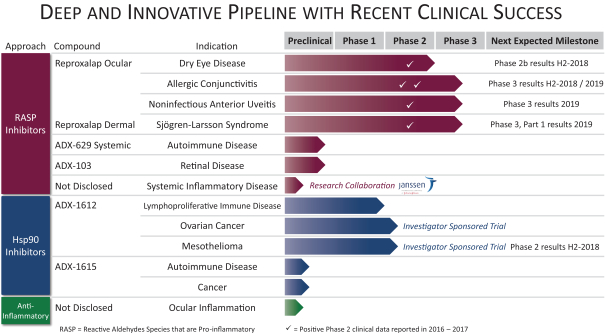
Results of Operations
We anticipate that our results of operations will fluctuate for the foreseeable future due to several factors, including the progress of our research and development efforts, the timing and outcome of clinical trials, and regulatory requirements. Our limited operating history makes predictions of future operations difficult or impossible. Since our inception, we have incurred significant losses.
Three months ended June 30, 2018 compared to three months ended June 30, 2017
Research and development expenses. Research and development expenses were $6.8 million for the three months ended June 30, 2018, compared to $3.8 million for the three months ended June 30, 2017. The increase of $3.0 million is primarily related to the increases in our external research and development expenditures, including clinical and manufacturing expenses and personnel costs including stock compensation, partially offset by a reduction in pre-clinical expenses.
General and administrative expenses.General and administrative expenses were $2.4 million for the three months ended June 30, 2018, and $1.5 million for the three months ended June 30, 2017. The increase of $0.9 million is primarily related to an increase in personnel cost including stock compensation and intellectual property legal expenses.
Other income (expense).Total other income (expense), net was $115,598 and $21,921 for the three months ended June 30, 2018 and 2017, respectively. For the three months ended June 30, 2018 and 2017, other income (expense) primarily consisted of interest income, which was partially offset by interest expense related to our Credit Facility.
Six months ended June 30, 2018 compared to six months ended June 30, 2017
Research and development expenses. Research and development expenses were $13.4 million for the six months ended June 30, 2018, compared to $7.2 million for the six months ended June 30, 2017. The increase of $6.2 million is primarily related to the increases in our external research and development expenditures, including clinical and manufacturing expenses and personnel costs including stock compensation, partially offset by a reduction in pre-clinical expenses.
General and administrative expenses.General and administrative expenses were $4.3 million for the six months ended June 30, 2018, compared to $3.2 million for the six months ended June 30, 2017. The increase of $1.1 million is primarily related to an increase in personnel costs, including stock-based compensation, and legal costs.
Other income (expense).Total other income (expense), net was $209,944 and $26,701 for the six months ended June 30, 2018 and 2017, respectively. For the six months ended June 30, 2018 and 2017, other income (expense) primarily consisted of interest income, which was partially offset by interest expense related to our credit facility.
Liquidity and Capital Resources
We have funded our operations primarily from the sale of equity securities and convertible equity securities and borrowings under our Credit Facility discussed below. Since inception, we have incurred operating losses and negative cash flows from operating activities, and have devoted substantially all of our efforts towards research and development. At June 30, 2018, we had total stockholders’ equity of approximately $37.0 million, and cash, cash equivalents, reverse repurchase agreements and marketable securities of $41.7 million. During the three months ended June 30, 2018, we had a net loss of approximately $9.1 million. We expect to generate operating losses for the foreseeable future.
We are a party to a loan and security agreement (the Credit Facility) with Pacific Western Bank (Pacific Western, formerly Square 1 Bank), which was originally entered into in April 2012 and has been subsequently amended. Pursuant to the Credit Facility, Pacific Western agreed to make term loans in a principal amount of up to $5.0 million available to us to fund expenses related to our clinical trials and general working capital purposes. The term loans were made available to us upon the following terms: (i) $2.0 million was made available in November 2014 (which was used in part to refinance then outstanding loans from Pacific Western); and (ii) $3.0 million became available to us in May 2016 following the satisfaction of certain conditions, including receipt of positive Phase 2 clinical trial results in noninfectious anterior uveitis. In November 2017, we amended our Credit Facility such that any term loan we draw is payable as interest only prior to October 2018 and thereafter is payable in monthly installments of principal plus accrued interest over 36 months. Each term loan accrues interest from its date of issue at a variable annual interest rate equal to the greater of 2.0% plus prime or 5.25% per annum. The annualized interest rate as of December 31, 2017 was 6.56%. The Credit Facility is collateralized by our assets, including our intellectual property. As of June 30, 2018 and December 31, 2017, $1.4 million was outstanding under the Credit Facility. At June 30, 2018 and December 31, 2017, the Credit Facility is shown net of a remaining debt discount of $52,000 and $59,000 respectively, which is being amortized using the effective interest method through the current maturity date of the Credit Facility, September 2021.
In February 2017, we closed an underwritten public offering in which we sold, 2,555,555 shares of our common stock, including 333,333 shares sold in connection with the exercise in full by the underwriters of their option to purchase additional shares. The net proceeds of the offering, including the full exercise of the option, were approximately $10.6 million after deducting underwriting discounts and commissions and the other offering expenses payable by us.
21