
Third Quarter 2018 Business Update November 8, 2018 Exhibit 99.2

Forward Looking Statements Safe Harbor Statement Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s business, discussion of anticipated clinical trials and future collaborations. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s strategy and overall approach to its business model; (ii) Intrexon’s ability to successfully enter new markets or develop additional products, whether with its collaborators or independently; (iii) Intrexon's ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (iv) actual or anticipated variations in Intrexon’s operating results; (v) actual or anticipated fluctuations in Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (vi) Intrexon’s cash position; (vii) market conditions in Intrexon’s industry; (viii) the volatility of Intrexon’s stock price; (ix) Intrexon’s ability, and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (x) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations and policies; (xi) the outcomes of pending and future litigation; (xii) the rate and degree of market acceptance of any products developed by Intrexon, its subsidiaries, collaborations or joint ventures; (xiii) Intrexon’s ability to retain and recruit key personnel; (xiv) Intrexon’s expectations related to the use of proceeds from its public offerings and other financing efforts; and (xv) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intrexon’s Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law. © 2018 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon.

intrexon and Engineered Biology Engineered biology: The largest industrial revolution the planet has ever seen and we are a technological leader in the field Unique toolbox two decades in the making: Our precision engineering capabilities allow for controllable, multi-genic payload capacity of our gene programs Attainable value of this revolution: Gives us the opportunity to pursue targets that are difficult technologically but promising for global health, and at the same time, we meter our approach by augmenting our platforms for use across multiple targets 3

Leveraging Microbes for Industrial and Therapeutic Applications Methane Bioconversion Platform (MBP) to produce high-value fuels and chemicals Yeast fermentation platform for production of compounds for medical use
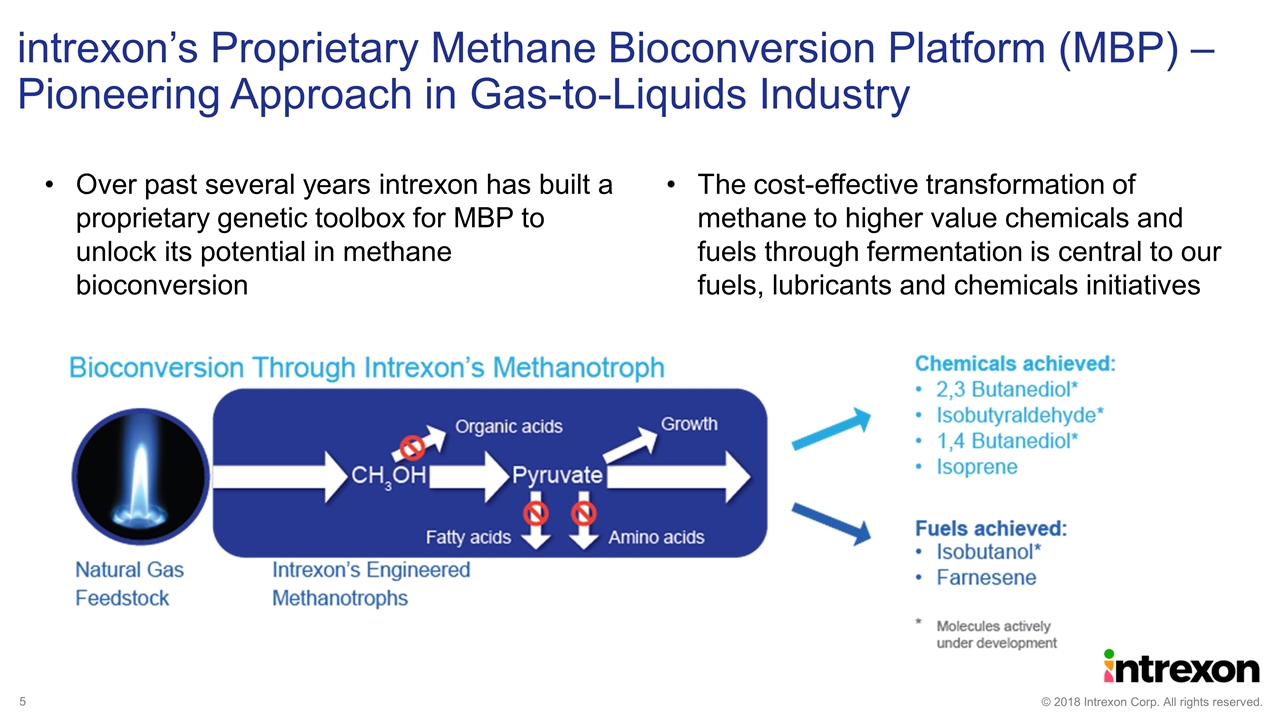
Over past several years intrexon has built a proprietary genetic toolbox for MBP to unlock its potential in methane bioconversion The cost-effective transformation of methane to higher value chemicals and fuels through fermentation is central to our fuels, lubricants and chemicals initiatives intrexon’s Proprietary Methane Bioconversion Platform (MBP) – Pioneering Approach in Gas-to-Liquids Industry
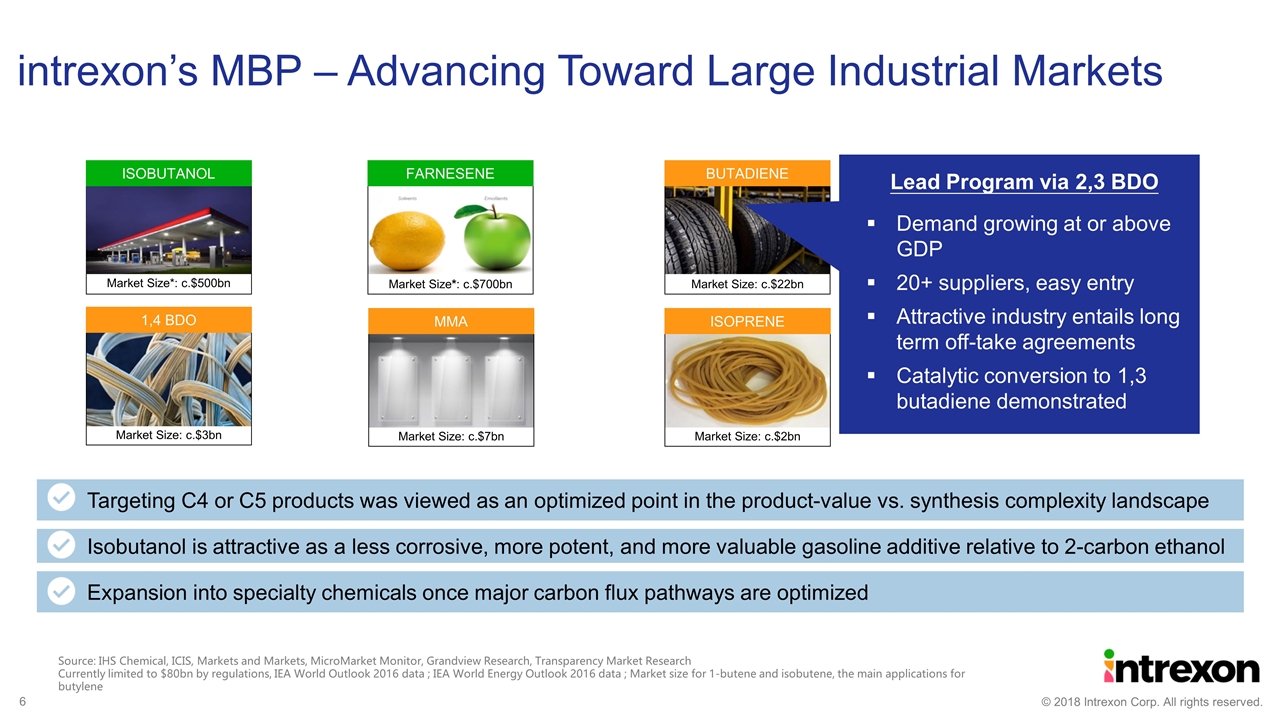
Source: IHS Chemical, ICIS, Markets and Markets, MicroMarket Monitor, Grandview Research, Transparency Market Research Currently limited to $80bn by regulations, IEA World Outlook 2016 data ; IEA World Energy Outlook 2016 data ; Market size for 1-butene and isobutene, the main applications for butylene intrexon’s MBP – Advancing Toward Large Industrial Markets Market Size*: c.$500bn ISOBUTANOL Market Size: c.$22bn BUTADIENE Market Size*: c.$700bn FARNESENE Market Size: c.$3bn 1,4 BDO Market Size: c.$7bn MMA Market Size: c.$2bn ISOPRENE Targeting C4 or C5 products was viewed as an optimized point in the product-value vs. synthesis complexity landscape Isobutanol is attractive as a less corrosive, more potent, and more valuable gasoline additive relative to 2-carbon ethanol Expansion into specialty chemicals once major carbon flux pathways are optimized Lead Program via 2,3 BDO Demand growing at or above GDP 20+ suppliers, easy entry Attractive industry entails long term off-take agreements Catalytic conversion to 1,3 butadiene demonstrated

Producing BDO from natural gas at roughly 50% of the theoretical final target yield Demonstrated performance at 500X scale-up Sustained production runs exceeding 1,000 hours without reduction in output Q3 Advances © 2018 Intrexon Corp. All rights reserved.

Robust Microbial Production of Cannabinoids for Medical Use North American Cannabis market estimated to be $47.3 billion by 2027 Volume and market will demand production routes beyond current plant-based methods intrexon’s proprietary yeast strains enable a transformative process for robust production of cannabinoids with consistent yield and purity Mixture of products Typically low product accumulation Difficult to isolate and purify Contamination possible Large-scale cultivation required Cannabis Plant Pure cannabinoid products High product accumulation Isolation and purification On-demand production Controlled process performed in standard assets Intrexon’s Yeast

Bioengineering Cells for Human Gene and Cellular Therapies Next generation targeted, controllable, multigenic therapeutic treatments across broad range of diseases Microbe-based delivery of biopharmaceuticals
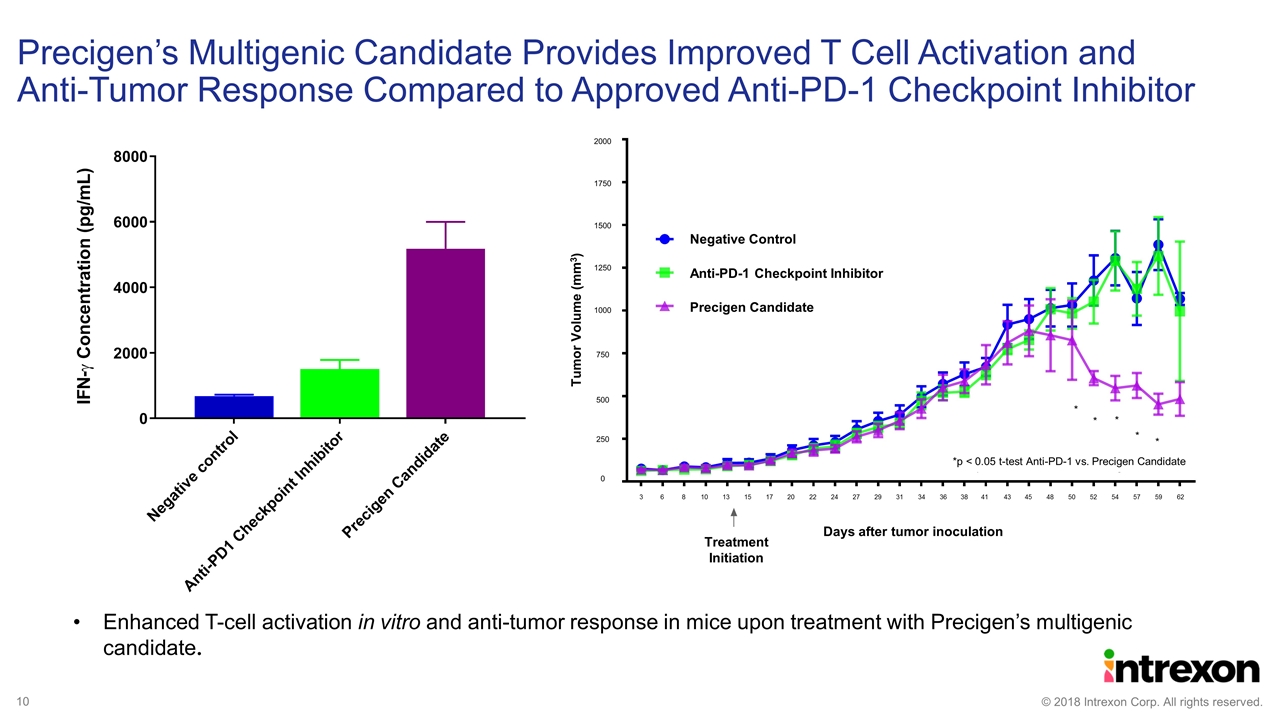
Enhanced T-cell activation in vitro and anti-tumor response in mice upon treatment with Precigen’s multigenic candidate. Precigen’s Multigenic Candidate Provides Improved T Cell Activation and Anti-Tumor Response Compared to Approved Anti-PD-1 Checkpoint Inhibitor 2000 1750 1500 1250 1000 750 500 Negative Control 250 Anti-PD-1 Checkpoint Inhibitor Precigen Candidate 0 3 6 8 10 13 15 17 20 22 24 27 29 31 34 36 38 41 43 45 48 50 52 54 57 59 62 Days after tumor inoculation Treatment Initiation Tumor Volume (mm3) *p < 0.05 t-test Anti-PD-1 vs. Precigen Candidate * * * * * 10
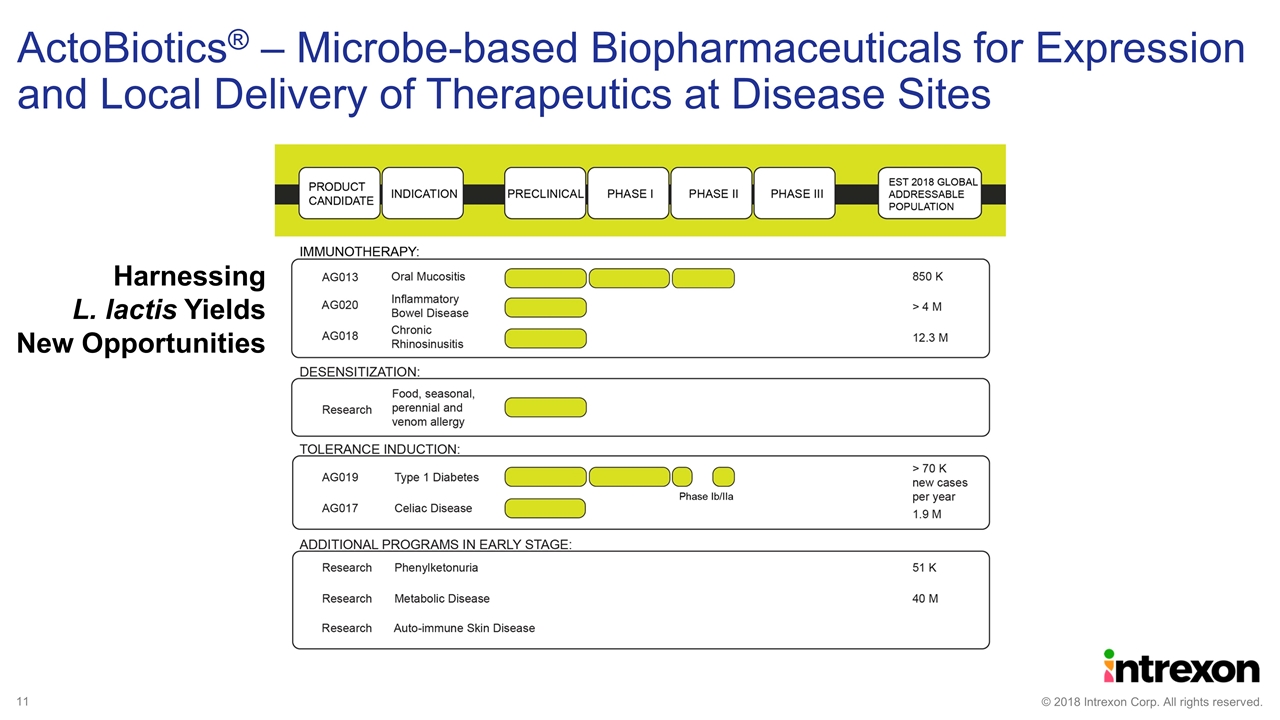
ActoBiotics® – Microbe-based Biopharmaceuticals for Expression and Local Delivery of Therapeutics at Disease Sites Harnessing L. lactis Yields New Opportunities
ActoBio Updates Oragenics, Inc. continues to enroll patients in the Phase IIb trial for AG013 in the treatment for Oral Mucositis ActoBio has dosed the first patient in the AG019 Ib/IIa trial in the treatment of early onset Type 1 Diabetes AG017, an immune tolerance approach, for the treatment of Celiac Disease is targeting a Q1 2019 IND

Engineering Biology for Food, Agriculture, Environmental, and Health Solutions Okanagan Specialty Fruits Trans Ova elite bovine genetics Exemplar MiniSwine research models EnviroFlight black soldier fly larvae for animal feed AquaBounty land-based salmon production

The 2018 harvest is complete yielding 2,100 bins, 10 times more than the 2017 harvest Targeting >500 retail outlets for our fresh sliced, whole apples and ApBitz™ snacks Consumer reception to the apples has been highly favorable Expected to plant ~1,000,000 trees in the spring of 2019; additional to the existing ~980,000 trees on 600 acres of orchards Scaling Arctic® Apple Plantings to Meet Consumer Demand

Trans Ova Genetics Sales of embryos with elite genetics continue to a growing number of customers domestically and abroad Created six bull calves that rank at the top of the global Holstein bull population Exemplar Genetics Demand for the MiniSwine Models continues to grow Expansion into regenerative medicine continues; through collaboration, the first pig has been born with the potential to produce organs for human transplant Trans Ova and Exemplar Q3 Updates © 2018 Intrexon Corp. All rights reserved. 15

Ongoing construction in Maysville facility Black Soldier Fly Larvae Commercial Facility to be Operational in Q4 Advancing on schedule toward opening the largest black soldier fly larvae facility in the US in Q4 2018 Modular facility – Phase one will have the ability to produce 900mt of product a year and is designed to scale up to 3,200mt Black soldier fly larvae was approved for poultry diet in September Orders for product from the new facility are being generated

Currently distributed throughout Canada November 19, 2015 – FDA approval for production, sale, and consumption in the U.S. April 27, 2018 – FDA approval to raise AquAdvantage® Salmon at land-based Indiana facility; conventional salmon is currently being raised at the facility AquaBounty granted a CA$2.0M loan from the Department of Economic Development of the Province of Prince Edward Island to complete the construction of a 250mt facility Production and sales of AquAdvantage® Salmon in U.S. await official labeling guidelines by the FDA Domestically-produced alternative to imported ocean cage reared salmon Sustainable AquAdvantage® Salmon Update 17

Innovations in Plant Biology and Vector Control

The Botticelli™ Advantage Advanced tissue culture technology that offers a sustainable, scalable, and more economical solution for growers to meet the rapidly expanding demand for premium quality products Indefinite replication of genetics and consistent product performance with “clean” plants Improved bottom line due to scalable and sustainable high quality plantlet production Speed to market and top line growth with new and improved varietals
Next Gen Tissue Culture Technology Starting Tissue Proprietary Culture Method Multiple Varietal Production Lettuce Tomato

Second Collaborative Agreement with the Bill & Melinda Gates Foundation Grant to develop Friendly™ Anopheles stephensi strain is being designed to combat malaria in India, Middle East and Horn of Africa Continue to Advance Oxitec’s Second Generation Aedes Technology Ongoing transition to 5034 in US, Brazil and Caymans Oxitec – Progressing Programs for Friendly™ Mosquitoes 21






















