected losses are measured at its expected lifetime. Expected credit losses are based on the contractual cash flows multiplied by the premium of a credit default swap according to the expected maturity of the con- tracting party (Level 1). In case the credit risk of a financial instrument has increased significantly since initial recognition, the Group measures impairment for that financial instrument at an amount equal to the life- time expected credit losses. The Group currently classifies an increase in credit risk on debt instruments as significant when the premium on a counterparty credit default swap has increased by 100 basis points since the initial recognition of the instrument or if the amount is more than 30 days overdue (Level 2). If there is an objective indication of impairment, the interest received must also be adjusted so that the interest as of this date is accrued based on the net carrying amount (carrying amount less risk provisions) of the financial instrument (Level 3). Financial Instruments according to Simplified Expected Credit Loss Model In the case of accounts receivable, the Group applies the simplified ap- proach, which requires expected lifetime losses to be recognized from the initial recognition of the receivables (Level 2). In the event of objec- tive evidence of impairment of trade receivables, such assets are reported as credit-impaired and the expected loss is calculated as the difference between the gross carrying amount and the present value of the ex- pected cash flows discounted at the original effective interest rate (Level 3). All accounts receivable were aggregated to measure the expected credit losses. All accounts receivable are currently due from customers in the pharmaceutical industry with similar credit risk profiles. The impair- ment is determined on the basis of the premium for an industry credit default swap. In the event that accounts receivable cannot be grouped together, they are measured individually. Objective indications of the impairment of financial instruments may re- sult from an overdue period of more than 90 days, significant financial difficulties on the part of the issuer or debtor, a breach of contract such as a default or delay in interest or principal payments, an increased proba- bility of insolvency or other reorganization proceedings, or the disap- pearance of an active market for a financial asset due to financial difficul- ties. Financial instruments are impaired if, based on a reasonable estimate, they are not expected to be realized and one of the objective indications occurs. An indicator that there is no reasonable expectation of recovery is, among other things, when internal or external information indicates that the Group will not receive the outstanding contractual amounts in full. This is generally assumed if financial instruments are more than two years overdue. Derecognition Financial assets are derecognized when the rights to receive cash flows from the financial assets have expired or have been transferred and the Group has transferred substantially all risks and rewards of ownership. Financial Liabilities Classification, Measurement and Disclosure Contracts for liabilities are examined to determine whether they are only equity or only debt in nature or contain components of both. If the eco- nomic substance of the contractual agreement contains both compo- nents, they are recognized separately as equity instruments and as fi- nancial liabilities. Financial liabilities are classified in the following categories: • Financial liabilities at fair value through profit or loss • Financial liabilities at amortized cost Subsequent measurement at fair value through profit or loss (FVTPL) can be irrevocably designated upon initial recognition or is performed for derivatives with a negative fair value. Gains or losses arising from changes in fair value are recognized in profit or loss in the financial re- sult. The Group does not make a designation for measurement at fair value. Financial liabilities measured at amortized cost (FLAC) are measured us- ing the effective interest method. Gains and losses are recognized in the income statement in other income/expenses or in the financial result us- ing the effective interest method. For financial liabilities measured at amortized cost, an assessment is made at initial recognition as to whether separable embedded derivatives have been agreed in the contract. Em- bedded derivatives must be separated and recognized separately at fair value through profit or loss unless their terms are closely related to the host contract. The Group’s financial liabilities measured at amortized cost include trade payables (part of the balance sheet item “Accounts Pay- 122 Financial Statements Notes

able and Accruals”), the balance sheet items “Financial Liabilities from Collaborations” and the balance sheet items “Financial Liabilities from Future Payments to Royalty Pharma”. For contracts with equity and liability components, the fair value of the liability component is determined at the time of initial recognition using the market interest rate applicable to comparable instruments. This amount is recognized as a financial liability measured at amortized cost until the contract is settled or becomes due. The component classified as equity is determined by the difference between the total value of the contract and the fair value of the liability component. The resulting amount, net of income tax effects, is recognized as part of equity in addi- tional paid-in capital and is not adjusted in subsequent periods. Transac- tion costs associated with the instrument are allocated between the two components based on the allocation of proceeds. Transaction costs attrib- utable to the debt component are deducted from the carrying amount of the debt component and are amortized over the life of the contract using the effective interest method. Such a contract includes the convertible bond in the balance sheet item “Bonds”. The exercise of the conversion option does not give rise to a gain or loss, but rather to a derecognition of the liability and a recognition of equity. All amounts on financial liabilities at amortized cost that are payable within the next twelve months, are reported as a current liability. For bonds the undiscounted cash flows within the next twelve are considered as current. For the financial liabilities from collaborations and the finan- cial liabilities from future payments to Royalty Pharma the planned pay- ments in the next twelve months are discounted to determine the current liability. Derecognition A financial liability is derecognized when the underlying obligation is discharged, cancelled or expires. 2.7.2 Income Tax Receivables and Other Receivables Income tax receivables mainly include receivables due from tax authori- ties in the context of capital gain taxes withheld to the nominal value without discount. 2.7.3 Inventories Inventories are measured at the lower value of production or acquisition cost and net realizable value under the first-in, first-out method. Acquisi- tion costs comprise all purchase costs, including those incurred in bring- ing the inventories into operating condition, and take purchase price re- ductions into account, such as bonuses and discounts. Manufacturing costs comprise all directly attributable costs as well as reasonably allo- cated overhead. Net realizable value is the estimated selling price less the estimated expenses necessary for completion and sale. Inventories are divided into the categories of raw materials and supplies, as well as unfinished and finished goods. Inventory of tafasitamab used for clinical trials or research activities are presented as other current assets and once it is used costs are recognized in the income statement under research and development expenses when consumed. 2.7.4 Prepaid Expenses and Other Assets Prepaid expenses include expenses resulting from an outflow of liquid assets prior to the reporting date that are only recognized as expenses in the subsequent financial year. Such expenses usually involve mainte- nance contracts, sublicenses and upfront payments for external labora- tory services not yet performed. Current other assets primarily consist of receivables from tax authorities from input tax surpluses, combination drugs as well as receivables from upfront payments. Measurement is at nominal value or acquisition cost less impairments. 2.7.5 Property, Plant and Equipment Property, plant and equipment are recorded at historical cost less accu- mulated depreciation (see Note 5.8*) and any impairment losses (see Note 2.7.9*). Historical cost includes expenditures directly related to the purchase at the time of the acquisition. Replacement purchases, building alterations and improvements are capitalized, whereas repair and main- tenance expenses are recognized as expenses as they are incurred. Prop- erty, plant and equipment are depreciated on a straight-line basis over its estimated useful life (see table below). Leasehold improvements are de- preciated on a straight-line basis over the shorter of either the asset’s estimated useful life or the remaining term of the lease. * cross-reference to page 137 and page 125 Asset Class Useful Life Depreciation Rates Office Equipment 8 years 13% Laboratory Equipment 4 years 25% Low-value Office and Laboratory Equipment Immediately 100% Computer Hardware 3 years 33% Permanent Improvements to Property/ Buildings 10 years 10% The residual values and useful lives of assets are reviewed at the end of each reporting period and adjusted when necessary. Borrowing costs that can be directly attributed to the acquisition, con- struction or production of a qualifying asset are not included in the ac- quisition or production costs. 2.7.6 Leases For lessees, a uniform approach is applied to the recognition of leases, according to which assets for the right-of-use assets of the leased assets and liabilities for the payment obligations entered into are required to be recognized in the balance sheet for all leases. At the time a leased asset becomes available for the Group’s use, a right-of-use asset and corre- sponding lease liability are recognized in the balance sheet. Right-of-use assets are measured at cost, which is calculated as the lease liability plus lease payments made at or before the date on which the as- set is made available for use, less lease incentives received and additional initial direct costs and dismantling obligations. Subsequent measure- ment of right-of-use assets is at amortized cost. The right-of-use assets are amortized on a straight-line basis over the shorter of either the useful life or the term of the lease agreement and the amortization is recognized in profit or loss. The lease liability is the present value of the fixed and variable lease payments that are paid during the term of the lease less any lease incen- tives receivable. The discounting is carried out based on the implied in- terest rate underlying the lease contract if the rate can be determined. If not, discounting is carried out based on the lessee’s incremental borrow- ing rate, i.e., the interest rate a lessee would need to pay to borrow over a similar term, and with a similar security, the funds necessary to obtain an asset of similar value and condition to the right-of-use asset in a simi- lar economic environment. Notes Financial Statements 123

In subsequent measurement, the carrying amount of the lease liability is increased to reflect the interest expense on the lease liability and re- duced to reflect the lease payments made. Each lease installment is sepa- rated into a repayment portion and a financing expense portion. Finance expenses are recognized in profit or loss over the term of the lease. The Group is exposed to potential future increases in variable lease pay- ments based on an index or rate, which are not included in the lease lia- bility until they take effect. When adjustments to lease payments based on an index or rate take effect, the lease liability is reassessed and ad- justed against the right-of-use asset. The payments for the redemption of lease liabilities and the payments attributable to the interest portion of the lease liabilities are allocated to cash flow from financing activities. For low-value leases and short-term leases (terms of less than twelve months), mainly technical equipment, use is made of the simplified appli- cation. Accordingly, no right-of-use assets or lease liabilities are recog- nized; instead, the lease payments are recognized as an expense over the term of the lease. Impairment losses are recognized in accordance with the principles de- scribed in Note 2.7.9*. * cross-reference to page 125 2.7.7 Intangible Assets Purchased intangible assets are capitalized at acquisition cost and exclu- sively amortized on a straight-line basis over their useful lives. Internally generated intangible assets are recognized to the degree the correspond- ing recognition criteria are met. Development costs are capitalized as intangible assets when the corre- sponding capitalization criteria have been met, namely, clear specifica- tion of the product or procedure, technical feasibility, intention of comple- tion, use, commercialization, coverage of development costs through future free cash flows, reliable determination of these free cash flows and availability of sufficient resources for completion of development and sale. Amortization of intangible assets is recorded in cost of sales or re- search and development expenses. Expenses to be classified as research expenses are allocated to research and development expenses. Subsequent expenditures for capitalized intangible assets are capital- ized only when they substantially increase the future economic benefit of the specific asset to which they relate. All other expenditures are ex- pensed as incurred. Patents Patents obtained by the Group are recorded at acquisition cost less accu- mulated amortization (see below) and any impairment (see Note 2.7.9*). Patent costs are amortized on a straight-line basis over the lower of the estimated useful life of the patent (ten years) or the remaining patent term. Amortization starts when the patent is issued. Technology identi- fied in the purchase price allocation for the acquisition of Sloning Bio- Technology GmbH was recorded at the fair value at the time of acquisi- tion, less accumulated amortization in subsequent measurement (useful life of 10 years). * cross-reference to page 125 Licenses The Group has acquired license rights from third parties by making up- front license payments, paying annual fees to maintain the license and paying fees for sublicenses. The Group amortizes upfront license pay- ments on a straight-line basis over the estimated useful life of the ac- quired license (8 to 13 years). The amortization period and method are reviewed at the end of each financial year. Sublicense fees are amortized on a straight-line basis over the term of the contract or the estimated useful life of the collaboration for contracts without a set duration. Licenses for Marketed Products The balance sheet item contains prepaid license fees and milestone pay- ments for Monjuvi that are subsequently paid after the milestones have been reached. The Group amortizes those payments over the estimated useful life of the acquired license. The duration and method of amortiza- tion are reviewed at the end of each financial year. In the case of trigger- ing events, the asset is tested for any impairment. Because the Group applies the cost accumulation approach, milestones in the near future are not taken into account. In-Process R&D Programs This line item contains capitalized payments from the in-licensing of compounds, as well as milestone payments for these compounds subse- quently paid as milestones were achieved. Additionally, intangible assets identified in a business combination are included in this balance sheet item. No market approvals have been granted for those compounds. Internally Generated Intangible Assets In 2021, certain development costs related to tafasitamab and Monjuvi have been capitalized as internally generated intangible assets for the first time, as the recognition criteria, as stated above, are met. The devel- opment of these assets is currently not yet completed and therefore they are not yet subject to amortization. Until the development activities are completed, the capitalized assets will undergo an annual impairment test. Software Software is recorded at acquisition cost less accumulated amortization (see below) and any impairment (see Note 2.7.9*). Amortization is recog- nized in profit or loss on a straight-line basis over the estimated useful life of three to five years. Software is amortized from the date the soft- ware is operational. * cross-reference to page 125 Intangible Asset Class Useful Life Amortization Rates Patents 10 years 10% Licenses and Licenses for Marketed Products 8 – 24 years 13% – 4% In-process R&D Programs and Internally Generated Intangible Assets Not yet amortized, Impairment Only — Software 3 to 5 years 33% – 20% 2.7.8 Goodwill Goodwill is recognized from business combinations. Goodwill is tested annually for impairment (see Notes 2.7.9* and 5.11*). * cross-reference to page 125 and page 140 124 Financial Statements Notes

2.7.9 Impairment of Non-Financial Assets The carrying amounts of the Group’s non-financial assets and inventories are reviewed at each reporting date for any indication of impairment. The non-financial asset’s recoverable amount and the inventory’s net realiz- able value are estimated if such indication exists. For goodwill and intan- gible assets that have indefinite useful lives or are not yet available for use, the recoverable amount is estimated at the same time each year or determined on an interim basis, if required. Impairment is recognized if the carrying amount of an asset or the cash-generating unit (CGU) ex- ceeds its estimated recoverable amount. The recoverable amount of an asset or CGU is the greater of its value-in- use or its fair value less the cost of disposal. In assessing value-in-use, the estimated future pre-tax cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assess- ments of the time value of money and the risks specific to the asset or CGU. For the purposes of impairment testing, assets that cannot be tested individually are grouped into the smallest group of assets that generates cash flows from ongoing use that are largely independent of the cash flows of other assets or CGUs. A ceiling test for the operating segment must be carried out for goodwill impairment testing. CGUs that have been allocated goodwill are aggregated so that the Level at which impair- ment testing is performed reflects the lowest Level at which goodwill is monitored for internal reporting purposes. Goodwill acquired in a busi- ness combination may be allocated to groups of CGUs that are expected to benefit from the combination’s synergies. The Group’s corporate assets do not generate separate cash flows and are utilized by more than one CGU. Corporate assets are allocated to CGUs on a reasonable and consistent basis and are tested for impairment as part of the impairment testing of the CGU that was allocated the corporate asset. Impairment losses are recognized in profit or loss. Goodwill impairment cannot be reversed. For all other assets, the impairment recognized in prior periods is assessed on each reporting date for any indications that the losses decreased or no longer exist. Impairment is reversed when there has been a change in the estimates used to determine the recover- able amount. Impairment losses can only be reversed to the extent that the asset’s carrying amount does not exceed the carrying amount net of depreciation or amortization that would have been determined if an im- pairment had not been recognized. 2.7.10 Accounts Payables, Accruals and Provisions Accounts payable are presented in Note 2.7.1* under financial liabilities at amortized cost. * cross-reference to page 121 Accruals are recognized for obligations to third parties arising from past events that are uncertain in their timing or amount. Furthermore, accru- als are only recognized for legal or factual obligations to third parties if the event’s occurrence is more likely than not. Accruals are recognized in the amount required to settle the respective obligation and discounted to the reporting date when the interest effect is material. The amount re- quired to meet the obligation also includes expected price and cost in- creases. The interest portion of the addition to accruals is recorded in the finance result. The measurement of accruals is based on past experience and considers the circumstances in existence on the reporting date. These non-financial liabilities with a maturity of more than one year are discounted to their present value. Provisions mainly include cash-settled share-based payments. 2.7.11 Contract Liabilities Upfront payments from customers for services to be rendered by the Group and revenue that must be recognized over a period of time are de- ferred and measured at the nominal amount of cash received. For current contract liabilities, the corresponding rendering of services and revenue recognition is expected to occur within a twelve-month period following the reporting date. 2.7.12 Tax Liabilities Tax liabilities are recognized and measured at their nominal value. Tax liabilities contain obligations from current taxes, excluding deferred taxes. Liabilities for trade taxes, corporate taxes and similar taxes on in- come are determined based on the taxable income of the consolidated entities less any prepayments made. 2.7.13 Deferred Taxes Deferred tax assets and liabilities are calculated using the liability method, which is commonly used internationally. Under this method, taxes expected to be paid or recovered in subsequent financial years are based on the applicable tax rate at the time of recognition. Deferred tax assets and liabilities are recorded separately in the balance sheet and take into account the future tax effect resulting from tempo- rary differences between carrying amounts in the balance sheet for as- sets and liabilities and tax loss carryforwards. Deferred tax assets are offset against deferred tax liabilities when the taxes are levied by the same taxation authority and their maturity and the entity has a legally enforceable right to offset current tax assets against current tax liabilities. Deferred tax assets and liabilities may not be discounted. Deferred tax assets on loss carryforwards and temporary differences are recognized and measured on the basis of projected future taxable in- come. They are only recognized if sufficient taxable income is available in the future to utilize the deferred tax assets. In assessing the recoverability of deferred tax assets, only the effects on earnings of the reversal of temporary differences arising from deferred tax liabilities, the planned results from operating activities, and possible tax strategies are taken into account. The planned results are based on internal forecasts of the future earnings situation of the respective Group company for the assessment of recoverability in the case of loss carryfor- wards and the long-term planning of the respective company for the as- sessment of recoverability in the case of temporary differences. If there are doubts about the realizability of the loss carryforwards, no corre- sponding deferred tax assets are recognized in individual cases, or de- ferred tax assets already recognized are impaired. The tax deferrals rec- ognized are subject to ongoing reviews of the underlying assumptions. Changes in assumptions or circumstances may necessitate adjustments, which may result in additional tax deferrals or their reversal. Deferred tax assets and liabilities are offset if they relate to the same tax authority, and the right to offset current tax assets and liabilities is legally enforce- able. Deferred tax assets and liabilities are recognized on an undis- counted basis. If the items underlying the temporary differences, or tax expenses and income respectively, are recognized directly in equity, this also applies to the current taxes or deferred tax assets and liabilities at- tributable thereto. Notes Financial Statements 125

2.7.14 Stockholders’ Equity Common Stock Ordinary shares are classified as stockholders’ equity. Incremental costs directly attributable to the issue of ordinary shares are recognized as a deduction from stockholders’ equity. Treasury Stock Repurchases of the Company’s own shares at prices quoted on an ex- change or at market value are recorded in this line item as a deduction from common stock. When common stock recorded as stockholders’ equity is repurchased, the amount of consideration paid, including directly attributable costs, is recognized as a deduction from stockholders’ equity net of taxes and classified as treasury shares. When treasury shares are subsequently sold or reissued, the proceeds are recognized as an increase in stockhold- ers’ equity, and any difference between the proceeds from the transac- tion and the initial acquisition costs is recognized in additional paid-in capital. The allocation of treasury shares to beneficiaries under long-term incen- tive plans (in this case: performance shares) is reflected in this line item based on the set number of shares to be allocated after the expiration of the four-year vesting period (quantity structure) and multiplied by the weighted-average purchase price of the treasury shares (value struc- ture). The adjustment is carried out directly in equity through a reduc- tion in the line item “treasury stock,” which is a deduction from common stock, while simultaneously reducing additional paid-in capital. Further information can be found in Notes 6.2.1* and 6.2.2*. * cross-reference to page 149 and page 150 Additional Paid-In Capital Additional paid-in capital mainly consists of personnel expenses result- ing from the grant of share-based payments, the conversion option of the convertible bonds classified as equity, as well as the proceeds from newly created shares in excess of their nominal value. Other Comprehensive Income Reserve The line item “Other Comprehensive Income Reserve” includes changes in the fair value of equity instruments that are recognized in other com- prehensive income and currency exchange differences that are not recog- nized in profit or loss. Accumulated Deficit The “Accumulated Deficit” line item consists of the Group’s accumulated consolidated net profits/losses. A separate measurement of this item is not made. 3 Business Combination In 2021 Constellation Pharmaceuticals Inc., Cambridge, Massachusetts, USA and its 100% subsidiary Constellation Securities Corp., Cambridge, Massachusetts, USA, (both together “Constellation”) was acquired via a cash tender offer. In this transaction, MorphoSys Development Inc. ac- quired the shares in Constellation. Following the acquisition, Constella- tion Pharmaceuticals Inc. was merged into MorphoSys Development Inc. Constellation Pharmaceuticals Inc. remained from this merger. As a clinical stage biopharmaceutical company, Constellation’s business activities uses its expertise in epigenetics to discover and develop novel therapeutics that address serious unmet medical needs in patients with various forms of cancer. As of the date of the acquisition Constellation does not generate any revenues. The cash tender offer, that started on June 16, 2021, to acquire all out- standing shares of Constellation for US$ 34.00 per share (equivalent to € 28.79) expired at the end of July 14, 2021. A total of 42,811,957 shares with a total value of US$ 1,456 million (equivalent to € 1,233 million) were acquired under this offer by MorphoSys Development Inc. (Dover, Delaware, USA). This represented about 89% of Constellation’s total out- standing 48,094,531 shares. The remaining shares, representing approx- imately 11% of the total outstanding shares, were also acquired after the merger in the context of an automatic squeeze-out procedure on July 15, 2021, at the same price per share in the amount of US$ 34.00 (equivalent to € 28.79). A total purchase price of US$ 1,635.2 million (€ 1,384.7 mil- lion) was paid in cash for the acquisition of the shares. The acquisition of Constellation was financed with the cash inflows re- ceived from Royalty Pharma in the amount of US$ 1,425.0 million (equiv- alent to € 1,206.7 million) as well as with cash and other financial assets from MorphoSys and Constellation. For further information on cash in- flows from Royalty Pharma, refer to Note 5.19*. This transaction had mul- tiple objectives, including the acceleration of the growth strategy and expansion of the clinical pipeline in hematology/oncology. The acquisi- tion date for accounting purposes is July 15, 2021, from which date Con- stellation and its sole subsidiary Constellation Securities Corp. have been fully consolidated into the MorphoSys Group. * cross-reference to page 144 In the period from acquisition to December 31, 2021, Constellation con- tributed revenues of € 0.0 million and a loss of € 60.4 million to the Group’s net loss. This loss does not contain the impairment of goodwill. If the acquisition had occurred on January 1, 2021, the consolidated pro- forma net loss for 2021 would have been € 645.7 million. The pro-forma net loss for 2021 includes the impairment of goodwill. In determining this amount, management assumed that the preliminary fair value ad- justments to the acquired assets at the acquisition date would also have been valid in the event of an acquisition on January 1, 2021. As of July 15, 2021, the acquired assets and liabilities resulting from the acquisition included the following items. in 000’ € Fair Value Cash and Cash Equivalents 178,090 Other Financial Assets 118,909 Property, Plant and Equipment 1,572 In-process R&D Programs 719,399 Deferred Tax Asset 145,900 Prepaid Expenses and Other Assets 10,971 Accounts Payable and Accruals (147,791) Tax Liabilities (33) Deferred Tax Liability (183,878) Fair Value of Identifiable Net Assets and Liabilities 843,139 Goodwill on Acquisition Date 541,561 Consideration Paid 1,384,700 Cash and Cash Equivalents Acquired (178,090) Net Cash Outflow 1,206,610 Business Combination 126 Financial Statements Notes

The goodwill is attributable to several preclinical programs, especially for further indications of the main compounds pelabresib, CPI-0209 as well as other small molecules being in a very early stage of the preclini- cal development. Potential future cash inflows, resulting solely from the commercialization of the drug candidates will be recognized by Constel- lation. Hence, Constellation is expected to benefit from the synergies of the business combination and therefore the Goodwill was allocated to this cash-generating units. Goodwill is not expected to be deductible for income tax purposes. In the context of the acquisition of Constellation directly attributable transaction costs in the amount of € 19.2 million were incurred and ex- pensed as general and administrative in 2021. The following amount of goodwill was recognized as a result of the acqui- sition. in 000’ € Consideration Paid 1,384,700 Fair Value of Identifiable Net Assets and Liabilities 843,139 Goodwill 541,561 The following table shows the effects on goodwill recognized in financial year. in 000’ € Balance as of January 1, 2021 0 Initial Recognition 541,561 Impairment (230,715) Exchange differences 23,108 Balance as of December 31, 2021 333,955 In addition, the following agreements, which are not attributable to the business combination and therefore not part of the purchase price, were concluded: 1. Royalty Purchase and Revenue Participation Agreements with Royalty Pharma The acquisition of Constellation also triggered the enforcement of the royalty purchase agreement and the revenue participation agreement with Royalty Pharma on July 15, 2021. The agreements primarily serve to finance the acquisition of Constellation and to further develop the MorphoSys and Constellation product pipelines. Please refer to section “5.19 Financial liabilities for future payments to Royalty Pharma” for de- tails of the contractual content and accounting effects. * cross-reference to page 144 2. Development Funding Bond Agreement with Royalty Pharma On July 15, 2021, the development funding bond agreement with Royalty Pharma became effective. Under the terms of this agreement, MorphoSys must draw at least US$ 150.0 million (equivalent to € 127.0 million) and can draw down a maximum of US$ 350.0 million (equivalent to € 296.4 million) within one year. Repayment will be made at 2.2 times the amount drawn according to a fixed payment schedule within ten years and nine months after the first drawdown without any repayment in the first two years after a drawdown. To date, no partial amount of the bond has been called. 3. Share-based payment programs for employees Constellation has implemented several share-based employee incentive plans (“Plans”) in previous years. These grant beneficiaries options, stock appreciation rights (“SARs”), restricted stock, restricted stock units and other stock-based awards (“Awards”), depending on the underlying contract. Beneficiaries are “all employees, officers and directors of Con- stellation and consultants to the Company (as defined and interpreted under the Securities Act of 1933, as amended) who are eligible to receive Awards under the Plans. These Plans specify that in the event of certain other events, such as “reorganization events,” the Management Board may grant beneficiaries a cash payment in respect of any Award in ex- change for the termination of the Award. The acquisition of Constellation by MorphoSys is considered to be such a reorganization event within the meaning of the program, which triggered the change-of-control clause. For the allocation of the awards to the beneficiaries, Constellation recog- nized a liability in the closing balance sheet as of July 14, 2021 to the beneficiaries of US$84.9 million (€71.9 million), which was settled in cash in August 2021. 4. Retention and Severance Agreements to certain employees Constellation has offered certain employees in key positions staggered retention bonuses depending on the term in order to bind them to the company at least for a certain period of time. Expenses totaling € 5.7 mil- lion were recognized for this until December 31, 2021 in Constellation’s statement of profit or loss. In contrast, certain employees were offered severance payments in order to achieve an early termination of the em- ployment relationship; expenses totaling € 7.3 million were recognized for this until December 31, 2021. 5. Milestone Payment to the Leukemia & Lymphoma Society (LLS-Milestone) Under a research, development and commercialization agreement en- tered into in 2012 with the Leukemia & Lymphoma Society, New York, U.S.A. (“LLS”), Constellation received research grants in the past for cer- tain research and development activities. The agreement requires Con- stellation to make repayments to LLS upon the achievement of certain milestones. The acquisition of Constellation by MorphoSys meets the re- quirement of such a milestone and triggered a payment to LLS in the amount of US$ 7.4 million (€ 6.3 million). Constellation therefore recog- nized a liability in the closing balance sheet as of July 14, 2021 to LLS. Notes Financial Statements 127

4 Notes to the Profit or Loss State- ment 4.1 Revenues in 000’ € 2021 2020 2019 Product Sales, Net 66,861 22,983 0 Royalties 65,576 42,467 31,788 License Fees 43 236,094 265 Milestone Payments 19,952 4,825 30,470 Service Fees 19,726 21,329 9,232 Other 7,454 0 0 Licenses, Milestones and Other 47,175 262,248 39,967 Total 179,612 327,698 71,755 The following overview shows the Group’s regional distribution of reve- nue on the basis of the customer location: in 000’ € 2021 2020 2019 Germany 0 0 145 Europe and Asia 23,328 8,640 39,322 USA and Canada 156,284 319,058 32,288 Total 179,612 327,698 71,755 The following overview shows the timing of the satisfaction of perfor- mance obligations: in 000’ € 2021 2020 2019 At a Point in Time 179,569 327,438 71,270 Over Time 43 260 485 Total 179,612 327,698 71,755 Of the total revenues generated in 2021, a total of € 85.5 million were recognized from performance obligations that were fulfilled in previous periods and related to milestone payments and royalties (2020: € 47.1 mil- lion; 2019: € 62.0 million). 4.2 Cost of Sales Cost of sales consisted of the following: in 000’ € 2021 2020 2019 Expensed Acquisition or Production Cost of Inventories 12,618 5,564 0 Personnel Expenses 11,630 11,054 3,233 Impairment (+) and Reversals of Impairment (–) on Inventories 0 (9,933) 8,685 Impairment, Amortization and Other Costs of Intangible Assets 7,409 2,251 0 External Services 289 128 49 Depreciation and Other Costs for Infrastructure 221 98 100 Other Costs 28 12 18 Total 32,195 9,174 12,085 Notes to the Profit or Loss Statement 128 Financial Statements Notes
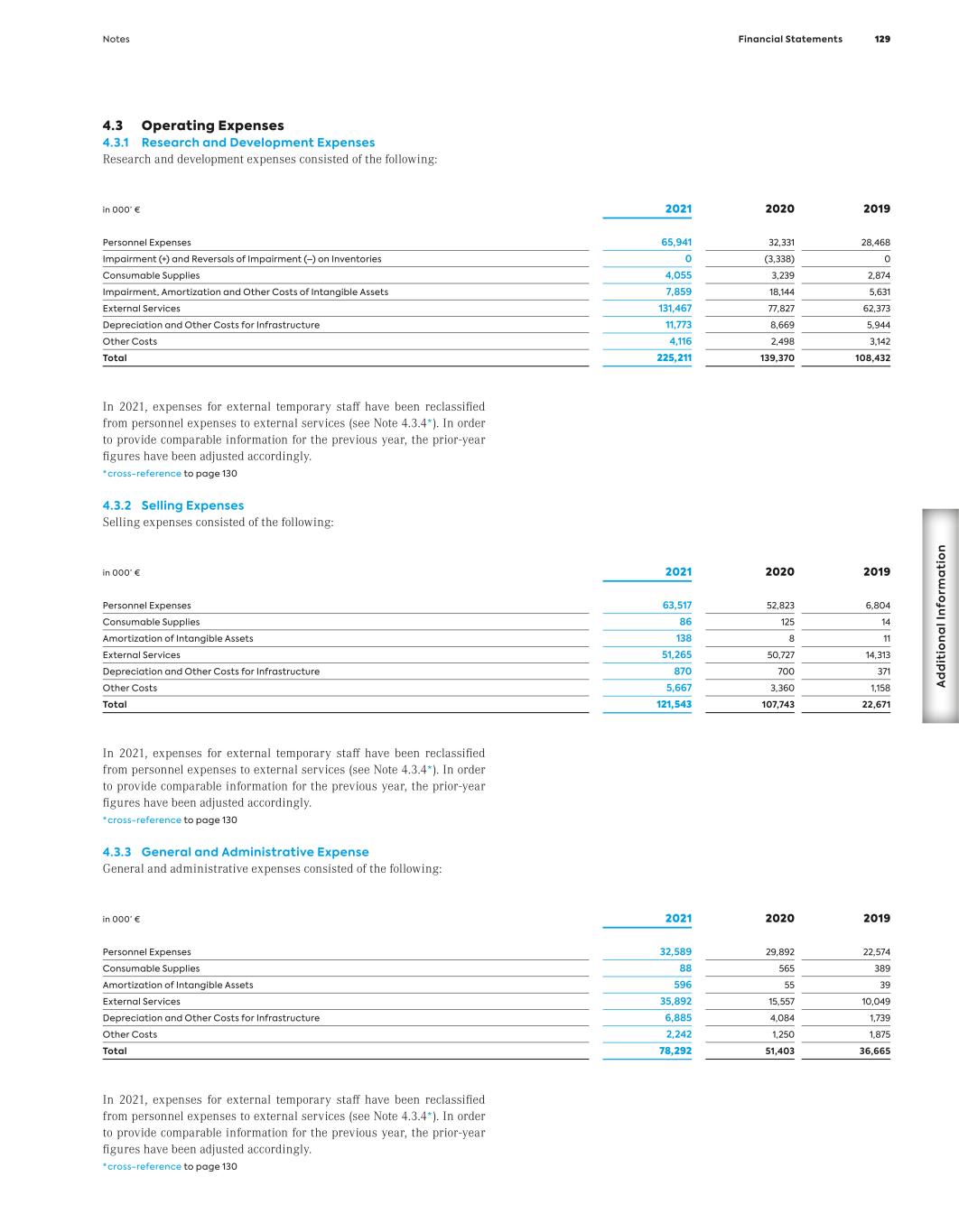
4.3 Operating Expenses 4.3.1 Research and Development Expenses Research and development expenses consisted of the following: in 000’ € 2021 2020 2019 Personnel Expenses 65,941 32,331 28,468 Impairment (+) and Reversals of Impairment (–) on Inventories 0 (3,338) 0 Consumable Supplies 4,055 3,239 2,874 Impairment, Amortization and Other Costs of Intangible Assets 7,859 18,144 5,631 External Services 131,467 77,827 62,373 Depreciation and Other Costs for Infrastructure 11,773 8,669 5,944 Other Costs 4,116 2,498 3,142 Total 225,211 139,370 108,432 In 2021, expenses for external temporary staff have been reclassified from personnel expenses to external services (see Note 4.3.4*). In order to provide comparable information for the previous year, the prior-year figures have been adjusted accordingly. * cross-reference to page 130 4.3.2 Selling Expenses Selling expenses consisted of the following: in 000’ € 2021 2020 2019 Personnel Expenses 63,517 52,823 6,804 Consumable Supplies 86 125 14 Amortization of Intangible Assets 138 8 11 External Services 51,265 50,727 14,313 Depreciation and Other Costs for Infrastructure 870 700 371 Other Costs 5,667 3,360 1,158 Total 121,543 107,743 22,671 In 2021, expenses for external temporary staff have been reclassified from personnel expenses to external services (see Note 4.3.4*). In order to provide comparable information for the previous year, the prior-year figures have been adjusted accordingly. * cross-reference to page 130 4.3.3 General and Administrative Expense General and administrative expenses consisted of the following: in 000’ € 2021 2020 2019 Personnel Expenses 32,589 29,892 22,574 Consumable Supplies 88 565 389 Amortization of Intangible Assets 596 55 39 External Services 35,892 15,557 10,049 Depreciation and Other Costs for Infrastructure 6,885 4,084 1,739 Other Costs 2,242 1,250 1,875 Total 78,292 51,403 36,665 In 2021, expenses for external temporary staff have been reclassified from personnel expenses to external services (see Note 4.3.4*). In order to provide comparable information for the previous year, the prior-year figures have been adjusted accordingly. * cross-reference to page 130 Notes Financial Statements 129
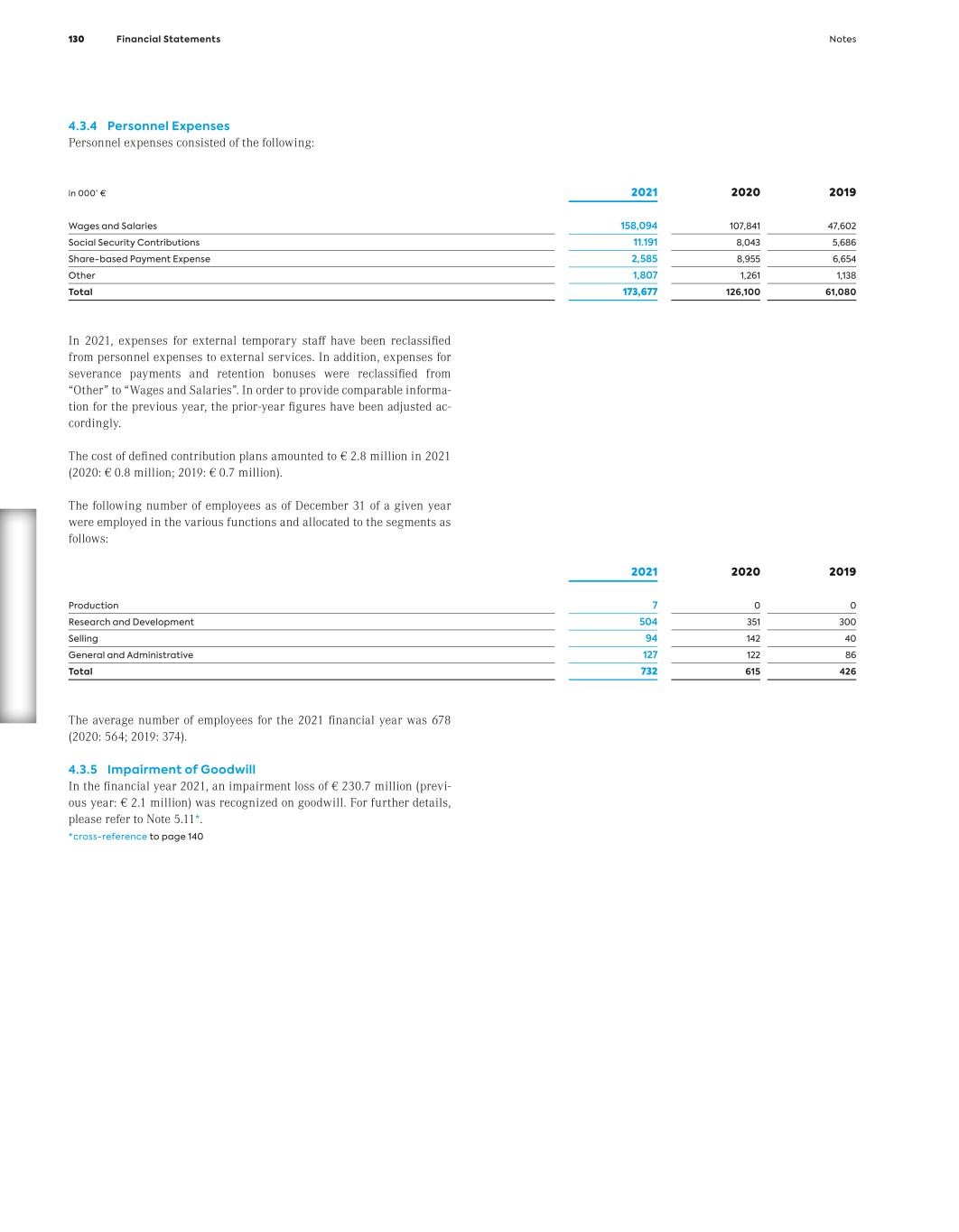
4.3.4 Personnel Expenses Personnel expenses consisted of the following: in 000’ € 2021 2020 2019 Wages and Salaries 158,094 107,841 47,602 Social Security Contributions 11.191 8,043 5,686 Share-based Payment Expense 2,585 8,955 6,654 Other 1,807 1,261 1,138 Total 173,677 126,100 61,080 In 2021, expenses for external temporary staff have been reclassified from personnel expenses to external services. In addition, expenses for severance payments and retention bonuses were reclassified from “Other” to “Wages and Salaries”. In order to provide comparable informa- tion for the previous year, the prior-year figures have been adjusted ac- cordingly. The cost of defined contribution plans amounted to € 2.8 million in 2021 (2020: € 0.8 million; 2019: € 0.7 million). The following number of employees as of December 31 of a given year were employed in the various functions and allocated to the segments as follows: 2021 2020 2019 Production 7 0 0 Research and Development 504 351 300 Selling 94 142 40 General and Administrative 127 122 86 Total 732 615 426 The average number of employees for the 2021 financial year was 678 (2020: 564; 2019: 374). 4.3.5 Impairment of Goodwill In the financial year 2021, an impairment loss of € 230.7 million (previ- ous year: € 2.1 million) was recognized on goodwill. For further details, please refer to Note 5.11*. * cross-reference to page 140 130 Financial Statements Notes

4.4 Other Income and Expenses, Finance Income and Finance Expense The other income is shown in the following overview. in 000’ € 2021 2020 2019 Gain from Deconsolidation of Lanthio Entities 0 379 0 Gain on Foreign Exchange 7,640 13,656 233 Grant Income 5 61 98 Income from Other Items 545 489 474 Other Income 8,190 14,585 805 The other expenses are shown in the following overview. in 000’ € 2021 2020 2019 Loss on Foreign Exchange (5,944) (4,581) (413) Expenses from Other Items (425) (594) (214) Other Expenses (6,369) (5,175) (627) The finance income is shown in the following overview. in 000’ € 2021 2020 2019 Foreign Exchange Gains 18.782 7.160 121 Gains from Measurement at Fair Value 15.231 83.654 2.456 Income from Carrying Amount Adjustments of Financial Liabilities at Amortized cost 61,876 0 0 Interest Income 723 1,233 223 Finance Income 96,612 92,047 2,799 The finance expenses are shown in the following overview. in 000’ € 2021 2020 2019 Foreign Exchange Losses (46,297) (31,694) (777) Losses from Measurement at Fair Value (4,247) (19,313) (442) Effective Interest Expenses from Financial Liabilities at Amortized Cost (62,252) (17,783) 0 Expenses from Carrying Amount Adjustments of Financial Liabilities at Amortized cost (64,846) (24,565) 0 Interest Expenses (2,415) (1,021) (91) Interest Expenses on Lease Liabilities (1,157) (1,174) (932) Bank Fees (242) (664) (31) Finance Expenses (181,456) (96,215) (2,273) Notes Financial Statements 131
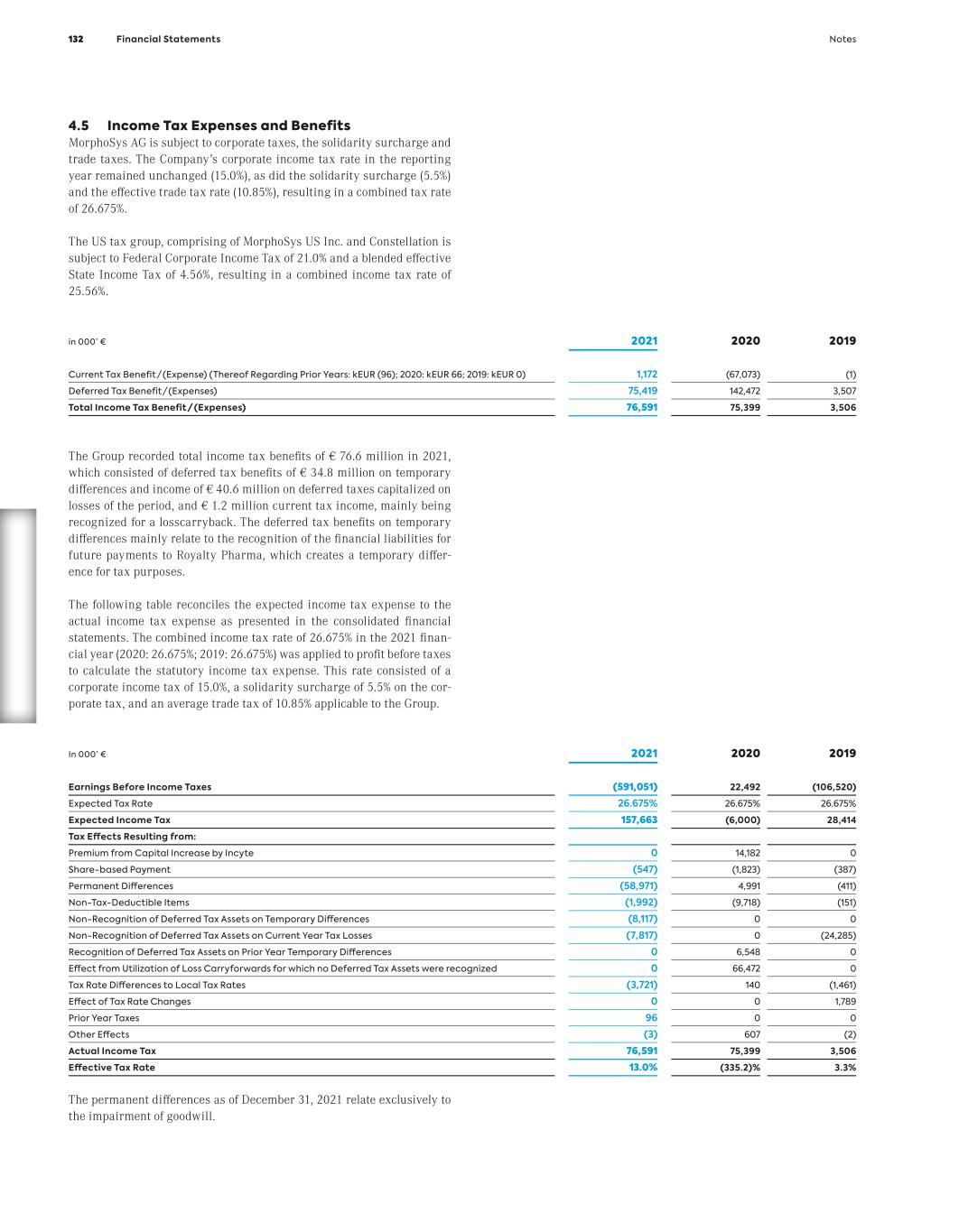
4.5 Income Tax Expenses and Benefits MorphoSys AG is subject to corporate taxes, the solidarity surcharge and trade taxes. The Company’s corporate income tax rate in the reporting year remained unchanged (15.0%), as did the solidarity surcharge (5.5%) and the effective trade tax rate (10.85%), resulting in a combined tax rate of 26.675%. The US tax group, comprising of MorphoSys US Inc. and Constellation is subject to Federal Corporate Income Tax of 21.0% and a blended effective State Income Tax of 4.56%, resulting in a combined income tax rate of 25.56%. in 000’ € 2021 2020 2019 Current Tax Benefit / (Expense) (Thereof Regarding Prior Years: kEUR (96); 2020: kEUR 66; 2019: kEUR 0) 1,172 (67,073) (1) Deferred Tax Benefit / (Expenses) 75,419 142,472 3,507 Total Income Tax Benefit / (Expenses) 76,591 75,399 3,506 The Group recorded total income tax benefits of € 76.6 million in 2021, which consisted of deferred tax benefits of € 34.8 million on temporary differences and income of € 40.6 million on deferred taxes capitalized on losses of the period, and € 1.2 million current tax income, mainly being recognized for a losscarryback. The deferred tax benefits on temporary differences mainly relate to the recognition of the financial liabilities for future payments to Royalty Pharma, which creates a temporary differ- ence for tax purposes. The following table reconciles the expected income tax expense to the actual income tax expense as presented in the consolidated financial statements. The combined income tax rate of 26.675% in the 2021 finan- cial year (2020: 26.675%; 2019: 26.675%) was applied to profit before taxes to calculate the statutory income tax expense. This rate consisted of a corporate income tax of 15.0%, a solidarity surcharge of 5.5% on the cor- porate tax, and an average trade tax of 10.85% applicable to the Group. In 000’ € 2021 2020 2019 Earnings Before Income Taxes (591,051) 22,492 (106,520) Expected Tax Rate 26.675% 26.675% 26.675% Expected Income Tax 157,663 (6,000) 28,414 Tax Effects Resulting from: Premium from Capital Increase by Incyte 0 14,182 0 Share-based Payment (547) (1,823) (387) Permanent Differences (58,971) 4,991 (411) Non-Tax-Deductible Items (1,992) (9,718) (151) Non-Recognition of Deferred Tax Assets on Temporary Differences (8,117) 0 0 Non-Recognition of Deferred Tax Assets on Current Year Tax Losses (7,817) 0 (24,285) Recognition of Deferred Tax Assets on Prior Year Temporary Differences 0 6,548 0 Effect from Utilization of Loss Carryforwards for which no Deferred Tax Assets were recognized 0 66,472 0 Tax Rate Differences to Local Tax Rates (3,721) 140 (1,461) Effect of Tax Rate Changes 0 0 1,789 Prior Year Taxes 96 0 0 Other Effects (3) 607 (2) Actual Income Tax 76,591 75,399 3,506 Effective Tax Rate 13.0% (335.2)% 3.3% The permanent differences as of December 31, 2021 relate exclusively to the impairment of goodwill. 132 Financial Statements Notes

As of December 31, 2021, the deferred tax assets of MorphoSys AG relat- ing to temporary differences as well as tax loss carry forwards created in 2021 have been capitalized. Deferred taxes of MorphoSys AG are capital- ized in full due to the long-term positive business development and the associated positive earnings forecasts of the legal entity. The forecast period is up to 2039 and in line with the accrual period of the financial liability from collaborations, and the respective analysis is based on long- term corporate planning and supports the assessment as strong evidence that the deferred tax assets will be realized. As far as the US tax group companies are concerned, the deferred tax assets relating to temporary differences as well as the tax losses incurred until year end, have been capitalized in the amounts where a future offset with deferred tax liabilities is assured. This takes into account any lim- itations on the offsetting of losses with deferred tax assets and liabilities, insofar the deferred tax liability from the purchase price allocation at acquisition date assures recoverability. For the period after the acquisi- tion date, due to uncertain forecasts, any additional deferred tax asset can only be capitalized to the same extent, namely that sufficient de- ferred tax liabilities assure future recoverability. Due to the history of losses being absorbed from Constellation Level, and the current uncer- tainties regarding the realization of planned taxable income, correspond- ing deferred tax assets on loss carry forwards were only recognized as outlined in the following table. in 000’ € Carry-Forward of Tax Losses Tax Losses from Prior Years 0 Tax Losses absorbed from Constellation 563,697 Tax Losses from Current Year 138,257 Foreign Currency Translation Differences 22,795 Total Tax Losses as of December 31, 2021 724,749 Expected Deferred Tax Assets on Total Tax Losses 186,945 Non-Recognition of Deferred Tax Assets on Current Year Tax Losses (7,817) Deferred Tax Assets on Tax Losses 179,128 The tax losses as of December 31, 2021 include losses of € 69,9 million with a limited utilization period, which relate to the US tax group and forfeit from 2037 until 2043. The deferred tax assets on temporary differences, which have not been capitalized in the period amount to € 8.1 million. Deferred tax assets and deferred tax liabilities consisted of the following: in 000’s €, as of December 31 Deferred Tax Asset 2021 Deferred Tax Asset 2020 Deferred Tax Liability 2021 Deferred Tax Liability 2020 Financial Assets /Liabilities from Collaborations 137,184 137,778 531 5,475 Financial Liabilities from Future Payments to Royalty Pharma 43,611 0 2,092 0 Bonds 507 113 11,260 13,653 Leases 802 824 976 787 Intangible Assets 6,549 8,753 195,371 517 Inventories 2,255 1,328 0 0 Receivables and Other Assets 890 1,099 1,988 211 Property, Plant and Equipment 0 0 108 381 Provisions 5,880 2,581 0 2,723 Other Liabilities 0 0 0 980 Tax Losses 179,128 0 0 0 Offsetting (190,261) (19,670) (190,261) (19,670) Total 186,545 132,806 22,065 5,057 Notes Financial Statements 133
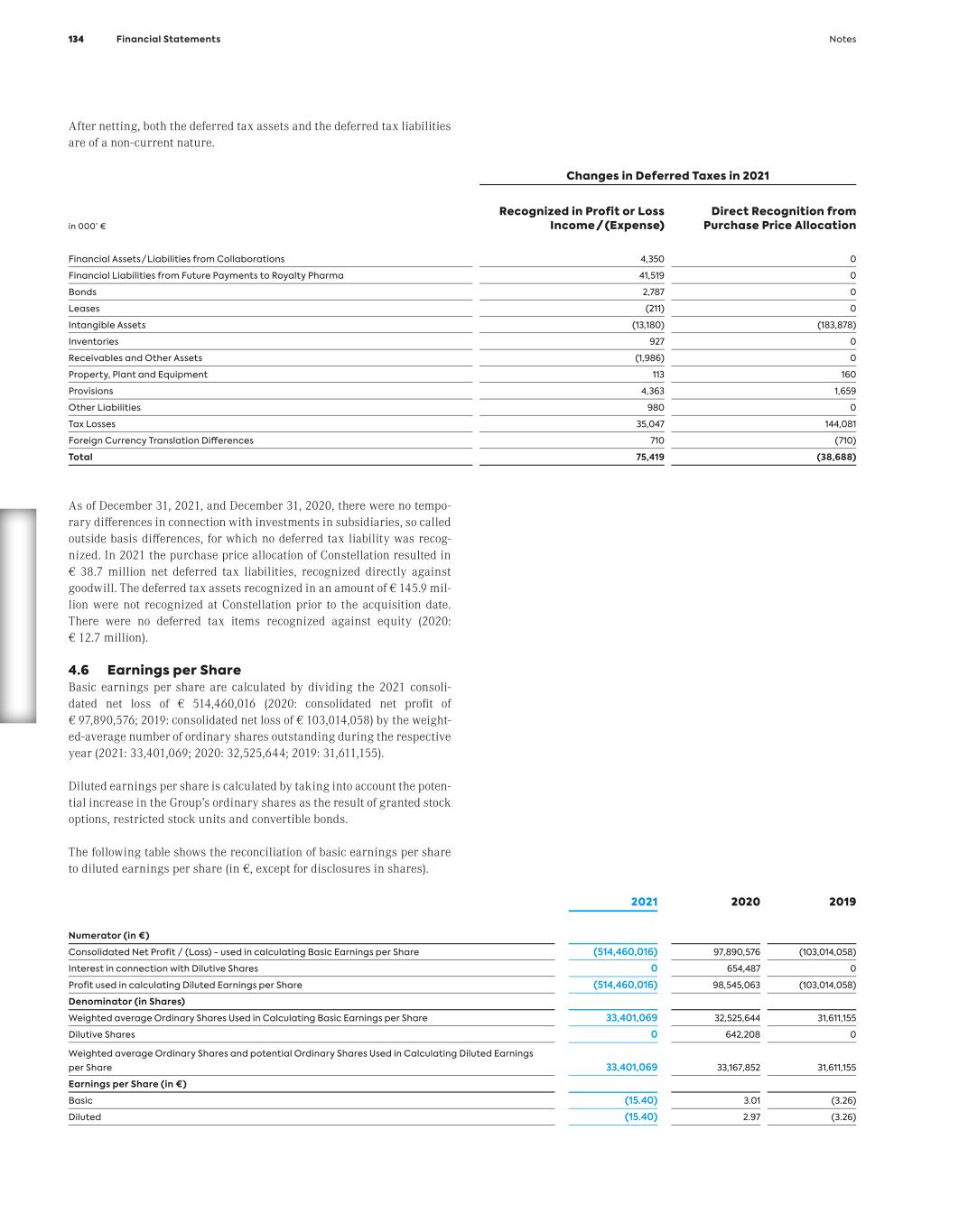
After netting, both the deferred tax assets and the deferred tax liabilities are of a non-current nature. Changes in Deferred Taxes in 2021 in 000’ € Recognized in Profit or Loss Income / (Expense) Direct Recognition from Purchase Price Allocation Financial Assets / Liabilities from Collaborations 4,350 0 Financial Liabilities from Future Payments to Royalty Pharma 41,519 0 Bonds 2,787 0 Leases (211) 0 Intangible Assets (13,180) (183,878) Inventories 927 0 Receivables and Other Assets (1,986) 0 Property, Plant and Equipment 113 160 Provisions 4,363 1,659 Other Liabilities 980 0 Tax Losses 35,047 144,081 Foreign Currency Translation Differences 710 (710) Total 75,419 (38,688) As of December 31, 2021, and December 31, 2020, there were no tempo- rary differences in connection with investments in subsidiaries, so called outside basis differences, for which no deferred tax liability was recog- nized. In 2021 the purchase price allocation of Constellation resulted in € 38.7 million net deferred tax liabilities, recognized directly against goodwill. The deferred tax assets recognized in an amount of € 145.9 mil- lion were not recognized at Constellation prior to the acquisition date. There were no deferred tax items recognized against equity (2020: € 12.7 million). 4.6 Earnings per Share Basic earnings per share are calculated by dividing the 2021 consoli- dated net loss of € 514,460,016 (2020: consolidated net profit of € 97,890,576; 2019: consolidated net loss of € 103,014,058) by the weight- ed-average number of ordinary shares outstanding during the respective year (2021: 33,401,069; 2020: 32,525,644; 2019: 31,611,155). Diluted earnings per share is calculated by taking into account the poten- tial increase in the Group’s ordinary shares as the result of granted stock options, restricted stock units and convertible bonds. The following table shows the reconciliation of basic earnings per share to diluted earnings per share (in €, except for disclosures in shares). 2021 2020 2019 Numerator (in €) Consolidated Net Profit / (Loss) - used in calculating Basic Earnings per Share (514,460,016) 97,890,576 (103,014,058) Interest in connection with Dilutive Shares 0 654,487 0 Profit used in calculating Diluted Earnings per Share (514,460,016) 98,545,063 (103,014,058) Denominator (in Shares) Weighted average Ordinary Shares Used in Calculating Basic Earnings per Share 33,401,069 32,525,644 31,611,155 Dilutive Shares 0 642,208 0 Weighted average Ordinary Shares and potential Ordinary Shares Used in Calculating Diluted Earnings per Share 33,401,069 33,167,852 31,611,155 Earnings per Share (in €) Basic (15.40) 3.01 (3.26) Diluted (15.40) 2.97 (3.26) 134 Financial Statements Notes

The 41,632 stock options and 108,576 restricted stock units still unvested as of December 31, 2021 and the 2,475,437 shares from the convertible bonds are potentially dilutive shares for 2021, but excluded from the cal- culation of dilutive earnings per share as it would result in a decline in the loss per share. 5 Notes to the Balance Sheet 5.1 Cash and Cash Equivalents in 000’ € 12/31/2021 12/31/2020 Bank Balances and Cash in Hand 123,248 109,797 Impairment 0 (2) Cash and Cash Equivalents 123,248 109,795 The presentation of the development of the expected twelve-month loss for cash and cash equivalents can be found in Note 7.4.1*. * cross-reference to page 165 5.2 Other Financial Assets Other Financial Assets include, on the one hand, money market funds classified as FVTPL and term deposits and bonds classified as AC. The financial assets at fair value, with changes recognized in profit or loss, are shown in the following overview. Unrealized in 000’ € Maturity Cost Gross Profit Losses Market Value December 31, 2021 Money Market Funds daily 8,874 1 0 8,875 Total 8,875 December 31, 2020 Money Market Funds daily 288,050 293 (405) 287,938 Total 287,938 Realized and unrealized gains and losses on money market funds were recognized in the finance result in profit or loss. The valuation of money market funds resulted in a net gain of € 0.6 million in 2021 (2020: net loss of € 6.1 million; 2019: net gain of € 0.4 million). The financial assets at amortized cost are shown in the following overview. in 000’ € Maturity Cost Effective Interest Income (+) / Expense (–) Impairment Carrying Amount December 31, 2021 Term Deposits, Current Portion 4 to 12 months 562,369 0 (491) 561,878 Bonds 4 to 12 months 285,144 (2,025) (185) 282,934 Total 844,812 December 31, 2020 Term Deposits, Current Portion 4 to 12 months 649,745 380 (412) 649,713 Bonds more than 12 months 197,827 (652) (587) 196,588 Total 846,301 Notes to the Balance Sheet Notes Financial Statements 135

As of December 31, 2021, these assets mainly consisted of term deposits with fixed or variable interest rates, as well as corporate bonds with fixed interest. Net interest expense from financial assets classified as “at amortized cost” amounted to € 1.7 million in 2021 (2020: € 0.5 million net interest expense; 2019: € 0.1 million net interest income) and was recognized in the finance result. The risk associated with these financial instruments results primarily from bank credit risks. Further information on the credit risk for term deposits and corporate bonds can be found in Note 7.4.1*. * cross-reference to page 165 5.3 Accounts Receivable All accounts receivable are non-interest-bearing and generally have pay- ment terms of between 30 and 180 days. As of December 31, 2021, ac- counts receivable mainly included receivables against Incyte from shared development costs as well as receivables from Monjuvi product sales. As of December 31, 2020, accounts receivable mainly consisted of royalty payments not yet received and receivables against Incyte from shared development costs. The Group‘s single most significant customer Incyte accounted for € 38.5 million of accounts receivables as of December 31, 2021 (Decem- ber 31, 2020: € 50.1 million), or 51% of the Group‘s total accounts receiv- able at the end of 2021 (December 31, 2020: 60%). The table below shows the accounts receivable by region as of the report- ing date. in 000’ € 12/31/2021 12/31/2020 Europe and Asia 6,368 4,452 USA and Canada 69,903 79,326 Impairment (360) (424) Total 75,911 83,354 The presentation of the development of the risk provisions in the 2021 and 2020 financial years for accounts receivable using the simplified im- pairment model can be found in Note 7.4.1*. * cross-reference to page 165 5.4 Other Receivables Other receivables as of December 31, 2021, mainly consisted of receiv- ables from creditors with debit accounts in the amount of € 1.1 million (December 31, 2020: € 1.2 million). As of December 31, 2021 and December 31, 2020, there were no impair- ments recognized on other receivables due to immateriality. 5.5 Inventories Inventories amounted to € 20.8 million as of December 31, 2021 (Decem- ber 31, 2020: € 10.0 million) and consisted of raw materials and supplies (€ 12.1 million; December 31, 2020: € 5.3 million), unfinished goods (€ 4.1 million; December 31, 2020: € 0.0 million) and finished goods (€ 4.5 million; December 31, 2020: € 4.7 million). There were no impairment losses to be recognized in 2021 and 2020. The impairment to a net realizable value of zero on antibody material (tafasitamab), which was recognized in cost of sales and research and development expenses in prior periods, was reversed due to the market approval of Monjuvi in 2020. At the time of the reversal tafasitamab was allocated only under inventories. The reversal resulted in a net gain of € 13.3 million in 2020, which was fully attributable to financial year 2019. The reversal of the impairment loss was recognized in cost of sales of € 9.9 million and in research and development expenses of € 3.3 mil- lion. 5.6 Income Tax Receivables As of December 31, 2021, income tax receivables amounted to € 1.1 mil- lion (December 31, 2020: 0.4 million) and consisted of receivables from capital gain taxes withheld. 5.7 Prepaid Expenses and Other Assets The current prepaid expenses and other assets are shown in the follow- ing table. in 000’ € 12/31/2021 12/31/2020 Combination Drugs 15,945 10,003 Receivables due from Tax Authorities from Input Tax Surplus 6,563 3,920 Upfront Fees for External Laboratory Services 1,724 1,210 Upfront Fees for Sublicenses 1,304 777 Other Prepayments 13,787 4,711 Total 39,323 20,621 An impairment of € 3.5 million was recognized on combination drugs in 2021 (December 31, 2020: € 0.5 million). Other prepayments mainly in- clude payments made in advance for maintenance contracts, insurances, sublicenses as well as external laboratory services. The non-current prepaid expenses and other assets are shown in the fol- lowing table. in 000’ € 12/31/2021 12/31/2020 Prepaid Expenses 9,192 183 Other Assets 4,059 1,384 Total 13,251 1,567 The non-current prepaid expenses mainly include prepayments for exter- nal services that will be utilized from 2023 onwards. The Group has classified certain items within other assets as “restricted cash” that is not available for operational purposes of the Group. As of December 31, 2021, the Group had non-current restricted cash of € 3.8 million for rental deposits issued (December 31, 2020: € 1.2 mil- lion). As of December 31, 2021, € 0.2 million were deposited as collateral for credit cards by MorphoSys US Inc. (December 31, 2020: € 0.2 million). 136 Financial Statements Notes
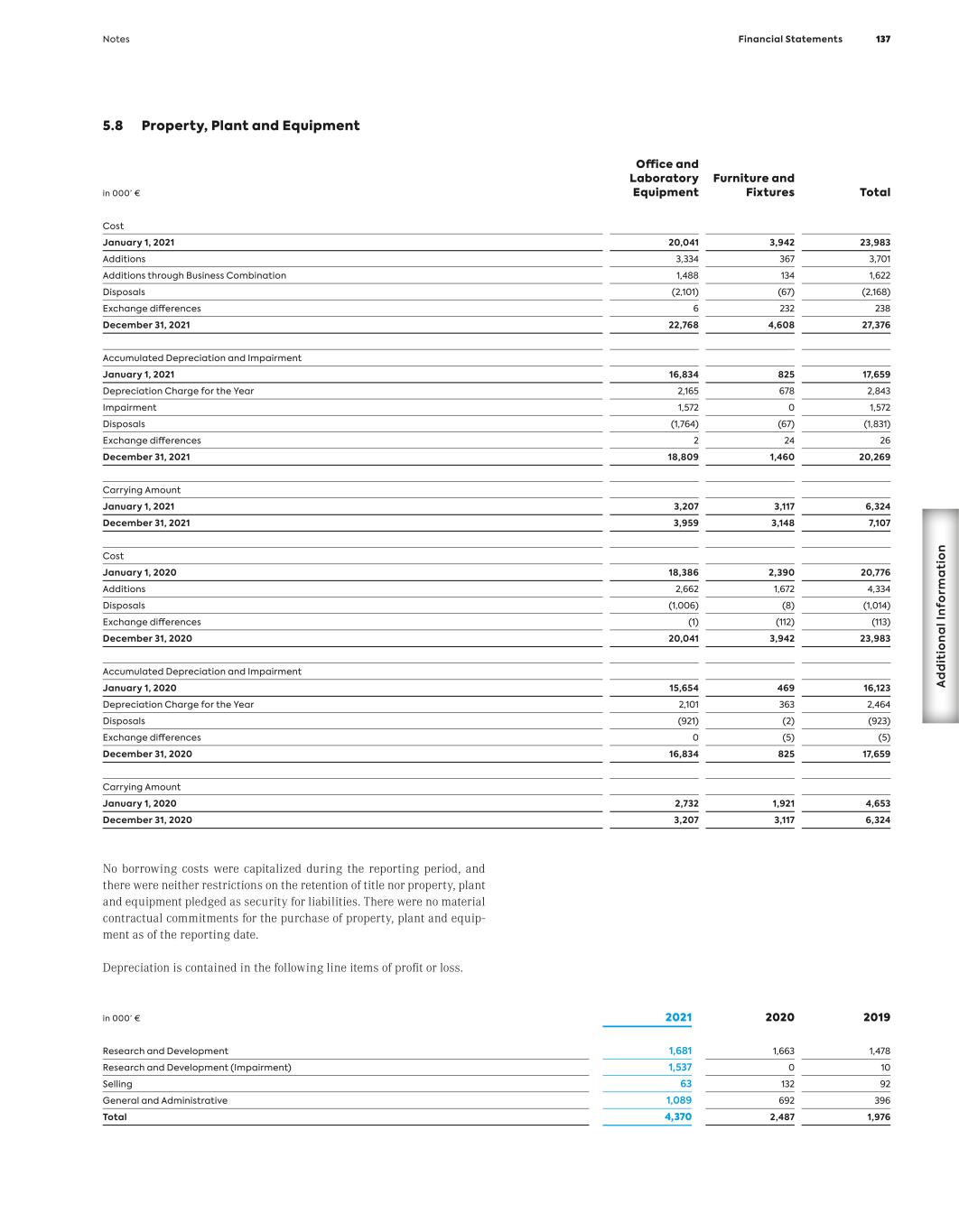
5.8 Property, Plant and Equipment in 000’ € Office and Laboratory Equipment Furniture and Fixtures Total Cost January 1, 2021 20,041 3,942 23,983 Additions 3,334 367 3,701 Additions through Business Combination 1,488 134 1,622 Disposals (2,101) (67) (2,168) Exchange differences 6 232 238 December 31, 2021 22,768 4,608 27,376 Accumulated Depreciation and Impairment January 1, 2021 16,834 825 17,659 Depreciation Charge for the Year 2,165 678 2,843 Impairment 1,572 0 1,572 Disposals (1,764) (67) (1,831) Exchange differences 2 24 26 December 31, 2021 18,809 1,460 20,269 Carrying Amount January 1, 2021 3,207 3,117 6,324 December 31, 2021 3,959 3,148 7,107 Cost January 1, 2020 18,386 2,390 20,776 Additions 2,662 1,672 4,334 Disposals (1,006) (8) (1,014) Exchange differences (1) (112) (113) December 31, 2020 20,041 3,942 23,983 Accumulated Depreciation and Impairment January 1, 2020 15,654 469 16,123 Depreciation Charge for the Year 2,101 363 2,464 Disposals (921) (2) (923) Exchange differences 0 (5) (5) December 31, 2020 16,834 825 17,659 Carrying Amount January 1, 2020 2,732 1,921 4,653 December 31, 2020 3,207 3,117 6,324 No borrowing costs were capitalized during the reporting period, and there were neither restrictions on the retention of title nor property, plant and equipment pledged as security for liabilities. There were no material contractual commitments for the purchase of property, plant and equip- ment as of the reporting date. Depreciation is contained in the following line items of profit or loss. in 000’ € 2021 2020 2019 Research and Development 1,681 1,663 1,478 Research and Development (Impairment) 1,537 0 10 Selling 63 132 92 General and Administrative 1,089 692 396 Total 4,370 2,487 1,976 Notes Financial Statements 137
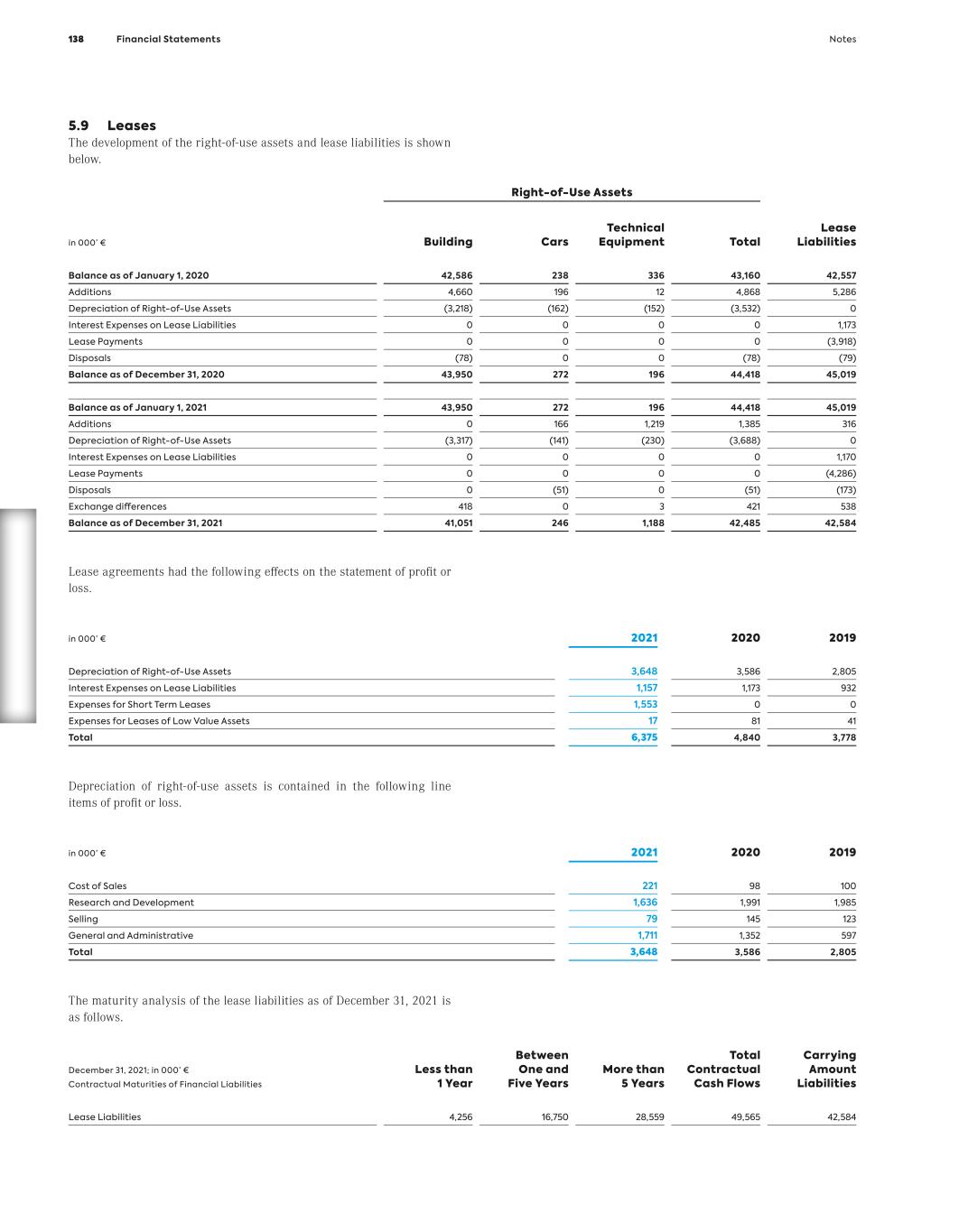
5.9 Leases The development of the right-of-use assets and lease liabilities is shown below. Right-of-Use Assets in 000’ € Building Cars Technical Equipment Total Lease Liabilities Balance as of January 1, 2020 42,586 238 336 43,160 42,557 Additions 4,660 196 12 4,868 5,286 Depreciation of Right-of-Use Assets (3,218) (162) (152) (3,532) 0 Interest Expenses on Lease Liabilities 0 0 0 0 1,173 Lease Payments 0 0 0 0 (3,918) Disposals (78) 0 0 (78) (79) Balance as of December 31, 2020 43,950 272 196 44,418 45,019 Balance as of January 1, 2021 43,950 272 196 44,418 45,019 Additions 0 166 1,219 1,385 316 Depreciation of Right-of-Use Assets (3,317) (141) (230) (3,688) 0 Interest Expenses on Lease Liabilities 0 0 0 0 1,170 Lease Payments 0 0 0 0 (4,286) Disposals 0 (51) 0 (51) (173) Exchange differences 418 0 3 421 538 Balance as of December 31, 2021 41,051 246 1,188 42,485 42,584 Lease agreements had the following effects on the statement of profit or loss. in 000’ € 2021 2020 2019 Depreciation of Right-of-Use Assets 3,648 3,586 2,805 Interest Expenses on Lease Liabilities 1,157 1,173 932 Expenses for Short Term Leases 1,553 0 0 Expenses for Leases of Low Value Assets 17 81 41 Total 6,375 4,840 3,778 Depreciation of right-of-use assets is contained in the following line items of profit or loss. in 000’ € 2021 2020 2019 Cost of Sales 221 98 100 Research and Development 1,636 1,991 1,985 Selling 79 145 123 General and Administrative 1,711 1,352 597 Total 3,648 3,586 2,805 The maturity analysis of the lease liabilities as of December 31, 2021 is as follows. December 31, 2021; in 000’ € Contractual Maturities of Financial Liabilities Less than 1 Year Between One and Five Years More than 5 Years Total Contractual Cash Flows Carrying Amount Liabilities Lease Liabilities 4,256 16,750 28,559 49,565 42,584 138 Financial Statements Notes

The rental conditions for leases are negotiated individually and include different terms. Leases are generally concluded for fixed periods but may include extension options. Such contractual conditions offer the Group the greatest possible operational flexibility. In determining the term of the lease, all facts and circumstances are taken into account that provide an economic incentive to exercise extension options. If extension options are exercised with sufficient certainty, they are taken into account when determining the term of the contract. The leases contain fixed and vari- able lease payments linked to an index. 5.10 Intangible Assets in 000’ € Patents Licenses Licenses for Marketed Products In-process R&D Programs Internally Generated Intangible Assets Software Total Cost January 1, 2021 18,214 35,396 56,449 0 0 5,847 115,906 Additions 345 0 0 10,429 11,517 205 22,496 Additions through Business Combination 0 0 0 719,399 0 16 719,415 Disposals (309) (1,000) 0 0 0 (3,447) (4,756) Exchange differences 0 0 0 30,679 0 0 30,679 December 31, 2021 18,250 34,396 56,449 760,507 11,517 2,621 883,740 Accumulated Amortization and Impairment January 1, 2021 16,276 23,560 963 0 0 5,731 46,530 Amortization Charge for the Year 235 986 2,312 0 0 94 3,627 Impairment 2 0 0 0 0 14 16 Disposals (309) (999) 0 0 0 (3,447) (4,755) December 31, 2021 16,204 23,547 3,275 0 0 2,392 45,418 Carrying Amount January 1, 2021 1,938 11,836 55,486 0 0 116 69,376 December 31, 2021 2,046 10,849 53,174 760,507 11,517 229 838,322 Cost January 1, 2020 18,034 23,896 0 52,159 0 5,758 99,847 Additions 290 12,000 0 32,501 0 90 44,881 Disposals (110) (500) 0 (28,211) 0 (1) (28,822) Reclassification 0 0 56,449 (56,449) 0 0 0 December 31, 2020 18,214 35,396 56,449 0 0 5,847 115,906 Accumulated Amortization and Impairment January 1, 2020 15,053 21,546 0 16,475 0 5,651 58,725 Amortization Charge for the Year 990 206 963 0 0 81 2,240 Impairment 233 2,000 0 11,736 0 0 13,969 Disposals 0 (192) 0 (28,211) 0 (1) (28,404) December 31, 2020 16,276 23,560 963 0 0 5,731 46,530 Carrying Amount January 1, 2020 2,981 2,350 0 35,684 0 107 41,122 December 31, 2020 1,938 11,836 55,486 0 0 116 69,376 There were no material contractual commitments for the purchase of in- tangible assets as of the reporting date. Notes Financial Statements 139
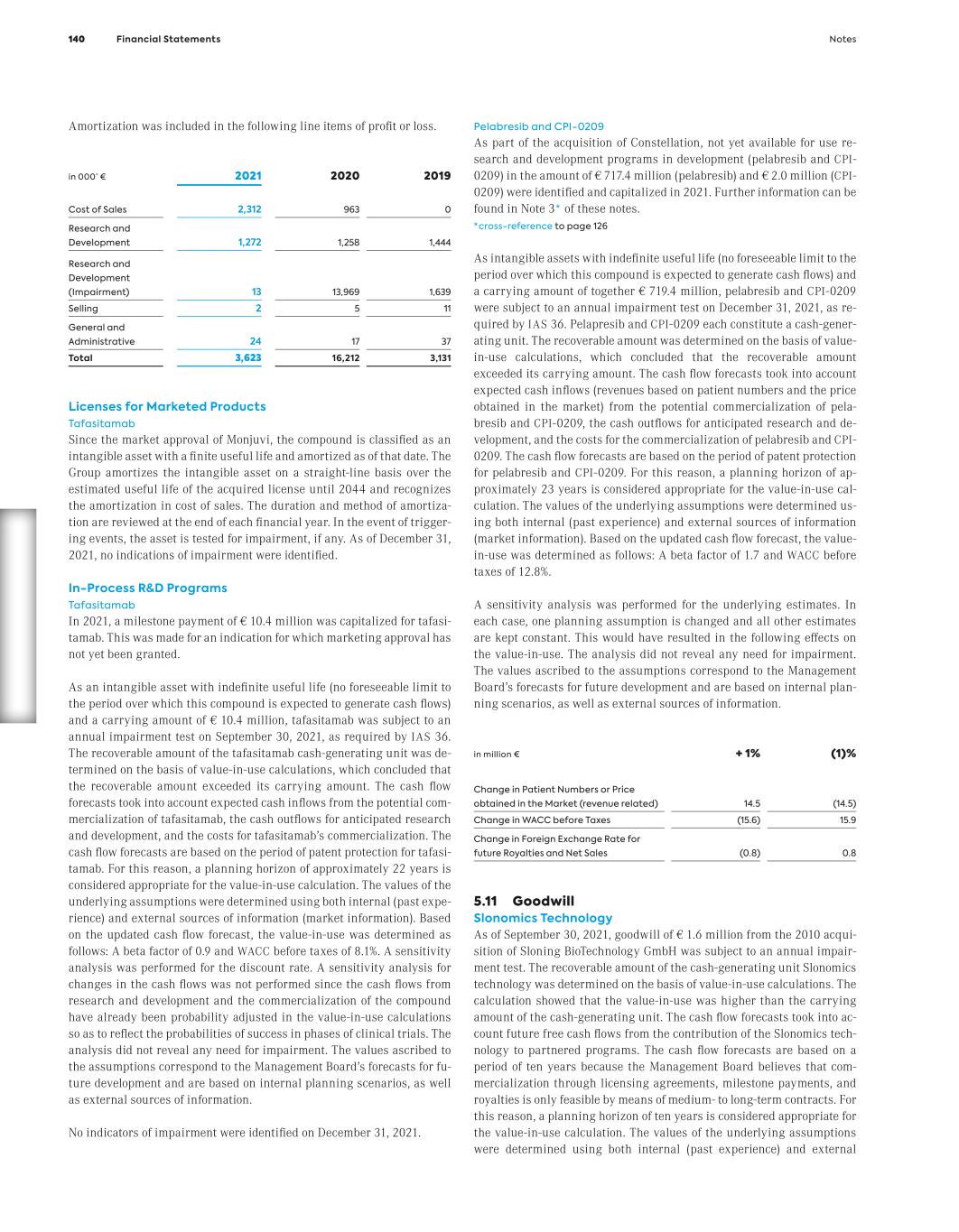
Amortization was included in the following line items of profit or loss. in 000’ € 2021 2020 2019 Cost of Sales 2,312 963 0 Research and Development 1,272 1,258 1,444 Research and Development (Impairment) 13 13,969 1,639 Selling 2 5 11 General and Administrative 24 17 37 Total 3,623 16,212 3,131 Licenses for Marketed Products Tafasitamab Since the market approval of Monjuvi, the compound is classified as an intangible asset with a finite useful life and amortized as of that date. The Group amortizes the intangible asset on a straight-line basis over the estimated useful life of the acquired license until 2044 and recognizes the amortization in cost of sales. The duration and method of amortiza- tion are reviewed at the end of each financial year. In the event of trigger- ing events, the asset is tested for impairment, if any. As of December 31, 2021, no indications of impairment were identified. In-Process R&D Programs Tafasitamab In 2021, a milestone payment of € 10.4 million was capitalized for tafasi- tamab. This was made for an indication for which marketing approval has not yet been granted. As an intangible asset with indefinite useful life (no foreseeable limit to the period over which this compound is expected to generate cash flows) and a carrying amount of € 10.4 million, tafasitamab was subject to an annual impairment test on September 30, 2021, as required by IAS 36. The recoverable amount of the tafasitamab cash-generating unit was de- termined on the basis of value-in-use calculations, which concluded that the recoverable amount exceeded its carrying amount. The cash flow forecasts took into account expected cash inflows from the potential com- mercialization of tafasitamab, the cash outflows for anticipated research and development, and the costs for tafasitamab’s commercialization. The cash flow forecasts are based on the period of patent protection for tafasi- tamab. For this reason, a planning horizon of approximately 22 years is considered appropriate for the value-in-use calculation. The values of the underlying assumptions were determined using both internal (past expe- rience) and external sources of information (market information). Based on the updated cash flow forecast, the value-in-use was determined as follows: A beta factor of 0.9 and WACC before taxes of 8.1%. A sensitivity analysis was performed for the discount rate. A sensitivity analysis for changes in the cash flows was not performed since the cash flows from research and development and the commercialization of the compound have already been probability adjusted in the value-in-use calculations so as to reflect the probabilities of success in phases of clinical trials. The analysis did not reveal any need for impairment. The values ascribed to the assumptions correspond to the Management Board’s forecasts for fu- ture development and are based on internal planning scenarios, as well as external sources of information. No indicators of impairment were identified on December 31, 2021. Pelabresib and CPI-0209 As part of the acquisition of Constellation, not yet available for use re- search and development programs in development (pelabresib and CPI- 0209) in the amount of € 717.4 million (pelabresib) and € 2.0 million (CPI- 0209) were identified and capitalized in 2021. Further information can be found in Note 3* of these notes. * cross-reference to page 126 As intangible assets with indefinite useful life (no foreseeable limit to the period over which this compound is expected to generate cash flows) and a carrying amount of together € 719.4 million, pelabresib and CPI-0209 were subject to an annual impairment test on December 31, 2021, as re- quired by IAS 36. Pelapresib and CPI-0209 each constitute a cash-gener- ating unit. The recoverable amount was determined on the basis of value- in-use calculations, which concluded that the recoverable amount exceeded its carrying amount. The cash flow forecasts took into account expected cash inflows (revenues based on patient numbers and the price obtained in the market) from the potential commercialization of pela- bresib and CPI-0209, the cash outflows for anticipated research and de- velopment, and the costs for the commercialization of pelabresib and CPI- 0209. The cash flow forecasts are based on the period of patent protection for pelabresib and CPI-0209. For this reason, a planning horizon of ap- proximately 23 years is considered appropriate for the value-in-use cal- culation. The values of the underlying assumptions were determined us- ing both internal (past experience) and external sources of information (market information). Based on the updated cash flow forecast, the value- in-use was determined as follows: A beta factor of 1.7 and WACC before taxes of 12.8%. A sensitivity analysis was performed for the underlying estimates. In each case, one planning assumption is changed and all other estimates are kept constant. This would have resulted in the following effects on the value-in-use. The analysis did not reveal any need for impairment. The values ascribed to the assumptions correspond to the Management Board’s forecasts for future development and are based on internal plan- ning scenarios, as well as external sources of information. in million € + 1% (1)% Change in Patient Numbers or Price obtained in the Market (revenue related) 14.5 (14.5) Change in WACC before Taxes (15.6) 15.9 Change in Foreign Exchange Rate for future Royalties and Net Sales (0.8) 0.8 5.11 Goodwill Slonomics Technology As of September 30, 2021, goodwill of € 1.6 million from the 2010 acqui- sition of Sloning BioTechnology GmbH was subject to an annual impair- ment test. The recoverable amount of the cash-generating unit Slonomics technology was determined on the basis of value-in-use calculations. The calculation showed that the value-in-use was higher than the carrying amount of the cash-generating unit. The cash flow forecasts took into ac- count future free cash flows from the contribution of the Slonomics tech- nology to partnered programs. The cash flow forecasts are based on a period of ten years because the Management Board believes that com- mercialization through licensing agreements, milestone payments, and royalties is only feasible by means of medium- to long-term contracts. For this reason, a planning horizon of ten years is considered appropriate for the value-in-use calculation. The values of the underlying assumptions were determined using both internal (past experience) and external 140 Financial Statements Notes

sources of information (market information). Based on the updated ten- year cash flow forecast, the value-in-use was determined as follows: A beta factor of 0.9 (2020: 0.9), WACC before taxes of 8.5% (2020: 8.5%) and a perpetual growth rate of 1.0% (2020: 0.9%). A sensitivity analysis was performed for the growth rate and the discount rate for calculating value- in-use. The sensitivity analysis took into account the change in one as- sumption, with the remaining assumptions remaining unchanged from the original calculation. A change in the pre-tax WACC of +1.0% would cause a € 0.2 million lower value-in-use of goodwill and an impairment by this amount would be necessary. A sensitivity analysis for changes in the cash flows has not been performed since the cash flows have already been probability-adjusted in the value-in-use calculations so as to reflect the probabilities of success in phases of clinical trials. This analysis did not reveal any need for impairment. The values ascribed to the assump- tions correspond to the Management Board’s forecasts for future develop- ment and are based on internal planning scenarios as well as external sources of information. No indication of impairment was identified as of December 31, 2021. Constellation As of December 31, 2021, goodwill of €564.7 million from the acquisition of Constellation was subject to an impairment test. Goodwill was allo- cated to the group of cash-generating units Constellation, as goodwill is monitored at this level. In addition, future potential cash flows of this group of cash-generating units will only be generated by Constellation’s own compounds, which are also recognized by these companies. MorphoSys decided in the last quarter of the reporting year 2021 to focus its research efforts on the most advanced discovery and technology pro- grams and to centralize all laboratory activities at its German research hub in Planegg, Germany. Consequently, all US-based activities relating to discovery biology and drug discovery departments were abandoned. Therefore, any early pipeline projects cannot be realized anymore and the expected cash flows from these projects will not materialize accord- ingly. Since the early pipeline was part of the goodwill acquired as of July 15, 2021, an impairment test was performed as of December 31, 2021, based on the latest cash flow projections. The recoverable amount of the group of cash-generating units Constella- tion was determined on the basis of value-in-use calculations. The calcu- lation showed that the value-in-use (€ 334.0 million) was lower than the carrying amount of this group of cash-generating units and an impair- ment of € 230.7 million was recognized as a result. The cash flow projec- tions included expected payments from the commercialization of pela- bresib and other compounds, the cash outflows for anticipated research and development, and the costs for pelabresib’s and the other compounds’ commercialization. The cash flow forecasts are based on the period of patent protection for pelabresib and the other compounds. For this rea- son, a planning horizon of approximately 23 years is considered appro- priate for the value-in-use calculation. The values of the underlying as- sumptions were determined using both internal (past experience) and external sources of information (market information). Based on the cash flow forecast, the value-in-use was determined as follows: A beta factor of 1.7 and WACC before taxes of 14.1%. A sensitivity analysis was performed for the underlying estimates. In each case, one planning assumption is changed and all other estimates are kept constant. This would have resulted in lower or higher impair- ment of goodwill. The values ascribed to the assumptions correspond to the Management Board’s forecasts for future development and are based on internal planning scenarios, as well as external sources of information. in million € + 1% (1)% Change in Patient Numbers or Price obtained in the Market 16.6 (16.6) Change in WACC before Taxes (19.1) 19.5 Change in Foreign Exchange Rate for future Royalties and Net Sales (0.8) 0.8 5.12 Deferred Tax Assets The Group recognized deferred tax assets of € 186.5 million in the 2021 financial year (December 31, 2020: € 132.8 million), the increase was mainly due to the capitalization of deferred tax assets on current year tax losses of MorphoSys AG and deferred tax assets on temporary differences on the financial liability from future payments to Royalty Pharma. 5.13 Accounts Payable and Accruals Accounts payable and licenses payable were non-interest-bearing and, under normal circumstances, have payment terms of no more than 30 days. Accounts payable and accruals are listed in the following table. In the fi- nancial reporting 2020, licenses payable were presented separately. These have been included in accounts payable in 2021. The prior year’s presentation of the figures has been adjusted accordingly in order to pro- vide comparable information for the previous years. in 000’ € 12/31/2021 12/31/2020 Accounts Payable 73,787 47,818 Accruals 113,055 79,200 Other Liabilities 1,235 1,536 Total 188,077 128,554 Accruals are shown in the following overview: in 000’ € 12/31/2021 12/31/2020 Accruals for External Laboratory Services 65,026 43,500 Accrued Personnel Expenses for Payments to Employees and Management 29,666 17,320 Accruals for Outstanding Invoices 12,515 15,236 Accruals for Revenue Deductions from Product Sales 1,998 943 Accruals for Legal Fees 169 472 Accruals for Audit Fees and other related Costs 703 683 Accruals for License Payments 2,978 1,046 Total 113,055 79,200 At the Company’s Annual General Meeting in May 2021, Pricewater- houseCoopers GmbH Wirtschaftsprüfungsgesellschaft (PwC GmbH), Munich, was appointed as the auditor. The Supervisory Board engaged PwC GmbH to audit the financial statements. Notes Financial Statements 141

The table below shows the total fees PwC GmbH received. in 000’ € 2021 2020 Audit Fees 2,141 1,561 Fees for Other Assurance Services 116 70 Tax Service Fees 0 0 Other Fees for Other Services 2 2 Total 2,258 1,633 The other assurance services comprised fees in connection with the non-financial group report as well as the audit of the content of the remu- neration report. 5.14 Tax Liabilities and Provisions As of December 31, 2021, the Group recorded tax liabilities and provi- sions of € 4.7 million (December 31, 2020: € 67.3 million). Tax liabilities included primarily provisions for income taxes. Provisions included mainly expenses for share-based payments when these are set- tled by other assets equivalent to the value of a certain number of shares or stock options (“cash settlement”), as well as personnel recruitment measures. The table below shows the development of tax liabilities and current and non-current provisions in the 2021 financial year. in 000’ € 1/1/2021 Additions Utilization Release 12/31/2021 Tax Liabilities 65,728 362 (65,562) 0 528 Provisions, current 0 2,549 0 0 2,549 Provisions, non-current 1,528 494 (445) 0 1,577 Total 67,256 3,405 (66,007) 0 4,654 5.15 Contract Liabilities Contract liabilities related to transaction prices paid by customers that were allocated to unfulfilled performance obligations as of December 31, 2021. It is expected that the realization of current contract liabilities will be in the 2022 financial year and non-current contract liabilities mainly in the 2023 financial year. The changes in this item are shown in the ta- ble below. in 000’ € 2021 2020 Opening Balance 2,616 1,686 Prepayments Received in the Financial Year 4,323 13,430 Revenues Recognized in the Reporting Period that was included in the Contract Liability at the Beginning of the Period (2,544) (1,571) Revenues Recognized for Received Prepayments and Services Performed in the Financial Year (4,142) (10,929) Closing Balance 253 2,616 thereof short-term 224 2,544 thereof long-term 29 72 5.16 Deferred Tax Liabilities As of December 31, 2021, deferred tax liabilities of € 22.1 million were recognized after offsetting (December 31, 2020: € 5.1 million), the in- crease is mainly due to the addition of the net deferred tax liabilities from the purchase price allocation of Constellation. 5.17 Bonds MorphoSys AG placed non-subordinated, unsecured convertible bonds in 2020 for a nominal amount of € 325.0 million, equal to 3,250 bonds with a nominal amount of € 100,000 each, and maturing on October 16, 2025. The convertible bonds were issued at 100% of their nominal amount and carry a coupon of 0.625% p.a. payable semi-annually. The conversion price is € 131.29. The convertible bonds are traded on the Open Market Segment (Freiverkehr) of the Frankfurt Stock Exchange. The convertible bonds are convertible between November 26, 2020 and the fortieth trading day prior to maturity. As of the maturity date, MorphoSys has the right to either pay the full amount in cash or to settle a certain amount through the delivery of shares. The convertible bonds are convertible into approximately 2,475,436 new or existing bearer ordi- nary shares MorphoSys. MorphoSys is entitled to redeem the convertible bonds at any time the market price of MorphoSys shares reaches at least 130% of the then appli- cable conversion price over a period of twenty trading days or when only 142 Financial Statements Notes

20% or less of the original total nominal amount of the convertible bond is still outstanding. Repayment is then made in the amount of the nomi- nal value plus accrued interest. The holders of the convertible bonds have a conditional call right should an investor directly or indirectly acquire at least 30% of the voting rights in MorphoSys (representing a change of control). In the event of such a change of control, each convertible bondholder has the right to call the bonds that have not yet been converted or redeemed. Repayment is then made in the amount of the nominal value plus accrued interest. The conversion right securitized in the convertible bond represents an equity instrument and was recognized in equity for an amount of € 49.2 million net of issuance costs attributable to the equity component. The equity component is not adjusted over time, and the liability compo- nent is classified as a financial liability at amortized cost. As of the date of initial recognition, the liability component amounted to € 270.7 mil- lion after the deduction of issuance costs. The difference between this amount and the nominal value of € 325.0 million is recognized as an in- terest expense over the term of the financial liability using the effective interest method. The early termination rights from MorphoSys (issuer call and clean-up call) and the put option of the convertible bondholders in the case of change of control all represent embedded derivatives that, however, have not been separated in accordance with IFRS 9, as they are considered to be closely related to the base contract. Accordingly, these components are included in the financial liability. There were no bond conversions in 2021 and 2020. 5.18 Financial Assets and Liabilities from Collaborations MorphoSys AG and Incyte Corporation signed a collaboration and license agreement in 2020 for the further global development and commercial- ization of MorphoSys’s proprietary anti-CD19 antibody tafasitamab. Un- der the terms of this agreement, MorphoSys could, among other things, pending on the achievement of certain developmental, regulatory, and commercial milestones, receive milestone payments amounting to up to US$1.1 billion (approximately € 971.2 million). MorphoSys also receives tiered royalties in a mid-teen to mid-twenties percentage of net sales of Monjuvi outside the US. In the US, MorphoSys and Incyte co-commercial- ize Monjuvi, with MorphoSys being responsible for the commercial rela- tionship with the end customer, which also comprises the deliveries of the drug and the collection of the related cash inflows. The revenues from product sales of Monjuvi are, therefore, recognized by MorphoSys, as it is the principal of the transaction. Incyte and MorphoSys are jointly respon- sible for the commercialization activities in the US and will equally share any profits and losses (50/50 basis). Outside the US, Incyte has received exclusive commercialization rights, determines the commercialization strategy and is responsible for the commercial relationship with the end customer, including the deliveries of the drug and the collection of the related cash inflows. Therefore, Incyte will recognize all revenues gener- ated from sales of tafasitamab outside the US and will pay royalties to MorphoSys on these sales. As part of the agreement, MorphoSys recorded the balance sheet items “Financial Assets from Collaborations” and “Financial Liabilities from Collaborations”. The financial asset represents MorphoSys’s current re- imbursement claim against Incyte from the expected future losses asso- ciated with the US commercialization activities (as Incyte has agreed to compensate MorphoSys for 50% of said losses) measured at fair value. The non-current financial liability, measured initially at fair value, rep- resents Incyte’s prepaid entitlement to future profit sharing on sales of Monjuvi in the US (as MorphoSys will share 50% of these profits with In- cyte). Incyte has already acquired this right with the payments made in 2020; therefore, a liability had to be recognized at that time. The basis for the initial valuation at fair value is the corporate planning and its shared profits and losses thereof in connection with the commercialization activ- ities of MorphoSys and Incyte in the United States for the years ahead. The financial asset is subsequently measured at fair value through profit or loss and the financial liability at amortized cost using the effective in- terest method. Any resulting effective interest is recognized in the fi- nance result. The basis for the valuation at fair value is the corporate planning and its shared profits and losses thereof in connection with the commercialization activities of MorphoSys and Incyte in the US for the years ahead. Cash flows from the profits and losses shared equally be- tween the two parties are generally recognized directly against the fi- nancial asset or financial liability. Differences between the planned and actual cash flows from the financial asset or financial liability are re- corded in the finance result. Effects resulting from changes in planning estimates regarding the expected net cash flows from financial assets and financial liabilities are also recognized in the finance result. The initial effective interest rate continues to be applied for the subsequent measurement of the financial liability, whereas the current yield curve is used for the financial assets. Foreign currency translation effects from the financial asset or financial liability are also recognized in the finance result. The planning assumptions are influenced by estimates and mainly com- prise revenues and costs for the production and sale of Monjuvi in the US, the discount rate and the expected term of cash flows. Revenues are af- fected by variable influencing factors such as patient numbers and the number of doses of Monjuvi administered, as well as the price that can be obtained in the market. Costs include the manufacturing costs for these doses of Monjuvi and other cost components for e.g. sale, transport, in- surance and packaging. To determine the fair value of financial assets from collaborations, expected cash inflows from Incyte‘s planned losses resulting from the co-promotion activities of Monjuvi in the USA are dis- counted using market interest rates of financial instruments with compa- rable currencies and maturities, taking into account Incyte‘s credit risk. The expected cash outflows are discounted using market interest rates of financial instruments with comparable currencies and maturities, taking into account the credit risk of MorphoSys The term is the estimated time period over which Monjuvi will generate benefits in the approved indica- tion and therefore the expected term of product sales in the US. These estimates are based on assumptions that are jointly arrived at and ap- proved quarterly by the responsible departments at MorphoSys and In- cyte. Financial assets and financial liabilities from collaborations are furthermore subject to significant uncertainties from currency exchange rate developments. As of December 31, 2021, US$ 18.9 million (€ 16.7 million) were recog- nized as a current financial asset and US$ 1.2 million (€ 1.1 million) as a current and US$ 581.3 million (€ 513.3 million) as a non-current financial liability as result of the collaboration with Incyte. MorphoSys and Incyte will also share the development costs for the jointly initiated worldwide and US-specific clinical trials at a ratio of 55% (Incyte) to 45% (MorphoSys). This 45% share of development costs borne by MorphoSys is included in research and development costs. Should MorphoSys provide services in excess of this 45% share, MorphoSys will be entitled to a compensation claim against Incyte, which will qualify as revenue in accordance with IFRS 15. Related expenses for the provision of the service are recognized as cost of sales. Conversely, MorphoSys has to bear additional research and development expenses if Incyte performs more than 55% of the total clinical trial services. In addition, Incyte will Notes Financial Statements 143

assume 100% of future development costs for clinical trials in countries outside the United States, which are conducted in Incyte’s own responsi- bility. Incyte has the option to obtain development services from MorphoSys for this purpose. If this option is exercised, the related in- come will be recognized as revenue. The financial assets from collaborations are measured FVTPL and their measurement is based on the above-mentioned partly unobservable pa- rameters. This results in a fair value classification in the Level 3 mea- surement hierarchy. The assets changed in 2021 as follows: in 000’ € 2021 2020 Balance as of January 1 42,870 0 Additions 0 45,090 Cash Receipts (40,004) (12,677) Through Profit or Loss (in Finance Result) 13,864 10,457 Balance as of December 31 16,730 42,870 If the expected sales revenues and cost components had changed by 1%, the fair value of the financial asset from collaborations would have been in a range of € 16.2 million to € 17.3 million. The estimates underlying the financial liabilities from collaboration are subject to a sensitivity analysis below. This would have resulted in the following effects on the carrying amount of the financial liabilities from collaborations as of December 31, 2021 and 2020. In each case, one plan- ning assumption is changed and all other estimates are kept constant. 12/31/2021 12/31/2020 in million € + 1% (1)% + 1% (1)% Change in Price obtained in the Market (revenue related) 9.7 (9.7) 11.2 (11.2) Change in Patient Numbers and Number of Doses administered (revenue related) 8.7 (8.7) 10.1 (10.1) Change in Manufacturing Costs and other Cost Components (cost related) (4.6) 4.6 (6.2) 6.2 Change in Patient Numbers and Number of Doses administered (cost related) (0.9) 0.9 (1.1) 1.1 5.19 Financial Liabilities from Future Payments to Royalty Pharma The acquisition of Constellation also triggered the enforcement of the royalty purchase agreement and the revenue participation agreement with Royalty Pharma on July 15, 2021. The agreements primarily serve to finance the acquisition of Constellation and to further develop the MorphoSys and Constellation product pipelines. Under the terms of the agreements, Royalty Pharma made a non-refund- able payment of US$ 1,425.0 million (equivalent to € 1,206.7 million) to MorphoSys. In addition, a contingent purchase price payment from Roy- alty Pharma to MorphoSys of up to US$ 100.0 million (€ 84.7 million) was agreed, which is subject to the achievement of certain clinical, regulatory and commercial milestones for otilimab from GSK, gantenumerab from Roche and pelabresib from Constellation. In return, MorphoSys has agreed in the royalty purchase agreement to pass on the following to Royalty Pharma: 100% of MorphoSys’ entitlement since April 1, 2021, for royalties from net sales of Tremfya from Janssen, 80% of future royalties as well as 100% of the future milestone payments for otilimab from GSK and 60% of future royalties for gantenerumab from Roche. Constellation will pass on 3% of future net sales of clinical-stage compounds (pelabresib and CPI-0209) to Royalty Pharma based on the revenue participation agreement. If net sales of pelabresib exceed US$ 30.00 million (€ 25.4 million) in any fiscal year, an additional pur- chase price of US$ 50.00 million (€ 42.3 million) will be due. However, the rights to the underlying intellectual property of pelabresib and CPI- 0209 will remain with MorphoSys. Currently, only Tremfya has market approval and generates royalties from net sales, which are to be passed on to Royalty Pharma. Otilimab, gantenumerab, pelabresib and CPI-0209 are currently in clinical develop- ment and it is uncertain whether MorphoSys will receive royalty or mile- stone payments or generate revenues from them in the future. In addition, Royalty Pharma has agreed to acquire equity in MorphoSys in the amount of up to US$ 100.0 million (€ 84.7 million) or a maximum of 3,289,004 shares. To this end, on July 16, 2021, MorphoSys, by resolu- tion of the Management Board and the Supervisory Board, carried out a capital increase from Authorized Capital 2021-II excluding the subscrip- tion rights of existing shareholders by means of a cash contribution. In the course of the capital increase, 1,337,552 shares (nominal amount € 1,337,552) were newly created on the Frankfurt Stock Exchange, regis- tered in the commercial register on July 29, 2021, and the capital increase became effective on that date. This resulted in an inflow of € 84.7 million 144 Financial Statements Notes

and corresponds to € 63.35 per share. The purchase price of the shares corresponds to the volume-weighted average price of 5 days and was € 6.85 per share above the share price at the time of the resolution. The capital increase thus includes a share premium of € 9.2 million. In con- nection with Royalty Pharma’s participation in MorphoSys’s equity, € 1.3 million (corresponding to a nominal value of € 1 per ordinary share) were recognized in subscribed capital and € 83.3 million (including de- duction of transaction costs) in additional paid-in capital as part of the capital increase. It was agreed with Royalty Pharma that the latter may not sell the acquired shares within one year of acquisition. As the agreed lock-up period is in the interest of both parties, it is assumed that the fair value of the capital contribution corresponds to the amount of cash paid by Royalty Pharma. On July 15, 2021, the development funding bond agreement with Royalty Pharma became effective. Under the terms of this agreement, MorphoSys must draw at least US$ 150.0 million (equivalent to € 127.0 million) and can draw down a maximum of US$ 350.0 million (equivalent to € 296.4 million) within one year. Repayment will be made at 2.2 times the amount drawn according to a fixed payment schedule within ten years and nine months after the first drawdown without any repayment in the first two years after a drawdown. To date, no partial amount of the bond has been called. The payment of US$ 1,425.0 million (€ 1,206.7 million) was recognized as non-current financial liabilities from future payments to Royalty Pharma taking into account directly attributable transaction costs of US$ 0.9 mil- lion (€ 0.8 million). The portion of the liability expected to be due to Roy- alty Pharma within the next 12 months after the balance sheet date is reported as part of current financial liabilities. As all of the agreements with Royalty Pharma described above were entered into on an arm’s length basis, it can be assumed that the consideration paid by Royalty Pharma corresponds in total to the fair value of the liabilities entered into. However, as the implied interest rate on the development funding bond individually is 14% which is higher than the market interest rate of 6.5%, it can be assumed that part of the consideration is to be considered as compensation for the market inequity (off-market component in the amount of the present value of the interest rate differential) on the devel- opment funding bond. Accordingly, the financial liabilities to Royalty Pharma were reduced by US$ 69.0 million (€ 58.4 million), and this amount was allocated to the development funding bond as compensation for the market inequity. The market inequity of the development funding bond is also presented in the balance sheet item “Financial Liabilities from Future Payments to Royalty Pharma”. The off-market component is amortized to interest expense over the term in accordance with the effec- tive interest rate method. As of December 31, 2021 the financial liability from future payments to Royalty Pharma contains a liability from the sale of future royalties as well as revenue participation of € 1,193.3 mil- lion and for the market inequity of the development funding bond of €62.9 million. The financial liabilities are subsequently measured at amortized cost us- ing the effective interest method. The resulting effective interest is rec- ognized in the financial result. As of December 31, 2021, the carrying amount of the current financial liability is € 88.4 million and the carry- ing amount of the non-current financial liability is € 1,167.8 million. The financial liabilities represent the obligation of MorphoSys to forward to Royalty Pharma certain future license income in the form of royalties and milestones of Tremfya, otilimab, gantenerumab and of shares of fu- ture net sales of the product candidates pelabresib and CPI-0209 (as de- scribed above) as well as the market inequity of the contractually agreed minimum amount of the development funding bond. There is no cash in- flow and outflow at MorphoSys, as the agreed royalty percentages and milestones are paid directly by Janssen, GSK and Roche to Royalty Pharma. The initial measurement at fair value was based on corporate planning and the resulting net sales for the coming years. The cash flows from the transfer of assigned license revenues are generally recognized directly against the financial liability. Deviations of the actual cash flows from the original planning are recognized in the financial result. Effects resulting from changes in the planning assumptions regarding the ex- pected net cash flows are also recognized in the financial result. The ini- tial effective interest rate continues to be used for the subsequent mea- surement of the financial liability. Foreign currency translation effects from the financial liabilities are also recognized in the financial result. Royalty revenue from any product sales will continue to be recognized by MorphoSys, which acts as the principal. The planning assumptions are influenced by estimates and mainly relate to the expected revenues from Tremfya, otilimab, gantenerumab, pela- bresib and CPI-0209, the initial discount rate and the expected term of the cash flows. Revenues are influenced by variable factors such as pa- tient numbers and the number of doses administered as well as the price that can be achieved in the market. The term represents the estimated period over which Tremfya in the approved indication and otilimab, gan- tenerumab and pelabresib will generate future cash inflows and there- fore the expected duration of product sales. The above estimates are weighted with an expected probability of obtaining regulatory approval. The cash inflows and outflows represent an estimate of future revenues and costs from the outlicensed products and are subject to a significant degree of judgment. These estimates are based on assumptions that are developed and approved by the responsible departments of MorphoSys on a quarterly basis. The estimates underlying the financial liability are subject to a sensitiv- ity analysis below. This would have resulted in the following effects on the fair value of the financial liabilities upon initial recognition. In each case, one planning assumption is changed and all other estimates are kept constant. in million € + 1% (1)% Change in variable Factors on Revenues 11.0 (11.0) Change in Foreign Exchange Rate for future Royalties and Net Sales (13.8) 14.1 As of December 31, 2021, percentage changes in significant estimates would have impacted the financial liabilities from future payments to Royalty Pharma measured at amortized costs as follows. in million € + 1% (1)% Change in variable Factors on Revenues 11.4 (11.4) Change in Foreign Exchange Rate for future Royalties and Net Sales (14,4) 14.8 5.20 Stockholders’ Equity 5.20.1 Common Stock As of December 31, 2021, the Company’s fully paid common stock, in- cluding treasury shares, amounted to € 34,231,943 and 34,231,943 shares, representing an increase of € 1,341,897 and 1,341,897 shares compared to € 32,890,046 and 32,890,046 shares as of December 31, 2020. Each no-par value share of common stock with a notional value of € 1 is entitled to dividends and grants one vote at the general meeting Notes Financial Statements 145

with the exception of the treasury shares held by the Company. The com- mon stock increased due to Royalty Pharma’s purchase of 1,337,552 shares, created from a capital increase from Authorized Capital 2021-II, as well as from the exercise of 4,345 stock options granted to employees amounting to € 4,345, or 4,345 shares. The weighted-average exercise price of the exercised stock options amounted to € 55.52. 5.20.2 Authorized Capital In comparison to December 31, 2020, the number of authorized ordinary shares decreased from 15,214,050 (€ 15,214,050) to 7,287,025 (€ 7,287,025). At the Annual General Meeting on May 19, 2021, Authorized Capital 2021-I in the amount of 4,861,376, Authorized Capital 2021-II in the amount of 3,289,004 and Authorized Capital 2021-III in the amount of 315,000 were newly created. The remaining Authorized Capital 2018-I in the amount of 11,768,314 and the remaining Authorized Capital 2020-I in the amount of 3,286,539 were canceled at this Annual General Meeting. The number was also reduced by the capital increase of 1,337,552 from the Authorized Capital 2021-II carried out in July 2021 under the agree- ment with Royalty Pharma. Under the Authorized Capital 2021-I, the Management Board is autho- rized, with the consent of the Supervisory Board, to increase the Compa- ny’s share capital on one or several occasions until and including May 18, 2026 against cash and/or non-cash contributions by a total of up to € 4,861,376 by issuing up to 4,861,376 new no-par-value bearer shares. Under the Authorized Capital 2021-II, the Management Board is autho- rized, with the consent of the Supervisory Board, to increase the Compa- ny’s share capital on one or several occasions until and including May 18, 2026 against cash contributions by a total of up to € 3,289,004 by issuing up to 3,289,004 new no-par-value bearer shares. Under the Authorized Capital 2021-III, the Management Board is autho- rized, with the consent of the Supervisory Board, to increase the Compa- ny’s share capital on one or several occasions until and including May 18, 2026 against cash contributions and/or contributions in kind by a total of up to € 315,000 by issuing up to 315,000 new no-par-value bearer shares. Pursuant to the Company’s articles of association, the shareholders may authorize the Management Board to increase the share capital with the consent of the Supervisory Board within a period of five years by issuing shares for a specific total amount referred to as authorized capital (Genehmigtes Kapital), which is a concept under German law that en- ables the company to issue shares without going through the process of obtaining an additional shareholders’ resolution. The aggregate nominal amount of the authorized capital created by the shareholders may not exceed half of the share capital existing at the time of registration of the authorized capital in the commercial register. 5.20.3 Conditional Capital In comparison to December 31, 2020, the number of ordinary shares of conditional capital increased from 7,630,728 (€ 7,630,728) to 7,816,101 (€ 7,816,101). At the Annual General Meeting on May 19, 2021, Condi- tional Capital 2021-I in the amount of 3,289,004 was newly created. In the course of this General Meeting, the Conditional Capital 2008-III in the amount of 13,415, the Conditional Capital 2016-I in the amount of 2,832,099 and the Conditional Capital 2016-III in the mount of 253,772 were reduced. The exercise of 4,345 stock options in 2021 from the Con- ditional Capital 2016-III had an offsetting effect as well. The reduction from the exercise of the 4,345 stock options was entered into the commer- cial register in January 2021. Although shareholders may resolve to amend or create conditional capital (Bedingtes Kapital), they may do so only to issue conversion or subscrip- tion rights to holders of convertible bonds in preparation for a merger with another company or to issue subscription rights to employees and members of the Management Board of the Company or of an affiliated company by way of consent or authorizing resolution. According to Ger- man law, the aggregate nominal amount of the conditional capital created at the shareholders’ meeting may not exceed half of the share capital ex- isting at the time of the shareholders’ meeting adopting such resolution. The aggregate nominal amount of the conditional capital created for the purpose of granting subscription rights to employees and members of the management of our Company or of an affiliated company may not exceed 10% of the share capital existing at the time of the shareholders’ meeting adopting such resolution. 5.20.4 Treasury Stock In the years 2021 and 2020, the Group did not repurchase any of its own shares. The composition and development of this line item are listed in the table below. Number of Shares Value As of 12/31/2018 281,036 10,398,773 Transfer in 2019 (55,236) (2,041,523) As of 12/31/2019 225,800 8,357,250 Transfer in 2020 (94,386) (3,488,506) As of 12/31/2020 131,414 4,868,744 Transfer in 2021 (48,260) (1,783,690) As of 12/31/2021 83,154 3,085,054 On December 31, 2021, the Company held 83,154 treasury shares with a value of 3,085,054€ – a decrease of € 1,783,690 compared to Decem- ber 31, 2020 (131,414 shares, € 4,868,744). The reason for this decrease was the transfer of 45,891 treasury shares amounting to € 1,696,131 to the Management Board and selected employees of the Company (benefi- ciaries) from the 2017 Long-Term Incentive Plan (LTI Plan). The vesting period for this LTI Plan expired on April 1, 2021 and offered beneficiaries a six-month period until October 13, 2021 to receive a total of 45,891 shares. In addition, 2,369 treasury shares for an amount of € 87,558 from the 2019 Long-Term Incentive Plan were transferred to certain employees of MorphoSys US Inc. Consequently, the number of MorphoSys shares owned by the Company as of December 31, 2021, was 83,154 (December 31, 2020: 131,414) and the number of outstanding shares amounted to 34,148,789 (December 31, 2020: 32,758,632). The repurchased shares may be used for all of the purposes named in the authorization granted by the Annual General Meeting on May 23, 2014, particularly for existing and future employee stock option programs and/or to finance acquisitions. The shares may also be redeemed. 5.20.5 Additional Paid-In Capital As of December 31, 2021, the capital reserve amounted to € 833,320,689 (December 31, 2020: € 748,978,506). The increase by a total of € 84,342,183 resulted mainly from the capital increase as a result of the issuance of shares to Royalty Pharma in the amount of € 83,301,053 after deducting transaction costs of € 91,417. Furthermore, the additional paid-in capital increased due to the addition of personnel expenses from share-based payments in the amount of € 2,587,931 and the exercise of stock options in the amount of € 236,889. This was offset by the decrease from reclassifications of treasury shares in connection with the alloca- 146 Financial Statements Notes

tion of shares from the MorphoSys AG 2017 Performance Share Plan in the amount of € 1,696,131 and from the MorphoSys US Inc. 2019 LTI Plan in the amount of € 87,558. 5.20.6 Other Comprehensive Income Reserve On December 31, 2021, this reserve included changes in the fair value of equity instruments of € –27,486 (December 31, 2020: € 1,260,132) recog- nized directly in equity, as well as currency translation differences from consolidation of € 52,785,077 (December 31, 2020: € 2,238,905). The cur- rency translation differences from consolidation included exchange rate differences from the revaluation of the financial statements of Group com- panies prepared in foreign currencies and differences between the ex- change rates used in the balance sheet and income statement. 5.20.7 Accumulated Deficit The consolidated net loss for the year of € 514,460,016 is reported under “accumulated deficit.” As a result, the accumulated deficit increased from € 157,889,210 in 2020 to € 672,349,226 in 2021. 6 Remuneration System for the Management Board and Employees of the Group 6.1 Stock Option Plans 6.1.1 2017 Stock Option Plan On April 1, 2017, MorphoSys established a stock option plan (SOP) for the Management Board and selected employees of the Company (beneficia- ries). The program is considered an equity-settled share-based payment and is accounted for accordingly. The vesting period/performance has ended on March 31, 2021. The performance criteria were set at 110%. Each stock option thus grants 1.1 subscription rights to shares in the Company. The number of subscription rights vested per year were calcu- lated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech In- dex and the TecDAX Index. The exercise price is € 55.52. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2024. Based on the performance criteria achieved, 72,650 stock options can be exercised; this corresponds to 79,935 shares. Of these, the Management Board can exercise 8,197 stock options (9,017 shares), the members of the Executive Committee can exercise 4,018 stock options (4,421 shares) and current and former employees of the Company can exercise 60,435 stock options (66,497 shares). As of December 31, 2021, 3,950 stock options have been exercised, representing 4,345 shares. In 2021, personnel expenses from stock options under the Group’s 2017 SOP amounted to € 2,757 based on the fair value on the grant date (2020: € 62,780; 2019: 252,393). 6.1.2 2018 Stock Option Plan On April 1, 2018, MorphoSys established a stock option plan (SOP) for the Management Board and selected Company employees (beneficiaries). The program is considered an equity-settled share-based payment and is ac- counted for accordingly. The grant date was April 1, 2018, and the vesting period/performance period is 4 years. Each stock option grants up to two subscription rights to shares in the Company. The subscription rights vest each year by 25% within the four-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is cal- culated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech In- dex and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achieve- ment for that year is 0%. The exercise price, derived from the average market price of the Compa- ny’s shares in the XETRA closing auction on the Frankfurt Stock Ex- change from the 30 trading days prior to the issue of the stock options, is € 81.04. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2016-III or by issu- ing treasury shares, or in cash should the exercise from Conditional Cap- ital 2016-III not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is March March 31, 2025. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit with- out entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the 4-year vesting period/performance period, 1/48 of the stock options granted are forfeited for each up to 30 days of absence. A period of ab- sence is defined as absence due to illness, continued payment of remuner- ation in the event of illness or a suspended service or employment rela- tionship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. In 2021, personnel expenses from stock options under the Group’s 2018 SOP amounted to € 52,795 based on the fair value on the grant date (2020: € 251,855; 2019: 704,954). 6.1.3 2019 Stock Option Plan On April 1, 2019, MorphoSys established a stock option plan (SOP) for the Management Board and selected employees of the Company (beneficia- ries). The program is considered an equity-settled share-based payment and is accounted for accordingly. The grant date was April 1, 2019, and the vesting period/performance period is four years. Each stock option grants up to two subscription rights to shares in the Company. The sub- scription rights vest each year by 25% within the four-year vesting pe- riod, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is calculated based on the key performance criteria of the abso- lute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achievement for that year is 0%. Notes Financial Statements 147

The exercise price, derived from the average market price of the Compa- ny’s shares in the XETRA closing auction on the Frankfurt Stock Ex- change from the 30 trading days prior to the issue of the stock options, is € 87.86. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2016-III, issuing treasury shares, or in cash should the exercise from Conditional Capital 2016-III not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2026. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit with- out entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, 1/48 of the stock op- tions granted are forfeited for each up to 30 days of absence. A period of absence is defined as absence due to illness, continued payment of remu- neration in the event of illness or a suspended service or employment relationship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. On October 1, 2019, MorphoSys established a further stock option plan (SOP plan) for one member of the Management Board. The terms and conditions were identical to those of the April 1, 2019 program, and the exercise price was € 106.16. The exercise period is three years after the end of the four-year vesting period/performance period, which is Sep- tember 30, 2023. In 2021, personnel expenses from stock options under the Group’s 2019 SOP amounted to € 625,806 based on the fair value on the grant date (2020: € 1,570,241; 2019: € 1,718,087). 6.1.4 2020 Stock Option Plan On April 1, 2020, MorphoSys established a stock option plan (SOP) for the Management Board and selected employees of the Company (beneficia- ries). The program is considered an equity-settled share-based payment and is accounted for accordingly. The grant date was April 21, 2020, and the vesting period/performance period is four years. Each stock option grants up to two subscription rights to shares in the Company. The sub- scription rights vest each year by 25% within the four-year vesting pe- riod, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is calculated based on the key performance criteria of the abso- lute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achievement for that year is 0%. The exercise price, derived from the average market price of the Compa- ny’s shares in the XETRA closing auction on the Frankfurt Stock Ex- change from the 30 trading days prior to the issue of the stock options, is € 93.66. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2016-III, through the issue of treasury shares, or in cash should the exercise from Condi- tional Capital 2016-III not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2027. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit with- out entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, 1/48 of the stock op- tions granted are forfeited for each up to 30 days of absence. A period of absence is defined as absence due to illness, continued payment of remu- neration in the event of illness or a suspended service or employment relationship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. In 2021, personnel expenses from stock options under the Group’s 2020 SOP amounted to € 1,033,944 based on the fair value on the grant date (2020: € 1,990,326). 148 Financial Statements Notes

The table below shows the development of the stock option plans in the financial year 2021. April 2017 Stock Option Plan April 2018 Stock Option Plan April 2019 Stock Option Plan October 2019 Stock Option Plan April 2020 Stock Option Plan Outstanding on January 1, 2021 72,650 64,255 73,183 57,078 107,042 Granted 0 0 0 0 0 Exercised (4,345) 0 0 0 0 Forfeited 0 (1,109) (3,512) 0 (6,692) Expired 0 0 0 0 0 Outstanding on December 31, 2021 68,305 63,146 69,671 57,078 100,350 Exercisable on December 31, 2021 68,305 0 0 0 0 Weighted-average Exercise Price (€) 55.52 81.04 87.86 106.16 93.66 The fair value of the stock options from the 2018, 2019 and 2020 stock option plans was determined using a Monte Carlo simulation. The ex- pected volatility is based on the development of the share volatility of the last four years. Furthermore, the calculation of fair value equally consid- ered the performance criteria of the absolute and relative performance of MorphoSys shares compared to the development of the Nasdaq Biotech Index and the TecDAX Index. The parameters and fair value of each pro- gram are listed in the table below. April 2018 Stock Option Plan April 2019 Stock Option Plan October 2019 Stock Option Plan April 2020 Stock Option Plan Share Price on Grant Date in € 81.05 85.00 98.10 94.90 Exercise Price in € 81.04 87.86 106.16 93.66 Expected Volatility of the MorphoSys share in % 35.95 37.76 38.02 39.86 Expected Volatility of the Nasdaq Biotech Index in % 25.10 18.61 18.17 25.32 Expected Volatility of the TecDAX Index in % 17.73 26.46 24.82 20.48 Performance Term of Program in Years 4.0 4.0 4.0 4.0 Dividend Yield in % n/a n/a n/a n/a Risk-free Interest Rate in % between 0.02 and 0.15 between 0.02 and 0.13 between 0.0 and 0.02 between (0.55) and (0.83) Fair Value on Grant Date in € 30.43 31.81 35.04 38.20 6.2 Long-Term Incentive Programs 6.2.1 2016 Long-Term Incentive Plan On April 1, 2016, MorphoSys established a Long-Term Incentive Plan (LTI Plan) for the Management Board and certain employees of the Company (beneficiaries). The vesting period for this LTI Plan expired on April 1, 2020. The program is considered an equity-settled share-based payment and is accounted for accordingly. The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the Supervisory Board. The per- formance criteria were based on a mathematical comparison of the abso- lute and relative performance of the MorphoSys share price against the Nasdaq Biotech Index and the TecDAX Index. Achievement of these crite- ria was set at 173.5%. In addition, the Supervisory Board set a “company factor” as 1, which determines the number of performance shares to be issued. Based on these conditions and the set factor, 91,037 performance shares of MorphoSys AG were transferred to the beneficiaries after the four-year vesting period in the period ending October 20, 2020. The Man- agement Board received 13,677 performance shares and the members of the Executive Committee received 8,754 performance shares. A total of 68,606 performance shares were granted to current and former employ- ees of the Company. In 2021, personnel expenses resulting from performance shares under the Group’s 2016 LTI Plan amounted to € 0 based on the fair value on the grant date (2020: € 4,921; 2019: € 141,473). Notes Financial Statements 149

6.2.2 2017 Long-Term Incentive Plan On April 1, 2017, MorphoSys established another Long-Term Incentive Plan (LTI Plan) for the Management Board and selected employees of the Company (beneficiaries). The vesting period for this LTI Plan expired on April 1, 2021. The program is considered an equity-settled share-based payment and is accounted for accordingly. The LTI Plan is a perfor- mance-related share plan and will be paid out in ordinary shares (perfor- mance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the Supervisory Board. The performance criteria were based on a mathematical compari- son of the absolute and relative performance of the MorphoSys share price against the Nasdaq Biotech Index and the TecDAX Index. Achieve- ment of these criteria was set at 130%. In addition, the Supervisory Board set a “company factor” as 1, which determines the number of perfor- mance shares to be issued. Based on these conditions and the set factor, 45,891 performance shares of MorphoSys AG were transferred to the beneficiaries after the four-year vesting period in the period ending Oc- tober 13, 2021. The Management Board received 4,143 performance shares (for further information, see the tables entitled “Shares” and “Per- formance Shares” in Note 6.7* “Related Parties”), and the members of the Executive Committee received 2,030 performance shares. A total of 39,718 performance shares were granted to current and former employ- ees of the Company. * cross-reference to page 157 In 2021, personnel expenses resulting from performance shares under the Group’s 2017 LTI Plan amounted to € 3,530 based on the fair value on the grant date (2020: € 80,383; 2019: € 323,165). 6.2.3 2018 Long-Term Incentive Plan On April 1, 2018, MorphoSys established another Long-Term Incentive Plan (LTI Plan) for the Management Board and selected employees of the Company (beneficiaries). This plan is considered a share-based payment program with settlement in equity instruments and is accounted for ac- cordingly. The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The grant date was April 1, 2018, and the vesting/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance shares become vested in each year of the four- year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The performance criteria can be met annually up to a maximum of 300% and up to 200% for the entire four-year period. If the specified performance criteria are met by less than 0% in one year, no shares will be earned for that year (entitlement). In any case, the maximum payout at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the Level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a specific allocation of performance shares under the LTI Plan, however, occurs only at the end of the four-year vesting/perfor- mance period. At the end of the four-year waiting period, there is a six- month exercise period during which the Company can transfer the per- formance shares to the beneficiaries. The beneficiaries can choose the allocation date within this exercise period. If the number of repurchased shares is not sufficient for servicing the LTI Plan, MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date. In the event of a departure from the Company, the beneficiaries are gen- erally entitled to the performance shares that have vested up to the date of their departure on a pro rata basis. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance shares forfeit without enti- tlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, the beneficiary is enti- tled to performance shares on a pro rata basis. A period of absence is de- fined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship with- out continued payment of remuneration. If a change of control occurs during the four-year vesting period, all per- formance shares will become fully vested. In this case, the right to re- ceive a specific allocation of performance shares under the LTI Plan oc- curs only at the end of the four-year vesting period. In 2021, personnel expenses resulting from performance shares under the Group’s 2019 LTI Plan amounted to € 54,967 based on the fair value on the grant date (2020: € 257,494; 2019: € 720,764). 6.2.4 2019 Long-Term Incentive Plan On April 1, 2019, MorphoSys established another Long-Term Incentive Plan (LTI Plan) for the Management Board and selected employees of the Company (beneficiaries). This plan is considered a share-based payment program with settlement in equity instruments and is accounted for ac- cordingly. The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The grant date was April 1, 2019, and the vesting/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance shares become vested in each year of the four- year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The performance criteria can be met annually up to a maximum of 300% and up to 200% for the entire four-year period. If the specified performance criteria are met by less than 0% in one year, no shares will be earned for that year (entitlement). In any case, the maximum payout at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the Level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a specific allocation of performance shares under the LTI Plan, however, occurs only at the end of the four-year vesting/perfor- mance period. At the end of the four-year vesting period, there is a six- month exercise period during which the Company can transfer the per- formance shares to the beneficiaries. The beneficiaries can choose the allocation date within this exercise period. 150 Financial Statements Notes

If the number of repurchased shares is not sufficient for servicing the LTI Plan, MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date. In the event of a departure from the Company, the beneficiaries are gen- erally entitled to the performance shares that have vested up to the date of their departure on a pro rata basis. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance shares forfeit without enti- tlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, the beneficiary is enti- tled to performance shares on a pro rata basis. A period of absence is de- fined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship with- out continued payment of remuneration. If a change of control occurs during the four-year vesting period, all per- formance shares will become fully vested. In this case, the right to re- ceive a specific allocation of performance shares under the LTI Plan oc- curs only at the end of the four-year vesting period. In 2021, personnel expenses resulting from performance shares under the Group’s 2019 LTI Plan amounted to € 190,767 based on the fair value on the grant date (2020: € 682,162; 2019: € 1,294,974). The table below shows the development of the LTI plans in the financial year 2021. April 2017 Long-Term Incentive Program April 2018 Long-Term Incentive Program April 2019 Long-Term Incentive Program Outstanding on January 1, 2021 29,838 19,371 21,783 Granted 0 0 0 Adjustment due to Performance Criteria 16,053 0 0 Exercised (45,891) 0 0 Forfeited 0 (794) (1,796) Expired 0 0 0 Outstanding on December 31, 2021 0 18,577 19,987 Exercisable on December 31, 2021 0 0 0 Weighted-average Exercise Price (€) n/a n/a n/a The fair value of the performance shares from the Long-Term Incentive Plans from 2018 and 2019 has been determined using a Monte Carlo sim- ulation. The expected volatility is based on the development of the share volatility of the last four years. Furthermore, the calculation of fair value equally considered the performance criteria of the absolute and relative performance of MorphoSys shares compared to the development of the Nasdaq Biotech Index and the TecDAX Index. The parameters and the fair value of each program are listed in the table below. April 2018 Long-Term Incentive Program April 2019 Long-Term Incentive Program Share Price on Grant Date in € 81.05 85.00 Exercise Price in € n/a n/a Expected Volatility of the MorphoSys share in % 35.95 37.76 Expected Volatility of the Nasdaq Biotech Index in % 25.10 18.61 Expected Volatility of the TecDAX Index in % 17.73 26.46 Performance Term of Program in Years 4.0 4.0 Dividend Yield in % n/a n/a Risk-free Interest Rate in % between 0.02 and 0.15 between 0.02 and 0.13 Fair Value on Grant Date in € 103.58 106.85 6.2.5 2020 Performance Share Unit Program On April 1, 2020, MorphoSys established a performance share unit pro- gram (PSU program) for the Management Board and certain employees of the Company (beneficiaries). The program is considered a cash-settled, share-based payment and is accounted for accordingly. The PSU program is a performance-based program and is paid out in cash subject to the fulfillment of predefined performance criteria. The grant date was April 21, 2020; the vesting period/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance share units become vested in each year of the four-year vesting period. The number of performance share units vested per year is calculated on the basis of the performance criteria of the absolute and relative development of the MorphoSys share price com- pared to the development of the Nasdaq Biotech Index and the TecDAX Index. The performance criteria can be met each year up to a maximum of 200%. If the defined performance criteria are met by less than 0% in any one year, no performance share units will be earned for that year. However, the right to receive a certain cash settlement from the PSU pro- gram does not arise until the end of the four-year vesting period/perfor- mance period. After the end of the four-year vesting period, there is a six-month period during which the performance shares can be trans- ferred from the Company to the beneficiaries. MorphoSys reserves the right to settle the PSU program at the end of the vesting period in MorphoSys AG’s own ordinary shares equal to the amount of the performance share units earned. The currently available treasury stock is not sufficient to settle the vested awards. MorphoSys therefore accounts for the plan only as a cash-settled share-based pay- ment. In the event of a departure from the Company, the beneficiaries generally retain the performance share units that have vested by the time of their departure. Notes Financial Statements 151

In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance share units forfeit without entitlement to compensation. If an accumulated period of absence of more than 12 months occurs during the four-year vesting period/performance period, 1/48 of the per- formance share units are forfeited for each month of absence. A period of absence is defined as an absence due to illness or a period of inactive service or employment without continued payment of remuneration. If a change of control occurs during the four-year vesting period, all per- formance share units will become fully vested. In this case, the right to receive a specific allocation of performance share units under the PSU program occurs only at the end of the four-year vesting period. On June 1, 2020, MorphoSys established another performance share unit program (PSU program) for one member of the Management Board. The terms and conditions were identical to those of the April 1, 2020 program. In March 2021, the terms of the Performance Share Unit Programs (PSU Programs) of April 1, 2020 and June 1, 2020 for the Management Board and certain employees of the Company (beneficiaries) were amended so that the number of Performance Share Units still to be vested for the re- maining three years is calculated on the basis of the performance criteria of the absolute performance of the MorphoSys share price and the rela- tive performance of the MorphoSys share price compared to the perfor- mance of the EURO STOXX Total Market Pharmaceuticals & Biotechnol- ogy Index. Previously, the number of performance share units earned in the first year was calculated on the basis of the performance criteria of the absolute and relative performance of the MorphoSys share price com- pared to the performance of the Nasdaq Biotech Index and the TecDAX Index. If the predefined performance criteria for the respective period are 100% met, 25% of the performance share units become vested in the first year, and 75% become vested during the remaining three-year vesting period. The modification of the program’s terms concerns the respective remaining vesting periods/performance periods of the programs for the subsequent three years as of April 1, 2021 and June 1, 2021. The approval of the Management Board and certain employees of the Company (bene- ficiaries) to the modified program terms was obtained by April 17, 2021. The modification of the programs had no material impact on the fair val- ues of the performance shares or on the period over which the personnel expenses are allocated. In 2021, personnel expenses under the Group’s 2020 performance share unit program amounted to –€ 1,083,058 (2020: € 1,166,194). 6.2.6 2021 Performance Share Unit Program On April 1, 2021, MorphoSys established a performance share unit pro- gram (PSU program) for the Management Board and certain employees of the Company (beneficiaries). The program is considered a cash-settled, share-based payment and is accounted for accordingly. The PSU program is a performance-based program and is paid out in cash subject to the fulfillment of predefined performance criteria. The grant date was April 19, 2021; the vesting period/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance share units become vested in each year of the four-year vesting period. The number of performance share units to be vested is calculated on the basis of the performance criteria of the abso- lute share price development of the MorphoSys share, the relative devel- opment of the MorphoSys share price compared to the EURO STOXX Total Market Pharmaceuticals & Biotechnology Index and an assessment of the employee engagement. The performance criteria can be met each year up to a maximum of 200%. If the defined performance criteria are met by less than 0% in any one year, no performance share units will be earned for that year. However, the right to receive a certain cash settlement from the PSU program does not arise until the end of the four-year vesting period/performance period. After the end of the four-year vesting period, there is a six-month period during which the performance shares can be transferred from the Company to the beneficiaries. MorphoSys reserves the right to settle the PSU program at the end of the vesting period in MorphoSys AG’s own ordinary shares equal to the amount of the performance share units earned. The currently available treasury stock is not sufficient to settle the vested awards. MorphoSys therefore accounts for the plan only as a cash-settled share-based pay- ment. In the event of a departure from the Company, the beneficiaries generally retain the performance share units that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance share units forfeit without entitlement to compensation. If an accumulated period of absence of more than 12 months occurs during the four-year vesting period/performance period, 1/48 of the per- formance share units are forfeited for each month of absence. A period of absence is defined as an absence due to illness or a period of inactive service or employment without continued payment of remuneration. If a change of control occurs during the four-year vesting period, all per- formance share units will become fully vested. In this case, the right to receive a specific allocation of performance share units under the PSU program occurs only at the end of the four-year vesting period. As of April 1, 2021, a total of 122,005 performance share units were granted to beneficiaries, consisting of 54,232 performance share units to the Management Board, 12,340 performance share units to other mem- bers of the Executive Committee and 55,433 performance share units to certain employees of the Company who are not members of the Executive Committee. For the calculation of the personnel expenses from share- based compensation, it was assumed for the PSU program 2021 that fif- teen beneficiaries would leave the Company during the four-year period. 152 Financial Statements Notes
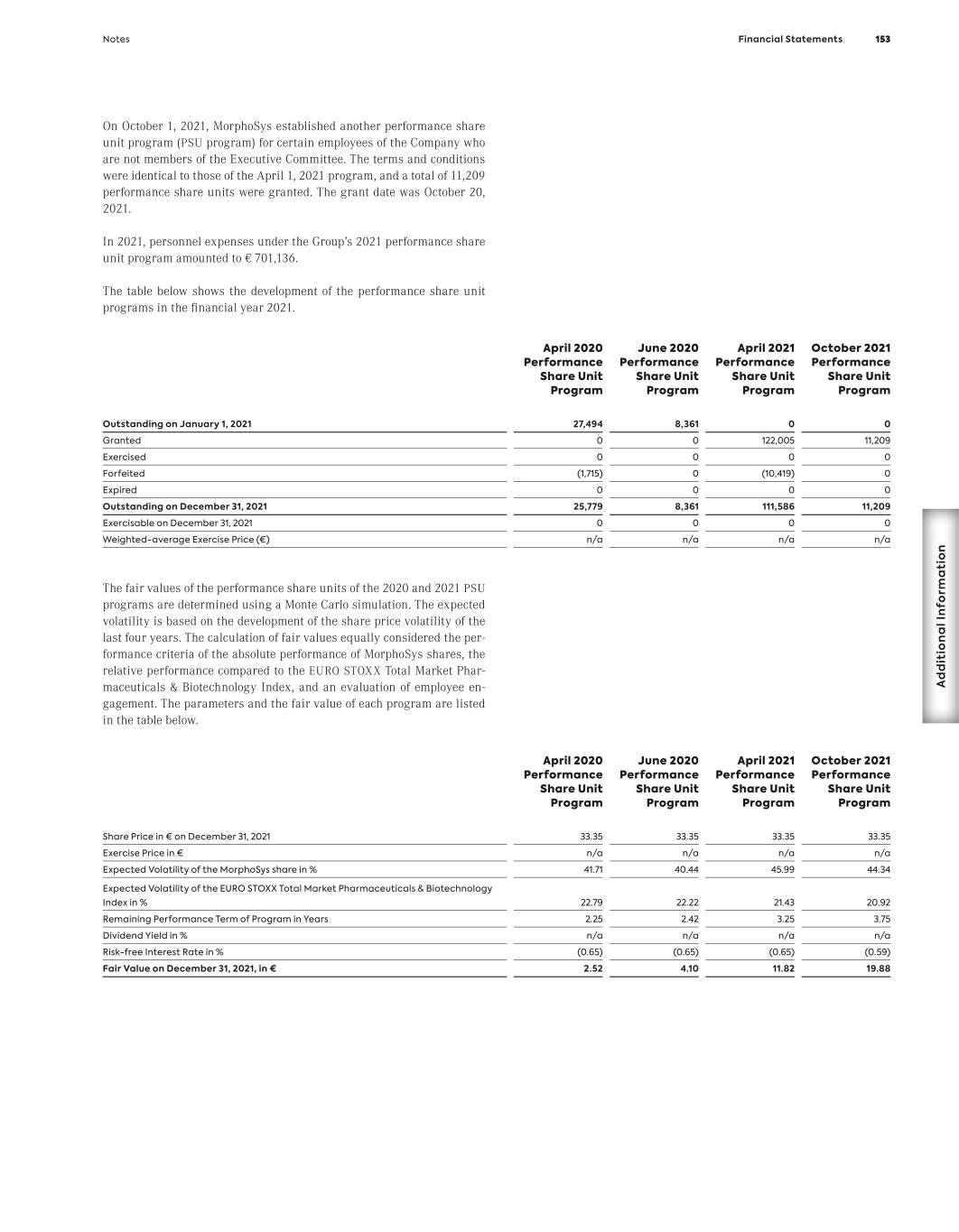
On October 1, 2021, MorphoSys established another performance share unit program (PSU program) for certain employees of the Company who are not members of the Executive Committee. The terms and conditions were identical to those of the April 1, 2021 program, and a total of 11,209 performance share units were granted. The grant date was October 20, 2021. In 2021, personnel expenses under the Group’s 2021 performance share unit program amounted to € 701,136. The table below shows the development of the performance share unit programs in the financial year 2021. April 2020 Performance Share Unit Program June 2020 Performance Share Unit Program April 2021 Performance Share Unit Program October 2021 Performance Share Unit Program Outstanding on January 1, 2021 27,494 8,361 0 0 Granted 0 0 122,005 11,209 Exercised 0 0 0 0 Forfeited (1,715) 0 (10,419) 0 Expired 0 0 0 0 Outstanding on December 31, 2021 25,779 8,361 111,586 11,209 Exercisable on December 31, 2021 0 0 0 0 Weighted-average Exercise Price (€) n/a n/a n/a n/a The fair values of the performance share units of the 2020 and 2021 PSU programs are determined using a Monte Carlo simulation. The expected volatility is based on the development of the share price volatility of the last four years. The calculation of fair values equally considered the per- formance criteria of the absolute performance of MorphoSys shares, the relative performance compared to the EURO STOXX Total Market Phar- maceuticals & Biotechnology Index, and an evaluation of employee en- gagement. The parameters and the fair value of each program are listed in the table below. April 2020 Performance Share Unit Program June 2020 Performance Share Unit Program April 2021 Performance Share Unit Program October 2021 Performance Share Unit Program Share Price in € on December 31, 2021 33.35 33.35 33.35 33.35 Exercise Price in € n/a n/a n/a n/a Expected Volatility of the MorphoSys share in % 41.71 40.44 45.99 44.34 Expected Volatility of the EURO STOXX Total Market Pharmaceuticals & Biotechnology Index in % 22.79 22.22 21.43 20.92 Remaining Performance Term of Program in Years 2.25 2.42 3.25 3.75 Dividend Yield in % n/a n/a n/a n/a Risk-free Interest Rate in % (0.65) (0.65) (0.65) (0.59) Fair Value on December 31, 2021, in € 2.52 4.10 11.82 19.88 Notes Financial Statements 153

6.3 MorphoSys US Inc. – 2019 Long-Term Incentive Program On April 1, 2019, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). This program is considered a share-based payment program with settle- ment in equity instruments and is accounted for accordingly. The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key perfor- mance criteria are achieved. The plan has a term of four years and com- prises four one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 25% of the performance shares become vested in each year. The number of shares vested per year is calculated based on key performance criteria of MorphoSys US Inc. during the annual performance period. The performance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. After the end of each one-year performance period, there is a six-month period during which the performance shares can be trans- ferred from the Company to the beneficiaries. If the number of repurchased shares is not sufficient for servicing the LTI Plan, MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the average market price of one share of the Company in the XETRA closing auction on the Frankfurt Stock Exchange during the 30 trading days pre- ceding the grant of the performance shares. In the event of a departure from the Company, the beneficiaries are gen- erally entitled to the performance shares that have vested up to the date of their departure on a pro rata basis. In the event of termination by a beneficiary for good cause, all perfor- mance shares will be forfeited without entitlement to compensation. After the end of the second one-year performance period, a target achievement of 77% was determined. Taking this target achievement into account, 2,369 performance shares of MorphoSys AG were transferred to the beneficiaries in the period from April 1, 2021 to October 18, 2021. The fair value of the performance shares on December 31, 2021 was € 33.35 per share. In 2021, personnel expenses of the Group from performance shares un- der the MorphoSys US Inc. 2019 LTI Plan amounted to –€ 503,206 based on the fair value on December 31, 2021. (2020: € 38,888; 2019: € 1,076,158). The table below shows the development of the performance shares under the MorphoSys US Inc. 2019 LTI Plan in the financial year 2021. MorphoSys US Inc. – 2019 Long-Term Incentive Program Outstanding on January 1, 2021 9,118 Granted 0 Exercised (2,369) Forfeited (4,041) Expired 0 Outstanding on December 31, 2021 2,708 Exercisable on December 31, 2021 0 Weighted-average Exercise Price (€) n/a 6.4 MorphoSys US Inc. – Restricted Stock Unit Plan (RSUP) 6.4.1 2019 Long-Term Incentive Program On October 1, 2019, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficia- ries). The program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI Plan is a restricted stock unit plan (RSUP) and is paid out in shares of MorphoSys AG that are to be created from authorized capital provided predefined performance criteria have been fulfilled. The term of the plan is three years and includes three one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 33.3% of the performance shares become vested in each year. The number of performance shares vested per year is calculated based on the key performance criteria of MorphoSys US Inc. and the MorphoSys share price performance during the annual performance period. The perfor- mance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. At the end of the total three-year performance period, the corresponding number of shares eventually vested is calcu- lated, and the shares created from authorized capital are transferred from the Company to the beneficiaries. MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash at the end of the performance period, equal to the value of the per- formance shares granted. If a beneficiary loses his office or terminates his employment with MorphoSys US Inc. prior to the end of a performance period, the benefi- ciary will generally be entitled to all vested restricted stock units for al- ready completed one-year performance periods. All remaining restricted stock units are forfeited without entitlement to compensation. The fair values of the performance shares according to the grant dates or measurement dates for each of the three performance periods were € 127.90 per share on December 13, 2019, € 94.14 per share on November 30, 2020, and € 44.63 per share on August 6, 2021. In 2021, personnel expenses of the Group from the MorphoSys US Inc. 2019 RSU Plan amounted to –€ 383,159 based on the fair values (2020: € 600,445; 2019: € 269,415). 154 Financial Statements Notes

6.4.2 2020 Long-Term Incentive Program On April 1, 2020, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). The program is considered a share-based payment program with settle- ment in equity instruments and is accounted for accordingly. The LTI Plan is a restricted stock unit plan (RSUP) and is paid out in shares of MorphoSys AG that are to be created from authorized capital provided predefined performance criteria have been fulfilled. The term of the plan is three years and includes three one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 33.3% of the performance shares become vested in each year. The number of performance shares vested per year is calculated based on the key performance criteria of MorphoSys US Inc. and the MorphoSys share price performance during the annual performance period. The perfor- mance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. At the end of the total three-year performance period, the corresponding number of shares eventually vested is calcu- lated, and the shares created from authorized capital are transferred from the Company to the beneficiaries. MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash at the end of the performance period, equal to the value of the per- formance shares granted. If a beneficiary loses his office or terminates his employment with MorphoSys US Inc. prior to the end of a performance period, the benefi- ciary will generally be entitled to all vested restricted stock units for al- ready completed one-year performance periods. All remaining restricted stock units are forfeited without entitlement to compensation. The fair value of the restricted shares granted on April 1, 2020, in accor- dance with the grant dates or measurement dates for each of the three performance periods were € 94.14 per share on November 30, 2020, € 44.63 per share on August 6, 2021 and € 33.35 per share on Decem- ber 31, 2021 . On October 1, 2020, MorphoSys established an additional Long-Term In- centive Plan in the form of a restricted stock unit plan (RSUP) for certain employees of MorphoSys US Inc. (beneficiaries). The terms and condi- tions were identical to those of the April 1, 2020 program. The fair value of the restricted shares granted on October 1, 2020, in ac- cordance with the grant dates or measurement dates for each of the three performance periods were € 94.14 per share as of November 30, 2020, € 44.63 per share on August 6, 2021 and € 33.35 per share as of Decem- ber 31, 2021. In 2021, personnel expenses of the Group from the MorphoSys US Inc. 2021 RSU Plan amounted to –€ 462,243 based on the fair values (2020: € 1,916,267). 6.4.3 2021 Long-Term Incentive Program On April 1, 2021, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). The program is considered a share-based payment program with settle- ment in equity instruments and is accounted for accordingly. The LTI Plan is a restricted stock unit plan (RSUP) and is paid out in shares of MorphoSys AG that are to be created from authorized capital provided predefined performance criteria have been fulfilled. The term of the plan is three years and includes three one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 33.3% of the performance shares become vested in each year. The number of performance shares vested per year is calculated based on the key performance criteria of MorphoSys US Inc. and the MorphoSys share price performance during the annual performance period. The perfor- mance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. At the end of the total three-year performance period, the corresponding number of shares eventually vested is calcu- lated, and the shares created from authorized capital are transferred from the Company to the beneficiaries. MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash at the end of the performance period, equal to the value of the per- formance shares granted. If a beneficiary loses his office or terminates his employment with MorphoSys US Inc. prior to the end of a performance period, the benefi- ciary will generally be entitled to all vested restricted stock units for al- ready completed one-year performance periods. All remaining restricted stock units are forfeited without entitlement to compensation. As of April 1, 2021, 67,724 restricted shares were granted to the benefi- ciaries. For the calculation of the personnel expenses from share-based compensation, it was assumed for the LTI Plan 2021 that thirty-two ben- eficiaries would leave the Company during the three-year period. The fair value of the restricted shares granted on April 1, 2021, in accor- dance with the grant dates or measurement dates for each of the three performance periods were € 44.63 per share on August 6, 2021 and € 33.35 per share as of December 31, 2021. On October 1, 2021, MorphoSys established an additional Long-Term In- centive Plan in the form of a restricted stock unit plan (RSUP) for certain employees of MorphoSys US Inc. (beneficiaries). The terms and condi- tions were identical to those of the April 1, 2021 program, except that the performance criteria can be met up to a maximum of 175% per year. 36,827 restricted shares were granted. For the calculation of the person- nel expenses from share-based compensation, it was assumed for the 2021 LTI Plan that twenty beneficiaries would leave the Company during the three-year period. The fair value of the restricted shares granted on October 1, 2021, in ac- cordance with the grant dates or measurement dates for each of the three performance periods were € 33.35 per share as of December 31, 2021. In 2021, personnel expenses of the Group from the MorphoSys US Inc. 2021 RSU Plan amounted to € 1,260,750.00 based on the fair values. Notes Financial Statements 155
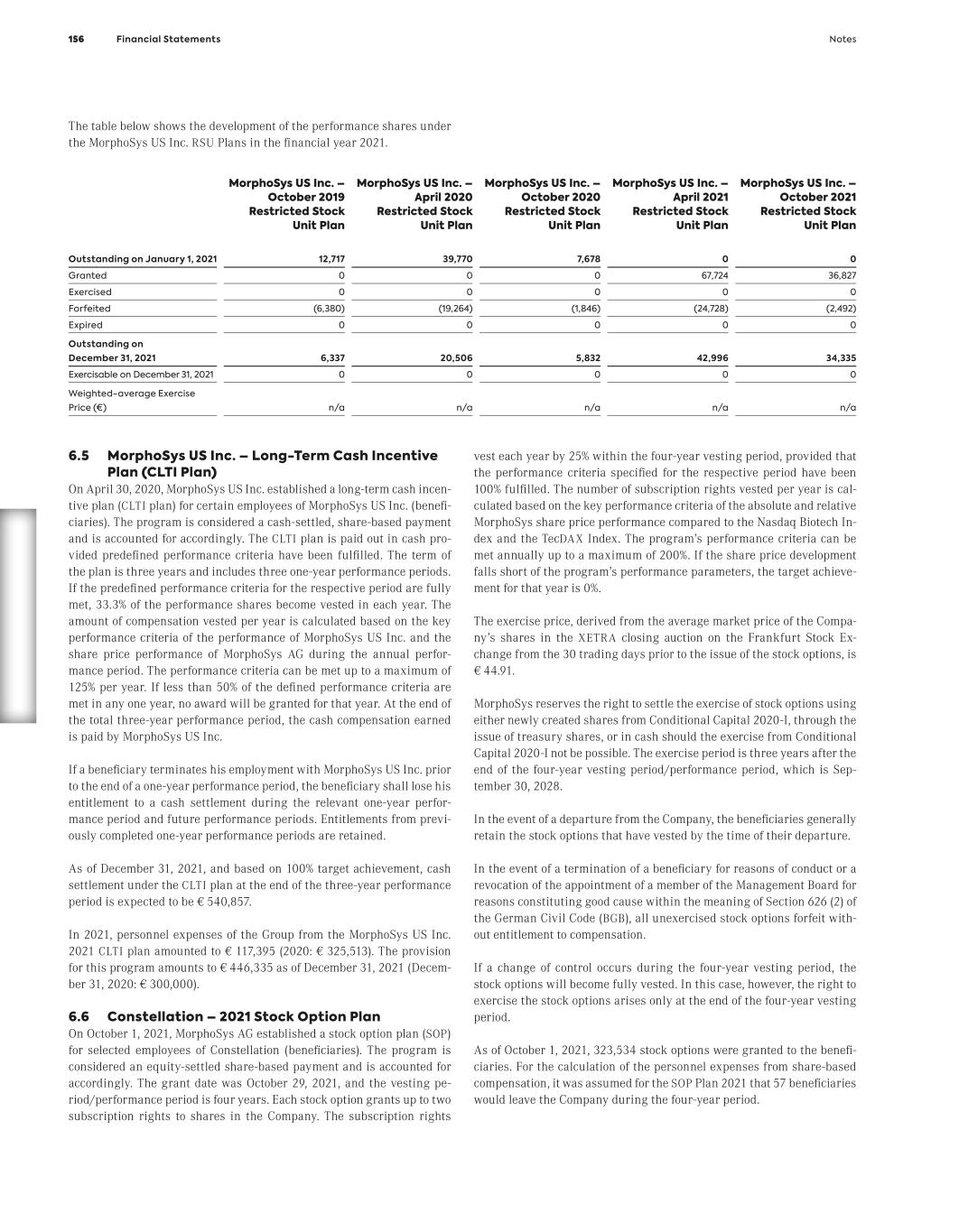
The table below shows the development of the performance shares under the MorphoSys US Inc. RSU Plans in the financial year 2021. MorphoSys US Inc. – October 2019 Restricted Stock Unit Plan MorphoSys US Inc. – April 2020 Restricted Stock Unit Plan MorphoSys US Inc. – October 2020 Restricted Stock Unit Plan MorphoSys US Inc. – April 2021 Restricted Stock Unit Plan MorphoSys US Inc. – October 2021 Restricted Stock Unit Plan Outstanding on January 1, 2021 12,717 39,770 7,678 0 0 Granted 0 0 0 67,724 36,827 Exercised 0 0 0 0 0 Forfeited (6,380) (19,264) (1,846) (24,728) (2,492) Expired 0 0 0 0 0 Outstanding on December 31, 2021 6,337 20,506 5,832 42,996 34,335 Exercisable on December 31, 2021 0 0 0 0 0 Weighted-average Exercise Price (€) n/a n/a n/a n/a n/a 6.5 MorphoSys US Inc. – Long-Term Cash Incentive Plan (CLTI Plan) On April 30, 2020, MorphoSys US Inc. established a long-term cash incen- tive plan (CLTI plan) for certain employees of MorphoSys US Inc. (benefi- ciaries). The program is considered a cash-settled, share-based payment and is accounted for accordingly. The CLTI plan is paid out in cash pro- vided predefined performance criteria have been fulfilled. The term of the plan is three years and includes three one-year performance periods. If the predefined performance criteria for the respective period are fully met, 33.3% of the performance shares become vested in each year. The amount of compensation vested per year is calculated based on the key performance criteria of the performance of MorphoSys US Inc. and the share price performance of MorphoSys AG during the annual perfor- mance period. The performance criteria can be met up to a maximum of 125% per year. If less than 50% of the defined performance criteria are met in any one year, no award will be granted for that year. At the end of the total three-year performance period, the cash compensation earned is paid by MorphoSys US Inc. If a beneficiary terminates his employment with MorphoSys US Inc. prior to the end of a one-year performance period, the beneficiary shall lose his entitlement to a cash settlement during the relevant one-year perfor- mance period and future performance periods. Entitlements from previ- ously completed one-year performance periods are retained. As of December 31, 2021, and based on 100% target achievement, cash settlement under the CLTI plan at the end of the three-year performance period is expected to be € 540,857. In 2021, personnel expenses of the Group from the MorphoSys US Inc. 2021 CLTI plan amounted to € 117,395 (2020: € 325,513). The provision for this program amounts to € 446,335 as of December 31, 2021 (Decem- ber 31, 2020: € 300,000). 6.6 Constellation – 2021 Stock Option Plan On October 1, 2021, MorphoSys AG established a stock option plan (SOP) for selected employees of Constellation (beneficiaries). The program is considered an equity-settled share-based payment and is accounted for accordingly. The grant date was October 29, 2021, and the vesting pe- riod/performance period is four years. Each stock option grants up to two subscription rights to shares in the Company. The subscription rights vest each year by 25% within the four-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is cal- culated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech In- dex and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achieve- ment for that year is 0%. The exercise price, derived from the average market price of the Compa- ny’s shares in the XETRA closing auction on the Frankfurt Stock Ex- change from the 30 trading days prior to the issue of the stock options, is € 44.91. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2020-I, through the issue of treasury shares, or in cash should the exercise from Conditional Capital 2020-I not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is Sep- tember 30, 2028. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit with- out entitlement to compensation. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. As of October 1, 2021, 323,534 stock options were granted to the benefi- ciaries. For the calculation of the personnel expenses from share-based compensation, it was assumed for the SOP Plan 2021 that 57 beneficiaries would leave the Company during the four-year period. 156 Financial Statements Notes

In 2021, personnel expenses from stock options under the Group’s 2021 SOP amounted to € 711,223 based on the fair value on the grant date. The table below shows the development of the stock options plans in the financial year 2021. Constellation – October 2021 Stock Option Plan Outstanding on January 1, 2021 0 Granted 323,534 Exercised 0 Forfeited (29,941) Expired 0 Outstanding on December 31, 2021 293,593 Exercisable on December 31, 2021 0 Weighted-average Exercise Price (€) 45 The fair value of the stock options from the 2021 stock option plans was determined using a Monte Carlo simulation. The expected volatility is based on the development of the share volatility of the last four years. Furthermore, the calculation of fair value equally considered the perfor- mance criteria of the absolute and relative performance of MorphoSys shares compared to the development of the Nasdaq Biotech Index and the TecDAX Index. The parameters and fair value of each program are listed in the table below. Constellation – October 2021 Stock Option Plan Share Price on Grant Date in € 40.75 Exercise Price in € 44.91 Expected Volatility of the MorphoSys share in % 40.51 Expected Volatility of the Nasdaq Biotech Index in % 24.95 Expected Volatility of the TecDAX Index in % 22.17 Performance Term of Program in Years 4.0 Dividend Yield in % n/a Risk-free Interest Rate in % between (0.70) and (0.22) Fair Value on Grant Date in € 16.67 6.7 Related Parties Related parties that can be influenced by the Group or can have a signifi- cant influence on the Group can be divided into subsidiaries, members of the Supervisory Board, members of management in key positions and other related entities. The Group engages in business relationships with members of the Man- agement Board and Supervisory Board as related parties responsible for the planning, management and monitoring of the Group. In addition to cash compensation, the Group has granted the Management Board per- formance shares. The tables below show the shares, stock options and performance shares held by the members of the Management Board and Supervisory Board, as well as the changes in their ownership during the 2021 financial year. Notes Financial Statements 157
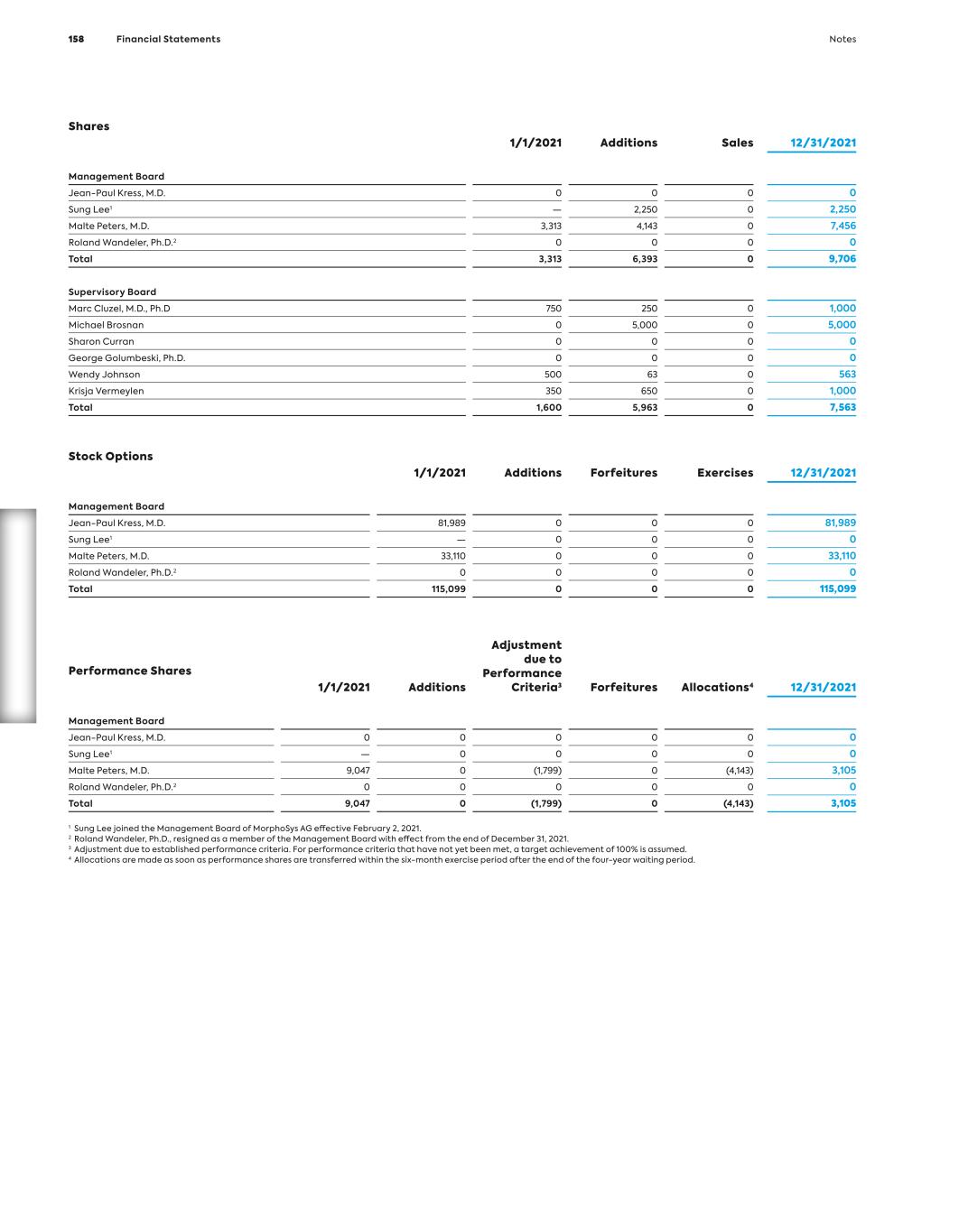
Shares 1/1/2021 Additions Sales 12/31/2021 Management Board Jean-Paul Kress, M.D. 0 0 0 0 Sung Lee1 — 2,250 0 2,250 Malte Peters, M.D. 3,313 4,143 0 7,456 Roland Wandeler, Ph.D.2 0 0 0 0 Total 3,313 6,393 0 9,706 Supervisory Board Marc Cluzel, M.D., Ph.D 750 250 0 1,000 Michael Brosnan 0 5,000 0 5,000 Sharon Curran 0 0 0 0 George Golumbeski, Ph.D. 0 0 0 0 Wendy Johnson 500 63 0 563 Krisja Vermeylen 350 650 0 1,000 Total 1,600 5,963 0 7,563 Stock Options 1/1/2021 Additions Forfeitures Exercises 12/31/2021 Management Board Jean-Paul Kress, M.D. 81,989 0 0 0 81,989 Sung Lee1 — 0 0 0 0 Malte Peters, M.D. 33,110 0 0 0 33,110 Roland Wandeler, Ph.D.2 0 0 0 0 0 Total 115,099 0 0 0 115,099 Performance Shares 1/1/2021 Additions Adjustment due to Performance Criteria3 Forfeitures Allocations4 12/31/2021 Management Board Jean-Paul Kress, M.D. 0 0 0 0 0 0 Sung Lee1 — 0 0 0 0 0 Malte Peters, M.D. 9,047 0 (1,799) 0 (4,143) 3,105 Roland Wandeler, Ph.D.2 0 0 0 0 0 0 Total 9,047 0 (1,799) 0 (4,143) 3,105 1 Sung Lee joined the Management Board of MorphoSys AG effective February 2, 2021. 2 Roland Wandeler, Ph.D., resigned as a member of the Management Board with effect from the end of December 31, 2021. 3 Adjustment due to established performance criteria. For performance criteria that have not yet been met, a target achievement of 100% is assumed. 4 Allocations are made as soon as performance shares are transferred within the six-month exercise period after the end of the four-year waiting period. 158 Financial Statements Notes

The Supervisory Board of MorphoSys AG does not hold any stock options or performance shares. The remuneration system for the Management Board is intended to pro- vide sustainable, results-oriented corporate governance. The Manage- ment Board’s total remuneration consists of several components, includ- ing fixed compensation, an annual cash bonus that is dependent upon the achievement of corporate targets (short-term incentives – STI), variable compensation components with long-term incentives (LTI) and other re- muneration components. Variable remuneration components with long- term incentive consist of long-term incentive plans (LTI Plan) from previ- ous years, stock option and performance share plans from previous years, and a performance share unit program and a stock option plan from the current year. The members of the Management Board addition- ally receive fringe benefits in the form of benefits in kind, essentially consisting of a company car and insurance premiums. All total remuner- ation packages are reviewed annually by the Remuneration and Nomina- tion Committee and compared to an annual Management Board remuner- ation analysis to check the scope and appropriateness of the remuneration packages. The amount of remuneration paid to members of the Manage- ment Board is based largely on the duties of the respective Management Board member, the financial situation and the performance and business outlook for the Company versus its competition. All resolutions on adjust- ments to the overall remuneration packages are passed by the plenum of the Supervisory Board. The Management Board’s total remuneration package and the index-linked pension contracts were thoroughly re- viewed and then adjusted by the Supervisory Board in 2021. If a Management Board member’s service contract terminates due to death, the member’s spouse or life partner is entitled to the fixed monthly salary for the month of death and the 12 months thereafter. In the event of a change of control, Management Board members are entitled to exer- cise their extraordinary right to terminate their service contracts and receive any outstanding fixed salary and the annual bonus for the re- mainder of the agreed contract period, but at least 200% of the annual gross fixed salary and the annual bonus. Moreover, in such a case, all stock options, performance share units and performance shares granted will become vested immediately and can be exercised after the expira- tion of the statutory vesting periods. A change of control has occurred when (i) MorphoSys transfers assets or a substantial portion of its assets to unaffiliated third parties, (ii) MorphoSys merges with an unaffiliated company, (iii) an agreement pursuant to Section 291 AktG is entered into with MorphoSys as a dependent company, MorphoSys is integrated under Section 319 AktG or (iv) a shareholder or third party holds 30% or more of MorphoSys’s voting rights. For the fiscal year 2021, the members of the Management Board were granted a total compensation of € 9,718,350 (2020 € 11,532,252), consist- ing of performance-unrelated remuneration of € 3,759,850 (2020: € 5,529,112), performance-related remuneration of € 2,680,000 (2020: € 2,478,346) as well as long-term incentive compensation of € 3,278,500 (2020: € 3,524,794) in the form of share-based compensation. Perfor- mance-unrelated compensation includes post-employment benefits in the amount of € 806,297 (2020: € 2,443,409) granted during the respective board membership terms. The Supervisory Board decided, that Roland Wandeler, Ph.D. would not forfeit on a pro-rate basis the long-term incentive plans despite his termi- nation of the employment before the end of the four-year vesting period. Because of this modification of terms and conditions, the respective per- sonnel expense from share-based compensation for the outstanding vest- ing periods was allocated to the remaining period of performance. The fair value was not affected by this modification. On the occasion of his departure from the Company with effect as of the end of December 31, 2021, Roland Wandeler, Ph.D., secured a severance payment in the amount of € 806,296, payable in 16 monthly installments. As of April 1, 2021, the Management Board was granted 54,232 Perfor- mance Share Units. The fair value as of December 31, 2021, amounts to € 11.82. For the individualized Management Board compensation, refer to the sep- arately available remuneration report. In the years 2021 and 2020, there were no other long-term benefits in accordance with IAS 24.17 (c) accruing to the Management Board or Su- pervisory Board. No benefits upon termination of service in accordance with IAS 24.17 (d) were accrued for the Supervisory Board in the years 2021 and 2020. Payments to former members of the Management Board amounted to € 4.6 million in 2021 (2020: € 0.6 million). The total compensation for key management personnel in 2021 and 2020 was as follows. in 000’ € 2021 2020 Total Short-Term Employee Benefits 7,336,167 7,261,119 Total Post-Employment Benefits 443,372 424,300 Total Termination Benefits 806,297 2,443,409 Total Share-Based Payment 4,278,500 4,125,979 Total Compensation 12,864,336 14,254,807 As of December 31, 2021, there were accrued personnel expenses of € 3.3 million for payments to key management personnel for perfor- mance-related remuneration and non-current provisions of € 0.5 million for long-term incentive compensation (December 31, 2020: € 3.0 million and € 0.8 million, respectively). Notes Financial Statements 159
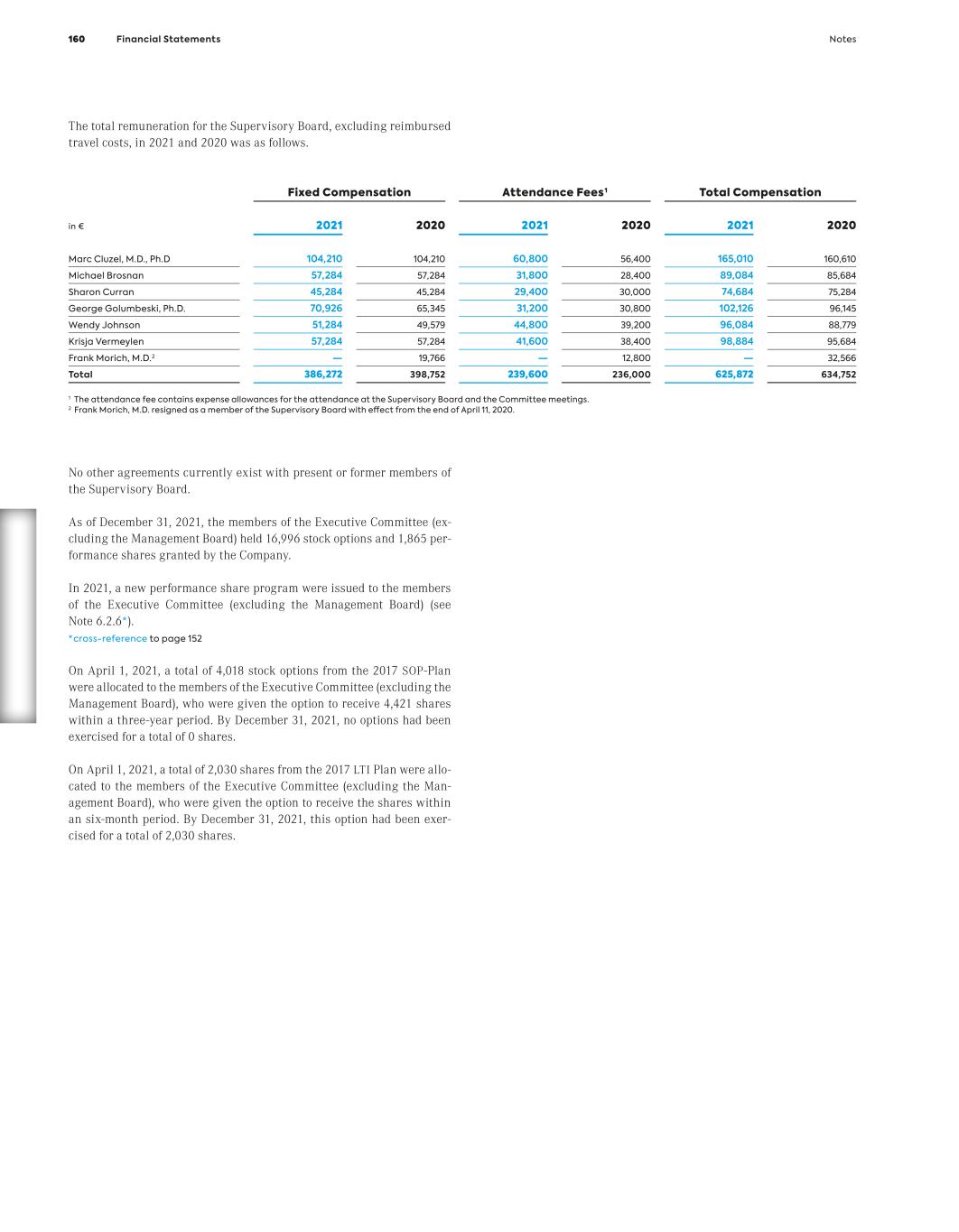
The total remuneration for the Supervisory Board, excluding reimbursed travel costs, in 2021 and 2020 was as follows. Fixed Compensation Attendance Fees1 Total Compensation in € 2021 2020 2021 2020 2021 2020 Marc Cluzel, M.D., Ph.D 104,210 104,210 60,800 56,400 165,010 160,610 Michael Brosnan 57,284 57,284 31,800 28,400 89,084 85,684 Sharon Curran 45,284 45,284 29,400 30,000 74,684 75,284 George Golumbeski, Ph.D. 70,926 65,345 31,200 30,800 102,126 96,145 Wendy Johnson 51,284 49,579 44,800 39,200 96,084 88,779 Krisja Vermeylen 57,284 57,284 41,600 38,400 98,884 95,684 Frank Morich, M.D.2 — 19,766 — 12,800 — 32,566 Total 386,272 398,752 239,600 236,000 625,872 634,752 1 The attendance fee contains expense allowances for the attendance at the Supervisory Board and the Committee meetings. 2 Frank Morich, M.D. resigned as a member of the Supervisory Board with effect from the end of April 11, 2020. No other agreements currently exist with present or former members of the Supervisory Board. As of December 31, 2021, the members of the Executive Committee (ex- cluding the Management Board) held 16,996 stock options and 1,865 per- formance shares granted by the Company. In 2021, a new performance share program were issued to the members of the Executive Committee (excluding the Management Board) (see Note 6.2.6*). * cross-reference to page 152 On April 1, 2021, a total of 4,018 stock options from the 2017 SOP-Plan were allocated to the members of the Executive Committee (excluding the Management Board), who were given the option to receive 4,421 shares within a three-year period. By December 31, 2021, no options had been exercised for a total of 0 shares. On April 1, 2021, a total of 2,030 shares from the 2017 LTI Plan were allo- cated to the members of the Executive Committee (excluding the Man- agement Board), who were given the option to receive the shares within an six-month period. By December 31, 2021, this option had been exer- cised for a total of 2,030 shares. 160 Financial Statements Notes
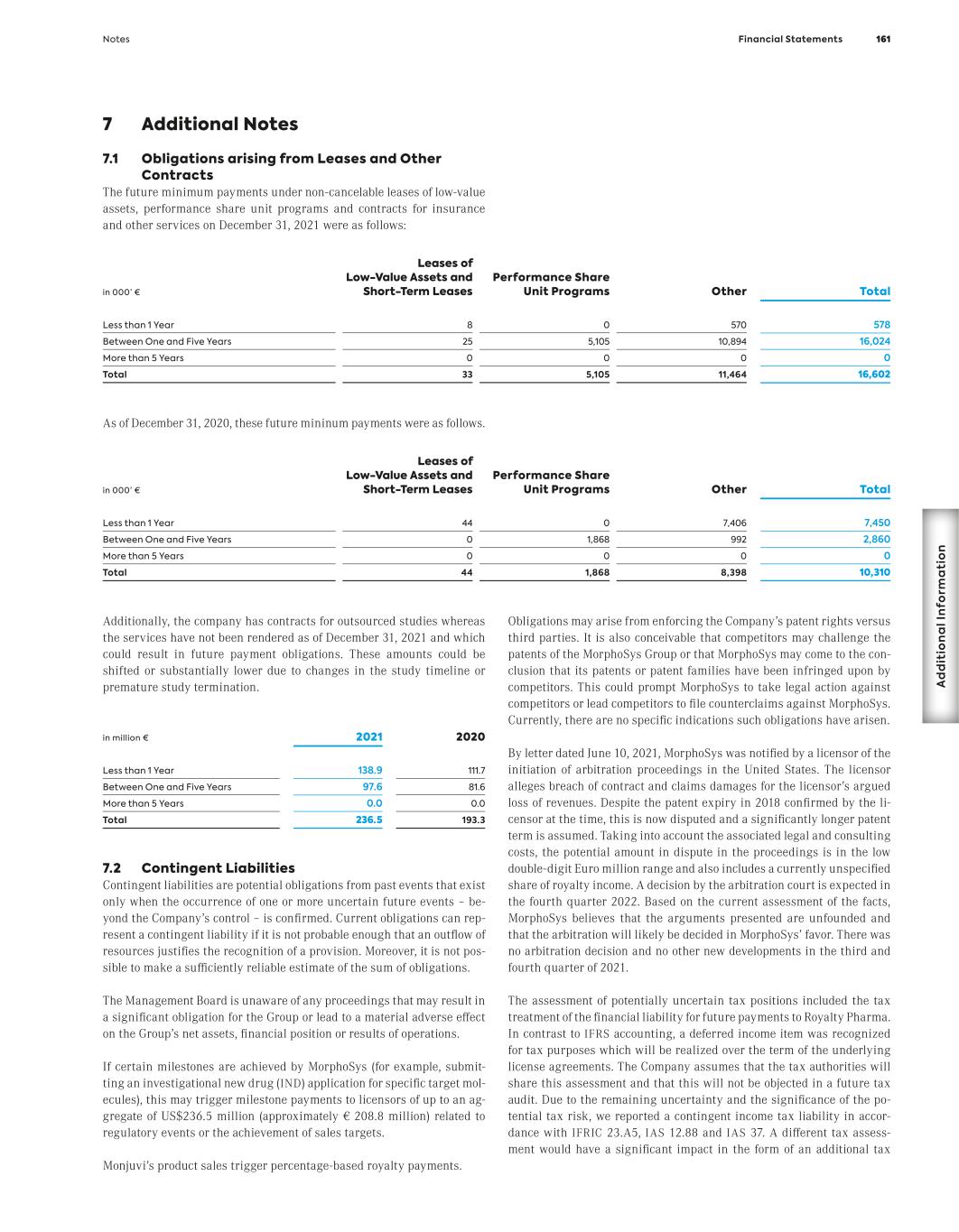
7 Additional Notes 7.1 Obligations arising from Leases and Other Contracts The future minimum payments under non-cancelable leases of low-value assets, performance share unit programs and contracts for insurance and other services on December 31, 2021 were as follows: in 000’ € Leases of Low-Value Assets and Short-Term Leases Performance Share Unit Programs Other Total Less than 1 Year 8 0 570 578 Between One and Five Years 25 5,105 10,894 16,024 More than 5 Years 0 0 0 0 Total 33 5,105 11,464 16,602 As of December 31, 2020, these future mininum payments were as follows. in 000’ € Leases of Low-Value Assets and Short-Term Leases Performance Share Unit Programs Other Total Less than 1 Year 44 0 7,406 7,450 Between One and Five Years 0 1,868 992 2,860 More than 5 Years 0 0 0 0 Total 44 1,868 8,398 10,310 Additionally, the company has contracts for outsourced studies whereas the services have not been rendered as of December 31, 2021 and which could result in future payment obligations. These amounts could be shifted or substantially lower due to changes in the study timeline or premature study termination. in million € 2021 2020 Less than 1 Year 138.9 111.7 Between One and Five Years 97.6 81.6 More than 5 Years 0.0 0.0 Total 236.5 193.3 7.2 Contingent Liabilities Contingent liabilities are potential obligations from past events that exist only when the occurrence of one or more uncertain future events – be- yond the Company’s control – is confirmed. Current obligations can rep- resent a contingent liability if it is not probable enough that an outflow of resources justifies the recognition of a provision. Moreover, it is not pos- sible to make a sufficiently reliable estimate of the sum of obligations. The Management Board is unaware of any proceedings that may result in a significant obligation for the Group or lead to a material adverse effect on the Group’s net assets, financial position or results of operations. If certain milestones are achieved by MorphoSys (for example, submit- ting an investigational new drug (IND) application for specific target mol- ecules), this may trigger milestone payments to licensors of up to an ag- gregate of US$236.5 million (approximately € 208.8 million) related to regulatory events or the achievement of sales targets. Monjuvi’s product sales trigger percentage-based royalty payments. Obligations may arise from enforcing the Company’s patent rights versus third parties. It is also conceivable that competitors may challenge the patents of the MorphoSys Group or that MorphoSys may come to the con- clusion that its patents or patent families have been infringed upon by competitors. This could prompt MorphoSys to take legal action against competitors or lead competitors to file counterclaims against MorphoSys. Currently, there are no specific indications such obligations have arisen. By letter dated June 10, 2021, MorphoSys was notified by a licensor of the initiation of arbitration proceedings in the United States. The licensor alleges breach of contract and claims damages for the licensor’s argued loss of revenues. Despite the patent expiry in 2018 confirmed by the li- censor at the time, this is now disputed and a significantly longer patent term is assumed. Taking into account the associated legal and consulting costs, the potential amount in dispute in the proceedings is in the low double-digit Euro million range and also includes a currently unspecified share of royalty income. A decision by the arbitration court is expected in the fourth quarter 2022. Based on the current assessment of the facts, MorphoSys believes that the arguments presented are unfounded and that the arbitration will likely be decided in MorphoSys’ favor. There was no arbitration decision and no other new developments in the third and fourth quarter of 2021. The assessment of potentially uncertain tax positions included the tax treatment of the financial liability for future payments to Royalty Pharma. In contrast to IFRS accounting, a deferred income item was recognized for tax purposes which will be realized over the term of the underlying license agreements. The Company assumes that the tax authorities will share this assessment and that this will not be objected in a future tax audit. Due to the remaining uncertainty and the significance of the po- tential tax risk, we reported a contingent income tax liability in accor- dance with IFRIC 23.A5, IAS 12.88 and IAS 37. A different tax assess- ment would have a significant impact in the form of an additional tax Additional Notes Notes Financial Statements 161

payment. For tax purposes, deferred income for the obligations to Royalty Pharma amounted to € 988.9 million as of December 31, 2021 and the associated contingent tax liability upon non-acceptance of the deferral amounts to € 223.1 million, determined utilizing deferred tax assets on loss carry forwards of €40.6 million, capitalized as of December 31, 2021. 7.3 Additional Disclosures for Financial Instruments Fair Value Hierarchy and Measurement Methods The fair value is the price that would be achieved for the sale of an asset in an arm’s length transaction between independent market participants or the price to be paid for the transfer of a liability (disposal or exit price). Fair value is measured by using the same assumptions and taking into account the same characteristics of the asset or liability as would an in- dependent market participant. Fair value is a market-based, not an entity- specific measurement. The fair value of non-financial assets is based on the best use of the asset by a market participant. For financial instruments, the use of bid prices for assets and ask prices for liabilities is permitted but not required if those prices best reflect the fair value in the respective circumstances. For simplification, mean rates are also permitted. MorphoSys applies the following hierarchy in determining and disclos- ing the fair value of financial instruments: Level 1: Quoted (unadjusted) prices in active markets for identical assets or liabilities to which the Company has access. Level 2: Inputs other than quoted prices included within Level 1 that are observable for assets or liabilities, either directly (i.e., as prices) or indirectly (i.e., derived from prices). Level 3: Inputs for asset or liability that are not based on observable mar- ket data (that is, unobservable inputs). The carrying amounts of certain financial assets and liabilities, such as financial assets at amortized cost, as well as accounts receivable and ac- counts payable, approximate their fair value because of their short- term maturities. Hierarchy Level 1 The fair value of financial instruments traded in active markets is based on the quoted market prices on the reporting date. A market is consid- ered active if quoted prices are available from an exchange, dealer, bro- ker, industry group, pricing service, or regulatory body that is easily and regularly accessible, and prices reflect current and regularly occurring market transactions at arm’s length conditions. For assets held by the Group, the appropriate quoted market price is the buyer’s bid price. Hierarchy Levels 2 and 3 The fair value of financial instruments not traded in active markets can be determined using valuation methods. In this case, fair value is esti- mated using the results of a valuation method that makes maximum use of market data and relies as little as possible on not observable market data. If all significant inputs required for measuring fair value by using valuation methods are observable, the instrument is allocated to Hierar- chy Level 2. If significant inputs are not based on observable market data, the instrument is allocated to Hierarchy Level 3. Hierarchy Level 2 contains foreign exchange forward agreements to hedge exchange rate fluctuations, term deposits and restricted cash as well as in 2020 the debt component of the convertible bond. Future cash flows for these foreign exchange forward agreements are determined based on forward exchange rate curves. The fair value of these instru- ments corresponds to their discounted cash flows. The fair value of the term deposits and restricted cash is determined by discounting the ex- pected cash flows at market interest rates. The fair value of the debt com- ponent of the convertible bonds was determined by calculating the pres- ent value of all cash flows associated with the liability using the applicable reference interest rate with an adjustment to reflect MorphoSys’ credit risk premium. Hierarchy Level 3 financial assets comprise equity investments, financial assets and financial liabilities from collaborations, in 2021 the debt com- ponent of the convertible bond as well as financial liabilities from future payments to Royalty Pharma. The underlying valuations are generally carried out by employees in the finance department who report directly to the Chief Financial Officer. The valuation process and results are re- viewed and discussed among the persons involved on a regular basis. The financial assets from collaborations represent MorphoSys’ current reimbursement claim against Incyte from the expected future losses as- sociated with the co-commercialization activities of Monjuvi as sec- ond-line treatment for relapsed or refractory diffuse large B-cell lym- phoma (“DLBCL”) in the U.S. (as Incyte has agreed to compensate MorphoSys for 50% of said losses). To determine the fair value of financial assets from collaborations, expected cash inflows are discounted using market interest rates of financial instruments with comparable curren- cies and maturities, taking into account Incyte’s credit risk. The reconciliation and sensitivity analysis for the Hierarchy Level 3 fi- nancial assets are presented in Note 5.18* and in the heading “equity investments” below. For further information on financial liabilities car- ried at amortized cost whose fair value is assigned to hierarchy Level 3, please refer to Notes 5.18* and 5.19*. * cross-reference to page 143 and page 144 Reclassifications between the hierarchy levels are generally taken into account as of the reporting dates. In 2021, the fair value measurement of the debt component of the convertible bond was reclassified from hierar- chy Level 2 to hierarchy Level 3, as the entity’s own credit risk is not ob- servable as a significant parameter for the fair value measurement. In 2020, no transfers were made between the fair value hierarchy levels. The carrying amounts of current financial assets and liabilities at amor- tized cost approximate their fair values given their short maturities. The table below shows the fair values of financial assets and liabilities and the carrying amounts presented in the consolidated balance sheet. 162 Financial Statements Notes
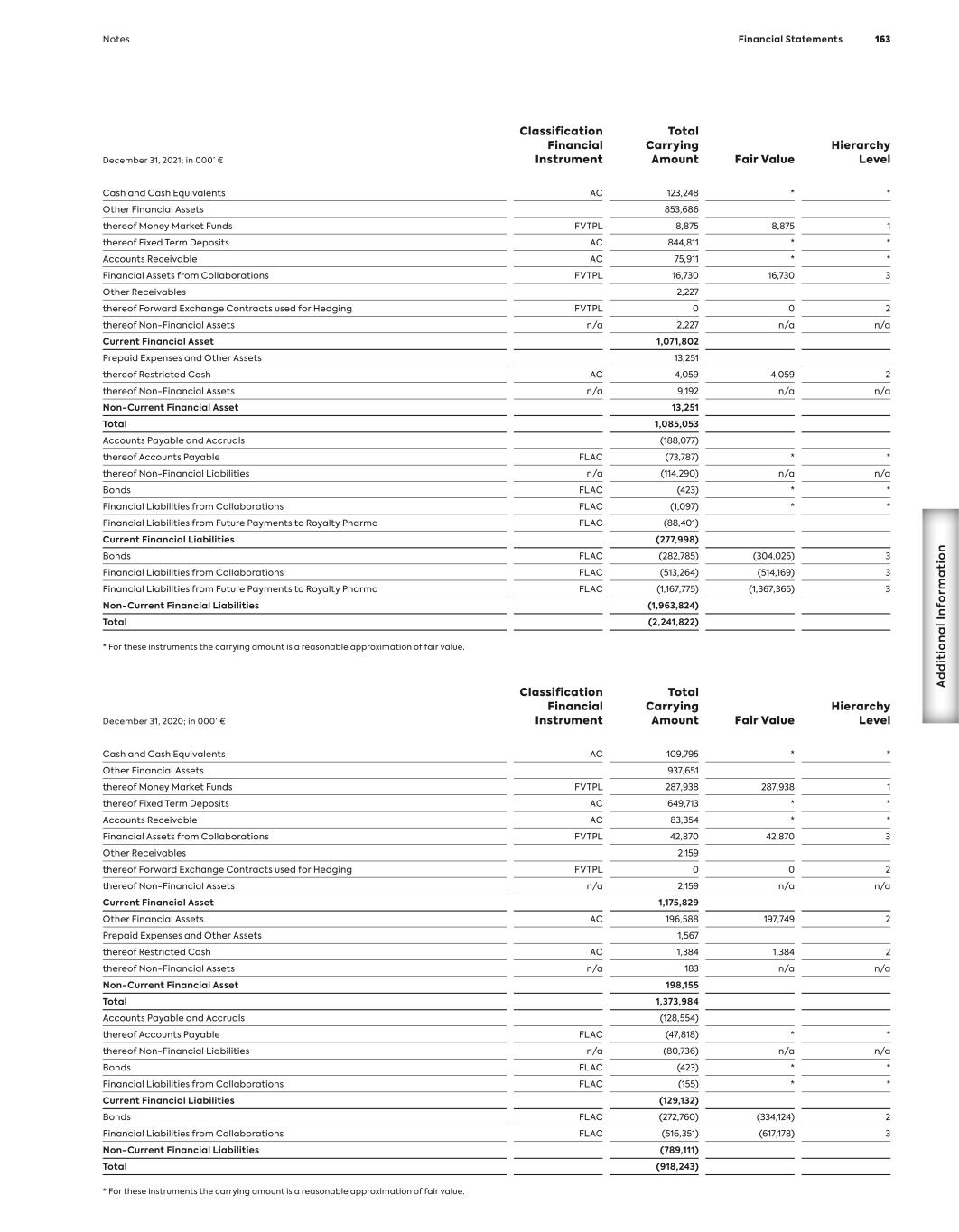
December 31, 2021; in 000’ € Classification Financial Instrument Total Carrying Amount Fair Value Hierarchy Level Cash and Cash Equivalents AC 123,248 * * Other Financial Assets 853,686 thereof Money Market Funds FVTPL 8,875 8,875 1 thereof Fixed Term Deposits AC 844,811 * * Accounts Receivable AC 75,911 * * Financial Assets from Collaborations FVTPL 16,730 16,730 3 Other Receivables 2,227 thereof Forward Exchange Contracts used for Hedging FVTPL 0 0 2 thereof Non-Financial Assets n/a 2,227 n/a n/a Current Financial Asset 1,071,802 Prepaid Expenses and Other Assets 13,251 thereof Restricted Cash AC 4,059 4,059 2 thereof Non-Financial Assets n/a 9,192 n/a n/a Non-Current Financial Asset 13,251 Total 1,085,053 Accounts Payable and Accruals (188,077) thereof Accounts Payable FLAC (73,787) * * thereof Non-Financial Liabilities n/a (114,290) n/a n/a Bonds FLAC (423) * * Financial Liabilities from Collaborations FLAC (1,097) * * Financial Liabilities from Future Payments to Royalty Pharma FLAC (88,401) Current Financial Liabilities (277,998) Bonds FLAC (282,785) (304,025) 3 Financial Liabilities from Collaborations FLAC (513,264) (514,169) 3 Financial Liabilities from Future Payments to Royalty Pharma FLAC (1,167,775) (1,367,365) 3 Non-Current Financial Liabilities (1,963,824) Total (2,241,822) * For these instruments the carrying amount is a reasonable approximation of fair value. December 31, 2020; in 000’ € Classification Financial Instrument Total Carrying Amount Fair Value Hierarchy Level Cash and Cash Equivalents AC 109,795 * * Other Financial Assets 937,651 thereof Money Market Funds FVTPL 287,938 287,938 1 thereof Fixed Term Deposits AC 649,713 * * Accounts Receivable AC 83,354 * * Financial Assets from Collaborations FVTPL 42,870 42,870 3 Other Receivables 2,159 thereof Forward Exchange Contracts used for Hedging FVTPL 0 0 2 thereof Non-Financial Assets n/a 2,159 n/a n/a Current Financial Asset 1,175,829 Other Financial Assets AC 196,588 197,749 2 Prepaid Expenses and Other Assets 1,567 thereof Restricted Cash AC 1,384 1,384 2 thereof Non-Financial Assets n/a 183 n/a n/a Non-Current Financial Asset 198,155 Total 1,373,984 Accounts Payable and Accruals (128,554) thereof Accounts Payable FLAC (47,818) * * thereof Non-Financial Liabilities n/a (80,736) n/a n/a Bonds FLAC (423) * * Financial Liabilities from Collaborations FLAC (155) * * Current Financial Liabilities (129,132) Bonds FLAC (272,760) (334,124) 2 Financial Liabilities from Collaborations FLAC (516,351) (617,178) 3 Non-Current Financial Liabilities (789,111) Total (918,243) * For these instruments the carrying amount is a reasonable approximation of fair value. Notes Financial Statements 163
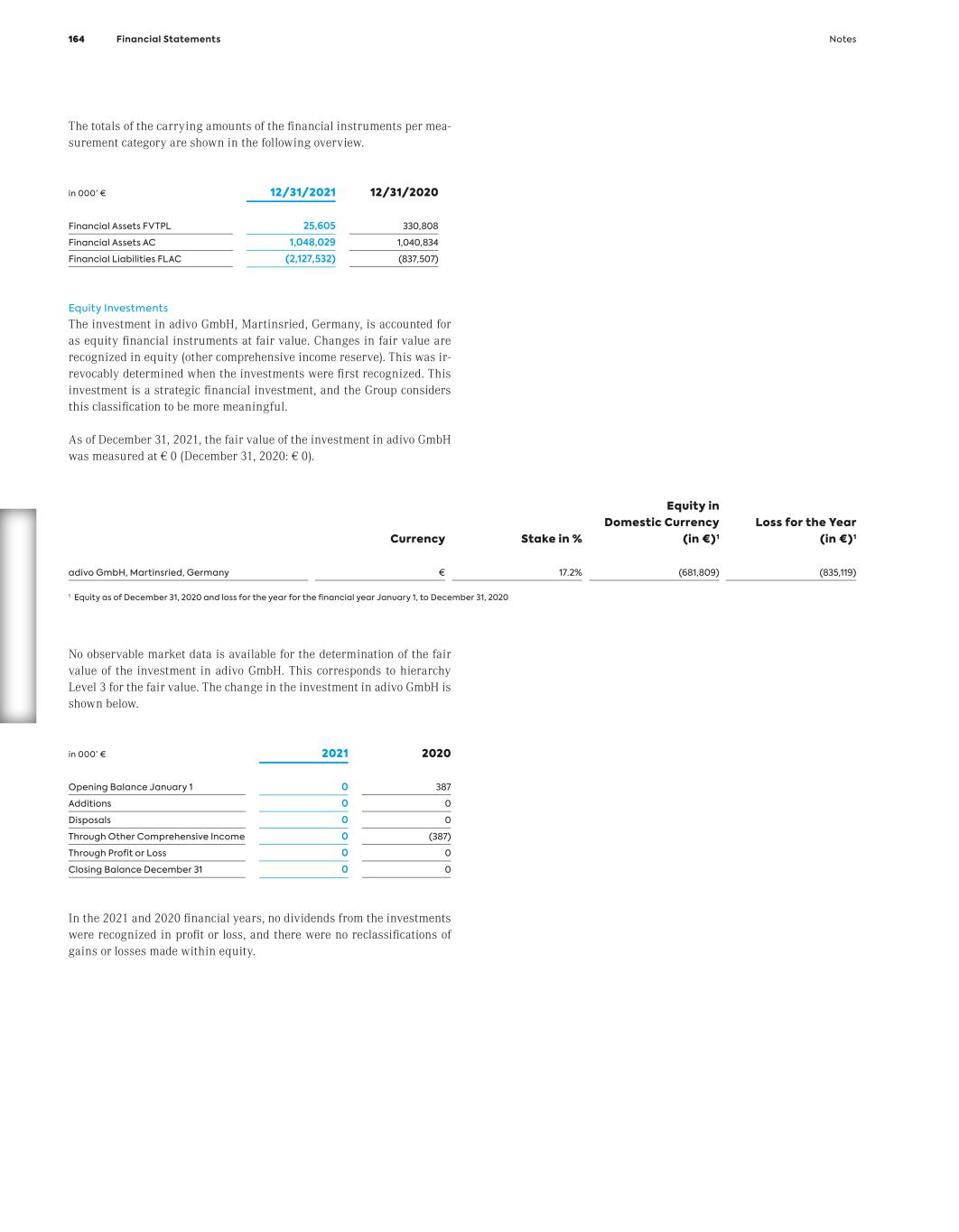
The totals of the carrying amounts of the financial instruments per mea- surement category are shown in the following overview. in 000’ € 12/31/2021 12/31/2020 Financial Assets FVTPL 25,605 330,808 Financial Assets AC 1,048,029 1,040,834 Financial Liabilities FLAC (2,127,532) (837,507) Equity Investments The investment in adivo GmbH, Martinsried, Germany, is accounted for as equity financial instruments at fair value. Changes in fair value are recognized in equity (other comprehensive income reserve). This was ir- revocably determined when the investments were first recognized. This investment is a strategic financial investment, and the Group considers this classification to be more meaningful. As of December 31, 2021, the fair value of the investment in adivo GmbH was measured at € 0 (December 31, 2020: € 0). Currency Stake in % Equity in Domestic Currency (in €)1 Loss for the Year (in €)1 adivo GmbH, Martinsried, Germany € 17.2% (681,809) (835,119) 1 Equity as of December 31, 2020 and loss for the year for the financial year January 1, to December 31, 2020 No observable market data is available for the determination of the fair value of the investment in adivo GmbH. This corresponds to hierarchy Level 3 for the fair value. The change in the investment in adivo GmbH is shown below. in 000’ € 2021 2020 Opening Balance January 1 0 387 Additions 0 0 Disposals 0 0 Through Other Comprehensive Income 0 (387) Through Profit or Loss 0 0 Closing Balance December 31 0 0 In the 2021 and 2020 financial years, no dividends from the investments were recognized in profit or loss, and there were no reclassifications of gains or losses made within equity. 164 Financial Statements Notes
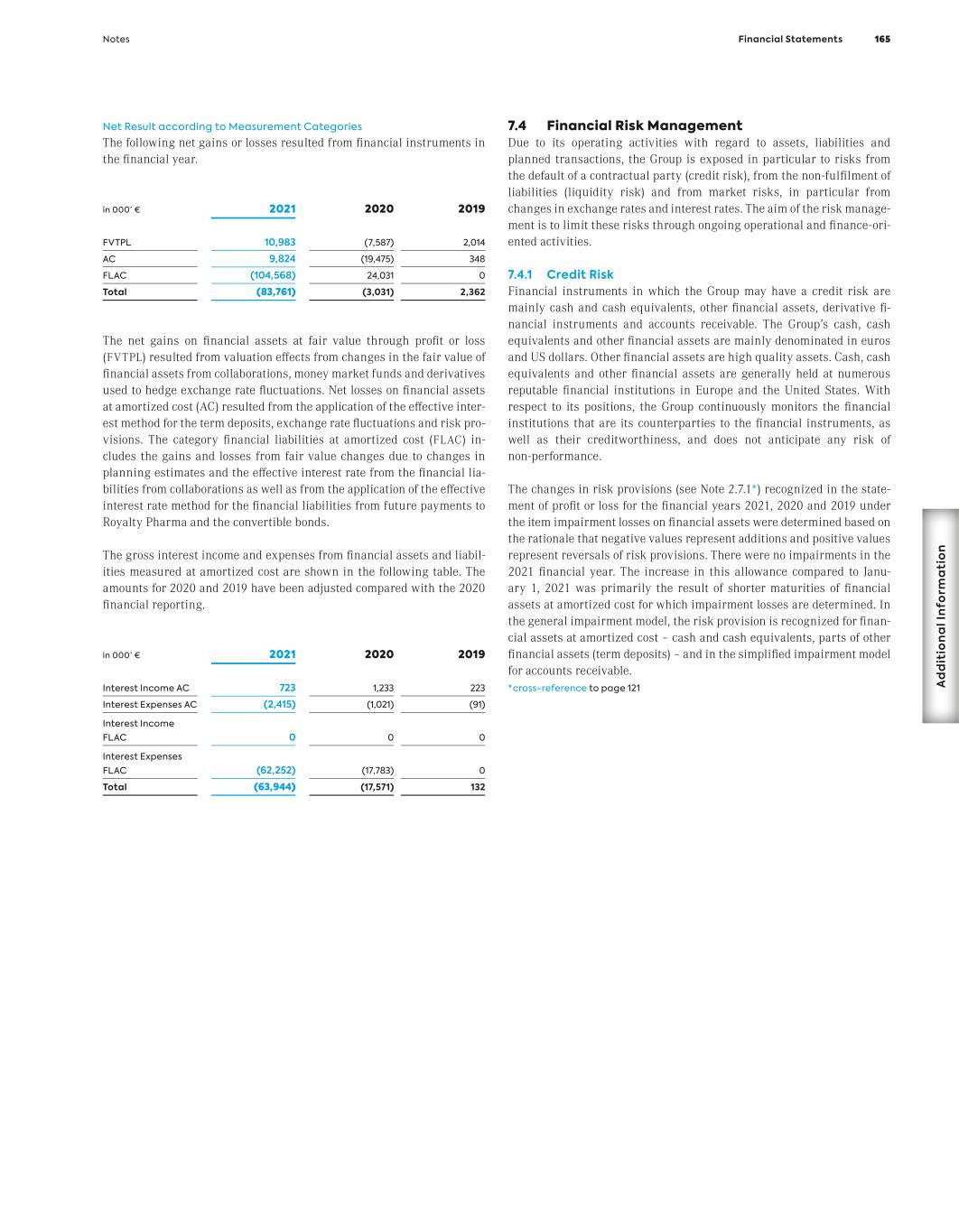
Net Result according to Measurement Categories The following net gains or losses resulted from financial instruments in the financial year. in 000’ € 2021 2020 2019 FVTPL 10,983 (7,587) 2,014 AC 9,824 (19,475) 348 FLAC (104,568) 24,031 0 Total (83,761) (3,031) 2,362 The net gains on financial assets at fair value through profit or loss (FVTPL) resulted from valuation effects from changes in the fair value of financial assets from collaborations, money market funds and derivatives used to hedge exchange rate fluctuations. Net losses on financial assets at amortized cost (AC) resulted from the application of the effective inter- est method for the term deposits, exchange rate fluctuations and risk pro- visions. The category financial liabilities at amortized cost (FLAC) in- cludes the gains and losses from fair value changes due to changes in planning estimates and the effective interest rate from the financial lia- bilities from collaborations as well as from the application of the effective interest rate method for the financial liabilities from future payments to Royalty Pharma and the convertible bonds. The gross interest income and expenses from financial assets and liabil- ities measured at amortized cost are shown in the following table. The amounts for 2020 and 2019 have been adjusted compared with the 2020 financial reporting. in 000’ € 2021 2020 2019 Interest Income AC 723 1,233 223 Interest Expenses AC (2,415) (1,021) (91) Interest Income FLAC 0 0 0 Interest Expenses FLAC (62,252) (17,783) 0 Total (63,944) (17,571) 132 7.4 Financial Risk Management Due to its operating activities with regard to assets, liabilities and planned transactions, the Group is exposed in particular to risks from the default of a contractual party (credit risk), from the non-fulfilment of liabilities (liquidity risk) and from market risks, in particular from changes in exchange rates and interest rates. The aim of the risk manage- ment is to limit these risks through ongoing operational and finance-ori- ented activities. 7.4.1 Credit Risk Financial instruments in which the Group may have a credit risk are mainly cash and cash equivalents, other financial assets, derivative fi- nancial instruments and accounts receivable. The Group’s cash, cash equivalents and other financial assets are mainly denominated in euros and US dollars. Other financial assets are high quality assets. Cash, cash equivalents and other financial assets are generally held at numerous reputable financial institutions in Europe and the United States. With respect to its positions, the Group continuously monitors the financial institutions that are its counterparties to the financial instruments, as well as their creditworthiness, and does not anticipate any risk of non-performance. The changes in risk provisions (see Note 2.7.1*) recognized in the state- ment of profit or loss for the financial years 2021, 2020 and 2019 under the item impairment losses on financial assets were determined based on the rationale that negative values represent additions and positive values represent reversals of risk provisions. There were no impairments in the 2021 financial year. The increase in this allowance compared to Janu- ary 1, 2021 was primarily the result of shorter maturities of financial assets at amortized cost for which impairment losses are determined. In the general impairment model, the risk provision is recognized for finan- cial assets at amortized cost – cash and cash equivalents, parts of other financial assets (term deposits) – and in the simplified impairment model for accounts receivable. * cross-reference to page 121 Notes Financial Statements 165

General Impairment Model Simplified Impairment Model Total in 000’ € Stage 1 Stage 2 Stage 3 Stage 2 Stage 3 Balance as of January 1, 2020 (299) 0 0 (80) 0 (379) Unused Amounts Reversed 299 0 0 80 0 379 Increase in Impairment Losses for Credit Risks recognized in Profit or Loss during the Year (1,001) 0 0 (424) 0 (1,425) Change between Impairment Stages 0 0 0 0 0 0 Amounts written off during the Year as uncollectible 0 0 0 0 0 0 Balance as of December 31, 2020 (1,001) 0 0 (424) 0 (1,425) Balance as of January 1, 2021 (1,001) 0 0 (424) 0 (1,425) Unused Amounts Reversed 1,001 0 0 424 0 1,425 Increase in Impairment Losses for Credit Risks recognized in Profit or Loss during the Year (685) 0 0 (360) 0 (1,045) Change between Impairment Stages 0 0 0 0 0 0 Amounts written off during the Year as uncollectible 0 0 0 0 0 0 Balance as of December 31, 2021 (685) 0 0 (360) 0 (1,045) The gross carrying amounts of the Group’s financial assets by credit risk rating class are as follows. Financial Assets as of December 31, 2021 Internal Credit Rating Basis for Recognition of Expected Credit Loss Provision Gross Carrying Amount (in 000’ €) Cash and Cash Equivalents low Expected Twelve-Month Loss 123,248 Term Deposits low Expected Twelve-Month Loss 845,488 Accounts Receivable low Lifetime Expected Credit Losses 76,270 Financial Assets as of December 31, 2020 Internal Credit Rating Basis for Recognition of Expected Credit Loss Provision Gross Carrying Amount (in 000’ €) Cash and Cash Equivalents low Expected Twelve-Month Loss 109,797 Term Deposits low Expected Twelve-Month Loss 847,300 Accounts Receivable low Lifetime Expected Credit Losses 83,778 The Group is also exposed to credit risk from debt instruments that are measured at fair value through profit or loss. This includes the items “Financial Assets at Fair Value through Profit or Loss” and “Financial Assets from Collaborations”. As of December 31, 2021, the maximum credit risk corresponded to the carrying amounts of these items amount- ing to € 25.6 million (December 31, 2020: € 330.8 million). One of the Group’s policies requires that all customers who wish to trans- act business on credit undergo a credit assessment based on external ratings. Nevertheless, the Group’s revenue and accounts receivable are still subject to credit risk from customer concentration. The Group’s sin- gle most significant customer accounted for € 38.5 million of accounts receivables as of December 31, 2021 (December 31, 2020: € 50.1 million), or 51% of the Group’s total accounts receivable at the end of 2021. The Group’s top three customers individually accounted for 36%, 14% and 9% of the total revenue in 2021. 166 Financial Statements Notes

As of December 31, 2020, 60% of the Group’s accounts receivable balance related to a single customer; of the total revenue in 2020, three customers individually accounted for 78%, 14% and 1%. On December 31, 2019, one customer had accounted for 53% of the Group’s accounts receivable, and the top three customers in 2019 individually accounted for 45%, 31% and 13% of the Group’s revenue. The maximum credit risk (equal to the carrying amount) for rent deposits and other deposits on the reporting date amounted to € 4.1 million (De- cember 31, 2020: € 1.4 million). 7.4.2 Liquidity Risk Liquidity risk arises primarily from accounts payable, lease liabilities (refer to Note 5.9*), bonds, financial liabilities from collaborations and fi- nancial liabilities from future payments to Royalty Pharma. Liquidity risk is managed on the basis of balance sheet and profit and loss figures. This is done by means of liquidity planning for the current year on a monthly basis, for the three subsequent years on an annual basis and a monthly target/actual comparison. The top priority is always to ensure sufficient liquidity so that all payment obligations can be met. * cross-reference to page 138 The following table shows the the maturities of the cash flows of accounts payable and bonds at the balance sheet date. For the financial liabilities from collaborations, the non-discounted, future planned half profit shar- ing payments from Incyte for the sales of Monjuvi in the USA are pre- sented. The financial liabilities from future payments to Royalty Pharma include the undiscounted, planned net sales in the coming years. There is no cash inflow and outflow at MorphoSys as the agreed percentage royalties and milestones are paid directly by Janssen, GSK and Roche to Royalty Pharma. As of December 31, 2021, financial liabilities from fu- ture payments to Royalty Pharma include an amount of € 1.5 million, which will result in a cash outflow for MorphoSys in 2022. Refer to Note 5.9* for the contractual cash flows of lease liabilities. * cross-reference to page 138 in ‘000 €; due on December 31, 2021 in Less than 1 Year Between One and Five Years More than 5 Years Total Accounts Payable 73,787 0 0 73,787 Bonds 2,031 331,094 0 333,125 Financial Liabilities from Collaborations 1,140 167,669 530,242 699,052 Financial Liabilities from Future Payments to Royalty Pharma 89,845 505,938 1,051,077 1,646,860 in ‘000 €; due on December 31, 2020 in Less than 1 Year Between One and Five Years More than 5 Years Total Accounts Payable 47,818 0 0 47,818 Bonds 2,031 333,125 0 335,156 Financial Liabilities from Collaborations 161 180,347 529,338 709,846 Compared with the 2020 financial reporting, accounts payable also in- clude licenses payable, which were presented separately in the previous year (refer to Note 5.13*). The prior year’s presentation of the figures has been adjusted accordingly in order to provide comparable information for the previous years. * cross-reference to page 141 There were no financial instruments pledged as collateral as of Decem- ber 31, 2021. Notes Financial Statements 167
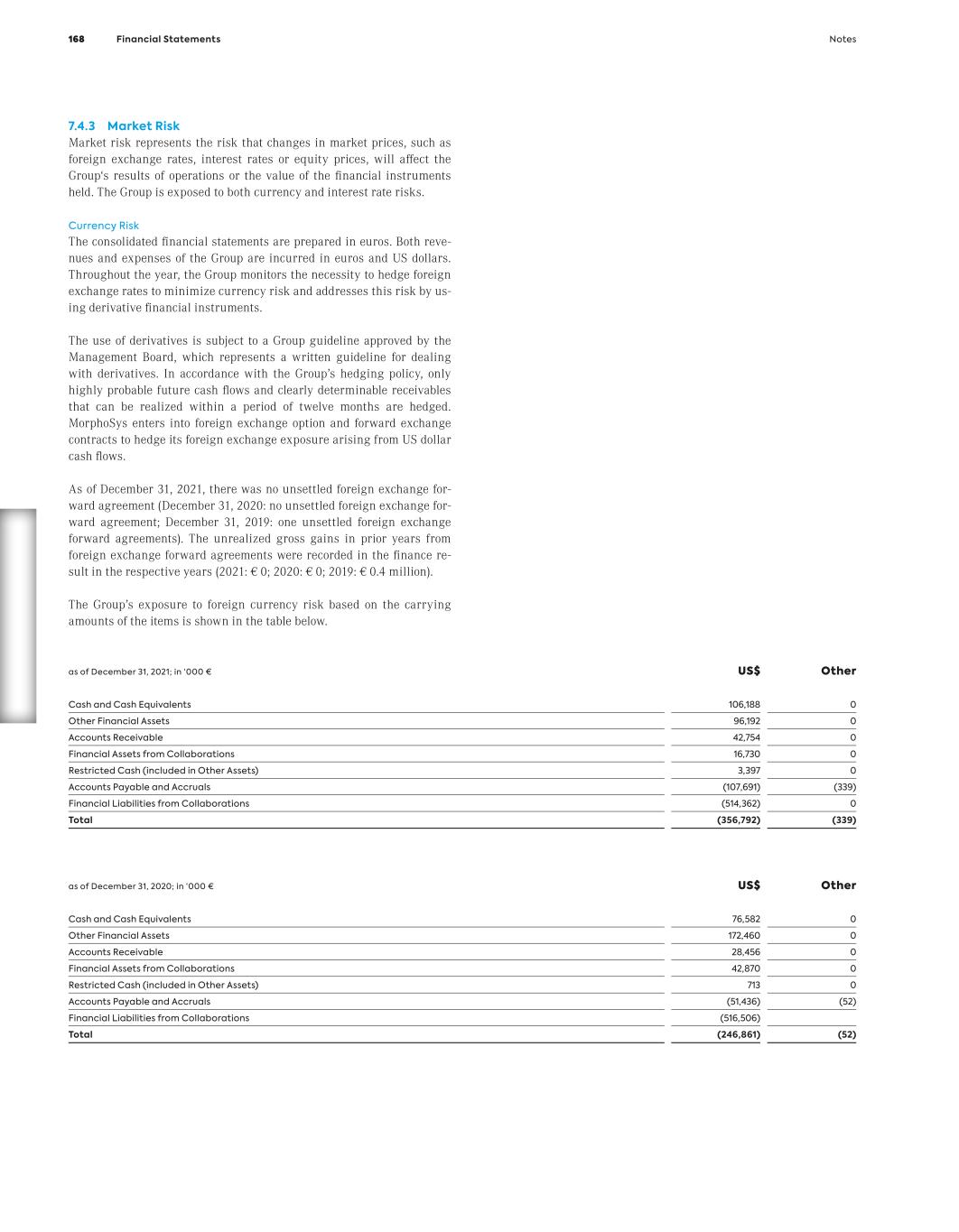
7.4.3 Market Risk Market risk represents the risk that changes in market prices, such as foreign exchange rates, interest rates or equity prices, will affect the Group‘s results of operations or the value of the financial instruments held. The Group is exposed to both currency and interest rate risks. Currency Risk The consolidated financial statements are prepared in euros. Both reve- nues and expenses of the Group are incurred in euros and US dollars. Throughout the year, the Group monitors the necessity to hedge foreign exchange rates to minimize currency risk and addresses this risk by us- ing derivative financial instruments. The use of derivatives is subject to a Group guideline approved by the Management Board, which represents a written guideline for dealing with derivatives. In accordance with the Group’s hedging policy, only highly probable future cash flows and clearly determinable receivables that can be realized within a period of twelve months are hedged. MorphoSys enters into foreign exchange option and forward exchange contracts to hedge its foreign exchange exposure arising from US dollar cash flows. As of December 31, 2021, there was no unsettled foreign exchange for- ward agreement (December 31, 2020: no unsettled foreign exchange for- ward agreement; December 31, 2019: one unsettled foreign exchange forward agreements). The unrealized gross gains in prior years from foreign exchange forward agreements were recorded in the finance re- sult in the respective years (2021: € 0; 2020: € 0; 2019: € 0.4 million). The Group’s exposure to foreign currency risk based on the carrying amounts of the items is shown in the table below. as of December 31, 2021; in ‘000 € US$ Other Cash and Cash Equivalents 106,188 0 Other Financial Assets 96,192 0 Accounts Receivable 42,754 0 Financial Assets from Collaborations 16,730 0 Restricted Cash (included in Other Assets) 3,397 0 Accounts Payable and Accruals (107,691) (339) Financial Liabilities from Collaborations (514,362) 0 Total (356,792) (339) as of December 31, 2020; in ‘000 € US$ Other Cash and Cash Equivalents 76,582 0 Other Financial Assets 172,460 0 Accounts Receivable 28,456 0 Financial Assets from Collaborations 42,870 0 Restricted Cash (included in Other Assets) 713 0 Accounts Payable and Accruals (51,436) (52) Financial Liabilities from Collaborations (516,506) Total (246,861) (52) 168 Financial Statements Notes
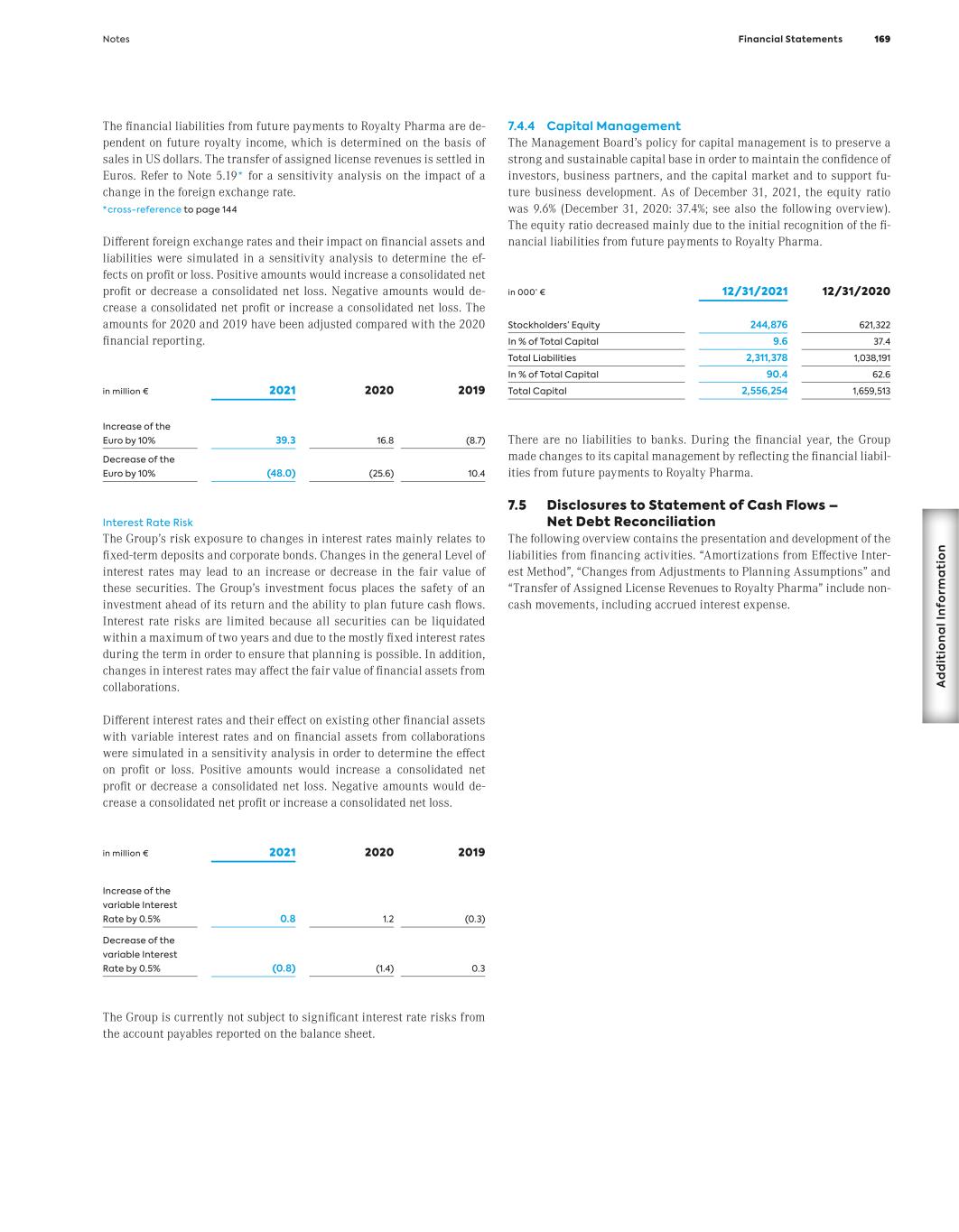
The financial liabilities from future payments to Royalty Pharma are de- pendent on future royalty income, which is determined on the basis of sales in US dollars. The transfer of assigned license revenues is settled in Euros. Refer to Note 5.19* for a sensitivity analysis on the impact of a change in the foreign exchange rate. * cross-reference to page 144 Different foreign exchange rates and their impact on financial assets and liabilities were simulated in a sensitivity analysis to determine the ef- fects on profit or loss. Positive amounts would increase a consolidated net profit or decrease a consolidated net loss. Negative amounts would de- crease a consolidated net profit or increase a consolidated net loss. The amounts for 2020 and 2019 have been adjusted compared with the 2020 financial reporting. in million € 2021 2020 2019 Increase of the Euro by 10% 39.3 16.8 (8.7) Decrease of the Euro by 10% (48.0) (25.6) 10.4 Interest Rate Risk The Group’s risk exposure to changes in interest rates mainly relates to fixed-term deposits and corporate bonds. Changes in the general Level of interest rates may lead to an increase or decrease in the fair value of these securities. The Group’s investment focus places the safety of an investment ahead of its return and the ability to plan future cash flows. Interest rate risks are limited because all securities can be liquidated within a maximum of two years and due to the mostly fixed interest rates during the term in order to ensure that planning is possible. In addition, changes in interest rates may affect the fair value of financial assets from collaborations. Different interest rates and their effect on existing other financial assets with variable interest rates and on financial assets from collaborations were simulated in a sensitivity analysis in order to determine the effect on profit or loss. Positive amounts would increase a consolidated net profit or decrease a consolidated net loss. Negative amounts would de- crease a consolidated net profit or increase a consolidated net loss. in million € 2021 2020 2019 Increase of the variable Interest Rate by 0.5% 0.8 1.2 (0.3) Decrease of the variable Interest Rate by 0.5% (0.8) (1.4) 0.3 The Group is currently not subject to significant interest rate risks from the account payables reported on the balance sheet. 7.4.4 Capital Management The Management Board’s policy for capital management is to preserve a strong and sustainable capital base in order to maintain the confidence of investors, business partners, and the capital market and to support fu- ture business development. As of December 31, 2021, the equity ratio was 9.6% (December 31, 2020: 37.4%; see also the following overview). The equity ratio decreased mainly due to the initial recognition of the fi- nancial liabilities from future payments to Royalty Pharma. in 000’ € 12/31/2021 12/31/2020 Stockholders’ Equity 244,876 621,322 In % of Total Capital 9.6 37.4 Total Liabilities 2,311,378 1,038,191 In % of Total Capital 90.4 62.6 Total Capital 2,556,254 1,659,513 There are no liabilities to banks. During the financial year, the Group made changes to its capital management by reflecting the financial liabil- ities from future payments to Royalty Pharma. 7.5 Disclosures to Statement of Cash Flows – Net Debt Reconciliation The following overview contains the presentation and development of the liabilities from financing activities. “Amortizations from Effective Inter- est Method”, “Changes from Adjustments to Planning Assumptions” and “Transfer of Assigned License Revenues to Royalty Pharma” include non- cash movements, including accrued interest expense. Notes Financial Statements 169

in 000’ € Lease Liabilities Bonds Financial Liabilities from Collaborations Financial Liabilities from Future Pay- ments to Roy- alty Pharma Total Balance as of 1. January 2020 (42,557) 0 0 0 (42,557) Cash Flows 3,918 (319,946) (542,599) 0 (858,627) New Leases (5,286) 0 0 0 (5,286) Exchange differences 0 0 66,379 0 66,379 Changes recognized in Equity 0 49,217 0 0 49,217 Amortizations from Effective Interest Method (1,094) (2,454) (15,329) 0 (18,877) Changes from Adjustments to Planning Assumptions 0 0 (24,956) 0 (24,956) Balance as of 31. December 2020 (45,019) (273,183) (516,506) 0 (834,708) Balance as of 1. January 2021 (45,019) (273,183) (516,506) 0 (834,708) Cash Flows 4,286 2,031 0 (1,205,911) (1,199,594) New Leases (316) 0 0 0 (316) Disposal Leases 173 0 0 0 173 Exchange differences (538) 0 (39,346) (7,499) (47,383) Amortizations from Effective Interest Method (1,170) (12,056) (20,386) (29,811) (63,422) Changes from Adjustments to Planning Assumptions 0 0 61,876 (64,846) (2,970) Transfer of Assigned License Revenues to Royalty Pharma 0 0 0 51,890 51,890 Balance as of 31. December 2021 (42,584) (283,208) (514,362) (1,256,176) (2,096,329) The “Transfer of Assigned License Revenues to Royalty Pharma” include transactions whereas Janssen directly transfers to Royalty Pharma the settlement amount without influence by MorphoSys on timing and/or amount. As MorphoSys has not received or paid cash for these assigned license revenues, the related amounts have neither been included in the operating nor in the financing cash flow, respectively. The changes from the bonds recognized in equity in 2020 relate initially to the transfer of the conversion right to additional paid-in capital and conversions in subsequent periods. 7.6 Geographical Disclosures A total of € 132.9 million (December 31, 2020: € 311.6 million) of the Group’s non-current assets, excluding deferred tax assets, are located in Germany and € 1,103.8 million in the USA (December 31, 2020: € 8.3 mil- lion). Of the Group’s investments, € 24.5 million (2020: € 47.6 million) were made in Germany and € 1.7 million (2020: € 1.6 million) in the USA. In accordance with internal definitions, investments solely include addi- tions to property, plant and equipment and intangible assets not related to leases and business combinations. 7.7 Corporate Governance The Group has submitted the Declaration of Conformity with the recom- mendations of the Government Commission on the German Corporate Governance Code for the 2021 financial year under Section 161 of the German Stock Corporation Act (AktG). This declaration was published on the Group’s website (https://www.morphosys.com/en/investors/ corporate-governance) on November 29, 2021 and made permanently available to the public. 7.8 Research and Development Agreements The Group has entered some research and development agreements. The following information describes the agreements that have a material ef- fect on the Group and the developments under the research and develop- ment agreements in the 2021 financial year. 7.8.1 Agreements Related to Proprietary Clinical Development Partnerships currently exist with (in alphabetical order) Incyte and Xencor. In January 2020, MorphoSys and Incyte announced that the companies had signed a collaboration and license agreement for the continued global development and commercialization of MorphoSys’s proprietary an- ti-CD19 antibody tafasitamab. A detailed description of the agreement can be found in Note 5.18*. * cross-reference to page 143 In June 2010, MorphoSys and the U.S.-based biopharmaceutical company Xencor signed an exclusive global licensing and cooperation agreement under which MorphoSys receives exclusive global licensing rights to taf- asitamab, the antibody for the treatment of cancer and other indications. The companies jointly conducted a phase 1/2a trial in the U.S. in patients with chronic lymphocytic leukemia. MorphoSys is solely responsible for the further clinical development after the successful completion of the phase 1 clinical trial and commercialization. Upon signing the license and cooperation agreement, Xencor received a payment of US$13.0 mil- lion (approximately € 10.5 million) from MorphoSys. Xencor also received milestone payments from MorphoSys totaling US$65.5 million (approxi- mately € 53.8 million). These payments were then capitalized under in-process R&D programs. Xencor is entitled to development, regulatory and commercially related milestone payments. Furthermore, Xencor is also eligible to receive tiered royalty payments of tafasitamab in the mid single-digit to sub-teen double-digit percentage range based upon net sales of licensed antibody sold by us or our licensees. Our royalty obliga- tions continue on a product-by-product and country-by-country basis un- til the later to occur of the expiration of the last valid claim in the licensed patent covering a licensed product in such country, or 11 years after the first sale of a licensed product following marketing authorization in such country. In November 2020, MorphoSys, Incyte and Xencor announced a clinical collaboration agreement to study the combination of tafasitamab, plam- otamab and lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), first-line DLBCL and relapsed or refrac- tory follicular lymphoma (FL). MorphoSys and Incyte will provide tafasi- 170 Financial Statements Notes

tamab for the studies. The studies are sponsored and funded by Xencor and are planned to be conducted in North America, Europe and the Asia-Pacific region. 7.8.2 Agreements Related to Clinical Development Through Partners Through some commercial partnerships, MorphoSys receives various types of payments that are spread over the duration of the agreements or recognized in full as revenue as predefined targets and milestones are reached. These payments include payments upon signature, annual li- cense fees in exchange for access to MorphoSys’s technologies and pay- ments for funded research to be performed by MorphoSys on behalf of the partner. MorphoSys is also entitled to development-related milestone payments and royalties on product sales for specific antibody programs. Prior to the 2021 financial year, active collaborations with a number of partners had already ended. However, drug development programs initi- ated in the active phase are designed so that they can be continued by the partner and, therefore, still result in performance-based payments for the achievement of the defined milestones. Partnerships (incl. partnerships for which the active collaboration has ended before the beginning of 2021 but where drug development pro- grams were still being pursued) include (in alphabetical order): advance- COR, Bayer AG, Boehringer Ingelheim, Fibron Ltd. (transfer of the con- tract from ProChon Biotech Ltd.), GeneFrontier Corporation/Kaneka, GlaxoSmithKline (GSK), I-Mab Biopharma, Janssen Research and Devel- opment LLC, LEO Pharma, Novartis, OncoMed Pharmaceuticals (fully acquired in April 2019 by Mereo BioPharma Group), Pfizer, Roche and Sosei Heptares. In June 2013, MorphoSys announced it had entered into a global agree- ment with GSK for the development and commercialization of otilimab. Otilimab is MorphoSys’s proprietary HuCAL antibody against the GM- CSF target molecule. Under the agreement, GSK assumes responsibility for the compound’s entire development and commercialization. MorphoSys received an upfront payment of € 22.5 million under this agreement and, next to tiered double-digit royalties on net sales, is eligi- ble to receive additional payments from GSK of up to € 423.0 million, de- pending on the achievement of certain developmental stages, as well as regulatory, commercial and revenue-related milestones. In July 2019, GSK initiated a phase 3 development program in rheumatoid arthritis called ContRAst. The treatment of the first patient in this program trig- gered a milestone payment of € 22.0 million to MorphoSys. In 2020, GSK also initiated a clinical trial (OSCAR) to evaluate the efficacy and safety of otilimab in patients with severe pulmonary COVID 19-associated dis- ease. The dosing of the first patient in the expanded OSCAR study trig- gered milestone payments totaling € 16.0 million to MorphoSys. In Octo- ber 2021, GSK provided an update that it had made the decision not to further explore otilimab as a potential treatment for severe pulmonary COVID-19 related disease in patients 70 years and older. In November 2017, MorphoSys announced it had signed an exclusive re- gional licensing agreement with I-Mab to develop and commercialize felzartamab in mainland China, Taiwan, Hong Kong and Macao. Felz- artamab is MorphoSys’s proprietary antibody targeting CD38. Under the terms of the agreement, I-Mab has the exclusive right for the later devel- opment and commercialization of felzartamab in the agreed regions. In November 2017, MorphoSys received a payment of US$20.0 million (ap- proximately 16.8 million) and until 2021 milestone payments of US$8.0 million (approximately € 7.1 million). MorphoSys is also entitled to receive additional success-based clinical and commercial milestone payments from I-Mab of up to roughly US$90.5 million (approximately € 79.9 million). In addition, MorphoSys will be entitled to receive dou- ble-digit, staggered royalties on the net sales of felzartamab in the agreed regions. I-Mab is investigating felzartamab in a phase 3 clinical study in Greater China in combination with lenalidomide plus dexameth- asone in r/r multiple myeloma. I-Mab is also evaluating felzartamab as a potential third-line therapy in r/r multiple myeloma in a phase 2 trial. Both studies are considered pivotal in the agreed regions. In November 2018, MorphoSys announced the signing of an exclusive strategic development collaboration and regional licensing agreement with I-Mab for MOR210/TJ210. MOR210/TJ210 is MorphoSys’ proprietary, preclinical-stage antibody directed against C5aR which has potential to be developed as an immuno-oncology agent. I-Mab has exclusive rights to develop and market MOR210/TJ210 in mainland China, Hong Kong, Ma- cao, Taiwan and South Korea, while MorphoSys retains the rights for the rest of the world. With the support of MorphoSys, I-Mab will undertake and fund all global development activities, including clinical trials in China and the United States, to clinical proof of concept in cancer medi- cine. In November 2018, MorphoSys received a payment of US$3.5 mil- lion (approximately € 3.1 million) and until 2020 milestone payments of US$1.0 million (approximately € 0.8 million). MorphoSys is further eligi- ble to receive performance-related clinical and sales-based milestone payments of up to US$ 99.0 million (approximately € 87.4 million). In addition, MorphoSys will receive tiered royalties in the mid-single-digit percentage range of net sales of MOR210/TJ210 in I-Mab’s teritorries. In return for conducting a successful clinical proof of concept trial, I-Mab is entitled to low-single-digit royalties on net sales of MOR210/TJ210 out- side the I-Mab territory, as well as staggered shares of proceeds from the further out-licensing of MOR210. In January 2021, MorphoSys and I-Mab announced the dosing of first patient in a U.S. phase 1 study. MorphoSys received a US$1.5 million (approx. € 1.2 million) payment from I-Mab for achieving this milestone. The Group’s alliance with Novartis AG for the research and development of biopharmaceuticals came to an end in November 2017. The collabora- tion began in 2004 and led to the creation of several ongoing therapeutic antibody programs against a number of diseases. MorphoSys receives performance-based milestones contingent upon the successful clinical development and regulatory approval of several products. In addition to these payments, MorphoSys is also entitled to royalties on any future product sales. 7.9 Subsequent Events No events that require reporting occurred. Planegg, March 15, 2022 Jean-Paul Kress, M.D. Sung Lee Chief Executive Officer Chief Financial Officer Malte Peters, M.D. Chief Research and Development Officer Notes Financial Statements 171

To the best of our knowledge, and in accordance with the applicable re- porting principles, the consolidated financial statements give a true and fair view of the Group’s net assets, financial position and results of oper- ations, and the group management report provides a fair review of the development and performance of the business and the position of the Group, together with a description of the principal opportunities and risks associated with the Group’s expected development. Planegg, March 15, 2022 Jean-Paul Kress, M.D. Sung Lee Chief Executive Officer Chief Financial Officer Malte Peters, M.D. Chief Research and Development Officer Responsibility Statement 172 Responsibility StatementAdditional Information

Executive Committee of MorphoSys AG Jean-Paul Kress, M.D. Chief Executive Officer Sung Lee Chief Financial Officer Charlotte Lohmann SVP General Counsel 180 Additional Information Executive Committee of MorphoSys AG

Malte Peters, M.D. Chief Research and Development Officer Barbara Krebs-Pohl, Ph.D. SVP Global Head of BD&L and Alliance Management Maria Castresana SVP Global Head of Human Resources Daniel Palmacci SVP Global Head of Technical Operations Joe Horvat General Manager MorphoSys US Executive Committee of MorphoSys AG Additional Information 181

Glossary A ADS – American Depositary Share; share of a non-U.S. company that is held by a U.S. depos- itary bank and is traded at a stock exchange in the U.S. Amyloid beta – Protein produced by the body that can be deposited in the brain and is asso- ciated with the development of Alzheimer’s disease Antibody library – A collection of genes that encode corresponding human antibodies Antigen – Foreign substance stimulating anti- body production; binding partner of antibody anti-PLA2R antibody positive membranous nephropathy – autoimmune kidney disease ASCT – Autologous stem cell transplantation; Treatment with stem cells from a patients own body for the treatment of lymphomas B B-cells – White blood cells, part of the immune system, capable of generation antibodies BLA – Biologics License Application; request to the FDA for permission to introduce, or deliver for introduction, a biologic product into inter- state commerce B-MIND – Study to evaluate bendamustine- tafasitamab in DLBCL C C5a – Part of the immune system; involved in growth of certain cancers C5aR – Receptor for C5a CAR-T – CD19 chimeric antigen receptor T-cell CD19 – Potential therapeutic target for immuno therapy CD38 – Potential therapeutic target for immu- notherapy Clinical trial – Clinical trials allow safety and efficacy data to be collected for new drugs or devices; depending on the type of product and the stage of its development, investigators en- roll healthy volunteers and/or patients into small pilot studies initially, followed by larg- er-scale studies in patients CLL – Chronic lymphocytic leukemia; most common type of cancer of the blood and bone marrow, affecting the B cells COSMOS – CLL patients assessed for ORR/ Safety in tafasitamab study CPI-0610 – Pelabresib CR – Complete response D DLBCL – Diffuse large B cell lymphoma, a sub- form of ›› NHL DoR – duration of response E EMA – European Medicines Agency EZH2 – enzyme that suppresses target gene expression F FDA – Food and Drug Administration; U.S. fed- eral agency for the supervision of food and drugs Felzartamab – MOR202; human monoclonal HuCAL-IgG1-antibody directed against the target molecule CD38 firstMIND – Clinical phase 1b study with tafasit amab in first-line patients with DLBCL FL – Follicular lymphoma frontMIND – Pivotal phase 3 study with tafasit- amab in first-line patients with DLBCL G GCP – Good clinical practice; an international ethical and scientific quality standard for de- signing, conducting, recording and reporting trials that involve the participation of human subjects GDP – Good distribution practice; guidelines on quality standards for distribution practice of pharmaceutical products GLP – Good laboratory practice; a formal framework for the implementation of safety tests on chemical products GM-CSF – Granulocyte-macrophage colony- stimulating factor; underlying target molecule of MOR103 program GMP – Good manufacturing practice; term for the control and management of manufactur- ing and quality control testing of pharmaceu- tical products and medical devices GVP – Good pharmacovigilance practice; quality standard for monitoring the safety of medicinal products GxP – General abbreviation for the “good practice” quality guidelines and regulations H HDC – Highdose chemotherapy Hemibody technology – Multispecific anti- body technology for the recruitment of effec- tor cells (T cell engager) HuCAL – Human Combinatorial Antibody Li- brary; proprietary antibody library enabling rapid generation of specific human antibodies for all applications 182 Additional Information Glossary

I IFRS – International Financial Reporting Stan- dards; accounting standards issued by the IASB and adopted by the EU IgAN – the most common form of glomerulo- nephritis IGNAZ – phase 2 trial evaluating felzartamab in patients with IgAN IND – Investigational New Drug; application for permission to test a new drug candidate on humans, i.e. in clinical studies inMIND – Pivotal phase 3 study with tafasitam- ab in patients with indolent lymphomas L L-MIND – Study to evaluate lenalidomide- tafasitamab in DLBCL M MAA – Marketing Authorization Application; application seeking permission to bring a me- dicinal product to the market in Europe Market capitalization – Value of a company’s outstanding shares, as measured by shares times current price MM – Multiple Myeloma; Type of cancer that develops in a subset of white blood cells called plasma cells formed in the bone marrow MN – membranous nephropathy Monjuvi® (tafasitamab-cxix) – First propri- etary drug on the market; approved in the U.S. in July 2020 in combination with lenalidomide for the treatment of adult patients with re- lapsed or refractory diffuse large B-cell lym- phoma (DLBCL) not otherwise specified, in- cluding DLBCL arising from low grade lymphoma, and who are not eligible for autolo- gous stem cell transplant (ASCT). Tafasitamab is co-marketed by Incyte and MorphoSys under the brand name Monjuvi® in the U.S., and mar- keted by Incyte under the brand name Minjuvi® in Europe and Canada M-PLACE – Phase 1/2 study with felzartamab in anti-PLA2R antibody positive membranous nephropathy MZL – Marginal zone lymphoma N NHL – Non-Hodgkin lymphoma; diverse group of blood cancers that include any kind of lym- phoma except Hodgkin lymphoma New-PLACE – Phase 2 study with felzartamab in anti-PLA2R antibody positive membranous nephropathy O ORR – Overall response rate OS – Overall survival Otilimab – formerly MOR103/GSK3196165 P Pelabresib – CPI-0610 PFS – Progression-free survival Pola-BR – polatuzumab vedotin plus benda- mustine and rituximab PR – partial response PsA – Psoriatic arthritis Chronic joint inflamma- tion that occures in connection with psoriasis Psoriasis – A chronic, non-contagious autoim- mune disease which affects the skin and joints R R2 – rituximab plus lenalidomide R-CHOP – Rituximab, Cyclophosphamid, Doxorubicin, Vincristin and Prednison; Combi- nation treatment with rituximab and combina- tion chemotherapy as standard first-line treatment of ›› DLBCL RE-MIND2 – Retrospective observational study to compare the efficacy of tafasitamab in combination with lenalidomide in the L-MIND study against the most frequently used treatments in adult patients with relapsed or refractory diffuse large B-cell lymphoma Rheumatoid arthritis – Inflammatory disease of the joints; abbreviation: RA Royalties – Percentage share of ownership of the revenue generated by drug products r/r – relapsed or refractory S SAE – serious adverse event SD – stable disease SLL – Small lymphocytic lymphoma Slonomics – DNA engineering and protein li- brary generation platform acquired by MorphoSys in 2010 SOX – Sarbanes-Oxley Act of 2002 Splenomegaly – increased spleen size T Tafasitamab – MOR208, formerly XmAb5574 Target – Target molecule for therapeutic in- tervention, e.g. on the surface of diseased cells T-cells – An abbreviation for T-lymphocytes; a subtype of white blood cells that together with B-lymphocytes are responsible for the body’s immune defense TEAE – treatment emergent adverse event topMIND – trial sponsored by Incyte evaluat- ing tafasitamab in combination with parsaclis- ib for adults with r/r B-cell malignancies Y Ylanthia – The novel next-generation anti- body platform of MorphoSys Glossary Additional Information 183

List of Figures and Tables Figures 01 Performance of the MorphoSys Share in 2021 28 02 Performance of the MorphoSys Share 2017–2021 28 03 Total Headcount of the MorphoSys Group 52 04 Employees By Gender 54 05 Revenues by Region 57 06 Revenues by Categories 58 07 Monjuvi Product Sales 58 08 Selected R&D Expenses 60 09 Risk and Opportunity Management System at MorphoSys 77 10 Compliance Management Program (CMP) 103 Tables 01 Key Data for the MorphoSys Share 27 02 Analyst Recommendations 29 03 Development of Key Financial Performance Indicators 38 04 Multi-Year Overview – Statement of Profit or Loss 62 05 Multi-Year Overview – Financial Situation 65 06 Multi-Year Overview – Balance Sheet Structure 67 07 Contractual Obligations 67 08 Comparison of Actual Business Results Versus Forecasts 68 09 Comparison of Old and New Integrated Opportunity & Risk Management System 76 10 Risk Assessment Categories 78 11 Summary of MorphoSys’ Key Opportunities 79 12 Overview of Risk Categories 81 13 Overview of MorphoSys’ Most Significant Risks 82 14 Composition of the Supervisory Board until Termination of the 2021 Annual General Meeting 92 15 Composition of the Supervisory Board since Termination of the 2021 Annual General Meeting 92 16 Participation of Supervisory Board Members 94 17 Managers’ Transactions 2021 100 184 Additional Information List of Figures and Tables

MorphoSys AG Semmelweisstrasse 7 82152 Planegg Germany Phone: +49-89-89927-0 Fax: +49-89-89927-222 Email: info@morphosys.com Website: www.morphosys.com/en Investor Relations Phone: +49-89-89927-404 Fax: +49-89-89927-5404 Email: investors@morphosys.com Concept and Design 3st kommunikation GmbH, Mainz Photography/Picture Credits Andreas Pohlmann, Munich Getty Images iStock Shutterstock Translation Klusmann Communications, Niedernhausen Editorial Office Götz Translations and Proofreading GmbH, Hamburg Typesetting and Lithography Knecht GmbH, Ockenheim Printer Woeste Druck + Verlag GmbH & Co. KG, Essen-Kettwig Publication date March 16, 2022 This Annual Report is also available in German and can be downloaded from the Company’s website. For better readability, this report uses the masculine form only but refers equally to all genders. HuCAL®, HuCAL GOLD®, HuCAL PLATINUM®, CysDisplay®, RapMAT®, Ylanthia®, 100 billion high potentials®, Slonomics®, CyCAT®, MONJUVI® and MINJUVI® are registered trademarks of the MorphoSys Group. Tremfya® is a registered trademark of Janssen Biotech, Inc. XmAb® is a registered trademark of Xencor Inc. National Comprehensive Cancer Network®, NCCN® and NCCN Guidelines® are registered trademarks of the National Comprehensive Cancer Network, Inc. Imprint

March 16 Publication of 2021 Year-End Results May 4 Publication of 2022 First Quarter Interim Statement May 18 2022 Annual General Meeting August 3 Publication of 2022 Half-Year Report November 16 Publication of 2022 Third Quarter Interim Statement Financial Calendar 2022 MorphoSys AG Semmelweisstrasse 7 82152 Planegg Germany Phone: +49-89-89927-0 Fax: +49-89-89927-222 www.morphosys.com/en A nn ua l R ep or t 20 21






















































































































































































