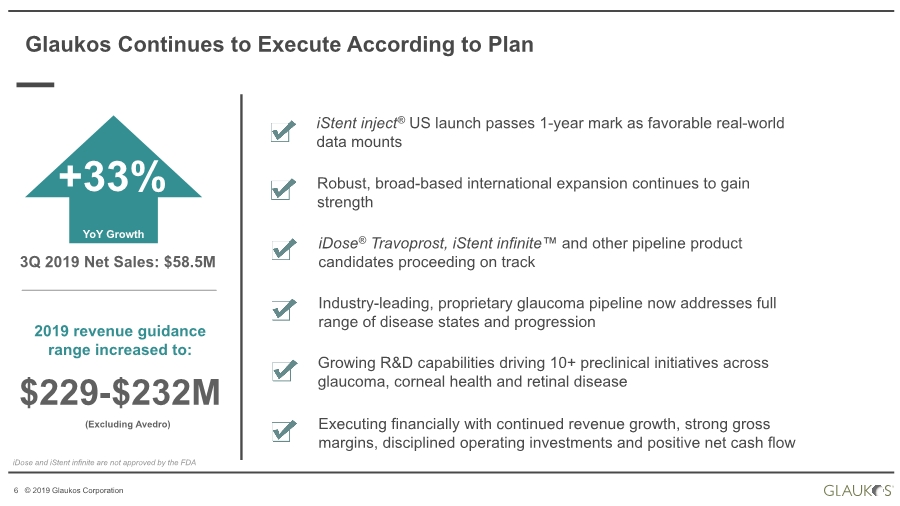
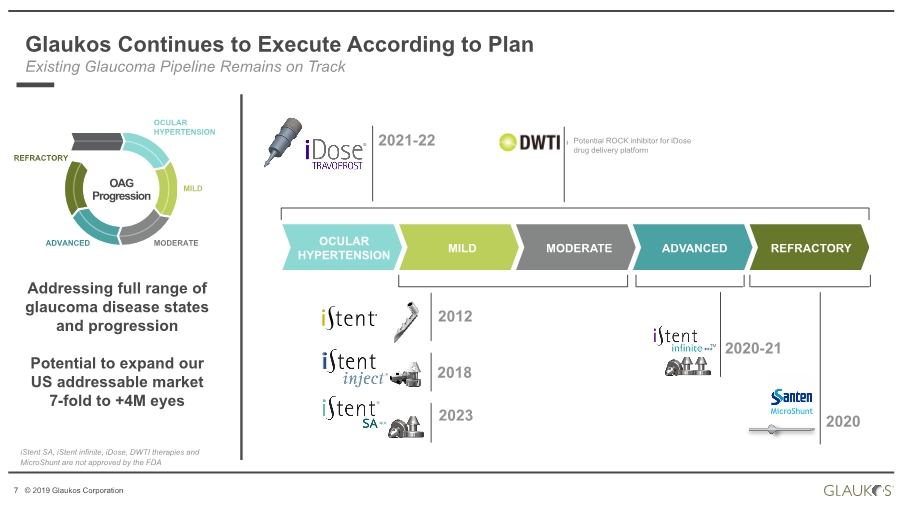
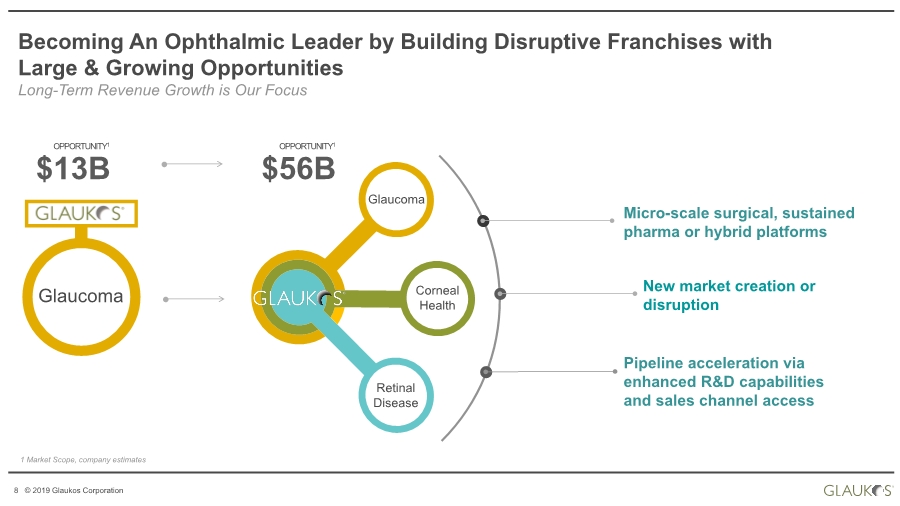
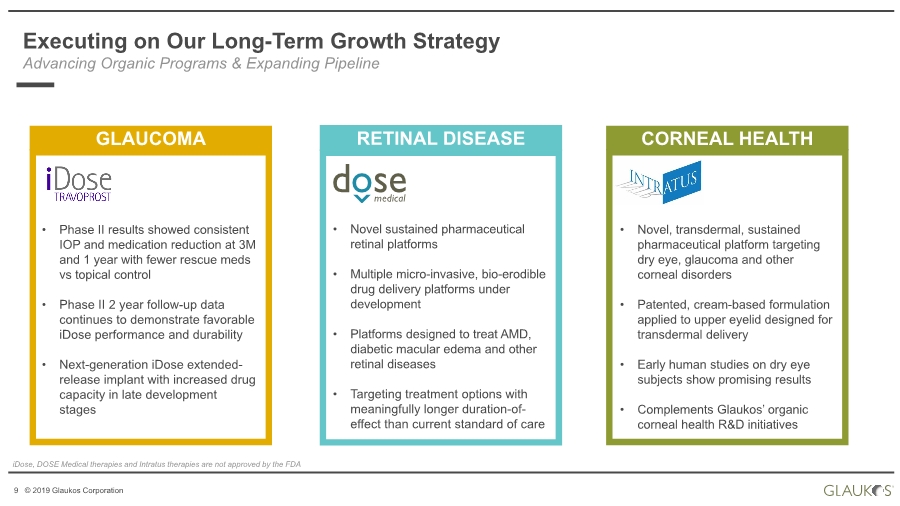
| 2 © 2019 Glaukos Corporation Use of Forward-Looking Statements This communication contains “forward-looking statements” within the meaning of federal securities laws. Forward-looking statements may contain words such as “believes”, “anticipates”, “estimates”, “expects”, “intends”, “aims”, “potential”, “will”, “would”, “could”, “considered”, “likely” and words and terms of similar substance used in connection with any discussion of future plans, actions or events identify forward-looking statements. All statements, other than historical facts, including statements regarding the expected timing of the closing of the proposed transaction and the expected benefits of the proposed transaction, are forward-looking statements. These statements are based on management’s current expectations, assumptions, estimates and beliefs. While Glaukos and Avedro believe these expectations, assumptions, estimates and beliefs are reasonable, such forward- looking statements are only predictions, and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: failure of Avedro to obtain stockholder approval as required for the proposed transaction; failure to satisfy the conditions to the closing of the proposed transaction; unexpected costs, liabilities or delays in connection with or with respect to the proposed transaction; the effect of the announcement of the proposed transaction on the ability of Avedro or Glaukos to retain and hire key personnel and maintain business relationships with customers, suppliers and others with whom Avedro or Glaukos does business, or on Avedro’s or Glaukos’ operating results, market price of common stock, and business generally; legal proceedings relating to the proposed transaction and the outcome of any such legal proceeding; (vii) the inherent risks, costs and uncertainties associated with integrating the businesses successfully and risks of not achieving all or any of the anticipated benefits of the proposed transaction, or the risk that the anticipated benefits of the proposed transaction may not be fully realized or take longer to realize than expected; competitive pressures in the markets in which Avedro and Glaukos operate; the occurrence of any event, change or other circumstances that could give rise to the termination of the merger agreement; other risks to the consummation of the proposed transaction, including the risk that the proposed transaction will not be consummated within the expected time period or at all; uncertainties about Glaukos’ ability to maintain profitability; Glaukos’ dependence on the success and market acceptance of the iStent®; Glaukos’ ability to leverage its sales and marketing infrastructure to increase market penetration and acceptance both in the United States and internationally of its products; Glaukos’ dependence on a limited number of third-party suppliers, some of which are single-source, for components of its products; the occurrence of a crippling accident, natural disaster or other disruption at Glaukos’ primary facility, which may materially affect its manufacturing capacity and operations; maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent or other products in development; Glaukos’ ability to properly train, and gain acceptance and trust from, ophthalmic surgeons in the use of its products; Glaukos’ ability to successfully develop and commercialize additional products; Glaukos’ ability to compete effectively in the highly competitive and rapidly changing medical device industry and against current and future competitors (including MIGS competitors) that are large public companies or divisions of publicly traded companies that have competitive advantages; the timing, effect and expense of navigating different regulatory approval processes as Glaukos develops additional products and penetrates foreign markets; the impact of any product liability claims against Glaukos and any related litigation; the effect of the extensive and increasing federal and Disclaimer |