August 28, 2008
VIA EDGAR AND FACSIMILE
Mr. Russell Mancuso
United States Securities and Exchange Commission
Division of Corporation Finance
100 F Street
Washington, DC 20549
Re: NewCardio, Inc.
Registration Statement on Form S-1 Amendment No. 6
Filed August 27, 2008
File No. 333-149166
Dear Mr. Mancuso:
On behalf of NewCardio, Inc. (the “Company”), the following are the Company’s responses to the corresponding numbered comments in the August 18, 2008 letter from the Securities and Exchange Commission (the “Commission”). The Company has filed on the EDGAR system an amended registration statement on Form S-1, entitled Form S-1/A (No. 7) (the “Amended Form S-1/A (No. 7)”) to conform the Amended Form S-1/A (No. 7) to the Commission’s comments in its above-referenced letter.
| 1. | Please ensure that your disclosure throughout your document is current. For example, we note: |
| · | your reference on page 27 indicates that a patent application needed to be filed by August 1, 2008, and |
| · | your Form 8-K filed August 21, 2008 indicates that you hired a new president and entered into an agreement with him, but your disclosure regarding your management is not updated with this information. |
Response:
| | The Company has added additional disclosure reflecting that the patent application has been filed on July 30, 2008, prior to the August 1, 2008 deadline. Furthermore, the Company has also added disclosure regarding its new president. |
Securities and Exchange Commission
August 28, 2008
Page 2
Clinical Development, page 32
| 2. | We note your disclosure that you completed an “independent” study and that you plan to publish your “external” study. |
| · | Please clarify what you mean by an “external” and “independent” study. It is unclear how a study could be conducted by you and at the same time be independent. |
| · | If the study was independent, please identify who conducted the study and file their consent to your prospectus summary of their report, including the summary here and on page 23. Please see Rule 436 and comment 31 in our letter dated April 30, 2008. |
| · | Please provide us a copy of the report. |
Response:
The Company has removed references to independent and external studies with regard to QTinno in both its Product Development Plan and in its Management’s Discussion and Analysis. We note that this disclosure is consistent with prior disclosure on the same matter which the Staff previously reviewed. A summary of the study's results is attached as Exhibit A. The results of the full study is in excess of 100 pages and we do not believe it would be comprehensible or beneficial for the Staff.
Quarter Ended June 30, 2008, page 34
| 3. | We note your references in this section to your use and compensation of consultants. For investors to understand the nature of your activities during the period, please discuss in this section the nature of the consulting services you purchased. We note, for example, your reference to an investor relations consultant for “public market incite” on page II-1. |
Response:
| | The Company has expanded its disclosure to clarify the nature of the consulting services it has purchased so that investors have a better understanding of its activities during the period. |
Securities and Exchange Commission
August 28, 2008
Page 3
Condensed Consolidated Balance Sheets, page F-2
| 4. | We note that you recorded deferred compensation in your stockholders’ equity (deficit) during the interim period ended June 30, 2008. Based on pages F-8 and F-9, it appears that you are accounting for certain share-based payments as deferred compensation. If that is the case, then please explain to us the facts and circumstances behind such share-based payments and why you believe your accounting and presentation for share-based payment transactions herein comply with applicable U.S GAAP. Also in your explanation, please include how you considered the impact of the guidance at paragraph 74 of SFAS 123(R) related to your presentation of unearned or deferred compensation (presented as a contra-equity account at June 30, 2008). |
Response:
The Company issued shares of its restricted common stock to non-employees for services to be rendered to the Company over the next several months. The Company’s policy is to value the shares at the date of issuance and recognize and measure the cost of the shares in the same periods in the same manner (i.e., expensed) as if the Company had paid cash for the services in accordance with the Task Force consensus in Issue EITF 00-18. The Company recognizes the expense over the period the services are provided and an offsetting credit to additional paid in capital.
The Company believes its accounting for non-employee equity-based deferred compensation is reasonable and in accordance with generally accepted accounting principles.
The Company has eliminated the deferred compensation (contra-equity account) and recorded the offsetting credit recognizing the expense for the quarter ended June 30, 2008 to additional paid in capital
The Company has amended its June 30, 2008 Form 10-Q accordingly.
Item 15. Recent Sales of Unregistered Securities, page II-1
| 5. | Please reconcile the disclosure in this section regarding 2008 transactions with the information in your updated Statement of Stockholder’s Deficit. |
Response:
| | As you are aware, this Item merely calls for disclosure of unregistered issuances of securities. Included in the Statement of Stockholder’s Deficit are both registered securities pertaining to S-8 issuances and option exercises and unregistered securities. These S-8 issuances and S-8 registered option exercises are therefore not reflected in Item 15. The difference between the Statement of Stockholder’s can be identified as follows: |
| · | 110,301 shares pertain to the preferred dividend, as listed in Item 15; |
| · | 25,000 shares pertain to option exercise; |
| · | 50,000 shares pertain to JFS Investments, as listed in Item 15; |
| · | 10,000 and 15,000 shares pertain to additional option exercises; |
| · | 5,000 and 3,000 shares pertain to grants from the S-8 plan; and |
| · | 50,000 shares pertain to First Montauk Securities Corp., as listed in Item 15. |
Securities and Exchange Commission
August 28, 2008
Page 4
The Company will file an acceleration request under separate cover today which seeks effectiveness of the S-1 tomorrow afternoon, in accordance with guidance received from Mr. Eckstein.
We hope the above has been responsive to the Commission’s comments and assists the Commission in evaluating the Amended Form S-1/A (No. 7). If you have any questions or require any additional information or documents, please telephone the undersigned at (212) 930-9700.
| | Sincerely, /s/ Thomas Rose Thomas Rose |
| | |
| | |
| cc: | Paula W. Barnett, Esq. |
Exhibit A
QTinno™ Third Validation Study
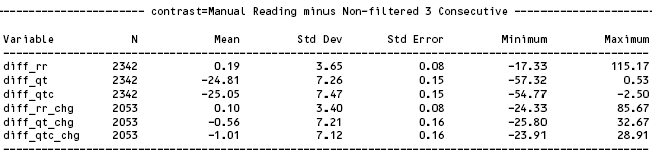
The study has compared NewCardio’s QTinno fully automated tool results with a single highly qualified manual reader. The primary end point will be discussed: 3 consecutive beats on the non-filtered signals. Manual reader was asked to measure QT interval on the same 3 most similar consecutive beats that QTinno has selected.
Shown in Table 1 are the results displaying mean and standard deviation and associated metrics for the primary endpoint for the Third Validation Study. N is the number of triplicate readings.
Table 1: Manual vs. non-filtered QTinno key results
The most interesting variable in the above table is indeed the time matched change from baseline (diff_qt_chg). The mean value for the change is only 0.56 msec. QTinno returned change that is a bit longer. The standard deviation of the difference between manual and QTinno is 7.21 msec.
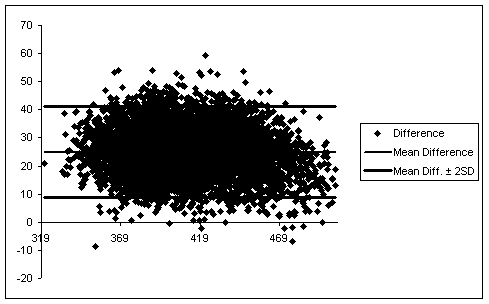
Below is Fig. 2, the Bland-Altman plot of the difference between the two methods.
Table 2: Bland-Altman Plots of Difference Between Methods
The variability is addressed in Table 3. While there are some differences between methods the overall conclusion is that variability is to the first order the same between QTinno (non-filtered signal) and a highly qualified manual reader.
| Third Validation Study: Estimated Between and Within Subject Variability by Method and Group |
| Method | Variability | QT | QTc | Change QT | Change QTc |
| Manual | Between | 27.0 | 16.4 | 9.8 | 6.4 |
| Within | 12.0 | 7.2 | 13.6 | 8.9 |
| Non_Filtered 3C | Between | 25.6 | 15.0 | 9.6 | 5.9 |
| Within | 12.2 | 7.3 | 14.1 | 9.0 |
*Mixed model including factors for dose, subject, day and hour and all interactions
In summary, QTinno has matched the highly qualified single manual reader exceptionally well in assessing the most important parameter: time matched pair-wise QT prolongation. Bland Altman plots show a very small standard deviation between the two methods.