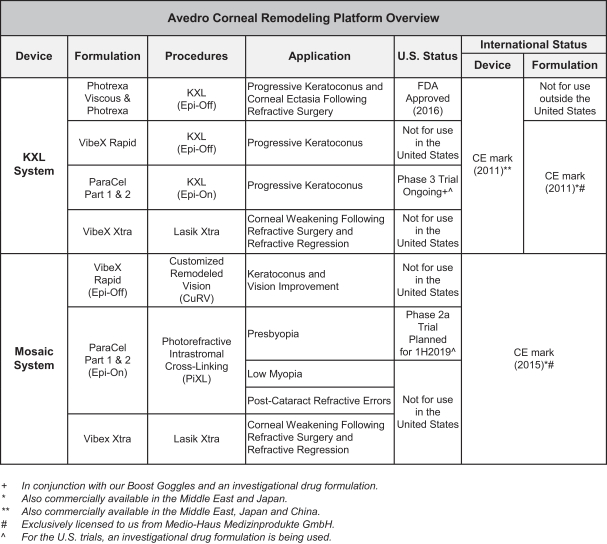
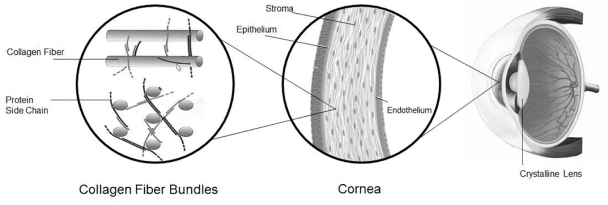
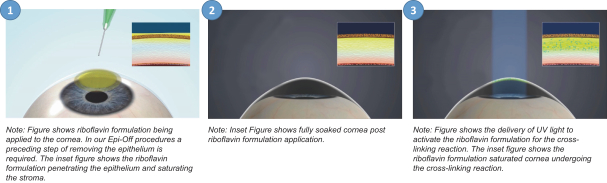
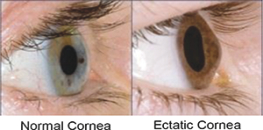
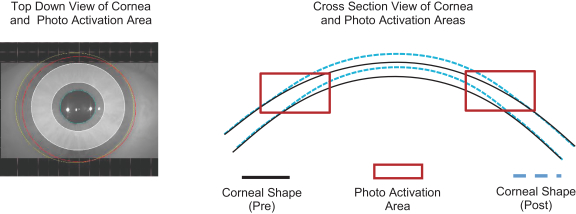
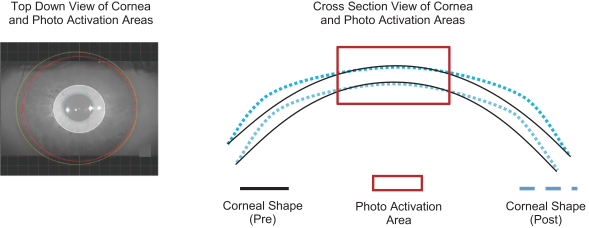
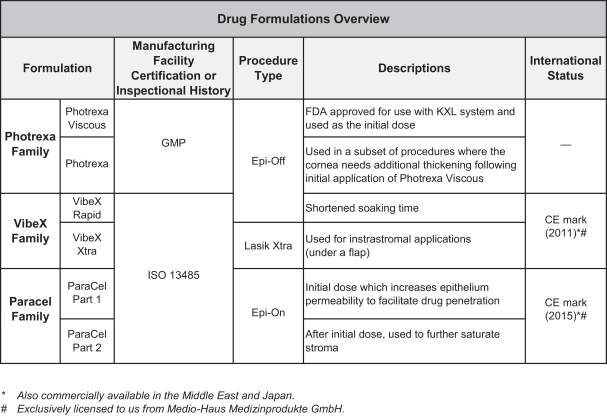
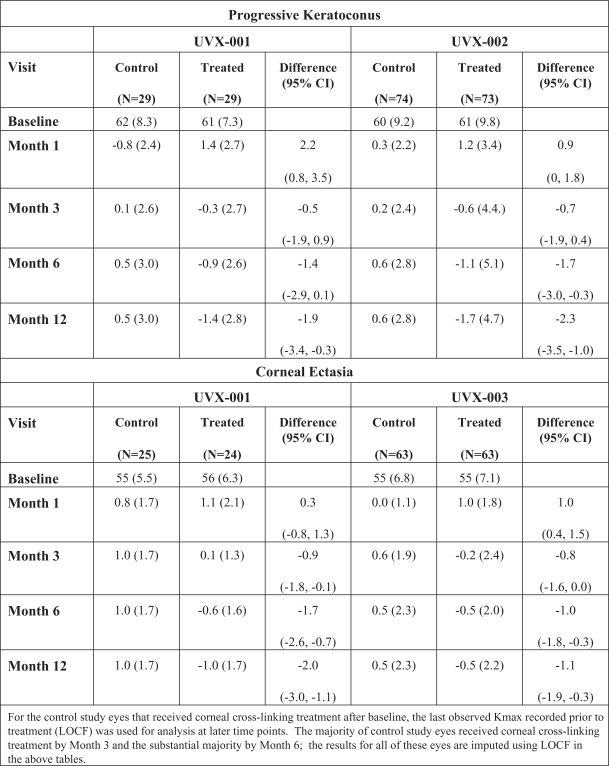
Intellectual Property Agreements
CalTech
In February 2015, we entered into a license agreement with the California Institute of Technology, or CalTech, which was amended and restated, or the Amended CalTech Agreement, in its entirety in July 2017. The Amended CalTech Agreement is an exclusive, royalty-bearing license in the United States to certain patent rights granting us the right to make, have made, import, use, sell and offer for sale any product, device, system, article of manufacture, machine, composition of matter or process or service in the field of corneal cross-linking through the use of UVA light. However, the license grant specifically excludes the use of visible light and cross-linking in anynon-corneal tissue such as the sclera. Under the Amended CalTech Agreement, we agreed to pay CalTech a high single digit royalty on net revenue derived from the sale or distribution of cross-linking agents for performing cross-linking procedures in the United States that are substantially concurrent with corneal surgically invasive corrective procedures, including, but not limited to, LASIK or PK, the royalty products, but excluding procedures for the treatment of keratoconus. The agreement includes certain other payments including (1) an initial payment in the low five digits, (2) a low single digit dollar royalty per treatment on the sale of cross-linking agents for treatment of keratoconus, as well as a minimum annual royalty in the low five digits for such procedures, (3) a percentage in themid-double digits of certainnon-royalty sublicensing revenue and (4) development and regulatory milestone payments in the low to mid six digits in the aggregate. Royalties are payable on aproduct-by-product basis until December 5, 2029.
Unless earlier terminated, the Amended CalTech Agreement expires on the later of the expiration, revocation, invalidation or unenforceability of the licensed patents and the date that royalties are no longer payable. Either party may terminate the Amended CalTech Agreement upon an uncured material breach of the other party. Additionally, CalTech may terminate the Amended CalTech Agreement upon an uncured payment default by us or our bankruptcy or insolvency.
IROC
In August 2014, we entered into an asset purchase and license agreement with IROC Innocross AG, or IROC, which we refer to as the IROC Asset Purchase Agreement, to acquire certain assets, including a list of customers and certain agreements, as well as a fullypaid-up, royalty-free, perpetual, worldwide, nonexclusive license to certain patents, granting us the right to import, manufacture, develop, use, advertise, merchandise, promote, publicize, sell and distribute devices and drugs used in corneal cross-linking. The total purchase price was CHF2.5 million, to be paid in specified delineated installments.
In April 2015, we entered into a patent license and purchase agreement with IROC, or the IROC IP Agreement, to expand the scope of the IROC Asset Purchase Agreement. The IROC IP Agreement granted us a worldwide, exclusive license to certain additional patents to research, develop, make, have made, use, import, offer for sale, sell and otherwise commercialize additional corneal cross-linking technologies. Additionally, under the IROC IP Agreement, upon the payment of the last remaining payment required under the agreement, which include (1) the $50,000 upfront payment and (2) milestone payments of up to $1.7 million in the aggregate, the additional patents will be transferred to us.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting and import and export of pharmaceutical products and medical devices. The processes for obtaining marketing approvals for drugs and devices in the United States and in foreign countries and jurisdictions, along with subsequent compliance with
138