Exhibit 99.1

CORPORATE PRESENTATION January 2025

FORWARD LOOKING STATEMENTS 2 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Statements contained in this presentation regarding matters that are not historical facts are "forward looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 . Protara may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “designed,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words or expressions referencing future events, conditions or circumstances that convey uncertainty of future events or outcomes to identify these forward - looking statements . Such forward - looking statements include but are not limited to, statements regarding Protara’s intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things : Protara’s business strategy, including its development plans for its product candidates and plans regarding the timing or outcome of existing or future clinical trials (including reporting initial data from 12 - month evaluable patients in mid - 2025 ) ; statements related to expectations regarding interactions with the FDA , Protara’s financial footing ; statements regarding the anticipated safety or efficacy of Protara’s product candidates ; and Protara’s outlook for the remainder of the year and future periods . Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward - looking statements . Factors that contribute to the uncertain nature of the forward - looking statements include : risks that Protara’s financial guidance may not be as expected, as well as risks and uncertainties associated with : Protara’s development programs, including the initiation and completion of non - clinical studies and clinical trials and the timing of required filings with the FDA and other regulatory agencies ; general market conditions ; changes in the competitive landscape ; changes in Protara’s strategic and commercial plans ; Protara’s ability to obtain sufficient financing to fund its strategic plans and development and commercialization efforts ; having to use cash in ways or on timing other than expected ; the impact of market volatility on cash reserves ; the loss of key members of management ; the impact of general U . S . and foreign, economic, industry, market, regulatory, political or public health conditions ; and the risks and uncertainties associated with Protara’s business and financial condition in general, including the risks and uncertainties described more fully under the caption “Risk Factors” and elsewhere in Protara's filings and reports with the United States Securities and Exchange Commission . All forward - looking statements contained in this presentation speak only as of the date on which they were made and are based on management's assumptions and estimates as of such date . Protara undertakes no obligation to update any forward - looking statements, whether as a result of the receipt of new information, the occurrence of future events or otherwise, except as required by law .

Promising NMIBC therapy C de - risked rare disease programs TARA - 002 in NMIBC • Dosing underway in Phase 2 STARBORN - 1 trial • TARA - 002 predecessor is standard of care in Japan • U.S. FDA granted Rare Pediatric Disease Designation – PRV eligible • Positive interim results from ADVANCED - 2 trial in NMIBC • Unique product characteristics anticipated to drive significant adoption • Expanding clinical program into BCG - naïve, combinations and systemic priming dosing Oncology Rare Disease TARA - 002 in LMs IV Choline for Parenteral Support • Enrolling pivotal study with PK endpoint • 30K patient population in the US 1 3 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute • FDA Orphan Drug and Fast Track Designations 1. Data on file
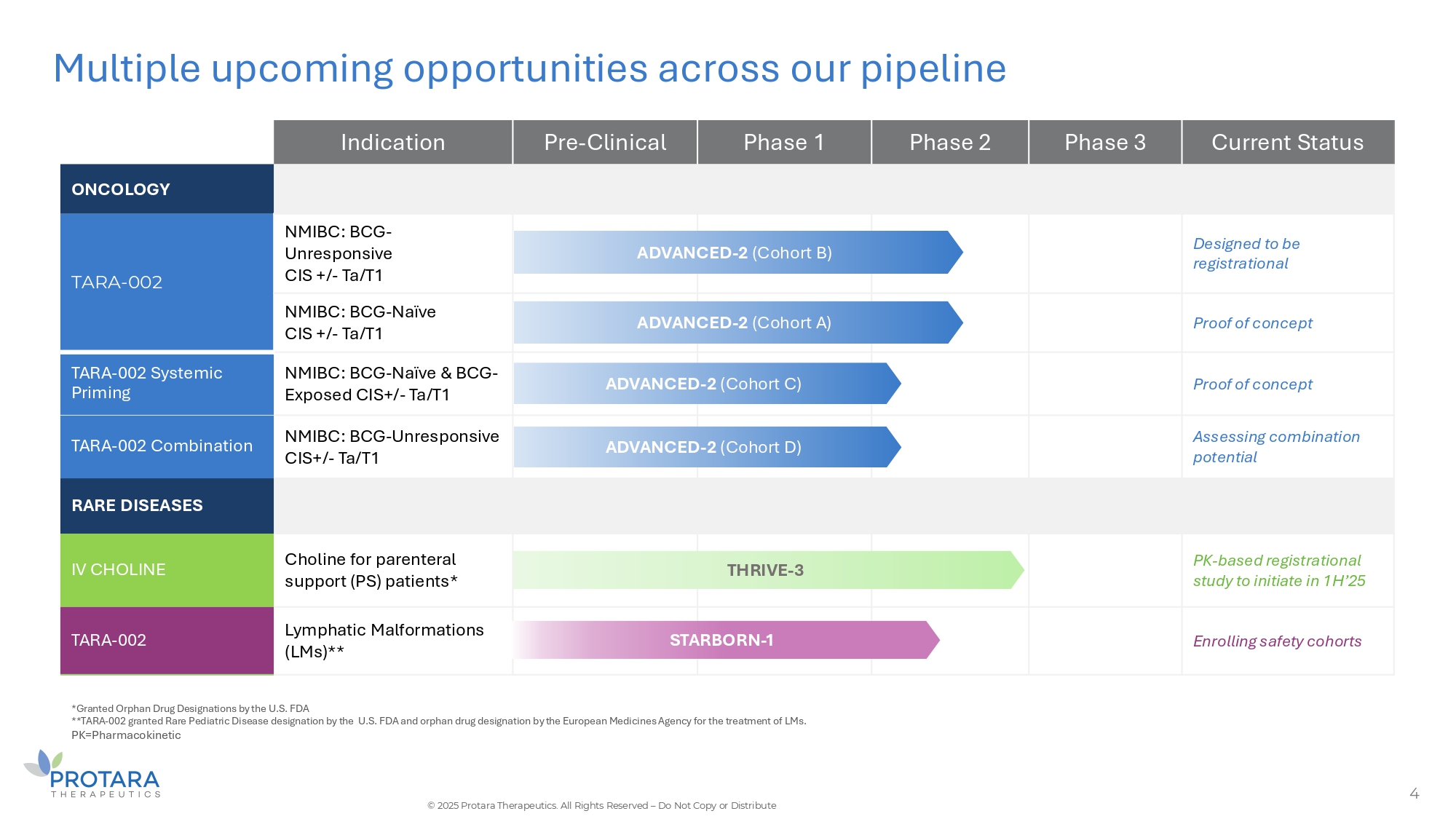
Multiple upcoming opportunities across our pipeline 4 *Granted Orphan Drug Designations by the U.S. FDA **TARA - 002 granted Rare Pediatric Disease designation by the U.S. FDA and orphan drug designation by the European Medicines Agency for the treatment of LMs. PK=Pharmacokinetic © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Current Status Phase 3 Phase 2 Phase 1 Pre - Clinical Indication ONCOLOGY Designed to be registrational ED - 2 (Cohort B) ADVANC NMIBC: BCG - Unresponsive CIS +/ - Ta/T1 TARA - 002 Proof of concept ED - 2 (Cohort A) ADVANC NMIBC: BCG - Naïve CIS +/ - Ta/T1 Proof of concept - 2 (Cohort C) ADVANCED NMIBC: BCG - Naïve C BCG - Exposed CIS+/ - Ta/T1 TARA - 002 Systemic Priming Assessing combination potential - 2 (Cohort D) ADVANCED NMIBC: BCG - Unresponsive CIS+/ - Ta/T1 TARA - 002 Combination RARE DISEASES PK - based registrational study to initiate in 1H’25 THRIVE - 3 Choline for parenteral support (PS) patients* IV CHOLINE Enrolling safety cohorts RBORN - 1 STA Lymphatic Malformations (LMs)** TARA - 002

Multiple anticipated near - term milestones 5 **Does not include 10.8M common warrants issued with the April 2024 private placement exercisable at a $5.25 per share at the earlier or April 10, 2027 or 90 days after public announcement of a minimum 42% six - month CR rate from at least 25 BCG - Unresponsive patients in the ADVANCED - 2 clinical trial. BALANCE SHEET: $81.5M of cash, cash equivalents and investments as of September 30, 2024. Cash runway into 2027 including $102.7M of gross proceeds from recent public offering COMMON SHARE EǪUIVALENTS (30.3M) ** : 20.6M Common + 8.0M Preferred + 1.7M Pre - funded warrants on as converted basis as of September 30, 2024 not including 14.1M Common and 2.3M Pre - Funded Warrants issued in recent public offering (46.7M common share equivalents) *Designed to be registrational aligned with U.S. FDA guidance on NMIBC clinical trials. ADVANCED - 2 BCG - Unresponsive Registrational* futility analysis THRIVE - 3 interim data STARBORN - 1 interim data ADVANCED - 2 interim data in 12 - month evaluable patients Initial systemic priming data 1H’2025 STARBORN - 1 interim data NMIBC IV Choline LMs 2H’2025 Initiate combination trial Ph 2 POC combo BCG - naïve trial design expect FDA feedback Initiate THRIVE - 3 registrational trial © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 Lyophilized, Inactivated Group A Streptococcus pyogenes

7 TARA - 002: Broad Immunopotentiator with significant potential TARA - 002 is an investigational, genetically distinct strain of Streptococcus pyogenes that is inactivated while retaining its immune - stimulating properties TARA - 002 is manufactured under cGMP conditions from the same Master Cell Bank as originator therapy OK - 432, (1) approved for LMs and a number of oncology indications in Japan There are close to 2,000 publications for OK - 432 in Pubmed Protara has worldwide rights, excluding Japan C Taiwan, for TARA - 002 / OK - 432 1. Marketed in Japan as Picibanil ® . LIVE CELLS INACTIVATED © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
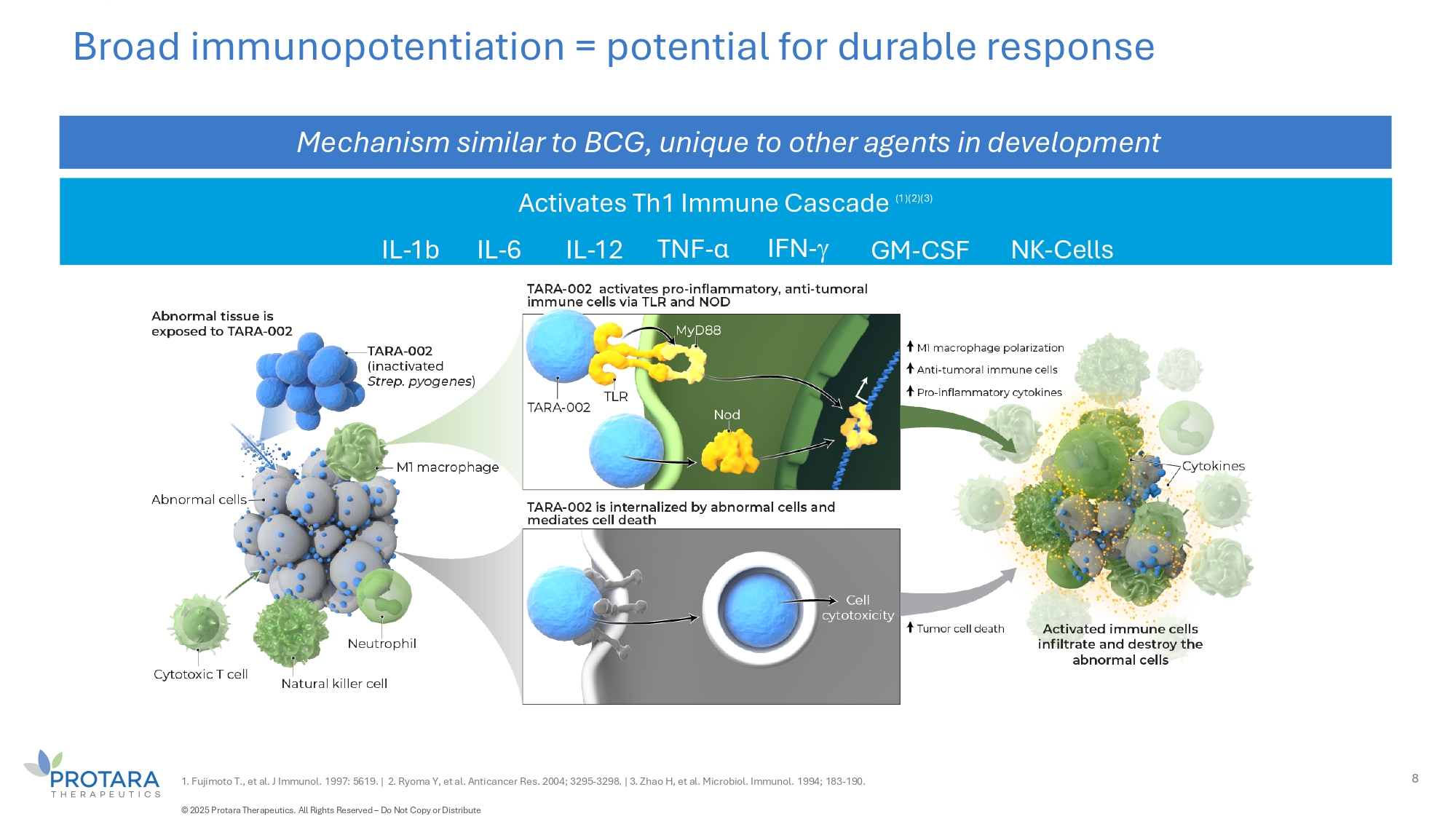
Activates Th1 Immune Cascade (1)(2)(3) Broad immunopotentiation = potential for durable response Mechanism similar to BCG, unique to other agents in development 1. Fujimoto T., et al. J Immunol. 1997: 5619. | 2. Ryoma Y, et al. Anticancer Res. 2004; 3295 - 3298. | 3. Zhao H, et al. Microbiol. Immunol. 1994; 183 - 190. 8 IFN - IL - 1b NK - Cells IL - 6 IL - 12 TNF - α GM - CSF © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 Non - Muscle Invasive Bladder Cancer

Anticipated low burden on physicians G patients Favorable safety G tolerability Encouraging interim ADAVNCED - 2 data • No additional administration procedures or safety protocols required • Fast administration typically performed by nurse • Dedicated to ensuring access with minimal burden • To date, no Grade 2 or greater treatment - related adverse events • To date, majority of adverse events are grade 1 and transient • Compelling response rates in BCG - UN and BCG - naïve • 100% durability observed from 3 - to 6 - months and 80% reinduction salvage rate seen across all patients Unique product characteristics anticipated to drive significant adoption 10 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

Bladder cancer: significant unmet need Currently approved therapies for BCG - unresponsive NMIBC were approved on the basis of single arm trials FDA BCG - UN; Significant unmet need 1. National Cancer Institute. SEER Bladder Cancer – Stat Facts. Accessed April 25, 2023. | 2. Anastasiadis et al. Therapeutic Advances in Urology, 2012. | 3. Campbell Walsh 11th edition, 2014, Elsevier. | 4. J Gual Frau et al. Arch Esp Urol. 2016. ~40 % to 50 % BCG failure rate radical cystectomy is the SOC after BCG failure 4 ~80,000 People diagnosed with bladder cancer annually in the U.S. 1 ~725,000 People estimated living with bladder cancer annually in the US 1 All Bladder Cancers ~75 % Of all bladder cancer diagnoses are NIMBC 2 80 % Estimated to recur in 3 years 3 Fat tissue CIS (carcinoma in situ) NMIBC Inner lining (urothelium) Connective Ta (urothelium) T1 (lamina propia) tissue (lamina propia) Superficial muscle Deep muscle 11 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Average age 73

TARA - 002 demonstrated 72% six - month CRR and 70% CRR at any time across BCG exposures BCG Unresponsive 12 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute BCG Naïve Combined Combined BCG Naïve BCG Un - responsive High - grade CR at Month 6 100 100% 80 72% 60 64% 40 20 0 N = 18 High - grade CR at any time 100 80 80% 70% 60 67% 40 20 0 N = 20 N = 20 N = 15 N = 5 (%) (%) (%) 14/20 (70.0) 10/15 (66.7) 4/5 (80.0) High - grade CR at any time High - grade CR at Timepoint 13/18 (72.2) 9/14 (64.3) 4/4 (100.0) Month 6 2/3 (66.7) 2/3 (66.7) N/A Month 9 High - grade CR at Month 6 by Baseline Dx 9/10 (90.0) 5/6 (83.3) 4/4 (100.0) CIS only 4/8 (50.0) 4/8 (50.0) N/A CIS + Ta/T1 Data cut - off date: 19 November 2024 Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ; Dx = diagnosis; NMIBC = non - muscle invasive bladder cancer Notes: At the time of data cutoff, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9; Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to dx progression or treatment failure. Subjects who have not yet completed week 12 visit as of study cut off date are not included; Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9.
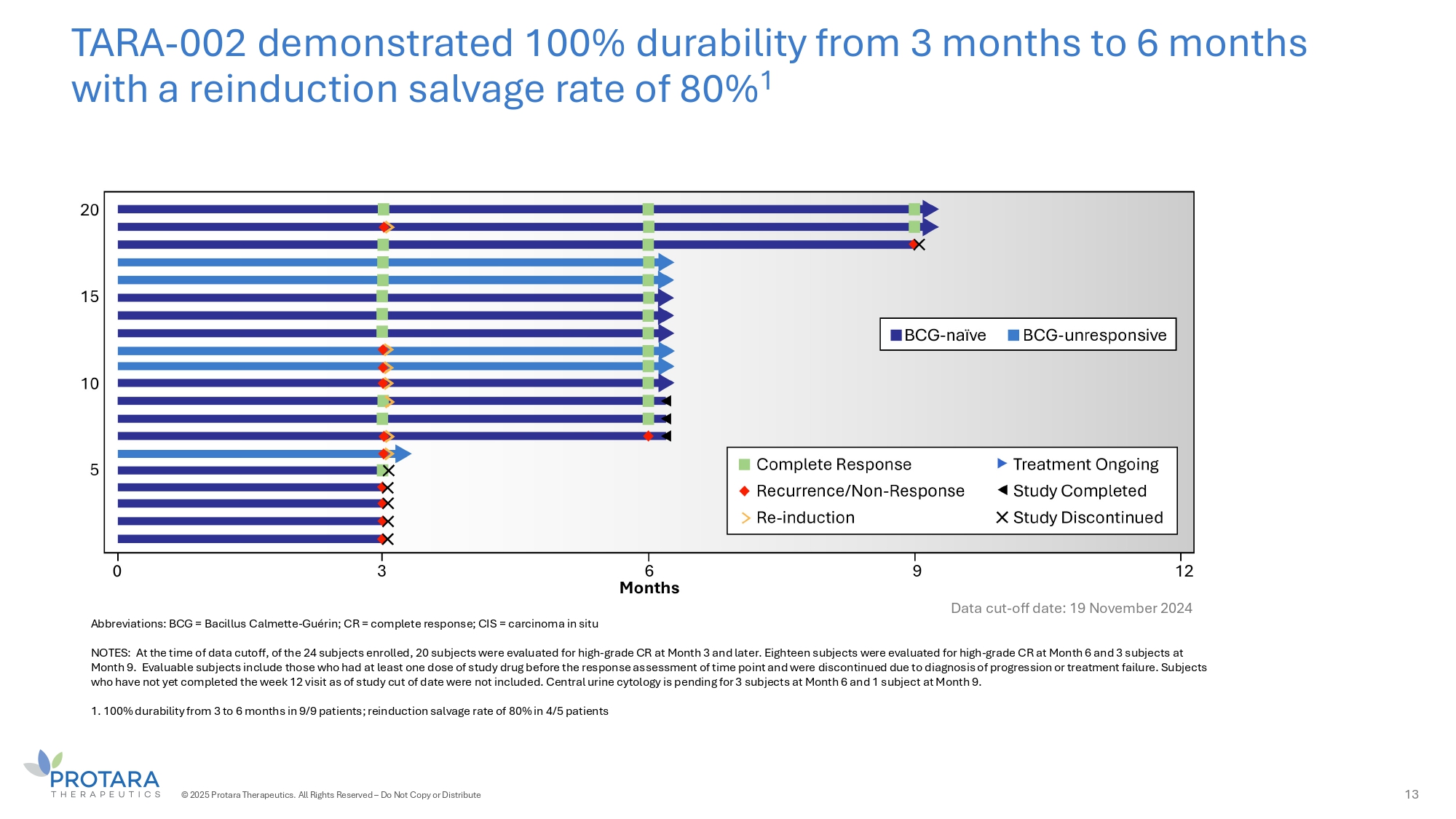
TARA - 002 demonstrated 100% durability from 3 months to 6 months with a reinduction salvage rate of 80% 1 Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ NOTES: At the time of data cutoff, of the 24 subjects enrolled, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9. Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to diagnosis of progression or treatment failure. Subjects who have not yet completed the week 12 visit as of study cut of date were not included. Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9. 1. 100% durability from 3 to 6 months in 9/9 patients; reinduction salvage rate of 80% in 4/5 patients 13 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Data cut - off date: 19 November 2024

14 TARA - 002 demonstrated favorable safety and tolerability in interim analysis of ADVANCED - 2 trial Data cut - off date: 19 November 2024 AEs reflect urinary tract instrumentation effects and known safety profile of an immune - potentiating drug Abbreviations: AE = adverse event; NMIBC = non - muscle invasive bladder cancer; TEAE = treatment emergent AE ^ Subjects may be counted in multiple categories + Non - drug related Serious TEAEs included urinary tract infection (UTI; N = 2) and urosepsis (N =1) Note: the safety population includes any patients who have had at least 1 dose of TARA - 002. The 24 patients in safety analysis include 3 patients who have not reached their week 12 assessment, and 1 patient withdrew consent prior to their week 12 assessment © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Grade 4/5 Grade 3 Grade 2 Grade 1 Any Grade N=24 0 3 (13) 7 (29) 11 (46) 16 (67) Number of Subjects with TEAEs , n^ (%) 0 0 0 6 (25) 6 (25) Number of Subjects with Related TEAEs ^, n (%) 0 0 0 3 (13) 3 (13) Dysuria 0 0 0 1 (4) 1 (4) Bladder Discomfort 0 0 0 1 (4) 1 (4) Bladder Spasm 0 0 0 1 (4) 1 (4) Chills 0 0 0 1 (4) 1 (4) Fatigue 0 0 0 1 (4) 1 (4) Hematuria 0 0 0 1 (4) 1 (4) Micturition Urgency 0 0 0 1 (4) 1 (4) Urinary Incontinence 0 2 (8) 1 (4) 0 3 (13) Number of Subjects with Serious TEAEs + , n (%) 0 0 0 0 0 Number of Subjects with TEAEs leading to Study Drug Withdrawal, n (%)
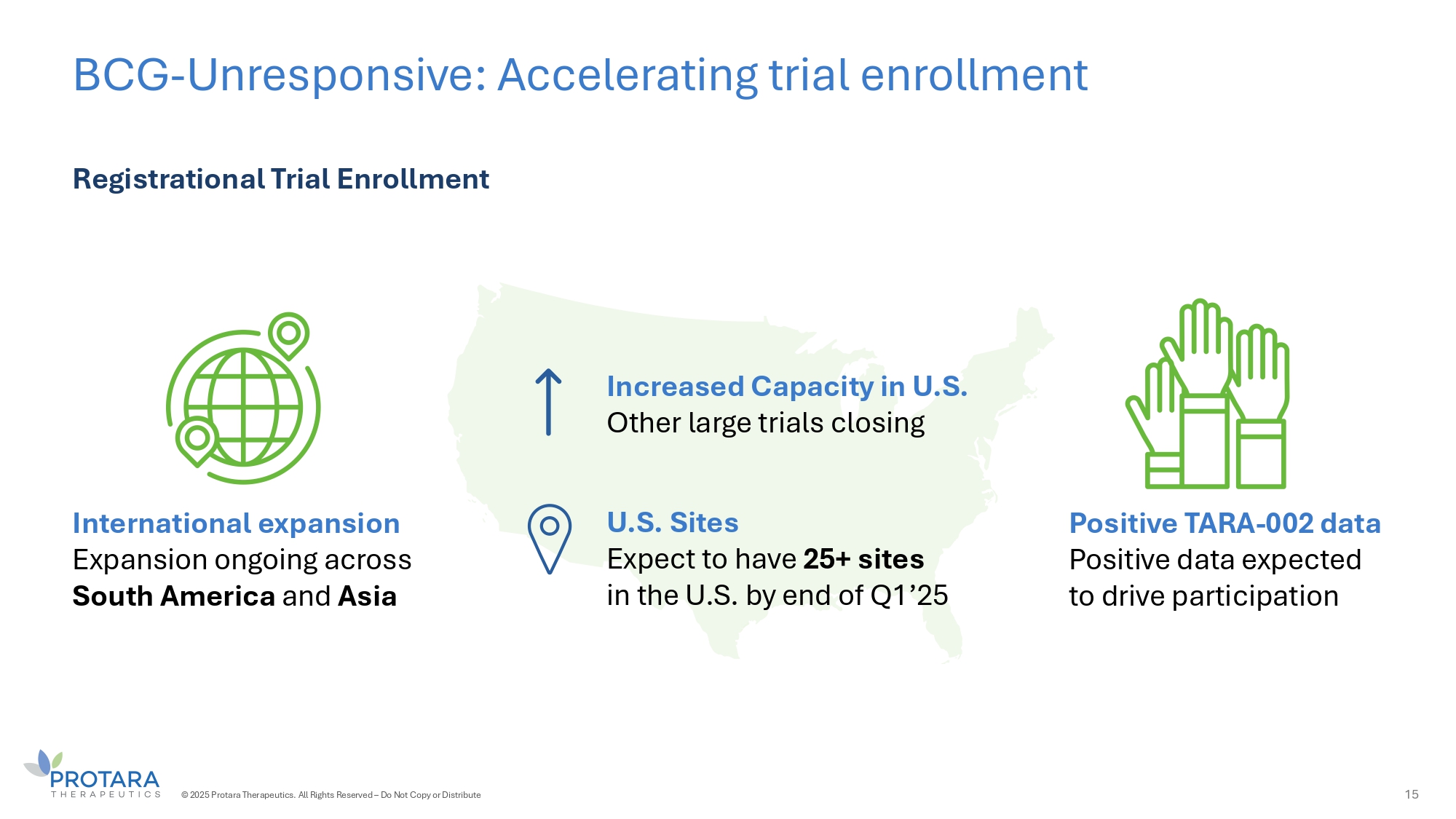
BCG - Unresponsive: Accelerating trial enrollment 15 Registrational Trial Enrollment International expansion Expansion ongoing across South America and Asia Positive TARA - 002 data Positive data expected to drive participation Increased Capacity in U.S. Other large trials closing U.S. Sites Expect to have 25+ sites in the U.S. by end of Ǫ1’25 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 BCG Cytokines + -- - IFN - γ +++ + TNF - α + = IL - 12p70 + + IL - 6 +++ +++ IL - 1β + = IL - 10 + + IL - 4 = = IL - 13 - = IL - 8 -- -- IL - 2 TARA - 002 treatment promotes higher release of pro - inflammatory TH1 - type cytokines than BCG in co - culture =, no change; +: 2 - 5 fold upregulation +++: ≥ 15 - fold upregulation; - : 2 - 5 fold upregulation; -- : 5 - 14 - fold upregulation; --- : ≥ 15 - fold upregulation 1 2 TARA - 002 (KE/mL) BCG (MOI) 1. Data from company pre - clinical studies 0 0.05 0.1 0.2 0.4 0.8 1.6 3.2 6.4 12.8 1 5 10 20 40 80 0 20 40 60 80 100 % of Cytotoxicity *** ** * * MBT2 cells TARA - 002 induces higher cytotoxicity than BCG in bladder cancer cells MB49 cells TARA - 002 demonstrates differentiated profile to BCG (1) © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 administration among the easiest of approved and experimental NMIBC treatments 17 Definitions: USPI – U.S. prescribing information; DDM - dodecyl maltoside; Data derived from product SOPIs and clinical trial publications © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute TARA - 002 No vial thaw, simple preparation; no pre/post treatment protocol EG - 70 No vial thaw, simple preparation; no pre/post treatment protocol N - 803/BCG - TICE Risk of infection for patient and caregivers; urine decontamination burden Adstiladrin Vial thaw introduces preparation bottleneck; elevated biosafety procedures required; additional pre/post burden for patients Cretostimogene Complex process with unclear vial thaw, biosafety, and urine decontamination requirements TARA - 002 has reduced burden for physicians and patients Preparation: can be conducted on open table or benchtop; no special handling Preparation: can be conducted on open table or benchtop; no special handling Preparation: mask/gown required to avoid infection 6 - hour period of required urine bleaching Antichollinergic premedication 3 – 10 hour vial thaw Preparation : “universal biosafety precautions” required ; USPI contains infection risk warning 48 - hour period of required urine bleaching Thaw process not disclosed Preparation: BSL2/2+ handling likely required Saline wash DDM wash Saline wash DDM re - infusion and dwell (15 min) Urine bleaching requirements not disclosed TAR - 200 Requires insertion every three weeks, potential for patients to feel device in bladder and removal requires cystoscopy Assembly of the device on open table or benchtop Use catheter after successful insertion Removal of the device requiring cystoscopy Repeat the same procedure every 3 weeks
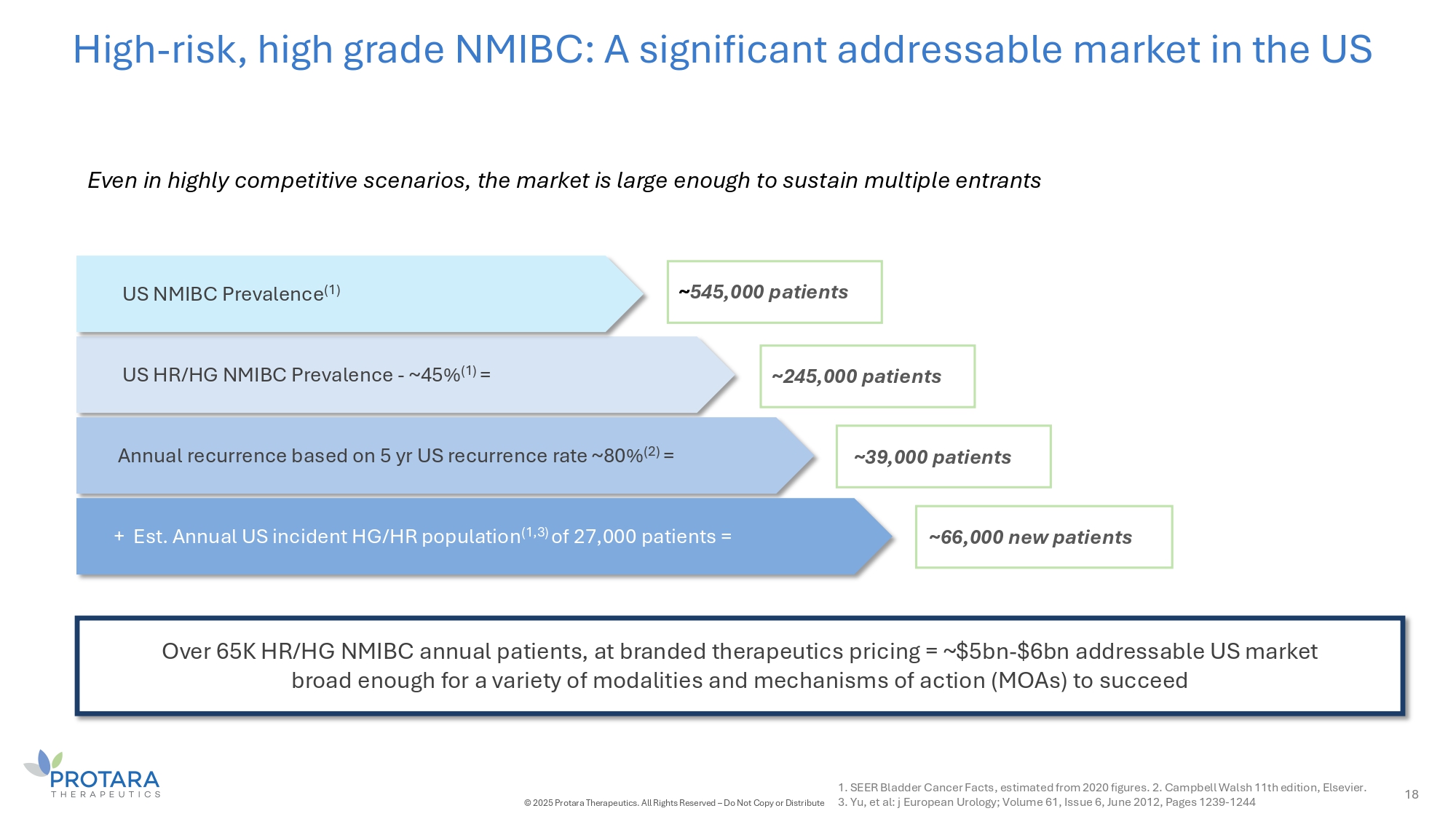
High - risk, high grade NMIBC: A significant addressable market in the US 18 Even in highly competitive scenarios, the market is large enough to sustain multiple entrants US HR/HG NMIBC Prevalence - ~45% (1) = Annual recurrence based on 5 yr US recurrence rate ~80% (2) = Over 65K HR/HG NMIBC annual patients, at branded therapeutics pricing = ~$5bn - $6bn addressable US market broad enough for a variety of modalities and mechanisms of action (MOAs) to succeed + Est. Annual US incident HG/HR population (1,3) of 27,000 patients = 1. SEER Bladder Cancer Facts, estimated from 2020 figures. 2. Campbell Walsh 11th edition, Elsevier. © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 3. Yu, et al: j European Urology; Volume 61, Issue 6, June 2012, Pages 1239 - 1244 ~ 545,000 patients ~33,000 patients ~CC,000 new patients US NMIBC Prevalence (1) ~245,000 patients
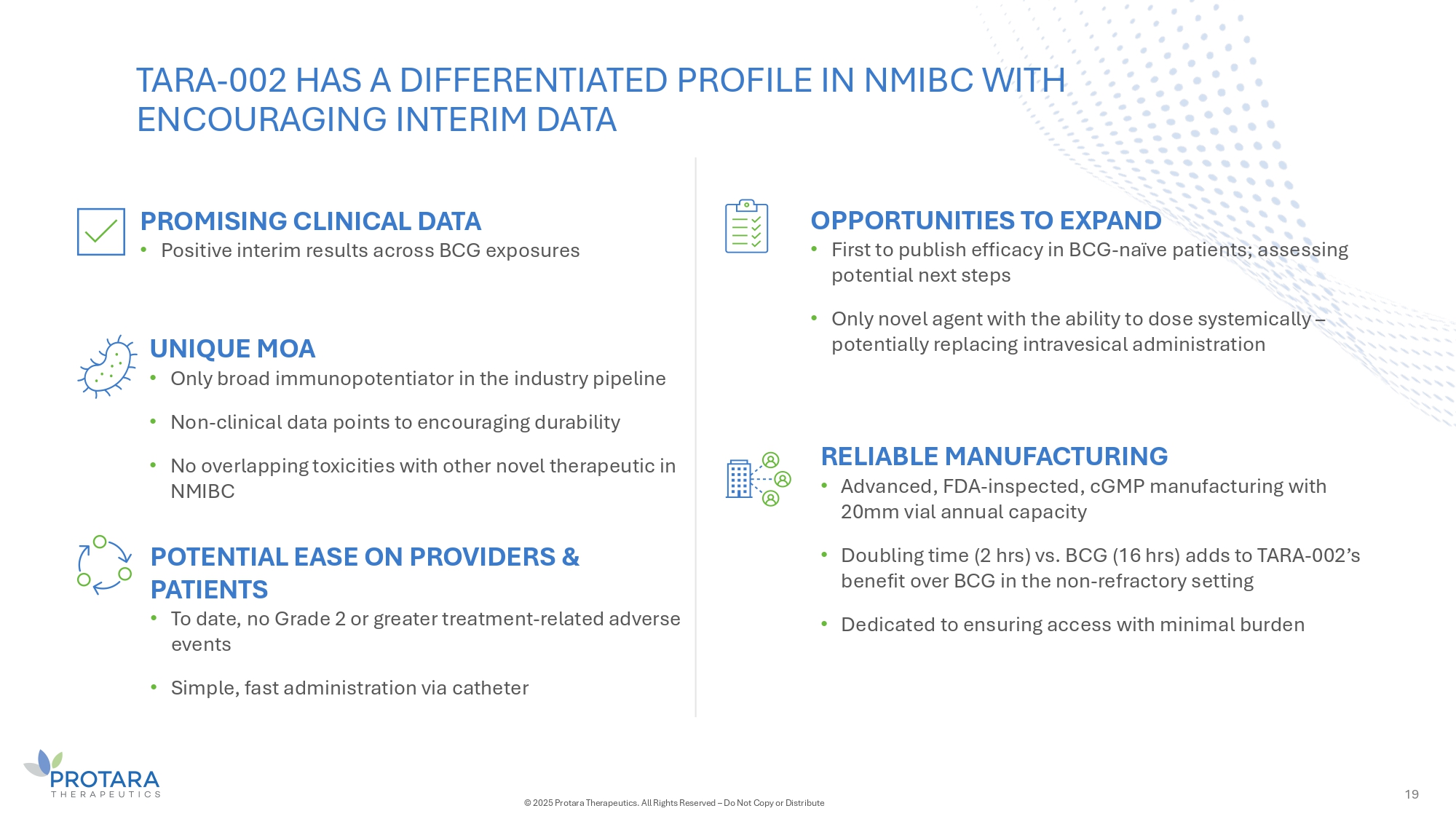
19 UNIǪUE MOA • Only broad immunopotentiator in the industry pipeline • Non - clinical data points to encouraging durability • No overlapping toxicities with other novel therapeutic in NMIBC OPPORTUNITIES TO EXPAND • First to publish efficacy in BCG - naïve patients; assessing potential next steps • Only novel agent with the ability to dose systemically – potentially replacing intravesical administration POTENTIAL EASE ON PROVIDERS G PATIENTS • To date, no Grade 2 or greater treatment - related adverse events • Simple, fast administration via catheter TARA - 002 HAS A DIFFERENTIATED PROFILE IN NMIBC WITH ENCOURAGING INTERIM DATA PROMISING CLINICAL DATA • Positive interim results across BCG exposures RELIABLE MANUFACTURING • Advanced, FDA - inspected, cGMP manufacturing with 20mm vial annual capacity • Doubling time (2 hrs) vs. BCG (16 hrs) adds to TARA - 002’s benefit over BCG in the non - refractory setting • Dedicated to ensuring access with minimal burden © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

IV CHOLINE CHLORIDE Phospholipid substrate replacement therapy for patients dependent on parenteral support (PS)
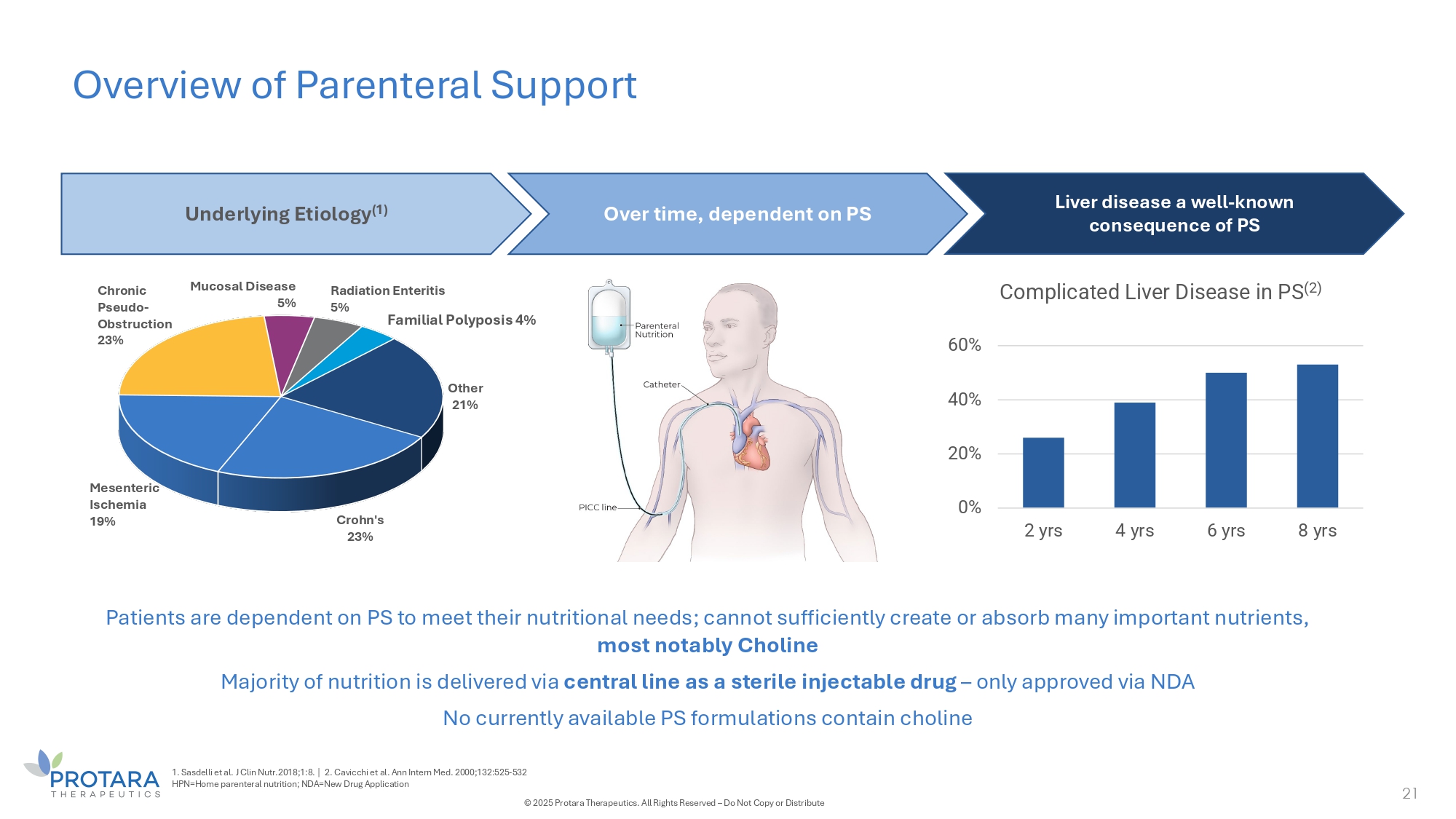
Overview of Parenteral Support 21 Crohn's 23% Mesenteric Ischemia 1G% Chronic Pseudo - Obstruction 23% Mucosal Disease 5% Other 21% Radiation Enteritis 5% Familial Polyposis 4% Underlying Etiology (1) Over time, dependent on PS Liver disease a well - known consequence of PS Patients are dependent on PS to meet their nutritional needs; cannot sufficiently create or absorb many important nutrients, most notably Choline Majority of nutrition is delivered via central line as a sterile injectable drug – only approved via NDA No currently available PS formulations contain choline 0% 20% 40% 60% 2 yrs 4 yrs 6 yrs 8 yrs Complicated Liver Disease in PS (2) 1. Sasdelli et al. J Clin Nutr.2018;1:8. | 2. Cavicchi et al. Ann Intern Med. 2000;132:525 - 532 HPN=Home parenteral nutrition; NDA=New Drug Application © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

Choline deficiency in PS is among the largest rare disease indications 22 ~30,000 1 PS patients in the U.S. and the majority are choline deficient • 78% of PS - dependent patients are choline - deficient and of those 63% have some degree of liver damage 2 • Data confirm choline deficiency results in liver, bone, muscle and cognitive impairment 3,4 Phase 2 study confirmed choline replacement restored normal levels • Independently conducted Phase 2 data demonstrated significant improvement in serum choline concentrations and a pronounced impact on steatosis 4 • Choline replacement is included in guidelines and recommendations by key PS professional associations FDA has cleared the way for “source of choline” label with single study • FDA granted a targeted indication of “source of choline for PS patients who are, or may become, choline - deficient” • Single study demonstrating an increase in choline levels required (already demonstrated in Ph 2 trial) • Both a compound patent and a method of treatment patent in U.S. to 2041 1. Data on file | 2. THRIVE - 1: A Multi - Center, Cross - Sectional, Observational Study to Assess the Prevalence of Choline Deficiency in Patients Dependent on Parenteral Nutrition. ESPEN. 2024 | 3. Chawla R, et al. Am J Clin Nutr. 1986 42:577 - 584. | 3. Zeisel S, et al. Neurology. 1980 30:1226 - 1229. | 4. Buchman et al. JPEN, 2002 - Protara Therapeutics re - analysis of patient CRFs, data on file. Definitions: IV, intravenous; PN, parenteral nutrition. © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
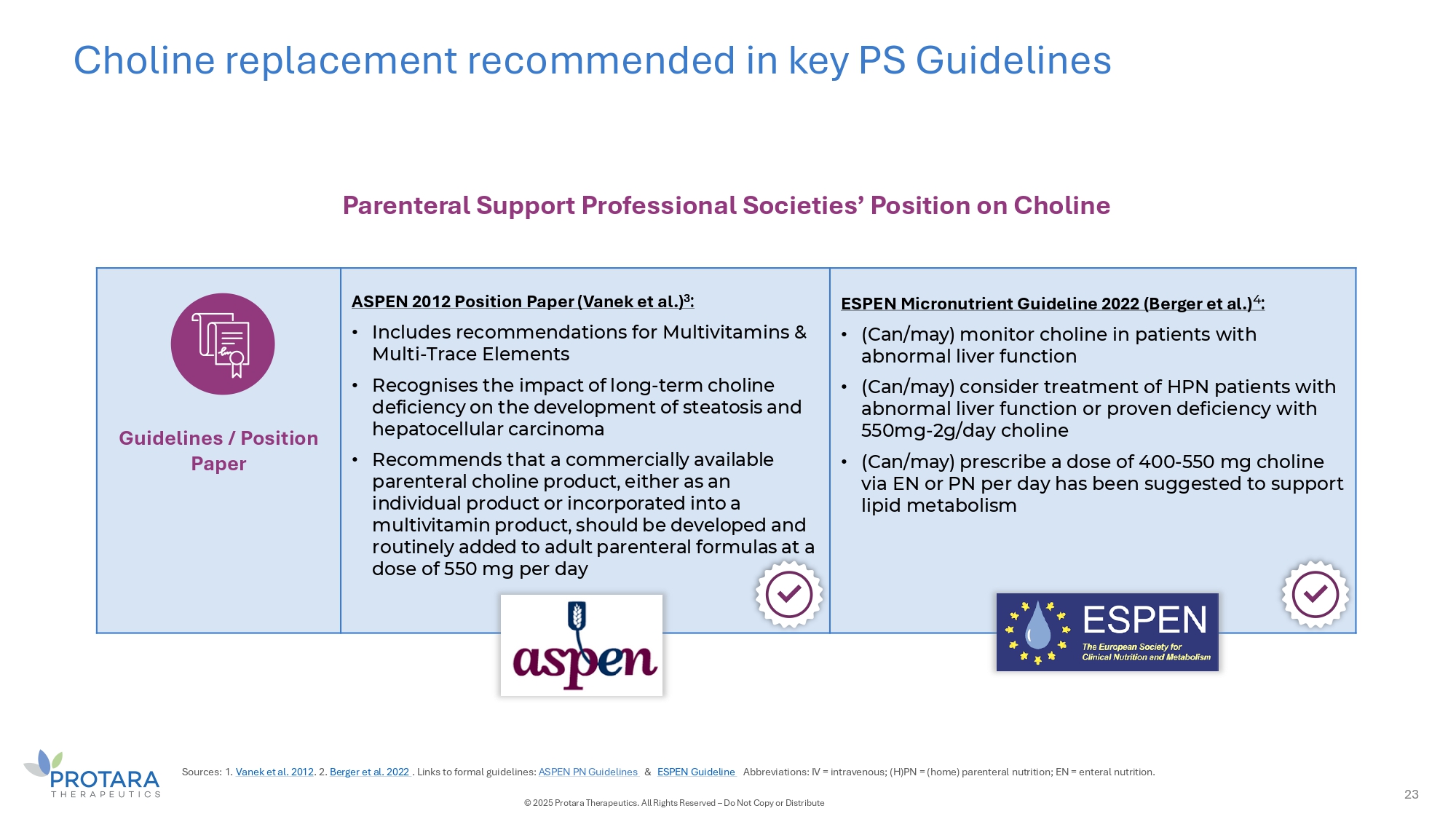
Choline replacement recommended in key PS Guidelines Parenteral Support Professional Societies’ Position on Choline Guidelines / Position Paper ASPEN 2012 Position Paper (Vanek et al.) 3 : • Includes recommendations for Multivitamins & Multi - Trace Elements • Recognises the impact of long - term choline deficiency on the development of steatosis and hepatocellular carcinoma • Recommends that a commercially available parenteral choline product, either as an individual product or incorporated into a multivitamin product, should be developed and routinely added to adult parenteral formulas at a dose of 550 mg per day ESPEN Micronutrient Guideline 2022 (Berger et al.) 4 : • (Can/may) monitor choline in patients with abnormal liver function • (Can/may) consider treatment of HPN patients with abnormal liver function or proven deficiency with 550mg - 2g/day choline • (Can/may) prescribe a dose of 400 - 550 mg choline via EN or PN per day has been suggested to support lipid metabolism Sources: 1. Vanek et al. 2012 . 2. Berger et al. 2022 . Links to formal guidelines: ASPEN PN Guidelines C ESPEN Guideline Abbreviations: IV = intravenous; (H)PN = (home) parenteral nutrition; EN = enteral nutrition. 23 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
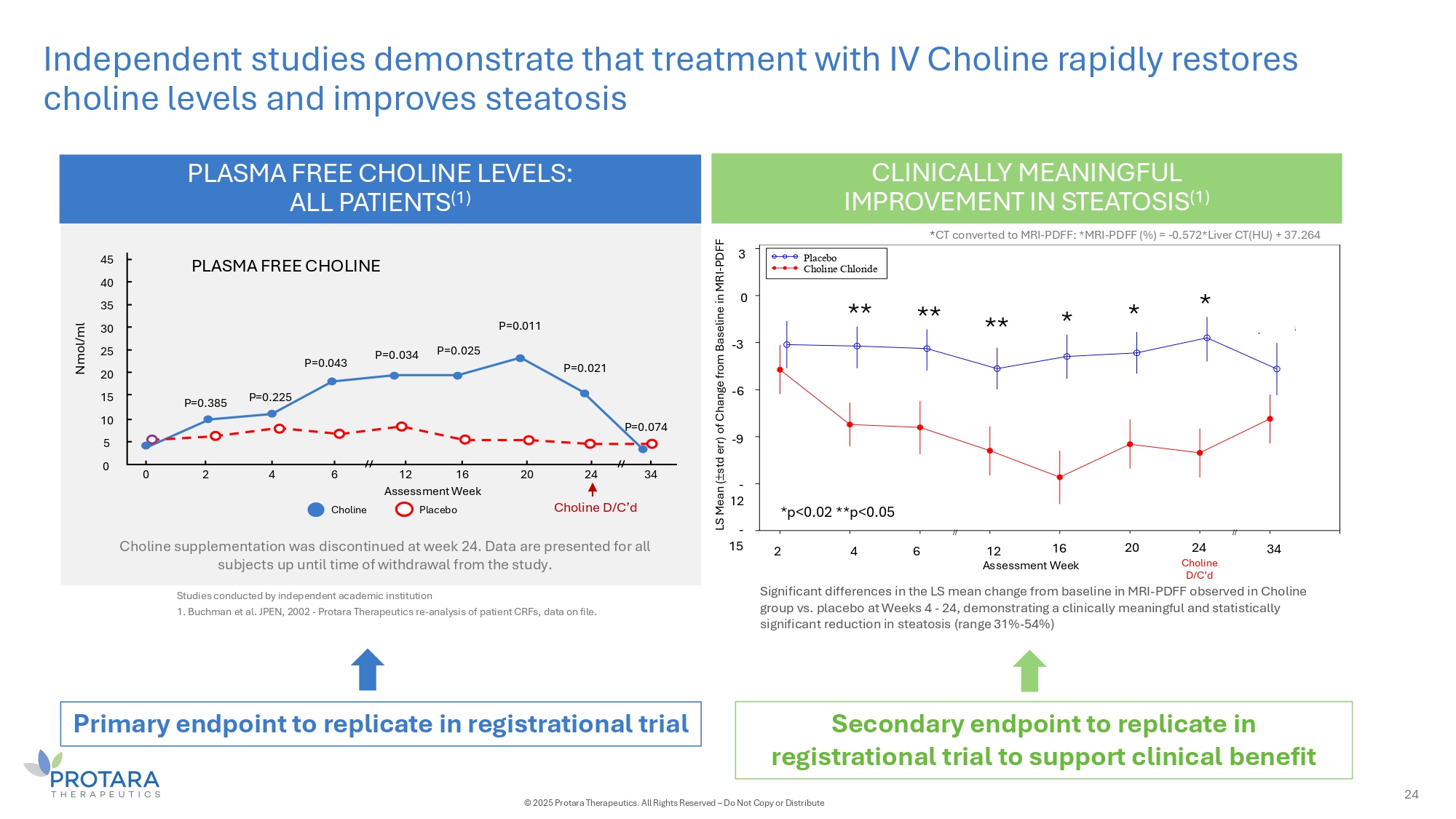
Independent studies demonstrate that treatment with IV Choline rapidly restores choline levels and improves steatosis PLASMA FREE CHOLINE LEVELS: ALL PATIENTS (1) Choline supplementation was discontinued at week 24. Data are presented for all subjects up until time of withdrawal from the study. PLASMA FREE CHOLINE Nmol/ml Choline D/C’d 0 2 4 6 20 24 34 P=0.225 P=0.043 P=0.034 P=0.025 P=0.011 P=0.021 P=0.074 45 40 35 30 25 20 15 10 5 0 Choline 12 16 Assessment Week Placebo // // CLINICALLY MEANINGFUL IMPROVEMENT IN STEATOSIS (1) P=0.385 Studies conducted by independent academic institution 1. Buchman et al. JPEN, 2002 - Protara Therapeutics re - analysis of patient CRFs, data on file. Choline D/C'd Assessment Week S Means tderr) ange aseli L (+/ - s of ch from B ne of MRI p= 0.4770 p= 0.0197 p= 0.0312 p= 0.0180 p= 0.0014 p= 0.0091 p= 0.0021 p= 0.1823 // // - 15 - 12 - 9 - 6 - 3 0 3 2 4 6 12 16 20 24 34 Placebo Choline Chloride * * * ** ** ** - 3 3 0 - 6 - 9 - 2 1 - 15 Assessment Week *p<0.02 **p<0.05 2 4 6 12 16 20 Choline D/C’d 34 24 Significant differences in the LS mean change from baseline in MRI - PDFF observed in Choline group vs. placebo at Weeks 4 - 24, demonstrating a clinically meaningful and statistically significant reduction in steatosis (range 31% - 54%) * CT converted to MRI - PDFF: *MRI - PDFF (%) = - 0.572*Liver CT(HU) + 37.264 LS Mean ( std err) of Change from Baseline in M - PD R F I - F PDFF Primary endpoint to replicate in registrational trial Secondary endpoint to replicate in registrational trial to support clinical benefit 24 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
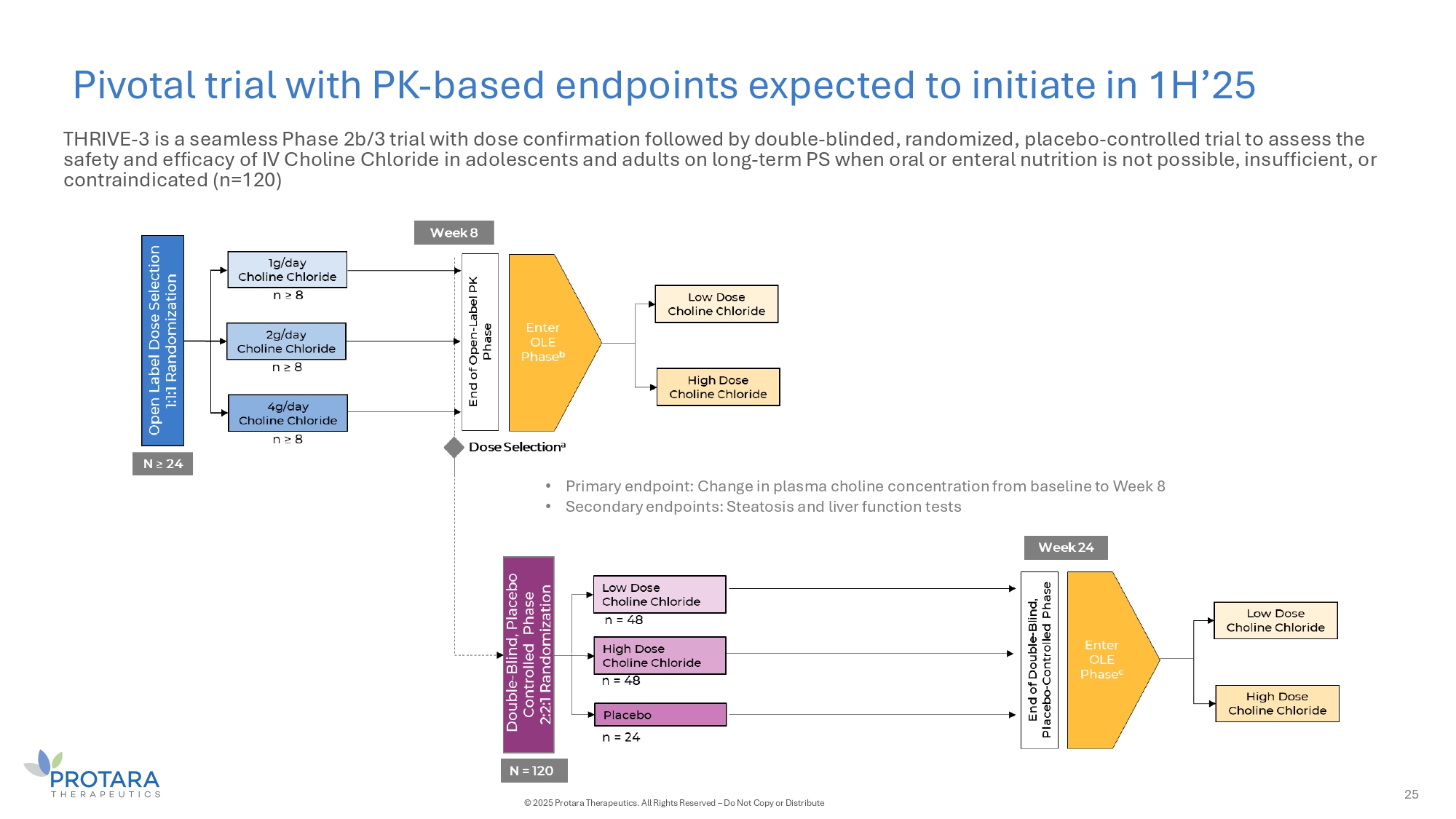
Pivotal trial with PK - based endpoints expected to initiate in 1H’25 25 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute • Primary endpoint: Change in plasma choline concentration from baseline to Week 8 • Secondary endpoints: Steatosis and liver function tests THRIVE - 3 is a seamless Phase 2b/3 trial with dose confirmation followed by double - blinded, randomized, placebo - controlled trial to assess the safety and efficacy of IV Choline Chloride in adolescents and adults on long - term PS when oral or enteral nutrition is not possible, insufficient, or contraindicated (n=120)

TARA - 002 Lymphatic Malformations (LMs)

27 TARA - 002 in LMs Lymphatic Malformations Rare, non - malignant lesions consisting of dilated, lymphatic fluid - filled sacs caused by abnormal development of the lymphatic endothelial system (1) Epidemiology: incidence of lymphatic malformations is ≈1,400 - 1,800 LM cases per year (2) Current treatment options Current treatment options include surgical excision with high complication (33%) and recurrence (55%) rates (3) as well as off - label use of sclerosants Potential for Priority Review Voucher upon approval Granted RPDD in 2021 Ongoing Ph 2 clinical trial Ph 2 STARBORN - 1 trial in pediatric LMs patients is ongoing Additional indications Historical literature and patient experience indicate that TARA - 002 could have the potential to treat other maxillofacial cysts 1. Brouillard P, et al. J Clin Invest. 2014;124:898 - 904. | 2. Internal company estimates | 3. Ha J, et al. Curr Ped Rev. 2014;10:238 - 248. © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
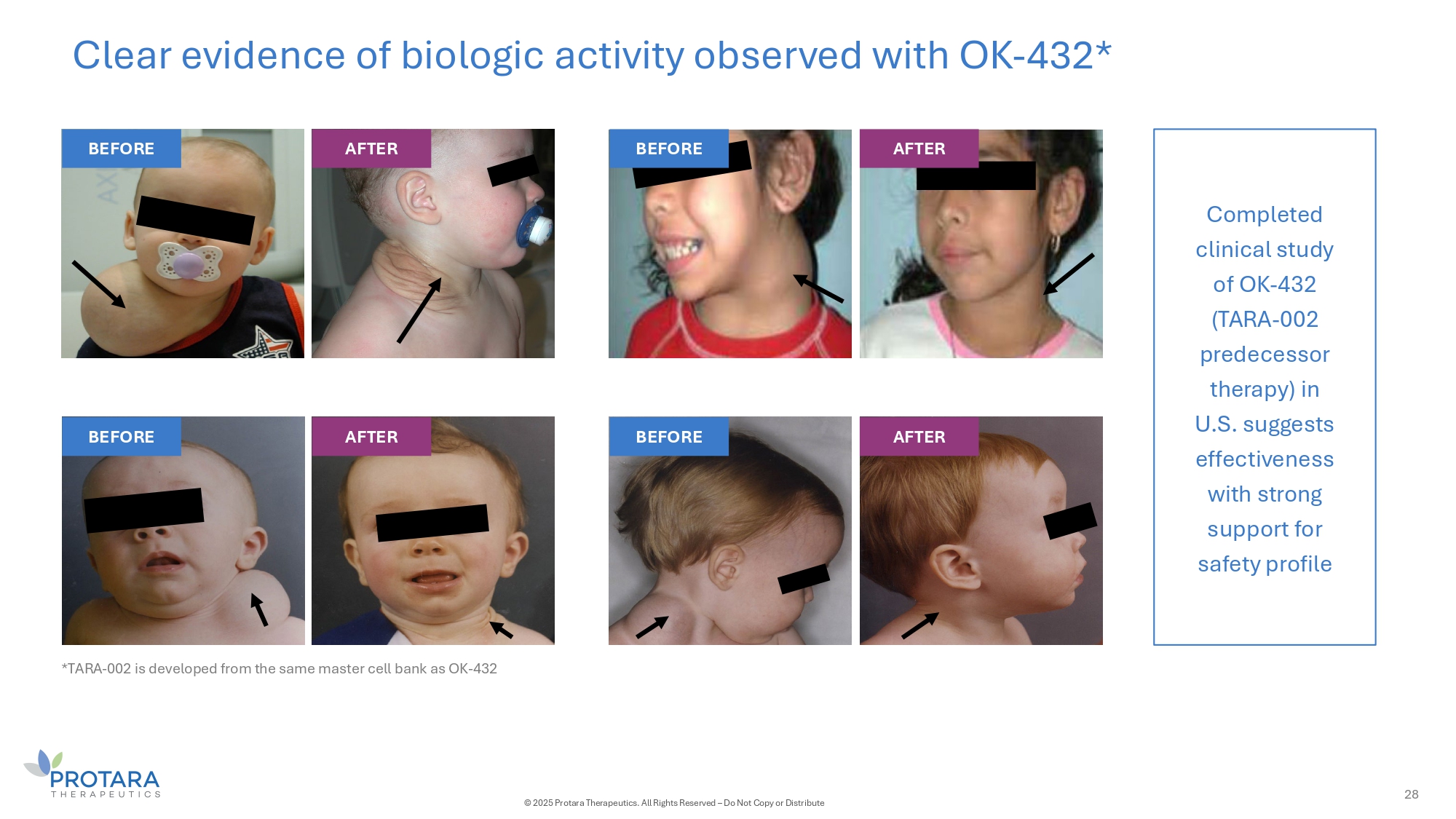
Clear evidence of biologic activity observed with OK - 432* Completed clinical study of OK - 432 (TARA - 002 predecessor therapy) in U.S. suggests effectiveness with strong support for safety profile BEFORE AFTER BEFORE AFTER BEFORE AFTER BEFORE 28 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute AFTER *TARA - 002 is developed from the same master cell bank as OK - 432
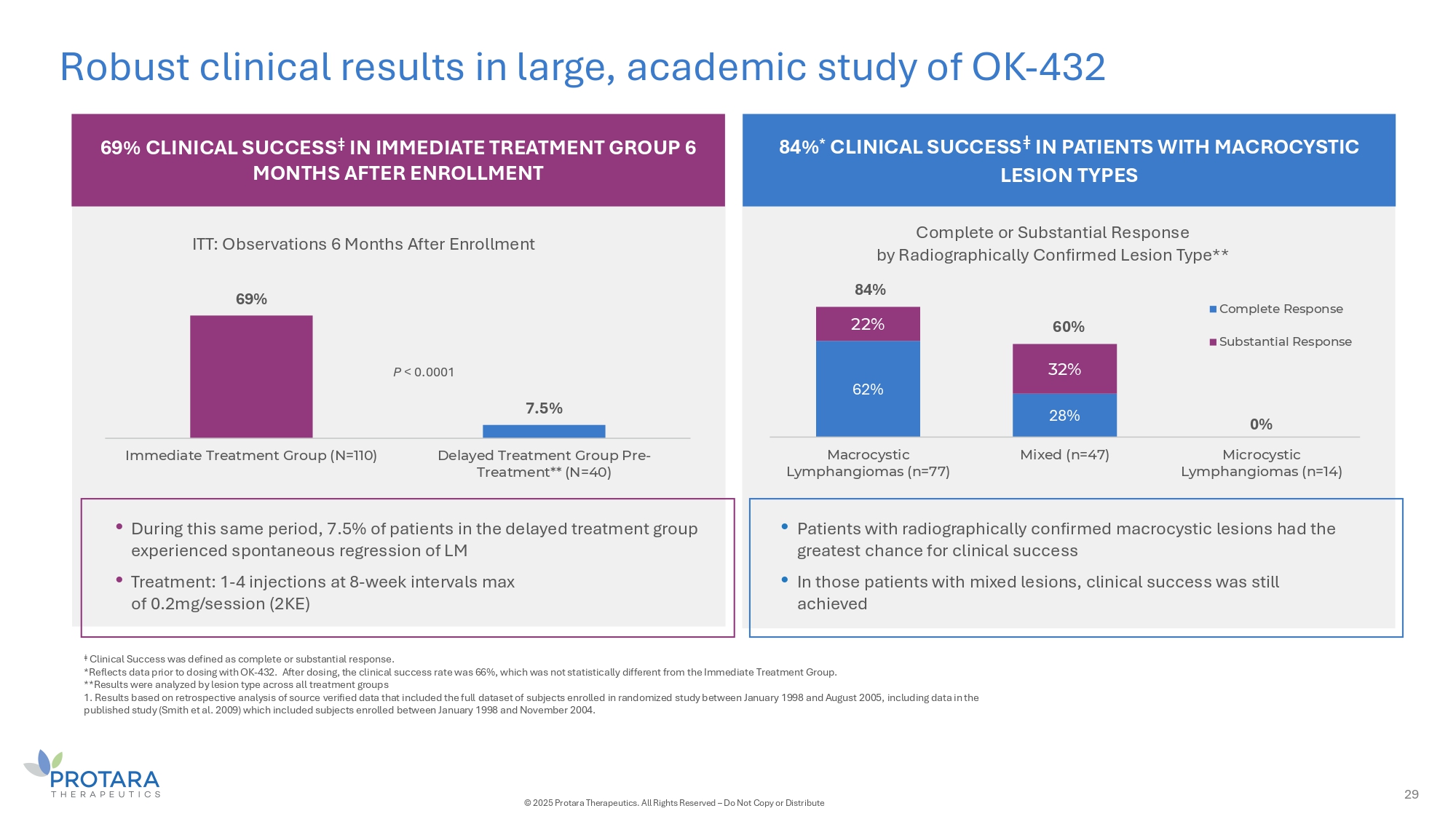
Robust clinical results in large, academic study of OK - 432 6G% CLINICAL SUCCESS ǂ IN IMMEDIATE TREATMENT GROUP 6 MONTHS AFTER ENROLLMENT 84% * CLINICAL SUCCESS ǂ IN PATIENTS WITH MACROCYSTIC LESION TYPES 6G% 7.5% Immediate Treatment Group (N=110) Delayed Treatment Group Pre - Treatment** (N=40) ITT: Observations 6 Months After Enrollment 62% 28% 0% 22% 32% Macrocystic Lymphangiomas (n=77) Mixed (n=47) Microcystic Lymphangiomas (n=14) Complete or Substantial Response by Radiographically Confirmed Lesion Type** Complete Response Substantial Response • Patients with radiographically confirmed macrocystic lesions had the greatest chance for clinical success • In those patients with mixed lesions, clinical success was still achieved • During this same period, 7.5% of patients in the delayed treatment group experienced spontaneous regression of LM • Treatment: 1 - 4 injections at 8 - week intervals max of 0.2mg/session (2KE) P < 0.0001 84% 60% ǂ Clinical Success was defined as complete or substantial response. *Reflects data prior to dosing with OK - 432. After dosing, the clinical success rate was 66%, which was not statistically different from the Immediate Treatment Group. **Results were analyzed by lesion type across all treatment groups 1. Results based on retrospective analysis of source verified data that included the full dataset of subjects enrolled in randomized study between January 1998 and August 2005, including data in the published study (Smith et al. 2009) which included subjects enrolled between January 1998 and November 2004. 29 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
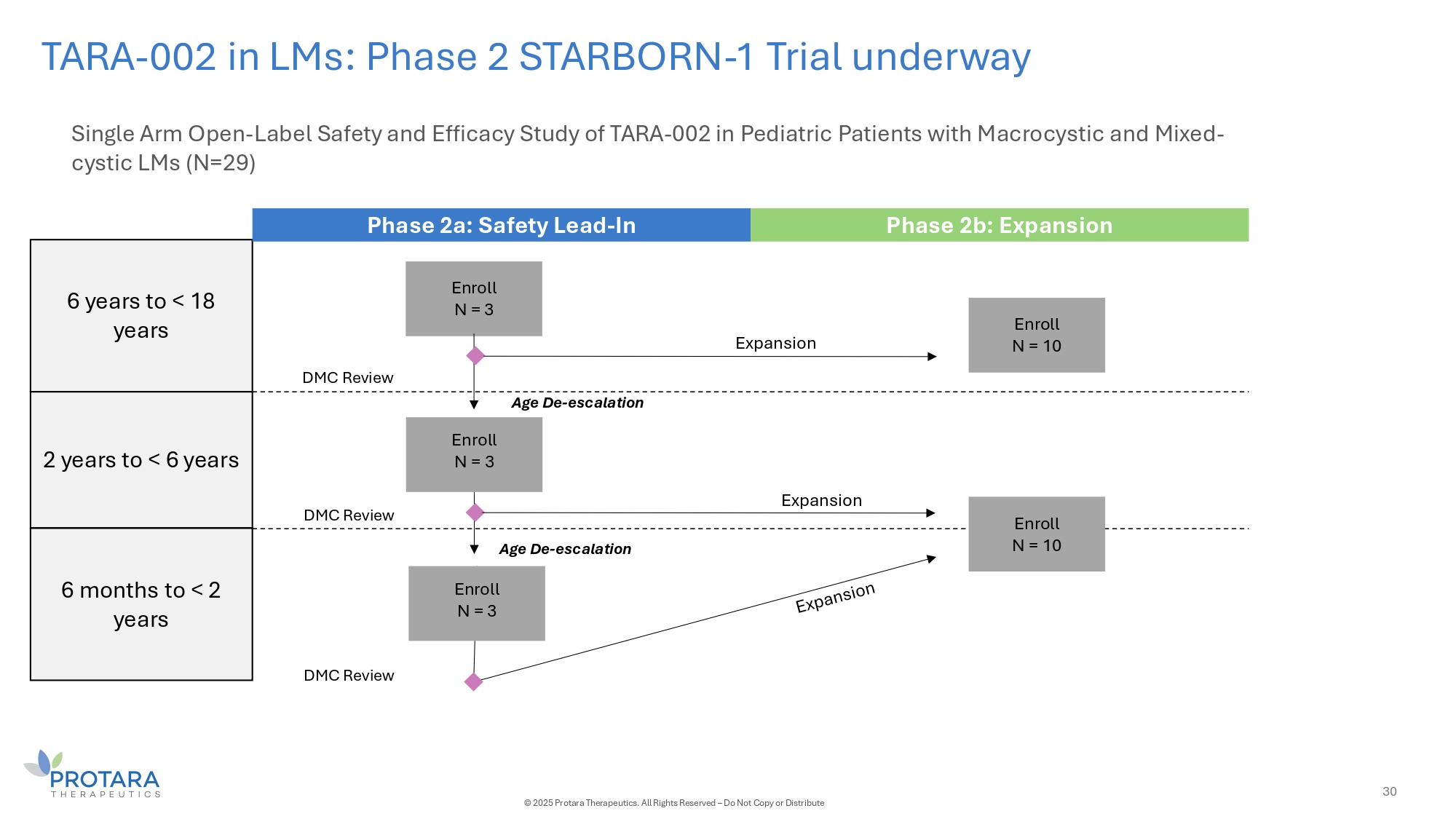
TARA - 002 in LMs: Phase 2 STARBORN - 1 Trial underway Single Arm Open - Label Safety and Efficacy Study of TARA - 002 in Pediatric Patients with Macrocystic and Mixed - cystic LMs (N=29) 6 years to < 18 years 2 years to < 6 years 6 months to < 2 years Phase 2a: Safety Lead - In Phase 2b: Expansion Enroll N = 3 Enroll N = 3 Expansion Age De - escalation Age De - escalation Expansion DMC Review DMC Review DMC Review Enroll N = 3 Enroll N = 10 Enroll N = 10 30 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

APPENDIX

Led by a team of experienced professionals Jesse Shefferman Co - founder, Director, Chief Executive Officer Pat Fabbio Chief Financial Officer Jacqueline Zummo, PhD, MPH, MBA Co - founder, Senior Vice President, Chief Scientific Operations Officer Justine O’Malley Senior Vice President, Investor Relations and Corporate Communications Mary Grendell General Counsel, Corporate Secretary 32 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 in NMIBC: ADVANCED - 2 clinical trial design RE - induction (if eligible*) Maintenance (months 6 - 18) Month 3 Month 6 Month 18 Month 60 Maintenance (months 6 - 18) Month 18 Protara Confidential Information Data cut - off date: 19 November 2024 33 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Month 60 Month 3 Month 6 Abbreviations: CR = complete response; CIS = carcinoma in situ *Aligned with the FDA’s 2024 BCG Unresponsive NMIBC: Developing Drugs and Biologics for Treatment Guidance for Industry. †Residual CIS and/or recurrence of HGTa Primary endpoint of high - grade complete response (CR) at any time at 6 months; Key secondary of 12 - month DOR

ADVANCED - 2 demographics and disease characteristics 34 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Data cut - off date: 19 November 2024 N = 24 N = 24 Prior BCG Status, n (%) Age (years) 17 (71) BCG Naïve 71 (10.9) Mean (SD) 2 (8) BCG Exposed 71 Median 5 (21) BCG Unresponsive 45, 92 Min, Max Prior No. of BCG Doses, n (%) Sex, n (%) 5 (21) ≥ 12 BCG doses 19 (79) Male 2 (8) < 12 BCG doses 5 (21) Female Prior non - BCG Treatment, n (%) Race, n (%) 2 (8) Gemcitabine/Docetaxel 24 (100) White 1 (4) Gemcitabine Ethnicity, n (%) 3 (12) Mitomycin 1 (4) Hispanic 2 (8) Other 23 (96) Non - Hispanic Prior TURBT Status, n (%) ECOG Score, n (%) 5 (21) > 3 TURBTs 18 (75) 0 19 (79) ≤ 3 TURBTs 5 (21) 1 1 (4) 2 Baseline Diagnosis, n (%) 14 (58) CIS only 6 (25) CIS + Ta 4 (17) CIS + T1

20M vial capacity ability to expand capacity 5X Efficient 2 hour doubling time with t wo - week batch completion 47 successful batches to date Completed FDA inspection without Form 483s TARA - 002: Manufacturing is a potential competitive advantage 35 © 2025 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute


































