
Half-Year Report JANUARY – JUNE 2024

Contents MorphoSys Group: Half-Year Report January – June 2024 3 Summary 6 Interim Group Management Report 6 Operating Business Performance 11 Strategy and Group Management 11 General Business and Market Environment 12 Intellectual Property 12 Human Resources 12 Financial Analysis 19 Interim Consolidated Financial Statements 19 Consolidated Statement of Profit or Loss (IFRS) 20 Consolidated Statement of Comprehensive Income (IFRS) 21 Consolidated Balance Sheet (IFRS) 23 Consolidated Statement of Changes in Stockholder’s Equity (IFRS) 25 Consolidated Statement of Cash Flows (IFRS) 27 Notes to the Consolidated Financial Statements 2 Group Interim Statement MorphoSys – II/2024

Summary of the Second Quarter of 2024 Corporate Developments • On February 5, 2024, MorphoSys announced that it entered into a Business Combination Agreement with Novartis BidCo AG (formerly trading as Novartis data42 AG) and Novartis AG according to which Novartis BidCo AG intended to submit a voluntary public takeover offer for all of MorphoSys’ outstanding common shares in exchange for payment of € 68.00 per share. The corresponding voluntary public takeover offer was then published on April 11, 2024, by Novartis BidCo AG at an offer price of € 68.00 per MorphoSys share following approval by the German Federal Financial Supervisory Authority (BaFin) on April 11, 2024. • Separately, MorphoSys entered into a purchase agreement (the "Purchase Agreement") with Incyte Corporation ("Incyte") to sell and transfer to Incyte all rights worldwide related to tafasitamab for a purchase price of US$ 25.0 million. MorphoSys and Incyte have been collaborating on the development and commercialization of tafasitamab since 2020. Prior to this agreement, tafasitamab was co-marketed in the U.S. by MorphoSys and Incyte as Monjuvi® (tafasitamab-cxix) and outside the U.S. by Incyte as Minjuvi®. • On March 22, 2024, MorphoSys announced the receipt of the last outstanding antitrust clearance in the U.S. in connection with takover offer of Novartis BidCo AG, following the receipt of antitrust clearance in Germany and Austria. • The acceptance period of the takeover offer of Novartis BidCo AG ended on May 13, 2024, and the additional acceptance period ended on May 30, 2024. Until expiration of the acceptance period on May 13, 2024, 25,610,813 MorphoSys shares were tendered, with the transfer of these MorphoSys shares to Novartis BidCo AG being completed on May 23, 2024. As a result, Novartis BidCo AG became the majority shareholder of MorphoSys, making MorphoSys a part of the Novartis Group. Together with the MorphoSys shares tendered during the additional acceptance period, ultimately 29,336,378 MorphoSys shares (corresponding to approximately 77.78% of the share capital of MorphoSys and approximately 77.89% of the voting share capital of MorphoSys) were tendered and transferred to Novartis BidCo AG in the course of the takeover offer. Through further acquisitions until June 16, 2024, Novartis BidCo AG has increased its stake in MorphoSys to 34,337,809 MorphoSys shares (corresponding to approximately 91.04% of the share capital, or approximately 91.17% of the voting share capital of MorphoSys). • On May 22, 2024, Biogen Inc. (Biogen) and Human Immunology Biosciences (HI-Bio), in which MorphoSys holds an investment in associates announced that the companies have entered into an agreement which stipulates that Biogen will acquire HI-Bio for US$ 1.15 billion (€ 1.07 billion) upfront and up to US$ 650 million (€ 607 million) in potential milestone payments. HI-Bio is investigating felzartamab which was originally developed by MorphoSys. The acquisition has been completed in July 2024. More details can be found in the section "Subsequent Events". • Following the closing of the takeover offer by Novartis BidCo AG to the shareholders of MorphoSys AG and the resignation of Marc Cluzel, M.D., Ph.D., George Golumbeski, Ph.D., Krisja Vermeylen, Michael Brosnan and Andrew Cheng, M.D., Ph.D. as members of the Supervisory Board, the Munich Local Court appointed Heinrich Moisa, Romain Lege and Silke Mainka as new members of the Supervisory Board. The newly composed Supervisory Board held its first meeting on June 6, 2024, and resolved to appoint Arkadius Pichota, Ph.D., and Lukas Gilgen as new members of the management board. Arkadius Pichota, Ph.D., who previously served as President, General Manager and Chairman of the Board of the Novartis company Navigate BioPharma Services, Inc., has been appointed as the new CEO, and Lukas Gilgen, who previously Group Interim Statement 3 MorphoSys – II/2024

served as Transaction Lead Enterprise Projects with Novartis International AG, has been appointed as the new CFO. At the same time, the membership of Jean-Paul Kress, M.D., and Lucinda Crabtree, Ph.D., on the Management Board ended on June 6, 2024. Heinrich Moisa, Romain Lege, Silke Mainka and Christian Diehl were proposed to the Annual General Meeting of MorphoSys convoked for August 27, 2024, for election as members of the Supervisory Board. • On June 20, 2024, MorphoSys announced that the company has entered into a delisting agreement with Novartis AG and Novartis BidCo AG in which it was agreed that Novartis BidCo AG publishes a public delisting purchase offer for all outstanding ordinary shares of MorphoSys not held by Novartis companies. Furthermore, on June 20, 2024 Novartis BidCo Germany AG, a subsidiary of Novartis BidCo AG informed MorphoSys of its intention to merge MorphoSys into Novartis BidCo Germany, Munich by initiating a squeeze-out of MorphoSys’ minority shareholders following the transfer of Novartis BidCo AG’s stake in MorphoSys of 34,337,809 MorphoSys shares (corresponding to approximately 91.04% of the share capital of MorphoSys) to Novartis BidCo Germany AG on June 19, 2024. R&D Highlights of the Second Quarter of 2024 • On May 31, 2024, MorphoSys presented new efficacy and safety data from the Phase 3 MANIFEST-2 trial of pelabresib, an investigational BET inhibitor, in combination with the JAK inhibitor ruxolitinib in JAK inhibitor-naïve patients with myelofibrosis during an oral presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. • On June 1, 2024, new data from the Phase 2 study of tulmimetostat, an investigational next-generation dual inhibitor of EZH2 and EZH1, in patients with advanced solid tumors or hematologic malignancies have been showcased in a poster presentation at ASCO 2024. Financial Results for the First Half-Year of 2024 • The financial results presented for the first half-year of 2024 related to continued business operations of MorphoSys and the figures in connection with "Discontinued Operations and Assets Held for Sale". Due to the announcement on February 5, 2024, to sell and transfer tafasitamab to Incyte, the entire tafasitamab business has been classified as discontinued operations in accordance with IFRS 5. Furthermore, on May 22, 2024 Biogen Inc. headquartered in Cambridge, Massachusetts, USA, announced that it had entered into an agreement to acquire Human Immunology Bioscience (HI-Bio). The share in HI-Bio is now classified as "held for sale" by MorphoSys and presented separately. Consequently, the figures reported for the first half-year of 2023 were adapted due to these changes in presentation. • Group revenues from continued operations amounted to € 62.2 million (H1 2023: € 50.9 million) and mainly included revenues from royalties and to a smaller extent from milestones. • Research and development expenses in the first half-year of 2024 amounted to € 134.8 million (H1 2023: € 105.0 million). In the first half-year of 2024 the combined expenses for selling and general and administration totaled € 233.3 million (H1 2023: € 35.5 million). • Cash and other financial assets totaled € 512.3 million as of June 30, 2024 (December 31, 2023: € 680.5 million). • As a consequence of the sale and transfer of tafasitamab to Incyte on February 5, 2024, MorphoSys' 2024 financial guidance published on January 30, 2024, could not be maintained and therefore was revoked. For the time being, MorphoSys will no longer make a forecast for revenues from product sales, as no such revenues are expected to be realized this year. • As a consequence of the Novartis takeover, MorphoSys is now including the effects from the implementation of the takeover in its guidance. Consequently, the Group now expects R&D expenses of € 205 million to € 225 million for the 2024 which mainly represent our investments in the development of pelabresib and tulmimetostat. Selling, administrative and general expenses are now expected to be between € 260 million and € 270 million. 4 Group Interim Statement MorphoSys – II/2024
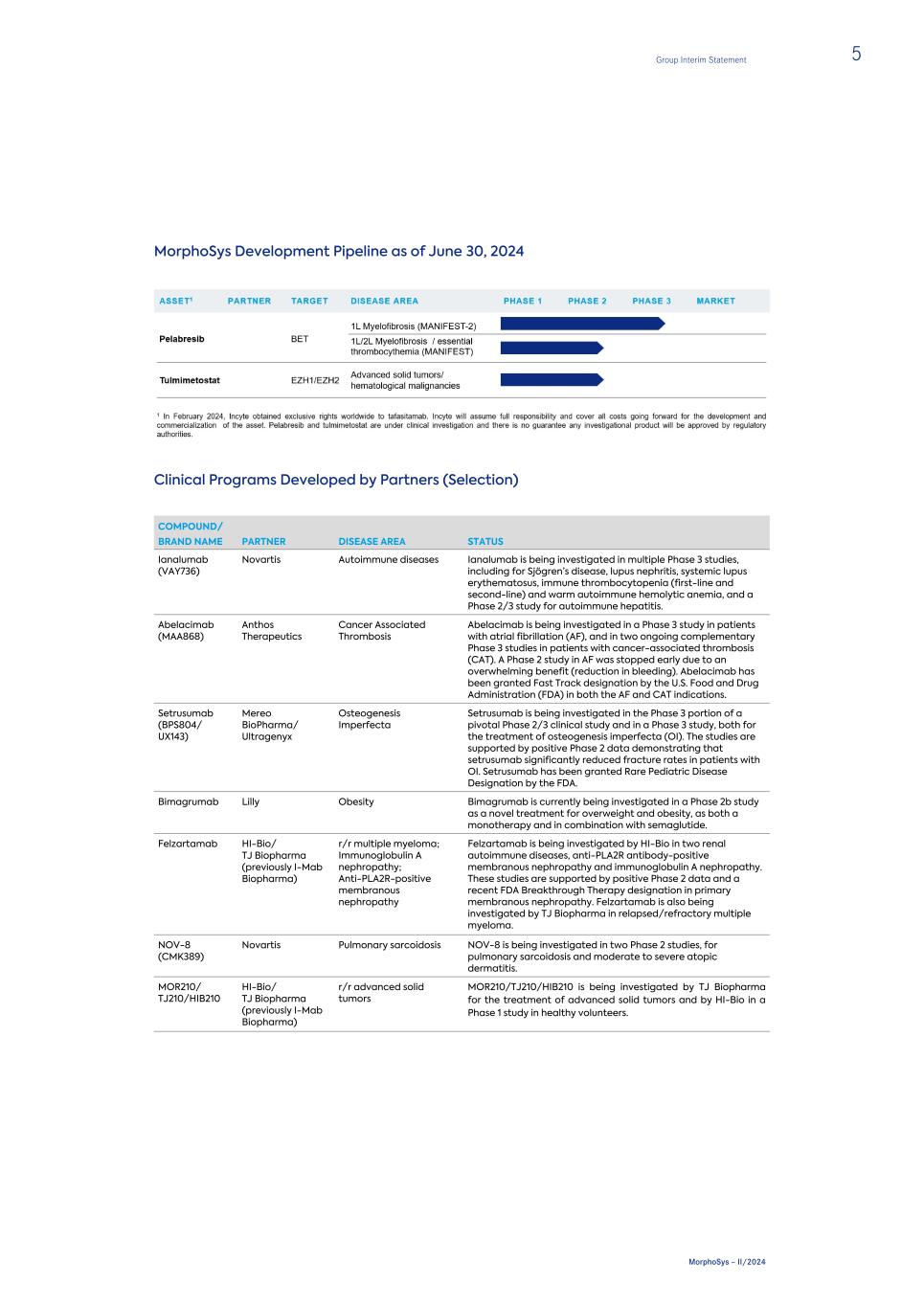
MorphoSys Development Pipeline as of June 30, 2024 Clinical Programs Developed by Partners (Selection) COMPOUND/ BRAND NAME PARTNER DISEASE AREA STATUS Ianalumab (VAY736) Novartis Autoimmune diseases Ianalumab is being investigated in multiple Phase 3 studies, including for Sjögren’s disease, lupus nephritis, systemic lupus erythematosus, immune thrombocytopenia (first-line and second-line) and warm autoimmune hemolytic anemia, and a Phase 2/3 study for autoimmune hepatitis. Abelacimab (MAA868) Anthos Therapeutics Cancer Associated Thrombosis Abelacimab is being investigated in a Phase 3 study in patients with atrial fibrillation (AF), and in two ongoing complementary Phase 3 studies in patients with cancer-associated thrombosis (CAT). A Phase 2 study in AF was stopped early due to an overwhelming benefit (reduction in bleeding). Abelacimab has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in both the AF and CAT indications. Setrusumab (BPS804/ UX143) Mereo BioPharma/ Ultragenyx Osteogenesis Imperfecta Setrusumab is being investigated in the Phase 3 portion of a pivotal Phase 2/3 clinical study and in a Phase 3 study, both for the treatment of osteogenesis imperfecta (OI). The studies are supported by positive Phase 2 data demonstrating that setrusumab significantly reduced fracture rates in patients with OI. Setrusumab has been granted Rare Pediatric Disease Designation by the FDA. Bimagrumab Lilly Obesity Bimagrumab is currently being investigated in a Phase 2b study as a novel treatment for overweight and obesity, as both a monotherapy and in combination with semaglutide. Felzartamab HI-Bio/ TJ Biopharma (previously I-Mab Biopharma) r/r multiple myeloma; Immunoglobulin A nephropathy; Anti-PLA2R-positive membranous nephropathy Felzartamab is being investigated by HI-Bio in two renal autoimmune diseases, anti-PLA2R antibody-positive membranous nephropathy and immunoglobulin A nephropathy. These studies are supported by positive Phase 2 data and a recent FDA Breakthrough Therapy designation in primary membranous nephropathy. Felzartamab is also being investigated by TJ Biopharma in relapsed/refractory multiple myeloma. NOV-8 (CMK389) Novartis Pulmonary sarcoidosis NOV-8 is being investigated in two Phase 2 studies, for pulmonary sarcoidosis and moderate to severe atopic dermatitis. MOR210/ TJ210/HIB210 HI-Bio/ TJ Biopharma (previously I-Mab Biopharma) r/r advanced solid tumors MOR210/TJ210/HIB210 is being investigated by TJ Biopharma for the treatment of advanced solid tumors and by HI-Bio in a Phase 1 study in healthy volunteers. Group Interim Statement 5 MorphoSys – II/2024

Interim Group Management Report: January 1 – June 30, 2024 Operating Business Performance MorphoSys AG (hereinafter also referred as "MorphoSys") focuses on advancing product candidates at various stages of development, positioning itself for long-term sustainable growth. The key measures of value for MorphoSys’ development activities include: • Advancement of development programs and product approvals • Clinical trial results • Regulatory interactions with (or feedback from) health authorities regarding the approval of new drug candidates or of marketed drugs for additional indications • Strong patent protection to secure MorphoSys’ market position Research and Development MorphoSys’ research and development activities are currently focused on the following clinical candidates: • Pelabresib1 (CPI-0610) is an investigational selective small-molecule BET inhibitor designed to promote anti-tumor activity by specifically inhibiting the function of BET proteins. The clinical development of pelabresib is currently focused on myelofibrosis (MF). MF is a form of bone marrow cancer that disrupts the body's normal production of blood cells and is characterized by four hallmarks: an enlarged spleen, anemia, bone marrow fibrosis and disease-associated symptoms. • Tulmimetostat (CPI‑0209) is an investigational small-molecule, second-generation dual EZH2 and EZH1 inhibitor with an epigenetic mechanism of action. Tulmimetostat was designed to improve on first generation EZH2 inhibitors through increased potency, longer residence time on target and a longer half- life, offering the potential for enhanced anti-tumor activity. Tulmimetostat is being investigated in a basket study of solid tumors and lymphomas. In addition to MorphoSys’ own pipeline, the following programs, among others, are being further developed by MorphoSys’ partners: • Ianalumab (VAY736) – a fully human IgG1/k mAb with a dual mode of action targeting B-cell lysis and BAFF-R blockade, developed by Novartis; • Abelacimab (MAA868) – an antibody directed against Factor XI, developed by Anthos Therapeutics; • Setrusumab (BPS804) – an antibody directed against sclerostin, developed by Ultragenyx and Mereo BioPharma; • Bimagrumab - an antibody binding to activin type II receptors, developed by Lilly; • Felzartamab – a therapeutic human monoclonal antibody directed against CD38, developed by HI-Bio and TJ Biopharma (previously I-Mab Biopharma); • MOR210/TJ210/HIB210 – a human antibody directed against C5aR1, the receptor of the complement factor C5a, developed by HI-Bio and TJ Biopharma (previously I-Mab Biopharma). In addition to the late-stage partnered programs listed above, there are several additional partnered programs in early to mid-stage research and development. 6 Group Interim Statement MorphoSys – II/2024 1The development of pelabresib was funded in part by The Leukemia and Lymphoma Society®.

Development of Tafasitamab On February 5, 2024, Incyte obtained exclusive rights worldwide to tafasitamab. Incyte assumes full responsibility and covers all costs going forward for the development and commercialization of the asset. From January 1, 2024 until February 5, 2024, Monjuvi® sales amounted to € 5.9 million and are classified under discontinued operations. Proprietary Clinical Development Studies of Pelabresib There are currently two ongoing trials evaluating pelabresib in myelofibrosis (MF), the Phase 2 MANIFEST trial and the Phase 3 MANIFEST-2 trial. MANIFEST is a global, multicenter, open-label Phase 2 study that evaluates pelabresib as a monotherapy or in combination with ruxolitinib (marketed as Jakafi®/Jakavi®), the current standard of care. MANIFEST‑2 is a global, multicenter, double-blind, randomized Phase 3 clinical study evaluating pelabresib plus ruxolitinib versus placebo plus ruxolitinib in JAK-inhibitor-naïve patients with primary MF or post- essential thrombocythemia (post-ET) or post-polycythemia vera (post-PV) MF who have splenomegaly and symptoms requiring therapy. On November 20, 2023, MorphoSys announced topline results from the Phase 3 MANIFEST-2 study. MANIFEST-2 met its primary endpoint, as the combination therapy demonstrated a statistically significant and clinically meaningful improvement in the proportion of patients achieving at least a 35% reduction in spleen volume (SVR35) at week 24. The key secondary endpoints assessing symptom improvement – proportion of patients achieving at least a 50% reduction in total symptom score (TSS50) and absolute change in total symptom score (TSS) from baseline at week 24 – showed a positive trend favoring the pelabresib and ruxolitinib combination. 430 JAK inhibitor-naïve adult patients with myelofibrosis were randomized for this study. On December 10, 2023, detailed findings of the MANIFEST-2 study were presented during an oral presentation at the 65th American Society for Hematology (ASH) Annual Meeting and Exposition: In the MANIFEST-2 study, pelabresib and ruxolitinib demonstrated a near doubling in the proportion of patients achieving a ≥35% reduction in spleen volume (SVR35) at 24 weeks, the primary endpoint, versus placebo plus ruxolitinib (p<0.001). For the first key secondary endpoint assessing symptom reduction, absolute change in total symptom score (TSS) at 24 weeks, there was a strong numerical improvement for patients receiving pelabresib and ruxolitinib versus placebo plus ruxolitinib. The response rate for the second key secondary endpoint, proportion of patients achieving ≥50% reduction in symptom score (TSS50) at 24 weeks, was also numerically greater for patients receiving pelabresib and ruxolitinib. The proportion of patients achieving both SVR35 and TSS50 at 24 weeks was doubled with pelabresib and ruxolitinib versus placebo plus ruxolitinib (40.2% vs. 18.5%, respectively). Group Interim Statement 7 MorphoSys – II/2024
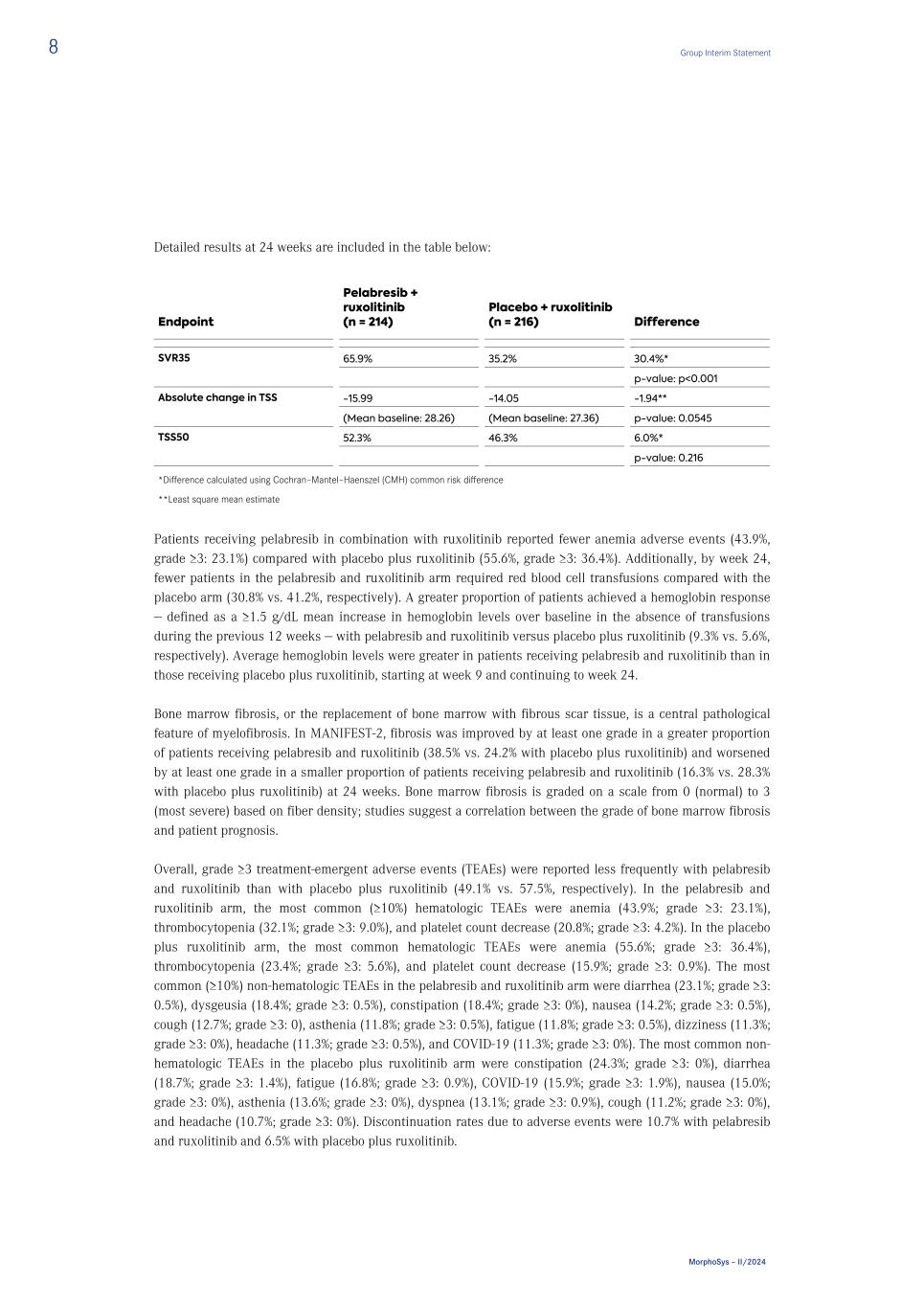
Detailed results at 24 weeks are included in the table below: Endpoint Pelabresib + ruxolitinib (n = 214) Placebo + ruxolitinib (n = 216) Difference SVR35 65.9% 35.2% 30.4%* p-value: p<0.001 Absolute change in TSS -15.99 -14.05 -1.94** (Mean baseline: 28.26) (Mean baseline: 27.36) p-value: 0.0545 TSS50 52.3% 46.3% 6.0%* p-value: 0.216 *Difference calculated using Cochran–Mantel–Haenszel (CMH) common risk difference **Least square mean estimate Patients receiving pelabresib in combination with ruxolitinib reported fewer anemia adverse events (43.9%, grade ≥3: 23.1%) compared with placebo plus ruxolitinib (55.6%, grade ≥3: 36.4%). Additionally, by week 24, fewer patients in the pelabresib and ruxolitinib arm required red blood cell transfusions compared with the placebo arm (30.8% vs. 41.2%, respectively). A greater proportion of patients achieved a hemoglobin response — defined as a ≥1.5 g/dL mean increase in hemoglobin levels over baseline in the absence of transfusions during the previous 12 weeks — with pelabresib and ruxolitinib versus placebo plus ruxolitinib (9.3% vs. 5.6%, respectively). Average hemoglobin levels were greater in patients receiving pelabresib and ruxolitinib than in those receiving placebo plus ruxolitinib, starting at week 9 and continuing to week 24. Bone marrow fibrosis, or the replacement of bone marrow with fibrous scar tissue, is a central pathological feature of myelofibrosis. In MANIFEST-2, fibrosis was improved by at least one grade in a greater proportion of patients receiving pelabresib and ruxolitinib (38.5% vs. 24.2% with placebo plus ruxolitinib) and worsened by at least one grade in a smaller proportion of patients receiving pelabresib and ruxolitinib (16.3% vs. 28.3% with placebo plus ruxolitinib) at 24 weeks. Bone marrow fibrosis is graded on a scale from 0 (normal) to 3 (most severe) based on fiber density; studies suggest a correlation between the grade of bone marrow fibrosis and patient prognosis. Overall, grade ≥3 treatment-emergent adverse events (TEAEs) were reported less frequently with pelabresib and ruxolitinib than with placebo plus ruxolitinib (49.1% vs. 57.5%, respectively). In the pelabresib and ruxolitinib arm, the most common (≥10%) hematologic TEAEs were anemia (43.9%; grade ≥3: 23.1%), thrombocytopenia (32.1%; grade ≥3: 9.0%), and platelet count decrease (20.8%; grade ≥3: 4.2%). In the placebo plus ruxolitinib arm, the most common hematologic TEAEs were anemia (55.6%; grade ≥3: 36.4%), thrombocytopenia (23.4%; grade ≥3: 5.6%), and platelet count decrease (15.9%; grade ≥3: 0.9%). The most common (≥10%) non-hematologic TEAEs in the pelabresib and ruxolitinib arm were diarrhea (23.1%; grade ≥3: 0.5%), dysgeusia (18.4%; grade ≥3: 0.5%), constipation (18.4%; grade ≥3: 0%), nausea (14.2%; grade ≥3: 0.5%), cough (12.7%; grade ≥3: 0), asthenia (11.8%; grade ≥3: 0.5%), fatigue (11.8%; grade ≥3: 0.5%), dizziness (11.3%; grade ≥3: 0%), headache (11.3%; grade ≥3: 0.5%), and COVID-19 (11.3%; grade ≥3: 0%). The most common non- hematologic TEAEs in the placebo plus ruxolitinib arm were constipation (24.3%; grade ≥3: 0%), diarrhea (18.7%; grade ≥3: 1.4%), fatigue (16.8%; grade ≥3: 0.9%), COVID-19 (15.9%; grade ≥3: 1.9%), nausea (15.0%; grade ≥3: 0%), asthenia (13.6%; grade ≥3: 0%), dyspnea (13.1%; grade ≥3: 0.9%), cough (11.2%; grade ≥3: 0%), and headache (10.7%; grade ≥3: 0%). Discontinuation rates due to adverse events were 10.7% with pelabresib and ruxolitinib and 6.5% with placebo plus ruxolitinib. 8 Group Interim Statement MorphoSys – II/2024

In May and June 2024, MorphoSys presented new efficacy and safety data from the MANIFEST-2 study during oral presentations at the American Society of Clinical Oncology (ASCO) and at the European Hematology Association (EHA) Annual Meetings. The updated data showed that the combination of pelabresib and ruxolitinib demonstrated rapid, deep and sustained SVR35 responses (a ≥35% reduction in spleen volume). More than twice as many patients receiving pelabresib and ruxolitinib achieved SVR35 (64.0%) at 12 weeks (the first post-treatment measurement of spleen volume) compared with those receiving placebo plus ruxolitinib (31.5%). Additionally, a higher proportion of responders maintained their SVR35 response in the pelabresib and ruxolitinib arm compared with the placebo plus ruxolitinib arm. The new insights from MANIFEST-2 build on MorphoSys’ understanding of the previously observed improvements in reducing spleen volume. The safety profile of the combination of pelabresib and ruxolitinib was generally comparable to the established safety profile of ruxolitinib, with fewer grade ≥3 events versus placebo plus ruxolitinib (49.1% vs. 57.0%, respectively). Leukemic transformation to accelerated or blast phase occurred in 3.3% of patients receiving pelabresib and ruxolitinib compared with 2.3% of patients receiving placebo plus ruxolitinib. As the trial is ongoing, MorphoSys continues to monitor the long-term efficacy and safety of the combination. Study of Tulmimetostat Patient enrollment in a Phase 1/2 clinical trial of tulmimetostat is ongoing. This Phase 1/2, open-label, multi- center, first-in-human study is designed to evaluate the safety and tolerability and preliminary clinical activity in patients with advanced solid tumors or lymphomas. The Phase 1 evaluated the dose escalation period in patients with advanced tumors and aimed to determine maximum tolerated dose (MTD) and/or recommended Phase 2 dose (RP2D) as a monotherapy in patients with advanced tumors or lymphomas. Patients are currently enrolled in two Phase 2 dose optimization cohorts in gynecological tumor indications. Safety and efficacy data from the ongoing Phase 2 study of tulmimetostat monotherapy in multiple advanced malignancies were presented during the ASCO Annual Meeting in June 2023. The data demonstrated disease stabilization or better across all solid tumor cohorts studied, including those with heavily pre-treated patients. Safety findings from the trial were consistent with the mechanism of EZH2 inhibition. In September 2023, the FDA granted Fast Track designation for tulmimetostat, for the treatment of patients with advanced, recurrent, or metastatic endometrial cancer harboring AT-rich interacting domain-containing protein 1A (ARID1A) mutations and who have progressed on at least one prior line of treatment. During the IGCS (International Gynecologic Cancer Society) 2023 Annual Global Meeting held in Seoul, South Korea, in November 2023, MorphoSys showcased in a featured poster abstract session, updated preliminary Phase 2 clinical data and first biomarker findings in a subset of patients with ARID1A-mutated ovarian clear- cell or endometrial carcinomas. Updated efficacy and safety data from the Phase 2 study of tulmimetostat in patients with advanced solid tumors or hematologic malignancies were further highlighted in a poster presentation at ASCO 2024. The presentation featured new information on the two dose optimization cohorts (M2 and M3) that are enrolling patients with ARID1A mutated ovarian clear cell carcinoma and endometrial cancer, showing preliminary signs of tulmimetostat’s anti-tumor activity with disease response and stabilization in heavily pre-treated patients with advanced/recurrent gynecological and other solid and hematological malignancies. Preliminary safety data from the M2 and M3 cohorts indicate improved tolerability with a trend towards decrease in incidence and severity of TEAEs and number of treatment discontinuations in 200mg and 300mg arms compared with 350mg. Group Interim Statement 9 MorphoSys – II/2024

Clinical Development Through Partners Studies of Ianalumab Ianalumab (VAY736) is a fully human IgG1/k mAb with a dual mode of action targeting B-cell lysis and BAFF-R blockade that is being investigated by Novartis in multiple indications within the immunology and hematology field. Ianalumab is currently in Phase 3 clinical development in lupus nephritis (LN), Sjögren’s disease, systemic lupus erythematosus (SLE), immune thrombocytopenia (1L and 2L ITP), and warm autoimmune hemolytic anemia (wAIHA). Ianalumab is also in Phase 2/3 clinical development in autoimmune hepatitis (AIH). MorphoSys is entitled to milestone payments and royalties upon approval and commercialization. Study of Abelacimab Abelacimab (MAA868) is an antibody directed against Factor XI that is being investigated by Anthos Therapeutics in two complementary Phase 3 clinical studies in cancer-associated thrombosis (CAT) for the prevention of venous thromboembolism (VTE) and in one Phase 3 study in high-risk patients with atrial fibrillation (AF). A Phase 2 study in AF was stopped early due to an overwhelming benefit (reduction in bleeding). The FDA granted fast track designation to abelacimab for both indications under study. MorphoSys is entitled to milestone payments and royalties upon approval and commercialization. Study of Setrusumab Setrusumab (BPS804/UX143) is a fully human monoclonal antibody inhibiting sclerostin that is currently being investigated by Ultragenyx and Mereo BioPharma in the Phase 3 portion of the pivotal Phase 2/3 clinical study and a Phase 3 study for the treatment of osteogenesis imperfecta (OI). On April 30, 2024, Ultragenyx announced that all patients have been enrolled across the Phase 3 Orbit and Cosmic studies evaluating setrusumab in pediatric and young adult patients with OI. MorphoSys is entitled to milestone payments and royalties upon approval and commercialization. Study of Bimagrumab Bimagrumab is a fully human monoclonal antibody against activin type II receptors that is currently in clinical development. Versanis Bio was investigating bimagrumab in a global Phase 2b study in patients with obesity and announced completion of enrollment in June 2023. Versanis Bio was acquired by Eli Lilly and Company, the transaction was completed in August 2023. MorphoSys is entitled to milestone payments and royalties upon approval and commercialization. Studies of Felzartamab Felzartamab is an investigational therapeutic human monoclonal antibody directed against CD38. Human Immunology Biosciences, Inc. (HI-Bio) obtained exclusive rights to develop and commercialize felzartamab across all indications worldwide, with the exception of Greater China. During a transition phase MorphoSys evaluated felzartamab for patients with two renal autoimmune diseases, anti-PLA2R antibody-positive membranous nephropathy (M-PLACE and New-PLACE trial) and immunoglobulin A nephropathy (IGNAZ trial) together with HI-Bio. On May 25, 2023, HI-Bio announced that the FDA has granted orphan drug designation (ODD) for felzartamab in development for the treatment of membranous nephropathy (MN). On May 22, 2024, Biogen and HI-Bio announced that the companies have entered into an agreement which stipulates that Biogen will acquire HI-Bio for US$ 1.15 billion (€ 1.07 billion) upfront and up to US$ 650 million (€ 607 million) in potential milestone payments. The acquisition has been completed in July 2024. More details can be found in the section "Subsequent Events". On May 24, 2024, HI-Bio presented positive interim results from the Phase 2 IGNAZ study of felzartamab in IgA nephropathy at the 61st European Renal Association (ERA) Congress. On May 25, 2024, HI-Bio announced positive results from a 10 Group Interim Statement MorphoSys – II/2024

Phase 2 investigator-sponsored clinical trial of felzartamab for late antibody-mediated rejection (AMR) in kidney transplant recipients. TJ Biopharma (previously I-Mab Biopharma) holds the exclusive rights to develop and commercialize felzartamab in Greater China for all indications and is studying felzartamab in relapsed/refractory multiple myeloma. MorphoSys will be eligible to receive payments on achievement of development, regulatory, and commercial milestones in addition to royalties on net sales of felzartamab. Studies of MOR210/TJ210/HIB210 MOR210/TJ210/HIB210 is an investigational human antibody directed against C5aR1, the receptor of the complement factor C5a. HI-Bio obtained exclusive worldwide rights to develop and commercialize MOR210 across all indications worldwide, with the exception of Greater China and South Korea. On July 11, 2023, HI-Bio announced that the first participants have been dosed in a Phase 1 healthy volunteer study of HIB210. TJ Biopharma (previously I-Mab Biopharma) holds the exclusive rights for MOR210 in Greater China and South Korea and is currently investigating MOR210 for the treatment of advanced solid tumors and exploring autoimmune disease. MorphoSys will be eligible to receive payments on achievement of development, regulatory, and commercial milestones in addition to royalties on net sales of MOR210/TJ210/HIB210. Other Programs In addition to the late-stage partnered programs listed above, there are several additional partnered programs in early to mid-stage research and development. Strategy and Group Management The Company aims to realize intermediate- and long-term growth through its focus on proprietary drug development. The Company prioritizes the lead development candidates pelabresib and tulmimetostat. MorphoSys is also pursuing the development of further clinical candidates as described in the Annual Report 2023 starting on page 33. The group management has been adjusted to reflect these operations. General Business and Market Environment Economic Trends In early 2024, the International Monetary Fund (IMF) projected a stabilization for the world economy — with the inflation falling faster than expected and the growth recovery. Meanwhile, the momentum on global disinflation has slowed. This reflects different sectoral dynamics: the persistence of higher-than-average inflation in services prices, tempered to some extent by stronger disinflation in the prices of goods. In its updated World Economic Outlook from July 16, 2024, the IMF projects a stable global growth with an estimated 3.2% in 2024 and 3.3% in 2025. The global inflation will continue to decline. Advanced economies are expected to see a stable growth, from 1.7% in 2024 to 1.8% in 2025. At the end of the first half of the year, the SDAX index closed 2.6 % higher, the TecDAX 0.3 % lower and the Nasdaq Biotech Index 4.0 % higher. The MorphoSys share started 2024 at € 34.00 and reached a high of € 69.35 on May 17, 2024. The paper closed the first half of 2024 at € 67.60 on June 28, 2024. Sector Developments In the first half of 2024, numerous medical conferences were held where companies in the sector presented their research results. The world’s largest oncology conference, the American Society of Clinical Oncology Group Interim Statement 11 MorphoSys – II/2024

(ASCO) Annual Meeting, was held in Chicago on May 31 — June 4, 2024 and the leading European conference in the field of hematology, the Annual Meeting of the European Hematology Association (EHA) was held on June 13 — 16, 2024. MorphoSys presented clinical results of pelabresib and tulmimetostat in oral presentations, posters and publications at these medical conferences. Intellectual Property In the first six months of 2024, we continued to reinforce the patent protection of our development programs and technology portfolio, which represent the core value drivers of our Company. Currently, the Company has more than 100 different proprietary patent families worldwide, in addition to the numerous patent families we are pursuing in collaboration with our partners. Human Resources On June 30, 2024, the MorphoSys Group had 446 employees (December 31, 2023: 524). During the first half- year of 2024, the MorphoSys Group employed an average of 475 people (H1 2023: 591). The decrease is caused by the decision to terminate the US sales force, which is related to the sale of tafasitamab to Incyte announced on February 5, 2024. Financial Analysis MorphoSys reports the key financial figures – research and development expenses as well as combined expenses for selling and general and administration – relevant for internal management purposes in its half- year financial statements. Their presentation is supplemented accordingly if other areas of the statement of profit or loss or balance sheet are affected by material business transactions during the quarter. Revenues Group revenues in the first half-year of 2024 amounted to € 62.2 million (H1 2023: € 50.9 million) and mainly included revenues from royalties in the amount of € 61.2 million (H1 2023: € 45.5 million). Additional group revenues were attributable to licenses, milestones, and other sources, amounting to € 1.0 million (H1 2023: € 5.4 million). On a regional basis, MorphoSys generated 100% or € 62.2 million of its revenues with biopharmaceutical companies in North America. In the same period last year, 98% (€ 49.8 million) of revenues were generated with customers in North America, and 2% (€ 1.2 million) with customers located in Europe. In the first half- year of 2024, 100% of the Group's revenues were generated with the customer Janssen (H1 2023: 98% with Janssen and HI-Bio). 12 Group Interim Statement MorphoSys – II/2024

Cost of Sales Cost of sales in the first half-year of 2024 amounted to € 4.1 million (H1 2023: € 2.8 million). The year-on-year increase resulted primarily from higher personnel costs from accelerated vesting of certain share-based payment programs. Operating Expenses Research and Development Expenses Research and development expenses amounted to € 134.8 million in the first half-year of 2024 (H1 2023: € 105.0 million) and consisted primarily of personnel expenses of € 90.3 million (H1 2023: € 42.7 million) and costs for external research and development services of € 34.5 million (H1 2023: € 51.9 million). In the first half-year of 2024, the increase in personnel expenses mainly resulted from effects of both an accelerated vesting of certain share-based payment programs, according to the terms and conditions of the share-based compensation plans, and the recognition of remuneration-related provisions following the acquisition by Novartis. In the first half-year of 2023, personnel expenses included a one-time effect resulting from severances in connection with the restructuring of the research area. Furthermore, the first half-year of 2023 comprised costs for external research and development services incurred due to the positive development of the patient recruitment in the major ongoing clinical studies of MorphoSys. The € 17.4 million decrease in expenses for external research and development services in the first half-year of 2024 is attributable to the regular progress of the clinical trial phase for the testing of pelabresib. Combined Expenses for Selling and General and Administration The combined expenses for selling and general and administration amounted to € 233.3 million in the first half-year of 2024 (H1 2023: € 35.5 million). This sum consisted mainly of personnel expenses of € 129.0 million (H1 2023: € 22.2 million) and expenses for external services of € 101.9 million (H1 2023: € 5.4 million). Selling expenses amounted to € 27.7 million in the first half-year of 2024 (H1 2023: € 8.1 million). This item consisted mainly of personnel expenses of € 22.7 million (H1 2023: € 2.2 million) and expenses for external services of € 5.2 million (H1 2023: € 1.5 million). The increase in selling expenses was mainly due to the effects of accelerated vesting of certain share-based payment programs, according to the terms and conditions of the share-based compensation plans, and the recognition of remuneration-related provisions following the acquisition by Novartis. In comparison to the same period of the previous year, general and administrative expenses increased to € 205.6 million (H1 2023: € 27.4 million). This line item mainly comprised personnel expenses amounting to € 106.4 million (H1 2023: € 20.0 million) and expenses for external services of € 96.6 million (H1 2023: € 3.9 million). The increase in general and administrative expenses was mainly due to the effects of accelerated vesting of certain share-based payment programs and the recognition of remuneration-related provisions following the acquisition by Novartis in accordance with terms and conditions of the share-based Group Interim Statement 13 MorphoSys – II/2024

compensation plans and individual employee contract terms. The increase in expenses resulting from external services was attributable to transaction costs in connection with the acquisition by Novartis. Finance Income / Finance Expenses Finance income totaled € 17.3 million in the first half-year of 2024 (H1 2023: € 57.6 million) and resulted from income from the investment of cash and cash equivalents and corresponding currency translation gains from investing of funds amounting to € 17.2 million (H1 2023: € 12.1 million). In the same period last year, finance income included measurement effects from deviations between underlying planning assumptions and actual numbers of financial liabilities from future payments to Royalty Pharma. (H1 2024: € 0.0 million; H1 2023: € 28.8 million) as well as effects from the repurchase of own convertible bonds (H1 2024: € 0.0 million; H1 2023 € 16.4 million). Finance expenses totaled € 100.7 million in the first half-year of 2024 (H1 2023: € 51.6 million). This increase was due to the measurement effects from financial liabilities from future payments to Royalty Pharma of € 80.0 million (H1 2023: € 43.8 million) mainly resulting from differences between underlying planning assumptions and actual figures, foreign currency effects and the application of the effective interest method. Furthermore, interest expenses on the convertible bond increased to € 17.5 million (H1 2023: € 5.7 million). It was expected that the bondholders will exercise their contractual right of termination due to the change of control by Novartis on May 23, 2024. Payment of the nominal amount plus interest up to the payment date is expected in the third quarter of 2024. As of June 30, 2024, the convertible bonds were written up to the nominal amount plus interest up to the payment date, resulting in additional interest expenses of € 14.9 million. The other interest expenses for convertible bonds were attributable to the regular application of the effective interest method until the change of control. Also included were finance expenses from the investment of liquid funds and foreign currency translation losses from financing activities in the amount of € 2.9 million (H1 2023: € 1.5 million). Financial Position Cash and Investments On June 30, 2024, the Group had cash and investments of € 512.3 million, compared to € 680.5 million on December 31, 2023. Cash and investments are presented in the balance sheet items "Cash and Cash Equivalents" and current and non-current "Other Financial Assets". The decrease in cash and investments resulted mainly from financing the operating activities in the first half- year of 2024 and from the settlement of transaction costs in connection with the acquisition by Novartis. Balance Sheet In contrast to the Consolidated Statement of Profit or Loss, as permitted, the previous year's balances within the Consolidated Balance Sheet were not split into Discontinued Operations and Assets Held for Sale. As a result, the balance sheet compared to the previous year balances show significant variances. 14 Group Interim Statement MorphoSys – II/2024

Assets Total assets as of June 30, 2024, amounted to € 1,705.0 million, a decrease of € 321.3 million compared to December 31, 2023 (€ 2,026.3 million). Current assets decreased by € 275.1 million from € 814.0 million as of December 31, 2023 to € 539.0 million as of June 30, 2024, mainly due to a € 142.2 million decrease in "Other Financial Assets" and a € 24.8 million decrease in “Cash and Cash Equivalents”. The changes were mainly due to the financing of operating activities in the first half-year of 2024 and from the settlement of transaction costs in connection with the acquisition by Novartis. The decrease in "Inventories" by € 62.1 million, in “Accounts Receivable” by € 30.6 million and in the balance sheet item “Prepaid Expenses and Other Assets” by € 15.6 million were due to the sale of tafasitamab to Incyte. Refer to note "11. Discontinued Operations and Assets Held for Sale " of the Notes to the Consolidated Financial Statement for more details. As of June 30, 2024, the balance sheet item "Discontinued Operations and Assets Held for Sale" consisted of accounts receivables in connection with tafasitamab in the amount of € 5.2 million as well as the Investment in Associates that shows a carrying amount of 0 €. In comparison to December 31, 2023, Non-Current Assets decreased by € 46.2 million to € 1,166.1 million. P&L-neutral fluctuations in currency exchange rates resulted in an increase in Goodwill by € 11.0 million and in Intangible Assets by € 24.8 million but were more than offset by effects stemming from discontinued operations, predominantely due to the transfer of intangible assets in the amount of € 74.8 million and Right- of-Use Assets for technical equipment in the amount of € 3.7 million to Incyte. Please refer to note "11. Discontinued Operations and Assets Held for Sale " of the Notes to the Consolidated Financial Statement for more details. Liabilities Current Liabilities increased by € 431.4 million from € 264.3 million as of December 31, 2023, to € 695.7 million as of June 30, 2024. The increase mainly resulted from effects related to the Novartis acquisition. Due to the change of control, bondholders can exercise their right of redemption and therefore a repayment of the nominal amount plus accrued interest in the third quarter is expected. This caused a reclassification of the non-current portion of the convertible bonds to current financial liabilities resulting in an increase in the line item "Bonds" in the amount of € 260.7 million. Furthermore, due to the effects of accelerated vesting of certain share-based payment programs and the recognition of current remuneration- related provisions the "Personnel-Related Provisions (Novartis Transaction)" increased to € 204.9 million. In addition, current "Financial Liabilities from Future Payments to Royalty Pharma" increased by € 31.0 million due to adjustments in planning assumptions. The effect was partially offset by a decrease in "Accounts Payable and Accruals" of € 66.0 million, the "Contract Liability" of € 19.4 million as well as the "Financial Liabilities from Collaborations" in the amount of € 5.5 million that are due to the sale of tafasitamab to Incyte. Refer to note "11. Discontinued Operations and Assets Held for Sale " of the Notes to the Consolidated Financial Statement for more details. As of June 30, 2024 the item "Liabilities directly associated with Discontinued Operations" in the amount of € 14.8 million contained accruals and accounts payables in connection with tafasitamab mainly as a result from the agreed transition activities. Group Interim Statement 15 MorphoSys – II/2024

Non-Current Liabilities decreased by € 368.9 million to € 1,344.0 million, compared to December 31, 2023. The decrease mainly resulted from effects related to the Novartis acquisition. Due to the change-of-control events, bondholders can exercise their right of redemption and therefore it is expected that the nominal amount plus accrued interest are repaid in the third quarter. This caused a reclassification of the non-current portion of the convertible bonds to current financial liabilities resulting in a decrease in the non-current line item "Bonds" in the amount of € 244.0 million. The item "Personnel-Related Provisions (Novartis Transaction)" decreased by € 16.6 million. By contrast, the "Financial Liabilities from Future Payments to Royalty Pharma" increased in the amount of € 5.3 million due to adjustments in planning assumptions. The decrease in "Financial Liabilities from Collaborations" by € 108.9 million was due to the sale of tafasitamab to Incyte. Stockholder’s Equity As of June 30, 2024, the Company’s common stock excluding treasury shares amounted to € 37,716,423 (December 31, 2023: € 37,655,137). As of June 30, 2024, the value of treasury shares was € 1,995,880 (December 31, 2023: € 1,995,880). The number of MorphoSys shares held by the Company as of June 30, 2024, still amounted to 53,685 shares (December 31, 2023: 53,685 shares). As of June 30, 2024, additional paid-in capital amounted to € 927,779,776 (December 31, 2023: € 938,088,474). The decrease totaling € 10,308,698 was attributable to the reclassification of personnel expenses from share-based payment programs previously recorded in additional paid-in capital to the balance sheet item Personnel-Related Provisions (Novartis Transaction)" in the amount of € 13,650,010 resulting from the execution of the Novartis Business Combination Agreement according to which all share-payment programs shall be settled in cash. In contrast, cash contributions to additional paid-in capital in the amount of € 3,341,313 from the exercising of stock option programs were recorded. On June 30, 2024, the other comprehensive income reserve mainly contained foreign currency translation differences from the consolidation of € 109,072,358 (December 31, 2023: € 88,435,451). The currency translation differences from the consolidation included exchange rate differences from the translation of the financial statements of Group companies prepared in foreign currencies and differences between the exchange rates used in the balance sheet and income statement. The consolidated net loss for the first six months of financial year 2024 of € 390,208,995 from continued operations, and € 3,946,859 from discontinued operations is reported under “accumulated deficit.” As a result, the accumulated deficit increased from € 1,013,133,943 on December 31, 2023 to € 1,407,289,797 on June 30, 2024. The development of the equity of the parent company MorphoSys AG (including the assessment with regard to the provision of section 92 German Stock Corporation Act) as well as of MorphoSys Group is closely monitored by the Management Board. In addition, the company is thoroughly monitoring the liquidity situation of MorphoSys Group. On June 20, 2024, Novartis BidCo AG, Basel, Switzerland, and MorphoSys AG concluded a Shareholder Loan Facility Agreement with a volume of up to € 500.0 million. With this agreement MorphoSys has access to sufficient liquid funds to ensure business operations for the forecast period which is subject to the going-concern assessment (at least twelve months from the issuance date of the interim consolidated financial statements) without requiring additional proceeds from external refinancing. At the 16 Group Interim Statement MorphoSys – II/2024

time of this report, the Management Board is not aware of any imminent risks that could affect the company as a going concern. Risks and Opportunities As already explained in the risk and opportunity report for the 2023 financial year, the risk situation changed in the first half of the year due to the completion of the takeover by Novartis and the sale of the tafasitamab business to Incyte. As a result, all risks relating to tafasitamab no longer apply as at the half-year reporting date compared to the presentation in the annual report as of December 31, 2023. In addition, the risk mentioned in the subsequent event report that the takeover by Novartis cannot be completed as planned has become obsolete due to the completion of the takeover on May 23, 2024. As a result of the takeover by Novartis, the financing of the MorphoSys Group companies is also secured, so that the risk of long-term corporate financing is no longer relevant. Taking into account the current developments on the relevant markets, the other risks and opportunities and their assessments remain unchanged in all material aspects compared to the situation described on pages 64 to 76 of the 2023 Annual Report. Outlook Expected Development of Financial Position As a consequence of the sale and transfer of tafasitamab to Incyte on February 5, 2024, MorphoSys' 2024 financial guidance published on January 30, 2024, could not be maintained and therefore was revoked. For the time being, MorphoSys will no longer make a forecast for revenues from product sales, as no such revenues are expected to be realized this year. As a consequence of the Novartis takeover, MorphoSys is now including the effects from the implementation of the takeover in its guidance. Consequently, the Group now expects R&D expenses of € 205 million to € 225 million for the 2024 which mainly represent our investments in the development of pelabresib and tulmimetostat. Selling, administrative and general expenses are now expected to be between € 260 million and € 270 million. The overall forecast is subject to a number of uncertainties, including inflation and foreign currency effects. The development of the equity of the parent company MorphoSys AG (including the assessment with regard to the provision of Section 92 German Stock Corporation Act) as well as of MorphoSys Group is closely monitored by the Management Board. In addition, the company is closely monitoring the liquidity situation of MorphoSys Group as well as for MorphoSys AG. On June 20, 2024, Novartis BidCo AG, Basel, Switzerland, and MorphoSys AG concluded a Shareholder Loan Facility Agreement with a volume of up to € 500.0 million. With this agreement MorphoSys has access to sufficient liquid funds to ensure business operations for the forecast period (at least twelve months from the issuance date of the interim consolidated financial statements), which is subject to the going-concern assessment, without requiring additional proceeds from external refinancing. At the time of this report, the Management Board is not aware of any imminent risks, neither individually nor collectively, that could affect the company as a going concern. Group Interim Statement 17 MorphoSys – II/2024

As of June 30, 2024, MorphoSys had cash and investments of € 512.3 million (December 31, 2023: € 680.5 million). 18 Group Interim Statement MorphoSys – II/2024

Consolidated Statement of Profit or Loss (IFRS) – (unaudited) in € Note Q2 2024 1 Q2 2023 1 H1 2024 H1 2023 Product Sales 2 0 0 0 0 Royalties 2 34,121,668 24,667,068 61,161,409 45,528,281 Licenses, Milestones and Other 2 522,110 1,893,878 1,022,110 5,379,373 Revenues 2 34,643,778 26,560,946 62,183,519 50,907,653 Cost of Sales (1,293,718) (1,721,614) (4,117,928) (2,759,521) Gross Profit 33,350,060 24,839,332 58,065,591 48,148,132 Operating Expenses Research and Development (49,658,680) (39,544,306) (134,810,150) (104,958,212) Selling (9,216,250) (4,672,196) (27,667,880) (8,093,955) General and Administrative (20,058,598) (16,776,815) (205,592,501) (27,370,642) Total Operating Expenses (78,933,528) (60,993,317) (368,070,531) (140,422,810) Operating Profit / (Loss) (45,583,468) (36,153,984) (310,004,940) (92,274,678) Other Income 1,227,852 603,682 2,064,511 2,711,622 Other Expenses (199,317) (533,830) (619,506) (2,366,521) Finance Income 7,714,167 6,800,000 17,277,381 57,579,480 Finance Expenses (43,828,838) (26,373,312) (100,662,941) (51,562,680) Income from Reversals of Impairment Losses / (Impairment Losses) on Financial Assets 45,000 45,967 154,000 590,967 Income Tax Benefit / (Expenses) 3 0 0 1,582,500 0 Consolidated Net Profit / (Loss) from Continued Operations (80,624,603) (55,611,478) (390,208,995) (85,321,810) Consolidated Net Profit / (Loss) from Discontinued Operations and Assets held for Sale 11 1,431,545 (18,355,682) (3,946,859) (33,076,989) Consolidated Net Profit / (Loss) (79,193,058) (73,967,160) (394,155,854) (118,398,799) Earnings per Share from Continued Operations, Basic and Diluted (in €) (2.14) (1.63) (10.37) (2.50) Earnings per Share (Total operations), Basic and Diluted (in €) (2.10) (2.16) (10.47) (3.47) Earnings per Share from Discontinued Operations and Assets held for Sale, Basic and Diluted (in €) 0.04 (0.54) (0.10) (0.97) Shares Used in Computing Earnings per Share, Basic and Diluted 37,662,738 34,166,655 37,640,439 34,166,401 1 The three month period is not part of the auditor’s review. Group Interim Statement 19 MorphoSys – II/2024

Consolidated Statement of Comprehensive Income (IFRS) – (unaudited) in € Q2 2024 1 Q2 2023 1 H1 2024 H1 2023 Consolidated Net Profit / (Loss) (79,193,058) (73,967,160) (394,155,854) (118,398,799) Items that will not be reclassified to Profit or Loss Change in Fair Value of Shares through Other Comprehensive Income 0 359,458 0 359,458 Items that may be reclassified to Profit or Loss Foreign Currency Translation Differences from Consolidation 6,142,825 99,042,501 20,636,907 (16,311,587) Other Comprehensive Income 6,142,825 99,401,959 20,636,907 (15,952,129) Total Comprehensive Income (73,050,233) 25,434,799 (373,518,947) (134,350,928) 1 The three month period is not part of the auditor’s review. 20 Group Interim Statement MorphoSys – II/2024

Consolidated Balance Sheet (IFRS) – (unaudited) in € Note 06/30/2024 12/31/2023 ASSETS Current Assets Cash and Cash Equivalents 5 133,670,902 158,499,651 Other Financial Assets 5 378,640,705 520,845,412 Accounts Receivable 5 1,487,592 32,093,682 Financial Assets from Collaborations 5 0 3,410,247 Income Tax Receivables 3,981,640 5,284,542 Other Receivables 5 1,269,509 1,496,489 Inventories 0 62,068,115 Prepaid Expenses and Other Assets 14,753,615 30,323,373 Discontinued Operations and Assets Held for Sale 11 5,153,976 0 Total Current Assets 538,957,939 814,021,511 Non-Current Assets Property, Plant and Equipment 2,986,905 3,890,162 Right-of-Use Assets 6,343,401 11,100,166 Intangible Assets 793,986,754 844,109,432 Goodwill 353,328,541 342,296,501 Other Financial Assets 5 0 1,133,982 Investment in Associates 11, 13 0 2,417,968 Prepaid Expenses and Other Assets 5 9,407,666 7,341,491 Total Non-Current Assets 1,166,053,267 1,212,289,702 TOTAL ASSETS 1,705,011,206 2,026,311,213 Group Interim Statement 21 MorphoSys – II/2024

in € Note 06/30/2024 12/31/2023 LIABILITIES AND STOCKHOLDERS’ EQUITY Current Liabilities Accounts Payable and Accruals 5 43,762,504 109,804,500 Lease Liabilities 3,777,981 3,628,433 Tax Liabilities 3 329,723 329,723 Provisions 14,887,312 4,127,121 Personnel-Related Provisions (Novartis Transaction) 204,912,015 0 Contract Liability 0 19,443,663 Bonds 14 262,369,281 1,638,125 Financial Liabilities from Collaborations 4, 5 0 5,526,679 Financial Liabilities from Future Payments to Royalty Pharma 4, 5 150,843,882 119,811,363 Liabilities directly associated with Discontinued Operations 11 14,802,019 0 Total Current Liabilities 695,684,717 264,309,607 Non-Current Liabilities Lease Liabilities 7,489,131 8,796,915 Provisions 122,041 3,794,171 Personnel-Related Provisions (Novartis Transaction) 7,980,649 24,568,963 Deferred Tax Liability 3 6,760,763 6,549,655 Bonds 14 0 244,020,955 Financial Liabilities from Collaborations 4, 5 0 108,868,561 Financial Liabilities from Future Payments to Royalty Pharma 4, 5 1,321,691,025 1,316,353,147 Total Non-Current Liabilities 1,344,043,609 1,712,952,367 Total Liabilities 2,039,728,326 1,977,261,974 Stockholders’ Equity Common Stock 6 37,716,423 37,655,137 Treasury Stock (53,685 and 53,685 shares for 2024 and 2023, respectively), at Cost (1,995,880) (1,995,880) Additional Paid-in Capital 6 927,779,776 938,088,474 Other Comprehensive Income Reserve 6 109,072,358 88,435,451 Accumulated Deficit 6 (1,407,289,797) (1,013,133,943) Total Stockholders’ Equity (334,717,120) 49,049,239 TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY 1,705,011,206 2,026,311,213 22 Group Interim Statement MorphoSys – II/2024
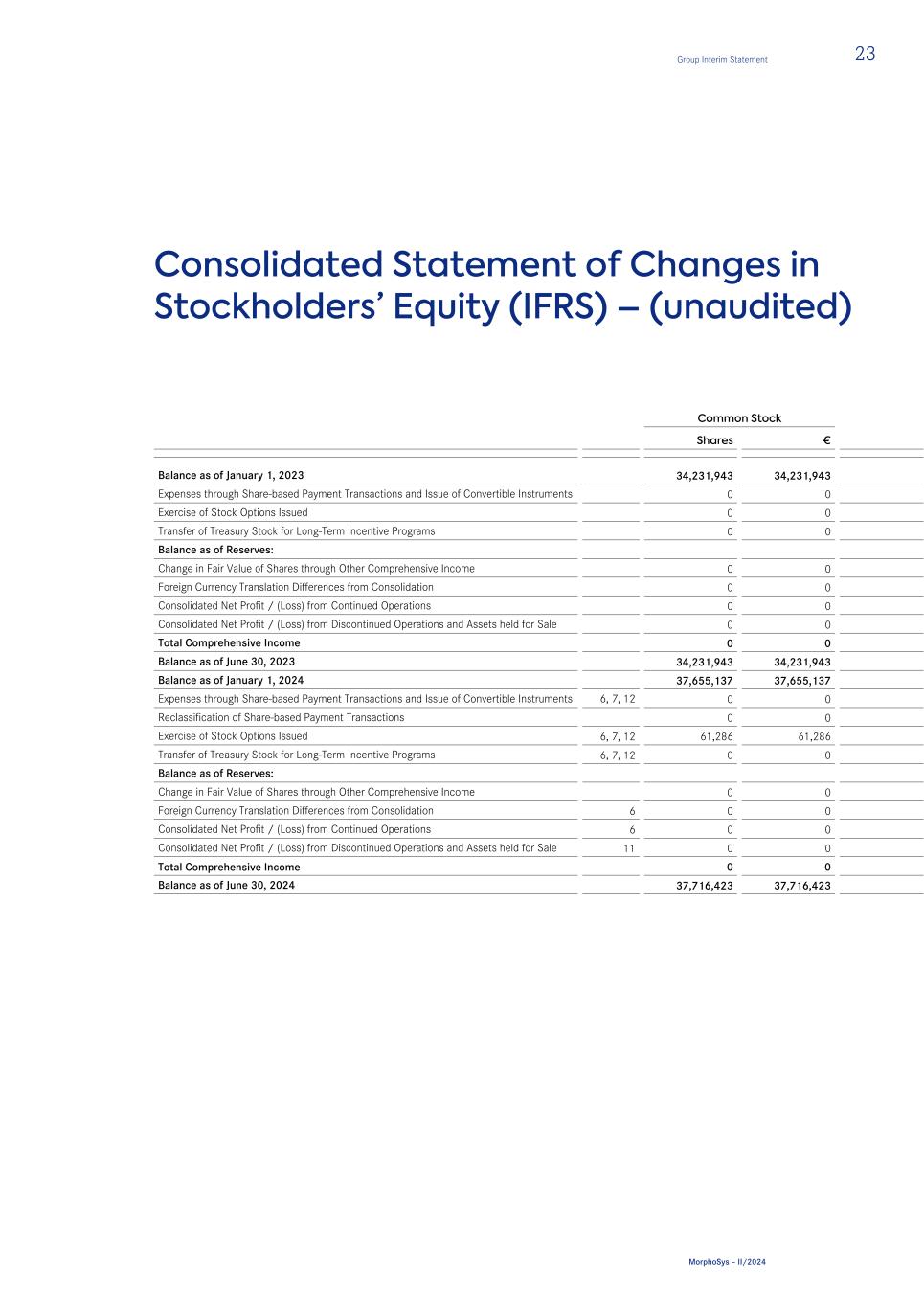
Consolidated Statement of Changes in Stockholders’ Equity (IFRS) – (unaudited) Common Stock Shares € Balance as of January 1, 2023 34,231,943 34,231,943 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 0 0 Exercise of Stock Options Issued 0 0 Transfer of Treasury Stock for Long-Term Incentive Programs 0 0 Balance as of Reserves: Change in Fair Value of Shares through Other Comprehensive Income 0 0 Foreign Currency Translation Differences from Consolidation 0 0 Consolidated Net Profit / (Loss) from Continued Operations 0 0 Consolidated Net Profit / (Loss) from Discontinued Operations and Assets held for Sale 0 0 Total Comprehensive Income 0 0 Balance as of June 30, 2023 34,231,943 34,231,943 Balance as of January 1, 2024 37,655,137 37,655,137 Expenses through Share-based Payment Transactions and Issue of Convertible Instruments 6, 7, 12 0 0 Reclassification of Share-based Payment Transactions 0 0 Exercise of Stock Options Issued 6, 7, 12 61,286 61,286 Transfer of Treasury Stock for Long-Term Incentive Programs 6, 7, 12 0 0 Balance as of Reserves: Change in Fair Value of Shares through Other Comprehensive Income 0 0 Foreign Currency Translation Differences from Consolidation 6 0 0 Consolidated Net Profit / (Loss) from Continued Operations 6 0 0 Consolidated Net Profit / (Loss) from Discontinued Operations and Assets held for Sale 11 0 0 Total Comprehensive Income 0 0 Balance as of June 30, 2024 37,716,423 37,716,423 Group Interim Statement 23 MorphoSys – II/2024

Treasury Stock Additional Paid- in Capital Other Comprehensive Income Reserve Accumulated Deficit Total Stockholders’ Equity Shares € € € € € 65,980 (2,450,303) 833,708,724 115,326,601 (823,407,416) 157,409,549 0 0 2,338,789 0 0 2,338,789 0 0 0 0 0 0 (4,149) 153,347 (153,347) 0 0 0 0 0 6,271,775 359,458 0 6,631,233 0 0 0 (16,311,588) 0 (16,311,588) 0 0 0 0 (85,321,810) (85,321,810) 0 0 0 0 (33,076,989) (33,076,989) 0 0 6,271,775 (15,952,130) (118,398,799) (128,079,154) 61,831 (2,296,956) 842,165,941 99,374,471 (941,806,215) 31,669,184 53,685 (1,995,880) 938,088,474 88,435,451 (1,013,133,943) 49,049,239 0 0 0 0 0 0 0 0 (13,650,010) 0 0 (13,650,010) 0 0 3,341,313 0 0 3,402,599 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 20,636,907 0 20,636,907 0 0 0 0 (390,208,995) (390,208,995) 0 0 0 0 (3,946,859) (3,946,859) 0 0 0 20,636,907 (394,155,854) (373,518,947) 53,685 (1,995,880) 927,779,776 109,072,358 (1,407,289,797) (334,717,120) 24 Group Interim Statement MorphoSys – II/2024

Consolidated Statement of Cash Flows (IFRS) – (unaudited) H1 (in €) Note 2024 2023 Operating Activities: Consolidated Net Profit / (Loss) from Continued Operations (390,208,995) (85,321,810) Adjustments to Reconcile Consolidated Net Profit / (Loss) to Net Cash Provided by / (Used in) Operating Activities: Impairments of Assets 379,225 0 Depreciation and Amortization of Tangible and Intangible Assets and of Right-of-Use Assets 2,248,312 5,121,631 Net (Gain) / Loss of Other Financial Assets (13,216,494) (10,461,167) (Income) from Reversals of Impairments / Impairments on Financial Assets (154,000) (590,967) Net (Gain) / Loss on Derivative Financial Instruments (100,682) 0 Non Cash Effective Net Change in Financial Liabilities from Future Payments to Royalty Pharma 18,862,937 (30,584,264) Interest Expense from Convertible Bond and Gain on Repurchase of Convertible Bond 17,529,263 (10,656,108) Net (Gain) / Loss on Sale of Property, Plant and Equipment (224,696) 0 Share-based Payment 10 141,973,192 12,807,771 Share of Loss of Associates accounted for using the Equity Method 0 0 Other Cash and Non-Cash Expenses (+) / Income (-) (1,489,755) (183,783) Income Tax (Benefit) / Expenses 3 (1,582,500) 0 Changes in Operating Assets and Liabilities: Accounts Receivable 294,477 7,038,682 Income Tax Receivables, Other Receivables, Inventories and Prepaid Expenses and Other Assets (2,669,731) 2,071,632 Accounts Payable and Accruals, Lease Liabilities, Tax Liabilities and Provisions 20,534,889 (29,692,055) Income Taxes Paid (-) / Received (+) 2,885,401 (132,740) Net Cash Provided by / (Used in) Operating Activities (204,939,157) (140,583,177) Group Interim Statement 25 MorphoSys – II/2024
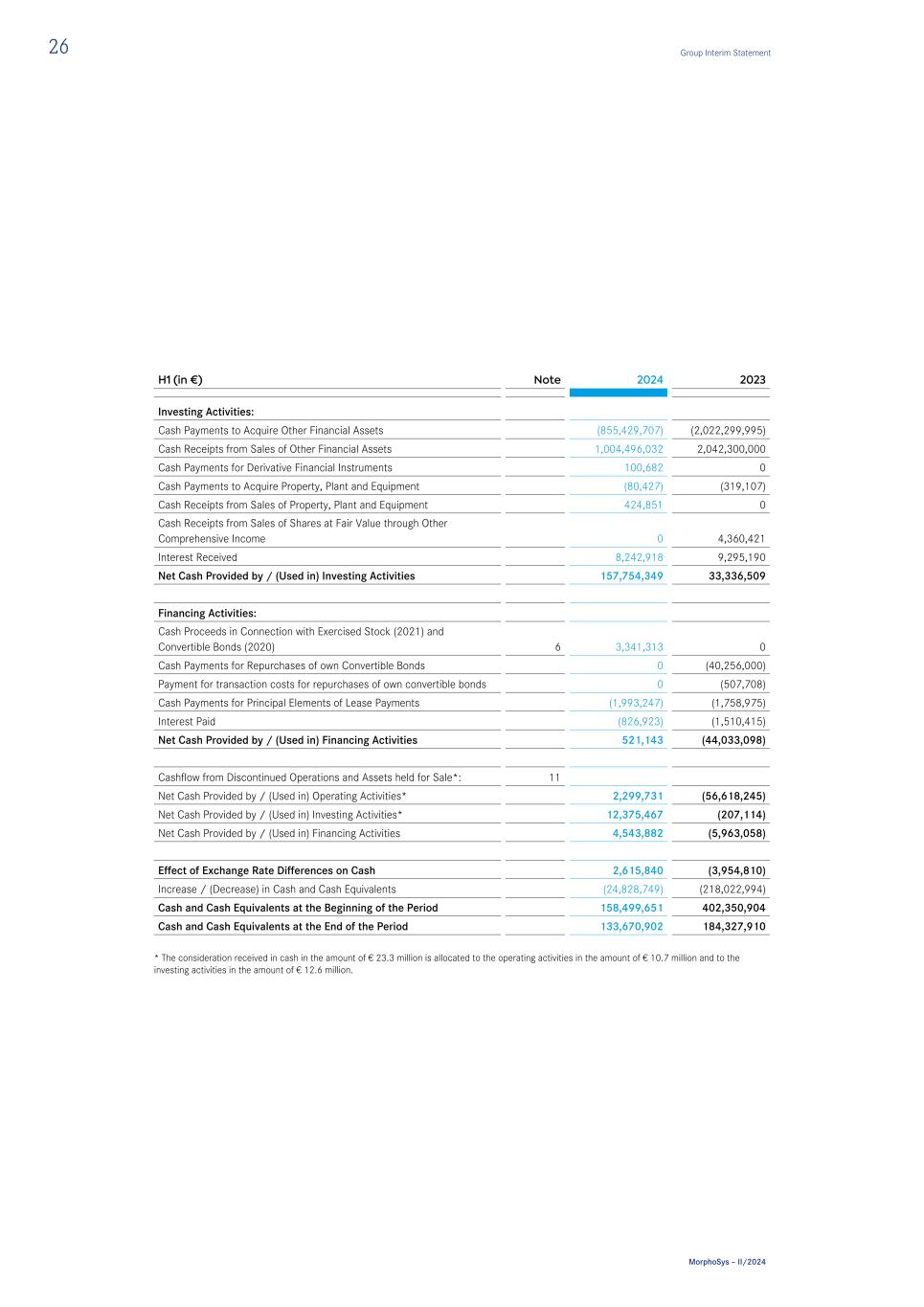
H1 (in €) Note 2024 2023 Investing Activities: Cash Payments to Acquire Other Financial Assets (855,429,707) (2,022,299,995) Cash Receipts from Sales of Other Financial Assets 1,004,496,032 2,042,300,000 Cash Payments for Derivative Financial Instruments 100,682 0 Cash Payments to Acquire Property, Plant and Equipment (80,427) (319,107) Cash Receipts from Sales of Property, Plant and Equipment 424,851 0 Cash Receipts from Sales of Shares at Fair Value through Other Comprehensive Income 0 4,360,421 Interest Received 8,242,918 9,295,190 Net Cash Provided by / (Used in) Investing Activities 157,754,349 33,336,509 Financing Activities: Cash Proceeds in Connection with Exercised Stock (2021) and Convertible Bonds (2020) 6 3,341,313 0 Cash Payments for Repurchases of own Convertible Bonds 0 (40,256,000) Payment for transaction costs for repurchases of own convertible bonds 0 (507,708) Cash Payments for Principal Elements of Lease Payments (1,993,247) (1,758,975) Interest Paid (826,923) (1,510,415) Net Cash Provided by / (Used in) Financing Activities 521,143 (44,033,098) Cashflow from Discontinued Operations and Assets held for Sale*: 11 Net Cash Provided by / (Used in) Operating Activities* 2,299,731 (56,618,245) Net Cash Provided by / (Used in) Investing Activities* 12,375,467 (207,114) Net Cash Provided by / (Used in) Financing Activities 4,543,882 (5,963,058) Effect of Exchange Rate Differences on Cash 2,615,840 (3,954,810) Increase / (Decrease) in Cash and Cash Equivalents (24,828,749) (218,022,994) Cash and Cash Equivalents at the Beginning of the Period 158,499,651 402,350,904 Cash and Cash Equivalents at the End of the Period 133,670,902 184,327,910 * The consideration received in cash in the amount of € 23.3 million is allocated to the operating activities in the amount of € 10.7 million and to the investing activities in the amount of € 12.6 million. 26 Group Interim Statement MorphoSys – II/2024

Notes to the Consolidated Financial Statements (unaudited) MorphoSys AG ("the Company" or "MorphoSys") is a biopharmaceutical company dedicated to the development and commercialization of therapeutic antibodies for patients suffering from various cancers. The Company has a proprietary portfolio of compounds and a pipeline of compounds developed with partners from the pharmaceutical and biotechnology industry. MorphoSys was founded as a German limited liability company in July 1992. In June 1998, MorphoSys became a German stock corporation. In March 1999, the Company completed its initial public offering on Germany’s "Neuer Markt": the segment of the Deutsche Börse designated, at that time, for high-growth companies. On January 15, 2003, MorphoSys AG was admitted to the Prime Standard segment of the Frankfurt Stock Exchange. On April 18, 2018, MorphoSys completed an IPO on the Nasdaq Global Market through the issue of American Depositary Shares (ADS). Each ADS represents 1/4 of a MorphoSys ordinary share. MorphoSys AG’s registered office is located in Planegg (district of Munich), and the registered business address is Semmelweisstrasse 7, 82152 Planegg, Germany. The MorphoSys AG consolidated and separate financial statements can be viewed at this address. The Company is registered in the Commercial Register B of the District Court of Munich under the number HRB 121023. On February 5, 2024, MorphoSys announced that it entered into a Business Combination Agreement with Novartis BidCo AG (formerly trading as Novartis data42 AG) and Novartis AG according to which Novartis BidCo AG intended to submit a voluntary public takeover offer for all of MorphoSys’ outstanding common shares in exchange for payment of € 68.00 per share. The corresponding voluntary public takeover offer was then published on April 11, 2024, by Novartis BidCo AG at an offer price of € 68.00 per MorphoSys share following approval by the German Federal Financial Supervisory Authority (BaFin) on April 11, 2024. The acceptance period of the takeover offer ended on May 13, 2024, and the additional acceptance period ended on May 30, 2024. Until expiration of the acceptance period on May 13, 2024, 25,610,813 MorphoSys shares were tendered, with the transfer of these MorphoSys shares to Novartis BidCo AG being completed on May 23, 2024. As a result, Novartis BidCo AG became the majority shareholder of MorphoSys, making MorphoSys a part of the Novartis Group. Together with the MorphoSys shares tendered during the additional acceptance period, ultimately 29,336,378 MorphoSys shares (corresponding to approximately 77.78% of the share capital of MorphoSys and approximately 77.89% of the voting share capital of MorphoSys) were tendered and transferred to Novartis BidCo AG in the course of the takeover offer. Through further acquisitions until June 16, 2024, Novartis BidCo AG has increased its stake in MorphoSys to 34,337,809 MorphoSys shares (corresponding to approximately 91.04% of the share capital, or approximately 91.17% of the voting share capital of MorphoSys). On June 20, 2024, MorphoSys announced that the company has entered into a delisting agreement with Novartis AG and Novartis BidCo AG in which it was agreed that Novartis BidCo AG publishes a public delisting purchase offer for all outstanding ordinary shares of MorphoSys not held by Novartis companies. Furthermore, on June 20, 2024, Novartis BidCo Germany AG, a subsidiary of Novartis BidCo AG, informed MorphoSys of its intention to merge MorphoSys into Novartis BidCo Germany AG by initiating a squeeze-out of MorphoSys’ minority shareholders, following the transfer of Novartis BidCo AG’s stake in MorphoSys of 34,337,809 MorphoSys shares (corresponding to approximately 91.04% of the share capital of MorphoSys) to Novartis BidCo Germany AG on June 19, 2024. Group Interim Statement 27 MorphoSys – II/2024

These interim consolidated financial statements were prepared in accordance with the International Financial Reporting Standards (IFRS) of the International Accounting Standards Board (IASB), taking into account the recommendations of the International Financial Reporting Standards Interpretations Committee (IFRS IC) as applicable in the European Union (EU). These interim consolidated financial statements comply with both the IFRSs published by the International Accounting Standards Board (IASB) and those adopted by the EU. These interim consolidated financial statements comply with IAS 34 "Interim Financial Reporting." The condensed interim consolidated financial statements do not contain all of the information and disclosures required for the financial year-end consolidated financial statements and therefore should be read in conjunction with the consolidated financial statements dated December 31, 2023. The condensed interim consolidated financial statements were approved for publication on August 28, 2024. The interim consolidated financial statements as of June 30, 2024, include MorphoSys AG as the parent company. MorphoSys AG has one wholly owned subsidiary, MorphoSys US Inc. (Boston, Massachusetts, USA). MorphoSys US Inc. in turn has a wholly owned subsidiary - Constellation Pharmaceuticals, Inc. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. also has a wholly owned subsidiary, Constellation Securities Corp. (Cambridge, Massachusetts, USA). Constellation Pharmaceuticals, Inc. and Constellation Securities Corp. are collectively referred to as “Constellation”, and all entities constitute the “MorphoSys Group” or the “Group”. 1. Summary of Material Accounting Policies Basis of Application The accounting and valuation principles applied to the consolidated financial statements for the financial year ending December 31, 2023, were the same as those applied to the first six months of 2024. The consolidated financial statements as of December 31, 2023, are available on the Company’s website at: https://www.morphosys.com/en/investors/financial-information. Changes in Accounting Policies and Disclosures New or Revised Standards and Interpretations Adopted for the First Time in the Financial Year Standard/Interpretation Mandatory Application for financial years starting on Adopted by the European Union Impact on MorphoSys IAS 1 (A) Classification of Liabilities as Current or Non- current, Non-current Liabilities with Covenants 1/1/2024 yes yes IAS 7 (A) and IFRS 7 (A) Supplier Finance Arrangements 1/1/2024 yes none IFRS 16 (A) Lease Liability in a Sale and Leaseback 1/1/2024 yes none (A) Amendments Standards with the remark "none" do not have a material impact on the consolidated financial statements. The impact on the consolidated financial statements of the amendments to IAS 1 is not considered material and therefore not explained separately. 28 Group Interim Statement MorphoSys – II/2024

New or Revised Standards and Interpretations not yet Mandatorily Applicable The following new and revised standards that were not yet mandatory in the reporting period and not yet adopted by the European Union were not applied in advance. Standards with the remark "yes" are likely to have an impact on the consolidated financial statements and are currently being assessed by the Group. Standards with the remark "none" are not expected to have a material impact on the consolidated financial statements. Standard/Interpretation Mandatory Application for financial years starting on Adopted by the European Union Possible Impact on MorphoSys IFRS 18 Presentation and Disclosure in Financial Statements 1/1/2027 no yes IFRS 19 Subsidiaries without Public Accountability: Disclosures 1/1/2027 no none IFRS 9/IFRS 7 (A) Classification and Measurement of Financial Instruments 1/1/2026 no none IAS 21 (A) The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability 1/1/2025 no none (A) Amendments Change in Presentation Provisions As of June 30, 2024, the current and non-current item "Personnel-Related Provisions (Novartis Transaction)" was presented separately in the balance sheet. This item exclusively comprises provisions for share-based payment programs and provisions for other amounts related to the change of control. The takeover by Novartis made it necessary to recognize significant amounts for these matters in the first six months of the 2024 financial year (see also sections 7 to 10 of the notes to the half-year financial statements); previously, any current and non-current amounts were reported in the balance sheet item "Provisions". To improve the comparability of the balance sheet item, any amounts as at December 31, 2023 have also been adjusted. Assets held for sale and discontinued operations The sale of tafasitamab to Incyte on February 5, 2024 was classified as "Discontinued Operations" in accordance with IFRS 5. In addition, the shares in the associated company Human Immunology Biosciences Inc. (HI-Bio) are classified as an "asset held for sale" after the acquisition of the company by Biogen Inc. was announced on May 22, 2024. Therefore, the Consolidated Statement of Profit or Loss as well as the Consolidated Statement of Cash Flows have been split into "Continued Operations" and "Discontinued Operations" and prior year amounts have been reclassified respectively. Balance sheet items related to "Discontinued Operations and Assets Held for Sale" were shown separately as of June 30, 2024. A corresponding allocation of the previous year's balances in the Consolidated Balance Sheet was not carried out as permitted. Group Interim Statement 29 MorphoSys – II/2024

2. Revenues in 000' € H1 2024 H1 2023 Product Sales, Net 0 0 Royalties 61,161 45,528 License Fees 0 152 Milestone Payments 1,000 1,500 Service Fees 22 3,728 Other 0 0 Licenses, Milestones and Other 1,022 5,379 Total 62,184 50,908 The following overview shows the Group’s regional distribution of revenue on the basis of the customer location: in 000' € H1 2024 H1 2023 Europe (excluding Germany) 0 1,151 USA 62,184 49,756 Total 62,184 50,908 The following overview shows the timing of the satisfaction of performance obligations: in 000' € H1 2024 H1 2023 At a Point in Time 62,161 49,908 Over Time 22 1,000 Total 62,184 50,908 Of the total revenues generated in the first half-year of 2024, a total of € 62.2 million were recognized from performance obligations that were fulfilled in previous periods and related to milestone payments and royalties (H1 2023: € 47.0 million). 3. Income Taxes In the first half-year of 2024, the Group recognized tax income in the amount of € 1.6 million (H1 2023: tax expenses of € 0.0 million). In 2023, there were neither tax expenses nor tax income and no deferred taxes. In the first half of 2024, tax income for previous years was recognized in profit or loss. No deferred taxes were recognized as of June 30, 2024, as the conditions for non-recognition of deferred taxes still continue to exist. The effective group tax rate for the first half-year of 2024 is 0.4% (H1 2023: —%). The change was mainly due to the recognition of tax income from previous years. 30 Group Interim Statement MorphoSys – II/2024

4. Significant Assumptions and Estimates on Financial Instruments Financial Assets and Liabilities from Collaborations The financial liabilities from collaborations represented Incyte's entitlement to future profit sharing for sales of Monjuvi as a second-line therapy in DLBCL in the USA (as MorphoSys shared 50% of these profits with Incyte). The planning assumptions were influenced by estimates and mainly comprised revenues and costs for the production and sale of Monjuvi in the US, the discount rate and the expected term of cash flows. Revenues are affected by variable influencing factors such as patient numbers and the number of doses of Monjuvi administered, as well as the price that can be obtained in the market. Costs include the manufacturing costs for these doses of Monjuvi and other cost components for e.g. sale, transport, insurance and packaging. The term is the estimated time period over which Monjuvi will generate benefits in the approved indication and therefore the expected term of product sales in the USA. These estimates are based on assumptions that are jointly arrived at and approved quarterly by the responsible departments at MorphoSys and Incyte. Financial liabilities from collaborations are furthermore subject to significant uncertainties from currency exchange rate developments. On February 5, 2024, the sale and transfer of tafasitamab to Incyte was announced. Effective with this date, Incyte received exclusive worldwide rights, assumed full responsibility and bears all costs for the development and commercialization of tafasitamab. In return, MorphoSys received a total amount of US$ 25.0 million (€ 23.3 million) in cash (purchase price). Furthermore, the Collaboration- and License Agreement with Incyte concluded in 2020 was terminated and consequently the Financial Liabilities from Collaborations were fully derecognized (June 30, 2024: € 0.0 million; December 31, 2023: € 114.4 million). Financial Liabilities from Future Payments to Royalty Pharma and from Development Funding Bond The non-current financial liabilities from future payments to Royalty Pharma represent the obligation of MorphoSys to forward to Royalty Pharma certain future license income in the form of royalties and milestones of Tremfya from Janssen and royalties on future net sales of the product candidates pelabresib and tulmimetostat. The planning assumptions are influenced by estimates and mainly relate to the expected revenues from Tremfya, pelabresib and tulmimetostat, the initial discount rate and the expected term of the cash flows. Revenues are influenced by variable factors such as patient numbers and the number of doses administered as well as the price that can be achieved in the market. The term represents the estimated period over which Tremfya in the approved indication and pelabresib will generate future cash inflows and therefore the expected duration of product sales. The above estimates are weighted with an expected probability of obtaining regulatory approval. The cash inflows and outflows represent an estimate of future revenues and costs from the out-licensed products and are subject to a significant degree of judgment. These estimates are based on assumptions that are developed and approved by the responsible departments of MorphoSys on a quarterly basis. Financial liabilities from future payments to Royalty Pharma are furthermore subject to significant uncertainties from currency exchange rate developments. Compared to December 31, 2023, financial liabilities for future payments to Royalty Pharma increased by € 36.4 million as of June 30, 2024. This was mainly due to the recognition of the amounts transferred to Royalty Pharma amounting to € 61.2 million. Offsetting effects resulted from the application of the effective Group Interim Statement 31 MorphoSys – II/2024

interest method and effects from changes in underlying assumptions totaling € 80.0 million and effects from foreign currency valuation in the amount of to € 17.4 million. The estimates underlying the financial liability are subject to a sensitivity analysis below. This would have resulted in the following effects on the carrying amount measured using the effective interest method of the financial liabilities from future payments to Royalty Pharma as of June 30, 2024, and December 31, 2023. In each case, one planning assumption is changed while all other estimates are kept constant. 6/30/2024 12/31/2023 in million € +1% (1) % +1% (1) % Change in variable Factors on Revenues 10.6 (10.6) 10.6 (10.6) Change in Foreign Exchange Rate for future Royalties and Net Sales 0.2 (0.2) 0.2 (0.2) The deferral relating to the financial liability of the development funding bond, which is recorded as a debit amount as part of the financial liability from future payments to Royalty Pharma, changed as follow in 2024 and 2023. in 000' € 2024 Balance as of January 1 46,485 Addition 0 Amortization (2,854) Foreign Currency Translation Differences from Consolidation 1,469 June 30 45,100 in 000' € 2023 Balance as of January 1 52,862 Addition 0 Amortization (4,640) Foreign Currency Translation Differences from Consolidation (1,737) Balance as of December 31 46,485 5. Fair Value Measurement of Financial Instruments MorphoSys uses the hierarchy below for determining and disclosing the fair value of financial instruments. Level 1: Quoted (unadjusted) prices in active markets for identical financial assets or liabilities to which the Company has access. Level 2: Inputs other than quoted prices included within Level 1 that are observable for the financial asset or financial liability, either directly (as prices) or indirectly (derived from prices). Level 3: Inputs for the financial asset or financial liability that are not based on observable market data (i.e., unobservable inputs). 32 Group Interim Statement MorphoSys – II/2024

Hierarchy Level 1 The fair value of financial instruments traded in active markets is based on the quoted market prices on the reporting date. A market is considered active if quoted prices are available from an exchange, dealer, broker, industry group, pricing service, or regulatory body that is easily and regularly accessible, and prices reflect current and regularly occurring market transactions at arm’s length conditions. For assets held by the Group, the appropriate quoted market price is the buyer’s bid price. Hierarchy Levels 2 and 3 The fair value of financial instruments not traded in active markets can be determined using valuation methods. In this case, fair value is determined using the results of a valuation method that makes use of market data. If all significant inputs required for measuring fair value by using valuation methods are observable, the instrument is allocated to Hierarchy Level 2. If significant inputs are not based on observable market data, the instrument is allocated to Hierarchy Level 3. Hierarchy Level 2 contains foreign exchange forward agreements to hedge exchange rate fluctuations, term deposits as well as restricted cash. Future cash flows for these foreign exchange forward agreements are determined based on forward exchange rate curves. The fair value of these instruments corresponds to their discounted cash flows. The fair value of the term deposits and restricted cash is determined by discounting the expected cash flows using term-specific and risk-adjusted market interest rates. Hierarchy Level 3 financial assets comprise equity investments, financial liabilities from collaborations, financial assets which is part of other receivables, financial assets from restricted escrow account, the debt component of the convertible bond as well as financial liabilities from future payments to Royalty Pharma. The underlying valuations are generally carried out by employees in the finance department who report directly to the Chief Financial Officer. The valuation process and results are reviewed and discussed among the persons involved on a regular basis. The fair value of the debt component of the convertible bond is determined based on the contractual cash flows (interest and principal), that are discounted using market interest rates of financial instruments with a comparable currency and maturities, taking into account MorphoSys' credit risk. In order to determine the fair value of the non-current financial liabilities from collaborations for disclosure purposes (these are accounted for at amortized cost using the effective interest method), the expected cash outflows are discounted using market interest rates of financial instruments with comparable currencies and maturities, taking into account MorphoSys' credit risk. For determining the fair value of the non-current financial liabilities for future payments to Royalty Pharma for disclosure purposes (these are accounted for at amortized cost using the effective interest method), the expected cash outflows from the planned royalty and milestone payments to Royalty Pharma are discounted using market interest rates of financial instruments with comparable currencies and maturities, taking into account MorphoSys' credit risk. For further information on the assumptions and estimates made to derive the cash flows from the liabilities from collaborations and the financial liabilities from future payments to Royalty Pharma, as well as a sensitivity analysis of the significant estimates and assumptions of the financial liabilities recognized at amortized cost whose fair value is assigned to hierarchy level 3, please refer to note 4. Group Interim Statement 33 MorphoSys – II/2024

For appraising the fair value of the financial assets from restricted escrow accounts (these are accounted for at fair value through profit or loss), the expected cash inflows were probability adjusted depending on the occurrence of certain conditions and discounted using market interest rates of the obligated contract party. Reclassifications between the hierarchy levels are generally taken into account as of the reporting dates. In 2024 and 2023, no transfers were made between the fair value hierarchy levels. The carrying amounts of current financial assets and liabilities at amortized cost approximate their fair values given their short maturities. 34 Group Interim Statement MorphoSys – II/2024
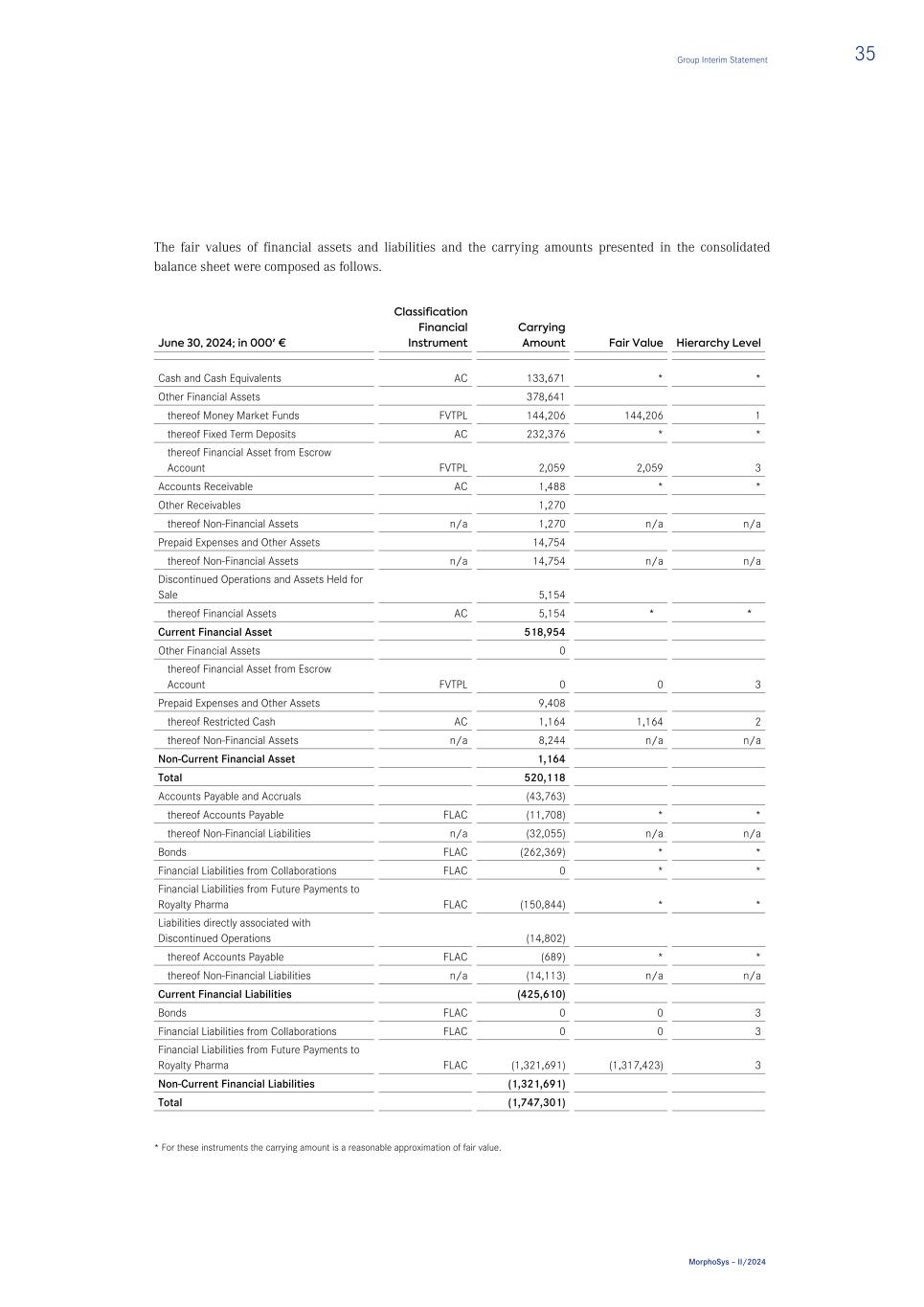
The fair values of financial assets and liabilities and the carrying amounts presented in the consolidated balance sheet were composed as follows. June 30, 2024; in 000' € Classification Financial Instrument Carrying Amount Fair Value Hierarchy Level Cash and Cash Equivalents AC 133,671 * * Other Financial Assets 378,641 thereof Money Market Funds FVTPL 144,206 144,206 1 thereof Fixed Term Deposits AC 232,376 * * thereof Financial Asset from Escrow Account FVTPL 0 0 0 2,059 2,059 3 Accounts Receivable AC 1,488 * * Other Receivables 1,270 thereof Non-Financial Assets n/a 1,270 n/a n/a Prepaid Expenses and Other Assets 14,754 thereof Non-Financial Assets n/a 14,754 n/a n/a Discontinued Operations and Assets Held for Sale 5,154 thereof Financial Assets AC 5,154 * * Current Financial Asset 518,954 Other Financial Assets 0 thereof Financial Asset from Escrow Account FVTPL 0 0 3 Prepaid Expenses and Other Assets 9,408 thereof Restricted Cash AC 1,164 1,164 2 thereof Non-Financial Assets n/a 8,244 n/a n/a Non-Current Financial Asset 1,164 Total 520,118 Accounts Payable and Accruals (43,763) thereof Accounts Payable FLAC (11,708) * * thereof Non-Financial Liabilities n/a (32,055) n/a n/a Bonds FLAC (262,369) * * Financial Liabilities from Collaborations FLAC 0 * * Financial Liabilities from Future Payments to Royalty Pharma FLAC (150,844) * * Liabilities directly associated with Discontinued Operations (14,802) thereof Accounts Payable FLAC (689) * * thereof Non-Financial Liabilities n/a (14,113) n/a n/a Current Financial Liabilities (425,610) Bonds FLAC 0 0 3 Financial Liabilities from Collaborations FLAC 0 0 3 Financial Liabilities from Future Payments to Royalty Pharma FLAC (1,321,691) (1,317,423) 3 Non-Current Financial Liabilities (1,321,691) Total (1,747,301) * For these instruments the carrying amount is a reasonable approximation of fair value. Group Interim Statement 35 MorphoSys – II/2024

December 31, 2023; in 000' € Classification Financial Instrument Carrying Amount Fair Value Hierarchy Level Cash and Cash Equivalents AC 158,500 * * Other Financial Assets 520,845 thereof Money Market Funds FVTPL 234,094 234,094 1 thereof Fixed Term Deposits AC 285,984 * * thereof Financial Asset from Escrow Account FVTPL 768 768 3 Accounts Receivable AC 32,094 * * Financial Assets from Collaborations FVTPL 3,410 3,410 3 Other Receivables 1,496 thereof Non-Financial Assets n/a 1,496 n/a n/a Prepaid Expenses and Other Assets 30,323 thereof Non-Financial Assets n/a 30,323 n/a n/a Current Financial Asset 714,849 Other Financial Assets AC 1,134 2 thereof Financial Asset from Escrow Account FVTPL 1,134 1,134 3 Prepaid Expenses and Other Assets 7,341 thereof Restricted Cash AC 1,217 1,217 2 thereof Non-Financial Assets n/a 6,124 n/a n/a Non-Current Financial Asset 2,351 Total 717,200 Accounts Payable and Accruals (109,805) thereof Accounts Payable FLAC (28,388) * * thereof Non-Financial Liabilities n/a (81,417) n/a n/a Bonds FLAC (1,638) * * Financial Liabilities from Collaborations FLAC (5,527) * * Financial Liabilities from Future Payments to Royalty Pharma FLAC (119,811) * * Current Financial Liabilities (155,364) Bonds FLAC (244,021) (244,818) 3 Financial Liabilities from Collaborations FLAC (108,869) (93,354) 3 Financial Liabilities from Future Payments to Royalty Pharma FLAC (1,316,353) (1,318,880) 3 Non-Current Financial Liabilities (1,669,243) Total (1,824,607) * For these instruments the carrying amount is a reasonable approximation of fair value. 36 Group Interim Statement MorphoSys – II/2024

The development of the fair values of financial assets measured at fair value and allocated to hierarchy level 3 is shown in the following reconciliation. in 000' € Financial Asset from Escrow Account Balance as of Balance as of January 1, 2024 1,901 Additions 28 Gains/(losses) recognized in other comprehensive income 0 Gains/(losses) recognized in profit or loss 130 Reclassification hierarchy levels 0 Disposals 0 Balance as of Balance as of June 30, 2024 2,059 in 000' € Financial Asset from Escrow Account Shares in Affiliated Companies < 20 % at Fair Value Balance as of Balance as of January 1 2023 1,854 0 Additions — 0 Gains/(losses) recognized in other comprehensive income — 6,272 Gains/(losses) recognized in profit or loss 47 0 Foreign Currency Translation Differences from Consolidation — 0 Reclassification hierarchy levels — 0 Disposals — -6,272 Balance as of Balance as of December 31, 2023 1,901 0 6. Changes in Stockholders’ Equity Common stock As of June 30, 2024, the Company’s common stock including treasury shares amounted to € 37,716,423 (December 31, 2023: € 37,655,137). As of June 30, 2024, the value of treasury shares remained unchanged (June 30, 2024: € 1,995,880 on December 31, 2023: € 1,995,880). The number of MorphoSys shares held by the Company as of June 30, 2024, amounted to 53,685 shares (December 31, 2023: 53,685 shares). Additional Paid-in Capital As of June 30, 2024, additional paid-in capital amounted to € 927,779,776 (December 31, 2023: € 938,088,474). The decrease totaling € 10,308,698 resulted from the reclassification of personnel expenses from certain share-based payment programs originally recorded in the capital reserve to the item "Personnel- Related Provisions (Novartis Transaction)" in the amount of € (13,650,010) due to the implementation of the Novartis Business Combination Agreement, according to which all share-based payment programs are to be settled in cash. This decrease was partially offset by € 3,341,313 in payments that were made to the capital reserve from the exercise of stock options. Group Interim Statement 37 MorphoSys – II/2024

Other Comprehensive Income Reserve On June 30, 2024, the other comprehensive income reserve amounted to € 109,072,358 (December 31, 2023: € 88,435,451) and mainly contained foreign currency translation differences from consolidation. The currency translation differences from consolidation included exchange rate differences from the translation of the financial statements of Group companies prepared in foreign currencies and differences between the exchange rates used in the balance sheet and income statement. Accumulated Deficit The consolidated net loss for the first six months of 2024 of € 394,155,854 was reported under “accumulated deficit.” As a result, the accumulated deficit increased from € 1,013,133,943 on December 31, 2023 to € 1,407,289,797 on June 30, 2024. 7. Development of Stock Options, Performance Share Units and Restricted Stock Units In the past, MorphoSys granted various share-based payment programs ("Long-Term Incentive Plans") (as refer to sections 5.1 and 5.2 of the notes to the consolidated financial statements 2023) to selected beneficiaries. As outlined in the Business Combination Agreement with Novartis, it is intended to cancel and compensate all Long-Term Incentive Plans which have been issued prior to the financial year 2024 (the “Pre-2024 Long-Term Incentive Plans”) against payment of the offer price of € 68.00 (minus the exercise price in case of stock options) paid in the course of the Novartis Takeover Offer. Further, the payout cap of 250% of the initial award amount applicable to the Performance Share Unit Programs of the Company shall be cancelled for active employees with a continuing employment relationship. Corresponding statements have been issued to all beneficiaries of the Pre-2024 Long-Term Incentive Plans on July 9, 2024. For certain Long-Term Incentive Plans for which MorphoSys assumed an equity settlement after the waiting period as of the grant date, this assumption has now been revised to a full cash-settlement, and the respective provisions for subsequent valuation are to be applied to these programs accordingly. The amounts previously recognized in equity in the amount of € 13.7 million were reclassified to personnel-related provisions with no effect on income. A total of € 133.9 million was recognized in profit or loss in the first six month for the aforementioned matters, resulting in a personnel-related provision totaling € 167.9 million as of June 30, 2024, presented as part of the balance sheet line item "Personnel-Related Provisions (Novartis Transaction)". The payment shall occur following the revocation of the admission of the Company’s shares to trading on the regulated market on the Frankfurt Stock Exchange becoming effective, which is expected to occur in the third quarter of 2024. Please refer to section "Subsequent Events" for further information. In the first six months of 2024, there were no stock options and convertible bonds issued to the Management Board or employees. In January 2024, 500,188 performance share units were issued under the 2024 Performance Share Unit Program (2024 PSU Program) to the Management Board and certain Company employees. Further details can be found in Note 8. 38 Group Interim Statement MorphoSys – II/2024

In January 2024, 233,497 restricted stock units were granted under the 2024 Restricted Stock Unit Plan (2024 RSU Program) to certain employees of MorphoSys US Inc. and Constellation Pharmaceuticals, Inc. Further details can be found in Note 9. In the first six months of 2024 61,286 shares from the 2017 SOP were exercised and transferred to the program's beneficiaries. As of June 30, 2024, 0 shares from the 2019 SOP were transferred to the program’s beneficiaries. At the end of the four-year performance period of the 2020 PSU Program, a target achievement of 0% was evaluated and accordingly no performance shares were allocated to the beneficiaries. After the end of the third one-year performance period, certain employees of MorphoSys US Inc. were granted a six-month period to receive a total of 9,934 performance shares under the 2021 RSU Program. The settlement in cash of the 2021 RSU Program took place on May, 24, 2024. 8. 2024 Performance Share Unit Program On January 22, 2024, MorphoSys established a performance share unit program (2024 PSU Program) for the Management Board and certain employees of the Company (beneficiaries). The 2024 PSU Program is considered a cash-settled share-based payment and is accounted for accordingly. The 2024 PSU Program is a performance-based program and is paid out in cash subject to the fulfillment of predefined market and performance criteria. The grant date was January 22, 2024; the waiting period/performance period is four years. Depending on the achievement of predefined market and performance criteria for the four-year period, the performance share units become vested in the four-year vesting period. The number of performance share units to be vested is calculated based on a combination of market and performance criteria: the relative development of the MorphoSys share price compared to the EURO STOXX Total Market Pharmaceuticals & Biotechnology Index, the achievement of Development Milestones and an assessment of the employee engagement. The market and performance criteria can be met up to a maximum of 200%. If the defined market and performance criteria are met by 0%, no performance share units will be earned for the four-year assessment period. The right to receive a certain cash settlement from the 2024 PSU Program does not arise until the end of the four-year waiting period/performance period. The performance share units are settled in cash within 90 days following the end of the four-year waiting period. MorphoSys reserves the right to settle the 2024 PSU Program at the end of the waiting period in MorphoSys AG’s treasury shares. The currently available treasury shares are likely not sufficient to settle the vested awards. MorphoSys therefore accounts for the 2024 PSU Program as a cash-settled share-based payment in accordance with IFRS 2. In the event of a departure from the Company, beneficiaries generally retain the performance share units that have vested by the time of their departure. In the event of a termination of a beneficiary for reasons of conduct or a revocation of the appointment of a member of the Management Board for reasons constituting good cause within the meaning of Section 626 (2) of the German Civil Code (BGB), all unexercised performance share units forfeit without entitlement to compensation. Group Interim Statement 39 MorphoSys – II/2024

As of January 22, 2024 a total of 500,188 performance share units were granted to beneficiaries, of which 124,746 performance share units to the Management Board, 90,906 performance share units to other members of the Executive Committee and 284,536 performance share units to certain employees of the Company who are not members of the Management Board or Executive Committee. In the period from January 22, 2024 to June 30, 2024, beneficiaries have left the Company, and therefore 6,762 performance share units have expired. For the 2024 PSU Program, the calculation of personnel expenses from share-based compensation was based on the assumption that beneficiaries would leave the Company during the four-year period, for which 25% of the performance share units granted are designated. The 2024 PSU Program shall now be converted into a cash program without performance targets. The performance share units shall generally continue to vest monthly at 1/48 (subject to a continuing employment relationship with the Company). On July 9, 2024, the Company has offered a corresponding amendment of the terms & conditions of the 2024 PSU Program to the beneficiaries. 9. MorphoSys US – 2024 Restricted Stock Unit Program On January 22, 2024, MorphoSys established a long-term incentive program in the form of a Restricted Stock Unit Program (2024 RSU Program) for certain employees of MorphoSys US Inc. and Constellation Pharmaceuticals. Inc. (beneficiaries). According to IFRS 2, the 2024 RSU Program is considered a share-based payment program with settlement in cash and is accounted for accordingly. The 2024 RSU Program is a performance-related share plan which is paid out in cash or in shares subject to the achievement of predefined key performance criteria. The plan has a term of three years and comprises three performance periods with a term of one year each. The restricted stock units vest each year subject to the achievement of certain predefined performance criteria. The number of restricted stock units vested per year is calculated based on key performance criteria of MorphoSys US entities during the annual performance period. The performance criteria can be met annually up to a maximum of 175%. If the specified performance criteria are met by less than 50% in one year, no restricted stock units will be earned for that year. After the end of the total three-year performance period, the restricted stock units are settled in shares of the Company or in cash. If a beneficiary ceases to hold office or is no longer employed at MorphoSys US Inc. or Constellation Pharmaceuticals. Inc. before the end of a one-year performance period, the beneficiary is generally entitled to all restricted stock units that have vested for previously completed one-year performance periods. All other restricted stock units will be forfeited without compensation. As of January 22, 2024, U.S. beneficiaries had been granted 233,497 restricted stock units. In the period from January 22, 2024 to June 30, 2024, U.S. beneficiaries have left MorphoSys US Inc. and Constellation Pharmaceuticals, Inc., and therefore 39,790 restricted stock units have expired. For the 2024 RSU Program, the calculation of personnel expenses from share-based compensation was based on the assumption that beneficiaries would leave the Company during the three-year period, for which 40% of the restricted stock units granted are designated. The 2024 RSU Program shall now be converted into a cash program without performance targets. The restricted stock units shall generally continue to vest yearly at 1/3 (subject to a continuing employment relationship with the Company). On July 9, 2024, the Company has offered a corresponding amendment of the terms & conditions of the 2024 RSU Program to the beneficiaries. 40 Group Interim Statement MorphoSys – II/2024

10. Personnel Expenses Resulting from Share-Based Payments In the first six months of 2024, personnel expenses resulting from share-based payments totaling € 142.0 million were recognized on the income statement (H1 2023: € 12.7 million). This is due in particular to the recognition of personnel expenses of € 133.9 million due to the accelerated vesting of certain share- based payment programs according to the terms and conditions of the share-based compensation plans in connection with the acquisition of control by Novartis. In addition, the pro rata personnel expenses in the amount of € 8.0 million for the share-based payment programs issued in 2024 were recognized. In 2024, this amount resulted from share-based payments settled with cash compensation. In 2023, the amount resulted from share-based payments settled with equity instruments and cash compensation. Of this amount, € 0.8 million was related to personnel expenses from stock options (H1 2023: € 0.1 million), € 45.8 million (H1 2023: € 2.3 million) to restricted stock units and € 95.3 million (H1 2023: € 10.3 million) to performance share units. 11. Discontinued Operations and Assets Held for Sale Assets Held for Sale In May 2024, the management undertook to sell all shares in HI-Bio in the amount of 11.5%, which were held as (Investment in Associates and accounted for using the at-equity-method in the past. The decision is accompanied by Biogen's announcement published on May 22, 2024 that it has entered into an agreement to acquire HI-Bio. In the first six months of 2024, the accumulated pro-rated Share of Loss of Associates accounted for using the Equity Method in the amount of € 2.4 million was recognized, which is fully attributable to the owners of MorphoSys. The cumulative loss recognized in HI-Bio as of June 30, 2024, resulted in a carrying amount of € 0.0 million, which was classified as an "Asset held for Sale". Discontinued Operations On February 5, 2024, the sale and transfer of tafasitamab to Incyte was announced. Effective with this date, Incyte received exclusive worldwide rights, assumed full responsibility and bears all costs for the development and commercialization of tafasitamab. In return, MorphoSys received a total amount of US$ 25.0 million (€ 23.3 million) in cash (purchase price). The sale and transfer of tafasitamab to Incyte is considered a discontinued operation in accordance with IFRS 5, and the consolidated income statement for the first half-year of the reporting year 2024 as well as for the comparative period have therefore been adjusted to present discontinued operation separately from continued operations. The loss of the discontinued operation tafasitamab for the first half-year 2024 in the amount of € 1.5 million is fully attributable to the owners of MorphoSys. Monjuvi® U.S. net product sales reached € 5.9 million ( US$ 6.4 million) until February 5, 2024 (H1 2023: € 41.1 million; US$ 44.4 million). In the first half-year of 2024, other revenue amounted to € 33.3 million (H1 2023: € 23.5 million), predominantly comprising additional sales of tafasitamab and transitional services provided to Incyte during the agreed transition period of 180 days. Group Interim Statement 41 MorphoSys – II/2024

As of February 5, 2024, MorphoSys transferred all intellectual property rights related to tafasitamab in the amount of € 74.8 million (Balance Sheet item: Intangible Assets) to Incyte. Furthermore, due to the sale of tafasitamab, all balances for commercial and clinical inventories associated with the production of tafasitamab in the amount of € 61.6 million, advance payments made in the amount of € 17.8 million and right-of-use assets for technical equipment in the amount of € 3.7 million were derecognized through profit or loss. As of February 5, 2024, MorphoSys had recognized current and non-current provisions related to tafasitamab, which ceased to exist due to the assumption of full responsibility by Incyte for the manufacturing and commercialization of tafasitamab going forward. As a result, current and non-current provisions in the amount of € 3.6 million and € 3.6 million, respectively, were derecognized through profit or loss. With the sale and transfer of tafasitamab, the Collaboration and License agreement concluded between MorphoSys and Incyte in 2020 was terminated. As a result, the related balance sheet item "Financial Liabilities from Collaborations" was derecognized through profit or loss (see section 4.19 of the notes to MorphoSys' Annual Report 2023 "Financial Liabilities from Collaborations"). This resulted in income from Discontinued Operations in the amount of € 118.0 million. As of June 30, 2024, the balance sheet item "Discontinued Operations and Assets Held for Sale" contained trade receivables in the amount of € 5.2 million related to tafasitamab and mainly stemmed from transitional services performed during the transition phase. Liabilities directly associated with Discontinued Operations related to tafasitamab business included trade payables and accruals in the amount of € 14.8 million in connection with the agreed transitional services as well as a € 2.0 million provision related to severance payments for employees working in the sales force in the U.S. Due to the Purchase Agreement with Incyte, the obligations from future payments in connection with contracts for outsourced studies (refer to section 6.1 in the Notes to MorphoSys' Annual Report 2023) were reduced by approx. € 129 million. Furthermore, MorphoSys is no longer obliged to pay milestone payments to licensors in the amount of US$ 236.5 million (€ 220.9 million), which were presented as contingent liabilities as of December 31, 2023. Result of Discontinued Operations and Assets held for Sale The following section outlines the key financial effects as of June 30, 2024, from the presentation of the Discontinued Operations and Assets Held for Sale. in T € 6M 2024 6M 2023 Revenues 39,279 64,576 Expenses (155,112) (92,346) Effects from the Valuation of Financial Liabilities from Collaborations 114,304 (1,002) Gain (+) / Loss (-) from Discontinued Operations (Tafasitamab) (1,529) (28,773) thereof Gain on Sale of Assets related to Discontinued Operations 10,032 Share of Loss of Associates accounted for using the Equity Method (Assets Held for Sale) (2,418) (4,304) Gain (+) / Loss (-) from Discontinued Operations and Assets held for Sale (3,947) (33,077) Income Tax Benefit / (Expenses) Profit (+) / Loss (-) from Discontinued Operations and Assets held for Sale, net of tax (3,947) (33,077) Diluted (in €) (0.10) (0.97) 42 Group Interim Statement MorphoSys – II/2024
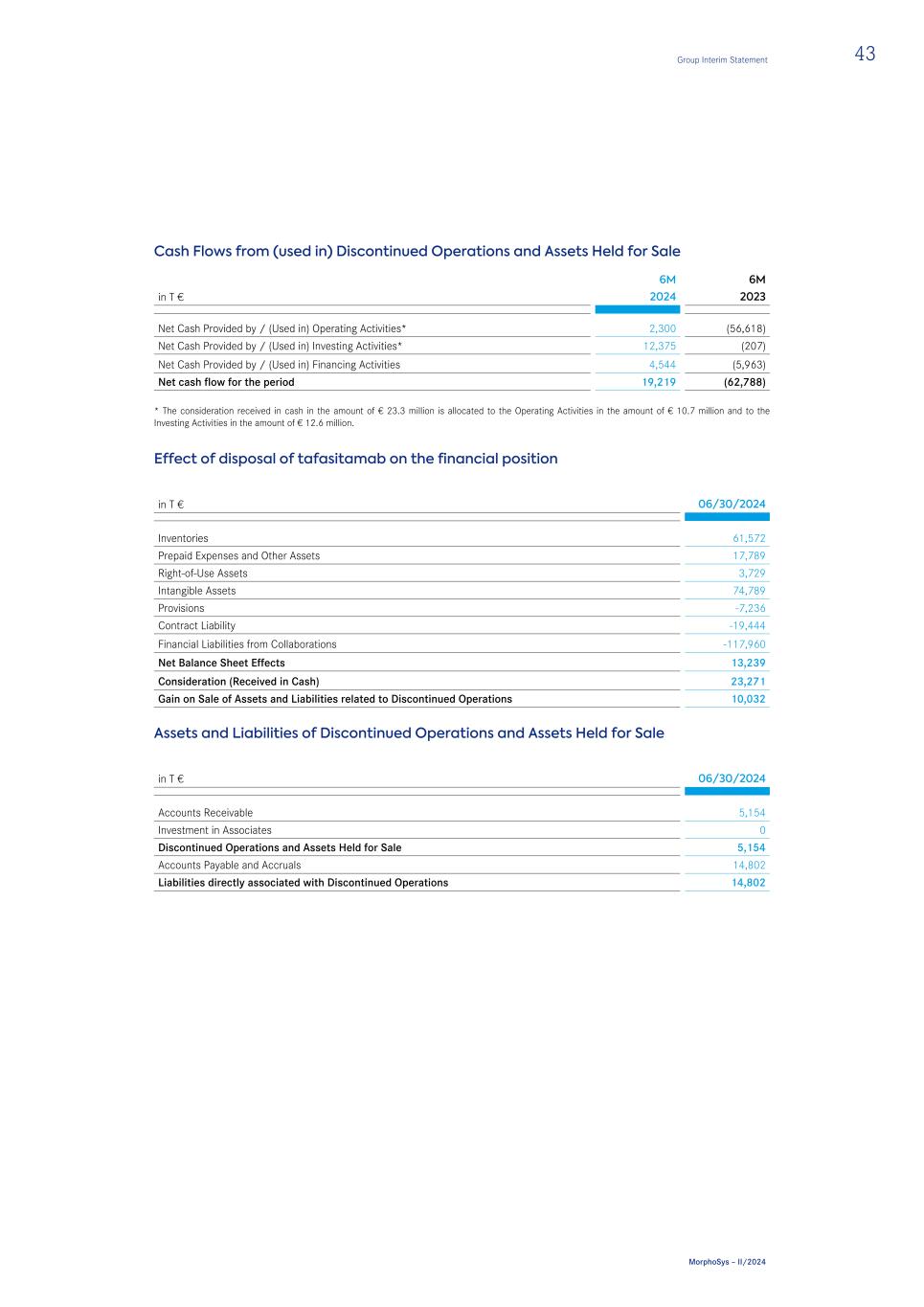
Cash Flows from (used in) Discontinued Operations and Assets Held for Sale in T € 6M 2024 6M 2023 Net Cash Provided by / (Used in) Operating Activities* 2,300 (56,618) Net Cash Provided by / (Used in) Investing Activities* 12,375 (207) Net Cash Provided by / (Used in) Financing Activities 4,544 (5,963) Net cash flow for the period 19,219 (62,788) * The consideration received in cash in the amount of € 23.3 million is allocated to the Operating Activities in the amount of € 10.7 million and to the Investing Activities in the amount of € 12.6 million. Effect of disposal of tafasitamab on the financial position in T € 06/30/2024 Inventories 61,572 Prepaid Expenses and Other Assets 17,789 Right-of-Use Assets 3,729 Intangible Assets 74,789 Provisions -7,236 Contract Liability -19,444 Financial Liabilities from Collaborations -117,960 Net Balance Sheet Effects 13,239 Consideration (Received in Cash) 23,271 Gain on Sale of Assets and Liabilities related to Discontinued Operations 10,032 Assets and Liabilities of Discontinued Operations and Assets Held for Sale in T € 06/30/2024 Accounts Receivable 5,154 Investment in Associates 0 Discontinued Operations and Assets Held for Sale 5,154 Accounts Payable and Accruals 14,802 Liabilities directly associated with Discontinued Operations 14,802 Group Interim Statement 43 MorphoSys – II/2024

12. Managers’ Transactions The Group engages in business relationships with members of the Management Board and Supervisory Board as related parties responsible for the planning, management and monitoring of the Group. In addition to cash compensation, the Group has granted the Management Board performance shares. The tables below show the shares held and equity-settled stock options and performance shares from LTI plans that are part of share- based plans by the members of the Management Board and Supervisory Board, as well as the changes in their ownership during the first half-year 2024. Shares 01/01/2024 Additions Sales 06/30/2024 Management Board Arkadius Pichota, Ph.D.1 — 0 0 0 Lukas Gilgen2 — 0 0 0 Jean-Paul Kress, M.D.3 0 — — — Lucinda Crabtree, Ph.D.4 0 — — — Total 0 0 0 0 Supervisory Board Heinrich Moisa — 0 0 0 Romain Lege — 0 0 0 Silke Mainka — 0 0 0 Sharon Curran 0 0 0 0 Total 0 0 0 0 Stock Options 01/01/2024 Additions Adjustment due to Performance Criteria Forfeitures Exercises 06/30/2024 Management Board Arkadius Pichota, Ph.D.1 — 0 0 0 0 0 Lukas Gilgen2 — 0 0 0 0 0 Jean-Paul Kress, M.D.3 57,446 — — — — — Lucinda Crabtree, Ph.D.4 0 — — — — — Total 57,446 0 0 0 0 0 44 Group Interim Statement MorphoSys – II/2024

Performance Shares from LTI plans 01/01/2024 Additions Adjustment due to Performance Criteria Forfeitures Conversion to Shares 06/30/2024 Management Board Arkadius Pichota, Ph.D.1 — 0 0 0 0 0 Lukas Gilgen2 — 0 0 0 0 0 Jean-Paul Kress, M.D.3 0 — — — — — Lucinda Crabtree, Ph.D.4 0 — — — — — Total 0 0 0 0 0 0 1 On June 6, 2024, Arkadius Pichota, Ph.D. was appointed to the Company’s Management Board. 2 On June 6, 2024, Lukas Gilgen was appointed to the Company’s Management Board. 3 Jean-Paul Kress's, M.D. membership of the Company’s Management Board ended on June 6, 2024. Changes after his departure from the Management Board are not presented. 4 Lucinda Crabtree's, Ph.D. membership of the Company’s Management Board ended on June 6, 2024. Changes after her departure from the Management Board are not presented. Members of the MorphoSys AG Supervisory Board do not hold any stock options, convertible bonds or performance shares. 13. Transactions with Related Parties Parent entity and ultimate controlling party On February 5, 2024, MorphoSys announced that it entered into a Business Combination Agreement with Novartis BidCo AG and its parent company Novartis AG according to which Novartis BidCo AG intended to submit a voluntary public takeover offer for all of MorphoSys’ outstanding common shares in exchange for payment of € 68.00 per share. As a result of the takeover offer and additional acquisitions on the stock exchange and over the counter, until June 16, 2024, Novartis BidCo AG acquired 34,337,809 MorphoSys shares (corresponding to approximately 91.04% of the share capital of MorphoSys). On June 20, 2024, Novartis BidCo Germany AG informed MorphoSys that it was holding 34,337,809 MorphoSys shares (corresponding to approximately 91.04% of the share capital of MorphoSys) since June 19, 2024, which had been transferred to Novartis BidCo Germany AG from its sole shareholder, Novartis BidCo AG. After deduction of the treasury shares held by MorphoSys, from which MorphoSys has no rights, this corresponds to a stake of approximately 91.17% of the voting share capital as of June 20, 2024. The ultimate controlling parent company is still Novartis AG. The ultimate controlling parent company is therefore Novartis AG. Group Interim Statement 45 MorphoSys – II/2024

With the exception of the transactions explained under "Managers’ Transactions" and the following transactions, there were no other related party transactions carried out in the first six months of 2024. Related Entity In the first half-year of 2024, revenues of € 0.0 million and cost reimbursements of € 0.7 million were recognized with associated companies under the underlying license agreements. As of June 30, 2024, there were trade receivables of € 0.4 million. Following the described takeover by Novartis and the inclusion of MorphoSys in the Novartis Group Financial Statements, the scope of related entities and related persons changed accordingly. On June 20, 2024, Novartis BidCo AG, Basel, Switzerland, and MorphoSys AG entered into a Shareholder Loan Facility Agreement (loan from the majority shareholder) with a volume of up to € 500.0 million. Under this agreement, MorphoSys can request advances on the loan amount at any time. The loan amounts bear variable interest at the 12-month EURIBOR rate plus a margin of 0.25%. The loan amounts can be repaid at any time, at the latest by the regular end of the agreement on December 31, 2026. As of June 30, no amounts had been drawn from the Shareholder Loan Facility Agreement. No other transactions were carried out with related parties in the first half of 2024. Related Person On June 30, 2024, the members of the Executive Committee (excluding the Management Board) held 6,026 stock options granted by the Company. In 2024, a new program of performance shares was issued to the Management Board and the members of the Executive Committee. The members of the Management Board received 0 performance share units. The members of the Executive Committee (excluding the Management Board) received 90,906 performance share units. Refer to Note 8. 2024 Performance Share Unit Program for details regarding the implications of the takeover by Novartis. In 2024, a new Restricted Stock Units Program was issued to a member of the Executive Committee (without Management Board). Hereby, one member of the Executive Committee received 12,488 Restricted Stock Units. Refer to Note 9. MorphoSys US – 2024 Restricted Stock Unit Program for details regarding the implications of the takeover by Novartis. By June 30, 2024, members of the Executive Committee (excluding the Management Board) had exercised 4,421 options from the 2017 SOP plan. 46 Group Interim Statement MorphoSys – II/2024
The total compensation for key management personnel (Management Board and members of the Executive Committee) in the first half-year of 2024 and in 2023 was as follows. in € H1 2024 H1 2023 Total Short-Term Employee Benefits 2,308,953 3,356,538 Total Post-Employment Benefits 160,269 212,653 Total Termination Benefits 0 0 Total Share-Based Payment 3,412,126 6,621,110 Total Compensation 5,881,348 10,190,301 As of June 30, 2024, there were accrued personnel expenses for payments to key management personnel for performance-related remuneration of € 0.7 million and provisions for share-based payments of € 31.2 million (June 30, 2024: € 0.9 million and € 8.4 million, respectively). 14. Further Significant Events and Transactions Changes to the Supervisory Board and Management Board Marc Cluzel, M.D., Ph.D., George Golumbeski, Ph.D., Krisja Vermeylen, Michael Brosnan and Andrew Cheng, M.D., Ph.D. resigned from the Company’s Supervisory Board following the closing of the takeover offer by Novartis BidCo AG to the shareholders of MorphoSys AG on May, 23, 2024. The Munich Local Court appointed Heinrich Moisa, Romain Lege and Silke Mainka as new members by order dated June 4, 2024. All new members waive their entitlement to Supervisory Board remuneration. Jean-Paul Kress's, M.D. and Lucinda Crabtree's, Ph.D. membership of the Company’s Management Board ended on June 6, 2024. On June 6, 2024, Arkadius Pichota, Ph.D. (CEO) and Lukas Gilgen (CFO) were appointed to the Company’s Management Board. Bonds In the event of a change of control, the terms and conditions of the convertible bond issued in 2020 provide that the bondholders may declare due all or some of its bonds not previously converted or redeemed on a certain date (Control Record Date). In return, the Company is obliged to repay the nominal amount stipulated in the terms of issue plus accrued interest. As the Company assumes that all outstanding convertible bonds will be repurchased within the next twelve months, the entire balance of € 262.4 million was recognized in short-term bonds as of June 30, 2024. Due to the voluntary public takeover offer by Novartis BidCo AG, which was completed on May 23, 2024, the Control Record Date was set at July 22, 2024. Convertible bonds of € 115.8 million including accrued interest were redeemed at the Control Record Date. Due to the transfer of all shares amounting to 91.04% of the share capital of MorphoSys from Novartis BidCo AG, Basel, Switzerland, to Novartis BidCo Germany AG, Munich, Germany, on June 19, 2024, the latter acquired control over MorphoSys AG. The Control Record Date was set for August 8, 2024. Convertible bonds of € 142.4 million including accrued interest were redeemed at the Control Record Date. Group Interim Statement 47 MorphoSys – II/2024

As of the publication date of this report, convertible bonds with a nominal value of € 4.3 million are still outstanding. The remaining convertible bonds will be terminated and repaid, including accrued interest, effective August 30, 2024. Other relevant transactions in connection with the Novartis takeover As of June 30, 2024, MorphoSys recognized transaction costs related to the change of control in the amount of € 92.7 million. As of June 30, 2024, provisions for personnel-related compensation claims in the amount of € 35.8 million due to contractual change-of-control clauses in the employment contracts of certain individuals were recognized and presented as part of the balance sheet item "Personnel-Related Provisions (Novartis Transaction)". Acquisition of HI-Bio by Biogen On May 22, 2024, Biogen Inc. announced that it has entered into a definite agreement to acquire Human Immunology Bioscience (HI-Bio). MorphoSys held 11.5% of shares in HI-Bio. Under the agreement, Biogen will pay US$ 1.15 billion (€ 1.07 billion) upfront and up to US$ 650 million (€ 607 million) in potential milestone payments. The transaction is expected to close in the third quarter of 2024, pending necessary regulatory approvals and customary closing conditions. The investment was presented as "Investment in Associates" and was reclassified as "Assets held for sale" in accordance with IFRS 5 since it is assumed that the transaction will close as announced after the clearance by the authorities is obtained. The book value amounts to € 0.0 million. The proceeds from the sale to Biogen represent contingent assets that must not be recognized in the balance sheet as of June 30, 2024. 15. Subsequent Events Merger Squeeze-out of MorphoSys AG's Minority Shareholders After Novartis BidCo Germany AG had informed the management board of MorphoSys of its intention to merge MorphoSys as transferring company into Novartis BidCo Germany AG with the simultaneous exclusion of minority shareholders against payment of an adequate cash compensation by way of a merger squeeze-out pursuant to Section 62 para. 5 of the German Transformation Act (Umwandlungsgesetz – UmwG) in conjunction with Section 327a para. 1 of the German Stock Corporation Act (Aktiengesetz – AktG) on June 20, 2024, Novartis BidCo Germany AG submitted the specified request (konkretisiertes Verlangen) specifying a cash compensation of € 68.00 per MorphoSys share to have the MorphoSys Annual General Meeting resolve on the transfer of the shares of MorphoSys’ minority shareholders to Novartis BidCo Germany AG as the majority shareholder against an adequate cash compensation set at € 68.00 per share on July 12, 2024. The court-appointed expert auditor has confirmed the cash compensation to be adequate. On August 27, 2024, the Annual General Meeting of MorphoSys resolved with the required majority to transfer the no-par value bearer shares of the remaining shareholders of MorphoSys (minority shareholders) pursuant to Section 62 para. 5 UmwG in conjunction with Sections 327a et seq. AktG to Novartis BidCo Germany AG against payment of an adequate cash compensation by Novartis BidCo Germany AG, Munich, in the amount of € 68.00 per no-par value bearer share of MorphoSys. 48 Group Interim Statement MorphoSys – II/2024

The merger squeeze-out will become effective once the transfer resolution and merger have been registered in the commercial register at the seat of MorphoSys, and the merger has been registered in the commercial register at the seat of Novartis BidCo Germany AG. Delisting On June 20, 2024, MorphoSys, Novartis AG and Novartis BidCo AG signed a delisting agreement in which it was agreed that Novartis BidCo AG publishes a public delisting purchase offer for all outstanding ordinary shares of MorphoSys not held by Novartis companies. On July 4, 2024, Novartis BidCo AG published the offer document for the delisting purchase offer following approval by the German Federal Financial Supervisory Authority (BaFin). The delisting purchase offer provided for a cash compensation of € 68.00 per MorphoSys share. Also on July 4, 2024, the management board and supervisory board of MorphoSys published a joint reasoned statement unanimously recommending its shareholders to accept the delisting purchase offer. By August 2, 2024, 24:00 hours, the delisting purchase offer was accepted for 1,037,601 MorphoSys shares and for an additional 179,325 MorphoSys shares represented by MorphoSys American Depositary Shares (“ADSs”), for a total of 1,216,926 MorphoSys shares. The 1,216,926 MorphoSys shares were transferred to Novartis BidCo Germany AG on August 14, 2024, meaning that Novartis BidCo Germany AG holds a total of 35,554,735 MorphoSys shares (corresponding to approximately 94.27% of the share capital). Subsequently, MorphoSys applied for revocation of the admission to trading of MorphoSys shares on the regulated market of the Frankfurt Stock Exchange. On July 12, 2024, MorphoSys also formally notified the Nasdaq Stock Market of its intention to voluntarily delist its ADSs from the Nasdaq Global Market. The delisting from the Frankfurt Stock Exchange became effective at the end of August 2, 2024. The delisting of the ADSs from the Nasdaq Global Market became effective prior to market opening on August 5, 2024. Since the delisting became effective, MorphoSys shares and ADSs are no longer traded on the regulated market of the Frankfurt Stock Exchange or on Nasdaq, and follow-up obligations from such a public listing do no longer apply. Despite having delisted from Nasdaq Global Market, MorphoSys will remain an SEC registrant and subject to SEC reporting obligations until such time as MorphoSys deregisters with the SEC. Deregistration is a separate process from delisting that will, among other things, eliminate SEC reporting obligations. The filing of a Form 15 with the SEC is required to suspend the reporting obligations under Section 15(d) of the Exchange Act 1934 and deregister MorphoSys with the SEC. Sale of Investment in Associate HI-Bio On May 22, 2024, Biogen Inc. (Biogen) and Human Immunology Biosciences (HI-Bio), in which MorphoSys holds an investment in associates announced that the companies have entered into an agreement which stipulates that Biogen will acquire HI-Bio for US$ 1.15 billion (€ 1.07 billion) upfront and up to US$ 650 million (€ 607 million) in potential milestone payments. On July 2, 2024, Biogen announced that the deal to acquire HI-Bio was completed after all outstanding regulatory approvals had been obtained by that date. Subsequent to the completion of the acquisition, MorphoSys received US$ 128 million (€ 118 million) in return for the sale of the total share in HI-Bio. Out of this amount, US$ 110 million (€ 103 million) had to be classified in profit or loss as Discontinued Operations and Assets Held for Sale in accordance with IFRS 5. The remainder in the amount of US$ 18 million (€ 17 million) was recorded as milestone revenue related to additional shares MorphoSys was entitled to upon the sale of HI-Bio. As of June 30, 2024, the sale was not yet classified as highly probable due to a lack of clearances for the transaction in the US by the respective authorities and a lack of visibility for MorphoSys management regarding the status of certain legal requirements. Group Interim Statement 49 MorphoSys – II/2024

Furthermore, MorphoSys is entitled to receive up to an additional amount of approximately US$ 96 million (€ 90 million), dependent on milestones and indemnification clauses related to the contingent consideration associated with the sale of HI-Bio. Issuance share-based compensation program With effect from August 1, 2024, a cash-settled share-based payment program (Performance Share Unit Program - PSU Program) was issued to the members of the Management Board. Changes in the composition of the Supervisory Board The Annual General Meeting of MorphoSys resolved on August 27, 2024, to appoint Heinrich Moisa, Romaine Lege, Silke Mainka and Christian Diehl as members of the Supervisory Board. Sharon Curran’s term of office ended at the close of the Annual General Meeting on August 27, 2024, thereby marking her departure from the Supervisory Board of MorphoSys. 50 Group Interim Statement MorphoSys – II/2024

Responsibility Statement “To the best of our knowledge, and in accordance with the applicable accounting principles for interim financial reporting, the interim consolidated financial statements give a true and fair view of the Group’s net assets, financial position and results of operations, and the group interim management report provides a fair view of the development and performance of the business and the position of the Group together with a description of the principal opportunities and risks associated with the Group’s expected development during the remainder of the financial year.” Planegg, August 28, 2024 Arkadius Pichota, Ph.D. Lukas Gilgen Chief Executive Officer Chief Financial Officer Group Interim Statement 51 MorphoSys – II/2024

Imprint MorphoSys AG Semmelweisstr. 7 82152 Planegg Germany Tel.: +49-89-89927-0 Fax: +49-89-89927-222 Email: info@morphosys.com Website: www.morphosys.com Investor Relations Tel.: +49-89-89927-404 Fax: +49-89-89927-5404 Email: investors@morphosys.com Published on August 29, 2024 This Half-Year Report is also available in German and can be downloaded from the Company’s website (PDF). For better readability, this report uses the masculine form only but refers equally to all genders. HuCAL® and Ylanthia® are registered trademarks of the MorphoSys Group. As of February 5, 2024, Monjuvi® and Minjuvi® are registered trademarks of Incyte. We also refer to trademarks of other corporations and organizations in this Report. MorphoSys – II/2024

Financial Calendar 2024 March 13, 2024 Publication of 2023 Year-End Results April 29, 2024 Publication of 2024 First Quarter Interim Statement August 27, 2024 2024 Annual General Meeting August 29, 2024 Publication of 2024 Half-Year Report MorphoSys AG Semmelweisstr. 7 82152 Planegg Germany Tel.: +498989927-0 Fax: +498989927-222 www.morphosys.com MorphoSys – II/2024




















































