Exhibit 10.4
Territory License Agreement
between
Molecular Insight Pharmaceuticals, Inc
and
BIOMEDICA Life Sciences, S.A.
TABLE OF CONTENTS
| | |
Article I | | 3 |
Definitions | | 3 |
Article II | | 9 |
License | | 9 |
Article III | | 11 |
Manufacture and Supply | | 11 |
Article IV | | 12 |
Development and Commercialization | | 12 |
Article V | | 15 |
Consideration | | 15 |
Article VI | | 21 |
Call Back Option and Right of First Discussions | | 21 |
Article VII | | 23 |
Intellectual Property | | 23 |
Article VIII | | 28 |
Representations and Warranties | | 28 |
Article IX | | 30 |
Confidentiality | | 30 |
Article X | | 31 |
Announcement and Publicity | | 31 |
Article XI | | 32 |
Term and Termination | | 32 |
Article XII | | 34 |
Indemnification and Insurance | | 34 |
Article XIII | | 36 |
Information on Clinical Safety and Pharmacovigilance | | 36 |
Article XIV | | 37 |
Governing Law and Jurisdiction | | 37 |
Article XV | | 37 |
Miscellaneous Provisions | | 37 |
2
TERRITORY LICENSE AGREEMENT
Molecular Insight Pharmaceuticals, Inc. / BIOMEDICA Life Sciences S.A.
This territory license agreement (“Agreement”), dated this 1st day of September, 2009 (the “Effective Date”) is entered into by and between Molecular Insight Pharmaceuticals, Inc. (referred to herein as “MIP”), a corporation organized and existing under the laws of The Commonwealth of Massachusetts and having its principal office at 160 Second Street, Cambridge, MA 02142 USA, and BIOMEDICA Life Sciences S.A., a corporation organized and existing under the laws of Greece, with offices at 4 Papanikoli Str., 15232 Halandri, Athens, Greece (referred to herein as “BIOMEDICA”), with Greek Tax ID of 094413470, from the tax office of FAEE Athens; each a “Party” and collectively the “Parties” hereto.
RECITALS
WHEREAS, MIP has experience with and has developed and controls certain intellectual property and technical information with respect to a pharmaceutical product known as Onalta™. In particular, MIP has licensed exclusive and non-exclusive worldwide rights from Novartis AG to certain intellectual property relating to radiolabeled somatostatin analogues;
WHEREAS, BIOMEDICA has expertise in the health care market, radiopharmaceuticals in particular, and is engaged in the innovation and R&D in the subject areas.
WHEREAS, BioMedica wishes to obtain, and MIP desires to grant, a territory license from MIP for the development, exploitation and commercialization of Onalta™;
WHEREAS, the Parties have executed a Letter of Intent (LOI) on March 26, 2009, in furtherance of this purpose.
NOW, THEREFORE, in consideration of the mutual promises, covenants and agreements hereinafter set forth, the sufficiency of which is hereby acknowledged, the Parties to this Agreement mutually agree as follows:
1. DEFINITIONS
| 1.1. | “Accounting Standards” shall mean, with respect to MIP, U.S. GAAP (United States Generally Accepted Accounting Principles) and, with respect to BIOMEDICA, IFRS (International Financial Reporting Standards). |
| 1.2. | “Affiliate” shall mean any entity that directly or indirectly controls or is controlled by or is under common Control with a Party to this Agreement. For purposes of this definition, “control” or “controlled” means ownership directly or through one or more Affiliates, of fifty percent (50%) or more of the shares of stock entitled to vote for the election of directors, in the case of a |
3
| | corporation, or fifty percent (50%) or more of the equity interest in the case of any other type of legal entity, status as a general partner in any partnership, or any other arrangement whereby a Party controls or has the right to control the board of directors or equivalent governing body of a corporation or other entity, or the ability to cause the direction of the management or policies of a corporation or other entity. |
| 1.3. | “Agreement” shall mean this Agreement together with all exhibits, schedules, and appendices attached to this Agreement, all as respectively amended, modified or supplemented by the Parties in accordance with the terms of this Agreement. |
| 1.4. | “BIOMEDICA Field” shall mean human oncology therapeutic use, and use of the Indium 111 labeled Compound for dosimetry purposes in relation to therapy administration of the Product. |
| 1.5. | “BIOMEDICA New Intellectual Property” shall exclude technology developed jointly by the Parties. |
| 1.6. | “Calendar Year” shall mean the calendar year, starting on January 1 and ending on December 31, in which the first sale of the Compound or the Product occurs and each successive calendar year. |
| 1.7. | “Change of Control” shall mean any of the following events: (a) any Third Party (or group of Third Parties acting in concert) becomes the beneficial owner, directly or indirectly, of fifty percent (50%) or more of the voting power of the stock then outstanding of a Party; (b) a Party consolidates with or merges into another corporation or entity, or any corporation or entity consolidates with or merges into the Party, in either event pursuant to a transaction in which fifty percent (50%) or more of the total voting power of the stock outstanding of the surviving entity normally entitled to vote is not held by the Parties holding more than fifty percent (50%) of the outstanding shares of a Party prior to such consolidation or merger; (c) any Third Party (or group of Third Parties acting in concert) obtains the power to direct or cause the direction of the management and policies of a Party by any lawful means whatsoever; or (d) a Party conveys, transfers or leases all or substantially all of its assets. |
| 1.8. | “Commercialization” or “Commercialize” shall mean activities conducted by a Party either by itself or through a Third Party and directed to marketing, promoting, distributing, importing, exporting, offering for sale or selling a Product, which may include pre-launch market preparation, whether undertaken by a Party alone or with a partner or a sub-licensee. When used as a verb, “Commercialize” means to engage in Commercialization. |
| 1.9. | “Commercially Reasonable Effort” shall mean the efforts and resources customarily used in the industry by a company of similar size for a product with an equivalent sales and profit potential to the Product. |
4
| 1.10. | “Compound” shall mean a lyophilized kit comprising the DOTA-chelated somatostatin peptide analogue known as edotreotide plus excipients provided. |
| 1.11. | “Confidential Information” shall mean and include any and all Know-How, data and information, not in the public domain. It shall also include, but not be limited to, information relating to the Product and/or Compound, or the business, research and development activities, results of clinical trials, regulatory proceedings, finances, contractual relationships and operations of the Parties. |
| 1.12. | “Controlled” or “Controls”, when used in reference to intellectual property, shall mean the legal authority or right of a Party hereto (or any of its Affiliates) to grant a license or sub-license of intellectual property rights to another Party, or to otherwise disclose proprietary or trade secret information to such other Party, without breaching the terms of any agreement with a Third Party, infringing upon the intellectual property rights of a Third Party, or misappropriating the proprietary or trade secret information of a Third Party. |
| 1.13. | “Development” shall mean all activities conducted by a Party either by itself or through a Third Party, as are necessary for an application for a Marketing Authorization. |
| 1.14. | “Development Costs” shall mean, with respect to the Compound or Product, expenses and other costs, including regulatory expenses, incurred by or on behalf of a Party in connection with the Development of the Compound or Product, including, the costs of clinical trials, the preparation, collation and/or validation of data from such clinical trials and the preparation of medical writing and publishing. |
| 1.15. | “Effective Date” shall mean the date first above written. |
| 1.16. | “EMEA” shall mean the European Medicines Agency or its successor agency. |
| 1.17. | “First Commercial Sale” shall mean the first sale of the Compound or the Product to a Third Party by BIOMEDICA or an Affiliate or sub-licensee of BIOMEDICA in a country in the Territory following applicable EMEA approval for or consent to compassionate use of the Compound or the Product and/or Marketing Authorization in one indication of the Product in the Territory. |
| 1.18. | “Good Clinical Practice” or “GCP” shall mean the generally accepted standard of Good Clinical Practice within the pharmaceutical industry for the design, conduct, performance, monitoring, auditing, recording, analyses and reporting of clinical trials that provides assurance that the data and reported results are clinical and accurate and that the rights, integrity and confidentiality of the trial subjects are protected. |
| 1.19. | “Good Manufacturing Practices” or “GMP” shall mean the then current Good Manufacturing Practices as such term is defined from time to time by the FDA or other relevant Governmental Authority having jurisdiction over the development, manufacture or sale of the Product in the Territory pursuant to its regulations, guidelines or otherwise. |
5
| 1.20. | “Initial Period” is the time from the effective date until the filing of a marketing application |
| 1.21. | “Interim Period” is the time from the filing of the marketing application to the final action by the EMEA. |
| 1.22. | “Marketing Authorization” shall mean, with respect to a country in the Territory, the approval by the appropriate authority necessary for the Commercialization of the Product in that country. For the sake of clarity, Marketing Authorization shall not include the reimbursement approval. |
| 1.23. | “NDA” or “New Drug Application” shall mean a new drug application and all amendments and supplements thereto filed with the EMEA, or any such Regulatory Authority requiring such filing, including all documents, data and other information concerning a pharmaceutical product that are necessary for gaining Marketing Authorization to market and sell such pharmaceutical product. |
| 1.24. | “NDA Acceptance” shall mean the date upon which the Product NDA submitted by BIOMEDICA is deemed filable by the EMEA or the equivalent dossier is accepted for review by any other country’s respective Regulatory Authority. |
| 1.25. | “Net Sales” shall mean with respect to the Compound and the Product the gross amount invoiced by or on behalf of BIOMEDICA and its Affiliates and sub-licensees for the Compound or Product sold to Third Parties other than sub-licensees in bona fide, arms-length transactions, less the following customary deductions, determined in accordance with the BIOMEDICA’s Accounting Standards as generally and consistently applied by BIOMEDICA, to the extent included in the gross invoiced sales price of any Compound or Product or otherwise directly paid or incurred by BIOMEDICA, its Affiliates or sub-licensees with respect to the sale of such Compound or Product: i) normal and customary trade and quantity discounts actually allowed and properly taken directly with respect to sales of the Compound or Product; ii) amounts actually repaid or credited by reasons of defects, rejection recalls, returns, rebates and allowances of goods; iii) chargebacks and other amounts paid on sale or dispensing of such Compound or Product; iv) amounts payable resulting from governmental mandated rebate programs; v) tariffs, duties, mandatory transaction or sales related type fees imposed by any governmental entity (even if paid to a third party), excise, sales, value- added and other taxes (other than taxes based on income); vi) retroactive price reductions specifically identifiable to the Compound or Product that are actually allowed or granted; vii) customary cash discounts for timely payment; viii) delayed ship order credits; ix) discounts pursuant to indigent patient programs and patient discount programs and coupon discounts; and x) all freight, postage and insurance included in the invoice price. |
| | 1.25.1. | Sales from BIOMEDICA to its Affiliates shall be disregarded for purposes of calculating Net Sales. Any of the items set forth above that would otherwise be deducted from the invoice price in the calculation of Net Sales but which are separately charged to Third Parties shall not be deducted from the invoice price in the calculation of Net Sales. |
6
| | 1.25.2. | In the case of any sale or other disposal of the Compound or the Product between or among BIOMEDICA and its Affiliates or sub-licensees, for resale, Net Sales shall be calculated as above only on the value charged or invoiced on the first arm’s-length sale thereafter to a Third Party; |
| | 1.25.3. | In the case of any sale which is not invoiced or is delivered before invoice, Net Sales shall be calculated at the time of shipment or when the Compound or the Product is paid for, if paid for before shipment or invoice; |
| | 1.25.4. | In the case of any sale or other disposal for value, such as barter or counter-trade, of any Compound or Product, or part thereof, other than in an arm’s length transaction exclusively for money, Net Sales shall be calculated as above on the value of the non-cash consideration received or the fair market price (if higher) of the Compound or Product in the country of sale or disposal; |
| | 1.25.5. | In the event the Compound and the Product is sold in a finished dosage form containing the Compound in combination with one or more other active ingredients (a “Combination Product”), the Net Sales of the Compound or Product, for the purposes of determining royalty payments, shall be determined by multiplying the Net Sales (as defined above in this Article) of the Combination Product by the fraction, A/(A+B) where A is the weighted (by sales volume) average sale price in a particular country of the Compound or Product when sold separately in finished form and B is the weighted average sale price in that country of the other product(s) sold separately in finished form. In the event that such average sale price cannot be determined for both the Compound, Product and the other product(s) in combination, Net Sales for purposes of determining royalty payments shall be agreed by the Parties based on the relative value contributed by each component, such agreement shall not be unreasonably delayed or withheld. |
| 1.26. | “Know-How” shall mean proprietary or non proprietary information relating to the Compound and/or the Product excluding the regulatory CMC information for the chemical manufacturing process. The information shall include the relevant specifications, technical data, inventions, discoveries, formulation, processes, trade secrets, expertise developments and regulatory information of the Product controlled by MIP as well as, chemical, stability, pharmacological, safety, clinical data, analytical and quality control data, whether or not protected under patent, trademark, copyright or other legal principles, to which MIP has rights at the Effective Date. For the sake of clarity, any information related to the manufacture of the Tyr-3 octreotide will not be disclosed to BIOMEDICA. |
7
| 1.27. | “Novartis License Agreement” shall mean the license agreement entered into by and between Novartis and MIP dated November 3, 2006 and any amendments thereto. |
| 1.28. | “Novartis” shall mean Novartis Pharma AG, a corporation organized and existing under the laws of Switzerland, and having its principal offices at Lichtstrasse 35, CH-4056 Basel, Switzerland. |
| 1.29. | “Novartis Patents” shall mean those patents and patent applications owned or Controlled by MIP during the term of this Agreement with a claim encompassing Compound, Product, or any other formulations of Compound, processes, uses and intermediates for the foregoing and shall include any patents or patent applications and any continuations, continuations-in-part, divisions, provisionals, substitutions, patents of addition, reissues, examination, renewals or extensions thereof (including any supplemental patent certificates) and any confirmation patent or registration patent and all foreign counterparts of any of the foregoing. |
| 1.30. | “Onalta” and “www.onalta.com” shall mean the trademarks owned and selected by MIP for the Product. |
| 1.31. | “Peptide” shall mean the DOTA-chelated somatostatin peptide analogue known as edotreotide. |
| 1.32. | “Product” shall mean the yttrium radiolabeled Compound for therapeutic use and indium-111 radiolabeled Compound for dosimetry purposes, both in a form ready for use in human clinical trials and/or by the ultimate consumer with the trademark of Onalta. |
| 1.33. | “Regulatory Authority” shall mean the EMEA or additional governmental or regulatory agencies in the Territory responsible for applicable Marketing Authorization. |
| 1.34. | “Royalty Term” shall have the meaning as set forth in article 5.6. |
| 1.35. | “Sub-licensee” shall mean a Third Party to whom BIOMEDICA may grant a right or license to use Novartis Patents or Know-How to make, use or sell any Product in the Territory. |
| 1.36. | “Term” shall have the meaning as set forth in article 11.1. |
| 1.37. | “Territory” shall mean those countries as of the Effective Date belonging to the European Union, the European Free Trade Association (Switzerland, Iceland, Lichtenstein and Norway), Eastern Europe, Russia and the other former Commonwealth of Independent States (CIS), the Middle East and Arabic States, Turkey, and the North Africa region. “Territory” shall specifically exclude the State of Israel unless and until such rights become available pursuant to article 2.4. |
8
| 1.38. | “Trademark” shall mean MIP’s “Onalta” related trademarks, logos, and tradenames. |
| 1.39. | “Valid Claim” shall mean an issued claim of a Novartis Patent which claim has not been held invalid or unenforceable by final decision of a court or other governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and which is not admitted to be invalid or unenforceable through reissue, disclaimer or otherwise. |
The following Schedules attached to this Agreement are hereby incorporated by reference:
Exhibit A: BIOMEDICA Business Plan
Exhibit B: Intellectual Property
Exhibit C: Examples of Contingent Milestone Payment Calculations
Exhibit D: Examples of Permitted Disclosures
2. LICENSE
| 2.1 | Grant to BIOMEDICA.Subject to the terms and conditions of this Agreement, MIP hereby grants to BIOMEDICA exclusively within the Territory, a royalty-bearing license under its rights in the Novartis Patents and Know-How necessary for BIOMEDICA to (i) develop, (ii) market, (iii) advertise, (iv) manufacture, (vi) have manufactured, (vii) import, (viii) export, (ix) distribute, and (x) commercialize the Compound and the Product, including securing all regulatory approvals and performing clinical studies. Such rights granted are expressly conditioned on and subject to the Novartis License Agreement. In addition, MIP hereby grants to BIOMEDICA a non-exclusive, non-transferable license to use the trademark ONALTA in connection with sale or distribution of the Product throughout the Territory subject to the terms and conditions set forth below. For the avoidance of doubt, the rights granted herein are for the Compound when used as a therapeutic Product, and BIOMEDICA shall not be granted any rights to use, sell or otherwise promote the Compound or Product as a diagnostic product. |
| 2.2 | MIP agrees that in the event that it grants future sublicenses under the Novartis License Agreement on more favorable terms than the terms set forth in this Agreement, MIP will offer such terms to BIOMEDICA provided such terms are not unfairly discriminatory to BIOMEDICA. More favorable payment terms to a third party licensee which are either not under MIP’s control (such as government imposed terms), or reasonable in light of the size and limited economic opportunity in the subject country or region, shall not constitute unfair discrimination. |
9
| 2.3 | Sub-license. BIOMEDICA shall have the right to grant, under the license granted in article 2.1, additional sub-licenses to Third Parties with respect to the Product, provided that the terms and conditions of sub-license agreements shall be consistent with the terms of this agreement. BIOMEDICA shall undertake to enforce the provisions of such sub-license agreements and shall remain responsible for the performance of sub-licensee’s obligations. BIOMEDICA shall cause each sub-licensee to execute any and all additional documents reasonably requested by MIP to reflect the conditions set forth in this agreement. |
| | 2.3.1 | BIOMEDICA’s right to grant Third Party sublicenses hereunder is expressly conditioned upon prior written notice and approval by MIP and by Novartis, which approval from MIP shall not be unreasonably withheld. |
| 2.4 | Limitations of Rights. It is acknowledged and agreed that no other license rights are granted by MIP to BIOMEDICA other than the license expressly granted by the provisions of Article 2.1, and that MIP expressly retains all other rights under any MIP Know-How and MIP Patents. |
| 2.5 | MIP-NOVARTIS Change in Payment Terms. To the extent that MIP and NOVARTIS, or in the event of material breach by Novartis enter into a new agreement reducing the payments to be paid to Novartis under the Novartis License Agreement and/or amending the payment structure, MIP agrees to extend the benefit of any such reduction or amendment to BIOMEDICA to the extent fair and reasonable. |
| 2.6 | Additional Countries. If exclusive sublicense rights to the Novartis Patents become available in ******, then MIP shall notify BIOMEDICA in writing of the existence of such rights, and the Parties shall undertake to include ****** within the Territory under this Agreement. Other than ******, if after the Effective Date but within the Term of this Agreement, if MIP desires to sublicense rights to the Novartis Patents for any country not included in the Territory, then MIP shall give BIOMEDICA reasonable notice of its intent and an opportunity to participate in negotiations for sublicense rights in such country. |
| 2.7 | Transfer of MIP Information and MIP Files. MIP shall use reasonable efforts to transfer to BIOMEDICA within ninety (90) days of the Effective Date or such longer period as reasonably required by MIP, instructions and training manuals for the radiolabeling and patient administration of the Compound, existing as of the Effective Date, on an “as is” basis. For the sake of clarity MIP shall not be requested to perform clinical trials or animal studies with the Compound or Product, and all data and files shall be transferred as is with no further obligations on MIP. The MIP information provided hereunder shall remain the property of MIP, but BIOMEDICA shall be free to use it in connection with the sublicense granted above. |
10
*Confidential Treatment Requested*
| 2.8 | Liaison. The primary contact persons (the “Contact”) at BIOMEDICA and MIP are set forth in Section 15.8, and these designated persons shall act as liaisons for the purpose of this Agreement, which role shall include but not be limited to receiving the reports set forth in Articles 4.2 and 5.8 and other notices under this Agreement. The Parties may change their respective Contact at any time by providing written notice to the other Party. |
3. MANUFACTURE AND SUPPLY
| 3.1 | Purchase of Compound and Product. During the Term of this Agreement BIOMEDICA shall purchase all of its requirements for the Compound and the Product exclusively and solely from MIP, a MIP designated third party manufacturer, and/or a BIOMEDICA designated third party manufacturer approved by MIP. BIOMEDICA shall not, without the written authorization from MIP, supply or otherwise distribute the Compound or Product to any third party outside of the Territory. |
| | 3.1.1 | Supply agreement. The Parties agree to begin good faith negotiations on a definitive supply agreement for the Compound and the Product immediately upon execution of this Agreement. It is the goal of the Parties to finalize the supply agreement within 14 days of the Effective Date of this Agreement. The Parties acknowledge that the effective date of the supply agreement shall be concurrent with the date MIP enters into a manufacturing agreement with an MIP designated third party, manufacturer. The Parties understand and acknowledge that the supply agreement will include an advance payment by BIOMEDICA for inventory on the following schedule: |
| | 3.1.2 | The acquisition of product from inventory will be at the agreed-to price for both the Compound and the Product (section 3.4). For the sake of clarity, the Parties agree that the supply agreement referenced above in Section 3.1.1 will include a minimum purchase requirement by BioMedica, with the amount(s) and frequency of such minimum purchase to be |
11
*Confidential Treatment Requested*
| | negotiated by the Parties acting in good faith. The inventory payments referred to in section 3.1.1 will be credited against purchases once purchases exceed milestones that will be defined in the supply agreement between MIP and BIOMEDICA. |
| 3.2 | Responsibility for Manufacturing Product. MIP, or the MIP designated third party manufacturer shall supply BIOMEDICA’s requirements for the Product for a period of ten years following the Effective Date of this Agreement upon terms to be agreed in the supply agreement. |
| 3.3 | Responsibility for Manufacturing Compound. MIP, or the MIP designated third party manufacturer shall supply BIOMEDICA’s requirements for the Compound for a period of five years following the Effective Date of this Agreement upon terms to be agreed in the supply agreement. |
| 3.4 | ****** License Termination: Immediately upon execution of this Agreement, MIP and BIOMEDICA jointly agree to develop and implement a Peptide distribution plan to be effective concurrent with MIP’s termination of the ****** Agreement (90 day notice required) in order to be able to seamlessly supply peptide within the Territory. |
| 3.5 | Pricing Commitment: MIP will provide the Compound for radiolabeling during the interim period until the Product, Onalta™, is available. BIOMEDICA will purchase the Compound at an agreed upon single unit dose price of ******. BIOMEDICA will be responsible for Y-90 isotope purchase for the production at radiopharmacies until the Product, Onalta™, is available. BIOMEDICA will purchase the Product at an agreed upon unit price of ******. |
| 3.6 | Training and Support: MIP will provide reasonable levels of technical support to BIOMEDICA at BIOMEDICA’s request on a time and materials basis. The Parties will enter into good faith discussions regarding the details of such support and associated costs upon request by BIOMEDICA. Such support will take place as soon as reasonably possible and primarily focused on training BIOMEDICA’s personnel. |
4. DEVELOPMENT AND COMMERCIALIZATION
| 4.1 | Development Costs. BIOMEDICA shall be solely responsible for all Development and Commercialization activities to be conducted pursuant to this Agreement from the Effective Date and all costs related thereto. BIOMEDICA has developed a Business Plan (the “Business Plan”) attached hereto Exhibit A that describes in detail the Development and Commercialization activities that BIOMEDICA will undertake in connection with the Compound and the Product. BIOMEDICA commits to implement the Development and Commercialization activities substantially in accordance with the “Business Plan” Development milestones and timelines shown on page 20 of Exhibit A. |
12
*Confidential Treatment Requested*
| 4.2 | Reports: BIOMEDICA shall provide to MIP reports detailing its Development and Commercialization activities as set forth below. If BIOMEDICA should elect a third party manufacturer of the Product pursuant to article 3.3, then the report shall include all manufacturing information, including but not limited to lot numbers, Product specifications, and GMP compliance. |
| | 4.2.1 | During the Initial Period, no later than thirty (30) days after the end of each calendar quarter, BIOMEDICA shall provide written reports to MIP containing specific and detailed information regarding BIOMEDICA’s performance under each of the Timelines and Milestones set forth in the Business development Plan, including but not limited to (i) a summary of the regulatory activities for the Product, including any material changes in such activities since the prior reporting period, (ii) a summary of the clinical study results for the Compound and the Product, including any material changes in the clinical activities since the prior reporting period, (iii) other development activities for the Licensed Product (which may include material changes in the projected timelines) as deemed appropriate by BIOMEDICA in its reasonable discretion, and (iv) a summary of material planned regulatory and clinical activities for the four (4) calendar quarters following such reporting period; the relevant reporting period shall be the three (3)-month period ending March 31, June 30, September 30 or December 31 of each calendar year preceding such report, as applicable; |
| | 4.2.2 | During the Interim Period, no later than sixty (60) days after the end of each six (6)-month period ending June 30 and December 31 of each calendar year (semi-annual), BIOMEDICA shall provide written reports to MIP containing the information specified in clause (i) above for the six (6)-month reporting period ending June 30 or December 31 of each calendar year preceding such report and a summary of material planned regulatory and clinical activities for the following two (2) six (6)-month periods ending December 31 and June 30; and |
| | 4.2.3 | Thereafter, no later than sixty (60) days after the end of each calendar year, BIOMEDICA shall provide written reports to MIP containing the information specified in clause (i) above for each calendar year preceding such report and a summary of material planned regulatory and clinical activities for the following calendar year (each report referred to in clauses (i), (ii) and (iii), a “Development Report”). MIP shall be permitted to disclose summaries of Development Reports provided by BIOMEDICA to MIP. |
| 4.3 | Development Efforts. BIOMEDICA shall use Commercially Reasonable Efforts to develop and register the Product and shall be solely responsible for compiling the NDA for each Product in the BIOMEDICA Field at its own expense. BIOMEDICA may request MIP’s involvement with any NDA, and BIOMEDICA shall pay all charges associated with MIP’s assistance on a time and materials basis for any requested involvement by MIP’s personnel. |
| | 4.3.1 | Medical Symposia, Advisory Boards or other Meetings with Key Opinion Leaders. BIOMEDICA agrees that, if it is planning to hold such a meeting, at least one member of MIP’s management shall have the right to attend (at MIP’s sole expense) |
13
| 4.4 | Development Committee. The parties shall establish a development committee to review and discuss current material clinical development activities and safety information relating to the Compound and the Product, and review and discuss as appropriate future material clinical development, medical symposia, publications in top peer reviewed journals and regulatory plans for the Compound and the Product. BIOMEDICA and MIP may each appoint up to four (4) members to the development committee. |
| | 4.4.1 | Development Committee Meetings. Beginning no later than ninety (90) days after the Effective Date, the Development Committee shall meet quarterly through the Initial Period. After the Initial Period the Development Committee will meet semi-annually during the Interim Period. Thereafter, the Development Committee shall meet annually. All meetings of the Development Committee will be timed to follow the receipt of the Reports (as defined in Section 4.2 above) and will be held in person at a mutually agreeable time and location. |
| 4.5 | Clinical Trials. BIOMEDICA shall be solely responsible for and shall bear all the costs related to the conduct of clinical trials required to file NDAs in the Territory. BIOMEDICA undertakes to run the clinical trial program in accordance with GCP. MIP retains the right to read and access data from BIOMEDICA’s clinical trial including supporting data for MIP’s NDAs in regions outside the territories. |
| 4.6 | Marketing Efforts - Market Launch. BIOMEDICA shall use Commercially Reasonable Efforts to launch and sell the Product in the Territory for as long as this Agreement is in effect. The parties agree to meet and discuss marketing and market launch activities with a post development committee as set forth below in section 4.7. |
| 4.7 | Post-Development Committee: Within six (6) months of the Effective Date, the parties shall establish a “Post-Development Committee” to provide information sharing and be a forum for creative discussion and problem solving relating to (i) BIOMEDICA’s pre-launch activities, timelines and pre-commercialization plan ii) sales force development plans and marketing plans (iii) an overview of the market penetration rates by territory and any other relevant issues. Each party shall appoint no more than three (3) members to the Post-Development Committee. |
| | 4.7.1 | Post-Development Committee Meetings: The first meeting of the Post-Development Committee shall occur within one (1) year of the Effective Date at which meeting time the committee meeting schedule will be determined. |
14
| | 4.7.2 | Post-Development Committee Meeting Expenses. Each party shall bear all expenses of their respective members relating to their participation on the Post-Development Committee. |
| 4.8 | Booking Sales. BIOMEDICA shall, at its own expense, book sales of the Compound and the Product as distributed by or on behalf of BIOMEDICA. |
| 4.9 | Pricing. BIOMEDICA shall be free to set prices of the Compound and the Product. |
| 4.10 | Product Name. Product sold or distributed pursuant to this Agreement shall be sold or distributed under the ONALTA Trademark and all such use shall be governed by the terms provided herein. |
| 4.11 | Compound Name. Compound sold or distributed pursuant to this Agreement shall be sold or distributed under the name edotreotide radiolabeling kit for intravenous use and all such use shall be governed by the terms provided herein. |
5. CONSIDERATION
| 5.1 | License Fee. Upon execution of this Agreement, in consideration of the exclusivity in negotiations enjoyed by BIOMEDICA prior to execution of this Agreement for the rights granted to BIOMEDICA hereunder, BIOMEDICA shall pay and shall cause to be released to MIP the sum of ****** that the Parties have previously escrowed. In addition, in further consideration of the rights granted herein, BIOMEDICA shall pay to MIP, the additional sum of ****** such payment due upon execution of this Agreement. These initial license payments shall not be credited against any royalty or other payments to be made pursuant to this Agreement, and shall not be refundable. |
| 5.2 | Milestone Payments. In consideration of the rights granted to BIOMEDICA by MIP hereunder, BIOMEDICA shall make the following milestone payments to MIP. These milestone payments shall not be credited against any royalty or other payments to be made pursuant to this Agreement, and shall not be refundable unless otherwise indicated. BIOMEDICA shall spontaneously notify MIP of such events upon occurrence thereof, whereupon MIP shall submit an invoice to BIOMEDICA requesting such milestone payment. The payment shall be received by MIP 60 days after the occurrence of such milestone. |
| | 5.2.1 | ****** shall be due three (3) months after a first sale of the Compound following EMEA approval for or consent to compassionate use of the Compound |
15
*Confidential Treatment Requested*
| | 5.2.2 | ****** shall be due upon submission of the first NDA by BIOMEDICA in the Territory. |
| | 5.2.3 | ****** shall be due upon NDA Acceptance in the Territory. |
| | 5.2.4 | In addition to the amount provided in article 5.2.3, ****** shall be due and payable upon the granting of Marketing Authorization by the EMEA. |
| | 5.2.5 | The payments under article 5.2.2 and 5.2.3 shall be reduced ****** going forward, or will be retroactively refunded in the amount of ****** of the stated sum if the milestone has already be achieved, upon MIP submitting an NDA or obtaining NDA Acceptance outside of the Territory. |
| | 5.2.6 | If subsequent to the Effective Date, pursuant to article 2.4 the Parties expand the Territory to include additional countries not specified as part of the Territory as of the Effective Date, and the additional countries generate an incremental increase in Net Sales greater than ****** over the original countries in the Territory,then BIOMEDICA shall not be entitled to the reduction or refund specified in article 5.2.5. In addition, to the extent any refund has been paid by MIP, then BIOMEDICA shall repay the corresponding refunded portion to MIP. |
| 5.3 | Additional Milestone Payments. The following milestone payments shall be in addition to those specified in Section 5.2, and shall be payable to MIP in the Calendar Quarter following that in which the milestone is achieved pursuant to article 5.10. The following additional milestones under this article 5.3 shall be cumulative. These additional milestone payments shall not be credited against any royalty or other payments to be made pursuant to this Agreement, and shall not be refundable. The payment shall be received by MIP 60 days after the occurrence of such milestone. |
| | 5.3.1 | ****** shall be due upon achieving cumulative Net Sales of the Compound and/or the Product in excess of ******; |
| | 5.3.2 | An additional ****** shall be due and payable upon achieving cumulative Net Sales of the Compound and/or the Product in excess of ******; |
16
*Confidential Treatment Requested*
| | 5.3.3 | An additional ****** shall be due and payable upon achieving cumulative Net Sales of the Compound and/or the Product in excess of ******; and |
| | 5.3.4 | An additional ****** shall be due and payable upon achieving cumulative Net Sales of the Compound and/or the Product in excess of ******. |
| 5.4 | Contingent Milestone Payments. MIP’s rights under the Novartis Patents, and by extension BIOMEDICA’s license rights thereto, are subject to a one-time Call-Back Option as defined by the Novartis and MIP License Agreement and as essentially set forth below in article 6 herein. The Call Back Option is triggered when aggregate worldwide Net Sales of Product in a Calendar Year first reach ******. |
| | 5.4.1 | BIOMEDICA shall provide MIP with reasonable advance notice if its sales forecasts project potential Net Sales of the Compound and/or the Product could reach ****** in a Calendar Year. |
| | 5.4.2 | If worldwide Net Sales of the Compound or the Product reach ****** in a Calendar Year, then BIOMEDICA agrees to fully cooperate with MIP as part of any Novartis due diligence inquiry. BIOMEDICA shall assume all costs associated with its involvement in such due diligence. |
| | 5.4.3 | Should Novartis decide not to exercise its Call Back Option, then BIOMEDICA shall pay the following contingent milestone payments to MIP on a pro-rata basis as determined by BIOMEDICA Net Sales as a proportion of worldwide Net Sales. Where a previously-triggered contingent milestone payment is triggered in a future year, then ****** shall govern an equitable contribution and credit back to any sublicensee having earlier contributed to such previously triggered contingent milestone payment. |
| | 5.4.3.1 | ****** once an annual worldwide Net Sales amount of ****** is achieved, payable in two equal installments, the first payment due by January 31st of the Calendar Year following the year in which the milestone is achieved and the second payment 12 months later. |
| | 5.4.3.2 | An additional ****** once an annual worldwide Net Sales of ****** is achieved, payable in two equal installments, the first payment due by January 31st of the Calendar Year following the year in which the milestone is achieved and the second payment 12 months later. |
| | 5.4.3.3 | An additional ****** once an annual worldwide Net Sales amount to ****** is achieved, payable in two equal installments, the first payment by January 31st of the Calendar Year following the year in which the milestone is achieved and the second payment 12 months later. |
17
*Confidential Treatment Requested*
| 5.5 | Royalties. In addition to the license fees set forth in article 5.1 and the milestone payments set forth in articles 5.2, 5.3 and 5.4, and as further consideration for the license rights granted to BIOMEDICA hereunder, BIOMEDICA further agrees to pay to MIP royalties on BIOMEDICA’s and/or its Sub-licensee’s aggregate Net Sales for both the Compound and/or the Product in the Territory, during the Royalty Term at a rate calculated according to the following: |
| | 5.5.1 | ****** of the annual Net Sales of the Compound and/or the Product for Net Sales amounting to less than ****** annually. |
| | 5.5.2 | ****** of the Net Sales of the Compound and/or the Product on the incremental sales between ****** and ****** annually. |
| | 5.5.3 | ****** of the Net sales of the Compound and/or the Product on the incremental sales over ******. |
| 5.6 | Royalty Term: Royalties shall be payable quarterly on a country-by-country basis from the First Commercial Sale of the Product until the occurrence of the later of: (i) the expiration of the latest to expire Novartis Patent including any extensions in each such country or (ii) 10 years from First Commercial Sale. Royalty payments shall be received by MIP no later than 30 days from the close of the quarter. |
| 5.7 | The Combination Royalty: In the event that the Peptide, Compound and / or Product (“MIP Component”) is formulated with a different or other active ingredient or a second product to comprise a fixed Combination Product, the “net sales” of the MIP Component, for purposes of determining royalty payments to Novartis and MIP, shall be determined by multiplying the Net Sales of the Combination Product by the fraction, A/(A+B), whereas A is the weighted average sales price of the Product (by sales volume) when sold independently and B is the weighted average sales price (by sales volume) of the other product-component when sold independently. In the event the weighted average sales price cannot be determined for either “A” or “B”, the Parties hereto shall agree on an alternative method for calculating “net sales” of the MIP Component of the Combination Product for purposes of calculating royalties to Novartis and MIP. In such event, the Parties shall agree on the methodology prior to submitting the registration dossier for the Combination Product, taking into account relevant factors such as, but not limited to, the relative cost and value contributed by each component weighted proportionally. |
18
*Confidential Treatment Requested*
| 5.8 | Equity/Option Rights. In addition to the license fees set forth in article 5.1, the milestone payments set forth in articles 5.2, 5.3 and 5.4, and the royalty payments in article 5.5, and as further consideration for the license rights granted to BIOMEDICA hereunder, MIP is entitled to the following stock option grant and conditions: |
| | 5.8.1 | BIOMEDICA grants MIP an option to have BIOMEDICA assign, transfer and convey a minority shareholder interest in BIOMEDICA to MIP upon conclusion of and execution of the Comprehensive Agreement: |
| | 5.8.2 | BIOMEDICA grants MIP an option to have BIOMEDICA assign, transfer and convey a minority shareholder interest in BIOMEDICA to MIP upon EMEA approval. |
| | 5.8.3 | The amount in equivalent shares which MIP shall have the right to exercise under the option grant in (i) and (ii) above, is exactly ****** of the total, non-diluted interest, in all classes of any issued and authorized shares outstanding in BIOMEDICA at the time of each exercise, assuming a fair market valuation of BIOMEDICA at ****** on the Effective Date of this Agreement; |
| | 5.8.4 | To the extent B BIOMEDICA’s grant of the option grant in (i) and (ii) above is subject to Board or shareholder approval, BIOMEDICA agrees to, in good faith, seek to secure all necessary approvals, or alternatively, to secure a commitment by a major shareholder for the transfer of its shares to MIP. |
| | 5.8.5. | BIOMEDICA represents that it believes that it has the legal right to make the above commitments. In the event that such commitments violate applicable Greek or E.U. securities laws or rules, then the above grants may, to the extent possible, be rewritten or amended, consistent with the intent of the parties, to cure any deficiencies therein, and if this is not possible, the commitments are automatically null and void. |
| | 5.8.6 | In the event of a change of control, bankruptcy or dissolution by MIP, or in the event that MIP desires to transfer or assign any equity interest that it may hold in BIOMEDICA, BIOMEDICA shall have a right of first refusal to buy back all or a portion of MIP’s interest in BIOMEDICA’s equity. |
| 5.9 | Sales Reports. After the First Commercial Sale of the Compound or the Product and during the Term of this Agreement, BIOMEDICA shall furnish or cause to be furnished to MIP on a quarterly basis a written report showing the Net Sales of Product in each country in the Territory. |
| | 5.9.1 | Timing. Each quarterly report shall be due within thirty (30) days following the close of each quarter. |
| | 5.9.2 | Records. BIOMEDICA shall keep accurate records in sufficient detail to enable the amounts due hereunder to be determined and to be verified by an independent certified public accountant mutually agreed upon by the Parties. |
19
*Confidential Treatment Requested*
| 5.10 | Currency Exchange. All royalty payments shall be made in United States Dollars (“USD”). With respect to sales or other dispositions of the Compound or the Product invoiced in a currency other than USD, the Net Sales and amounts due to MIP hereunder will be expressed in USD equivalent calculated on a monthly basis in the currency of the country of sale and converted to their dollar equivalent using the Daily Spot Rate on the last day of the period, published in the Reuters System - Reuters Daily Rates between 09:00 a.m. and 10 a.m. CET. |
| 5.11 | Payment Due Date; Accrual.Payments shall be made by wire transfer as directed by the receiving Party or to the address designated herein. Milestones and Royalties which have accrued during any Calendar Year and are required to be shown on a sales report provided under this Article 5 of the Agreement shall be due and payable as set forth in the relevant sections in this Article 5 pertaining to such Milestones and Royalties. |
| | 5.11.1 | BIOMEDICA and Sub-licensees shall keep for three (3) years from the date of each payment of milestones and royalties, complete and accurate records of sales by BIOMEDICA and Sub-licensees of the Compound and the Product in sufficient detail to allow the accruing payments to be determined accurately. |
| | 5.11.2 | MIP shall have the right for a period of three (3) years after receiving any report or statement with respect to royalties or milestones due and payable to appoint an independent certified public accountant reasonably acceptable to BIOMEDICA to inspect the relevant records of BIOMEDICA and its Affiliates and Sub-licensees to verify such report or statement. MIP may exercise this right once with respect to each year’s Net Sales. If the right is not exercised during the three (3) year period described, the report shall be deemed accepted. MIP may exercise this right only once in any year. |
| | 5.11.3 | BIOMEDICA and Sub-licensees shall each make its records available for inspection by such independent certified public accountant during regular business hours at such place or places where such records are customarily kept, upon reasonable notice from MIP, solely to verify the accuracy of the reports and payments. Such inspection right shall not be exercised more than once in any Calendar Year. |
| | 5.11.4 | MIP agrees to hold in strict confidence and use only for the purpose described in this Article 5 all information concerning payments and reports, and all information learned in the course of any audit or inspection (and not to make copies of such reports and information), except to the extent necessary for MIP to reveal such information in order to enforce its rights under this Agreement or if disclosure is required by law, regulation or judicial order. The results of each inspection, if any, shall be binding on both Parties. |
| | 5.11.5 | MIP shall pay for such inspections, except that in the event there is any upward adjustment in aggregate royalties payable for any year shown by such inspection of more than ****** of the amount paid, BIOMEDICA shall pay for such inspection. Any overpayments shall be fully creditable against amounts payable in subsequent payment periods. |
20
*Confidential Treatment Requested*
| | 5.11.6 | Any upward adjustment in aggregate royalties payable for any year shown by such inspection of not more than ****** of the amount paid shall not be deemed a Material Breach according to article 11.4, but shall be repayable by BIOMEDICA in full with interest as set forth in article 5.12 and promptly be paid upon MIP’s request. |
| | 5.11.7 | BIOMEDICA shall include in each sub-license or commercialization agreement entered into by it pursuant to this Agreement a provision requiring the Sub-licensee or commercialization partner to keep and maintain adequate records of sales made pursuant to such sub-license or commercialization agreement and to grant access to such records by the aforementioned independent public accountant for the reasons specified in this article. |
| 5.12 | Tax Withholding. All payments pursuant to this Agreement are exclusive of any applicable Value Added Tax. Withholding tax applied by a government of any country of the Territory on payments made by BIOMEDICA to MIP hereunder shall be borne by MIP. BIOMEDICA, its Affiliates and Sub-licensees, shall cooperate with MIP to enable MIP to claim exemption therefore under any double taxation or similar agreement in force and shall provide to MIP proper evidence of payments of withholding tax and assist MIP by obtaining or providing in as far as reasonably possible the required documentation for the purpose of MIP’s returns. BIOMEDICA undertakes to make all payments due under this Agreement that are subject to a withholding tax treatment, including double taxation or similar agreement, from the USA. |
| 5.13 | Interest Due. In case of any delay in payment (including underpayment) by BIOMEDICA to MIP not occasioned by Force Majeure, interest on the overdue payment shall accrue at an annual interest rate, compounded monthly, equal to the ******, as determined for each month on the last day of that month, assessed from the day payment was initially due. The foregoing interest shall be due from BIOMEDICA without any special notice. |
6. CALL BACK OPTION AND
RIGHT OF FIRST DISCUSSIONS.
| 6.1 | Call Back Option. Subject to the provisions of this article 6, Novartis shall have a one time call back option to re-acquire the rights to the Compound and Product in the event that worldwide Net Sales of the Product first reaches ****** in a calendar year (“Call Back Option”). |
| 6.2 | Information. In order to enable Novartis to determine whether it wishes to exercise the Call Back Option, MIP and/or Novartis shall be entitled to perform a full due diligence of all the information reasonably available to BIOMEDICA, at MIP’s and/or |
21
*Confidential Treatment Requested*
| | Novartis’ expense, such information shall include, but not be limited to all relevant clinical data, manufacturing data, marketing data, and other data reasonably needed by MIP for reviewing its interest in exercising the Call Back Option. BIOMEDICA shall give access to MIP or Novartis to all information necessary to complete the due diligence. |
| 6.3 | Exercise of Call-Back Option. If Novartis exercises the Call-Back Option (“Call Back Exercise”), the following rights and obligations shall apply as between the Parties: |
| | 6.3.1 | MIP shall immediately notify BIOMEDICA in writing that Novartis has initiated the Call-Back Exercise. |
| | 6.3.2 | This Agreement, including the licenses and rights granted by MIP to BIOMEDICA shall terminate and BIOMEDICA shall grant to MIP an exclusive, worldwide license in the BIOMEDICA Field under all BIOMEDICA Know-How and BIOMEDICA’s interest in any Third Party intellectual property if such interest may be conveyed, as necessary for MIP or Novartis to develop, make, use, sell, offer for sale and import the Compound and/or Products, and MIP or Novartis shall conduct (or have conducted) under its control the Commercialization of the Product. BIOMEDICA undertakes to use Commercially Reasonable Efforts to secure sub-license rights under any Third Party intellectual property. |
| | 6.3.3 | In return for the termination of this Agreement and for the grant of the license in article 6.3.2, following MIP’s receipt of payment by Novartis, MIP shall pay to BIOMEDICA a portion of such Novartis payment in an amount equal to BIOMEDICA’s percent of worldwide Net Sales contribution generated, such payment to BIOMEDICA not to exceed a maximum amount of ****** of the total Novartis payment to MIP. MIP acknowledges and agrees that should a portion of the Novartis payment is due to an additional sublicense of MIP such payment will be owed and paid by MIP. |
| | 6.3.4 | In the event that Novartis at its sole discretion, decides to involve BIOMEDICA in the further Development of the Compound after Novartis has exercised the Call Back Option, BIOMEDICA and Novartis may discuss the nature and scope of such BIOMEDICA involvement and agree in writing the nature of any further BIOMEDICA involvement, but there shall be no obligation on MIP to do so. |
| | 6.3.5 | All obligations on BIOMEDICA to pay Milestones as set forth in article 5 which at the time of the Call Back Exercise, are not already due and payable pursuant to article 5, shall lapse. |
22
*Confidential Treatment Requested*
| | 6.3.6 | For the sake of clarity MIP will not pay BIOMEDICA any other payments or royalties. |
| | 6.3.7 | Any Third Party sub-licenses granted by BIOMEDICA will be transferred to Novartis. |
| 6.4 | Non-Exercise of Call Back Option. If Novartis does not exercise the Call Back Option, this Agreement shall continue but the Contingent Milestones shall be thereafter be due as set forth in article 5.4. |
7. INTELLECTUAL PROPERTY
| 7.1 | Inventions and Know-How. |
| | 7.1.1 | MIP Patent Prosecution. MIP agrees it will not abandon, to the extent under MIP control, any Novartis Patent in any country or region of the Territory. |
| 7.2 | Infringement Claims by Third Parties |
| | 7.2.1 | Notice. If the manufacture, use or sale of Product results in a claim or a threatened claim by a Third Party against a Party hereto for patent infringement or for inducing or contributing to patent infringement (“Infringement Claim”), the Party first having notice of an Infringement Claim shall promptly notify the other in writing. The notice shall set forth the facts of the Infringement Claim in reasonable detail. |
| | 7.2.2 | Third Party Licenses. In the event the Parties agree that practicing under the Novartis Patents in connection with manufacture, use or sale of the Compound or the Product in a country would infringe a Third Party Patent and a license to such Third Party Patent is available and BIOMEDICA in its sole discretion determines that it requires such a license, the Parties agree that: |
| | 7.2.2.1 | BIOMEDICA will use commercially reasonable efforts to obtain any such required licenses under the Third Party’s Patents; and |
| | 7.2.2.2 | In the event that BIOMEDICA obtain any such required licenses from Third Party under which BIOMEDICA shall pay the Third Party certain royalties, BIOMEDICA may deduct such Third Party payment from its royalty payments to MIP under article 5 but in no event will such deductions reduce the Royalty payments in any quarter to MIP by greater than ******. |
23
*Confidential Treatment Requested*
| | 7.2.3 | Litigation. If a Third Party asserts that a patent, trademark or other intellectual properties owned by it is infringed by the importation, manufacture, offer for sale, use or sale of Compound or Product, or if either Party learns of a claim or assertion that the manufacture, use, marketing, promotion, importation, offer for sale, distribution or sale of Compound or Product infringes or otherwise violates the intellectual property rights of any Third Party, then such Party will promptly notify the other Party in writing. MIP and/or Novartis will have the first right, but not the obligation, to control such defense at their own expense. If MIP/Novartis does not assume control of such defense within 120 days of the notice described above, then BIOMEDICA shall have the right to control such defense at its own expense. In any event, the Party not controlling such defense will have the right to be represented in any such action by counsel of its choosing at its own expense; this includes Novartis. The Party controlling such defense shall keep the other Party advised of the status of such action and shall consider recommendations made by the other Party in respect thereto. The Party not controlling such defense will assist and cooperate in any such infringement litigation at the defending Party’s reasonable request and expense. The costs and expenses (including attorneys’ fees) of any suit brought in accordance with this Article shall be borne by the Party controlling the prosecution of such suit. Any costs borne by BIOMEDICA in accordance with this article shall however be entirely off-set against any payments due to MIP under Article 5. |
| 7.3 | Infringement Claims Against Third Parties |
| | 7.3.1 | Notice. If any Novartis Patents are infringed by a Third Party, the Party to this Agreement first having knowledge of such infringement, or knowledge of a reasonable probability of such infringement, shall promptly notify the other in writing. The notice shall set forth the facts of such infringement in reasonable detail. |
| | 7.3.2 | Institution of Proceedings. MIP and/or Novartis shall have the primary right, but not the obligation, to institute, prosecute, and control with its own counsel at its own expense any action or proceeding with respect to infringement of the claims of such Novartis Patents. In the event MIP/Novartis does not take any action with respect to any such infringement within 120 days after receiving notice of such infringement thereof, BIOMEDICA may request in writing permission to undertake such defense thereof, at its own expense. The Party undertaking the defense shall have the sole charge and direction of the defense of any such suit or action and the other Party including Novartis shall have the right, |
24
| | but not the obligation and at its own expense, to be represented in such action by its own counsel action. Each Party agrees to cooperate fully in the prosecution of any such suit or action undertaken hereunder by the other Party and to provide all evidence in its reasonable control. |
| | 7.3.3 | Division of Damages Award. Each Party shall recover their respective actual out-of-pocket expenses, or equitable proportions thereof, associated with any litigation or settlement thereof from any recovery made by any Party. Any excess amount allocated as a damage award shall be shared between the Parties as follows: MIP shall be allocated an amount equal to its royalty loss and BIOMEDICA shall be allocated the remainder of damages; any amount allocated as a penalty shall be shared equally between the Parties. |
| | 7.3.4 | Settlement. The Parties shall keep each other informed of the status of and of their respective activities regarding any litigation or settlement thereof concerning Product; provided, however, that no settlement or consent judgment or other voluntary final disposition of a suit under this article 7 may be undertaken without the consent of the other Party if such settlement would require the other Party to be subject to an injunction or to make a monetary payment or would otherwise adversely affect the other Party’s rights under this Agreement. |
| 7.4 | Notice of Certification. MIP and BIOMEDICA each shall immediately give notice to the other of any certification filed under the “U.S. Drug Price Competition and Patent Term Restoration Act of 1984” (or its foreign equivalent) claiming that a Novartis Patent is invalid or that infringement will not arise from the manufacture, use or sale of any Product by a Third Party. |
| | 7.4.1 | If MIP decides not to bring infringement proceedings against the entity making such a certification, MIP shall give notice to BIOMEDICA of its decision not to bring suit within twenty-one (21) days after receipt of notice of such certification. |
| | 7.4.2 | BIOMEDICA may then, but is not required to, bring suit against the Party that filed the certification at its own expense. |
| | 7.4.3 | Any suit by BIOMEDICA or MIP shall either be in the name of BIOMEDICA or in the name of MIP, or jointly in the name of BIOMEDICA and MIP, and further including Novartis as may be required by law. |
25
| | 7.4.4 | For this purpose, the Party not bringing suit shall execute such legal papers necessary for the prosecution of such suit as may be reasonably requested by the Party bringing suit. |
| 7.5 | Patent Term Extensions. The Parties shall cooperate in good faith with each other in gaining patent term extension wherever applicable to the Compound or Product. All filings for such extension shall be made by MIP, provided, however, that in the event that if MIP elects not to file for an extension, it shall (i) inform BIOMEDICA of its intention not to file and (ii) BIOMEDICA shall have the right to file for such extension. |
| 7.6 | BIOMEDICA New Intellectual Property. |
| | 7.6.1 | Ownership of BIOMEDICA New Intellectual Property. During the Term of the Agreement, New Intellectual Property shall be owned by BIOMEDICA. BIOMEDICA shall inform MIP of new patent applications and action on them in the report provided by BIOMEDICA to MIP pursuant to section 4.2. |
| | 7.6.2 | BIOMEDICA shall have the sole right to file, prosecute, and maintain all of the patents within the BIOMEDICA New Intellectual Property included herein, and shall have the right to determine whether or not, and where, to file a patent application, to abandon the prosecution of any patent or patent application, or to discontinue the maintenance of any patent application. |
| | 7.6.3 | Should MIP terminate this Agreement due to a breach by BIOMEDICA of its obligation hereunder, BIOMEDICA shall grant to MIP a perpetual, world-wide, royalty-bearing, exclusive license to such BIOMEDICA New Intellectual Property, with the right to grant sublicenses, to use any BIOMEDICA New Intellectual Property invented or generated by persons obliged to assign their rights to BIOMEDICA in the BIOMEDICA Field. The royalty rate on the MIPs’ Net Sales of the product shall be determined by the Parties upon termination of the Agreement, shall be based on reasonable commercial terms and in no event shall exceed ****** of the royalty rates payable by BIOMEDICA under section 5.5 of this Agreement. |
| | 7.6.4 | BIOMEDICA New Intellectual Product that relates to the Product or the Compound may be subject to rights by Novartis, during the Term of this Agreement. |
| | 7.7.1 | Generalities. MIP is the owner of all right and title in and to the ONALTA Trademark throughout the Territory including but not limited to all applications therefore and registrations thereof and all goodwill associated therewith. |
26
*Confidential Treatment Requested*
| | 7.7.2 | Use of the Trademarks. BIOMEDICA and/or its Sub-licensee shall promote, market, sell and distribute the Product in the Territory under the Trademark during the Term. |
| | 7.7.3 | Quality Control Standards; Compliance with Laws: Upon MIP prior written request, BIOMEDICA shall furnish to MIP a sample of products and materials bearing the TRADEMARK. If MIP reasonably and in good faith believes the samples bearing the TRADEMARK do not meet a Minimum Quality Threshold (as defined below), MIP shall notify BIOMEDICA in writing, and MIP shall have a reasonable period of time (but in no event more than 30 days from the date of receipt of notice) to make the changes and/or corrections that the parties mutually agree are necessary to protect the TRADEMARK. For purposes of this Agreement, “Minimum Quality Threshold” shall mean, with respect to each product or advertising material bearing the TRADEMARK, the level of quality necessary to comply: (a) in all material respects, with the respective specifications and technical requirements of MIP’s customers applicable to such product; and (b) in all respects, with all statutory and regulatory standards applicable to such product or product advertising. |
| | 7.7.4 | BIOMEDICA agrees to cooperate with MIP in facilitating MIP’s control of such nature and quality, to permit reasonable inspection of BIOMEDICA operations, and to supply MIP with specimens of all uses of the ONALTA mark upon request. BIOMEDICA shall comply with all applicable laws and regulations and obtain all appropriate government approvals pertaining to the sale, distribution and advertising of goods and services covered by this license. |
| | 7.7.5 | BIOMEDICA agrees to use the ONALTA mark only in the form and manner and with appropriate legends as prescribed from time to time by MIP, and not to use any other trademark or service mark in combination with the ONALTA mark without prior written approval of MIP. |
| | 7.7.6 | Domain Name and Website Agreement provision. The Parties shall enter into a separate Domain name and website agreement within 120 days of the Effective Date I connection with the Onalta domain name. |
| | 7.7.7 | BIOMEDICA agrees to notify MIP of any unauthorized use of the ONALTA mark by others promptly as it comes to BIOMEDICA’s attention. MIP shall have the sole right and discretion to bring infringement or other proceedings involving the ONALTA mark. BIOMEDICA agrees to cooperate in any investigation or suit in the Territory relating to the ONALTA mark at the expense of MIP. |
27
8. REPRESENTATIONS AND WARRANTIES
| 8.1 | MIP Representations and Warranties |
| | 8.1.1 | This Agreement has been duly executed and delivered by MIP and constitutes the valid and binding obligation of MIP, enforceable against MIP in accordance with its terms except as enforceability may be limited by bankruptcy, fraudulent conveyance, insolvency, reorganization, moratorium and other laws relating to or affecting creditors’ rights generally and by general equitable principles. The execution, delivery and performance of this Agreement have been duly authorized by all necessary action on the part of MIP, its officers and directors. |
| | 8.1.2 | As of the Effective Date MIP owns or Controls or otherwise possesses adequate licenses or other rights necessary to make, use and sell the Compound and the Product covered by the Novartis Patents and to grant the sublicenses provided herein; and the granting of the license to BIOMEDICA hereunder does not violate any right known to MIP of a Third Party. |
| | 8.1.3 | The execution, delivery and performance of this Agreement by MIP does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, and to the best of its knowledge, does not violate any material law or regulation of any court, governmental body or administrative or other agency having authority over it. |
| | 8.1.4 | MIP has disclosed to BIOMEDICA any and all material information in its Control pertaining to the suitability of Compound as a pharmaceutical candidate. |
| | 8.1.5 | MIP is not currently a Party to, and during the term of this Agreement will not enter into, any agreements, oral or written, that are inconsistent with its obligations under this Agreement. MIP has disclosed to BIOMEDICA that ****** is currently unavailable. Should ****** become available, BIOMEDICA shall have the right, at its option, to expand its Territory to include ******. No upfront payments will be required for BIOMEDICA to exercise this option. |
28
*Confidential Treatment Requested*
| | 8.1.6 | MIP is duly organized and validly existing under the laws of the country of the United States and has full legal power and authority to enter into this Agreement. |
| | 8.1.7 | MIP is not subject to any order, decree or injunction by a court of competent jurisdiction which prevents or materially delays the consummation of the transactions contemplated by this Agreement. |
| 8.2 | BIOMEDICA Representations and Warranties |
| | 8.2.1 | This Agreement has been duly executed and delivered by BIOMEDICA and constitutes the valid and binding obligation of BIOMEDICA, enforceable against BIOMEDICA in accordance with its terms except as enforceability may be limited by bankruptcy, fraudulent conveyance, insolvency, reorganization, moratorium and other laws relating to or affecting creditors’ rights generally and by general equitable principles. The execution, delivery and performance of this Agreement have been duly authorized by all necessary action on the part of BIOMEDICA, its officers and directors; |
| | 8.2.2 | BIOMEDICA is not currently a Party to, and during the term of this Agreement will not enter into, any agreements, oral or written, that are inconsistent with its obligations under this Agreement; |
| | 8.2.3 | BIOMEDICA is duly organized and validly existing under the laws of Greece and has full legal power and authority to enter into this Agreement; and |
| | 8.2.4 | BIOMEDICA is not subject to any order, decree or injunction by a court of competent jurisdiction which prevents or materially delays the consummation of the transactions contemplated by this Agreement. |
| | 8.2.5 | BIOMEDICA has sufficient credit commitments and/or capital commitments, such that it will be able to develop and commercialize Products within all countries in the Territory, consistent with the scope of and pursuant to the license granted herein. |
| | 8.2.6 | BIOMEDICA has obtained any approval or consent needed from BIOMEDICA’s shareholders or its Board of Directors, to execute this Agreement and perform the activities specified herein. |
| | 8.2.7 | BIOMEDICA acknowledges and agrees that MIP is the owner of the ONALTA mark and further agrees that it will do nothing inconsistent with such ownership and that all use of the ONALTA mark by BIOMEDICA shall inure to the benefit of and be on behalf of MIP. BIOMEDICA will not apply to register the ONALTA mark in its own name but shall assist MIP in filing any applications to register the ONALTA mark in the name of MIP or its designated agents, to |
29
| | the extent the same have not already been filed, and in recording this Agreement with appropriate government authorities. BIOMEDICA agrees that nothing in this Trademark License or any related document shall give BIOMEDICA any right, title or interest in the ONALTA mark other than the right to use the ONALTA mark in the Territory and consistent with this Trademark License and BIOMEDICA agrees that it will not attack the title of MIP in the ONALTA mark or the validity of this Trademark License. |
| 8.3 | THE LIMITED WARRANTIES CONTAINED IN THIS ARTICLE ARE THE SOLE WARRANTIES GIVEN BY THE PARTIES AND ARE MADE EXPRESSLY IN LIEU OF AND EXCLUDE ANY IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, TITLE, INFRINGEMENT OR OTHERWISE, AND ALL OTHER EXPRESS OR IMPLIED REPRESENTATIONS AND WARRANTIES PROVIDED BY COMMON LAW, STATUTE OR OTHERWISE ARE HEREBY DISCLAIMED BY BOTH PARTIES. |
9. CONFIDENTIALITY
| 9.1 | Confidentiality. Subject to the exercise of the licenses granted in Article 2, during the Term of this Agreement, and for a period of five (5) years thereafter, each Party hereto will maintain in confidence all information generated under this Agreement as well as any information disclosed by the other Party hereto (“Confidential Information”). Neither Party shall use, disclose or grant use of such Confidential Information except as required under this Agreement. Each Party shall use the same standard of care as it uses to protect its own Confidential Information to ensure that its and its Affiliates’ employees, agents, consultants, Sub-licensee(s) and clinical investigators only make use of Confidential Information for the purpose of this Agreement and do not disclose or make any unauthorized use of such Confidential Information. Each Party shall promptly notify the other upon discovery of any unauthorized use or disclosure of Confidential Information. |
| | 9.1.1 | Confidential Information shall not include any information which and to the extent: was already known to the receiving Party, other than under an obligation of confidentiality, at the time of disclosure by the other Party; was generally available to the public or otherwise part of the public domain at the time of its disclosure to the other Party; becomes generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement; was disclosed to the receiving Party, by a Third Party who had no obligation to the other Party not to disclose such information; or was independently developed by the receiving Party without reference to the disclosure by the other Party. |
30
| 9.2 | The Parties agree that the material financial terms of the Agreement and the reports described in article 4.2 shall be considered the Confidential Information of both Parties. |
| 9.3 | Each Party may disclose the Confidential Information to the extent such disclosure is reasonably necessary in filing or prosecuting patent applications, prosecuting or defending litigation, or complying with any applicable statute or governmental regulation provided such Party has given the disclosing Party prompt written notice allowing it to limit such disclosure. In addition, either Party may disclose Confidential Information to its Affiliates and to its Sub-licensees; provided, however, in connection with any such disclosure the disclosing Party shall secure confidential treatment of such Confidential Information. |
| 9.4 | The Parties shall undertake to ensure that all their employees who have access to Confidential Information of the other Party are under obligations of confidentiality fully consistent with those provided in this article. |
| 9.5 | BIOMEDICA agrees that the Confidential Information it receives, including but not limited to the financial information, may be disclosed by MIP to Novartis without further notice, provided that Novartis shall be subject to the same confidential obligations as MIP, as set forth herein. |
10. ANNOUNCEMENT, PUBLICITY AND PUBLICATIONS
| 10.1 | Except with the prior written consent of the other Party not to be unreasonably withheld, neither Party hereto shall make any disclosure to any third Party except Affiliates concerning the terms of this Agreement. Each Party will inform the other prior to the disclosure of this Agreement or any of its terms to any government authorities or agencies and will observe all reasonable requirements of the other in regard to any such disclosure. In addition, MIP may make a public statement, including in analyst meetings, concerning this Agreement or the progress of the Product, at MIP’s discretion including but not limited to the events described in Exhibit D. The restrictions on disclosure specified herein shall not apply to announcements required by law or regulations or stock exchange rules, including announcements required by law, regulations or stock exchange rules to be made by either Party to their respective shareholders. It is, however, the Parties’ intent that they will co-ordinate to such extent as may be reasonably possible with respect to the wording of any such announcements and that the financial terms of this Agreement shall not be made public. |
| 10.2 | BIOMEDICA will, and will cause its Sub-licensees to obtain MIP’s prior written permission to use MIP’s name, symbols and any other marks in any form of publicity. |
31
| 10.3 | None of BIOMEDICA, its Affiliates, or any of its or its Affiliates’ or present any information, including, without limitation, the results of any work performed under the Agreement, or preclinical or clinical studies with respect to the Compound or Product, without MIP’s prior written consent. Neither party shall have the right to publish or present Confidential Information of the other party. |
11. TERM AND TERMINATION
| 11.1 | Term. Unless earlier terminated as provided under article 6, 11.2 or 11.3 the term of this Agreement shall commence as of the Effective Date and shall remain in full force and effect on a country-by-country basis until the end of the last to expire payment obligations of BIOMEDICA under article 5.6 (“Term”). |
| 11.2 | Accrued Obligations. Except where explicitly provided elsewhere herein or in any applicable laws, termination of this Agreement for any reason, or expiration of this Agreement, will not affect: (i) obligations, including the payment of any royalties or other sums which have accrued as of the date of termination or expiration, and (ii) rights and obligations which, from the context thereof, are intended to survive termination or expiration of this Agreement. |
| 11.3 | Termination For Insolvency. Either Party may terminate this Agreement immediately upon delivery of written notice to the other Party (a) upon the institution by or against the other Party of insolvency, receivership or bankruptcy proceedings or any other proceedings for the settlement of the other Party’s debts; provided, however with respect to involuntary proceedings, that such proceedings are not dismissed within one hundred and twenty (120) days; (b) upon the other Party’s making an assignment for the benefit of creditors; (c) upon the other Party’s dissolution or ceasing to do business. |
| 11.4 | Material Breach. If either Party is in breach of any material obligation hereunder and, in the case of a breach capable of remedy, it shall not have been remedied by the defaulting Party within sixty (60) days of written notice specifying the breach and requiring its remedy, the Party not in breach of the material obligation may forthwith terminate this Agreement by notice without prejudice to the accrued rights of either Party. |
| 11.5 | Change of Control.MIP may be forced to terminate this Agreement in the event that Novartis exercises its right in the MIP_NOVARTIS License Agreement to terminate caused by change of control of MIP by a Direct Competitor of Novartis. MIP shall upon signing of this Agreement use its best efforts to seek to obtain a commitment from NOVARTIS that this Sub-license Agreement and any similar sub-license agreements related to the Compound and the Product shall survive any termination of the MIP-NOVARTIS Agreement under this section so as to reasonably protect the investment and expectations of all sub-licensees. |
32
| 11.6 | Effect Of Termination. |
| | 11.6.1 | Effect On License. Upon the expiration or earlier termination of this Agreement, the rights licensed under this Agreement shall be treated as follows: |
| | 11.6.1.1 | Upon expiration of the Term, BIOMEDICA shall have a fully paid-up, perpetual, irrevocable, royalty-free, transferable, worldwide, non-exclusive right and license under the Novartis Patents and Know-How existing as of the date of such expiration to make, use, offer to sell, import and sell Product in the Territory. |
| | 11.6.1.2 | Upon early termination pursuant to article 6, 11.2, 11.3 or 11.4, all rights to the Compound and Product shall revert to MIP, including but not limited to the licenses granted under article 2.1 and 2.2. |
| | 11.6.2 | Effect On Trademark. |
| | 11.6.2.1 | Upon expiration of the Term, BIOMEDICA and/or its Sub-licensee may continue to use the Trademark in connection with the sale of the Product. |
| | 11.6.2.2 | Upon early termination of this Agreement by MIP pursuant to article 11.2, 11.3 or 11.4, all the rights to use the Trademark(s) shall revert to MIP. BIOMEDICA agrees to immediately discontinue all use of the ONALTA mark and any term confusingly similar thereto, and to delete the same from its corporate or business name, to cooperate with MIP or its appointed agent to apply to the appropriate authorities to cancel recording of any trademark license from all government records, to destroy all printed materials bearing the ONALTA mark or return the same to MIP at MIP’s sole discretion, and that all rights in the ONALTA mark and the good will connected therewith shall remain the property of MIP. |
| | 11.6.3 | Ongoing Obligations. |
| | 11.6.3.1 | Upon expiration or termination of this Agreement for any reason, each Party shall immediately return to the other Party or destroy any Confidential Information disclosed by the other Party, except for one copy which may be retained to determine its continuing obligations pursuant to this Agreement. |
33
| | 11.6.3.2 | Upon early termination by MIP pursuant to article 11.2, 11.3 or 11.4 above, BIOMEDICA shall have no further right to use or refer to the Know-How, and BIOMEDICA shall transfer to MIP all documentation embodying or referring to the Know-How. |
| | 11.6.3.3 | Upon early termination by MIP pursuant to articles 11.2, 11.3 or 11.4 above and at MIP’ election, BIOMEDICA will immediately transfer ownership of all Marketing Authorizations and shall give an access right to all clinical development, regulatory and manufacturing data, as well as to all other relevant information to MIP, which shall be free to use it. |
| | 11.6.3.4 | Upon early termination by MIP pursuant to article 11.2, 11.3 or 11.4 above, BIOMEDICA shall transfer to MIP upon MIP’ request all its stock of Compound and Product at cost. |
| | 11.6.3.5 | Upon early termination of this Agreement by MIP pursuant to article 11.2, 11.3 or 11.4 above and at MIP’s election, BIOMEDICA shall grant and hereby grants to MIP, the right to use any BIOMEDICA pre-clinical and clinical trial results, regulatory files and filings related to Product and Compound. In addition, BIOMEDICA shall provide at MIP’ request, the reasonable assistance of appropriate BIOMEDICA personnel in connection with the transfer therewith. |
| | 11.6.3.6 | Inventory. Notwithstanding the foregoing, upon early termination of this Agreement pursuant to articles 11.2 or 11.3 but not under article 6, BIOMEDICA shall have the right to sell all remaining Product in its inventory within six (6) months after the date of termination, subject to the payment to MIP of the amounts specified in article 5. Thereafter, BIOMEDICA agrees to destroy any remaining supply of Product at MIP’s request. |
12. INDEMNIFICATION AND INSURANCE
| 12.1 | BIOMEDICA will indemnify, defend and hold MIP and Novartis harmless from and against any and all costs, losses, claims, liabilities, fines, penalties, damages, expenses, court costs, interest, and reasonable fees and disbursements of counsel, |
34
| | consultants, and expert witnesses (“Damages”) incurred or suffered by MIP arising out of or resulting from: (i) BIOMEDICA’s breach of a material term if this Agreement; (ii) BIOMEDICA’s breach of any of its representations or warranties hereunder; or (iii) the development, handling, use, marketing, sale or other disposition, of Compound and/or Product by any of BIOMEDICA, its Affiliates, Sub-licensees, and their contractors in the BIOMEDICA Field except to the extent that such Damages are due to MIP’s or MIP’s directors, officers, employees, or Affiliates negligence or willful misconduct. |
| 12.2 | MIP will indemnify, defend and hold BIOMEDICA harmless from and against any and all Damages incurred or suffered by BIOMEDICA arising out of or resulting from: (i) MIP’ breach of a material term if this Agreement; (ii) MIP’s breach of any of its representations or warranties hereunder or failure to perform duly and punctually any of its covenants, agreements, or undertakings contained in this Agreement; except to the extent that such Damages are due to BIOMEDICA’s or BIOMEDICA’s directors, officers, employees, or Affiliates negligence or willful misconduct. |
| 12.3 | The Parties agree as follows: |
| | 12.3.1 | Each Party shall give the other Party prompt written notice of any claim or threat of claim it receives with respect to any matter for which it may be entitled to indemnification, and the indemnifier shall thereafter defend or settle any such claim at the indemnifier’s sole expense, with counsel selected by the indemnifier. In the defense or settlement of any such claim, the indemnified Party shall co-operate with and assist the indemnifier to the extent reasonably possible, but the indemnifier shall bear and pay any and all expenses incurred by the indemnified Party in providing such co-operation and assistance, either directly or upon request of the indemnified Party who has incurred such expense. Failure to give notice shall not constitute a defense, in whole or in part, to any claim by any indemnified person hereunder except to the extent the rights of the indemnifier are materially prejudiced by such failure to give notice. |
| | 12.3.2 | Notwithstanding the foregoing, upon any claim being made by a person not a Party to this Agreement (and not an Affiliate of a Party) with respect to any matter to which the foregoing indemnities relate, the indemnified Party may make settlement of such claim on not less than 30 days prior written notice of the proposed terms thereof to the indemnifier; provided, however, that if within said 30-day period the indemnifier shall have requested the indemnified Party not to settle such claim and to deny such claim, the indemnified Party will promptly comply and the indemnifier shall have the right to defend the claim at the indemnifier’s sole expense and with counsel reasonably acceptable to the indemnified Party. In the event that the indemnifier has not responded to such notice within such 30-day period, such absence of response shall be deemed a written consent to the proposed settlement. |
35
| | 12.3.3 | Notwithstanding that the indemnifier has assumed the defense of any claim with counsel selected by the indemnifier, the indemnified Party shall have the right to employ its own counsel, at its sole expense. If, in good faith, an indemnified Party concludes that there are specific defenses available to the indemnified Party which are different from or in addition to those available to the indemnifier with respect to the scope of the foregoing indemnities, then such indemnified Party shall have the right to direct the defense of any such defense of any such claim and each Party shall pay all its own costs, fees and damages. |
| | 12.3.4 | Neither Party will conduct itself in a way that could prejudice the defense of any such claims or threats. |
| 12.4 | References in this Article 12 to a Party that may be entitled to indemnification shall also include its Affiliates and its and their officers, directors, employees and agents. |
| 12.5 | The Parties agree to maintain insurance, including but not limited to product liability insurance and Clinical Trial insurance in the case of BIOMEDICA, with respect to their activities hereunder. Such insurance shall be in such amounts and subject to such deductibles based upon standards prevailing in the industry at the time. The Parties may satisfy its obligations under this article through self-insurance to the same extent. |
| 12.6 | Limitation of Damages.Neither Party nor its affiliates shall have any liability for any special, incidental, or consequential damages, including, but not limited to the loss of opportunity, revenue or profit, in connection with or arising out of this Agreement, even if it shall have been advised of the possibility of such damages. |
| | 12.6.1 | In the event of a material breach by MIP of this Agreement then BIOMEDICA’s royalty rate under this Agreement to MIP is reduced by ******. |
13. INFORMATION ON CLINICAL SAFETY
AND PHARMACOVIGILANCE
| 13. | BIOMEDICA shall be fully responsible for ensuring regulatory compliance with all pharmacovigilance obligations, including the holding and maintaining of a safety database for the Compound and the Product including post marketing surveillance. Within thirty days (30) of the Effective date, MIP will provide a copy of any existing global safety database relating to the |
36
*Confidential Treatment Requested*
| | Product to BIOMEDICA. BIOMEDICA will be responsible to communicate and forward all drug safety information including those events which require expedited reporting under FDA and EMEA regulations, relating to both the Compound and the Product to MIP and to the relevant regulatory authorities as dictated by their safety report regulations. BIOMEDICA will provide on an annual basis commencing one year from the Effective Date a summary report of the clinical and non-clinical safety experience, format to be provided by MIP. Conversely, MIP will provide reciprocate communication of all ongoing and future drug safety data. |
14. GOVERNING LAW AND JURISDICTION
| 14.1 | The construction, validity and performance of this Agreement will be governed in all respects by the laws of Switzerland. All disputes arising out of or affecting this Agreement which cannot be resolved amicably shall be submitted to the exclusive jurisdiction of the courts of the Commonwealth of Massachusetts, U.S.A. if the defendant is MIP, or to the exclusive jurisdiction of the courts of Athens, Greece, should BIOMEDICA be the defendant. If Novartis is joined or is otherwise named as a defendant in any suit under this Agreement, then the Parties agree to waive their rights to defend such suit in the above venues, and instead submit to exclusive jurisdiction in the courts of Basel-Stadt, Switzerland and further agree that Swiss Law shall control interpretation of this Agreement in such proceeding. |
15. MISCELLANEOUS PROVISIONS
| 15.1 | Waiver. The failure on the part of BIOMEDICA or MIP to exercise or enforce any rights conferred upon it hereunder shall not be deemed to be a waiver of any such rights nor operate to bar the exercise or enforcement thereof at any time or times thereafter. The observance of any term of this Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively) by the Party entitled to enforce such term, but any such waiver shall be effective only if in writing signed by the non-waiving Party. |
| 15.2 | Force Majeure. Neither Party shall be held liable or responsible to the other Party nor be deemed to have defaulted under or breached this Agreement for failure or delay in fulfilling or performing any term of this Agreement, other than an obligation to make a payment, when such failure or delay is caused by or results from fire, floods, embargoes, government regulations, prohibitions or interventions, war, acts of war (whether war be declared or not), insurrections, riots, civil commotions, strikes, |
37
| | lockouts, or any other cause beyond the reasonable control of the affected Party. If a force majeure circumstance persists for more than 12 months, the Parties shall discuss in good faith about termination of this Agreement. |
| 15.3 | Severability. It is the intention of the Parties to comply with all applicable laws domestic or foreign in connection with the performance of its obligations hereunder. In the event that any provision of this Agreement, or any part hereof, is found invalid or unenforceable, the remainder of this Agreement will be binding on the Parties hereto, and will be construed as if the invalid or unenforceable provision or part thereof had been deleted, and the Agreement shall be deemed modified to the extent necessary to render the surviving provisions enforceable to the fullest extent permitted by law. |
| 15.4 | Government Acts. In the event that any act, regulation, directive, or law of a government, including its departments, agencies or courts, should make impossible or prohibit, restrain, modify or limit any material act or obligation of BIOMEDICA or MIP under this Agreement, the Party, if any, not so affected shall have the right, at its option, to suspend or terminate this Agreement as to such country, if good faith negotiations between the Parties to make such modifications to this Agreement as may be necessary to fairly address the impact thereof, after a reasonable period of time are not successful in producing mutually acceptable modifications to this Agreement. |
| 15.5 | Assignment. This Agreement and any NDA or Marketing Authorization described herein may not be assigned or otherwise transferred by either Party without the prior written consent of the other Party; provided, however, that either Party may assign this Agreement, without the consent of the other Party, (i) to any of its Affiliates, if the assigning Party guarantees the full performance of its Affiliates’ obligations hereunder or (ii) in connection with the transfer or sale of all or substantially all of its assets or business or in the event of its merger or consolidation with another company. In all cases the assigning Party shall provide the other Party with prompt notice of any such assignment. Any purported assignment in contravention of this article shall, at the option of the non assigning Party, be null and void and of no effect. No assignment shall release either Party from responsibility for the performance of any accrued obligation of such Party hereunder. |
| 15.6 | Counterparts. This Agreement may be executed in two copies, both of which shall be deemed to be originals, and all of which shall constitute one and the same Agreement. |
| 15.7 | No Agency. Nothing herein contained shall be deemed to create an agency, joint venture, amalgamation, partnership or similar relationship between MIP and BIOMEDICA. Notwithstanding any of the provisions of this Agreement, neither Party shall at any time enter into, incur, or hold itself out to third Parties as having authority to enter into or incur, on behalf of the other Party, any commitment, expense, or liability whatsoever, and all contracts, expenses and liabilities undertaken or incurred by |
38
| | one Party in connection with or relating to the development, manufacture or sale Compound or Product shall be undertaken, incurred or paid exclusively by that Party, and not as an agent or representative of the other Party. |
| 15.8 | Notice. All communications between the Parties with respect to any of the provisions of this Agreement will be sent to the addresses set out below, or to other addresses as designated by one Party to the other by notice pursuant hereto, by internationally recognized courier or by prepaid certified, air mail (which shall be deemed received by the other Party on the seventh Day following deposit in the mails), or by facsimile transmission or other electronic means of communication (which shall be deemed received when transmitted), with confirmation by letter given by the close of business on or before the next following day. |
Molecular Insight Pharmaceuticals, Inc.
160 Second Street
Cambridge, MA 02142 USA
Attn: Paul Granger, General Counsel
| | 15.8.2 | If to BIOMEDICA at: |
BioMedica Life Sciences, Ltd.
4 Papanikoli Str.,
15232 Halandri, Athens, Greece
Attn: Ioannis Vitsaras, CEO
| 15.9 | Survival. Except where explicitly provided elsewhere herein, termination of this Agreement for any reason, or expiration of this Agreement, will not affect: (i) obligations, including the payment of any sums which have accrued as of the date of termination or expiration, and (ii) rights and obligations which, from the context thereof, are intended to survive termination or expiration of this Agreement. |
| 15.10 | Headings. The paragraph headings are for convenience only and will not be deemed to affect in any way the language of the provisions to which they refer. |
| 15.11 | Costs. Each Party shall be responsible for its own costs and expenses incurred in connection with negotiation and execution of this Agreement. Unless otherwise specifically provided for herein, each Party shall bear all costs and expenses it incurs in its’ performance of this Agreement. |
39
| 15.12 | Entire Agreement. This Agreement shall supersede any prior agreement between the Parties and constitutes the entire understanding of the Parties relating to the matters referred to herein, and may only be amended by a written document, duly executed on behalf of the respective Parties. However the terms of the Novartis License Agreement shall prevail over any conflicting terms contained in this territory license Agreement or the LOI. |
—Signature Page to Follow—
40
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed by their duly authorized representatives as of the day and year first above written.
| | | | | | | | |
| BioMedica Life Sciences S.A. | | | | Molecular Insight Pharmaceuticals Inc. |
| | | | |
| Name: | | /s/ Yannis Vitsaras | | | | Name: | | /s/ Daniel Peters |
| | | | |
| Title: | | President & CEO | | | | Title: | | President & CEO |
41
EXHIBIT A
BIOMEDICA BUSINESS PLAN
CONFIDENTIAL-UNAUTHORIZED
DUPLICATION PROHIBITED

Onalta™ Europe Business Plan
Prepared For

Biomedica Life Sciences S.A.
4, Papanikoli Str
15232, Halandri
Athens, Greece
Phone: +30.210.689.9801
Fax: +30.210.689.9809
Contact:
Yannis Vitsaras, CEO
Phone: +30.210.689.9807
Vitsaras@biomedica.gr
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 1 of 31 | | CONFIDENTIAL |

Table of Contents
| | |
Overview of BioMedica Life Sciences S.A. | | 4 |
Company Profile | | 4 |
Key Products | | 4 |
Radiopharmaceutical Products | | 4 |
Cold Kits | | 5 |
Radioimmunoassay (RIA) Products | | 5 |
Precursors for Medical Imaging | | 5 |
Crystal Probe 2000 | | 5 |
Current R&D Programs | | 5 |
Corporate Structure | | 6 |
BioGenomica S.A. | | 6 |
BioRP | | 6 |
Demokritos National Scientific Research Center | | 6 |
Management Team | | 7 |
Key Financials | | 8 |
Projected 2009 Sales & EBIT (without Onalta™) | | 8 |
Current Debt Structure | | 8 |
Cash Position | | 8 |
Cash Flow | | 8 |
Historical Financials | | 9 |
Projected Cash Flows (pre-Onalta™) | | 10 |
BioMedica’s Five Year Business Plan | | 11 |
Vision Statement | | 11 |
Tactics to be Employed by BioMedica | | 11 |
Role of Onalta™ in Five Year Business Plan | | 12 |
Role of Demogastrin™ and Other Products in BioMedica’s Five Year Business Plan | | 13 |
Demogastrin™ | | 13 |
BioGenomica | | 13 |
Onalta™ Revenue Projections | | 14 |
Source of Funds Required for Development and Commercialization of Onalta™ | | 17 |
Primary Immediate Source of Funds | | 18 |
Secondary Near-term Source of Funds | | 18 |
Tertiary, Longer-term Source of Funds | | 19 |
Onalta™ Development and Commercialization Plan | | 20 |
Onalta™ Development Plan | | 20 |
Timeline & Budget | | 20 |
Phase 3 Clinical Study | | 21 |
Proposed Phase 3 Study Sites | | 21 |
Commencement of Development Activities | | 22 |
Organizational Structure & Staffing | | 22 |
MIP involvement in the European Phase 3 Clinical Development of Onalta™ | | 23 |
Named Patient Program | | 23 |
Overview and Responsibilities | | 23 |
Pricing | | 24 |
Timing | | 25 |
MIP Involvement | | 25 |
Regulatory Affairs | | 25 |
Manufacturing and Distribution | | 26 |
Sales and Marketing | | 27 |
Reimbursement Strategy | | 30 |
Consolidated Cash Flow | | 31 |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 2 of 31 | | CONFIDENTIAL |

Table of Figures:
| | |
Figure 1. BioMedica Historical Financials | | 9 |
| |
Figure 2. Cash & Cash Equivalents at end of Period | | 10 |
| |
Figure 3. Onalta™ Revenue Projections | | 16 |
| |
Figure 4. Forecasted Cash Requirements | | 17 |
| |
Figure 5. Cash Requirements vs. Secured Financing | | 19 |
| |
Figure 6. Onalta™ Clinical Development Timeline and Costs | | 20 |
| |
Figure 7. Proposed Phase 3 Clinical Trial Centers | | 21 |
| |
Figure 8. Named Patient Program Pricing | | 24 |
| |
Figure 9. Named Patient Program Event Timeline | | 25 |
| |
Figure 10. Manufacturing & Distribution Timeline | | 27 |
| |
Figure 11. Designated Hub Countries in Europe and Middle East | | 28 |
| |
Figure 12. Type-2 Hub Countries and their Respective Spoke Countries | | 28 |
| |
Figure 13. Type-1 Hub Countries and their Respective Spoke Countries | | 29 |
| |
Figure 14. Sales and Marketing Critical Event Timeline | | 29 |
| |
Figure 15. Onalta™ Product Pricing | | 30 |
| |
Figure 16. BioMedica Consolidated Cash Flows (w/ Onalta™) | | 31 |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 3 of 31 | | CONFIDENTIAL |
Overview of BioMedica Life Sciences S.A.
Company Profile
Established in 1987 in Athens, Greece, BioMedica Life Sciences S.A. is a privately held research, early and late stage development focused company in the emerging field of nuclear medicine. Since its inception over two decades ago, BioMedica has grown to become Hellenic market leader in diagnostic and therapeutic radiopharmaceutical products and services.
Three strategic imperatives drive BioMedica’s business efforts:
| | • | | Development and commercialization of innovative radiopharmaceutical products and services through internal R&D efforts |
| | • | | Sourcing late stage radiopharmaceutical diagnostics and therapies for development and commercialization in Europe and The Middle East |
| | • | | Strengthening research partnerships with Greek academic institutions and research centers |
Key Products
BioMedica is the Greek license holder of Octreoscan™ (Covidien), an111In molecular imaging agent for scintigraphic localization of primary and metastatic neuroendocrine tumors bearing somatostatin receptors. BioMedica is also sole EU license holder of Rebone™ (MDS-Nordion)186Re, indicated for palliative treatment of painful bone metastases.
Radiopharmaceutical Products
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 4 of 31 | | CONFIDENTIAL |

Cold Kits
| | • | | TechneScan™DMSA : Renal cortex scintigraphy |
| | • | | TechneScan™ DTPA : Nephrological and urological disorders scintigraphy |
| | • | | TechneScan™ HDP : Bone scintigraphy |
| | • | | TechneScan™ LyoMAA :Lung perfusion scintigraphy |
| | • | | TechneScan™MAG3: Renal scintigraphy |
| | • | | TechneScan™PYP : Gastrointestinal bleeding scintigraphy |
Radioimmunoassay (RIA) Products
| | • | | BC 19000 Free Testosterone |
| | • | | BC 1012 Human Growth Hormone |
Precursors for Medical Imaging
Crystal Probe 2000
| | • | | Laparoscopic – Intra-Operational Gamma-Ray Detection Probe |
Current R&D Programs
BioMedica has a proven track record in radiopharmaceutical product and clinical development as demonstrated by its successful, ongoing, clinical programs:
| | • | | Demogastrin™: Minigastrin analog labeled with99MTc |
| | • | | First Greek company to file a successful EMEA submission |
| | • | | Granted EMEA orphan drug designation in 2006 |
| | • | | Initiating Phase II clinical trials in Europe |
| | • | | Demobesin-6: 99mTc-labeled bombesin analogs (antagonists) |
| | • | | In Phase I clinical trials in Europe |
| | • | | RASP™: Retinoid conjugates |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 5 of 31 | | CONFIDENTIAL |

| | • | | In pre-clinical evaluation |
| | • | | Development of novel resins for solid phase peptide synthesis |
Corporate Structure
In addition to its core radiopharmaceuticals business, BioMedica is actively involved in the development and commercialization of complementary products and services through its subsidiaries, as well as strategic alliances with Hellenic academic and research centers.
|
| BioGenomica S.A. |
|
| BioMedica is majority shareholder of BioGenomica, a biotechnology company established in 2004 as a spin-off from The National Scientific Research Center (Demokritos) to provide a wide range of highly specialized genetic testing services: |
| | • | | Molecular analysis of disease predisposing genes |
| | • | | Molecular cytogenetic analysis |
|
| BioRP |
|
| BioMedica is the majority shareholder of BioRP, a biotechnology company established in 2007 as a spin-off from The National Scientific Research Center (Demokritos). It is engaged in development and production of novel radiopharmaceutical imaging and therapeutic products based on innovative peptide derivative research. |
|
| Demokritos National Scientific Research Center |
|
| Strategic alliance with BioMedica since 2001 focuses on research and development of novel peptides with commercial applications in nuclear medicine. Demokritos is the oldest and largest scientific research center in Greece and operates the country’s single nuclear reactor. |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 6 of 31 | | CONFIDENTIAL |

Management Team
BioMedica has a highly experienced and seasoned senior management team with a successful track record in nuclear medicine.
| | • | | Major share holder of Biomedica. Previously Sales Manager of Medgenix Greece. Studied mathematics and trained in radiopharmacy |
| | • | | Dr. S. Georgopoulou MD: CSO |
| | • | | Medical pathologist with over 12 years experience. Previously Medical Director of Roche Greece |
| | • | | Mr. D. Protogirou MSc Eng. MBA: CFO |
| | • | | Over 10 years experience as CFO in several telecommunication companies |
| | • | | Dr. N. Tsiakkas MD: Regulatory |
| | • | | Previously Regulatory Director at Astra Zeneca. Founded Medworks, leading CRO in Greece |
| | • | | Ms. M. Anagnou: Marketing |
| | • | | Has held management positions in marketing at Roche prior to joining BioMedica |
| | • | | Dr. D. Psimadas: Radiopharmacist |
| | • | | Post Doctoral Fellow at Demokritos National Scientific Research Center |
| | • | | Prof. P. Kordopatis: Peptide Chemistry |
| | • | | Full Professor of Pharmacology at Patras University. Specialized in Peptide Chemistry |
| | • | | Dr. P. Kaldrimides: Endocrinologist |
| | • | | Director of Endocrinology Clinic of METAXA Anticancer Hospital of Athens |
| | • | | Prof. V. Georgiou: Nuclear Medicine |
| | • | | Full Professor of Medical Physics at Athens University |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 7 of 31 | | CONFIDENTIAL |

Key Financials
BioMedica has grown into a high-growth, sustainable, and profitable business in the two decades since its formation in 1987. BioMedica has increased annual sales turnover by ****** over this period has been driven by improved sales and marketing efforts for existing radiopharmaceutical products and services, as well as a growing contribution from BioGenomica’s high-growth, high-margin, genetic testing business. This business is expected to contribute ****** of revenue in 2009.
Projected 2009 Sales & EBIT (without Onalta™)
BioMedica forecasts ****** in 2009 sales delivering an estimated EBIT of ******.
Current Debt Structure
| | • | | Long-term debt of ****** loan to complete construction of BioMedica’s new headquarters in Athens, Greece |
| | • | | Short-term debt of ****** to “factor” payables: owed to banks unconditionally backed by receivables from the Government of Greece(see cash flow) |
Cash Position
On July 27, 2009 BioMedica had ****** in cash & cash equivalents with an additional ****** in available credit.
Cash Flow
The Government of Greece is the sole payer of healthcare product and service reimbursements in Greece. It is an accepted, albeit burdensome, cost of doing business in the Greek healthcare sector that providers of products and services are required to factor receivables from the Government of Greece through banks at an historic rate of approximately 5%-6% per annum.
This arrangement creates an apparent negative cash flow according to GAAP accounting principles since receivables are all factored under this arrangement which appears in financial statements as short term debt.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 8 of 31 | | CONFIDENTIAL |

Historical Financials
Figure 1. BioMedica Historical Financials
*****
BioMedica has demonstrated consistent growth in turnover and gross operating income over the past five years. Net results have been positive throughout the period.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 9 of 31 | | CONFIDENTIAL |
Projected Cash Flows (pre-Onalta™)
Figure 2. Cash & Cash Equivalents at end of Period
*****
The growth trajectory of BioMedica’s existing product portfolio will drive an increasing availability of cash to fund operations and drive expansion through in-licensing, acquisition and internally driven innovation.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 10 of 31 | | CONFIDENTIAL |
BioMedica’s Five Year Business Plan
Vision Statement
BioMedica is committed to transformation, within the next five years, from the leading radiopharmaceutical sales and distribution company in the Southern European/Hellenic region to the leading Pan-European, radiopharmaceutical development, sales & marketing, and distribution company, focused in PET, SPECT, and therapeutic products delivered by targeting molecules
Tactics to be Employed by BioMedica
| | 1. | Successful launch and commercialization of Onalta™ in the targeted territories in Europe, Middle East and Mediterranean Rim |
| | 2. | Completion of Demogastrin™ clinical development and successful European launch ***** |
| | 3. | Ongoing external sourcing of products, both by in-licensing and acquisition, to expand BioMedica’s radiopharmaceutical portfolio and contribute to increasing credibility in development and commercialization capabilities |
| | 4. | Leveraging its current domain-knowledge and extensive participation in radiopharmaceuticals associated with fourteen medical specialties |
| | 5. | Initiation of internal development programs for radiopharmaceutical products for European markets |
| | 6. | Continued growth and expansion of BioGenomica, its current high margin genomic testing subsidiary business, in key European territories to provide funds for investment in the radiopharmaceutical business |
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 11 of 31 | | CONFIDENTIAL |

Role of Onalta™ in Five Year Business Plan
Pan-European growth in its business resulting from the successful development and commercialization of Onalta™ is a critical component in BioMedica’s strategy to transform from a Southern European/Hellenic centric company to a Pan European leader in its markets. Onalta’s success will be dependent upon a flawless execution by Biomedica of three critical objectives:
| | 1. | Roll-out of compassionate use program throughout the targeted territories |
| | a. | Complete shut-down of existing DOTATOC supply channels and strict ongoing enforcement of such closure, through enforcement of patents and supply agreements |
| | b. | Smooth transfer of all current DOTATOC users to purchase of product from BioMedica as the sole source supplier |
| | c. | Consistent and timely supply of DOTATOC through named patient programs in the licensed territories |
| | 2. | Excellence and detailed compliance to protocol in clinical development |
| | a. | Execution of Phase 3 Clinical Trial in Europe in compliance with protocol(s), endpoints and guidelines through tight management of experienced radiopharmaceutical CRO(s). This program will be coordinated with MIP as part of a global development effort |
| | b. | EMEA MAA submission coordinated with MIP and the global development objectives for Onalta™. This submissions will meet or exceed the guidelines set forth for the Onalta™ study |
| | 3. | Effective, phased, coordinated commercialization throughout the targeted territories upon approval by respective European regulatory agencies |
| | a. | Expansion of distribution capabilities |
| | b. | Successful negotiation of country by country reimbursement, utilizing leaders in the filed and advancing reimbursement from “high price” to “lower priced” markets |
Biomedica projects that the successful execution of these key elements will result in near-term, longer-term, and sustainable value to BioMedica and will result in the phased roll-out of critical resources and competencies necessary for the planned expansion of Biomedica in Europe:
| | 1. | Specific clinical development expertise and the demonstrated ability to manage development stage products through the development process, leading to European marketing approval of radiopharmaceutical products |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 12 of 31 | | CONFIDENTIAL |

| | 2. | Regulatory expertise, and the demonstrated ability to navigate European regulatory approval processes successfully, confirming the ability to manage an expanding product development and commercialization effort |
| | 3. | Product sourcing and distribution capabilities for radiopharmaceuticals throughout Europe and rest of world |
By building upon the success of Onalta™, BioMedica plans to exploit its experience to target new diseases, develop novel treatment options for these diseases, and commercialize new products for patients on a pan-European scale.
Role of Demogastrin™ and Other Products in BioMedica’s Five Year Business Plan
Demogastrin™
Demogastrin™, BioMedica’s radiopharmaceutical product directed at the diagnosis of medullary thyroid carcinoma, is entering phase II/III clinical trials. Marketing approval and commercialization in Europe is expected within *****, at which point Demogastrin™ will utilize the operational sales, marketing and distribution channels created for Onalta™ to improve the operating margins of Demogastrin™ and Onalta™. Commercialization of Demogastrin™ will significantly enrich BioMedica’s product portfolio, further confirm the clinical development and regulatory skills and experience of the company, and establish BioMedica’s position as an innovator in radiopharmaceuticals.
BioGenomica
BioMedica currently enjoys a leading position in the genomic testing market in Southern Europe, with strong growth expected from BioGenomica, the company’s high margin genetic testing subsidiary. Revenue growth is expected to reach *****, enjoying operating profit margins of *****, and contributing ***** to BioMedica’s overall EBIT each year. BioGenomica’s cash contributions will be utilized to support BioMedica’s growth in radiopharmaceuticals business in Europe.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 13 of 31 | | CONFIDENTIAL |

Onalta™ Revenue Projections
BioMedica projects Onalta™ sales will reach *****. The following assumptions underpin these projections:
Assumptions
| | 2. | COGs: Projections have utilized the higher end of the acceptable sector benchmark of ***** of sales across all markets, and all products |
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 14 of 31 | | CONFIDENTIAL |

| | 3. | Administrative Expenses: |
| | a. | *****: Limited resources are required to achieve sales objectives. BioMedica will establish European presence by opening an office in Germany and begin recruitment of key management |
| | b. | ***** administrative offices and facilities will be fully-recruited, with costs covered from operating revenues and capital commitments secured for Onalta™ development and commercialization. Sales and marketing staff will be hired as growth objectives dictate, with payroll costs reflecting usual and customary market rates |
| | 4. | Sales and Distribution Expenses |
| | 5. | Clinical Development Expenses: Established as highest priority for Biomedica and is fully funded by dedicated funds raised |
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 15 of 31 | | CONFIDENTIAL |

Figure 3. Onalta™ Revenue Projections
******
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 16 of 31 | | CONFIDENTIAL |

Source of Funds Required for Development and Commercialization of Onalta™
Forecasted capital expenditures upon execution of the Onalta™ comprehensive agreement total ***** over the initial *****. Cash requirements in 2009 are projected to be ***** and includes the payment additional upfront obligations to Molecular Insight Pharmaceuticals, compassionate use licensing fees, clinical development payments as well as costs associated with salaries, facilities and marketing. Cash requirements for 2010 are projected to be ***** and include payment for clinical development, and costs associated with marketing, salaries and facilities.
Figure 4. Forecasted Cash Requirements
*****
Funding to meet forecasted cash requirements has been secured through multiple sources and provides cash in immediate, near-term and intermediate-term time frames. Expenses will be covered by the secured funding and interest payments will be covered from BioMedica operating revenues from its core business (projected 2010 EBIT of ***** excluding contributions from Onalta™ sales for the initial period of *****.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 17 of 31 | | CONFIDENTIAL |

Primary Immediate Source of Funds
BioMedica has secured immediate funding from three institutions to cover the cash requirements for Onalta™ development. These funds will become available upon execution of the Onalta™ comprehensive agreement with MIP.
| | 1. | ***** of Greece has committed to provide ***** |
| | a. | Letter of commitment will be issued by July 31, 2009 |
| | i. | ***** is earmarked to pay Licensee fees due upon signature of final agreement with MIP |
| | ii. | ***** is earmarked to cover first quarter cash requirements for Onalta™ development. |
| | 2. | ***** of Greece has committed to provide ***** to BioMedica to support Onalta™ development |
| | 3. | ***** of Greece has committed to provide ***** to BioMedica to support Onalta™ development |
Secondary Near-term Source of Funds
BioMedica has secured additional sources of funding which will become available upon the execution of the Onalta™ Comprehensive Agreement and will accessed to supplement primary sources of funds as circumstances demand. The committed institutions include:
| | 1. | ***** - available by early November 2009 (commitment expected in early September) |
| | 2. | ***** in additional funding upon request (commitment in place) |
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 18 of 31 | | CONFIDENTIAL |

| | 3. | BioMedica EBIT: BioMedica has committed to utilize its free-cash flow from its projected annual ***** |
Tertiary, Longer-term Source of Funds
An additional ***** will be available to BioMedica upon execution of Onalta™ comprehensive agreement, and as needed in the longer term, from leading private and institutional banks in Europe and Greece. These commitments will be announced in late 2009 and 2010.
| | 2. | Other Leading Institutional Banks in Greece and Europe |
Figure 5. Cash Requirements vs. Secured Financing
*****
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 19 of 31 | | CONFIDENTIAL |

Onalta™ Development and Commercialization Plan
Onalta™ Development Plan
The successful clinical development and regulatory approval strategy elements for Onalta™ in the EU involve completion of a pivotal Phase 3 clinical trial utilizing oversight by a clinical research organization (CRO such as ABX) that specializes in execution of radiopharmaceutical clinical development programs in Europe. The organization selected will assist in completion of any additional non-clinical studies required for MAA approval.
Timeline & Budget
Figure 6. Onalta™ Clinical Development Timeline and Costs
*****
The Phase 3 clinical trial is scheduled to commence Q1, 2010, will have a 19 month enrollment plan (approximately 10 patients/month), with last patient enrollment projected to take place in Q3, 2011. The study protocol requires a 10 month follow-up period and is projected to complete in Q2, 2012. The projected overall cost for the Phase 3 trial is *****, including drug supply costs. Preparation of the non-clinical regulatory package is planned to begin Q4, 2010 and complete in Q2, 2012. This activity is budgeted at a total cost of *****. Within eight months of study completion, all CSRs will be complete. MAA filing with EMEA is projected to occur in Q4, 2012, and EMEA approval is expected in late 2013.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 20 of 31 | | CONFIDENTIAL |

Although patent expiry occurs in 2015, orphan drug status grants EU market exclusivity for Onalta™ for 10 years post approval. It is therefore expected that market exclusivity for Onalta™ will continue through late 2023.
Phase 3 Clinical Study
BioMedica intends to follow MIP’s Phase 3 study protocol (ON-C301), a protocol that has been submitted to, and approved by EMEA (see Appendix for protocol details)
Proposed Phase 3 Study Sites
BioMedica will execute the proposed Phase 3 trial at 18-28 centers in 10 European countries with the goal to recruit 255 patients within the 19 month recruiting timeframe described above. A sample of proposed centers is identified in figure 7.
Figure 7. Proposed Phase 3 Clinical Trial Centers
| | |
Proposed Clinical Trial Centers | | Location |
| University Hospital of Basel | | Basel, Switzerland |
| |
Royal Marsden Hospital NHS Foundation Trust | | Sutton (Surrey), United Kingdom |
| |
Erasmus Medical Center | | Rotterdam, The Netherlands |
| |
Universitätsklinikum Innsbruck | | Innsbruck, Austria |
| |
Central Hospital of Bad Berka GmbH: | | Berka, Germany |
| |
University Hospital of Heidelberg | | Heidelberg, Germany |
| |
European Institute of Oncological Research | | Milan, Italy |
| |
University of Louvain Medical School | | Brussels, Belgium |
| |
University of Mainz Johannes Gutenberg | | Mainz, Germany |
| |
University of Bonn | | Bonn, Germany |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 21 of 31 | | CONFIDENTIAL |

Commencement of Development Activities
BioMedica will immediately start Onalta™ development activities upon execution of the comprehensive Onalta™ agreement. BioMedica will expedite the initial development program activities to ensure timely completion of the Phase 3 clinical program in Europe on time and in compliance with the protocol. Activities that have been identified include:
| | 1. | Identification and recruitment of study centres: |
| | a. | Submit protocol summary |
| | b. | Distribute feasibility questionnaires |
| | c. | Select initial study centres |
| | d. | Contract selected centres |
| | e. | Continue expansion of study centre enrolment until full complement is reached |
| | 2. | Identification and recruitment of independent review committee |
| | 3. | Identification, qualification and selection of electronic data capture vendor |
| | 4. | Appointment of CRO for study monitoring (European CRO with oncology expertise – see below for examples) |
| | 5. | Set up and validate pharmacovigilance systems |
| | 6. | Initiate manufacture of investigational Onalta™ drug and establish distribution and compliance programs |
| | 7. | Finalize drug packaging and labelling for investigational drug |
| | 8. | Establish and test drug distribution logistics and finalize logistics program |
Organizational Structure & Staffing
BioMedica will initiate staffing of its Onalta™ development organization immediately following completion of the definitive Onalta™ license agreement with MIP. It will plan commencing with the recruitment of a qualified, industry experienced, Head of clinical development. BioMedica intends its clinical development function for Onalta™ to be located in Germany and staffed by professionals located in Germany. Subsequent to the recruitment of the head of clinical development, BioMedica will recruit a clinical development manager and a clinical research associates (CRAs). Final selection of the clinical research organization (CRO) will be done by the head of clinical development, in close association with MIP. At present, BioMedica has identified two companies with the appropriate credentials and experience to successfully manage the Onalta™ Phase 3 program: IBIS and ABX.
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 22 of 31 | | CONFIDENTIAL |

MIP involvement in the European Phase 3 Clinical Development of Onalta™
BioMedica considers MIP’s support in the development of Onalta™ in Europe as critical to its success, and as the means to fully coordinate the global development of the product. BioMedica intends to utilize relevant MIP staff to assist in setting up, training and executing critical components of the Phase 3 trial at the selected study centers. The expenses and costs associated with MIP assistance will be fully funded by BioMedica, and have been included in the Phase 3 budget. It is expected that the comprehensive agreement between BioMedica and MIP will include the mechanisms for defining and quantifying the elements of the services and resources provided by MIP, the terms of reference for their use, and their cost.
Moreover, BioMedica intends to inform and seek advice from MIP throughout the Phase 3 trial and intends to incorporate MIP input in the final trial design, primary endpoint confirmation, and patient recruitment, as the clinical program progresses.
Named Patient Program
Overview and Responsibilities
BioMedica has initiated negotiations with IDIS Limited (“IDIS”) for exclusive distribution of pre-approved Onalta™ through a named patient program in Europe. IDIS, based in the United Kingdom, is widely accepted as having unique capabilities and significant experience in the initiation, management and execution of pre-approval, named patient programs, and the distribution of un-licensed products on a named patient supply basis within the territories that have been targeted for Onalta™ distribution, which include:
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 23 of 31 | | CONFIDENTIAL |

As the sole distributor of pre-approved Onalta™, IDIS will be responsible for:
| | 1. | Distribution of Onalta™ “kits” and Onalta™ final product in all targeted territories to approved patients |
| | 2. | Compliance with all applicable regulations and laws that apply to the distribution of Onalta™ to indentified and approved patients |
| | 3. | Compliance with the cGDP guidelines and the maintenance of an inclusive and compliant quality management system to monitor the performance of the distribution |
| | 4. | Training of all staff with valid standard operation procedures and further regulations of BioMedica |
| | 5. | Management and execution of invoicing for drugs supplied to approved patients, and the reimbursement for their use within each country |
| | 6. | Management of product storage, and storage facilities in accordance with cGDP |
| | 7. | Maintenance of all applicable regulatory files and treatment records as required by law |
Pricing
Fixed prices governing the sale of the Onalta™ “kits” and the Onalta™ finished product to IDIS have been determined and are as shown in figure 8:
Figure 8. Named Patient Program Pricing
| | | | |
Product | | Service Fee Paid to
IDIS by BioMedica | | Patient Price per
Dose |
Onalta™ “kits” (ready to infuse excluding90Y) | | ***** | | ***** |
Onalta™ finished product | | ***** | | ***** |
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 24 of 31 | | CONFIDENTIAL |

BioMedica will pay a one time set up fee of ***** for the program following agreement sign-off. In addition, a management fee of ***** per month will be charged by IDIS during the period that Onalta™ “kits” are distributed. This fee will decline to ***** per month when Onalta™ finished product is distributed through this program.
Timing
The proposed named patient program will be operational within eight weeks of execution of the distribution agreement, with roll-out to 15-18 key treatment centers by the end of 2009. Within 24 months, IDIS intends to target >100 centers throughout Europe.
Figure 9. Named Patient Program Event Timeline
| | | | |
Event | | Timeline | | |
Signing of Named Patient Program Distribution Agreement | | August ‘09 | | |
| | |
Roll-out of Named Patient Program | | October ‘09 | | |
| | |
Target key European Centers (15-18) | | December ‘09 | | |
| | |
Roll-out to include >100 centers in Europe | | October ‘11 | | |
MIP Involvement
As part of its continuing relationship with MIP, BioMedica intends to access the technical expertise resident at MIP, and utilize specific MIP resources to prepare training materials for physicians and pharmacists governing the administration and dosing of Onalta™, as well as to provide pharmacovigilance support during the initial roll-out period.
BioMedica intends, and has budgeted for, payments of all approved expenses and costs associated with MIP assistance. BioMedica expects that the comprehensive agreement with MIP will include the mechanisms for defining and quantifying these payments, and the means to approve and pay for such resource use.
Regulatory Affairs
Building upon the experience gained from its successful EMEA Phase 1 filing of Demogastrin™, BioMedica will recruit a world-class Head of regulatory affairs with a proven track record of radiopharmaceutical filing with EMEA, to lead the regulatory program for Onalta™. BioMedica intends to complete recruit this key executive by Q4, 2010. In the interim, BioMedica intends to actively utilize, MedWorks, its long-standing regulatory affairs consultant, in addition to seeking the guidance, support and recommendations from MIP.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 25 of 31 | | CONFIDENTIAL |

MIP Involvement
As part of its continuing relationship with MIP, BioMedica intends to utilize the Regulatory Affairs expertise resident at MIP and views this resource as a critical component to the success of securing Onalta™ EMEA MAA.
BioMedica intends, and has budgeted for, payments of all approved expenses and costs associated with MIP assistance in regards to regulatory affairs. BioMedica expects that the comprehensive agreement with MIP will include the mechanisms for defining and quantifying these payments, and the means to approve and pay for such resource use.
Manufacturing and Distribution
Under the final terms to be agreed upon at the execution of the final comprehensive agreement MIP will retain the rights and obligations to manufacture and distribute Onalta™ for a period of at least three years, with an automatic extension of this obligation of two years granted to BioMedica. Upon expiry of the three/five year manufacturing and distribution contract, BioMedica may opt to assume the rights and obligations to manufacture and distribute Onalta™, or negotiate further extension to its agreement with MIP. Provision for these matters will be incorporated into in the manufacturing and distribution section of the definitive Onalta™ license agreement with MIP.
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 26 of 31 | | CONFIDENTIAL |

With the execution of a manufacturing and distribution agreement with MIP, the following critical activities are to be carried out by BioMedica, IDIS Pharma, and MIP:
Figure 10. Manufacturing & Distribution Timeline
| | | | |
Activity | | Timeline | | Responsibility |
Terminate Bachem peptide distribution | | Upon Contract Signing | | MIP |
Begin supply of90Y kits to IDIS Pharma | | Upon Contract Signing | | MIP |
Convert kits to lyophilized “ready to infuse” vial form to be labeled with90Y by radiopharmacists at treatment centers | | September-October ‘09 | | MIP |
Enforce the purchase and use of “ready to infuse” kits from IDIS Pharma at treatment centers | | October ‘09-Until EMEA Compassionate Use Approval | | IDIS Pharma & BioMedica |
Enforce use of Onalta™ finished product at Treatment Centers | | Upon Compassionate Use Approval | | IDIS Pharma & BioMedica |
Sales and Marketing
Overview
Upon completion of a detailed sales and marketing study in Q1, 2009, and after further interaction with external consultants knowledgeable of the field and specific attributes of its proposed commercialization strategy, BioMedica has created a sales and marketing plan it believes will deliver the revenues and cash flows detailed in this business plan, and which are based on the following assumptions:
| | • | | Economics and pricing based on a luxury goods pricing model/paradigm |
| | • | | BioMedica will price Onalta™ at ***** per dose in all sublicensed territories, aligning Onalta™ pricing in Europe reasonably in line with MIP’s suggested price of ***** per dose |
| | • | | Market penetration assumptions are based on the following parameters |
| | • | | Population distribution and probabilities to access market at suggested price per dose price |
| | • | | Market penetrations based on social and economic criteria |
| | • | | Targeted sales approaches based on hub and spoke model |
| | • | | Intensive pre-marketing activities to develop the markets |
| | • | | Onalta™ sales regions will be divided into two broad categories that represent probable sales penetration rates |
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 27 of 31 | | CONFIDENTIAL |

| | • | | Type-2: High sales penetration countries |
| | • | | Type-1: Low sales penetration countries |
| | • | | Sales and Marketing effort will concentrate onhub countries in Europe and the Middle East that will have oversight overspoke countries in respective European and Middle Eastern countries/regions |
Figure 11. Designated Hub Countries in Europe and Middle East
| | | | | | |
HubCountries in Europe | | Type | | Hub Countries in Middle East | | Type |
GERMANY | | 2 | | U.A.E. | | 1 |
U.K. | | 2 | | EGYPT | | 1 |
FRANCE | | 2 | | IRAN | | 1 |
ITALY | | 2 | | | | |
SPAIN | | 1 | | | | |
POLAND | | 1 | | | | |
GREECE | | 1 | | | | |
RUSSIA | | 1 | | | | |
TURKEY | | 1 | | | | |
Figure 12. Type-2 Hub Countries and their Respective Spoke Countries
| | | | | | |
TYPE-2Hub Countries & Respective Oversight ofSpoke Countries (red text) |
France | | Germany | | Italy | | U.K. |
| Monaco | | Austria | | Bosnia & Herzegovina | | Iceland |
| San Mar. | | Hungary | | Croatia | | Ireland |
| | Czech Republic | | Malta | | |
| | Denmark | | Montenegro | | |
| | Finland | | Serbia | | |
| | Norway | | Slovakia | | |
| | Sweden | | Slovenia | | |
| | Switzerland | | | | |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 28 of 31 | | CONFIDENTIAL |

Figure 13. Type-1 Hub Countries and their Respective Spoke Countries
| | | | | | | | |
TYPE-1Hub Countries & Respective Oversight ofSpoke Countries (red text) |
Belgium | | Greece | | Poland | | Russia | | Spain Turkey |
| Luxembourg | | Albania | | Estonia | | Armenia | | Portugal |
| Netherlands | | Bulgaria | | Georgia | | Azerbaijan | | |
| | Cyprus | | Latvia | | Belarus | | |
| | Republic of Macedonia | | Lithuania | | Kazakhstan | | |
| | Romania | | Ukraine | | | | |
| | MIDDLE EAST | | | | | | |
Timeline of Critical Sales, Marketing and Commercialization Activities
Critical to the success of BioMedica’s Onalta™ “kit” business, its named patient program, and Onalta™ final product penetration and sales is the ability to initiate a comprehensive sales, marketing and commercialization effort.
In planning the sales and marketing effort for Onalta™ BioMedica has identified critical activities that will be executed upon completion of the comprehensive license agreement with MIP:
Figure 14. Sales and Marketing Critical Event Timeline
| | |
Event | | Target
Completion Date |
Hire Head of Sales & Marketing | | Q1. 2010 |
Hire and train six initial sales reps. targeted at core EU clinical centers | | Q2. 2010 |
Establish Onalta™ KOL Advisory Board | | Q3. 2010 |
Hire and train 16 additional sales reps. targeted at remaining territories | | Q4. 2011 |
Enter into exclusive distribution agreements for Israel and Turkey | | Q2. 2010 |
Hire and train additional 25-28 sales reps (50 total) | | Upon EMEA MAA |
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 29 of 31 | | CONFIDENTIAL |

Onalta™ Product Pricing
BioMedica has completed an exhaustive pricing analysis for Onalta™ and will deploy product pricing at launch according to the schedule outlined in figure 14:
Figure 15. Onalta™ Product Pricing
| | | | |
Product | | Single Dose Price | | Treatment Price
(three doses) |
Onalta™ Kit (ready to infuse excluding90Y) | | ***** | | ***** |
Onalta™ Final Product | | ***** | | ***** |
Reimbursement Strategy
| | • | | As part of its contractual agreement with BioMedica, IDIS Pharma will assume responsibility for reimbursement strategy and implementation during the period from license and through EMEA MAA approval |
| | • | | BioMedica intends to retain IDIS and other external reimbursement consultants to aggressively secure approval for reimbursement of Onalta™ final product at the targeted price of ***** per dose |
MIP Involvement
Although all significant sales and marketing activities will be executed by BioMedica and its Onalta™ team, it is BioMedica’s intention of to seek MIP’s counsel, in relationship to pricing so as to maintain a coherent global pricing platform for Onalta™.
* Confidential Treatment Requested *
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 30 of 31 | | CONFIDENTIAL |
Consolidated Cash Flow
Figure 16. BioMedica Consolidated Cash Flows (w/ Onalta™)
*****
* Confidential Treatment Requested*
| | | | |
| BioMedica Onalta™ Business Plan© 29.07.09 | | Page 31 of 31 | | CONFIDENTIAL |


Onalta™ Europe Business Plan
APPENDIX
July 29, 2009
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 1 - | | CONFIDENTIAL |

Appendix
Index of Figures
| | |
Figure 1. BioMedica Historical Growth | | 3 |
| |
Figure 2. BioMedica Project Cash Flow (w/out Onalta) | | 3 |
| |
Figure 3. Project Onalta Revenues | | 4 |
| |
Figure 4. BioMedica Projected Consolidated Cash Flow (w Onalta) | | 5 |
| |
Figure 5. Clinical Development Activities Descriptions | | 6 |
| |
Figure 6. Plan for ON-C301 (Phase 3) | | 7 |
| |
Figure 7. Normalized Development Plan Activities Time Table | | 8 |
| |
Figure 8. NAMED PATIENT SUPPLY AND DISTRIBUTION AGREEMENT | | 9 |
| |
Figure 9. Technical Agreement for Distribution Services | | 32 |
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 2 - | | CONFIDENTIAL |

Figure 1. BioMedica Historical Growth
******
Figure 2. BioMedica Project Cash Flow (w/out Onalta)
******
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 3 - | | CONFIDENTIAL |
* Confidential Treatment Requested *

Figure 3. Project Onalta Revenues
******
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 4 - | | CONFIDENTIAL |
* Confidential Treatment Requested *

Figure 4. BioMedica Projected Consolidated Cash Flow (w Onalta)
******
| | | | |
| BioMedica OnaltaTM Appendix 29.07.09 | | - 5 - | | CONFIDENTIAL |
* Confidential Treatment Requested *

Figure 5. Clinical Development Activities Descriptions
| 1. | Study Protocol and Informed Consent |
Study protocol writing/development, review and approval
Development of subject information sheet and informed consent form
Translation of subject information sheet and informed consent form
Prepare info package w feasibility questionnaire. Communicate to centers
CRF development, review, approval and send to sites
Select electronic CRF vendor
| 3. | Selection of Study Sites |
Proposal of study sites
Pre-Study site evaluation visits
Study site approval based upon due diligence information
| 4. | Submission to Authorities & IRB/ECs |
Arrange for insurance
Prepare Master CTA - general part
Collection of regulatory documents for to CA and EC
Submission to ECs
Receive all approvals for study start (CA, EC, CTX etc)
| 5. | Study Set-up Activities |
Set up of the study tracking system
Development/Update of study Manual of Operations (MOP)
Hospital & Investigator grant/case fee negotiation
Hospital & Investigator contract preparation and signature
Selection of Independent Review Committee
Selection of various other vendors, incl. contract preparation
Selection/Definition of committees and board members
| 6. | Study Drug and Supplies |
Labeling and packaging of study drug and supplies
Arrange logistics of drug shipment
Distribution of study drug and supplies to study sites
Waste management
| 7. | Central Patient Randomisation (IVRS) |
Contract IVRS provider
| 8. | Investigator/Team Meeting |
Organization and conduct of investigator meetings
Organization of CRA training meetings
Conduct of site initiation visits
DCF generation and clearance
Conduct of Study close down visits
| 10. | Drug Safety / SAE Reporting |
Establish PV System
Ongoing data clean
Final review of all data
Data base freeze
| 12. | Medical and Statistical Report Writing |
Identify study Statistician
Interim analysis
Statistical analysis
Issuance of statistical report
Medical writing
| 13. | Submission to EMEA review process |
MAA dossier submission
Review time
Granting of MAA
National CA submission
Granting of national authorization
Set-up of audit plan with CQA
Set-up of audit action plan
Audit of study sites
Audit of CRO (if applicable)
Contracting CRO
Overseeing if milestones are reached/expectations met
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 6 - | | CONFIDENTIAL |

Figure 6. Plan for ON-C301 (Phase 3)
Plan for ON-C301 (Phase 3)
Study Title.A Phase 3 randomized, controlled, open label, multi-center study evaluating the efficacy of90Y-edotreotide administered by intravenous infusion to adults with metastatic, progressive somatostatin-receptor positive carcinoid tumors versus Octreotide acetate (Sandostatin LAR®)
ON-C301 is a two-arm, randomized, controlled, open label, multi-center study to evaluate the efficacy and safety of90Y-edotreotide versus octreotide acetate (Sandostatin LAR®) in subjects with metastatic, progressive, somatostatin-receptor positive carcinoid cancer. The proposed study is composed of a screening period followed by randomization into one of two arms: Either (three cycles of90Y-edotreotide given approximately eight weeks apart) or octreotide acetate (40 mg, intramuscularly (i.m.), depot formulation; Sandostatin LAR®). Subject follow up will continue until documented disease progression, start of an alternative therapy or 36 months post enrollment. Subjects will be assessed for tumor burden every other month for the first six months of the study. Thereafter, responding or stable subjects will be assessed every four months (16 weeks) until documented disease progression or 36 months post enrollment. If a subject randomized to the octreotide acetate (Sandostatin LAR®) arm experiences progressive disease, based on RECIST criteria and confirmed by blinded independent readers, they may receive three cycles of90Y-edotreotide given approximately eight weeks apart following the procedures stipulated in this protocol. Progression-free survival will be assessed via measurement of tumor response (determined via RECIST criteria). Secondary efficacy assessments include overall survival, overall tumor response, change of biomarkers, quality of life assessed with EORTC QLQ-C30 and GI.NET21 and safety of the treatment regimens.
The primary endpoint is the improvement in the period of progression free survival observed in patients receiving90Y-edotreotide or Octreotide acetate (Sandostatin LAR®). Approximately 194 subjects will receive90Y-edotreotide or Octreotide acetate (Sandostatin LAR®). At enrollment, subjects will be randomized in a 1:1 ratio to receive either investigational drug or control. Only subjects determined to be eligible will be randomized. There is no blinding for this study. The sample size estimate assumes a hazard ratio of 0.625 with 90% power to detect a 37.5% effect size.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 7 - | | CONFIDENTIAL |

Figure 7. Normalized Development Plan Activities Time Table
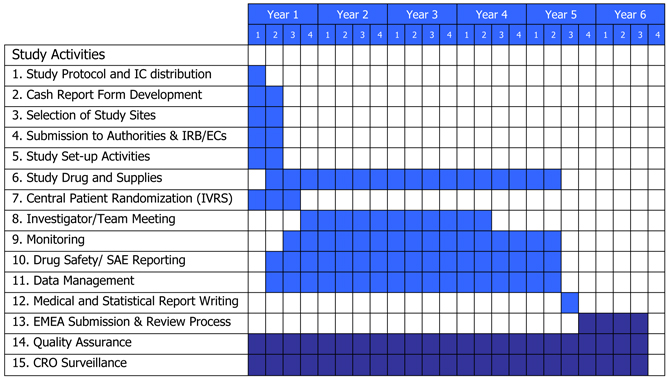
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 8 - | | CONFIDENTIAL |

Figure 8. NAMED PATIENT SUPPLY AND DISTRIBUTION AGREEMENT
| | |
| Dated | | 2009 |
| |
| (1) | | BioMedica Life Sciences S.A. |
| |
| (2) | | IDIS LIMITED |
NAMED PATIENT SUPPLY AND DISTRIBUTION AGREEMENT
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 9 - | | CONFIDENTIAL |

Contents
| | | | |
| 1 | | DEFINITIONS AND INTERPRETATION | | 11 |
| 2 | | APPOINTMENT AND RESTRICTIONS | | 13 |
| 3 | | TERM | | 13 |
| 4 | | SUPPLY OF THE PRODUCTS | | 14 |
| 5 | | PRICING | | 14 |
| 6 | | PURCHASE ORDERS | | 14 |
| 7 | | DELIVERY | | 15 |
| 8 | | REJECTION | | 16 |
| 9 | | PAYMENT | | 16 |
| 10 | | RISK AND PROPERTY | | 17 |
| 11 | | RIGHTS AND DUTIES OF IDIS | | 17 |
| 12 | | RIGHTS AND DUTIES OF THE MANUFACTURER | | 18 |
| 13 | | WARRANTIES | | 19 |
| 14 | | INDEMNITIES | | 19 |
| 15 | | INTELLECTUAL PROPERTY | | 20 |
| 16 | | TERMINATION | | 21 |
| 17 | | CONSEQUENCES OF TERMINATION | | 22 |
| 18 | | CONFIDENTIAL INFORMATION | | 22 |
| 19 | | FORCE MAJEURE | | 23 |
| 20 | | NOTICE | | 23 |
| 21 | | GENERAL | | 24 |
| 22 | | DISPUTES | | 25 |
| 23 | | GOVERNING LAW AND JURISDICTION | | 25 |
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 10 - | | CONFIDENTIAL |

PARTIES
| (1) | BioMedica Life Sciences S.A. (registered number 094413470) whose registered office is at 4 Papanikoli Str., Chalandri, Attica, Greece |
(“the Manufacturer”)
| (2) | IDIS LIMITED, a company incorporated in England (registered number 2143039), whose registered office is at IDIS House, Churchfield Road, Weybridge, Surrey, KT13 8DB, United Kingdom |
(“IDIS”)
INTRODUCTION
| A) | The Manufacturer wishes to supply the Products in the Territory through an entity with experience in the distribution of unlicensed products on a Named Patient Supply basis. |
| B) | IDIS has capability in the distribution of unlicensed products on a Named Patient Supply basis,within each country in the Territory and wishes to act as the Manufacturer’s distributor of the Products in the Territory. |
| C) | The Manufacturer appoints IDIS as its Exclusive distributor of the Products in the Territory on a Named Patient Supply basis on the terms set out in this Agreement. |
OPERATIVE PROVISIONS
Definitions and interpretation
In this Agreement the following words have the following meanings:
“Business Day” means any day other than a Saturday or Sunday or a public or bank holiday in England;
“Commencement Date” means the date of signature of this Agreement by both parties;
“Confidential Agreements” means any and all confidentiality agreements entered into between the parties either before or after the Commencement Date relating to the subject matter of this Agreement;
“Confidential Information” means all information which is commercially sensitive or of a secret nature, or information which is marked confidential, or which is orally stated to be confidential, relating to any and all aspects of the business of either party, including any confidential information set out in Confidentiality Agreements. Such information may be expressed in any form including orally, as an idea, as prices, price lists, plans, customer lists or details or information concerning either party’s relationships with actual or potential clients or customers and the needs and requirements of such persons;
“Contract” has the meaning set out inClause 6.2;
“Exclusive” means a right granted under this Agreement which the Manufacturer will not itself exercise and will not authorise any other person to exercise;
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 11 - | | CONFIDENTIAL |

“Fax Address” means the fax address of the relevant party given inSchedule 4;
“Force Majeure” means any circumstances beyond the reasonable control of the relevant party (including, without limitation, any strike, lock-out or other form of industrial action, acts of God, war or national emergency, an act of terrorism, riot, civil commotion, malicious damage, compliance with any law or government order, rule, regulation or direction, accident, fire, flood or storm) which prevents that party from complying with any or all of its obligations under this Agreement;
“Indication” means the symptoms, condition, or disease for which the Product has been prescribed for a particular Named Patient;
“Initial Period” means the period of three Years commencing on the Commencement Date;
“Intellectual Property Rights” means all intellectual and industrial property rights including patents, know-how, registered trade marks, registered designs, utility models, applications for and rights to apply for any of the foregoing, unregistered design rights, unregistered trade marks, rights to prevent passing off for unfair competition and copyright, database rights, topography rights and any other rights in any invention, discovery or process, in each case in the United Kingdom and all other countries in the world and together with all renewals and extensions;
“Marketing Authorisation” means an authorisation for the sale and placing on the market of a Product within the Territory;
“Named Patient” means the patient for whom the Product(s) have been prescribed;
“Named Patient Supply” means the supply of Products which do not have a Marketing Authorisation for the Indication in the country of destination and are supplied to meet the special needs of a specific patient or patients under the order of a medical practitioner or other person lawfully permitted to prescribe such Products to a specific patient or patients in the Territory or relevant part of it;
“Orders” has the meaning set out inClause 6.1;
“Prices” means the price to be paid by IDIS to the Manufacturer for the Products as set out inSchedule 1 of this Agreement, or as varied in accordance withClause 5.1;
“Products” means the product or products listed inSchedule 1 and additionally any further products of the Manufacturer offered to be supplied to IDIS by the Manufacturer in writing from to time to time after the Commencement Date and accepted in writing by IDIS;
“Service Address” means the address for service of the relevant party given inSchedule 4 of this Agreement;
“Technical Agreement” means the technical agreement set out inSchedule 5;
“Territory” means those countries set out inSchedule 2 of this Agreement and additionally any further countries agreed between the parties in writing from time to time after the Commencement Date;
“Trade Marks” means the trade marks and trade names of the Manufacturer listed inSchedule 3 and such other trade marks as the Manufacturer notifies to IDIS in writing from time to time after the Commencement Date;
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 12 - | | CONFIDENTIAL |

“Year” means the period of 12 (twelve) months beginning on the Commencement Date and each subsequent period of 12 (twelve) months commencing on the anniversary of the Commencement Date during the continuance of this Agreement.
Headings to the Clauses of and Schedules to this Agreement are for convenience only and shall not affect its construction or interpretation.
References to Clauses and Schedules are to the Clauses of and Schedules of this Agreement.
The Schedules are deemed to be incorporated and form part of this Agreement and the term “Agreement” shall be construed accordingly. In the event of conflict between any of the terms of this main part of the Agreement and the Schedules, the former shall prevail.
The word “indemnify” in this Agreement will mean to indemnify, keep indemnified and hold harmless the indemnified party from and against all costs (including the cost of enforcement), expenses, liabilities (including any tax liability), injuries, damages, claims, demands, proceedings or legal costs (on a full indemnity basis) and judgements which the indemnified party incurs or suffers and “indemnity”, “indemnities” and “indemnifies” have a corresponding meaning.
Any reference to a “month” is a reference to the period of a calendar month.
Any reference to “person” means a natural or legal person, firm or unincorporated association.
Words importing the singular include the plural and vice versa.
Appointment and Restrictions
The Manufacturer hereby grants to IDIS the Exclusive right to distribute on its own account the Products in the Territory and IDIS agrees to act in this capacity subject to the terms of this Agreement.
During the continuance of this Agreement, the Manufacturer undertakes not to market or sell the Products directly or indirectly to any other person in the Territory without first obtaining IDIS’s express written consent (such consent not to be unreasonably withheld or delayed).
During the continuance of this Agreement, IDIS undertakes not to seek customers or promote sales of the Products outside the Territory.
IDIS undertakes that it shall not unless otherwise approved in writing by the Manufacturer and for 5 (five) Years from the Commencement Date:
manufacture any goods that compete with the Products in the Territory;
obtain its supplies of the Products for distribution within the Territory other than from the Manufacturer; provided that the Manufacturer is able and willing to supply the same on the terms of this Agreement.
Term
This Agreement shall commence on the Commencement Date and subject to early termination in accordance with its terms shall continue in force in respect of each Product until that Product have been granted a Marketing Authorisation for the Indication within the Territory.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 13 - | | CONFIDENTIAL |

Supply of the Products
The Manufacturer shall supply the Products to IDIS in accordance with the terms and conditions of this Agreement to the exclusion of any terms and conditions of sale submitted at any time by the Manufacturer and whether printed or sent with any order form, delivery note, invoice or otherwise.
The Manufacturer shall not supply IDIS with any Products with a remaining shelf life of less than 12 (twelve) months or 75% of the full shelf life, whichever is greater. If the Manufacturer is unable to comply with thisClause 4.2 it shall notify IDIS immediately providing details of the remaining unexpired shelf lives of the available Products and, in such event, the Manufacturer shall not proceed with the Order until it has received written confirmation from IDIS that the Order may proceed at which time the Contract shall be formed.
Pricing
The Products will be supplied on a consignment basis and shall be sold by the Manufacturer to IDIS at the Prices. The Prices shall remain fixed for the duration of the Agreement but may be varied by the Manufacturer by giving IDIS not less than 60 (sixty) days notice; save that nothing in thisClause 5.1 shall give the Manufacturer the right to vary Prices in respect of Orders of Products placed by IDIS with the Manufacturer prior to the date of receipt of any notice of Price variation.
All Prices are inclusive of packaging but exclusive of any applicable value added or any other sales tax for which IDIS shall be additionally liable.
The Manufacturer may recommend in writing to IDIS a sale price for each of the Products or impose a maximum selling price at any time; provided that that price does not amount to a minimum selling price or retail price maintenance. For the avoidance of doubt:
where the Manufacturer has recommended a selling price to IDIS, IDIS shall be free to distribute the Products at any price it so chooses; and
where the Manufacturer has set a maximum price, IDIS shall be obliged to distribute the Products at no more than that price; provided that does not amount to a minimum selling price or retail price maintenance.
Without prejudice to any other provision of this Agreement, the Manufacturer shall advise IDIS immediately if any Price given for Products in an Order is incorrect and, in such event where the Price is incorrect the Manufacturer shall not proceed with the Order until it has notified IDIS of that fact and received written confirmation from IDIS that the Order may proceed, at which time the Contract shall be formed.
Purchase Orders
IDIS shall submit, from time to time, written purchase orders (“Orders”) to the Manufacturer for the supply of the Products. Each Order shall stipulate the Products’ names, the Products’ codes, the quantity required and the total price of the Order (excluding VAT).
The receipt by the Manufacturer of an Order during the term of this Agreement shall constitute a contract (“Contract”), subject to the terms and conditions of this Agreement.
NotwithstandingClause 6.2 and Clause 21.4, before delivery IDIS may vary, add or omit any or all of the Products in an Order by notice in writing to the Manufacturer. The Manufacturer shall not vary, add or omit any of the Products or any part of them from an Order without the express consent of IDIS.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 14 - | | CONFIDENTIAL |

An update of all outstanding Orders placed by IDIS with the Manufacturer shall be provided by the Manufacturer at least once a week or as often as reasonably requested by IDIS.
Delivery
Within 1 (one) Business Day of the receipt of an Order the Manufacturer shall provide IDIS with an estimated date for delivery. The Manufacturer will in any event give IDIS reasonable notice of the date of delivery.
The Manufacturer shall use all commercially reasonable endeavours to meet delivery dates and shall:
notify IDIS as soon as reasonably practicable of any anticipated or actual delays it experiences or anticipates experiencing in meeting an estimated delivery date;
provide IDIS with such details of the causes of such delays as IDIS reasonably requires; and
update IDIS at least once a week until the causes of such delays are rectified or lapse.
IDIS may, but is not obliged to, grant such extension of time as it considers in its sole opinion is appropriate for the Manufacturer to deliver the Products on time without breaching the Contract or this Agreement. Subject to the foregoing and without prejudice to any other rights or remedies available to IDIS, failure to meet the estimated delivery date or any subsequently agreed date or notify IDIS of any actual or anticipated delay shall entitle IDIS to terminate the Contract and/or the Agreement immediately.
Unless otherwise agreed, delivery of the Products shall take place at IDIS’ premises DDP (ICC Incoterms 2000) at Unit 3 Canada Road, Byfleet, Surrey, KT14 7JL (or such other premises as IDIS may notify to the Manufacturer from time to time) and the Manufacturer shall at its cost arrange for suitable transport to IDIS’ premises at Unit 3 Canada Road, Byfleet, Surrey, KT14 7JL (or such other premises as have been notified by IDIS to Manufacturer) and arrange insurance therefore.
Where the Products are to be delivered in instalments, without prejudice toClause 6.2, each instalment shall constitute a separate Contract and without prejudice to any other rights or remedies available to IDIS, failure by the Manufacturer to deliver any one or more of the instalments in accordance with the terms and conditions of the Agreement shall entitle IDIS to treat the Contract as a whole as repudiated and terminate the Contract as a whole immediately.
The Manufacturer shall:
fax to IDIS at its Fax Address a copy of the delivery note for each delivery or instalment on the day of delivery and supply a copy of the delivery note with the delivered Products;
inform IDIS if an Order for Products exceeds 30kg by weight when providing IDIS with the estimated date for delivery underClause 7.1.
On delivery the Products shall be marked in accordance with IDIS’s instructions and properly packed and secured so as to reach their destination in an undamaged condition in the ordinary course of events.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 15 - | | CONFIDENTIAL |

Rejection
Notwithstanding any other provision of this Agreement, acceptance of Products shall not occur until IDIS or its agent or representative has been given a reasonable time to inspect the Products for compliance with the terms and conditions of this Agreement following delivery or, if later, within a reasonable time after any latent defect in any of the Products has become apparent; provided that latent defect has become apparent before the expiry of the warranty period set out inClause 13.1.
In the event of a breach of the Manufacturer’s warranties inClauses 13.1 or13.2, or where IDIS becomes aware of a defect or latent defect pursuant toClause 8.1, IDIS may, within a reasonable time of becoming aware of such breach or defect, reject such Products by notice to the Manufacturer specifying the nature and quantity of the defective Products. Within 30 (thirty) days of receipt of such notice the Manufacturer, shall, collect the defective Products from IDIS at the Manufacturer’s expense (including, without limitation, costs of carriage, insurance, export/import duties), and:
where such Products have not yet been distributed by IDIS, replace the defective Products with Products that meet the warranties inClauses 13.1 and13.2at its own expense; or
where such Products have been distributed by IDIS, as the case may be, deduct the relevant amount from the invoice to be raised for such distribution or credit to IDIS’s account the purchase price invoiced and any applicable value added or other sales tax (where these have been paid) for such defective Products plus costs of carriage, insurance and other fees incurred by IDIS (including, without limitation, export/import duties) plus such amounts equal to the profit IDIS would have made on those Products.
Where the Manufacturer fails to collect defective Products pursuant toClause 8.2 within 14 (fourteen) days, IDIS may store defective Products on its premises or with a third party at the Manufacture’s risk and expense. Where the Manufacturer fails to collect defective Products pursuant toClause 8.2 within 30 (thirty) days IDIS may destroy the defective Products with the consent of the Manufacturer.
Payment
Within the first 5 (five) Business Days of each month IDIS shall provide the Manufacturer with a monthly sales report for the just concluded month which shall set out the quantity of Products sold by IDIS in that just concluded month and such other information as the Manufacturer may reasonably request.
Upon receipt of a monthly sales report the Manufacturer may invoice IDIS within 90 (ninety) days for the Price of the Products sold by IDIS as stated in the sales report.
Upon delivery of a monthly sales report IDIS may invoice the Manufacturer at any time for the monthly service fee and variable service fee as stated in the sales report. The amount due for both service fees shall be paid by the Manufacturer to IDIS within 30 (thirty) days from the date of the invoice.
Unless otherwise agreed, undisputed invoices shall be paid within 90 (ninety) days of the date of the Manufacturer’s invoice (in the currency stated on the Manufacturer’s invoice) by transfer to such bank account as the Manufacturer may from time to time notify in writing to IDIS.
If an invoice is disputed by IDIS, IDIS shall pay the undisputed amount in accordance withClause 9.4 and the parties shall attempt to settle the disputed part of that invoice in accordance withClause 22. Once the dispute is resolved, and if payment is due by IDIS to the Manufacturer the payment shall be made within 30 (thirty) days of the date of resolution.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 16 - | | CONFIDENTIAL |

IDIS reserves the right to decide appropriate credit terms for it’s customers or to invoice customers on a pro-forma basis, if it deems that these customers pose a high credit risk. In the event that a customer is deemed a high credit risk and cannot pay on a pro-forma basis or has an account in arrears, IDIS reserves the right not to fulfil orders for that customer unless a specific agreement is reached with the Manufacturer in accordance withClause 9.7.
Where agreed by both Parties and for specific customer orders, IDIS will not pay the Manufacturer the Price until IDIS has received payment from the customer. Where agreement is reached under thisClause 9.7, IDIS shall separately identify in the monthly sales report the customer orders to which thisClause 9.7 applies and shall promptly inform the Manufacturer of receipt of payment in respect of the relevant customer orders. Invoices issued by the Manufacturer for the Price of Products sold pursuant to the specific customer orders that are subject toClause 9.7 shall be paid by IDIS within thirty (30) days of the date of payment.
Risk and Property
Risk of damage to or loss of the Products shall pass to IDIS on delivery. Title will pass to IDIS on receipt of an order from a customer.
Rights and duties of IDIS
During the continuance of this Agreement IDIS shall:
hold and maintain all relevant wholesale dealers and import licences and comply with all applicable legal and regulatory requirements in relation to each of the Products and in particular those relating to the storage and distribution of the Products in the Territory on a Named Patient Supply basis;
unless otherwise prohibited by the applicable legal and regulatory requirements, maintain at all times a minimum stock of Products as it shall reasonably require (such minimum to be agreed between the parties from time to time);
inform the Manufacturer of customer complaints, requirements and suggestions regarding the Products and any information which comes into its possession which IDIS reasonably considers may prejudice or enhance sales of the Products in the Territory;
inform the Manufacturer of any adverse reaction(s) to the Products reported to them by customers or any other person;
if the Manufacturer or a competent regulatory authority recalls a Product, notify all customers who have purchased the relevant Product and in such circumstances and at the Manufacturer’s expense (including price and carriage charge actually and reasonably reimbursed by IDIS to the customer and any transport, an additional fixed charge of €400 per recall and €250 per Order will be charged to coveradministration and handling costs actually and reasonably incurred by IDIS), IDIS shall implement a recall and arrange for the return of the recalled Products from all relevant customers and at the Manufacturer’s option either return the same to the Manufacturer or destroy them;
not remove the Products from the packages designed for delivery to customers without the Manufacturer’s prior written approval;
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 17 - | | CONFIDENTIAL |

be entitled to describe itself as the Manufacturer’s authorised distributor of the Products in the Territory but shall not hold itself out as the Manufacturer’s agent.
Rights and duties of the Manufacturer
During the continuance of this Agreement the Manufacturer shall:
hold and maintain all relevant licences to manufacture, assemble, package and label, export from the country of manufacture (if relevant) and/or supply the Products to IDIS pursuant to this Agreement, including, without limitation, on a Named Patient Supply basis, and will comply with all relevant legal and regulatory requirements in relation to the manufacture, assembly, packaging, packing, labelling, export from the country of manufacture (if applicable) and/or supply of each of the Products;
notify and identify to IDIS any Product which becomes the subject of a Marketing Authorisation for the Indication after the Commencement Date;
reply to requests for information by IDIS regarding the Products, whether related to a technical query or otherwise, as soon as practicable but in any event no later than one Business Day after the request was received;
from time to time provide IDIS with such samples, catalogues, brochures and up to date information (including Prices) concerning the Products as IDIS may reasonably require to assist IDIS with the distribution and sale of the Products in the Territory;
shall ensure that any quotation regarding Products shall always contain its brand name, generic name, form, strength, pack size, availability and any other information reasonably requested by IDIS.
If the Manufacturer wishes to or a competent authority requires the recall of any Product, the Manufacturer shall immediately notify IDIS by telephone, fax or email (with confirmation in writing) of the recall, its urgency, providing details of the specific problem known to it, the batch number of the Products concerned and the details of any alternative Products and their prices that are available from the Manufacturer.
In the event IDIS notifies the Manufacturer of a reported adverse reaction(s) to a Product received from a customer or any other person, the Manufacturer will henceforth assume responsibility for taking any and all actions relating to such adverse reaction(s) including, without limitation, dealing with (i) all reporting aspects of pharmacovigilance to the relevant competent authority, and (ii) allegations or findings of product and/or strict liability that may be required or result from an adverse reaction(s), and shall confirm to IDIS in writing that it has done so.
The Manufacturer shall at all times supply IDIS with the minimum stock agreed by the parties pursuant toClause 11.1.2 of this Agreement. Notwithstanding the above, the Manufacturer shall use its best endeavours to supply Products to IDIS in respect of orders submitted above that agreed threshold.
The Manufacturer shall upon 60 (sixty) days written notice to IDIS be entitled to:
discontinue or vary the manufacture and assembly and/or supply of any of the Products or any substance or ingredient included in the Products;
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 18 - | | CONFIDENTIAL |

make changes in the formulation and/or composition of the Products, provided the Manufacturer is able to supply any Orders of affected Products made by IDIS prior to the date of receipt by IDIS of the notice of any such discontinuation or variation, in their unvaried form and prior to discontinuation.
Warranties
The Manufacturer accepts complete responsibility for the manufacture and assembly of the Products and their packaging and labelling, and represents and warrants to IDIS that for a period of at least 12 (twelve) months after the supply of any Products to IDIS and, if longer, up to the expiry date of any Products, the Products shall be of merchantable quality, free from defects in composition, formulation, material and workmanship.
The Manufacturer represents and warrants that:
it has title to the Products;
the Products shall conform to their description, specification and data sheet or summary of product characteristics (if any);
the Products, their manufacture, assembly, packaging and labelling, export from the country of manufacture (if relevant), use, supply to IDIS and sale, supply or distribution of the Products in the Territory by IDIS will not infringe the Intellectual Property Rights of any third party;
it is authorised to manufacture, assemble, package and label, export from the country of manufacture (if applicable) and supply the Products to IDIS for IDIS to distribute on a Named Patient Supply basis within the Territory.
IDIS represents and warrants to the Manufacturer that it is authorised to sell, supply and distribute the Products in the Territory on a Named Patient Supply basis, where permitted, in each country within the Territory as set out inSchedule 2.
Each party represents and warrants to the other that it will comply with its obligations set out in this Agreement and associated Schedules.
Indemnities
The Manufacturer shall indemnify IDIS in respect of:
the manufacture and assembly, packaging and labelling of the Products;
the sale, supply or distribution of the Products in the Territory;
failure to provide any information required to be provided by the Manufacturer to IDIS under the terms of this Agreement within the applicable time frame.
The Manufacturer shall indemnify IDIS in respect of any allegation or finding that the sale of Products by IDIS in the Territory infringes the Intellectual Property Rights of any third party or any rights of any third party unless such allegation or finding is attributable to any mark or method of packaging or get up of the Product or written material or directions relating to the Product applied, used or given by IDIS otherwise than as directed by the Manufacturer.
Each party shall indemnify the other party in respect of any breach by it of that party’s representations and warranties given in this Agreement.
The indemnities contained inClauses 14.1,14.2 and14.3 above shall be conditional in each case upon the indemnified party:
promptly giving written notice of any claim to be indemnified to the indemnifying party;
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 19 - | | CONFIDENTIAL |

providing the indemnifying party with the absolute discretion to conduct, take or resist any proceedings as it sees fit at its own expense;
providing the indemnifying party on request with such information and assistance in relation to such proceedings as it may reasonably require, subject to the indemnifying party indemnifying the other party against all costs reasonably incurred by it in the provision of such information or assistance; and
does not make any settlement, compromise or prejudicial admission in relation to such claim without the prior consent of the indemnifying party (such consent not to be unreasonably withheld or delayed).
Intellectual Property
The Manufacturer grants IDIS a royalty free licence to use the Trade Marks in the Territory on or in relation to the Products for the purposes only of exercising its rights and performing its obligations under this Agreement (including, without limitation, distributing and selling the Products).
IDIS shall not without the prior written consent of the Manufacturer:
make any modifications to the Products or their packaging or labelling;
alter, remove or tamper with any Trade Marks, numbers, or other means of identification used on or in relation to the Products;
use any of the Trade Marks in any way which might prejudice their distinctiveness or validity or the goodwill of the Manufacturer therein; or
use in relation to the Products any trade marks other than the Trade Marks without obtaining the prior written consent of the Manufacturer.
Except as provided in this Agreement IDIS shall have no rights in respect of any trade names or Trade Marks used by the Manufacturer in relation to the Products or of the goodwill associated therewith, and IDIS hereby acknowledges that, except as expressly provided in this agreement, it shall not acquire any rights in respect of any trade names or Trade Marks and that all such rights and goodwill are, and shall remain, vested in the Manufacturer.
IDIS shall, at the expense of the Manufacturer, take all such steps as the Manufacturer may reasonably require to assist the Manufacturer in maintaining the validity and enforceability of the Intellectual Property Rights of the Manufacturer during the continuance of this Agreement.
Without prejudice to the rights of IDIS or any third party to challenge the validity of any Intellectual Property Rights of the Manufacturer, IDIS shall not knowingly do or authorise any third party to do any act which would invalidate or be inconsistent with any Intellectual Property Rights of the Manufacturer and shall not knowingly omit or authorise any third party to omit to do any act which, by its omission, would have that effect or character.
Without prejudice toClause 14.4, IDIS shall notify the Manufacturer of any actual or threatened infringement in the Territory of any Intellectual Property Rights of the Manufacturer which comes to IDIS’s notice, and of any claim by any third party so coming to its notice that the importation of the Products into the Territory, or their sale in the Territory, infringes any rights of any other person, and IDIS shall at the request and expense of the Manufacturer do all such things as may be reasonably required to assist the Manufacturer in taking or resisting any proceedings in relation to any such infringement or claim.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 20 - | | CONFIDENTIAL |

Termination
Subject toClause 3.1, after the Initial Period, either party may terminate this Agreement in respect of any or all Products for any reason by giving not less than 90 (ninety) days written notice to the other to that effect, such notice to expire at or any time after the end of the Initial Period.
Before or after the expiry of the Initial Period, as the case may be, either party shall have the right to terminate this Agreement immediately by notice to the other if the other party:
commits any material or persistent breach of any of the provisions of this Agreement and, in the case of a breach capable of remedy, that party fails to remedy such breach within 30 (thirty) days after receipt of a notice giving full particulars of the breach and requiring it to be remedied;
being a company, summons a meeting of its creditors, makes a proposal for a voluntary arrangement, becomes subject to any voluntary arrangement, is unable to pay its debts within the meaning of section 123 Insolvency Act 1986, has a receiver, manager or administrative receiver appointed over any of its assets, undertakings or income, has passed a resolution for its winding-up (save for the purpose of a voluntary reconstruction or amalgamation previously approved in writing by the party serving notice), is subject to a petition presented to any Court for its winding-up (save for the purpose of a voluntary reconstruction or amalgamation previously approved in writing by the party serving notice), has a provisional liquidator appointed, has a proposal made for a scheme of arrangement under section 425 Companies Act 1985, has an administrator appointed in respect of it or is the subject of an application for administration filed at any court or a notice of appointment of an administrator filed at any court or a notice of intention to appoint an administrator given by any person or is the subject of a notice to strike off the register at Companies House or any of the foregoing ;
the other party has any distrait, execution or other process levied or enforced on any of its property;
the other party ceases, or threatens to cease to carry on business;
has any proceedings analogous to those referred to inClauses 16.2.2 to16.2.4 occurs in relation to the other party or its assets in accordance with the jurisdiction to which the other party or its assets are subject;
in the circumstances contemplated byClause 19.3 there is no agreement reached by the parties within 30 (thirty) days after discussions for that purpose began or ought to have begun.
For the purposes ofClause 16.2.1 a breach shall be considered capable of remedy if the party in breach can comply with the provision in question in all respects save as to the time of performance (provided that time of performance is not of the essence).
The rights to terminate this Agreement given by thisClause 16.2 shall be without prejudice to any other right or remedy of either party in respect of the breach concerned (if any) or any other breach.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 21 - | | CONFIDENTIAL |

Consequences of termination
Upon termination of this Agreement for any reason:
save as otherwise agreed, including, without limitation, to effectClause 17.1.3, IDIS shall immediately cease to make use of the Trade Marks;
IDIS shall be entitled to complete all Orders placed with the Manufacturer prior to the date of termination;
at the request of a party and at that party’s expense, the other party shall return or destroy any Confidential Information provided by the first party;
IDIS shall have no claim against the Manufacturer for loss of distribution rights, goodwill or other similar loss.
In the event of termination by either party underClause 16.1 or the Manufacturer underClause 16.2.1:
IDIS shall, if the Manufacturer so requests, within 10 (ten) days of the date of termination of this Agreement return all or any part of the stocks of the Products then held by IDIS to the Manufacturer at the Manufacturer’s cost of transportation and insurance, and risk. IDIS shall be responsible for arranging transportation and insurance;
IDIS shall be entitled to sell all remaining stocks that the Manufacturer does not wish to reclaim underClause 17.2.1 and for those purposes and to that extent, the provisions of this Agreement shall continue in full force and effect.
In the event of termination by IDIS underClause 16.2.1 IDIS may at its option:
within 10 (ten) days of the date of termination of this Agreement require the Manufacturer to collect all or any part of the stocks of the Products then held by IDIS at the Manufacturer’s cost of transportation and insurance and risk. IDIS shall be responsible for arranging transportation and insurance;
sell all or any remaining stocks the Manufacturer is not required to collect underClause 17.3.1 and for those purposes and to that extent, the provisions of this Agreement shall continue in full force and effect.
Clauses 13.1,13.2,14,17,18 and21 shall survive termination of this Agreement.
Confidential Information
Except as provided byClauses 18.2 and18.3, each party shall at all times during the continuance of this Agreement and after its termination:
use its best endeavours to keep all Confidential Information of the other party secret and confidential and accordingly not disclose any Confidential Information of the other party to any other person; and
not use any Confidential Information of the other party for any purpose other than for the performance of its obligations under this Agreement.
Any Confidential Information may be disclosed by a party to:
any governmental or relevant competent authority pursuant to a statutory or regulatory requirement but then only to the extent of such required disclosure;
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 22 - | | CONFIDENTIAL |

its professional advisers, lawyers, auditors and bankers; or
any of its directors or employees or of any of the aforementioned persons, to such extent only as is necessary for the purposes contemplated by this Agreement, or as is required by law and subject in each case to that party ensuring that the person in question keeps the same confidential and does not use the same except for the purposes for which the disclosure is made.
Any Confidential Information may be used by a party for any purpose, or disclosed to any other person, to the extent only that:
it is on the Commencement Date, or later becomes, public knowledge through no fault of that party (provided that in doing so that party shall not disclose any Confidential Information which is not public knowledge); or
it can be shown by that party, to the reasonable satisfaction of the other party, to have been known to the first party other than as a result of a breach of thisClause 18 prior to the same being disclosed by the other party to the first party;
that information has been lawfully obtained after the Commencement Date free of any duty of confidentiality otherwise than directly or indirectly from the other party;
the other party has given its prior written consent to such use and/or disclosure.
Force Majeure
If either party is affected by Force Majeure it shall forthwith notify the other party of the nature and extent thereof.
Neither party shall be deemed to be in breach of this Agreement, or otherwise be liable to the other, by reason of any delay in performance, or non-performance, of any of its obligations under this Agreement to the extent that such delay or non-performance is due to any Force Majeure of which it has notified the other party, and the time for performance of that obligation shall be extended accordingly.
If the Force Majeure in question prevails for a continuous period in excess of three months, the parties shall enter into bona fide discussions with a view to alleviating its effects, or to agreeing upon such alternative arrangements as may be fair and reasonable in the circumstances.
Notice
Any notice to be served by one party on the other shall be in writing and shall be served by sending it by pre-paid recorded delivery (or by pre-paid registered air mail where appropriate) to the Service Address or by fax to the Fax Address or to such other address as is notified by that party to the other from time to time in accordance with thisClause 20.
Notice shall be deemed received in the case of pre-paid recorded delivery, two days from the date of posting, in the case of registered airmail, five days from the date of posting, and in the case of fax, at the time of transmission.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 23 - | | CONFIDENTIAL |

In proving service it shall be sufficient to prove that the envelope containing such notice was correctly addressed and delivered or the notice was transmitted by fax to the Fax Address and a successful transmission sheet exists.
General
Neither party shall without the prior written consent of the other sub-contract, assign or transfer, or purport to sub-contract, assign or transfer to any other person any of its rights or obligations under this Agreement without the prior written consent of the other party, save that this Agreement will be binding upon and inure to any successor(s) to the business of either party.
Neither party nor its agents or employees shall be deemed to be an agent of the other for any purpose whatsoever, and neither party shall have, nor shall it represent itself as having, any authority to make contracts or obligations in the name of or binding upon the other party, to pledge the other party’s credit, or to extend credit to anyone in the other party’s name.
This Agreement and the documents referred to in it, including Confidentiality Agreements, contains the entire agreement between the parties in respect of the subject matter of the Agreement, and supersede all prior written or oral agreements, representations or understandings between the parties in respect thereto. The parties acknowledge that this Agreement has not been entered into wholly or partly in reliance on, nor has either party been given any warranty, statement, promise or representation made by or on their behalf other than as expressly set out in this Agreement. To the extent that any such warranties, statements, promises or representations have been given the recipient party unconditionally and irrevocably waives any claims, rights or remedies which it might otherwise have had in relation to them. Nothing in thisClause 21.3 will exclude any liability which one party would otherwise have to the other party in respect of any statements made fraudulently.
This Agreement may not be modified except in writing signed by the duly authorised representatives of each party.
In the event that any of the provisions of this Agreement or the application of any such provisions to the parties shall be held by a court of competent jurisdiction to be contrary to law, the remaining portions of this Agreement shall remain in full force and effect.
The failure or delay by either party to this Agreement in exercising any right, power or remedy of that party under this Agreement will not in any circumstances impair such right, power or remedy nor operate as a waiver of it. The single or partial exercise by either party to this Agreement of any right, power or remedy under this Agreement will not in any circumstances preclude any other or further exercise of it or the exercise of any other right, power or remedy.
Subject as expressly provided in this Agreement, the rights, powers and remedies provided in this Agreement are cumulative and not exclusive of any rights, powers and remedies provided by law.
No waiver by either party of any breach of this Agreement by the other shall be considered as a waiver of any subsequent breach of the same or any other provisions.
The parties to this Agreement do not intend that any of its terms will be enforceable by virtue of the Contracts (Rights of Third Parties) Act 1999 by any person not a party to it.
This Agreement may be executed in any number of counterparts, each of which when executed shall be construed as an original, but together will constitute one and the same instrument.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 24 - | | CONFIDENTIAL |

Disputes
Subject toClause 23, if any dispute arises out of this Agreement, in the first instance, the parties’ account managers shall attempt to resolve the dispute amicably within 30 (thirty) days.
If a dispute cannot be resolved by the parties’ account managers, the parties shall promptly refer the matter to their respective Chief Executive Officers. If either party refuses to make such referral or participate in good faith in this dispute resolution procedure and in any event, if the dispute is not resolved within 30 (thirty) days of such referral or the date such referral could have been made, then either party may commence proceedings in accordance withClause 23.
Where either party reasonably believes that a dispute relates to a material breach or potential breach of this Agreement or a Contract, that party shall notify its and the other party’s account managers and each party shall then refer the dispute directly to their Chief Executive Officers and the provisions ofClause 22.2 shall apply.
Governing Law and Jurisdiction
This Agreement shall be governed by, and construed in all respects in accordance with the laws of England.
Subject toClause 22, the courts of England will have exclusive jurisdiction to settle any disputes which may arise out of or in connection with this Agreement. The parties irrevocably agree to submit to that jurisdiction except that either party may seek injunctive relief in any court of competent jurisdiction.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 25 - | | CONFIDENTIAL |

IN WITNESS of which the parties have signed this Agreement on the date set out above.
|
| SIGNED for and on behalf of |
| MANUFACTURER |
|
|
| [signature] |
|
|
| [printed name] |
|
|
| [position in company] |
|
|
| [date] |
|
| SIGNED for and on behalf of |
| IDIS LIMITED |
|
|
| [signature] |
|
|
| [printed name] |
|
|
| [position in company] |
|
|
| [date] |
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 26 - | | CONFIDENTIAL |

SCHEDULE 1
Products
| | | | |
PRODUCT | | IDIS SERVICE FEE** | | SELLING PRICE TO
TERRITORY*** |
Onalta Kit* | | ****** | | ****** |
Onalta Radiopharmaceutical | | ****** | | ****** |
*Onalta kit to be sent in minimum order quantities of 3
** This service fee does not include carriage and packaging which will be charged at cost to the customer.
** If the parties agree to include countries in the Territory that are non-Euro (€) countries, the sales price will be quoted in $ (US) or £ sterling and will be converted from Euros at the exchange rate published in the Financial Times on the first day of the calendar month of customer invoice. These sales will then be identified in the monthly sales report provided by IDIS to the Manufacturer and invoiced by the Manufacturer to IDIS in the same currency and at the same exchange rate relative to the Euro as invoiced to the customer.
Initial Set Up Fee
IDIS will invoice the manufacturer a one off set up fee to encompass the set up of the Onalta Kit and of the Onalta Radiopharmacuetical of ****** following agreement sign-off.
Monthly Management Fee
IDIS will invoice the Manufacturer a management fee of ****** per month whilst providing access to the Onalta kit reducing to a management fee of ****** per month when it is only the Onalta Radiopharmaceutical that is being provided access via this programme. The services provided would include consultancy, provision of information and similar services and will commence at point of agreement sign off. This fee will be incorporated into the reporting as referenced inClause 9.1.
Annual Escalation
All IDIS costs (either monthly management fees or service fees) will be subject to an annual inflationary increase up to a maximum of ******. This increase will be applied on the 1st January on an annual basis throughout the duration of this Agreement.
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 27 - | | CONFIDENTIAL |
* Confidential Treatment Requested *

SCHEDULE 2
Territory
UK
France
Italy
Germany
Switzerland
Austria
France
Netherlands
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 28 - | | CONFIDENTIAL |

SCHEDULE 3
Trade Marks
Onalta
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 29 - | | CONFIDENTIAL |

SCHEDULE 4
Service
| | |
| |
| Manufacturer’s Service Address | | |
| |
| Manufacturer’s Telephone Number(s) | | |
| |
| Manufacturers Fax Address | | |
| |
| IDIS’s Service Address | | IDIS House |
| | Churchfield Road |
| | Weybridge |
| | Surrey |
| | KT13 8DB |
| | United Kingdom |
| |
| IDIS’ Telephone Number(s) | | [+44 1932 824000] |
| |
| IDIS’ Fax Address | | [+44 1932 824200] |
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 30 - | | CONFIDENTIAL |

SCHEDULE 5
Technical Agreement
| | | | |
| BioMedica Onalta™ Appendix 29.07.09 | | - 31 - | | CONFIDENTIAL |

Figure 9. Technical Agreement for Distribution Services
Technical Agreement for Distribution Services
| | |
| Contract Giver: | | Biomedica Life Sciences S.A |
| |
| Name and Address: | | Biomedica Life Sciences S.A |
| |
| | 4 Papanikoli Str |
| |
| | Chalandri |
| |
| | Attica |
| |
| | Greece |
|
| (Hereafter referred to as “Biomedica”) |
| |
| Contract Acceptor: | | IDIS Limited |
| Name and Address: | | IDIS House |
| | Churchfield Road |
| | Weybridge |
| | Surrey |
| | KT13 8DB |
| | United Kingdom |
|
| (Hereafter referred to as “IDIS”) |
| 2. | Changes to this Agreement |
| | 2.1. | All changes to this agreement must be agreed by both parties following written submission by the requesting party. |
| | | | |
No | | Details of changes / Reason | | Date |
| 1. | | | | |
| 2. | | | | |
| 3. | | | | |
| 3. | Purpose of the Agreement |
| | 3.1. | The purpose of this agreement is to describe the technical aspects of the storage and the distribution of certain finished products of Biomedica carried out by IDIS. |
| | 3.2. | The regulations of this agreement apply to all orders referring to the product list (see Schedule 1), which have been agreed upon signing the Technical Agreement and before the notice has taken effect. They also apply to orders, which have already been given to IDIS on behalf of Biomedica, as long as they have not yet been carried out. |
| | 3.3. | Biomedica is responsible for marketing the product. |
| | 4.1. | IDIS shall comply with the legal regulations that apply to its area of responsibility. |
| | 4.2. | IDIS is in the possession of a valid wholesale dealer’s licence issued by the UK Medicines and Healthcare Products Regulatory Agency. IDIS shall maintain its systems to support its licence. |
| | 4.3. | Biomedica and IDIS appoint responsible contact persons (refer to Schedule 2). |
Both must inform each other of every amendment to the agreement in writing. Amendments are also part of this agreement.
| | 4.4. | IDIS shall implement and update an effective Quality Management System (QMS), including the performance of internal audits and the implementation of the resulting corrective actions. |
| | | | |
| Biomedica Technical Agreement | | - 32 - | | 29/7/2009 |

| | 4.5. | IDIS shall ensure that all staff affected by this agreement shall be appropriately trained with the valid standard operating procedures (SOP) and further regulations of Biomedica and incorporating any necessary amendments into IDIS SOPs which are validated by Biomedica. |
| | 4.6. | IDIS shall allow the responsible persons of Biomedica to carry out quality assurance audits providing reasonable notice has been given. |
| | 4.6.1. | These audits shall be limited in scope to the area’s and systems covered by this agreement |
| | 5.1. | Biomedica is responsible for: |
| | 5.1.1. | Ensuring product and packaging elements meet the appropriate regulatory requirements. |
| | 5.1.2. | Placing all relevant information at the disposal of IDIS as well as requirements of the authorities which are necessary for the correct product handling |
5.1.2.1. All packaging shall reflect Biomedica livery.
| | 5.1.3. | Making sure that the site of manufacturer is appropriately licensed. |
| | 5.2. | IDIS is responsible for: |
| | 5.2.1. | Complying with the cGDP (current Good Distribution Practices) guidelines and ensuring that the correct products are delivered to the consignee at the right time |
| | 5.2.2. | A cGDP compliant handling of the returned products |
| | 5.2.3. | Implementing an effective system for Recall |
| | 5.2.4. | Performing visual inspection on received goods (e.g. integrity of shipment containers, completeness of shipment against shipment documents etc.) |
| | 5.2.5. | IDIS shall inform Biomedica of any negative quality or safety issues regarding Biomedica products should they become aware of any. |
| | 5.2.6. | Additional activities/requirements as defined in the ‘Supply and Distribution Agreement’: maintenance of the named patient program database, processes of a controlled distribution, and aspects with regard to drug |
| | 5.2.7. | Managing product in line with the process flow in schedule 4 |
| | 5.2.8. | Reporting any received adverse events notifications to Biomedica in accordance with the process detailed in schedule 6 |
| | 6.1. | IDIS shall manage the warehouse in accordance with cGDP as well as storing the Biomedica products delivered on behalf of Biomedica duly. |
| | 6.2. | IDIS shall protect the Biomedica products from damaging impacts and from access by unauthorized persons. |
Damaging impacts are in particular:
| | • | | damaging variations in temperature |
| | 6.3. | IDIS shall inform Biomedica immediately if Biomedica products become visibly damaged or risk getting visibly damaged. |
| | 6.4. | IDIS shall maintain the storage rooms and facilities in accordance with GDP and to calibrating measuring instruments (e.g. temperature loggers) on a regular basis in accordance with cGDP. |
| | 6.5. | IDIS shall keep the storage rooms in an orderly and tidy condition. Cleaning is done following a written cleaning program. |
| | 6.6. | IDIS shall appoint reliable personnel for the distribution orders, having the required theoretical and technical qualifications. |
| | 6.7. | IDIS shall obtain approval from Biomedica if the stored goods are transferred into another warehouse other than the one in Unit 3, Canada Road, Byfleet, Surrey, United Kingdom. The same applies to changes of locations or any change within the warehouse, which may affect the storage, and handling of the products. |
| | | | |
| Biomedica Technical Agreement | | - 33 - | | 29/7/2009 |

| | 7.1. | Biomedica shall forward IDIS the relevant product data necessary for carrying out the tasks well in advance and for keeping IDIS updated with current product information should there be any changes or amendments |
| | 7.2. | IDIS shall maintain product related data in line with regulatory requirements. |
| 8. | Storage, Delivery and Transportation |
| | 8.1. | The handling of processes is defined in the existing QMS of IDIS based on SOPs. These are: |
| | 8.1.1. | Incoming goods: Quarantine, release, documentation |
| | 8.1.2. | Handling of quality deficiencies |
| | 8.1.3. | Incoming and outgoing of goods: According to the FEFO principle (first expiry - first out), or the FIFO principle (first in - first out), respectively |
| | 8.1.4. | Commissioning and distribution of products |
| | 8.1.6. | Destruction of damaged or expired goods |
| | 8.1.7. | Drug safety procedures |
| | 9.1. | IDIS must immediately inform Biomedica if: |
| | 9.1.1. | An inspection by an authority regarding the contracting products is announced |
| | 9.1.2. | Measures are taken by competent authorities regarding Biomedica products |
| | 9.1.3. | IDIS learns about a serious product safety, quality of efficacy problem/issue |
| | 9.2. | Biomedica must inform IDIS of the supply chain details e.g. site of manufacturer, packaging/assembly, storage prior to product arriving at IDIS. This shall be listed in Schedule 4 |
| | 9.3. | Biomedica shall provide IDIS with copies of licenses held by Biomedica nominated manufacturer, packaging/assembly site or storage site. |
| | 10.1 | Information shared by either party concerning the Products will remain confidential between the parties even after the termination of this Agreement. Refer to the Confidentiality Agreement. |
| | 11.1. | Biomedica shall be responsible for full product testing in accordance with their internal procedures, specifications, methods of analysis and any relevant Marketing Authorisation of the Products detailed in Schedule 1. |
| | 11.2. | Biomedica shall be responsible for forwarding a Certificate of Analysis for each batch to IDIS. |
| | 11.3. | Biomedica shall be responsible for forwarding a Certificate of Compliance for each batch to IDIS. |
| | 12.1. | With the exception of courier services, IDIS shall not subcontract any activities effecting quality, safety and efficacy of the product without prior consent of Biomedica. |
| 13. | Table of Responsibilities |
| | | | | | |
13.1. | | Responsibilities | | Biomedica | | IDIS |
| 1 | | Adverse event notification to pharmaceutical company | | | | X |
| 2 | | Adverse event notification to local competent authority | | X | | |
| | | | |
| Biomedica Technical Agreement | | - 34 - | | 29/7/2009 |

| | | | | | |
13.2. | | Product Data & Quality Control | | Biomedica | | IDIS |
| 1 | | Transmission of product-related logistic | | X | | |
| 2 | | Correct handover and maintenance of product data | | X | | X |
| 3 | | Provide TSE Certification regarding product status to IDIS | | X | | |
| 4 | | Provide Certificate of Analysis for each product batch | | X | | |
| 5 | | Provide Certificate of Compliance for each product batch | | X | | |
| | | |
13.3. | | Storage, Delivery and Transportation | | Biomedica | | IDIS |
| 1 | | Delivery of products in conformity with the local market as nominated by Biomedica | | | | X |
| 2 | | Determination of the storage conditions and way of shipment | | X | | |
| 3 | | Adherence to storage conditions and way of shipment to user | | | | X |
| 4 | | Compliance with cGDP guidelines | | | | X |
| 5 | | Handling of returned goods | | | | X |
| 6 | | Handling of technical product complaints | | X | | X |
| 7 | | Recall procedures | | X | | X |
| 8 | | Incoming goods, control, inventory update | | X | | X |
| 9 | | Adherence to FEFO principle | | | | X |
| 10 | | Shipments of products | | | | X |
| 11 | | Destruction of damaged, returned and expired goods | | | | X |
| | | | |
| Biomedica Technical Agreement | | - 35 - | | 29/7/2009 |

IN WITNESS whereof this Agreement has been entered into the day and year written.
| | |
| SIGNED for and on behalf of |
| Biomedica |
|
|
| Name: | | [ ] |
| Title: | | [ ] |
| Date: | | |
|
| SIGNED for and on behalf of |
| IDIS (TRADING AS IDIS LIMITED) |
|
|
| Name: | | Maria Kempshall |
| Title: | | Responsible Person |
| Date: | | |
|
|
| Name: | | Tony Dutta |
| Title: | | General Manager |
| Date: | | |
| | | | |
| Biomedica Technical Agreement | | - 36 - | | 29/7/2009 |

Schedule 1 – Product List
| | |
| Brand Name: | | Onalta Kit |
| |
| Generic Name: | | |
| |
| Strength: | | |
| |
| Presentation: | | |
| |
| Form: | | |
| |
| Storage: | | |
| |
| Pack size: | | |
| |
| Therapeutic Area: | | |
| |
| Product codes: | | |
| |
| Brand Name: | | Onalta |
| |
| Generic Name: | | 90Y-edotreotide |
| |
| Strength: | | 100 ml glass vial containing 80 mL of stabilized aqueous solution. |
| | Radioactivity concentration: 1.2-1.8 mCi / mL (TOC) |
| |
| Presentation: | | Liquid in Vial |
| |
| Form: | | Aqueous solution |
| |
| Storage: | | 90Y-edotreotide is shipped at ambient temperature, should be stored at room temperature (25 ± 2°C), and should be used by the expiration date and time printed on the label. It is Radioactive. DGR. |
| |
| | The 90Y-edotreotide vial will be labelled and packaged within a lead shield to reduce radiation emission and in accordance with radioactive pharmaceutical requirements. |
| |
| Pack size: | | One Vial |
| |
| Therapeutic Area: | | Oncology |
| |
| Product codes: | | |
| | | | |
| Biomedica Technical Agreement | | - 37 - | | 29/7/2009 |

Schedule 2 – Contact Persons
| | | | |
| Contract Acceptor | | Contract Giver |
| Tony Dutta |
| General Manager |
|
| IDIS House |
| Churchfield Road |
| Weybridge |
| Surrey |
| KT13 8DB |
| United Kingdom |
|
| Telephone No: 01932 824000 |
| Fax No: 01932 824200 |
|
| Maria Kempshall |
| Responsible Person |
|
| At above address |
|
| Direct Telephone No: 01932 824031 |
| Email address:mkempshall@idispharma.com |
|
| Alliance Development |
|
| Colleen Grant |
| Alliance Manager |
|
| At above address |
| Tel: | | 01932 824 147 |
| E-mail: | | cgrant@idispharma.com |
|
| Alliance Management |
|
| Maxine Hamilton |
| Global Alliance Manager |
|
| At above address |
| Tel: | | 01932 824 105 |
| Mobile: | | 07740 720 233 | | |
| E-mail: | | mhamilton@idispharma.com |
| | | | |
| Biomedica Technical Agreement | | - 38 - | | 29/7/2009 |

Schedule 3 – Supply Chain Details
Onalta Kit
| | |
| Site of manufacturer: | | (require full postal address) |
| |
| Site of packaging/assembly: | | (require full postal address) |
| | |
|
| Site of storage prior to product arriving at IDIS: (Please enter details) |
|
| Contact for placement of purchase orders: |
| | |
| Product lead-time from placement of purchase order: | | please advise |
| |
| Contact for Invoicing: | | please advise |
| |
| Contact for provision of monthly sales report: | | Please advise of further names |
Onalta
| | |
| Site of manufacturer: | | (require full postal address) |
| |
| Site of packaging/assembly: | | (require full postal address) |
| | |
| |
| Site of storage prior to dispatch to hospitals: | | (Please enter details) |
| |
| Contact for placement of purchase orders: | | |
| | |
| Product lead-time from placement of purchase order: | | please advise |
| |
| Contact for Invoicing: | | please advise |
| |
| Contact for provision of monthly sales report: | | Please advise of further names |
| | | | |
| Biomedica Technical Agreement | | - 39 - | | 29/7/2009 |

Schedule 4
Order Management Process Flow Onalta Kit
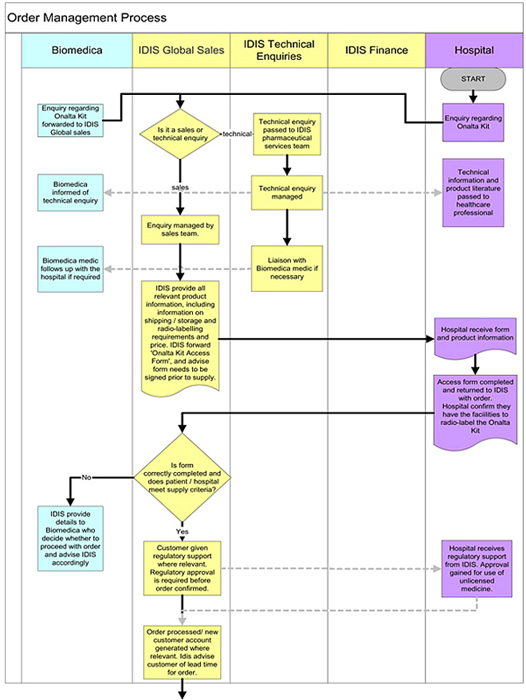
| | | | |
| Biomedica Technical Agreement | | - 40 - | | 29/7/2009 |

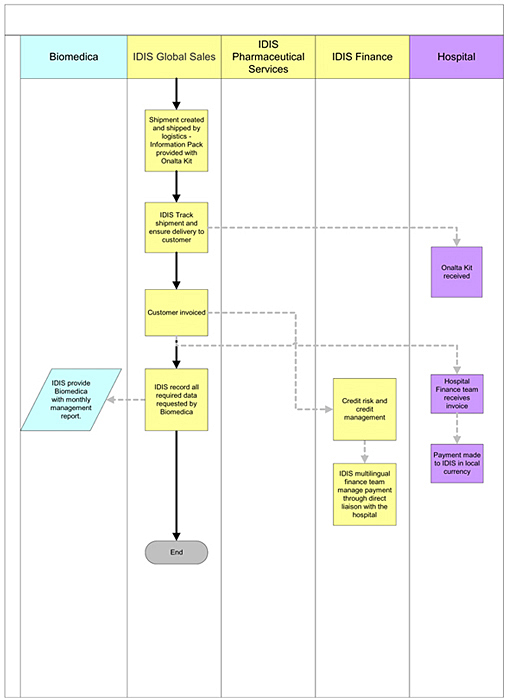
| | | | |
| Biomedica Technical Agreement | | - 41 - | | 29/7/2009 |

Order Management Process Flow Onalta
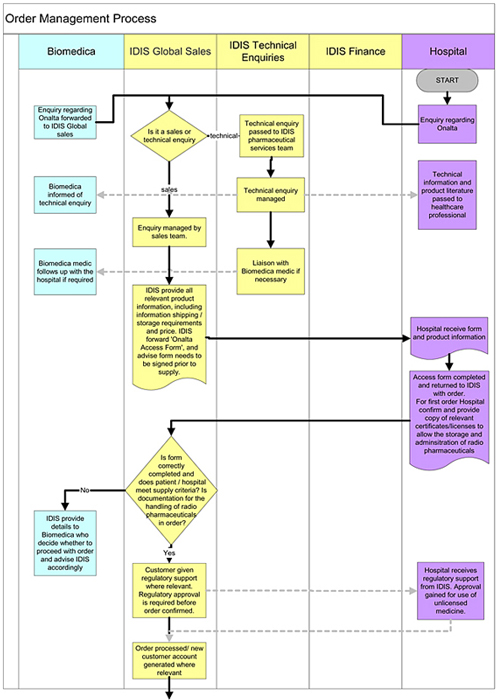
| | | | |
| Biomedica Technical Agreement | | - 42 - | | 29/7/2009 |

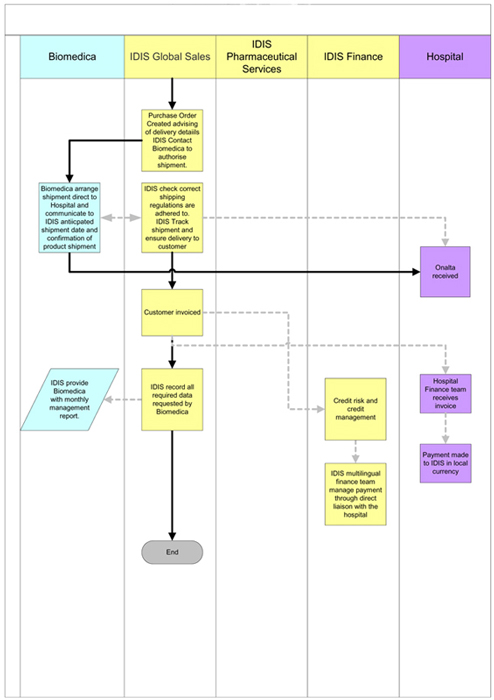
| | | | |
| Biomedica Technical Agreement | | - 43 - | | 29/7/2009 |

Schedule 5
Process Map: Product Management Onalta Kit
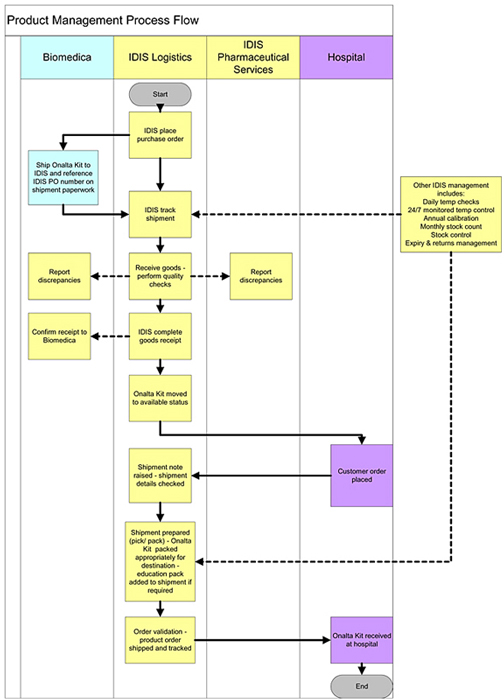
| | | | |
| Biomedica Technical Agreement | | - 44 - | | 29/7/2009 |

Process Map: Product Management Onalta
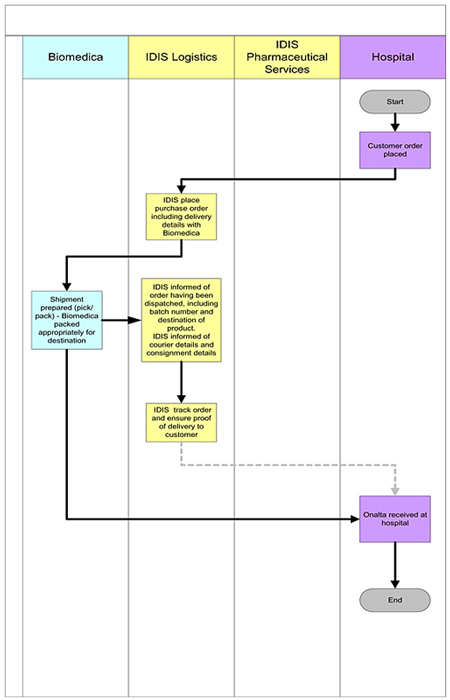
| | | | |
| Biomedica Technical Agreement | | - 45 - | | 29/7/2009 |

Schedule 6 – Pharmacovigilance
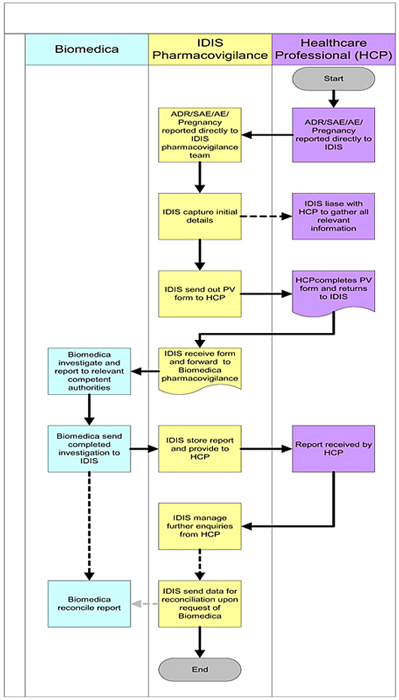
| | | | |
| Biomedica Technical Agreement | | - 46 - | | 29/7/2009 |

| | |
Biomedica Pharmacovigilance Representatives |
| |
Contacts: | | (TBC – please complete) |
| |
Address: | | |
| |
Telephone No: | | + |
| |
Fax No: | | + |
| |
Email: | | |
|
IDIS Pharmacovigilance Representatives |
| |
Telephone : | | +44 (0) 1932 824 026 |
| |
Fax : | | +44 (0) 1932 824 226 - FAO Technical Enquiries |
| |
Email : | | ps@idispharma.com |
| | | | |
| Biomedica Technical Agreement | | - 47 - | | 29/7/2009 |
EXHIBIT B
INTELLECTUAL PROPERTY
Generic Patent (100-7382)
| | | | | | |
Country | | Grant. Date | | Patent No. | | Exp. Date |
Australia | | 04.06.93 | | 633859 | | 04.12.09 |
Austria | | 09.12.97 | | 403476 | | 30.11.09 |
Belgium | | 20.11.90 | | 1002296 | | 05.12.09 |
Canada | | 22.02.00 | | 2004532 | | 04.12.09 |
Cyprus | | 01.12.95 | | 1893 | | 01.12.09 |
Denmark | | 30.08.04 | | 175338 | | 05.12.09 |
Finland | | 30.09.98 | | 101967 | | 11.07.14 |
Finland | | 31.12.98 | | 102540 | | 04.12.09 |
France | �� | 21.04.95 | | 8915993 | | 04.12.09 |
Germany | | 20.04.06 | | 3991505 | | 30.11.09 |
Great Britain | | 20.01.93 | | 2225579 | | 01.12.09 |
Greece | | 26.11.96 | | 1002475 | | 05.12.09 |
Hong Kong | | 21.12.95 | | 1899/95 | | 01.12.09 |
Hungary | | 20.09.95 | | 211468 | | 01.12.09 |
Ireland | | 05.12.94 | | 62091 | | 04.12.09 |
Israel | | 25.09.94 | | 92534 | | 04.12.09 |
Italy | | 19.10.93 | | 1239285 | | 05.12.09 |
Japan | | 10.06.05 | | 3686503 | | 04.12.09 |
Japan | | 05.12.97 | | 2726320 | | 04.12.09 |
Korea-South | | 22.07.98 | | 156541 | | 22.07.13 |
Luxemburg | | 18.09.91 | | 87633 | | 05.12.09 |
Malaysia | | 31.03.95 | | 106120 | | 31.03.10 |
Netherlands | | 03.04.03 | | 194828 | | 04.12.09 |
New Zealand | | 20.01.94 | | 231623 | | 04.12.09 |
Nigeria | | 13.04.93 | | 10732 | | 05.12.09 |
Pakistan | | 21.03.92 | | 132014 | | 05.12.00 |
Philippines | | 07.05.96 | | 29649 | | 07.05.13 |
Poland | | 28.09.93 | | 163432 | | 04.12.09 |
Portugal | | 20.10.95 | | 92487 | | 20.10.10 |
Saudi Arabia* | | | | | | 01.01.09 |
Singapore | | 15.04.97 | | 38709 | | 01.12.09 |
South Africa | | 28.08.91 | | 89/9285 | | 05.12.09 |
Spain | | 22.11.91 | | 2023533 | | 05.12.09 |
Sweden | | 09.11.98 | | 8904087-7 | | 04.12.09 |
Switzerland | | 30.08.91 | | 678329-6 | | 30.11.09 |
Tanganyika | | 30.09.97 | | 2533 | | 01.12.09 |
Trinidad+Tobago | | 14.11.95 | | 78/95 | | 01.12.09 |
USA | | 19.05.98 | | 5753627 | | 06.06.15 |
USA | | 07.07.98 | | 5776894 | | 07.07.15 |
| * | Saudi Arabia: patent application still pending, filing number 95160495 |
- 1 -
Additional Use Patent (118-7595)
| | | | | | |
Country | | Grant. Date | | Patent No. | | Exp. Date |
Belgium | | 05.01.93 | | 1004645 | | 19.02.11 |
France | | 20.01.95 | | 9101993 | | 18.02.11 |
Germany* | | | | | | 13.02.11 |
Great Britain | | 20.04.94 | | 2241167 | | 18.02.11 |
Hong Kong | | 10.04.97 | | 434/97 | | 18.02.11 |
Italy | | 15.07.94 | | 1244496 | | 19.02.11 |
Japan | | 15.03.02 | | 3288055 | | 21.02.11 |
Switzerland | | 28.02.94 | | 683318-4 | | 19.02.11 |
USA | | 26.09.00 | | 6123916 | | 26.09.17 |
| * | Germany: patent application still pending. Filing number: P4104308.1 |
Novartis Specific Patents
| | | | | | | | | | |
Country | | Appl. Date | | Appl. No. | | Grant. Date | | Patent No. | | Exp. Date |
Argentina | | 05.09.95 | | 333413 | | 22.03.04 | | AR256010M | | 05.09.15 |
Australia | | 04.09.95 | | 30414/95 | | 24.06.99 | | 703057 | | 04.09.15 |
Austria | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Belgium | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Brazil | | 05.09.95 | | PI9503936-8 | | 22.06.04 | | PI9503936-8 | | 05.09.15 |
Canada | | 05.09.95 | | 2157530 | | | | | | 05.09.15 |
Chile | | 05.09.95 | | 1351/95 | | 10.10.00 | | 40732 | | 10.10.15 |
China | | 05.09.95 | | 115610/95 | | 19.01.05 | | ZL95115610.1 | | 05.09.15 |
Colombia | | 05.09.95 | | 95040393 | | 30.07.01 | | 27134 | | 05.09.15 |
Czech Republic | | 04.09.95 | | PV1995-2263 | | 14.06.00 | | 287012 | | 04.09.15 |
Denmark | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Ecuador | | 05.09.95 | | SP 95-1528 | | 20.06.97 | | PI 97-1158 | | 05.09.15 |
Europe | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Finland | | 04.09.95 | | 4147/95 | | 13.10.06 | | 117424 | | 04.09.15 |
France | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Germany | | 04.09.95 | | 69520256.1 | | 07.03.01 | | 714911 | | 04.09.15 |
Great Britain | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Greece | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Hungary | | 04.09.95 | | 2577/95 | | 10.05.00 | | 218284 | | 04.09.15 |
India | | 24.08.95 | | 1092/MAS/95 | | | | | | 24.08.15 |
Ireland | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
- 2 -
| | | | | | | | | | |
Country | | Appl. Date | | Appl. No. | | Grant. Date | | Patent No. | | Exp. Date |
Israel | | 04.09.95 | | 115154 | | 14.11.00 | | 115154 | | 04.09.15 |
Italy | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Japan | | 05.09.95 | | 227906/95 | | 07.04.00 | | 3054346 | | 05.09.15 |
Korea-South | | 05.09.95 | | 28905/95 | | 27.11.02 | | 364111 | | 05.09.15 |
Luxemburg | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Malaysia | | 05.09.95 | | PI95002626 | | | | | | 05.09.15 |
Mexico | | 04.09.95 | | 9503785 | | 29.03.00 | | 195731 | | 04.09.15 |
Netherlands | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
New Zealand | | 04.09.95 | | 272919 | | 17.04.97 | | 272919 | | 04.09.15 |
Norway | | 04.09.95 | | 19953457 | | 23.02.04 | | 316569 | | 04.09.15 |
Pakistan | | 03.09.95 | | 467/95 | | 03.09.97 | | 134791 | | 06.09.10 |
Peru | | 04.09.95 | | 277844 | | 28.02.01 | | 001736 | | 04.09.15 |
Philippines | | 04.09.95 | | 51237 | | 29.10.04 | | 1-1995-51237 | | 29.10.21 |
Poland | | 04.09.95 | | P310274 | | 10.05.01 | | 182434 | | 04.09.15 |
Portugal | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Russia | | 04.09.95 | | 95114740 | | 13.04.00 | | 2156774 | | 04.09.15 |
Singapore | | 04.09.95 | | 9501282-9 | | 16.11.01 | | 50356 | | 04.09.15 |
Slovak Republic | | 04.09.95 | | 1088/95 | | 04.11.03 | | 283774 | | 04.09.15 |
Slovenia | | 04.09.95 | | P9530491 | | 07.03.01 | | 714911 | | 04.09.15 |
South Africa | | 06.09.95 | | 95/7475 | | 28.05.97 | | 7475/95 | | 06.09.15 |
Spain | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Sweden | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Switzerland | | 04.09.95 | | 95810545.4 | | 07.03.01 | | 714911 | | 04.09.15 |
Taiwan | | 08.09.95 | | 84-109395 | | 16.08.00 | | 114095 | | 08.09.15 |
Thailand | | 04.09.95 | | 27824/95 | | 08.01.02 | | 11623 | | 04.09.15 |
Turkey | | 06.09.95 | | 1094/95 | | 22.04.02 | | TR199501094B | | 06.09.15 |
USA | | 23.04.97 | | 09/609844 | | 21.08.01 | | 6277356 | | 01.09.15 |
USA | | 23.04.97 | | 08/842125 | | 06.02.01 | | 6183721 | | 01.09.15 |
Venezuela | | 06.09.95 | | 1572-95 | | 08.01.99 | | | | 06.09.15 |
- 3 -
EXHIBIT C
FORMULAIC DISTRIBUTION OF CONTIGENT MILESTONE PAYMENT
CALCULATIONS
The apportionment of the payments, should they become due, will be as follows:
Milestone payment A (5.4.3.1): Proportionately paid by all companies contributing to the generation of ***** in annual revenue. If only one company has generated revenue then the entire ***** will be due from that company. If two or more companies have generated the revenue then the payment will be divided in direct proportion to the revenues generated by that company, in that year.
EXAMPLE: Company A generates ***** revenue and Company B generates ***** revenue.
Milestone payment due from Company A: *****
Milestone payment due from Company B: *****
Milestone payment B (5.4.3.2): Proportionately paid by all companies contributing to the generation of ***** in revenue. If only one company has generated revenue then the entire ***** will be due from that company. If two or more companies have generated the revenue then the payment will be divided in direct proportion to the revenues generated by that company, in that year.
EXAMPLE: Company A generated ***** revenue and Company B generated ***** revenue, Company C *****
Payment due from Company A: *****
Payment due from Company B: *****
Payment due from Company C: *****
Since Company C did not participate in the payment of Milestone Payment (A) it will be required to contribute funds back to Companies A & B to compensate them for their initial development of the market, thus enabling the growth to *****. The amount that will be contributed is equal to the proportion generated by Company C in generating ***** multiplied by Milestone (A).
Total required payment to Companies A & B from Company C: *****
* Confidential Treatment Requested *
- 1 -
Distribution of Payment from Company C will be in the same proportion that Milestone Payment (A) was paid.
Funds paid to Company A: *****
Funds paid to Company B: *****
Milestone payment C (5.4.3.3): Proportionately paid by all companies contributing to the generation of ***** in revenue. If only one company has generated revenue then the entire ***** will be due from that company. If two or more companies have generated the revenue then the payment will be divided in direct proportion to the revenues generated by that company, in that year.
EXAMPLE 1: Company A generated ***** revenue and Company B generated ***** revenue, Company C *****
Payment due from Company A: *****
Payment due from Company B: *****
Payment due from Company C: *****
Since all three companies have paid for both Milestone (A) and Milestone (B) no further payment would be due in this example.
EXAMPLE 2: If a fourth company had contributed to the achievement of the milestone, it would be required to make a market development payment to the other three companies for its share of both Milestone (A) and Milestone (B). The methodology for determining the payment due, for each milestone, would be the same as that employed in the Milestone (B) example. The methodology for distribution of the payment would be as follows:
Milestone (A) Distribution
To Company A :*****
To Company B: *****
To Company C: *****
Milestone (B) Distribution
To Company A: *****
To Company B: *****
To Company C: *****
* Confidential Treatment Requested *
- 2 -
EXHIBIT D
EXAMPLES OF PERMITTED DISCLOSURES
1) Phase 3 Study:
2) Acceptance by EMEA of the MAA application
3) EMEA approval of the MAA
4) Any presentation or publication of data from the clinical development of Onalta™ by BIOMEDICA.
5) Any public disclosure of trial results (e.g. safety, efficacy, primary or secondary endpoints) by BIOMEDICA
6) Final approval by any EU Regulatory Authority subsequent to EMEA approval.
7) Commencement of commercialization of Onalta.
- 3 -












