Exhibit 99.2

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. 1 Acquisition of Adamas Pharmaceuticals, Inc. Transaction Overview October 11, 2021

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward - looking statements within the meaning of the federal securities laws. These statements, among other things, relate to Supernus’ business strategy, goals and expectations concerning its product candidates, ability to integrate the acquired portfolio into its infrastructure, future operations, prospects, plans and objectives of management. The words “anticipate”, “believe”, “could”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict“, “project”, “will“, and similar terms and phrases are used to identify forward - looking statements in this presentation. Supernus’ operations involve risks and uncertainties, many of which are outside its control, including the potential impact of COVID - 19, and any one of which, or a combination of which, could materially affect its results of operations and whether the forward - looking statements ultimately prove to be correct. Supernus assumes no obligation to update any forward - looking statements except as required by applicable law. Supernus has filed with the U.S. Securities and Exchange Commission (SEC) reports and other documents required by Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended. Before you purchase any Supernus securities, you should read such reports and other documents to obtain more complete information about the company’s operations and business and the risks and uncertainties that it faces in implementing its business plan. You may get these documents for free by visiting EDGAR on the SEC website at http://www.sec.gov . 2

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Additional Information About the Tender Offer and Where to Find it The tender offer for the outstanding common stock of Adamas Pharmaceuticals, Inc. (“Adamas”) has not been commenced. This filing does not constitute a recommendation, an offer to purchase or a solicitation of an offer to sell Adamas securities. At the time the tender offer is commenced, Supernus Pharmaceuticals, Inc. (“Supernus”) and Supernus Reef, Inc., a direct wholly owned subsidiary of Supernus (“Purchaser”), will file a Tender Offer Statement on Schedule TO (including an Offer to Purchase) with the Securities and Exchange Commission (the “SEC”) and thereafter, Adamas will file a Solicitation/Recommendation Statement on Schedule 14D - 9 with the SEC, in each case, with respect to the tender offer. The solicitation and offer by Supernus to purchase shares of Adamas common stock will only be made pursuant to such Offer to Purchase and related materials. Once filed, investors and security holders are urged to read these materials (including the Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents, as each may be amended or supplemented from time to time) carefully since they will contain important information that Adamas investors and security holders should consider before making any decision regarding tendering their common stock, including the terms and conditions of the tender offer. The Tender Offer Statement, Offer to Purchase, Solicitation/Recommendation Statement and related materials will be filed with the SEC, and Adamas investors and security holders may obtain a free copy of these materials (when available) and other documents filed by Supernus, Purchaser and Adamas with the SEC at the website maintained by the SEC at www.sec.gov. In addition, the Tender Offer Statement and other documents that Supernus and Purchaser file with the SEC will be made available to all investors and security holders of Adamas free of charge from the information agent for the tender offer. Investors may also obtain, at no charge, the documents filed with or furnished to the SEC by (i) Supernus under the “Investor Relations” section of Supernus’s website at https://www.adamaspharma.com and (ii) by Adamas under the Adamas under the “Investors & Media” section of Adamas’s website at https://www.adamaspharma.com/. 3

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Jack Khattar President and CEO Timothy Dec Senior Vice President, CFO Bryan Roecklein, Ph.D Senior Vice President, Corporate Development Presenters 4
© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. 5 Overview of Transaction Details Total consideration of up to $9.10/share Upfront payment of $8.10/share ‒ Approximately $400 million upfront (fully - diluted basis) (1) Commercial milestone payments of up to $1.00/share ‒ $0.50/share payable if in any four consecutive quarters between closing and the end of 2024, net sales of GOCOVRI achieve $150 million ‒ $0.50/share payable if in any four consecutive quarters between closing and the end of 2025, net sales of GOCOVRI achieve $225 million All cash consideration, funded through existing cash on balance sheet Transaction expected to close in late Q4 2021 / early Q1 2022 Acquisition of Adamas Pharmaceuticals, Inc. (1) Includes 45.691mm common shares outstanding, 4.844mm options outstanding, 2.570mm RSUs outstanding, dilution calculated u sin g Treasury Stock Method. Excludes options available for future grant.
© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. 6 Adding Commercial Stage Neurology Assets With Significant Revenue 2020 Net Sales: $71.2M 1H2021 Net Sales: $37.7M; 16.3% growth All trademarks are the property of their respective owners For the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa - based therapy, with or without concomitant dopaminergic medications As adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease experiencing “off” episodes Durable IP and exclusivity Incremental royalty stream on net sales of Namzaric by AbbVie through 2024 Osmolex ER is indicated for the treatment of: ‒ Parkinson’s disease ‒ Drug - induced extrapyramidal reactions in adult patients Rights acquired in January 2021 ®

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. 7 Strategic Fit and Rationale This acquisition fits squarely with Supernus’ corporate development strategy of adding commercial and late - stage neurology and psychiatry assets Two marketed products Strong strategic fit Strengthens Leading CNS and Movement Disorder Portfolio GOCOVRI as a growth driver Adds a New Growth Catalyst Approximately mid - teens percent increase in revenue (1) Reduces the reliance on Trokendi XR ® Diversifies and Increases Revenue Base and Cash Flow $60M to $80M in potential synergies in year one (2) Significant Synergies from Strong Overlap with Existing Infrastructure Significantly accretive to EPS in Year One Strong Return on Capital Investment (1) On a 2021 annual proforma basis. (2) Excluding costs to achieve synergies.
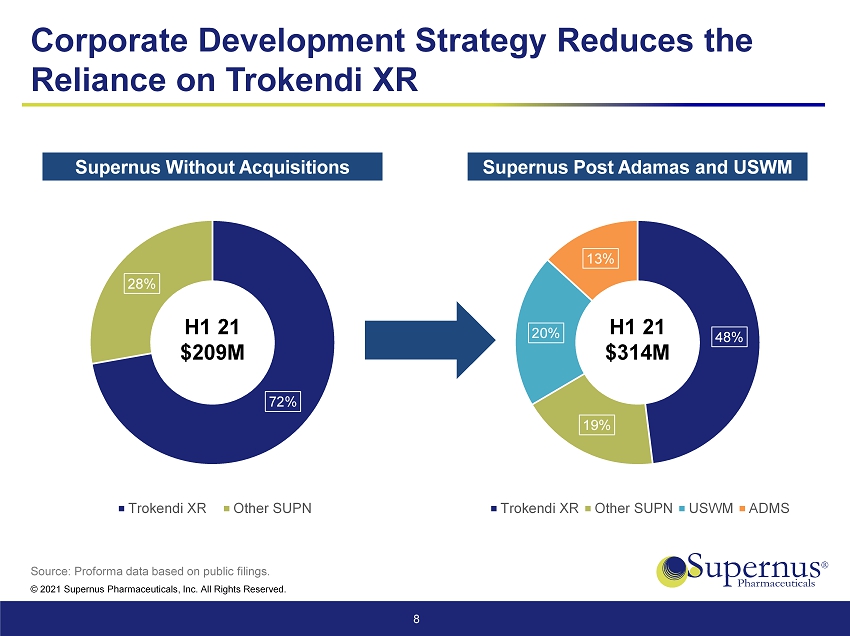
© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. 72% 28% Trokendi XR Other SUPN 48% 19% 20% 13% Trokendi XR Other SUPN USWM ADMS Corporate Development Strategy Reduces the Reliance on Trokendi XR 8 Source: Proforma data based on public filings. H1 21 $209M H1 21 $314M Supernus Without Acquisitions Supernus Post Adamas and USWM
© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Parkinson’s Disease (PD) Market U.S. PD Market is anticipated to grow from $1.5B to $6.2B by 2026 (1) Second most common chronic progressive neurodegenerative disorder, affecting 1 - 2% of individuals 65 years and older (2) Number of U.S. PD Patients in 2020 is ~1M with an annual growth rate of approximately 2.5% (1) PD occurs when cells in the brain, which produce dopamine, become impaired or die The mainstay for therapy is levodopa with effectiveness wearing off resulting in “OFF” periods 9 (1) Global Data Parkinson’s Disease Global Drug Forecast and Market Analysis 2026. (2) Saxton JM. Exercise and Chronic Disease: an Evidence - Based Approach. London, Routledge, 2011.

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. GOVOCRI Addresses Significant Unmet Need of Parkinson’s Patients Unique formulation Proven efficacy Differentiated value 10 (1) Kim H - J, et al., Mov Disord , 2020. (2) Mizuno Y et al., Journal of Neural Transmission, 2018. GOCOVRI potential addressable U.S. patient population – 400,000 to 500,000 based on market research 1,000,000 PD PATIENTS DIAGNOSED IN U.S. 800,000 DIAGNOSED AND TREATED PATIENTS 700,000 LEVODOPA - TREATED PATIENTS PATIENTS WITH DYSKINESIA +/ - OFF ~200,000 ~200,000 - 300,000 + PATIENTS WITH OFF Over 50% of people with PD experience OFF episodes, dyskinesia or both within 5 years, and up to 100% after 10 years (1)(2)

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Addressing Patient Needs at Different Stages of Parkinson’s Disease 11 Stage 1 Stage 1.5 Stage 2 Stage 2.5 Stage 3 Stage 4 Stage 5 Generic Levodopa Initial Symptoms Monotherapy L - dopa Adjuncts On - demand Therapy Advanced continuous treatments Pen SPN - 830 All trademarks are the property of their respective owners
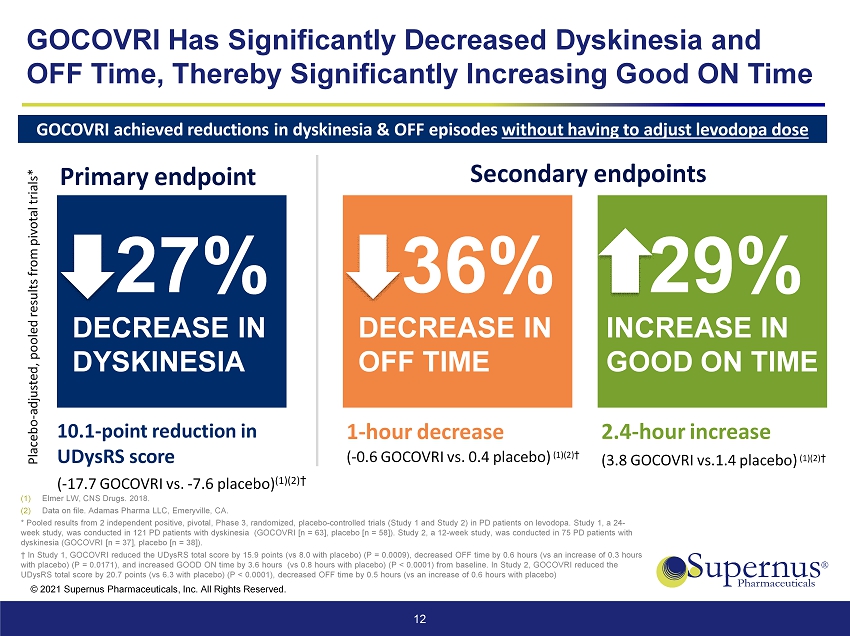
© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. GOCOVRI Has Significantly Decreased Dyskinesia and OFF Time, Thereby Significantly Increasing Good ON Time 12 (1) Elmer LW, CNS Drugs. 2018 . (2) Data on file. Adamas Pharma LLC, Emeryville, CA. * Pooled results from 2 independent positive, pivotal, Phase 3, randomized, placebo - controlled trials (Study 1 and Study 2) in P D patients on levodopa. Study 1, a 24 - week study, was conducted in 121 PD patients with dyskinesia (GOCOVRI [n = 63], placebo [n = 58]). Study 2, a 12 - week study, wa s conducted in 75 PD patients with dyskinesia (GOCOVRI [n = 37], placebo [n = 38]). † In Study 1, GOCOVRI reduced the UDysRS total score by 15.9 points (vs 8.0 with placebo) (P = 0.0009), decreased OFF time by 0. 6 hours (vs an increase of 0.3 hours with placebo) (P = 0.0171), and increased GOOD ON time by 3.6 hours (vs 0.8 hours with placebo) (P < 0.0001) from baseline. In Study 2, GOCOVRI reduced the UDysRS total score by 20.7 points (vs 6.3 with placebo) (P < 0.0001), decreased OFF time by 0.5 hours (vs an increase of 0.6 hou rs with placebo) GOCOVRI achieved reductions in dyskinesia & OFF episodes without having to adjust levodopa dose Placebo - adjusted, pooled results from pivotal trials* 27% DECREASE IN DYSKINESIA 10.1 - point reduction in UDysRS score ( - 17.7 GOCOVRI vs. - 7.6 placebo) ( 1 )( 2 ) † Primary endpoint 36% DECREASE IN OFF TIME 1 - hour decrease ( - 0.6 GOCOVRI vs. 0.4 placebo) (1)(2) † 29% INCREASE IN GOOD ON TIME 2.4 - hour increase (3.8 GOCOVRI vs.1.4 placebo) (1)(2) † Secondary endpoints C
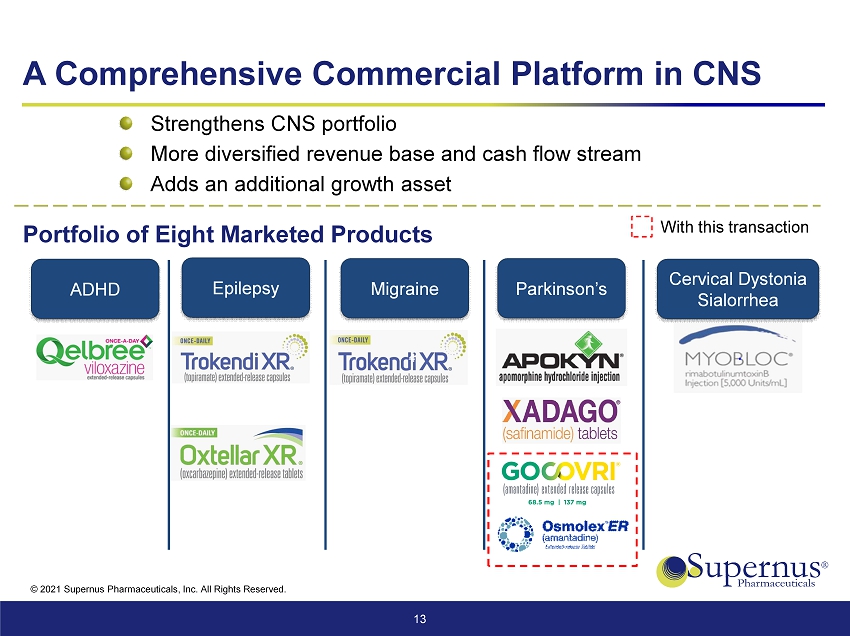
© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. A Comprehensive Commercial Platform in CNS 13 Strengthens CNS portfolio More diversified revenue base and cash flow stream Adds an additional growth asset Epilepsy Migraine Cervical Dystonia Sialorrhea Parkinson’s ADHD Portfolio of Eight Marketed Products With this transaction

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Diversified CNS Portfolio Qelbree Œ , Oxtellar XR ® , Trokendi XR ® , APOKYN ® , MYOBLOC ® , XADAGO ® , GOCOVRI ® , Osmolex ® ER Innovative Pipeline in CNS Qelbree Œ ADHD (adult) SPN - 830 Parkinson’s Disease MYOBLOC Neurological Disorders SPN - 820 Depression SPN - 817 Severe Epilepsy Positioned For Long - Term Growth 14

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Q&A 15

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. Appendix 16

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. 17 GOCOVRI WHAT IS GOCOVRI? GOCOVRI ® (amantadine) extended release capsules is a prescription medicine used: ‒ for the treatment of dyskinesia (sudden uncontrolled movements) in people with Parkinson’s disease who are treated with levodopa therapy or levodopa therapy with other medicines that increase the effects of dopamine in the brain. ‒ with levodopa and carbidopa in people with Parkinson’s disease who are having “off” episodes. ‒ It is not known if GOCOVRI is safe and effective in children. WHAT ARE THE POSSIBLE SIDE EFFECTS OF GOCOVRI? Falling asleep during normal activities. Activities may include driving, talking, or eating. You may fall asleep without being drowsy or warning. Suicidal thoughts or actions and depression. Tell your doctor if you have new or sudden changes in mood, behaviors, thoughts, or feelings, including thoughts about hurting yourself or ending your life. Hallucinations. GOCOVRI can cause or worsen hallucinations (seeing or hearing things that are not real) or psychotic behavior. Feeling dizzy, faint or lightheaded, especially when you stand up (orthostatic hypotension) . Lightheadedness or fainting may happen when getting up too quickly after long periods of time, when first starting GOCOVRI, or if your dose has been increased. Unusual urges . Examples include gambling, sexual urges, spending money, binge eating, and the inability to control them. The most common side effects of GOCOVRI include dry mouth, swelling of legs and feet, constipation, and falls. If you or your family notices that you are developing any new, unusual or sudden changes in behavior or related symptoms, tell your healthcare provider right away. See Full Prescribing Information: https://www.gocovrihcp.com/pdf/Gocovri_Prescribing_Information.pdf

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. NAMZARIC 18 NAMZARIC is a prescription medicine approved to treat moderate to severe Alzheimer’s disease in patients who are taking donepezil hydrochloride 10 mg, the active ingredient in Aricept ® . There is no evidence that NAMZARIC prevents or slows the underlying disease process in patients with Alzheimer's disease. What are the possible side effects of NAMZARIC? NAMZARIC may cause serious side effects, including: muscle problems in patients given anesthesia slow heartbeat and fainting. This happens more often in people with heart problems. Call the doctor right away if the patient faints while taking NAMZARIC. more stomach acid. This raises the chance of ulcers and bleeding especially when taking NAMZARIC. The risk is higher for patients who have had ulcers, or take aspirin or other NSAIDs. nausea and vomiting difficulty passing urine seizures worsening of lung problems in people with asthma or other lung disease The most common side effects of memantine HCl include: headache, diarrhea, and dizziness. The most common side effects of donepezil HCl include: diarrhea, not wanting to eat (anorexia), and bruising. These are not all the possible side effects of NAMZARIC. See Full Prescribing Information: https://media.allergan.com/actavis/actavis/media/allergan - pdf - documents/product - prescribing/2019 - Jan - Namzaric - USPI - Clean.pdf

© 2021 Supernus Pharmaceuticals, Inc. All Rights Reserved. OSMOLEX ER 19 OSMOLEX® ER (amantadine) extended - release tablets is indicated for the treatment of Parkinson’s disease and for the treatment of drug - induced extrapyramidal reactions in adult patients. OSMOLEX ER is contraindicated in patients with end - stage renal disease (i.e., creatinine clearance below 15 mL/min/1.73 m 2). What are the possible side effects of OSMOLEX ER? Falling Asleep During Activities of Daily Living and Somnolence: Patients treated with amantadine have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes has resulted i n accidents. Suicidality and Depression: Suicide, suicide attempts, and suicidal ideation have been reported in patients with and without prior history of psychiatric illness while treated with amantadine. Hallucinations/Psychotic Behavior: Patients with a major psychotic disorder should ordinarily not be treated with OSMOLEX ER due to the risk of exacerbating psychosis. Dizziness and Orthostatic Hypotension Withdrawal - Emergent Hyperpyrexia and Confusion: Abrupt discontinuation of OSMOLEX ER may cause an increase in the symptoms of Parkinson’s disease or cause delirium, agitation, delusions, hallucinations, paranoid reaction, stupor, anxiety, depression, or slurred speech. Impulse Control/Compulsive Behaviors: Patients can experience increased sexual urges, and intense urges to gamble, spend money, binge eat, and/or other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, including OSMOLEX ER. The most common adverse reactions reported in ≥5% of patients at the recommended dosage of immediate - release amantadine were nausea, dizziness/lightheadedness, and insomnia. See Full Prescribing Information: https://www.osmolexhcp.com/wp - content/uploads/2021/01/Prescribing_Information.pdf


















