
Exhibit 99.2 Precigen, Inc. 2Q-2020 Business Update 10 August 2020 1Exhibit 99.2 Precigen, Inc. 2Q-2020 Business Update 10 August 2020 1

Forward-looking Statements Some of the statements made in this presentation are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Precigen's current expectations and projections about future events and generally relate to plans, objectives and expectations for the development of Precigen's business and can be identified by forward-looking words such as “may,” “will,” “potential,” “seek,” “expect,” “believe,” “anticipate,” “intend,” “continue,” “opportunity,” “groundwork,” “poised,” “future,” “update” and similar expressions. Examples of forward-looking statements in this presentation, include statements about the timing, pace and progress of preclinical and clinical trials and discovery programs, potential benefits of platforms and product candidates including in comparison to competitive platforms and products, and future plans for the company’s remaining non-healthcare assets. Although management believes that the plans, objectives and results reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) the impact of the COVID-19 pandemic on our businesses, operating results, cash flows and/or financial condition, (ii) ongoing transition efforts following the company’s recent divestment of several assets and businesses, (iii) Precigen’s strategy and overall approach to its business model, its recent efforts to realign its business, and its ability to exercise more control and ownership over the development process and commercialization path; (iv) the ability to successfully enter new markets or develop additional products, including the expected timing and results of investigational studies and preclinical and clinical trials, including any delays or potential delays as a result of the COVID-19 pandemic, whether with its collaborators or independently; (v) the ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (vi) the ability to hold or generate significant operating capital, including through partnering, asset sales and operating cost reductions; (vii) actual or anticipated variations in operating results; (viii) actual or anticipated fluctuations in competitors’ or collaborators’ operating results or changes in their respective growth rates; (ix) cash position; (x) market conditions in the company’s industry; (xi) the volatility of Precigen’s stock price; (xii) the ability, and the ability of collaborators, to protect Precigen’s intellectual property and other proprietary rights and technologies; (xiii) the ability, and the ability of collaborators, to adapt to changes in laws or regulations and policies, including federal, state, and local government responses to the COVID-19 pandemic; (xiv) outcomes of pending and future litigation; (xv) the rate and degree of market acceptance of any products developed by Precigen, its subsidiaries, collaborations or joint ventures; (xvi) the ability to retain and recruit key personnel; (xvii) expectations related to the use of proceeds from public offerings and other financing efforts; (xviii) estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xix) the challenges inherent in leadership transitions. For a discussion of other risks and uncertainties, and other important factors, any of which could cause actual results to differ from those contained in the forward-looking statements, see the section entitled Risk Factors in Precigen’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Precigen’s subsequent filings with the Securities and Exchange Commission. All informationin thispresentationisasof the date itscover page,and Precigen undertakes no duty to update this information unless required by law. This presentation includes reference to Segment Adjusted EBITDA, which is a non-GAAP financial measure. This measure is provided as additional information, not as an alternative to GAAP measures, and is intended to enhance an overall understanding of Precigen’s financial performance. A reconciliation of Segment AEBITDA to net loss from continuing operations before income taxes has been furnished on an exhibit to Precigen’s current report on Form 8-K shortly prior to this presentation. All of the pharmaceutical products described in this presentation are investigational new drugs, which are currently undergoing pre-clinical and/or human clinical trial testing. As a result, none of them have had their safety or efficacy established or are approved by the U.S. Food and Drug Administration or any other regulatory agency. © 2020 Precigen, Inc. All rights reserved. 2Forward-looking Statements Some of the statements made in this presentation are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Precigen's current expectations and projections about future events and generally relate to plans, objectives and expectations for the development of Precigen's business and can be identified by forward-looking words such as “may,” “will,” “potential,” “seek,” “expect,” “believe,” “anticipate,” “intend,” “continue,” “opportunity,” “groundwork,” “poised,” “future,” “update” and similar expressions. Examples of forward-looking statements in this presentation, include statements about the timing, pace and progress of preclinical and clinical trials and discovery programs, potential benefits of platforms and product candidates including in comparison to competitive platforms and products, and future plans for the company’s remaining non-healthcare assets. Although management believes that the plans, objectives and results reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) the impact of the COVID-19 pandemic on our businesses, operating results, cash flows and/or financial condition, (ii) ongoing transition efforts following the company’s recent divestment of several assets and businesses, (iii) Precigen’s strategy and overall approach to its business model, its recent efforts to realign its business, and its ability to exercise more control and ownership over the development process and commercialization path; (iv) the ability to successfully enter new markets or develop additional products, including the expected timing and results of investigational studies and preclinical and clinical trials, including any delays or potential delays as a result of the COVID-19 pandemic, whether with its collaborators or independently; (v) the ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (vi) the ability to hold or generate significant operating capital, including through partnering, asset sales and operating cost reductions; (vii) actual or anticipated variations in operating results; (viii) actual or anticipated fluctuations in competitors’ or collaborators’ operating results or changes in their respective growth rates; (ix) cash position; (x) market conditions in the company’s industry; (xi) the volatility of Precigen’s stock price; (xii) the ability, and the ability of collaborators, to protect Precigen’s intellectual property and other proprietary rights and technologies; (xiii) the ability, and the ability of collaborators, to adapt to changes in laws or regulations and policies, including federal, state, and local government responses to the COVID-19 pandemic; (xiv) outcomes of pending and future litigation; (xv) the rate and degree of market acceptance of any products developed by Precigen, its subsidiaries, collaborations or joint ventures; (xvi) the ability to retain and recruit key personnel; (xvii) expectations related to the use of proceeds from public offerings and other financing efforts; (xviii) estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xix) the challenges inherent in leadership transitions. For a discussion of other risks and uncertainties, and other important factors, any of which could cause actual results to differ from those contained in the forward-looking statements, see the section entitled Risk Factors in Precigen’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Precigen’s subsequent filings with the Securities and Exchange Commission. All informationin thispresentationisasof the date itscover page,and Precigen undertakes no duty to update this information unless required by law. This presentation includes reference to Segment Adjusted EBITDA, which is a non-GAAP financial measure. This measure is provided as additional information, not as an alternative to GAAP measures, and is intended to enhance an overall understanding of Precigen’s financial performance. A reconciliation of Segment AEBITDA to net loss from continuing operations before income taxes has been furnished on an exhibit to Precigen’s current report on Form 8-K shortly prior to this presentation. All of the pharmaceutical products described in this presentation are investigational new drugs, which are currently undergoing pre-clinical and/or human clinical trial testing. As a result, none of them have had their safety or efficacy established or are approved by the U.S. Food and Drug Administration or any other regulatory agency. © 2020 Precigen, Inc. All rights reserved. 2

Q2 2020 Financial Highlights Improvement in quarter over quarter Segment Adjusted EBITDA Reduction in capital requirements driven by suspension of MBP Titan operations and streamlining corporate functions Trans Ova Genetics and Precigen Exemplar cash flow positive in H1 2020 Current cash on hand sufficient to fund operations into late 2021 3Q2 2020 Financial Highlights Improvement in quarter over quarter Segment Adjusted EBITDA Reduction in capital requirements driven by suspension of MBP Titan operations and streamlining corporate functions Trans Ova Genetics and Precigen Exemplar cash flow positive in H1 2020 Current cash on hand sufficient to fund operations into late 2021 3
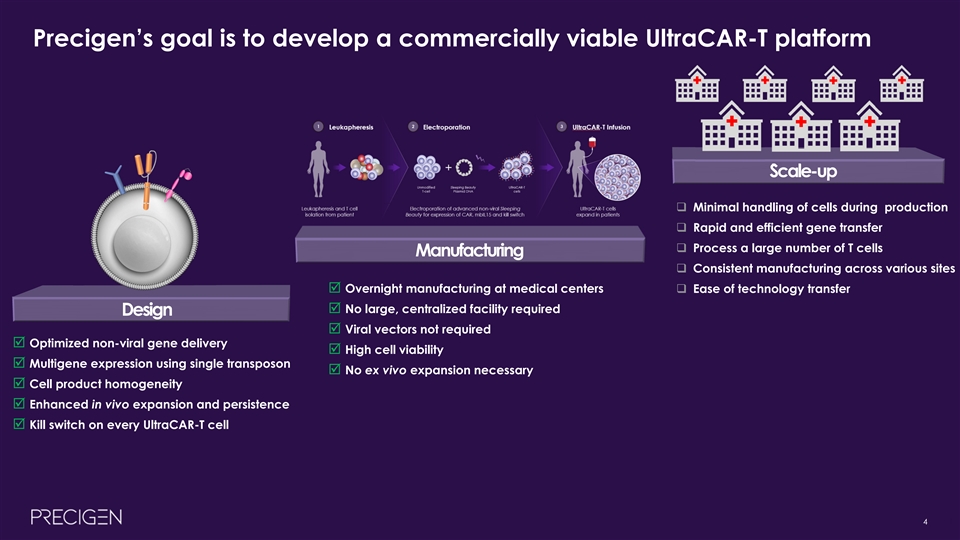
Precigen’s goal is to develop a commercially viable UltraCAR-T platform Scale-up q Minimal handling of cells during production q Rapid and efficient gene transfer q Process a large number of T cells Manufacturing q Consistent manufacturing across various sites þ Overnight manufacturing at medical centersq Ease of technology transfer þ No large, centralized facility required Design þ Viral vectors not required þ Optimized non-viral gene delivery þ High cell viability þ Multigene expression using single transposon þ No ex vivo expansion necessary þ Cell product homogeneity þ Enhanced in vivo expansion and persistence þ Kill switch on every UltraCAR-T cell 4 4Precigen’s goal is to develop a commercially viable UltraCAR-T platform Scale-up q Minimal handling of cells during production q Rapid and efficient gene transfer q Process a large number of T cells Manufacturing q Consistent manufacturing across various sites þ Overnight manufacturing at medical centersq Ease of technology transfer þ No large, centralized facility required Design þ Viral vectors not required þ Optimized non-viral gene delivery þ High cell viability þ Multigene expression using single transposon þ No ex vivo expansion necessary þ Cell product homogeneity þ Enhanced in vivo expansion and persistence þ Kill switch on every UltraCAR-T cell 4 4

Today’s electroporation devices have scale-up limitations § Relatively low throughput § Processing of one cuvette at a time § Labor and time intensive process § Multiple batches needed for manufacturing a dose § Manual handling may increase contamination risk 5Today’s electroporation devices have scale-up limitations § Relatively low throughput § Processing of one cuvette at a time § Labor and time intensive process § Multiple batches needed for manufacturing a dose § Manual handling may increase contamination risk 5

™ UltraPorator is designed to commercially scale-up UltraCAR-T manufacturing § Precigen’s proprietary system § Electroporation optimized for UltraCAR-T § Semi-closed system minimizes manual handling § Rapid, high efficiency gene transfer protocol § High throughput system handles a large number of T cells per batch 6™ UltraPorator is designed to commercially scale-up UltraCAR-T manufacturing § Precigen’s proprietary system § Electroporation optimized for UltraCAR-T § Semi-closed system minimizes manual handling § Rapid, high efficiency gene transfer protocol § High throughput system handles a large number of T cells per batch 6
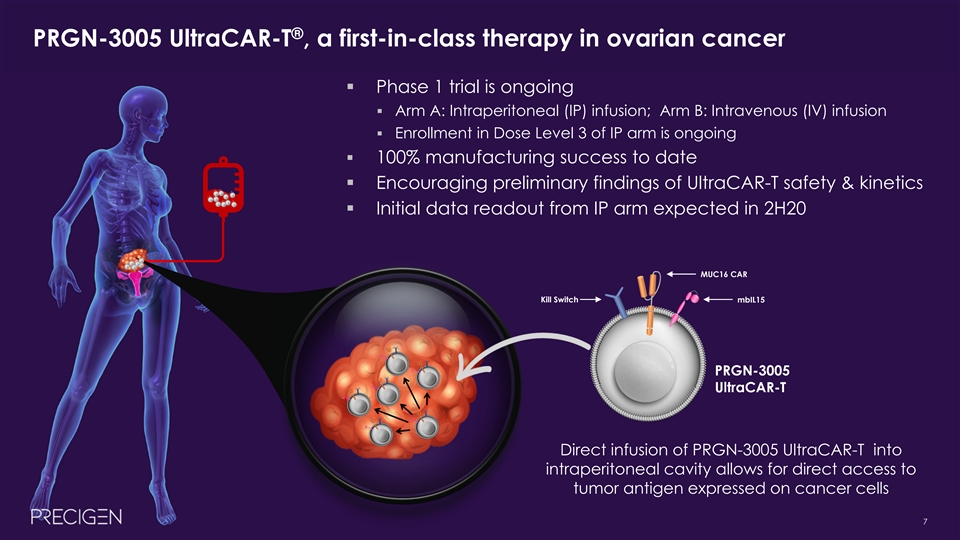
® PRGN-3005 UltraCAR-T , a first-in-class therapy in ovarian cancer § Phase 1 trial is ongoing § Arm A: Intraperitoneal (IP) infusion; Arm B: Intravenous (IV) infusion § Enrollment in Dose Level 3 of IP arm is ongoing § 100% manufacturing success to date § Encouraging preliminary findings of UltraCAR-T safety & kinetics § Initial data readout from IP arm expected in 2H20 MUC16 CAR Kill Switch mbIL15 PRGN-3005 UltraCAR-T Direct infusion of PRGN-3005 UltraCAR-T into intraperitoneal cavity allows for direct access to tumor antigen expressed on cancer cells 7® PRGN-3005 UltraCAR-T , a first-in-class therapy in ovarian cancer § Phase 1 trial is ongoing § Arm A: Intraperitoneal (IP) infusion; Arm B: Intravenous (IV) infusion § Enrollment in Dose Level 3 of IP arm is ongoing § 100% manufacturing success to date § Encouraging preliminary findings of UltraCAR-T safety & kinetics § Initial data readout from IP arm expected in 2H20 MUC16 CAR Kill Switch mbIL15 PRGN-3005 UltraCAR-T Direct infusion of PRGN-3005 UltraCAR-T into intraperitoneal cavity allows for direct access to tumor antigen expressed on cancer cells 7
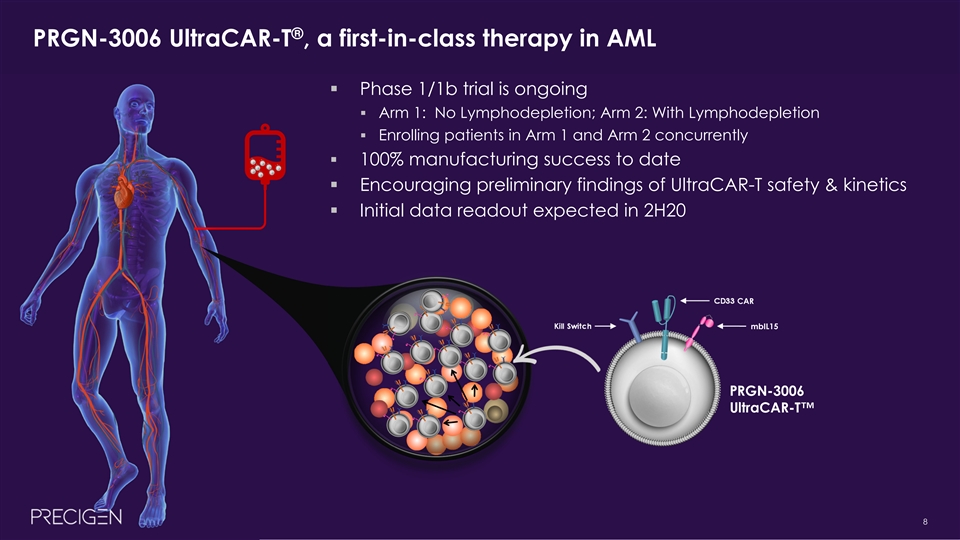
® PRGN-3006 UltraCAR-T , a first-in-class therapy in AML § Phase 1/1b trial is ongoing § Arm 1: No Lymphodepletion; Arm 2: With Lymphodepletion § Enrolling patients in Arm 1 and Arm 2 concurrently § 100% manufacturing success to date § Encouraging preliminary findings of UltraCAR-T safety & kinetics § Initial data readout expected in 2H20 CD33 CAR Kill Switch mbIL15 PRGN-3006 UltraCAR-T™ 8® PRGN-3006 UltraCAR-T , a first-in-class therapy in AML § Phase 1/1b trial is ongoing § Arm 1: No Lymphodepletion; Arm 2: With Lymphodepletion § Enrolling patients in Arm 1 and Arm 2 concurrently § 100% manufacturing success to date § Encouraging preliminary findings of UltraCAR-T safety & kinetics § Initial data readout expected in 2H20 CD33 CAR Kill Switch mbIL15 PRGN-3006 UltraCAR-T™ 8
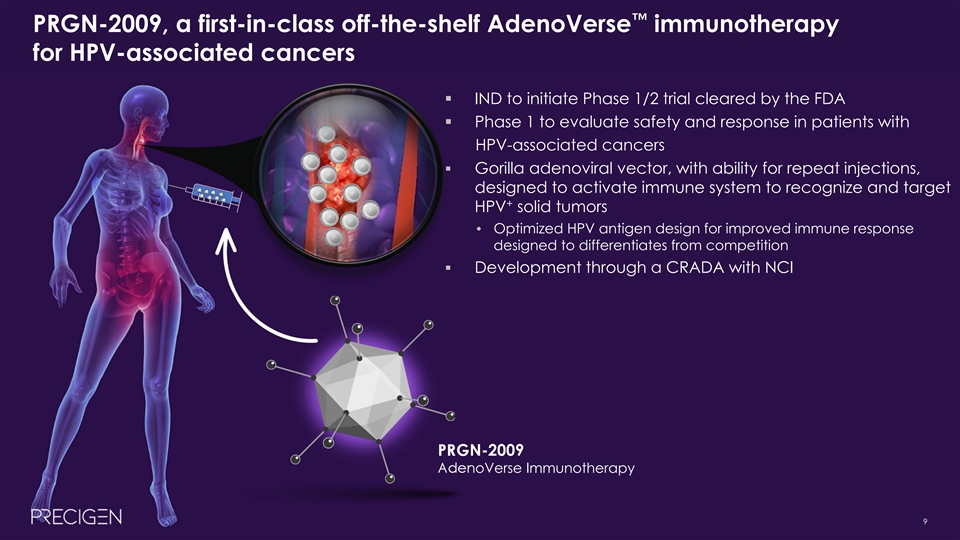
™ PRGN-2009, a first-in-class off-the-shelf AdenoVerse immunotherapy for HPV-associated cancers § IND to initiate Phase 1/2 trial cleared by the FDA § Phase 1 to evaluate safety and response in patients with HPV-associated cancers § Gorilla adenoviral vector, with ability for repeat injections, designed to activate immune system to recognize and target + HPV solid tumors • Optimized HPV antigen design for improved immune response designed to differentiates from competition § Development through a CRADA with NCI PRGN-2009 AdenoVerse Immunotherapy 9™ PRGN-2009, a first-in-class off-the-shelf AdenoVerse immunotherapy for HPV-associated cancers § IND to initiate Phase 1/2 trial cleared by the FDA § Phase 1 to evaluate safety and response in patients with HPV-associated cancers § Gorilla adenoviral vector, with ability for repeat injections, designed to activate immune system to recognize and target + HPV solid tumors • Optimized HPV antigen design for improved immune response designed to differentiates from competition § Development through a CRADA with NCI PRGN-2009 AdenoVerse Immunotherapy 9
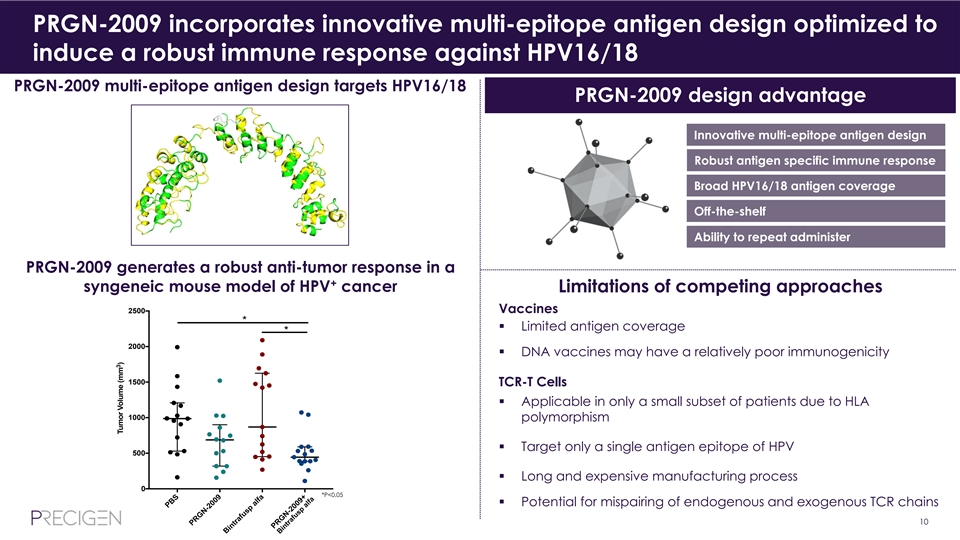
PRGN-2009 incorporates innovative multi-epitope antigen design optimized to induce a robust immune response against HPV16/18 PRGN-2009 multi-epitope antigen design targets HPV16/18 PRGN-2009 design advantage Innovative multi-epitope antigen design Robust antigen specific immune response Broad HPV16/18 antigen coverage Off-the-shelf Ability to repeat administer PRGN-2009 generates a robust anti-tumor response in a + syngeneic mouse model of HPV cancer Limitations of competing approaches 2500 Vaccines * § Limited antigen coverage * 2000 § DNA vaccines may have a relatively poor immunogenicity 1500 TCR-T Cells § Applicable in only a small subset of patients due to HLA 1000 polymorphism § Target only a single antigen epitope of HPV 500 § Long and expensive manufacturing process 0 *P<0.05 § Potential for mispairing of endogenous and exogenous TCR chains 10 PBS PRGN-2009 B intrafusp alfa PRGN-2009+ 3 Tumor Volume (mm )PRGN-2009 incorporates innovative multi-epitope antigen design optimized to induce a robust immune response against HPV16/18 PRGN-2009 multi-epitope antigen design targets HPV16/18 PRGN-2009 design advantage Innovative multi-epitope antigen design Robust antigen specific immune response Broad HPV16/18 antigen coverage Off-the-shelf Ability to repeat administer PRGN-2009 generates a robust anti-tumor response in a + syngeneic mouse model of HPV cancer Limitations of competing approaches 2500 Vaccines * § Limited antigen coverage * 2000 § DNA vaccines may have a relatively poor immunogenicity 1500 TCR-T Cells § Applicable in only a small subset of patients due to HLA 1000 polymorphism § Target only a single antigen epitope of HPV 500 § Long and expensive manufacturing process 0 *P<0.05 § Potential for mispairing of endogenous and exogenous TCR chains 10 PBS PRGN-2009 B intrafusp alfa PRGN-2009+ 3 Tumor Volume (mm )
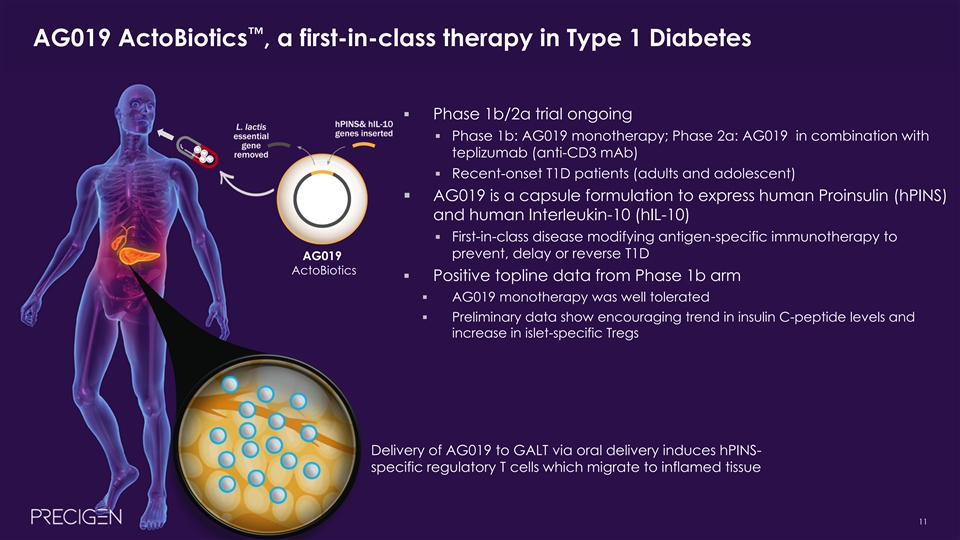
™ AG019 ActoBiotics , a first-in-class therapy in Type 1 Diabetes § Phase 1b/2a trial ongoing § Phase 1b: AG019 monotherapy; Phase 2a: AG019 in combination with teplizumab (anti-CD3 mAb) § Recent-onset T1D patients (adults and adolescent) § AG019 is a capsule formulation to express human Proinsulin (hPINS) and human Interleukin-10 (hIL-10) § First-in-class disease modifying antigen-specific immunotherapy to prevent, delay or reverse T1D AG019 ActoBiotics § Positive topline data from Phase 1b arm § AG019 monotherapy was well tolerated § Preliminary data show encouraging trend in insulin C-peptide levels and increase in islet-specific Tregs Delivery of AG019 to GALT via oral delivery induces hPINS- specific regulatory T cells which migrate to inflamed tissue 11™ AG019 ActoBiotics , a first-in-class therapy in Type 1 Diabetes § Phase 1b/2a trial ongoing § Phase 1b: AG019 monotherapy; Phase 2a: AG019 in combination with teplizumab (anti-CD3 mAb) § Recent-onset T1D patients (adults and adolescent) § AG019 is a capsule formulation to express human Proinsulin (hPINS) and human Interleukin-10 (hIL-10) § First-in-class disease modifying antigen-specific immunotherapy to prevent, delay or reverse T1D AG019 ActoBiotics § Positive topline data from Phase 1b arm § AG019 monotherapy was well tolerated § Preliminary data show encouraging trend in insulin C-peptide levels and increase in islet-specific Tregs Delivery of AG019 to GALT via oral delivery induces hPINS- specific regulatory T cells which migrate to inflamed tissue 11
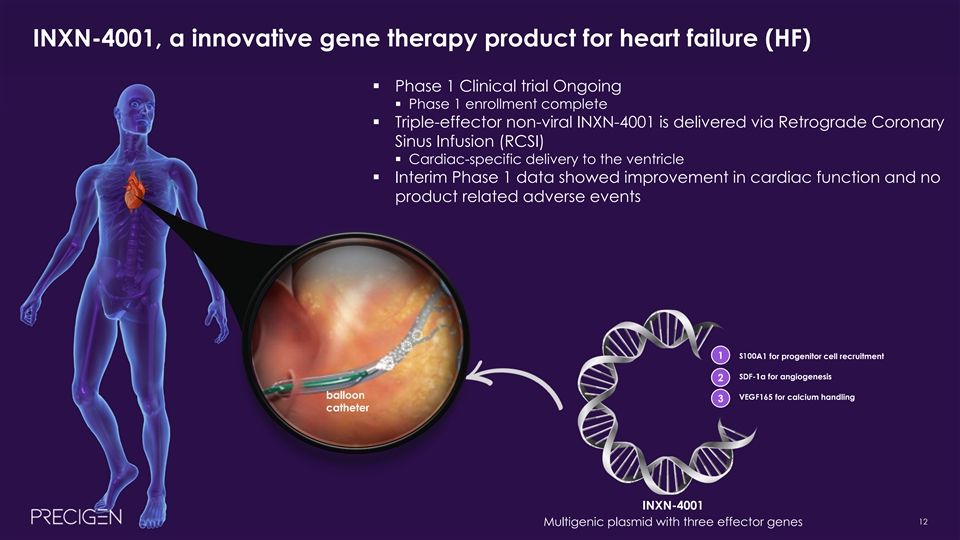
INXN-4001, a innovative gene therapy product for heart failure (HF) § Phase 1 Clinical trial Ongoing § Phase 1 enrollment complete § Triple-effector non-viral INXN-4001 is delivered via Retrograde Coronary Sinus Infusion (RCSI) § Cardiac-specific delivery to the ventricle § Interim Phase 1 data showed improvement in cardiac function and no product related adverse events 1 1. S100A1 for progenitor cell recruitment 2. SDF-1α for angiogenesis 2 balloon 3. VEGF165 for calcium handling 3 catheter INXN-4001 12 Multigenic plasmid with three effector genes INTERNAL & CONFIDENTIALINXN-4001, a innovative gene therapy product for heart failure (HF) § Phase 1 Clinical trial Ongoing § Phase 1 enrollment complete § Triple-effector non-viral INXN-4001 is delivered via Retrograde Coronary Sinus Infusion (RCSI) § Cardiac-specific delivery to the ventricle § Interim Phase 1 data showed improvement in cardiac function and no product related adverse events 1 1. S100A1 for progenitor cell recruitment 2. SDF-1α for angiogenesis 2 balloon 3. VEGF165 for calcium handling 3 catheter INXN-4001 12 Multigenic plasmid with three effector genes INTERNAL & CONFIDENTIAL

1313












