
Intrexon Health Overview May 2019 Exhibit 99.2

Forward Looking Statements © 2019 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon. Safe Harbor Statement Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s business, discussion of anticipated clinical trials and future collaborations. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s strategy and overall approach to its business model and its ability to exercise more control and ownership over the development process and commercialization path; (ii) Intrexon’s ability to successfully enter new markets or develop additional products, whether with its collaborators or independently; (iii) Intrexon's ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (iv) actual or anticipated variations in Intrexon’s operating results; (v) actual or anticipated fluctuations in Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (vi) Intrexon’s cash position; (vii) market conditions in Intrexon’s industry; (viii) the volatility of Intrexon’s stock price; (ix) Intrexon’s ability, and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (x) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations and policies; (xi) the outcomes of pending and future litigation; (xii) the rate and degree of market acceptance of any products developed by Intrexon, its subsidiaries, collaborations or joint ventures; (xiii) Intrexon’s ability to retain and recruit key personnel; (xiv) Intrexon’s expectations related to the use of proceeds from its public offerings and other financing efforts; and (xv) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intrexon’s Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law. All of the pharmaceutical products described in this presentation are investigational new drugs, which are currently undergoing pre-clinical and/or human clinical trial testing. As a result, none of them have had their safety or efficacy established or are approved by the U.S. Food and Drug Administration or any other regulatory agency.

Intrexon Health Investment Overview Possessing the broadest and deepest gene and cell therapy technologies and capabilities Addressing unmet medical needs with therapeutics that are Targeted, Controllable, and Multigenic Eight clinical stage assets across diverse indications, more coming to the clinic Offering significantly improved COGS as compared with current generations Multiple near-term catalysts for value recognition

Board of Directors Randal J. Kirk Chairman Chairman & CEO of Intrexon Former Chairman/CEO of New River Pharmaceuticals, Inc. and Chairman of Clinical Data, Inc. Cesar Alvarez Senior Chairman of Greenberg Traurig Recognized as one of the “100 Most Influential Lawyers in America” Vinita Gupta CEO of Lupin Limited Named 2015 Ernst & Young Entrepreneur of the Year Jeffrey Kindler CEO of Centrexion Corporation Former Chairman/CEO of Pfizer, Inc. Dean Mitchell Executive Chairman and Board member of Covis Pharma Holdings S.a.r.l. Former CEO, Alpharma Robert Shapiro Lead Independent Chairman, Managing Director, and Co-Founder of Sandbox Industries, LLC Former Chairman/CEO of Monsanto Company Steve Frank Chairman of Global Healthcare Investment Banking at J.P. Morgan Former head of Bear Stearns Worldwide Health Care Investment Banking Group James Turley Director of Citigroup and Emerson Electric Co. Former Chairman/CEO of Ernst & Young Fred Hassan Partner and Managing Director of Warburg Pincus LLC Former Chairman/CEO of Schering-Plough

Executive Leadership Co-Founder & COO of General Injectables and Vaccines (now the medical business of Henry Schein) Co-founder and first President of King Pharmaceuticals (now part of Pfizer) Board member and largest shareholder of Scios, Inc. (now part of Johnson & Johnson) Founder, Chairman, CEO and largest shareholder of New River Pharmaceuticals (now part of Takeda) Chairman and largest shareholder of Clinical Data, Inc. (now part of Allergan) Board member (11 years) and largest shareholder of Halozyme (traded on Nasdaq) Chairman, CEO and largest shareholder of Intrexon Randal J. Kirk Chairman and CEO Helen Sabzevari, PhD President, Precigen, Inc. President of Precigen Inc., a dedicated discovery and clinical stage biopharmaceutical company advancing the next generation of gene and cellular therapies Co-founder and Chief Science Officer of Compass Therapeutics, a fully-integrated drug discovery and development company focused on manipulating the immune system to treat human disease Global Head of Immuno-Oncology Global Research and Early Development at EMD Serono (a subsidiary of Merck KGaA, Darmstadt, Germany). Led discovery and development of the anti-PD-L1 checkpoint inhibitor Bavencio® (avelumab), designated by the US FDA as a breakthrough therapy and approved to treat metastatic Merkel cell carcinoma and urothelial carcinoma. Led the Molecular Immunology Group at the Laboratory of Tumor Immunology and Biology at the US National Cancer Institute PhD in cell and molecular immunology and postdoctoral fellow at the department of immunology at the Scripps Research Institute
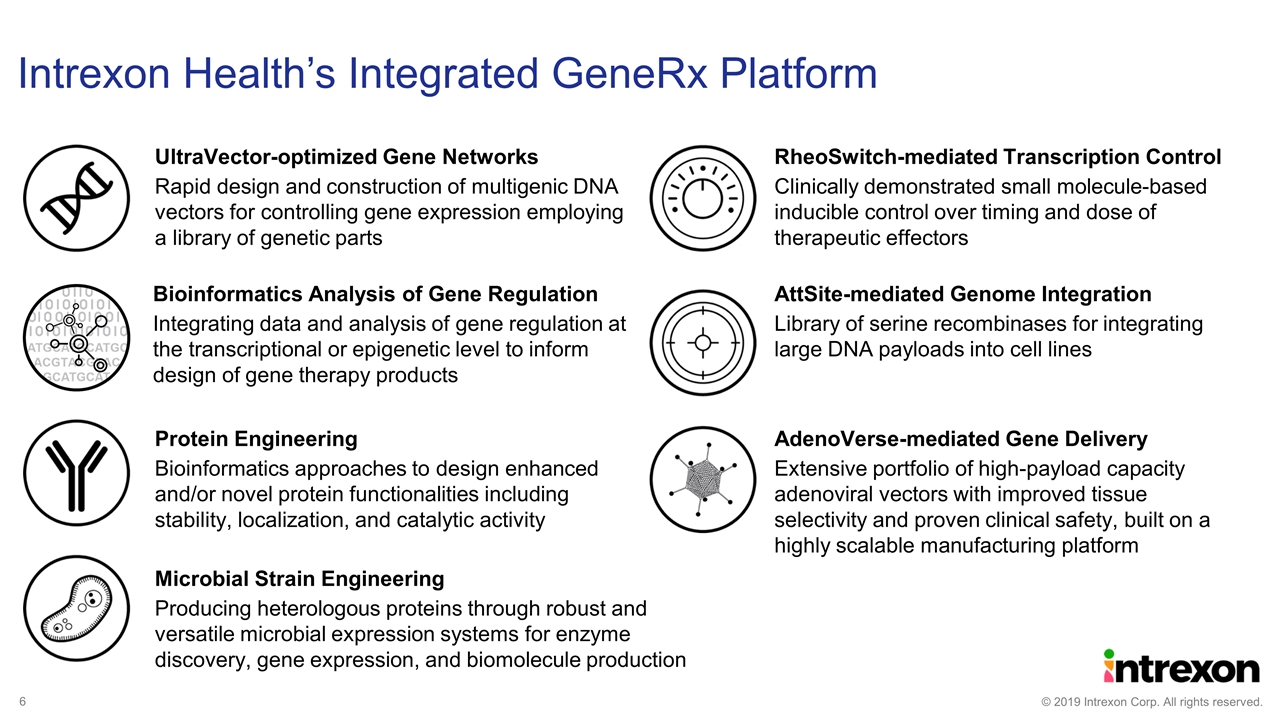
Intrexon Health’s Integrated GeneRx Platform AttSite-mediated Genome Integration Library of serine recombinases for integrating large DNA payloads into cell lines RheoSwitch-mediated Transcription Control Clinically demonstrated small molecule-based inducible control over timing and dose of therapeutic effectors AdenoVerse-mediated Gene Delivery Extensive portfolio of high-payload capacity adenoviral vectors with improved tissue selectivity and proven clinical safety, built on a highly scalable manufacturing platform Protein Engineering Bioinformatics approaches to design enhanced and/or novel protein functionalities including stability, localization, and catalytic activity Microbial Strain Engineering Producing heterologous proteins through robust and versatile microbial expression systems for enzyme discovery, gene expression, and biomolecule production UltraVector-optimized Gene Networks Rapid design and construction of multigenic DNA vectors for controlling gene expression employing a library of genetic parts Bioinformatics Analysis of Gene Regulation Integrating data and analysis of gene regulation at the transcriptional or epigenetic level to inform design of gene therapy products

Intrexon Therapeutics are Targeted, Controllable, Multigenic Intrexon’s technology and expertise facilitate the creation of sophisticated solutions designed to treat complex diseases Targeted: We employ cell and genome delivery systems designed to match disease and/or injury-related specifications. Our platforms include viral or non-viral gene delivery, engineered microbes, exosome-mediated RNA delivery, transient or persistent genome modifications, and autologous or universal cell therapies. Controllable: We create modulated gene systems (including RheoSwitch® technology), in concert with DNA/RNA/protein-based regulatory motifs, to enable spatiotemporal controls over one or more therapeutic effector molecules. Multigenic: We optimize multi-effector therapies to advance treatment or cure underlying disease etiologies and also to ameliorate effects of chronic comorbid tissue damage.
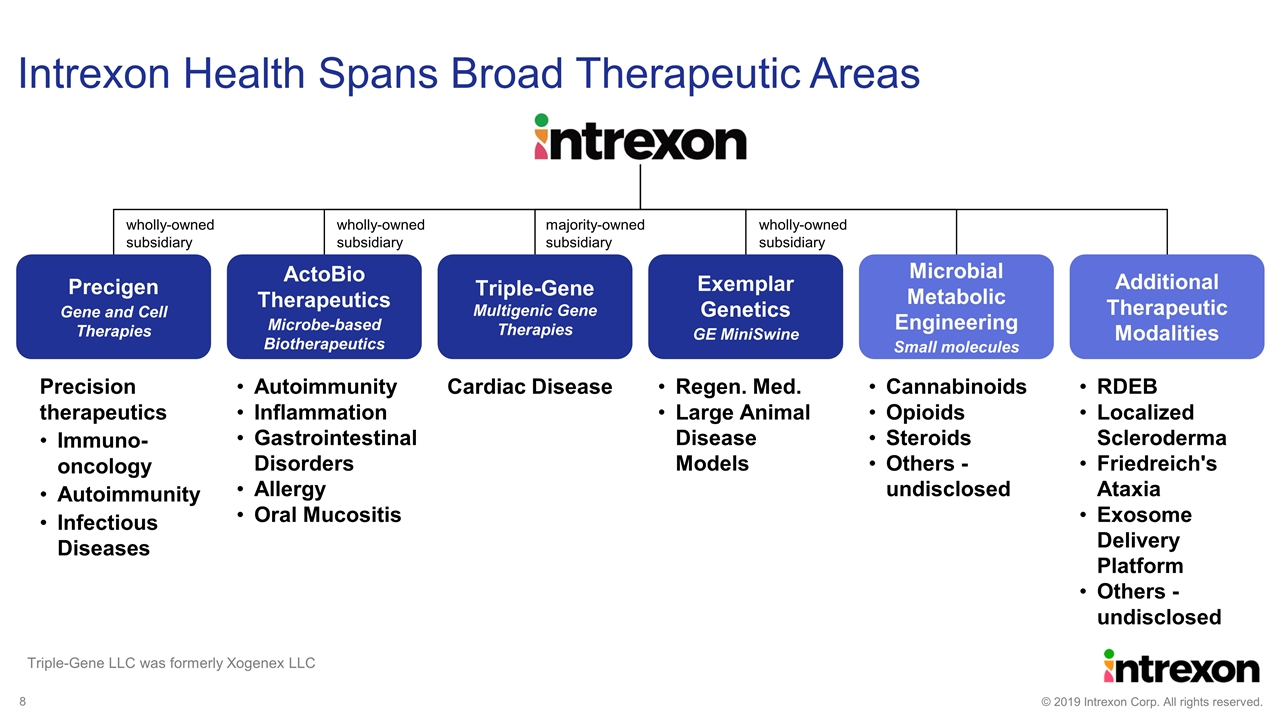
Intrexon Health Spans Broad Therapeutic Areas Precision therapeutics Immuno-oncology Autoimmunity Infectious Diseases Autoimmunity Inflammation Gastrointestinal Disorders Allergy Oral Mucositis RDEB Localized Scleroderma Friedreich's Ataxia Exosome Delivery Platform Others - undisclosed Regen. Med. Large Animal Disease Models Cannabinoids Opioids Steroids Others - undisclosed Precigen Gene and Cell Therapies ActoBio Therapeutics Microbe-based Biotherapeutics Additional Therapeutic Modalities Exemplar Genetics GE MiniSwine Microbial Metabolic Engineering Small molecules Cardiac Disease Triple-Gene Multigenic Gene Therapies wholly-owned subsidiary wholly-owned subsidiary wholly-owned subsidiary majority-owned subsidiary Triple-Gene LLC was formerly Xogenex LLC
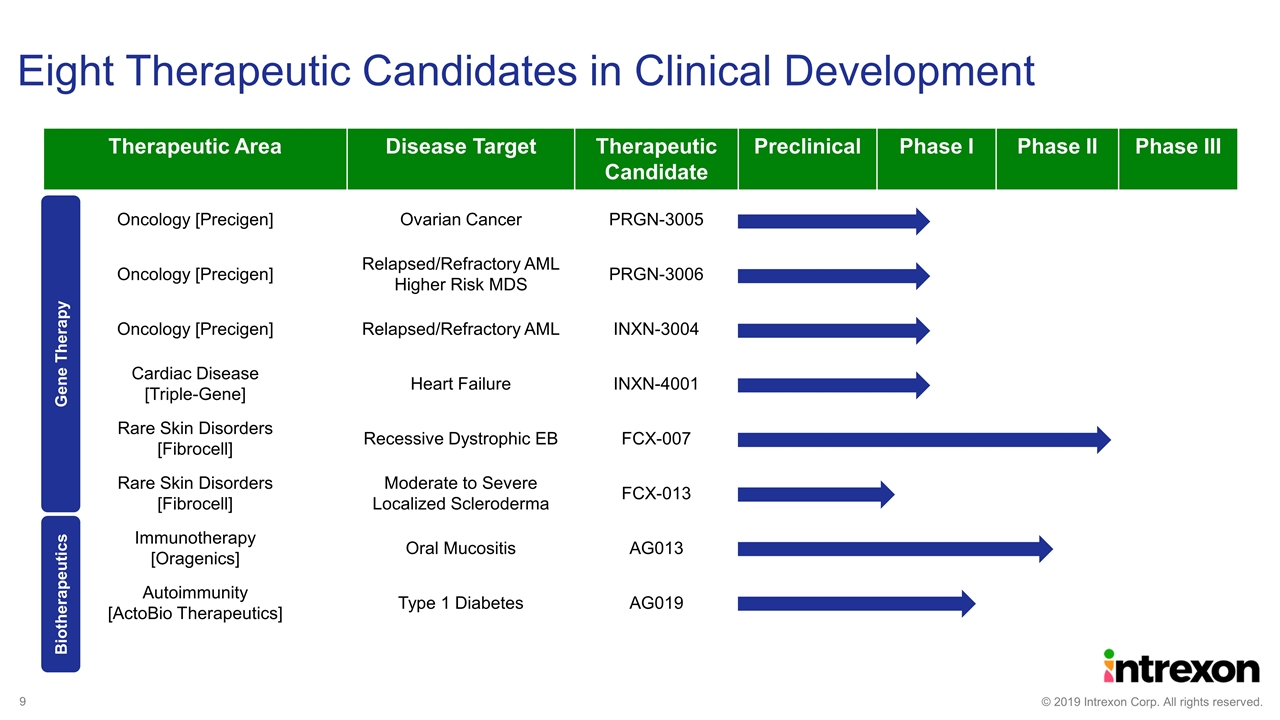
Therapeutic Area Disease Target Therapeutic Candidate Preclinical Phase I Phase II Phase III Oncology [Precigen] Ovarian Cancer PRGN-3005 Oncology [Precigen] Relapsed/Refractory AML Higher Risk MDS PRGN-3006 Oncology [Precigen] Relapsed/Refractory AML INXN-3004 Cardiac Disease [Triple-Gene] Heart Failure INXN-4001 Rare Skin Disorders [Fibrocell] Recessive Dystrophic EB FCX-007 Rare Skin Disorders [Fibrocell] Moderate to Severe Localized Scleroderma FCX-013 Immunotherapy [Oragenics] Oral Mucositis AG013 Autoimmunity [ActoBio Therapeutics] Type 1 Diabetes AG019 Eight Therapeutic Candidates in Clinical Development Gene Therapy Biotherapeutics
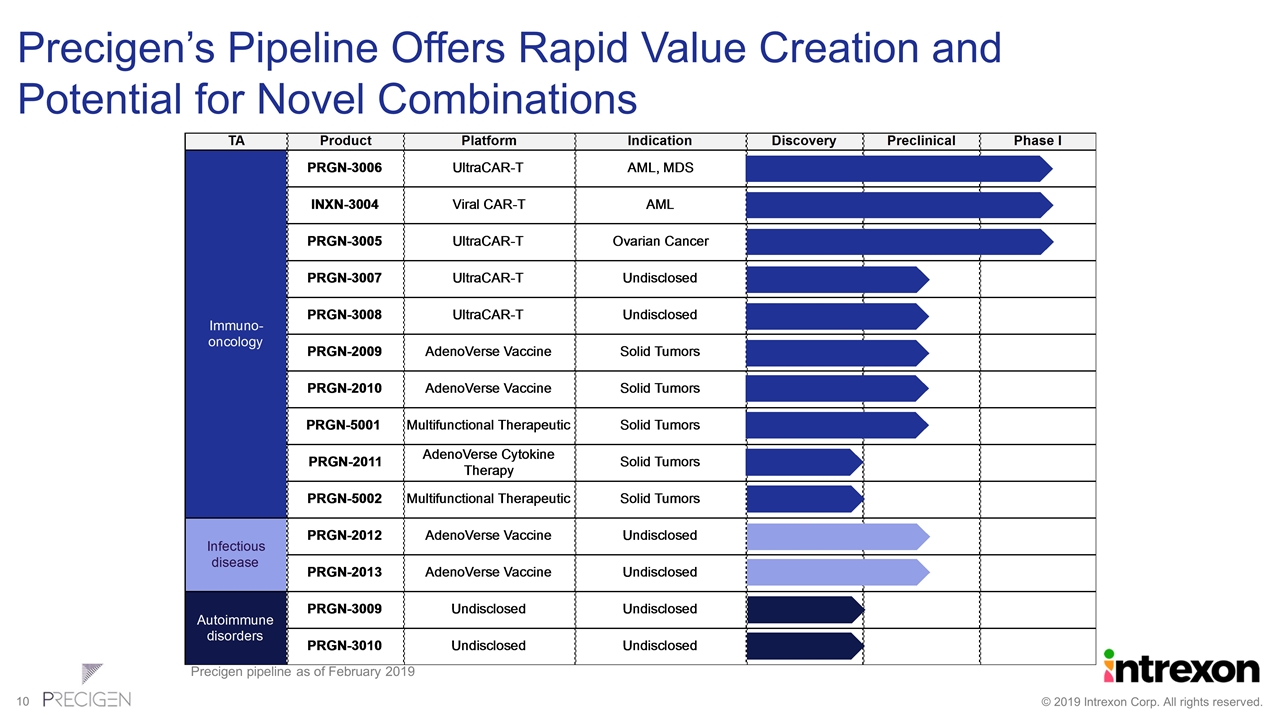
Precigen’s Pipeline Offers Rapid Value Creation and Potential for Novel Combinations Precigen pipeline as of February 2019 TA Product Platform Indication Discovery Preclinical Phase I Immuno-oncology PRGN-3006 UltraCAR-T AML, MDS INXN-3004 Viral CAR-T AML PRGN-3005 UltraCAR-T Ovarian Cancer PRGN-3007 UltraCAR-T Undisclosed PRGN-3008 UltraCAR-T Undisclosed PRGN-2009 AdenoVerse Vaccine Solid Tumors PRGN-2010 AdenoVerse Vaccine Solid Tumors PRGN-5001 Multifunctional Therapeutic Solid Tumors PRGN-2011 AdenoVerse Cytokine Therapy Solid Tumors PRGN-5002 Multifunctional Therapeutic Solid Tumors Infectious disease PRGN-2012 AdenoVerse Vaccine Undisclosed PRGN-2013 AdenoVerse Vaccine Undisclosed Autoimmune disorders PRGN-3009 Undisclosed Undisclosed PRGN-3010 Undisclosed Undisclosed PRGN-3005 UltraCAR-T PRGN-3006 UltraCAR-T PRGN-2009 PRGN-2010 PRGN-2008 PRGN-3007 (Autologous cell therapy) PRGN-3008 (Autologous cell therapy) PRGN-3009 (Autologous cell therapy) PRGN-5001 (Bi-specific antibody) PRGN-5001 (Bi-specific antibody) PRGN-3007 UltraCAR-T PRGN-3008 UltraCAR-T PRGN-2011 PRGN-3009 (Autologous cell therapy) PRGN-3010 (Autologous cell therapy)
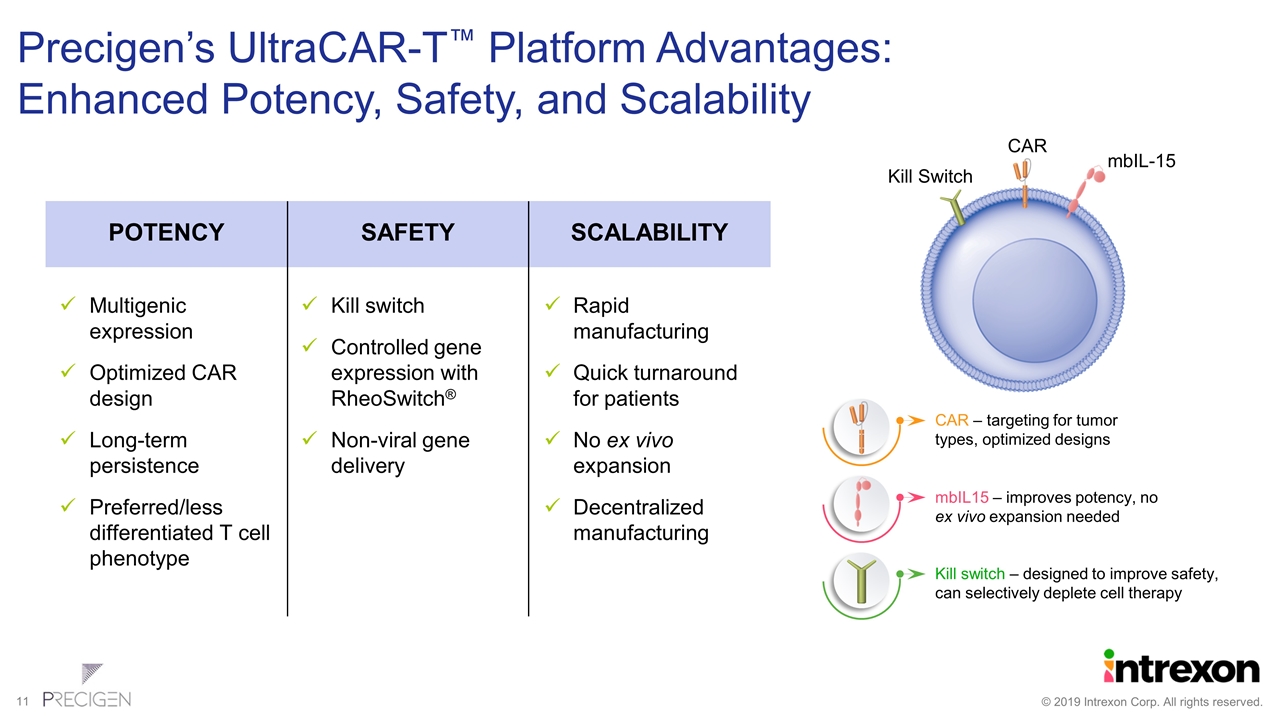
Precigen’s UltraCAR-T™ Platform Advantages: Enhanced Potency, Safety, and Scalability CAR mbIL-15 Kill Switch CAR – targeting for tumor types, optimized designs mbIL15 – improves potency, no ex vivo expansion needed Kill switch – designed to improve safety, can selectively deplete cell therapy Multigenic expression Optimized CAR design Long-term persistence Preferred/less differentiated T cell phenotype POTENCY Kill switch Controlled gene expression with RheoSwitch® Non-viral gene delivery SAFETY Rapid manufacturing Quick turnaround for patients No ex vivo expansion Decentralized manufacturing SCALABILITY
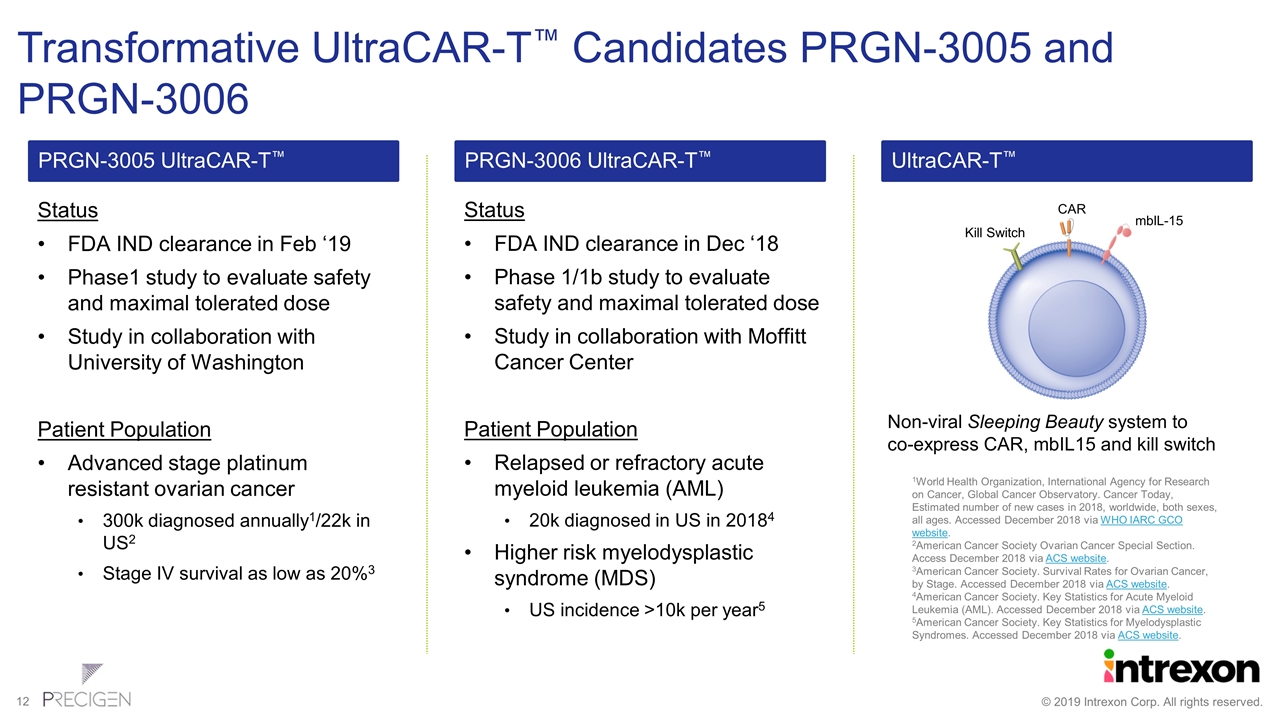
Transformative UltraCAR-T™ Candidates PRGN-3005 and PRGN-3006 Status FDA IND clearance in Feb ‘19 Phase1 study to evaluate safety and maximal tolerated dose Study in collaboration with University of Washington Patient Population Advanced stage platinum resistant ovarian cancer 300k diagnosed annually1/22k in US2 Stage IV survival as low as 20%3 PRGN-3005 UltraCAR-T™ 1World Health Organization, International Agency for Research on Cancer, Global Cancer Observatory. Cancer Today, Estimated number of new cases in 2018, worldwide, both sexes, all ages. Accessed December 2018 via WHO IARC GCO website. 2American Cancer Society Ovarian Cancer Special Section. Access December 2018 via ACS website. 3American Cancer Society. Survival Rates for Ovarian Cancer, by Stage. Accessed December 2018 via ACS website. 4American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML). Accessed December 2018 via ACS website. 5American Cancer Society. Key Statistics for Myelodysplastic Syndromes. Accessed December 2018 via ACS website. Status FDA IND clearance in Dec ‘18 Phase 1/1b study to evaluate safety and maximal tolerated dose Study in collaboration with Moffitt Cancer Center Patient Population Relapsed or refractory acute myeloid leukemia (AML) 20k diagnosed in US in 20184 Higher risk myelodysplastic syndrome (MDS) US incidence >10k per year5 PRGN-3006 UltraCAR-T™ Non-viral Sleeping Beauty system to co-express CAR, mbIL15 and kill switch UltraCAR-T™ CAR mbIL-15 Kill Switch
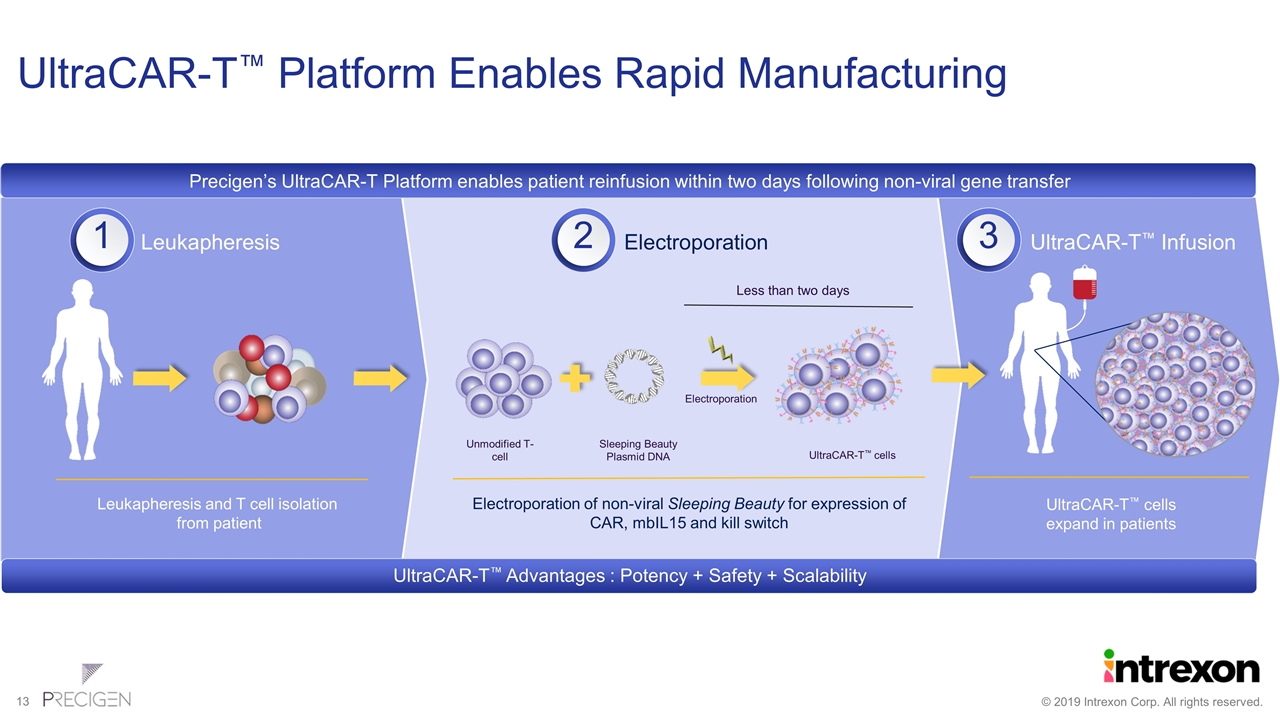
UltraCAR-T™ Platform Enables Rapid Manufacturing Electroporation Leukapheresis UltraCAR-T™ Infusion Less than two days Leukapheresis and T cell isolation from patient UltraCAR-T™ cells expand in patients UltraCAR-T™ cells Electroporation of non-viral Sleeping Beauty for expression of CAR, mbIL15 and kill switch Electroporation Unmodified T-cell Sleeping Beauty Plasmid DNA UltraCAR-T Advantages : Potency + Safety + Scalability Precigen’s UltraCAR-T Platform enables patient reinfusion within two days following non-viral gene transfer 1 2 3 UltraCAR-T™ Advantages : Potency + Safety + Scalability
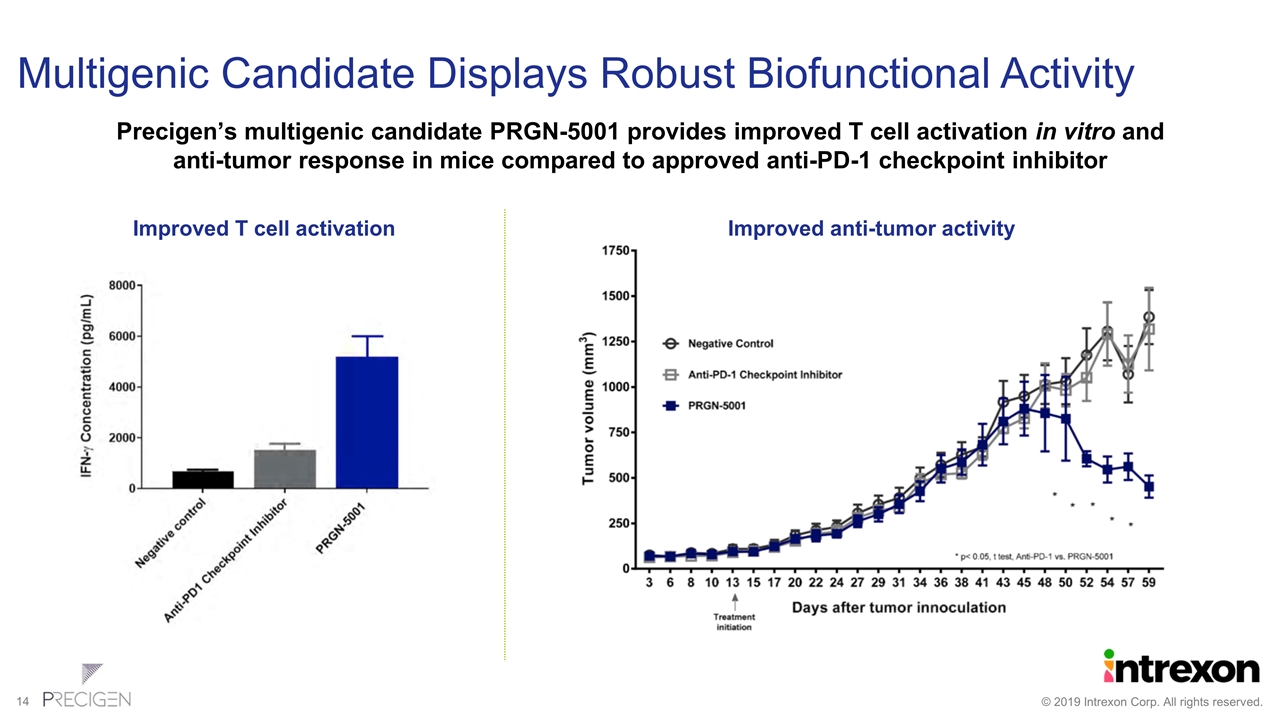
Multigenic Candidate Displays Robust Biofunctional Activity Precigen’s multigenic candidate PRGN-5001 provides improved T cell activation in vitro and anti-tumor response in mice compared to approved anti-PD-1 checkpoint inhibitor Improved T cell activation Improved anti-tumor activity

Vaccine Candidates Generate Superior Immune Responses Novel antigen designs for infectious disease vaccine candidates produce robust antigen-specific response AdenoVerse™ vaccine (PRGN-2013) produces superior immune response Precigen AdenoVerse™ vaccine candidates and technology platform generate superior immune responses in a mouse model system compared to competitor vaccines Antigen specific immune response In vivo screening
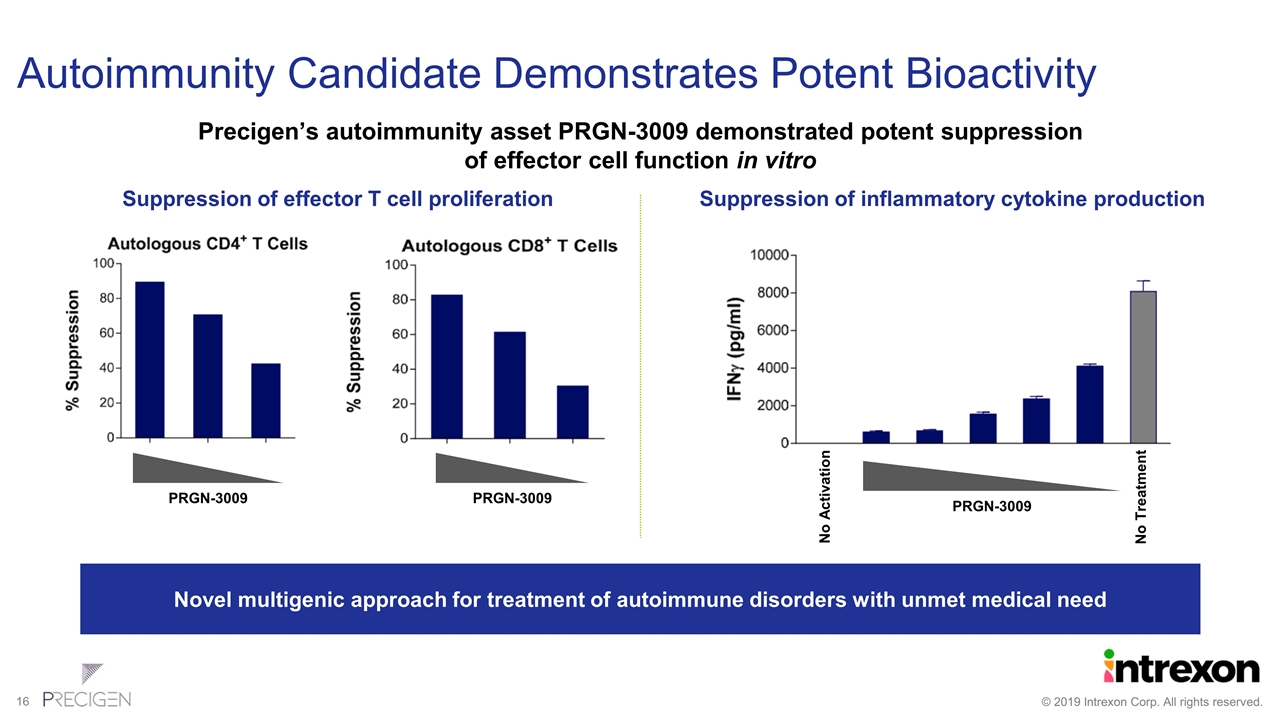
Autoimmunity Candidate Demonstrates Potent Bioactivity Precigen’s autoimmunity asset PRGN-3009 demonstrated potent suppression of effector cell function in vitro PRGN-3009 PRGN-3009 Suppression of effector T cell proliferation Novel multigenic approach for treatment of autoimmune disorders with unmet medical need PRGN-3009 No Activation No Treatment Suppression of inflammatory cytokine production
ActoBiotics® Platform – A Novel Class of Oral Biotherapeutics ActoBiotics® is a fully integrated, cost-effective & unique food microbe-based delivery platform for therapeutics Potential for superior efficacy and safety through oral or local targeted delivery Platform based on using Lactococcus lactis Safe, food grade, living, lactic acid bacteria Ongoing animal and human studies Not a gut commensal: no replication in the GI tract Designed to perform specific biological interventions Accelerates development and validated regulatory path for new IND candidates Scalable and low cost of manufacturing
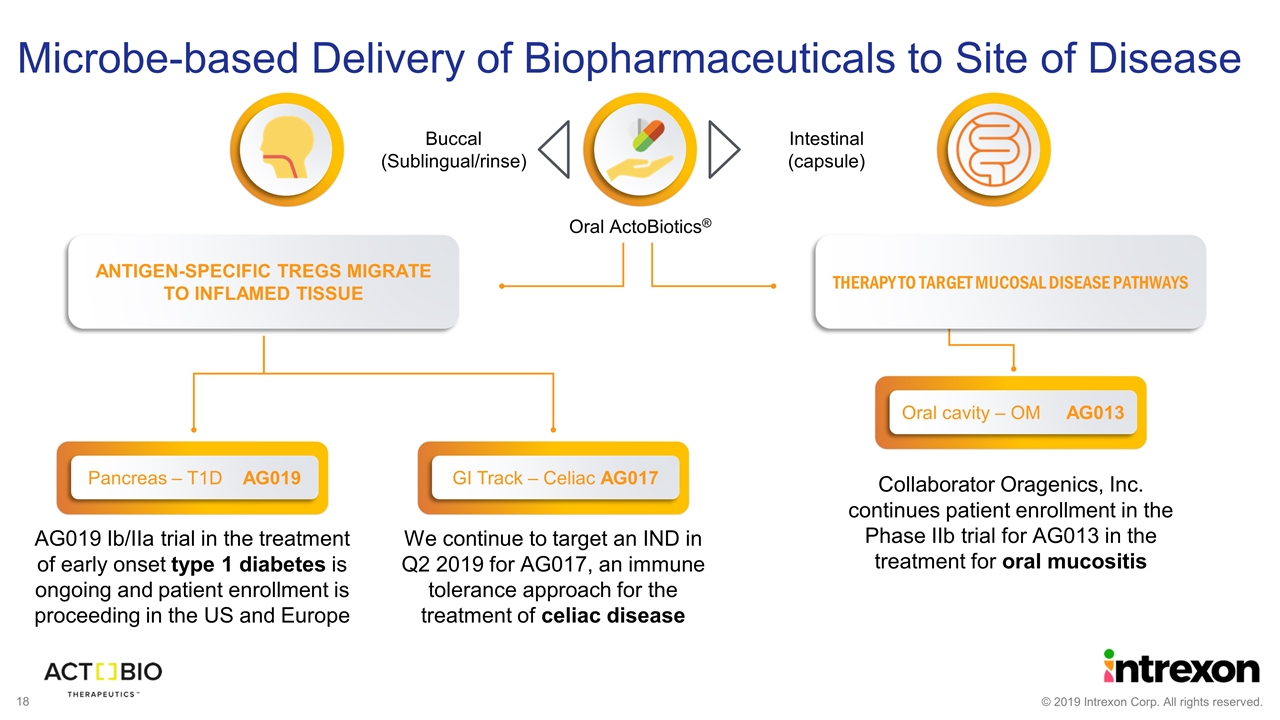
AG019 Ib/IIa trial in the treatment of early onset type 1 diabetes is ongoing and patient enrollment is proceeding in the US and Europe Collaborator Oragenics, Inc. continues patient enrollment in the Phase IIb trial for AG013 in the treatment for oral mucositis ANTIGEN-SPECIFIC TREGS MIGRATE TO INFLAMED TISSUE THERAPY TO TARGET MUCOSAL DISEASE PATHWAYS Oral cavity – OM AG013 Microbe-based Delivery of Biopharmaceuticals to Site of Disease Oral ActoBiotics® Intestinal (capsule) Buccal (Sublingual/rinse) GI Track – Celiac AG017 We continue to target an IND in Q2 2019 for AG017, an immune tolerance approach for the treatment of celiac disease Pancreas – T1D AG019
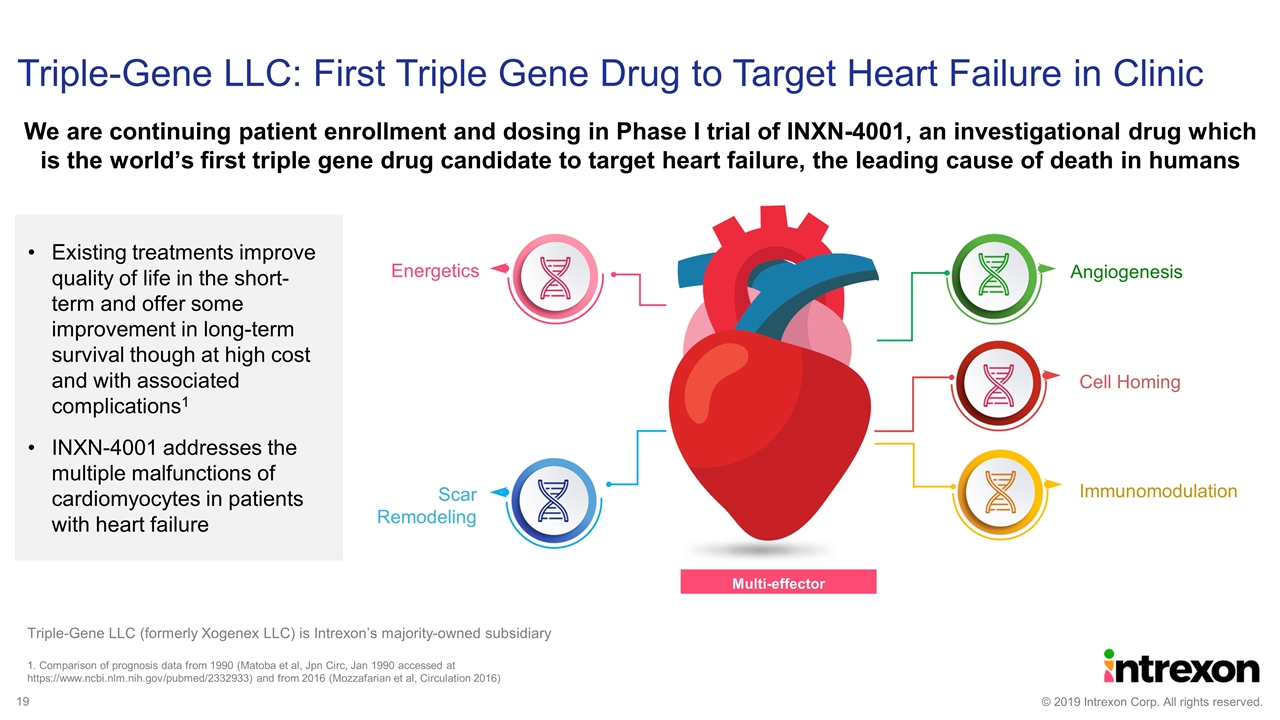
We are continuing patient enrollment and dosing in Phase I trial of INXN-4001, an investigational drug which is the world’s first triple gene drug candidate to target heart failure, the leading cause of death in humans Triple-Gene LLC: First Triple Gene Drug to Target Heart Failure in Clinic Angiogenesis Cell Homing Immunomodulation Scar Remodeling Energetics Multi-effector therapeutics 1. Comparison of prognosis data from 1990 (Matoba et al, Jpn Circ, Jan 1990 accessed at https://www.ncbi.nlm.nih.gov/pubmed/2332933) and from 2016 (Mozzafarian et al, Circulation 2016) Existing treatments improve quality of life in the short-term and offer some improvement in long-term survival though at high cost and with associated complications1 INXN-4001 addresses the multiple malfunctions of cardiomyocytes in patients with heart failure Triple-Gene LLC (formerly Xogenex LLC) is Intrexon’s majority-owned subsidiary
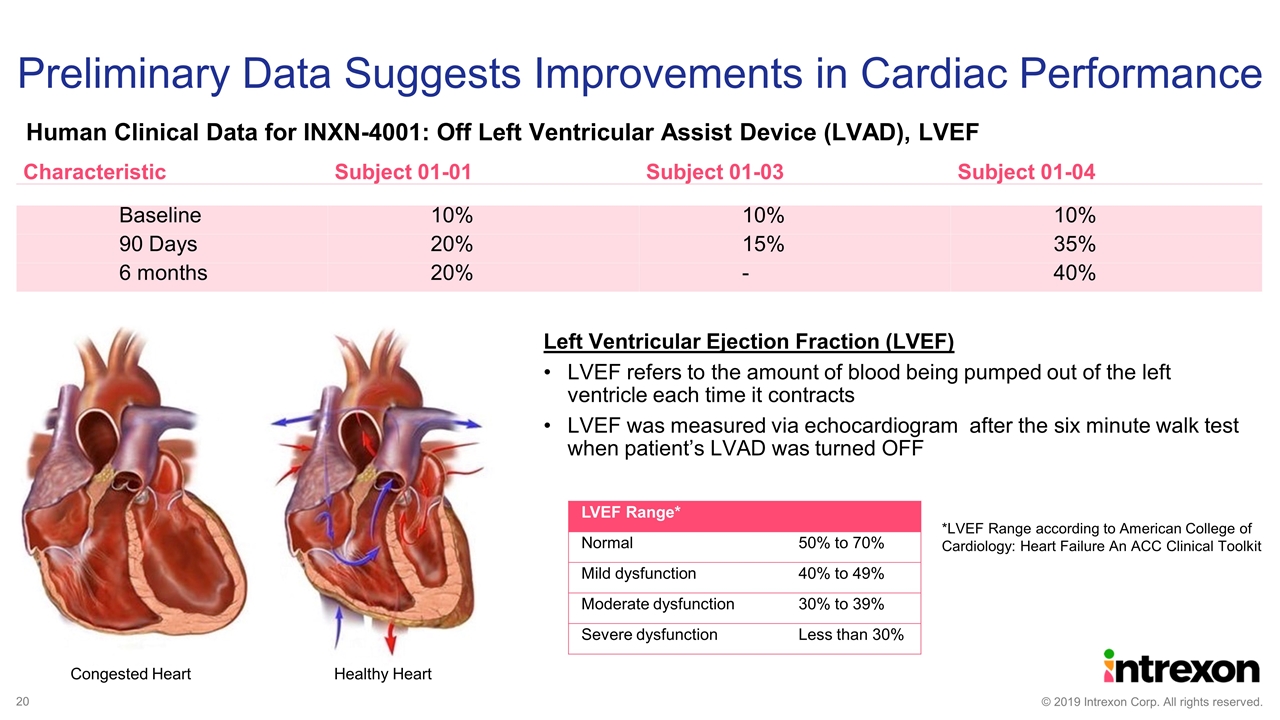
Preliminary Data Suggests Improvements in Cardiac Performance Characteristic Subject 01-01 Subject 01-03 Subject 01-04 Baseline 10% 10% 10% 90 Days 20% 15% 35% 6 months 20% - 40% Left Ventricular Ejection Fraction (LVEF) LVEF refers to the amount of blood being pumped out of the left ventricle each time it contracts LVEF was measured via echocardiogram after the six minute walk test when patient’s LVAD was turned OFF Congested Heart Healthy Heart LVEF Range* Normal 50% to 70% Mild dysfunction 40% to 49% Moderate dysfunction 30% to 39% Severe dysfunction Less than 30% *LVEF Range according to American College of Cardiology: Heart Failure An ACC Clinical Toolkit Human Clinical Data for INXN-4001: Off Left Ventricular Assist Device (LVAD), LVEF

Exemplar: Advancing Swine for Regenerative Medicine and Research Focus on regenerative medicine continues with Exemplar and the Mayo Clinic launching a joint venture, Cytotheryx, to develop high-quality source of human liver cells to advance medical research and address an estimated $250 million annual market opportunity Exemplar Genetics is developing porcine research models, which more accurately replicate human pathology as compared to traditional research models

Cannabinoids for Medical Use Intrexon’s proprietary yeast strains enable a transformative process for robust production of cannabinoids with consistent yield and purity Platform is designed to enable production of multiple target cannabinoids Intrexon Labs Hungary: Robust Microbial Production of Therapeutic Compounds Active Pharmaceutical Ingredients (APIs) Microbial production of APIs avoids resource intensive isolation from plant and animal sources offering potential for better consistency and purity of final product Intrexon’s proprietary pathway engineering has enabled development of microbial strains producing APIs, including the opioid intermediate thebaine
Diverse Delivery Modalities for Innovative Therapeutics FCX-007: An autologous dermal fibroblast genetically modified to express functional Type VII collagen (COL7) that is missing or deficient in patients with Recessive Dystrophic Epidermolysis Bullosa (RDEB) is currently in a Phase 1/2 clinical trial FCX-013: An autologous fibroblast genetically modified using lentivirus and encoded for matrix metalloproteinase 1 (MMP-1), a protein responsible for breaking down collagen to treat moderate to severe localized scleroderma is currently enrolling Phase 1 portion of a Phase 1/2 clinical trial Autologous Gene Therapies for Rare Skin Disorders Human exosomes developed for delivering diverse payloads to treat a broad array of diseases. Lead programs are focused on delivering bioactive RNAs to treat leukemias. Exosome-based Delivery of Bioactive Molecules UltraVector-optimized adeno-associated virus (AAV)-Frataxin for treatment of Friedreich’s Ataxia Gene Replacement Therapies for Rare Neurological Diseases Intrexon is collaborating with Fibrocell Science, Inc. (NASDAQ: FCSC) on FCX-007 and FCX-013; AAV-Frataxin is licensed to PTC Therapeutics, Inc. (NASDAQ: PTCT) Cell Manufacturing Photo courtesy of Fibrocell Science
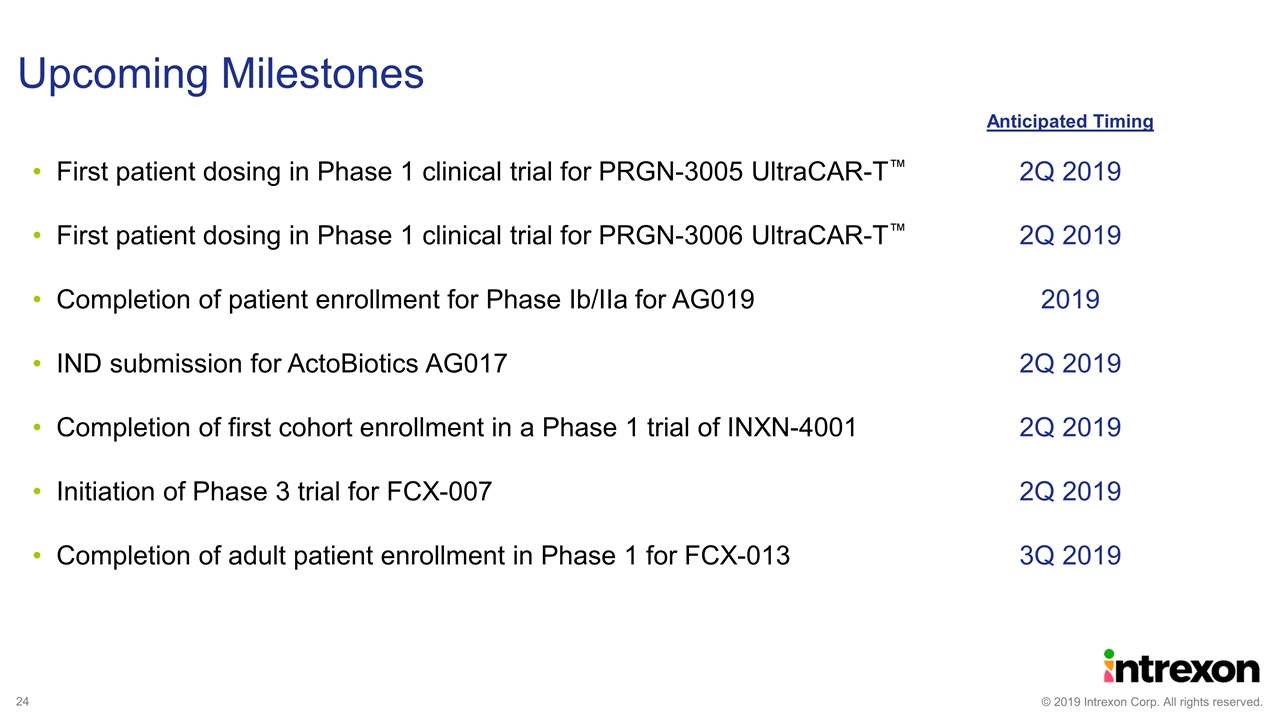
Upcoming Milestones 2Q 2019 2Q 2019 2019 2Q 2019 2Q 2019 2Q 2019 3Q 2019 Anticipated Timing First patient dosing in Phase 1 clinical trial for PRGN-3005 UltraCAR-T™ First patient dosing in Phase 1 clinical trial for PRGN-3006 UltraCAR-T™ Completion of patient enrollment for Phase Ib/IIa for AG019 IND submission for ActoBiotics AG017 Completion of first cohort enrollment in a Phase 1 trial of INXN-4001 Initiation of Phase 3 trial for FCX-007 Completion of adult patient enrollment in Phase 1 for FCX-013
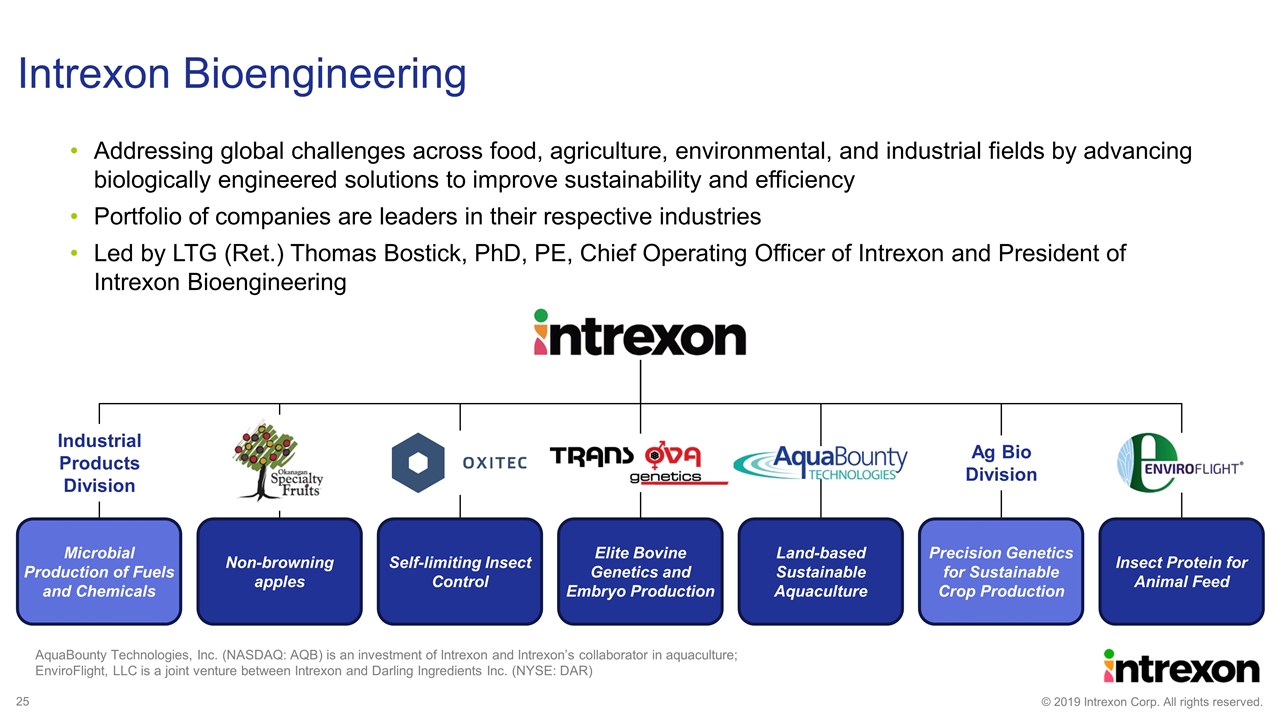
Intrexon Bioengineering Elite Bovine Genetics and Embryo Production Land-based Sustainable Aquaculture Non-browning apples Microbial Production of Fuels and Chemicals Precision Genetics for Sustainable Crop Production Insect Protein for Animal Feed Self-limiting Insect Control Ag Bio Division Industrial Products Division AquaBounty Technologies, Inc. (NASDAQ: AQB) is an investment of Intrexon and Intrexon’s collaborator in aquaculture; EnviroFlight, LLC is a joint venture between Intrexon and Darling Ingredients Inc. (NYSE: DAR) Addressing global challenges across food, agriculture, environmental, and industrial fields by advancing biologically engineered solutions to improve sustainability and efficiency Portfolio of companies are leaders in their respective industries Led by LTG (Ret.) Thomas Bostick, PhD, PE, Chief Operating Officer of Intrexon and President of Intrexon Bioengineering

Intrexon Corporate Highlights We are realigning to focus on Healthcare by forming Intrexon Health and Intrexon Bioengineering while streamlining management We believe the company will end the year with approximately the same net cash and short-term investment position that it held on April 3, 2019, achieving this through a combination of partnering, asset sales and operating cost reductions Numerous processes and conversations underway relative to asset sales and partnering We prepare for an IPO of Precigen, pending data and/or transaction

























