
Intrexon Second Quarter 2019 Business Update August 2019 Exhibit 99.2

Forward Looking Statements Safe Harbor Statement Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s business, discussion of anticipated clinical trials and future collaborations, and possible other transactions. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s strategy and overall approach to its business model, its efforts to realign its business, and its ability to exercise more control and ownership over the development process and commercialization path; (ii) Intrexon’s ability to successfully enter new markets or develop additional products, including the expected timing and results of investigational studies and preclinical and clinical trials, whether with its collaborators or independently; (iii) Intrexon's ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (iv) Intrexon’s ability to hold or generate significant operating capital, including through partnering, asset sales and operating cost reductions; (v) actual or anticipated variations in Intrexon’s operating results; (vi) actual or anticipated fluctuations in Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (vii) Intrexon’s cash position; (viii) market conditions in Intrexon’s industry; (ix) the volatility of Intrexon’s stock price; (x) Intrexon’s ability, and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (xi) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations and policies; (xii) the outcomes of pending and future litigation; (xiii) the rate and degree of market acceptance of any products developed by Intrexon, its subsidiaries, collaborations or joint ventures; (xiv) Intrexon’s ability to retain and recruit key personnel; (xv) Intrexon’s expectations related to the use of proceeds from its public offerings and other financing efforts; and (xvi) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intrexon’s Annual Report on Form 10-K for the fiscal year ended December 31, 2018 and subsequent reports filed with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law. All of the pharmaceutical products described in this presentation are investigational new drugs, which are currently undergoing pre-clinical and/or human clinical trial testing. As a result, none of them have had their safety or efficacy established or are approved by the U.S. Food and Drug Administration or any other regulatory agency. © 2019 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon.

First Patients Dosed with Precigen’s Transformative PRGN-3005 and PRGN-3006 UltraCAR-T™ Therapies Status Phase1 study to evaluate safety and maximal tolerated dose is recruiting patients Study in collaboration with University of Washington and Fred Hutchinson Cancer Center Patient Population Advanced stage platinum resistant ovarian cancer 300k diagnosed annually1/22k in US2 Stage IV survival as low as 20%3 PRGN-3005 UltraCAR-T™ 1World Health Organization, International Agency for Research on Cancer, Global Cancer Observatory. Cancer Today, Estimated number of new cases in 2018, worldwide, both sexes, all ages. Accessed December 2018 via WHO IARC GCO website. 2American Cancer Society Ovarian Cancer Special Section. Access December 2018 via ACS website. 3American Cancer Society. Survival Rates for Ovarian Cancer, by Stage. Accessed December 2018 via ACS website. 4American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML). Accessed December 2018 via ACS website. 5American Cancer Society. Key Statistics for Myelodysplastic Syndromes. Accessed December 2018 via ACS website. Status Phase 1/1b study to evaluate safety and maximal tolerated dose is recruiting patients Study in collaboration with H. Lee Moffitt Cancer Center Patient Population Relapsed or refractory acute myeloid leukemia (AML) 20k diagnosed in US in 20184 Higher risk myelodysplastic syndrome (MDS) US incidence >10k per year5 PRGN-3006 UltraCAR-T™ Non-viral Sleeping Beauty system to co-express CAR, mbIL15 and kill switch UltraCAR-T™ CAR mbIL-15 Kill Switch
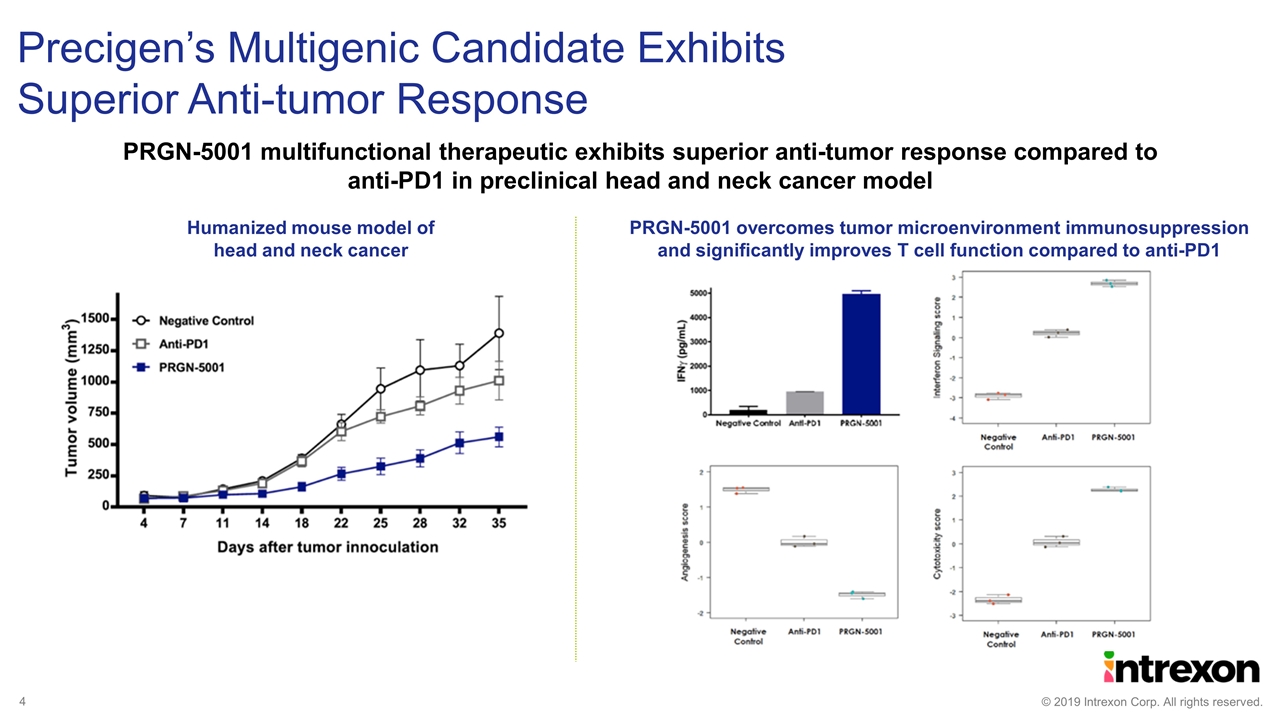
Precigen’s Multigenic Candidate Exhibits Superior Anti-tumor Response PRGN-5001 multifunctional therapeutic exhibits superior anti-tumor response compared to anti-PD1 in preclinical head and neck cancer model Humanized mouse model of head and neck cancer PRGN-5001 overcomes tumor microenvironment immunosuppression and significantly improves T cell function compared to anti-PD1
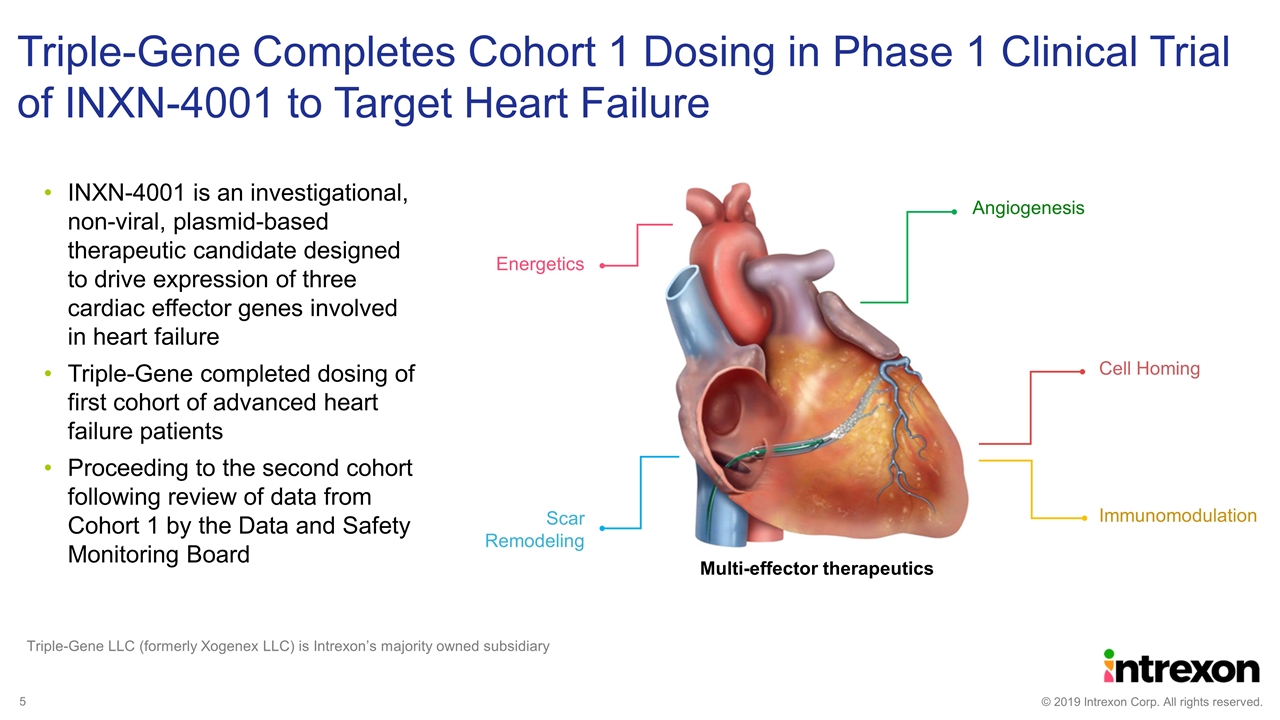
Triple-Gene Completes Cohort 1 Dosing in Phase 1 Clinical Trial of INXN-4001 to Target Heart Failure Triple-Gene LLC (formerly Xogenex LLC) is Intrexon’s majority owned subsidiary INXN-4001 is an investigational, non-viral, plasmid-based therapeutic candidate designed to drive expression of three cardiac effector genes involved in heart failure Triple-Gene completed dosing of first cohort of advanced heart failure patients Proceeding to the second cohort following review of data from Cohort 1 by the Data and Safety Monitoring Board Immunomodulation Cell Homing Energetics Scar Remodeling Multi-effector therapeutics Angiogenesis
ActoBio Therapeutics Continues Clinical Progress on Microbe-based Delivery of Biopharmaceuticals Initiated enrollment of next two patient cohorts of Phase Ib/IIa clinical trial for investigational drug AG019 for treatment of Type 1 Diabetes AG019 dosing in adolescents (patients 12-17 years of age) Phase IIa arm combination dosing of AG019 plus teplizumab (PRV-031) in adults Partner Oragenics nearing completion of enrollment of patients in the Phase IIb clinical trial for AG013 for the treatment of Oral Mucositis Intestinal (capsule) Buccal (Sublingual/rinse) Oral ActoBiotics®

Okanagan Specialty Fruits (OSF) Expanding Market Plans Placed fresh sliced Arctic® apples in select markets, as well as dehydrated ApBitz™ apple snack on Amazon and in retail stores Planted 955,000 new Arctic® apple trees on 650 acres in Washington State, including first 80 acres of Arctic® Fujis, which received approval from the US FDA in April Expecting to crop 217 acres of orchard in Sep/Oct with an estimated 10,000 bins or 8 million lbs of apples anticipated (5-fold increase over 2018) Planning on increasing product range to include food service and additional fresh slice retail with 2019 go-to-market plan

Oxitec 2nd Gen Friendly™ Aedes Advancing Pilot Programs Completed the first pilot project of its 2nd Generation Friendly™ Aedes aegypti technology in the city of Indaiatuba, Brazil Demonstrated the new strain’s effectiveness (treatment area compared to untreated control area) in suppressing populations of Aedes aegypti in four densely populated urban communities throughout the city of Indaiatuba Submitted an Experimental Use Permit (EUP) to the Environmental Protection Agency (EPA) for the first US-based pilot project with its 2nd generation mosquito 2nd Gen Friendly™ Aedes is designed to enable greater cost-effectiveness and scalability over Oxitec’s 1st generation mosquito

Operational Updates Identification and implementation of significant operating cost reductions Concentrating focus on overall net cash and short-term investment position Transactions and Cost Savings: Prospective acquisition of Exemplar Genetics Considering offers for sale of Trans Ova Genetics Implemented targeted reductions of non-essential programs including closure of Animal Sciences Division Expected Closing 30 days Q4 completed










