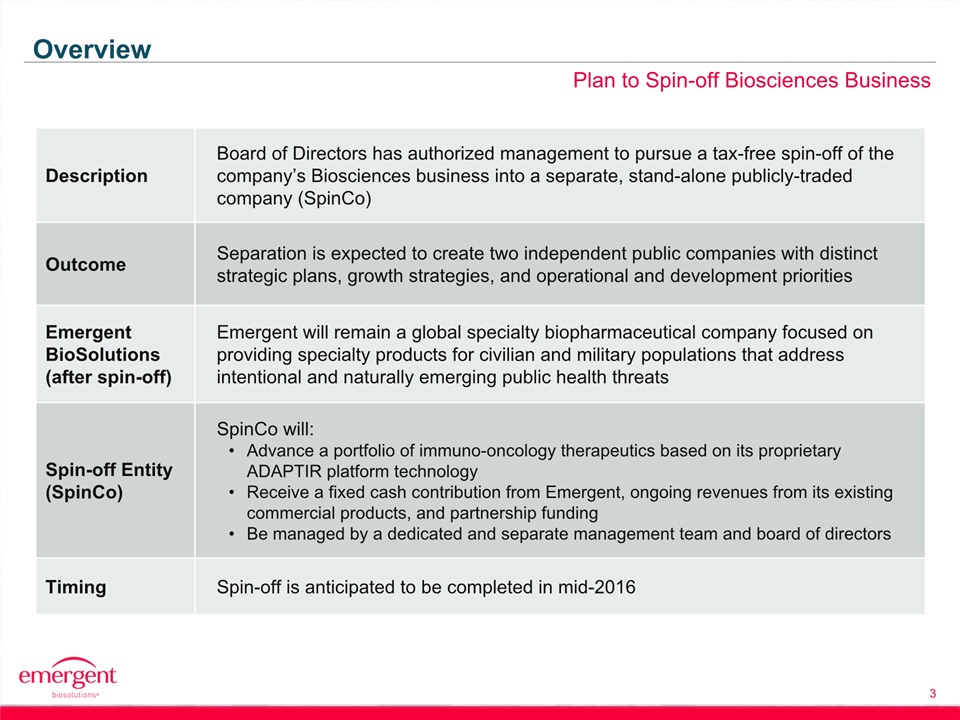
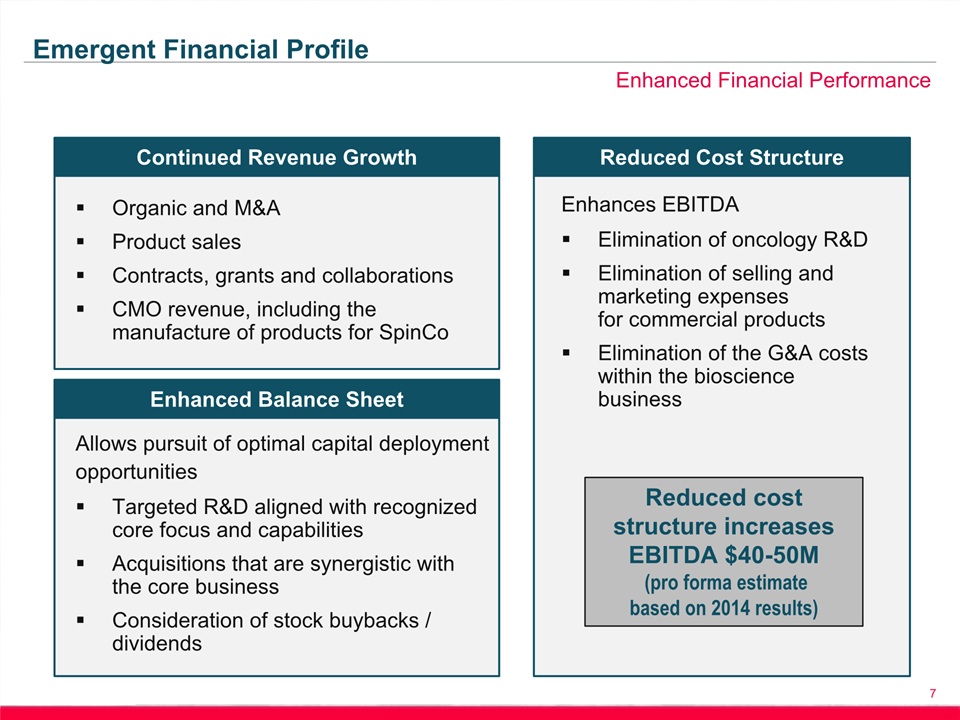
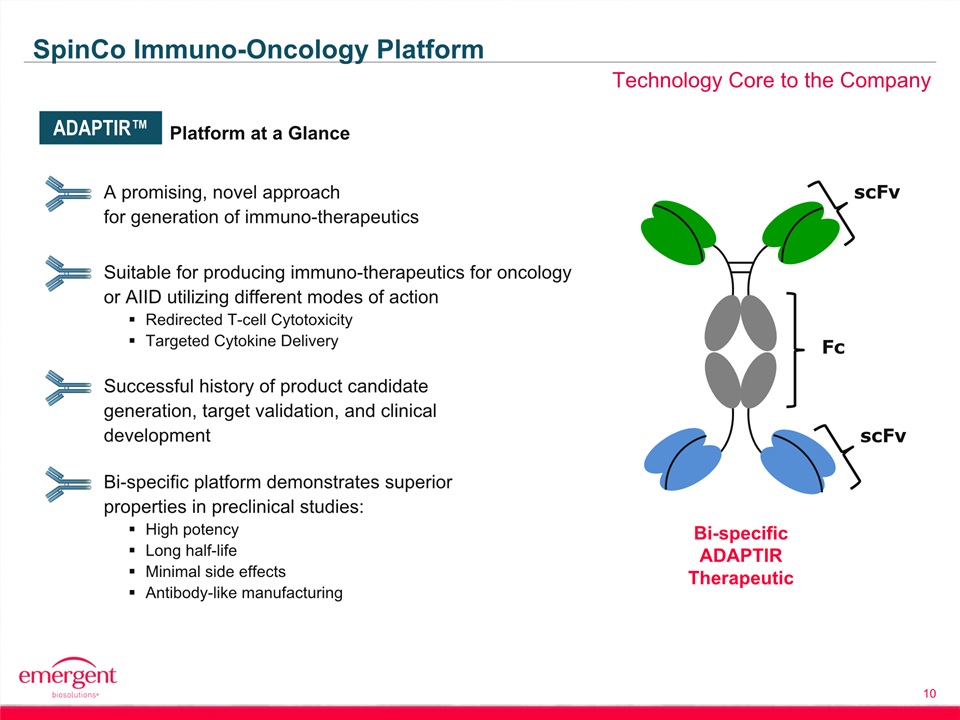
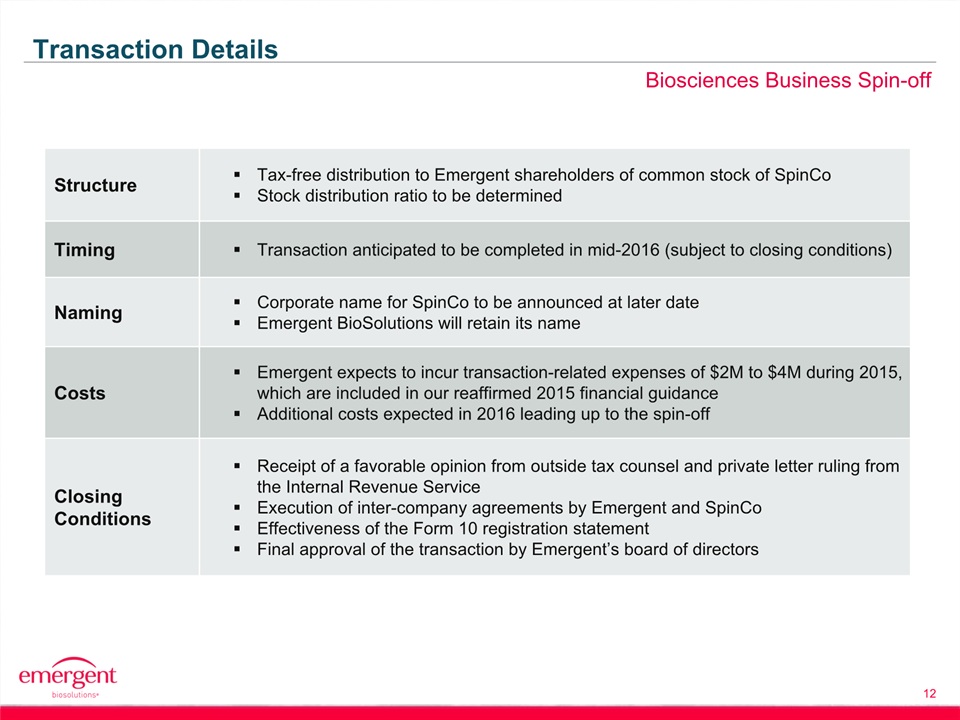
Safe Harbor Statement 2 This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including, without limitation, statements regarding our financial guidance, statements regarding the planned spin-off of our biosciences business, the timing of any such spin-off, the future earnings and performance of Emergent or any of its businesses, including the biodefense and biosciences businesses on a standalone basis if the spin-off is completed, and any other statements containing the words “believes”, “expects”, “anticipates”, “intends”, “plans”, “forecasts”, “estimates” and similar expressions in conjunction with, among other things, discussions of financial performance or financial condition, strategy, product sales, manufacturing capabilities, product development, regulatory approvals or expenditures are forward-looking statements. These forward-looking statements are based on our current intentions, beliefs and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events or circumstances.There are a number of important factors that could cause the company’s actual results to differ materially from those indicated by such forward-looking statements, including whether the planned spin-off of the biosciences business is completed, as expected or at all, and the timing of any such spin-off; whether the conditions to the spin-off can be satisfied; whether the operational, marketing and strategic benefits of the spin-off can be achieved; whether the costs and expenses of the spin-off can be controlled within expectations; appropriations for BioThrax procurement; our ability to perform under our contracts with the U.S. government related to BioThrax, including the timing of deliveries; our ability to obtain new BioThrax sales contracts or modifications to existing contracts; the availability of funding for our U.S. government grants and contracts; our ability to successfully execute our growth strategy and achieve our financial and operational goals; our ability to successfully integrate and develop the products or product candidates, programs, operations and personnel of any entities or businesses that we acquire; our ability to perform under our contract with the U.S. government to develop and obtain regulatory approval for large-scale manufacturing of BioThrax in Building 55, our large-scale vaccine manufacturing facility in Lansing, Michigan; our ability to identify and acquire companies or in-license products or late-stage product candidates that satisfy our selection criteria; our ability to realize synergies and benefits from acquisitions or in-licenses within expected time periods or at all; our ability to selectively enter into collaboration arrangements; our ability to achieve milestones in our out-license and collaboration contracts; our ability to obtain and maintain intellectual property protection for our products and product candidates; our ability and plans to expand our manufacturing facilities and capabilities; our ability and the ability of our contractors and suppliers to maintain compliance with cGMP and other regulatory obligations; the results of regulatory inspections; our ability to meet operating and financial restrictions placed on us and our subsidiaries under our senior secured credit facility; the rate and degree of market acceptance and clinical utility of our products; the success of our ongoing and planned development programs, non-clinical activities and clinical trials of our product candidates; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing.The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the SEC, when evaluating our forward-looking statements.