UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
(Amendment No.__)
Filed by the Registrantþ
Filed by a Party other than the Registranto
Check the appropriate box:
| o | | Preliminary Proxy Statement |
| |
| o | | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| |
| o | | Definitive Proxy Statement |
| |
| o | | Definitive Additional Materials |
| |
| þ | | Soliciting Material Pursuant to § 240.14a-12 |
Alexza Pharmaceuticals, Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box)
| þ | | No fee required. |
| |
| o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| |
| 1. | | Title of each class of securities to which transaction applies: |
| 2. | | Aggregate number of securities to which transaction applies: |
| 3. | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): |
| 4. | | Proposed maximum aggregate value of transaction: |
| o | | Fee paid previously with preliminary materials. |
| o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| 6. | | Amount Previously Paid: |
| 7. | | Form, Schedule or Registration Statement No.: |
On June 23, 2009 Alexza Pharmaceuticals, Inc. will make the following presentation at the Piper Jaffray Fourth Annual Europe Conference:
| Alexza Pharmaceuticals, Inc. |
1

| Forward-Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including statements relating to the initiation and completion of clinical trials, the potential advantages of Alexza’s product candidates and the Company’s capital needs. Actual events could differ materially from those projected and the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, the presentation. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Company’s results may be affected by its effectiveness at managing its financial resources, difficulties or delays in developing manufacturing processes for its product candidates, preclinical and toxicology testing and regulatory developments. Delays in clinical programs, whether caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, could adversely affect the Company’s financial position and prospects. Prior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. The Company may not enter into any additional strategic partnership agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing of and outcomes of clinical trials, competitive developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue one or more of its drug development or discovery research programs. Alexza is at an early stage of development and may not ever have any products that generate significant revenue. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of Alexza’s Annual Report on Form 10-K for the period ended December 31, 2008 and the Company’s periodic reports on Form 10-Q and 8-K. Alexza undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result |
2
| Alexza Pharmaceuticals Advantaged Therapies for CNS Conditions Accelerated relief for acute & intermittent CNS conditions – revolutionary Staccato aerosol technology – strong, positive Phase 3 data from two AZ-004 studies – > 650 patients in two pivotal trials (schizophrenia and bipolar patients) – 10 minute onset established in both patient populations – on the path for NDA submission in early 2010• Rapid, efficient, lower-risk product development – demonstrated rapid clinical development, 6 INDs in four years – > 2,000 patients dosed in 5 different therapeutic areas – > 165 US and International patents, issued and allowed strong pipeline, with solid clinical data in migraine, pain and sleep June 2009 |
3
| AZ-004 (Staccato loxapine) Rationally Designed to Treat Agitation Changing the Face of Medicine |
4
| What is Agitation? Agitation is a complex set of behavioral disturbances that occur in a number of psychiatric disorders1 – exceeding restlessness associated with mental distress – excessive motor activity associated with a feeling of inner tension• Agitation is not a treatment failure, but an integral part of the disease and the patient’s life...which needs to be specifically treated 1J Clin Psychiatry, 2006, 67 (suppl 10), 13 June 2009 |
5
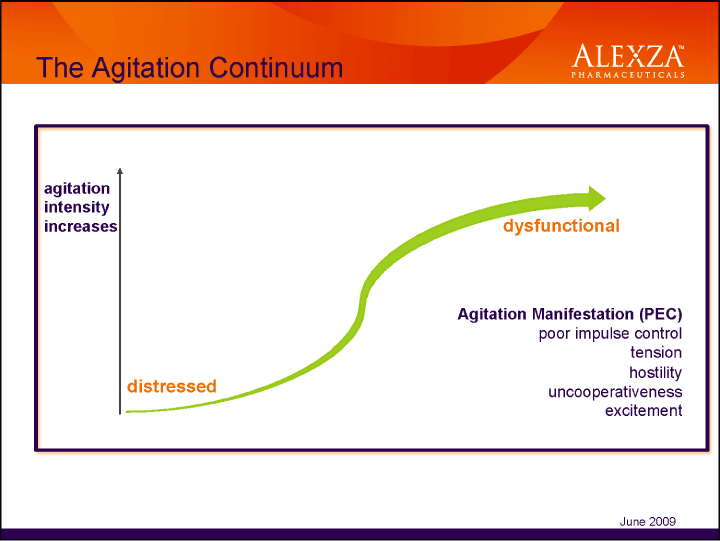
| The Agitation Continuum The Agitation Continuum agitation intensity increases Agitation Manifestation (PEC) poor impulse control tension hostility uncooperativeness excitement June 2009 |
6
| Who has Agitation? Occurs in Many Patients and Many Diseases |
7
| A Novel, New Anti-Agitation Agent Initial Indication for AZ-004 (StaccatoLoxapine) US target population is 8.5 million adults – 2.5 million adult schizophrenia patients 6.0 million adult bipolar disorder patients Agitation is common symptom – 90% of patients have acute agitation each year – patients average 11 — 12 agitation episodes / year > $200mm US market potential for initial indication Profound product opportunity for AZ-004 – no current product meets the clinical Consensus Guidelines for acute agitation – injections too invasive oral tablets too slow and unpredictable June 2009 |
8
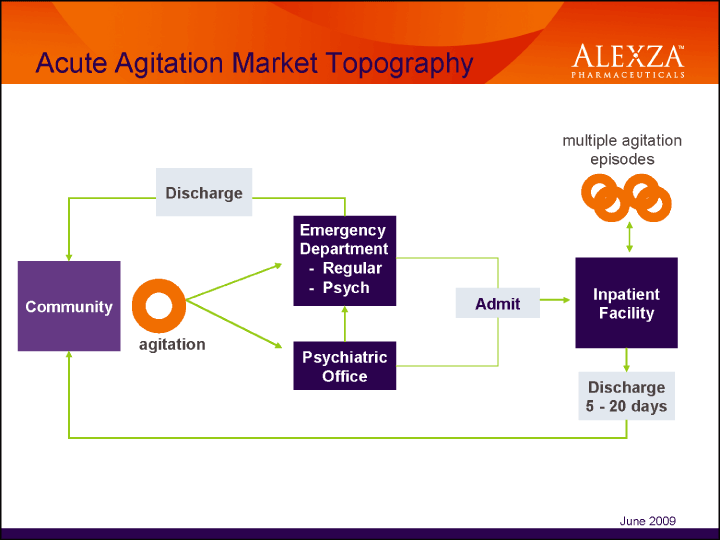
| Acute Agitation Market Topography June 2009 |
9
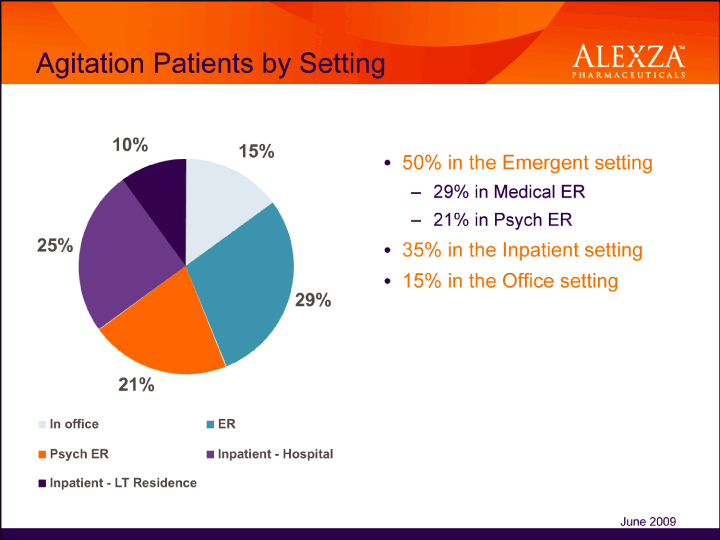
| Agitation Patients by Setting• 50% in the Emergent setting – 29% in Medical ER – 21% in Psych ER• 35% in the Inpatient setting• 15% in the Office setting |
10
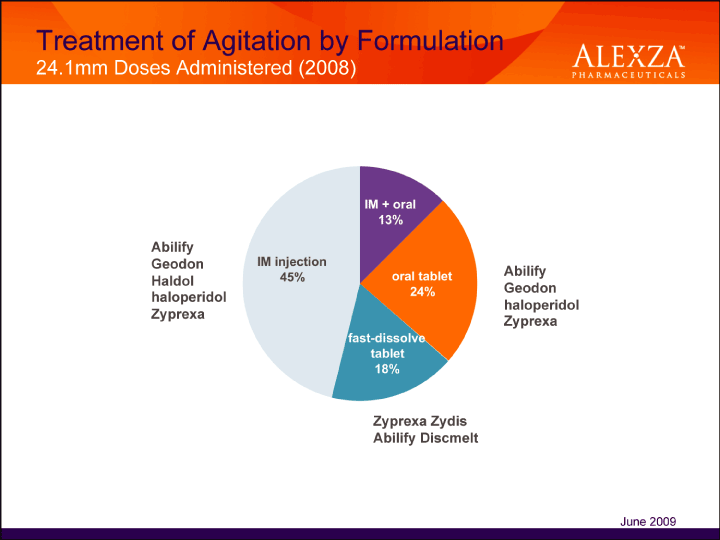
| Treatment of Agitation by Formulation 24.1mm Doses Administered (2008) Abilify Geodon Haldol haloperidol Zyprexa Abilify Geodon haloperidol Zyprexa Zyprexa Zydis Abilify Discmelt June 2009 |
11
| AZ-004 Market Not Fully Developed Highly fragmented treatment• Agitation considered a symptom rather than a disease• Market not easily defined• IM forms developed only as product line extensions for orals• Marginalized and disenfranchised patients Profound and unique opportunity to create, build and own “Agitation”! June 2009 |
12
| AZ-004 Positioning 1st time needle-free respectfully efficiently anti-agitation treatment calm & control quickly June 2009 |
13
| AZ-004 Positioning Optimal Choice AZ-004 is the optimal choice specifically designed as the fastest, non-invasive anti-agitation treatment, providing an immediate calming effect, thus restoring patient control and promptly facilitating resumption of the patient - - treater relationship and treatment plan June 2009 |
14
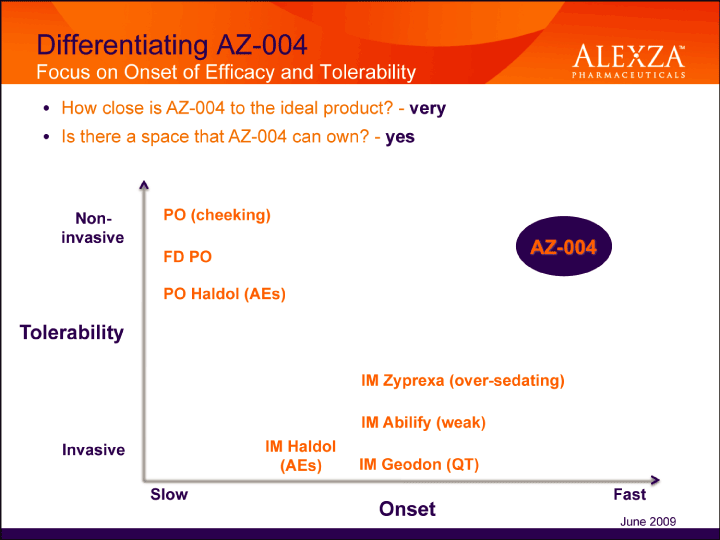
| Differentiating AZ-004 Focus on Onset of Efficacy and Tolerability Tolerability Noninvasive Invasive• How close is AZ-004 to the ideal product? — very• Is there a space that AZ-004 can own? – yes June 2009 |
15
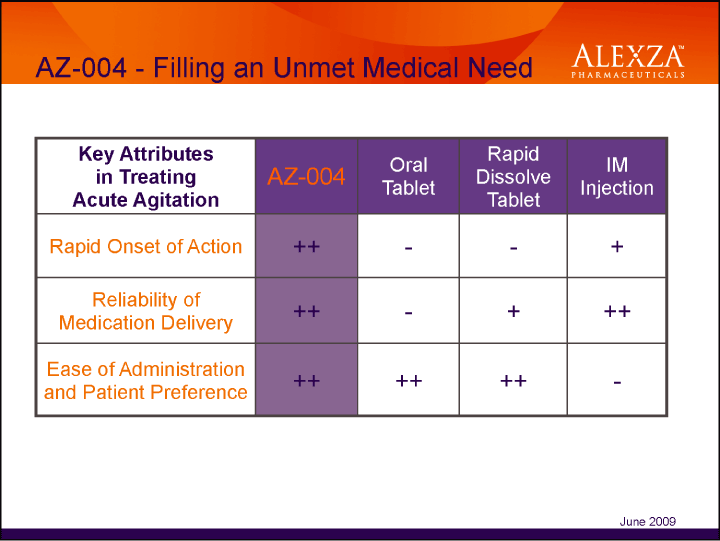
| AZ-004 — Filling an Unmet Medical Need Key Attributes in Treating Acute Agitation AZ-004 Oral Tablet Rapid Dissolve Tablet IM Injection Rapid Onset of Action ++ — - + Reliability of Medication Delivery ++ — + ++ Ease of Administration and Patient Preference ++ ++ June 2009 |
16
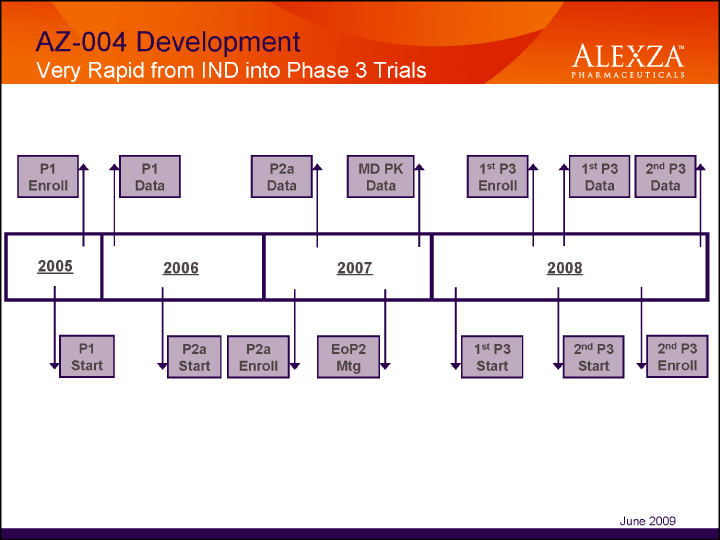
| AZ-004 Development Very Rapid from IND into Phase 3 Trials June 2009 |
17
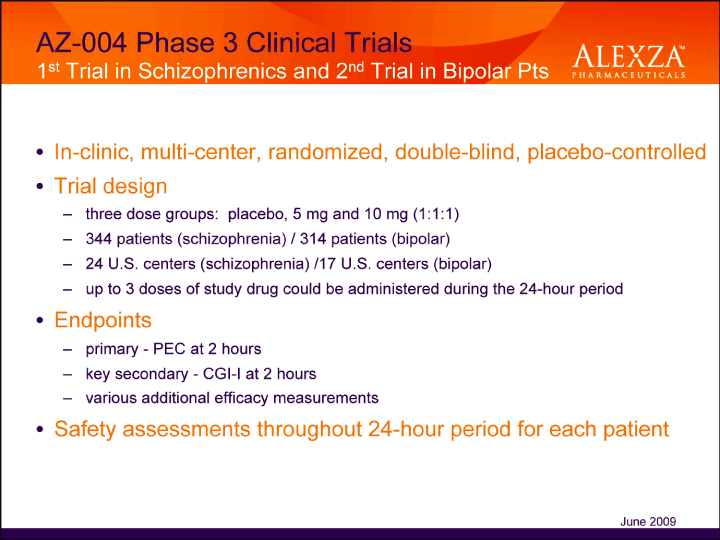
| AZ-004 Phase 3 Clinical Trials 1st Trial in Schizophrenics and 2nd Trial in Bipolar Pts In-clinic, multi-center, randomized, double-blind, placebo-controlled• Trial design – three dose groups: placebo, 5 mg and 10 mg (1:1:1) – 344 patients (schizophrenia) / 314 patients (bipolar) – 24 U.S. centers (schizophrenia) /17 U.S. centers (bipolar) – up to 3 doses of study drug could be administered during the 24-hour period• Endpoints – primary — PEC at 2 hours – key secondary — CGI-I at 2 hours – various additional efficacy measurements• Safety assessments throughout 24-hour period for each patient June 2009 |
18
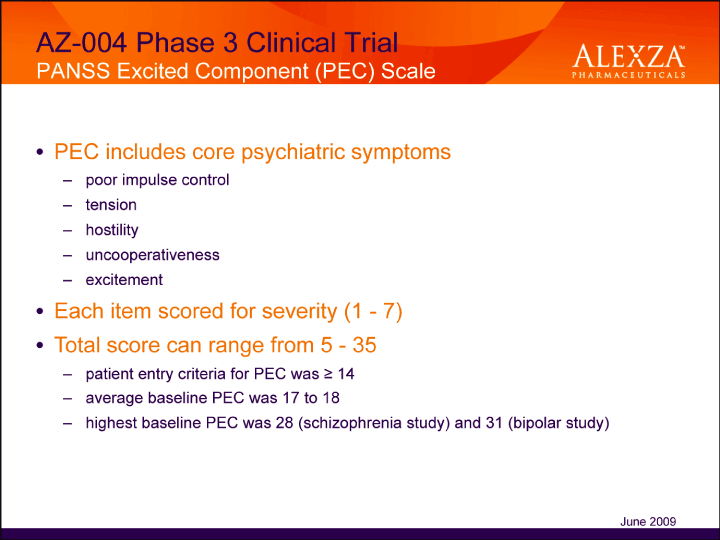
| AZ-004 Phase 3 Clinical Trial PANSS Excited Component (PEC) Scale• PEC includes core psychiatric symptoms – poor impulse control – tension – hostility – uncooperativeness – excitement• Each item scored for severity (1 — 7)• Total score can range from 5 — 35 – patient entry criteria for PEC was = 14 – average baseline PEC was 17 to 18 highest baseline PEC was 28 (schizophrenia study) and 31 (bipolar study) June 2009 |
19
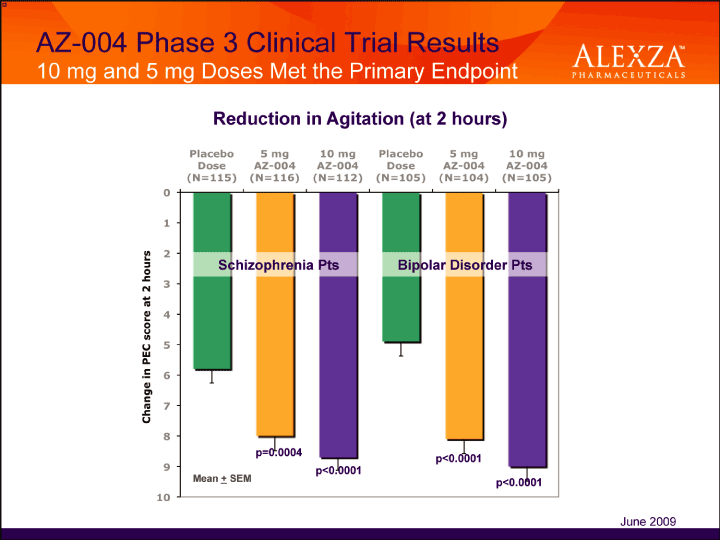
| AZ-004 Phase 3 Clinical Trial Results 10 mg and 5 mg Doses Met the Primary Endpoint Reduction in Agitation (at 2 hours) June 2009 |
20
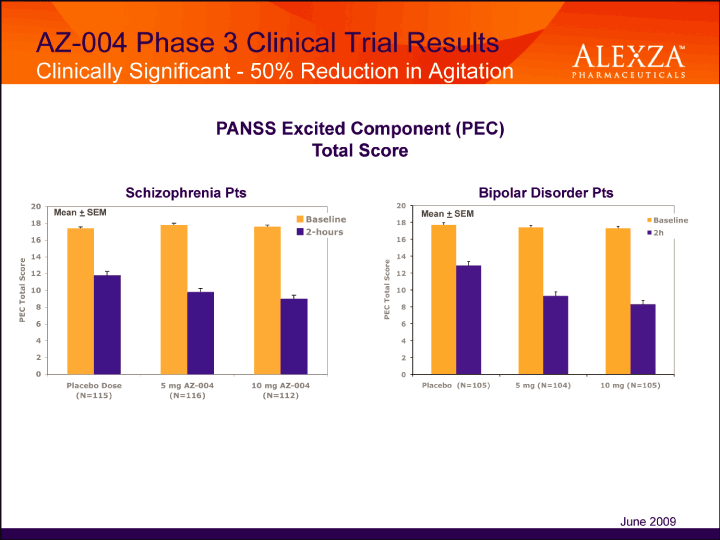
| AZ-004 Phase 3 Clinical Trial Results Clinically Significant — 50% Reduction in Agitation PANSS Excited Component (PEC) Total Score p=0.0004 p<0.0001 Schizophrenia Pts Bipolar June 2009 |
21
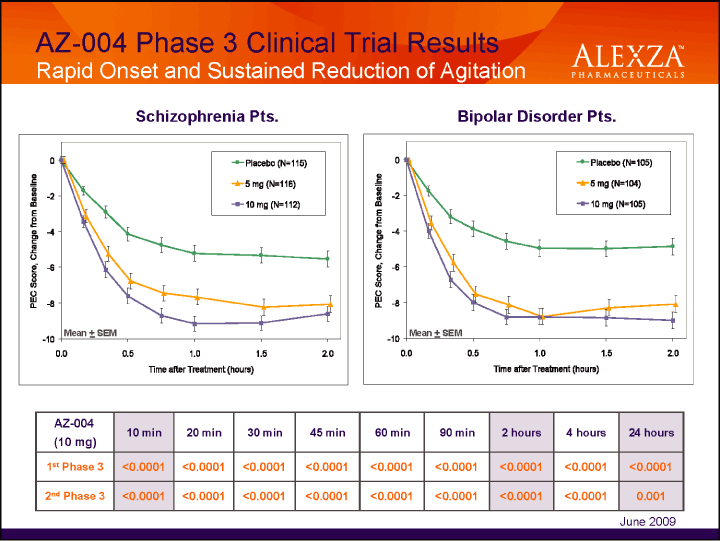
| AZ-004 Phase 3 Clinical Trial Results Rapid Onset and Sustained Reduction of Agitation Schizophrenia Pts. Bipolar Disorder Pts AZ-004 (10 mg) 10 min 20 min 30 min 45 min 60 min 90 min 2 hours 4 hours 24 hours 1st Phase 3 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 2nd Phase 3 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 0.001 June 2009 |
22
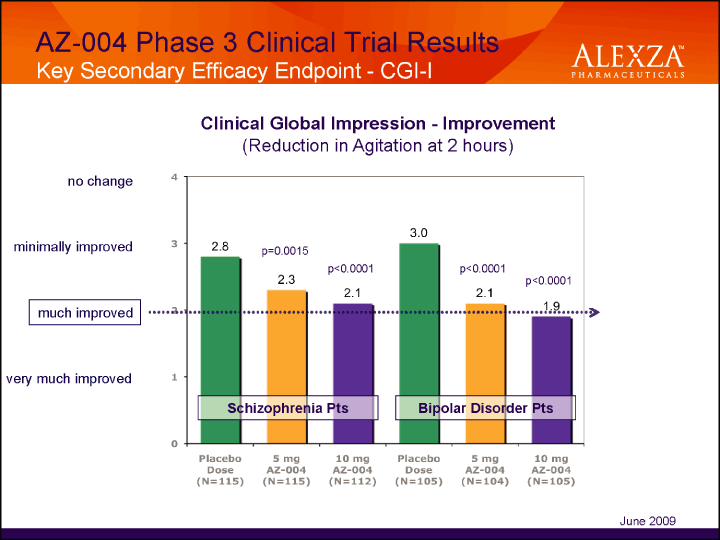
| AZ-004 Phase 3 Clinical Trial Results Key Secondary Efficacy Endpoint — CGI-I Clinical Global Impression — Improvement (Reduction in Agitation at 2 hours) June 2009 |
23
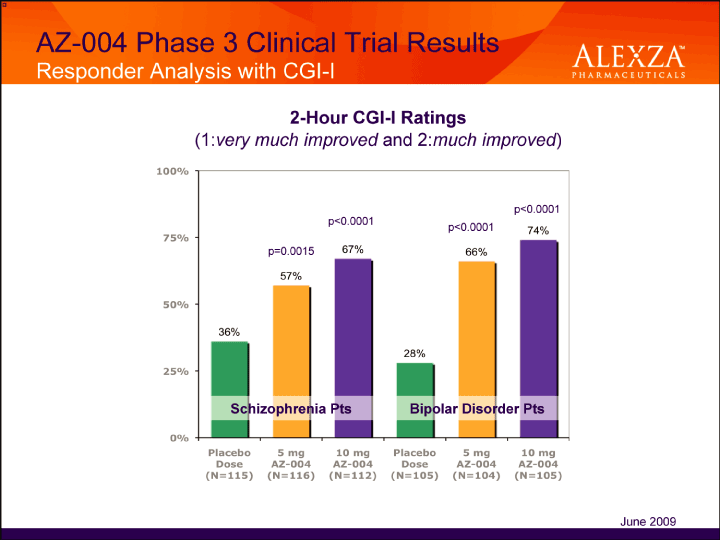
| AZ-004 Phase 3 Clinical Trial Results Responder Analysis with CGI-I 2-Hour CGI-I Ratings (1:very much improved and 2:much improved June 2009 |
24
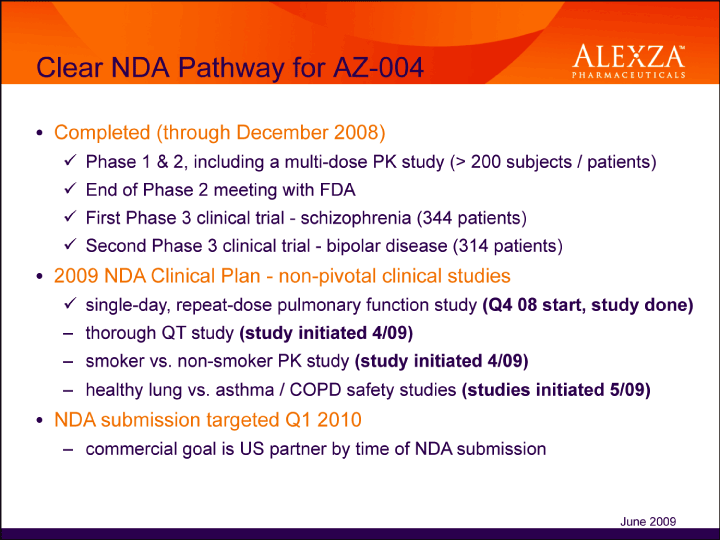
| Clear NDA Pathway for AZ-004 Completed (through December 2008)üPhase 1 & 2, including a multi-dose PK study (> 200 subjects / patients)üEnd of Phase 2 meeting with FDAüFirst Phase 3 clinical trial — schizophrenia (344 patients)üSecond Phase 3 clinical trial — bipolar disease (314 patients)• 2009 NDA Clinical Plan — non-pivotal clinical studiesüsingle-day, repeat-dose pulmonary function study (Q4 08 start, study done) – thorough QT study (study initiated 4/09) – smoker vs. non-smoker PK study (study initiated 4/09) – healthy lung vs. asthma / COPD safety studies (studies initiated 5/09)• NDA submission targeted Q1 2010 – commercial goal is US partner by time of NDA submission June 2009 |
25
| AZ-004 Commercial Strategy• US Strategy – broader opportunity than originally thought – combination of in-hospital (non-psych and psych MDs) and office (psychs) – already existing sales force a plus, with a focus in psych or CNS – key success factor — “market creation” model, not “market capture” model• EU Strategy – psychiatry focus, with a majority of the patients treated as in-patients – target partner — mid-sized pharma company, with regional strengths – key success factor — to build a new “anti-agitation” market with AZ-004• Ongoing discussions for both geographic territories June 2009 |
26

| AZ-004 Commercial Strategy Acquisition of Symphony Allegro• Acquisition of Symphony Allegro, announced June 15, 2009 – 10mm shares of ALXA Common Stock – 5mm share warrant, 5-year term, exercise price of $2.26 – sharing of cash from future partnering deals (AZ-004, AZ-104, AZ-002)• Proxy to be filed shortly• Shareholder vote / transaction projected to close — Q3, 09 RULE 14a-12 LEGEND — Participants in Solicitation Alexza Pharmaceuticals, Inc. and its directors and officers may be deemed to be participants in the solicitation of proxies from Alexza stockholders in connection with the exercise of the option to acquire the Symphony Allegro equity. Information about the directors and executive officers of Alexza and their ownership of Alexza’s stock is set forth in the proxy statement for Alexza’s 2009 Annual Meeting of Stockholders. Investors can obtain more information when the proxy statement relating to stockholder approval of the issuance of shares in connection with the exercise of the option becomes available. This proxy statement, and any other documents filed by Alexza with the SEC, may be obtained free of charge at the SEC web site at www.sec.gov. Investors should read the proxy statement carefully, when it becomes available, before making any voting decision June 2009 |
27
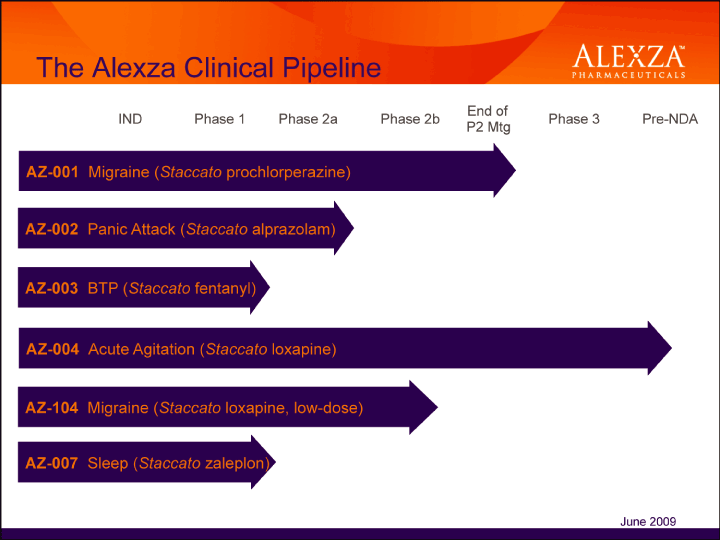
| The Alexza Clinical Pipeline AZ-001 Migraine (Staccato prochlorperazine) AZ-002 Panic Attack (Staccato alprazolam) AZ-104 Migraine (Staccato loxapine, low-dose) AZ-004 Acute Agitation (Staccato loxapine) AZ-007 Sleep (Staccato zaleplon) AZ-003 BTP (Staccato fentanyl) End of IND Phase 1 Phase 2a Phase 2b P2 Mtg Phase 3 June 2009 |
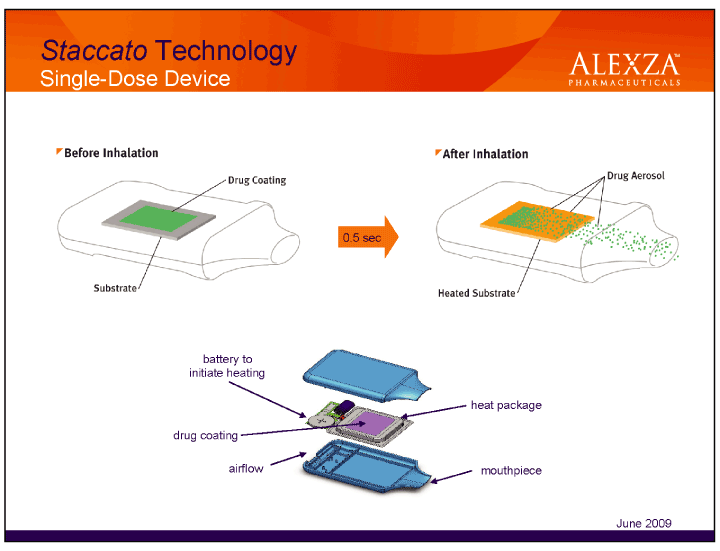
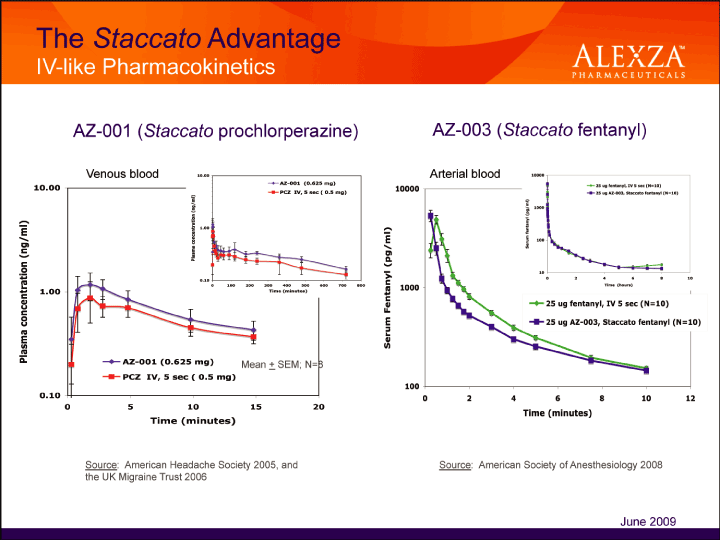
28
| Staccato Technology Single-Dose Device June 2009 The Staccato Advantage IV-like Pharmacokinetics AZ-001 (Staccato prochlorperazine) AZ-003 (Staccato fentanyl) Source: American Headache Society 2005, and the UK Migraine Trust 2006 Source: American Society of Anesthesiology 2008 June 2009 |
29
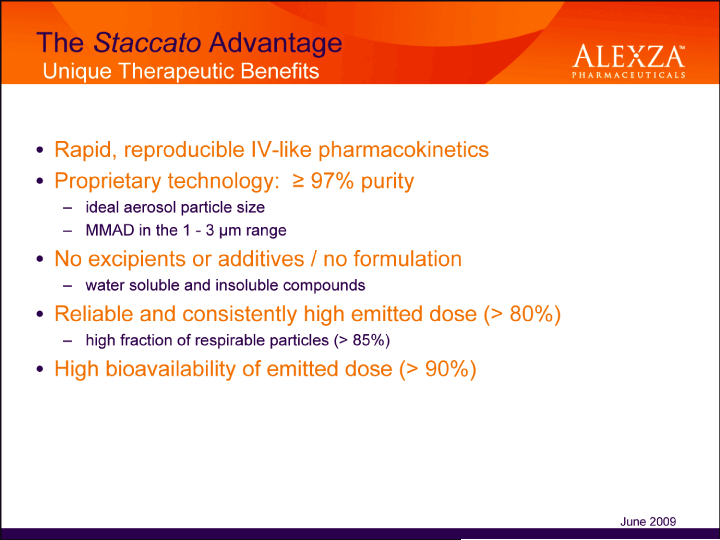
| The Staccato Advantage Unique Therapeutic Benefits Rapid, reproducible IV-like pharmacokinetics• Proprietary technology: = 97% purity – ideal aerosol particle size – MMAD in the 1 — 3 µm range• No excipients or additives / no formulation – water soluble and insoluble compounds• Reliable and consistently high emitted dose (> 80%) – high fraction of respirable particles (> 85%)• High bioavailability of emitted dose (> 90%) June 2009 |
31
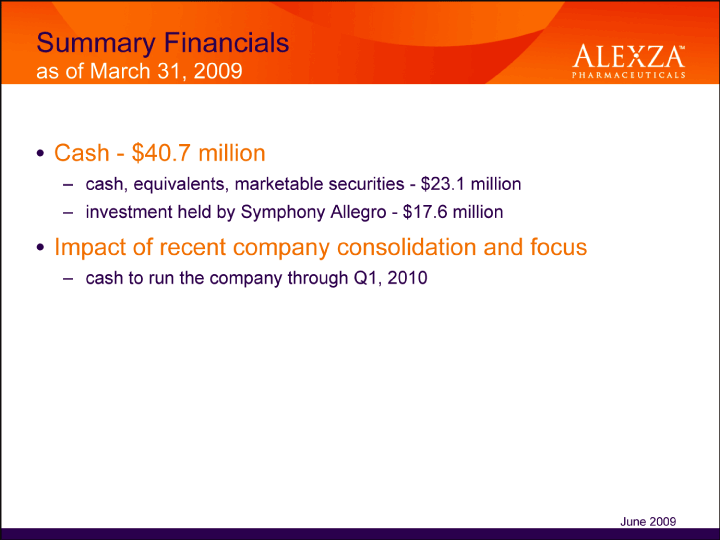
| Summary Financials as of March 31, 2009• Cash — $40.7 million – cash, equivalents, marketable securities — $23.1 million – investment held by Symphony Allegro — $17.6 million• Impact of recent company consolidation and focus cash to run the company through Q1, 2010 June 2009 |
32
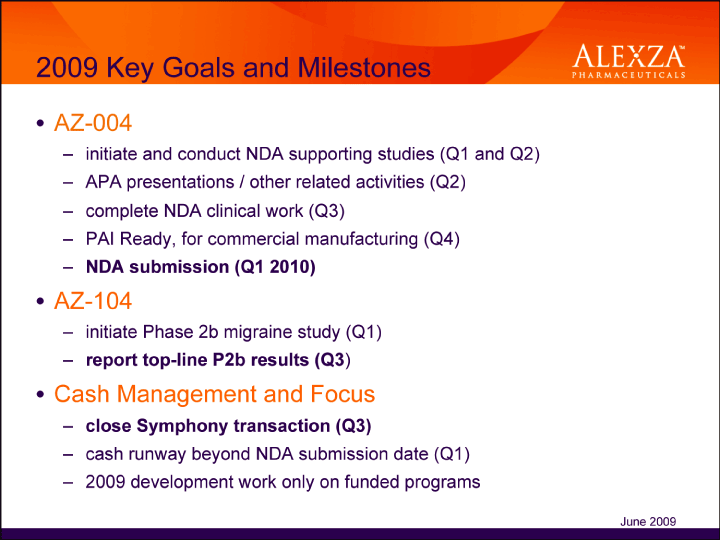
| 2009 Key Goals and Milestones AZ-004 – initiate and conduct NDA supporting studies (Q1 and Q2) – APA presentations / other related activities (Q2) – complete NDA clinical work (Q3) – PAI Ready, for commercial manufacturing (Q4) – NDA submission (Q1 2010)• AZ-104 – initiate Phase 2b migraine study (Q1) – report top-line P2b results (Q3)• Cash Management and Focus – close Symphony transaction (Q3) – cash runway beyond NDA submission date (Q1) 2009 development work only on funded programs June 2009 |
33