
Management Report and Financial Statements of MorphoSys AG as of December 31, 2021 MorphoSys AG, Planegg

2 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021

Management Report 3 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Management Report Significant developments in financial year 2021 In 2021, MorphoSys continued its work to discover, develop and commercialize, innovative therapies for patients, with a focus on cancer and autoimmune diseases. In July 2021, we completed the acquisition of Constellation Pharmaceuticals, Inc. ("Constellation"). The addition of Constellation offers a transformational growth opportunity for MorphoSys, expanding our pipeline in a meaningful way with two clinical-stage cancer drug candidates. In addition to this major corporate event, we continued to make progress with our existing programs. At the time of the Constellation acquisition, we also entered into a funding agreement with Royalty Pharma plc. Our lead program, tafasitamab, is already on the market in the U.S. under the brand name Monjuvi (tafasitamab-cxix). Monjuvi (tafasitamab-cxix) has been approved under accelerated approval by the U.S. FDA in July 2020. Together with Incyte, we are co-promoting Monjuvi in the U.S. Incyte holds exclusive rights for development and commercialization outside the U.S. In August 2021, the European Commission (EC) granted conditional marketing authorization for Minjuvi® (tafasitamab) in Europe in combination with lenalidomide, followed by Minjuvi monotherapy, for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) who are not eligible for autologous stem cell transplant (ASCT). Also in August 2021, Health Canada granted Incyte a Notice of Compliance with conditions for Minjuvi (tafasitamab) in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, who are not eligible for ASCT. In January 2021, a marketing authorization application for tafasitamab was accepted for review by regulatory authorities in Switzerland. To further broaden the tafasitamab opportunity, during 2021, MorphoSys initiated the frontMIND study, a pivotal phase 3 trial in patients with first-line DLBCL. Also, MorphoSys and Incyte announced the first patient dosed in inMIND, a phase 3 study in patients with r/r follicular lymphoma or r/r marginal zone lymphoma (MZL). In June 2021, MorphoSys and Incyte announced new three-year follow-up data from the phase 2 L- MIND study of tafasitamab in combination with lenalidomide in adult patients with r/r DLBCL. The new results built on previous findings showing durable responses and a consistent safety profile of tafasitamab in combination with lenalidomide followed by tafasitamab monotherapy. In December 2021, additional results from RE-MIND2 dataset comparing tafasitamab and lenalidomide outcomes to those observed in matched cohorts of 1) polatuzumab vedotin plus bendamustine and rituximab (pola-BR), 2) rituximab plus lenalidomide (R2); and 3) CAR-T therapies were presented. The findings suggested that tafasitamab plus lenalidomide may improve health outcomes compared to pola-BR and R2, with a prolonged survival benefit for r/r DLBCL patients. Comparable overall survival (OS) between tafasitamab and lenalidomide and CAR-T therapies was observed. Pelabresib is a late-stage proprietary program that MorphoSys acquired through the Constellation acquisition. We are currently conducting two clinical trials of pelabresib for the treatment of myelofibrosis (MF): MANIFEST, our ongoing, open-label phase 2 clinical trial evaluating pelabresib both as a monotherapy and in combination with ruxolitinib, and MANIFEST-2, our global, double-blinded, randomized pivotal phase 3 study evaluating pelabresib in combination with ruxolitinib versus placebo in JAK-inhibitor-naïve MF patients. Exploratory data from the MANIFEST trial were presented at the European Hematology Association (EHA) Annual Meeting in June 2021. Translational data across all three arms of the study illustrated the effect of pelabresib on key cytokines associated with MF and its impact on bone marrow fibrosis. Taken together,

4 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 these data support our hypothesis that pelabresib may have a potential disease-modifying activity in MF. At the American Society of Hematology (ASH) Annual Meeting in December 2021, we presented updated interim clinical and translational data from the ongoing MANIFEST trial, which included 54 more patients and longer- term follow-up than previously reported data. We believe that the latest interim data from MANIFEST underscore the potential of pelabresib in the treatment of MF. With respect to MANIFEST-2, MorphoSys has optimized the study design and implemented measures to accelerate patient recruitment since the acquisition of Constellation. Progress was also made with programs in earlier stages of clinical development. Interim data from the phase 1/2 proof-of-concept M-PLACE trial that evaluates felzartamab in anti-PLA2R antibody positive membranous nephropathy (MN) were presented in November 2021. Early efficacy data were presented: of the 27 treated patients with evaluable results, 24 showed an initial rapid reduction of anti-PLA2R antibody levels one week after the first treatment. The safety profile was shown to be consistent with the proposed mechanism of action of felzartamab. The trial was fully enrolled in November 2021. Two additional trials with felzartamab were initiated during 2021 – New-PLACE, a phase 2 study evaluating different treatment schedules to identify the regimen for a pivotal study in patients with anti-PLA2R antibody positive MN, and the phase 2 IGNAZ trial evaluating felzartamab in patients with immunoglobulin A nephropathy (IgAN). CPI-0209 is another product candidate acquired through Constellation. CPI-0209 is a small molecule designed to promote anti-tumor activity by specifically inhibiting the enzymatic function of enhancer of zeste homolog 2 (EZH2) protein. We are currently conducting a phase 1/2 clinical trial of CPI-0209 in patients with solid tumors and hematological malignancies. Our partners responsible for clinical development of licensed programs also continued their activities during 2021. For example, in June I-Mab announced that the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) had approved the Investigational New Drug (IND) application to initiate a phase 1b study with felzartamab in patients with systemic lupus erythematosus (SLE), the most common form of lupus. In October, licensing partner Roche received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) for gantenerumab for the treatment of people living with Alzheimer’s disease. Also in October 2021, licensing partner GSK announced that it had made the decision not to further explore otilimab as a potential treatment for severe pulmonary COVID-19- related disease in patients aged of 70 years and older. Clinical development of otilimab in rheumatoid arthritis remains ongoing. In 2021, MorphoSys continued to transform the Company with activities focused on advancing and positioning the Company for long-term success. We grew sales of Monjuvi, while also broadening our pipeline through a transformative acquisition.

Management Report 5 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Fundamentals of MorphoSys AG Organizational Structure and Business Model MorphoSys AG discovers and develops innovative therapies for patients suffering from cancer and autoimmune diseases. MorphoSys AG, as the ultimate parent company, is located in Planegg, near Munich. MorphoSys AG has one wholly owned subsidiary, MorphoSys US Inc. (Boston, Massachusetts, USA). MorphoSys US Inc. in turn has a wholly owned subsidiary - Constellation Pharmaceuticals, Inc. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. also has a wholly owned subsidiary, Constellation Securities Corp. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. and Constellation Securities Corp. are collectively referred to as “Constellation”, and all entities constitute the “MorphoSys Group” or “Group”. The Planegg site MorphoSys AG houses the central functions such as accounting, controlling, human resources, legal, patents, purchasing, corporate communications and investor relations, as well as the scientific research departments and laboratories. MorphoSys US Inc. is responsible for advancing tafasitamab’s commercialization. Constellation focuses its activities on the clinical development of drug candidates and the related administrative departments. Legal structure of the MorphoSys: Company Management and supervision The parent company of the MorphoSys Group is MorphoSys AG, a German stock corporation listed in the Prime Standard segment of the Frankfurt Stock Exchange and on the NASDAQ Global Market. In accordance with the German Stock Corporation Act, the Company has a dual management structure with the Management Board as the governing body. The four members of the Management Board are appointed and supervised by the Supervisory Board. Following the departure of Roland Wandeler, Ph.D., Chief Operating Officer, effective December 31, 2021, the Management Board consists of only three members. The Supervisory Board of MorphoSys AG is elected by the Annual General Meeting and currently consists of six members. Detailed information on the Company’s management and supervision and its corporate governance principles can be found in the Corporate Governance Report. Targets and Strategy MorphoSys mission is to discover, develop and commercialize innovative therapies for patients. MorphoSys is a fully integrated commercial biopharmaceutical company. Its activities in 2021 focused on hematology/oncology and autoimmune diseases. The Company aims to realize intermediate- and long-term growth through its focus on proprietary drug development and commercialization. Through the acquisition of Constellation, the Company has rapidly expanded its pipeline in the hematology/oncology area. Our priority is on the Company’s lead development candidates pelabresib and tafasitamab; continuing to make progress with the commercialization of Monjuvi and obtaining approvals in additional indications; bringing pelabresib to the market as well as continuing to develop other clinical candidates. MorphoSys is now primarily advancing the clinical development of its own compounds, with further antibody candidates being clinically developed by partners. During the clinical phases, decisions are made on a case-

6 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 by-case basis as to whether and at what point a partnership for further development and commercialization should be pursued. Drug candidates can be either fully out-licensed, developed on a proprietary basis or with a partner (co-development). The development of drug candidates on behalf of other companies is no longer a focus of MorphoSys’ business activities. As such, the previous segment reporting for the Proprietary Development and Partnered Discovery segments was discontinued as of the first quarter of 2021. The development of drug candidates is broadly based on MorphoSys’ innovative technologies. These include our established antibody and technology platforms HuCAL®, Ylanthia® and Slonomics®, as well as the bispecific technology CyCAT®. Under the agreement signed with Cherry Biolabs in November 2020, MorphoSys was granted exclusive access to the Hemibody technology for several targets, which will be used to develop a novel multispecific antibody technology for effector cell recruitment (T-cell engager). We continue to leverage our resources and know-how to expand and develop these technologies. This may include the expansions of our portfolio not only through our own proprietary research and development activities but also through in-licensing and acquisitions. Company’s Management and Performance Indicators MorphoSys AG uses financial indicators to steer the Company. These indicators help to monitor the success of strategic decisions and give the Company the opportunity to take quick corrective action when necessary. The Company’s management also monitors and evaluates selected early indicators so that it can thoroughly assess a project’s progress and promptly take the appropriate actions should a problem occur. No most important non-financial performance indicators are used for steering the Company. Material non-financial aspects are taken into account in a separate “Non-Financial Group Report", which is available on our website. Financial Performance Indicators The development of the financial performance indicators in the reporting year is described in detail in the chapter “Analysis of Net Assets, Financial Position and Results of Operations”. The most important financial indicators used to measure the Company’s operating performance are revenues, operating expenses (total sum of cost of sales, research and development expenses, selling expenses and general administration expenses) and research and development expenses as percentage of total operating expenses. In future periods, research and development expenses as well as selling, general and administrative expenses will be used as most important financial performance indicators for steering the Company. MorphoSys’ business performance is additionally influenced by liquidity. This indicator is also routinely analyzed and evaluated. Liquidity position is not considered to be part of the key financial performance indicators The budget for the respective financial year is approved by the Management Board and Supervisory Board. Subsequent to the approval of the budget, a forecast is made two times within the year, to assess if the Company is on track to achieve its financial goals and progress towards financial guidance. The forecast informs decision making and enables management to take actions to achieve its goals. Non-Financial Aspects With its own marketed drug in the U.S., MorphoSys has completed its transformation from a technology provider to a fully integrated biopharmaceutical company. The Company’s core mission has not changed in

Management Report 7 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 this process: We strive to develop new drugs for the well-being of patients with serious diseases. To ensure sustainable business success in this endeavor, we take selected non-financial aspects into account in addition to financial performance indicators. At MorphoSys, innovation in research and development remains a central aspect. Our research and development strategy focuses on indications with high unmet medical need, where patients’ lives depend on new treatment options. Our goal is to improve the lives of these patients by focusing on therapeutic areas that best fit our expertise while making optimal use of our resources. The acquisition of Constellation in July 2021 represents a significant investment in the development of new drugs for patients with limited treatment options in the field of hematology/oncology. Leading Indicators MorphoSys follows a variety of leading indicators to monitor the macroeconomic environment, the industry and the Company itself. At the Company level, economic data is gathered on the progress of individual programs. MorphoSys uses general market data and external financial reports to acquire information on leading macroeconomic indicators such as industry transactions, changes in the legal environment and the availability of research funding and reviews these data carefully. Market analyses that assess the medical need for innovative therapies for serious diseases focused not only on cancer and autoimmune diseases, but also generally with regard to new technologies in the market, serve as early indicators in the area of business development. By continuously monitoring the market, MorphoSys can respond to trends and requirements quickly and initiate its own activities and partnerships. For active collaborations, a joint steering committee meets regularly (usually two to four times per year) to update and monitor the programs’ progress. These ongoing reviews give the Company a chance to intervene at an early stage if there are any negative developments and provide it with information about expected interim goals and related milestone payments well in advance. Partners in non-active collaborations regularly provide (once per year) MorphoSys with written reports so that the Company can follow the progress of active therapeutic programs. Commercialization In July 2018, MorphoSys established a subsidiary in the United States – MorphoSys US Inc. – in preparation for the potential marketing approval of tafasitamab. The subsidiary’s registered office is located in Boston, Massachusetts, USA. At the end of 2021, MorphoSys US Inc. had 93 people employed as part of, or to support, its commercial structure. On July 31, 2020, Monjuvi (tafasitamab-cxix) in combination with lenalidomide was approved under accelerated approval by the U.S. FDA for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT). This was the first U.S. FDA approval of a second-line treatment for adult patients with r/r DLBCL in the U.S. The safety and tolerability profile supports a paradigm shift towards treating patients to progression, which could enable long-term disease control. Monjuvi is accessible to patients in both community care and academic settings as an off-the- shelf intravenous infusion that does not require hospitalization or heavy monitoring. Upon approval, MorphoSys and Incyte launched 'My Mission Support', a robust patient support program offering financial

8 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 assistance, ongoing education and other resources to eligible patients who are prescribed Monjuvi in the U.S. The program was launched to support patients throughout their treatment journeys and to help lower patient access barriers. Monjuvi has been included in the National Comprehensive Cancer Network® Clinical Practice Guidelines (NCCN Guidelines®) in Oncology for B-cell Lymphomas since August 2020. The NCCN Guidelines in the United States were updated to include Monjuvi in combination with lenalidomide with a Category 2A designation as an option for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are not eligible for ASCT. Inclusion in these guidelines increases awareness of a product within the oncology community and also drives certain formulary decisions. As of April 1, 2021, Monjuvi was granted a J-code, further simplifying reimbursement for some treatment centers. Business Performance During 2021, MorphoSys focused on advancing product candidates at various stages of development. The acquisition of Constellation represents a transformation for MorphoSys, expanding its clinical development pipeline and positioning the Company for long-term sustainable growth. The key measures of value for MorphoSys’ research and development activities include: • Project launches and the advancement of individual development programs • Clinical and preclinical research results • Regulatory guidance of healthcare authorities for the approval of individual therapeutic programs • Collaborations, partnerships and M&A activities with other companies to expand the technology base and expand the drug pipeline, as well as to commercialize the therapeutic programs • Strong patent protection to secure MorphoSys’ market position MorphoSys announced on June 2, 2021, its plans to acquire Constellation Pharmaceuticals, Inc. for US$ 34.00 (equivalent to € 28.79) per share in cash, representing a total equity value of US$1,635.2 million (equivalent to €1,384.7 million). The transaction was unanimously approved by the Management Board and Supervisory Board of MorphoSys as well as by the Board of Directors of Constellation. The acquisition was completed on July 15, 2021. Constellation is a clinical-stage biopharmaceutical company that discovers and develops novel product candidates to address serious unmet medical needs in patients with cancers associated with abnormal gene expression or drug resistance. Constellation’s two lead product candidates, pelabresib (CPI- 0610), a BET inhibitor, and CPI-0209, a second-generation EZH2 inhibitor, are in mid- to late-stage clinical development. MorphoSys expects the following benefits from the acquisition: • Accelerates growth strategy with promising mid- to late-stage product candidates: The transaction accelerates MorphoSys’ strategy to grow through proprietary drug development and commercialization. Constellation’s lead product candidates, pelabresib and CPI-0209, are in phase 3 and phase 2, respectively, and may offer broad potential for a range of oncology indications. They fit well with MorphoSys’ proven clinical development, regulatory and commercial capabilities, and MorphoSys is well positioned to rapidly advance and unlock the potential of the Constellation portfolio. • Strengthens position in hematology/oncology and expands into solid tumors: Constellation adds an attractive, complementary pipeline of highly innovative mid- to late-stage cancer therapy candidates, augmenting MorphoSys’ existing pipeline in hematologic malignancies and expanding into potential therapies for solid tumors.

Management Report 9 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 At the time of the Constellation acquisition, MorphoSys also entered into a funding agreement with Royalty Pharma plc (“Royalty Pharma”). Under the terms of this agreement, Royalty Pharma made a US$ 1,300.0 million (€1,100.9 million) upfront payment to MorphoSys and also provided the MorphoSys group access to up to US$ 350.0 million (€ 296.4 million) in development funding bonds with the flexibility to draw over a one-year period. Royalty Pharma also invested US$ 100.0 million (€ 84.7 million) in a cash capital increase of MorphoSys under an authorization to exclude subscription rights of existing shareholders and will make additional payments of up to US$ 100.0 million (€ 84.7 million) upon reaching clinical, regulatory and commercial milestones for otilimab and gantenerumab. Royalty Pharma gained rights to receive 100% of MorphoSys’ royalties on net sales of Tremfya, 80% of future royalties and 100% of future milestone payments on otilimab and 60% of future royalties on gantenerumab. Research and Development As of December 31, 2021, MorphoSys’ research and development activities are currently focused on the following clinical candidates: • Tafasitamab (MOR208, formerly XmAb5574) is a humanized Fc-modified monoclonal antibody directed against CD19. CD19 is selectively expressed on the surface of B-cells, which belong to a group of white blood cells. CD19 enhances B-cell receptor signaling, which is an important factor in B-cell survival and growth. CD19 is a potential target structure for the treatment of B-cell malignancies. • Pelabresib (CPI-0610) is an investigational selective small molecule BET inhibitor with an epigenetic mechanism of action that has been designed to promote anti-tumor activity by specifically inhibiting the function of BET proteins, which normally enhance target gene expression. The FDA and the EMA granted orphan drug designation to pelabresib for the treatment of myelofibrosis in November 2019 and February 2020 respectively. We believe there is an opportunity to address serious unmet medical needs in patients with myelofibrosis. As part of MorphoSys’ agreement with Royalty Pharma, Royalty Pharma is entitled to receive 3% of future net sales of pelabresib. • Felzartamab (MOR202/TJ202) is an investigational human monoclonal HuCAL-IgG1-antibody directed against a unique epitope of the target molecule CD38. CD38 is a surface antigen broadly expressed on malignant myeloma cells as well as on antibody-producing plasmablasts and plasma cells, the latter playing an important role in the pathogenesis of antibody-mediated autoimmune diseases. • CPI‑0209 is an investigational small molecule, second-generation EZH2 inhibitor with an epigenetic mechanism of action that has been designed to achieve comprehensive target coverage through increased on-target residence time. Data from in vitro preclinical models of multiple cancer types suggested that CPI- 0209 may bind to EZH2 more durably and with higher affinity than first-generation EZH2 inhibitors. CPI- 0209 was designed to eliminate auto-induction of metabolism, which has been an issue with other EZH2 inhibitors. Royalty Pharma is entitled to receive 3% of future net sales of CPI-0209. In addition to MorphoSys’ own pipeline, the following programs, among others, are being further developed by MorphoSys’ partners: • Felzartamab (see above) is also being further developed by I-Mab for mainland China, Taiwan, Hong Kong and Macao, where, if approved, it may also be commercialized. I-Mab is currently pursuing development in multiple myeloma (MM) and systemic lupus erythematosus (SLE).

10 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 • Gantenerumab, a HuCAL antibody targeting amyloid beta, is being developed by Roche as a potential treatment for Alzheimer’s disease. As part of the agreement with Royalty Pharma, MorphoSys will retain 40% of future royalties on gantenerumab and will provide Royalty Pharma with 60% of future royalties. • Otilimab (formerly MOR103/GSK3196165) is a HuCAL antibody directed against granulocyte-monocyte colony-stimulating factor (GM-CSF). Due to its diverse functions in the immune system, GM-CSF can be considered a target for a broad range of anti-inflammatory therapies such as rheumatoid arthritis (RA). Otilimab was fully out-licensed to GlaxoSmithKline (GSK) in 2013. MorphoSys will retain 20% of future royalties on otilimab and, as part of the agreement with Royalty Pharma, will provide Royalty Pharma with 80% of future royalties and 100% of future milestone payments. • Tremfya® is a HuCAL antibody targeting the p19 subunit of IL-23 that is being developed and commercialized by Janssen. It is the first commercial product based on MorphoSys’ proprietary technology. Royalty Pharma is entitled to receive 100% of MorphoSys’ royalties on net sales of Tremfya, commencing with the second quarter of 2021. • MOR210/TJ210 is an antibody directed against C5aR, derived from MorphoSys’ HuCAL library. C5aR, the receptor of complement factor C5a, is being investigated as a potential new drug target in the fields of immuno-oncology, immune and chronic inflammatory diseases. In November 2018, MOR210/TJ210 was out-licensed to I-Mab for Greater China and South Korea. • In addition to the programs listed above, MorphoSys and its partners are pursuing several programs in various stages of research and clinical development. Proprietary Clinical Development Tafasitamab Overview Tafasitamab (MOR208, formerly XmAb5574) is a humanized Fc-modified monoclonal antibody directed against CD19. CD19 is selectively expressed on the surface of B-cells, which belong to a group of white blood cells. CD19 enhances B-cell receptor signaling, which is an important factor in B-cell survival and growth, making CD19 a potential target structure for the treatment of B-cell malignancies. The clinical development of tafasitamab is currently focused on B-cell non-Hodgkin’s lymphoma (NHL), particularly diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and marginal zone lymphoma (MZL). In addition, we will initiate the MINDway study, in which we are investigating an optimized treatment regimen to reduce the frequency of drug administration and thereby reduce the burden on the patient. Lymphomas collectively represent approximately 5% of all cancers diagnosed in the United States. The group of NHL diseases is the most prevalent of all lymphoproliferative diseases. According to the National Cancer Institute, there were an estimated 81,560 new cases in the United States in 2021 and an estimated 20,720 deaths due to this disease (“Cancer Stat Facts 2021: Non-Hodgkin’s Lymphoma”). DLBCL is the most common type of NHL in adults and accounts for approximately one-third of all NHL cases globally. The current first- line treatment of B-cell lymphomas, including DLBCL, most commonly consists of a combination chemotherapy regimen plus the antibody rituximab, also referred to commonly as R-CHOP (R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone). Yet, despite the therapeutic success of frontline R-CHOP in DLBCL, up to 40% of patients either do not respond to the treatment (are refractory) or relapse after initial treatment with fast disease progression. The market research and consulting firm GlobalData expects the therapeutic market for non-Hodgkin’s lymphoma (NHL) to reach approximately € 8 billion (approximately US$ 9 billion) in 2024 (report “B-cell NHL: Opportunity Analysis 2017–2027”). We currently forecast an opportunity as a second- and later-line treatment in r/r DLBCL of approximately 10,000 eligible patients per year in the U.S. and approximately 14,000 eligible patients per year in Europe who are not eligible for high-dose chemotherapy (HDC) and ASCT. As a potential first-line treatment in

Management Report 11 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 DLBCL, we believe there is currently a market opportunity of 30,000 patients in the U.S. and 40,000 patients in Europe. Operational Development Tafasitamab is being developed pursuant to a collaboration and license agreement entered into with Xencor, Inc. (Xencor) in June 2010. Under this agreement, Xencor granted MorphoSys an exclusive worldwide license to tafasitamab for all indications. MorphoSys also has a collaboration and license agreement for the global further development and commercialization of tafasitamab with Incyte, signed in January 2020. Under the terms of the agreement, MorphoSys and Incyte will develop tafasitamab broadly in relapsed or refractory (r/r) DLBCL and first-line DLBCL, as well as in additional indications beyond DLBCL, such as follicular lymphoma (FL), and marginal zone lymphoma (MZL). MorphoSys is responsible for conducting frontMIND, a pivotal phase 3 study in first-line DLBCL. Incyte is responsible for conducting inMIND, a pivotal phase 3 study in r/r FL/MZL. Incyte is also responsible for conducting a phase 1b combination study of its PI3K delta inhibitor parsaclisib with tafasitamab in various r/r B-cell malignancies. MorphoSys and Incyte share responsibility for initiating additional global clinical trials. MorphoSys and Incyte are co-commercializing Monjuvi in the United States. Monjuvi in combination with lenalidomide was approved in the U.S. in July 2020 for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low- grade lymphoma, and who are not eligible for autologous stem cell transplantation (ASCT). This was the first FDA approval of a second-line therapy for adult patients with r/r DLBCL in the United States. Monjuvi was approved by the FDA under an accelerated approval process based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). On August 26, 2021, MorphoSys and Incyte announced that the European Commission (EC) had granted conditional marketing authorization for tafasitamab (brand name Minjuvi) in combination with lenalidomide, followed by tafasitamab monotherapy, for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) who are not eligible for ASCT. In July 2021, the Committee for Orphan Medicinal Products (COMP) confirmed the orphan drug designation status of Minjuvi, agreeing that sufficient justification had been provided that Minjuvi may be of significant benefit to patients with this disease. On August 24, 2021, Health Canada granted conditional marketing authorization to Incyte for Minjuvi in combination with lenalidomide for the treatment of adults with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, who are not eligible for ASCT. Related to these ex-U.S. regulatory approvals, in the third quarter 2021, MorphoSys received, for the first time, royalty revenue for Minjuvi sales outside of the U.S. pursuant to the agreement with Incyte. On January 5, 2021, MorphoSys and Incyte announced that the Swiss Agency for Therapeutic Products (Swissmedic) had accepted the marketing authorization application (MAA) for tafasitamab. The MAA seeks approval for tafasitamab, in combination with lenalidomide, followed by tafasitamab monotherapy, for the treatment of adult patients with r/r DLBCL, including DLBCL arising from low-grade lymphoma, who are not candidates for ASCT. The MAA is being reviewed as part of the U.S. Food and Drug Administration’s (FDA) modified Project Orbis, which provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators. Collaboration among international regulators may allow patients with cancer to receive earlier access to products in other countries.

12 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 On August 17, 2021, Incyte announced that it had entered into a collaboration and license agreement with a subsidiary of InnoCare for tafasitamab in Greater China. Under the terms of the agreement, InnoCare received the rights to develop and exclusively commercialize tafasitamab in hematology/oncology in mainland China, Hong Kong, Macao and Taiwan. Incyte holds the development and commercialization rights for tafasitamab outside the U.S., and MorphoSys receives tiered royalties on ex-U.S. net sales. Studies of Tafasitamab The clinical development of tafasitamab is focused on non-Hodgkin’s lymphoma (NHL). In DLBCL, MorphoSys aims to position tafasitamab as a backbone therapy for patients suffering from this disease, regardless of treatment line or potential combination therapy. Treatment options for patients with r/r DLBCL who are not candidates for high-dose chemotherapy (HDC) and ASCT were limited prior to the U.S. approval of tafasitamab. In June 2021, MorphoSys and Incyte announced new three-year follow-up data from the ongoing phase 2 L- MIND study of tafasitamab (Monjuvi) in combination with lenalidomide in adult patients with r/r DLBCL. The new results, based on an October 30, 2020 data cut-off, built on previous findings showing durable responses and a consistent safety profile of tafasitamab in combination with lenalidomide followed by tafasitamab monotherapy. A total of 80 out of 81 enrolled study patients receiving tafasitamab plus lenalidomide were included in the efficacy analysis at approximately three years follow-up (≥35 months). The long-term analysis, as assessed by an independent review committee (IRC), showed that patients treated with tafasitamab plus lenalidomide had an overall response rate (ORR) of 57.5%, including a complete response (CR) rate of 40%. Additionally, the median duration of response (DoR) was 43.9 months, with a median overall survival (OS) of 33.5 months and median progression-free survival (PFS) of 11.6 months. In December 2021, additional results from the RE-MIND2 study were presented at the 2021 American Society of Hematology (ASH) Annual Meeting. The study matched L-MIND trial patients receiving tafasitamab in combination with lenalidomide followed by tafasitamab monotherapy with real-world adult patients who received the most frequently used treatments for r/r DLBCL. These treatments included 1) polatuzumab vedotin plus bendamustine and rituximab (pola-BR); 2) rituximab plus lenalidomide (R2); and 3) CD19 chimeric antigen receptor T-cell (CAR-T) therapies. Specifically, the study showed the following results: • A significant improvement in median overall survival (OS) was observed for tafasitamab plus lenalidomide with 20.1 months compared to pola-BR with 7.2 months (p = 0.038), and 24.6 months for tafasitamab plus lenalidomide compared to 7.4 months for R2 (p = 0.014). • A comparable median OS benefit was observed with tafasitamab plus lenalidomide with 22.5 months compared to CAR-T with 15 months, however, these results were not statistically significant. • ORR, a key secondary endpoint, was statistically significantly higher for tafasitamab plus lenalidomide with 63.6% versus R2 with 30.3% (p = 0.013). • Tafasitamab plus lenalidomide also achieved a significantly higher CR rate, a key secondary endpoint, with 39.4% versus 15.2% for R2 (p = 0.0514). • While safety endpoints were not included in this study, the most common adverse events (AEs) associated with tafasitamab plus lenalidomide were feeling tired or weak, diarrhea, cough, fever, swelling of lower legs or hands, respiratory tract infection and decreased appetite. Warnings and Precautions for Monjuvi included infusion-related reactions (6%), serious or severe myelosuppression (including neutropenia (50%), thrombocytopenia (18%), and anemia (7%)), infections (73%) and embryo-fetal toxicity. Neutropenia led to treatment discontinuation in 3.7% of patients. The most common adverse reactions (≥ 20%) were neutropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, pyrexia, peripheral edema, respiratory tract infection, and decreased appetite.

Management Report 13 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The phase 2/3 study, B-MIND, is evaluating the safety and efficacy of tafasitamab in combination with the chemotherapeutic agent bendamustine in comparison to rituximab plus bendamustine in patients with r/r DLBCL who are not candidates for HDC and ASCT. The study has been fully recruited as of June 2021. The regulatory significance of the B-MIND study has decreased as both FDA and EMA have approved Monjuvi and Minjuvi, respectively, based on L-MIND data. Long-term safety data of B-MIND are required by the EMA as an obligation for the conditional marketing authorization. As such, the event-driven primary analysis has been removed from the planned analyses; all final analyses of primary and secondary endpoints will be performed in mid-2024. In addition to clinical development in r/r DLBCL, on May 11, 2021 MorphoSys announced that the first patient had been dosed in frontMIND, a pivotal phase 3 trial of tafasitamab in first-line DLBCL: frontMIND is evaluating tafasitamab and lenalidomide in combination with R-CHOP compared to R-CHOP alone as first-line treatment for high-intermediate and high-risk patients with untreated DLBCL. The study is planned to enroll up to 880 patients. Updated preliminary data presented at ASH 2021, from firstMIND, a phase 1b, open-label, randomized study on the safety and efficacy of R-CHOP plus either tafasitamab or tafasitamab plus lenalidomide for patients with newly diagnosed DLBCL, showed a preliminary overall response rate of 90.9% versus 93.9%, respectively, in a patient population that had an overall poor prognosis. The combination of tafasitamab, lenalidomide and R-CHOP had an acceptable and manageable safety profile. These results supported further investigation of the tafasitamab plus lenalidomide combination in the frontMIND study. On November 11, 2021, MorphoSys provided an update on the frontMIND study, indicating that enrollment was going well and that additional sites were being added in the United States to satisfy investigator and patient interests. Topline data from the trial are expected in the second half of 2025. On April 19, 2021, MorphoSys and Incyte announced that the first patient had been dosed in the phase 3 inMIND study. InMIND is a global, double-blind, placebo-controlled, randomized phase 3 study evaluating whether tafasitamab and lenalidomide as an add-on to rituximab provides improved clinical benefit compared with lenalidomide alone as an add-on to rituximab in patients with r/r FL Grade 1 to 3a or r/r nodal, splenic or extranodal MZL. The study is expected to enroll over 600 adult patients with r/r FL or r/r MZL. The primary endpoint of the study is PFS in the FL population, and the key secondary endpoints are PFS and OS in the overall population as well as positron emission tomography complete response (PET-CR) at the end of treatment (EOT) in the FL population. Topline data from the inMIND trial are expected in the second half of 2023. Initiated in late 2021 and sponsored by Incyte, the topMIND trial is a single-arm, open-label, phase 1b/2a, multicenter basket study to evaluate whether tafasitamab and parsaclisib can be safely combined at the recommended phase 2 dose and dosing regimen that was established for each of the two compounds as a treatment option for adult participants with r/r B-cell malignancies. Participants will be assigned to disease- specific cohorts based on the histology of their underlying disease: Cohort 1: r/r DLBCL, Cohort 2: r/r MCL, Cohort 3: r/r FL, Cohort 4: r/r MZL, and Cohort 5: r/r CLL/SLL. The primary outcomes of the phase 1b part of the trial will be the number of TEAEs and incidence of dose-limiting toxicities. Key secondary objectives include ORR for the phase 2a part and various PK measures. Pelabresib Overview Pelabresib, also known as CPI-0610, is a small molecule designed to promote anti-tumor activity by selectively inhibiting the function of BET proteins to decrease the expression of abnormally expressed genes in cancer. The clinical development of pelabresib is currently focused on myelofibrosis (MF). MF is a form of bone marrow cancer that disrupts the body's normal production of blood cells. It causes fibrosis (scarring) of

14 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 the bone marrow, leading to severe anemia as well as thrombocytopenia. MF have enlarged spleens as well as many other physical symptoms, including abdominal discomfort, bone pain and extreme fatigue. Approximately 4–6 per 100,000 people in the U.S. are diagnosed with MF, most of whom are intermediate- or high-risk patients. There are limited treatment options for patients with MF. We believe there are approximately 30,000 to 35,000 intermediate- or high-risk MF patients in the United States and Europe that are eligible for systemic treatment, including ruxolitinib. Incyte, which markets ruxolitinib (Jakafi®), has estimated that about half of these eligible patients in the United States receive treatment with ruxolitinib. Ruxolitinib, a JAK1/2 inhibitor, is the current standard of care for intermediate- and high-risk MF patients. Many of these eligible patients do not initially receive treatment with ruxolitinib. For example, patients with low red blood cell or platelet counts are ineligible to receive ruxolitinib. Fedratinib is a second JAK1/2 inhibitor approved for use in treating MF. Patients who become refractory to, or discontinue therapy with, ruxolitinib and fedratinib generally have a poor survival prognosis. Currently approved drugs for the treatment of patients suffering from MF offer symptomatic improvement and are generally not considered to be disease-modifying. As part of MorphoSys’ agreement with Royalty Pharma, Royalty Pharma is entitled to receive 3% of future net sales of pelabresib. Studies of Pelabresib Pelabresib is currently in two clinical trials for the treatment of MF, the phase 2 MANIFEST trial and the phase 3 MANIFEST-2 trial. MANIFEST is a global, multicenter, open-label, phase 2 study that evaluates pelabresib as monotherapy or in combination with ruxolitinib, the current standard of care. In Arm 3 of this study, pelabresib is being evaluated in combination with ruxolitinib, in JAK-inhibitor-naïve MF patients, with a primary endpoint of the proportion of patients with a ≥35% spleen volume reduction from baseline (SVR35) after 24 weeks of treatment. Pelabresib is also being evaluated in a second-line setting (2L) either as a monotherapy in patients who are resistant to, intolerant of, or ineligible for ruxolitinib and no longer on the drug (Arm 1), or as add-on therapy to ruxolitinib in patients with a sub-optimal response to ruxolitinib or MF progression (Arm 2). Patients in Arms 1 and 2 are being stratified based on transfusion-dependent (TD) status. The primary endpoint for the patients in cohorts 1A and 2A, who were TD at baseline, is conversion to transfusion independence for 12 consecutive weeks. The primary endpoint for patients in cohorts 1B and 2B, who were not TD at baseline, is the proportion of patients with a SVR35 after 24 weeks of treatment. On June 11, 2021, Constellation announced that interim data from the MANIFEST trial were presented at the European Hematology Association (EHA) annual meeting. The data were based on a data cut-off of September 29, 2020. In Arm 3 of the study, an interim efficacy subgroup analysis in JAK-inhibitor-naïve patients was presented. Forty-two of 63 evaluable patients (67%) achieved a SVR35 at 24 weeks, achieving the primary endpoint for Arm 3. Thirty-four of 60 evaluable patients (57%) achieved a ≥50% reduction in Total Symptom Scores (TSS50) at 24 weeks. Strong response was observed with pelabresib, irrespective of baseline risk status or demographic and disease characteristics. Central pathology review of 27 1L patient bone marrow samples showed at least a one-grade improvement in bone marrow fibrosis in 9 out of 27 patients (33%); in all of these patients, improvement was observed within six months of starting treatment. Sixteen out of 27 patients (59%) showed stabilization of bone marrow fibrosis, while only one out of 27 patients (4%) showed worsening. An interim analysis of Arms 1 and 2 suggested that pelabresib monotherapy in JAK-inhibitor- experienced or -ineligible patients, and with pelabresib in combination with ruxolitinib in ruxolitinib- experienced patients, may result in improvements in anemia.

Management Report 15 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 In December 2021, updated data from MANIFEST were presented at the 2021 ASH Annual Meeting. At this meeting, the latest interim data from Arm 3 of MANIFEST evaluating pelabresib as a first-line combination with ruxolitinib for patients with MF who had not previously been treated with a JAK inhibitor (JAK inhibitor- naïve) were presented. As of September 10, 2021, the data cut-off, a total of 84 JAK inhibitor-naïve patients had been enrolled in Arm 3 and received the combination. Based on the interim data, 68% (n=57) of patients treated with the combination achieved an SVR35 response at week 24 and 60% (n=47) had SVR35 response at week 48. Most patients also saw their symptoms reduced, with 56% (n = 46) achieving TSS50 from baseline at week 24. At the time of the data cut-off, 53 patients (63% of the 84 patients) were still on treatment. No new safety signals were identified in the study. The most common hematologic adverse events were thrombocytopenia (12%, grade 3/4) and anemia (34%, grade 3/4). Non-hematological events included dyspnea (5%, grade 3) and respiratory tract infections (8%, grade 3/4). Additional data from Arm 1 of the ongoing MANIFEST trial were also presented in an oral presentation at the 2021 ASH Annual Meeting: pelabresib is being evaluated as a monotherapy in patients with advanced MF who are ineligible to receive, intolerant of, or refractory to JAK inhibitors, a population with very limited therapeutic options. Patients were divided into two cohorts, TD and non-TD. For the TD cohort, the primary endpoint was conversion to transfusion independence for 12 consecutive weeks. In the non-TD cohort, the primary endpoint was SVR35 at week 24. At week 24, 11% (n = 7) of patients reached SVR35. In addition, 31% of patients had a spleen volume reduction of 25% or more (n = 20) at week 24. Across all cohorts, 28% (n = 18) of patients achieved TSS50. No new safety signals were identified in the study. The most common hematologic adverse events were thrombocytopenia (23%, grade 3/4) and anemia (15%, grade 3). Non- hematological events included diarrhea (6%, grade 3) and respiratory tract infections (5%, grade 3). MANIFEST‑2, a global, double-blinded, randomized phase 3 clinical study, is evaluating pelabresib plus ruxolitinib versus placebo plus ruxolitinib in JAK-inhibitor-naïve patients with primary MF or post-essential thrombocythemia (post-ET) or post-polycythemia (post-PV) MF who have splenomegaly and symptoms requiring therapy. Since the acquisition of Constellation, MorphoSys has optimized the study’s design by increasing the number of trial participants to 400 patients. Measures have also been taken to improve the speed of enrollment, including adding new contract research organizations (CROs), improving the interaction with investigators, and expanding the number of countries and sites, as well as other measures. With these activities in place, MorphoSys expects to report primary analysis data from this study in the first half of 2024. Felzartamab Overview Felzartamab is an investigational human monoclonal HuCAL-IgG1-antibody directed against a unique epitope of the target molecule CD38. CD38 is a surface antigen broadly expressed on malignant myeloma cells as well as on antibody-producing plasmablasts and plasma cells, the latter playing an important role in the pathogenesis of antibody-mediated autoimmune diseases. Preclinical and clinical results suggest that felzartamab may have therapeutic activity in autoantibody-mediated autoimmune diseases, and clinical trials are ongoing in two such diseases – membranous nephropathy (MN) and immunoglobulin A nephropathy (IgAN). MN occurs when the small blood vessels in a part of the kidney, called glomeruli, which filter wastes from the blood, become inflamed and thickened. Around 80% of MN cases are primary and mediated by autoantibodies, with phospholipase A2 receptor (PLA2R) antibody positive MN accounting for up to 85% of all primary MN (Trujillo, 2019; Pozdizk, 2018; Couser 2017). MN is a leading cause of nephrotic syndrome in adults worldwide (Couser, 2017). Nephrotic syndrome results from excreting too much protein in urine due to a kidney disorder. Although 30–40% of MN patients may experience spontaneous remission, 30% of patients

16 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 experience persistent proteinuria with long-term preservation of renal function, and another 30–50% progress to renal failure within 10–15 years (Trujillo, 2019; Heaf, 1999; Troyanov, 2004). Even if patients do not progress to renal failure, they have an increased risk of life-threatening thromboembolic and cardiovascular events, and are subject to infections (Wagner, 1983; Heaf, 1999; Lee, 2016). In the U.S., the incidence of MN is estimated at 1.2 per 100,000; about 3,000 adults are newly diagnosed every year (McGrogan, 2011). There currently is no approved standard treatment for MN. IgAN is the most common form of glomerulonephritis, a group of renal disorders that causes damage to the glomeruli, hindering their ability to carry out their essential functions. In IgAN, a combination of genetic and environmental factors causes patients to produce galactose-deficient IgA (Gd-IgA), whereupon the patients’ immune system reacts by producing specific autoantibodies. The binding of these IgG autoantibodies to Gd- IgA leads to the formation of immune complexes in the circulation. The immune complexes then accumulate in the glomerular mesangium where they induce local inflammation, mesangial proliferation, glomerulosclerosis and loss of renal function. Patients with IgAN may experience different symptoms including blood and/or protein leaking into the urine, high blood pressure, interstitial lung disease, glomerulosclerosis (scarring of the kidneys’ blood vessels) and a slow progression to chronic kidney disease. About 40% of patients with IgAN progress to end-stage renal disease within 20 years of diagnosis. Worldwide IgAN incidence is estimated at 2.5 per 100,000. Currently there are no approved treatments that can specifically prevent the production of Gd-IgA nor its corresponding autoantibody. According to Data Bridge Market Research, the U.S. membranous nephropathy market is projected to grow at a CAGR of 5.0% between 2021 and 2028 and is expected to reach US$ 153.1 million (€ 135.2 million) by 2028. According to Research and Markets, the IgAN market in the seven major markets (United States, Germany, Spain, Italy, France, United Kingdom and Japan) was US$ 109.3 million (€ 96.5 million) in 2020 and the prevalence has been shown to increase over time. Studies of Felzartamab In October 2019, MorphoSys initiated a phase 1/2 trial in anti-PLA2R antibody positive MN. The proof-of- concept trial called M-PLACE is an open-label, multicenter trial primarily assessing the safety and tolerability of felzartamab. On November 4, 2021, MorphoSys presented interim results from M-PLACE at the 2021 Annual Meeting of the American Society of Nephrology (ASN). The study included 31 patients with primarily medium or high levels of anti-PLA2R antibody titers at baseline and/or patients who were refractory to previous treatments. Of the 27 treated patients with evaluable results, 24 showed an initial rapid reduction of anti-PLA2R antibody levels one week after the first treatment. After 12 weeks of treatment, most patients showed a substantial reduction in autoantibody titer. The observed titer reduction was independent of cohort and suggests successful depletion of CD38-positive plasma cells. The safety profile was consistent with the proposed mechanism of action of felzartamab. An early assessment of urine protein: creatinine ratio (UPCR) results at six months of treatment showed a decrease in six of ten patients, with four patients having a decrease of >=50% from baseline. The first patient who had already reached the 12-month time point showed a complete immunologic response and a partial clinical response. Also in November 2021, MorphoSys reported that the M-PLACE trial was fully enrolled. Additional data from the study are expected to be available in the second half of 2022.In February 2021, the first patient was dosed in the New-PLACE study, a phase 2 study evaluating different treatment schedules to identify the regimen for a pivotal study in patients with anti-PLA2R antibody positive MN. Enrollment in this study was completed at the end of 2021, and topline data are expected in the second half of 2022.

Management Report 17 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 In October 2021, the first patient was dosed in the phase 2 IGNAZ trial evaluating felzartamab in patients with IgAN. This multicenter, randomized, double-blind, parallel-group, placebo-controlled trial is planned to enroll approximately 48 patients and is designed to assess the efficacy, safety and pharmacokinetics (PK)/pharmacodynamics (PD) of felzartamab in patients with IgAN. The primary objective of this study is to evaluate the efficacy of felzartamab compared to placebo. The primary endpoint is the relative change in UPCR and will be assessed for each patient nine months after treatment initiation. Study sites are located in Europe, North America and Asia-Pacific, excluding Greater China. Proof-of-concept data from the IGNAZ trial are expected in the fourth quarter of 2022. CPI-0209 Overview CPI-0209 is a small molecule designed to promote anti-tumor activity by specifically inhibiting EZH2, an enzyme that suppresses target gene expression. We believe that targeting EZH2 may have the potential for therapeutic application in various tumor types. Royalty Pharma is entitled to receive 3% of future net sales of CPI-0209. Studies of CPI-0209 Patient enrollment in a phase 1/2 clinical trial of CPI‑0209 is ongoing. The phase 1 portion of the trial evaluated CPI‑0209 as a monotherapy in patients with advanced solid tumors. After determining the recommended phase 2 dose of 350 mg (oral, once-daily), patients are currently being dosed in the phase 2 expansion cohorts in select tumor indications (urothelial carcinoma (ARID1A mutant), ovarian clear cell carcinoma (ARID1A mutant), endometrial carcinoma (ARID1A mutant), lymphoma, mesothelioma, metastatic castration resistant prostate cancer), and data from this part of the trial are expected in 2022. As of the data cut off of March 9, 2021, of the 4 BAP1 loss mesothelioma patients, one patient had a PR after four cycles of treatment and two had SD. The high levels of target engagement observed preclinically were corroborated clinically. All 40 patients were evaluated for safety. Across all dose cohorts, 43% of patients had at least one Grade 3 or greater treatment emergent adverse event (TEAE), 28% of patients had at least one serious adverse event (SAE). The most common TEAEs (≥15%) included thrombocytopenia (reversible and dose dependent), diarrhea, asthenic conditions, nausea, anemia, dysgeusia, abdominal pain and alopecia. 23% of patients reported a TEAE that led to dose reduction or interruption. Four patients discontinued treatment because of TEAEs. One patient in the highest dose cohort (375mg) experienced Grade 4 thrombocytopenia, and one patient experienced a Grade 5 adverse event due to progressive disease. Based on this preliminary data, CPI-0209 appeared to be generally well tolerated. We expect to report additional results from the trial in 2022. Clinical Development through Partners The most advanced programs being developed by partners are outlined below. Felzartamab Overview MorphoSys has an exclusive regional licensing agreement for felzartamab with I-Mab for Greater China, where development is currently focused on multiple myeloma (MM), a blood cancer that develops in mature plasma cells in the bone marrow. MM is the second most common form of blood cancer worldwide. According to GLOBOCAN 2020 statistics, there were an estimated 4.6 million cancer cases, more than 21,000 MM cases and more than 16,000 deaths in China in 2020. In China, the incidence of MM is projected to continue to increase at least through 2040. Current therapies are associated with serious side effects and limited efficacy.

18 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Regional Agreement with I-Mab MorphoSys has an exclusive regional licensing agreement for felzartamab with I-Mab. Under the terms of the agreement, signed in November 2017, I-Mab has the exclusive rights to develop and commercialize felzartamab in mainland China, Taiwan, Hong Kong and Macao. Upon signing the agreement, MorphoSys received an immediate upfront payment of US$ 20 million (€ 18 million). MorphoSys is also entitled to receive additional success-based clinical and commercial milestone payments from I-Mab of up to US$ 100 million (€ 88 million), as well as tiered double-digit royalties on net sales of felzartamab in the agreed regions. Studies of Felzartamab I-Mab is conducting a phase 3 clinical trial in Greater China to evaluate felzartamab in combination with lenalidomide plus dexamethasone in patients with r/r MM. This study is a randomized, open-label, parallel- controlled, multi-center study to evaluate the efficacy and safety of the combination of felzartamab, lenalidomide and dexamethasone versus the combination of lenalidomide and dexamethasone in patients with r/r MM who have received at least one prior line of treatment. The study was initiated in April 2019 at sites in Taiwan and started in mainland China in April 2020 as part of a coordinated effort to accelerate the study. In October 2021, I-Mab announced that patient enrollment in this pivotal phase 3 trial has been completed. I-Mab is also evaluating felzartamab as a third-line therapy in patients with r/r MM in a pivotal phase 2 trial that started in March 2019. At the end of August 2021, I-Mab announced that topline data met primary and secondary endpoints. On June 25, 2021, I-Mab announced that the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) had approved the Investigational New Drug (IND) application to initiate a phase 1b study with felzartamab in patients with systemic lupus erythematosus (SLE). SLE, the most common type of lupus, is an autoimmune disease in which the immune system attacks its own tissues, causing widespread inflammation and tissue damage in the affected organs. It can affect the joints, skin, brain, lungs, kidneys and blood vessels. There is no cure for SLE. The phase 1b multi-center trial is evaluating the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of felzartamab in patients with SLE in China. The SLE study start date is scheduled for Q1 2022. Gantenerumab Overview Gantenerumab is a HuCAL antibody targeting amyloid beta, and is being developed by licensing partner Roche as a potential treatment for Alzheimer’s disease (AD). Amyloid beta refers to a group of peptides that play an important role in Alzheimer’s disease as they are the main component of the amyloid plaques found in the brain of Alzheimer’s patients. Gantenerumab binds to the N-terminus and a section in the middle of the amyloid beta peptide. The antibody removes amyloid beta via microglia-mediated phagocytosis. It has been designed to promote clearance of amyloid plaques in the brain, a pathological hallmark of AD, and has shown downstream effects on multiple biomarkers of AD pathology and neurodegeneration in clinical trials. According to the market research and consulting company Decision Resources, the value of the global market for the treatment of Alzheimer’s disease is expected to reach approximately € 35 billion (approximately US$ 40 billion) in 2030 (report titled “Market Forecast Assumption Alzheimer’s Disease 2020–2030”). According to figures from the Alzheimer’s Association, more than six million people in the United States live with Alzheimer’s disease. Deaths from Alzheimer’s disease increased 16% during the COVID-19 pandemic (https://www.alz.org/alzheimers-dementia/facts-figures). In 2019, Alzheimer’s disease was the sixth-leading cause of death in the United States (https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm).

Management Report 19 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Operational Development Under the agreement announced in June 2021 between MorphoSys and Royalty Pharma, Royalty Pharma has the right to receive 60% of future royalties on gantenerumab. On October 11, 2021, MorphoSys announced that Roche had received Breakthrough Therapy Designation by the U.S. FDA for gantenerumab for the treatment of Alzheimer’s disease. This designation was based on data showing that gantenerumab significantly reduced brain amyloid plaque, a pathological hallmark of this disease, in the SCarlet RoAD and Marguerite RoAD open-label extension trials, as well as other studies. Studies of Gantenerumab In June 2018, Roche initiated a new phase 3 development program for patients with Alzheimer’s disease. The program consists of the GRADUATE 1 and GRADUATE 2 phase 3 trials. The two multicenter, randomized, double-blinded, placebo-controlled studies are investigating the efficacy and safety of gantenerumab in more than 2,000 patients with early (prodromal to mild) Alzheimer’s disease and follow them for over two years. The primary endpoint for both studies is the assessment of the signs and symptoms of dementia, measured as the clinical dementia rating sum of boxes (CDR-SOB) score. Learnings from the SCarlet RoAD and Marguerite RoAD studies were incorporated into the optimized design of the phase 3 GRADUATE trials, with patients receiving a significantly higher dose of gantenerumab as a subcutaneous injection than in Roche’s previous trials. The GRADUATE 1 and 2 trials are expected to be completed in Q4 2022. Otilimab Overview Otilimab (formerly MOR103/GSK3196165) is a HuCAL-IgG1-antibody directed against granulocyte- macrophage colony-stimulating factor (GM-CSF). Due to its diverse functions in the immune system, GM-CSF can be considered a target for a broad spectrum of anti-inflammatory therapies, such as those in rheumatoid arthritis (RA). RA is a chronic inflammatory disease that affects the synovial membrane of the joints and is accompanied by painful swelling that can lead to bone destruction and joint deformity. MorphoSys discovered and advanced otilimab to clinical development. In June 2013, MorphoSys announced that it had entered into a worldwide agreement with GSK for the development and commercialization of otilimab. Under the terms of the agreement, GSK assumes responsibility for all further development and commercialization of the compound. Under the terms of the agreement, MorphoSys received an upfront payment of € 22.5 million. Depending on the achievement of certain development, regulatory, commercial and revenue milestones, MorphoSys is eligible to receive further payments from GSK of up to € 423 million, as well as tiered double-digit royalties on net sales. Under the agreement between MorphoSys and Royalty Pharma, Royalty Pharma is entitled to 80% of future royalties and 100% of future milestone payments for otilimab. The total market for RA drugs is growing steadily. According to the market research and consulting firm Decision Resources, the market for RA drugs was projected to reach € 28 billion (US$ 32 billion) in 2022 in G7 countries (report titled “Market Forecast Assumptions Rheumatoid Arthritis 2020–2030”). Studies of Otilimab In July 2019, GSK launched a phase 3 program for RA called ContRAst. The treatment of the first patient in this program triggered a milestone payment of € 22 million to MorphoSys. Data from the ContRAst program studies are expected by the end of 2022.

20 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 GSK also initiated a clinical trial (OSCAR) in 2020 to evaluate the efficacy and safety of otilimab in patients with severe pulmonary COVID-19-associated disease. The event of the first patient dosed in the expanded OSCAR study triggered milestone payments totaling € 16 million to MorphoSys in financial year 2021. In October 2021, GSK provided an update that it had made the decision not to further explore otilimab as a potential treatment for severe pulmonary COVID-19 related disease in patients aged of 70 years and older. Tremfya® (Guselkumab) Overview Tremfya is a human HuCAL antibody targeting the p19 subunit of IL-23 that is being developed and commercialized by Janssen. It is the first commercial product based on MorphoSys’ proprietary technology. It is approved for the treatment of patients with moderate to severe psoriasis (plaque psoriasis) in the United States, Canada, the European Union (EU), Japan, China and a number of other countries. In the U.S. and elsewhere, it is also approved for the treatment of adults with active psoriatic arthritis and in the EU for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or have not tolerated prior disease-modifying antirheumatic drug (DMARD) therapy. In Japan, Tremfya is approved for the treatment of patients with various forms of psoriasis, psoriatic arthritis and palmoplantar pustulosis. Under an agreement with Janssen, MorphoSys receives royalties on net sales of Tremfya and is also entitled to milestone payments on selected future development activities. Under the agreement between MorphoSys and Royalty Pharma, Royalty Pharma is entitled to 100% of future Tremfya royalties starting with royalties for the second quarter of 2021. MOR210/TJ210 Overview MOR210/TJ210 is a human antibody directed against C5aR, derived from MorphoSys' HuCAL technology. C5aR, the receptor of complement factor C5a, is being investigated as a potential new drug target in the fields of immuno-oncology and autoimmune diseases. Tumor cells generate high levels of C5a, which is believed to contribute to an immuno-suppressive and, consequently, tumor growth-promoting microenvironment by recruiting and activating myeloid suppressor cells (MDSCs). MOR210/TJ210 is engineered to neutralize the immuno-suppressive function of MDSCs by blocking the interaction between C5a and its receptor and enabling the immune system to fight the tumor. Regional Agreement with I-Mab In November 2018, MorphoSys announced that the Company had entered into an exclusive strategic collaboration and regional licensing agreement with I-Mab. Under the agreement, I-Mab has exclusive rights to develop and commercialize MOR210/TJ210 in mainland China, Hong Kong, Macao, Taiwan and South Korea, while MorphoSys retains rights in the rest of the world. The agreement deepened the existing partnership with I-Mab and built on the existing collaboration to develop MOR210/TJ210. Under the agreement, I-Mab has exclusive rights to develop and commercialize MOR210/TJ210 in the territories covered by the agreement. With MorphoSys’ support, I-Mab is to conduct and fund all worldwide development activities for MOR210/TJ210, including clinical trials in China and the U.S., up to proof-of-concept in oncology. Study of MOR210/TJ210 On January 25, 2021, MorphoSys and I-Mab announced the dosing of the first patient in the U.S. in a phase 1 dose-finding study evaluating the safety, tolerability, PK and PD of MOR210/TJ210 as monotherapy in

Management Report 21 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 patients with r/r advanced solid tumors. The phase 1 clinical trial is an open-label, multiple-dose group, dose- finding study in various centers across the U.S. I-Mab has announced another phase 1 clinical trial to evaluate the dose-finding and safety for the treatment of patients with advanced solid tumors in 2022 in China. Other Business Activities Technologies MorphoSys has developed a number of technologies that provide direct access to human antibodies for the treatment of diseases. MorphoSys has historically used these technologies for proprietary and partnered programs but is now primarily focused on expanding its own pipeline with these and other technologies. MorphoSys’ most important technologies include HuCAL, a collection of several billion fully human antibodies, and a system for their optimization. Another important and, compared to HuCAL, further optimized platform is Ylanthia: a large antibody library representing the next generation of antibody technologies. Ylanthia is based on an innovative concept for generating highly specific and fully human antibodies. With Ylanthia, MorphoSys has set a new standard in therapeutic antibody development and will continue to preferentially use this technology to identify antibody candidates for its proprietary pipeline. With Slonomics, MorphoSys has a patent-protected, fully automated gene synthesis and modification technology to generate highly diverse gene libraries in a controlled process, for example to improve antibody properties. MorphoSys also has a licensing agreement with Cherry Biolabs, a spin-off of the University Hospital of Würzburg, Germany, granting MorphoSys the rights to apply Cherry Biolabs’ innovative, multispecific Hemibody technology to six exclusive targets. Combined with MorphoSys’ expertise in antibody technologies, the Hemibody technology offers the potential to generate novel T-cell-engaging medicines with higher precision and better safety profiles for the treatment of cancer patients. MorphoSys intends to further develop the Hemibody technology in the context of MorphoSys’ CyCAT dual-targeting platform to advance novel Hemibody-based treatment options for patients with hematological and solid cancers. Drug Development MorphoSys has a broad development pipeline and develops drugs using its own research and development and in collaboration with pharmaceutical and biotechnology partners as well as academic institutions. Our core business is the development of new therapies for patients suffering from serious diseases. Our first proprietary program to receive marketing approval is tafasitamab – brand name Monjuvi, which was first approved in the U.S. in July 2020 in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are not eligible for ASCT. Tafasitamab under the brand name Minjuvi has also been approved for marketing in the EU and Canada. We have become a fully integrated biopharmaceutical company developing and commercializing proprietary medicines. Our activities focus on cancer treatments, but we also conduct select programs in inflammatory diseases. The ability of monoclonal antibodies to bind to specific antigens on tumors or activate the immune system against cancer to unleash a therapeutic effect in patients has led to their dominant role in targeted cancer therapies. According to the report “2021 Global Oncology Trends” published by the IQVIA Institute, the surge

22 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 in innovation treatments in recent years, accompanied by a strong focus across health systems to increase early diagnosis and expanded patient access to treatments, has resulted in global spending on oncology drugs reaching US$ 164 billion in 2020 and an estimated US$ 269 billion by 2025 even as annual growth rates ease to about 10%. Chronic inflammatory and autoimmune diseases affect millions of patients worldwide and impose an enormous social and economic burden. MorphoSys’ most advanced proprietary clinical programs are described in the Research and Development section. Clinical-stage programs developed through partners are entirely under the control of our partners. These programs include not only those in our core area of oncology but also in indications where we have not established proprietary expertise. Programs, which are the most advanced, are outlined in the Research and Development section. Influential Factors Good public medical care is a political goal in many countries. The need for new forms of therapy is growing as a result of demographic change. Certain cost containment measures in Europe and the U.S. risk limiting access to innovation for patients and could slow the industry’s investment in the development of new therapies. Regulatory approval processes in the U.S., Europe and elsewhere are lengthy, time-consuming and largely unpredictable. Approval-related laws, regulations and policies and the type and amount of information necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. MorphoSys recognized the impact of the global COVID‑19 pandemic on healthcare systems and society worldwide, as well as the resulting potential impact on preclinical and clinical programs, specifically clinical trials. In spring 2020, MorphoSys activated its existing business continuity plans to minimize any disruptions to ongoing operations caused by the COVID‑19 pandemic and to take the necessary actions to protect its employees. In addition, MorphoSys continuously monitors the situation as a whole as well as each clinical program individually and decides on the necessary course of action to ensure the safety of patients, personnel and other stakeholders, as well as on the collection of data. The Company makes adjustments where necessary to comply with regulatory, institutional and governmental requirements and guidelines related to COVID‑19. The top priority is the safety of all clinical program participants and ensuring that the studies in which they participate are conducted in accordance with the study protocol. Despite the rapid changes in conditions worldwide and the potential impact they may have on clinical trials, MorphoSys continues to work diligently to maintain its drug development plans. The continuation of the commercialization of Monjuvi had incorporated the use of digital channels. In addition, the sales and medical teams are using a combination of virtual and face-to-face communication to market Monjuvi, which enables them to take the right response to the uncertainty caused by the COVID‑19 pandemic in the U.S. MorphoSys continuously monitors the development of the global COVID-19 pandemic and the emergence of any new virus variants and decides on a case-by-case basis on the necessary course of action and measures to ensure the safety of employees and patients.

Group Management Report 23 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Patents Our proprietary technologies and resulting drug candidates are our most valuable assets. It is therefore crucial to our success that we protect these assets with appropriate measures such as patents and patent applications. This is the only way we can exclusively utilize these assets and the reason our Intellectual Property (IP) department seeks out the most optimal strategy to protect our products and technologies. The rights of third parties are also actively monitored and respected. Our core technologies form the basis of the Company’s success. All our technologies are protected by numerous patent families. For our Ylanthia antibody library, patents have been granted in all major territories, including Europe, the U.S. and Asian markets. For other technologies, such as the dual targeting- based CyCAT concept, patents have been in-licensed to ensure we have freedom to act. Our development programs are also protected by numerous patent families. In addition to patents protecting the drug candidates themselves, we have filed further patent applications that cover other aspects of the programs. The relevant patents for our development candidates otilimab (out-licensed to GSK) and felzartamab (out-licensed to I-Mab for China, Hong Kong, Macao and Taiwan) will not expire before 2026. This date does not take into account potential added protection of up to five years that could be gained through supplementary protection certificates or patent term extensions. The main patents for pelabresib run until 2032 (U.S.) and 2031 (Europe), not including possible extension through supplemental protection certificates or term extensions. In addition, the use of pelabresib for the treatment of myelofibrosis is patent-protected in the U.S. until 2039. The main patents for CPI-0209 have a term until 2039. Here, too, a possible extension through supplementary protection certificates or term extensions is not included. The tafasitamab program is also protected by diverse patents. The core patents are scheduled to expire in 2029 (U.S.) and 2027 (Europe), without taking into account the additional protection of up to five years available through supplementary protection certificates or patent term extensions. A corresponding application for patent term extension (PTE) has been filed to extend the patent term in the U.S., while an application for a supplementary protection certificate (SPC) in Europe is being prepared based on the European approval and will be filed in early 2022. The patents for the tafasitamab program are being advanced in close coordination with our partner Incyte. Regulatory exclusivities are also in place for all development programs. The programs that are co-developed with or for partner companies are also extensively patent-protected. Our patent department works closely with the relevant partners. The patents for these drug development programs have terms that far exceed the terms of the underlying technology patents. We also monitor our competitors’ activities so we can take action when necessary. In the 2021 financial year, we continued to reinforce the patent protection of our development programs and growing technology portfolio, which represent the core value drivers of our Company. We have more than 110 different proprietary patent families worldwide, in addition to the numerous patent families we are pursuing in collaboration with our partners.

24 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Corporate Developments In 2021, MorphoSys continuously monitored the development of the global COVID-19 pandemic and the emergence of new viral variants, and decided on a case-by-case basis the measures necessary to ensure the safety of employees and patients. This allowed MorphoSys to continue to maintain its drug development plans despite the rapidly changing conditions worldwide and their potential impact on clinical trials. On January 6, 2021, MorphoSys announced the appointment of Sung Lee as the Company’s Chief Financial Officer (CFO), effective February 2, 2021. Mr. Lee has more than 20 years of financial leadership experience in the biopharmaceutical and technology businesses. As a member of the Management Board of MorphoSys AG, he is responsible for all financial areas of the Company. He is based in Planegg, Germany. On May 19, 2021, MorphoSys AG’s Annual General Meeting reelected Marc Cluzel, M.D., Ph.D., Sharon Curran and Krisja Vermeylen to the Company’s Supervisory Board. Due to the ongoing COVID-19 restrictions, the 2021 Annual General Meeting was also held as a virtual meeting without the physical attendance of shareholders or their proxies and made available as an audio/video broadcast on the Internet to registered shareholders. On June 2, 2021, MorphoSys entered into an agreement with Constellation under which MorphoSys would acquire Constellation for US$34.00 (€28.79) per share in cash, representing a total equity value of €1,635.2 million (€1,384.7 million). The transaction was unanimously approved by the Management and Supervisory Boards of MorphoSys and Constellation’s Board of Directors. MorphoSys also announced it had entered into a financing agreement with Royalty Pharma in conjunction with the Constellation transaction. On July 15, 2021, MorphoSys announced the successful completion of its all-cash tender offer, announced on June 2, 2021, to acquire all outstanding shares of Constellation for US$34.00 per share, net in cash. 42,811,957 shares of Constellation were validly tendered and not validly withdrawn in the tender offer, representing approximately 89% of Constellation’s outstanding shares on the date of the tender offer’s expiration. Under the terms of the merger agreement between Constellation, MorphoSys and MorphoSys Development Inc., all shares validly tendered and not validly withdrawn were accepted for payment. MorphoSys immediately completed the acquisition of Constellation in a second step merger of MorphoSys Development Inc. with Constellation, resulting in Constellation surviving as an indirect wholly owned subsidiary of MorphoSys. On July 16, 2021, MorphoSys announced that the Management Board, with the consent of the Supervisory Board, had resolved an increase in the share capital of MorphoSys AG by issuing 1,337,552 new ordinary shares from Authorized Capital 2021-II. The capital increase excluded the preemptive rights of existing shareholders to enable the purchase of 1,337,552 new ordinary shares by Royalty Pharma. Following the capital increase, the new ordinary shares represented 3.9% of MorphoSys’ registered share capital. The share purchase from Royalty Pharma totaling US$ 100 million is part of the financing agreement with MorphoSys for the completed acquisition of Constellation. The agreement became effective upon the merger’s closing on July 15, 2021. On November 9, 2021, MorphoSys announced the decision of Roland Wandeler, Ph.D., to step down as Chief Operating Officer (COO) and Management Board member of MorphoSys, effective December 31, 2021, in order to pursue new opportunities. Following his departure, the ongoing management of the marketing and

Group Management Report 25 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 sales organization remain with General Manager Joe Horvat in the U.S., who reports directly to the Chief Executive Officer, Jean-Paul Kress, M.D. Headcount Development On December 31, 2021, the MorphoSys AG had 455 employees (December 31, 2020: 464). MorphoSys employed an average of 456 people in 2021 (2020: 430). Of the current 455 employees, 7 worked in the production (included in cost of sales), 340 worked in research and development, 21 in selling and 87 in general and administrative positions. We do not have collective wage agreements with our employees, and there were no employee strikes during the reporting year. To compete successfully for the top talent, MorphoSys conducts an annual comparison of the Company’s compensation with that paid by other companies in the biotech industry and similar sectors and adjusts the salary structure when necessary. The remuneration system consists of fixed compensation and a variable annual bonus linked to the achievement of corporate targets. Individual targets promote the employees’ personal development and the achievement of overriding corporate goals. A “spot bonus” is also awarded on the spot to employees for exceptional performance. This instrument was used frequently again to reward employees during the reporting year.

26 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Macroeconomic and Sector- Specific Conditions Changes in the Business Environment In January 2022, the International Monetary Fund (IMF) forecast that the global economy would grow by 4.4% for 2021 (report “World Economic Outlook Update January 2022”). Following from the IMF’s previous report in October 2021, supply disruptions continued into the fourth quarter, hindering global manufacturing, especially in Europe and the United States. A resurgence in COVID-19 cases (particularly in Europe) also held back a broader recovery. Although there were signs of a global turnaround in November with a pickup in international trade and upside surprises for services activity and industrial production data, this only partially offset earlier declines. The IMF’s growth forecast for the advanced economies in 2021 was +5.0%, compared to a decline of 4.5% in 2020, and the forecast for the emerging and developing economies was +6.5% (2020: –2.0%). The IMF’s forecast for growth in the euro area in 2021 was +5.2% (2020: –6.4%), compared to +2.7% for Germany (2020: –4.6%); +5.6% for the U.S. (2020: –3.4%); +8.1% for China (2020: +2.3%); +4.5% for Russia (2020: –2.7%) and +4.7% for Brazil (2020: –3.9%). When managing its business activities, MorphoSys takes a number of potential macroeconomic risks and opportunities into consideration. Currency Development The EUR/USD exchange rate has fluctuated between 1.22 and 1.12 over the last year, currently around 1.12, with inflation expectations and interest rate differences being the main drivers, in addition to trade conflicts and ongoing geopolitical tensions. The majority of our business transactions are conducted in euros and U.S. dollars. With the acquisition of Constellation we have significantly expanded our footprint in the US. Primarily driven by the additional ongoing clinical studies, U.S. dollar expenses are expected to exceed the U.S. dollar revenues for the next financial year. Therefore, strengthening of the U.S. dollar against the euro, all other things remaining equal, would have a negative impact on our operating result. We manage this risk through various mechanisms, such as optimizing our U.S. dollar assets against our U.S. dollar liabilities and maintaining an adequate (currently around 20%) amount of U.S. dollars in our bank accounts.

Management Report 27 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Analysis of Net Assets, Financial Position and Results of Operations Revenues Revenues in comparison to the prior year decreased by 49% to € 128.1 million (2020: € 252.1 million). In 2021, the major portion of external revenues was generated from antibody collaboration and license agreements with Janssen, Incyte and GSK (2021: € 96.0 million; 2020: € 236.1 million from Incyte, Janssen and I-Mab Biopharma). The major portion of the decrease resulted from the revenues in 2020 from the collaboration and license agreement with Incyte in the amount of € 183.5 million. Revenues from royalties on net sales of Tremfya amounted to € 54.7 million (2020: € 42.5 million) and from Milestones to € 20.0 million (2020: € 4.8 million). Revenues with affiliated companies amounted to € 22.1 million (2020: € 13.8 million), mainly from Monjuvi product sales. Of total revenues, € 104.4 million (2020: € 243.0 million) were attributed to biotechnology and pharmaceutical companies and non-profit organizations based in North America and revenues in other European countries and Asia (excluding Germany) amounted to € 23.3 million (2020: € 8.6 million). Domestic revenues mainly resulted from staff canteen and amounted to € 0.4 million (2020: € 0.5 million). Cost of Sales Cost of sales, which mainly consisted of costs of inventories, increased by € 18.9 million to € 33.3 million (2020: € 14.4 million without research and development costs). This change was primarily driven by higher material costs (2021: € 13.8 million; 2020: € -0.7 million). The increase is mainly due to the reversal of impairment of inventories upon market approval of Monjuvi in the amount of € 13.3 million in 2020. Research and Development Research and development expenses of € 177.7 million (2020: € 126.8 million) included acquisition and production costs for inventories and research and development costs recognized as an expense. These comprised primarily costs for external services of € 118.4 million (2020: € 75.9 million) and personnel costs of € 39.5 million (2020: € 30.9 million). Costs for external services increased mainly due to higher expenses for external laboratory services in connection with the research and development of tafasitamab. The increase in personnel costs was mainly due to higher salary expenses due to an increase of employee numbers (see also the section "Personnel expenses"). In 2021, no impairment losses were recognized for licenses for concessions, industrial property rights and similar rights and assets (2020: € 2.0 million). Selling Expenses Selling expenses increased by € 28.0 million to € 69.8 million (2020: € 41.9 million). This change was mainly related to higher expenses for external services and higher personnel cost in connection with the commercialization activities from Monjuvi in the US.

28 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 General and Administrative Expenses General and administrative expenses amounted to € 36.9 million (2020: € 41.2 million). This decrease mainly resulted from lower personnel costs (2021: € 17.0 million; 2020: € 28.2 million). This effect was partially compensated by higher expenses for external services (2021: € 13.7 million; 2020: € 9.6 million) as well as by higher depreciation and other costs for infrastructure (2021: € 4.3 million; 2020: € 2.4 million). Other Operating Income, Other Operating Expenses, Other Interest and Similar Income as well as Other Interest and Similar expenses Other operating income amounted to € 38.6 million, equaling a € 7.9 million increase compared to 2020. This item mainly included effects from foreign currency gains in the amount of € 25.0 million (2020: € 14.9 million), from the taxation of non-cash benefits in connection with the exercise of share-based payment programs by Company employees of € 1.2 million (2020: € 8.7 million) and from income from the reversal of provisions in the amount of € 10.9 million (2020: € 3.8 million). Other operating expenses decreased from € 47.3 million in 2020 to € 13.2 million in 2021. The main reasons for the decrease were lower losses from foreign currencies (2021: € 9.1 million; 2020: € 30.9 million), lower losses realized from forward exchange transactions (forward rate agreements) of € 3.5 million (2020: € 5.0 million) as well as the discontinuation of losses from the sale of total shares of affiliated companies (2021: € 0.0 million; 2020: € 11.7 million). Other interest and similar income amounting to € 30.9 million (2020: € 46.6 million) included primarily effects from the subsequent valuation of the provision associated with the collaboration and license agreement with Incyte amounting to € 25.0 million (2020: € 41.8 million), interest income from affiliated companies amounting to € 4.7 million (2020: € 3.6 million) as well as from bank balances and financial investments classified as other assets in the amount of € 0.6 million (2020: € 1.2 million). Other interest and similar expenses decreased from € 21.9 million in 2020 to € 21.1 million in 2021 and mainly included expenses from interest on the nominal value of convertible bonds in the amount of € 2.0 million (2020: € 1.9 million), effects from discounting the provision associated with the collaboration and license agreement with Incyte amounted to € 16.6 million (2020: € 13.4 million) as well as interest expenses of financial investments classified as other assets in the amount of € 2.4 million (2020: € 1.6 million). Income and Losses from Other Securities and Loans Presented under Financial Assets Income from other securities and loans presented under financial assets amounted to € 1.7 million (€ 0.9 million) and included realized gains from the sale of marketable securities. Losses from other securities and loans presented under financial assets amounted to € 0.7 million in 2021 (2020: € 14.5 million) and comprised unrealized losses from the valuation as well as realized losses from the sale of marketable securities.

Management Report 29 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Impairment of Financial Assets and Current Securities In 2021, the shares in MorphoSys US Inc. was impaired in the amount of € 128.1 million to reflect the reduced fair value. MorphoSys decided to focus its research efforts on the most advanced discovery and technology programs and also to centralize all laboratory activities at its German research hub in Planegg, Germany. Consequently, all US-based activities relating to discovery biology and drug discovery departments were abandoned. Therefore, any early pipeline projects in the indirect subsidiary Constellation Pharmaceuticals, Inc. cannot be realized anymore and the expected cash flows from these projects will not materialize accordingly. In 2020, an impairment of € 0.4 million was recognized for the shares in adivo GmbH. Expenses from Contribution agreements In 2021, the expenses from contribution agreements included the absorption of start-up costs incurred in 2021 and a contribution for operating costs to the affiliated company MorphoSys US Inc. totaling € 30.2 million (2020: € 65.7 million). Result after Taxes / Net Loss The aforementioned effects and the tax income of the reporting year of € 1.3 million lead to a result after taxes of € -310.5 million (2020: € -108.6 million) and a net loss in the amount of € 310.5 million (2020: € 108.6 million). The change in taxes resulted mainly from a tax loss carryback from the current fiscal year to the previous year, while in the previous year income tax expense was incurred from the taxation of the upfront payment from the collaboration and license agreement with Incyte. Financial Position Principles of Financial Management At MorphoSys, the primary goal of financial management is to ensure sufficient liquidity reserves at all times for the Company’s continued growth. The most important sources of this liquidity are the commercial operations of the individual business units and the related cash inflows. Cash flow projections and scenarios are used to determine the level of liquidity needed. Investments MorphoSys’s investments in property, plant and equipment amounted to € 2.2 million (2020: € 2.2 million). Depreciation of property, plant and equipment amounted to € 2.0 million in 2021 (2020: € 1.7 million). In 2021, the Company invested € 0.3 million (2020: € 21.4 million) in intangible assets, namely licenses. Amortization of intangible assets increased in comparison to the previous year and amounted to € 3.4 million in 2021 (2020: € 1.4 million). In 2021, no impairment loss was recognized for licenses (2021: € 0.0 million; 2020: € 2.0 million).

30 Liquidity As of December 31, 2021, the Company held liquid funds, bank deposits, other securities presented under current assets and other financial assets in the amount of € 824.2 million, compared to € 1,190.9 million on December 31, 2020. The decrease in liquidity resulted mainly from the cash outflow in connection with the acquisition of the indirect subsidiary Constellation as well as from the use of cash for operating activities in 2021. Net Assets Assets Total assets increased by € 789.6 million to € 2,277.4 million as December 31, 2021, compared to € 1,487.8 million as of December 31, 2020. The increase was primarily due to increased Shares in affiliated Companies (€ +1,150.7 million) and inventories (€ +11.4 million). This effect was partially offset by a decrease of securities (€ -278.4 million), receivables and other assets (€ -96.3 million), of intangible assets (€ -3.1 million) and cash on hand and cash at banks (€ -0.5 million). The increase in the Shares in affiliated Companies resulted from a contribution to MorphoSys US Inc.’s capital reserves paid by MorphoSys AG on July 15, 2021 in the amount of € 1,278.8 million, which was partially offset by an impairment of MorphoSys US Inc.'s shares of € 128.1 million. The changes in securities, other assets and liquid funds resulted from the reallocation of cash investments within the scope of optimizing the portfolio and the use of liquid funds for operating activities. Provisions, Liabilities and Deferred Income As of December 31, 2021, provisions totaled € 629.9 million, compared to € 673.5 million in the prior year. The increase in other provisions from € 608.6 million to € 629.6 million was primarily due to the recognition of the collaboration and license agreement with Incyte (2021: 550.5 million, 2020: € 527.0 million) as well as to expenses for external laboratory services (2021: 50.0 million, 2020: € 43.5 million). This effect was compensated by the utilization of provisions for milestone payments in connection with the commercialization of tafasitamab (2021: 0.0 million, 2020: € 10.2 million). Tax provisions decreased from € 64.9 million to € 0.3 million million due to a loss carryback and a payment of income taxes for 2020. Liabilities increased from € 378.4 million by € 67.7 million to € 446.1 million. This increase mainly resulted from increased liabilities to affiliated companies and from the change in liabilities that were not yet due as of the reporting date. Liabilities to affiliated companies mainly included the compensation obligation for Constellation in the amount of € 68.7 million for the excess interest of the development funding bond agreement with Royalty Pharma (December 31, 2020: € 0.0 million), which were partly netted with receivables from Constellation in the amount of € 22.1 million (December 31, 2020: € 0.0 million). Deferred income increased from € 0.1 million by € 988.8 million to € 988.9 million. The increase is mainly due to the non-refundable payment related to the agreement with Royalty Pharma. Equity On December 31, 2021, equity amounted to € 212.5 million, compared to € 435.8 million on December 31, 2020.

Management Report 31 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The number of shares issued as of December 31, 2021 totaled 34,231,943 of which 34,148,789 shares were outstanding (December 31, 2020: 32,890,046 and 32,758,632 shares, respectively). In comparison to December 31, 2020, the number of authorized ordinary shares decreased from 15,214,050 to 7,287,025. At the Annual General Meeting on May 19, 2021, Authorized Capital 2021-I in the amount of 4,861,376, Authorized Capital 2021-II in the amount of 3,289,004 and Authorized Capital 2021-III in the amount of 315,000 were newly created. The remaining Authorized Capital 2018-I in the amount of 11,768,314 and the remaining Authorized Capital 2020-I in the amount of 3,286,539 were canceled at this Annual General Meeting. The number was also reduced by the capital increase of 1,337,552 from the Authorized Capital 2021-II carried out in July 2021 under the agreement with Royalty Pharma. In comparison to December 31, 2020, the number of ordinary shares of conditional capital increased from 7,630,728 to 7,816,101. At the Annual General Meeting on May 19, 2021, Conditional Capital 2021-I in the amount of 3,289,004 was newly created. In the course of this General Meeting, the Conditional Capital 2016-I in the amount of 2,832,099 and the Conditional Capital 2016-III in the amount of 253,772 were reduced. The exercise of 4,345 stock options in 2021 from the Conditional Capital 2016-III had an offsetting effect as well. On December 31, 2021, the Company held 83,154 treasury shares with a value of 3,085,054€ – a decrease of €1,783,690 compared to December 31, 2020 (131,414 shares, €4,868,744). The reason for this decrease was the transfer of 45,891 treasury shares amounting to €1,696,131 to the Management Board and selected employees of the Company (beneficiaries) from the 2017 Long-Term Incentive Plan (LTI Plan). The vesting period for this LTI Plan expired on April 1, 2021 and offered beneficiaries a six-month period until October 13, 2021 to receive a total of 45,891 shares. In addition, 2,369 treasury shares for an amount of €87,558 from the 2019 Long-Term Incentive Plan were transferred to certain employees of MorphoSys US Inc. Consequently, the number of MorphoSys shares owned by the Company as of December 31, 2021, was 83,154 (December 31, 2020: 131,414). As of December 31, 2021, additional paid-in capital amounted to € 835.6 million, compared to € 751.2 million as of December 31, 2020. The increase in additional paid-in capital of € 84.4 million mainly resulted from the capital increase under the agreement with Royalty Pharma. The net loss for 2021 of € 310.5 million increased the accumulated deficit carried forward from 2020 of € 370.4 million to a total of € 680.8 million. Financing The Company's equity ratio as of December 31, 2021, amounted to 9%, compared to a level of 29% on December 31, 2020. The equity ratio decreased mainly due to the initial recognition of the deferred income from future payments to Royalty Pharma and to the net loss of the reporting year 2021. Currently, the Company does not have any financial liabilities to financial institutions.

32 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Off-Balance-Sheet Financing MorphoSys does not use any off-balance-sheet financing instruments such as the sale of receivables, asset- backed securities, sale-and-leaseback transactions or contingent liabilities in combination with non- consolidated special-purpose entities. Comparison of Actual Business Results versus Forecasts A detailed comparison of the Company’s forecasts versus the actual results can be found in Table 01. Tab. 01: Comparison of Actual Business Results versus Forecasts 2021 Targets 2021 Results Financial targets Revenues between € 95 million and € 145 million Group revenues of € 128.1 million Operating expenses between € 260 million and 290 million thereof R&D Expenses of 65-70% Operating expenses of € 317.7 million; deviation from guidance mainly due to self-constructed intangible assets, for which the option to capitalize according to section 248 para. 2 sent. 1 HGB was not used as well as higher cost of sales thereof R&D Expenses of 56%

Management Report 33 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 2021 Targets 2021 Results Proprietary Clinical Development Tafasitamab • Continue phase 1b study with tafasitamab in previously untreated DLBCL (firstMIND) • Initiate pivotal phase 3 study of tafasitamab in previously untreated DLBCL (frontMIND) • Initiate pivotal phase 3 study (inMIND) of tafasitamab in patients with indolent lymphoma (r/r FL/MZL) • Evaluate (in collaboration with Incyte and Xencor) tafasitamab, plamotamab and lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), first-line DLBCL and relapsed or refractory follicular lymphoma (FL) • Continue L-MIND study of tafasitamab and evaluate long-term efficacy and safety data • Continue pivotal phase 3 trial (B-MIND) with tafasitamab in combination with bendamustine for r/r DLBCL • Continue phase 2 COSMOS study with tafasitamab in CLL/SLL in combination with idelalisib and venetoclax • Support Incyte in its initiated regulatory submissions to the EMA, Swissmedic and Health Canada for tafasitamab in combination with lenalidomide for r/r DLBCL • Support Incyte in submitting marketing authorization applications in additional markets Tafasitamab • Phase 1b study firstMIND continued as planned • The first patient in the pivotal phase 3 frontMIND study was dosed in May • The first patient was dosed in the pivotal phase 3 inMIND study in April • Preparations continued for a possible study (in collaboration with Incyte and Xencor) of tafasitamab, plamotamab and lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), first-line DLBCL and relapsed or refractory follicular lymphoma (FL) • Three-year results from the phase 2 L-MIND study of tafasitamab in combination with lenalidomide for the treatment of relapsed or refractory DLBCL were presented in June at the American Society of Clinical Oncology (ASCO) Annual Meeting 2021 • The phase 3 B-MIND trial continued and has been fully recruited as of June; the significance of the B-MIND study has decreased as both FDA and EMA have approved Monjuvi based on L-MIND data. Consequently, the event-driven primary analysis has been removed from the planned analyses • The COSMOS study was completed • Tafasitamab in combination with lenalidomide was conditionally approved for r/r DLBCL by the EMA and Health Canada in August • Support provided to Incyte for its initiated regulatory submission to Swissmedic for tafasitamab in combination with lenalidomide for r/r DLBCL Felzartamab • Generate data from the phase 1/2 M-PLACE (proof-of-concept) study of felzartamab for the treatment of anti-PLA2R antibody positive membranous nephropathy • Continue dose schedule finding study (New-PLACE) in membranous nephropathy Felzartamab • Interim results from the M-PLACE study of felzartamab were presented at the Annual Meeting of the American Society of Nephrology in November • The New-PLACE study was continued in 2021 Continue and/or initiate development programs for antibody identification and preclinical development Early-stage drug discovery programs continued Clinical Development Through Partners Felzartamab/I-Mab • Support partner I-Mab in the regulatory filing (BLA) for felzartamab for multiple myeloma in China Otilimab/GSK • Publish preliminary results of OSCAR study of otilimab for the treatment of severe pulmonary COVID-19-associated disease by partner GSK in February 2021 Felzartamab/I-Mab • Continue to support partner I-Mab in the regulatory filing (BLA) for felzartamab for multiple myeloma in China Otilimab/GSK • GSK announced preliminary results from the OSCAR trial of otilimab for the treatment of severe pulmonary COVID-19-related disease in March • In October 2021, GSK provided an update that it had made the decision not to further explore otilimab as a potential treatment for severe pulmonary COVID-19 related disease in patients aged of 70 years and older

34 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The Management Board's General Assessment of Business Performance The 2021 financial year was a transformative year for MorphoSys and its employees. We worked together to transform MorphoSys from a research partner to the pharmaceutical industry into a fully integrated biopharmaceutical company. As part of this process, the corporate activities in 2021 focused on three important areas: • Executing on the commercialization of Monjuvi in the U.S. • Expanding the development pipeline by acquiring Constellation • Advancing the clinical development of our proprietary product candidates The co-commercialization of tafasitamab with Incyte in the U.S. gained momentum during the year after headwinds from the COVID-19 pandemic in the first quarter. The number of prescribing health centers grew steadily through the year, treating approximately 2,000 patients with relapsed or refractory diffuse large B- cell lymphoma (DLBCL) since launch in August 2020. Monjuvi’s net product sales for full-year 2021 equaled US$ 79.1 million. Further milestones were also achieved with tafasitamab. In August, Health Canada granted conditional marketing approval for Minjuvi (tafasitamab) in combination with lenalidomide for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma. The European Commission also granted conditional marketing approval for Minjuvi (tafasitamab) in combination with lenalidomide, followed by monotherapy with tafasitamab, for the treatment of adult DLBCL patients who are ineligible for autologous stem cell transplantation (ASCT). With the completion of the Constellation acquisition in mid-July 2021, MorphoSys strengthened its position in hematology/oncology. Constellation’s lead product candidate, pelabresib (CPI-0610), a BET inhibitor, is currently in a phase 3 clinical trial for the treatment of myelofibrosis (MF). Following the acquisition by MorphoSys, improvements were made to the study design of the phase 3 trial, and measures were taken to accelerate recruitment. In December 2021, MorphoSys presented the latest data from the MANIFEST phase 2 study evaluating the potential of pelabresib in the treatment of myelofibrosis at the 63rd Annual Meeting of the American Society of Hematology. These data supported the further development of pelabresib in combination with ruxolitinib in MANIFEST-2, a global, phase 3, randomized, double-blind study in JAK-inhibitor-naïve MF patients. The following advances were made in our proprietary clinical development programs during the financial year: In January 2021, the U.S. Food and Drug Administration (FDA) granted orphan drug designation to tafasitamab for the treatment of follicular lymphoma (FL), with the first patient dosed in the phase 3 inMIND study in April 2021. The study is evaluating the safety and efficacy of tafasitamab compared to placebo in combination with lenalidomide and rituximab in patients with relapsed or refractory follicular lymphoma (FL) or marginal zone lymphoma (MZL). In 2021, the phase 1b firstMIND study of tafasitamab, initiated in December 2019, continued as planned. The study is evaluating the safety and preliminary efficacy of tafasitamab in combination with R-CHOP compared to tafasitamab and lenalidomide in addition to R-CHOP in adult patients with newly diagnosed, previously untreated DLBCL.

Management Report 35 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 In May 2021, the first patient was dosed in the pivotal phase 3 frontMIND study. The study is evaluating tafasitamab plus lenalidomide in combination with R-CHOP compared to R-CHOP as first-line treatment for patients in moderate to high-risk groups with untreated diffuse large B-cell lymphoma (DLBCL). In June 2021, three-year results from the phase 2 L-MIND trial of tafasitamab in combination with lenalidomide for the treatment of relapsed or refractory DLBCL were presented at the 2021 Annual Meeting of the American Society of Clinical Oncology (ASCO). MorphoSys and Incyte presented real-world evidence results in December from the RE-MIND2 study of tafasitamab (Monjuvi) in combination with lenalidomide for the treatment of relapsed or refractory diffuse large B-cell lymphoma at the American Society of Hematology Annual Meeting (ASH 2021). RE-MIND2 compares outcomes of patients from the pivotal L-MIND trial to comparable patient populations treated with NCCN/ESMO recommended therapies. The results of the retrospective cohort analysis showed a significant improvement in overall survival compared to pola-BR and R2, two other treatments for DLBCL, and should further increase Monjuvi’s acceptance among treating doctors and patients. The CD38 antibody felzartamab, a proprietary development based on our HuCAL antibody technology, could be used against autoimmune diseases, among others. In the first half of 2021, first data from the phase 1/2 M- PLACE (proof-of-concept) study in membranous nephropathy (MN) were presented at the Annual Meeting of the American Society of Nephrology. In this study, felzartamab demonstrated the potential to rapidly and significantly reduce anti-PLA2R antibody levels in patients with anti-PLA2R antibody positive membranous nephropathy.In October 2021, the first patient was treated in a new phase 2 trial (IGNAZ) evaluating felzartamab in patients with immunoglobulin A nephropathy (IgAN). IgAN is a chronic and debilitating autoimmune disease that affects the kidneys and is the most common glomerular disease worldwide. In conclusion, MorphoSys made major strides forward in advancing its business during 2021. This included the first full year of commercializing Monjuvi in the U.S. with our partner Incyte, while working together to further broaden the opportunity for this important product via new market approvals and clinical trials in areas beyond the lead indication. We completed a transformative M&A deal, acquiring Constellation to grow our late-stage oncology pipeline with compelling product candidates that fit well with our existing pipeline, which we also moved forward during the year. We are well positioned to continue to advance and grow our business in the months and years ahead. In the 2021 financial year, revenues decreased to € 128.1 million and Net Loss decreased to € 310.5 million. Revenues consisted primarily of external revenues from antibody collaboration and license agreements with Janssen, Incyte and GSK. Revenues from royalties on net sales of Tremfya amounted to € 54.7 million. The change in Net Loss compared to the previous year resulted from lower sales compared to the previous year as well as increased expenses for the development and commercialization of tafasitamab and the higher expenses from compensation agreements for expenses of the U.S. subsidiary. Our cash and cash equivalents of € 824.2 million are a confirmation of the strength of the Company’s financial resources. MorphoSys recognized the impact of the global COVID‑19 pandemic on healthcare systems and society worldwide, as well as the resulting potential impact on preclinical and clinical programs, specifically clinical trials. In spring 2020, MorphoSys activated its existing business continuity plans to minimize any disruptions to ongoing operations caused by the COVID‑19 pandemic and to take the necessary actions to protect its employees. In addition, MorphoSys continuously monitors the situation as a whole as well as each clinical program individually and decides on the necessary course of action to ensure the safety of patients, personnel and other stakeholders, as well as on the collection of data.

36 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Outlook and Forecast The acquisition of Constellation was an important step on MorphoSys’ path to becoming a leading biopharmaceutical company in hematology/oncology. To be successful, MorphoSys must rapidly develop new therapies with first-in-class and/or best-in-class potential and make them available to patients. In order to accomplish this, MorphoSys plans to prioritize its capital allocation to late stage clinical studies. MorphoSys’ own development activities focus mainly on therapies for the treatment of blood cancers, which the Company intends to bring to market maturity and commercialize. Other drug candidates, such as felzartamab, which MorphoSys is currently testing in clinical trials for autoimmune diseases, could be further developed with partners or fully out-licensed due to its focus on hematology/oncology. General Statement on Expected Development MorphoSys has defined three strategic value drivers: • Revenues from the commercialization of proprietary products, such as Monjuvi® • New marketing approvals for advanced drug candidates • Further development of mid-stage drug candidates with the option to partner or out-license them The Management Board expects the following to be among the developments taking place in 2022: • Higher sales of Monjuvi in the U.S., with commercialization driven by the Company’s own capabilities and its partner Incyte • Expand Monjuvi to additional disease indications and advance proprietary clinical development of product candidates: pelabresib, felzartamab and CPI-0209 The expected developments and progress of the pipeline are presented in detail below in the section “Future Research and Development and Expected Business Performance.” Strategic Outlook MorphoSys invests a significant portion of its financial resources in the clinical development of its own drug candidates. The Company is focused on diseases in the hematology/oncology area. The Management Board believes a focus on proprietary drug development and commercialization offers the best path to achieving a sustainable increase in the Company’s value. The Management Board has prioritized the further clinical development of tafasitamab and pelabresib as well as the financing of pivotal clinical trials. To this end, revenues from the commercialization of Monjuvi are expected to contribute as well as partnerships to leverage the full potential of the Company's own development candidates. Increasing direct revenues from the commercialization of Monjuvi is a core component of MorphoSys’ value creation strategy. Following the 2020 approval and launch of Monjuvi in the U.S., the processes are also underway for launches in Europe and Canada through MorphoSys’ partner Incyte. Additional regions are expected to follow, such as Switzerland, where Incyte could also commercialize tafasitamab, with MorphoSys entitled to royalties on sales. MorphoSys and Incyte have also identified significant unmet medical need and commercial opportunities for tafasitamab outside of DLBCL in non-Hodgkin’s lymphoma. The Management Board believes tafasitamab could offer considerable future potential, not only as a first-line therapy in DLBCL, but also in other

Management Report 37 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 indications such as r/r follicular lymphoma (FL) and r/r marginal zone lymphoma (MZL). Tafasitamab could become the future backbone in DLBCL therapy and other therapies. Pelabresib is viewed by the Management Board, as well as by leading medical experts, as a promising drug that may have the potential to fundamentally improve the treatment of myelofibrosis. In ongoing clinical trials, pelabresib is demonstrating that the mechanism of action of the BET inhibitor has significant ameliorative effects on all four major disease characteristics in myelofibrosis, such as spleen size, anemia, fibrosis of bone marrow and the patient’s overall physical condition. With felzartamab, MorphoSys has another proprietary development candidate in advanced clinical development studies in the field of autoimmune diseases. MorphoSys’ focus on hematology/oncology agents may lead to the Company choosing not to continue developing and commercializing felzartamab on its own but instead develop it further within the framework of a partnership or by out-licensing it to another company with expertise in the autoimmune field. Partnerships can also help generate value through milestone payments and royalties in the event of market approval (revenue sharing). Partnered programs such as gantenerumab with Roche, felzartamab in multiple myeloma with I-Mab or otilimab with GSK are the next candidates that could reach the market. In order to accomplish the overriding aim of being a leader in hematology/oncology, continually investing in the Company’s further development is not only sensible, but also essential. Expected Economic Development In its January 2022 report, the International Monetary Fund (IMF) projected global economic growth of 4.4% in 2022, compared to 5.9% for the year 2021. According to the IMF, the global economy entered 2022 in a weaker position than previously expected. As the new COVID-19 variant Omicron has spread, countries have reimposed mobility restrictions. Additionally, rising energy prices and supply disruptions have resulted in higher and more broad-based inflation than anticipated, notably in the United States and many emerging markets and developing economies. Uncertainty remains for the year ahead. The emergence of new COVID-19 variants could prolong the pandemic and induce renewed economic disruptions. Moreover, supply chain disruptions, energy price volatility, and localized wage pressures translate into a high level of uncertainty around inflation and policy paths. Adding to the unknown, geopolitical tensions remain high, particularly with the recent invasion of Ukraine by Russia. This conflict is expected to take a strong human and economic toll far beyond the Ukrainian border, particularly in Europe. Given these many challenges, international cooperation is critical, including developing an effective global health strategy to address the now two-year- old pandemic. Growth in advanced economies is anticipated to reach 3.9% in 2022, compared to 5.0% for 2021. The IMF expects growth in the euro area to be 3.9% in 2022 compared to 5.2% for 2021. Growth in Germany is anticipated to rise to 3.8% in 2022 (2021: 2.7%), and the IMF projection for U.S. economic growth in 2022 is 4.0% (2021: 5.6%). The IMF’s 2022 growth forecast for emerging and developing countries is 4.8% (2021: 6.5%), and growth in China in the coming year is projected at 4.8% (2021: 8.1%). Russia’s economy is anticipated to grow by 2.8% in 2022 (2021: 4.5%), and Brazil is expected to barely grow at 0.3% for 2022 (2021: 4.7%). MorphoSys AG has implemented a business continuity plan to largely prevent the collapse of critical business processes and ensure their resumption in the case of a natural disaster, public health emergency such as the novel coronavirus, or other serious events. However, depending on the severity of the situation, it may be difficult or, in some cases, impossible to avoid an interruption in our business for a significant period of time. Our contingency plans for disaster recovery and business continuity may prove inadequate in the event of a

38 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 serious disaster or similar event, and we may incur substantial costs that could have a material adverse effect on our business. Expected Development of the Life Sciences Sector In mid-January 2022, BioCentury published an article “Valuations could get investors, acquirers hunting for opportunities,” interviewing 15 investors and bankers to get their views on the year ahead. With biotech valuations beaten down in 2021 and a number of new companies debuting, investors believe there are plenty of buying opportunities heading into 2022. However, the IPO flow is predicted to slow as poor IPO performance translates to less crossover activity. The article reports that sentiment largely remains negative for the sector, but the low valuations could help change this. The conditions for M&A remain strong given the lower valuations and the large amounts of cash that large biopharma companies have on hand. In a related article, “Buysiders eye 2022 as the year of immuno-oncology’s next big act,” investors indicated that cancer immunotherapy is poised for long-awaited breakthroughs in 2022, which promises a full slate of regulatory and clinical milestones that could reignite buysider excitement while drawing in generalists. Both checkpoint inhibitors as well as bispecific antibodies could emerge from a seven-year regulatory lull in 2022. Investors also expect interest in neurology — ignited by the Aduhelm (aducanumab) approval last year — to carry over into 2022, with multiple catalysts anticipated in this area. The high level of innovation in the biotechnology sector is reflected in the number of new product approvals in 2021. Despite the ongoing challenges posed by the COVID-19 crisis, 50 new compounds were approved by the U.S. FDA in 2021, down slightly from the 53 approved in 2020. In addition, there were ten Biologics License Application (BLA) approvals in 2021. In the EU, a record 52 new drugs and vaccines were authorized for marketing in 2021, compared to 42 in 2020. The record in 2021 was in part due to the approval of four new vaccines and three new therapeutics for COVID-19. According to the report by PricewaterhouseCoopers (PwC), entitled: “Pharmaceutical & Life Sciences: Deals 2022 Outlook,” M&A activity in 2022 is projected to be between US$ 350–400 billion for the year, driven by all subsectors. This would be an increase compared to 2021, when the total value of deals was US$ 269.4 billion, a 46% increase compared to 2020. Biotech deals in the range of US$ 5 billion to US$ 15 billion, combined with mid-sized pharma and medical device deals, are expected to drive significant investment money into M&A activity. PwC also predicts that the sector could see a large (US$ 100 billion or higher) deal that is part of a “transact-to-transform” strategy. Tailwinds around the need to invest in growth as well as access to capital are expected to more than offset potential negative factors such as drug pricing, a recently active U.S. Federal Trade Commission agenda and tax rate increases. Future Research and Development and Expected Business Performance MorphoSys will continue to invest in research and development, with the majority of funds directed towards developing the Company’s proprietary drug candidates tafasitamab, pelabresib, felzartamab and CPI-0209. Most of these funds will be used in the short to medium term for advancing the broad clinical development of tafasitamab and pelabresib. Investments aimed at identifying target molecules, developing the corresponding antibodies, and in technology are also possible. The investments planned in proprietary drug candidates and technologies are expected to continue to lead to the progressive maturity of the pipeline’s product candidates.

Management Report 39 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The following events and development activities, among others, are planned in 2022: • First proof-of-concept data from the ongoing clinical phase 2 study of CPI-0209 in solid tumors and blood cancer; • Additional data from the phase 1/2 M-PLACE (proof-of-concept) study of felzartamab for the treatment of anti-PLA2R antibody positive membranous nephropathy (MN); • First data from the phase 2 study (IGNAZ) to evaluate felzartamab in patients with immunoglobulin A nephropathy (IgAN); • MorphoSys’ partner Roche expects a pivotal data readout of the GRADUATE 1 and GRADUATE 2 trials with gantenerumab in the second half of 2022. Roche initiated these phase 3 development program for patients with Alzheimer’s disease in 2018; • Initiation of a combination study (in collaboration with Incyte and Xencor) of tafasitamab, plamotamab and lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), first-line DLBCL and relapsed or refractory follicular lymphoma (FL). The following events and development activities, among others, are planned beyond 2022: • The primary analysis data from the pivotal phase 3 study (inMIND) of tafasitamab in patients with indolent lymphoma (r/r FL/MZL) in the second half of 2023; • The primary analysis data from the pivotal phase 3 study (MANIFEST-2) of pelabresib in myelofibrosis (MF) in the first half of 2024; • The primary analysis data from the pivotal phase 3 study (frontMIND) of tafasitamab in previously untreated DLBCL in the second half of 2025. We also expect individual product candidates developed by partners to continue to mature in programs where MorphoSys benefits from royalties and milestone payments if successful. Whether, when and to what extent any news is published after the studies’ primary completion is solely at the discretion of our partners. Expected Development of the Financial Position and Liquidity MorphoSys transformed from a research- and technology-driven company to a commercial biopharmaceutical company with the launch of its first product in 2020. With the change in our business model and a certain degree of added complexity to our income statement from our partnership with Incyte and the agreement with Royalty Pharma, our focus is on the forecast parameters that are the most important to our external stakeholders. These parameters highlight our key business drivers: revenue growth from product sales, ongoing investment in expanding our pipeline, and supporting the launch of Monjuvi. In concrete terms, these parameters focus on Monjuvi’s net sales in the U.S., the associated gross margin, and the R&D and SG&A expenses. In business year 2022, research and development expenses are expected to be in the range of € 165 million to € 185 million while selling, general and administrative expenses are expected to be in the range of € 80 million to € 95 million. The guidance is subject to a number of uncertainties, including but not limited to the ongoing COVID-19 pandemic and its impact on MorphoSys’ business. Milestone payments and royalties related to the achievement of market maturity of HuCAL and Ylanthia antibodies could have an impact on the Company’s net assets and financial position in the years ahead. Such events could lead to exceeding our financial targets. Failures in drug development could also have an adverse

40 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 effect on MorphoSys. Negative effects from another COVID-19-like pandemic or COVID-19 variants are also possible, and cannot be excluded. Revenue growth in the short to medium term will depend on the Company’s ability to successfully continue to commercialize Monjuvi. At the end of the 2021 year, MorphoSys had cash and investments (consisting of Cash on Hand and Cash at Banks, Other Securities presented under Current Assets and Other Financial Assets) of € 824.2 million (December 31, 2020: € 1,190.9 million). The liquid funds are predominantly required to finance and advance the development of the proprietary portfolio to key clinical milestones, including pivotal data readouts for tafasitamab and pelabresib. Dividend The separate financial statements of MorphoSys AG, prepared in accordance with German Generally Accepted Accounting Principles (German Commercial Code), show an accumulated deficit, which prevents the Company from distributing a dividend for the 2021 financial year. In view of the anticipated losses in 2022, the Company expects to continue to report an accumulated loss for the 2022 financial year. MorphoSys plans to invest further in the development of proprietary drugs and commercialization of Monjuvi. Based on these plans, MorphoSys does not expect to pay a dividend in the foreseeable future. This outlook takes into account all known factors at the time of preparing this report and is based on the Management Board’s assumptions about events that could affect the Company’s business in 2022 and beyond. Future results may differ from the expectations described in the section “Outlook and Forecast.” The most significant risks are described in the risk report.

Management Report 41 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Risk and Opportunity Report We operate in an industry characterized by constant change and innovation. The challenges and opportunities in the pharmaceutical and biotechnology industry are influenced by a variety of factors. Global demographic changes, medical advances and the desire to improve the quality of life offer excellent growth opportunities. Companies must also, however, grapple with the growing regulatory requirements in the areas of drug development and commercialization, as well as the cost pressures weighing on healthcare systems. We make every effort to systematically identify new opportunities and leverage our business success to generate a sustainable increase in the Company’s enterprise value. In our industry, entrepreneurial success is not achievable without conscious risk-taking. Our integrated risk and opportunity management system identifies the relevant issues, assesses them and takes suitable action to avert threats so we can achieve our corporate objectives. A periodic strategy review ensures there is a balance between risks and opportunities. We assume a risk only when it involves an opportunity to increase the Company’s value. Principles of Integrated Risk and Opportunity Management We continually encounter both risks and opportunities that could have a potential material impact on our net assets and financial position, as well as a direct effect on intangible assets, such as our image in the sector or our brand name. We define risk as internal or external events that could have a direct adverse impact on the achievement of our corporate objectives. Opportunities represent positive deviations from our corporate planning and are in direct relation to risks. Our integrated risk and opportunity management system is therefore an integral part of our corporate governance practices and ensures adherence to the principles of good corporate governance and compliance with the regulatory requirements. We have a comprehensive system in place to recognize, assess, communicate and manage our risks, and to identify our opportunities at an early stage. The Company-wide integrated risk and opportunity management system focuses on major risks which alone or in combination with other risks could potentially jeopardize the Company. Risks and opportunities that do not meet this criterion are deliberately excluded by the system and managed and monitored on a decentralized basis at the level of the respective organizational unit. The integrated risk and opportunity management system is described in a risk manual containing all the key elements of the process. During the 2021 financial year, we continued to develop our risk and opportunity management system to better reflect the business model of an integrated biopharmaceutical company and our associated internationalization. Table 02 provides an overview of significant changes in comparison to prior years.

42 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Tab. 02: Comparison of Old and New Integrated Opportunity & Risk Management System Organization of Integrated Risk and Opportunity Management Our Management Board is responsible for the integrated risk and opportunity management system and ensures that all risks and opportunities are evaluated, monitored and presented in their entirety. The system’s Company-wide coordination, implementation and further development are the responsibility of the central Global Risk Management function, which is part of the Group Financial Planning & Analysis (FP&A) Department and reports directly to the Chief Financial Officer. The Supervisory Board has tasked the Audit Committee with monitoring the effectiveness of our risk management system. The Audit Committee reports its findings to the entire Supervisory Board and the Management Board twice a year. Risk ownership is generally assigned at the level of the respective Executive Committee member as well as to selected executives with overriding responsibility. This group is defined as “risk owners.” As part of the integrated risk and opportunity management process, risk owners receive support from “risk agents.” Risk agents are experienced employees and generally members of the Global Leadership Group. They identify the risks in their respective areas in close coordination with the central Global Risk Management function. The distinction between the responsibilities of risk owners and risk agents is based on MorphoSys’ global management and operating model. The central Global Risk Management function initiates and directs the systematic risk identification process. Its organizational integration into the Group’s FP&A unit ensures that there is a tight link between risk and opportunity management and corporate planning. Global Risk Management plays an important role in analyzing the interdependencies of risks and objectifying risk assessment. The Corporate Internal Audit Department is closely involved in the risk and opportunity management process. In addition to an ongoing exchange with the Global Risk Management function, the Internal Audit

Management Report 43 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Department receives the audit reports in order to incorporate the findings into its risk-based audit plan. In accordance with this plan, the Internal Audit Department also conducts audits relating to integrated risk and opportunity management at irregular intervals. Figure 01 provides an overview of the organization and responsibilities of our integrated risk and opportunity management system, which is based on the globally recognized “Three Lines of Defense” model and meets the statutory requirements for the responsibilities of the Management Board and supervisory bodies. Fig. 01: Risk and Opportunity Management System at MorphoSys Process and Reporting of Integrated Risk and Opportunity Management As part of our integrated risk and opportunity management process, all our major risks are identified and assessed by the relevant departments and reported in a structured form to Global Risk Management. This routine process takes place twice a year as what is called a “risk run”. For significant changes in material risks between the risk runs, the risk owners and risk agents are required to submit their respective reports to Global Risk Management via an ad hoc process. Various quality assurance measures have been implemented to ensure that the departments involved initially assess and record the risks as objectively as possible. These measures include a kick-off meeting to present the key aspects of the integrated risk and opportunity manual, as well as the close monitoring of the reporting process by Global Risk Management. After receiving the feedback from the risk agents, Global Risk Management carries out an initial review to identify the principal risks and highlight the interdependencies between identified risks. A workshop is held with selected risk agents in which the key risks and opportunities are calibrated based on the initial feedback and the key statements, and detailed in the report to the Management Board and supervisory bodies.

44 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The risk assessment is derived from an evaluation of the risk’s probability of occurrence and impact using a five-point scale, as shown in table 03. In terms of impact, MorphoSys distinguishes between financial and non-financial impact. Financial impact is defined as a negative deviation from the budgeted operating result, whereby the impact on liquidity is equally considered for non-profit items. Both the short-term (12 months) and medium-term (three years) financial impact is taken into consideration. Non-financial risks in our integrated opportunity and risk management system are circumstances and defined as those having no initial direct impact on the operating result or liquidity during the planning period, but still have a negative impact on the achievement of the Company’s targets. Examples include the loss of reputation or key employees, both of which can have a sustained impact on the Company’s potential for success. Another example specific to our industry is the impact of delays in patient recruitment for clinical trials. Such delays initially lead to lower costs, which from a purely mechanical standpoint represent an opportunity when compared to initial planning, but in the long term have a negative effect causing a delay in the development plan, which outweigh the short-term benefit of lower costs. In view of the increasing importance clinical programs have on the Company’s value, we continued to evolve our integrated opportunity and risk management system in the reporting year to optimally reflect these Company- and industry-specific characteristics. The integrated opportunity and risk management system addresses both opportunities and risks of the Company, with systematic quantification and aggregation only for risks. Tab. 03: Risk Assessment Categories Accounting-Related Internal Control System To ensure accurate bookkeeping and accounting and maintain reliable financial reporting in the financial statements of MorphoSys AG and management report, we use internal controls through our financial reporting, which we have expanded pursuant to the provisions of Section 404 of the Sarbanes-Oxley Act of 2002 (SOX 404), in addition to Company-wide reporting guidelines and other measures, such as employee training and ongoing professional education. This essential component of Company accounting consists of preventative, monitoring and detection measures intended to ensure adequate security and control in accounting and operating functions.

Management Report 45 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Further detailed information about the internal control system for financial reporting can be found in the Corporate Governance Report. Description of Key Opportunities Increasing life expectancy in industrialized countries and the changes in income and lifestyle in the emerging markets are expected to drive the demand for new and innovative treatments and advanced technologies. Progress in science and medicine has led to a better understanding of the biological processes of disease. This, in turn, paves the way for new therapeutic approaches in bi- and multispecific antibody development and other technology platforms. Advanced antibody discovery and protein engineering technologies and a broad portfolio of validated clinical programs have made us one of the globally recognized biotechnology companies in the therapeutic antibody field. The class of monoclonal antibodies is one of today’s most successful, top-selling therapies in cancers and immune diseases. Our key opportunities are described in table 04 and ranked according to their expected potential value contribution and strategic relevance. Tab. 04: Summary of MorphoSys' Key Opportunities Opportunities Full realization of pelabresib’s potential in product development Full realization of tafasitamab’s potential in product development and commercialization Further advancement of current phase 2 studies of felzartamab and CPI-0209 Additional income from milestones and royalties from partnered programs Maximization of value proposition from promising investigational compounds Full Realization of Pelabresib's Potential in Product Development We believe pelabresib has the potential to become the standard of care in myelofibrosis. This assessment was underlined by the presentation of confirmatory phase 2 data (MANIFEST) at the American Society of Hematology conference at the end of the last financial year. The approval of pelabresib could unlock significant positive and transformative potential for MorphoSys in an indication where there is a high need for improved treatment options for approximately 30,000 to 35,000 patients in the U.S. and Europe. To intensify further product development, MorphoSys has already adapted the study’s design and plans to enroll more patients in the active phase 3 study. One of the Company-wide strategic priorities, in addition to the activities already completed, is to ensure the active study’s smooth and prompt completion. In addition, the Company is contemplating expanding the geographic availability, although the clear focus is on the U.S. and European markets. Full Realization of Potential of Monjuvi (Tafasitamab) in Product Development and Commercialization Monjuvi (tafasitamab-cxix) is our first commercial product and represents a significant opportunity as it is currently the only FDA-approved drug in the second line setting for patients with r/r DLBCL in combination with lenalidomide. MorphoSys is focusing on commercializing Monjuvi in the U.S. market with its partner Incyte. MorphoSys will receive royalties for the commercialization outside the U.S., which will be handled by

46 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Incyte. Data from the L-MIND study published in 2021 have underpinned the existing long-term treatment outcomes, and approximately 2,000 patients have been treated with Monjuvi in the U.S. since its launch. We are therefore concentrating our efforts on Monjuvi’s further commercialization where we believe many more patients could benefit from treatment, which would directly lead to higher sales revenues. In addition to the focus on Monjuvi’s commercialization, we are also prioritizing further development in DLBCL and beyond, particularly within the scope of our active phase 3 trial in first-line DLBCL, tafasitamab’s development in FL, and through combination studies with other promising drugs. If approval is granted in important markets after completion of the clinical phases, there is a chance of a significant increase in medium- and long-term sales potential. Further Advancement of Current Phase 2 Studies of Felzartamab and CPI-0209 The two phase 2 compounds, felzartamab and CPI-0209, complement MorphoSys’ proprietary clinical pipeline. CPI-0209 is a potentially best-in-class EZH2 inhibitor currently in phase 2 development for advanced solid tumors and blood cancer. Results from the ongoing feasibility study are expected in 2022. Felzartamab is a CD38 antibody that, based on data from two active phase 2 studies, may have strong therapeutic potential for autoimmune diseases.The focus for both compounds is to continue their development and gain further insight from the data generated. Further in-house development, co-development with a partner and out-licensing are all conceivable options to accomplish this. Additional Income from Milestones and Royalties from Partnered Programs As previously described, our business focus during the past few years has significantly shifted away from traditional contract research towards proprietary product development and commercialization, especially since our acquisition of Constellation and the agreement with Royalty Pharma. Due to programs partnered in the past, however, MorphoSys may still be entitled to substantial cash inflows from milestones and/or licensing income in the future. This is the case, for example, for otilimab and gantenerumab that are covered under the agreement with Royalty Pharma, despite the fact that a portion of the royalties for these product candidates has been assigned. MorphoSys’ partners, such as Novartis, with whom the Company has had a long-standing research collaboration, also have other drugs in development. Maximization of Value of Promising Investigational Compounds in the Discovery Phase We have already demonstrated our ability to develop promising drugs and technologies. Our in-house research department could help us in the future to increase the pace and success rate of our proprietary drug development programs, and new technologies could open up new disease areas with completely novel modes of action. The Company’s technology development is driven by a team of scientists focused on advancing our technologies. In addition to our in-house technology development, we also rely on external sources to bolster our efforts in this area. One example is our licensing agreement with Cherry Biolabs, which grants us the rights to apply their innovative, multispecific Hemibody technology in the context of our CyCAT dual- targeting platform. Interesting development candidates are discussed regularly by portfolio management committees and the Executive Committee. Description of Key Risks In this report describing the key risks, we explain the financial and non-financial risks that we consider to be most relevant for the achievement of the Company’s targets in 2022 and beyond. The risk and opportunity profile of MorphoSys has changed significantly in the past year as a result of the acquisition of Constellation on July 15, 2021, and the associated financing agreement with Royalty Pharma. We have therefore revised

Management Report 47 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 our allocation of risks to risk categories compared to the previous year’s presentation to better reflect the evolution of our business model. The following overview provides an explanation and summary of the different risk categories and a description of the items generally included in these categories. Tab. 05: Overview of Risk Categories The assessment of risk relevance is not distinguished according to category, but instead by impact and probability of occurrence. For this reason, the major risks listed in table 06 do not always include risks from all five categories.
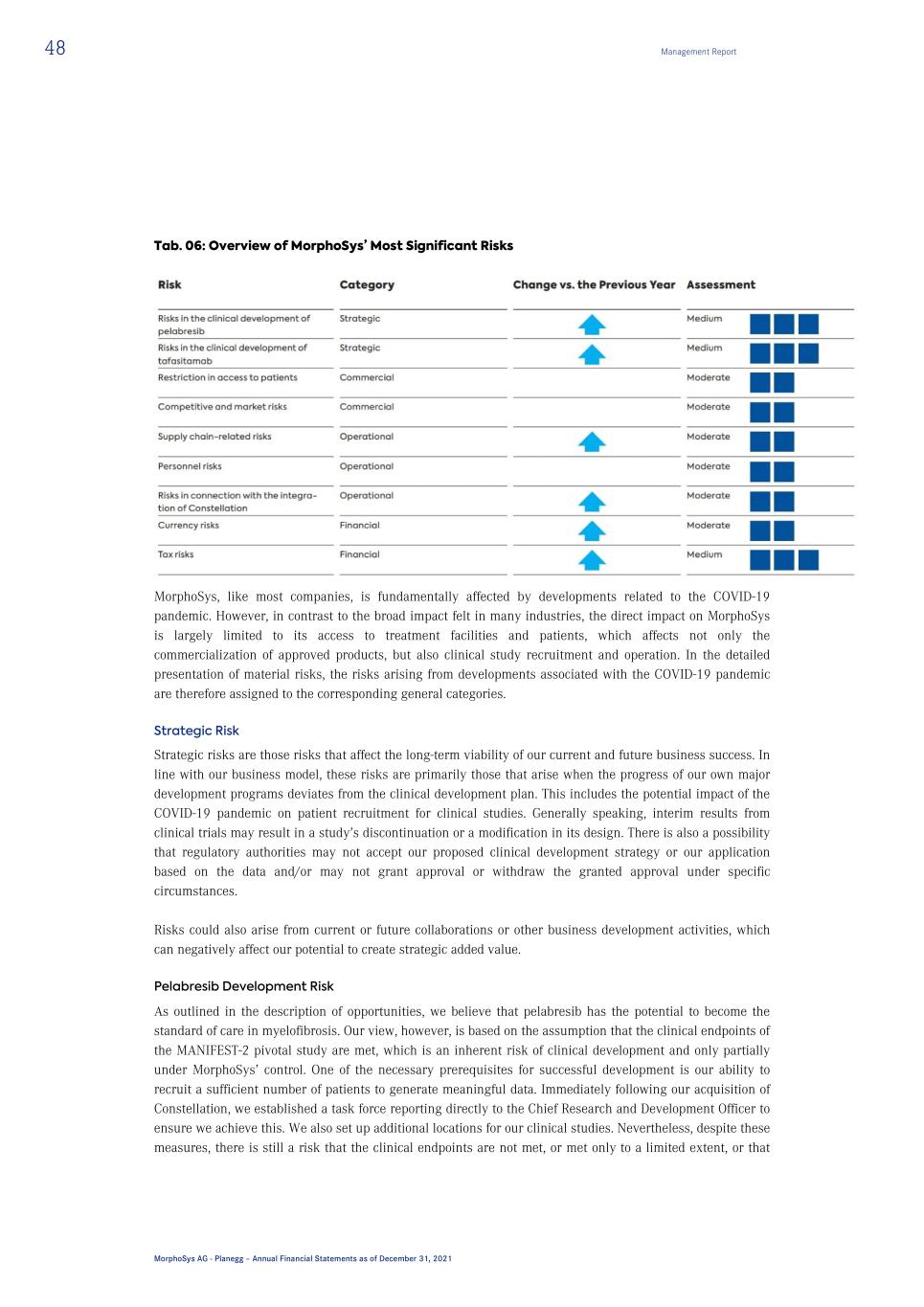
48 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Tab. 06: Overview of MorphoSys’ Most Significant Risks MorphoSys, like most companies, is fundamentally affected by developments related to the COVID-19 pandemic. However, in contrast to the broad impact felt in many industries, the direct impact on MorphoSys is largely limited to its access to treatment facilities and patients, which affects not only the commercialization of approved products, but also clinical study recruitment and operation. In the detailed presentation of material risks, the risks arising from developments associated with the COVID-19 pandemic are therefore assigned to the corresponding general categories. Strategic Risk Strategic risks are those risks that affect the long-term viability of our current and future business success. In line with our business model, these risks are primarily those that arise when the progress of our own major development programs deviates from the clinical development plan. This includes the potential impact of the COVID-19 pandemic on patient recruitment for clinical studies. Generally speaking, interim results from clinical trials may result in a study’s discontinuation or a modification in its design. There is also a possibility that regulatory authorities may not accept our proposed clinical development strategy or our application based on the data and/or may not grant approval or withdraw the granted approval under specific circumstances. Risks could also arise from current or future collaborations or other business development activities, which can negatively affect our potential to create strategic added value. Pelabresib Development Risk As outlined in the description of opportunities, we believe that pelabresib has the potential to become the standard of care in myelofibrosis. Our view, however, is based on the assumption that the clinical endpoints of the MANIFEST-2 pivotal study are met, which is an inherent risk of clinical development and only partially under MorphoSys’ control. One of the necessary prerequisites for successful development is our ability to recruit a sufficient number of patients to generate meaningful data. Immediately following our acquisition of Constellation, we established a task force reporting directly to the Chief Research and Development Officer to ensure we achieve this. We also set up additional locations for our clinical studies. Nevertheless, despite these measures, there is still a risk that the clinical endpoints are not met, or met only to a limited extent, or that

Management Report 49 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 there is a delay in comparison to the original development plan, any of which could have a significant impact on the Company’s potential for future value creation. Tafasitamab Development Risk Similar risks exist for clinical trials in other indications as well as for approvals for tafasitamab, which we are working on together with our collaboration partner Incyte. We initiated new studies in the reporting year and have implemented measures to ensure that we can promptly enroll patients. The achievement of the clinical endpoint is again beyond MorphoSys’ control and is an inherent risk of clinical development. Development Risks Associated with Other Clinical Programs In addition to our two main clinical programs, we have two other programs, CPI-0209 and felzartamab, in phase 2 clinical development. These active studies are considered proof-of-concept studies, which means there are further opportunities for clinical development if the outcome is successful. These studies also carry the risk that the clinical endpoints are not achieved to a satisfactory extent. Business Development Risk Due to the high cost of clinical trials, we are not able to conduct all scientifically feasible development projects independently and need to prioritize our investments based on business decision models despite our strong liquidity. Collaborations with other partners may be an alternative for development projects investigating our product candidates in new indications. Should such collaborations fail to materialize, there is a risk that we will not be able to realize the Company’s potential to create value. However, this does not represent a risk compared to our forecast, as the latter does not include such an assumption due to the uncertainty of the conclusion or the conditions of possible collaborations. Commercial Risk In July 2020, MorphoSys received accelerated FDA approval for the commercialization of Monjuvi in the U.S. Since that time, the relative importance of revenues generated from our own commercialization of the product with our partner Incyte has been steadily increasing. From a risk standpoint, we distinguish the short-term risk arising from potential COVID-19 restrictions from the more mid- to long-term risk of the market and competitive environment. Pandemic-Related Limitations in Access to Patients and Facilities The ebb and flow in COVID-19 infections since the start of the pandemic have impacted patient access to treatment facilities in the U.S. Therapies planned in “hot spot” regions, for example, may be postponed due to a lack of capacity. Furthermore, safety protocols implemented at various sites of care may restrict the ability of our sales force to engage in-person with medical personnel. As a result, there is a risk that we will not achieve the revenue planned from the sale of Monjuvi in the U.S. We assess any potential impact of this risk, however, as moderate. Competitive and Market Risk Despite our innovative products, we operate in a competitive environment not only for existing therapies but also unapproved therapeutic alternatives still in clinical research. We meet these challenges through a combination of education about our product and additional data from ongoing clinical studies. Nevertheless, there is a risk that the therapies preferred change over time, competitive products are approved, or existing therapies gain market share at our expense.

50 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 There is also significant pressure to contain healthcare costs in the European and North American markets, and payers have taken actions that may result in access restrictions or lead directly and indirectly to price reductions for our products. We expect these efforts to increase and expand over time and continuously monitor the related discussions. However, due to the political situation in the U.S., our core sales market, we do not expect any significant impact from such regulatory measures during the forecast period. Operational Risk Operational risk includes material risks that are attributable to the Company’s operations, specifically those related to the execution of processes such as maintaining business operations in the event of catastrophic events or cybersecurity incidents. Risks in connection with the integration of Constellation are also included in this category. Supply Chain Risk MorphoSys does not produce its own active pharmaceutical ingredients but outsources this manufacturing to contract manufacturing organizations (“CMOs”), which is typical for a number of comparable companies in our industry. MorphoSys addresses the risk of material procurement through contractual agreements and continual monitoring. The impact of the COVID-19 pandemic on global supply chains has resulted in an increased risk of bottlenecks in the availability of consumables and raw materials compared to prior years. MorphoSys is addressing this risk by securing a safety backlog, resulting in the risk from delays in the supply of products for active clinical trials and commercial use during the forecast period being assessed as low risk. Personnel Risk MorphoSys’ key asset is its employees. The Company’s future success largely depends on its ability to attract, develop and retain key employees over time. This risk has increased due to the outbreak of the COVID-19 pandemic and the higher demand for personnel in pharmaceutical companies that has resulted. MorphoSys has offices in Germany, a country with a high demand for personnel and a correspondingly large number of competing biotechnology companies. To maintain its image as an attractive employer for skilled personnel, MorphoSys offers competitive compensation and a range of options for personnel development. Succession planning for key positions ensures that there is no significant risk arising from the level of employee turnover that is typical for the industry and the Company’s location. Integration Risk A “risk run” in the fall of 2021 identified a short-term, moderate organizational risk related to the operational integration of Constellation. Should MorphoSys be unable to integrate the acquired company into its structures and processes within a reasonable period of time, there is a risk that potential synergies may fail to be realized as planned. This risk also includes the potential departure of employees in key positions with specific background knowledge. To mitigate this risk, a project team has been formed consisting of experienced Constellation and MorphoSys employees from various departments that is focused on key aspects of the integration. By the end of the 2021 financial year, significant progress had already been made in integrating the companies’ operations. A global operating model was rolled out to manage major functions across locations and facilitate the business and decision-making processes. While the measures taken have greatly reduced integration risk, a financial risk exists that potential synergies will not be leveraged as planned. IT and Cybersecurity Risk Cyber risks encompass all risks to computer and information networks, IT infrastructure and IT-based business and production processes from exposure to sabotage, espionage or other criminal acts. Should the

Management Report 51 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 established security measures fail, MorphoSys could suffer reputational damage as well as payment obligations arising from contractual and legal claims from customers, contractual partners and authorities. An increase in the professionalization of cyberattacks has become evident in the past several years, with social engineering techniques increasingly being used in addition to purely technological attacks. MorphoSys has implemented extensive safeguards in information technology and cybersecurity. Internal controls and quality assurance procedures have been rolled out across all major applications and underlying networks and infrastructure. We have advanced systems to prevent unauthorized intrusions and support the timely monitoring of attacks on our IT systems. A qualified Computer Emergency Response Team (CERT) has also been established in addition to extensive preventive training and awareness-raising measures for employees. Further details on our IT and cybersecurity measures can also be found in the Information Technology section in the Corporate Governance Report. Business Continuity Risk MorphoSys has implemented a business continuity plan to prevent the widespread collapse of critical business processes and ensure their resumption should a natural disaster, health-related crisis (such as the coronavirus), or other serious event occur. However, depending on the severity, it may be difficult or impossible for us to continue our business for a significant length of time. Our disaster recovery and business continuity plans may prove inadequate should a severe disaster or similar event occur. We also may incur significant costs that could have a material adverse effect on our business. In almost two years since the onset of the coronavirus pandemic, mobile working has allowed operations to continue at MorphoSys. Except for a few tasks that require an on-site presence, business can continue off-site without significant restrictions. As a result, business continuity risk is classified as low. Financial Risk Our financial risk management aims to mitigate financial risks and balance these risks with the needs arising from our business activities. As part of our financial risk management, we continuously monitor current developments in the tax legislation of our sales markets and operating sites so that we can identify and address tax risks at an early stage. Liquidity Risk Unexpected fluctuations in revenues, unplanned adverse developments in expenses and external events and changes in the business environment can all have a negative impact on our short- to medium-term liquidity and profitability. To ensure our short-term liquidity, we invest a sufficient share of our financial assets in short-term financial instruments. We also have a comprehensive liquidity plan based on our corporate planning that includes the simulated effects of various scenarios so we can determine our medium- and long- term liquidity requirements. To further reduce our financial risk, we take the outcome of the liquidity plan into account when prioritizing research and development projects and determining the financing requirements and the resulting concrete financing activities of the Management Board. MorphoSys also has access to other non-dilutive financing options, such as utilizing the development funding bonds provided by Royalty Pharma. Currency Risk MorphoSys generates a large percentage of its revenues in U.S. dollars, and this percentage is expected to increase with the expected increase in revenues from Monjuvi. U.S. commercialization costs and research and development costs are also incurred in U.S. dollars, and the proportion of these costs have increased following the acquisition of Constellation. As long as the costs in U.S. dollars exceed U.S. dollar revenues, a further

52 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 depreciation in the EUR/USD exchange rate represents a short- and medium-term risk for MorphoSys. The fluctuation in macroeconomic development in recent months has caused this risk to increase compared to the previous year. The Financial Planning & Analysis and Corporate Treasury departments continuously monitor changes in the EUR/USD exchange rate. A strategy for investing in U.S. dollar financial products has been developed in consultation with the Chief Financial Officer and in line with the internal guidelines for investing in financial products. Interest Rate and Default Risk As a result of the ongoing, tense economic situation in Europe, the potential insolvency of banking institutions continues to represent a financial risk. We are therefore continuing to invest, when possible, only in funds and products of banks that are considered safe and have a high rating or are backed by a strong partner. We diversify and invest in lower-risk money market funds in order to limit our exposure to individual financial institutions. A strategy that excludes all risks of potential bank insolvencies would be too expensive and impractical. German government bonds, for example, are a very safe investment, but currently trade at a negative interest rate. On the other hand, earning adequate interest on financial investments poses a risk, particularly as the key interest rate is at a negative level. We have limited investment options if we want to avoid negative interest rates while staying within the Company’s investment guidelines. We invest, when possible, in instruments that yield positive interest, but there is no guarantee that such investments will remain available. Tax Risk The accounting treatment of the payment that MorphoSys AG received from Royalty Pharma in the third quarter of 2021 could be examined by the tax authorities under German tax law in the context of a future tax audit. This examination is considered standard given the amount of the payment. Based on the Company’s knowledge of German tax law and supported by tax experts, the Company has concluded that the tax risk assessment is medium in accordance with the Company's internal risk valuation system. Regulatory and Compliance Risk Regulatory and compliance-related risks include risks arising from failing to comply with laws and equivalent regulations. Of particular relevance are risks related to industry-specific regulations in the area of healthcare compliance, GxP-relevant issues, and risks concerning the protection of intellectual property (IP). MorphoSys has implemented extensive systems and processes to minimize these risks. Due to the implemented countermeasures, these risks in the financial year were classified as low overall. Compliance Risk In the area of healthcare compliance, the focus is on combating bribery and corruption and the key regulations governing commercialization activities in the U.S., such as the Anti-Kickback Statute, False Claims Act, Open Payments Act, and the Food, Drug, and Cosmetic Act. A relevant compliance risk is that the Company fails to fully grasp operational challenges and, as a result, the compliance management program (CMP) is not established in accordance with regulatory requirements and industry standards. To address this risk, we have implemented a risk-based compliance management program that takes into account all of the current trends and applicable requirements, including the Code of Conduct; the Global Anti-Bribery Policy; the Global Policy on Interactions with Healthcare Professionals, Healthcare Organizations, Patients and Patient Organizations; the Global Fair Market Value Policy; the Global Policy on Transparency and Disclosure of Transfers of Value to Healthcare Professionals, Healthcare Organizations, Patients and Patient Organizations; and the relevant U.S. and German guidelines.

Management Report 53 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 We also have a global Compliance Committee that meets quarterly and makes informed decisions on the further development of the CMP. Regular training sessions are held, which are aimed at all employees as well as specific employee groups. A guide for the sales force has also been developed to help the sales team implement the guidelines in their daily work. An extensive onboarding program is offered to new employees in both Germany and the U.S. A compliance risk assessment is conducted annually, in which feedback is gathered from more than 60 of the Company’s executives to evaluate and minimize risks. Our control activities feed into our training and communication priorities. All these measures would not be possible without a clear message from the management: Our Management Board members emphasize the importance of compliance regularly, including at events during the annual Compliance Week, which took place again in the reporting year. Further details on our CMP can be found in the Corporate Governance Report in the section “Compliance management program.” GxP-Related Risk Companies that research, develop and produce drugs and active ingredients for commercial use are subject to comprehensive regulations known as GxP regulations. Compliance with these regulations is essential to receive approval from regulatory authorities. GxP-relevant risks can arise from a number of business areas if quality standards are not met. To counter these risks, we are committed to meeting the highest quality standards in our business operations, as outlined in our separate “Non-Financial Group Report.”* Certain risks may arise when the internal quality management system fails to meet legal requirements or fails to implement internal systems to detect quality issues. If internal controls are unable to detect guideline violations of Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), Good Laboratory Practice (GLP), Good Distribution Practice (GDP), or Good Pharmacovigilance Practice (GVP), this would also represent a compliance risk. To minimize risk, the internal quality management system is also regularly reviewed by external experts and subjected to recurring audits by an internal, independent quality assurance department. * This information is not part of the management report that is subject to audit. Intellectual Property Risk The patent protection of our proprietary technologies and active ingredients is vitally important to realizing the expected benefits. To mitigate risks in this area, we monitor new patents as well as patent applications and analyze the corresponding results. We also develop strategies to ensure that third-party patents and patent applications do not restrict our own activities. In doing so, we try to safeguard our freedom of action with regard to our proprietary technology platforms and products as much as possible. Risks in this area can arise from the potential for third-party patents or patent applications to fail to be recognized or incorrectly assessed. Risks may also arise from enforcing our property rights against third parties. The respective processes may involve high costs and require considerable resources. There is also a risk that a third party may file a counterclaim. A further risk may also arise from a changing regulatory environment. We minimize this risk through the ongoing training of the relevant groups and discussions with external experts. It is also conceivable that competitors may attack our patents, or that our patents or patent families are infringed upon, which in turn could lead us to take legal action against competitors. Such proceedings are associated with high costs and represent a significant financial risk, particularly in the U.S. By letter dated June 10, 2021, MorphoSys was notified by a licensor of the initiation of arbitration proceedings in the United States. The licensor alleges breach of contract and claims damages for the licensor’s argued loss of revenues. Despite the patent expiry in 2018 confirmed by the licensor at the time, this is now disputed and

54 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 a significantly longer patent term is assumed. Taking into account the associated legal and consulting costs, the potential amount in dispute in the proceedings is in the low double-digit Euro million range and also includes a currently unspecified share of royalty income. A decision by the arbitration court is expected in the fourth quarter 2022. Based on the current assessment of the facts, MorphoSys believes that the arguments presented are unfounded and that the arbitration will likely be decided in MorphoSys’ favor. There was no arbitration decision and no other new developments in the third and fourth quarter of 2021. The Management Board’s Evaluation of the Company's Overall Risk Situation Our Management Board considers our overall risk as manageable and trusts in the effectiveness of the integrated risk and opportunity management system to keep up with changes in the environment and the needs of the ongoing business. It is the Management Board’s view that the Company’s continued existence is not jeopardized. This statement also applies in the unlikely event that several of the material risks occur cumulatively, as even in such a scenario the risk-bearing capacity defined by the Board of Management is not undercut. The Management Board’s conclusion is based on the following considerations: • The Company’s high liquidity base • The Management Board’s conviction that the Company is well-positioned to cope with any adverse events that may occur • The Company’s comprehensive portfolio of proprietary preclinical and clinical programs • The Company's extensive portfolio of partnerships with a number of large pharmaceutical companies and a base of technologies to expand its proprietary portfolio Despite these factors, it is impossible to influence, control or rule out risk in its entirety.

Management Report 55 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Subsequent Events For details on events after the reporting date please refer to the notes to the Annual Financial Statements of MorphoSys AG.

56 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Statement on Corporate Governance, Group Statement on Corporate Governance and Report on Corporate Governance The Statement on Corporate Governance and the Group Statement on Corporate Governance, as well as the Report on Corporate Governance, are available on our website under Investors – Corporate Governance. Statement on Corporate Governance pursuant to Section 289f HGB and Group Statement on Corporate Governance pursuant to Section 315d HGB for the 2021 Financial Year In the Statement on Corporate Governance pursuant to Section 289f of the German Commercial Code (HGB) and the Group Statement on Corporate Governance pursuant to Section 315d HGB, the Management Board and the Supervisory Board present information on the most essential components of our corporate governance. The components include the annual Declaration of Conformity pursuant to Section 161 of the German Stock Corporation Act (AktG), the relevant information on corporate governance practices and other aspects of corporate governance that include, above all, a description of the working practices of the Management Board and Supervisory Board. Declaration of Conformity of the Management Board and Supervisory Board of MorphoSys AG with regard to the German Corporate Governance Code (“Code”) The Management Board and the Supervisory Board of MorphoSys AG declare pursuant to Section 161 of the German Stock Corporation Act: 1. From November 29, 2020, the date of its most recent Declaration of Conformity in the version as amended and updated on March 11, 2021, MorphoSys AG has complied – with the exceptions described below – with the recommendations of the “Government Commission on the German Corporate Governance Code” in the Code version dated December 16, 2019 (“GCGC 2020”): • MorphoSys AG does not comply with the recommendation C.4 of the GCGC 2020, according to which a Supervisory Board member, who is not a member of any Management Board of a listed company, shall not accept more than five Supervisory Board mandates at non-group listed companies or comparable functions (in a listed or non-listed company), with an appointment as chair of the Supervisory Board being counted twice. The member of the Supervisory Board Dr. George Golumbeski currently holds in aggregate four comparable functions in pharmaceutical and biotechnological companies in Ireland and the United States of America, thereof two functions as chairman of the board of directors. Dr. Golumbeski’s positions have at no time in the past affected the fulfillment of his duties as a member of the Supervisory Board of MorphoSys AG. MorphoSys AG continuously ensures that Dr. Golumbeski’s positions will not distract his focus on MorphoSys AG’s business and that Mr. Golumbeski has sufficient time to perform his duties as a member of the Supervisory Board of MorphoSys AG with due regularity and care. • MorphoSys AG does not comply with the recommendation C.5 of the GCGC 2020, according to which members of the Management Board of a listed company shall not accept the chairmanship of

Management Report 57 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 a Supervisory Board in a non-group listed company. The Chief Executive Officer (CEO) of MorphoSys AG, Dr. Jean-Paul Kress, holds a position as chairman of the Board of Directors of a French biopharmaceutical company, which he had already accepted prior to his appointment as a member of the Management Board of MorphoSys AG and which has at no time in the past affected the fulfillment of his duties as CEO of MorphoSys AG. MorphoSys AG continuously ensures that Dr. Kress’ position as chairman of the Board of Directors of such company will not distract his focus on MorphoSys AG’s business and that Dr. Kress has sufficient time to perform his duties as CEO of MorphoSys AG with due regularity and care. 2. MorphoSys AG will continue to comply – with the exceptions described above under item 1 – with the recommendations of the GCGC 2020. Planegg, this November 29, 2021 MorphoSys AG For the Management Board: For the Supervisory Board: Dr. Jean-Paul Kress Dr. Marc Cluzel Chief Executive Officer Chairman of the Supervisory Board Relevant Information on Corporate Governance Practices We ensure compliance with the law and the highest ethical standards in particular through the Group-wide enforcement of the Code of Conduct, the Compliance Management Handbook, and other internal policies and guidelines. Our Code of Conduct sets out the fundamental principles and key policies and practices for business behavior. The Code is a valuable tool for our employees and executives, particularly in business, legal and ethical dilemmas. The Code of Conduct reinforces our transparent and sound management principles and fosters the trust placed in us by the public, business partners, employees and financial markets. Compliance with the Code of Conduct is carefully monitored. The Group-wide implementation of the Code is overseen by the Global Compliance Committee. The Code of Conduct itself is routinely reviewed and updated, provided to all new employees, and can be downloaded in German or English from our website under the section Investors – Corporate Governance. The Compliance Management Handbook describes our compliance management program (CMP) and is intended to ensure compliance with all regulations and prescribe high ethical standards that apply to both the management and all employees. The Management Board has overall responsibility for the CMP and is required to report regularly to the Audit Committee of the Supervisory Board. In carrying out its compliance responsibility, the Management Board has assigned the relevant tasks to various functions at MorphoSys. The Global Compliance Committee consists of our three members of the Management Board (Chief Executive Officer, Chief Research and Development Officer, and Chief Financial Officer) and senior representatives from various departments. In 2021 the Chief Integration Officer and Site Head of Constellation has also been included as a member of the Global Compliance Committee to ensure gradient integration of Constellation into MorphoSys’ Compliance Management Program. It meets quarterly and supports the Head of Global Compliance in implementing and monitoring the CMP. The Global Compliance Committee is specifically responsible for the identification and discussion of all compliance-relevant issues, and thus makes it possible

58 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 for the Head of Global Compliance and the other members of the Global Compliance Committee to periodically verify our compliance status and, if necessary, update the CMP. The Head of Global Compliance monitors our existing CMP and updates it in accordance with the decisions of the Management Board and Global Compliance Committee. Compliance colleagues are the first point of contact for all employees regarding all compliance matters. For more information on our compliance management program, please see the Report on Corporate Governance. Composition of the Management Board and Supervisory Board Management Board In the 2021 financial year, the Management Board of MorphoSys AG consisted of a Chief Executive Officer and three other members. By resolution of the Supervisory Board on January 18, 2021, Sung Lee was appointed as a member of the Management Board and Chief Financial Officer effective February 2, 2021. Roland Wandeler, Ph.D., resigned from the MorphoSys AG Management Board effective December 31, 2021. Therefore, as of January 1, 2022, the Management Board consists of a Chief Executive Officer and two additional members. The various areas of responsibility are currently defined in the business allocation plan as follows: • Jean-Paul Kress, M.D., Chief Executive Officer, responsible for the areas of Strategy & Planning, Business Development & Alliance Management, Human Resources, Legal, Compliance & Intellectual Property, Corporate Communications, Technical Operations, Information Technology & Facilities, Quality Assurance & Internal Audit, global responsibility for U.S. operations, Strategic Marketing & Market Access; Forecasting & Insights, coordination of responsibilities of Management Board members; representative of Management Board to the Supervisory Board and the public • Sung Lee, Chief Financial Officer (as of February 2, 2021), responsible for Accounting & Taxes, Global Controlling & Internal Controls, Corporate Development & M&A, Central Purchasing & Logistics, Investor Relations, and Environmental Social Governance (ESG) • Malte Peters, M.D., Chief Research and Development Officer, responsible for Research, Preclinical Development, Clinical Development, Clinical Operations, Biostatistics & Data Management, Drug Safety & Pharmacovigilance, Regulatory Affairs, Medical Affairs, and Global Program Teams • Roland Wandeler, Ph.D., Chief Operating Officer (until December 31, 2021), responsible globally for U.S. operations, Strategic Marketing & Market Access, and Forecasts & Insights Supervisory Board Our Supervisory Board consists of six members who oversee and advise the Management Board. Sharon Curran, Krisja Vermeylen and Marc Cluzel were reelected as members of the Supervisory Board at the 2021 Annual General Meeting. The current Supervisory Board consists of professionally qualified members who represent our shareholders. The Chair of the Supervisory Board, Marc Cluzel, M.D., Ph.D., coordinates the Board’s activities, chairs the Supervisory Board meetings and represents the interests of the Supervisory Board externally. All Supervisory Board members are independent as per the definition in the German Corporate Governance Code (“Code”) and the NASDAQ Listing Rules and have many years of experience in the biotechnology and pharmaceutical industries. The Chair of the Supervisory Board is not a former member of our Management Board. The detailed composition of the Supervisory Board, including its members and committees, is listed in the tables below.

Management Report 59 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Tab. 07: Composition of the Supervisory Board until Termination of the 2021 Annual General Meeting Name Position Initial Appointment End of Term Audit Committee Remuneration and Nomination Committee Science and Technology Committee Marc Cluzel, M.D., Ph.D. Chairman 2012 2021 George Golumbeski, Ph.D. Deputy Chairman 2018 2023 Krisja Vermeylen Member 2017 2021 Michael Brosnan Member 2018 2023 Wendy Johnson Member 2015 2022 Sharon Curran Member 2019 2021 Independent financial expert Chairperson Member Tab. 08: Composition of the Supervisory Board since Termination of the 2021 Annual General Meeting Name Position Initial Appointment End of Term Audit Committee Remuneration and Nomination Committee Science and Technology Committee Marc Cluzel, M.D., Ph.D. Chairman 2012 2024 George Golumbeski, Ph.D. Deputy Chairman 2018 2023 Krisja Vermeylen Member 2017 2024 Michael Brosnan Member 2018 2023 Wendy Johnson Member 2015 2022 Sharon Curran Member 2019 2024 Independent financial expert Chairperson Member Working Practices of the Management Board, Supervisory Board and Executive Committee To ensure good corporate governance, a guiding principle of the cooperation between our Management Board and our Supervisory Board is the open, comprehensive and regular communication of information. The dual board system prescribed by the German Stock Corporation Act clearly differentiates between the Company’s management and its supervision. The responsibility of both Boards is clearly stipulated by the legislator and the Articles of Association as well as the Boards’ rules of procedure. The boards work closely together to make decisions and take actions for the Company’s benefit. Their stated objective is to sustainably increase the Company’s value.

60 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Management Board members have their own separate areas of responsibility, as defined in the schedule of responsibilities, and regularly report to the other Management Board members. Cooperation among Management Board members is governed by the rules of procedure. The Supervisory Board approves both the schedule of responsibilities and the rules of procedure. The Company has also established the so-called “Executive Committee.” Under the leadership of the Chief Executive Officer, the Executive Committee is responsible for the development of the strategy, for the commercialization, the operational management of the Company and the achievement of its targets and results. The Executive Committee prepares the decisions for the Management Board’s resolutions and adopts resolutions jointly with the Management Board, provided this is not the sole responsibility of the Management Board by law or by resolution of the Supervisory Board. The Executive Committee consists of the members of the Management Board and senior executives from the Company’s core areas such as Business Development & Licensing and Alliance Management, Technical Operations, Human Resources, Legal, and Compliance & Intellectual Property. In addition to the members of the Management Board, the current members of the Executive Committee are Barbara Krebs-Pohl, Ph.D. (Senior VP, Head of Global BD&L and Alliance Management), Daniel Palmacci (Senior VP, Global Head of Technical Operations), Maria Castresana (Senior VP, Global Head of Human Resources), Charlotte Lohmann (Senior VP, General Counsel, Legal, Compliance & IP) and Joe Horvat (US General Manager) . Executive Committee meetings are generally held weekly and at least once every two weeks and when necessary in the interest of the Company. Separate Management Board meetings are generally held when this is in the interest of the Company or legally required. During these meetings, resolutions are passed concerning measures and transactions that, under the rules of procedure of the Management Board, require the approval of the entire Management Board. At least half of the Management Board’s members must be present to pass a resolution. Management Board resolutions are passed by a simple majority and, in the event of a tied vote, the Chief Executive Officer’s vote decides. In case of material events, each Management Board or Supervisory Board member can call an extraordinary meeting of the entire Management Board. Management Board resolutions can also be adopted outside of meetings orally, by telephone or in writing (also by e-mail). Written minutes are taken for each meeting of the full Management Board and Executive Committee and are submitted for approval to the full Management Board and Executive Committee, as well as for the signature of the Chief Executive Officer, at the following meeting. The Management Board promptly and comprehensively informs the Supervisory Board in writing and at Supervisory Board meetings about planning, business development, the Company’s position, risk management and other compliance issues. Extraordinary meetings of the Supervisory Board are also convened in case of material events. The Management Board involves the Supervisory Board in the strategy, planning and all fundamental Company issues. The Management Board’s rules of procedure specify that material business transactions require the approval of the Supervisory Board. Detailed information on the cooperation of the Management Board and Supervisory Board and important items of discussion during the 2021 financial year can be found in the Report of the Supervisory Board. The Supervisory Board holds a minimum of two meetings during each calendar half-year. In addition to the Articles of Association, the Supervisory Board has adopted rules of procedure for the Supervisory Board. In accordance with these rules of procedure, the Chairperson of the Supervisory Board coordinates the activities of the Supervisory Board, chairs the Supervisory Board meetings and represents the interests of the Supervisory Board externally. The Supervisory Board generally adopts its resolutions in meetings, but resolutions may also be passed outside of meetings in writing (also by e-mail), by telephone or video conference.

Management Report 61 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The Supervisory Board has a quorum when at least two-thirds of its members participate in the vote. Resolutions of the Supervisory Board are generally passed with a simple majority. In the event of a tied vote, the Chairperson’s vote decides. The Supervisory Board meetings are recorded in minutes. Resolutions passed outside of meetings are also documented in writing. A copy of the Supervisory Board’s minutes is made available to all Supervisory Board members. In accordance with recommendation D.13 of the Code, the Supervisory Board assesses at regular intervals how effective the Supervisory Board in its entirety and its committees perform their tasks. The members of the Management Board also participate in this review. The last review was carried out by the Supervisory Board in December 2021 and was based on a questionnaire completed by the members of both the Supervisory Board and the Management Board. The results were then discussed and evaluated in a subsequent Supervisory Board meeting. Composition and Working Practices of the Management Board and Supervisory Board Committees The Management Board has not formed any committees. The Supervisory Board has three permanent committees: the Audit Committee, the Remuneration and Nomination Committee, and the Science and Technology Committee. The members of the three committees formed by the Supervisory Board are professionally qualified. Tab. 09: Participation of Supervisory Board Members Supervisory Board Meetings Video conference Video conference On-site On-site Video conference Video conference Name 01/18/ 2021 03/11/ 2021 05/18/ 2021 05/19/ 2021 05/26/ 2021 06/01/ 2021 Marc Cluzel, M.D., Ph.D. X X X X X X Wendy Johnson X X X X X X Krisja Vermeylen X X X X X X George Golumbeski, Ph.D. X X X (via Video) X (via Video) X X Michael Brosnan X X X (via Video) X (via Video) X X Sharon Curran X X X (via Video) X (via Video) X X Video conference Video conference On-site On-site On-site (strategy meeting) Video conference Name 06/02/ 2021 07/16/2021 07/27/2021 11/08/ 2021 11/09/ 2021 12/14/ 2021 Marc Cluzel, M.D., Ph.D. X X X X X X Wendy Johnson X X X X X X Krisja Vermeylen X X X X X X George Golumbeski, Ph.D. X X X (via Video) X X X Michael Brosnan X X X X X X Sharon Curran X X X X X X
62 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Meetings of the Audit Committee Video conference Video conference On-site Video conference On-site Name 03/10/2021 05/04/2021 07/27/2021 09/30/2021 11/08/2021 Krisja Vermeylen X X X X X Michael Brosnan X X X X (via Video) X Sharon Curran X X X X (via Video) X Meetings of the Remuneration and Nomination Committee Video conference Video conference Video conference On-site Video conference Video conference Video conference Video conference Name 01/15/ 2021 03/01/ 2021 05/05/ 2021 05/18/ 2021 07/22/ 2021 09/22/ 2021 10/29/ 2021 12/13/ 2021 Marc Cluzel, M.D., Ph.D. X X X X X X X X Krisja Vermeylen X X X X X X X X Wendy Johnson X X X X X X X X George Golumbeski, Ph.D.* X (via Video) X X X Meetings of the Science and Technology Committee Video conference Video conference Video conference On-site On-site On-site Name 03/09/ 2021 03/11/ 2021 04/30/ 2021 05/17/ 2021 07/26/ 2021 11/08/ 2021 Wendy Johnson X X X X X X George Golumbeski, Ph.D. X X X X (via Video) X (via Video) X Michael Brosnan* X X Marc Cluzel, M.D., Ph.D.* X *Guest participation Audit Committee The main task of the Audit Committee is to support the Supervisory Board in fulfilling its supervisory duties with respect to the accuracy of the annual and consolidated financial statements, the activities of the auditor and internal control functions, such as risk management, compliance and internal auditing. The Audit Committee submits a recommendation to the Supervisory Board for the election at the Annual General Meeting of an independent auditor. The members of the Audit Committee are Michael Brosnan (Chair), Sharon Curran and Krisja Vermeylen. Currently, Michael Brosnan meets the prerequisite of an independent financial expert. Remuneration and Nomination Committee The Remuneration and Nomination Committee is responsible for the preparation and the annual review of the Management Board’s remuneration system prior to its final approval. When necessary, the Committee searches for suitable candidates to appoint to the Management Board and Supervisory Board and submits

Management Report 63 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 appointment proposals to the Supervisory Board. The Committee also prepares the service agreements with Management Board members. The members of the Remuneration and Nomination Committee are Krisja Vermeylen (Chair), Marc Cluzel, M.D., Ph.D., and Wendy Johnson. Science and Technology Committee The Science and Technology Committee advises the Supervisory Board on matters concerning proprietary drug and technology development and prepares the relevant Supervisory Board resolutions. The members of the Science and Technology Committee are George Golumbeski, Ph.D. (Chair) and Wendy Johnson. Ad Hoc Deal Committee The members of the Science and Technology Committee also serve as members of the Ad Hoc Deal Committee, which meets in this capacity as required. In 2021, the Ad Hoc Deal Committee dealt with the acquisition of Constellation Pursuant to recommendation C.14 of the Code, the curriculum vitae of the members of the Supervisory Board are published on our website under About us – Leadership – Supervisory Board. Remuneration System and Compensation of the Members of the Management Board and Supervisory Board On March 11, 2021, the Supervisory Board resolved a remuneration system for the members of the Management Board which is in line with the requirements for management board remuneration as amended by the Act Implementing the Second Shareholders’ Rights Directive (ARUG II) and the Code in its version as amended on December 16, 2019. The remuneration system does not yet apply to any member of the Management Board. The remuneration report pursuant to Section 162 AktG, including the auditor’s report, can be found on our website under Investors – Corporate Governance. The applicable remuneration system for the members of the Management Board and the most recent resolution of the Annual General Meeting 2021 on the remuneration of Supervisory Board members can be found on our website under Investors – Annual General Meeting 2021. Report on Corporate Governance At MorphoSys, responsible, sustainable and value-oriented corporate governance is a high priority. Good corporate governance is an essential aspect of our corporate management and forms the framework for the Company’s management and supervision, including the Company’s organization, commercial principles and tools for its guidance and control. The Code provides a standard for the transparent monitoring and management of companies that strongly emphasizes shareholder interests. The German Federal Ministry of Justice originally published the Code in 2002. On December 16, 2019, the Government Commission on the German Corporate Governance Code adopted a new version of the Code, which entered into force upon its publication in the German Federal Gazette on March 20, 2020. The Code contains recommendations and suggestions with regard to the management and supervision of German companies listed on a stock exchange. It is based on domestic and internationally recognized standards for good and responsible corporate governance. The Code aims to make the German system of corporate governance transparent for investors. It contains recommendations and suggestions on corporate governance with regard to shareholders and the Annual General Meeting, the Management Board and Supervisory Board, transparency, accounting and valuation principles, and auditing.

64 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 There is no obligation to comply with the recommendations and suggestions of the Code. The German Stock Corporation Act only requires the management boards and supervisory boards of listed German companies to publish a declaration each year, (i) either confirming that the company has complied with the recommendations of the Code or (ii) listing the recommendations the company has not complied with and the reasons for the deviation from the recommendations of the Code. In addition, a listed company must also state in its annual declaration whether it intends to comply with the recommendations or must list the recommendations it does not intend to comply with in the future. These declarations must be published permanently on the company’s website. If the company changes its position on certain recommendations between two annual declarations, it must disclose this fact and state the reasons for the deviation from the recommendations. If suggestions from the Code are not complied with, this does not have to be disclosed. Many of the corporate governance principles contained in the Code have been practiced at MorphoSys for many years. Our corporate governance principles are outlined in the Statement on Corporate Governance pursuant to Sections 289f and 315d HGB. The statement also contains the annual Declaration of Conformity, relevant information on corporate governance practices and a description of the Management Board and Supervisory Board’s working practices. Additional information can be found in this Report on Corporate Governance. Communication with the Capital Market A key principle of corporate communication at MorphoSys is to simultaneously and fully inform institutional investors, private shareholders, financial analysts, employees and all other stakeholders of the Company’s situation through regular, transparent and timely communication. The Company is firmly committed to following a fair information policy. Regular meetings with analysts and investors in the context of roadshows and individual meetings play a central role in investor relations at MorphoSys. Conference calls are publicly webcast and follow the publications of quarterly and annual results and give analysts an immediate opportunity to ask questions about the Company’s development. Presentations from conferences and similar events are made available to those interested on the MorphoSys website, as are visual and audio recordings of other important events. The Company’s website www.morphosys.com/en serves as a central platform for current information on the Company and its development. Financial reports, analyst meetings and conference presentations, as well as press releases and ad hoc statements, are also available. The important regularly scheduled publications and events (annual reports, interim reports, annual general meetings and press and analyst conferences) are published in the Company’s financial calendar well in advance. Competence Profile, Diversity Concept and Objectives for the Composition The Company’s Supervisory Board has updated its competence profile and objectives for its composition based on the new Code recommendations and has prepared a diversity concept in accordance with Section 289f (2) no. 6 of the German Commercial Code. According to this concept, the Supervisory Board of MorphoSys AG shall be composed in such a way that the Supervisory Board in its entirety possesses the knowledge, skills and professional experience necessary to perform its duties properly and ensure that it appropriately supervises and advises the Management Board of MorphoSys AG while taking diversity into account. When electing Supervisory Board members, the candidates who are proposed to the Annual General Meeting fulfill the overall competence profile based on their professional competence, experience, integrity, commitment, independence and character. Proposals to the Annual General Meeting also take the objectives for the composition of the Supervisory Board into consideration.

Management Report 65 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Competence Profile The members of the Supervisory Board shall in its entirety possess the professional competence and experience to fulfill the tasks of the Supervisory Board of MorphoSys AG as an internationally operating biopharmaceutical company. The Supervisory Board in particular considers the following skills and expertise to be essential for the composition of the Supervisory Board of MorphoSys AG: • Members should have a general knowledge of the industry in which the Company operates in order to make sufficient and substantive contributions at Supervisory Board meetings • At least one member must have experience in drug development • At least one member must have experience in commercialization • At least one member must have expertise in the fields of accounting or auditing (Section 100 (5) AktG) • At least one member must have experience with personnel issues concerning Management Board matters Diversity Concept for the Supervisory Board of MorphoSys AG The Supervisory Board strives to ensure an appropriate level of diversity with respect to age, gender, internationality and professional background, as well as regarding professional expertise, experience and personality, in order to achieve a diverse composition of the Supervisory Board and enable it, in its entirety, to base its decisions on different cultural and professional perspectives and wide experiences. The Supervisory Board gives particular consideration to the following criteria: • At least two members of the Supervisory Board shall have extensive international experience or an international background • At least one member of the Supervisory Board shall be under the age of 60 at the time of the member’s appointment • At least two members of the Supervisory Board shall have different professional backgrounds and experience With respect to the proportion of women on the Supervisory Board, the Supervisory Board has set target figures as well as deadlines for their achievement in accordance with Section 111 (5) AktG, to which reference is made. Other Targets in the Composition of the Supervisory Board Age Limit At the time of their appointment by the Annual General Meeting, Supervisory Board members should not be more than 70 years of age. The Supervisory Board may, however, decide to make an exception in specific cases. Duration of Appointment The uninterrupted length of the term of office of a Supervisory Board member shall generally not exceed 12 years. However, the Supervisory Board may resolve an exception to this rule in certain cases.

66 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Independence The Supervisory Board of MorphoSys AG considers a number of at least four independent members to be an appropriate number of independent members, taking into account the shareholder structure. According to the Code, a Supervisory Board member is considered to be independent of MorphoSys AG, its Management Board and any controlling shareholders when he or she has no personal or business relationship with the Company, the Management Board or a controlling shareholder. The Supervisory Board’s assessment of the independence of Supervisory Board members is, amongst others, based on the recommendations of the Code. Consequently, a Supervisory Board member is generally not considered independent if such member, or a close member of his or her family • was a member of the Management Board of MorphoSys AG in the two years preceding his or her appointment to the Supervisory Board of MorphoSys AG; • maintains or has maintained a material business relationship (directly or indirectly) with MorphoSys AG or a Group company of MorphoSys AG in the year preceding his or her appointment; • is a close family member of a Management Board member; or • has been a member of the Supervisory Board for more than 12 years. Significant and lasting conflicts of interest should be avoided, particularly those resulting from functions carried out for major competitors. It must be taken into account, however, that certain conflicts of interest cannot generally be excluded. Possible conflicts of interest must be disclosed to the Chairperson of the Supervisory Board and will be resolved by appropriate measures. This could lead to the termination of the Supervisory Board mandate of the member concerned if the conflict of interest is not merely temporary. Availability All members of the Supervisory Board must ensure that they have sufficient time available to properly perform their Supervisory Board duties at MorphoSys AG. Therefore, as a rule, it is required that: • the Supervisory Board member is able to personally attend at least four ordinary Supervisory Board meetings per year, for which a reasonable amount of preparation time is required in each case; in the event of exceptional circumstances to be determined by the Supervisory Board’s Chairperson, the participation of one or more Supervisory Board members in ordinary Supervisory Board meetings by other means (such as video conference) shall also be sufficient; • the Supervisory Board member is able to attend extraordinary meetings of the Supervisory Board, if necessary, to deal with specific topics; • the Supervisory Board member is able to attend the Annual General Meeting; • the Supervisory Board member has sufficient time to review the annual and consolidated financial statements; and • the Supervisory Board member allocates additional time to prepare for and attend committee meetings, in accordance with his or her membership in one or more of the Supervisory Board’s current three permanent committees. Current Composition of the Supervisory Board The Supervisory Board of MorphoSys AG is composed in accordance with the above objectives. It is composed of an appropriate number of independent members with an international background. As the Supervisory Board as a whole currently has six members, of which three are women, an appropriate proportion of women has been achieved.

Management Report 67 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Target Values for the Proportion of Women In the Supervisory Board The Supervisory Board of MorphoSys AG consists of six members, three of whom are women, representing a proportion of 50%. The Supervisory Board of MorphoSys AG has set the target value for the proportion of women on the Supervisory Board at 33.33%, meaning at least two out of six members shall be women. This target figure shall apply until June 30, 2025. In the Management Board The Management Board of MorphoSys AG consisted of four male members until December 31, 2021, and has consisted of three male members since January 1, 2022. As a result, the current proportion of women on the Company’s Management Board is 0%. Since the Supervisory Board of MorphoSys AG is of the opinion that, despite the continuing efforts to increase the proportion of women within the Management Board, the best possible qualification of a candidate for the Management Board must be assessed according to a variety of applied diversity criteria, in July 2020, the Supervisory Board has set the target value for the proportion of women on the Company’s Management Board at 0%. This target value shall apply until June 30, 2023. The Supervisory Board nevertheless aims to increase the proportion of women within the Management Board. In the course of the next resolution upon a target value for the proportion of women within the Management Board, the Supervisory Board will take this objective into account. In the First and Second Management Level below the Management Board 1. Target value for the first management level below the Management Board In 2020, the Management Board confirmed its resolution for a target value of 30% of women in the first management level below the Management Board as of July 2017 and intends to maintain a minimum proportion of women of 30% in the first management level below the Management Board until June 30, 2025. As of the date of the resolution on the target value, the first management level below the Management Board of MorphoSys AG (line managers reporting directly to the Management Board) consisted of 21 members, of which nine are women, corresponding to a proportion of women of 42.86%. MorphoSys AG continuously complied with this requirement in the reporting year. 2. Target value for the second management level below the Management Board In 2020, the Management Board confirmed its resolution for a target value of 30% women in the second management level below the Management Board as of July 2017 and intends to maintain a minimum proportion of women of 30% in the second management level below the Management Board until June 30, 2025. As of the date of the resolution on the target, the second management level below the Management Board of MorphoSys AG (line managers reporting directly to the first management level below the Management Board) consisted of 53 members, 22 of whom are women, corresponding to a proportion of women of 41.51%. MorphoSys AG continuously complied with this requirement in the reporting year. Diversity Concept for the Management Board of MorphoSys AG Pursuant to Section 289f (2) No. 6 of the German Commercial Code, the Supervisory Board has determined the following diversity concept for the composition of the Management Board of MorphoSys AG. The aim of the diversity concept for the Management Board is to consciously use diversity for the further success of the Company. The Supervisory Board believes that diversity in terms of different perspectives,

68 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 competencies and backgrounds of experience is an important prerequisite for competitiveness and sustainable corporate success. Together with the Management Board, the Supervisory Board ensures long-term succession planning for the Management Board. In the search for candidates for the position of a member of the Management Board of MorphoSys AG, the decisive selection criteria include, amongst others, professional qualifications for the position to be taken over, leadership qualities, previous performance, and acquired skills and knowledge of the business of MorphoSys AG. In the composition of the Management Board, the Supervisory Board also particularly takes the following aspects into account: • The members of the Management Board shall, in their entirety, have the necessary knowledge, skills and professional experience required for their tasks. • Where possible, the members of the Management Board should have different levels of educational and professional experience. • The members of the Management Board shall, in their entirety, be familiar with the market environment, the individual business fields and the market segment in which MorphoSys AG operates. • The members of the Management Board shall, in their entirety, have relevant experience in leading a publicly listed company. • There should be a sufficient age mix among the members of the Management Board. • With regard to the proportion of women on the Management Board, the Supervisory Board has set target values, as well as deadlines for their achievement, in accordance with Section 111 (5) AktG, to which reference is made. The above criteria were taken into account in the appointment of the Management Board members. Other Targets in the Composition of the Management Board Age Limit At the time of their appointment, Management Board members should not be more than 67 years of age. The Supervisory Board may, however, decide to make an exception in specific cases. The age limit of 67 is currently complied with. Managers' Transactions The members of the Management Board and the Supervisory Board of MorphoSys AG, as well as persons closely associated with them, are required to disclose trading in MorphoSys shares in accordance with the requirements set forth in the relevant legal provisions (Article 19 (1a) of the Market Abuse Regulation (MAR)). During the reporting year, MorphoSys received notifications pursuant to Article 19 (1a) MAR, which are shown in the table below.

Management Report 69 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Tab. 10: Managers' Transactions 2021 Party Subject to the Notification Requirement Function Date of Transaction Type of Transaction Aggregated Share Price Aggregated Volume Place of Transaction Sung Lee Chief Financial Officer 12/03/2021 Acquisition of shares US$ 9.36 US$ 14,040.00 Nasdaq Sung Lee Chief Financial Officer 11/23/2021 Acquisition of shares US$ 9.55 US$ 4,775.00 Nasdaq Sung Lee Chief Financial Officer 11/22/2021 Acquisition of shares US$ 9.80 US$ 19,600.00 Nasdaq Sung Lee Chief Financial Officer 10/01/2021 Acquisition of shares US$ 11.67 US$ 23,340.00 Nasdaq Sung Lee Chief Financial Officer 08/02/2021 Acquisition of shares US$ 13.70 US$ 27,400.00 Nasdaq Michael Brosnan Member of the Supervisory Board 07/30/2021 Acquisition of shares US$ 13.96 US$ 69.788,58 Nasdaq Krisja Vermeylen Member of the Supervisory Board 07/29/2021 Acquisition of shares € 47.25 € 16,538.66 Xetra Michael Brosnan Member of the Supervisory Board 06/03/2021 Acquisition of shares US$ 19.58 US$ 97,906.28 Nasdaq Malte Peters, M.D. Chief Research and Development Officer 04/14/2021 Allocation of 4,143 shares as part of his remuneration as member of the Management Board (Performance Share Plan 2017) (issuer’s own not numerable not numerable Outside a trading venue Sung Lee Chief Financial Officer 03/23/2021 Acquisition of shares US$ 22.75 US$ 22,750.00 Nasdaq Wendy Johnson Member of the Supervisory Board 03/17/2021 Acquisition of shares US$ 23.35 US$ 5,837.50 Nasdaq Krisja Vermeylen Member of the Supervisory Board 03/17/2021 Acquisition of shares € 75.90 € 22,770.00 Xetra C&F Consulting EURL Person closely associated 03/16/2021 Acquisition of shares € 76.00 € 19,000.00 Xetra Michael Brosnan Member of the Supervisory Board 03/16/2021 Acquisition of shares US$ 22.73 US$ 227,300.00 Nasdaq Avoiding Conflicts of Interest The members of the Management Board and the Supervisory Board are obligated to refrain from actions that could lead to conflicts of interest with their responsibilities at MorphoSys AG. Such transactions or sideline activities of the Management Board must be disclosed to the Supervisory Board without undue delay and require the Supervisory Board’s approval. The Supervisory Board, in turn, must inform the Annual General Meeting of any conflicts of interest that arise and disclose how they were dealt with. No conflict of interest arose in the Supervisory Board in the 2021 financial year. Share Repurchases By resolution of the Annual General Meeting on May 23, 2014, MorphoSys was authorized, in accordance with Section 71 (1) no. 8 of the German Stock Corporation Act (AktG), to repurchase treasury shares in an amount of up to 10% of the existing share capital up to and including April 30, 2019. Following the

70 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 authorization’s expiry, no new authorization was proposed to the Annual General Meeting; therefore, no such authorization currently exists. Information Technology The transition from working remotely due to COVID-19 to a hybrid and highly flexible work model was accompanied by an integrative technology update of our physical and virtual meeting rooms and a new collaboration and booking platform. We began with the technical integration and consolidation of IT systems following the acquisition of Constellation and will complete this process in 2022. In 2021, the focus was on the interoperability of all systems to ensure the fastest possible collaboration. This year, we will exploit the full potential of all synergies and establish a global, harmonized IT landscape. The specialist IT teams have already been combined to form a joint IT organization. A special focus was placed on the further digitalization and automation of business processes. With the introduction of electronic signatures using DocuSign™, we were able to significantly accelerate signature circulation and automate processes. A new, global learning management system forms the basis for the digital education strategy, which relies on e-learning and remote training. We are advancing our innovation using artificial intelligence through tools such as Aily™, which will make it possible to foresee ways to optimize recruitment for clinical trials. We are also investing in the expansion of our Veeva™ system landscape for unified management of quality and regulatory information, which is crucial for rapidly launching products (e.g., pelabresib) and maintaining their marketing approval. In the area of IT security, we continued to optimize our cyber defense measures and progressed through the integration phase with Constellation with increased awareness. An automated penetration testing and validation platform was deployed to review our technical security controls and identify potential vulnerabilities. We continued to train and raise our employees’ awareness of their own contributions to the Company's IT security. Our Computer Emergency Response Team (CERT) has not detected any serious security incidents during the reporting year. Information on the Internal Control and Risk Management System with regard to the Accounting Process under Section 289 (4) and Section 315 (4) HGB In the 2021 reporting year, we completed a routine update of the documentation for our existing internal control and risk management system for maintaining adequate internal control over financial reporting, which we have expanded based on the provisions of Section 404 of the Sarbanes-Oxley Act of 2002 (SOX 404). This ensures the existence of essential controls designed to report financial figures as precisely and accurately as possible. Our internal controls over financial reporting are based on the globally recognized COSO 2013 Internal Control – Integrated Framework, defined by the COSO organization (Committee of Sponsoring Organizations of the Treadway Commission). We use this framework, which is the most commonly used framework for the internal control over financial reporting. System constraints make it impossible to give absolute assurance that internal controls will always prevent or completely detect all misrepresentations made in the context of financial reporting. Internal controls can only provide sufficient assurance that financial reporting is reliable and verify that the financial statements were

Management Report 71 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 prepared in accordance with the applicable IFRS standards endorsed by the European Union (EU) for external purposes. The financial statements are subject to a number of preparation, auditing and control processes to ensure that they are submitted to the market and the shareholders in a timely, complete and quality manner. All internal controls over financial reporting are defined and rolled out for all companies by the central Global Internal Controls function in close coordination with the departments involved. These process-integrated measures include the separation of planning, posting and execution of financial transactions within the framework of a strict four eyes principle. The separation of functions is significantly enhanced by the appropriate allocation rights for the IT systems. Internal guidelines and procedures also exist to regulate the implementation of process activities and controls and must be complied with at all times by the employees involved. The transactional controls are flanked by target/actual comparisons and further downstream plausibility checks. In addition to internal controls integrated into the processes, a separate independent monitoring process is also carried out by the Internal Audit Department, which is bundled in the Corporate Internal Audit central function. Due to the obligations of SOX 404 and in order to comply with the requirements of Section107 (3) of the German Stock Corporation Act, Internal Audit performs an annual independent audit of all significant internal controls for financial reporting, supported by a qualified and independent external service provider. As part of its regular communication with the supervisory bodies, the Internal Audit Department reports every six months to the Chief Financial Officer and the Audit Committee on the results of the structural and functional audits of the accounting-related internal control system. Predictions of future events in the narrower sense are not part of our internal control and risk management system. Nevertheless, we have implemented a risk management system that ensures early identification and assessment of business-specific risks. Appropriate countermeasures are taken to eliminate identified risks or at least reduce them to an acceptable level. Particular attention is paid to those risks which could endanger the existence of the company. The Management Board ensures that risks are dealt with responsibly on an ongoing basis and keeps the Supervisory Board informed of existing risks and their development. Detailed information on our opportunities and risks can be found in the “Risk and Opportunity Report.” Accounting and External Audit We prepare our annual financial statements in accordance with the provisions of the German Commercial Code (HGB) and the German Stock Corporation Act (AktG). The consolidated financial statements are prepared in accordance with International Financial Reporting Standards (IFRS) and in compliance with the recommendations of the International Financial Reporting Standards Interpretations Committee (IFRS IC). We have applied all standards and interpretations that were in force on December 31, 2021 and adopted by the EU into European law. As of December 31, 2021, there were no standards or interpretations with an impact on our consolidated financial statements as of December 31, 2021 and 2020 that had entered into force but had not yet been adopted into European law. Therefore, our consolidated financial statements comply with both the IFRS published by the International Accounting Standards Board (IASB) and the IFRS adopted by the EU. In addition, our consolidated financial statements take into account the supplementary provisions of German commercial law that are to be applied in accordance with Section 315e (1) HGB). For the election of our auditor, the Audit Committee of the Supervisory Board submits a nomination proposal to the Supervisory Board. At the 2021 Annual General Meeting, PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft was appointed as auditor for the 2021 financial year. As proof of its

72 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 independence, the auditor submitted an Independence Declaration to the Supervisory Board. The lead auditor of these financial statements was Holger Lutz, who has audited the financial statements since 2019. PricewaterhouseCoopers GmbH has been our auditor since the 2011 financial year. Information on audit- related fees and all other fees provided by PricewaterhouseCoopers GmbH to us during the 2021 financial year can be found in the Notes to the Annual Financial Statements of MorphoSys AG. Compliance Management Program The separate “Non-Financial Group Report”* sets out the basic mechanisms of our compliance management program (CMP). The report is available on our website under https://csr.morphosys.com/2021. The identification and assessment of compliance risks are an important part of the CMP and are incorporated into the program’s overall strategic development. Our main compliance-relevant risk areas are evaluated using a systematic approach and taking into account our current business strategy and priorities. During the reporting year, we carried out an annual compliance risk assessment that included anti-bribery and other relevant risk areas. Risk mitigation measures were initiated for the areas of action identified. Within the scope of the CMP, employees are given the opportunity to report potential compliance issues within the MorphoSys Group in a protected and, if desired, anonymous manner through the MorphoSys Integrity Line reporting system. In addition to an annual compliance risk analysis, we have developed other appropriate guidelines and have monitored compliance. In order to prevent compliance breaches, employees were routinely trained in topics relevant for compliance. Traditional compliance refresher trainings have been provided to the employees, as well as newly developed trainings on thoughtful communications and investigator-initiated trials. In November 2021, MorphoSys held a Compliance Week that included a number of engaging activities for employees of MorphoSys AG, MorphoSys US Inc. and, for the first time, Constellation under the motto “Integrity in All We Do.” Compliance-related discussions and analyses at all levels of the Company lead to a continuous improvement in managing and mitigating risk at MorphoSys. In conjunction with the EU General Data Protection Regulation (Regulation [EU] 2016/679 – “GDPR”), which entered into force on May 25, 2018, we have implemented various procedures since 2018 to ensure compliance with the GDPR. More details can be found in the separate “Non-Financial Group Report.”* * This information is not part of the management report that is subject to audit.

Management Report 73 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Fig. 02: Compliance Management Program (CMP) Internal Audit Department Our Internal Audit department is an essential element of the Corporate Governance structure. The department assists us in accomplishing our objectives by prescribing a systematic approach to evaluating and improving

74 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 the effectiveness of our risk management, internal control and other corporate governance processes. The activities of the Internal Audit function are supported by co-sourcing partner Protiviti, an independent consulting firm with expertise in internal audit, risk and compliance. The Internal Audit department executes a risk-based audit plan that includes the requirements and recommendations of the Management Board, as well as those of the Supervisory Board’s Audit Committee. The Internal Audit department is also responsible for performing management testing in accordance with the requirements of the U.S. Sarbanes-Oxley Act, Section 404 (SOX). This procedure involves independently testing the appropriateness and effectiveness of internal controls in the business processes relevant to financial reporting. The outcome of each internal audit is communicated to the CEO and the relevant members of the Executive Committee. In addition, the Head of Internal Audit reports to the Audit Committee of the Supervisory Board on the results of the internal audits and SOX management testing twice a year or immediately if necessary. Three audits were carried out in the year 2021. Some areas for action were identified, resulting in the adoption of corresponding corrective plans of action. The internal audit plan for 2022 envisages four audits.

Management Report 75 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Disclosures pursuant to Section 289a (1), Section 315a (1) HGB and Explanatory Report of the Management Board pursuant to Section 176 (1) Sentence 1 AktG Composition of Share Capital On December 31, 2021, the Company’s share capital amounted to €34,231,943, divided into 34,231,943 no- par-value bearer shares. With the exception of the 83,154 treasury shares held by the Company, these bearer shares possess voting rights, with each share granting one vote at the Annual General Meeting. As of December 31, 2021, the Company’s registered share capital amounted to €34,227,598, divided into 34,227,598 no-par-value bearer shares. This amount of registered share capital does not yet reflect the increase in share capital and the number of shares resulting from the exercise of 4,345 subscription rights to shares in the Company (stock options) in 2021. On January 17, 2022, the Supervisory Board of the Company resolved to amend the wording of the Articles of Association to reflect the higher share capital of €34,231,943 and filed this amendment for entry in the Commercial Register. The entry in the Commercial Register was made on January 29, 2022. Restrictions Affecting Voting Rights and the Transfer of Shares Our Management Board is not aware of any restrictions that may affect voting rights or the transfer of shares, or any restrictions that may emerge from agreements between shareholders. Voting rights restrictions may also arise from the provisions of the German Stock Corporation Act (AktG), such as those pursuant to Section 136 AktG, or the provisions for treasury shares pursuant to Section 71b AktG. Shareholdings in Common Stock Exceeding 10% of Voting Rights We have not been made aware or notified of any direct or indirect interests in the Company’s share capital that exceed 10% of the voting rights. Shares with Special Rights Conferring Powers of Control Shares with special rights conferring powers of control do not exist. Control over Voting Rights with regard to Employee Ownership of Capital Employees who hold shares in the Company exercise their voting rights directly in accordance with the statutory provisions and the Articles of Association, as do other shareholders. Appointment and Dismissal of Management Board Members and Amendments to the Articles of Association In accordance with Section 6 of the Articles of Association and Section 84 of the German Stock Corporation Act (AktG), the Supervisory Board determines the number of members on the Management Board, appoints and revokes members and nominates the Chairman. Until December 31, 2021, the Management Board consisted of the Chairman and three further members. Since the departure of Roland Wandeler, Ph.D., the Management Board consists only of the Chairman of the Management Board and two further members. Members of the Management Board can be appointed for a maximum term of five years. Reappointments and extensions in the term of office are allowed for a maximum term of five years in each case. The Supervisory Board may revoke the appointment of a Management Board member or Chairman of the Management Board

76 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 for good cause as defined by Section 84 (3) AktG. Where the Management Board lacks a required member, a Management Board member will be appointed by the court in urgent cases pursuant to Section 85 AktG. As a rule, the Articles of Association can only be amended by a resolution of the General Meeting in accordance with Section 179 (1) sentence 1 AktG. Pursuant to Section 179 (2) sentence 2 AktG in conjunction with Section 20 of the Articles of Association, our Annual General Meeting resolves on amendments to the Articles of Association generally with a simple majority of the votes cast and a simple majority of the share capital represented. If the law stipulates a higher mandatory majority of votes or capital, this shall apply. Amendments to the Articles of Association that only affect their wording can be resolved by the Supervisory Board in accordance with Section 179 (1) sentence 2 AktG in conjunction with Section 12 (3) of the Articles of Association. Power of the Management Board to Issue Shares The Management Board’s power to issue shares is granted under Section 5 (5) through (6i) of the Company’s Articles of Association and the statutory provisions. The Supervisory Board is authorized to amend the wording of the Articles of Association in accordance with the scope of the capital increase from conditional or authorized capital. 1. Authorized Capital In the case of an authorized capital increase, the Management Board is authorized with the consent of the Supervisory Board to determine the further details of the capital increase and its implementation. a. Pursuant to Article 5 (5) of the Articles of Association, the Management Board is authorized with the consent of the Supervisory Board to increase the Company’s share capital against contribution in cash and/or contribution in kind on one or several occasions by a total of up to €4,861,376 by issuing up to 4,861,376 new, no-par-value bearer shares until and including May 18, 2026 (Authorized Capital 2021-I). b. In case of capital increases, shareholders are principally entitled to subscription rights. The shares may also be subscribed to by one or several credit institutions with the obligation to offer the shares to shareholders for subscription. The Management Board with the Supervisory Board’s consent is, however, authorized to exclude shareholders’ subscription rights in the following cases: aa) in the case of a capital increase against contribution in cash, to the extent necessary to avoid fractional amounts; or bb) in the case of a capital increase against contribution in kind; or cc) in the case of a capital increase against contribution in cash to the extent the new shares shall be placed on a foreign stock exchange in the context of an IPO. The total number of shares to be issued by way of a capital increase against contribution in cash and/or in kind, excluding subscription rights and based on the above authorizations, shall not exceed 10% of the share capital, calculated either based on the authorizations’ effective date or the time they are exercised, whichever amount is lower. The 10% limit mentioned above shall take into account (i) treasury shares sold with the exclusion of subscription rights after the effective date of these authorizations, (ii) shares issued on the basis of other authorized capital with the exclusion of subscription rights during the period in which these authorizations are in effect, and (iii) shares to be issued to service convertible bonds and/or bonds with

Management Report 77 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 warrants, insofar as the convertible bonds and/or bonds with warrants have been issued with the exclusion of shareholders’ subscription rights while these authorizations are in effect but in respect of items (i), (ii) and/or (iii) in each case only insofar as the shares are not used to service claims by members of governing bodies and/or employees of the Company and/or its affiliated companies under employee participation programs. b) Pursuant to Section 5 (6) of the Articles of Association, the Management Board is authorized with the consent of the Supervisory Board to increase the Company’s share capital against contribution in cash on one or several occasions by a total of up to € 1,951,452 by issuing up to 1,951,452 new no-par-value bearer shares until and including May 18, 2026 (Authorized Capital 2021-II). In case of capital increases, shareholders are principally entitled to subscription rights. The shares may also be subscribed to by one or several credit institutions with the obligation to offer the shares to shareholders for subscription. The Management Board is, however, authorized to exclude shareholder subscription rights, with the Supervisory Board’s consent, in the following cases: aa) to the extent such exclusion is necessary to avoid fractional amounts; or bb) if the issue price of the new shares is not significantly below the market price of shares of the same class already listed and the total number of shares issued against contribution in cash, excluding subscription rights, during the term of this authorization does not exceed 10% of the share capital on the date this authorization takes effect or at the time it is exercised, in accordance with or in the respective application of Section 186 (3) sentence 4 AktG. This 10% limit shall take into account treasury shares of the Company, which are sold during the term of this authorization with the exclusion of shareholders’ subscription rights in accordance with section 71 para. 1 no. 8 sentence 5 clause 2 AktG in conjunction with section 186 para. 3 sentence 4 AktG. Furthermore, shares issued or to be issued to service convertible bonds and/or bonds with warrants shall be included in this 10% limit of the share capital, provided that these convertible bonds and/or bonds with warrants were issued during the term of this authorization with the exclusion of subscription rights in the respective application of section 186 para. 3 sentence 4 AktG. In addition, shares issued excluding shareholders’ subscription rights during the term of this authorization on the basis of other capital measures in direct or mutatis mutandis application of section 186 para. 3 sentence 4 AktG shall be included in this 10% limit of the share capital. The maximum limit reduced in accordance with the above sentences of this paragraph shall be increased again when a new authorization to exclude shareholders’ subscription rights resolved by the Annual General Meeting takes effect in accordance with section 186 para. 3 sentence 4 AktG after the reduction, in the amount of the new authorization, up to a maximum of 10% of the share capital in accordance with the requirements of sentence 1 of this paragraph bb). The total number of shares to be issued by way of a capital increase against contribution in cash, excluding subscription rights and based on the authorizations mentioned above shall not exceed 10% of the share capital when calculated based on the authorizations’ effective date or exercise, whichever amount is lower. The aforementioned 10% limit shall include (i) treasury shares sold with exclusion of subscription rights after the effective date of these authorizations, (ii) shares issued on the basis of other authorized capital with the exclusion of subscription rights during the period in which these authorizations are in effect and (iii) shares to be issued to service convertible bonds and/or bonds with warrants, insofar as the convertible bonds and/or bonds with warrants have been issued with the exclusion of shareholders’ subscription rights while these authorizations are in effect but in respect of items (i), (ii) and/or (iii) in each case only insofar as the shares are not used to service claims of members of the Management Board and/or employees of the Company and/or its affiliated companies under employee participation programs. The maximum limit reduced in accordance with the above sentences of this paragraph shall be increased again when a new authorization to

78 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 exclude shareholders’ subscription rights resolved by the Annual General Meeting takes effect after the reduction, in the amount of the new authorization, up to a maximum of 10% of the share capital in accordance with the requirements of sentence 1 of this paragraph. c. Pursuant to Article 5 (6a) of the Articles of Association, the Management Board is authorized with the consent of the Supervisory Board to increase the Company’s share capital against contribution in cash and/or contribution in kind on one or several occasions up to and including May 18, 2026 by up to a total of € 315,000 by issuing up to 315,000 new no-par-value bearer shares (Authorized Capital 2021-III). The subscription rights of shareholders are excluded. The Authorized Capital 2021-III serves the purpose of delivering shares of the Company against the contribution of payment claims resulting from Restricted Stock Units (RSUs) in order to fulfill RSUs that were granted in accordance with the terms and conditions of the Restricted Stock Unit Program 2021 of the Company (RSUP 2021) exclusively to senior managers and employees (including directors and officers) of MorphoSys US Inc. The issue price of the new shares must amount to at least € 1.00 and can be paid either by way of a cash contribution and/or contribution in kind, including in particular the contribution of claims against the Company under the RSUP 2021. The Management Board is authorized to determine the further details of the capital increase and its implementation with the consent of the Supervisory Board; this also includes the determination of the profit participation of the new shares, which may, in deviation from Section 60 (2) AktG, also participate in the profit of an already completed financial year, provided that no resolution on the appropriation of profits has yet been adopted for the fiscal year in question. d. Pursuant to Article 5 (6h) of the Articles of Association, the Management Board is authorized with the consent of the Supervisory Board to increase the Company’s share capital on one or several occasions by a total of up to € 159,197 by issuing up to 159,197 new no-par-value bearer shares against cash contribution and/or contribution in kind until and including April 30, 2024 (Authorized Capital 2019-I). The subscription rights of shareholders are excluded. The Authorized Capital 2019-I serves the purpose of delivering shares of the Company against the contribution of payment claims resulting from Restricted Stock Units (RSUs) in order to fulfill RSUs that were granted in accordance with the terms and conditions of the Company’s Restricted Stock Unit Program (RSUP) exclusively to senior managers and employees (including directors and officers) of MorphoSys US Inc. The issue price of the new shares must amount to at least € 1.00 and may be paid either by way of a cash contribution and/or contribution in kind, including in particular the contribution of claims against the Company under the RSUP. The Management Board is authorized with the consent of the Supervisory Board to determine the further details of the capital increase and its implementation; this also includes the determination of the profit participation of the new shares, which may, in deviation from Section 60 para. 2 AktG, also participate in the profit of an already completed financial year, provided that no resolution on the appropriation of profits has yet been adopted for the fiscal year in question. 2. Conditional Capital a. Pursuant to Article 5 (6b) of the Articles of Association, the Company’s share capital is conditionally increased by up to € 2,475,437 through the issue of up to 2,475,437 no-par-value bearer shares (Conditional Capital 2016-I). The conditional capital increase serves solely as a means to grant new shares to the holders of conversion or warrant rights, which will be issued by the company or companies in which the Company has a direct or indirect majority interest according to the authorizing resolution of the Annual General Meeting on June 2, 2016, under Agenda Item 7 letter a). The shares will be issued at the respective

Management Report 79 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 conversion or exercise price to be determined in accordance with the resolution above. The conditional capital increase will only be carried out to the extent that the holders of conversion or warrant rights exercise these rights or fulfill conversion obligations under such bonds. The shares will be entitled to dividends as of the beginning of the previous financial year, provided they were issued before the start of the Company’s Annual General Meeting, or as of the beginning of the financial year in which they were issued. b. Pursuant to Article 5 (6c) of the Articles of Association, the Company’s share capital is conditionally increased by up to €3,289,004 by issuing up to 3,289,004 new no-par-value bearer shares (Conditional Capital 2021-I). The conditional capital increase serves exclusively to grant new shares to the holders of conversion or warrant rights issued by the Company or by companies in which the Company directly or indirectly holds a majority interest in accordance with the authorization resolution of the Annual General Meeting of May 19, 2021 under Agenda Item 10 a). The shares shall be issued at the conversion or warrant price to be determined in each case in accordance with the aforementioned resolution. The conditional capital increase shall only be carried out to the extent that the holders of conversion or warrant rights exercise their conversion or warrant rights or fulfill conversion obligations under such bonds. The shares shall participate in profits – to the extent they come into existence by the beginning of the Annual General Meeting of the Company – from the beginning of the preceding financial year, otherwise from the beginning of the financial year in which they come into existence. c. Pursuant to Article 5 (6g) of the Articles of Association, the share capital is increased conditionally by up to € 741,390 through the issue of up to 741,390 new no-par-value bearer shares of the Company (Conditional Capital 2016-III). The conditional capital serves to meet the obligations of subscription rights that have been issued and exercised based on the authorization resolved by the Annual General Meeting of June 2, 2016 under Agenda Item 9 letter a). The conditional capital increase will only be executed to the extent that holders of subscription rights exercise their right to subscribe to shares of the Company. The shares will be issued at the exercise price set in each case as the issue price in accordance with Agenda Item 9 letter a) subparagraph (8) of the Annual General Meeting’s resolution dated June 2, 2016; Section 9 (1) AktG remains unaffected. The new shares are entitled to dividends for the first time for the financial year for which there has been no resolution by the Annual General Meeting on the appropriation of profits at the time of the shares’ issue. The Management Board, and the Supervisory Board where members of the Management Board are concerned, is authorized to determine the additional detail of the conditional capital increase and its execution. d. Pursuant to Article 5 (6i) of the Articles of Association, the Company’s share capital is increased conditionally by up to € 1,314,615 by issuing up to 1,314,615 new no-par-value bearer shares (Conditional Capital 2020-I). The conditional capital serves to fulfill subscription rights that were issued and exercised on the basis of the authorization resolved by the Annual General Meeting on May 27, 2020, under Agenda Item 11, letter a). The conditional capital increase will only be implemented to the extent that holders of subscription rights exercise their subscription rights to subscribe to shares of the Company. The shares will be issued at the exercise price determined in accordance with the resolution of the Annual General Meeting of May 27, 2020, under Agenda Item 11, letter a) subparagraph (8) as the issue price; Section9 (1) AktG remains unaffected. The new shares are entitled to dividends for the first time for the financial year for which, at the time of their issue, no resolution by the Annual General Meeting on the appropriation of profits has yet been passed. The Management Board, or, insofar as members of the Management Board are affected, the Supervisory Board are authorized to determine the further details of the conditional capital increase and its implementation. Power of Management Board to Repurchase Shares

80 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The Management Board is currently not authorized to repurchase the Company’s shares. Material Agreements Made by the Company that fall under the Condition of a Change of Control after a Takeover Bid A change of control as a result of a takeover bid could have an impact on our convertible bond issued in October 2020, the underlying contract of which contains customary change-of-control clauses. According to these clauses, bondholders can demand early repayment of the outstanding amounts in the event of a change of control. The Company has not entered into any further material agreements that are subject to a change of control following a takeover bid.

Management Report 81 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Compensation Agreements Concluded by the Company with Management Board Members and Employees in the Event of a Takeover Bid In accordance with the service agreements in force in the reporting period, the Management Board members may terminate their contract following a change of control and claim the compensation still outstanding up to the regular end of the service contract, but at least the compensation for two years, as a severance payment. In the case of Sung Lee, severance payments in the event of premature termination of the service contract due to a change of control are capped at 200% of the annual compensation in line with the new requirements of the Code. In addition, the plan terms of the long-term variable compensation programs provide that, in the event of a change of control, all granted stock options, performance shares and other comparable direct or indirect interests in MorphoSys with compensation character vest with immediate effect and can be exercised after the expiry of the statutory waiting periods. Following a change of control, some executives may terminate their service contracts and demand a severance payment in the amount of one annual gross fixed salary and the full contractual bonus for the calendar year in which the termination is effected. A target achievement rate of 100% is applied. In such a case, all stock options and performance shares granted will vest immediately and may be exercised after the statutory vesting periods and blackout periods have expired. The following cases are considered to be a change of control: (i) MorphoSys transfers all or substantially all of its corporate assets to a non-affiliated company, (ii) MorphoSys merges with a non-affiliated company, (iii) MorphoSys AG, as a controlled company, becomes a party to an agreement pursuant to Section 291 of the German Stock Corporation Act (AktG), or MorphoSys is integrated in accordance with Section 319 of the German Stock Corporation Act (AktG), or (iv) a shareholder or third party directly or indirectly holds 30% or more of the voting rights of MorphoSys, or at least 30% of the voting rights are attributed to the shareholder or third party.

82 Management Report MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 List of Figures and Tables • Fig. 01: Risk and Opportunity Management System at MorphoSys • Fig. 02: Compliance Management Program (CMP) • Tab. 01: Comparison of Actual Business Results versus Forecasts • Tab. 02: Comparison of Old and New Integrated Opportunity & Risk Management System • Tab. 03: Risk Assessment Categories • Tab. 04: Summary of MorphoSys' Key Opportunities • Tab. 05: Overview of Risk Categories • Tab. 06: Overview of MorphoSys’ Most Significant Risks • Tab. 07: Composition of the Supervisory Board until Termination of the 2021Annual General Meeting • Tab. 08: Composition of the Supervisory Board since Termination of the 2021 Annual General Meeting • Tab. 09: Participation of Supervisory Board Members • Tab. 10: Managers' Transactions 2021


MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Annual Financial Statements of MorphoSys AG as of December 31, 2021 (German GAAP) MorphoSys AG, Planegg


84 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Balance Sheet as of December 31, 2021 12/31/2021 12/31/2021 12/31/2020 ASSETS in € in € A. FIXED ASSETS I. Intangible Assets Paid Concessions, Commercial Property Rights and similar Rights and Assets and Licenses to such Rights and Assets 74,374,006 74,374,006 77,450,077 II. Property, Plant and Equipment 1 Land, Leasehold Rights and Buildings, including Leasehold Improvements 400,932 421,106 2 Other Equipment, Furniture and Fixtures 3,579,281 3,369,703 3,980,213 3,790,809 III. Financial Assets 1 Shares in affiliated Companies 1,152,260,363 1,538,439 1,152,260,363 1,538,439 1,230,614,582 82,779,325 B. CURRENT ASSETS I. Inventories 1 Raw materials, Supplies and Production Materials 12,125,722 5,307,573 2 Unfinished Goods 4,089,282 0 3 Finished Goods 0 1,746,054 4 Advance payments 2,204,394 0 18,419,398 7,053,627 II. Receivables and Other Assets 1 Trade Accounts Receivable (thereof due over one year 0, prior year: EUR 0) 44,842,910 65,560,079 2 Receivables due from affiliated Companies (thereof due over one year 106,772,080, prior year: EUR 95,006,590) 106,772,080 95,006,590 3 Other Assets (thereof due after one year 0, prior year: EUR 0) 601,760,298 689,068,446 753,375,288 849,635,115 III. Securities Other securities 195,801,237 474,184,111 195,801,237 474,184,111 IV. Cash on Hand and Cash at Banks 66,074,945 66,074,945 66,543,115 1,033,670,868 1,397,415,968 C. PREPAID EXPENSES 13,150,065 13,150,065 7,600,961 2,277,435,515 1,487,796,254

85 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 12/31/2021 12/31/2021 12/31/2020 LIABILITIES AND STOCKHOLDERS’ EQUITY in € in € in € A. Stockholders’ Equity I. Common Stock (Nominal Value of the Conditional Capital as of December 31, 2021: € 7,816,101; December 31, 2020: € 7,630,728) 34,231,943 32,890,046 Treasury Stock (83,154) (131,414) 34,148,789 32,758,632 II. Additional Paid-in Capital 835,555,661 835,555,661 751,201,728 III. Earnings Reserves Other Earnings Reserves 23,632,500 23,632,500 22,182,157 IV. Accumulated Deficit (680,842,191) (680,842,191) (370,359,955) 212,494,759 435,782,562 B. Provisions 1 Tax Provisions 329,723 64,897,998 2 Other Provisions 629,567,475 608,595,959 629,897,198 673,493,957 C. LIABILITIES 1 Bonds (thereof convertible 325,000,000, prior year: EUR 325,000,000) 325,000,000 325,000,000 2 Prepayments Received on Orders 180,765 2,500,806 3 Accounts Payable 64,558,175 44,845,812 4 Liabilities due to Affiliated Companies 53,218,232 3,672,174 5 Other Liabilities (thereof due within one year EUR 3,145,431, prior year: EUR 2,386,017) (thereof for taxes EUR 1,189,188, prior year: EUR 1,499,090) 3,145,431 2,386,017 446,102,603 378,404,809 D. DEFERRED INCOME 988,940,955 988,940,955 114,926 2,277,435,515 1,487,796,254
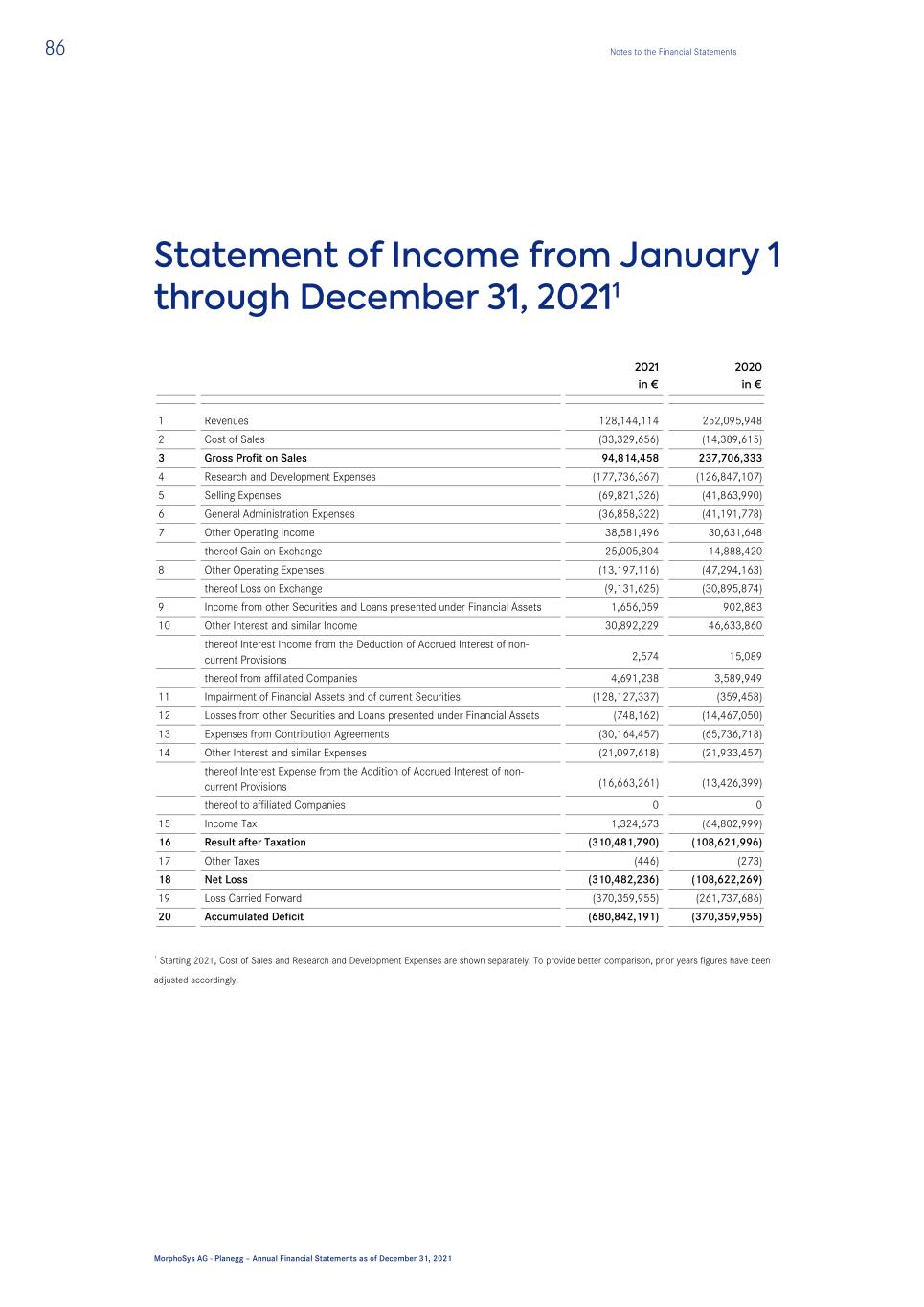
86 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Statement of Income from January 1 through December 31, 20211 2021 2020 in € i € in € i € 1 Revenues 128,144,114 252,095,948 2 Cost of Sales (33,329,656) (14,389,615) 3 Gross Profit on Sales 94,814,458 237,706,333 4 Research and Development Expenses (177,736,367) (126,847,107) 5 Selling Expenses (69,821,326) (41,863,990) 6 General Administration Expenses (36,858,322) (41,191,778) 7 Other Operating Income 38,581,496 30,631,648 thereof Gain on Exchange 25,005,804 14,888,420 8 Other Operating Expenses (13,197,116) (47,294,163) thereof Loss on Exchange (9,131,625) (30,895,874) 9 Income from other Securities and Loans presented under Financial Assets 1,656,059 902,883 10 Other Interest and similar Income 30,892,229 46,633,860 thereof Interest Income from the Deduction of Accrued Interest of non- current Provisions 2,574 15,089 thereof from affiliated Companies 4,691,238 3,589,949 11 Impairment of Financial Assets and of current Securities (128,127,337) (359,458) 12 Losses from other Securities and Loans presented under Financial Assets (748,162) (14,467,050) 13 Expenses from Contribution Agreements (30,164,457) (65,736,718) 14 Other Interest and similar Expenses (21,097,618) (21,933,457) thereof Interest Expense from the Addition of Accrued Interest of non- current Provisions (16,663,261) (13,426,399) thereof to affiliated Companies 0 0 15 Income Tax 1,324,673 (64,802,999) 16 Result after Taxation (310,481,790) (108,621,996) 17 Other Taxes (446) (273) 18 Net Loss (310,482,236) (108,622,269) 19 Loss Carried Forward (370,359,955) (261,737,686) 20 Accumulated Deficit (680,842,191) (370,359,955) 1 Starting 2021, Cost of Sales and Research and Development Expenses are shown separately. To provide better comparison, prior years figures have been adjusted accordingly.

Notes to the Financial Statements 87 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Notes to the Financial Statements General Information These annual financial statements were prepared in accordance with Section 242 et seq. and Section 264 et seq. of the German Commercial Code (HGB), the corresponding provisions of the German Stock Corporation Act (AktG) and the Company's Articles of Association. The shares of MorphoSys AG ("MorphoSys" and the "Company") are listed for trading in the Regulated Market (Prime Standard segment) of the Frankfurt Stock Exchange. On April 18, 2018, MorphoSys completed an IPO on the Nasdaq Global Market through the issue of American Depositary Shares (ADS). Each ADS represents 1/4 of a MorphoSys ordinary share. These annual financial statements were prepared in accordance with the regulations for large corporations. The statement of income has been structured in accordance with the cost of sales method for the purposes of comparison with the consolidated financial statements prepared pursuant to IFRS. The financial year corresponds to the calendar year. MorphoSys AG prepares the consolidated financial statements for the largest and the smallest consolidated group. The Company's registered office is located at Semmelweisstrasse 7, 82152 Planegg, Germany. The MorphoSys AG consolidated and separate financial statements can be viewed at this address. The Company is recorded in the Commercial Register B of the District Court of Munich, Germany, under the number HRB 121023. Accounting and Valuation Principles These annual financial statements were prepared on the basis of the following accounting and valuation principles. Due to the managements increased focus on cost of sales since market approval of Monjuvi, cost of sales and research & development costs will be presented separately within statement of income from fiscal year 2021 onwards. For better comparability, prior years figures have been adjusted accordingly. Acquired intangible assets are capitalized with their acquisition costs and amortized using the straight-line method over the course of their expected useful lives. Acquired in-process research and development programs are recognized at acquisition cost and are only subject to amortization when the studies on the efficacy of the respective antibody program are fully completed, and a marketing authorization has been obtained. From the time of market approval, these are recognized as licenses for marketed products. Prior to receiving marketing authorization, the values of these assets are reviewed at the reporting date and carried at the lower of their carrying amount or fair value. The option to capitalize self-constructed intangible assets was not called upon according to section 248 para. 2 sent. 1 HGB.

88 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Asset Class Useful Life Amortization Rates Licenses 8 to 10 years 13% - 10% In-process R&D Programs not yet subject for amortization - Licenses for Marketed Products 24 years 4% Software 3 to 5 years 33% - 20% Tangible assets are carried at acquisition cost and depreciated on a straight-line basis over their expected useful lives. Low-value assets up with values between € 250 and € 800 are fully depreciated in the year they are acquired. Asset Class Useful Life Depreciation Rates Land, Leasehold Rights and Buildings, including Leasehold Improvements 10 years 10% Other Equipment, Furniture and Fixtures 3 to 8 years 33% - 13% Financial assets are recognized according to the strict lower of cost or market principle at the lower of their acquisition cost or fair value. The fair value corresponds to the market price from an active market. If no active market exists, fair value is determined using generally accepted valuation methods such as the discounted cash flow method. Inventories include raw materials, supplies and production materials as well as unfinished goods, and are stated at the lower of cost or market value, applying permitted valuation simplification procedures. In addition to the direct cost, the production cost also include appropriate components of the necessary material and production overhead as well as production-related depreciation. Impairments are recognized for inventory risks resulting from increased storage periods or reduced usability. Inventories are not subject to third-party rights, except for the customary retention of title. Receivables and other assets are recognized at nominal value. Risks are taken into account by means of write- downs or impairments. Other securities are recognized at the lower of acquisition cost or fair value in accordance with Section 253 (4) HGB. Cash and cash equivalents are carried at their nominal value as of the reporting date. Prepayments are recognized as prepaid expenses on the reporting date insofar as they represent expenses for a certain period subsequent to the reporting date. Common stock is carried at nominal value. The nominal value of the shares repurchased is offset against common stock in accordance with Section 272 (1a) HGB, while the remaining amount of the total purchase price is offset against the other earnings reserves within equity. Provisions cover all identifiable risks and uncertain obligations and are recognized at the settlement amount required according to prudent business judgment. In the case of provisions with a remaining term of more than one year, future price and cost increases are taken into account in the amount of the general inflation rate and discounted to the reporting date. The discount rates used are the average market interest rates of the

Notes to the Financial Statements 89 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 past seven financial years corresponding to the remaining terms of the provisions, as determined and published monthly by the German Central Bank (Deutsche Bundesbank) in accordance with the German Regulation on the Discounting of Provisions (‘Rückstellungsabzinsungsverordnung’). A currency-matching (US dollar) discount rate for the payment weighted remaining term of the provision relating to the collaboration and license agreement with Incyte is also determined in accordance with this same regulation. As of December 31, 2021, an interest rate of 3.11 % was determined with an underlying duration of 7.87 years. Refer to section “Collaboration and License Agreement with Incyte” for further information. Provisions have been recognized on a pro rata basis for personnel expenses resulting from long-term incentive plans established in 2018, 2019, 2020 and 2021 because the repurchase of treasury shares for servicing the incentive plans and cash settlement of the performance share unit program constitutes a financial burden on the Company. The measurement of forward rate agreements qualifying as derivative financial instruments is based on the change in forward exchange curves. Recognition and measurement follow the imparity principle. Negative valuation effects as of balance sheet date are shown as liabilities. Valuation units were not formed in the past financial year. Liabilities are measured at the settlement amount. The imparity principle is applied to non-current liabilities. Prepayments received on orders are measured at the settlement amount. Deferred revenue consists of payments received prior to the reporting date to the extent these payments represent income for a specific period after this date. Since the purchase price received from Royalty Pharma represents consideration for future license income that arise over a period of several years, it has to be assumed that the time value of money has been taken into account. In order to avoid differences in the accounting according to German GAAP and the German Income Tax, which would result in additional deferred taxes to be recognized, MorphoSys chose to not consider discounting effects separately. The release into revenue is recognized in line with the actual license income realized in the period in relation to the sum of the undiscounted expected license income. The recognition of revenue for income from collaboration and research agreements is carried on the basis of the contractual terms and takes into account the realization principle of Section 252 (1) no. 4 HGB and the accrual-based method of Section 250 (2) HGB based on the contract period. Upfront payments made at the time of the conclusion of a contract for the out-licensing of antibody programs and the transfer of beneficial ownership of a distribution license are recognized as revenue at the time of the transfer to the licensee, provided that no material performance obligations have to be provided in the future. Revenue from milestone payments is recognized upon the achievement of certain success criteria (for example, the achievement of specified clinical phases, certain approvals and the number of patients treated). Service fees related to research and development collaborations are recognized in the period the services were rendered. Royalties from product sales are recognized in the period in which the corresponding sales are generated by the partner. Revenues from product sales are recognized upon completion of transfer of risk. This is case, once the customer obtains control of the product. The deferred income from the purchase price paid by Royalty Pharma for the forfaiting of future receivables is released over the duration of the underlying license agreements.

90 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Cost of sales includes acquisition and production costs of inventories recognized as an expense, mainly consisted of costs for external services, personnel costs, material costs, infrastructure costs, operating costs, depreciation and amortization and other expenses. Research and development costs primarily comprised costs for external services, personnel costs, material costs, infrastructure costs, operating costs, impairment losses, depreciation and amortization and other expenses. They also included reasonable research and development-related expenses for voluntary social benefits and company pension plans. Negative interest on financial assets and marketable securities is reported under other interest and similar expenses. Any total tax charge that results from a difference between the carrying amounts of assets, liabilities, accruals and deferrals prescribed by commercial law and these items' tax carrying amounts that are likely to diminish in subsequent financial years is recognized as a deferred tax liability in the balance sheet in accordance with Section 274 HGB. Any total tax relief that results is not recognized as deferred tax assets in the balance sheet pursuant to the option granted in Section 274 (1) sent. 2 HGB. The amount of the resulting tax charge and relief is measured at the Company-specific tax rates, applicable at the time the differences are reversed and are not discounted. The line items reported are reversed as soon as the tax charge or benefit occurs or is no longer expected. The income or expense from changes in deferred tax assets or liabilities is recorded separately in the statement of income under the line item "income tax." All amounts in this report are rounded to the nearest euro, thousand euros or million euros. Foreign Currency Translation Current receivables and liabilities denominated in foreign currencies are translated on the basis of the mean spot exchange rate prevailing on the day of the transaction or the reporting date pursuant to Section 256a HGB. The Company did not recognize any non-current receivables or liabilities denominated in foreign currencies. Notes to the Balance Sheet Fixed Assets The development of the individual line items under fixed assets and the respective depreciation in the financial year are presented in the statement of fixed assets. Intangible Assets Acquired concessions, industrial property rights and similar rights and assets, as well as licenses to such rights and assets, amounted to € 74,374k as of December 31, 2021 (December 31, 2020: € 77,450k). This decrease resulted mainly from scheduled amortization of acquired in-process research and development programs in the amount of € 2,312k and acquired licenses in the amount of € 986k . As of the reporting date, intangible assets were tested for impairment, and no impairment loss (December 31, 2020: € 2,000k) was recognized for licenses no longer in use. The development of intangible assets and the respective amortization in the financial year are presented in the statement of fixed assets.

Notes to the Financial Statements 91 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Financial Assets At the reporting date December 31, 2021, the Company recognized shares in affiliated companies in the amount of € 1,152,260k (December 31, 2020: € 1,538k), which are attributable to the total shares of MorphoSys US Inc. The increase in the carrying amount of the share in MorphoSys US Inc. by € 1,150,722k resulted from a contribution to MorphoSys US Inc.’s capital reserves paid by MorphoSys AG on July 15, 2021 in the amount of € 1,278,849k, which was partially offset by an impairment of these shares of € 128,127k. At the reporting date of December 31, 2021, the company held shares in shares in adivo GmbH at a value of € 0 (December 31, 2020: € 0). Direct and indirect shares in affiliated companies and investments are listed individually in the following overview: Currency Stake in % Equity (in €) Profit / Loss for the Year (in €) Constellation Pharmaceuticals, Inc., Cambridge, Massachusetts, USA 1 $ 2 100 1,002,503,861 (1,569,762,195) Constellation Securities Corp., Cambridge, Massachusetts, USA 1 $ 2 100 105,606,736 37,270 MorphoSys US Inc., Boston, Massachusetts, USA $ 2 100 892,839,341 (341,853,874) adivo GmbH, Martinsried, Germany 3 € 17.2 (681,809) (835,119) 1 Indirect subsidiary via MorphoSys US Inc. 2 As of December 31, 2021, fx-rate for 1 $ to 1 €: 0.8829 3 Equity as of December 31, 2020 and loss for the year for the financial year January 1, to December 31, 2020 Following the acquisition on July 15, 2021, Constellation Pharmaceuticals, Inc. was merged into MorphoSys Development Inc., which was incorporated as a wholly owned subsidiary of MorphoSys US Inc. on May 28, 2021, in accordance with the merger agreement. From this upward merger, Constellation Pharmaceuticals, Inc. remained as a wholly owned subsidiary of MorphoSys US Inc. Inventories As of the reporting date, inventories of € 18,419k (December 31, 2020: € 7,054k) consisted of raw materials and supplies of € 12,126k (December 31, 2020: € 5,308k), unfinished goods (Monjuvi) of € 4,089k and down payments for inventories of € 2,204k (December 31, 2020: € 0k). As of December 31, 2021, no finished goods (Monjuvi) were accounted for at MorphoSys AG (December 31, 2020: € 1,746k). Trade Account Receivable As of December 31, 2021, MorphoSys AG recorded trade accounts receivables of € 44,843k (December 31, 2020: € 65,560k). All trade accounts receivables are due within one year. Based on the Management Board's assessment, valuation allowances were not made in the 2021 and 2020 financial years

92 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Receivables Due From Affiliated Companies On December 31, 2021, receivables due from affiliated companies amounted to € 106,772k (December 31, 2020: € 95,007k), resulting from receivables under a master loan agreement with MorphoSys US Inc. In 2020 and in 2021 no trade accounts receivable due from affiliated companies were included. Other Assets Other assets totaled € 601,760k as of December 31, 2021 (December 31, 2020: € 689,068k). As of December 31, 2021, the Company held financial assets of € 562,369k. These were recorded under other assets and comprised various fixed deposits (December 31, 2020: € 650,125k). The risk associated with these financial instruments is primarily bank credit risk. There was no indication of impairment in the 2021 financial year. Realized claims from the equal share in losses with Incyte in the amount of € 14,738k were recognized in this line item for the first time as of December 31, 2021 (December 31, 2020: € 22,344k). Refer to section “Collaboration and License Agreement with Incyte” for further details. In addition to combination compounds amounting to € 11,910k (December 31, 2020: € 10,003k) and compounds for clinical studies amounting to € 4,035k (December 31, 2020: € 0k), other assets also included rent deposits amounting to € 671k (December 31, 2020: € 671k). Other assets also contained a receivable due from tax authorities from excess VAT payments of € 6,563k (December 31, 2020: € 3,920k). An impairment on other assets was recognized in 2021 in the amount of € 3,533k (December 31, 2020: € 454k) for combination compounds. Securities Securities consisted of marketable securities in the amount of € 195,801k (December 31, 2020: € 474,184k). As of December 31, 2021, impairments due to unrealized losses on marketable securities amounted to € 0k (December 31, 2020: € 405k). The change of € 405k was recognized in profit and loss. Prepaid Expenses Prepaid Expenses in the amount of € 13,150k (December 31, 2020: € 7,601k) comprised payments in advance mainly for maintenance contracts, insurances, sublicenses as well as upfront payments for external laboratory services. Compared to the previous year, the amount increased mainly due to higher prepayments for external laboratory services for tafasitamab. Common Stock As of December 31, 2021, the Company’s fully paid common stock, including treasury shares, amounted to €34,231,943 and 34,231,943 shares, representing an increase of €1,341,897 and 1,341,897 shares compared to €32,890,046 and 32,890,046 shares as of December 31, 2020. Each no-par value share of common stock with a notional value of €1 is entitled to dividends and grants one vote at the general meeting with the exception of the treasury shares held by the Company. The common stock increased due to Royalty Pharma’s purchase of 1,337,552 shares, created from a capital increase from Authorized Capital 2021-II, as well as from the exercise of 4,345 stock options granted to employees amounting to €4,345, or 4,345 shares. The weighted-average exercise price of the exercised stock options amounted to €55.52.

Notes to the Financial Statements 93 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Treasury Stock The nominal value of the Company's treasury stock is offset against the common stock. The development of treasury stock is shown below. Number of Shares Value of Capital Subscribed in € As of 12/31/2019 225,800 225,800 Transfer in 2020 (94,386) (94,386) As of 12/31/2020 131,414 131,414 Transfer in 2021 (48,260) (48,260) As of 12/31/2021 83,154 83,154 As of December 31, 2021, treasury stock amounted to 0.24% (December 31, 2020: 0.40%) of common stock. The cause of this decline was the transfer of 45,891 of the Company's own shares to the Management Board and certain Company employees under the performance-based 2017 Long-Term Incentive Plan (LTI Plan) amounting to € 1,783,690. The vesting period for this LTI Plan expired on April 1, 2021 and provides or provided beneficiaries with a six-month option to acquire a total of 45,891 shares. In addition, 2,369 shares of treasury stock in the amount of € 87,558 from the 2019 Long-Term Incentive Plan were transferred to certain employees of MorphoSys US Inc. As a result, the number of MorphoSys shares held by the Company as of December 31, 2021 amounted to 83,154 shares (December 31, 2020: 131,414 shares). The repurchased shares can be used for all purposes specified in the authorization of the Annual General Meeting of May 23, 2014, and specifically for existing and future employee participation programs and/or to finance acquisitions. They may also be canceled. Authorized and Conditional Capital In comparison to December 31, 2020, the number of authorized ordinary shares decreased from 15,214,050 (or € 15,214,050) to 7,287,025 or € 7,287,025. At the Annual General Meeting on May 19, 2021, Authorized Capital 2021-I in the amount of € 4,861,376, Authorized Capital 2021-II in the amount of € 3,289,004 and Authorized Capital 2021-III in the amount of € 315,000 were newly created. The remaining Authorized Capital 2018-I in the amount of € 11,768,314 and the remaining Authorized Capital 2020-I in the amount of € 3,286,539 were canceled at this Annual General Meeting. The number was also reduced by the capital increase of 1,337,552 from the Authorized Capital 2021-II carried out in July 2021 under the agreement with Royalty Pharma. Under the Authorized Capital 2021-I, the Management Board is authorized, with the consent of the Supervisory Board, to increase the Company’s share capital on one or several occasions until and including May 18, 2026 against cash and/or non-cash contributions by a total of up to € 4,861,376 by issuing up to 4,861,376 new no-par-value bearer shares. Under the Authorized Capital 2021-II, the Management Board is authorized, with the consent of the Supervisory Board, to increase the Company’s share capital on one or several occasions until and including May 18, 2026 against cash contributions by a total of up to €3,289,004 by issuing up to 3,289,004 new no- par-value bearer shares.

94 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Under the Authorized Capital 2021-III, the Management Board is authorized, with the consent of the Supervisory Board, to increase the Company’s share capital on one or several occasions until and including May 18, 2026 against cash contributions and/or contributions in kind by a total of up to €315,000 by issuing up to 315,000 new no-par-value bearer shares. In comparison to December 31, 2020, the number of ordinary shares of conditional capital increased from 7,630,728 or € 7,630,728 to 7,816,101 or € 7,816,101. At the Annual General Meeting on May 19, 2021, Conditional Capital 2021-I in the amount of 3,289,004 was newly created. In the course of this General Meeting, the Conditional Capital 2008-III in the amount of 13,415, the Conditional Capital 2016-I in the amount of 2,832,099 and the Conditional Capital 2016-III in the amount of 253,772 were reduced. The exercise of 4,345 stock options in 2021 from the Conditional Capital 2016-III had an offsetting effect as well. The reduction from the exercise of the 4,345 stock options was entered into the commercial register in January 2021. Additional Paid-In Capital In the 2021 financial year, additional paid-in capital developed as follows: in 000' € Additional Paid-in Capital as of January 01, 2021 751,202 Addition in connection with Capital Increase 83,392 Additions in connection with the Exercise of Stock Options 237 Additions in connection with the Transfer of Treasury Stock 1,676 Correction Additions in connection with the Transfer of Treasury Stock prior years (951) Additional Paid-in Capital as of December 31, 2021 835,556 The additional paid in capital consisted of € 834,075k in accordance with Section 272 (2) no. 1 HGB and € 1,481k in accordance with section 272 (2) no. 2 HGB. In 2021, a correction of € 951k was recognized in connection transfer of treasury stock in previous years, resulting from the valuation of stock-based compensation. Earnings Reserves Other earnings reserves amounted to € 23,632k (December 31, 2020: € 22,182k) and developed in the 2021 financial year as follows: in 000' € Other Earnings Reserve as of 1/1/2021 22,182 Settlement with the difference from transfer of Treasury Stock by Allocation to Other Earnings Reserves (Reclassification from Other Provisions) 1,735 Correction of settlement with the difference from transfer of Treasury Stock by Allocation to Other Earnings Reserves prior years (Reclassification from Other Provisions) (285) Additional Paid-in Capital as of December 31, 2021 23,632 In 2021, a correction of € 285k was recognized in connection with the settlement with the difference from transfer of Treasury Stock by Allocation to Other Earnings Reserves prior years, resulting from the valuation of stock-based compensation.

Notes to the Financial Statements 95 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Accumulated Deficit The prior year's accumulated deficit developed in the reporting year as follows: in 000' € Accumulated Deficit as of 1/1/2021 (370,360) Net Loss (310,482) Accumulated Deficit as of 12/31/2021 (680,842) The Accumulated Deficit includes the Company's net loss for the 2021 financial year of € -310,482k. Consequently, the Accumulated Deficit increased from € -370,360k in 2020 to € -680,842k in 2021. Stock Option Plans 2017 Stock Options Plans On April 1, 2017 MorphoSys established a stock option plan (SOP) for the Management Board and selected employees of the Company (beneficiaries). The vesting period/performance has ended on March 31, 2021. The performance criteria were set at 110%. . Each stock option thus grants 1.1 subscription rights to shares in the Company. The number of subscription rights vested per year were calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The exercise price is €55.52. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2024. Based on the performance criteria achieved, 72,650 stock options can be exercised; this corresponds to 79,935 shares. Of these, the Management Board can exercise 8,197 stock options (9,017 shares), the members of the Executive Committee can exercise 4,018 stock options (4,421 shares) and current and former employees of the Company can exercise 60,435 stock options (66,497 shares). As of December 31, 2021, 3,950 stock options have been exercised, representing 4,345 shares. 2018 Stock Option Plan On April 1, 2018, MorphoSys established a stock option plan (SOP) for the Management Board and selected Company employees (beneficiaries). The grant date was April 1, 2018, and the vesting period/performance period is 4 years. Each stock option grants up to two subscription rights to shares in the Company. The subscription rights vest each year by 25% within the 4-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achievement for that year is 0%. The exercise price, derived from the average market price of the Company’s shares in the XETRA closing auction on the Frankfurt Stock Exchange from the 30 trading days prior to the issue of the stock options, is €81.04. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2016-III or by issuing treasury shares, or in cash should the exercise from Conditional

96 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Capital 2016-III not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2025. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit without entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, 1/48 of the stock options granted are forfeited for each up to 30 days of absence. A period of absence is defined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. As of December 31, 2021, 63,146 stock options are outstanding. In 2021, 1,109 stock options forfeited. 2019 Stock Option Plan On April 1, 2019, MorphoSys established a stock option plan (SOP) for the Management Board and selected employees of the Company (beneficiaries). The grant date was April 1, 2019, and the vesting period/performance period is four years. Each stock option grants up to two subscription rights to shares in the Company. The subscription rights vest each year by 25% within the four-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achievement for that year is 0%. The exercise price, derived from the average market price of the Company’s shares in the XETRA closing auction on the Frankfurt Stock Exchange from the 30 trading days prior to the issue of the stock options, is €87.86. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2016-III, issuing treasury shares, or in cash should the exercise from Conditional Capital 2016-III not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2026. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit without entitlement to compensation.

Notes to the Financial Statements 97 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, 1/48 of the stock options granted are forfeited for each up to 30 days of absence. A period of absence is defined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. As of December 31, 2021, 69,671 stock options are outstanding. In 2021, 3,512 stock options forfeited. On October 01, 2019, MorphoSys established a further stock option plan (SOP plan) for one member of the Management Board. The terms and conditions were identical to those of the April 1, 2019 program, and the exercise price was 106.16. As of December 31, 2021, 57,078 stock options of this SOP plan are outstanding. In 2021, 0 stock options forfeited. 2020 Stock Option Plan On April 1, 2020, MorphoSys established a stock option plan (SOP) for the Management Board and selected employees of the Company (beneficiaries). The grant date was April 21, 2020, and the vesting period/performance period is four years. Each stock option grants up to two subscription rights to shares in the Company. The subscription rights vest each year by 25% within the four-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achievement for that year is 0%. The exercise price, derived from the average market price of the Company’s shares in the XETRA closing auction on the Frankfurt Stock Exchange from the 30 trading days prior to the issue of the stock options, is €93.66. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2016-III, through the issue of treasury shares, or in cash should the exercise from Conditional Capital 2016-III not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is March 31, 2027. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit without entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, 1/48 of the stock options granted are forfeited for each up to 30 days of absence. A period of absence is defined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship without continued payment of remuneration.

98 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. As of December 31, 2021, 100,350 stock options are outstanding. In 2021, 6,692 stock options forfeited. Long-Term Incentive Programs 2017 Long-Term Incentive Plan On April 1, 2017, MorphoSys established another Long-Term Incentive Plan (LTI Plan) for the Management Board and selected employees of the Company (beneficiaries). The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. These criteria are evaluated annually by the Supervisory Board. The performance criteria were based on a mathematical comparison of the absolute and relative performance of the MorphoSys share price against the Nasdaq Biotech Index and the TecDAX Index. Achievement of these criteria was set at 130%. In addition, the Supervisory Board set a “company factor” as 1, which determines the number of performance shares to be issued. Based on these conditions and the set factor, 45,891 performance shares of MorphoSys AG were transferred to the beneficiaries after the four-year vesting period in the period ending October 13, 2021. The Management Board received 4,143 performance shares, and the members of the Executive Committee received 2,030 performance shares. A total of 39,718 performance shares were granted to current and former employees of the company. In 2021, personnel expenses resulting from performance shares under the Company's 2017 LTI Plan amounted to € 265k (2020: € 516k). 2018 Long-Term Incentive Plan On April 1, 2018, MorphoSys established another Long-Term Incentive Plan (LTI Plan) for the Management Board and selected employees of the Company (beneficiaries). The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The grant date was April 1, 2018, and the vesting/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance shares become vested in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The performance criteria can be met annually up to a maximum of 300% and up to 200% for the entire four-year period. If the specified performance criteria are met by less than 0% in one year, no shares will be earned for that year (entitlement). In any case, the maximum payout at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a specific allocation of performance shares under the LTI Plan, however, occurs only at the end of the four-year vesting/performance period. At the end of the four-year waiting period, there is a six-month exercise period during which the Company can transfer the performance shares to the beneficiaries. The beneficiaries can choose the allocation date within this exercise period. If the number of repurchased shares is not sufficient for servicing the LTI Plan, MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.

Notes to the Financial Statements 99 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 In the event of a departure from the Company, the beneficiaries are generally entitled to the performance shares that have vested up to the date of their departure on a pro rata basis. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance shares forfeit without entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, the beneficiary is entitled to performance shares on a pro rata basis. A period of absence is defined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, all performance shares will become fully vested. In this case, the right to receive a specific allocation of performance shares under the LTI Plan occurs only at the end of the four-year vesting period. As of December 31, 2021, 18,577 performance shares are outstanding. In 2021, 794 performance shares forfeited. In 2021, personnel expenses resulting from performance shares under the Company's 2018 LTI Plan amounted to € (206)k (2020: € 551k). 2019 Long-Term Incentive Plan On April 1, 2019, MorphoSys established another Long-Term Incentive Plan (LTI Plan) for the Management Board and selected employees of the Company (beneficiaries). The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The grant date was April 1, 2019, , and the vesting/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance shares become vested in each year of the four-year vesting period. The number of performance shares vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The performance criteria can be met annually up to a maximum of 300% and up to 200% for the entire four-year period. If the specified performance criteria are met by less than 0% in one year, no shares will be earned for that year (entitlement). In any case, the maximum payout at the end of the four-year period is limited by a factor determined by the Group, which generally amounts to 1. However, in justified cases, the Supervisory Board may set this factor freely between 0 and 2, for example, if the level of payment is regarded as unreasonable in view of the general development of the Company. The right to receive a specific allocation of performance shares under the LTI Plan, however, occurs only at the end of the four-year vesting/performance period. At the end of the four-year vesting period, there is a six-month exercise period during which the Company can transfer the performance shares to the beneficiaries. The beneficiaries can choose the allocation date within this exercise period. If the number of repurchased shares is not sufficient for servicing the LTI Plan, MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the fair value of the performance shares on the grant date.

100 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 In the event of a departure from the Company, the beneficiaries are generally entitled to the performance shares that have vested up to the date of their departure on a pro rata basis. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance shares forfeit without entitlement to compensation. If an accumulated period of absence of more than 90 days occurs during the four-year vesting period/performance period, the beneficiary is entitled to performance shares on a pro rata basis. A period of absence is defined as absence due to illness, continued payment of remuneration in the event of illness or a suspended service or employment relationship without continued payment of remuneration. If a change of control occurs during the four-year vesting period, all performance shares will become fully vested. In this case, the right to receive a specific allocation of performance shares under the LTI Plan occurs only at the end of the four-year vesting period. As of December 31, 2021, 19,987 performance shares are outstanding. In 2021, 1,796 performance shares forfeited. In 2021, personnel expenses resulting from performance shares under the Company's 2019 LTI Plan amounted to € (41)k (2020: € 618k). 2020 Performance Share Unit Program On April 1, 2020, MorphoSys established a performance share unit program (PSU program) for the Management Board and certain employees of the Company (beneficiaries). The PSU program is a performance-based program and is paid out in cash subject to the fulfillment of predefined performance criteria. The grant date was April 21, 2020; the vesting period/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance share units become vested in each year of the four-year vesting period. The number of performance share units vested per year is calculated on the basis of the performance criteria of the absolute and relative development of the MorphoSys share price compared to the development of the Nasdaq Biotech Index and the TecDAX Index. The performance criteria can be met each year up to a maximum of 200%. If the defined performance criteria are met by less than 0% in any one year, no performance share units will be earned for that year. However, the right to receive a certain cash settlement from the PSU program does not arise until the end of the four-year vesting period/performance period. After the end of the four-year vesting period, there is a six-month period during which the performance shares can be transferred from the Company to the beneficiaries. MorphoSys reserves the right to settle the PSU program at the end of the vesting period in MorphoSys AG’s own ordinary shares equal to the amount of the performance share units earned. The currently available treasury stock is not sufficient to settle the vested awards. MorphoSys therefore accounts for the plan only as a cash-settled share-based payment. In the event of a departure from the Company, the beneficiaries generally retain the performance share units that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance share units forfeit without entitlement to compensation.

Notes to the Financial Statements 101 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 If an accumulated period of absence of more than 12 months occurs during the four-year vesting period/performance period, 1/48 of the performance share units are forfeited for each month of absence. A period of absence is defined as an absence due to illness or a period of inactive service or employment without continued payment of remuneration. If a change of control occurs during the four-year vesting period, all performance share units will become fully vested. In this case, the right to receive a specific allocation of performance share units under the PSU program occurs only at the end of the four-year vesting period. On June 1, 2020, MorphoSys established another performance share unit program (PSU program) for one member of the Management Board. The terms and conditions were identical to those of the April 1, 2020 program. In March 2021, the terms of the Performance Share Unit Programs (PSU Programs) of April 1, 2020 and June 1, 2020 for the Management Board and certain employees of the Company (beneficiaries) were amended so that the number of Performance Share Units still to be vested for the remaining three years is calculated on the basis of the performance criteria of the absolute performance of the MorphoSys share price and the relative performance of the MorphoSys share price compared to the performance of the EURO STOXX Total Market Pharmaceuticals & Biotechnology Index. Previously, the number of performance share units earned in the first year was calculated on the basis of the performance criteria of the absolute and relative performance of the MorphoSys share price compared to the performance of the Nasdaq Biotech Index and the TecDAX Index. If the predefined performance criteria for the respective period are 100% met, 25% of the performance share units become vested in the first year, and 75% become vested during the remaining three-year vesting period. The modification of the program’s terms concerns the respective remaining vesting periods/performance periods of the programs for the subsequent three years as of April 1, 2021 and June 1, 2021. The approval of the Management Board and certain employees of the Company (beneficiaries) to the modified program terms was obtained by April 17, 2021. The modification of the programs had no material impact on the fair values of the performance shares or on the period over which the personnel expenses are allocated. As of December 31, 2021, 25,779 performance shares are outstanding. In 2021, 1,715 performance shares forfeited. In 2021, personnel expenses resulting from performance shares under the Company's 2020 PSU program amounted to € (308)k (2020: € 337k ). 2021 Performance Share Unit Program On April 1, 2021, MorphoSys established a performance share unit program (PSU program) for the Management Board and certain employees of the Company (beneficiaries). The PSU program is a performance-based program and is paid out in cash subject to the fulfillment of predefined performance criteria. The grant date was April 19, 2021; the vesting period/performance period is four years. If the predefined performance criteria for the respective period are 100% met, 25% of the performance share units become vested in each year of the four-year vesting period. The number of performance share units to be vested is calculated on the basis of the performance criteria of the absolute share price development of the MorphoSys share, the relative development of the MorphoSys share price compared to the EURO STOXX Total Market Pharmaceuticals & Biotechnology Index and an assessment of the employee engagement. The performance criteria can be met each year up to a maximum of 200%. If the defined performance criteria are met by less than 0% in any one year, no performance share units will be earned for that year. However, the

102 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 right to receive a certain cash settlement from the PSU program does not arise until the end of the four-year vesting period/performance period. After the end of the four-year vesting period, there is a six-month period during which the performance shares can be transferred from the Company to the beneficiaries. MorphoSys reserves the right to settle the PSU program at the end of the vesting period in MorphoSys AG’s own ordinary shares equal to the amount of the performance share units earned. The currently available treasury stock is not sufficient to settle the vested awards. MorphoSys therefore accounts for the plan only as a cash-settled share-based payment. In the event of a departure from the Company, the beneficiaries generally retain the performance share units that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all performance share units forfeit without entitlement to compensation. If an accumulated period of absence of more than 12 months occurs during the four-year vesting period/performance period, 1/48 of the performance share units are forfeited for each month of absence. A period of absence is defined as an absence due to illness or a period of inactive service or employment without continued payment of remuneration. If a change of control occurs during the four-year vesting period, all performance share units will become fully vested. In this case, the right to receive a specific allocation of performance share units under the PSU program occurs only at the end of the four-year vesting period. As of April 1, 2021, a total of 122,005 performance share units were granted to beneficiaries, consisting of 54,232 performance share units to the Management Board, 12,340 performance share units to other members of the Executive Committee and 55,433 performance share units to certain employees of the Company who are not members of the Executive Committee. For the calculation of the personnel expenses from share-based compensation, it was assumed for the PSU program 2021 that fifteen beneficiaries would leave the Company during the four-year period. As of December 31, 2021, 111,586 performance shares are outstanding. In 2021, 10,419 performance shares forfeited. October 1, 2021, MorphoSys established another performance share unit program (PSU program) for certain employees of the Company who are not members of the Executive Committee. The terms and conditions were identical to those of the April 1, 2021 program, and a total of 11,209 performance share units were granted. The grant date was October 20, 2021. In 2021, personnel expenses under the Group’s 2021 performance share unit program amounted to € 247k. MorphoSys US Inc. — 2019 Long-Term Incentive Plan On April 1, 2019, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). The LTI Plan is a performance-related share plan and will be paid out in ordinary shares (performance shares) of MorphoSys AG if predefined key performance criteria are achieved. The plan has a term of four years and comprises four one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 25% of the performance shares become vested in each year. The number of shares vested per year is calculated based on key performance criteria of

Notes to the Financial Statements 103 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 MorphoSys US Inc. during the annual performance period. The performance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. After the end of each one-year performance period, there is a six-month period during which the performance shares can be transferred from the Company to the beneficiaries. If the number of repurchased shares is not sufficient for servicing the LTI Plan, MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash in the amount of the performance shares at the end of the vesting period, provided the cash amount does not exceed 200% of the average market price of one share of the Company in the XETRA closing auction on the Frankfurt Stock Exchange during the 30 trading days preceding the grant of the performance shares. In the event of a departure from the Company, the beneficiaries are generally entitled to the performance shares that have vested up to the date of their departure on a pro rata basis. In the event of termination by a beneficiary for good cause, all performance shares will be forfeited without entitlement to compensation. After the end of the second one-year performance period, a target achievement of 77% was determined. Taking this target achievement into account, 2,369 performance shares of MorphoSys AG were transferred to the beneficiaries in the period from April 1, 2021 to October 18, 2021. A target achievement of 77% is assumed for the remaining performance periods. As of December 31, 2021, 2,708 performance shares are outstanding. In 2021, 4,041 performance shares forfeited. The personnel expenses from performance shares from the Company's 2019 LTI Plan will be charged to MorphoSys US Inc. at an arm's length premium. MorphoSys US Inc. — Restricted Stock Unit Plan (RSUP) MorphoSys US Inc. — 2019 Long-Term Incentive Plan On October 1, 2019, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). The program is considered a share-based payment program with settlement in equity instruments and is accounted for accordingly. The LTI Plan is a restricted stock unit plan (RSUP) and is paid out in shares of MorphoSys AG that are to be created from authorized capital provided predefined performance criteria have been fulfilled. The term of the plan is three years and includes three one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 33.3% of the performance shares become vested in each year. The number of performance shares vested per year is calculated based on the key performance criteria of MorphoSys US Inc. and the MorphoSys share price performance during the annual performance period. The performance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. At the end of the total three-year performance period, the corresponding number of shares eventually vested is calculated, and the shares created from authorized capital are transferred from the Company to the beneficiaries. MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash at the end of the performance period, equal to the value of the performance shares granted. If a beneficiary loses his office or terminates his employment with MorphoSys US Inc. prior to the end of a performance period, the beneficiary will generally be entitled to all vested restricted stock units for already

104 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 completed one-year performance periods. All remaining restricted stock units are forfeited without entitlement to compensation. As of December 31, 2021, 6,337 restricted stock units are outstanding. In 2021, 6,380 restricted stock units forfeited. The personnel expenses from the 2019 RSUP of MorphoSys US Inc. will be charged to MorphoSys US Inc. at an arm's length premium. MorphoSys US Inc. — 2020 Long-Term Incentive Plan On April 1, 2020, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). The LTI Plan is a restricted stock unit plan (RSUP) and is paid out in shares of MorphoSys AG that are to be created from authorized capital provided predefined performance criteria have been fulfilled. The term of the plan is three years and includes three one-year performance periods. If the predefined performance criteria for the respective period are 100% met, 33.3% of the performance shares become vested in each year. The number of performance shares vested per year is calculated based on the key performance criteria of MorphoSys US Inc. and the MorphoSys share price performance during the annual performance period. The performance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. At the end of the total three-year performance period, the corresponding number of shares eventually vested is calculated, and the shares created from authorized capital are transferred from the Company to the beneficiaries. MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash at the end of the performance period, equal to the value of the performance shares granted. If a beneficiary loses his office or terminates his employment with MorphoSys US Inc. prior to the end of a performance period, the beneficiary will generally be entitled to all vested restricted stock units for already completed one-year performance periods. All remaining restricted stock units are forfeited without entitlement to compensation. On 1. April 2020 , MorphoSys established an additional Long-Term Incentive Plan in the form of a restricted stock unit plan (RSUP) for certain employees of MorphoSys US Inc. (beneficiaries). Taking a target achievement of 100% into account, 42,307 performance shares of MorphoSys AG were transferred to employees of MorphoSys US Inc. As of December 31, 2021, 20,506 restricted stock units are outstanding. In 2021, 19,264 restricted stock units forfeited. On October 1, 2020, MorphoSys established an additional Long-Term Incentive Plan in the form of a restricted stock unit plan (RSUP) for certain employees of MorphoSys US Inc. (beneficiaries). The terms and conditions were identical to those of the April 1, 2020 program. Taking a target achievement of 100% into account, 7,678 performance shares of MorphoSys AG were transferred to employees of MorphoSys US Inc. As of December 31, 2021, 5,832 restricted stock units are outstanding. In 2021, 1,846 restricted stock units forfeited. The personnel expenses from the 2020 RSUP of MorphoSys US Inc. will be charged to MorphoSys US Inc. at an arm's length premium.

Notes to the Financial Statements 105 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 MorphoSys US Inc. — 2021 Long-Term Incentive Plan On April 1, 2021, MorphoSys AG established a Long-Term Incentive Plan (LTI Plan) for selected employees of MorphoSys US Inc. (beneficiaries). The LTI Plan is a restricted stock unit plan (RSUP) and is paid out in shares of MorphoSys AG that are to be created from authorized capital provided predefined performance criteria have been fulfilled. The term of the plan is 100% met, 33.3% of the performance shares become vested in each year. The number of performance shares vested per year is calculated based on the key performance criteria of MorphoSys US Inc. and the MorphoSys share price performance during the annual performance period. The performance criteria can be met up to a maximum of 125% per year. If less than 0% of the defined performance criteria are met in any one year, no shares will be vested for that year. At the end of the total three-year performance period, the corresponding number of shares eventually vested is calculated, and the shares created from authorized capital are transferred from the Company to the beneficiaries. MorphoSys reserves the right to pay a specific amount of the LTI Plan in cash at the end of the performance period, equal to the value of the performance shares granted. If a beneficiary loses his office or terminates his employment with MorphoSys US Inc. prior to the end of a performance period, the beneficiary will generally be entitled to all vested restricted stock units for already completed one-year performance periods. All remaining restricted stock units are forfeited without entitlement to compensation. As of April 1, 2021, taking a target achievement of 100% into account, 67,724 restricted shares were granted to the beneficiaries of MorphoSys US Inc. As of December 31, 2021, 42,996 restricted stock units are outstanding. In 2021, 24,728 restricted stock units forfeited. On October 1, 2021, MorphoSys established an additional Long-Term Incentive Plan in the form of a restricted stock unit plan (RSUP) for certain employees of MorphoSys US Inc. (beneficiaries). The terms and conditions were identical to those of the April 1, 2021 program, except that the performance criteria can be met up to a maximum of 175% per year. 36,827 restricted shares were granted. For the calculation of the personnel expenses from share-based compensation, it was assumed for the December 31, 2021 LTI Plan that twenty beneficiaries would leave the Company during the three-year period. As of December 31, 2021, 34,335 restricted stock units are outstanding. In 2021 2,492 restricted stock units forfeited. The personnel expenses from the 2021 RSUP of MorphoSys US Inc. will be charged to MorphoSys US Inc. at an arm's length premium. Constellation 2021 Stock Option Plan On October 1, 2021 MorphoSys AG established a stock option plan (SOP) for selected employees of Constellation (beneficiaries). The grant date was October 29, 2021, and the vesting period/performance period is four years. Each stock option grants up to two subscription rights to shares in the Company. The subscription rights vest each year by 25% within the four-year vesting period, provided that the performance criteria specified for the respective period have been 100% fulfilled. The number of subscription rights vested per year is calculated based on the key performance criteria of the absolute and relative MorphoSys share price performance compared to the Nasdaq Biotech Index and the TecDAX Index. The program’s performance criteria can be met annually up to a maximum of 200%. If the share price development falls short of the program’s performance parameters, the target achievement for that year is 0%.

106 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The exercise price, derived from the average market price of the Company’s shares in the XETRA closing auction on the Frankfurt Stock Exchange from the 30 trading days prior to the issue of the stock options, is €44.91. MorphoSys reserves the right to settle the exercise of stock options using either newly created shares from Conditional Capital 2020-I, through the issue of treasury shares, or in cash should the exercise from Conditional Capital 2020-I not be possible. The exercise period is three years after the end of the four-year vesting period/performance period, which is September 30, 2028. In the event of a departure from the Company, the beneficiaries generally retain the stock options that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised stock options forfeit without entitlement to compensation. If a change of control occurs during the four-year vesting period, the stock options will become fully vested. In this case, however, the right to exercise the stock options arises only at the end of the four-year vesting period. As of October 1, 2021, 323,534 stock options were granted to the beneficiaries. For the calculation of the personnel expenses from share-based compensation, it was assumed for the SOP Plan 2021 that 57 beneficiaries would leave the Company during the four-year period. As of December 31, 2021 293,593 performance shares are outstanding. In 2021 29,941 performance shares are forfeited. The personnel expenses from the 2021 SOP Plan of Constellation will be charged to Constellation at an arm's length premium. Tax Provisions As of December 31, 2021, MorphoSys AG recognized tax provisions in the amount of € 330k (December 31, 2020: € 64,898 k). Other Provisions The provisions cover all identifiable risks and uncertain liabilities. They mainly consisted of the recognition of the collaboration and license agreement with Incyte (2021: € 550,515k; 2020: € 527,034k), expenses for external laboratory services (2021: € 49,991k; 2020: € 43,500k), personnel expenses from performance shares from the LTI plans and for the cash settlement of the performance share unit programs (2021: € 3,256k; 2020: € 6,535k), bonus payments (2021: € 9,189k; 2020: € 6,439k), legal advice (2021: € 36k; 2020: € 620k), consulting services (2021: € 1,960k; 2020: € 3,457k), outstanding vacation entitlements (2021: € 814k; 2020: € 935k) and license and inventor compensation (2021: € 2,978k; 2020: € 1,046k). As of December 31, 2020, a provision for milestone payments in connection with the commercialization of tafasitamab, which were highly probable, was recognized in the amount of € 10,187k. This provision has been utilized in 2021.

Notes to the Financial Statements 107 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 As of December 31, 2021, there were provisions of € 2,427k for contracts in connection with expenses from settlement agreements (December 31, 2020: € 4,541k) as well as € 2,549k for onerous contracts in connection with close-out activities for a clinical study that was stopped in 2021. In accordance with the Company's hedging policy, highly probable future cash flows and clearly identifiable foreign currency receivables that are expected to be collected within a 12-month period are reviewed for hedging requirements. As of December 31, 2021 and as of December 31, 2020, there was no forward rate agreement. Collaboration And License Agreement With Incyte MorphoSys AG and Incyte Corporation signed a collaboration and license agreement in 2020 for the further global development and commercialization of MorphoSys’s proprietary anti-CD19 antibody tafasitamab. Under the terms of this agreement, MorphoSys could, among other things, pending on the achievement of certain developmental, regulatory, and commercial milestones, receive milestone payments amounting to up to US$1.1 billion (approximately € 971.2 million). MorphoSys also receives tiered royalties in a mid-teen to mid-twenties percentage of net sales of Monjuvi outside the US. In the US, MorphoSys and Incyte co- commercialize Monjuvi, with MorphoSys being responsible for the commercial relationship with the end customer, which also comprises the deliveries of the drug and the collection of the related cash inflows. The revenues from product sales of Monjuvi are, therefore, recognized by MorphoSys, as it is the principal of the transaction. Incyte and MorphoSys are jointly responsible for the commercialization activities in the US and will equally share any profits and losses (50/50 basis). Outside the US, Incyte has received exclusive commercialization rights, determines the commercialization strategy and is responsible for the commercial relationship with the end customer, including the deliveries of the drug and the collection of the related cash inflows. Therefore, Incyte will recognize all revenues generated from sales of tafasitamab outside the US and will pay royalties to MorphoSys on these sales. As part of the agreement, MorphoSys recorded a provision. This provision represents Incyte's entitlement to future profit and loss sharing on sales of Monjuvi in the US (as MorphoSys will share 50% of these profits with Incyte). The basis for the valuation is the corporate planning and its shared profits and losses thereof in connection with the commercialization activities of MorphoSys and Incyte in the United States for the years ahead. Subsequently, the provision will be compounded, and the interest effect will be recognized in other interest and similar expenses. Cash flows from the equally shared losses and profits are generally recognized directly in equity against the provision and, as soon as they are realized, reported in other assets, if a claim by MorphoSys arises. Differences between actual cash flows from the provision and original projections as well as effects resulting from changes in planning assumptions on the expected net cash flows from the provision are recognized in other interest and similar income or expenses. For the subsequent measurement of the provision, the respective current discount rate calculated on the basis of the provisions of the German Regulation on the Discounting of Provisions is used. MorphoSys and Incyte will also share the development costs for the jointly initiated worldwide and US- specific clinical trials at a ratio of 55% (Incyte) to 45% (MorphoSys). This 45% share of development costs borne by MorphoSys is included in research and development costs. Should MorphoSys provide services in excess of this 45% share, MorphoSys will be entitled to a compensation claim against Incyte, which will qualify as revenue. Related expenses for the provision of the service are recognized as cost of sales. Conversely, MorphoSys has to bear additional research and development expenses if Incyte performs more than 55% of the total clinical trial services. In addition, Incyte will assume 100% of future development costs for clinical trials in countries outside the United States, which are conducted in Incyte’s own responsibility.
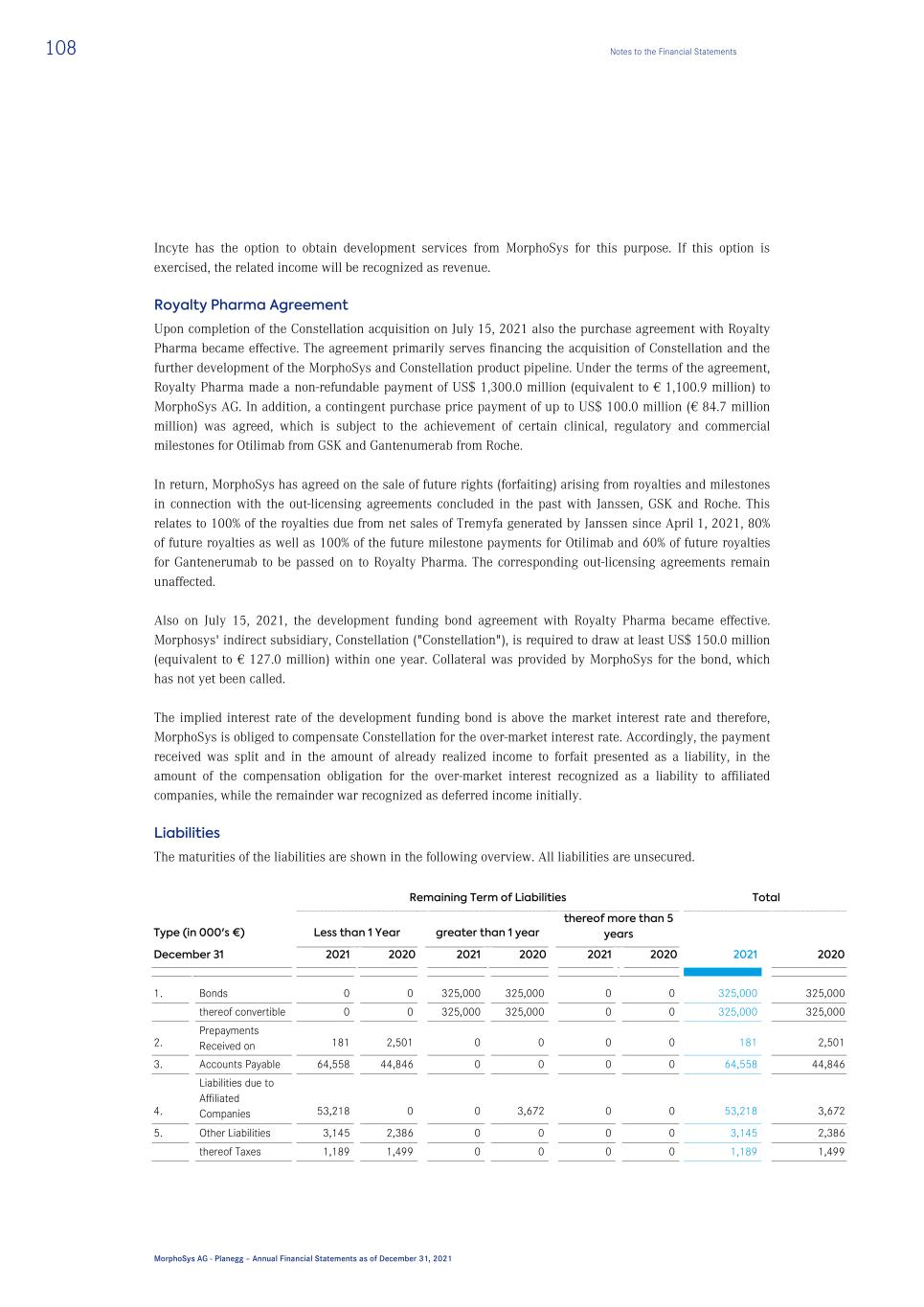
108 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Incyte has the option to obtain development services from MorphoSys for this purpose. If this option is exercised, the related income will be recognized as revenue. Royalty Pharma Agreement Upon completion of the Constellation acquisition on July 15, 2021 also the purchase agreement with Royalty Pharma became effective. The agreement primarily serves financing the acquisition of Constellation and the further development of the MorphoSys and Constellation product pipeline. Under the terms of the agreement, Royalty Pharma made a non-refundable payment of US$ 1,300.0 million (equivalent to € 1,100.9 million) to MorphoSys AG. In addition, a contingent purchase price payment of up to US$ 100.0 million (€ 84.7 million million) was agreed, which is subject to the achievement of certain clinical, regulatory and commercial milestones for Otilimab from GSK and Gantenumerab from Roche. In return, MorphoSys has agreed on the sale of future rights (forfaiting) arising from royalties and milestones in connection with the out-licensing agreements concluded in the past with Janssen, GSK and Roche. This relates to 100% of the royalties due from net sales of Tremyfa generated by Janssen since April 1, 2021, 80% of future royalties as well as 100% of the future milestone payments for Otilimab and 60% of future royalties for Gantenerumab to be passed on to Royalty Pharma. The corresponding out-licensing agreements remain unaffected. Also on July 15, 2021, the development funding bond agreement with Royalty Pharma became effective. Morphosys' indirect subsidiary, Constellation ("Constellation"), is required to draw at least US$ 150.0 million (equivalent to € 127.0 million) within one year. Collateral was provided by MorphoSys for the bond, which has not yet been called. The implied interest rate of the development funding bond is above the market interest rate and therefore, MorphoSys is obliged to compensate Constellation for the over-market interest rate. Accordingly, the payment received was split and in the amount of already realized income to forfait presented as a liability, in the amount of the compensation obligation for the over-market interest recognized as a liability to affiliated companies, while the remainder war recognized as deferred income initially. Liabilities The maturities of the liabilities are shown in the following overview. All liabilities are unsecured. Remaining Term of Liabilities Total Type (in 000's €) Less than 1 Year greater than 1 year thereof more than 5 years December 31 2021 2020 2021 2020 2021 2020 2021 2020 1. Bonds 0 0 325,000 325,000 0 0 325,000 325,000 thereof convertible 0 0 325,000 325,000 0 0 325,000 325,000 2. Prepayments Received on 181 2,501 0 0 0 0 181 2,501 3. Accounts Payable 64,558 44,846 0 0 0 0 64,558 44,846 4. Liabilities due to Affiliated Companies 53,218 0 0 3,672 0 0 53,218 3,672 5. Other Liabilities 3,145 2,386 0 0 0 0 3,145 2,386 thereof Taxes 1,189 1,499 0 0 0 0 1,189 1,499

Notes to the Financial Statements 109 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Bonds Bonds amounted to € 325,000k as of December 31, 2021. The non-subordinated, unsecured convertible bonds placed by MorphoSys AG in 2020 for a nominal amount of € 325,000k , equal to 3,250 bonds with a nominal amount of € 100k each, and maturing on October 16, 2025 amounted as of December 31, 2021 € 325,000k. As of December 31, 2021, the remaining term of the convertible bond is less than 4 years. There was no bond conversion in 2021 and 2020. Prepayments Received On Orders Prepayments received on orders amounted to € 181k as of December 31, 2021 (December 31, 2020: € 2,501) and consisted mainly of payments made in advance by customers for services to be rendered in 2022. Trade Accounts Payable As of December 31, 2021, MorphoSys AG had trade accounts payable of € 64,558k (December 31, 2020: € 44,846k). The year-on-year increase resulted from a higher level of liabilities for external laboratory services. Liabilities Due To Affiliated Companies Liabilities due to affiliated companies amounted after netting with receivables due from affiliated companies to € 53,218k as of December 31, 2021 (December 31, 2020: € 3,672k) and included liabilities due to Constellation in the amount of € 71,667k for the excess interest of the development funding bond agreement with Royalty Pharma (December 31, 2020: € 0k) as well as liabilities due to MorphoSys US Inc. and Constellation from the allocation of share-based remuneration in the amount of € 3,652k (December 31, 2020: € 3,672k). This amount was partly offset by receivables from Constellation in the amount of € 22,101k. Other Liabilities Other liabilities as of December 31, 2021, amounted to € 3,145k (December 31, 2020: € 2,386k) and mainly included liabilities to tax authorities for the deduction and payment of income tax in the amount of € 1,189k (December 31, 2020: € 1,499k), a liability in connection with the transfer of payments to Royalty Pharma in the amount of € 1,492k (December 31, 2020: € 0k) and accumulated interest on the convertible bond in the amount of € 423k (December 31, 2020: € 423k). As of December 31, 2021, other liabilities included € 3k for social security payments (December 31, 2020: € 0k). Deferred Income Deferred income consists of payments received from customers and of the agreement with Royalty Pharma for which a service was not yet rendered. In the years 2021 and 2020, deferred income developed as follows: in 000' € 2021 2020 Opening Balance 115 1,686 Prepayments Received 1,016,571 0 Revenue Recognized through Release of Prepayments in line with Services Performed (27,745) (1,571) Closing Balance 988,941 115

110 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The prepayments received as well as the revenue recognized are mainly related to the forfaiting of future receivables to Royalty Pharma. The deferred income is released over the term of the underlying license agreements, and the release is based on a specific release factor that relates the realized license income in the respective period to the sum of the undiscounted expected license income. Contingent Liabilities As of December 31, 2021 the contingent liabilities from guarantees amounted to 132.4 Mio. €. (December 31, 2020: 0,0 Mio. €) and relate to the development funding bond of Royalty Pharma, in the amount the indirect subsidiary Constellation is obligated to draw until July 15, 2022. To date, the bond has not been drawn. Other Financial Obligations The following overview shows other financial obligations from rental and lease agreements, performance share unit programs, insurance and other services as of December 31, 2021. Other services mainly comprise insurance contracts and other service contracts. in 000' € Rent and Leasing Performance Share Unit Programs Other Total 2022 4,270 0 10,415 14,686 2023 4,222 0 602 4,824 2024 4,192 1,105 0 5,297 2025 4,186 4,000 0 8,186 2026 4,134 0 0 4,134 more 2,809 0 0 2,809 Total 23,814 5,105 11,018 39,936 In addition, future payments may become due from outsourced studies after December 31, 2021. These amounts could be substantially lower or incurred at different times if a study were to be terminated prematurely or delayed. in million € Total 2021 Less than 1 Year 111.7 Between One and Five Years 81.6 More than 5 Years 0.0 Total 193.3 If certain milestones are achieved by MorphoSys (for example, submitting an investigational new drug (IND) application for specific target molecules), this may trigger milestone payments to licensors of up to an aggregate of 236.5 (approximately 208.8) related to regulatory events or the achievement of sales targets. Obligations may arise from enforcing the Company’s patent rights versus third parties. It is also conceivable that competitors may challenge the patents of the MorphoSys Group or that MorphoSys may come to the conclusion that its patents or patent families have been infringed upon by competitors. This could prompt MorphoSys to take legal action against competitors or lead competitors to file counterclaims against MorphoSys. Currently, there are no specific indications such obligations have arisen.

Notes to the Financial Statements 111 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 By letter dated June 10, 2021, MorphoSys was notified by a licensor of the initiation of arbitration proceedings in the United States. The licensor alleges breach of contract and claims damages for the licensor’s argued loss of revenues. Despite the patent expiry in 2018 confirmed by the licensor at the time, this is now disputed and a significantly longer patent term is assumed. Taking into account the associated legal and consulting costs, the potential amount in dispute in the proceedings is in the low double-digit Euro million range and also includes a currently unspecified share of royalty income. A decision by the arbitration court is expected in the fourth quarter 2022. Based on the current assessment of the facts, MorphoSys believes that the arguments presented are unfounded and that the arbitration will likely be decided in MorphoSys’ favor. There was no arbitration decision and no other new developments in the third and fourth quarter of 2021. Since the 2019 financial year, a master loan agreement with an annual interest rate of 4.65% has been in place between MorphoSys AG and its wholly owned subsidiary MorphoSys US Inc. for a potential total volume of up to € 166.0 million, of which € 106.8 million had been utilized by December 31, 2021 (December 31, 2020: € 95.0 million). For the development funding bond agreement with Royalty Pharma, for which Constellation is required to draw at least US$ 150.0 million (equivalent to € 127.0 million) until July 15, 2022, collateral was provided by MorphoSys. The bond has not yet been called.

112 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Notes to the Statement of Income Revenues Revenues in the 2021 financial year amounted to € 128,144k (2020: € 252,096k). In the 2021 financial year, the majority of external revenues were generated from the antibody collaborations and license agreements with Janssen, Incyte and GSK (2021: € 95,955k, 2020: € 232,349k from Incyte, Janssen and I-Mab Biopharma). This includes the release of deferred revenue of € 27,702k from Royalty Pharma from the sale of rights (forfaiting) arising from royalties and milestones in connection with the out-licensing agreements concluded in the past. The major portion of the decrease resulted from the revenues in 2020 from the collaboration and license agreement with Incyte in the amount of € 183,549k. Revenues from supply relationships with affiliated companies in the financial year amounted to € 22,057k (2020: € 13,774k). Revenues from milestones from GSK amounted to € 16,000k and revenues from royalties on net sales of Tremfya amounted to € 54,745k (2020: € 42,468k). Of total revenues, € 104,384k (2020: € 242,985k) was attributed to biotechnology and pharmaceutical companies and non-profit organizations based in North America and revenues in other European countries and Asia (excluding Germany) amounted to € 23,328k (2020: € 8,558k). Domestic revenues mainly resulted from staff canteen and amounted to € 432k (2020: € 453k). Cost Of Sales The cost of sales of € 33,330k (2020: € 14,390k) consisted of acquisition and production costs for inventories which have been recognized as an expense. These comprised costs for external services of € 438k (2020: € 791k), personnel costs of € 11,606k (2020: € 12,044k), costs related to intangible assets of € 7,409k (2020: € 2,251k), cost of materials of € 13,844k (2020: € (740)k), infrastructure costs of € 0k (2020: € 6,734k) and other costs of € 33k (2020: € 44k). The increase is mainly due to the reversal of impairment of inventories upon market approval of Monjuvi in the amount of € 13,271k in 2020 as well as increased regular amortization of acquired licenses. Due to the now separately presented research and development costs, cost of sales were reduced by € 126,847k compared prior years reported value of € 141,237k. Research & Development Research and development expenses of € 177,736k (2020: € 126,847k) included acquisition and production costs for inventories and research and development costs recognized as an expense. These comprised costs for external services of € 118,411k (2020: € 75,890k), personnel costs of € 39,527k (2020: € 30,855k), costs related to intangible assets of € 5,422k (2020: € 5,516k), cost of materials of € 2,410k (2020: € 3,123k), infrastructure costs of € 8,729k (2020: € 9,070k) and other costs of € 3,239k (2020: € 2,393k). Costs for external services increased mainly due to higher expenses for external laboratory services in connection with the research and development of tafasitamab. The increase in personnel costs was mainly due to higher higher salary expenses due to an increase of employee numbers (see also the section "Personnel expenses"). In 2021, no impairment losses were recognized for licenses for concessions, industrial property rights and similar rights and assets (2020: € 2,024k). Selling Expenses Selling expenses of € 69,821k (2020: € 41,864k) consisted mainly of personnel costs in the amount of € 38,992k (2020: € 23,377k), costs for external services of € 29,036k (2020: € 17,165k) and other costs of € 739k (2020: € 562k).

Notes to the Financial Statements 113 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 General Administration Expenses General and administrative expenses of € 36,858k (2020: € 41,192k) contained primarily personnel costs of € 17,030k (2020: € 28,150k), costs for external services of € 13,702k (2020: € 9,556k), infrastructure of € 4,295k (2020: € 2,350k) and other costs of € 1,157k (2020: € 992k). Personnel Expenses Personnel expenses of € 107,154k (2020: € 95,583k) consisted of wages and salaries of € 97,630k (2020: € 79,428k), social security contributions of € 6,004k (2020: € 5,305k), pension costs of € 1,137k (2020: 1,078k) and other costs of € 2,383k (2020: 7,030k). In 2021, other personnel expenses mainly included costs related to severance payments. The increase in personnel expenses was driven mainly by higher salary expenses (€ 18,472k) as a result of the average headcount. Although MorphoSys AG executes the taxation of the non-cash benefits for active employees from the allocation and exercise of share-based remuneration, the employees are obliged to refund MorphoSys for this tax payment. In order to technically execute this taxation over the payroll, the basis for the assessment must be recorded under personnel expenses. For accounting purposes, this expense is offset by other operating income (see "Other Operating Income"). In 2021, this amount was € 1,161k (2020: € 8,708k). The decrease in the assessment basis in 2021 was due to the lower volume of transactions versus the prior year. Material Expenses The cost of materials of € 16,368k (2020: € 2,508k) related mainly to expenses for the production of finished products (Monjuvi) and the purchase of raw materials and supplies of € 13,101k (2020: € -741k). The cost of materials in 2021 and 2020 did not include any purchased services. Other Operating Income Other operating income amounted to € 38,581k, compared with € 30,632k in 2020. This amount mainly included effects from foreign currency gains in the amount of € 25,006k (2020: € 14,888k) and effects from refunded taxes paid as well as for the correction of the assessment base for the taxation of non-cash benefits (see the explanations under "Personnel expenses") in the amount of € 1,479k (2020: € 9,147k). Other operating income also included income related to prior periods from the correction of effects connected to share-based compensation of € 1,236k (2021: € 0k) and the reversal of provisions recognized in the previous year of € 10,853k (2020: € 3,758k). Other Operating Expenses Other operating expenses amounted to € 13,197k, compared with € 47,294k in 2020. The main reasons for the decrease were lower losses from foreign currencies (2021: € 9,132k; 2020: € 30,896k), lower losses on forward exchange contracts (forward rate agreements) in the amount of € 3,496k (2020: € 4,950k) as well as the discontinuation of losses from the sale of total shares of affiliated companies (2021: € 0k; 2020 € 11,057k). Other Interest And Similar Income This line item amounting to € 30,892k (2020: € 46,634k) included primarily effects from the subsequent valuation of the provision associated with the collaboration and license agreement with Incyte amounting to € 24,958k (2020: € 41,809), interest income from affiliated companies amounting to € 4,691k (2020: € 3,590k), from bank balances and financial investments classified as other assets in the amount of € 633k

114 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 (2020: € 1,220k) and from the discounting of a non-current provision for personnel expenses from performance shares under the LTI Plan in the amount of € 3k (2020: € 15k). Losses (Income) From Other Securities And Loans Presented Under Financial Assets In the financial year, income from other securities and loans held as financial assets in the financial year amounted to € 1,656k (2020: € 903k) comprised realized income from the sale of marketable securities and bonds. In 2021, € 748k losses from other securities and loans held as financial assets were accounted for, whereas in 2020, losses in the amount of € 14,467k comprised unrealized losses from measurement and realized losses from the sale of marketable securities and bonds. Impairment of Financial Assets and Securities held as Current Assets In 2021, the shares in MorphoSys US Inc. was impaired in the amount of € 128,127k to reflect the reduced fair value. MorphoSys decided to focus its research efforts on the most advanced discovery and technology programs and also to centralize all laboratory activities at its German research hub in Planegg, Germany. Consequently, all US-based activities relating to discovery biology and drug discovery departments were abandoned. Therefore, any early pipeline projects in the indirect subsidiary Constellation Pharmaceuticals, Inc. cannot be realized anymore and the expected cash flows from these projects will not materialize accordingly. In 2020, an impairment of € 359k was accounted for the Company's shares in adivo GmbH. Expenses from Contribution Agreements In 2021 the expenses incurred are related to a contribution for operating costs to the affiliated company MorphoSys US Inc. totaling € 30,164k (2020: € 65,737k). Other Interest And Similar Expenses The interest expense of € 21,098k (2020: € 21,934k) mainly included expenses from interest on the nominal value of convertible bonds in the amount of € 2,031k (2020: € 1,903k), effects from discounting the provision associated with the collaboration and license agreement with Incyte in the amount € 16,648k (2020: € 13,396k) as well as interest expenses of financial investments classified as other assets in the amount of € € 2,403k (2020: € 1,550k). Taxes on Income After a tax expense of € 64,803k in 2020, a tax income of € 1,325k was recognized in 2021, due to the losses incurred in the period and relating to a loss carry back. Differences between commercial and tax regulations led to the recognition of temporary differences in the balance sheet of MorphoSys AG, based on a tax rate of 26.675%. The Company has elected to offset the deferred tax assets and liabilities. The deferred differences existing on December 31, 2021 result from temporary differences, and would be the basis for recognizing deferred tax assets. The differences are primarily related to the different recognition of provisions, predominantly from the collaboration and license agreement with Incyte.
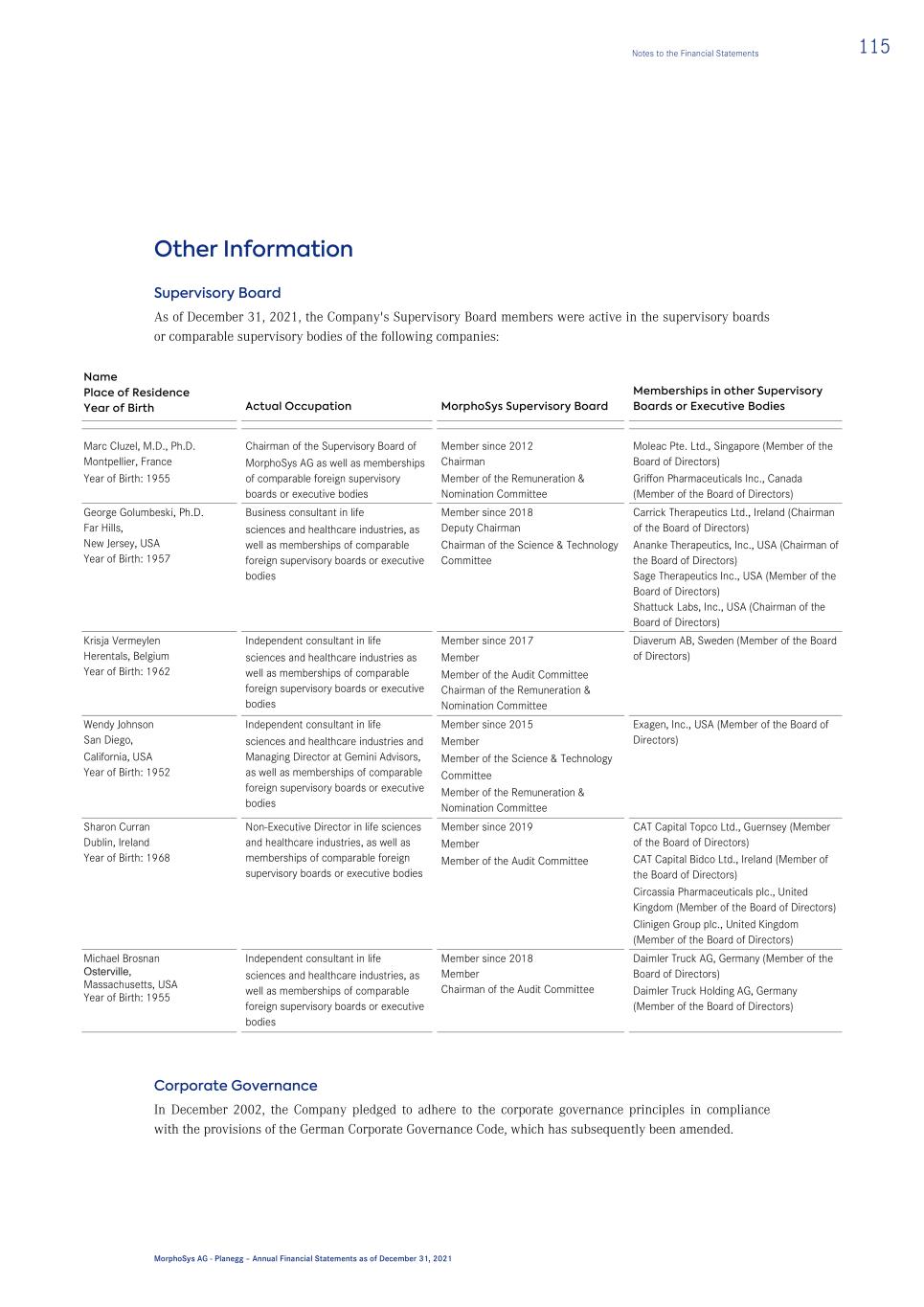
Notes to the Financial Statements 115 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Other Information Supervisory Board As of December 31, 2021, the Company's Supervisory Board members were active in the supervisory boards or comparable supervisory bodies of the following companies: Name Place of Residence Year of Birth Actual Occupation MorphoSys Supervisory Board Memberships in other Supervisory Boards or Executive Bodies Marc Cluzel, M.D., Ph.D. Montpellier, France Year of Birth: 1955 Chairman of the Supervisory Board of MorphoSys AG as well as memberships of comparable foreign supervisory boards or executive bodies Member since 2012 Chairman Member of the Remuneration & Nomination Committee Moleac Pte. Ltd., Singapore (Member of the Board of Directors) Griffon Pharmaceuticals Inc., Canada (Member of the Board of Directors) George Golumbeski, Ph.D. Far Hills, New Jersey, USA Year of Birth: 1957 Business consultant in life sciences and healthcare industries, as well as memberships of comparable foreign supervisory boards or executive bodies Member since 2018 Deputy Chairman Chairman of the Science & Technology Committee Carrick Therapeutics Ltd., Ireland (Chairman of the Board of Directors) Ananke Therapeutics, Inc., USA (Chairman of the Board of Directors) Sage Therapeutics Inc., USA (Member of the Board of Directors) Shattuck Labs, Inc., USA (Chairman of the Board of Directors) Krisja Vermeylen Herentals, Belgium Year of Birth: 1962 Independent consultant in life sciences and healthcare industries as well as memberships of comparable foreign supervisory boards or executive bodies Member since 2017 Member Member of the Audit Committee Chairman of the Remuneration & Nomination Committee Diaverum AB, Sweden (Member of the Board of Directors) Wendy Johnson San Diego, California, USA Year of Birth: 1952 Independent consultant in life sciences and healthcare industries and Managing Director at Gemini Advisors, as well as memberships of comparable foreign supervisory boards or executive bodies Member since 2015 Member Member of the Science & Technology Committee Member of the Remuneration & Nomination Committee Exagen, Inc., USA (Member of the Board of Directors) Sharon Curran Dublin, Ireland Year of Birth: 1968 Non-Executive Director in life sciences and healthcare industries, as well as memberships of comparable foreign supervisory boards or executive bodies Member since 2019 Member Member of the Audit Committee CAT Capital Topco Ltd., Guernsey (Member of the Board of Directors) CAT Capital Bidco Ltd., Ireland (Member of the Board of Directors) Circassia Pharmaceuticals plc., United Kingdom (Member of the Board of Directors) Clinigen Group plc., United Kingdom (Member of the Board of Directors) Michael Brosnan Osterville, Massachusetts, USA Year of Birth: 1955 Independent consultant in life sciences and healthcare industries, as well as memberships of comparable foreign supervisory boards or executive bodies Member since 2018 Member Chairman of the Audit Committee Daimler Truck AG, Germany (Member of the Board of Directors) Daimler Truck Holding AG, Germany (Member of the Board of Directors) Corporate Governance In December 2002, the Company pledged to adhere to the corporate governance principles in compliance with the provisions of the German Corporate Governance Code, which has subsequently been amended.

116 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 On November 29, 2021, the Company published the Declaration of Conformity of the Management Board and Supervisory Board pursuant to Section 161 AktG and made it permanently available to its shareholders. This declaration can be found on the Company's website (www.morphosys.com). Management Board Jean-Paul Kress, M.D., physician, Boston, MA, USA (Chief Executive Officer) and chairman of the Board of Directors of Erytech Pharma SA, Lyon, France (a publicly listed company) Malte Peters, M.D., physician, Munich, Germany (Chief Research and Development Officer) and member of the Board of Directors of Tango Therapeutics, Cambridge, MA, USA (a publicly listed company) Sung Lee, Master of Business Taxation, Munich, Germany (Chief Financial Officer from February 2, 2021) Roland Wandeler, Ph.D., chemical engineer, Westlake Village, California, USA (Chief Operating Officer until the end of December 31, 2021) Total Remuneration of the Management Board And Supervisory Board The Supervisory Board of MorphoSys AG does not hold any stock options or performance shares. The remuneration system for the Management Board is intended to provide sustainable, results-oriented corporate governance. The Management Board’s total remuneration consists of several components, including fixed compensation, an annual cash bonus that is dependent upon the achievement of corporate targets (short-term incentives – STI), variable compensation components with long-term incentives (LTI) and other remuneration components. Variable remuneration components with long-term incentive consist of long-term incentive plans (LTI Plan) from previous years, stock option and performance share plans from previous years, and a performance share unit program and a stock option plan from the current year. The members of the Management Board additionally receive fringe benefits in the form of benefits in kind, essentially consisting of a company car and insurance premiums. All total remuneration packages are reviewed annually by the Remuneration and Nomination Committee and compared to an annual Management Board remuneration analysis to check the scope and appropriateness of the remuneration packages. The amount of remuneration paid to members of the Management Board is based largely on the duties of the respective Management Board member, the financial situation and the performance and business outlook for the Company versus its competition. All resolutions on adjustments to the overall remuneration packages are passed by the plenum of the Supervisory Board. The Management Board’s total remuneration package and the index-linked pension contracts were thoroughly reviewed and then adjusted by the Supervisory Board in 2021. If a Management Board member’s service contract terminates due to death, the member’s spouse or life partner is entitled to the fixed monthly salary for the month of death and the 12 months thereafter. In the event of a change of control, Management Board members are entitled to exercise their extraordinary right to terminate their service contracts and receive any outstanding fixed salary and the annual bonus for the remainder of the agreed contract period, but at least 200% of the annual gross fixed salary and the annual bonus. Moreover, in such a case, all stock options, performance share units and performance shares granted will become vested immediately and can be exercised after the expiration of the statutory vesting periods. A change of control has occurred when (i) MorphoSys transfers assets or a substantial portion of its assets to unaffiliated third parties, (ii) MorphoSys merges with an unaffiliated company, (iii) an agreement pursuant to Section 291 AktG is entered into with MorphoSys as a dependent company, MorphoSys is integrated under Section 319 AktG or (iv) a shareholder or third party holds 30% or more of MorphoSys’s voting rights.
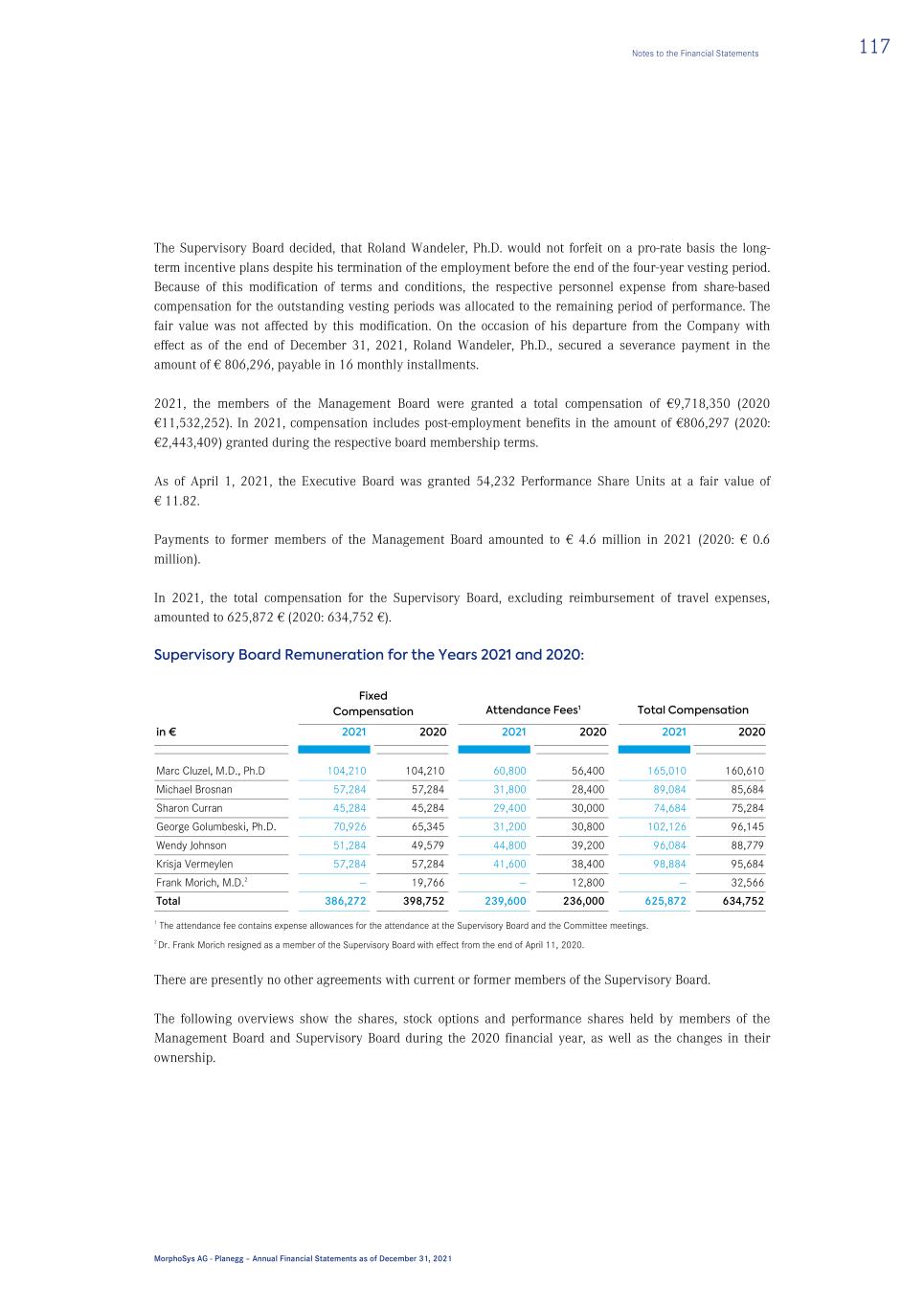
Notes to the Financial Statements 117 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 The Supervisory Board decided, that Roland Wandeler, Ph.D. would not forfeit on a pro-rate basis the long- term incentive plans despite his termination of the employment before the end of the four-year vesting period. Because of this modification of terms and conditions, the respective personnel expense from share-based compensation for the outstanding vesting periods was allocated to the remaining period of performance. The fair value was not affected by this modification. On the occasion of his departure from the Company with effect as of the end of December 31, 2021, Roland Wandeler, Ph.D., secured a severance payment in the amount of € 806,296, payable in 16 monthly installments. 2021, the members of the Management Board were granted a total compensation of €9,718,350 (2020 €11,532,252). In 2021, compensation includes post-employment benefits in the amount of €806,297 (2020: €2,443,409) granted during the respective board membership terms. As of April 1, 2021, the Executive Board was granted 54,232 Performance Share Units at a fair value of € 11.82. Payments to former members of the Management Board amounted to € 4.6 million in 2021 (2020: € 0.6 million). In 2021, the total compensation for the Supervisory Board, excluding reimbursement of travel expenses, amounted to 625,872 € (2020: 634,752 €). Supervisory Board Remuneration for the Years 2021 and 2020: Fixed Compensation Attendance Fees1 Total Compensation in € 2021 2020 2021 2020 2021 2020 Marc Cluzel, M.D., Ph.D 104,210 104,210 60,800 56,400 165,010 160,610 Michael Brosnan 57,284 57,284 31,800 28,400 89,084 85,684 Sharon Curran 45,284 45,284 29,400 30,000 74,684 75,284 George Golumbeski, Ph.D. 70,926 65,345 31,200 30,800 102,126 96,145 Wendy Johnson 51,284 49,579 44,800 39,200 96,084 88,779 Krisja Vermeylen 57,284 57,284 41,600 38,400 98,884 95,684 Frank Morich, M.D.2 — 19,766 — 12,800 — 32,566 Total 386,272 398,752 239,600 236,000 625,872 634,752 1 The attendance fee contains expense allowances for the attendance at the Supervisory Board and the Committee meetings. 2 Dr. Frank Morich resigned as a member of the Supervisory Board with effect from the end of April 11, 2020. There are presently no other agreements with current or former members of the Supervisory Board. The following overviews show the shares, stock options and performance shares held by members of the Management Board and Supervisory Board during the 2020 financial year, as well as the changes in their ownership.

118 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Shares 01/01/2021 Additions Sales 12/31/2021 Management Board Jean-Paul Kress, M.D. 0 0 0 0 Sung Lee1 — 2,250 0 2,250 Malte Peters, M.D. 3,313 4,143 0 7,456 Roland Wandeler, Ph.D. 2 0 0 0 0 Total 3,313 6,393 0 9,706 Supervisory Board Marc Cluzel, M.D., Ph.D 750 250 0 1,000 Michael Brosnan 0 5,000 0 5,000 Sharon Curran 0 0 0 0 George Golumbeski, Ph.D. 0 0 0 0 Wendy Johnson 500 63 0 563 Krisja Vermeylen 350 650 0 1,000 Total 1,600 5,963 0 7,563 Stock Options 01/01/2021 Additions Forfeitures Exercises 12/31/2021 Management Board Jean-Paul Kress, M.D. 81,989 0 0 0 81,989 Sung Lee1 — 0 0 0 0 Malte Peters, M.D. 33,110 0 0 0 33,110 Roland Wandeler, Ph.D. 2 0 0 0 0 0 Total 115,099 0 0 0 115,099 Performance Shares 01/01/2021 Additions Adjustment due to Performance Criteria3 Forfeitures Allocations4 12/31/2021 Management Board Jean-Paul Kress, M.D. 0 0 0 0 0 0 Sung Lee1 0 0 0 0 0 0 Malte Peters, M.D. 9,047 0 (1,799) 0 (4,143) 3,105 Roland Wandeler, Ph.D. 2 0 0 0 0 0 0 Total 9,047 0 (1,799) 0 (4,143) 3,105 1Sung Lee joined the Management Board of MorphoSys AG effective February 2, 2021. 2Roland Wandeler, Ph.D., resigned as a member of the Management Board with effect from the end of December 31, 2021. 3Adjustment due to established performance criteria. For performance criteria that have not yet been met, a target achievement of 100% is assumed. 4 Allocations are made as soon as performance shares are transferred within the six-month exercise period after the end of the four-year waiting period. The MorphoSys AG Supervisory Board members do not hold any stock options or performance shares.

Notes to the Financial Statements 119 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Compensation of the Auditor At the Company’s Annual General Meeting in May 2021, PricewaterhouseCoopers GmbH Wirtschaftsprüfungsgesellschaft (PwC GmbH), Munich, was appointed as the auditor. The Supervisory Board engaged PwC GmbH to audit the financial statements. The table below shows the total fees PwC GmbH received.2021 financial year. in 000' € 2021 2020 Audit Fees 2,141 1,561 Fees for Other Assurance Services 116 70 Tax Service Fees 0 0 Other Fees for Other Services 2 2 Total 2,258 1,633 The other assurance services comprised fees in connection with the non-financial group report as well as the audit of the content of the remuneration report. Human Resources As of December 31, 2021, MorphoSys AG engaged a total of 455 employees (December 31, 2020: 464) in addition to the 3 Management Board members and 12 trainees (December 31, 2020: 3 Management Board members and 11 trainees). Of these 455 employees, 7 were employed in production, 340 in research and development, 21 in sales and 87 in general and administration (December 31, 2020: 338 in R&D and 126 in sales, general and administration). The average number of employees in the 2021 financial year was 456 (2020: 430). Of this number, a total 7 persons were employed in production, 23 in research and development, 23 in selling and 90 in general and administration in 2021. Dividends The net loss in 2021 was offset against the prior year's accumulated deficit, resulting in an accumulated deficit as of December 31, 2021. In line with the standard practice in the biotechnology industry, MorphoSys does not expect to pay a dividend in the foreseeable future. The majority of the Company's potential future profit is expected to be reinvested in the operating business, particularly in the area of proprietary drug development, in order to create additional shareholder value and to take advantage of growth opportunities.

120 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Mandatory disclosures in accordance with the German Securities Trading ACT (WpHG) The Company published the following notifications of shareholdings that require reporting in accordance with Section 33 (1) of the German Securities Trading Act (WpHG) (status as of December 31, 2021): BAILLIE GIFFORD & CO, ON MARCH 23, 2020 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights Other reason: voluntary group notification due to crossing a threshold on subsidiary level 3. Details of person subject to the notification obligation Baillie Gifford & Co, Edinburgh, UK 5. Date on which threshold was crossed or 03/16/2020 6. Total position New % of voting rights attached to shares (total of 7.a.) 6.23% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 6.23% Total number of voting rights pursuant to Sec. 41 WpHG 32890046 Previous notification % of voting rights attached to shares (total of 7.a.) 6.26% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 6.26% 7. Details on total position a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolut – indirect (Sec. 34 WpHG) 2048414 In % - indirect (Sec. 34 WpHG) 6.23% Total - Absolut 2048414 Total - in % 6.23% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more Baillie Gifford & Co % Baillie Gifford Overseas Limited 4.9996% FMR LLC, ON MAY 5, 2020 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights

Notes to the Financial Statements 121 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 3. Details of person subject to the notification obligation FMR LLC, Wilmington, Delaware, USA 5. Date on which threshold was crossed or reached 04/30/2020 6. Total position New % of voting rights attached to shares (total of 7.a.) 2.82% % of voting rights through instruments (total of 7.b.1+7.b.2) 0,10% Total of both in % (7.a.+7.b.) 2.92% Total number of voting rights pursuant to Sec. 41 WpHG 32890046 Previous notification % of voting rights attached to shares (total of 7.a.) 3.99% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.15% Total of both in % (7.a.+7.b.) 4.14% 7. Details on total position a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolut – indirect (Sec. 34 WpHG) 927821 In % - indirect (Sec. 34 WpHG) 2.82% Total - Absolut 927821 Total - in % 2.82% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG Type of instrument Lent Securities (right to recall) Total Voting rights absolut 33875 Total Voting rights in % 0.10 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more FMR LLC % Fidelity Management & Research Company % FMR LLC % FIAM Holdings LLC % Fidelity Institutional Asset Management Trust Company % FMR LLC % FIAM Holdings LLC % FIAM LLC % FMR LLC % Fidelity Advisory Holdings LLC % Strategic Advisers LLC. %

122 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 MINISTRY OF FINANCE ON BEHALF OF THE STATE OF NORWAY, ON JUNE 25, 2020 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation Ministry of Finance on behalf of the State of Norway, Oslo, Norway 5. Date on which threshold was crossed or reached 06/23/2020 6. Total position New % of voting rights attached to shares (total of 7.a.) 2.62% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.49% Total of both in % (7.a.+7.b.) 3.10% Total number of voting rights pursuant to Sec. 41 WpHG 32890046 Previous notification % of voting rights attached to shares (total of 7.a.) 3.09% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.49% Total of both in % (7.a.+7.b.) 3.58% 7. Details on total position a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolut – indirect (Sec. 34 WpHG) 860304 In % - indirect (Sec. 34 WpHG) 2.62% Total - Absolut 860304 Total - in % 2.62% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG Type of instrument Shares on Loan (right to recall) Total Voting rights absolute 106398 Total Voting rights in % 0.32% b.2. Instruments according to Sec. 38 (1) no. 2 WpHG Type of instrument Contract for Difference Cash or physical settlement Cash Total - Voting rights absolut 54084 Total Voting rights in % 0.16% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more State of Norway % Norges Bank %

Notes to the Financial Statements 123 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 AIM INTERNATIONAL MUTUAL FUNDS (INVESCO MUTUAL FUNDS), ON OCTOBER 28, 2020 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation AIM INTERNATIONAL MUTUAL FUNDS (INVESCO INTERNATIONAL MUTUAL FUNDS), Wilmington, Delaware, USA 5. Date on which threshold was crossed or reached 10/23/2020 6. Total position New % of voting rights attached to shares (total of 7.a.) 2.88% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 2.88% Total number of voting rights pursuant to Sec. 41 WpHG 32890046 Previous notification % of voting rights attached to shares (total of 7.a.) 4.92% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 4.92% 7. Details on total position a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolut – indirect (Sec. 34 WpHG) 947139 In % - indirect (Sec. 34 WpHG) 2.88% Total - Absolut 947139 Total - in % 2.88% 8. Information in relation to the person subject of the notification obligation Person subject to the notification obligation is not controlled nor does it control any other undertaking(s) that directly or indirectly hold(s) an interest in the (underlying) issuer (1.). T. ROWE PRICE INTERNATIONAL FUNDS, INC., ON April 19, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of instruments 3. Details of person subject to the notification obligation T. Rowe Price International Funds, Inc., Baltimore, Maryland, United States of America 5. Date on which threshold was crossed or reached 04/13/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2.57%

124 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 % of voting rights through instruments (total of 7.b.1+7.b.2) 0.96% Total of both in % (7.a.+7.b.) 3.53% Total number of voting rights pursuant to Sec. 41 WpHG 32890046 Previous notification % of voting rights attached to shares (total of 7.a.) 3.01% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 3.01% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 843705 In % - indirect (Sec. 34 WpHG) 2.57% Total - Absolute 843705 Total - in % 2.57% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG lSIN DE0006632003 Type of instrument Shares on loan Total Voting rights absolute 317289 Total Voting rights in % 0.96% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more T. Rowe Price International Funds, Inc. % -T. Rowe Price International Stock Fund % - T. Rowe Price International Funds, Inc. % -T. Rowe Price International Discovery Fund % - T. Rowe Price International Funds, Inc. % -T. Rowe Price European Stock Fund %

Notes to the Financial Statements 125 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 T. ROWE PRICE GROUP, INC., ON JUNE 07, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation T. Rowe Price Group, Inc., Baltimore, Maryland, United States of America 5. Date on which threshold was crossed or reached 06/01/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 4.05% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.81% Total of both in % (7.a.+7.b.) 4.86% Total number of voting rights pursuant to Sec. 41 WpHG 32890689 Previous notification % of voting rights attached to shares (total of 7.a.) 4.71% % of voting rights through instruments (total of 7.b.1+7.b.2) 1.54% Total of both in % (7.a.+7.b.) 6.25% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 1332425 In % - indirect (Sec. 34 WpHG) 4.05% Total - Absolute 1332425 Total - in % 4.05% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG lSIN DE0006632003 Type of instrument Shares on loan Total Voting rights absolute 267333 Total Voting rights in % 0.81% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights (if at least 3% or more) T. Rowe Price Group, Inc. % T. Rowe Price Associates, Inc. % T. Rowe Price International, Ltd %

126 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 INVESCO LTD., ON JULY 21, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation Invesco Ltd., Hamilton, Bermuda 5. Date on which threshold was crossed or reached 03/29/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2.98% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 2.98% Total number of voting rights pursuant to Sec. 41 WpHG 32890689 Previous notification % of voting rights attached to shares (total of 7.a.) 3.01% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 3.01% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 979174 In % - indirect (Sec. 34 WpHG) 2.98% Total - Absolute 979174 Total - in % 2.98% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights (if at least 3% or more) -Invesco Ltd. % -Invesco UK Limited % -Invesco Asset Management Limited % - -Invesco Ltd. % -Invesco Holding Company Limited % -Invesco Holding Company (US), Inc. % -Oppenheimer Acquisition Corporation % -OppenheimerFunds, Inc. % -Invesco Group Services, Inc. % -Invesco Capital Management LLC % - -Invesco Ltd. %

Notes to the Financial Statements 127 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 -Invesco Holding Company Limited % -Invesco Holding Company (US), Inc. % -Oppenheimer Acquisition Corporation % -OppenheimerFunds, Inc. % -Invesco Group Services, Inc. % -Invesco Advisers, Inc. % - -Invesco Ltd. % -Invesco UK Limited % -Invesco International Holdings Limited % -Invesco Asset Management Deutschland GmbH %

128 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 PABLO LEGORRETA (ROYALTY PHARMA INVESTMENTS 2019 ICAV), On August 2, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation Pablo Legorreta, Date of birth: 10/30/1963 4. Name(s) of shareholder(s) Royalty Pharma Investments 2019 ICAV 5. Date on which threshold was crossed or reached 07/29/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 3.91% % of voting rights through instruments (total of 7.b.1+7.b.2) 0,00% Total of both in % (7.a.+7.b.) 3.91% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) n/a % of voting rights through instruments (total of 7.b.1+7.b.2) n/a Total of both in % (7.a.+7.b.) n/a 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 1337552 In % - indirect (Sec. 34 WpHG) 3.91% Total - Absolute 1337552 Total - in % 3.91% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more Pablo Legorreta % RP Management, LLC 3.91%

Notes to the Financial Statements 129 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 ROYALTY PHARMA PLC ((ROYALTY PHARMA INVESTMENTS 2019 ICAV), ON August 2, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation Royalty Pharma PLC, Bristol, United Kingdom of Great Britain and Nothern Ireland 4. Name(s) of shareholder(s) Royalty Pharma Investments 2019 ICAV 5. Date on which threshold was crossed or reached 07/29/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 3.91% % of voting rights through instruments (total of 7.b.1+7.b.2) 0,00% Total of both in % (7.a.+7.b.) 3.91% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) n/a % of voting rights through instruments (total of 7.b.1+7.b.2) n/a Total of both in % (7.a.+7.b.) n/a 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 1337552 In % - indirect (Sec. 34 WpHG) 3.91% Total - Absolute 1337552 Total - in % 3.91% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more Royalty Pharma PLC % Royalty Pharma Holdings Ltd. % Royalty Pharma Investments 2019 ICAV 3.91%

130 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 ARTISAN PARTNERS FUNDS, INC., ON SEPTEMBER 20, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation Artisan Partners Funds, Inc., Madison, Wisconsin, United States of America 5. Date on which threshold was crossed or reached 09/15/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2.93% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 2.93% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 3.02% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 3.02% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 1003630 In % - indirect (Sec. 34 WpHG) 2.93% Total - Absolute 1003630 Total - in % 2.93% 8. Information in relation to the person subject of the notification obligation Person subject to the notification obligation (3.) is not controlled nor does it control any other undertaking(s) holding directly or indirectly an interest in the (underlying) issuer (1.).

Notes to the Financial Statements 131 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 ARTISAN PARTNERS ASSET MANAGEMENT INC., ON SEPTEMBER 23, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation Artisan Partners Asset Management Inc., Wilmington, Delaware, United States of America 5. Date on which threshold was crossed or reached 09/20/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2.95% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 2.95% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 3.04% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.00% Total of both in % (7.a.+7.b.) 3.04% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 1010913 In % - indirect (Sec. 34 WpHG) 2.95% Total - Absolute 1010913 Total - in % 2.95% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights (if at least 3% or more) Artisan Partners Asset Management Inc. % Artisan Partners Holdings LP % Artisan Investments GP LLC % Artisan Partners Limited Partnership % JPMORGAN CHASE & CO., ON OCTOBER 13, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation JPMorgan Chase & Co., Wilmington, Delaware, United States of America 5. Date on which threshold was crossed or reached 10/06/2021

132 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 1.79% & of voting rights through instruments (total of 7.b.1+7.b.2) 3.34% Total of both in % (7.a.+7.b.) 5.12% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 1.68% % of voting rights through instruments (total of 7.b.1+7.b.2) 3.21% Total of both in % (7.a.+7.b.) 4.88% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 611445 In % - indirect (Sec. 34 WpHG) 1.79% Total - Absolute 611445 Total - in % 1.79% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG Type of instrument Internal right to recall shares lent out Voting rights absolute 361631 Voting rights in % 1.06% Type of instrument Convertible Bond due 2025 Voting rights absolute 393021 Voting rights in % 1.15% Total Voting rights absolute 754652 Total Voting rights in % 2.20% b.2. Instruments according to Sec. 38 (1) no. 2 WpHG Type of instrument Equity Swap Cash or physical settlement Cash Total Voting rights absolute 388114 Total Voting rights in % 1.13% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights (if at least 3% or more) JPMorgan Chase & Co. % JPMorgan Chase Bank, National Association % J.P. Morgan International Finance Limited % J.P. Morgan Capital Holdings Limited % J.P. Morgan Securities plc % -

Notes to the Financial Statements 133 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 JPMorgan Chase & Co. % JPMorgan Chase Holdings LLC % J.P. Morgan Broker-Dealer Holdings Inc. % J.P. Morgan Securities LLC % - JPMorgan Chase & Co. % JPMorgan Chase Holdings LLC % J.P. Morgan Broker-Dealer Holdings Inc. % J.P. Morgan Securities LLC % J.P. Morgan Prime Inc. % THE GOLDMAN SACHS GROUP, INC., ON DECEMBER 14, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of instruments 3. Details of person subject to the notification obligation The Goldman Sachs Group, Inc., Wilmington, Delaware, United States of America 5. Date on which threshold was crossed or reached 12/08/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 0.04% % of voting rights through instruments (total of 7.b.1+7.b.2) 5.26% Total of both in % (7.a.+7.b.) 5.30% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 0.31% % of voting rights through instruments (total of 7.b.1+7.b.2) 4.57% Total of both in % (7.a.+7.b.) 4.88% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 10665 In % - indirect (Sec. 34 WpHG) 0.03% a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN US6177602025 Absolute – indirect (Sec. 34 WpHG) 4020 In % - indirect (Sec. 34 WpHG) 0.01% Total - Absolute 14685% Total - in % 0.04%

134 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 b.1. Instruments according to Sec. 38 (1) no. 1 WpHG Type of instrument Right To Recall Voting rights absolute 492476 Voting rights in % 1.44% Type of instrument Right Of Use Voting rights absolute 158876 Voting rights in % 0.46% Type of instrument Convertible Bond Voting rights absolute 239165 Voting rights in % 0.70% Type of instrument Call Option Voting rights absolute 23070 Voting rights in % 0.07% Total Voting rights absolute 913587 Total Voting rights in % 2.67% b.2. Instruments according to Sec. 38 (1) no. 2 WpHG Type of instrument Swap Cash or physical settlement Cash Voting rights absolute 677933 Voting rights in % 1.98% Type of instrument Call Warrant Cash or physical settlement Cash Voting rights absolute 86058 Voting rights in % 0.25% Type of instrument Put Option Cash or physical settlement Physical Voting rights absolute 122190 Voting rights in % 0.36% Total Voting rights absolute 886181 Total Voting rights in % 2.59% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights (if at least 3% or more) The Goldman Sachs Group, Inc. % GSAM Holdings LLC % Goldman Sachs Asset Management, L.P. % - The Goldman Sachs Group, Inc. % Goldman Sachs Bank USA % Goldman Sachs Bank Europe SE % - The Goldman Sachs Group, Inc. % Goldman Sachs (UK) L.L.C. % Goldman Sachs Group UK Limited %

Notes to the Financial Statements 135 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Goldman Sachs International Bank % - The Goldman Sachs Group, Inc. % Folio Financial, Inc. % Folio Investments Inc. % - The Goldman Sachs Group, Inc. % Goldman Sachs & Co. LLC % - The Goldman Sachs Group, Inc. % Goldman Sachs (UK) L.L.C. % Goldman Sachs Group UK Limited % Goldman Sachs International % BLACKROCK, INC., ON DECEMBER 23, 2021 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation BlackRock, Inc., Wilmington, Delaware, USA 5. Date on which threshold was crossed or 12/17/2021 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2.45% % of voting rights through instruments (total of 7.b.1+7.b.2) 1.04% Total of both in % (7.a.+7.b.) 3.49% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 3.07% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.95% Total of both in % (7.a.+7.b.) 4.02% 7. Details on total position a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 828218 In % - indirect (Sec. 34 WpHG) 2.42% a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN US6177602025 Absolute – indirect (Sec. 34 WpHG) 10174

136 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 In % - indirect (Sec. 34 WpHG) 0.03% Total - Absolute 838392 Total - in % 2.45% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG Type of instrument Lent Securities (right to recall) Total Voting rights absolute 357718 Total Voting rights in % 1.04% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights in % if at least held 3% or more BlackRock, Inc. % Trident Merger LLC % BlackRock Investment Management, LLC % BlackRock Investment Management, LLC % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % - BlackRock, Inc. % Trident Merger LLC % BlackRock Investment Management, LLC % Amethyst Intermediate LLC % Aperio Holdings LLC % Aperio Group, LLC % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock Holdco 4, LLC % BlackRock Holdco 6, LLC % BlackRock Delaware Holdings Inc. % BlackRock Fund Advisors % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock Holdco 4, LLC % BlackRock Holdco 6, LLC % BlackRock Delaware Holdings Inc. % BlackRock Institutional Trust Company, National Association % - BlackRock, Inc. %

Notes to the Financial Statements 137 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Australia Holdco Pty. Ltd. % BlackRock Investment Management (Australia) Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Canada Holdings LP % BlackRock Canada Holdings ULC % BlackRock Asset Management Canada Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock (Singapore) Holdco Pte. Ltd. % BlackRock HK Holdco Limited % BlackRock Lux Finco S. a r.l. % BlackRock Japan Holdings GK % BlackRock Japan Co., Ltd. % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Finance Europe Limited % BlackRock Advisors (UK) Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. %

138 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Luxembourg Holdco S.a.r.l. % BlackRock (Luxembourg) S.A. % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock International Limited % BlackRock Life Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Finance Europe Limited % BlackRock Investment Management (UK) Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited %

Notes to the Financial Statements 139 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Luxembourg Holdco S.a.r.l. % BlackRock Investment Management Ireland Holdings Limited % BlackRock Asset Management Ireland Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Luxembourg Holdco S.a.r.l. % BlackRock UK Holdco Limited % BlackRock Asset Management Schweiz AG % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Finance Europe Limited % BlackRock Investment Management (UK) Limited % BlackRock Fund Managers Limited % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited %

140 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 BlackRock Finance Europe Limited % BlackRock (Netherlands) B.V. % BlackRock Asset Management Deutschland AG % - BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Finance Europe Limited % BlackRock Investment Management (UK) Limited % BlackRock Asset Management Deutschland AG % - BlackRock, Inc. % BlackRock Holdco 2, Inc. % BlackRock Financial Management, Inc. % BlackRock International Holdings, Inc. % BR Jersey International Holdings L.P. % BlackRock Holdco 3, LLC % BlackRock Cayman 1 LP % BlackRock Cayman West Bay Finco Limited % BlackRock Cayman West Bay IV Limited % BlackRock Group Limited % BlackRock Finance Europe Limited % BlackRock Investment Management (UK) Limited % BlackRock Asset Management Deutschland AG % iShares (DE) I Investmentaktiengesellschaft mit Teilgesellschaftsvermögen % - After the end of the reporting period (December 31, 2021), the Company published the following notifications of shareholdings that require reporting in accordance with Section 33 (1) of the German Securities Trading Act (WpHG) (status as of March 15, 2022):
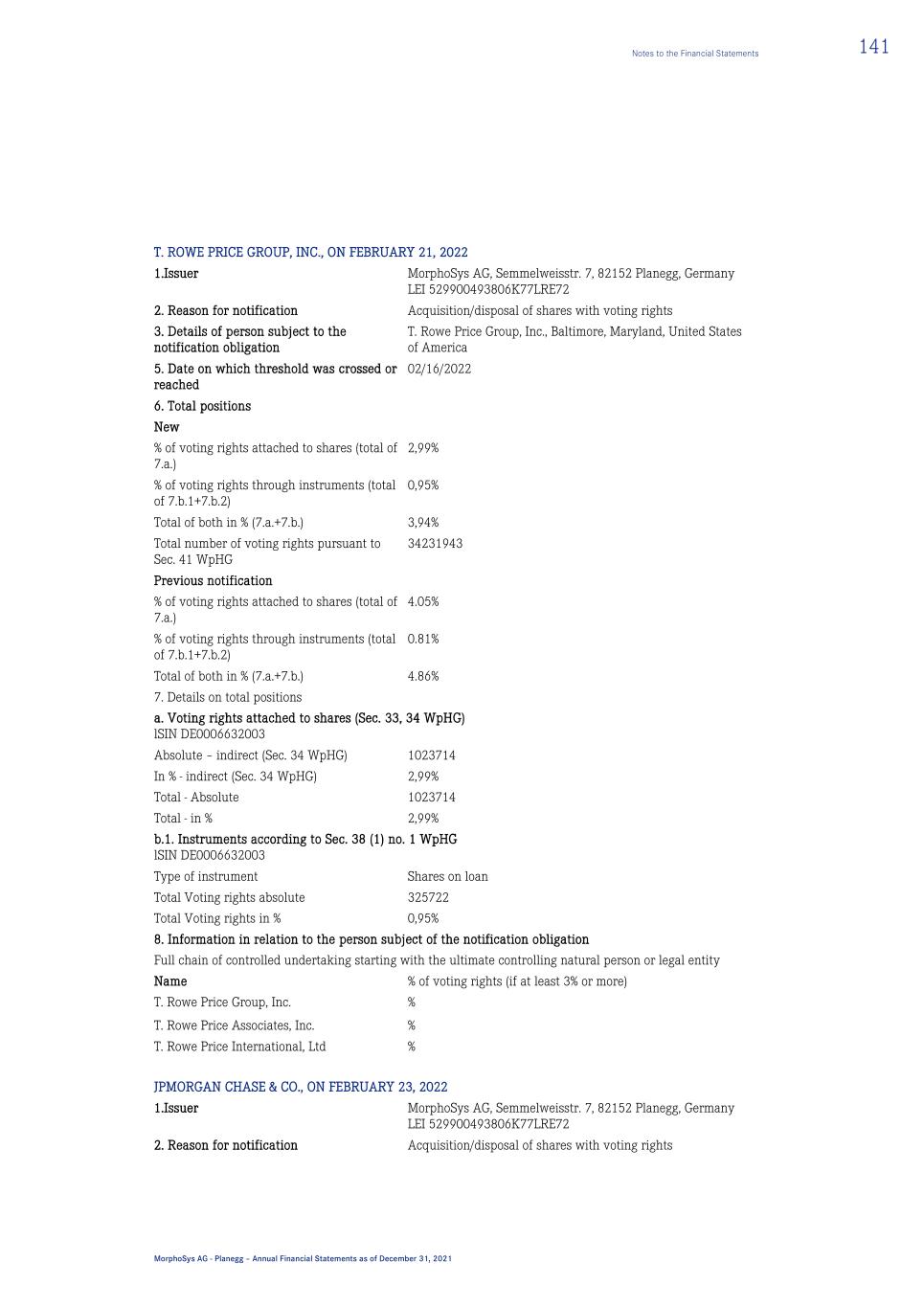
Notes to the Financial Statements 141 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 T. ROWE PRICE GROUP, INC., ON FEBRUARY 21, 2022 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights 3. Details of person subject to the notification obligation T. Rowe Price Group, Inc., Baltimore, Maryland, United States of America 5. Date on which threshold was crossed or reached 02/16/2022 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2,99% % of voting rights through instruments (total of 7.b.1+7.b.2) 0,95% Total of both in % (7.a.+7.b.) 3,94% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 4.05% % of voting rights through instruments (total of 7.b.1+7.b.2) 0.81% Total of both in % (7.a.+7.b.) 4.86% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 1023714 In % - indirect (Sec. 34 WpHG) 2,99% Total - Absolute 1023714 Total - in % 2,99% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG lSIN DE0006632003 Type of instrument Shares on loan Total Voting rights absolute 325722 Total Voting rights in % 0,95% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity Name % of voting rights (if at least 3% or more) T. Rowe Price Group, Inc. % T. Rowe Price Associates, Inc. % T. Rowe Price International, Ltd % JPMORGAN CHASE & CO., ON FEBRUARY 23, 2022 1.Issuer MorphoSys AG, Semmelweisstr. 7, 82152 Planegg, Germany LEI 529900493806K77LRE72 2. Reason for notification Acquisition/disposal of shares with voting rights

142 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 3. Details of person subject to the notification obligation JPMorgan Chase & Co., Wilmington, Delaware, United States of America 5. Date on which threshold was crossed or reached 02/17/2022 6. Total positions New % of voting rights attached to shares (total of 7.a.) 2.72% & of voting rights through instruments (total of 7.b.1+7.b.2) 3.93% Total of both in % (7.a.+7.b.) 6.65% Total number of voting rights pursuant to Sec. 41 WpHG 34231943 Previous notification % of voting rights attached to shares (total of 7.a.) 2.72% % of voting rights through instruments (total of 7.b.1+7.b.2) 3.93% Total of both in % (7.a.+7.b.) 7.36% 7. Details on total positions a. Voting rights attached to shares (Sec. 33, 34 WpHG) lSIN DE0006632003 Absolute – indirect (Sec. 34 WpHG) 931528 In % - indirect (Sec. 34 WpHG) 2.72% Total - Absolute 931528 Total - in % 2.72% b.1. Instruments according to Sec. 38 (1) no. 1 WpHG Type of instrument Internal right to recall shares lent out Voting rights absolute 346146 Voting rights in % 1.01% Type of instrument Right to recall shares lent out Voting rights absolute 2025 Voting rights in % 0.01% Type of instrument Convertible Bond due 2025 Voting rights absolute 408254 Voting rights in % 1.19% Total Voting rights absolute 756425 Total Voting rights in % 2.21% b.2. Instruments according to Sec. 38 (1) no. 2 WpHG Type of instrument Equity Swap Cash or physical settlement Cash Total Voting rights absolute 587512 Total Voting rights in % 1.72% 8. Information in relation to the person subject of the notification obligation Full chain of controlled undertaking starting with the ultimate controlling natural person or legal entity

Notes to the Financial Statements 143 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Name % of voting rights (if at least 3% or more) JPMorgan Chase & Co. % JPMorgan Chase Bank, National Association % J.P. Morgan International Finance Limited % J.P. Morgan Capital Holdings Limited % J.P. Morgan Securities plc % - JPMorgan Chase & Co. % JPMorgan Chase Holdings LLC % J.P. Morgan Broker-Dealer Holdings Inc. % J.P. Morgan Securities LLC % Subsequent Events No events that require reporting occurred. Planegg, March 15, 2022 Jean-Paul Kress, M.D. Sung Lee Chief Executive Officer Chief Financial Officer Malte Peters, M.D. Chief Research and Development Officer

144 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Statement of Fixed Assets Aquisition and Production Cost 01.01.2021 Additions Disposals 31.12.2021 in € in € in € in € A. FIXED ASSETS I. Intangible Assets 1. Paid Concessions, Commercial Property Rights and similar Rights and Assets and Licenses to such Rights and Assets 128,048,722 297,419 4,433,310 123,912,831 128,048,722 297,419 4,433,310 123,912,831 II. Property, Plant and Equipment 1. Land, Leasehold Rights and Buildings, including Leasehold Improvements 644,949 52,610 0 697,559 2. Other Equipment, Furniture and Fixtures 20,467,458 2,102,749 984,958 21,585,249 21,112,407 2,155,359 984,958 22,282,808 III. Financial Assets 1. Shares in affiliated Companies 1,538,439 1,278,849,261 0 1,280,387,700 2. Shares in Participations 359,458 0 0 359,458 1,897,897 1,278,849,261 0 1,280,747,158 151,059,026 1,281,302,039 5,418,268 1,426,942,797
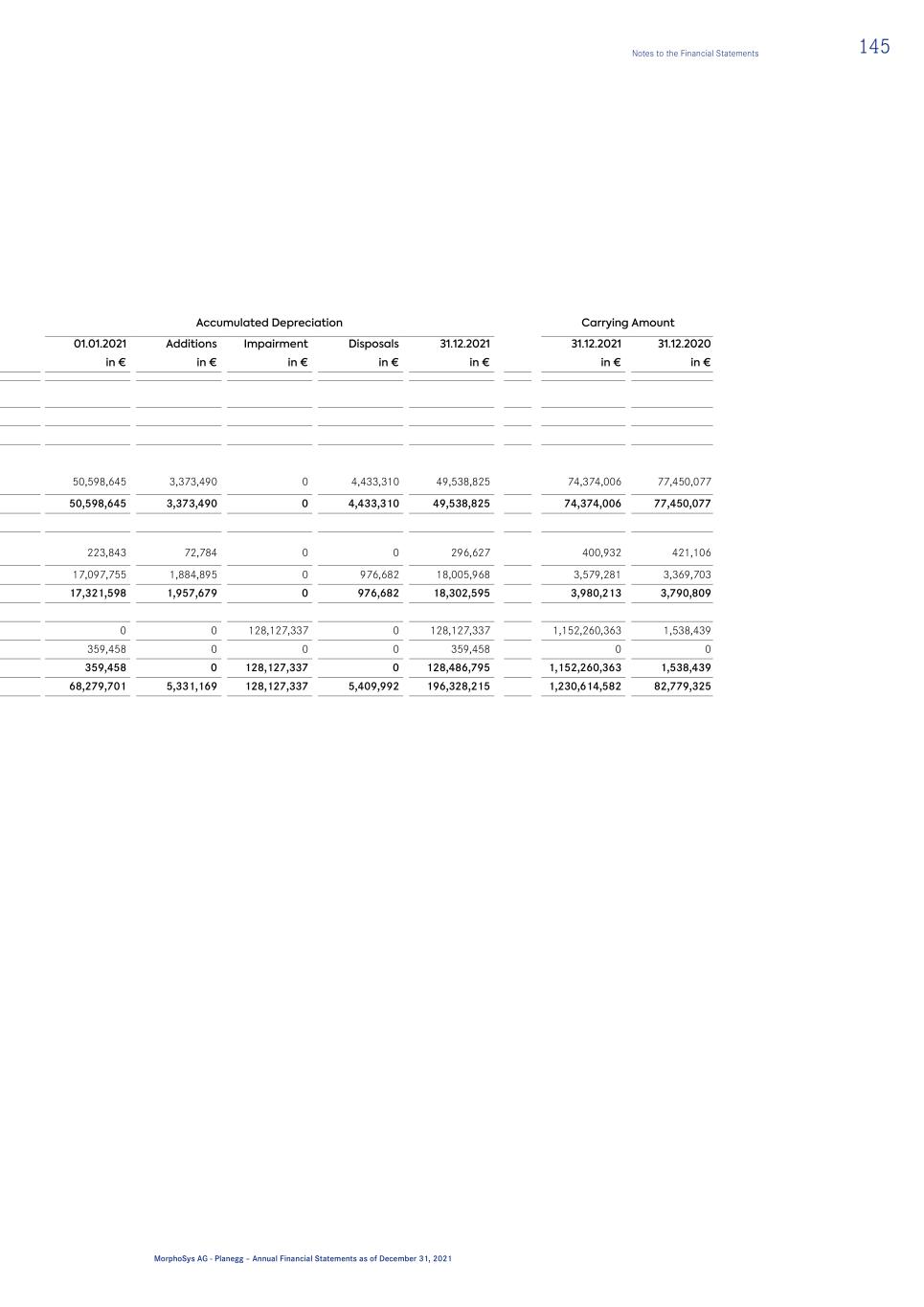
Notes to the Financial Statements 145 MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Accumulated Depreciation Carrying Amount 01.01.2021 Additions Impairment Disposals 31.12.2021 31.12.2021 31.12.2020 in € in € in € in € in € in € in € 50,598,645 3,373,490 0 4,433,310 49,538,825 74,374,006 77,450,077 50,598,645 3,373,490 0 4,433,310 49,538,825 74,374,006 77,450,077 223,843 72,784 0 0 296,627 400,932 421,106 17,097,755 1,884,895 0 976,682 18,005,968 3,579,281 3,369,703 17,321,598 1,957,679 0 976,682 18,302,595 3,980,213 3,790,809 0 0 128,127,337 0 128,127,337 1,152,260,363 1,538,439 359,458 0 0 0 359,458 0 0 359,458 0 128,127,337 0 128,486,795 1,152,260,363 1,538,439 68,279,701 5,331,169 128,127,337 5,409,992 196,328,215 1,230,614,582 82,779,325

146 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Responsibility Statement To the best of our knowledge, and in accordance with the applicable reporting principles, the financial statements give a true and fair view of the Company's net assets, financial position and results of operations, and the management report provides a fair review of the development and performance of the business and the position of the Company together with a description of the principal opportunities and risks associated with the Company's expected development. Planegg, March 15, 2022 Jean-Paul Kress, M.D. Sung Lee Chief Executive Officer Chief Financial Officer Malte Peters, M.D. Chief Research and Development Officer

156 Notes to the Financial Statements MorphoSys AG - Planegg – Annual Financial Statements as of December 31, 2021 Imprint Contact Information Investor Relations Phone.: +49 89 89927-404 Fax: +49 89 89927-5404 E-Mail: investors@morphosys.com MorphoSys AG Semmelweisstraße 7 82152 Planegg Germany Phone.: +49 89 89927-0 Fax: +49 89 89927-222 E-Mail: info@morphosys.com Website: www.morphosys.com/en These annual financial statements are also available in German and can be downloaded from the Company's website. HuCAL®, HuCAL GOLD®, HuCAL PLATINUM®, CysDisplay®, RapMAT®, Ylanthia®, 100 billion high potentials®, Slonomics®, CyCAT®, MONJUVI® and MINJUVI® are registered trademarks of the MorphoSys Group. Tremfya® is a registered trademark of Janssen Biotech, Inc. XmAb® is a registered trademark of Xencor Inc. National Comprehensive Cancer Network®, NCCN® and NCCN Guidelines® are registered trademarks of the National Comprehensive Cancer Network, Inc.




















































































































































