EXHIBIT 99.1

FOR IMMEDIATE RELEASE

Investors Contact:
Robert G. Burrows
Vice President, Investor Relations
301-795-1877
BurrowsR@ebsi.com
Media Contact:
Tracey Schmitt
Vice President, Corporate Communications
301-795-1800
SchmittT@ebsi.com
EMERGENT BIOSOLUTIONS REPORTS FINANCIAL RESULTS FOR SECOND QUARTER AND FIRST SIX MONTHS OF 2009
• | 2Q and six month 2009 total revenues of $73.2 and $137.7 million, respectively |
• | 2Q 2009 net income of $14.8 million, or $0.49 per share |
• | Six month 2009 net income of $26.0 million, or $0.86 per share |
• | June 30, 2009 cash and cash equivalents of $102.5 million |
• | 2009 financial guidance reaffirmed—revenue range maintained at $225 to $240 million, net income in excess of $20 million |
ROCKVILLE, MD, August 6, 2009—Emergent BioSolutions Inc. (NYSE: EBS) announced today its financial results for the second quarter and six months ended June 30, 2009.
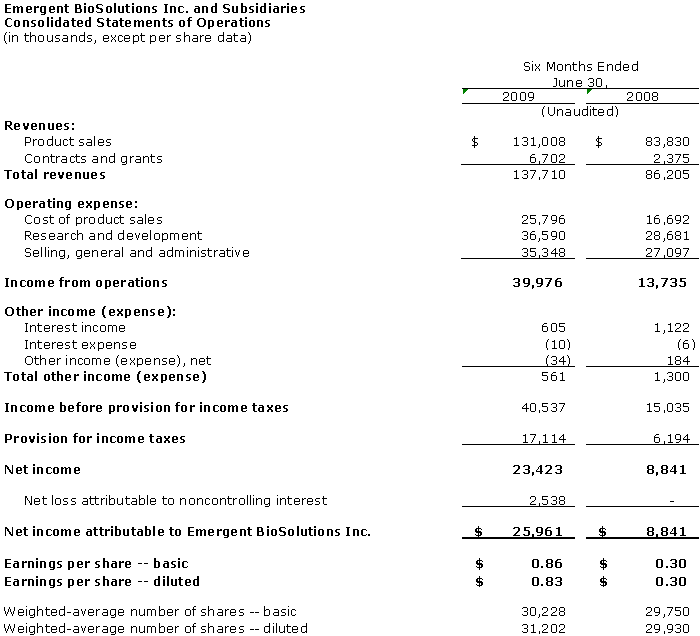
Total revenues for the second quarter and six months of 2009 were $73.2 million and $137.7 million, respectively. Net income for the second quarter and six months of 2009 was $14.8 million or $0.49 per share, and $26.0 million or $0.86 per share, respectively. Such performance was primarily driven by growth in sales of BioThrax® (Anthrax Vaccine Adsorbed), the Company’s FDA licensed vaccine for the prevention of anthrax, as well as a lump-sum payment from HHS related to the granting by FDA of four-year expiry dating for BioThrax®.
R. Don Elsey, chief financial officer of Emergent BioSolutions, stated, “Our financial performance for the second quarter and first six months of 2009 reflects the continued strength of our core BioThrax® business and our ability to manufacture and deliver doses of our anthrax vaccine into the SNS under contract with the U.S. government. Recently, we completed our commitments under the current contract and look forward to seamlessly transitioning into initiating deliveries under a follow on contract that provides sales through late 2011. Beyond the follow on contract, we expect that the U.S. government will continue to procure BioThrax® for the strategic national stockpile.”
Continuing, Mr. Elsey stated, “The multiple vaccine and therapeutic candidates in our biodefense franchise, focused on anthrax and botulism, continue to advance as we see increasing interest on the part of the U.S. government in implementing a multi-supplier, multi-product strategy for building the nation’s biopreparedness. Our commercial pipeline also continues to move forward as we, both on our own and in partnership with government and non-governmental institutions, pursue programs that address global unmet medical needs, most notably tuberculosis.”
2Q 2009 Key Operational Accomplishments
• | Received FDA approval extending shelf life of BioThrax® to 4 years, triggering a $29.6 million lump-sum payment from HHS; and, |
• | Initiated a Phase IIb proof-of-concept trial in South Africa for the Company’s advanced TB vaccine candidate, largely funded by The Aeras Global TB Vaccine Foundation and the Wellcome Trust. |
2Q 2009 Key Financial Results
Product Sales
For 2Q 2009, product sales were $69.3 million, an increase of $27.0 million, or 64 percent, from $42.3 million in 2Q 2008. The increase was primarily due to a lump-sum payment from HHS of $29.6 million related to the approval of four-year expiry dating for BioThrax®.
For the six month period of 2009, product sales increased by $47.2 million, or 56 percent, to $131.0 million from $83.8 million for the comparable period of 2008, primarily due to a 21 percent increase in the number of doses of BioThrax® delivered and the $29.6 million lump-sum payment from HHS.
Contracts and Grants Revenues
For 2Q 2009, contracts and grants revenue was $3.9 million, an increase of $2.7 million, or 233 percent, from $1.2 million in 2Q 2008. For the six month period of 2009, contracts and grants revenue increased by $4.3 million, or 182 percent, to $6.7 million from $2.4 million for the comparable period of 2008. Contracts and grants revenue for 2Q 2009 and the six month period of 2009 consisted of development revenue from NIAID and BARDA.
Cost of Product Sales
For 2Q 2009, cost of product sales was $10.4 million, an increase of $1.7 million, or 20 percent, from $8.7 million in 2Q 2008. Cost of product sales for 2Q 2009 primarily reflects an increase in the cost per dose sold associated with reduced production yield in the period during which the doses sold were produced.
For the six month period of 2009, cost of product sales increased by $9.1 million, or 55 percent, to $25.8 million from $16.7 million for the comparable period of 2008. This increase was attributable to a 21 percent increase in the number of doses of BioThrax® delivered and increased cost associated with reduced production yield.
Research and Development
For 2Q 2009, research and development expenses were $20.7 million, an increase of $3.5 million, or 20 percent, from $17.2 million in 2Q 2008. This increase reflects additional personnel and contract service costs, and includes increased expenses of $3.1 million related to our biodefense programs and $1.7 million in other research and development expenses, partially offset by decreased expenses of $1.3 million related to our commercial programs.
For the six month period of 2009, research and development expenses increased by $7.9 million, or 28 percent, to $36.6 million from $28.7 million for the comparable period of 2008. This increase reflects additional personnel and contract service costs, and includes increased expenses of $7.3 million related to our biodefense programs and $2.6 million in other research and development expenses, partially offset by decreased expenses of $2.0 million related to our commercial programs.
Selling, General and Administrative
For 2Q 2009, selling, general and administrative expenses were $19.4 million, an increase of $4.3 million, or 29 percent, from $15.0 million in 2Q 2008. This increase primarily reflects a $3.8 million non-cash impairment charge associated with the Company’s Frederick, Maryland facilities and an increase of $0.8 million in additional personnel and increased professional services to support growth of the business, partially offset by decreased expenses of $0.3 million in sales and marketing expenses.
For the six month period of 2009, selling, general and administrative expenses increased by $8.3 million, or 30 percent, to $35.3 million from $27.1 million for the comparable period of 2008. This increase primarily reflects an increase of approximately $3.5 million resulting from the addition of personnel and increased professional services for the Company’s headquarters organization, a $3.8 million non-cash impairment charge associated with the Company’s Frederick, Maryland facilities and a $1.4 million non-cash charge associated with acquisitions that were in progress but not completed as of December 31, 2008, partially offset by a decrease of $0.4 million in sales and marketing expenses.
Financial Condition and Liquidity
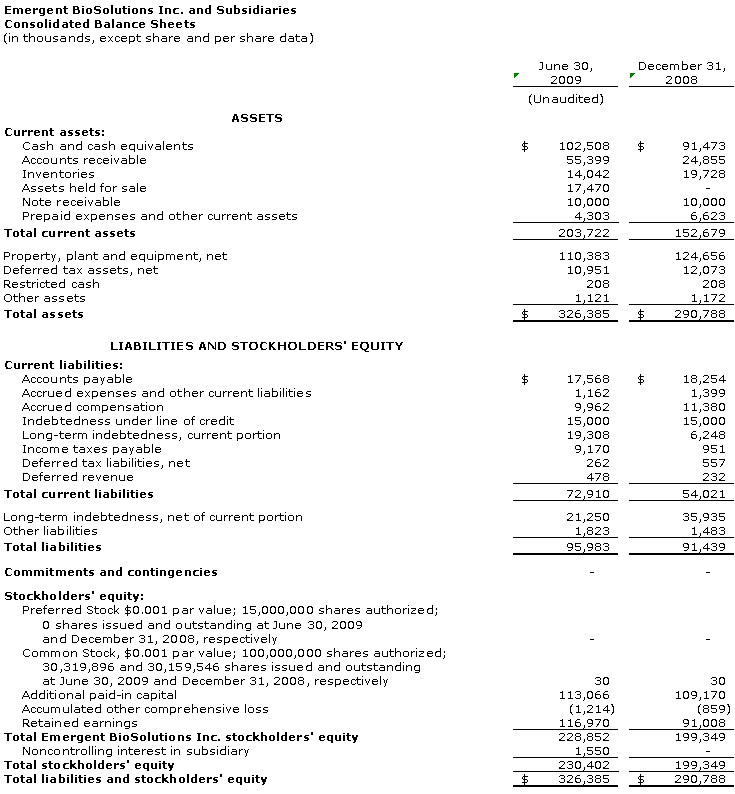
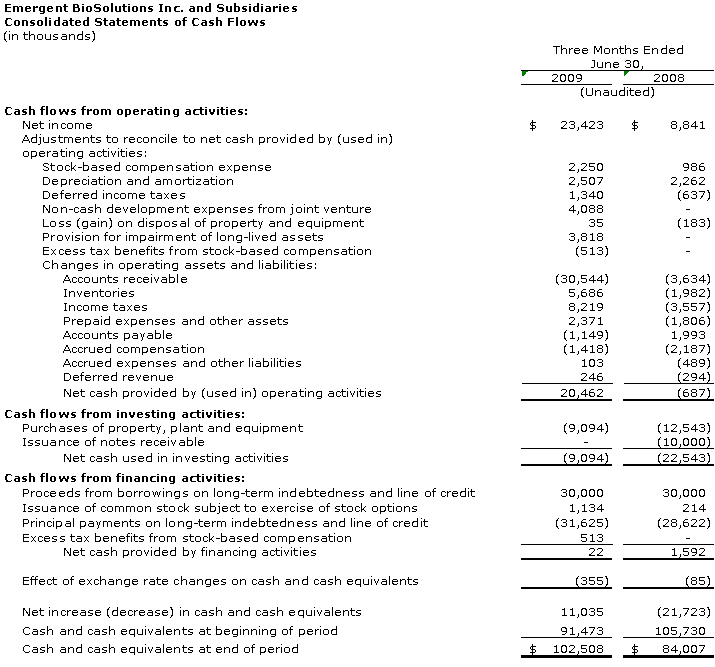
Cash and cash equivalents at June 30, 2009 was $102.5 million compared to $91.5 million at December 31, 2008. Additionally, at June 30, 2009, the accounts receivable balance was $55.4 million, which primarily includes the lump-sum payment from HHS related to the approval of four-year expiry dating for BioThrax® as well as an unpaid balance due from the U.S. government for doses of BioThrax® delivered in 2Q 2009. Payment of most of this accounts receivable balance was received by the Company in early 3Q 2009.
2009 Financial Outlook
For 2009, the Company is reaffirming its financial outlook and is forecasting 25% to 35% growth in year-over-year total revenue to approximately $225 to $240 million. The Company also anticipates 2009 net income in excess of $20 million.
Conference Call and Webcast
Company management will host a conference call at 5:00 pm Eastern on August 6, 2009 to discuss these financial results, recent business developments and the outlook for the second half of 2009. The conference call will be accessible by dialing 888/680-0878 or 617/213-4855 (international) and providing passcode 93911203. A webcast of the conference call will be accessible from the Company’s website at www.emergentbiosolutions.com, under “Investors”.
A replay of the conference call will be accessible, approximately one hour following the conclusion of the call, by dialing 888/286-8010 or 617/801-6888 and using the passcode 81565297. The replay will be available through August 20. The webcast will be archived on the Company’s website, www.emergentbiosolutions.com, under “Investors”.
About Emergent BioSolutions Inc.
Emergent BioSolutions Inc. is a biopharmaceutical company focused on the development, manufacture and commercialization of vaccines and therapeutics that assist the body’s immune system to prevent or treat disease. Emergent’s marketed product, BioThrax® (Anthrax Vaccine Adsorbed), is the only vaccine approved by the U.S. Food and Drug Administration for the prevention of anthrax disease. Emergent’s development pipeline includes programs focused on anthrax, botulism, tuberculosis, typhoid, hepatitis B and chlamydia. Additional information may be found at www.emergentbiosolutions.com.
Safe Harbor Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including
statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, including any potential future securities offering, our expected revenue growth and net earnings for 2009, and any other statements containing the words “believes”, “expects”, “anticipates”, “plans”, “estimates” and similar expressions, are forward-looking statements. There are a number of important factors that could cause the Company’s actual results to differ materially from those indicated by such forward-looking statements, including appropriations for BioThrax® procurement; our ability to obtain new BioThrax® sales contracts; our plans to pursue label expansions and improvements for BioThrax®; our plans to expand our manufacturing facilities and capabilities; the rate and degree of market acceptance and clinical utility of our products; our ongoing and planned development programs, preclinical studies and clinical trials; our ability to identify and acquire or in license products and product candidates that satisfy our selection criteria; the potential benefits of our existing collaboration agreements and our ability to enter into selective additional collaboration arrangements; the timing of and our ability to obtain and maintain regulatory approvals for our other product candidates; our commercialization, marketing and manufacturing capabilities and strategy; our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and other factors identified in the Company’s Quarterly Report on Form 10-Q for the year ended March 31, 2009 and subsequent reports filed with the SEC. The Company disclaims any intention or obligation to update any forward-looking statements as a result of developments occurring after the date of this press release.
Financial Statements Follow