
Certain identified information has been excluded from the exhibit because it is both (i) not material and (ii) would likely cause competitive harm to the Company, if publicly disclosed. Double asterisks denote omissions. Exhibit 10.48 AWARD/CONTRACT 1. THIS CONTRACT IS A RATED ORDER RATING PAGE OF UNDER DPAS (15 CFR 700) 1 26 2. CONTRACT (Proc. Inst. Ident.) NO. 3. EFFECTIVE DATE 4. REQUISITION/PURCHASE REQUEST/PROJECT NO. 75A50119C00071 08/30/2019 OS247977 5. ISSUED BY CODE ASPR-BARDA 6. ADMINISTERED BY (If other than Item 5) CODE ASPR-BARDA02 ASPR-BARDA US DEPT OF HEALTH & HUMAN SERVICES 200 Independence Ave., S.W. Room ASPR/ORM 640-G O’NEILL HOUSE OFFICE BUILDING Washington DC 20201 Washington DC 20515 8. DELIVERY 7. NAME AND ADDRESS OF CONTRACTOR (No., street, county, State and ZIP Code) SCD-C. EMERGENT PRODUCT DEVELOPMENT GAITHERSBURG INC. 1365869 OTHER (See below) Attn: Mike Mann FOB ORIGIN 300 PROFESSIONAL DRIVE 9. DISCOUNT FOR PROMPT PAYMENT SUITE 100 GAITHERSBURG MD 208793419 10. SUBMIT INVOICES (4 copies unless otherwise specified) ITEM TO THE ADDRESS SHOWN IN CODE 1365869 FACILITY CODE 11. SHIP TO/MARK FOR CODE OS 12. PAYMENT WILL BE MADE BY CODE ASPR-BARDA02 Office of the Secretary ASPR-BARDA02 200 Independence Ave. S.W. 200 C St SW Washington DC 20201 Washington DC 20201 13. AUTHORITY FOR USING OTHER THAN FULL AND OPEN COMPETITION 14. ACCOUNTING AND APPROPRIATION DATA 10 U.S.C. 2304 (c)( ) 41 U.S.C. 3304(a)( ) 2019.199SNS1.26088 15C. 15D. 15A. ITEM NO 15B. SUPPLIES/SERVICES 15E. UNIT PRICE 15F. AMOUNT QUANTITY UNIT (Base Year) ACAM Doses [**] $[**] $169,988,000.00 Continued 15G. TOTAL AMOUNT OF CONTRAC $2,007,468,366.00 16. TABLE OF CONTENTS (X) SEC DESCRIPTION PAGE(S) (X) SEC DESCRIPTION PAGE(S) PART I – THE SCHEDULE PART II – CONTRACT CLAUSES X A SOLICITATION/CONTRACT FORM 1 X I CONTRACT CLAUSES 23 X B SUPPLIES OR SERVICES AND 5 PART III – LIST OF DOCUMENTS, EXHIBITS AND OTHER PRICES/COSTS ATTACH. X C DESCRIPTION/SPECS./WORK 9 X J LIST OF ATTACHMENTS 26 STATEMENT X D PACKAGING AND MARKING 16 PART IV – REPRESENTATIONS AND INSTRUCTIONS X E INSPECTION AND ACCEPTANCE 16 K REPRESENTATIONS, CERTIFICATIONS X F DELIVERIES OR PERFORMANCE 17 AND OTHER STATEMENTS OF OFFERORS X G CONTRACT ADMINISTRATION DATA 18 L INSTRS., CONDS., AND NOTICES TO OFFERORS X H SPECIAL CONTRACT REQUIREMENTS 19 M EVALUATION FACTORS FOR AWARD CONTRACTING OFFICER WILL COMPLETE ITEM 17 (SEALED-BID OR NEGOTIATED PROCUREMENT) OR 18 (SEALED-BID PROCUREMENT) AS APPLICABLE 17. Contractor’s Negotiated Agreement (Contractor is required to sign 18. SEALED-BID AWARD (Contractor is not required to sign this this document and return one copy to issuing office.) Contractor agrees to document.) Your bid on Solicitation Number ________, including the furnish and deliver all items or perform all the services set forth or additions or changes made by you which additions or changes are set otherwise identified above and on any continuation sheets for the forth in full above, is hereby accepted as to the items listed above and on consideration stated herein. The rights and obligations of the parties to any continuation sheets. This award consummates the contract which this contract shall be subject to and governed by the following consists of the following documents: (a) the Government’s solicitation and documents: (a) this award/contract, (b) the solicitation, if any, and (c) your bid, and (b) this award/contract.

such provisions, representation, certifications, and specifications, as are No further contractual document is necessary. (Block 18 should be attached or incorporated by reference herein. (Attachments are listed checked only when awarding a sealed-bid contract.) herein.) 19A. NAME AND TITLE OF SIGNER (Type or print) 20A. NAME OF CONTRACTING OFFICER Adam Havey, EVP Operations [**] 19B. NAME OF CONTRACTOR 19C. DATE SIGNED 20B. UNITED STATES OF AMERICA 20C. DATE SIGNED 16 Dec 19 BY /s/ [**] BY /s/ Adam Havey Digitally signed by (Signature of person authorized to sign) [**] – S Date: 2019.12.17 09:22:47-05’00’ (Signature of the Contracting Officer) AUTHORIZED FOR LOCAL REPRODUCTION Previous edition is NOT usable ACAM 10-Yr 75A50119C00071 Page 2 of 33

CONTINUATION SHEET REFERENCE NO. OF DOCUMENT BEING CONTINUED PAGE OF 75A50119C00071 2 26 NAME OF OFFEROR OR CONTRACTOR EMERGENT PRODUCT DEVELOPMENT GAITHERSBURG INC. 1365869 ITEM No. SUPPLIES/SERVICES QUANTITY UNIT UNIT PRICE AMOUNT (A) (B) (C) (D) (E) (F) Tax ID Number: [**] DUNS Number: [**] ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) Delivery: 12/30/2019 Appr. Yr.: 2019 CAN: 199SNS1 Object Class: 26402 Period of Performance: 08/30/2019 to 08/29/2029 - All Dollar Amounts based on Target Amounts of Products CLINs X0001 through X0004 as stated on Page 3. 1 Base Year ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. Obligated Amount: $169,988,000.00 2 Option Year 1 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 3 Option Year 2 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 4 Option Year 3 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 5 Option Year 4 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 6 Option Year 5 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 7 Option Year 6 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 8 Option Year 7 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] 9 Option Year 8 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) ACAM 10-Yr 75A50119C00071 Page 3 of 33

See Section B below for further details. NTE: $[**] 10 Option Year 9 ACAM2000, Smallpox (Vaccinia) Vaccine, Live (ACAM) See Section B below for further details. NTE: $[**] This Contract award document finalizes the Notice of Award Letter issued by Contracting Officer [**] on August 30, 2019, which established an effective date of August 30, 2019. AUTHORIZED FOR LOCAL REPR OPTIONAL FORM 336 (4-86) Sponsored by GSA FAR (48 CFR) 53.11 ACAM 10-Yr 75A50119C00071 Page 4 of 33

Table of Contents Section B - Supplies or Services and Cost/Price ..........................................................................8 Section C - Statement of Work (SOW) ......................................................................................12 C.1 – Background and Need ..................................................................................................12 C.2 – Purpose .........................................................................................................................12 C.3 – Scope of Work ..............................................................................................................12 C.4 – Task 1: Manufacturing and Delivery of ACAM2000 Vaccine ...................................13 Manufacture of Bulk Vaccine ............................................................................................13 Vaccine Fill and Finish ......................................................................................................13 Requirements for Packaging & Delivery ...........................................................................13 SNS Locations ...................................................................................................................14 Warranties ..........................................................................................................................15 Historical Lots - Testing Program and Quarantine ............................................................15 Task 2: Diluent Replacement .............................................................................................17 Task 3: Syringe Replacement ............................................................................................17 Task 4: ACAM2000 Vaccine Limited Re-Labeling Activities ........................................17 C-5 – Reporting Requirements (Emergent) ...........................................................................19 C.6 – Reporting Requirements (SNS) ....................................................................................19 C.7. – Delivery Notifications .................................................................................................20 C.8 – Quality Inspections .......................................................................................................20 Section D - Packaging And Marking ..........................................................................................20 Section E - Inspection And Acceptance .....................................................................................20 E.1. – FAR 52.252-2 Clauses Incorporated by Reference (1998) .....................................20 E.2. – Inspection and Acceptance .....................................................................................21 E.3. – Shipment Acceptance..............................................................................................21 Section F - Deliveries Or Performance ......................................................................................22 F.1. – Period of Performance .................................................................................................22 F.2. – Delivery Locations.......................................................................................................22 Section G - Contract Administration Data ................................................................................22 G.1. – Electronic Subcontracting Reporting System (eSRS) .................................................22 G.2. – Invoice Submission .....................................................................................................23 G.3. – Contracting Officer .....................................................................................................23 G.4. – Contracting Officer’s Representative ..........................................................................24 G.5 – Contract Communications/Correspondence ................................................................24 ACAM 10-Yr 75A50119C00071 Page 5 of 33

Section H - Special Contract Requirements ..............................................................................24 H.1. – Evaluation of Contractor Performance Utilizing CPARS (April 2015) .....................24 H.2. – CDC37.0001 Non-Personal Services (April 2015) .....................................................25 H.3. – Restrictions on Disclosure of Information and Rights in Data ...................................26 H.4. – Liability Protection under the PREP Act ....................................................................26 H.5. – SNS Responsibilities ...................................................................................................27 H.6. – Rights of the Contractor to Market Smallpox Vaccine ...............................................27 H.7. – Expired Doses in the SNS ...........................................................................................27 H.8 – Testing of Quarantined Product ...................................................................................27 Section I - Contract Clauses ........................................................................................................29 I.1 - Clauses Incorporated By Reference ...............................................................................29 I.1 - Clauses Incorporated By Reference ...............................................................................30 FAR 52.217-9 Option to Extend the Term of the Contract (Mar 2000) .........................32 Section J - List Of Attachments ..................................................................................................33 Table 8: Kit Reference (including Shelf Life) ..........................................................................33 ACAM 10-Yr 75A50119C00071 Page 6 of 33

Tables Table 1: Overall Price Summary .....................................................................................................5 Table 2: Task 1 - ACAM2000 Vaccine Manufacture and Provision to the SNS ...........................6 Table 3: Task 2 - Diluent Replacement and Provision to the SNS .................................................7 Table 4: Task 3 - Transfer Syringe Replacement and Provision to the SNS1F ..............................7 Table 5: Task 4 - Vaccine Limited Re-Labeling Program ..............................................................7 Table 6: Strategy for managing expiring ACAM2000 vaccine ....................................................12 Table 7: Re-labeling of Rockville MD Manufactured Lots with [**] expiry ...............................14 Table 8: Kit Reference (including Shelf Life) ..............................................................................26 ACAM 10-Yr 75A50119C00071 Page 7 of 33

Section B - Supplies or Services and Cost/Price Table 1: Overall Price Summary ITEM CLIN X001- Doses CLIN X002- Diluent CLIN X003- Syringe CLIN X004- Replacement Replacement Re-labelling Grand Total Contract Year ACAM Doses EXTENDED Diluent EXTENDED Transfer EXTENDE Target PRICE Vials PRICE Syringes D PRICE 1 Base Year [**] $169,988,000 $169,988,000 100X Option Year 1 [**] [**] [**] [**] 200X Option Year 2 [**] [**] [**] [**] 300X Option Year 3 [**] [**] [**] 400X Option Year 4 [**] [**] [**] [**] [**] [**] [**] [**] 500X Option Year 5 [**] [**] [**] [**] [**] [**] [**] 600X Option Year 6 [**] [**] [**] [**] [**] [**] [**] [**] 700X Option Year 7 [**] [**] [**] [**] [**] [**] [**] 800X Option Year 8 [**] [**] [**] [**] [**] [**] [**] [**] 900X Option Year 9 [**] [**] [**] [**] [**] [**] [**] Overall [**] [**] [**] [**] [**] [**] [**] $2,007,468,366 Note: Values based on delivery of target amounts of product and relabeling of inventory at the time of contract preparation. ACAM 10-Yr 75A50119C00071 Page 8 of 33
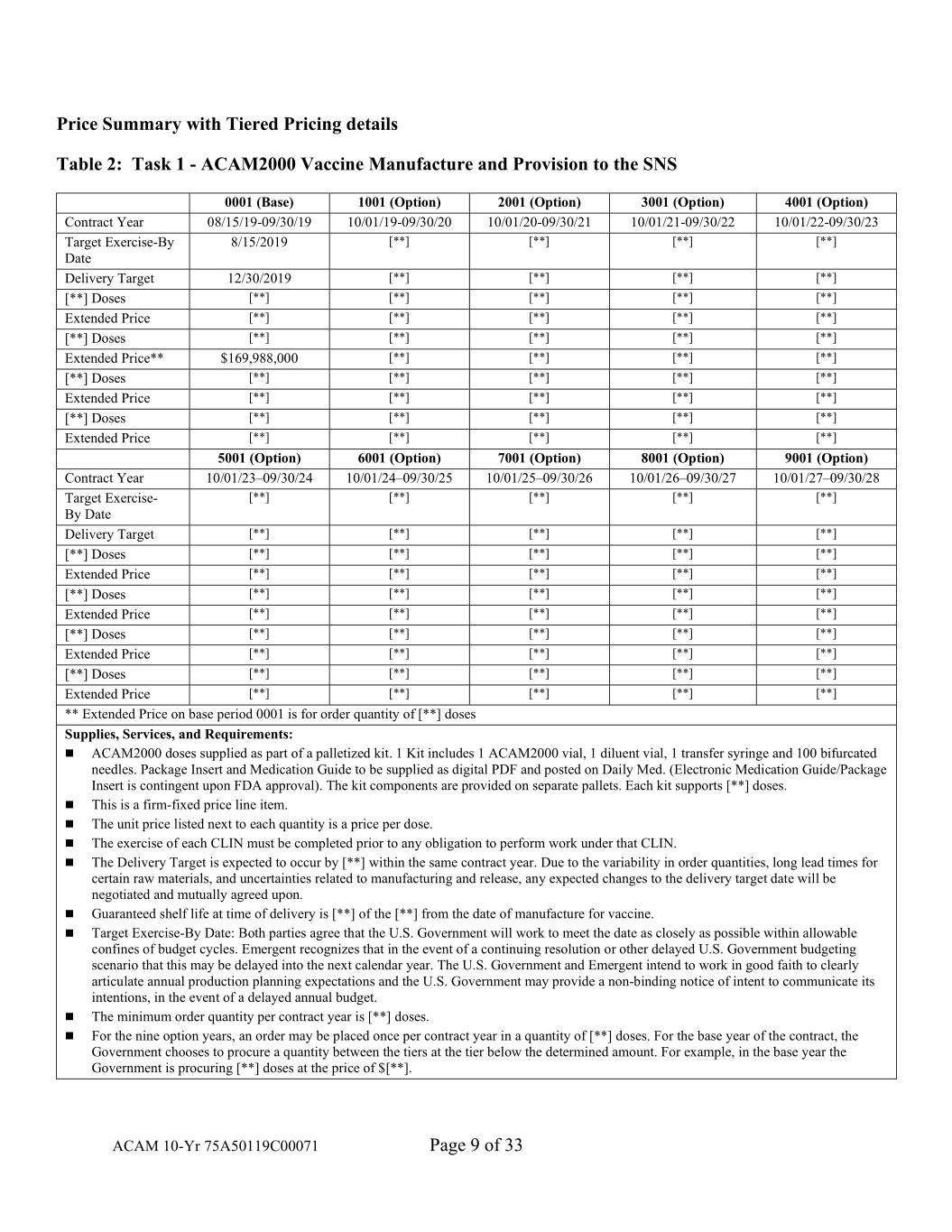
Price Summary with Tiered Pricing details Table 2: Task 1 - ACAM2000 Vaccine Manufacture and Provision to the SNS 0001 (Base) 1001 (Option) 2001 (Option) 3001 (Option) 4001 (Option) Contract Year 08/15/19-09/30/19 10/01/19-09/30/20 10/01/20-09/30/21 10/01/21-09/30/22 10/01/22-09/30/23 Target Exercise-By 8/15/2019 [**] [**] [**] [**] Date Delivery Target 12/30/2019 [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price** $169,988,000 [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] 5001 (Option) 6001 (Option) 7001 (Option) 8001 (Option) 9001 (Option) Contract Year 10/01/23–09/30/24 10/01/24–09/30/25 10/01/25–09/30/26 10/01/26–09/30/27 10/01/27–09/30/28 Target Exercise- [**] [**] [**] [**] [**] By Date Delivery Target [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] ** Extended Price on base period 0001 is for order quantity of [**] doses Supplies, Services, and Requirements: ACAM2000 doses supplied as part of a palletized kit. 1 Kit includes 1 ACAM2000 vial, 1 diluent vial, 1 transfer syringe and 100 bifurcated needles. Package Insert and Medication Guide to be supplied as digital PDF and posted on Daily Med. (Electronic Medication Guide/Package Insert is contingent upon FDA approval). The kit components are provided on separate pallets. Each kit supports [**] doses. This is a firm-fixed price line item. The unit price listed next to each quantity is a price per dose. The exercise of each CLIN must be completed prior to any obligation to perform work under that CLIN. The Delivery Target is expected to occur by [**] within the same contract year. Due to the variability in order quantities, long lead times for certain raw materials, and uncertainties related to manufacturing and release, any expected changes to the delivery target date will be negotiated and mutually agreed upon. Guaranteed shelf life at time of delivery is [**] of the [**] from the date of manufacture for vaccine. Target Exercise-By Date: Both parties agree that the U.S. Government will work to meet the date as closely as possible within allowable confines of budget cycles. Emergent recognizes that in the event of a continuing resolution or other delayed U.S. Government budgeting scenario that this may be delayed into the next calendar year. The U.S. Government and Emergent intend to work in good faith to clearly articulate annual production planning expectations and the U.S. Government may provide a non-binding notice of intent to communicate its intentions, in the event of a delayed annual budget. The minimum order quantity per contract year is [**] doses. For the nine option years, an order may be placed once per contract year in a quantity of [**] doses. For the base year of the contract, the Government chooses to procure a quantity between the tiers at the tier below the determined amount. For example, in the base year the Government is procuring [**] doses at the price of $[**]. ACAM 10-Yr 75A50119C00071 Page 9 of 33

Table 3: Task 2 - Diluent Replacement and Provision to the SNS 4002 (Option) 5002 (Option) 6002 (Option) 7002 (Option) 8002 (Option) 9002 (Option) Contract Year 10/01/22– 10/01/23– 10/01/24– 10/01/25– 10/01/26– 10/01/27– 09/30/23 09/30/24 09/30/25 09/30/26 09/30/27 09/30/28 Target Exercise-By [**] [**] [**] [**] [**] [**] Date Delivery Target [**] [**] Vials [**] [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] [**] Vials [**] [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] [**] Vials [**] [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] [**] Vials [**] [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Supplies, Services, and Requirements: Diluent replacement for ACAM2000 vaccine kits provided under this contract. Expiry is [**] from date of manufacture. This is a firm-fixed price line item. Guaranteed shelf life from delivery is [**] from the date of manufacture for vials of diluent. Provision of diluent replacement under prior contracts is available under separate contract. Table 4: Task 3 - Transfer Syringe Replacement and Provision to the SNS1 4003 (Option) 5003 (Option) 6003 (Option) 7003 (Option) 8003 (Option) 9003 (Option) Contract Year 10/01/22– 10/01/23– 10/01/24– 10/01/25– 10/01/26– 10/01/27– 09/30/23 09/30/24 09/30/25 09/30/26 09/30/27 09/30/28 Target Exercise-By [**] [**] [**] [**] [**] [**] Date Delivery Target [**] [**] Transfer [**] [**] [**] [**] [**] [**] Syringes Extended Price [**] [**] [**] [**] [**] [**] [**] Transfer [**] [**] [**] [**] [**] [**] Syringes Extended Price [**] [**] [**] [**] [**] [**] [**] Transfer [**] [**] [**] [**] [**] [**] Syringes Extended Price [**] [**] [**] [**] [**] [**] [**] Transfer [**] [**] [**] [**] [**] [**] Syringes Extended Price [**] [**] [**] [**] [**] [**] Supplies, Services, and Requirements: Transfer syringe replacement for ACAM2000 vaccine kits. Expiry is [**] from date of manufacture. This is a firm-fixed price line item. Guaranteed shelf life from delivery is [**] from the date of manufacture for transfer syringes. Provision of transfer syringe replacement under prior contracts is available under separate contract. ACAM 10-Yr 75A50119C00071 Page 10 of 33

Table 5: Task 4 - Vaccine Limited Re-Labeling Program 1004 (Option) 2004 (Option) 4004 (Option) 6004 (Option) 8004 (Option) Contract Year 10/01/19–09/30/20 10/01/20–09/30/21 10/01/22–09/30/23 10/01/24–09/30/25 10/01/26–09/30/27 Target Exercise-By [**] [**] [**] [**] [**] Date Delivery Target [**] Number of Vials [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] Unit Price (per vial) [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] Supplies, Services, and Requirements: This is a firm-fixed price line item. Complete prior to expiry at site; the number of vials is approximate. Annual stability testing of representative lots is incorporated into the unit price for ACAM2000 vaccine. Notes: B.1 As shown in the chart above, the Government agrees to purchase at least [**] doses of product in the base year for a total amount of $169,988,000, and to purchase a minimum of [**] doses (with a target amount of [**] doses) in each option year, if exercised. The Government maintains the right to purchase up to [**] doses per option year. B.2 As shown in the chart above, the pricing accounts for both annual escalation and tiered pricing, which is based on the quantity of the Government’s order. B.3 Delivery of product shall be FOB Destination. An updated and detailed delivery schedule will be provided to the SNS within [**] of annual award. Following delivery, SNS will provide Emergent written notification that it either accepts or rejects the delivered lot(s) within [**] of receipt. If formal acceptance or rejection is not received in the [**] period it shall be deemed to have been accepted. ACAM 10-Yr 75A50119C00071 Page 11 of 33

Section C - Statement of Work (SOW) Title: Warm based manufacturing and license maintenance for ACAM2000 Vaccine C.1 – Background and Need As a direct result of the September 11, 2001 tragedy and the increased perceived threat of bio- terrorist activities against the United States, the Department of Health and Human Services (DHHS) and the Assistant Secretary for Preparedness and Response (ASPR) have a need to maintain a contract with the experienced manufacturer to supply its Food & Drug Administration (FDA) licensed ACAM2000, Smallpox (Vaccinia) Vaccine, Live; the only domestically- produced such vaccine, to maintain its ability to manufacture and deliver the licensed vaccine up to [**] doses annually. C.2 – Purpose The purpose of this project is to maintain the ability to manufacture and deliver licensed ACAM2000, Smallpox (Vaccinia) Vaccine, Live. The ACAM2000 vaccine shall be manufactured within the United States (US) in accordance with current Good Manufacturing Practices (cGMP) guidelines and palletized kits of ACAM2000 Vaccine, diluent, bifurcated needles and transfer syringes delivered to the ASPR Strategic National Stockpile (SNS). C.3 – Scope of Work The Contractor, as an independent organization and not as an agent of the Government, shall furnish all labor, materials, supplies, facilities, equipment, transportation and travel necessary to manufacture and deliver listed doses of licensed ACAM2000 vaccine, in accordance with C.10. The contractor shall deliver an equivalent quantity of ancillaries (ACAM2000 diluent, bifurcated needles and transfer syringes for use in the administration of the ACAM2000 vaccine. The contractor shall comply with the following objectives: • (Task 1) Manufacture and deliver, Food & Drug Administration (FDA) licensed ACAM2000 under Biologic License Application (BLA) Submission Tracking Number (STN) 125158 from approved facilities with the timeframe and quantities in accordance with Task 1. • (Task 1) Maintain the agency-approved Stability testing structure which assesses the long-term safety and efficacy of the ACAM2000 Lyophilized Drug Product at the Strategic National Stockpile and supports [**] of shelf life [**] from the time of product manufacture. • (Task 1) Perform qualified vaccinia vaccine potency assay on ACAM2000. • (Task 2) Replace expiring ACAM2000 diluent as related to new doses supplied under this contract • (Task 3) Replace expiring transfer syringes as related to new doses supplied under this contract • (Task 4) Relabel vaccine to [**] shelf-life ACAM 10-Yr 75A50119C00071 Page 12 of 33

• (C.5.) Provide quarterly reports and update meetings by the [**] following the end of the quarter. C.4 – Task 1: Manufacturing and Delivery of ACAM2000 Vaccine The Contractor shall manufacture and deliver, in accordance with this Task 1, licensed ACAM2000, including ancillary components which shall be delivered to the SNS [**], and produced entirely in the US in accordance with this Statement of Work. The product shall meet all requirements as specified in the approved FDA license and any approved supplements or amendments thereto. Ancillary components include diluent, syringe and bifurcated needle for reconstitution of vaccine. One vial of diluent will be provided for USP for each vial of vaccine; one syringe for reconstitution of each vial of vaccine; and one bifurcated needle for each dose of vaccine. The vaccine and diluent will be labeled and packaged as outlined in the BLA and subsequently approved FDA license. Manufacture of Bulk Vaccine The contractor shall manufacture the cell cultures required for the domestic production of ACAM2000 at its US bulk vaccine manufacturing facility at the [**] bioreactor scale. The contractor shall perform all Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) on any equipment required to produce the vaccine. The domestic manufacturing of the ACAM2000 vaccine in Vero cells grown on micro carrier beads at the [**] scale shall be in accordance with the approved ACAM2000 BLA on file with the FDA. Vaccine Fill and Finish The Contractor shall maintain a validated filling, lyophilization, labeling and packaging of ACAM2000 at its US fill and finish facility and shall perform all IQ, OQ, and PQ on any additional equipment required at that facility and associated process development. Requirements for Packaging & Delivery The products shall be delivered as follows: ACAM2000, (Smallpox (Vaccinia) Vaccine, Live), shall be provided in a multiple dose [**]. Each vial shall contain [**]. Each vaccine box shall contain [**]. Each case shall contain [**]. The ACAM2000 vaccine shall have >[**] shelf life remaining at the time of delivery. ACAM2000 Diluent shall be provided as [**]. Each diluent box shall contain [**]. Each case shall contain [**]. The diluent shall have a [**] shelf life. The diluent should have > [**] of shelf life remaining at the time of delivery Bifurcated needles shall be supplied in boxes containing [**] needles each. Each case shall contain [**] bifurcated needles with an expiry date of [**] from date of manufacture. Transfer Syringes shall be provided as [**]needles for vaccine reconstitution. Each box shall contain [**] syringes and [**] syringes per case. Transfer syringes have an expiry date of [**] from the date ACAM 10-Yr 75A50119C00071 Page 13 of 33

of manufacture. The transfer syringes should have ≥[**] of shelf life remaining at the time of delivery. Complete, unopened cases of bifurcated needles and syringes will be provided. The additional quantity required to provide complete cases (rounded up quantity in excess of kit requirements) will be provided at no additional cost to the Government). The provided kit summary should note both the Emergent lot number and the manufacturer’s lot number for needles and syringes. FDA-approved ACAM2000 Package Insert and Medication Guides shall be provided per the license as a compact disk (CD). Emergent will work with the FDA to phase-out the CDs and provide a suitable electronic solution. The contractor shall notify the Contracting Officer’s Representative (COR) when the Package Insert or Medication Guide are revised and will ship the current PDF version with each delivery order. Boxes, cases and shelf cartons shall not contain mixed lot numbers. All pallets are to have the identical TiHi stack pattern except for the last final pallet per lot number. All ACAM2000 vaccine product shall be delivered on standard 48" by 40" pallet, not to exceed 60” in height, stretch wrapped and secured to pallet for safe transport. Prior to an ACAM2000 vaccine and ancillary delivery, the Kit Component Inventory Summary Sheet which provides the ACAM2000 Kit (vaccine, diluent, bifurcated needle and transfer syringe) Inventory Summary, shall be provided outlining the products in each delivery; lot numbers of each items; number of full and partial boxes, cases and pallets; and the total quantities of each items in vials or each. The contractor shall provide Certificates of Analysis and Certificates of Conformance for the vaccine at least [**] prior to shipment arriving at the SNS facility. The contractor shall also provide Certificates of Analysis and Vendor Certificate of Manufacturing (or Conformance) for the diluent prior to shipment arriving at the SNS facility. Driver information for each delivery truck shall be provided as soon as available to the SNS site. After the delivery, a documented review of the temperature data from the vaccine and diluent shipments will be provided. SNS Locations Delivery location shall be in accordance with United States Government (USG) instructions provided [**] prior to the shipment and are specific exclusively to Continental US (CONUS) locations. Locations outside CONUS may incur additional shipping/validation charges which will result in a contract modification to be performed prior to shipment. No more than [**] shipments [**]. Vaccine relabeling shall occur at current inventory locations unless otherwise agreed upon. Delivery shall also include ancillary items for reconstitution of each vial of vaccine (diluent, transfer syringe) as well as a bifurcated needle for administration of each dose of vaccine. Packaging, labeling and delivery shall be done according to the methodology and specifications ACAM 10-Yr 75A50119C00071 Page 14 of 33

outlined in the current BLA filed with the FDA and as outlined in Section C.6, C.8. Section D, Section E. Warranties A warranty shall be provided for vaccines, diluent, transfer syringes, and bifurcated needles delivered to the USG during this 10-year contract. • Vaccine will have ≥ [**] of shelf-life remaining at time of delivery • Diluent will have >[**] of shelf-life remaining at time of delivery. • The transfer syringes should have ≥[**] of shelf life remaining at the time of delivery. • Transfer Syringes and Needles are commercial products. In the event of supplier and/or delivery issues that result in shelf life not meeting minimum requirements, the parties will develop suitable alternatives in delivery and pricing. For ACAM2000 delivered during this contract, if it is determined that the ACAM2000 vials delivered do not meet the shelf life standard of [**] expiry from the date of manufacture (with a minimum of [**] of shelf life remaining at the time of delivery) and that USG has met its storage and handling obligations for such products pursuant to a separate Quality Agreement, then the Contractor will provide an equitable remedy based on the remaining shelf life of the non- conforming product(s), which will include one of the following: (a) replacement product (only during the contract period), (b) a credit against future purchases under the contract, or (c) reimbursement. Historical Lots-Testing Program and Quarantine Doses manufactured and distributed prior to 2018 had labeled expiry periods of less than the current [**] expiry. As these lots approach their labeled expiry dates, Emergent will coordinate with SNS and the SNS sites to re-label impacted lots with [**] expiry, per STN 103821/203 (approved 15 Dec 2017). Once lots have exceeded the FDA approved [**] expiry, they will be quarantined by SNS and will not be distributed for any use. Given the commitment for a long-term contract to replace the stockpile, Emergent will agree to test representative lots of quarantined product on an [**] basis and provide the data as For Information Only (FIO) to SNS until replenished stockpile inventory levels meet the desired threat assessment level. This information will be provided as soon as it is available and following the Government placing its annual optional CLIN order for at least [**] ACAM2000 doses. Under this Vaccine Testing Program, Emergent understands that, in the event of an emergency, the SNS would be responsible for seeking an appropriate regulatory mechanism to deploy for use quarantined product. • Emergent would follow our procedures in the event of an OOS (out of specification) result. Upon confirmation of an OOS, that information would be shared with the SNS; • Testing would continue only with the concurrent exercise of the option for product delivery; ACAM 10-Yr 75A50119C00071 Page 15 of 33
• Testing would only continue until the stockpile reaches the threat assessment level ([**] doses); • Based on the lots tested and the proposed delivery schedule, the maximum that lots would be tested is anticipated to be no more than [**] from the date of manufacture; The product expiration will remain at [**]. This testing will not be used to support any further extension to the product expiry dating. Under this agreement, the product maintained in the SNS stockpile beyond the shelf life of [**] is no longer considered to be under our license. Emergent is not making any representation or guarantees that the testing will support extensions to the shelf life and the testing data is not being provided to the SNS in support of expiry extensions. As a result, Emergent will also no longer be performing any activities beyond the submission of the data to the SNS. The testing data is not being provided to the SNS in support of expiry extensions, and any discussions with the FDA regarding the use of the stockpile in an emergency event or any use of this product will be captured under a different regulatory mechanism outside of Emergent’ s license and will be the responsibility of the SNS. The following table provides a summary detailing the strategy for managing expiring ACAM2000 vaccine: Table 6: Strategy for managing expiring ACAM2000 vaccine Category Proposed Strategy Older lots with < [**] expiry All lots can be managed as having [**] expiry per STN labeling and < [**] since date of 125158/203 (approved 15 Dec 2017). These lots will be manufacture re-labeled with [**] expiry per the schedule in Section C below. Once these lots exceed [**], the lots will be quarantined by SNS, per below. Older lots that have ≥ [**] since These lots have, or will have, exceeded the FDA approved date of manufacture expiry period and will be quarantined by SNS. The lots will not be distributed by ASPR for any purpose (vaccination, research, etc.) except under an approved appropriate regulatory mechanism. Once SNS has achieved its inventory goal for ACAM2000 vaccine, all quarantined expired doses should be destroyed. New lots with [**] expiry* All newly manufactured lots under this contract will be *It is expected the U.S. labeled with [**] expiry. Therefore, none of these lots will Government will deploy in date expire during the 10- year period of this contract. When vaccine first and quarantined the lots do expire in the future, the expired materials vaccine would only be deployed should be destroyed. after all in date product is depleted (and with the appropriate regulatory oversight). ACAM 10-Yr 75A50119C00071 Page 16 of 33

Task 2: Diluent Replacement The contractor shall replace expiring ACAM2000 diluent related this contract (see Task 2 table). Government to provide forecast [**] to confirm the quantity to be delivered Options may be exercised after the base year is awarded through year 10 at the quantities also outlined herein. If the quantity of diluent requiring replacement changes from the quantity outlined in Section C.6, both parties shall agree to the change. Note timing listed in C.6 are approximations as replacement timing is dependent of the manufacturing date of shipped quantities. The contractor shall deliver [**]. The diluent shall be packaged in [**] per case. The diluent shall have a [**] shelf life. At the time of delivery to the SNS the diluent should have >[**] of shelf life remaining. Task 3: Syringe Replacement • Transfer Syringe quantities will be in increments of a full case box (divisible by [**]). • Replacement of transfer syringes sold prior to this contract are outside the scope of this proposal and can be quoted separately upon request. Task 4: ACAM2000 Vaccine Limited Re-Labeling Activities The ACAM2000 vaccine currently has an expiration date of [**]. Emergent will re-label vaccine lots located at the Strategic National Stockpile (SNS) for lots produced by the [**] facility which have less than an expiry of [**] as they approach their current labeled expiration. (See table below). Quantities are estimates and will be verified prior to relabeling efforts. ACAM 10-Yr 75A50119C00071 Page 17 of 33

Table 7: Re-labeling of [**] Manufactured Lots with [**] expiry Over-label Expire Lot Grand Location 2020 2021 2023 2025 2027 Year Date Number Total [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] Grand [**] [**] [**] [**] [**] [**] [**] [**] Total ACAM 10-Yr 75A50119C00071 Page 18 of 33

C-5 – Reporting Requirements (Emergent) The contractor shall submit a Quarterly Progress Report, which shall include the information listed below that is applicable for the performance period during the quarter being reported. The contractor shall provide the Contracting Officer’s Representative (COR) with one electronic copy of the Quarterly Progress Report via e-mail. Any attachments to the report shall be submitted in Microsoft Word, Adobe Acrobat, or similar files. The contractor shall meet with the COR quarterly to discuss the Quarterly Progress Report. The contractor shall submit the Quarterly Reports and schedule the meetings by the [**] following the end of the quarter. The following shall be included in the quarterly report. 1. Quarterly Reporting Requirements Manufacturing and Delivery of ACAM2000® Kits a. Procurement and Production b. Quality Control Testing and Potency c. Quality Manufacturing Deviations (major) 2. FDA inspections, consultation results or recommendations and any files to the FDA concerning the ACAM2000 BLA 3. Security Assessment 4. Stability Program Assessment (Provided [**]) 5. Overall Project Assessment a. Delivery Summary b. Projected Deliveries for next reporting period c. Plan vs. Actual and Specific problems to address C.6 – Reporting Requirements (SNS) SNS shall provide the following information to Emergent at the frequency described below: 1. Doses delivered from SNS. This number is needed for safety reporting ([**]) 2. Destruction of expired lots of ACAM Vaccine. ([**] for record retention) 3. ACAM Vaccine lots in inventory, number of vials, and quantity in quarantine. ([**] for over-labelling planning) 4. ACAM Diluent lots in inventory, number of vials. ([**]) ACAM 10-Yr 75A50119C00071 Page 19 of 33

5. Note: Updated inventory on specific lots may be required as part of investigations throughout the contract. C.7. – Delivery Notifications Emergent shall notify the COR of the total quantity of product(s) and pallet count that will be delivered utilizing the Kit Component Inventory Summary Sheet, which provides the ACAM2000 Kit (vaccine, diluent, bifurcated needle and transfer syringe) Inventory Summary to the SNS at least [**] prior to each delivery. C.8 – Quality Inspections • Site Visits/Audits: The Government shall perform [**] site visits/security audits as deemed necessary by the Government throughout the period of performance of the contract. • Quality: The Government reserves the right to visit the contractor’s site for purposes of assessing quality on an [**] basis or as deemed necessary by the Government throughout the period of performance of the contract. • Notice: The Government will provide [**] advance notice prior to the Contractor of all site visits and audits. The notice will include a statement concerning the intended scope of the audit and a list of the required documents or access to personnel. Note: Facilities with live vaccine is in use (core production/testing areas) require vaccinations or waivers prior to entry. • All audits will be conducted between normal business hours i.e. 8 a.m. through 4 p.m., Monday through Friday. • Report to be provided by the Government as to any observations associated with site visits/audits. The government reserves the right to inspect any contractor or subcontractor facility used for the manufacture, packaging, storage, transportation or any other handling of products ordered as a result of this solicitation without prior notice. These inspections do not replace any required inspections conducted by the FDA but are in addition to such inspections. The contractor will be required to respond to any finding’s resultant from these inspections with remediation plans or an explanation of why no remediation is required. Section D - Packaging And Marking Packaging shall be consistent with the FDA approved labeling and packaging for this product at the time of manufacture. Section E - Inspection And Acceptance E.1. – FAR 52.252-2 Clauses Incorporated by Reference (1998) ACAM 10-Yr 75A50119C00071 Page 20 of 33

This contract incorporates one or more clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available. Also, the full text of a clause may be accessed electronically at this/these address (es): https://www.acquisition.gov FAR SOURCE TITLE AND DATE 52.246-1 Contractor Inspection Requirements (Apr 1984) 52.246-2 Inspection of Supplies – Fixed Price (Aug 1996) 52.246-4 Inspection of Services – Fixed Price (Aug 1996) 52.246-16 Responsibility for Supplies (Apr 1984) E.2. – Inspection and Acceptance Inspection and acceptance of the articles, services, and documentation called for herein shall be accomplished by the Contracting Officer, or his duly authorized representative (who for the purposes of this contract shall be the Project Officer/COR) at the destination of the articles, services or documentation. The Contractor shall only tender for acceptance those items that conform to the requirements of this contract. The Government reserves the right to inspect or test any supplies or services that have been tendered for acceptance. The Government may require repair or replacement of nonconforming supplies or re-performance of nonconforming services at no increase in contract price. The Government must exercise its post-acceptance rights: • Within a reasonable time after the defect was discovered or should have been discovered; and • Before any substantial change occurs in the condition of the item, unless the change is due to the defect in the item. • Goods damaged by Government personnel during product receipt is not covered under warranty. • Note: Needles and Syringes are provided as complete, unopened cases. As such, excess quantities required due to rounding to the nearest whole case will be supplied as no cost to the government. E.3. – Shipment Acceptance Shipment Acceptance is defined by two activities: 1) Signed review of delivered materials by SNS (FRM041013 or other written notification) • Confirmation of quantity of all kit components • Confirmation that the materials are labeled correctly (with lot #, date of manufacture and/or expiration date) ACAM 10-Yr 75A50119C00071 Page 21 of 33

• Confirmation that CoAs are available for each lot • Confirmation there is no damage, leakage or moisture, or any other defects associated with the pallets and boxes. 2) Review of shipping temperature for the ACAM2000 vaccine and diluent by Emergent Quality Assurance. (FRM044234) Emergent may provide staff to assist in the delivery inspection at no additional cost to the government. This activity is not a requirement of delivery. SNS will provide Emergent written notification (either by signed FRM041013 or other written notification) that it either accepts or rejects the delivered lot(s) within [**] of receipt. If formal acceptance or rejection is not received in the [**] period, it shall be deemed the shipment is accepted. Section F - Deliveries Or Performance FAR SOURCE TITLE AND DATE 52.211-17 Delivery of Excess Quantities (Sept 1989) 52.242-15 Stop-Work Order (Aug 1989) 52.242-17 Government Delay of Work (Apr 1984) 52.247-34 FOB Destination (Nov 1991) F.1. – Period of Performance The period of performance of this contract shall be a base period and nine 12-month option periods as follows: 08/30/2019 – 08/29/2020 (Base) 08/30/2024 – 08/29/2025 (Option Year 5) 08/30/2020 – 08/29/2021 (Option Year 1) 08/30/2025 – 08/29/2026 (Option Year 6) 08/30/2021 – 08/29/2022 (Option Year 2) 08/30/2026 – 08/29/2027 (Option Year 7) 08/30/2022 – 08/29/2023 (Option Year 3) 08/30/2027 – 08/29/2028 (Option Year 8) 08/30/2023 – 08/29/2024 (Option Year 4) 08/30/2028 – 08/29/2029 (Option Year 9) F.2. – Delivery Locations Finished products shall be delivered by the Contractor to any one of the [**] current SNS sites. Site Locations to be provided [**] in advance of delivery. Section G - Contract Administration Data G.1. – Electronic Subcontracting Reporting System (eSRS) The Contractor shall register with the Electronic Subcontracts Reporting System (eSRS) for the submission of its Individual Subcontract Report (SF 294) and the Annual Summary Reports (SF 295). Before registering in eSRS, the Contractor information must be correct in the System for ACAM 10-Yr 75A50119C00071 Page 22 of 33

Award Management database. The eSRS is a world wide web-based application available at: http://www.esrs.gov. The eSRS website provides training and instruction for data submission. G.2. – Invoice Submission Invoices should be submitted electronically (.pdf) simultaneously to [**], Contracting Officer (CO) at ([**]), [**], Contracting Officer’s Representative (COR) at ([**]), and the Program Support Center (PSC) at psc_invoices@psc.hhs.gov. Invoice requirements (from FAR 32.905) (a) General. Payment will be based on receipt of a proper invoice and satisfactory contract performance. (b) content of invoices. (1) A proper invoice must include the following items: (i) Name and address of the contractor. (ii) invoice date and invoice number. (Contractors should date invoices as close as possible to the date of mailing or transmission.) (iii) Contract number or other authorization for supplies delivered or services performed (including order number and line item number). (iv) Description, quantity, unit of measure, unit price, and extended price of supplies delivered, or services performed. (v) Shipping and payment terms (e.g., shipment number and date of shipment, discount for prompt payment terms). Bill of lading number and weight of shipment will be shown for shipments on government bills of lading. (vi) Name and address of contractor official to whom payment is to be sent (must be the same as that in the contract or in a proper notice of assignment). (vii) Name (where practicable), title, phone number, and mailing address of person to notify in the event of a defective invoice. (viii) Taxpayer identification number (TIN). The contractor must include its tin on the invoice. (ix) Electronic funds transfer (EFT) banking information. G.3. – Contracting Officer The Contracting Officer is the only individual who can legally commit the Government to the expenditure of public funds. No person other than the Contracting Officer can make any changes to the terms, conditions, general provisions, or other stipulations of this contract. No information, ACAM 10-Yr 75A50119C00071 Page 23 of 33

other than that which may be contained in an authorized modification to this contract, duly issued by the Contracting Officer, which may be received from any person employed by the United States Government, or otherwise, shall be considered grounds for deviation from any stipulation of this contract. G.4. – Contracting Officer’s Representative Performance of the work hereunder shall be subject to the technical directions of the designated Contracting Officer’s Representative (COR) for this contract. As used herein, technical directions are directions to the Contractor which fill in details, suggests possible lines of inquiry, or otherwise completes the general scope of work set forth herein. These technical directions must be within the general scope of work and may not alter the scope of work or cause changes of such a nature as to justify an adjustment in the stated contract price/cost, or any stated limitation thereof. In the event that the Contractor feels that full implementation of any of these directions may exceed the scope of the contract, he or she shall notify the originator of the technical direction and the Contracting Officer in a letter separate of any required report(s) within [**] of the date of receipt of the technical direction and no action shall be taken pursuant to the direction. If the Contractor fails to provide the required notification within the said [**] period that any technical direction exceeds the scope of the contract, then it shall be deemed for purposes of this contract that the technical direction was within the scope. No technical direction, nor its fulfillment, shall alter or abrogate the rights and obligations fixed in this contract. The Government COR is not authorized to change any of the terms and conditions of this contract. Changes shall be made only by the Contracting Officer by properly written modification(s) to the contract. The Government will provide the Contractor with a copy of the delegation memorandum for the COR. Any changes in COR delegation will be made by the Contracting Officer in writing with a copy being furnished to the Contractor. G.5 – Contract Communications/Correspondence The Contractor shall identify all correspondence, reports, and other data pertinent to this contract by imprinting thereon the contract number from Page 1 of the contract 75A50119C00071. Section H - Special Contract Requirements H.1. – Evaluation of Contractor Performance Utilizing CPARS (April 2015) In accordance with FAR 42.15, the SNS will review and evaluate contract performance. FAR 42.1502 and 42.1503 requires agencies to prepare evaluations of contractor performance and submit them to the Past Performance Information Retrieval System (PPIRS). The SNS utilizes the Department of Defense (DOD) web-based Contractor Performance Assessment Reporting System (CPARS) to prepare and report these contractor performance evaluations. All information contained in these assessments may be used by the Government, within the limitations of FAR 42.15, for future source selections in accordance with FAR 15.304 where past performance is an evaluation factor. ACAM 10-Yr 75A50119C00071 Page 24 of 33

The CPARS system requires a contractor representative to be assigned so that the contractor has appropriate input into the performance evaluation process. The CPARS contractor representative will be given access to CPARS and will be given the opportunity to concur or not-concur with performance evaluations before the evaluations are complete. The CPARS contractor representative will also have the opportunity to add comments to performance evaluations. The assessment is not subject to the Disputes clause of the contract, nor is it subject to appeal beyond the review and comment procedures described in the guides on the CPARS website. Refer to: www.cpars.gov for details and additional information related to CPARS, CPARS user access, how contract performance assessments are conducted, and how Contractors participate. Access and training for all persons responsible for the preparation and review of performance assessments is also available at the CPARS website. The contractor must provide the SNS contracting office with the name, e-mail address, and phone number of their designated CPARS representative who will be responsible for logging into CPARS and reviewing and commenting on performance evaluations. The contractor must maintain a current representative to serve as the contractor representative in CPARS. It is the contractor’s responsibility to notify the SNS contracting office, in writing (letter or email), when their CPARS representative information needs to be changed or updated. Failure to maintain current CPARS contractor representative information will result in the loss of an opportunity to review and comment on performance evaluations. H.2. – CDC37.0001 Non-Personal Services (April 2015) Personal services shall not be performed under this contract. Although the Government may provide sporadic or occasional instructions within the scope of the contract, the Contractor is responsible for control and supervision of its employees. If the Contractor (including its employees) believes any Government action or communication has been given that would create a personal services relationship between the Government and any Contractor employee, the Contractor shall promptly notify the Contracting Officer of this communication or action. The contractor shall comply with, and ensure their employees and subcontractors comply with, SNS Policy titled “Identification of Contractors' Employees and Safeguarding Government Information.” No Contractor employee shall hold him or herself out to be a Government employee, agent, or representative. No Contractor employee shall state orally or in writing at any time that he or she is acting on behalf of the Government. In all communications with third parties in connection with this contract, Contractor employees shall identify themselves as Contractor employees and specify the name of the company for which they work. The contractor is limited to performing the services identified in the contract statement of work and shall not interpret any communication with anyone as a permissible change in contract scope or as authorization to perform work not described in the contract. All contract changes will be incorporated by a modification signed by the Contracting Officer. The Contractor shall ensure that all of its employees and subcontractor employees working on this contract are informed of the substance of this clause. The Contractor agrees that this is a non-personal services contract; and that for all the purposes of the contract, the Contractor is not, nor shall it hold itself out to be an agent or partner of, or joint venture with, the Government. The ACAM 10-Yr 75A50119C00071 Page 25 of 33

Contractor shall notify its employees that they shall neither supervise nor accept supervision from Government employees. The substance of this clause shall be included in all subcontracts at any tier. Nothing in this clause shall limit the Government's rights in any way under any other provision of the contract, including those related to the Government's right to inspect and accept or reject the services performed under this contract. H.3. – Restrictions on Disclosure of Information and Rights in Data Information made available to the Contractor by the Government for the performance or administration of this effort shall be used only for those purposes and shall not be used in any other way without the written agreement of the Contracting Officer. The Contractor agrees to assume responsibility for protecting the confidentiality of Government records, which are not public information. Each Contractor or employee of the Contractor to whom information may be made available or disclosed shall be notified in writing by the Contractor that such information may be disclosed only for a purpose and to the extent authorized herein. Contractor and/or contractor personnel shall not divulge or release data or information developed or obtained in performance of this effort, until made public by the Government, except to authorize Government personnel or upon written approval of the Contracting Officer or COR. The contractor shall not use, disclose, or reproduce proprietary data that bears a restrictive legend, other than as required in the performance of this effort. Nothing herein shall preclude the use of any data independently acquired by the contractor without such limitations or prohibit an agreement at no cost to the Government between the contractor and the data owner which provides for greater rights to the contractor. H.4. – Liability Protection under the PREP Act The Public Readiness & Emergency Preparedness Act (PREP Act), Pub. L. 109-148, Division C, 119 Stat. 2818 to 2832, amended the Public Health Service Act, 42, U.S.C. 243 et seq., to provide targeted liability protections. The Government agrees that the medical countermeasure delivered by the Contractor under this contract will be administered in humans, in accordance with the declaration under the PREP Act issued by the Secretary of the Department of Health and Human Services on December 9, 2015 pursuant to section 319F-3(b) of the Public Health Service Act, 42, U.S.C 247-d-6d regarding Smallpox Medical Countermeasures- Amendment. The declaration provides targeted liability protections for smallpox countermeasures based on a credible risk that the threat of exposure to smallpox and the resulting disease constitutes a public health emergency. For purposes of this provision, “Vaccine” means ACAM2000 vaccine with its labeling, packaging, diluent, needles, and any other of their respective components as well as any other deliverable under this contract. The Vaccine may not be used outside of the United States until the Contractor and the Government agree to reasonable liability protections for outside the United States and the Contract has been modified to reflect that agreement; such contract modification will be a change for which the Contractor will be entitled to an equitable adjustment in accordance with FAR 52.243-1. Additionally, the USG further agrees, in ACAM 10-Yr 75A50119C00071 Page 26 of 33

accordance with Federal rules and regulations, to provide written notice to the Contractor of any intended use of the Vaccine delivered under this contract. In the event that a claim or suit for damages is brought against the Contractor by a third party arising out of its performance of this contract, the USG will provide reasonable and timely access to documents and information potentially relevant to the Contractor’s assertion of defenses to any dispute with a third party, including, but not limited to, a claim for bodily injury or other damages allegedly arising out of the use of the Vaccine delivered to the USG under this contract to the extent permitted by Federal rules and regulations. The USG will consider any request from the Contractor to assist the Contractor in litigation, including a request to support the Contractor’s assertion of appropriate defenses. If and when appropriate, the USG will file papers in support of the Contractor’s assertion of its defenses in such disputes. H.5. – SNS Responsibilities Please refer to QUAL40041 (Quality Agreement, SNS) for Emergent and USG responsibilities regarding the availability of the most current FDA approved Package Insert and Medication Guide. Emergent and SNS agree that this Quality Agreement is applicable to this contract. Note that after contract award the Quality Agreement will be updated to reflect any changes in the SNS organizational structure, COR and items no longer applicable to SNS. H.6. – Rights of the Contractor to Market Smallpox Vaccine The Contractor is not prohibited from marketing the Smallpox Vaccine being sold to SNS under this Contract provided that such marketing does not interfere with performance under this Contract. H.7. – Expired Doses in the SNS During the term of this contract, any and all expired doses will be held in quarantine by SNS and will not be distributed for any purpose (e.g., vaccination, research, etc.) except under an appropriate regulatory mechanism. SNS will be solely responsible for obtaining said appropriate regulatory mechanism and maintaining it as may be required. Once SNS inventory needs are met with non-expired doses, SNS will be responsible for the destruction of the expired doses under the appropriate regulatory mechanism and any future expiring lots of ACAM2000 vaccine. This Provision does not diminish any Contractor protection granted by Special Provision H.4 above and is in addition to it. Accordingly, all protections to Emergent granted by Special Provision H.4 are fully applicable to any and all Government use of such expired doses held in quarantine by SNS. H.8 – Testing of Quarantined Product Given the commitment for a long-term contract to replace the stockpile, Emergent will agree to test representative lots of quarantined product on an [**] basis and provide the data as “For Information Only” (FIO) to SNS until replenished stockpile inventory levels meet the desired threat assessment level. Under this Vaccine Testing Program, Emergent understands that, in the event of an emergency, the SNS would be responsible for seeking an appropriate regulatory mechanism from the FDA to deploy for use Quarantined product. ACAM 10-Yr 75A50119C00071 Page 27 of 33

• Emergent would follow our procedures in the event of an OOS result. Upon confirmation of an OOS, that information would be shared with the SNS. • Testing would continue only with the concurrent exercise of the option for product delivery. • Testing would only continue until the stockpile reaches the threat assessment level. • Based on the lots tested and the proposed delivery schedule, the maximum that lots would be tested is anticipated to be no more than [**] from the date of manufacture. • The product expiration will remain at [**]. This testing will not be used to support any further extension to the product expiry dating. Under this agreement, the product maintained in the SNS stockpile beyond the shelf life of [**] is no longer considered to be under our license. Emergent is not making any representation or guarantees that the testing will support extensions to the shelf life and the testing data is not being provided to the SNS in support of expiry extensions. As a result, Emergent will also no longer be performing any activities beyond the submission of the data to the SNS. Any discussions with the FDA regarding the use of the stockpile in an emergency event or any use of this product will be captured under a different regulatory mechanism outside of Emergent’ s license and will be the responsibility of the SNS. ACAM 10-Yr 75A50119C00071 Page 28 of 33
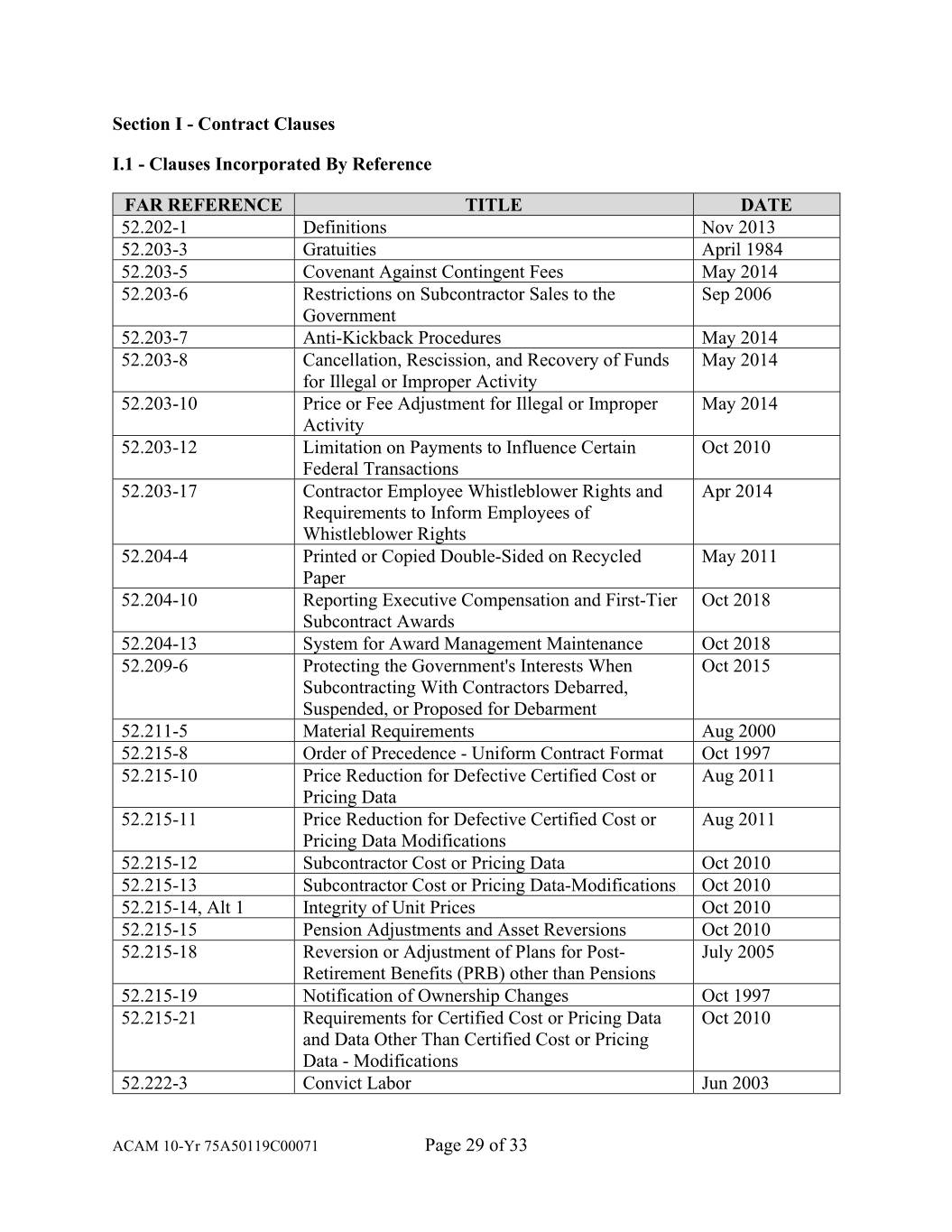
Section I - Contract Clauses I.1 - Clauses Incorporated By Reference FAR REFERENCE TITLE DATE 52.202-1 Definitions Nov 2013 52.203-3 Gratuities April 1984 52.203-5 Covenant Against Contingent Fees May 2014 52.203-6 Restrictions on Subcontractor Sales to the Sep 2006 Government 52.203-7 Anti-Kickback Procedures May 2014 52.203-8 Cancellation, Rescission, and Recovery of Funds May 2014 for Illegal or Improper Activity 52.203-10 Price or Fee Adjustment for Illegal or Improper May 2014 Activity 52.203-12 Limitation on Payments to Influence Certain Oct 2010 Federal Transactions 52.203-17 Contractor Employee Whistleblower Rights and Apr 2014 Requirements to Inform Employees of Whistleblower Rights 52.204-4 Printed or Copied Double-Sided on Recycled May 2011 Paper 52.204-10 Reporting Executive Compensation and First-Tier Oct 2018 Subcontract Awards 52.204-13 System for Award Management Maintenance Oct 2018 52.209-6 Protecting the Government's Interests When Oct 2015 Subcontracting With Contractors Debarred, Suspended, or Proposed for Debarment 52.211-5 Material Requirements Aug 2000 52.215-8 Order of Precedence - Uniform Contract Format Oct 1997 52.215-10 Price Reduction for Defective Certified Cost or Aug 2011 Pricing Data 52.215-11 Price Reduction for Defective Certified Cost or Aug 2011 Pricing Data Modifications 52.215-12 Subcontractor Cost or Pricing Data Oct 2010 52.215-13 Subcontractor Cost or Pricing Data-Modifications Oct 2010 52.215-14, Alt 1 Integrity of Unit Prices Oct 2010 52.215-15 Pension Adjustments and Asset Reversions Oct 2010 52.215-18 Reversion or Adjustment of Plans for Post- July 2005 Retirement Benefits (PRB) other than Pensions 52.215-19 Notification of Ownership Changes Oct 1997 52.215-21 Requirements for Certified Cost or Pricing Data Oct 2010 and Data Other Than Certified Cost or Pricing Data - Modifications 52.222-3 Convict Labor Jun 2003 ACAM 10-Yr 75A50119C00071 Page 29 of 33
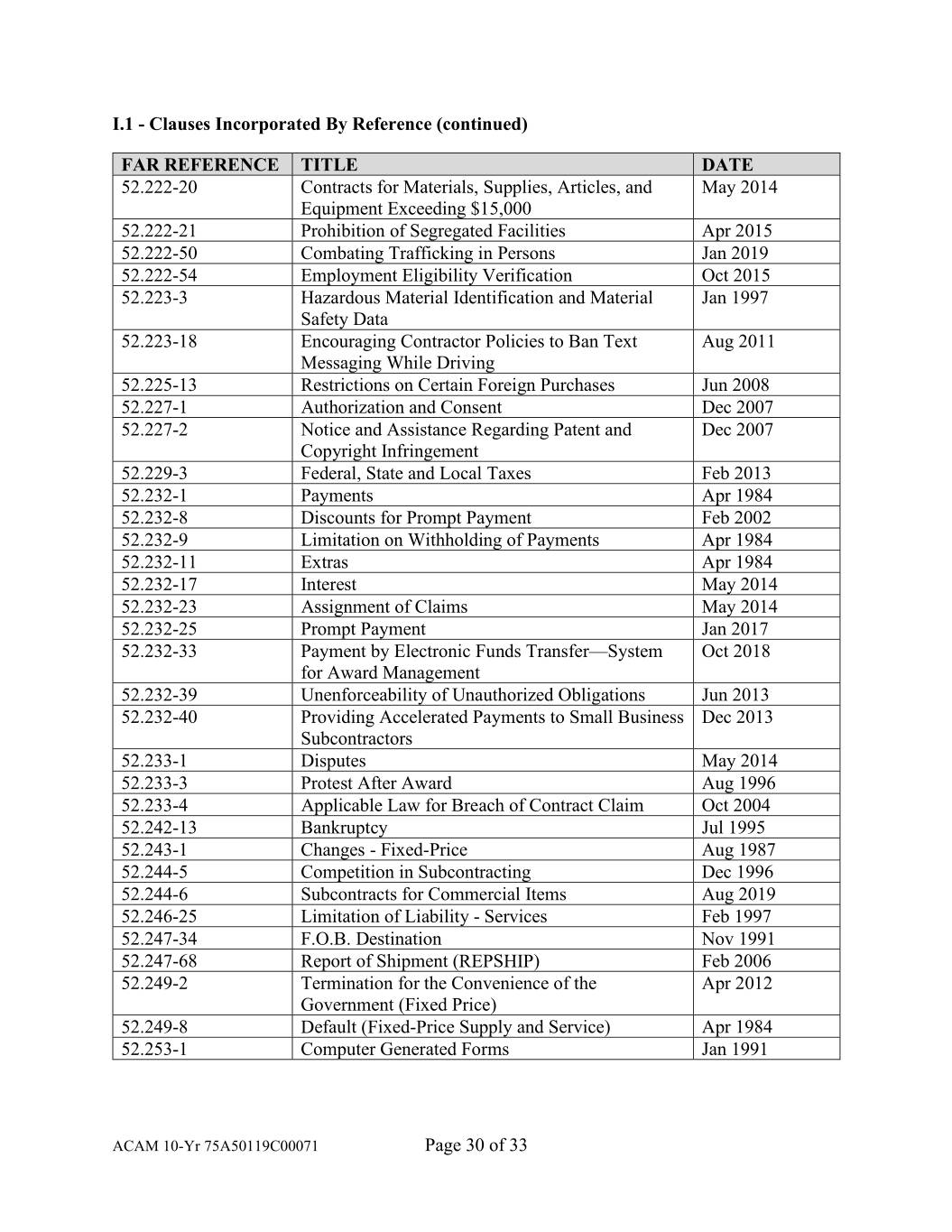
I.1 - Clauses Incorporated By Reference (continued) FAR REFERENCE TITLE DATE 52.222-20 Contracts for Materials, Supplies, Articles, and May 2014 Equipment Exceeding $15,000 52.222-21 Prohibition of Segregated Facilities Apr 2015 52.222-50 Combating Trafficking in Persons Jan 2019 52.222-54 Employment Eligibility Verification Oct 2015 52.223-3 Hazardous Material Identification and Material Jan 1997 Safety Data 52.223-18 Encouraging Contractor Policies to Ban Text Aug 2011 Messaging While Driving 52.225-13 Restrictions on Certain Foreign Purchases Jun 2008 52.227-1 Authorization and Consent Dec 2007 52.227-2 Notice and Assistance Regarding Patent and Dec 2007 Copyright Infringement 52.229-3 Federal, State and Local Taxes Feb 2013 52.232-1 Payments Apr 1984 52.232-8 Discounts for Prompt Payment Feb 2002 52.232-9 Limitation on Withholding of Payments Apr 1984 52.232-11 Extras Apr 1984 52.232-17 Interest May 2014 52.232-23 Assignment of Claims May 2014 52.232-25 Prompt Payment Jan 2017 52.232-33 Payment by Electronic Funds Transfer—System Oct 2018 for Award Management 52.232-39 Unenforceability of Unauthorized Obligations Jun 2013 52.232-40 Providing Accelerated Payments to Small Business Dec 2013 Subcontractors 52.233-1 Disputes May 2014 52.233-3 Protest After Award Aug 1996 52.233-4 Applicable Law for Breach of Contract Claim Oct 2004 52.242-13 Bankruptcy Jul 1995 52.243-1 Changes - Fixed-Price Aug 1987 52.244-5 Competition in Subcontracting Dec 1996 52.244-6 Subcontracts for Commercial Items Aug 2019 52.246-25 Limitation of Liability - Services Feb 1997 52.247-34 F.O.B. Destination Nov 1991 52.247-68 Report of Shipment (REPSHIP) Feb 2006 52.249-2 Termination for the Convenience of the Apr 2012 Government (Fixed Price) 52.249-8 Default (Fixed-Price Supply and Service) Apr 1984 52.253-1 Computer Generated Forms Jan 1991 ACAM 10-Yr 75A50119C00071 Page 30 of 33

HHSAR Reference TITLE DATE HHSAR 352.203-70 Anti-Lobbying Dec 2015 HHSAR 352.208-70 Printing and Duplication Dec 2015 HHSAR 352.224-71 Confidential Information Dec 2015 HHSAR 352.239-74 Electronic and Information Technology Dec 2015 Accessibility ACAM 10-Yr 75A50119C00071 Page 31 of 33

FAR 52.217-8 Option to Extend Services (NOV 1999) The Government may require continued performance of any services within the limits and at the rates specified in the contract. These rates may be adjusted only as a result of revisions to prevailing labor rates provided by the Secretary of Labor. The option provision may be exercised more than once, but the total extension of performance hereunder shall not exceed [**]. The Contracting Officer may exercise the option by written notice to the Contractor within [**]. (End of Clause) FAR 52.217-9 Option to Extend the Term of the Contract (Mar 2000) (a) The Government may extend the term of this contract by written notice to the Contractor within [**] provided that the Government gives the Contractor a preliminary written notice of its intent to extend at least [**] before the contract expires. The preliminary notice does not commit the Government to an extension. (b) If the Government exercises this option, the extended contract shall be considered to include this option clause. (c) The total duration of this contract, including the exercise of any options under this clause, shall not exceed [**]. (End of Clause) ACAM 10-Yr 75A50119C00071 Page 32 of 33
Section J - List Of Attachments Table 8: Kit Reference (including Shelf Life) Component Quantity Expiry Shelf life Part#/ Box and Case Pallet Product at time of Supplier Location and Delivery Temperature Requirements ACAM2000 [**] [**] [**] [**] [**] [**] [**] Vaccine [**] ACAM2000 [**] [**] [**] [**] [**] [**] [**] Diluent [**] Bifurcated [**] [**] [**] [**] [**] [**] [**] Needle Transfer [**] [**] [**] [**] [**] [**] [**] Syringe [**] ACAM 10-Yr 75A50119C00071 Page 33 of 33
































