
mesoblast Global Leader in Allogeneic Cellular Medicines for Inflammatory Diseases February 2025 ASX: MSB; Nasdaq: MESO Financial Results and Operational Update for the Half Year Ended December 31, 2024 Exhibit 99.2

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward- looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements other than statements of historical facts contained in this presentation are forward-looking statements. Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “targets,” “likely,” “will,” “would,” “could,” and similar expressions or phrases identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and future events , recent changes in regulatory laws, and financial trends that we believe may affect our financial condition, results of operation, business strategy and financial needs. These statements may relate to, but are not limited to: expectations regarding the safety or efficacy of, or potential applications for, Mesoblast's adult stem cell technologies; expectations regarding the strength of Mesoblast's intellectual property, the timeline for Mesoblast's regulatory approval process, and the scalability and efficiency of manufacturing processes; expectations about Mesoblast's ability to grow its business and statements regarding its relationships with current and potential future business partners and future benefits of those relationships; statements concerning Mesoblast's share price or potential market capitalization; and statements concerning Mesoblast's capital requirements and ability to raise future capital, among others. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. You should read this presentation together with our financial statements and the notes related thereto, as well as the risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast's actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, include, without limitation: risks inherent in the development and commercialization of potential products; uncertainty of clinical trial results or regulatory approvals or clearances; government regulation; the need for future capital; dependence upon collaborators; and protection of our intellectual property rights, among others. Accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise.

Our Mission Mesoblast is committed to bringing to market innovative off-the-shelf allogeneic cellular medicines to treat serious and life-threatening inflammatory illnesses

4 m e s o b l a s t Global Markets Global leader in allogeneic cellular medicines for inflammatory diseases ✓World leader in developing allogeneic (off-the- shelf) cellular medicines for the treatment of severe and life-threatening inflammatory conditions ✓ Locations in Australia, the United States and Singapore ✓ Listed on the ASX (MSB) and NASDAQ (MESO) ✓ Developing product candidates for distinct indications based on its remestemcel-L and rexlemestrocel-L stromal cell technology platforms ✓ Extensive global intellectual property portfolio with protection extending through to at least 2041 in all major markets ✓ FDA-inspected commercial scale manufacturing process and facilities ONE product FDA approved more than 1,100 patents & applications Phase 3 trials in TWO major indications
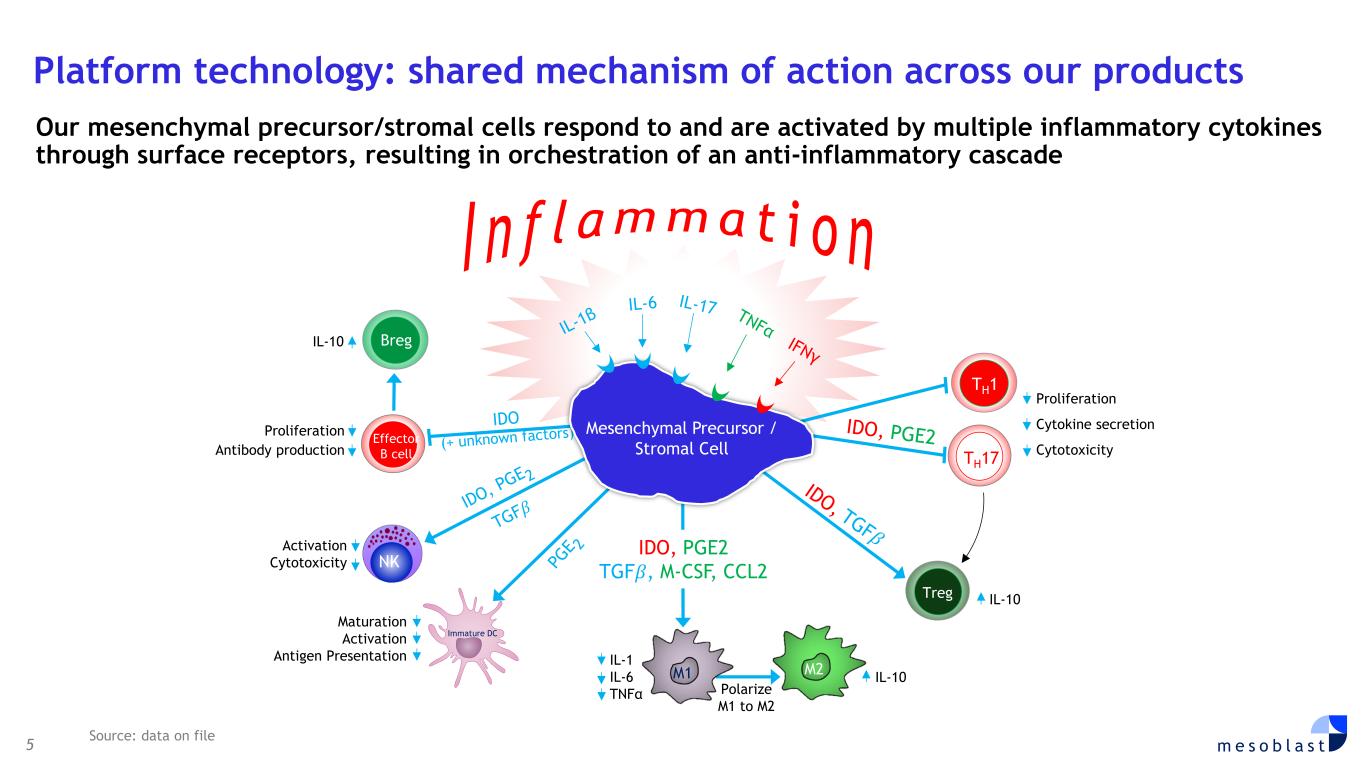
5 m e s o b l a s t Platform technology: shared mechanism of action across our products Our mesenchymal precursor/stromal cells respond to and are activated by multiple inflammatory cytokines through surface receptors, resulting in orchestration of an anti-inflammatory cascade Effector B cell M2 Breg Treg I n f l a m m a t i o n NK Activation Cytotoxicity Maturation Activation Antigen Presentation Proliferation Antibody production IDO, PGE2 TGF𝛽, M-CSF, CCL2 IL-10 TH17 Proliferation Cytokine secretion Cytotoxicity IL-10 TH1 M1 Immature DC IL-1 IL-6 TNFα IL-10 Mesenchymal Precursor / Stromal Cell Polarize M1 to M2 Source: data on file
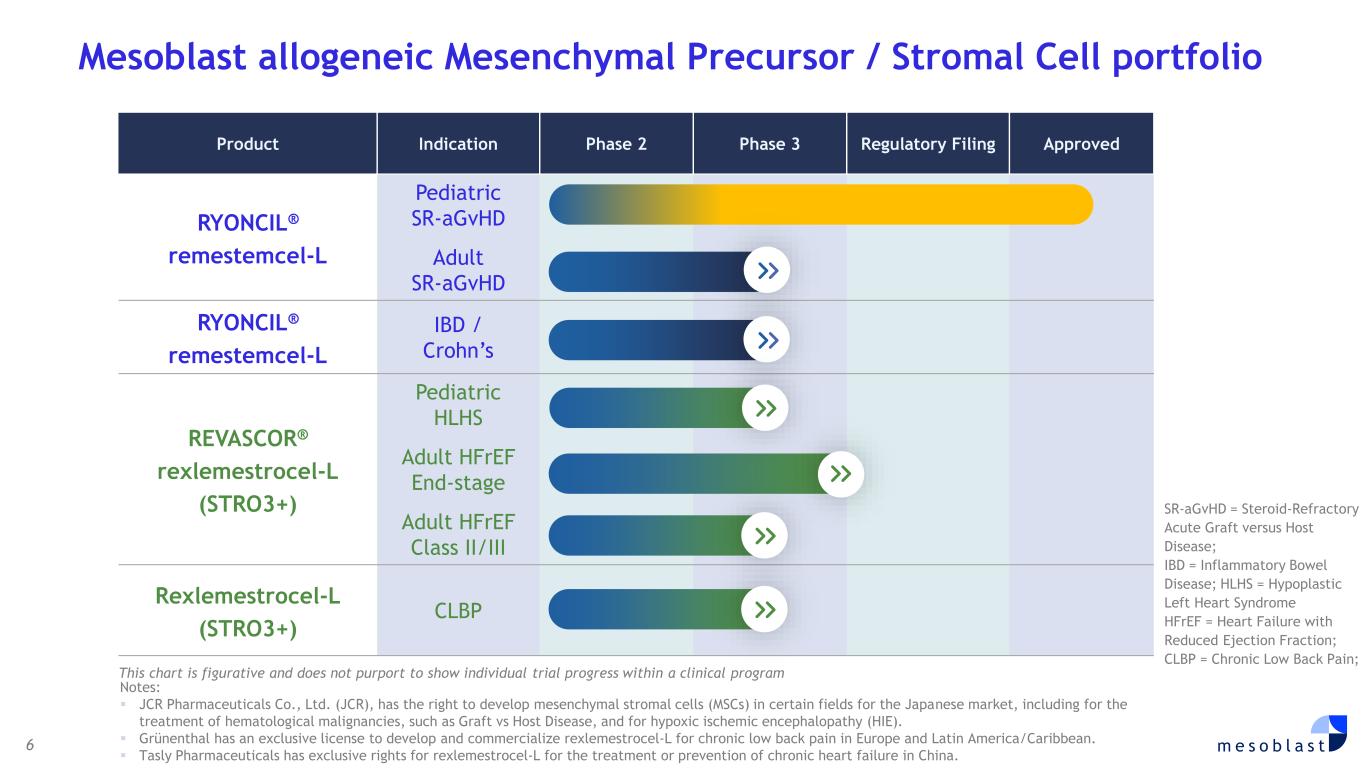
6 m e s o b l a s t Mesoblast allogeneic Mesenchymal Precursor / Stromal Cell portfolio Product Indication Phase 2 Phase 3 Regulatory Filing Approved RYONCIL® remestemcel-L Pediatric SR-aGvHD Adult SR-aGvHD RYONCIL® remestemcel-L IBD / Crohn’s REVASCOR® rexlemestrocel-L (STRO3+) Pediatric HLHS Adult HFrEF End-stage Adult HFrEF Class II/III Rexlemestrocel-L (STRO3+) CLBP SR-aGvHD = Steroid-Refractory Acute Graft versus Host Disease; IBD = Inflammatory Bowel Disease; HLHS = Hypoplastic Left Heart Syndrome HFrEF = Heart Failure with Reduced Ejection Fraction; CLBP = Chronic Low Back Pain; This chart is figurative and does not purport to show individual trial progress within a clinical program Notes: ▪ JCR Pharmaceuticals Co., Ltd. (JCR), has the right to develop mesenchymal stromal cells (MSCs) in certain fields for the Japanese market, including for the treatment of hematological malignancies, such as Graft vs Host Disease, and for hypoxic ischemic encephalopathy (HIE). ▪ Grünenthal has an exclusive license to develop and commercialize rexlemestrocel-L for chronic low back pain in Europe and Latin America/Caribbean. ▪ Tasly Pharmaceuticals has exclusive rights for rexlemestrocel-L for the treatment or prevention of chronic heart failure in China.

7 m e s o b l a s t Financial Results © Lonza, reproduced with permission Manufacturing Remestemcel-L for the Period Ended December 31, 2024
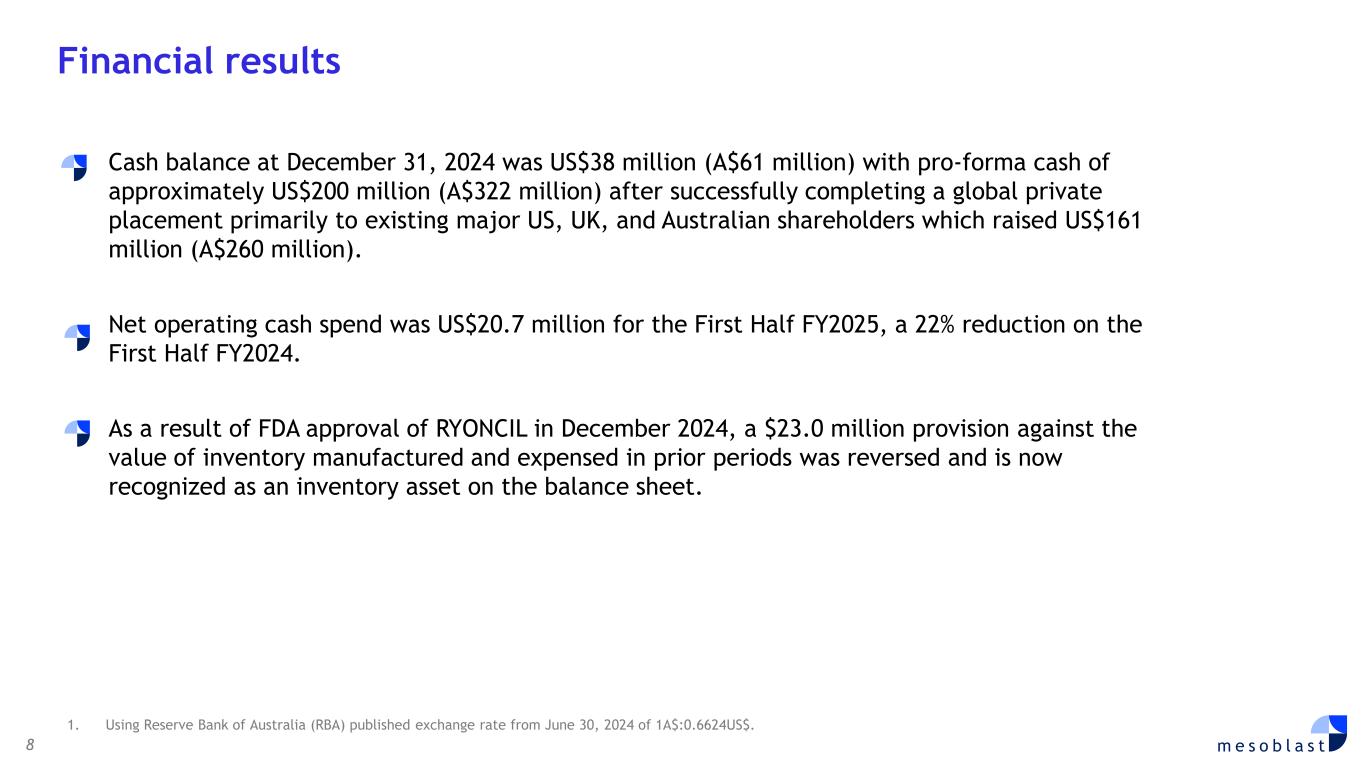
8 m e s o b l a s t Cash balance at December 31, 2024 was US$38 million (A$61 million) with pro-forma cash of approximately US$200 million (A$322 million) after successfully completing a global private placement primarily to existing major US, UK, and Australian shareholders which raised US$161 million (A$260 million). Net operating cash spend was US$20.7 million for the First Half FY2025, a 22% reduction on the First Half FY2024. As a result of FDA approval of RYONCIL in December 2024, a $23.0 million provision against the value of inventory manufactured and expensed in prior periods was reversed and is now recognized as an inventory asset on the balance sheet. Financial results 1. Using Reserve Bank of Australia (RBA) published exchange rate from June 30, 2024 of 1A$:0.6624US$.
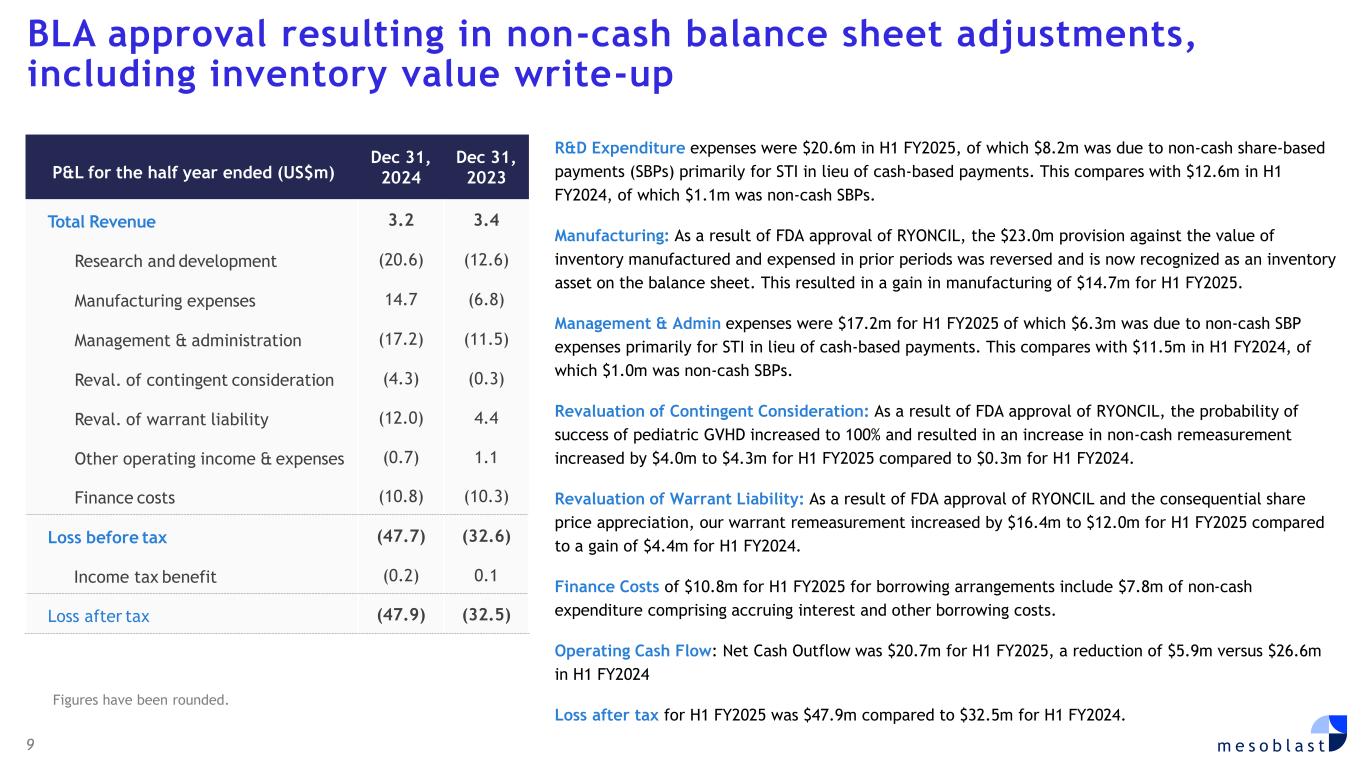
9 m e s o b l a s t BLA approval resulting in non-cash balance sheet adjustments, including inventory value write-up R&D Expenditure expenses were $20.6m in H1 FY2025, of which $8.2m was due to non-cash share-based payments (SBPs) primarily for STI in lieu of cash-based payments. This compares with $12.6m in H1 FY2024, of which $1.1m was non-cash SBPs. Manufacturing: As a result of FDA approval of RYONCIL, the $23.0m provision against the value of inventory manufactured and expensed in prior periods was reversed and is now recognized as an inventory asset on the balance sheet. This resulted in a gain in manufacturing of $14.7m for H1 FY2025. Management & Admin expenses were $17.2m for H1 FY2025 of which $6.3m was due to non-cash SBP expenses primarily for STI in lieu of cash-based payments. This compares with $11.5m in H1 FY2024, of which $1.0m was non-cash SBPs. Revaluation of Contingent Consideration: As a result of FDA approval of RYONCIL, the probability of success of pediatric GVHD increased to 100% and resulted in an increase in non-cash remeasurement increased by $4.0m to $4.3m for H1 FY2025 compared to $0.3m for H1 FY2024. Revaluation of Warrant Liability: As a result of FDA approval of RYONCIL and the consequential share price appreciation, our warrant remeasurement increased by $16.4m to $12.0m for H1 FY2025 compared to a gain of $4.4m for H1 FY2024. Finance Costs of $10.8m for H1 FY2025 for borrowing arrangements include $7.8m of non-cash expenditure comprising accruing interest and other borrowing costs. Operating Cash Flow: Net Cash Outflow was $20.7m for H1 FY2025, a reduction of $5.9m versus $26.6m in H1 FY2024 Loss after tax for H1 FY2025 was $47.9m compared to $32.5m for H1 FY2024. P&L for the half year ended (US$m) Dec 31, 2024 Dec 31, 2023 Total Revenue 3.2 3.4 Research and development (20.6) (12.6) Manufacturing expenses 14.7 (6.8) Management & administration (17.2) (11.5) Reval. of contingent consideration (4.3) (0.3) Reval. of warrant liability (12.0) 4.4 Other operating income & expenses (0.7) 1.1 Finance costs (10.8) (10.3) Loss before tax (47.7) (32.6) Income tax benefit (0.2) 0.1 Loss after tax (47.9) (32.5) Figures have been rounded.

10 m e s o b l a s t First mesenchymal stromal cell (MSC) therapy approved by FDA
11 m e s o b l a s t Press Release available at www.mesoblast.com 1. Please see the full Prescribing Information at www.ryoncil.com RYONCIL® is the first FDA-approved, off- the-shelf cell therapy for children aged 2 months and older, including adolescents and teenagers, with steroid-refractory acute graft versus host disease (SR- aGvHD), a life-threatening condition with high mortality rates.1

12 m e s o b l a s t Opportunity to address critical unmet need in children 2 months and older, including adolescents & teenagers with SR-aGVHD ~1,500 Children & adolescents undergo allogeneic BMT in US annually Approx. 10,000 allogeneic BMTs performed in the US annually Acute GvHD occurs in ~50% of patients1 with approx. half failing to respond to steroids SR-aGvHD has high mortality1,4 and significant extended hospital stay costs2 ~50% Incidence of acute GvHD ~375pts Per year with SR-aGvHD 1. Westin, J., Saliba, RM., Lima, M. (2011) Steroid-refractory acute GVHD: predictors and outcomes. Advances in Hematology. 2. Niederwieser D, Baldomero H, Szer J. (2016) Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. 3. HRSA Transplant Activity Report, CIBMTR, 2020 4. Axt L, Naumann A, Toennies J (2019) Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. >30,000 allogeneic BMTs performed globally (>20K US/EU) annually, ~20% pediatric2,3 Corticosteroids are first-line therapy for aGvHD RYONCIL is the only approved therapy for SR-aGvHD in children 2 months and older
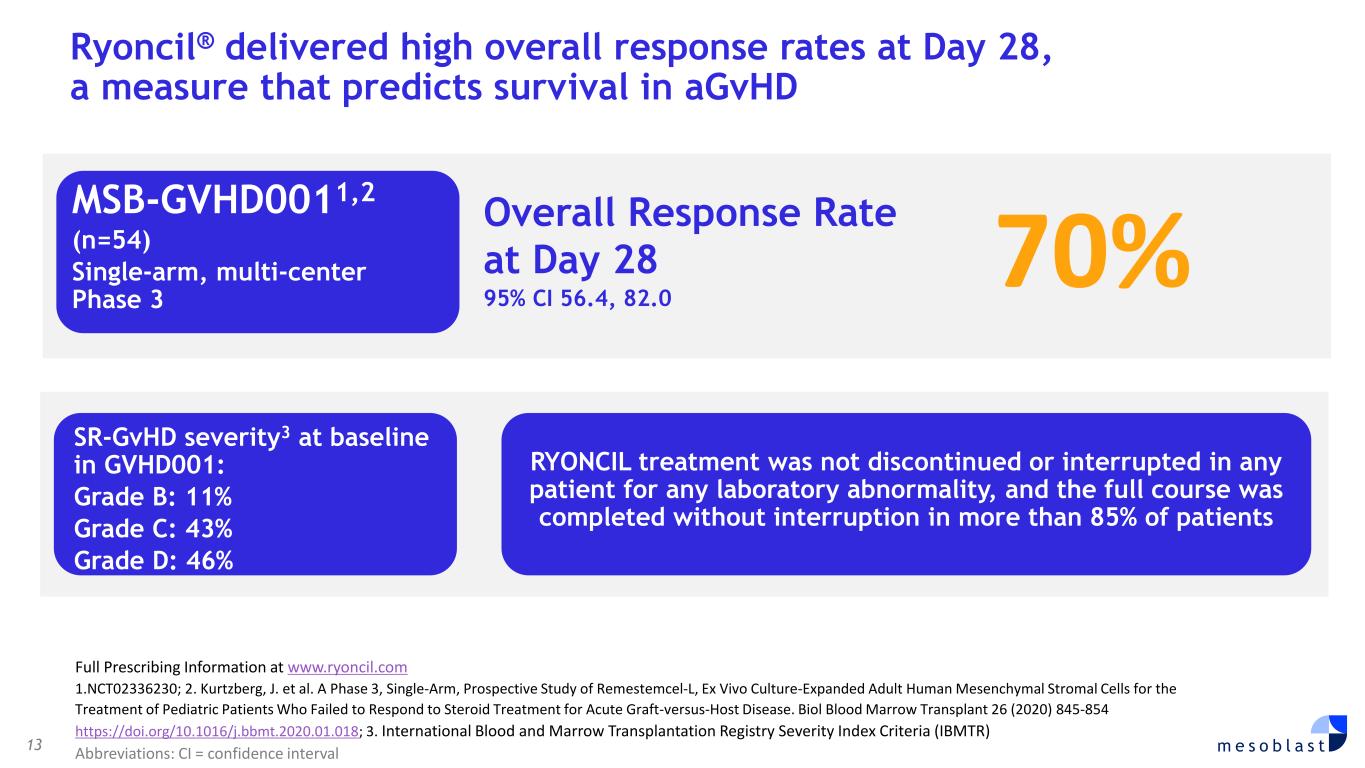
13 m e s o b l a s t Ryoncil® delivered high overall response rates at Day 28, a measure that predicts survival in aGvHD Overall Response Rate at Day 28 95% CI 56.4, 82.0 MSB-GVHD0011,2 (n=54) Single-arm, multi-center Phase 3 70% Full Prescribing Information at www.ryoncil.com 1.NCT02336230; 2. Kurtzberg, J. et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant 26 (2020) 845-854 https://doi.org/10.1016/j.bbmt.2020.01.018; 3. International Blood and Marrow Transplantation Registry Severity Index Criteria (IBMTR) Abbreviations: CI = confidence interval SR-GvHD severity3 at baseline in GVHD001: Grade B: 11% Grade C: 43% Grade D: 46% RYONCIL treatment was not discontinued or interrupted in any patient for any laboratory abnormality, and the full course was completed without interruption in more than 85% of patients

14 m e s o b l a s t High cost of treating child who dies from SR-aGvHD The cost of treating a child who dies of SR-aGVHD within 12 months of transplant is: ➢ Approximately $2.5M ➢ $1.8M higher than for those with SR aGvHD who remain alive1 1. Grabner M et al. Economic burden of acute steroid-refractory graft-versus-host disease in commercially insured pediatric patients. J Manag Care Spec Pharm.2021;27(5):607-14

15 m e s o b l a s t Ryoncil® long-term survival free from aGvHD Year 2 Survival: Long-term follow- up of Ryoncil by the Center for International Blood and Marrow Transplant Research (CIBMTR) 51% Year 4 Survival: 49% Data on file Children from GVHD001 N=51 88% Grade C/D Only 14% (N=7) died due to aGvHD through 4 years
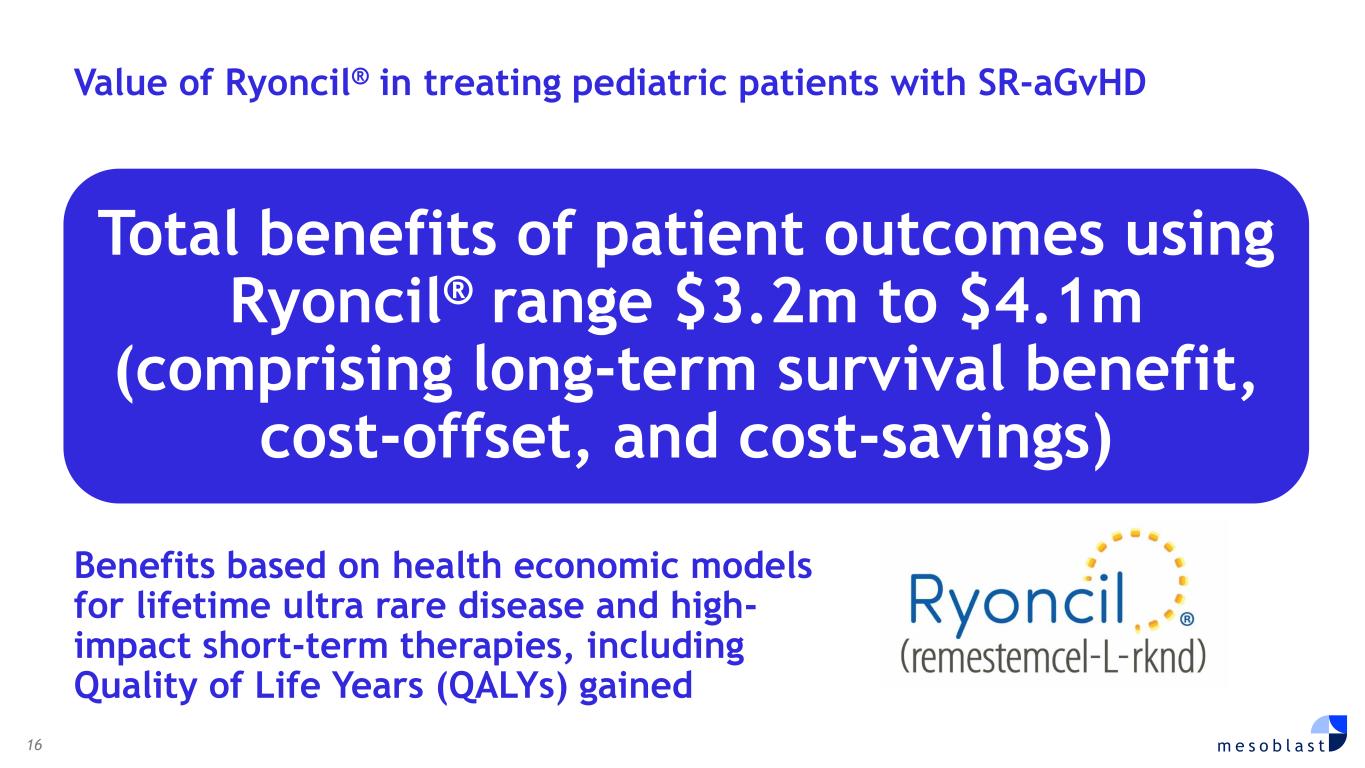
16 m e s o b l a s t Benefits based on health economic models for lifetime ultra rare disease and high- impact short-term therapies, including Quality of Life Years (QALYs) gained Total benefits of patient outcomes using Ryoncil® range $3.2m to $4.1m (comprising long-term survival benefit, cost-offset, and cost-savings) Value of Ryoncil® in treating pediatric patients with SR-aGvHD

17 m e s o b l a s t Ryoncil® for treating pediatric patients with SR-aGvHD The recommended dosage of Ryoncil® for treatment of pediatric SR-aGvHD is 2×106 MSC/kg body weight per intravenous infusion given twice per week for 4 consecutive weeks The wholesale acquisition cost (WAC) of Ryoncil® is $194,000 per intravenous infusion, irrespective of body weights
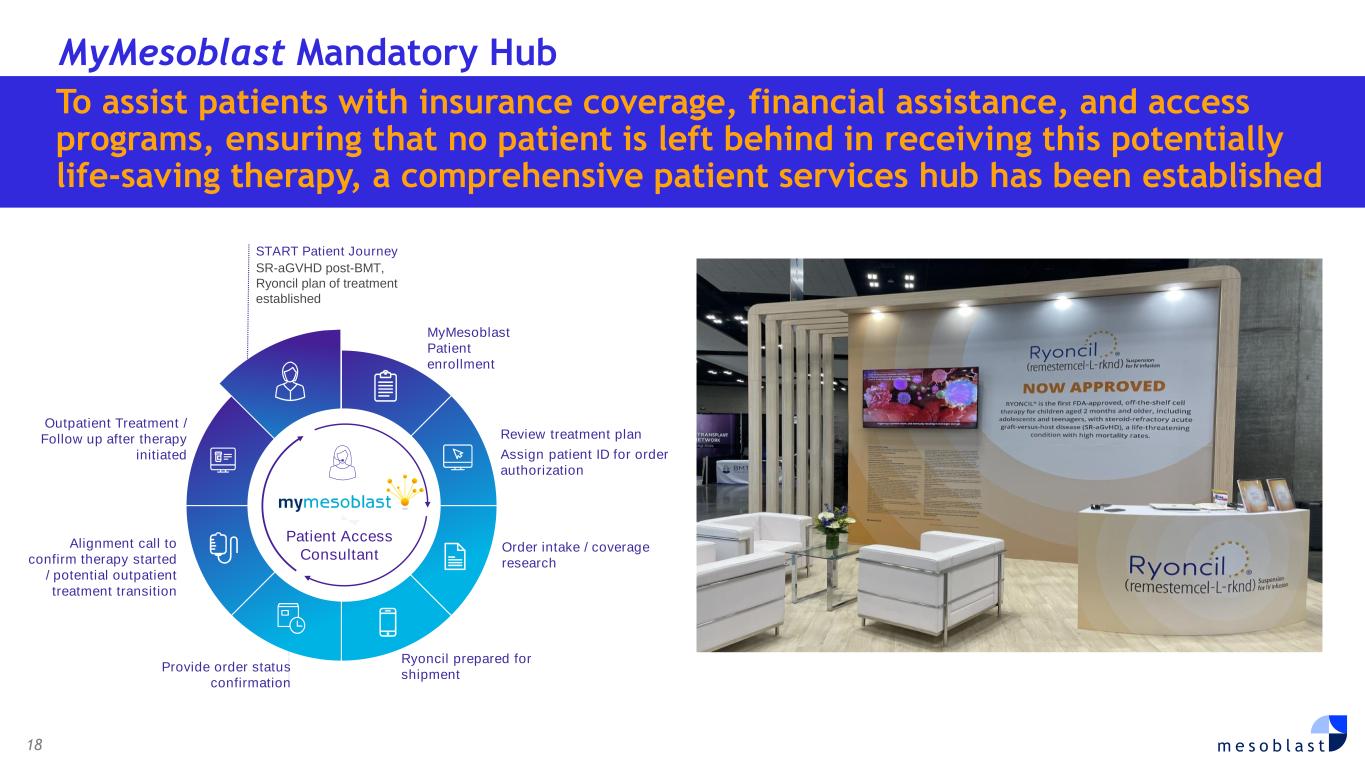
18 m e s o b l a s t MyMesoblast Mandatory Hub START Patient Journey SR-aGVHD post-BMT, Ryoncil plan of treatment established MyMesoblast Patient enrollment Review treatment plan Assign patient ID for order authorization Order intake / coverage research Ryoncil prepared for shipment Outpatient Treatment / Follow up after therapy initiated Provide order status confirmation Patient Access Consultant Alignment call to confirm therapy started / potential outpatient treatment transition To assist patients with insurance coverage, financial assistance, and access programs, ensuring that no patient is left behind in receiving this potentially life-saving therapy, a comprehensive patient services hub has been established
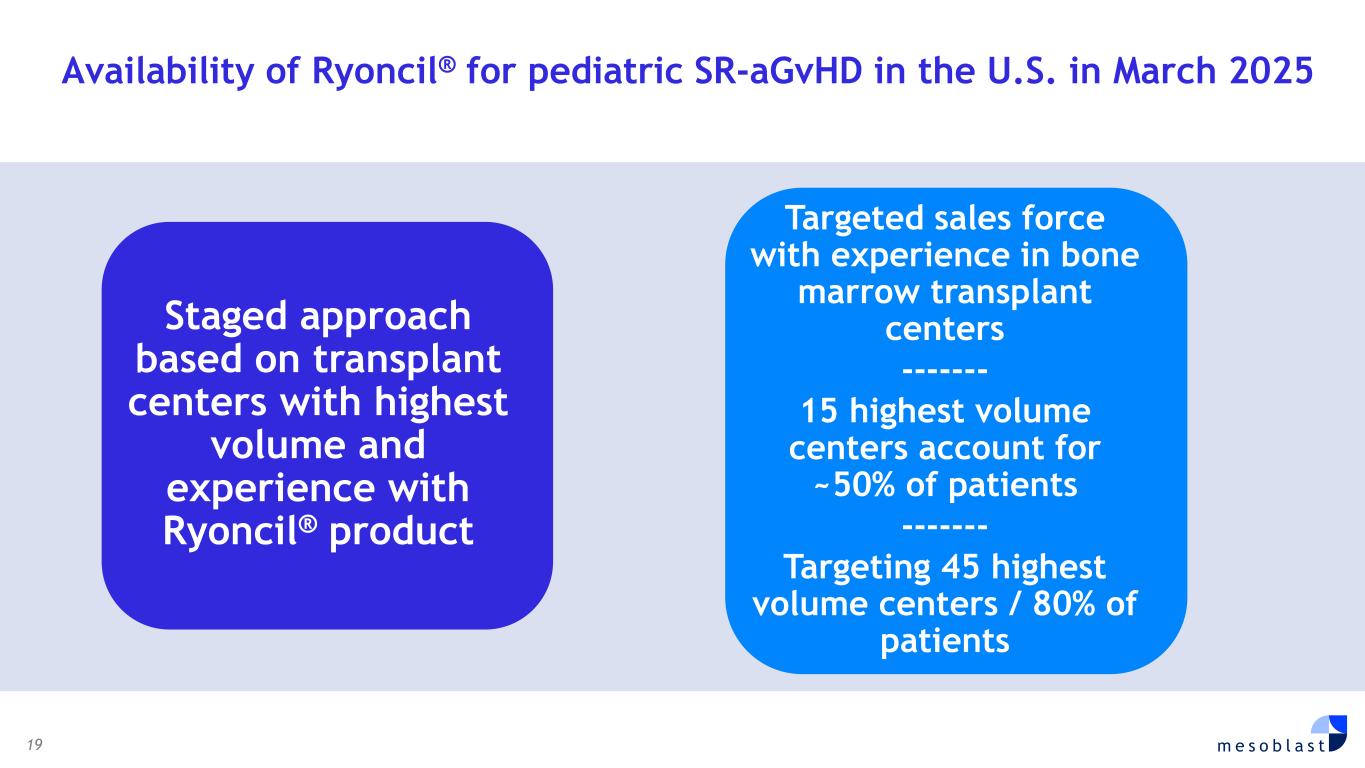
19 m e s o b l a s t Availability of Ryoncil® for pediatric SR-aGvHD in the U.S. in March 2025 Staged approach based on transplant centers with highest volume and experience with Ryoncil® product Targeted sales force with experience in bone marrow transplant centers ------- 15 highest volume centers account for ~50% of patients ------- Targeting 45 highest volume centers / 80% of patients

20 m e s o b l a s t Ryoncil® Expanding the Label Expanding the Label
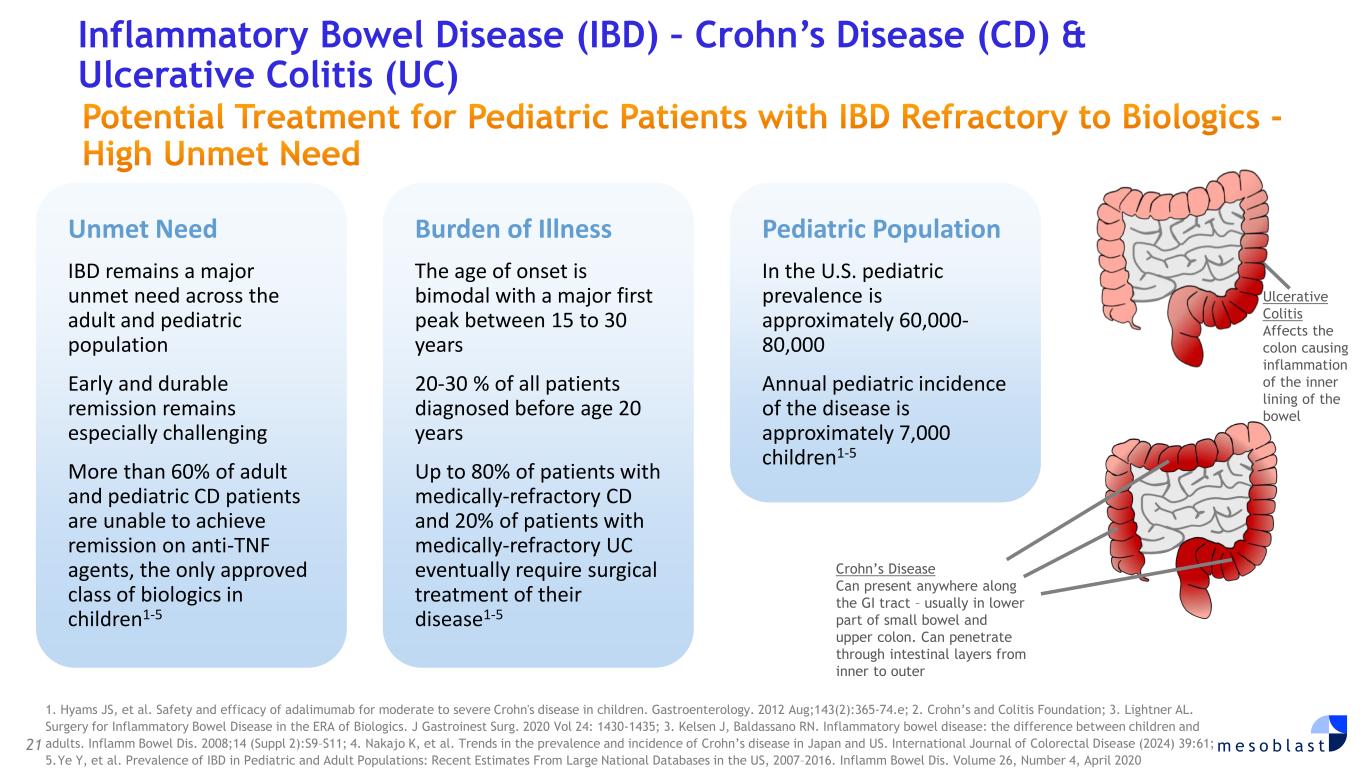
21 m e s o b l a s t 1. Hyams JS, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012 Aug;143(2):365-74.e; 2. Crohn’s and Colitis Foundation; 3. Lightner AL. Surgery for Inflammatory Bowel Disease in the ERA of Biologics. J Gastroinest Surg. 2020 Vol 24: 1430-1435; 3. Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14 (Suppl 2):S9–S11; 4. Nakajo K, et al. Trends in the prevalence and incidence of Crohn’s disease in Japan and US. International Journal of Colorectal Disease (2024) 39:61; 5. Ye Y, et al. Prevalence of IBD in Pediatric and Adult Populations: Recent Estimates From Large National Databases in the US, 2007–2016. Inflamm Bowel Dis. Volume 26, Number 4, April 2020 Inflammatory Bowel Disease (IBD) – Crohn’s Disease (CD) & Ulcerative Colitis (UC) Potential Treatment for Pediatric Patients with IBD Refractory to Biologics - High Unmet Need Ulcerative Colitis Affects the colon causing inflammation of the inner lining of the bowel Crohn’s Disease Can present anywhere along the GI tract – usually in lower part of small bowel and upper colon. Can penetrate through intestinal layers from inner to outer Unmet Need IBD remains a major unmet need across the adult and pediatric population Early and durable remission remains especially challenging More than 60% of adult and pediatric CD patients are unable to achieve remission on anti-TNF agents, the only approved class of biologics in children1-5 Burden of Illness The age of onset is bimodal with a major first peak between 15 to 30 years 20-30 % of all patients diagnosed before age 20 years Up to 80% of patients with medically-refractory CD and 20% of patients with medically-refractory UC eventually require surgical treatment of their disease1-5 Pediatric Population In the U.S. pediatric prevalence is approximately 60,000- 80,000 Annual pediatric incidence of the disease is approximately 7,000 children1-5
22 m e s o b l a s t A recent pilot study in adults demonstrated positive outcomes (rapid mucosal healing and disease remission) in biologic refractory patients receiving remestemcel-L by direct endoscopic injection to areas of inflammation. In addition, Mesoblast has data showing that remestemcel-L induces early remission in CD adults who have failed a single anti-TNF agent following a course of intravenous treatment. Given the effectiveness of Ryoncil® in treating children with GI-related SR-aGvHD, and the exisiting data on adult CD, Mesoblast plans to further evaluate the immunomodulatory effects of Ryoncil on GI inflammation in treating medically-refractory pediatric CD patients. Label expansion strategy for Ryoncil® in pediatric & adult patients with biologic-refractory IBD 1. Lightner A, et al. A Phase IB/IIA study of remestemcel-L, an allogeneic bone marrow derived mesenchymal stem cell product, for the treatment of medically refractory Crohn’s colitis: A preliminary analysis. Abstracts of the 17th Congress of ECCO - European Crohn’s and Colitis Organisation. Poster P428. 2. Lightner A, et al. A Phase IB/IIA study of remestemcel-L, an allogeneic bone marrow derived mesenchymal stem cell product, for the treatment of medically refractory ulcerative colitis: An interim analysis. Abstracts of the 17th Congress of ECCO - European Crohn’s and Colitis Organisation. Poster P407.
23 m e s o b l a s t Continued unmet need in adults with SR-aGVHD who fail ruxolitinib (>40% of treated patients). Survival in these patients who fail ruxolitinib remains a dismal 20-30% by 100 days, a patient population with no approved therapies.1,2 In contrast, 100-day survival was 73% after RYONCIL treatment was used under expanded access in 25 adults with SR-aGVHD who failed to respond to at least one additional agent, such as ruxolitinib. Mesoblast intends to commence a Phase 3 trial of RYONCIL in adults with SR-aGVHD who are refractory to a second line agent such as ruxolitinib. Mesoblast is collaborating with the Blood and Marrow Transplant Clinical Trials Network (BMT CTN), a NIH-funded body responsible for approximately 80% of all US transplants, to conduct the trial. Label extension strategy for Ryoncil® in adult patients with SR-aGvHD 1. Jagasia M et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020 May 14; 135(20): 1739–1749. 2. Abedin S, et al. Ruxolitinib resistance or intolerance in steroid-refractory acute graft versus-host disease — a real-world outcomes analysis. British Journal of Haematology, 2021;195:429–43.
24 m e s o b l a s t Rexlemestrocel-L Chronic Low Back Pain due to Degenerative Disc Disease (CLBP)
25 m e s o b l a s t 1. Williams, J., NG, Nawi, Pelzter, K. (2015) Risk factors and disability associated with low back pain in older adults in low-and middle-income countries. Results from the WHO Study on global ageing and adult health (SAGE). PloS One. 2015; 10(6): e0127880., 2.Decision Resources: Chronic Pain December 2015., 3. LEK & NCI opinion leader interviews, and secondary analysis., 4. Navigant: Commercial Assessment for a Proprietary Cell-Based Therapy for DDD in the U.S. and the EU3 – August 2014. Chronic low back pain due to degenerative disc disease (CLBP) impacts 7M+ Over 7m patients are estimated to suffer from CLBP due to degenerative disc disease (DDD) in each of the U.S. and E.U.5 2-4 Minimal treatment options for patients with chronic low back pain (CLBP) who fail conservative therapy include opioids and surgery 50% of opioid prescriptions are for CLBP2 Durable improvement in pain has potential to reduce opioid use and prevent surgical intervention Burden of Illness Treatment Options Market Opportunity Back pain causes more disability than any other condition1 Inflicts substantial direct and indirect costs on the healthcare system,1 including excessive use of opioids in this patient population
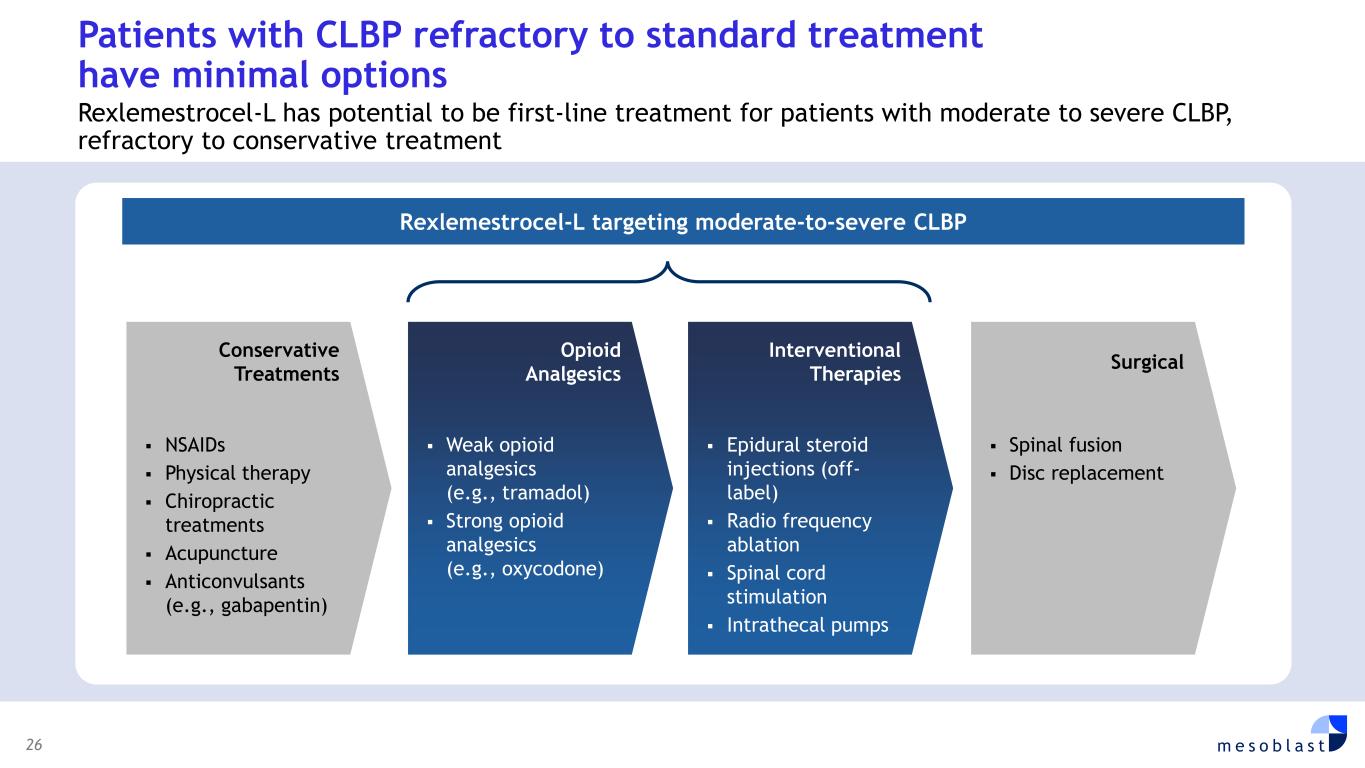
26 m e s o b l a s t Patients with CLBP refractory to standard treatment have minimal options Rexlemestrocel-L has potential to be first-line treatment for patients with moderate to severe CLBP, refractory to conservative treatment Rexlemestrocel-L targeting moderate-to-severe CLBP ▪ NSAIDs ▪ Physical therapy ▪ Chiropractic treatments ▪ Acupuncture ▪ Anticonvulsants (e.g., gabapentin) Conservative Treatments ▪ Weak opioid analgesics (e.g., tramadol) ▪ Strong opioid analgesics (e.g., oxycodone) Opioid Analgesics ▪ Epidural steroid injections (off- label) ▪ Radio frequency ablation ▪ Spinal cord stimulation ▪ Intrathecal pumps Interventional Therapies ▪ Spinal fusion ▪ Disc replacement Surgical
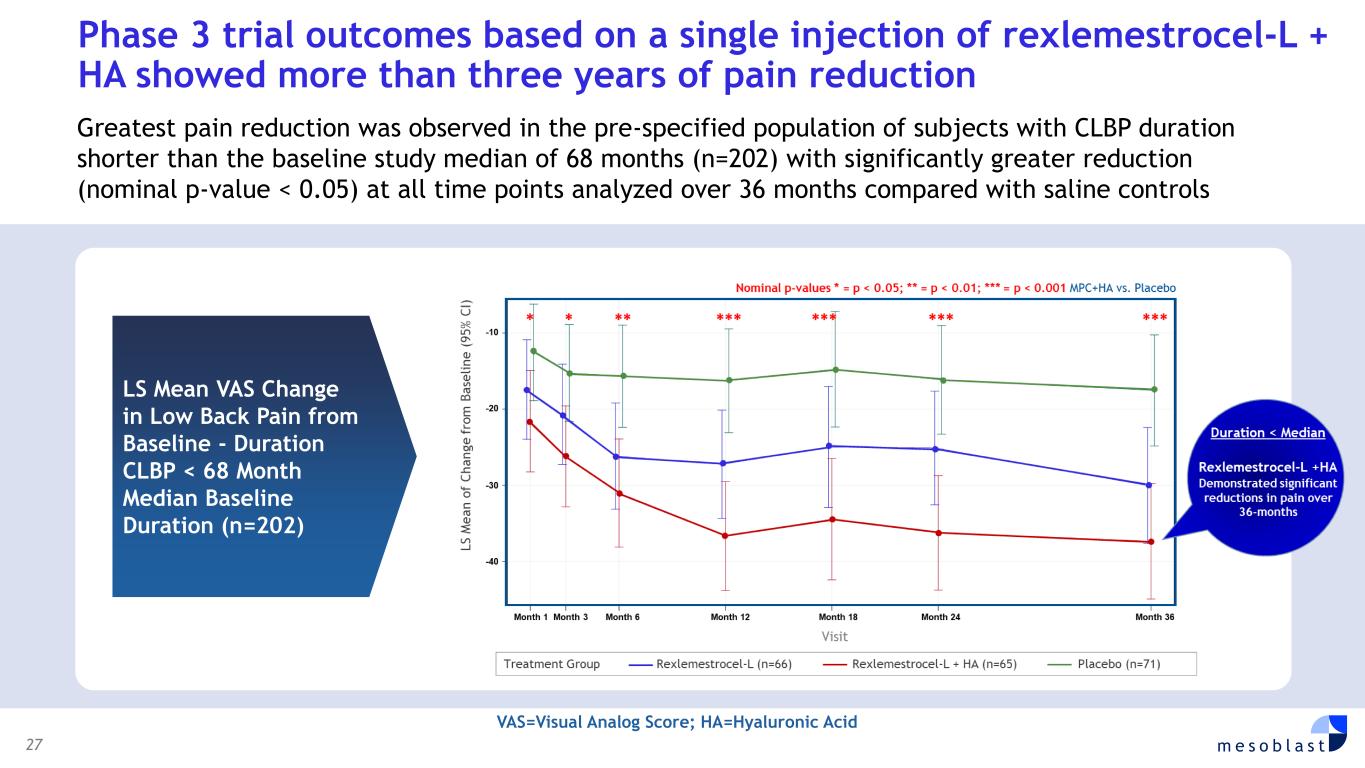
27 m e s o b l a s t LS Mean VAS Change in Low Back Pain from Baseline - Duration CLBP < 68 Month Median Baseline Duration (n=202) Phase 3 trial outcomes based on a single injection of rexlemestrocel-L + HA showed more than three years of pain reduction Greatest pain reduction was observed in the pre-specified population of subjects with CLBP duration shorter than the baseline study median of 68 months (n=202) with significantly greater reduction (nominal p-value < 0.05) at all time points analyzed over 36 months compared with saline controls VAS=Visual Analog Score; HA=Hyaluronic Acid

28 m e s o b l a s t Rexlemestrocel-L Heart Failure

29 m e s o b l a s t Adult: Heart failure with low ejection fraction (HFrEF) and underlying ischemia is increasing in prevalence and associated with high risk of mortality, heart attacks and strokes Heart failure affects 6.5 million patients in the US alone, with prevalence increasing.1 Chronic heart failure (CHF) is a progressive disease with a high mortality that approaches 50% at 5 years1,2 and at least 75% after an initial hospitalization.3 Heart failure with low ejection fraction (HFrEF) is associated with greater mortality, occurs in approximately 50% of all patients. Over 60% of HFrEF patients have underlying ischemia and these are at highest risk of recurrent major adverse cardiac events involving large vessels (heart attacks / strokes). 1. United States Food & Drug Administration. Treatment for Heart Failure: Endpoints for Drug Development. Draft Guidance. June 2019. 2. Taylor CJ, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. 2019;364:I223. 3. Shah KS, et al. Heart Failure with Preserve, Borderline, and Reduced Ejection Fraction; 5-Year Outcomes. JACC. 2017;Nov12. Target market: approximately one million patients with ischemic HFrEF and inflammation in the U.S.
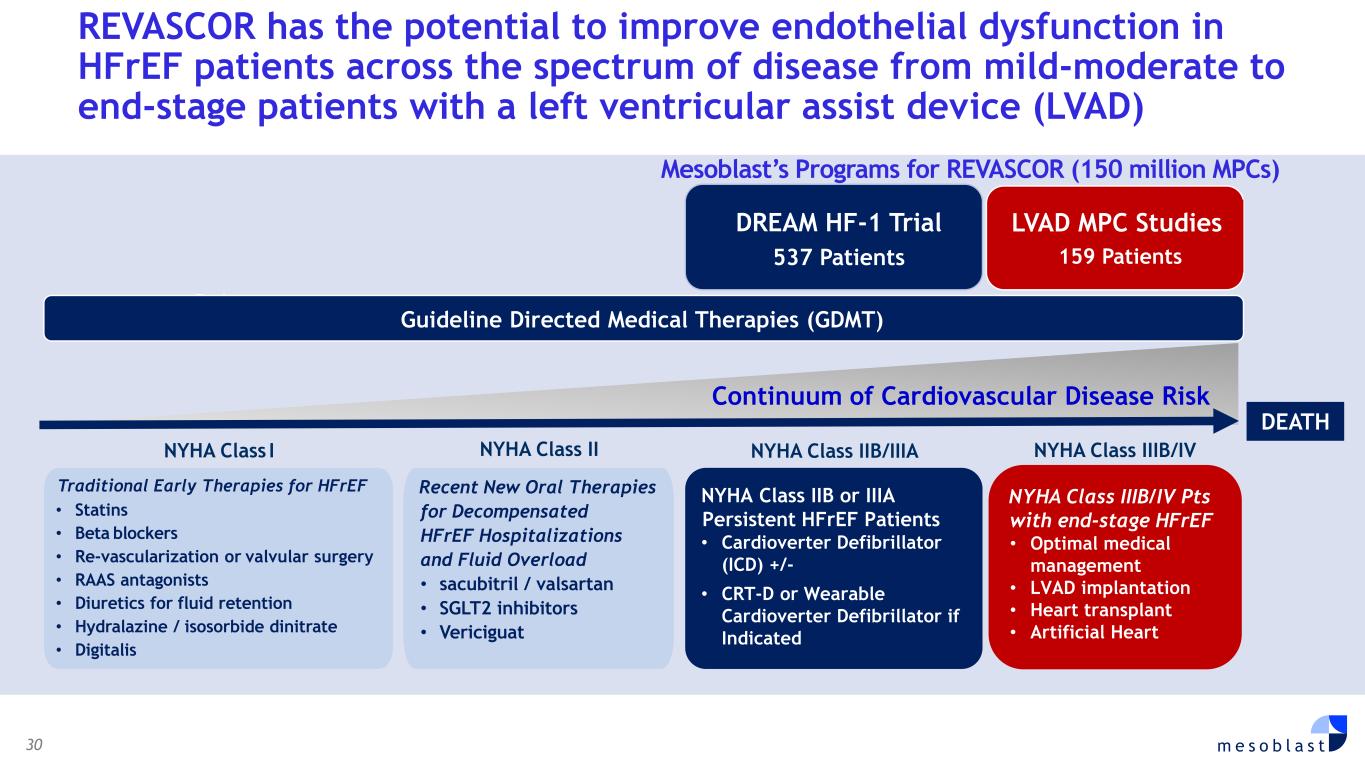
30 m e s o b l a s t REVASCOR has the potential to improve endothelial dysfunction in HFrEF patients across the spectrum of disease from mild-moderate to end-stage patients with a left ventricular assist device (LVAD) DEATH Early • Statins • Beta blockers • Re-vascularization or valvular surgery • RAAS antagonists • Diuretics for fluid retention • Hydralazine / isosorbide dinitrate • Digitalis NYHA Class I NYHA Class IIIB/IVNYHA Class II NYHA Class IIB/IIIA NYHA Class IIIB/IV Pts with end-stage HFrEF • Optimal medical management • LVAD implantation • Heart transplant • Artificial Heart NYHA Class IIB or IIIA Persistent HFrEF Patients • Cardioverter Defibrillator (ICD) +/- • CRT-D or Wearable Cardioverter Defibrillator if Indicated Traditional Early Therapies for HFrEF DREAM HF-1 Trial 537 Patients Recent New Oral Therapies for Decompensated HFrEF Hospitalizations and Fluid Overload • sacubitril / valsartan • SGLT2 inhibitors • Vericiguat Guideline Directed Medical Therapies (GDMT) LVAD MPC Studies 159 Patients Continuum of Cardiovascular Disease Risk Mesoblast’s Programs for REVASCOR (150 million MPCs)
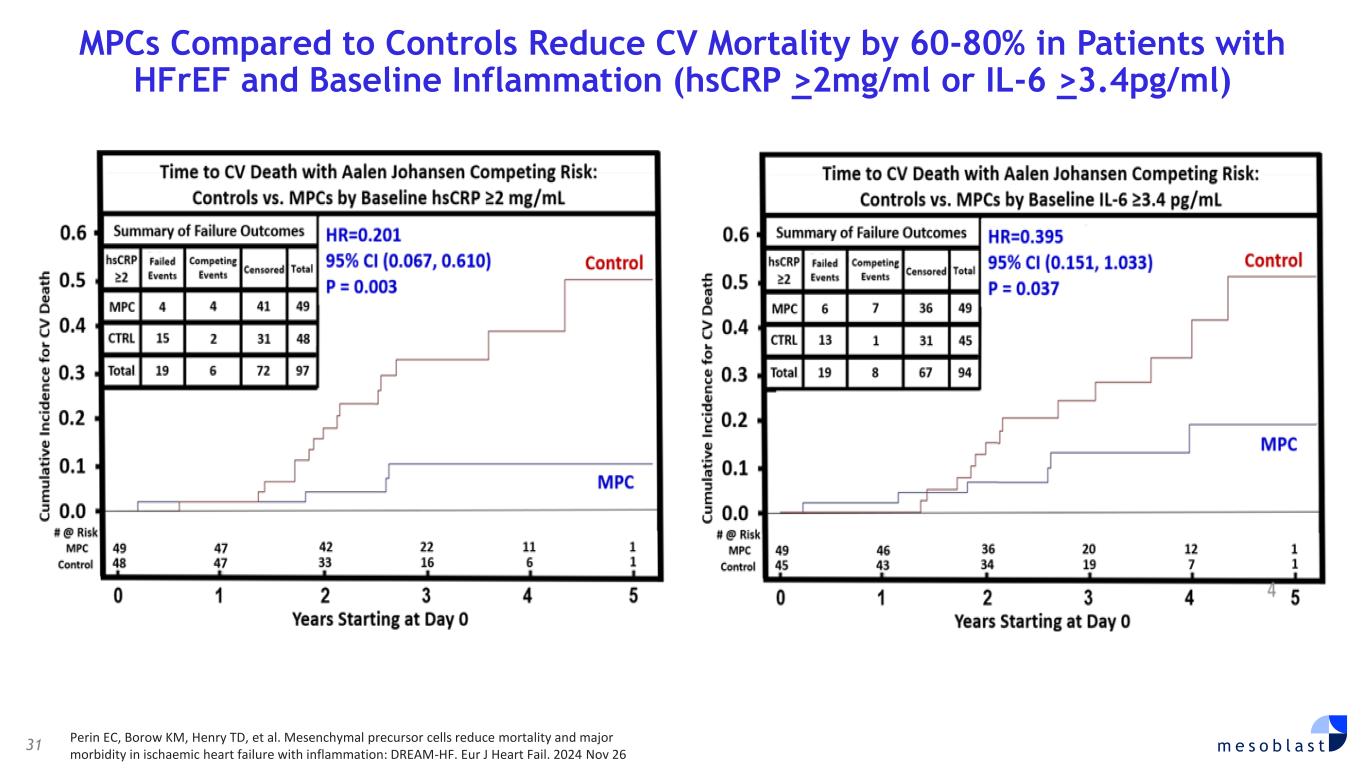
31 m e s o b l a s t MPCs Compared to Controls Reduce CV Mortality by 60-80% in Patients with HFrEF and Baseline Inflammation (hsCRP >2mg/ml or IL-6 >3.4pg/ml) Perin EC, Borow KM, Henry TD, et al. Mesenchymal precursor cells reduce mortality and major morbidity in ischaemic heart failure with inflammation: DREAM-HF. Eur J Heart Fail. 2024 Nov 26
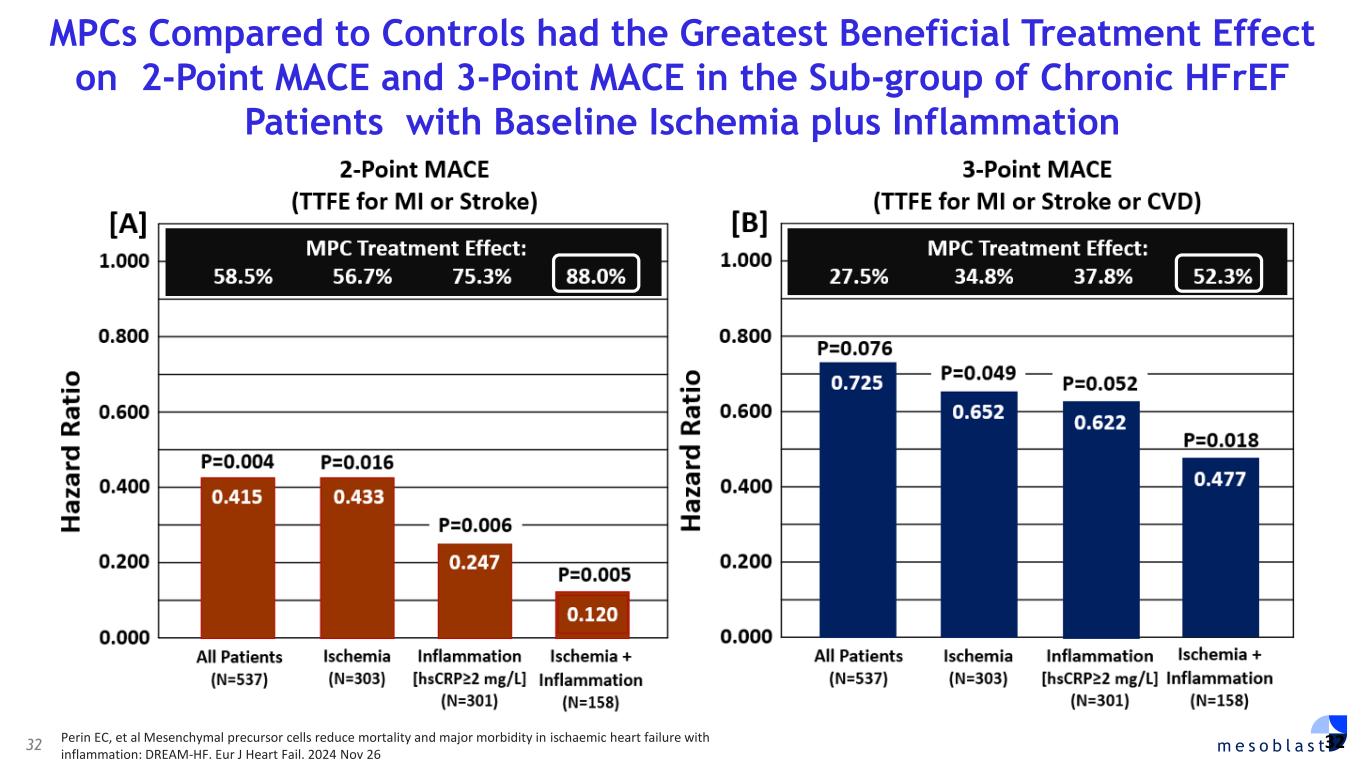
32 m e s o b l a s t MPCs Compared to Controls had the Greatest Beneficial Treatment Effect on 2-Point MACE and 3-Point MACE in the Sub-group of Chronic HFrEF Patients with Baseline Ischemia plus Inflammation 32 Perin EC, et al Mesenchymal precursor cells reduce mortality and major morbidity in ischaemic heart failure with inflammation: DREAM-HF. Eur J Heart Fail. 2024 Nov 26

33 m e s o b l a s t Pathway to accelerated approval for REVASCOR in adults with HFrEF DREAM-HF Trial over a mean follow-up of 30 months showed significant reduction in 3-Point MACE in ischemic HFrEF patients (n=158). LVAD-MPC Study #2, over 12 months of follow-up, showed significant increase in proportion of LVAD recipients with ischemic HFrEF etiology successfully weaned (n=70), with significant reduction in hospitalizations and mortality. At Type B meeting in Q1 2024, FDA informed Mesoblast that the totality of the trial results from these studies may support an accelerated approval pathway for REVASCOR in ischemic HFrEF patients. Mesoblast intends to request a pre-BLA meeting with FDA to discuss data presentation, timing and FDA expectations for an accelerated approval filing in ischemic HFrEF patients.

34 m e s o b l a s t FDA granted REVASCOR, Rare Pediatric Disease Designation (RPDD), Regenerative Medicine Advanced Therapy (RMAT) designation, and Orphan Drug Designation (ODD) during 2024. This followed submission of results from the randomized controlled trial in children with hypoplastic left heart syndrome (HLHS), a potentially life-threatening congenital heart condition. RPDD demonstrates that the disease is serious or life-threatening and the manifestations primarily affect individuals aged from birth to 18 years, including age groups often called neonates, infants, children, and adolescents, and that the disease is a rare disease or condition. On FDA approval of a BLA for REVASCOR for the treatment of HLHS, Mesoblast may be eligible to receive a Priority Review Voucher (PRV) that can be redeemed for any subsequent marketing application or may be sold or transferred to a third party. Mesoblast plans to meet with FDA to discuss whether the randomized controlled study can be used to obtain regulatory approval for REVASCOR in children with this life-threatening condition. FDA awarded Rare Pediatric Disease Designation, RMAT and Orphan Drug designation to REVASCOR for hypoplastic left heart syndrome
35 m e s o b l a s t Key Corporate Milestones

36 m e s o b l a s t REVASCOR Heart Failure Mesoblast expects to substantially advance its multiple product pipeline toward FDA approvals over the next six to twelve months RYONCIL Pediatric & Adult Inflammatory Diseases 1 Ryoncil® available for use in SR-aGvHD at U.S. hospitals this quarter Studies to commence in pediatric and adult label extension indications Program Key Objectives Rexlemestrocel-L Chronic Low Back Pain 2 3 Heart failure in adults with low ejection fraction heart failure (HFrEF), and children with congenital heart disease Preparing for accelerated approval filing CLBP Phase 3 trial actively enrolling at multiple sites across the U.S. The 300-patient randomized, placebo-controlled trial has a 12-month primary endpoint of pain reduction

37 m e s o b l a s t mesoblast Thank You




































