
Noninfectious Anterior Uveitis Phase II Results and Ocular Clinical Development Update May 9, 2016 Exhibit 99.2

Disclaimers and Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations and expenses, business strategies and plans, research and development plans or expectations, trends, market sizing, competitive position, industry environment and potential growth opportunities, among other things. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements include, among others, the timing of commencement, enrollment and completion of Aldeyra’s clinical trials; the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; the ability to obtain and maintain regulatory approval to commercialize Aldeyra’s product candidates, and the labeling for any approved products; the scope, progress, expansion, and costs of developing and commercializing Aldeyra’s product candidates; the size and growth of the potential markets for Aldeyra’s product candidates and the ability to serve those markets; Aldeyra’s expectations regarding its expenses and revenue, the sufficiency or use of Aldeyra’s cash resources and needs for additional financing; the rate and degree of market acceptance of any of Aldeyra’s product candidates; Aldeyra’s expectations regarding competition; Aldeyra’s anticipated growth strategies; Aldeyra’s ability to attract or retain key personnel; Aldeyra’s ability to establish and maintain development partnerships; Aldeyra’s expectations regarding federal, state and foreign regulatory requirements; regulatory developments in the United States and foreign countries; Aldeyra’s ability to obtain and maintain intellectual property protection for its product candidates; the anticipated trends and challenges in Aldeyra’s business and the market in which it operates; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2016, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at www.sec.gov. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of May 9, 2016, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.
Aldehydes Are Mediators of Inflammation NS2 is a first-in-class aldehyde trap and is the lead compound in a novel anti-inflammatory platform. Aldehydes Protein Signaling via Thiol and Amine Binding Inflammasome , NF-KB Activation Cytokine Release
The Aldehyde Trap Ocular Anti-Inflammatory Platform ü ü NS2 has demonstrated activity in the two major types of inflammation, and has the potential to be efficacious in a wide variety of inflammatory disease. Allergy (TH2 Cytokines) Atopic Kerato -Conjunctivitis, etc. Allergic Conjunctivitis Autoimmune (TH1 Cytokines) Cyclitis , Iritis, Scleritis , DES, etc. Uveitis
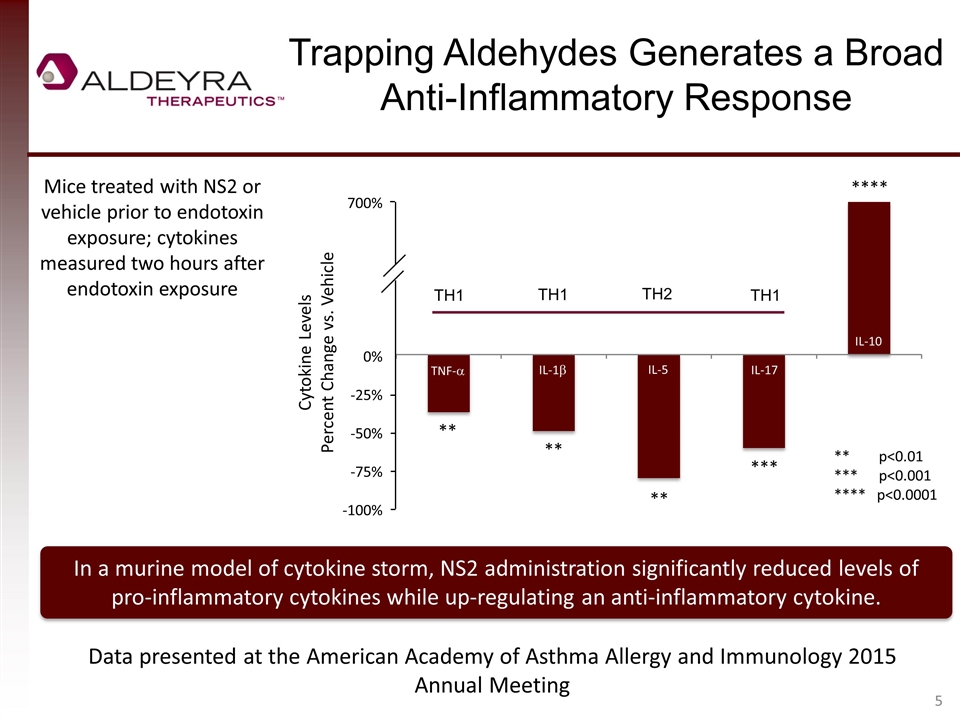
Trapping Aldehydes Generates a Broad Anti-Inflammatory Response In a murine model of cytokine storm, NS2 administration significantly reduced levels of pro-inflammatory cytokines while up-regulating an anti-inflammatory cytokine. Mice treated with NS2 or vehicle prior to endotoxin exposure; cytokines measured two hours after endotoxin exposure ** p<0.01 *** p<0.001 **** p<0.0001 ** ** *** ** Data presented at the American Academy of Asthma Allergy and Immunology 2015 Annual Meeting **** 700% 0% -25% -50% -75% -100% TNF-a IL-1b IL-5 IL-17 IL-10 Cytokine Levels Percent Change vs. Vehicle TH1 TH2 TH1 TH1
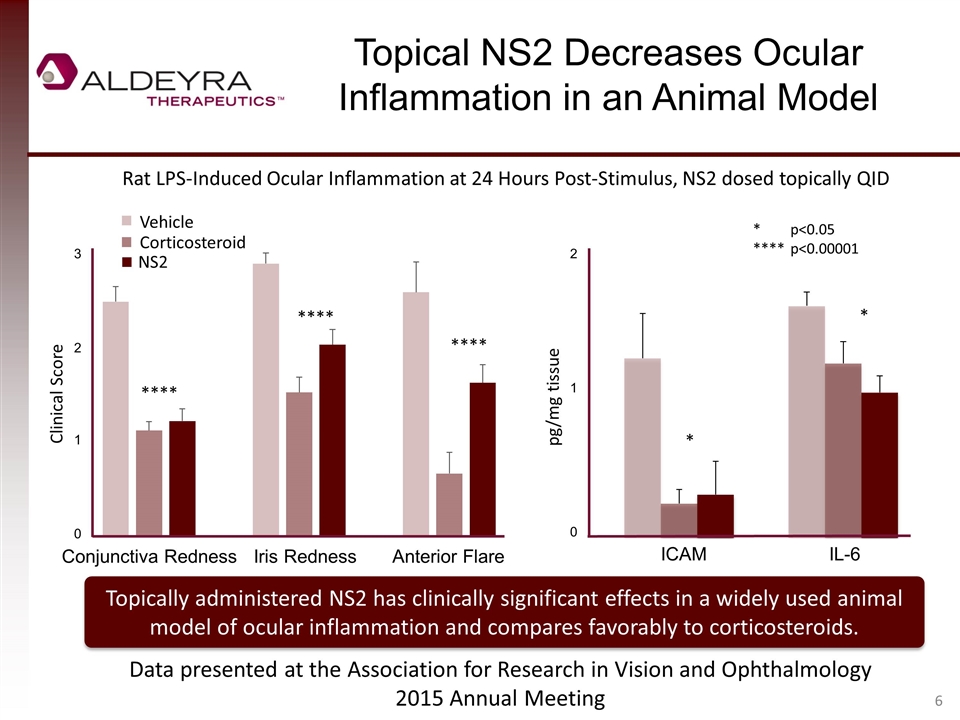
Topical NS2 Decreases Ocular Inflammation in an Animal Model pg/mg tissue * *p<0.05 ****p<0.00001 Rat LPS-Induced Ocular Inflammation at 24 Hours Post-Stimulus, NS2 dosed topically QID Topically administered NS2 has clinically significant effects in a widely used animal model of ocular inflammation and compares favorably to corticosteroids. Clinical Score Data presented at the Association for Research in Vision and Ophthalmology 2015 Annual Meeting * 3 2 1 0 Conjunctiva RednessIris Redness Anterior Flare 2 1 0 ICAM IL-6 **** **** **** NS2 Corticosteroid Vehicle
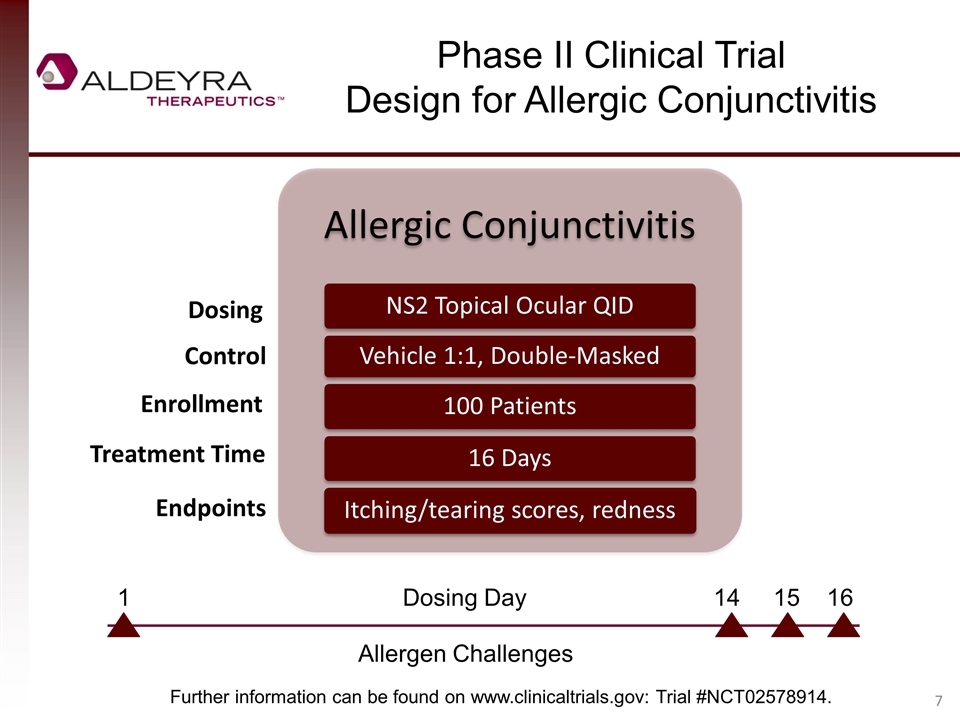
Phase II Clinical Trial Design for Allergic Conjunctivitis Dosing Control Enrollment Treatment Time Endpoints Further information can be found on www.clinicaltrials.gov: Trial #NCT02578914. Allergen Challenges 1 Dosing Day 14 15 16 Allergic Conjunctivitis NS2 Topical Ocular QID Vehicle 1:1, Double-Masked 100 Patients 16 Days Itching/tearing scores, redness
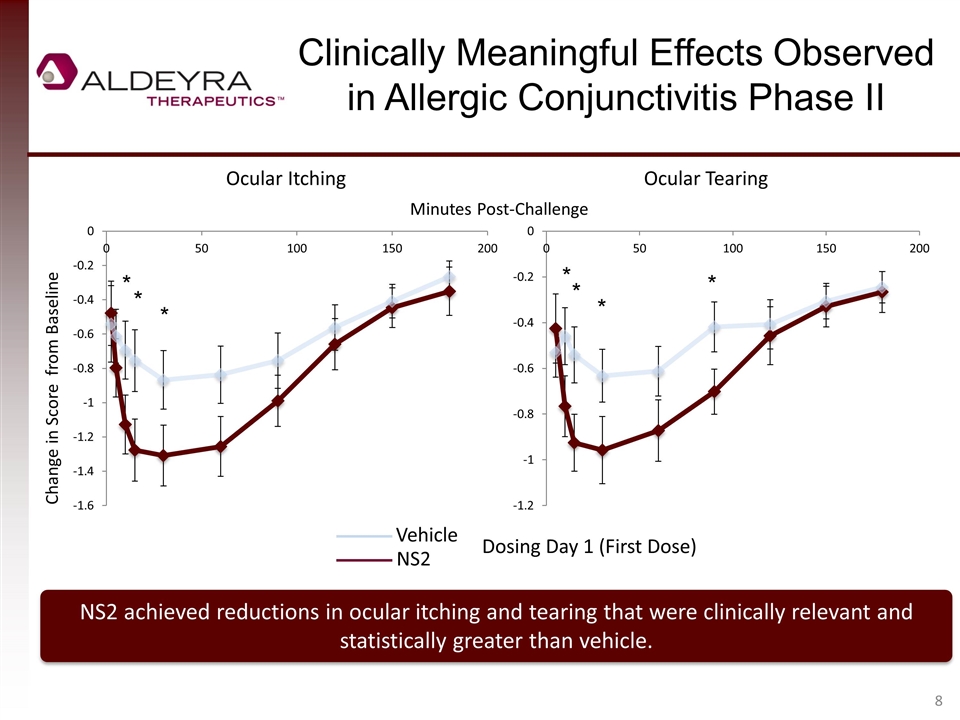
Clinically Meaningful Effects Observed in Allergic Conjunctivitis Phase II NS2 achieved reductions in ocular itching and tearing that were clinically relevant and statistically greater than vehicle. Change in Score from Baseline Ocular Itching Ocular Tearing Vehicle NS2 Dosing Day 1 (First Dose) Minutes Post-Challenge * * * * * * *
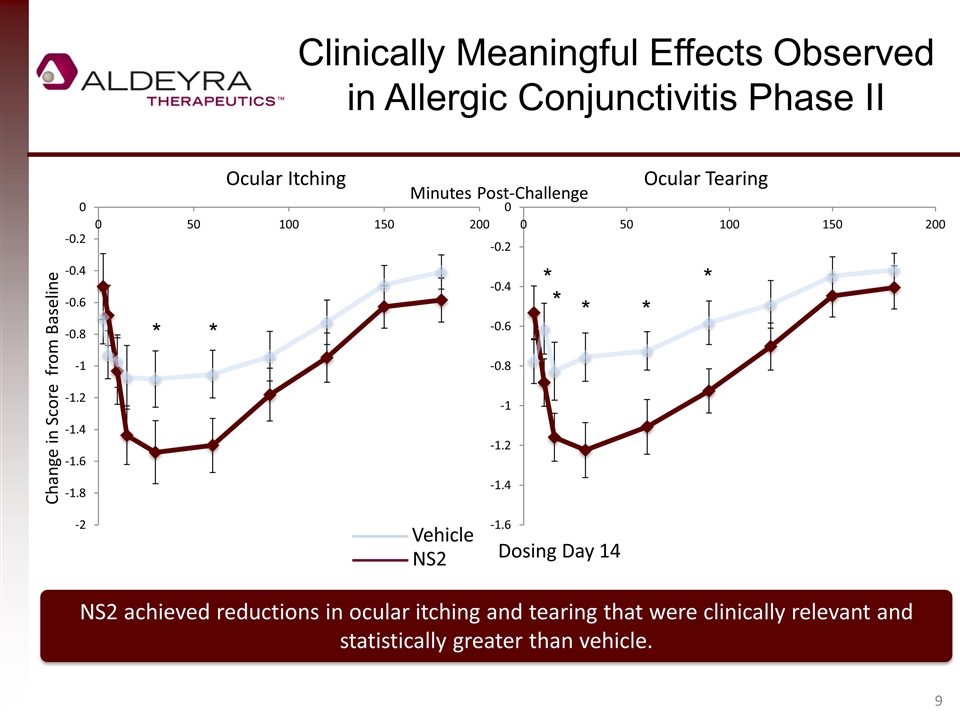
Clinically Meaningful Effects Observed in Allergic Conjunctivitis Phase II NS2 achieved reductions in ocular itching and tearing that were clinically relevant and statistically greater than vehicle. Change in Score from Baseline Ocular Itching Ocular Tearing Vehicle NS2 Dosing Day 14 Minutes Post-Challenge * * * * * * *
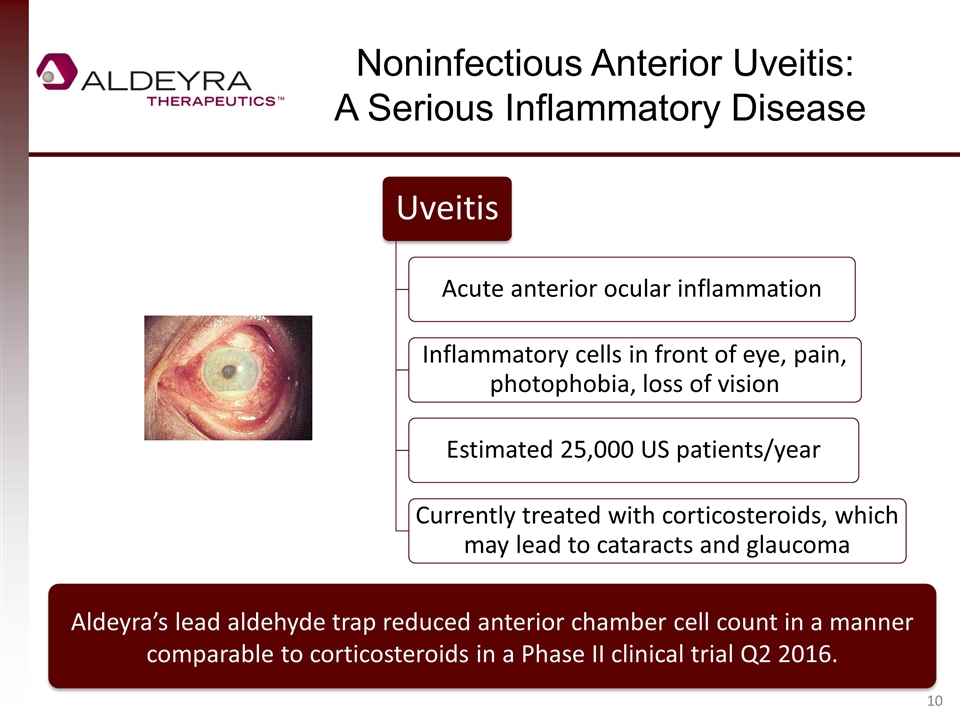
Noninfectious Anterior Uveitis: A Serious Inflammatory Disease Aldeyra’s lead aldehyde trap reduced anterior chamber cell count in a manner comparable to corticosteroids in a Phase II clinical trial Q2 2016. Uveitis Acute anterior ocular inflammation Inflammatory cells in front of eye, pain , photophobia, loss of vision Estimated 25,000 US patients/year Currently treated with corticosteroids , which may lead to cataracts and glaucoma
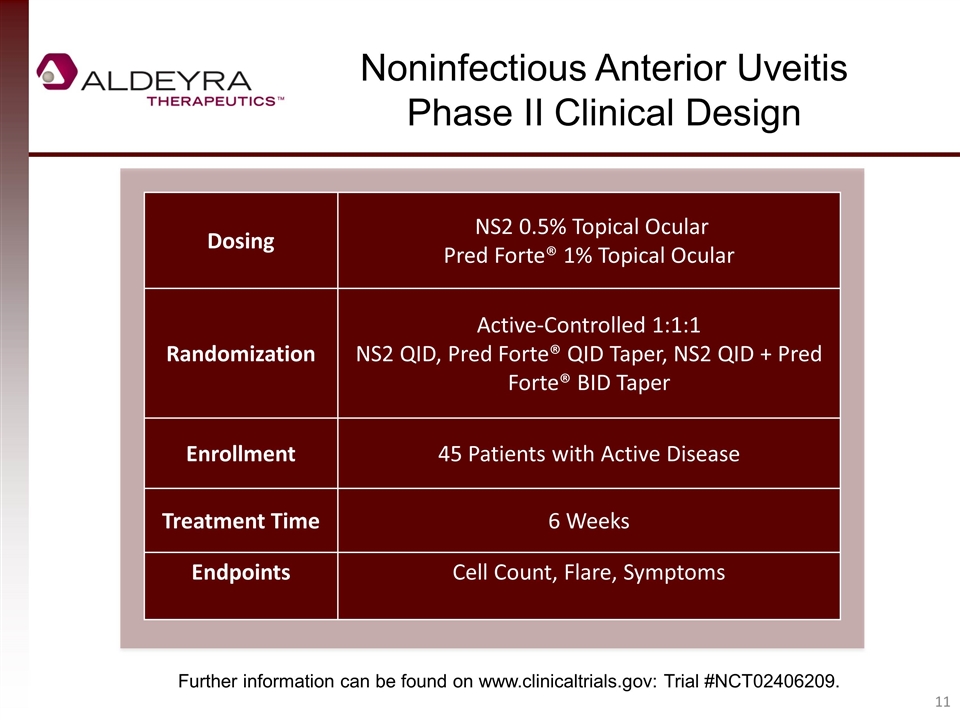
Noninfectious Anterior Uveitis Phase II Clinical Design Dosing NS2 0.5% Topical Ocular Pred Forte® 1% Topical Ocular Randomization Active-Controlled 1:1:1 NS2 QID, Pred Forte® QID Taper, NS2 QID + Pred Forte® BID Taper Enrollment 45 Patients with Active Disease Treatment Time 6 Weeks Endpoints Cell Count, Flare, Symptoms Further information can be found on www.clinicaltrials.gov: Trial #NCT02406209.
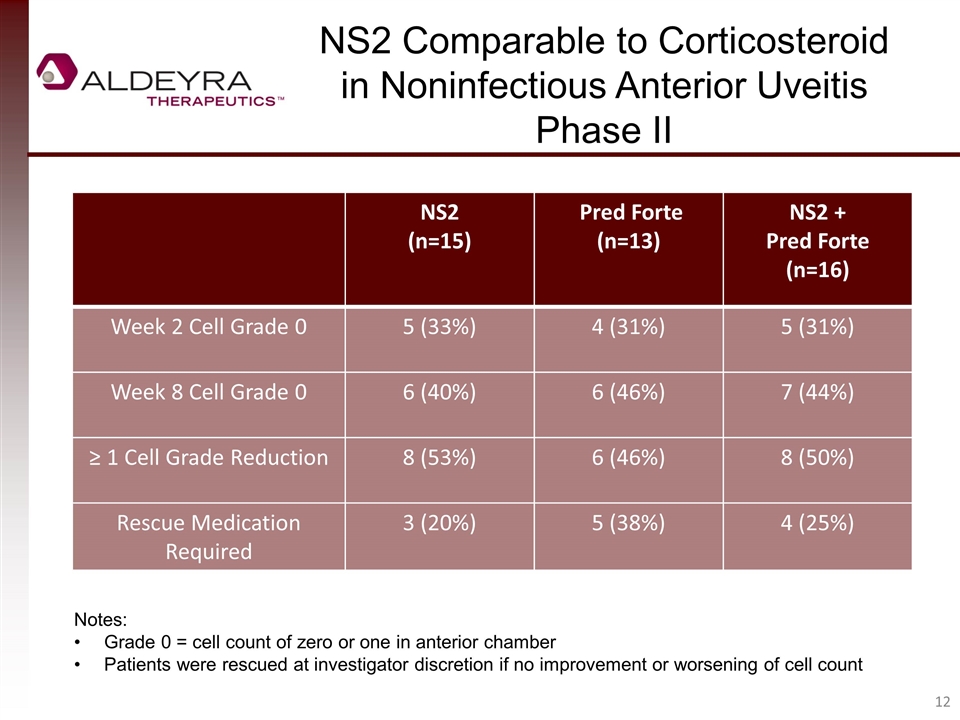
NS2 (n=15) Pred Forte (n=13) NS2 + Pred Forte (n=16) Week 2 Cell Grade 0 5 (33%) 4 (31%) 5 (31%) Week 8 Cell Grade 0 6 (40%) 6 (46%) 7 (44%) ≥ 1 Cell Grade Reduction 8 (53%) 6 (46%) 8 (50%) Rescue Medication Required 3 (20%) 5 (38%) 4 (25%) NS2 Comparable to Corticosteroid in Noninfectious Anterior Uveitis Phase II Notes: Grade 0 = cell count of zero or one in anterior chamber Patients were rescued at investigator discretion if no improvement or worsening of cell count
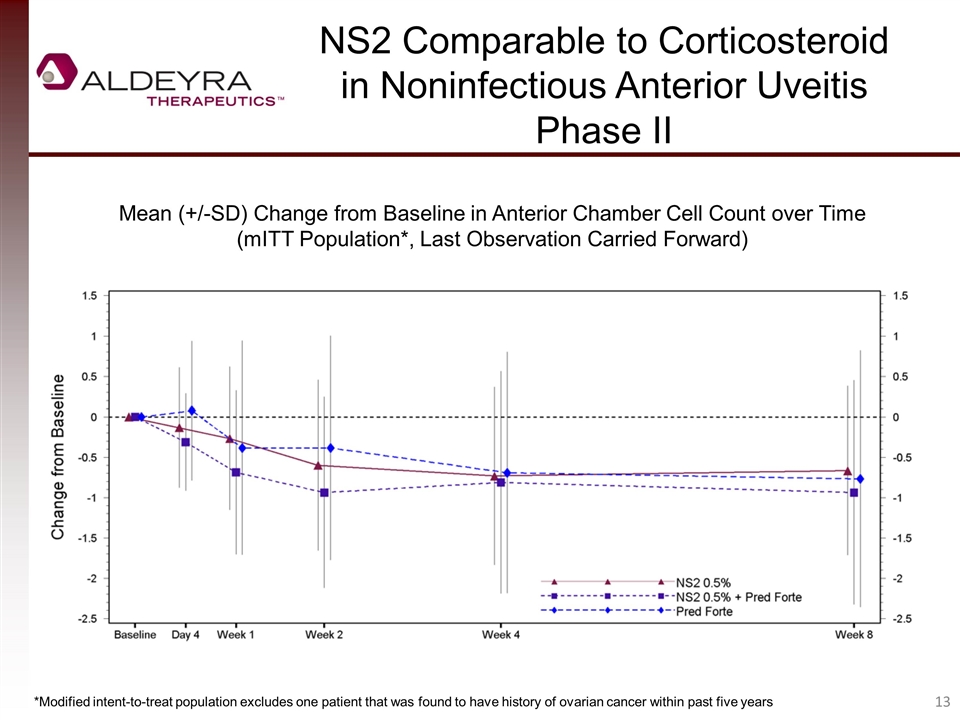
NS2 Comparable to Corticosteroid in Noninfectious Anterior Uveitis Phase II Mean (+/-SD) Change from Baseline in Anterior Chamber Cell Count over Time (mITT Population*, Last Observation Carried Forward) *Modified intent-to-treat population excludes one patient that was found to have history of ovarian cancer within past five years
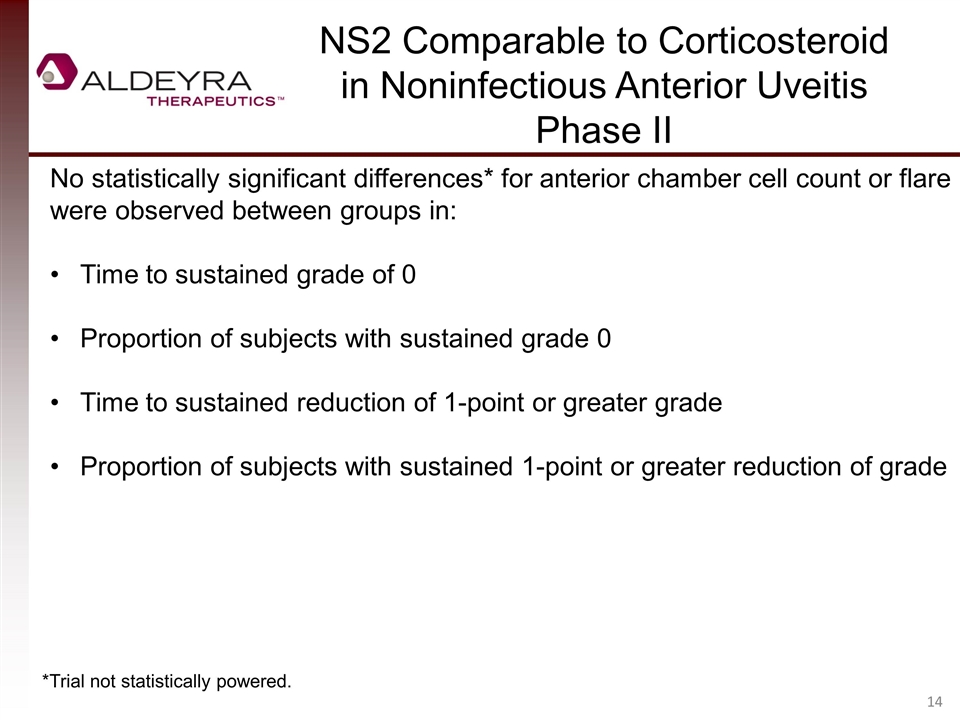
No statistically significant differences* for anterior chamber cell count or flare were observed between groups in: Time to sustained grade of 0 Proportion of subjects with sustained grade 0 Time to sustained reduction of 1-point or greater grade Proportion of subjects with sustained 1-point or greater reduction of grade NS2 Comparable to Corticosteroid in Noninfectious Anterior Uveitis Phase II *Trial not statistically powered.

No significant differences observed in symptom scores No SAEs in any group Eye stinging/burning was reported in the NS2 treated groups 4 subjects in NS2 group, leading to one discontinuation 3 subjects in NS2+Corticosteroid group, leading to one discontinuation Overall, NS2 was well tolerated with no safety issues raised for future studies of topical ocular NS2 NS2 Generally Well-Tolerated in Noninfectious Anterior Uveitis Phase II
Clinical/Regulatory Development Plans for Topical Ocular NS2 Allergic Conjunctivitis Pre-IND Meeting and IND Filing (prior Phase II was under CTA) Phase II/III Dose-Ranging Noninfectious Anterior Uveitis Regulatory Advice Meeting Phase II/III Dose-Ranging Other ocular inflammation Phase II trials under review, both in rare and common diseases All Development Plans Are Contingent on FDA Interaction
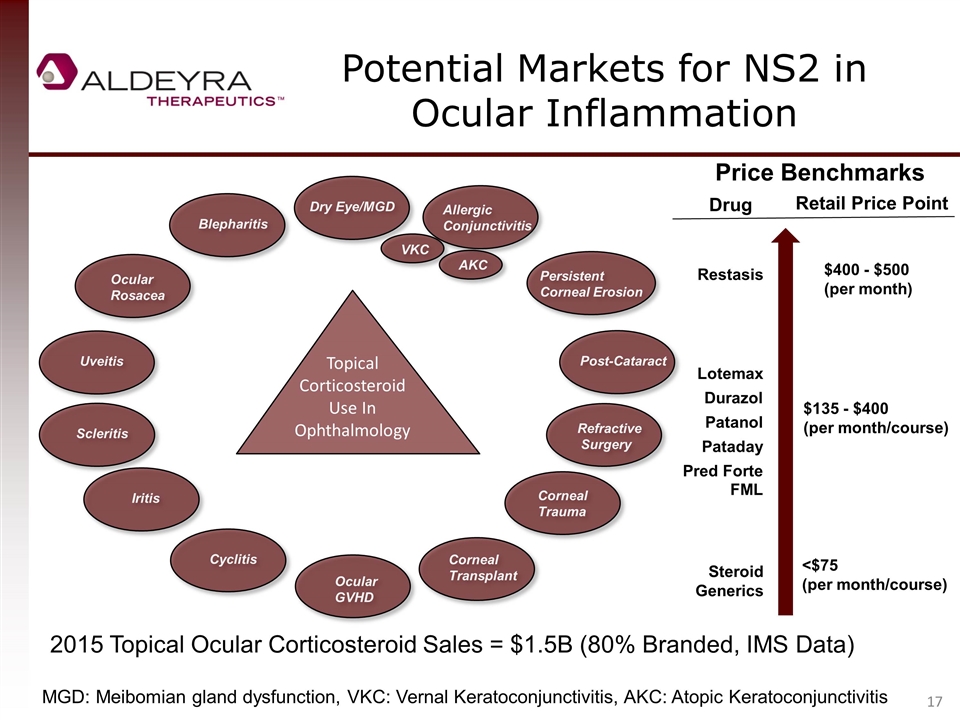
Potential Markets for NS2 in Ocular Inflammation VKC AKC Topical Corticosteroid Use In Ophthalmology Dry Eye/MGD Allergic Conjunctivitis Refractive Surgery Post-Cataract Ocular Rosacea Blepharitis Iritis Cyclitis Corneal Trauma Ocular GVHD Persistent Corneal Erosion Uveitis Scleritis Corneal Transplant Price Benchmarks Restasis Lotemax Patanol Durazol Pred Forte FML Steroid Generics Drug Retail Price Point $400 - $500 (per month) $135 - $400 (per month/course) <$75 (per month/course) Pataday 2015 Topical Ocular Corticosteroid Sales = $1.5B (80% Branded, IMS Data) MGD: Meibomian gland dysfunction, VKC: Vernal Keratoconjunctivitis, AKC: Atopic Keratoconjunctivitis
















