
Program update of Bardoxolone Methyl in CKD Exhibit 99.2

Forward-Looking Statements This presentation contains certain “forward-looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward-looking statements, including statements regarding our future financial condition, business strategy, and plans and objectives of management for future operations. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “aim,” “assume,” “anticipate,” “contemplate,” “model,” “intend,” “target,” “project,” “should,” “plan,” “expect,” “predict,” “could,” “possible,” “seek,” “goal”, “potential,” “hypothesize,” “likely” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecast in these statements. Any differences could be caused by a number of factors including but not limited to: the success, cost, and timing of our product development activities and clinical trials; our ability to advance our NRF2 Activators and other technologies; our ability to obtain and maintain regulatory approval of our product candidates, and limitations and warnings in the label of an approved product candidate; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the commercialization of our product candidates, if approved; the rate and degree of market acceptance of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to identify target patient populations and serve those markets, especially for diseases with small patient populations; the success of competing therapies that are or may become available; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; our ability to attract collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; and regulatory developments in the United States and foreign countries. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. All forward-looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward-looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Outline for Bardoxolone CKD Program Update Review of Phase 2 CARDINAL data Review of Phase 2 TSUBAKI data Other pharmacologic effects of bardoxolone in CKD patients Overview of PHOENIX program Questions

Cardinal phase 2 data review
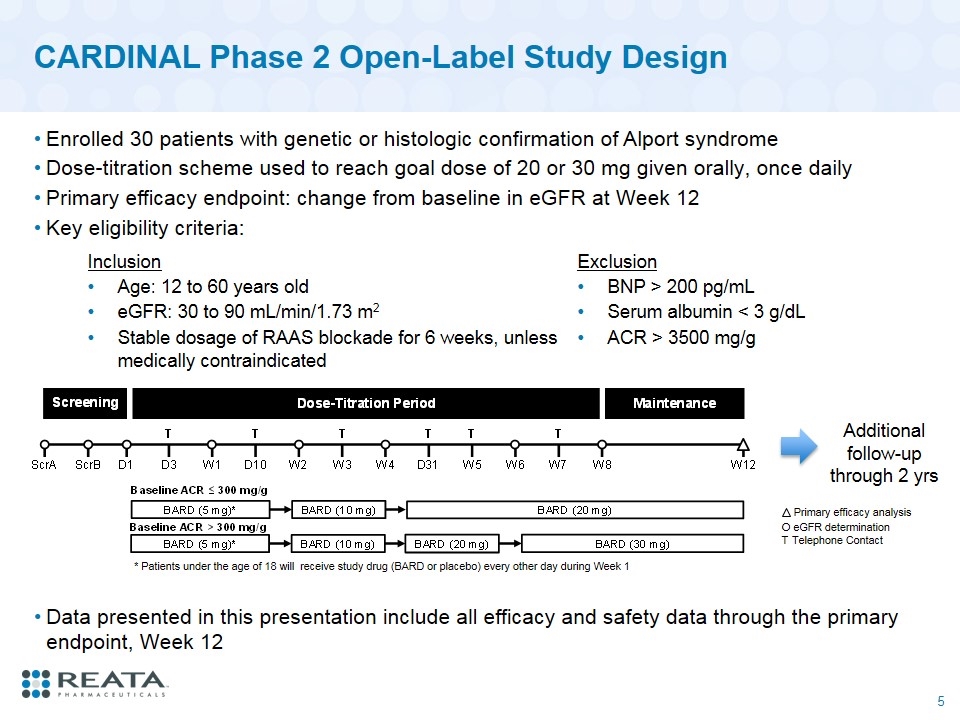
CARDINAL Phase 2 Open-Label Study Design Enrolled 30 patients with genetic or histologic confirmation of Alport syndrome Dose-titration scheme used to reach goal dose of 20 or 30 mg given orally, once daily Primary efficacy endpoint: change from baseline in eGFR at Week 12 Key eligibility criteria: O eGFR determination T Telephone Contact Primary efficacy analysis * Patients under the age of 18 will receive study drug (BARD or placebo) every other day during Week 1 Inclusion Exclusion Age: 12 to 60 years old BNP > 200 pg/mL eGFR: 30 to 90 mL/min/1.73 m2 Serum albumin < 3 g/dL Stable dosage of RAAS blockade for 6 weeks, unless medically contraindicated ACR > 3500 mg/g Additional follow-up through 2 yrs Data presented in this presentation include all efficacy and safety data through the primary endpoint, Week 12
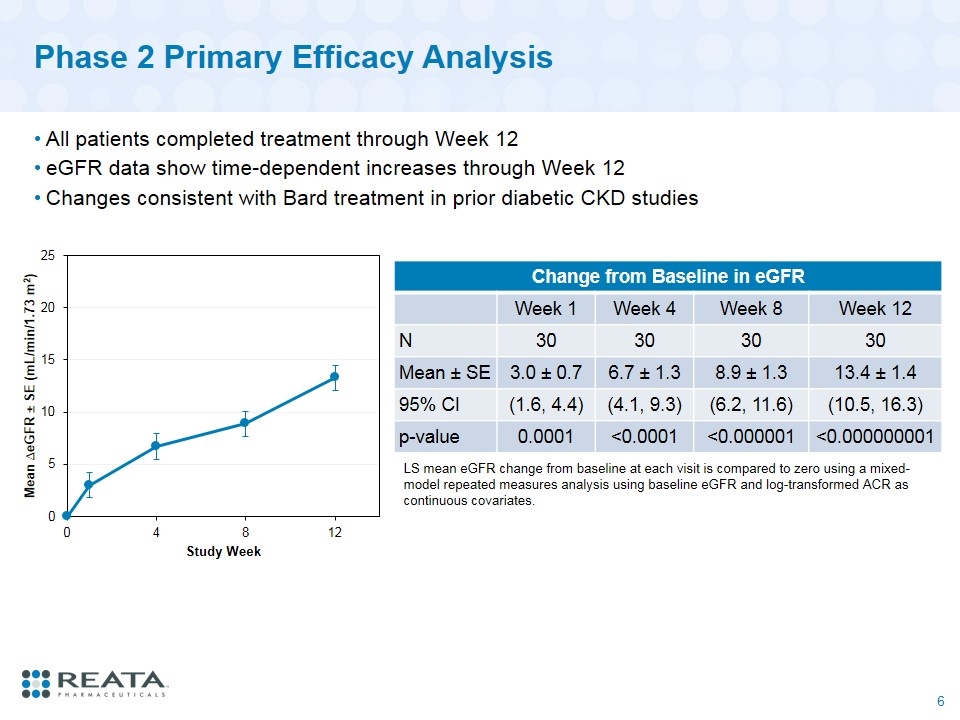
Phase 2 Primary Efficacy Analysis All patients completed treatment through Week 12 eGFR data show time-dependent increases through Week 12 Changes consistent with Bard treatment in prior diabetic CKD studies Change from Baseline in eGFR Week 1 Week 4 Week 8 Week 12 N 30 30 30 30 Mean ± SE 3.0 ± 0.7 6.7 ± 1.3 8.9 ± 1.3 13.4 ± 1.4 95% CI (1.6, 4.4) (4.1, 9.3) (6.2, 11.6) (10.5, 16.3) p-value 0.0001 <0.0001 <0.000001 <0.000000001 LS mean eGFR change from baseline at each visit is compared to zero using a mixed-model repeated measures analysis using baseline eGFR and log-transformed ACR as continuous covariates.
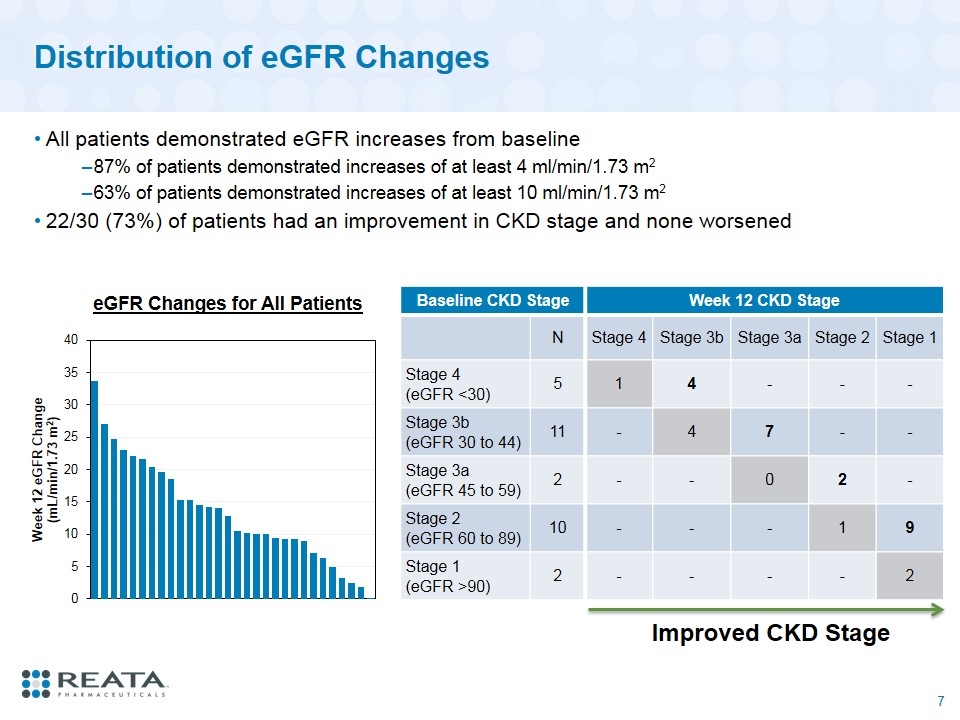
Distribution of eGFR Changes All patients demonstrated eGFR increases from baseline 87% of patients demonstrated increases of at least 4 ml/min/1.73 m2 63% of patients demonstrated increases of at least 10 ml/min/1.73 m2 22/30 (73%) of patients had an improvement in CKD stage and none worsened Baseline CKD Stage Week 12 CKD Stage N Stage 4 Stage 3b Stage 3a Stage 2 Stage 1 Stage 4 (eGFR <30) 5 1 4 - - - Stage 3b (eGFR 30 to 44) 11 - 4 7 - - Stage 3a (eGFR 45 to 59) 2 - - 0 2 - Stage 2 (eGFR 60 to 89) 10 - - - 1 9 Stage 1 (eGFR >90) 2 - - - - 2 Improved CKD Stage eGFR Changes for All Patients
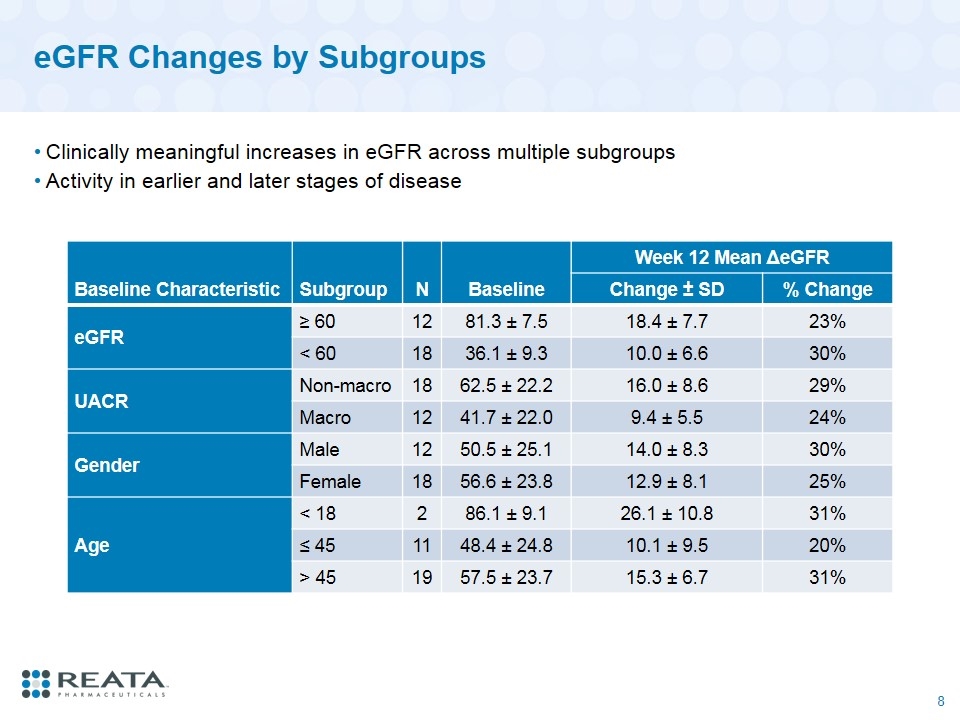
eGFR Changes by Subgroups Clinically meaningful increases in eGFR across multiple subgroups Activity in earlier and later stages of disease Baseline Characteristic Subgroup N Baseline Week 12 Mean ΔeGFR Change ± SD % Change eGFR ≥ 60 12 81.3 ± 7.5 18.4 ± 7.7 23% < 60 18 36.1 ± 9.3 10.0 ± 6.6 30% UACR Non-macro 18 62.5 ± 22.2 16.0 ± 8.6 29% Macro 12 41.7 ± 22.0 9.4 ± 5.5 24% Gender Male 12 50.5 ± 25.1 14.0 ± 8.3 30% Female 18 56.6 ± 23.8 12.9 ± 8.1 25% Age < 18 2 86.1 ± 9.1 26.1 ± 10.8 31% ≤ 45 11 48.4 ± 24.8 10.1 ± 9.5 20% > 45 19 57.5 ± 23.7 15.3 ± 6.7 31%
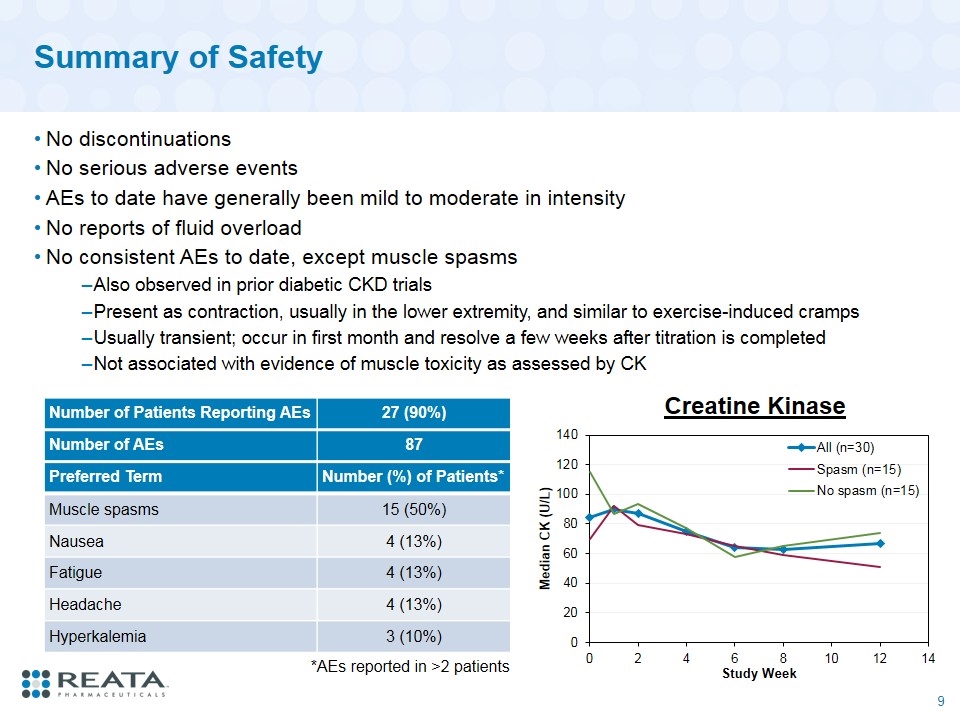
Summary of Safety No discontinuations No serious adverse events AEs to date have generally been mild to moderate in intensity No reports of fluid overload No consistent AEs to date, except muscle spasms Also observed in prior diabetic CKD trials Present as contraction, usually in the lower extremity, and similar to exercise-induced cramps Usually transient; occur in first month and resolve a few weeks after titration is completed Not associated with evidence of muscle toxicity as assessed by CK Number of Patients Reporting AEs 27 (90%) Number of AEs 87 Preferred Term Number (%) of Patients* Muscle spasms 15 (50%) Nausea 4 (13%) Fatigue 4 (13%) Headache 4 (13%) Hyperkalemia 3 (10%) *AEs reported in >2 patients Creatine Kinase
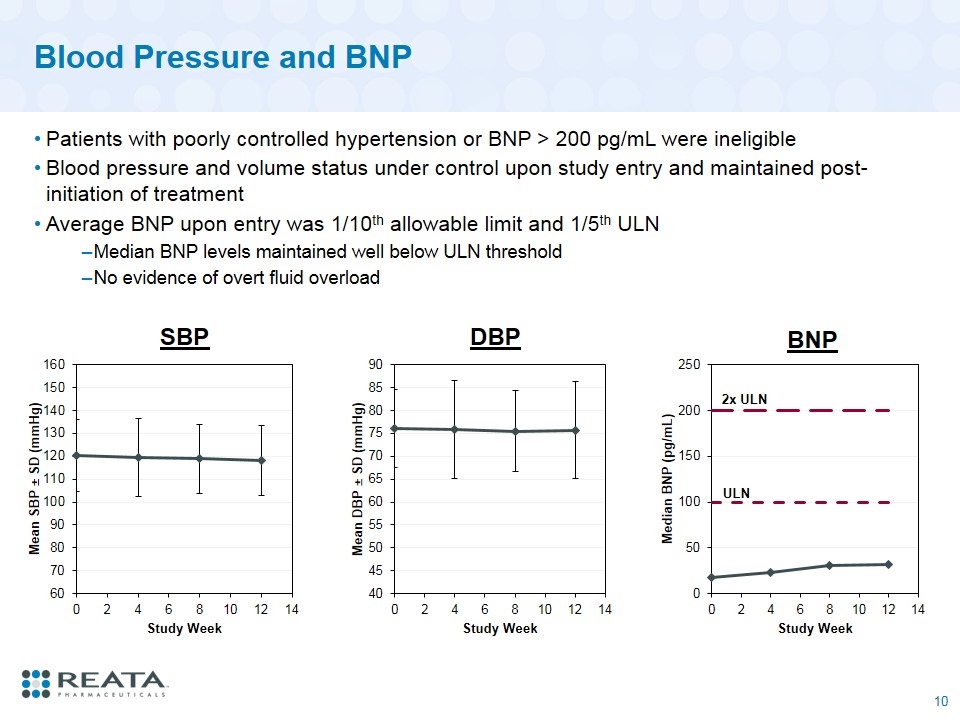
Blood Pressure and BNP Patients with poorly controlled hypertension or BNP > 200 pg/mL were ineligible Blood pressure and volume status under control upon study entry and maintained post-initiation of treatment Average BNP upon entry was 1/10th allowable limit and 1/5th ULN Median BNP levels maintained well below ULN threshold No evidence of overt fluid overload SBP DBP 2x ULN BNP ULN
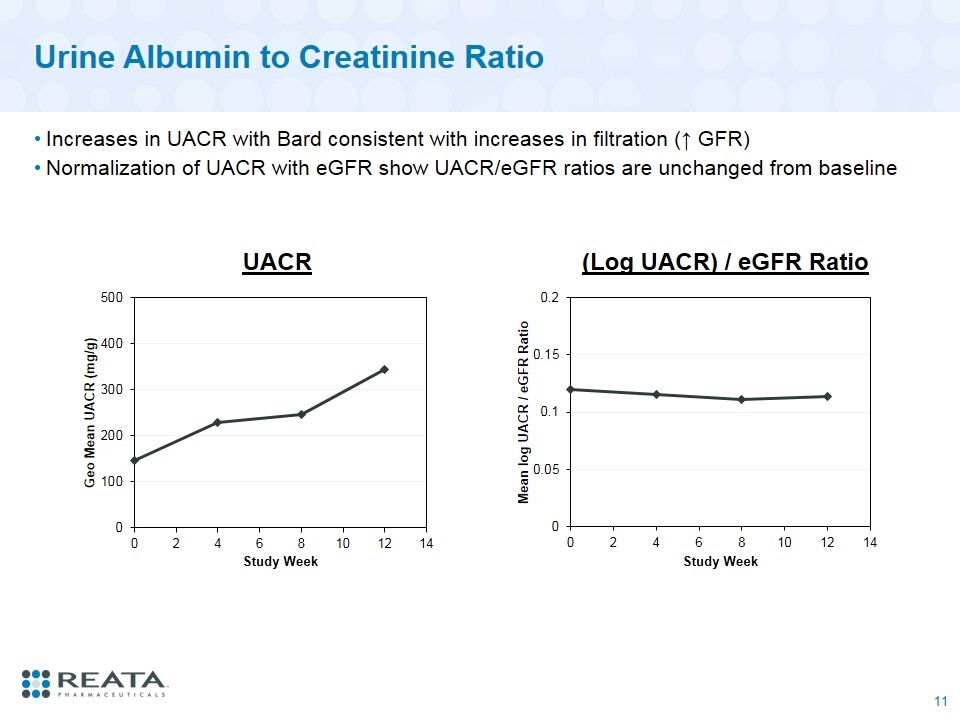
Urine Albumin to Creatinine Ratio Increases in UACR with Bard consistent with increases in filtration (↑ GFR) Normalization of UACR with eGFR show UACR/eGFR ratios are unchanged from baseline UACR (Log UACR) / eGFR Ratio
Conclusions Phase 2 CARDINAL study demonstrates bardoxolone methyl significantly increases eGFR in patients with Alport syndrome after 12 weeks of treatment eGFR increases in CARDINAL were observed over full range of baseline eGFR values (range: 28 to 94 mL/min/1.73 m2) and across multiple subgroups Most patients demonstrated improvements in CKD stage Increases are similar in magnitude to those previously observed in patients with type 2 diabetes and Stage 3b-4 CKD Bard was well tolerated in patients with Alport syndrome No discontinuations from study No serious adverse events No effect on blood pressure When normalized by change in eGFR, urinary protein was unchanged from baseline AEs to date have been mild to moderate in intensity Muscle spasms were most commonly reported AE and not associated with evidence of muscle toxicity Phase 3 portion of CARDINAL study is actively enrolling

tsubaki phase 2 data review
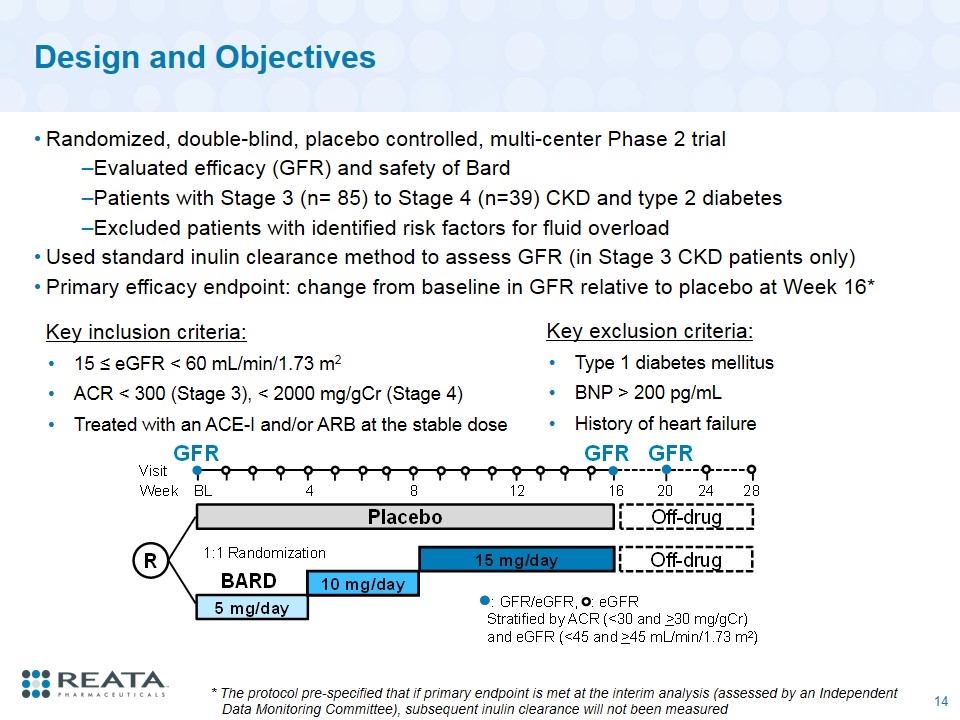
Design and Objectives Randomized, double-blind, placebo controlled, multi-center Phase 2 trial Evaluated efficacy (GFR) and safety of Bard Patients with Stage 3 (n= 85) to Stage 4 (n=39) CKD and type 2 diabetes Excluded patients with identified risk factors for fluid overload Used standard inulin clearance method to assess GFR (in Stage 3 CKD patients only) Primary efficacy endpoint: change from baseline in GFR relative to placebo at Week 16* Key inclusion criteria: 15 ≤ eGFR < 60 mL/min/1.73 m2 ACR < 300 (Stage 3), < 2000 mg/gCr (Stage 4) Treated with an ACE-I and/or ARB at the stable dose Key exclusion criteria: Type 1 diabetes mellitus BNP > 200 pg/mL History of heart failure * The protocol pre-specified that if primary endpoint is met at the interim analysis (assessed by an Independent Data Monitoring Committee), subsequent inulin clearance will not been measured
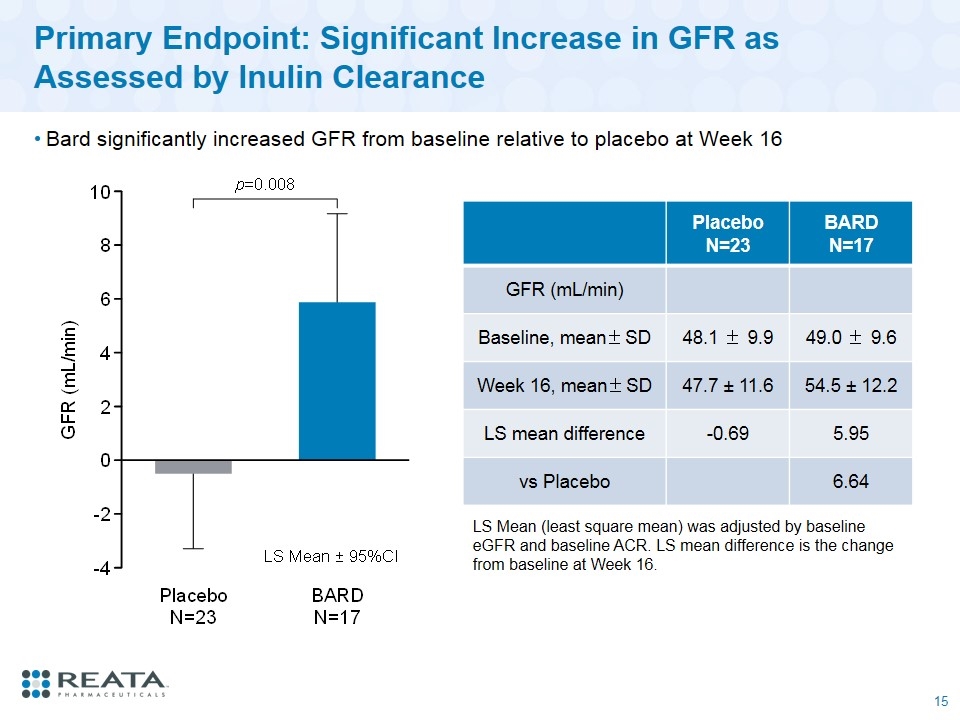
Primary Endpoint: Significant Increase in GFR as Assessed by Inulin Clearance Bard significantly increased GFR from baseline relative to placebo at Week 16 Placebo N=23 BARD N=17 GFR (mL/min) Baseline, mean±SD 48.1 ± 9.9 49.0 ± 9.6 Week 16, mean±SD 47.7 ± 11.6 54.5 ± 12.2 LS mean difference -0.69 5.95 vs Placebo 6.64 LS Mean (least square mean) was adjusted by baseline eGFR and baseline ACR. LS mean difference is the change from baseline at Week 16.
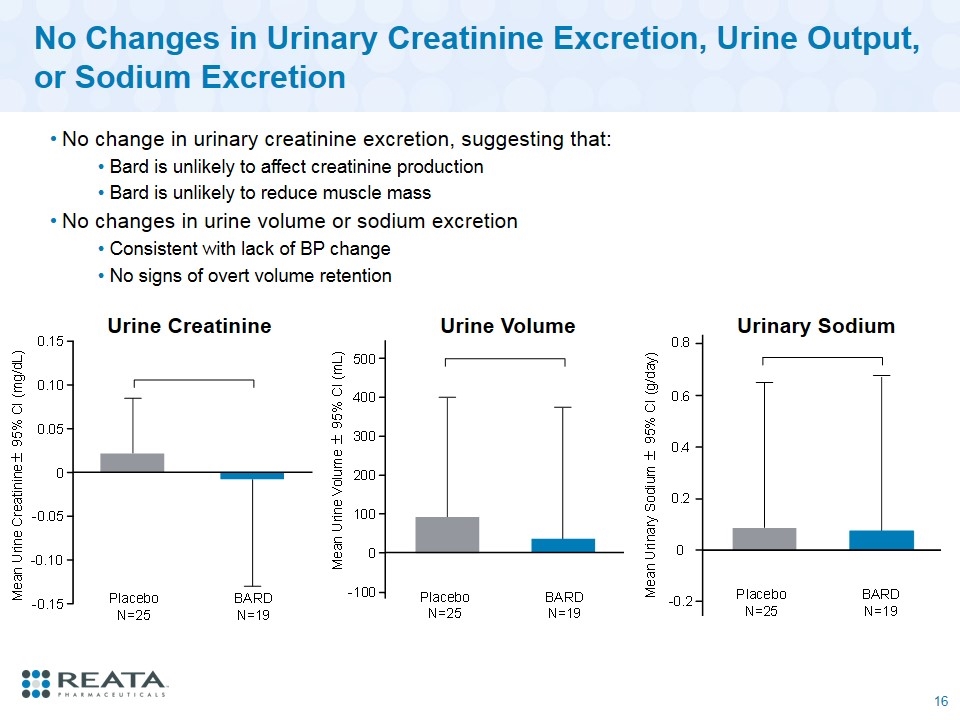
No Changes in Urinary Creatinine Excretion, Urine Output, or Sodium Excretion No change in urinary creatinine excretion, suggesting that: Bard is unlikely to affect creatinine production Bard is unlikely to reduce muscle mass No changes in urine volume or sodium excretion Consistent with lack of BP change No signs of overt volume retention Urine Creatinine Urine Volume Urinary Sodium
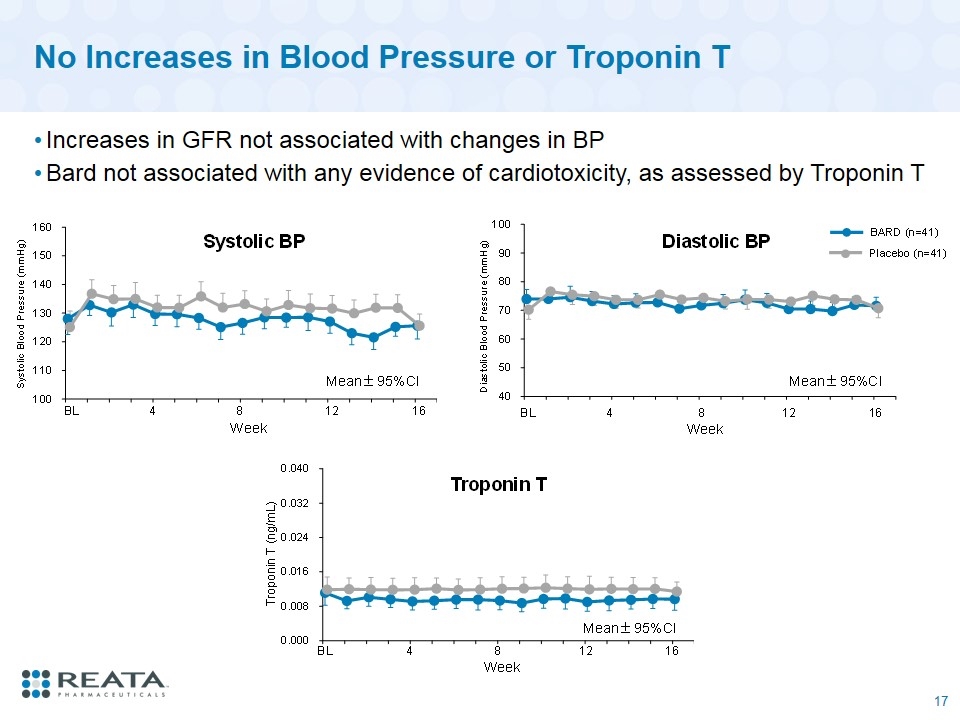
No Increases in Blood Pressure or Troponin T Increases in GFR not associated with changes in BP Bard not associated with any evidence of cardiotoxicity, as assessed by Troponin T
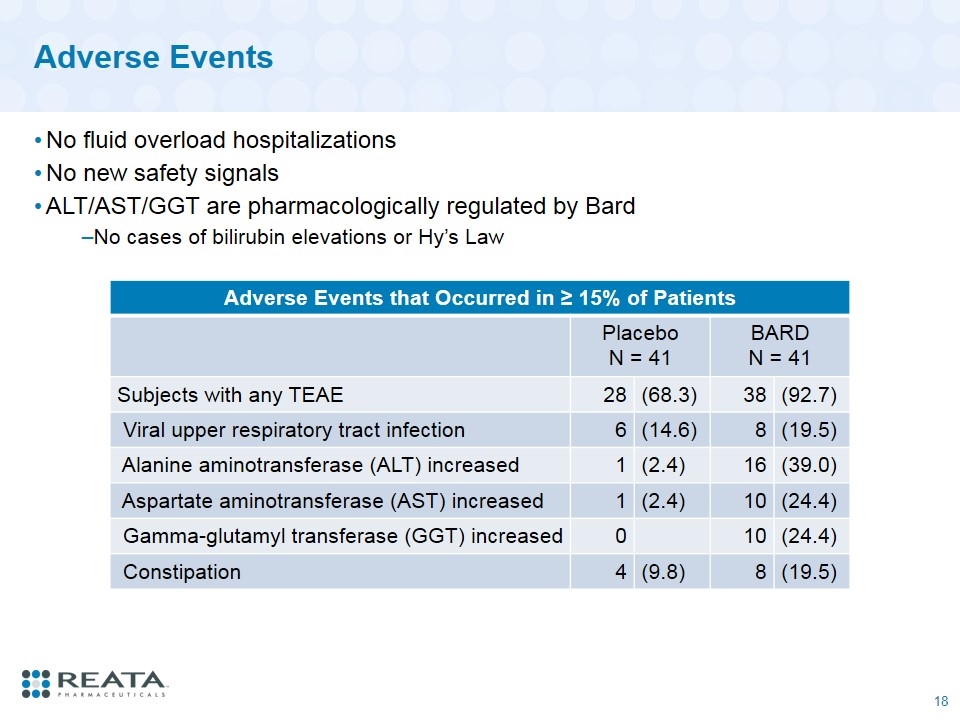
Adverse Events Adverse Events that Occurred in ≥ 15% of Patients Placebo N = 41 BARD N = 41 Subjects with any TEAE 28 (68.3) 38 (92.7) Viral upper respiratory tract infection 6 (14.6) 8 (19.5) Alanine aminotransferase (ALT) increased 1 (2.4) 16 (39.0) Aspartate aminotransferase (AST) increased 1 (2.4) 10 (24.4) Gamma-glutamyl transferase (GGT) increased 0 10 (24.4) Constipation 4 (9.8) 8 (19.5) No fluid overload hospitalizations No new safety signals ALT/AST/GGT are pharmacologically regulated by Bard No cases of bilirubin elevations or Hy’s Law
Conclusions and Summary BARD significantly improved renal function (GFR) as assessed by inulin clearance By prospectively enrolling patients who were not at-risk for fluid retention, BARD appeared well tolerated without any major safety concerns No fluid overload-related hospitalizations No new safety signals Trends in efficacy and safety parameters for Stage 4 CKD patients similar to those observed in Stage 3 CKD patients KHK is planning to initiate a large Phase 3 trial in diabetic CKD patients in Japan to further evaluate safety and efficacy in 2018

Other Pharmacologic Effects of bardoxolone in ckd
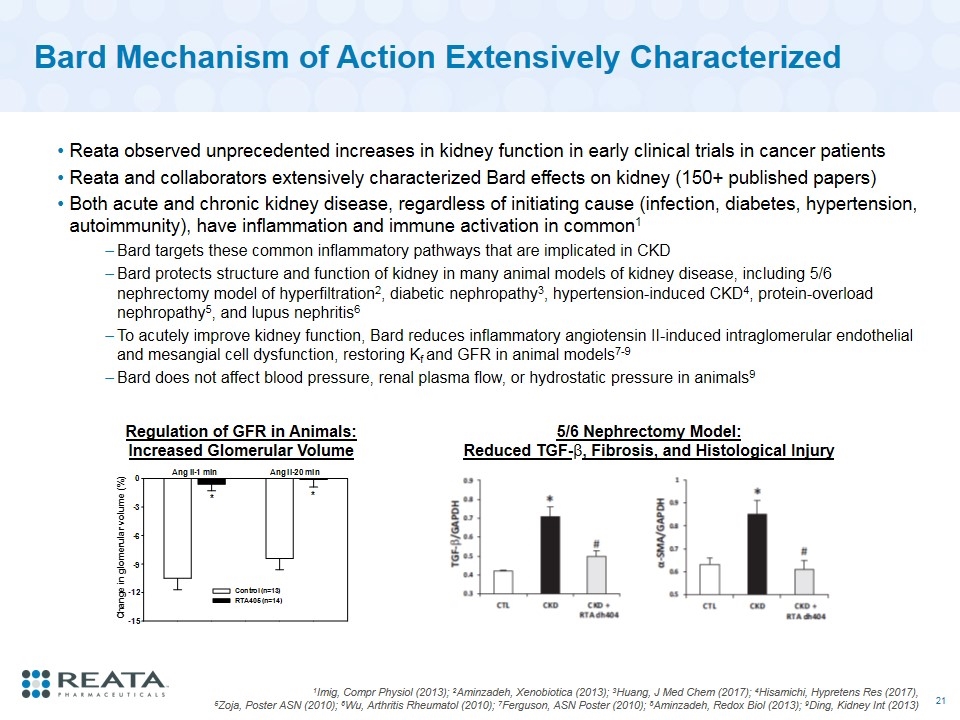
Bard Mechanism of Action Extensively Characterized Reata observed unprecedented increases in kidney function in early clinical trials in cancer patients Reata and collaborators extensively characterized Bard effects on kidney (150+ published papers) Both acute and chronic kidney disease, regardless of initiating cause (infection, diabetes, hypertension, autoimmunity), have inflammation and immune activation in common1 Bard targets these common inflammatory pathways that are implicated in CKD Bard protects structure and function of kidney in many animal models of kidney disease, including 5/6 nephrectomy model of hyperfiltration2, diabetic nephropathy3, hypertension-induced CKD4, protein-overload nephropathy5, and lupus nephritis6 To acutely improve kidney function, Bard reduces inflammatory angiotensin II-induced intraglomerular endothelial and mesangial cell dysfunction, restoring Kf and GFR in animal models7-9 Bard does not affect blood pressure, renal plasma flow, or hydrostatic pressure in animals9 1Imig, Compr Physiol (2013); 2Aminzadeh, Xenobiotica (2013); 3Huang, J Med Chem (2017); 4Hisamichi, Hypretens Res (2017), 5Zoja, Poster ASN (2010); 6Wu, Arthritis Rheumatol (2010); 7Ferguson, ASN Poster (2010); 8Aminzadeh, Redox Biol (2013); 9Ding, Kidney Int (2013) Regulation of GFR in Animals: Increased Glomerular Volume 5/6 Nephrectomy Model: Reduced TGF-β, Fibrosis, and Histological Injury
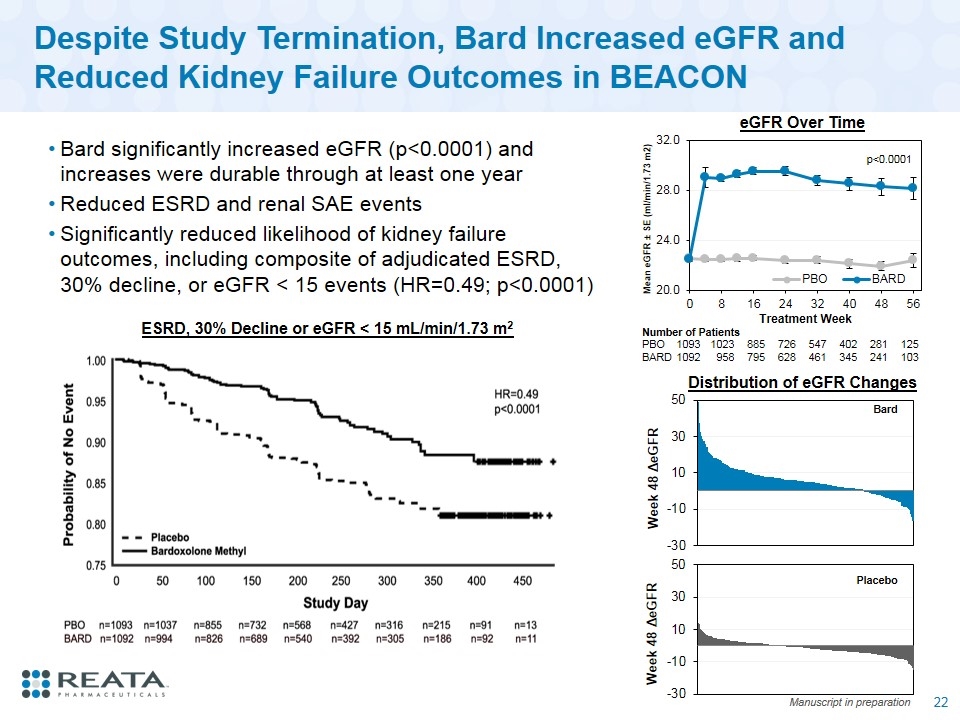
Despite Study Termination, Bard Increased eGFR and Reduced Kidney Failure Outcomes in BEACON Bard significantly increased eGFR (p<0.0001) and increases were durable through at least one year Reduced ESRD and renal SAE events Significantly reduced likelihood of kidney failure outcomes, including composite of adjudicated ESRD, 30% decline, or eGFR < 15 events (HR=0.49; p<0.0001) ESRD, 30% Decline or eGFR < 15 mL/min/1.73 m2 Number of Patients PBO 1093 1023 885 726 547 402 281 125 BARD 1092 958 795 628 461 345 241 103 eGFR Over Time Manuscript in preparation Bard Placebo Distribution of eGFR Changes p<0.0001
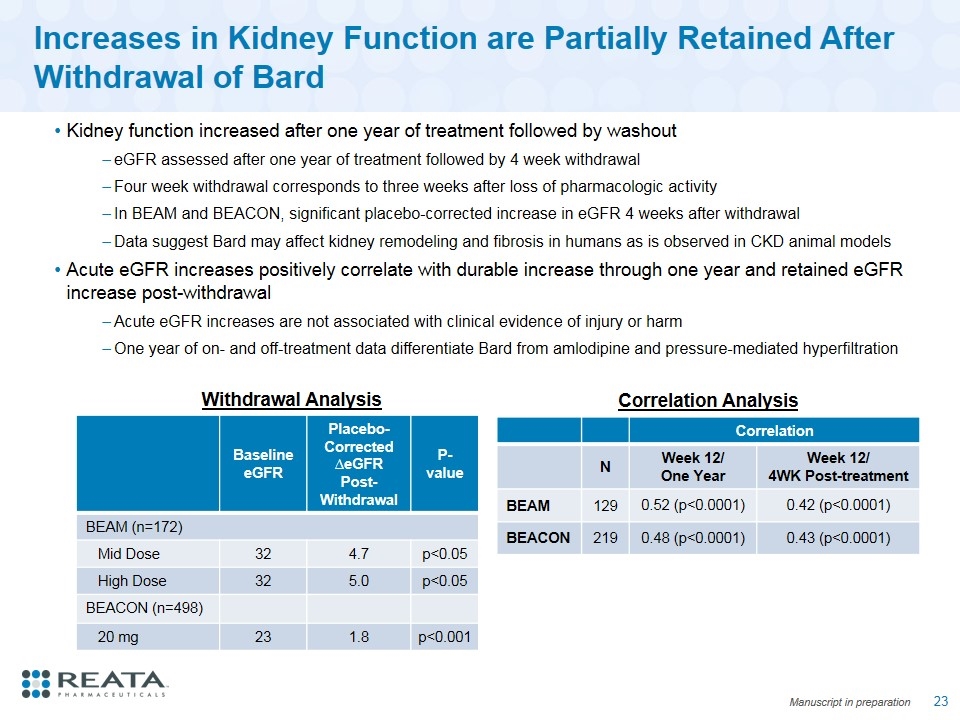
Increases in Kidney Function are Partially Retained After Withdrawal of Bard Kidney function increased after one year of treatment followed by washout eGFR assessed after one year of treatment followed by 4 week withdrawal Four week withdrawal corresponds to three weeks after loss of pharmacologic activity In BEAM and BEACON, significant placebo-corrected increase in eGFR 4 weeks after withdrawal Data suggest Bard may affect kidney remodeling and fibrosis in humans as is observed in CKD animal models Acute eGFR increases positively correlate with durable increase through one year and retained eGFR increase post-withdrawal Acute eGFR increases are not associated with clinical evidence of injury or harm One year of on- and off-treatment data differentiate Bard from amlodipine and pressure-mediated hyperfiltration Correlation N Week 12/ One Year Week 12/ 4WK Post-treatment BEAM 129 0.52 (p<0.0001) 0.42 (p<0.0001) BEACON 219 0.48 (p<0.0001) 0.43 (p<0.0001) Withdrawal Analysis Baseline eGFR Placebo-Corrected ∆eGFR Post-Withdrawal P-value BEAM (n=172) Mid Dose 32 4.7 p<0.05 High Dose 32 5.0 p<0.05 BEACON (n=498) 20 mg 23 1.8 p<0.001 Correlation Analysis Manuscript in preparation
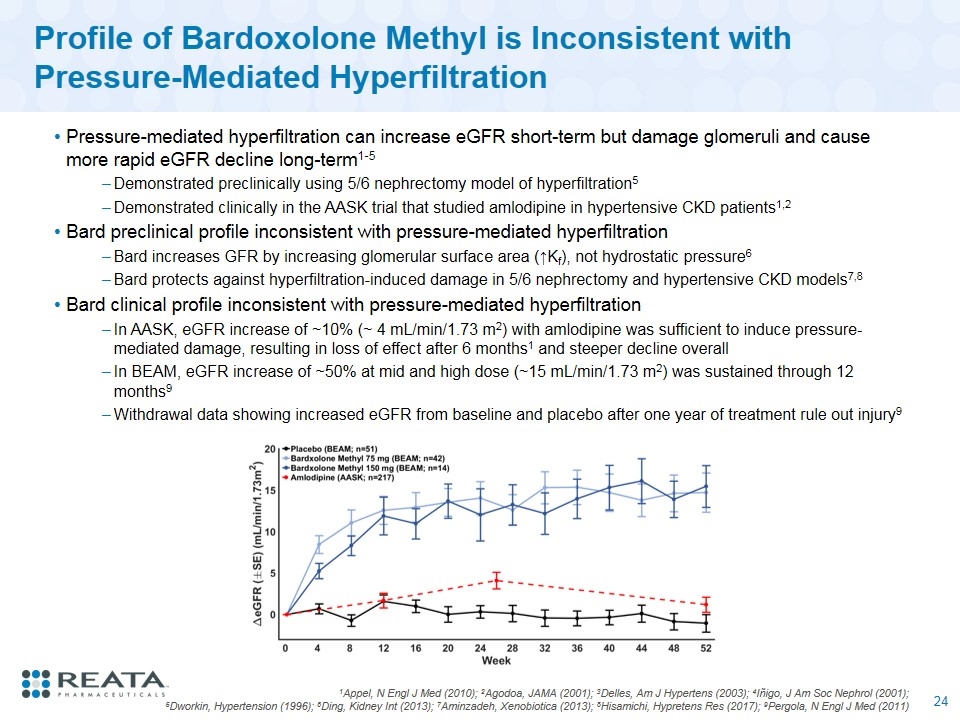
Profile of Bardoxolone Methyl is Inconsistent with Pressure-Mediated Hyperfiltration Pressure-mediated hyperfiltration can increase eGFR short-term but damage glomeruli and cause more rapid eGFR decline long-term1-5 Demonstrated preclinically using 5/6 nephrectomy model of hyperfiltration5 Demonstrated clinically in the AASK trial that studied amlodipine in hypertensive CKD patients1,2 Bard preclinical profile inconsistent with pressure-mediated hyperfiltration Bard increases GFR by increasing glomerular surface area (↑Kf), not hydrostatic pressure6 Bard protects against hyperfiltration-induced damage in 5/6 nephrectomy and hypertensive CKD models7,8 Bard clinical profile inconsistent with pressure-mediated hyperfiltration In AASK, eGFR increase of ~10% (~ 4 mL/min/1.73 m2) with amlodipine was sufficient to induce pressure-mediated damage, resulting in loss of effect after 6 months1 and steeper decline overall In BEAM, eGFR increase of ~50% at mid and high dose (~15 mL/min/1.73 m2) was sustained through 12 months9 Withdrawal data showing increased eGFR from baseline and placebo after one year of treatment rule out injury9 1Appel, N Engl J Med (2010); 2Agodoa, JAMA (2001); 3Delles, Am J Hypertens (2003); 4Iñigo, J Am Soc Nephrol (2001); 5Dworkin, Hypertension (1996); 6Ding, Kidney Int (2013); 7Aminzadeh, Xenobiotica (2013); 8Hisamichi, Hypretens Res (2017); 9Pergola, N Engl J Med (2011)
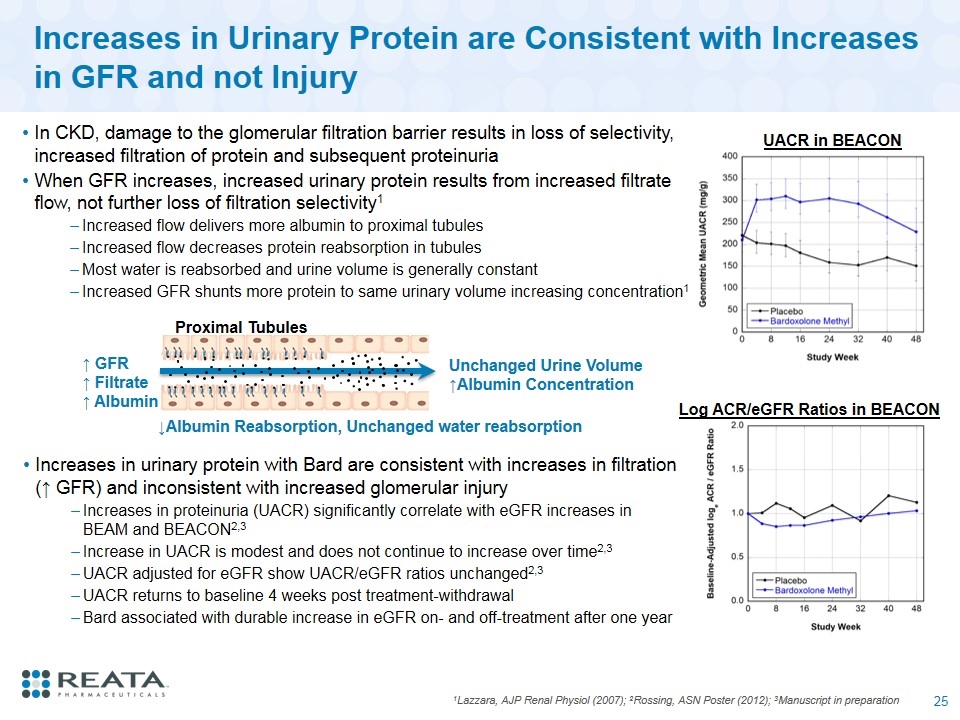
Increases in Urinary Protein are Consistent with Increases in GFR and not Injury 1Lazzara, AJP Renal Physiol (2007); 2Rossing, ASN Poster (2012); 3Manuscript in preparation In CKD, damage to the glomerular filtration barrier results in loss of selectivity, increased filtration of protein and subsequent proteinuria When GFR increases, increased urinary protein results from increased filtrate flow, not further loss of filtration selectivity1 Increased flow delivers more albumin to proximal tubules Increased flow decreases protein reabsorption in tubules Most water is reabsorbed and urine volume is generally constant Increased GFR shunts more protein to same urinary volume increasing concentration1 Log ACR/eGFR Ratios in BEACON UACR in BEACON ↑ GFR ↑ Filtrate ↑ Albumin Proximal Tubules ↓Albumin Reabsorption, Unchanged water reabsorption Unchanged Urine Volume ↑Albumin Concentration Increases in urinary protein with Bard are consistent with increases in filtration (↑ GFR) and inconsistent with increased glomerular injury Increases in proteinuria (UACR) significantly correlate with eGFR increases in BEAM and BEACON2,3 Increase in UACR is modest and does not continue to increase over time2,3 UACR adjusted for eGFR show UACR/eGFR ratios unchanged2,3 UACR returns to baseline 4 weeks post treatment-withdrawal Bard associated with durable increase in eGFR on- and off-treatment after one year

phoenix phase 2 program overview
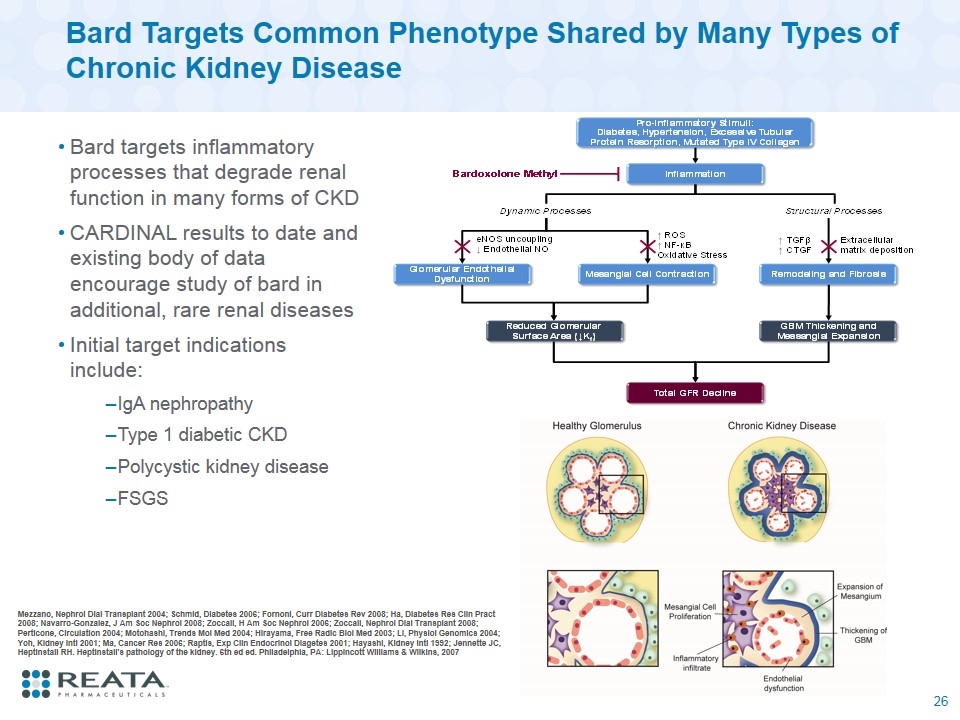
Bard Targets Common Phenotype Shared by Many Types of Chronic Kidney Disease Bard targets inflammatory processes that degrade renal function in many forms of CKD CARDINAL results to date and existing body of data encourage study of bard in additional, rare renal diseases Initial target indications include: IgA nephropathy Type 1 diabetic CKD Polycystic kidney disease FSGS Mezzano, Nephrol Dial Transplant 2004; Schmid, Diabetes 2006; Fornoni, Curr Diabetes Rev 2008; Ha, Diabetes Res Clin Pract 2008; Navarro-Gonzalez, J Am Soc Nephrol 2008; Zoccali, H Am Soc Nephrol 2006; Zoccali, Nephrol Dial Transplant 2008; Perticone, Circulation 2004; Motohashi, Trends Mol Med 2004; Hirayama, Free Radic Biol Med 2003; Li, Physiol Genomics 2004; Yoh, Kidney Intl 2001; Ma, Cancer Res 2006; Raptis, Exp Clin Endocrinol Diagetes 2001; Hayashi, Kidney Intl 1992; Jennette JC, Heptinstall RH. Heptinstall's pathology of the kidney. 6th ed ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007 26
PHOENIX Program Underway Similar to CARDINAL Phase 2 design: 12 weeks of open-label treatment, titration to goal dose of 20 or 30 mg of Bard orally, once daily Key eligibility criteria similar to CARDINAL design Stable doses of background meds (ACEi, ARB, insulin, prednisone, etc.) Will enroll 20-30 patients per indication powered to detect a change of 3.4 mL/min/1.73 m2 Each indication represents a substantial market expansion opportunity for Reata Disease U.S. Prevalence Disease Characteristics IgA Nephropathy ~100,000 IgA deposits inflame and damage glomeruli, causing blood and protein leakage and scarring that progresses to ESRD T1 Diabetic CKD ~100,000 Chronic hyperglycemia results in GBM thickening, inflammation, mesangial expansion ADPKD 500,000 to 600,000 Cyst growth damages kidney tissue resulting in inflammation, fibrosis, and renal function decline FSGS ~ 40,000 Proteinuria and nephrotic syndrome with worsening hypertension and progressive loss of renal function; patients with massive proteinuria (>10 g/day) develop ESRD within 5 years

Upcoming Key CKD Program Milestones Phase 3 portion of the CARDINAL trial began enrollment during August 2017 and one year data are expected 2H 2019 Site activations in PHOENIX Phase 2 trial to study Bard in four additional rare kidney indications are underway Enrolling patients with Autosomal Dominant Polycystic Kidney Disease, IgA nephropathy, type 1 diabetic CKD, and FSGS Data will be available from 2H 2018 through 2019 If positive data from PHOENIX, will plan to rapidly initiate additional Phase 3 trial(s) KHK is planning to initiate a large Phase 3 trial in diabetic CKD patients in Japan to further evaluate safety and efficacy in 2018

Q&A





























