
Reproxalap Phase 2b Dry Eye Disease Results September 2018 NASDAQ: ALDX ©Aldeyra Therapeutics, Inc. 2018 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, research and development plans or expectations, trends, the structure, timing and success of Aldeyra’s planned or pending clinical trials, expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development, clinical and regulatory plans or expectations for Aldeyra’s product candidates and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development timelines may be subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, and other factors that could delay the initiation, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of September 26, 2018, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.
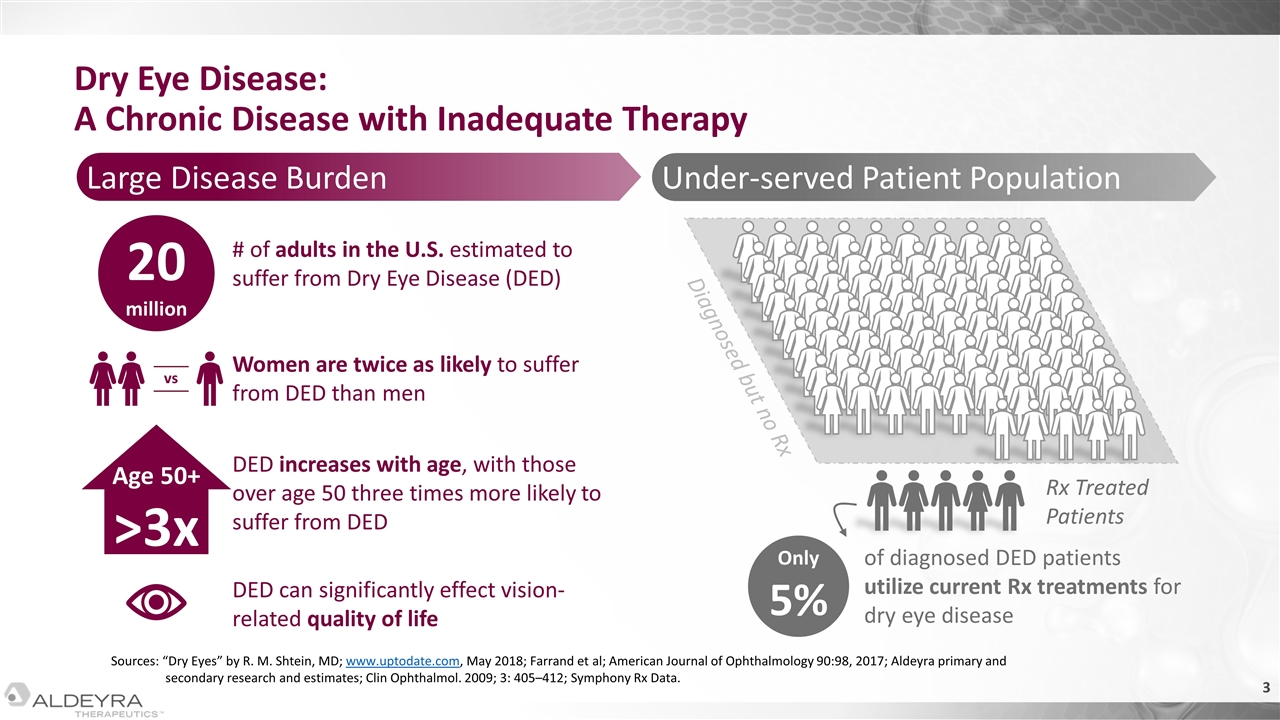
Under-served Patient Population Only 5% of diagnosed DED patients utilize current Rx treatments for dry eye disease Diagnosed but no Rx Rx Treated Patients Sources: “Dry Eyes” by R. M. Shtein, MD; www.uptodate.com, May 2018; Farrand et al; American Journal of Ophthalmology 90:98, 2017; Aldeyra primary and secondary research and estimates; Clin Ophthalmol. 2009; 3: 405–412; Symphony Rx Data. Large Disease Burden Dry Eye Disease: A Chronic Disease with Inadequate Therapy 20 million Age 50+ >3x # of adults in the U.S. estimated to suffer from Dry Eye Disease (DED) Women are twice as likely to suffer from DED than men vs DED increases with age, with those over age 50 three times more likely to suffer from DED DED can significantly effect vision-related quality of life
Positive Phase 2b Clinical Trial Results Reproxalap: A Novel Drug Candidate For the Treatment of Dry Eye Disease Primary objective achieved: Endpoint selection and sample size powering confirmed for Phase 3 clinical trials Reproxalap demonstrated statistically significant improvements versus vehicle across multiple symptom and sign measures, consistent with novel and broad mechanism of action Pathway to registration trials confirmed with ocular dryness symptom score, ocular staining score, and 0.25% reproxalap dose Improvements in symptoms and signs observed as early as two weeks, consistent with prior reproxalap clinical trial results and supportive of differentiated product profile Aldeyra plans to discuss results with regulatory authorities, and expects to initiate Phase 3 clinical trials in 2019 Rigorous clinical data demonstrate the efficacy and safety of reproxalap in dry eye disease and allergic conjunctivitis, two medical conditions with considerable overlap
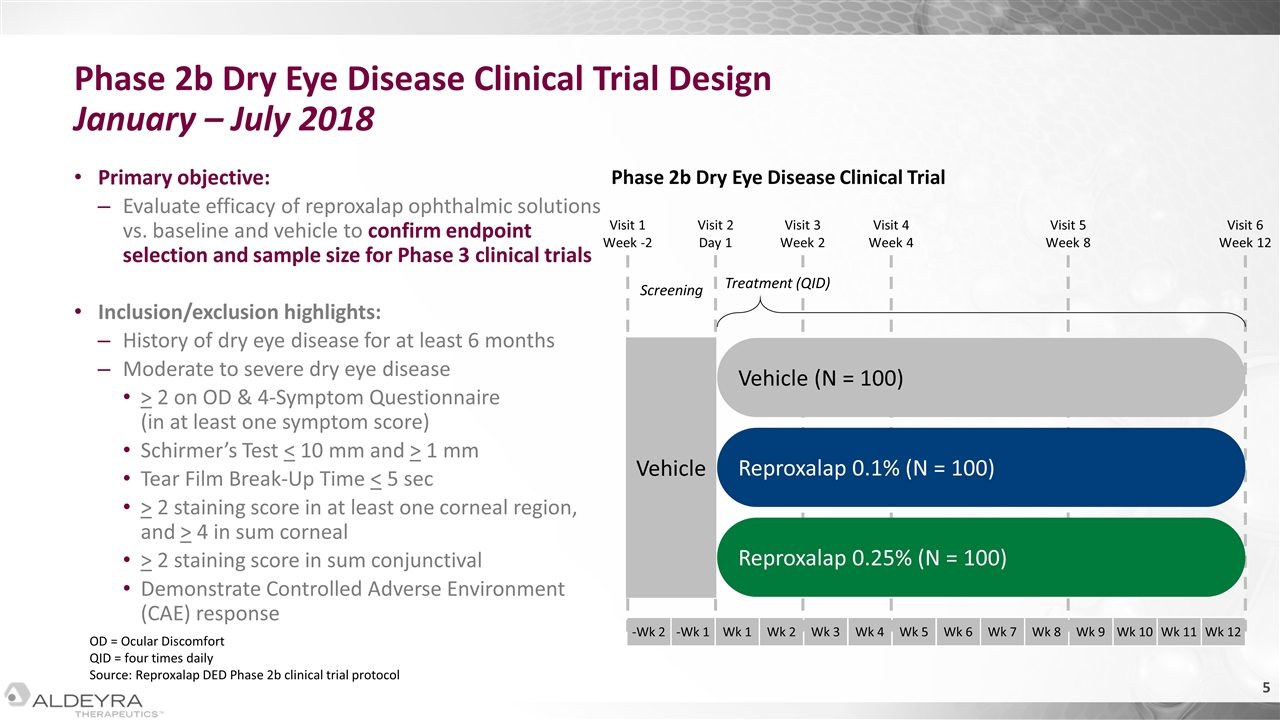
Primary objective: Evaluate efficacy of reproxalap ophthalmic solutions vs. baseline and vehicle to confirm endpoint selection and sample size for Phase 3 clinical trials Inclusion/exclusion highlights: History of dry eye disease for at least 6 months Moderate to severe dry eye disease > 2 on OD & 4-Symptom Questionnaire (in at least one symptom score) Schirmer’s Test < 10 mm and > 1 mm Tear Film Break-Up Time < 5 sec > 2 staining score in at least one corneal region, and > 4 in sum corneal > 2 staining score in sum conjunctival Demonstrate Controlled Adverse Environment (CAE) response OD = Ocular Discomfort QID = four times daily Source: Reproxalap DED Phase 2b clinical trial protocol Phase 2b Dry Eye Disease Clinical Trial Design January – July 2018 Vehicle -Wk 2 -Wk 1 Wk 1 Wk 2 Wk 3 Wk 4 Wk 5 Wk 6 Wk 7 Wk 8 Wk 9 Wk 10 Wk 11 Wk 12 Reproxalap 0.25% (N = 100) Visit 1 Week -2 Visit 2 Day 1 Visit 3 Week 2 Visit 4 Week 4 Visit 5 Week 8 Visit 6 Week 12 Reproxalap 0.1% (N = 100) Vehicle (N = 100) Screening Treatment (QID) Phase 2b Dry Eye Disease Clinical Trial
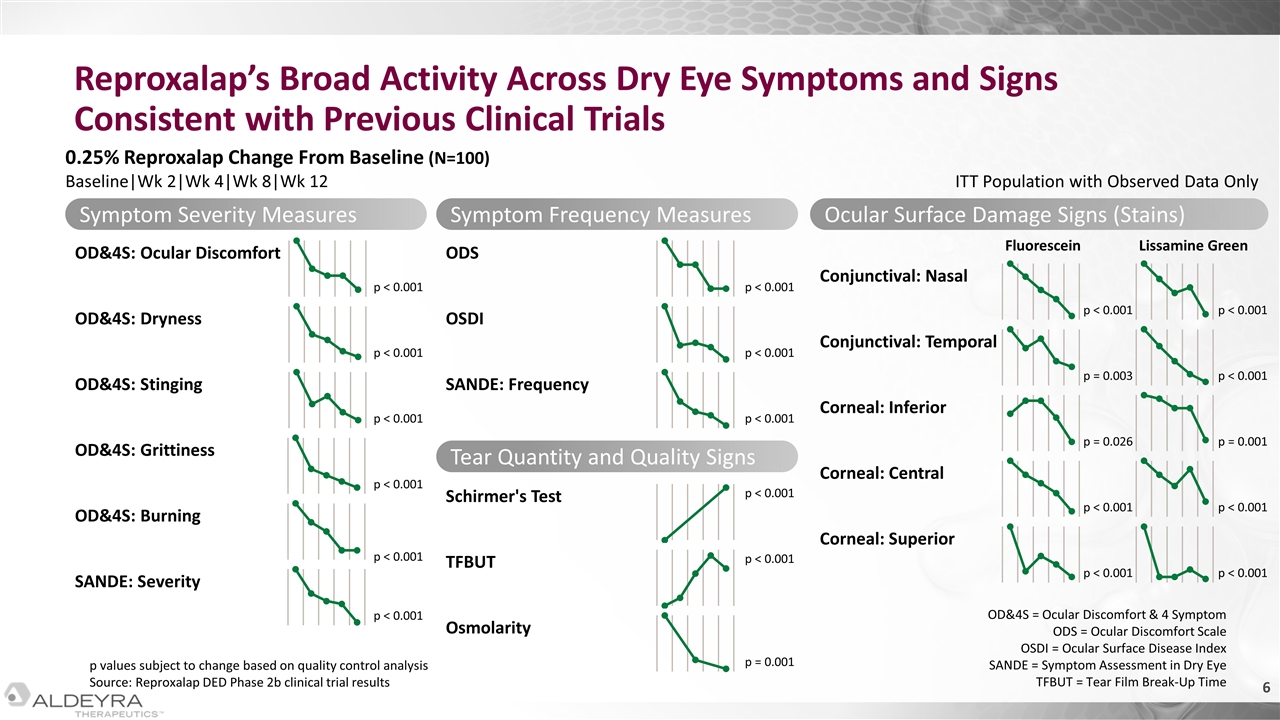
p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results ODS OSDI SANDE: Frequency Reproxalap’s Broad Activity Across Dry Eye Symptoms and Signs Consistent with Previous Clinical Trials Symptom Severity Measures OD&4S: Ocular Discomfort OD&4S: Dryness OD&4S: Stinging OD&4S: Grittiness OD&4S: Burning SANDE: Severity p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 p < 0.001 Symptom Frequency Measures Tear Quantity and Quality Signs TFBUT Schirmer's Test Osmolarity Ocular Surface Damage Signs (Stains) Conjunctival: Nasal Conjunctival: Temporal Corneal: Inferior Corneal: Central Corneal: Superior Fluorescein Lissamine Green p < 0.001 p < 0.001 p = 0.001 p < 0.001 p < 0.001 p = 0.026 p = 0.003 p < 0.001 p < 0.001 p < 0.001 p = 0.001 p < 0.001 p < 0.001 0.25% Reproxalap Change From Baseline (N=100) Baseline|Wk 2|Wk 4|Wk 8|Wk 12 OD&4S = Ocular Discomfort & 4 Symptom ODS = Ocular Discomfort Scale OSDI = Ocular Surface Disease Index SANDE = Symptom Assessment in Dry Eye TFBUT = Tear Film Break-Up Time ITT Population with Observed Data Only
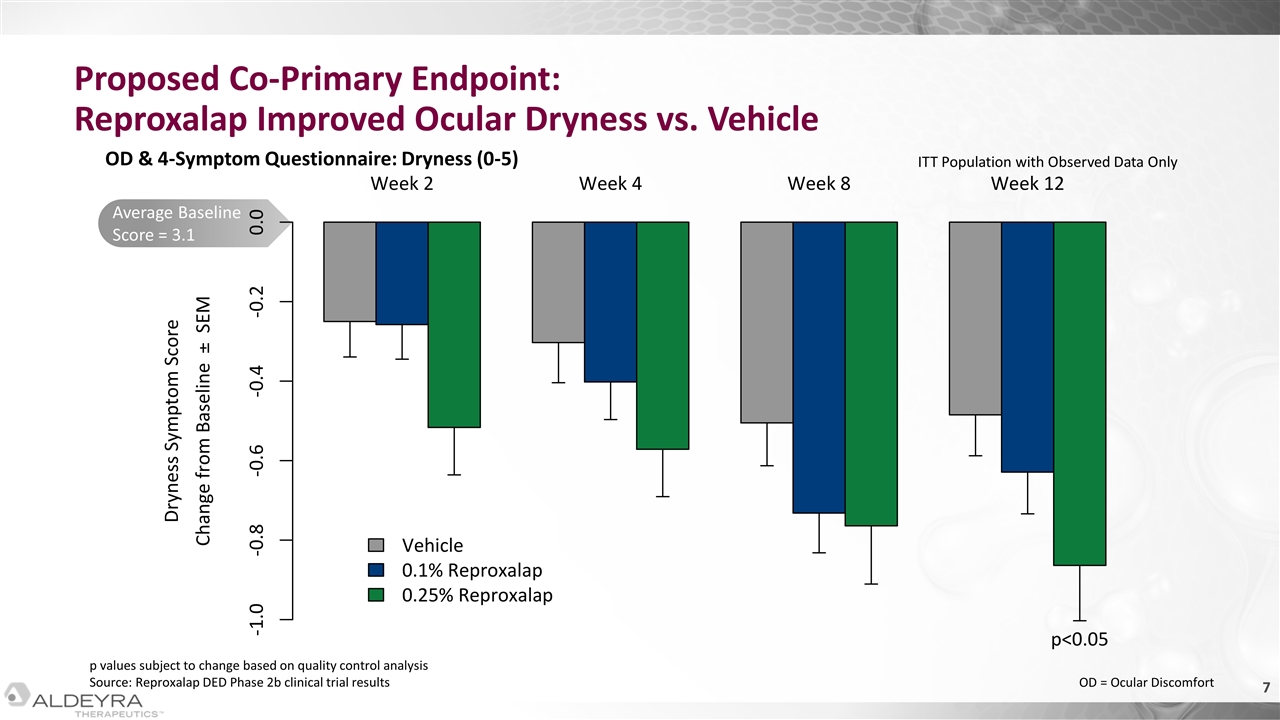
Average Baseline Score = 3.1 Proposed Co-Primary Endpoint: Reproxalap Improved Ocular Dryness vs. Vehicle OD & 4-Symptom Questionnaire: Dryness (0-5) p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results ITT Population with Observed Data Only OD = Ocular Discomfort
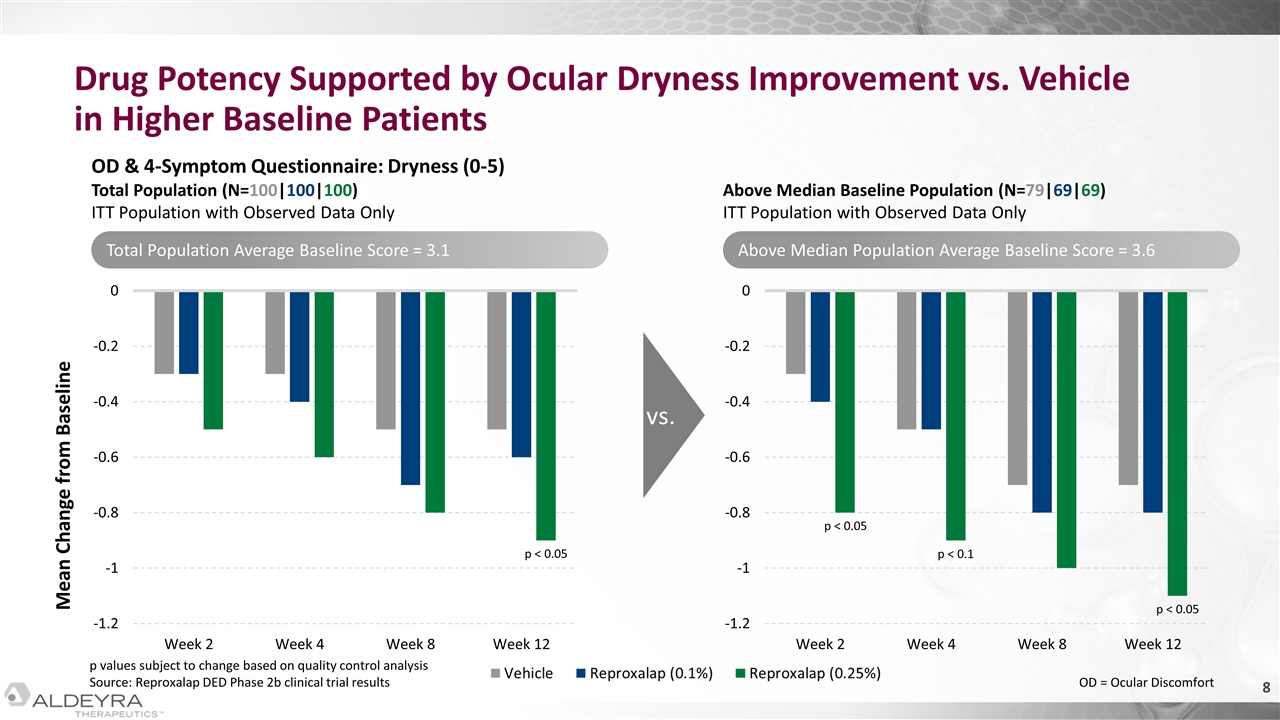
Drug Potency Supported by Ocular Dryness Improvement vs. Vehicle in Higher Baseline Patients Mean Change from Baseline OD & 4-Symptom Questionnaire: Dryness (0-5) Total Population (N=100|100|100) ITT Population with Observed Data Only Above Median Baseline Population (N=79|69|69) ITT Population with Observed Data Only vs. p < 0.1 p < 0.05 p < 0.05 p < 0.05 Total Population Average Baseline Score = 3.1 Above Median Population Average Baseline Score = 3.6 p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results OD = Ocular Discomfort
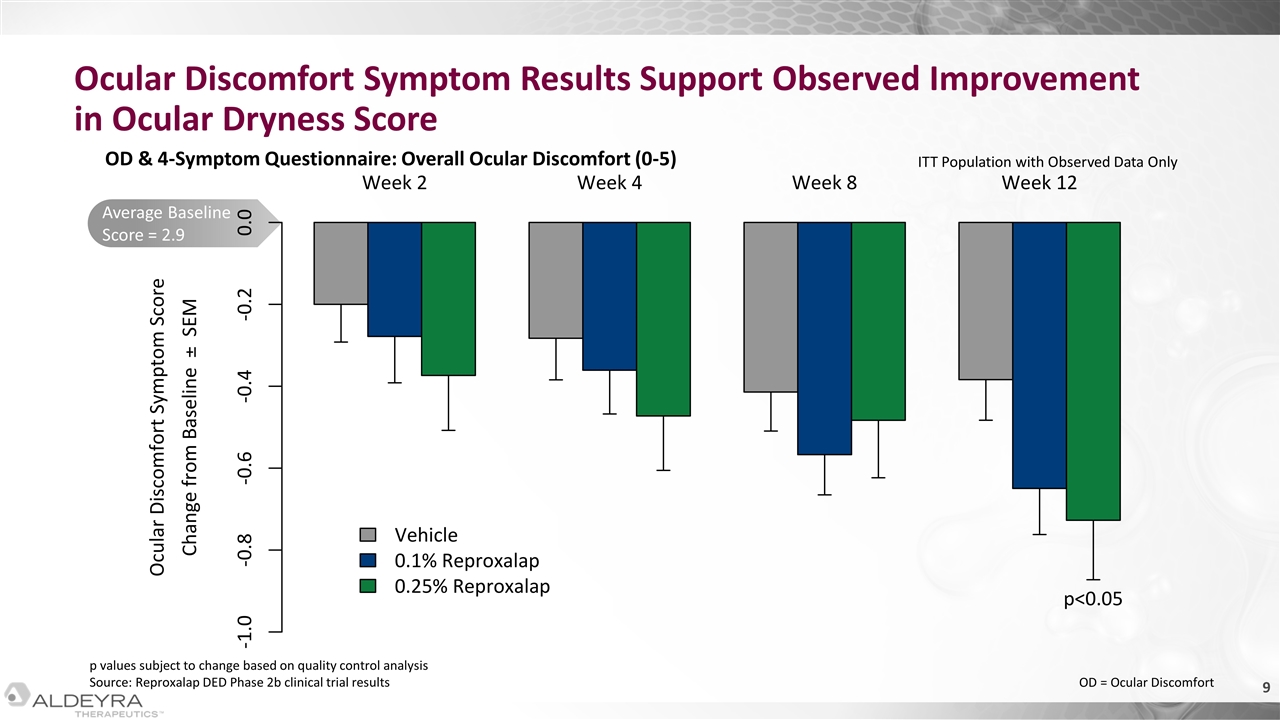
Average Baseline Score = 2.9 Ocular Discomfort Symptom Results Support Observed Improvement in Ocular Dryness Score OD & 4-Symptom Questionnaire: Overall Ocular Discomfort (0-5) ITT Population with Observed Data Only OD = Ocular Discomfort p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results
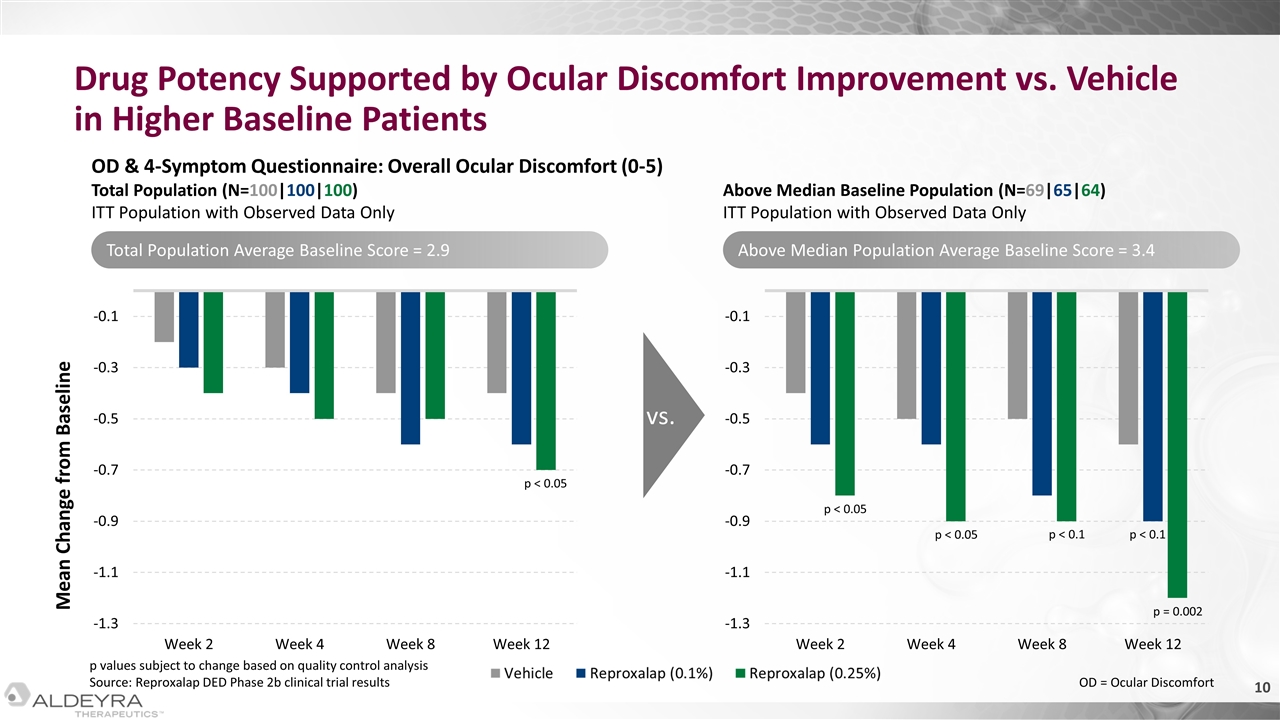
Drug Potency Supported by Ocular Discomfort Improvement vs. Vehicle in Higher Baseline Patients Mean Change from Baseline OD & 4-Symptom Questionnaire: Overall Ocular Discomfort (0-5) Total Population (N=100|100|100) ITT Population with Observed Data Only Above Median Baseline Population (N=69|65|64) ITT Population with Observed Data Only vs. p < 0.05 p < 0.05 p < 0.05 p < 0.1 p < 0.1 p = 0.002 Total Population Average Baseline Score = 2.9 Above Median Population Average Baseline Score = 3.4 p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results OD = Ocular Discomfort
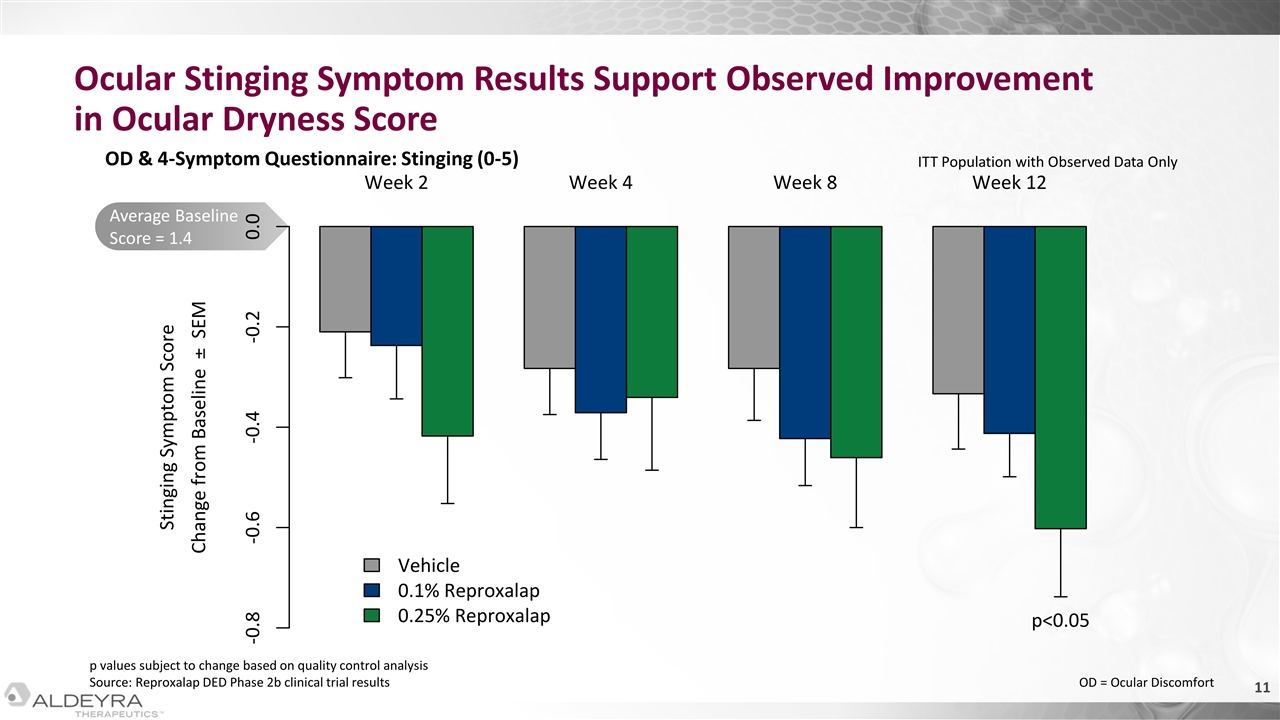
Average Baseline Score = 1.4 Ocular Stinging Symptom Results Support Observed Improvement in Ocular Dryness Score OD & 4-Symptom Questionnaire: Stinging (0-5) ITT Population with Observed Data Only OD = Ocular Discomfort p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results
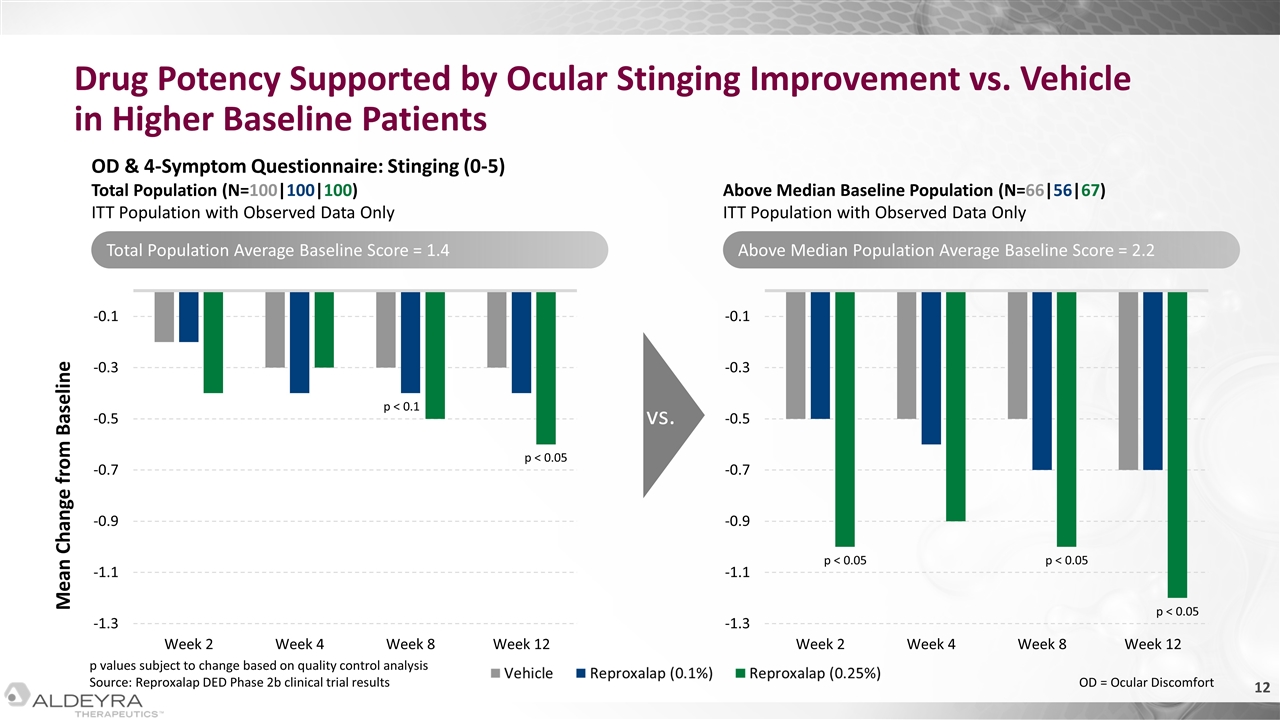
Drug Potency Supported by Ocular Stinging Improvement vs. Vehicle in Higher Baseline Patients Mean Change from Baseline OD & 4-Symptom Questionnaire: Stinging (0-5) Total Population (N=100|100|100) ITT Population with Observed Data Only Above Median Baseline Population (N=66|56|67) ITT Population with Observed Data Only vs. p < 0.05 p < 0.05 p < 0.05 p < 0.05 Total Population Average Baseline Score = 1.4 Above Median Population Average Baseline Score = 2.2 p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results OD = Ocular Discomfort p < 0.1
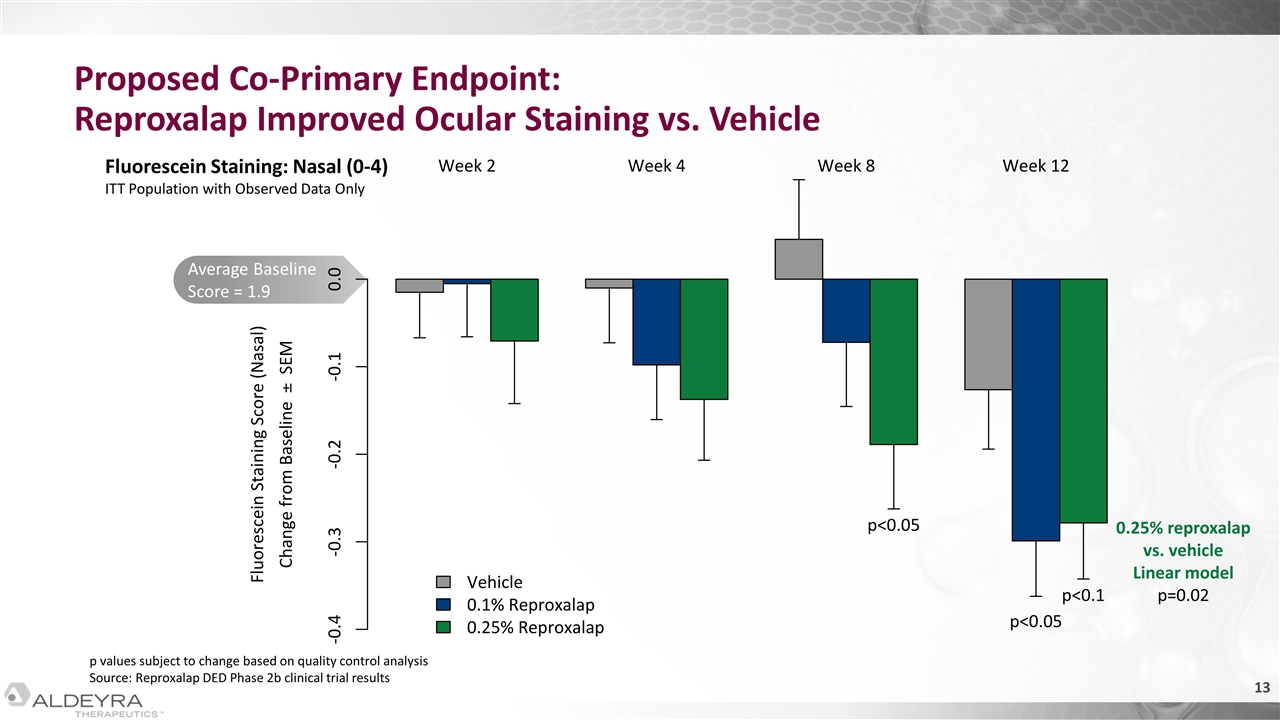
Average Baseline Score = 1.9 Proposed Co-Primary Endpoint: Reproxalap Improved Ocular Staining vs. Vehicle Fluorescein Staining: Nasal (0-4) ITT Population with Observed Data Only p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results 0.25% reproxalap vs. vehicle Linear model p=0.02
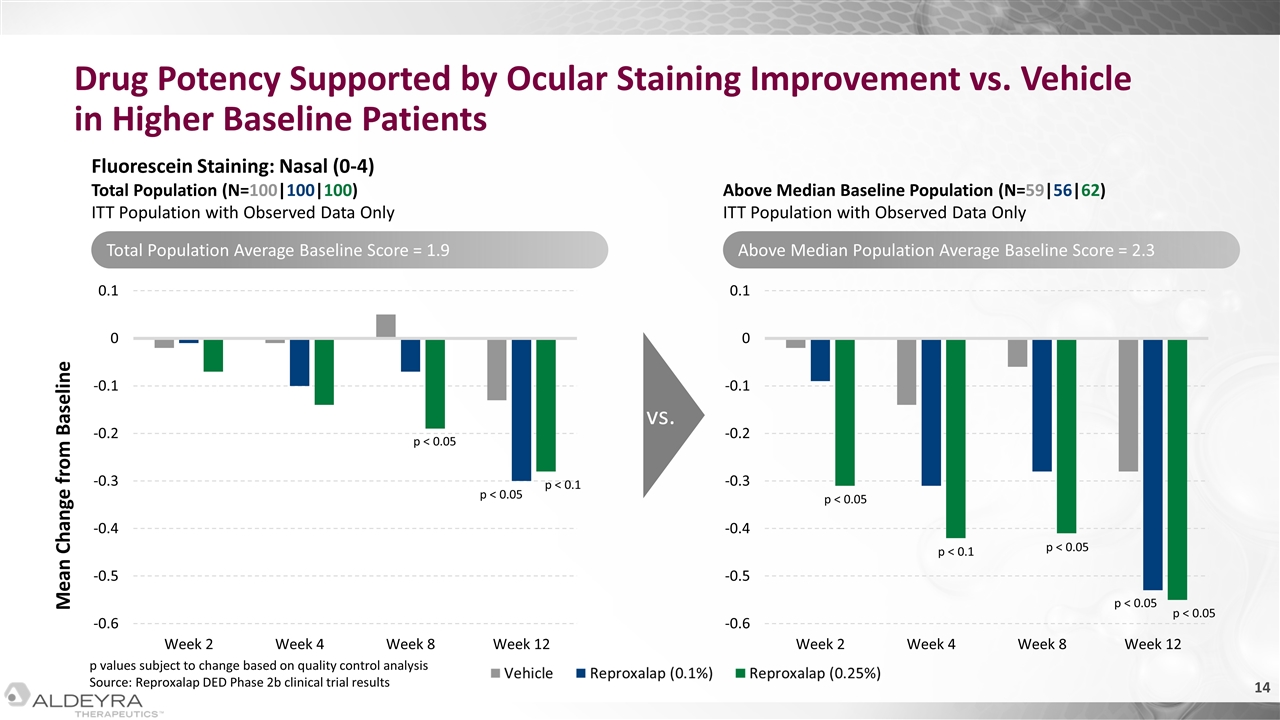
Drug Potency Supported by Ocular Staining Improvement vs. Vehicle in Higher Baseline Patients Mean Change from Baseline Fluorescein Staining: Nasal (0-4) Total Population (N=100|100|100) ITT Population with Observed Data Only Above Median Baseline Population (N=59|56|62) ITT Population with Observed Data Only vs. p < 0.1 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.1 Total Population Average Baseline Score = 1.9 Above Median Population Average Baseline Score = 2.3 p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results
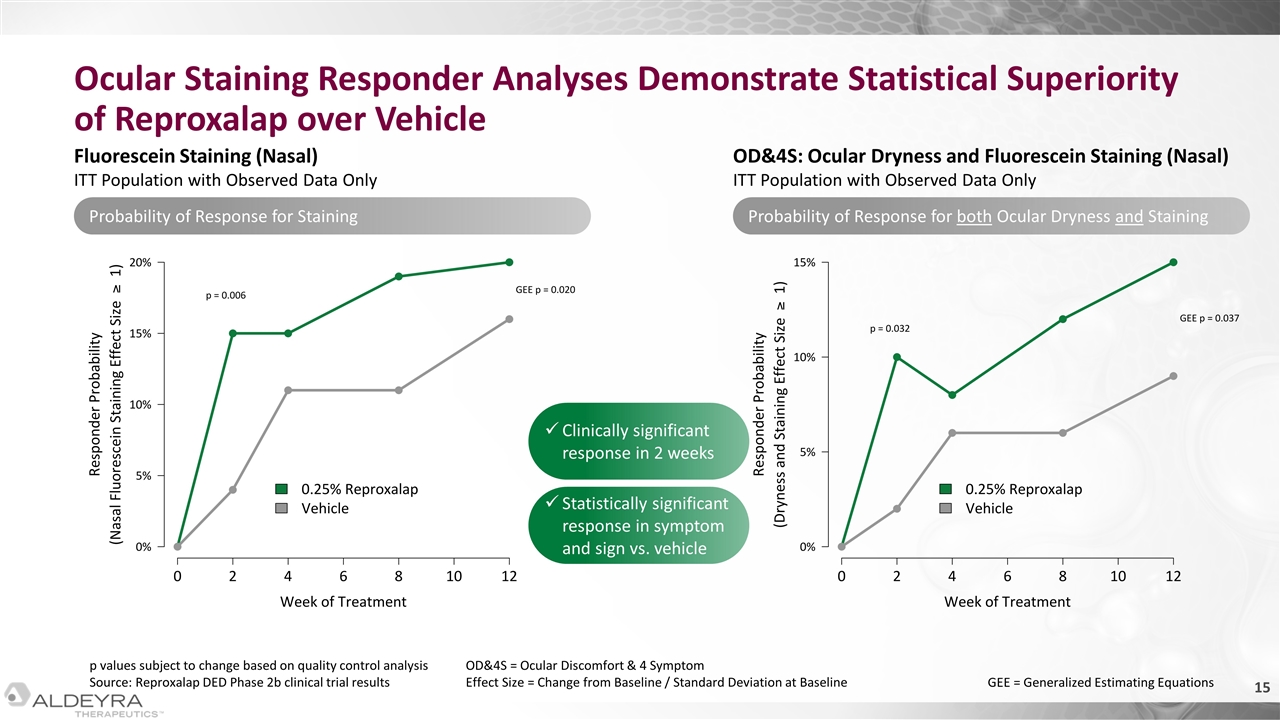
Ocular Staining Responder Analyses Demonstrate Statistical Superiority of Reproxalap over Vehicle p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results OD&4S = Ocular Discomfort & 4 Symptom Effect Size = Change from Baseline / Standard Deviation at Baseline GEE = Generalized Estimating Equations Clinically significant response in 2 weeks Statistically significant response in symptom and sign vs. vehicle Probability of Response for Staining Probability of Response for both Ocular Dryness and Staining Fluorescein Staining (Nasal) ITT Population with Observed Data Only OD&4S: Ocular Dryness and Fluorescein Staining (Nasal) ITT Population with Observed Data Only
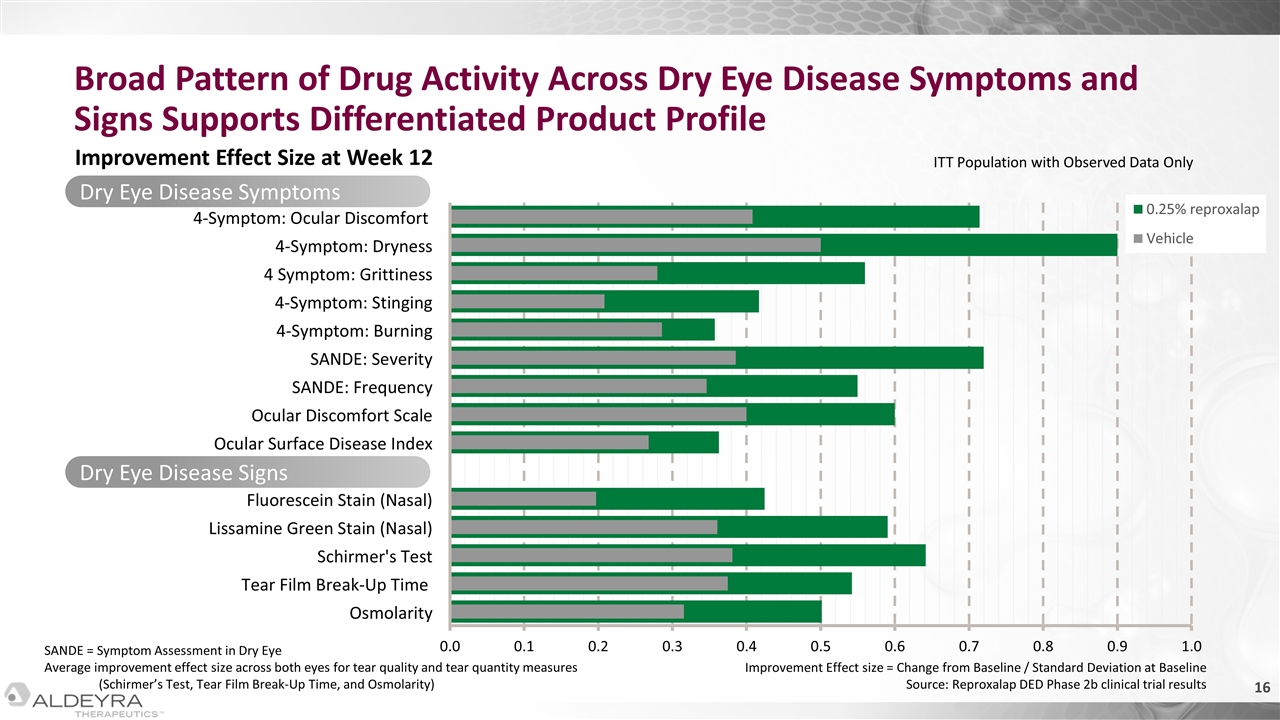
Dry Eye Disease Symptoms Broad Pattern of Drug Activity Across Dry Eye Disease Symptoms and Signs Supports Differentiated Product Profile Improvement Effect Size at Week 12 Dry Eye Disease Signs Improvement Effect size = Change from Baseline / Standard Deviation at Baseline Source: Reproxalap DED Phase 2b clinical trial results SANDE = Symptom Assessment in Dry Eye Average improvement effect size across both eyes for tear quality and tear quantity measures (Schirmer’s Test, Tear Film Break-Up Time, and Osmolarity) ITT Population with Observed Data Only
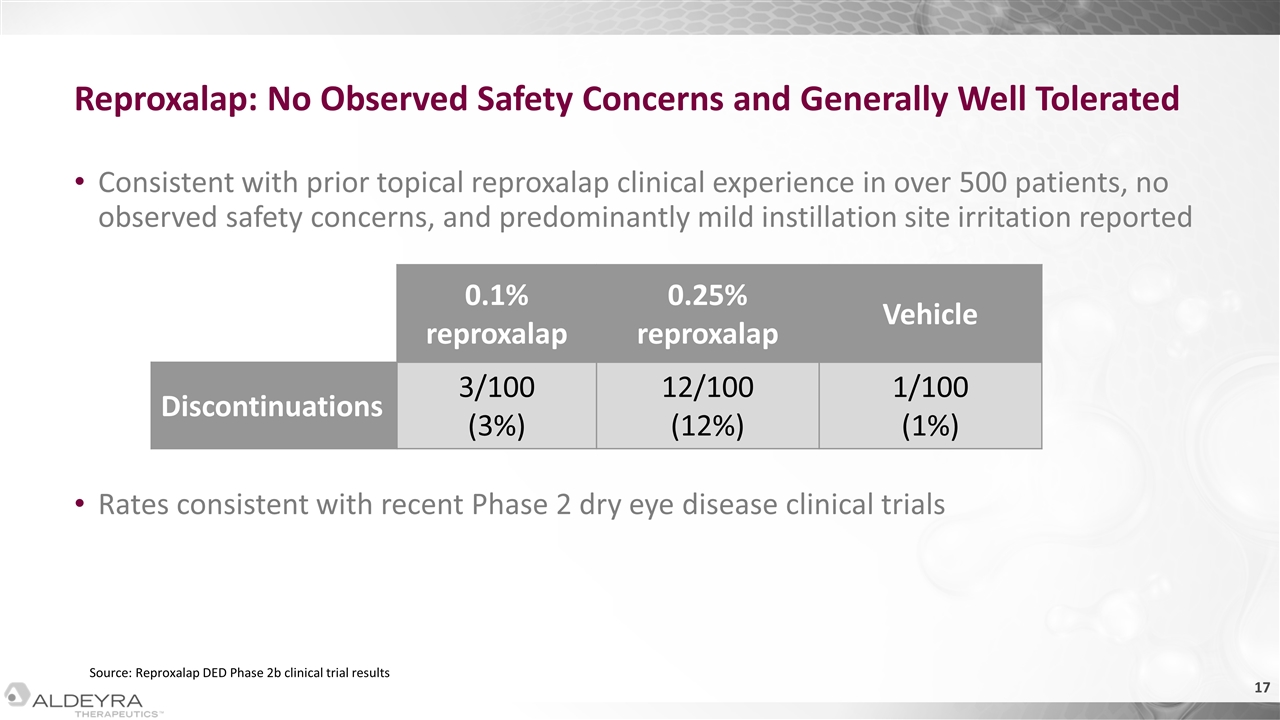
Reproxalap: No Observed Safety Concerns and Generally Well Tolerated Consistent with prior topical reproxalap clinical experience in over 500 patients, no observed safety concerns, and predominantly mild instillation site irritation reported Rates consistent with recent Phase 2 dry eye disease clinical trials 0.1% reproxalap 0.25% reproxalap Vehicle Discontinuations 3/100 (3%) 12/100 (12%) 1/100 (1%) Source: Reproxalap DED Phase 2b clinical trial results
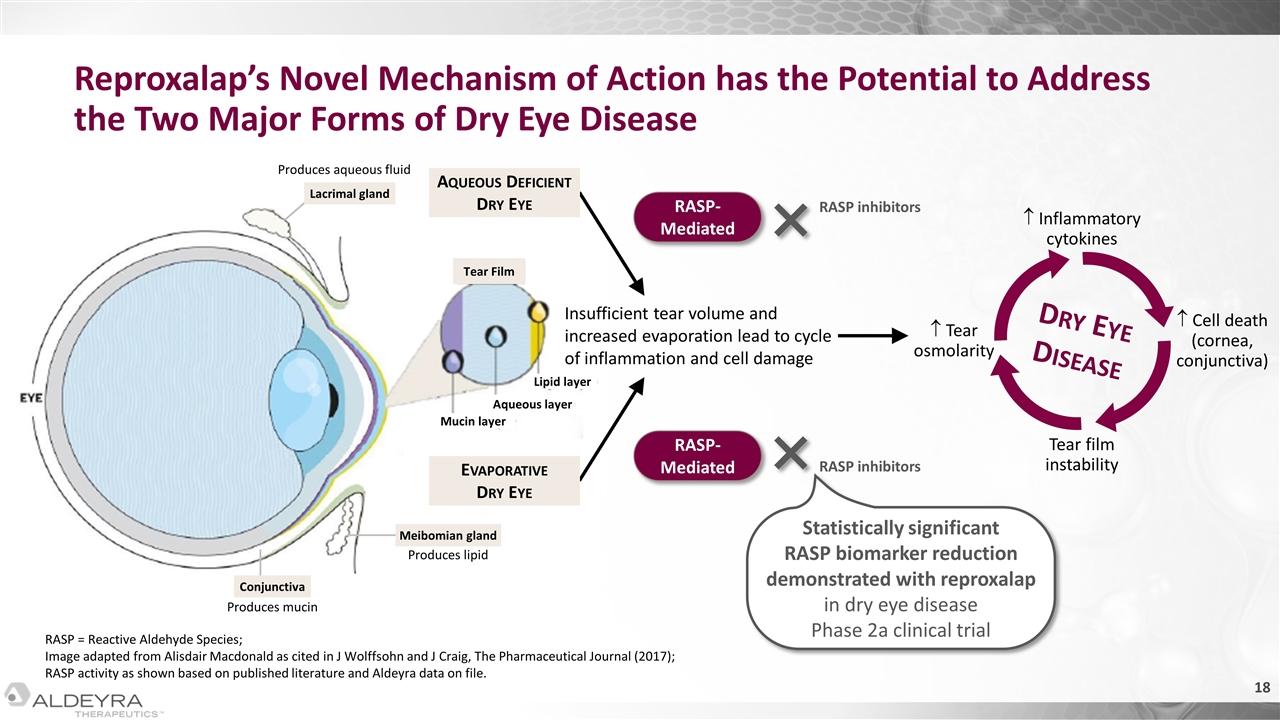
Produces aqueous fluid Lacrimal gland Tear Film Produces mucin Conjunctiva Produces lipid Meibomian gland Mucin layer Aqueous layer Lipid layer Reproxalap’s Novel Mechanism of Action has the Potential to Address the Two Major Forms of Dry Eye Disease RASP = Reactive Aldehyde Species; Image adapted from Alisdair Macdonald as cited in J Wolffsohn and J Craig, The Pharmaceutical Journal (2017); RASP activity as shown based on published literature and Aldeyra data on file. Insufficient tear volume and increased evaporation lead to cycle of inflammation and cell damage RASP- Mediated RASP- Mediated RASP inhibitors RASP inhibitors Dry Eye Disease Inflammatory cytokines Cell death (cornea, conjunctiva) Tear film instability Tear osmolarity Statistically significant RASP biomarker reduction demonstrated with reproxalap in dry eye disease Phase 2a clinical trial Aqueous Deficient Dry Eye Evaporative Dry Eye
Positive Phase 2b Clinical Trial Results Reproxalap: A Novel Drug Candidate For the Treatment of Dry Eye Disease Primary objective achieved: Endpoint selection and sample size powering confirmed for Phase 3 clinical trials Reproxalap demonstrated statistically significant improvements versus vehicle across multiple symptom and sign measures, consistent with novel and broad mechanism of action Pathway to registration trials confirmed with ocular dryness symptom score, ocular staining score, and 0.25% reproxalap dose Improvements in symptoms and signs observed as early as two weeks, consistent with prior reproxalap clinical trial results and supportive of differentiated product profile Aldeyra plans to discuss results with regulatory authorities, and expects to initiate Phase 3 clinical trials in 2019 Rigorous clinical data demonstrate the efficacy and safety of reproxalap in dry eye disease and allergic conjunctivitis, two medical conditions with considerable overlap

Reproxalap Planned Phase 3 Dry Eye Disease Program Two Phase 3 clinical trials expected to be initiated in 2019, following discussion with regulatory authorities First Phase 3 clinical trial is an adaptive two-stage design; expected initiation in 2019 Stage 1: Protocol optimization and sample size confirmation (12 weeks) Stage 2: Randomized, double masked, two-arm, parallel-group design, reproxalap vs vehicle (12 weeks) Primary endpoints: Ocular dryness score and ocular staining Secondary endpoints include ocular itch, based on positive reproxalap allergic conjunctivitis program results and high comorbidity of allergic conjunctivitis in dry eye disease patients Estimated sample size of 400-500 per arm with approximately 90% statistical power Second Phase 3 clinical trial expected to initiate in 2019 Contingent on funding, regulatory review, and other factors.
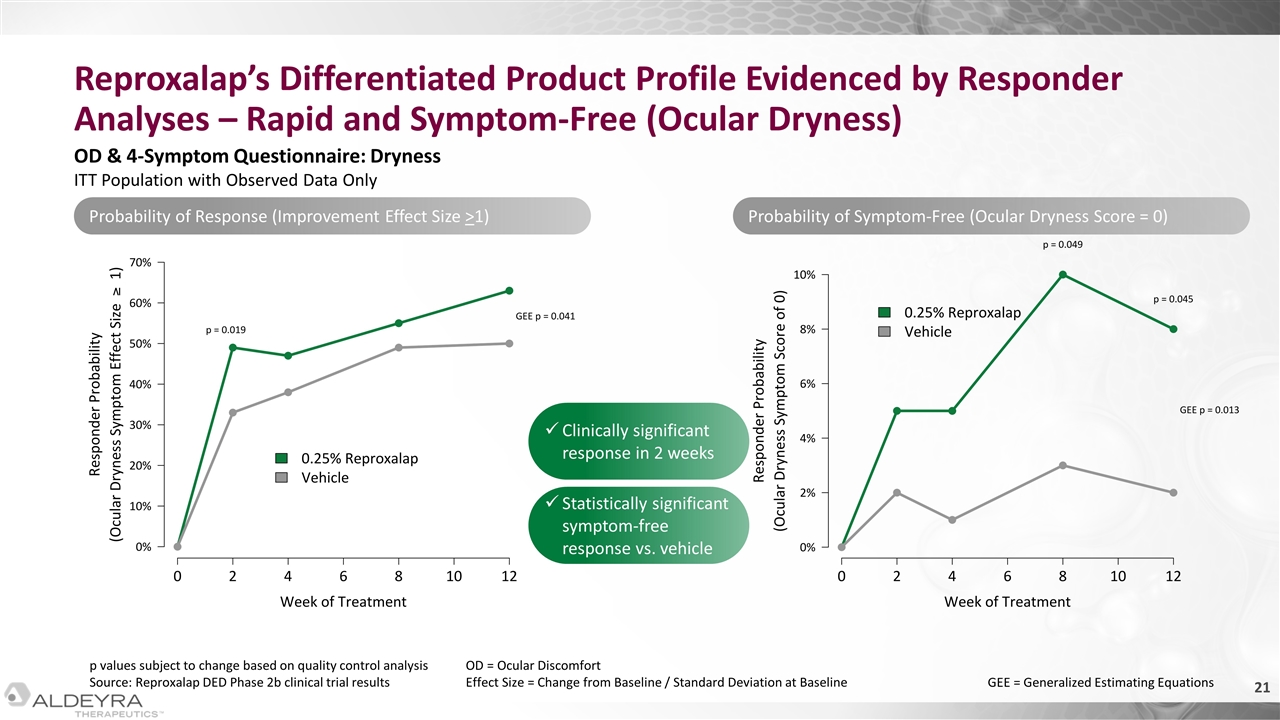
Reproxalap’s Differentiated Product Profile Evidenced by Responder Analyses – Rapid and Symptom-Free (Ocular Dryness) p values subject to change based on quality control analysis Source: Reproxalap DED Phase 2b clinical trial results OD & 4-Symptom Questionnaire: Dryness ITT Population with Observed Data Only OD = Ocular Discomfort Effect Size = Change from Baseline / Standard Deviation at Baseline GEE = Generalized Estimating Equations Clinically significant response in 2 weeks Statistically significant symptom-free response vs. vehicle Probability of Response (Improvement Effect Size >1) Probability of Symptom-Free (Ocular Dryness Score = 0)

A Unique Opportunity Early and consistent symptom improvements in Phase 2b clinical trial Patients & Physicians Not Satisfied Reproxalap: A Unique and Novel Product Candidate For Dry Eye Disease Sources: Aldeyra primary patient market research, primary physician market research, secondary market research, and estimates; Clin Ophthalmol. 2009; 3: 405–412. Up to 75% of patients with DED are not satisfied with current prescription options Up to 50% of patients treated for DED with current therapies fail and discontinue according to prescribing physicians Reproxalap Current prescription options may take up to six weeks or longer to have an effect Novel mechanism of action and differentiated approach to treat DED Broad symptom and sign improvements in Phase 2b clinical trial
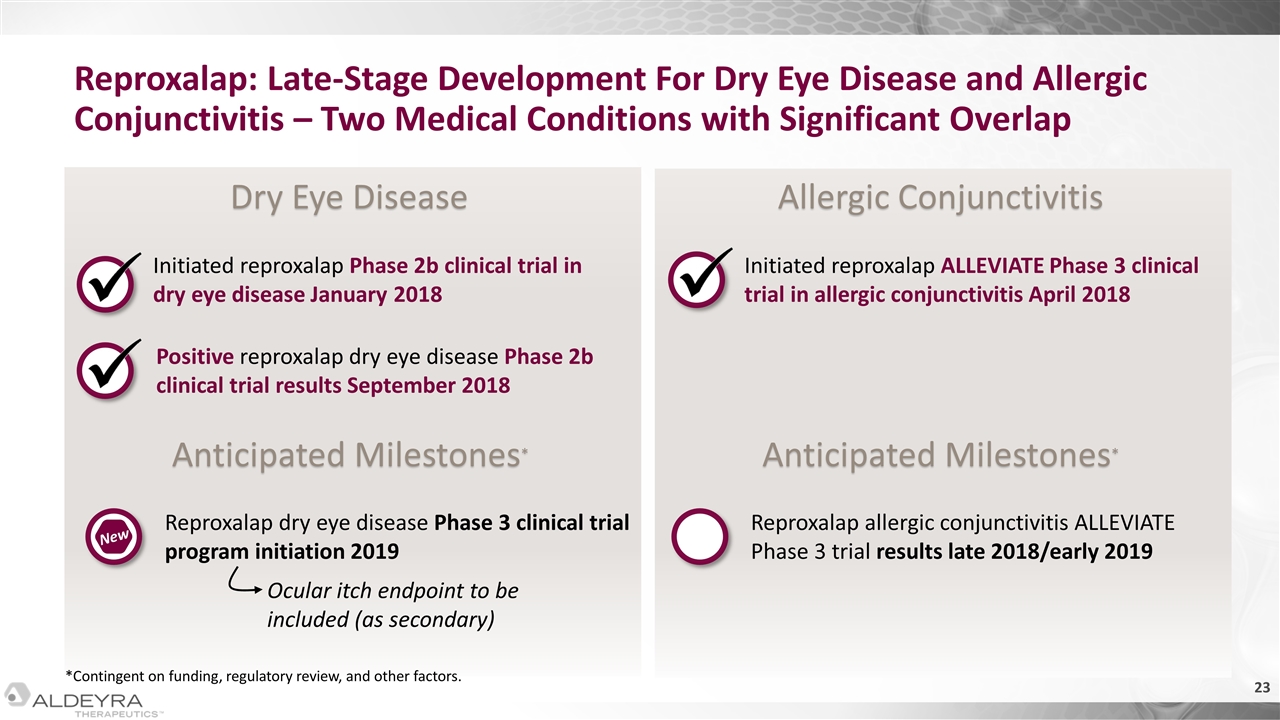
Anticipated Milestones* Anticipated Milestones* Dry Eye Disease Allergic Conjunctivitis Reproxalap: Late-Stage Development For Dry Eye Disease and Allergic Conjunctivitis – Two Medical Conditions with Significant Overlap Initiated reproxalap Phase 2b clinical trial in dry eye disease January 2018 Positive reproxalap dry eye disease Phase 2b clinical trial results September 2018 Reproxalap dry eye disease Phase 3 clinical trial program initiation 2019 New Initiated reproxalap ALLEVIATE Phase 3 clinical trial in allergic conjunctivitis April 2018 Reproxalap allergic conjunctivitis ALLEVIATE Phase 3 trial results late 2018/early 2019 *Contingent on funding, regulatory review, and other factors. Ocular itch endpoint to be included (as secondary)

Deep and Innovative Pipeline Not Disclosed Reproxalap Ocular Reproxalap Dermal ADX-629 Systemic ADX-103 ADX-1612 Dry Eye Disease Allergic Conjunctivitis Noninfectious Anterior Uveitis Sjögren-Larsson Syndrome Autoimmune Disease Retinal Disease PTLD Phase 3 initiation 2019 Phase 3 results late 2018 / 2019 Phase 3 results 2019 Phase 3, Part 1 results 2019 Systemic Inflammatory Disease RASP Inhibitors Hsp90 Inhibitors Anti-Inflammatory Ovarian Cancer Mesothelioma ADX-1615 Autoimmune Disease Cancer Not Disclosed Ocular Inflammation Research Collaboration Investigator-Sponsored Trial Indication Compound Mechanism ü ü ü ü Preclinical Phase 1 Phase 2 Phase 3 Next Expected Milestone Phase 2 initiation 2019 = Positive Phase 2 clinical trial data reported in 2016 – 2018 ü RASP = Reactive Aldehyde Species PTLD = Post-Transplant Lymphoproliferative Disorder Research Collaboration (undisclosed) ü ü ü

Two Mechanisms of action in development Seven Successful Phase 2 Clinical Trials 2016-2018 Phase 2 count includes mesothelioma investigator-sponsored trial; Phase 3 trials contingent on funding, regulatory review, and other factors. Four Phase 3 Clinical Trials Ongoing or Expected to Initiate
























