
July 2022 Corporate Presentation Free Writing Prospectus dated July 25, 2022 Filed Pursuant to Rule 433 of the Securities Act of 1933, as amended Registration Statement No. 333-265769

Forward Looking Statements Statements in this presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans, expected near-term Phase 2 and Phase 3 milestones (including but not limited to, enrollment completion), hypothesis leading to adhesion formation, severity and incidence of adhesions, mode of function of other adhesion treatments, potential of LB1148 to reduce adhesions, potential of LB1148 to reduce types of adhesions, potential for LB1148 to transform the current standard of care, strategy, potential of LB1148 to be covered by a patient’s pharmacy benefit, ability to leverage certain regulatory pathways, timing of studies, competitors, regulatory matters, market size and opportunity and our ability to complete certain milestones, including completion of subject enrollment. Words such as “believe,” “anticipate,” “could,” “estimate,” “aim,” “target,” “plan,” “expect,” “intend,” “will,” “may,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the beliefs of management of Palisade Bio, Inc. (the “Company”) as well as assumptions that may never materialize or prove to be incorrect. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company, including, without limitation, risks inherent in developing pharmaceutical products, future results from the Company’s ongoing and planned clinical trials, the Company’s ability to obtain adequate financing to fund its planned clinical trials and other expenses, trends in the industry, changes in the competitive landscape, delays or disruptions due to the COVID-19 pandemic, the legal and regulatory framework for the industry and future expenditures. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. Other factors that may cause the Company's actual results to differ from current expectations are discussed in the Company's filings with the Securities and Exchange Commission, including the section titled “Risk Factors” contained therein. These forward-looking statements should not be taken as forecasts or promises, nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. Except as required by law, Palisade Bio assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. This presentation discusses product candidates that are under clinical study, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates. Caution should be exercised when interpreting results from separate trials involving separate product candidates. Differences exist between trial designs and subject demographics, which limit the conclusions that can be drawn from comparisons across different trials. This presentation includes statistical and other industry and market data that we obtained from industry publications, third-party research, surveys and studies. The information has been obtained from sources believed to be reliable, although there is no guarantee regarding the accuracy or completeness of such information. The trademarks included herein are the property of the owners thereof and are used for reference purposes only.

Free Writing Prospectus This presentation highlights basic information about the Company and the offering. Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in our Company. Except as otherwise indicated, this presentation speaks only as of the date hereof. This presentation does not constitute an offer to sell, nor a solicitation of an offer to buy, any securities by any person in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. Neither the United States Securities and Exchange Commission (the “SEC”) nor any other regulatory body has approved or disapproved of our securities or passed upon the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offense. This presentation includes industry and market data that we obtained from industry publications and journals, third-party studies and surveys, internal company studies and surveys, and other publicly available information. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein. We have filed a registration statement on Form S-1 (File No. 333-265769) with the SEC, including a preliminary prospectus dated July 22, 2022 (the “Preliminary Prospectus”), with respect to the offering of our securities to which this communication relates. Before you invest, you should read the Preliminary Prospectus (including the risk factors described therein) in the registration statement and, when available, the final prospectus relating to the offering, and the other documents we have filed with the SEC, for more complete information about the Company and the offering. You may obtain these documents, including the Preliminary Prospectus, for free by visiting EDGAR on the SEC website at http://www.sec.gov. Alternatively, copies of the prospectus may be obtained, when available, from: Ladenburg Thalmann & Co. Inc. by written request addressed to Syndicate Department, 640 5th Avenue, 4th Floor, New York, NY 10019 (telephone number 1-800-573-2541) or by emailing prospectus@ladenburg.com.

Risk Factors Investing in our securities stock involves a high degree of risk. You should carefully consider the risks described below, as well as other information included in our Registration Statement on Form S-1 (File No. 333-265769), Annual Report on Form 10-K filed with the SEC on March 17, 2022, and Quarterly Report on Form 10-Q filed with the SEC on May 13, 2022, including our financial statements and the related notes, and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” any of which may be relevant to decisions regarding an investment in or ownership of our securities. The occurrence of any of these risks could have a significant adverse effect on our reputation, business, financial condition, results of operations, growth and ability to accomplish our strategic objectives. We have organized the description of these risks into groupings in an effort to enhance readability, but many of the risks interrelate or could be grouped or ordered in other ways, so no special significance should be attributed to the groupings or order below. Risks include but are not limited to: The Company has conducted clinical trials in China; consequently, the FDA and other regulatory authorities may delay, limit or deny approval or require the Company to conduct additional trials in the United States in order to seek marketing authorization. The Company’s business depends on the successful clinical development, regulatory approval and commercialization of LB1148. Some of the initial indications in which the Company plans to pursue development of LB1148 are indications for which there are no FDA-approved therapies. This makes it difficult to predict the timing and costs of clinical development for LB1148 in these indications, as well as the regulatory approval path. The development and commercialization strategy for the Company’s lead product candidate LB1148 depends, in part, on published scientific literature and the FDA’s prior findings regarding the safety and efficacy of tranexamic acid. If the Company is not able to pursue this strategy, it may be delayed in receiving regulatory authority approval. The Company’s product candidates may cause undesirable side effects or have other unexpected properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in post-approval regulatory action. The Company relies on and expects to continue to rely on third-party CROs and other third parties to conduct and oversee its clinical trials. If these third parties do not meet the Company’s requirements or otherwise conduct the trials as required, the Company may not be able to satisfy its contractual obligations or obtain regulatory approval for, or commercialize, its product candidates. If the Company is not able to comply with the applicable continued listing requirements or standards of The Nasdaq Capital Market, Nasdaq could delist its common stock. The Company has expressed substantial doubt about its ability to continue as a going concern. The Company will need to raise additional financing in the future to fund its operations, which may not be available to it on favorable terms or at all.
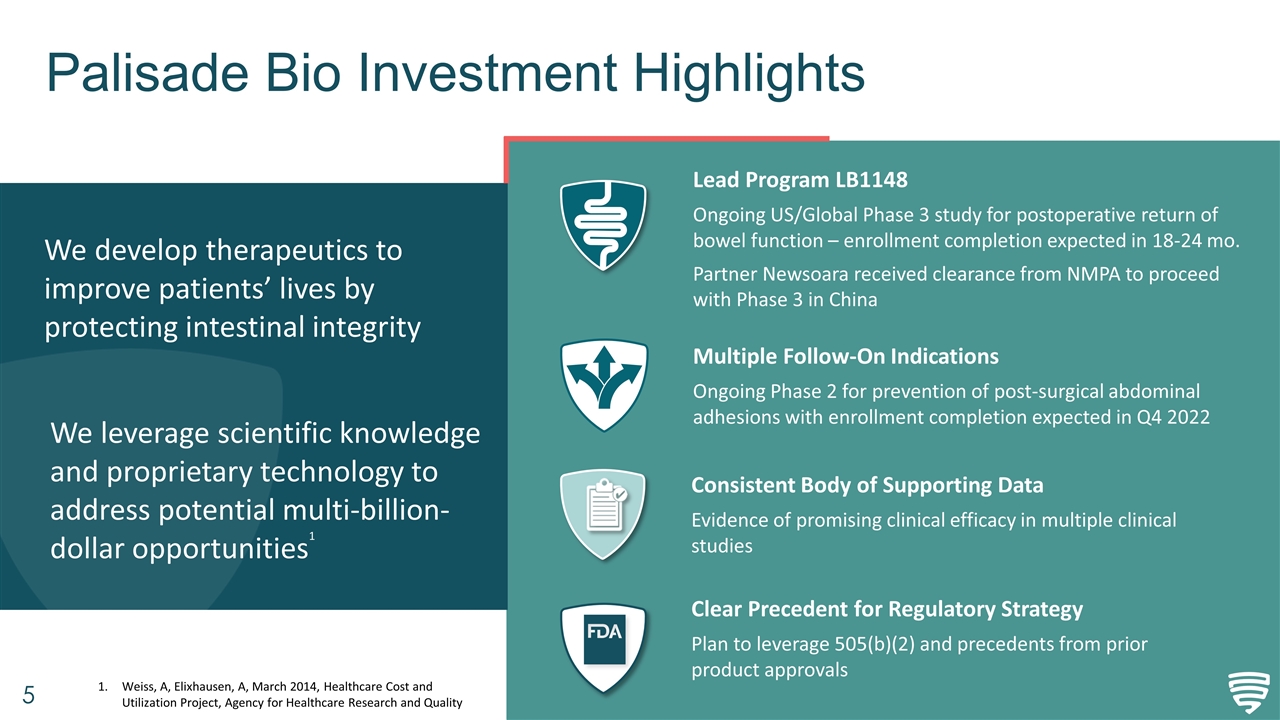
Palisade Bio Investment Highlights We develop therapeutics to improve patients’ lives by protecting intestinal integrity We leverage scientific knowledge and proprietary technology to address potential multi-billion-dollar opportunities1 Clear Precedent for Regulatory Strategy Plan to leverage 505(b)(2) and precedents from prior product approvals Consistent Body of Supporting Data Evidence of promising clinical efficacy in multiple clinical studies Lead Program LB1148 Ongoing US/Global Phase 3 study for postoperative return of bowel function – enrollment completion expected in 18-24 mo. Partner Newsoara received clearance from NMPA to proceed with Phase 3 in China Weiss, A, Elixhausen, A, March 2014, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality Multiple Follow-On Indications Ongoing Phase 2 for prevention of post-surgical abdominal adhesions with enrollment completion expected in Q4 2022
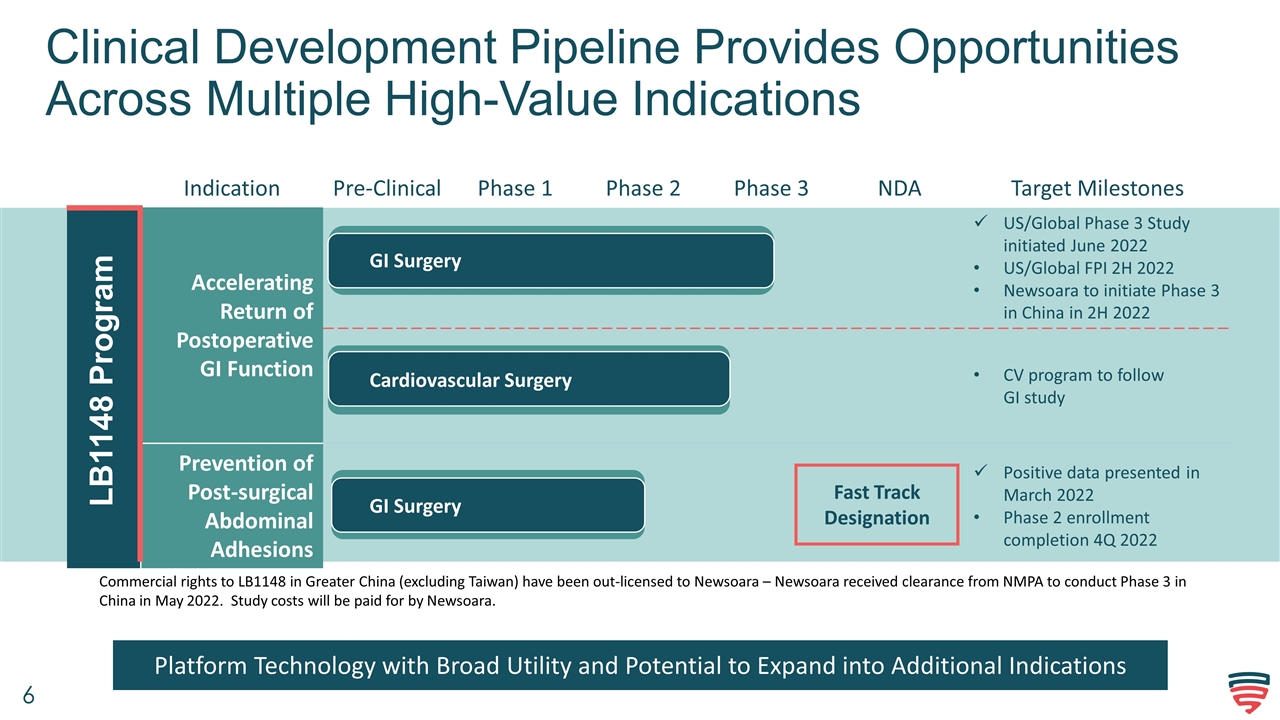
Clinical Development Pipeline Provides Opportunities Across Multiple High-Value Indications Indication Pre-Clinical Phase 1 Phase 2 Phase 3 NDA Target Milestones Accelerating Return of Postoperative GI Function US/Global Phase 3 Study initiated June 2022 US/Global FPI 2H 2022 Newsoara to initiate Phase 3 in China in 2H 2022 CV program to follow GI study Prevention of Post-surgical Abdominal Adhesions Positive data presented in March 2022 Phase 2 enrollment completion 4Q 2022 LB1148 Program GI Surgery Cardiovascular Surgery GI Surgery Fast Track Designation Platform Technology with Broad Utility and Potential to Expand into Additional Indications Commercial rights to LB1148 in Greater China (excluding Taiwan) have been out-licensed to Newsoara – Newsoara received clearance from NMPA to conduct Phase 3 in China in May 2022. Study costs will be paid for by Newsoara.
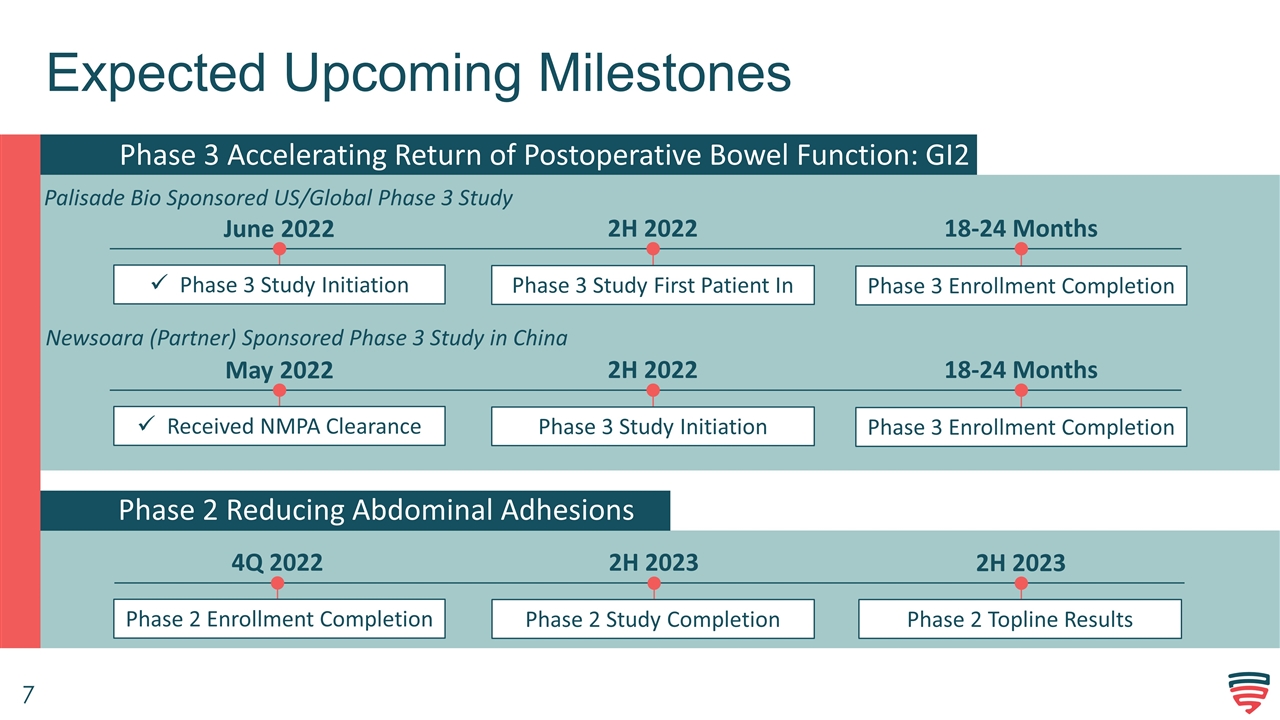
Expected Upcoming Milestones Phase 3 Accelerating Return of Postoperative Bowel Function: GI2 June 2022 Phase 3 Study Initiation 2H 2022 Phase 3 Study First Patient In Phase 2 Reducing Abdominal Adhesions Phase 2 Enrollment Completion 4Q 2022 Phase 2 Study Completion Phase 2 Topline Results 2H 2023 2H 2023 Phase 3 Enrollment Completion 18-24 Months Palisade Bio Sponsored US/Global Phase 3 Study Newsoara (Partner) Sponsored Phase 3 Study in China May 2022 Received NMPA Clearance 2H 2022 Phase 3 Study Initiation Phase 3 Enrollment Completion 18-24 Months
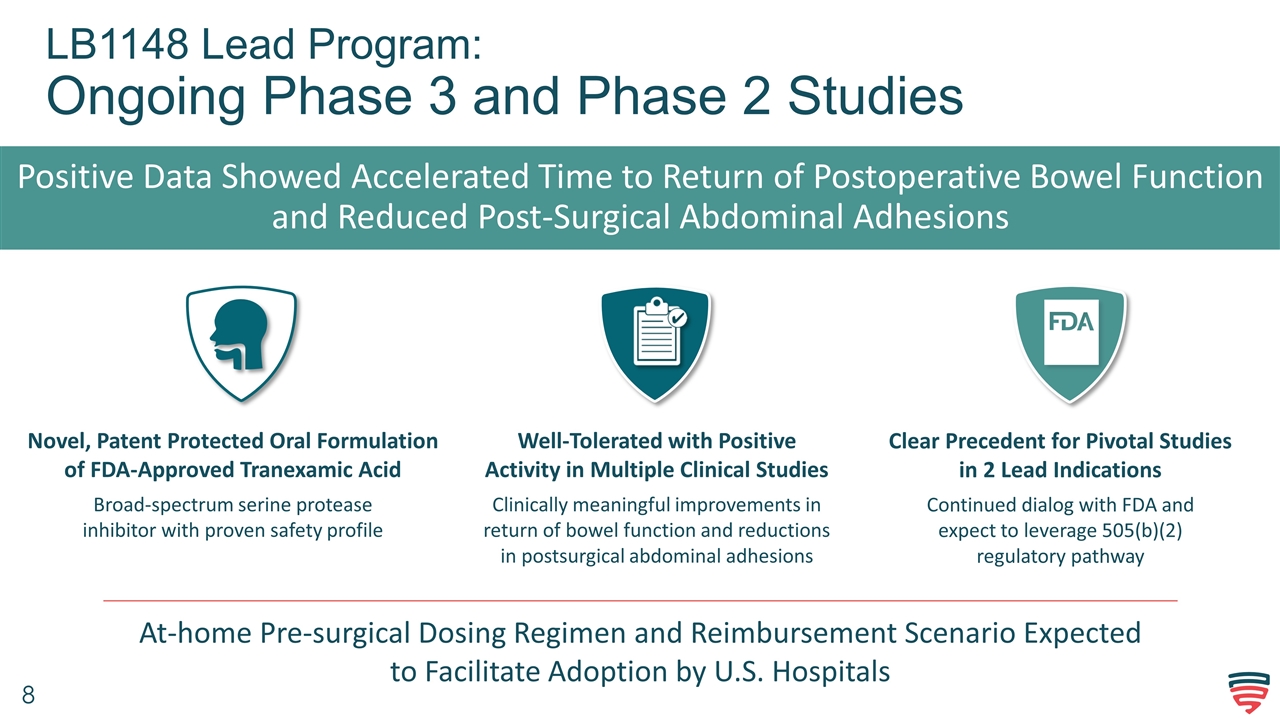
LB1148 Lead Program: Ongoing Phase 3 and Phase 2 Studies Positive Data Showed Accelerated Time to Return of Postoperative Bowel Function and Reduced Post-Surgical Abdominal Adhesions Novel, Patent Protected Oral Formulation of FDA-Approved Tranexamic Acid Broad-spectrum serine protease inhibitor with proven safety profile Well-Tolerated with Positive Activity in Multiple Clinical Studies Clinically meaningful improvements in return of bowel function and reductions in postsurgical abdominal adhesions Clear Precedent for Pivotal Studies in 2 Lead Indications Continued dialog with FDA and expect to leverage 505(b)(2) regulatory pathway At-home Pre-surgical Dosing Regimen and Reimbursement Scenario Expected to Facilitate Adoption by U.S. Hospitals
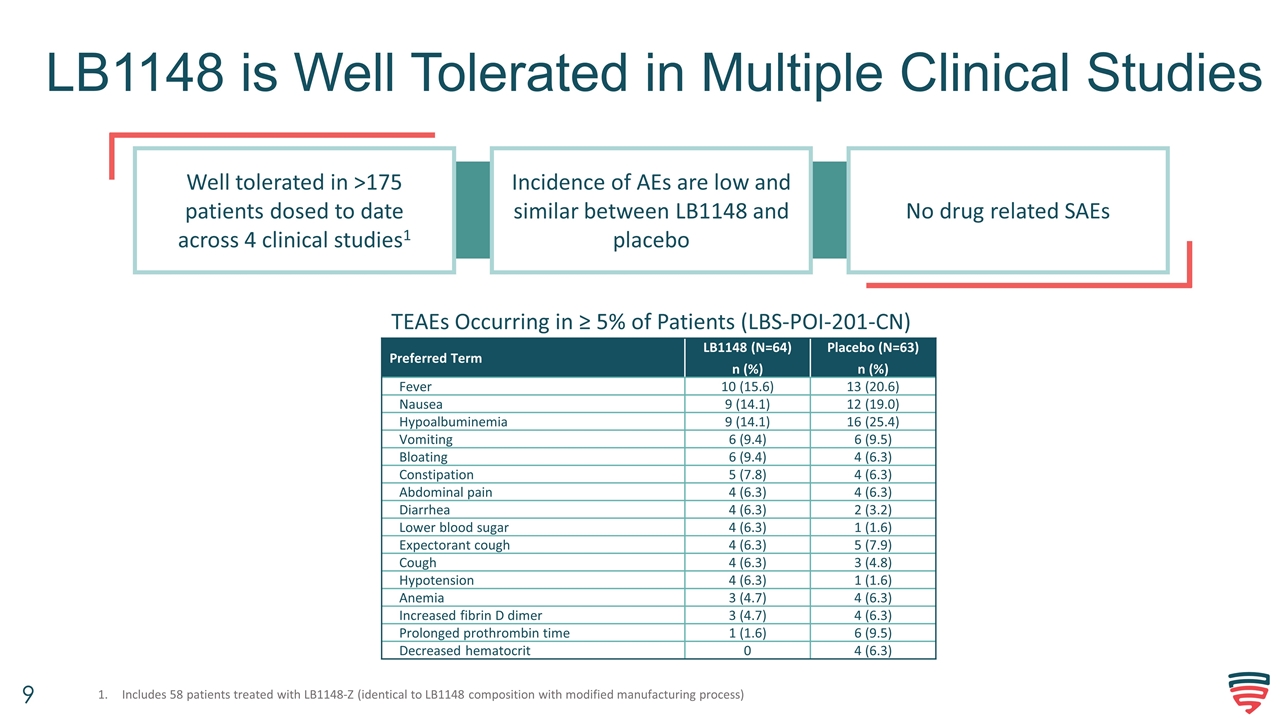
LB1148 is Well Tolerated in Multiple Clinical Studies No drug related SAEs Incidence of AEs are low and similar between LB1148 and placebo Well tolerated in >175 patients dosed to date across 4 clinical studies1 Preferred Term LB1148 (N=64) n (%) Placebo (N=63) n (%) Fever 10 (15.6) 13 (20.6) Nausea 9 (14.1) 12 (19.0) Hypoalbuminemia 9 (14.1) 16 (25.4) Vomiting 6 (9.4) 6 (9.5) Bloating 6 (9.4) 4 (6.3) Constipation 5 (7.8) 4 (6.3) Abdominal pain 4 (6.3) 4 (6.3) Diarrhea 4 (6.3) 2 (3.2) Lower blood sugar 4 (6.3) 1 (1.6) Expectorant cough 4 (6.3) 5 (7.9) Cough 4 (6.3) 3 (4.8) Hypotension 4 (6.3) 1 (1.6) Anemia 3 (4.7) 4 (6.3) Increased fibrin D dimer 3 (4.7) 4 (6.3) Prolonged prothrombin time 1 (1.6) 6 (9.5) Decreased hematocrit 0 4 (6.3) TEAEs Occurring in ≥ 5% of Patients (LBS-POI-201-CN) Includes 58 patients treated with LB1148-Z (identical to LB1148 composition with modified manufacturing process)
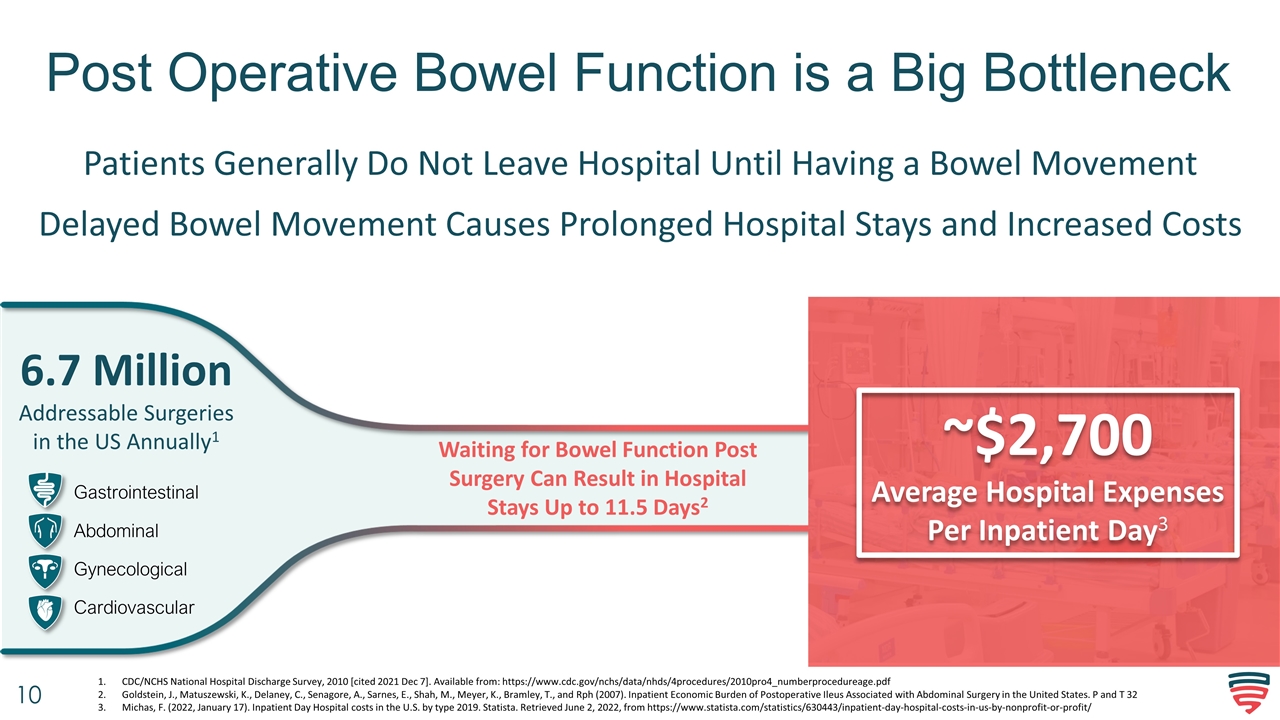
Post Operative Bowel Function is a Big Bottleneck Waiting for Bowel Function Post Surgery Can Result in Hospital Stays Up to 11.5 Days2 ~$2,700 Average Hospital Expenses Per Inpatient Day3 Patients Generally Do Not Leave Hospital Until Having a Bowel Movement Delayed Bowel Movement Causes Prolonged Hospital Stays and Increased Costs CDC/NCHS National Hospital Discharge Survey, 2010 [cited 2021 Dec 7]. Available from: https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf Goldstein, J., Matuszewski, K., Delaney, C., Senagore, A., Sarnes, E., Shah, M., Meyer, K., Bramley, T., and Rph (2007). Inpatient Economic Burden of Postoperative Ileus Associated with Abdominal Surgery in the United States. P and T 32 Michas, F. (2022, January 17). Inpatient Day Hospital costs in the U.S. by type 2019. Statista. Retrieved June 2, 2022, from https://www.statista.com/statistics/630443/inpatient-day-hospital-costs-in-us-by-nonprofit-or-profit/ 6.7 Million Addressable Surgeries in the US Annually1 Gastrointestinal Abdominal Gynecological Cardiovascular
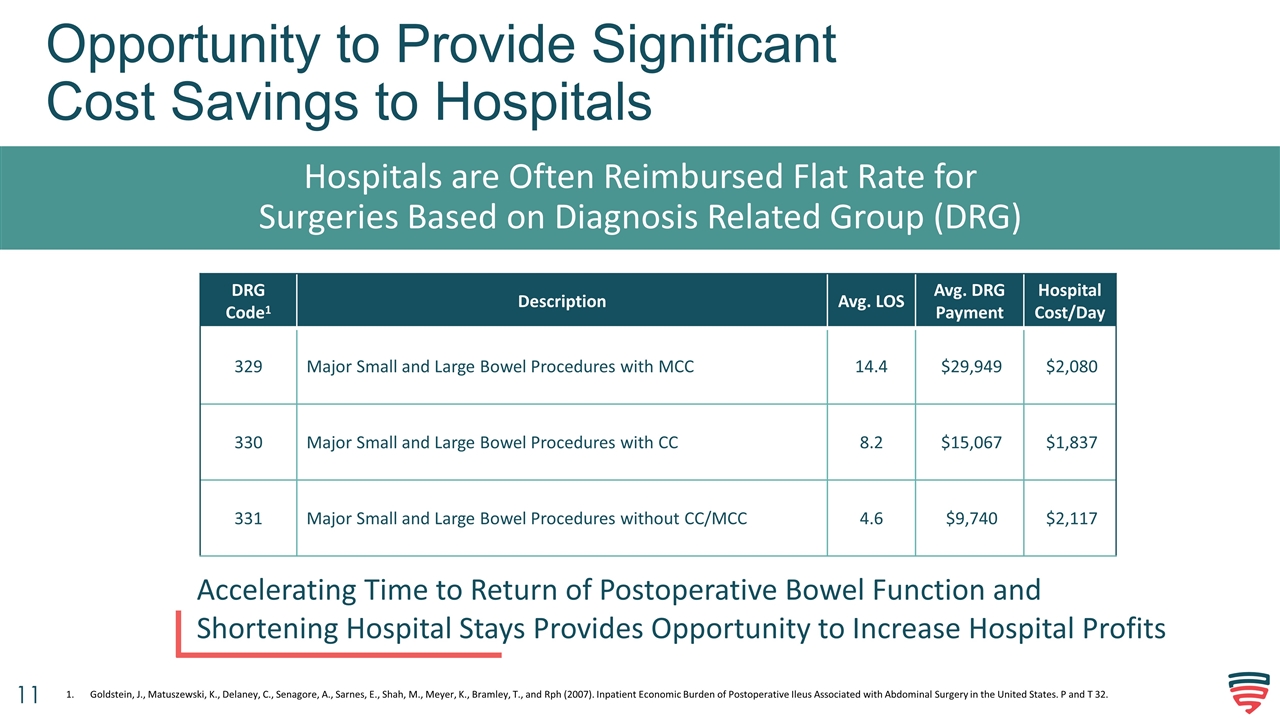
Opportunity to Provide Significant Cost Savings to Hospitals Hospitals are Often Reimbursed Flat Rate for Surgeries Based on Diagnosis Related Group (DRG) DRG Code1 Description Avg. LOS Avg. DRG Payment Hospital Cost/Day 329 Major Small and Large Bowel Procedures with MCC 14.4 $29,949 $2,080 330 Major Small and Large Bowel Procedures with CC 8.2 $15,067 $1,837 331 Major Small and Large Bowel Procedures without CC/MCC 4.6 $9,740 $2,117 Accelerating Time to Return of Postoperative Bowel Function and Shortening Hospital Stays Provides Opportunity to Increase Hospital Profits Goldstein, J., Matuszewski, K., Delaney, C., Senagore, A., Sarnes, E., Shah, M., Meyer, K., Bramley, T., and Rph (2007). Inpatient Economic Burden of Postoperative Ileus Associated with Abdominal Surgery in the United States. P and T 32.
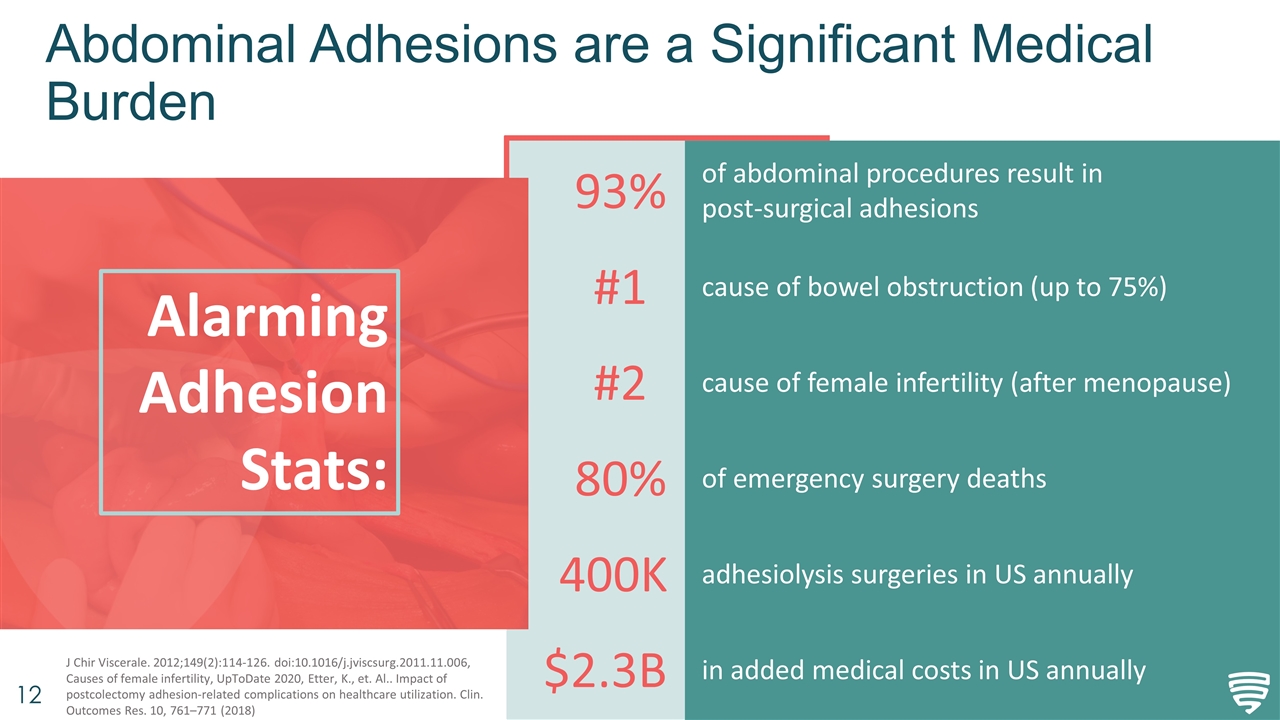
Abdominal Adhesions are a Significant Medical Burden Alarming Adhesion Stats: J Chir Viscerale. 2012;149(2):114-126. doi:10.1016/j.jviscsurg.2011.11.006, Causes of female infertility, UpToDate 2020, Etter, K., et. Al.. Impact of postcolectomy adhesion-related complications on healthcare utilization. Clin. Outcomes Res. 10, 761–771 (2018) 93% #1 #2 80% 400K $2.3B of abdominal procedures result in post-surgical adhesions cause of bowel obstruction (up to 75%) cause of female infertility (after menopause) of emergency surgery deaths adhesiolysis surgeries in US annually in added medical costs in US annually

LB1148: Protecting Intestinal Barrier Health Mechanism of Action
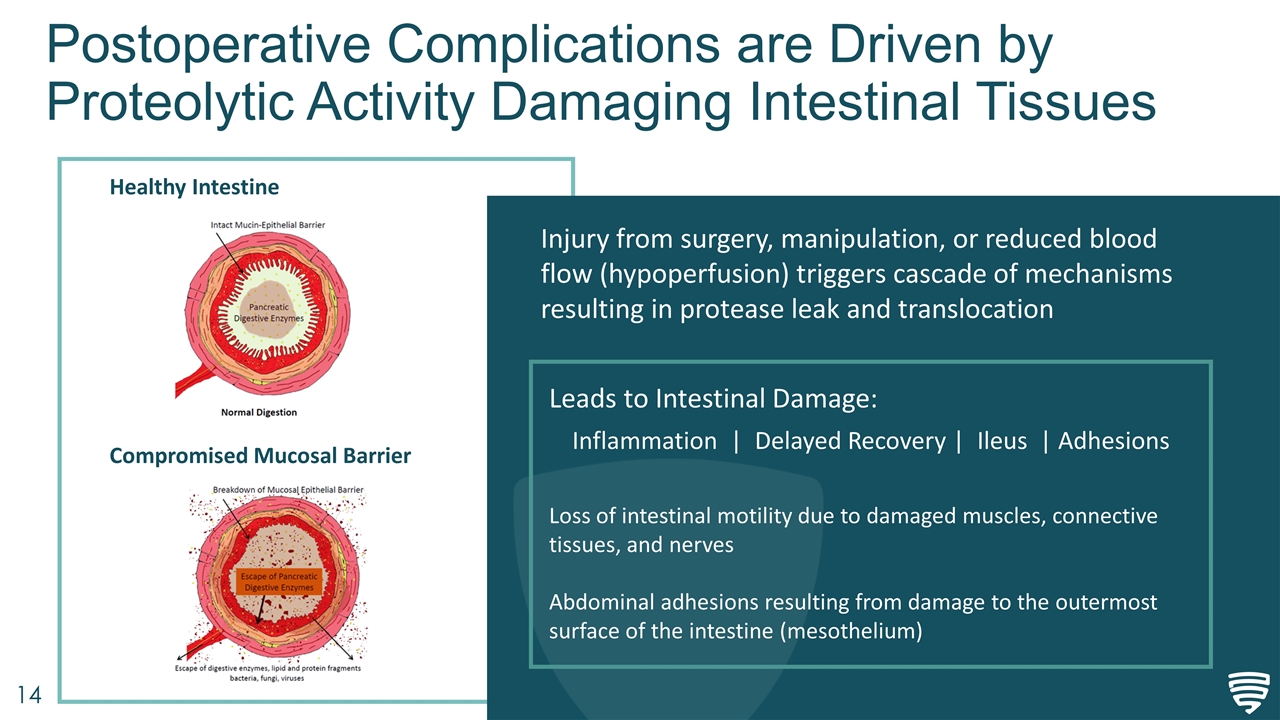
Postoperative Complications are Driven by Proteolytic Activity Damaging Intestinal Tissues Healthy Intestine Compromised Mucosal Barrier Injury from surgery, manipulation, or reduced blood flow (hypoperfusion) triggers cascade of mechanisms resulting in protease leak and translocation Leads to Intestinal Damage: Inflammation | Delayed Recovery | Ileus | Adhesions Loss of intestinal motility due to damaged muscles, connective tissues, and nerves Abdominal adhesions resulting from damage to the outermost surface of the intestine (mesothelium)
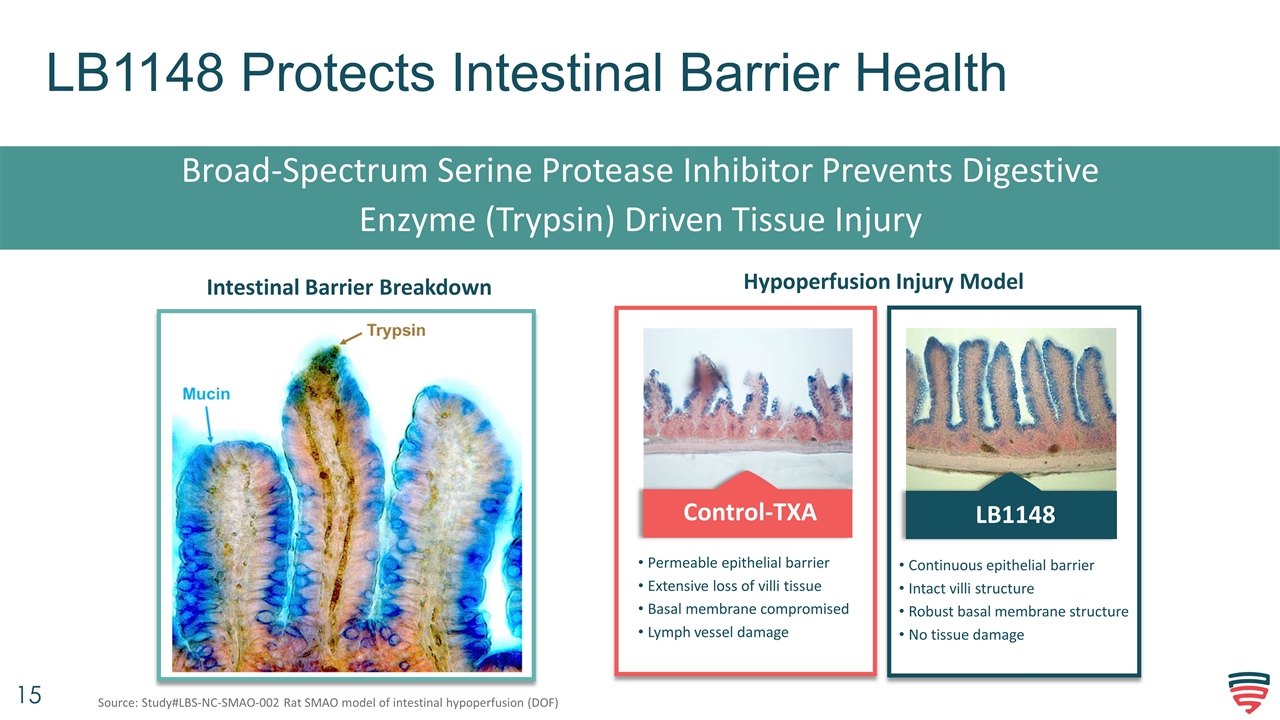
LB1148 Protects Intestinal Barrier Health Broad-Spectrum Serine Protease Inhibitor Prevents Digestive Enzyme (Trypsin) Driven Tissue Injury Intestinal Barrier Breakdown Control-TXA Permeable epithelial barrier Extensive loss of villi tissue Basal membrane compromised Lymph vessel damage LB1148 Continuous epithelial barrier Intact villi structure Robust basal membrane structure No tissue damage Hypoperfusion Injury Model Source: Study#LBS-NC-SMAO-002 Rat SMAO model of intestinal hypoperfusion (DOF)

LB1148: Accelerating Return of Bowel Function Development Path to Registration
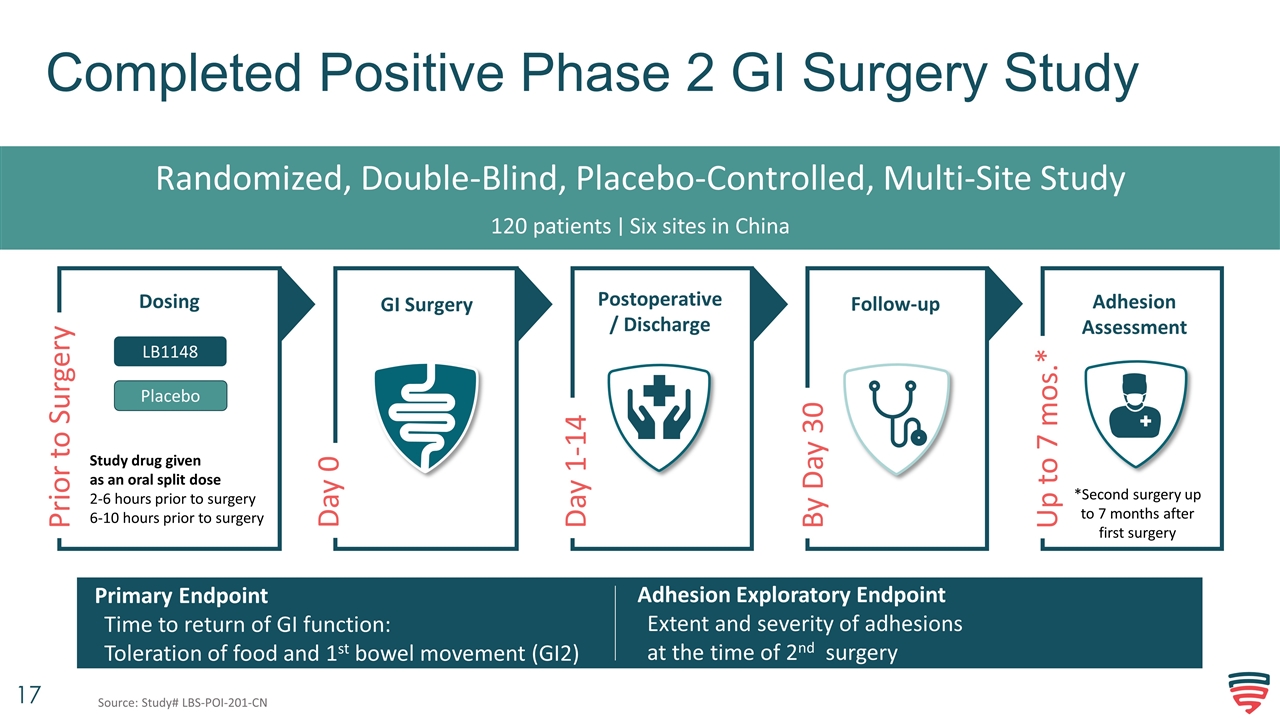
Completed Positive Phase 2 GI Surgery Study Randomized, Double-Blind, Placebo-Controlled, Multi-Site Study 120 patients ∣ Six sites in China Adhesion Exploratory Endpoint Extent and severity of adhesions at the time of 2nd surgery Primary Endpoint Time to return of GI function: Toleration of food and 1st bowel movement (GI2) Day 0 GI Surgery Follow-up Dosing Postoperative/ Discharge Prior to Surgery Day 1-14 By Day 30 LB1148 Study drug given as an oral split dose 2-6 hours prior to surgery 6-10 hours prior to surgery Placebo Adhesion Assessment *Second surgery up to 7 months after first surgery Up to 7 mos.* Source: Study# LBS-POI-201-CN
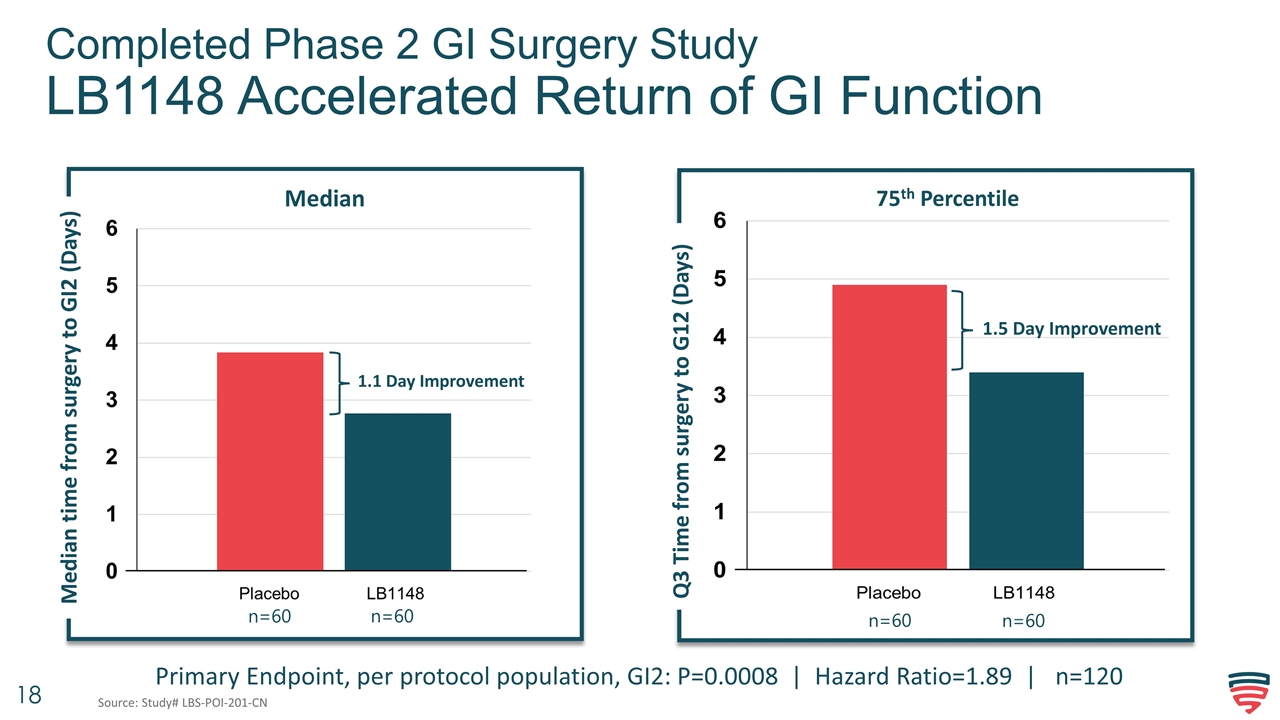
Completed Phase 2 GI Surgery Study LB1148 Accelerated Return of GI Function 1.1 Day Improvement Median 1.5 Day Improvement 75th Percentile Primary Endpoint, per protocol population, GI2: P=0.0008 | Hazard Ratio=1.89 | n=120 Median time from surgery to GI2 (Days) Q3 Time from surgery to G12 (Days) Q3 Time from surgery to G12 (Days) n=60 n=60 n=60 n=60 Source: Study# LBS-POI-201-CN
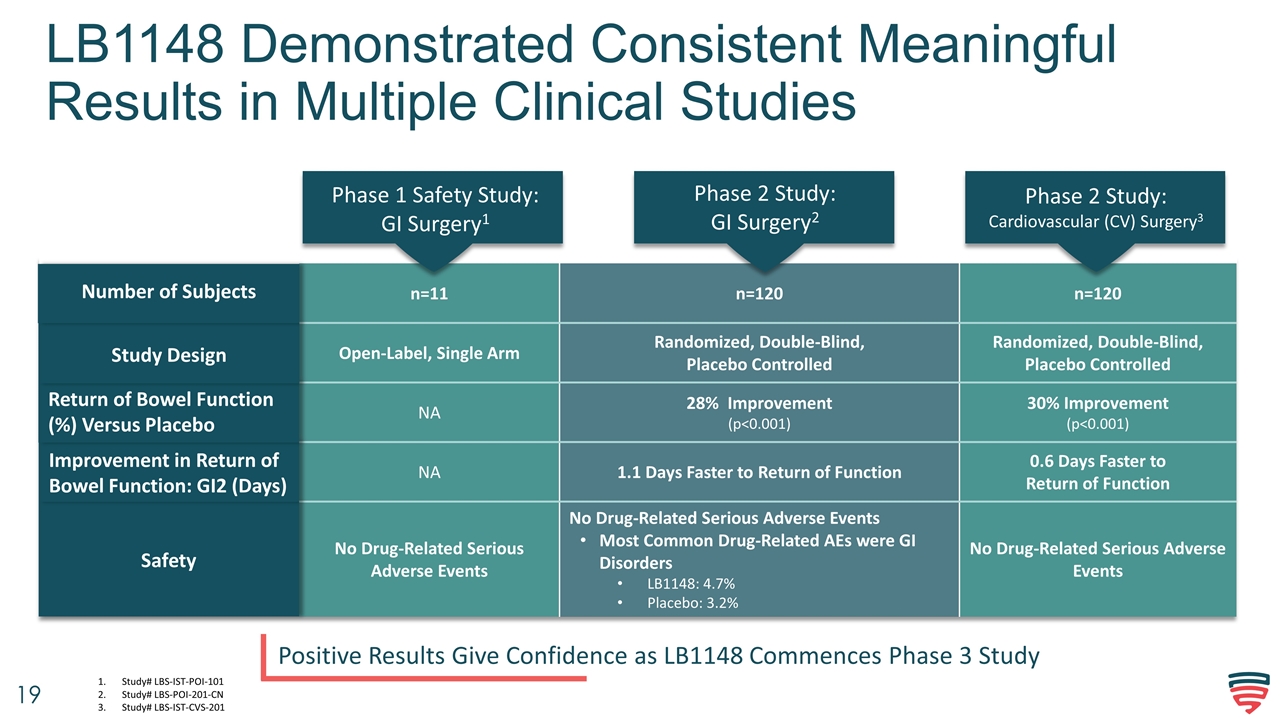
LB1148 Demonstrated Consistent Meaningful Results in Multiple Clinical Studies Positive Results Give Confidence as LB1148 Commences Phase 3 Study n=11 n=120 n=120 Open-Label, Single Arm Randomized, Double-Blind, Placebo Controlled Randomized, Double-Blind, Placebo Controlled NA 28% Improvement (p<0.001) 30% Improvement (p<0.001) NA 1.1 Days Faster to Return of Function 0.6 Days Faster to Return of Function No Drug-Related Serious Adverse Events No Drug-Related Serious Adverse Events Most Common Drug-Related AEs were GI Disorders LB1148: 4.7% Placebo: 3.2% No Drug-Related Serious Adverse Events Safety Improvement in Return of Bowel Function: GI2 (Days) Return of Bowel Function (%) Versus Placebo Study Design Phase 1 Safety Study: GI Surgery1 Phase 2 Study: GI Surgery2 Phase 2 Study: Cardiovascular (CV) Surgery3 Number of Subjects Study# LBS-IST-POI-101 Study# LBS-POI-201-CN Study# LBS-IST-CVS-201
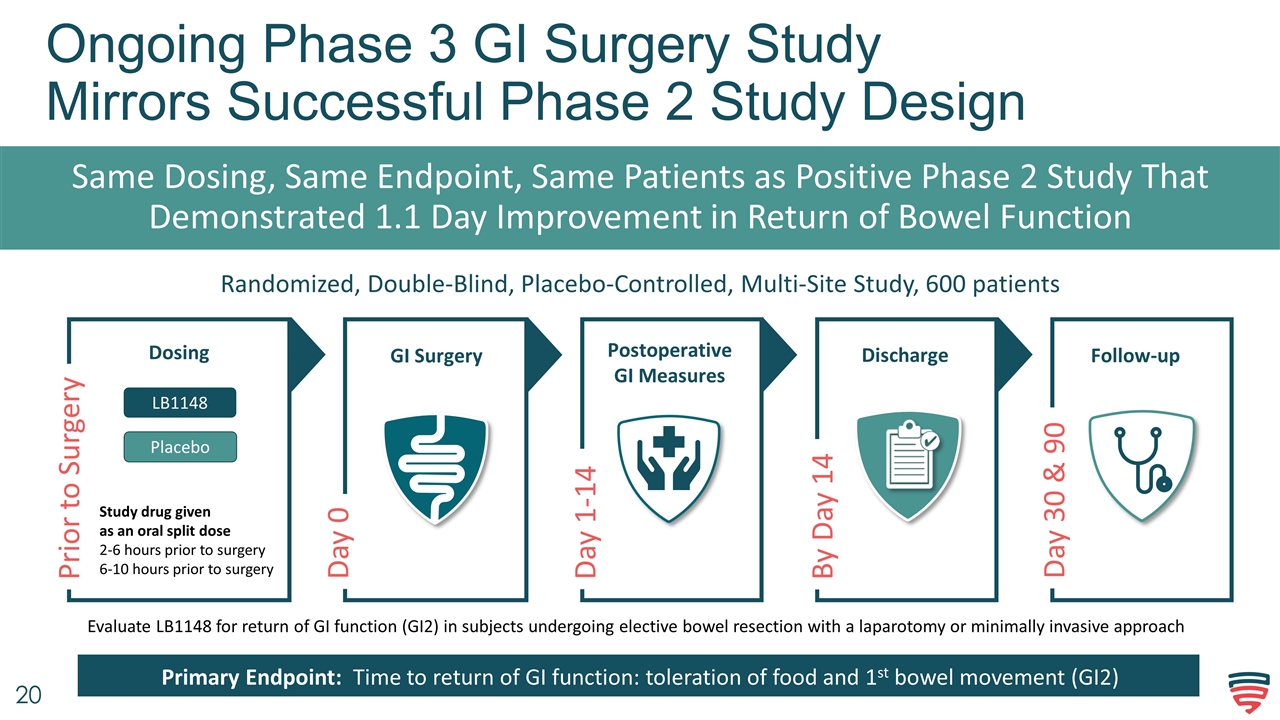
Day 0 Ongoing Phase 3 GI Surgery Study Mirrors Successful Phase 2 Study Design Same Dosing, Same Endpoint, Same Patients as Positive Phase 2 Study That Demonstrated 1.1 Day Improvement in Return of Bowel Function GI Surgery Discharge Follow-up Randomized, Double-Blind, Placebo-Controlled, Multi-Site Study, 600 patients Dosing Postoperative GI Measures Prior to Surgery Day 1-14 By Day 14 Day 30 & 90 Evaluate LB1148 for return of GI function (GI2) in subjects undergoing elective bowel resection with a laparotomy or minimally invasive approach Primary Endpoint: Time to return of GI function: toleration of food and 1st bowel movement (GI2) LB1148 Study drug given as an oral split dose 2-6 hours prior to surgery 6-10 hours prior to surgery Placebo

Advancing Second Program Towards Potential FDA Approval LB1148: Preventing Post-Surgical Adhesions
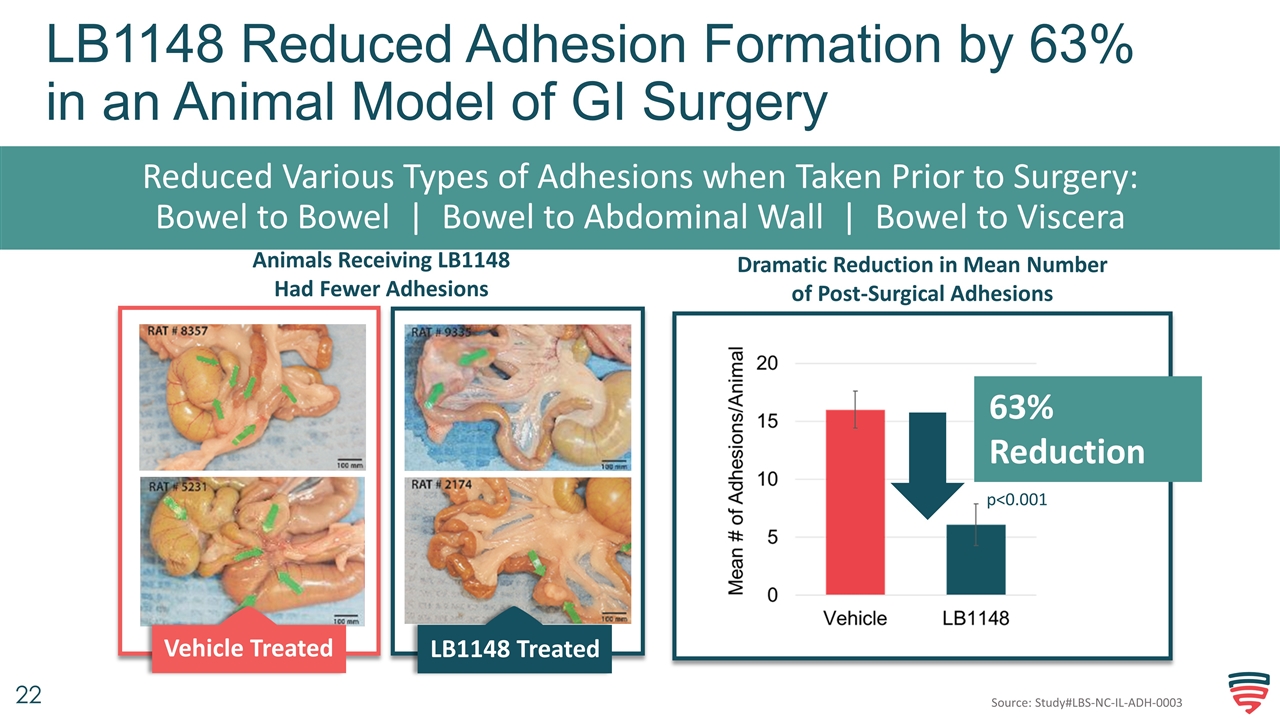
LB1148 Reduced Adhesion Formation by 63% in an Animal Model of GI Surgery Reduced Various Types of Adhesions when Taken Prior to Surgery: Bowel to Bowel | Bowel to Abdominal Wall | Bowel to Viscera Animals Receiving LB1148 Had Fewer Adhesions Vehicle Treated LB1148 Treated p<0.001 63% Reduction Dramatic Reduction in Mean Number of Post-Surgical Adhesions 63% Reduction Source: Study#LBS-NC-IL-ADH-0003
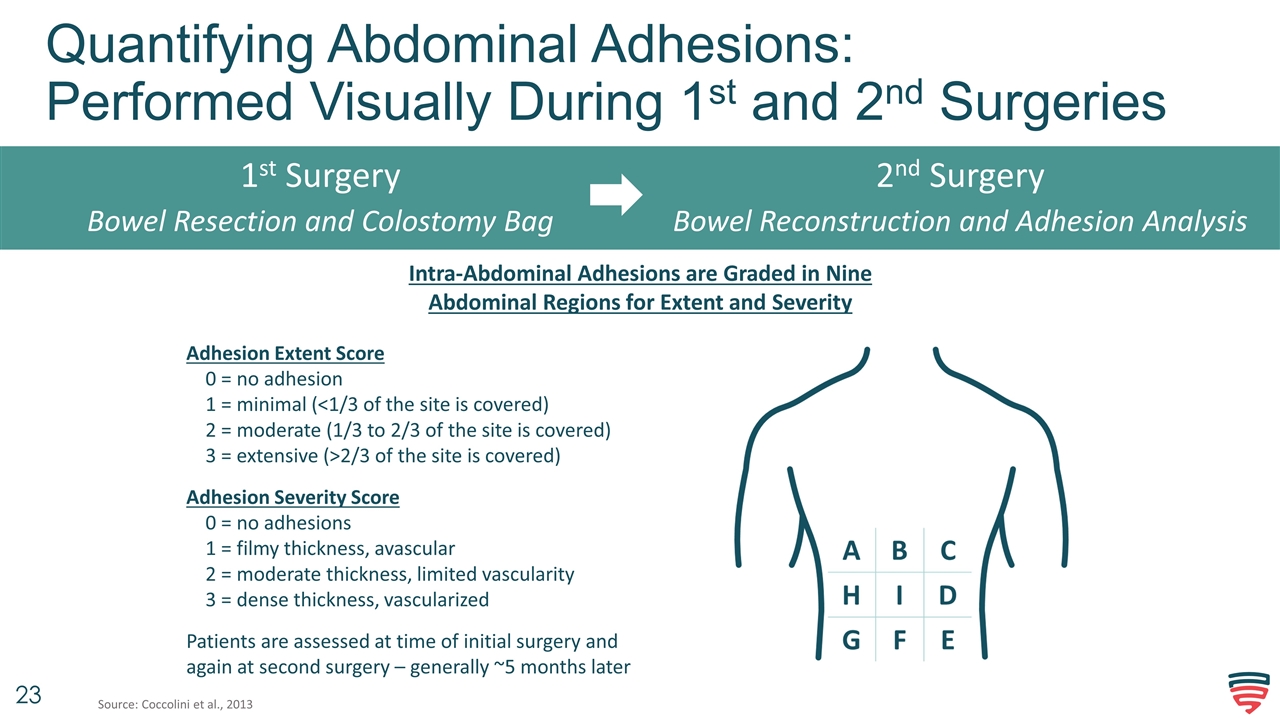
Quantifying Abdominal Adhesions: Performed Visually During 1st and 2nd Surgeries 1st Surgery Bowel Resection and Colostomy Bag Adhesion Extent Score 0 = no adhesion 1 = minimal (<1/3 of the site is covered) 2 = moderate (1/3 to 2/3 of the site is covered) 3 = extensive (>2/3 of the site is covered) Adhesion Severity Score 0 = no adhesions 1 = filmy thickness, avascular 2 = moderate thickness, limited vascularity 3 = dense thickness, vascularized Patients are assessed at time of initial surgery and again at second surgery – generally ~5 months later Source: Coccolini et al., 2013 2nd Surgery Bowel Reconstruction and Adhesion Analysis Intra-Abdominal Adhesions are Graded in Nine Abdominal Regions for Extent and Severity
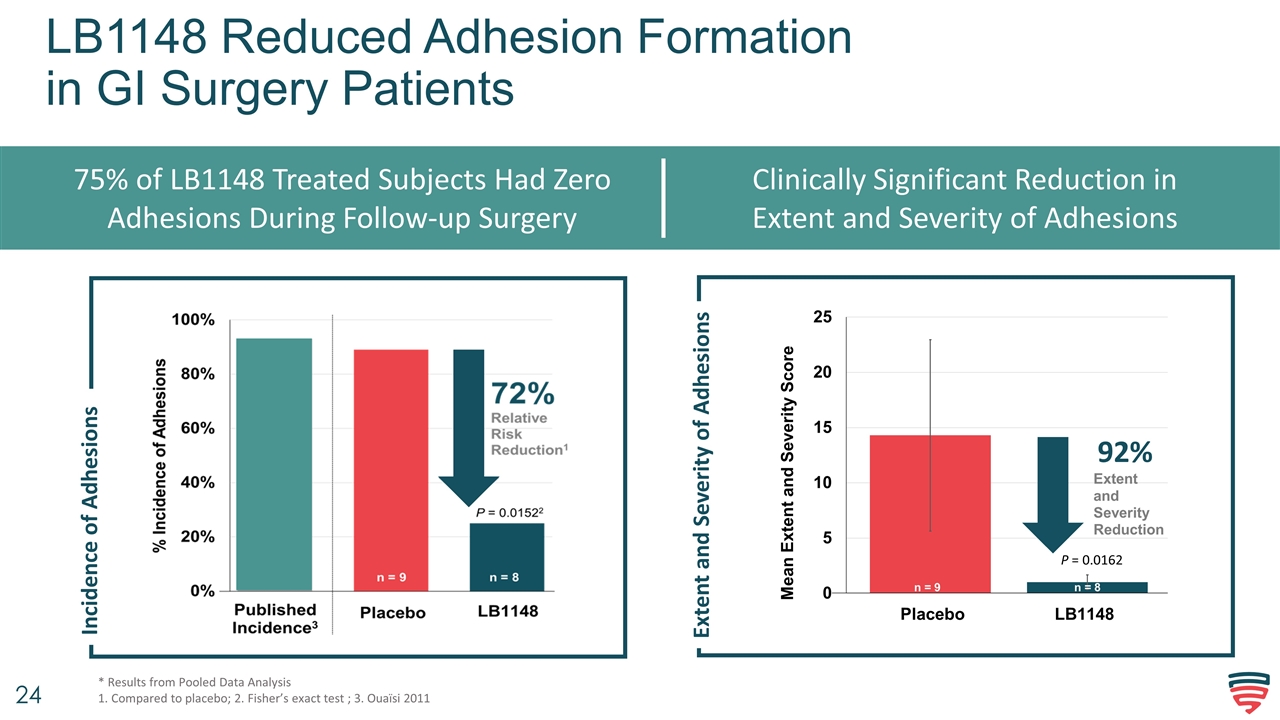
LB1148 Reduced Adhesion Formation in GI Surgery Patients 75% of LB1148 Treated Subjects Had Zero Adhesions During Follow-up Surgery Clinically Significant Reduction in Extent and Severity of Adhesions * Results from Pooled Data Analysis 1. Compared to placebo; 2. Fisher’s exact test ; 3. Ouaïsi 2011 Incidence of Adhesions Extent and Severity of Adhesions P = 0.0162 92%
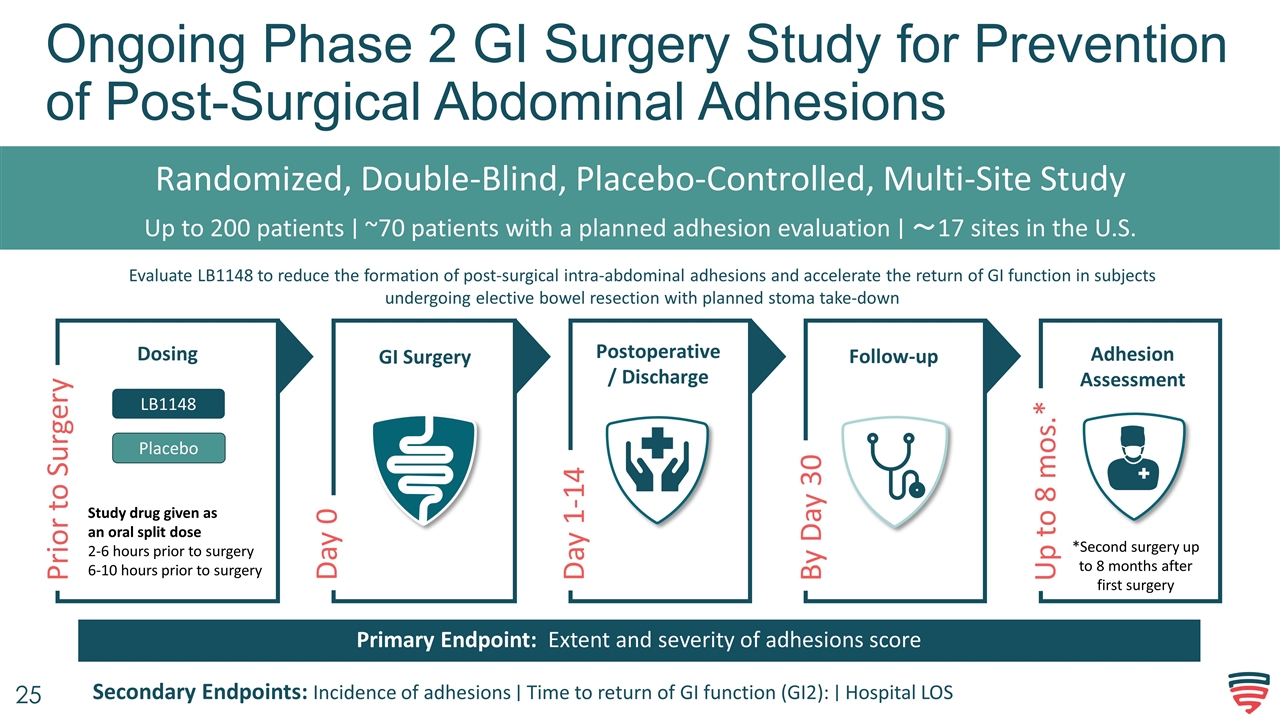
Ongoing Phase 2 GI Surgery Study for Prevention of Post-Surgical Abdominal Adhesions Randomized, Double-Blind, Placebo-Controlled, Multi-Site Study Up to 200 patients ∣ ~70 patients with a planned adhesion evaluation ∣ ~17 sites in the U.S. Evaluate LB1148 to reduce the formation of post-surgical intra-abdominal adhesions and accelerate the return of GI function in subjects undergoing elective bowel resection with planned stoma take-down Primary Endpoint: Extent and severity of adhesions score Secondary Endpoints: Incidence of adhesions ∣ Time to return of GI function (GI2): ∣ Hospital LOS Day 0 GI Surgery Follow-up Dosing Postoperative/ Discharge Prior to Surgery Day 1-14 By Day 30 LB1148 Study drug given as an oral split dose 2-6 hours prior to surgery 6-10 hours prior to surgery Placebo Adhesion Assessment *Second surgery up to 8 months after first surgery Up to 8 mos.*

LB1148: Commercial Opportunity Potential to Reduce Costs for Hospitals and Improve Outcomes
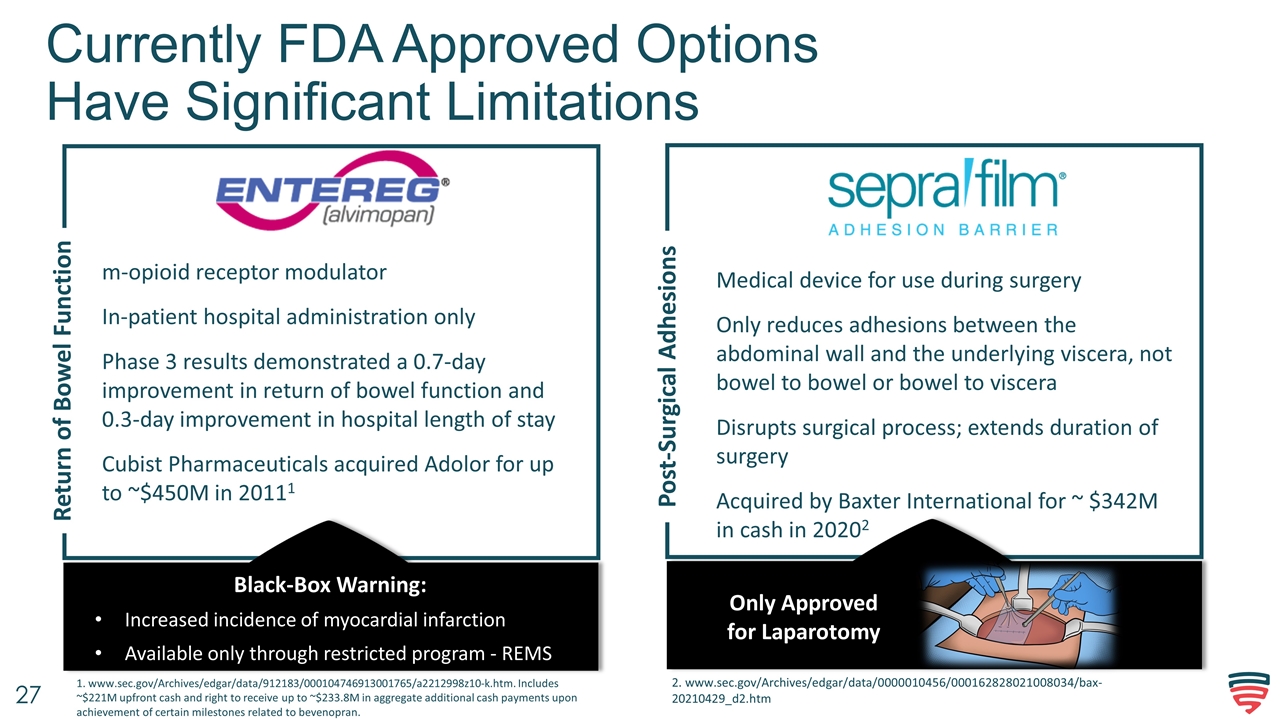
Currently FDA Approved Options Have Significant Limitations Return of Bowel Function Post-Surgical Adhesions m-opioid receptor modulator In-patient hospital administration only Phase 3 results demonstrated a 0.7-day improvement in return of bowel function and 0.3-day improvement in hospital length of stay Cubist Pharmaceuticals acquired Adolor for up to ~$450M in 20111 Medical device for use during surgery Only reduces adhesions between the abdominal wall and the underlying viscera, not bowel to bowel or bowel to viscera Disrupts surgical process; extends duration of surgery Acquired by Baxter International for ~ $342M in cash in 20202 Black-Box Warning: Increased incidence of myocardial infarction Available only through restricted program - REMS Only Approved for Laparotomy 2. www.sec.gov/Archives/edgar/data/0000010456/000162828021008034/bax-20210429_d2.htm 1. www.sec.gov/Archives/edgar/data/912183/000104746913001765/a2212998z10-k.htm. Includes ~$221M upfront cash and right to receive up to ~$233.8M in aggregate additional cash payments upon achievement of certain milestones related to bevenopran.
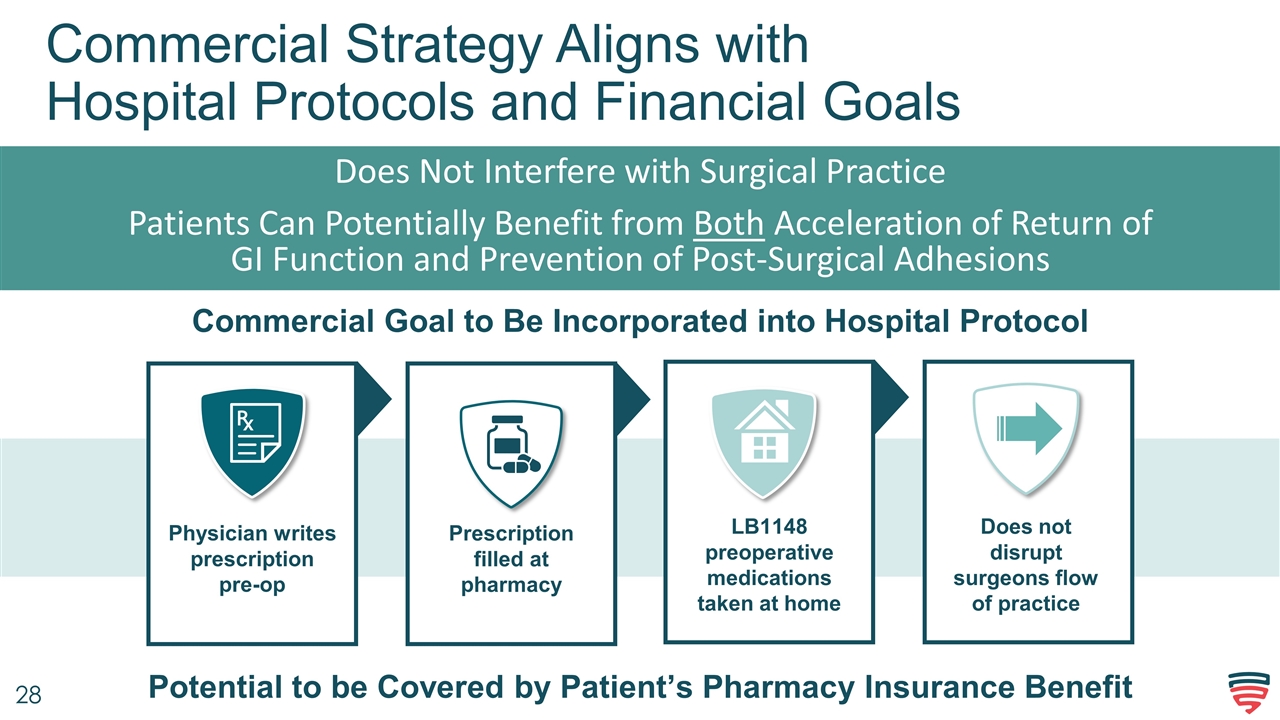
Commercial Strategy Aligns with Hospital Protocols and Financial Goals Does Not Interfere with Surgical Practice Patients Can Potentially Benefit from Both Acceleration of Return of GI Function and Prevention of Post-Surgical Adhesions Commercial Goal to Be Incorporated into Hospital Protocol Potential to be Covered by Patient’s Pharmacy Insurance Benefit Physician writes prescription pre-op Prescription filled at pharmacy LB1148 preoperative medications taken at home Does not disrupt surgeons flow of practice
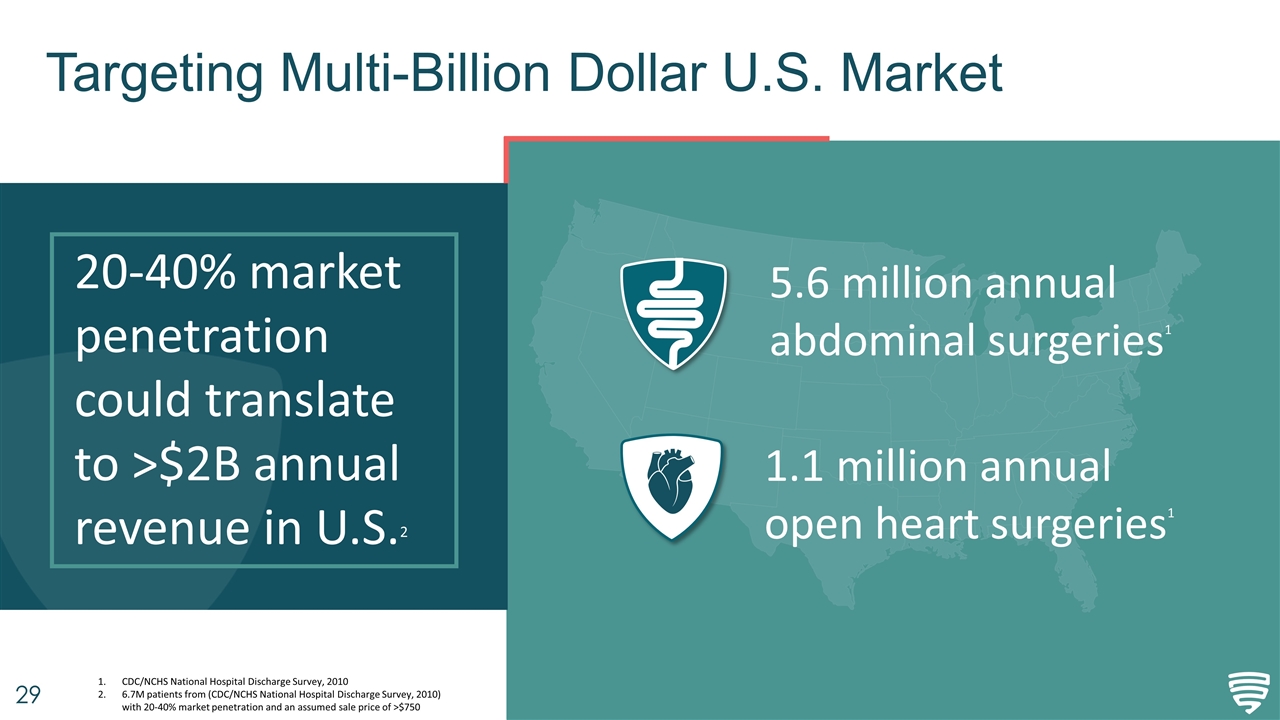
Targeting Multi-Billion Dollar U.S. Market 20-40% market penetration could translate to >$2B annual revenue in U.S.2 5.6 million annual abdominal surgeries1 1.1 million annual open heart surgeries1 CDC/NCHS National Hospital Discharge Survey, 2010 6.7M patients from (CDC/NCHS National Hospital Discharge Survey, 2010) with 20-40% market penetration and an assumed sale price of >$750

Corporate Overview
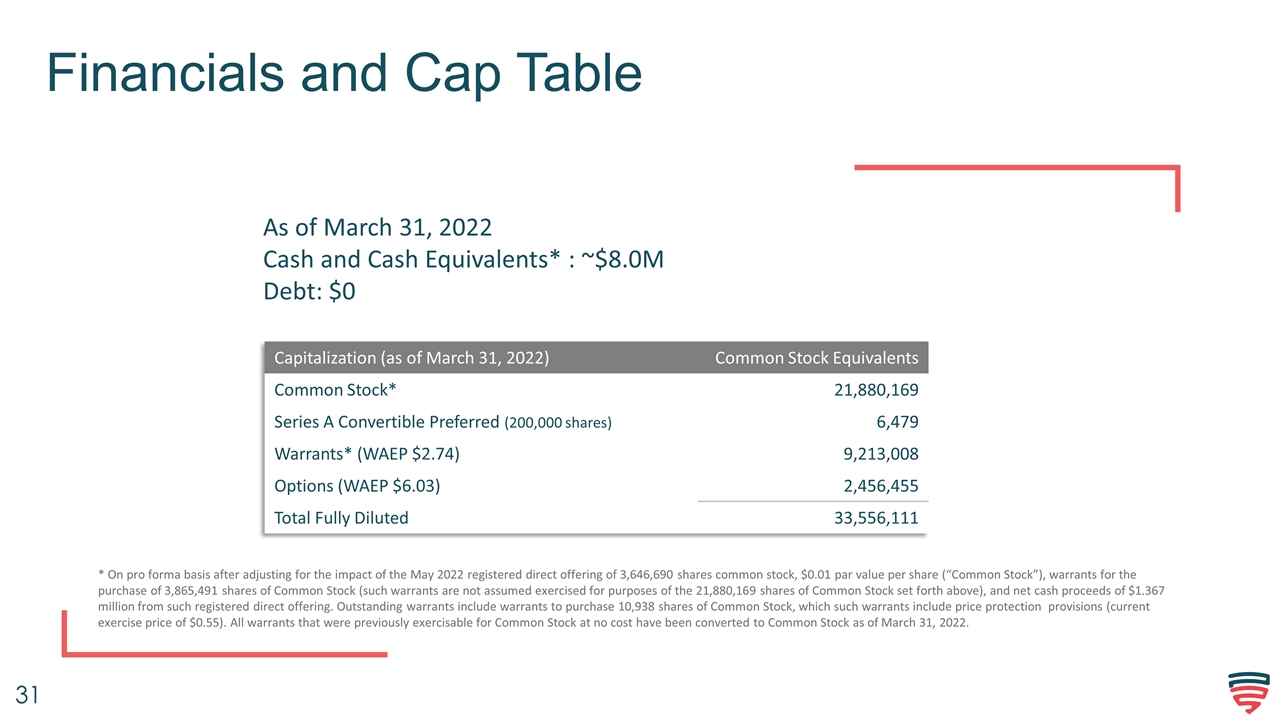
Financials and Cap Table Capitalization (as of March 31, 2022) Common Stock Equivalents Common Stock* 21,880,169 Series A Convertible Preferred (200,000 shares) 6,479 Warrants* (WAEP $2.74) 9,213,008 Options (WAEP $6.03) 2,456,455 Total Fully Diluted 33,556,111 As of March 31, 2022 Cash and Cash Equivalents* : ~$8.0M Debt: $0 * On pro forma basis after adjusting for the impact of the May 2022 registered direct offering of 3,646,690 shares common stock, $0.01 par value per share (“Common Stock”), warrants for the purchase of 3,865,491 shares of Common Stock (such warrants are not assumed exercised for purposes of the 21,880,169 shares of Common Stock set forth above), and net cash proceeds of $1.367 million from such registered direct offering. Outstanding warrants include warrants to purchase 10,938 shares of Common Stock, which such warrants include price protection provisions (current exercise price of $0.55). All warrants that were previously exercisable for Common Stock at no cost have been converted to Common Stock as of March 31, 2022.
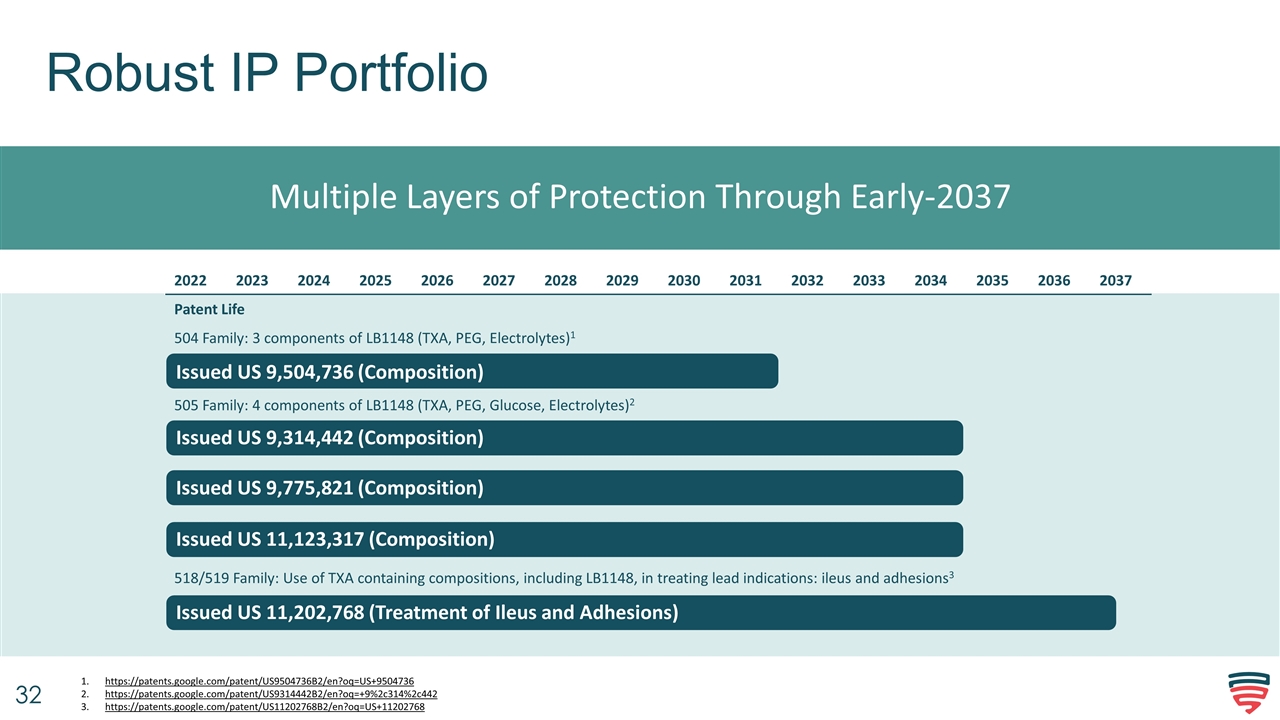
Robust IP Portfolio Multiple Layers of Protection Through Early-2037 Issued US 9,314,442 (Composition) Issued US 9,775,821 (Composition) Issued US 11,123,317 (Composition) Issued US 9,504,736 (Composition) Issued US 11,202,768 (Treatment of Ileus and Adhesions) https://patents.google.com/patent/US9504736B2/en?oq=US+9504736 https://patents.google.com/patent/US9314442B2/en?oq=+9%2c314%2c442 https://patents.google.com/patent/US11202768B2/en?oq=US+11202768 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 Patent Life 504 Family: 3 components of LB1148 (TXA, PEG, Electrolytes)1 505 Family: 4 components of LB1148 (TXA, PEG, Glucose, Electrolytes)2 518/519 Family: Use of TXA containing compositions, including LB1148, in treating lead indications: ileus and adhesions3

We Are Working to Establish New Standards of Care Rapidly Advancing Lead Asset in Global Phase 3 Program for Postoperative Return of Bowel Function Commercial Strategy Aligns with Hospitals Financial Goals US reimbursement system incentivizes hospitals to accelerate patient discharge and reduce readmission rates Targeting Multi-Billion Dollar Market Opportunities 6.7 million addressable surgeries in the US Annually1 Clinically Meaningful Results in Multiple Clinical Studies Improved time to return of bowel GI function, reduction in abdominal adhesions and was well-tolerated with positive benefit to risk ratio has the Potential to Establish a New Standard of Care LB1148 CDC/NCHS National Hospital Discharge Survey, 2010.


































