
What’s next….. How can we quickly restore T-cell mediated immunity and, thus, further protect patients from life- threatening pathogens in the first 3 months after HSCT, while preventing GvHD occurrence?


Study sponsor: Bellicum Pharmaceuticals Participating Centers: Rome, Freiburg, London, Newcastle PI: F. Locatelli Co-PIs: A. Bertaina, P. Merli, B. Lucarelli, B. Strahm, W. Qasim, M. Slatter Phase I/II study of CaspaCide T cells from an HLA- partially matched family donor after negative selection of TCR αβ+ T cells in pediatric patients affected by hematological disorders ClinicalTrials.gov identifier: NCT02065869

CaspaCide T cell Clinical Schema 4

Trial design • This is a phase I-II open label study. The trial design consists of 3 cohorts, receiving escalation doses of BPX-501 of 2.5 x 105, 5 x105, and 1x106 cells/kg, respectively. Dose escalation will occur according to a 3+3 design. • If none of the initial 3 patients in a cohort experiences a dose- limiting toxicity, another 3 patients will be treated at the next higher dose level. If one of the first three patients experiences a DLT, three more patients will be treated at the same dose level. Dose escalation will continue until at least 2 patients in a cohort of 3 to 6 patients experience dose limiting toxicities. • A Phase 2 extension will occur after dose escalation, enrolling at the highest tolerated dose for a maximum of 60 paediatric patients total, enrolled over a period of 12 months and the minimum active study follow-up for each patient will be 2 years. The maximum duration of the study will be 3 years.


Ospedale Pediatrico Bambino Gesù – Protocol Tαβ depletion non malignant- update September 2, 2015 First 30 patients included 15 with malignant disease and 15 with non malignant disorders Non malignant disorders included in the study 15 Severe combined immunodeficiencey (SCID) 4 Wiskott-Aldrich syndrome (WAS) 3 Fanconi Anemia 4 Beta Thalassemia 3 Hemophagocytic lymphohistiocytosis 1

Clinical outcome of initial 15 children with non- malignant disorders in the study so far • BPX-501 T cells engraft and expand in all patients • In no patients given BPX-501 cells did secondary graft failure occur • Grade II skin-only GvHD was observed in 1 patient, but it promptly responded to topical steroids; • None of the patients so far have developed chronic GvHD; • No patient died from transplantation-related complications and all children are alive and disease-free
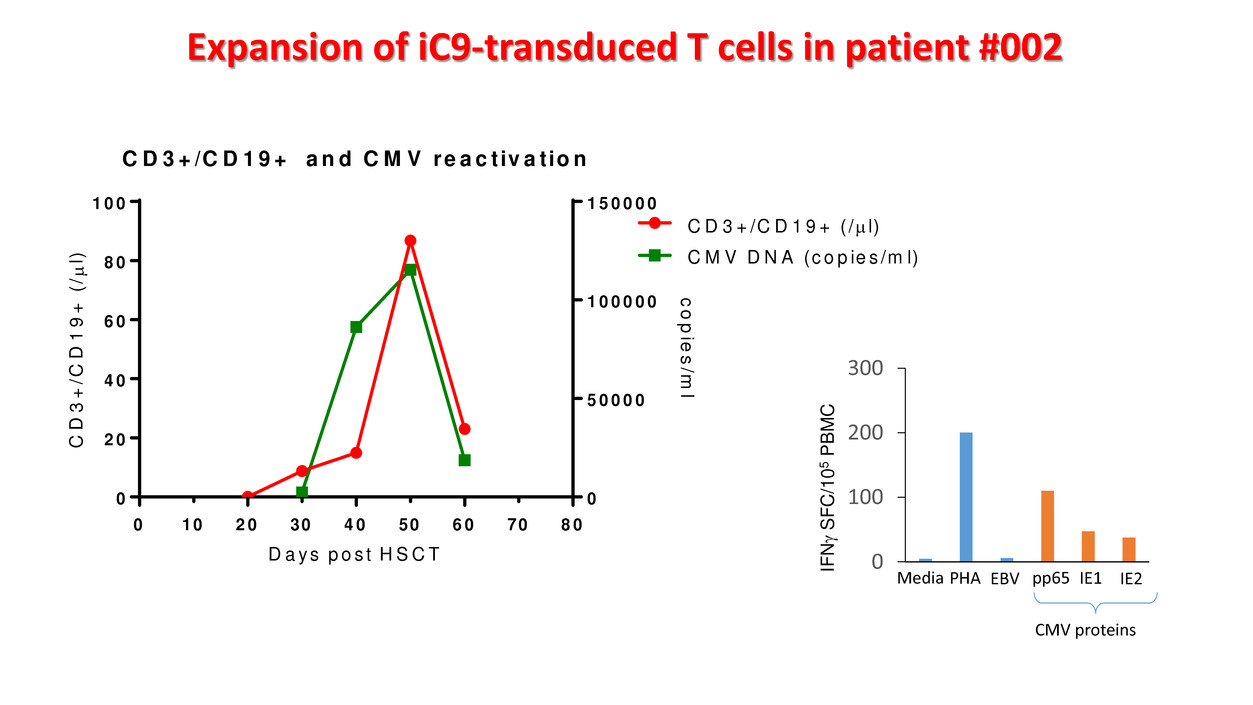
0 2 0 4 0 6 0 8 0 0 2 0 4 0 6 0 8 0 1 0 0 0 5 0 0 0 0 1 0 0 0 0 0 1 5 0 0 0 0 C D 3 + /C D 1 9 + a n d C M V re a c t iv a t io n D a y s p o s t H S C T C D 3 + /C D 1 9 + ( / µ l) c o p ie s /m l C D 3 + /C D 1 9 + ( /µ l) C M V D N A (c o p ie s /m l) 30 50 7010 Expansion of iC9-transduced T cells in patient #002 0 100 200 300 Media PHA EBV pp65 IE1 IE2 CMV proteins IF N γ S FC /1 05 PB M C