
Non-Financial Group Report 2022
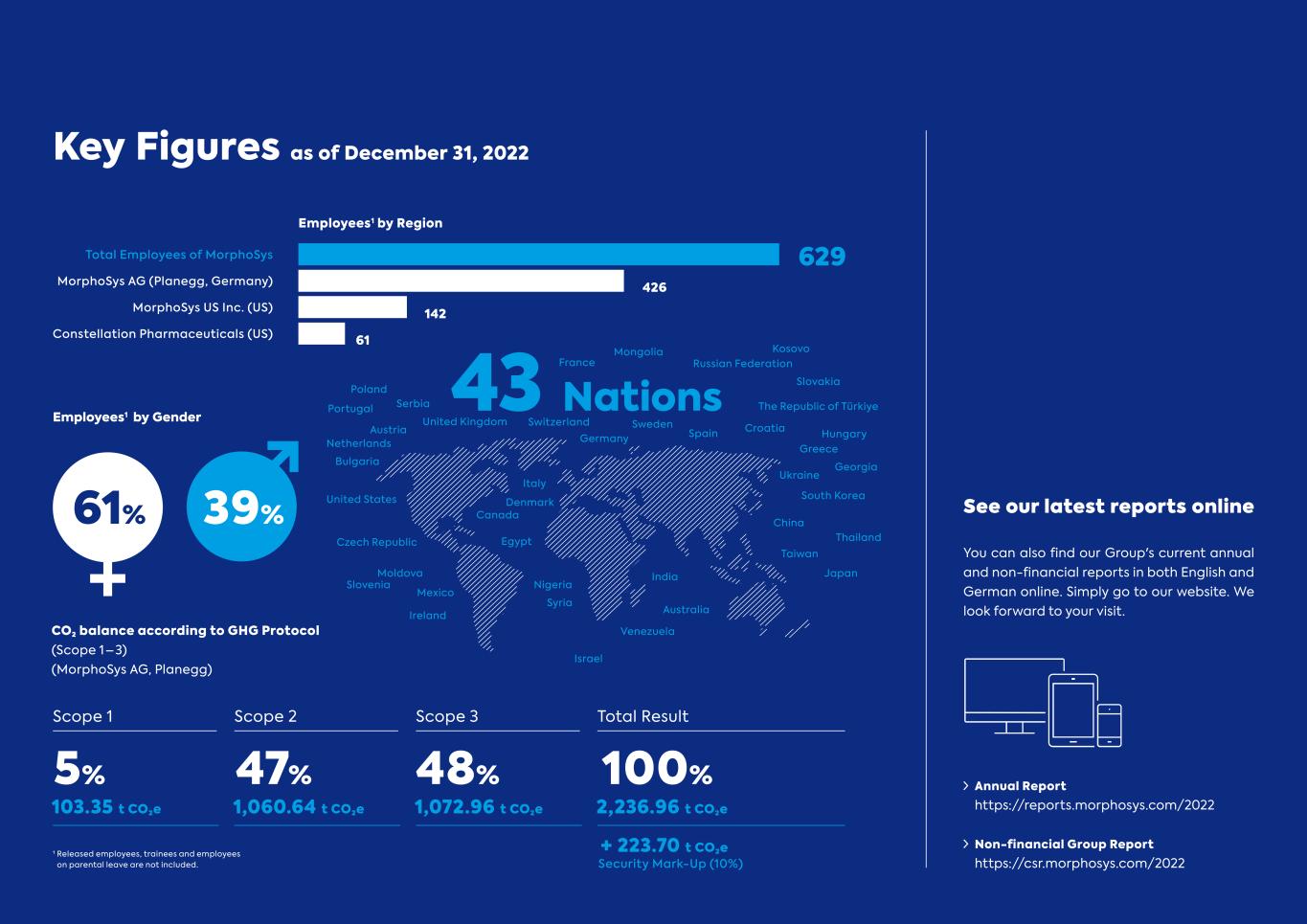
See our latest reports online You can also find our Group's current annual and non-financial reports in both English and German online. Simply go to our website. We look forward to your visit. Annual Report https://reports.morphosys.com/2022 Non-financial Group Report https://csr.morphosys.com/2022 Key Figures as of December 31, 2022 Employees1 by Region Total Employees of MorphoSys MorphoSys AG (Planegg, Germany) MorphoSys US Inc. (US) Constellation Pharmaceuticals (US) 629 426 142 61 Employees1 by Gender 61% 39% 43 Nations CO₂ balance according to GHG Protocol (Scope 1 – 3) (MorphoSys AG, Planegg) 5% 47% 48% 100% Scope 1 Scope 2 Scope 3 Total Result 103.35 t CO₂e 1,060.64 t CO₂e 1,072.96 t CO₂e 2,236.96 t CO₂e + 223.70 t CO₂e Security Mark-Up (10%) Mongolia Russian Federation China Thailand Venezuela Australia Croatia The Republic of Türkiye Ireland Egypt Moldova Canada Israel United States United Kingdom Nigeria India Taiwan Japan Greece Ukraine South Korea Czech Republic Slovenia Mexico France Germany SwedenSwitzerland Italy Austria Netherlands Spain Bulgaria Poland Kosovo Slovakia Denmark Hungary Georgia Portugal Syria Serbia 1 Released employees, trainees and employees on parental leave are not included.

We are conscious of the responsibil- ity we share for present and future generations and see sustainable ac- tion as a prerequisite for long-term business success. We are focused on creating long-term value and weigh our actions in terms of their impact on the environment, society, patients and employees.

01 Contents Additional Information 30 EU Taxonomy Regulation 31 Independent Practitioners' Limited Assurance Report 36 Imprint 38 Our Sustainability Approach 05 Business Ethics and Compliance 08 Social Matters 14 Employee Matters 19 Introduction 05 About This Non-Financial Group Report 06 Our Understanding of Sustainability 06 Our Business Model 06 Our Non-Financial Risk Analysis 06 Our Materiality Analysis 06 Compliance Organization and Anti-Corruption 09 Bioethics in Clinical Development 11 Selling Practices and Labeling 12 Quality of Products 15 Access to Medicine 16 Innovation in Research and Development (R&D) 17 Data Protection and IT Security 17 Employer Attractiveness 20 Diversity and Equal Opportunities 22 Employee Engagement 23 Employee Development 24 Occupational Health and Safety (OHS) 26 02 03 Environmental Matters 28 Emissions 29 04 05 04

Introduction Dear Ladies and Gentlemen, I am pleased to introduce MorphoSys’ 2022 Non-Financial Group Report addressing environmental, social and gov- ernance topics and highlight here a few key points from the report on patients, employees, environment and society as a whole. Ensuring patient access to our medicines. At MorphoSys, we are driven by the urgency to deliver med- icines that can improve the quality of life for people living with cancer. Critical to this is ensuring that patients have access to these treatments. In 2022, MorphoSys continued in the US the work through My MISSION Support to help pa- tients access appropriate and necessary care. As MorphoSys advances additional therapies in its pipeline, the Company is looking to enhance the current support offerings to ad- dress new indications and products. Spotlight on our people, our most important asset. In 2022, MorphoSys increased the focus on another essential part of its business – the employees. The acquisition of Con- stellation Pharmaceuticals, Inc. in 2021 and the ongoing challenging environment for hiring talent make this area a specific priority. The Company strengthened its commit- ment to transparency and equal opportunity in job vacan- cies, to employee development, and to enhancing the posi- tive working environment. In fact, in 2022 MorphoSys added employee development as topic to evaluate and assess its performance in this key area. Important in focusing on our people is listening to what they have to say. Thus, the annual employee survey was made a top priority, adding the results to MorphoSys’ key perfor- mance indicators and making employee engagement an important success factor. The Company was gratified to see that, while there is always room for improvement, it had positive employee scores in all three components of ESG. Making our world more sustainable. With the increasingly visible impact of climate change and fewer resources available for more people, sustainability has become an ever more pressing issue. We are conscious of the responsibility MorphoSys shares for present and future generations. To put weight behind our words, for the first time, MorphoSys is publishing data on its carbon footprint. The greenhouse gas emissions data provided in this report will help to set environmental goals and further enhance the measure- ments already in use. It is necessary that MorphoSys views its activities through both a narrow and a broad lens. The Company will continue to look carefully at how its actions impact patients, employ- ees, the environment, and society as a whole. Sincerely Sharon Curran Member of the Supervisory Board "MorphoSys is highly aware of the responsibility for present and future generations. The Company is looking closely at how to measure the impact the activities have on patients, employees, the environ- ment and society as a whole and how it can continually improve in all areas of ESG." Sharon Curran Member of the Supervisory Board 05Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach ›› Introduction

Our Sustainability Approach About This Non-Financial Group Report With the following separate Non-Financial Group Report, MorphoSys AG provides information pursuant to Section 315b and Section 315c in conjunction with Section 289c to 289e HGB (German Commercial Code) on material non- financial aspects for the Group’s 2022 financial year (January 1, 2022, to December 31, 2022) and thus on those aspects relevant for an understanding of the Group’s business development, re- sults of operations, Group management, and the effects of its business activities, and pursuant to Article 8 of Regulation (EU) 2020/852 of the European Parliament and of the Council of June 18, 2020, on establishing a framework to facilitate sustainable investment and amending Regulation (EU) 2019/2088 (hereinafter the “EU Taxonomy Regulation”). The requirements of the Corporate Sustainability Reporting Directive Implementation Act (CSR-RUG) were taken into account in the preparation of the Non-Financial Report. In particular, the analysis of the material aspects and the de- scription of the concepts were additionally guided by the Global Reporting Initiative (GRI) standards. A full application of the GRI standards is too extensive for the MorphoSys Group at the current time and therefore not expedient. Unless otherwise stated, the report applies to the entire MorphoSys Group according to the Scope of consolidation for financial reporting purposes. In July 2021, we completed the acquisition of Constellation Pharmaceuticals Inc. (here- inafter “Constellation Pharmaceuticals”). The transaction added two clinical-stage cancer drug candidates that complement and enhance MorphoSys’ own proprietary pipeline. Constellation Pharmaceuticals is therefore also in- cluded in the Scope of this report, and statements relating only to Constellation Pharmaceuticals are shown accordingly. PricewaterhouseCoopers GmbH Wirtschaftsprüfungs- gesellschaft (PwC) has been engaged on a voluntary basis to perform a limited-assurance report on the separate Non-Financial Group Report, hereinafter the “Non-Finan- cial Report,” in accordance with ISAE 3000 (Revised). The report can be found ›› here. References made in this Non-Financial Report to informa- tion outside of the Annual Report are additional information and are therefore not part of the assurance engagement. Our Understanding of Sustainability We are conscious of the responsibility we share for present and future generations and see sustainable action as a prerequisite for long-term business success. MorphoSys’ mission is to develop and commercialize innovative thera- pies for patients. MorphoSys is a fully integrated commercial biopharmaceutical company. Its activities in 2022 focused on hematology and oncology diseases. To ensure sustaina- ble business success, we incorporate environmental, social, and governance (ESG) principles into our daily business and base our business model on sustainable growth that is aligned with the interests of stakeholders. We are focused on creating long-term value and weigh our actions in terms of their impact on the environment, society, patients, and employees. Our Business Model Information on our business model can be found in the 2022 Annual Report on ›› page 31. Our Non-Financial Risk Analysis According to the CSR-RUG on the disclosure of non-financial information, companies must, in addition to reporting on material aspects, also disclose related risks that are linked to their own business activities, business relationships, prod- ucts, and services, and that are very likely to have or will have serious negative effects on the material aspects ac- cording to Section 289c (2) HGB. The Group has not identi- fied any such risks in the financial year under review on a net basis in accordance with Section 289c (3) nos. 3 and 4 HGB. Further information on opportunities and risks can be found on ›› page 64 of the 2022 Annual Report (“Risk and Opportu- nity Report”). Our Materiality Analysis Our report represents the material non-financial aspects that have been determined according to their business rele- vance and the Group’s impact on the aspects according to Section 289c (3) HGB. In 2020 we conducted a first business analysis involving the responsible departments as well as MorphoSys’ Executive Committee. 06Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach ›› About This Non-Financial Group Report ›› Our Understanding of Sustainability ›› Our Business Model ›› Our Non-Financial Risk Analysis ›› Our Materiality Analysis

In 2022 we reviewed the analysis of all identified non-finan- cial aspects of sustainability at MorphoSys. A yearly review is necessary to consider all current developments in selecting the most material topics for our Non-Financial Report and to adjust the priorities as necessary. The validation process for 2022 included an online questionnaire to identify aspects from last year’s report and any additional topics that could be in Scope for this year’s report. The questionnaire was filled out by internal experts and approved by our Manage- ment Board. The analysis showed that our key focus topics – business ethics and compliance, social matters, and em- ployee matters – are still our highest key focus topics. But our analysis also showed that due to the upcoming require- ments of the EU Taxonomy Regulation as well as the upcom- ing implementation of the Corporate Sustainability Report- ing Directive, environmental matters, in particular emissions, are coming into focus for us at MorphoSys. We are therefore adding environmental matters to our Non-Financial Report starting with the reporting year 2022. In addition, we have made an adjustment of the employee matters accordingly and identified employee development as a new aspect. With the launch of our first own product in 2020 and the acquisition of Constellation Pharmaceuticals in 2021, in 2022 we wanted to increase the focus on our em- ployees. The report is therefore based on the four areas that have been identified as most relevant in our analysis: business ethics and compliance, social matters, employee matters, and environmental matters, with their respective subcategories. Results of MorphoSys` Materiality Analysis Materiality scoring on impact and business relevance for identified material aspects per topic • • • • highest score among material aspects of the respective topic, descending by points Business Ethics and Compliance • • • Compliance Organization and Anti-Corruption • • • Selling Practices and Labeling • • • Bioethics in Clinical Development Environmental Matters • • • Emissions 04 Employee Matters • • • • Employee Engagement • • • Employer Attractiveness • • • Diversity and Equal Opportunities • • • Employee Development • • Occupational Health and Safety (OHS) 03 Material non-financial aspects at MorphoSys 01 Social Matters • • • • Quality of Products • • • • Data Protection and IT Security • • • Innovation in Research and Development (R&D) • • • Access to Medicine 02 07 ›› Our Materiality Analysis Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Business Ethics and Compliance Compliance Organization and Anti-Corruption 09 Bioethics in Clinical Development 11 Selling Practices and Labeling 12 01 08Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Compliance-Management-Program (CMP) Business Ethics and Compliance This chapter deals with MorphoSys’ compliance organiza- tion and anti-corruption strategy and clinical development, and selling practices and labeling. Compliance Organization and Anti-Corruption We are committed to good corporate governance practices, which include the highest standards in business ethics and compliance as set out in our ›› Code of Conduct. For further information please also see our latest ›› Corporate Governance Report. With the acquisition of Constellation Pharmaceuticals in July 2021, we established the same compliance standards for all three legal entities. The Global Compliance Committee and the Head of Global Compliance are now overseeing the compliance management program (CMP) for MorphoSys AG, MorphoSys US Inc., and Constellation Pharmaceuticals. We recognize that it is critically important to ensure the same commitment to business ethics across all three com- panies. MorphoSys AG, MorphoSys US Inc., and Constellation Pharmaceuticals share a new Code of Conduct launched in March 2022, and have been under the Scope of MorphoSys global compliance policies since July 2022 to help achieve this goal. MorphoSys’ CMP adheres to the industry standards and comprises all necessary elements, as indicated in the guid- ance documents from various authorities. The CMP ad- dresses the needs of various functions across the Company, including research, development, commercial, medical af- fairs, and others. reports to Head of Global Compliance Chief Executive Officer General Counsel, Member of the Executive Committee reports, if required, to Chairperson of the Audit Committee leading the global CMP and managing the interfaces between different compliance streams Anti-Bribery, Due Diligence of Third Parties Trainings & Awareness Integrity Line Compliance Documents Compliance Committee Transparency & Disclosure Compliance Management Program CMP Compliance Risk Management Review and Approval of Key Initiatives Monitoring & Continuous Improvement Credo Code of Conduct 09 ›› Compliance Organization and Anti-Corruption Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Our Global Compliance Committee comprises of two mem- bers of the MorphoSys AG Management Board, the Chief Research & Development Officer, the Chief Business Officer, the General Manager of MorphoSys US Inc., the General Counsel, the Head of Global HR, the U.S. General Counsel, and Head of U.S. Compliance, and is chaired by the Head of Global Compliance. The Committee meets quarterly and is available to our employees as a point of contact at all times. Our U.S. Compliance Committee has representation from U.S. business heads and meets quarterly to discuss U.S.-spe- cific activities and compliance with applicable laws and regulations. The U.S. Compliance Committee is chaired by the U.S. General Counsel and Head of U.S. Compliance. Our Compliance Subcommittee with Incyte meets quarterly as well to discuss compliance matters related to co-com- mercialization. Additionally, the Head of Global Compliance provides a re- port twice a year to the Supervisory Board’s Audit Commit- tee (in 2022 in August and November) and coordinates vari- ous improvements to MorphoSys’ CMP based on the feedback. Our maxim “Integrity in all we do” sets the direction for all our business activities. Our CMP addresses anti-bribery and anti-corruption topics in line with our corporate culture, our values, and applicable internal and external regulations. It is set up to protect patients, investors, other stakeholders, and MorphoSys’ reputation, thereby supporting business conti- nuity and sustainable growth. Our goal is to nurture a culture of integrity and compliance and prevent compliance violations as far as possible through continuous risk assessment, monitoring of our activities, and training of all our employees. Focus in 2022 In 2022 our main focus was on implementing the same com- pliance standards across all MorphoSys entities, supporting commercial efforts related to Monjuvi® (tafasitamab-cxix), and building pre-launch capabilities for pelabresib. The new Code of Conduct was rolled out in March 2022 and an e-learning course covering the Code of Conduct and the Anti-Bribery Policy was launched for all MorphoSys employ- ees using our learning management system Learn4MOR in Q2 2022. In line with the new Code of Conduct, the Compliance de- partment launched a conflict-of-interest screening cover- ing employees of all MorphoSys entities in September 2022. We kept working on the mitigation measures to address the 2021 compliance risk assessment. The mitigation measures included live and online trainings, policy updates, live moni- toring and desk reviews, compliance awareness initiatives, and due diligence screening. In Q4 2022 MorphoSys launched a new cycle of the compli- ance risk assessment, this time also covering Constellation Pharmaceuticals assets, to inform our strategy for 2023. The compliance risk assessment conducted in Q4 2022 did not reveal any high-risk areas, and the results were in line with overall industry practice. MorphoSys continues to ad dress the risks related to interactions with healthcare pro- fessionals, social media use, data privacy, and due diligence of third parties, among other risks. " Integrity in all we do." 10 ›› Compliance Organization and Anti-Corruption Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Training also remains an important focus of our CMP. It is our goal to ensure that our employees receive relevant compli- ance training in line with our values, culture, and ethical standards. Examples of compliance training delivered in 2022 include updated e-learning on appropriate use of so- cial media in Q3 2022, compliance with transparency regu- lations, and compliant interactions with healthcare profes- sionals and other stakeholders. The U.S. organization also conducted numerous training and employee engagement activities on U.S.-specific laws and associated compliance policies. In November 2022 we held Compliance Week 2022, which highlighted the importance of compliance topics; it included a lunch hosted by the Compliance department in the Boston office, a social media post, and a brochure, “Meet the Com- pliance Team,” distributed to all MorphoSys employees. The global compliance team also held an off-site meeting in Boston on November 15–16, 2022, dedicating time to cross-functional exchange with the Finance, Internal Audit, and Business departments, as well as internal strategic dis- cussions and building a robust plan for 2023. Maintaining open lines of communication is also a funda- mental aspect of our CMP. All MorphoSys employees and external parties have access to MorphoSys’ Integrity Line. This electronic incident management system is hosted by an external provider, and allows employees to report any com- pliance concerns in three languages, along with having the option to remain anonymous. MorphoSys frequently informs employees about the MorphoSys Integrity Line through a variety of channels, including training, communication, and other awareness initiatives. We make clear that retaliation or harassment against anyone who makes a report in good faith is prohibited. The MorphoSys Compliance department reviews potential compliance cases, escalates them to the responsible local or global Compliance Committee, where necessary, and manages investigations and follow-up ac- tions, where required, in line with the respective policies. MorphoSys structures its CMP on several key regulations and guidelines. Notably, we use the “Seven Elements of a Compli- ance Management Program” as communicated by the Of- fice of Inspector General (OIG), the updated 2020 guidance of the U.S. Department of Justice, and applicable EU direc- tives and regulations. In addition, there are entity level con- trols in the framework of the Sarbanes-Oxley Act (SOX), which address key compliance elements on a regular basis. These indicators are constantly monitored and improved. Our key priorities for 2023 will be maintaining an efficient CMP, rolling out an updated Compliance Management Handbook and Fair Market Value Policy, developing tailored trainings for customer-facing departments, considering implementation of an advanced healthcare professional (HCP) engagement approval tool, refreshing U.S. policies and providing respective trainings, and monitoring our busi- ness activities to ensure compliance and continuous im- provement. Bioethics in Clinical Development We conduct clinical trials in accordance with the ICH Har- monized Tripartite Guidelines for Good Clinical Practice (ICH-GCP), applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki. At MorphoSys, we make it a priority to protect the rights, safety, and well-being of all participants involved in clinical trials. Clinical trials are only initiated after the Independent Ethics Committee (IEC)/Institutional Review Board (IRB) and/or regulatory authorities give written approval or a favorable opinion as required. In addition, written informed consent of clinical trial participants must be obtained prior to their participation. Most advanced development stage Program Indication Tafasitamab1 L-MIND / Relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) frontMIND / First-line DLBCL inMIND / r/r follicular lymphoma / marginal zone lymphoma B-MIND / r/r DLBCL firstMIND / First-line DLBCL Pelabresib MANIFEST-2 / Myelofibrosis MANIFEST / Myelofibrosis Tulmimetostat (CPI-0209) Advanced solid tumors / Hematologic malignancies P H A S E 1 P H A S E 2 P H A S E 3 L A U N C H E D 2 1 Global Collaboration and License Agreement with Incyte Corporation; co-commercialization in the U.S.; Incyte has exclusive commercialization rights outside the U.S. 2 Not conducted, as not necessary. Our Clinical Pipeline 11 ›› Bioethics in Clinical Development›› Compliance Organization and Anti-Corruption Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Focus in 2022 Integration of Constellation Pharmaceuticals’ develop- ment activities into MorphoSys’ global development or- ganization continued in 2022, by leveraging the current established standard operating procedures and MorphoSys quality management system. In 2022, the focus was on the execution of clinical development plans, in particular patient enrollment into ongoing pivotal Phase 3 clinical trials of tafasitamab and pelabresib programs. Following the entry of MorphoSys and Human Immunology Biosciences, Inc. (HI-Bio), a biotechnology company based in south San Francisco that focuses on discovering and develop- ing precision medicines for autoimmune and inflammatory diseases, into equity participation and license agreements for felzartamab and MOR210, transfer of completed and ongoing development activities to HI-Bio has been initiated. Despite the improving situation of the global COVID-19 pan- demic in 2022, restrictions on visits to healthcare facilities, increased demands on healthcare services, and changes in the availability of study personnel are still present. MorphoSys continuously monitors the situation and decides how to best proceed depending on the situation to ensure the safety of patients, study personnel, and other stakeholders, as well as to safeguard data integrity in the conduct of all ongoing studies of tafasitamab, felzartamab, pelabresib, and tulmi- metostat programs. Information about our programs can be found on our website at https://www.morphosys.com/en/our-pipeline. Selling Practices and Labeling In 2020 the U.S. Food and Drug Administration (FDA) ap- proved our immunotherapy Monjuvi® (tafasitamab-cxix) under accelerated approval. MorphoSys US Inc. and Incyte have a partnership to co-commercialize Monjuvi® in the U.S. Outside of the U.S., Incyte has exclusive commercialization rights for tafasitamab-cxix, which is sold under the trade name Minjuvi®. As Monjuvi® is co-commercialized, a joint multidisciplinary review committee (RC) was established in 2020 to review and approve all commercial promotional materials. The joint RC consists of legal, medical, and regu- latory functional reviewers representing both, MorphoSys US Inc. and Incyte. For commercial materials not covered by the co-commercialization agreement with Incyte, MorphoSys US Inc. has an independent RC, which consists of the same functional representatives. All new Monjuvi® promotional materials, including any previ- ously approved pieces requiring updates, are submitted for review by the business owner. The joint RC convenes on a weekly basis to review these materials. The joint RC either provides feedback of necessary changes or approves the materials. Due to Monjuvi’s® Subpart E accelerated approval with the FDA, a 30-day review period is required for all Mon- juvi® sales and marketing materials. All materials are ap- proved by the joint RC with expiration dates that are moni- tored in our Veeva platform. In advance of the expiration, the business owner is notified to either resubmit the piece for review and expiration extension or to decommission the promotional piece. When materials used by the field sales team are scheduled to be decommissioned, a message is sent to the field sales team informing them and all materials are pulled from the field sales ordering portal. The formal training of our sales representatives is an essential element of our commercial operations, which are aligned to business ethics and compliance policies. Upon commencing employment at MorphoSys US Inc., each sales representative completes detailed training on the product and disease state. Successful certification, including competency in ap- proved verbal messaging, is required before engagement with any healthcare professionals. A learning management system and live verbalization sessions with our Head of Train- ing tracks training progress and certification. In addition, our sales representatives are trained on all relevant compliance and legal policies by the MorphoSys compliance and legal teams, including Code of Conduct training, promotional speaker bureau training, social media engagement training, and HCP interactions training. As we are constantly evaluat- ing the evolving landscape and the effectiveness of our pro- motional materials, enhancements may be made to our materials, including the use of new data, as appropriate. Subsequent and continuous training and certification of the sales representatives on how to appropriately educate cus- tomers using new materials is always planned and completed prior to actual use. The balance of efficacy and safety consistent with product labeling is always displayed throughout all direct and non-personal promotional materials. As this is the primary information that can be shared with healthcare providers, we attach great importance to ensure all relevant informa- tion is well balanced with efficacy and safety information. 12 ›› Bioethics in Clinical Development ›› Selling Practices and Labeling Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach
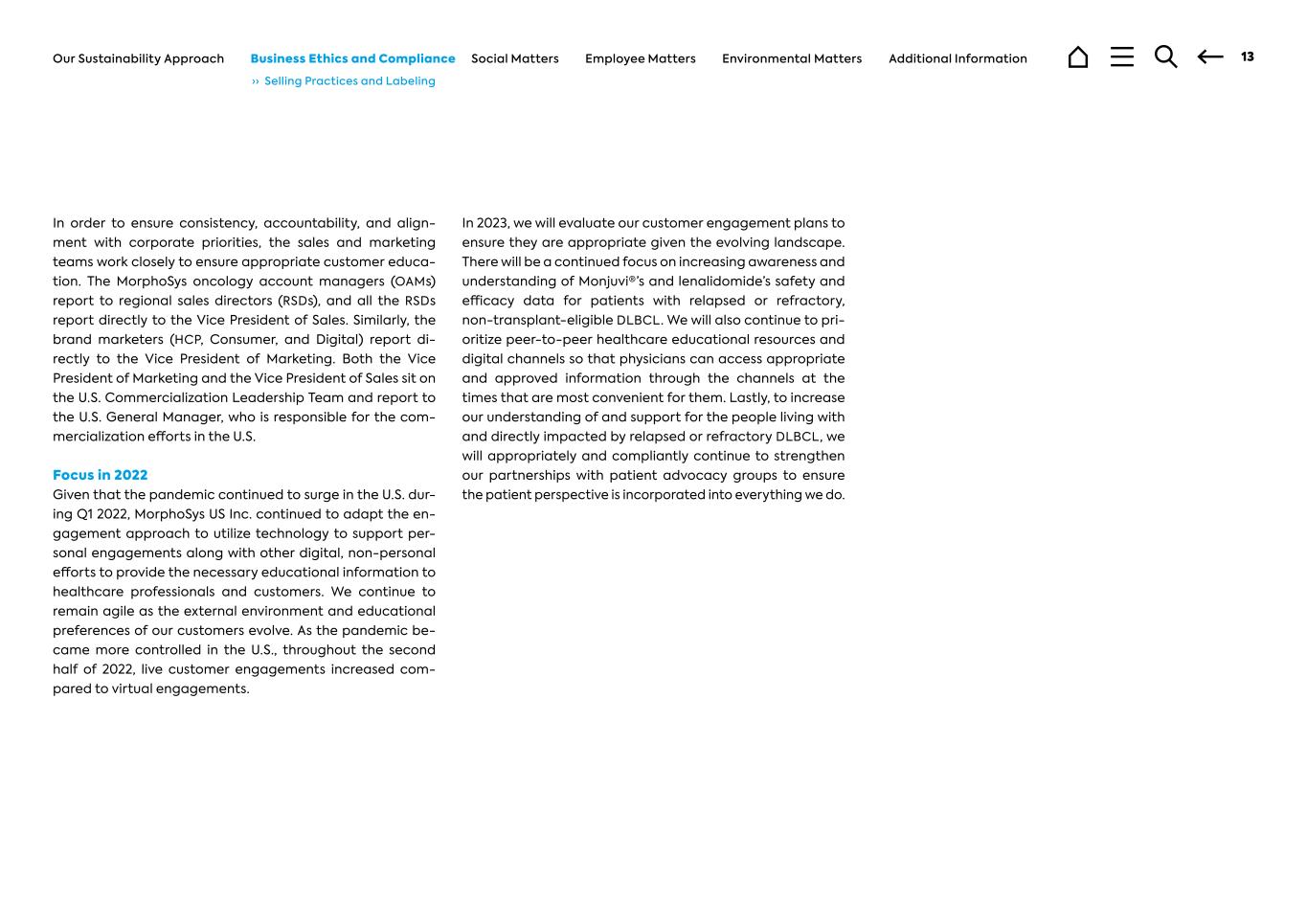
In order to ensure consistency, accountability, and align- ment with corporate priorities, the sales and marketing teams work closely to ensure appropriate customer educa- tion. The MorphoSys oncology account managers (OAMs) report to regional sales directors (RSDs), and all the RSDs report directly to the Vice President of Sales. Similarly, the brand marketers (HCP, Consumer, and Digital) report di- rectly to the Vice President of Marketing. Both the Vice President of Marketing and the Vice President of Sales sit on the U.S. Commercialization Leadership Team and report to the U.S. General Manager, who is responsible for the com- mercialization efforts in the U.S. Focus in 2022 Given that the pandemic continued to surge in the U.S. dur- ing Q1 2022, MorphoSys US Inc. continued to adapt the en- gagement approach to utilize technology to support per- sonal engagements along with other digital, non-personal efforts to provide the necessary educational information to healthcare professionals and customers. We continue to remain agile as the external environment and educational preferences of our customers evolve. As the pandemic be- came more controlled in the U.S., throughout the second half of 2022, live customer engagements increased com- pared to virtual engagements. In 2023, we will evaluate our customer engagement plans to ensure they are appropriate given the evolving landscape. There will be a continued focus on increasing awareness and understanding of Monjuvi®’s and lenalidomide’s safety and efficacy data for patients with relapsed or refractory, non-transplant-eligible DLBCL. We will also continue to pri- oritize peer-to-peer healthcare educational resources and digital channels so that physicians can access appropriate and approved information through the channels at the times that are most convenient for them. Lastly, to increase our understanding of and support for the people living with and directly impacted by relapsed or refractory DLBCL, we will appropriately and compliantly continue to strengthen our partnerships with patient advocacy groups to ensure the patient perspective is incorporated into everything we do. 13 ›› Selling Practices and Labeling Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

02 Social Matters Quality of Products 15 Access to Medicine 16 Innovation in Research and Development (R&D) 17 Data Protection and IT Security 17 14Additional InformationEnvironmental MattersEmployee MattersBusiness Ethics and ComplianceOur Sustainability Approach Social Matters
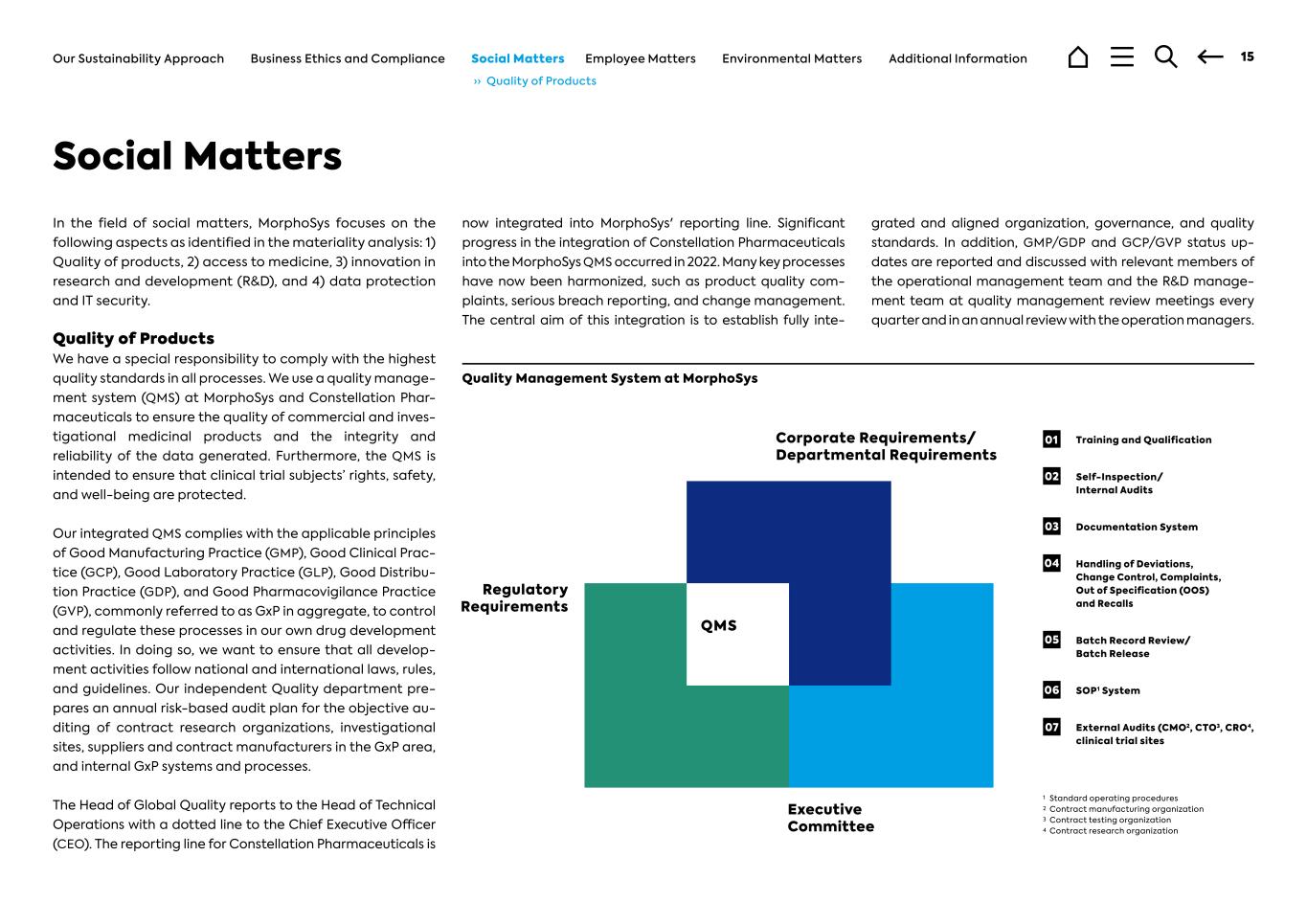
QMS Corporate Requirements/ Departmental Requirements Executive Committee Regulatory Requirements Quality Management System at MorphoSys 1 Standard operating procedures 2 Contract manufacturing organization 3 Contract testing organization 4 Contract research organization 01 Training and Qualification 02 Self-Inspection/ Internal Audits 03 Documentation System 04 Handling of Deviations, Change Control, Complaints, Out of Specification (OOS) and Recalls 05 Batch Record Review/ Batch Release 06 SOP1 System 07 External Audits (CMO2, CTO3, CRO4, clinical trial sites Social Matters In the field of social matters, MorphoSys focuses on the following aspects as identified in the materiality analysis: 1) Quality of products, 2) access to medicine, 3) innovation in research and development (R&D), and 4) data protection and IT security. Quality of Products We have a special responsibility to comply with the highest quality standards in all processes. We use a quality manage- ment system (QMS) at MorphoSys and Constellation Phar- maceuticals to ensure the quality of commercial and inves- tigational medicinal products and the integrity and reliability of the data generated. Furthermore, the QMS is intended to ensure that clinical trial subjects’ rights, safety, and well-being are protected. Our integrated QMS complies with the applicable principles of Good Manufacturing Practice (GMP), Good Clinical Prac- tice (GCP), Good Laboratory Practice (GLP), Good Distribu- tion Practice (GDP), and Good Pharmacovigilance Practice (GVP), commonly referred to as GxP in aggregate, to control and regulate these processes in our own drug development activities. In doing so, we want to ensure that all develop- ment activities follow national and international laws, rules, and guidelines. Our independent Quality department pre- pares an annual risk-based audit plan for the objective au- diting of contract research organizations, investigational sites, suppliers and contract manufacturers in the GxP area, and internal GxP systems and processes. The Head of Global Quality reports to the Head of Technical Operations with a dotted line to the Chief Executive Officer (CEO). The reporting line for Constellation Pharmaceuticals is now integrated into MorphoSys' reporting line. Significant progress in the integration of Constellation Pharmaceuticals into the MorphoSys QMS occurred in 2022. Many key processes have now been harmonized, such as product quality com- plaints, serious breach reporting, and change management. The central aim of this integration is to establish fully inte- grated and aligned organization, governance, and quality standards. In addition, GMP/GDP and GCP/GVP status up- dates are reported and discussed with relevant members of the operational management team and the R&D manage- ment team at quality management review meetings every quarter and in an annual review with the operation managers. 15Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach ›› Quality of Products

Focus in 2022 MorphoSys conducted GxP audits in 2022. Due to COVID-19 pandemic constraints, some of the audits were conducted virtual; however, most audits were conducted on-site. Audit findings were categorized as critical, major, or minor as per the MorphoSys standard operating procedures (SOPs) and were either addressed by the site or by establishing appro- priate corrective and preventive actions (CAPAs). MorphoSys was subject to a routine Postmarketing Adverse Drug Expe- rience (PADE) Inspection by FDA in 2022 and the Establish- ment Inspection Report (EIR) is pending. To make our organization more agile and lean, the integra- tion of Constellation Pharmaceuticals has been a key prior- ity since 2021. The electronic quality management system (QMS), which encompasses many key quality processes, was rolled out in 2022 for MorphoSys and Constellation Pharma- ceuticals. Since the acquisition of Constellation Pharma- ceuticals, the Good Clinical Practices (GCP) procedures and processes have been fully integrated into MorphoSys and the focus in 2023 will be on Good Manufacturing Practices (GMP) processes. In addition, in 2021 we implemented online inspection readiness training for all employees at MorphoSys AG and MorphoSys US Inc. as part of our prepa- ration for hosting regular inspections by the U.S. FDA or local authorities. Access to Medicine Ensuring access to our medicines is a critical priority for MorphoSys, and we make considerable investments in devel- oping potential medicines for patients in need. MorphoSys does so without a guarantee of clinical and commercial suc- cess, as many products in research and development phases do not achieve market authorization. Sustainable revenues from approved and commercially viable medicines allow for future investments into our research and development efforts. At MorphoSys, our philosophy is to responsibly price our medicines by balancing the value of the outcomes and in- novation they bring to patients and the healthcare system. There are patients who do not have third-party coverage in several countries around the world. For this reason, access to medicine also involves a social, charitable commitment to help patients without insurance coverage. MorphoSys is dedicated to supporting patients throughout their treat- ment journeys, and we are working together to help remove patient access barriers. The responsible department consists of a centralized Value, Access and Policy team responsible for setting the strategic direction for value, access, and policy for all investigational agents and approved products (including Constellation Pharmaceuticals assets) across all relevant markets and a team to execute tactics in the U.S. The reporting line structure is directly to the General Manager of MorphoSys US Inc. with regular updates through a quarterly business review, to the Management Board. As part of MorphoSys’ and Incyte’s commitment to support- ing patients, the ›› My MISSION Support program was launched in 2020. My MISSION Support is a robust patient support program offering financial assistance, ongoing education, and other resources to eligible patients who are prescribed Monjuvi® (tafasitamab-cxix) in the U.S. The My MISSION Support program has been able to support 296 patients in initiating treatment with Monjuvi® since FDA approval, by helping them understand their insurance benefits and offer- ing financial assistance to those who qualify. Additionally, My MISSION Support, the non-profit MorphoSys Foundation (“the Foundation”) was established in 2020 in the U.S. Its purpose is to help patients access appropriate and necessary care by administering a free drug patient assistant program (PAP). All patients must meet certain eli- gibility requirements, and be either uninsured, have insur- ance that does not cover Monjuvi®, or be unable to afford the cost-sharing for the drug under the policies set by their insurance. Furthermore, the Foundation provides charitable donations to independent charitable organizations that provide assistance to patients undergoing treatment for a particular disease. Focus in 2022 MorphoSys is committed to assisting patients and will con- tinue to operate the My MISSION Support program in 2023. As we look forward to the future with additional therapies in our pipeline that are currently under investigation, we are planning to enhance the current offerings. For oral thera- pies, we understand that there are different needs, and we will be prepared to assist patients in the evolving healthcare environment. For example, as we look to the future, we plan to add specialty pharmacies to our distribution network to ensure that patients have dedicated partners helping them to understand their insurance benefits and out-of-pocket expenses, and to provide appropriate resources. 16 ›› Quality of Products ›› Access to Medicine Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Innovation in Research and Development (R&D) At MorphoSys, our ambition is to redefine how cancer is treated. Our research and development activities address areas of high unmet medical need where people’s lives de- pend on novel, more effective, and differentiated treatment options. We aim to make a real difference in patients’ lives by focusing on therapeutic areas that best fit our expertise and make the best use of our resources. This includes hema- tological and solid tumor indications. Our core aim is to dis- cover, develop, and deliver innovative medicines, and to make them accessible to patients – a commitment to a sustainable contribution to society’s health. Focus in 2022 The further development of the clinical pipeline, with par- ticular regard to hematology and oncology, was the main strategic focus in 2022. Research activities were focused on the most advanced programs, and all laboratory activities were subsequently centralized at our German research hub in Planegg, Germany. Consequently, all U.S.-based activities relating to research were discontinued and therefore the expected cash flows from the discontinued programs will not materialize accordingly. We are now optimally positioned to develop highly differen- tiated cancer medicines, and the combined R&D organiza- tion operates in an integrated, global organizational setup. Both the Head of Research and Head of Clinical Develop- ment report directly to the Chief Research & Development Officer (CR&DO), ensuring full alignment and close collabo- ration between the organizations. Our cross-functional governance body, the Portfolio Innovation Board (PIB), builds the platform to elevate and advance key strategic questions, in order to ensure an effective and globally aligned execution of our R&D strategy. Significant advances were also made to use patient-derived materials and in vitro disease models to minimize the need for animal testing. For more information on ongoing clinical trials with our in- vestigational products, please visit www.clinicaltrials.gov. Data Protection and IT Security As a biopharmaceutical company, we constantly work with personal data of patients, employees, partners, and other stakeholders. The protection of this data is important. We implemented various procedures to safeguard compliance with the EU’s General Data Protection Regulation (GDPR) as well as U.S. requirements for the protection and confidential handling of protected health information (PHI), and are continuously working on further enhancements. Our team in the U.S. is offered training on compliance with the Health- care Insurance Portability and Accountability Act (HIPAA) and the appropriate use of PHI. MorphoSys AG continues to have an external Data Protec- tion Officer (eDPO) in line with the GDPR and the German Data Protection Act. The eDPO summarizes results in a re- port. A defined reporting process comes into force immedi- ately in the event of suspicious incidents. Focus in 2022 Our previous GDPR e-learning tool was replaced by a new version provided via MorphoSys’ internal online training platform. It comprises the following topics: personal data and data processing, the principles of data protection, rights of data subjects, common data breaches, data secu- rity, the role of the eDPO. It also includes an interactive test to ensure that the foregoing principles are understood by our colleagues. This training is for all MorphoSys AG employ- ees, all Constellation Pharmaceuticals employees, and MorphoSys US Inc. employees involved in clinical develop- ment activities. Data protection management software was implemented to further ensure compliance with the requirements of the GDPR at MorphoSys. An important area of focus was the implementation of the new EC standard contractual clauses for international transfers of personal data to third countries as required by the Commission Implementing Decision (EU) 2021/914 of June 4, 2021. There were no reportable data protection incidents at MorphoSys AG in 2022. Data protection via IT security measures continued to be a key topic in the reporting year. Part of reorganizing the Technical Operations division was to incorporate the IT or- ganization into the CFO organization. The Company utilized an automated penetration testing and validation platform to verify the technical security controls and detect potential 17 ›› Innovation in Research and Development (R&D) ›› Data Protection and IT Security Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach
weaknesses. No serious weaknesses were identified. Through special trainings, phishing simulations, and a monthly secu- rity newsletter, employees learned about their joint respon- sibility and essential contribution to IT security in our Com- pany. As part of the ongoing shift to leverage cloud services, MorphoSys’ security information and event management (SIEM) system was migrated to the cloud and expanded with security orchestration, automation, and response (SOAR) capabilities. Furthermore, we implemented enhanced tech- nologies to detect and prevent data leakage.. Our internal Computer Emergency Response Team (CERT) did not detect any serious security incidents during the re- porting year. The key project for 2023 is to onboard an exter- nal security operations center (SOC) provider to ensure consistent 24/7 coverage in responding to security-related events. Finally, various platforms and measures in the area of email and identity security were introduced or further developed in order to optimize our cyberdefense measures and to ensure data integrity and protection. 18 ›› Data Protection and IT Security Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

03 Employee Matters Employer Attractiveness 20 Diversity and Equal Opportunities 22 Employee Engagement 23 Employee Development 24 Occupational Health and Safety (OHS) 26 19Additional InformationEnvironmental MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Employee Matters

Employee Matters Our Human Resources (HR) department manages all topics related to employer attractiveness, diversity and equal op- portunities, employee engagement, and employee develop- ment. The Head of Global Human Resources reports directly to our CEO and is part of our Executive Committee. Our Health and Safety department, integrated into the Technical Operations division, takes care of all aspects of occupational health and safety (OHS). Our aspiration is to give more hope to people with cancer, and our employees are crucial to our success. In an industry such as biotechnology, where success largely depends on the innovation capability and commitment of our employees, aspects such as employee attraction, retention, and en- gagement are critical success factors. The Management Board has made it a key priority to focus on employee en- gagement. Employee satisfaction is part of our short and long-term goals, and this commitment is reflected in the in- clusion of employee engagement as a key performance indi- cator for the Management Board and selective employee groups’ 2022 Long-Term Incentive Plan, which covers the pe- riod up to 2026. In 2022, we assessed ESG topics for the second time as part of our annual employee survey to identify areas for improvement. MorphoSys is aiming for sustainable man- agement practices across all three areas, and moving for- ward we will measure progress annually. Employer Attractiveness In our three entities MorphoSys AG, MorphoSys US Inc., and Constellation Pharmaceuticals Inc. our workforce stabilized at 6291 employees at the end of 2022, a reduction of 14% ver- sus the end of 2021 following the integration of Constellation Pharmaceuticals and synergy measures. As a combined company, we are focusing on the needs of patients and are inspired by our company values – courage, urgency, innova- tion, and collaboration – in everything we do. Our employer proposition is based on our strong commitment to patients and our employees. Focus in 2022 We wanted MorphoSys to be seen as bold, unique, collabo- rative, and passionate – underscoring our shift from an R&D technology provider to a company that develops and deliv- ers innovative cancer medicines. Our new mission, “More life for people with cancer,” which was introduced in 2022, underscores that our work does not stop when the research is done, or the discovery made, while also highlighting our aspirations to redefine how cancer is treated. After our new mission was established in January 2022, we developed a comprehensive communications strategy and launch plan that translated how we would bring this new aspiration to life and amplify it. This included: 1 Released employees, trainees and employees on parental leave are not included. " Courage, Urgency, Innovation and Collaboration" A launch strategy that linked the new brand and mission with new content and programming released throughout 2022 An enhanced narrative, brand guidelines, and templates A consolidated, user-friendly, and modern website that visually embodies our new focus A refreshed creative and social media strategy to showcase the work we do every day to support cancer pa- tients and their families A new program – MOR Champions – to encourage employee advocacy and instill pride An internal campaign and updated office branding 1 2 3 4 5 6 20Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach ›› Employer Attractiveness
In 2022 the Company’s Life and Job pages on LinkedIn were visited by more than 16,000 users. This is an increase of 23% compared to 2021. 56% of the visitors viewed the Jobs page and 36% of new hires visited the Life or Jobs pages, showing a high degree of success in our employer branding and ex- ternal outreach. We are committed to transparency and equal opportunities in our job vacancies, development of employees, and a positive working environment. All our open job opportunities are advertised worldwide. MorphoSys is focused on attracting skilled employees in all technical areas as well as people with leadership compe- tencies, since leadership is directly connected to employee engagement, sustainable management, and our overall Company success. Our Global Leadership Group is com- prised of more than 10% of leaders from all departments across the Company. The Global Leadership Group worked with the Management Board on enabling the implementa- tion of our strategy, balancing a growing organizational structure with reduced complexity and cross-functional collaboration and the launch and implementation of ESPRIT in 2021. We view a leadership environment characterized by strong values, empowerment, and accountability as essen- tial to achieving our goals. This work proved vital in 2022, when the talent market was exceptionally challenging in 2022. It became very clear from the beginning of the year that this market is candi- date-driven. Post-COVID dynamics, demographic develop- ments, and the scarcity of specialized oncology skill sets made it increasingly difficult to find qualified employees. Challenges ranged from getting in touch with the right candidates, to candidates’ desire to flexibly decide how and where to work, to their ideas about relevant salary expecta- tions. One trend that became obvious is that people are no longer mainly interested in the salary itself. People’s expec- tations of remuneration are as varied as their lives. In a candidate-driven market one concept for all no longer ap- plies. We have been very successful with our hiring due to the range of customizable components in our salary packages. The outlook for 2023 is that due to demographic factors this trend will not change, and may even become stronger. At the beginning of 2022, we began implementing a new, Company-wide applicant tracking system, Greenhouse, to replace our two legacy ATS platforms. Our goal is to have a single hiring and engagement platform for MorphoSys to engage with candidates. To that end, the Company launched Greenhouse, a recruiting platform designed to el- evate the entire recruiting process for all users, from stake- holders to candidates, in December 2022. It uses process automation to increase in-house efficiency and candidate experience and also supports the harmonization of our hir- ing experience. Some examples of its enhanced efficiency are a “self-scheduling” tool that allows candidates to schedule their own interviews at convenient times and the option for candidates to record the pronunciation of their name ahead of the interview to allow interviewers to ad- dress them correctly. Greenhouse places a strong emphasis on reducing inter- viewer bias in the hiring process and provides hiring manag- ers with an option to create interview toolkits that help them to develop their key assessment criteria, define hiring teams, and create a uniform interview process for all applicants. Every MorphoSys employee will have access to Greenhouse and be able to recommend candidates for open positions within the system and track their success throughout the interview process. They will also have the opportunity to link their LinkedIn profile to the system and directly share MorphoSys job opportunities on their social media accounts. In April 2022, we launched an updated onboarding format for our new employees: the “Welcome Days” for MorphoSys AG employees. Our goal is to provide our new employees with a smooth and welcoming start. During the onboarding, we enable employees to get to know the Company better in their first two days of employment and to receive important information firsthand. This involves different departments collaborating with each other. The new employees first get to know each other in a round of introductions and then receive insights into the corporate culture and organization from our Global Head of Human Resources and an Executive Committee member. This is followed by an initial IT and HR introduction. On the second day, they receive a guided tour through our laboratories and get to know the building. Our new starters receive useful tips from our Compliance department as well as a safety briefing. Our Research and Development depart- ment will give them a first insight into our product pipeline. A similar format was also launched in our U.S. affiliates, tailored to U.S. geographic needs. 21 ›› Employer Attractiveness Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information

Diversity and Equal Opportunities Valuing diversity and ensuring equal opportunities are firmly anchored in our corporate culture. We believe that every single colleague needs to be heard and plays an important role in contributing to our success. We are therefore commit- ted to policies that prevent discrimination in our recruitment, hiring, training, promotion, or other employment practices for reasons of race, color, religion, gender, gender identity, national origin, age, sexual orientation, marital or protected veteran status, medical conditions, pregnancy, disability, or any other legally protected status. We aim for an open work- ing environment where creativity and innovation can flourish. In 2020 our CEO Jean-Paul Kress, M.D., signed the “CEO Pledge for a More Equitable and Inclusive Life Sciences In- dustry,” initiated by the Massachusetts Biotechnology Coun- cil, to demonstrate the commitment of MorphoSys and the whole biotechnology industry to diversity and inclusion. Our diversity definition pursuant to the German Commercial Code (HGB) can be found in our ›› Corporate Governance Report. It is of paramount importance to MorphoSys to create a culture of collaboration and inclusion of different perspec- tives, where everyone can contribute and be at their best. At the end of 2022, 61% of our employees and 44% of our ex- ecutives2 were women. The proportion of women in the Company’s workforce thus remains at a consistently high level. In addition, we proudly employed individuals of 43 dif- ferent nationalities, which adds to our identity as a truly global organization. Total Employees (in%) Employees1 by Gender (December 31, 2022) Executives2 (Number) Employees1 by Region (December 31, 2022) Total Employees of MorphoSys 1 Released employees, trainees and employees on parental leave are not included. 2 Executives in the first and second manage- ment levels. MorphoSys AG (Planegg, Germay) MorphoSys US Inc. (U.S.) Constellation Pharmaceuticals (U.S.) 2021 59 41 61 39 2022 female male 2021 32 44 31 24 2022 629 426 142 43 Nations 61 22 ›› Diversity and Equal Opportunities Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information

75 %Overall ESG score Focus in 2022 During 2022 the goal was to focus on further enhancing our diversity and inclusion efforts by driving focused initiatives with employee resource groups (ERGs), diversified supplier networks, and social responsibility initiatives in our communities. Our Women’s Network Group and our Pride Network Group championed a number of initiatives to foster awareness around inclusion topics from women and LGBTQ+ groups. Breakfast meetings with speakers such as Supervisory Board member Krisja Vermeylen, information sessions, and exter- nal media activities during Pride Month emphasized MorphoSys’ commitment to a diverse and inclusive environ- ment. MorphoSys network groups are grassroots-founded groups sponsored by one of our Executive Committee members. In May 2022, MorphoSys signed the Charta der Vielfalt, one of the largest employer initiatives in Germany to promote diversity in companies and institutions, which is supported by the Federal Government Commissioner for Migration, Refugees, and Integration. In the context of the implementation of Greenhouse, our new system for job vacancies and application management, and the launch of our new careers site, particular focus was placed on ensuring an equal opportunity environment, in- cluding specified checks and balances in the recruitment process such as non-biased wording in job postings and di- verse recruitment teams. In the 2022 ESG employee survey, 77% of responses rated diversity and inclusion favorably, up by 7% from 2021. In 2023 we will continue to support initiatives related to ERGs and workplace inclusion initiatives. Employee Engagement As we are striving to create a working environment that embraces sustainability and social responsibility, the Man- agement Board has made our employee survey a top priority and included the results in our KPIs to make sure they are measured and reviewed regularly. In addition, employee engagement is an important success factor, which gives in- sights into the degree to which employees identify with the values of MorphoSys and the Company practices, and how strong their bond with the Company is. Focus in 2022 In 2022 we conducted an employee survey for all MorphoSys employees in the U.S. and Germany to evaluate ESG aspects. We achieved a participation rate of 84%, which is a very strong response rate and was even higher than the previous year. All three ESG dimensions were rated positively1 at 75% overall. The high participation rate and the favorable re- sponses to many questions are evidence of an open feed- back culture and of employees’ high degree of identification with and interest in ESG topics. A total of 31 questions were asked. Environmental questions were related to safety in the workplace and environmentally friendly practices at MorphoSys. The overall environment score was 81%. Social questions were related to the commit- ment to patients, delivering excellence and quality stand- ards, and career development and training. The overall so- cial score was 80%. Questions on governance related to diversity and inclusion, ethics and compliance, and a learn- ing culture. The overall governance score was 67%. We in- creased by 3% to 6% in all three dimensions. All environment scores have increased or stabilized since 2021, with the two largest increases being seen in questions that scored lowest in 2021 (environmental practices). Social scores have also increased across almost all questions, with the largest in- crease being in understanding of socially and environmen- tally responsible actions. In the governance dimension, the ESG scores per dimension 81% 80% 67% Environment Social Governance 1 Overall scores: average favorable score of all 31 questions asked in the 2022 survey. 23 ›› Diversity and Equal Opportunities ›› Employee Engagement Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information

most significant gains were in management showing appre- ciation for employees. Our strongest results highlighted the connection between our employees’ work and our purpose of helping people living with cancer, our strong commitment to delivering excellence for patients, our supportive and collaborative working environment, and our commitment to employees’ health and safety. Based on these first-time baseline results we had the oppor- tunity to gather valuable feedback, identify potential for improvement, and formulate the following measures for 2023: We will be focusing on providing clarity on career de- velopment opportunities at MorphoSys, continuing to build trust in management, and giving more clarity on MorphoSys’ environmental practices including our carbon footprint. The ESG metric is derived from the ESG survey and reflects employee engagement. The 2022 employee engagement score was 69%, an increase of 6% compared to 2021. Em- ployees are significantly more engaged than in 2021, with improvements across all elements of workforce engage- ment. The most notable increase is in the willingness to rec- ommend MorphoSys as a place to work. In 2022 our employees demonstrated a high degree of in- volvement in their communities by participating in various charity events. In Germany we partnered with Gerets- ried-Wolfratshausen Tafel for a food drive and collaborated with the Stiftung Aktion Knochenmarkspende Bayern for a bone marrow registry drive. The U.S. team participated in awareness-raising events such as “Light the Night” organ- ized by the Leukemia and Lymphoma Society and supported the Boston Health Care’s Homeless Program by collecting needed items. In December 2022 MorphoSys organized a Christmas charity campaign in the U.S. and Germany. Our colleagues in Boston donated gifts to support families in need in the Boston area. The German team partnered with an orphanage in Munich, which provides a temporary home for children and young people from different social and cultural backgrounds. Our employees bought children living at the orphanage things from their wish lists. In 2023 our goal is to continue to engage employees with a combination of communication, discussion forums, and so- cial events in virtual, hybrid, and in-person settings to en- courage connections and a sense of belonging across our entire workforce. In addition, we will continue our commu- nity outreach efforts and promote awareness initiatives around cancer and its impact on society. Employee Development We know that our people are our best asset. In 2022 we identified employee development as a key priority at MorphoSys. It supports the growth of our employees and brings the best out of them. It will therefore always be a key component of our Company’s success. Employee development covers three main areas: individual development, leadership development, and team develop- ment, which all contribute to the healthy development of our organization as a whole. Starting with individual development, we agree that most development happens on the job. That is why we keep improving the way we identify our talents (via our annual Talent Talks) and promote internal mobility. Upon request, employees can have access to 360° assessments as well as to coaching and dedicated training if a need has been identified. In order to facilitate the integration in our multi- cultural teams, we also offer English and German classes, which take place on a weekly basis. 24 ›› Employee Engagement ›› Employee Development Individual Development Leadership Development Team Development Employee Development Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information

Focus 2022 In 2022 we designed and rolled out three global programs for new or current leaders: a comprehensive accelerated development program for first-line leaders, a mentoring program, and a short employee engagement program with two modules. The Accelerated Leadership Program’s first cohort was launched in May 2022. A second one started in September, and going forward two cohorts will be starting each year for a six-month hybrid journey, with ten to twelve employees per cohort. The mentoring program kicked off this year in April. It is an annual initiative that will start each year in Q2 and finish in Q1 the year after. The first cohort started with 15 mentoring pairs. The two employee engagement workshop modules supported our managers with having conversations about drivers and development goals with their team members, accelerating new hire onboarding, and supporting overall employee growth. The feedback so far has been excellent. Here is a tes- timonial given by one participant when he was asked what he enjoyed most about the program: “Taking the time to reflect on employee engagement. Getting to know people I’ve never previously interacted with at MorphoSys. The relaxed and open atmosphere in the group.” With a focus on strengthening our leaders and their team members we revamped our ESPRIT communication around our yearly priority-setting, feedback, development, and compensation planning processes. The new communication campaign includes three new videos, two of which were launched at the end of 2022, with the remaining one sched- uled to be launched in 2023. Following the opportunity to conduct group meetings after the relaxation of COVID-19 measures, there was a high in- crease in requests for team development workshops. The post-pandemic corporate world sees a growing need for people and teams to reconnect and work better to- gether. By supporting these initiatives, which are highly praised by participants, in 2023, MorphoSys shows that it has understood that high-performing teams are much more than the sum of their parts – and that investing in them is the best way to prepare for the future. "Taking the time to reflect on employee engagement. Getting to know people I have never interacted with so far at MorphoSys." 25 ›› Employee Development Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information

Occupational Health and Safety (OHS) MorphoSys believes it has a responsibility to provide a safe, healthy, and clean work environment in accordance with the provisions of our Code of Conduct, while complying with all applicable health, safety, and environmental laws and regulations, Company standards, and best practices. The Health and Safety department reports monthly to the Head of Facilities, who in turn reports the listed KPIs to the Head of Technical Operations. The New Work model introduced in 2021 was well received by our employees and became firmly entrenched during the year. The introduction of hybrid meetings, making it possible to participate remotely when working from home or during quarantine, has proved successful. MorphoSys offers employees highly flexible working condi- tions, both when working from home and when working from the Company’s offices. This flexibility is supported by training, tips, and a wide range of information for remote working (intranet). Focus in 2022 Although the pandemic-related restrictions were largely lifted at the beginning of 2022, the Health and Safety team continues to meticulously monitor the pandemic’s contin- ued progress. Employees continue to be provided with self-testing kits and masks and kept regularly up to date on the pandemic situation as well as on legal regulations. In Boston, vaccination was made mandatory for all employ- ees. The pandemic has now transitioned into an endemic in the U.S. Principles for Occupational Safety at MorphoSys Only certified companies are authorized by MorphoSys to dispose of chemical waste Only specially trained employees are allowed to work with toxic substances Pathogenic organisms are processed in labora- tories with particular safety standards Lowest possible amounts of hazardous substances used Introduction of hazardous biological materials for R&D purposes at MorphoSys AG • A dedicated biosafety team as defined by the “Gentechnik Sicherheitsverordnung” (German Genetic Engineering Safety Directive) and Infek- tionsschutzgesetz (infection control act ) • ▪ Safety professionals perform an internal audit to assess the risk involved • ▪ Specific safety training for the employees working with the substances • ▪ Assurance that all safety measures are implemented before actual work commences 26 ›› Occupational Health and Safety (OHS) Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information
As part of our business continuity plan in the event of a crisis, MorphoSys AG and MorphoSys US Inc. have established a Company-wide health emergency plan that includes a local plan for each site to comply with the varying legal require- ments and regulations in Germany and the United States. The health contingency plan and the above measures, along with continued compliance with all applicable COVID-19 regulations, allowed us to maintain normal business opera- tions. The implementation of operational reintegration was trans- ferred from the Human Resources department to the Health and Safety department in Q2 2022. We have optimized both the process and the reporting and have successfully imple- mented the new procedures. This also facilitates better monitoring of long-term illnesses in order to achieve a last- ing improvement in adapting preventative measures to current needs. Principles of occupational safety at MorphoSys AG – “Safety in all we do!” • Establishing a biosafety team in accordance with the In- fection Protection Act (IfSG) to conduct internal audits to assess the associated risks • Providing specific risk assessments, operating instruc- tions, and safety training for employees working with hazardous substances • Ensuring all safety measures are implemented by both in- ternal and external workers before actual work begins • Contracting only with certified companies to dispose of chemical and all other waste and sending waste (fat, oil, paper, cardboard, electronics, used office furniture, bat- teries/rechargeable batteries, Styrofoam, garden waste) for recycling when possible • Using the lowest possible amount of hazardous sub- stances and substituting them where possible • Ensuring work with infectious pathogens takes place in laboratories with special safety standards • Permitting only specially trained employees to handle hazardous substances • Making sure all laboratory employees have a very high level of training and are continuously trained and in- structed (annual trainings for the office, Laboratory S1, Laboratory S2, special technical infrastructure training, new starters, and pregnant employees) • Maintaining MorphoSys’ high safety standards through regular internal inspections conducted by safety officers and the biosafety team • A virtual audit of the genetic engineering laboratories by the Government of Upper Bavaria did not result in any deficiencies. • A building inspection by the owner's insurer revealed only minor defects. • Ensuring the Company’s fire protection plan continues to include regular briefings, evacuation assistance training, evacuation drills (annually) for all employees, and the presence of a trained fire protection officer (one external officer and, since 2022, an internal officer) During the reporting year, there were three reportable occu- pational accidents, 3 of them were commuting accidents, at MorphoSys AG, which means the number of occupational accidents remains at a very low level again this year. The number is also significantly lower than the comparable aver- age for the chemical industry in Germany (15.38 reportable occupational accidents per 1,000 full-time employees ac- cording to the latest survey by the Employer’s Liability Insur- ance Association for the Raw Materials and Chemical Industry (BG RCI) in 2021; a reportable accident is defined by the BG RCI as an occupational or commuting accident that causes more than three calendar days of incapacity to work). We intend to focus on several areas in 2023. These include taking the logical next step in our project development by hosting a health week centered on a variety of topics, such as resilience, healthy eating, athletics, and bicycle cam- paigns. Our goal is to bring down the commuting accident rate by educating our employees through information leaflets, supported by a road safety event. Particular focus will also be placed on preparing for MorphoSys’ occupa- tional safety certification with BG RCI. 27 ›› Occupational Health and Safety (OHS) Environmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Additional Information

Environmental Matters Emissions 29 04 28Additional InformationEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach Environmental Matters

Environmental Matters Emissions By signing the Paris Agreement, all EU member states have agreed to make their economies and societies climate-neu- tral by 2050. The German Federal Government made an amendment to the Climate Change Act to tighten climate regulations and enshrine in law the goal of achieving greenhouse gas neutrality by 2045. We at MorphoSys are aware of our responsibility on the path to climate neutrality. In our materiality analysis, we identified environmental matters as a new material issue, beginning with this year’s non-financial report. Baseline for MorphoSys AG’s Greenhouse Gas Emissions in 2022 For the first time we have evaluated our Greenhouse Gas (GHG) emissions for MorphoSys AG’s headquarters in Planegg, Germany, in accordance with the standards of the GHG Protocol methodology. We have not evaluated the data from MorphoSys US Inc. in Boston, Massachusetts, for this baseline reporting, as the majority of emissions are generated at MorphoSys AG in Planegg, Germany. Our total 2022 result for the CO₂ balance in accordance with the GHG Protocol (Scope 1 – 3) was 2,236.96 metric tons of CO₂e (100%). The total result including the security mark-up (10%) was 2,460.65 metric tons CO₂e. We measure and report our Scope 1 and Scope 2 emissions in accordance with the GHG Protocol operational control approach. A total of 103.35 metric tons of CO₂e (5%) are Scope 1 and a total of 1,060.64 metric tons of CO₂e (47%) are Scope 2. Our Scope 1 data includes direct GHG emissions from mo- bile combustion and fugitive emissions. These are emissions that are directly generated by MorphoSys AG. Our Scope 2 data includes indirect GHG emissions from purchased elec- tricity that are not directly generated by us but are a result of our activities. We also disclose the following categories for our Scope 3 emissions, that include other GHG indirect emissions: cate- gory 1 purchased goods and services (without transporta- tion (up to Tier 1), category 3 fuel- and energy-related ac- tivities, category 5 waste generated in operations, category 8 upstream leased assets. These emissions result from our activities that are outside of our control or ownership and result in a total of 1,072.96 metric tons of CO₂e (48%) for Scope 3 emissions. This data published for 2022 serves as a basis and as com- parative values for subsequent years. This baseline reporting is intended to create transparency about our company’s environmental impacts. We will use the data as a basis for further considerations regarding our environmental goals and strategies. For the calculation of our Scope 3.6 and 3.7 emissions we have data available, but this data is not yet optimal for presentation with the required quality and the respective completeness. For the 2023 financial year, we plan to evaluate the data for MorphoSys US Inc. in Boston, Massachusetts, and to include additional Scope 3 emissions so as to further increase the level of transparency. CO₂ balance according to GHG Protocol (Scope 1 – 3) (MorphoSys AG, Planegg) 5% 47% 48% 100% Scope 1 Scope 2 Scope 3 Total Result 103.35 t CO₂e 1,060.64 t CO₂e 1,072.96 t CO₂e 2,236.96 t CO₂e • Mobile Combustion • Fugitive Emissions • Purchased Electricity • Cat. 1: Purchased goods and services • Cat. 3: Fuel- and energy- related activities • Cat. 5: Waste generated in operations • Cat. 8: Upstream leased assets + 223.70 t CO₂e Security mark-up (10%) 2,460.65 t CO₂e Total result incl. security mark-up 29Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach ›› Emissions

05 Additional Information EU Taxonomy Regulation 31 Independent Practitioners' Limited Assurance Report 36 Imprint 38 30Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Background and objectives of the regulation On June 22, 2020, the EU Taxonomy Regulation was pub- lished in the Official Journal of the European Union (EU). It entered into force on July 12, 2020. The basis for this is the Sustainable Finance Action Plan, which is one of the four pillars of the European Green Deal. To achieve the EU’s 2050 climate and energy targets, it is necessary to redirect capi- tal flows toward a more sustainable economy. The EU Taxonomy is a classification system for environmen- tally sustainable economic activities. The intention is to create greater transparency with regard to the degree of sustainability of corporate activities, investments, and op- erating expenditures. Reporting for the 2022 financial year According to Article 8 EU Taxonomy Regulation, all compa- nies that are required to prepare a non-financial group report pursuant to Section 315b HGB, must provide informa- tion on the share of their group turnover, capital expenditure (CapEx), and operating expenditure (OpEx) for the 2022 re- porting period that is associated with Taxonomy-eligible and Taxonomy-aligned economic activities related to the first two environmental objectives (climate change mitiga- tion and climate change adaptation). Basis of reporting Identification of Taxonomy-eligible and Taxonomy- aligned activities For the identification of Taxonomy-eligible and Taxonomy- aligned activities, MorphoSys reviewed the activity descrip- tions of the Climate Act for consistency with our business activities, taking into account the NACE (nomenclature statistique des activités économiques dans la Communauté) codes. As the business model of MorphoSys is to develop and commercialize innovative therapies for patients, we have not identified any Taxonomy-eligible and Taxonomy-aligned economic activities based on our business model. MorphoSys is therefore not covered by the Climate Delegated Act and not identified as a relevant source of greenhouse gas emissions. Accounting policy The specification of the key performance indicators (KPIs) is determined in accordance with Annex 1 to Article 8 Climate Delegated Act. We determine the Taxonomy-eligible and Taxonomy-aligned KPIs in accordance with the legal re- quirements. In the following section we describe the ac- counting policy for turnover, CapEx, and OpEx. Turnover KPI Definition The turnover KPI is defined as Taxonomy-eligible and Taxon- omy-aligned turnover from product sales, license fees, milestone payments, service fees, and royalties (numerator) divided by our total Group turnover (denominator). For further details on our accounting policies regarding our total turnover, please see ›› page 108 of our 2022 Annual Report. As we have not identified any Taxonomy-eligible and Taxon- omy-aligned activities for the 2022 financial year, this results in a share of Taxonomy-eligible economic activities in our total turnover of 0%. Reconciliation Our consolidated turnover is reflected in our Consolidated Statement of Profit or Loss (IFRS) on ›› page 98 of our 2022 Annual Report (“Revenues”). Capital expenditure (CapEx) KPI Definition The CapEx KPI is defined as Taxonomy-eligible and Taxon- omy-aligned CapEx (numerator) divided by our total CapEx (denominator). The denominator comprises addi- tions to property, plant, and equipment and intangible as- sets during the financial year under review before depreci- ation, amortization, and remeasurements, including those resulting from revaluations and impairments, and exclud- ing changes in fair value. It includes additions to fixed assets (IAS 16), intangible assets (IAS 38), and right-of-use assets (IFRS 16). Additions resulting from business combinations are also included. Goodwill is not included in CapEx as it is not defined as an intangible asset under IAS 38. As the numerator is zero, there is no risk of double counting of economic activities. For further details on our accounting policies regarding our CapEx, please see ›› pages 113 and 114 of our 2022 Annual Report. EU Taxonomy Regulation 31 ›› EU Taxonomy Regulation Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Reconciliation Our total CapEx can be reconciled to our Consolidated Balance Sheet (IFRS) on ›› page 100 of our 2022 Annual Report (“Property, Plant, and Equipment”, “Intangible Assets”) and to our notes to the balance sheet on ›› page 128 (“4.8 Property, Plant, and Equipment – Additions”), on ›› page 130 (“4.9 Leases – Additions”), and on ›› page 133 (“4.10 Intangible Assets – Additions”). Operating expenditure (OpEx) KPI Definition The OpEx KPI is defined as Taxonomy-eligible and Taxono- my-aligned OpEx (numerator) divided by our total OpEx as defined by the EU Taxonomy (denominator). Our OpEx has been determined via the following accounts as of the reporting date, December 31, 2022: non-capital- ized research and development costs, non-capitalized building renovation costs, short-term leases, maintenance and repair costs. This does not include depreciation, amortization, impair- ment, and raw material costs. For further details on our ac- counting policies regarding our OpEx, please see ›› page 109 of our 2022 Annual Report. Explanations on the numerators of the CapEx KPI and the OpEx KPI As MorphoSys has not identified any significant Taxonomy- eligible and Taxonomy-aligned economic activities for the 2022 reporting period (in total <1% in Taxonomy-eligible expenses), we do not record CapEx and OpEx related to Taxonomy-eligible and Taxonomy-aligned economic activ- ities in the numerators of the CapEx and OpEx KPIs. For CapEx and OpEx, only “category c” applies to MorphoSys. This refers to the purchase of products and services from Taxonomy-eligible or Taxonomy-aligned economic activi- ties, as well as individual measures through which the target activities are carried out in a low-carbon manner or the emission of greenhouse gases is reduced (Section 1.1.2.2. (c) of Annex 1 to Article 8 Climate Delegated Act). No expenses were incurred in the 2022 financial year. MorphoSys is committed to sustainability but according to the current EU Taxonomy requirements, we do not have Taxonomy-eligible and Taxonomy-aligned economic activ- ities. Currently we do not have sustainable CapEx or OpEx. MorphoSys has no economic activities in the area of fossil gas and nuclear energy. Therefore, the disclosure of these specific tables is omitted. 32 ›› EU Taxonomy Regulation Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Proportion of turnover from products or services associated with Taxonomy-aligned economic activities - disclosure covering year 2022 Substantial contribution criteria DNSH criteria ('Does Not Significantly Harm') Economic activities (1) C o d e( s) ( 2) A b so lu te t ur n ov er ( 3) Pr o p o rt io n of t ur n ov er (4 ) C lim a te c h a n g e m it ig a ti o n (5 ) C lim a te c h a n g e a d a p ti o n (6 ) W a te r a n d m a ri n e re so ur ce s (7 ) C ir cu la r ec o n o m y (8 ) Po llu ti o n (9 ) B io d iv er si ty a n d ec os ys te m s (1 0 ) C lim a te c h a n g e m it ig a ti o n (1 1) C lim a te c h a n g e a d a p ti o n (1 2) W a te r a n d m a ri n e re so ur ce s (1 3) C ir cu la r ec o n o m y ( 14 ) Po llu ti o n (1 5) B io d iv er si ty a n d ec os ys te m s (1 6) M in im um s a fe g ua rd s (1 7) Taxonomy- aligned proportion of turnover, year 2022 (18) Taxonomy- aligned proportion of tunover, year 2021 (19) Category (enabling activity or) (20) Category '(transiti- onal acti- vity)' (21) in million euro % % % % % % % Y/N Y/N Y/N Y/N Y/N Y/N Y/N Percent Percent E T A. TAXONOMY ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) 0 0 % 0 % 0 % n/a n/a n/a n/a n/a n/a n/a n/a n/a n/a n/a 0 % n/a n/a n/a A.2. Taxonomy-eligible but not environ- mentally sustainable activities (not Taxonomy-aligned activities) Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 0 0 % 0 % n/a n/a n/a Total (A.1 + A.2) 0 0 % 0 % n/a n/a n/a B. TAXONOMY-NON-ELIGIBLE ACTIVITIES Turnover of Taxonomy-non-eligible activities (B) 278.3 100 % Total (A + B) 278.3 100 % 33 ›› EU Taxonomy Regulation Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Proportion of CapEx from products or services associated with Taxonomy-aligned economic activities - disclosure covering year 2022 Substantial contribution criteria DNSH criteria ('Does Not Significantly Harm') Economic activities (1) C o d e( s) ( 2) A b so lu te C a p E x (3 ) Pr o p o rt io n of C a p E x (4 ) C lim a te c h a n g e m it ig a ti o n (5 ) C lim a te c h a n g e a d a p ti o n (6 ) W a te r a n d m a ri n e re so ur ce s (7 ) C ir cu la r ec o n o m y (8 ) Po llu ti o n (9 ) B io d iv er si ty a n d ec os ys te m s (1 0 ) C lim a te c h a n g e m it ig a ti o n (1 1) C lim a te c h a n g e a d a p ti o n (1 2) W a te r a n d m a ri n e re so ur ce s (1 3) C ir cu la r ec o n o m y ( 14 ) Po llu ti o n (1 5) B io d iv er si ty a n d ec os ys te m s (1 6) M in im um s a fe g ua rd s (1 7) Taxonomy- aligned proportion of CapEx, year 2022 (18) Taxonomy- aligned proportion of CapEx, year 2021 (19) Category (enabling activity or) (20) Category '(transi ti- onal acti- vity)' (21) in million euro % % % % % % % Y/N Y/N Y/N Y/N Y/N Y/N Y/N Percent Percent E T A. TAXONOMY ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) 0 0 % 0 0 n/a n/a n/a n/a n/a n/a n/a n/a n/a n/a n/a 0 % n/a n/a n/a A.2. Taxonomy-eligible but not environ- mentally sustainable activities (not Taxonomy-aligned activities) CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 0 0 % 0 % n/a n/a n/a Total (A.1 + A.2) 0 0 % 0 % n/a n/a n/a B. TAXONOMY-NON-ELIGIBLE ACTIVITIES CapEx of Taxonomy-non-eligible activities (B) 21.5 100 % Total (A + B) 21.5 100 % 34 ›› EU Taxonomy Regulation Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Proportion of OpEx from products or services associated with Taxonomy-aligned economic activities - disclosure covering year 2022 Substantial contribution criteria DNSH criteria ('Does Not Significantly Harm') Economic activities (1) C o d e( s) ( 2) A b so lu te O p E x (3 ) Pr o p o rt io n of O p E x (4 ) C lim a te c h a n g e m it ig a ti o n (5 ) C lim a te c h a n g e a d a p ti o n (6 ) W a te r a n d m a ri n e re so ur ce s (7 ) C ir cu la r ec o n o m y (8 ) Po llu ti o n (9 ) B io d iv er si ty a n d ec os ys te m s (1 0 ) C lim a te c h a n g e m it ig a ti o n (1 1) C lim a te c h a n g e a d a p ti o n (1 2) W a te r a n d m a ri n e re so ur ce s (1 3) C ir cu la r ec o n o m y ( 14 ) Po llu ti o n (1 5) B io d iv er si ty a n d ec os ys te m s (1 6) M in im um s a fe g ua rd s (1 7) Taxonomy- aligned proportion of OpEx, year 2022 (18) Taxonomy- aligned proportion of OpEx, year 2021 (19) Category (enabling activity or) (20) Category '(transiti- onal acti- vity)' (21) in million euro % % % % % % % Y/N Y/N Y/N Y/N Y/N Y/N Y/N Percent Percent E T A. TAXONOMY ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) 0 0 % 0 0 n/a n/a n/a n/a n/a n/a n/a n/a n/a n/a n/a 0 % n/a n/a n/a A.2. Taxonomy-eligible but not environ- mentally sustainable activities (not Taxonomy-aligned activities) OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 0 0 % 0 % n/a n/a n/a Total (A.1 + A.2) 0 0 % 0 % n/a n/a n/a B. TAXONOMY-NON-ELIGIBLE ACTIVITIES OpEx of Taxonomy-non-eligible activities (B) 272.7 100 % Total (A + B) 272.7 100 % 35 ›› EU Taxonomy Regulation Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach

Imprint MorphoSys AG Semmelweisstrasse 7 82152 Planegg Germany Phone: +49-89-89927-0 Fax: +49-89-89927-222 Email: info@morphosys.com Website: www.morphosys.com/en Investor Relations Phone: +49-89-89927-404 Fax: +49-89-89927-5404 Email: investors@morphosys.com Concept and Design 3st kommunikation GmbH, Mainz Photography/Picture Credits Getty Images MorphoSys Translation Klusmann Communications, Niedernhausen Editorial Office Götz Translations and Proofreading GmbH, Hamburg Typesetting and Lithography 3st kommunikation GmbH, Mainz Publication date March 15, 2023 This Non-Financial Report is also published in German and is available to download on our website. HuCAL®, HuCAL GOLD®, HuCAL PLATINUM®, CysDisplay®, Ylanthia®, 100 billion high potentials®, MONJUVI®, and MINJUVI® are registered trademarks of the MorphoSys Group. 38 ›› Imprint Additional InformationEnvironmental MattersEmployee MattersSocial MattersBusiness Ethics and ComplianceOur Sustainability Approach



































