
INVESTOR DAY | NOVEMBER 12, 2015 Exhibit 99.1

Forward Looking Statements Special Note Regarding Forward-Looking Statements Some of the matters discussed in this presentation may constitute forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements relate to expectations, beliefs, projections, future plans and strategies, anticipated events or trends and similar expressions concerning matters that are not historical facts. The forward-looking statements are based on Intrexon’s beliefs, assumptions and expectations of its future performance, taking into account all information currently available to it. These beliefs, assumptions and expectations can change as a result of many possible events or factors, not all of which are known to Intrexon or are within its control. The following factors, among others, could cause actual results to vary from the forward-looking statements: These risks and uncertainties include, but are not limited to, (i) our ability to enter into new exclusive channel collaborations (ECCs), joint ventures, license agreements and other collaborations and agreements; (ii) developments concerning our collaborators and licensees; (iii) our ability to successfully enter new markets or develop additional products, whether with our collaborators or independently; (iv) competition from existing technologies and products or new technologies and products that may emerge; (v) actual or anticipated variations in our operating results; (vi) actual or anticipated fluctuations in our competitors' or our collaborators' and licensees' operating results or changes in their respective growth rates; (vii) our cash position; (viii) market conditions in our industry; (ix) our ability, and the ability of our collaborators and licensees, to protect our intellectual property and other proprietary rights and technologies; (x) our ability, and the ability of our collaborators and licensees, to adapt to changes in laws or regulations and policies; (xi) the ability of our collaborators and licensees to secure any necessary regulatory approvals to commercialize any products developed under the ECCs and license agreements; (xii) the rate and degree of market acceptance of any products developed by a collaborator under an ECC or through a joint venture or license under a license agreement; (xiii) our ability to retain and recruit key personnel; (xiv) our expectations related to the use of proceeds from our public offerings and other financing efforts;… (Continued on next slide) © 2015 Intrexon Corp. All rights reserved.

Forward Looking Statements (xv) our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xvi) our expectations relating to AquaBounty, Trans Ova Genetics and Oxitec Limited. Intrexon does not undertake any obligation to update any forward-looking statements to reflect circumstances or events that occur after the date on which such statements were made except as required by law. Additional information about factors affecting Intrexon is available in Intrexon’s Annual Report on Form 10-K for the year ended December 31, 2014, and Quarterly Report on Form 10-Q for the period ended September 30, 2015, and other filings with the SEC, which are available at www.sec.gov. Use of Non-GAAP Financial Measures This presentation presents Adjusted EBITDA and Basic Adjusted EBITDA per share, which are non-GAAP financial measures within the meaning of applicable rules and regulations of the Securities and Exchange Commission (SEC). For a reconciliation of Adjusted EBITDA to net loss attributable to Intrexon in accordance with generally accepted accounting principles and for a discussion of the reasons why the company believes that these non-GAAP financial measures provide information that is useful to investors please refer to Intrexon’s earnings release dated November 9, 2015. Such information is provided as additional information, not as an alternative to our consolidated financial statements presented in accordance with GAAP, and is intended to enhance an overall understanding of our current financial performance. © 2015 Intrexon Corp. All rights reserved.

Company Introduction and Overview Randal J. Kirk Chairman and Chief Executive Officer
Five Key Takeaways for Today Expansive addressable markets Proprietary technologies and platforms Strong pipeline and substantial growth potential Capital efficient model for long-term value Strong management/ technical team © 2015 Intrexon Corp. All rights reserved.
Engineering Biology Intrexon: Engineering Biology © 2015 Intrexon Corp. All rights reserved.

Differentiated Synthetic Biology Platform Thomas D. Reed, Ph.D. Founder and Chief Science Officer
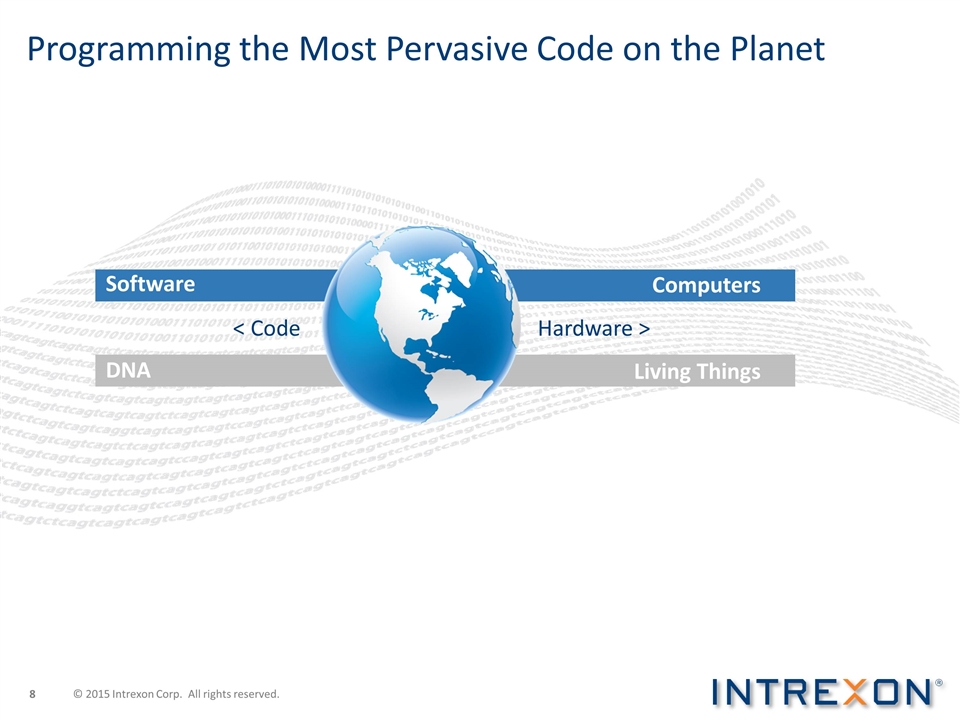
Programming the Most Pervasive Code on the Planet Software DNA Computers Living Things < Code Hardware > © 2015 Intrexon Corp. All rights reserved.
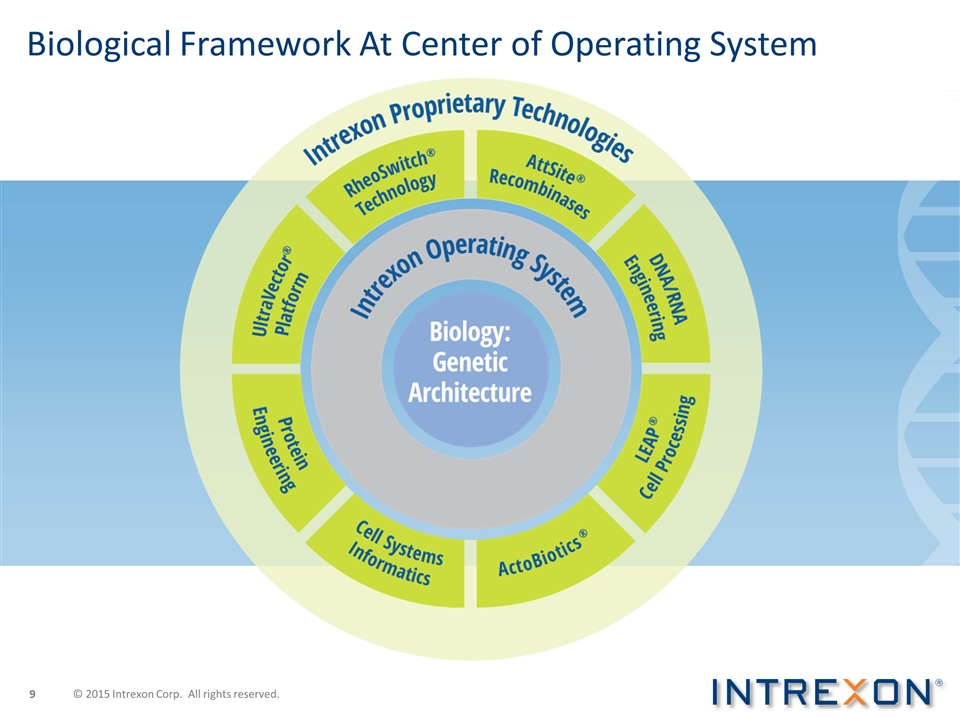
Biological Framework At Center of Operating System © 2015 Intrexon Corp. All rights reserved.
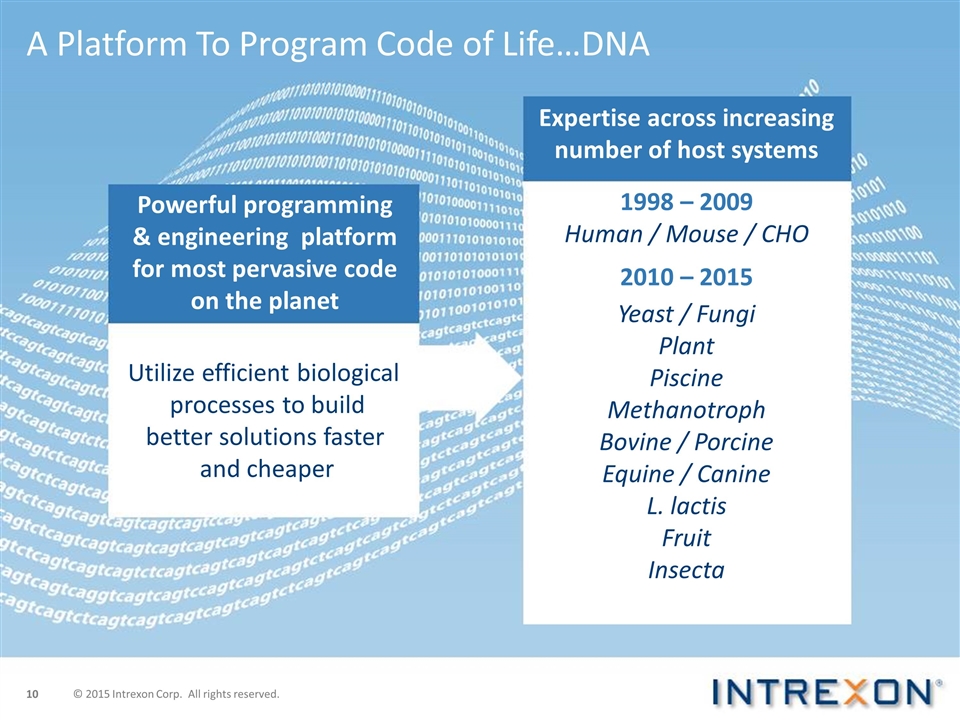
A Platform To Program Code of Life…DNA © 2015 Intrexon Corp. All rights reserved. Powerful programming & engineering platform for most pervasive code on the planet Utilize efficient biological processes to build better solutions faster and cheaper Expertise across increasing number of host systems 1998 – 2009 Human / Mouse / CHO 2010 – 2015 Yeast / Fungi Plant Piscine Methanotroph Bovine / Porcine Equine / Canine L. lactis Fruit Insecta
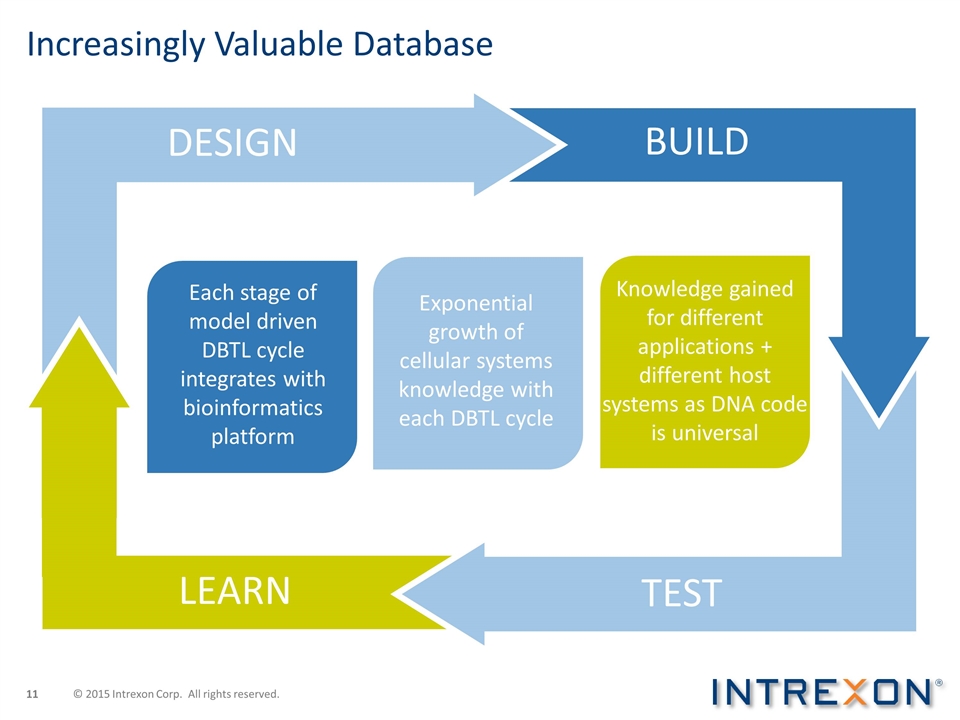
BUILD LEARN Each stage of model driven DBTL cycle integrates with bioinformatics platform TEST Exponential growth of cellular systems knowledge with each DBTL cycle Knowledge gained for different applications + different host systems as DNA code is universal Increasingly Valuable Database DESIGN © 2015 Intrexon Corp. All rights reserved.
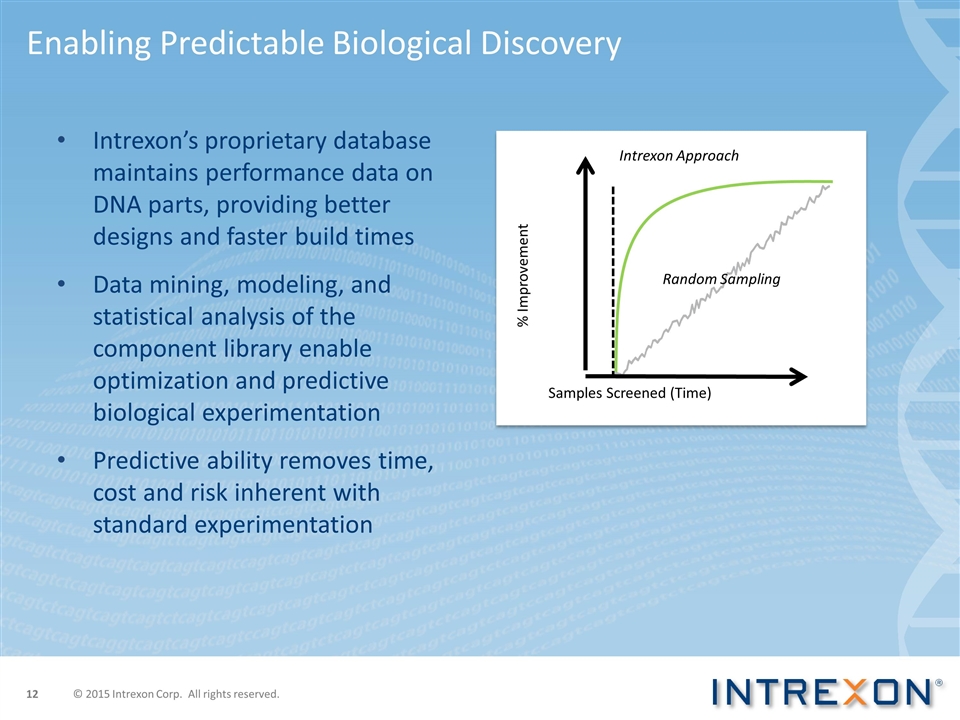
Intrexon’s proprietary database maintains performance data on DNA parts, providing better designs and faster build times Data mining, modeling, and statistical analysis of the component library enable optimization and predictive biological experimentation Predictive ability removes time, cost and risk inherent with standard experimentation Enabling Predictable Biological Discovery Samples Screened (Time) % Improvement Random Sampling Intrexon Approach © 2015 Intrexon Corp. All rights reserved.
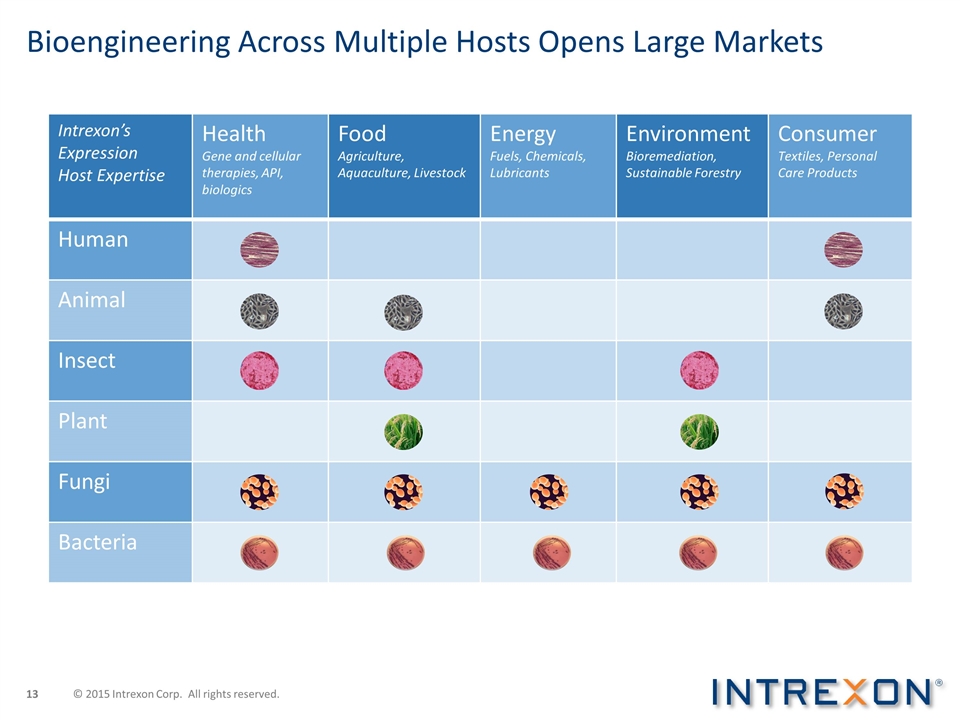
Bioengineering Across Multiple Hosts Opens Large Markets Intrexon’s Expression Host Expertise Health Gene and cellular therapies, API, biologics Food Agriculture, Aquaculture, Livestock Energy Fuels, Chemicals, Lubricants Environment Bioremediation, Sustainable Forestry Consumer Textiles, Personal Care Products Human Animal Insect Plant Fungi Bacteria © 2015 Intrexon Corp. All rights reserved.
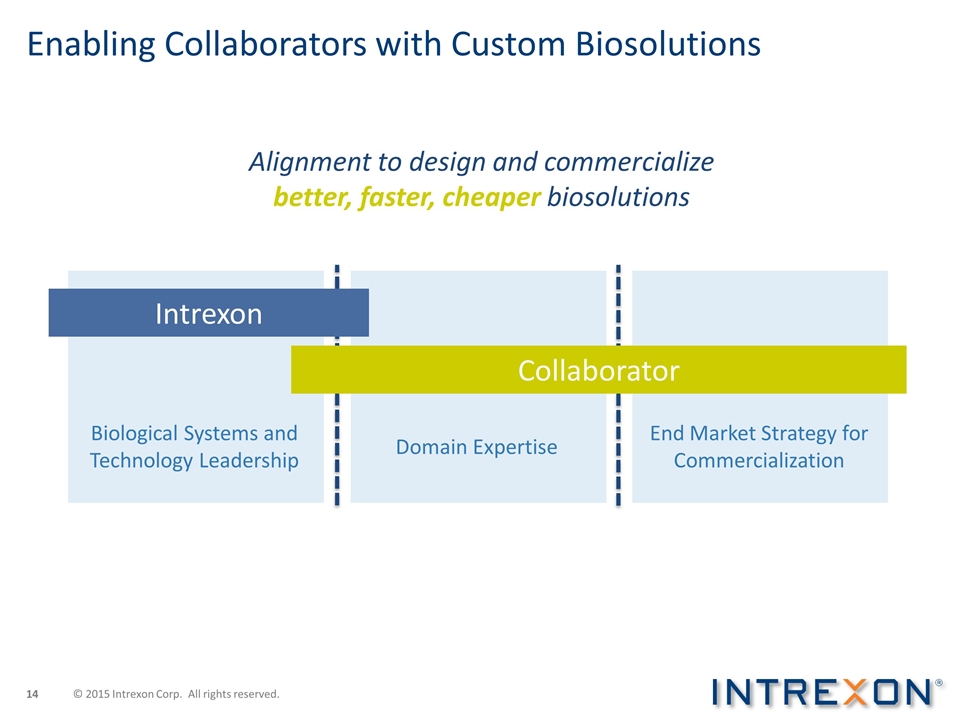
Enabling Collaborators with Custom Biosolutions Intrexon Collaborator Domain Expertise End Market Strategy for Commercialization Biological Systems and Technology Leadership Alignment to design and commercialize better, faster, cheaper biosolutions © 2015 Intrexon Corp. All rights reserved.

Accelerating Concept to Candidate with Informatics Mike Kubal Senior Manager, Informatics
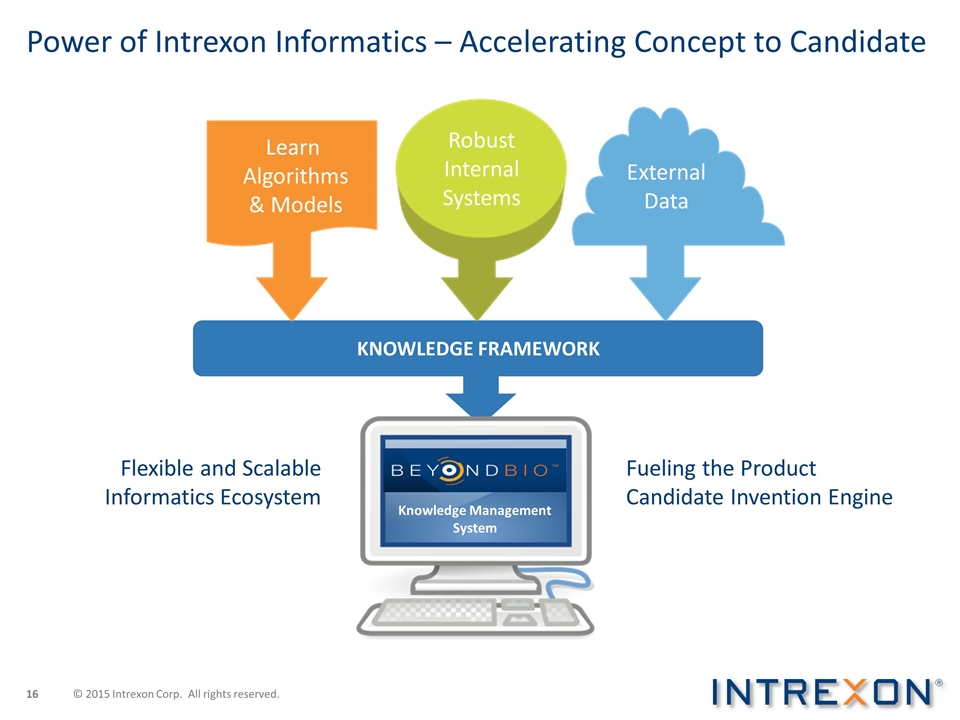
Power of Intrexon Informatics – Accelerating Concept to Candidate KNOWLEDGE FRAMEWORK Knowledge Management System Learn Algorithms & Models External Data Robust Internal Systems Fueling the Product Candidate Invention Engine Flexible and Scalable Informatics Ecosystem © 2015 Intrexon Corp. All rights reserved.
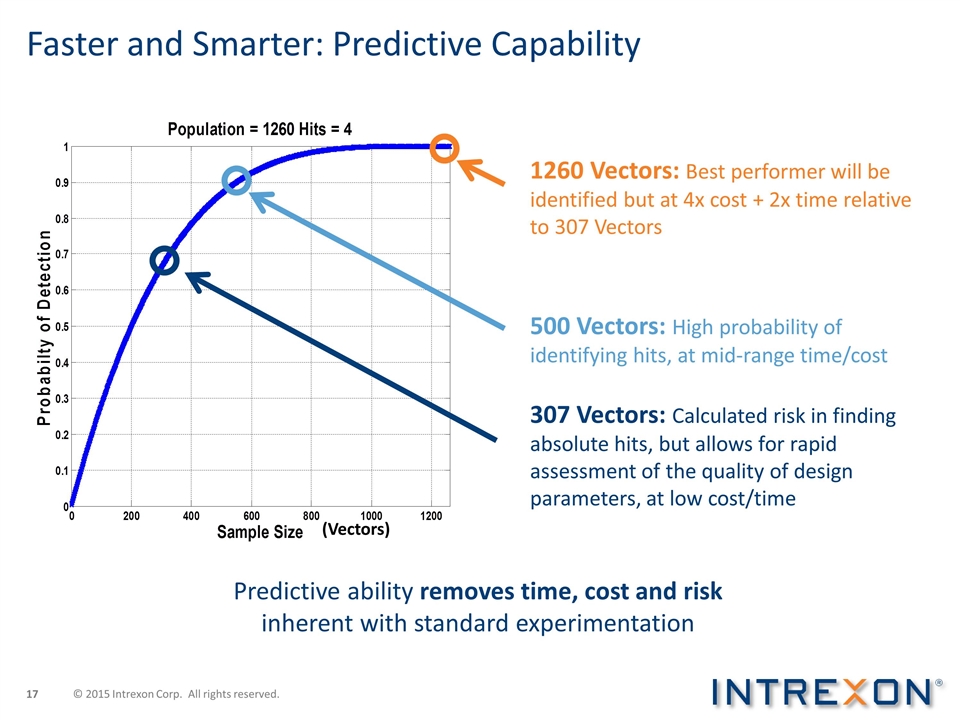
Predictive ability removes time, cost and risk inherent with standard experimentation 1260 Vectors: Best performer will be identified but at 4x cost + 2x time relative to 307 Vectors 500 Vectors: High probability of identifying hits, at mid-range time/cost 307 Vectors: Calculated risk in finding absolute hits, but allows for rapid assessment of the quality of design parameters, at low cost/time (Vectors) Faster and Smarter: Predictive Capability © 2015 Intrexon Corp. All rights reserved.
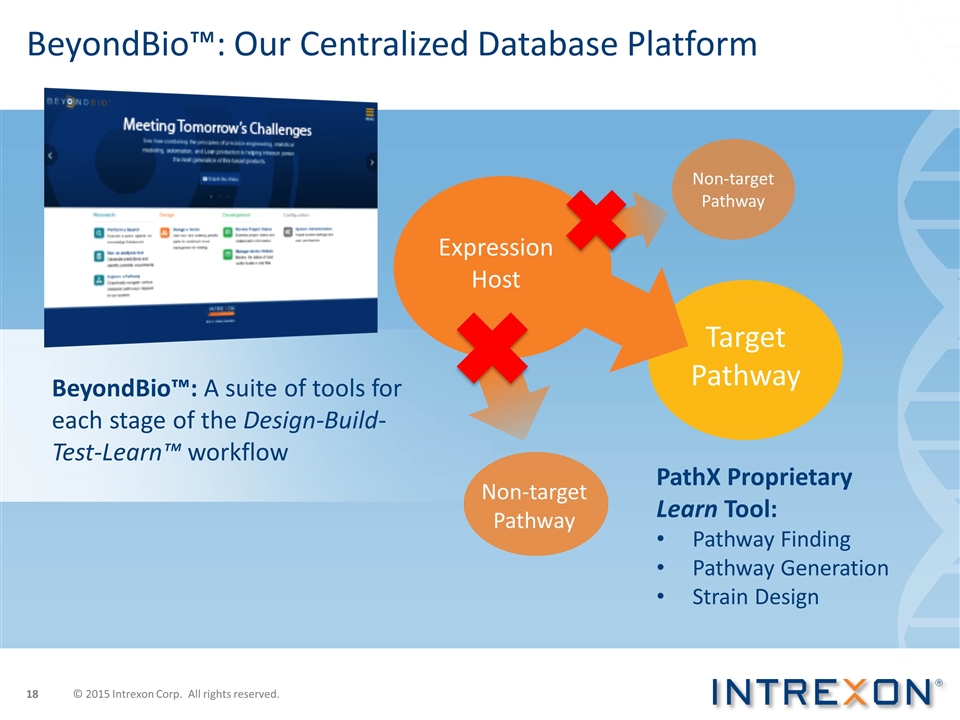
BeyondBio™: Our Centralized Database Platform BeyondBio™: A suite of tools for each stage of the Design-Build-Test-Learn™ workflow PathX Proprietary Learn Tool: Pathway Finding Pathway Generation Strain Design Target Pathway Non-target Pathway Non-target Pathway Expression Host © 2015 Intrexon Corp. All rights reserved.
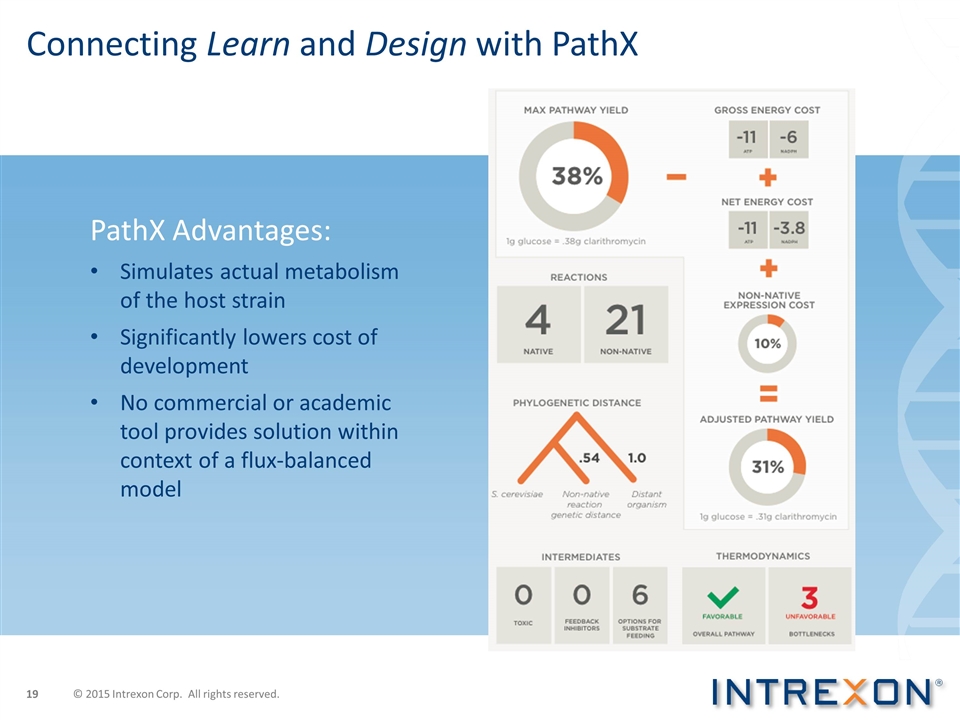
Connecting Learn and Design with PathX PathX Advantages: Simulates actual metabolism of the host strain Significantly lowers cost of development No commercial or academic tool provides solution within context of a flux-balanced model © 2015 Intrexon Corp. All rights reserved.

Connecting Design with Commercial Targets for Partners PathX allows Intrexon to quickly screen and prioritize vast numbers of potential targets for many industries from both a scientific and business perspective. Today PathX has identified potential biological routes for many target compounds with attractive financials. As our knowledge framework grows, the number of potential targets expands exponentially. © 2015 Intrexon Corp. All rights reserved.

Beyond the Borders of Health with Better DNA® Samuel Broder, M.D. Senior Vice President, Health Sector
A Single Molecule at the Center of Biology © 2015 Intrexon Corp. All rights reserved.
Better DNA® at the Center of Many Industries © 2015 Intrexon Corp. All rights reserved.
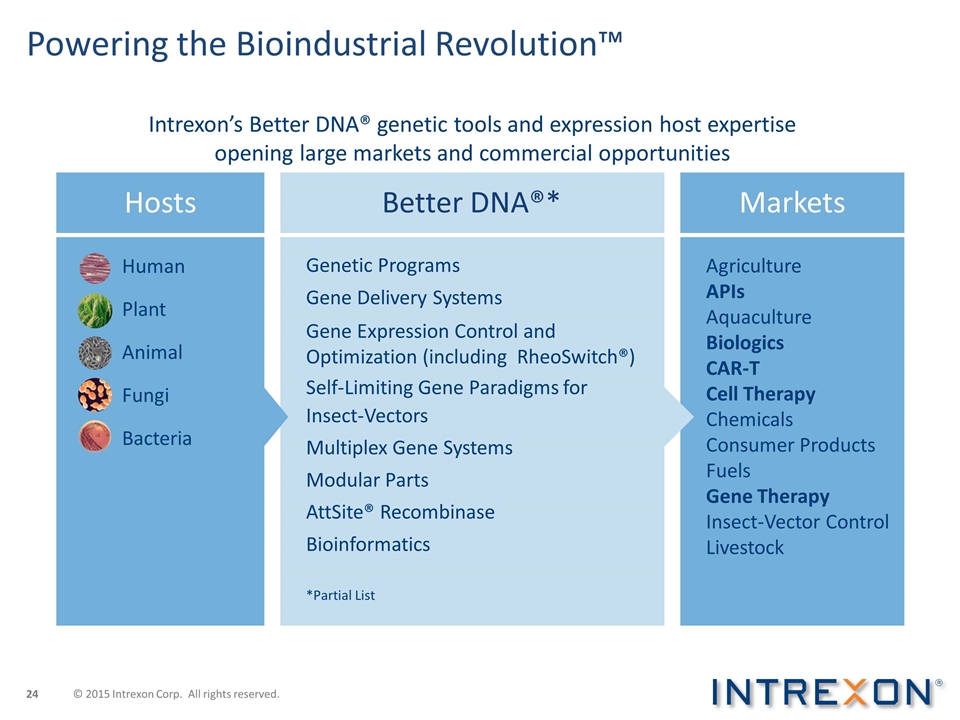
Powering the Bioindustrial Revolution™ Hosts Better DNA®* Markets Human Plant Animal Bacteria Fungi Agriculture APIs Aquaculture Biologics CAR-T Cell Therapy Chemicals Consumer Products Fuels Gene Therapy Insect-Vector Control Livestock Genetic Programs Gene Delivery Systems Gene Expression Control and Optimization (including RheoSwitch®) Self-Limiting Gene Paradigms for Insect-Vectors Multiplex Gene Systems Modular Parts AttSite® Recombinase Bioinformatics *Partial List Intrexon’s Better DNA® genetic tools and expression host expertise opening large markets and commercial opportunities © 2015 Intrexon Corp. All rights reserved.
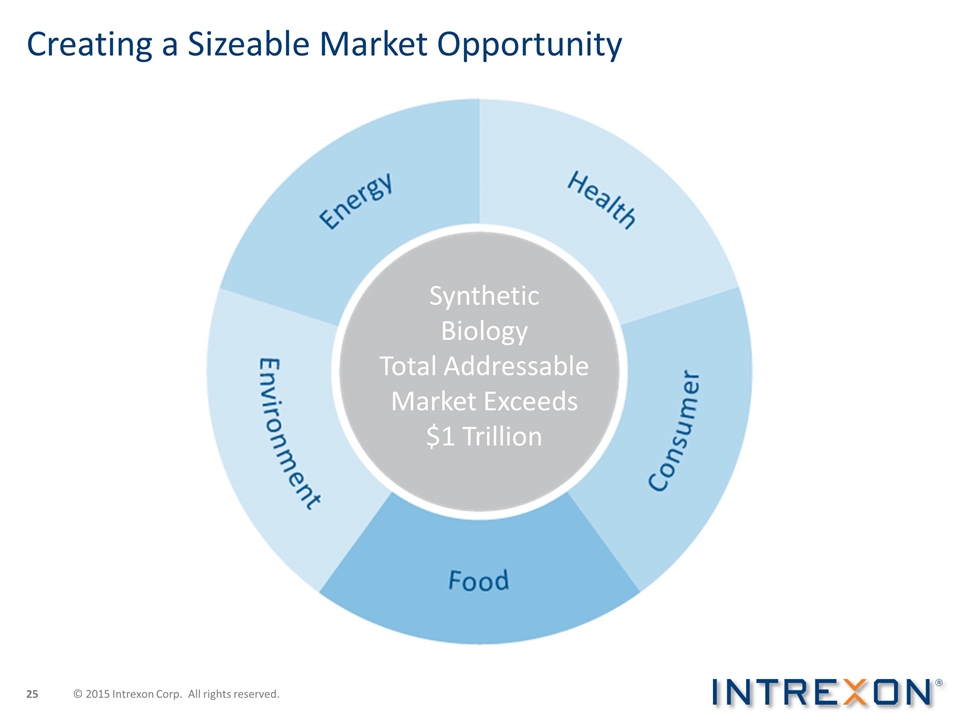
Creating a Sizeable Market Opportunity Synthetic Biology Total Addressable Market Exceeds $1 Trillion © 2015 Intrexon Corp. All rights reserved.

Proprietary Approach for Gene Expression and Regulation Peter Emtage, Ph.D. Vice President, Immunology
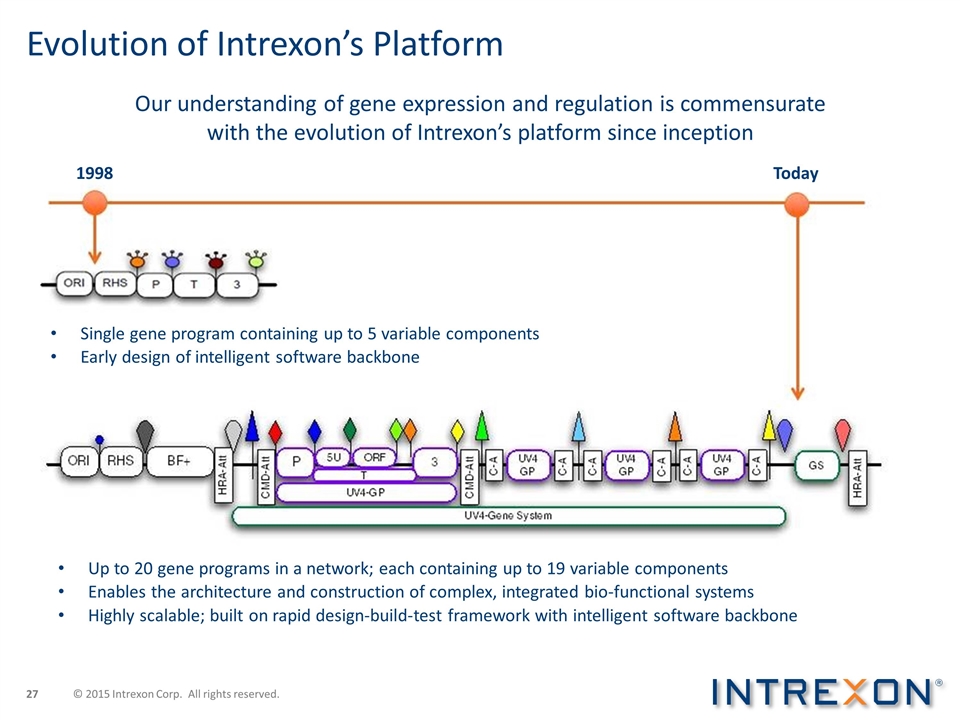
Evolution of Intrexon’s Platform Up to 20 gene programs in a network; each containing up to 19 variable components Enables the architecture and construction of complex, integrated bio-functional systems Highly scalable; built on rapid design-build-test framework with intelligent software backbone Single gene program containing up to 5 variable components Early design of intelligent software backbone Our understanding of gene expression and regulation is commensurate with the evolution of Intrexon’s platform since inception © 2015 Intrexon Corp. All rights reserved. 1998 Today
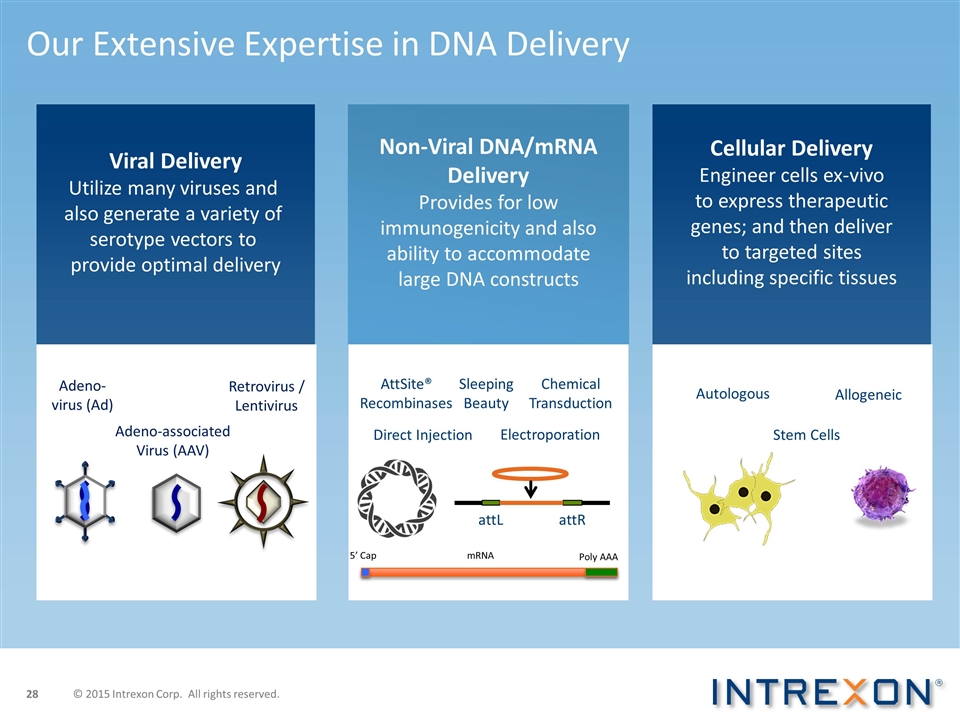
Our Extensive Expertise in DNA Delivery Electroporation Chemical Transduction Direct Injection Autologous Allogeneic Adeno- virus (Ad) Adeno-associated Virus (AAV) Retrovirus / Lentivirus AttSite® Recombinases Sleeping Beauty attR attL 5’ Cap Poly AAA mRNA Stem Cells Viral Delivery Utilize many viruses and also generate a variety of serotype vectors to provide optimal delivery Non-Viral DNA/mRNA Delivery Provides for low immunogenicity and also ability to accommodate large DNA constructs Cellular Delivery Engineer cells ex-vivo to express therapeutic genes; and then deliver to targeted sites including specific tissues © 2015 Intrexon Corp. All rights reserved.
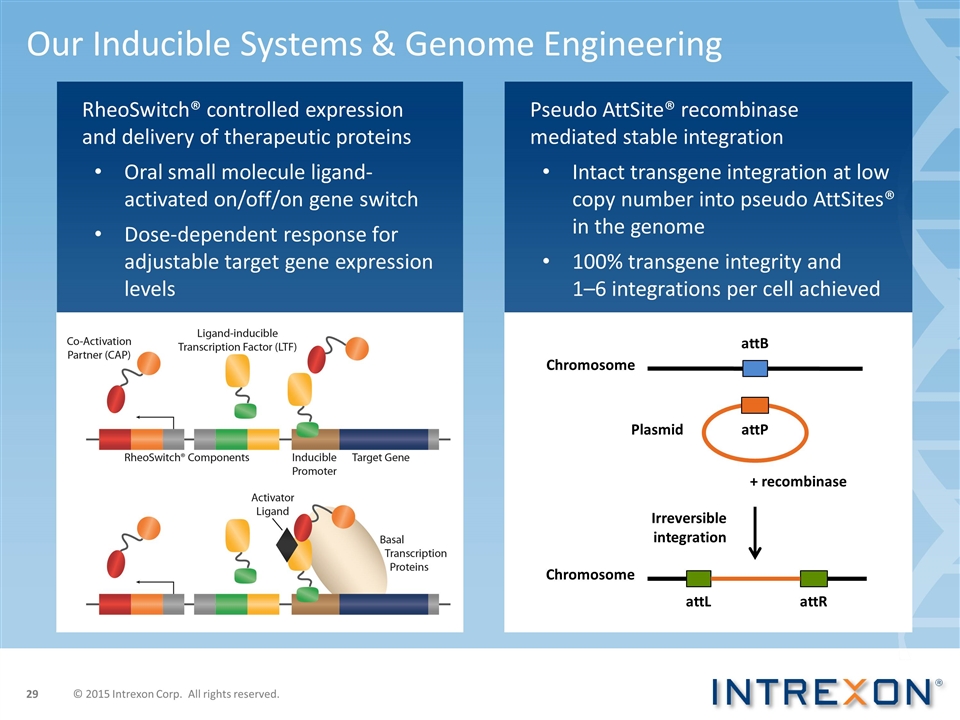
Pseudo AttSite® recombinase mediated stable integration Intact transgene integration at low copy number into pseudo AttSites® in the genome 100% transgene integrity and 1–6 integrations per cell achieved Our Inducible Systems & Genome Engineering + recombinase Irreversible integration Chromosome attB attR attL Chromosome attP Plasmid RheoSwitch® controlled expression and delivery of therapeutic proteins Oral small molecule ligand- activated on/off/on gene switch Dose-dependent response for adjustable target gene expression levels © 2015 Intrexon Corp. All rights reserved.
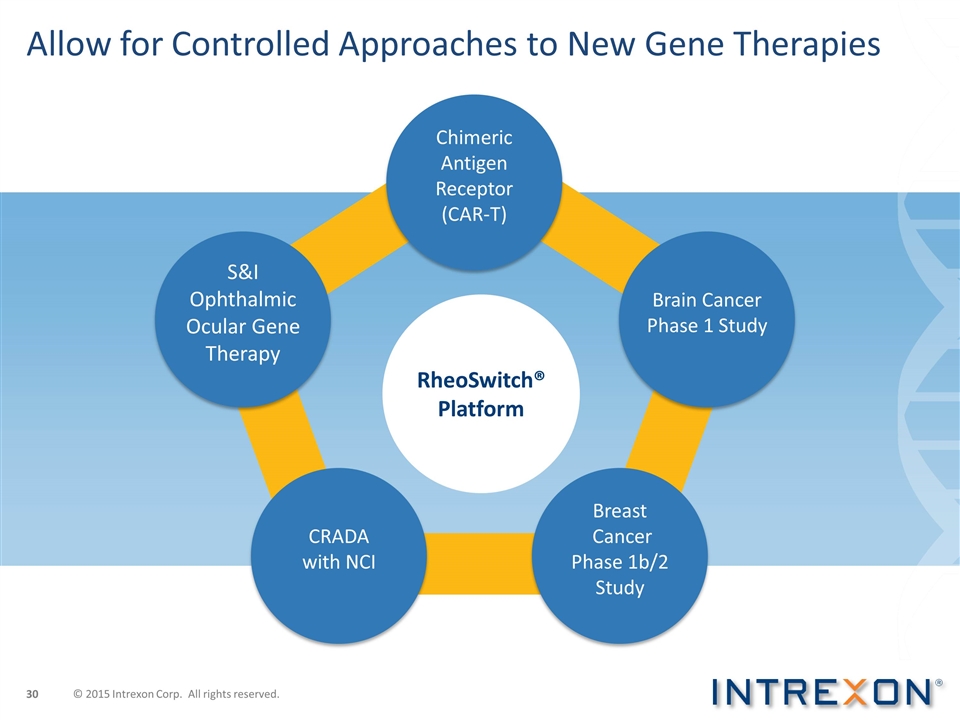
RheoSwitch® Platform Allow for Controlled Approaches to New Gene Therapies S&I Ophthalmic Ocular Gene Therapy Chimeric Antigen Receptor (CAR-T) Brain Cancer Phase 1 Study CRADA with NCI Breast Cancer Phase 1b/2 Study © 2015 Intrexon Corp. All rights reserved.
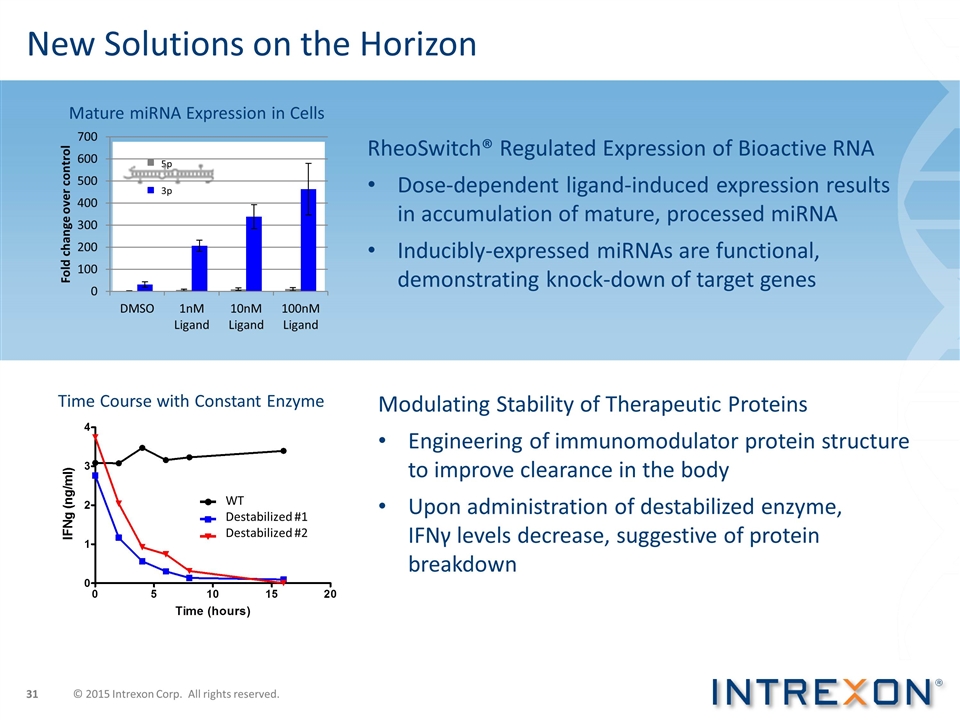
RheoSwitch® Regulated Expression of Bioactive RNA Dose-dependent ligand-induced expression results in accumulation of mature, processed miRNA Inducibly-expressed miRNAs are functional, demonstrating knock-down of target genes New Solutions on the Horizon Time Course with Constant Enzyme Destabilized #1 Destabilized #2 WT Modulating Stability of Therapeutic Proteins Engineering of immunomodulator protein structure to improve clearance in the body Upon administration of destabilized enzyme, IFNγ levels decrease, suggestive of protein breakdown 5p 3p Mature miRNA Expression in Cells © 2015 Intrexon Corp. All rights reserved.
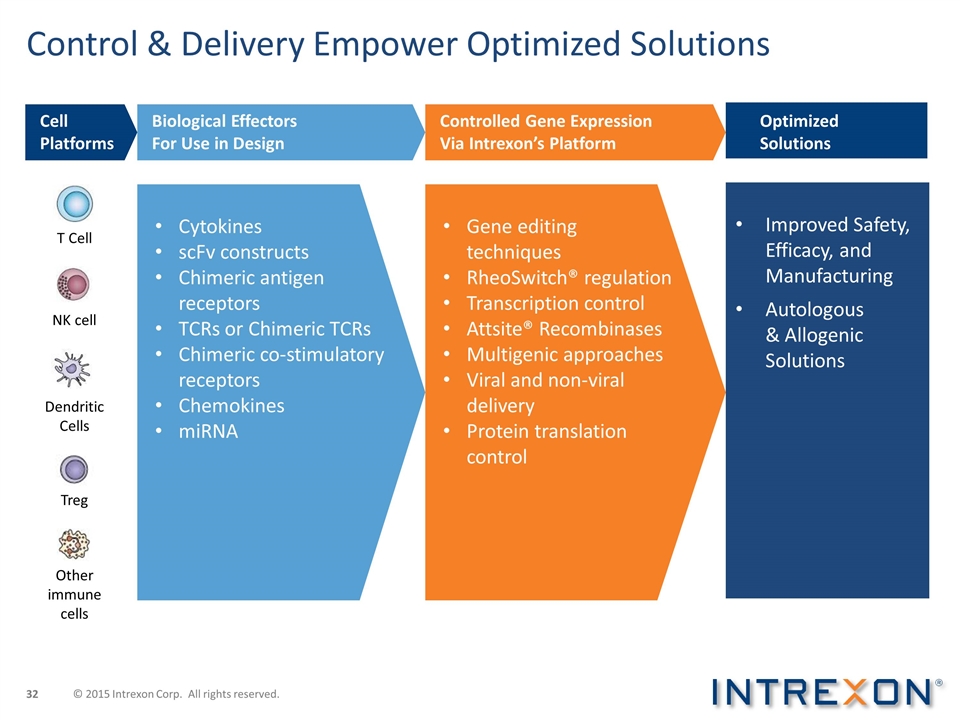
Control & Delivery Empower Optimized Solutions Improved Safety, Efficacy, and Manufacturing Autologous & Allogenic Solutions Cytokines scFv constructs Chimeric antigen receptors TCRs or Chimeric TCRs Chimeric co-stimulatory receptors Chemokines miRNA Gene editing techniques RheoSwitch® regulation Transcription control Attsite® Recombinases Multigenic approaches Viral and non-viral delivery Protein translation control Dendritic Cells T Cell NK cell Treg Other immune cells Cell Platforms Biological Effectors For Use in Design Controlled Gene Expression Via Intrexon’s Platform Optimized Solutions © 2015 Intrexon Corp. All rights reserved.

Intrexon’s Synthetic Immunology Efforts Expanding Team of Scientists Viral & Non-viral gene delivery / Biological Effectors / Controlled Expression Knowledge Framework Driving Partner Solutions Through our Innovative DNA Engine © 2015 Intrexon Corp. All rights reserved.
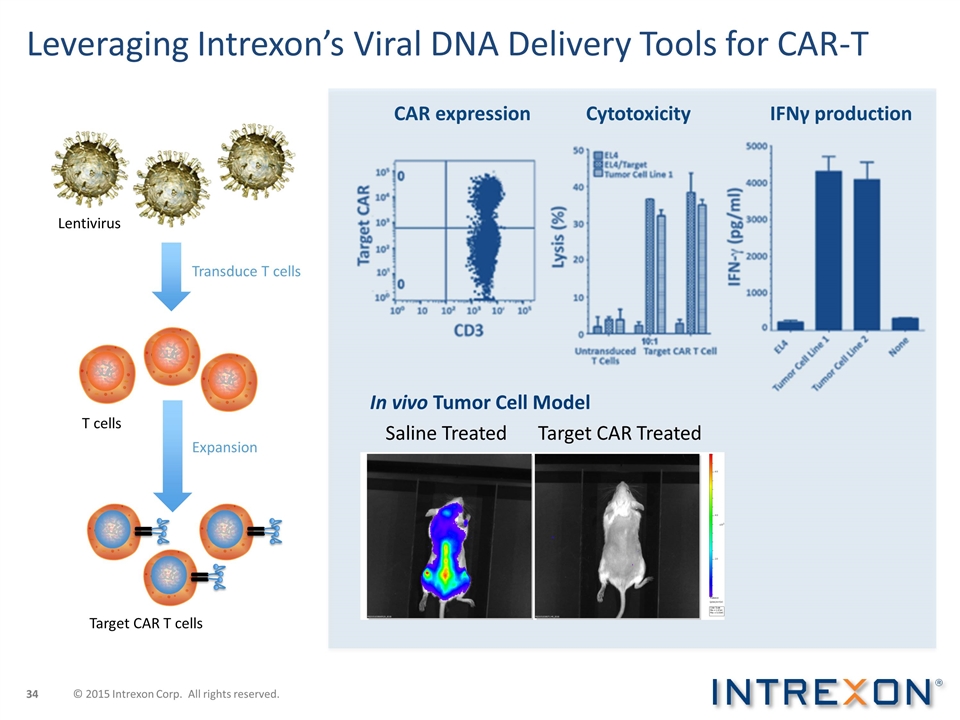
Leveraging Intrexon’s Viral DNA Delivery Tools for CAR-T Target CAR T cells T cells Expansion Transduce T cells Lentivirus Cytotoxicity IFNγ production CAR expression Saline Treated Target CAR Treated In vivo Tumor Cell Model © 2015 Intrexon Corp. All rights reserved.
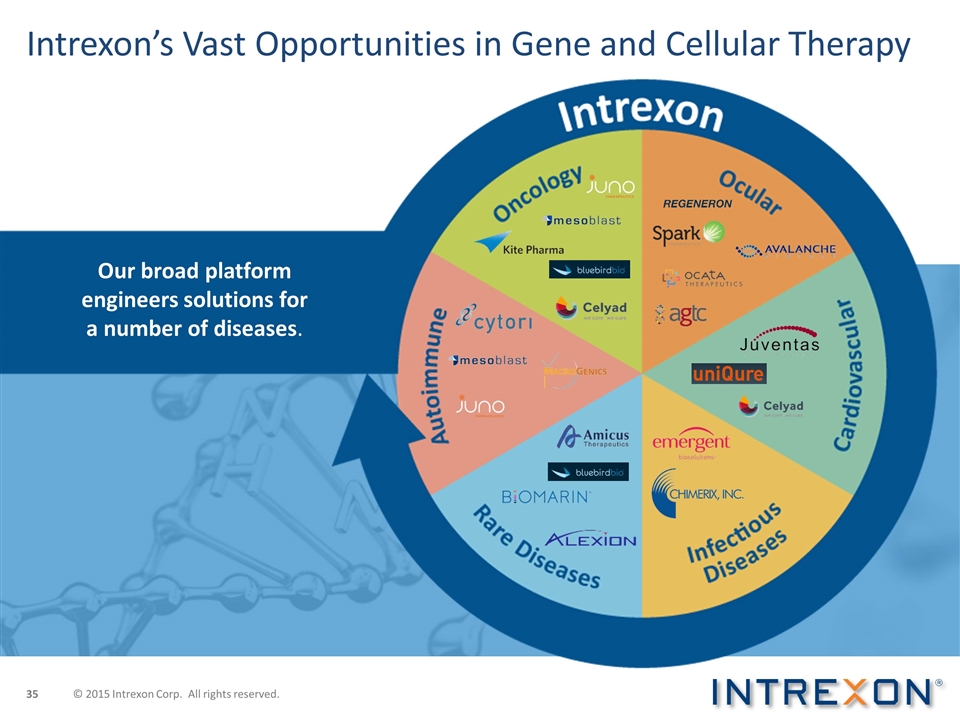
Our broad platform engineers solutions for a number of diseases. Intrexon’s Vast Opportunities in Gene and Cellular Therapy © 2015 Intrexon Corp. All rights reserved.

Harnessing the power of the immune system to fight cancer Laurence Cooper M.D., Ph.D. Chief Executive Officer, ZIOPHARM Oncology, Inc.
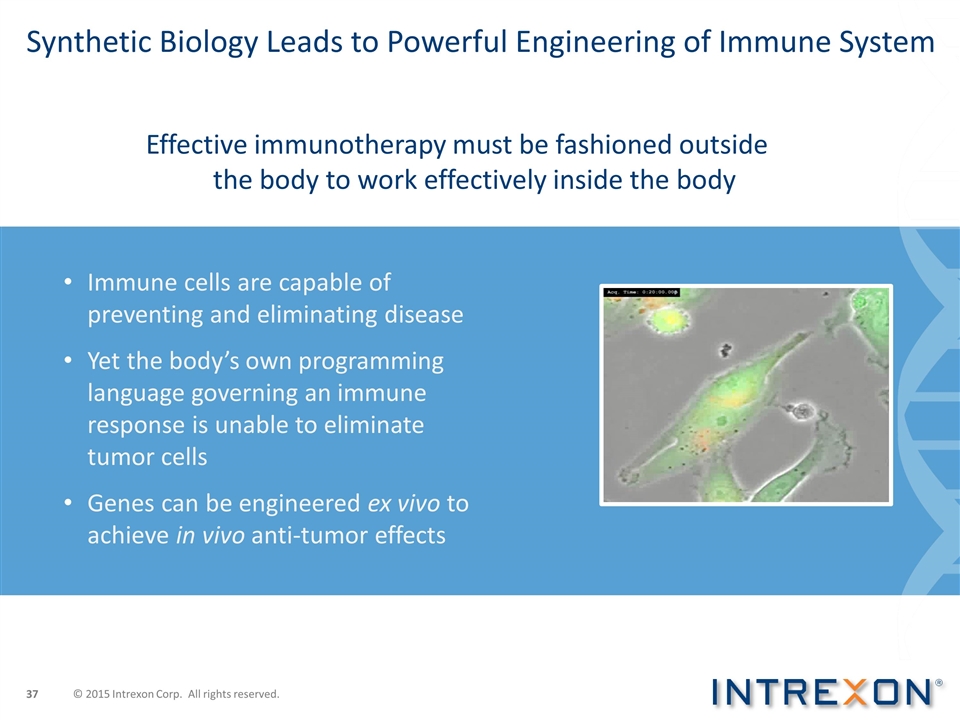
Synthetic Biology Leads to Powerful Engineering of Immune System Immune cells are capable of preventing and eliminating disease Yet the body’s own programming language governing an immune response is unable to eliminate tumor cells Genes can be engineered ex vivo to achieve in vivo anti-tumor effects Effective immunotherapy must be fashioned outside the body to work effectively inside the body © 2015 Intrexon Corp. All rights reserved.
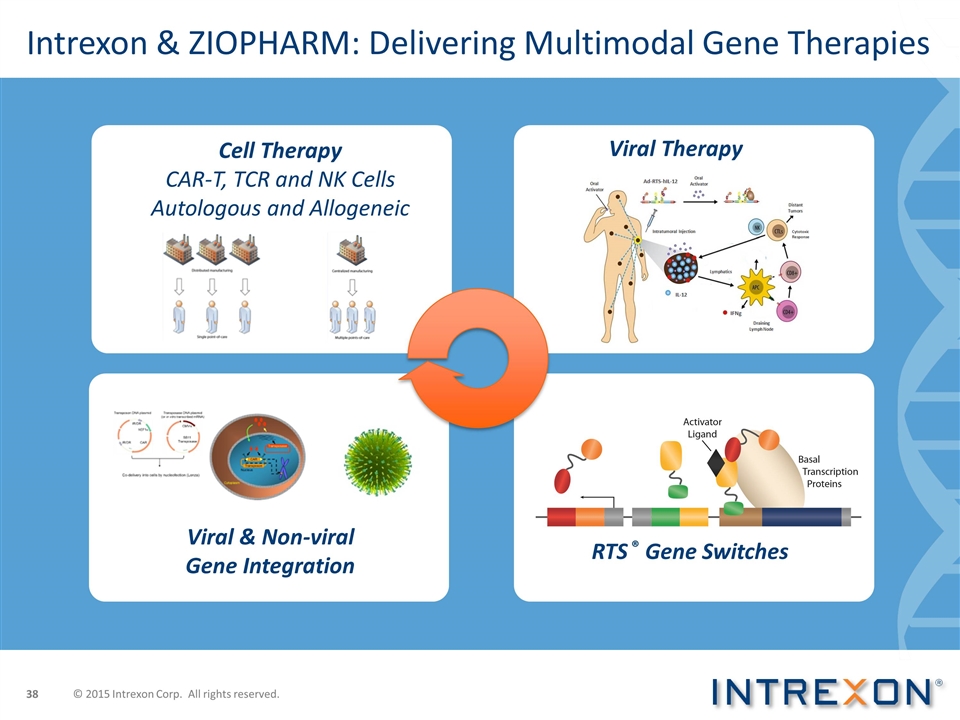
Intrexon & ZIOPHARM: Delivering Multimodal Gene Therapies Viral Therapy RTS ® Gene Switches Cell Therapy CAR-T, TCR and NK Cells Autologous and Allogeneic Viral & Non-viral Gene Integration © 2015 Intrexon Corp. All rights reserved.
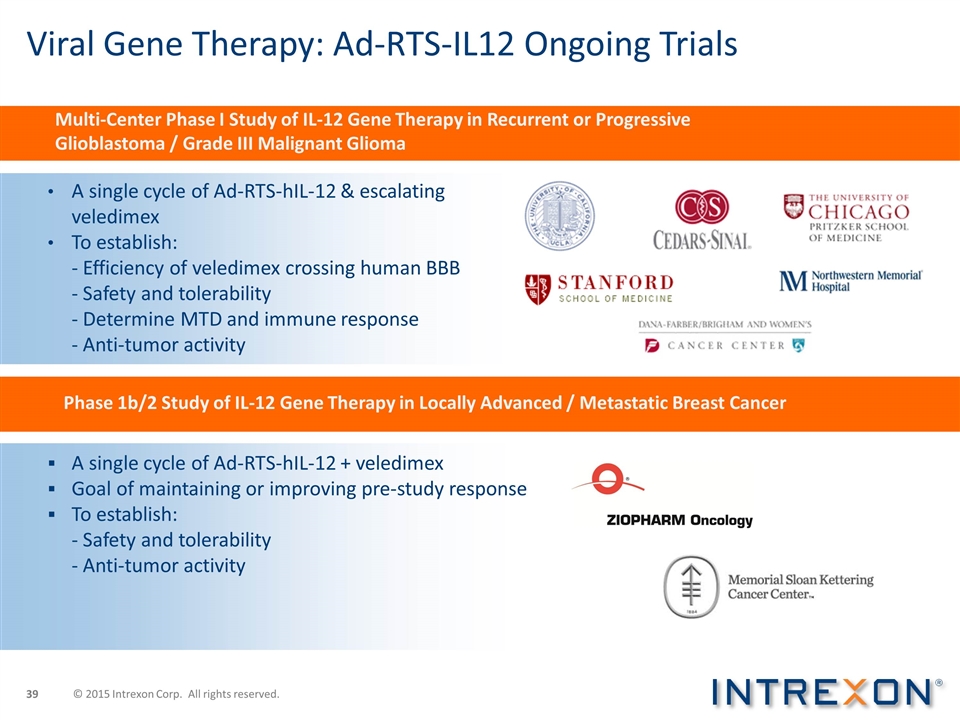
Viral Gene Therapy: Ad-RTS-IL12 Ongoing Trials A single cycle of Ad-RTS-hIL-12 & escalating veledimex To establish: - Efficiency of veledimex crossing human BBB - Safety and tolerability - Determine MTD and immune response - Anti-tumor activity A single cycle of Ad-RTS-hIL-12 + veledimex Goal of maintaining or improving pre-study response To establish: - Safety and tolerability - Anti-tumor activity Multi-Center Phase I Study of IL-12 Gene Therapy in Recurrent or Progressive Glioblastoma / Grade III Malignant Glioma Phase 1b/2 Study of IL-12 Gene Therapy in Locally Advanced / Metastatic Breast Cancer © 2015 Intrexon Corp. All rights reserved.
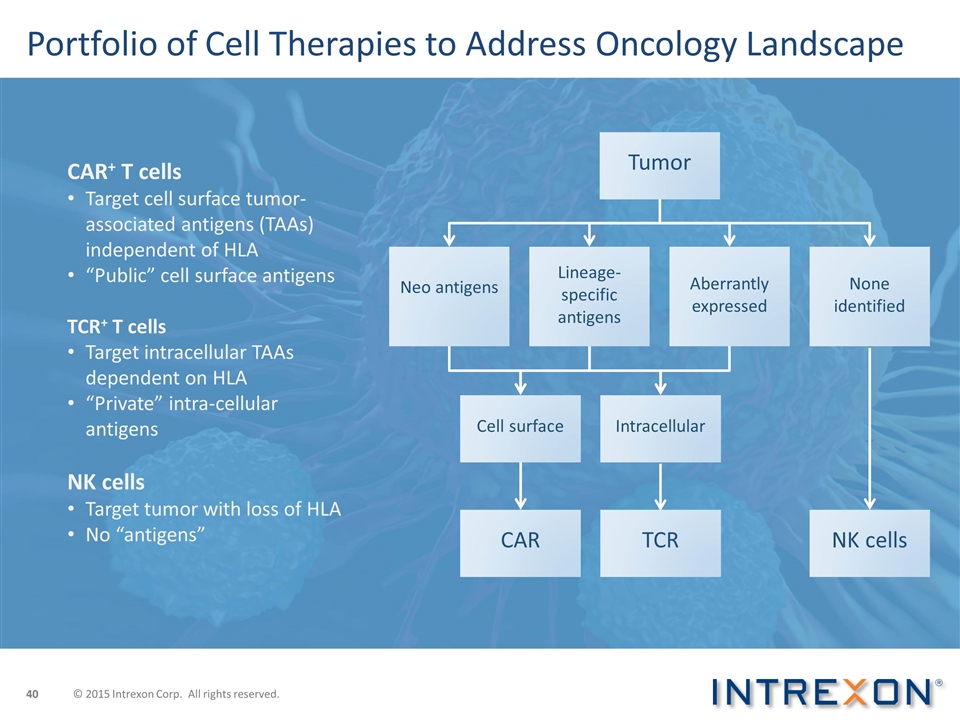
Portfolio of Cell Therapies to Address Oncology Landscape CAR+ T cells Target cell surface tumor-associated antigens (TAAs) independent of HLA “Public” cell surface antigens TCR+ T cells Target intracellular TAAs dependent on HLA “Private” intra-cellular antigens NK cells Target tumor with loss of HLA No “antigens” Tumor Neo antigens Lineage-specific antigens Aberrantly expressed None identified Cell surface Intracellular CAR TCR NK cells © 2015 Intrexon Corp. All rights reserved.
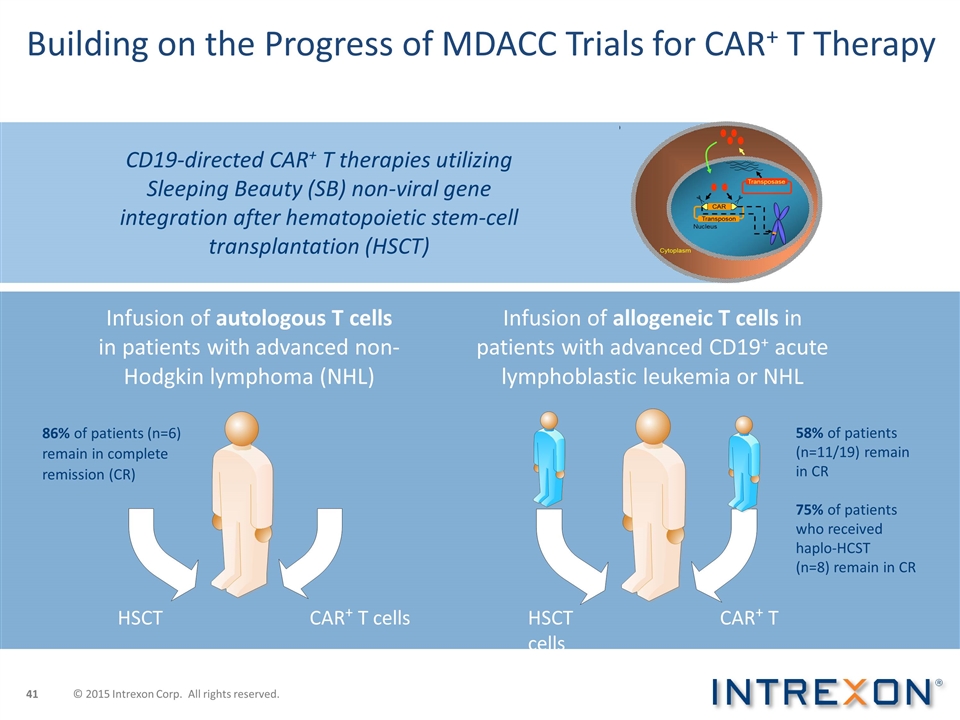
Building on the Progress of MDACC Trials for CAR+ T Therapy Infusion of autologous T cells in patients with advanced non-Hodgkin lymphoma (NHL) Infusion of allogeneic T cells in patients with advanced CD19+ acute lymphoblastic leukemia or NHL CD19-directed CAR+ T therapies utilizing Sleeping Beauty (SB) non-viral gene integration after hematopoietic stem-cell transplantation (HSCT) HSCT CAR+ T cells HSCTCAR+ T cells 86% of patients (n=6) remain in complete remission (CR) 58% of patients (n=11/19) remain in CR 75% of patients who received haplo-HCST (n=8) remain in CR © 2015 Intrexon Corp. All rights reserved.
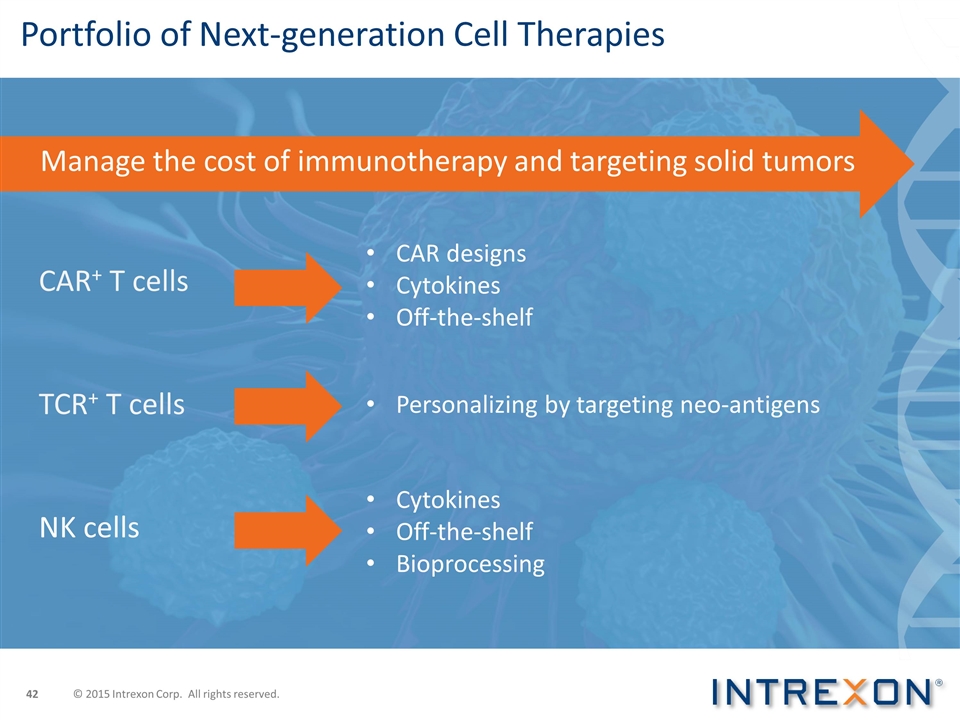
Portfolio of Next-generation Cell Therapies CAR designs Cytokines Off-the-shelf Personalizing by targeting neo-antigens CAR+ T cells TCR+ T cells NK cells Cytokines Off-the-shelf Bioprocessing Manage the cost of immunotherapy and targeting solid tumors © 2015 Intrexon Corp. All rights reserved.
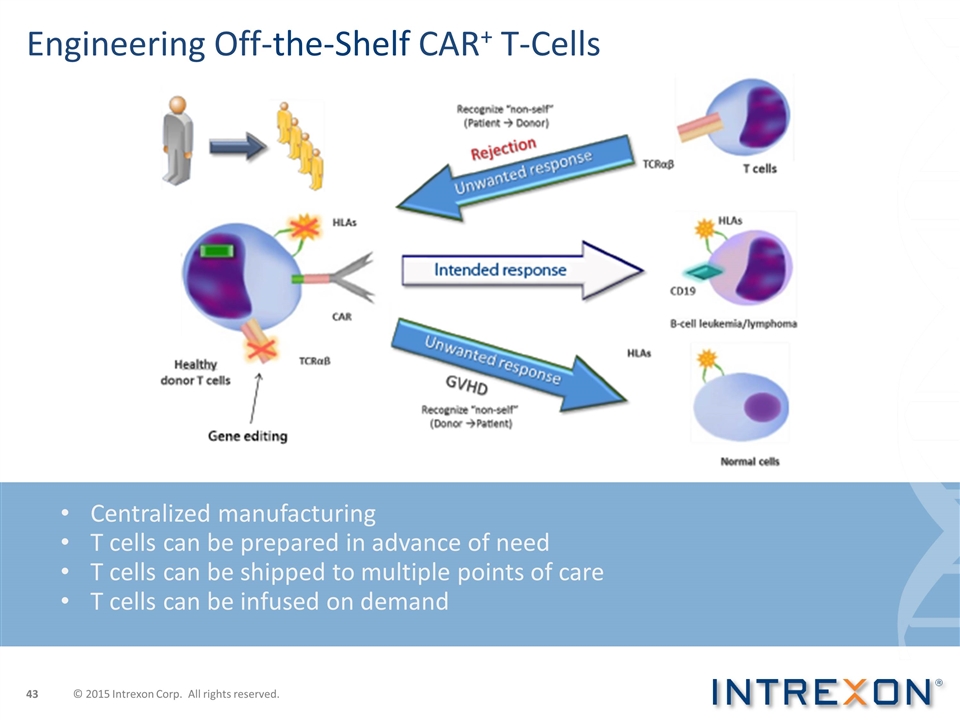
Engineering Off-the-Shelf CAR+ T-Cells Centralized manufacturing T cells can be prepared in advance of need T cells can be shipped to multiple points of care T cells can be infused on demand © 2015 Intrexon Corp. All rights reserved.
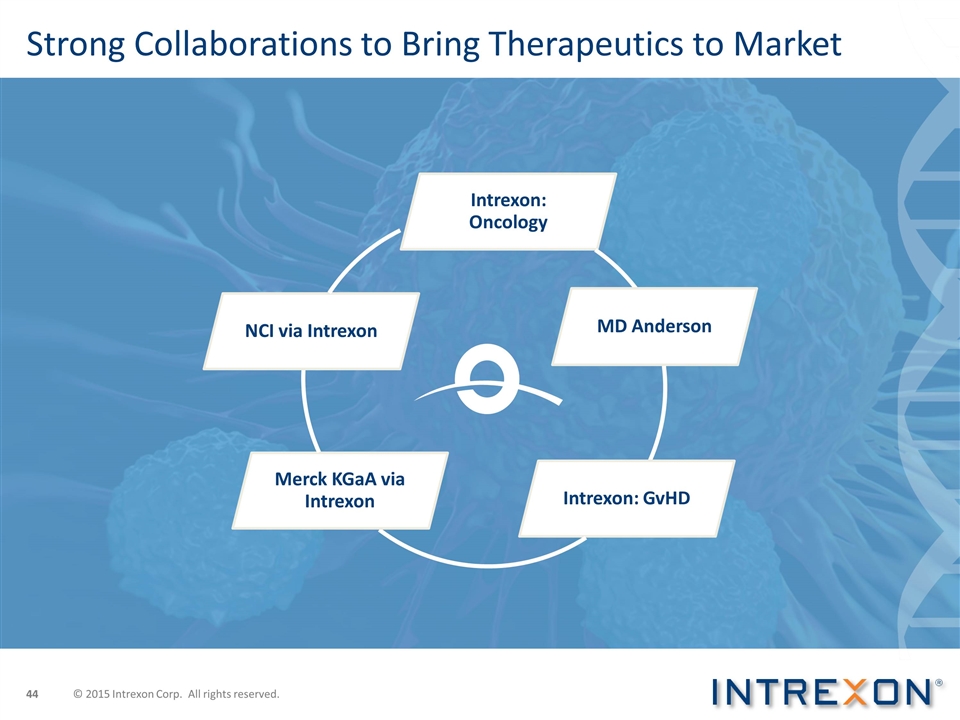
Strong Collaborations to Bring Therapeutics to Market © 2015 Intrexon Corp. All rights reserved. Intrexon : Oncology Intrexon: GvHD Merck KGaA via Intrexon NCI via Intrexon MD Anderson
Differentiated Platforms to Fight Cancer Intrexon Engine Ziopharm Implementation Harnessing synthetic biology to develop and implement a pipeline of therapeutics Update on clinical trials by year end 2015 New trials anticipated in 2016 © 2015 Intrexon Corp. All rights reserved.
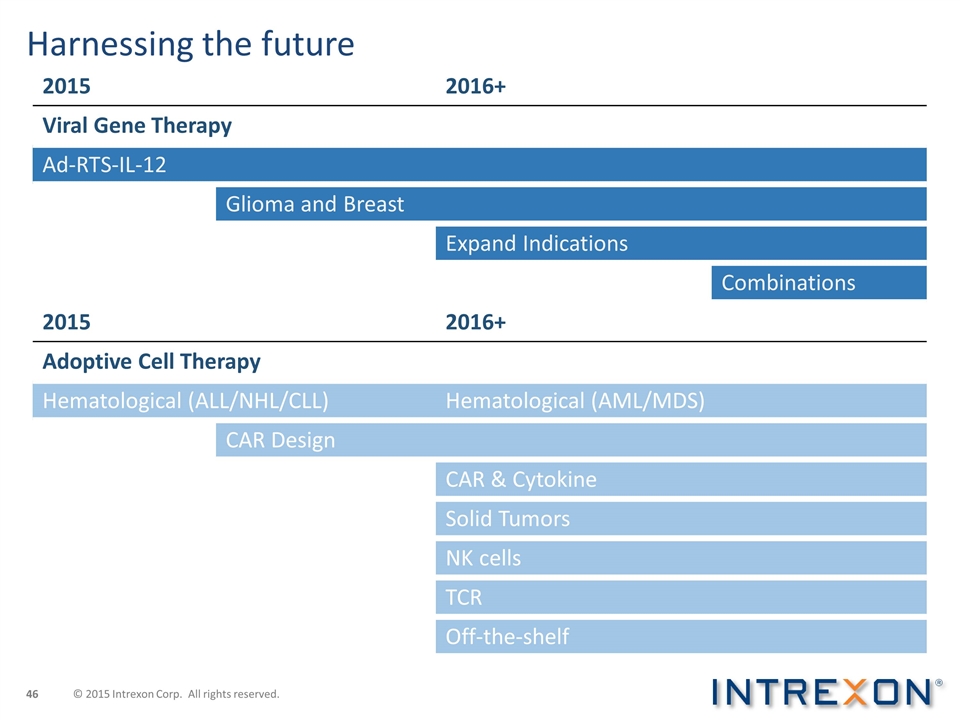
Harnessing the future 2015 2016+ Viral Gene Therapy Ad-RTS-IL-12 Glioma and Breast Expand Indications Combinations 2015 2016+ Adoptive Cell Therapy Hematological (ALL/NHL/CLL) Hematological (AML/MDS) CAR Design CAR & Cytokine Solid Tumors NK cells TCR Off-the-shelf © 2015 Intrexon Corp. All rights reserved.

Therapeutic Approaches Beyond Cancer Suma Krishnan Senior Vice President, Product Development & Head of Human Therapeutics Division
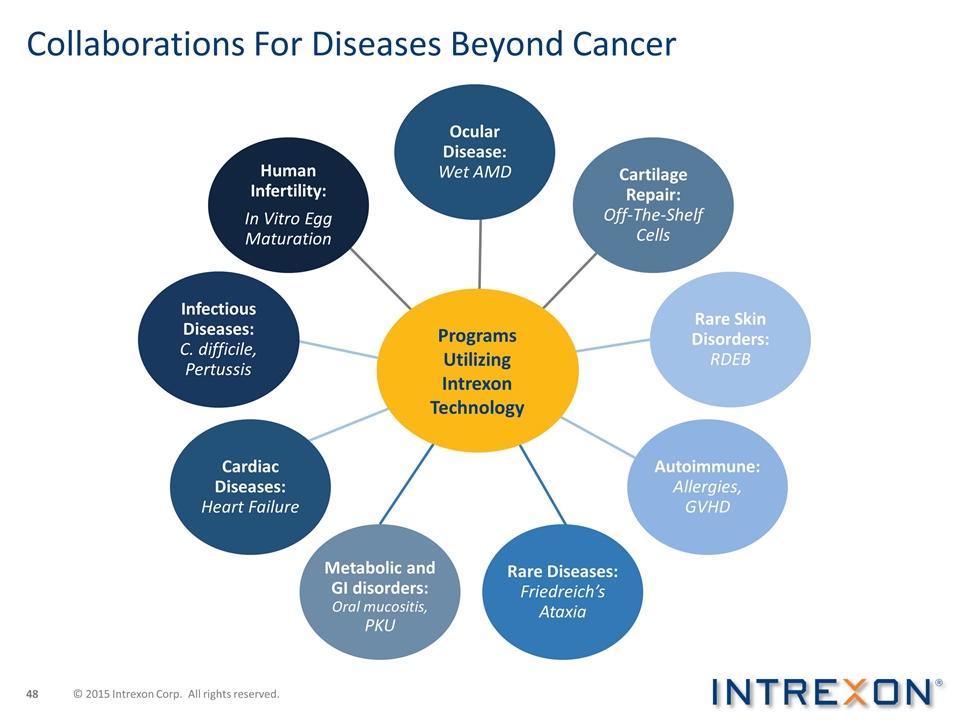
Collaborations For Diseases Beyond Cancer Ocular Disease: Wet AMD Cartilage Repair: Off-The-Shelf Cells Rare Skin Disorders: RDEB Autoimmune: Allergies, GVHD Rare Diseases: Friedreich’s Ataxia Metabolic and GI disorders: Oral mucositis, PKU Cardiac Diseases: Heart Failure Infectious Diseases: C. difficile, Pertussis Human Infertility: In Vitro Egg Maturation Programs Utilizing Intrexon Technology © 2015 Intrexon Corp. All rights reserved.
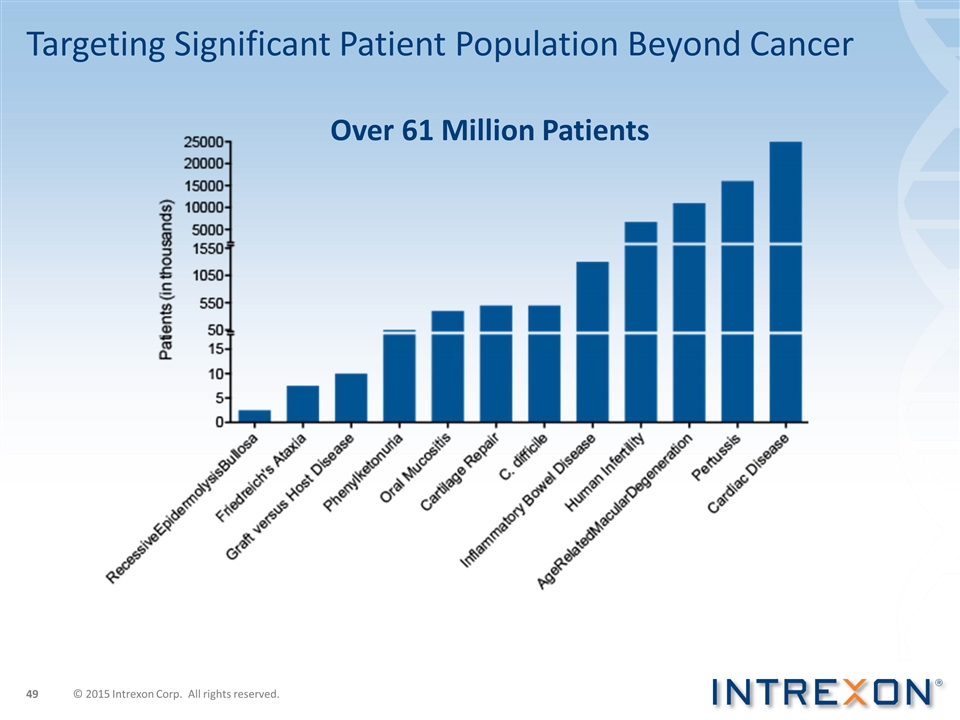
Over 61 Million Patients Targeting Significant Patient Population Beyond Cancer © 2015 Intrexon Corp. All rights reserved.
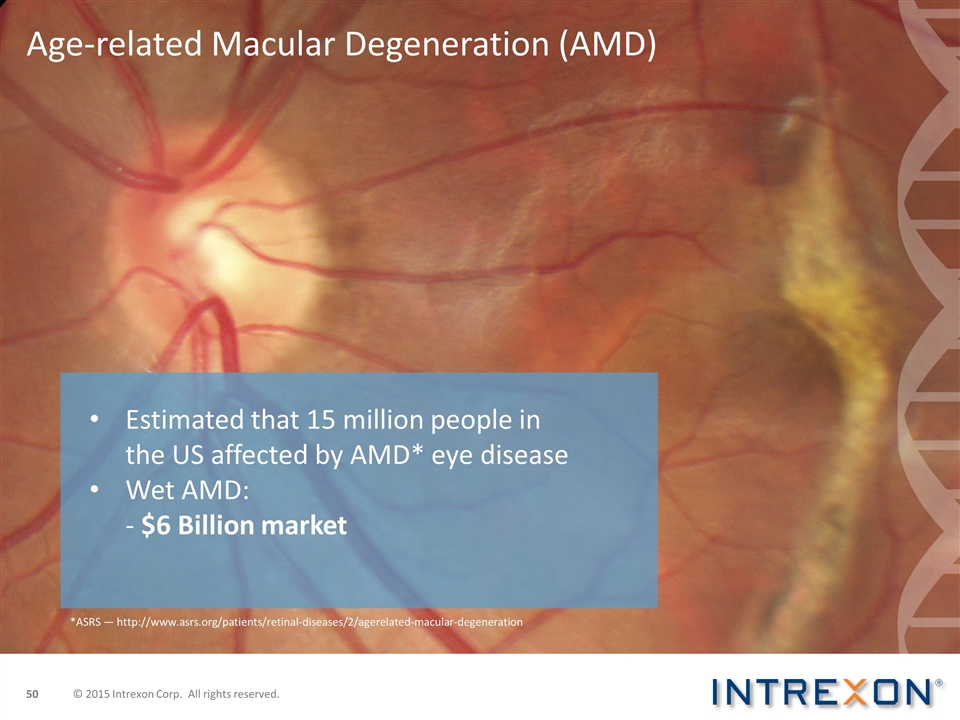
Age-related Macular Degeneration (AMD) Estimated that 15 million people in the US affected by AMD* eye disease Wet AMD: - $6 Billion market *ASRS — http://www.asrs.org/patients/retinal-diseases/2/agerelated-macular-degeneration © 2015 Intrexon Corp. All rights reserved.
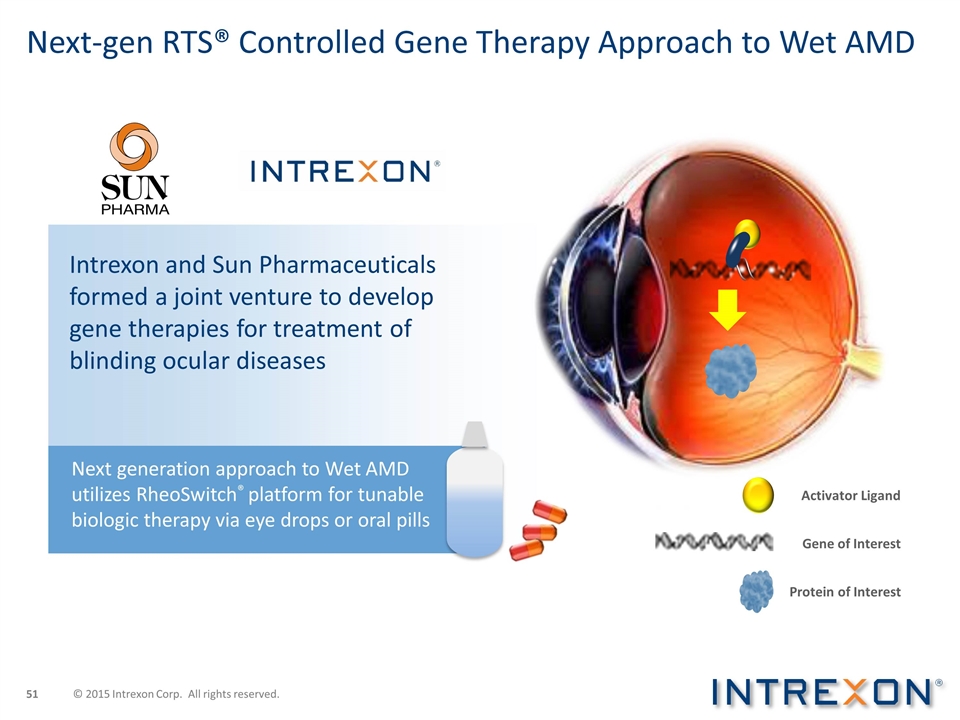
Next-gen RTS® Controlled Gene Therapy Approach to Wet AMD Intrexon and Sun Pharmaceuticals formed a joint venture to develop gene therapies for treatment of blinding ocular diseases Activator Ligand Protein of Interest Gene of Interest Next generation approach to Wet AMD utilizes RheoSwitch® platform for tunable biologic therapy via eye drops or oral pills © 2015 Intrexon Corp. All rights reserved.
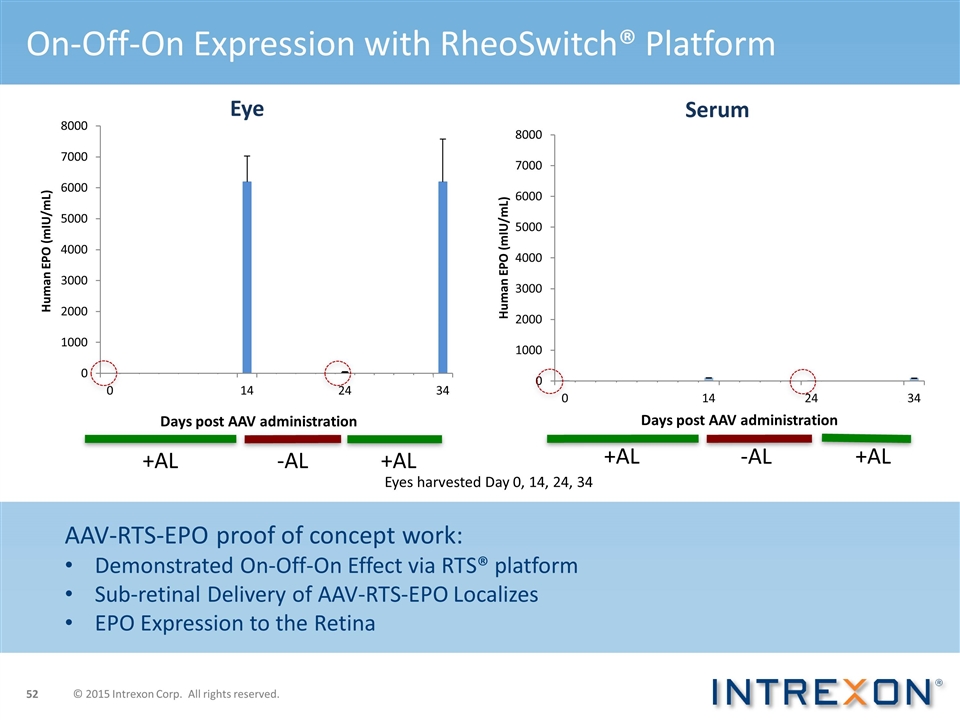
+AL +AL -AL +AL -AL +AL Eyes harvested Day 0, 14, 24, 34 On-Off-On Expression with RheoSwitch® Platform AAV-RTS-EPO proof of concept work: Demonstrated On-Off-On Effect via RTS® platform Sub-retinal Delivery of AAV-RTS-EPO Localizes EPO Expression to the Retina
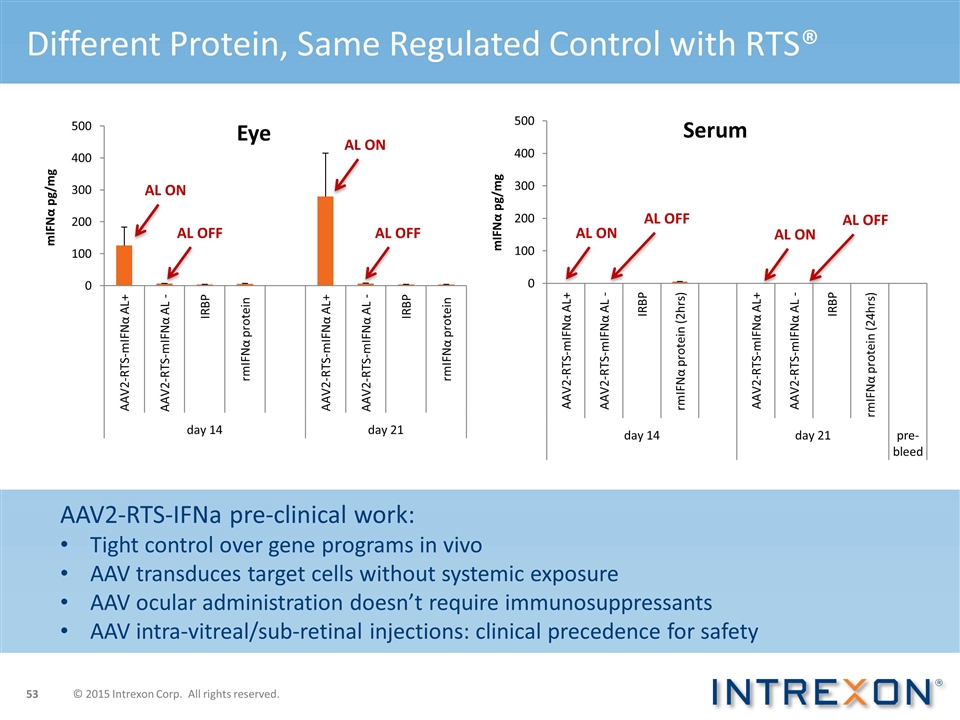
AL ON AL OFF AL OFF AL ON AL ON AL OFF AL ON AL OFF Different Protein, Same Regulated Control with RTS® AAV2-RTS-IFNa pre-clinical work: Tight control over gene programs in vivo AAV transduces target cells without systemic exposure AAV ocular administration doesn’t require immunosuppressants AAV intra-vitreal/sub-retinal injections: clinical precedence for safety © 2015 Intrexon Corp. All rights reserved.
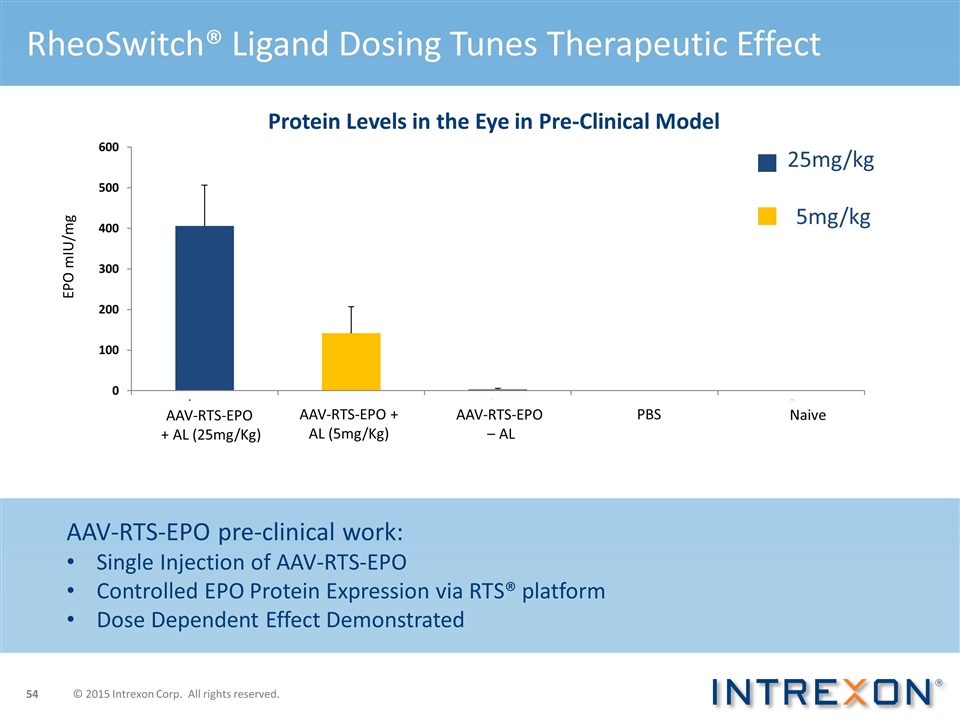
AAV-RTS-EPO + AL (25mg/Kg) AAV-RTS-EPO + AL (5mg/Kg) AAV-RTS-EPO – AL PBS Naive RheoSwitch® Ligand Dosing Tunes Therapeutic Effect Protein Levels in the Eye in Pre-Clinical Model AAV-RTS-EPO pre-clinical work: Single Injection of AAV-RTS-EPO Controlled EPO Protein Expression via RTS® platform Dose Dependent Effect Demonstrated 25mg/kg 5mg/kg © 2015 Intrexon Corp. All rights reserved.
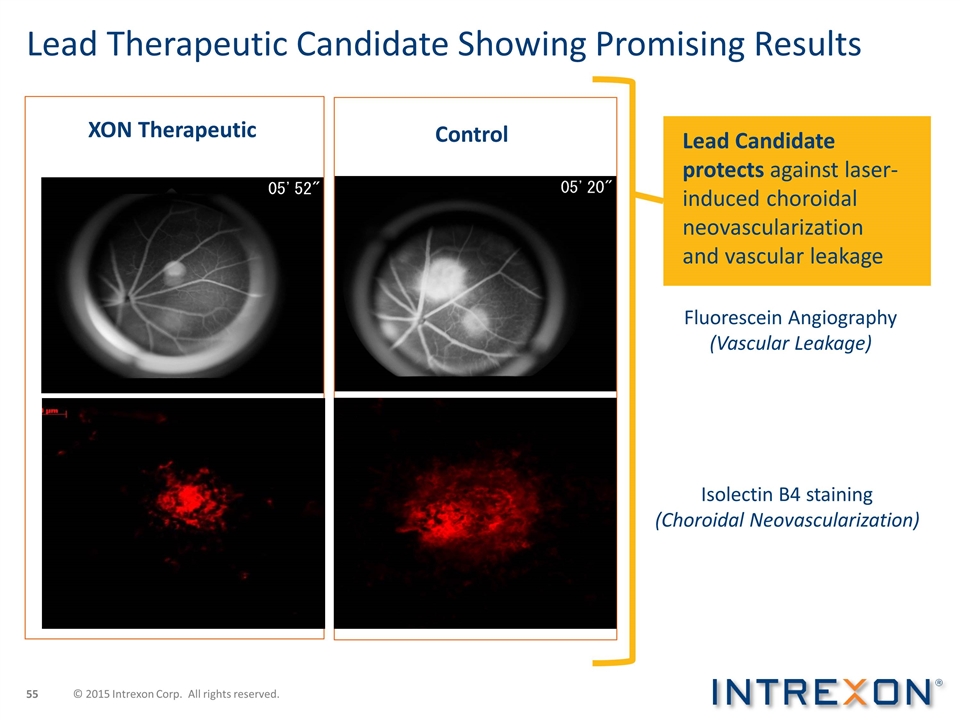
Lead Therapeutic Candidate Showing Promising Results XON Therapeutic Control Isolectin B4 staining (Choroidal Neovascularization) Fluorescein Angiography (Vascular Leakage) Lead Candidate protects against laser-induced choroidal neovascularization and vascular leakage © 2015 Intrexon Corp. All rights reserved.

CONFIDENTIAL © 2015 Intrexon Corp. All rights reserved.
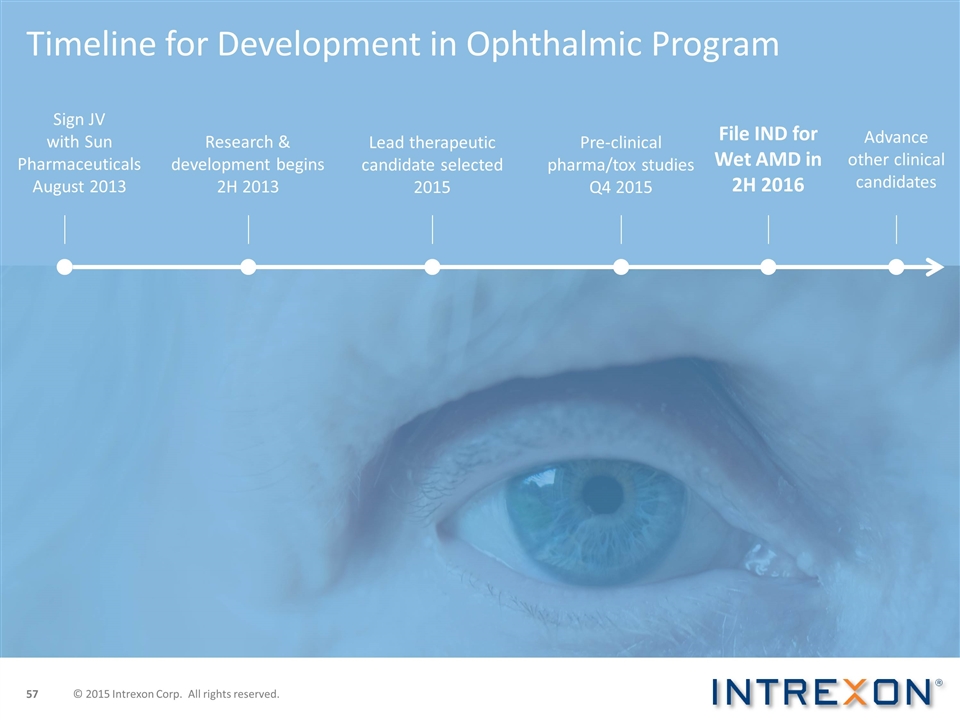
Timeline for Development in Ophthalmic Program Sign JV with Sun Pharmaceuticals August 2013 File IND for Wet AMD in 2H 2016 Lead therapeutic candidate selected 2015 Research & development begins 2H 2013 Pre-clinical pharma/tox studies Q4 2015 Advance other clinical candidates © 2015 Intrexon Corp. All rights reserved.

Xogenex: Targeting Heart Failure with Novel Multigenic Approaches Amit Patel, M.D. Director of Clinical Regenerative Medicine and Tissue Engineering at the University of Utah
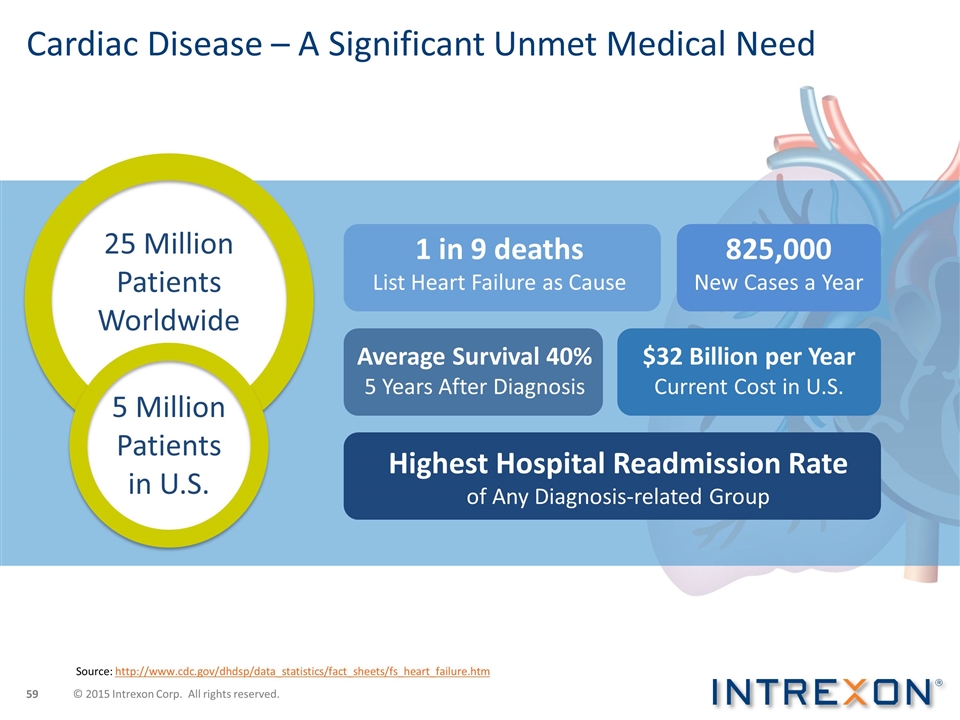
Cardiac Disease – A Significant Unmet Medical Need Source: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm 25 Million Patients Worldwide 1 in 9 deaths List Heart Failure as Cause 825,000 New Cases a Year $32 Billion per Year Current Cost in U.S. Average Survival 40% 5 Years After Diagnosis Highest Hospital Readmission Rate of Any Diagnosis-related Group 5 Million Patients in U.S. © 2015 Intrexon Corp. All rights reserved.
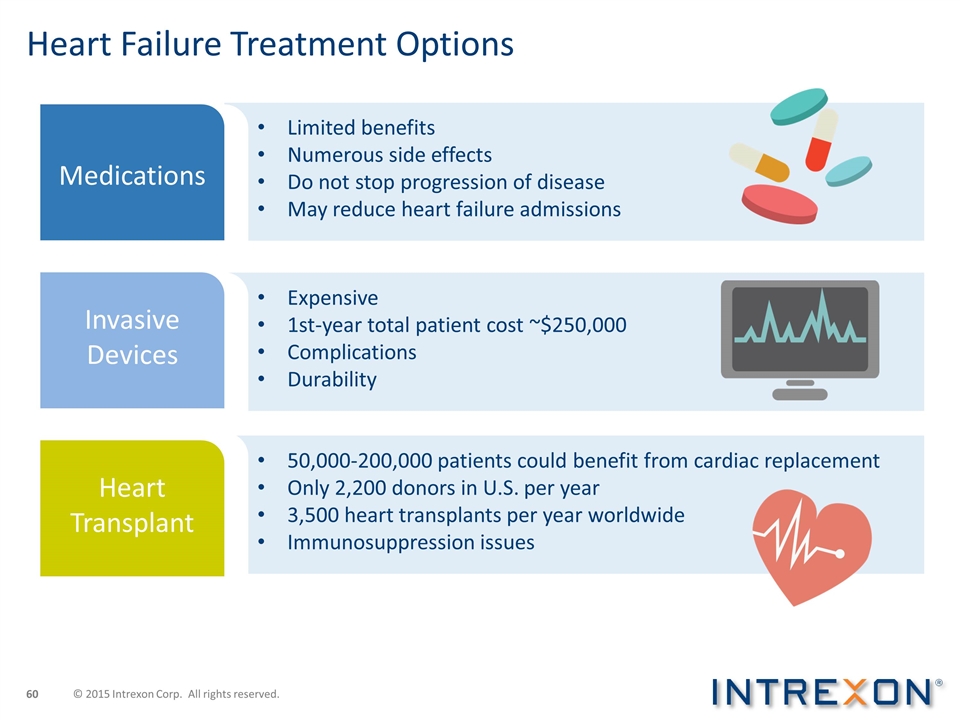
50,000-200,000 patients could benefit from cardiac replacement Only 2,200 donors in U.S. per year 3,500 heart transplants per year worldwide Immunosuppression issues Heart Failure Treatment Options Limited benefits Numerous side effects Do not stop progression of disease May reduce heart failure admissions Expensive 1st-year total patient cost ~$250,000 Complications Durability Medications Invasive Devices Heart Transplant © 2015 Intrexon Corp. All rights reserved.

Limited Success to Date with Heart Failure Biologics Stem Cells Costly approach Currently in clinical trials Mechanism of action unknown After 15 years no approved product or large scale positive trial Gene Therapy Currently in clinical trials Defined mechanism Current generation viral or single target approach After 20 years no approved product or large scale positive trial © 2015 Intrexon Corp. All rights reserved.
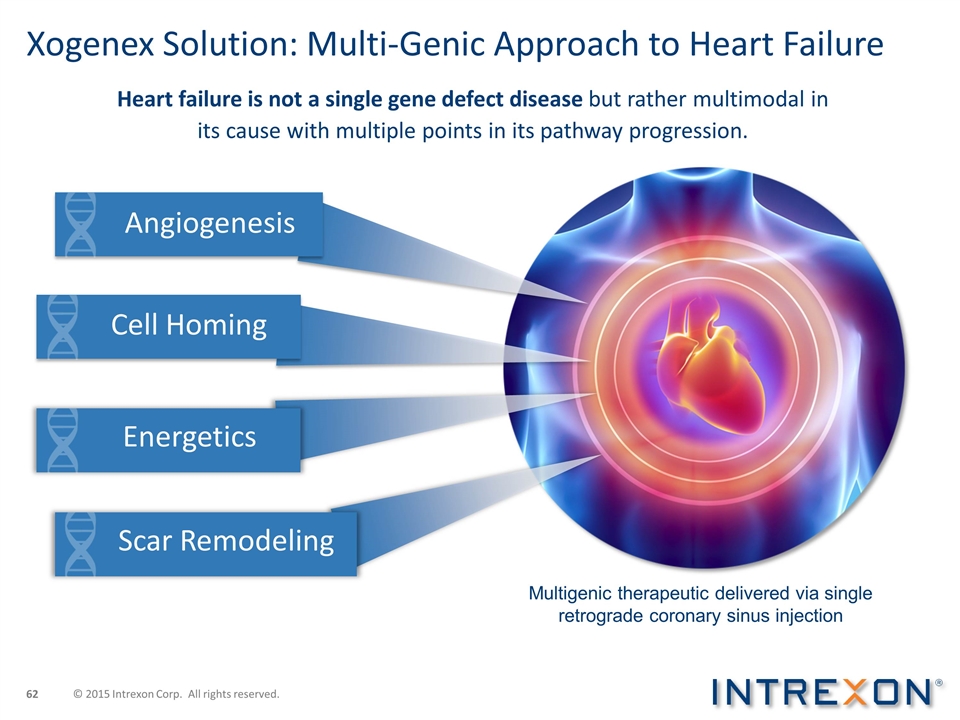
Xogenex Solution: Multi-Genic Approach to Heart Failure Heart failure is not a single gene defect disease but rather multimodal in its cause with multiple points in its pathway progression. Multigenic therapeutic delivered via single retrograde coronary sinus injection Angiogenesis Cell Homing Energetics Scar Remodeling © 2015 Intrexon Corp. All rights reserved.
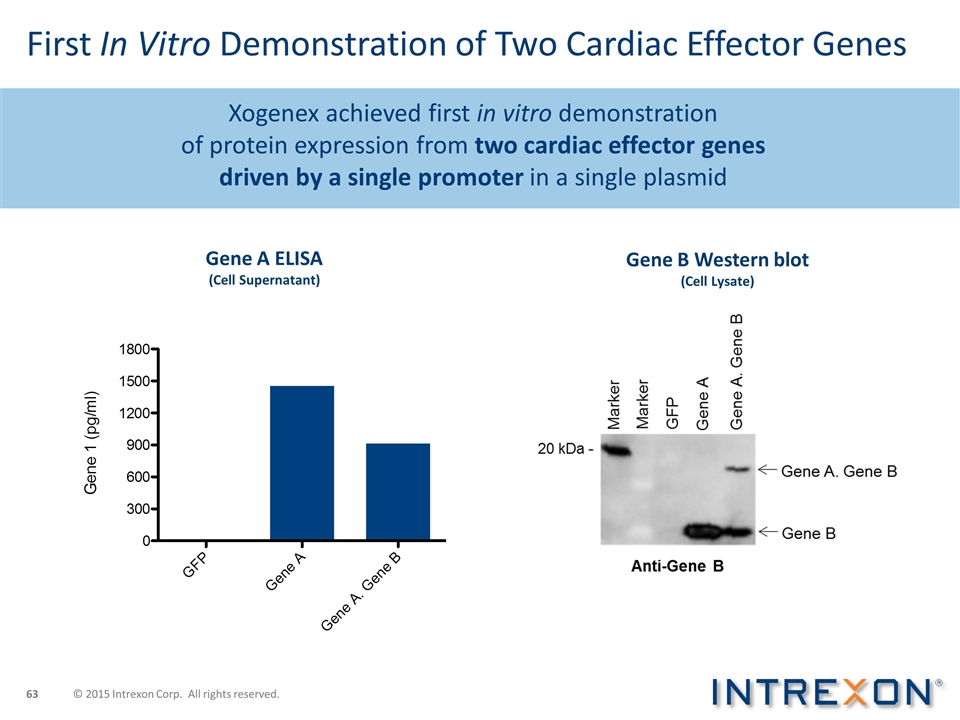
Xogenex achieved first in vitro demonstration of protein expression from two cardiac effector genes driven by a single promoter in a single plasmid First In Vitro Demonstration of Two Cardiac Effector Genes Gene B Western blot (Cell Lysate) Gene A ELISA (Cell Supernatant) © 2015 Intrexon Corp. All rights reserved.
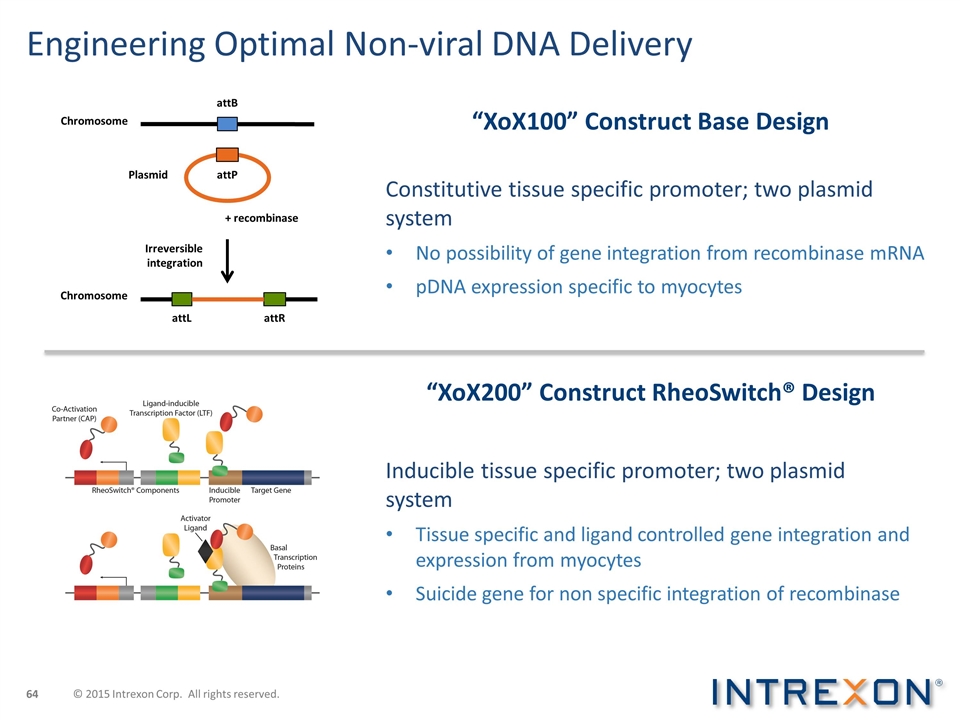
Engineering Optimal Non-viral DNA Delivery “XoX100” Construct Base Design Constitutive tissue specific promoter; two plasmid system No possibility of gene integration from recombinase mRNA pDNA expression specific to myocytes Inducible tissue specific promoter; two plasmid system Tissue specific and ligand controlled gene integration and expression from myocytes Suicide gene for non specific integration of recombinase “XoX200” Construct RheoSwitch® Design + recombinase Irreversible integration Chromosome attB attR attL Chromosome attP Plasmid © 2015 Intrexon Corp. All rights reserved.
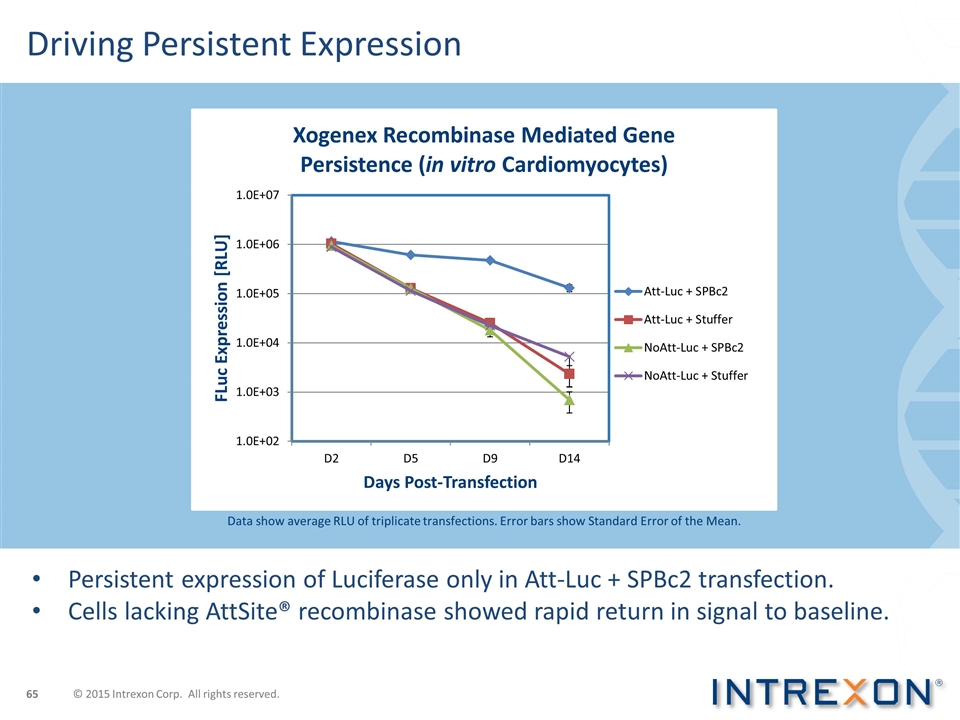
Driving Persistent Expression Data show average RLU of triplicate transfections. Error bars show Standard Error of the Mean. Persistent expression of Luciferase only in Att-Luc + SPBc2 transfection. Cells lacking AttSite® recombinase showed rapid return in signal to baseline. © 2015 Intrexon Corp. All rights reserved.
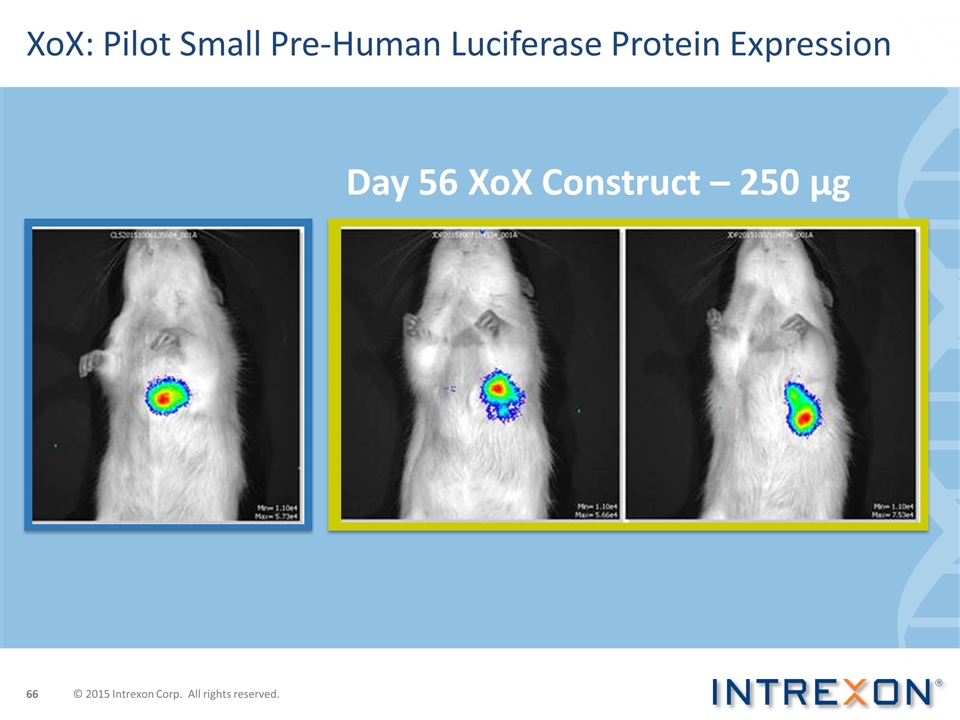
Day 56 XoX Construct – 250 µg XoX: Pilot Small Pre-Human Luciferase Protein Expression © 2015 Intrexon Corp. All rights reserved.

XoX: Pilot Large Pre-Human Data Biodistribution of XoX Construct in large Pre-Human model Blue dye demonstrates both macro and micro distribution Reproducible © 2015 Intrexon Corp. All rights reserved.
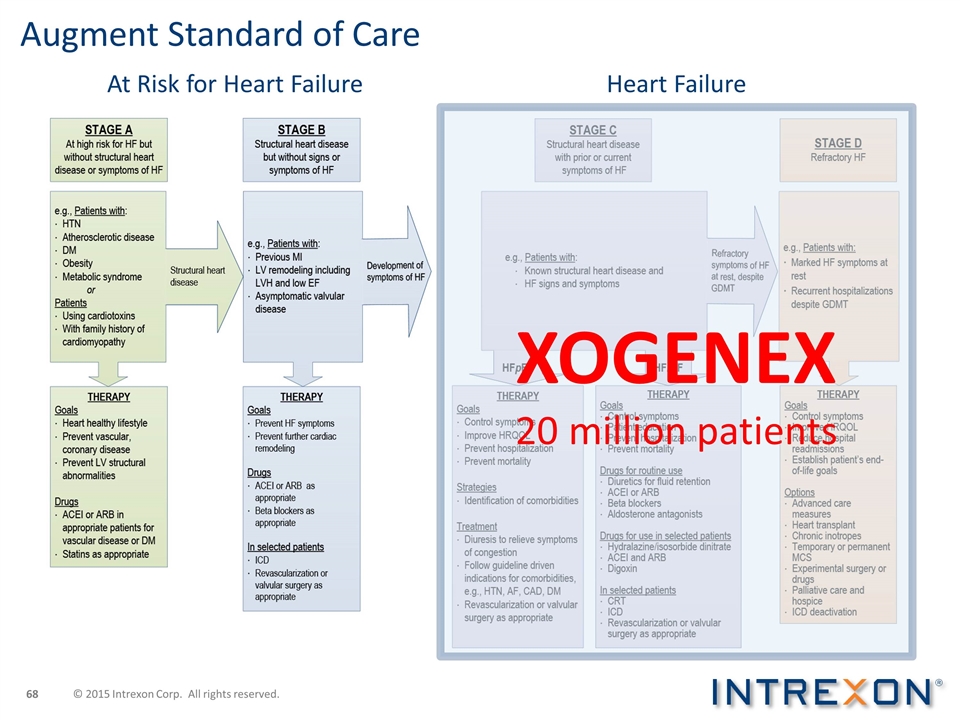
Augment Standard of Care XOGENEX 20 million patients At Risk for Heart Failure Heart Failure © 2015 Intrexon Corp. All rights reserved.
Summary Novel multi-target approach for heart failure Multi-target approach 825,000 new cases annually Disease costs US approximately $32 Billion per Year Simple, reproducible, with possible re-dosing options Scientific Advisory Board experienced in retrograde biologics delivery in U.S. and globally Anticipate IND Filing by 1H 2017 © 2015 Intrexon Corp. All rights reserved.

Third Generation Microbial Fermentation For Commercial Energy and Industrial Products Robert Walsh, Senior Vice President Head of Industrial Products Division
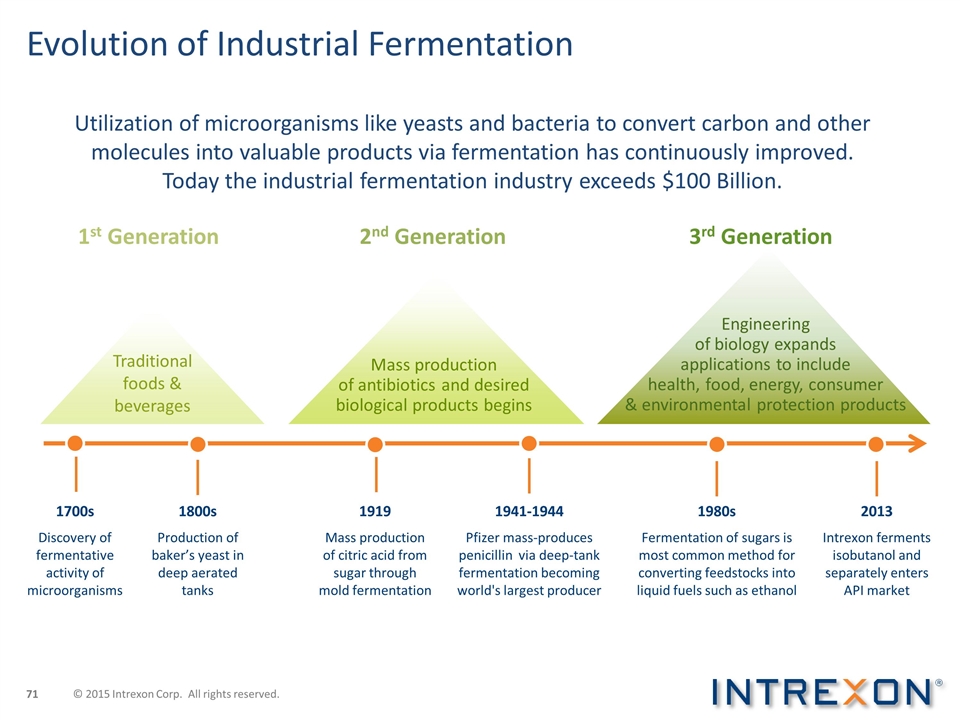
Evolution of Industrial Fermentation Utilization of microorganisms like yeasts and bacteria to convert carbon and other molecules into valuable products via fermentation has continuously improved. Today the industrial fermentation industry exceeds $100 Billion. 1st Generation 2nd Generation 3rd Generation 1928 1941-1944 Pfizer mass-produces penicillin via deep-tank fermentation becoming world's largest producer 1800s Production of baker’s yeast in deep aerated tanks 1919 Mass production of citric acid from sugar through mold fermentation 1700s Discovery of fermentative activity of microorganisms 1980s Fermentation of sugars is most common method for converting feedstocks into liquid fuels such as ethanol 2013 Intrexon ferments isobutanol and separately enters API market Traditional foods & beverages Mass production of antibiotics and desired biological products begins Engineering of biology expands applications to include health, food, energy, consumer & environmental protection products © 2015 Intrexon Corp. All rights reserved.
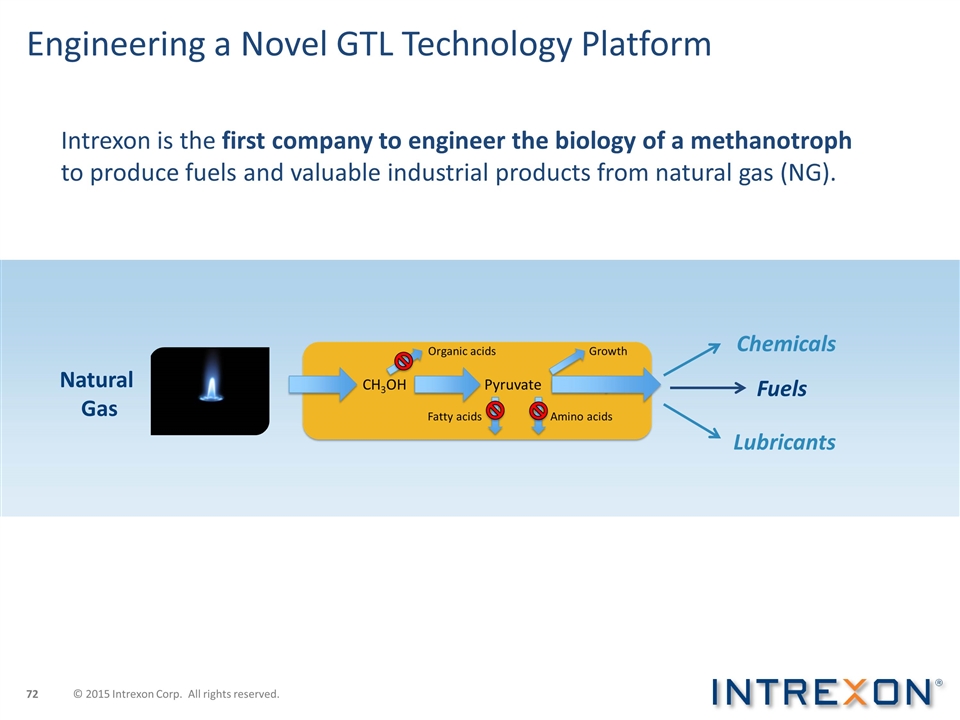
Engineering a Novel GTL Technology Platform Intrexon is the first company to engineer the biology of a methanotroph to produce fuels and valuable industrial products from natural gas (NG). Natural Gas Fuels Chemicals Lubricants © 2015 Intrexon Corp. All rights reserved.
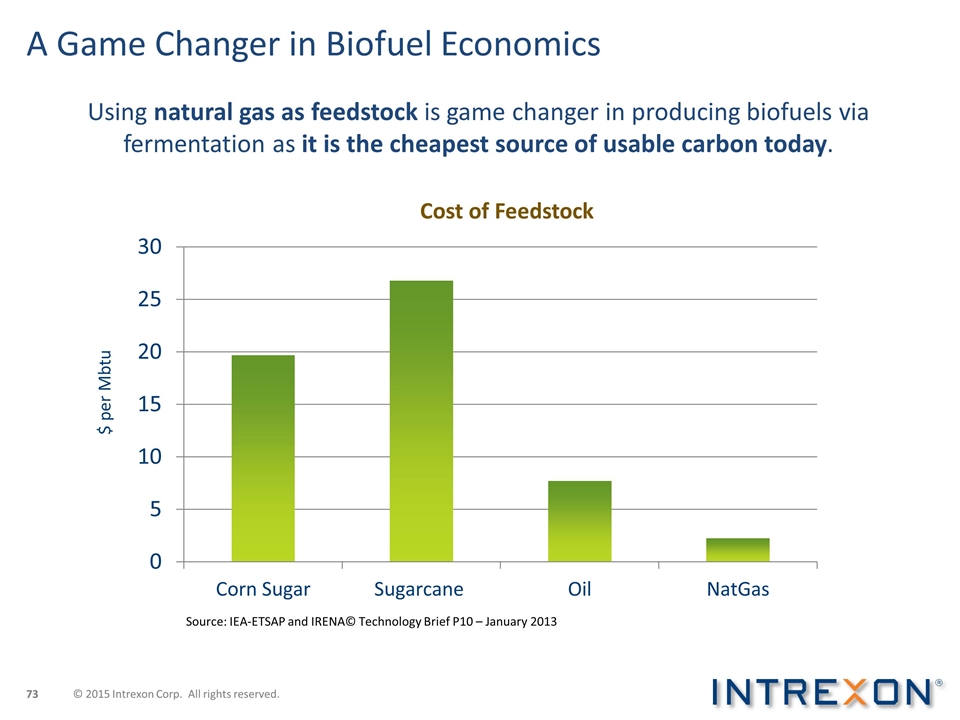
A Game Changer in Biofuel Economics Using natural gas as feedstock is game changer in producing biofuels via fermentation as it is the cheapest source of usable carbon today. Source: IEA-ETSAP and IRENA© Technology Brief P10 – January 2013 $ per Mbtu © 2015 Intrexon Corp. All rights reserved.

A More Attractive ‘Drop-in Fuel’ The first target for Intrexon’s bioconversion platform is isobutanol, an attractive biofuel with competitive advantages versus other “drop in" gasoline replacement fuels or major additives 98% Energy Density of Gas Well Above Other Oxygenated Fuels No Corrosion to Engines or Pipelines Reduces Carbon Monoxide Emissions Natural gas feedstock not connected to food supplies prevents a fuel-price/food-price relationship like corn & sugar © 2015 Intrexon Corp. All rights reserved.
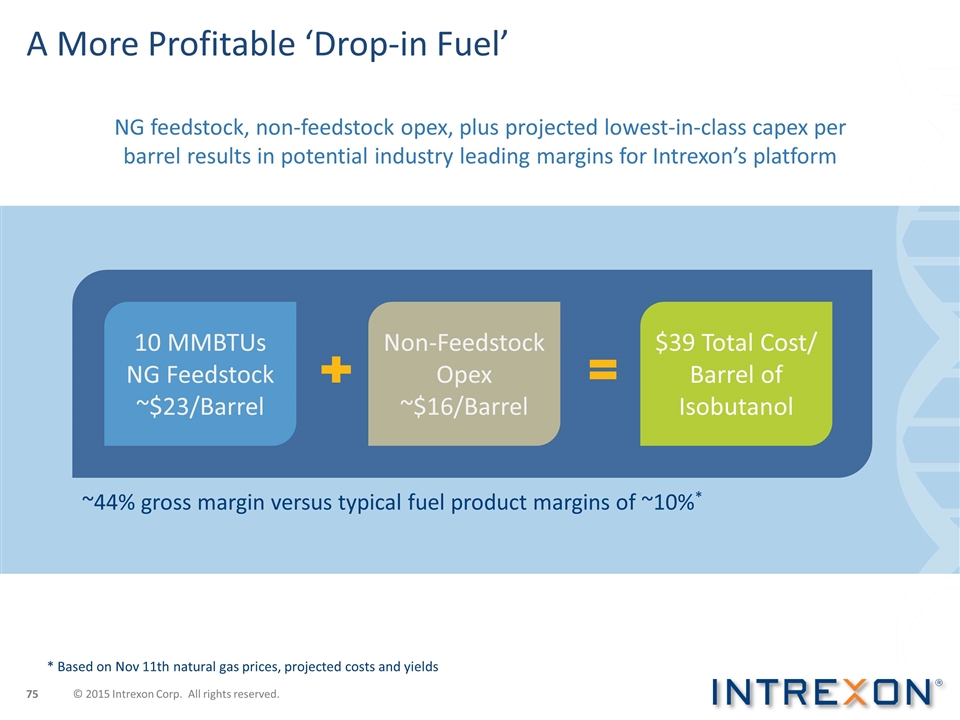
A More Profitable ‘Drop-in Fuel’ * Based on Nov 11th natural gas prices, projected costs and yields ~44% gross margin versus typical fuel product margins of ~10%* 10 MMBTUs NG Feedstock ~$23/Barrel Non-Feedstock Opex ~$16/Barrel $39 Total Cost/ Barrel of Isobutanol NG feedstock, non-feedstock opex, plus projected lowest-in-class capex per barrel results in potential industry leading margins for Intrexon’s platform © 2015 Intrexon Corp. All rights reserved.
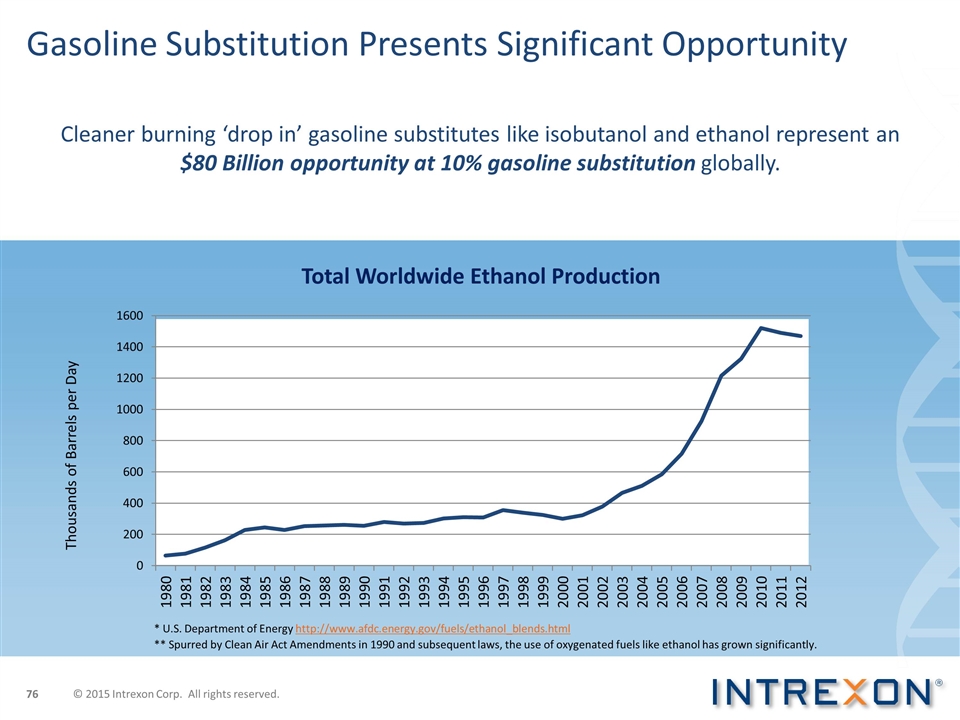
Gasoline Substitution Presents Significant Opportunity Cleaner burning ‘drop in’ gasoline substitutes like isobutanol and ethanol represent an $80 Billion opportunity at 10% gasoline substitution globally. Total Worldwide Ethanol Production * U.S. Department of Energy http://www.afdc.energy.gov/fuels/ethanol_blends.html Thousands of Barrels per Day ** Spurred by Clean Air Act Amendments in 1990 and subsequent laws, the use of oxygenated fuels like ethanol has grown significantly. © 2015 Intrexon Corp. All rights reserved.
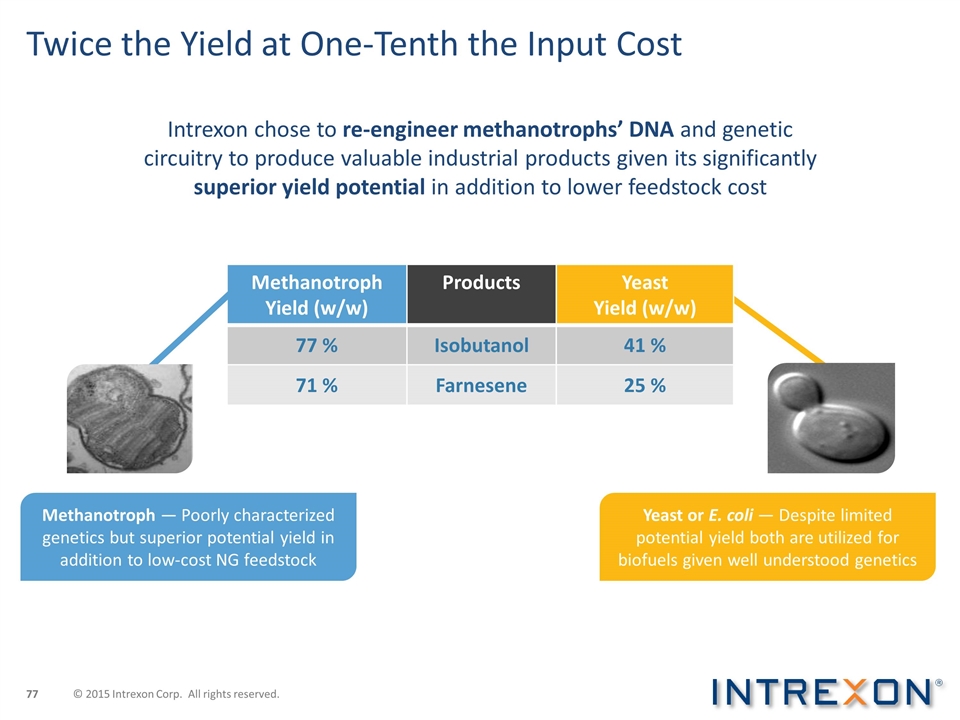
Twice the Yield at One-Tenth the Input Cost Intrexon chose to re-engineer methanotrophs’ DNA and genetic circuitry to produce valuable industrial products given its significantly superior yield potential in addition to lower feedstock cost Methanotroph Yield (w/w) Products Yeast Yield (w/w) 77 % Isobutanol 41 % 71 % Farnesene 25 % Methanotroph — Poorly characterized genetics but superior potential yield in addition to low-cost NG feedstock Yeast or E. coli — Despite limited potential yield both are utilized for biofuels given well understood genetics © 2015 Intrexon Corp. All rights reserved.
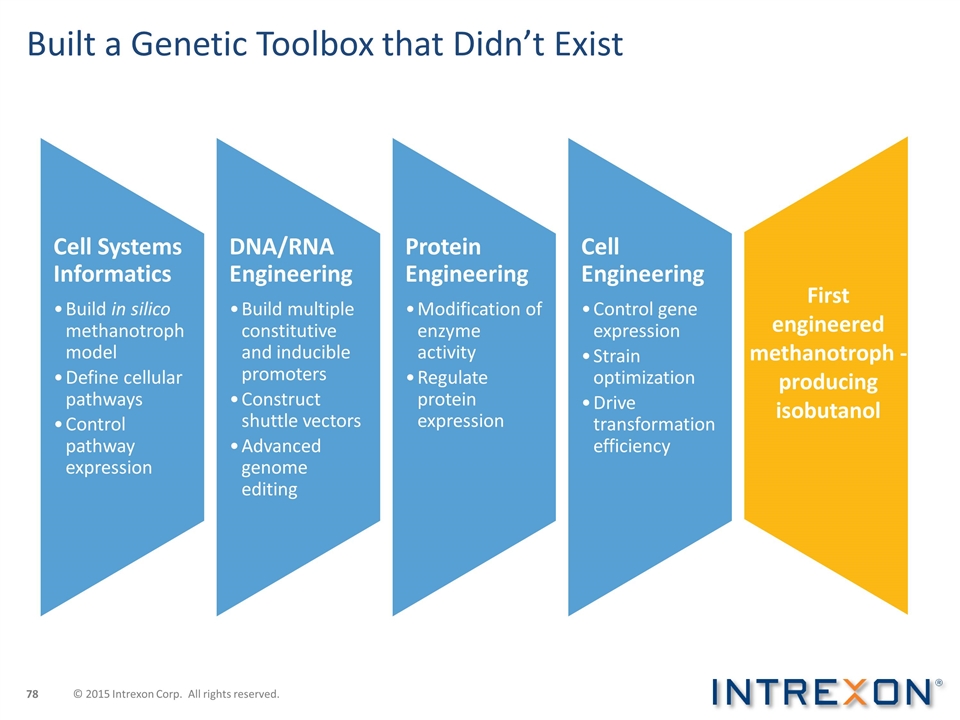
Built a Genetic Toolbox that Didn’t Exist Cell Systems Informatics Build in silico methanotroph model Define cellular pathways Control pathway expression DNA/RNA Engineering Build multiple constitutive and inducible promoters Construct shuttle vectors Advanced genome editing Protein Engineering Modification of enzyme activity Regulate protein expression Cell Engineering Control gene expression Strain optimization Drive transformation efficiency First engineered methanotroph -producing isobutanol © 2015 Intrexon Corp. All rights reserved.
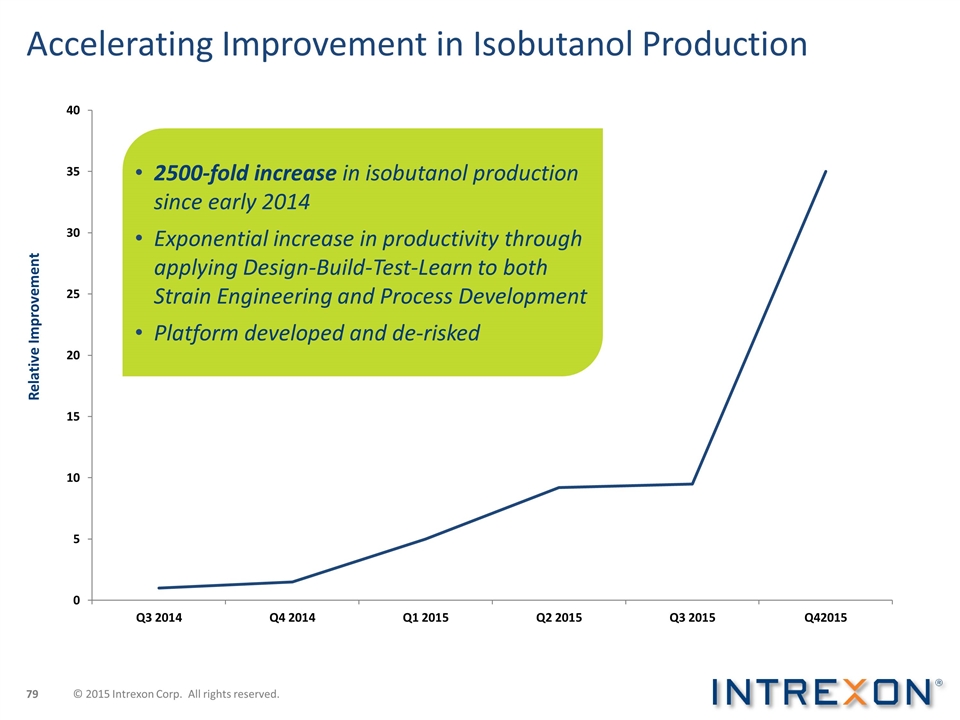
Accelerating Improvement in Isobutanol Production Relative Improvement 2500-fold increase in isobutanol production since early 2014 Exponential increase in productivity through applying Design-Build-Test-Learn to both Strain Engineering and Process Development Platform developed and de-risked © 2015 Intrexon Corp. All rights reserved.
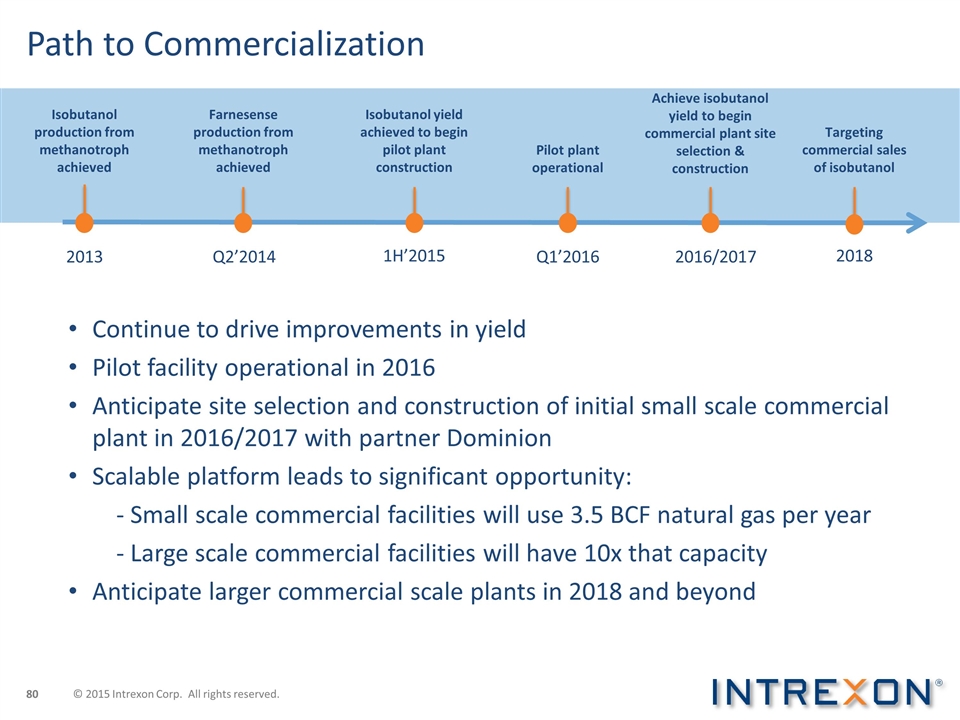
Path to Commercialization Continue to drive improvements in yield Pilot facility operational in 2016 Anticipate site selection and construction of initial small scale commercial plant in 2016/2017 with partner Dominion Scalable platform leads to significant opportunity: - Small scale commercial facilities will use 3.5 BCF natural gas per year - Large scale commercial facilities will have 10x that capacity Anticipate larger commercial scale plants in 2018 and beyond Isobutanol production from methanotroph achieved 2013 Farnesense production from methanotroph achieved Isobutanol yield achieved to begin pilot plant construction Q2’2014 1H’2015 Pilot plant operational Q1’2016 2016/2017 Achieve isobutanol yield to begin commercial plant site selection & construction 2018 Targeting commercial sales of isobutanol © 2015 Intrexon Corp. All rights reserved.
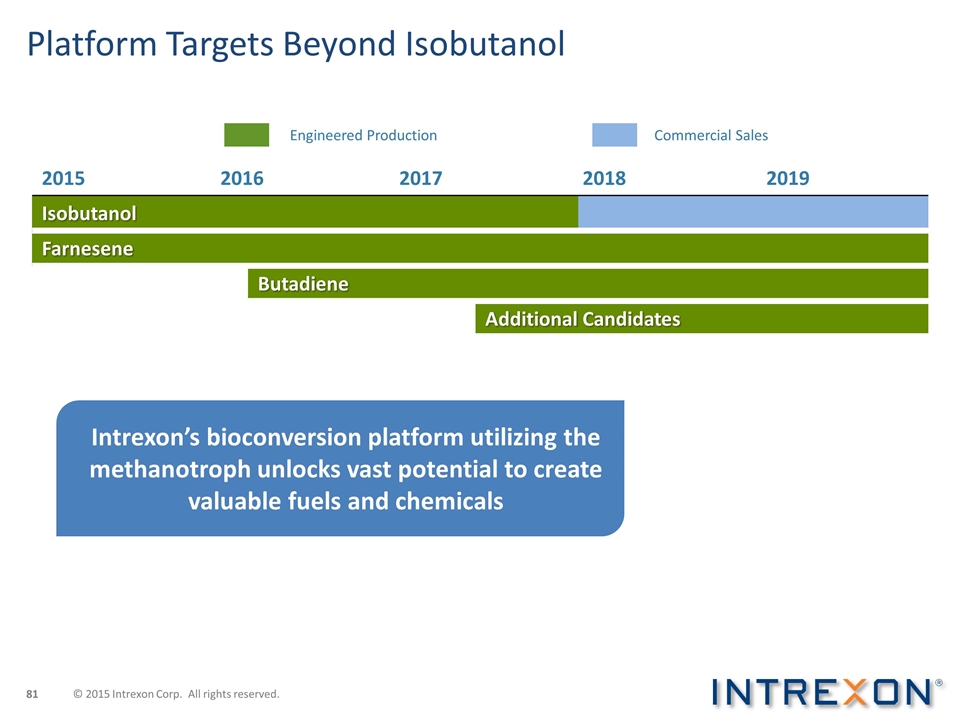
Platform Targets Beyond Isobutanol 2015 2016 2017 2018 2019 Isobutanol Farnesene Butadiene Additional Candidates Engineered Production Commercial Sales Intrexon’s bioconversion platform utilizing the methanotroph unlocks vast potential to create valuable fuels and chemicals © 2015 Intrexon Corp. All rights reserved.

Driving Innovation in Food and Agriculture Corey Huck Senior Vice President, Food Sector

Global Population Is the Growth Engine of Food Sector 9B 2050 7B 2015 © 2015 Intrexon Corp. All rights reserved.

Accelerating Food and Agricultural Innovation CONFIDENTIAL 75% More Food By 2050 Continued innovation required to meet population growth and market demands $797B Animal Protein Market by 2050* * FAO World Livestock 2011 © 2015 Intrexon Corp. All rights reserved.
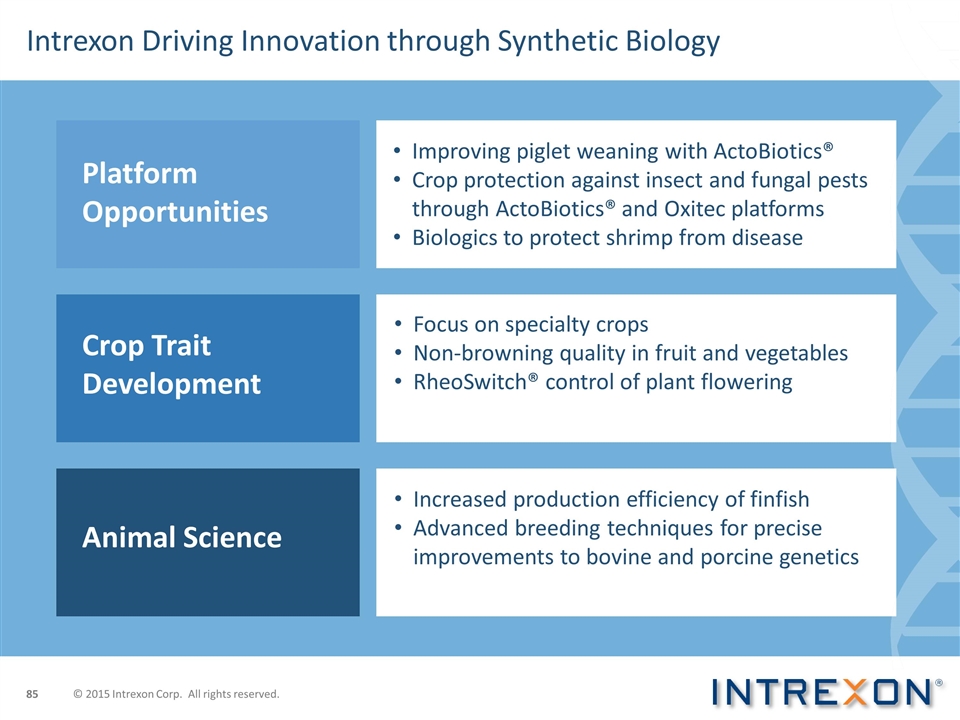
Platform Opportunities Crop Trait Development Animal Science Improving piglet weaning with ActoBiotics® Crop protection against insect and fungal pests through ActoBiotics® and Oxitec platforms Biologics to protect shrimp from disease Focus on specialty crops Non-browning quality in fruit and vegetables RheoSwitch® control of plant flowering Increased production efficiency of finfish Advanced breeding techniques for precise improvements to bovine and porcine genetics Intrexon Driving Innovation through Synthetic Biology © 2015 Intrexon Corp. All rights reserved.

Building Market Leadership Position Game changing tech Drive consumer benefit Create new categories Drive participation across produce verticals Leading reproductive technologies Multiply elite genetics Expand to porcine Enable more protein Safe and sustainable Improve productivity Accelerate current category growth More for less © 2015 Intrexon Corp. All rights reserved.

Driving unparalleled productivity advancements for efficient, high-quality food production David Faber, DVM President CEO of Trans Ova
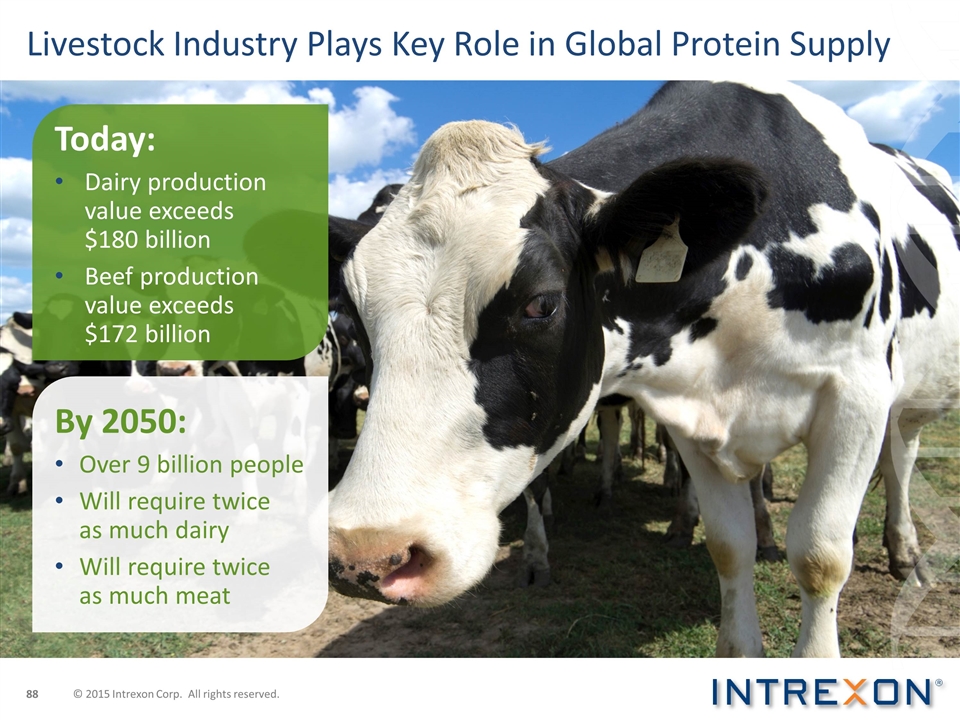
Livestock Industry Plays Key Role in Global Protein Supply Today: Dairy production value exceeds $180 billion Beef production value exceeds $172 billion By 2050: Over 9 billion people Will require twice as much dairy Will require twice as much meat © 2015 Intrexon Corp. All rights reserved.

Trans Ova #1 Driver of Bovine Genetics in North America Trans Ova at forefront of expanding genetic gains through elite cows Preeminent market share in IVF and Embryo Transfer Largest supplier/producer of bovine embryos in the U.S. Produces elite dairy heifers and top breeding bulls © 2015 Intrexon Corp. All rights reserved.
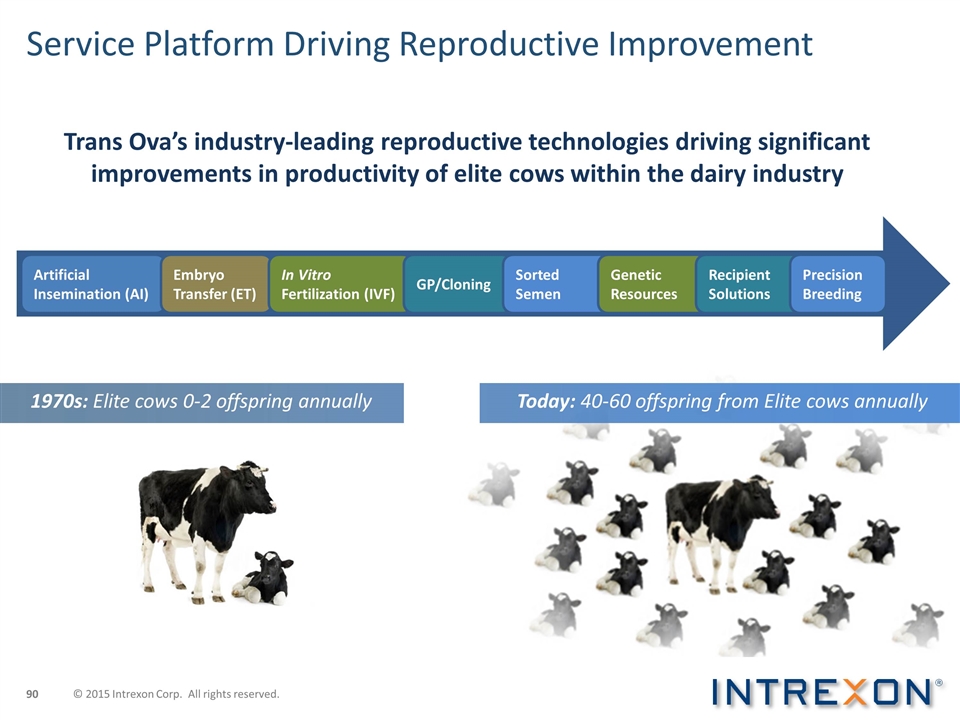
Service Platform Driving Reproductive Improvement 1970s: Elite cows 0-2 offspring annually Today: 40-60 offspring from Elite cows annually Trans Ova’s industry-leading reproductive technologies driving significant improvements in productivity of elite cows within the dairy industry Artificial Insemination (AI) Embryo Transfer (ET) In Vitro Fertilization (IVF) GP/Cloning Sorted Semen Genetic Resources Recipient Solutions Precision Breeding © 2015 Intrexon Corp. All rights reserved.
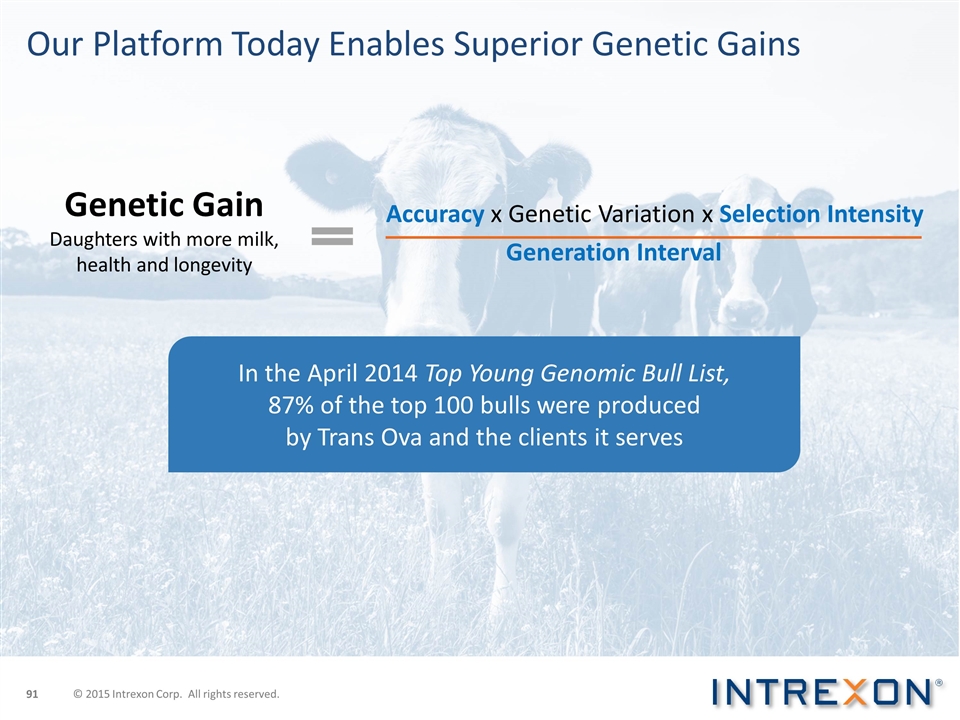
In the April 2014 Top Young Genomic Bull List, 87% of the top 100 bulls were produced by Trans Ova and the clients it serves Our Platform Today Enables Superior Genetic Gains Genetic Gain Daughters with more milk, health and longevity Accuracy x Genetic Variation x Selection Intensity Generation Interval © 2015 Intrexon Corp. All rights reserved.
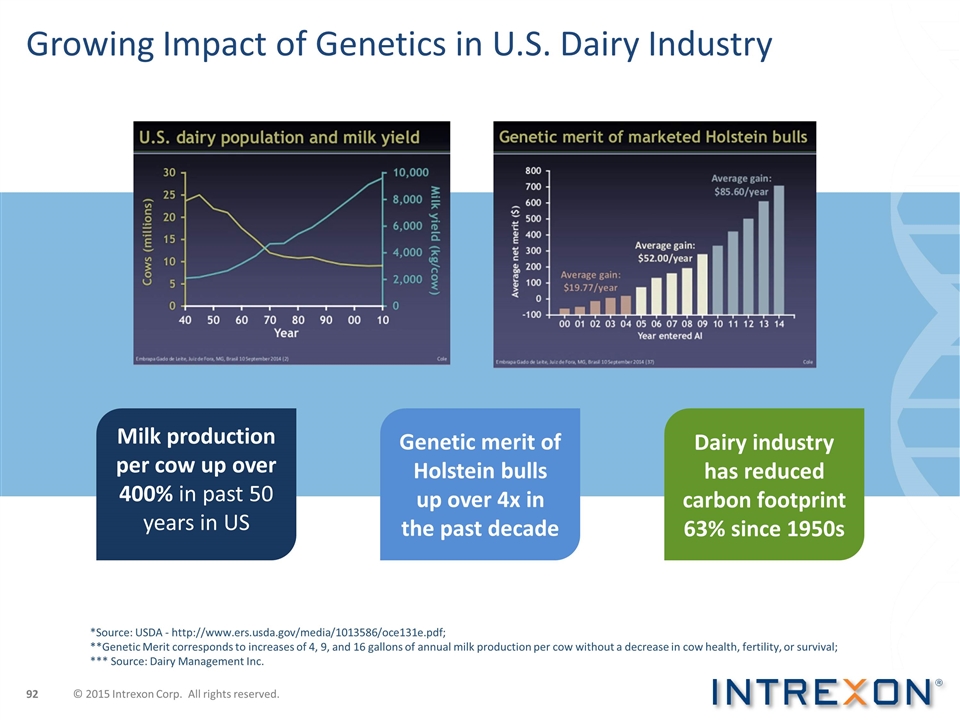
Growing Impact of Genetics in U.S. Dairy Industry *Source: USDA - http://www.ers.usda.gov/media/1013586/oce131e.pdf; **Genetic Merit corresponds to increases of 4, 9, and 16 gallons of annual milk production per cow without a decrease in cow health, fertility, or survival; *** Source: Dairy Management Inc. Milk production per cow up over 400% in past 50 years in US Dairy industry has reduced carbon footprint 63% since 1950s Genetic merit of Holstein bulls up over 4x in the past decade © 2015 Intrexon Corp. All rights reserved.
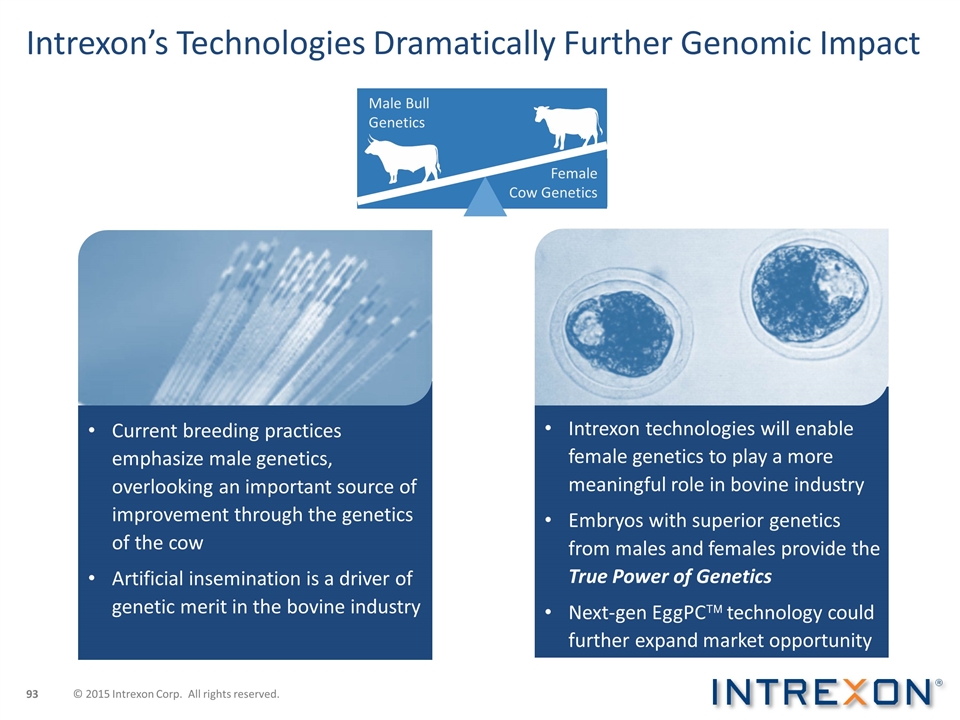
Intrexon’s Technologies Dramatically Further Genomic Impact Current breeding practices emphasize male genetics, overlooking an important source of improvement through the genetics of the cow Artificial insemination is a driver of genetic merit in the bovine industry Intrexon technologies will enable female genetics to play a more meaningful role in bovine industry Embryos with superior genetics from males and females provide the True Power of Genetics Next-gen EggPCä technology could further expand market opportunity Male Bull Genetics Female Cow Genetics © 2015 Intrexon Corp. All rights reserved.
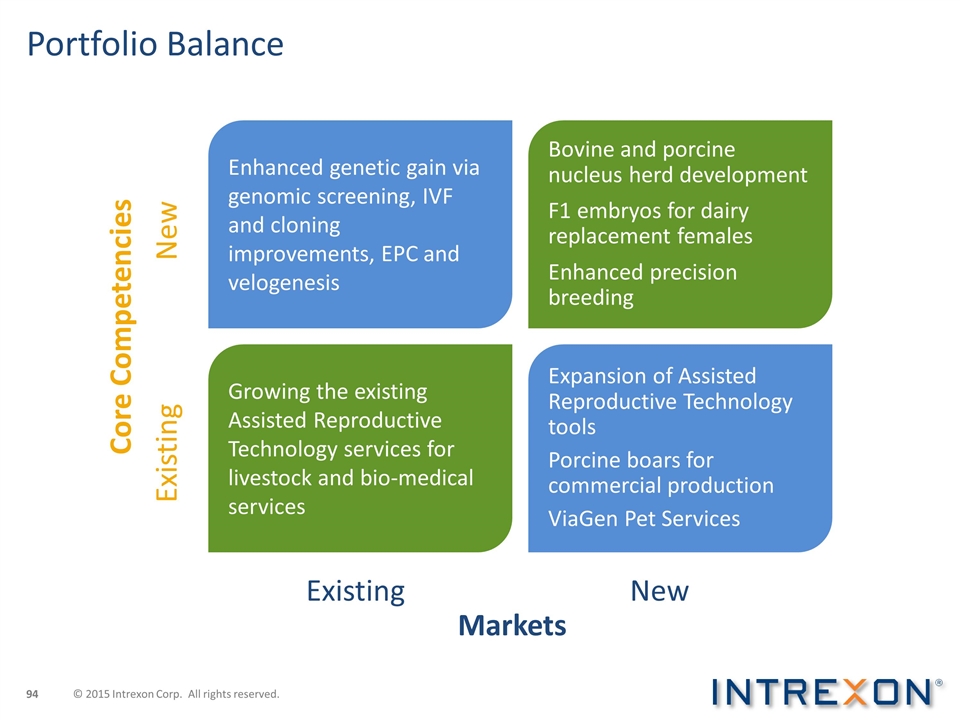
Portfolio Balance Existing New Core Competencies Markets Existing New Enhanced genetic gain via genomic screening, IVF and cloning improvements, EPC and velogenesis Bovine and porcine nucleus herd development F1 embryos for dairy replacement females Enhanced precision breeding Growing the existing Assisted Reproductive Technology services for livestock and bio-medical services Expansion of Assisted Reproductive Technology tools Porcine boars for commercial production ViaGen Pet Services © 2015 Intrexon Corp. All rights reserved.
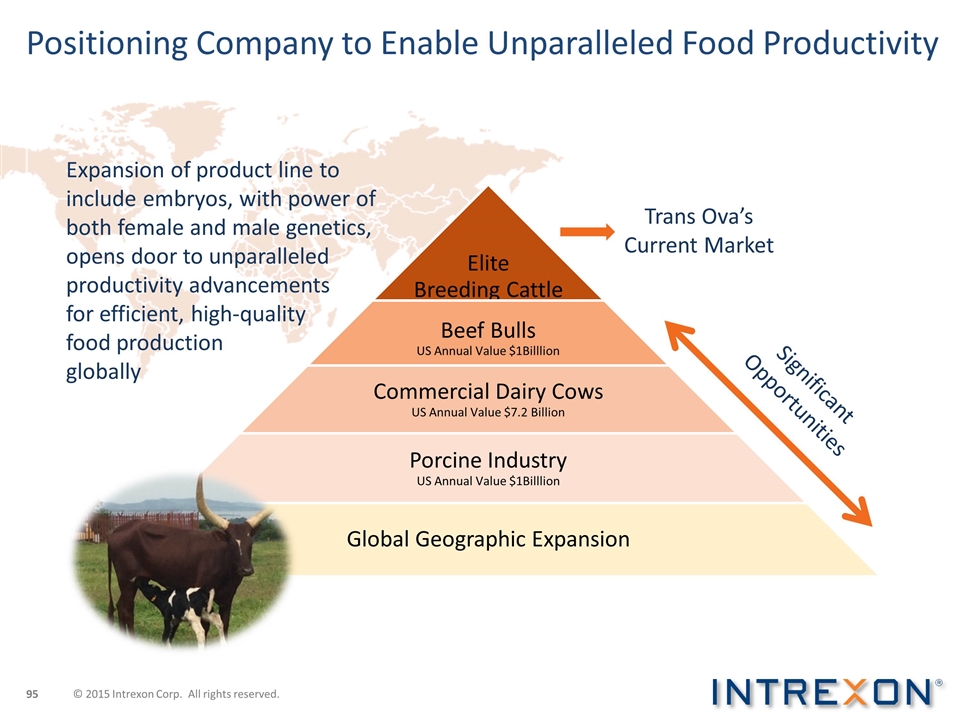
Positioning Company to Enable Unparalleled Food Productivity Expansion of product line to include embryos, with power of both female and male genetics, opens door to unparalleled productivity advancements for efficient, high-quality food production globally Significant Opportunities Trans Ova’s Current Market © 2015 Intrexon Corp. All rights reserved. Elite Breeding Cattle Beef Bulls US Annual Value $1Billlion Commercial Dairy Cows US Annual Value $7.2 Billion Porcine Industry US Annual Value $1Billlion Global Geographic Expansion

NON-BROWNING ARCTIC® APPLES A Game-Changer for Fresh Produce Neal Carter President & Founder, Okanagan Specialty Fruits
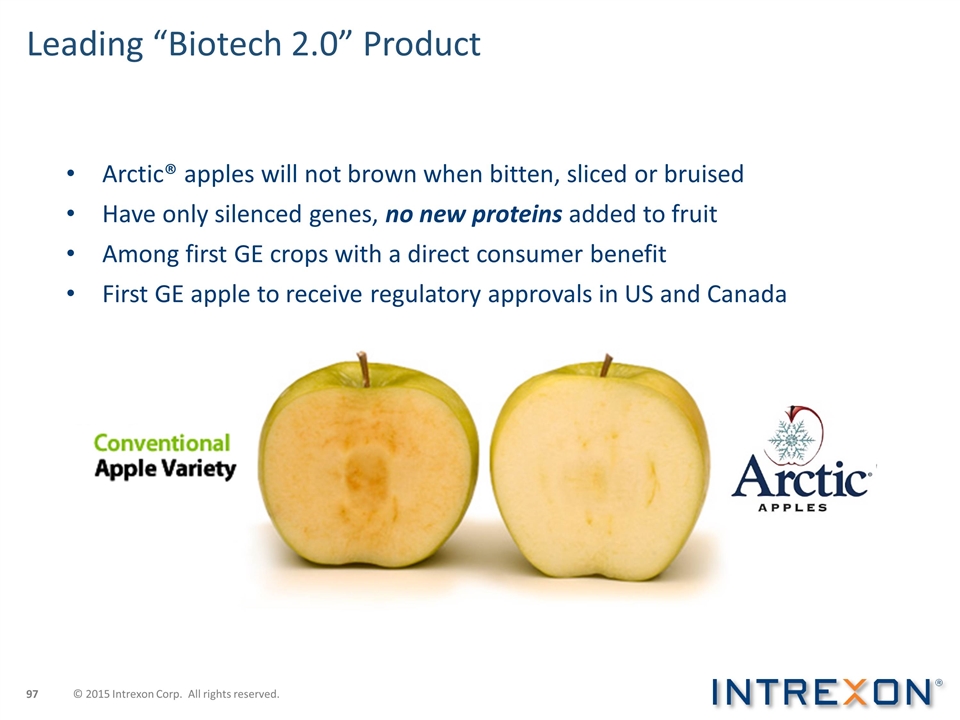
Leading “Biotech 2.0” Product Arctic® apples will not brown when bitten, sliced or bruised Have only silenced genes, no new proteins added to fruit Among first GE crops with a direct consumer benefit First GE apple to receive regulatory approvals in US and Canada © 2015 Intrexon Corp. All rights reserved.
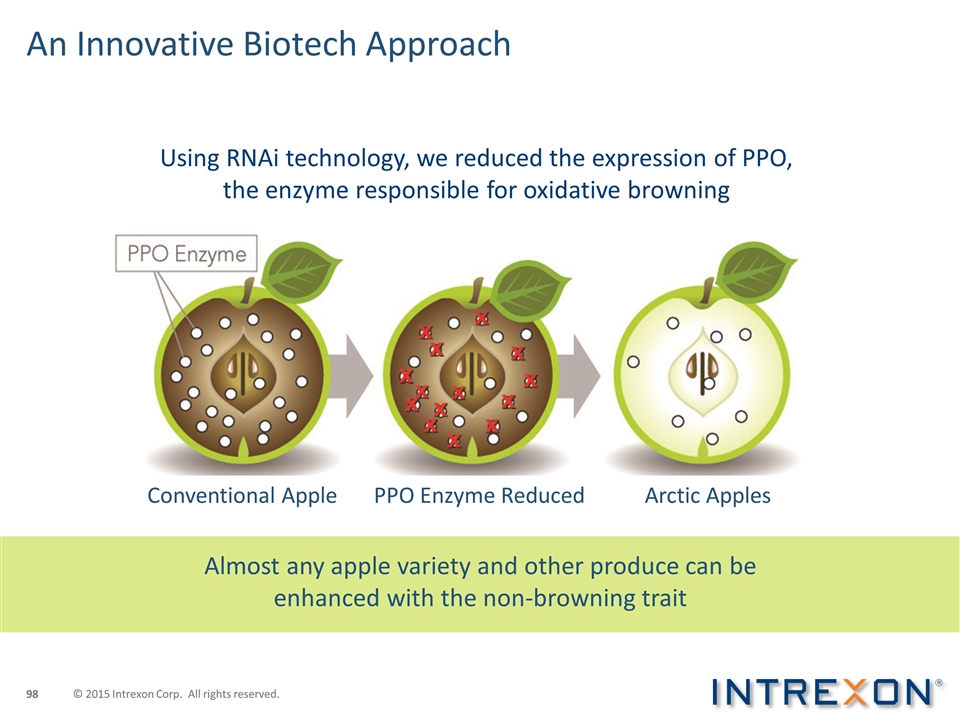
An Innovative Biotech Approach Using RNAi technology, we reduced the expression of PPO, the enzyme responsible for oxidative browning Almost any apple variety and other produce can be enhanced with the non-browning trait Conventional Apple PPO Enzyme Reduced Arctic Apples © 2015 Intrexon Corp. All rights reserved.
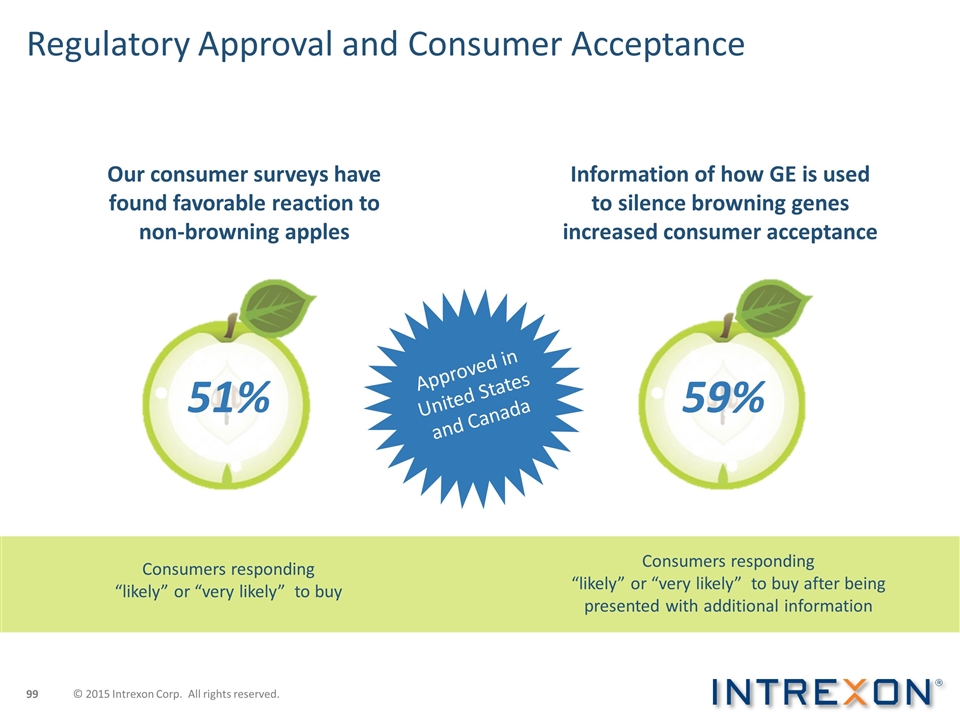
Approved in United States and Canada Regulatory Approval and Consumer Acceptance Our consumer surveys have found favorable reaction to non-browning apples Information of how GE is used to silence browning genes increased consumer acceptance 51% 59% Consumers responding “likely” or “very likely” to buy Consumers responding “likely” or “very likely” to buy after being presented with additional information

Non-Browning Benefits Across Supply Chain 55% of consumers consider browning to be a significant issue1 Per capita U.S. apple consumption declined 20% between 1991 and 20112; this trend continues At least 40% of apples produced are never consumed; among most wasted foods3 1OSF (2013) 2USDA ERS (2012) 3Tesco (2013) © 2015 Intrexon Corp. All rights reserved.
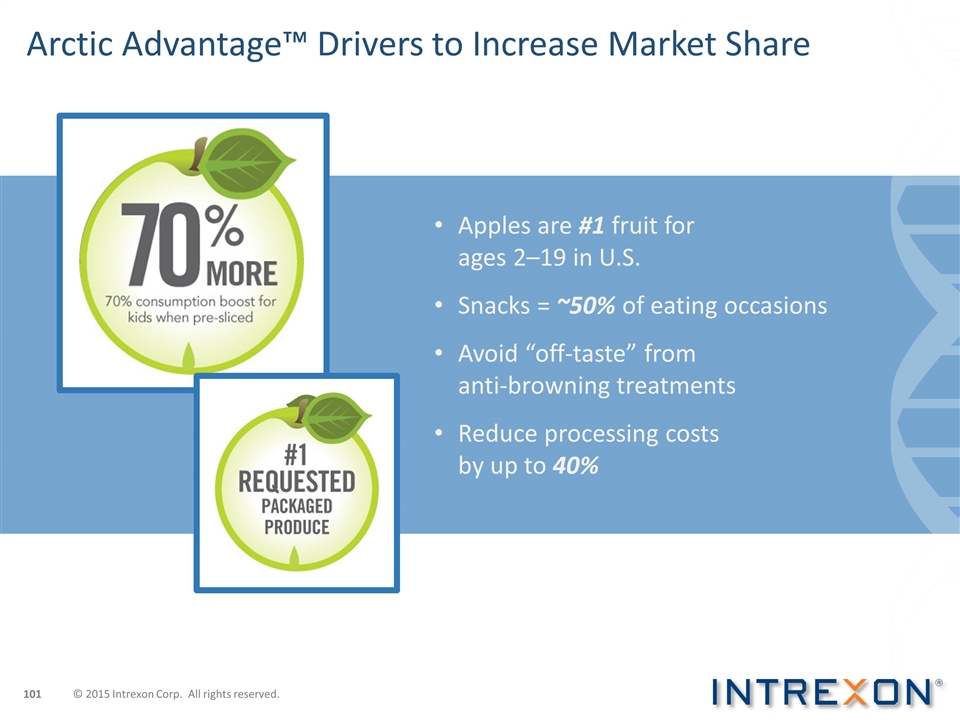
Arctic Advantage™ Drivers to Increase Market Share Apples are #1 fruit for ages 2–19 in U.S. Snacks = ~50% of eating occasions Avoid “off-taste” from anti-browning treatments Reduce processing costs by up to 40% © 2015 Intrexon Corp. All rights reserved.
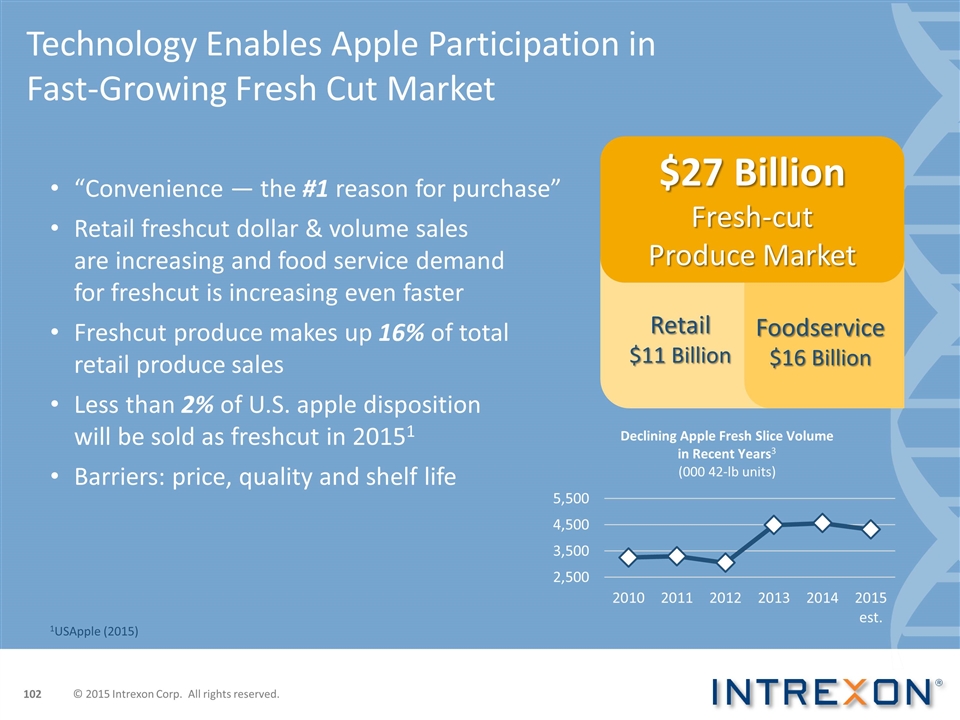
Technology Enables Apple Participation in Fast-Growing Fresh Cut Market “Convenience — the #1 reason for purchase” Retail freshcut dollar & volume sales are increasing and food service demand for freshcut is increasing even faster Freshcut produce makes up 16% of total retail produce sales Less than 2% of U.S. apple disposition will be sold as freshcut in 20151 Barriers: price, quality and shelf life $27 Billion Fresh-cut Produce Market Retail $11 Billion Foodservice $16 Billion 1USApple (2015) © 2015 Intrexon Corp. All rights reserved.

Arctic® Apples – Creating A New Food Category Arctic® Leather Arctic® Leather Arctic® Apple Slices Arctic® Smoothie

Arctic® Platform Beyond Apples Arctic® platform will create consumption and profit triggers in untapped markets The non-browning trait will allow for new innovative fresh apple products Consumer acceptance helps open the door for the use of biotech in specialty crops Intrexon is expanding the Arctic® platform and currently developing additional Arctic® apple varieties; plus, non-browning avocados, lettuce, pears and cherries. Other traits and products are in the pipeline © 2015 Intrexon Corp. All rights reserved.
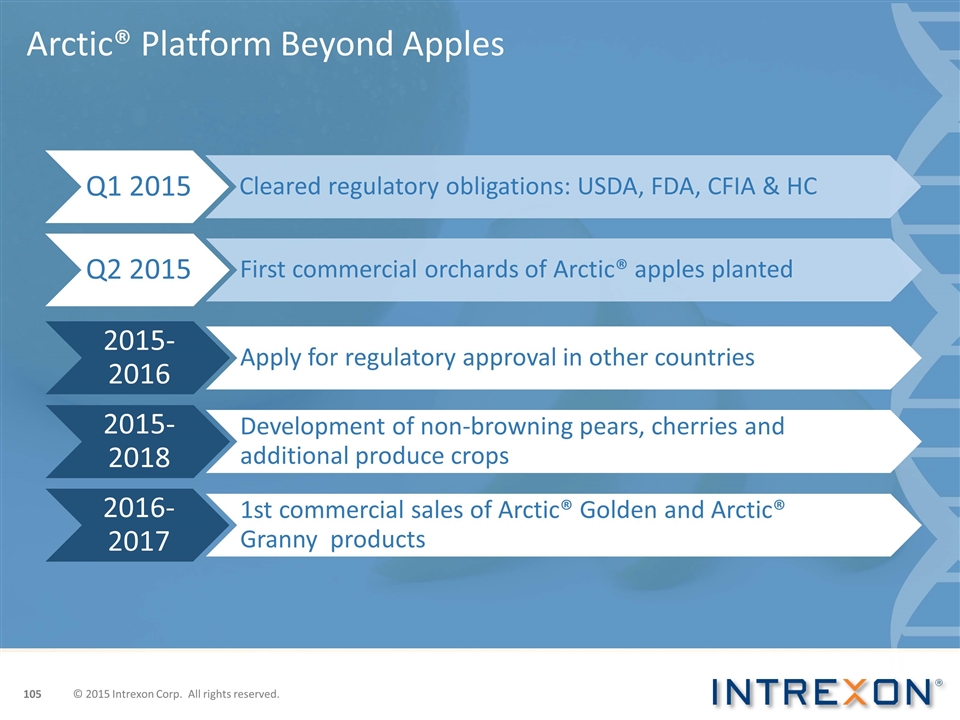
Arctic® Platform Beyond Apples © 2015 Intrexon Corp. All rights reserved. Q1 2015 Cleared regulatory obligations: USDA, FDA, CFIA & HC Q2 2015 First commercial orchards of Arctic ® apples planted Apply for regulatory approval in other countries 2015-2018 Development of non-browning pears, cherries and additional produce crops 2015-2016 2016-2017 1st commercial sales of Arctic ® Golden and Arctic ® Granny products

Environmentally Friendly Insect Control Solutions for Major Global Disease and Agricultural Challenges Hadyn Parry Chief Executive Officer, Oxitec

The Challenge: Mosquito Borne Diseases “Today, dengue ranks as the most important mosquito-borne viral disease in the world. Everywhere the human and economic costs are staggering” Dr Margaret Chan, 2012 Director General, WHO Dengue fever © 2015 Intrexon Corp. All rights reserved.

Our Enemy © 2015 Intrexon Corp. All rights reserved.
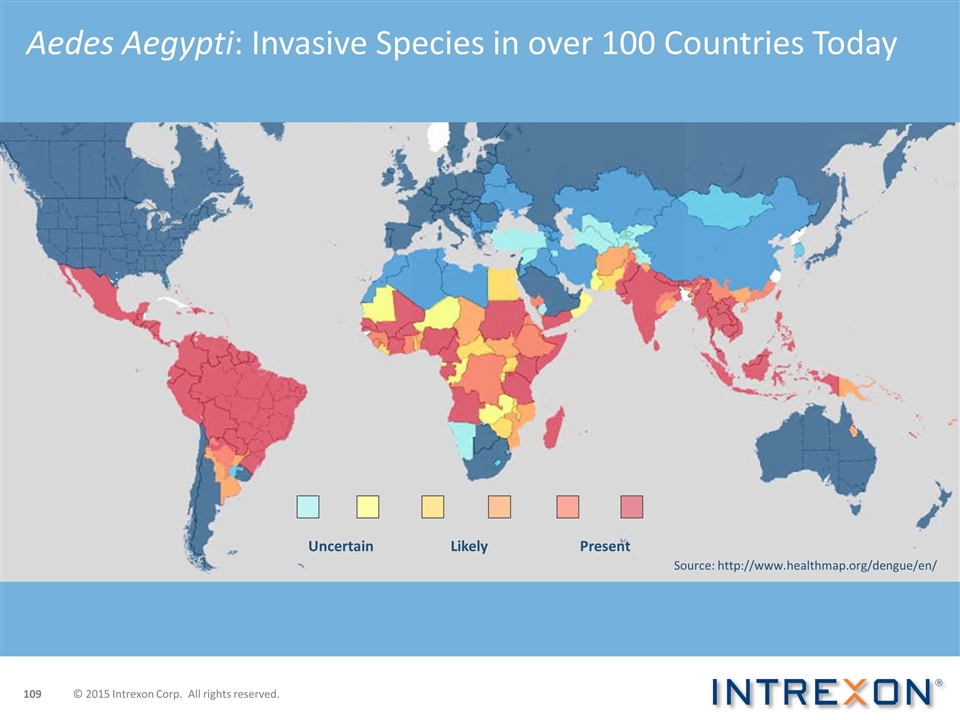
Aedes Aegypti: Invasive Species in over 100 Countries Today Uncertain Likely Present Source: http://www.healthmap.org/dengue/en/ © 2015 Intrexon Corp. All rights reserved.

Current ‘Modern’ Practice © 2015 Intrexon Corp. All rights reserved.

Our Solution © 2015 Intrexon Corp. All rights reserved.
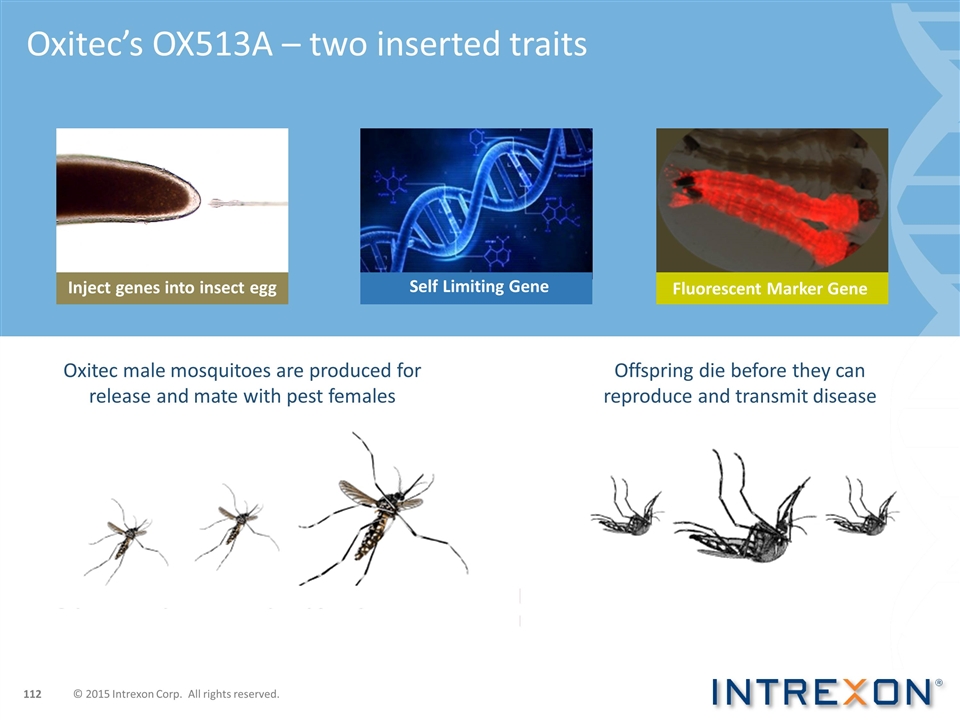
Self Limiting Gene Oxitec’s OX513A – two inserted traits Fluorescent Marker Gene Oxitec male mosquitoes are produced for release and mate with pest females Offspring die before they can reproduce and transmit disease Inject genes into insect egg © 2015 Intrexon Corp. All rights reserved.

Production and Release More than 100 million Oxitec mosquitoes released worldwide © 2015 Intrexon Corp. All rights reserved.
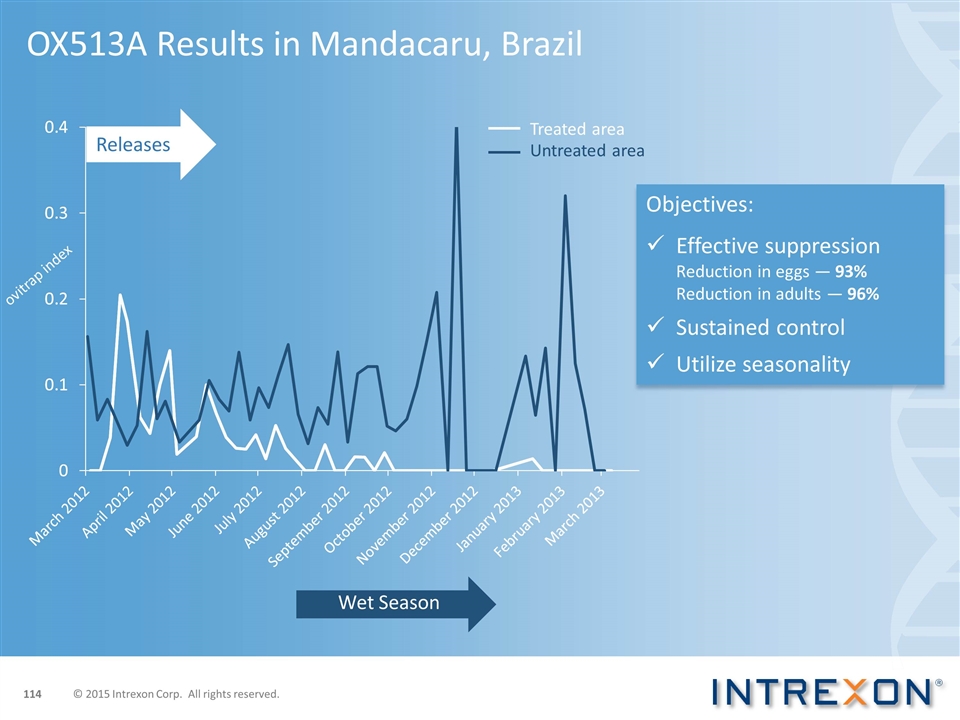
OX513A Results in Mandacaru, Brazil Objectives: Effective suppression Reduction in eggs — 93% Reduction in adults — 96% Sustained control Utilize seasonality Releases ovitrap index Treated area Untreated area Wet Season © 2015 Intrexon Corp. All rights reserved.
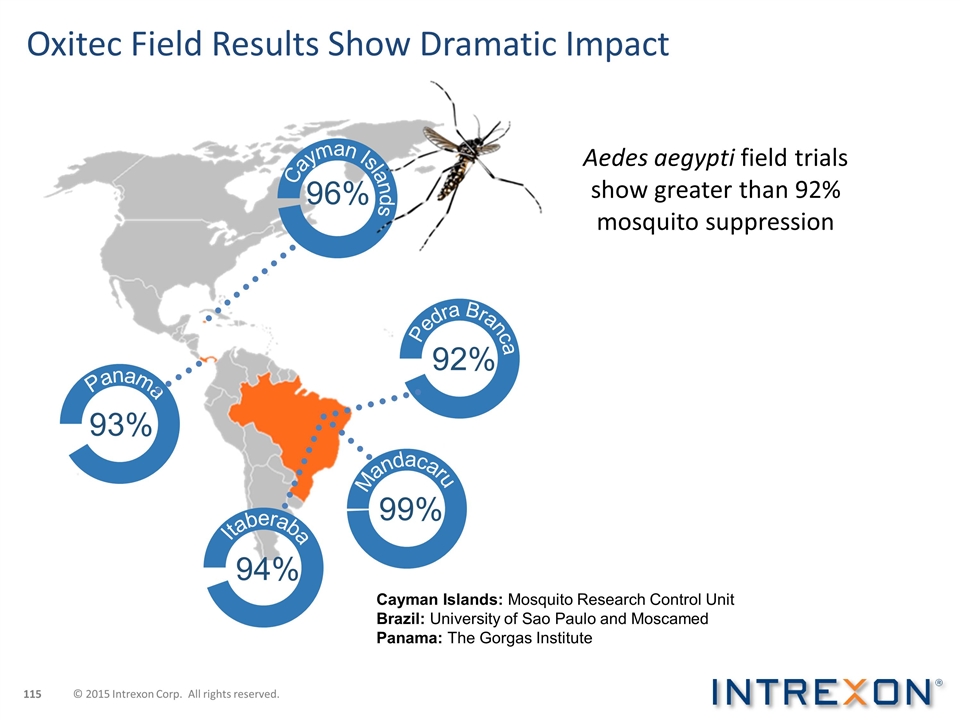
Oxitec Field Results Show Dramatic Impact Cayman Islands Pedra Branca Mandacaru Itaberaba Panama 96% 92% 99% 94% 93% Aedes aegypti field trials show greater than 92% mosquito suppression Cayman Islands: Mosquito Research Control Unit Brazil: University of Sao Paulo and Moscamed Panama: The Gorgas Institute © 2015 Intrexon Corp. All rights reserved.

Large Opportunities in Agriculture 40% Global Food Production Lost to Insect Related Crop Damage $11 Billion Spent Annually on Conventional Insecticides © 2015 Intrexon Corp. All rights reserved.

Brazil: A Case Study Early 1970s No Aedes aegypti No dengue Brazilian press describe our product as Aedes aegypti do bem: ‘the good mosquito’ 96% of local Piracicaba residents support the Oxitec program Recent years 0.5m — 1.5m cases of dengue per year Over $1 billion spent on the dengue vector control program annually Hundreds of deaths Chikungunya and Zika virus entering Oxitec Trials 2011–13 National Biosafety Approval granted Oxitec do Brasil established Piracicaba — world’s first municipal partnership releasing Oxitec mosquitoes © 2015 Intrexon Corp. All rights reserved.

Synergistic Solutions with Value in Large, Growing Markets Adjacent Market Examples Losses due to insects Cattle flies $3.6B Pig house flies$0.3B Culex $1.0B Agriculture Insecticide market $11B Losses due to insects DBM$ 4B Corn earworm$14B Soya stinkbugs$ 7B 1.http://www.idcostcalc.org/contents/dengue/index.html In addition to OX513A for dengue, Oxitec is working on new solutions for additional mosquito-borne diseases and has more than five of the most damaging agricultural pests in various stages of development including field trials. Health — Dengue Vector control and community$5B Productivity loss1 $6B Healthcare1 $5B Mortality1 $2B Total Impact$17 Billion Before loss of tourism © 2015 Intrexon Corp. All rights reserved.
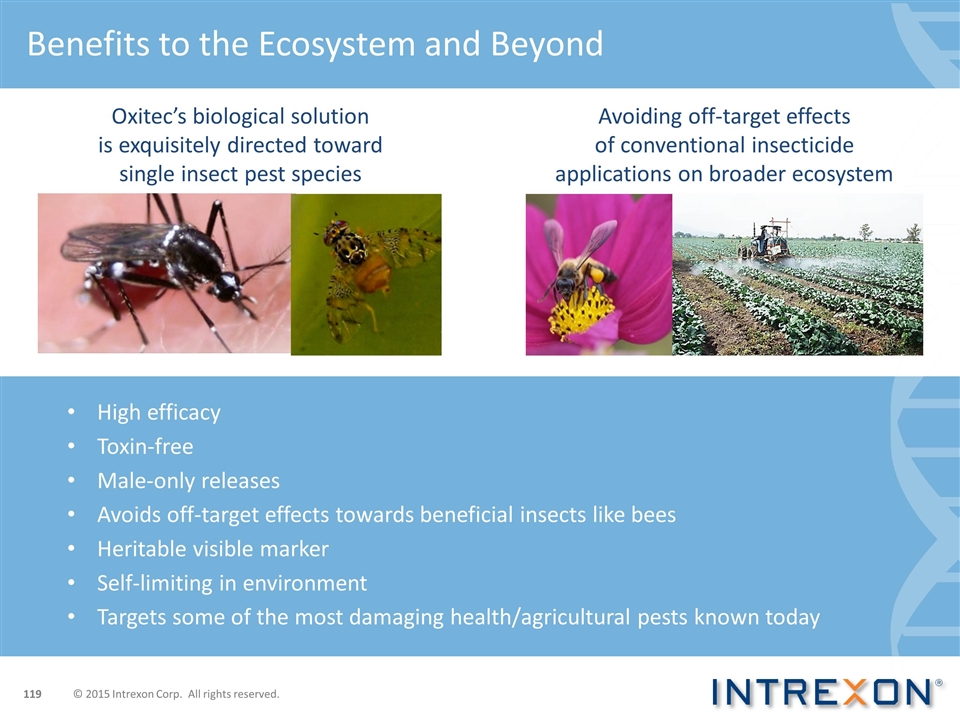
Benefits to the Ecosystem and Beyond Oxitec’s biological solution is exquisitely directed toward single insect pest species High efficacy Toxin-free Male-only releases Avoids off-target effects towards beneficial insects like bees Heritable visible marker Self-limiting in environment Targets some of the most damaging health/agricultural pests known today Avoiding off-target effects of conventional insecticide applications on broader ecosystem © 2015 Intrexon Corp. All rights reserved.

Multiple Paths to Shareholder Value Joel Liffmann Senior Vice President, Finance
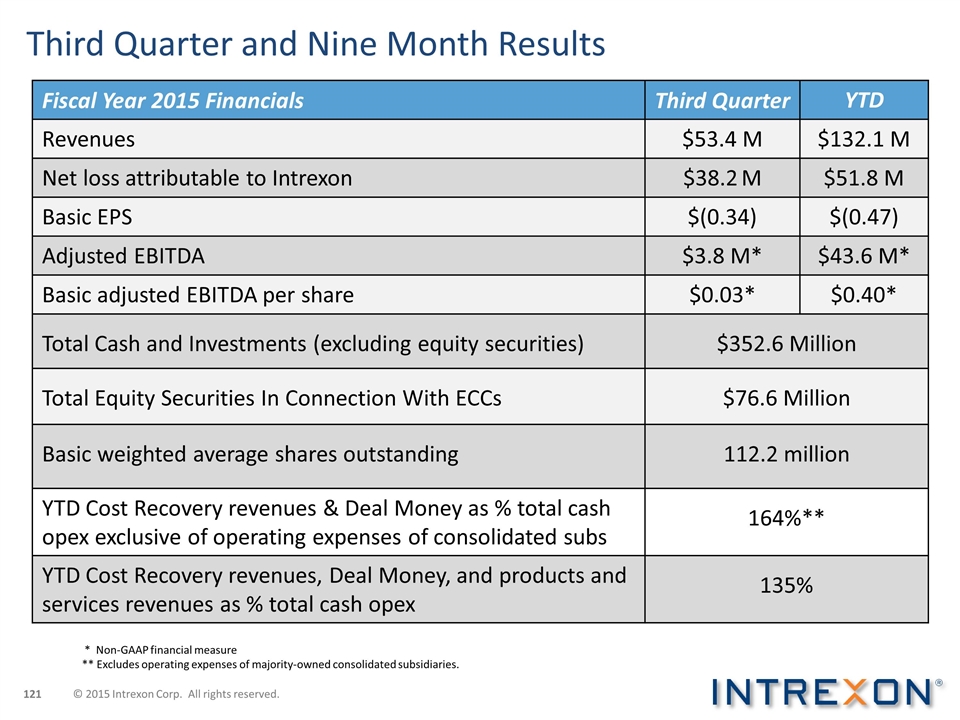
Third Quarter and Nine Month Results Fiscal Year 2015 Financials Third Quarter YTD Revenues $53.4 M $132.1 M Net loss attributable to Intrexon $38.2 M $51.8 M Basic EPS $(0.34) $(0.47) Adjusted EBITDA $3.8 M* $43.6 M* Basic adjusted EBITDA per share $0.03* $0.40* Total Cash and Investments (excluding equity securities) $352.6 Million Total Equity Securities In Connection With ECCs $76.6 Million Basic weighted average shares outstanding 112.2 million YTD Cost Recovery revenues & Deal Money as % total cash opex exclusive of operating expenses of consolidated subs 164%** YTD Cost Recovery revenues, Deal Money, and products and services revenues as % total cash opex 135% * Non-GAAP financial measure ** Excludes operating expenses of majority-owned consolidated subsidiaries. © 2015 Intrexon Corp. All rights reserved.
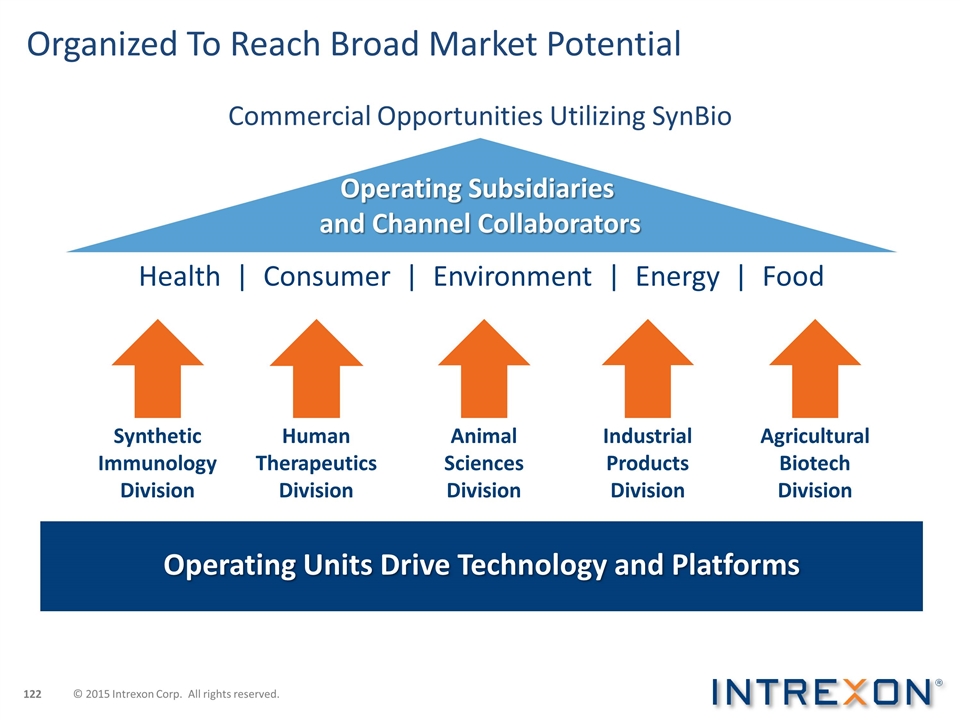
Organized To Reach Broad Market Potential Operating Subsidiaries and Channel Collaborators Operating Units Drive Technology and Platforms Health | Consumer | Environment | Energy | Food Synthetic Immunology Division Agricultural Biotech Division Human Therapeutics Division Animal Sciences Division Industrial Products Division Commercial Opportunities Utilizing SynBio © 2015 Intrexon Corp. All rights reserved.
Flexible and Financially Disciplined Business Model Exclusive Channel Collaborations (ECCs) Joint Ventures (JVs) Start Ups M&A Opportunities © 2015 Intrexon Corp. All rights reserved.
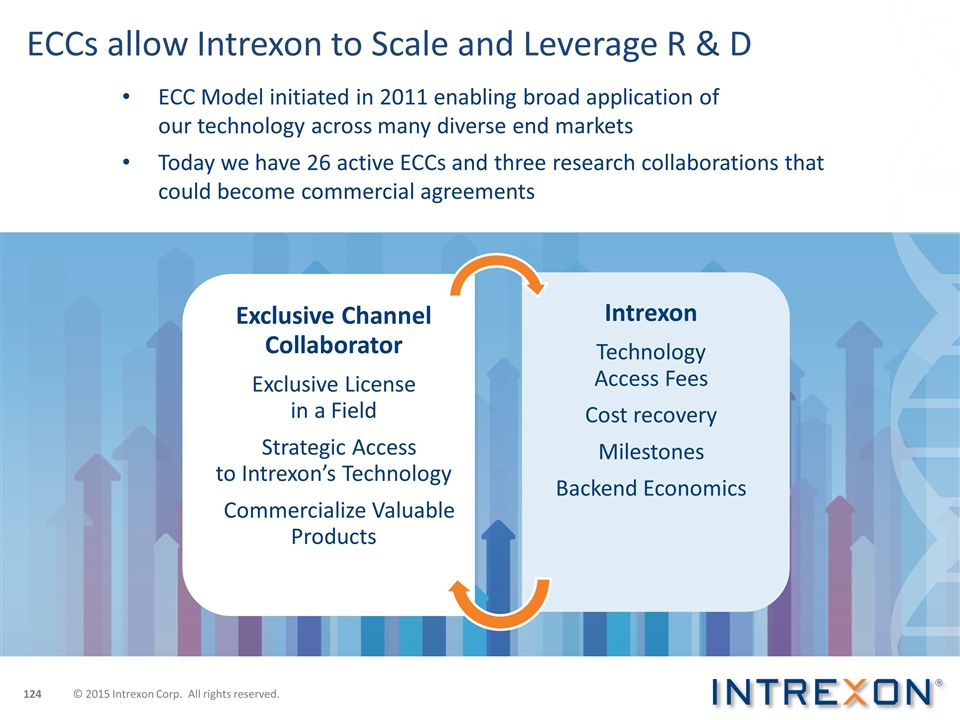
ECCs allow Intrexon to Scale and Leverage R & D ECC Model initiated in 2011 enabling broad application of our technology across many diverse end markets Today we have 26 active ECCs and three research collaborations that could become commercial agreements © 2015 Intrexon Corp. All rights reserved. Exclusive Channel Collaborator Exclusive License in a Field Strategic Access to Intrexon’s Technology Commercialize Valuable Products Intrexon Technology Access Fees Cost recovery Milestones Backend Economics
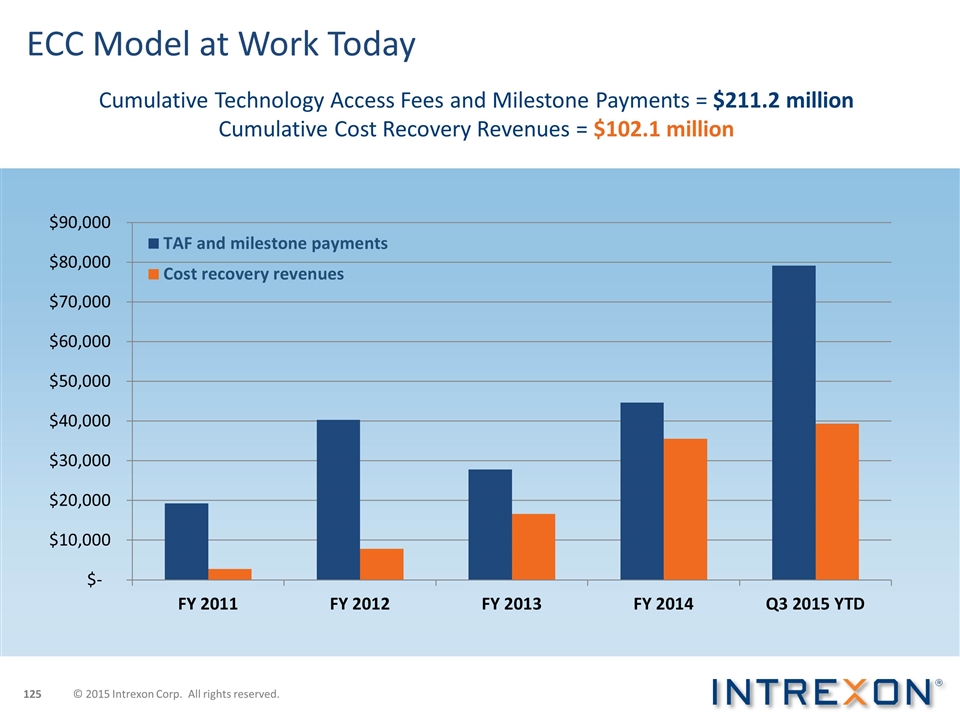
ECC Model at Work Today Cumulative Technology Access Fees and Milestone Payments = $211.2 million Cumulative Cost Recovery Revenues = $102.1 million © 2015 Intrexon Corp. All rights reserved.
ECC Model at Work Tomorrow Growing portfolio of backend economics with royalties ranging from mid-single digits of net sales to 50% of net operating profits © 2015 Intrexon Corp. All rights reserved.

JVs Provide Valuable Future Growth Opportunities Joint Venture Partner / Intrexon Shared Economics Shared Costs Defined Field JV Model initiated in 2014 3 joint ventures today in which we share of operating costs and future profits with our partners © 2015 Intrexon Corp. All rights reserved.
Successful M&A Strategy: Proactive, Deliberate and Focused Target sectors that are positioned to gain from the disruptive potential of bioengineering Identify companies: With attractive stand alone financial and commercial profiles That add complimentary technologies to our resources; and/or That can utilize our existing technologies and resources and can provide access to large end market opportunities Market Leadership Position Synergistic Large Market Opportunities Paradigm Shift Potential © 2015 Intrexon Corp. All rights reserved.

Start Up Investments: Visibility to Next-Gen Opportunities First ECC: Thrive Agrobiotics, Inc., Sept 2015 Why: Thrive will access Intrexon’s ActoBiotics® platform to develop animal feed supplements Market need: Pork is the most consumed meat in the world today, playing an important role in the supply of animal protein to humans Goal: Promote growth performance, feed efficiency and a more sustainable food supply of porcine animals Harvest Capital Strategies LLC Investment Fund Fund dedicated to the inventions and discoveries of a single company, Intrexon Intrexon will provide proposals across its five sectors that are suitable for pursuit by a start up Intrexon currently sees potential to form up to ten new companies per year © 2015 Intrexon Corp. All rights reserved.
Summary Intrexon is well positioned to effectively capitalize on the opportunities within Synthetic Biology Capital efficient ECC model lowers risk and delivers shareholder value Strong financial position to execute strategic plan Significant potential for growth Disciplined business model and approach to efficiently capture market share across sizable markets © 2015 Intrexon Corp. All rights reserved. First mover advantage Best-in-class human capital in synbio Deliver high value for partners Uniquely diversified portfolio Capital Efficient Model

INVESTOR DAY | NOVEMBER 12, 2015


































































































































