- PALI Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Palisade Bio (PALI) 8-KRegulation FD Disclosure
Filed: 23 May 13, 12:00am
World Leader in Neural Stem Cell Science Corporate Overview May 2013

Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem , Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com . Safe Harbor
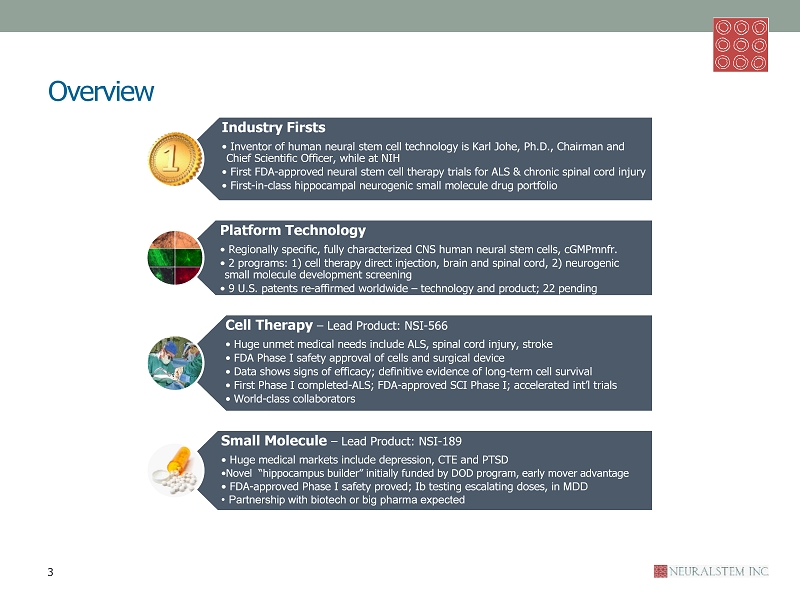
Overview Industry Firsts • Inventor of human neural stem cell technology is Karl Johe , Ph.D., Chairman and Chief Scientific Officer, while at NIH • First FDA - approved neural stem cell therapy trials for ALS & chronic spinal cord injury • First - in - class hippocampal neurogenic small molecule drug portfolio Platform Technology • Regionally specific, fully characterized CNS human neural stem cells, cGMPmnfr . • 2 programs: 1) cell therapy direct injection, brain and spinal cord, 2) neurogenic small molecule development screening • 9 U.S. patents re - affirmed worldwide – technology and product; 22 pending Cell Therapy – Lead Product: NSI - 566 • Huge unmet medical needs include ALS, spinal cord injury, stroke • FDA Phase I safety approval of cells and surgical device • Data shows signs of efficacy; definitive evidence of long - term cell survival • First Phase I completed - ALS; FDA - approved SCI Phase I; accelerated int’l trials • World - class collaborators Small Molecule – Lead Product: NSI - 189 • Huge medical markets include depression, CTE and PTSD • Novel “hippocampus builder” initially funded by DOD program, early mover advantage • FDA - approved Phase I safety proved; Ib testing escalating doses, in MDD • Partnership with biotech or big pharma expected 3
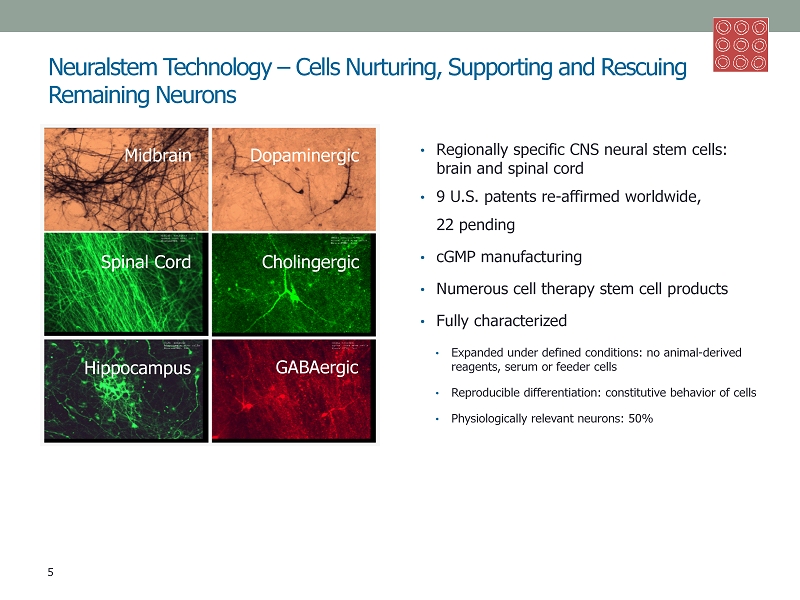
Neuralstem Technology – Cells Nurturing, Supporting and Rescuing Remaining Neurons • Regionally specific CNS neural stem cells: brain and spinal cord • 9 U.S. p atents re - affirmed worldwide, 22 pending • cGMP manufacturing • Numerous cell therapy stem cell products • Fully characterized • Expanded under defined conditions: no animal - derived reagents, serum or feeder cells • Reproducible differentiation: constitutive behavior of cells • Physiologically relevant neurons: 50% 5 Midbrain Dopaminergic Spinal Cord Cholingergic Hippocampus GABAergic
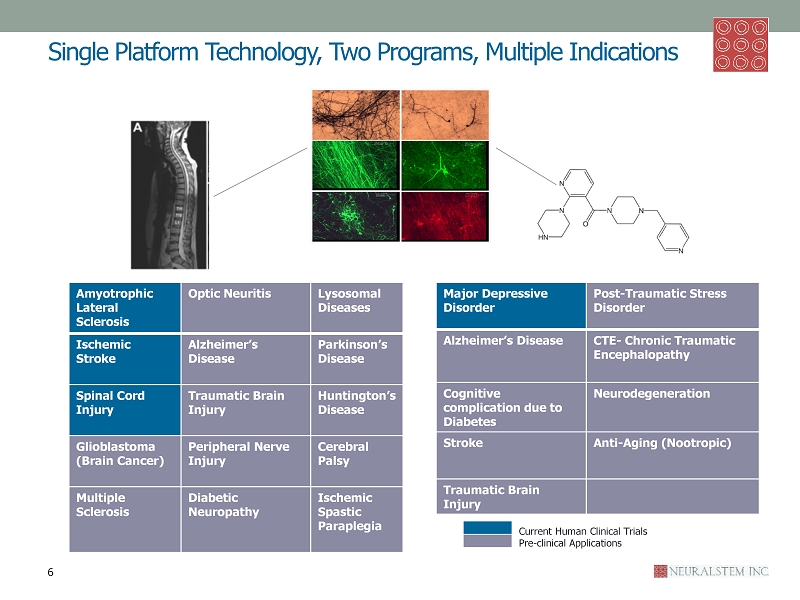
Single Platform Technology, Two Programs, Multiple Indications Ischemic Spastic Paraplegia Amyotrophic Lateral Sclerosis Optic Neuritis Lysosomal Diseases Ischemic Stroke Alzheimer’s Disease Parkinson’s Disease Spinal Cord Injury Traumatic Brain Injury Huntington’s Disease Glioblastoma (Brain Cancer) Peripheral Nerve Injury Cerebral Palsy Multiple Sclerosis Diabetic Neuropathy Ischemic Spastic Paraplegia Major Depressive Disorder Post - Traumatic Stress Disorder Alzheimer’s Disease CTE - Chronic Traumatic Encephalopathy Cognitive complication due to Diabetes Neurodegeneration Stroke Anti - Aging ( Nootropic ) Traumatic Brain Injury 6 Current Human Clinical Trials Pre - clinical Applications

ALS – Amyotrophic Lateral Sclerosis • NSI - 566 FDA Orphan Designation • No C ure • Unmet Medical Need: • Est. 30,000 Americans have disease at any given time (ALSA) • Est. 5,600+ people diagnosed each year in the U.S. (ALSA) • Est. n early 120,000 cases diagnosed worldwide each year (Int’l Alliance of ALS/MND Assns . ) • Progressive neurodegenerative disease • Affects nerve cells in the brain and spinal cord • Leads to complete paralysis, and eventually, death “Now this is a disease that doesn't get better. So we don't see patients who spontaneously get better. It just doesn't happen.” -- Neuralstem ALS Trial Site Principal Investigator and Emory University neurologist Dr . Jonathan Glass, referring to pre - trial disease status 8
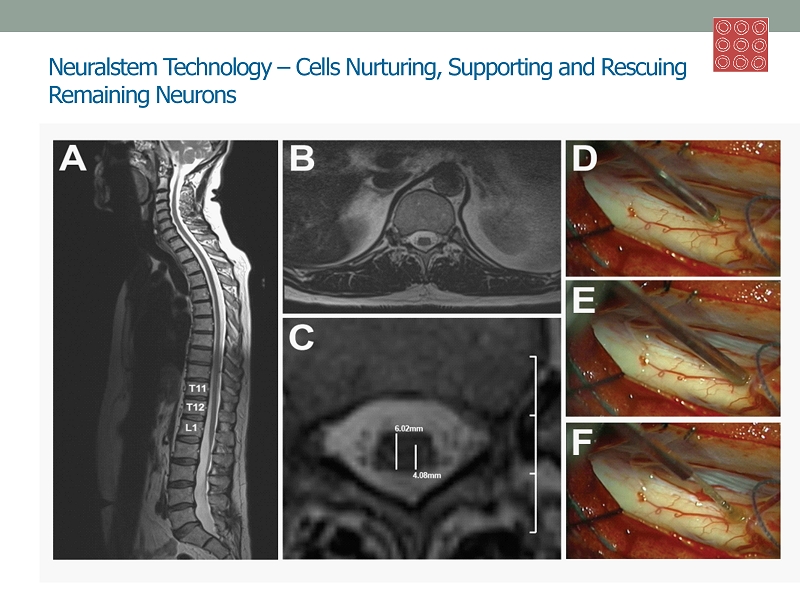
Neuralstem Technology – Cells Nurturing, Supporting and Rescuing Remaining Neurons • Regionally specific CNS neural stem cells: brain and spinal cord • 9 U.S. p atents re - affirmed worldwide, 22 pending • cGMP manufacturing • Numerous cell therapy stem cell products • Fully characterized • Expanded under defined conditions: no animal - derived reagents, serum or feeder cells • Reproducible differentiation: constitutive behavior of cells • Physiologically relevant neurons: 50% 5 Midbrain Dopaminergic Spinal Cord Cholingergic Hippocampus GABAergic
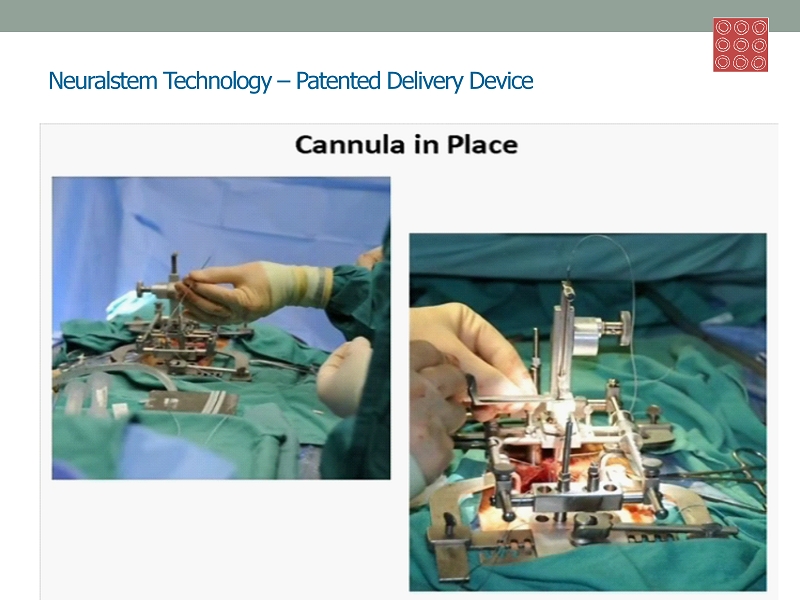
Neuralstem Technology – Patented Delivery Device 5 Midbrain Dopaminergic Spinal Cord Cholingergic Hippocampus GABAergic

Neuralstem Technology – Patented Delivery Device 5 Midbrain Dopaminergic Spinal Cord Cholingergic Hippocampus GABAergic
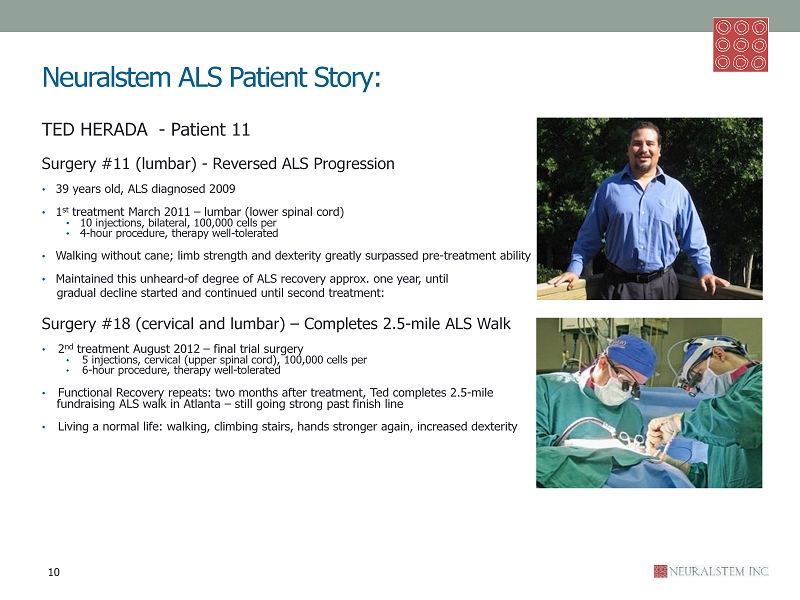
Neuralstem ALS Patient Story: TED HERADA - Patient 11 Surgery #11 (lumbar) - Reversed ALS Progression • 39 years old, ALS diagnosed 2009 • 1 st treatment March 2011 – lumbar (lower spinal cord) • 10 injections, bilateral, 100,000 cells per • 4 - hour procedure, therapy well - tolerated • Walking without cane; limb strength and dexterity greatly surpassed pre - treatment ability • Maintained this unheard - of degree of ALS recovery approx. one year, until gradual decline started and continued until second treatment: Surgery #18 (cervical and lumbar) – Completes 2.5 - mile ALS Walk • 2 nd treatment August 2012 – final trial surgery • 5 injections, cervical (upper spinal cord), 100,000 cells per • 6 - hour procedure, therapy well - tolerated • Functional Recovery repeats: two months after treatment, Ted completes 2.5 - mile fundraising ALS walk in Atlanta – still going strong past finish line • Living a normal life: walking, climbing stairs, hands stronger again, increased dexterity 10

NSI - 566 Human Proof of Concept, Safety, and Signs of Efficacy • Successful , safe injection of neural stem cells • 15 patients, 18 surgeries • L umbar and/or cervical regions of spinal cords • No complications related to cell delivery • No toxicity related to cells • Definitive evidence of long - term cell survival via DNA fingerprinting “ With the transplantation of these neural stem cells, we are exploring a paradigm shift in the treatment of ALS. We have demonstrated that intraspinal transplantation is feasible and well - tolerated. Although this phase of the trial was not powered to demonstrate efficacy, we appear to have interrupted the progression of the disease in one subgroup of patients. We are anxious to move to future trial phases to examine therapeutic efficacy.” Principal Investigator: Eva L. Feldman, M.D., Ph.D. Professor of Neurology & Director, A. Alfred Taubman Medical Research Inst. of the University of Michigan Medical School; President of American Neurological Association 7 Amyotrophic Lateral Sclerosis Ischemic Stroke Spinal Cord Injury

NSI - 566/ALS Phase I Success: Proven Safe, Signs of Efficacy Five of six ambulatory patients showed improvement or very slow progression of the disease post surgery ALS Functional Rating Scale – Revised ( ALSFRS - R) monitors changes in a patient over time with ten measures including speech , swallowing , walking and breathing, total score of 48. 0 baseline: Treatment 9 ALSFRS - R 0 5 10 15 20 25 30 35 40 45 50 -300 -200 -100 0 100 200 300 400 500 600 700 800 ALSFRS - R Score Days After Stem Cell Transplantation #210 #211 #212
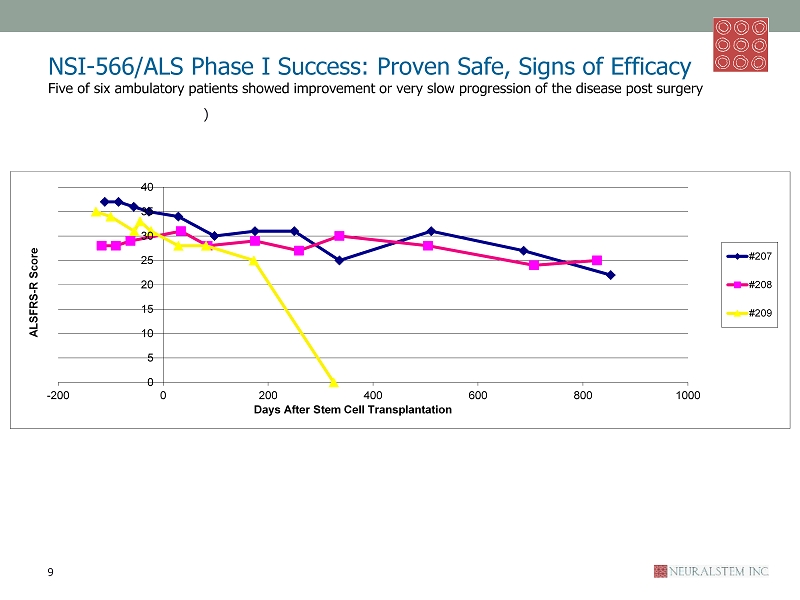
NSI - 566/ALS Phase I Success: Proven Safe, Signs of Efficacy Five of six ambulatory patients showed improvement or very slow progression of the disease post surgery ) ALS Functional Rating Scale – Revised ( ALSFRS - R) monitors changes in a patient over time with ten measures including speech , swallowing , walking and breathing, total score of 48. 0 baseline: Treatment 9 ALSFRS - R 0 5 10 15 20 25 30 35 40 -200 0 200 400 600 800 1000 ALSFRS - R Score Days After Stem Cell Transplantation #207 #208 #209

NSI - 566/ALS - Next Phase: Increase Dosage, Evaluate Efficacy and Safety • U.S. Phase II expected to commence in second half of 2013 • Emory announced significant NIH grant to cover majority of Phase II trial cost • Pending FDA approval of new protocol; IND filed 4Q12; official Phase I completion date mid - Feb 2013 • Collaborators: Eva L. Feldman, M.D., Ph.D. and Jonathan D. Glass, M.D. • Goal: maximum safe tolerated dose; evaluate e fficacy for clinical proof - of - concept to advance to Phase III ALS Phase II Trial: Recommended Protocol • 15 ambulatory patients • Accelerated dosing schedule, cervical injections • 200,000 - 400,000 cells per injection • 5 - 20 injections, escalated dosing per cohort (requested protocol) • Dual centers , escalated timeline • Emory University Hospital • A. Alfred Taubman Medical Research Inst. of the University of Michigan Medical School • 6 - month observation period per patient 11
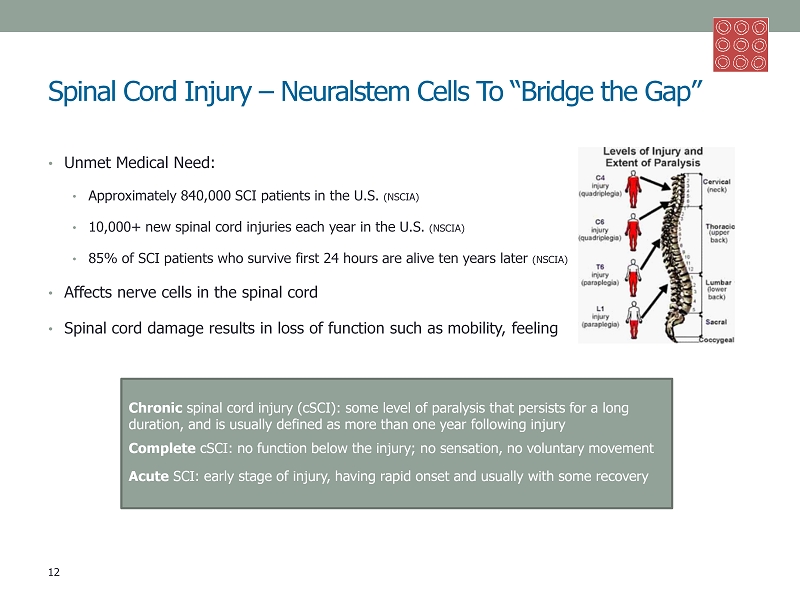
Spinal Cord Injury – Neuralstem Cells To “Bridge the Gap” • Unmet Medical Need: • Approximately 840,000 SCI patients in the U.S. (NSCIA) • 10,000+ new spinal cord injuries each year in the U.S. (NSCIA) • 85% of SCI patients who survive first 24 hours are alive ten years later (NSCIA) • Affects nerve cells in the spinal cord • Spinal cord damage results in loss of function such as mobility, feeling Chronic spinal cord injury ( cSCI ): some level of paralysis that persists for a long duration, and is usually defined as more than one year following injury Complete cSCI : no function below the injury; no sensation, no voluntary movement Acute SCI: early stage of injury, having rapid onset and usually with some recovery 12

NSI - 566/SCI Trials – Same Cells and Procedure • U.S. - NSI - 566/ cSCI Phase I Expected to Commence in 2 nd Half of 2013 • FDA approved trial in January 2013 • One - year Phase I completion goal ; trial study period ends six months post - surgery for each patient • Principal investigator: Keith Tanney , M.D., Ph.D., Shepherd Center • Seoul - NSI - 566/Acute SCI Phase I with Partner • CJ CheilJedang , IND Expected 1Q13 • Trial expected to start in 2 nd half of 2013 – four sites, following Korea Food and Drug Administration (KFDA) approval of trial • Goal is return of motor/sensory function to patients • South Korean partner CJ CheilJedang , whose pharma division has 1,200 employees and $400 million USD annual revenue from a wide breadth of products, has exclusive option agreement for cell therapy products in six South Asian countries : South Korea, Indonesia, Malaysia, Philippines, Singapore and Vietnam • 8 cSCI patients • T2 - T12 injuries – complete thoracic, no cervical • American Spinal Injury Assn. (ASIA) level A impairment – complete paralysis – one - to - two years post - injury • Multiple centers ( 4) – clinical trial institution agreements expected 2Q13 • All dosing completed six months from trial commencement • Six injections in, or around, the injury site • 100,000 cells per, first four patients; 200,000 cells per, second four patients • Primary objective : safety and toxicity of NSI - 566 • Secondary e ndpoints: evaluate graft survival in the transplant site by MRI; effectiveness of transient immunosuppression . Exploratory objectives include: evaluate ability of NSI - 566 transplantation to positively affect AIS level, ISNC SCI motor and sensory index scores, bowel and bladder function, pain, UAB IMR scores, SCIM scores, evoked sensory and motor potentials, and electromyogram (EMG) • Patients to receive physical therapy post - surgery to guide newly formed nerves to proper connections & functionality • Patients to also receive immunosuppressive therapy, which will be for three months, as tolerated 13
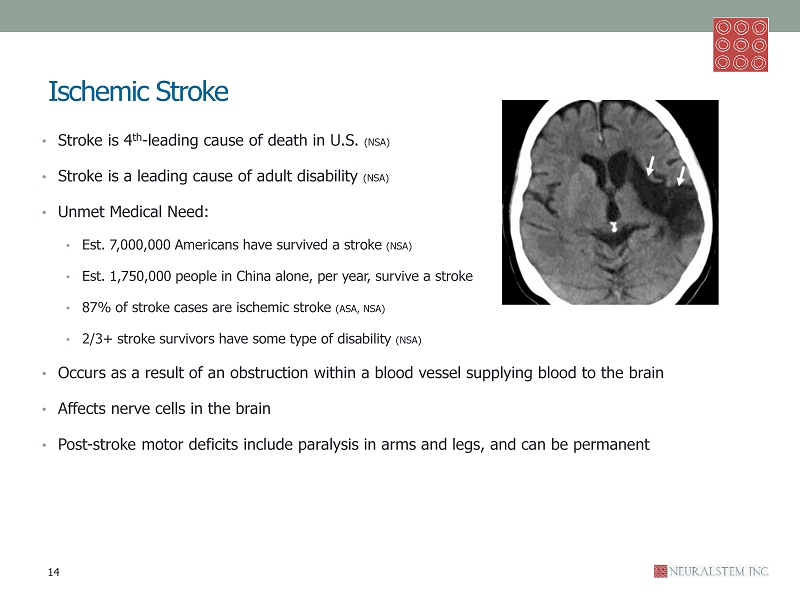
Ischemic Stroke • Stroke is 4 th - leading cause of death in U.S. (NSA) • Stroke is a leading cause of adult disability (NSA) • Unmet Medical Need: • Est. 7,000,000 Americans have survived a stroke (NSA) • Est. 1,750,000 people in China alone, per year, survive a stroke • 87% of stroke cases are ischemic stroke (ASA, NSA) • 2/3+ stroke survivors have some type of disability (NSA) • Occurs as a result of an obstruction within a blood vessel supplying blood to the brain • Affects nerve cells in the brain • Post - stroke motor deficits include paralysis in arms and legs, and can be permanent 14

NSI - 566/Ischemic Stroke – Rebuilding Neural Circuitry and Promoting Repair and Recovery of Surviving Tissue • Combined Phase I / II Trial expected to commence in 1Q13 – approved September 2012 • Wholly owned subsidiary: Neuralstem China 神 脑生物医药公司 ( Suzhou Neuralstem Biopharmaceutical Company, Ltd.) • Collaborator: BaYi Brain Hospital, in Beijing, world - class research facilities and one of China’s premier neurological hospitals • Expected duration : approximately two years, combined Phase I/II study, including patient monitoring and data collection 15 Trial protocol developed for FDA approval in the U.S., advanced preclinical program • One - time treatment of direct intracerebral injections of cells into the stroke area, using well - accepted stereotactic injection procedures • Up to 118 Patients: ischemic stroke with chronic residual motor disorder, 4 to 24 months post - stroke • Phase I: safety , determine m aximum safe dose • Open - label , up to 18 patients assigned to three cohorts • Each cohort receives ascending doses of NSI - 566: • 3 - 5 deposits on each of 5 tracks • 40,000 - 80,000 cells per deposit • Phase II/III: proof - of - concept , evaluates efficacy and safety • Multi - site, r andomized , controlled , single - blind study • Up to 100 stroke patients with stable paralysis for at least four months • 50 % receive one - time treatment, with physical therapy • 50 % receive physical therapy with no surgery • Outcome measures conducted in single - blinded manner
NSI - 566 Cell Therapy – Additional Applications • Glioblastoma (Brain Cancer ) • Research program initiated with U.S. DOD funding: $1.6 M award • Preclinical: Developing and Engineering neural stem cell technology to express anti - cancer agents • Collaborator: John H. Zhang, M.D. Ph.D., Loma Linda University • Multiple Indications – • Further clinical development pending NSI - 566 ALS Phase I Data, ongoing trial • Multiple Sclerosis – est. 2.5 million worldwide, 400,000 U.S. (NMSS) • Optic Neuritis – rare • Alzheimer’s Disease -- est. 5.4 million U.S. (AA/Alzheimer’s Assn ) • Traumatic Brain Injury – est.1.7 million U.S. per year; approx. 5.3 million live with disability in U.S. alone (AANS) • Peripheral Nerve Injury – extensive, 100+types, network of 43 pairs of motor and sensory nerves • Diabetic Neuropathy – affects approx. 60 % of est. 25.8 million both type 1 and type 2 diabetes patients (ADA) • Lysosomal Diseases – rare, varies; group of 50 distinct genetic diseases (NCBI, NIH) • Parkinson’s Disease – est. 1.5 million Americans, with est. 60K new cases per year (AANS) • Huntington’s Disease – est. 30,000 U.S. (HDSA) • Cerebral Palsy – est. 764K U.S. (CP) • Ischemic Spastic Paraplegia – can result from complication from surgery to repair aortic aneurysms 16
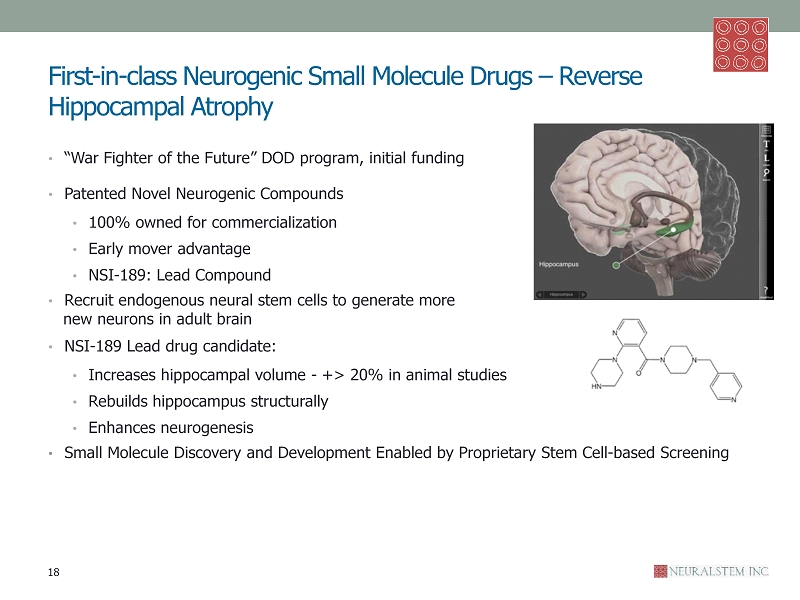
First - in - class Neurogenic Small Molecule Drugs – Reverse Hippocampal Atrophy • “War Fighter of the Future” DOD program, initial funding • Patented Novel Neurogenic Compounds • 100% owned for commercialization • Early mover advantage • NSI - 189: Lead Compound • R ecruit endogenous neural stem cells to generate more new neurons in adult brain • NSI - 189 Lead drug candidate: • Increases hippocampal volume - +> 20% in animal studies • Rebuilds hippocampus structurally • Enhances neurogenesis • Small Molecule Discovery and Development Enabled by Proprietary Stem Cell - based Screening 18

Major Depressive Disorder (MDD) • NSI - 189 Lead Drug Candidate First Application • Challenging Medical Need: • Est. 14.8 million American adults (NIMH) • Leading Cause of Disability in U.S. for ages 15 - 44 (NIMH) • New Theory of How To Treat: • Brain physiology in the disease rather than brain chemistry alone • Researchers now know depressed patients have reduced hippocampal volume • NSI - 189, by stimulating the generation of new neurons in the hippocampus, could potentially address the pathology of the depression itself • Current MDD oral medications, including worldwide - widely prescribed selective serotonin reuptake inhibitors, modulate levels of different neurotransmitters in the brain • MDD, also termed “major depression”: combination of symptoms interferes with ability to work, sleep, study, eat, and enjoy once - pleasurable activities 19

NSI - 189/MDD – Phase Ib Escalated Dosing, Topline Data Expected 3Q13 • Established drug safety in Phase Ia h ealthy volunteers : 41 volunteers received escalating doses of single oral administration (completed October 2011 ) • Psychiatric clinical trial consultant : Maurizio Fava, M.D., Slater Family Professor of Psychiatry at Harvard Medical School and Executive Vice Chair of the Department of Psychiatry at Massachusetts General Hospital 20 Ongoing NSI - 189/MDD Phase Ib Trial • Completion and topline data expected 3Q13 (commenced June 2012 ) • Randomized, double - blind, placebo - controlled, multiple - dose escalating trial. • 24 patients with recurrent MDD: three sets of eight patients (six treated, two placebo per set) • Escalating dose per set, daily for 28 days: 40 mg. q.d ., 40 mg. b.i.d ., 40 mg. t.i.d . • Two - month recovery follow - up, including MRI to monitor hippocampal volume • Secondary Phase Ib Endpoints: • Tolerability • Pharmacokinetics • Pharmacodynamic effects - hippocampal volume by MRI, BDNF/CRH/other factors in blood, urine biomarkers, cortisol in saliva, qEEG , MADRS, NGH Depression Questionnaire, Columbia Suicidal
1 2 - Month Clinical Trial Catalysts NSI - 566/ALS • Phase II U.S. Trial • NIH - major funding • Increased dosing, all cervical • Dual center, accelerated timeline • Data 6 months after final surgery • Orphan Status Designation • Est. 30,000 ALS patients, est. 5,600 diagnosed per year, U.S. • Phase I/II Mexico City Trial • Accelerated timeline and approvals • Pending partnership agreement NSI - 566/Chronic Spinal Cord Injury • Phase I U.S. Trial • FDA Approved Jan 2013 • Same Cells, Procedure as ALS • One - year timeline goal • Multiple sites, agreements expected 2Q13 • Est. 840K SCI patients, 10K+ new SCI injuries per year, U.S. • Phase I/II Seoul Trial • Partner CJ CheilJedang • Acute SCI patients • IND expected 1Q13, trial mid - year NSI - 566/Ischemic Stroke ( Neuralstem China) • Phase I/II China Trial • World - class Bayi Brain Hospital • Same Cells, injected in brain • Phase I - determine max safe dose, up to 18 Patients • Phase II - 100 patients, efficacy • China stroke pop est : 1.75 million+ NSI - 189/Major Depressive Disorder • Phase Ib Data, MDD patients • Phase II application • Partnership deal with Biotech or Big Pharma • Est. 14.8 million MDD patients, U.S. • NSI - 189 proven safe in Phase I; add’l applications expected to commence testing at Phase II 4

Appendix • Management • Patents: Technology and Products • Proprietary Surgical Devices: Cell Therapy • Published Papers • World - Class Clinical Partners 23

Management: Dedicated, Driven Team Karl Johe , Ph.D., Chairman of the Board and Chief Scientific Officer • Discovered and developed human neural stem cell technology, while at NIH • Successful neural stem cell therapy is career - long goal • Early work for four years as staff scientist at the NIH Laboratory of Molecular Biology of the National Institute of Neurological Disorders and Stroke in Bethesda (1993 - 1997) • Education: Post Doctoral, Molecular Genetics, University of California San Francisco; Ph.D., Biochemistry, Albert Einstein College of Medicine: Masters and B.A., Chemistry, University of Kansas Richard Garr, JD , President and CEO • Teamed legal and business expertise with friend Karl Johe’s science background to co - found Neuralstem (1996) • Focus on the business of science for 17 years; corporate legal, regulatory and patent experience • Previous corporate and commercial law practice: Beli , Weil & Jacobs, the B&G Companies, and Circle Management Companies • Education: J.D., Columbus School of Law, The Catholic University of America; B.A., Psychology, Drew University • Co - founder and Director, First Star Foundation; Mid - Atlantic Chapter Co - founder, The Starlight Foundation; Former Honorary Chairman, Brain Tumor Society Thomas G. Hazel, Ph.D., Senior Vice President • Served as Neuralstem’s Stem Cell Discovery Program Director, 2000 - 2004; Senior Scientist, 1998 - 2000 • Staff Scientist at the NIH Laboratory of Molecular Biology of the National Institute of Neurological Disorders and Stroke in Bethesda (1996 - 1998); IRTA fellow (1993 - 1996) • Education: Ph.D., Genetics, University of Illinois College of Medicine; B.A., Biology, Kalamazoo College 24
Technology & Product U.S . Patents Issued and Reaffirmed Worldwide • U. S. Pat. No. 8,236,299 (August 2012) • Transplantation of human neural cells for treatment of neurodegenerative conditions • U.S. Pat. No. 8,058,434 (November 2011) • Compositions to effect neuronal growth • U.S. Pat. No. 8,030,492 (October 2011) • Compositions to effect neuronal growth • U.S. Pat. No. 7,691,629 (April 2010) • Transplantation of human neural cells for treatment of neurodegenerative conditions • U.S. Pat. Nos. 7,560,553 and 7,858,628 (July 2009, December 2010) • Use of fused nicotinamides to promote neurogenesis (div) • U.S. Pat. No. 7,544,511 (June 2009) • Stable neural stem cell line methods • U.S. Pat. No. 6,284,539 (September 2001) • Method for generating dopaminergic cells derived from neural precursors • U.S. Pat. No. 6,040,180 (March 2000) • In vitro generation of differentiated neurons from cultures of mammalian multipotential CNS stem cells • U.S. Pat. No. 5,753,506 (May 1998) • Isolation propagation and directed differentiation of stem cells from embryonic and adult central nervous system of mammals (div) • 2 2 Patents Pending Neuralstem.com Live Map Includes Worldwide I.P . 25

Exclusive Worldwide Licenses: Breakthrough Cell Therapy Surgical Devices • Spinal Platform and Floating Cannula • Proprietary breakthrough medical devices proven safe in 18 surgeries • New standard in industry and research community for intraspinal procedures • Designed by ALS trial neurosurgeon, Nicholas M. Boulis , M.D., specifically for the world's first delivery of cells directly into the gray matter of the spinal cord • Safety of device first reported in data presented at the American Association of Neurologists' 2011 Annual Meeting • Patent - protected: • U.S. Patent No. 8,092,495 (January 2012) • Spinal Platform and Method for Delivering a Therapeutic Agent to a Spinal Cord Target • Issued & Pending Patents: U.S. Patent No. 7,833,217 (November 2010 ) ; U.S . Application No. 12/913,527 • Floating Spinal Cannula and Method of Use 26

Published Papers • Long - Distance Growth and Connectivity of Neural Stem Cells after Severe Spinal Cord Injury. • Paul Lu, Yaozhi Wang, Lori Graham, Karla McHale, Mingyong Gao , Di Wu, John Brock, Armin Blesch , Ephron S. Rosenzweig , Leif A. Havton , Binhai Zheng , James M. Conner, Martin Marsala , Mark H. Tuszynski • Cell , Volume 150, Issue 6, 14 September 2012, Pages 1264 - 1273 • Lumbar Intraspinal Injection of Neural Stem Cells in Patients with ALS: Results of a Phase I Trial in 12 Patients. • Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman, EL, Department of Neurology, Emory University School of Medicine, Atlanta • Stem Cells , 2012 Jun;30(6):1144 - 51. • Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. • Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE , Department of Pathology, Division of Neuropathology, The Johns Hopkins Medical Institutions, Baltimore • The Journal of Comparative Neurology , 2009 Jun 1;514(4):297 - 309. • Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. • Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE , Department of Pathology, Division of Neuropathology, The Johns Hopkins Medical Institutions, Baltimore • PLoS Medicine , 2007 Feb;4(2):e39. • Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. • Cizkova D, Kakinohana O, Kucharova K, Marsala S, Johe K, Hazel T, Hefferan MP, Marsala M , Institute of Neurobiology, Centrum of Excellence, Slovak Academy of Science, Kosice, Soltesovej 4, Slovakia. • Neuroscience , 2007 Jun 29;147(2):546 - 60. Epub 2007 May 23. • Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. • Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE, Department of Pathology, Neuropathology Division, The Johns Hopkins University School of Medicine, Baltimore • Stem cells (Dayton, Ohio) , 2006 Aug;24(8):1976 - 85. Epub 2006 Apr 27. 27

World - Class Clinical P artners • NSI - 566/ALS Trial Clinical Investigators: • Principal Investigator: Eva L. Feldman, M.D., Ph.D., Professor of Neurology & Director A. Alfred Taubman Medical Research Inst. of the University of Michigan Medical School; President of American Neurological Assn. • Site Principal Investigator: Jonathan D. Glass, M.D., Professor of Neurology & Director Emory ALS Center, Emory University • Co - Investigator & Neurosurgeon: Nicholas M. Boulis M.D., Assist. Professor Neurosurgery, Emory University • NSI - 566/ALS Trial Advisory Board: • Zachary Simmons M.D., Chairman, SMB , Professor of Neurology Penn State University Hershey Medical Center and Director Neuromuscular Program and ALS Center • Mark Bromberg M.D., Ph.D. Professor of Neurology & Director of the Motor Neuron Disease/ALS Clinic University of Utah School of Medicine • Lucie Bruijn , Ph.D., Chief Scientist & Sr. VP of Research and Development ALS Association • Thomas Freeman M.D., Professor, Dept of Neurosurgery & Medical Director, Center for Aging and Brain Repair University of South Florida College of Medicine • Clifton L. Gooch, M.D., Professor & Chairman Department of Neurology & Director Division of Neuromuscular Disease University of South Florida College of Medicine • Hiroshi Mitsumoto M.D., D.Sc., Professor of Neurology Columbia University & Director of the Eleanor and Lou Gehrig MDA/ALS Research Center and Neuromuscular Division at the Neurological Institute of New York • Paul Park M.D., Assistant Professor & Neurosurgeon University of Michigan School of Medicine • Mike Vogelbaum M.D., Ph.D. , Associate Director, Brain Tumor and Neuro - Oncology Center Cleveland Clinic • NSI - 566/ Glioblastoma (Brain Cancer) Principal Investigator John Zhang, M.D., Ph.D., Professor of Neurosurgery, Loma Linda University, CA • NSI - 566/Spinal Cord Injury Lead Collaborator Martin Marsala , M.D., Ph.D., University of California San Diego • NSI - 189/Psychiatric/Small Molecule Trial Consultant Maurizio Fava, M.D., Slater Family Professor of Psychiatry at Harvard Medical School and Executive Vice Chair of the Department of Psychiatry at Massachusetts General Hospital 28