
Management call to discuss interim phoenix data Exhibit 99.3

Forward-Looking Statements This presentation contains certain “forward-looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward-looking statements, including statements regarding our future financial condition, business strategy, and plans and objectives of management for future operations. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “aim,” “assume,” “anticipate,” “contemplate,” “model,” “intend,” “target,” “project,” “should,” “plan,” “expect,” “predict,” “could,” “possible,” “seek,” “goal”, “potential,” “hypothesize,” “likely” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecast in these statements. Any differences could be caused by a number of factors including but not limited to: the success, cost, and timing of our product development activities and clinical trials; our ability to advance our NRF2 activators and other technologies; our ability to obtain and maintain regulatory approval of our product candidates, and limitations and warnings in the label of an approved product candidate; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the commercialization of our product candidates, if approved; the rate and degree of market acceptance of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to identify target patient populations and serve those markets, especially for diseases with small patient populations; the success of competing therapies that are or may become available; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; our ability to attract collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; and regulatory developments in the United States and foreign countries. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. All forward-looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward-looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
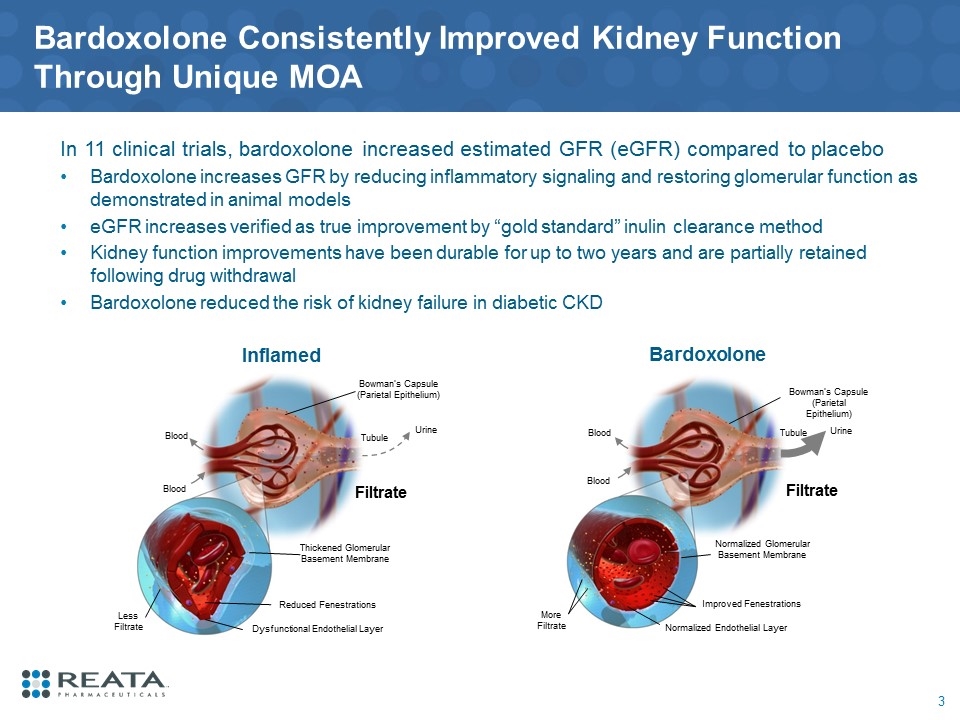
Bowman’s Capsule (Parietal Epithelium) Tubule Filtrate Urine Blood Blood Normalized Glomerular Basement Membrane Normalized Endothelial Layer Bardoxolone Improved Fenestrations More Filtrate Urine Bowman’s Capsule (Parietal Epithelium) Tubule Filtrate Blood Blood Thickened Glomerular Basement Membrane Dysfunctional Endothelial Layer Inflamed Reduced Fenestrations Less Filtrate Bardoxolone Consistently Improved Kidney Function Through Unique MOA 3 In 11 clinical trials, bardoxolone increased estimated GFR (eGFR) compared to placebo Bardoxolone increases GFR by reducing inflammatory signaling and restoring glomerular function as demonstrated in animal models eGFR increases verified as true improvement by “gold standard” inulin clearance method Kidney function improvements have been durable for up to two years and are partially retained following drug withdrawal Bardoxolone reduced the risk of kidney failure in diabetic CKD
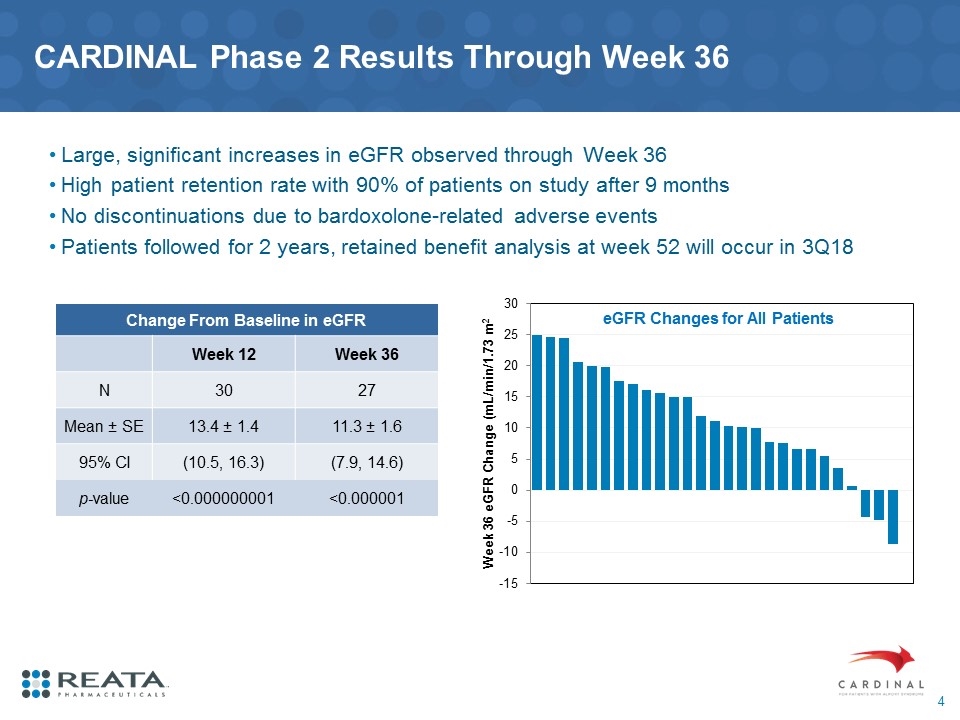
CARDINAL Phase 2 Results Through Week 36 Large, significant increases in eGFR observed through Week 36 High patient retention rate with 90% of patients on study after 9 months No discontinuations due to bardoxolone-related adverse events Patients followed for 2 years, retained benefit analysis at week 52 will occur in 3Q18 eGFR Changes for All Patients Change From Baseline in eGFR Week 12 Week 36 N 30 27 Mean ± SE 13.4 ± 1.4 11.3 ± 1.6 95% CI (10.5, 16.3) (7.9, 14.6) p-value <0.000000001 <0.000001
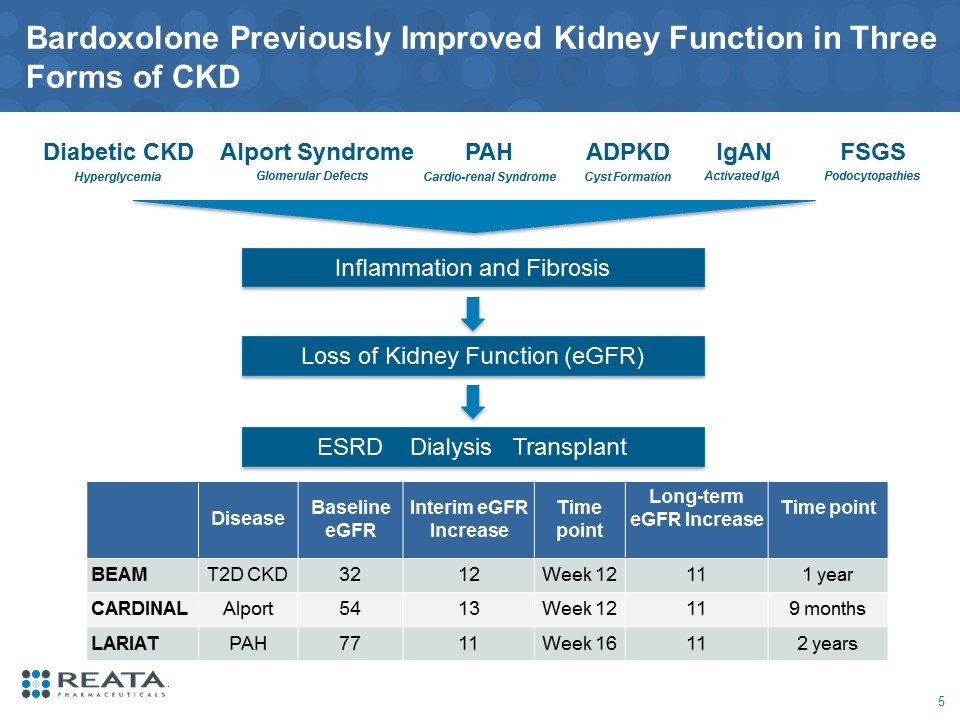
Disease Baseline eGFR Interim eGFR Increase Time point Long-term eGFR Increase Time point BEAM T2D CKD 32 12 Week 12 11 1 year CARDINAL Alport 54 13 Week 12 11 9 months LARIAT PAH 77 11 Week 16 11 2 years Bardoxolone Previously Improved Kidney Function in Three Forms of CKD 5 Alport Syndrome Glomerular Defects FSGS Podocytopathies ADPKD Cyst Formation IgAN Activated IgA Diabetic CKD Hyperglycemia PAH Cardio-renal Syndrome Inflammation and Fibrosis Loss of Kidney Function (eGFR) ESRD Dialysis Transplant
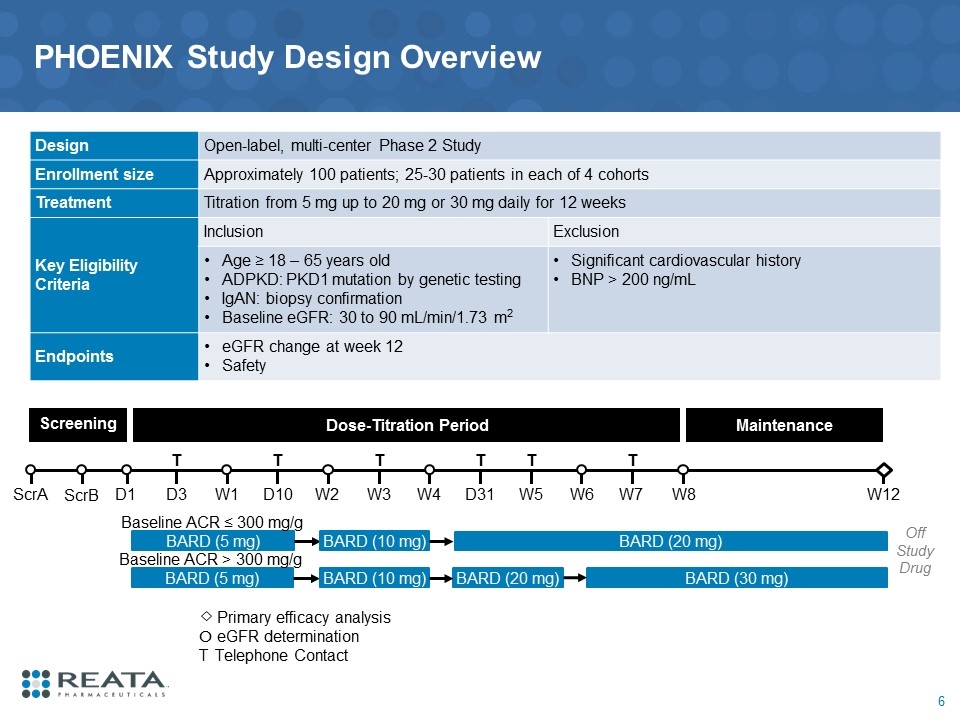
PHOENIX Study Design Overview Design Open-label, multi-center Phase 2 Study Enrollment size Approximately 100 patients; 25-30 patients in each of 4 cohorts Treatment Titration from 5 mg up to 20 mg or 30 mg daily for 12 weeks Key Eligibility Criteria Inclusion Exclusion Age ≥ 18 – 65 years old ADPKD: PKD1 mutation by genetic testing IgAN: biopsy confirmation Baseline eGFR: 30 to 90 mL/min/1.73 m2 Significant cardiovascular history BNP > 200 ng/mL Endpoints eGFR change at week 12 Safety Screening Dose-Titration Period W2 W1 W8 ScrA D1 D3 T D10 T W3 T W4 Maintenance D31 T W5 T W6 W7 T BARD (5 mg) BARD (20 mg) W12 BARD (10 mg) Primary efficacy analysis O eGFR determination T Telephone Contact ScrB BARD (5 mg) BARD (20 mg) BARD (10 mg) BARD (30 mg) Baseline ACR ≤ 300 mg/g Baseline ACR > 300 mg/g Off Study Drug
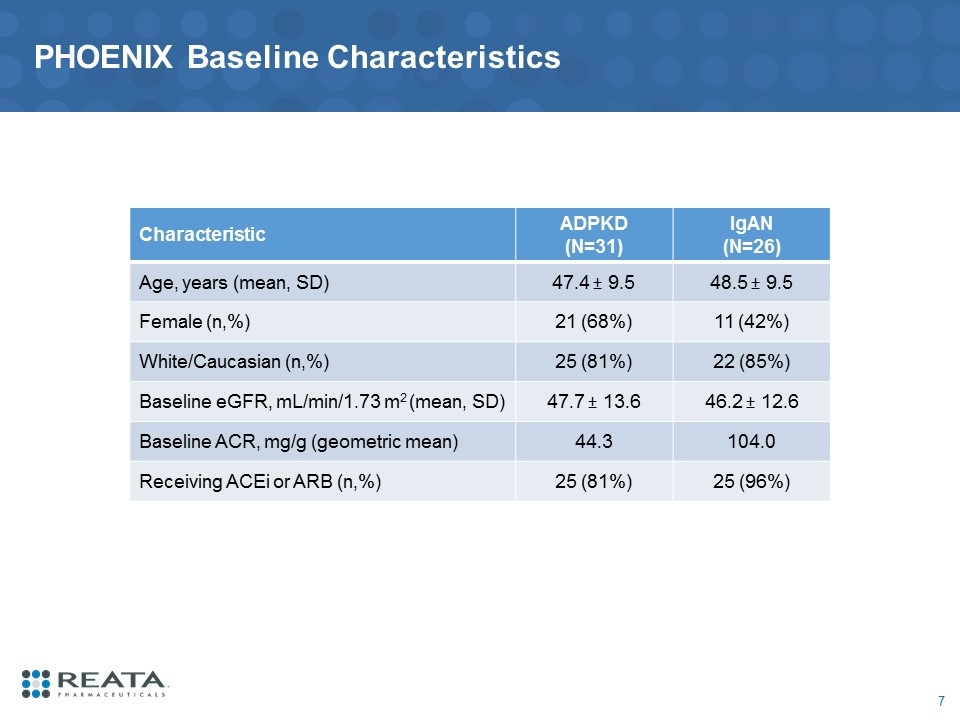
PHOENIX Baseline Characteristics Characteristic ADPKD (N=31) IgAN (N=26) Age, years (mean, SD) 47.4 ± 9.5 48.5 ± 9.5 Female (n,%) 21 (68%) 11 (42%) White/Caucasian (n,%) 25 (81%) 22 (85%) Baseline eGFR, mL/min/1.73 m2 (mean, SD) 47.7 ± 13.6 46.2 ± 12.6 Baseline ACR, mg/g (geometric mean) 44.3 104.0 Receiving ACEi or ARB (n,%) 25 (81%) 25 (96%)
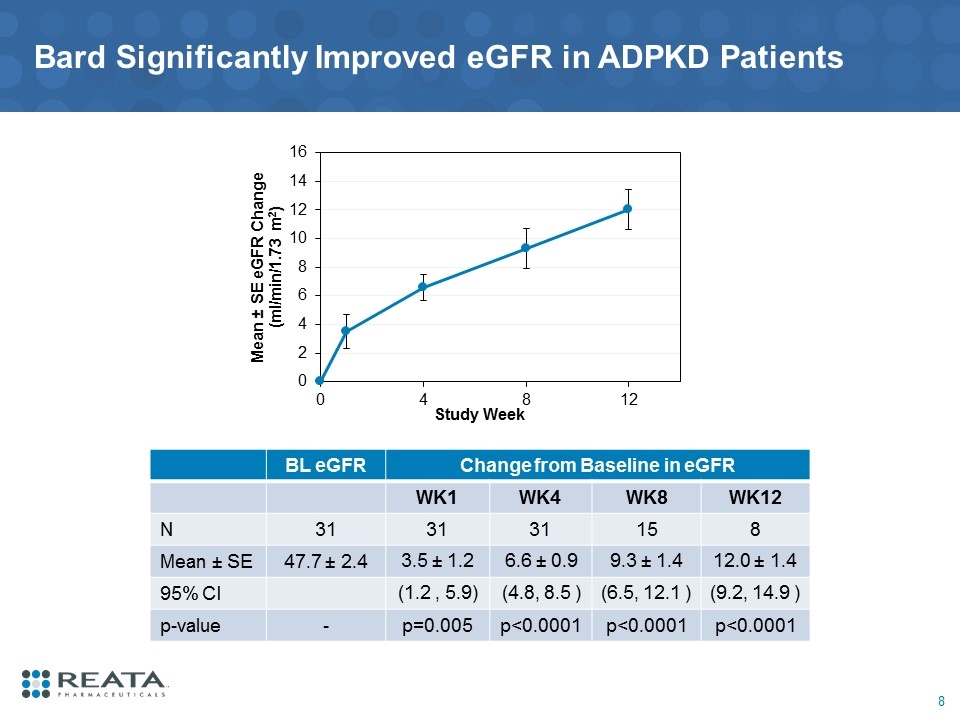
Bard Significantly Improved eGFR in ADPKD Patients BL eGFR Change from Baseline in eGFR WK1 WK4 WK8 WK12 N 31 31 31 15 8 Mean ± SE 47.7 ± 2.4 3.5 ± 1.2 6.6 ± 0.9 9.3 ± 1.4 12.0 ± 1.4 95% CI (1.2 , 5.9) (4.8, 8.5 ) (6.5, 12.1 ) (9.2, 14.9 ) p-value - p=0.005 p<0.0001 p<0.0001 p<0.0001
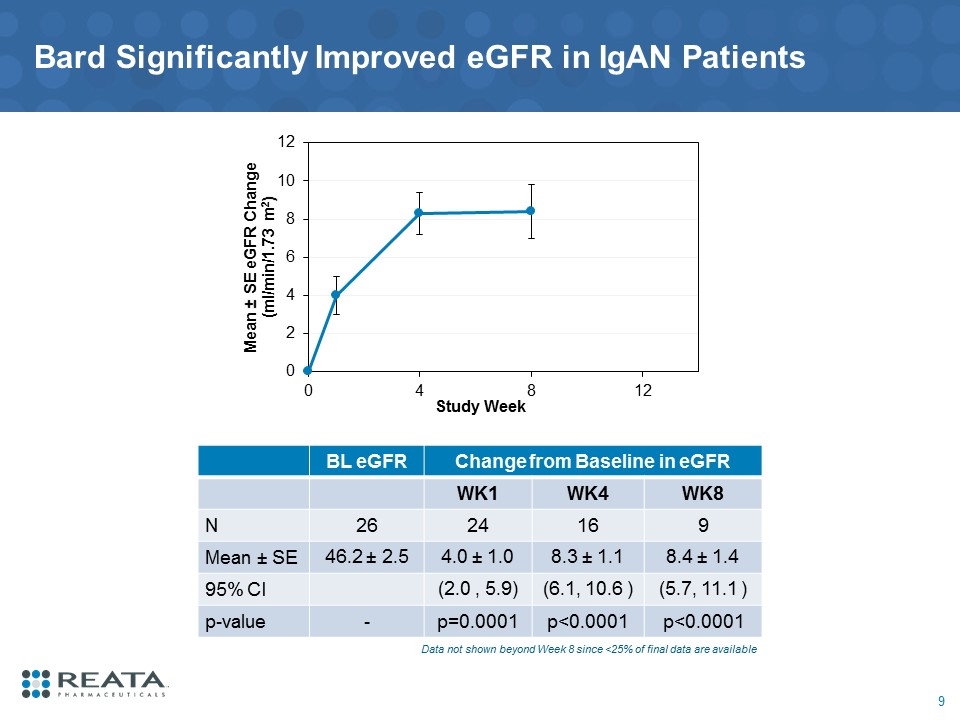
Bard Significantly Improved eGFR in IgAN Patients BL eGFR Change from Baseline in eGFR WK1 WK4 WK8 N 26 24 16 9 Mean ± SE 46.2 ± 2.5 4.0 ± 1.0 8.3 ± 1.1 8.4 ± 1.4 95% CI (2.0 , 5.9) (6.1, 10.6 ) (5.7, 11.1 ) p-value - p=0.0001 p<0.0001 p<0.0001 Data not shown beyond Week 8 since <25% of final data are available
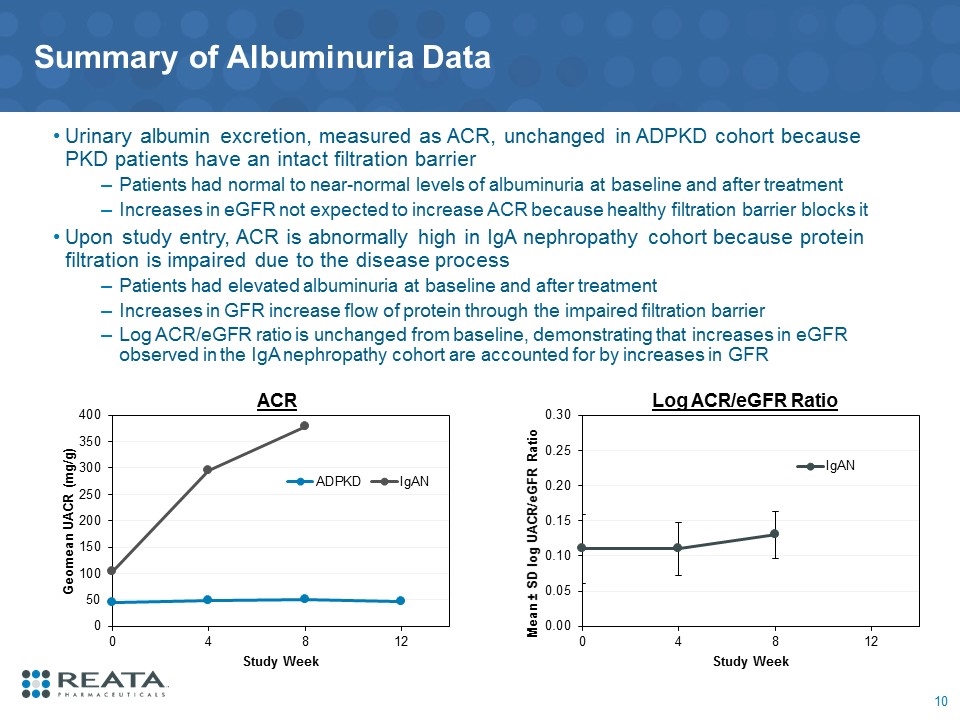
Summary of Albuminuria Data Urinary albumin excretion, measured as ACR, unchanged in ADPKD cohort because PKD patients have an intact filtration barrier Patients had normal to near-normal levels of albuminuria at baseline and after treatment Increases in eGFR not expected to increase ACR because healthy filtration barrier blocks it Upon study entry, ACR is abnormally high in IgA nephropathy cohort because protein filtration is impaired due to the disease process Patients had elevated albuminuria at baseline and after treatment Increases in GFR increase flow of protein through the impaired filtration barrier Log ACR/eGFR ratio is unchanged from baseline, demonstrating that increases in eGFR observed in the IgA nephropathy cohort are accounted for by increases in GFR ACR Log ACR/eGFR Ratio
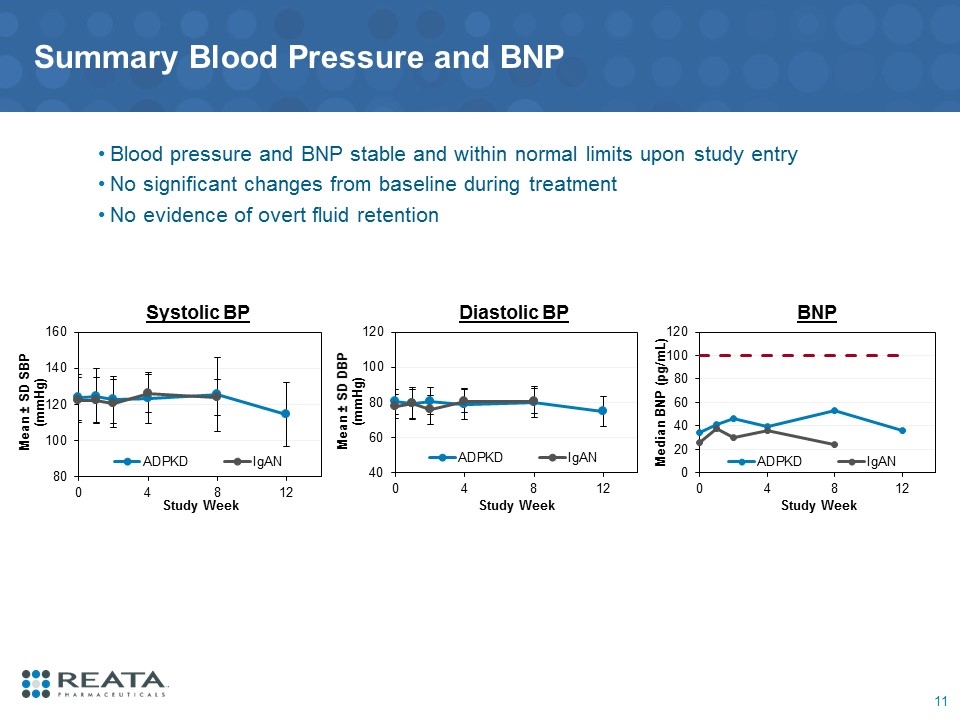
Summary Blood Pressure and BNP Blood pressure and BNP stable and within normal limits upon study entry No significant changes from baseline during treatment No evidence of overt fluid retention BNP Diastolic BP Systolic BP
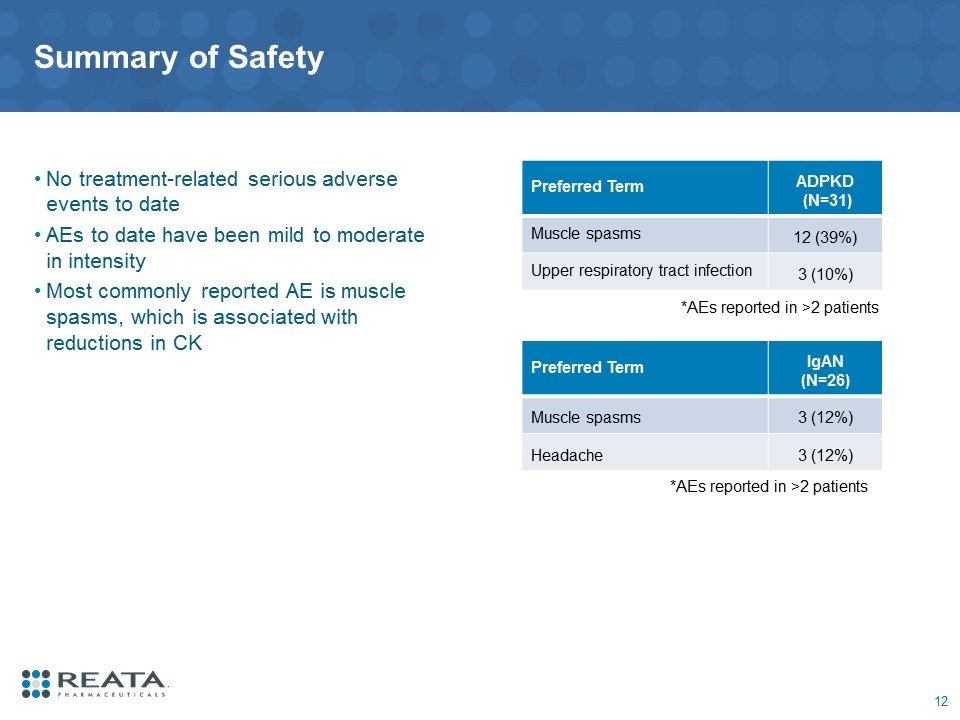
Summary of Safety No treatment-related serious adverse events to date AEs to date have been mild to moderate in intensity Most commonly reported AE is muscle spasms, which is associated with reductions in CK *AEs reported in >2 patients Preferred Term ADPKD (N=31) Muscle spasms 12 (39%) Upper respiratory tract infection 3 (10%) *AEs reported in >2 patients Preferred Term IgAN (N=26) Muscle spasms 3 (12%) Headache 3 (12%)
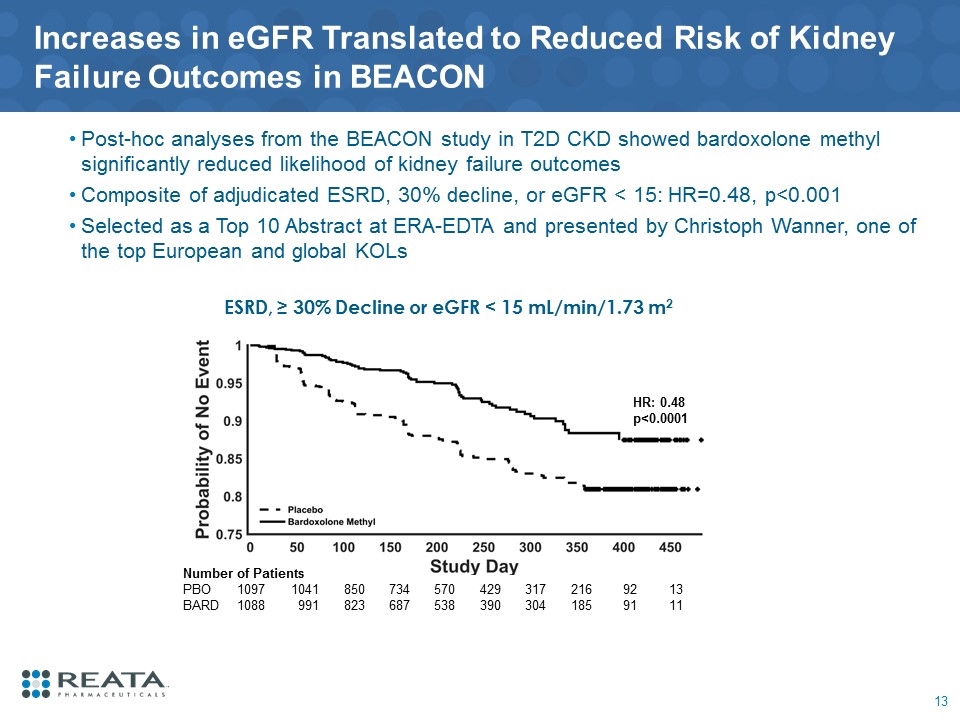
Increases in eGFR Translated to Reduced Risk of Kidney Failure Outcomes in BEACON Post-hoc analyses from the BEACON study in T2D CKD showed bardoxolone methyl significantly reduced likelihood of kidney failure outcomes Composite of adjudicated ESRD, 30% decline, or eGFR < 15: HR=0.48, p<0.001 Selected as a Top 10 Abstract at ERA-EDTA and presented by Christoph Wanner, one of the top European and global KOLs ESRD, ≥ 30% Decline or eGFR < 15 mL/min/1.73 m2 Number of Patients PBO 1097 1041 850 734 570 429 317 216 92 13 BARD 1088 991 823 687 538 390 304 185 91 11 HR: 0.48 p<0.0001
Future Development and Commercialization Large unmet medical need exists in ADPKD and IgA nephropathy In the U.S., ADPKD is the leading inheritable cause of CKD and the 4th leading cause of ESRD U.S. prevalence of ADPKD is approximately 400,000 people; 116,000 patients are diagnosed 50% of patients progress to ESRD; ADPKD is responsible for 5% of all ESRD IgA nephropathy is the most prevalent primary chronic glomerular disease worldwide U.S. prevalence of IgA nephropathy is approximately 120,000 people 50% of patients progress to ESRD; Leading cause of ESRD in young Caucasian adults Bardoxolone may substantially impact the cost burden of ADPKD and IgAN Bardoxolone significantly reduced the likelihood of kidney failure outcomes in BEACON The average per-patient cost burden for ADPKD at ESRD is 3x the cost burden at stage 3 U.S. Medicare spending alone totaled $64B for CKD and $34B for ESRD in 2015 Significant upcoming news flow for bardoxolone in CKD Full week 12 data for ADPKD, IgAN and T1D CKD in 3Q18 52-week retained benefit data from Phase 2 of CARDINAL in 3Q18 KHK commences pivotal T2D CKD trial in Japan in 2018 Full week 12 data for FSGS in 1H19 Pivotal CARDINAL data in 2H19

Q&A














