Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10
GENERAL FORM FOR REGISTRATION OF SECURITIES
Pursuant to Section 12(b) or (g) of The Securities Exchange Act of 1934
| WORLDWIDE STRATEGIES INC. |
| (Exact name of registrant as specified in its charter) |
Nevada | | 41-0946897 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
1961 NW 150 Avenue Suite 205 Pembroke Pines, Florida | | 33028 |
| (Address of principal executive office) | | (Zip Code) |
Registrant’s telephone number including area code: 844-500-9974
Securities to be registered pursuant to Section 12(b) of the Act:
| None | | None |
| (Title of class) | | Name of each exchange on which each class is to be registered |
Securities to be registered pursuant to Section 12(g) of the Act:
| Common Stock, par value $0.001 per share | | None |
| (Title of class) | | Name of each exchange on which each class is to be registered |
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
TABLE OF CONTENTS
EXPLANATORY NOTE
Worldwide Strategies Inc. is filing this General Form for Registration of Securities on Form 10, or this “registration statement,” to register its common stock, par value $0.001 per share (“Common Stock”), pursuant to Section 12(g) of the Securities Exchange Act of 1934. Unless otherwise mentioned or unless the context requires otherwise, when used in this registration statement, the terms “Company,” “we,” “us,” “our” and “WWSG” refer to Worldwide Strategies Inc.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This following information specifies certain forward-looking statements of management of our Company. Forward-looking statements are statements that estimate the happening of future events and are not based on historical fact. Forward-looking statements may be identified by the use of forward-looking terminology, such as may, shall, could, expect, estimate, anticipate, predict, probable, possible, should, continue, or similar terms, variations of those terms, or the negative of those terms. The forward-looking statements specified in the following information have been compiled by our management on the basis of assumptions made by management and considered by management to be reasonable. Our future operating results, however, are impossible to predict and no representation, guaranty, or warranty is to be inferred from those forward-looking statements.
The assumptions used for purposes of the forward-looking statements specified in the following information represent estimates of future events and are subject to uncertainty as to possible changes in economic, legislative, industry, and other circumstances. As a result, the identification and interpretation of data and other information and their use in developing and selecting assumptions from and among reasonable alternatives require the exercise of judgment. To the extent that the assumed events do not occur, the outcome may vary substantially from anticipated or projected results, and, accordingly, no opinion is expressed on the achievability of those forward-looking statements.
The market data and other statistical information contained in this registration statement are based on internal Company estimates of our past experience in the industry, general market data, and public information which was not commissioned by us for this filing.
ITEM 1. BUSINESS
Overview
We are a science based direct to consumer (DTC) health company offering products and services focused on aging biology wellness and longevity. Our program is based on the book the Kaufmann Protocol® authored by our co-founder Dr. Sandra Kaufmann, M.D., and on identifying and offering individual specific services, recommendations and treatments designed to improve our customers’ lifespan and health-span. Whereas lifespan represents the total number of years we live, and health-span is how many of those years we remain healthy, active, energetic and free from disease. Our goal is to use science and technology, current and emerging treatments, for our customers to lengthen lifespan and maximize health-span.
We operate a DTC sales model, which means we market our products directly to our target consumers. We currently sell our book, the Kaufmann Protocol online, we offer, the Kaufmann Protocol, a mobile application on the iOS platform, and plan to commercialize and market a line of products, including our own branded molecular agents, health and wellness testing kits and services, as well as published and multimedia content.

Kaufmann Protocol
In the book The Kaufmann Protocol, Dr. Kaufmann explores the multifactorial causes of aging and presents strategies where aging is curtailed, these strategies are hereinafter referred to as the “Protocol”.
At the most basic level organisms age because their component cells age, the Protocol addresses the seven known theories, or the “Kaufmann Seven Tenets” of cellular aging into tenets, which are: Information Systems (DNA), Cellular Energy, Cellular Pathways, Quality Control, Immune System, Individual Cells and Waste Management.
| 1. | Information Systems (DNA). DNA is our information depot. Issues with aging in this category include epigenetic modification, accumulation of DNA damage, and telomeric integrity. Epigenetic modification encompasses changes to the “packaging” of the DNA, including methylation, histone modification and the like. Telomeres, the caps or ends of DNA, are known to shorten over time and are broadly correlated to the length of a life. |
| 2. | Cellular Energy. Mitochondria, cellular organelles, serve as our energy source. These organelles are rate limiting over time as their output declines, second to either damage from free radicals or simply declining availability of raw materials. |
| 3. | Cellular Pathways. The pathways are our aging or anti-aging pathways, such as the AMP Kinase, the Sirtuin or the mTOR pathways. These are like enzymatic dominoes that can either direct your cells and tissues to age or not age. |
| 4. | Quality Control. This category includes the DNA and Protein Repair mechanisms, which are key to repairing the ongoing damage inside your cells. As you get older and the damage becomes more extensive, these mechanisms get a bit stressed. This category also includes intracellular autophagy, a mechanism for cellular recycling. |
| 5. | Immune System. The cells that compose the immune system constitute your security system. Over time, unfortunately, this system becomes problematic and causes the body to be in a state of chronic and systemic inflammation. In addition, the failing immune system causes an increase in infection and cancer. |
| 6. | Individual Cells. Depending on the lifespan of particular cells, some live for days while some last a life time, their particular needs can be specialized. Some require an increased pool of nutrients, while others have more issues with trash accumulation. |
| 7. | Waste Management. Every cell has requirements for living, such as oxygen and glucose. Unfortunately, these can lead to increased aging. As an example, glucose forms molecular complexes called Advanced Glycation Endproducts (AGEs), which are very destructive. As well, longer lived cells produce cellular waste, called lipofuscin, that accumulates and eventually causes space issues. |
Our Products
Based on the Seven Tenets, the Protocol has identified a series of molecular agents, certain of which are dietary supplements and or prescription medications that can be used to combat aging and improve health-span. Many of the molecular agents we recommend have been used in eastern medicine for thousands of years based on their curative effects, and are generally available, none of which are proprietary to us. We do however identify and present individualized and in certain instances dosage specific individualized recommendations, based on algorithmic outputs of our software, based on individual biology, diet, lifestyle and desired outcomes. Three generalized, and our most popular regimens are:
| · | The Panacea; The General Strategy |
| · | The Sweet Tooth; The Anti-Glycation Strategy |
| · | The Ache Remedy; The Anti-Inflammatory Strategy |
We do not currently offer the molecular agents that makeup our Strategies. Our designed strategies, include a regimen of molecular agents, which currently must be sourced from third parties, however it is our plan to source and market the products which make up our proprietary strategies under our own brand, the Kaufmann Protocol, which we plan to offer through our website and mobile application.
Our Mobile Application
The mobile application guides users through a specific set of questions relating to your age and aging concerns and will use our proprietary algorithm to design a regimen scientifically calculated to achieve optimum results addressing your needs, based on the users’ responses. We expect our mobile and web applications will serve as a sales tool, for initial sales, reorders and as a means to connect with our customers to track their progress, deliver relevant and meaningful content and serve as a platform for future growth and expansion of our DTC product offerings.
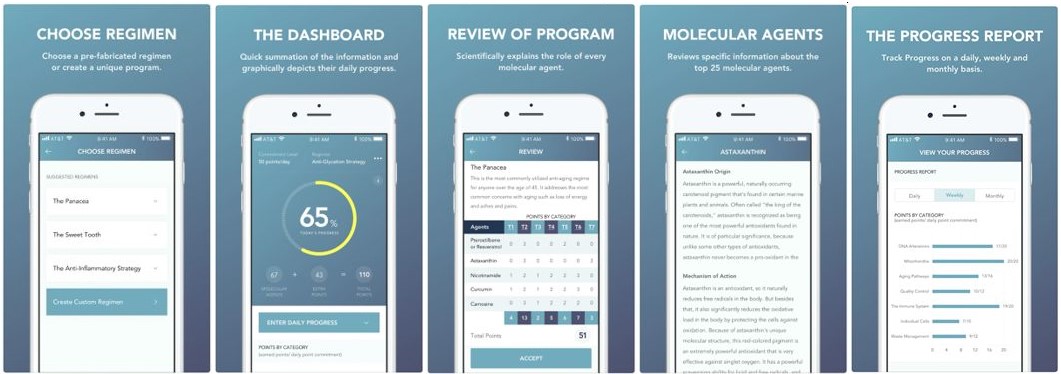
Market for our Products and Sales Strategy
Our target market spans “Baby Boomers” (71.2 million, US Census 2019), “Generation X” (65.2 million, US Census 2019) and the “Silent Generation” (23 million US Census 2019). We expect that our target market, within our customer cohort skews towards college, post graduate with significant disposable income and are actively seeking health & wellness solutions with a view towards maximizing quality of life, elongating lifespan and maximizing health-span.
We believe that there is a large audience of people who are interested in a science-based approach to healthy aging and lifespan enhancing strategies. Since it was published, the Kaufmann Protocol book has sold approximately ten thousand copies with no marketing or advertising spend, Dr. Kaufmann has participated in approximately 40 podcasts, and is consistently asked to speak and has spoken at leading anti-aging industry conferences.
We plan to source and market the molecular agents that make up our primary protocols, the Panacea, the Sweet Tooth and the Anti-Inflammatory and sell those products on our website, through our mobile application and on third party ecommerce platforms, specifically on Amazon through the Fulfillment by Amazon program, if we are accepted. Moreover, we intend to sell personalized protocols directly to users based on the results of our personalized evaluations which users will be able to access through our mobile application, and eventually through our website. We plan to drive traffic to our sales channels through paid advertising campaigns, content we generate and distribute directly through our own social media channels and in partnership with influencers who we may seek to engage. We expect that Dr. Kaufmann’s continued appearance on podcasts, as a speaker at industry conferences and in content we develop and distribute through our own distribution channels and on social media, will be effective at driving awareness of our products and as a result drive sales.
Competition.
We operate in the health & wellness industry and plan to generate revenues through the sale of nutritional supplements and health & wellness related content. Both the health & wellness and nutritional supplement industries are highly fragmented, and intensely competitive. We are an early-stage company and most of our competitors have longer operating histories, established customer bases with greater marketing reach and visibility, they have more operating experience and greater financial resources than we do. Nutritional supplements are available through mass-market retailers, drug stores, supermarkets, discount stores, health food stores, mail order companies, and direct sales organizations. We will also compete with, bio-hackers, authors and other content providers who have written books on anti-aging, specialized anti-aging companies, medical doctors and practices specializing in longevity medicine, and med-spas and facilities which offer anti-aging treatments, each of whom have longer operating histories, may be more capitalized and better positioned than we are. We expect that in each category named above there are formidable competitors and competition is intense.
Sources and Availability of Raw Materials and the Names of Principal Suppliers.
We have no present commitments or agreements with respect to the purchase of any raw materials needed for the production of our molecular agent and or dietary supplement-based products; however, management believes that there is an adequate available supply of these materials from various suppliers and there is and will be no constraint on us in sourcing the raw materials we need to make our finished products. We may have to purchase raw materials based on certain minimum quantities; however, we believe that purchasing said quantities and any supply agreements that require minimum purchase commitments will be available to us at reasonable and commercial terms. We plan to enter exclusive supply agreements and manufacturing agreements to protect our products, regulate product costs, and help ensure quality control standards.
Intellectual Property
We hold a fully pre-paid license, hereinafter the “Kaufmann License,” of the intellectual property including the book “the Kaufmann Protocol” the associated copyrights and trademarks, relating to the book together the “Kaufmann Protocol IP,” and all media, websites, recordings, podcasts, software, apps, customer lists, images, marketing, promoting and advertising the Kaufmann Protocol IP and any and all improvements and additions thereto. The license was contributed to us by Dr. Kaufmann in connection with her co-founding our company.
Government Regulations
The FDA regulates the formulation, manufacturing, packaging, storage, labeling, promotion, distribution, and sale of foods, dietary supplements, over-the-counter drugs, medical devices, and pharmaceuticals. In January 2000, the FDA issued a final rule called “Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of the Body”. In the rule and its preamble, the FDA distinguished between permitted claims under the Federal Food, Drug and Cosmetic Act (the “FFDC Act”) relating to the effect of dietary supplements on the structure or functions of the body, and impermissible direct or implied claims of the effect of dietary supplements on any disease. In June 2007, the FDA issued a rule, as authorized under the FFDC Act, that defined current Good Manufacturing Practices in the manufacture and holding of dietary supplements. Effective January 1, 2006, legislation required specific disclosures in labeling where a food, including a dietary supplement, contains an ingredient derived from any of eight named allergens. Legislation passed at the end of 2006 requires the reporting to the FDA any reports of “serious adverse events” associated with the use of a dietary supplement or an over-the-counter drug that is not covered by new drug approval reporting. The FDA created the Office of Dietary Supplements (“ODSP”) on December 21, 2015. The creation of this new office elevates the FDA’s program from its previous status as a division under the Office of Nutrition and Dietary Supplements. ODSP will continue to monitor the safety of dietary supplements.
The Dietary Supplement Health and Education Act of 1994, referred to as DSHEA, revised the provisions of the FFDC Act concerning the composition and labeling of dietary supplements and statutorily created a new class entitled “dietary supplements.” Dietary supplements include vitamins, minerals, herbs, amino acids, and other dietary substances used to supplement diets. A majority of our products are considered dietary supplements as outlined in the FFDC Act, which requires us to maintain evidence that a dietary supplement is reasonably safe. A manufacturer of dietary supplements may make statements concerning the effect of a supplement or a dietary ingredient on the structure or any function of the body, in accordance with the regulations described above. As a result, we will be required to make such statements with respect to any of the products we offer or may offer in the future. In some cases, such statements must be accompanied by a statutory statement that the claim has not been evaluated by the FDA and that the product is not intended to treat, cure, mitigate, or prevent any disease, and the FDA must be notified of such claim within 30 days of first use.
The FDA oversees product safety, manufacturing, and product information, such as claims on a company's website, product’s label, package inserts, and accompanying literature. The FDA has promulgated regulations governing the labeling and marketing of dietary and nutritional supplement products. The regulations include:
| · | the identification of dietary or nutritional supplements and their nutrition and ingredient labeling; |
| · | requirements related to the wording used for claims about nutrients, health claims, and statements of nutritional support; |
| · | labeling requirements for dietary or nutritional supplements for which “high potency,” “antioxidant,” and “trans-fatty acids” claims are made; |
| · | notification procedures for statements on dietary and nutritional supplements; and |
| · | pre-market notification procedures for new dietary ingredients in nutritional supplements. |
In certain markets, including the United States, specific claims made with respect to a product may change the regulatory status of a product. For example, a product sold as a dietary supplement but marketed as a treatment, prevention, or cure for a specific disease or condition would likely be considered by the FDA or other regulatory bodies as unapproved and thus an illegal drug. To maintain a product’s status as a dietary supplement, its labeling and marketing must comply with the provisions in DSHEA and the FDA’s extensive regulations.
Dietary supplements are also subject to the Nutrition, Labeling and Education Act and various other acts that regulate health claims, ingredient labeling, and nutrient content claims that characterize the level of nutrients in a product. These acts prohibit the use of any specific health claim for dietary supplements unless the health claim is supported by significant scientific research and is pre-approved by the FDA.
The FTC and other regulators regulate marketing practices and advertising of a company and its products. Regulators have instituted and continue to bring enforcement actions against numerous dietary supplement companies for false and/or misleading marketing practices, as well as misleading advertising of products. These enforcement actions have resulted in consent decrees and significant monetary judgments against the companies and/or individuals involved. Regulators require a company to convey product claims clearly and accurately and further require marketers to maintain adequate substantiation for their claims. More specifically, the FTC requires such substantiation to be competent and reliable scientific evidence and requires a company to have a reasonable basis for the expressed and implied product claim before it disseminates an advertisement. A reasonable basis is determined based on the claims made, how the claims are presented in the context of the entire advertisement, and how the claims are qualified. The FTC’s standard for evaluating substantiation is designed to ensure that consumers are protected from false and/or misleading claims by requiring scientific substantiation of product claims at the time such claims are first made. The failure to have this substantiation violates the Federal Trade Commission Act.
Due to the diverse scope of regulations applicable to our planned products and the various regulators enforcing these requirements, determining how to conform to all requirements is often open to interpretation and debate. However, our policy is and will continue to be, to fully cooperate with any regulatory agency in connection with any inquiries or other investigations. We can make no assurances that regulators will not question our actions in the future, even though we continue to make efforts to comply with all applicable regulations, inquiries, and investigations.
History of Our Company
Worldwide Strategies Incorporated ("we", "us", or "our") was originally incorporated in the State of Nevada on April 6, 1998 as Boyd Energy Corporation, on July 17, 2001 the corporation’s name was changed to Barnett Energy Corporation and on June 15, 2005, pursuant to a business combination with Worldwide Business Solutions Incorporated, a Colorado corporation ("WBSI"), WBSI became a wholly-owned subsidiary of the company and the corporation’s name was changed to Worldwide Strategies Inc. On July 31, 2007, we acquired 100% of the issued and outstanding shares of Centric Rx, Inc., a Nevada corporation which was merged out of existence in connection with the share exchange. We subsequently ceased operations in 2015 and the Company has fully impaired all assets since the shutdown of its operations in 2015 and recorded the effects of this impairment as part of its discontinued operations. As a result of our discontinuation of operations, on August 1, 2017 and January 2, 2018, respectively our two subsidiaries were dissolved for non-payment of annual fees. Therefore, Worldwide Business Solutions Incorporated, a Colorado corporation and Worldwide Business Solutions Limited, a United Kingdom corporation, a subsidiary of Worldwide Business Solutions Incorporated, are no longer subsidiaries of the Company.
On May 7, 2019, the Eighth Judicial District Court of Nevada appointed Small Cap Compliance, LLC (“Custodian”) as custodian for Worldwide Strategies Inc., and on May 8, 2019, the Custodian appointed an executive officer and board member, who on July 10, 2019, filed a certificate of reinstatement of WWSG with the state of Nevada. On October 16, 2019, the Eighth Judicial District Court of Nevada discharged Small Cap Compliance, LLC as custodian for Worldwide Strategies Inc. On July 10, 2019 the Custodian appointed board member and sole executive officer, appointed a new member to the board of directors and subsequently resigned from the board and as the company’s sole executive officer. The board of directors subsequently appointed the current management team, who are reorganizing the business as a health technology company.
Employees
As of June 21, 2021, we had two employees, our CEO and CFO, each of whom are part-time employees and each of whom are our founders.
ITEM 1A. RISK FACTORS.
RISK FACTORS
Risks Relating to Our Business and Industry.
We Have a limited Operating History Within this Industry, and we may not Succeed.
We have limited specific operating history or experience in procuring, marketing and selling anti-aging products and as such, within this industry we may not succeed. Moreover, we are subject to all risks inherent in a developing a new business enterprise. Our likelihood of success must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with establishing a new business and the competitive environment in which we operate. For example, we will need to develop supply chains for our molecular agents, develop systems and processes for fulfillment, logistics and customer service and we will need to develop and execute effective communications, marketing and sales campaigns.
You should further consider, among other factors, our prospects for success in light of the risks and uncertainties encountered by companies that, like us, are in their early stages. For example, unanticipated expenses, delays and or complications with sourcing raw materials and managing time-sensitive inventories. We may not successfully address these risks and uncertainties or successfully implement our operating strategies. If we fail to do so, it could materially harm our business to the point of having to cease operations and could impair the value of our common stock to the point our investors may lose their entire investment
We have not yet commenced operations which will generate revenue for the company.
We plan to generate revenue through the commercialization of the Protocol described in the book the Kaufmann Protocol, which includes driving sales of the book and the molecular agents identified in Dr. Kaufmann’s book. While we believe that our products will benefit our customers, and we believe that our fundraising efforts will enable us to execute our business plan, there are no assurances when we will begin generating revenue, how much revenue we will generate and if the revenues we generate will be sufficient to cover our operations, or if we will generate revenues at all. If we are unable to generate revenues from the sale of Protocol related products and content, we would be hard pressed to identify a new business model within the anti-aging space that would generate revenues in a reasonable amount of time, if at all.
We are smaller and less diversified than many of our competitors.
Many of the producers of anti-aging, health & wellness and longevity products with which we compete are part of large diversified corporate groups with a variety of other operations, more extensive product lines, which provide stable sources of earnings that may allow them to better offset fluctuations in the financial performance of their operations. In addition, larger media and health & wellness companies have more resources with which to compete for customers. The resources of larger companies may also give them an advantage in scaling marketing campaigns, procuring lower prices for raw materials as well as finished products.
We depend upon key personnel, the loss of which could seriously harm our business.
Our operating performance is substantially dependent on the continued services of our executive officers, Mr. Pavan Charan, Mr. Adam Laufer, who also serve on our Board of Directors, as well as our board member, Dr. Sandra Kaufmann. We believe our executives collective knowledge and experience would be difficult to replicate. We have not entered into an employment agreement with either Messrs. Charan, Laufer or Dr. Kaufmann and, although we are considering doing so. We have not secured any key-person life insurance on our officers or directors. The unexpected loss of the services of Dr. Kaufmann, Messrs. Charan or Laufer could have a material adverse effect on our business, operations, financial condition and operating results, as well as the value of our common stock.
If we are unable to obtain and maintain intellectual property protection for our technology, content and products, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize technology, content and products similar or identical to ours, and our ability to successfully commercialize our existing intellectual property and any intellectual property we may develop in the future, may be adversely affected.
Our commercial success will depend in large part on our ability to obtain and maintain appropriate intellectual property protections, including but not limited to patent, trademark, trade secret and other intellectual property protection of our content, products and other technology including our protocols, and methods of treatment, as well as successfully defending our patent and other intellectual property rights against third-party challenges. It is difficult and costly to protect our technology and products, and we may not be able to ensure their protection. Our ability to stop unauthorized third parties from making, using, selling, offering to sell, importing or otherwise commercializing our products or products similar or indistinguishable from ours is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these assets could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Any significant disruption in the computer systems of third parties that we utilize in our operations could result in a loss or degradation of service and could adversely impact our business.
Our reputation and ability to sell our products and serve our customers through our websites and applications is dependent upon the reliable performance of the computer systems of third parties that we utilize in our operations. These systems may be subject to damage or interruption from earthquakes, adverse weather conditions, other natural disasters, terrorist attacks, power loss, telecommunications failures, computer viruses, computer denial of service attacks or other attempts to harm these systems. Interruptions in these systems or to the internet in general, could make our services unavailable or impair our ability to sell our products and or serve our customers.
If we violate governmental regulations or fail to obtain necessary regulatory approvals, our operations could be adversely affected.
Our operation is subject to extensive laws, governmental regulations, administrative determinations, court decisions, and similar constraints at the federal, state, and local levels in our domestic and foreign markets. These regulations primarily involve the following:
| · | the formulation, manufacturing, packaging, labeling, distribution, importation, sale, and storage of our products; |
| · | the health and safety of dietary supplements; |
| · | our product claims and advertising; |
| · | the assessment of customs duties; |
| · | further taxation of our independent associates, which may obligate us to collect additional taxes and maintain additional records; and |
| · | export and import restrictions. |
Any unexpected new regulations or changes in existing regulations could significantly restrict our ability to continue operations, which could adversely affect our business. For example, changes regarding health and safety and food and drug regulations for our nutritional products could require us to reformulate our products to comply with such regulations.
Increased regulatory scrutiny of nutritional supplements as well as new regulations that are being adopted in some of our markets with respect to nutritional supplements could result in more restrictive regulations and harm our results if our supplements or advertising activities are found to violate existing or new regulations or if we are not able to effect necessary changes to our products in a timely and efficient manner to respond to new regulations.
There has been an increasing movement in the United States and other markets to increase the regulation of dietary supplements, which could impose additional restrictions or requirements on us and increase the cost of doing business. On February 11, 2019, the FDA issued a statement from FDA Commissioner, Dr. Scott Gottlieb, regarding the agency's efforts to strengthen the regulation of dietary supplements. The FDA will be prioritizing and focusing resources on misbranded products bearing unproven claims to treat, cure, or mitigate disease. Commissioner Gottlieb established a Dietary Supplement Working Group tasked with reviewing the agency's organizational structure, process, procedures, and practices to identify opportunities to modernize the oversight of dietary supplements. Additionally, on December 21, 2015, the FDA created the Office of Dietary Supplements (“ODSP”). The creation of this new office elevates the FDA’s program from its previous status as a division under the Office of Nutrition and Dietary Supplements. ODSP will continue to monitor the safety of dietary supplements. In markets outside of the United States, prior to commencing operations or marketing new products, we may be required to obtain approvals, registrations, licenses, or certifications from an agency comparable to the FDA for the specific market. Approvals or registration may require reformulation of our products or may be unavailable to us with respect to certain products or ingredients. We must also comply with product labeling regulations, which vary by jurisdiction.
In August 2016, the FDA published its revised draft guidance on Dietary Supplements: New Dietary Ingredient Notifications and Related Issues. If a company sells a dietary supplement containing an ingredient that FDA considers either not a dietary ingredient or a new dietary ingredient (“NDI”) that needs an NDI notification, the agency may threaten or initiate enforcement against the Company. For example, it might send a warning letter that can trigger consumer lawsuits, demand a product recall, or even work with the Department of Justice to bring a criminal action. Our operations could be harmed if new guidance or regulations require us to reformulate products or effect new registrations, if regulatory authorities make determinations that any of our products do not comply with applicable regulatory requirements, if the cost of complying with regulatory requirements increases materially, or if we are not able to effect necessary changes to our products in a timely and efficient manner to respond to new regulations. In addition, our operations could be harmed if governmental laws or regulations are enacted that restrict the ability of companies to market or distribute nutritional supplements or impose additional burdens or requirements on nutritional supplement companies.
If our outside suppliers and manufacturers fail to supply products in sufficient quantities and in a timely fashion, our business could suffer.
Outside manufacturers will make all of our products. We will be dependent on outside suppliers and manufacturers to supply us with products in a timely and cost-efficient manner. We believe there are dependable suppliers for all of the ingredients we require for the products we plan to sell, however if we are unable to find and retain suppliers and or our suppliers are unable to perform and we are unable to find replacement suppliers, our business operations would be adversely affected.
The loss of suppliers or shortages of raw materials could have an adverse effect on our business, financial condition, or results of operations.
We will depend on outside suppliers for raw materials. We expect that some if not all of our contract manufacturers will acquire the raw materials for manufacturing our products from third-party suppliers. In the event we were to lose any significant suppliers and have trouble in finding or transitioning to alternative suppliers, it could result in product shortages or product back orders, which could harm our business. There can be no assurance that suppliers will be able to provide our contract manufacturers the raw materials in the quantities and at the appropriate level of quality that we request or at a price that we are willing to pay. We are also subject to delays caused by any interruption in the production of these materials including weather, disease, crop conditions, climate change, transportation interruptions and natural disasters or other catastrophic events. For example, in December 2019, COVID-19 was first identified in Wuhan, Hubei Province, China. While initially the outbreak was largely concentrated in China and caused significant disruptions to its economy, it has now spread to several other countries and infections have been reported globally. The extent to which COVID-19 impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others. In particular, the continued spread of COVID-19 globally could adversely impact our operations, including among others, our manufacturing and supply chain, sales and marketing and clinical trial operations and could have an adverse impact on our business and our financial results.
The occurrence of natural or man-made disasters could result in declines in business that could adversely affect our financial condition, results of operations and cash flows.
We are exposed to various risks arising out of natural disasters, including earthquakes, hurricanes, fires, floods, landslides, tornadoes, typhoons, tsunamis, hailstorms, explosions, climate events or weather patterns and pandemic health events (such as the recent pandemic spread of the novel corona virus known as COVID-19 virus, duration and full effects of which are still uncertain), as well as man-made disasters, including acts of terrorism, military actions, cyber-terrorism, explosions and biological, chemical or radiological events. The continued threat of terrorism and ongoing military actions may cause significant volatility in global financial markets, and a natural or man-made disaster could trigger an economic downturn in the areas directly or indirectly affected by the disaster. These consequences could, among other things, result in a decline in business. Disasters also could disrupt public and private infrastructure, including communications and financial services, which could disrupt our normal business operations. A natural or man-made disaster also could disrupt the operations of our partners and counterparties or result in increased prices for the products and services they provide to us.
If we are exposed to product liability claims, we may be liable for damages and expenses, which could affect our overall financial condition.
We could face financial liability from product liability claims if the use of our products results in significant loss or injury. We can make no assurances that we will not be exposed to any substantial future product liability claims. Such claims may include claims that our products contain contaminants, that we provide consumers with inadequate instructions regarding product use, or that we provide inadequate warnings concerning side effects or interactions of our products with other substances. We believe that, our suppliers, and our manufacturers maintain adequate product liability insurance coverage, and we believe that product liability insurance will be available to us at reasonable terms. However, a substantial future product liability claim could exceed the amount of insurance coverage or could be excluded under the terms of an existing insurance policy, which could adversely affect our overall future financial condition.
In recent years, a discovery of Bovine Spongiform Encephalopathy (“BSE”), which is commonly referred to as “Mad Cow Disease”, has caused concern among the general public. As a result, some countries have banned the importation or sale of products that contain bovine materials sourced from locations where BSE has been identified. We have changed the vast majority of our capsules to a vegetable base. However, if a vegetable base is not available or practical for use, certifications are required to ensure the capsule material is BSE-free. The higher costs could affect our financial condition, results of operations, and our cash flows.
The global nutrition and skin care industries are intensely competitive and the strengthening of any of our competitors could harm our business.
The global nutrition and skin care industries are intensely fragmented and competitive. We compete with other global nutrition and skin care industries. Many of our competitors have greater name recognition and financial resources, which may give them a competitive advantage. Our competitors may also be able to devote greater resources to marketing, promotional, and pricing campaigns to lead customers to buy products from competitors rather than from us. Such competition could adversely affect our business.
A downturn in the economy, including as a result of COVID-19, could affect consumer purchases of discretionary items such as the health and wellness products that we offer, which could have an adverse effect on our business, financial condition, profitability, and cash flows.
A downturn in the economy, including as a result of COVID-19, could adversely impact consumer purchases of discretionary items such as health and wellness products. The United States and global economies may slow dramatically as a result of a variety of problems, including turmoil in the credit and financial markets, concerns regarding the stability and viability of major financial institutions, the state of the housing markets, and volatility in worldwide stock markets. In the event of such economic downturn, the U.S. and global economies could become significantly challenged in a recessionary state for an indeterminate period of time. These economic conditions could negatively affect demand for our products for some time, which in turn could harm our business by adversely affecting our revenues, results of operations, cash flows and financial condition. We cannot predict these economic conditions or the impact they would have on our consumers or business.
Adverse or negative publicity could cause our business to suffer.
Our business depends, in part, on the public’s perception of our integrity and the safety and quality of our products. Any adverse publicity could negatively affect the public’s perception about our industry, our products, or our reputation and could result in a significant decline in our operations. Specifically, we are susceptible to adverse or negative publicity regarding:
| · | the nutritional supplements industry; |
| · | skeptical consumers; |
| · | competitors; |
| · | the safety and quality of our products and/or our ingredients; |
| · | regulatory investigations of our products or our competitors’ products; |
If our information technology system fails or if the implementation of new information technology systems is not executed efficiently and effectively, our business, financial position, and operating results could be adversely affected.
Like many companies, our business is heavily dependent upon our information technology infrastructure to effectively manage and operate many of our key business functions, including:
| · | order processing; |
| · | supply chain management; |
| · | customer service; |
| · | product distribution; |
| · | cash receipts and payments; and |
| · | financial reporting. |
These systems and operations are vulnerable to damage and interruption from fires, earthquakes, telecommunications failures, and other events. They are also subject to break-ins, sabotage, intentional acts of vandalism and similar misconduct. Although we maintain an extensive security system and business continuity program that was developed under the guidelines published by the National Institute of Standards of Technology, a long-term failure or impairment of any of our information technology systems could adversely affect our ability to conduct day-to-day business.
Occasionally information technology systems must be upgraded or replaced and if this system implementation is not executed efficiently and effectively, the implementation may cause interruptions in our primary management information systems, which may make our website or services unavailable thereby preventing us from processing transactions, which would adversely affect our financial position or operating results.
The regulatory climate for data privacy and protection continues to grow in scope and complexity both domestically and in the international markets in which we operate. Although there is no single federal law in the United States imposing a cross-sectoral data breach notification obligation, virtually every state has enacted breach notification requirements. Additionally, many of the international countries in which we operate have proposed or enacted laws or regulations on the appropriate use and disclosure of financial and personal data. The European Union (“EU”) adopted the General Data Protection Regulation (“GDPR”) on April 27, 2016. The GDPR went into effect on May 25, 2018. The GDPR applies to organizations based in the EU and organizations based outside of the EU that offer products or services to individuals in the EU or that otherwise monitor individuals in the EU. While U.S. state laws generally cover specific categories of sensitive personal data (e.g., social security numbers, bank account numbers, and credit card numbers), the GDPR notification requirements will apply to incidents involving any personal data, meaning any data related to an identified person. In Canada, the Personal Information Protection and Electronic Documents Act (“PIPEDA”) went into effect on November 1, 2018. PIPEDA applies to foreign organizations with a real and substantial link to Canada that collect, use, or disclose the personal information of Canadians in the course of their commercial activities. Under PIPEDA, an organization must notify individuals of any breach of the security of safeguards involving their personal information if it is reasonable to believe that the breach creates a “real risk of significant harm.” Concurrently, the organization must also report to the Privacy Commissioner of Canada. As noted above, many states have enacted data protection requirements. Most recently, the California Consumer Privacy Act ("CCPA"), a state statute signed into law on June 28, 2018 and effective on January 1, 2020, provides enhanced data privacy protections to California residents. The CCPA applies to companies with annual gross revenues in excess of $25 million. Our failure or inability to comply with data protection regimes domestically and in foreign countries could result in fines, penalties, injunctions, or material litigation expenditures.
With increased frequency in recent years, cyber-attacks against companies have resulted in breaches of data security. Our business requires the storage and transmission of suppliers’ data and customers’ personal, credit card, and other confidential information. Our information technology systems are susceptible to a growing and evolving threat of cybersecurity risk. Any substantial compromise of our data security, whether externally or internally, or misuse of associate, customer, or employee data, could cause considerable damage to our reputation, cause the public disclosure of confidential information, and result in lost sales, significant costs, and litigation, which would negatively affect our financial position and results of operations. We currently do not have insurance to protect us from claims surrounding the protection of sensitive data, and we have no assurances that we will be able to insure against these risks, and if we are if the cost of insurance will be available at reasonable terms and if such coverage would be adequate, and there can be no assurances that we will not be subject to such claims in the future.
There is no established market four our stock and our common stock is not listed on a stock exchange; as a result, stockholders may not be able to resell their shares at or above the price paid for them.
There is not established market for our common stock. Our common stock is listed on the OTC market under ticker symbol WWSG; stocks which trade on the OTC markets tend to be illiquid and volatile and could be subject to significant fluctuations due to changes in sentiment in the market regarding our operations or business prospects, among other factors. Further, our common stock is not listed on a stock exchange, nor do we currently intend to list the common stock on a stock exchange. There are no assurances that an active public market for our common stock will develop. Therefore, stockholders may not be able to sell their shares at or above the price they paid for them.
Other factors that could affect our stock price are:
| · | broad market fluctuations and general economic conditions; |
| · | fluctuations in our financial results; |
| · | future securities offerings; |
| · | changes in the market’s perception of our products or our business, including false or negative publicity; |
| · | governmental regulatory actions; |
| · | the outcome of any lawsuits; |
| · | financial and business announcements made by us or our competitors; |
| · | the demand and daily trading volume of our shares; |
| · | the general condition of the industry; and |
| · | the sale of large amounts of stock by insiders. |
In addition, the stock market has experienced extreme price and volume fluctuations in recent years that have significantly affected the quoted prices of the securities of many companies. The changes sometimes appear to occur without regard to specific operating performance. The price of our common stock in the open market could fluctuate based on factors that have little or nothing to do with us or that are outside of our control. For example, general economic conditions, such as recession or interest rate or currency rate fluctuations in the United States or abroad, could negatively affect the market price of our common stock in the future.
Our management controls a large block of our common stock that will allow them to control us.
As of June 21, 2021, while members of our management team and affiliates beneficially don’t own any of our outstanding common stock, they do own shares of our preferred stock, which are convertible to 90.92% of our common stock. As such, management owns approximately 90.92% of our voting power and controls the Company. As a result, management has the ability to control substantially all matters submitted to our stockholders for approval including:
| · | election of our board of directors; |
| · | removal of any of our directors; |
| · | removal of any of our directors; |
| · | amendment of our articles of incorporation or bylaws; and |
| · | adoption of measures that could delay or prevent a change in control or impede a merger, takeover or other business combination involving us. |
In addition, management's stock ownership may discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
Sales by our stockholders of a substantial number of shares of our common stock in the public market could adversely affect the market price of our common stock.
A substantial portion of our total outstanding shares of common stock may be sold into the market by our principal stockholders, who are also executive officers, and while we believe that such holders have no current intention to sell a significant number of shares of our stock, if our principal stockholders were to decide to sell large amounts of stock over a short period of time such sales could cause the market price of our common stock to drop significantly, even if our business is doing well.
Further, the market price of our common stock could decline as a result of the perception that such sales could occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and price that we deem appropriate. We currently have 19,830,679 shares of common stock outstanding, 3,106,228 of which are freely tradable without restriction under the Securities Act.
We are not required to pay dividends, and our Board of Directors may decide not to declare dividends in the future.
The declaration of dividends on our common stock is solely within the discretion of our Board of Directors, subject to limitations under Texas law stipulating that dividends may not be paid if payment therefore would cause the corporation to be insolvent or if the amount of the dividend would exceed the surplus of the corporation. Our Board of Directors may decide not to declare dividends or we could be prevented from declaring a dividend because of legal or contractual restrictions. The failure to pay dividends could reduce our stock price.
Going concern report of independent certified public accountants.
Our limited history of operations and our absence of revenues to date raise substantial doubt about our ability to continue as a going concern. In this regard, see the Report of Independent Certified Public Accountants accompanying our audited financial statements appearing elsewhere herein which cites substantial doubt about our ability to continue as a going concern. There can be no assurance that we will achieve profitability or generate positive cash flow in the future. As a result of these and other factors, there can be no assurance that our proposed activities will be successful or that the Company will be able to achieve or maintain profitable operations. If we fail to achieve profitability, our growth strategies could be materially and adversely affected.
In the event that we do not generate adequate cash flow from operations, we will need to raise money through a debt or equity financing, if available, or curtail operations.
If we are unsuccessful in generating positive cash flow from operations, we could exhaust whatever cash resources we may have on hand, if any, and be required to secure additional funding through a debt or equity financing, significantly scale back our operations, and/or discontinue many of our activities, which could negatively affect our business and prospects. Additional funding may not be available or may only be available on unfavorable terms.
If we experience rapid growth, we may not manage our growth effectively, execute our business plan as proposed or adequately address competitive challenges.
If we are successful in executing our business plan and grow at an accelerated rate beyond our expectation, such growth could place a significant strain on our management, administrative, operational and financial infrastructure. Our long-term success will depend, in part, on our ability to manage this growth effectively, grow our internal resources as required, including management and staff personnel. To manage the expected growth of our operations and personnel, we also will need to increase our internal operational, financial and management controls, and our reporting systems and procedures. Failure to effectively manage growth could result in an ability to meet customer orders in a timely manner, if at all, and possibly damaging our reputation, resulting in the loss of existing and or potential customers, wasting of financial resources, and realizing lost opportunities. Any of these difficulties could adversely impact our business financial condition, operating results, liquidity and prospects.
To be successful, we need to attract and retain qualified personnel.
Our success will depend to a significant extent on our ability to identify, attract, hire, train and retain qualified professional and managerial personnel. Competition for qualified employees is significant. We cannot assure you that we will be successful in identifying, attracting, hiring, training and retaining such personnel in the future. If we were unable to hire, assimilate and retain qualified personnel in the future, such inability could have a material adverse effect on our business, financial condition, operating results, liquidity and prospects.
The spread of COVID-19 underscores certain risks we face, and the rapid development and fluidity of this situation precludes any prediction as to the ultimate adverse impact to us of COVID-19.
In December 2019, COVID-19 was reported to have surfaced in Wuhan, China. COVID-19 has since spread to over 100 countries, including every state in the United States. On March 11, 2020 the World Health Organization declared COVID-19 a pandemic, and on March 13, 2020 the United States declared a national emergency with respect to COVID-19. The spread of COVID-19 underscores certain risks we face in our business that are described in this disclosure document. Governmental and non-governmental organizations may not effectively combat the spread and severity of COVID-19, which could adversely impact our profitability. The adverse economic effects of COVID-19 may materially decrease demand for our products based on changes in consumer behavior or the restrictions in place by governments trying to curb the outbreak. For example, we have rescheduled corporate sponsored events, and in some cases, our associates have canceled sales meetings. This could lead to adverse impacts on our sales in fiscal year 2020 and our overall liquidity.
The spread of COVID-19, or actions taken to mitigate this spread, could have material and adverse effects on our ability to operate effectively, including as a result of the complete or partial closure of certain businesses and the inability of our associates to market our products as a result of “shelter-in-place” and similar policies that may be implemented in an effort to mitigate the spread of COVID-19. Furthermore, the outbreak of COVID-19 has severely impacted global economic activity, and caused significant volatility and negative pressure in the financial markets. We have started to experience challenges in getting raw materials and ingredients to our contract manufacturers and finished products to our distribution centers resulting from reductions in global transportation capacity.
The rapid development and fluidity of this situation precludes any prediction as to the ultimate adverse impact to us of COVID-19. We are continuing to monitor the spread of COVID-19 and related risks. The magnitude and duration of the pandemic and its impact on our business, results of operations, financial position, and cash flows is uncertain as this continues to evolve globally. However, if the spread continues on its current trajectory, such impact could grow and our business, results of operations, financial position, and cash flows could be materially adversely affected.
Having no independent directors on our board limits our ability to establish effective independent corporate governance procedures.
We do not have any independent directors on our board of directors nor do we maintain a standing audit committee, compensation committee or nominating and governance committee. Accordingly, without independent directors, we cannot establish effective standing board committees to oversee functions such as audit, compensation and corporate governance. In addition, our executive officers are also directors. This structure gives our executive officers significant control over all corporate issues.
Unless and until we have a larger board of directors that would include a majority of independent members, there will be limited oversight of our executive officers' decisions and activities and little ability for you to challenge or reverse those activities and decisions, even if they are not in your best interests.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results. As a result, we could become subject to sanctions or investigations by regulatory authorities and/or stockholder litigation, which could harm our business and have an adverse effect on our stock price.
As a public reporting company, we are required to comply with the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC, including periodic reports, disclosures and more complex accounting rules. As directed by Section 404 of Sarbanes-Oxley, the SEC adopted rules requiring public companies to include a report of management on a company's internal control over financial reporting in their Annual Report on Form 10-K. Based on current rules, we are required to report under Section 404(a) of Sarbanes-Oxley regarding the effectiveness of our internal control over financial reporting.
Requirements associated with being a reporting public company will require significant company resources and management attention.
Subsequent to effectiveness of this registration statement, we will be required to comply with the reporting requirements as promulgated under the Securities Exchange Act of 1934 which will require that we retain legal, accounting and financial advisors to ensure adequate disclosure and control systems to manage our growth and our obligations as a company that files reports with the SEC. These areas include corporate governance, internal control, internal audit, disclosure controls and procedures and financial reporting and accounting systems. However, we cannot assure you that these and other measures we may take will be sufficient to allow us to satisfy our obligations as an SEC reporting company on a timely basis.
In addition, compliance with reporting and other requirements applicable to SEC reporting companies will create additional costs for us, will require the time and attention of management and will require the hiring of additional personnel and legal, audit and other professionals. We cannot predict or estimate the amount of the additional costs we may incur, the timing of such costs or the impact that our management's attention to these matters will have on our business.
Our officers and directors have limited liability, and we are required in certain instances to indemnify our officers and directors for breaches of their fiduciary duties.
We have adopted provisions in our Articles of Incorporation and Bylaws, which limit the liability of our officers and directors and provide for indemnification by us of our officers and directors to the full extent permitted by Nevada corporate law. Our articles generally provide that our officers and directors shall have no personal liability to us or our shareholders for monetary damages for breaches of their fiduciary duties as directors, except for breaches of their duties of loyalty, acts or omissions not in good faith or which involve intentional misconduct or knowing violation of law, acts involving unlawful payment of dividends or unlawful stock purchases or redemptions, or any transaction from which a director derives an improper personal benefit. Such provisions substantially limit our shareholders' ability to hold officers and directors liable for breaches of fiduciary duty, and may require us to indemnify our officers and directors.
No audit or compensation committee
Because we do not have an audit or compensation committee, stockholders will have to rely on our entire Board of Directors, none of which are independent, to perform these functions. We do not have an audit or compensation committee comprised of independent directors. Indeed, we do not have any audit or compensation committee. These functions are performed by our Board of Directors as a whole. No members of our Board of Directors are independent directors. Thus, there is a potential conflict in that Board members who are also part of management will participate in discussions concerning management compensation and audit issues that may affect management decisions.
If we fail to establish and maintain proper and effective internal control over financial reporting, our operating results and our ability to operate our business could be harmed.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles. Currently we do not have appropriate controls in place, due in part to our size and lack of resources, however as soon as practicable, we intend to begin the process of documenting, reviewing and improving our internal controls and procedures for compliance with Section 404 of the Sarbanes-Oxley Act of 2002, or SOX, which will require annual management assessment of the effectiveness of our internal control over financial reporting.
Implementing any appropriate changes to our internal controls may distract our officers and employees, entail substantial costs to modify our existing processes and take significant time to complete. These changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate financial statements on a timely basis, could increase our operating costs and harm our business. In addition, investors’ perceptions that our internal controls are inadequate or that we are unable to produce accurate financial statements on a timely basis may harm our common share price and have an adverse effect on how we are perceived by customers, current and potential investors.
Any future litigation could have a material adverse impact on our results of operations, financial condition and liquidity, particularly since we do not currently have director and officer insurance. Our lack of D&O insurance may also make it difficult for us to retain and attract talented and skilled directors and officers.
From time to time, we may be subject to litigation, including potential stockholder derivative actions. Risks associated with legal liability are difficult to assess and quantify, and their existence and magnitude can remain unknown for significant periods of time. To date we have not procured directors and officers liability ("D&O") insurance to cover such risk exposure for our directors and officers. Such insurance generally pays the expenses (including amounts paid to plaintiffs, fines, and expenses including attorneys' fees) of officers and directors who are the subject of a lawsuit as a result of their service to the Company. While we are currently seeking such insurance, there can be no assurance that we will be able to do so at reasonable rates or at all, or in amounts adequate to cover such expenses should such a lawsuit occur. While neither Nevada law nor our articles of incorporation or bylaws require us to indemnify or advance expenses to our officers and directors involved in such a legal action, we expect that we would do so to the extent permitted by Nevada law. Without D&O insurance, the amounts we would pay to indemnify our officers and directors should they be subject to legal action based on their service to the Company could have a material adverse effect on our financial condition, results of operations and liquidity. Further, our lack of D&O insurance may make it difficult for us to retain and attract talented and skilled directors and officers, which could adversely affect our business.
Risks Related to the Market for our Stock
The reduced disclosure requirements applicable to us as a "smaller reporting company" may make our common stock less attractive to investors.
We are a "smaller reporting company" as defined in Rule 12b-2 of the Exchange Act. As a smaller reporting company, we prepare and file SEC forms similar to other SEC reporting companies; however, the information disclosed may differ and be less comprehensive. If some investors find our common stock less attractive as a result of less comprehensive information we may disclose pursuant to the exemptions available to us as a smaller reporting company, there may be a less active trading market for our common stock and our stock price may be more volatile than that of an otherwise comparable company that does not avail itself of the same or similar exemptions.
Circumstances and conditions may change. Accordingly, additional risks and uncertainties not currently known, or that we currently deem not material, may also adversely affect our business operations.
The OTC and share value
Our Common Stock trades over the counter, which may deprive stockholders of the full value of their shares. Our stock is quoted via the Over-The-Counter (“OTC”) Pink Sheets. Therefore, our Common Stock is expected to have fewer market makers, lower trading volumes, and larger spreads between bid and asked prices than securities listed on an exchange such as the New York Stock Exchange or the NASDAQ Stock Market. These factors may result in higher price volatility and less market liquidity for our Common Stock.
Volatility in our common stock price may subject us to securities litigation.
The market for our common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities to us and could divert our management's attention and resources from managing our operations and business.
Low market price
A low market price would severely limit the potential market for our Common Stock. Our Common Stock may trade at a price below $5.00 per share, subjecting trading in the stock to certain Commission rules requiring additional disclosures by broker-dealers. These rules generally apply to any non-NASDAQ equity security that has a market price share of less than $5.00 per share, subject to certain exceptions (a “penny stock”). Such rules require the delivery, prior to any penny stock transaction, of a disclosure schedule explaining the penny stock market and the risks associated therewith and impose various sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and institutional or wealthy investors. For these types of transactions, the broker-dealer must make a special suitability determination for the purchaser and have received the purchaser’s written consent to the transaction prior to the sale. The broker-dealer also must disclose the commissions payable to the broker-dealer, current bid and offer quotations for the penny stock and, if the broker-dealer is the sole market maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Such information must be provided to the customer orally or in writing before or with the written confirmation of trade sent to the customer. Monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. The additional burdens imposed upon broker-dealers by such requirements could discourage broker-dealers from effecting transactions in our Common Stock.
Lack of market and state blue sky laws
Investors may have difficulty in reselling their shares due to the lack of market or state Blue Sky laws. The holders of our shares of Common Stock and persons who desire to purchase them in any trading market that might develop in the future should be aware that there may be significant state law restrictions upon the ability of investors to resell our shares. Accordingly, even if we are successful in having the shares available for trading on the OTC, investors should consider any secondary market for our securities to be a limited one. We intend to seek coverage and publication of information regarding our Company in an accepted publication which permits a “manual exemption.” This manual exemption permits a security to be distributed in a particular state without being registered if the company issuing the security has a listing for that security in a securities manual recognized by the state. However, it is not enough for the security to be listed in a recognized manual. The listing entry must contain (1) the names of issuers, officers, and directors, (2) an issuer’s balance sheet, and (3) a profit and loss statement for either the fiscal year preceding the balance sheet or for the most recent fiscal year of operations. We may not be able to secure a listing containing all of this information. Furthermore, the manual exemption is a non-issuer exemption restricted to secondary trading transactions, making it unavailable for issuers selling newly issued securities. Most of the accepted manuals are those published in Standard and Poor’s, Moody’s Investor Service, Fitch’s Investment Service, and Best’s Insurance Reports, and many states expressly recognize these manuals. A smaller number of states declare that they “recognize securities manuals” but do not specify the recognized manuals. The following states do not have any provisions and therefore do not expressly recognize the manual exemption: Alabama, Georgia, Illinois, Kentucky, Louisiana, Montana, South Dakota, Tennessee, Vermont, and Wisconsin.
Accordingly, our shares of Common Stock should be considered totally illiquid, which inhibits investors’ ability to resell their shares.
Penny stock regulations
We will be subject to penny stock regulations and restrictions and you may have difficulty selling shares of our Common Stock. The Commission has adopted regulations which generally define so-called “penny stocks” to be an equity security that has a market price less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. We anticipate that our Common Stock will become a “penny stock”, and we will become subject to Rule 15g-9 under the Exchange Act, or the “Penny Stock Rule”. This rule imposes additional sales practice requirements on broker-dealers that sell such securities to persons other than established customers. For transactions covered by Rule 15g-9, a broker-dealer must make a special suitability determination for the purchaser and have received the purchaser’s written consent to the transaction prior to sale. As a result, this rule may affect the ability of broker-dealers to sell our securities and may affect the ability of purchasers to sell any of our securities in the secondary market.
For any transaction involving a penny stock, unless exempt, the rules require delivery, prior to any transaction in a penny stock, of a disclosure schedule prepared by the Commission relating to the penny stock market. Disclosure is also required to be made about sales commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements are required to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
We do not anticipate that our Common Stock will qualify for exemption from the Penny Stock Rule. In any event, even if our Common Stock were exempt from the Penny Stock Rule, we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the Commission the authority to restrict any person from participating in a distribution of penny stock, if the Commission finds that such a restriction would be in the public interest.
Rule 144 Risks
Sales of our Common Stock under Rule 144 could reduce the price of our stock. Our affiliates hold preferred shares that can be converted, to 291,926,606 shares of our Common Stock, which Rule 144 of the Securities Act defines as restricted securities.
These shares will be subject to the resale restrictions of Rule 144, should we hereinafter cease being deemed a “shell company”. In general, persons holding restricted securities, including affiliates, must hold their shares for a period of at least six months, may not sell more than 1.0% of the total issued and outstanding shares in any 90-day period, and must resell the shares in an unsolicited brokerage transaction at the market price. The availability for sale of substantial amounts of Common Stock under Rule 144 could reduce prevailing market prices for our securities.
Because we may be deemed a shell company, it will likely be difficult for us to obtain additional financing by way of private offerings of our securities or retain qualified employees or advisers.
We may be deemed a “shell company” within the meaning of Rule 405, promulgated pursuant to Securities Act, because we have nominal assets and nominal operations. Accordingly, the holders of securities purchased in private offerings of our securities we make to investors will not be able to rely on the safe harbor from being deemed an underwriter under SEC Rule 144 in order to resell their securities. This will likely make it more difficult for us to attract additional capital through subsequent unregistered offerings because purchasers of securities in such unregistered offerings will not be able to resell their securities in reliance on Rule 144, a safe harbor on which holders of restricted securities usually rely to resell securities. Furthermore, if we are deemed a “shell company,” we will be unable to utilize Form S-8 as a registration statement for automatic effectiveness for employees or advisers, thereby hampering our ability to hire or retain qualified employees or advisers.
If we are deemed a shell company, the shares we issue, if any, will be restricted from resale under Rule 144.
These shares are currently restricted from trading under Rule 144. They will only be available for resale, within the limitations of Rule 144, to the public if:
(i) We are no longer a shell company as defined under section 12b-2 of the Exchange Act. A “shell company” is defined as a company with no or nominal operations, and with no or nominal assets or assets consisting solely of cash and cash equivalents;
(ii) We have filed all Exchange Act reports required for at least 12 consecutive months; and
(iii) If applicable, at least one year has elapsed from the time that we file current Form 10-type of information on Form 8-K or other report changing our status from a shell company to an entity that is not a shell company.
Security laws exposure
We are subject to compliance with securities laws, which exposes us to potential liabilities, including potential rescission rights. We may offer to sell our shares of our Common Stock to investors pursuant to certain exemptions from the registration requirements of the Securities Act, as well as those of various state securities laws. The basis for relying on such exemptions is factual; that is, the applicability of such exemptions depends upon our conduct and that of those persons contacting prospective investors and making the offering. We may not seek any legal opinion to the effect that any such offering would be exempt from registration under any federal or state law. Instead, we may elect to relay upon the operative facts as the basis for such exemption, including information provided by investor themselves.
If any such offering did not qualify for such exemption, an investor would have the right to rescind its purchase of the securities if it so desired. It is possible that if an investor should seek rescission, such investor would succeed. A similar situation prevails under state law in those states where the securities may be offered without registration in reliance on the partial preemption from the registration or qualification provisions of such state statutes under the National Securities Markets Improvement Act of 1996. If investors were successful in seeking rescission, we would face severe financial demands that could adversely affect our business and operations. Additionally, if we did not in fact qualify for the exemptions upon which we have relied, we may become subject to significant fines and penalties imposed by the Commission and state securities agencies.
We have never paid dividends on our Common Stock and We do not expect to pay any cash dividends in the foreseeable future.
We have never paid dividends on our Common Stock and we intend to retain our future earnings, if any, in order to reinvest in the development and growth of our business and, therefore, do not intend to pay dividends on our common stock for the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, and such other factors as our board of directors deems relevant. Accordingly, investors may need to sell their shares of our common stock to realize a return on their investment, and they may not be able to sell such shares at or above the price paid for them.
Our board of directors can issue additional shares of common and preferred stock which will dilute existing shareholders.
We can sell additional shares of common stock without consulting stockholders and without offering shares to existing stockholders, which would result in dilution of existing stockholders' interests in our company and could depress our stock price.
Our articles of incorporation, as amended authorize 975,000,000 shares of common stock, of which 19,830,679 are outstanding as of June 21, 2021, and 25,000,000 shares of preferred stock, of which 270,000 Convertible Series B and 5,000,000 Convertible Series A, are outstanding. Our Board of Directors is authorized to issue additional shares of our common stock and preferred stock. Although our Board of Directors intend to utilize its reasonable business judgment to fulfill its fiduciary obligations to our stockholders in connection with any future issuance of our capital stock, the future issuance of additional shares of our common stock or preferred stock convertible into common stock would cause immediate, and potentially substantial, dilution to our existing stockholders, which could also have a material effect on the market value of the shares.
Our board could issue "blank check" preferred stock without stockholder approval with the effect of diluting existing stockholders and impairing their voting rights, and provisions in our charter documents could discourage a takeover that stockholders may consider favorable.
Our certificate of incorporation authorize the issuance of "blank check" preferred stock with designations, rights and preferences as may be determined from time to time by our board of directors. Our board is empowered, without stockholder approval, to issue a series of preferred stock with dividend, liquidation, conversion, voting or other rights which could dilute the interest of, or impair the voting power of, our common stockholders. The issuance of a series of preferred stock could be used as a method of discouraging, delaying or preventing a change in control. For example, it would be possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to effect a change in control of our company.
Our bylaws also allow our board of directors to fix the number of directors. Our stockholders do not have cumulative voting in the election of directors.
Any aspect of the foregoing, alone or together, could delay or prevent unsolicited takeovers and changes in control or changes in our management.
ITEM 2. FINANCIAL INFORMATION
Management’s Plan of Operation
The following discussion contains forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. They use of words such as “anticipate”, “estimate”, “expect”, “project”, “intend”, “plan”, “believe”, and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. From time to time, we also may provide forward-looking statements in other materials we release to the public.
Overview
We are a science based direct-to-consumer (DTC) health company offering products and services focused on aging biology wellness and longevity. Our program is based on the book the Kaufmann Protocol® authored by our co-founder Dr. Sandra Kaufmann, M.D., and on identifying and offering individual specific services, recommendations and treatments designed to improve our customers’ lifespan and health-span. Whereas lifespan represents the total number of years we live, and health-span is how many of those years we remain healthy, active, energetic and free from disease. Our goal is to use science and technology, current and emerging treatments, for our customers to lengthen lifespan and maximize health-span.
We operate a DTC sales model, which means we market our products directly to our target consumers. We currently sell our book, the Kaufmann Protocol online, we offer, a mobile application, and plan to commercialize and market a line of products, including our own branded molecular agents, health and wellness testing kits and services, as well as published and multimedia content.
Significant Recent Developments Regarding COVID-19
During March 2020, a global pandemic was declared by the World Health Organization related to the rapidly spreading outbreak of a novel strain of coronavirus designated COVID-19. The pandemic has significantly impacted economic conditions in the United States. The long-term impact of COVID-19 on the economy and on our business remains uncertain, the duration and scope of which cannot currently be predicted. Please refer to the matters discussed under the caption “Risk Factors”.
Results of Operations During the Year Ended July 31, 2020 As Compared to The Year Ended July 31, 2019
Revenue
For the years ended July 31, 2020 and 2019, we generated no revenue.
Expenses
For the year ended July 31, 2020 we incurred interest expense of approximately $48,000 in relation to the promissory notes outstanding.
For the year ended July 31, 2019, we incurred interest expense of approximately $48,000 in relation to promissory notes outstanding and stock compensation expense of $5.4 million. Stock compensation expense related to the granting of stock to satisfy all outstanding obligations and debts owed to the custodian for costs associated with the custodianship proceedings, and all expenses incurred by the custodian in reinstating the company under Nevada state law, and settling all outstanding balances with the Company’s transfer agent.
Net Loss
For the years ended July 31, 2020 and 2019 we incurred net losses of approximately $48,000, and $5.4 million respectively.
Liquidity
Currently, we rely on our management to provide us with the capital needed to run our business on a day-to-day basis.
For the years ended July 31, 2020 and 2019 we incurred net losses of approximately $48,000, and $5.4 million respectively. As of July 31, 2020, we had no cash on hand and current liabilities of $0.9 million. As of July 31, 2019, we had no cash on hand and current liabilities of $0.8 million.
We will seek additional funds through equity or debt financing, collaborative or other arrangements with corporate partners, licensees or others, and from other sources, which may have the effect of diluting the holdings of existing shareholders. The Company has no current arrangements with respect to, or sources of, such additional financing and we do not anticipate that existing shareholders will provide any portion of our future financing requirements.
No assurance can be given that additional financing will be available when needed or that such financing will be available on terms acceptable to the Company. If adequate funds are not available, we may be required to delay or terminate expenditures for certain of its programs that it would otherwise seek to develop and commercialize. This would have a material adverse effect on the Company.
Going Concern
The report of our independent registered public accounting firm on the financial statements for the years ended July 31, 2020 and 2019, includes an explanatory paragraph relating to the uncertainty of our ability to continue as a going concern. We have incurred recurring losses, incurred liabilities in excess of assets over the past year, and have an accumulated deficit of $14 million. Based upon current operating levels, we will be required to obtain additional capital in order to sustain our operations through July 31, 2022.
Critical Accounting Policies and Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Fair Value of Financial Instruments
On August 1, 2012, the Company adopted ASC 820, Fair Value Measurements and Disclosures. ASC 820 defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures. The three levels are defined as follows:
| · | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| · | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| · | Level 3 inputs to valuation methodology are unobservable and significant to the fair measurement. |
Off-Balance Sheet Arrangements
As of July 31, 2020 and 2019, we did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Securities Act of 1934.
Contractual Obligations and Commitments
As of July 31, 2020 and 2019, we did not have any contractual obligations.
ITEM 3. PROPERTIES.
Our principal business address is 1961 NW 150 Avenue, Suite 205 Pembroke Pines, FL 33028. The office space we are currently occupying is currently being provided to us an no cost to the company by our CFO. We expect this arrangement to continue until our operations require expansion. We currently do not own or lease any other property.
ITEM 4. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT.
The following table sets forth as of June 21, 2021 the number of shares of the Company’s common stock and preferred stock owned on record or beneficially by each person known to be the beneficial owner of 5% or more of the issued and outstanding shares of the Company’s voting stock, and by each of the Company’s directors and executive officers and by all its directors and executive officers as a group. Beneficial ownership representing less than one percent is denoted with an “*.” Unless otherwise indicated, the address for each person is our address at 1961 NW 150 Avenue, Pembroke Pines, Suite 205 Florida 33028.
| | | Shares Beneficially Owned | | | | |
| Name of Beneficial Owner | | Common Stock | | | Class A
Preferred Stock | | | Class B
Preferred Stock | | | | |
| | | Shares | | % | | | Shares | | | % | | | Shares | | | % | | | %Total
Voting
Power (1) | |
| Officers and Directors | | | | | | | | | | | | | | | | | | | | |
| Sandra Kaufmann | | | 0 | | * | | | | 1,169,419 | | | | 23.38 | | | | 90,000 | | | | 33.3 | | | | 30.30 | |
| Adam Laufer | | | 0 | | * | | | | 1,169,419 | | | | 23.38 | | | | 90,000 | | | | 33.3 | | | | 30.30 | |
| Pavan Charan | | | 0 | | * | | | | 1,169,419 | | | | 23.38 | | | | 90,000 | | | | 33.3 | | | | 30.30 | |
| All executive officers and directors as a group (3 persons) | | | 0 | | * | | | | 3,508,257 | | | | 70.16 | | | | 270,000 | | | | 100 | | | | 90.92 | |
| 5% Security Holders | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sandra Kaufmann | | | 0 | | * | | | | 1,169,419 | | | | 23.38 | | | | 90,000 | | | | 33.3 | | | | 30.30 | |
| Adam Laufer | | | 0 | | * | | | | 1,169,419 | | | | 23.38 | | | | 90,000 | | | | 33.3 | | | | 30.30 | |
| Pavan Charan | | | 0 | | * | | | | 1,169,419 | | | | 23.38 | | | | 90,000 | | | | 33.3 | | | | 30.30 | |
| (1) | | Percentage total voting power represents voting power with respect to all shares of our common stock, class A Preferred stock and class B Preferred Stock, as a single class. Each share of Class A preferred stock shall be entitled to 6.25 votes per share of common stock and each share of Class B preferred stock shall be entitled to one thousand votes per share of common stock on all matters submitted to our stockholders for a vote. The common stock, class A Preferred stock and class B preferred stock vote together as a single class on all matters submitted to a vote of our stockholders, except as may otherwise be required by law. |
ITEM 5. DIRECTORS AND EXECUTIVE OFFICERS.
| Name | | Age | | Position(s) |
| Adam Laufer | | 47 | | CEO, Director |
| Pavan Charan | | 45 | | CFO, Director |
| Dr. Sandra Kaufmann | | 53 | | Director |
| Rhonda Keaveney | | 53 | | Prior CEO, Secretary, Treasurer, and Director |
Adam Laufer, Since July 20, 2019, Mr. Laufer has served as our CEO and a member of our Board of Directors. Mr. Laufer leads the strategic vision of our company and oversees the implementation of our developing strategy and expansion as a direct-to-consumer health-tech company. Mr. Laufer is responsible for our acquisition, financing and growth strategies. From February 10, 2014 until On December 15, 2017, Mr. Laufer, Mr. Laufer served as chief executive officer and a director of MJ Holdings, Inc. a publicly traded real estate holding company. From January 2009 until his resignation in 2013, Mr. Laufer served as chairman and chief executive officer of Soleil Capital L.P., a publicly traded company. In 2013, prior to his resignation as an executive officer and a director of Soleil Capital L.P., Mr. Laufer successfully negotiated and executed the acquisition of a portfolio of electronic cigarette and personal vaporizer patents. Mr. Laufer co-founded Vapor Corp., an electronic cigarette company, and from 2009-2013, Mr. Laufer served the company as an advisor and general counsel; consulting on matters of corporate strategy and regulatory issues related to electronic cigarette products, during which time the company’s revenues grew from $1M to $23M. Mr. Laufer has significant experience in working with start-up and development stage businesses in defining their corporate strategy, identifying funding and growth opportunities, and in implementing liquidity strategies. Mr. Laufer is a member in good standing of the Florida Bar.
Pavan Charan. Pavan (Satyaketu) Charan has served as our CFO and a member of our board of directors since July 2019. Mr. Charan is a serial entrepreneur and has over twenty-five years of finance and accounting experience within the United States, Europe, Latin America and the Caribbean. Pavan has served in in the CFO capacity for several fast-growing technology companies to enable rapid, repeatable and scalable growth and has also been involved in financial reporting, finance transformation and capital market transactions. His experience spans various industries from healthcare, distribution and technology. Earlier in his career, Pavan was a Senior Manager at KPMG, LLP where he provided audit and advisory services to publicly and privately held clients as well as private equity groups. Pavan began his career at Price Waterhouse, is a Chartered Accountant (UK) and a Certified Public Accountant (inactive).
Dr. Sandra Kaufmann M.D. Sandra Kaufmann, M.D., a member of our board of directors since June 2021. Dr. Kaufmann is the creator of the Kaufmann Protocol and the author of the book The Kaufmann Protocol. Dr. Kaufmann, is currently the chief of pediatric anesthesiology at Joe DiMaggio Children’s Hospital. Dr. Kaufmann earned her Medical Degree at the University of Maryland School of Medicine in 1996, and completed a residency and fellowship at Johns Hopkins in the field of pediatric anesthesiology in 2002. She is board-certified in both Anesthesiology and Pediatric Anesthesiology from the American Board of Anesthesiology and earned her Bachelor’s Degree of Science from the University of Miami in 1990 followed by a Master’s Degree from the University of Connecticut in Tropical Ecology and Evolutionary Biology. Dr. Kaufmann was recognized as “Best in Medicine” by the American Health Council.
Rhonda Keaveney. Ms. Keaveney holds a Juris Doctor degree and a Master Certificate in Project Management. She has extensive knowledge in the areas of FINRA corporate filings, OTC Markets filings, and SEC compliance filings. She has had over 20 years working with small cap companies. She is the owner of Small Cap Compliance, LLC which was the Custodian of the Company between May 7, 2019 until its discharge on October 16, 2019. From May 8, 2019 until July 10, 2019, Rhonda was the CEO, Secretary, Treasurer and Director of the Company. She resigned all positions from the Company on July 10, 2019.
Board Committees
Our board does not have a standing audit committee, a compensation committee or a nominating and governance committee.
ITEM 6. EXECUTIVE COMPENSATION.
No executive compensation was paid during the fiscal years ended July 31, 2020 and 2019. The Company has no employment agreement with any of its officers and directors.
ITEM 7. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
On May 7, 2019, the Eight Judicial District Court of Nevada appointed Small Cap Compliance, LLC as custodian for Worldwide Strategies Inc., proper notice having been given to the officers and directors of Worldwide Strategies Inc. There was no opposition.
On July 10, 2019, the Company filed a Certificate of Reinstatement with the state of Nevada. Also, on July 10, 2019, the Company issued to the Custodian 270,000 shares of Convertible Series B preferred stock to satisfy all outstanding obligations and debts owed to Custodian for costs associated with the custodianship proceedings, and all expenses incurred by the custodian in reinstating the company under Nevada state law, and settling all outstanding balances with the company’s transfer agent.
On June 10, 2021, the Company reorganized itself as a health & wellness company and entered into a license agreement with Dr. Sandra Kaufmann M.D. covering certain intellectual property, databases, media rights, copyrights and trademarks, in connection therewith, our chief executive officer contributed 90,000 shares of convertible Class B preferred stock to Dr. Kaufmann for the benefit of the Company. Additionally, our CEO contributed 90,000 shares of convertible Class B preferred stock to our CFO, in connection with the reformation of our new business.
Our CFO, has provided us with office space at no charge.
Our CEO, CFO in their respective capacities, as executive officers and board members and Dr. Kaufmann, as a board member are providing their services to us without compensation.
Board Composition and Director Independence
Our business and affairs are managed under the direction of the board of directors. Our board of directors is currently comprised of three members, Messrs. Charan and Laufer and Dr. Kaufmann. Because of their relationships with us, none of them are "independent" under the rules of any national securities exchange or Rule 10A-3 under the Securities Exchange Act of 1934, or the Exchange Act.
ITEM 8. LEGAL PROCEEDINGS.
There are no legal proceedings which are pending or have been threatened against us or any of our officers, directors or control persons of which management is aware.
ITEM 9. MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS.
Our common stock is currently quoted on the OTC market "Pink Sheets" under the symbol WWSG. For the periods indicated, the following table sets forth the high and low bid prices per share of common stock. The below prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions.
| | | Price Range |
| Period | | High | | Low |
| Year ended July 31, 2019 | | | | |
| First Quarter | | $0.0053 | | 0.0053 |
| Second Quarter | | $0.006 | | 0.004 |
| Third Quarter | | $0.01 | | 0.0041 |
| Fourth Quarter | | $0.028 | | 0.008 |
| | | | | |
| Year ended July 31, 2020 | | | | |
| First Quarter | | $0.0393 | | 0.0095 |
| Second Quarter | | $0.03 | | 0.0102 |
| Third Quarter | | $0.026 | | 0.0088 |
| Fourth Quarter | | $0.0648 | | 0.0215 |
| | | | | |
| Year ended July 31, 2021 | | | | |
| First Quarter | | $0.06 | | 0.013 |
| Second Quarter | | $0.045 | | 0.013 |
| Third Quarter | | $0.1099 | | 0.275 |
ITEM 10. RECENT SALES OF UNREGISTERED SECURITIES.
On June 7, 2021 we issued 1,169,419 shares each to Adam Laufer, Sandy Kaufmann and Pavan Charan, of our convertible Class A preferred stock, as founder stock, in connection with the reorganization of the company.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. Unless otherwise stated, the sales of the above securities were deemed to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act (and Regulation D promulgated thereunder). The recipients of the securities in each of these transactions represented their status as an accredited investor and their respective intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed on the share certificates issued in these transactions. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
ITEM 11. DESCRIPTION OF REGISTRANT’S SECURITIES TO BE REGISTERED.
Common Stock
We are authorized to issue 975,000,000 common shares at a par value of $0.001. As of June 21, 2021, there are 19,830,679 common shares outstanding. Each holder of Common Stock shall be entitled to one vote per share.
Preferred Stock
We are authorized to issue 25,000,000 preferred shares at a par value of $0.001. The Certificate of Incorporation, as amended, of the Corporation expressly vests in the Board of Directors of the Corporation the authority provided therein to issue any or all of said shares in one or more series and by resolution or resolutions, the designation, number, full or limited voting powers, or the denial of voting powers, preferences and relative, participating, optional, and other special rights and the qualifications, limitations, restrictions, and other distinguishing characteristics of each series to be issued.
We have two classes of preferred stock authorized and issued and outstanding. On December 15, 2008 we filed a certificate of designation with the Nevada Secretary of State, in which we designated and authorized to issuance 5,000,000 shares of Convertible Series A Preferred Stock at a par value of $0.001 and on July 10, 2019 we filed a certificate of designation with the Nevada Secretary of State, in which we designated and authorized to issuance 5,000,000 shares of Convertible Series B Preferred Stock at a par value of $0.001. As of June 21, 2021, we have 5,000,000 share of Convertible Series A Preferred Stock and 270,000 shares of Convertible Series B Preferred Stock at a par value of $0.001, issued and outstanding respectively.
Series A Preferred Shares
Designation and Number of Shares. Series A Convertible Preferred Stock (the “Series A”) shall consist of 5,000,000 shares, $0.001 par value per share. Shares of the Series A which are redeemed, retired, converted into shares of the Company’s common stock, $0.001 par value per share (the “Common Stock”), purchased or otherwise acquired by the Company shall be cancelled (and thereafter shall not be re-issued as shares of Series A) and shall revert to the status of authorized but unissued preferred stock, undesignated as to series and subject to reissuance by the Company as shares of preferred stock of any one or more series as permitted by the Articles of Incorporation.
Redemption. Shares of Series A may be redeemed by the Company for $0.50 per share (the “Series A Redemption Price”). In the event of the Company’s election to redeem the shares of Series A, the Company shall provide notice of such election to each holder of Series A shares (the “Redemption Notice”), which notice shall (i) be sent via first-class U.S. mail at least fifteen (15) days prior to the termination of the Series A Conversion Rights and (ii) state the Series A Redemption Price. Upon the sixteenth (16th) day after mailing of the Redemption Notice, the Company will mail the Series A Redemption Price to the holder of Series A shares at the holder’s address of record on the books and records of the Company.
Dividends. Shares of Series A will not be entitled to dividends unless the Company pays dividends, in cash or other property, to holders of outstanding Common Stock. In the event the Company declares and pays a dividend to Common Stock holders, five percent (5%) of the value of such dividend shall be paid to the holders of outstanding Series A shares (the “Series A 5% Preference”). After payment of the Series A 5% Preference, each outstanding Series A share will participate in the distribution of the remaining 95% of the dividend with the holders of Common Stock, as if each outstanding Series A share were one share of Common Stock. Any dividend payable to holders of Series A shares will have the same record and payment date and terms as the dividend payable on the Common Stock.
Conversion. The holders of Series A shall have the following conversion rights (the “Series A Conversion Rights”):
Right to Convert. At any time on or after the issuance of the Series A, each share of Series A will be convertible into 1 share of Common Stock, which may be adjusted from time to time pursuant to Section 5 herein (the “Series A Conversion Rate”). At any time on or after the issuance of Series A shares, any holder of Series A may, at such holder’s option, subject to the limitation set forth in Section 7 herein, elect to convert all or any portion of the Series A shares held by such person into that number of fully paid and nonassessable shares of Common Stock equal to (i) the number of Series A shares to be converted (ii) multiplied by 6.25 and (iii) rounded up to the nearest whole share of Common Stock (a “Conversion”). In the event of a redemption, liquidation, dissolution or winding up of the Company, the Series A Conversion Rights shall terminate at the close of business on the last full day preceding the date fixed for the payment of any amounts distributable on such event to the holders of Series A.
Adjustments to Conversion Rate and Certain Other Adjustments. The Series A Conversion Rate for the number of shares of Common Stock into which the Series A shall be converted shall be subject to adjustment from time to time as hereinafter set forth, notice of which shall be promptly provided to the Series A holders:
Stock Dividends, Recapitalization, Reclassification, Split-Ups. If, prior to or on the date of a Series A Conversion, the number of outstanding shares of Common Stock is increased by a stock dividend payable in shares of Common Stock or any right to acquire Common Stock or by a split-up, recapitalization or reclassification of shares of Common Stock or other similar event, then, on the effective date thereof, the Series A Conversion Rate will be adjusted so that the number of shares of Common Stock issuable on such Conversion of the Series A shall be increased in proportion to such increase in outstanding shares of Common Stock.
Aggregation of Shares. If prior to or on the date of a Conversion, the number of outstanding shares of Common Stock is decreased by a consolidation, combination or reclassification of shares of Common Stock or other similar event, then, upon the effective date thereof, the number of shares of Common Stock issuable on Conversion of the Series A shall be decreased in proportion to such decrease in outstanding shares of Common Stock.
Mergers or Consolidations. If at any time or from time to time prior to the date of a Conversion there is a merger, consolidation or similar capital reorganization of the Common Stock (other than a recapitalization, subdivision, combination, reclassification, exchange or substitution of shares provided for in Section 5(a) or 5(b) above) (each a “Reorganization”), then as a part of such capital reorganization, provision shall be made so that each holder of outstanding Series A at the time of such reorganization shall thereafter be entitled to receive, upon Conversion of the Series A, the number of shares of stock or other securities or property of the Company to which a holder of the number of shares of Common Stock deliverable upon Conversion of such holder’s Series A would be entitled on such capital reorganization, subject to adjustment in respect of such stock or securities by the terms thereof. In any such case, the resulting or surviving corporation (if not the Company) shall expressly assume the obligations to deliver, upon the exercise of the conversion privilege, such securities or property as the holders of Series A remaining outstanding (or of other convertible preferred stock received by such holders in place thereof) shall be entitled to receive pursuant to the provisions hereof, and to make provisions for the protection of the conversion rights as provided above. If this Section 5(c) applies to a Reorganization, Sections 5(a) and 5(b) shall not apply to such Reorganization.
Successive Changes. The provisions of this Section shall similarly apply to successive reclassifications, reorganizations, mergers or consolidations, sales or other transfers.
Voting Rights. The holders of shares of Series A shall be entitled to the following voting rights:
| · | Those voting rights required by applicable law; |
| · | The right to vote together with the holders of the Common Stock as a single class, upon all matters submitted to holders of Common Stock for a vote, with each share of Series A carrying a number of votes equal to the number of shares of Common Stock issuable upon Conversion of one share of Series A based on the then applicable Conversion Rate, and each holder of Series A shall be entitled to notice of any stockholders’ meeting in accordance with the bylaws of the Company; and |
| · | Whenever holders of Series A are required or permitted to take any action by vote taken by separate class or series, such action may be taken without a meeting by written consent, setting forth the action so taken and signed by the holders of the outstanding capital stock of the Company having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. |
No Impairment. The Company will not, by amendment of its Articles of Incorporation or through any reorganization, recapitalization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other action, avoid or seek to avoid the observance or performance of any of the terms to be observed or performed hereunder by the Company, but will at all times in good faith assist in the carrying out of all the provisions of this Certificate of Designation and in the taking of all such action as may be necessary or appropriate in order to protect the conversion rights of the holders of Series A against impairment.
No Charge for Conversion. The issuance of certificates for shares of Common Stock upon the conversion of shares of Series A shall be made without charge to the converting holders for such certificates and without any tax in respect of the issuance of such certificates.
Reservation of Shares. On and after the initial issuance of the Series A, the Corporation shall at all times reserve and keep available out of any stock held as treasury stock or out of its authorized but unissued Common Stock, or both, solely for the purpose of effecting the conversion of the shares of Series A, no less than one hundred percent (100%) of the aggregate number of shares of Common Stock then issuable upon the conversion of all outstanding shares of Series A. The Corporation shall immediately, in accordance with the laws of the State of Nevada, increase the authorized amount of its Common Stock if, at any time, the authorized amount of its Common Stock remaining unissued shall not be sufficient to permit the conversion of all shares of Series A.
Return of Status as Authorized Shares. Upon a Conversion or any other redemption or extinguishment of the Series A, the shares converted, redeemed or extinguished will be cancelled (and may not be reissued as shares of Series A) and automatically returned to the status of authorized and unissued shares of preferred stock, available for future designation and issuance pursuant to the terms of the Articles of Incorporation.
Amendment. This Certificate of Designation constitutes an agreement between the Company and the holders of the Series A. For as long as any shares of Series A are outstanding, the terms hereof may be amended, modified, repealed or waived only by the affirmative vote or written consent of holders of seventy five percent (75%) of the then outstanding shares of Series A, voting together as a class and series.
Series B Preferred Shares
Designation and Number of Shares. Series B Convertible Preferred Stock (the “Series B”) shall consist of 5,000,000 shares, $0.001 par value per share.
Liquidation Rights. In the event of any liquidation, dissolution or winding up of the Corporation, either voluntary or involuntary, after setting apart or paying in full the preferential amounts due to Holders of senior capital stock, if any, the Holders of Preferred Class B Stock and parity capital stock, if any, shall be entitled to receive, prior and in preference to any distribution of any of the assets or surplus funds of the Corporation to the Holders of junior capital stock, including Common Stock, an amount equal to $0.001 per share [the "Liquidation Preference"]. If upon such liquidation, dissolution or winding up of the Corporation, the assets of the Corporation available for distribution to the Holders of the Preferred Class B Stock and parity capital stock, if any, shall be insufficient to permit in full the payment of the Liquidation Preference, then all such assets of the Corporation shall be distributed ratably among the Holders of the Preferred Class B Stock and parity capital stock, if any. Neither the consolidation or merger of the Corporation nor the sale, lease or transfer by the Corporation of all or a part of its assets shall be deemed a liquidation, dissolution or winding up of the Corporation for purposes of this Section (c).
Dividends. The Preferred Class B Stock is not entitled to receive any dividends in any amount during which such shares are outstanding.
Conversion Rights. Each one share of Preferred Class B Stock shall be convertible, at the option of the Holder, into one thousand fully paid and non-assessable shares of the Corporation's Common Stock. The foregoing conversion calculation shall be hereinafter referred to as the "Conversion Ratio."
Conversion Procedure. Upon written notice to the Holder, the Holder shall effect conversions by surrendering the certificate(s) representing the Preferred Class B Stock to be converted to the Corporation, together with a form of conversion notice satisfactory to the Corporation, which shall be irrevocable. Not later than five [5] business days after the conversion date, the Corporation will deliver to the Holder, (i) a certificate or certificates, which shall be subject to restrictive legends, representing the number of shares of Common Stock being acquired upon the conversion; provided, however, that the Corporation shall not be obligated to issue such certificates until the Preferred Class B Stock is delivered to the Corporation. If the Corporation does not deliver such certificate(s) by the date required under this paragraph (e) (i), the Holder shall be entitled by written notice to the Corporation at any time on or before receipt of such certificate(s), to receive 100 Preferred Class B Stock shares for every week the Corporations fails to deliver Common Stock to the Holder.
Adjustments on Stock Splits, Dividends and Distributions. If the Corporation, at any time while any Preferred Class B Stock is outstanding, (a) shall pay a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock payable in shares of its capital stock [whether payable in shares of its Common Stock or of capital stock of any class], (b) subdivide outstanding shares of Common Stock into a larger number of shares, (c) combine outstanding shares of Common Stock into a smaller number of shares, or (d) issue reclassification of shares of Common Stock for any shares of capital stock of the Corporation, the Conversion Ratio shall be adjusted by multiplying the number of shares of Common Stock issuable by a fraction of which the numerator shall be the number of shares of Common Stock of the Corporation outstanding after such event and of which the denominator shall be the number of shares of Common Stock outstanding before such event. Any adjustment made pursuant to this paragraph (e)(iii) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or reclassification. Whenever the Conversion Ratio is adjusted pursuant to this paragraph, the Corporation shall promptly mail to the Holder a notice setting forth the Conversion Ratio after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
Adjustments on Reclassifications. Consolidations and Mergers. In case of reclassification of the Common Stock, any consolidation or merger of the Corporation with or into another person, the sale or transfer of all or substantially all of the assets of the Corporation or any compulsory share exchange pursuant to which the Common Stock is converted into other securities, cash or property, then each Holder of Preferred Class B Stock then outstanding shall have the right thereafter to convert such Preferred Class B Stock only into the shares of stock and other securities and property receivable upon or deemed to be held by Holders of Common Stock following such reclassification, consolidation, merger, sale, transfer or share exchange, and the Holder shall be entitled upon such event to receive such amount of securities or property as the shares of the Common Stock into which such Preferred Class B Stock could have been converted immediately prior to such reclassification, consolidation, merger, sale, transfer or share exchange would have been entitled. The terms of any such consolidation, merger, sale, transfer or share exchange shall include such terms so as to continue to give to the Holder the right to receive the securities or property set forth in this paragraph (e)(iv) upon any conversion following such consolidation, merger, sale, transfer or share exchange. This provision shall similarly apply to successive reclassifications, consolidations, mergers, sales, transfers or share exchanges.
Fractional Shares; Issuance Expenses. Upon a conversion of Preferred Class B Stock, the Corporation shall not be required to issue stock certificates representing fractions of shares of Common Stock, but shall issue that number of shares of Common Stock rounded to the nearest whole number. The issuance of certificates for shares of Common Stock on conversion of Preferred Class B Stock shall be made without charge to the Holder for any documentary stamp or similar taxes that may be payable in respect of the issue or delivery of such certificate, provided that the Corporation shall not be required to pay any tax that may be payable in respect of any transfer involved in the issuance and delivery of any such certificate upon conversion in a name other than that of the Holder, and the Corporation shall not be required to issue or deliver such certificates unless or until the person or persons requesting the issuance thereof shall have paid to the Corporation the amount of such tax or shall have established to the satisfaction of the Corporation that such tax has been paid.
Voting Rights. Except as otherwise expressly provided herein or as required by law, the Holders of shares of Preferred Class B Stock shall be entitled to vote on any and all matters considered and voted upon by the Corporation's Common Stock. The Holders of the Preferred Class B Stock shall be entitled to one thousand votes per share of Preferred Class B Stock.
Reservation of Shares of Common Stock. The Corporation covenants that it will at all times reserve and keep available out of its authorized and unissued Common Stock solely for the purpose of issuance upon conversion of Preferred Class B Stock as herein provided, free from preemptive rights or any other actual contingent purchase rights of persons other than the Holders of Preferred Class B Stock, such number of shares of Common Stock as shall be issuable upon the conversion of the outstanding Preferred Class B Stock. If at any time the number of authorized, but unissued shares of Common Stock shall not be sufficient to effect the conversion of all outstanding Preferred Class B Stock, the Corporation will take such corporate action necessary to increase its authorized shares of Common Stock to such number as shall be sufficient for such purpose. The Corporation covenants that all shares of Common Stock that shall be so issuable shall, upon issue, be duly and validly authorized, issued and fully paid and non-assessable.
Dividends
Dividends, if any, will be contingent upon our revenues and earnings, if any, capital requirements and financial conditions. The payment of dividends, if any, will be within the discretion of our board of directors and paid subject to the designated rights of each series and or class of stock authorized and outstanding. We intend to retain earnings, if any, for use in our business operations and accordingly, the board of directors does not anticipate declaring any dividends prior to an acquisition transaction, nor can there be any assurance that any dividends will be paid following any acquisition.
ITEM 12. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Under the corporate laws of the State of Nevada and specifically under article IX of our articles of incorporation, no Director, Officer, or Agent, to include counsel, shall be personally liable to the Corporation or its stockholders for monetary damages for any breach or alleged breach of fiduciary or professional duty by such person acting in such capacity. It shall be presumed that in accepting the position as an Officer, Director, Agent, or Counsel, said individual relied upon and acted in reliance upon the terms and protections provided for by this Article. Notwithstanding the foregoing, a person specifically covered by Article IX of our articles of incorporation, shall be liable to the extent provided by applicable law, for acts or omissions which involve intentional misconduct, fraud, or a knowing violation of law, or for the payment of dividends in violation of NRS 78.300. Additionally, Article VI of our Bylaws provide for indemnification of our directors and executive officers, and permissive indemnification of our employees and agents, to the fullest extent permissible under Nevada law.
We intend to procure liability insurance policies that indemnify our directors and officers against various liabilities, including certain liabilities under arising under the Securities Act and the Exchange Act, which may be incurred by them in their capacity as such.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ITEM 13. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
INDEX TO FINANCIAL STATEMENTS

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Shareholders of Worldwide Strategies, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Worldwide Strategies, Inc. (the Company) as of July 31, 2020 and 2019, and the related statements of operations, changes in stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended July 31, 2020, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of July 31, 2020 and 2019 and the results of its operations and its cash flows for each of the years in the two-year period ended July 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company suffered a net loss from operations and has no source of revenue, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that are communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Going Concern Analysis
As discussed in Note 3, the Company has a going concern due to lack of source of revenue and net loss from operations during the audited periods.
Auditing management’s evaluation of a going concern can be a significant judgement given the fact that the Company uses management estimates on future revenues and expenses which are not able to be substantiated.
To evaluate the appropriateness of the lack of going concern, we examined and evaluated the financial information that was the initial cause along with management’s plans to mitigate the going concern and managements lack of disclosure on going concern.
/s/ M&K CPAS, PLLC
We have served as the Company’s auditor since 2021.
Houston, TX
June 21, 2021
Worldwide Strategies, Inc.
Balance Sheets
July 31, 2020 and 2019
| | | July 31, 2020 | | | July 31, 2019 | |
| Assets | | | | | | | | |
| Current Assets: | | | | | | | | |
| Cash | | $ | – | | | $ | – | |
| Total assets | | $ | – | | | $ | – | |
| Liabilities and Stockholders' Deficit | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable | | $ | 42,967 | | | $ | 42,967 | |
| Accrued liabilities | | | 327,904 | | | | 280,227 | |
| Convertible notes payable, in default | | | 492,406 | | | | 492,406 | |
| Total current liabilities | | | 863,277 | | | | 815,600 | |
| | | | | | | | | |
| Stockholders' deficit: | | | | | | | | |
| Preferred Stock; $.001 par value; 25,000,000 shares authorized | | | | | | | | |
| Series A, 1,491,743 shares issued and outstanding | | | 1,492 | | | | 1,492 | |
| Series B, 270,000 shares issued and outstanding | | | 270 | | | | 270 | |
| Common stock, $.001 par value, 975,000,000 shares authorized 19,830,679 shares issued and outstanding, respectively | | | 19,831 | | | | 19,831 | |
| Additional paid-in capital | | | 13,185,185 | | | | 13,185,185 | |
| Accumulated deficit | | | (14,070,055 | ) | | | (14,022,378 | ) |
| Total Stockholders' Deficit | | | (863,277 | ) | | | (815,600 | ) |
| Total Liabilities and Stockholders' Deficit | | $ | – | | | $ | – | |
Worldwide Strategies, Inc.
Statement of Operations
For the years ended July 31, 2020 and 2019
| | | For The Year Ended July 31, | |
| | | 2020 | | | 2019 | |
| Operating expenses: | | | | | | | | |
| Other general and administrative expenses | | $ | – | | | $ | 5,400,000 | |
| Total operating expenses | | | – | | | | 5,400,000 | |
| Loss from operations | | | – | | | | (5,400,000 | ) |
| Other expense: | | | | | | | | |
| Interest expense | | | (47,677 | ) | | | (47,672 | ) |
| Loss before income taxes | | | (47,677 | ) | | | (5,447,672 | ) |
| Income tax provision | | | – | | | | – | |
| Net loss | | $ | (47,677 | ) | | $ | (5,447,672 | ) |
| | | | | | | | | |
| Basic and diluted loss per share | | $ | (0.00 | ) | | $ | (0.27 | ) |
| | | | | | | | | |
| Basic and diluted weighted average common shares outstanding | | | 19,830,679 | | | | 19,830,679 | |
Worldwide Strategies, Inc.
Statement of Changes in Stockholders’ Equity (Deficit)
For the years ended July 31, 2020 and 2019
| | | Preferred Stock | | | Common Stock | | | | | | | | | | |
| | | Series A | | | Series B | | | | | | | | | | | | | | | | |
| | | Shares | | | Par Value | | | Shares | | | Par Value | | | Shares | | | Par Value | | | Additional Paid-In Capital | | | Accumulated Deficit | | | Total | |
| Balance at July 31, 2018 | | | 1,491,743 | | | $ | 1,492 | | | | – | | | $ | – | | | | 19,830,679 | | | $ | 19,831 | | | $ | 7,785,455 | | | $ | (8,574,706 | ) | | $ | (767,928 | ) |
| Net Loss | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | (5,447,672 | ) | | | (5,447,672 | ) |
| Stock issued for services | | | | | | | – | | | | 270,000 | | | | 270 | | | | – | | | | – | | | | 5,399,730 | | | | – | | | | 5,400,000 | |
| Balance July 31, 2019 | | | 1,491,743 | | | $ | 1,492 | | | | 270,000 | | | $ | 270 | | | | 19,830,679 | | | $ | 19,831 | | | $ | 13,185,185 | | | $ | (14,022,378 | ) | | $ | (815,600 | ) |
| Net Loss | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | | | | | (47,677 | ) | | | (47,677 | ) |
| Balance July 31, 2020 | | | 1,491,743 | | | $ | 1,492 | | | | 270,000 | | | $ | 270 | | | | 19,830,679 | | | $ | 19,831 | | | $ | 13,185,185 | | | $ | (14,070,055 | ) | | $ | (863,277 | ) |
Worldwide Strategies, Inc.
Statement of Cash Flows
For the years ended July 31, 2020 and 2019
| | | For The Year Ended July 31, | |
| | | 2020 | | | 2019 | |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (47,677 | ) | | $ | (5,447,672 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Stock Based Compensation | | | | | | | 5,400,000 | |
| Accrued liabilities | | | 47,677 | | | | 47,672 | |
| Net cash used in operating activities | | | – | | | | – | |
| | | | | | | | | |
| Cash, beginning of period | | | – | | | | – | |
| Cash, end of period | | $ | – | | | $ | – | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information: | | | | | | | | |
| | | | | | | | | |
| Cash paid for taxes | | $ | – | | | $ | – | |
| | | | | | | | | |
| Cash paid for interest | | $ | – | | | $ | – | |
Worldwide Strategies Inc.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEAR ENDED JULY 31, 2020 and 2019
(Audited)
Note 1 – Organization and Basis of Presentation,
Organization and Basis of Presentation
Worldwide Strategies Incorporated (“WWSG” or the “Company”) was incorporated under the laws of the State of Nevada on April 6, 1998 and ceased operations in 2015. The Company fully impaired all assets since the shutdown of its operations in 2015. On May 7, 2019, the eight judicial District Court of Nevada appointed Small Cap Compliance, LLC (“Custodian”) as custodian for Worldwide Strategies Incorporated., proper notice having been given to the officers and directors of Worldwide Strategies Incorporated with no opposition. On July 10, 2019, the Company filed a Certificate of Reinstatement with the state of Nevada.
The accompanying financial statements are prepared on the basis of accounting principles generally accepted in the United States of America (“GAAP”) and have been prepared assuming the continuation of the Company as a going concern. The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs and is dependent on debt and equity financing to fund its operations. Management of the Company is making efforts to raise additional funding until a registration statement relating to an equity funding facility is in effect. While management of the Company believes that it will be successful in its capital formation and planned operating activities, there can be no assurance that the Company will be able to raise additional equity capital or be successful in the development and commercialization of the products it develops or initiates collaboration agreements thereon. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
Note 2 – Summary of significant accounting policies
Cash and Cash Equivalents
The Company doesn’t maintain any bank accounts and does not have any cash in hand. For day-to-day business activities, the Company depends upon the directors’ personal accounts.
For purposes of reporting within the statements of cash flows, the Company considers all cash on hand, cash accounts not subject to withdrawal restrictions or penalties, and all highly liquid debt instruments purchased with a maturity of three months or less to be cash and cash equivalents.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Loss per Common Share
Net loss per common share is computed by dividing net loss by the weighted average number of common shares outstanding for the period. As a result, diluted loss per common share is the same as basic loss per common share for the years ended July 31, 2020 and 2019. Excluded from the weighted average common shares outstanding amount is convertible preferred stock equivalent to 279,323,394 common shares as the effect of these on the computation of net loss per share would have been anti-dilutive.
Income Taxes
The Company accounts for income taxes pursuant to FASB ASC Topic 740, Income Taxes. Under FASB ASC Topic 740, deferred tax assets and liabilities are determined based on temporary differences between the bases of certain assets and liabilities for income tax and financial reporting purposes. The deferred tax assets and liabilities are classified according to the financial statement classification of the assets and liabilities generating the differences.
The Company maintains a valuation allowance with respect to deferred tax assets. The Company establishes a valuation allowance based upon the potential likelihood of realizing the deferred tax asset and taking into consideration the Company’s financial position and results of operations for the current period. Future realization of the deferred tax benefit depends on the existence of sufficient taxable income within the carry-forward period under the Federal tax laws.
Changes in circumstances, such as the Company generating taxable income, could cause a change in judgment about the reliability of the related deferred tax asset. Any change in the valuation allowance will be included in income in the year of the change in estimate.
Fair Value of Financial Instruments
On August 1, 2012, the Company adopted ASC 820, Fair Value Measurements and Disclosures. ASC 820 defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures. The three levels are defined as follows:
| · | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| · | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| · | Level 3 inputs to valuation methodology are unobservable and significant to the fair measurement. |
The following tables represent our assets and liabilities by level measured at fair value on a recurring basis at July 31, 2020 and July 31, 2019:
| | | Fair Value Measurements at July 31, 2020 | |
| | | Level 1 | | | Level 2 | | | Level 3 | |
| Description | | | | | | | | | | | | |
| Convertible Debt | | $ | – | | | $ | 492,406 | | | $ | – | |
| Total Liabilities | | | – | | | | 492,406 | | | | – | |
| Totals | | $ | – | | | $ | 492,406 | | | $ | – | |
| | | Fair Value Measurements at July 31, 2019 | |
| | | Level 1 | | | Level 2 | | | Level 3 | |
| Description | | | | | | | | | | | | |
| Convertible Debt | | $ | – | | | $ | 492,406 | | | $ | – | |
| Total Liabilities | | | – | | | | 492,406 | | | | – | |
| Totals | | $ | – | | | $ | 492,406 | | | $ | – | |
Recent Accounting Pronouncements
The Company reviewed all the recently issued, but not yet effective, accounting pronouncements and we do not believe any of these pronouncements will have a material impact on the Company.
Note 3- Going Concern
For the years ended July 31, 2020 and 2019 we incurred net losses of approximately $48,000, and $5.4 million respectively. As of July 31, 2020, we had no cash on hand and current liabilities of $0.9 million. As of July 31, 2019, we had no cash on hand and current liabilities of $0.8 million. These losses combined with our current liabilities cast significant doubt on the company’s ability to operate under the going concern. The Company is filing a Registration Statement; Form-10 and will become effective 60 days post filing. Management believes that this plan provides an opportunity for the Company to continue as a going concern. The ability to continue as a going concern is dependent upon the Company generating profitable operations in the future and/or obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. Management intends to finance operating costs over the next twelve months with loans from directors and/or private placement of common stock. The failure to achieve the necessary levels of profitability or obtaining additional funding would be detrimental to the Company.
Note 4 – Related party transactions
The Company’s CFO has provided office space at no cost to the Company. As of July 31, 2020 and 2019, our CEO owned 270,000 shares of convertible Class B preferred stock.
Note 5 – Convertible Notes Payable
The Company has convertible promissory notes that in the aggregate result in a principal outstanding balance of $200,750 as of July 31, 2020 and 2019, respectively. Interest on these notes range from nine to ten percent per annum and such notes had maturity dates of July 31, 2015. The principal and accrued interest is convertible, at the option of the holder, into common shares at $.01 per share.
The Company has convertible promissory notes that in the aggregate result in a principal outstanding balance of $157,945 as of July 31, 2020 and 2019, respectively. Interest on these notes range from eight to ten percent per annum and such notes had maturity dates of July 31, 2015. The principal and accrued interest is convertible, at the option of the holder, into common shares at $.04 per share.
The Company has convertible promissory notes that in the aggregate result in a principal outstanding balance of $50,000 as of July 31, 2020 and 2019, respectively. Interest on these notes are 8% per annum and such notes had maturity date of March 31, 2015. The principal and accrued interest is convertible, at the option of the holder, into non-restricted common stock in an amount equal to the total sum due, based on a mutually agreed discount (not to exceed 50%) to the then market price.
The Company has convertible promissory notes that in the aggregate result in a principal outstanding balance of $44,711 as of July 31, 2020 and 2019, respectively. Interest on these notes are 10% per annum and such notes had maturity dates ranging from July 31, 2015 to December 31, 2015. The principal and accrued interest is convertible, at the option of the holder, into common shares at $.07 per share.
The Company has convertible promissory notes that in the aggregate result in a principal outstanding balance of $39,000 as of July 31, 2020 and 2019, respectively. Interest on these notes are 10% per annum and such notes had maturity dates ranging from July 31, 2015 to December 31, 2015. The principal and accrued interest is convertible, at the option of the holder, into common shares at $.10 per share.
Accrued interest on such notes total $327,904 and $280,227 as of July 31, 2020 and 2019, respectively and are included within accrued liabilities on the accompanying balance sheet. Based on the maturity dates of the promissory notes, all promissory notes are in default.
Note 6 – Shareholders’ Equity
Preferred stock
The Company has two classes of preferred stock and is authorized to issue 25,000,000 shares of $.001 par value preferred stock. The Company's Board of Directors may divide and issue the preferred shares in series. Each Series, when issued, shall be designated to distinguish them from the shares of all other series. The relative rights and preferences of these series include preference of dividends, redemption terms and conditions, amount payable upon shares of voluntary or involuntary liquidation, terms and condition of conversion as well as voting powers.
Series A Preferred Stock
On December 15, 2008 the Company filed a certificate of designation with the Nevada Secretary of State, in which it was designated and authorized to issue 5,000,000 shares of Convertible Series A Preferred Stock at a par value of $0.001. Each share of Series A Preferred Stock is convertible into 6.25 shares of common stock at the election of the holder. Each Series A share is entitled to 6.25 votes in any vote of the common stock holders. Series A shares are redeemable by the Company at $.50 per share with 15 days written notice. Series A shares are entitled to a 5% dividend preference and a participation interest in the remaining 95% dividend.
Series B Preferred Stock
On July 10, 2019 the Company filed a certificate of designation with the Nevada Secretary of State, in which it was designated and authorized to issue 5,000,000 shares of Convertible Series B Preferred Stock at a par value of $0.001. Each share of Series B Preferred Stock is convertible into 1,000 shares of common stock at the election of the holder. On July 10, 2019, the Company filed a Certificate of Reinstatement with the state of Nevada and issued to the Custodian 270,000 shares of Convertible Series B preferred stock to satisfy all outstanding obligations and debts owed to Custodian for costs associated with the custodianship proceedings, and all expenses incurred by the custodian in reinstating the company under Nevada state law, and settling all outstanding balances with the company’s transfer agent. These shares were valued using the underlying stock price at the date of issuance which resulted in the Company recording stock compensation expense of $5.4 million.
Common stock
The Company is authorized to issue 33,333,333 shares of common stock as of July 31, 2020 and 2019. Total shares outstanding at July 31, 2020 and 2019 were 19,830,679, respectively. See Note 8 Subsequent Events.
Note 7 - Income taxes
The Company accounts for income taxes under FASB ASC Topic 740, which requires use of the liability method. FASB ASC Topic 740 provides that deferred tax assets and liabilities are recorded based on the differences the tax basis of assets and liabilities and their carrying amounts for financial reporting purposes, referred to as temporary differences.
As of July 31, 2020, the Company incurred a net operating loss and, accordingly, no provision for income taxes has been recorded. In addition, no benefit for income taxes has been recorded due to the uncertainty of the realization of any tax assets. The Company has approximately $3.8 million and $3.7 million of federal net operating loss carry forwards at July 31, 2020 and 2019, respectively. In addition, the Company had gross deferred tax assets of $0.8 million as of July 31, 2020 and 2019 for which a full valuation allowance has provided.
Based on the available objective evidence, including the Company's history of losses, management believes it is more likely than not, the net deferred tax assets will not be fully realizable. Accordingly, the Company provided for a full valuation allowance against its net deferred tax assets at July 31, 2020 and 2019. The Company had no uncertain tax positions as of July 31, 2020 and 2019.
Note 8 – Subsequent Events
On May 26, 2021, the Company increased the authorized amount of common stock to be issued to 975,000,000.
On May 29, 2021 we entered into a binding letter of intent to acquire a company in the health and fitness industry, the acquisition is subject to a financing contingency and customary due diligence review.
On June 7, 2021 we issued an aggregate of 3,508,257 shares of our convertible series A preferred stock to our founders, Adam Laufer, Pavan Charan and Dr. Sandra Kaufmann, as founder stock in connection with the reorganization of our business.
ITEM 14. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
In its two most recent fiscal years, the Company has had no disagreements with its independent accountants.
ITEM 15. FINANCIAL STATEMENTS AND EXHIBITS.
Exhibits Schedule
The following exhibits are filed with this Form 10:
| Exhibit No. | | Description |
| | | |
| 2.1 | | Notice of Entry of Order Appointing, Eight Judicial District Court, Clark County, Nevada, Case No.: A-19-791451-P dated May 7, 2019. |
| 2.2 | | Notice of Entry of Order Discharging, Eight Judicial District Court, Clark County, Nevada, Case No.: A-19-791451-P dated October 16, 2019. |
| 3.1 | | Amended and Restated Articles of Incorporation (Filed as an exhibit to Form SB-2, File No. 333-129398, on November 2, 2005). |
| 3.2 | | Amended Bylaws (Filed as an exhibit to Form SB-2, File No. 333-129398, on November 2, 2005). |
| .3.3 | | Certificate of Change Pursuant to NRS 78.209 effective July 31, 2007 (Filed as an exhibit to the Form 8-K dated July 31, 2007, filed August 6, 2007). |
| 3.4 | | Certificate of Designation Pursuant to NRS 78.1955 effective December 8, 2008 (Filed as an exhibit to Form 8-K dated December 8, 2008, filed December 10, 2008). |
| 3.5 | | Amendment to Certificate of Designation Pursuant to NRS 78.1955 effective December 15, 2008 (Filed as an exhibit to the Form 8-K dated December 15, 2008, filed December 17, 2008). |
| 3.6 | | Certificate of Reinstatement dated July 10, 2019. |
| 3.7 | | Certificate of Designation dated July 10, 2019. |
| 3.8 | | Certificate of Amendment by Custodian filed July 10, 2019. |
| 3.9 | | Certificate of Amendment Filed July 10, 2019. |
| 3.10 | | Amended and Restated Bylaws of the Company. (Filed as an Exhibit to the Form SB-2, filed on November 2, 2005, and incorporated herein by reference). |
| 3.11 | | Certificate of Amendment to the Articles of Incorporation Filed May 26, 2021. |
| 10 | | Intellectual Property License Agreement Between Worldwide Strategies Incorporated and Dr. Sandra Kaufmann |
| 10.1 | | 2005 Stock Plan (Filed as an exhibit to the initial filing of the registration statement on Form SB-2, File No. 333-129398, on November 2, 2005). |
| 23 | | Consent of Independent Auditor |
| 24 | | Power of Attorney |
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities Exchange Act of 1934, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, hereunto duly authorized.
| | WORLDWIDE STRATEGIES INC. |
| | |
| | |
| Date: June 21, 2021 | /s/ Pavan Charan |
| | Name: Pavan Charan |
| | Chief Financial Officer and Director |
| | (Principal Financial Officer) |


