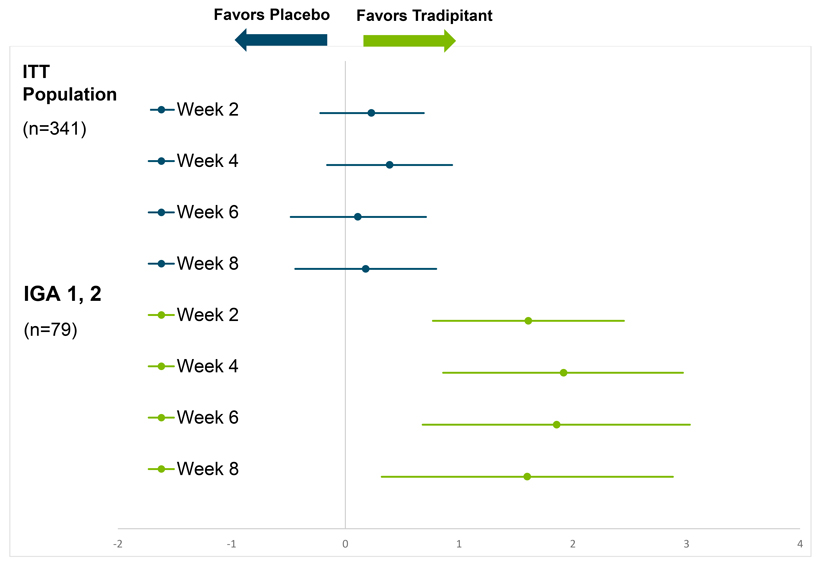
pruritus as measured by the Worst Itch Numeric Rating Scale(WI-NRS), but while the tradipitant magnitude of improvement was greater than that of placebo, the difference between treatment groups was not statistically significant. A significant interaction was observed between baseline disease severity (IGA1-4) and treatment (p=0.0004). This suggests that study participants with different baseline disease severity experienced different treatment outcomes. When accounting for baseline disease severity and treatment interaction, a significantly larger improvement inWI-NRS was seen with tradipitant at thepre-specified endpoint of Week 8 in the full trial population (p=0.0217). Similar effects were seen throughout the treatment periods at all post-randomization visits comprising weeks 2, 4, 6 and 8 (Table 1).
Given the observed significant interaction between baseline disease severity and treatment, a subgroup analysis showed that patients with mild disease severity (23% of study patients, IGA 1, 2) experienced the largest improvement over placebo. Specifically, in the mild AD group, tradipitant significantly improvedWI-NRS over placebo at every visit (Table 1, Figure 1). The categoricalWI-NRS responder analysis (>4 point improvement) showed that 72.5% of tradipitant patients had a clinically meaningful response as compared to 33.3% of placebo patients.
These results suggest a large and significant antipruritic effect of tradipitant in mild AD, and were confirmed with patient daily diary entries. For mild AD patients, a time course of response also showed that the antipruritic effect was seen immediately after the first full day of tradipitant dosing, suggesting a large and immediate therapeutic effect. Similar improvement was observed for nighttime sleep, often disrupted in patients with severe pruritus.
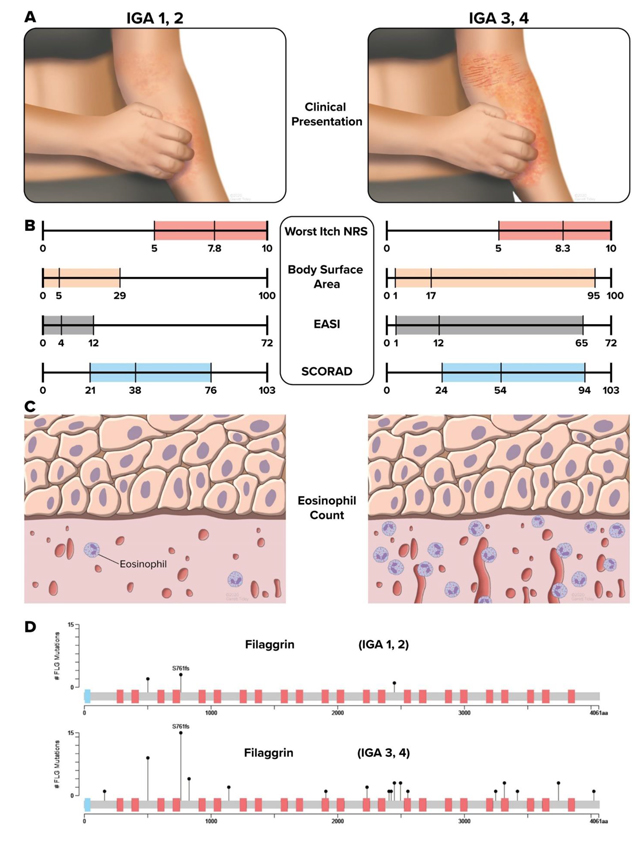
Results from the EPIONE study (Figure 2) and scientific literature suggest that mild and severe AD appear to be distinct endotypes with different sets of causative factors and course.3, 4 The American Academy of Dermatology (AAD) atopic dermatitis guidelines lists pruritus as an essential feature of atopic dermatitis.5The significant pruritus associated with mild AD and the worsening of lesions through scratching, along with the sleep disruption, continue to represent a significant unmet medical need.
“The majority of AD patients across all age groups from children to seniors suffer from a form of atopic dermatitis characterized by mild lesions. Yet these patients might still have severe pruritus and suffer from impacts to quality of life, as well as sleep. With a beneficial safety profile and assuming this significant improvement in itch in the mild-type atopic dermatitis is confirmed in a future study, this therapy would be of interest to all these mild-type AD patients,” said Dr. Sonja Stander, professor of Dermatology and Neurodermatology at the Department of Dermatology, and head of the Interdisciplinary Center for Chronic Pruritus (KCP) of the University Hospital Münster, Germany.
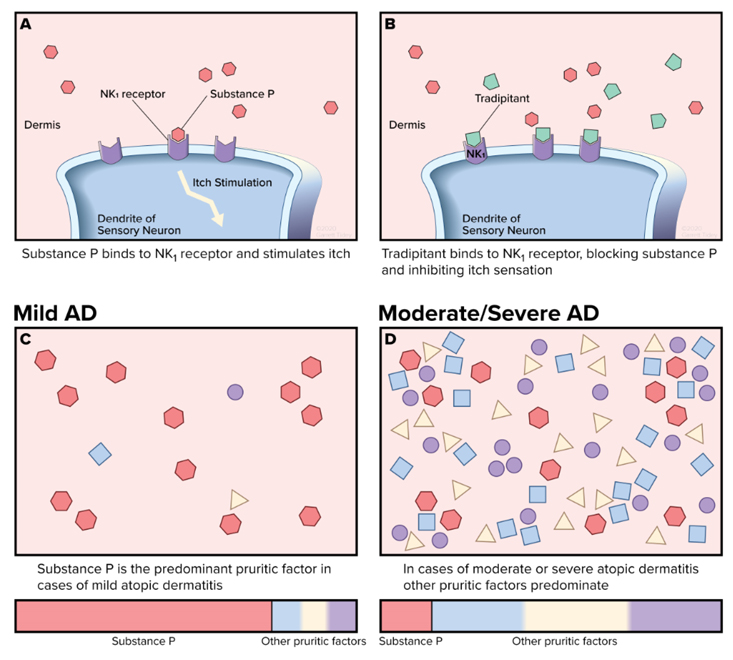
Dr. Stander added, “we also see patients withmoderate-to-severe disease where immunomodulatory therapies may have a profound effect on healing lesions in the short term but they still suffer from significant itch. TheNK-1 antagonist mechanism may work well for this population where only the severe itch is left and their disease is mild or almost clear after steroids, an interleukin inhibitor, or another immunomodulatory therapy has been used.”