Exhibit 99.01

NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF

SUMMARY OF OPPORTUNITY : PROGRAMS AND INDICATIONS NSI - 566 is an allogeneic neural stem cell line that is currently being evaluated in China in a placebo - controlled Phase II clinical trial for chronic ischemic stroke, and has been evaluated in the US in a Phase II trial for amyotrophic lateral sclerosis (ALS) and a Phase I trial for chronic spinal cord injury (cSCI) . NSI - 566 has been granted orphan drug designation by the US FDA for treatment of ALS . Seneca Biopharma, Inc . owns all rights to NSI - 566 and is seeking an asset sale, out - license, or global development partnership to further NSI - 566 development . Seneca Biopharma, Inc . , (NASDAQ : SNCA) is a clinical - stage biopharmaceutical company developing novel treatments for diseases of unmet medical need . NSI - 566 is the lead asset in Seneca’s pipeline and was developed using the Company’s proprietary neural stem cell technology . Seneca has generated a large patent estate in support of this technology, including approximately 50 issued and pending US and foreign patents with protection through 2023 to 2035 . The therapeutic basis and clinical benefits of NSI - 566 have been documented in over 30 peer - reviewed publications . NSI - 566 PIPELINE OVERVIEW : ALLOGENEIC, OFF - THE - SHELF THERAPY IN THREE INDICATIONS NSI - 566 is an allogeneic off - the - shelf neural stem cell line that has been evaluated in three clinical indications, each of which represents a substantial unmet medical need . MULTIPLE UPCOMING & ALREADY - ACHIEVED MILESTONES FOR NSI - 566 There are recently completed and near - term milestones for NSI - 566 across all 3 indications: I. Chronic Ischemic Stroke Phase I & II (n=31): Phase I trial demonstrated safety and preliminary evidence of clinical improvement in motor function from baseline levels. - Placebo - controlled study in China scheduled to complete 3Q 2020 II. ALS Phase I & II (n=30): demonstrated preliminary evidence of clinical benefit against historical data. - Pivotal study in the US in planning stages - FDA meeting March 2020 to provide feedback on pivotal study design & development plan III. cSCI Phase I (n=7): demonstrated safety, along with modest neurological improvements in some subjects. - Phase I completed 2019 Indication Preclinical Phase I Phase II Phase III Amyotrophic Lateral Sclerosis (ALS) Chronic Ischemic Stroke Chronic Spinal Cord Injury NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF 1

MARKET OPPORTUNITY & KEY DATA BY INDICATION CHRONIC ISCHEMIC STROKE - MULTI - BILLION DOLLAR GLOBAL OPPORTUNITY Ischemic stroke is a leading cause of long - term disability and death worldwide . Ischemic strokes account for approximately 75 % of all strokes and occur as a result of an obstruction within a vessel supplying blood to the brain . Post - stroke motor deficits include paralysis or weakness in arms and legs and oftentimes are permanent . The National Stroke Association estimates there are 7 million stroke survivors in the US and literature reports indicate a total of > 80 million worldwide . Currently, there are no approved interventional therapies . Over 700 , 000 individuals suffer stroke in the US each year, and it is calculated that over 5 million people in China suffer stroke annually . There are no approved therapies to restore motor function, and therapeutic intervention is limited to rehabilitation . Conservatively, Seneca estimates at least 175 , 000 patients each year in the US could benefit from treatment with NSI - 566 . With a modest 10 % market penetration Seneca would reach 15 , 000 – 20 , 000 patients annually . This creates an attractive market opportunity in excess of $ 1 billion annually in the US alone . Globally, chronic stroke represents a multi - billion - dollar opportunity . NSI - 566 may provide an effective treatment for restoring motor deficits resulting from chronic ischemic stroke by creating new circuitry in the area of injury and by repairing and/or nurturing damaged neurons to improve function in patients . Seneca has completed a Phase I study and is currently conducting a placebo - controlled Phase II study at BaYi Brain Hospital in Beijing, China in 22 subjects with motor deficits due to ischemic stroke . Subject enrollment has been completed and topline data readout is expected in Q 4 of 2020 . SUMMARY OF THE PHASE I STUDY AND RESULTS : Subjects received one - time intracerebral injections of 12 , 24 , or 72 million cells adjacent to the area of the stroke lesion . Injections of NSI - 566 were found to be safe, and subjects showed a persistent improvement in motor function as measured by the motor scale of the Fugl - Meyer Assessment (Figure 1 , top) . When combined, subjects from the three cohorts of the trial showed a mean improvement of 16 points from their original baseline score on the Fugl - Meyer Motor Scale (FMMS) at 12 months after surgery . An improvement of > 10 points is seen as a meaningful clinical improvement . Subjects showed continued evidence of tissue regeneration in the area of the lesion two years after surgery (Figure 1 a, below, and Figure 1 b, next page) . FUGL - MEYER MOTOR SCORE Change from Baseline (+/ - SEM) - One - time administration: 12 - 72 million cells - Direct injections into lesion area of brain - 4 weeks of immunosuppression - Evidence of long - term graft survival (at least 2 years) - Evidence for tissue regeneration - Shows potential for motor improvement NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF 2 Post Treatment (months, 0 - 12) Figure 1 a . Summary of Phase I trial in ischemic stroke . Subjects receiving cells showed a measurable and stable improvement relative to baseline in Fugl - Meyer motor scores for the 12 months following NSI - 566 transplantation (top right) . An improvement of 10 points or more in this index is considered clinically meaningful .
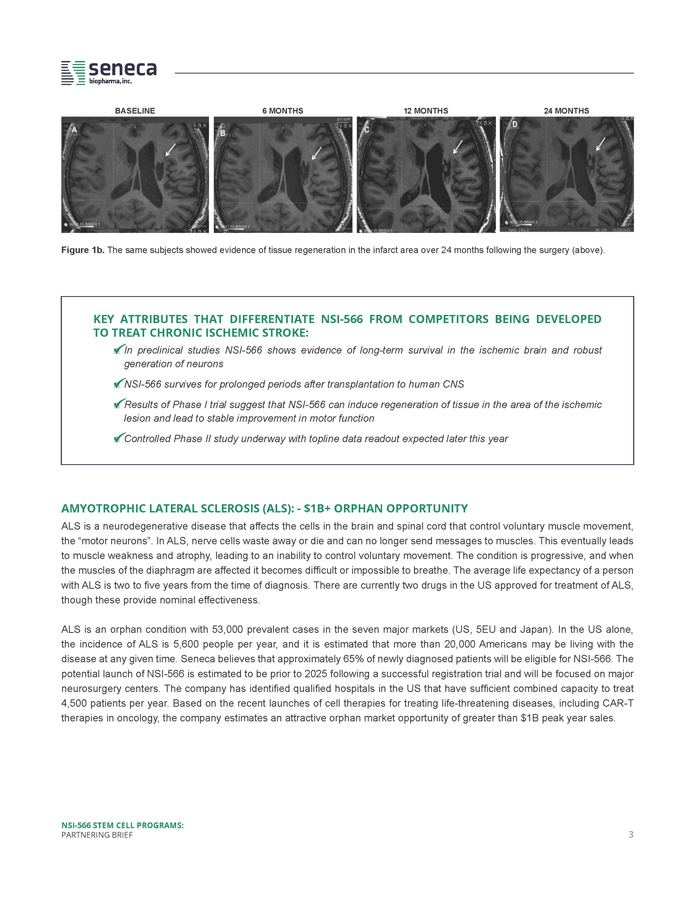
Figure 1b. The same subjects showed evidence of tissue regeneration in the infarct area over 24 months following the surgery (above). BASELINE 6 MONTHS 12 MONTHS 24 MONTHS KEY ATTRIBUTES THAT DIFFERENTIATE NSI - 566 FROM COMPETITORS BEING DEVELOPED TO TREAT CHRONIC ISCHEMIC STROKE: □ In preclinical studies NSI - 566 shows evidence of long - term survival in the ischemic brain and robust generation of neurons □ NSI - 566 survives for prolonged periods after transplantation to human CNS □ Results of Phase I trial suggest that NSI - 566 can induce regeneration of tissue in the area of the ischemic lesion and lead to stable improvement in motor function □ Controlled Phase II study underway with topline data readout expected later this year AMYOTROPHIC LATERAL SCLEROSIS (ALS) : - $ 1 B+ ORPHAN OPPORTUNITY ALS is a neurodegenerative disease that affects the cells in the brain and spinal cord that control voluntary muscle movement, the “motor neurons” . In ALS, nerve cells waste away or die and can no longer send messages to muscles . This eventually leads to muscle weakness and atrophy, leading to an inability to control voluntary movement . The condition is progressive, and when the muscles of the diaphragm are affected it becomes difficult or impossible to breathe . The average life expectancy of a person with ALS is two to five years from the time of diagnosis . There are currently two drugs in the US approved for treatment of ALS, though these provide nominal effectiveness . ALS is an orphan condition with 53 , 000 prevalent cases in the seven major markets (US, 5 EU and Japan) . In the US alone, the incidence of ALS is 5 , 600 people per year, and it is estimated that more than 20 , 000 Americans may be living with the disease at any given time . Seneca believes that approximately 65 % of newly diagnosed patients will be eligible for NSI - 566 . The potential launch of NSI - 566 is estimated to be prior to 2025 following a successful registration trial and will be focused on major neurosurgery centers . The company has identified qualified hospitals in the US that have sufficient combined capacity to treat 4 , 500 patients per year . Based on the recent launches of cell therapies for treating life - threatening diseases, including CAR - T therapies in oncology, the company estimates an attractive orphan market opportunity of greater than $ 1 B peak year sales . NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF 3
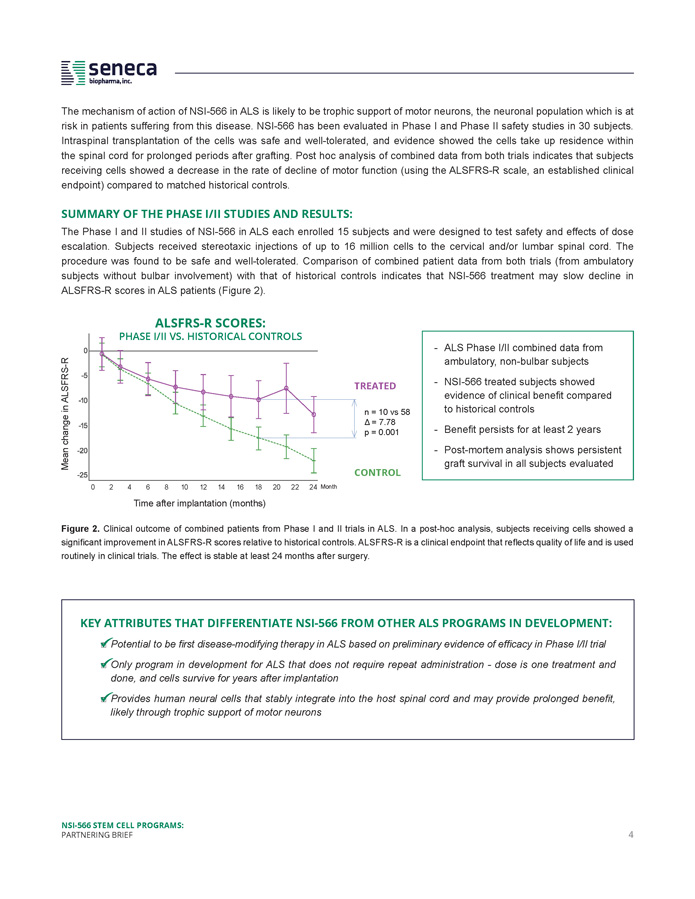
The mechanism of action of NSI - 566 in ALS is likely to be trophic support of motor neurons, the neuronal population which is at risk in patients suffering from this disease . NSI - 566 has been evaluated in Phase I and Phase II safety studies in 30 subjects . Intraspinal transplantation of the cells was safe and well - tolerated, and evidence showed the cells take up residence within the spinal cord for prolonged periods after grafting . Post hoc analysis of combined data from both trials indicates that subjects receiving cells showed a decrease in the rate of decline of motor function (using the ALSFRS - R scale, an established clinical endpoint) compared to matched historical controls . SUMMARY OF THE PHASE I/II STUDIES AND RESULTS : The Phase I and II studies of NSI - 566 in ALS each enrolled 15 subjects and were designed to test safety and effects of dose escalation . Subjects received stereotaxic injections of up to 16 million cells to the cervical and/or lumbar spinal cord . The procedure was found to be safe and well - tolerated . Comparison of combined patient data from both trials (from ambulatory subjects without bulbar involvement) with that of historical controls indicates that NSI - 566 treatment may slow decline in ALSFRS - R scores in ALS patients (Figure 2 ) . KEY ATTRIBUTES THAT DIFFERENTIATE NSI - 566 FROM OTHER ALS PROGRAMS IN DEVELOPMENT: □ Potential to be first disease - modifying therapy in ALS based on preliminary evidence of efficacy in Phase I/II trial □ Only program in development for ALS that does not require repeat administration - dose is one treatment and done, and cells survive for years after implantation □ Provides human neural cells that stably integrate into the host spinal cord and may provide prolonged benefit, likely through trophic support of motor neurons ALSFRS - R SCORES: PHASE I/II VS. HISTORICAL CONTROLS TREATED CONTROL Mean change in ALSFRS - R 8 10 12 14 16 18 20 22 24 Month 0 2 4 6 Time after implantation (months) Figure 2 . Clinical outcome of combined patients from Phase I and II trials in ALS . In a post - hoc analysis, subjects receiving cells showed a significant improvement in ALSFRS - R scores relative to historical controls . ALSFRS - R is a clinical endpoint that reflects quality of life and is used routinely in clinical trials . The effect is stable at least 24 months after surgery . n = 10 vs 58 Δ = 7.78 p = 0.001 NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF 4 - ALS Phase I/II combined data from ambulatory, non - bulbar subjects - NSI - 566 treated subjects showed evidence of clinical benefit compared to historical controls - Benefit persists for at least 2 years - Post - mortem analysis shows persistent graft survival in all subjects evaluated
CHRONIC SPINAL CORD INJURY (cSCI) - GLOBAL OPPORTUNITY As a truly unmet medical need, chronic spinal cord injury (cSCI) is an opportunity with significant potential . SCI is a long term, chronic and disabling neurological condition that may result from any trauma to the spinal cord, but most frequently occurs as a result of vehicle accidents and falls . The US prevalence of SCI is 249 , 000 - 363 , 000 with an annual incidence of ~ 17 , 000 new cases, and the WHO cites annual incidence of 250 , 000 - 500 , 000 new cases globally . Chronic SCI refers to the window after recovery has plateaued, beginning approximately 6 - 12 months after injury . cSCI disproportionally affects younger men, two - thirds of whom survive for at least 20 years . Patients are treated symptomatically during this time and have minimal prospects for functional improvement, leading to devastating effects on quality of life for patients and caregivers . There are currently no approved therapies to restore neurological function for these patients . Due to the large global incidence and gap in neurological treatment options, this creates a significant third market opportunity for NSI - 566 . NSI - 566 may provide an effective treatment for cSCI by “bridging the gap” in the spinal cord circuitry created in traumatic SCI and providing new cells to help transmit the signal from the brain to points at or below the level of injury . The company has recently completed a Phase I trial to test the safety and feasibility of NSI - 566 as a therapy for cSCI . SUMMARY OF PHASE I STUDY AND RESULTS : Subjects who had suffered thoracic (cohort 1 ) or cervical (cohort 2 ) spinal cord injury at least 12 months prior to surgery were administered 1 . 2 million cells by stereotaxic intraspinal injection . Neurological evaluation comprised recognized endpoints including ISNCSCI scores, pain scores, and electromyography (EMG), and subjects are being followed for up to 5 years . Improvements in sensory and motor function were apparent below the pre - surgery (baseline) level of injury in two of the four subjects in cohort 1 (improvement from T 8 neurological level to T 10 in subject 001 and from T 5 to T 6 in subject 010 ; Figure 3 ) . Figure 3. Clinical outcome of Cohort 1 from SCI trial. Two of four subjects experienced stable neurological improvements, as indicated by the change in diagnosed neurological level of injury. Subject Baseline 6 months 12 months 18 months 001 T8 T1 0 T1 0 T1 0 006 T7 - T7 T7 008 T2 - T2 - 010 T5 T6 T6 T6 KEY ATTRIBUTES THAT DIFFERENTIATE NSI - 566 FROM OTHER cSCI PROGRAMS IN DEVELOPMENT: □ NSI - 566 has the potential to be disease - modifying for cSCI □ NSI - 566 is capable of stably integrating into the patient’s spinal cord and surviving for years □ Preliminary evidence from Phase I trial suggests NSI - 566 may confer persistent neurological improvement □ Studies in non - human primates indicate NSI - 566 generates neurons that can integrate functionally into the CNS and bridge the gap between corticospinal tracts above and below the area of injury NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF 5

CONTACT FOR BUSINESS DEVELOPMENT INQUIRIES: NSI - 566 STEM CELL PROGRAMS: PARTNERING BRIEF 6 CONCLUSION AND PARTNERING OPPORTUNITY NSI - 566 is a promising and unique partnership opportunity, driven by several key factors . It is distinguished from competitors by its unique mechanism of action as one of very few neural lineage restorative therapies in development . Further, strong evidence of safety along with preliminary evidence of clinical benefit in multiple indications provide a broad foundation for future partners or acquirers of the asset to advance into development . NSI - 566 is addressing unmet medical needs for significant populations worldwide in all three core indications : ischemic stroke, ALS and chronic spinal cord injury . In each indication this asset has demonstrated the potential for disease modifying efficacy . Moreover, NSI - 566 has been granted orphan drug designation for ALS by the US FDA and could be by approved for this indication as early as 2025 pending successful completion of a single pivotal study . The company is currently seeking global or regional partnerships or outright asset sale opportunities . By integrating the NSI - 566 asset with a partner’s development and commercialization capabilities and resources, it will be possible to accelerate development and approval and maximize the value of NSI - 566 in its core indications . SUMMARY OF PARTNERING PROCESS & TIMELINE Seneca is currently initiating a process for asset sale, out - license, global development partnerships or other transactions . The company’s current management team that will facilitate any form of transaction or partnership includes senior executives with extensive therapeutic R&D experience that have shepherded the NSI - 566 programs to date . Seneca is open to various structures : Investment, license/co - development, asset sale, or JV . The anticipated asset sale and out - license process with timelines is included below : - Q1 2020: Initial outreach to interested parties and initial due diligence - Q2 2020: Completion of diligence and soliciting term sheets by end of Q2 - Q3 2020: Transaction close, begin of technology transfer as required Business Development Contact: Sia Anagnostou Hibiscus BioVentures bd@hibiscusbio.com Seneca Biopharma, Inc. Contact: Kenneth Carter, PhD Executive Chairman Seneca Biopharma, Inc. kcarter@senecabio.com






