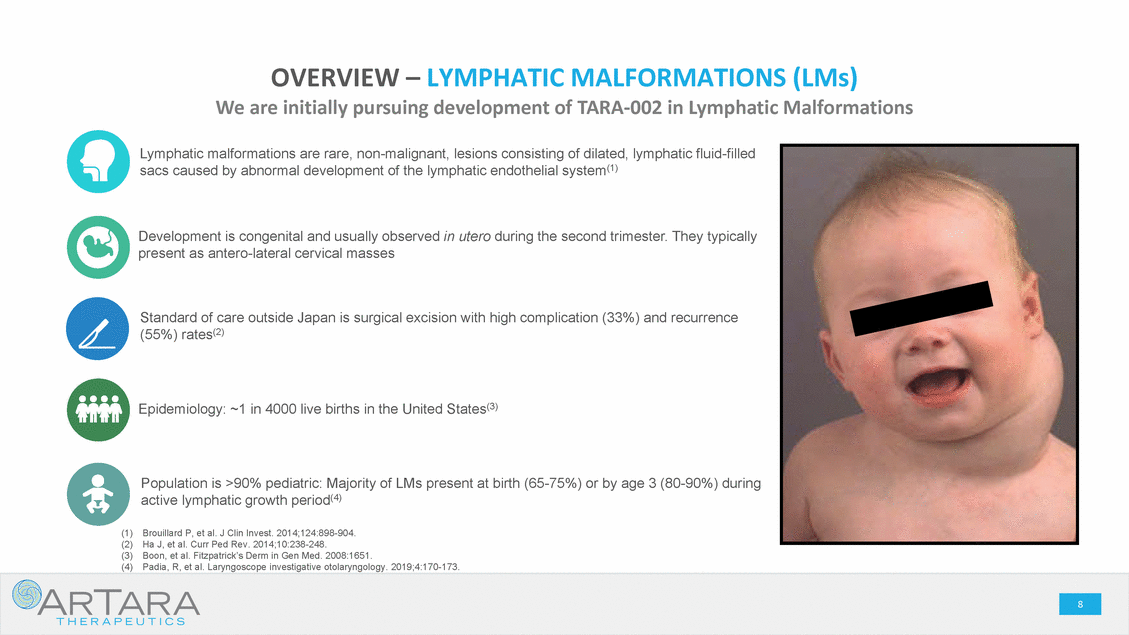
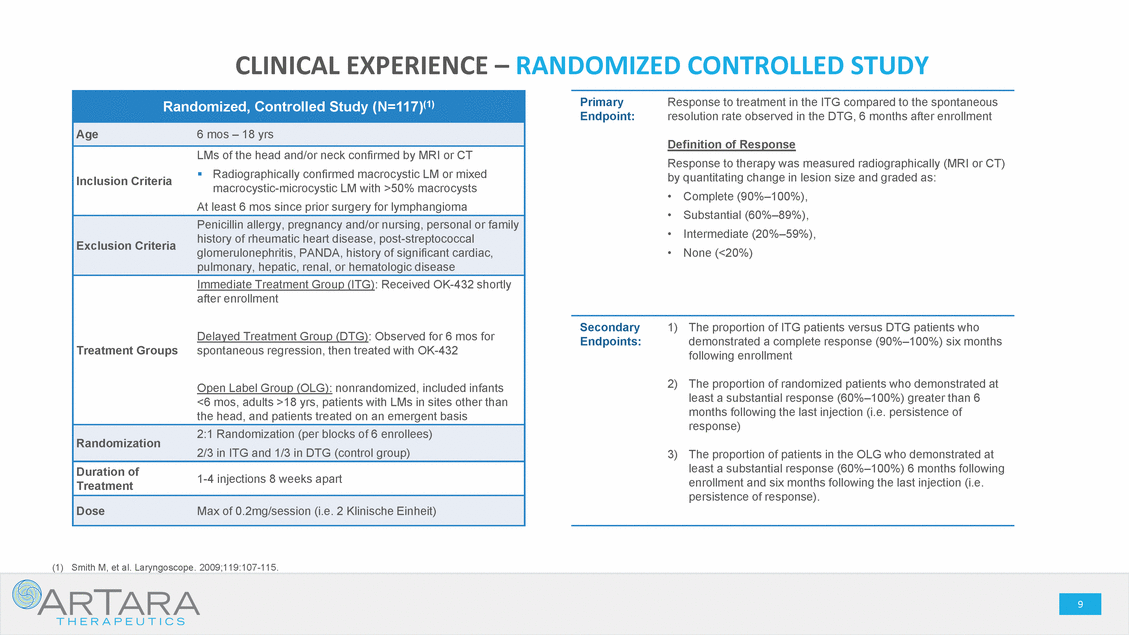
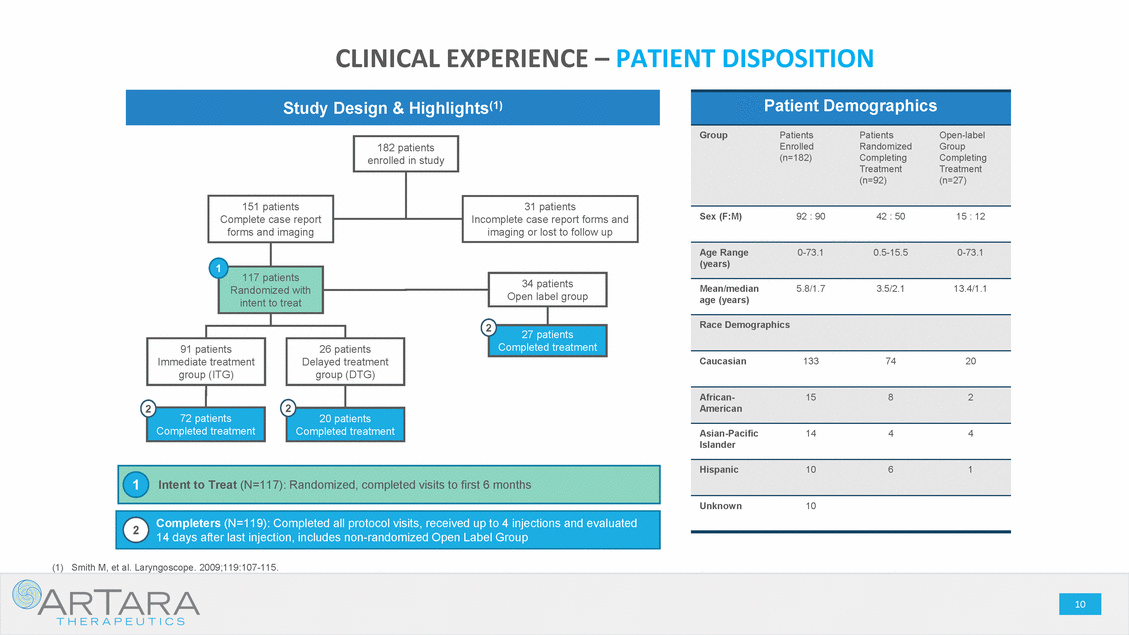
CAUTIONARY STATEMENT - FORWARD LOOKING STATEMENTS This presentation is being made in respect of a proposed transaction involving ArTara Therapeutics, Inc. (“ArTara”) and Proteon Therapeutics, Inc. (“Proteon” or “we” or “our”). Certain statements contained in this presentation regarding matters that are not historical facts are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, stockholders are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. Such forward-looking statements are based on management expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the forward-looking statements due to a number of factors, including, but not limited to, risks relating to the completion of the proposed transaction, including the need for Proteon’s and ArTara’s stockholder approval and the satisfaction of certain closing conditions; the anticipated financing to be completed concurrently with the closing of the proposed transaction; the cash balance of the combined company following the closing of the proposed transaction and the financing, and expectations with respect thereto; the potential benefits of the proposed transaction; the business and prospects of the combined company following the proposed transaction; and the ability of Proteon to remain listed on the Nasdaq Global Market. Risks and uncertainties that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to: the closing of the proposed transaction; ArTara’s plans to develop and commercialize its product candidates, including TARA-002, and Choline Chloride; the timing, costs and outcomes of ArTara’s planned clinical trials; expectations regarding potential market size; the timing of the availability of data from ArTara’s clinical trials; the timing of any planned investigational new drug application or new drug application; ArTara’s plans to research, develop and commercialize its current and future product candidates; ArTara’s ability to successfully collaborate with existing collaborators or enter into new collaborations, and to fulfill its obligations under any such collaboration agreements; the clinical utility, potential benefits and market acceptance of ArTara’s product candidates; ArTara’s commercialization, marketing and manufacturing capabilities and strategy; ArTara’s ability to identify additional products or product candidates with significant commercial potential; developments and projections relating to ArTara’s competitors and industry; the impact of government laws and regulations; ArTara’s ability to protect its intellectual property position; and ArTara’s estimates regarding future revenue, expenses, capital requirements, and the need for and timing of additional financing following the proposed transaction. These risks, as well as other risks associated with the proposed transaction, will be more fully discussed in the proxy statement/prospectus that will be included in the registration statement on Form S-4 that will be filed by Proteon with the U.S. Securities and Exchange Commission (the “SEC”) in connection with the proposed transaction. Additional risks and uncertainties are identified and discussed in the “Risk Factors” section of Proteon’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other documents filed from time to time with the SEC. Forward-looking statements included in this presentation are based on information available to Proteon and ArTara as of the date of this presentation. Neither Proteon nor ArTara undertakes any obligation to update such forward-looking statements to reflect events or circumstances after the date of this presentation. No Offer or Solicitation: This presentation does not constitute an offer to sell, or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No public offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Additional Information About the Proposed Transaction and Where to Find it This presentation is being made in respect of a proposed transaction involving ArTara and and Proteon, and Proteon intends to file a registration statement on Form S-4 with the SEC, which will contain a proxy statement/prospectus and other relevant materials, and plans to file with the SEC other documents regarding the proposed transaction. The final proxy statement/prospectus will be sent to the stockholders of Proteon in connection with the Proteon’s special meeting of stockholders to be held to vote on matters relating to the proposed transaction. The proxy statement/prospectus will contain information about Proteon, ArTara, the proposed transaction, and related matters. STOCKHOLDERS OF PROTEON ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS THERETO) AND OTHER DOCUMENTS FILED WITH THE SEC CAREFULLY IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE, AS THEY WILL CONTAIN IMPORTANT INFORMATION THAT STOCKHOLDERS OF PROTEON SHOULD CONSIDER BEFORE MAKING A DECISION ABOUT THE PROPOSED TRANSACTION AND RELATED MATTERS. In addition to receiving the proxy statement/prospectus and proxy card by mail, Proteon stockholders will also be able to obtain the proxy statement/prospectus, as well as other filings containing information about Proteon, without charge, from the SEC’s website at www.sec.gov or, without charge, by directing a written request to: Proteon Therapeutics, Inc., 200 West St. Waltham, MA 02451, Attention: Investor Relations. Participants in the Solicitation Proteon, ArTara and their respective executive officers, directors, certain members of management and certain employees may be deemed, under the SEC rules, to be participants in the solicitation of proxies from Proteon stockholders with respect to the matters relating to the proposed transaction. Information regarding Proteon’s executive officers and directors is available in Proteon’s proxy statement on Schedule 14A for its 2018 annual meeting of stockholders, filed with the SEC on April 26, 2018 and Proteon’s Annual Report on Form 10-K and the amendment thereto for the year-ended December 31, 2018. These documents are available free of charge at the SEC’s website at www.sec.gov or by going to Proteon’s investor and media page on its corporate website at www.proteontherapeutics.com. Additional information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of proxies in connection with the proposed transaction, and a description of their direct and indirect interests in the proposed transaction, which may differ from the interests of Proteon’s stockholders generally, will be set forth in the proxy statement/prospectus that Proteon intends to file with the SEC in connection with its stockholder vote on matters relating to the proposed transaction. Proteon stockholders will be able to obtain this information by reading the proxy statement/prospectus when it becomes available. . 2