|
Exhibit 99.1 
|
Powering the Bioindustrial Revolution with Better DNA™
Corporate Presentation
May 2015
Forward-Looking Statements
Safe Harbor Statement
Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the Safe harbor Provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s current and future ECCs and joint ventures; (ii) Intrexon’s ability to successfully enter new markets or develop additional products, whether with its collaborators or independently; (iii) actual or anticipated variations in Intrexon’s operating results; (iv) actual or anticipated fluctuations in Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (v) Intrexon’s cash position; (vi) market conditions in Intrexon’s industry; (vii) Intrexon’s ability, and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (viii) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations and policies; (ix) the rate and degree of market acceptance of any products developed by a collaborator under an ECC or through a joint venture; (x) Intrexon’s ability to retain and recruit key personnel; (xi) Intrexon’s expectations related to the use of proceeds from its public offerings and other financing efforts; (xii) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xiii) Intrexon’s expectations relating to its subsidiaries and other affiliates. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intrexon’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Intrexon’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law.
Non-GAAP Financial Measures
This presentation presents Adjusted EBITDA and Adjusted EBITDA earnings per share, which are non-GAAP financial measures within the meaning of applicable rules and regulations of the Securities and Exchange Commission (SEC). For a reconciliation of Adjusted EBITDA to net loss attributable to Intrexon in accordance with generally accepted accounting principles and for a discussion of the reasons why the company believes that these non-GAAP financial measures provide information that is useful to investors see the tables below under “Reconciliation of GAAP to Non-GAAP Measures.” Such information is provided as additional information, not as an alternative to Intrexon’s consolidated financial statements presented in accordance with GAAP, and is intended to enhance an overall understanding of the Company’s current financial performance.
© 2015 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon.
2 © 2015 Intrexon Corp. All rights reserved.
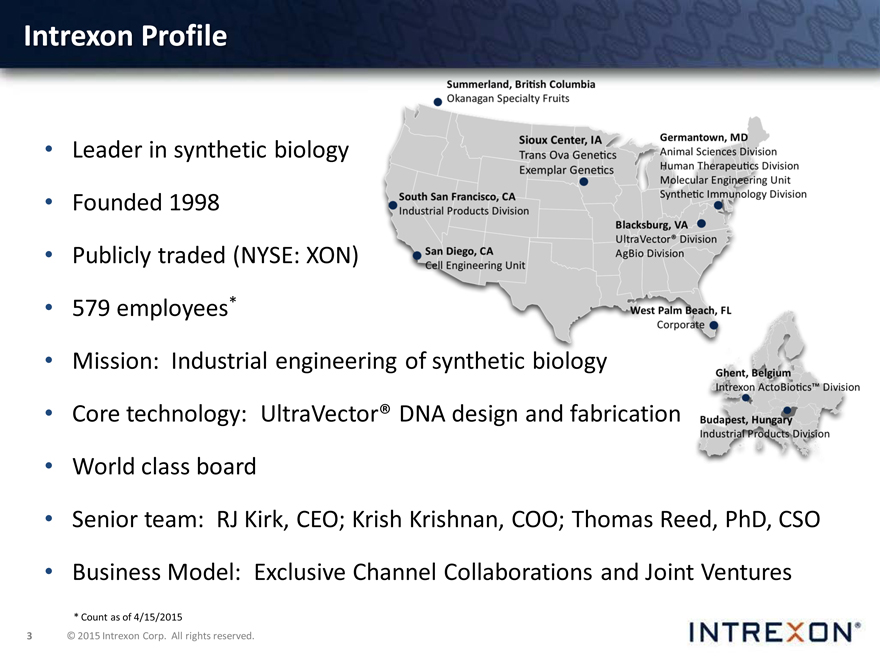
Intrexon Profile
Leader in synthetic biology
Founded 1998
Publicly traded (NYSE: XON)
579 employees*
Mission: Industrial engineer
Core technology: UltraVecto
World class board
Senior team: RJ Kirk, CEO; Krish Krishnan, COO; Thomas Reed, PhD, CSO
Business Model: Exclusive Channel Collaborations and Joint Ventures
* Count as of 4/15/2015
3 © 2015 Intrexon Corp. All rights reserved.
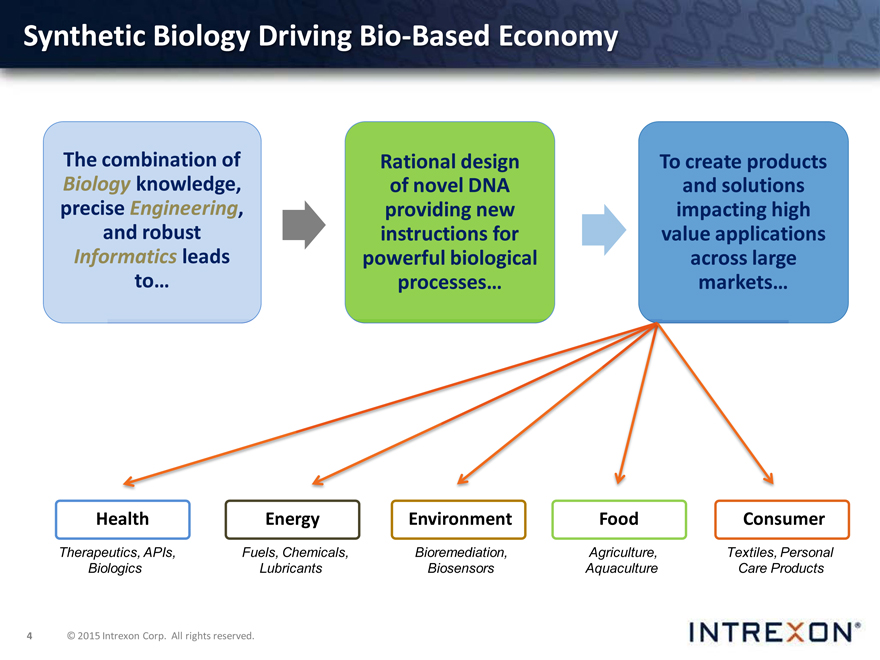
Synthetic Biology Driving Bio-Based Economy
The combination of
Biology knowledge,
precise Engineering,
and robust
Informatics leads
to…
Rational design
of novel DNA
providing new
instructions for
powerful biological
processes…
To create products and solutions impacting high value applications across large markets…
Health
Therapeutics, APIs,
Biologics
Energy
Fuels, Chemicals,
Lubricants
Environment
Bioremediation, Biosensors
Food
Agriculture, Aquaculture
Consumer
Textiles, Personal Care Products
4 © 2015 Intrexon Corp. All rights reserved.
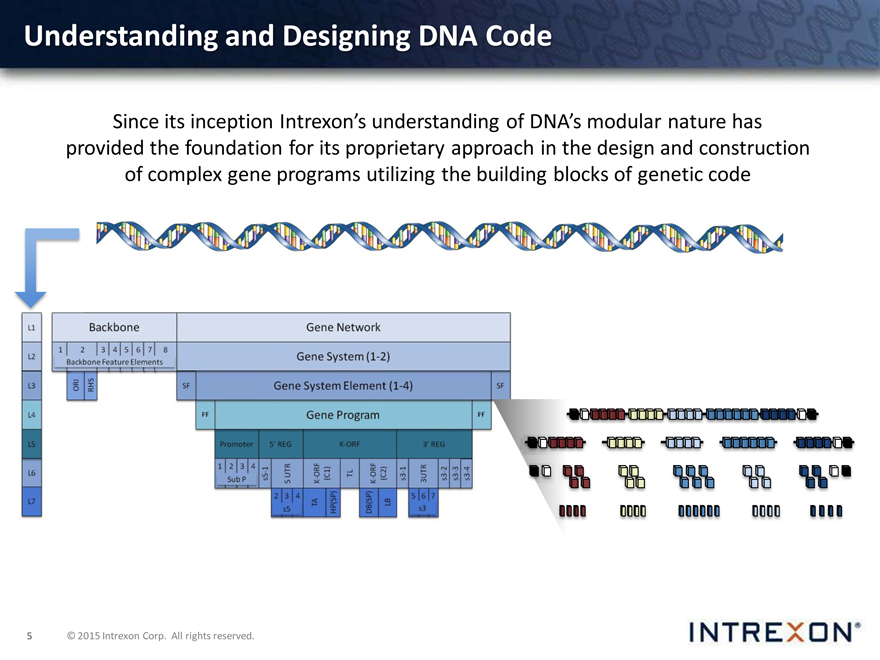
Understanding and Designing DNA Code
Since its inception Intrexon’s understanding of DNA’s modular nature has provided the foundation for its proprietary approach in the design and construction of complex gene programs utilizing the building blocks of genetic code
5 © 2015 Intrexon Corp. All rights reserved.
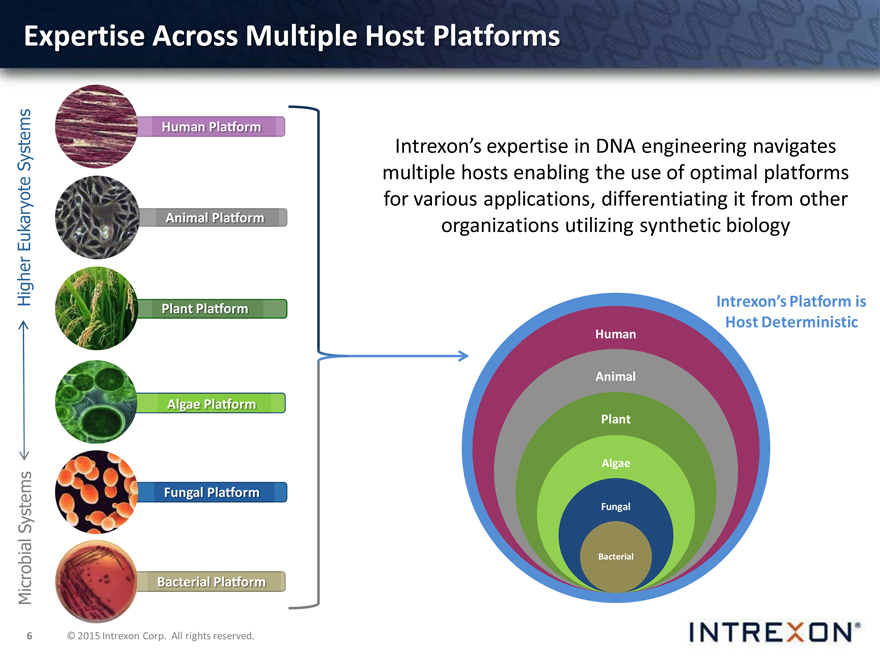
Expertise Across Multiple Host Platforms
Intrexon’s expertise in DNA engineering navigates multiple hosts enabling the use of optimal platforms for various applications, differentiating it from other organizations utilizing synthetic biology
Intrexon’s Platform is Host Deterministic
Human Animal Plant
Algae
Fungal
Bacterial
Systems Human Platform
Eukaryote Animal Platform
Higher Plant Platform
Algae Platform
Systems Fungal Platform
Microbial Bacterial Platform
6 © 2015 Intrexon Corp. All rights reserved.
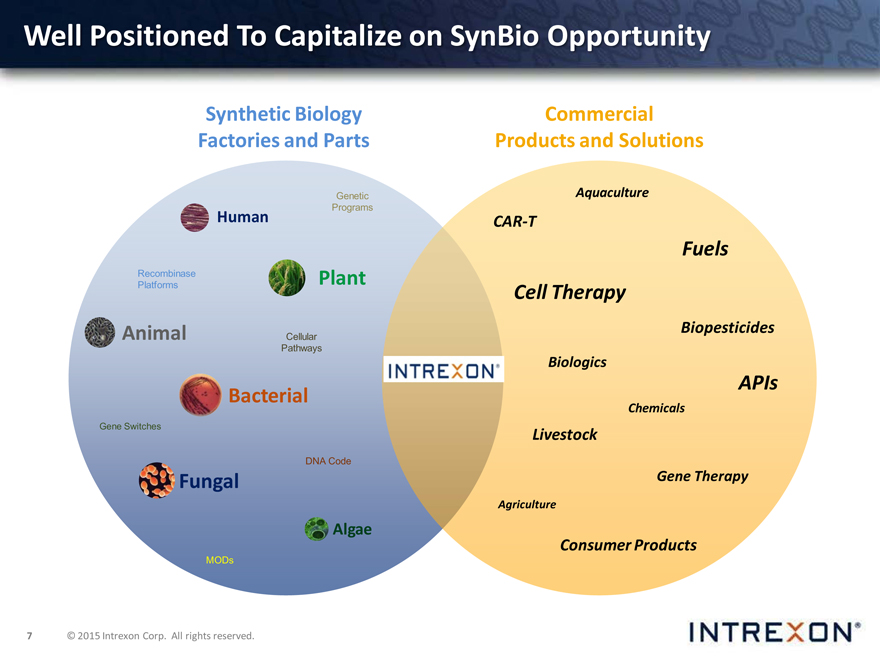
Well Positioned To Capitalize on SynBio Opportunity
Synthetic Biology Commercial Factories and Parts Products and Solutions
Genetic Human Programs
Recombinase Plant Platforms
Animal Cellular Pathways
Bacterial
Gene Switches
DNA Code
Fungal
Algae
MODs
Products and Solutions
Aquaculture
CAR-T
Fuels Cell Therapy
Biopesticides
Biologics
APIs
Chemicals
Livestock
Gene Therapy
Agriculture
Consumer Products
7 © 2015 Intrexon Corp. All rights reserved.
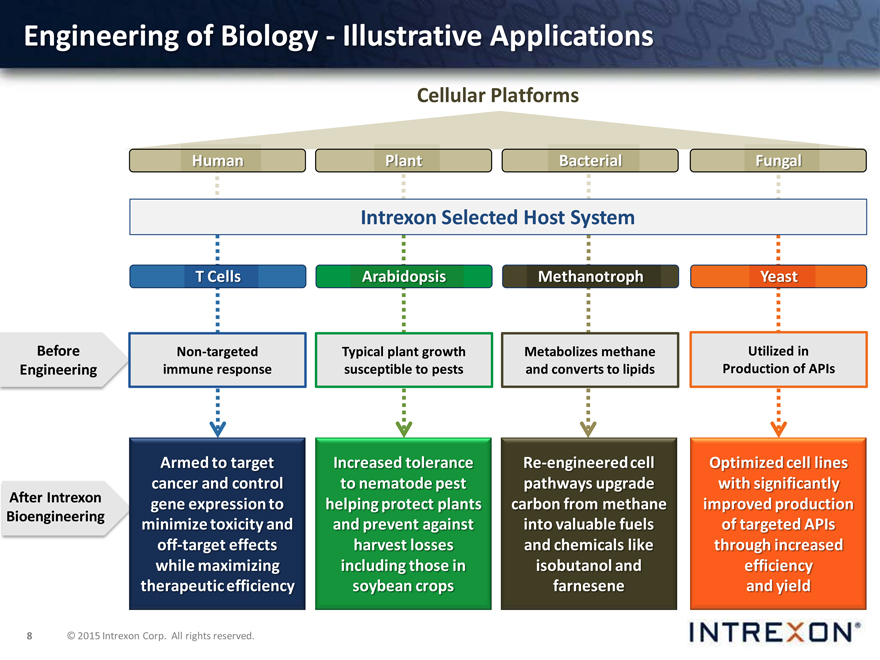
Engineering of Biology—Illustrative Applications
Cellular Platforms
Human Plant Bacterial Fungal
Intrexon Selected Host System
T Cells Arabidopsis Methanotroph Yeast
Before Non-targeted Typical plant growth Metabolizes methane Utilized in Engineering immune response susceptible to pests and converts to lipids Production of APIs
After Intrexon Bioengineering
Armed to target cancer and control gene expression to minimize toxicity and off-target effects while maximizing therapeutic efficiency
Increased tolerance to nematode pest helping protect plants and prevent against harvest losses including those in soybean crops
Re-engineered cell pathways upgrade carbon from methane into valuable fuels and chemicals like isobutanol and farnesene
Optimized cell lines with significantly improved production of targeted APIs through increased efficiency and yield
8 © 2015 Intrexon Corp. All rights reserved.
Integrated Technology Suite
UltraVector® is a modular, object-oriented DNA design & manufacturing platform enabling rapid construction of complex DNA RheoSwitch Therapeutic System® provides inducible regulation of level and timing of gene expression AttSite® Recombinases enable genome engineering and delivery technologies for targeted gene integration plus expression DNA/RNA Engineering of genetic components to regulate transcription and modulate RNA persistence and translation Protein Engineering utilizes bioinformatics to enhance stability, localization, and catalytic activity of proteins Cell Systems Informatics guide selection of optimal pathways for genomic modification
LEAP® Cell Processing provides imaging and laser-based purification of high value cells ActoBiotics™ provide for in situ expression and secretion of novel biotherapeutic proteins and peptides through microbes
Intrexon has built, acquired and integrated a suite of technologies creating a one-stop-shop for start-to-finish conceptualization, engineering and production of bio-based solutions
9 © 2015 Intrexon Corp. All rights reserved.
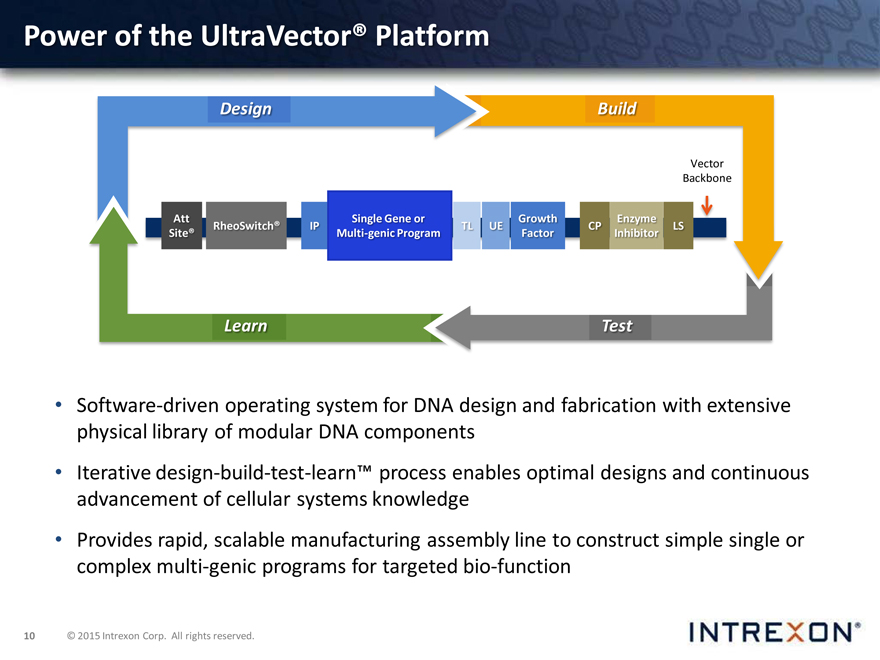
Power of the UltraVector® Platform
Design Build
Learn Test
Software-driven operating system for DNA design and fabrication with extensive physical library of modular DNA components
Iterative design-build-test-learn™ process enables optimal designs and continuous advancement of cellular systems knowledge
Provides rapid, scalable manufacturing assembly line to construct simple single or complex multi-genic programs for targeted bio-function
10 © 2015 Intrexon Corp. All rights reserved.
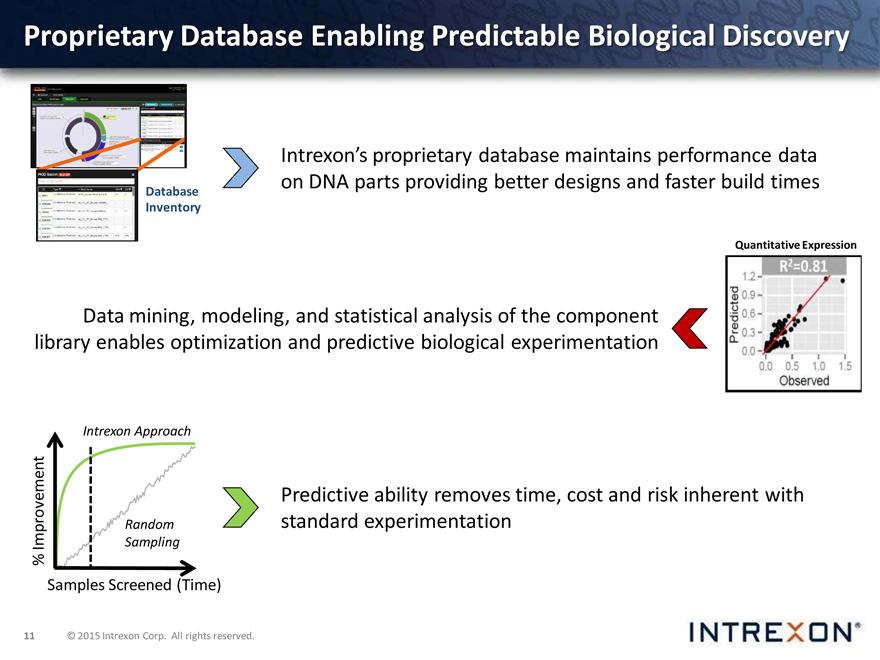
Proprietary Database Enabling Predictable Biological Discovery
Intrexon’s proprietary database maintains performance data on DNA parts providing better designs and faster build times
Database Inventory
Quantitative Expression
Data mining, modeling, and statistical analysis of the component library enables optimization and predictive biological experimentation
Intrexon Approach
Predictive ability removes time, cost and risk inherent with Random standard experimentation
Improvement Sampling % Samples Screened (Time)
11 © 2015 Intrexon Corp. All rights reserved.
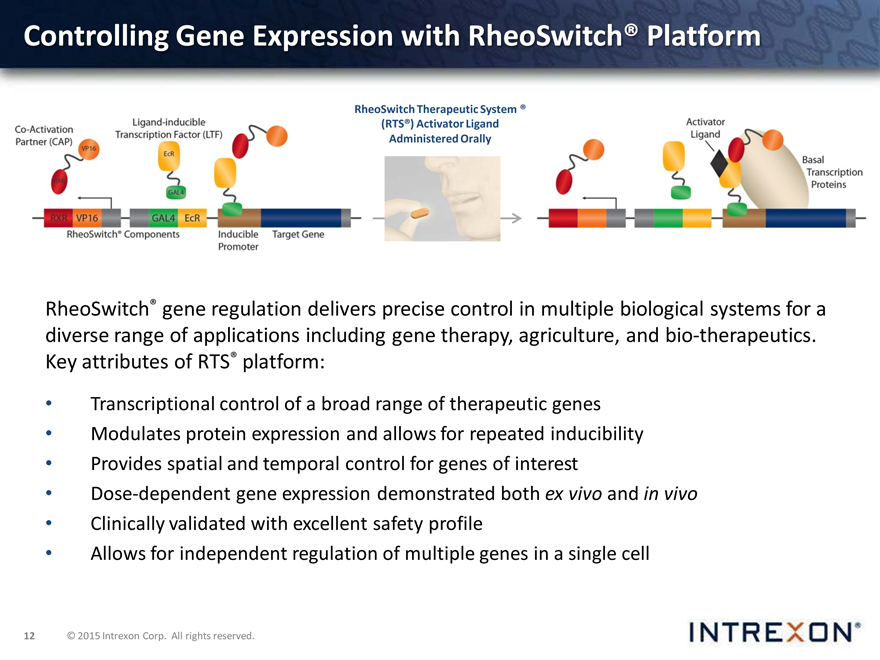
Controlling Gene Expression with RheoSwitch® Platform
RheoSwitch Therapeutic System ® (RTS®) Activator Ligand Administered Orally
RheoSwitch® gene regulation delivers precise control in multiple biological systems for a diverse range of applications including gene therapy, agriculture, and bio-therapeutics. attributes of RTS® platform: Key
Transcriptional control of a broad range of therapeutic genes
Modulates protein expression and allows for repeated inducibility
Provides spatial and temporal control for genes of interest
Dose-dependent gene expression demonstrated both ex vivo and in vivo
Clinically validated with excellent safety profile
Allows for independent regulation of multiple genes in a single cell
12 © 2015 Intrexon Corp. All rights reserved.
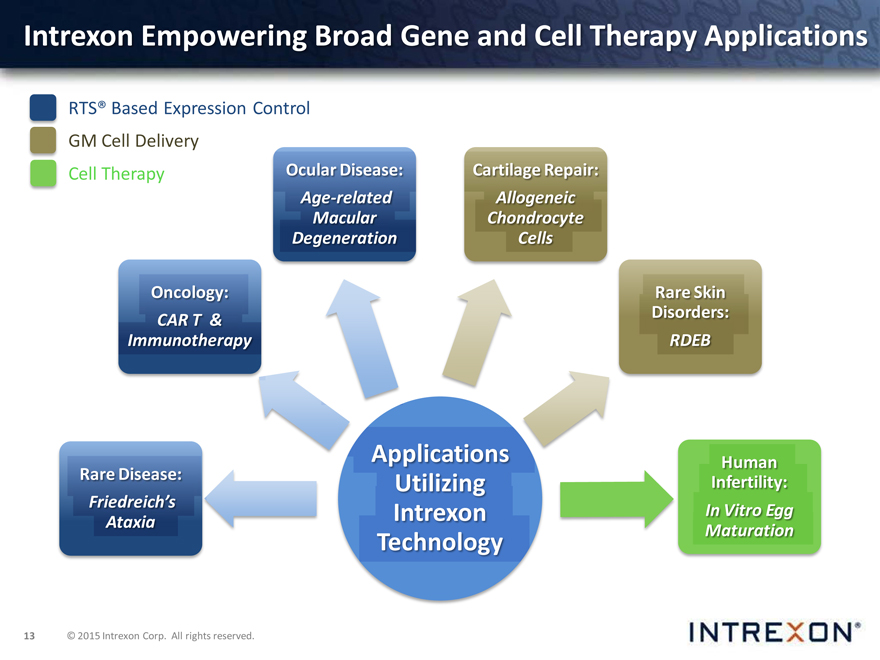
Intrexon Empowering Broad Gene and Cell Therapy Applications
RTS® Based Expression Control GM Cell Delivery
Cell Therapy Ocular Disease: Cartilage Repair: Age-related Allogeneic Macular Chondrocyte Degeneration Cells
Oncology: Rare Skin CAR T & Disorders:
Immunotherapy RDEB
Rare Disease: Applications Human Utilizing Infertility: Friedreich’s Intrexon In Vitro Egg Ataxia Maturation
Technology
13 © 2015 Intrexon Corp. All rights reserved.
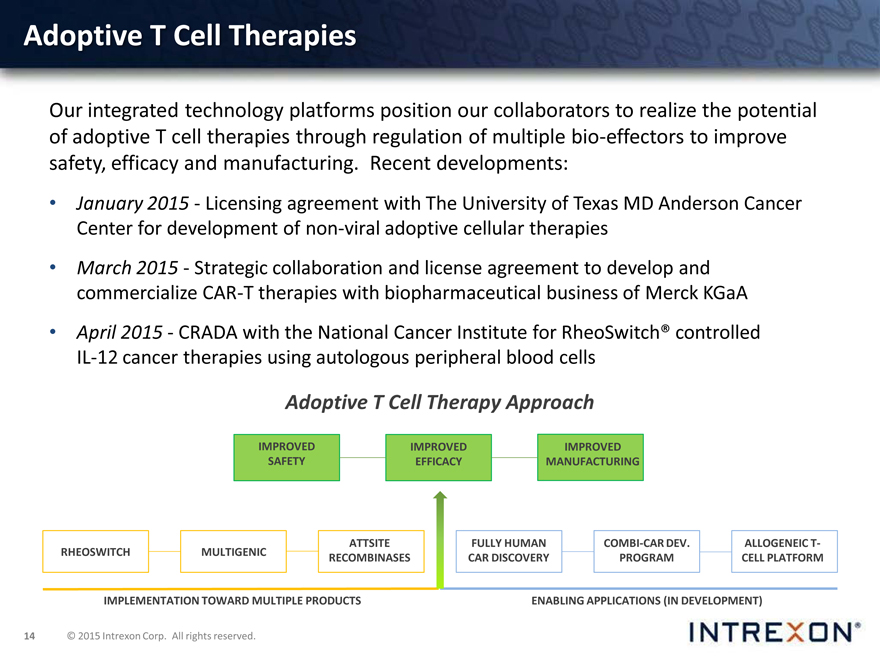
Adoptive T Cell Therapies
Our integrated technology platforms position our collaborators to realize the potential of adoptive T cell therapies through regulation of multiple bio-effectors to improve safety, efficacy and manufacturing. Recent developments:
January 2015—licensing agreement with The University of Texas MD Anderson Cancer Center for development of non-viral adoptive cellular therapies
March 2015—strategic collaboration and license agreement to develop and commercialize CAR-T therapies with biopharmaceutical business of Merck KGaA
April 2015— CRADA with the National Cancer Institute for RheoSwitch® controlled IL-12 cancer therapies using autologous peripheral blood cells
Adoptive T Cell Therapy Approach
IMPROVED IMPROVED IMPROVED SAFETY EFFICACY MANUFACTURING
ATTSITE FULLY HUMAN COMBI-CAR DEV. ALLOGENEIC T-RHEOSWITCH MULTIGENIC RECOMBINASES CAR DISCOVERY PROGRAM CELL PLATFORM
IMPLEMENTATION TOWARD MULTIPLE PRODUCTS ENABLING APPLICATIONS (IN DEVELOPMENT)
14 © 2015 Intrexon Corp. All rights reserved.
Cell-based Treatments for Rare Skin Diseases
Collaboration with Fibrocell Science to develop genetically-modified human dermal fibroblasts expressing collagen VII for treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB)
RDEB is a debilitating disease with no approved treatment and is often lethal before the age of 30
Plan to file an IND application with the U.S. FDA in 1H of 2015 for drug candidate, GM-HDF-COL7
Also pursuing Linear scleroderma, a skin-hardening disease affecting movement and development
15 © 2015 Intrexon Corp. All rights reserved.
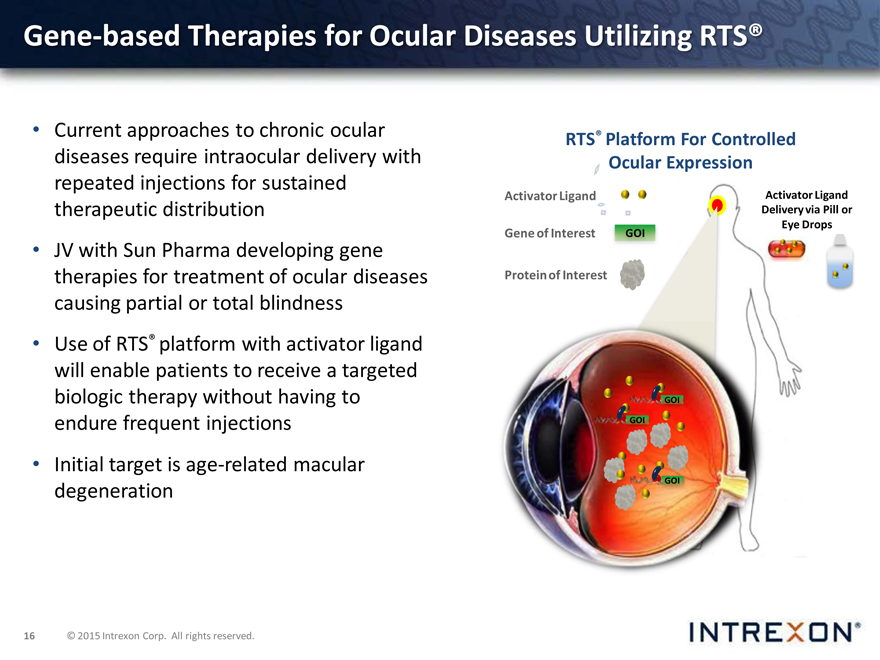
Gene-based Therapies for Ocular Diseases Utilizing RTS®
Current approaches to chronic ocular RTS® Platform For Controlled diseases require intraocular delivery with Ocular Expression repeated injections for sustained
Activator Ligand
therapeutic distribution
Gene of Interest GOI
JV with Sun Pharma developing gene therapies for treatment of ocular diseases Protein of Interest causing partial or total blindness
Use of RTS® platform with activator ligand will enable patients to receive a targeted biologic therapy without having to GOI endure frequent injections GOI
Initial target is age-related macular degeneration GOI
16 © 2015 Intrexon Corp. All rights reserved.
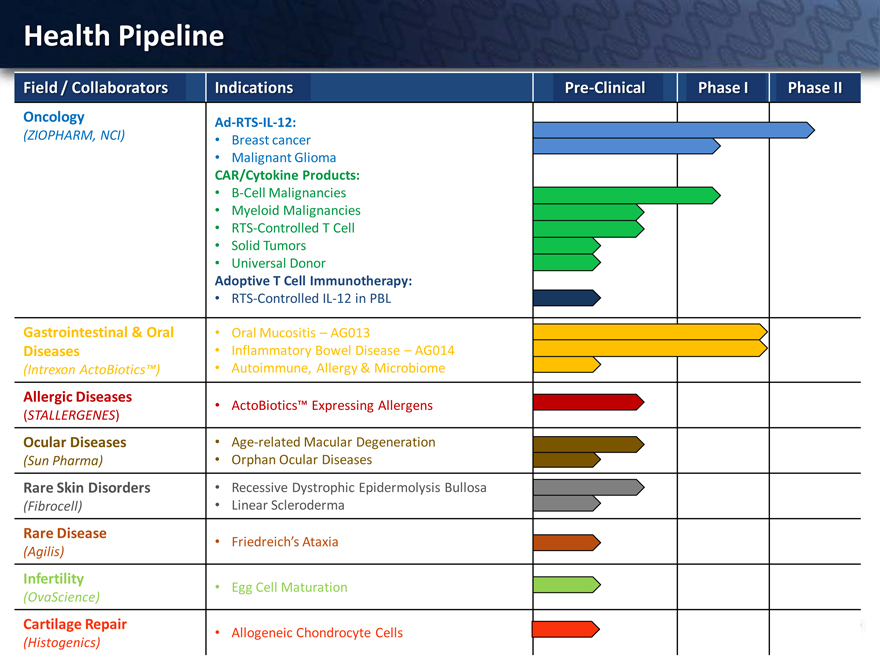
Health Pipeline
Field / Collaborators Indications Pre-Clinical Phase I Phase II
Oncology Ad-RTS-IL-12:
(ZIOPHARM, NCI) • Breast cancer
• Malignant Glioma
CAR/Cytokine Products:
• B-Cell Malignancies
• Myeloid Malignancies
• RTS-Controlled T Cell
• Solid Tumors
• Universal Donor
Adoptive T Cell Immunotherapy:
• RTS-Controlled IL-12 in PBL
Gastrointestinal & Oral • Oral Mucositis – AG013
Diseases • Inflammatory Bowel Disease – AG014
(Intrexon ActoBiotics™) • Autoimmune, Allergy & Microbiome
Allergic Diseases • ActoBiotics™ Expressing Allergens
(STALLERGENES)
Ocular Diseases • Age-related Macular Degeneration
(Sun Pharma) • Orphan Ocular Diseases
Rare Skin Disorders • Recessive Dystrophic Epidermolysis Bullosa
(Fibrocell) • Linear Scleroderma
Rare Disease • Friedreich’s Ataxia
(Agilis)
Infertility • Egg Cell Maturation
(OvaScience)
Cartilage Repair • Allogeneic Chondrocyte Cells
(Histogenics)
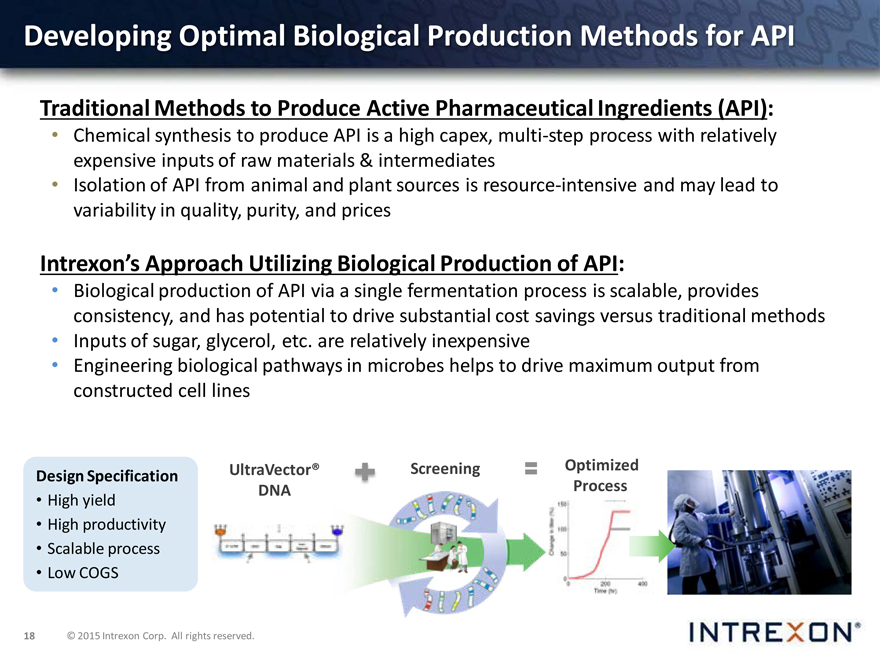
Developing Optimal Biological Production Methods for API
Traditional Methods to Produce Active Pharmaceutical Ingredients (API):
Chemical synthesis to produce API is a high capex, multi-step process with relatively expensive inputs of raw materials & intermediates
Isolation of API from animal and plant sources is resource-intensive and may lead to variability in quality, purity, and prices
Intrexon’s Approach Utilizing Biological Production of API:
Biological production of API via a single fermentation process is scalable, provides consistency, and has potential to drive substantial cost savings versus traditional methods
Inputs of sugar, glycerol, etc. are relatively inexpensive
Engineering biological pathways in microbes helps to drive maximum output from constructed cell lines
Design Specification
High yield
High productivity
Scalable process
Low COGS
UltraVector® DNA
Screening
Optimized Process
18 © 2015 Intrexon Corp. All rights reserved.
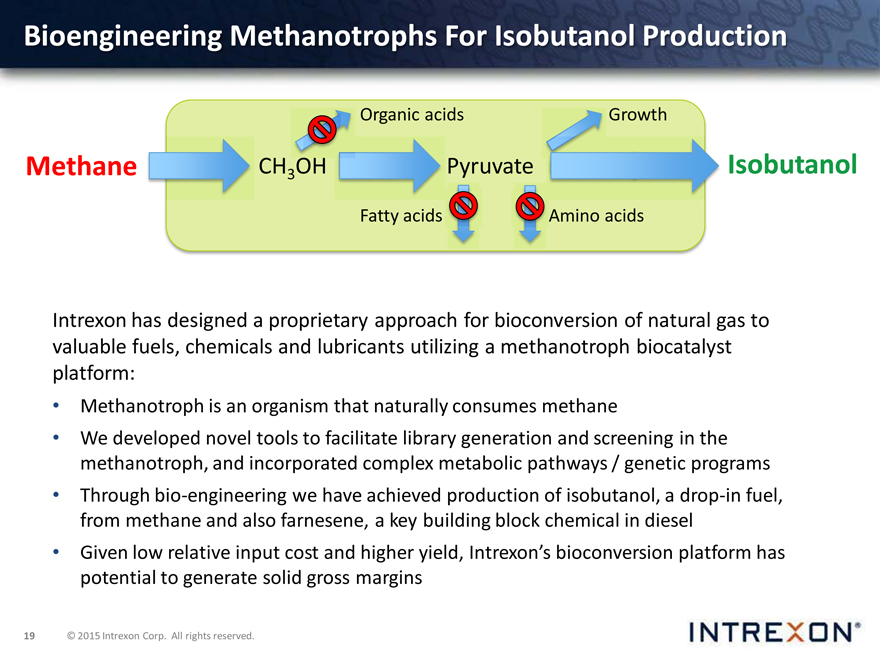
Bioengineering Methanotrophs For Isobutanol Production
Organic acids Growth
Methane CH3OH Pyruvate Isobutanol
Fatty acids Amino acids
Intrexon has designed a proprietary approach for bioconversion of natural gas to valuable fuels, chemicals and lubricants utilizing a methanotroph biocatalyst platform:
Methanotroph is an organism that naturally consumes methane
We developed novel tools to facilitate library generation and screening in the methanotroph, and incorporated complex metabolic pathways / genetic programs
Through bio-engineering we have achieved production of isobutanol, a drop-in fuel, from methane and also farnesene, a key building block chemical in diesel
Given low relative input cost and higher yield, Intrexon’s bioconversion platform has potential to generate solid gross margins
19 © 2015 Intrexon Corp. All rights reserved.
Trans Ova Genetics—Leading Bovine Reproductive Technologies
Trans Ova is a wholly owned subsidiary of Intrexon and the largest producer and supplier of bovine embryos in the U.S.
In the April 2014 Top Young Genomic Bull List, 87% of the top 100 bulls were produced by Trans Ova and its clients
With Intrexon’s technologies, Trans Ova is positioned to enable unparalleled productivity advancements for efficient, high-quality food production
20 © 2015 Intrexon Corp. All rights reserved.
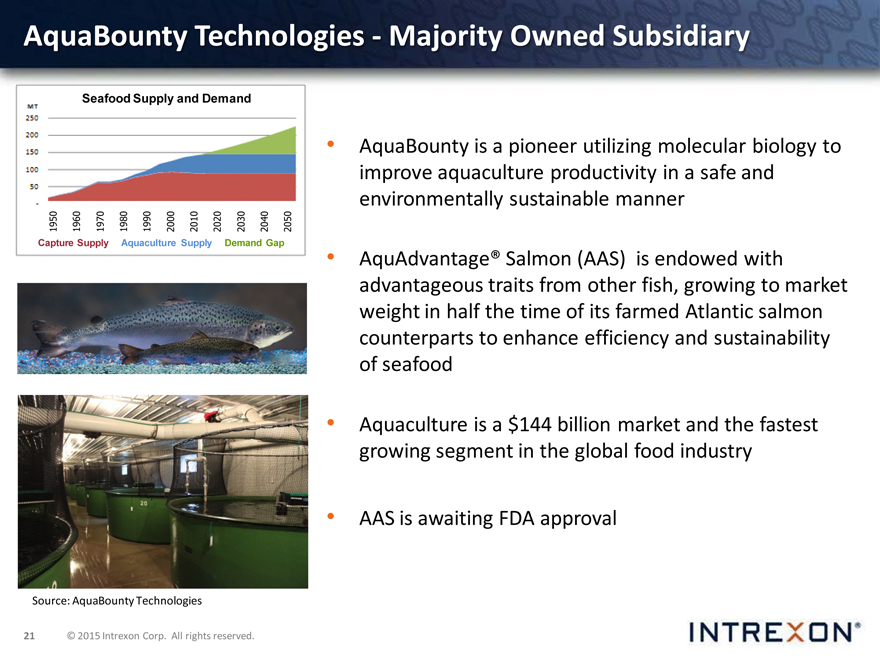
AquaBounty Technologies—Majority Owned Subsidiary
AquaBounty is a pioneer utilizing molecular biology to improve aquaculture productivity in a safe and environmentally sustainable manner
AquAdvantage® Salmon (AAS) is endowed with advantageous traits from other fish, growing to market weight in half the time of its farmed Atlantic salmon counterparts to enhance efficiency and sustainability of seafood
Aquaculture is a $144 billion market and the fastest growing segment in the global food industry
AAS is awaiting FDA approval
Capture 1950
1960
Supply 1970
1980 Seafood
1990
Aquaculture 2000 Supply
2010 and
Supply
2020
2030 Demand
Demand Gap 2040
2050
Source: AquaBounty Technologies
21 © 2015 Intrexon Corp. All rights reserved.
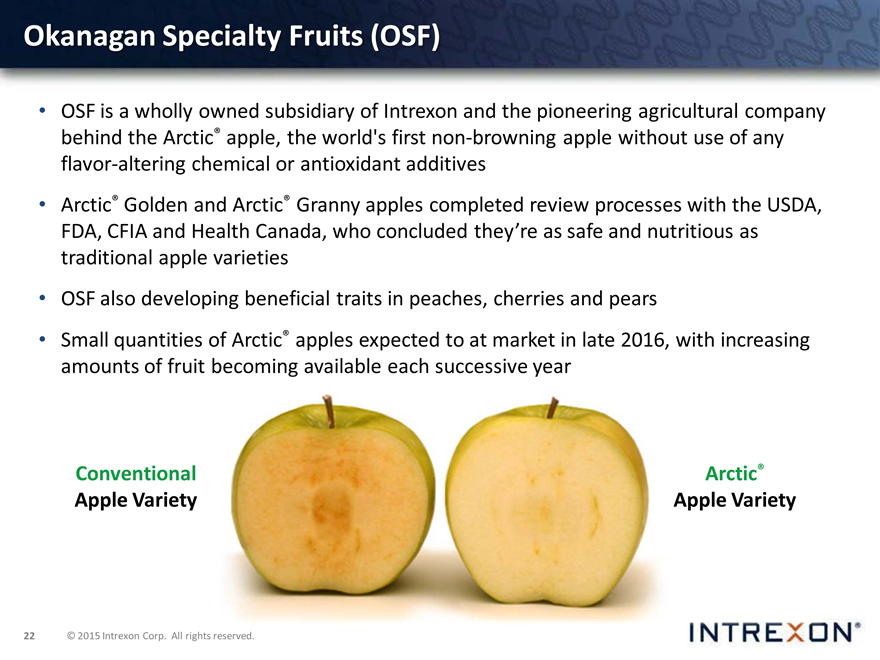
Okanagan Specialty Fruits (OSF)
OSF is a wholly owned subsidiary of Intrexon and the pioneering agricultural company behind the Arctic® apple, the world’s first non-browning apple without use of any flavor-altering chemical or antioxidant additives
Arctic® Golden and Arctic® Granny apples completed review processes with the USDA, FDA, CFIA and Health Canada, who concluded they’re as safe and nutritious as traditional apple varieties
OSF also developing beneficial traits in peaches, cherries and pears
Small quantities of Arctic® apples expected to at market in late 2016, with increasing amounts of fruit becoming available each successive year
Conventional Arctic® Apple Variety Apple Variety
22 © 2015 Intrexon Corp. All rights reserved.
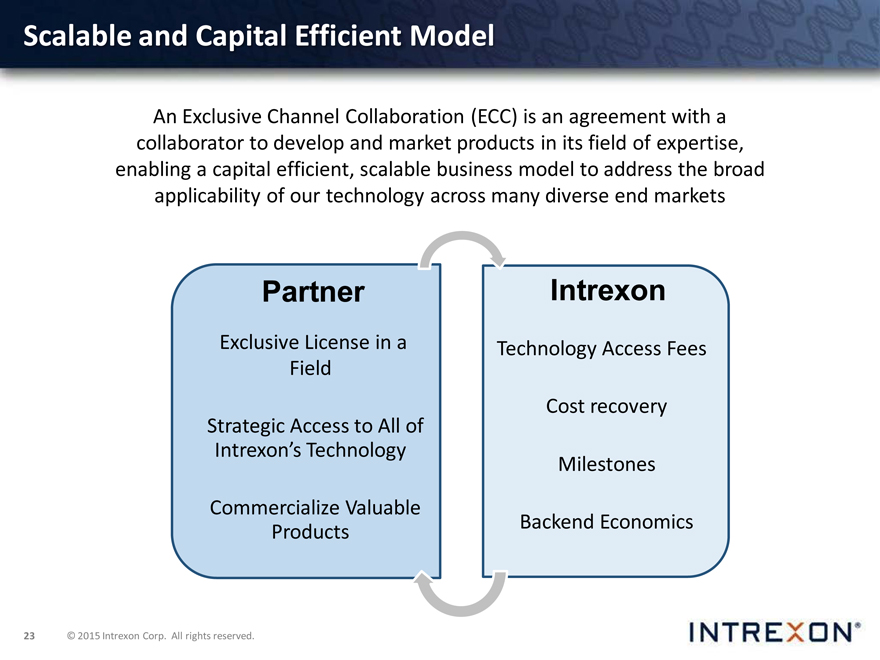
Scalable and Capital Efficient Model
An Exclusive Channel Collaboration (ECC) is an agreement with a collaborator to develop and market products in its field of expertise, enabling a capital efficient, scalable business model to address the broad applicability of our technology across many diverse end markets
Partner
Exclusive License in a Field
Strategic Access to All of Intrexon’s Technology
Commercialize Valuable Products
Intrexon
Technology Access Fees Cost recovery Milestones Backend Economics
23 © 2015 Intrexon Corp. All rights reserved.
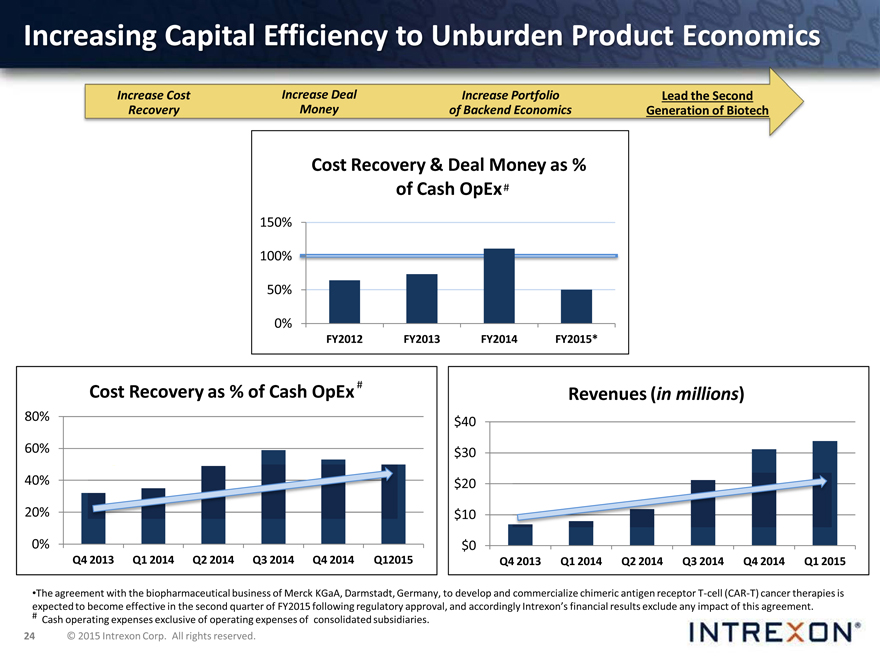
Increasing Capital Efficiency to Unburden Product Economics
Increase Cost Increase Deal Increase Portfolio Lead the Second Recovery Money of Backend Economics Generation of Biotech
Cost Recovery & Deal Money as % of Cash OpEx
FY2012, FY2013, FY2014
150% 100% 50%
0%
FY2012 FY2013 FY2014 FY2015*
Cost Recovery as % of Cash OpEx
80%
60% 40%
20%
0%
Q4 2013 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q12015
Revenues (in millions)
$40 $30 $20 $10 $0
Q4 2013 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015
* The agreement with the biopharmaceutical business of Merck KGaA, Darmstadt, Germany, to develop and commercialize chimeric antigen receptor T-cell (CAR-T) cancer therapies expected to become effective in the second quarter of FY2015 following regulatory approval, and accordingly Intrexon’s financial results exclude any impact of this agreement.
# Cash operating expenses exclusive of operating expenses of consolidated subsidiaries.
24 © 2015 Intrexon Corp. All rights reserved.
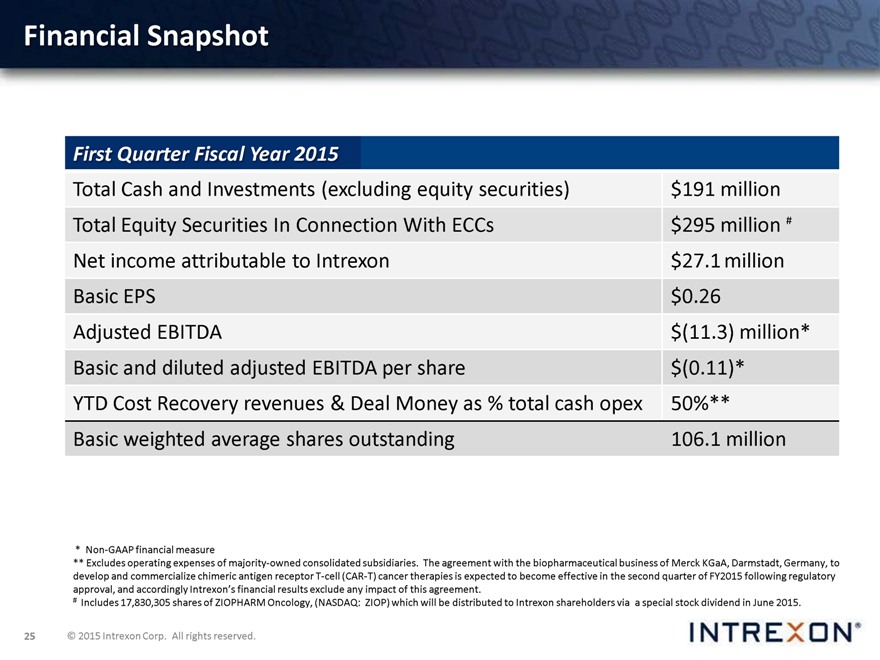
Financial Snapshot
First Quarter Fiscal Year 2015
Total Cash and Investments (excluding equity securities) $191 million Total Equity Securities In Connection With ECCs $295 million Net income attributable to Intrexon $27.1 million Basic EPS $0.26 Adjusted EBITDA $(11.3) million* Basic and diluted pro forma adjusted EBITDA per share $(0.11)* YTD Cost Recovery revenues & Deal Money as % total cash opex 50%** Basic weighted average shares outstanding 106.1 million
* Non-GAAP financial measure
** Excludes operating expenses of majority-owned consolidated subsidiaries. The agreement with the biopharmaceutical business of Merck KGaA, Darmstadt, Germany, to develop and commercialize chimeric antigen receptor T-cell (CAR-T) cancer therapies is expected to become effective in the second quarter of FY2015 following regulatory approval, and accordingly Intrexon’s financial results exclude any impact of this agreement.
# Includes 17,830,305 shares of ZIOPHARM Oncology, (NASDAQ: ZIOP) which will be distributed to Interxon shareholders via a special stock dividend in June 2015.
25 © 2015 Intrexon Corp. All rights reserved.
Driving Scale & Efficiency Through BeyondBio™ Portal
BeyondBio™ portal will facilitate more efficient collaboration providing partners increased flexibility to tap into our knowledge base remotely and develop customizable bio-based solutions from their own facilities with an integrated end-to-end capability
26 © 2015 Intrexon Corp. All rights reserved.
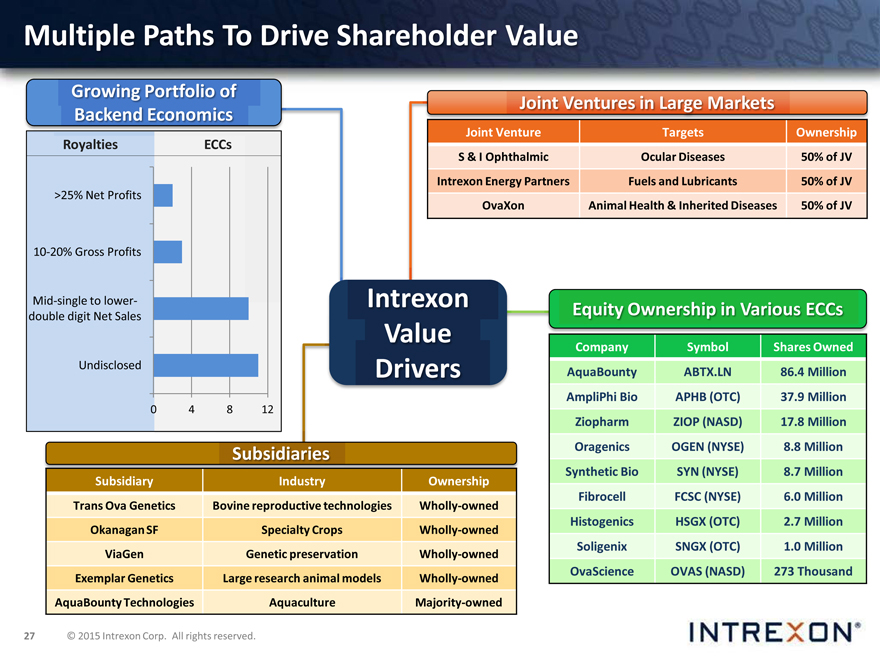
Multiple Paths To Drive Shareholder Value
Growing Portfolio of Backend Economics
Royalties ECCs
>25% Net Profits
10-20% Gross Profits
Mid-single to lower-double digit Net Sales
Undisclosed
0 4 8 12
Intrexon Value Drivers
Joint Ventures in Large Markets
Joint Venture Targets Ownership
S & I Ophthalmic Ocular Diseases 50% of JV Intrexon Energy Partners Fuels and Lubricants 50% of JV
OvaXon Animal Health & Inherited Diseases 50% of JV
Equity Ownership in Various ECCs
Company Symbol Shares Owned AquaBounty ABTX.LN 86.4 Million AmpliPhi Bio APHB (OTC) 37.9 Million Ziopharm ZIOP (NASD) 17.8 Million Oragenics OGEN (NYSE) 8.8 Million Synthetic Bio SYN (NYSE) 8.7 Million Fibrocell FCSC (NYSE) 6.0 Million Histogenics HSGX (OTC) 2.7 Million Soligenix SNGX (OTC) 1.0 Million OvaScience OVAS (NASD) 273 Thousand
Subsidiaries
Subsidiary Industry Ownership
Trans Ova Genetics Bovine reproductive technologies Wholly-owned Okanagan SF Specialty Crops Wholly-owned ViaGen Genetic preservation Wholly-owned Exemplar Genetics Large research animal models Wholly-owned AquaBounty Technologies Aquaculture Majority-owned
27 © 2015 Intrexon Corp. All rights reserved.
Intrexon Summary
We apply engineering principles to artisanal biological systems in large and established markets with built-in demand using a scalable capital efficient business model to develop imperative high value applications
for present and future generations while delivering
meaningful returns to our shareholders.
28 © 2015 Intrexon Corp. All rights reserved.
Appendix A
Intrexon Corporation and Subsidiaries
Reconciliation of GAAP to Non-GAAP Measures (Unaudited)
Adjusted EBITDA and Adjusted EBITDA per share. To supplement Intrexon’s financial information presented in accordance with U.S. generally accepted accounting principles (“GAAP”), Intrexon presents Adjusted EBITDA and Adjusted EBITDA per share. A reconciliation of Adjusted EBITDA to Intrexon’s net income or loss attributable to Intrexon under GAAP appears below. Adjusted EBITDA is a non-GAAP financial measure that Intrexon calculates as net income or loss attributable to Intrexon adjusted for income tax expense or benefit, interest expense, depreciation and amortization, stock-based and executive incentive compensation, contribution of services by shareholder, noncash research and development expenses related to the acquisition of our license agreement with the University of Texas MD Anderson Cancer Center, unrealized appreciation or depreciation in the fair value of equity securities, equity in net loss of affiliate and the change in deferred revenue related to upfront and milestone payments. Adjusted EBITDA and Adjusted EBITDA per share are key metrics for Intrexon’s management and Board of Directors for evaluating the Company’s financial and operating performance, generating future operating plans and making strategic decisions about the allocation of capital. Management and the Board of Directors believe that Adjusted EBITDA and Adjusted EBITDA per share are useful to understand the long-term performance of Intrexon’s core business and facilitates comparisons of the Company’s operating results over multiple reporting periods. Intrexon is providing this information to investors and others to assist them in understanding and evaluating the Company’s operating results in the same manner as its management and board of directors. While Intrexon believes that these non-GAAP financial measures are useful in evaluating its business, and may be of use to investors, this information should be considered as supplemental in nature and is not meant as a substitute for the related financial information prepared in accordance with GAAP. In addition, these non-GAAP financial measures may not be the same as non-GAAP financial measures presented by other companies. Adjusted EBITDA and Adjusted EBITDA per share are not measures of financial performance under GAAP, and are not intended to represent cash flows from operations nor earnings per share under GAAP and should not be used as an alternative to net income or loss as an indicator of operating performance or to represent cash flows from operating, investing or financing activities as a measure of liquidity. Intrexon compensates for the limitations of Adjusted EBITDA and Adjusted EBITDA per share by using them only to supplement the Company’s GAAP results to provide a more complete understanding of the factors and trends affecting the Company’s business. Adjusted EBITDA and Adjusted EBITDA per share have limitations as an analytical tool and you should not consider them in isolation or as a substitute for analysis of Intrexon’s results as reported under GAAP.
In addition to the reasons stated above, which are generally applicable to each of the items Intrexon excludes from its non-GAAP financial measure, Intrexon believes it is appropriate to exclude certain items from the definition of Adjusted EBITDA for the following reasons: Interest expense may be subject to changes in interest rates which are beyond Intrexon’s control; Depreciation of Intrexon’s property and equipment and amortization of acquired identifiable intangibles can be affected by the timing and magnitude of business combinations and capital asset purchases; Stock-based compensation expense is a noncash expense and may vary significantly based on the timing, size and nature of awards granted and also because the value is determined using formulas which incorporate variables, such as market volatility. Executive incentive compensation expenses are determined by the compensation committee of the Board of Directors at the end of the year and may be based on, among other items, the results of Adjusted EBITDA, exclusive of such expenses. Contribution of services by shareholder is a noncash expense which Intrexon excludes in evaluating its financial and operating performance; Unrealized appreciation or depreciation in the fair value of securities which Intrexon holds in its collaborators may be significantly impacted by market volatility and other factors which are outside of the Company’s control in the short term and Intrexon intends to hold these securities over the long term except as provided above; Equity in net loss of affiliate reflects Intrexon’s proportionate share of the income or loss of entities over which the Company has significant influence, but not control, and accounts for using the equity method of accounting. Our acquisition of the license agreement with the University of Texas MD Anderson Cancer Center was a noncash expense we incurred to obtain access to specific technologies which are strategic to us. Intrexon believes excluding the impact of such losses or gains on these types of strategic investments from its operating results is important to facilitate comparisons between periods; and GAAP requires Intrexon to account for its collaborations as multiple-element arrangements. As a result, the Company defers certain collaboration revenues because certain of its performance obligations cannot be separated and must be accounted for as one unit of accounting. The collaboration revenues that Intrexon so defers arise from upfront and milestone payments received from the Company’s collaborators, which Intrexon recognizes over the future performance period even though the Company’s right to such consideration is neither contingent on the results of Intrexon’s future performance nor refundable in the event of nonperformance. In order to evaluate Intrexon’s operating performance, its management adjusts for the impact of the change in deferred revenue for these upfront and milestone payments in order to include them as a part of adjusted EBITDA when the transaction is initially recorded. The adjustment for the change in deferred revenue removes the noncash revenue recognized during the period and includes the cash and stock received from collaborators for upfront and milestone payments during the period. Intrexon believes that adjusting for the impact of the change in deferred revenue in this manner is important since it permits the Company to make quarterly and annual comparisons of the Company’s ability to consummate new collaborations or to achieve significant milestones with existing collaborators. Further, Intrexon believes it is useful when evaluating its financial and operating performance, generating future operating plans and making strategic decisions about the allocation of capital. Continued on next slide…
29 © 2015 Intrexon Corp. All rights reserved.
Appendix A continued
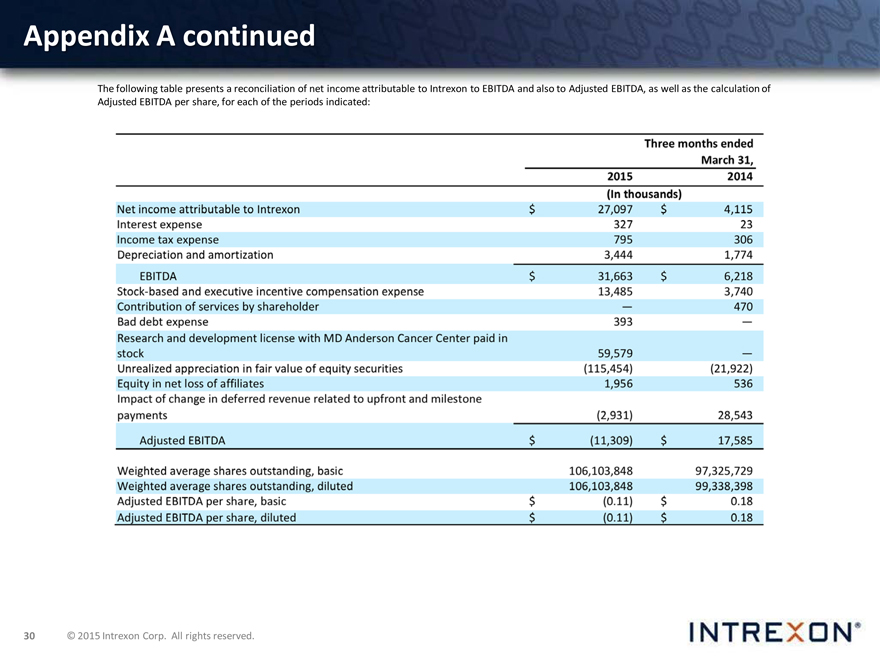
The following table presents a reconciliation of net income attributable to Intrexon to EBITDA and also to Adjusted EBITDA, as well as the calculation of Adjusted EBITDA per share, for each of the periods indicated:
30 © 2015 Intrexon Corp. All rights reserved.