
May 10th, 2017 First Quarter 2017 Financial Results and Business Update Exhibit 99.2

Safe Harbor Statement Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the Safe harbor Provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s current and future ECCs and joint ventures; (ii) Intrexon’s ability to successfully enter new markets or develop additional products, whether with its collaborators or independently; (iii) actual or anticipated variations in Intrexon’s operating results; (iv) actual or anticipated fluctuations in Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (v) Intrexon’s cash position; (vi) market conditions in Intrexon’s industry; (vii) the volatility of Intrexon’s stock price; (viii) Intrexon’s ability, and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (ix) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations and policies; (x) the outcomes of pending and future litigation; (xi) the rate and degree of market acceptance of any products developed by a collaborator under an ECC or through a joint venture; (xii) Intrexon’s ability to retain and recruit key personnel; (xiii) Intrexon’s expectations related to the use of proceeds from its public offerings and other financing efforts; (xiv) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xv) Intrexon’s expectations relating to its subsidiaries and other affiliates. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the forward-looking statements, see the section entitled ”Risk Factors“ in Intrexon’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Intrexon’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law. Non-GAAP Financial Measures This presentation presents Adjusted EBITDA, which are non-GAAP financial measures within the meaning of applicable rules and regulations of the Securities and Exchange Commission (SEC). For a reconciliation of Adjusted EBITDA to net loss attributable to Intrexon in accordance with generally accepted accounting principles and for a discussion of the reasons why the company believes that these non-GAAP financial measures provide information that is useful to investors see the tables below under “Reconciliation of GAAP to Non-GAAP Measures.” Such information is provided as additional information, not as an alternative to Intrexon’s consolidated financial statements presented in accordance with GAAP, and is intended to enhance an overall understanding of the Company’s current financial performance. © 2017 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon. Forward-Looking Statements

Marketable Products & Health Andrew Last, Ph.D. – Chief Operating Officer
The Next-Generation of Industrial Factories Human Platform Plant Platform Animal Platform Bacterial Platform Fungal Platform Insect Platform Multi-Cellular Organisms Microbes Intrexon’s platform encompasses the next-generation of biofactories to tackle numerous challenges & deliver on the promise of synthetic biology

Marketable Products Marketable Products Arctic® Apple First USDA approved non-browning apple without use of chemical or antioxidant treatments AquAdvantage® Salmon First engineered food animal approved by US FDA/Health Canada Friendly™ Aedes Scalable enviro-friendly mosquito solution to suppress the disease-carrying Aedes aegypti

Advantages of the Arctic® Apple Arctic® apples drive benefits across the entire supply chain Convenience for consumer major driver of value in rapidly growing healthy snack market Potential to impact estimated 40% of apples wasted each year Marketable Products
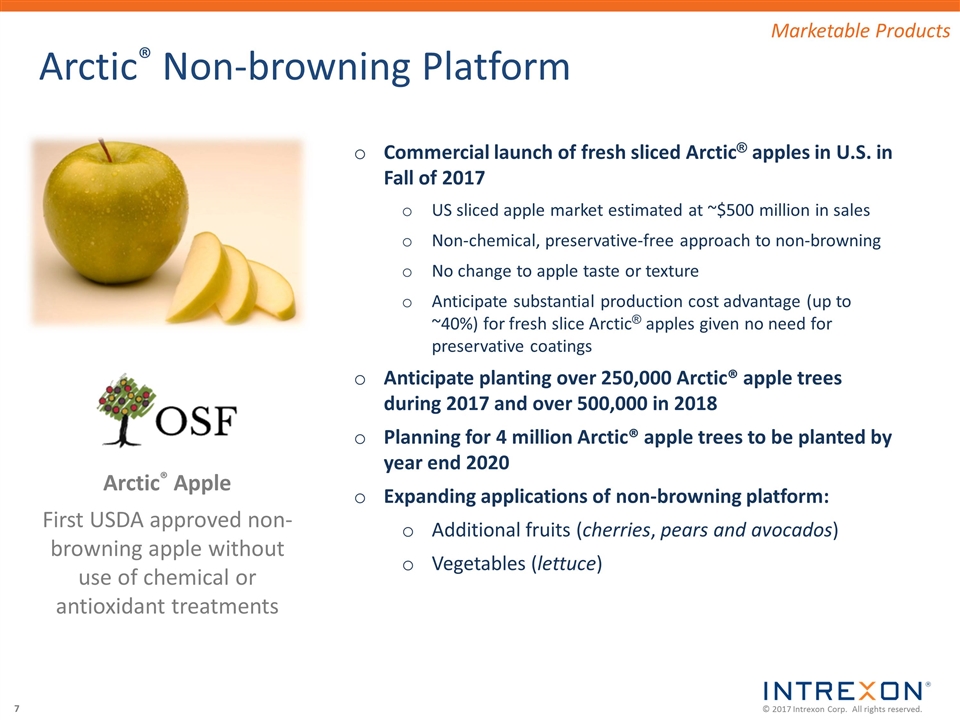
Arctic® Non-browning Platform Marketable Products Arctic® Apple First USDA approved non-browning apple without use of chemical or antioxidant treatments Commercial launch of fresh sliced Arctic® apples in U.S. in Fall of 2017 US sliced apple market estimated at ~$500 million in sales Non-chemical, preservative-free approach to non-browning No change to apple taste or texture Anticipate substantial production cost advantage (up to ~40%) for fresh slice Arctic® apples given no need for preservative coatings Anticipate planting over 250,000 Arctic® apple trees during 2017 and over 500,000 in 2018 Planning for 4 million Arctic® apple trees to be planted by year end 2020 Expanding applications of non-browning platform: Additional fruits (cherries, pears and avocados) Vegetables (lettuce)

Friendly™ Aedes Marketable Products Friendly™ Aedes Scalable enviro-friendly mosquito solution to suppress the disease-carrying Aedes aegypti Ongoing geographic expansion in 2017: India – Initiated outdoor caged trials in India to demonstrate the efficacy of Friendly™ mosquitoes in collaboration with Gangabishan Bhikulal Investment and Trading Limited Colombia - Announced memorandum of understanding to deploy Friendly™ Aedes in the Comuna 16 region, an area of over 104,000 residents, in the Municipality of Santiago de Cali, Colombia Expect to initiate new deployments for Friendly™ Aedes in multiple new countries including the United States Working to broaden engagements in existing countries for Friendly™ Aedes including: Brazil – Expand into new states/cities/municipalities Cayman Islands – Expand to island-wide deployment on Grand Cayman India – Move from large cage to open field trials during late 2017 / early 2018
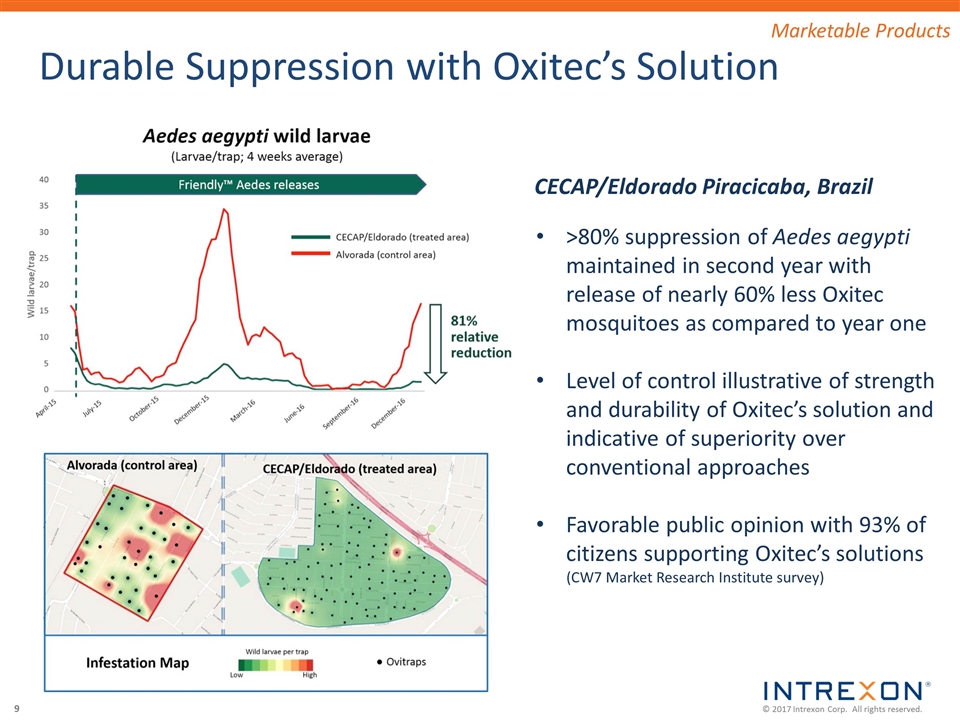
Durable Suppression with Oxitec’s Solution >80% suppression of Aedes aegypti maintained in second year with release of nearly 60% less Oxitec mosquitoes as compared to year one Level of control illustrative of strength and durability of Oxitec’s solution and indicative of superiority over conventional approaches Favorable public opinion with 93% of citizens supporting Oxitec’s solutions (CW7 Market Research Institute survey) CECAP/Eldorado Piracicaba, Brazil Marketable Products

AquAdvantage® Salmon Marketable Products AquAdvantage® Salmon First engineered food animal approved by US FDA/Health Canada Advantages of AquAdvantage® platform Lower production cost driven by faster growth rate and ~25% less feed requirement No vaccines or antibiotics Land-based production enables “production anywhere” Potential for lower supply fluctuations and steady prices Intrexon’s majority-owned subsidiary AquaBounty Technologies, Inc. (NASDAQ: AQB): Completed listing on NASDAQ Intrexon distributed special stock dividend to shareholders while maintaining majority ownership in AquaBounty Atlantic salmon market in U.S. estimated at >$2 billion (~50% food service / ~50% direct retail) Almost all commercially available Atlantic salmon is farmed US alone imports over 200,000 tons of farmed Atlantic Salmon annually
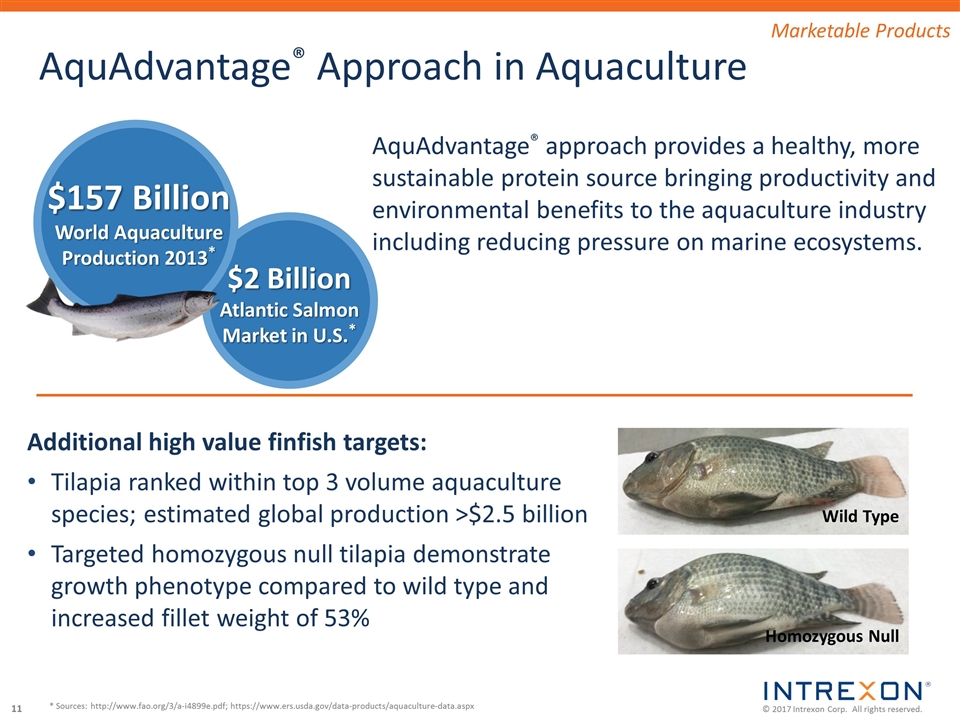
$2 Billion Atlantic Salmon Market in U.S.* AquAdvantage® Approach in Aquaculture $157 Billion World Aquaculture Production 2013* AquAdvantage® approach provides a healthy, more sustainable protein source bringing productivity and environmental benefits to the aquaculture industry including reducing pressure on marine ecosystems. * Sources: http://www.fao.org/3/a-i4899e.pdf; https://www.ers.usda.gov/data-products/aquaculture-data.aspx Additional high value finfish targets: Tilapia ranked within top 3 volume aquaculture species; estimated global production >$2.5 billion Targeted homozygous null tilapia demonstrate growth phenotype compared to wild type and increased fillet weight of 53% Wild Type Homozygous Null Marketable Products

Health Sector & Precigen
Precigen Precigen Precigen: Focused Gene/Cell Therapy Company Autoimmune & Inflammatory Disease Cardiac Disease Cartilage Repair Human Infertility Infectious Disease Metabolic & GI Disorders Neurodegenerative Disease Neuropathic Pain Oncology Ophthalmology Rare Diseases Respiratory Infection Oral Mucositis Cardiac Disease Neuropathic Pain Ophthalmology Infectious Disease Rare Skin Disorders Rare Diseases Infertility Autoimmune Diseases Cartilage Repair Oncology Cartilage Repair Respiratory Infection Diabetes Arthritis
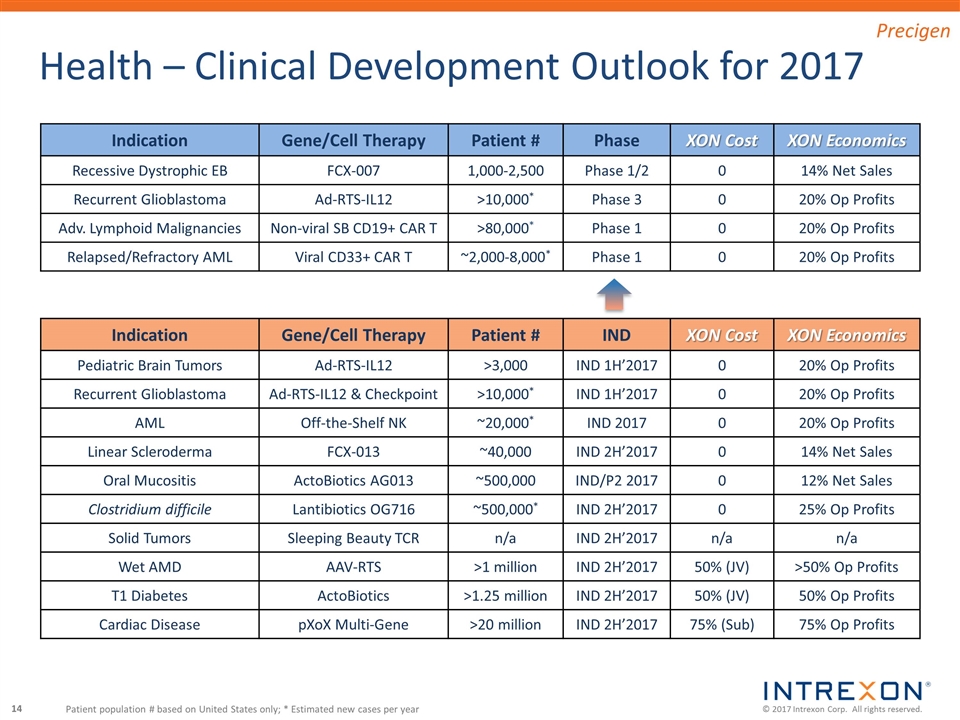
Indication Gene/Cell Therapy Patient # Phase XON Cost XON Economics Recessive Dystrophic EB FCX-007 1,000-2,500 Phase 1/2 0 14% Net Sales Recurrent Glioblastoma Ad-RTS-IL12 >10,000* Phase 3 0 20% Op Profits Adv. Lymphoid Malignancies Non-viral SB CD19+ CAR T >80,000* Phase 1 0 20% Op Profits Relapsed/Refractory AML Viral CD33+ CAR T ~2,000-8,000* Phase 1 0 20% Op Profits Health – Clinical Development Outlook for 2017 Patient population # based on United States only; * Estimated new cases per year Indication Gene/Cell Therapy Patient # IND XON Cost XON Economics Pediatric Brain Tumors Ad-RTS-IL12 >3,000 IND 1H’2017 0 20% Op Profits Recurrent Glioblastoma Ad-RTS-IL12 & Checkpoint >10,000* IND 1H’2017 0 20% Op Profits AML Off-the-Shelf NK ~20,000* IND 2017 0 20% Op Profits Linear Scleroderma FCX-013 ~40,000 IND 2H’2017 0 14% Net Sales Oral Mucositis ActoBiotics AG013 ~500,000 IND/P2 2017 0 12% Net Sales Clostridium difficile Lantibiotics OG716 ~500,000* IND 2H’2017 0 25% Op Profits Solid Tumors Sleeping Beauty TCR n/a IND 2H’2017 n/a n/a Wet AMD AAV-RTS >1 million IND 2H’2017 50% (JV) >50% Op Profits T1 Diabetes ActoBiotics >1.25 million IND 2H’2017 50% (JV) 50% Op Profits Cardiac Disease pXoX Multi-Gene >20 million IND 2H’2017 75% (Sub) 75% Op Profits Precigen
Next-Gen CAR+ T Platform under RTS® Control Intrexon, ZIOPHARM Oncology, and biopharmaceutical business of Merck KGaA advancing unique approach to develop therapeutic candidates for two CAR+ T targets expressed on a wide range of tumor types, including hematologic malignancies and solid tumors Distinctive method centers on proprietary RTS® platform to regulate expression of membrane-bound interleukin-15 co-expressed with CARs and Sleeping Beauty non-viral gene integration IL-15 is key driver of therapeutic effect in CAR+ T therapy* RTS® gene switch regulates expression of mbIL15 in CAR-modified T cells, providing a new paradigm in controlling T cells after administration Anticipate entering the clinic in 2018 *J Clin Oncol. 2017 Mar 14:JCO2016713024: Lymphoma remissions caused by CD19-specific CAR+ T cells are associated with elevated serum interleukin-15 levels Precigen
Targeting Neoantigens in Solid Tumors Nat Med. 2016 Jan;22(1):26-36; Mol Ther. 2016 Jun;24(6):1078-89 ZIOPHARM and Intrexon Announce CRADA with National Cancer Institute (NCI) Utilizing Sleeping Beauty System to Generate T cells Targeting Neoantigens January 2016 June 2016 January 2017 Targeting Neoantigens in Solid Tumors With TCR-Modified T Cells Using Non-Viral Sleeping Beauty System Precigen
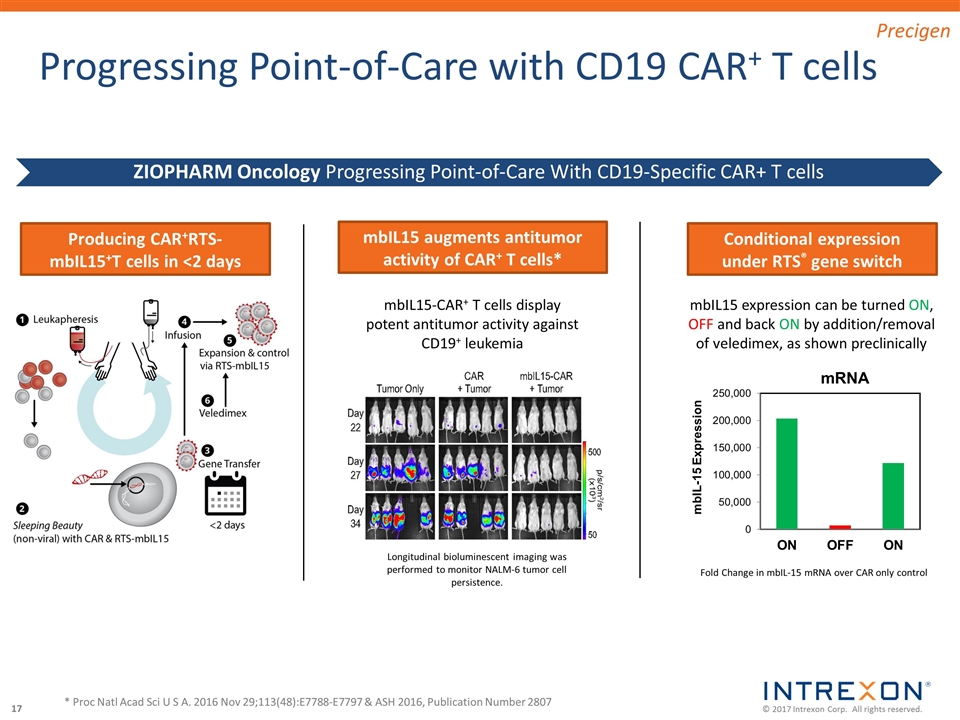
Progressing Point-of-Care with CD19 CAR+ T cells * Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7788-E7797 & ASH 2016, Publication Number 2807 Conditional expression under RTS® gene switch mbIL15 expression can be turned ON, OFF and back ON by addition/removal of veledimex, as shown preclinically mbIL15 augments antitumor activity of CAR+ T cells* Longitudinal bioluminescent imaging was performed to monitor NALM-6 tumor cell persistence. mbIL15-CAR+ T cells display potent antitumor activity against CD19+ leukemia Producing CAR+RTS-mbIL15+T cells in <2 days Fold Change in mbIL-15 mRNA over CAR only control ZIOPHARM Oncology Progressing Point-of-Care With CD19-Specific CAR+ T cells Precigen

Clinical Trials Update Intra-tumoral IL-12 RheoSwitch® program (Ad-RTS-hIL-12 + veledimex): In collaboration with investigators and regulators, ZIOPHARM assessing protocol design options for pivotal trial of Ad-RTS-hIL-12 + veledimex in recurrent glioblastoma (GBM) and details of pivotal Phase 3 trial will be made available following evaluation and completion of discussions with clinical advisors and regulators Update on clinical data from Phase 1 of Ad-RTS-hIL-12 + veledimex for recurrent GBM at ASCO in June CAR+ T programs (2nd Gen Non-viral CD19-specific CAR+ T and CD33-specific CAR+ T): Continue Phase 1 second-generation Sleeping Beauty non-viral clinical study of CD19-specific CAR+ T for lymphoid malignancies Initiating Phase 1 study of CD33-specific CAR+ T for relapsed/refractory acute myeloid leukemia (AML) with first patient expected to begin treatment in Q3’2017 Rare Disease program (FCX-007): Collaborator Fibrocell Science, Inc. received fast track designation from FDA for FCX-007 for treatment of recessive dystrophic epidermolysis bullosa (RDEB) Announced dosing of first patient in Phase I/II clinical trial of FCX-007 gene therapy in February Data from multiple patients treated in the trial are expected in the third quarter Precigen
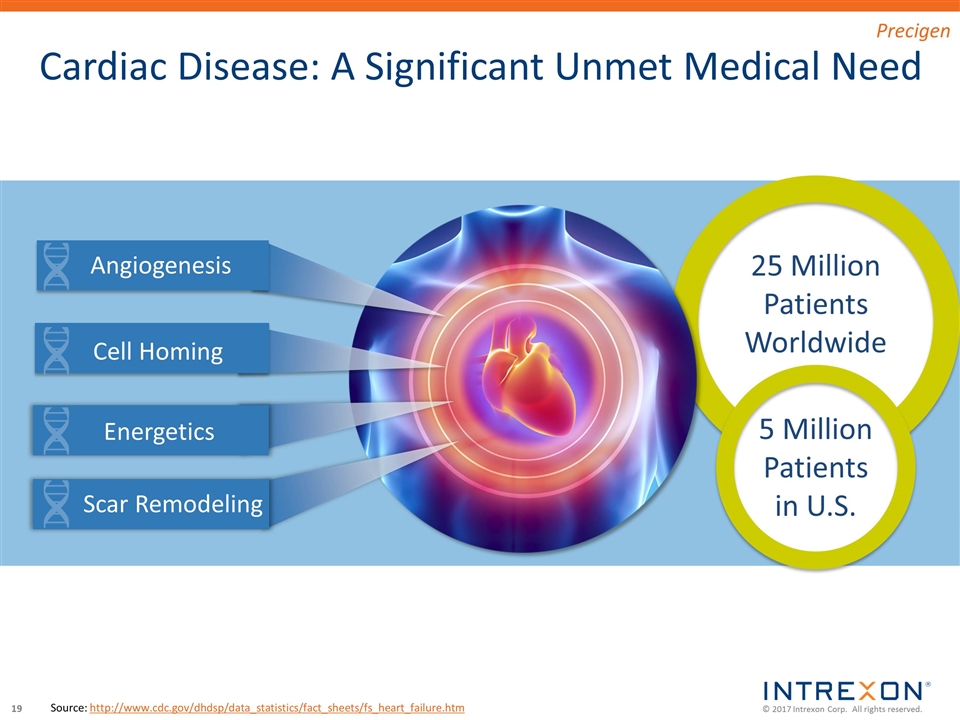
Source: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm 25 Million Patients Worldwide 5 Million Patients in U.S. Cardiac Disease: A Significant Unmet Medical Need Angiogenesis Cell Homing Energetics Scar Remodeling Precigen
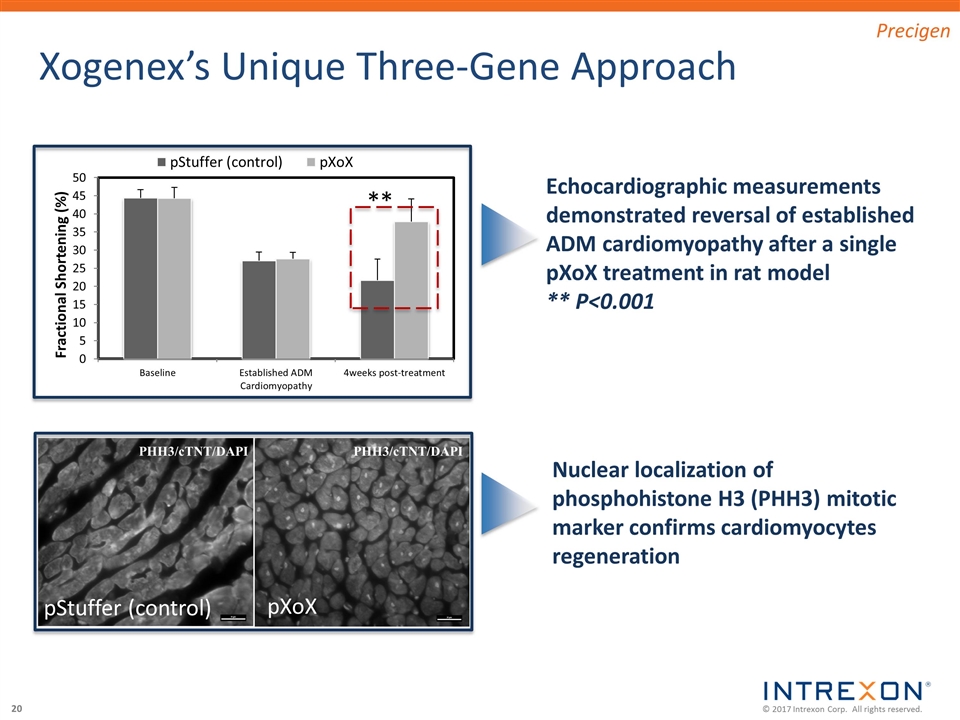
Xogenex’s Unique Three-Gene Approach Echocardiographic measurements demonstrated reversal of established ADM cardiomyopathy after a single pXoX treatment in rat model ** P<0.001 PHH3/cTNT/DAPI PHH3/cTNT/DAPI pXoX pStuffer (control) Nuclear localization of phosphohistone H3 (PHH3) mitotic marker confirms cardiomyocytes regeneration Precigen

Methanotroph Bioconversion Platform (MBP) Robert Walsh – Senior Vice President, Head of Energy Sector

Intrexon Methane Bioconversion Platform Intrexon has developed disruptive MBP technology that enables the profitable use of low cost natural gas to replace oil as the feedstock for several high value industrial products. Need for cleaner burning fuels in automotive and other industries Gas-to-liquids conversion currently relies on costly, energy-intensive processes Oil supplies destined for depletion Negative eco-impact from gas flaring High Capex & High Opex Industrial Products Division
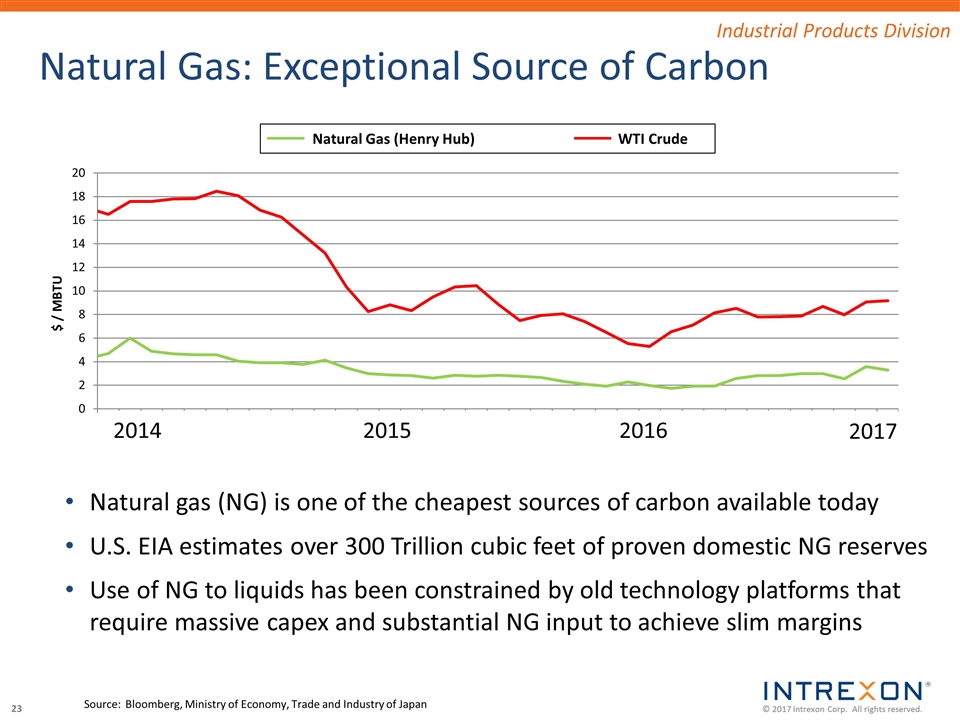
Natural Gas: Exceptional Source of Carbon Source:Bloomberg, Ministry of Economy, Trade and Industry of Japan Industrial Products Division Natural gas (NG) is one of the cheapest sources of carbon available today U.S. EIA estimates over 300 Trillion cubic feet of proven domestic NG reserves Use of NG to liquids has been constrained by old technology platforms that require massive capex and substantial NG input to achieve slim margins $ / MBTU Natural Gas (Henry Hub) WTI Crude 2014 2015 2016 2017
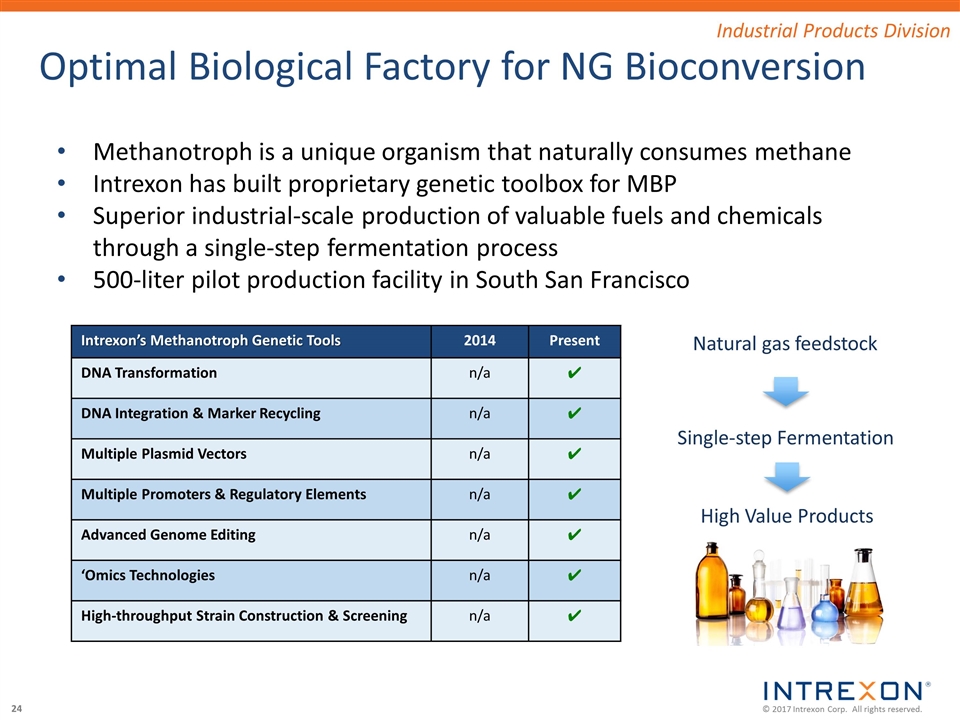
Optimal Biological Factory for NG Bioconversion Industrial Products Division Intrexon’s Methanotroph Genetic Tools 2014 Present DNA Transformation n/a ✔ DNA Integration & Marker Recycling n/a ✔ Multiple Plasmid Vectors n/a ✔ Multiple Promoters & Regulatory Elements n/a ✔ Advanced Genome Editing n/a ✔ ‘Omics Technologies n/a ✔ High-throughput Strain Construction & Screening n/a ✔ Methanotroph Single-step Fermentation Natural gas feedstock High Value Products Methanotroph is a unique organism that naturally consumes methane Intrexon has built proprietary genetic toolbox for MBP Superior industrial-scale production of valuable fuels and chemicals through a single-step fermentation process 500-liter pilot production facility in South San Francisco
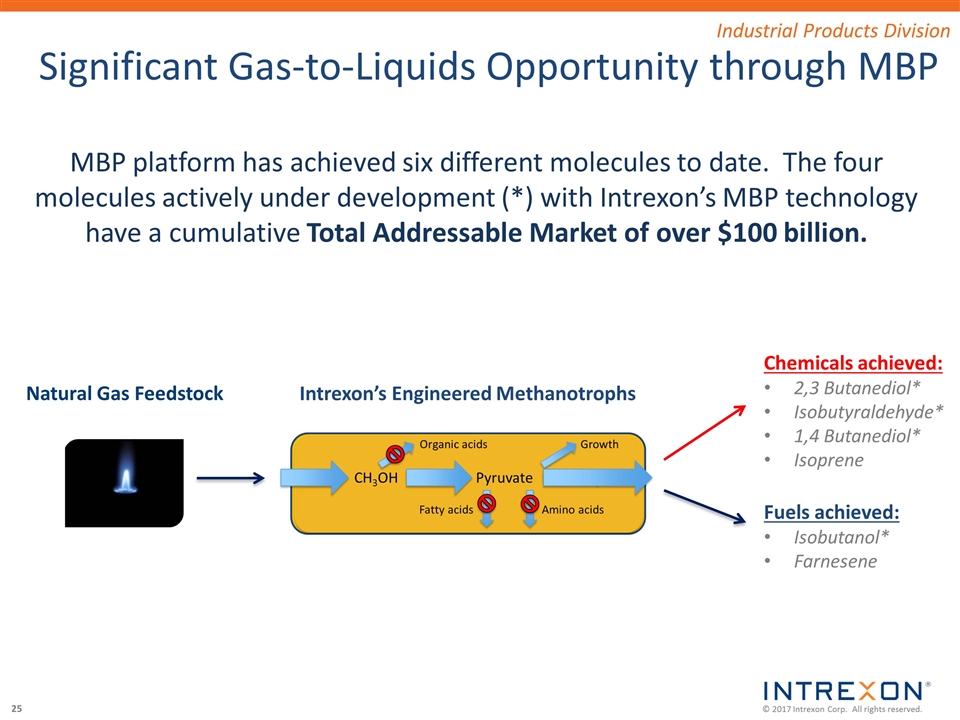
Significant Gas-to-Liquids Opportunity through MBP Intrexon’s Engineered Methanotrophs Chemicals achieved: 2,3 Butanediol* Isobutyraldehyde* 1,4 Butanediol* Isoprene Natural Gas Feedstock Fuels achieved: Isobutanol* Farnesene MBP platform has achieved six different molecules to date. The four molecules actively under development (*) with Intrexon’s MBP technology have a cumulative Total Addressable Market of over $100 billion. Industrial Products Division
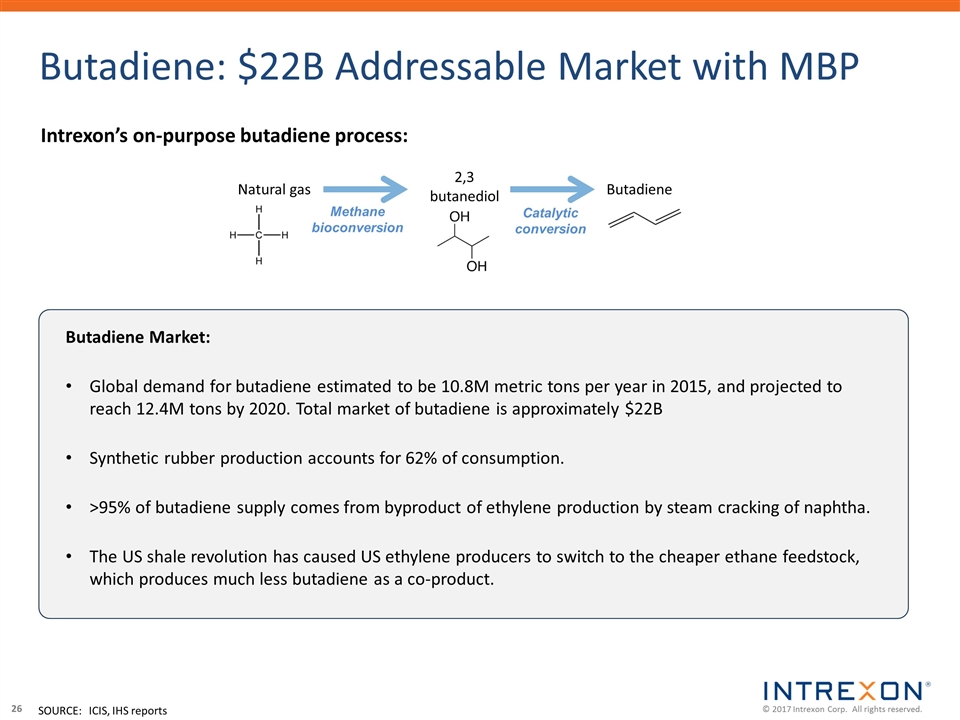
Butadiene: $22B Addressable Market with MBP Intrexon’s on-purpose butadiene process: 2,3 butanediol Methane bioconversion Catalytic conversion Natural gas Butadiene SOURCE:ICIS, IHS reports Butadiene Market: Global demand for butadiene estimated to be 10.8M metric tons per year in 2015, and projected to reach 12.4M tons by 2020. Total market of butadiene is approximately $22B Synthetic rubber production accounts for 62% of consumption. >95% of butadiene supply comes from byproduct of ethylene production by steam cracking of naphtha. The US shale revolution has caused US ethylene producers to switch to the cheaper ethane feedstock, which produces much less butadiene as a co-product.
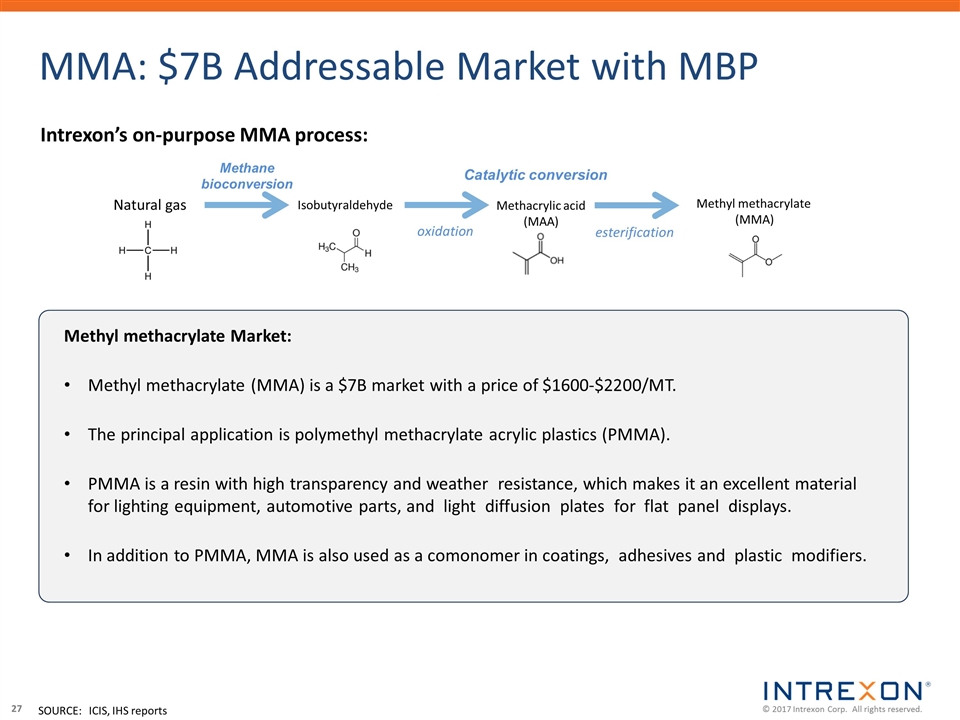
MMA: $7B Addressable Market with MBP esterification Methyl methacrylate Market: Methyl methacrylate (MMA) is a $7B market with a price of $1600-$2200/MT. The principal application is polymethyl methacrylate acrylic plastics (PMMA). PMMA is a resin with high transparency and weather resistance, which makes it an excellent material for lighting equipment, automotive parts, and light diffusion plates for flat panel displays. In addition to PMMA, MMA is also used as a comonomer in coatings, adhesives and plastic modifiers. Methyl methacrylate (MMA) Methacrylic acid (MAA) oxidation Catalytic conversion Isobutyraldehyde Intrexon’s on-purpose MMA process: Methane bioconversion Natural gas SOURCE:ICIS, IHS reports

Financial Overview Joel Liffmann – Senior Vice President, Finance
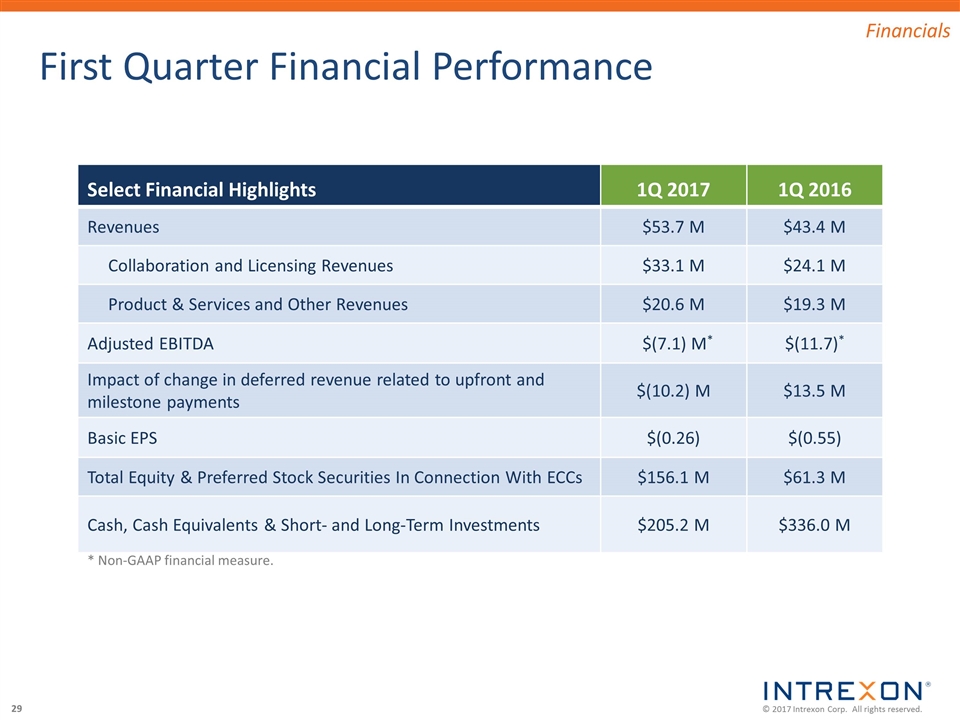
First Quarter Financial Performance * Non-GAAP financial measure. Select Financial Highlights 1Q 2017 1Q 2016 Revenues $53.7 M $43.4 M Collaboration and Licensing Revenues $33.1 M $24.1 M Product & Services and Other Revenues $20.6 M $19.3 M Adjusted EBITDA $(7.1) M* $(11.7)* Impact of change in deferred revenue related to upfront and milestone payments $(10.2) M $13.5 M Basic EPS $(0.26) $(0.55) Total Equity & Preferred Stock Securities In Connection With ECCs $156.1 M $61.3 M Cash, Cash Equivalents & Short- and Long-Term Investments $205.2 M $336.0 M Financials
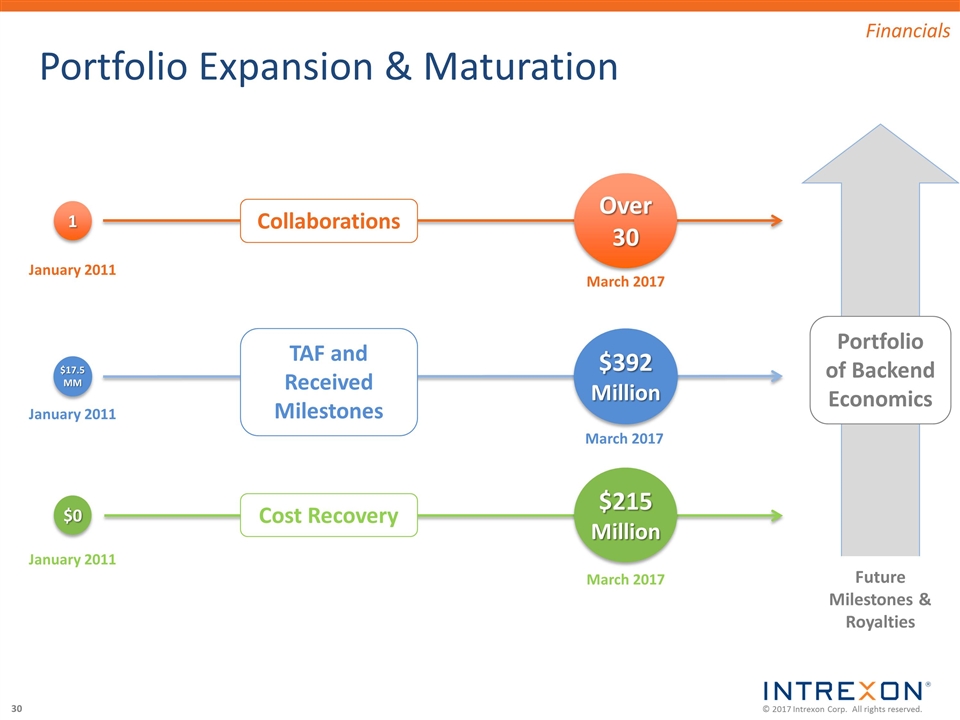
Portfolio Expansion & Maturation March 2017 1 Over 30 January 2011 Collaborations March 2017 $17.5MM $392Million January 2011 TAF and Received Milestones March 2017 $0 $215 Million January 2011 Cost Recovery Portfolio of Backend Economics Future Milestones & Royalties Financials

Summary Over 30 collaborations in place capitalizing on our leadership position in the engineering of biology Strong cash position with $205 million in cash, cash equivalents, and short-term investments, and equity securities and preferred stock valued at $156 million Developing high value bio-solutions with our collaborators in large established markets with built-in demand Positioned to deliver meaningful returns to our shareholders through our scalable, capital efficient model and successful execution of current & future opportunities

1Q 2017 Financial Results and Business Update Q&A Session May 10th, 2017































