
Non-Financial Group Report 2023

We are conscious of the responsibility we share for present and future generations and see sustainable action as a prerequisite for long-term business success. We are focused on creating long-term value and weigh our actions in terms of their impact on the environment, society, patients, and employees. CONTENTS 3 Introduction 4 Our Sustainability Approach 6 Business Ethics and Compliance 11 Social Matters 14 Employee Matters 22 Environmental Matters 24 Additional Information 2

“MorphoSys is making an impact on society, not only through its paradigm-shifting medicines but also through a culture that promotes its employees and protects the environment.” Sharon Curran Member of the Supervisory Board Introduction I am pleased to share MorphoSys’ 2023 Non-Financial Group Report detailing environmental, social, and governance topics. This report will highlight the Company’s latest progress and initiatives related to patients, employees, the environment, and society. Improving Patient Outcomes MorphoSys is driven by our mission: More life to people with cancer. Pelabresib, our investigational BET inhibitor, represents an opportunity to substantially improve the standard of care for myelofibrosis, a debilitating and often deadly disease. MorphoSys also strengthened its patient- centric approach by establishing a Patient Advisory Council in 2023, ensuring that the patient perspective is incorporated into our Company initiatives. MorphoSys continued to lower patient access barriers through My MISSION Support, a holistic yet personalized support services program that provides financial assistance, educational resources, and other practical tools to help patients and families navigate the treatment journey. Making the MorphoSys Mission Possible Clinical milestones achieved in 2023 would not have been possible without the dedication of MorphoSys’ team of experts. The Company has built an open culture and prioritizes employee feedback through its annual employee survey. As previous employee feedback showed an increased interest in both physical and mental health, in May 2023 MorphoSys began providing free access to a specialized health app and offered a check-up with individualized recommendations for building a healthier lifestyle. MorphoSys also enhanced employee personal and professional development opportunities by initiating the developMOR U.S. pilot program. Paving the Way for a More Sustainable World MorphoSys understands the responsibility it shares for present and future generations, as well as the urgency of sustainable solutions to address the increasingly visible impacts of climate change. The greenhouse gas emissions data provided in this report have been expanded to include our operations at MorphoSys US Inc. in Boston, Massachusetts. Additionally, for the first time, we assessed Scope 3 emissions resulting from upstream and downstream logistics to provide further transparency about the environmental impact of activities outside our direct control or ownership. Last but not least, we have initiated preparations for launching a Sustainability Committee in 2024. We are excited and proud of MorphoSys’ accomplishments in 2023. The Company remains committed to robust corporate governance and continues to closely evaluate the impact of its actions on society as a whole. With warm regards, Sharon Curran Member of the Supervisory Board Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 3 ›› Introduction

Our Sustainability Approach CONTENTS 4 About This Non-Financial Group Report 4 Our Understanding of Sustainability 4 Our Business Model 5 Our Non-Financial Risk Analysis 5 Our Materiality Analysis About This Non-Financial Group Report With the following separate Non-Financial Group Report, MorphoSys AG provides information pursuant to Sections 315b and 315c in conjunction with Sections 289c to 289e HGB (German Commercial Code) on material non-financial aspects for the Group’s 2023 financial year (January 1, 2023, to December 31, 2023) and thus on those aspects relevant to an understanding of the Group’s business development, results of operations, Group management, and the effects of its business activities, and pursuant to Article 8 of Regulation (EU) 2020/852 of the European Parliament and the Council of June 18, 2020, on establishing a framework to facilitate sustainable investment and amending Regulation (EU) 2019/2088 (hereinafter the “EU Taxonomy Regulation”). The requirements of the Corporate Sustainability Reporting Directive Implementation Act (CSR-RUG) were taken into account in the preparation of the Non-Financial Group Report. In particular, the analysis of the material aspects and the description of the concepts were additionally guided by the Global Reporting Initiative (GRI) standards. A full application of the GRI standards, particularly in light of the Corporate Social Responsibility Directive (CSRD)/ European Sustainability Reporting Standards (ESRS), is not expedient for the MorphoSys Group at the current time. Unless otherwise stated, the report applies to the entire MorphoSys Group according to the scope of consolidation for financial reporting purposes. Constellation Pharmaceuticals, Inc. (hereinafter “Constellation Pharmaceuticals”) is also included in the scope of this report, and statements relating only to Constellation Pharmaceuticals are shown accordingly. PricewaterhouseCoopers GmbH Wirtschaftsprüfungs- gesellschaft (PwC) has been engaged on a voluntary basis to perform a limited-assurance review on the separate Non-Financial Group Report, hereinafter the “Non-Financial Report”, in accordance with ISAE 3000 (Revised). The report can be found ›› here. References made in this Non-Financial Report to information outside of the Annual Report are additional information and are therefore not part of the assurance engagement to obtain limited assurance. Our Understanding of Sustainability We are conscious of the responsibility we share for present and future generations and see sustainable action as a prerequisite for long-term business success. MorphoSys’ purpose is to develop and commercialize innovative cancer medicines for patients. To ensure sustainable business success, we incorporate environmental, social, and governance (ESG) principles into our daily business and base our business model on sustainable growth that is aligned with the interests of stakeholders. We are focused on creating long-term value and weigh our actions in terms of their impact on the environment, society, patients, and employees. The responsibility for the preparation of our Non-Financial Report lies with the Investor Relations & Sustainability department, which deals with all related topics. Overall responsibility for Non-Financial reporting lies with MorphoSys’ Management Board. Depending on the further development of the Business Combination Agreement with Novartis, we plan to set up a Sustainability Committee. Our Business Model Information on our business model can be found in the 2023 Annual Report ›› page 30. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 4 ›› About This Non-Financial Group Report ›› Our Understanding of Sustainability ›› Our Business Model

Our Non-Financial Risk Analysis According to the CSR-RUG on the disclosure of non- financial information, companies must, in addition to reporting on material aspects, also disclose related risks that are linked to their own business activities, business relationships, products, and services, and that are very likely to have or will have serious negative effects on the material aspects according to Section 289c (2) HGB. The Group has not identified any such risks in the financial year under review on a net basis in accordance with Section 289c (3) nos. 3 and 4 HGB. Further information on opportunities and risks can be found on ›› page 64 of the 2023 Annual Report (“Risk and Opportunity Report”). Our Materiality Analysis Our report represents the material non-financial aspects that have been determined according to their business relevance and the Group’s impact on the aspects according to Section 289c (3) HGB. In 2020 we conducted a first business analysis involving the responsible departments as well as MorphoSys’ Executive Committee. Each year, we review all identified non-financial aspects of sustainability at MorphoSys. A yearly review is necessary to take account of all current developments when selecting the most material topics for our Non-Financial Report and to adjust the priorities as necessary. The validation process included an online questionnaire to identify aspects from last year’s report and any additional topics that could be in scope for next year’s report. The questionnaire was filled out by internal experts and approved by our Management Board. The report is therefore based on the four areas that have been identified as most relevant in our analysis: business ethics and compliance, social matters, employee matters, and environmental matters, including relevant subcategories. The material aspects were retained for 2023 and confirmed by the Management Board. In preparation for the CSRD, we started the double materiality analysis in 2023 and will report on this for the first time in 2024. Results of MorphoSys’ Materiality Analysis Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 5 ›› Our Non-Financial Risk Analysis ›› Our Materiality Analysis

Business Ethics and Compliance 01 CONTENTS 6 Compliance Organization and Anti- Corruption 8 Bioethics in Clinical Development 9 Selling Practices and Labeling This chapter deals with MorphoSys’ compliance organization and anti-corruption strategy, bioethics in clinical development, and selling practices and labeling. Compliance Organization and Anti-Corruption We are committed to good corporate governance practices, which include the highest standards in business ethics and compliance as set out in our ›› Code of Conduct. For further information please also see our latest ›› Report on Corporate Governance. We established the same compliance standards for all MorphoSys’ legal entities. The Global Compliance Committee and the Head of Global Compliance are overseeing the compliance management program (CMP) for MorphoSys AG, MorphoSys US Inc., and Constellation Pharmaceuticals. MorphoSys structures its CMP based on several key regulations and guidelines. Notably, we use the “Seven Elements of a Compliance Management Program” as communicated by the Office of Inspector General (OIG), the updated 2020 guidance of the U.S. Department of Justice, and applicable EU directives and regulations. MorphoSys’ CMP is oriented towards the industry standards and addresses the needs of various functions across the Company, including research, development, commercial, and medical affairs. All elements of the MorphoSys CMP are reflected in the 2023 Compliance Management Handbook. To maintain a great corporate culture, MorphoSys implemented a Code of Conduct (current version updated and launched in March 2022) and several important policies addressing ethical business conduct, prevention of bribery and corruption, interactions with healthcare professionals (HCP´s), due diligence of third parties, reporting and responding to the cases of non-compliance, whistleblower protection, and other matters. MorphoSys conducts a regular compliance risk assessment to identify risks and opportunities for improvement. More than 50 Company leaders complete an online questionnaire and share their perspectives on potential compliance risks. Additionally, a comprehensive monitoring program is being run across all legal entities. Our Global Compliance Committee comprises two members of the MorphoSys AG Management Board, the Chief Research and Development Officer, the Chief Business Officer, the General Manager of MorphoSys US Inc., the Chief Legal & Human Resource (HR) Officer, the U.S. General Counsel & Head of U.S. Compliance, and is chaired by the Head of Global Compliance. The Committee generally meets quarterly and is available to our employees as a point of contact at all times. Our U.S. Compliance Committee has representation from U.S. business heads and meets quarterly to discuss U.S.- specific activities and compliance with applicable laws and regulations. The U.S. Compliance Committee is chaired by the U.S. General Counsel and the Head of U.S. Compliance. Our Compliance Subcommittee met quarterly with our partner Incyte to discuss compliance matters related to co- commercialization matters. Additionally, the Head of Global Compliance provides a report twice a year to the Supervisory Board’s Audit Committee and coordinates various improvements to MorphoSys’ CMP based on feedback. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 6 ›› Compliance Organization and Anti-Corruption

Compliance-Management-Program (CMP) “ Integrity in all we do.” In 2020, MorphoSys implemented an Integrity Line available to our internal employees and external stakeholders. The link to the website is reflected in our Code of Conduct, which is available on the MorphoSys corporate website. Cases of non-compliance can be reported online or by phones, including anonymously. All reported cases are promptly addressed. MorphoSys prohibits retaliation against people reporting cases of non-compliance in good faith. MorphoSys’ Supervisory Board Audit Committee and Global Compliance Committee are regularly informed of all cases of potential non-compliance. In 2023, there were no cases related to bribery and corruption. Our maxim “Integrity in all we do” sets the direction for all our business activities. Our CMP is set up to protect patients, investors, other stakeholders, and MorphoSys’ reputation, thereby supporting business continuity and sustainable growth. Our goal is to nurture a culture of integrity and compliance and prevent compliance violations as far as possible through continuous risk assessment, monitoring of our activities, and training of all our employees. Focus in 2023 In 2023, our main focus was on maintaining high compliance standards across all MorphoSys entities, supporting commercial efforts related to Monjuvi® (tafasitamab-cxix), and building prelaunch capabilities for the pelabresib launch. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 7 ›› Compliance Organization and Anti-Corruption

The compliance risk assessment conducted at the end of 2022 contributed to our 2023 compliance strategy. It did not reveal any high-risk areas, and the results were in line with overall industry practice. At the beginning of the year, we undertook an assessment of our CMP, leveraging all recent legislative developments and best practices. Additionally, we revised a few policies related to our interactions with HCP´s and updated all U.S. compliance policies up to the modern standard. Training also remains an important focus of our CMP. It is our goal to ensure that our employees receive relevant compliance training every year in line with our values, culture, and ethical standards and reach a 95–100% completion rate. Examples of compliance training topics delivered in 2023 include the Code of Conduct and anti- bribery, appropriate use of social media, compliance with transparency regulations, healthcare compliance, and congress activities. The U.S. organization also conducted numerous training and employee engagement activities on U.S.-specific laws and associated compliance policies. Our key priorities for 2024 will be maintaining an efficient CMP, including leveraging the OIG’s recent General Compliance Program Guidance issued in November 2023, elaborating on enhanced due diligence of third parties, implementing transparency regulations for new countries, as appropriate, developing IT systems, and streamlining our processes. Based on the CSRD, we have evaluated the following key figures: Disclosure Requirement G1-3 – Prevention and Detection of Corruption and Bribery1 Each year, we provide a 40-minute Code of Conduct and anti-bribery e-learning course to all MorphoSys employees (100%). It covers our Credo, the main provisions of the Code of Conduct, and the Anti-Bribery Policy. Disclosure Requirement G1-4 – Incidents of Corruption or Bribery2 Number of convictions and the amount of fines for violation of anti-corruption and anti-bribery laws: 0 Total number and nature of confirmed incidents of corruption or bribery: 0 Number of confirmed incidents in which own workers were dismissed or disciplined for corruption or bribery-related incidents: 0 Number of confirmed incidents relating to contracts with business partners that were terminated or not renewed due to violations related to corruption or bribery: 0 There were no public legal cases regarding corruption or bribery brought against the undertaking and its own workers during the reporting period. Disclosure Requirement G1-5 – Political Influence and Lobbying Activities2 MorphoSys has no activities and commitments related to exerting its political influence, including lobbying activities related to its material impacts, risks, and opportunities. 1 Based on the number of employees of MorphoSys as of December 31, 2023 2 For the MorphoSys Group Bioethics in Clinical Development We conduct clinical trials in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice (ICH-GCP), applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki. At MorphoSys, we make it a priority to protect the rights, safety, and well-being of all participants involved in clinical trials. Clinical trials are only initiated after internal approval by functional forums and executive committees (as per MorphoSys’ business governance) and after the Independent Ethics Committee (IEC)/Institutional Review Board (IRB) and/or regulatory authorities give written approval or a favorable opinion, as required. In addition, informed consent must be obtained in writing from clinical trial participants prior to their participation. At MorphoSys, clinical trials are carried out by the Clinical Trial Team (CTT), which outsources services to qualified third-party vendors (TPVs) and contract research organizations (CROs). The CTT is a cross-functional team, which is led by the clinical trial leader and provides oversight to TPVs and CROs to ensure clinical trial progress according to approved development plans and compliance with internal and external standards and requirements. Members of the CTT belong to the global Clinical Development and Technical Operations departments, which are located at MorphoSys’ global offices in Boston, U.S., and Planegg, Germany. Senior members from Clinical Development and Technical Operations provide leadership to CTTs and governance to TPVs and CROs, as required. In addition, a risk assessment is carried out for each clinical trial prior to initiation, which is reviewed and updated on a regular basis, as required. During the conduct of clinical trials, ICH-GCP audits of third-party vendors and clinical centers are scheduled to further ensure patient safety, data integrity, and compliance with applicable regulatory requirements and standards. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 8 ›› Compliance Organization and Anti-Corruption ›› Bioethics in Clinical Development

Our Clinical Pipeline Most advanced development stage Program1 Indication – P H AS E 1 – P H AS E 2 – P H AS E 3 – L AU N C H ED Pelabresib MANIFEST-2 / Myelofibrosis l l l ¡ MANIFEST / Myelofibrosis/essential thrombocythemia l l ¡ ¡ Tulmimetostat Advanced solid tumors/ hematologic malignancies l l ¡ ¡ 1 In February 2024, Incyte obtained exclusive rights worldwide to tafasitamab. Incyte will assume full responsibility and cover all costs going forward for the development and commercialization of the asset. Focus in 2023 In 2023, the focus was on the execution of clinical development plans, in particular completion of patient enrollment into pivotal Phase 3 clinical trials of tafasitamab and pelabresib programs, which was accomplished in March 2023. Furthermore, there was a focus on the collection and review of data from enrolled patients, continuation of patient enrollment in Phase 1 or 2 clinical trials of tafasitamab and tulmimetostat programs, and setting up new clinical trials for the pelabresib program. Following the entry of MorphoSys and Human Immunology Biosciences, Inc. (HI-Bio), a biotechnology company based in San Francisco that focuses on discovering and developing precision medicines for autoimmune and inflammatory diseases, into equity participation and license agreements for felzartamab and MOR210/TJ210/HIB210, transfer of completed and ongoing development activities to HI-Bio was successfully completed in June 2023. In 2023, a corporate audit was conducted with no findings related to compliance with the Anti-Bribery Policy and accruals of third-party vendor costs, and some non-critical findings and recommendations related to business continuity planning, in response to a corrective and preventive action plan was put in place. Information about our programs can be found on our website at https://www.morphosys.com/en/our-pipeline. Selling Practices and Labeling In 2020, the U.S. Food and Drug Administration (FDA) approved Monjuvi® (tafasitamab-cxix) under accelerated approval. MorphoSys US Inc. and Incyte had a partnership to co-commercialize Monjuvi® in the U.S. Outside of the U.S., Incyte had exclusive commercialization rights for tafasitamab-cxix, which is sold under the trade name Minjuvi®. As Monjuvi® was co-commercialized, a joint multidisciplinary review committee (RC) was established in 2020 to review and approve all commercial promotional materials. The joint RC consisted of legal, medical, and regulatory functional reviewers representing both MorphoSys US Inc. and Incyte. For commercial materials not covered by the co-commercialization agreement with Incyte, MorphoSys US Inc. had an independent RC, which consisted of the same functional representatives. All new Monjuvi® promotional materials, including any previously approved pieces requiring updates, were submitted for review by the business owner. The joint RC convened on a weekly basis to review these materials. The joint RC either provided feedback on necessary changes or approved the materials. Due to Monjuvi’s® Subpart E status (promotional material requirements under an accelerated approval) with the FDA, a 30-day review period was required for all Monjuvi® sales and marketing materials. All materials were approved by the joint RC with expiration dates that were monitored in our Veeva platform. In advance of the expiration, the business owner was notified to either resubmit the piece for review and expiration extension or to decommission the promotional piece. When materials used by the field sales team were scheduled to be decommissioned, a message was sent to the field sales team informing them and all materials were pulled from the field sales ordering portal. The formal training of our sales representatives was an essential element of our commercial operations, which are aligned to business ethics and compliance policies. Upon commencing employment at MorphoSys US Inc., each sales representative completed detailed training on the product and disease state. Successful certification, including competency in approved verbal messaging, was required before engagement with any HCP´s. A learning management system and live verbalization sessions with our Head of Training tracked training progress and certification. In addition, our sales representatives were trained on all relevant compliance and legal policies by the MorphoSys compliance and legal teams, including Code of Conduct training, promotional speaker bureau training, social media engagement training, and HCP interactions training. The appropriate balance of efficacy and safety consistent with product labeling was always displayed throughout all direct and non-personal promotional materials. As this was the primary information that could be shared with healthcare providers, we attached great importance to ensure all relevant information pertinent to appropriate prescribing behavior, was well balanced. In order to ensure consistency, accountability, and alignment with corporate priorities, the sales and marketing teams worked closely to ensure appropriate customer education. The MorphoSys oncology account managers (OAMs) reported to regional sales directors (RSDs), and all the RSDs reported directly to the Vice President of Sales. Similarly, the brand marketing teams (HCP, Consumer, and Digital marketers and field-based thought leader liaisons (TLLs)) reported directly to the Vice President of Marketing. Both the Vice President of Marketing and the Vice President of Sales sat on the U.S. Commercialization Leadership Team and reported to the U.S. General Manager, who was responsible for the commercialization efforts in the U.S. Focus in 2023 In 2023, the extreme impacts of the pandemic on the healthcare industry and patient care were reduced; however, many of the access issues and engagement Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 9 ›› Bioethics in Clinical Development ›› Selling Practices and Labeling

preferences among HCPs and large institutions were sustained. MorphoSys US Inc. continued to adapt the engagement approach to utilize technology to support personal engagements along with other digital, non- personal efforts to provide the necessary educational information to HCP´s and other appropriate customers. We continued to remain agile as the external environment and educational preferences of our customers evolved. Throughout 2023, in-person customer engagements increased compared with virtual engagements. In April 2023, MorphoSys presented final long-term five-year follow-up data analysis of the registrational L-MIND study in relapsed or refractory (r/r) DLBCL. In order to maintain our leading position as the most prescribed second-line treatment option for non-transplant-eligible DLBCL, the team focused its efforts on preparing for the education and execution of the updated five-year data and continued to reinforce Monjuvi’s competitive value proposition. We evaluated our engagement plans to ensure they were appropriate given the changing landscape. We also continued to prioritize peer-to-peer healthcare educational resources and digital channels so that physicians could access appropriate and approved information through these channels at the times that are most convenient for them. Lastly, to increase our understanding of and support for the people living with and directly impacted by r/r DLBCL, we appropriately and compliantly continued to strengthen our partnerships with patient advocacy groups and created a Patient Advisory Council to ensure the patient perspective is incorporated where appropriate. In February 2024, Incyte obtained exclusive rights worldwide to tafasitamab. Incyte will assume full responsibility and cover all costs going forward for the development and commercialization of the asset. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 10 ›› Selling Practices and Labeling

Social Matters 02 CONTENTS 11 Quality of Products 12 Access to Medicine 13 Innovation in Research and Development (R&D) 13 Data Protection and IT Security In the field of social matters, MorphoSys focuses on the following aspects as identified in the materiality analysis: 1) quality of products, 2) access to medicine, 3) innovation in research and development (R&D), and 4) data protection and IT security. Quality Management System at MorphoSys Quality of Products We have a special responsibility to comply with the highest quality standards in all processes. We use a quality management system (QMS) at MorphoSys to ensure the quality of commercial and investigational medicinal products and the integrity and reliability of the data generated. Furthermore, the QMS is intended to ensure that clinical trial subjects’ rights, safety, and well-being are protected. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 11 ›› Quality of Products

Our integrated QMS complies with the applicable principles of Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), Good Laboratory Practice (GLP), Good Distribution Practice (GDP), and Good Pharmacovigilance Practice (GVP), commonly referred to as GxP in aggregate, to control and regulate these processes in our own drug development activities. In doing so, we want to ensure that all development activities follow national and international laws, rules, and guidelines. Our independent Quality department prepares an annual risk-based audit plan for the objective auditing of contract research organizations, investigational sites, suppliers and contract manufacturers in the GxP area, and internal GxP systems and processes. The Head of Global Quality reports to the Head of Technical Operations with a dotted line to the Chief Executive Officer (CEO). The majority of key quality processes, such as deviation and change management and serious breach reporting, are harmonized acrosss MorphoSys,, using one electronic system (Veeva) and the workflows established therein. According to international standards, such as from ICH, GMP/GDP, and GCP/GVP, status updates are reported and discussed with relevant members of the Technical Operations (TO) management team and the R&D management team at quality management review meetings every half-year. Focus in 2023 MorphoSys conducted or managed GxP audits either directly or with vendors in 2023. In early 2023 an audit plan (including internal and external audits) following a risk- based approach was approved, and the execution followed accordingly over the year. In the reporting year 2022, these audits were conducted as a mix between on-site and virtual visits, with a clear focus on on-site audits of the most strategic and important partners. Audit findings were categorized as critical, major, or minor as per the MorphoSys standard operating procedures (SOPs). MorphoSys as an organization was subject to a routine surveillance inspection by the Oberbayern local government in January 2023 and a partner audit of Incyte in October 2023. The outcome of these audits was satisfactory with no critical findings. All other observations were addressed either by the sites/CxOs (any kind of contract organization) or by establishing appropriate corrective and preventive actions (CAPAs) within the MOR eQMS system. To leverage the full potential of harmonization the integration of Constellation Pharmaceuticals processes has been a key priority since 2021. The electronic quality management system (MOR eQMS, Veeva), which encompasses many key quality processes, was supplemented in 2023 by the Veeva Quality Docs and Trainings module, simplifying the system landscape and enabling better visibility and tracking of trainings status. Following the acquisition of Constellation Pharmaceuticals, GCP procedures and processes were fully integrated into MorphoSys by the end of 2022. The focus in 2023 was on GMP processes. This activity will continue until 2024 and is planned to be finalized then. Access to Medicine Ensuring access to our medicines is a critical priority for MorphoSys, and we make considerable investments in developing potential medicines for patients in need. MorphoSys does so without a guarantee of clinical and commercial success, as many products in research and development phases do not achieve market authorization. Sustainable revenues from approved and commercially viable medicines allow for future investments in our research and development efforts. At MorphoSys, our philosophy is to responsibly price our medicines by balancing the value of the outcomes and innovation they bring to patients and the healthcare system. For this reason, access to medicine also involves a social, charitable commitment to help patients without insurance coverage. MorphoSys is dedicated to supporting patients throughout their treatment journeys, and we are working together to help remove patient access barriers. The responsible department consists of a centralized Value, Access, and Policy team responsible for setting the strategic direction for value, access, and policy for all investigational agents and approved products across all relevant markets and a team to execute tactics in the U.S. The reporting line is directly to the General Manager of MorphoSys US Inc. with regular updates to the Management Board of MorphoSys through a quarterly business review. As part of MorphoSys’ and Incyte’s commitment to supporting patients, a support program was launched. My MISSION Support, MorphoSys’ Patient Support Program (previously administered by the MorphoSys Foundation and then by MorphoSys US Inc.) (“the Patient Support Program”), was established in 2020 in the U.S. The Patient Support Program offered educational resources, financial assistance, and practical tools to help providers and patients navigate the treatment journey. In addition, the Patient Support Program helped patients access appropriate and necessary care by administering a free drug patient assistance program (PAP). All patients must have met certain eligibility requirements, and either had to be uninsured, to have insurance that did not cover Monjuvi®, or to be unable to afford the cost-sharing for the drug under the policies set by their insurance. The My MISSION Support program had received 388 patient enrollment requests for Monjuvi® since FDA approval, thus helping patients understand their insurance benefits and offering financial assistance to those who qualified. Focus in 2023 MorphoSys was committed to assisting patients via the My MISSION Support program in 2023. As we look forward to the future with additional therapies in our pipeline that are currently under investigation, we are planning to enhance the current offerings. For oral therapies, we understand that there are different needs, and we will be prepared to assist patients in the evolving healthcare environment. For example, as we look to the future, we plan to evaluate the benefits and challenges that offering an oral oncolytic to patients brings. In 2024, we will evolve our strategies, model and services to provide appropriate support and resources for future therapies. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 12 ›› Quality of Products ›› Access to Medicine

Innovation in Research and Development (R&D) At MorphoSys, our ambition is to redefine how cancer is treated. Our research and development activities address areas of high unmet medical need where people’s lives depend on novel, more effective, and differentiated treatment options. We aim to make a real difference in patients’ lives by focusing on therapeutic areas that best fit our expertise and make the best use of our resources. Focus in 2023 The focus remained on the advancement of our clinical development pipeline, with particular focus on blood cancers and solid tumors. In March 2023, MorphoSys terminated its preclinical research programs and discontinued all related activities and focused its resources on its mid- to late-stage oncology pipeline. We continue to develop highly differentiated cancer medicines, and our R&D organization operates in an integrated, global organizational setup. The Chief Research and Development Officer (CR&DO) oversees all preclinical and clinical activities, ensuring full alignment and close collaboration between the organizations. Our cross- functional governance body, the Portfolio Innovation Board (PIB), builds the platform to elevate and advance key strategic questions, in order to ensure an effective and globally aligned execution of our R&D strategy. For more information on ongoing clinical trials with our investigational products, please visit www.clinicaltrials.gov. Data Protection and IT Security As a global biopharmaceutical company, we constantly work with the personal data of patients, employees, partners, and other stakeholders. The protection of this data is important. We have implemented various procedures to safeguard compliance with the EU’s General Data Protection Regulation (GDPR) as well as U.S. requirements for the protection and confidential handling of protected health information (PHI), and are continuously working on further enhancements. Our new employees in the U.S. are offered training on compliance with the Healthcare Insurance Portability and Accountability Act (HIPAA) and the appropriate use of PHI. In November 2022 all employees were trained. MorphoSys AG continues to have an external Data Protection Officer (eDPO) in line with the GDPR and the German Data Protection Act. The eDPO summarizes results in a report. A defined reporting process comes into force immediately in the event of suspicious incidents. In addition to the eDPO, MorphoSys AG has an in-house legal team dedicated to data protection. The in-house legal data protection team reports to MorphoSys’ Chief Legal and HR Officer. Focus in 2023 In 2023, we consolidated our eDPO services for activities conducted within the European Union by our affiliate Constellation Pharmaceuticals with the MorphoSys AG eDPO. This necessary step upheld our high level of compliance with GDPR and other relevant data protection laws. This consolidation involved a comprehensive review of our existing contracts, forms, sample texts, privacy policies, and processes. This thorough review process allowed us to ensure consistency in our data protection practices and reinforce our commitment to safeguarding personal data. At MorphoSys AG we continue to use the data protection management software implemented in 2022. This software plays an important role in maintaining the record of processing activities, ensuring that we have an up-to-date understanding of our data protection operations. In 2023 we also undertook a review of our data protection notices (DPNs) and informed consent forms (ICFs). These reviews ensured that these important documents remain current and comprehensive, reflecting any changes in our data processing activities and applicable laws. Where necessary, updates were made. Furthermore, we updated the cookie consent management tool for our dedicated United States websites. This update was carried out to ensure compliance with the California Consumer Protection Act, demonstrating our commitment to respecting the privacy rights of all data subjects, regardless of their location. There were no reportable data protection incidents at MorphoSys in 2023. Data protection via IT security measures continued to be a key topic in the reporting year. The Head of IT reports to the Head of Technical Operations, which is part of the CEO organization. The Company utilizes an external penetration testing consultancy to verify the technical security controls and detect potential weaknesses. No serious weaknesses were identified. Through special trainings, phishing simulations, and a monthly security newsletter, employees learn about their joint responsibility and essential contribution to IT security in our Company. All new hires receive one-time security awareness training. Additionally, all employees including managers have to attend our security awareness training once a year. The attendance rate of our yearly security awareness training for 2023 was 90.46%. Our key project for 2023, the onboarding of an external security operations center (SOC) provider to ensure consistent 24/7 coverage in responding to security-related events, is ongoing and expected to be completed in Q1/2024. All security-related technology, processes, and measures that we implement cover MorphoSys. Our internal Computer Emergency Response Team (CERT) did not detect any serious security incidents during the reporting year. The key project for 2024 is strengthening the authentication security of accounts by implementing a phishing-resistant multi-factor authentication technology. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 13 ›› Innovation in Research and Development (R&D) ›› Data Protection and IT Security

Employee Matters 03 CONTENTS 14 Employer Attractiveness 15 Diversity and Equal Opportunities 17 Employee Engagement 18 Employee Development 19 Occupational Health and Safety (OHS) Our HR department manages all topics related to employer attractiveness, diversity and equal opportunities, employee engagement, and employee development. The Chief Legal and HR Officer reports directly to our CEO and is part of our Executive Committee. Our Health and Safety department, which is integrated into the Technical Operations division, deals with all aspects of occupational health and safety (OHS). Our ambition is to redefine how cancer is treated, and our employees are crucial to our success. In an industry such as biotechnology, where success largely depends on the innovation capability and commitment of our employees, aspects such as employee attraction, retention, and engagement are critical success factors. The Management Board has made it a key priority to focus on employee engagement. Employee satisfaction is part of our short and long-term goals, and this commitment is reflected in the inclusion of employee engagement as a key performance indicator for the Management Board and selective employee groups’ Long-Term Incentive Plans. In 2023, we assessed ESG topics for the third time as part of our annual employee survey to identify areas for improvement. Employer Attractiveness As of December 31, 2023, the number of employees in our three entities MorphoSys AG, MorphoSys US Inc., and Constellation Pharmaceuticals Inc. stood at 524.1,2 As a combined company, we are focusing on the needs of patients and are inspired by our Company values – courage, urgency, innovation, and collaboration – in everything we do. Our employer proposition is based on our strong commitment to patients and our employees. 1 Released employees, trainees, and employees on parental leave are not included. 2 Numbers in headcount Focus in 2023 The results of our employee survey from the previous year revealed an increased interest in physical and mental health. As a result, the focus this year was on our internal employer branding, particularly in the area of well-being. Since May 2023, MorphoSys AG employees have had access to a free health app that includes sports sessions to strengthen the body, healthy recipes, and guided meditations that help them to relax, reduce their stress, and improve their concentration. The demands of everyday working life are changing and require more flexibility and resilience. All MorphoSys AG employees have therefore been able to take advantage of a free health check-up since 2023. The check-ups are carried out by a team of doctors at various locations. The program was specially developed to show each participant personal ways to lead a healthier lifestyle. The examinations are scientifically sound, suitable for everyday use, and, above all, sustainable. Our employees receive a personal action plan based on the test results and their state of health. The employees of MorphoSys US Inc. also benefited from a new well-being initiative in 2023. They can now put together an individual well-being program and submit expenses of up to US$ 600 per year. They can choose between a gym membership, the purchase of sports equipment for home use, or a subscription for meditation or wellness. This gives everyone the opportunity to focus on their individual needs and work on them. We are committed to transparency and equal opportunities in our job vacancies, development of employees, and a positive working environment. All our open job opportunities are advertised worldwide. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 14 ›› Employer Attractiveness
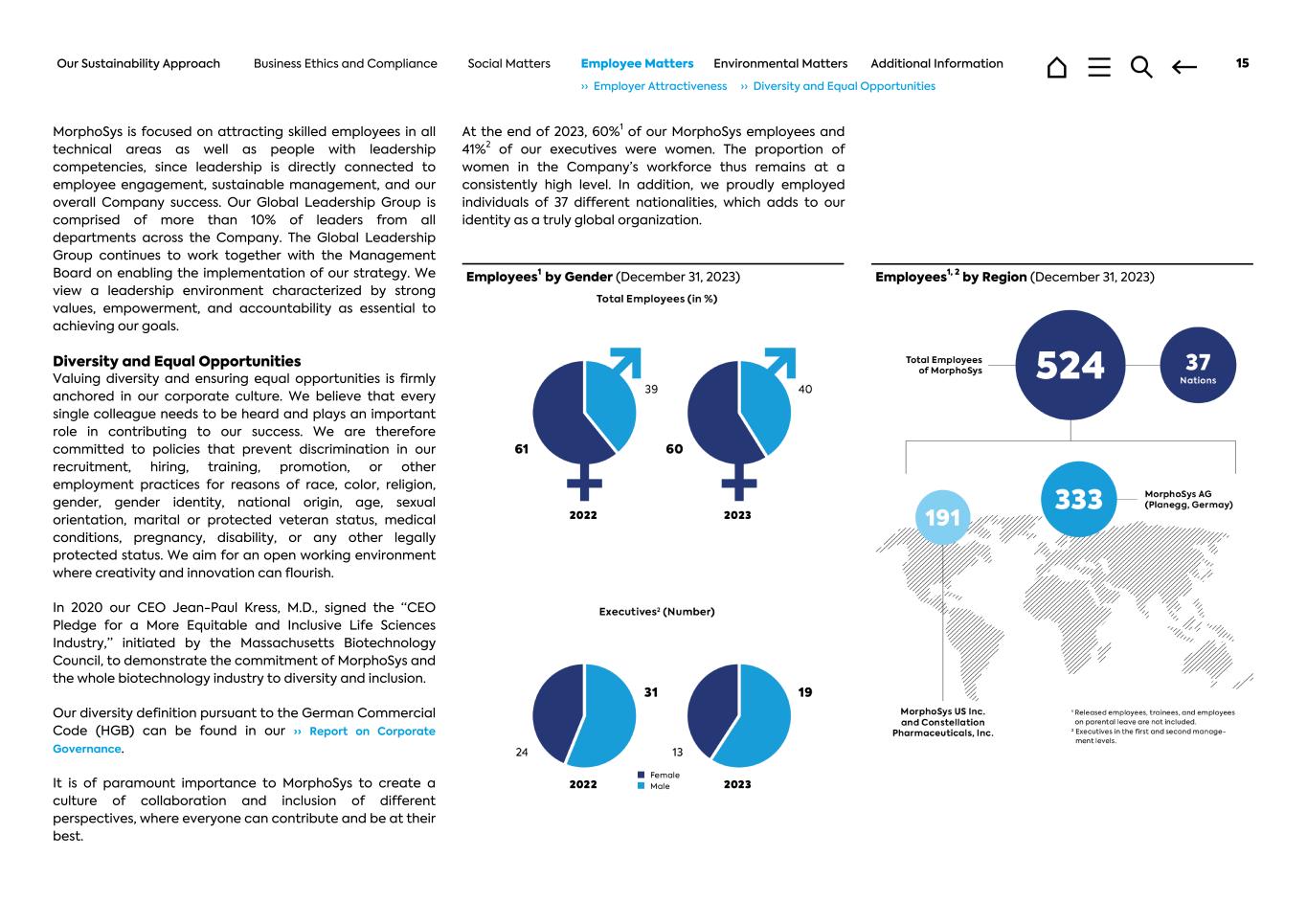
MorphoSys is focused on attracting skilled employees in all technical areas as well as people with leadership competencies, since leadership is directly connected to employee engagement, sustainable management, and our overall Company success. Our Global Leadership Group is comprised of more than 10% of leaders from all departments across the Company. The Global Leadership Group continues to work together with the Management Board on enabling the implementation of our strategy. We view a leadership environment characterized by strong values, empowerment, and accountability as essential to achieving our goals. Diversity and Equal Opportunities Valuing diversity and ensuring equal opportunities is firmly anchored in our corporate culture. We believe that every single colleague needs to be heard and plays an important role in contributing to our success. We are therefore committed to policies that prevent discrimination in our recruitment, hiring, training, promotion, or other employment practices for reasons of race, color, religion, gender, gender identity, national origin, age, sexual orientation, marital or protected veteran status, medical conditions, pregnancy, disability, or any other legally protected status. We aim for an open working environment where creativity and innovation can flourish. In 2020 our CEO Jean-Paul Kress, M.D., signed the “CEO Pledge for a More Equitable and Inclusive Life Sciences Industry,” initiated by the Massachusetts Biotechnology Council, to demonstrate the commitment of MorphoSys and the whole biotechnology industry to diversity and inclusion. Our diversity definition pursuant to the German Commercial Code (HGB) can be found in our ›› Report on Corporate Governance. It is of paramount importance to MorphoSys to create a culture of collaboration and inclusion of different perspectives, where everyone can contribute and be at their best. At the end of 2023, 60%1 of our MorphoSys employees and 41%2 of our executives were women. The proportion of women in the Company’s workforce thus remains at a consistently high level. In addition, we proudly employed individuals of 37 different nationalities, which adds to our identity as a truly global organization. Employees1 by Gender (December 31, 2023) Employees1, 2 by Region (December 31, 2023) Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 15 ›› Employer Attractiveness ›› Diversity and Equal Opportunities
Focus in 2023 During 2023 the goal was still to focus on further enhancing our diversity and inclusion efforts by driving focused initiatives with employee networks, diversified supplier networks, and social responsibility initiatives in our communities. Our Women’s Network and our LGBTQ+ Network "MOR Pride" championed a number of initiatives to foster awareness and understanding for diversity, equity and inclusion issues. MorphoSys is committed to a diverse and inclusive environment. A highlight in 2023 was a keynote and a panel discussion in honor of Pride Month on the importance of developing and maintaining a welcoming, inclusive, and respectful work environment, organized by the MOR Pride and Women´s Network. The keynote speaker, Samet Akti, Principal/Lead of Diversity & Inclusion Strategy at Zalando, shared his personal experiences and offered guidance on how to identify unconscious biases, create a thriving workplace culture, avoid stereotyping, be an ally, and successfully address discrimination at work. MorphoSys´ employee networks are grassroots groups sponsored by one of our Executive Committee members. In May 2022, MorphoSys signed the Charta der Vielfalt, one of the biggest employer initiatives in Germany to promote diversity in companies and institutions, which is supported by the Federal Government Commissioner for Migration, Refugees, and Integration. In 2024, we will be working on further initiatives to strengthen our open and inclusive culture. Based on the CSRD, we have evaluated the following key figures: Disclosure Requirement S1-6 – Characteristics of the Undertaking’s Employees These data relate to MorphoSys AG, MorphoSys US Inc., and Constellation Pharmaceuticals as of the reporting date of December 31, 2023, including inactive employees. Gender Number of employees (headcount) Male 220 Female 338 Other Not reported Total employees 558 Country Number of employees (headcount) Germany 367 USA 191 2023 Female Male Other* Not disclosed Total Number of employees (FTE) 321.9 219 540.9 Number of permanent employees (FTE) 315.4 211.5 526.9 Number of temporary employees (FTE) 6.5 7.5 14 Number of non-guaranteed-hours employees (FTE) 0 0 0 Number of full-time employees (FTE) 278 216 494 Number of part-time employees (FTE) 43.9 3 46.9 *Gender, as indicated by the employees themselves. Total number of employees who left the Company during the reporting period and the rate of employee turnover in the reporting period (01/01/2023 to 12/31/2023) in headcount: 168/27%. The changes in headcount are related to attrition and the termination of work and operations on pre-clinical research programs. Disclosure Requirement S1-7 – Characteristics of Non- Employees in the Undertaking’s Own Workforce Total number of non-employees in the Company’s workforce, i.e., either people with contracts with the Company to supply labor (“self-employed people”) or people provided by undertakings primarily engaged in “employment activities” (NACE Code N78): 51. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 16 ›› Diversity and Equal Opportunities

Disclosure Requirement S1-9 – Diversity Metrics This information relates only to MorphoSys AG including inactive employees. Gender distribution in number and percentage at top management level: 8 female (35%) / 15 male (65%) Distribution of employees by age group: Under 30 years old: 28 employees 30–50 years old: 267 employees Over 50 years old: 72 employees The top management level is defined as the first and second level below the Management Board. Employee Engagement As we are striving to create a working environment that embraces sustainability and social responsibility, the Management Board has made our employee survey a top priority and included the results in our KPIs to make sure they are measured and reviewed regularly. In addition, employee engagement is an important success factor, which gives insights into employee satisfaction and commitment. Focus in 2023 In 2023 we again conducted an employee survey for all MorphoSys employees in the U.S. and Germany to evaluate ESG aspects. The participation rate remained unchanged at 84%. All three dimensions, environment, social, and governance, were rated positively1 at 77% overall. The high participation rate and the favorable responses to many questions are evidence of an open feedback culture and of employees’ high degree of identification with and interest in ESG topics. 1 Overall scores: average favorable score of all 31 questions asked in the 2023 survey. Overall result per dimension (in %) A total of 31 questions were asked. Environmental questions were related to safety in the workplace and employee satisfaction with environmentally friendly practices at MorphoSys. The overall environment score was 81%. Social questions were related to the commitment to patients, quality standards, and career development and training. The overall social score was 81%. Questions on governance related to diversity and inclusion, ethics and compliance, and a learning culture. The overall governance score was 69%. We improved in the dimensions social and governance by 1% and 2% respectively. The environment score has remained the same. Based on these results we had the opportunity to gather valuable feedback, identify potential for improvement, and formulate the following measures for 2024: We will be focusing on providing clarity on career development opportunities at MorphoSys and continuing more information sharing by senior management. The ESG metric is derived from the employee survey and reflects employee engagement. The 2023 score was 72%, an increase of 3% compared with 2022. Improvements were recorded across all areas, the highest increase being in the question about being proud to work for the Company. In 2023 our employees demonstrated a high degree of involvement in their communities by participating in various charity events. In Germany we continued partnering with Tafel Geretsried-Wolfratshausen. MorphoSys AG also donated an emergency power generator for Ukraine to the association humedica e. V. and organized a blood donation drive for employees together with Bayrisches Rotes Kreuz. The U.S. team once again participated in awareness-raising events such as “Light the Night,” organized by the Leukemia and Lymphoma Society, and supported the Boston Health Care’s Homeless Program by collecting needed items. During the national meeting in March, our employees packed and donated comfort items for cancer patients at the Ironwood Cancer Center in Arizona. In December 2023 MorphoSys organized a Christmas charity campaign in the U.S. and Germany. Our colleagues in Boston donated gifts to support families in need in the Boston area. The German team partnered again with an orphanage in Munich, which provides a temporary home for children and young people from different social and cultural backgrounds. MorphoSys AG also donated an additional amount to the Ronald McDonald House in Munich, which has been committed to the health and well-being of children in Germany since 1987. In 2024 we also want to work on further initiatives to engage employees with a combination of communication, discussion forums, and social events in virtual, hybrid, and in- person settings to encourage connections and a sense of belonging across our entire workforce. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 17 ›› Diversity and Equal Opportunities ›› Employee Engagement

Employee Development Employee development remained a key priority at MorphoSys in 2023 and will always be a key component of our Company’s success, covering three main areas: individual development, leadership development, and team development, which all contribute to the healthy development of our organization as a whole. We believe that most development happens on the job, for example by taking on a new job internally or through project work. That is why we keep improving the way we identify our talents (via our annual Talent Talks) and promote internal mobility. Upon request, employees can still have access to 360° assessments as well as to coaching and dedicated training if a need has been identified together with the line manager and in coordination with Human Resources. In order to facilitate the integration in our multicultural teams, we also offer weekly English and German classes. Focus in 2023 In 2023 we continued and further developed the two global programs for new or current leaders: a comprehensive accelerated development program for first-line leaders and a mentoring program. In addition a new module was added to our employee engagement training and a new series of workshops on the topic “Ways of Working” was held for all employees. The third Accelerate Leadership Program cohort started in March 2023. Due to the great success and demand, the fourth, fifth, and sixth cohorts of the Accelerate Leadership Program were also launched in 2023: a total of four cohorts with a total of 50 participants. Of these 50 participants, 26 have already successfully completed this six-month hybrid program in 2023. The other cohorts will be completed next year and a further cohort with 12 participants is already planned for next year. “Meaningful and enjoyable interactions and hands-on experiences, application of good standard models, and rich content.” The mentoring program kicked off in May 2023 and is an annual initiative that will start each year in Q2 and finish in Q1 the year after. This year’s cohort started with 17 mentoring pairs. The employee engagement workshop modules trained our managers in having conversations about drivers and development goals with their team members, accelerating new hire onboarding, and supporting overall employee growth. Due to the positive feedback, another module was repeated at the beginning of 2023 and a third module on “Intercultural Career Conversations” was launched. This third module aims to raise managers´awareness of cultural differences and provide them with tools to navigate the intercultural working environment. MorphoSys continuously supports its employees in adapting to new ways of working, particularly with regard to the new hybrid working situation. In this context, the “Ways of Working” workshop series was established, which included four different mini-workshops ranging from “Facilitating Engaging Online Meetings and Workshops” to “Designing Your Career with Agility” and was offered alternately online and on-site. These courses, led by external trainers with mixed groups from different functions and departments, reached more than 150 participants. In 2023, two further pilot projects were launched as part of continuous employee development: LinkedIn Learning and the “2023 developMOR program.” With the launch of LinkedIn Learning, we are pursuing the goal of learning anytime, anywhere, and at your own pace. The launch of LinkedIn Learning was a great success this year. Employees could take advantage of a variety of courses that are relevant to them both personally and professionally. The ability to learn online allows easy access from anywhere. The “2023 developMOR program” is a U.S. pilot program for even more personal and professional growth opportunities: As part of this pilot program, colleagues are supported in developing their careers through important experiences in the workplace. The program includes a pre-internship in a business unit outside of their current role that promotes personal experience and interaction with another team or in a different function. The pilot program started with two opportunities for an eight-week rotation in a different functional area. We have revised our “ESPRIT” communication around our annual prioritization, feedback, development, and remuneration planning processes with the aim of empowering our managers and their team members. The new communication campaign, which was launched in 2022, was completed in 2023. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 18 ›› Employee Development

MorphoSys continues to recognize the need for personal development and cross-departmental sharing and learning to help people and teams work better together. By supporting these initiatives, which were highly praised by participants, MorphoSys demonstrated in 2023 that the Company understands that high-performing teams are far more than the sum of their members – and that investing in them is the best way to prepare us for the future. Occupational Health and Safety (OHS) MorphoSys believes it has a responsibility to provide a safe, healthy, and clean work environment in accordance with the provisions of our Code of Conduct, while complying with all applicable health, safety, and environmental laws and regulations, Company standards, and best practices. The Health and Safety department reports quarterly to the Head of Facilities, who in turn reports the listed KPIs to the Head of Technical Operations. The New Work model introduced in 2021 was well received by our employees and has become very well established over the past few years. MorphoSys offers its employees a high degree of flexibility in terms of working conditions, with the option to work either remotely or in the office. This flexibility is supported by training, tips, and a wide range of information about remote working (intranet). Hybrid working also makes it possible to take part in meetings from home and also prevent the spread of possible infections. Focus in 2023 Even though the coronavirus pandemic is over, the Health and Safety team continues to meticulously monitor the progress of the global infection situation. In addition, employees continue to be provided with self-testing kits and masks and are regularly informed about the current situation and legal regulations. As part of our business continuity plan in the event of a crisis, in 2021 MorphoSys AG and MorphoSys US Inc. established a Company-wide health emergency plan that includes a local plan for each site to comply with the varying legal requirements and regulations in Germany and the United States. This is reviewed annually. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 19 ›› Employee Development ›› Occupational Health and Safety (OHS)

Principles of Occupational Safety at MorphoSys Introduction of hazardous biological materials for R&D purposes at MorphoSys AG • A dedicated biosafety team as defined by the “Gentechnik Sicherheitsverordnung” (German Genetic Engineering Safety Directive) and Infektionsschutzgesetz (Infection Control Act) • Safety professionals perform an internal audit to assess the risk involved • Specific safety training for the employees working with the substances • Assurance that all safety measures are implemented before actual work commences Only certified companies are authorized by MorphoSys to dispose of chemical waste Lowest possible amounts of hazardous substances used Hazardous organisms and chemicals are processed in laboratories with particular safety standards Only specially trained employees are allowed to work with toxic substances Principles of occupational safety at MorphoSys AG – “Safety in all we do!”: • Establishing a biosafety team in accordance with the Infection Control Act (IfSG) to conduct internal audits to assess the associated risks • Providing specific risk assessments, operating instructions, and safety training for employees working with hazardous substances • Ensuring all safety measures are implemented by both internal and external workers before actual work begins • Contracting only with certified companies to dispose of chemical and all other waste and sending waste (fat, oil, paper, cardboard, electronics, used office furniture, batteries/rechargeable batteries, Styrofoam, garden waste) for recycling when possible • Using the lowest possible amount of hazardous substances and substituting them where possible • Ensuring work with infectious pathogens takes place in laboratories with special safety standards • Permitting only specially trained employees to handle hazardous substances • Making sure all laboratory employees have a very high level of training and are continuously trained and instructed (annual trainings for the office, Laboratory S1, Laboratory S2, IT S2, special technical infrastructure training, new starters, and pregnant employees) • Maintaining MorphoSys’ high safety standards through regular internal inspections conducted by safety officers and the biosafety team • An on-site audit of the genetic engineering laboratories by the Oberbayern local government did not find any deficiencies. • A building inspection by the owner’s insurer revealed only minor defects. • Ensuring the Company’s fire protection plan continues to include regular briefings, evacuation assistance training, evacuation drills (annually) for all employees, and the presence of trained fire protection officers (one external officer and, since 2022, an internal officer) Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 20 ›› Occupational Health and Safety (OHS)

A particular focus in 2023 was on the successful implementation of the Health Week in May 2023 at the Planegg site. This included the following activities: • Cycling (ergonomics, bike fitting and leasing, provision of a “bicycle first aid station”), • Health (heart rate variability test, sugar and cholesterol check, pupillography, health check, special food offers from our Company restaurant, food exhibition), • Two keynote speeches about resilience, • Sport (yoga presentation and exercise break), and • A team competition. During the reporting year, there were two reportable occupational accidents, both of them commuting accidents, at MorphoSys AG, which means the number of occupational accidents remains at a very low level again this year. The number is also significantly lower than the comparable average for the chemical industry in Germany (13.97 reportable occupational accidents per 1,000 full-time employees according to the latest survey by the Employer’s Liability Insurance Association for the Raw Materials and Chemical Industry (BG RCI) in 2022; a reportable accident is defined by the BG RCI as an occupational or commuting accident that causes more than three calendar days of incapacity to work). For 2024 we intend to focus on several areas: • As the Health Week in 2023 was very successful we are planning to spread the health campaigns for 2024 over several days a year with different focus topics: resilience, healthy eating, ergonomics and sport • Updating the transfer of occupational health and safety duties to managers in accordance with DGUV Regulation 1 • Further training for safety officers and inclusion in the occupational health and safety organization • Digitalization of Company medical appointments and instructions Based on the CSRD, we evaluated the following key figures for MorphoSys AG in relation to the number of employees as of December 31, 2023: Disclosure Requirement S1-14 – Health and Safety Metrics a) The percentage of the Company’s own workers who are covered by the Company’s health and safety management system based on legal requirements and/or recognized standards or guidelines: 100% b) The number of fatalities as a result of work-related injuries and work-related ill health: 0 c) The number and rate of recordable work-related accidents: 2 (0.60%) d) The number of cases of recordable work-related ill health, subject to legal restrictions on the collection of data: 0. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 21 ›› Occupational Health and Safety (OHS)

Environmental Matters 04 CONTENTS 22 Emissions Emissions By signing the Paris Agreement, all EU member states have agreed to make their economies and societies climate- neutral by 2050. The German Federal Government made an amendment to the Climate Change Act to tighten climate regulations and enshrine in law the goal of achieving greenhouse gas neutrality by 2045. We at MorphoSys are aware of our responsibility on the path to climate neutrality. Despite our business model of being a biotechnology company and not having our own production sites, we can also make a contribution. CO₂ Balance 2023 according to GHG Protocol (Scope 1 – 3) 2022 Reporting In 2022 we evaluated our greenhouse gas (GHG) emissions for MorphoSys AG’s headquarters in Planegg, Germany, in accordance with the standards of the GHG Protocol methodology. We have not evaluated the data from MorphoSys US Inc. (Boston, Massachusetts, USA) for the baseline reporting. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 22 ›› Emissions

Our total 2022 result for the CO₂ balance in accordance with the GHG Protocol (Scope 1, Scope 2 (market-based) and Scope 3) was 2,236.96 metric tons of CO₂e (100%). The total result including the security mark-up (10%) was 2,460.65 metric tons of CO₂e. 2023 Reporting For 2023, we have included the data from MorphoSys US Inc. in Boston, Massachusetts, in the evaluation of our emissions for the first time. In 2023, we expanded the evaluation of Scope 3 emissions to include emissions from upstream and downstream logistics. As a result, the total value of our Scope 3 emissions has increased significantly compared to 2022. A comparison with the previous year's figures is therefore not possible. We measure and report our Scope 1 and Scope 2 emissions in accordance with the GHG Protocol operational control approach. The emissions in Scope 1 correspond to a total of 101.82 tons of CO₂e and Scope 2 (location-based) to a total of 1,029.12 tons of CO₂e Scope 2 (market-based) corresponds to a total of 1.34 tons of CO₂e. Our Scope 1 data include direct GHG emissions from mobile and stationary combustion as well as fugitive emissions. These are emissions that are directly generated by MorphoSys. Our Scope 2 (market-based) data include indirect GHG emissions from purchased electricity that are not directly generated by us but are a result of our activities. In 2023, we switched our electricity to green electricity, which reduced our Scope 2 (market-based) emissions. Scope 3 emissions, including other indirect GHG emissions, result from our activities that are outside of our control or ownership and result in a total of 18,753.31 metric tons of CO₂e in Scope 3 emissions. An overview of the individual categories: Corporate Carbon Footprint (Scope 3 Details) – Total Result (in t CO₂e) With our extended reporting for 2023, including MorphoSys US Inc. and extended Scope 3 reporting, we want to create more transparency about our Company’s environmental impacts. We will use the data as a basis for further deliberations about our environmental goals and strategies. For the 2024 financial year, we plan to improve our data quality. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 23 ›› Emissions

Additional Information 05 CONTENTS 24 EU Taxonomy Regulation 30 Independent Practitioner’s Report 32 Imprint EU Taxonomy Regulation Background and Objectives of the Regulation The EU Taxonomy Regulation, which was published in the Official Journal of the European Union (EU) on June 22, 2020, and entered into force on July 12, 2020, is a key component of the Action Plan on Financing Sustainable Growth, which is one of the pillars of the European Green Deal. Its main objective is to steer capital flows towards a more sustainable economy in order to achieve the EU’s 2050 climate and energy targets. The EU Taxonomy acts as a classification system for environmentally sustainable economic activities. It aims to increase transparency regarding the degree of sustainability of turnover, investments, and operating expenditures. Reporting for the 2023 Financial Year According to Article 8 of the EU Taxonomy Regulation, all companies that are obliged to provide non-financial group reporting in accordance with Section 315b of the German Commercial Code (HGB) are subject to certain requirements related to the EU Taxonomy. These include the disclosure of the Taxonomy-eligible and Taxonomy-aligned share of group turnover, capital expenditure (CapEx), and operating expenditure (OpEx) for the 2023 reporting period associated with economic activities related to the following environmental targets: I. Climate change mitigation II. Climate change adaptation III. Sustainable use and protection of water and marine resources IV. Transition to a circular economy V. Pollution prevention and control VI. Protection and restoration of biodiversity and ecosystems The publication of the new Delegated Acts on the EU Taxonomy on June 13, 2023, marks a significant milestone. The new Delegated Acts encompass economic activities and technical screening criteria (TSCs) for environmental objectives III–VI, which were previously not covered (Environmental Delegated Act) and amendments to the existing Delegated Regulations on content and presentation and on the TSCs for the climate-related environmental objectives (Climate Delegated Act). Disclosure of taxonomy-eligible economic activities related to environmental objectives III to VI is required for the 2023 reporting period. In addition, disclosure of the Taxonomy- eligibility of new activities in relation to environmental objectives I and II as well as disclosure of the Taxonomy- eligibility and Taxonomy-alignment of existing activities in relation to environmental objectives I and II, including any changes, must be published. Disclosure of Taxonomy- aligned economic activities for environmental objectives III to VI is not required for the reporting period. Basis of Reporting Identification of Taxonomy-Eligible and Taxonomy- Aligned Activities MorphoSys has based the identification of Taxonomy- eligible and Taxonomy-aligned economic activities on the Delegated Acts related to objectives I–II and adaptations to objectives I–II (Climate Delegated Act) as well as the Delegated Act related to objectives III–VI (Environmental Delegated Act). An economic activity is considered Taxonomy-eligible if it is defined in the Delegated Acts for one of the six environmental objectives. This is independent of whether the economic activity described fulfills the TSCs defined in the Delegated Acts. As a first step, MorphoSys reviewed the activity descriptions of the Delegated Acts for consistency with our business activities, taking into account the NACE (Nomenclature statistique des activités économiques dans la Communauté) codes in order to identify Taxonomy- eligible economic activities. As MorphoSys’ business model is to develop and commercialize innovative therapies for patients, our core business activities are not covered by the Delegated Acts. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 24 ›› EU Taxonomy Regulation

In a second step, we performed an analysis of our capital expenditures for the reporting year. As a result, individually Taxonomy-eligible CapEx related to environmental target I was identified that is considered Taxonomy-eligible in accordance with item 6.5 of the EU Taxonomy (“Transport by motorbikes, passenger cars and light commercial vehicles”). Individually Taxonomy-eligible CapEx is capital expenditure that is related to the purchase of products and services from Taxonomy-eligible or Taxonomy-aligned economic activities, as well as individual measures through which the target activities are carried out in a low-carbon manner or the emission of greenhouse gases is reduced (section 1.1.2.2. (c) of Annex 1 to Article 8, Climate Delegated Act). These capital expenditures relate to leased Company vehicles available to selected employees of MorphoSys AG. In order to achieve Taxonomy alignment, a test scheme consisting of TSCs must be passed. These TSCs consist of criteria for a significant contribution to achieving the respective environmental goal. But at the same time, it must be ensured that none of the other five environmental goals are significantly impaired (Do No Significant Harm, DNSH). The TSCs also contain specific information on this. Separate TSCs are defined for each environmental objective. Furthermore, minimum protection requirements need to be adhered to. We have analyzed the Taxonomy alignment of the identified Taxonomy-eligible CapEx. Although our electric / hybrid vehicles produce low emissions during operation and could therefore potentially meet the requirements for a significant contribution to the environmental objective of climate change mitigation, we are currently not in a position to fully comply with the strict requirements of the EU Taxonomy. Simultaneous compliance with the criteria for fuel efficiency and rolling noise of the tires is a significant requirement of DNSH. For this reason, we analyzed the specific characteristics of the tires for each significant vehicle added in the reporting year using the EPREL database and determined whether the tires would fulfill the criteria to substantially contribute to environmental objective V in accordance with DNSH. Due to the fact that the tires in question do not meet the highest classes for fuel efficiency and external rolling noise as defined in the respective product classes, they do not meet the requirements for Taxonomy alignment. Accounting Policy The specification of the key performance indicators (KPIs) is determined in accordance with Annex 1 to Article 8 of the Climate Delegated Act. We determine the Taxonomy- eligible and Taxonomy-aligned KPIs in accordance with the legal requirements. In the following section we describe the accounting policy for turnover, CapEx, and OpEx. Turnover KPI Definition The turnover KPI is defined as Taxonomy-eligible and Taxonomy-aligned turnover from product sales, license fees, milestone payments, service fees, and royalties (numerator) divided by our total Group turnover (denominator). For further details on our accounting policies regarding our total turnover, please see ›› page 109 of our 2023 Annual Report. As we have not identified any Taxonomy-eligible and Taxonomy-aligned revenue-generating activities for the 2023 financial year, this results in a share of Taxonomy- eligible economic activities in our total turnover of 0%. As the numerator is zero, there is no risk of double counting of economic activities. Reconciliation Our consolidated turnover is reflected in our Consolidated Statement of Profit or Loss (IFRS) on ›› page 99 of our 2023 Annual Report (“Revenues”). Capital Expenditure (CapEx) KPI Definition The CapEx KPI is defined as Taxonomy-eligible and Taxonomy-aligned CapEx (numerator) divided by our total CapEx (denominator). The denominator comprises additions to property, plant, and equipment and intangible assets during the financial year under review before depreciation, amortization, and remeasurements, including those resulting from revaluations and impairments, and excluding changes in fair value. It includes additions to fixed assets (IAS 16), intangible assets (IAS 38), and right-of-use assets (IFRS 16). Additions resulting from business combinations are also included. Goodwill is not included in CapEx as it is not defined as an intangible asset under IAS 38. Due to the small number of leased vehicles underlying the Taxonomy-eligible CapEx and the allocation to only one environmental target, there is no risk of double counting of economic activities. For further details on our accounting policies regarding our CapEx, please see ›› pages 115 - 117 of our 2023 Annual Report. Reconciliation Our total CapEx can be reconciled to our Consolidated Balance Sheet (IFRS) on ›› page 101 of our 2023 Annual Report (“Property, plant, and equipment,” “Intangible assets”) and to our Notes to the Balance Sheet on ›› page 127 (“4.8 Property, plant, and equipment – additions”), on ›› page 128 (“4.9 Leases – additions”), and on ›› page 130 (“4.10 Intangible assets – additions”). Operating Expenditure (OpEx) KPI Definition The OpEx KPI is defined as Taxonomy-eligible and Taxonomy-aligned OpEx (numerator) divided by our total OpEx as defined by the EU Taxonomy (denominator). Our OpEx has been determined via the following accounts as of the reporting date, December 31, 2023: non- capitalized research and development costs, non- capitalized building renovation costs, short-term leases, maintenance and repair costs. This does not include depreciation, amortization, impairment, and raw material costs. For further details on our accounting policies regarding our OpEx, please see ›› page 110 - 111 of our 2023 Annual Report. As the numerator is zero, there is no risk of double counting of economic activities. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 25 ›› EU Taxonomy Regulation

Explanations of the Numerators of the Turnover KPI, the CapEx KPI, and the OpEx KPI As MorphoSys has not identified any significant Taxonomy- eligible and Taxonomy-aligned Group turnover and OpEx for the reporting year 2023, we do not recognize any Group turnover or OpEx in the numerator of the corresponding key figures. For MorphoSys, only “Category C” applies with regard to CapEx. In the 2023 reporting year, we identified category C CapEx of € 106,380. MorphoSys has no economic activities in the area of fossil gas and nuclear energy. Template 1 Nuclear and fossil gas related activities Nuclear energy related activities 1 The undertaking carries out, funds or has exposures to research, development, demonstration and deployment of innovative electricity generation facilities that produce energy from nuclear processes with minimal waste from the fuel cycle. NO 2 The undertaking carries out, funds or has exposures to construction and safe operation of new nuclear installations to produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production, as well as their safety upgrades, using best available technologies. NO 3 The undertaking carries out, funds or has exposures to safe operation of existing nuclear installations that produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production from nuclear energy, as well as their safety upgrades. NO Fossil gas related activities 4 The undertaking carries out, funds or has exposures to construction or operation of electricity generation facilities that produce electricity using fossil gaseous fuels. NO 5 The undertaking carries out, funds or has exposures to construction, refurbishment, and operation of combined heat/cool and power generation facilities using fossil gaseous fuels NO 6 The undertaking carries out, funds or has exposures to construction, refurbishment and operation of heat generation facilities that produce heat/cool using fossil gaseous fuels. NO Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 26 ›› EU Taxonomy Regulation

Proportion of Turnover from Products or Services Associated with Taxonomy-Aligned Economic Activities – Disclosure Covering Year 2023 Fiscal year 2023 Substantial contribution criteria DNSH criteria (“Do No Significant Harm”) Economic Activities (1) C od e (2 ) Tu rn ov er (3 ) Pr op or ti on o f t ur no ve r, y ea r 20 23 (4 ) C lim at e ch an ge m it ig at io n (5 ) C lim at e ch an ge ad ap ta ti on (6 ) W at er (7 ) Po llu ti on (8 ) C ir cu la r e co no m y (9 ) Bi od iv er si ty (1 0) C lim at e ch an ge m it ig at io n (1 1) C lim at e ch an ge ad ap ta ti on (1 2) W at er (1 3) Po llu ti on ( 14 ) C ir cu la r e co no m y (1 5) Bi od iv er si ty (1 6) M in im um s af eg ua rd s (1 7) Proportion of Taxonomy- aligned (A.1.) or -eligible (A.2.) turnover, year 2023 (18) Category enabling activity (19) Category transitional activity (20) In million euro % Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1.) 0 0% 0% 0% 0% 0% 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% Of which enabling 0 0% 0% 0% 0% 0% 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% n/a Of which transitional 0 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% n/a A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2.) 0 0% 0% 0% 0% 0% 0% 0% 0% A. Turnover of Taxonomy-eligible activities (A.1.+A.2.) 0 0% 0% 0% 0% 0% 0% 0% 0% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES Turnover of Taxonomy-non-eligible activities 238.3 100% Total 238.3 100% Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 27 ›› EU Taxonomy Regulation

Proportion of CapEx from Products or Services Associated with Taxonomy-Aligned Economic Activities – Disclosure Covering Year 2023 Fiscal year 2023 Substantial contribution criteria DNSH criteria (“Do No Significant Harm”) Economic Activities (1) C od e (2 ) C ap Ex (3 ) Pr op or ti on o f C ap Ex , y ea r 20 23 (4 ) C lim at e ch an ge m it ig at io n (5 ) C lim at e ch an ge ad ap ta ti on (6 ) W at er (7 ) Po llu ti on (8 ) C ir cu la r e co no m y (9 ) Bi od iv er si ty (1 0) C lim at e ch an ge m it ig at io n (1 1) C lim at e ch an ge ad ap ta ti on (1 2) W at er (1 3) Po llu ti on ( 14 ) C ir cu la r e co no m y (1 5) Bi od iv er si ty (1 6) M in im um s af eg ua rd s (1 7) Proportion of Taxonomy- aligned (A.1.) or -eligible (A.2.) CapEx, year 2023 (18) Category enabling activity (19) Category transitional activity (20) In million euro % Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1.) 0 0% 0% 0% 0% 0% 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% Of which enabling 0 0% 0% 0% 0% 0% 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% n/a Of which transitional 0 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% n/a A.2. Taxonomy-eligible but not environ- mentally sustainable activities (not Taxonomy-aligned activities) EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2.) 6.5 0.1 2% 2% 0% 0% 0% 0% 0% 0% A. CapEx of Taxonomy-eligible activities (A.1.+A.2.) 0.1 2% 2% 0% 0% 0% 0% 0% 0% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES CapEx of Taxonomy-non-eligible activities 4.3 98% Total 4.4 100% Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 28 ›› EU Taxonomy Regulation
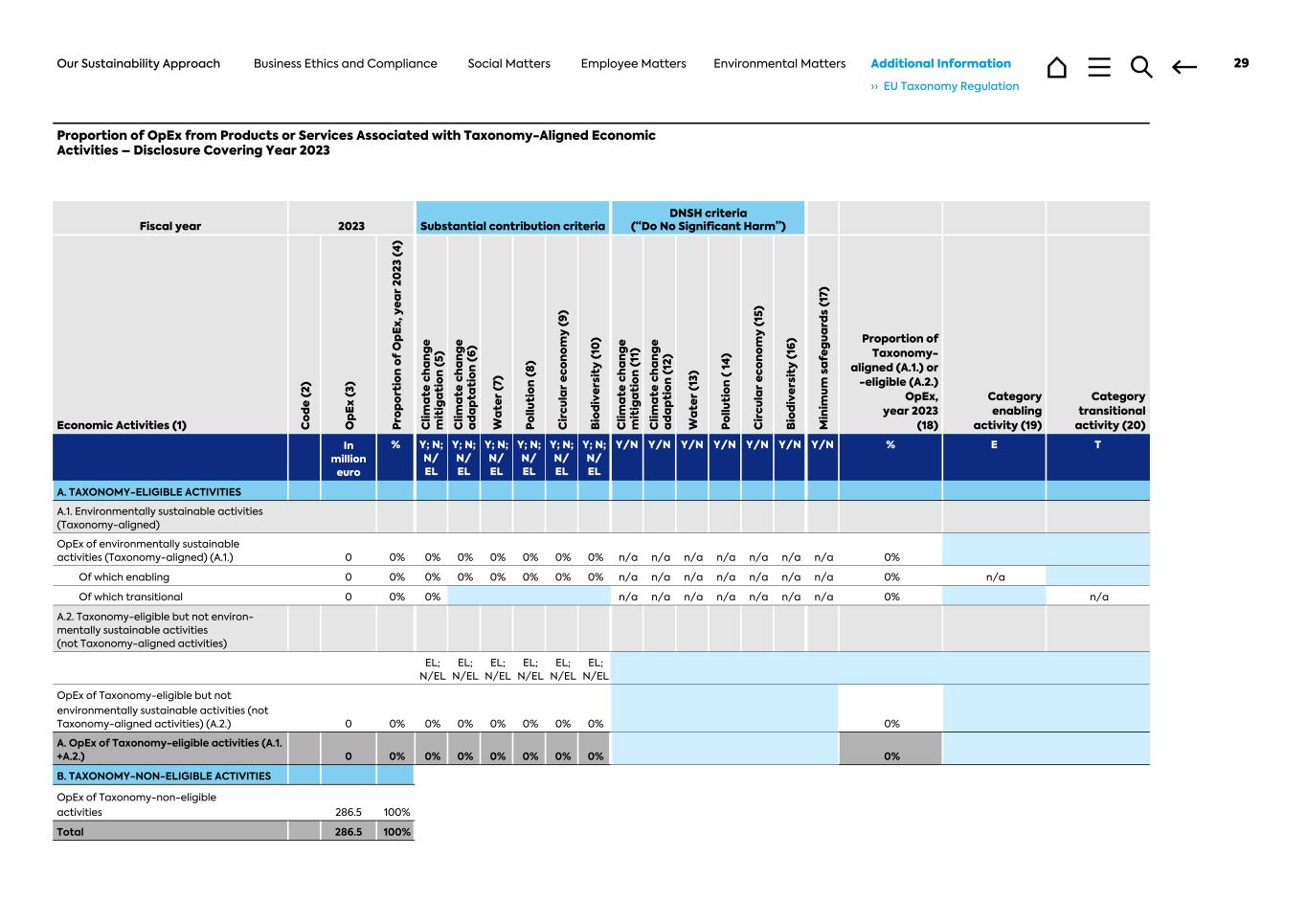
Proportion of OpEx from Products or Services Associated with Taxonomy-Aligned Economic Activities – Disclosure Covering Year 2023 Fiscal year 2023 Substantial contribution criteria DNSH criteria (“Do No Significant Harm”) Economic Activities (1) C od e (2 ) O pE x (3 ) Pr op or ti on o f O pE x, y ea r 2 02 3 (4 ) C lim at e ch an ge m it ig at io n (5 ) C lim at e ch an ge ad ap ta ti on (6 ) W at er (7 ) Po llu ti on (8 ) C ir cu la r e co no m y (9 ) Bi od iv er si ty (1 0) C lim at e ch an ge m it ig at io n (1 1) C lim at e ch an ge ad ap ti on (1 2) W at er (1 3) Po llu ti on ( 14 ) C ir cu la r e co no m y (1 5) Bi od iv er si ty (1 6) M in im um s af eg ua rd s (1 7) Proportion of Taxonomy- aligned (A.1.) or -eligible (A.2.) OpEx, year 2023 (18) Category enabling activity (19) Category transitional activity (20) In million euro % Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y; N; N/ EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1.) 0 0% 0% 0% 0% 0% 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% Of which enabling 0 0% 0% 0% 0% 0% 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% n/a Of which transitional 0 0% 0% n/a n/a n/a n/a n/a n/a n/a 0% n/a A.2. Taxonomy-eligible but not environ- mentally sustainable activities (not Taxonomy-aligned activities) EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2.) 0 0% 0% 0% 0% 0% 0% 0% 0% A. OpEx of Taxonomy-eligible activities (A.1. +A.2.) 0 0% 0% 0% 0% 0% 0% 0% 0% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES OpEx of Taxonomy-non-eligible activities 286.5 100% Total 286.5 100% Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 29 ›› EU Taxonomy Regulation
Imprint MorphoSys AG Semmelweisstrasse 7 82152 Planegg Germany Phone: +49-89-89927-0 Fax: +49-89-89927-222 Email: info@morphosys.com Website: www.morphosys.com/en Investor Relations Phone: +49-89-89927-404 Fax: +49-89-89927-5404 Email: investors@morphosys.com Concept and Design 3st kommunikation GmbH, Mainz Photography/Picture Credits Getty Images Editorial Office Götz Translations and Proofreading GmbH, Hamburg Typesetting and Lithography 3st kommunikation GmbH, Mainz Publication date March 13, 2024 This Non-Financial Report is also published in German and is available to download on our website. For better readability, this report uses the masculine form only but refers equally to all genders. HuCAL® and Ylanthia® are registered trademarks of the MorphoSys Group. As of February 5, 2024, Monjuvi® and Minjuvi® are registered trademarks of Incyte. Our Sustainability Approach Business Ethics and Compliance Social Matters Employee Matters Environmental Matters Additional Information 32 ›› Imprint





























