
Investor Presentation Building a powerful new future in cellular IO October 2020 Exhibit 99.2

Forward Looking Statement This presentation contains estimates, projections and other forward-looking statements, concerning, among other things: our research and development activities relating to our GoCAR™ platform, and related technologies; our product candidates including BPX-601, BPX-603, and rimiducid; the timing and success of our current and planned clinical trials, including the timing of receipt of data from such clinical trials and the timing of our reports of such data; the possible range of applications of our cell therapy programs and potential curative effects and safety in the treatment of diseases, including as compared to other treatment options and competitive therapies; and our near-term restructuring plan, including focus of our clinical and research and development activities, repayment of our outstanding obligations under our credit facility with Oxford Finance, reduction in employee headcount and reduction in cash utilization. Our estimates, projections and other forward-looking statements are based on management's current assumptions and expectations of future events and trends, which affect or may affect our business, strategy, operations or financial performance. Although we believe that these estimates, projections and other forward-looking statements are based upon reasonable assumptions, they are subject to numerous known and unknown risks and uncertainties and are made in light of information currently available to us. Many important factors, in addition to the factors described in this presentation, may adversely and materially affect our results as indicated in forward-looking statements. All statements other than statements of historical fact are forward-looking statements. Estimates, projections and other forward-looking statements speak only as of the date they were made, and, except to the extent required by law, we undertake no obligation to update any forward-looking statement. These statements are also subject to a number of material risks and uncertainties that are described more fully in Bellicum’s filings with the Securities and Exchange Commission, including without limitation our annual report on Form 10-K for the year ended December 31, 2019 and our quarterly report on Form 10-Q for the period ended June 30, 2020.
Power Re-ignites the host immune response, unleashing the power to combat tumor tolerance and intensify tumor killing Proliferation Boosts effector cell proliferation and extends survival, potentially leading to more durable responses Persistence Enhances effector cell functional persistence by resisting exhaustion and inhibitory signals from the tumor environment Performance Molecular switch technology enables superior control over GoCAR cells Building a Powerful New Future in Cellular IO Our GoCAR platform is engineered to break through the limitations of current cell therapies
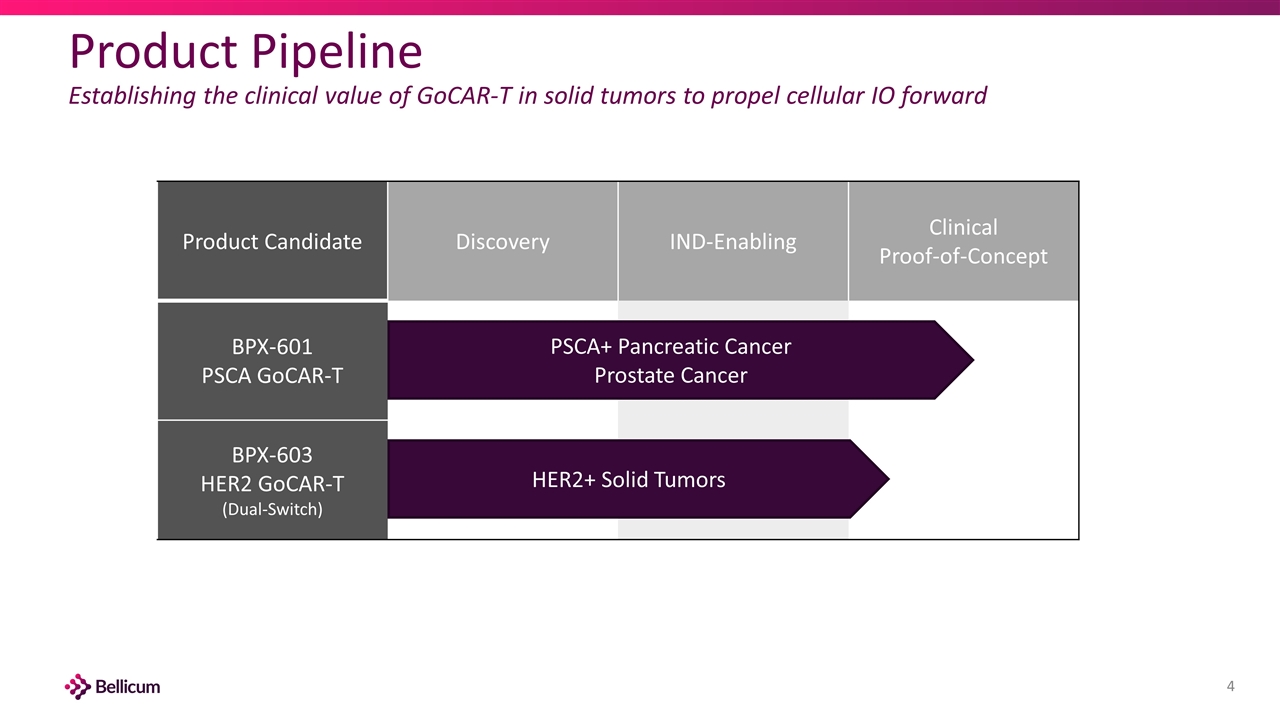
Product Candidate Discovery IND-Enabling Clinical Proof-of-Concept BPX-601 PSCA GoCAR-T BPX-603 HER2 GoCAR-T (Dual-Switch) Product Pipeline Establishing the clinical value of GoCAR-T in solid tumors to propel cellular IO forward PSCA+ Pancreatic Cancer Prostate Cancer HER2+ Solid Tumors

Technology Overview
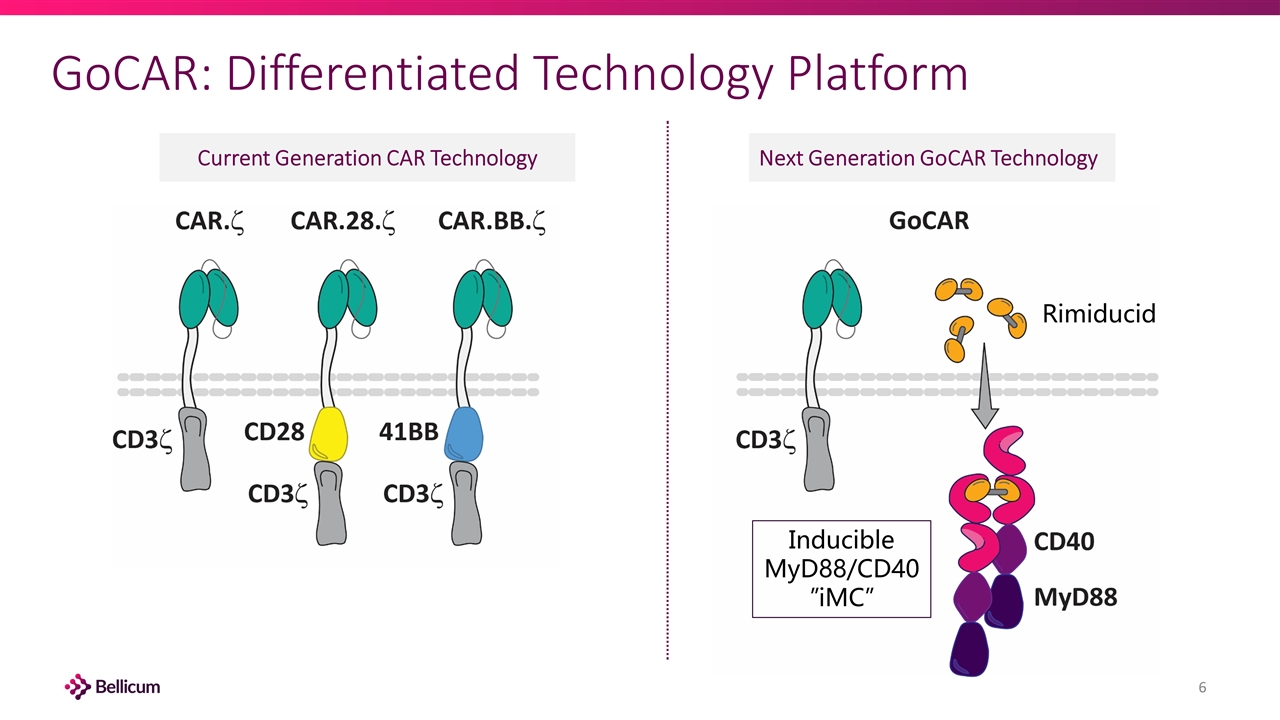
GoCAR: Differentiated Technology Platform Current Generation CAR Technology Next Generation GoCAR Technology Rimiducid Inducible MyD88/CD40 ”iMC”
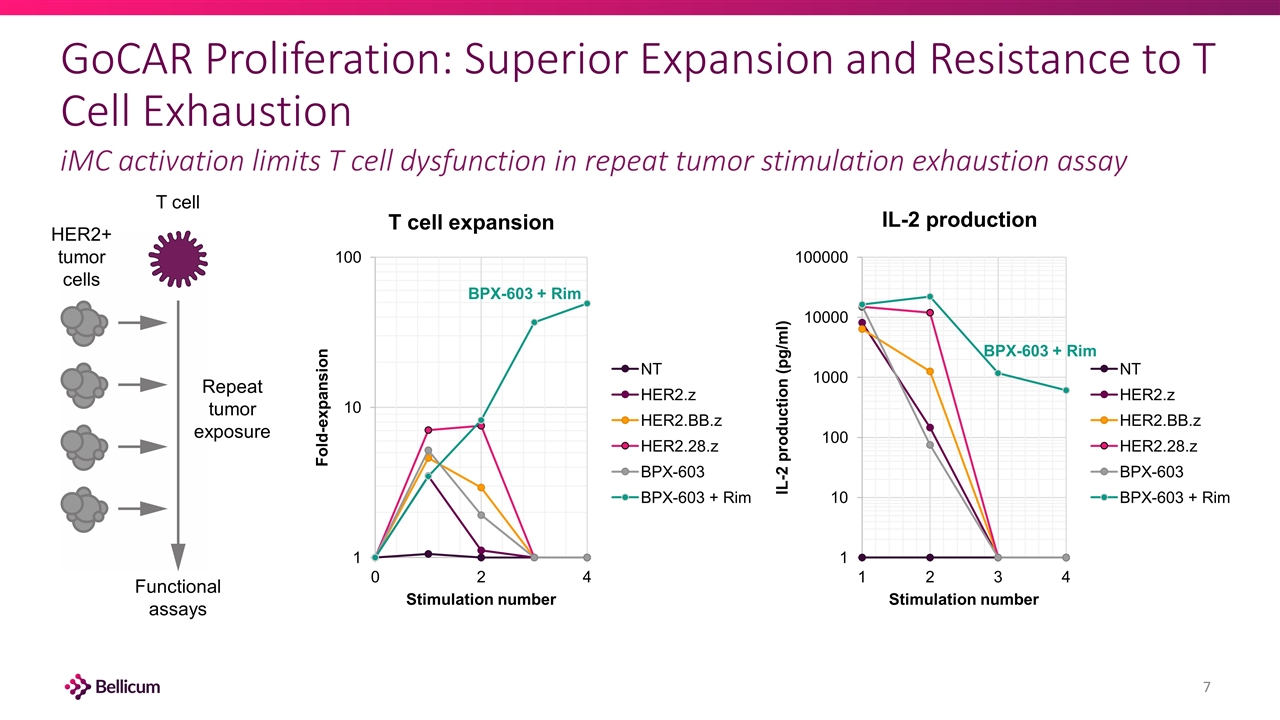
GoCAR Proliferation: Superior Expansion and Resistance to T Cell Exhaustion iMC activation limits T cell dysfunction in repeat tumor stimulation exhaustion assay Repeat tumor exposure T cell Functional assays HER2+ tumor cells BPX-603 + Rim BPX-603 + Rim
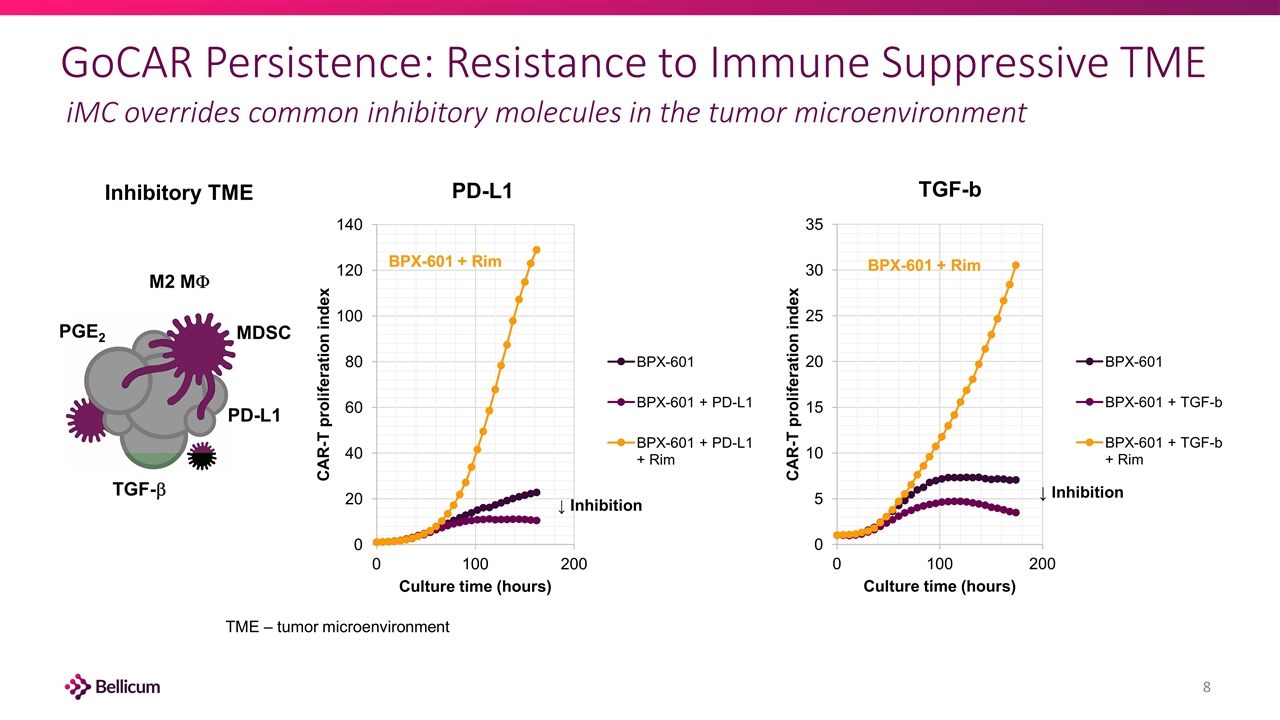
GoCAR Persistence: Resistance to Immune Suppressive TME iMC overrides common inhibitory molecules in the tumor microenvironment PD-L1 TGF-b PGE2 M2 MF MDSC BPX-601 + Rim BPX-601 + Rim ↓ Inhibition ↓ Inhibition Inhibitory TME TME – tumor microenvironment

BPX-601 PSCA GoCAR-T
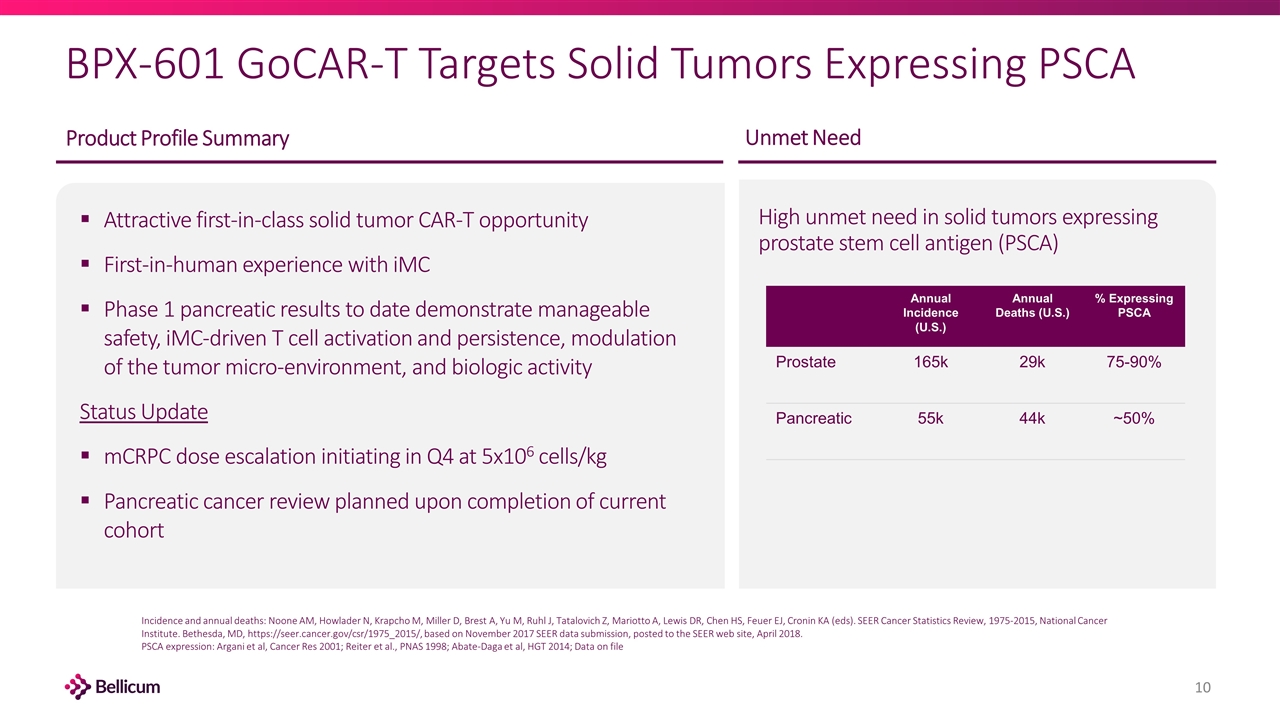
BPX-601 GoCAR-T Targets Solid Tumors Expressing PSCA High unmet need in solid tumors expressing prostate stem cell antigen (PSCA) Attractive first-in-class solid tumor CAR-T opportunity First-in-human experience with iMC Phase 1 pancreatic results to date demonstrate manageable safety, iMC-driven T cell activation and persistence, modulation of the tumor micro-environment, and biologic activity Status Update mCRPC dose escalation initiating in Q4 at 5x106 cells/kg Pancreatic cancer review planned upon completion of current cohort Unmet Need Product Profile Summary Annual Incidence (U.S.) Annual Deaths (U.S.) % Expressing PSCA Prostate 165k 29k 75-90% Pancreatic 55k 44k ~50% Incidence and annual deaths: Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. PSCA expression: Argani et al, Cancer Res 2001; Reiter et al., PNAS 1998; Abate-Daga et al, HGT 2014; Data on file
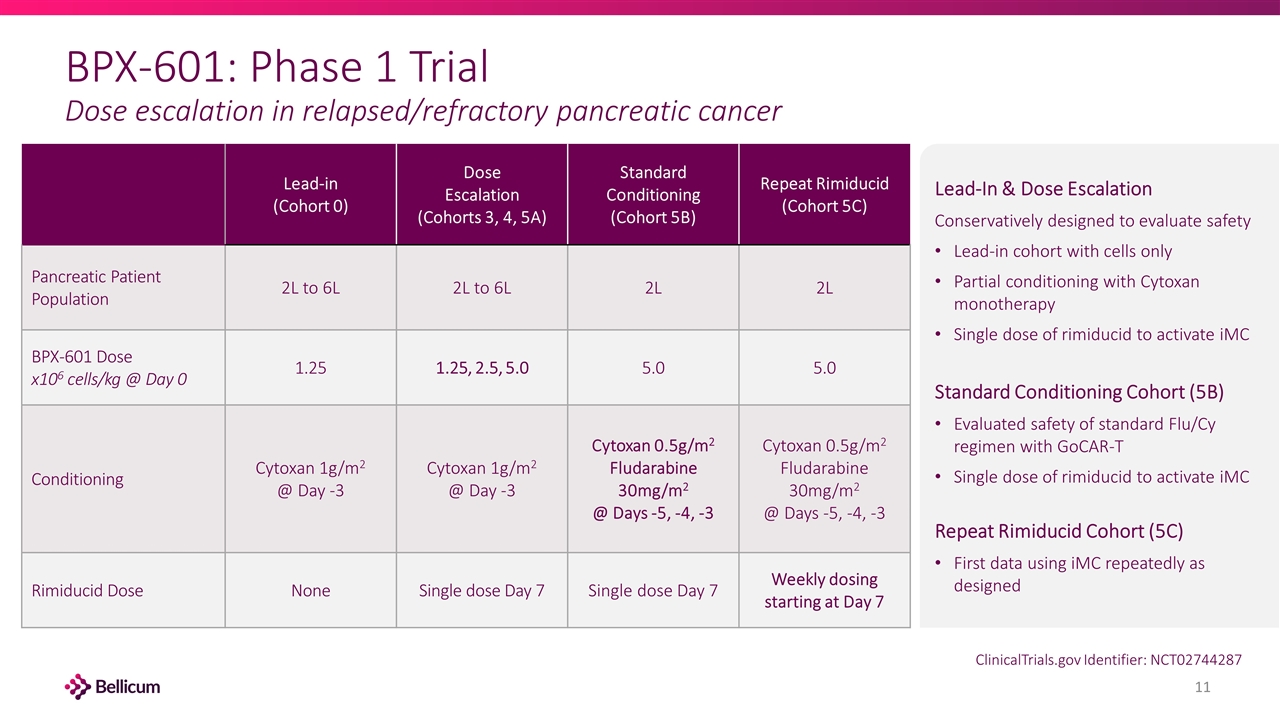
BPX-601: Phase 1 Trial Dose escalation in relapsed/refractory pancreatic cancer Lead-in (Cohort 0) Dose Escalation (Cohorts 3, 4, 5A) Standard Conditioning (Cohort 5B) Repeat Rimiducid (Cohort 5C) Pancreatic Patient Population 2L to 6L 2L to 6L 2L 2L BPX-601 Dose x106 cells/kg @ Day 0 1.25 1.25, 2.5, 5.0 5.0 5.0 Conditioning Cytoxan 1g/m2 @ Day -3 Cytoxan 1g/m2 @ Day -3 Cytoxan 0.5g/m2 Fludarabine 30mg/m2 @ Days -5, -4, -3 Cytoxan 0.5g/m2 Fludarabine 30mg/m2 @ Days -5, -4, -3 Rimiducid Dose None Single dose Day 7 Single dose Day 7 Weekly dosing starting at Day 7 Lead-In & Dose Escalation Conservatively designed to evaluate safety Lead-in cohort with cells only Partial conditioning with Cytoxan monotherapy Single dose of rimiducid to activate iMC Standard Conditioning Cohort (5B) Evaluated safety of standard Flu/Cy regimen with GoCAR-T Single dose of rimiducid to activate iMC Repeat Rimiducid Cohort (5C) First data using iMC repeatedly as designed ClinicalTrials.gov Identifier: NCT02744287
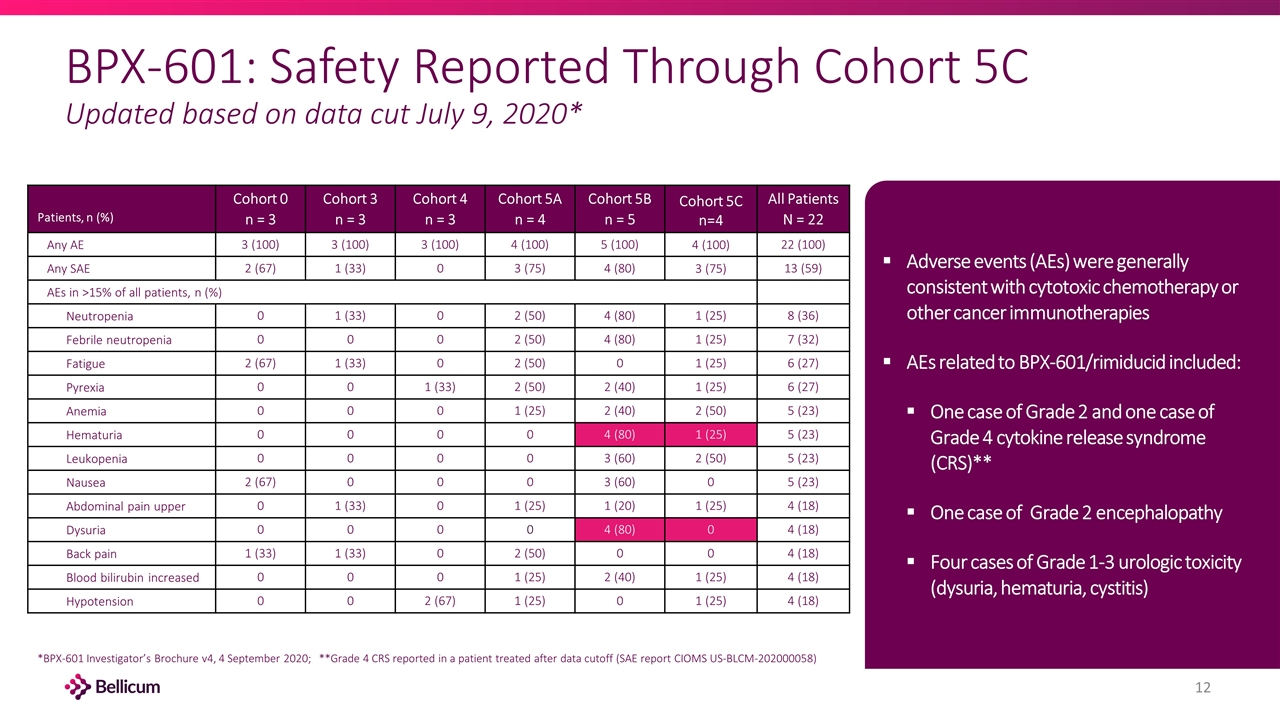
BPX-601: Safety Reported Through Cohort 5C Updated based on data cut July 9, 2020* Patients, n (%) Cohort 0 n = 3 Cohort 3 n = 3 Cohort 4 n = 3 Cohort 5A n = 4 Cohort 5B n = 5 Cohort 5C n=4 All Patients N = 22 Any AE 3 (100) 3 (100) 3 (100) 4 (100) 5 (100) 4 (100) 22 (100) Any SAE 2 (67) 1 (33) 0 3 (75) 4 (80) 3 (75) 13 (59) AEs in >15% of all patients, n (%) Neutropenia 0 1 (33) 0 2 (50) 4 (80) 1 (25) 8 (36) Febrile neutropenia 0 0 0 2 (50) 4 (80) 1 (25) 7 (32) Fatigue 2 (67) 1 (33) 0 2 (50) 0 1 (25) 6 (27) Pyrexia 0 0 1 (33) 2 (50) 2 (40) 1 (25) 6 (27) Anemia 0 0 0 1 (25) 2 (40) 2 (50) 5 (23) Hematuria 0 0 0 0 4 (80) 1 (25) 5 (23) Leukopenia 0 0 0 0 3 (60) 2 (50) 5 (23) Nausea 2 (67) 0 0 0 3 (60) 0 5 (23) Abdominal pain upper 0 1 (33) 0 1 (25) 1 (20) 1 (25) 4 (18) Dysuria 0 0 0 0 4 (80) 0 4 (18) Back pain 1 (33) 1 (33) 0 2 (50) 0 0 4 (18) Blood bilirubin increased 0 0 0 1 (25) 2 (40) 1 (25) 4 (18) Hypotension 0 0 2 (67) 1 (25) 0 1 (25) 4 (18) Adverse events (AEs) were generally consistent with cytotoxic chemotherapy or other cancer immunotherapies AEs related to BPX-601/rimiducid included: One case of Grade 2 and one case of Grade 4 cytokine release syndrome (CRS)** One case of Grade 2 encephalopathy Four cases of Grade 1-3 urologic toxicity (dysuria, hematuria, cystitis) *BPX-601 Investigator’s Brochure v4, 4 September 2020; **Grade 4 CRS reported in a patient treated after data cutoff (SAE report CIOMS US-BLCM-202000058)
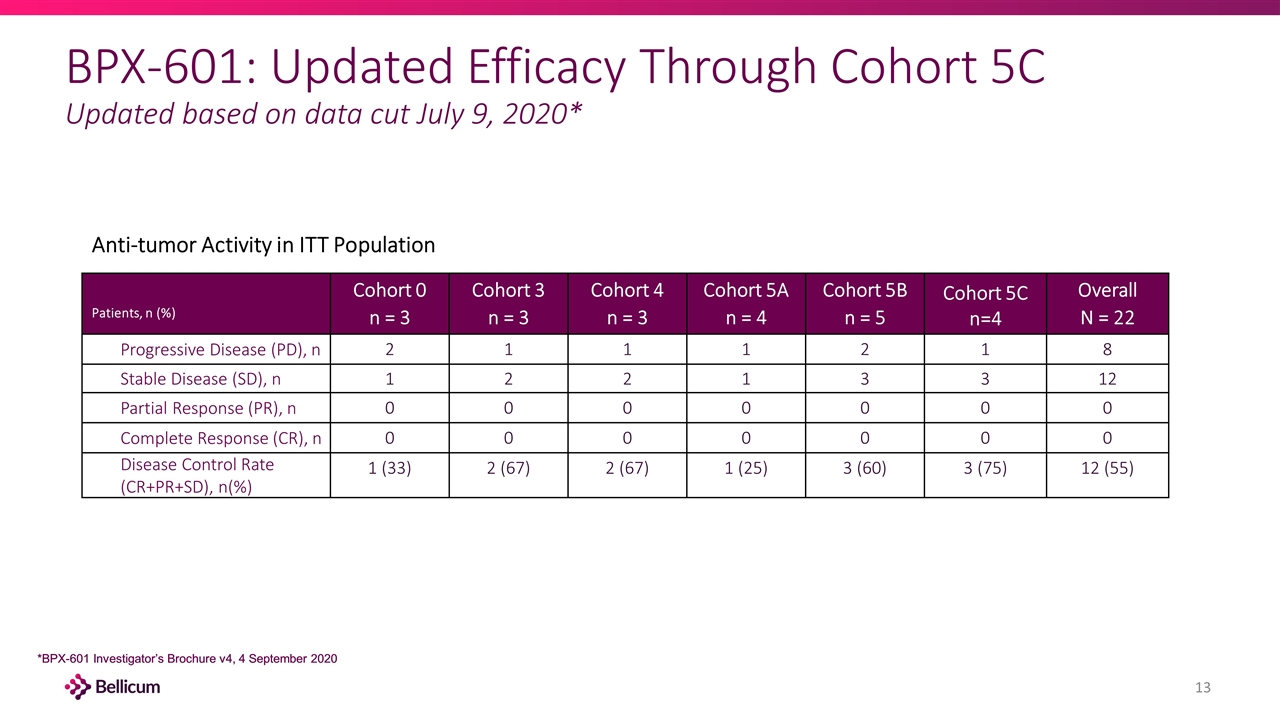
*BPX-601 Investigator’s Brochure v4, 4 September 2020 BPX-601: Updated Efficacy Through Cohort 5C Updated based on data cut July 9, 2020* *BPX-601 Investigator’s Brochure v4, 4 September 2020 Patients, n (%) Cohort 0 n = 3 Cohort 3 n = 3 Cohort 4 n = 3 Cohort 5A n = 4 Cohort 5B n = 5 Cohort 5C n=4 Overall N = 22 Progressive Disease (PD), n 2 1 1 1 2 1 8 Stable Disease (SD), n 1 2 2 1 3 3 12 Partial Response (PR), n 0 0 0 0 0 0 0 Complete Response (CR), n 0 0 0 0 0 0 0 Disease Control Rate (CR+PR+SD), n(%) 1 (33) 2 (67) 2 (67) 1 (25) 3 (60) 3 (75) 12 (55) Anti-tumor Activity in ITT Population
Interim Update: BPX-601 Cohort 5C As of July 6, 2020, four patients treated with BPX-601 followed by 2-11 doses of rimiducid in cohort 5C Administration of BPX-601 and repeat doses of rimiducid was tolerated No treatment related adverse events ≥Grade 2 observed in these four patients; one treatment-related SAE (Grade 4 cytokine release syndrome) was reported in a patient treated after data cutoff One genitourinary toxicity event reported (Grade 1 intermittent hematuria) Four events of Grade 1 neurotoxicity reported in two patients (neurotoxicity, dysgraphia, and confusion x2) Safety profile otherwise consistent with previous reports Best Overall Response: 3 Stable Disease and 1 Progressive Disease Evidence of repeat rimiducid-mediated CAR-T cell activation was observed Rimiducid administration was associated with increased serum cytokine levels, including IL-5, TNF-�, and IFN-� Rimiducid treatment was also associated with increased expression of activation markers (e.g. CD25) on peripheral CD4+ and CD8+ T cells, indicative of systemic immune modulation via BPX-601 iMC activation In two evaluable subjects receiving >2 doses of rimiducid, repeat dosing was not shown to increase peak or AUC circulating BPX-601 cells relative to single-dose rimiducid Consistent with previous cohorts, rimiducid administration was associated with a transient decline followed by partial recovery in circulating BPX-601 cells
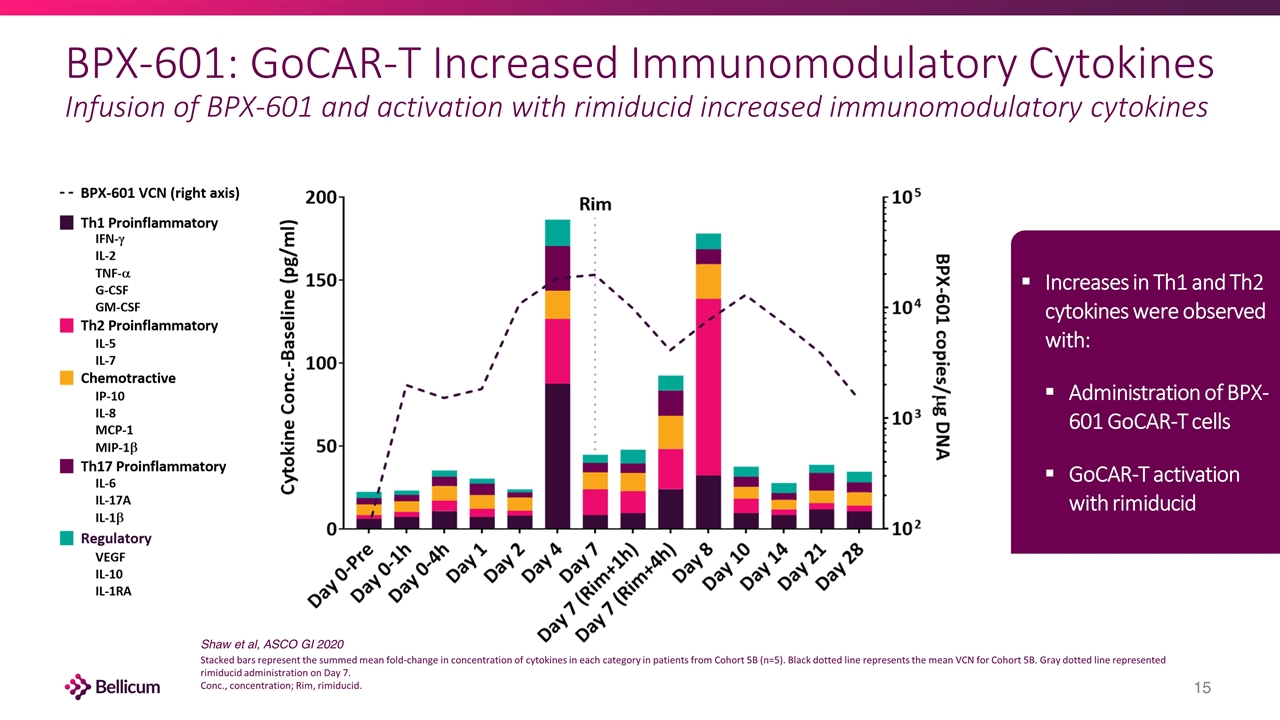
BPX-601: GoCAR-T Increased Immunomodulatory Cytokines Infusion of BPX-601 and activation with rimiducid increased immunomodulatory cytokines Increases in Th1 and Th2 cytokines were observed with: Administration of BPX-601 GoCAR-T cells GoCAR-T activation with rimiducid Stacked bars represent the summed mean fold-change in concentration of cytokines in each category in patients from Cohort 5B (n=5). Black dotted line represents the mean VCN for Cohort 5B. Gray dotted line represented rimiducid administration on Day 7. Conc., concentration; Rim, rimiducid. Shaw et al, ASCO GI 2020
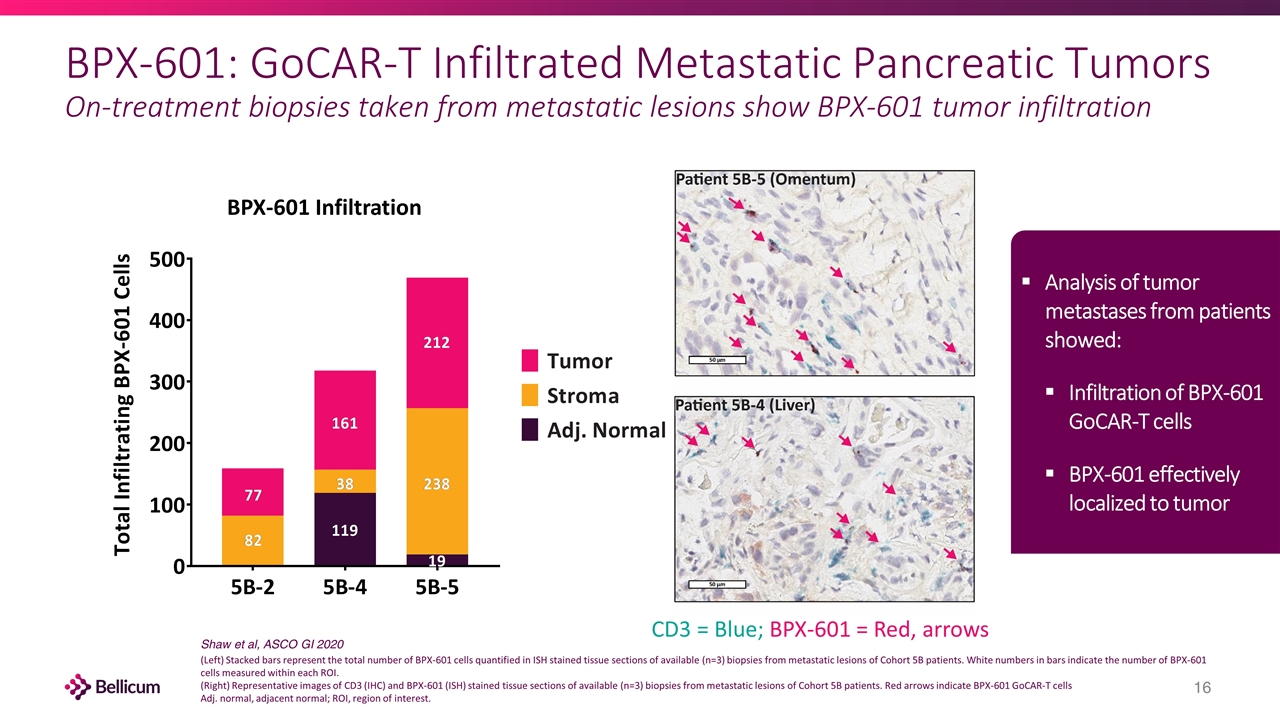
BPX-601: GoCAR-T Infiltrated Metastatic Pancreatic Tumors On-treatment biopsies taken from metastatic lesions show BPX-601 tumor infiltration CD3 = Blue; BPX-601 = Red, arrows (Left) Stacked bars represent the total number of BPX-601 cells quantified in ISH stained tissue sections of available (n=3) biopsies from metastatic lesions of Cohort 5B patients. White numbers in bars indicate the number of BPX-601 cells measured within each ROI. (Right) Representative images of CD3 (IHC) and BPX-601 (ISH) stained tissue sections of available (n=3) biopsies from metastatic lesions of Cohort 5B patients. Red arrows indicate BPX-601 GoCAR-T cells Adj. normal, adjacent normal; ROI, region of interest. Shaw et al, ASCO GI 2020 BPX-601 Infiltration Analysis of tumor metastases from patients showed: Infiltration of BPX-601 GoCAR-T cells BPX-601 effectively localized to tumor
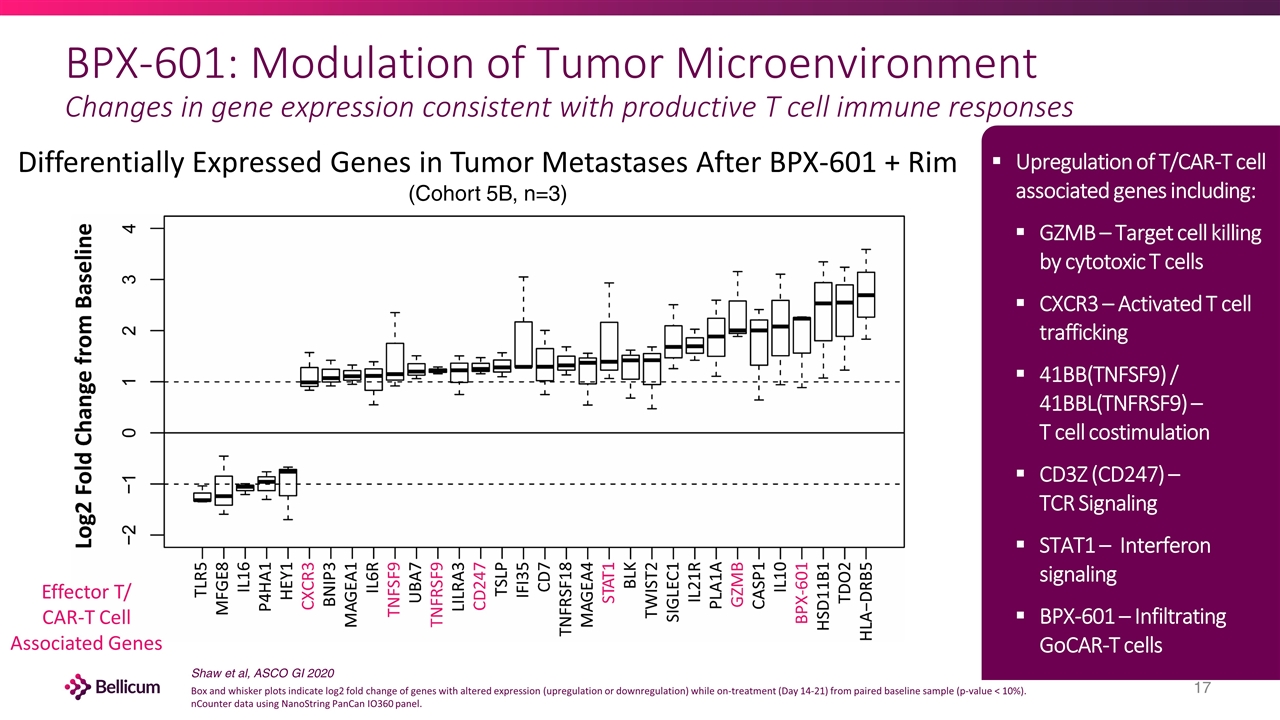
BPX-601: Modulation of Tumor Microenvironment Changes in gene expression consistent with productive T cell immune responses Differentially Expressed Genes in Tumor Metastases After BPX-601 + Rim (Cohort 5B, n=3) Effector T/ CAR-T Cell Associated Genes Upregulation of T/CAR-T cell associated genes including: GZMB – Target cell killing by cytotoxic T cells CXCR3 – Activated T cell trafficking 41BB(TNFSF9) / 41BBL(TNFRSF9) – T cell costimulation CD3Z (CD247) – TCR Signaling STAT1 – Interferon signaling BPX-601 – Infiltrating GoCAR-T cells Shaw et al, ASCO GI 2020 Box and whisker plots indicate log2 fold change of genes with altered expression (upregulation or downregulation) while on-treatment (Day 14-21) from paired baseline sample (p-value < 10%). nCounter data using NanoString PanCan IO360 panel.

BPX-603 HER-2 GoCAR-T
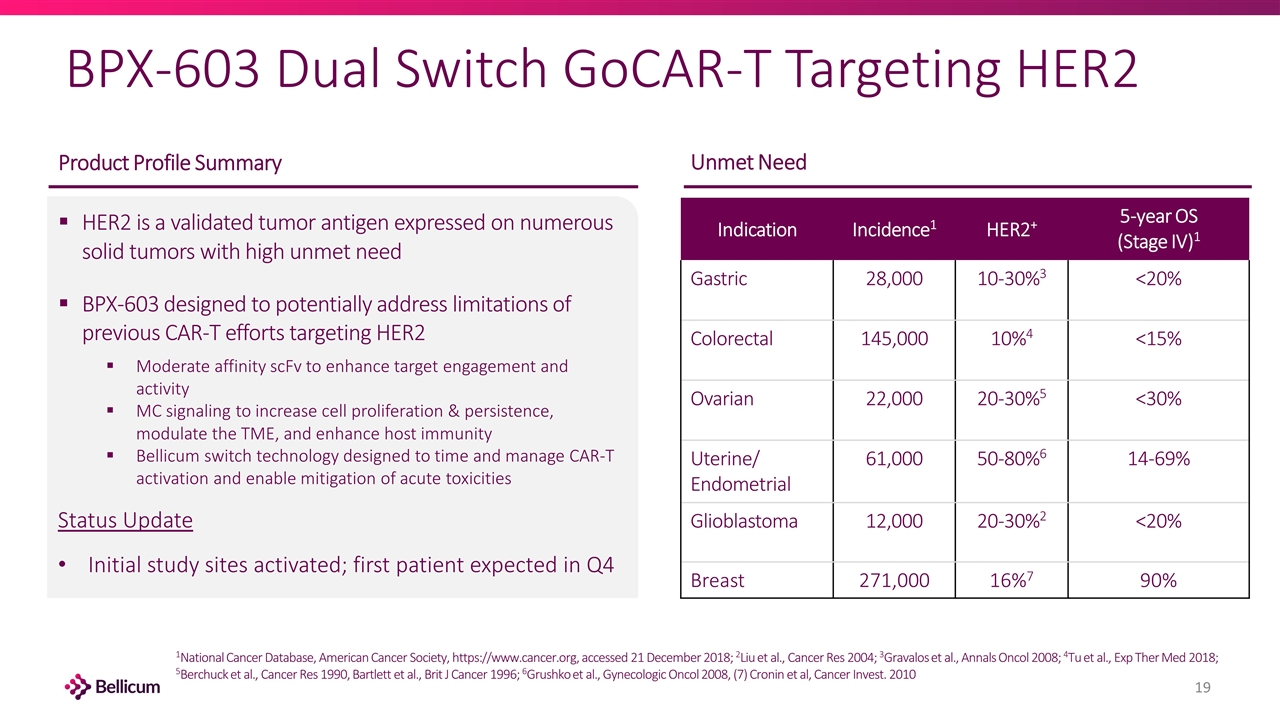
BPX-603 Dual Switch GoCAR-T Targeting HER2 Indication Incidence1 HER2+ 5-year OS (Stage IV)1 Gastric 28,000 10-30%3 <20% Colorectal 145,000 10%4 <15% Ovarian 22,000 20-30%5 <30% Uterine/ Endometrial 61,000 50-80%6 14-69% Glioblastoma 12,000 20-30%2 <20% Breast 271,000 16%7 90% Unmet Need Product Profile Summary 1National Cancer Database, American Cancer Society, https://www.cancer.org, accessed 21 December 2018; 2Liu et al., Cancer Res 2004; 3Gravalos et al., Annals Oncol 2008; 4Tu et al., Exp Ther Med 2018; 5Berchuck et al., Cancer Res 1990, Bartlett et al., Brit J Cancer 1996; 6Grushko et al., Gynecologic Oncol 2008, (7) Cronin et al, Cancer Invest. 2010 HER2 is a validated tumor antigen expressed on numerous solid tumors with high unmet need BPX-603 designed to potentially address limitations of previous CAR-T efforts targeting HER2 Moderate affinity scFv to enhance target engagement and activity MC signaling to increase cell proliferation & persistence, modulate the TME, and enhance host immunity Bellicum switch technology designed to time and manage CAR-T activation and enable mitigation of acute toxicities Status Update Initial study sites activated; first patient expected in Q4
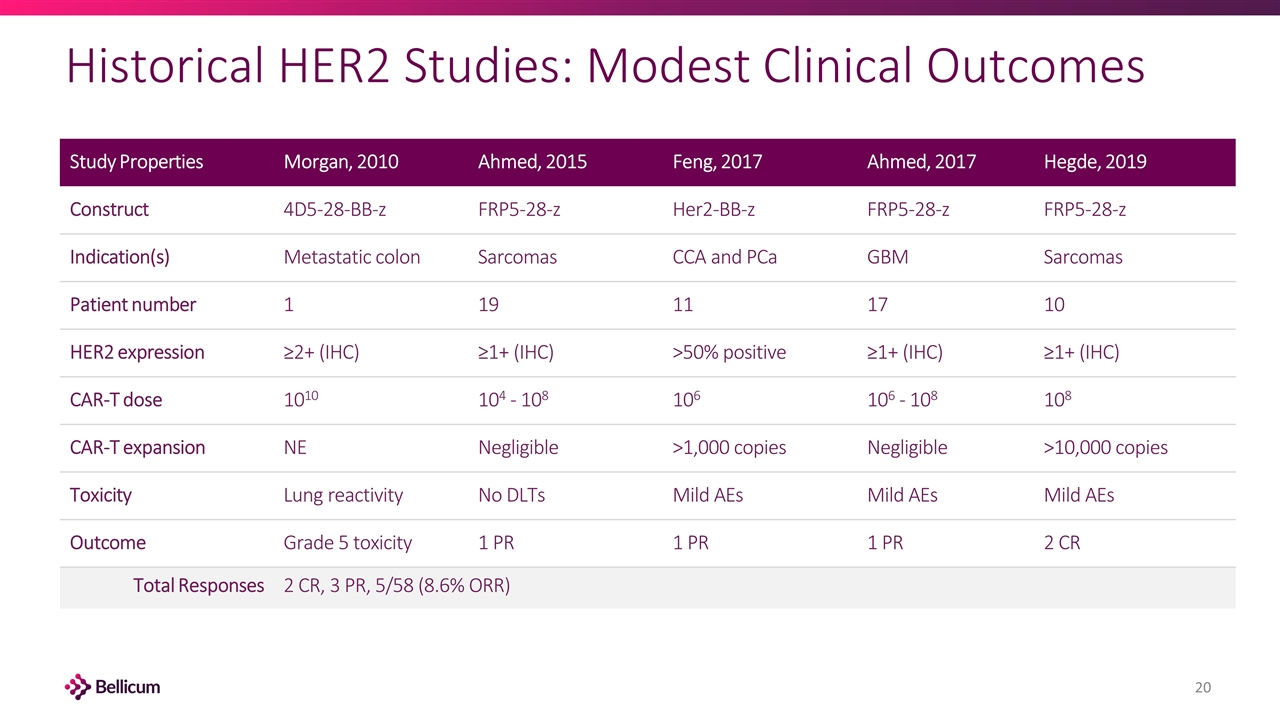
Historical HER2 Studies: Modest Clinical Outcomes Study Properties Morgan, 2010 Ahmed, 2015 Feng, 2017 Ahmed, 2017 Hegde, 2019 Construct 4D5-28-BB-z FRP5-28-z Her2-BB-z FRP5-28-z FRP5-28-z Indication(s) Metastatic colon Sarcomas CCA and PCa GBM Sarcomas Patient number 1 19 11 17 10 HER2 expression ≥2+ (IHC) ≥1+ (IHC) >50% positive ≥1+ (IHC) ≥1+ (IHC) CAR-T dose 1010 104 - 108 106 106 - 108 108 CAR-T expansion NE Negligible >1,000 copies Negligible >10,000 copies Toxicity Lung reactivity No DLTs Mild AEs Mild AEs Mild AEs Outcome Grade 5 toxicity 1 PR 1 PR 1 PR 2 CR Total Responses 2 CR, 3 PR, 5/58 (8.6% ORR)
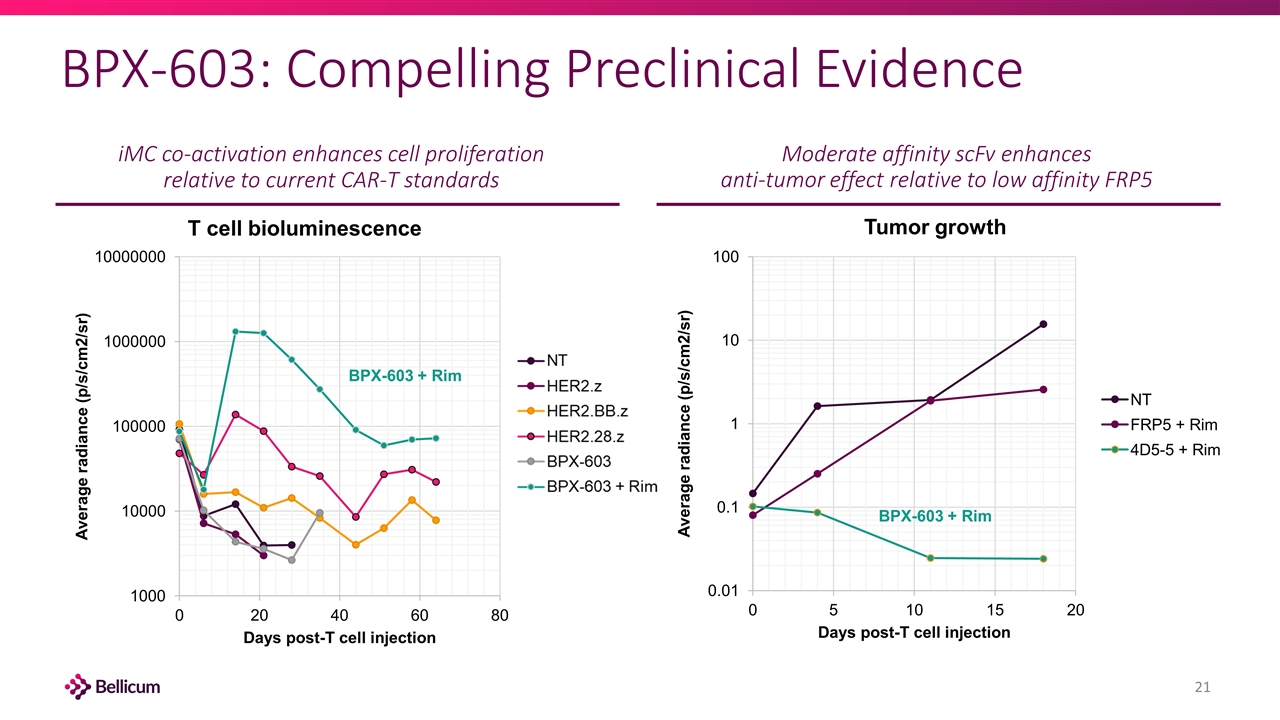
BPX-603: Compelling Preclinical Evidence iMC co-activation enhances cell proliferation relative to current CAR-T standards BPX-603 + Rim BPX-603 + Rim Moderate affinity scFv enhances anti-tumor effect relative to low affinity FRP5
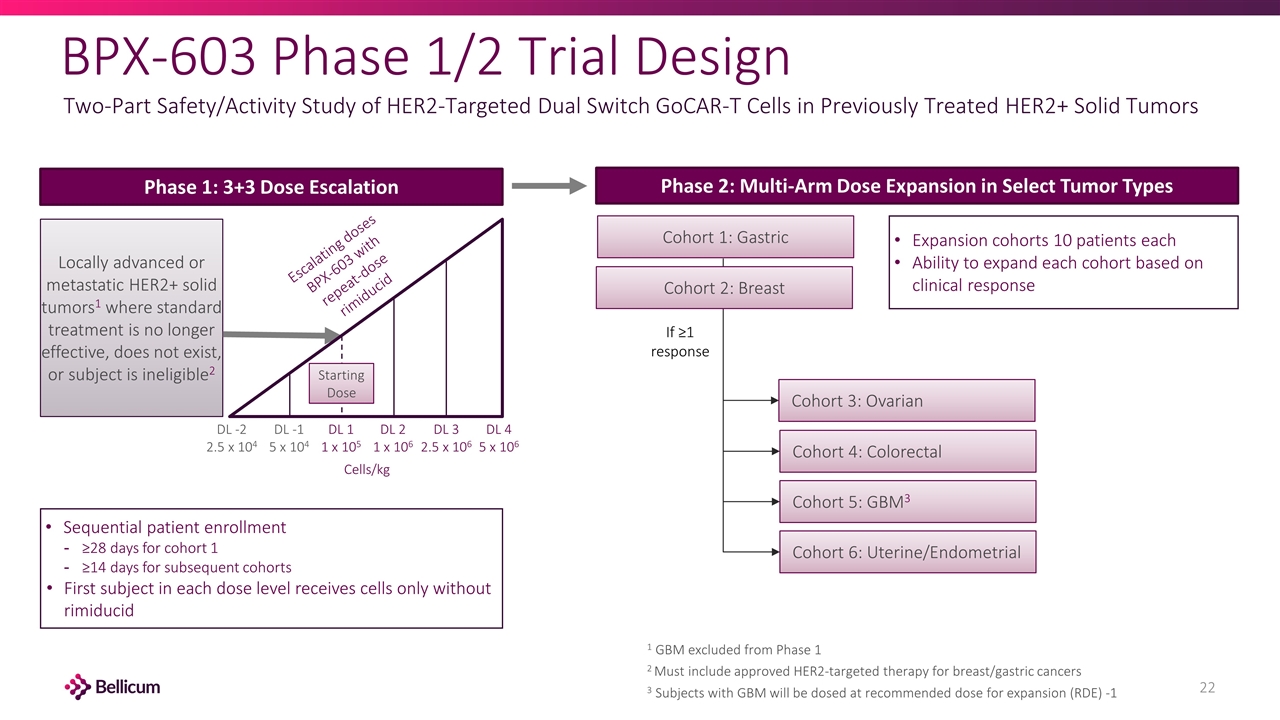
BPX-603 Phase 1/2 Trial Design Two-Part Safety/Activity Study of HER2-Targeted Dual Switch GoCAR-T Cells in Previously Treated HER2+ Solid Tumors Locally advanced or metastatic HER2+ solid tumors1 where standard treatment is no longer effective, does not exist, or subject is ineligible2 Escalating doses BPX-603 with repeat-dose rimiducid Phase 1: 3+3 Dose Escalation Phase 2: Multi-Arm Dose Expansion in Select Tumor Types Cohort 3: Ovarian If ≥1 response DL -2 2.5 x 104 DL -1 5 x 104 DL 1 1 x 105 DL 2 1 x 106 DL 3 2.5 x 106 DL 4 5 x 106 Cohort 1: Gastric Cohort 2: Breast Cohort 4: Colorectal Cohort 5: GBM3 Cohort 6: Uterine/Endometrial 1 GBM excluded from Phase 1 2 Must include approved HER2-targeted therapy for breast/gastric cancers 3 Subjects with GBM will be dosed at recommended dose for expansion (RDE) -1 Expansion cohorts 10 patients each Ability to expand each cohort based on clinical response Sequential patient enrollment ≥28 days for cohort 1 ≥14 days for subsequent cohorts First subject in each dose level receives cells only without rimiducid Starting Dose Cells/kg

Summary

Near-Term Restructuring Plan Focus on clinical development of BPX-601 and BPX-603 Pause BCMA GoCAR-NK program and discontinue discovery research and new product development Reduce headcount by 81%, from 68 to 13 full-time employees during Q4 2020, with estimated severance costs of ~$2 million Settle Oxford Finance debt obligations of ~$28 million in Q4 2020 Reduce annual operating cash utilization to $25 to $30 million

Goals & Milestones Planned Timing Phase 1 data update – mCRPC & Pancreatic Phase 1 data update - mCRPC 2H’21 2022 Initiate Phase 1/2 trial Initial Phase 1 data Phase 1 data update Q4’20 2H’21 2022 Anticipated Key Program Goals & Milestones BPX-601 BPX-603
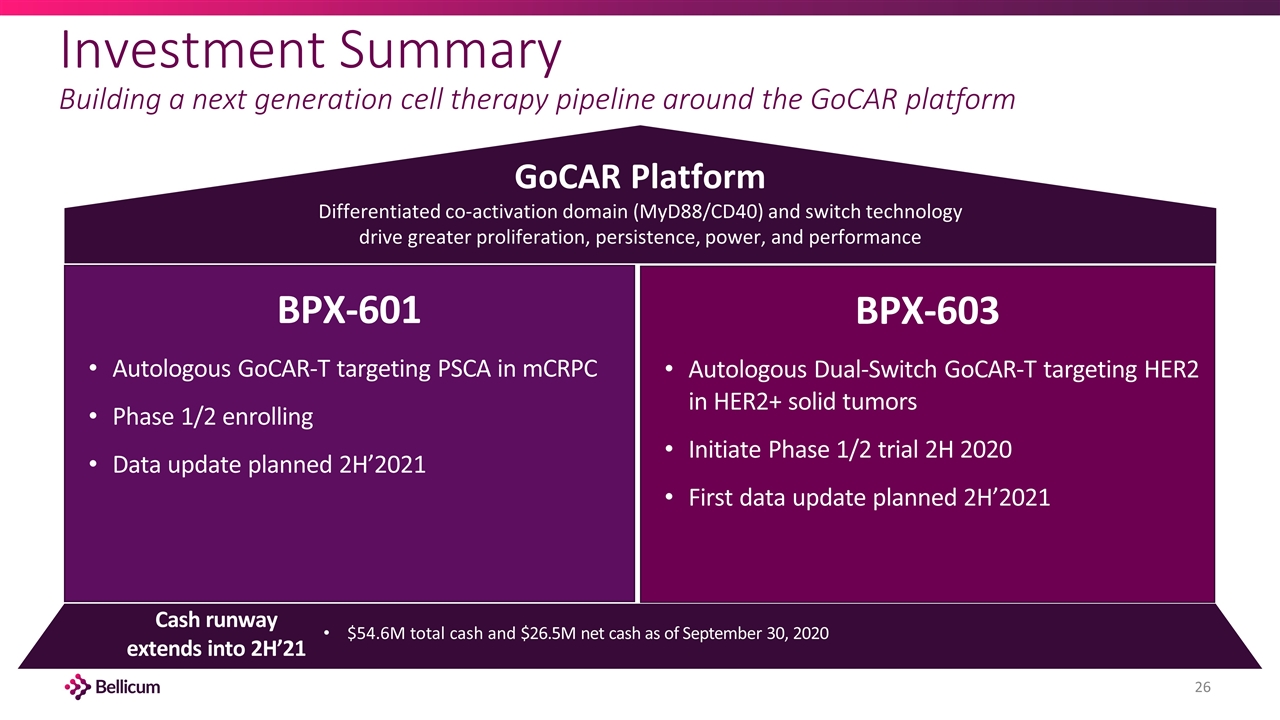
Investment Summary Building a next generation cell therapy pipeline around the GoCAR platform GoCAR Platform Differentiated co-activation domain (MyD88/CD40) and switch technology drive greater proliferation, persistence, power, and performance Cash runway extends into 2H’21 $54.6M total cash and $26.5M net cash as of September 30, 2020 BPX-601 Autologous GoCAR-T targeting PSCA in mCRPC Phase 1/2 enrolling Data update planned 2H’2021 BPX-603 Autologous Dual-Switch GoCAR-T targeting HER2 in HER2+ solid tumors Initiate Phase 1/2 trial 2H 2020 First data update planned 2H’2021

























