Exhibit 10.1
AWARD/CONTRACT | | 1. THIS CONTRACT IS A RATED ORDER | 
| | RATING | | |
| | UNDER DPAS (15 CFR 350) | | D0-C9 | | | |
2. CONTRACT (Proc. Inst. Indent.) NO. | | 3. EFFECTIVE DATE | | 4. REQUISITION/PURCHASE REQUEST/PROJECT NO. |
W9113M-08-C-0028 | | 2 Jan 2008 | | | | |
| | | | |
5. ISSUED BY | CODE W9113M | | 6. ADMINISTERED BY (If other than Item 5) | CODE |
US ARMY SPACE & MISSILE DEFENSE COMMAND
SMDC-RDCM
PO BOX 1500
HUNTSVILLE AL 35807-3801 | | |
| | |
7. NAME AND ADDRESS OF CONTRACTOR (No, street, city, county, State and Zip Code) | | 8. DELIVERY |
| | |
| OSIRIS THERAPEUTICS, INC
7015 ALBERT EINSTEIN DRIVE
COLUMBIA MD 21046-1707 | | o FOB ORIGIN | x OTHER (See below |
| | | 9. DISCOUNT FOR PROMPT PAYMENT
Net 30 |
| | | | |
CODE 06JU9 | | FACILITY CODE | | 10. SUBMIT INVOICES
(4 copies unless other-
wise specified) TO THE
ADDRESS SHOWN IN: | 
| ITEM
G |
| | | |
11. SHIP TO/MARK FOR | CODE | | 12. PAYMENT WILL BE MADE BY | CODE | HQ0302 |
See Schedule | | | DFAS-ROME
DFAS-RO-A, 325 BROOKS ROAD PHONE 800-553-0527
ROME NY 13441-4527 |
| | | |
13. AUTHORITY FOR USING OTHER THAN FULL AND OPEN COMPETION: | | 14. ACCOUNTING AND APPROPRIATION DATA
|
o 10 U.S.C. 2304(c) ( ) | o 41 U.S.C. 253(c) ( ) | | See Schedule |
| | |
15A. ITEM NO. | | 15B. SUPPLIES/SERVICES | | 15C. QUANTITY | | 15D. UNIT | | 15E. UNIT PRICE | | 15F. AMOUNT |
| | | | | | | | | | |
| | SEE SCHEDULE | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | 15G. TOTAL AMOUNT OF CONTRACT | 
| | $4,253,390.80 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
x | SEC. | | DESCRIPTION | | PAGE(S) | | x | SEC. | | DESCRIPTION | | PAGE(S) |
| | | PART I - THE SCHEDULE | | | | | | | PART II - CONTRACT CLAUSES |
x | A | | SOLICITATION/CONTRACT FORM | | 1-3 | | x | I | | CONTRACT CLAUSES | | 19-25 |
x | B | | SUPPLIES OR SERVICES AND PRICES/COSTS | | 4-10 | | PART III - LIST OF DOCUMENTS, EXHIBITS AND OTHER ATTACH. |
x | C | | DESCRIPTION/SPECS./WORK STATEMENT | | 11 | | x | J | | LIST OF ATTACHMENTS | | 26 |
x | D | | PACKAGING AND MARKING | | 12 | | | PART IV REPRESENTATIONS AND INSTRUCTIONS |
x | E | | INSPECTION AND ACCEPTANCE | | 13 | | | K | | REPRESENTATIONS, CERTIFICATIONS AND OTHER STATEMENTS OF OFFERORS | | |
x | F | | DELIVERIES OR PERFORMANCE | | 14-15 | | | | |
x | G | | CONTRACT ADMINISTRATION DATA | | 16 | | | L | | INSTRS., CONDS., AND NOTICES TO OFFERORS |
x | H | | SPECIAL CONTRACT REQUIREMENTS | | 17-18 | | | M | | EVALUATION FACTORS FORAWARD | | |
| | | | | | | | | | | | | | | | | | | |
CONTRACTING OFFICER WILL COMPLETE ITEM 17 OR 18 AS APPLICABLE
17. x CONTRACTOR’S NEGOTIATED AGREEMENT (Contractor is required in sign this document and return copies to issuing office.) Contractor agrees to furnish and deliver all items or perform all the services set forth of otherwise identified above and on any continustion sheets for the consideration stated herain. The rights and obligations of the parties to this contract shall be subject to and governed by the following documents: (a) this award/contract, (b) the solicitation, if any, and (c) such provisions, representations, certifications, and specifications, as are attached or incorporated by reference herein. (Attachments are listed herein) | | 18. o AWARD (Contractor is not required to sign this document) Your offer on solicitation Number including the additions or changes made by you which additions or changes are set forth in full above, is hereby accepted as to the items listed above and on any continuation sheets. This award the consummates which consists of the following documents: (a) the Government’s solicitation and your offer, and (b) this award/contract. No further contractual document is necessary. |
19A. NAME AND TITLE OF SIGNER (Typs or print) | 20A. NAME OF CONTRACTING OFFICER |
C. Randal Mills, President | LYNN M. SELFRIDGE |
| |
19B. NAME OF CONTRACTOR | 19C. DATE SIGNED | 20B. UNITED STATES OF AMERICA | 20C. DATE SIGNED |
BY | /s/ C. Randal Mills | 12/31/07 | BY | /s/ Lynn M. Selfridge | 1/3/08 |
| (Signature of person authorized to sign) | | | (Signature of Contracting Officer |
NSN 7540-01-152-8069 PREVIOUS EDITION UNUSABLE | 26-107 | STANDARD FORM 26 (REV. 4-85 Presented by GSA
FAR (46 CFR 53.214(a) | USAPPCV 1.00 |
1
W9113M-08-C-0028
Page 2 of 26
Section A - Solicitation/Contract Form
CONTINUATION OF FORM 26
Award is hereby made for the Development and Delivery of an FDA Approved Medical Radiation Countermeasure (MRC) product.
The Osiris Therapeutics Inc. proposal dated August 10, 2007 as revised on September 6, 2007, is incorporated into contract No. W9113M-08-C-0028 with the following revisions.
1. Contract Data Requirements List (CDRL) A001, A003, A004 and A008 are requirements associated with SLINs 0001A and 0001AB, not 0001AD. Costs associated with these CDRLs are removed from option SLIN 0001AD and added into SLINs 0001AA and option 0001AB as shown in Section B.
2. SubCLINs (SLINs) 0004AA-0004AB are hereby decreased to a quantity of 5,000 each, and SubCLINs 0004AC, and 0004AD are hereby added with a quantity of 5,000 each. Prior to the first exercise of these optional line items, the Government reserves the right to renegotiate with Osiris the $10,000 unit price for these optional line items at a price not to exceed (i) $10,000 or (ii) the lowest “most favored end user customer” commercial price (including consideration of any discount or rebate arrangement) at the time of negotiation; whichever is lower. If at any time any of these options are exercised the product is commercially available to the public (as defined by FAR part 2.101), the price shall not exceed the (i) the lowest “most favored end user customer” commercial price paid or offered within 6 months of the option exercise or (ii) $10,000; whichever is lower.
3. All costs identified through the revised proposal of September 6, 2007, include any costs that are a result of the Osiris and Genzyme collaborative agreement.
4. Osiris Therapeutics Inc.’s shall implement all recommendations as identified in DCAA Audit Report No. 6311-2007D17740064, within 3 months of contract award to have a fully compliant accounting system audited by the DCAA and approved by the assigned ACO. If at the end of the 3 months, Osiris has failed to fulfill this requirement, the Government shall suspend acceptance of additional vouchers until the requirements are completed.
5. CLIN 0002 is removed.
6. Section E. Inspection/Acceptance terms have been added.
7. Section F. Manpower Reporting requirement is removed.
8. Section H. Unamed clause after 52.234-4 is removed.
9. Section I.
The following clauses are added by reference:
52.211-5, Material Requirements (Aug 2000)
52.232-1, Payments - Fixed Price (Apr 1984)
52.242-15, Stop Work Order (Aug 1989)
The following clauses are incorporated by full text:
52.217-6, Option for Increased Quantity (Mar 1989)
252.211-7003, Item Identification and Valuation (Jun 2005)
The following clauses are revised to include the most current version:
52.203-6 (Sep 2006)
52.204-7 (Jul 2006)
W9113M-08-C-0028
Page 3 of 26
52.209-6 (Sep 2006)
52.222-26 (Mar 2007)
52.222-35 (Sep 2006)
52.244-6 (Mar 2007)
252.209-7004 (Dec 2006)
252.244-7000 (Jan 2007)
The following clauses are deleted:
52.230-2
52.225-5 DEV
10. Section J:
CDRLs A001-A008 are corrected to include the correct Contract Line Item Number.
W9113M-08-C-0028
Page 4 of 26
Section B - Supplies or Services and Prices
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0001 | | | | | | | | | | | |
| | MRC Development and Delivery | | | | | | | |
| | CPIF | | | | | | | | | |
| | (Information CLIN only) | | | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | TARGET COST | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | TARGET FEE | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | TOTAL TGT COST+ FEE | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | MINIMUM FEE | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | MAXIMUM FEE | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | SHARE RATIO ABOVE TARGET | | | |
| | | | | | | | | | | |
| | | | | | | | SHARE RATIO BELOW TARGET | | | |
| | | | | | | | | | | |
W9113M-08-C-0028
Page 5 of 26
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | | |
0001AA | | | | | | | | | | | | |
| | CPIF | | | | | | | | | | |
| | Technology Development phase of the Advanced Development, Demonstration, and Delivery of a FDA-Approved therapeutic Medical Radiation Countermeasure (MRC) product in accordance with the contractor’s Statement of Work (SOW) dated August 10, 2007. (As provided in response to the Government’s Statement of Objectives (SOO) located as an attachment to Section J generally, and the activities described in paragraph C.3.1 of the SOO specifically) to include delivery to the Government of, at a minimum, one dose of an Investigational New Drug (IND) that has 1) successfully undergone Phase 1 safety testing acceptable for a therapeutic MRC indication and 2) has received FDA authorization to proceed and is not subject to a clinical hold, and 3) has successfully completed two years of International Conference on Harmonisation-compliant stability testing, and 4) has undergone all other work specified in the Contractor’s SOW. | | | | |
| | FOB: Destination | | | | | | | | | | |
| | TARGET COST | | $3,866,719.00 | |
| | | | | |
| | TARGET FEE | | $386,671.80 | |
| | | | | |
| | TOTAL TGT COST+ FEE | | $4,253,390.80 | |
| | | | | |
| | MINIMUM FEE | | $0.00 | |
| | | | | |
| | MAXIMUM FEE | | $773,343.78 | |
| | | | | |
| | SHARE RATIO ABOVE TARGET | | 70/30 | |
| | | | | |
| | SHARE RATIO BELOW TARGET | | 70/30 | |
| | | | | |
ACRN AA | | | | $4,253,390.80 | |
CIN: 000000000000000000000000000000 | | | |
| | | | | | | | | | | | | | | | | |
W9113M-08-C-0028
Page 6 of 26
| | | | | | | | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | | |
0001AA | | | | | | | | | | | | |
OPTION | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | CPIF | | | | | | | | | | |
| | System Development and Demonstration phase of the Advanced Development, Demonstration, and Delivery of a FDA-Approved therapeutic MRC product in accordance with the Contractor’s Statement of Work (SOW) dated August 10, 2007. (As provided in response to the Governments Statement of Objectives (SOO) located as an attacment to Section J generally, and the activities described in paragraph C.3.2 of the SOO specifically), to include delivery to the Government of, at a minimum, one dose of the FDA-approved MRC. | | | | |
| | FOB: Destination | | | | | | | | | | |
| | | | | | |
| | TARGET COST | | $16,815,380.00 | | |
| | | | | | |
| | TARGET FEE | | $1,681,538.00 | | |
| | | | | | |
| | TOTAL TGT COST+ FEE | | $18,496,918.00 | | |
| | | | | | |
| | MINIMUM FEE | | $0.00 | | |
| | | | | | |
| | MAXIMUM FEE | | $3,363,076.00 | | |
| | | | | | |
| | SHARE RATIO ABOVE TARGET | | 70/30 | | |
| | | | | | |
| | SHARE RATIO BELOW TARGET | | 70/30 | | |
| | | | | | |
| | | | | | |
| | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0001AC | | | | | | | | | | | |
OPTION | | | | | | | | | | | |
| | | | | | | | | | | |
| | CPIF | | | | | | | | | |
| | Implementation of an Earned Value Management (EVM) System in accordance | | | |
with DFARS 252.242-7002. | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | TARGET COST | | $241,229.00 | | |
| | | | | | |
| | TARGET FEE | | $24,123.00 | | |
| | | | | | |
| | TOTAL TGT COST+ FEE | | $265,352.00 | | |
| | | | | | |
| | MINIMUM FEE | | $0.00 | | |
| | | | | | |
| | MAXIMUM FEE | | $48,246.00 | | |
| | | | | | |
| | SHARE RATIO ABOVE TARGET | | 70/30 | | |
| | | | | | |
| | SHARE RATIO BELOW TARGET | | 70/30 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
W9113M-08-C-0028
Page 7 of 26
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0001AD | | | | | | | | | | | |
OPTION | | | | | | | | | | | |
| | | | | | | | | | | |
| | CPIF | | | | | | | | | |
| | Monthly Contract Performance Report in accordance with the relevant DD Form 1423, Contract Data Requirement List (CDRL). See Exhibit A005, attached in Section J. | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | TARGET COST | | $801,485.00 | |
| | | | | |
| | TARGET FEE | | $80,149.20 | |
| | | | | |
| | TOTAL TGT COST+ FEE | | $881,634.20 | |
| | | | | |
| | MINIMUM FEE | | $0.00 | |
| | | | | |
| | MAXIMUM FEE | | $160,297.00 | |
| | | | | |
| | SHARE RATIO ABOVE TARGET | | 70/30 | |
| | | | | |
| | SHARE RATIO BELOW TARGET | | 70/30 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0001AE | | | | | | | | | | | |
OPTION | | | | | | | | | | | |
| | | | | | | | | | | |
| | CPIF | | | | | | | | | |
| | Quarterly Contract Funding Status Report in accordance with the relevant DD Form 1423, Exhibit A002 attached in Section J. | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | TARGET COST | | $407,345.00 | |
| | | | | |
| | TARGET FEE | | $40,734.00 | |
| | | | | |
| | TOTAL TGT COST+ FEE | | $448,079.00 | |
| | | | | |
| | MINIMUM FEE | | $0.00 | |
| | | | | |
| | MAXIMUM FEE | | $81,469.00 | |
| | | | | |
| | SHARE RATIO ABOVE TARGET | | 70/30 | |
| | | | | |
| | SHARE RATIO BELOW TARGET | | 70/30 | |
| | | | | | | | | | | | | | | | |
W9113M-08-C-0028
Page 8 of 26
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0001AF | | | | | | | | | | | |
OPTION | | | | | | | | | | | |
| | | | | | | | | | | |
| | CPIF | | | | | | | | | |
| | Technical Data Package to be delivered after FDA approval of MRC | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | TARGET COST | | $190,582.00 | |
| | | | | | | | | | | |
| | | | | | | | TARGET FEE | | $19,058.00 | |
| | | | | | | | | | | |
| | | | | | | | TOTAL TGT COST+ FEE | | $209,640.00 | |
| | | | | | | | | | | |
| | | | | | | | MINIMUM FEE | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | MAXIMUM FEE | | $38,116.00 | |
| | | | | | | | | | | |
| | | | | | | | SHARE RATIO ABOVE TARGET | | 70/30 | |
| | | | | | | | | | | |
| | | | | | | | SHARE RATIO BELOW TARGET | | 70/30 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0003 | | | | 1 | | Lot | | $116,610.00 | | $116,610.00 | |
OPTION | | | | | | | | | | | |
| | FFP | | | | | | | | | |
| | Drug Master File. Exercised at Milestone B and shall be completed no later than | | | |
| | 90 days once the option is exercised. | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | NET AMT | | $116,610.00 | |
| | | | | | | | | | | |
W9113M-08-C-0028
Page 9 of 26
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0004 | | | | | | | | | | | |
OPTION | | Production End Items | | | | | | | |
| | FFP | | | | | | | | | |
| | HOSPITAL-BASED Medical Radiation Countermeasure (MRC): An MRC | | | |
| | suitable for delayed administration after diagnosis of the GI syndrome of ARS is | | | |
| | made and used at medical treatment facilities in the battle space and the sustaining | | | |
| | base, and under the supervision of a Physician. (SubCLINS 0004AA through | | | |
| | 0004AD below). | | | |
| | | | | | | | | | | |
| | (Information CLIN Only) | | | | | | | |
| | | | | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | NET AMT | | $0.00 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0004AA | | | | 5,000 | | Each | | $10,000.00 | | $50,000,000.00 | |
OPTION | | | | | | | | | | | |
| | HOSPITAL-BASED MRC | | | | | | | |
| | FFP | | | | | | | | | |
| | 5,000 TED | | | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | NET AMT | | $50,000,000.00 | |
W9113M-08-C-0028
Page 10 of 26
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0004AB | | | | 5,000 | | Each | | $10,000.00 | | $50,000,000.00 | |
OPTION | | | | | | | | | | | |
| | HOSPITAL-BASED MRC | | | | | | | |
| | FFP | | | | | | | | | |
| | 5,000 TED | | | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | NET AMT | | $50,000,000.00 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0004AC | | | | 5,000 | | Each | | $10,000.00 | | $50,000,000.00 | |
OPTION | | | | | | | | | | | |
| | HOSPITAL-BASED MRC | | | | | | | |
| | FFP | | | | | | | | | |
| | 5,000 TED | | | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | NET AMT | | $50,000,000.00 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
ITEM NO | | SUPPLIES/SERVICES | | QUANTITY | | UNIT | | UNIT PRICE | | AMOUNT | |
0004AD | | | | 5,000 | | Each | | $10,000.00 | | $50,000,000.00 | |
OPTION | | | | | | | | | | | |
| | HOSPITAL-BASED MRC | | | | | | | |
| | FFP | | | | | | | | | |
| | 5,000 TED | | | | | | | | | |
| | FOB: Destination | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | NET AMT | | $50,000,000.00 | |
W9113M-08-C-0028
Page 11 of 26
Section C - Descriptions and Specifications
SECTION C
Contractor’s Statement of Work (SOW). See Section J, List of Attachments, in response to Government’s Statement of Objectives (SOO).
W9113M-08-C-0028
Page 12 of 26
Section D - Packaging and Marking
SECTION D
Packaging and Marking shall be in accordance with the Contractor’s Statement of Work, as provided in response to the Government’s Statement of Objectives (SOO).
W9113M-08-C-0028
Page 13 of 26
Section E - Inspection and Acceptance
INSPECTION AND ACCEPTANCE TERMS
All Supplies/services will be inspected/accepted by the government at Destination.
CLAUSES INCORPORATED BY REFERENCE
52.246-2 | | Inspection Of Supplies-Fixed Price | | AUG 1996 |
52.246-3 | | Inspection Of Supplies Cost-Reimbursement | | MAY 2001 |
52.246-8 | | Inspection Of Research And Development Cost Reimbursement | | MAY 2001 |
52.246-16 | | Responsibility For Supplies | | APR 1984 |
252.246-7000 | | Material Inspection And Receiving Report | | MAR 2003 |
W9113M-08-C-0028
Page 14 of 26
Section F - - Deliveries or Performance
DELIVERY INFORMATION
CLIN | | DELIVERY DATE | | QUANTITY | | SHIP TO ADDRESS | | UIC |
| | | | | | | | |
0001 | | N/A | | N/A | | N/A | | N/A |
| | | | | | | | |
0001AA | | 5 yrs after Award | | N/A | | JPM-MITS | | N/A |
| | | | | | 64 Thomas Johnson Drive | | |
| | | | | | Frederick, MD 21702 | | |
| | | | | | | | |
0001AB | | 5 yrs after option is exercised | | N/A | | Same as above | | N/A |
| | | | | | | | |
0001AC | | Implemented 6 mos of option exercised | | N/A | | Same as above | | N/A |
| | | | | | | | |
0001AD | | Monthly after option exercised | | N/A | | Same as above | | N/A |
| | | | | | | | |
| | | | | | | | |
0001AE | | Quarterly after option exercised | | N/A | | Same as above | | N/A |
| | | | | | | | |
0001AF | | One yr after option is exercised | | N/A | | Same as above | | N/A |
| | | | | | | | |
0003 | | 90 days after option is exercised | | N/A | | Same as above | | N/A |
| | | | | | | | |
0004 | | N/A | | N/A | | Same as above | | N/A |
| | | | | | | | |
0004AA | | Within 6 mos of exercise of option | | 5,000 | | Same as above | | N/A |
| | | | | | | | |
| | | | | | | | |
0004AB | | Within 6 mos of exercise of option | | 5,000 | | Same as above | | N/A |
| | | | | | | | |
0004AC | | Within 6 mos of exercise of option | | 5,000 | | Same as above | | N/A |
| | | | | | | | |
0004AD | | Within 6 mos of exercise of option | | 5,000 | | Same as above | | N/A |
| | | | | | | | |
CLASSES INCORPORATED BY REFERENCE
52.242-15 | | Stop-Work Order | | AUG 1989 |
52.242-15 Alt I | | Stop-Work Order (Aug 1989) - Alternate I | | APR 1984 |
W9113M-08-C-0028
Page 15 of 26
52.247-34 | | F.O.B. Destination | | NOV 1991 |
W9113M-08-C-0028
Page 16 of 26
Section G - Contract Administration Data
PAYMENT/INVOICING
In accordance with DFARS Clause 252.232-7003, Electronic Submission of Payment Requests, the contractor shall submit invoices electronically. A copy of the invoice shall be submitted to the Contracting Officer’s Representative (COR) listed below to facilitate the process for payment. Payment shall be made by the Defense Finance and Accounting Office, upon acceptance by the COR.
Government Point of Contact:
US Army Space & Missile Defense Command
Attn: SMDC-RDCM-SB/Sandra O’Connell
64 Thomas Johnson Drive
Frederick, MD 21702
(301)619-2895
sandra.oconnell@us.army.mil
Contracting Officer’s Representative
MAJ Eric Wagar
64 Thomas Johnson Drive
Frederick, MD 21702
(301)619-8424
eric.wagar@us.army.mil
ACCOUNTING AND APPROPRIATION DATA
AA: 977040026017Y5YCM406038BP000255Y12YMMRW90GXK72270154YMMR12044008
AMOUNT: $4,253,390.80
CIN 000000000000000000000000000000: $4,253,390.80
W9113M-08-C-0028
Page 17 of 26
Section H - Special Contract Requirements
SPECIAL REQUIREMENT
“The Sponsor shall seek permission from the FDA to re-label and use consistency lot product as FDA-approved product and if allowed by the FDA, the Government reserves the option to apply these doses in the fulfillment of the production quantities to be obtained under the optional production SUBCLIN(s) with the right to obtain a pro rata reduction in price under the exercised production SUBCLIN(s) (taking into account the number of individual doses per TED) using the effective unit price bid under the exercised production SUBCLIN(s) as designated by the Government for the purposes of this special provision.”
CLAUSES INCORPORATED BY FULL TEXT
52.234-4 EARNED VALUE MANAGEMENT SYSTEM (JUL 2006)
(a) The Contractor shall use an earned value management system (EVMS) that has been determined by the Cognizant Federal Agency (CFA) to be compliant with the guidelines in ANSI/EIA Standard - 748 (current version at the time of award) to manage this contract. If the Contractor’s current EVMS has not been determined compliant at the time of award, see paragraph (b) of this clause. The Contractor shall submit reports in accordance with the requirements of this contract.
(b) If, at the time of award, the Contractor’s EVM System has not been determined by the CFA as complying with EVMS guidelines or the Contractor does not have an existing cost/schedule control system that is compliant with the guidelines in ANSI/EIA Standard - 748 (current version at time of award), the Contractor shall—
(1) Apply the current system to the contract; and
(2) Take necessary actions to meet the milestones in the Contractor’s EVMS plan approved by the Contracting Officer.
(c) The Government will conduct an Integrated Baseline Review (IBR). If a pre-award IBR has not been conducted, a post award IBR shall be conducted as early as practicable after contract award.
(d) The Contracting Officer may require an IBR at-
(1) Exercise of significant options; or
(2) Incorporation of major modifications.
(e) Unless a waiver is granted by the CFA, Contractor proposed EVMS changes require approval of the CFA prior to implementation. The CFA will advise the Contractor of the acceptability of such changes within 30 calendar days after receipt of the notice of proposed changes from the Contractor. If the advance approval requirements are waived by the CFA, the Contractor shall disclose EVMS changes to the CFA at least 14 calendar days prior to the effective date of implementation.
(f) The Contractor shall provide access to all pertinent records and data requested by the Contracting Officer or a duly authorized representative as necessary to permit Government surveillance to ensure that the EVMS conforms, and continues to conform, with the performance criteria referenced in paragraph (a) of this clause.
(g) The Contractor shall require the subcontractors specified below to comply with the requirements of this clause: (Insert list of applicable subcontractors.)
W9113M-08-C-0028
Page 18 of 26
---------------------------
---------------------------
---------------------------
(End of clause)
W9113M-08-C-0028
Page 19 of 26
Section 1 - Contract Clauses
CLAUSES INCORPORATED BY REFERENCE
52.202-1 | | Definitions | | JUL 2004 |
52.203-3 | | Gratuities | | APR 1984 |
52.203-5 | | Covenant Against Contingent Fees | | APR 1984 |
52.203-6 | | Restrictions On Subcontractor Sales To The Government | | SEP 2006 |
52.203-7 | | Anti-Kickback Procedures | | JUL 1995 |
52.203-8 | | Cancellation, Rescission, and Recovery of Funds for Illegal or Improper Activity | | JAN 1997 |
52.203-10 | | Price Or Fee Adjustment For Illegal Or Improper Activity | | JAN 1997 |
52.203-12 | | Limitation On Payments To Influence Certain Federal Transactions | | SEP 2005 |
52.204-4 | | Printed or Copied Double-Sided on Recycled Paper | | AUG 2000 |
52.204-7 | | Central Contractor Registration | | JUL 2006 |
52.209-6 | | Protecting the Government’s Interest When Subcontracting With Contractors Debarred, Suspended, or Proposed for Debarment | | SEP 2006 |
52.211-5 | | Material Requirements | | AUG 2000 |
52.215-2 | | Audit and Records—Negotiation | | JUN 1999 |
52.215-8 | | Order of Precedence—Uniform Contract Format | | OCT 1997 |
52.215-11 | | Price Reduction for Defective Cost or Pricing Data—Modifications | | OCT 1997 |
52.215-15 | | Pension Adjustments and Asset Reversions | | OCT 2004 |
52.215-19 | | Notification of Ownership Changes | | OCT 1997 |
52.219-6 | | Notice Of Total Small Business Set-Aside | | JUN 2003 |
52.219-8 | | Utilization of Small Business Concerns | | MAY 2004 |
52.219-14 | | Limitations On Subcontracting | | DEC 1996 |
52.222-3 | | Convict Labor | | JUN 2003 |
52.222-21 | | Prohibition Of Segregated Facilities | | FEB 1999 |
52.222-26 | | Equal Opportunity | | MAR 2007 |
52.222-35 | | Equal Opportunity For Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans | | SEP 2006 |
52.222-36 | | Affirmative Action For Workers With Disabilities | | JUN 1998 |
52.223-6 | | Drug-Free Workplace | | MAY 2001 |
52.223-14 | | Toxic Chemical Release Reporting | | AUG 2003 |
52.225-13 | | Restrictions on Certain Foreign Purchases | | FEB 2006 |
52.227-11 | | Patent Rights—Retention By The Contractor (Short Form) | | JUN 1997 |
52.227-14 | | Rights in Data—General | | JUN 1987 |
52.227-23 | | Rights to Proposal Data (Technical) | | JUN 1987 |
52.228-7 | | Insurance—Liability To Third Persons | | MAR 1996 |
52.232-1 | | Payments | | APR 1984 |
52.232-17 | | Interest | | JUN 1996 |
52.232-20 | | Limitation of Cost | | APR 1984 |
52.232-23 Alt I | | Assignment of Claims (Jan 1986) - Alternate I | | APR 1984 |
52.232-25 | | Prompt Payment | | OCT 2003 |
52.232-33 | | Payment by Electronic Funds Transfer—Central Contractor Registration | | OCT 2003 |
52.233-1 | | Disputes | | JUL 2002 |
52.233-3 Alt I | | Protest After Award (Aug 1996) - Alternate I | | JUN 1985 |
52.242-1 | | Notice of Intent to Disallow Costs | | APR 1984 |
52.242-3 | | Penalties for Unallowable Costs | | MAY 2001 |
W9113M-08-C-0028
Page 20 of 26
52.242-4 | | Certification of Final Indirect Costs | | JAN 1997 |
52.242-13 | | Bankruptcy | | JUL 1995 |
52.243-1 Alt V | | Changes--Fixed Price (Aug 1987) - Alternate V | | APR 1984 |
52.243-2 | | Changes--Cost Reimbursement | | AUG 1987 |
52.244-5 | | Competition In Subcontracting | | DEC 1996 |
52.244-6 | | Subcontracts for Commercial Items | | MAR 2007 |
52.249-2 | | Termination For Convenience Of The Government (Fixed-Price) | | MAY 2004 |
52.249-6 | | Termination (Cost Reimbursement) | | MAY 2004 |
52.249-14 | | Excusable Delays | | APR 1984 |
52.253-1 | | Computer Generated Forms | | JAN 1991 |
252.203-7001 | | Prohibition On Persons Convicted of Fraud or Other Defense-Contract-Related Felonies | | DEC 2004 |
252.204-7004 Alt A | | Central Contractor Registration (52.204-7) Alternate A | | NOV 2003 |
252209-7004 | | Subcontracting With Firms That Are Owned or Controlled By The Government of a Terrorist Country | | DEC 2006 |
252.211-7003 | | Item Identification and Valuation | | JUN 2005 |
252.215-7000 | | Pricing Adjustments | | DEC 1991 |
252.232-7003 | | Electronic Submission of Payment Requests | | MAR 2007 |
252.235-7010 | | Acknowledgment of Support and Disclaimer | | MAY 1995 |
252.235-7011 | | Final Scientific or Technical Report | | NOV 2004 |
252.243-7002 | | Requests for Equitable Adjustment | | MAR 1998 |
252.244-7000 | | Subcontracts for Commercial Items and Commercial Components (DoD Contracts) | | JAN 2007 |
252.247-7023 | | Transportation of Supplies by Sea | | MAY 2002 |
252.247-7024 | | Notification Of Transportation Of Supplies By Sea | | MAR 2000 |
CLAUSES INCORPORATED BY FULL TEXT
52.216-7 ALLOWABlE COST AND PAYMENT (DEC 2002)
(a) Invoicing.
(I) The Government will make payments to the Contractor when requested as work progresses, but (except for small business concerns) not more often than once every 2 weeks, in amounts determined to be allowable by the Contracting Officer in accordance with Federal Acquisition Regulation (FAR) subpart 31.2 in effect on the date of this contract and the terms of this contract. The Contractor may submit to an authorized representative of the Contracting Officer, in such form and reasonable detail as the representative may require, an invoice or voucher supported by a statement of the claimed allowable cost for performing this contract.
(2) Contract financing payments are not subject to the interest penalty provisions of the Prompt Payment Act. Interim payments made prior to the final payment under the contract are contract financing payments, except interim payments if this contract contains Alternate I to the clause at 52.232-25.
(3) The designated payment office will make interim payments for contract financing on the 30th (Contracting Officer insert day as prescribed by agency head; if not prescribed, insert “30th”) day after the designated billing office receives a proper payment request.
In the event that the Government requires an audit or other review of a specific payment request to ensure compliance with the terms and conditions of the contract, the designated payment office is not compelled to make payment by the specified due date.
W9113M-08-C-0028
Page 21 of 26
(b) Reimbursing costs. (1) For the purpose of reimbursing allowable costs (except as provided in subparagraph (b)(2) of the clause, with respect to pension, deferred profit sharing, and employee stock ownership plan contributions), the term “costs” includes only—
(i) Those recorded costs that, at the time of the request for reimbursement, the Contractor has paid by cash, check, or other form of actual payment for items or services purchased directly for the contract;
(ii) When the Contractor is not delinquent in paying costs of contract performance in the ordinary course of business, costs incurred, but not necessarily paid, for—
(A) Supplies and services purchased directly for the contract and associated financing payments to subcontractors, provided payments determined due will be made—
(1) In accordance with the terms and conditions of a subcontract or invoice; and
(2) Ordinarily within 30 days of the submission of the Contractor’s payment request to the Government;
(B) Materials issued from the Contractor’s inventory and placed in the production process for use on the contract;
(C) Direct labor;
(D) Direct travel;
(E) Other direct in-house costs; and
(F) Properly allocable and allowable indirect costs, as shown in the records maintained by the Contractor for purposes of obtaining reimbursement under Government contracts; and
(iii) The amount of financing payments that have been paid by cash, check, or other forms of payment to subcontractors.
(2) Accrued costs of Contractor contributions under employee pension plans shall be excluded until actually paid unless—
(i) The Contractor’s practice is to make contributions to the retirement fund quarterly or more frequently; and
(ii) The contribution does not remain unpaid 30 days after the end of the applicable quarter or shorter payment period (any contribution remaining unpaid shall be excluded from the Contractor’s indirect costs for payment purposes).
(3) Notwithstanding the audit and adjustment of invoices or vouchers under paragraph (g) of this clause, allowable indirect costs under this contract shall be obtained by applying indirect cost rates established in accordance with paragraph (d) of this clause.
(4) Any statements in specifications or other documents incorporated in this contract by reference designating performance of services or furnishing of materials at the Contractor’s expense or at no cost to the Government shall be disregarded for purposes of cost-reimbursement under this clause.
(c) Small business concerns. A small business concern may receive more frequent payments than every 2 weeks.
(d) Final indirect cost rates. (1) Final annual indirect cost rates and the appropriate bases shall be established in accordance with Subpart 42.7 of the Federal Acquisition Regulation (FAR) in effect for the period covered by the indirect cost rate proposal.
W9113M-08-C-0028
Page 22 of 26
(2)(i) The Contractor shall submit an adequate final indirect cost rate proposal to the Contracting Officer (or cognizant Federal agency official) and auditor within the 6-month period following the expiration of each of its fiscal years. Reasonable extensions, for exceptional circumstances only, may be requested in writing by the Contractor and granted in writing by the Contracting Officer. The Contractor shall support its proposal with adequate supporting data.
(ii) The proposed rates shall be based on the Contractor’s actual cost experience for that period. The appropriate Government representative and the Contractor shall establish the final indirect cost rates as promptly as practical after receipt of the Contractor’s proposal.
(3) The Contractor and the appropriate Government representative shall execute a written understanding setting forth the final indirect cost rates. The understanding shall specify (i) the agreed-upon final annual indirect cost rates, (ii) the bases to which the rates apply, (iii) the periods for which the rates apply, (iv) any specific indirect cost items treated as direct costs in the settlement, and (v) the affected contract and/or subcontract, identifying any with advance agreements or special terms and the applicable rates. The understanding shall not change any monetary ceiling, contract obligation, or specific cost allowance or disallowance provided for in this contract. The understanding is incorporated into this contract upon execution.
(4) Failure by the parties to agree on a final annual indirect cost rate shall be a dispute within the meaning of the Disputes clause.
(5) Within 120 days (or longer period if approved in writing by the Contracting Officer) after settlement of the final annual indirect cost rates for all years of a physically complete contract, the Contractor shall submit a completion invoice or voucher to reflect the settled amounts and rates.
(6)(i) If the Contractor fails to submit a completion invoice or voucher within the time specified in paragraph (d)(5) of this clause, the Contracting Officer may—
(A) Determine the amounts due to the Contractor under the contract; and
(B) Record this determination in a unilateral modification to the contract.
(ii) This determination constitutes the final decision of the Contracting Officer in accordance with the Disputes clause.
(e) Billing rates. Until final annual indirect cost rates are established for any period, the Government shall reimburse the Contractor at billing rates established by the Contracting Officer or by an authorized representative (the cognizant auditor), subject to adjustment when the final rates are established. These billing rates—
(1) Shall be the anticipated final rates; and
(2) May be prospectively or retroactively revised by mutual agreement, at either party’s request, to prevent substantial overpayment or underpayment.
(f) Quick-closeout procedures. Quick-closeout procedures are applicable when the conditions in FAR 42.708(a) are satisfied.
(g) Audit. At any time or times before final payment, the Contracting Officer may have the Contractor’s invoices or vouchers and statements of cost audited. Any payment may be (1) Reduced by amounts found by the Contracting Officer not to constitute allowable costs or (2) Adjusted for prior overpayments or underpayments.
(h) Final payment. (1) Upon approval of a completion invoice or voucher submitted by the Contractor in accordance with paragraph (d)(4) of this clause, and upon the Contractor’s compliance with all terms of this contract, the Government shall promptly pay any balance of allowable costs and that part of the fee (if any) not previously paid.
W9113M-08-C-0028
Page 23 of 26
(2) The Contractor shall pay to the Government any refunds, rebates, credits, or other amounts (including interest, if any) accruing to or received by the Contractor or any assignee under this contract, to the extent that those amounts are properly allocable to costs for which the Contractor has been reimbursed by the Government. Reasonable expenses incurred by the Contractor for securing refunds, rebates, credits, or other amounts shall be allowable costs if approved by the Contracting Officer. Before final payment under this contract, the Contractor and each assignee whose assignment is in effect at the time of final payment shall execute and deliver—
(i) An assignment to the Government, in form and substance satisfactory to the Contracting Officer, of refunds, rebates, credits, or other amounts (including interest, if any) properly allocable to costs for which the Contractor has been reimbursed by the Government under this contract; and
(ii) A release discharging the Government, its officers, agents, and employees from all liabilities, obligations, and claims arising out of or under this contract, except—
(A) Specified claims stated in exact amounts, or in estimated amounts when the exact amounts are not known;
(B) Claims (including reasonable incidental expenses) based upon liabilities of the Contractor to third parties arising out of the performance of this contract; provided, that the claims are not known to the Contractor on the date of the execution of the release, and that the Contractor gives notice of the claims in writing to the Contracting Officer within 6 years following the release date or notice of final payment date, whichever is earlier; and
(C) Claims for reimbursement of costs, including reasonable incidental expenses, incurred by the Contractor under the patent clauses of this contract, excluding, however, any expenses arising from the Contractor’s indemnification of the Government against patent liability.
(End of clause)
52.216-10 INCENTIVE FEE (MAR 1997)
(a) General. The Government shall pay the Contractor for performing this contract a fee determined as provided in this contract.
(b) Target cost and target fee. The target cost and target fee specified in the Schedule are subject to adjustment if the contract is modified in accordance with paragraph (d) below.
(1) “Target cost,” as used in this contract, means the estimated cost of this contract as initially negotiated, adjusted in accordance with paragraph (d) below.
(2) “Target fee,” as used in this contract, means the fee initially negotiated on the assumption that this contract would be performed for a cost equal to the estimated cost initially negotiated, adjusted in accordance with paragraph (d) below.
(c) Withholding of payment. Normally, the Government shall pay the fee to the Contractor as specified in the Schedule. However, when the Contracting Officer considers that performance or cost indicates that the Contractor will not achieve target, the Government shall pay on the basis of an appropriate lesser fee. When the Contractor demonstrates that performance or cost clearly indicates that the Contractor will earn a fee significantly above the target fee, the Government may, at the sole discretion of the Contracting Officer, pay on the basis of an appropriate higher fee. After payment of 85 percent of the applicable fee, the Contracting Officer may withhold further payment of fee until a reserve is set aside in an amount that the Contracting Officer considers necessary to protect the Government’s interest. This reserve shall not exceed 15 percent of the applicable fee or $100,000, whichever is less. The Contracting Officer shall release 75 percent of all fee withholds under this contract after receipt of the certified
W9113M-08-C-0028
Page 24 of 26
final indirect cost rate proposal covering the year of physical completion of this contract, provided the Contractor has satisfied all other contract terms and conditions, including the submission of the final patent and royalty reports, and is not delinquent in submitting final vouchers on prior years’ settlements. The Contracting Officer may release up to 90 percent of the fee withholds under this contract based on the Contractor’s past performance related to the submission and settlement of final indirect cost rate proposals.
(d) Equitable adjustments. When the work under this contract is increased or decreased by a modification to this contract or when any equitable adjustment in the target cost is authorized under any other clause, equitable adjustments in the target cost, target fee, minimum fee, and maximum fee, as appropriate, shall be stated in a supplemental agreement to this contract.
(e) Fee payable. (1) The fee payable under this contract shall be the target fee increased by 30 . [Contracting Officer insert Contractor’s participation] cents for every dollar that the total allowable cost is less than the target cost or decreased by 30 [Contracting Officer insert Contractor’s participation] cents for every dollar that the total allowable cost exceeds the target cost. In no event shall the fee be greater than 20 [Contracting Officer insert percentage] percent or less than . . . . . 0 . . . . .[Contracting Officer insert percentage] percent of the target cost.
(2) The fee shall be subject to adjustment, to the extent provided in paragraph (d) above, and within the minimum and maximum fee limitations in subparagraph (1) above, when the total allowable cost is increased or decreased as a consequence of (i) payments made under assignments or (ii) claims excepted from the release as required by paragraph (h)(2) of the Allowable Cost and Payment clause.
(3) If this contract is terminated in its entirety, the portion of the target fee payable shall not be subject to an increase or decrease as provided in this paragraph. The termination shall be accomplished in accordance with other applicable clauses of this contract.
(4) For the purpose of fee adjustment, “total allowable cost” shall not include allowable costs arising out of—
(i) Any of the causes covered by the Excusable Delays clause to the extent that they are beyond the control and without the fault or negligence of the Contractor or any subcontractor;
(ii) The taking effect, after negotiating the target cost, of a statute, court decision, written ruling, or regulation that results in the Contractor’s being required to pay or bear the burden of any tax or duty or rate increase in a tax or duty;
(iii) Any direct cost attributed to the Contractor’s involvement in litigation as required by the Contracting Officer pursuant to a clause of this contract, including furnishing evidence and information requested pursuant to the Notice and Assistance Regarding Patent and Copyright Infringement clause;
(iv) The purchase and maintenance of additional insurance not in the target cost and required by the Contracting Officer, or claims for reimbursement for liabilities to third persons pursuant to the Insurance Liability to Third Persons clause;
(v) Any claim, loss, or damage resulting from a risk for which the Contractor has been relieved of liability by the Government Property clause; or
(vi) Any claim, loss, or damage resulting from a risk defined in the contract as unusually hazardous or as a nuclear risk and against which the Government has expressly agreed to indemnify the Contractor.
(5) All other allowable costs are included in “total allowable cost” for fee adjustment in accordance with this paragraph (e), unless otherwise specifically provided in this contract.
(f) Contract modification. The total allowable cost and the adjusted fee determined as provided in this clause shall be evidenced by a modification to this contract signed by the Contractor and Contracting Officer.
W9113M-08-C-0028
Page 25 of 26
(g) Inconsistencies. In the event of any language inconsistencies between this clause and provisioning documents or Government options under this contract, compensation for spare parts or other supplies and services ordered under such documents shall be determined in accordance with this clause.
(End of clause)
52.217-6 OPTION FOR INCREASED QUANTITY (MAR 1989)
The Government may increase the quantity of supplies called for in the Schedule at the unit price specified. The Contracting Officer may exercise the option by written notice to the Contractor within 60 days. Delivery of the added items shall continue at the same rate as the like items called for under the contract, unless the parties otherwise agree.
(End of clause)
52.233-4 APPLICABLE LAW FOR BREACH OF CONTRACT CLAIM (OCT 2004)
United States law will apply to resolve any claim of breach of this contract.
(End of clause)
W9113M-08-C-0028
Page 26 of 26
Section J - List of Documents, Exhibits and Other Attachments
LIST OF ATTACHMENTS
No. | | Description | | Date | | No Pages |
| | | | | | |
I | | Contractor’s Statement of Work | | Apr 23, 2007 | | 15 |
| | | | (with revisions dated August 10, 2007) |
Exhibits — Contract Data Requirements List (CDRL) DD 1423
A001 | | Contractor’s Progress, Status, and Management Report |
A002 | | Contracts Funds Status Report (CFSR) |
A003 | | Contract Work Breakdown Structure (CWBS) |
A004 | | Integrated Master Schedule (IMS) |
A005 | | Contract Performance Report (CPR) |
A006 | | Regulatory Submission File |
A007 | | Technical Data Package (TDP) |
A008 | | Interim Program Review MS Powerpoint Presentation |
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AA, 0001AB | B. EXHIBIT A001 | C. CATEGORY: TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A001 | 2. TITLE OF DATA ITEM Contractor’s Progress, Status and Management Report | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.) DI-MGMT-80227 | 5. CONTRACT REFERENCE C SOW paragraph C.4.4 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED
TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
Yes | REQUIRED | Monthly | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| No | Award Date | TBD | | | | | | |
16. REMARKS
This report shall be submitted monthly. The Contractor’s Progress, Status, and Management Report indicates the progress of the work, status of the program and of the assigned tasks. The report shall include cost updates, and information relating to existing or potential problem areas and proposed action to resolve the problems. (A draft report is due on the first Tuesday of the month. Issues and questions from the draft report will be discussed at a teleconference to be conducted on the second Tuesday of the month. A final report, with incorporated changes, corrections, and minutes of the teleconference, are due within a week following the teleconference.)
Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr. Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL  | 2 | 3 | 0 | | |
G. PREPARED BY E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007
| I. APPROVED BY 
| J. DATE
NOV 27 2007
| | |
| | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AD | B. EXHIBIT A002 | C. CATEGORY: TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A002 | 2. TITLE OF DATA ITEM Contract Funds Status Report (CFSR) | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.) DI-MGMT-81468 | 5. CONTRACT REFERENCE C SOW paragraph C.4.5 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
Yes | REQUIRED | Quarterly | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| No | Award Date | TBD | | | | | | |
16. REMARKS
A draft CFSR (DD Form 1568) is due on the first Tuesday of the first month of a fiscal year quarter (federal fiscal year begins Oct 1). Issues and questions from the draft report shall be discussed at a teleconference on the second Tuesday of the month. This teleconference may be the same as for the Contractor’s Progress, Status and Management Report, A001. A final report, with incorporated changes, corrections, and minutes of the teleconference, are due five working days following the teleconference.
Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr.
Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL  | 2 | 3 | 0 | | |
G. PREPARED BY E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007
| I. APPROVED BY
 | J. DATE
NOV 27 2007
| | |
| | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| | Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searcing existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AA, 0001AB | B. EXHIBIT A003 | C. CATEGORY: TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A003 | 2. TITLE OF DATA ITEM Contract Work Breakdown Structure (CWBS) | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.) DI-MGMT-81334B | 5. CONTRACT REFERENCE C SOW paragraph C.3.3 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED
TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
Yes | REQUIRED | Monthly | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| No | Award Date | TBD | | | | | | |
16. REMARKS
The CWBS and the CWBS dictionary shall be updated quarterly. The revised CWBS and CWBS dictionary are due on the first Tuesday of the first month. Issues and questions from the CWBS will be discussed at a teleconference to be conducted on the second Tuesday of the month. This teleconference may be the same as for the Contractor’s Progress, Status and Management Report, A001. A final report, with incorporated changes, corrections, and minutes of the teleconference, are due within a week following the teleconference. The Offeror shall extend the selected CWBS elements to define the complete contract scope. The CWBS will be to a depth and breadth necessary to accurately describe the Offeror’s proposed effort, to a minimum of Level 4. The CWBS shall be structured to correlate with the Government’s Statement of Objectives.
Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr.
Frederick, MD 21702. | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL  | 2 | 3 | 0 | | |
G. PREPARED BY
E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007 | I. APPROVED BY
 | J. DATE NOV 27 2007 | | |
| | | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| | Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection at information if it does not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AA, 0001AB | B. EXHIBIT A004 | C. CATEGORY: TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A004 | 2. TITLE OF DATA ITEM Integrated Master Schedule (IMS) | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.) DI-MGMT-81650 | 5. CONTRACT REFERENCE C SOW paragraph C.3.3 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED
TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
YES | REQUIRED | Monthly | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| NO | Award Date | TBD | | | | | | |
16. REMARKS
The IMS shall be updated monthly. The revised IMS is due on the first Tuesday of the month. Issues and questions from the IMS draft report shall be discussed at a teleconference on the second Tuesday of the month. This teleconference may be the same as for the Contractor’s progress, Status and Management Report, A001. The IMS shall be structured to correlate with the Statement of Objectives (SOO). A final report, with incorporated changes, corrections, and minutes of the teleconference, are due within a week following the teleconference. Address to: Joint Product Manager-Medical Identification Treatment Systems (JPM-MITS)
64 Thomas Johnson Dr. Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL  | 2 | 3 | 0 | | |
G. PREPARED BY
E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007 | I. APPROVED BY
 | J. DATE NOV 27 2007 | | |
| | | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing date sources, gathering and maintaining the date needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate {0704-0188}. Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it do not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AD | B. EXHIBIT A005 | C. CATEGORY: TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A005 | 2. TITLE OF DATA ITEM Contract Performance Report (CPR) | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.) DI-MGMT-81466A | 5. CONTRACT REFERENCE C SOW paragraph C.4.4 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
YES | REQUIRED | Monthly | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| NO | Award Date | TBD | | | | | | |
16. REMARKS The CPR shall be submitted monthly. The CPR shall contain Formats 1-5 containing data for measuring Contractors’ cost and schedule performance. These reports shall be integrated with the CWBS (A003) and IMS (A004). A draft report is due on the first Tuesday of the month. Issues and questions from the draft report will be discussed at a teleconference to be conducted on the second Tuesday of the month. This teleconference may be the same as for the Contractor’s Progress, Status and management Report (A001). A final report, with incorporated changes, corrections, and minutes of the teleconference, are due within a week following the teleconference. Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr. Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL | 2 | 3 | 0 | | |
G. PREPARED BY
E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007
| I. APPROVED BY
 | J. DATE
NOV 27 2007
| | |
| | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing date sources, gathering and maintaining the date needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection at information if it down not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AA, 0001AB | B. EXHIBIT A006 | C. CATEGORY: TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A006 | 2. TITLE OF DATA ITEM Regulatory Submission File | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquinition Document No.) DI-MGMT-81466A | 5. CONTRACT REFERENCE C SOW paragraph C.3.1 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
YES | REQUIRED | As Required | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| No | Award Date | TBD | | | | | | |
16. REMARKS
This data package shall contain copies of Investigational New Drug (IND) submissions, annual reports, New Drug Application (NDA) submissions, and other communications with the U.S. Food and drug Administration (FDA). The data package shall also contain all FDA-initiated correspondence, to include meeting minutes, requests for additional information, etc. The preferred format for these regulatory documents is electronic. The Contractor shall send copies of regulatory documents to the Government at the same time they are sent to the FDA. Copies of regulatory communications received from the FDA shall be sent within three to five working days of receipt.
Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr. Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
| | | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL | 2 | 3 | 0 | | |
G. PREPARED BY
E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007
| I. APPROVED BY
 | J. DATE
NOV 27 2007
| | |
| | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| | Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense. Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AF | B. EXHIBIT A007 | C. CATEGORY:
TDP TM OTHER X �� | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A007 | 2. TITLE OF DATA ITEM Technical Data Package (TDP) | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.)
| 5. CONTRACT REFERENCE C SOW paragraph C.3.4 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED
TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
Yes | REQUIRED | One Time | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| No | Award Date | TBD | | | | | | |
16. REMARKS
The Government shall direct preparation of a Technical Data Package that includes all necessary documentation and data for the Government, or its designee, to continue the development or production of the product. At any time the product ceases to be marketed and is retired or in the event the Contractor defaults and fails to remedy said default before or after approval, the Contractor shall transfer the TDP to the Government or its designee. The Contractor shall also assist in the technical transfer as determined by the Government. The draft TDP shall be grammatically and typographically correct. The corrected TDP with recommended changes made, is due within ten working days after receipt of the Government’s comments.
Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr. Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL  | 2 | 3 | 0 | | |
G. PREPARED BY
E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007 | I. APPROVED BY
 | J. DATE NOV 27 2007 | | |
| | | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0
CONTRACT DATA REQUIREMENTS LIST
(1 Data Item)
| Form Approved
OMB No. 0704-0188
| |
The public reporting burden for this collection of information is estimated to average 110 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing the burden, to the Department of Defense, Executive Services Directorate (0704-0188). Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. Please do not return your form to the above organization. Send completed form to the Government Issuing Contracting Officer for the Contract/PR No. listed in Block E. | |
A. CONTRACT LINE ITEM NO. 0001AA, 0001AB | B. EXHIBIT A008 | C. CATEGORY:
TDP TM OTHER X | |
D. SYSTEM/ITEM Reports | E. CONTRACT/PR NO. W9113M-08-C-0028 | F. CONTRACTOR | |
| | | | |
1. DATA ITEM NO. A008 | 2. TITLE OF DATA ITEM
Interim Program Review MS PowerPoint Presentation | 3. SUBTITLE | | 17. PRICE GROUP |
4. AUTHORITY (Data Acquisition Document No.)
| 5. CONTRACT REFERENCE C SOW paragraph C.4.5 | 6. REQUIRING OFFICE MITS JPMO | | 18. ESTIMATED
TOTAL PRICE |
7. DD 250 REQ | 9. DIST STATEMENT | 10. FREQUENCY | 12. DATE OF FIRST SUBMISSION | 14. DISTRIBUTION | | |
Yes | REQUIRED | Quarterly | TBD | | b. COPIES | | |
8. APP CODE | | 11. AS OF DATE | 13. DATE OF SUBSEQUENT | a. ADDRESSEE | Draft | Final | | |
| | | SUBMISSION | | Reg | Repro | | |
| No | Award Date | TBD | | | | | | |
16. REMARKS
A draft Microsoft PowerPoint presentation shall be submitted to the Government by the Contractor or Subcontractor ten days prior to the quarterly Interim Program Review. This gives the Government the opportunity to review the slides and present any changes to be made prior to the review with JPM-MITS.
Address to: Joint Product Manager-Medical Identification Treatment Systems
(JPM-MITS)
64 Thomas Johnson Dr. Frederick, MD 21702 | MITS/COR | 2 | 3 | | | |
See address in | | | | | |
block 16 | | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| 15. TOTAL  | 2 | 3 | 0 | | |
G. PREPARED BY
E. J. Wagar
MAJ, U.S. Army | H. DATE
27 NOV 2007 | I. APPROVED BY
 | J. DATE
NOV 27 2007 | | |
| | | | | | | | | | | | | | | | | | |
DD FORM 1423-1, FEB 2001 PREVIOUS EDITION MAY BE USED. Page of Pages
& nbsp; Adobe Professional 7.0

| | Attachment 1
W9113M-08-C-0028
Page 1 of 15 |
Solicitation Number: W9113M-07-R-0002
2.1 Statement of Work (SOW)
Osiris Therapeutics, Inc. is a stem cell company manufacturing adult-derived human mesenchymal stem cells (hMSCs) intended for “off-the-shelf” therapeutic use to repair tissue damage and reduce tissue inflammation. The lead stem cell product, ProchymalTM, is currently being tested in two Phase III clinical trials to treat Gastrointestinal (GI) Diseases (Graft versus Host Disease (GvHD) and Crohn’s Disease). Both preclinical and clinical evidence suggest that ProchymalTM could be used to repair GI damage from Acute Radiation Syndrome (ARS) to restore the anatomic integrity and normal physiological functioning of the GI tract. Data suggest that ProchymalTM may impact overall survival because it is a cellular product that can repair tissue damage resulting from cell death, which is the hallmark of radiation exposure.
Osiris plans to develop a post-exposure Medical Radiation Countermeasure (MRC) that will increase survival and decrease incapacity of military forces so that these forces can maintain operational effectiveness within a contaminated area following radiation exposure, irrespective of radiation source. Osiris plans to develop the hMSCs product for treatment of GI-ARS through product approval/licensure with FDA and sell quantities of the MRC to the government sufficient to achieve the Initial Operating Capability (IOC).
Osiris has already met several of the work items listed in the Statement of Objectives. The work that has been completed has been included in this SOW for completeness; this completed work is indicated by italicized text.
INSERTED TEXT
A summary of Osiris’ progress in completing sections C.3.1 (CLIN 0001AA) and C.3.2 (CLIN 0001AB) of the Statement of Work is provided in the table below.
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-1

| | Attachment 1
W9113M-08-C-0028
Page 2 of 15 |
Solicitation Number: W9113M-07-R-0002
INSERTED TABLE
Solicitation Number W9113M-07-R-0002
CLIN 0001AA | SOO
Paragraph | SOO Element | Completion
Date |
C.3.1 | IND Submission | 4Q 2007 |
C.3.1.1 | Development & Qualification of Small Scale GMP Manufacturing Process | COMPLETE |
C.3.1.2 | Manufacture GMP MRC for Toxicology Studies & Clinical Trial | COMPLETE |
C.3.1.3 | Develop Product Formulations Suitable for Humans | COMPLETE |
C.3.1.4 | Develop & Validate Product Characterization & Lot Release Assays for Large-Scale Manufacturing Process | COMPLETE |
C.3.1.5 | Conduct GLP Acute Toxicology Studies | COMPLETE |
C.3.1.6 | Carry Out Efforts to Ensure Successful Submission of IND Application | 4Q 2007 |
C.3.1.7 | Support Submission of EUA | 2Q 2008 |
C.3.1.8 | Conduct Phase I Clinical Trial | COMPLETE |
C.3.1.9 | Store Phase I Product for Two Years | COMPLETE |
C.3.1.10 | Carry Out Stability Testing for Phase I Product | COMPLETE |
CLIN 0001AB | SOO
Paragraph | SOO Element | Completion
Date |
C.3.2 | Conduct Activities Leading to FDA Approval | 3Q 2010 |
C.3.2.1 | Qualification and Validation of Large-Scale GMP Manufacturing Process | COMPLETE |
C.3.2.2 | Demonstrate Lot Consistency for Large-Scale GMP Manufacturing Process | COMPLETE |
C.3.2.3 | Refine Product Formulation and Develop Delivery System Suitable for Human Use | COMPLETE |
C.3.2.4 | Develop & Validate Product Characterization & Lot Release Assays for Large-Scale Manufacturing Process | COMPLETE |
C.3.2.5 | Active Pharmaceutical Ingredient Stability Testing (2yr Interim Report) | 3Q 2009 |
C.3.2.6 | GMP Formulated Product Stability Testing for Shelf Life (2yr Interim Report) | 3Q 2009 |
C.3.2.7 | Operational Storage / Military Scenario Stability Studies | 4Q 2009 |
C.3.2.8 | Nonclinical Studies to Support BLA Submission | COMPLETE |
C.3.2.9 | GLP Animal Efficacy Studies | 2Q 2009 |
C.3.2.10 | GCP Phase II Safety Clinical Study | COMPLETE |
C.3.2.11 | BLA Submission | 1Q 2010 |
C.3.2.12 | Delivery of 1 TED of FDA-Approved MRC | 4Q 2010 |
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-1a

| | Attachment 1
W9113M-08-C-0028
Page 3 of 15 |
Solicitation Number: W9113M-07-R-0002
Osiris shall be the sponsor and remain the sponsor for so long as the product is marketed for the gastrointestinal component of ARS (GI-ARS). If the product ceases to be marketed for GI-ARS and in the event that Osiris defaults and fails to remedy said default, the sponsorship shall be transferred to the government to include all data related to GI-ARS product development and manufacture, as follows: upon written request from the Contracting Officer, Osiris agrees to immediately request approval, in writing, from FDA to effectuate transfer of the Investigational New Drug (IND) application and/or New Drug Application (NDA) Biological License Application (BLA) to the Government or its designee, and Osiris agrees to use its best efforts in obtaining such approval, including but not limited to, the execution of any and all documents which are reasonably necessary to effectuate such a transfer. In the case of where Osiris would be required to transfer sponsorship to the government, the government shall only use the technology for GI-ARS and shall not use the technology for other indications. All such transfers of technical data that may be required under this paragraph will be in accordance with relevant Federal Acquisition Regulations and terms of the final contract.
Human Mesenchymal Stem Cell Manufacturing Process Description
Osiris is providing a description of the manufacturing process flow to provide a reference point for statements made about manufacturing, stability testing, and product release testing in this SOW.
Figure 2.1-1 is an overview of the ex vivo cultured hMSC manufacturing process. The flow starts with the BMA, continues with the production of the Drug Substance (Donor cell bank, DCB, in-process intermediate), and ends with the generation of the Medicinal Product (Product dose, PD) shipped for use in clinical trials. The DCB is an in-process intermediate and a major in-process control for the overall manufacturing
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-1b

| | Attachment 1
W9113M-08-C-0028
Page 4 of 15 |
Solicitation Number: W9113M-07-R-0002
Figure 2.1-1. Overall Schematic of the Ex Vivo Cultured hMSC Manufacturing Process
process for making the PD. One single BMA can generate numerous PD lots. Please note that this is a representative diagram that does not reflect the actual number of DCBs and PDs generated during the manufacturing process.
1. Donor Cell Bank, DCB - The DCB is a process intermediate of cryopreserved, partially expanded hMSCs, representing the first two cell passages (P0→P1, P1→P2). The DCB is used as the starting material for Product Dose.
2. Product Dose, PD — The PD is the final packaged product, representing the three cell passages (P2→P3, P3→P4, P4→P5). The PD is ultimately released for distribution and use in clinical trials.
C.3. Contract Objectives
C.3.1. Investigational New Drug (IND)
Osiris shall provide all labor and materials to prepare and submit an IND for ARS. Osiris shall submit copies of regulatory documents to the government in accordance with CDRL A006.
Osiris has completed several INDs demonstrating a strong safety profile. These INDs will be used to support the IND for ARS. The IND designation and current clinical status for each investigational agent that supports the IND for ARS is given in Figure 2.1-2.
Prochymal™ is the product that is intended for use in treating ARS. The Prochymal™ formulation is identical to that of the development-stage Osiris product Provacel™. Both are intended for IV delivery. The current cryopreserved Chondrogen™ formulation is also identical to Prochymal™. However, further processing steps are required prior to Chondrogen™ use, and the agent is injected directly into the knee joint.
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-2

| | Attachment 1
W9113M-08-C-0028
Page 5 of 15 |
Solicitation Number: W9113M-07-R-0002
Product Name | IND Number | Disease Category | Indication | Clinical Status |
Prochymal™ | BB-IND-7939 | Immune | grade II-IV acute GvHD | Phase II completed |
Prochymal™ | BB-IND-7939 | Immune | treatment refractory acute GvHD | Phase II completed |
Prochymal™ | BB-IND-7939 | Immune | steroid refractory acute GvHD | Phase III initiated |
Prochymal™ | BB-IND-12770 | Immune | Crohn’s Disease | Phase II completed |
Provacel™ | BB-IND-10419 | Cardiovascular | myocardial infarction | Phase I completed |
Chondrogen™ | BB-IND-10441 | Orthopedics | meniscal repair (knee) | Phase I/II completed |
| | | | OMR-025 |
Figure 2.1-2. ProchymalTM’s advanced regulatory approval status reduce the risk fo approval for its us in ARS
Osiris is far along in the clinical development of ProchymalTM with the completion of three Phase II human trials. Pivotal ARS animal trials following the animal rule are all that remain before product approval, as shown in Figure 2.1-3.
C.3.1.1. Conduct Small Scale Manufacturing for cGMP MRC
Osiris has completed small-scale process development and qualification for Prochymal™ and currently manufactures Prochymal™ for clinical trials in three cGMP compliant manufacturing facilities. This is the same product that would be used for treating radiation exposure.
The manufacturing process for ex vivo cultured hMSCs has been designed and implemented in a manner consistent with International Conference on Harmonization (ICH) and Food and Drug Administration (FDA)’s regulatory guidelines. Specific considerations are given to cGMP regulations (21 CFR Part 210 and 211) and practicing aseptic techniques as defined in Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing - cGMPs. For the manipulation of human-derived tissue material, consideration is given to FDA policies on human-derived products: 21 CFR 1271 Human Cells, Tissues, and Cellular and Tissue Based Products and Guidance for the Submission of Chemistry, Manufacturing and Controls Information and Establishment Description for Autologous Somatic Cell Therapy Products (US Federal Register Docket No. 95N-0200).
CHANGE
“Rats” to “Small Animal”
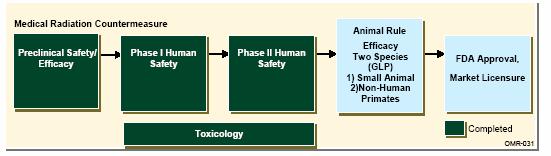
Figure 2.1-3. Osiris has completed the clinical development objectives for this contract through Phase II human trials. Pivotal minimal efficacy studies are all that remain for FDA approval
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-3

| | Attachment 1
W9113M-08-C-0028
Page 6 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.1.2. Manufacture cGMP for Nonclinical Toxicology and Phase I
Osiris has completed the Phase I clinical trial that shall be used to support the ARS IND (see section C.3.1.8) Osiris has completed non-clinical toxicology studies that shall be used to support the ARS IND (see section C.3.1.5).
C.3.1.3. Develop Product Formulations Suitable for Administration in Humans
Osiris has completed development of a product formulation that is suitable for administration in humans and a delivery system that is suitable for military use. The final product formulation contains 100 x 10(6) viable hMSCs plus the following excipients: HSA, DMSO and PlasmaLyte-A®. These excipients are required to maintain stable, viable cells through the freezing and thawing process. DMSO is a cryopreservative to assist in freezing. HSA is protein that acts a cellular stabilizer and provides protection from shearing forces in agitated cell culture systems. PlasmaLyte-A® is an electrolyte solution that acts as the diluent. All of the excipients are compatible with the hMSCs. There are no novel excipients in the formulation.
At the final stage of production, the product is filled into bags and cryopreserved at the manufacturing facility. The product is then shipped to the clinical site where it is stored, thawed, diluted and administered intravenously.
Osiris shall continue product development efforts towards achieving ease of administration in an operational military environment (see section C.3.2.3).
C.3.1.4. Develop and Validate Analytical Assays For Small-Scale Lot Release
Osiris has completed development and validation of analytical assays for cGMP compliant product characterization and lot release. The lot release specifications were established in cooperation with FDA. The lot release specs are currently being used to release product for a Phase III clinical trial to treat GvHD. Lot release is performed on the in-process intermediate (Donor Cell Bank, DCB) and on the final product (Product Dose, PD). The lot release specs for DCB include tests for cell phenotype, potency, appearance, viruses, cell line species identity, karotyping, sterility, endotoxin and mycoplasma. The lot release specs for PD include tests for viability, cell phenotype, potency, appearance, sterility, endotoxin, and mycoplasma.
INSERTED TEXT
Osiris believes that the current potency assay used for product release of Prochymal™ for the treatment of GvHD is also relevant for use as a release assay for Prochymal™ for the treatment of ARS. Osiris will discuss the the utility of the TNFR release assay for product release for the ARS indication with FDA. In the event that FDA requires an alternate assay, Osiris will proceed with development of an ARS-specific release assay accordingly. For product release of animal MSCs for use in preclinical studies, Osiris will develop a corresponding assay targeting the analog molecular marker.
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-4

| | Attachment 1
W9113M-08-C-0028
Page 7 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.1.5. Conduct Appropriate Nonclinical GLP Acute Toxicology Studies
Osiris has completed a GLP acute toxicology study to support ProchymalTM. This study was reviewed and verbally approved by FDA and shall support the BLA filing for ProchymalTM for ARS.
C.3.1.6. Perform Efforts to Ensure the Successful Submission of an IND
Osiris shall perform the necessary and appropriate efforts to assure the successful submission of an IND for ARS.
C.3.1.7. Support the Submission of an EUA with of Letters of Cross-Reference
Osiris shall support the submission of an EUA by preparation of letters that cross-reference the IND file and/or other regulatory documentation.
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-4a

| | Attachment 1
W9113M-08-C-0028
Page 8 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.1.8. Conduct Phase I Clinical Study in Accordance with FDA GCP
Osiris has completed a Phase I dose escalation clinical safety study in a relevant human subject population in accordance with FDA Good Clinical Practices (GCP). This was Osiris Protocol 401. Osiris Protocol 401 was a safety study testing hMSC treatment for people who had suffered an acute myocardial infarction (AMI). This Phase I study shall be used to support pivotal animal trials for ProchymalTM-ARS. To date, Osiris has completed several clinical trials for IV administration of hMSCs and is currently conducting a Phase III clinical trial for treating GvHD (see Figure 2.1-2). IRB approval was necessary at all institutions that participated in the clinical trials.
INSERTED TEXT
Osiris believes the current safety profile for Prochymal™ will support a BLA filing for the use of Prochymal™ as an ARS therapy. However, Osiris will arrange a Type B meeting with FDA to confirm the sufficiency of current human safety data for an ARS BLA.
C.3.1.9. Store Phase 1 Product for Two Years Following Clinical Trial
INSERTED TEXT
Osiris shall store Phase I product under cGMP-controlled conditions for two years following the clinical trial. Osiris has stored Phase I product under cGMP-controlled conditions from a Phase I safety study completed in October 2001(Protocol 201).
Osiris’ most recent Phase I safety study (Protocol 401) was completed in May 2006. Osiris shall store this product until May 2008.
C.3.1.10. Perform an ICH-Compliant Stability Testing Program
Osiris shall perform an ICH-compliant stability program on ProchymalTM. The stability program is currently being reviewed by FDA and is planned to capture stability data for 30 months.
C.3.2. Conduct Activities Leading to FDA Approval of the Product
INSERTED TEXT
Osiris shall conduct activities to lead to FDA approval of ProchymalTM for ARS. Osiris plans to file for approval under the animal rule. However, data collected in GLP animal studies will be supplemented with human data obtained in a Phase II clinical trial to evaluate the safety and efficacy of Prochymal™ therapy for the treatment of radiation-induced enteropathy.
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-5

| | Attachment 1
W9113M-08-C-0028
Page 9 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.2.1. Scale-up Manufacturing Processes
Osiris shall scale-up the manufacturing process to achieve a cGMP compliant large-scale manufacturing process. Osiris is currently scaling-up ProchymalTM production to meet clinical trial demands. In order to meet clinical trial demands, and in preparation for eventual product marketing, Osiris has contracted outside manufacturing facilities to manufacture ProchymalTM. More manufacturing facilities shall be contracted if necessary to produce product for this contract. Osiris shall conduct product comparability studies to demonstrate cGMP-compliant product comparability between contract manufacturers.
Presently, MSC manufacturing takes place at the following three GMP compliant manufacturing facilities.
1. Osiris Therapeutics, Inc.
2001 Aliceanna Street,
Baltimore, MD 21231
2. Lonza Bioscience
8830 Biggs Ford Road
Walkersville, MD 21793
3. AppTec
4751 League Island Blvd.
Philadelphia, PA 19112
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-5a

| | Attachment 1
W9113M-08-C-0028
Page 10 of 15 |
Solicitation Number: W9113M-07-R-0002
FDA granted Osiris comparability approval for product manufactured at Osiris and at Lonza Bioscience.
Osiris shall conduct qualification and validation studies of the manufacturing process. These studies are currently underway to support licensure of ProchymalTM- GvHD. The same manufacturing process shall be used for ProchymalTM-ARS.
C.3.2.2. Manufacture cGMP Product Suitable for Expanded Nonclinical Safety Studies, Definitive Animal Efficacy Studies, and Phase II Clinical Trials
Osiris has completed non-clinical safety and toxicology studies. FDA has reviewed these studies and has deemed them acceptable for supporting a BLA filing for ProchymalTM-GvHD.
Osiris shall contract out cell production for definitive animal efficacy studies.
Osiris has completed Phase II clinical trials that support the safety profile for ProchymalTM.
Osiris has completed an FDA approved comparability study on consistency lots, which demonstrated comparability between Osiris and Lonza Bioscience cGMP manufactured product.
Osiris shall cooperate with the FDA Pre-Approval Inspection as required for product approval.
C.3.2.3. Refine and Select a Final Product Formulation Suitable for Military Use
Osiris has completed development of a product formulation that is suitable for administration in humans and a delivery system that is suitable for military use (see section C.3.1.3).
For the military setting, we foresee a scenario where the product would be stored in liquid nitrogen freezers at a secure location anywhere in the world. If a radiological situation were to occur, the product could be shipped to the treatment location. At the treatment location, the product could be thawed, diluted with an electrolyte solution using a syringe, and administered intravenously. There is no need for working in a sterile environment and there is no necessity for hospital beds. The bags of cells would just have to be thawed, spiked, diluted, and hung for intravenous delivery.
INSERTED TEXT
Osiris shall continue development efforts for improving product storage and delivery techniques. These activities will include studies to support alternate packaging and storage conditions appropriate for the military setting.
Study Cell Delivery Technique
Osiris shall test new techniques for transferring the product from the thawed product bag into the IV bag with minimal manipulation.
C.3.2.4. Develop and Validate Analytical Assays for Large-Scale Lot Release
Osiris has completed development and validation of analytical assays for cGMP compliant product characterization and lot release. The lot release specifications were established in cooperation with FDA. The lot release specs are currently being used to release product for a Phase III clinical trial to treat GvHD (see section C.3.1.4).
Lot release specifications shall be updated if required by FDA for the scale-up manufacturing process.
August 10, 2007
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-6

| | Attachment 1
W9113M-08-C-0028
Page 11 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.2.5. Perform an ICH-Compliant Stability Testing Program on Bulk API
Osiris shall perform an ICH-compliant stability testing program on bulk API. The active pharmaceutical ingredient (API) for ProchymalTM manufacturing is termed the Donor Cell Bank (DCB). An ICH-compliant stability protocol for DCB is currently under review with FDA.
Preliminary data from a development study suggest that DCB is stable for a minimum of 35 months under the storage conditions of < -135°C.
C.3.2.6. Perform an ICH-Compliant Testing Program on Formulated Product
Osiris shall perform an ICH-compliant stability testing program on the cGMP formulated product from consistency lots produced from initial large-scale manufacturing to establish a shelf life for the product. The formulated medicinal product is termed Product Dose (PD). An ICH-compliant stability protocol for PD consistency lots is currently under review with FDA
Preliminary data from a development study suggest that the PD is stable for at least 18 months under the storage conditions of < -135°C.
C.3.2.7. Stability Testing to Establish Operational Storage and Distribution Temperature Ranges and Extremes
Osiris shall conduct stability testing to establish operational storage and distribution ranges as well as temperature extremes. One major benefit of ProchymalTM is that it can be administered several days after radiation exposure, and therefore does not need to be carried on the warfighter. The product can be shipped anywhere in the world within this dosing window. The product cannot be carried on the warfighter because it needs to be stored at ultra-low temperatures. Osiris suggests storing the product in remote liquid nitrogen freezers until a radiological incident occurs. When a radiation exposure occurs, the product would just need to be removed from remote liquid nitrogen freezers and shipped to arrive on site as soon as possible. Osiris requests assistance from the military in determining the optimal logistical plan for shipping the product following a radiological incident.
With the current envisioned logistical scenario in mind, Osiris shall perform studies to establish operational storage and distribution temperature ranges, as well as temperature extremes expected for use in the field.
Study 1. Shipping Stability
Osiris shall test product stability in shipping containers at temperature extremes.
Study 2. Product Stability After Thaw
Osiris shall test post thaw product stability at temperature extremes that are possible in a military setting.
C.3.2.8. Conduct Appropriate Nonclinical Studies in Accordance with GLP Regulations and Guidelines to Support Submission of a BLA/NDA
Osiris has completed appropriate nonclinical studies in accordance with GLP regulations and guidelines to support submission of a BLA for ProchymalTM- GvHD. This nonclinical package shall be used for ProchymalTM-ARS.
August 10, 2007 & #160;
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-7

| | Attachment 1
W9113M-08-C-0028
Page 12 of 15 |
Solicitation Number: W9113M-07-R-0002
On February 21st 2007, FDA agreed that the package of preclinical studies submitted and underway was appropriate to support BLA for ProchymalTM-GvHD. The package of studies included acute infusional toxicity, cardiovascular safety, pulmonary safety, immunological study, ectopic foci formation, and biodistribution.
A technical hurdle exists for PK and absorption, distribution, metabolism, and excretion (ADME) studies for cell therapies. There is currently no good cell tracking technique. This topic has been addressed with FDA. Osiris’ clinical and non-clinical studies shall support the submission of a BLA.
C.3.2.9. Conduct Necessary Definitive Efficacy Studies in Relevant Animal Models in Accordance with GLP Regulations and Guidelines
Osiris shall perform efficacy studies in relevant animal models in accordance with GLP regulations and guidelines. Osiris shall discuss these studies with FDA for product approval under the animal rule.
REPLACED TEXT
“Rats” to “small animal”, studies include tissue repair.
Osiris shall perform the following studies:
1. Small animal studies using total body irradiation to evaluate survival and tissue repair in Prochymal™-treated and control subjects. GI data shall be analyzed.
2. Non-human primate survival study using total body irradiation. GI data shall be analyzed.
3. Non-human primate GI-efficacy study using targeted abdominal irradiation. This study shall only be conducted if targeted abdominal irradiation is needed for FDA approval.
C.3.2.10. Conduct a Phase II Expanded Safety Clinical Study in a Relevant Human Subject Population in Accordance with GCP
Osiris has completed two Phase II studies in relevant human subject populations in accordance with cGCP. The human subjects in these trials were people with GI damage resulting from GvHD. The damage to the intestinal mucosa from GvHD and irradiation is the same. IRB approval was obtained at all clinical sites involved in these clinical trials.
INSERTED TEXT
To further supplement data collected under the Animal Rule, Osiris will conduct a Phase II clinical study to explore the safety and efficacy of Prochymal™ therapy for treating normal tissue damage in the GI tract in patients that have received radiotherapy. This development strategy will mitigate risks associated with product approval under the Animal Rule. FDA representatives have recently commented publicly that, for products evaluated under the Animal Rule, supplementation of animal data with human data collected in clinical trials would be considered “highly valued”.
C.3.2.11. Submit a NDA/BLA for FDA Review
Osiris shall prepare and submit a BLA for FDA review for treating ARS. Osiris has completed a Pre-IND meeting with FDA on February 26th to discuss an ARS IND.
Osiris shall prepare and submit responses to requests for additional information from FDA during the review process.
August 10, 2007 & #160;
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-8

| | Attachment 1
W9113M-08-C-0028
Page 13 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.2.12. Deliver One Dose of the FDA-Approved MRC to the Government
Osiris shall deliver a minimum of one dose of the FDA approved countermeasure as part of CLIN 00001.
C.3.3. Prepare a SOW, IMS, CWBS, CWBS Dictionary, and Integrated Risk Management Plan
Osiris shall submit a SOW, IMS (CDRL A004), CWBS (CDRL A003), CWBS dictionary and Integrated Risk Management Plan for the proposal that encompasses the entire scope of the project. The IMS and CWBS shall be updated monthly. The CWBS elements shall define the complete scope of the contract to a breadth and depth necessary to describe the proposed effort to a minimum of Level 4.
August 10, 2007 & #160;
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-8a

| | Attachment 1
W9113M-08-C-0028
Page 14 of 15 |
Solicitation Number: W9113M-07-R-0002
C.3.4. Prepare a Technical Data Package (TDP)
Osiris shall prepare a TDP (CDRL A007) solely for the use of ProchymalTM to treat Acute Radiation Syndrome. This TDP shall include technical information pertaining to the manufacture, safety, and known efficacy of ProchymalTM for the sole purpose of treating complications from Acute Radiation Syndrome.
C.3.5. Option: Prepare and Deliver a DMF
Osiris has completed a Master File with FDA for the ex vivo manufacture of human mesenchymal stem cells (MF-13146).
C.3.6. Option: Produce 50,000 — 500,000 Troop Equivalent Doses (TED) of the MRC
If the option is exercised by the government, Osiris shall produce no fewer that 50,000 and not to exceed 500,000 Troop Equivalent Doses (TED) of the MRC and shall deliver product to the government as directed.
C.4. General
C.4.1. Provide the Necessary Qualified Personnel, Facilities, Equipment, Supplies, Services, Subcontractors and Related Administrative and Information Technology Support
Osiris shall provide the necessary qualified personnel, facilities, equipment, supplies, services, subcontractors, and related administrative information technology support to accomplish the objectives.
C.4.2. Allow Access to Records Files and Other Data for FDA and/or MITS JPMO Audit
Osiris shall allow access to records, files, and other data derived from this work for the purposes of audit by the FDA and/or by MITS JPMO or its designees.
C.4.3. Allow the Government or its Designee to Audit the Contractor and/or its Subcontractor for Regulatory Compliance and Quality Assurance Purposes
Osiris shall allow the Government or its designee to audit Osiris and/or its subcontractors for regulatory compliance and quality assurance purposes.
C.4.4. Provide Monthly Contractor’s Progress, Status and Management Reports
Osiris shall provide monthly Progress, Status, and Management Reports (CDRL A001) that describe progress made within the period, summarize projected vs. actual progress, report costs, and inform the government of existing or potential problem areas. Invoices shall be submitted during the report month. Osiris shall submit a monthly Contract Performance Report (CPR) in accordance with CDRL A005.
C.4.5. Provide Quarterly Contract Funds Status Reports Using DD Form 1568
Osiris shall provide quarterly Contract Funds Status Reports using DD form 1568. Osiris shall participate in a periodic teleconference in accordance with CDRL A002. Osiris shall submit a MS PowerPoint presentation for quarterly Interim Program Reviews in accordance with CDRL A008.
August 10, 2007 & #160;
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-9

| | Attachment 1
W9113M-08-C-0028
Page 15 of 15 |
Solicitation Number: W9113M-07-R-0002
C.4.6. Utilize an Earned Value Management (EVM) System Complying With The Criteria Provided in the American National Standards Institute/Electronic Industries Alliance (ANSI/EIA) Standard 748
Osiris shall use an Earned Value Management System (EVMS) recognized by the Administrative Contracting Officer (ACO) as complying with the criteria provided in American National Standards Institute (ANSI)/Electronic Industries Alliance (EIA) EVMS standard (ANSI/EIA-748), DoD 5000.2-R, Mandatory Procedures for Major Defense Acquisition Program (MDAPs) and Major Automated Information System (MAIS) Acquisition programs (DFARS 252.242-7001 and DFARS 252.242-7002) as well as the policy letter, “Revision to DoD Earned Value Management Policy” dated March 7, 2005.
Osiris shall hold an Integrated Baseline Review (IBR) to assess the realism and accuracy of the integrated performance measurement baseline. The IBR shall be initiated no later than 6 months from the contract award, the exercise of significant contract options, and the incorporation of major modifications or as otherwise agreed upon. Osiris shall provide monthly EVM reports.
C.4.7. Provide a Summary Report of Events That Will Cause More Than a Two Week Delay in Schedule or an Increase in Cost Estimate At Completion
Osiris shall provide a Summary Report within three working days of events that will cause more than a two-week delay in schedule, or an increase in cost Estimate at Completion.
C.4.8. Participate in Teleconference as Needed
Osiris shall participate in teleconference with the government when needed. Osiris shall provide minutes of the teleconferences within one week.
C.4.9. Employ an Automate Information System (AIS)
Osiris shall employ an automated information system (AIS) in accordance with electronic common technical document (eCTD) and ICH guidance for electronic data submissions to FDA.
Osiris currently submits all documents to FDA electronically through the secure electronic submission gateway.
Osiris shall allow MITS JPMO read only remote access via Osiris’ AIS to clinical and non-clinical data pertaining to ARS following quality assurance review and acceptance.
C.4.10. Report All Contractor Manpower (Including Subcontractor Manpower) Required for Performance of this Contract
Osiris shall report all contract manpower (including subcontractor manpower) required for performance of this contract. Osiris shall completely fill in information using the Army data collection site at (URL: https://contractormanpower.army.pentagon.mil).
Osiris shall provide estimated total cost (if any) incurred to comply within this reporting requirement. The reporting period will be the period of performance not to exceed twelve months ending September 30 of the Government’s Fiscal Year and must be reported by October 31 of each subsequent Fiscal Year.
August 10, 2007 & #160;
Use or disclosure of data contained on this sheet is subject to the restriction on the title page of this proposal.
2.1-10

