
4th Quarter and Full Year 2021 Financial Results and Update on Clinical Development Programs February 28, 2022 Exhibit 99.2

This presentation contains certain “forward-looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward-looking statements, including statements regarding our future financial condition, future revenues, projected costs, prospects, business strategy, and plans and objectives of management for future operations, including our plans to submit for regulatory filings. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “might,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “model,” “should,” “would,” “plan,” “expect,” “predict,” “could,” “seek,” “goal,” “potential,” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, and are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecasted in these statements. Any differences could be caused by a number of factors including but not limited to: our expectations regarding the timing, costs, conduct, and outcome of our clinical trials, including statements regarding the timing of the initiation and availability of data from such trials; the timing and likelihood of regulatory filings and approvals for our product candidates; whether regulatory authorities determine that additional trials or data are necessary in order to obtain approval; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the commercialization of our product candidates, if approved; the rate and degree of market acceptance of our product candidates; our expectations regarding the potential market size and the size of the patient populations for our product candidates, if approved for commercial use, and the potential market opportunities for commercializing our product candidates; the success of competing therapies that are or may become available; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to maintain and establish relationships with third parties, such as contract research organizations, contract manufacturing organizations, suppliers, and distributors; our ability to maintain and establish collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; our expectations related to the use of our available cash; our ability to develop, acquire, and advance product candidates into, and successfully complete, clinical trials; the initiation, timing, progress, and results of future preclinical studies and developments and projections relating to our competitors and our industry; the impact of governmental laws and regulations and regulatory development in the United States and foreign countries; the impact of the coronavirus disease (COVID-19) on our clinical trials, our supply chain, and our operations; and other risks and uncertainties, including those described under the heading “Risk Factors” included in our most recent Annual Report on Form 10-k for the year ended December 31, 2021, filed with the U.S. Securities and Exchange Commission (SEC) on February 28, 2022. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. All forward-looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward-looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Omaveloxolone, bardoxolone methyl, and RTA 901 are investigational drugs, and their safety and efficacy have not been established by any agency.

Today’s Agenda Development Update |

Regulatory Update on Omaveloxolone for Patients with Friedreich’s Ataxia NDA: New Drug Application Obtained Fast Track Designation
Update on Bardoxolone Program CRL: Complete Response Letter; FDA: United States Food and Drug Administration We will continue to work with the FDA to confirm our next steps on our Alport syndrome program FALCON protocol amendment submitted to FDA Type A meeting requested

Omaveloxolone in Friedreich’s Ataxia
Friedreich’s Ataxia: No Approved Therapy 1Rummey C, et.al. Neurol Genet. 2019; 2Rummey C, et.al. E Clinical Medicine. 2020; 3Tsou AY, et. al. J Neurol Sci. 2011; 42020 IQVIA claims data and projected diagnosed; 5Estimated by Reata and the Friedreich’s Ataxia Research Alliance (FARA) based on publicly available data and from Vankan P, J Neurochem 2013 Patients progressively lose motor function Typically diagnosed in teens,1 wheelchair-bound in twenties2 Mean survival in mid-thirties3 Caused by epigenetic silencing of frataxin Impaired mitochondrial function, suppressed Nrf2 expression, which impairs energy production Most common recessive form of ataxia In U.S., an estimated 4,000 patients are diagnosed with Friedreich’s ataxia4 out of 5,000 total5
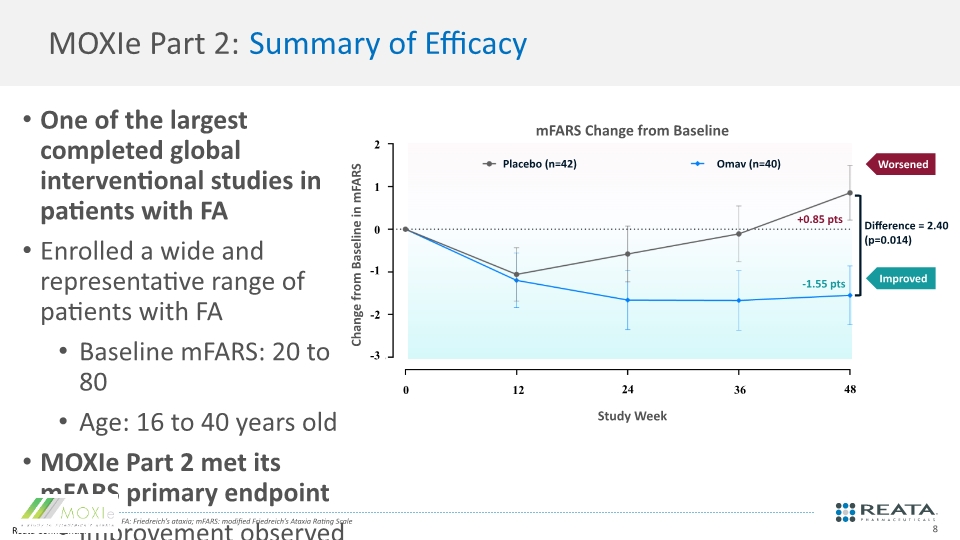
MOXIe Part 2: Summary of Efficacy One of the largest completed global interventional studies in patients with FA Enrolled a wide and representative range of patients with FA Baseline mFARS: 20 to 80 Age: 16 to 40 years old MOXIe Part 2 met its mFARS primary endpoint Improvement observed in all subsections relative to placebo Major subgroups and all analysis populations favored omaveloxolone Improvement in several other efficacy measures assessed as secondary endpoints FA: Friedreich’s ataxia; mFARS: modified Friedreich’s Ataxia Rating Scale mFARS Change from Baseline Worsened Improved 2 1 0 -1 -2 -3 0 12 24 36 48 Placebo (n=42) Omav (n=40) +0.85 pts -1.55 pts Difference = 2.40 (p=0.014) Change from Baseline in mFARS Study Week
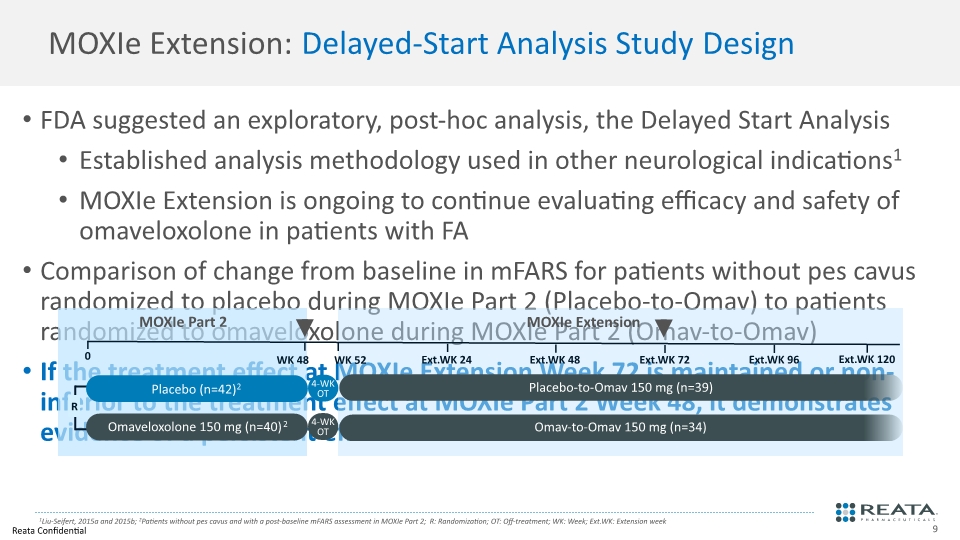
MOXIe Extension: Delayed-Start Analysis Study Design FDA suggested an exploratory, post-hoc analysis, the Delayed Start Analysis Established analysis methodology used in other neurological indications1 MOXIe Extension is ongoing to continue evaluating efficacy and safety of omaveloxolone in patients with FA Comparison of change from baseline in mFARS for patients without pes cavus randomized to placebo during MOXIe Part 2 (Placebo-to-Omav) to patients randomized to omaveloxolone during MOXIe Part 2 (Omav-to-Omav) If the treatment effect at MOXIe Extension Week 72 is maintained or non-inferior to the treatment effect at MOXIe Part 2 Week 48, it demonstrates evidence of a persistent effect on disease course 1Liu-Seifert, 2015a and 2015b; 2Patients without pes cavus and with a post-baseline mFARS assessment in MOXIe Part 2; R: Randomization; OT: Off-treatment; WK: Week; Ext.WK: Extension week
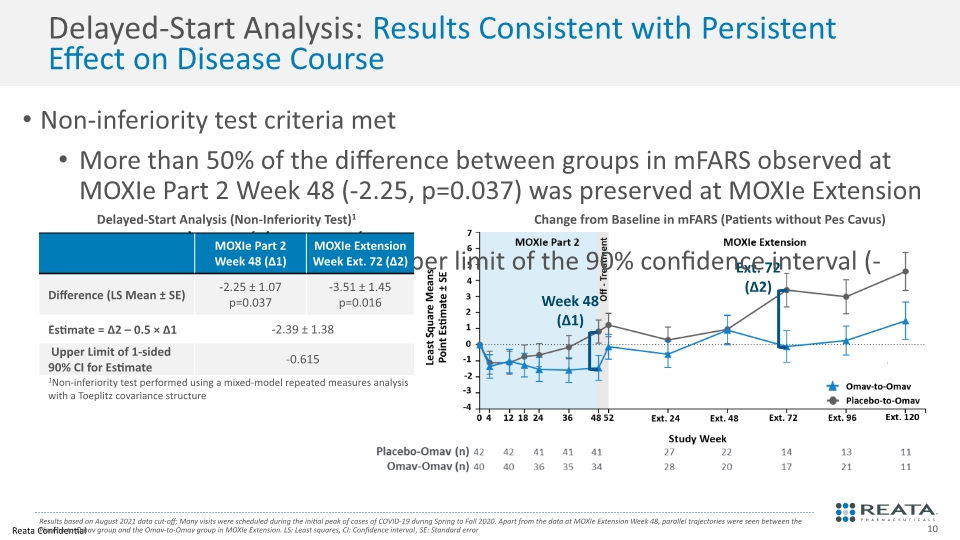
Ext. 72 (Δ2) Delayed-Start Analysis: Results Consistent with Persistent Effect on Disease Course Non-inferiority test criteria met More than 50% of the difference between groups in mFARS observed at MOXIe Part 2 Week 48 (-2.25, p=0.037) was preserved at MOXIe Extension Week 72 (-3.51, p=0.016) Point estimate (-2.39) and upper limit of the 90% confidence interval (-0.615) less than zero Results based on August 2021 data cut-off; Many visits were scheduled during the initial peak of cases of COVID-19 during Spring to Fall 2020. Apart from the data at MOXIe Extension Week 48, parallel trajectories were seen between the Placebo-to-Omav group and the Omav-to-Omav group in MOXIe Extension. LS: Least squares, CI: Confidence interval, SE: Standard error Delayed-Start Analysis (Non-Inferiority Test)1 Change from Baseline in mFARS (Patients without Pes Cavus) Week 48 (Δ1)
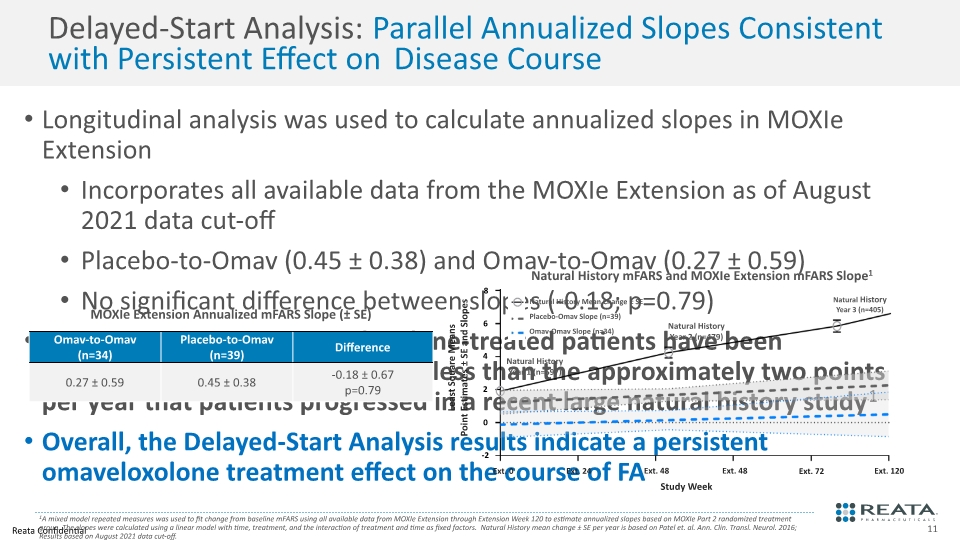
Delayed-Start Analysis: Parallel Annualized Slopes Consistent with Persistent Effect on Disease Course Longitudinal analysis was used to calculate annualized slopes in MOXIe Extension Incorporates all available data from the MOXIe Extension as of August 2021 data cut-off Placebo-to-Omav (0.45 ± 0.38) and Omav-to-Omav (0.27 ± 0.59) No significant difference between slopes (-0.18; p=0.79) In MOXIe Extension, omaveloxolone-treated patients have been progressing at a rate that is >75% less than the approximately two points per year that patients progressed in a recent large natural history study1 Overall, the Delayed-Start Analysis results indicate a persistent omaveloxolone treatment effect on the course of FA 1A mixed model repeated measures was used to fit change from baseline mFARS using all available data from MOXIe Extension through Extension Week 120 to estimate annualized slopes based on MOXIe Part 2 randomized treatment group. The slopes were calculated using a linear model with time, treatment, and the interaction of treatment and time as fixed factors. Natural History mean change ± SE per year is based on Patel et. al. Ann. Clin. Transl. Neurol. 2016; Results based on August 2021 data cut-off. Ext. 48 Study Week Least Square Means Point Estimates ± SE and Slopes Ext. 0 Ext. 24 Ext. 48 Ext. 72 Ext. 120 Natural History Year 2 (n=479) Natural History Year 3 (n=405) Natural History Year 1 (n=597) Natural History mFARS and MOXIe Extension mFARS Slope1 6 4 2 0 -2 8
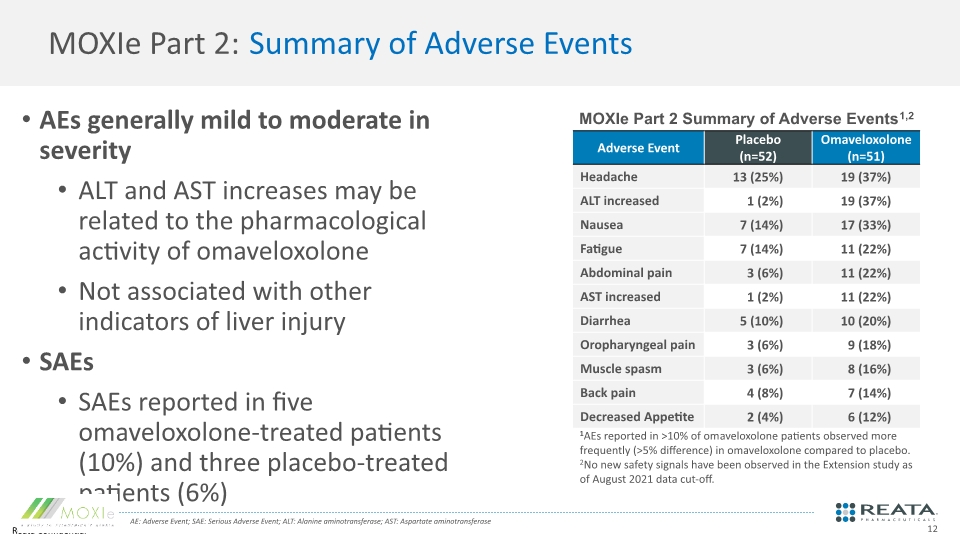
MOXIe Part 2: Summary of Adverse Events AEs generally mild to moderate in severity ALT and AST increases may be related to the pharmacological activity of omaveloxolone Not associated with other indicators of liver injury SAEs SAEs reported in five omaveloxolone-treated patients (10%) and three placebo-treated patients (6%) MOXIe Part 2 Summary of Adverse Events1,2 AE: Adverse Event; SAE: Serious Adverse Event; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase
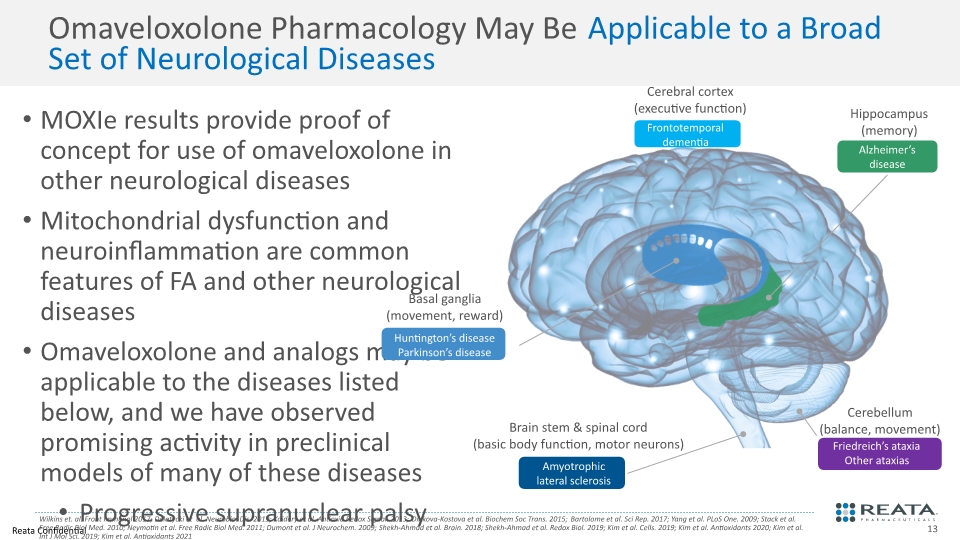
MOXIe results provide proof of concept for use of omaveloxolone in other neurological diseases Mitochondrial dysfunction and neuroinflammation are common features of FA and other neurological diseases Omaveloxolone and analogs may be applicable to the diseases listed below, and we have observed promising activity in preclinical models of many of these diseases Progressive supranuclear palsy Amyotrophic lateral sclerosis Parkinson’s disease Frontotemporal dementia Huntington’s disease Alzheimer’s disease Epilepsy Omaveloxolone Pharmacology May Be Applicable to a Broad Set of Neurological Diseases Wilkins et. al. Front Immunol 2017; Delatycki et al. Neurobiol Dis. 2019; Kaidery et al. Antioxid Redox Signal. 2013; Dinkova-Kostova et al. Biochem Soc Trans. 2015; Bartolome et al. Sci Rep. 2017; Yang et al. PLoS One. 2009; Stack et al. Free Radic Biol Med. 2010; Neymotin et al. Free Radic Biol Med. 2011; Dumont et al. J Neurochem. 2009; Shekh-Ahmad et al. Brain. 2018; Shekh-Ahmad et al. Redox Biol. 2019; Kim et al. Cells. 2019; Kim et al. Antioxidants 2020; Kim et al. Int J Mol Sci. 2019; Kim et al. Antioxidants 2021 Cerebral cortex (executive function) Brain stem & spinal cord (basic body function, motor neurons) Basal ganglia (movement, reward) Hippocampus (memory) Cerebellum (balance, movement)

Chronic Kidney Disease Program Updates

AYAME Phase 3 Diabetic CKD Outcomes Trial in Japan Kyowa Kirin, our strategic collaborator in Japan is sponsoring AYAME Randomized, double-blind, placebo-controlled, registrational trial of bardoxolone in patients with diabetic CKD in Japan Primary endpoint: time to onset of ≥ 30% decline in eGFR or ESKD Eligibility criteria eGFR ≥ 15 and < 60 mL/min/1.73 m2 20 to 79 years old No history of heart failure or BNP > 200 pg/mL More than 1,000 patients enrolled Kyowa Kirin expects last patient visit in 2H 2022 Three years of data on all patients expected CKD: Chronic kidney disease; eGFR: Estimated glomerular filtration rate; ESKD: End-stage kidney disease; BNP: Brain natriuretic peptide
International, multi-center, randomized, double-blind, placebo-controlled Phase 3 trial of bardoxolone in patients with ADPKD Protocol amendment filed with the FDA: Endpoints Primary: off-treatment eGFR change from baseline at Week 108 No treatment discontinuation at Year 1 Exploratory : additional visit added to measure off-treatment eGFR at Week 112 Sample size Increased from 550 to 850 patients Patient population Addition of adolescents ages 12 to 17 years (expanding age range - 12 to 70 years) Others Ambulatory blood pressure monitoring sub-study added >500 patients currently enrolled Requested a Type A meeting with FDA to discuss overall ADPKD program including protocol amendment FALCON Phase 3 Study of Bardoxolone in Patients with ADPKD ADPKD: Autosomal dominant polycystic kidney disease

RTA 901 HSP90 Modulator for Diabetic Neuropathy
RTA 901: Phase 2 Study in Patients with Diabetic Peripheral Neuropathic Pain (DPNP) RTA 901 is a novel, small-molecule, orally bioavailable C-terminal Hsp90 inhibitor and Hsp70 inducer1 DPNP is a serious complication of diabetes mellitus2-4 Associated with substantial morbidity, including often debilitating peripheral neuropathic pain Approximately 4 million patients in the U.S. are affected with moderate or severe DPNP 1Burlison et al. J Am Chem Soc. 2006; 2Xu et al. Anesth Analg. 2022; 3Price et al. Neurology. 2022; 4U.S. Food and Drug Administration. The Voice of the Patient Neuropathic Pain Associated with Peripheral Neuropathy. February 2017; PK: Pharmacokinetic; AAN: American Academy of Neurology; Hsp90: Heat shock protein 90; Hsp70: Heat shock protein 70; HSF1: Heat shock factor 1 Phase 1 study in healthy volunteers is complete Well tolerated with no safety signals, drug discontinuations, or SAEs Favorable PK profile Additional Phase 1 clinical pharmacology studies expected to begin in the first half of 2022 Phase 2 randomized, placebo-controlled study in DPNP expected to begin enrolling in the second half of 2022

Financial & Operational Update
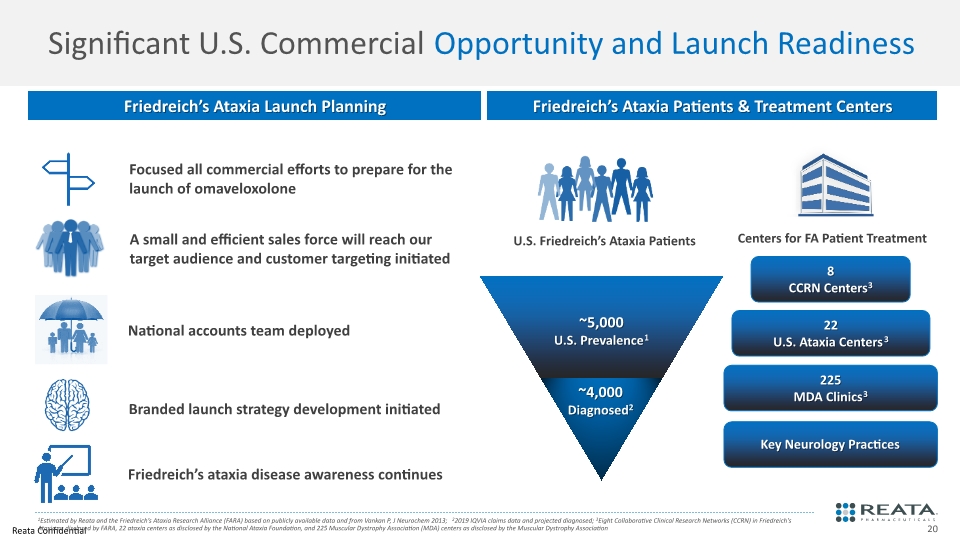
Significant U.S. Commercial Opportunity and Launch Readiness 1Estimated by Reata and the Friedreich’s Ataxia Research Alliance (FARA) based on publicly available data and from Vankan P, J Neurochem 2013; 22019 IQVIA claims data and projected diagnosed; 3Eight Collaborative Clinical Research Networks (CCRN) in Friedreich's Ataxia as disclosed by FARA, 22 ataxia centers as disclosed by the National Ataxia Foundation, and 225 Muscular Dystrophy Association (MDA) centers as disclosed by the Muscular Dystrophy Association Friedreich’s Ataxia Patients & Treatment Centers 8 CCRN Centers3 22 U.S. Ataxia Centers3 225 MDA Clinics3 Friedreich’s Ataxia Launch Planning Key Neurology Practices
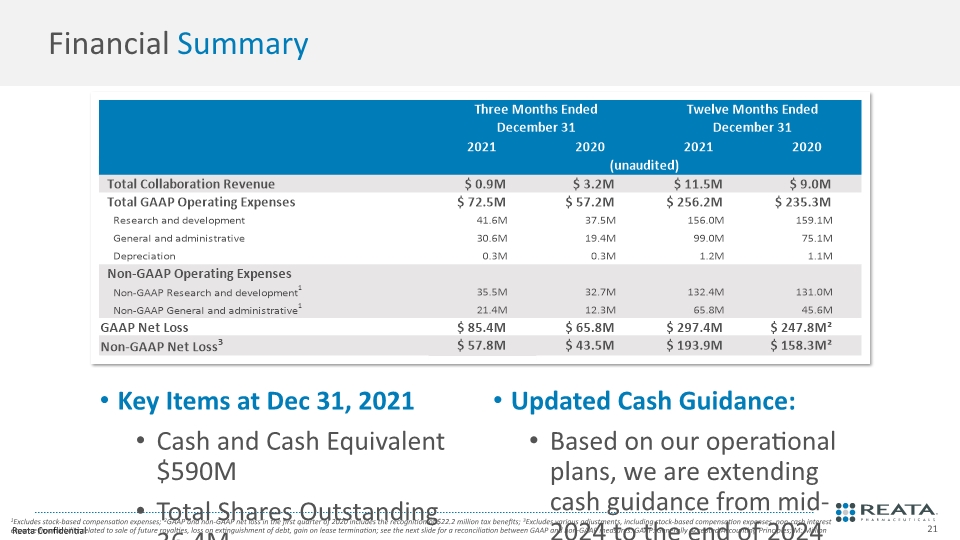
Key Items at Dec 31, 2021 Cash and Cash Equivalent $590M Total Shares Outstanding 36.4M 31.5M class A shares outstanding 4.9M class B shares outstanding Updated Cash Guidance: Based on our operational plans, we are extending cash guidance from mid-2024 to the end of 2024 Financial Summary 1Excludes stock-based compensation expenses; 2GAAP and non-GAAP net loss in the first quarter of 2020 includes the recognition of $22.2 million tax benefits; 3Excludes various adjustments, including stock-based compensation expenses, non-cash interest expense from liability related to sale of future royalties, loss on extinguishment of debt, gain on lease termination; see the next slide for a reconciliation between GAAP and non-GAAP measures. GAAP: Generally Accepted Accounting Principles; M: Million
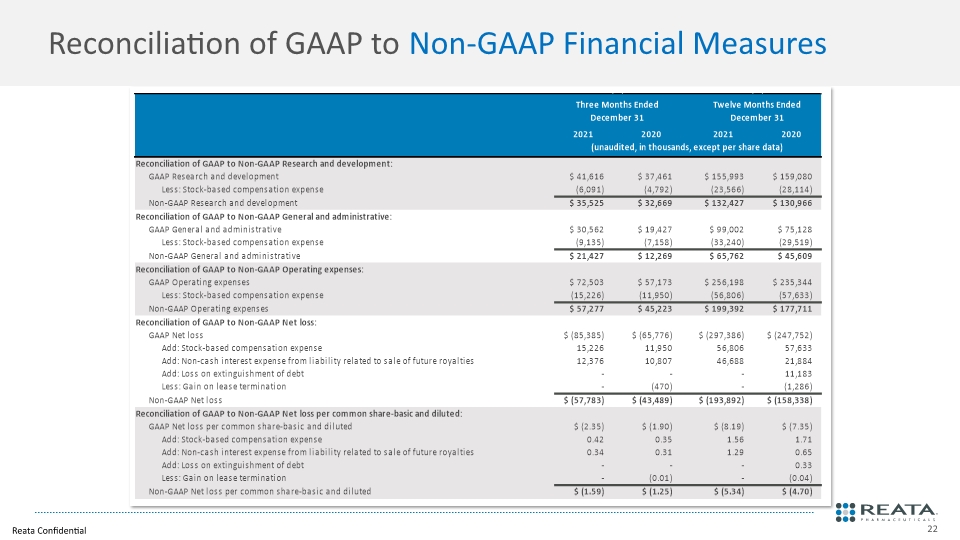
Reconciliation of GAAP to Non-GAAP Financial Measures

Concluding Remarks
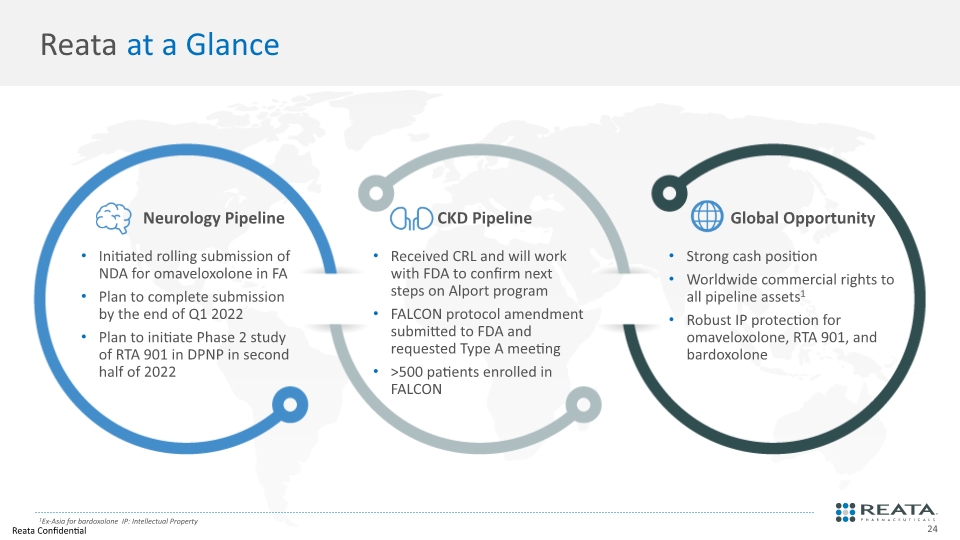
Reata at a Glance Initiated rolling submission of NDA for omaveloxolone in FA Plan to complete submission by the end of Q1 2022 Plan to initiate Phase 2 study of RTA 901 in DPNP in second half of 2022 Received CRL and will work with FDA to confirm next steps on Alport program FALCON protocol amendment submitted to FDA and requested Type A meeting >500 patients enrolled in FALCON Strong cash position Worldwide commercial rights to all pipeline assets1 Robust IP protection for omaveloxolone, RTA 901, and bardoxolone Neurology Pipeline CKD Pipeline Global Opportunity 1Ex-Asia for bardoxolone IP: Intellectual Property

Thank you
























