
Restoring Intestinal Barrier Health Corporate Presentation | January 2022 PALI (NASDAQ) | palisadebio.com

Forward Looking Statements Statements in this presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans, strategy, regulatory matters, market size and opportunity and our ability to complete certain milestones. Words such as “believe,” “anticipate,” “could,” “estimate,” “aim,” “target,” “plan,” “expect,” “intend,” “will,” “may,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the beliefs of the Company’s management as well as assumptions that may never materialize or prove to be incorrect. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing pharmaceutical products, future results from the Company’s ongoing and planned clinical trials, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, trends in the industry, changes in the competitive landscape, delays or disruptions due to the COVID-19 pandemic, the legal and regulatory framework for the industry and future expenditures. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. Other factors that may cause the Company's actual results to differ from current expectations are discussed in the Company's filings with the Securities and Exchange Commission, including the section titled “Risk Factors” contained therein. These forward-looking statements should not be taken as forecasts or promises, nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. Except as required by law, Palisade Bio assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates. Caution should be exercised when interpreting results from separate trials involving separate product candidates. Differences exist between trial designs and subject demographics, which limit the conclusions that can be drawn from comparisons across different trials. This presentation includes statistical and other industry and market data that we obtained from industry publications, third-party research, surveys and studies. The information has been obtained from sources believed to be reliable, although there is no guarantee regarding the accuracy or completeness of such information. The trademarks included herein are the property of the owners thereof and are used for reference purposes only.
Palisade Bio – Developing Targeted Inhibitors of Digestive Proteases Palisade Bio develops drugs treating diseases caused by digestive-enzyme leak that produce inflammation and tissue damage Scientific Platform Technology LB1148 is an oral liquid protease inhibitor administered at home as a bowel prep for major surgeries, and has demonstrated positive clinical results in two large indications: accelerating the return of postoperative bowel function, and reducing post-surgical adhesions Lead Drug Candidate Advancing to Phase 3

Lead Drug: LB1148
Lead Drug Candidate LB1148 – Highlights of Potential Path to Commercialize for Surgery Patients Oral digestive enzyme inhibitor has demonstrated clinical benefit in multiple surgical studies Clinical Safety & Efficacy Prior approvals provide precedent pathway for pivotal trial design and regulatory reviews; LB1148 advancing to pivotal studies LB1148 can be taken at home prior to surgery; ideal reimbursement scenario for commercializing a very large surgical market (20% market share could lead to over $1.5 billion in revenues) Path to Approval Large Commercial Opportunity
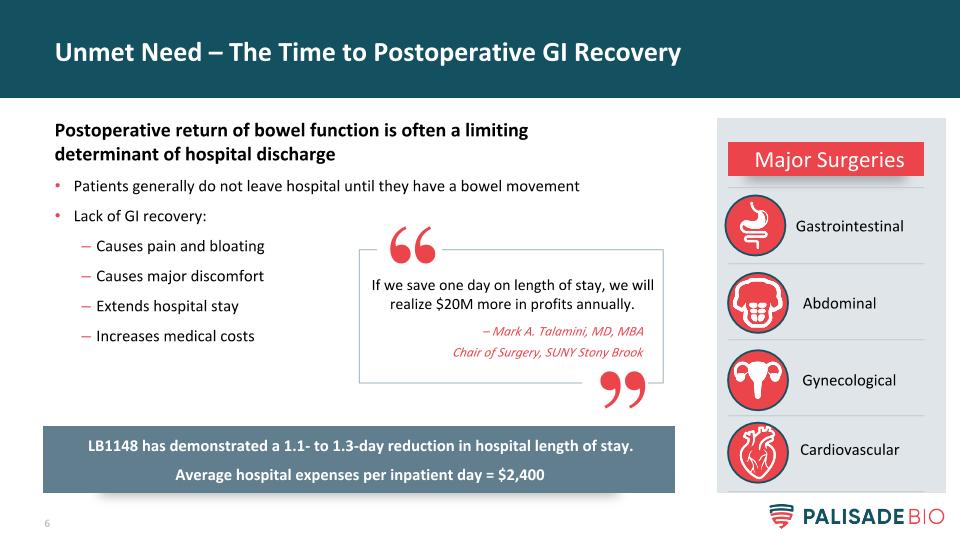
Unmet Need – The Time to Postoperative GI Recovery Postoperative return of bowel function is often a limiting determinant of hospital discharge Patients generally do not leave hospital until they have a bowel movement Lack of GI recovery: Causes pain and bloating Causes major discomfort Extends hospital stay Increases medical costs If we save one day on length of stay, we will realize $20M more in profits annually. – Mark A. Talamini, MD, MBA Chair of Surgery, SUNY Stony Brook Major Surgeries Cardiovascular Gastrointestinal Abdominal Gynecological LB1148 has demonstrated a 1.1- to 1.3-day reduction in hospital length of stay. Average hospital expenses per inpatient day = $2,400
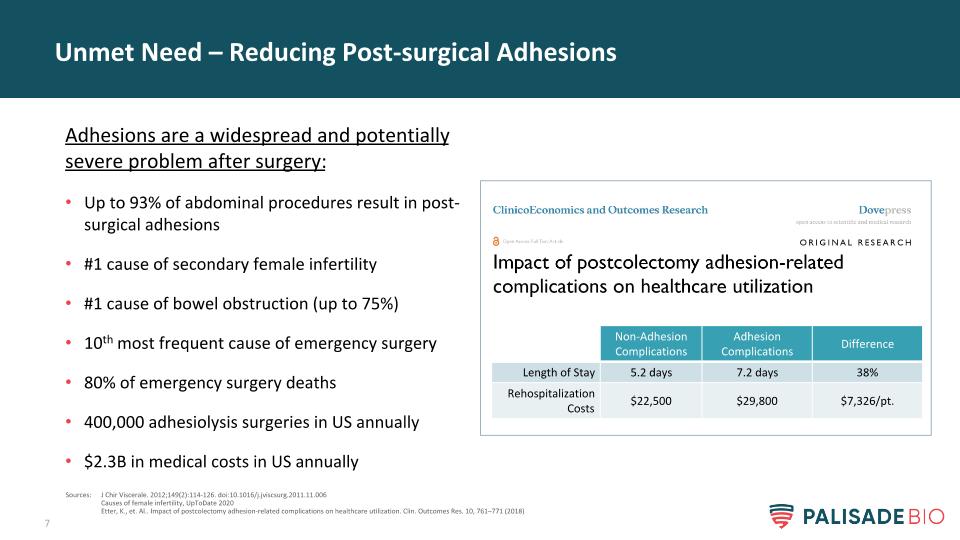
Unmet Need – Reducing Post-surgical Adhesions Adhesions are a widespread and potentially severe problem after surgery: Up to 93% of abdominal procedures result in post-surgical adhesions #1 cause of secondary female infertility #1 cause of bowel obstruction (up to 75%) 10th most frequent cause of emergency surgery 80% of emergency surgery deaths 400,000 adhesiolysis surgeries in US annually $2.3B in medical costs in US annually Sources: J Chir Viscerale. 2012;149(2):114-126. doi:10.1016/j.jviscsurg.2011.11.006 Causes of female infertility, UpToDate 2020 Etter, K., et. Al.. Impact of postcolectomy adhesion-related complications on healthcare utilization. Clin. Outcomes Res. 10, 761–771 (2018) Non-Adhesion Complications Adhesion Complications Difference Length of Stay 5.2 days 7.2 days 38% Rehospitalization Costs $22,500 $29,800 $7,326/pt.
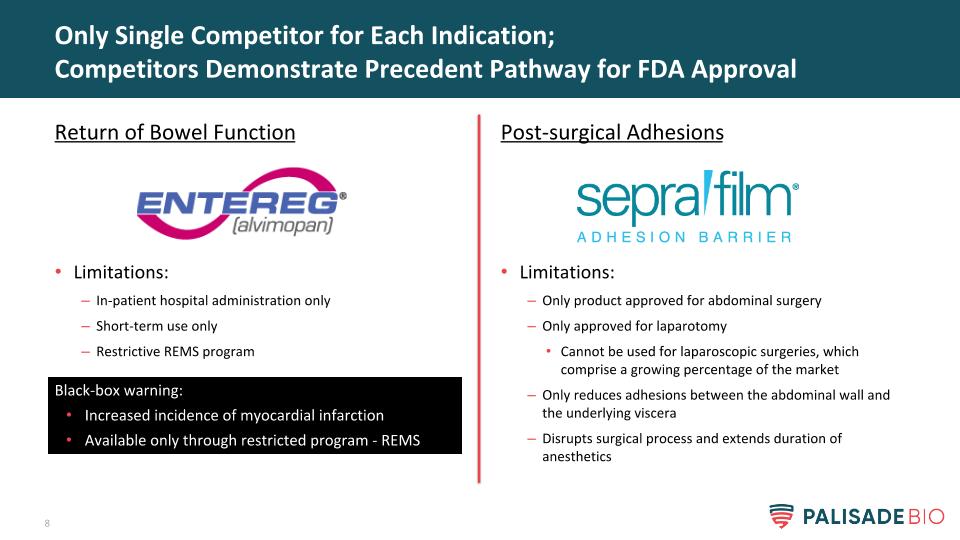
Post-surgical Adhesions Limitations: Only product approved for abdominal surgery Only approved for laparotomy Cannot be used for laparoscopic surgeries, which comprise a growing percentage of the market Only reduces adhesions between the abdominal wall and the underlying viscera Disrupts surgical process and extends duration of anesthetics Only Single Competitor for Each Indication; Competitors Demonstrate Precedent Pathway for FDA Approval Return of Bowel Function Limitations: In-patient hospital administration only Short-term use only Restrictive REMS program Black-box warning: Increased incidence of myocardial infarction Available only through restricted program - REMS
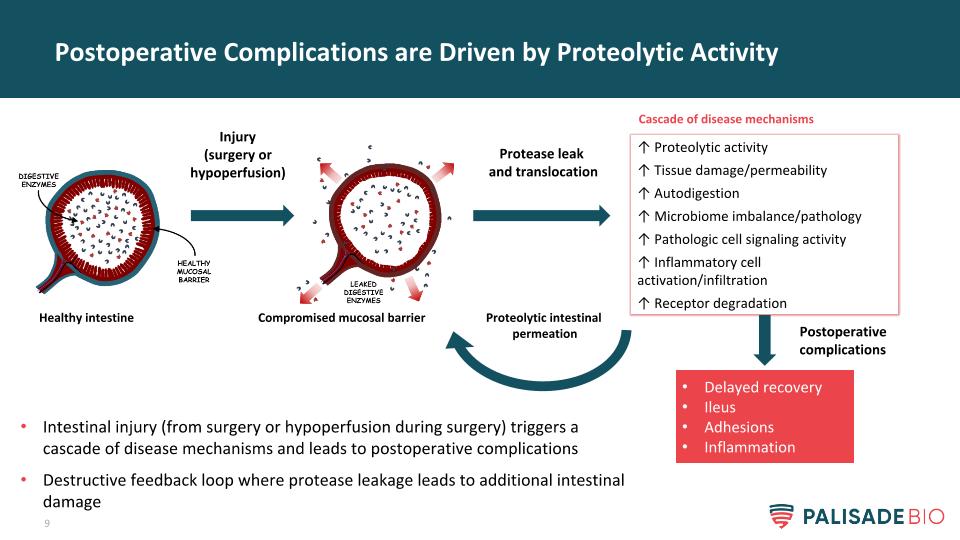
Postoperative Complications are Driven by Proteolytic Activity Injury (surgery or hypoperfusion) Compromised mucosal barrier Healthy intestine Protease leak and translocation ↑ Proteolytic activity ↑ Tissue damage/permeability ↑ Autodigestion ↑ Microbiome imbalance/pathology ↑ Pathologic cell signaling activity ↑ Inflammatory cell activation/infiltration ↑ Receptor degradation Delayed recovery Ileus Adhesions Inflammation Cascade of disease mechanisms Proteolytic intestinal permeation Intestinal injury (from surgery or hypoperfusion during surgery) triggers a cascade of disease mechanisms and leads to postoperative complications Destructive feedback loop where protease leakage leads to additional intestinal damage Postoperative complications
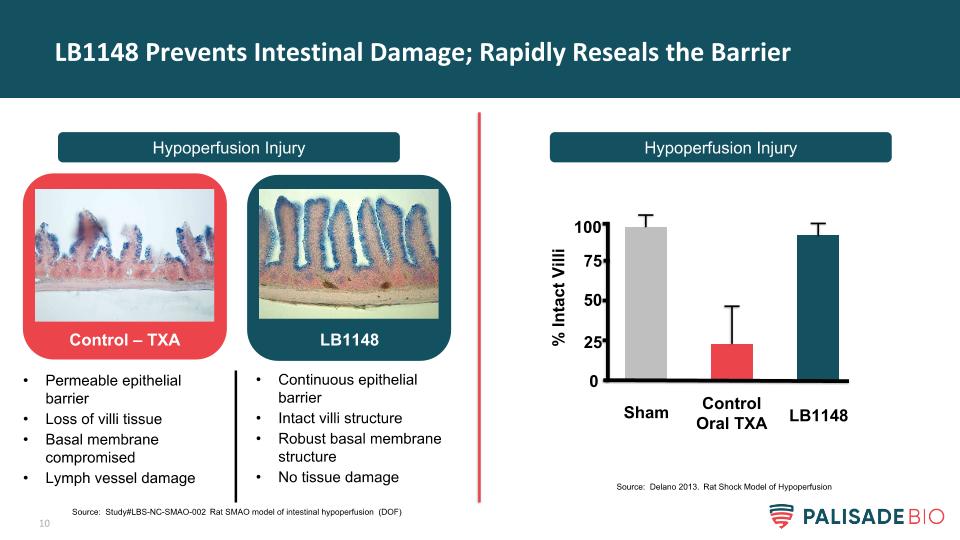
LB1148 Prevents Intestinal Damage; Rapidly Reseals the Barrier Hypoperfusion Injury Hypoperfusion Injury Control – TXA LB1148 Permeable epithelial barrier Loss of villi tissue Basal membrane compromised Lymph vessel damage Continuous epithelial barrier Intact villi structure Robust basal membrane structure No tissue damage % Intact Villi Sham Control Oral TXA LB1148 0 50 75 100 25 Source: Study#LBS-NC-SMAO-002 Rat SMAO model of intestinal hypoperfusion (DOF) Source: Delano 2013. Rat Shock Model of Hypoperfusion

LB1148 Overview: Pipeline in a Product – Multiple Indications Drug/Class Indication Research Pre-clinical Phase 1 Phase 2 Phase 3 LB1148* Oral broad-spectrum protease inhibitor* Postoperative return of bowel function GI Surgery Neonatal cardiac surgery Prevention of post-surgical abdominal adhesions *Commercial rights to LB1148 in Greater China (excluding Taiwan) have been out-licensed to Newsoara

Accelerating Return of Bowel Function
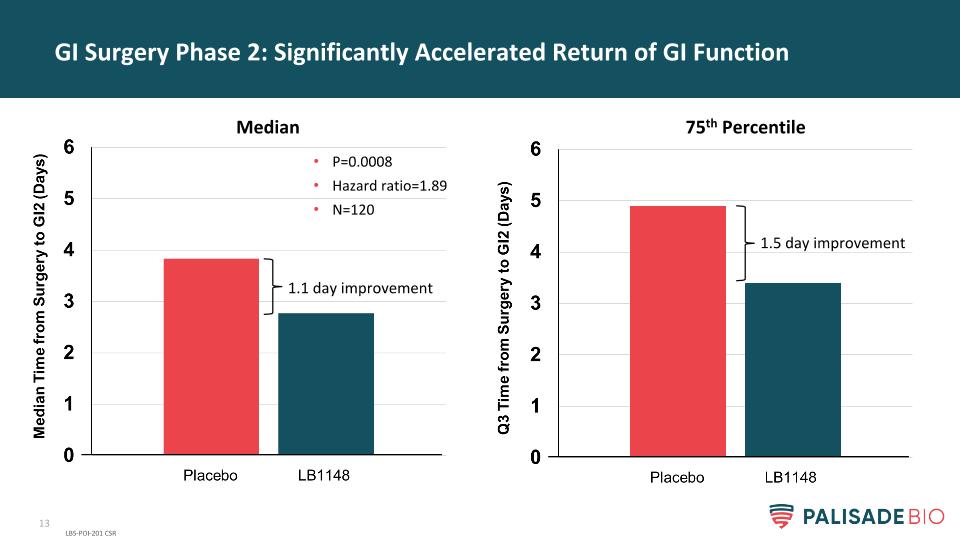
GI Surgery Phase 2: Significantly Accelerated Return of GI Function LBS-POI-201 CSR P=0.0008 Hazard ratio=1.89 N=120 1.1 day improvement 1.5 day improvement Median 75th Percentile
LB1148 Accelerates Return of GI Function – Advancing to Phase 3 Consistent statistically significant clinical efficacy in three completed clinical studies 48% improvement in time to return of bowel function after GI surgery in phase 1 (vs. placebo in Entereg study 314*) 1.3-day reduction in hospital LOS vs. expected LOS at time of admission (p<0.03) Zero post-surgical abdominal adhesions documented in 2nd look surgeries 28% improvement in time to return of bowel function after GI surgery in phase 2 (p<0.001) 30% improvement in time to return of bowel function after cardiovascular (CV) surgery phase 2 (p<0.001) 1.0-day reduction in ICU length of stay (LOS) (ns) 1.1-day reduction in hospital LOS (ns) Fast Track designation granted for pediatric cardiac surgery program and post-surgical adhesions Strong IP portfolio, including issued patents on LB1148 drug product 6.7 million addressable patients in the US Source: Entereg Prescribing Information

Preventing Post-surgical Adhesions
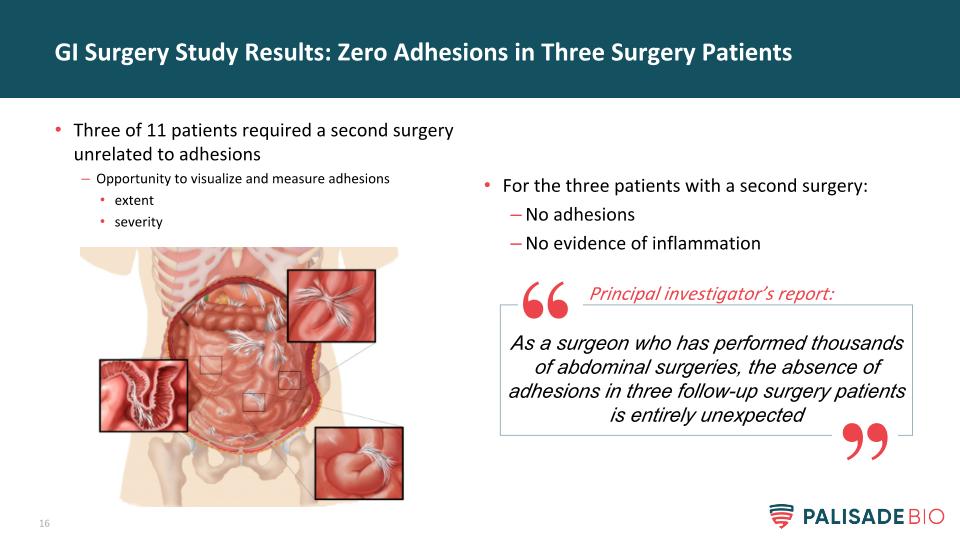
For the three patients with a second surgery: No adhesions No evidence of inflammation GI Surgery Study Results: Zero Adhesions in Three Surgery Patients Three of 11 patients required a second surgery unrelated to adhesions Opportunity to visualize and measure adhesions extent severity As a surgeon who has performed thousands of abdominal surgeries, the absence of adhesions in three follow-up surgery patients is entirely unexpected Principal investigator’s report:

LB1148 Commercial Opportunity
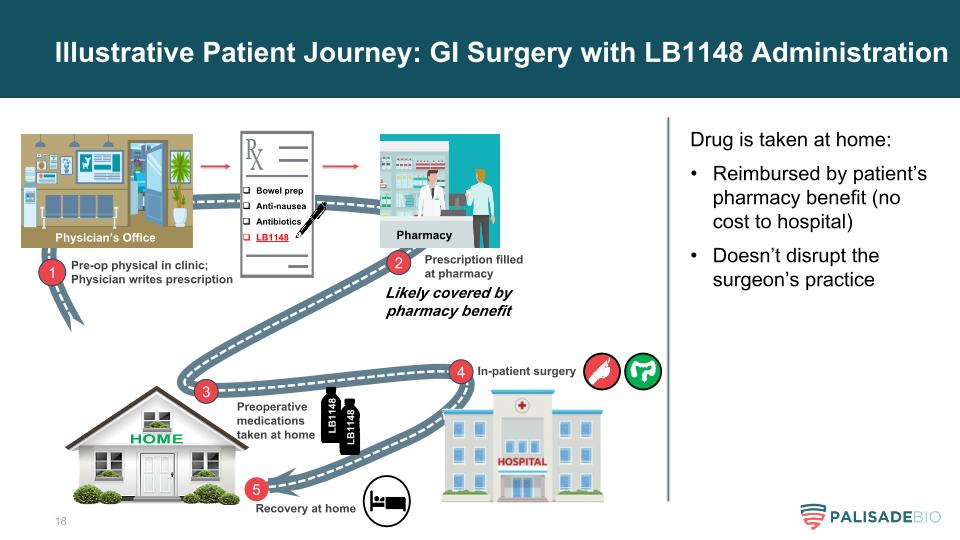
Illustrative Patient Journey: GI Surgery with LB1148 Administration 1 Pre-op physical in clinic; Physician writes prescription Bowel prep Anti-nausea Antibiotics LB1148 Likely covered by pharmacy benefit 2 Prescription filled at pharmacy 3 Preoperative medications taken at home LB1148 4 In-patient surgery 5 Recovery at home Drug is taken at home: Reimbursed by patient’s pharmacy benefit (no cost to hospital) Doesn’t disrupt the surgeon’s practice LB1148 Physician’s Office Pharmacy
LB1148 Has Large Global Market Opportunity US Market 1.1 million open heart surgeries 5.6 million abdominal surgeries 20-40% market penetration could translate to >$2B revenue Partnered China: w/ Newsoara EU and ROW Opportunities Potential to partner with US rights or independently USA Strategy Commercialize by marketing to 5,000 hospitals M&A – Shows Value of Adhesion Market Baxter acquired the medical device Seprafilm from Sanofi for $350M in 2020 3.5X sales

5800 Armada Dr, Suite 210 Carlsbad, CA 92008 858-704-4900 IR@palisadebio.com PALI (Nasdaq) www.palisadebio.com

Appendix Slides

Novel oral formulation comprised of FDA-approved components Broad-spectrum serine protease inhibitor (tranexamic acid) Known safety profile Plan to utilize 505(b)(2) pathway for approval Fast-track designation for Reducing surgical adhesions Reducing postoperative GI dysfunction for pediatric patients undergoing open heart surgery Issued patents on drug product No new approved drug for postoperative ileus since 2008 Entereg has austere black box warning, onerous REMS protocols, and limited utilization LB1148 - Expeditious Regulatory Pathway; Limited Competition
Entereg: Only Drug Approved for Accelerating Return of GI Function Provides a precedent pathway to FDA approval Developed by GSK/Adolor/Cubist and now owned by Merck Indicated to improve post-op recovery of bowel function Phase 3 demonstrated*: 17-hour improvement in return of bowel function 7-hour improvement in hospital length of stay Entereg MOA carries safety risks LB1148 has completely different MOA Limitations include*: In-patient hospital administration only Short-term use only Restrictive REMS program Black Box warning for severe side effects Utilized in few hospitals * Entereg Prescribing Information Black-box warning: Increased incidence of myocardial infarction Available only through restricted program - REMS Entereg’s FDA approval provides regulatory roadmap LB1148 clinical trial design

LB1148 Program Overview Pipeline in a Product – Multiple Indications Accelerate Return of Postoperative GI Function Reduce Post-surgical Adhesions Cardiovascular Surgery Gastrointestinal Surgery Abdominal Surgery

Cardiovascular Surgery (Hypoperfusion Injury)
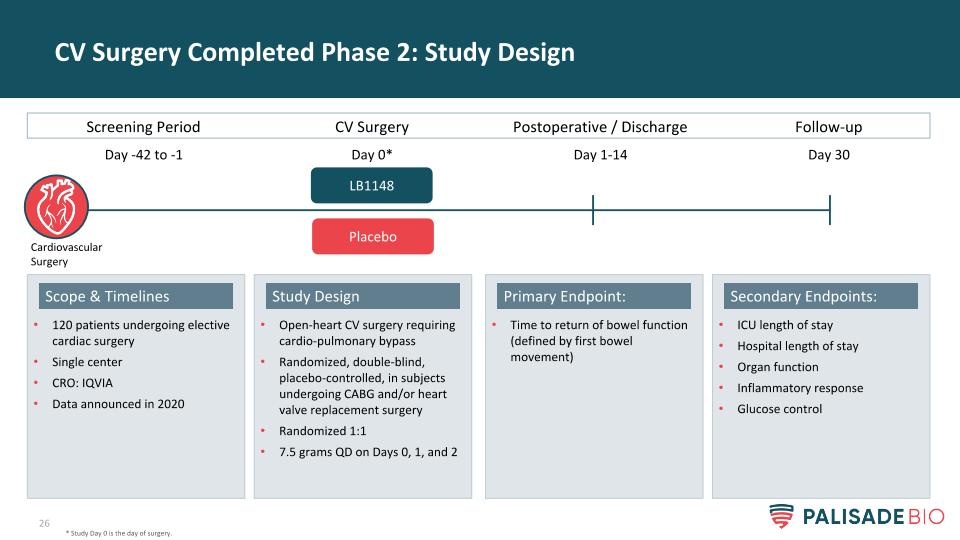
CV Surgery Completed Phase 2: Study Design * Study Day 0 is the day of surgery. Screening Period CV Surgery Postoperative / Discharge LB1148 Day -42 to -1 Day 0* Day 1-14 Follow-up Day 30 Placebo Cardiovascular Surgery Open-heart CV surgery requiring cardio-pulmonary bypass Randomized, double-blind, placebo-controlled, in subjects undergoing CABG and/or heart valve replacement surgery Randomized 1:1 7.5 grams QD on Days 0, 1, and 2 Study Design 120 patients undergoing elective cardiac surgery Single center CRO: IQVIA Data announced in 2020 Scope & Timelines ICU length of stay Hospital length of stay Organ function Inflammatory response Glucose control Secondary Endpoints: Time to return of bowel function (defined by first bowel movement) Primary Endpoint:
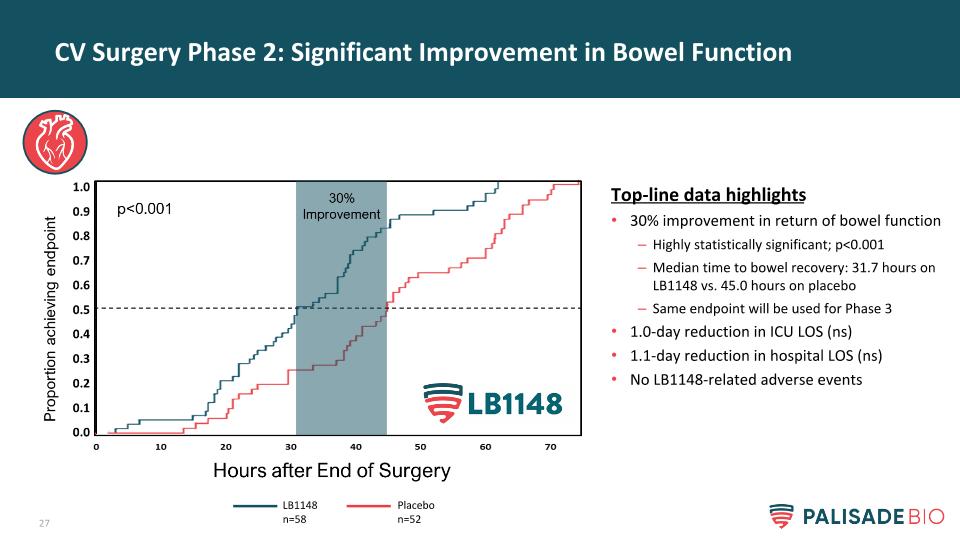
CV Surgery Phase 2: Significant Improvement in Bowel Function Top-line data highlights 30% improvement in return of bowel function Highly statistically significant; p<0.001 Median time to bowel recovery: 31.7 hours on LB1148 vs. 45.0 hours on placebo Same endpoint will be used for Phase 3 1.0-day reduction in ICU LOS (ns) 1.1-day reduction in hospital LOS (ns) No LB1148-related adverse events LB1148 n=58 Placebo n=52

GI Surgery: Return of Bowel Function (Physical Injury)
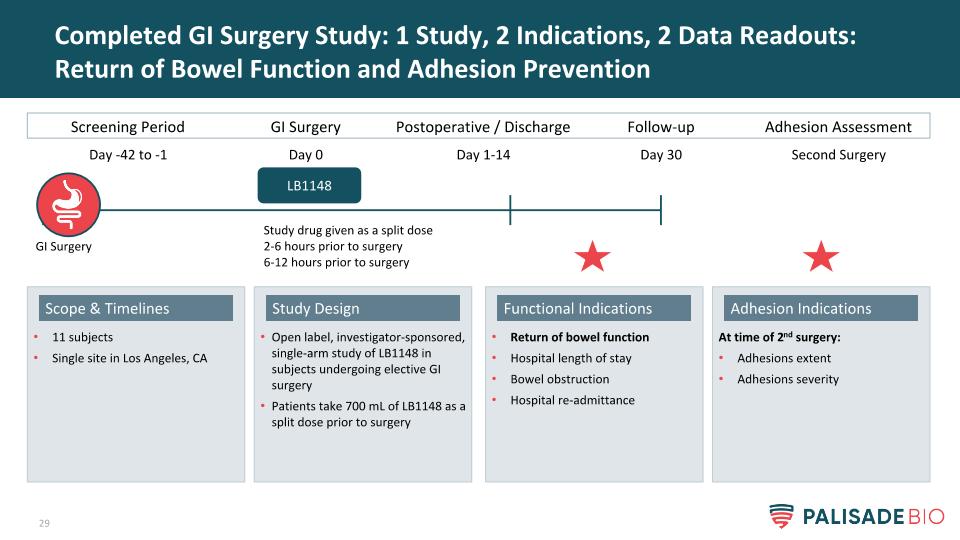
Completed GI Surgery Study: 1 Study, 2 Indications, 2 Data Readouts: Return of Bowel Function and Adhesion Prevention Open label, investigator-sponsored, single-arm study of LB1148 in subjects undergoing elective GI surgery Patients take 700 mL of LB1148 as a split dose prior to surgery Study Design 11 subjects Single site in Los Angeles, CA Scope & Timelines At time of 2nd surgery: Adhesions extent Adhesions severity Adhesion Indications Return of bowel function Hospital length of stay Bowel obstruction Hospital re-admittance Functional Indications Screening Period GI Surgery Postoperative / Discharge LB1148 Day -42 to -1 Day 0 Day 1-14 Follow-up Day 30 Study drug given as a split dose 2-6 hours prior to surgery 6-12 hours prior to surgery Adhesion Assessment Second Surgery GI Surgery
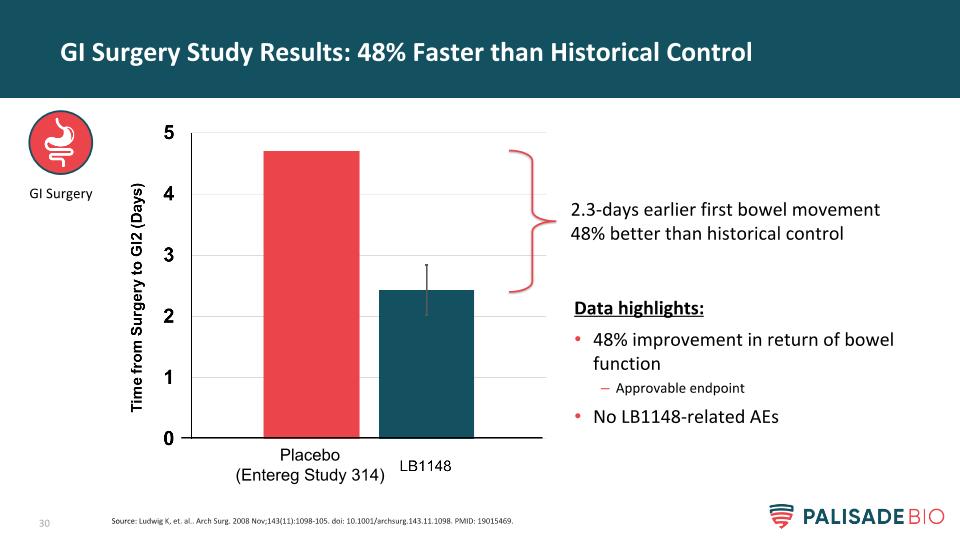
GI Surgery Study Results: 48% Faster than Historical Control Data highlights: 48% improvement in return of bowel function Approvable endpoint No LB1148-related AEs 2.3-days earlier first bowel movement 48% better than historical control GI Surgery Source: Ludwig K, et. al.. Arch Surg. 2008 Nov;143(11):1098-105. doi: 10.1001/archsurg.143.11.1098. PMID: 19015469. Placebo (Entereg Study 314)
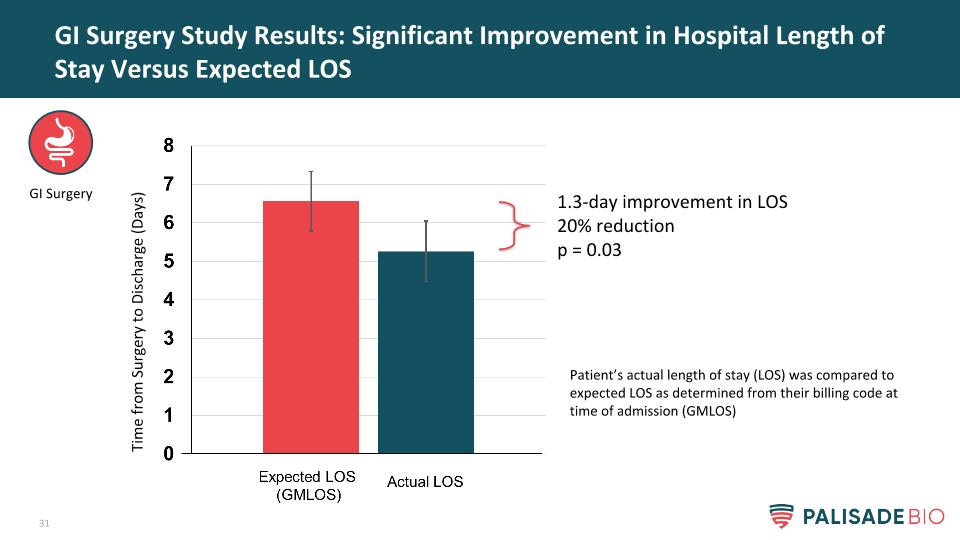
GI Surgery Study Results: Significant Improvement in Hospital Length of Stay Versus Expected LOS GI Surgery Time from Surgery to Discharge (Days) Patient’s actual length of stay (LOS) was compared to expected LOS as determined from their billing code at time of admission (GMLOS) 1.3-day improvement in LOS 20% reduction p = 0.03
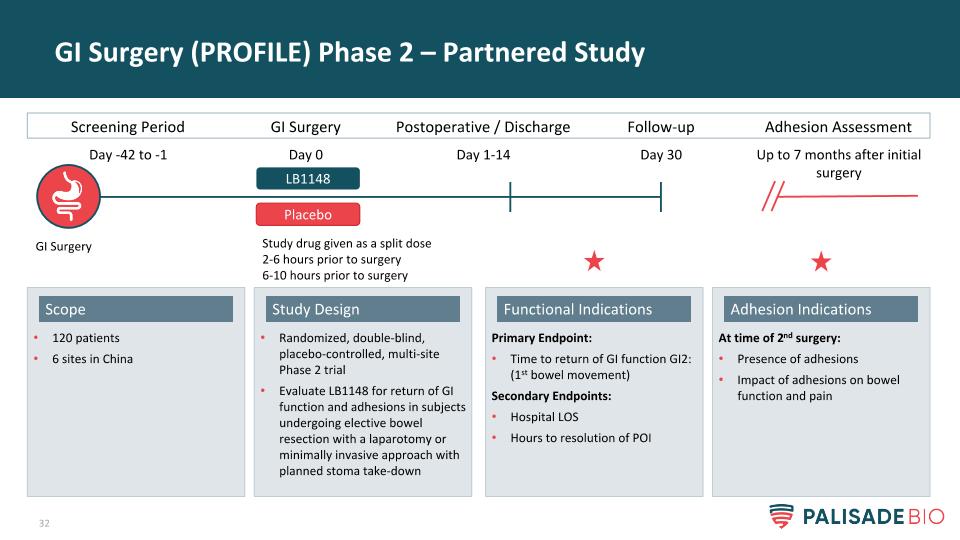
GI Surgery (PROFILE) Phase 2 – Partnered Study GI Surgery Randomized, double-blind, placebo-controlled, multi-site Phase 2 trial Evaluate LB1148 for return of GI function and adhesions in subjects undergoing elective bowel resection with a laparotomy or minimally invasive approach with planned stoma take-down Study Design 120 patients 6 sites in China Scope At time of 2nd surgery: Presence of adhesions Impact of adhesions on bowel function and pain Adhesion Indications Primary Endpoint: Time to return of GI function GI2: (1st bowel movement) Secondary Endpoints: Hospital LOS Hours to resolution of POI Functional Indications Screening Period GI Surgery Postoperative / Discharge LB1148 Day -42 to -1 Day 0 Day 1-14 Follow-up Day 30 Study drug given as a split dose 2-6 hours prior to surgery 6-10 hours prior to surgery Adhesion Assessment Up to 7 months after initial surgery Placebo
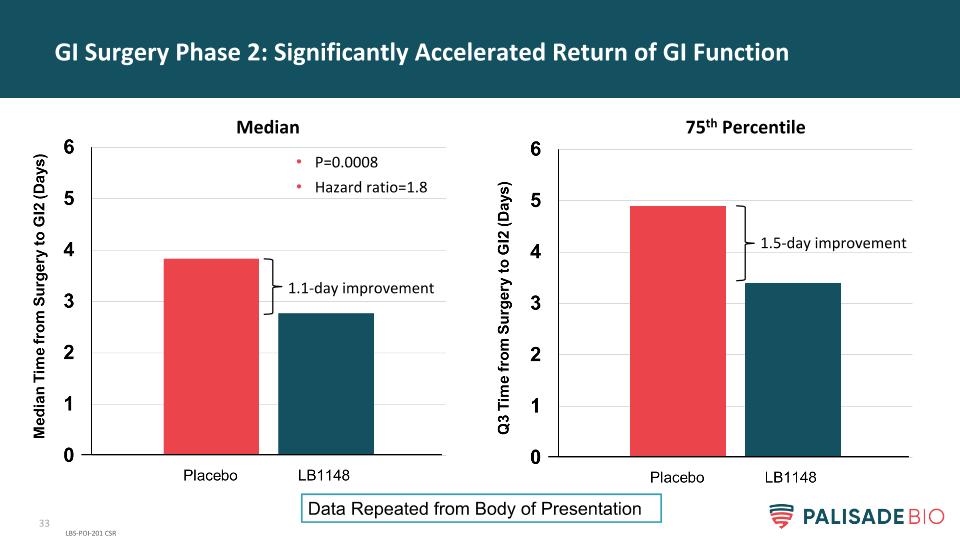
GI Surgery Phase 2: Significantly Accelerated Return of GI Function LBS-POI-201 CSR P=0.0008 Hazard ratio=1.8 1.1-day improvement 1.5-day improvement Median 75th Percentile Data Repeated from Body of Presentation

GI Surgery Phase 2: Safe and Well Tolerated LB1148 was well tolerated Drug-related adverse events LB1148 = 10.9% Placebo = 4.8% The most common drug-related AEs were GI disorders LB1148 4.7% Placebo 3.2% No drug-related serious adverse events occurred in the trial

GI Surgery: Reduction of Adhesions (Physical Injury)
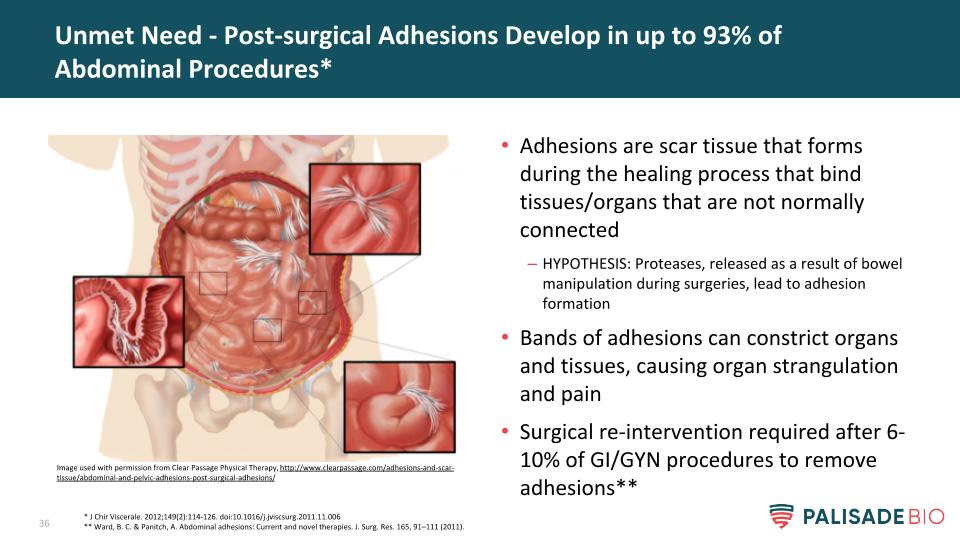
Adhesions are scar tissue that forms during the healing process that bind tissues/organs that are not normally connected HYPOTHESIS: Proteases, released as a result of bowel manipulation during surgeries, lead to adhesion formation Bands of adhesions can constrict organs and tissues, causing organ strangulation and pain Surgical re-intervention required after 6-10% of GI/GYN procedures to remove adhesions** Unmet Need - Post-surgical Adhesions Develop in up to 93% of Abdominal Procedures* * J Chir Viscerale. 2012;149(2):114-126. doi:10.1016/j.jviscsurg.2011.11.006 ** Ward, B. C. & Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 165, 91–111 (2011). Image used with permission from Clear Passage Physical Therapy, http://www.clearpassage.com/adhesions-and-scar-tissue/abdominal-and-pelvic-adhesions-post-surgical-adhesions/
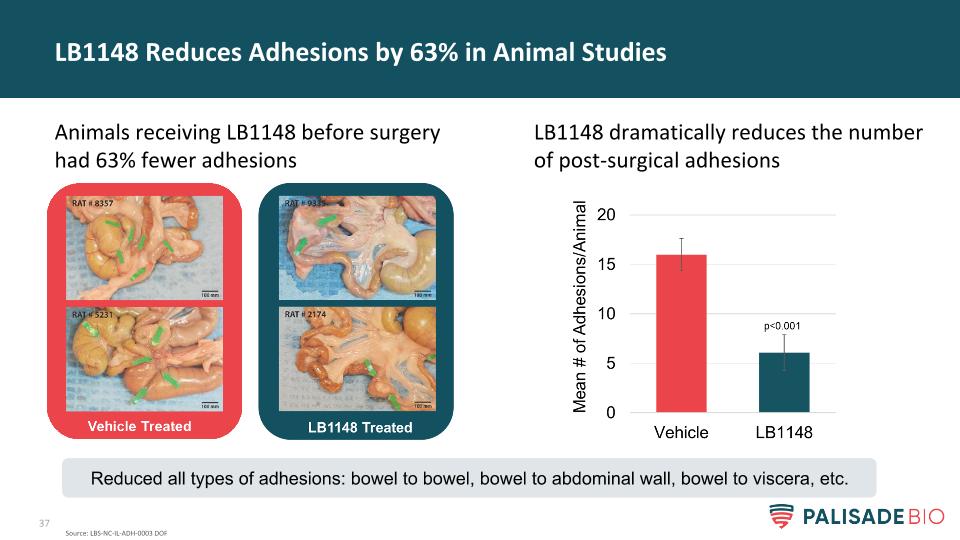
LB1148 dramatically reduces the number of post-surgical adhesions LB1148 Reduces Adhesions by 63% in Animal Studies Animals receiving LB1148 before surgery had 63% fewer adhesions Source: LBS-NC-IL-ADH-0003 DOF Reduced all types of adhesions: bowel to bowel, bowel to abdominal wall, bowel to viscera, etc.
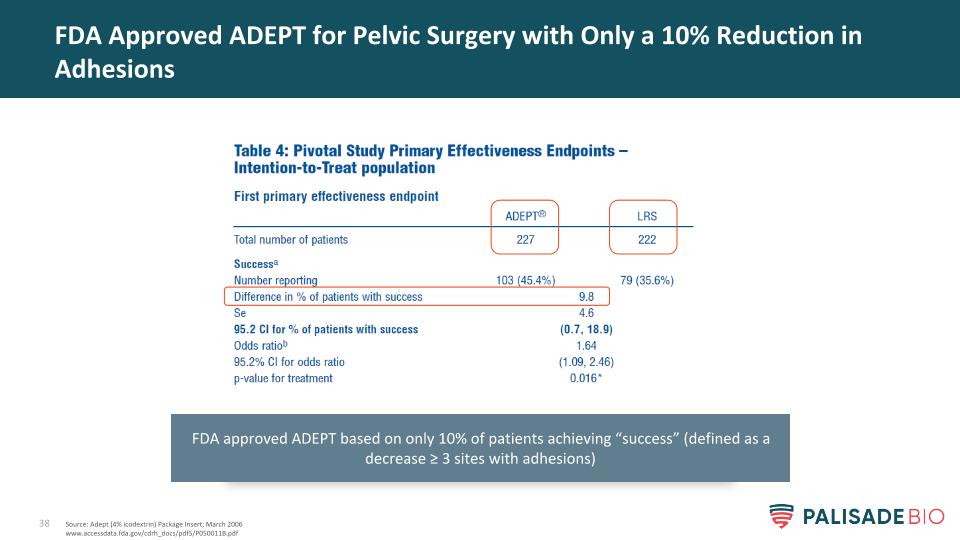
FDA Approved ADEPT for Pelvic Surgery with Only a 10% Reduction in Adhesions Source: Adept (4% icodextrin) Package Insert; March 2006 www.accessdata.fda.gov/cdrh_docs/pdf5/P050011B.pdf FDA approved ADEPT based on only 10% of patients achieving “success” (defined as a decrease ≥ 3 sites with adhesions)

5800 Armada Dr, Suite 210 Carlsbad, CA 92008 858-704-4900 IR@palisadebio.com PALI (Nasdaq) www.palisadebio.com