
SKYCLARYS™ Approval Call February 28, 2023 Exhibit 99.3
This presentation contains certain “forward‐looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward‐looking statements, including statements regarding our future financial condition, future revenues, projected costs, prospects, business strategy, and plans and objectives of management for future operations, including our plans to submit for regulatory filings, and plans and objectives of management for the commercialization of SKYCLARYS, including anticipated pricing and reimbursement, marketing costs, revenues, licenses (if any) and the production, manufacture and distribution of SKYCLARYS. In some cases, you can identify forward‐looking statements by terminology such as “believe,” “will,” “may,” “might,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “model,” “should,” “would,” “plan,” “expect,” “predict,” “could,” “seek,” “goal,” “potential,” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, and are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward‐looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecasted in these statements. Any differences could be caused by a number of factors including but not limited to: our expectations regarding the timing, costs, conduct, and outcome of our clinical trials, including statements regarding the timing of the initiation and availability of data from such trials; the timing and likelihood of regulatory filings and approvals for our product candidates; whether regulatory authorities determine that additional trials or data are necessary in order to obtain approval; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the success of our launch and commercialization of SKYCLARYS as well as the commercialization of our other product candidates, if approved; our ability to successfully build our commercial infrastructure to manufacture, market and sell our products, including the successful development and implementation of our sales and marketing campaigns for SKYCLARYS; the rate and degree of market acceptance of SKYCLARYS and our other product candidates; our expectations regarding the potential market size and the size of the patient populations for SKYCLARYS and our other product candidates, if approved, for commercial use, and the potential market opportunities for commercializing SKYCLARYS and our other product candidates; our ability to obtain or maintain coverage and reimbursement for SKYCLARYS from third-party payors, such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators, including whether such payors will reimburse for SKYCLARYS at a price that is profitable to us; product quality, efficacy or safety concerns relating to SKYCLARYS resulting in complaints, adverse events, product recalls or regulatory action; the adequacy of our pharmacovigilance and drug safety reporting processes; the ability of our third-party suppliers and contract manufacturers to manufacture SKYCLARYS at the required quality and quantities and in compliance with applicable laws and regulations; the effect of increased scrutiny by federal, state and foreign national governments on the pricing of pharmaceutical products, including government price controls or other changes in pricing regulation that would restrict the amount we are able to charge for SKYCLARYS and our other product candidates, if approved; our ability to successfully manufacture, market and distribute SKYCLARYS while maintaining compliance with applicable federal and state laws, rules and regulations; our ability to comply with ongoing regulatory requirements with respect to SKYCLARYS (or any product candidate for which we obtain approval in the future) by the FDA, the EMA and other comparable international regulatory authorities; the success of competing therapies that are or may become available, including competing therapies for SKYCLARYS in the treatment of Friedreich’s ataxia; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to maintain and establish relationships with third parties, such as contract research organizations, contract manufacturing organizations, suppliers, and distributors; our ability to maintain and establish collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; our expectations related to the use of our available cash; our ability to develop, acquire, and advance product candidates into, and successfully complete, clinical trials; the initiation, timing, progress, and results of future preclinical studies and developments and projections relating to our competitors and our industry; the impact of governmental laws and regulations and regulatory development in the United States and foreign countries; the impact of the coronavirus disease (COVID‐19) on our clinical trials, our supply chain, and our operations; and other risks and uncertainties, including those described under the heading “Risk Factors” included in our most recent Annual Report on Form 10‐K for the year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (SEC) on February 24, 2023. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward‐looking statements. All forward‐ looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward‐looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward‐looking statements, whether as a result of new information, future events, or otherwise. Bardoxolone methyl and Cemdomespib are investigational drugs, and their safety and efficacy have not been established by any agency.

Today’s Agenda Opening Remarks | Warren Huff, Chief Executive Officer SKYCLARYS Label | Colin Meyer, MD, Chief Innovation Officer SKYCLARYS PMR & Registry | Seemi Khan, MD, Chief Medical Officer SKYCLARYS Commercial Launch | Dawn Bir, Chief Commercial Officer Operational & Financial Update | Manmeet Soni, President, COO, CFO Concluding Remarks | Warren Huff, Chief Executive Officer

SKYCLARYSTM First and Only FDA Approved Therapy Indicated for Patients with Friedreich’s Ataxia 1Please see full Prescribing Information at SKYCLARYS.com; FDA: U.S. Food and Drug Administration; FA: Friedreich’s ataxia

Looking Forward mFARS: modified Friedreich’s Ataxia Rating Scale; MAA: Marketing Authorization Application SKYCLARYS is indicated for the treatment of FA in patients aged 16 years and older No contraindications or limitations based on pes cavus, cardiovascular status, ambulation, mFARS score, or older age Planning to engage with the FDA about possible label expansion for pediatric patients younger than 16 years of age We are prepared for SKYCLARYS launch Medical affairs and pharmacovigilance infrastructure is in place Commercial team is hired and trained Access to therapy is an important responsibility MAA for omaveloxolone submitted in fourth quarter of 2022 Strong balance sheet and no outstanding funded debt Our commitment to patients is to bring life-changing medicine to those who need them the most

SKYCLARYS Label
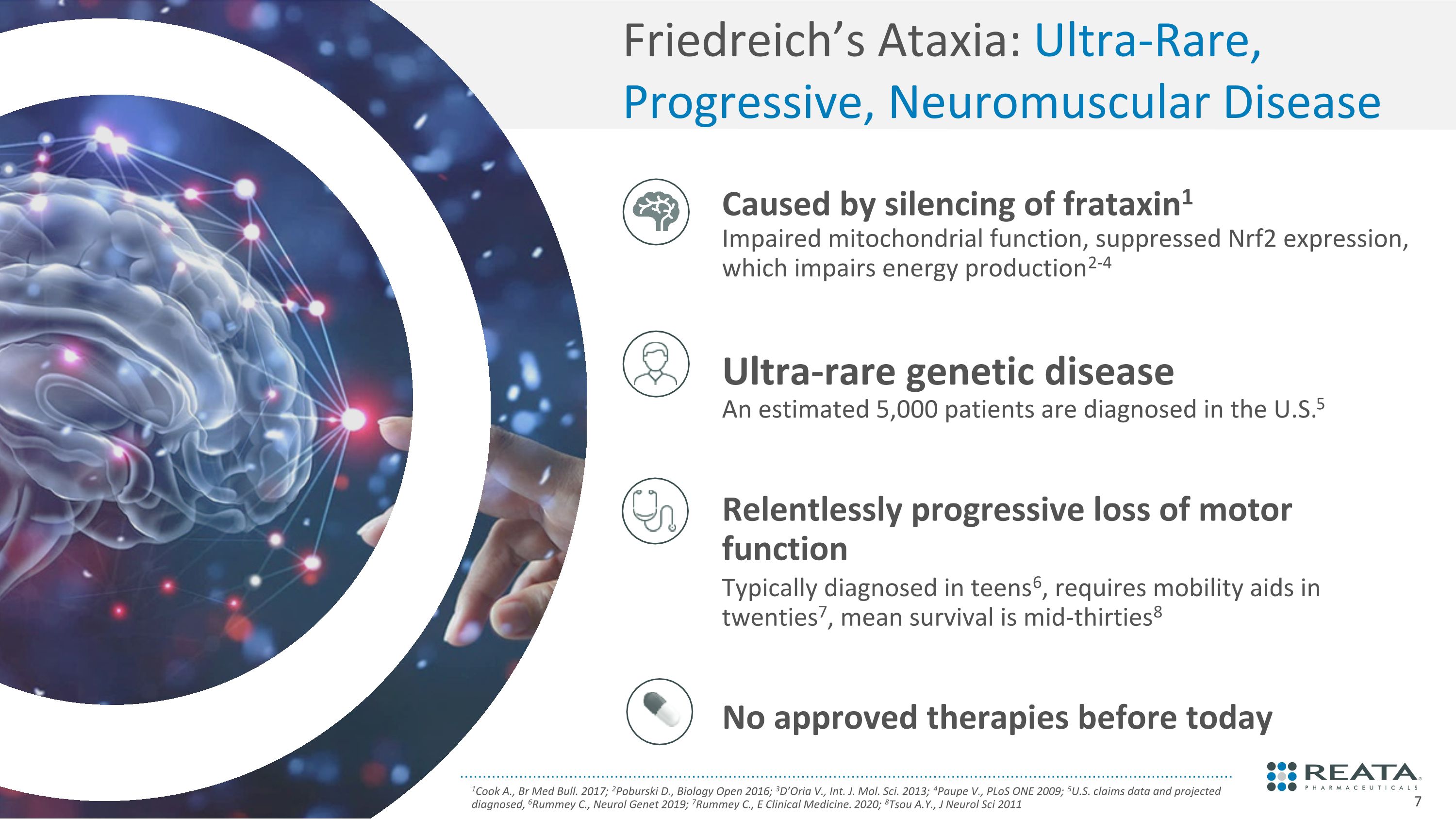
Friedreich’s Ataxia: Ultra-Rare, Progressive, Neuromuscular Disease 1Cook A., Br Med Bull. 2017; 2Poburski D., Biology Open 2016; 3D’Oria V., Int. J. Mol. Sci. 2013; 4Paupe V., PLoS ONE 2009; 5U.S. claims data and projected diagnosed, 6Rummey C., Neurol Genet 2019; 7Rummey C., E Clinical Medicine. 2020; 8Tsou A.Y., J Neurol Sci 2011 Caused by silencing of frataxin1 Impaired mitochondrial function, suppressed Nrf2 expression, which impairs energy production2-4 Ultra-rare genetic disease�An estimated 5,000 patients are diagnosed in the U.S.5 Relentlessly progressive loss of motor function Typically diagnosed in teens6, requires mobility aids in twenties7, mean survival is mid-thirties8 No approved therapies before today
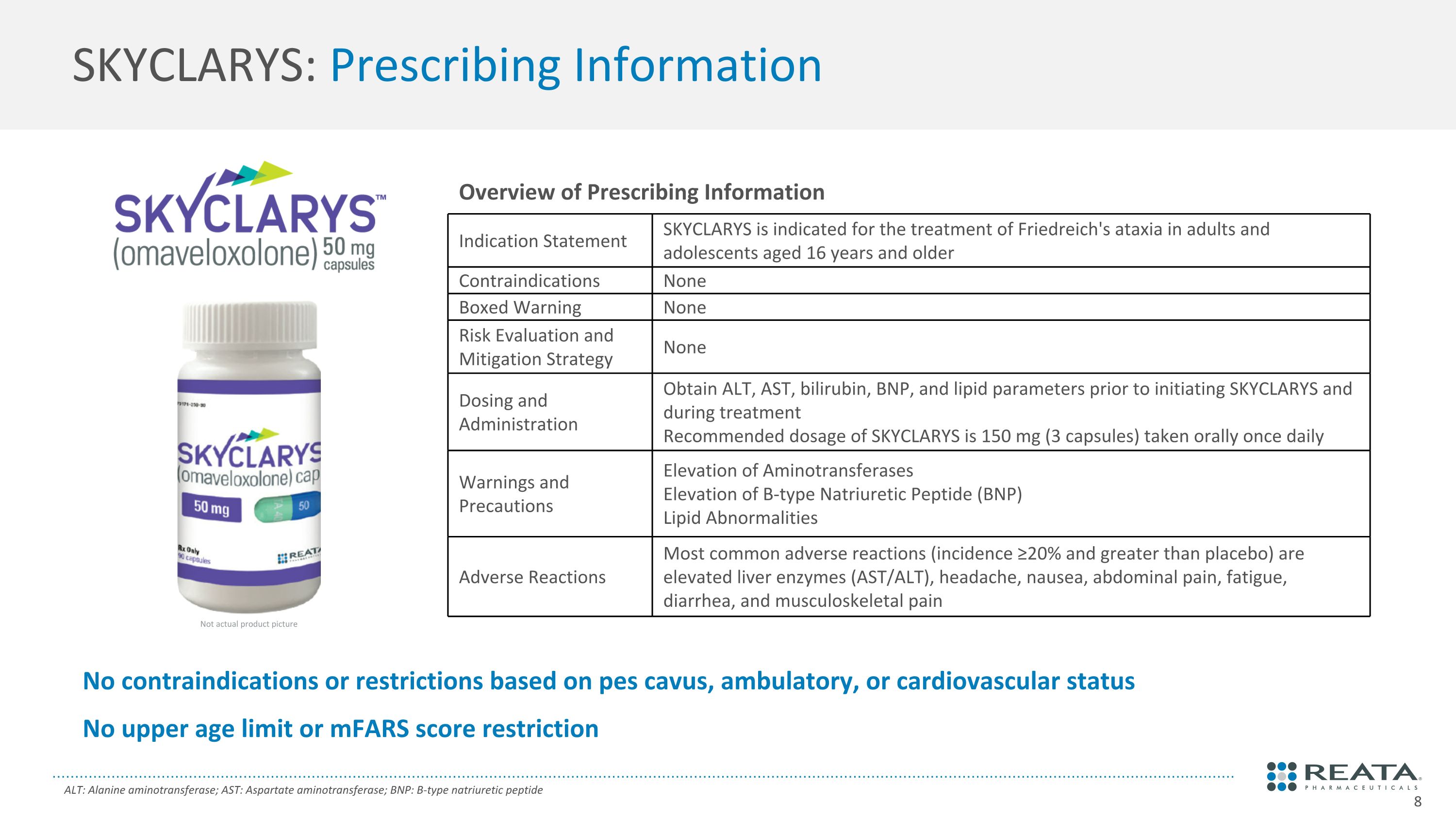
SKYCLARYS: Prescribing Information ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BNP: B-type natriuretic peptide No contraindications or restrictions based on pes cavus, ambulatory, or cardiovascular status No upper age limit or mFARS score restriction Overview of Prescribing Information Indication Statement SKYCLARYS is indicated for the treatment of Friedreich's ataxia in adults and adolescents aged 16 years and older Contraindications None Boxed Warning None Risk Evaluation and Mitigation Strategy None Dosing and Administration Obtain ALT, AST, bilirubin, BNP, and lipid parameters prior to initiating SKYCLARYS and during treatment Recommended dosage of SKYCLARYS is 150 mg (3 capsules) taken orally once daily Warnings and Precautions Elevation of Aminotransferases Elevation of B-type Natriuretic Peptide (BNP) Lipid Abnormalities Adverse Reactions Most common adverse reactions (incidence ≥20% and greater than placebo) are elevated liver enzymes (AST/ALT), headache, nausea, abdominal pain, fatigue, diarrhea, and musculoskeletal pain Not actual product picture
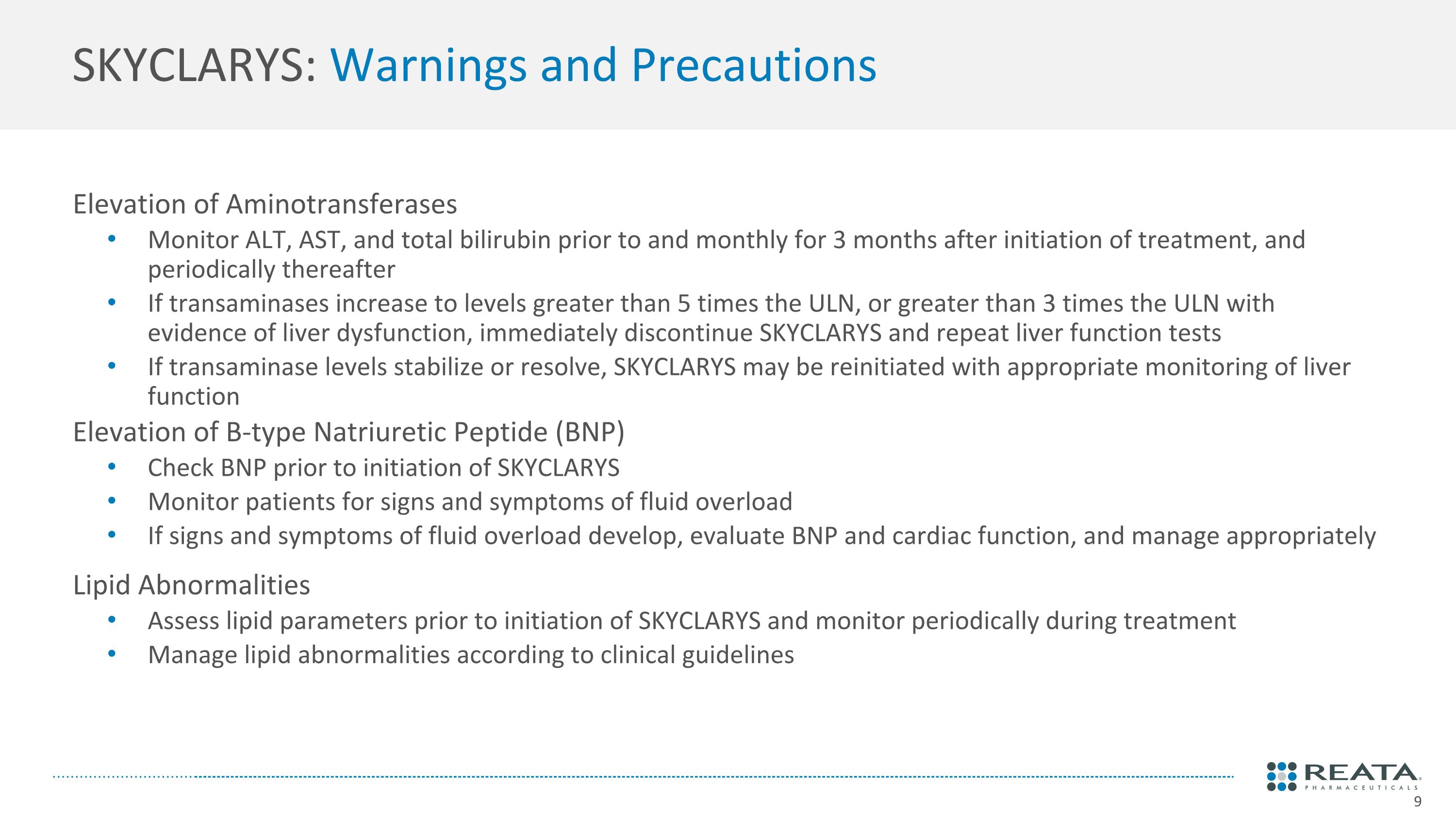
SKYCLARYS: Warnings and Precautions Elevation of Aminotransferases Monitor ALT, AST, and total bilirubin prior to and monthly for 3 months after initiation of treatment, and periodically thereafter If transaminases increase to levels greater than 5 times the ULN, or greater than 3 times the ULN with evidence of liver dysfunction, immediately discontinue SKYCLARYS and repeat liver function tests If transaminase levels stabilize or resolve, SKYCLARYS may be reinitiated with appropriate monitoring of liver function Elevation of B-type Natriuretic Peptide (BNP) Check BNP prior to initiation of SKYCLARYS Monitor patients for signs and symptoms of fluid overload If signs and symptoms of fluid overload develop, evaluate BNP and cardiac function, and manage appropriately Lipid Abnormalities Assess lipid parameters prior to initiation of SKYCLARYS and monitor periodically during treatment Manage lipid abnormalities according to clinical guidelines
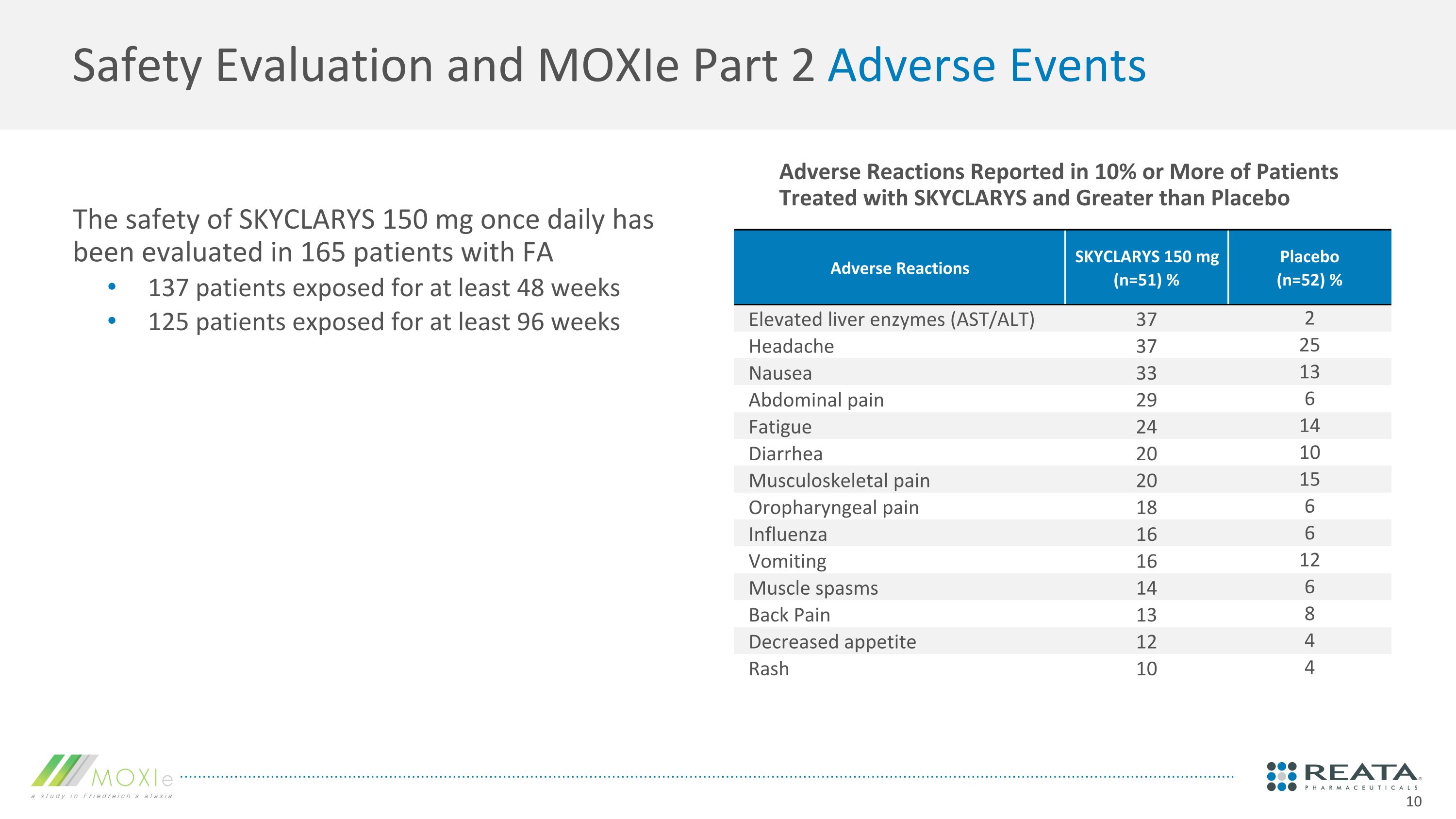
Adverse Reactions Reported in 10% or More of Patients Treated with SKYCLARYS and Greater than Placebo Safety Evaluation and MOXIe Part 2 Adverse Events The safety of SKYCLARYS 150 mg once daily has been evaluated in 165 patients with FA 137 patients exposed for at least 48 weeks 125 patients exposed for at least 96 weeks Adverse Reactions SKYCLARYS 150 mg�(n=51) % Placebo �(n=52) % Elevated liver enzymes (AST/ALT) 37 2 Headache 37 25 Nausea 33 13 Abdominal pain 29 6 Fatigue 24 14 Diarrhea 20 10 Musculoskeletal pain 20 15 Oropharyngeal pain 18 6 Influenza 16 6 Vomiting 16 12 Muscle spasms 14 6 Back Pain 13 8 Decreased appetite 12 4 Rash 10 4
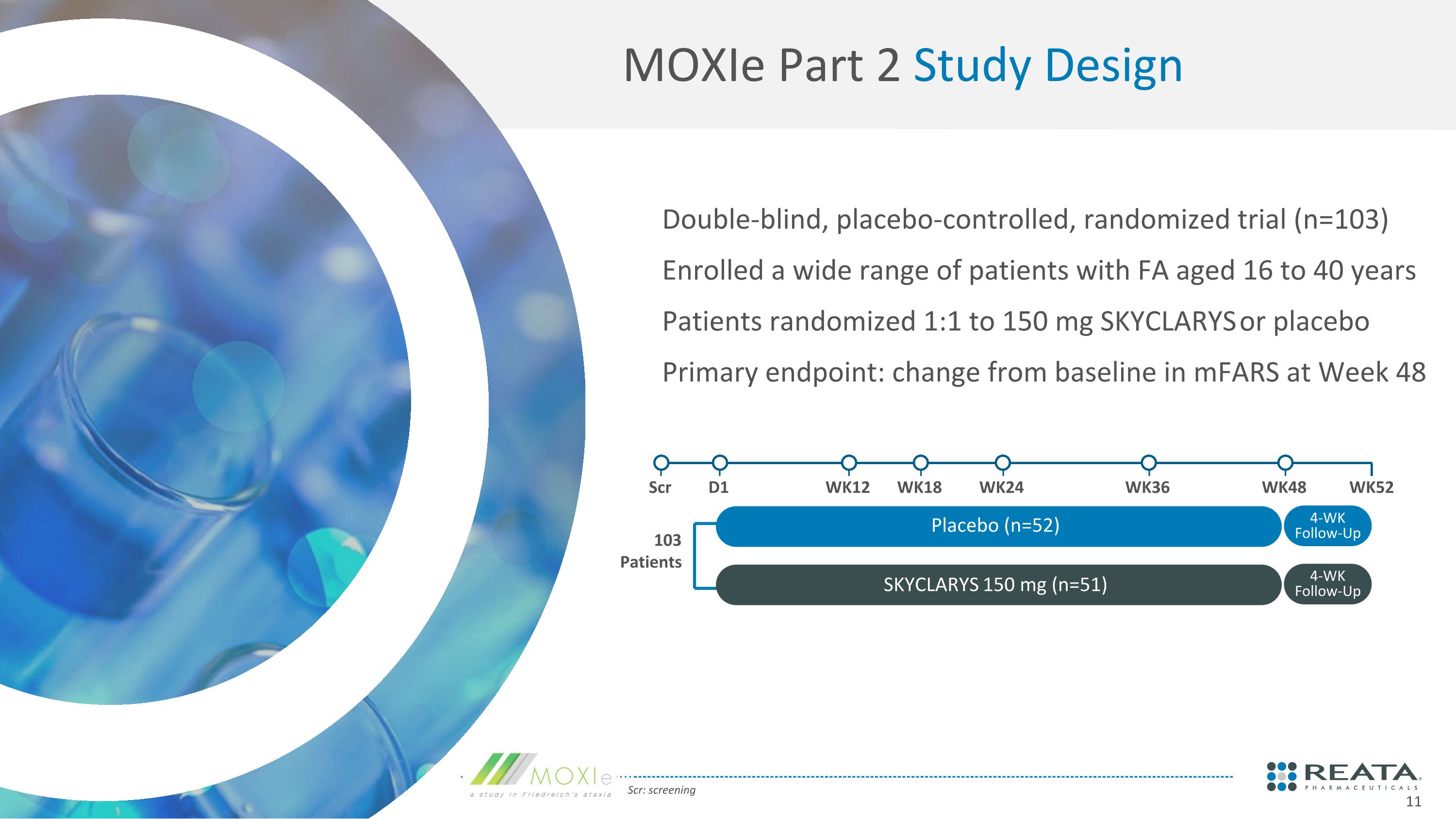
MOXIe Part 2 Study Design Scr: screening Double-blind, placebo-controlled, randomized trial (n=103) Enrolled a wide range of patients with FA aged 16 to 40 years Patients randomized 1:1 to 150 mg SKYCLARYS or placebo Primary endpoint: change from baseline in mFARS at Week 48 103 Patients Scr D1 WK12 WK18 WK24 WK36 WK48 Placebo (n=52) SKYCLARYS 150 mg (n=51) 4-WK Follow-Up 4-WK Follow-Up WK52
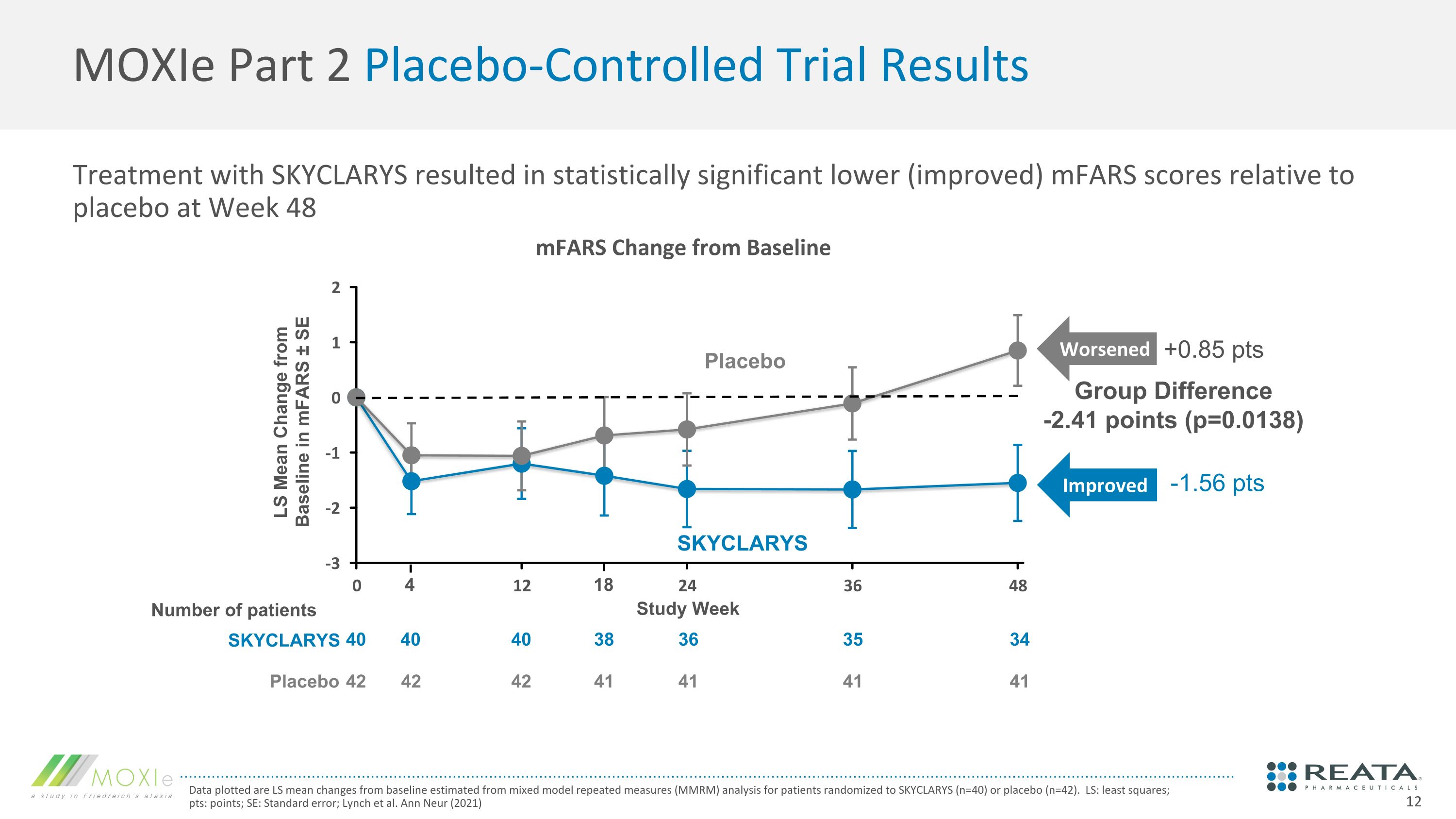
Treatment with SKYCLARYS resulted in statistically significant lower (improved) mFARS scores relative to placebo at Week 48 MOXIe Part 2 Placebo-Controlled Trial Results Data plotted are LS mean changes from baseline estimated from mixed model repeated measures (MMRM) analysis for patients randomized to SKYCLARYS (n=40) or placebo (n=42). LS: least squares; pts: points; SE: Standard error; Lynch et al. Ann Neur (2021) 40 42 40 42 36 41 35 41 34 41 40 42 38 41 Placebo SKYCLARYS Group Difference -2.41 points (p=0.0138) LS Mean Change from Baseline in mFARS ± SE 18 4 Study Week Number of patients SKYCLARYS Placebo mFARS Change from Baseline +0.85 pts -1.56 pts Worsened Improved
MOXIe Extension: Post Hoc Propensity-Matched Analysis FA-COMS: Friedreich’s Ataxia Clinical Outcome Measures Study A post hoc Propensity-Matched Analysis was conducted using data from the open-label MOXIe Extension study and external natural history data from FA-COMS as a comparator FA-COMS is a global, multi-center, longitudinal, prospective, observational study Enrolled more than 1,250 patients Clinical outcome measures, including mFARS, assessed annually Patients followed for up to 25 years Lower mFARS scores were observed in patients treated with SKYCLARYS after 3 years relative to a matched set of untreated patients in FA-COMS These exploratory analyses should be interpreted cautiously given the limitations of data collected outside of a controlled study, which may be subject to confounding

SKYCLARYS Post-Marketing Requirements �& Registry Study

Post-Marketing Requirements Drug-drug interaction study Thorough QT study Lactation study (milk only) Pregnancy and lactation surveillance study Additional nonclinical studies

SKYCLARYS: Post-Approval Registry Study In addition to the post-marketing requirements, Reata will sponsor a post-marketing registry study Prospective, observational, multinational study Patients with FA treated with SKYCLARYS commercially Objective is to evaluate long-term safety in the real-world setting

Pharmacovigilance and Medical Affairs Established infrastructure to support pharmacovigilance and safety obligations for SKYCLARYS in the U.S. Medical Information call center has been established Medical affairs team hired and trained to support SKYCLARYS launch

SKYCLARYS Commercial Launch Plans
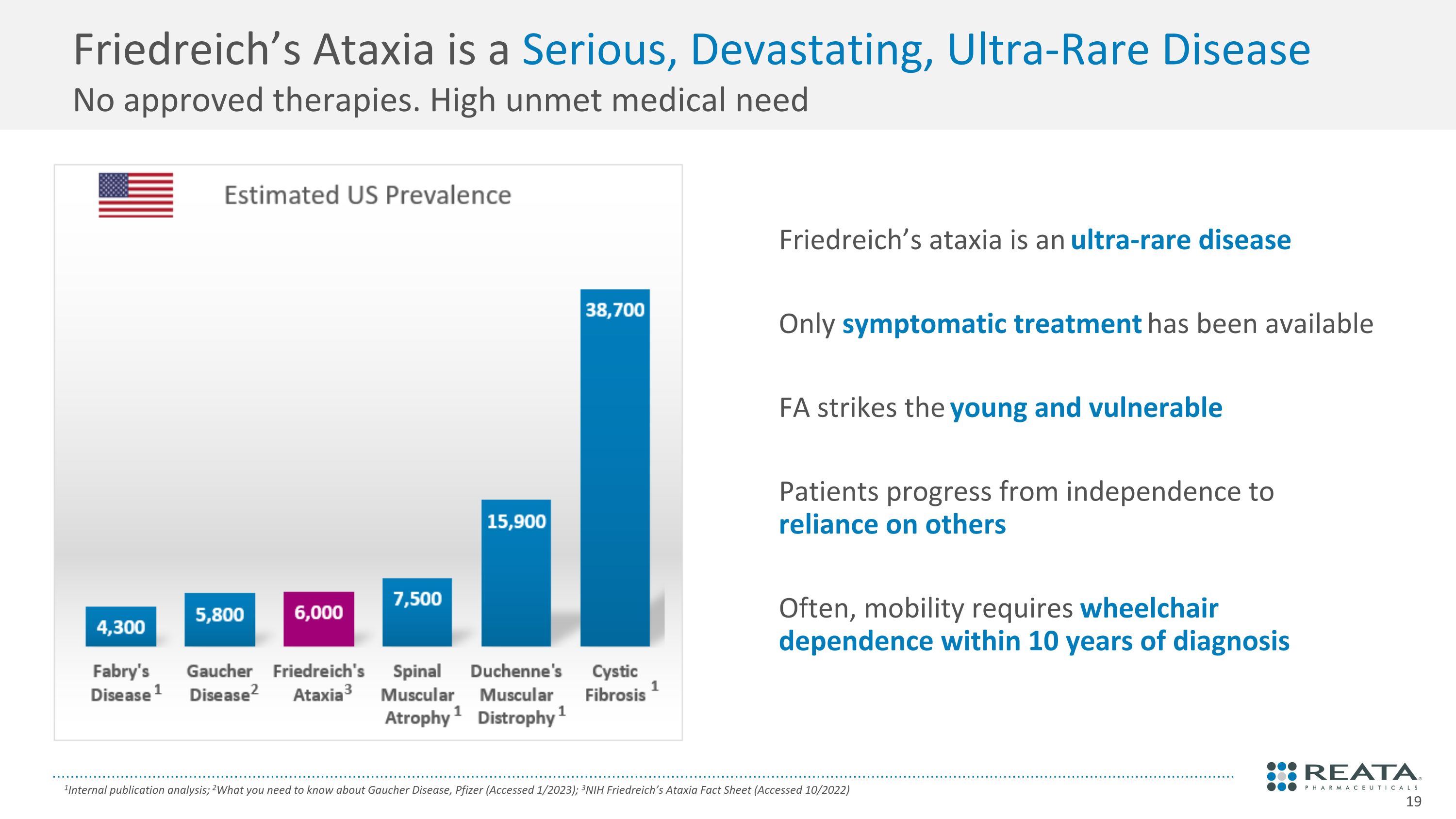
Friedreich’s ataxia is an ultra-rare disease Only symptomatic treatment has been available FA strikes the young and vulnerable Patients progress from independence to reliance on others Often, mobility requires wheelchair dependence within 10 years of diagnosis Friedreich’s Ataxia is a Serious, Devastating, Ultra-Rare Disease �No approved therapies. High unmet medical need 1Internal publication analysis; 2What you need to know about Gaucher Disease, Pfizer (Accessed 1/2023); 3NIH Friedreich’s Ataxia Fact Sheet (Accessed 10/2022)
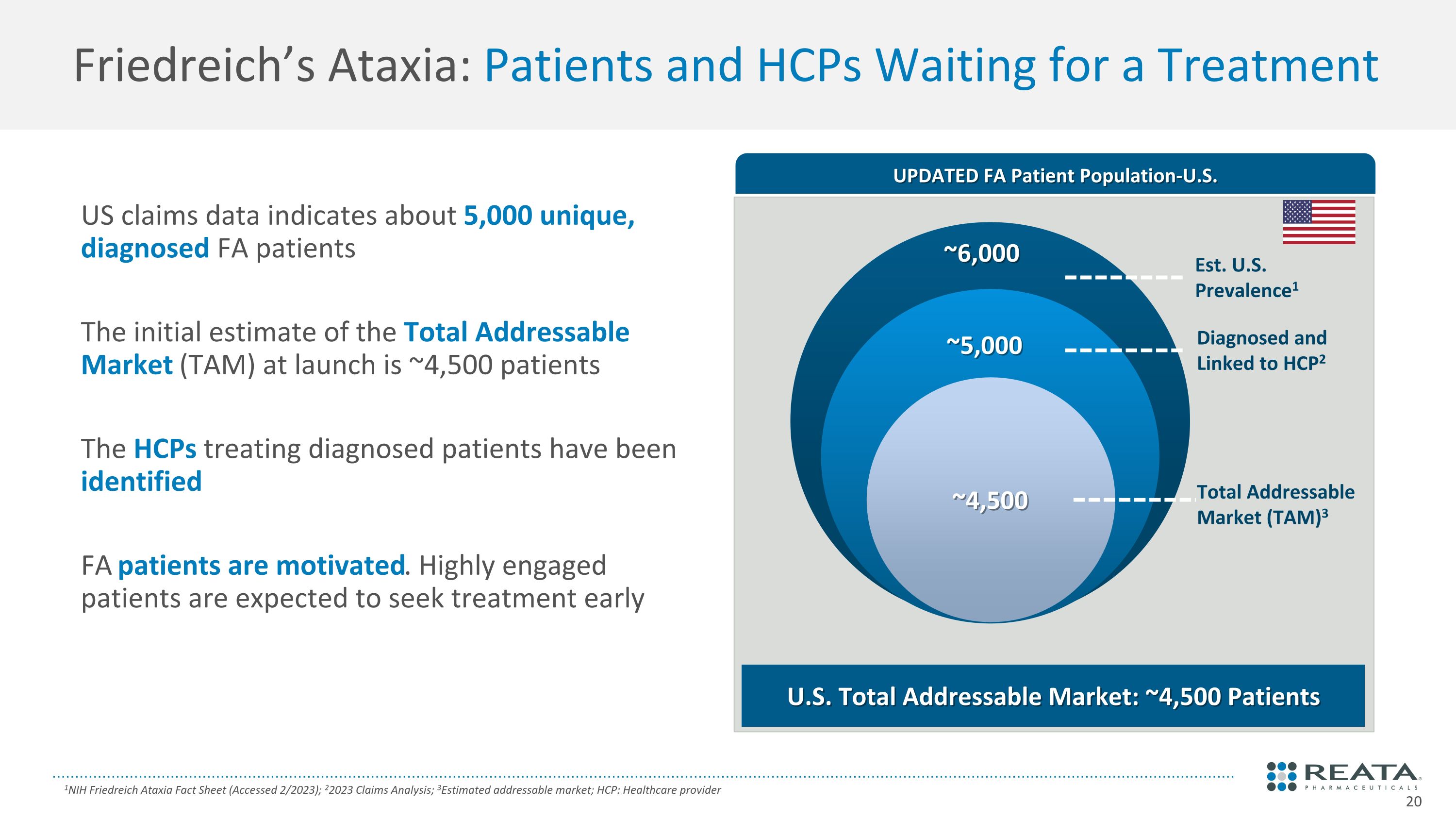
Friedreich’s Ataxia: Patients and HCPs Waiting for a Treatment 1NIH Friedreich Ataxia Fact Sheet (Accessed 2/2023); 22023 Claims Analysis; 3Estimated addressable market; HCP: Healthcare provider US claims data indicates about 5,000 unique, diagnosed FA patients The initial estimate of the Total Addressable Market (TAM) at launch is ~4,500 patients The HCPs treating diagnosed patients have been identified FA patients are motivated. Highly engaged patients are expected to seek treatment early UPDATED FA Patient Population-U.S. Est. U.S. Prevalence1 Diagnosed and Linked to HCP2 U.S. Total Addressable Market: ~4,500 Patients ~5,000 ~6,000 Total Addressable Market (TAM)3 ~4,500

Communicate the value of SKYCLARYS to �HCPs who treat FA Activate FA patients to proactively seek SKYCLARYS treatment Facilitate coverage, access and affordability Commercial Launch Strategy First and only FDA approved treatment for FA in adults and adolescents aged 16 years and older Establish SKYCLARYS as the first effective and safe treatment approved for Friedreich’s ataxia 1 2 3
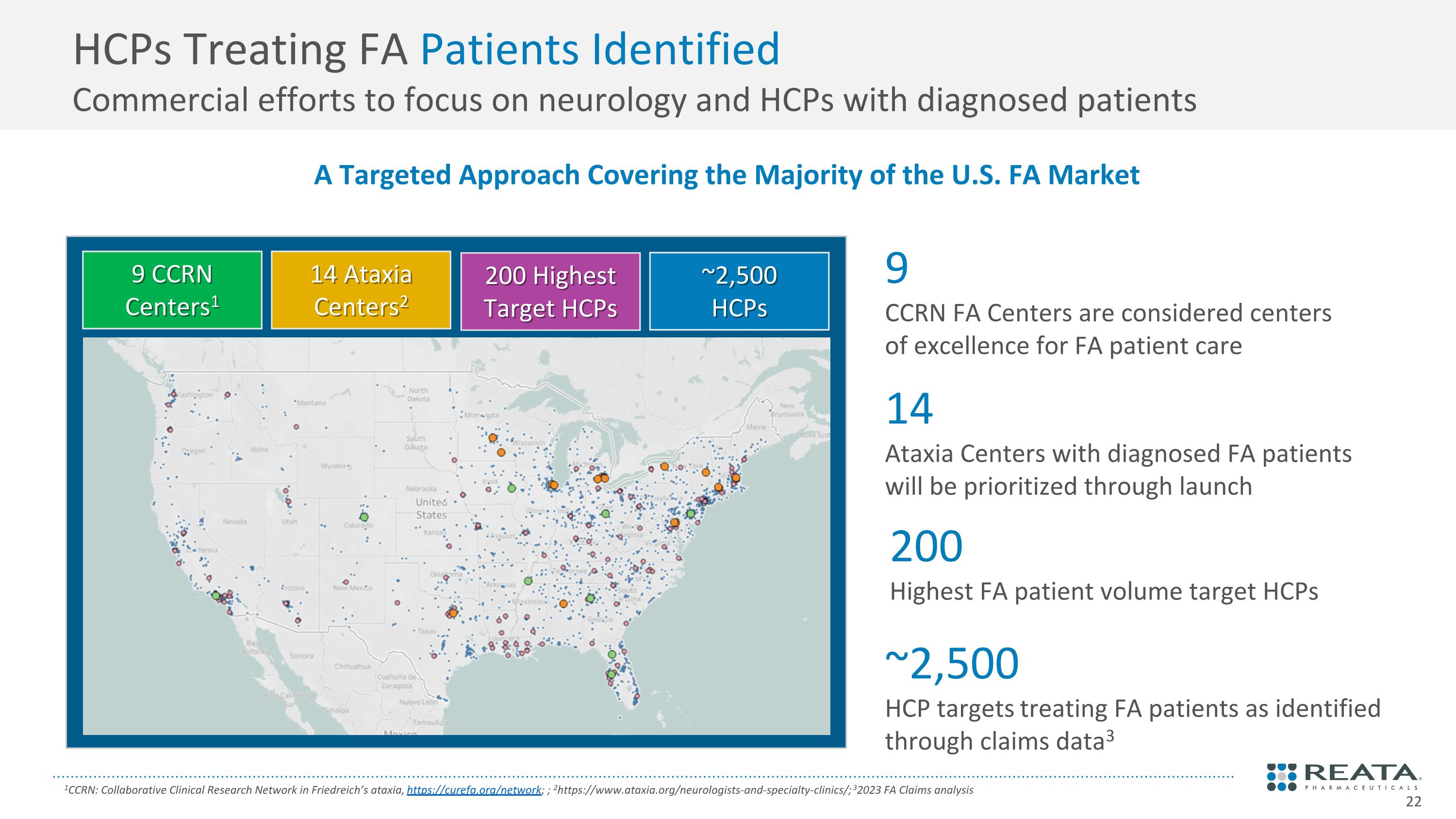
HCPs Treating FA Patients Identified�Commercial efforts to focus on neurology and HCPs with diagnosed patients 1CCRN: Collaborative Clinical Research Network in Friedreich’s ataxia, https://curefa.org/network; ; 2https://www.ataxia.org/neurologists-and-specialty-clinics/; 32023 FA Claims analysis A Targeted Approach Covering the Majority of the U.S. FA Market ~2,500 HCPs 9 CCRN Centers1 14 Ataxia Centers2 ~2,500 HCP targets treating FA patients as identified through claims data3 9 CCRN FA Centers are considered centers of excellence for FA patient care 14 Ataxia Centers with diagnosed FA patients will be prioritized through launch 200 Highest Target HCPs 200 Highest FA patient volume target HCPs
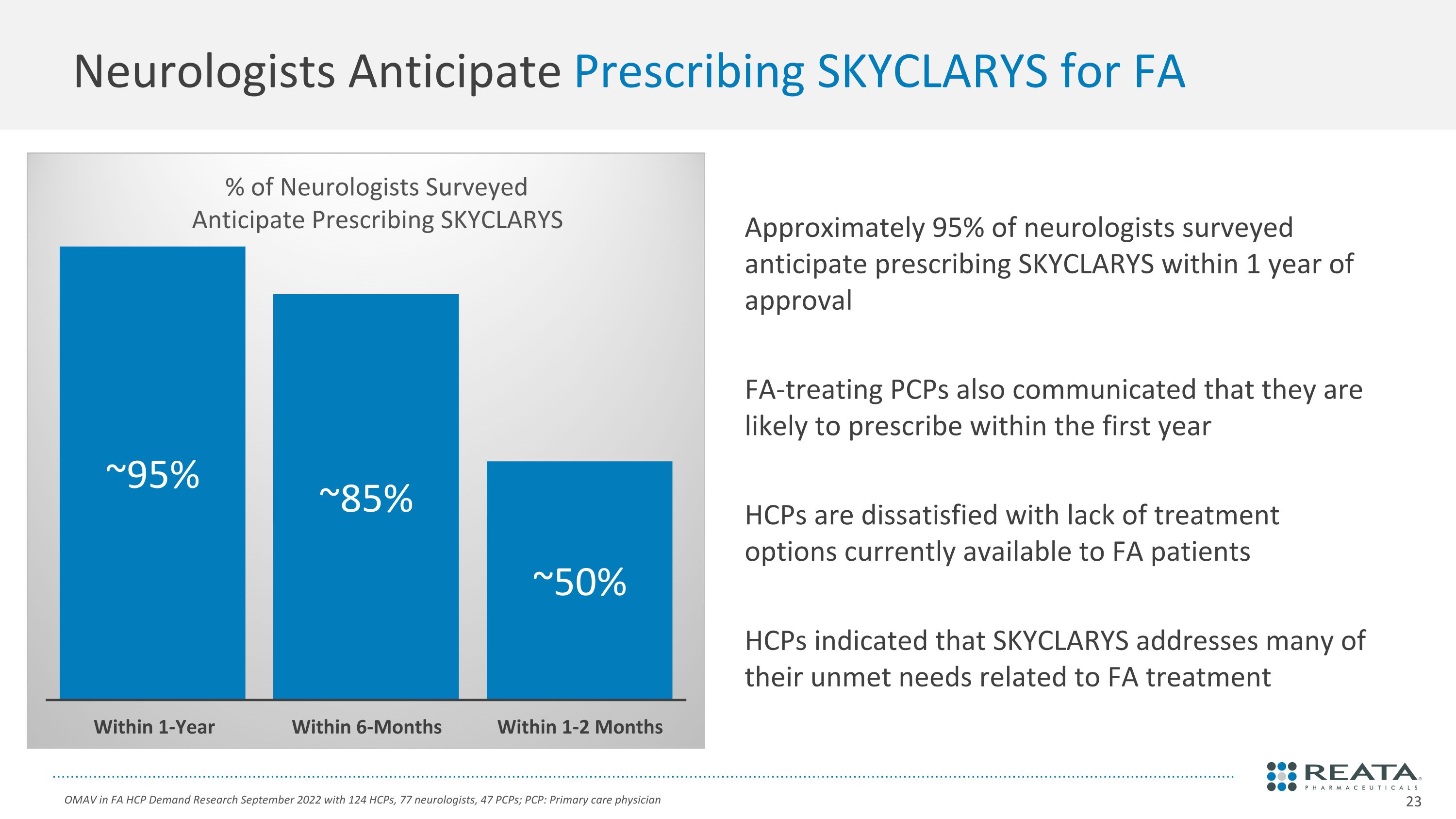
Approximately 95% of neurologists surveyed anticipate prescribing SKYCLARYS within 1 year of approval FA-treating PCPs also communicated that they are likely to prescribe within the first year HCPs are dissatisfied with lack of treatment options currently available to FA patients HCPs indicated that SKYCLARYS addresses many of their unmet needs related to FA treatment Neurologists Anticipate Prescribing SKYCLARYS for FA OMAV in FA HCP Demand Research September 2022 with 124 HCPs, 77 neurologists, 47 PCPs; PCP: Primary care physician % of Neurologists Surveyed Anticipate Prescribing SKYCLARYS
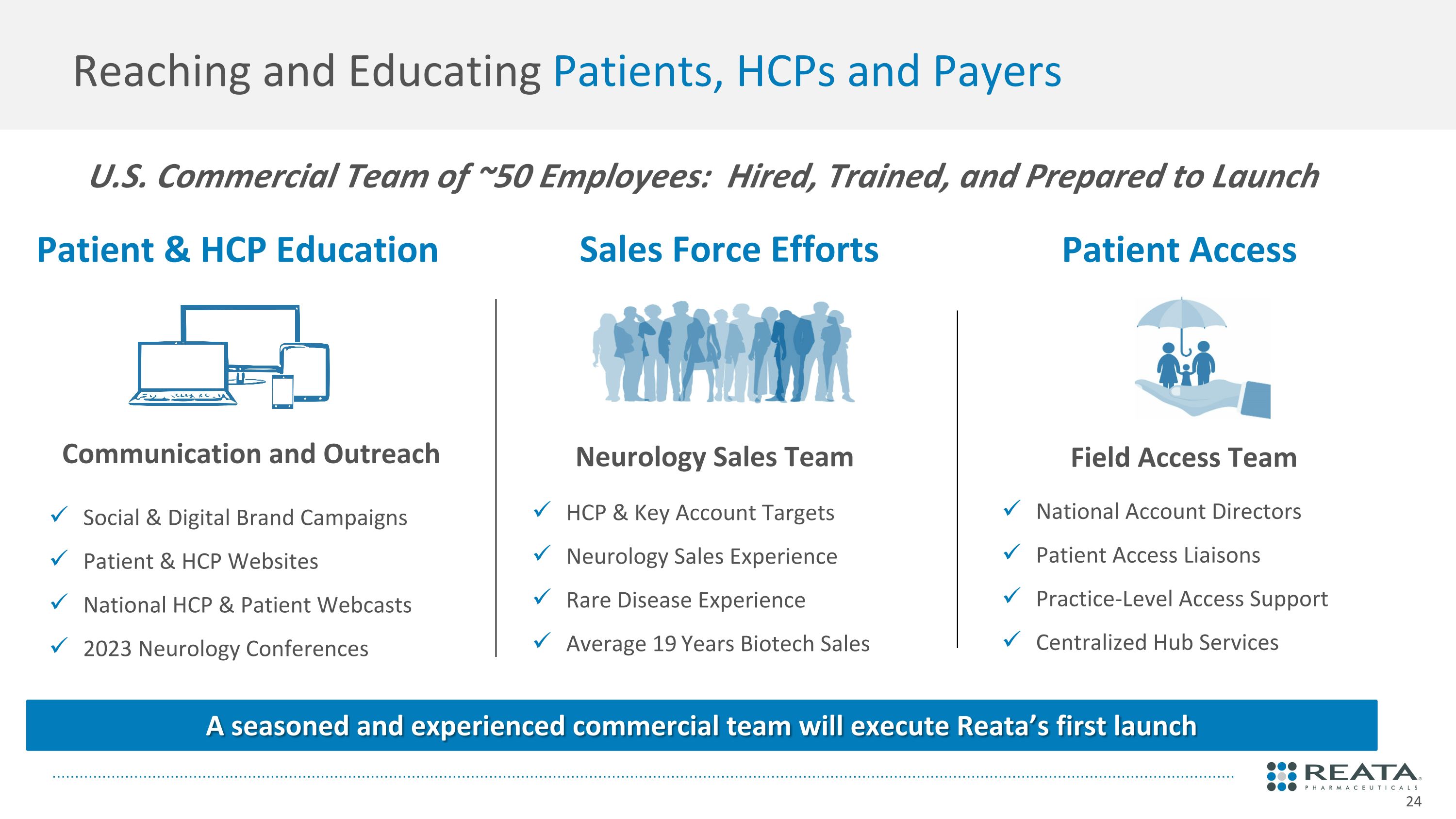
Reaching and Educating Patients, HCPs and Payers Patient & HCP Education Sales Force Efforts Patient Access Neurology Sales Team National Account Directors Patient Access Liaisons Practice-Level Access Support Centralized Hub Services HCP & Key Account Targets Neurology Sales Experience Rare Disease Experience Average 19 Years Biotech Sales Social & Digital Brand Campaigns Patient & HCP Websites National HCP & Patient Webcasts 2023 Neurology Conferences Field Access Team U.S. Commercial Team of ~50 Employees: Hired, Trained, and Prepared to Launch Communication and Outreach A seasoned and experienced commercial team will execute Reata’s first launch
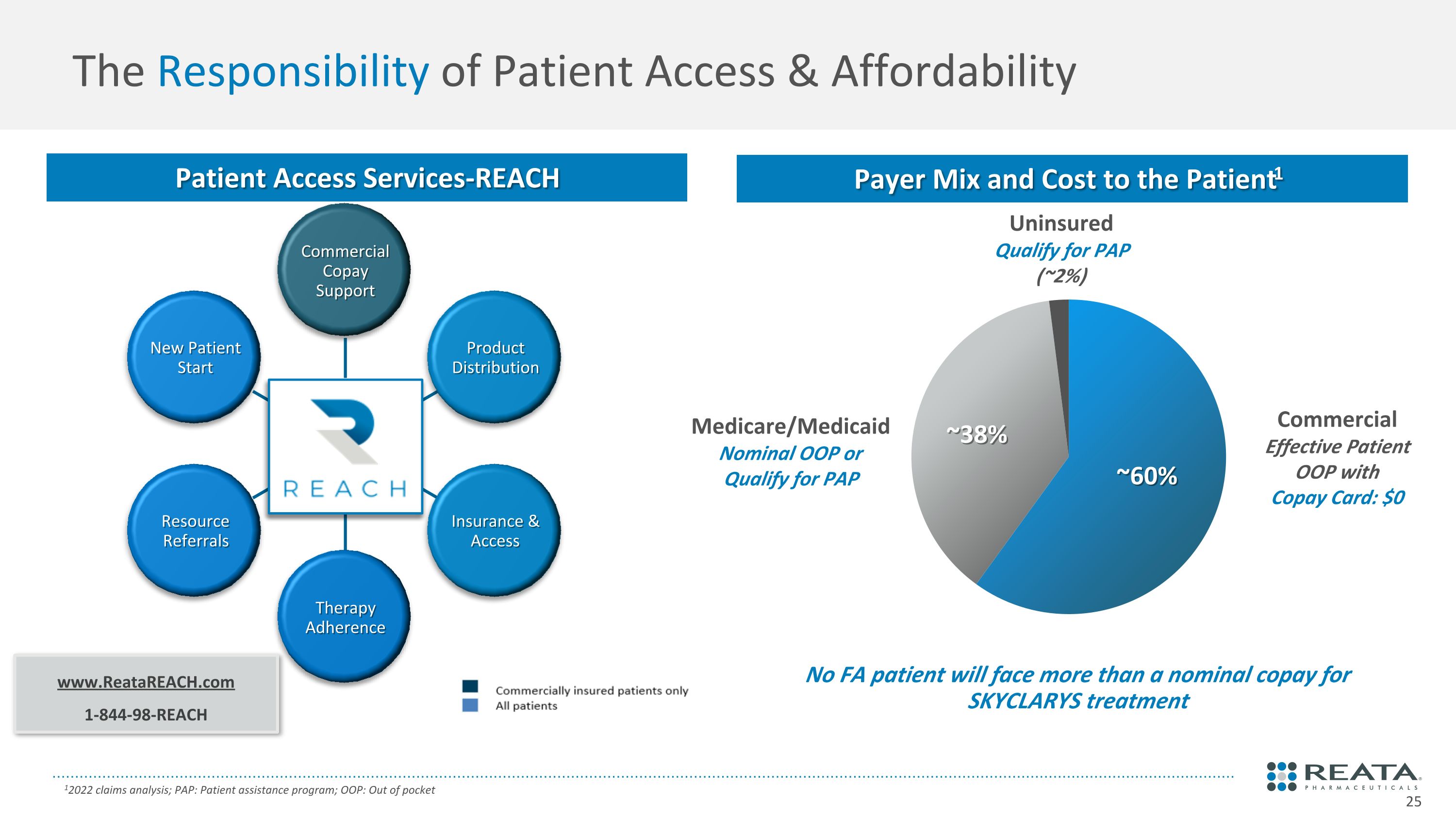
www.ReataREACH.com 1-844-98-REACH No FA patient will face more than a nominal copay for SKYCLARYS treatment The Responsibility of Patient Access & Affordability 12022 claims analysis; PAP: Patient assistance program; OOP: Out of pocket Commercial Copay Support Product Distribution Insurance & Access Therapy Adherence Resource Referrals New Patient Start Payer Mix and Cost to the Patient1 Patient Access Services-REACH Commercial Effective Patient OOP with Copay Card: $0 Medicare/Medicaid Nominal OOP or Qualify for PAP Uninsured Qualify for PAP (~2%) ~60% ~38%
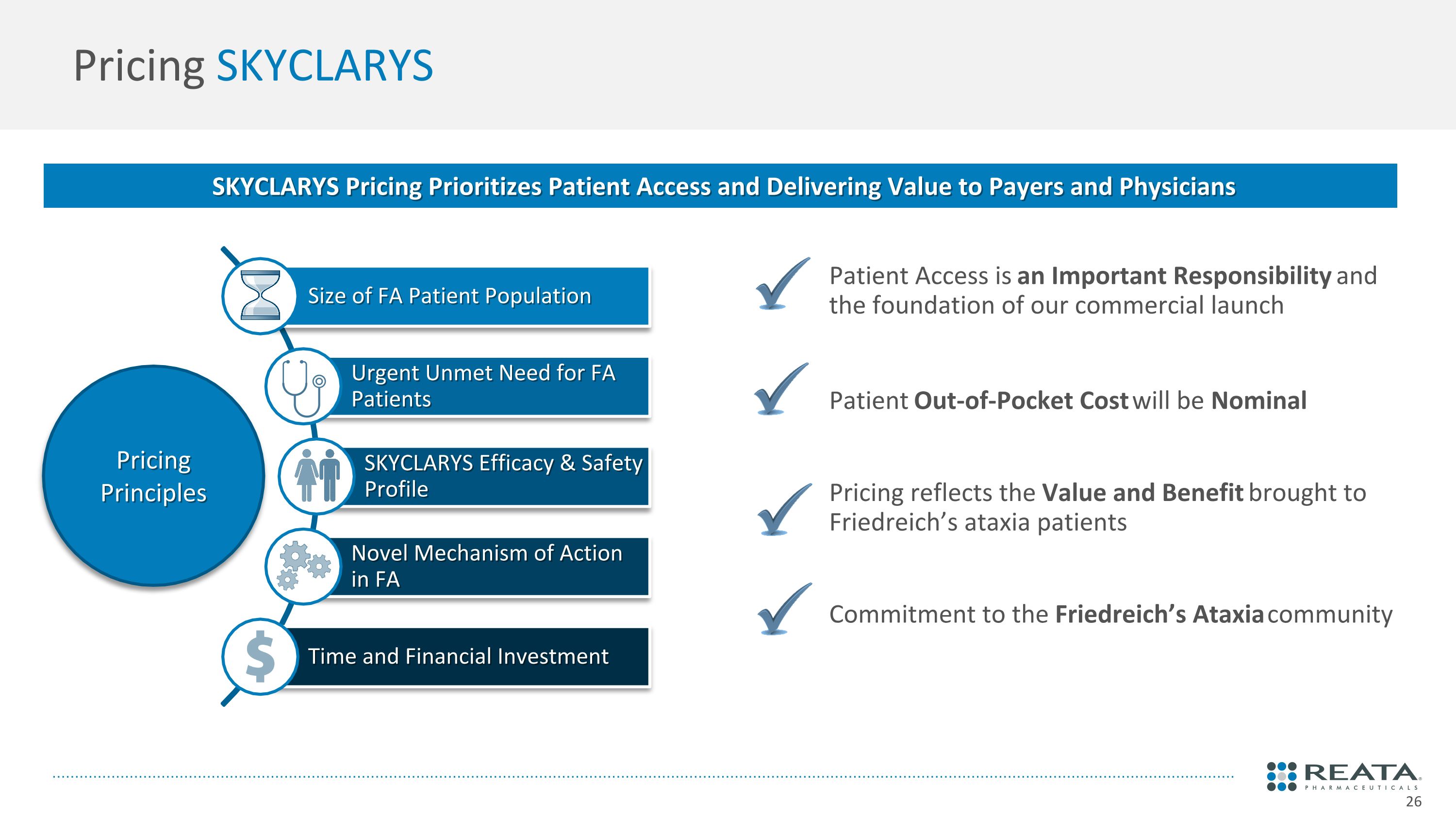
Pricing SKYCLARYS SKYCLARYS Pricing Prioritizes Patient Access and Delivering Value to Payers and Physicians Pricing Principles Size of FA Patient Population Urgent Unmet Need for FA Patients SKYCLARYS Efficacy & Safety Profile Novel Mechanism of Action in FA Time and Financial Investment Patient Access is an Important Responsibility and the foundation of our commercial launch Patient Out-of-Pocket Cost will be Nominal Pricing reflects the Value and Benefit brought to Friedreich’s ataxia patients Commitment to the Friedreich’s Ataxia community

Beginning Treatment with SKYCLARYS REACH Patient Access Services now active New Patient Start Forms available on the REACH website: www.reataREACH.com 1-844-98-REACH Find information about SKYCLARYS through the brand website: www.SKYCLARYS.com (Websites active within 24 hours) SKYCLARYS Commercial Product Available in Q2 2023 SKYCLARYS New Patient Start Form Not actual product picture

Operational and Financial Update

Operational Update Anticipate commercial drug supply to be available in the second quarter of 2023 Accelerating build up of our EU infrastructure following submission of MAA SKYCLARYS intellectual property Composition of Matter patents granted in U.S., Europe, Japan, China, and more than 20 other territories Could be extended 14 years post approval to 2037 in U.S. Could be extended as late as 2038 in Europe Not actual product picture

Financial Update Strong balance sheet Cash and marketable securities $387.5 million as of December 31, 2022 Option to monetize rare pediatric disease priority review voucher No outstanding funded debt Cash guidance Based on our operational plans, we reaffirm our cash guidance runway through the end of 2024

Closing Thoughts
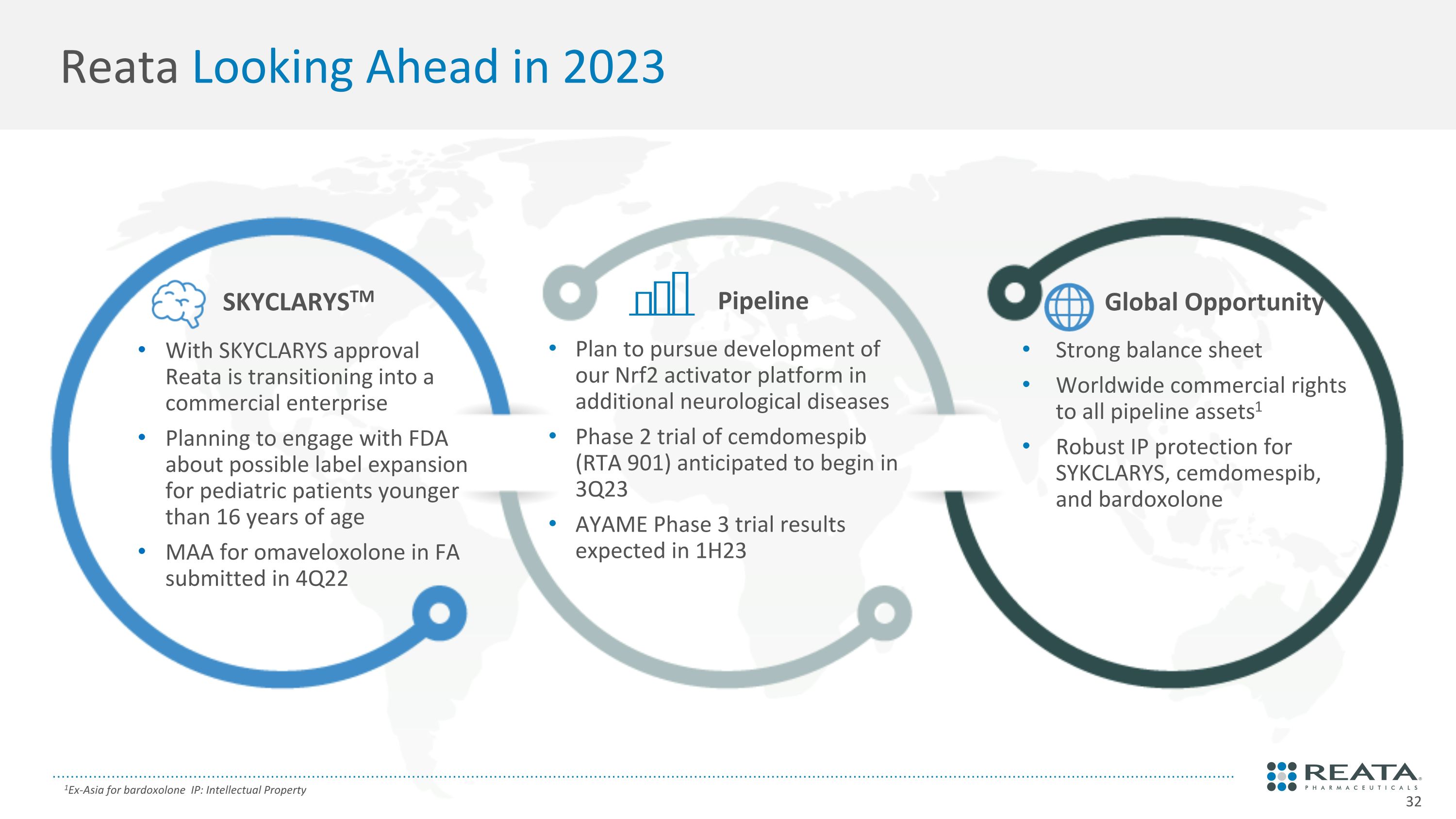
Reata Looking Ahead in 2023 With SKYCLARYS approval Reata is transitioning into a commercial enterprise Planning to engage with FDA about possible label expansion for pediatric patients younger than 16 years of age MAA for omaveloxolone in FA submitted in 4Q22 Plan to pursue development of our Nrf2 activator platform in additional neurological diseases Phase 2 trial of cemdomespib (RTA 901) anticipated to begin in 3Q23 AYAME Phase 3 trial results expected in 1H23 Strong balance sheet Worldwide commercial rights to all pipeline assets1 Robust IP protection for SYKCLARYS, cemdomespib, and bardoxolone 1Ex-Asia for bardoxolone IP: Intellectual Property SKYCLARYSTM Pipeline Global Opportunity

Q&A
































