Exhibit 99.1

Dry Eye Disease Chamber Phase 3 Clinical Trial of Reproxalap REPROXALAP CLINICAL DEVELOPMENT AND REGULATORY UPDATE August 8, 2024 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2024 TOP - LINE RESULTS

2 Disclaimers and Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Sec urities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, stat eme nts regarding Aldeyra's future expectations, plans and prospects, including, without limitation, statements regarding: FDA agreement with the clinical development and regulatory plan for reproxalap; the outcome and timing of the FDA’s review, acceptance, and/or approval of a potential NDA resubmission for reproxalap and the ad equ acy of the data included in the potential NDA resubmission or the supplemental responses to the FDA; the potential for and timing of regulatory approval; Aldeyra’s expecta tio ns regarding the exercise of the AbbVie Option; Aldeyra's goals as to the potential profile and benefit of reproxalap in dry eye disease and allergic conjunctivitis, and its other pro duc t candidates in the indications for which they are developed; anticipated clinical or regulatory milestones for ADX - 2191, ADX - 248, ADX - 743, ADX - 631, and ADX - 629, including expectations regarding the results of scheduled FDA meetings and discussions , clinical trial initiations and completions, and the timing and nature of NDA or other submissions to the FDA; Aldeyra's bus ine ss, research, development and regulatory plans or expectations; political, economic, legal, social, and health risks that may affect Aldeyra’s business or the global economy; the structure, timing, and success of Aldeyra’s planned or pending clinical trials ; and expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry envir onm ent, and potential growth opportunities, among other things . The results of earlier preclinical or clinical trials may not be predictive of future results. Forward - looking statements incl ude all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” "could," “can,” “woul d,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or th e n egative of these terms, and similar expressions intended to identify forward - looking statements. Forward - looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual resu lts, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. These sta tements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development of, and clinical an d regulatory plans or expectations for Aldeyra’s investigational new drugs (including reproxalap, ADX - 2191, ADX - 248, ADX - 743, ADX - 631, and ADX - 629), and systems - based approaches , later developments with the FDA that may be inconsistent with Aldeyra’s expectations and beliefs, including the risk that the results from earlier clinical trials, porti ons of clinical trials, or pooled clinical data may not accurately predict results of subsequent trials or the remainder of a clinical trial for the same or different indications, inconsistent expecta tio ns regarding FDA acceptance and review of the company’s filings and submitted data sets, and Aldeyra’s continuing or post - hoc review and quality control analysis of clinical data. Important fa ctors that could cause actual results to differ materially from those reflected in Aldeyra's forward - looking statements are described in Aldeyra’s most recent Annual Report on Form 10 - K and Qu arterly Report on Form 10 - Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development plans and timelines may be subje ct to adjustment depending on funding, recruitment rate, regulatory review, which regulatory review timeline may be flexible and subject to change based on the regulator's workload a nd other potential review issues, preclinical and clinical results, regulatory developments in the United States and other countries, and other factors any of which could result in changes to Aldeyra’s development pla ns and programs or delay the initiation, enrolment, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors als o could affect Aldeyra's results. No forward - looking statements can be guaranteed, and actual results may differ materially from such statements. The information in this presentation is pro vid ed only as of August 8 , 2024, and Aldeyra undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.

3 The Phase 3 Clinical Trial of Reproxalap in a Dry Eye Chamber was Designed to Satisfy the Requirements for NDA Resubmission † z • Randomized, double - masked, vehicle - controlled dry eye chamber challenge Design • Visit 1: Medical screening • Visit 2: Vehicle dry eye chamber (dosing just before and 50 minutes after entry) • Visit 3: Four doses of randomized treatment (reproxalap or vehicle) • Visit 4: Randomized dry eye chamber (dosing just before and 50 minutes after entry) Dosing 132 dry eye disease patients: 66 randomized to reproxalap, 66 randomized to vehicle Size Ocular discomfort score from 80 to 100 minutes Primary Endpoint Safety Other Endpoints Vehicle Chamber Vehicle Chamber Vehicle Chamber Drug Chamber Treatment Arm A: Treatment Arm B: Visit 2 Visit 4 Randomization † NDA submission requirements depend, in part, on regulatory feedback. R egulatory review and discussion timelines are flexible and subject to change based on the regulator's workload and other potential review issues. Topical ocular reproxalap is an investigational new drug candidate that has been st udied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical tria ls. NDA = New Drug Application.
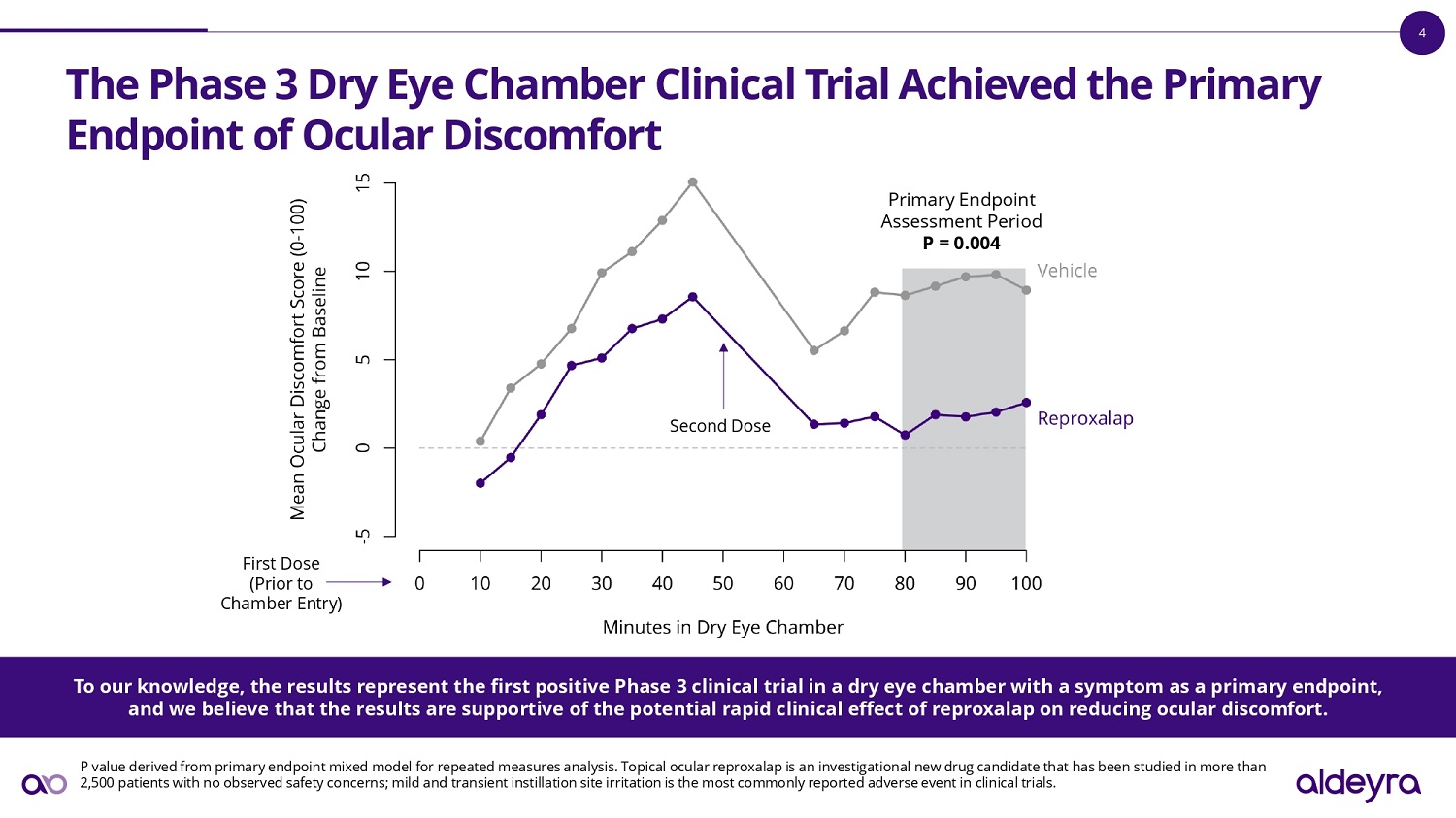
4 The Phase 3 Dry Eye Chamber Clinical Trial Achieved Success for the Primary Endpoint of Ocular Discomfort P value derived from primary endpoint mixed model for repeated measures analysis. Topical ocular reproxalap is an investigati ona l new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reporte d a dverse event in clinical trials. First Dose (Prior to Chamber Entry) Second Dose Primary Endpoint Assessment Period P = 0.004 To our knowledge, the results represent the first positive Phase 3 clinical trial in a dry eye chamber with a symptom as a pr ima ry endpoint, and we believe that the results are supportive of the potential rapid clinical effect of reproxalap on reducing ocular discom for t.
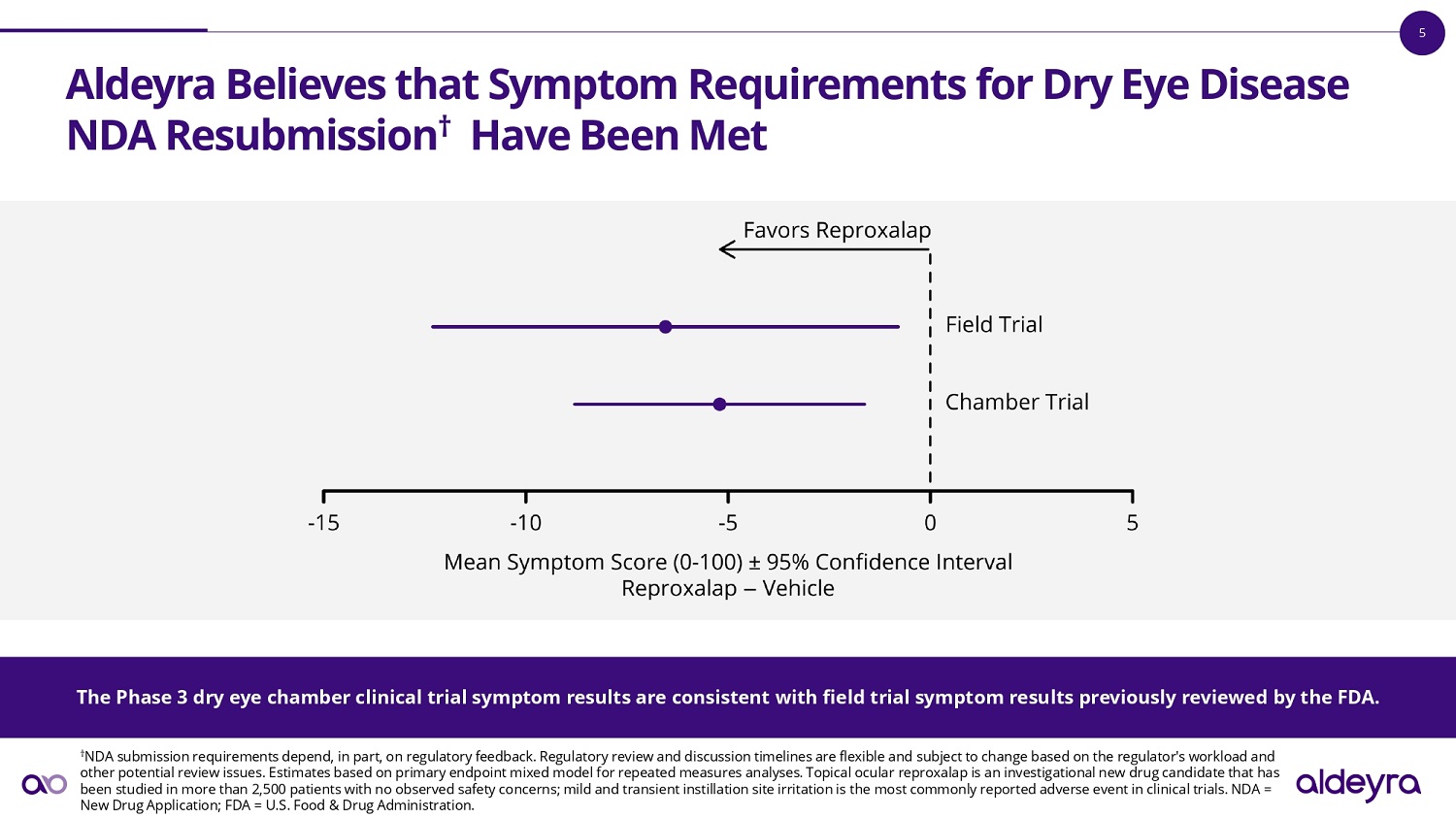
5 Aldeyra Believes that Symptom Requirements for Dry Eye Disease NDA Resubmission Have Been Met The Phase 3 dry eye chamber clinical trial symptom results are consistent with field trial symptom results previously reviewe d b y the FDA. Estimates based on mixed model for repeated measures analysis adjusted for baseline and other factors specified in the Statis tic al Analysis Plans. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild a nd transient instillation site irritation is the most commonly reported adverse event in clinical trials. FDA = U.S. Food & Drug Administration.

6 Aldeyra Believes that Positive Results from the Phase 3 Clinical Trial Enable NDA Resubmission † of Reproxalap for Dry Eye Disease • The dry eye chamber clinical trial was designed to satisfy the FDA’s single resubmission requirement of “at least one additional adequate and well - controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye.” • Through the Special Protocol Assessment process and written Advice Letter comments, the FDA provided feedback on the clinical trial protocol and statistical plan. • Aldeyra believes the Phase 3 clinical trial satisfies the FDA’s NDA resubmission requirement. NDA resubmission is anticipated in 2024. Based on FDA guidance, the anticipated review period for the potential NDA resubmission is expected to be six months. † NDA submission requirements depend, in part, on regulatory feedback. Regulatory review and discussion timelines are flexible and subject to change based on the regulator's workload and other potential review issues. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no obs erved safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical tria ls. NDA = New Drug Application; FDA = U.S. Food & Drug Administration.
7 Aldeyra has Entered into an Exclusive Option Agreement with AbbVie Inc. for License to Develop and Commercialize Reproxalap Option for AbbVie to obtain: • Co - exclusive license to develop, manufacture, and commercialize reproxalap in the U.S. • Exclusive license to develop, manufacture, and commercialize outside the U.S. Financial terms of license if option exercised: • Upfront payment of $100 million less option fees • $100 million milestone payment upon U.S. FDA approval in dry eye disease • $200 million in additional regulatory and commercial milestones • Profit and loss share (60% for AbbVie/40% for Aldeyra) from commercialization in U.S. • Tiered royalties on net sales outside of U.S. Key Terms of Reproxalap Option Agreement The option terminates on the earlier of (a) the 10th business day after the date on which Aldeyra received approval from the U.S . FDA of the NDA for reproxalap in dry eye disease and (b) the date that is 18 months after October 31, 2023. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients w ith no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials.

8 Reproxalap Represents a Novel Potential Therapeutic Approach in Dry Eye Disease with Rapid Activity in Clinical Trials Potential advantages for patients and healthcare providers could effect a paradigm shift relative to standard of care. † Company estimates and Am J Ophthalmol . 2014;157(4):799 - 806. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,5 00 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adve rse event in clinical trials. Dry Eye Disease Afflicts 39 Million or More Adults in the U.S. † Rapid and sustained symptom improvement Broad symptomatic activity Acute reduction of ocular redness







