39th Annual J.P. Morgan Healthcare Conference 13 January 2021 Helen Sabzevari, PhD President & CEO EXHIBIT 99.1

Forward-looking Statements Some of the statements made in this presentation are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Precigen's current expectations and projections about future events and generally relate to plans, objectives and expectations for the development of Precigen's business and can be identified by forward-looking words such as “may,” “will,” “potential,” “seek,” “expect,” “believe,” “anticipate,” “intend,” “continue,” “opportunity,” “groundwork,” “poised,” “future,” “update” and similar expressions. Examples of forward-looking statements in his presentation, include statements about the timing, pace and progress of preclinical and clinical trials and discovery programs, and potential benefits of platforms and product candidates including in comparison to competitive platforms and products. Although management believes that the plans, objectives and results reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to: (i) the impact of the COVID-19 pandemic on Precigen’s businesses, operating results, cash flows and/or financial condition; (ii) Precigen’s strategy and overall approach to its business model; (iii) the uncertain timing and results of investigational studies and preclinical and clinical trials, including any delays or potential delays as a result of the COVID-19 pandemic; (iv) the fact that interim and preliminary results may change as more data becomes available and are subject to procedures that could result in changes to the final data, and results in early-stage clinical trials may not be indicative of results in later-stage clinical trials; (v) the lengthy and expensive clinical development process and the potential difficulty in enrolling patients; (vi) the lengthy and unpredictable nature of the regulatory approval process; (vii) Precigen’s limited experience designing and implementing clinical trials; (viii) the ability to successfully enter into optimal strategic relationships with its subsidiaries and operating companies that it may form in the future; (ix) the ability to hold or generate significant operating capital, including through partnering, asset sales and operating cost reductions; (vii) actual or anticipated variations in operating results; (x) cash position; (xi) market conditions in the company’s industry; (xii) the volatility of Precigen’s stock price; (xiii) the ability, and the ability of collaborators, to protect Precigen’s intellectual property and other proprietary rights and technologies; (xiv) the ability, and the ability of collaborators, to adapt to changes in laws or regulations and policies, including federal, state, and local government responses to the COVID-19 pandemic; (xv) outcomes of pending and future litigation; (xvi) the ability to retain and recruit key personnel; and (xvii) expectations related to the use of proceeds from public offerings and other financing efforts. For a discussion of other risks and uncertainties, and other important factors, any of which could cause actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in Precigen’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Precigen’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of its cover page, and Precigen undertakes no duty to update this information unless required by law. This presentation contains market data and industry statistics and forecasts based on studies and clinical trials sponsored by third parties, independent industry publications and other publicly available information. Although Precigen believes these sources are reliable, it does not guarantee the accuracy or completeness of this information and has not verified this data. All of the pharmaceutical products described in this presentation are investigational new drugs, which are currently undergoing pre-clinical and/or human clinical trial testing. As a result, none of them have had their safety or efficacy established or are approved by the U.S. Food and Drug Administration or any other regulatory agency. © 2021 Precigen, Inc. All rights reserved.
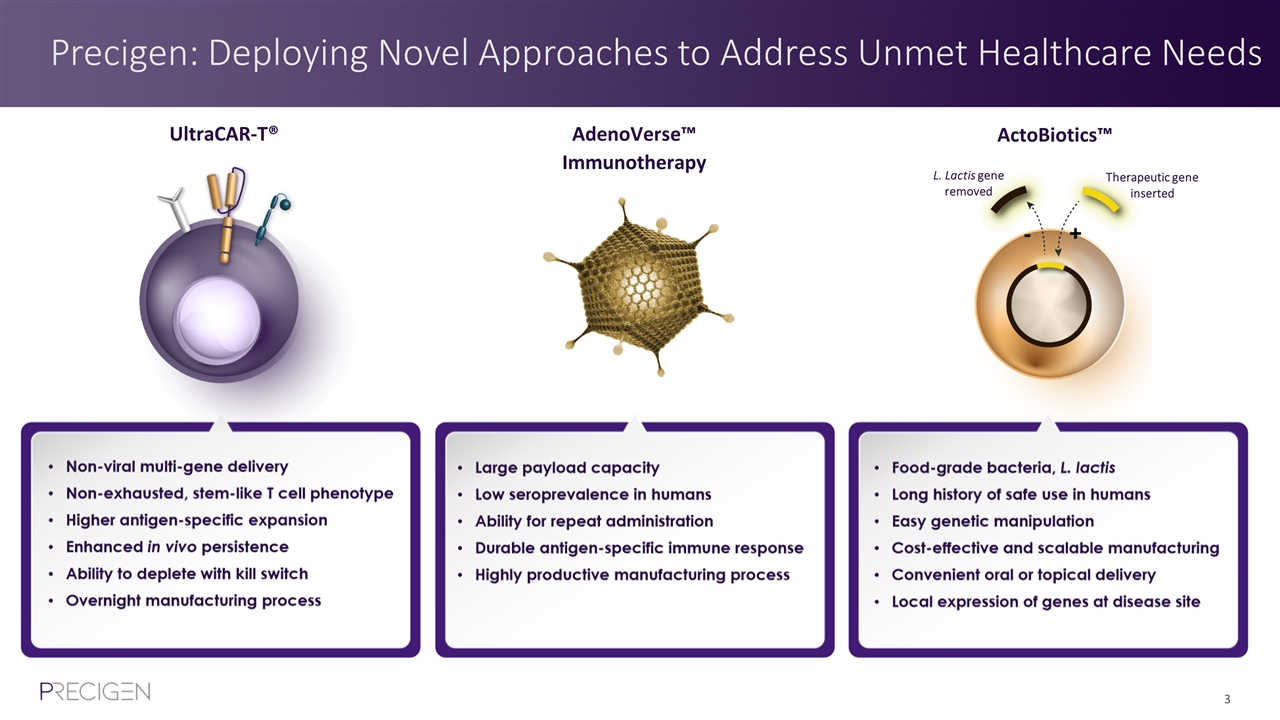
Precigen: Deploying Novel Approaches to Address Unmet Healthcare Needs L. Lactis gene removed Therapeutic gene inserted UltraCAR-T® AdenoVerse™ Immunotherapy ActoBiotics™
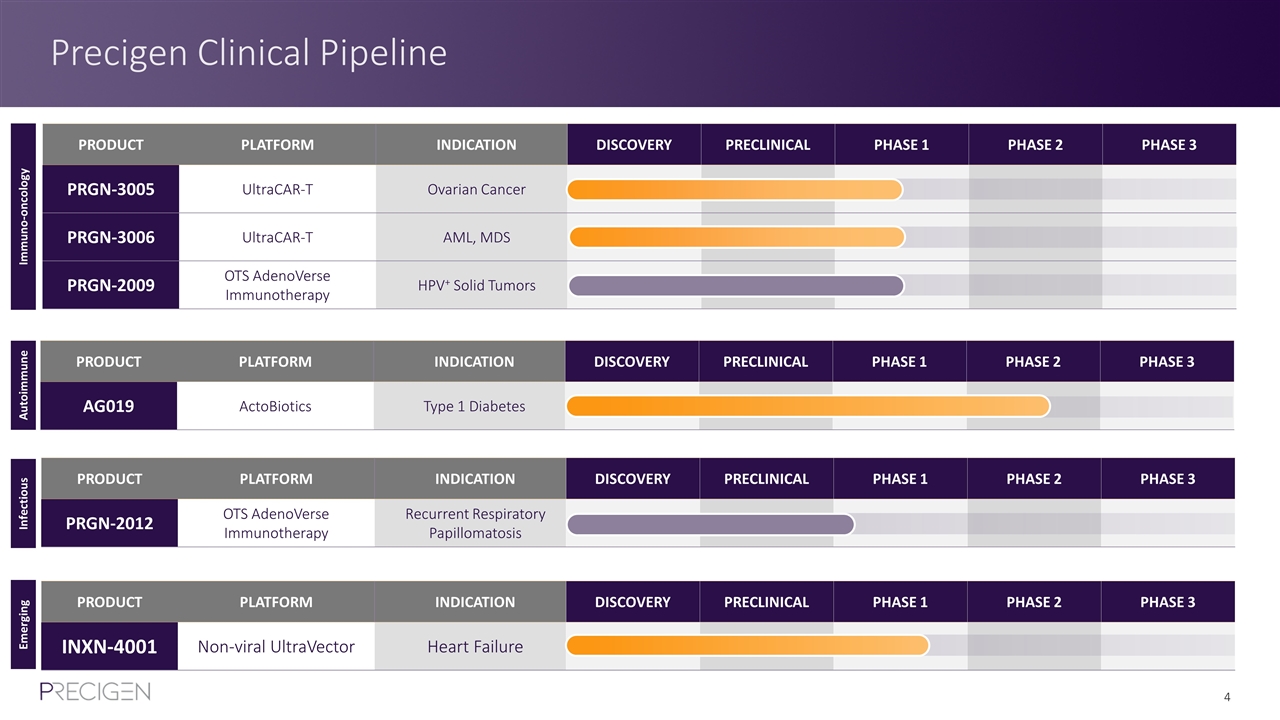
Precigen Clinical Pipeline PRODUCT PLATFORM INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PRGN-3005 UltraCAR-T Ovarian Cancer PRGN-3006 UltraCAR-T AML, MDS PRGN-2009 OTS AdenoVerse Immunotherapy HPV+ Solid Tumors PRODUCT PLATFORM INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 AG019 ActoBiotics Type 1 Diabetes PRODUCT PLATFORM INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 INXN-4001 Non-viral UltraVector Heart Failure PRODUCT PLATFORM INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PRGN-2012 OTS AdenoVerse Immunotherapy Recurrent Respiratory Papillomatosis Immuno-oncology Autoimmune Infectious Emerging

ActoBiotics®Platform
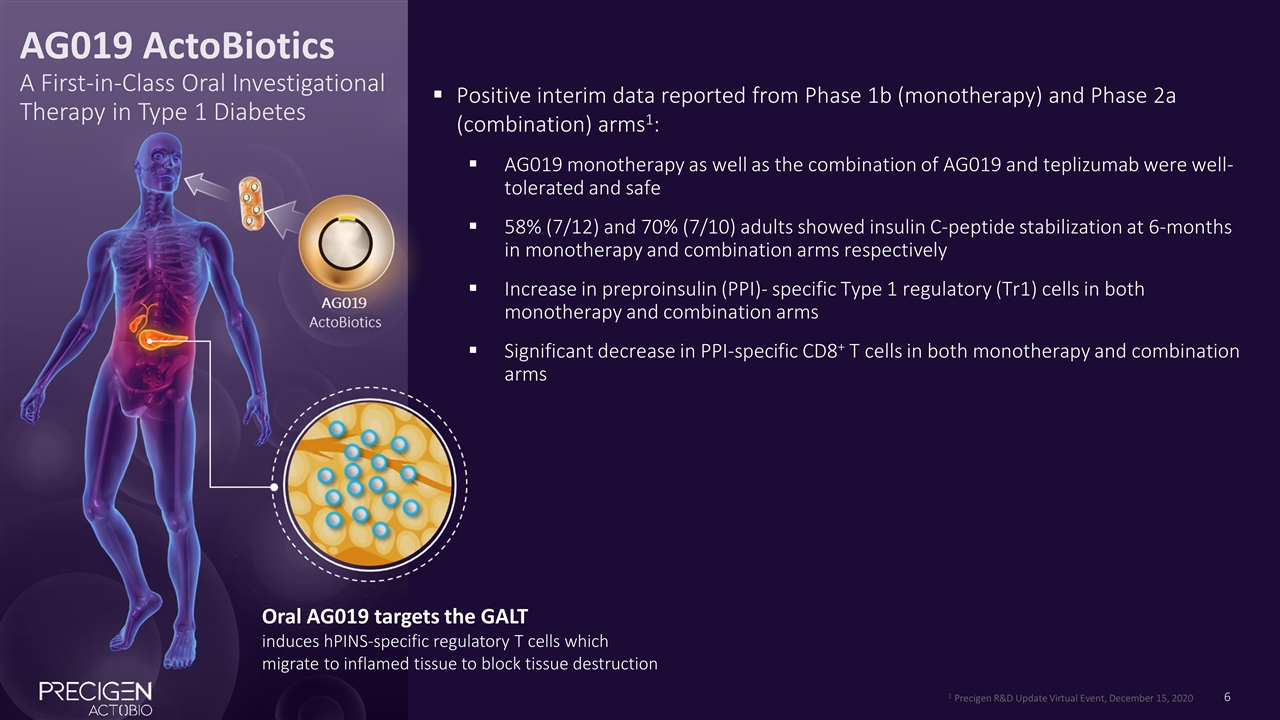
Positive interim data reported from Phase 1b (monotherapy) and Phase 2a (combination) arms1: AG019 monotherapy as well as the combination of AG019 and teplizumab were well-tolerated and safe 58% (7/12) and 70% (7/10) adults showed insulin C-peptide stabilization at 6-months in monotherapy and combination arms respectively Increase in preproinsulin (PPI)- specific Type 1 regulatory (Tr1) cells in both monotherapy and combination arms Significant decrease in PPI-specific CD8+ T cells in both monotherapy and combination arms Oral AG019 targets the GALT induces hPINS-specific regulatory T cells which migrate to inflamed tissue to block tissue destruction AG019 ActoBiotics A First-in-Class Oral Investigational Therapy in Type 1 Diabetes 1 Precigen R&D Update Virtual Event, December 15, 2020

UltraCAR-T® Platform
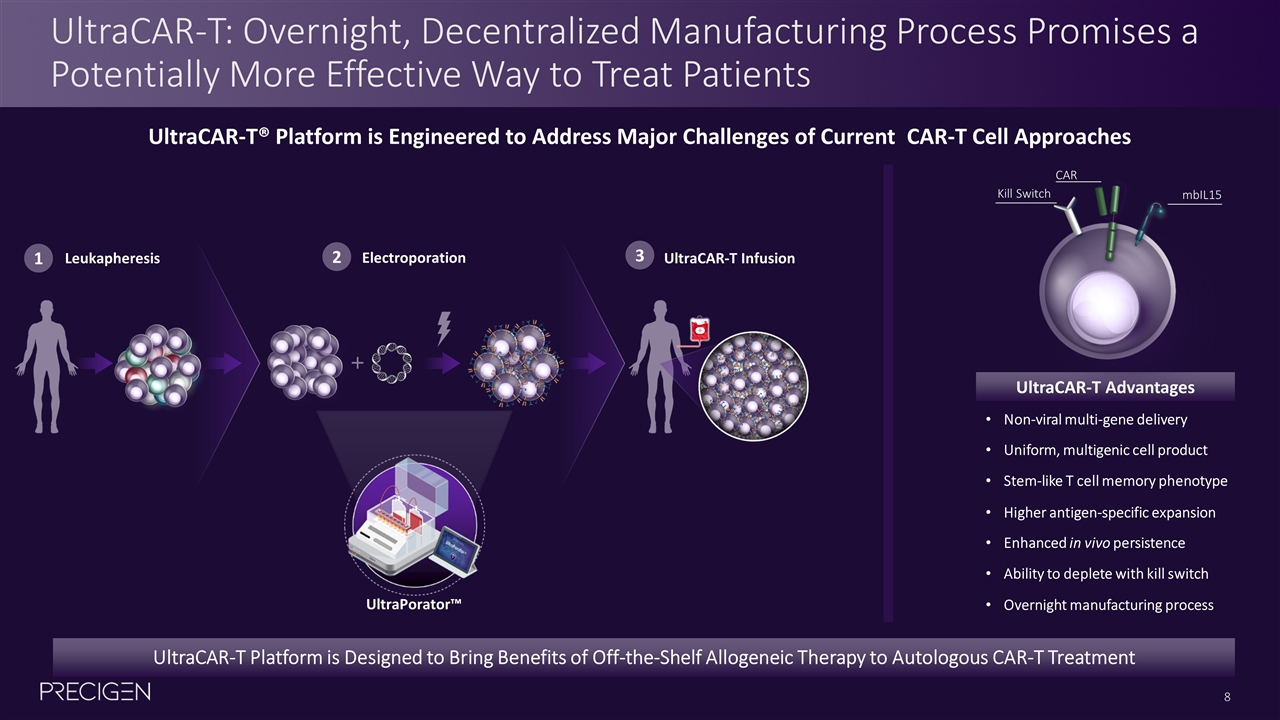
UltraCAR-T: Overnight, Decentralized Manufacturing Process Promises a Potentially More Effective Way to Treat Patients UltraCAR-T Platform is Designed to Bring Benefits of Off-the-Shelf Allogeneic Therapy to Autologous CAR-T Treatment Non-viral multi-gene delivery Stem-like T cell memory phenotype Overnight manufacturing process Ability to deplete with kill switch Enhanced in vivo persistence Higher antigen-specific expansion Uniform, multigenic cell product UltraCAR-T Advantages CAR mbIL15 Kill Switch UltraCAR-T® Platform is Engineered to Address Major Challenges of Current CAR-T Cell Approaches 1 Leukapheresis 2 Electroporation 3 UltraCAR-T Infusion UltraPorator™
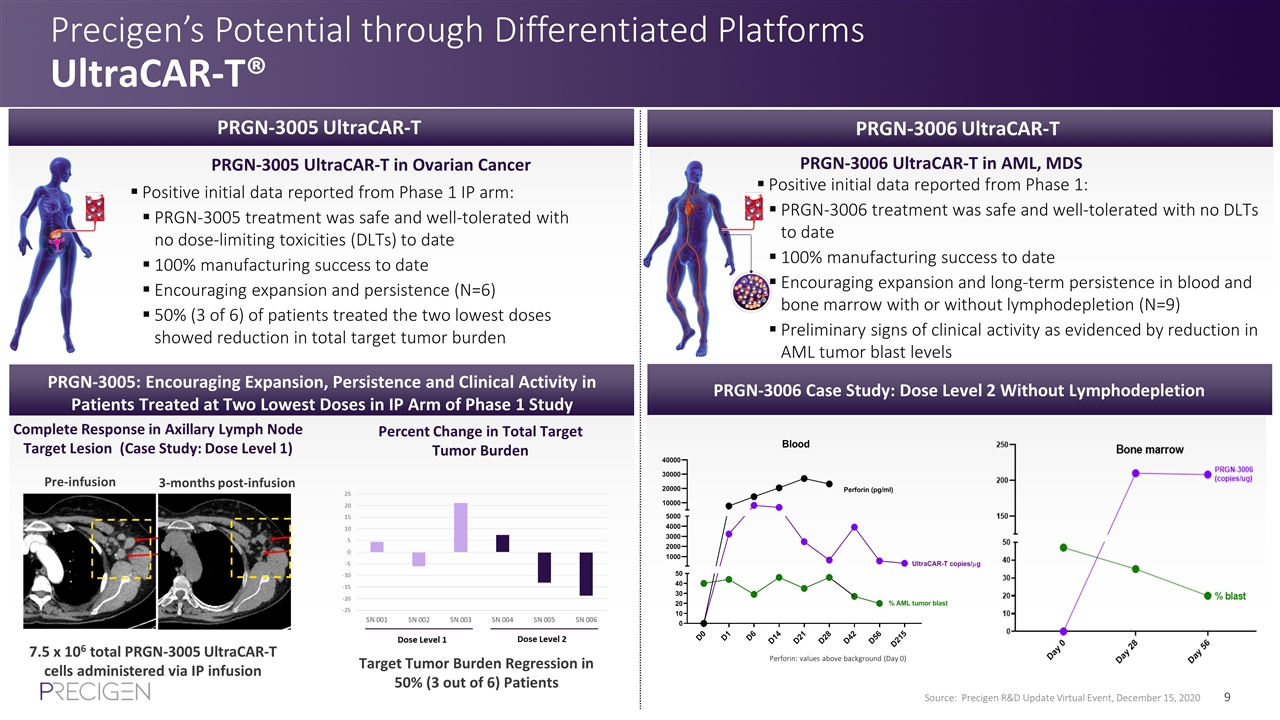
Precigen’s Potential through Differentiated Platforms UltraCAR-T® PRGN-3005 UltraCAR-T in Ovarian Cancer Positive initial data reported from Phase 1 IP arm: PRGN-3005 treatment was safe and well-tolerated with no dose-limiting toxicities (DLTs) to date 100% manufacturing success to date Encouraging expansion and persistence (N=6) 50% (3 of 6) of patients treated the two lowest doses showed reduction in total target tumor burden Positive initial data reported from Phase 1: PRGN-3006 treatment was safe and well-tolerated with no DLTs to date 100% manufacturing success to date Encouraging expansion and long-term persistence in blood and bone marrow with or without lymphodepletion (N=9) Preliminary signs of clinical activity as evidenced by reduction in AML tumor blast levels PRGN-3005 UltraCAR-T PRGN-3006 UltraCAR-T in AML, MDS PRGN-3005: Encouraging Expansion, Persistence and Clinical Activity in Patients Treated at Two Lowest Doses in IP Arm of Phase 1 Study Complete Response in Axillary Lymph Node Target Lesion (Case Study: Dose Level 1) Source: Precigen R&D Update Virtual Event, December 15, 2020 3-months post-infusion Pre-infusion 7.5 x 106 total PRGN-3005 UltraCAR-T cells administered via IP infusion Target Tumor Burden Regression in 50% (3 out of 6) Patients Percent Change in Total Target Tumor Burden PRGN-3006 Case Study: Dose Level 2 Without Lymphodepletion PRGN-3006 UltraCAR-T Perforin: values above background (Day 0)
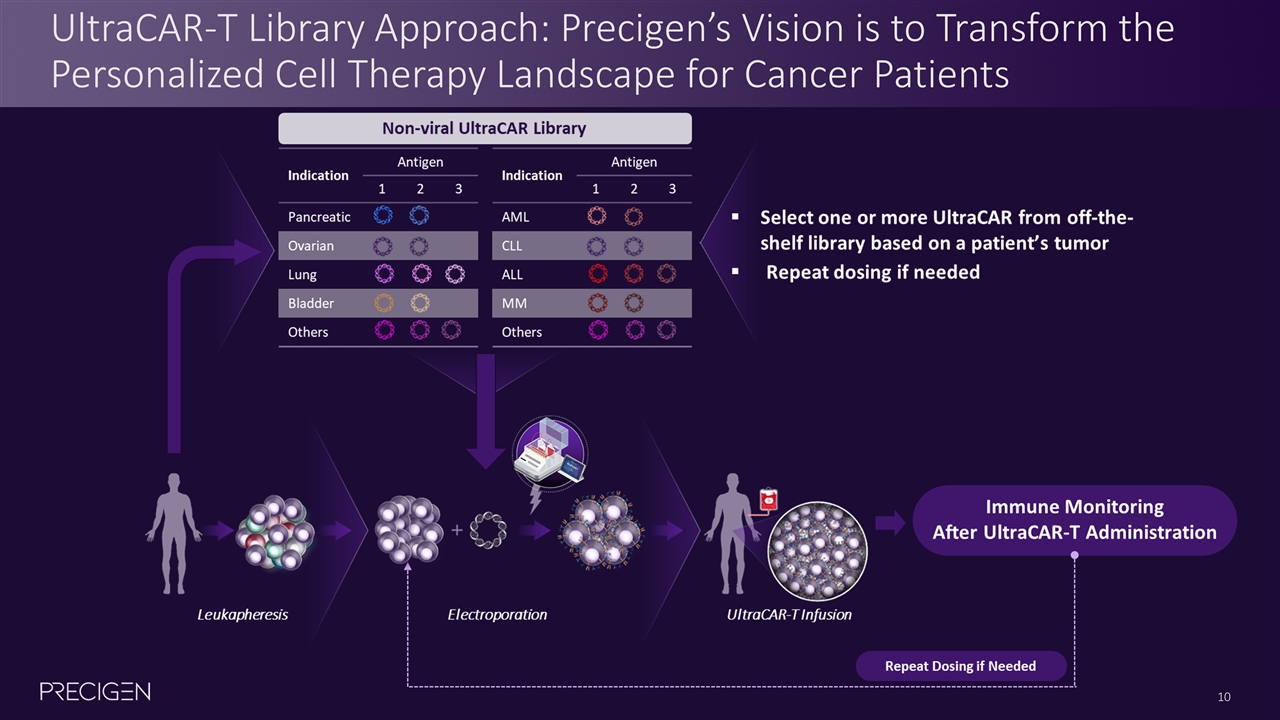
UltraCAR-T Library Approach: Precigen’s Vision is to Transform the Personalized Cell Therapy Landscape for Cancer Patients

AdenoVerse™ Immunotherapy Platform
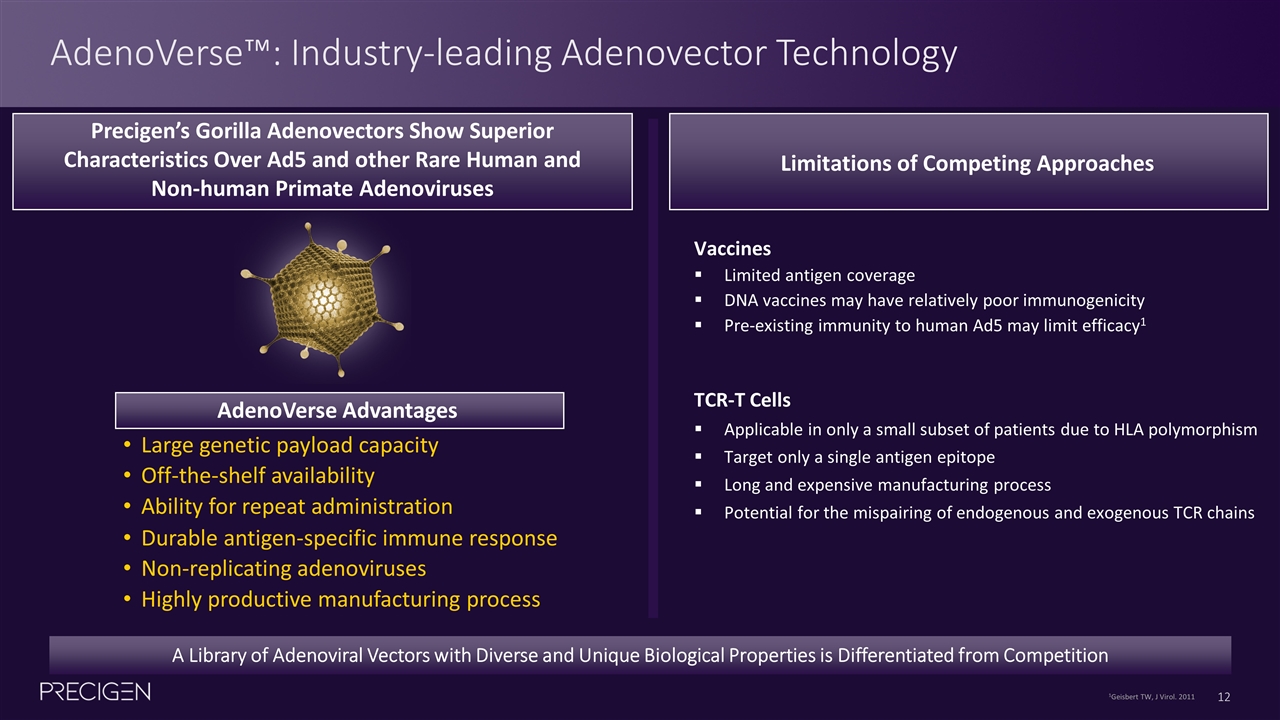
AdenoVerse™: Industry-leading Adenovector Technology A Library of Adenoviral Vectors with Diverse and Unique Biological Properties is Differentiated from Competition Large genetic payload capacity Ability for repeat administration Highly productive manufacturing process Non-replicating adenoviruses Durable antigen-specific immune response Off-the-shelf availability AdenoVerse Advantages Precigen’s Gorilla Adenovectors Show Superior Characteristics Over Ad5 and other Rare Human and Non-human Primate Adenoviruses Limitations of Competing Approaches Vaccines Limited antigen coverage DNA vaccines may have relatively poor immunogenicity Pre-existing immunity to human Ad5 may limit efficacy1 TCR-T Cells Applicable in only a small subset of patients due to HLA polymorphism Target only a single antigen epitope Long and expensive manufacturing process Potential for the mispairing of endogenous and exogenous TCR chains 1Geisbert TW, J Virol. 2011
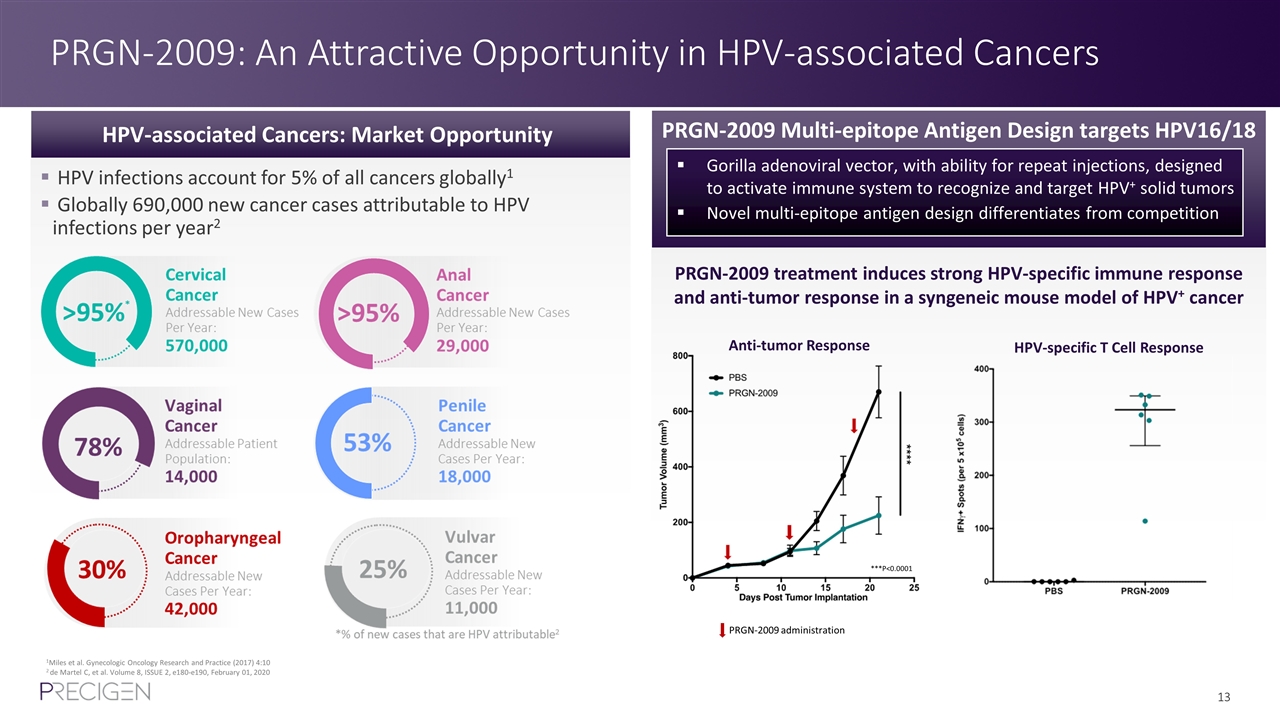
PRGN-2009: An Attractive Opportunity in HPV-associated Cancers 1Miles et al. Gynecologic Oncology Research and Practice (2017) 4:10 2 de Martel C, et al. Volume 8, ISSUE 2, e180-e190, February 01, 2020 HPV-associated Cancers: Market Opportunity PRGN-2009 Multi-epitope Antigen Design targets HPV16/18 Gorilla adenoviral vector, with ability for repeat injections, designed to activate immune system to recognize and target HPV+ solid tumors Novel multi-epitope antigen design differentiates from competition HPV infections account for 5% of all cancers globally1 Globally 690,000 new cancer cases attributable to HPV infections per year2 PRGN-2009 treatment induces strong HPV-specific immune response and anti-tumor response in a syngeneic mouse model of HPV+ cancer Anti-tumor Response HPV-specific T Cell Response ***P<0.0001 PRGN-2009 administration
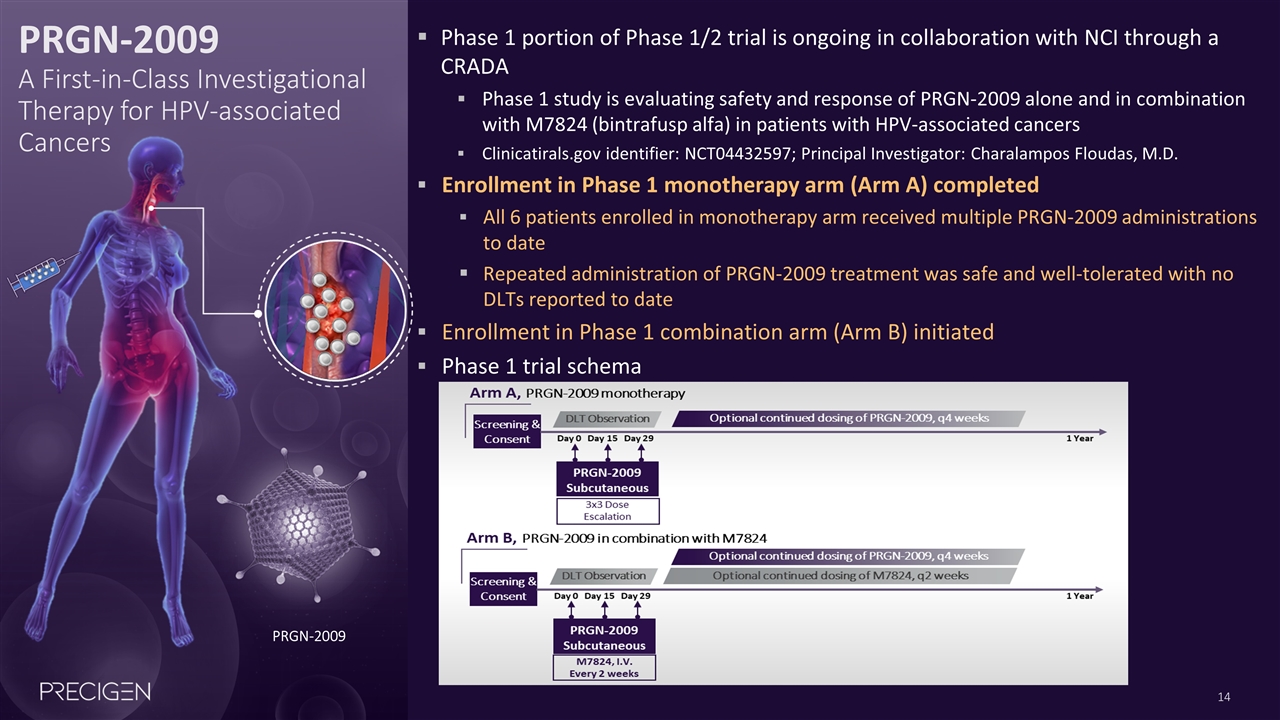
PRGN-2009 A First-in-Class Investigational Therapy for HPV-associated Cancers Phase 1 portion of Phase 1/2 trial is ongoing in collaboration with NCI through a CRADA Phase 1 study is evaluating safety and response of PRGN-2009 alone and in combination with M7824 (bintrafusp alfa) in patients with HPV-associated cancers Clinicatirals.gov identifier: NCT04432597; Principal Investigator: Charalampos Floudas, M.D. Enrollment in Phase 1 monotherapy arm (Arm A) completed All 6 patients enrolled in monotherapy arm received multiple PRGN-2009 administrations to date Repeated administration of PRGN-2009 treatment was safe and well-tolerated with no DLTs reported to date Enrollment in Phase 1 combination arm (Arm B) initiated Phase 1 trial schema PRGN-2009
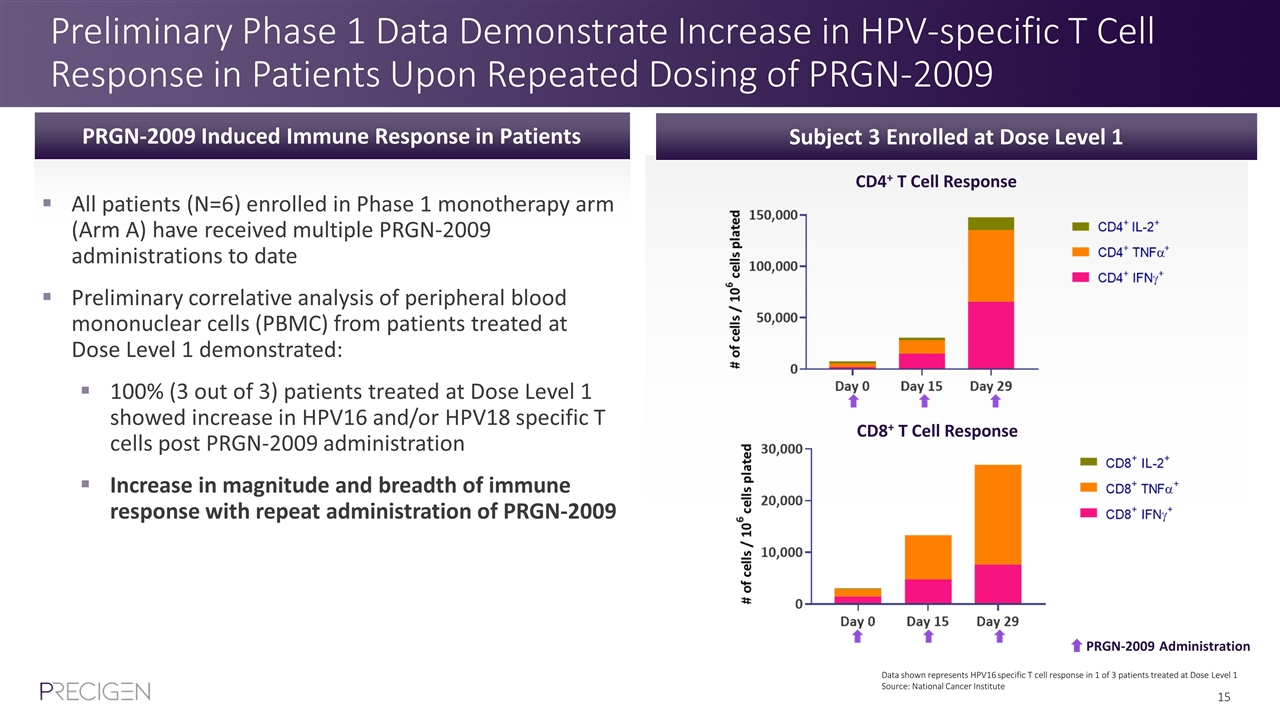
Preliminary Phase 1 Data Demonstrate Increase in HPV-specific T Cell Response in Patients Upon Repeated Dosing of PRGN-2009 Subject 3 Enrolled at Dose Level 1 PRGN-2009 Administration CD4+ T Cell Response CD8+ T Cell Response All patients (N=6) enrolled in Phase 1 monotherapy arm (Arm A) have received multiple PRGN-2009 administrations to date Preliminary correlative analysis of peripheral blood mononuclear cells (PBMC) from patients treated at Dose Level 1 demonstrated: 100% (3 out of 3) patients treated at Dose Level 1 showed increase in HPV16 and/or HPV18 specific T cells post PRGN-2009 administration Increase in magnitude and breadth of immune response with repeat administration of PRGN-2009 PRGN-2009 Induced Immune Response in Patients Data shown represents HPV16 specific T cell response in 1 of 3 patients treated at Dose Level 1 Source: National Cancer Institute
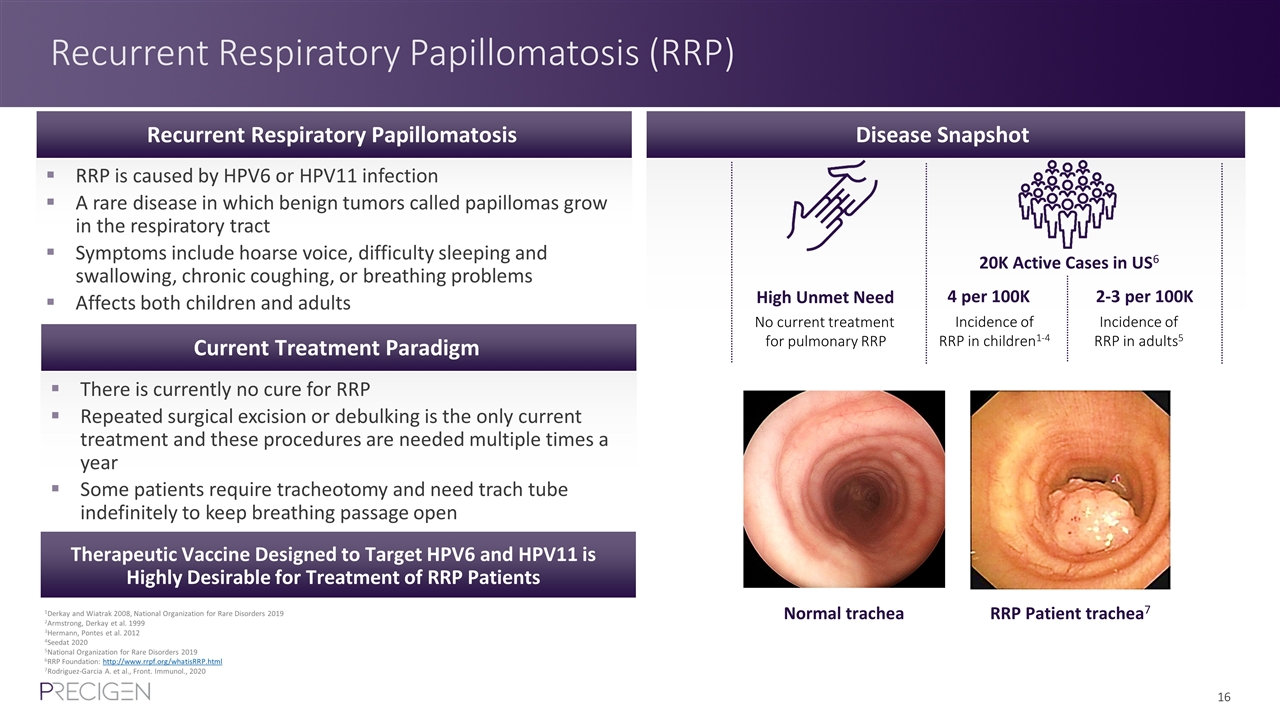
Recurrent Respiratory Papillomatosis (RRP) 1Derkay and Wiatrak 2008, National Organization for Rare Disorders 2019 2Armstrong, Derkay et al. 1999 3Hermann, Pontes et al. 2012 4Seedat 2020 5National Organization for Rare Disorders 2019 6RRP Foundation: http://www.rrpf.org/whatisRRP.html 7Rodriguez-Garcia A. et al., Front. Immunol., 2020 PRGN-3006 Targets CD33 Disease Snapshot High Unmet Need 4 per 100K No current treatment for pulmonary RRP Incidence of RRP in children1-4 2-3 per 100K Incidence of RRP in adults5 RRP is caused by HPV6 or HPV11 infection A rare disease in which benign tumors called papillomas grow in the respiratory tract Symptoms include hoarse voice, difficulty sleeping and swallowing, chronic coughing, or breathing problems Affects both children and adults There is currently no cure for RRP Repeated surgical excision or debulking is the only current treatment and these procedures are needed multiple times a year Some patients require tracheotomy and need trach tube indefinitely to keep breathing passage open Recurrent Respiratory Papillomatosis Current Treatment Paradigm 20K Active Cases in US6 Therapeutic Vaccine Designed to Target HPV6 and HPV11 is Highly Desirable for Treatment of RRP Patients Normal trachea RRP Patient trachea7
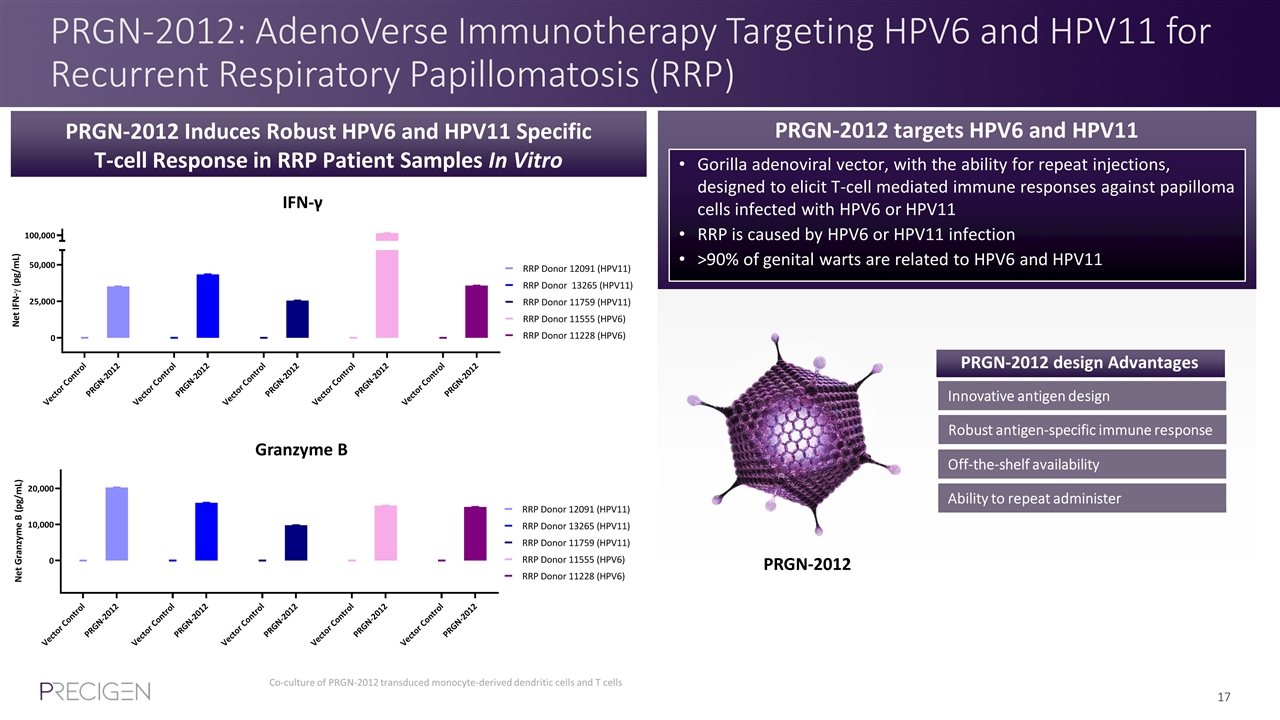
PRGN-2012: AdenoVerse Immunotherapy Targeting HPV6 and HPV11 for Recurrent Respiratory Papillomatosis (RRP) PRGN-2012 Induces Robust HPV6 and HPV11 Specific T-cell Response in RRP Patient Samples In Vitro PRGN-2012 targets HPV6 and HPV11 Gorilla adenoviral vector, with the ability for repeat injections, designed to elicit T-cell mediated immune responses against papilloma cells infected with HPV6 or HPV11 RRP is caused by HPV6 or HPV11 infection >90% of genital warts are related to HPV6 and HPV11 Innovative antigen design Robust antigen-specific immune response Ability to repeat administer Off-the-shelf availability PRGN-2012 design Advantages Co-culture of PRGN-2012 transduced monocyte-derived dendritic cells and T cells IFN-γ Granzyme B PRGN-2012
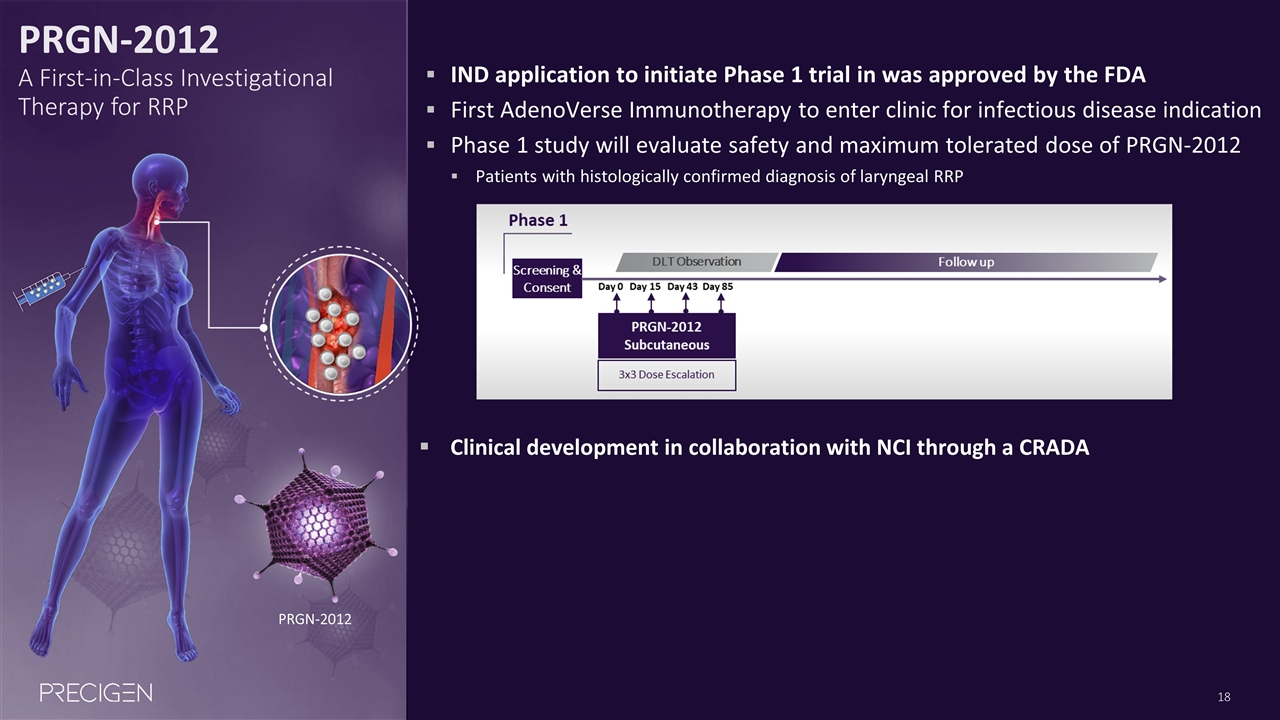
PRGN-2012 A First-in-Class Investigational Therapy for RRP PRGN-2012 IND application to initiate Phase 1 trial in was approved by the FDA First AdenoVerse Immunotherapy to enter clinic for infectious disease indication Phase 1 study will evaluate safety and maximum tolerated dose of PRGN-2012 Patients with histologically confirmed diagnosis of laryngeal RRP Clinical development in collaboration with NCI through a CRADA
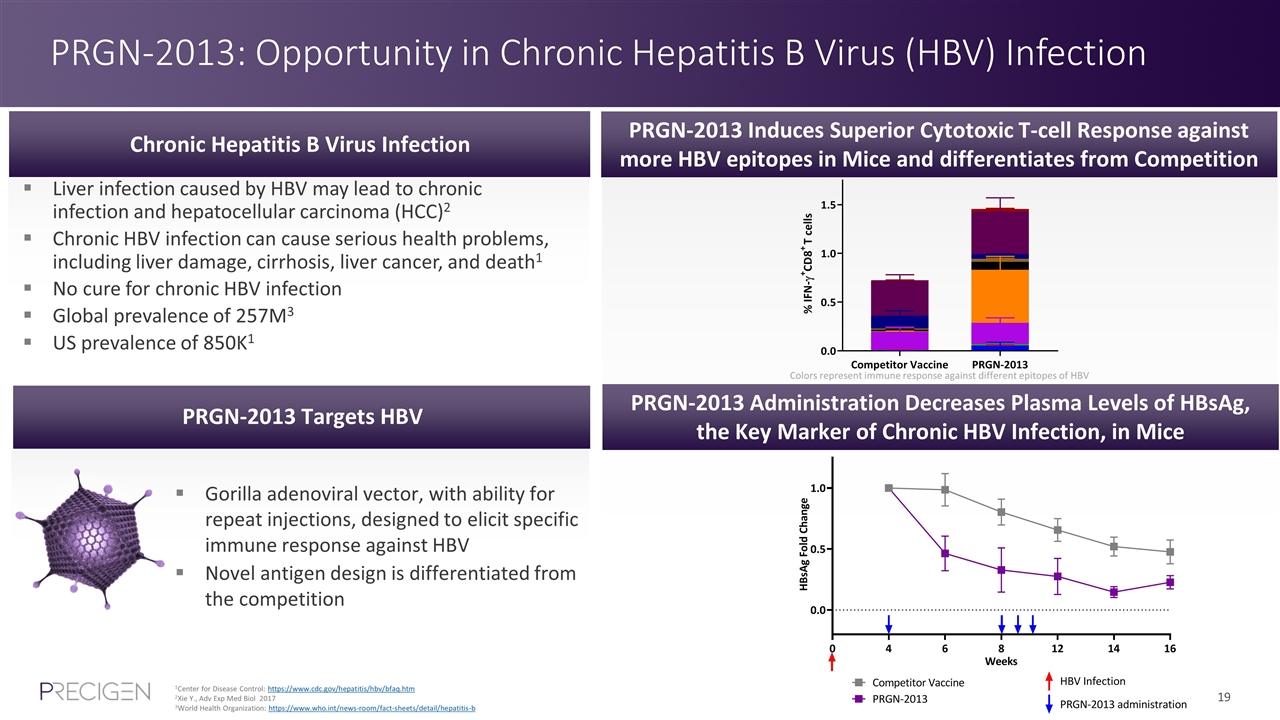
PRGN-2013: Opportunity in Chronic Hepatitis B Virus (HBV) Infection 1Center for Disease Control: https://www.cdc.gov/hepatitis/hbv/bfaq.htm 2Xie Y., Adv Exp Med Biol 2017 3World Health Organization: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Liver infection caused by HBV may lead to chronic infection and hepatocellular carcinoma (HCC)2 Chronic HBV infection can cause serious health problems, including liver damage, cirrhosis, liver cancer, and death1 No cure for chronic HBV infection Global prevalence of 257M3 US prevalence of 850K1 Chronic Hepatitis B Virus Infection PRGN-2013 Targets HBV Gorilla adenoviral vector, with ability for repeat injections, designed to elicit specific immune response against HBV Novel antigen design is differentiated from the competition PRGN-2013 Induces Superior Cytotoxic T-cell Response against more HBV epitopes in Mice and differentiates from Competition PRGN-2013 Administration Decreases Plasma Levels of HBsAg, the Key Marker of Chronic HBV Infection, in Mice Colors represent immune response against different epitopes of HBV

Summary

Precigen in 2020: Achieved All Anticipated Clinical Milestones Initial data released from the intraperitoneal (IP) arm of PRGN-3005 UltraCAR-T Phase 1 trial in Ovarian Cancer Initial data released from PRGN-3006 UltraCAR-T Phase 1 trial in AML and MDS Interim data released from Phase 2 trial of AG013 ActoBiotics in Oral Mucositis Interim data released from Phase 1b/2a trial of AG019 ActoBiotics in Type 1 Diabetes Phase 1 study of INXN-4001 completed in Heart Failure patients with LVAD Initiated Phase 1 trial of PRGN-2009 off-the-shelf AdenoVerse immunotherapy in HPV+ cancers

Precigen in 2021: Multiple Upcoming Milestones Complete dose escalation phase and initiate expansion phase of PRGN-3005 UltraCAR-T IP arm in Ovarian Cancer. Initiate the IV arm of PRGN-3005 Phase 1 trial. Present corresponding interim data. Present interim data from PRGN-3006 UltraCAR-T Phase 1 trial in AML and MDS and initiate dose expansion phase Submit IND application for a new UltraCAR-T candidate Present interim data from the Phase 1 trial of PRGN-2009 in HPV-associated cancers Initiate dosing patients in Phase 1 trial of PRGN-2012 in Recurrent Respiratory Papillomatosis Initiate IND-enabling studies for PRGN-2013 in chronic HBV infection Present data from AG019 Phase 1b/2a trial in Type 1 Diabetes























