Filed Pursuant to Rule 424(b)(5)
Registration No. 333-263705
PROSPECTUS SUPPLEMENT
(To Prospectus dated April 26, 2022)

756,317 Shares of Common Stock
We are offering 756,317 shares (“Shares”) of our common stock, par value $0.01 per share (“common stock”) pursuant to this prospectus supplement and accompanying prospectus (“Public Offering”). The Shares are being sold at a public offering price of $2.64.
In a concurrent private placement (the “Private Placement”), we are also selling an aggregate of: (i) 455,242 shares of common stock (each an “Unregistered Share” and collectively the “Unregistered Shares”) and (ii) 1,061,164 Prefunded Warrants (each a “Prefunded Warrant” and collectively the “Prefunded Warrants”) to purchase 1,061,164 shares of common stock. The Unregistered Shares and Prefunded Warrants are being sold at a price of $2.64 and $2.6399, respectively. Please see the section of this Prospectus Supplement entitled “Private Placement Transaction” for a further description of the Private Placement.
Additionally, pursuant to the Private Placement, we are issuing one (1) common stock purchase warrant (“Warrant(s)”) for each Share, Unregistered Share or Prefunded Warrant purchased in the Public Offering and Private Placement, as applicable, or an aggregate of 2,272,723 Warrants. The Warrants have a term of five (5) years and an exercise price of $2.64 per share. The Unregistered Shares, Prefunded Warrants, and Warrants are being offered pursuant to the exemption provided in Section 4(a)(2) under the Securities Act of 1933, as amended, or the Securities Act, and Rule 506(b) promulgated thereunder and are not being registered under the Securities Act at this time or offered pursuant to this prospectus supplement and the accompanying prospectus.
Our common stock is traded on The Nasdaq Capital Market under the symbol “PALI.” On April 4, 2023, the last reported closing sale price of our common stock on The Nasdaq Capital Market was $203 share.
As of the date of this prospectus supplement, the aggregate market value of our outstanding shares of common stock held by non-affiliates was $15,854,125, based on 4,543,977 outstanding shares of common stock, of which 4,504,013 shares were held by non-affiliates, and a price of $3.52 per share, which was the last reported sale price of our common stock on The Nasdaq Capital Market on March 31, 2023. As of the date of this prospectus supplement, we have sold $3,288,029 of securities pursuant to General Instruction I.B.6. of Form S-3 during the prior 12-month calendar period that ends on, and includes, the date of this prospectus (but excluding this offering). In no event will we sell securities pursuant to such registration statement in a public primary offering with value exceeding more than one-third of our public float in any 12-month calendar period so long as our public float remains below $75.0 million and General Instruction I.B.6 of Form S-3 continues to apply to us.
This investment involves a high degree of risk. See “Risk Factors” on page S-8 of this prospectus supplement and any similar section contained in the accompanying prospectus and in the documents that are incorporated by reference herein and therein.
We have engaged Ladenburg Thalmann & Co. Inc., or the placement agent, as our exclusive placement agent in connection with this offering. The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay the placement agent the placement agent fees set forth in the table below. See “Plan of Distribution” beginning on page S-13 of this prospectus supplement for more information regarding these arrangements.
| | | Per Share | | | Total | |
| Offering price (Per share) | | $ | 2.64 | | | $ | 1,996,676.88 | |
| Placement agent’s fees(1) | | $ | 0.2046 | | | $ | 154,742.46 | |
| Proceeds, before expenses, to us(2) | | $ | 2.4354 | | | $ | 1,841,934.42 | |
| (1) | In addition, we have agreed to pay the placement agent a 7.75% commission on the proceeds from our concurrent private placement and for certain of its expenses and to issue to the placement agent (or its designees) warrants to purchase shares of common stock equal to 6.0% of the aggregate number of Shares, Unregistered Shares, and Prefunded Warrants issued in this offering. See “Plan of Distribution” beginning on page S-13 of this prospectus supplement for more information on placement agent compensation. |
| (2) | The amount of the offering proceeds to us presented in this table does not take into account the proceeds from the Private Placement or the exercise of any of the Prefunded Warrants or Warrants or any of the placement agent warrants. |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Delivery of the shares of common stock is expected to be made on or about April 5, 2023, subject to the satisfaction of certain closing conditions.
Ladenburg Thalmann
The date of this prospectus supplement is April 3, 2023.
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is the prospectus supplement, including the documents incorporated by reference, which describes the specific terms of this offering. The second part, the accompanying prospectus, including the documents incorporated by reference, provides more general information. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. Before you invest, you should carefully read this prospectus supplement, the accompanying prospectus, all information incorporated by reference herein and therein, as well as the additional information described under “Where You Can Find More Information” of page S-15 and “Incorporation of Certain Information by Reference” on page S-15 of this prospectus supplement. These documents contain information you should consider when making your investment decision. This prospectus supplement may add, update or change information contained in the accompanying prospectus. To the extent that any statement that we make in this prospectus supplement is inconsistent with statements made in the accompanying prospectus or any documents incorporated by reference, the statements made in this prospectus supplement will be deemed to modify or supersede those made in the accompanying prospectus and such documents incorporated by reference.
You should rely only on the information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus and in any free writing prospectuses we authorize for use in connection with this offering. Neither we nor the placement agent have authorized any other person to provide you with any information that is different. If anyone provides you with different or inconsistent information, you should not rely on it. We are offering to sell, and seeking offers to buy, the securities offered hereby only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the offering of the securities offered hereby in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement must inform themselves about, and observe any restrictions relating to, the offering of the securities offered hereby and the distribution of this prospectus supplement outside the United States. This prospectus supplement does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
The information appearing in this prospectus supplement, the accompanying prospectus or any related free writing prospectus that we authorize for use in connection with this offering is accurate only as of the date on the front of the document and any information we have incorporated by reference is accurate only as of the date of such document incorporated by reference, regardless of the time of delivery of this prospectus supplement or any related free writing prospectus, or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
This prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein contain summaries of certain provisions contained in some of the documents described herein and therein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus supplement and the accompanying prospectus are a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
Except as otherwise indicated or unless the context otherwise requires, references to “Company,” “we,” “us,” “our,” “Palisade Bio,” or “Palisade,” refer to Palisade Bio, Inc. and its subsidiaries.
Our name “Palisade Bio,” the Palisade logo and other trademarks or service marks of Palisade Bio, Inc. appearing in this prospectus supplement, the accompanying prospectus and any related free writing prospectus and the information incorporated by reference herein or therein are the property of Palisade Bio, Inc. Other trademarks, service marks or trade names appearing in this prospectus supplement, the accompanying prospectus and any related free writing prospectus and the information incorporated by reference herein or therein are the property of their respective owners. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, these other companies.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information about us, this offering and the information appearing elsewhere in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus supplement, the accompanying prospectus and any related free writing prospectus that we authorize for use in connection with this offering, including the risks of investing in our securities discussed under the heading “Risk Factors” beginning on page S-8 of this prospectus supplement and under similar headings in the documents incorporated by reference. You should also carefully read the information incorporated by reference into this prospectus supplement and the accompanying prospectus, including our financial statements and related notes, and the exhibits to the registration statement of which this prospectus supplement and the accompanying prospectus are a part, before making your investment decision.
Overview
We are a biopharmaceutical company focused on developing therapeutics that protect the integrity of the intestinal barrier. We utilize over three decades of research and established science that links the role of intestinal barrier biology and human disease to develop novel therapeutics that target and improve the integrity of the intestinal barrier.
Our approach is founded on the discovery that damage to the intestinal epithelial barrier can result in the leakage of digestive enzymes from the gastrointestinal (“GI”) tract into the peritoneal cavity that can damage tissues and promote inflammation, causing a broad array of acute and chronic conditions. Our goal is to be an industry leader in developing therapies to prevent or treat conditions resulting from intestinal barrier dysfunction and to improve the lives of patients suffering from such conditions.
Our lead therapeutic candidate, LB1148, is a novel oral liquid formulation of the well-characterized digestive enzyme inhibitor tranexamic acid (“TXA”) that is currently being developed for administration prior to surgeries that are at risk of disrupting the intestinal epithelial barrier. By inhibiting the activity of digestive proteases, we believe that LB1148 has the potential to reduce the formation of postoperative adhesions between intra-abdominal tissues and accelerate the time to the return of normal GI function.
We believe that LB1148, if successfully developed and approved, may have the ability to become a suitable treatment option across a broad range of acute and chronic conditions associated with GI barrier dysfunction. Our strategy is to maintain a capital efficient organization focused on pursuing the approval of LB1148 for the reduction of postoperative adhesions following major surgeries. As part of our strategy, we are exploring possible indication expansion, partnering, and out-licensing opportunities and, if advantageous opportunities arise, in-licensing and partnering of other product candidates.
Postoperative Adhesions
Intra-abdominal adhesions are bands of scar tissue that form inside the abdomen. The fibrous bands form between two or more organs and/or surfaces that are not normally connected, causing the surfaces to become bound together. Intra-abdominal adhesions can lead to kinking, twisting, pulling (traction), or compression of the intestines and other organs in the abdomen, causing symptoms and complications, such as pain, bloating, intestinal obstruction or blockage.
Abdominal adhesions are common and often develop after open or laparoscopic abdominal surgery. In surgery with an open approach, the surgeon makes a large incision to open the abdominal cavity, whereas in laparoscopic surgery, the surgeon makes small openings in the abdomen and inserts special tools to view, remove, or repair organs and tissues. Adhesions may arise during these abdominal surgeries by a variety of mechanisms. We believe that injuries resulting from incisions, sutures, surgical manipulation, bleeding, and hypoperfusion can lead to leakage of digestive proteases. Digestive enzymes that escape from the intestine may create proteolytic damage to mesothelial surfaces. The body’s response is to generate scar tissue to heal such damage. As the new scar tissue grows it can connect these surfaces with adhesions. It is estimated that postoperative intra-abdominal adhesions may develop in up to 93% of patients undergoing abdominal or pelvic surgery.
Although many patients with intra-abdominal adhesions are asymptomatic, a significant portion of patients will develop “adhesive disease,” a symptomatic state inclusive of chronic, highly distressing, and even life-threatening symptoms. Approximately 6% to 10% of these cases require follow-up medical care. Abdominal adhesions are the most common cause of obstruction of the small intestine and can lead to the death of intestinal tissues, peritonitis (an infection of the lining of the abdominal cavity) and, in severe cases, death. In fact, although adhesion related bowel obstruction is the number ten cause of emergent surgery, intestinal obstruction from adhesions is one of the top causes of emergency surgery death in the United States. In women, abdominal adhesions in the abdomen and pelvis can compress, deform, or block parts of the reproductive system and lead to infertility.
Data from preclinical and clinical studies suggest that LB1148 administration may prevent postoperative adhesions in surgical patients. Postoperative adhesions are (i) costly for patients and hospitals; (ii) the number one cause of secondary infertility in women; (iii) the most common cause of bowel obstruction, accounting for up to 75% of cases; and (iv) the tenth most frequent cause of emergency surgeries. They also account for approximately 80% of emergency surgery deaths and more than 400,000 adhesion lysis surgeries annually in the United States.
By preventing or minimizing adhesions in abdominal and pelvic surgery patients, we believe that LB1148 may minimize numerous medical complications and reduce the need for additional surgeries or other treatments, benefiting both patients and providers.
Postoperative Ileus and Return of Bowel Function in Adults
Patients undergoing GI or cardiovascular (“CV”) surgery often experience some degree of GI dysfunction, or delayed return of GI function, manifested by a transient cessation of bowel motility, termed postoperative ileus (“POI”). Bowel function typically returns three to five days after abdominal surgery. However, about 8.5% of abdominal surgery patients experience severe POI that delays the return of bowel function by six or more days. Some procedures result in ileus incident rates of over 20%.
Prolonged POI is a serious complication of GI or CV surgery, resulting in increased morbidity, longer hospital stays, and higher costs. Patients experience bloating and major abdominal pain and, with extended lengths of stay in the hospital, may be at increased risk of hospital acquired infections. The mechanism of POI is likely multifactorial, involving digestive proteases, the nervous system (specifically the autonomic and enteric nervous systems), inflammation (mast cell inflammatory process), hormones, neuropeptides, anesthesia, and when used, narcotics.
There are key criteria for patients to meet prior to discharge following major surgery, which may include return of bowel function, infection source control and pain management. Antibiotics and analgesics can greatly help achieve two of these criteria, yet there is still an unmet need for therapeutics to help improve return of GI function.
Preliminary data from preclinical and clinical studies seem to indicate that LB1148 may protect the mucosal barrier and neutralizes digestive enzyme leakage, and promote return of bowel function after surgery.
By potentially accelerating return of bowel function and thereby reducing length of stay in surgical patients, we believe LB1148 may be able to improve patient outcomes, decrease health care costs, and increase operating margins for providers. Furthermore, we believe that these benefits may extend to patients undergoing GI/abdominal and CV surgery, expediting bowel recovery and return to normal feedings to improve long-term outcomes.
LB1148 has been granted Fast Track designation from the FDA for the treatment of postoperative GI dysfunction (which may present as feeding intolerance, ileus, necrotizing enterocolitis (“NEC”), etc.) associated with gut hypoperfusion injury in pediatric patients who have undergone congenital heart disease repair surgery.
Our Lead Product Candidate, LB1148
Our lead therapeutic candidate, LB1148, is a novel oral liquid formulation of the well-characterized digestive enzyme inhibitor, TXA, intended to inhibit digestive enzyme activity and preserve gut integrity during intestinal stress resulting from, among other things, reduced blood flow to the intestine, infections, or due to surgery. Peer reviewed publications of third-party research suggest that digestive enzyme leakage from the GI tract increases the incidence of GI and organ dysfunction following these events.
LB1148 is formulated as an aqueous solution for oral (enteral) administration. In addition to TXA, the patented LB1148 formulation contains polyethylene glycol, carbohydrates, and electrolytes. The components of LB1148 are provided as dry powders for reconstitution in water prior to administration. Such reconstitution may be carried out in a pharmacy (by a pharmacist), or in an outpatient setting (by a patient).
The potential of LB1148 relies on its formulation as a liquid composition for oral administration, which is designed to stop the downstream effects of a disruption of the intestinal mucosal barrier. We are not aware of any other approved oral TXA-containing liquid compositions in the marketplace suitable for such administration.
Prevention of Postoperative Abdominal Adhesions: GI Surgery
Adhesion prevalence is reported to be >90% in patients who have undergone abdominal surgery and represents a significant contributory factor to serious complications such as small bowel obstruction, infertility, chronic abdominal pain, subsequent surgery, and other morbidities. On March 16, 2022 we announced data from a pooled-analysis of studies LBS-IST-POI-101 and LBS-POI-201-CN (PROFILE-CN) at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) 2022 Annual Meeting. The results from the pooled analysis showed that 8/9 (89%) of subjects in the placebo group versus 2/8 (25%) in the LB1148 group had adhesions observed during a second follow-up surgery, representing a relative risk reduction of 72% (p = 0.0152). The mean total adhesion score which measures both the extent and severity of adhesions was 1.0 (8/8) for LB1148 and 14.3 (129/9) for placebo, representing relative risk reduction of 93% (p = 0.0162). We believe the reduction in the incidence of postoperative intra-abdominal adhesions as well as the reduction in the extent and severity of adhesions provides preliminary evidence of the clinically meaningful efficacy of LB1148 to reduce postoperative adhesions when compared to placebo.
In December 2022, we concluded enrollment of a randomized, double-blind, placebo-controlled, Phase 2 clinical trial of LB1148 in patients undergoing elective bowel resection surgery in the United States to evaluate if patients treated with LB1148 experience fewer postoperative intra-abdominal adhesions compared to placebo treated patients. We have enrolled a total of 35 of the planned 70 patients in this Phase 2 study. Of the patients enrolled, as of March 2, 2023, 31 patients had completed a first surgery, and 19 patients had completed a second surgery, which is primary assessment endpoint for data under the current study protocol. The Company believes that the data collected to date is sufficient for its evaluation purposes, including an evaluation of its risk profile, and for such reason, the Company voluntarily ceased enrollment in the trial. The Company expects to report topline data from the 35 patients in the second quarter of 2023.
The Company is currently planning a dose optimization study for all indications to determine if a different dosing protocol in healthy volunteers would enhance the risk profile of LB 1148 while simultaneously providing efficacy. It is anticipated that this study will generate pharmacokinetic and pharmacodynamic data across multiple doses in patients, with enrollment expected to commence in the second quarter of 2023.
Postoperative Return of Bowel Function: GI Surgery
On July 29, 2021, we and our co-development partner Newsoara announced topline data from a Phase 2 clinical trial (LBS-POI-201-CN (PROFILE-CN)) demonstrating that LB1148 had a statistically significant (p=0.001) effect in accelerating the return of bowel function in patients undergoing elective bowel resection surgery.
Results from the trial include:
| ● | A 1.1-day improvement in GI recovery in patients receiving LB1148 vs placebo. The median time to return of bowel function was 2.77 days in patients treated with LB1148 and 3.83 days in those receiving placebo (hazard ratio = 1.886; p = 0.0008). |
| ● | The difference between groups increased at the 3rd quartile (75th percentile), with LB1148 (3.4 days) demonstrating a 1.5-day faster recovery of bowel function compared to placebo (4.9 days). |
| ● | LB1148 was well tolerated with 10.9% and 4.8% of patients in the LB1148 group and placebo group, respectively, experiencing a drug-related adverse event. |
| ● | The most common drug-related adverse events were GI disorders (LB1148 4.7% vs. placebo 3.2%). |
| ● | No drug-related serious adverse events occurred in the trial. |
In May 2022, the Company’s co-development partner in China received clearance from the Center for Drug Evaluation (“CDE”) of the National Medical Products Administration (“NMPA”) of the People’s Republic of China to proceed with their Phase 3 clinical trial to evaluate LB1148 for accelerated return of bowel function in adult patients undergoing gastrointestinal surgery. In June 2022, based on data generated by this co-development partner in its earlier Phase 2 study, the Company initiated a Phase 3 clinical trial in the U.S. evaluating LB1148 to accelerate the return of bowel function in adult patients undergoing gastrointestinal surgery. LB1148 also received Fast Track designation from the FDA in November 2022 for the acceleration of time to return of bowel function, as defined as upper and lower GI recovery in adult patients undergoing abdominal surgery.
In late September of 2022, the Board, in connection with a special clinical subcommittee it appointed, initiated a review of the Company’s operations, including its ongoing clinical programs. As part of the review, the Company engaged the services of independent third-party clinical development experts to assist in the review. In October of 2022, the review identified that in 2020, a former member of the Company’s management received unblinded clinical data related to bowel function from a subset of patients in the Company’s ongoing U.S. Phase 2 study.
Upon discovery of this information, the special clinical subcommittee of the Board commenced a thorough review of the Company’s ongoing clinical programs. As a result of the review, the Company determined that the current U.S. Phase 3 study protocol required additional standardization across sites and further clarification in the definition of endpoints to permit an adequate assessment of the efficacy of LB1148 to recover GI function. The Company does not believe that the favorable safety and tolerability profiles of LB1148 were impacted by these findings.
Prior Regulatory History of Third-Party Products with TXA Active Ingredients
The active ingredient in LB1148, TXA, is a marketed drug that has been evaluated in human clinical trials and in tens of thousands of patients. Supporting these observations is also over 40 years of post-marketing data from approved TXA products. Studies and regulatory bodies have suggested that TXA administration, while accompanied by a potential increased risk of thrombosis and rare hypersensitivity, may be generally safe and well-tolerated. TXA is an over-the-counter medicine for treating heavy menses in multiple countries, including the United Kingdom, Canada, Japan, and Sweden.
Company Information
We were originally incorporated in 2001 in the State of Delaware under the name Neuralstem, Inc. In October 2019, we changed our name from Neuralstem, Inc. to Seneca Biopharma, Inc., or Seneca. In April 2021, we effected a merger transaction with Leading Biosciences, Inc., or LBS, whereby LBS became a wholly owned subsidiary of Seneca. In April 2021, we changed our name from Seneca Biopharma, Inc. to Palisade Bio, Inc. Our principal executive offices are located at 7750 El Camino Real #5200, Carlsbad, CA, 92009, our telephone number is (858) 704-4900 and our website address is www.palisadebio.com. The information contained in or accessible through our website does not constitute part of this prospectus supplement or the accompanying prospectus.
Subsidiaries
We conduct our operations through LBS, our wholly owned subsidiary.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our shares of common stock held by non-affiliates does not equal or exceed $250.0 million as of the prior June 30th, or (2) our annual revenues did not equal or exceed $100.0 million during such completed fiscal year and the market value of our shares of common stock held by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of any reduced disclosure obligations, it may make the comparison of our financial statements with other public companies difficult or impossible.
Reverse Stock Split
Effective at 5:00 p.m. Eastern Time on Tuesday November 15, 2022, Palisade effected a reverse stock split of its outstanding common stock (“Reverse Split”). As a result of the Reverse Split, each of the Company’s shareholders received one (1) new share of common stock for every fifty (50) shares such shareholder held immediately prior to the effective time of the Reverse Split. The Reverse Split affected all of the Company’s issued and outstanding shares of common stock equally. The Reverse Split also affected the Company’s outstanding stock options, warrants and other exercisable or convertible securities and resulted in the shares underlying such instruments being reduced and the exercise price being increased proportionately. No fractional shares were issued as a result of the Reverse Split. Any fractional shares that would have otherwise resulted from the Reverse Split was paid in cash, at an amount equal to the resulting fractional interest in one (1) share of the common stock to which the shareholder would otherwise be entitled, multiplied by the closing trading price of the Common Stock on November 15, 2022.
The Offering
| Securities Offered by Us | | 756,317 shares of common stock. |
| | | |
| Offering price per share | | $2.64 |
| | | |
| Shares of common stock to be outstanding immediately after this offering | | 5,300,294 (1) shares (excluding the Unregistered Shares issued in the Private Placement and assuming no exercise of the Prefunded Warrants or Warrants issued in the Private Placement). |
| | | |
Concurrent Private Placement of Unregistered Shares, Prefunded Warrants and Warrants to purchasers in this offering | | In the Private Placement, we are also selling an aggregate of: (i) 455,242 shares of common stock (each an “Unregistered Share” and collectively the “Unregistered Shares”) and (ii) 1,061,164 Prefunded Warrants (each a “Prefunded Warrant” and collectively the “Prefunded Warrants”) to purchase 1,061,164 shares of common stock. The Unregistered Shares and Prefunded Warrant are being sold at a price of $2.64 and $2.6399, respectively. Additionally, pursuant to the Private Placement, we are issuing one (1) common stock purchase warrant (“Warrant(s)”) for each Share, Unregistered Share or Prefunded Warrant (or 2,272,723 Warrants to purchase 2,272,723 shares of common stock) purchased in the Public Offering or Private Placement. The Warrants have a term of five (5) years and an exercise price of $2.64. The Unregistered Shares, Prefunded Warrants, and Warrants are being offered pursuant to the exemption provided in Section 4(a)(2) under the Securities Act of 1933, as amended, or the Securities Act, and Rule 506(b) promulgated thereunder and are not being registered under the Securities Act at this time or offered pursuant to this prospectus supplement and the accompanying prospectus. Pursuant to the securities purchase agreement, dated April 3, 2023, by and among us and the purchasers in the Private Placement, we have agreed to file within 30 days following the date of the transaction documents (April 3, 2023), a registration statement on Form S-1 providing for the resale by holders of (i) the Unregistered Shares and (ii) shares of our common stock issuable upon the exercise of the Prefunded Warrants and the Warrants, and we will be required to have such resale registration statement effective by the 60th day subsequent to the date of the transaction documents. Further, we agreed to use best efforts to keep such registration statement effective at all times until such date that the Unregistered Shares and shares underlying the Prefunded Warrants and Warrants either (i) have been sold, or (ii) may be sold without volume or manner-of-sale restrictions pursuant to Rule 144 and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144. |
| | | |
| Use of proceeds | | We estimate that our net proceeds from this offering (including the Private Placement) will be approximately $5.35 million after deducting placement agent fees and other estimated offering expenses payable by us. We intend to use the net proceeds from this offering for working capital and general corporate purposes. See the section titled “Use of Proceeds” on page S-10 in this prospectus supplement. |
| | | |
| Risk factors | | Investment in our securities involves a high degree of risk. You should read the section titled “Risk Factors” on page S-8 in this prospectus supplement, the accompanying prospectus and in the documents incorporated by reference herein and therein for a discussion of factors to consider before investing in our securities. |
| | | |
| Nasdaq Capital Market symbol | | “PALI” |
| (1) | The above discussion is based on 2,944,306 shares of common stock outstanding as of December 31, 2022, and adjusted for the following subsequent issuances: |
| ● | 513,842 shares of common stock issued in our January 2023 registered direct offering (“January 2023 Registered Offering”) (including the issuance of 37,000 shares pursuant to the exercise of a registered prefunded warrant thereunder); and |
| ● | 1,085,829 shares of common stock issued upon the exercise of outstanding warrants that have settled between December 31, 2022 through April 3, 2023. |
The above discussion excludes the following:
| | ● | 9,464 shares of common stock issuable upon exercise of outstanding stock options as of March 31, 2023 granted under the LBS 2013 Amended and Restated Employee, Director, and Consultant Equity Incentive Plan, as amended and restated, or the 2013 Plan, with a weighted-average exercise price of $1,059.26 per share; |
| | | |
| | ● | 44,380 shares of common stock issuable upon exercise of outstanding stock options as of March 31, 2023, granted under our 2021 Equity Incentive Plan, as amended, or the 2021 Plan, with a weighted-average exercise price of $37.71 per share; |
| | | |
| | ● | 8,426 shares of common stock issuable upon exercise of outstanding stock options as of March 31, 2023 granted under our 2021 Inducement Plan, with a weighted average exercise price of $51.08 per share; |
| | | |
| | ● | 42,153 shares of common stock issuable upon vesting of restricted stock units outstanding as of March 31, 2023, granted under our 2021 Plan. |
| | | |
| | ● | An aggregate of 209,700 shares of common stock underlying (i) 81,500 common stock purchase options, (ii) 59,500 restricted stock units, and (iii) 68,700 restricted performance stock units; all of which were issued under our 2021 Plan, and which were all made on a conditional basis, subject to the receipt of shareholder approval with respect to such grants. |
| | | |
| | ● | 67,955 shares of common stock reserved for future issuance under the 2021 Plan as of March 31, 2023, as well as any future automatic increases in the number of shares of common stock reserved for future issuance under the 2021 Plan; |
| | | |
| | ● | 34,602 shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan, or the ESPP, as of March 31, 2023, as well as any automatic increases in the number of shares of common stock reserved for future issuance under the ESPP; |
| | | |
| | ● | 6,440 shares of common stock reserved for issuance under our 2021 Inducement Plan as of March 31 2023; |
| | | |
| | ● | 1,624,422 shares of common stock issuable upon exercise of outstanding warrants as of March 31, 2023 with a weighted-average exercise price of $18.06 per share |
| | | |
| | ● | 129 shares of common stock issuable upon conversion of the 200,000 outstanding shares of our Series A 4.5% Convertible Preferred Stock as of March 31, 2023, as well as any future shares of common stock issuable upon conversion of additional shares of Series A 4.5% Convertible Preferred Stock that may be issued as payment-in-kind dividends thereon in accordance with their terms; and |
| | | |
| | ● | 455,252 Unregistered Shares sold in the concurrent Private Placement; and |
| | | |
| | ● | 3,333,887 shares of common stock issuable upon exercise of Prefunded Warrants and Warrants issued in the concurrent Private Placements; and |
| | | |
| | ● | 136,363 placement agent warrants issued in the concurrent Private Placement. |
| | | |
Unless otherwise indicated, all information in this prospectus supplement assumes no exercise of options or warrants and no conversion of the Series A 4.5% Convertible Preferred Stock described above and no exercise of the Warrants (or the Prefunded Warrants) issued to the purchasers in the concurrent Private Placement or the placement agent warrants to be issued as compensation to the placement agent for this offering.
RISK FACTORS
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks and uncertainties described below, together with the risks and uncertainties discussed under the heading “Risk Factors” contained in our Annual Report on Form 10-K for the year ended December 31, 2022 as well as our subsequent filings with the SEC, and as incorporated by reference herein, and as the same may be amended, supplemented or superseded by the risks and uncertainties described under similar headings in the other documents that are filed after the date hereof and incorporated by reference herein. The risks described in these documents are not the only ones we face, but those that we consider to be material. There may be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be seriously harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment. Please also read carefully the section herein titled “Special Note Regarding Forward-Looking Statements” on page S-8.
Risks Related to this Offering
Our management will have broad discretion in the use of the net proceeds from this offering and may invest or spend the proceeds in ways with which you do not agree and in ways that may not yield a return.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section titled “Use of Proceeds” on page S-10 and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary from their currently intended use. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in investment-grade, interest-bearing securities. These investments may not yield a favorable return to our securityholders.
A substantial number of shares of common stock may be sold in the market following this offering, which may depress the market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market following this offering could cause the market price of our common stock to decline. A substantial majority of the outstanding shares of our common stock are, and the shares of our common stock offered by this prospectus will be, freely tradable without restriction or further registration under the Securities Act.
Holders of the Prefunded Warrants and Warrants will have no rights as common stockholders until such holders exercise their Prefunded Warrants or Warrants.
Until holders of Prefunded Warrants or Warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our common stock underlying the Prefunded Warrants or Warrants, except to the extent that holders of the Prefunded Warrants or Warrants have certain rights to participate in distributions or dividends paid on our common stock as set forth in the Prefunded Warrants or Warrants, as applicable. Upon exercise of the Prefunded Warrants or Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the documents that we incorporate by reference herein and therein, contain “forward-looking statements” within the meaning of Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are not limited to, statements about:
| | ● | estimates about the size and growth potential of the markets for our product candidates, and our ability to serve those markets, including any potential revenue generated; |
| | ● | future regulatory, judicial, and legislative changes or developments in the United States (“U.S.”) and foreign countries and the impact of these changes; |
| | | |
| | ● | our ability to build a commercial infrastructure in the U.S. and other markets; |
| | | |
| | ● | our ability to compete effectively in a competitive industry; |
| | | |
| | ● | our ability to identify and qualify additional manufacturers to provide API and manufacture drug product; |
| | | |
| | ● | our ability to enter into commercial supply agreements; |
| | | |
| | ● | the success of competing technologies that are or may become available; |
| | | |
| | ● | our ability to attract and retain key scientific or management personnel; |
| | | |
| | ● | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
| | | |
| | ● | our ability to obtain funding for our operations; |
| | | |
| | ● | our ability to attract collaborators and strategic partnerships; and |
| | | |
| | ● | the impact of the COVID-19 pandemic on our business, and operations, and supply. |
| | | |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “intend,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and are subject to risks and uncertainties. As such, our actual results may differ significantly from those expressed in any forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
We discuss many of these risks in greater detail under the heading “Risk Factors” in this prospectus supplement and the accompanying prospectus, in the “Risk Factors,” “Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections incorporated by reference from our most recent Annual Report on Form 10-K and in our Quarterly Reports on Form 10-Q for the quarterly periods ended subsequent to our filing of such Annual Report on Form 10-K, as well as any amendments thereto reflected in subsequent filings with the U.S. Securities and Exchange Commission, or SEC.
The discussion of risks and uncertainties set forth in those filings is not necessarily a complete or exhaustive list of all risks facing us at any particular point in time. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus supplement and the accompanying prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. Forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable statement. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. You should read this prospectus supplement, the accompanying prospectus, and the documents that we have filed with the SEC that are incorporated by reference and any free writing prospectus we have authorized for use in connection with this offering, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
This prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein contain market data and industry statistics and forecasts that are based on independent industry publications and other publicly available information. Although we believe that these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. Although we are not aware of any misstatements regarding the market and industry data presented in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein, these estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” contained in this prospectus supplement, the accompanying prospectus and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus supplement and the accompanying prospectus. Accordingly, investors should not place undue reliance on this information.
USE OF PROCEEDS
We estimate that the net proceeds from this offering (including the Private Placement), after deducting placement agent fees and estimated offering expenses payable by us, will be approximately $5.35 million. We intend to use the net proceeds from this offering for working capital, and general corporate purposes, including the development of our lead therapeutic candidate, LB1148. The amounts and timing of our use of the net proceeds from this offering will depend on a number of factors, such as the timing and progress of our research and development efforts for LB1148, the timing and progress of any partnering and commercialization efforts, technological advances and the competitive environment for LB1148. Accordingly, our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments.
DESCRIPTION OF SECURITIES WE ARE OFFERING
Description of common stock
The material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common stock are described under the heading “Description of Capital Stock” in the accompanying prospectus.
PRIVATE PLACEMENT TRANSACTIONS
In the concurrent Private Placement, we shall issue (i) to each purchaser receiving common stock pursuant to this prospectus supplement, a Warrant to purchase common stock for each share of common stock purchased and (ii) to each purchaser receiving Unregistered Shares or Prefunded Warrants, a Warrant to purchase common stock for each Unregistered Share or Prefunded Warrant purchased. Accordingly, we will issue (i) 455,242 Unregistered Shares, (ii) Prefunded Warrants to purchase 1,064,164 shares of common stock and (iii) a total 2,272,723 Warrants in the Private Placement.
The Warrants will be exercisable immediately following the date of issuance and expire five (5) years from the date of issuance. The Prefunded Warrants will also be exercisable immediately following the date of issuance.
The Unregistered Shares, Prefunded Warrants, and the Warrants and the shares of common stock issuable upon exercise of the Prefunded Warrants and Warrants, are being offered pursuant to the exemption from registration provided in Section 4(a)(2) under the Securities Act and Rule 506(b) promulgated thereunder and are not being registered under the Securities Act at this time or offered pursuant to this prospectus supplement and the accompanying prospectus. Accordingly, the purchasers in this offering may only sell the Unregistered Shares, Prefunded Warrants, and Warrants and the shares of our common stock issued upon exercise of the Prefunded Warrants and Warrants pursuant to an effective registration statement under the Securities Act covering the resale of those shares, an exemption under Rule 144 under the Securities Act or another applicable exemption under the Securities Act. Pursuant to the securities purchase agreement and associated registration rights agreement, each dated April 3, 2023, by and among us and the purchaser signatories thereto, within 30 days following the date of such agreements, we will file a registration statement on Form S-1 providing for the resale by holders of Unregistered Shares and shares of our common stock issuable upon the exercise of the Prefunded Warrants and the Warrants and agree to have such registration statement effective by the 60th day from the date of such agreements and will use our best efforts to keep such registration statement effective at all times until such date that the shares underlying the Prefunded Warrants and Warrants either (i) have been sold, or (ii) may be sold without volume or manner-of-sale restrictions pursuant to Rule 144 and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144.
Unregistered Shares of Common Stock
The material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common stock are described under the heading “Description of Capital Stock” in the accompanying prospectus.
Description of Prefunded Warrants
The following is a brief summary of certain terms and provisions of the Prefunded Warrants being sold in the Private Placement and is subject in all respects to the provisions contained in the Prefunded Warrants.
Exercisability. The Prefunded Warrants will be exercisable immediately following the date of issuance, and at any time thereafter in perpetuity. The Prefunded Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and by payment in full in immediately available funds for the number of shares of our common stock purchased upon such exercise. At any time, the Prefunded Warrants may also be exercised, in whole or in part, by means of a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of our common stock determined according to the formula set forth in the Prefunded Warrants.
Exercise Limitation. A holder will not have the right to exercise any portion of the Prefunded Warrant if the holder would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon notice from the holder to us, the holder may increase or decrease the beneficial ownership limitation up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Prefunded Warrants, provided that any increase in such beneficial ownership limitation shall not be effective until 61 days following notice from the holder to us.
Exercise Price; Adjustments. The Prefunded Warrants have an exercise price of $0.0001 per share. The exercise price and the number of shares of common stock issuable upon exercise are subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting shares of our common stock. In addition, if we grant, issue or sell certain securities pro rata to the record holders of our common stock, other than certain exempt issuances, or if we declare or make any dividend or other distribution of our assets, including cash, stock or other property to the holders of our common stock, then the holders of the Prefunded Warrants will be entitled to participate in such transactions to the same extent such holder would have participated in such transaction if it held the number of shares of common stock issuable upon exercise of the Prefunded Warrants without regard to any limits on exercise contained in the Prefunded Warrants.
Transferability. Subject to applicable laws, the Prefunded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. There is no established trading market for the Prefunded Warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Prefunded Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Prefunded Warrants will be limited.
Fundamental Transactions. If a fundamental transaction (as defined in the Prefunded Warrants) occurs, then the successor entity will succeed to, and be substituted for us, and may exercise every right and power that we may exercise and will assume all of our obligations under the Prefunded Warrants with the same effect as if such successor entity had been named in the Prefunded Warrant itself. Following such fundamental transaction, the holders of the Prefunded Warrants will be entitled to receive upon exercise of the Prefunded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Prefunded Warrants immediately prior to such fundamental transaction without regard to any limits on exercise contained in the Prefunded Warrants. If holders of shares of our common stock are given a choice as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the same choice as to the consideration it receives upon any exercise of the Prefunded Warrant following such fundamental transaction. In addition, in certain circumstances, upon a fundamental transaction, the holder will have the right to require us or the successor entity to repurchase, with such payment to occur in common stock of the Company or its successor, its Prefunded Warrant at its fair value using the Black Scholes option pricing formula; provided, however, that, if the fundamental transaction is not within our control, including not approved by our board of directors, then the holder shall only be entitled to receive the same type or form of consideration (and in the same proportion), at the Black Scholes value per share of common stock in the fundamental transaction for each share of common stock underlying a Prefunded Warrant, that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of common stock are given the choice to receive from among alternative forms of consideration in connection with the fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the Prefunded Warrants or by virtue of such holder’s ownership of shares of our common stock, the holder of a Prefunded Warrant does not have the rights or privileges of a holder of shares of our common stock, including any voting rights, until the holder exercises the Prefunded Warrant.
Warrants
The following is a brief summary of certain terms and provisions of the Warrants being sold in the Private Placement and is subject in all respects to the provisions contained in the Warrants.
Exercisability. The Warrants will be exercisable immediately following the date of issuance, and at any time thereafter up to five (5) years from the initial issuance date. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and by payment in full in immediately available funds for the number of shares of our common stock purchased upon such exercise. Beginning six (6) months following the issuance date of the Warrants, if at the time of exercise there is no effective registration statement registering the Warrants, then the Warrants may also be exercised, in whole or in part, at such time by means of a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of our common stock determined according to the formula set forth in the Warrants.
Exercise Limitation. A holder will not have the right to exercise any portion of the Warrant if the holder would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon notice from the holder to us, the holder may increase or decrease the beneficial ownership limitation up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants, provided that any increase in such beneficial ownership limitation shall not be effective until 61 days following notice from the holder to us.
Exercise Price; Adjustments. The Warrants have an exercise price of $2.64 per share. The exercise price and the number of shares of common stock issuable upon exercise are subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting shares of our common stock. In addition, if we grant, issue or sell certain securities pro rata to the record holders of our common stock, other than certain exempt issuances, or if we declare or make any dividend or other distribution of our assets, including cash, stock or other property to the holders of our common stock, then the holders of the Warrants will be entitled to participate in such transactions to the same extent such holder would have participated in such transaction if it held the number of shares of common stock issuable upon exercise of the Warrants without regard to any limits on exercise contained in the Warrants.
Transferability. Subject to applicable laws, the Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. There is no established trading market for the Warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Warrants will be limited.
Fundamental Transactions. If a fundamental transaction (as defined in the Warrants) occurs, then the successor entity will succeed to, and be substituted for us, and may exercise every right and power that we may exercise and will assume all of our obligations under the Warrants with the same effect as if such successor entity had been named in the Warrant itself. Following such fundamental transaction, the holders of the Warrants will be entitled to receive upon exercise of the Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Warrants immediately prior to such fundamental transaction without regard to any limits on exercise contained in the Warrants. If holders of shares of our common stock are given a choice as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the same choice as to the consideration it receives upon any exercise of the Warrant following such fundamental transaction. In addition, in certain circumstances, upon a fundamental transaction, the holder will have the right to require us or the successor entity to repurchase, with such payment to occur in common stock of the Company or its successor, its Warrant at its fair value using the Black Scholes option pricing formula; provided, however, that, if the fundamental transaction is not within our control, including not approved by our board of directors, then the holder shall only be entitled to receive the same type or form of consideration (and in the same proportion), at the Black Scholes value per share of common stock in the fundamental transaction for each share of common stock underlying a Warrant, that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of common stock are given the choice to receive from among alternative forms of consideration in connection with the fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of shares of our common stock, the holder of a Warrant does not have the rights or privileges of a holder of shares of our common stock, including any voting rights, until the holder exercises the Warrant.
PLAN OF DISTRIBUTION
Pursuant to a placement agency agreement, dated as of April 3, 2023, we have retained Ladenburg Thalmann & Co. Inc., or the placement agent, to act as our exclusive placement agent in connection with this offering. Under the terms of the placement agency agreement, the placement agent is not purchasing the securities offered by us in this offering and is not required to sell any specific number or dollar amount of securities but will assist us in this offering on a reasonable best-efforts basis. The terms of this offering were subject to market conditions and negotiations between us, the placement agent and prospective investors. The placement agent will have no authority to bind us by virtue of the agreement. We may not sell the entire amount of the shares of our common stock offered pursuant to this prospectus supplement.
The placement agent proposes to arrange for the sale of the shares we are offering pursuant to this prospectus supplement and accompanying prospectus to certain institutional and accredited investors through a securities purchase agreement directly between each investor and us. We will only sell to such investors who have entered into the securities purchase agreement with us.
Delivery of the shares of our common stock, Unregistered Shares, Prefunded Warrants, and Warrants offered hereby and pursuant to the Private Placement is expected to occur on or about April 5, 2023, subject to satisfaction of certain closing conditions.
Fees and Expenses
We have agreed to pay the placement agent a cash fee equal to 7.75% of the aggregate gross proceeds raised in this offering and our concurrent private placement and to reimburse the placement agent’s expenses up to an aggregate of $85,000. We estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent’s fees and expenses, will be approximately $100,000. In addition, the placement agent will also receive warrants that have substantially the same terms as the Warrants issued in the concurrent private placement to the purchasers in this offering to purchase that number of shares of our common stock equal to 6.0% of the aggregate number of shares of our common stock, Unregistered Shares, and Prefunded Warrants sold in this offering, or an aggregate of 136,363 shares of common stock, at an exercise price of $3.30 per share. The placement agent warrants will be exercisable immediately following the date of issuance and will expire five (5) years after the commencement of sales.
| | | Per Share | | | Total | |
| Offering price (Per share) | | $ | 2.64 | | | $ | 1,996,676.88 | |
| Placement agent’s fees(1) | | $ | 0.2046 | | | $ | 154,742.46 | |
| Proceeds, before expenses, to us(2) | | $ | 2.4354 | | | $ | 1,841,934.42 | |
| (1) | In addition, we have agreed to pay the placement agent a 7.75% commission on the proceeds from our concurrent private placement and for certain of its expenses and to issue to the placement agent (or its designees) warrants to purchase shares of common stock equal to 6.0% of the aggregate number of Shares, Unregistered Shares, and Prefunded Warrants issued in this offering and concurrent Private Placement. See “Plan of Distribution” beginning on page S-13 of this prospectus supplement for more information on placement agent compensation. |
| (2) | The amount of the offering proceeds to us presented in this table does not take into account the proceeds from the Private Placement or the exercise of any of the Prefunded Warrants or Warrants or any of the placement agent warrants. |
Indemnification
We have agreed to indemnify the placement agent and specified other persons against certain liabilities relating to or arising out of the placement agent’s activities under the placement agency agreement and the investment banking agreement and to contribute to payments that the placement agent may be required to make in respect of such liabilities.
Regulation M
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of shares of our common stock by the placement agent acting as principal. Under these rules and regulations, the placement agent:
| | ● | may not engage in any stabilization activity in connection with our securities; and |
| | | |
| | ● | may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution. |
The securities purchase agreement has been included as an exhibit to a Current Report on Form 8-K that we will file with the SEC on April 5, 2023 and will be incorporated by reference into the registration statement of which this prospectus supplement forms a part.
Right of First Refusal.
In addition, if at any time during the nine (9) months following the closing of this offering, but only in the event that such offering results in gross proceeds of at least $5 million, the Company proposes to effect a further financing, we must offer the placement agent the opportunity to participate as a sole bookrunner or exclusive placement agent or exclusive sales agent with respect to such financing, except that we may designate one co-manager, placement agent, or sales agent who may participate in up to 30% of such future transaction..
Other Relationships
From time to time, the placement agent may provide in the future various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. Except as disclosed in this prospectus supplement and as provided in the investment banking agreement, we have no present arrangements with the placement agent for any further services.
Transfer Agent and Registrar
The transfer agent for our common stock is American Stock Transfer & Trust Company, LLC.
Common Stock Listing
Our shares of common stock are listed on The Nasdaq Capital Market under the symbol “PALI.”
LEGAL MATTERS
The validity of the securities offered by this prospectus supplement and the accompanying prospectus will be passed upon for us by Silvestre Law Group, P.C.
EXPERTS
The consolidated financial statements of Palisade Bio, Inc. as of December 31, 2021 and for the year then ended incorporated by reference in this Prospectus and in the Registration Statement have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and accounting. The report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
The financial statements of Palisade Bio, Inc. as of and for the year ended December 31, 2022 incorporated by reference in this Prospectus and in the Registration Statement has been audited by Baker Tilly US, LLP, an independent registered public accounting firm, as set forth in their report thereon, and are incorporated by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing. The report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus supplement and the accompanying prospectus are part of a registration statement on Form S-3 we filed with the SEC and do not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus supplement and the accompanying prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on information contained in this prospectus supplement, the accompanying prospectus and incorporated by reference herein and therein. We have not authorized any person to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus supplement is accurate as of any date other than the date on the front page of this prospectus supplement, regardless of the time of delivery of this prospectus supplement or any sale of the securities offered by this prospectus supplement.
Because we are subject to the information and reporting requirements of the Exchange Act, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, including any amendments to those reports, and other information that we file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act can also be accessed free of charge on the Investor section of our website. These filings will be available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Our website address is www. palisadebio.com. Information contained on or accessible through our website is not a part of this prospectus and is not incorporated by reference herein, and the inclusion of our website address in this prospectus is an inactive textual reference only.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information to you by referring to those documents. The information incorporated by reference is an important part of this prospectus supplement and the accompanying prospectus, and information that we file later with the SEC will automatically update and supersede this information.
We incorporate by reference the following documents we filed with the SEC pursuant to Section 13 of the Exchange Act and any future filings we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of this prospectus supplement until the termination of the offering of the shares covered by this prospectus supplement and the accompanying prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K):
| | ● | our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 22, 2023; |
| | | |
| | ● | our Current Reports Form 8-K filed with the SEC on January 4, 2023, February 8, 2023, and March 13, 2023; and |
| | | |
| | ● | the description of our common stock which is registered under Section 12 of the Exchange Act, in our registration statement on Form 8-A filed with the SEC on July 1, 2015, including any amendments or reports filed for the purpose of updating such description, including Exhibit 4.2 to our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 17, 2022. |
We will furnish without charge to each person, including any beneficial owner, to whom this prospectus supplement and the accompanying prospectus is delivered, upon written or oral request, a copy of any document incorporated by reference into this prospectus supplement and the accompanying prospectus. Requests should be addressed to 7750 El Camino Real, Suite 5200, Carlsbad, CA 92009, Attn: Secretary or may be made telephonically at (858) 704-4900.
You should rely only on the information incorporated by reference or provided in this prospectus supplement and the accompanying prospectus. We have not authorized anyone to provide you with different information. You should not assume that the information contained in this prospectus supplement or the accompanying prospectus is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus supplement and the accompanying prospectus is delivered or securities are sold on a later date.
PROSPECTUS

$100,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
From time to time, we may offer and sell up to an aggregate amount of $100,000,000 of any combination of the securities described in this prospectus, either individually or in combination, at prices and on terms described in one or more supplements to this prospectus. We may also offer common stock or preferred stock upon conversion of debt securities, or common stock upon conversion of preferred stock, or common stock, preferred stock or debt securities upon the exercise of warrants.
This prospectus describes some of the general terms that may apply to an offering of our securities. We will provide the specific terms of these offerings and securities in one or more supplements to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before you invest in any of the securities being offered.
This prospectus may not be used to consummate a sale of securities unless accompanied by a prospectus supplement.
As of March 17, 2022, the aggregate market value of our outstanding common stock held by non-affiliates pursuant to General Instruction I.B.6 of Form S-3 was approximately $19.5 million, which is based on 15,886,679 shares of common stock held by non-affiliates as of such date and a price of $1.23 per share, the last reported sales price of our common stock on February 1, 2022. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities registered on the registration statement of which this prospectus is a part with a value of more than one-third of the aggregate market value of our common stock held by non-affiliates in any 12-month period, so long as the aggregate market value of our common stock held by non-affiliates is less than $75,000,000. As of the date hereof, we have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the 12 calendar months prior to and including the date of this prospectus.
Securities may be sold by us to or through underwriters or dealers, directly to purchasers or through agents designated from time to time on a continuous or delayed basis. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus and in the applicable prospectus supplement. If any agents or underwriters are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such agents or underwriters and any applicable fees, commissions, discounts or options to purchase additional securities will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth in a prospectus supplement.
Our common stock is listed on The Nasdaq Capital Market under the trading symbol “PALI.” On March 17, 2022, the last reported sale price of our common stock was $0.995 per share. The applicable prospectus supplement will contain information, where applicable, as to other listings, if any, on the Nasdaq Capital Market or other securities exchange of the securities covered by the prospectus supplement.
Investing in our securities involves a high degree of risk. Before making an investment decision, you should review carefully the risks described under the heading “Risk Factors” on page S-8 of this prospectus and under similar headings in any amendment or supplement to this prospectus or in any filing with the Securities and Exchange Commission that is incorporated by reference herein.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is April 26, 2022
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration process. Under this shelf registration statement, we may from time to time, offer and sell, either individually or in combination, in one or more offerings up to a total dollar amount of $100,000,000 of any combination of the securities described in this prospectus. This prospectus provides you with a general description of the securities we may offer.
Each time we offer securities under this prospectus, we will provide a prospectus supplement that will contain more specific information about the terms of that offering. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change any of the information contained in this prospectus or in the documents that we have incorporated by reference into this prospectus. We urge you to carefully read both this prospectus, any applicable prospectus supplement and any free writing prospectuses we have authorized for use in connection with a specific offering, together with the information incorporated by reference as described under the heading “Incorporation of Certain Information by Reference,” before buying any of the securities being offered.
This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
You should rely only on the information contained in, or incorporated by reference into, this prospectus, any related prospectus supplement and in any free writing prospectus that we may authorize for use in connection with an offering. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are not making an offer to sell or soliciting an offer to buy our securities in any jurisdiction in which an offer or solicitation is not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information appearing in this prospectus, any related prospectus supplement and the documents incorporated by reference into this prospectus, any related prospectus supplement and in any free writing prospectus that we may authorize for use in connection with this offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus, any related prospectus supplement, the documents incorporated by reference into this prospectus, and any free writing prospectus that we may authorize for use in connection with this offering, in their entirety before making an investment decision. You should also read and consider the information in the documents to which we have referred you in the sections of this prospectus captioned “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The distribution of this prospectus and any related prospectus supplement and the offering of our securities in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus and any related prospectus supplement must inform themselves about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus and any related prospectus supplement outside the United States. This prospectus and any related prospectus supplement does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus and any related prospectus supplement by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
We obtained the industry and market data in this prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections,
assumptions and estimates of our future performance and the future performance of the industry in which we operate is necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and elsewhere in this prospectus, any related prospectus supplement and documents incorporated by reference into this prospectus and any related prospectus supplement. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
For investors outside the United States: We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
Unless otherwise stated, all references in this prospectus to “we,” “us,” “our,” “Palisade,” the “Company” and similar designations refer to Palisade Bio, Inc. The Palisade Bio, Inc. logo is a trademark of Palisade Bio, Inc. We use “Palisade Bio” as a trademark in the United States and other countries and have applied to register the trademark in the United States and other countries. This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
PROSPECTUS SUMMARY
This summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated by reference into this prospectus. This summary is not complete and does not contain all of the information that you should consider before making an investment decision. For a more complete understanding of our company, you should read and consider carefully the more detailed information included or incorporated by reference in this prospectus and any applicable prospectus supplement, including the factors described under the heading “Risk Factors” beginning on page 8 of this prospectus, as well as the information incorporated by reference from our most recent Annual Report on Form 10-K before making an investment decision.
Company Overview
We are a clinical-stage biopharmaceutical company focused on discovering, developing, and commercializing innovative oral therapies that target serious diseases associated with the breakdown of the mucosal barrier protecting the gastrointestinal (“GI”) tract. Our goal is to be an industry leader in developing therapies to treat these diseases and to improve the lives of patients suffering from such diseases.
Our approach is founded on the discovery that damage to the intestinal epithelial barrier can result in the leakage of digestive enzymes from the GI tract that can damage tissues and promote inflammation, causing a broad array of acute and chronic conditions.
We are focused on developing a portfolio of oral product candidates to treat conditions driven by protease (intestinal enzymes) leakage through the intestinal epithelial barrier, including by surgical complications and inflammatory conditions. The below graphic illustrates the protease leakage resulting from a compromised intestinal epithelial barrier:
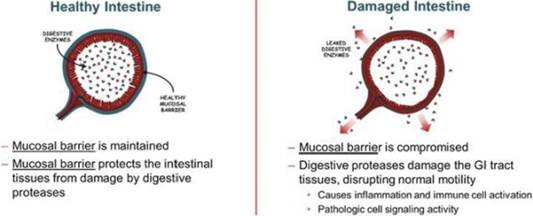
Our lead therapeutic candidate, LB1148, is an oral liquid formulation of the well-characterized digestive enzyme inhibitor, tranexamic acid, intended to inhibit digestive enzyme activity and preserve gut integrity during intestinal stress resulting from, among other things, reduced blood flow to the intestine, infections, or due to surgery. Peer reviewed publications of third-party research suggest that digestive enzyme leakage from the GI tract increases incidents of GI and organ dysfunction following these events.
Our pipeline of LB1148 is illustrated in this chart:
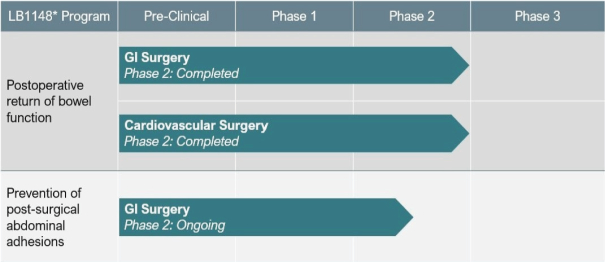
| * | Commercial rights to LB1148 in Greater China (excluding Taiwan) have been out-licensed to Newsoara Biopharma Co., Ltd. (“Newsoara”). |
We are initially developing LB1148 to be administered to patients prior to major surgeries that risk disrupting the intestinal mucosal barrier. As announced in March 2020, a randomized, double-blind, parallel, placebo-controlled Phase 2 investigator-sponsored clinical trial of LB1148 in 120 patients undergoing coronary artery bypass grafting and/or heart valve replacement surgery requiring cardiopulmonary bypass was completed. Patients were randomized to receive LB1148 or placebo in conjunction with surgery. The trial’s primary endpoint was time to return of bowel function. Secondary endpoints include Intensive Care Unit (“ICU”) length of stay, hospital length of stay, organ function changes, inflammatory response and glucose control. LB1148 provided an approximately 30% improvement in the time to normal bowel function following cardiovascular (“CV”) surgery (p<0.001) compared to placebo. The treatment group also had an average 1.0-day shorter length of stay in the ICU and an average 1.1-day shorter hospital stay. Generally, treatment with LB1148 was well tolerated. Adverse events were similar between the treatment groups and not considered unexpected for the subject population. None of the adverse events or serious adverse events reported were considered drug-related by the sponsor-investigator. One of the primary factors in discharging patients from the hospital following surgery is the return of bowel function. LB1148 has been granted Fast Track designation from the U.S. Food and Drug Administration (“FDA”) for the treatment of postoperative GI dysfunction (which may present as feeding intolerance, ileus, necrotizing enterocolitis (“NEC”), etc.) associated with gut hypoperfusion injury in pediatric patients who have undergone congenital heart disease repair surgery.
On July 20, 2021, we and our co-development partner Newsoara announced topline Phase 2 clinical trial data demonstrating that LB1148 had a statistically significant (p=0.0008) effect in accelerating the return of bowel function in patients undergoing elective bowel resection surgery.
Results from the trial include:
| | ● | A 1.1-day improvement in GI recovery in patients receiving LB1148 vs placebo. The median time to return of bowel function was 2.77 days in patients treated with LB1148 and 3.83 days in those receiving placebo (hazard ratio = 1.886; p = 0.0008). |
| | | |
| | ● | The difference between groups increased at the 3rd quartile (75th percentile), with LB1148 (3.4 days) demonstrating a 1.5-day faster recovery of bowel function compared to placebo (4.9 days). |
| | | |
| | ● | LB1148 was well tolerated with 10.9% and 4.8% of patients in the LB1148 group and placebo group, respectively, experiencing a drug-related adverse event. |
| | ● | The most common drug-related adverse events were GI disorders (LB1148 4.7% vs. placebo 3.2%). |
| | | |
| | ● | No drug-related serious adverse events occurred in the trial. |
Results from this trial are expected to be reported at upcoming surgical-focused medical conferences. We and Newsoara intend to advance LB1148 to pivotal Phase 3 clinical trials for accelerating the return of bowel function for major surgical indications. LB1148 has received Fast Track designation from the FDA for reduction of adhesions following abdominal and pelvic surgery.
Adhesion prevalence is reported to be >90% in patients who have abdominal surgery and represents a significant contribution to serious complications such as small bowel obstruction, infertility, chronic abdominal pain, subsequent surgery, and other morbidities. On March 16, 2022 we announced data from a pooled-analysis of studies LBS-IST-POI-101 and LBS-POI-201-CN (PROFILE-CN) at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) 2022 Annual Meeting. The results from the pooled analysis showed that 8/9 (89%) of subjects in the placebo group versus 2/8 (25%) in the LB1148 group had adhesions observed during a second follow-up surgery, representing a relative risk reduction of 72% (p = 0.0152). The mean total adhesion score which measures both the extent and severity of adhesions was 1.0 (8/8) for LB1148 and 14.3 (129/9) for placebo, representing relative risk reduction of 93% (p = 0.0162). We believe the reduction in the incidence of post-surgical intra-abdominal adhesions as well as the reduction in the extent and severity of adhesions provides preliminary evidence of the clinically meaningful efficacy of LB1148 to reduce post-surgical adhesions when compared to placebo.
We are also currently conducting a randomized, double-blind, placebo-controlled, proof-of-concept Phase 2 clinical trial of LB1148 in patients undergoing elective bowel resection surgery in the United States. This trial will also evaluate whether or not patients treated with LB1148 experience fewer postoperative intra-abdominal adhesions and quicker return of bowel function following surgery.
LB1148 contains a broad-spectrum serine protease inhibitor, tranexamic acid (“TXA”), and is formulated as an aqueous solution for oral (or enteral) administration. In addition to TXA, the patented LB1148 formulation contains polyethylene glycol, carbohydrates, and electrolytes. The components of LB1148 are provided as dry powders for reconstitution in water prior to administration. Such reconstitution may be carried out in a pharmacy (by a pharmacist), or in an outpatient setting (by a patient).
The potential of LB1148 relies on its formulation as a liquid composition for oral administration, which is designed to stop the downstream effects of a disruption of the intestinal mucosal barrier. We are not aware of any other approved oral TXA-containing liquid compositions in the marketplace suitable for such administration.
We believe that LB1148, if successfully developed and approved, may have the ability to become the standard of care across a broad range of acute and chronic conditions associated with GI barrier dysfunction.
Beyond our initial therapeutic focus on GI-related pathology triggered by major surgeries, we believe that protease-based therapeutics hold promise in meeting a number of unmet needs resulting from chronic protease leak. By leveraging our expertise in protease-mediated diseases and dysregulation of the intestinal epithelia barrier, our strategy is to create a broad portfolio of innovative oral therapeutics that target serious diseases associated with the breakdown of this barrier.
Selected Risks Affecting Our Business
Below is a summary of the principal factors that make an investment in our securities speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks and uncertainties summarized in this risk factor summary, and other risks and uncertainties that we face, are set forth below under the heading “Risk Factors” contained in this prospectus, any accompanying prospectus supplement or free writing prospectus, and under similar headings in the documents that are incorporated by reference into this prospectus.
| | ● | The Company’s business depends on the successful clinical development, regulatory approval and commercialization of the Company’s lead drug candidate, LB1148. |
| | | |
| | ● | Some of the initial indications in which the Company plans to pursue development of LB1148 are indications for which there are no U.S. Food and Drug Administration-approved therapies. This makes it difficult to predict the timing and costs of clinical development for LB1148 in these indications, as well as the regulatory approval path. |
| | | |
| | ● | The development and commercialization strategy for the Company’s product candidate LB1148 depends, in part, on published scientific literature and prior findings of the FDA regarding the safety and efficacy of tranexamic acid. If the Company is not able to pursue this strategy, it may be delayed in receiving regulatory authority approval. |
| | | |
| | ● | Clinical drug development is very expensive, time-consuming, and uncertain. |
| | | |
| | ● | The results of previous clinical trials may not be predictive of future results, and the results of the Company’s current and planned clinical trials may not satisfy the requirements of the FDA or non-U.S. regulatory authorities. |
| | | |
| | ● | Even if the Company receives marketing approval for LB1148, or any future product candidate, it may not be able to successfully commercialize its product candidates due to unfavorable pricing regulations or third-party coverage and reimbursement policies, which could make it difficult for the Company to sell its product candidates profitably. |
| | | |
| | ● | The Company’s product candidates may cause undesirable side effects or have other unexpected properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in post-approval regulatory action. |
| | | |
| | ● | The Company may in the future conduct clinical trials for its product candidates outside the United States, and the FDA and applicable foreign regulatory authorities may not accept data from such trials or the data may be insufficient to achieve regulatory approvals. |
| | | |
| | ● | The Company expects to rely on third-party Contract Research Organizations and other third parties to conduct and oversee its clinical trials. If these third parties do not meet the Company’s requirements or otherwise conduct the trials as required, the Company may not be able to satisfy its contractual obligations or obtain regulatory approval for, or commercialize, its product candidates. |
| | | |
| | ● | The Company will need to raise additional financing in the future to fund its operations, which may not be available to it on favorable terms or at all. |
| | | |
| | ● | The Company currently has no products approved for sale, and it may never obtain regulatory approval to commercialize any of its product candidate. |
| | | |
| | ● | The Company’s or third party’s clinical trials may fail to demonstrate the safety and efficacy of its product candidates, or serious adverse or unacceptable side effects may be identified during their development, which could prevent or delay marketing approval and commercialization, increase the Company’s costs or necessitate the abandonment or limitation of the development of the product candidate. |
| | | |
| | ● | The Company has expressed substantial doubt about its ability to continue as a going concern. |
| | | |
| | ● | The Company’s product candidates, if approved, will face significant competition and their failure to compete effectively may prevent them from achieving significant market penetration. |
| | | |
| | ● | Any adverse developments that occur during any clinical trials conducted by Newsoara may affect the Company’s ability to obtain regulatory approval or commercialize LB1148. |
| | ● | The Company has a very limited operating history and has never generated any revenues from product sales. |
| | | |
| | ● | If the Company is not able to comply with the applicable continued listing requirements or standards of The Nasdaq Capital Market, Nasdaq could delist its common stock. |
| | | |
| | ● | The Company may not be able to protect its intellectual property rights throughout the world. |
| | | |
| | ● | The Company’s board of directors (the “Board”) has broad discretion to issue additional securities, which might dilute the net tangible book value per share of our common stock for existing stockholders. |
| | | |
| | ● | The Company currently has no marketing capabilities and no sales organization. If the Company is unable to establish sales and marketing capabilities on its own or through third parties, the Company will be unable to successfully commercialize its product candidates, if approved, or generate product revenue. |
| | | |
| | ● | Failure to remediate a material weakness in internal accounting controls could result in material misstatements in the Company’s consolidated financial statements. |
| | | |
| | ● | The Company may not be able to obtain, maintain or enforce global patent rights or other intellectual property rights that cover its product candidates and technologies that are of sufficient breadth to prevent third parties from competing against the Company. |
| | | |
| | ● | Obtaining and maintaining the Company’s patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by governmental patent agencies, and its patent protection could be reduced or eliminated for non-compliance with these requirements. |
| | | |
| | ● | If the Company fails to comply with its obligations under its intellectual property license agreements, it could lose license rights that are important to its business. Additionally, these agreements may be subject to disagreement over contract interpretation, which could narrow the scope of its rights to the relevant intellectual property or technology or increase its financial or other obligations to its licensors. |
| | | |
| | ● | The Company’s business could be adversely affected by the effects of health pandemics or epidemics, including the recent COVID-19 pandemic, especially where overwhelming patient hospitalizations disrupt routine medical practices, clinical studies, and ability for appropriate follow-up of patients enrolled in clinical studies or supply chain constraints associated with such pandemics impact the availability of the components needed to manufacture LB1148. |
Corporate Information
We were originally incorporated in 2001 in the State of Delaware under the name Neuralstem, Inc. In October 2019, we changed our name from Neuralstem, Inc. to Seneca Biopharma, Inc. In April 2021, we effected a merger transaction with Leading Biosciences, Inc. (“LBS”), whereby LBS became a wholly owned subsidiary of Seneca. In April 2021, we changed our name from Seneca Biopharma, Inc. to Palisade Bio, Inc. Our principal executive offices are located at 5800 Armada Drive, Suite 210, Carlsbad, California 92008, our telephone number is (858) 704-4900 and our website address is www.palisadebio.com. The information contained in or accessible through our website does not constitute part of this prospectus.
Subsidiaries
We have two wholly owned subsidiaries, Suzhou Neuralstem Biopharmaceutical Co., Ltd., organized under the laws of the People’s Republic of China (“Suzhou”), and LBS. Suzhou was established by Seneca Biopharma, Inc. to sponsor a clinical trial of NSI-566 that was conducted between 2013 and 2016. At this time, Suzhou has limited operations and exists for the sole purpose of conducting observational follow-up for a small group of remaining patients from the completed clinical trial, which it does through the engagement of a consultant. Suzhou has no employees or other operations. We believe that all Suzhou operations will cease in 2022.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our shares of common stock held by non-affiliates does not equal or exceed $250.0 million as of the prior June 30th, or (2) our annual revenues did not equal or exceed $100.0 million during such completed fiscal year and the market value of our shares of common stock held by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of any reduced disclosure obligations, it may make comparison of our financial statements with other public companies difficult or impossible.
Merger Transaction
On April 27, 2021, pursuant to the Agreement and Plan of Merger (the “Merger Agreement”), dated as of December 16, 2020, by and among Palisade Bio, Inc., formerly known as Seneca Biopharma, Inc. (the “Company”), Leading Biosciences, Inc. (“LBS”) and Townsgate Acquisition Sub 1, Inc., a wholly owned subsidiary of the Company (“Merger Sub”), the Company completed the previously announced merger transaction with LBS, pursuant to which Merger Sub merged with and into LBS, with LBS surviving such merger as a wholly owned subsidiary of the Company (the “Merger”). In connection with the Merger, and immediately prior to the effective time of the Merger, the Company effected a reverse stock split of the Company Common Stock at a ratio of 1-for-6 (the “Reverse Stock Split”). Unless otherwise noted, all references to share and per share amounts in this Prospectus reflect the Reverse Stock Split. Also, in connection with the closing of the Merger, the Company changed its name from “Seneca Biopharma, Inc.” to “Palisade Bio, Inc.” and the business conducted by the Company became primarily the business conducted by LBS, which is a clinical-stage biopharmaceutical company focused on advancing LBS’s clinical program and developing a therapeutic to combat the interruption of gastrointestinal function following major surgery for which there is currently a significant unmet need for safe and effective therapies.
THE SECURITIES WE MAY OFFER
We may offer shares of our common stock and preferred stock, various series of debt securities and/or warrants to purchase any of such securities, either individually or in combination, with a total dollar amount up to $100,000,000 from time to time under this prospectus, together with the applicable prospectus supplement and any related free writing prospectus, at prices and on terms to be determined by market conditions at the time of any offering. We may also offer common stock, preferred stock and/or debt securities upon the exercise of warrants. This prospectus provides you with a general description of the securities we may offer. Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities, including, to the extent applicable:
| | ● | designation or classification; |
| | | |
| | ● | aggregate principal amount or aggregate offering price; |
| | | |
| | ● | maturity date, if applicable; |
| | | |
| | ● | original issue discount; |
| | | |
| | ● | rates and times of payment of interest or dividends; |
| | | |
| | ● | redemption, conversion, exercise, exchange or sinking fund terms; |
| | | |
| | ● | ranking; |
| | | |
| | ● | restrictive covenants; |
| | | |
| | ● | voting or other rights; |
| | | |
| | ● | conversion or exchange prices or rates and, if applicable, any provisions for changes to or adjustments in the conversion or exchange prices or rates and in the securities or other property receivable upon conversion or exchange; and |
| | | |
| | ● | a discussion of material United States federal income tax considerations, if any. |
The applicable prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change any of the information contained in this prospectus or in the documents we have incorporated by reference. However, no prospectus supplement or free writing prospectus will offer a security that is not registered and described in this prospectus at the time of the effectiveness of the registration statement of which this prospectus is a part.
This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
We may sell the securities directly to investors or to or through agents, underwriters or dealers. We, and our agents, underwriters or dealers reserve the right to accept or reject all or part of any proposed purchase of securities. If we do offer securities to or through agents, underwriters or dealers, we will include in the applicable prospectus supplement:
| | ● | the names of those agents, underwriters or dealers; |
| | | |
| | ● | applicable fees, discounts and commissions to be paid to them; |
| | | |
| | ● | details regarding over-allotment options, if any; and |
| | | |
| | ● | the net proceeds to us, if any. |
Common Stock
We may issue shares of our common stock from time to time. Our common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and does not have cumulative voting rights. Our Amended and Restated Bylaws (the “Bylaws”) establish a classified Board that is divided into three classes with staggered three-year terms. Only the directors in one class will be subject to election by a plurality of the votes cast at each annual meeting of our stockholders, with the directors in the other classes continuing for the remainder of their respective three-year terms. Except as otherwise expressly provided in our Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) or required by applicable law, all shares of common stock have the same rights and privileges and rank equally, share ratably, and are identical in all respects for all matters, including those described below. Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the Board out of funds legally available for that purpose. In the event of our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably in the assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock then outstanding. Holders of our common stock have no preemptive or conversion rights or other subscription rights and there are no redemption or sinking funds provisions applicable to our common stock. The rights, preferences, and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future. In this prospectus, we have summarized certain general features of the common stock under “Description of Capital Stock—Common Stock.” We urge you, however, to read the applicable prospectus supplement (and any related free writing prospectus that we may authorize to be provided to you) related to any common stock being offered.
Preferred Stock
We may issue shares of our preferred stock from time to time, in one or more series. Our Board has the authority, without further action by our stockholders, to issue up to 7,000,000 shares of preferred stock in one or more series pursuant to a resolution or resolutions providing for such issue duly adopted by our Board. Our Board is further authorized, subject to limitations prescribed by law, to fix by resolution or resolutions the designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, of any wholly unissued series of preferred stock, including without limitation authority to fix by resolution or resolutions the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), redemption price or prices, and liquidation preferences of any such series, and the number of shares constituting any such series and the designation thereof, or any of the foregoing. Our Board may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of our common stock. We have no current plans to issue any shares of preferred stock.
If we sell any series of preferred stock under this prospectus, we will fix the designations, voting powers, preferences and rights of such series of preferred stock, as well as the qualifications, limitations or restrictions thereof, in the certificate of designation relating to that series. We will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of any certificate of designation that describes the terms of the series of preferred stock that we are offering before the issuance of the related series of preferred stock. In this prospectus, we have summarized certain general features of the preferred stock under the heading “Description of Capital Stock—Preferred Stock.” We urge you to read the applicable prospectus supplement (and any free writing prospectus that we may authorize to be provided to you) related to the series of preferred stock being offered, as well as the complete certificate of designation that contains the terms of the applicable series of preferred stock.
Debt Securities
We may issue debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. The senior debt securities will rank equally with any other unsecured and unsubordinated debt. The subordinated debt securities will be subordinate and junior in right of payment, to the extent and in the manner described in the instrument governing the debt, to all of our senior indebtedness. Convertible or exchangeable debt securities will be convertible into or exchangeable for our common stock or our other securities. Conversion or exchange may be mandatory or optional (at our option or the holders’ option) and would be at prescribed conversion or exchange rates.
Any debt securities issued under this prospectus will be issued under one or more documents called indentures, which are contracts between us and a national banking association or other eligible party, as trustee. In this prospectus, we have summarized certain general features of the debt securities under the heading “Description of Debt Securities.” We urge you, however, to read the applicable prospectus supplement (and any free writing prospectus that we may authorize to be provided to you) related to the series of debt securities being offered, as well as the complete indenture and any supplemental indentures that contain the terms of the debt securities. A form of indenture has been filed as an exhibit to the registration statement of which this prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered will be filed as exhibits to the registration statement of which this prospectus is a part or will be incorporated by reference from reports that we file with the SEC.
Warrants
We may issue warrants for the purchase of common stock, preferred stock and/or debt securities in one or more series. We may issue warrants independently or in combination with common stock, preferred stock and/or debt securities offered by any prospectus supplement. In this prospectus, we have summarized certain general features of the warrants under the heading “Description of Warrants.” We urge you, however, to read the applicable prospectus supplement (and any related free writing prospectus that we may authorize to be provided to you) related to the particular series of warrants being offered, as well as any warrant agreements and warrant certificates, as applicable, that contain the terms of the warrants. We have filed forms of the warrant agreements and forms of warrant certificates containing the terms of the warrants that may be offered as exhibits to the registration statement of which this prospectus is a part. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of warrant and/or the warrant agreement and warrant certificate, as applicable, that contain the terms of the particular series of warrants we are offering, and any supplemental agreements, before the issuance of such warrants.
Any warrants issued under this prospectus may be evidenced by warrant certificates. Warrants also may be issued under an applicable warrant agreement that we enter into with a warrant agent. We will indicate the name and address of the warrant agent, if applicable, in the prospectus supplement relating to the particular series of warrants being offered.
RISK FACTORS
Investing in our common stock involves a high degree of risk. Before making an investment decision, you should carefully consider the risks described below and described in the sections entitled “Risk Factors” in our most recent Annual Report on Form 10-K, as filed with the SEC, which is incorporated herein by reference in its entirety, as well as any amendment or updates to our risk factors reflected in subsequent filings with the SEC, including any applicable prospectus supplement. Our business, financial condition, results of operations or prospects could be materially adversely affected by any of these risks. The trading price of our securities could decline due to any of these risks, and you may lose all or part of your investment. This prospectus and the documents incorporated herein by reference also contain forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks mentioned elsewhere in this prospectus. For more information, see the section entitled “Where You Can Find Additional Information.” Please also read carefully the section entitled “Special Note Regarding Forward-Looking Statements.”
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and any applicable prospectus supplement or free writing prospectus, including the documents that we incorporate by reference herein and therein, contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are not limited to, statements about:
| | ● | | estimates about the size and growth potential of the markets for our product candidates, and our ability to serve those markets; |
| | | | |
| | ● | | the impact of the COVID-19 pandemic on our business, and operations, and supply; |
| | | | |
| | ● | | the rate and degree of market acceptance of our products; |
| | | | |
| | ● | | our ability to build and expand our sales organization to address effectively existing and new markets that we intend to target; |
| | | | |
| | ● | | future regulatory, judicial, and legislative changes or developments in the United States (“U.S.”) and foreign countries and the impact of these changes; |
| | | | |
| | ● | | our ability to build a commercial infrastructure in the U.S. and other markets; |
| | | | |
| | ● | | our ability to compete effectively in a competitive industry; |
| | | | |
| | ● | | our ability to identify and qualify additional manufacturers to provide API and manufacture drug product; |
| | | | |
| | ● | | our ability to enter into longer term commercial supply agreements; |
| | | | |
| | ● | | the success of competing technologies that are or may become available; |
| | | | |
| | ● | | our ability to attract and retain key scientific or management personnel; |
| | | | |
| | ● | | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
| | | | |
| | ● | | our ability to obtain funding for our operations; and |
| | | | |
| | ● | | our ability to attract collaborators and strategic partnerships. |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “intend,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and are subject to risks and uncertainties. As such, our actual results may differ significantly from those expressed in any forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
We discuss many of these risks in greater detail under the heading “Risk Factors” in this prospectus, in the “Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections incorporated by reference from our most recent Annual Report on Form 10-K, as well as any amendments thereto reflected in subsequent filings with the SEC.
The discussion of risks and uncertainties set forth in those filings is not necessarily a complete or exhaustive list of all risks facing us at any particular point in time. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. Forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable statement. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. You should read this prospectus, any applicable prospectus supplement, together with the documents that we have filed with the SEC that are incorporated by reference and any free writing prospectus we have authorized for use in connection with this offering, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
USE OF PROCEEDS
Except as described in any applicable prospectus supplement or in any free writing prospectuses we have authorized for use in connection with a specific offering, we currently intend to use the net proceeds from the sale of securities offered by us hereunder, if any, for working capital and general corporate purposes, which may include, among other things, funding research and development, vendor payables, hiring additional personnel, and capital expenditures.
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock, certain provisions of our Amended and Restated Certificate of Incorporation (Certificate of Incorporation”), Amended and Restated Bylaws (“Bylaws”), Certificate of Designation of Preferences, Rights and Limitations of Series A 4.5% Convertible Preferred Stock (“Certificate of Designation”), and certain provisions of Delaware law are summaries. The following description is not complete and is subject to and qualified in its entirety by our Certificate of Incorporation, Bylaws and Certificate of Designation, which are filed as exhibits to the registration statement of which this prospectus is a part, as well as the relevant provisions of the Delaware General Corporation Law (“DGCL”).
As of the date of this prospectus, our authorized capital stock consists of 300,000,000 shares of common stock, par value $0.01 per share, and 7,000,000 shares of preferred stock, par value $0.01 per share.
Common Stock
Fully Paid and Non-Assessable
All outstanding shares of common stock are duly authorized, validly issued, fully paid, and nonassessable. All authorized but unissued shares of our common stock are available for issuance by our Board without any further stockholder action, except as required by the listing standards of the Nasdaq Stock Market.
Voting Rights
Our common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and does not have cumulative voting rights. Our Bylaws establish a classified Board that is divided into three classes with staggered three-year terms. Only the directors in one class will be subject to election by a plurality of the votes cast at each annual meeting of our stockholders, with the directors in the other classes continuing for the remainder of their respective three-year terms.
Economic Rights
Except as otherwise expressly provided in our Certificate of Incorporation or required by applicable law, all shares of common stock have the same rights and privileges and rank equally, share ratably, and are identical in all respects for all matters, including those described below.
Dividends and Distributions. Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the Board out of funds legally available for that purpose.
Liquidation Rights. In the event of our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably in the assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock then outstanding.
Holders of our common stock have no preemptive or conversion rights or other subscription rights and there are no redemption or sinking funds provisions applicable to our common stock. The rights, preferences, and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our Certificate of Incorporation, our Board has the authority, without further action by our stockholders, to issue up to 7,000,000 shares of preferred stock in one or more series pursuant to a resolution or resolutions providing for such issue duly adopted by our Board. Our Board is further authorized, subject to limitations prescribed by law, to fix by resolution or resolutions the designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, of any wholly unissued series of preferred stock, including without limitation authority to fix by resolution or resolutions the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), redemption price or prices, and liquidation preferences of any such series, and the number of shares constituting any such series and the designation thereof, or any of the foregoing.
Our Board may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of our common stock. We have no current plans to issue any shares of preferred stock.
We will fix the designations, voting powers, preferences and rights of the preferred stock of each series we issue under this prospectus, as well as the qualifications, limitations or restrictions thereof, in the certificate of designation relating to that series. We will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering. We will describe in the applicable prospectus supplement the terms of the series of preferred stock being offered, including, to the extent applicable:
| | ● | the title and stated value; |
| | | |
| | ● | the number of shares we are offering; |
| | | |
| | ● | the liquidation preference per share; |
| | | |
| | ● | the purchase price; |
| | | |
| | ● | the dividend rate, period and payment date and method of calculation for dividends; |
| | | |
| | ● | whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
| | | |
| | ● | the procedures for any auction and remarketing; |
| | | |
| | ● | the provisions for a sinking fund, if any; |
| | | |
| | ● | the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
| | | |
| | ● | any listing of the preferred stock on any securities exchange or market; |
| | | |
| | ● | whether the preferred stock will be convertible into our common stock, and, if applicable, the conversion price, or how it will be calculated, and the conversion period; |
| | | |
| | ● | whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price, or how it will be calculated, and the exchange period; |
| | | |
| | ● | voting rights of the preferred stock, if any; |
| | | |
| | ● | preemptive rights, if any; |
| | | |
| | ● | restrictions on transfer, sale or other assignment, if any; |
| | | |
| | ● | whether interests in the preferred stock will be represented by depositary shares; |
| | | |
| | ● | a discussion of any material United States federal income tax considerations applicable to the preferred stock; |
| | ● | the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; |
| | | |
| | ● | any limitations on the issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and |
| | | |
| | ● | any other specific terms, preferences, rights or limitations of, or restrictions on, the preferred stock. |
If we issue shares of preferred stock under this prospectus, the shares will be fully paid and non-assessable.
The issuance of preferred stock could adversely affect the voting power of holders of our common stock, and reduce the likelihood that holders of our common stock will receive dividend payments and payments upon liquidation. The issuance could have the effect of decreasing the market price of the common stock. The issuance of preferred stock also could have the effect of delaying, deterring or preventing a change in control of us.
Series A 4.5% Convertible Preferred Stock
In December 2016, a series of our preferred stock was designated as Series A 4.5% Convertible Preferred Stock (the “Series A Preferred Stock”), consisting of 1,000,000 designated shares (which is subject to increase without the consent of all of the holders of the Series A Preferred Stock in the event such additional shares of Series A Preferred Stock are issued solely to the holders as payment of accrued dividends).
As of March 17, 2022, we had outstanding 200,000 shares of Series A Preferred Stock with a stated value of $12.79 per share and which are immediately convertible into an aggregate of 6,479 shares of common stock. The Series A Preferred Stock have no provisions regarding subsequent securities issuances or so called “price protection provisions.” The holders of Series A Preferred Stock shall be entitled to receive dividends in cash or additional shares of Series A Preferred Stock if and when declared by our Board in preference to the payment of any dividends on our common stock. The holders of Series A Preferred Stock shall have no voting rights but shall be entitled to appoint one member to our Board. This right to appoint a member of the Board will terminate when there are less than 200,000 shares of Series A Preferred Stock outstanding. As long as any shares of Series A Preferred Stock are outstanding, we shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series A Preferred Stock, alter or change adversely the powers, preferences or rights given to the Series A Preferred Stock or alter or amend the Certificate of Designation, other than to authorize and issue additional shares of Series A Preferred Stock. In addition, holders of Series A Preferred Stock are subject to beneficial ownership limitations.
Options
As of March 17, 2022, stock options to purchase an aggregate of 796,719 shares of common stock were outstanding under the LBS 2013 Employee, Director, and Consultant Equity Incentive Plan, or 2013 Plan. No further awards will be made under the 2013 Plan. As of March 17, 2022, stock options to purchase an aggregate of 1,409,736 shares of common stock were outstanding under our 2021 Equity Incentive Plan, or 2021 Plan, and (ii) 662,414 shares remained available for future issuance under the 2021 Plan.
As of March 17, 2022, stock options to purchase an aggregate of 250,000 shares of common stock were outstanding under our 2021 Inducement Plan, or 2021 Inducement Plan, and (ii) 500,000 shares remained available for future issuance under the 2021 Inducement Plan.
Warrants
As of March 17, 2022, our outstanding warrants consist of warrants to purchase 5,348,289 shares of common stock, each with an exercise price ranging from $1.10 to $4,695.60 per share and generally expire between five and ten years after the date of issuance.
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
Some provisions of Delaware law, our Certificate of Incorporation and our Bylaws contain provisions that could make the following transactions more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise; or the removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions which provide for payment of a premium over the market price for our shares.
These provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our Board. We believe that the benefits of the increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Section 203 of the DGCL
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, subject to certain exceptions.
Amended and Restated Bylaws
Our Bylaws provide for our Board to be divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, the holders of a plurality of the voting power of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors, can elect all of the directors standing for election, if they so choose, other than any directors that holders of any preferred stock we have or may issue may be entitled to elect. Our Bylaws also provide that subject to the rights of the holders of any series of preferred stock then outstanding, any director or the entire Board may be removed from office at any time, with or without cause, by the affirmative vote of the holders of at least a majority of the voting power of the issued and outstanding shares of capital stock of the Company then entitled to vote in the election of directors.
Our Bylaws also provides that a special meeting of stockholders may be called only by our chairperson of the Board, chief executive officer or president, the secretary or any two directors.
Our Bylaws also establishes advance notice procedures with respect to certain stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors.
The Board is expressly empowered to adopt, amend or repeal the Bylaws. The stockholders shall also have power to adopt, amend or repeal the Bylaws; provided, however, that, in addition to any vote of the holders of any class or series of stock of the Company required by law or by the Certificate of Incorporation, the affirmative vote of the holders of at least a majority of the voting power of all of the then outstanding shares of capital stock of the Company entitled to vote generally in the election of directors, voting together as a single class, shall be required to adopt, amend or repeal any provision of the Bylaws.
The provisions of Delaware law, our Certificate of Incorporation and our Bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in the composition of our Board and management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Choice of Forum
Our Bylaws provide that unless we consent in writing to the selection of an alternative forum, the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders, (iii) any action asserting a claim arising pursuant to any provision of the DGCL or the Certificate of Incorporation or Bylaws (as either may be amended from time to time), or (iv) any action asserting a claim governed by the internal affairs doctrine shall be the Court of Chancery in the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware). If any action the subject matter of which is within the scope of the preceding sentence is filed in a court other than a court located within the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (i) the personal jurisdiction of the state and federal courts located within the State of Delaware in connection with any action brought in any such court to enforce the preceding sentence and (ii) having service of process made upon such stockholder in any such action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC. We act as the transfer agent and registrar for our Series A Preferred Stock. The transfer agent for any series of preferred stock that we may offer under this prospectus will be named and described in the prospectus supplement related to that series.
Listing on the Nasdaq Capital Market
Our common stock is listed on The Nasdaq Capital Market under the symbol “PALI.” The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any, on The Nasdaq Capital Market or any securities market or other exchange of the preferred stock covered by such prospectus supplement.
DESCRIPTION OF DEBT SECURITIES
We may issue debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. While the terms we have summarized below will apply generally to any debt securities that we may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities offered under a prospectus supplement may differ from the terms described below. Unless the context requires otherwise, whenever we refer to the indenture, we also are referring to any supplemental indentures that specify the terms of a particular series of debt securities.
We will issue the debt securities under the indenture that we will enter into with the trustee named in the indenture. The indenture will be qualified under the Trust Indenture Act of 1939, as amended, or the Trust Indenture Act. We have filed the form of indenture as an exhibit to the registration statement of which this prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered will be filed as exhibits to the registration statement of which this prospectus is a part or will be incorporated by reference from reports that we file with the SEC.
The following summary of material provisions of the debt securities and the indenture is subject to, and qualified in its entirety by reference to, all of the provisions of the indenture applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the debt securities that we may offer under this prospectus, as well as the complete indenture that contains the terms of the debt securities.
General
The indenture does not limit the amount of debt securities that we may issue. It provides that we may issue debt securities up to the principal amount that we may authorize and may be in any currency or currency unit that we may designate. Except for the limitations on consolidation, merger and sale of all or substantially all of our assets contained in the indenture, the terms of the indenture do not contain any covenants or other provisions designed to give holders of any debt securities protection against changes in our operations, financial condition or transactions involving us.
We may issue the debt securities issued under the indenture as “discount securities,” which means they may be sold at a discount below their stated principal amount. These debt securities, as well as other debt securities that are not issued at a discount, may be issued with “original issue discount,” or OID, for U.S. federal income tax purposes because of interest payment and other characteristics or terms of the debt securities. Material U.S. federal income tax considerations applicable to debt securities issued with OID will be described in more detail in any applicable prospectus supplement.
We will describe in the applicable prospectus supplement the terms of the series of debt securities being offered, including:
| | ● | the title of the series of debt securities; |
| | | |
| | ● | any limit upon the aggregate principal amount that may be issued; |
| | | |
| | ● | the maturity date or dates; |
| | | |
| | ● | the form of the debt securities of the series; |
| | | |
| | ● | the applicability of any guarantees; |
| | | |
| | ● | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| | | |
| | ● | whether the debt securities rank as senior debt, senior subordinated debt, subordinated debt or any combination thereof, and the terms of any subordination; |
| | ● | if the price (expressed as a percentage of the aggregate principal amount thereof) at which such debt securities will be issued is a price other than the principal amount thereof, the portion of the principal amount thereof payable upon declaration of acceleration of the maturity thereof, or if applicable, the portion of the principal amount of such debt securities that is convertible into another security or the method by which any such portion shall be determined; |
| | | |
| | ● | the interest rate or rates, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; |
| | | |
| | ● | our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| | | |
| | ● | if applicable, the date or dates after which, or the period or periods during which, and the price or prices at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; |
| | | |
| | ● | the date or dates, if any, on which, and the price or prices at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; |
| | | |
| | ● | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; |
| | | |
| | ● | any and all terms, if applicable, relating to any auction or remarketing of the debt securities of that series and any security for our obligations with respect to such debt securities and any other terms which may be advisable in connection with the marketing of debt securities of that series; |
| | | |
| | ● | whether the debt securities of the series shall be issued in whole or in part in the form of a global security or securities; the terms and conditions, if any, upon which such global security or securities may be exchanged in whole or in part for other individual securities; and the depositary for such global security or securities; |
| | | |
| | ● | if applicable, the provisions relating to conversion or exchange of any debt securities of the series and the terms and conditions upon which such debt securities will be so convertible or exchangeable, including the conversion or exchange price, as applicable, or how it will be calculated and may be adjusted, any mandatory or optional (at our option or the holders’ option) conversion or exchange features, the applicable conversion or exchange period and the manner of settlement for any conversion or exchange; |
| | | |
| | ● | if other than the full principal amount thereof, the portion of the principal amount of debt securities of the series which shall be payable upon declaration of acceleration of the maturity thereof; |
| | | |
| | ● | additions to or changes in the covenants applicable to the particular debt securities being issued, including, among others, the consolidation, merger or sale covenant; |
| | | |
| | ● | additions to or changes in the events of default with respect to the securities and any change in the right of the trustee or the holders to declare the principal, premium, if any, and interest, if any, with respect to such securities to be due and payable; |
| | | |
| | ● | additions to or changes in or deletions of the provisions relating to covenant defeasance and legal defeasance; |
| | | |
| | ● | additions to or changes in the provisions relating to satisfaction and discharge of the indenture; |
| | | |
| | ● | additions to or changes in the provisions relating to the modification of the indenture both with and without the consent of holders of debt securities issued under the indenture; |
| | | |
| | ● | the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S. dollars; |
| | ● | whether interest will be payable in cash or additional debt securities at our or the holders’ option and the terms and conditions upon which the election may be made; |
| | | |
| | ● | the terms and conditions, if any, upon which we will pay amounts in addition to the stated interest, premium, if any and principal amounts of the debt securities of the series to any holder that is not a “United States person” for federal tax purposes; |
| | | |
| | ● | any restrictions on transfer, sale or assignment of the debt securities of the series; and |
| | | |
| | ● | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, any other additions or changes in the provisions of the indenture, and any terms that may be required by us or advisable under applicable laws or regulations. |
Conversion or Exchange Rights
We will set forth in the applicable prospectus supplement the terms on which a series of debt securities may be convertible into or exchangeable for our common stock or our other securities. We will include provisions as to settlement upon conversion or exchange and whether conversion or exchange is mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of shares of our common stock or our other securities that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger or Sale
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the indenture will not contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose of our assets as an entirety or substantially as an entirety. However, any successor to or acquirer of such assets (other than a subsidiary of ours) must assume all of our obligations under the indenture or the debt securities, as appropriate.
Events of Default under the Indenture
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the following are events of default under the indenture with respect to any series of debt securities that we may issue:
| | ● | if we fail to pay any installment of interest on any series of debt securities, as and when the same shall become due and payable, and such default continues for a period of 90 days; provided, however, that a valid extension of an interest payment period by us in accordance with the terms of any indenture supplemental thereto shall not constitute a default in the payment of interest for this purpose; |
| | | |
| | ● | if we fail to pay the principal of, or premium, if any, on any series of debt securities as and when the same shall become due and payable whether at maturity, upon redemption, by declaration or otherwise, or in any payment required by any sinking or analogous fund established with respect to such series; provided, however, that a valid extension of the maturity of such debt securities in accordance with the terms of any indenture supplemental thereto shall not constitute a default in the payment of principal or premium, if any; |
| | | |
| | ● | if we fail to observe or perform any other covenant or agreement contained in the debt securities or the indenture, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive written notice of such failure, requiring the same to be remedied and stating that such is a notice of default thereunder, from the trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and |
| | | |
| | ● | if specified events of bankruptcy, insolvency or reorganization occur. |
If an event of default with respect to debt securities of any series occurs and is continuing, other than an event of default specified in the last bullet point above, the trustee or the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series, by notice to us in writing, and to the trustee if notice is given by such holders, may declare the unpaid principal of, premium, if any, and accrued interest, if any, due and payable immediately. If an event of default specified in the last bullet point above occurs with respect to us, the principal amount of and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice or other action on the part of the trustee or any holder.
The holders of a majority in principal amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indenture, if an event of default under an indenture shall occur and be continuing, the trustee will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such holders have offered the trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee, or exercising any trust or power conferred on the trustee, with respect to the debt securities of that series, provided that:
| | ● | the direction so given by the holder is not in conflict with any law or the applicable indenture; and |
| | | |
| | ● | subject to its duties under the Trust Indenture Act, the trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
A holder of the debt securities of any series will have the right to institute a proceeding under the indenture or to appoint a receiver or trustee, or to seek other remedies only if:
| | ● | the holder has given written notice to the trustee of a continuing event of default with respect to that series; |
| | ● | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request; |
| | ● | such holders have offered to the trustee indemnity satisfactory to it against the costs, expenses and liabilities to be incurred by the trustee in compliance with the request; and |
| | ● | the trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the trustee regarding our compliance with specified covenants in the indenture.
Modification of Indenture; Waiver
We and the trustee may change an indenture without the consent of any holders with respect to specific matters:
| | ● | to cure any ambiguity, defect or inconsistency in the indenture or in the debt securities of any series; |
| | | |
| | ● | to comply with the provisions described above under the heading “Description of Debt Securities—Consolidation, Merger or Sale”; |
| | | |
| | ● | to provide for uncertificated debt securities in addition to or in place of certificated debt securities; |
| | ● | to add to our covenants, restrictions, conditions or provisions such new covenants, restrictions, conditions or provisions for the benefit of the holders of all or any series of debt securities, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default or to surrender any right or power conferred upon us in the indenture; |
| | | |
| | ● | to add to, delete from or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue, authentication and delivery of debt securities, as set forth in the indenture; |
| | | |
| | ● | to make any change that does not adversely affect the interests of any holder of debt securities of any series in any material respect; |
| | | |
| | ● | to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided above under the heading “Description of Debt Securities—General” to establish the form of any certifications required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities; |
| | | |
| | ● | to evidence and provide for the acceptance of appointment under any indenture by a successor trustee; or |
| | | |
| | ● | to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act. |
In addition, under the indenture, the rights of holders of a series of debt securities may be changed by us and the trustee with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, we and the trustee may make the following changes only with the consent of each holder of any outstanding debt securities affected:
| | ● | extending the fixed maturity of any debt securities of any series; |
| | | |
| | ● | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption of any series of any debt securities; or |
| | | |
| | ● | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver. |
Discharge
Each indenture provides that we can elect to be discharged from our obligations with respect to one or more series of debt securities, except for specified obligations, including obligations to:
| | ● | provide for payment; |
| | | |
| | ● | register the transfer or exchange of debt securities of the series; |
| | | |
| | ● | replace stolen, lost or mutilated debt securities of the series; |
| | | |
| | ● | pay principal of and premium and interest on any debt securities of the series; |
| | | |
| | ● | maintain paying agencies; |
| | | |
| | ● | hold monies for payment in trust; |
| | | |
| | ● | recover excess money held by the trustee; |
| | | |
| | ● | compensate and indemnify the trustee; and |
| | | |
| | ● | appoint any successor trustee. |
In order to exercise our rights to be discharged, we must deposit with the trustee money or government obligations sufficient to pay all the principal of, any premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series only in fully registered form without coupons and, unless we provide otherwise in the applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indenture provides that we may issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company, or DTC, or another depositary named by us and identified in the applicable prospectus supplement with respect to that series. To the extent the debt securities of a series are issued in global form and as book-entry, a description of terms relating to any book-entry securities will be set forth in the applicable prospectus supplement.
At the option of the holder, subject to the terms of the indenture and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the indenture and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will impose no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt securities of each series.
If we elect to redeem the debt securities of any series, we will not be required to:
| | ● | issue, register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or |
| | | |
| | ● | register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Trustee
The trustee, other than during the occurrence and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture, the trustee must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the trustee is under no obligation to exercise any of the powers given it by the indenture at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur.
Payment and Paying Agents
Unless we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for the interest.
We will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except that unless we otherwise indicate in the applicable prospectus supplement, we will make interest payments by check that we will mail to the holder or by wire transfer to certain holders. Unless we otherwise indicate in the applicable prospectus supplement, we will designate the corporate trust office of the trustee as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter may look only to us for payment thereof.
Governing Law
The indenture and the debt securities will be governed by and construed in accordance with the internal laws of the State of New York, except to the extent that the Trust Indenture Act of 1939 is applicable.
DESCRIPTION OF WARRANTS
The following description, together with the additional information we may include in any applicable prospectus supplement and free writing prospectus, summarizes the material terms and provisions of the warrants that we may offer under this prospectus, which may consist of warrants to purchase common stock, preferred stock or debt securities and may be issued in one or more series. Warrants may be offered independently or in combination with common stock, preferred stock or debt securities offered by any prospectus supplement. While the terms we have summarized below will apply generally to any warrants that we may offer under this prospectus, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. The following description of warrants will apply to the warrants offered by this prospectus unless we provide otherwise in the applicable prospectus supplement. The applicable prospectus supplement for a particular series of warrants may specify different or additional terms.
We have filed forms of the warrant agreements and forms of warrant certificates containing the terms of the warrants that may be offered as exhibits to the registration statement of which this prospectus is a part. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of warrant and/or the warrant agreement and warrant certificate, as applicable, that contain the terms of the particular series of warrants we are offering, and any supplemental agreements, before the issuance of such warrants. The following summaries of material terms and provisions of the warrants are subject to, and qualified in their entirety by reference to, all the provisions of the form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements applicable to a particular series of warrants that we may offer under this prospectus. We urge you to read the applicable prospectus supplement related to the particular series of warrants that we may offer under this prospectus, as well as any related free writing prospectus, and the complete form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements, that contain the terms of the warrants.
General
We will describe in the applicable prospectus supplement the terms of the series of warrants being offered, including:
| | ● | the offering price and aggregate number of warrants offered; |
| | | |
| | ● | the currency for which the warrants may be purchased; |
| | | |
| | ● | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| | | |
| | ● | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise; |
| | | |
| | ● | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; |
| | | |
| | ● | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; |
| | | |
| | ● | the terms of any rights to redeem or call the warrants; |
| | | |
| | ● | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| | | |
| | ● | the dates on which the right to exercise the warrants will commence and expire; |
| | | |
| | ● | the manner in which the warrant agreements and warrants may be modified; |
| | ● | a discussion of any material or special U.S. federal income tax considerations of holding or exercising the warrants; |
| | | |
| | ● | the terms of the securities issuable upon exercise of the warrants; and |
| | | |
| | ● | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
| | ● | in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; or |
| | | |
| | ● | in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any. |
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. The warrants may be exercised as set forth in the prospectus supplement relating to the warrants offered. Unless we otherwise specify in the applicable prospectus supplement, warrants may be exercised at any time up to the close of business on the expiration date set forth in the prospectus supplement relating to the warrants offered thereby. After the close of business on the expiration date, unexercised warrants will become void.
Upon receipt of payment and the warrant or warrant certificate, as applicable, properly completed and duly executed at the corporate trust office of the warrant agent, if any, or any other office, including ours, indicated in the prospectus supplement, we will, as soon as practicable, issue and deliver the securities purchasable upon such exercise. If less than all of the warrants (or the warrants represented by such warrant certificate) are exercised, a new warrant or a new warrant certificate, as applicable, will be issued for the remaining warrants.
Governing Law
Unless we otherwise specify in the applicable prospectus supplement, the warrants and any warrant agreements will be governed by and construed in accordance with the laws of the State of New York.
Enforceability of Rights by Holders of Warrants
Each warrant agent, if any, will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, its warrants.
LEGAL OWNERSHIP OF SECURITIES
We can issue securities in registered form or in the form of one or more global securities. We describe global securities in greater detail below. We refer to those persons who have securities registered in their own names on the books that we or any applicable trustee, depositary or warrant agent maintain for this purpose as the “holders” of those securities. These persons are the legal holders of the securities. We refer to those persons who, indirectly through others, own beneficial interests in securities that are not registered in their own names, as “indirect holders” of those securities. As we discuss below, indirect holders are not legal holders, and investors in securities issued in book-entry form or in street name will be indirect holders.
Book-Entry Holders
We may issue securities in book-entry form only, as we will specify in the applicable prospectus supplement. This means securities may be represented by one or more global securities registered in the name of a financial institution that holds them as depositary on behalf of other financial institutions that participate in the depositary’s book-entry system. These participating institutions, which are referred to as participants, in turn, hold beneficial interests in the securities on behalf of themselves or their customers.
Only the person in whose name a security is registered is recognized as the holder of that security. Securities issued in global form will be registered in the name of the depositary or its participants. Consequently, for securities issued in global form, we will recognize only the depositary as the holder of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the payments it receives to its participants, which in turn pass the payments along to their customers who are the beneficial owners. The depositary and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so under the terms of the securities.
As a result, investors in a global security will not own securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other financial institution that participates in the depositary’s book-entry system or holds an interest through a participant. As long as the securities are issued in global form, investors will be indirect holders, and not legal holders, of the securities.
Street Name Holders
We may terminate a global security or issue securities in non-global form. In these cases, investors may choose to hold their securities in their own names or in “street name.” Securities held by an investor in street name would be registered in the name of a bank, broker or other financial institution that the investor chooses, and the investor would hold only a beneficial interest in those securities through an account he or she maintains at that institution.
For securities held in street name, we or any applicable trustee or depositary will recognize only the intermediary banks, brokers and other financial institutions in whose names the securities are registered as the holders of those securities, and we or any applicable trustee or depositary will make all payments on those securities to them. These institutions pass along the payments they receive to their customers who are the beneficial owners, but only because they agree to do so in their customer agreements or because they are legally required to do so. Investors who hold securities in street name will be indirect holders, not holders, of those securities.
Legal Holders
Our obligations, as well as the obligations of any applicable trustee and of any third parties employed by us or a trustee, run only to the legal holders of the securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect means. This will be the case whether an investor chooses to be an indirect holder of a security or has no choice because we are issuing the securities only in global form.
For example, once we make a payment or give a notice to the legal holder, we have no further responsibility for the payment or notice even if that legal holder is required, under agreements with its participants or customers or by law, to pass it along to the indirect holders but does not do so. Similarly, we may want to obtain the approval of the legal holders to amend an indenture, to relieve us of the consequences of a default or of our obligation to comply with a particular provision of the indenture or for other purposes. In such an event, we would seek approval only from the holders, and not the indirect holders, of the securities. Whether and how the legal holders contact the indirect holders is up to the legal holders.
Special Considerations for Indirect Holders
If you hold securities through a bank, broker or other financial institution, either in book-entry form because the securities are represented by one or more global securities or in street name, you should check with your own institution to find out:
| | ● | how it handles securities payments and notices; |
| | | |
| | ● | whether it imposes fees or charges; |
| | | |
| | ● | how it would handle a request for the holders’ consent, if ever required; |
| | | |
| | ● | whether and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in the future; |
| | | |
| | ● | how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect their interests; and |
| | | |
| | ● | if the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters. |
Global Securities
A global security is a security that represents one or any other number of individual securities held by a depositary. Generally, all securities represented by the same global securities will have the same terms.
Each security issued in book-entry form will be represented by a global security that we issue to, deposit with and register in the name of a financial institution or its nominee that we select. The financial institution that we select for this purpose is called the depositary. Unless we specify otherwise in the applicable prospectus supplement, DTC will be the depositary for all securities issued in book-entry form.
A global security may not be transferred to or registered in the name of anyone other than the depositary, its nominee or a successor depositary, unless special termination situations arise. We describe those situations below under the heading “Special Situations When a Global Security Will Be Terminated.” As a result of these arrangements, the depositary, or its nominee, will be the sole registered owner and legal holder of all securities represented by a global security, and investors will be permitted to own only beneficial interests in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial institution that in turn has an account with the depositary or with another institution that does. Thus, an investor whose security is represented by a global security will not be a legal holder of the security, but only an indirect holder of a beneficial interest in the global security.
If the prospectus supplement for a particular security indicates that the security will be issued in global form only, then the security will be represented by a global security at all times unless and until the global security is terminated. If termination occurs, we may issue the securities through another book-entry clearing system or decide that the securities may no longer be held through any book-entry clearing system.
Special Considerations for Global Securities
The rights of an indirect holder relating to a global security will be governed by the account rules of the investor’s financial institution and of the depositary, as well as general laws relating to securities transfers. We do not recognize an indirect holder as a holder of securities and instead deal only with the depositary that holds the global security.
If securities are issued only in the form of a global security, an investor should be aware of the following:
| | ● | an investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her interest in the securities, except in the special situations we describe below; |
| | | |
| | ● | an investor will be an indirect holder and must look to his or her own bank, broker or other financial institution for payments on the securities and protection of his or her legal rights relating to the securities, as we describe above; |
| | | |
| | ● | an investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required by law to own their securities in non-book-entry form; |
| | | |
| | ● | an investor may not be able to pledge his or her interest in a global security in circumstances where certificates representing the securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective; |
| | | |
| | ● | the depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in a global security; |
| | | |
| | ● | we and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in a global security, nor do we or any applicable trustee supervise the depositary in any way; |
| | | |
| | ● | the depositary may, and we understand that DTC will, require that those who purchase and sell interests in a global security within its book-entry system use immediately available funds, and your bank, broker or other financial institution may require you to do so as well; and |
| | | |
| | ● | financial institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in a global security, may also have their own policies affecting payments, notices and other matters relating to the securities. |
There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries.
Special Situations When a Global Security Will Be Terminated
In a few special situations described below, the global security will terminate and interests in it will be exchanged for physical certificates representing those interests. After that exchange, the choice of whether to hold securities directly or in street name will be up to the investor. Investors must consult their own banks, brokers or other financial institutions to find out how to have their interests in securities transferred to their own name, so that they will be direct holders. We have described the rights of holders and street name investors above.
Unless we provide otherwise in the applicable prospectus supplement, the global security will terminate when the following special situations occur:
| | ● | if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security and we do not appoint another institution to act as depositary within 90 days; |
| | | |
| | ● | if we notify any applicable trustee that we wish to terminate that global security; or |
| | | |
| | ● | if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived. |
The applicable prospectus supplement may also list additional situations for terminating a global security that would apply only to the particular series of securities covered by the applicable prospectus supplement. When a global security terminates, the depositary, and not we or any applicable trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
PLAN OF DISTRIBUTION
We may sell the securities from time to time pursuant to underwritten public offerings, direct sales to the public, negotiated transactions, block trades or a combination of these methods. A distribution of these securities offered by this prospectus may also be effected through the issuance of derivative securities, including, without limitation, warrants. We may sell the securities to or through underwriters or dealers, through agents, or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
| | ● | at a fixed price or prices, which may be changed; |
| | | |
| | ● | at market prices prevailing at the time of sale; |
| | | |
| | ● | at prices related to the prevailing market prices; or |
| | | |
| | ● | at negotiated prices. |
We may also sell equity securities covered by this registration statement in an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act. Such offering may be made into an existing trading market for such securities in transactions at other than a fixed price, either:
| | ● | on or through the facilities of The Nasdaq Capital Market or any other securities exchange or quotation or trading service on which such securities may be listed, quoted or traded at the time of sale; and/or |
| | | |
| | ● | to or through a market maker other than on The Nasdaq Capital Market or such other securities exchanges or quotation or trading services. |
Such at-the-market offerings, if any, may be conducted by underwriters acting as principal or agent.
Each time we offer and sell securities covered by this prospectus, we will provide a prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) that will describe the method of distribution and set forth the terms of the offering, including:
| | ● | the name or names of any underwriters, dealers or agents, if any; |
| | | |
| | ● | the amounts of securities underwritten or purchased by each of them; |
| | | |
| | ● | the purchase price of securities or other consideration therefore, and the proceeds, if any, we will receive from the sale; |
| | | |
| | ● | any over-allotment options under which underwriters may purchase additional securities from us; |
| | | |
| | ● | any underwriting discounts or commissions or agency fees and other items constituting underwriters’ or agents’ compensation; |
| | | |
| | ● | any public offering price of the securities; |
| | | |
| | ● | any indemnification of the underwriters or their controlling persons against liability arising under the Securities Act; |
| | | |
| | ● | any discounts, commissions or concessions allowed or reallowed or paid to dealers; and |
| | | |
| | ● | any securities exchange or market on which the securities may be listed. |
Only underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement, other than securities covered by any over-allotment option. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities, and we will describe any commissions and other compensation we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We may provide agents and underwriters with indemnification against civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
All securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any agents or underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities. There is currently no market for any of the offered securities, other than our common stock which is listed on The Nasdaq Capital Market. We have no current plans for listing of the preferred stock, debt securities or warrants on any securities exchange or quotation system; any such listing with respect to any particular preferred stock, debt securities or warrants will be described in the applicable prospectus supplement or other offering materials, as the case may be.
Any underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Rule 103 of Regulation M under the Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any agents and underwriters who are qualified market makers on The Nasdaq Capital Market may engage in passive market making transactions in the securities on The Nasdaq Capital Market in accordance with Regulation M under the Exchange Act, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
In compliance with guidelines of the Financial Industry Regulatory Authority, or FINRA, the maximum consideration or discount to be received by any FINRA member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus and any applicable prospectus supplement.
EXPERTS
The consolidated financial statements of Palisade Bio, Inc. as of December 31, 2021 and 2020 and for the years then ended incorporated by reference in this Prospectus and in the Registration Statement have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and accounting. The report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
LEGAL MATTERS
Certain legal matters, including the validity of the shares of common stock offered pursuant to this registration statement, will be passed upon for us by Cooley LLP, San Diego, California.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of the registration statement on Form S-3 we filed with the SEC under the Securities Act and does not contain all the information set forth or incorporated by reference in the registration statement. Whenever a reference is made in this prospectus to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated by reference into this prospectus for a copy of such contract, agreement or other document. Because we are subject to the information and reporting requirements of the Exchange Act, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, including any amendments to those reports, and other information that we file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act can also be accessed free of charge on the Investor section of our website. These filings will be available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Our website address is www. palisadebio.com. Information contained on or accessible through our website is not a part of this prospectus and is not incorporated by reference herein, and the inclusion of our website address in this prospectus is an inactive textual reference only.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information to you by referring to those documents. The information incorporated by reference is an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede this information.
We incorporate by reference the following documents we filed with the SEC pursuant to Section 13 of the Exchange Act and any future filings we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of this prospectus until the termination of the offering of the shares covered by this prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K):
| | ● | our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 17, 2022, including the information that will be incorporated by reference therein upon the filing of our Definitive Proxy Statement on Schedule 14A to be filed with the SEC; |
| | | |
| | ● | our Definitive Proxy Statement on Schedule 14A, filed with the SEC on October 7, 2021 (other than the portions thereof which are furnished and not filed); |
| | ● | our Current Reports on Form 8-K filed on February 1, 2022 and February 24, 2022; |
| | | |
| | ● | the description of our common stock which is registered under Section 12 of the Exchange Act, in our registration statement on Form 8-A filed with the SEC on July 1, 2015, including any amendments or reports filed for the purpose of updating such description, including Exhibit 4.2 to our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 17, 2022; |
We will furnish without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request, a copy of any document incorporated by reference. Requests should be addressed to 5800 Armada Drive, Suite 210, Carlsbad, CA 92008, Attn: Secretary or may be made telephonically at (858) 704-4900.
You should rely only on the information incorporated by reference or provided in this prospectus or any prospectus supplement. We have not authorized anyone to provide you with different information. You should not assume that the information contained in this prospectus or the accompanying prospectus supplement is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus and any accompanying prospectus supplement is delivered or securities are sold on a later date.

756,317 Shares of Common Stock
PROSPECTUS SUPPLEMENT
Ladenburg Thalmann
April 3, 2023


